
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 24 January 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.759086
This article is part of the Research Topic New Progresses of Fish Meal Replacement in Aquatic Animals View all 16 articles
This study was conducted to investigate the effects of substitution of dietary fishmeal (FM) by compound plant protein supplemented with essential amino acids on growth performance, plasma physiology, and muscle growth-related genes of gibel carp (Carassius auratus gibelio). Four diets with equal digestible protein were prepared, where 30FM (control feed) contained 30% FM and land animal protein as a protein source, 10FM contained 10% FM, PMAa contained full plant meal (PM) supplemented with crystalline amino acid, and PM contained full PM feed. There was no significant difference in the specific growth rate (SGR) with 30FM, 10FM, and PMAa diets (p > 0.05); however, the SGR of PM group was the lowest with significant difference (p < 0.05). Feed efficiency of the PM group was the lowest with significant difference (p < 0.05). The whole-body crude protein content of fish in PMAa group was significantly higher than that in each group with additional FM (p < 0.05). There were no significant differences in plasma total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) or low-density lipoprotein cholesterol (LDL-C), and free amino acid profile of 30FM, 10FM, and PMAa groups (p > 0.05); however, they were significantly higher than that in PM group (p < 0.05). The expression of key genes in the TOR signaling pathway (tor, s6k1, 4e-bp2, and eif4e), autophagy (ulk1 and atg13), and ubiquitin–proteasome (trim63 and znrf2) system of the PMAa group was similar to that of the FM group (p > 0.05), indicating that the muscle growth-related biomarker genes were positively regulated by the appropriate dietary amino acid composition at the transcriptional level. These results suggest that FM in gibel carp diet can be totally replaced by PM without negative impact on growth performance and muscle growth-related biomarkers at the transcriptional level, which provided the requirement of digestible protein and balanced amino acid profile is satisfied.
Over the past decades, aquaculture has been growing rapidly (Verhoeven et al., 2016) and has been a large contributor of animal protein for humans. Fishmeal (FM), which is not only a superior protein source in aquaculture but also a limited resource, plays an important role in aquaculture feed industry (Bu et al., 2018). The long-term sustainability of FM is a serious concern in the aquaculture industry. For sustainable development of aquaculture, more attention has been focused on replacing FM with plant feedstuffs (Benedito-Palos et al., 2007).
Previously, particularly over the past two decades, several studies have been conducted to determine alternative protein sources for substituting FM (Dossou et al., 2018; Inanan and Acar, 2019). It has been confirmed that the content of dietary FM could be reduced using plant ingredients in many cultured species. The addition of FM is low in the commercial feed formulation for omnivorous species. However, studies shown that FM was still the most optimal protein source in the diet and leads to the best growth performance for omnivorous species, such as tilapia (Oreochromis niloticus, Ahmad et al., 2020; Ismail et al., 2020) and carp (Cyprinus carpio, Wang et al., 2020), even herbivorous species, such as grass carp (Ctenopharyngodon idella, Jiang et al., 2016). Few reports are available to guide the total replacement of FM by plant ingredients in Cyprinidae species, which are widely cultured in China. The use of plant protein sources has been reported to induce intestinal inflammation in gibel carp (Liu et al., 2017) and grass carp (Duan et al., 2019), and abnormal cholesterol metabolism in blunt snout bream (Megalobrama amblycephala) (Xu et al., 2017). There are several factors restricting the usage of plant materials in aquatic feeds, such as amino acid imbalances, antinutritional factors (Tibbetts et al., 2017), and low mineral and high cellulose content (Hansen et al., 2007).
Several technologies have been developed to further increase the plant content in feeds, such as the use of mixed plant ingredients, supply of amino acids, steam modulation, and extrusion technology. Multiple usages of several techniques can ensure the supply of the essential amino acid, increase palatability of the diets, and decrease the levels of antinutritional factors (Gao et al., 2019). Albrektsen et al. (2006) used a complex protein source substitution (a mixture of soybean meal and corn gluten) and found that it has no effect on the growth and feed intake of farmed fish. Some studies have shown that by supplementing feed with exogenous essential amino acids, a few plant protein sources can partially or completely replace dietary FM (Kaushik et al., 1995, 2004; Jiang et al., 2016) and can promote growth performance by improving palatability and protein synthesis (Hansen et al., 2007). Several studies have revealed the importance of feed processing in aquaculture, and that the content of antinutritional factors in feed can be reduced by some processing techniques such as dry, wet heating, and extrusion (Li et al., 2018).
Feed is the main source of amino acids, which are important substrates for protein synthesis and other nitrogenous compounds, which are also considered as signaling molecules that participate in the regulation of major metabolic pathways (Gomes et al., 1993; National Research Council [NRC], 2011). Many studies have demonstrated that the target of rapamycin (TOR) signaling pathway plays a key role in nutrient perception and energy and protein metabolism (Wullschleger et al., 2006; Crovetto, 2010; Sun et al., 2019). Amino acids can activate the TOR complex 1 (tor) (Jobgen et al., 2006; Xia et al., 2016), then upregulate the downstream ribosomal protein S6 kinase 1 (s6k1), and suppress the expression level of eukaryotic translation factor 4E-binding protein 2 (4e-bp2) and eukaryotic translation initiation factor 4E (eif4e) (Zhang et al., 2018).
In addition, imbalanced amino acid concentrations can result in protein degradation (Hansen et al., 2007), which helps to redistribute nutrients from unnecessary processes to critical processes to maintain the quality of proteins (Akiko et al., 2004; Yuan et al., 2019). There are two main protein degradation pathways in living organisms: autophagy and ubiquitin–proteasome system. Autophagy-related protein 13-like (atg13) and serine/threonine-protein kinase ULK1 (ulk1) play important roles in the autophagy pathway (Chan et al., 2009; Uddin et al., 2012). E3 ubiquitin-protein ligase TRIM63 (trim63) and E3 ubiquitin-protein ligase ZNRF2 (znrf2) genes are the important biomarkers in the ubiquitin–proteasome system.
China produced about 2.7 million tons of Carassius spp. each year, about 2 million tons of them are gibel carp (Fisheries Bureau, Department of Agriculture of China, 2020). Gibel carp is an economical freshwater fish species that is known for its high yield, fast growth, and stronger resistibility and is widely cultivated in China (Gui and Zhou, 2010). Although gibel carp is a typical omnivorous fish, Xie et al. (2001) and Liu et al. (2016) have revealed that the best growth performance of gibel carp is obtained with diets containing high FM because of balanced amino acid profile and the absence of terrestrial, plant-derived, antinutritional factors. Thus, the purpose of this study is to reduce the amount of FM in gibel carp diets based on amino acid requirement and investigate the effects of low- or no-FM diets on growth performance, plasma physiology, the content of free amino acids in dorsal muscles and blood, and the expression of some genes involved in muscle protein synthesis and degradation.
Four diets with equal digestible protein and crude lipid were prepared. FM, chicken meal, bone meal, and blood meal were used as the animal protein sources. Seven plant proteins [soybean meal, rapeseed meal, cottonseed meal, distillers dried grains with soluble (DDGS), wheat gluten, soy protein concentrates, and corn gluten meal] were used as the plant protein sources. The chemical compositions and crude protein digestibility of the protein sources are shown in Table 1.
Diet formulations are shown in Table 2. Diet 30FM contained 30% FM and 22% terrestrial animal protein. Diet 10FM contained 10% FM and 45.4% vegetable protein. Diet PMAa and PM both contained 53.9% vegetable protein. Each vegetable protein source was added restrictedly to reduce antinutritional factors. Blood meal was added to low-FM diets to compensate for low amino acid content. Crystalline amino acids (L-lysine, L-methionine, and L-threonine) were added to PMAa to meet the requirements of the fish. Yeast hydrolyzate and spirulina (Arthrospira platensis) were added to 10FM, PMAa, and PM as immunopotentiators.
All ingredients of each diet were mixed, ground through a particle size of 100 mesh, completely mixed again, and then extruded using a twin-screwed extruder (Jinan Dingrun Machinery Co., Ltd., Jinan, Shandong, China). The pellets were stored at 4°C after drying in an oven at 60°C. The amino acid compositions of the diets are shown in Table 3.
All experimental animal care protocols were approved by the Ethics Committee of the Institute of Hydrobiology, Chinese Academy of Sciences. The feeding trial was conducted at Shishou Original Seed Stock Farm of Four Major Carps (Shishou, China). The gibel carp was obtained from Guanqiao hatchery of Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, Hubei, China). The experimental fishes were acclimated for 4 weeks before the feeding trial. All fish were fed with commercial feed two times a day during acclimation. At the beginning of the trial, fish were fasted for 24 h. Fish with apparent healthy and similar size (initial body weight: 14.98 ± 0.04 g) were randomly distributed into 12 floating net cages (2.0 m × 2.0 m × 2.0 m, water depth: 1.7 m) in the center of pond (70 m × 30 m × 3.5 m, water depth: 3 m), and 60 fishes were allocated in each cage. The diets were randomly assigned to triplicate floating net cages. During the feeding experiment, fish were hand-fed to apparent satiation two times a day at 8:30 and 16:30 for 45 days. The water temperature was recorded daily and maintained from 27 to 32°C during the trial. A part of the pond water was renewed every day. The dissolved oxygen was 5.0–7.5 mg/L and PH was 7.2–7.3, ammonia nitrogen kept under 0.1 mg/L throughout the feeding trial. The photoperiod was subjected to nature.
At the beginning of the trial, three fishes of each net cage were sampled randomly and stored at −20°C for initial body composition analysis. At the end of the experiment, all fish from each cage were weighed after depriving of food for 1 day. A total of two fishes of each cage were sampled randomly and also stored at −20°C for final body composition analysis. Another two individuals were anesthetized with MS-222 (100 mg L–1 tricaine methane sulfonate, Argent Chemical Laboratories Inc., Redmond, WA, United States). The tail venous blood of two fishes was drew from a 2-ml heparinized injector, and the blood samples were centrifuged at 3,500 g for 10 min. Plasma samples were frozen and stored at −80°C for further analysis. After blood sampling, samples of liver and dorsal muscles were dissected from those fish on ice, and these samples also kept at −80°C until analysis.
The sample of experimental fish and diets was conducted for proximate composition following the method of the Association of Official Analytical Chemists (AOAC, 2003). The moisture content was determined by oven drying at 105°C until a constant weight. The content of ash was quantified by incineration in a muffle furnace at 550°C for 12 h. A Soxtec system (Soxtec™ 2055, FOSS Tecator, Höganäs, Sweden) was used to analyze the content of crude lipid. Crude protein was tested through a Kjeltec Analyzer Unit (FOSS Tecator 8400, Höganäs, Sweden). Automatic biochemical analyzer (Mindray BS-460, Shenzhen, China) was used to determine the content of plasma total protein (TP), glucose (GLU), total triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), using standard kits according to the instructions [TP (P/N:105-000451-00), GLU (P/N:105-000949-00), TG (P/N:105-000449-00), TC (P/N:105-000448-00), LDL-C (P/N:105-000464-00), and HDL-C (P/N:105-000463-00)]. The hydrolyzation method of dietary amino acids was according to Liu et al. (2016). The separation method of free amino acids from muscle and plasma was according to Xu et al. (2015). The total amino acid levels of the experimental diets and the free amino acid levels in the dorsal muscle and plasma were with an amino acid analyzer (A300, MembraPure GmbH, Germany).
The primers for RT-qPCR were designed based on the National Center for Biotechnology Information (NCBI) primer BLAST service and from Tu et al. (2015; Table 4). According to the manufacturer’s instructions, total RNA in the dorsal muscle of gibel carp was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). The NanoDrop® ND-2000 UV–Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE, United States) was used to evaluate the concentration and quantity. Meanwhile, the integrity of the RNA sample was tested by a 1% denaturing agarose gel. The RNA was reverse-transcribed into cDNA through an M-MLV First-Strand Synthesis Kit (Invitrogen, Shanghai, China) following the protocol of the manufacturer. RT-qPCR was conducted using LightCycler 480 II system (Roche, Basel, Switzerland). Every sample with three biological duplicates ef1α and β-actin was selected as the internal reference gene. The relative expression levels of target genes were calculated following with Vandesompele et al. (2002).
All data of different treatments were analyzed using SPSS (IBM SPSS Statistics 20.0, IBM, United States). The homogeneity of variance was analyzed by Levene’s test. Then, one-way ANOVA and Duncan’s multiple range test were conducted to analyze the differences among treatments. The differences were considered significant at p < 0.05. The results were presented as the mean ± standard error (SE).
Chemical compositions of the protein sources used in the experimental diet are shown in Table 1. Protein apparent digestibility coefficient of the ingredients of gibel carp was measured in our laboratory, previously, according to the method described by Dong et al. (2012). The chemical composition and amino acid content of the experimental diets are shown in Tables 3, 5, respectively. The crude protein content of PMAa and PM diets was higher than that of 30FM and 10FM diets. The digestible protein content was almost the same in all diets (Table 5). The limiting amino acids in PM diet were lysine, methionine, and threonine. The essential amino acid requirements of gibel carp were in accordance with our previous work (Zhou et al., 2006; Li, 2009; Li et al., 2010; Ma, 2009; Ma et al., 2010; Jia et al., 2013; Tu et al., 2015). The total contents of essential amino acids were the highest in PMAa and the lowest in PM (Table 3).
The survival rate of fish in all the dietary treatments was 100%. The results of growth performance of gibel carp are shown in Table 6. The final body weight and specific growth rate (SGR) of fish fed 30FM, 10FM, and PMAa diets were not significantly different (p > 0.05), but were significantly higher than those of fish fed PM diet (p < 0.05). Feeding rate in PM group was higher than others though there were no significant differences. However, it was observed that feeding efficiency in PMAa was significantly higher than PM group (p < 0.05) and no significant difference with other groups (p > 0.05). There was no significant difference in protein retention efficiency (PRE) and protein efficiency ratio (PER) among all treatment groups except for PM group (p > 0.05). The PRE and PER of the PM group were significantly lower than those of the other groups (p < 0.05).
Whole-body compositions of gibel carp are presented in Table 7. The moisture and protein contents in the whole body of gibel carp were not significantly different among all groups (p > 0.05). The fish in the PM group had lower whole-body lipid content than that in the other groups (p < 0.05). There were no significant differences in whole-body ash content among all groups (p > 0.05).
There were no significant differences in TP and GLU in the plasma among all diet groups (Table 8). However, PM group had the lowest TG, TC, LDL-C, and HDL-C levels. Significant differences (p < 0.05) were observed in TG between PM and 30FM groups, in TC and HDL-C between PM and the other three groups, and in LDL-C between PM and the two groups containing dietary FM. The levels of TG, TC, LDL-C, and HDL-C in PMAa group were similar to those in 30FM and 10FM groups; TC and HDL-C concentrations in PMAa group were higher than in PM group (p < 0.05).
Free amino acid composition of plasma of gibel carp after the feeding trial is presented in Table 9. The fish from PMAa treatment had similar levels of all free amino acid concentrations with 30FM and 10FM treatments (p > 0.05). The essential free amino acids in the plasma of the PM treatment were also similar to those in other treatments and showed a downward trend (p > 0.05). However, the plasma aspartic acid, serine, alanine, and tyrosine concentrations in the PM group were significantly lower than those in the 30FM group (p < 0.05). The PM group also had the lowest plasma total essential amino acids compared to other groups (p > 0.05).
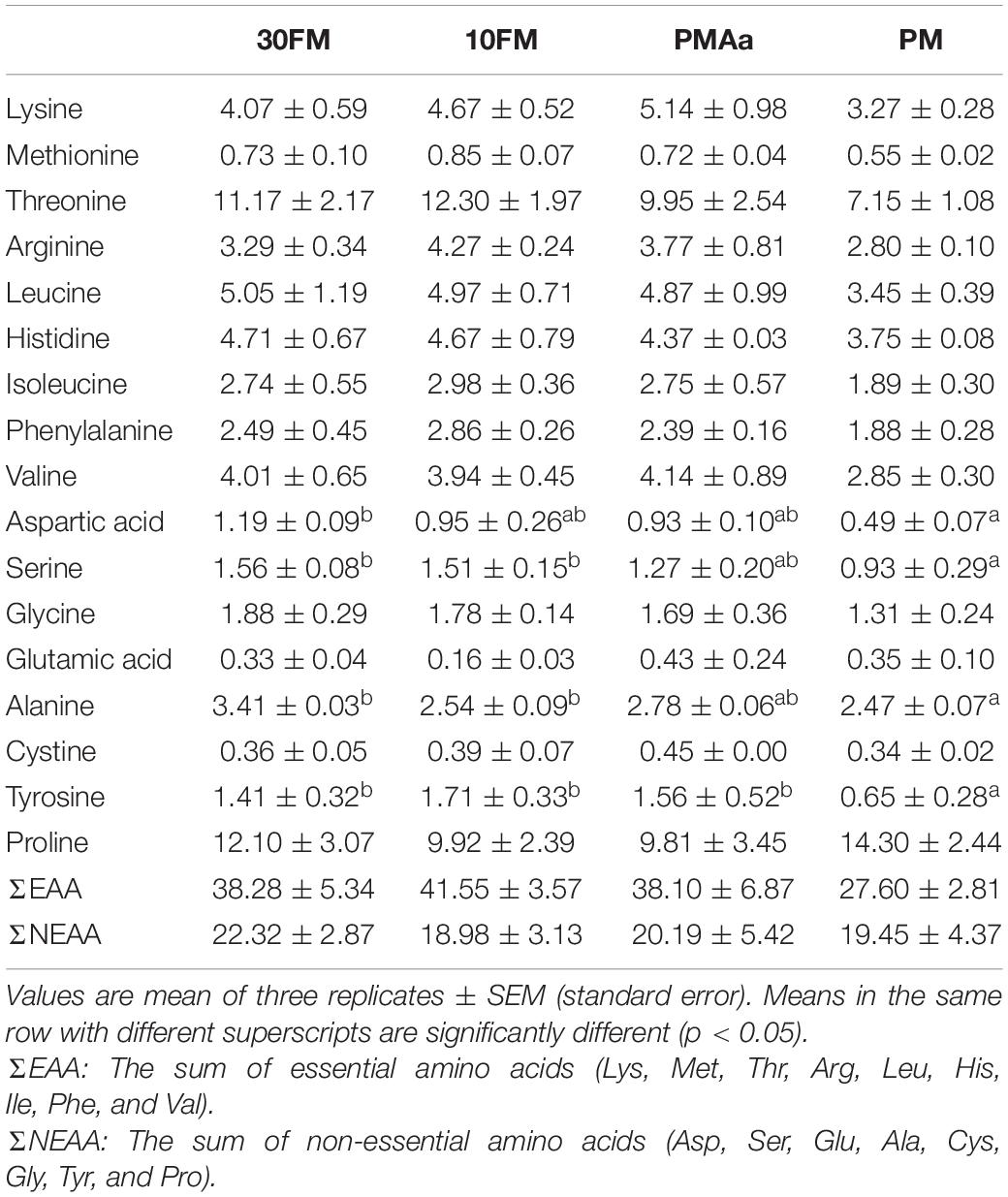
Table 9. Plasma free amino acid concentrations (mg/100 ml) in gibel carp fed dietary with different amino acid pattern about 45 days.
Results of dorsal muscle-free amino acid concentrations of gibel carp are listed in Table 10. There was a remarkable difference in the levels of free amino acids such as arginine, histidine, and glycine; total essential amino acid; total non-essential amino acid; and sum of flavor amino acids among the dietary groups. Even though there was no significant difference in lysine, methionine, and threonine among the four treatments, PMAa and PM treatments had lower levels of lysine and threonine than 30FM and 10FM treatments (p > 0.05). The arginine concentrations of 10FM, PM, and PMAa groups were significantly lower than that of 30FM group (p < 0.05). As for the contents of non-essential free amino acid in gibel carp dorsal muscle, the contents of glycine and glutamic acid of PM group were significantly lower than that of 30FM group (p < 0.05). However, PMAa and PM groups’ proline content was the highest among all groups. Notably, the total flavor amino acid levels of 30PM and 10FM were significantly higher than that of PM (p < 0.05) and was similar to that of PMAa (p > 0.05).
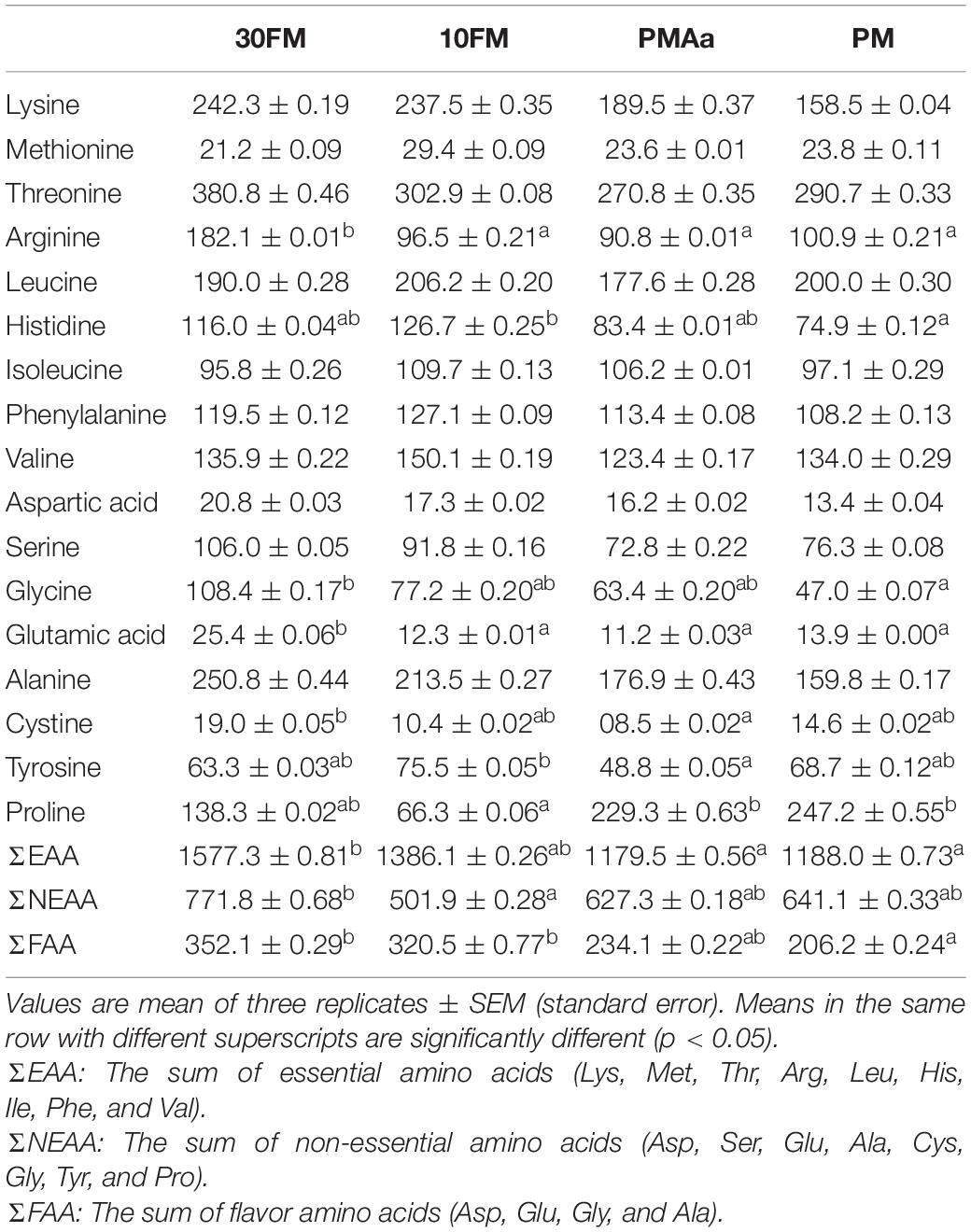
Table 10. Dorsal muscle free amino acid concentrations (μg/g) in gibel carp fed dietary with different amino acid pattern about 45 days.
The relative transcriptional levels of genes involved in muscle protein synthesis are presented in Figure 1. There were no significant differences observed in tor and eif4e expression among all experimental groups. Meanwhile, the expression of s6k1 in fish muscles in PMAa group was notably higher than that in PM group (p < 0.05); s6k1 expression in PM group was the lowest. The transcriptional levels of 4e-bp2 in PM group were the highest (p < 0.05) and were not significantly different among the remaining groups (p > 0.05).
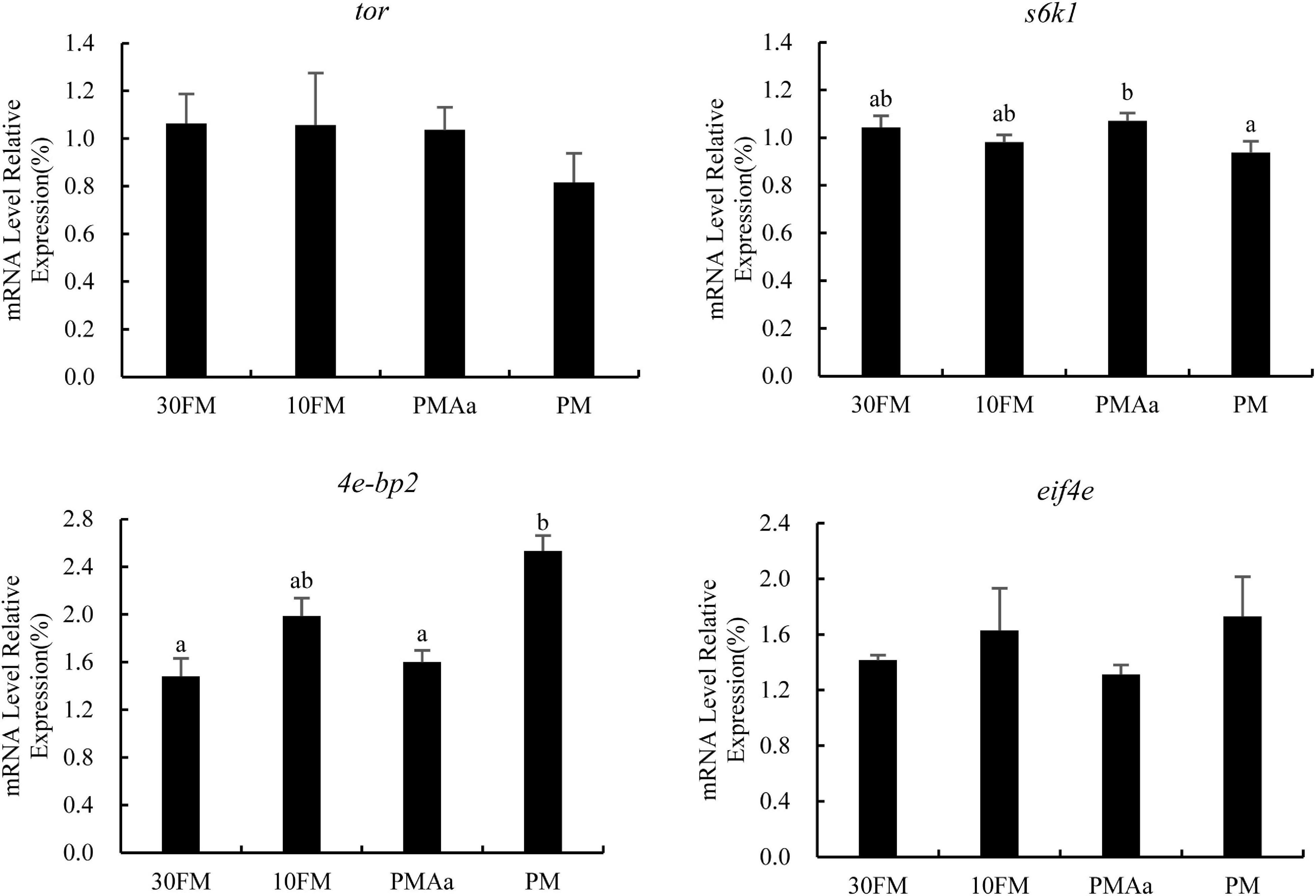
Figure 1. Relative expression of protein synthesis genes (tor, s6k1, 4e-bp2, and eif4e) in the muscle of gibel carp β-actin and ef1α was used as reference gene for the assessment of expression. Columns represent the mean ± SEM (n = 6). For each index, bars without sharing a common letter indicate significant differences (p < 0.05).
The relative transcriptional expression of genes involved in muscle protein degradation is illustrated in Figure 2. The relative expression of atg13 in PM group was significantly higher than that in 10FM group (p < 0.05). The relative expression level of ulk1 in the PM group was also significantly higher than that in 30FM and 10FM groups (p < 0.05). However, the relative expression of trim63 in 10FM group was the highest value among all groups (p < 0.05). There were no significant differences in the expression of znrf2 with all treatments (p > 0.05).
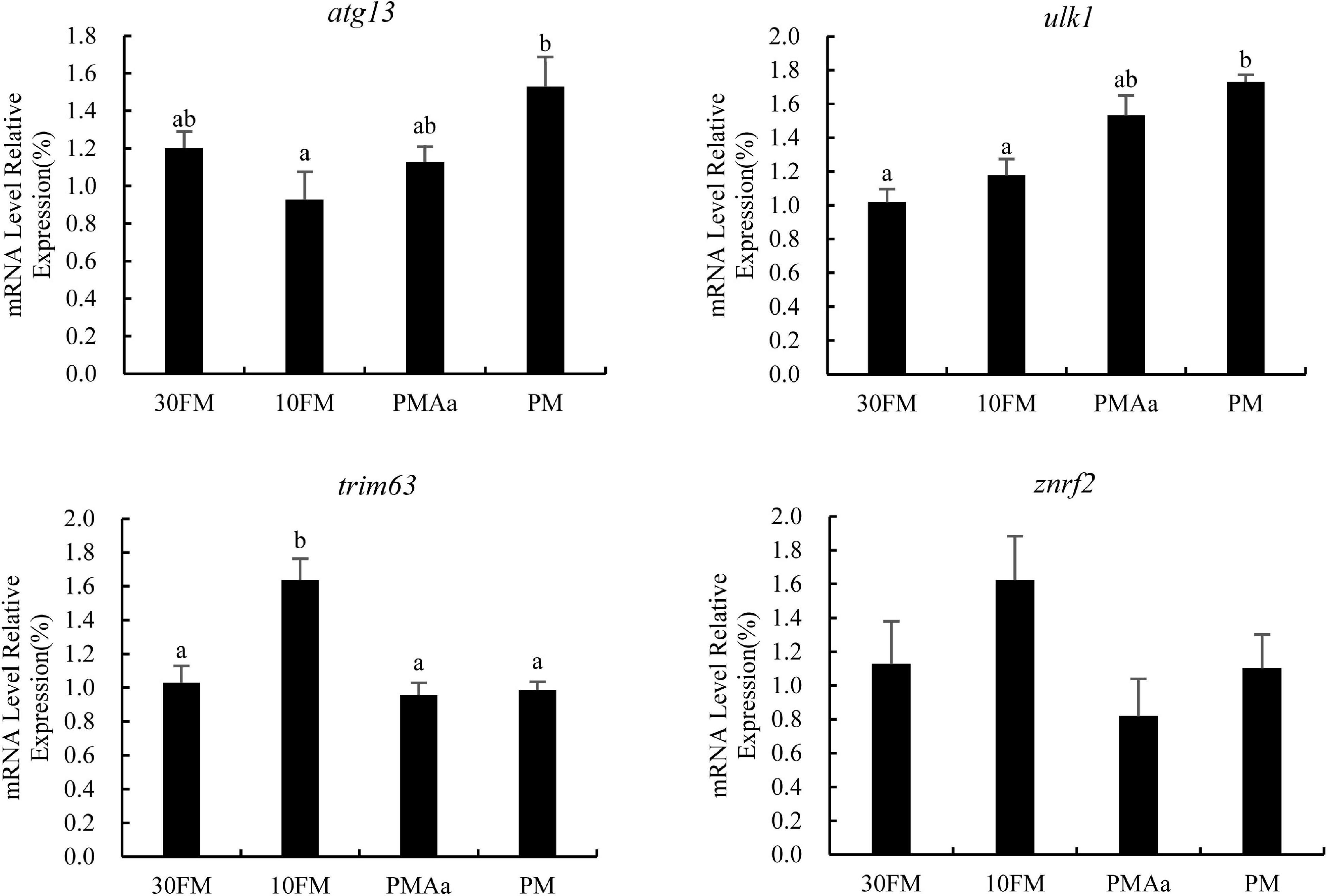
Figure 2. Relative expression of protein degradation genes (atg13, ulk1, trim63, and znrf2) in the muscle of gibel carp β-actin and ef1α was used as reference gene for the assessment of expression. Columns represent the mean ± SEM (n = 6). For each index, bars without sharing a common letter indicate significant differences (p < 0.05).
In this study, the possibility of replacing FM with mixed plant protein in the diet of gibel carp was investigated. The results showed that 20% of dietary FM and 22% of terrestrial animal protein could be replaced by mixed plant protein if digestible protein requirement in the diet was satisfied, the antinutritional factors from oil seed meals (soybean meal, rapeseed meal, and cottonseed meal) was low, and single-cell protein (blood meal, yeast hydrolyzate, spirulina) was supplement. All FM and terrestrial animal protein can be replaced by mixed plant protein if essential amino acid requirements are satisfied. Blood meal and spirulina were added to the plant-supplied diet because of their high lysine and threonine contents. Yeast hydrolyzate and spirulina were used as attractants and immunopotentiators. However, without the supplementation of essential amino acids, growth performance decreased significantly.
Many previous studies have revealed that poor growth performance of cultured fishes could result from the high level replacement of FM with single-plant ingredients (Watanabe et al., 1998; Gomez-Requeni et al., 2004; Kaushik et al., 2004; Panserat et al., 2008), such as soybean meal (Alexis and Nengas, 2001), rapeseed meal (Burel et al., 2000), and cottonseed meal (Luo et al., 2012), whether in carnivorous (Trachinotus ovatus, Wu et al., 2015; Lates calcarifer, Ma et al., 2019) or in herbivorous (Ctenopharyngodon Idella, Jiang et al., 2016) fish. Therefore, using a combination of protein ingredients with various nutritive properties can improve amino acid profile, dilute or even mitigate antinutrition factors, and consequently allow higher inclusion of alternative protein sources in the diet of cultured fish. However, in this study, PM diet, in which the dietary FM content was totally replaced by plant protein combination, showed lower growth performance in fish than 30FM, 10FM, and PMAa diet. The ability of fish to utilize crystalline amino acids is well reported (Kaushik and Seiliez, 2010), and limiting amino acid supplementation in plant-based diets is necessary to complement the low amino acid profile. We found that the growth performance and feed utilization of fish receiving PMAa treatment, in which plant protein combination was supplemented with limiting amino acids, were not significantly different from those of fish receiving 30FM and 10FM treatments and were much higher than those of fish receiving PM treatment. Similar observations have been reported in rainbow trout (Kaushik et al., 1995; Watanabe et al., 1998) and sea bass (Dicentrarchus labrax) (Tibaldi et al., 1999), where FM was replaced by a mixture of vegetable protein and adequate supplement of limiting amino acids without negative effects on growth. There have been several experiments in which the replacement of FM with high proportions or complete plant proteins in diets did not only result in decreased feed intake and growth performance but also caused oxidative stress or subhealth status (Sitjà-Bobadilla et al., 2005; Hansen et al., 2007). Single-cell proteins, such as spirulina and yeast, contain various water-soluble fractions, such as free amino acids, small peptides, nucleotides, vitamins, and minerals. In this study, spirulina and yeast hydrolyzate, which were used as attractants and immunopotentiators to cover for the shortage of FM replacement (Burgents et al., 2004; Sheikhzadeh et al., 2019), were supplied in the low-FM diet. Based on the results, total replacement of FM with compound plant proteins in the diet of gibel carp is a possibility, and it provides a promising approach for the sustainable development of aquaculture.
The whole-body proximate composition of gibel carp did not show considerable variability with the replacement of FM, except for the treatment containing PM only and without amino acid supplement (PM), with which fish had reduced protein and lipid contents. High proportion of dietary FM (200 g kg–1 diet) can be replaced with compound plant proteins without having significant effect on body composition. Similar results have been reported in trout (Santigosa et al., 2008), silvery-black porgy (Sparidentex hasta) (Yaghoubi et al., 2016), and gilthead sea bream (Sitjà-Bobadilla et al., 2005). PRE also decreased in the PM group. This phenomenon could probably be attributed to the unbalanced amino acid composition in the diet, which leads to increased protein degradation (Langar et al., 1993). Besides, when dietary amino acids that are absorbed into the body cannot match the profile needed for protein synthesis, the excess amino acids would result in increased oxidation and catabolism of amino acids (Kim et al., 1983; Fauconneau et al., 1992). The body protein levels and PRE of fish fed the PMAa diet supplemented with limiting amino acids were similar to those of fish fed 30FM diet (Table 7). A similar phenomenon was reported for other cultured fish, such as hybrid grouper (Epinephelus) (Wu et al., 2017) and large yellow croaker (Larimichthys crocea) (Li et al., 2013). Decreased lipid content of fish associated with very high dietary inclusion levels of PM has also been reported for Indian major carp (Cirrhinus mrigala) (Hasan et al., 1997) and Japanese flounder (Paralichthys olivaceus) (Kikuchi, 1999).
Serum parameters reflect the physiological stress response of fish to nutritional changes (Dawood et al., 2015) and have been used to assess the health of fish (Hossain et al., 2016; Dawood et al., 2017; Inanan et al., 2021). There are many kinds of literature that have reported the possible physiological effects of dietary plant protein intake on fish (Dias, 1999; Regost, 1999; Kaushik et al., 2004). In this study, plasma TG, TC, LDL-C, and HDL-C concentrations decreased with an increase in dietary PM. A previous study also showed that high level of dietary plant content causes hypocholesterolemic symptoms in rat, which presented low level of plasma cholesterol (de Schrijver, 1990). A significant hypocholesterolemic effect of soybean meal diet was also observed in rainbow trout by Kaushik et al. (1995), who speculated that the problem was in response to the withdrawal of FM rather than the addition of plant protein (Kaushik et al., 2004). However, Sitjà-Bobadilla et al. (2005) inferred that fish hypocholesterolemia may result from increased excretion of bile salts caused by dietary plant protein supply, which inhibits the absorption of cholesterol in the intestine. Plasma TC and HDL-C concentrations of fish fed PMAa diet were slightly lower than those of fish fed 30FM diet (p > 0.05) and were significantly higher than those of fish fed PM diet (p > 0.05). This phenomenon confirmed that lysine, methionine, and threonine supplement could relieve hypocholesterolemic symptoms (Hirche et al., 2006). The specific mechanism of plant proteins involved in the synthesis and metabolism of cholesterol needs further study (Kaushik et al., 2004; Sitjà-Bobadilla et al., 2005).
The content of amino acids in diets could affect plasma amino acid pool, which could also be used to judge the adequacy of dietary amino acids and reflects nitrogen balance (Mente et al., 2003; Hansen et al., 2007). In this study, we also found that the free amino acid concentrations in the dorsal muscles reflected the dietary amino acid profile. The concentrations of free essential amino acids and non-essential amino acids in the plasma were high in 30FM, 10FM, and PMAa diets but were low in PM diet. Accordingly, in the dorsal muscle, free essential amino acids and flavor amino acids also decreased when a high proportion of dietary FM was replaced with mixed PM. Amino acids in muscles are associated with the synthesis of muscle proteins (Hansen et al., 2007). It is worth mentioning that the concentration of dorsal-free lysine in PMAa group was higher than that in PM group. Lower lysine content in the dietary treatments also resulted in lower free lysine content in the dorsal muscle of the fish; this outcome agrees with that reported by Gomez-Requeni et al. (2004). Carter et al. (2000) suggested that the efficiency of protein synthesis and retention was limited by the lowest relative concentration of an essential amino acid pool. Lysine, methionine, and threonine are recognized as limiting amino acids for fish (Hansen et al., 2007). In addition to other results of this study, the positive growth performance in the PMAa group was achieved by satisfying the requirement of limiting amino acids.
Target of rapamycin signaling system pathway senses amino acid levels in cells and plays a key role in nutrient perception and energy regulation (Wullschleger et al., 2006). It also plays a key role in protein synthesis both in mammals (Dodd and Tee, 2012; Hernandez-García et al., 2016) and in fishes (Liang et al., 2016). Generally, the replacement of a high proportion of FM with PM may result in TOR signaling system pathway not being effectively activated, and the response of sensing stress pathway being activated (Xu et al., 2015; Song et al., 2016). The suppression effect of antinutritional factors in the TOR pathway was also reported by He and Klionsky (2017), who found that gossypol and soybean saponins suppress tor and promote catabolism, and that concanavalin-A causes inhibited insulin/IGF1/Akt signaling that consequently leads to apoptosis. In this study, compound plant ingredients were used to reduce the content of antinutritional substances. This may be the reason why the expressions of tor and eif4e genes were not significantly different among all treatments. This phenomenon was also reported for juvenile cobia (Rachycentron canadum) and large yellow croaker larvae (Luo et al., 2012; Li et al., 2013). The supplementation of essential amino acids can activate TOR pathway (Chen G. F. et al., 2012; Zhao et al., 2012). In the TOR signaling pathway, there are two downstream sites (4E-BPs and s6k1) for tor. The PMAa treatment had a significantly higher expression level of s6k1 than that in the PM treatment, which gene was positively regulated by the TOR complex. Furthermore, the reverse was found in the 4e-bp2 mRNA levels of the muscle of gibel carp in the PM treatments. These results were justifiable as tor promotes cap-dependent translation initiation through the inactivation of its downstream effector (namely 4E-BPs) or increases the translation of 5′-TOP mRNAs via the activation of S6K1 (Wullschleger et al., 2006). Similar results were reported that dietary supplement of essential amino acids, such as lysine (Cai et al., 2018), threonine (Habte-Tsion et al., 2015), and methionine (Pan et al., 2016), could promote the expression of s6k1 and depress the expression of 4e-bp2 in cultured fish species.
Growth performance can be partly reflected in the dynamic balance of muscle protein synthesis and degradation (Zeitz et al., 2019). Skeletal muscle protein turnover is the ratio between protein synthesis and protein breakdown rates (Burd et al., 2009). When positive, protein synthesis is higher than breakdown, and results in muscle mass gain. Autophagy and ubiquitin–proteasome system are the main pathways of protein degradation in the skeletal muscle of vertebrates (Sandri, 2010). This study found that the atg13 and ulk1 genes in PM group were highly expressed and indicated that the dorsal protein degradation rate in the PM treatment was higher than those in the other treatments. Ulk1 is a fundamental protein activated by AMP-activated protein kinase (AMPK) for survival under conditions of reduced nutrient availability (Bach et al., 2011). The high protein breakdown rate in the dorsal muscle of PM treatment explains the low growth performance. TRIM63-encoding muscle ring finger 1 maintains muscle protein homeostasis by tagging the sarcomere proteins with ubiquitin for subsequent degradation by the ubiquitin–proteasome system (Chen S. N. et al., 2012). In this study, the trim63 was highly expressed in 10FM treatment, which diet was not supplied with crystal amino acids. These phenomena suggest that the nutritional requirement of gibel carp could not be satisfied by compound plant ingredients without limiting amino acid supplements.
In summary, this study comprehensively evaluated the possibility of totally replacing FM with PM in the feed of gibel carp. The growth performance, feed utilization, and plasma biochemical composition in PMAa treatment were not different from those of the 30FM treatment. The key genes in the TOR signaling pathway and autophagy and ubiquitin–proteasome system corresponded with the dietary amino acid profile. We conclude that replacing FM with total plant protein is feasible and provides a reference for cost optimization design of feed formulation for gibel carp.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The animal study was reviewed and approved by the Ethics Committee of the Institute of Hydrobiology, Chinese Academy of Sciences.
WC and HL made major contributions to this work. WC was analysis of the data and draft of the manuscript. HL reviewed and corrected the manuscript. SX, XZ, YY, DH, and JJ participated in experiment design and sample collection. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key R&D Program of China (2019YFD0900200 and 2018YFD0900400), China Agriculture Research System (CARS-45-09), Science and Technology Project of Wuhan (2019020701011459), and Poverty Alleviation through Agricultural Projects from the Agricultural Office of Chinese Academy of Sciences and the Fund Project of the State Key Laboratory of Freshwater Ecology and Biotechnology (2019FBZ02 and 2019FBZ05).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Guanghan Nie for his technical assistance.
Ahmad, N., Siddiqui, P. J. A., Khan, K. M., Ali, A., Tahir, M., Akbar, N. U., et al. (2020). Effects of partial substitution of fishmeal by soybean meal in Nile tilapia (Oreochromis niloticus). J. Anim. Plant Sci. 30, 364–370. doi: 10.36899/JAPS.2020.2.0058
Akiko, K., Masahiko, H., Makoto, M., Akitsugu, Y., Haruaki, N., Tamotsu, Y., et al. (2004). The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036. doi: 10.1038/nature03029
Albrektsen, S., Mundheim, H., and Aksnes, A. (2006). Growth, feed efficiency, digestibility and nutrient distribution in Atlantic cod (Gadus morhua) fed two different fish meal qualities at three dietary levels of vegetable protein sources. Aquaculture 261, 626–640. doi: 10.1016/j.aquaculture.2006.08.031
Alexis, M. N., and Nengas, I. (2001). Current State of Knowledge Concerning the Use of Soy Products in Diets for Feeding Sea Bass and Seabream. Brussels: American Soybean Association, 32.
AOAC (2003). Official Methods of Analysis of the Association of Official Analytical Chemists, 17th Edn. Arlington, VA: Association of Official Analytical Chemists.
Bach, M., Larance, M., James, D. E., and Ramm, G. (2011). The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem. J. 440, 283–291. doi: 10.1042/BJ20101894
Benedito-Palos, L., Saera-Vila, A., and Calduch-Giner, J. A. (2007). Combined replacement of fish meal and oil in practical diets for fast growing juveniles of gilthead sea bream (Sparus aurata L.): networking of systemic and local components of GH/IGF axis. Aquaculture 267, 199–212. doi: 10.1016/j.aquaculture.2007.01.011
Bu, X. Y., Wang, Y. Y., Chen, F. Y., Tang, B. B., Luo, C. Z., Wang, Y., et al. (2018). An evaluation of replacing fishmeal with rapeseed meal in the diet of Pseudobagrus ussuriensis: growth, feed utilization, nonspecific immunity, and growth-related gene expression. J. World Aquac. Soc. 49, 1068–1080. doi: 10.1111/jwas.12470
Burd, N. A., Tang, J. E., Moore, D. R., and Phillips, S. M. (2009). Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J. Appl. Physiol. 106, 1692–1701. doi: 10.1152/japplphysiol.91351.2008
Burel, C., Boujard, T., Escaffre, A. M., Kaushik, S. J., Boeuf, G., Mol, K. A., et al. (2000). Dietary low-glucosinolate rapeseed meal affects thyroid status and nutrient utilization in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 83, 653–664. doi: 10.1017/S0007114500000830
Burgents, J. E., Burnett, K. G., and Burnett, L. E. (2004). Disease resistance of Pacific white shrimp, Litopenaeus vannamei, following the dietary administration of a yeast culture food supplement. Aquaculture 231, 1–8. doi: 10.1016/j.aquaculture.2003.09.003
Cai, W. C., Liu, W. B., Jiang, G. Z., Wang, K. Z., Sun, C. X., and Li, X. F. (2018). Lysine supplement benefits the growth performance, protein synthesis, and muscle development of Megalobrama amblycephala fed diets with fish meal replaced by rice protein concentrate. Fish Physiol. Biochem. 44, 1159–1174. doi: 10.1007/s10695-018-0503-3
Carter, C. G., Houihan, D. F., and He, Z. Y. (2000). Changes in tissue free amino acid concentrations in Atlantic salmon, Salmo salar L., after consumption of a low ration. Fish Physiol. Biochem. 23, 295–306. doi: 10.1023/a:1011187026997
Chan, E. Y. W., Longatti, A., McKnight, N. C., and Tooze, S. A. (2009). Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol. Cell. Biol. 29, 157–171. doi: 10.1128/MCB.01082-08
Chen, G. F., Feng, L., Kuang, S. Y., Liu, Y., Jiang, J., Hu, K., et al. (2012). Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Br. J. Nutr. 108, 195–207. doi: 10.1017/s0007114511005459
Chen, S. N., Czernuszewicz, G., Tan, Y., Lombardi, R., Jin, J., Willerson, J. T., et al. (2012). Human molecular genetic and functional studies identify TRIM63, encoding muscle RING finger protein 1, as a novel gene for human hypertrophic cardiomyopathy. Circ. Res. 111, 907–919. doi: 10.1161/circresaha.112.270207
Crovetto, G. M. (2010). Energy and Protein Metabolism and Nutrition. Wageningen: Wageningen Academic Publishers. doi: 10.3920/978-90-8686-781-3
Dawood, M. A. O., Koshio, S., Ishikawa, M., El-Sabagh, M., Yokoyama, S., Wang, W. L., et al. (2017). Physiological response, blood chemistry profile and mucus secretion of red sea bream (Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress. Fish Physiol. Biochem. 43, 179–192. doi: 10.1007/s10695-016-0277-4
Dawood, M. A. O., Koshio, S., Ishikawa, M., and Yokoyama, S. (2015). Effects of partial substitution of fishmeal by soybean meal with or without heat-killed Lactobacillus plantarum (lp20) on growth performance, digestibility, and immune response of amberjack, Seriola dumerili juveniles. Biomed Res. Int. 2015:514196. doi: 10.1155/2015/514196
de Schrijver, R. (1990). Cholesterol metabolism in mature and immature rats fed animal or plant protein. J. Nutr. 120, 1624–1632. doi: 10.1093/jn/120.12.1624
Dias, J. (1999). Lipid Deposition in Rainbow Trout (Oncorhynchus mykiss) and European seabass (Dicentrarchus labrax L.): Nutritional Regulation of Hepatic Lipogenesis. Doctoral thesis. Porto: University of Porto, 190.
Dodd, K. M., and Tee, A. R. (2012). Leucine and MTORC1: a complex relationship. Am. J. Physiol. Endocrinol. Metab. 302, 1329–1342. doi: 10.1152/ajpendo.00525.2011
Dong, X. L., Xie, S. Q., Lei, W., Zhu, X. M., Han, D., and Yang, Y. X. (2012). Effect of faeces collection methods on estimation of apparent digestibility coefficient in gibel carp (Carassius auratus gibelio). Acta Hydrobiol. Sin. 36, 450–456. doi: 10.1007/s11783-011-0280-z
Dossou, S., Koshio, S., Ishikawa, M., Yokoyama, S., Dawood, M. A., El Basuini, M. F., et al. (2018). Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture 490, 228–235. doi: 10.1016/j.aquaculture.2018.02.010
Duan, X. D., Jiang, W. D., Wu, P., Liu, Y., Jiang, J., Tan, B. P., et al. (2019). Soybean βconglycinin caused intestinal inflammation and oxidative damage in association with NF-κB, TOR and Nrf2 in juvenile grass carp (Ctenopharyngodon idella): varying among different intestinal segments. Fish Shellfish Immunol. 95, 105–116. doi: 10.1016/j.fsi.2019.10.021
Fauconneau, B., Basseres, A., and Kaushik, S. J. (1992). Oxidation of phenylalanine and threonine in response to dietary arginine supply in rainbow trout (Salmo gairdneri R.). Comp. Biochem. Physiol. 101A, 395–401. doi: 10.1016/0300-9629(92)90552-2
Fisheries Bureau, Department of Agriculture of China (2020). China Fishery Statistical Yearbook. Beijing: China Agriculture Press, 24.
Gao, S. Y., Jin, J. Y., Liu, H. K., Han, D., Zhu, X. M., Yang, Y. X., et al. (2019). Effects of pelleted and extruded feed of different ingredients particle sizes on feed quality and growth performance of gibel carp (Carassius gibelio var. CAS V). Aquaculture 511:734236. doi: 10.1016/j.aquaculture.2019.734236
Gomes, E. F., Corraze, G., and Kaushik, S. (1993). Effects of dietary incorporation of a coextruded plant protein (rapeseed and peas) on growth, nutrient utilization and muscle fatty-acid composition of rainbow-trout (Oncorhynchus-Mykiss). Aquaculture 113, 339–353. doi: 10.1016/0044-8486(93)90404-M
Gomez-Requeni, P., Mingarro, M., Calduch-Giner, J. A., Medale, F., Martin, S. A. M., Houlihan, D. F., et al. (2004). Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture 232, 493–510. doi: 10.1016/S0044-8486(03)00532-5
Gui, J. F., and Zhou, L. (2010). Genetic basis and breeding application of clonal diversity and dual reproduction modes in polyploid Carassius auratus gibelio. Sci. China Life Sci. 53, 409–415. doi: 10.1007/s11427-010-0092-6
Habte-Tsion, H. M., Ge, X. P., Liu, B., Xie, J., Ren, M. C., Zhou, Q. L., et al. (2015). A deficiency or an excess of dietary threonine level affects weight gain, enzyme activity, immune response and immune-related gene expression in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 42, 439–446. doi: 10.1016/j.fsi.2014.11.021
Hansen, A. C., Rosenlund, G., Karlsen, Ø, Koppe, W., and Hemre, G. I. (2007). Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I — effects on growth and protein retention. Aquaculture 272, 599–611. doi: 10.1016/j.aquaculture.2007.08.034
Hasan, M. R., Haq, M. S., Das, P. M., and Mowlah, G. (1997). Evaluation of poultry-feather meal as a dietary protein source for Indian major carp, Labeo rohita fry. Aquaculture 151, 47–54. doi: 10.1016/S0044-8486(96)01498-6
He, C., and Klionsky, D. J. (2017). Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93. doi: 10.1146/annurev-genet-102808-114910
Hernandez-García, A. D., Columbus, D. A., Manjarín, R., Nguyen, H. V., Suryawan, A., Orellana, R. A., et al. (2016). Leucine supplementation stimulates protein synthesis and reduces degradation signal activation in muscle of newborn pigs during acute endotoxemia. Am. J. Physiol. Endocrinol. Metab. 311, 791–801. doi: 10.1007/s00726-015-2078-y
Hirche, F., Schroder, A., Knoth, B., Stangl, G. I., and Eder, K. (2006). Effect of dietary methionine on plasma and liver cholesterol concentrations in rats and expression of hepatic genes involved in cholesterol metabolism. Br. J. Nutr. 95, 879–888. doi: 10.1079/BJN20061729
Hossain, M. S., Koshio, S., Ishikawa, M., Yokoyama, S., Sony, N. M., Ono, S., et al. (2016). Comparison of the effects of inosine and inosine monophosphate on growth, immune response, stress resistance and gut morphology of juvenile red sea bream, Pagrus major. Aquaculture 57, 96–106. doi: 10.1016/j.aquaculture.2016.02.032
Inanan, B. E., and Acar, U. (2019). Evaluation of sugar beet leave extracts in goldfish (Carassius auratus) diets: effects on blood and semen parameters. Acta Aquat. Turcica 4, 458–468. doi: 10.22392/actaquatr.556299
Inanan, B. E., Acar, U., and Inanan, T. (2021). Effects of dietary Ferula elaeochytris root powder concentrations on haematology, serum biochemical parameters, spermatozoa parameters, and oxidative status in tissues of male goldfish (Carassius auratus). Aquaculture 544:737087. doi: 10.1016/j.aquaculture.2021.737087
Ismail, T., Hegazi, E., Dawood, M. A. O., Nassef, E., Bakr, A., Paray, B. A., et al. (2020). Using of betaine to replace fish meal with soybean or/and corn gluten meal in Nile tilapia (Oreochromis niloticus) diets: histomorphology, growth, fatty acid, and glucose-related gene expression traits. Aquac. Rep. 17:100376. doi: 10.1016/j.aqrep.2020.100376
Jia, P., Xue, M., Zhu, X., Liu, H. Y., Wu, X. F., Wang, J., et al. (2013). Effects of dietary methionine levels on the growth performance of juvenile gibel carp (Carassius auratus gibelio). Acta Hydrobiol. Sin. 37, 217–226.
Jiang, J., Shi, D., Zhou, X. Q., Feng, L., Liu, Y., Jiang, W. D., et al. (2016). Effects of lysine and methionine supplementation on growth, body composition and digestive function of grass carp (Ctenopharyngodon idella) fed plant protein diets using high-level canola meal. Aquac. Nutr. 22, 1126–1133. doi: 10.1111/anu.12339
Jobgen, W. S., Fried, S. K., Fu, W. J., Meininger, C. J., and Wu, G. (2006). Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 17, 571–588. doi: 10.1016/j.jnutbio.2005.12.001
Kaushik, S. J., Covès, D., Dutto, G., and Blanc, D. (2004). Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 230, 391–404. doi: 10.1016/S0044-8486(03)00422-8
Kaushik, S. J., Cravedi, J. P., Lalles, J. P., Sumpter, J., Fauconneau, B., and Laroche, M. (1995). Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 133, 257–274. doi: 10.1016/0044-8486(94)00403-b
Kaushik, S. J., and Seiliez, I. (2010). Protein and amino acid nutrition and metabolism in fish: current knowledge and future needs. Aquac. Res. 41, 322–332. doi: 10.1111/j.1365-2109.2009.02174.x
Kikuchi, K. (1999). Use of defatted soybean meal as a substitute for fish meal in diets of Japanese flounder (Paralichthys olivaceus). Aquaculture 179, 3–11. doi: 10.1016/s0044-8486(99)00147-7
Kim, K., McMillan, I., and Bayley, H. S. (1983). Determination of amino acid requirements of young pigs using an indicator amino acid. Br. J. Nutr. 50, 369–382. doi: 10.1079/bjn19830104
Langar, H., Guillaume, J., Metailler, R., and Fauconneau, B. (1993). Augmentation of protein synthesis and degradation by poor dietary amino acid balance in European sea bass (Dicentrarchus labrax). J. Nutr. 123, 1754–1761. doi: 10.1093/jn/123.10.1754
Li, G. M. (2009). Studies on Dietary Requirements of Threonine, Leucine, Valine and Isoleucine for Juvenile Gibel Carp (Carassius auratus gibelio). Ph.D. thesis. Wuhan: Institute of Hydrobiology, Chinese Academy of Sciences. (In Chinese with English abstract).
Li, G. M., Xie, S. Q., Lei, W., Zhu, X. M., Han, D., and Yang, Y. X. (2010). Dietary valine requirement for juvenile gibel carp (Carassius auratus gibelio). Acta Hydrobiol. Sin. 34, 1157–1165. doi: 10.3724/SP.J.1035.2010.01157
Li, W. J., Ai, Q. H., Mai, K. S., Xu, W., Luo, Y. W., and Zhang, Y. J. (2013). Effects of dietary amino acid patterns on growth and protein metabolism of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 406–407, 1–8. doi: 10.1016/j.aquaculture.2013.04.029
Li, X. Q., Xu, H. B., Sun, W. T., Xu, X. Y., Xu, Z., and Leng, X. J. (2018). Grass carp fed a fishmeal-free extruded diet showed higher weight gain and nutrient utilization than those fed a pelleted diet at various feeding rates. Aquaculture 493, 283–288. doi: 10.1016/j.aquaculture.2018.04.058
Liang, H. L., Ren, M. C., Habte-Tsion, H. M., Ge, X. P., Xie, J., Mi, H. F., et al. (2016). Dietary arginine affects growth performance, plasma amino acid contents and gene expressions of the TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 461, 1–8. doi: 10.1016/j.aquaculture.2016.04.009
Liu, H. K., Jin, J. Y., Zhu, X. M., Han, D., Yang, Y. X., and Xie, S. Q. (2017). Effect of substitution of dietary fishmeal by soybean meal on different sizes of gibel carp (Carassius auratus gibelio): digestive enzyme gene expressions and activities, and intestinal and hepatic histology. Aquac. Nutr. 23, 129–147. doi: 10.1111/anu.12375
Liu, H. K., Zhu, X. M., Yang, Y. X., Han, D., Jin, J., and Xie, S. Q. (2016). Effect of substitution of dietary fishmeal by soya bean meal on different sizes of gibel carp (Carassius auratus gibelio): nutrient digestibility, growth performance, body composition and morphometry. Aquac. Nutr. 22, 142–157. doi: 10.1111/anu.12239
Luo, Y. W., Ai, Q. H., Mai, K. S., Zhang, W. B., Xu, W., and Zhang, Y. J. (2012). Effects of dietary rapeseed meal on growth performance, digestion and protein metabolism in relation to gene expression of juvenile cobia (Rachycentron canadum). Aquaculture 368, 109–116. doi: 10.1016/j.aquaculture.2012.09.013
Ma, Z., Hassan, M. M., Allais, L., He, T., Leterme, S., Ellis, A., et al. (2019). Comparison of partial replacement of fishmeal with soybean meal and EnzoMeal on growth performance of Asian seabass Lates calcarifer. Comp. Biochem. Phys. C 216, 29–37. doi: 10.1016/j.cbpc.2018.10.006
Ma, Z. Y. (2009). Studies on Dietary Requirements of Arginine, Histidine, Phenylalanine, Tryptophan and Taurine for Carassius auratus gibelio. Ph.D. thesis. Wuhan: Institute of Hydrobiology, Chinese Academy of Sciences. (In Chinese with English abstract)
Ma, Z. Y., Zhu, X. M., Xie, S. Q., Yang, Y. X., and Han, D. (2010). Dietary phenylalanine requirement of juvenile gibel carp. Acta Hydrobiol. Sin. 34, 1012–1021. doi: 10.3724/SP.J.1035.2010.01012
Mente, E., Deguara, S., Santos, M. B., and Houlihan, D. F. (2003). White muscle free amino acid concentrations following feeding a maize gluten dietary protein in Atlantic salmon (Salmo salar L.). Aquaculture 225, 133–147. doi: 10.1016/s0044-8486(03)00285-0
National Research Council [NRC] (2011). Nutrient Requirements of Fish. Washington, DC: National Academy of Sciences, 218.
Pan, F. Y., Feng, L., Jiang, W. D., Jiang, J., Wu, P., Kuang, S. Y., et al. (2016). Methionine hydroxy analogue enhanced fish immunity via modulation of NF-κB, TOR, MLCK, MAPKs and Nrf2 signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 56, 208–228. doi: 10.1016/j.fsi.2016.07.020
Panserat, S., Kolditz, C., Richard, N., Plagnes-Juan, E., Piumi, F., Esquerré, D., et al. (2008). Hepatic gene expression profiles in rainbow trout (Oncorhynchus mykiss) fed fish meal or fish oil-free diets. Br. J. Nutr. 100, 953–967. doi: 10.1017/S0007114508981411
Regost, C. (1999). Partial or total replacement of fish meal by corn gluten meal in diet for turbot (Psetta maxima). Aquaculture. 180, 99–117. doi: 10.1016/s0044-8486(99)00026-5
Sandri, M. (2010). Autophagy in skeletal muscle. FEBS Lett. 584, 1411–1416. doi: 10.1016/j.febslet.2010.01.056
Santigosa, E. J., Sánchez, F., Médale, F., Kaushik, S., Perez-Sanchez, J., and Gallardo, M. A. (2008). Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 252, 68–74. doi: 10.1016/j.aquaculture.2008.06.007
Sheikhzadeh, N., Mousavi, S., Hamidian, G., Firouzamandi, M., Khani Oushani, A., and Mardani, K. (2019). Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture 510, 1–8. doi: 10.1016/j.aquaculture.2019.05.009
Sitjà-Bobadilla, A., Peña-Llopis, S., Gómez-Requeni, P., Médale, F., Kaushik, S., and Pérez-Sánchez, J. (2005). Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 249, 387–400. doi: 10.1016/j.aquaculture.2005.03.031
Song, Z. D., Li, H. Y., Wang, J. Y., Li, P. Y., and Zhang, L. M. (2016). Effects of replacement fishmeal with soy protein hydrolysates on growth, blood biochemistry, body composition of juvenile starry flounder Platichthys stellatus pallas. Acta Hydrobiol. Sin. 40, 165–172. doi: 10.7541/2016.23
Sun, H., Jiang, W. D., Wu, P., Liu, Y., Jiang, J., Yang, Q. H., et al. (2019). Betaine supplementations enhance the intestinal immunity of on-growing grass carp (Ctenopharyngodon idella): partly related to TOR and NF-κB signaling pathways. Aquaculture 2019:734846. doi: 10.1016/j.aquaculture.2019.734846
Tibaldi, E., Tulli, F., and Amerio, M. (1999). “Feed intake and growth responses of sea bass (D. labrax) fed different plant-protein sources are not affected by supplementation with a feeding stimulant,” in Recent Progress in Animal Production Science: I. Proceedings of the A.S.P.A. XIII Congress, Piacenza, Italy, 21–24 June 1999, eds G. Piva, G. Bertoni, S. Satoh, P. Bani, and L. Calamari (Piacenza: Assn. Sci. Anim. Production), 752–754.
Tibbetts, S. M., Mann, J., and Dumas, A. (2017). Apparent digestibility of nutrients, energy, essential amino acids and fatty acids of juvenile Atlantic salmon (Salmo salar L.) diets containing whole-cell or cell-ruptured Chlorella vulgaris meals at five dietary inclusion levels. Aquaculture 481, 25–39. doi: 10.1016/j.aquaculture.2017.08.018
Tu, Y. Q., Xie, S. Q., Han, D., Yang, Y. X., Jin, J., and Zhu, X. M. (2015). Dietary arginine requirement for gibel carp (Carassis auratus gibelio var. CAS III) reduces with fish size from 50 g to 150 g associated with modulation of genes involved in TOR signaling pathway. Aquaculture 449, 37–47. doi: 10.1016/j.aquaculture.2015.02.031
Uddin, M. N., Nishio, N., Ito, S., Suzuki, H., and Isobe, K. (2012). Autophagic activity in thymus and liver during aging. Age 34, 75–85. doi: 10.1007/s11357-011-9221-9
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:research0034.1. doi: 10.1186/gb-2002-3-7-research0034
Verhoeven, J., Salvo, F., Hamoutene, D., and Dufour, S. (2016). Bacterial community composition of flocculent matter under a salmonid aquaculture site in Newfoundland, Canada. Aquac. Environ. Interact. 8, 637–646. doi: 10.3354/aei00204
Wang, T. T., Xu, M. M., Wang, J. T., Wan, W. J., Guan, D. Y., Han, H. J., et al. (2020). A combination of rapeseed, cottonseed and peanut meal as a substitute of soybean meal in diets of Yellow River carp Cyprinus carpio var. Aquac. Nutr. 26, 1520–1532. doi: 10.1111/anu.13099
Watanabe, T., Verakunpiriya, V., Watanabe, K., Viswanath, K., and Satoh, S. (1998). Feeding of rainbow trout with non-fish meal diets. Fish. Sci. 63, 258–266. doi: 10.2331/fishsci.63.258
Wu, M. J., Lu, S. D., Wu, X. Y., Jiang, S. T., Luo, Y., and Yao, W. (2017). Effects of dietary amino acid patterns on growth, feed utilization and hepatic IGF-I, TOR gene expression levels of hybrid grouper (Epinephelus fuscoguttatus ♀× Epinephelus lanceolatus ♂) juveniles. Aquaculture 468, 508–514. doi: 10.1016/j.aquaculture.2016.11.019
Wu, Y., Han, H., Qin, J., and Wang, Y. (2015). Replacement of fish meal by soy protein concentrate with taurine supplementation in diets for golden pompano (Trachinotus ovatus). Aquac. Nutr. 21, 214–222. doi: 10.1111/anu.2015.21.issue-2
Wullschleger, S., Loewith, R., and Hall, M. N. (2006). TOR signaling in growth and metabolism. Cell 124, 471–484. doi: 10.1016/j.cell.2006.01.016
Xia, J., Wang, R., Zhang, T. L., and Ding, J. P. (2016). Structural insight into the arginine-binding specificity of CASTOR1 in amino acid-dependent mTORC1 signaling. Cell Discov. 2:16035. doi: 10.1038/celldisc.2016.35
Xie, S. Q., Zhu, X. M., Cui, Y. B., and Yang, Y. X. (2001). Utilization of several plant proteins by gibel carp (Carassius auratus gibelio). J. Appl. Ichthyol. 17, 70–76. doi: 10.1046/j.1439-0426.2001.00275.x
Xu, D. D., He, G., Mai, K. S., Zhou, H. H., Xu, W., and Song, F. (2015). Postprandial nutrient-sensing and metabolic responses after partial dietary fishmeal replacement by soyabean meal in turbot (Scophthalmus maximus L.). Br. J. Nutr. 115, 379–388. doi: 10.1017/S0007114515004535
Xu, W. N., Qian, Y., Li, X. F., Li, J. Y., Li, P. F., Cai, D. S., et al. (2017). Effects of dietary biotin on growth performance and fatty acids metabolism in blunt snout bream, Megalobrama amblycephala fed with different lipid levels diets. Aquaculture 479, 790–797. doi: 10.1016/j.aquaculture.2017.07.018
Yaghoubi, M., Mozanzadeh, M. T., Marammazi, J. G., Safari, O., and Gisbert, E. (2016). Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 464, 50–59. doi: 10.1016/j.aquaculture.2016.06.002
Yuan, X. Y., Liu, M. Y., Cheng, H. H., Huang, Y. Y., Dai, Y. J., Liu, W. B., et al. (2019). Replacing fish meal with cottonseed meal protein hydrolysate affects amino acid metabolism via AMPK/SIRT1 and TOR signaling pathway of Megalobrama amblycephala. Aquaculture 510, 225–233. doi: 10.1016/j.aquaculture.2019.05.056
Zeitz, J. O., Käding, S. K., Niewald, I. R., Most, E., Dorigam, J. C., de, P., et al. (2019). The influence of dietary leucine above recommendations and fixed ratios to isoleucine and valine on muscle protein synthesis and degradation pathways in broilers. Poult. Sci. 98, 6772–6786. doi: 10.3382/ps/pez396
Zhang, P., Fu, L., Liu, H., Huda, N. U, Zhu, X., Han, D., et al. (2019). Effects of inosine 5′-monophosphate supplementation in high fishmeal and high soybean diets on growth, immune-related gene expression in gibel carp (Carassius auratus gibelio var. CAS III), and its challenge againstAeromonas hydrophila infection. Fish Shellfish Immunol. 86, 913–921. doi: 10.1016/j.fsi.2018.12.016
Zhang, Y. L., Wang, P., Lin, S., Mercier, Y., Yin, H. J., Song, Y. M., et al. (2018). mTORC1 signaling-associated protein synthesis in porcine mammary glands was regulated by the local available methionine depending on methionine sources. Amino Acids 50, 105–115. doi: 10.1007/s00726-017-2496-0
Zhao, J., Liu, Y., Jiang, J., Wu, P., Chen, G. F., Jiang, W. D., et al. (2012). Effects of dietary isoleucine on growth, the digestion and absorption capacity and gene expression in hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 368-369, 117–128. doi: 10.1016/j.aquaculture.2012.09.019
Keywords: plant protein, fishmeal, digestible protein, amino acid balance, TOR pathway
Citation: Cai W, Liu H, Han D, Zhu X, Jin J, Yang Y and Xie S (2022) Complete Replacement of Fishmeal With Plant Protein Ingredients in Gibel Carp (Carassius auratus gibelio) Diets by Supplementation With Essential Amino Acids Without Negative Impact on Growth Performance and Muscle Growth-Related Biomarkers. Front. Mar. Sci. 8:759086. doi: 10.3389/fmars.2021.759086
Received: 15 August 2021; Accepted: 24 September 2021;
Published: 24 January 2022.
Edited by:
Jin Niu, Sun Yat-sen University, ChinaReviewed by:
Zhigang Zhou, Feed Research Institute, Chinese Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2022 Cai, Liu, Han, Zhu, Jin, Yang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haokun Liu, bGl1aGFva3VuQGloYi5hYy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.