
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 28 September 2021
Sec. Global Change and the Future Ocean
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.751983
This article is part of the Research TopicImmunity and Disease of Aquatic Organisms Under the Combined Impact of Anthropogenic Stressors: Mechanisms and Disease OutcomesView all 7 articles
Marine bivalves are frequently exposed to multiple co-occurring challenges such as temperature extremes and anthropogenic pollution. These stressors can elicit negative effects on several biological pathways, including antioxidant and neuroendocrine-immune (NEI) systems, leading to immune disorders and altered immunocytes functionality. Since interactive mechanisms of action and resulting outcomes are still scarcely explored, we examined the single and combined effects of increased temperature (+5°C) and cadmium (20 μg/L) in the Mediterranean mussel Mytilus galloprovincialis. Analyzed parameters included cholinergic system in gills and hemolymph (acetylcholinesterase activity, AChE), total oxyradical scavenging capacity in gills and key functional processes in hemocytes, including lysosomal membrane stability, hemocytes subpopulations ratio, phagocytosis capacity, and onset of genotoxic damage. Results highlighted interactive inhibition of AChE activity along to a concomitant increased total oxyradical scavenging capacity, confirming neuroendocrine-immune system (NEI) disturbance and oxidative pressure. In hemocytes, lysosomal membrane stability and granulocytes:hyalinocytes ratio revealed additive effects of stressors, while a consistent reduction of phagocytosis was caused by temperature stress, with a slightly antagonistic effect of cadmium. Pearson’s correlation statistics provided either positive or negative relationships between investigated parameters and stressors, allowing to hypothesize putative mechanism of immune system functional alterations. The overall results suggest that the occurrence of short-term events of increased temperature and concomitant metal exposure could elicit interactive and negative effects on immune system efficiency of marine organisms.
Marine bivalves living in coastal areas are currently subjected to multiple co-occurring stressors: organisms are exposed, among others, to relevant loads of inorganic pollutants deriving from land-based human activities (e.g., trace metals, EEA, 2019; Bates et al., 2021) and frequent events of thermal stress caused by ongoing ocean warming and increasing intensity of marine heatwaves (Oliver et al., 2018, 2019; IPCC, 2019). At the molecular and biological level, these stressors can interact through complex mechanisms and each stressor may reciprocally alter the susceptibility of the organism toward the others (Kroeker et al., 2017), triggering harsh outcomes at lower levels of biological organization which climax with mass mortality events with loss of biodiversity (Giuliani et al., 2013; Seuront et al., 2019; Sarà et al., 2021). Current knowledge on the interactions between thermal stress and trace metals on marine ectotherms biology suggests additive or synergic mechanisms, with thermal stress enhancing deleterious effects of metals and vice versa: temperature can trigger metal uptake through increased metabolism and membranes permeability, while, trace metals can impair oxygen metabolism and affect thermal tolerance (Lannig et al., 2008; Sokolova and Lannig, 2008; Lan et al., 2020).
Both trace metals and thermal stress are known to disrupt bivalves optimal physiological functioning through the inhibition of defense mechanisms, as the capability to counteract onset of oxidative conditions and to maintain the functionality of immune system. Trace metals enhance oxidative pressure through the impairment of electron transport chains or by catalyzing Fenton-like and Haber-Weiss reactions, thus increasing intracellular production of reactive oxygen species (ROS, Regoli and Giuliani, 2014; Benedetti et al., 2021); at the same time, both the amount and the efficiency of antioxidant defenses can be affected by trace metals, weakening the capability to scavenge ROS (Regoli and Principato, 1995; Regoli et al., 1998). Oxidative unbalance is promoted also by acute temperature elevation through boosted cellular ROS production caused by increased metabolic rate and progressive mitochondrial damage during and after thermal stress (Benedetti et al., 2021). The thresholds and limits of antioxidant network efficiency are typically influenced by species-specific, seasonal and ecological history traits. For instance, after 1 week of exposure to temperature elevation, the production of reactive oxygen and nitrogen species (ROS and RNS, respectively) was increased in Crassostrea virginica, but this was not paralleled by activation of antioxidant defenses at the higher temperature, suggesting homeostatic responses limits (Rahman and Rahman, 2021). A similar antioxidants block was described in Tridacna crocea exposed to acute heat stress (Zhou et al., 2021). Moreover, Mytilus congeners with different ecological history and adaptation to thermal stress showed different proteome responsiveness toward heat stress, with the less adapted species reducing aerobic metabolism as a trade-off to compensate the lower levels of chaperones and antioxidants (Tomanek, 2014). When oxidative pressure is not properly counteracted by the antioxidants network, the onset of oxidative damages to lipids, proteins, and DNA is observed in tissues of marine organisms (Izagirre et al., 2014; Múgica et al., 2015; Coppola et al., 2017).
In bivalves, the responsiveness and adaptive mechanisms toward external stressors stimuli are often regulated through the neuroendocrine-immune system (NEI), which consists in a plethora of hormones, neurotransmitters, receptors, and enzymes playing a fundamental role on immunocompetence, energy allocation, growth, and locomotion (Liu et al., 2018a). The cholinergic system is one of the most conserved pathways across all phyla: the neurotransmitter acetylcholine (ACh), released at synaptic level and hydrolyzed by acetylcholinesterase (AChE), has been demonstrated to play an immunomodulatory action in bivalves species (Shi et al., 2012; Chen et al., 2015; Liu et al., 2018b; Du et al., 2020). Disturbance of this system, as AChE inhibition promoted by several environmental stressors (Solé et al., 2009), could be thus hypothesized to affect immunocompetence in bivalves.
The cellular-mediated immune function in bivalves is carried out by hemocytes (Balbi et al., 2021), which are clustered in two main sub-populations based on their morphotypes and function: the granulocytes host several granules of lysosomal origin in the cytoplasm and are mainly involved in phagocytosis, while the hyalinocytes are involved in tissue repair mechanisms, hemopoiesis and as precursors of granulocytes (Bouallegui, 2019; Rey-Campos et al., 2019). Hemocytes functionality is highly susceptible to external disturbance, as hypoxia, microplastics, algal toxins, and pharmaceuticals (Gorbi et al., 2013; Avio et al., 2015; Mezzelani et al., 2018; Andreyeva et al., 2019): a transcriptomic and functional investigation revealed the upregulation of antioxidant, stress-response and apoptosis genes (GST, SOD, HSP70, MRP1, CAS8) linked to reduced phagocytosis in hemocytes of M. edulis exposed in vitro to Cd (Granger Joly de Boissel et al., 2017). On the other hand, bivalves exposed to thermal stress revealed immunological disorders as altered phagocytosis and hemocytes lysosomal membrane stability (Hégaret et al., 2003; Wu et al., 2016; Parisi et al., 2017; Rahman et al., 2019).
In a global scenario of frequent extreme temperature events and continuous inputs of anthropogenic pollutants, it is crucial to understand mechanisms of action and reciprocal effects of these stressors on immunocompetence of marine organisms. With this aim, the present study investigated the outcomes of single and combined exposure to acute thermal stress (+5°C) and cadmium (20 μg/L) in the Mediterranean mussel M. galloprovincialis. Despite this species is considered highly resilient to environmental stressors (Bitter et al., 2019; Gerdol et al., 2020), its tolerance toward ongoing ocean changes and multiple co-occurring stressors still needs to be fully elucidated. In this respect, several biological responses were investigated including biochemical, cellular, and functional alterations, as the activity of acetylcholinesterase in hemolymph and gills, the total oxyradical scavenging capacity in gills, hemocytes lysosomal membranes stability, subpopulations ratio (granulocytes:hyalinocytes), phagocytosis activity, and onset of genotoxic damages in terms of micronuclei frequency. The overall results were further elaborated to hypothesize putative mechanisms of cause-effect relationships between stressors and biological parameters. Results were expected to provide knowledge on immunological interactive effects of thermal and chemical stress, and on the mechanisms underlying the susceptibility of marine species to combined ongoing ocean changes and anthropogenic pollution.
Mussels, M. galloprovincialis (6.15 ± 0.6 shell length), were collected from an unpolluted area of Central Adriatic Sea (Bocchetti and Regoli, 2006). After collection, mussels were acclimated for 7 days to laboratory conditions in artificial seawater at local seasonal temperature (20°C) and salinity 37. Mussels were then exposed in 20 L tanks (20 organisms each) for 14 days to clean or Cd-contaminated seawater (20 μg/L) under two temperature scenarios (control = 20°C or heat stress, HS = 25°C), resulting in four experimental treatments: CTL (20°C, no Cd); Cd (20°C, 20 μg/L Cd); HS (25°C, no Cd); HS + Cd (25°C, 20 μg/L Cd). Thermal stress condition (25°C) was chosen to simulate a short-term heatwave scenario based on past events of marine heatwaves in the Mediterranean sea,1,2 while cadmium concentration was based on environmentally realistic scenarios of polluted coastal areas (Neff, 2002; Beiras et al., 2003; Martín-Díaz et al., 2005; AbdAllah and Moustafa, 2007; Süren et al., 2007). During experimental phase, organisms were fed with a zooplankton mixture for marine filter-feeders (50–300 μm) 12 h before the water renewal, planned every other day, when cadmium was re-dosed at nominal concentration. At the end of the exposure, hemolymph was withdrawn from the adductor muscle and gills excised: 5 samples were prepared each constituted by tissues of 4 individuals for every experimental condition. Collected samples were immediately used for in vivo analyses (hemocytes lysosomal membrane stability, granulocytes:hyalinocytes ratio, phagocytosis rate), flash frozen in liquid nitrogen and stored at −80°C for measurement of acetylcholinesterase activity and total oxyradical scavenging capacity or fixed in Carnoy solution 1:3 acetic acid:methanol for micronuclei frequency determination.
Acetylcholinesterase activity (AChE) was measured in the hemolymph (supernatant obtained from centrifugation at 3,000 g for 5 min) and gills (homogenated in TrisHCl 100 mM pH 7.2 and 250 mM sucrose and centrifuged at 10,000 g for 10 min). Enzyme activity was spectrophotometrically determined through Ellman’s reaction according to Solé et al. (2009), using acetylthiocholine and 5,5′-dithiobis (2-nitrobenzoic acid), normalized to protein concentration, determined according to Lowry method with bovine serum albumine (BSA) as standard.
The overall capability of cellular antioxidants to neutralize peroxyl (ROO•) and hydroxyl (HO•) radicals was measured through the total oxyradical scavenging capacity (TOSC) assay, by evaluating the inhibition of 0.2 mM α-keto-γ-methiolbutyric acid (KMBA) oxidation to ethylene gas (Regoli and Winston, 1998; Regoli et al., 2002). Thermal homolysis of 20 mM 2-2-azo-bis-(2-methylpropionamide)-dihydrochloride (ABAP) and Fenton reaction of 1.8 μM iron-3.6 μM EDTA plus 180 μM ascorbate in 100 mM phosphate buffer pH 7.4 were used to generate peroxyl (ROO•) and hydroxyl (HO•) radicals, respectively. Gas-chromatographic analyses were used to determine ethylene formation at 10 min intervals in each sample and control reaction and used in the equation: TOSC = 100−(∫SA/∫CA × 100), where ∫SA and ∫CA are the integral areas calculated from the ethylene kinetic curve measured during the reaction, for sample (SA) and control (CA) reactions, respectively. For each sample, a specific TOSC value was calculated by normalizing the experimental TOSC to the relative protein concentration.
Aliquots (50 μL) of hemolymph were dispersed on glass slides and dried for 15 min. Slices were then placed for 15 min in Baker’s Fixative. After fixation, slides were stained with Hematoxylin-Eosin, mounted with Eukitt and observed at bright-field microscope; granulocytes:hyalinocytes ratio was determined by counting and evaluating at least 200 cells in each sample (Mezzelani et al., 2018).
Phagocytosis assay was performed by dispersing 50 μL of hemolymph on glass slides and allowing cells adhesion for 15 min at 4°C in a dark chamber. After adhesion, 50 μL of Fluorescein-labeled Zymosan A solution (Bioparticles—Invitrogen, 2.7 × 106 particles/mL) were added to each slide and samples were incubated for 2 h at 4°C in a dark room. After washing and fixation in Baker’s Fixative, slides were observed at fluorescence microscope counting the percentage of positive cells (those that internalized at least 3 fluorescent Zymosan particles) on a total of at least 300 cells for each sample (Gorbi et al., 2013).
The lysosomal membrane stability was assessed by the neutral red retention time assay (Gorbi et al., 2013). Hemolymph aliquots (50 μL) were dispersed on glass slides with 50 μL of Neutral Red working solution (2 μl/ml filtered seawater from a stock solution of 20 mg neutral red dye dissolved in 1 ml of dimethyl sulfoxide) and examined every 20 min to count the cells that had released the dye from lysosomes to the cytosol. Results are expressed as the time (in minutes) at which 50% of examined cells had Neutral Red in the cytosol.
The frequency of micronuclei was evaluated on 50 μL hemolymph aliquots previously fixed in Carnoy solution (1:3 acetic acid, methanol), placed on glass slides and stained with DAPI (4′,6-diamidino-2-phenylindole, 100 ng/mL). In each sample, 2000 cells were observed and the frequency of micronuclei determined, as the number of round structures clearly separated from the main nucleus and smaller than 1/3 of this on the total cells count (Regoli et al., 2014).
All statistical analyses were performed using RStudio (version 1.2.5033). Two-way analysis of variance (ANOVA) was applied to test the hypothesis that Cd-exposure, heat stress and their interaction had no significant effect on analyzed parameters. F- and p-values are shown in Table 1. Normal distribution and homogeneity of variances were checked through Shapiro-Wilk and Levene tests, respectively; significant differences among treatments were highlighted through Tukey HSD post hoc (p < 0.05).
Pearson correlation analyses were conducted on whole dataset of biological parameters, including Cd exposure and temperature as potential explanatory variables. Principal components analysis was applied to visualize relationships between treatments and contribution of variables to separation.
AChE activity was significantly reduced in hemolymph by increased temperature (Table 1, F = 11.82, p < 0.01) with a synergistic effect observed during co-exposure to temperature and cadmium (Figure 1A, HS + Cd vs. CTL). Temperature (Table 1, F = 14.56, p < 0.01) significantly affected AChE activity also in the gills (Figure 1B), but in this tissue co-exposure with Cd slightly softened this effect.
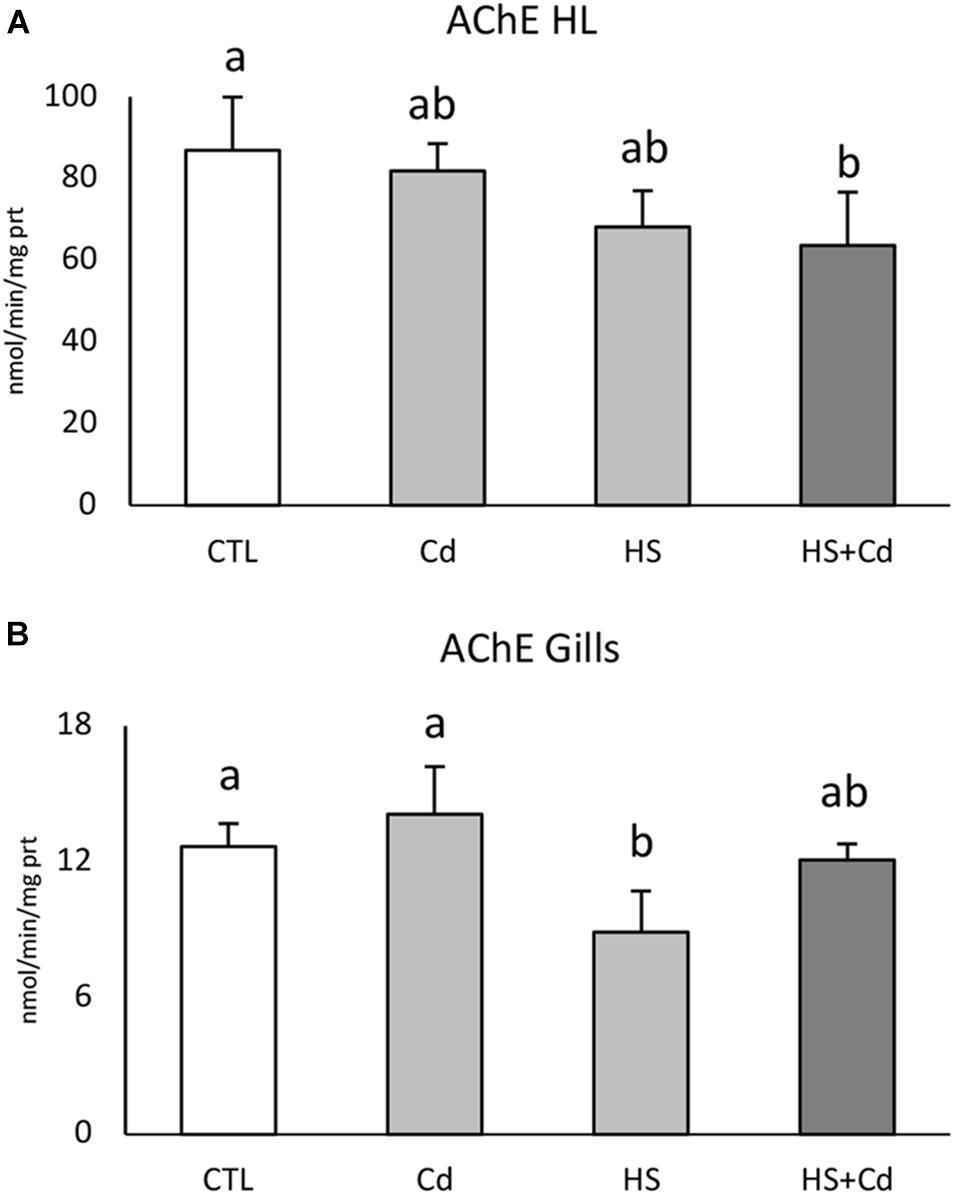
Figure 1. Acetylcholinesterase activity in hemolymph (A) and gills (B) of exposed mussels. Values are given as nmol/min/mg of protein, mean ± standard deviation, n = 4. Letters are used to highlight significant differences between group of means, CTL, Control; Cd, Cadmium exposure; HS, heat stress; HS + Cd, Cadmium exposure under heat stress.
Temperature significantly affected TOSC ROO• in gills (Figure 2A and Table 1, F = 20.14, p < 0.001) with no additional modulation during co-exposure with Cd. An increased capability to counteract HO• was observed in organisms exposed to Cd and higher temperature, both alone and in combination, with significant interaction of stressors (Figure 2B and Table 1, F = 19.32, p < 0.001).
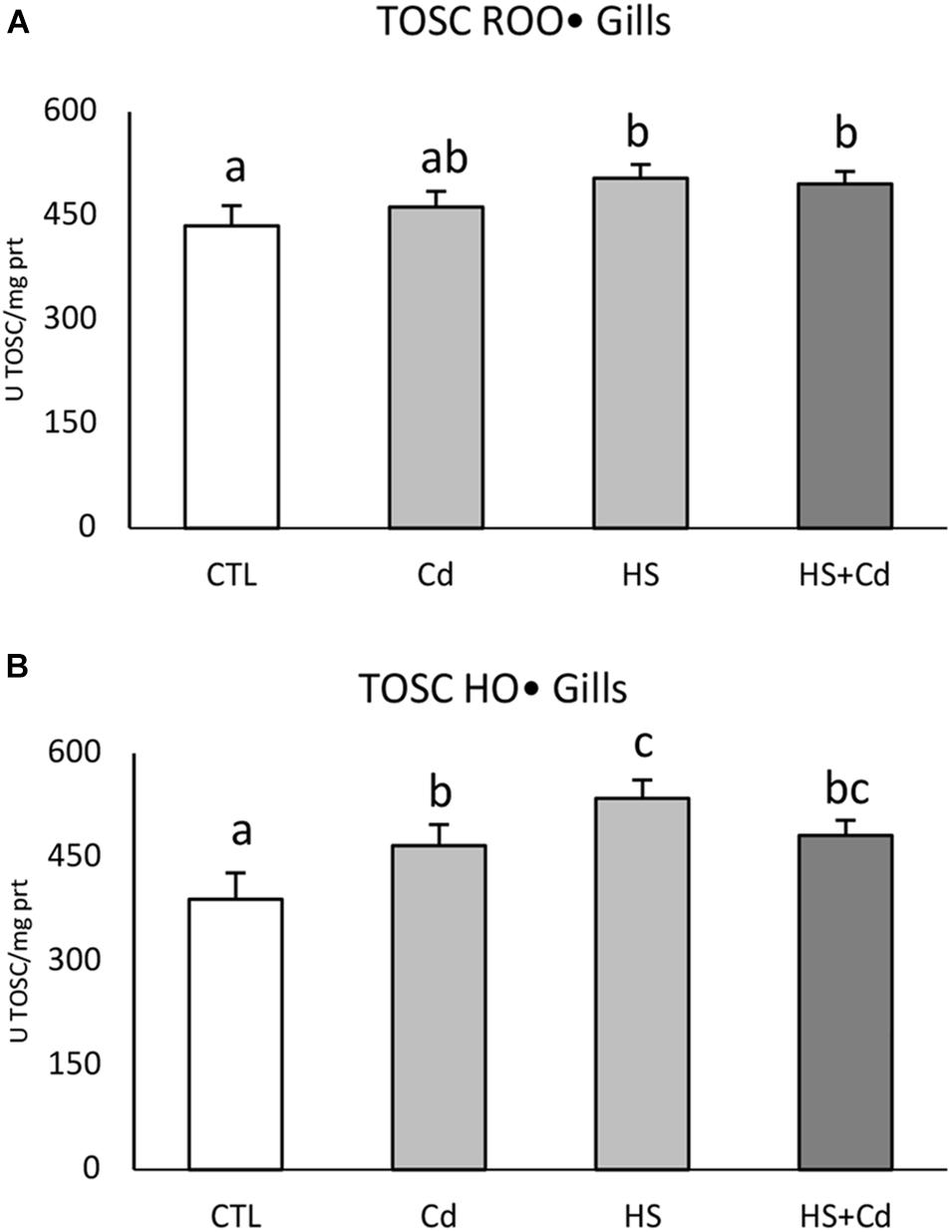
Figure 2. Total oxyradical scavenging capacity toward peroxyl (TOSC ROO•, A) and hydroxyl (TOSC HO•, B) radicals in gills of exposed organisms. Values are given as TOSC units/mg of proteins, mean ± standard deviation, n = 4. Letters are used to highlight significant differences between group of means, CTL, Control; Cd, Cadmium exposure; HS, heat stress; HS + Cd, Cadmium exposure under heat stress.
Cd-exposure significantly affected granulocytes:hyalinocytes ratio, but an antagonistic effect of temperature in co-exposed organisms revealed synergistic interaction of stressors (Figure 3A and Table 1, F = 4.821, p < 0.05). Two-way analysis of variance revealed a significant modulation of temperature on phagocytosis rate (Figure 3B and Table 1, F = 92.27, p < 0.001) which was lowered in HS treated organisms either with or without Cd. Lysosomal membrane stability was reduced by both Cd and higher temperature with a cumulative significant effect in co-exposed organisms (Figure 3C). Cd-exposure increased MN frequency (Figure 3D and Table 1, F = 4.8, p < 0.05), with no significant differences between treatments.
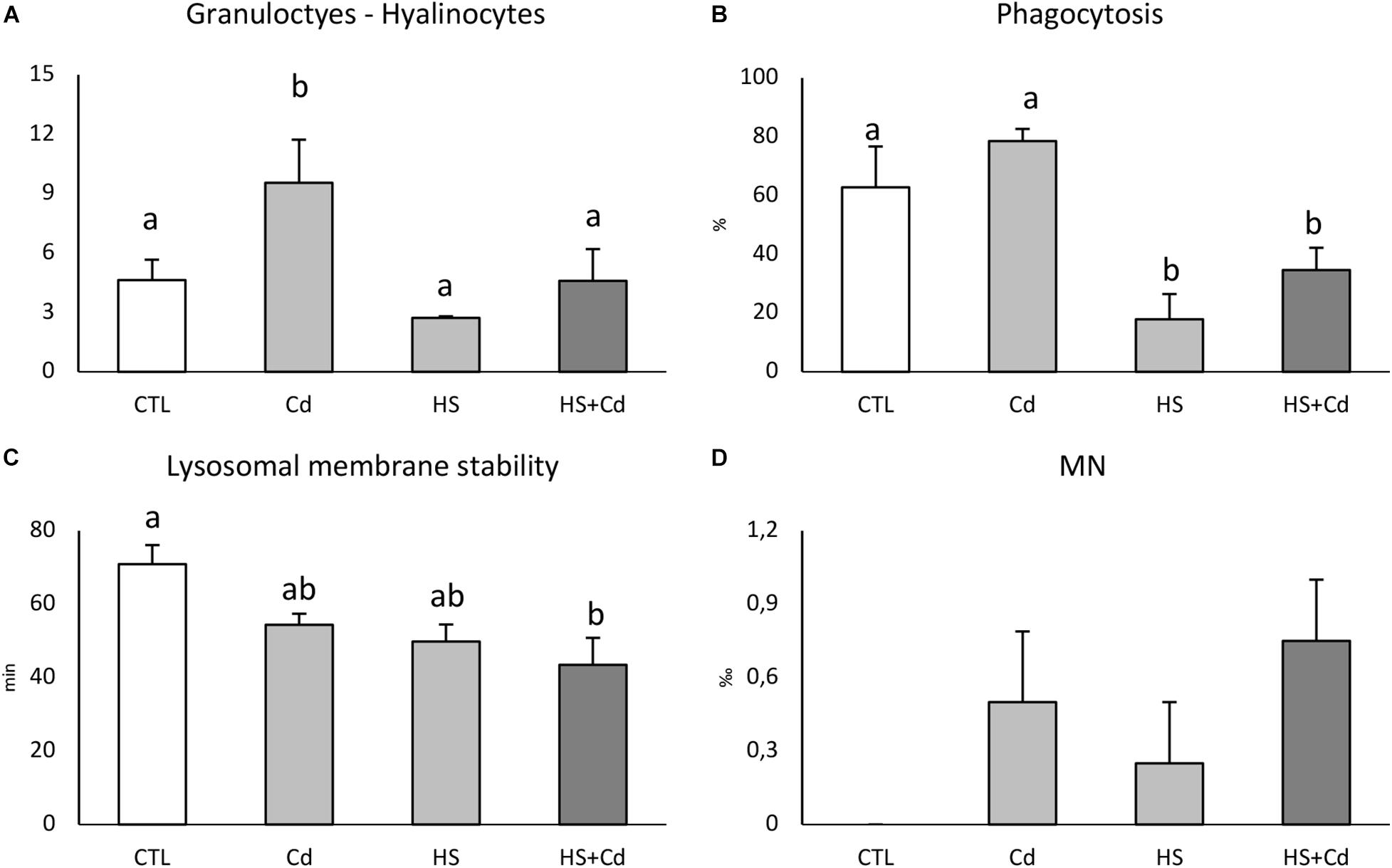
Figure 3. Hemocytes functional analyses of exposed mussels. Granulocytes:hyalinocytes ratio (A), phagocytosis rate (B), lysosomal membrane stability (C), and micronuclei frequency (D). Values are given as mean ± standard deviation, n = 4. Letters are used to highlight significant differences between group of means, CTL, Control; Cd, Cadmium exposure; HS, heat stress; HS + Cd, Cadmium exposure under heat stress.
Correlation analyses (Figure 4) revealed that temperature scenario was negatively correlated with immune parameters as phagocytosis (R = −0.89), granulocytes:hyalinocytes ratio (R = −0.61), lysosomal membrane stability (R = −0.58) and with acetylcholinesterase activity in both gills and hemolymph (R = −0.63 and R = −0.69, respectively). At the same time, a positive relationship was observed between temperature and both TOSC ROO• (R = 0.76) and TOSC HO• (R = 0.69). Conversely, Cd-exposure was positively related only with AChE activity in gills (R = 0.5), granulocytes:hyalinocytes ratio (R = 0.6) and micronuclei frequency (R = 0.52). Among biological parameters, phagocytosis was negatively correlated with TOSC ROO• (R = −0.7) and TOSC HO• (R = −0.68), while positively correlated with granulocytes:hyalinocytes ratio (R = 0.77) and AChE activity in gills (R = 0.7); TOSC ROO• and TOSC HO• were negatively correlated with lysosomal membrane stability (R = −0.55 and R = −0.51, respectively). Other relevant correlations were found between TOSC ROO• and AChE activity in hemolymph and in gills (R = −0.62 and R = −0.54, respectively), TOSC ROO• and TOSC HO• (R = 0.82), and between AChE activity in gills and granulocytes:hyalinocytes (R = 0.73).
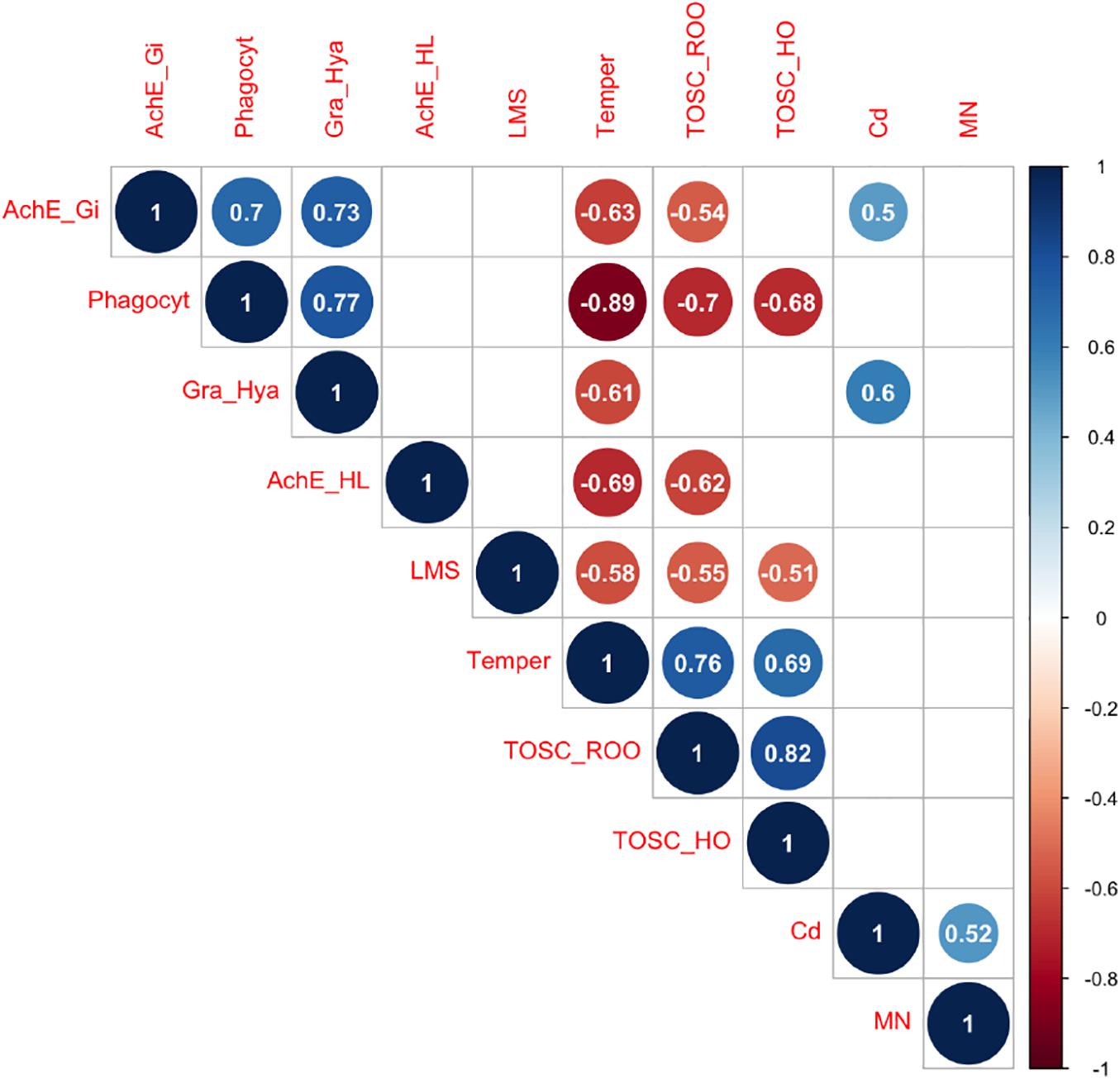
Figure 4. Pearson correlation heatmap showing correlation coefficients—blue and red indicate positive and negative correlation, respectively. Only significant correlations (p < 0.05) are shown.
Principal components analysis provided a two-dimensional pattern explaining 71% of total variance (Figure 5): a clear separation occurred along Dim1 (50.3%) between organisms maintained at control temperature (CTL and Cd) and those at higher temperature (HS and HS + Cd): the main parameters contributing to this separation along Dim1 were TOSC ROO•, phagocytosis, TOSC HO•, and AChE in gills. A further discrimination was revealed along Dim2 (20.7%), especially between organisms exposed at higher temperature alone (HS) or with Cd (HS + Cd), mainly due to micronuclei frequency, lysosomal membrane stability and granulocytes:hyalinocytes ratio.
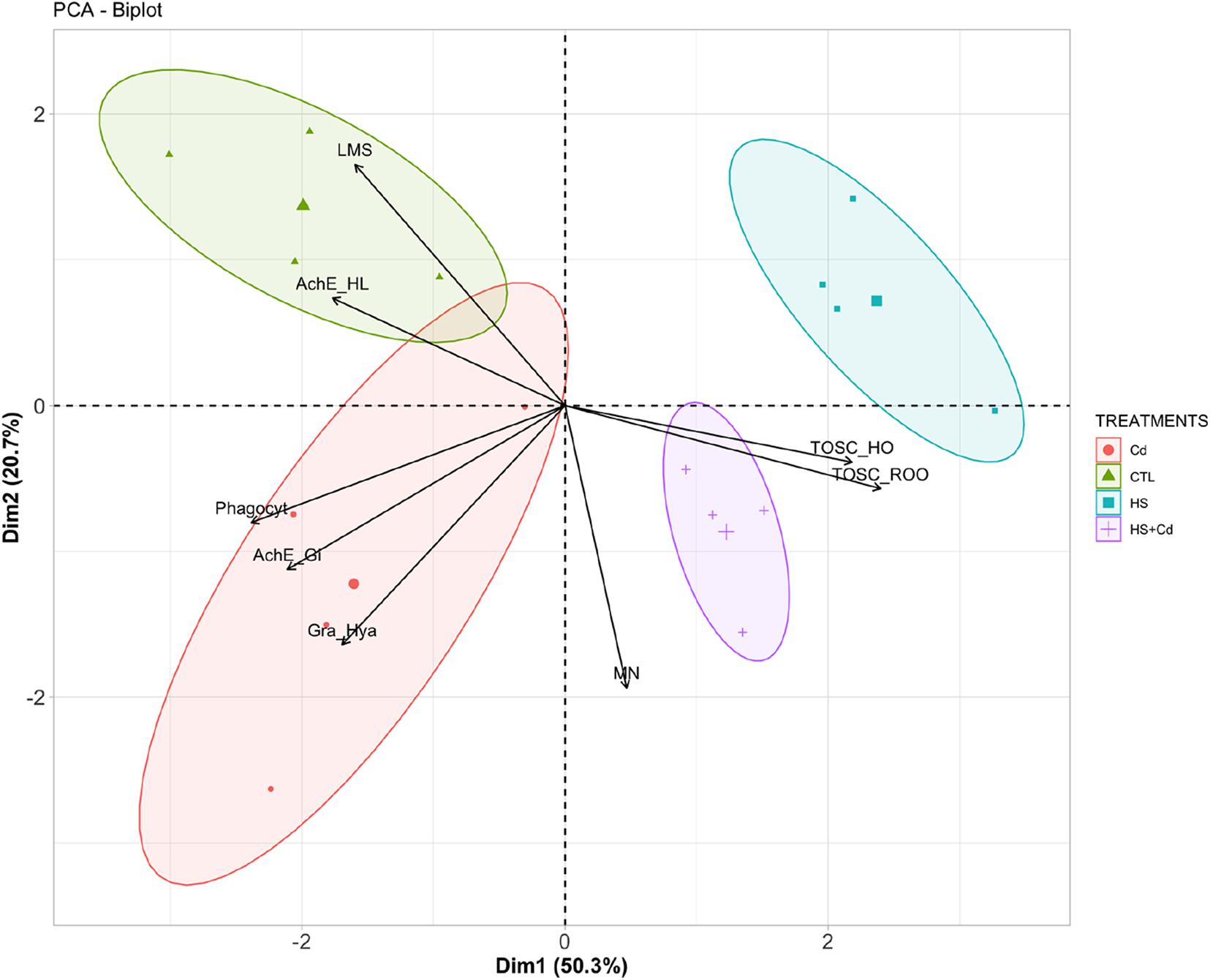
Figure 5. Graphical representation of principal component analysis. CTL, Control; Cd, Cadmium (20 μg/L) at control temperature; HS, heat stress (25°C); HS + Cd, heat stress (25°C) + cadmium (20 μg/L)—each variable contribution to the separation is represented by arrows.
Correlation coefficients of significant relationships (p < 0.05) were used to hypothesize putative cascade of relationships among tested stressors and investigated biological parameters (Figure 6), with the aim of providing a visual overview of results and discussed hypotheses.
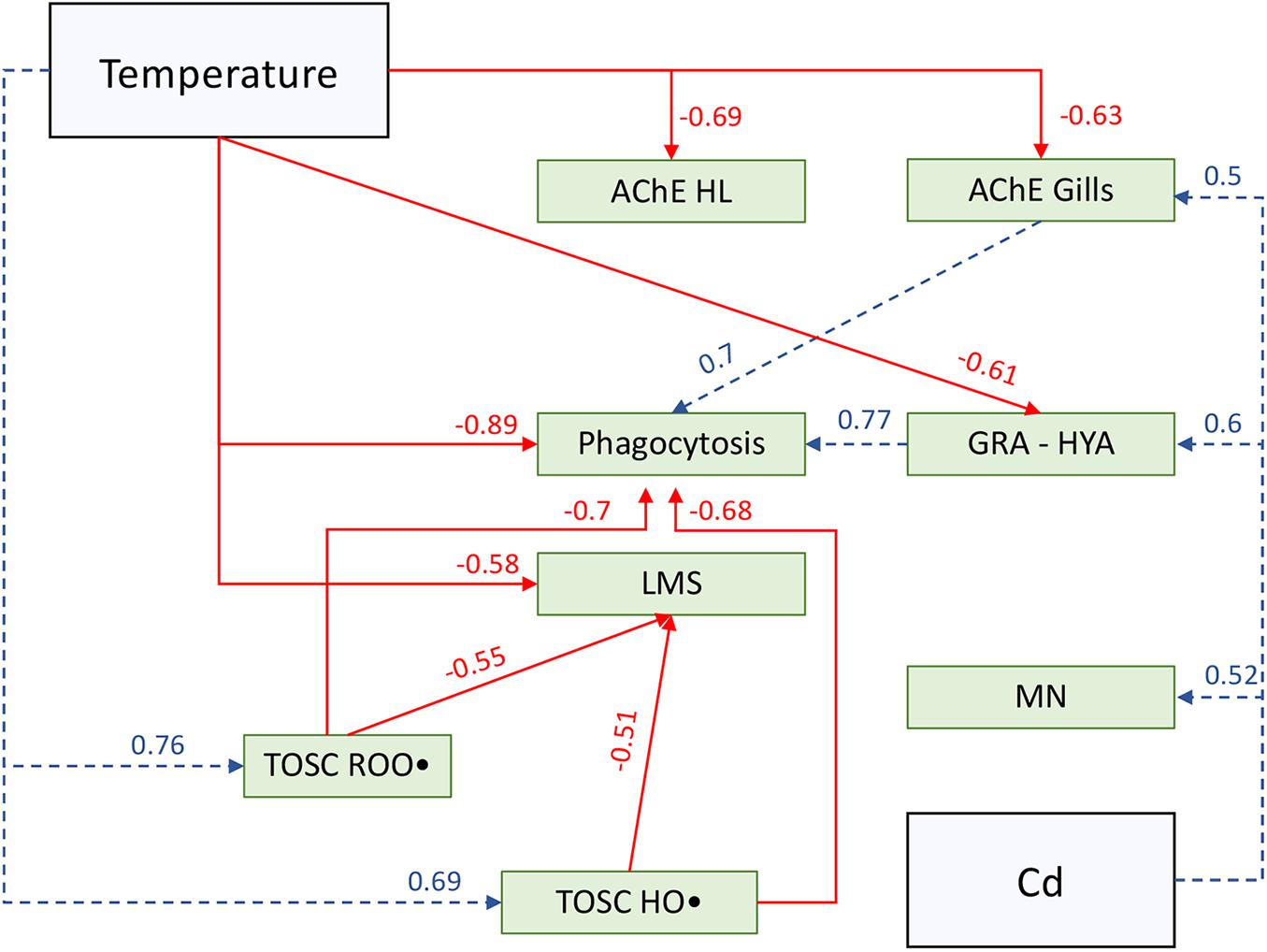
Figure 6. Schematic overview of putative cascade of effects among tested stressors (blue squares) and biological parameters (green squares) based on significant (p < 0.05) correlation coefficient (Pearson correlation analysis) and discussed hypotheses. Solid red lines represent significant negative correlations, while dotted blue lines represent significant positive correlations; arrows direction is based on putative cause-effect relationships. AChE HL, acetylcholinesterase activity in hemolymph; AChE Gills, acetylcholinesterase activity in gills; Phagocytosis, hemocytes phagocytosis rate; GRA-HYA, granulocytes on hyalinocytes ratio; LMS, hemocytes lysosomal membrane stability; MN, hemocytes micronuclei frequency; TOSC ROO•, total oxyradical scavenging capacity toward ROO• in gills; TOSC HO•, total oxyradical scavenging capacity toward HO• in gills; Temperature, exposure temperature scenario; Cd, cadmium exposure.
Marine organisms are challenged by multiple co-occurring stressors that interact through complex mechanisms on key biological processes, including immune system competence. The present investigation provides insights on the sensitivity of the Mediterranean mussel M. galloprovincialis revealing the responsiveness and possible interactions between antioxidant, cholinergic and immune systems under combined conditions of thermal stress and cadmium exposure, allowing to provide putative cause-effect mechanisms.
Our study revealed a certain responsiveness of the cholinergic system toward tested stressors: increased temperature lowered acetylcholinesterase activity in both hemolymph and gills, with different tissue responsiveness to additional stressors. In hemolymph, additive negative effects were observed for temperature and cadmium, while in the gills the effect of temperature was stronger than that of cadmium. Despite the mechanisms of cholinergic reduction by temperature increase are still to be clarified, previous studies showed the possibility for AChE to be inactivated above the optimal thermal range: our results are in accordance with the negative relationship between temperature and acetylcholinesterase activity described for M. galloprovincialis and M. arenaria (Greco et al., 2011; Kamel et al., 2012). On the other hand, several classes of pollutants have been shown to alter AChE activity, including organophosphorus and carbamate pesticides which directly interact with the active site of the enzyme, or trace metals exposure which also provoke AChE activity inhibition in marine organisms (Lehtonen and Leiniö, 2003; Bonacci et al., 2008; Attig et al., 2010).
The cellular innate immune response showed a consistent sensitivity in terms of both subpopulations composition and their functionality, with stressor-dependent effects. Cd-exposure increased the granulocytes:hyalinocytes ratio at control temperature, without a parallel variation of phagocytosis activity: this suggests either a decrease of hyalinocytes subpopulation or a lower phagocytosis efficiency of granulocytes, main actors of this defense mechanisms (Andreyeva et al., 2019). Conversely, phagocytosis, but not granulocytes:hyalinocytes ratio, was consistently weakened in organisms exposed at higher temperature, allowing to hypothesize a temperature-mediated mechanism on granulocytes function, as already described in C. virginica and M. coruscus subjected to 1-week thermal stress (Hégaret et al., 2003; Wu et al., 2016).
Given the negative correlation observed between temperature and both phagocytosis and acetylcholinesterase activity, and the positive relationship between branchial AChE and phagocytosis, is possible to hypothesize that the effects of temperature on immune system are mediated through the combined disturbance of neuroendocrine and immune system, as summarized in Figure 6. Indeed, the cholinergic system is part of the neuroendocrine-immune regulatory network and thus plays a fundamental role in immunoregulation processes in bivalves (Liu et al., 2018b). Thermal stress is known to cause incorrect proteins folding and is thus likely that the lower AChE activity may be, at least partially, due to temperature-mediated damage to proteins. The consequences of the inhibition of AChE activity, resulting in excess accumulation of ACh, may not be limited to impairment of neurotransmission. It has been recently demonstrated that incubation with the neurotransmitter ACh significantly reduced hemocytes phagocytosis in Tegillarca granosa by disrupting Ca2+ homeostasis and altering NF-κB signaling pathway (Du et al., 2020); conversely, incubation of Chlamys farreri hemocytes with immune-stimulants LPS and TNF-α up-regulated the expression of AChE mRNA (Shi et al., 2012). Overall, available literature suggests that ACh turnover influences a prompt immune response in bivalves: according to this, our results allow to hypothesize that thermal stress may disturb mussels NEI by affecting neurotransmitters metabolism, thus representing a threat for mussels pathogens response.
Nonetheless, hemocytes showed a reduced lysosomal membrane integrity following the combined action of temperature stress and cadmium exposure, revealing additive negative effects of multiple stressors on such cellular organelles (Figure 6). Lysosomes are fundamental for granulocytes phagocytosis (Pipe, 1990; Cajaraville and Pal, 1995; Pipe et al., 1997), and lysosomal membrane destabilization has been previously addressed in several marine bivalves as a marker of disturbance by thermal stress (Marigómez et al., 2017; Parisi et al., 2017), metals (Regoli, 1992; Calisi et al., 2008), and combined effects of these stressors (Izagirre et al., 2014). Mechanisms underlying this sensitivity can involve either membrane disruption caused by oxidative stress or Ca2+, caspase-3, p38-MAPK, and JNK signaling pathways (Canesi et al., 2007; Yao and Somero, 2012). The activation of these pathways has been even suggested to be involved in micronuclei formation (Decordier et al., 2005), which in our study increased in all experimental treatments, particularly in Cd-exposed organisms (Figure 6). Micronuclei formation may be promoted by pro-oxidative challenge triggered by tested stressors either directly, with an increased ROS production, or indirectly, affecting antioxidant defenses efficiency (Regoli and Giuliani, 2014; Benedetti et al., 2021). In our study, exposure at higher temperature was significantly related to an increased capability to counteract peroxyl radical, with no additional effect of cadmium-exposure (Figure 6); on the other hand, hydroxyl radical scavenging capacity was subjected to synergistic interactions between temperature and cadmium, despite a stronger modulation by thermal stress was further highlighted. These results suggest an increased demand to maintain the oxyradical metabolism in M. galloprovincialis, confirming the pro-oxidant nature of investigated stressors. A similar trend of increased antioxidant defenses in M. galloprovincialis was highlighted in the same temperature range by Rahman et al. (2019), but in that study this was coupled with an increased phagocytosis activity in hemocytes. Conversely, in our study, mussels appeared to counteract the onset of thermal oxidative challenge by enhancing the total oxyradical scavenging capacity toward both ROO• and HO• which however, did not prevent the loss of hemocytes lysosomal membranes stability (LMS), thus indicating a greater level of oxidative disfunction that may have contributed to a lower phagocytosis activity (Figure 6).
The two-dimensional pattern provided by principal components analysis confirmed synergistic effects of investigated stressors, with clear divergence between Cd-exposed and non-exposed organisms (along Dimension 2) further enhanced at higher temperature (HS and HS + Cd) compared to control temperature scenario (CTL and Cd).
On the whole, results highlighted synergistic-stress mechanisms on the co-occurring alteration of NEI and activation of antioxidant system in M. galloprovincialis exposed to thermal stress and cadmium. Putative cross-talk between NEI and antioxidant system suggests that complex mechanisms modulate organisms physiological functions. Immune efficiency of M. galloprovincialis might be highly susceptible under short-term extreme temperature events and contaminants exposure, but field observations and more in-depth assessments through novel molecular approaches are needed to fully enlighten compensation mechanisms and long-term susceptibility.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AN, MB, and FR: design of the study. AN: data collection, statistical analyses, and original draft writing. MB and SG: data elaboration supervision and critical review of the manuscript. AN and FR: results discussion. FR: activities supervision, critical review of the manuscript, and final draft writing. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbdAllah, A. T., and Moustafa, M. A. (2007). Accumulation of lead and cadmium in the marine prosobranch Nerita saxtilis, chemical analysis, light and electron microscopy. Environ. Pollut. 116, 185–191. doi: 10.1016/S0269-7491(01)00137-3
Andreyeva, A. Y., Efremova, E. S., and Kukhareva, T. A. (2019). Morphological and functional characterization of hemocytes in cultivated mussel (Mytilus galloprovincialis) and effect of hypoxia on hemocyte parameters. Fish Shellfish Immunol. 89, 361–367. doi: 10.1016/j.fsi.2019.04.017
Attig, H., Dagnino, A., Negri, A., Jebali, J., Boussetta, H., Viarengo, A., et al. (2010). Uptake and biochemical responses of mussels Mytilus galloprovincialis exposed to sublethal nickel concentrations. Ecotoxicol. Environ. Saf. 73, 1712–1719. doi: 10.1016/j.ecoenv.2010.08.007
Avio, C. G., Gorbi, S., Milan, M., Benedetti, M., Fattorini, D., D’Errico, G., et al. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 198, 211–222. doi: 10.1016/j.envpol.2014.12.021
Balbi, T., Auguste, M., Ciacci, C., and Canesi, L. (2021). Immunological responses of marine bivalves to contaminant exposure: contribution of the-omics approach. Front. Immunol. 12:618726. doi: 10.3389/fimmu.2021.618726
Bates, E. H., Alma, L., Ugrai, T., Gagnon, A., Maher, M., McElhany, P., et al. (2021). Evaluation of the effect of local water chemistry on trace metal accumulation in puget sound shellfish shows that concentration varies with species, size, and location. Front. Mar. Sci. 8:636170. doi: 10.3389/fmars.2021.636170
Beiras, R., Bellas, J., Fernández, N., Lorenzo, J. I., and Cobelo-García, A. (2003). Assessment of coastal marine pollution in Galicia (NW Iberian Peninsula); metal concentrations in seawater, sediments and mussels (Mytilus galloprovincialis) versus embryo-larval bioassays using Paracentrotus lividus and Ciona intestinalis. Mar. Environ. Res. 56, 531–553. doi: 10.1016/S0141-1136(03)00042-4
Benedetti, M., Giuliani, M. E., Mezzelani, M., Nardi, A., Pittura, L., Gorbi, S., et al. (2021). Emerging environmental stressors and oxidative pathways in marine organisms: current knowledge on regulation mechanisms and functional effects. Biocell. doi: 10.32604/biocell.2021.017507
Bitter, M. C., Kapsenberg, L., Gattuso, J.-P., and Pfister, C. A. (2019). Standing genetic variation fuels rapid adaptation to ocean acidification. Nat. Commun. 10:5821. doi: 10.1038/s41467-019-13767-1
Bocchetti, R., and Regoli, F. (2006). Seasonal variability of oxidative biomarkers, lysosomal parameters, metallothioneins and peroxisomal enzymes in the Mediterranean mussel Mytilus galloprovincialis from Adriatic Sea. Chemosphere 65, 913–921. doi: 10.1016/j.chemosphere.2006.03.049
Bonacci, S., Corsi, I., and Focardi, S. (2008). Cholinesterase activities in the scallop Pecten jacobaeus: characterization and effects of exposure to aquatic contaminants. Sci. Total Environ. 392, 99–109. doi: 10.1016/j.scitotenv.2007.11.029
Bouallegui, Y. (2019). Immunity in mussels: an overview of molecular components and mechanisms with a focus on the functional defenses. Fish Shellfish Immunol. 89, 158–169. doi: 10.1016/j.fsi.2019.03.057
Cajaraville, M. P., and Pal, S. G. (1995). Morphofunctional study of the haemocytes of the bivalve mollusc Mytilus galloprovincialis with emphasis on the endolysosomal compartment. Cell Struct. Funct. 20, 355–367. doi: 10.1247/csf.20.355
Calisi, A., Lionetto, M. G., Caricato, R., Giordano, M. E., and Schettino, T. (2008). Morphometric alterations in Mytilus galloprovincialis granulocytes : a new biomarker. Environ. Toxicol. Chem. 27, 1435–1441. doi: 10.1897/07-396
Canesi, L., Lorusso, L. C., Ciacci, C., Betti, M., Regoli, F., Poiana, G., et al. (2007). Effects of blood lipid lowering pharmaceuticals (bezafibrate and gemfibrozil) on immune and digestive gland functions of the bivalve mollusc, Mytilus galloprovincialis. Chemosphere 69, 994–1002. doi: 10.1016/j.chemosphere.2007.04.085
Chen, H., Wang, L., Zhou, Z., Hou, Z., Liu, Z., Wang, W., et al. (2015). The comprehensive immunomodulation of NeurimmiRs in haemocytes of oyster Crassostrea gigas after acetylcholine and norepinephrine stimulation. BMC Genomics 16:942. doi: 10.1186/s12864-015-2150-8
Coppola, F., Almeida, Â, Henriques, B., Soares, A. M. V. M., Figueira, E., Pereira, E., et al. (2017). Biochemical impacts of Hg in Mytilus galloprovincialis under present and predicted warming scenarios. Sci. Total Environ. 601–602, 1129–1138. doi: 10.1016/j.scitotenv.2017.05.201
Decordier, I., Cundari, E., and Kirsch-Volders, M. (2005). Influence of caspase activity on micronuclei detection: a possible role for caspase-3 in micronucleation. Mutagenesis 20, 173–179. doi: 10.1093/mutage/gei025
Du, X., Tang, Y., Han, Y., Ri, S., Kim, T., Ju, K., et al. (2020). Acetylcholine suppresses phagocytosis via binding to muscarinic- and nicotinic-acetylcholine receptors and subsequently interfering Ca2+- and NFκB-signaling pathways in blood clam. Fish Shellfish Immunol. 102, 152–160. doi: 10.1016/j.fsi.2020.04.030
EEA (2019). The European environment–state and outlook 2020. Knowledge for transition to a sustainable Europe. Luxembourg: Publications Office of the European Union. doi: 10.2800/96749 ISBN 978-92-9480-090-9.
Gerdol, M., Moreira, R., Cruz, F., Gómez-Garrido, J., Vlasova, A., Rosani, U., et al. (2020). Massive gene presence-absence variation shapes an open pan-genome in the Mediterranean mussel. Genome Biol. 21:275. doi: 10.1186/s13059-020-02180-3
Giuliani, M. E., Benedetti, M., Arukwe, A., and Regoli, F. (2013). Transcriptional and catalytic responses of antioxidant and biotransformation pathways in mussels, Mytilus galloprovincialis, exposed to chemical mixtures. Aquat. Toxicol. 13, 120–127. doi: 10.1016/j.aquatox.2013.03.012
Gorbi, S., Avio, G. C., Benedetti, M., Totti, C., Accoroni, S., Pichierri, S., et al. (2013). Effects of harmful dinoflagellate Ostreopsis cf. ovata exposure on immunological, histological and oxidative responses of mussels Mytilus galloprovincialis. Fish Shellfish Immunol. 35, 941–950. doi: 10.1016/j.fsi.2013.07.003
Granger Joly de Boissel, P., Fournier, M., Rodriguez-Lecompte, J. C., McKenna, P., Kibenge, F., and Siah, A. (2017). Functional and molecular responses of the blue mussel Mytilus edulis’ hemocytes exposed to cadmium-An in vitro model and transcriptomic approach. Fish Shellfish Immunol. 67, 575–585. doi: 10.1016/j.fsi.2017.06.001
Greco, L., Pellerin, J., Capri, E., Garnerot, F., Louis, S., Fournier, M., et al. (2011). Physiological effects of temperature and a herbicide mixture on the soft-shell clam Mya arenaria (Mollusca. Bivalvia). Environ. Toxicol. Chem. 30, 132–141. doi: 10.1002/etc.359
Hégaret, H., Wikfors, G. H., and Soudant, P. (2003). Flow cytometric analysis of haemocytes from eastern oysters, Crassostrea virginica, subjected to a sudden temperature elevation II. Haemocyte functions: aggregation, viability, phagocytosis, and respiratory burst. J. Exp. Mar. Biol. Ecol. 293, 249–265. doi: 10.1016/S0022-0981(03)00235-1
IPCC (2019). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, eds D. C. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, K. Mintenbeck, et al. Geneva: Intergovernmental Panel on Climate Change.
Izagirre, U., Errasti, A., Bilbao, E., Múgica, M., and Marigómez, I. (2014). Combined effects of thermal stress and Cd on lysosomal biomarkers and transcription of genes encoding lysosomal enzymes and HSP70 in mussels, Mytilus galloprovincialis. Aquat. Toxicol. 149, 145–156. doi: 10.1016/j.aquatox.2014.01.013
Kamel, N., Attig, H., Dagnino, A., Boussetta, H., and Banni, M. (2012). Increased temperatures affect oxidative stress markers and detoxification response to benzo[a]pyrene exposure in mussel Mytilus galloprovincialis. Arch. Environ. Contam. Toxicol. 63, 534–543. doi: 10.1007/s00244-012-9790-3
Kroeker, K. J., Kordas, R. L., and Harley, C. D. G. (2017). Embracing interactions in ocean acidification research: confronting multiple stressor scenarios and context dependence. Biol. Lett. 13:20160802. doi: 10.1098/rsbl.2016.0802
Lan, W.-R., Huang, X.-G., Lin, L.-X., Li, S.-X., and Liu, F.-J. (2020). Thermal discharge influences the bioaccumulation and bioavailability of metals in oysters: implications of ocean warming. Environ. Pollut. 259:113821. doi: 10.1016/j.envpol.2019.113821
Lannig, G., Cherkasov, A. S., Pörtner, H.-O., Bock, C., and Sokolova, I. M. (2008). Cadmium-dependent oxygen limitation affects temperature tolerance in eastern oysters (Crassostrea virginica Gmelin). Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1338–R1346. doi: 10.1152/ajpregu.00793.2007
Lehtonen, K. K., and Leiniö, S. (2003). Effects of exposure to copper and malathion on metallothionein levels and acetylcholinesterase activity of the mussel Mytilus edulis and the clam Macoma balthica from the Northern Baltic Sea. Bull. Environ. Contam. Toxicol. 71, 489–496. doi: 10.1007/s00128-003-8853-6
Liu, Z., Li, M., Yi, Q., Wang, L., and Song, L. (2018a). The neuroendocrine-immune regulation in response to environmental stress in marine bivalves. Front. Physiol. 9:1456. doi: 10.3389/fphys.2018.01456
Liu, Z., Wang, L., Lv, Z., Zhou, Z., Wang, W., Li, M., et al. (2018b). The cholinergic and adrenergic autocrine signaling pathway mediates immunomodulation in oyster Crassostrea gigas. Front. Immunol. 9:284. doi: 10.3389/fimmu.2018.00284
Marigómez, I., Múgica, M., Izagirre, U., and Sokolova, I. M. (2017). Chronic environmental stress enhances tolerance to seasonal gradual warming in marine mussels. PLoS One 12:e0174359. doi: 10.1371/journal.pone.0174359
Martín-Díaz, M. L., Blasco, J., González De Canales, M., Sales, D., and DelValls, T. Á (2005). Bioaccumulation and toxicity of dissolved heavy metals from the Guadalquivir Estuary after the Aznalcóllar mining spill using Ruditapes philippinarum. Arch. Environ. Contam. Toxicol. 48, 233–241. doi: 10.1007/s00244-003-9202-9
Mezzelani, M., Gorbi, S., Fattorini, D., d’Errico, G., Consolandi, G., Milan, M., et al. (2018). Long-term exposure of Mytilus galloprovincialis to diclofenac, ibuprofen and ketoprofen: insights into bioavailability, biomarkers and transcriptomic changes. Chemosphere 198, 238–248. doi: 10.1016/j.chemosphere.2018.01.148
Múgica, M., Izagirre, U., and Marigómez, I. (2015). Lysosomal responses to heat-shock of seasonal temperature extremes in Cd-exposed mussels. Aquat. Toxicol. 164, 99–107. doi: 10.1016/j.aquatox.2015.04.020
Oliver, E. C. J., Burrows, M. T., Donat, M. G., Sen Gupta, A., Alexander, L. V., Perkins-Kirkpatrick, S. E., et al. (2019). Projected marine heatwaves in the 21st century and the potential for ecological impact. Front. Mar. Sci. 6:734. doi: 10.3389/fmars.2019.00734
Oliver, E. C. J., Donat, M. G., Burrows, M. T., Moore, P. J., Smale, D. A., Alexander, L. V., et al. (2018). Longer and more frequent marine heatwaves over the past century. Nat. Commun. 9:1324. doi: 10.1038/s41467-018-03732-9
Parisi, M. G., Mauro, M., Sarà, G., and Cammarata, M. (2017). Temperature increases, hypoxia, and changes in food availability affect immunological biomarkers in the marine mussel Mytilus galloprovincialis. J. Comp. Physiol. B 187, 1117–1126. doi: 10.1007/s00360-017-1089-2
Pipe, R. K. (1990). Hydrolytic enzymes associated with the granular haemocytes of the marine mussel Mytilus edulis. Histochem. J. 22, 595–603. doi: 10.1007/BF01072941
Pipe, R. K., Farley, S. R., and Coles, J. A. (1997). The separation and characterisation of haemocytes from the mussel Mytilus edulis. Cell Tissue Res. 289, 537–545. doi: 10.1007/s004410050899
Rahman, M. A., Henderson, S., Miller-Ezzy, P., Li, X. X., and Qin, J. G. (2019). Immune response to temperature stress in three bivalve species: pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol. 86, 868–874. doi: 10.1016/j.fsi.2018.12.017
Rahman, M. S., and Rahman, M. S. (2021). Effects of elevated temperature on prooxidant-antioxidant homeostasis and redox status in the American oyster: signaling pathways of cellular apoptosis during heat stress. Environ. Res. 196:110428. doi: 10.1016/j.envres.2020.110428
Regoli, F. (1992). Lysosomal responses as a sensitive stress index in biomonitoring heavy metal pollution. Mar. Ecol. Prog. Ser. 84, 63–69. doi: 10.3354/meps084063
Regoli, F., and Giuliani, M. E. (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 93, 106–117. doi: 10.1016/j.marenvres.2013.07.006
Regoli, F., and Principato, G. (1995). Glutathione, glutathione-dependent and antioxidant enzymes in mussels, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat. Toxicol. 31, 1463–1464.
Regoli, F., and Winston, G. W. (1998). Applications of a new method for measuring the total oxyradical scavenging capacity in marine invertebrates. Mar. Environ. Res. 46, 439–442. doi: 10.1016/S0141-1136(97)00119-0
Regoli, F., Hummel, H., Amiard-Triquet, C., Larroux, C., and Sukhotin, A. (1998). Trace metals and variations of antioxidant enzymes in arctic bivalve populations. Arch. Environ. Contam. Toxicol. 35, 594–601. doi: 10.1007/s002449900421
Regoli, F., Nigro, M., Chiantore, M., and Winston, G. W. (2002). Seasonal variations of susceptibility to oxidative stress in Adamussium colbecki, a key bioindicator species for the Antarctic marine environment. Sci. Total Environ. 289, 205–211. doi: 10.1016/S0048-9697(01)01047-6
Regoli, F., Pellegrini, D., Cicero, A. M., Nigro, M., Benedetti, M., Gorbi, S., et al. (2014). A multidisciplinary weight of evidence approach for environmental risk assessment at the Costa Concordia wreck: integrative indices from Mussel Watch. Mar. Environ. Res. 96, 92–104. doi: 10.1016/j.marenvres.2013.09.016
Rey-Campos, M., Moreira, R., Gerdol, M., Pallavicini, A., Novoa, B., and Figueras, A. (2019). Immune tolerance in Mytilus galloprovincialis hemocytes after repeated contact with Vibrio splendidus. Front. Immunol. 10:1894. doi: 10.3389/fimmu.2019.01894
Sarà, G., Giommi, C., Giacoletti, A., Conti, E., Mulder, C., and Mangano, M. C. (2021). Multiple climate-driven cascading ecosystem effects after the loss of a foundation species. Sci. Total Environ. 770:144749. doi: 10.1016/j.scitotenv.2020.144749
Seuront, L., Nicastro, K. R., Zardi, G. I., and Goberville, E. (2019). Decreased thermal tolerance under recurrent heat stress conditions explains summer mass mortality of the blue mussel Mytilus edulis. Sci. Rep. 9:17498. doi: 10.1038/s41598-019-53580-w
Shi, X., Zhou, Z., Wang, L., Yue, F., Wang, M., Yang, C., et al. (2012). The immunomodulation of acetylcholinesterase in Zhikong scallop Chlamys farreri. PLoS One 7:e30828. doi: 10.1371/journal.pone.0030828
Sokolova, I. M., and Lannig, G. (2008). Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Clim. Res. 37, 181–201. doi: 10.3354/cr00764
Solé, M., Kopecka-Pilarczyk, J., and Blasco, J. (2009). Pollution biomarkers in two estuarine invertebrates, Nereis diversicolor and Scrobicularia plana, from a Marsh ecosystem in SW Spain. Environ. Int. 35, 523–531. doi: 10.1016/j.envint.2008.09.013
Süren, E., Yilmaz, S., Türkoglu, M., and Kaya, S. (2007). Concentrations of cadmium and lead heavy metals in Dardanelles seawater. Environ. Monit. Assess. 125, 91–98. doi: 10.1007/s10661-006-9242-5
Tomanek, L. (2014). Proteomics to study adaptations in marine organisms to environmental stress. J. Proteomics 105, 92–106. doi: 10.1016/j.jprot.2014.04.009
Wu, F., Lu, W., Shang, Y., Kong, H., Li, L., Sui, Y., et al. (2016). Combined effects of seawater acidification and high temperature on hemocyte parameters in the thick shell mussel Mytilus coruscus. Fish Shellfish Immunol. 56, 554–562. doi: 10.1016/j.fsi.2016.08.012
Yao, C.-L., and Somero, G. N. (2012). The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and Mytilus californianus): DNA damage, membrane integrity, apoptosis and signaling pathways. J. Exp. Biol. 215(Pt 24), 4267–4277. doi: 10.1242/jeb.073577
Keywords: multiple stressors, global change, pollution, bivalves, heatwaves, oxidative stress, immunity
Citation: Nardi A, Benedetti M, Gorbi S and Regoli F (2021) Interactive Immunomodulation in the Mediterranean Mussel Mytilus galloprovincialis Under Thermal Stress and Cadmium Exposure. Front. Mar. Sci. 8:751983. doi: 10.3389/fmars.2021.751983
Received: 02 August 2021; Accepted: 06 September 2021;
Published: 28 September 2021.
Edited by:
Anett Kristin Larsen, Arctic University of Norway, NorwayReviewed by:
Stefanos Dailianis, University of Patras, GreeceCopyright © 2021 Nardi, Benedetti, Gorbi and Regoli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Regoli, Zi5yZWdvbGlAdW5pdnBtLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.