- 1State Key Laboratory of Marine Environmental Science, College of Ocean and Earth Sciences, Xiamen University, Xiamen, China
- 2Environmental Defense Fund, Boston, MA, United States
- 3Dongshan Swire Marine Station, College of Ocean and Earth Sciences, Xiamen University, Xiamen, China
The spotted box crab Calappa philargius (Calappidae) is an increasingly consumed species in China, mainly sourced from a claw-only fishery. Being a not well-characterized species in the literature, this study is warranted to inform sustainable management approaches. Here we report on the first in-depth overview on biology and fishery dynamics of C. philargius in the southern Taiwan Strait of China. Whole body crabs (N = 1,009) were collected monthly from January to December 2019 from trawlers, operating in the southern Taiwan Strait; sample collection was absent from May to July because of the national fishing moratorium regulation. Sex ratio, growth pattern, size at maturity and fecundity were estimated. The overall male: female ratio was 1: 1.47, significantly differing from the 1: 1 (p < 0.01); monthly sex ratio variations were also significant (p < 0.01), suggesting possible seasonal sexual segregation. We found the carapace widths (CW) of males were significantly larger than those of females (p < 0.01). Based on the occurrence and percentages of berried females, we identified twin spawning events in January–April and August–October with the overall peak occurring in February. Size at 50% female maturity was 11.47 cm CW. Female absolute fecundity was significantly related to CW via a power function relationship (N = 14, p < 0.01). The estimated average claw yield was 36.28 ± 3.07% of the whole body weight (N = 95), irrespective of sex and size (p > 0.5). Capture and trade data of C. philargius using trawl and trap fishing gears were documented to characterize claw-only fishery dynamics for future research and sustainable use.
Introduction
Claw-only crab fisheries have been developed since the 1970s and are commonly considered as renewable fisheries. Typically, after the collection of claws on board, the declawed live bodies are released. This fishery mode includes the red crab Chaceon affinis (Geryonidae) in the Northeast Atlantic (Robinson, 2008), the brown crab Cancer pagurus (Cancridae) in Ireland (Fahy et al., 2004), the stone crabs Menippe spp. (Menippidae) in the United States (Savage and Sullivan, 1978; Duermit et al., 2017), Jonah crab Cancer borealis (Cancridae) in the United States (Goldstein and Carloni, 2021), and the European fiddler crab Uca tangeri (synonyms of Afruca tangeri) (Ocypodidae) for males only in southern Portugal (Oliveira et al., 2000), all mainly caught in trap fisheries. However, the proportion of crabs that regenerate claws and re-enter commercial landings is minimal; for example, only 7–13% regenerated claws entered the M. mercenaria fishery in Florida, the United States (Simonson and Hochberg, 1986; Wilber, 1995; Muller et al., 2011). The mortality rate associated with two-claw removal is high in M. mercenaria and C. borealis, 28–100% and 70%, respectively, and higher with increasing temperatures under laboratory conditions (Davis et al., 1978; Simonson and Hochberg, 1986; Gandy et al., 2016; Goldstein and Carloni, 2021). The estimated mortality rate for single-claw removal in M. mercenaria and C. borealis is reduced around 35–50% compared to two-claw removal in the fishery (Davis et al., 1978; Duermit et al., 2017; Goldstein and Carloni, 2021). However, voluntary adherence to one-claw fishing regulations is not universal, and the seasonal closure to protect spawning stocks can be a better management measure (Duermit et al., 2017).
China is one of the most important crab fisheries nations, contributing to more than 40% of total global marine crab capture production (Lin et al., 2021). Available Chinese national marine crab capture fishery yield statistics date back to 1987. Since 2003, these data have been divided into finer taxonomic resolution. National crab yield exceeded 800,000 t in 2014, and has since exhibited a gradual decline. More than 80% of the domestic Chinese crab yield is from species in the family Portunidae. With the change from target fisheries to multispecies fisheries and the overexploitation of traditionally important food crab species in China (Kang et al., 2018) and the increasing demands for crab consumption, some non-traditional food crab species are becoming utilized in China, such as the spanner crab Ranina ranina (Raninidae), the box crabs Calappa species (Calappidae), and the stone crabs Menippe and Etisus species (Xanthoidae) (Ng, 1998; Jiang and Yu, 2012; Shao et al., 2015; Liu and Lin, 2019; Lin et al., 2021). Furthermore, traditional Chinese crab pastes, made from small-size species from families Ocypodidae, Sesarmidae, and Varunidae, have been promoted through food documentaries and Wechat/online retail markets.
The box crab Calappa philargius is widely distributed in the Indo-Pacific Oceans from Australia and Thailand to Korea and Japan, and commonly found in southern China (Dai et al., 1986; Ng, 1998; Wisespongpand et al., 2016). In China, it usually inhabits nearshore shallow waters (10–100 m) on sandy and muddy bottoms, and mainly feeds on mollusks and hermit crabs (Dai et al., 1986; Ng, 1998; Shao et al., 2015). The species can be caught in trawl, trap, and gill net fisheries in the southern East China Sea and South China Sea. Historically, C. philargius was considered a non-food crab and typically discarded at sea (Ng, 1998). In less than one decade since, however, claw-only consumption of C. philargius is becoming popular in domestic markets (Liu and Lin, 2019). As a newly developing, claw-only fishery in China, the biology and fishery dynamics of C. philargius are poorly known.
In the southern Taiwan Strait of China, C. philargius is a common crab species in both trawl and trap catches (Liu and Lin, 2019). In 2019, we conducted the first in situ study to improve the understanding of C. philargius population structure, sex ratio, size variation, reproductive biology in terms of size at maturity, spawning season and fecundity, and basic fishery dynamics including claw yield, capture production and proportion in both trawl and trap fisheries. The results will provide crucial data for further stock assessment and data-limited fishery management for this newly developing claw-only crab fishery species in China.
Materials and Methods
Calappa philargius Collection
Commercial bottom trawl and trap vessels from Dongshan County (Fujian Province, China) operate exclusively in the fishing grounds of the southern Taiwan Strait (21.50–24.50° N, 116.00–119.00° E) (see Figure 2 in Lin et al., 2021). Monthly sampling of whole body crabs was conducted during January–April 2019 and August–December 2019 at the landing ports of Dongshan County. Owing to the national annual summer fishing moratorium regulation (i.e., no trawl fishing permitted from May 1st to August 15th, and no trap fishing from May 1st to August 1st) in China, no crabs were available for sampling spanning May–July 2019.

Figure 1. Calappa philargius. (A) Fresh whole body crabs from trawl fishery. (B) Claw-only landings from trap fishery. (C) Cleaned bodies.
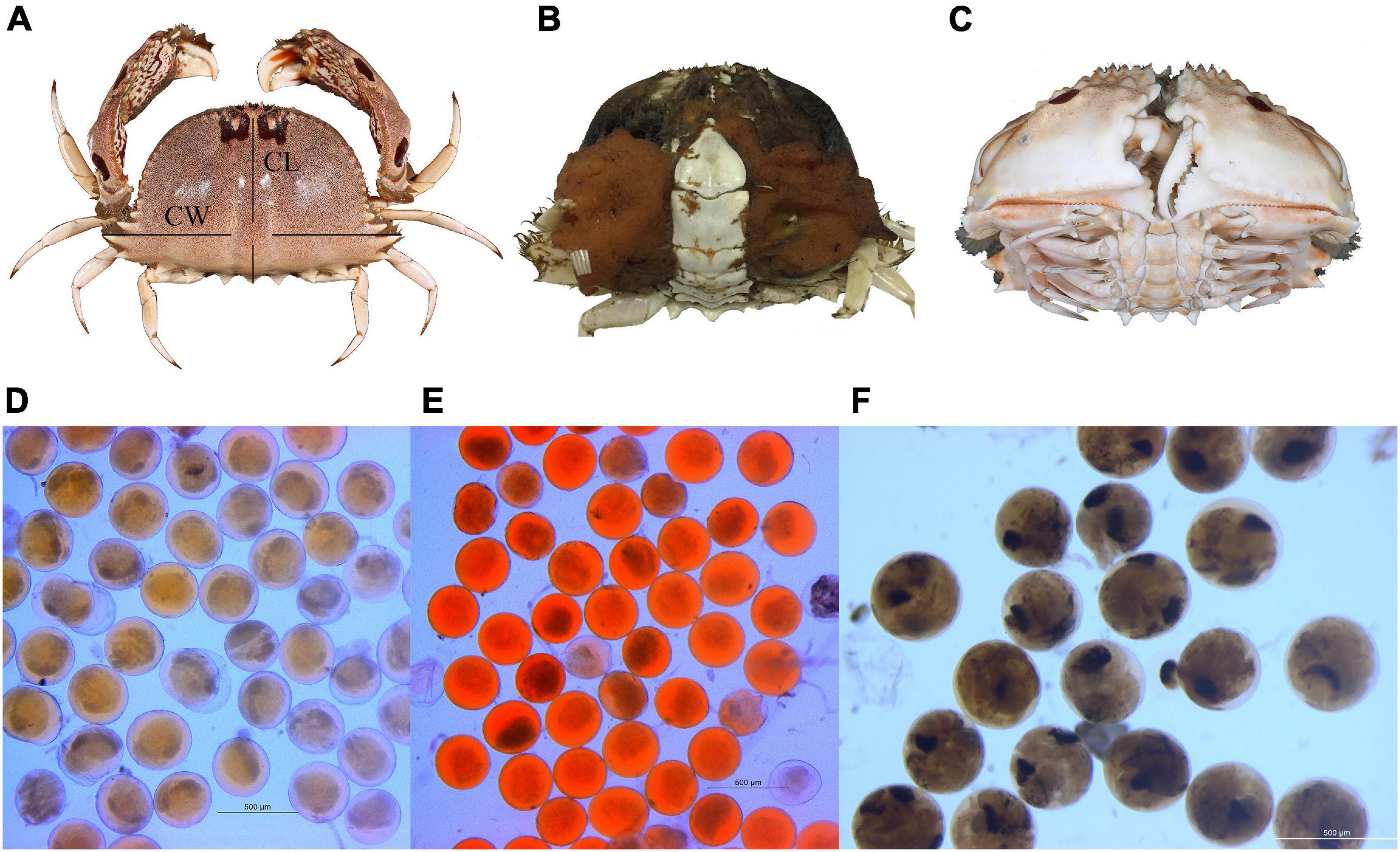
Figure 2. (A) Dorsal view of Calappa philargius for carapace width (CW) and carapace length (CL) measurements. Sex determination by abdomen morphology: (B) female and (C) male. (D) Yellow eggs, (E) orange-red eggs and (F) brown eggs measurement under a microscope.
Approximate 15–35 kg (about 1–2 baskets) C. philargius samples were randomly collected from at least five bottom trawl vessels in each sampling month (Figure 1A). About 1 kg of “feed-grade fish” (see definition in Zhang et al., 2020) from the same trawl vessels were also collected to obtain smaller C. philargius samples. All samples were transferred to Dongshan Swire Marine Station (Xiamen University) for further analysis.
No C. philargius samples were collected from trap vessels because they typically only land the large first-paired claws (Figure 1B); the declawed live hard bodies were discarded at sea.
Crab Measurement and Examination
For every whole body crab collected, carapace width (CW, to 0.1 cm), carapace length (CL, to 0.1 cm), and body weight (BW, wet weight, to 0.01 g) were measured (Figure 2A). Based on abdomen morphology, sexes were determined, and berried females were considered fully mature (Figures 2B,C). Consistent with literature on other similar crabs, embryonic developmental stages from early to late were noted by the yellow, orange-red and brown coloration, respectively (Arshad et al., 2006; Hisam et al., 2018; Lin et al., 2021).
Female fecundity, defined as the number of eggs carried by a female, was estimated empirically following standard methodologies (Lin et al., 2021). The absolute fecundity (Fa) was calculated as: Fa = W ∗ m/n, where W is the total weight of the whole egg mass for a female, m is the average weight of the five egg sub-samples (approximate 0.02 g per sub-sample), and n is the average number of eggs from the five sub-samples (Johnson et al., 2010; Soundarapandian et al., 2013). The relative fecundity (Fr) was calculated as: Fr = Fa/CW and Fr = Fa/BW. Egg diameters (to 1 μm) from different colors (5 individuals for yellow eggs, 5 individuals for orange-red eggs, and 4 individuals for brown eggs) were randomly measured using a Leica M165 FC fluorescence stereo microscope (Figures 2D–F).
Crab Capture Data Collection
Information on the total capture production (kg/vessel), C. philargius production (kg/vessel), and the number of fishing days at sea (days/vessel) was collected in the landing ports from the same trawl vessels during monthly sampling. Data collection was through captain and crew interviews, landing port estimation directly (i.e., landing volumes by species or species group level), and trade notebooks (i.e., captain’s wholesale information including weight and price on the per-trip level documented for revenue purposes).
Information collected from the trap vessels was comparable to that of the trawl vessels. However, due to the claw-only characteristics in trap vessel landings, C. philargius capture production (kg/vessel) was back-calculated from claw production estimation (Figure 1B). Briefly, both female and male whole body crabs were randomly selected after measurement and examination above. The claw yield (%) per crab was calculated as: Wclaws/BW ∗ 100, where Wclaws is the wet weight of the paired claws, which are typically broken off proximal of the merus. Then, the average claw yield (%) of C. philargius was calculated. Finally, whole crab capture production of C. philargius from trap vessels was estimated by dividing the total claw landings (kg) by the claw yield percentage.
Data Analyses
The percentage of berried females each month, irrespective of egg coloration, was calculated as: the number of berried females/the total number of females ∗ 100. The spawning season was defined as the months with berried females, and the peak spawning season was defined as the month(s) with at least 50% of berried females (Sadovy, 1996).
We estimated the size at 50% female maturity (CW50) by fitting a logistic regression (probity link) to the percentage of berried females in 0.5 cm CW size bins using females from the peak spawning month determined above.
Differences in sex ratio from 1: 1 monthly and overall were analyzed by student’s t-test. Differences in sizes (CW) of females and males monthly and overall were analyzed via one-way ANOVA and non-parametric analysis (Mann-Whitney U-test). The relationships of CW—BW, CW—CL, Fa—CW, and Fa—BW were all analyzed via one-way ANCOVA. The statistical analyses were conducted using R (R Core Team, 2020), SPSS 13.1 and Microsoft Excel 2019 software, by setting statistical significance at the level of p ≤ 0.05.
Results
Population Structure
A total of 1,009 whole body crabs with 408 males and 601 females were collected from trawl vessels; 58–183 crabs each month (Table 1). The overall male: female ratio was 1: 1.47 (χ2 = 36.54, df = 1, p < 0.01). Significant monthly differences (p < 0.01) from a 1: 1 ratio were observed from January, February, August, October, and November. For the rest of the sampling months there were no significant differences from an equal sex ratio (p > 0.05). Data showed a significant male-bias in August 2019, and a significant female-bias in January, February, October, and November 2019 (Figure 3). CWs in the trawl landings ranged from 1.8 to 15.7 cm (12.12 ± 1.66 cm CW, mean ± SD, N = 408) for males, and 1.2–16.0 cm (11.60 ± 1.63 cm, N = 601) for females (Figure 4). Males were mainly landed in size classes between 11.0 and 14.9 cm CW (80.15%) and females between 9.0 and 13.9 cm CW (91.18%), determined by the size frequencies > 10% (Figure 4). Males were significantly larger than females in CW (t = 4.23, df = 1,007, p < 0.01). Monthly variations in sizes of males (F = 4.80, df = 407, p < 0.01) and females (F = 6.95, df = 600, p < 0.01) were also significant (Table 1). Smaller crabs < 5 cm CW were found in feed-grade fish samples in August (1.2 cm CW, N = 1), October (3.2–4.5 cm CW, N = 3), and December (1.8 cm CW, N = 1).

Table 1. The variation of mean, maximum and minimum values of carapace width (CW, cm) and body weight (BW, g) of Calappa philargius sampled from January to December 2019 by trawl vessels in the southern Taiwan Strait, China.
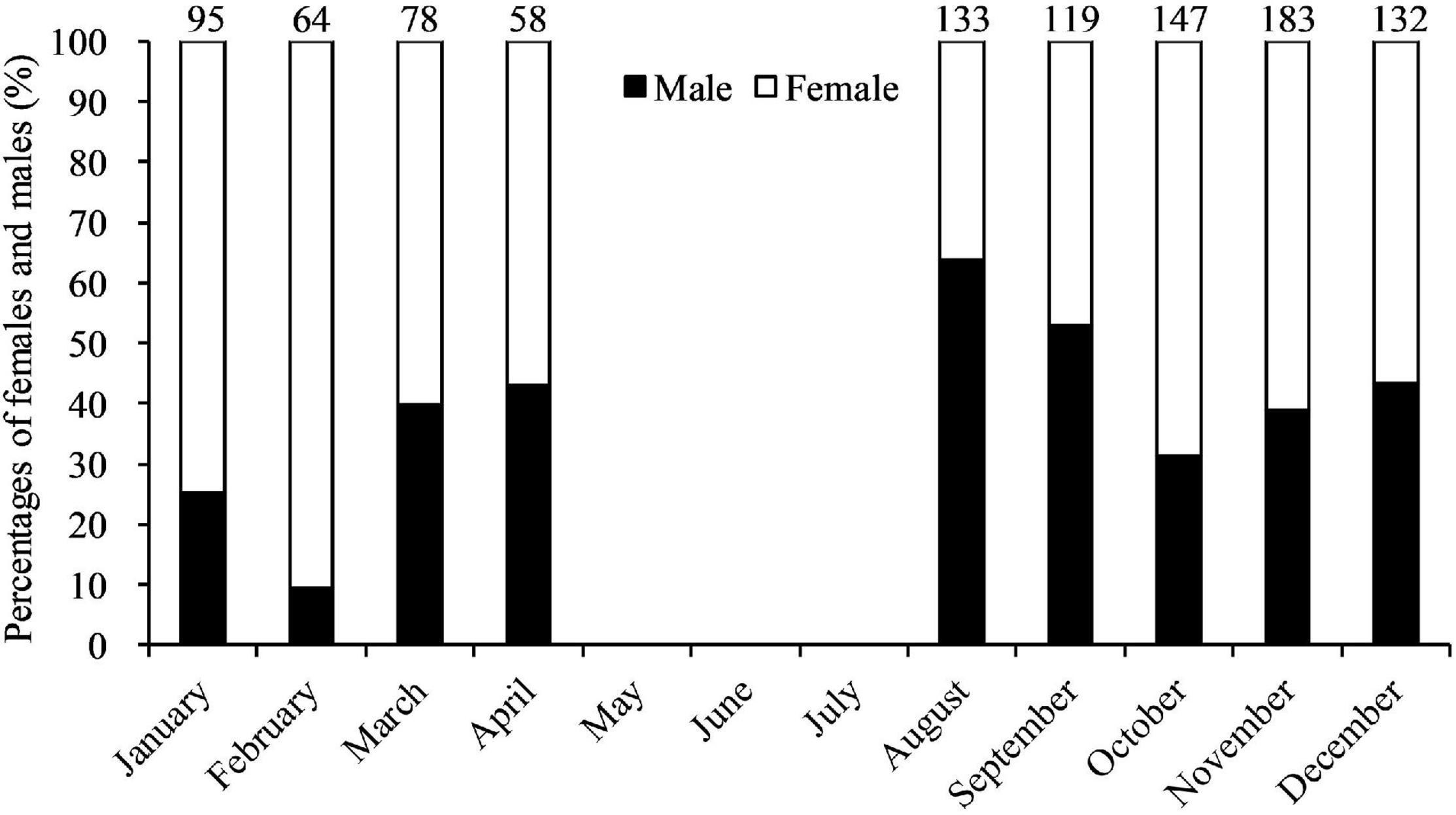
Figure 3. Percentages of Calappa philargius males and females monthly from January to December 2019. No samples in May–July 2019. Numbers above the bars referred to sample sizes.
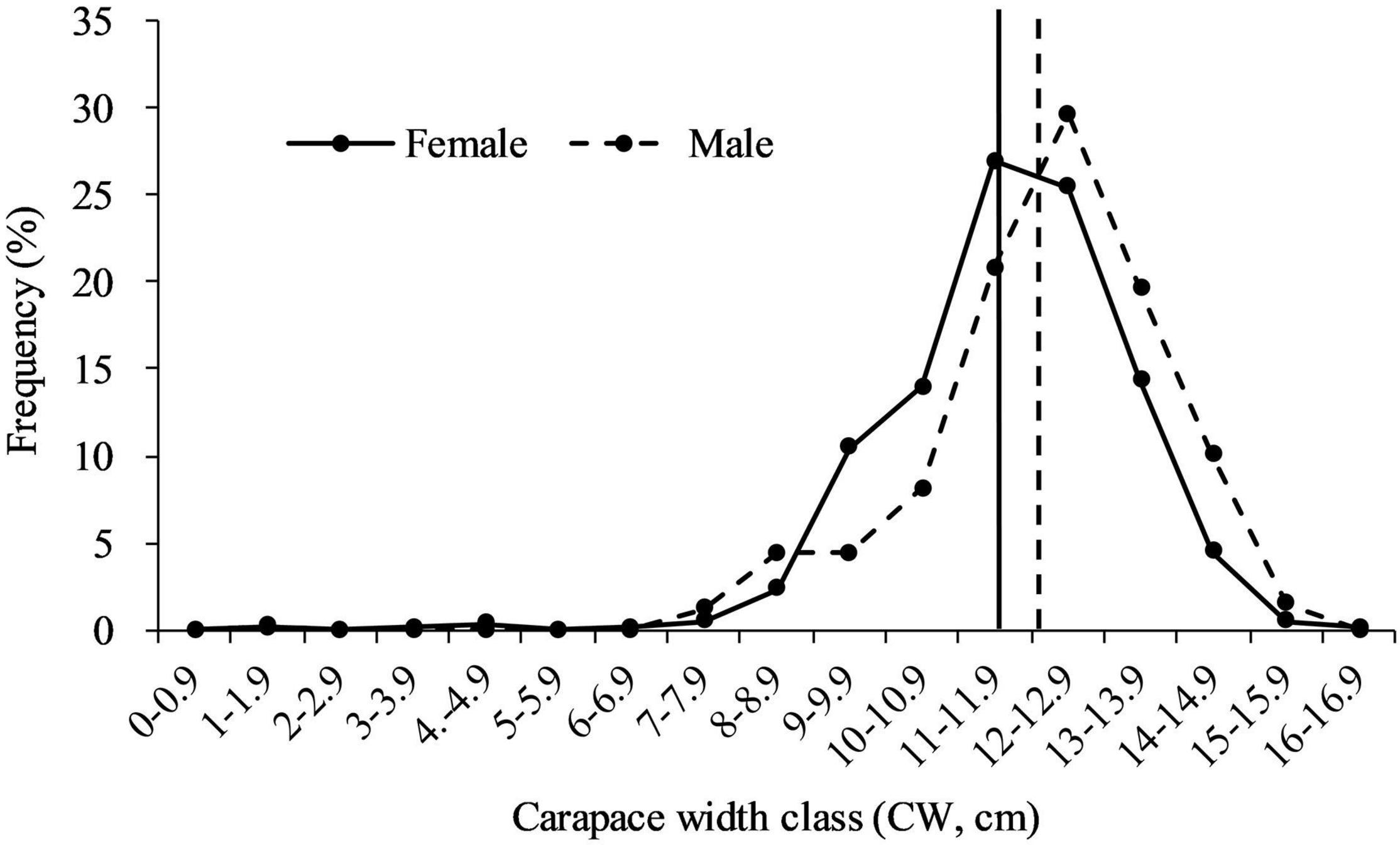
Figure 4. Size (carapace width, cm) frequency (%) of Calappa philargius males (N = 408) and females (N = 601), collected from January to December 2019. No samples in May–July 2019. Vertical lines indicate the average sizes of males and females.
The sex specific CW—BW relationships were not different significantly (F = 0.776, df = 1, p > 0.05). The regression coefficient b of C. philargius differed significantly (p < 0.01) from isometric growth (b = 3), with both males and females exhibiting negative growth allometry (Figure 5A and Table 2). The CW—CL relationships between females and males were significantly different (F = 3.875, df = 1, p < 0.05) (Figure 5B). The CW—CL relationships were highly correlated for males (R2 = 0.8329, p < 0.01) and females (R2 = 0.7158, p < 0.01).
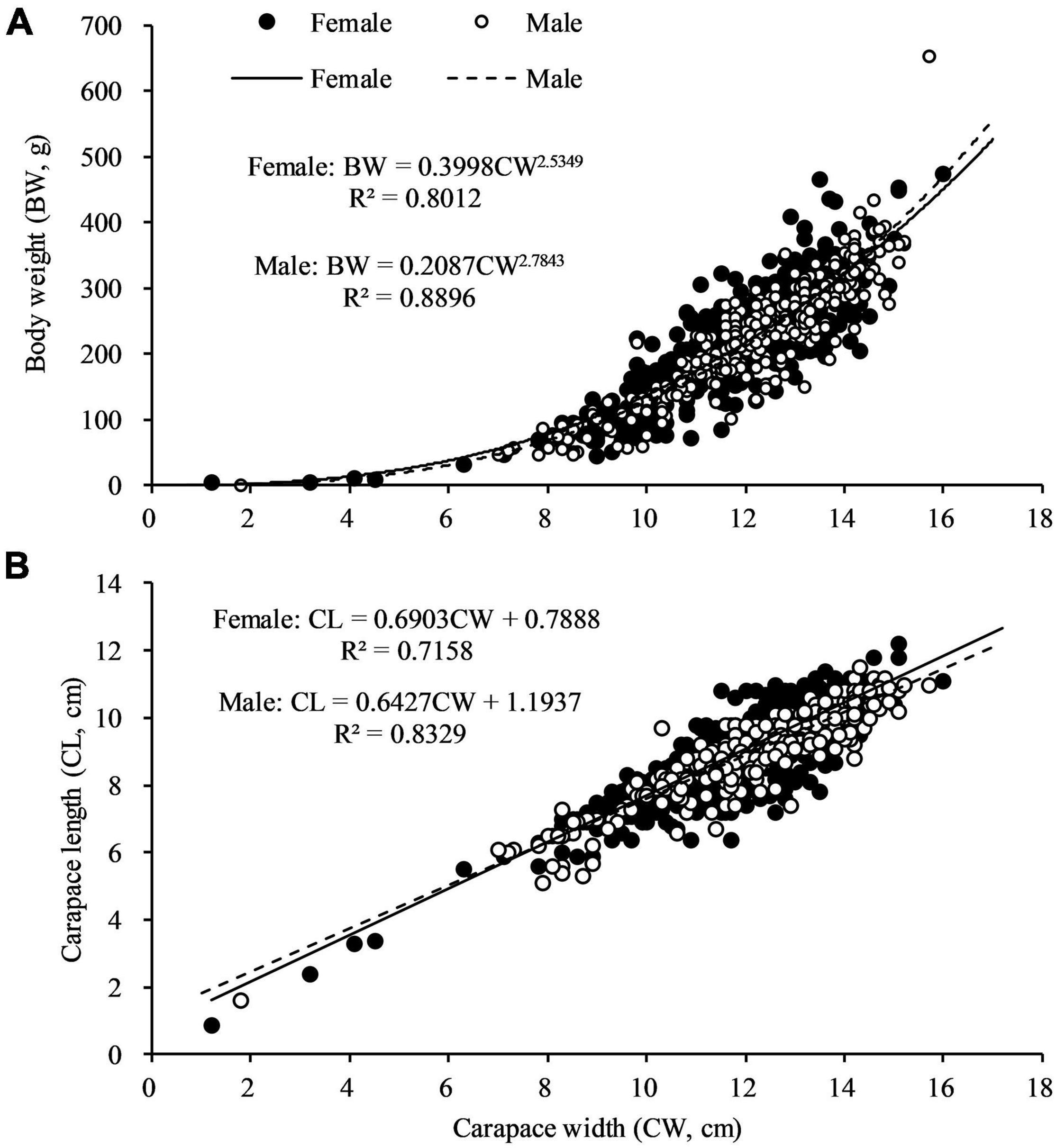
Figure 5. (A) Body weight (BW) and carapace width (CW) relationship, and (B) carapace length (CL) and carapace width (CW) relationship for Calappa philargius males (N = 408) and females (N = 601).
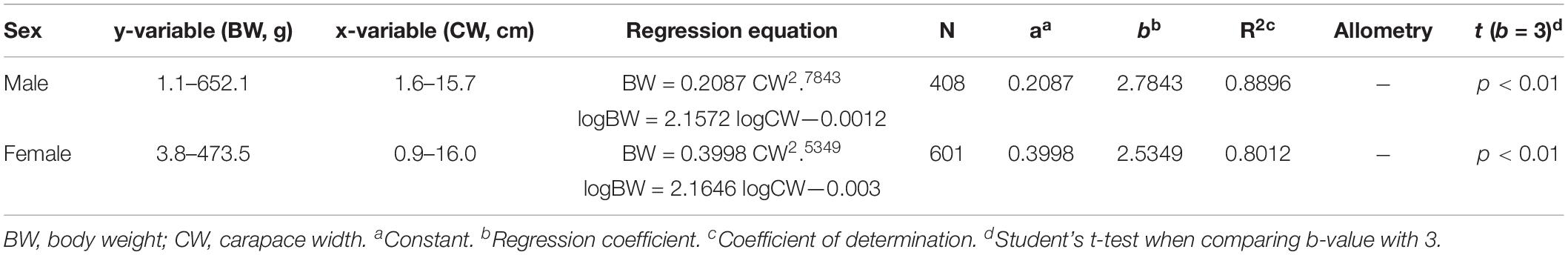
Table 2. Regression equations and estimated parameters of length–weight relationships for Calappa philargius by sex sampled in the southern Taiwan Strait, China.
Reproductive Dynamics
A total of 112 berried females were collected (Figure 6A). Two spawning seasons were determined, in January–April and in August–October, with the peak spawning month occurring in February (Figure 6B). It is unclear to what extent spawning occurs in May–July due to lack of data.
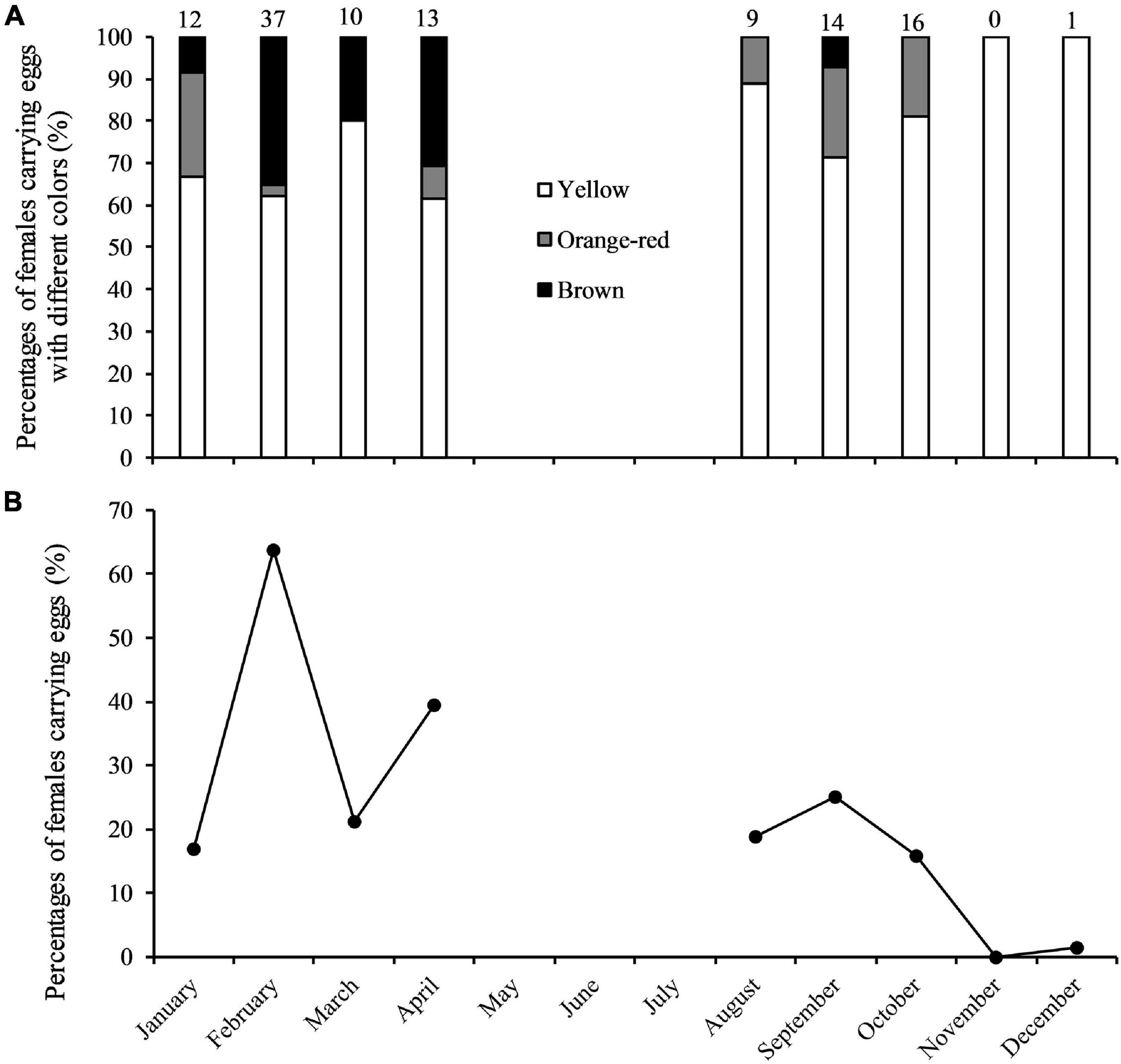
Figure 6. (A) Percentages of Calappa philargius berried females (N = 112) by month and egg color, and (B) percentages of berried females (irrespective of egg coloration) by month from January to December 2019. No samples in May–July 2019. Numbers above the bars referred to sample sizes (Table 3).
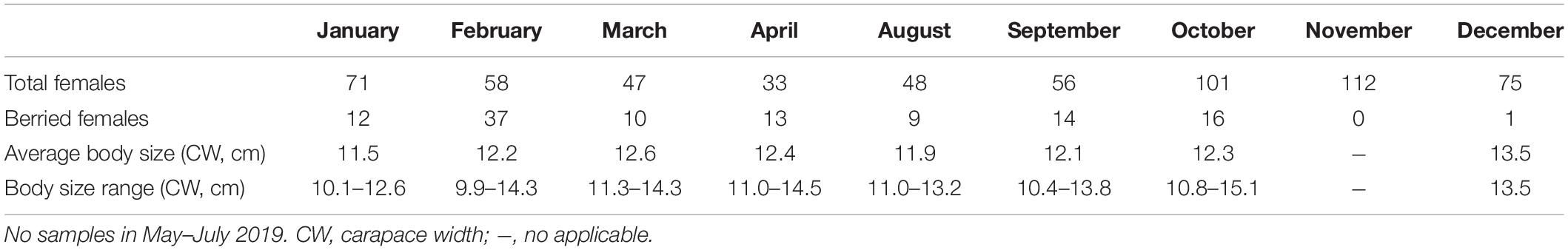
Table 3. Body size variation of Calappa philargius berried females, sampled from January to December 2019 by trawl vessels in the southern Taiwan Strait, China.
The minimum and maximum sizes for berried females were 9.9 cm CW (7.1 cm CL) found in February and 15.1 cm CW (11.8 cm CL) in October, respectively (Table 3). The CW50 for females was estimated as 11.47 cm CW (8.7 cm CL) based on females sampled in the peak spawning month, February (Figure 7). The dominant size classes for berried females were 11.0–13.9 cm CW (86.61%). Small berried females (< 11.0 cm CW) were mainly caught in February.
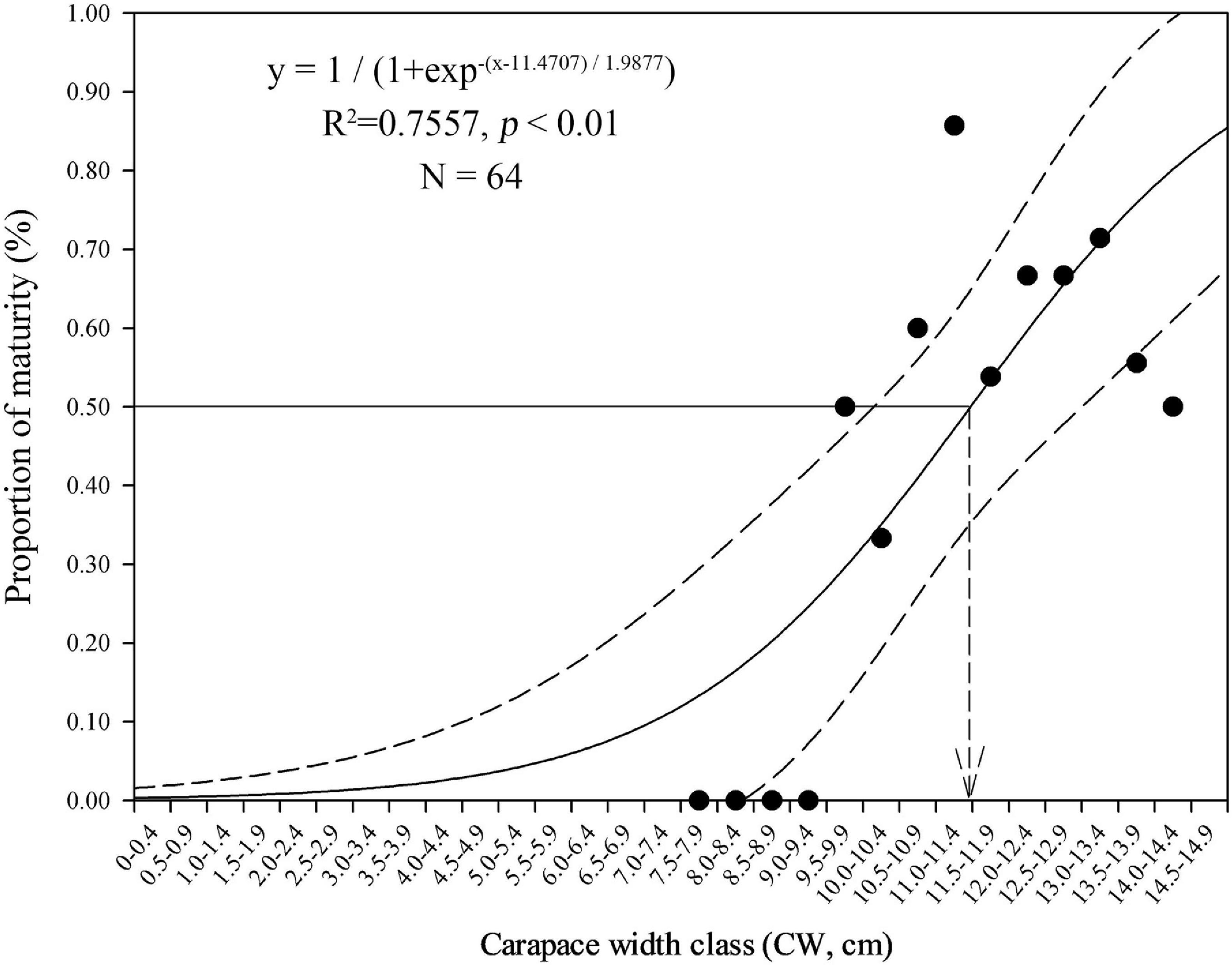
Figure 7. Size (carapace width, CW) at 50% female maturity for Calappa philargius based on all females sampled from the peak spawning month, February 2019 (N = 64). Bootstrapped 95% confidence intervals shown with dashed lines (N = 9,999).
From the sampled ovigerous females, 14 were selected to estimate fecundity (Fa) (10.0–12.4 cm CW), 5 with yellow eggs, 5 with orange-red eggs, and 4 with brown eggs. Fa ranged between 441,742 and 1,025,607 eggs (734,947 ± 181,397, N = 14), and Fr ranged between 42,071 and 82,710 eggs cm–1 CW (63,417 ± 12,486, N = 14), and between 2,227 and 4,232 eggs g–1 BW (3,159 ± 534, N = 14). A significant power relationship was found between Fa and CW (R2 = 0.83, p < 0.01) (Figure 8).
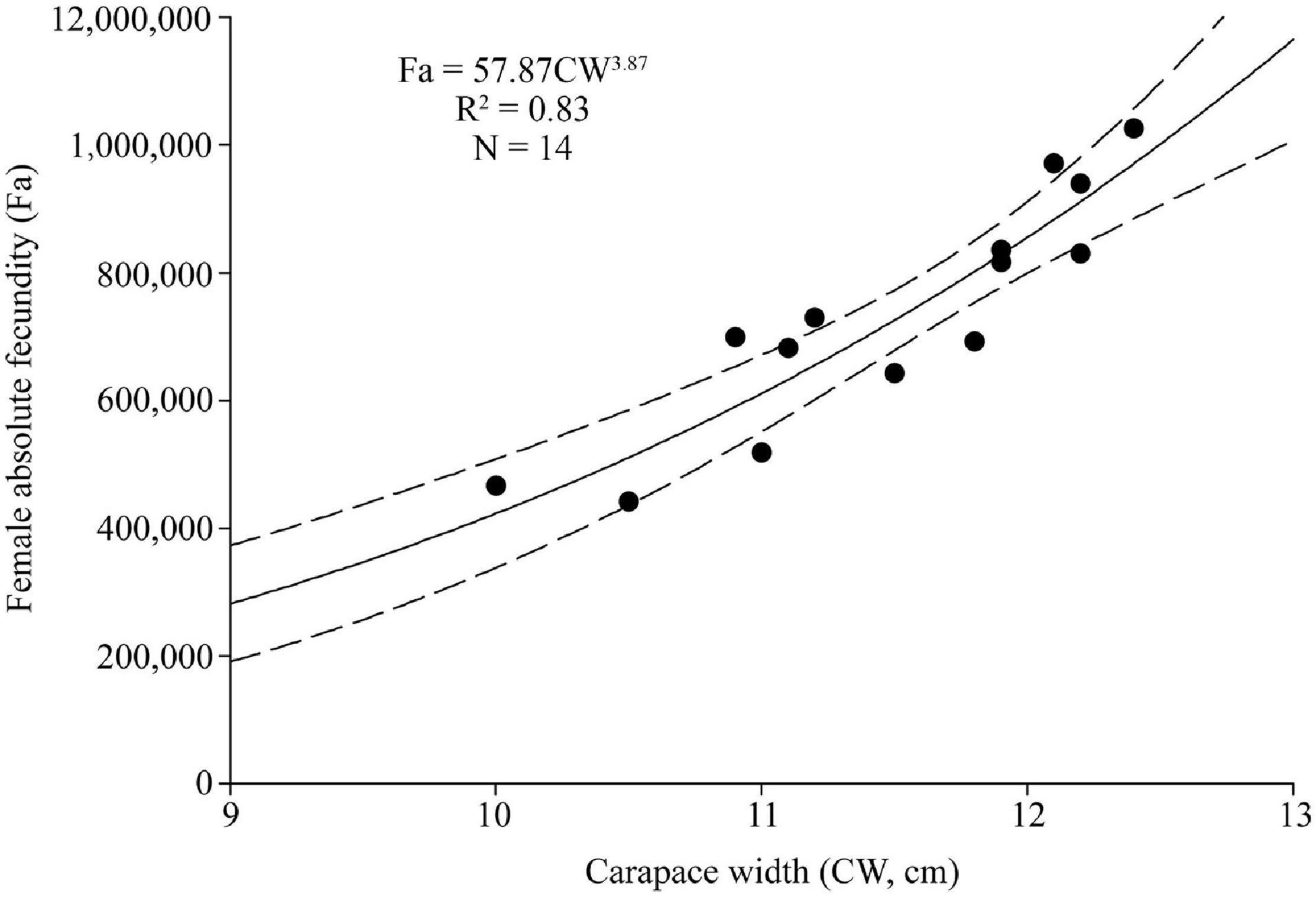
Figure 8. Absolute fecundity (Fa) and size (carapace width, CW) relationship for Calappa philargius (N = 14). Bootstrapped 95% confidence intervals shown with dashed lines (N = 9,999).
Egg diameters were 210–418 μm (332 ± 29, N = 145), with 312 ± 23 μm for yellow eggs (N = 49), 327 ± 22 μm for orange-red eggs (N = 53) and 362 ± 18 μm for brown eggs (N = 43). Egg diameters were significantly different among three embryonic developmental stages, increased with the increase of embryonic development (F = 64.50, df = 142, p < 0.01).
Capture Fishery Dynamics and Marketing
All 95 C. philargius samples collected in January 2019, i.e., 71 females (8.3–13.6 cm CW) and 24 males (7.9–14.2 cm CW), were used for the claw yield assessment. The claw yield ranged between 28.45% and 44.60% of the total wet body weight (36.28 ± 3.07%, N = 95), and showed no significant difference between sexes (t = 3.63, df = 93, p > 0.5). The claw yield also showed no difference with CW (F = 0.044, df = 93, p > 0.5). The average claw yield of 36.28% was used to estimate the C. philargius capture production in the trap fishery below.
From January to December in 2019, we surveyed a total of 127 vessels (77 trawl vessels and 50 trap vessels) at the landing ports of Dongshan County, all operating in the southern Taiwan Strait (Tables 4, 5). The estimated total capture production of C. philargius was 1,652 t; 63.65% from the 77 trawl vessels and 36.35% from the 50 trap vessels. Trawl vessels usually spent 3–12 days at sea per trip, while trap vessels operated longer at sea, usually 20–30 days per trip. However, trap vessel catches were delivered to transfer vessels approximately every 1–11 days. The CPUEs (kg day–1) of C. philargius were significantly higher in the trap fishery than the trawl fishery (Z = 2.782, p < 0.01). In trawl fishery, the CPUEs were mainly < 10 kg day–1, while in trap fishery, the CPUE averaged > 25 kg day–1 in January, April and September–December. C. philargius was less significant in the trawl fishery in terms of capture volume (wet weight, kg), all < 4% and mainly < 1%. C. philargius made up a substantial component of the total capture production in the trap fishery, e.g., about 13.7% in January and 25.6% in April.
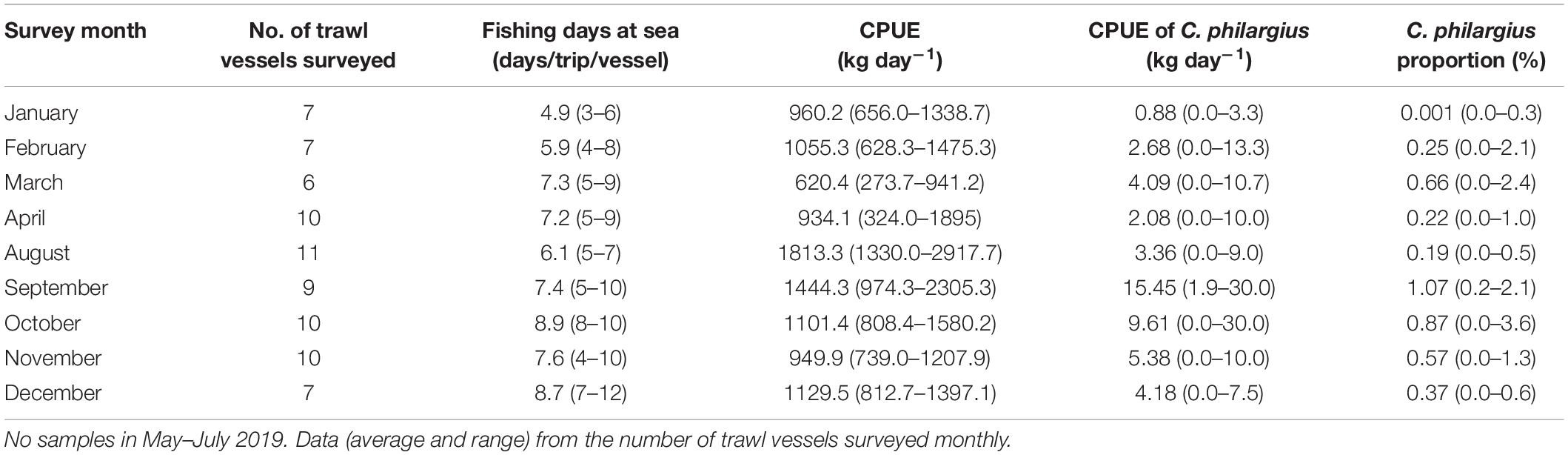
Table 4. Fishing days at sea per trip, catch per unit effort (CPUE, kg day–1) (total and Calappa philargius), and proportions (%) of C. philargius productions in the total capture productions of the trawl fishery from January to December 2019 in the southern Taiwan Strait, China.
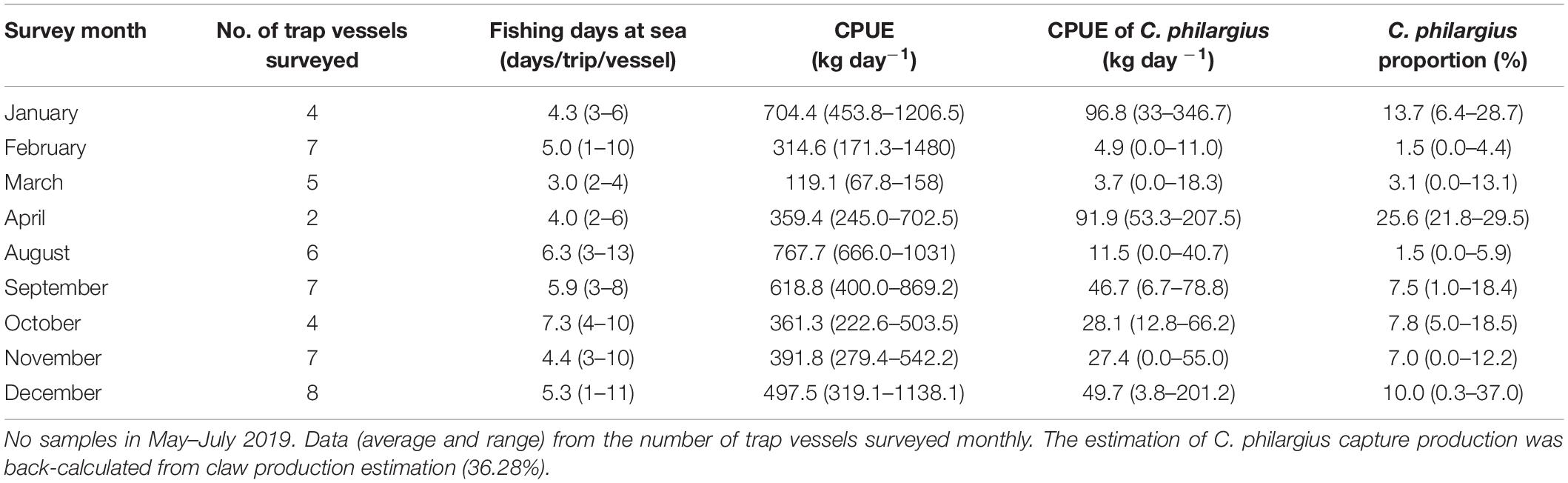
Table 5. Fishing days at sea per trip, catch per unit effort (CPUE, kg day–1) (total and Calappa philargius), and proportions (%) of C. philargius productions in the total capture productions of the trap fishery from January to December 2019 in the southern Taiwan Strait, China.
Based on captain, crew, and trader interviews at the landing ports of Dongshan County, the Chinese C. philargius claw market is largely domestic and has developed in less than one decade. In Dongshan County, the wholesale price for whole C. philargius caught from the trawl fishery is commonly around 6 RMB kg–1, which is typically further processed into claws (Figure 1A). The wholesale price of claws (processed on board) from the trap fishery is typically around 14 RMB kg–1 (Figure 1B). The claw price is nearly double in the wet and night food markets.
Since 2019, the domestic consumption market for C. philargius has continued to expand. Hard cleaned bodies of C. philargius are delivered to inland tourism cities of China (Figure 1C), and commonly cooked as spicy cuisine. Live whole crabs are also becoming popular in local provincial markets, particularly for dining on the ripe ovaries. The wholesale price of live whole crabs is around 10 RMB kg–1.
Discussion
Population Structure
Marine crustaceans commonly have a skewed sex-ratio, and the existence of sex-specific habit segregation and single-sex migration may explain the phenomenon (Wenner, 1972). The overall sex ratio of C. philargius habiting in the southern Taiwan Strait showed a strong predominance of females (p < 0.01), collected by trawlers; monthly variation from the 1: 1 was also observed (p < 0.01) in January, February, August, October, and November. The same overall female-bias trend was also reported for C. philargius in Thailand, collected by gill nets (Wisespongpand et al., 2016). In C. philargius, a strong female-bias occurred in the peak spawning month (February), suggesting that C. philargius females may also migrate from deeper waters to spawn nearshore. A recent study on M. haanii from the same fishing grounds showed a similar pattern, i.e., a strong female-bias in peak spawning months (February and March) (Lin et al., 2021).
Crabs often show sexual size dimorphism. Males of C. philargius were significantly larger than females in CW (p < 0.01). The pattern of sexual size dimorphism, however, is largely species specific. For example, Callinectes danae, C. sapidus, C. philargius, Chaceon macphersoni, Chaceon affinis, Chionoecetes tanneri, Cancer borealis Johngarthia lagostoma, M. haanii, Platyxanthus crenulatus, and Ranina ranina (Krajangdara and Watanabe, 2005; Hartnoll et al., 2009; Sforza et al., 2010; Keller et al., 2012; Groeneveld et al., 2013; Farias et al., 2014; Biscoito et al., 2015; Jivoff et al., 2017; Truesdale, 2018; Lin et al., 2021; this study) are female-biased in terms of sexual size dimorphism whereas Cancer pagurus, P. sanguinolentus, and P. trituberculatus (Oh, 2011; Yang et al., 2014; Öndes et al., 2017) are male-biased. Meanwhile, sexual size dimorphism could also exhibit spatial variation within the same species. For instance, Scylla olivacea, S. paramamosain, and S. tranquebarica (Ikhwanuddin et al., 2011; Waiho et al., 2016, 2021; Fazhan et al., 2021; Rouf et al., 2021). Therefore, sexual size dimorphism in crabs merits further investigation.
Sexual selection in decapod crustacean fisheries can influence both sex ratio and sexual size dimorphism in the population (Orensanz et al., 1998; Fenberg and Roy, 2008; Alborés et al., 2019). For instance, the earlier fishery regulation for larger C. affinis males in the Northeast Atlantic eventually resulted in the change of sex ratio (female: male) from 1.24: 1 to 2.08: 1 and variation shifting from males being larger, to females being larger after 30 years of heavy exploitation (Wahle et al., 2008). For the claw-only C. philargius fishery, large males are preferred in trade due to large claws and quality of meat. As a newly developing claw-only fishery, C. philargius males are significantly larger than females in CW with female-bias sex ratio (female: male = 1.47: 1) in the southern Taiwan Strait. Intensive and continuous fishing pressure may be changing the population structure of C. philargius in southern Taiwan Strait. Long term population monitoring for C. philargius is needed to evaluate the sex ratio and size variation pattern by sex under sexual selection fishery that is largely driven by specific claw-only market demand.
The sex specific CW—BW relationships were consistent (p > 0.05); however, we found sex-specific CW—CL relationships were significantly different (p < 0.05), i.e., females were typified by a more rounded and less oval carapace shape (Figure 5B). Size difference by sexes may reflect their difference in growth rates during different developmental stages or be associated with female fecundity. In C. philargius, females and males showed an intersection at 8.6 cm CW, where at that point females may input their energy in growing for maturation, particularly for increasing CL, as a larger carapace size is able to accommodate more eggs (Oh, 2011; Yang et al., 2014).
Reproduction
Studies in the southern Chinese waters suggest there is a common spawning season in February–April for many crabs, e.g., C. philargius (this study), the red swimming crab Monomia haanii (Lin et al., 2021), the blue swimming crab Portunus pelagicus (Liu et al., 2014), and the three-spot swimming crab P. sanguinolentus (Yang et al., 2014). However, it is unclear to what extent spawning takes place into May–July for all the aforementioned species, because the national annual summer fishing moratorium regulation typically disallows fishing and biological sampling. Some crabs demonstrate long spawning seasons, e.g., the ghost crab Ocypode retundata with one spawning season from May to October in the Persian Gulf (Naderi et al., 2018), and P. pelagicus from November through July in southeastern Australia (Johnson et al., 2010). For C. philargius, there could be a continuous spring–summer–autumn spawning period (Figure 6). Several small C. philargius juveniles (1.2–4.5 cm CW) were found in feed-grade fish samples in August, October and December, suggesting they were hatched in the summer closure period (May–July). On the other hand, no small juveniles found in January to April, supporting that C. philargius stops spawning activities in winter (November and December) (Figure 6). An extended spawning season in C. philargius might increase the number of breeding clutches to enhance the population recruitment. In the future, C. philargius samples from the missing months (May –July), and gonad examination before egg ovulation are needed to further understand this developing claw-only fishery in China.
The logistic estimation of CW50 for C. philargius showed an imperfect curve without 100% mature groups in larger female sizes. Two possible factors may lead to this result. First, some berried females may be discarded at sea because of their light meat weight and poor meat quality. Second, the logistic estimation does not extend to those mature females with fully developed ovaries inside the carapace which have not yet released eggs and attached them to their pleopods.
Fecundities of decapod crustaceans often have positive linear or power function correlation with body size or body weight (Erdman and Blake, 1988; Hisam et al., 2018; Lin et al., 2021). Fa had a strong power correlation with CW (p < 0.01) in C. philargius, similar to M. haanii (Lin et al., 2021), O. punctatus (Jiang and Yu, 2012), O. rotundata (Naderi et al., 2018), and P. sanguinolentus (Yang et al., 2014; Figure 9). For Fr, C. philargius has higher Fr than some swimming crabs, such as M. haanii (Lin et al., 2021), P. sanquinolentus (Yang et al., 2014), and O. rotundata (Naderi et al., 2018), but smaller than O. punctatus (Jiang and Yu, 2012). C. philargius females carry egg mass on their narrow pleopods ventrally, and the elongated pleopod appendages result in the capacity to bear more eggs in one clutch (Figure 2B). Fa of the same species may show large variation in different regions. For example, Fa of C. philargius caught in the southern Taiwan Strait (734,946 ± 181,396 eggs) is higher than that in Thailand (average 307,714 eggs) (Wisespongpand et al., 2016).
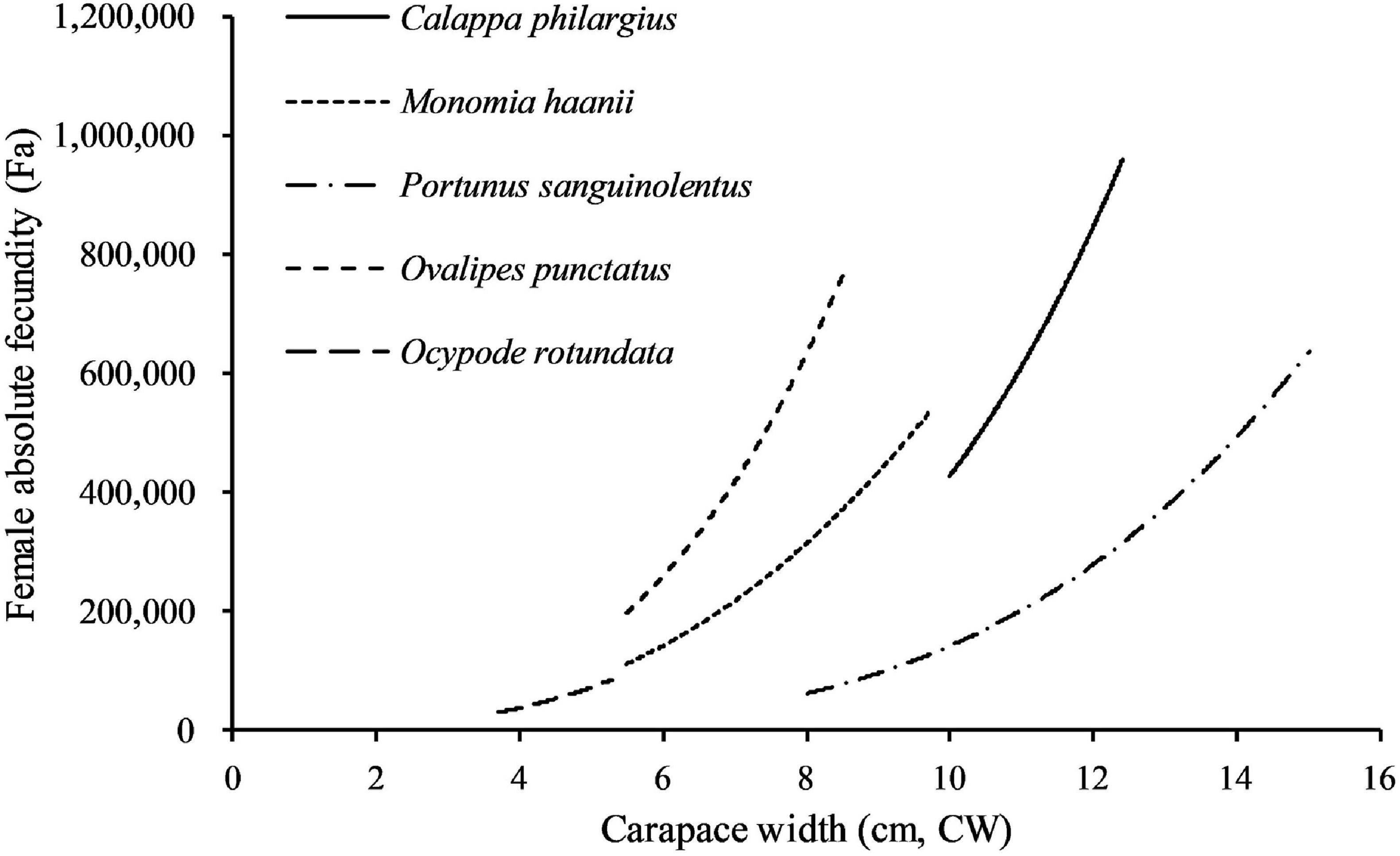
Figure 9. Comparison of the absolute fecundity (Fa, eggs/female) and size (carapace width, CW) relationship of crabs studied.
Claw-Only Fishery
There are some debates on the biological merits of claw-only fishery development. On one hand, a claw-only fishery can sustain crab capture production while causing less mortality than traditional crustacean fisheries. It is believed that at least some declawed crabs do survive and grow after their return to sea, and therefore regain possible breeding opportunities and re-enter into the fisheries. Clearly, declawed females expend energy on claw regeneration, which may negatively impact reproductive success and shorter spawning seasons (Savage and Sullivan, 1978; Hogan and Griffen, 2007). For males, a declawed status may negatively affect breeding success through reducing an individual’s competitive prowess as well as by compromising defensive ability (higher natural mortality) (Duermit et al., 2015).
In practice, only 7–13% regenerated claws entered the M. mercenaria market fishery in Florida, the United States (Simonson and Hochberg, 1986; Wilber, 1995; Muller et al., 2011). The mortality rate associated with two-claw removal is high in M. mercenaria and C. borealis, ranging from 28 to 100% and 70%, respectively, and higher with increasing temperatures under laboratory conditions (Davis et al., 1978; Simonson and Hochberg, 1986; Gandy et al., 2016; Goldstein and Carloni, 2021). The recent developing one-claw fishing regulations on M. mercenaria and C. borealis showed an around 35–50% reduction in mortality rate when compared to two-claw removal in the fishery (Davis et al., 1978; Duermit et al., 2017; Goldstein and Carloni, 2021). However, the application of one-claw fishing strategy in practice is difficult to monitor and highly depends on voluntary adherence (Duermit et al., 2017). The impacts of one-claw and two-claw removal fisheries of C. philargius merit further investigation on feeding ability, survival rate, claw regeneration, mating success, and rates of re-entrance into a fishery for further management consideration and policy-making.
Obtaining the claw yield (%) contributes significantly to estimate the C. philargius capture productions in the field surveys. The average claw yield for C. philargius was 36.28%, which is higher than that of C. affinis (15% in Robinson, 2008), but lower than those of U. tangeri (40% in Crane, 1975) and M. mercenaria (50% in Davis et al., 1978). Capture productions of C. philargius in the southern Taiwan Strait varies by fishing gear and month. The production proportions of C. philargius were generally less than 1% of the total catches in trawl fishery, but made up a considerable portion (> 10%) of the trap fishery landings (Tables 4, 5).
Higher C. philargius catches occurred in September and October in the trawl fishery, and in January, April and December for the trap fishery. One possible explanation is the differences in fishing zones in the southern Taiwan Strait by fishing gears over time and potential migration behavior in C. philargius during their life history. Among the C. philargius trawl catches in the southern Taiwan Strait, about 95% individuals were ≥ 9.0 cm CW, and about 75% individuals ≥ 11.0 cm CW. Individuals < 9.0 cm CW are usually discarded at sea in both trawl and trap fisheries based on interviews because of the low prices offered for small claws. Based on our estimated CW50 (11.47 cm CW and 8.7 cm CL), at least 25% of our sampled C. philargius catch was sexually immature.
Fishery Management
To date, only one national and two provincial (Zhejiang and Fujian Provinces) regulations have been implemented in China on the minimum catch sizes and landing proportions of juveniles. Collectively, these cover 36 commercially important species including 28 fin fishes, two shrimps, four crabs, and two cephalopods.1, 2, 3 Currently, there are landing prohibitions on P. trituberculatus juveniles (individuals < 70 g) and berried females as well as bans on sale in food markets in Zhejiang Province between April 1st and September 16th, i.e., throughout the entire national summer fishing moratorium period.4 This management strategy could also be suggested to apply to other crab species in China for protecting the spawning populations during the peak spawning seasons.
As a newly developing claw-only fishery for C. philargius, no management measures are set for the species except the general national summer fishing moratorium. However, the critical peak spawning months (February and April) for C. philargius fall outside of the national summer fishing moratorium. About 60% of C. philargius catches in the trawl fishery are females, and moreover, high female trawl catches occurred in the highest peak spawning month, February, indicating high exploitation of spawning female stocks. Recently, a seasonal spawning closure has been proposed in lieu of the one claw fishing regulation based on biological and practical tradeoffs between strategies (Duermit et al., 2017). For C. philargius, February needs to be considered in the spawning seasonal closure measure.
A recent bioeconomic models reviewed that simple regulatory measure adjustments to the M. haanii fishery in the southern Taiwan Strait, the same fishing areas as C. philargius, can provide multiple biological and economic benefits (Boenish et al., 2021). Furthermore, a total allowable catch (TAC) pilot project for the P. trituberculatus fishery in Zhejiang Province is evaluating the utility of e-logbooks to improve response time and monitoring accuracy for Chinese crab fisheries (Zhu et al., 2021).
Conclusion
In conclusion, the current national fishing moratorium regulation, i.e., season closure in May-July, is not enough for C. philargius stock protection because its peak spawning month (February) and dual spawning seasons (January–April and August–October) are vulnerable to high fishing pressure. Management strategies, such as TAC, the spawning season closure, fishing input control by reducing the number of fishing vessels, and IUU fishing monitoring, should be considered as parts of a long-term comprehensive sustainable management plan. Significant outcomes of this study will help to evaluate strategies for the processing sector, domestic trade and ultimately fishery sustainability for C. philargius.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Xiamen University of China.
Author Contributions
B-AL and YJ wrote the first draft. ML and RB revised the manuscript. B-AL, YJ, and RB performed the data analyses. B-AL, YJ, QX, and ML conducted commercial sampling, interviews, and measurement. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Fisheries Institute’s (NFI) Red Crab Council and Ocean Outcomes (O2) of United States, and Qingdao Marine Conservation Society (QMCS) of China. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dongshan Swire Marine Station, Xiamen University (XMU) for facility and logistics support, and undergraduates and postgraduates from XMU and fishermen for laboratory and field assist.
Footnotes
- ^ http://hyyyj.fujian.gov.cn/
- ^ http://www.zj.gov.cn/
- ^ http://www.moa.gov.cn/
- ^ http://nynct.zj.gov.cn/art/2021/2/26/art_1694969_58931103.html
References
Alborés, I., García-Soler, C., and Fernández, L. (2019). Reproductive biology of the slipper lobster Scyllarus arctus in Galicia (NW Spain): implications for fisheries management. Fish. Res. 212, 1–11. doi: 10.1016/j.fishres.2018.12.001
Arshad, A., Efrizal, E., Kamarudin, M. S., and Saad, C. R. (2006). Study on fecundity, embryology and larval development of blue swimming crab Portunus pelagicus (Linnaeus, 1758) under laboratory conditions. Res. J. Fish. Hydrobiol. 1, 35–44.
Biscoito, M., Freitas, M., Pajuelo, J. G., Triay-Portella, R., Santana, J. I., Costa, A. L., et al. (2015). Sex structure, depth distribution, intermoult period and reproductive pattern of the deep-sea red crab Chaceon affinis (Brachyura. Geryonidae) in two populations in the north-eastern Atlantic. Deep Sea Res. 1 95, 99–114. doi: 10.1016/j.dsr.2014.10.010
Boenish, R., Lin, B., Kritzer, J. P., Wilberg, M. J., Shen, C., Jiang, Y., et al. (2021). A bioeconomic approach towards improved fishery management of Monomia haanii in the southern Taiwan Strait, China. Fish. Res. 240:105969. doi: 10.1016/j.fishres.2021.105969
Crane, J. (1975). Fiddler Crabs Of The World (Ocypodidae: Genus Uca). Princeton, NJ: Princeton University Press.
Davis, G. E., Baughman, D. S., Chapman, J. D., MacArthur, D., and Price, A. C. (1978). Mortality Associated With Declawing Stone Crabs, Menippe Mercenaria. South Florida Research Center Report T-522. Florida, FL: U.S. National Park Service.
Duermit, E., Kingsley-Smith, P. R., and Wilber, D. H. (2015). The consequences of claw removal on stone crabs Menippe spp. and the ecological and fishery implications. N. Am. J. Fish. Manag. 35, 895–905. doi: 10.1080/02755947.2015.1064836
Duermit, E., Shervette, V., Whitaker, J. D., Kingsley-Smith, P. R., and Wilber, D. (2017). A field assessment of claw removal impacts on the movement and survival of stone crabs Menippe spp. Fish. Res. 193, 43–50. doi: 10.1016/j.fishres.2017.03.019
Erdman, R. B., and Blake, N. J. (1988). Reproductive ecology of female golden crabs, Geryon fenneri Manning and Holthuis, from southeastern Florida. J. Crustac. Biol. 8, 392–400. doi: 10.2307/1548278
Fahy, E., Hickey, J., Perella, N., Hervas, A., Carroll, J., and Andray, C. (2004). Bionomics of Brown Crab Cancer Pagurus In The South East Ireland Inshore Fishery. Irish Fisheries Investigations No. 12. Ireland: Marine Institute.
Farias, N. E., Luppi, T. A., and Spivak, E. D. (2014). Habitat use, relative growth and size at maturity of the purple stone crab Platyxanthus crenulatus (Decapoda: Brachyura), calculated under different models. Sci. Mar. 78, 567–578. doi: 10.3989/scimar.04108.10A
Fazhan, H., Waiho, K., Fujaya, Y., Rukminasari, N., Ma, H., and Ikhwanuddin, M. (2021). Sexual dimorphism in mud crabs: a tale of three sympatric Scylla species. PeerJ 9:e10936. doi: 10.7717/peerj.10936
Fenberg, P. B., and Roy, K. (2008). Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Mol. Ecol. 17, 209–220. doi: 10.1111/j.1365-294X.2007.03522.x
Gandy, R., Crowley, C., Chagaris, D., and Crawford, C. (2016). The effect of temperature on release mortality of declawed Menippe mercenaria in the Florida stone crab fishery. Bull. Mar. Sci. 92, 1–15. doi: 10.5343/bms.2015.1036
Goldstein, J. S., and Carloni, T. J. (2021). Assessing the implications of live claw removal on Jonah crab (Cancer borealis), an emerging fishery in the Northwest Atlantic. Fish. Res. 243:106046. doi: 10.1016/j.fishres.2021.106046
Groeneveld, J. C., Everett, B. I., Fennessy, S. T., Kirkman, S. P., Santos, J., and Robertson, D. (2013). Spatial distribution patterns, abundance and population structure of deep-sea crab Chaceon macphersoni, based on complementary analyses of trap and trawl data. Mar. Freshw. Res. 64, 507–517. doi: 10.1071/MF12263
Hartnoll, R. G., Broderick, A. C., Godley, B. J., and Saunders, K. E. (2009). Population structure of the land crab Johngarthia lagostoma on Ascension Island. J. Crustac. Biol. 29, 57–61. doi: 10.1651/08-2992.1
Hisam, F., Hajisamae, S., Ikhwanuddin, M., Aziz, N. A. N., Naimullah, M., and Hassan, M. (2018). Study on the reproductive biology of the blue swimming crab, Portunus pelagicus females from Pattani coastal waters, Thailand. AACL Bioflux 11, 1776–1791.
Hogan, J. M., and Griffen, B. D. (2007). The dietary and reproductive consequences of fishery-related claw removal for the stone crab Menippe spp. J. Shellfish Res. 33, 795–804. doi: 10.2983/035.033.0314
Ikhwanuddin, M., Azmie, G., Juariah, H. M., Zakaria, M. Z., and Ambak, M. A. (2011). Biological information and population features of mud crab, genus Scylla from mangrove areas of Sarawak, Malaysia. Fish. Res. 108, 299–306. doi: 10.1016/j.fishres.2011.01.001
Jiang, X. Q., and Yu, C. G. (2012). Reproductive biology of Ovalipes punctatus in the East China Sea. J. Zhejiang Ocean Univ. 31, 285–289.
Jivoff, P. R., Smith, J. M., Sodi, V. L., Vanmorter, S. M., Faugno, K. M., Werda, A. L., et al. (2017). Population structure of adult blue crabs, Callinectes sapidus, in relation to physical characteristics in barnegat Bay, New Jersey. Estuaries Coast 40, 235–250. doi: 10.1007/s12237-016-0127-8
Johnson, D. D., Gray, C. A., and Macbeth, W. G. (2010). Reproductive biology of Portunus pelagicus in a south-east Australian estuary. J. Crustac. Biol. 30, 200–205. doi: 10.1651/08-3076.1
Kang, B., Liu, M., Huang, X. X., Li, J., Yan, Y. R., Han, C. C., et al. (2018). Fisheries in Chinese seas: what can we learn from controversial official fisheries statistics? Rev. Fish Biol. Fish. 28, 503–519. doi: 10.1007/s11160-018-9518-1
Keller, A. A., Harms, J. H., and Buchanan, J. C. (2012). Distribution, biomass and size of grooved tanner crabs (Chionoecetes tanneri) from annual bottom trawl surveys (2003-2010) along the U.S. west coast (Washington to California). Deep Sea Res. 1 67, 44–54. doi: 10.1016/j.dsr.2012.05.002
Krajangdara, T., and Watanabe, S. (2005). Growth and reproduction of the red frog crab, Ranina ranina (Linnaeus, 1758), in the Andaman Sea off Thailand. Fish. Sci. 71, 20–28. doi: 10.1111/j.1444-2906.2005.00926.x
Lin, B., Boenish, R., Kritzer, J. P., Jiang, Y., Wang, S., and Liu, M. (2021). Reproductive dynamics of a swimming crab (Monomia haanii) in the world’s crab basket. Fish. Res. 236:105828. doi: 10.1016/j.fishres.2020.105828
Liu, M., and Lin, B. (2019). Chinese Red Swimming Crab (Portunus Haanii) Fishery Improvement Project (FIP) in Zhangzhou City, Fujian Province, China (August 2018-April 2019). Mainland: Xiamen University.
Liu, Z., Wu, X., Wang, W., Yan, B., and Cheng, Y. (2014). Size distribution and monthly variation of ovarian development for the female blue swimmer crab, Portunus pelagicus in Beibu Gulf, off south China. Sci. Mar. 78, 257–268. doi: 10.3989/scimar.03919.24A
Muller, R. G., Chagaris, D., Bert, T. M., Crawford, C., and Gandy, R. L. (2011). The 2011 Stock Assessment Update For The Stone Crab, Menippe Spp., fishery in Florida. Florida, FL: Florida Fish and Wildlife Conservation Commission, IHR 2011-003.
Naderi, M., Abbas, S. H., Pazooki, J., Hedayati, A., Zare, P., and Lastra, M. (2018). Reproductive biology of the ghost crab, Ocypode rotundata Miers, 1882 (Decapoda, Ocypodidae) at Qeshm Island, Persian Gulf. Crustaceana 91, 1039–1059. doi: 10.1163/15685403-00003804
Ng, P. K. L. (1998). “Crabs,” in FAO Species Identification Guide For Fishery Purposes. The Living Marine Resources Of The Western Central Pacific. Cephalopods, crustaceans, holothurians and sharks, Vol. 2, eds K. E. Carpenter and V. H. Niem (Rome: Food and Agriculture Organization), 1045–1155.
Oh, C. (2011). Population biology of the swimming crab Portunus trituberculatus (Miers, 1876) (Decapoda, Brachyura) on the western coast of Korea, Yellow Sea. Crustaceana 84, 1251–1267. doi: 10.1163/001121611X586675
Oliveira, R. F., Machado, J. L., Jordão, J. M., Burford, F. L., Latruffe, C., and McGregor, P. K. (2000). Human exploitation of male fiddler crab claws: behavioural consequences and implications for conservation. Anim. Conserv. 3, 1–5. doi: 10.1111/j.1469-1795.2000.tb00081.x
Öndes, F., Emmerson, J. A., Kaiser, M. J., Murray, L. G., and Kennington, K. (2017). The catch characteristics and population structure of the brown crab (Cancer pagurus) fishery in the Isle of Man, Irish Sea. J. Mar. Biol. Assoc. U.K. 99, 119–133. doi: 10.1017/S0025315417001849
Orensanz, J. M., Armstrong, J., Armstrong, D., and Hilborn, R. (1998). Crustacean resources are vulnerable to serial depletion-the multifaceted decline of crab and shrimp fisheries in the Greater Gulf of Alaska. Rev. Fish Biol. Fish. 8, 117–176. doi: 10.1023/A:1008891412756
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Robinson, M. (2008). Minimum landing size for Northeast Atlantic stocks of deep-water red crab, Chaceon affinis (Milne Edward and Bouvier, 1894). ICES J. Mar. Sci. 65, 148–154. doi: 10.1093/icesjms/fsm189
Rouf, M. A., Shahriar, S. I. M., Antu, A.-H., and Siddiqui, M. N. (2021). Population parameters of the orange mud crab Scylla olivacea (Herbst, 1796) from the Sundarban mangrove forest in Bangladesh. Heliyon 7:e06223. doi: 10.1016/j.heliyon.2021.e06223
Sadovy, Y. J. (1996). Reproduction Of Reef Fishery Species, eds N. V. C. Polunin and C. M. Roberts (London: Reef Fisheries. Chapman & Hall), 15–59. doi: 10.1007/978-94-015-8779-2_2
Savage, T., and Sullivan, J. R. (1978). Growth And Claw Regeneration Of The Stone Crab, Menippe Mercenaria. Florida, FL: Florida Marine Research Publications.
Sforza, R., Nalesso, R. C., and Joyeux, J. C. (2010). Distribution and population structure of Callinectes danae (decapoda: Portunidae) in a tropical Brazilian estuary. J. Crustacean Biol. 30, 597–606. doi: 10.1651/09-3223.1
Shao, K. T., Chang, J. S., Jeng, M. S., Tu, T. H., Ho, C. W., Chiu, Y. W., et al. (2015). A Guide Book Of Common Economic Aquatic Animals And Plant In Taiwan. Taiwan: Fishery Agency, Council of Agriculture.
Simonson, J. L., and Hochberg, R. J. (1986). Effects of air exposure and claw breaks on survival of stone crabs Menippe mercenaria. Trans. Am. Fish. Soc. 115, 471–477. doi: 10.1577/1548-8659(1986)115<471:EOAEAC>2.0.CO;2
Soundarapandian, P., Varadharajan, D., and Boopathi, A. (2013). Reproductive biology of the commercially important Portunid crab, Portunus sanguinolentus (Herbst). J. Mar. Sci. Res. Dev. 3, 1–9. doi: 10.4172/2155-9910.1000124
Truesdale, C. L. (2018). Fishery and biological characteristics of Jonah crab (Cancer borealis) in Rhode Island Sound. Open Access Master’s Theses, (University of Rhode Island), aer1206. doi: 10.1016/j.fishres.2018.10.030
Wahle, R. A., Bergeron, C. E., Chute, A. S., Jacobson, L. D., and Chen, Y. (2008). The Northwest Atlantic deep-sea red crab (Chaceon quinquedens) population before and after the onset of harvesting. ICES J. Mar. Sci. 65, 862–872. doi: 10.1093/icesjms/fsn058
Waiho, K., Fazhan, H., and Ikhwanuddin, M. (2016). Size distribution, length–weight relationship and size at the onset of sexual maturity of the orange mud crab. Scylla olivacea, in Malaysian waters. Mar. Biol. Res. 12, 726–738. doi: 10.1080/17451000.2016.1200726
Waiho, K., Ikhwanuddin, M., Abualreesh, M. H., Shu-Chien, A. C., Ishak, S. D., and Jalilah, M. (2021). Intra- and interspecific variation in sexual dimorphism patterns of mud crab genus Scylla along the equatorial region. Front. Mar. Sci. 8:690836. doi: 10.3389/fmars.2021.690836
Wenner, A. M. (1972). Sex ratio as a function of size in marine Crustacea. Am. Nat. 106, 32–50. doi: 10.1086/282774
Wilber, D. H. (1995). Claw regeneration among north Florida stone crabs (genus Menippe) and its implications to the southwest Florida fishery. Bull. Mar. Sci. 56, 296–302.
Wisespongpand, P., Jaingam, W., Thamrongnawasawat, T., and Khaodon, K. (2016). “Life history of shame-faced crab Calappa philargius (Linnaeus, 1758) and shellfish feeding behavior. Abstract from conference poster: Agricultural Innovation for Global Value Chain,” in Proceedings of 54th Kasetsart University Annual Conference, 2-5 February 2016, Kasetsart University, Thailand. Vol. 1, Plants, Animals, Veterinary Medicine, Fisheries, Agricultural Extension and Home Economics 2016 pp.922-930 ref.12. (Accession number 20163376083), Thailand.
Yang, C. P., Li, H. X., Li, L., Xu, J., and Yan, Y. (2014). Population structure, morphometric analysis and reproductive biology of Portunus sanguinolentus (Herbst, 1783) (Decapoda: Brachyura: Portunidae) in Honghai Bay. South China Sea. J. Crustac. Biol. 34, 722–730. doi: 10.1163/1937240X-00002273
Zhang, W., Liu, M., Sadovy de Mitcheson, Y., Cao, L., Leadbitter, D., Newton, R., et al. (2020). Fishing for feed in China: facts, impacts and implications. Fish Fish. 21, 47–62. doi: 10.1111/faf.12414
Keywords: Calappidae, crab fisheries, fecundity, sex ratio, spawning season
Citation: Lin B-a, Jiang Y, Boenish R, Xu Q and Liu M (2021) Population, Reproductive and Fishery Dynamics of Spotted Box Crab (Calappa philargius), a New Claw-Only Fishery Species, in the Southern Taiwan Strait, China. Front. Mar. Sci. 8:751790. doi: 10.3389/fmars.2021.751790
Received: 02 August 2021; Accepted: 28 September 2021;
Published: 21 October 2021.
Edited by:
Brett W. Molony, Oceans and Atmosphere (CSIRO), AustraliaReviewed by:
Khor Waiho, University of Malaysia Terengganu, MalaysiaHao Zhen Lin, Dalian Ocean University, China
Copyright © 2021 Lin, Jiang, Boenish, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Liu, bWlubGl1eG1AeG11LmVkdS5jbg==
†Present address: Yan Jiang, State Key Laboratory of Marine Pollution, City University of Hong Kong, Kowloon, Hong Kong, SAR China
 Bai-an Lin
Bai-an Lin Yan Jiang
Yan Jiang Robert Boenish
Robert Boenish Qing Xu
Qing Xu Min Liu
Min Liu