
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 06 December 2021
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.717360
This article is part of the Research Topic The Physiological and Molecular Response of Aquatic Animals to Environmental Stresses View all 23 articles
The present work aimed to investigate the causes of summer mortality syndrome affecting cultured European seabass (Dicentrarchus labrax) by examining physiochemical farm water characteristics, isolation, and identification of recovered bacterial pathogens from diseased fish studying the effect of water temperature on stress biomarkers and disease severity. Studied water parameters were normal except ammonia and dissolved oxygen was higher and lower than the standard value. Sixty-two bacterial isolates were recovered from moribund fish and identified as 31 Vibrio fluvialis, 23 Pseudomonas aeruginosa and 8 Staphylococcus aureus isolates. The calculated LD50 of V. fluvialis, P. aeruginosa and S. aureus for D. labrax fingerlings were 4.67 × 107, 2.37 × 106 and 1.38 × 107, respectively. There was a direct correlation between water temperature and mortality rate of fish challenged with V. fluvialis as the mortality rate was 44.44, 50, 66.66, and 83.33% for fish maintained at 27, 30, 33, and 36°C. Plasma cortisol, superoxide dismutase, catalase and malondialdehyde significantly increased when the water temperature exceeded 30°C. The experimentally infected fish showed similar clinical signs and postmortem lesions of naturally diseased fish with no boundary between different pathogens. Antibiogram test indicated that florfenicol was the most effective antibiotic against all the recovered bacterial isolates while all isolates resisted sulfamethoxazole-trimethoprim. Massive degenerative changes observed in the hepatopancreas, posterior kidney and gill tissues of experimentally infected fish.
– Automatic biochemical identification of 62 bacterial isolates are: 31 Vibrio fluvialis, 23 Pseudomonas aeruginosa and 8 Staphylococcus aureus for the first time in Egypt from diseased farmed seabass (Dicentrarchus labrax).
– Isolated bacteria were highly pathogenic for D. labrax fingerlings with LD50 estimated by 4.67 × 107, 2.37 × 106 and 1.38 × 107 for V. fluvialis, P. aeruginosa and S. aureus, respectively.
– Water temperature higher than 30°C plays a critical role as a primary stress factor by increasing stress biomarkers (cortisol and oxidative stress markers) and increasing disease susceptibility and mortality rates.
– Florfenicol was the most effective antibiotic against all the isolated pathogens.
– High toxic ammonia and low dissolved oxygen have a potential role in summer mortality syndrome.
European seabass (Dicentrarchus labrax) is considered the most economically important fish in Mediterranean mariculture (Vandeputte et al., 2019). Egyptian production of cultured D. labrax has reached 24,914 thousand tons in 2018 (GAFRD, 2020). Egypt produces 14% of cultured D. labrax and Sparus aurata in the Mediterranean region, and it is considered the third major producer after Turkey and Greece (Muniesa et al., 2019). Many bacterial pathogens are responsible for severe diseases affecting farmed seabass in the Mediterranean. Muniesa et al. (2019) reported that Vibriosis was the most frequently reported bacterial infection in D. labrax, followed by tenacibaculosis and photobacteriosis. Uzun and Ogut (2015) isolated Aeromonas veronii, Photobacterium damselae subsp. damselae, Vibrio vulnificus, V. harveyi and V. rotiferianus from cage cultured D. labrax. V. anguillarum, V. harvey, Photobacterium damsela subsp. piscicida, Tenacibaculum, Aeromonas spp. and Mycobacterium spp. represents the primary pathogens in European seabass aquaculture in Europe (Zrncic, 2020).
The optimum water temperature for European seabass growth is between 22 and 28°C, as reported by Barnabe (1990), Requena et al. (1997), and Lanari et al. (2002). They also recorded a sharp increase in the metabolic rate above 28°C due to high energy requirements for ventilation and blood circulation.
All marine fish farms rely on semi-intensive aquaculture, which is the most dominant system in Egypt. The semi-intensive system represents about 80% of the total production (Soliman and Yacout, 2016; Kaleem and Sabi, 2021), while the other systems represent only 20%.
Egyptian mariculture farms have suffered from annually disease attacks, mainly during the summer season, termed summer mortality syndrome. Each outbreak is characterized by appearance of general signs of septicemia on affected fish (Ali et al., 2019). The estimated direct economic losses in cultured sea bass were 163, 146 and 544 million Egyptian pounds during 2017, 2018, and 2019. The mortality rate ranged between 30 and 70%; in some cases, it reached up to 100% of stocked fish (Ali and Aboyadak, 2021).
Summer mortality syndrome was the common name used to characterize the unknown phenomenon affecting cultured Nile tilapia (Oreochromis niloticus) in Egyptian. This phenomenon occurred during the summer season and was characterized by a high mortality rate and general signs of septicemia on moribund and dead fish (Nicholson et al., 2017; Shaalan et al., 2018; Ali et al., 2020). The first scientific reporting of summer mortality syndrome was performed by Fathi et al. (2017), while this phenomenon started to hit tilapia farms in 2012. Mass mortalities were first observed in affected marine fish farms in 2014; meanwhile, the outbreak that hit farms in 2019 was the severest one (Eissa et al., 2021). The present work is considered the first scientific reporting of summer mortality syndrome in the Egyptian cultured sea bass farms.
Climate change, particularly global warming, negatively affects fish productivity through extreme temperature events like heatwaves that induce thermal stress with acute physiological consequences (Levitus et al., 2012; Currie and Schulte, 2014).
Cortisol is the principal glucocorticoid in fish; it is secreted by the steroidogenic cells present in the anterior kidney of the teleost fish; under stress conditions, cortisol is released through activation of the hypothalamus-pituitary inter renal axis (Martinez-Porchas et al., 2009). Cortisol is released in response to acute or chronic exposure to high temperatures in many fish species, including D. labrax (Alfonso et al., 2020).
Thermal stress-inducing reactive oxygen species (ROS) production ROS is responsible for oxidative damage in different biomolecules and tissues (Kamyab et al., 2017). Superoxide dismutase (SOD), malondialdehyde (MDA) and catalase are the most crucial oxidative stress markers. They act as a good indicator for monitoring the oxidative stress response induced by thermal stress.
Dissolved oxygen is considered one of the main limiting factors in fish farming; low dissolved oxygen negatively affects fish behavior, physiology and immunology (Abdel-Tawwab et al., 2019). There is a direct relationship between metabolic rates in fish and water temperature; increased water temperature results in the activation of many cellular enzymes responsible for most fish species physiological function and biochemical processes with a subsequent increase in oxygen demand (McNally and Mehta, 2004).
Cheng et al. (2015) reported that toxic ammonia induces intracellular reactive oxygen species and subsequently induces oxidative stress. Ammonia also interrupts intracellular calcium homeostasis and lead to DNA damage and cell apoptosis.
The present research aimed to identify the bacterial pathogens responsible for summer mortality syndrome affecting cultured seabass farms in Egypt, clarify the potential role of global warming and the physiochemical farm water characteristics as a predisposing factor for this syndrome.
Fish samples and the experimental fish used in the present research were handled, transported, examined and euthanized following the National Advisory Committee for Laboratory Animals Research (NACLAR, 2004) and CCAC (2005) guidelines for the care and use of fish in teaching and research. NIOF Committee for Institutional Care of Aquatic Organisms and Experimental Animals (NIOF-IACUC) has approved this work under certificate number (NIOF-AQ2-F-21-P-001).
Three European seabass (D. labrax) fish farms suffering from a high mortality rate reached up to 70% of the stocked fish. Studied farms located at Barbahary, Burullus district, Kafrelsheikh governorate closed to the Mediterranean Sea coast. All the studied farms used the semi-intensive fish culture system; each farm was about 2.5 ha; stocking density ranged between 16,000–20,000 fish in each farm.
Clinically diseased fish collected live (10 fish from each farm); each was rinsed with sterile water and packed in a sterile polystyrene bag. After that, collected samples were immediately transported in an icebox to the fish diseases Lab., National Institute of Oceanography and Fisheries, Alexandria branch. Fish samples were ranged between (380–433) g in body weight and (34.5–37) cm in total length.
Physiochemical water parameters were analyzed immediately during farm visits. According to the manufacturer's instructions, the multi-parameter electrochemical analyzer model, C6030 Consort, Belgium, was used to determine water temperature, hydrogen ion concentration, dissolved oxygen, and salinity. Total ammonia, nitrite, total hardness and alkalinity level were assayed using HI83399 photometer, HANNA instruments, Italy. The assay was performed using ammonia low range (HI93700-1), Nitrite low range (HI93707-01), total hardness low range (HI93735-00) and marine alkalinity (HI755-26) test kits according to the reference methods indicated by APHA (2018). The unionized (toxic) ammonia level was calculated using online the free ammonia-nitrogen calculator (Alleman, 1998).
Bacterial isolation was performed as described by El-Bahar et al. (2019) with some modification. Briefly, in the microbiological safety cabinet, each fish sample was rinsed with sterile normal saline; after that the external surface was disinfected with a large cotton piece soaked in 70% ethyl. Small piece of hepatopancreas, spleen, heart and a posterior kidney scrap were taken in a sterile falcon tube, tissue samples homogenized with 10 ml−1 of 0.1% peptone water at 6,000 rpm for 5 min. After homogenization, the falcon tube was centrifuged at 3,000 rpm for 2 min. One milliliter from the supernatant of each tube was added to 9 ml of sterile brain heart infusion broth and incubated at 35°C for 18 h. A single lopeful from each broth tube was streaked carefully over a brain heart infusion agar plate to obtain a single pure colony; plates were incubated at 35°C for 18 h.
Each single morphologically characteristic colony was picked up from the agar plate then incubated in brain heart infusion broth; after that, it was re-cultured on brain heart infusion agar; this process repeated till all the recovered isolates became in a pure form.
The recovered bacterial isolates were identified by VITEK 2 system as described by Ali et al. (2018) with few modifications. Briefly, a single colony was picked up and subjected to Gram staining (Black and Black, 2015) for determining the suitable identification card (GP or GN).
Few bacterial colonies were picked up using a sterile glass rod from fresh bacterial culture during the lag phase (about 12 h). Colonies were suspended in 5 ml of 0.5 % sodium chloride by vortex mixer; after that, the optical density of the solution was adjusted to 0.6 McFarland standards using the DensiCHEK Plus calibrator.
The identification cards were placed in VITEK 2 system cassette together with bacterial suspension tubes, and then the system logged for data entry of each isolate. Identification cards were automatically inoculated with bacterial suspension by the integrated vacuum apparatus, followed by automatic incubation and monitoring of the biochemical profile for each isolate.
The recovered V. fluvialis, P. aeruginosa and S. aureus isolates were cultured on thiosulphate citrate bile salts sucrose agar (TCBS), Pseudomonas agar base medium and mannitol salt agar for confirmation.
Five-hundred D. labrax fingerlings were used in the pathogenicity and challenge test; experimental fish were transported to the wet laboratory (NIOF) under the optimum condition as mentioned by Bosworth and Small (2004). During fish transportation, water temperature is reduced by 5° than the ambient using ice to reduce fish metabolic rate and activity. TRICAINE-S® (Tricaine methanesulfonate) is used for fish tranquillization at a dose of 25 mg L−1 as indicated by Wang et al. (2019). Continuous aeration was maintained during fish transportation using pure oxygen cylinders. Experimental fish were ranged between (14–16) cm in the total length and (27.8–30) g in body weight.
After transportation, fingerlings were kept in 5,000 L fiberglass aquaria and maintained off-food for 24 h. Fish were observed for 15 days for acclimatization with continuous water change at a rate of 1,000 L per day.
Pathogenicity test was performed according to Saleh et al. (2021) for a single randomly selected isolate from each recovered bacterial species to satisfy Koch's postulates and ensure the virulence for D. labrax fingerlings.
A single bacterial colony was picked up from specific media, then incubated on brain heart infusion broth at 35°C for 12 h. Bacterial growth was harvested by centrifugation at 5,000 rpm for 3 min. The bacterial pellet was suspended in 0.1% peptone water and adjusted to the absorbance of 0.451 at 600 nm equivalent to the second McFarland standard (6 × 108) CFU ml−1. One ml of sterile phosphate buffer saline was added to 5 ml of bacterial suspension to achieve a final concentration of (5 × 108) CFU ml−1. Tenfold serial dilution was performed three consecutive times to obtain the following concentrations (5 × 107, 5 × 106 and 5 × 105) CFU ml−1.
Two-hundred and seventy fish were used in the pathogenicity test. Each 90 fingerlings were challenged with a particular bacterial isolate after being randomly divided into five groups in triplicates (18 fish in each group and 6 fish per replicate). Fish in the first group were received 0.2 ml of normal saline and maintained as a negative control; fish in the remaining four groups were intraperitoneally inoculated with 0.2 ml of bacterial suspension as presented in Table 3. Each replicate was maintained in 100 L capacity glass aquarium; water temperature was thermostatically controlled at 24°C with continuous water change at a rate of 5 L h−1. Feeding was restricted for 24 h before infection and resumed at 12 h post-infection. All fish groups were kept under observation for 7 days to record abnormal clinical signs, postmortem lesions and mortality rate; each dead fish was considered only after re-isolation of the challenged bacterial isolate. Lethal dose fifty (LD50) was calculated as described by Aboyadak and Ali (2021).
Seventy-two healthy fingerlings were intraperitoneally inoculated with bacterial suspension containing 4.5 × 107 CFU of V. fluvialis fish−1. Inoculated fish were subdivided into four equal groups in triplicates, each subgroup maintained at a different water temperature in which groups 6, 7, 8 and 9 were maintained at 27, 30, 33, 36°C, respectively as shown in Table 4. For simulating the fluctuations in water temperature throughout the day, the aquarium water temperature was increased from 27°C by 0.1°C min−1 to reach 30, 33 and 36°C after that it was maintained constant for 6 h then left to decrease normally and kept at 27°C. Fish groups were observed for 5 days (the average heatwave period) with daily monitoring of the mortality rate and disease signs.
For estimating the effect of thermal stress on normal healthy D. labrax fingerlings maintained at different water temperatures, stress markers including plasma cortisol and oxidative stress markers were assayed. Seventy-two healthy fingerlings were subdivided into four equal groups in triplicates, each subgroup maintained at a different water temperature in which groups 10, 11, 12 and 13 were maintained at 27, 30, 33, 36°C, respectively as shown in Table 5. Aquarium water temperature was increased from 27°C by 0.1°C min−1 to reach 30, 33 and 36°C; after that it was maintained constant for 6 h.
Blood samples were collected from 6 fish in each group at 6-h post-application of thermal stress and under anesthesia (water temperature remains peaked in summer months at least for 6 h each day). Blood samples were collected via caudal venipuncture using 25-gauge needle. The needle was inserted with 45° slope, and blood was drowned slowly to avoid hemolysis. Half ml of the blood sample was placed in a heparinized tube for cortisol determination, while the other part was placed in Eppendorf tubes and kept at 8°C in the refrigerator for 30 min for clotting. Samples were centrifuged at 3,000 rpm for 15 min till complete separation, collected plasma and serum samples were preserved frozen at −85°C.
Plasma cortisol was extracted according to Fatira et al. (2014) with some modifications. Briefly, 150 μl of plasma sample was vortex mixed with 1.5 ml of diethyl ether for 2 min, the mixture was left for separation. Then 150 μl of the organic layer was evaporated in a test tube at 45°C; after that, residue was reconstituted in 150 μl of 1X EIA extraction buffer (bioPLUS™). The sample was further diluted 50 times before analysis; the obtained result was multiplied by 500. Plasma cortisol assayed by Ao Microplate Reader, Azure Biosystems, USA; absorbance was measured at 415 nm using 96 well cortisol ELISA kit, Cayman Chemical, USA.
Superoxide dismutase (SOD), catalase, and malondialdehyde (MDA) were assayed using V-750 UV-Visible spectrophotometer, Jasco, Japan, according to the methods described by Ghneim et al. (2016), Hadwan (2018), and Leon and Borges (2020), respectively.
Naturally infected fish were examined on the farm to determine any external abnormalities. The challenged fish were observed twice daily throughout the experiment period according to the method described by Austin and Austin (2016). Naturally infected and challenged fish were dissected during the microbiological examination as described by El-Bahar et al. (2019), any gross internal lesions were reported.
The antimicrobial susceptibility test was performed for three randomly selected isolates from each type of the identified bacteria. Bacterial susceptibility was investigated for doxycycline (DO 30 μg), oxytetracycline (OTC 30 μg), sulfamethoxazole-trimethoprim (STX 25 μg), florfenicol (F 10 μg), amoxicillin (AX 25), spiramycin (SP 100 μg) and erythromycin (E 15 μg). Agar disk diffusion test performed on Mueller-Hinton agar, Oxoid® according to the method described by Clinical and Laboratory Standards Institute (CLSI) (2016). Overnight seeded Mueller-Hinton broth was adjusted to 1.5 × 108 CFU/ml−1 by spectrophotometer. Two milliliters were spread on the agar plate surface with rotation movement to ensure the even distribution; then, the excess fluid was removed. The agar plate was allowed to stand in an inverted position on a flat surface for 10 min to absorb the excess moisture. Antimicrobial discs were gently fixed into the agar surface by fine forceps. The agar plates were incubated at 35°C for 24 h; Escherichia coli ATCC 25922 was used as a control strain. The inhibition zone was measured to the nearest mm using a digital caliper and interpreted according to breakpoints mentioned by Clinical and Laboratory Standards Institute (CLSI) (2016) and shown in Table 6.
The histopathological examination was performed as described by Suvarna et al. (2018). Small tissue pieces from the hepatopancreas, gills and posterior kidney of V. fluvialis infected fish were fixed in buffered formalin 10% solution then dehydrated in ascending grade ethyl alcohol; after that, was cleared in xylene. The cleared samples were impeded in soft then hard paraffin wax, sectioned to 5 μm thickness using Leica RM2235 microtome (Lecia, Germany). Tissue sections were mounted over glass slides and stained with hematoxylin and eosin. Stained sections were examined and photographed by Olympus microscope with digital camera.
Plasma cortisol and oxidative stress markers values were checked for normality by the Shapiro-Wilk test using the online version implemented by Simon (2009). After that, data were statistically analyzed for variance (ANOVA) at least significant difference (LSD) as described by Snedecor and Cochran (1989) using IBM SPSS Statistics for Windows, Version 22 (2013), Armonk, NY: IBM Corp, Data were considered statistically significant at P ≤ 0.05.
The tested water parameters were in the acceptable range for European seabass aquaculture except, dissolved oxygen was lower than the optimum concentration (5 mg L−1) and unionized (toxic) ammonia was higher than the acceptable value (0.05 mg L−1) as presented in Table 1.
A total of 62 pure isolates were identified biochemically as 31 V. fluvialis, 23 P. aeruginosa and 8 S. aureus using Vitek 2 system, the biochemical profile of recovered isolates represented in Table 2.
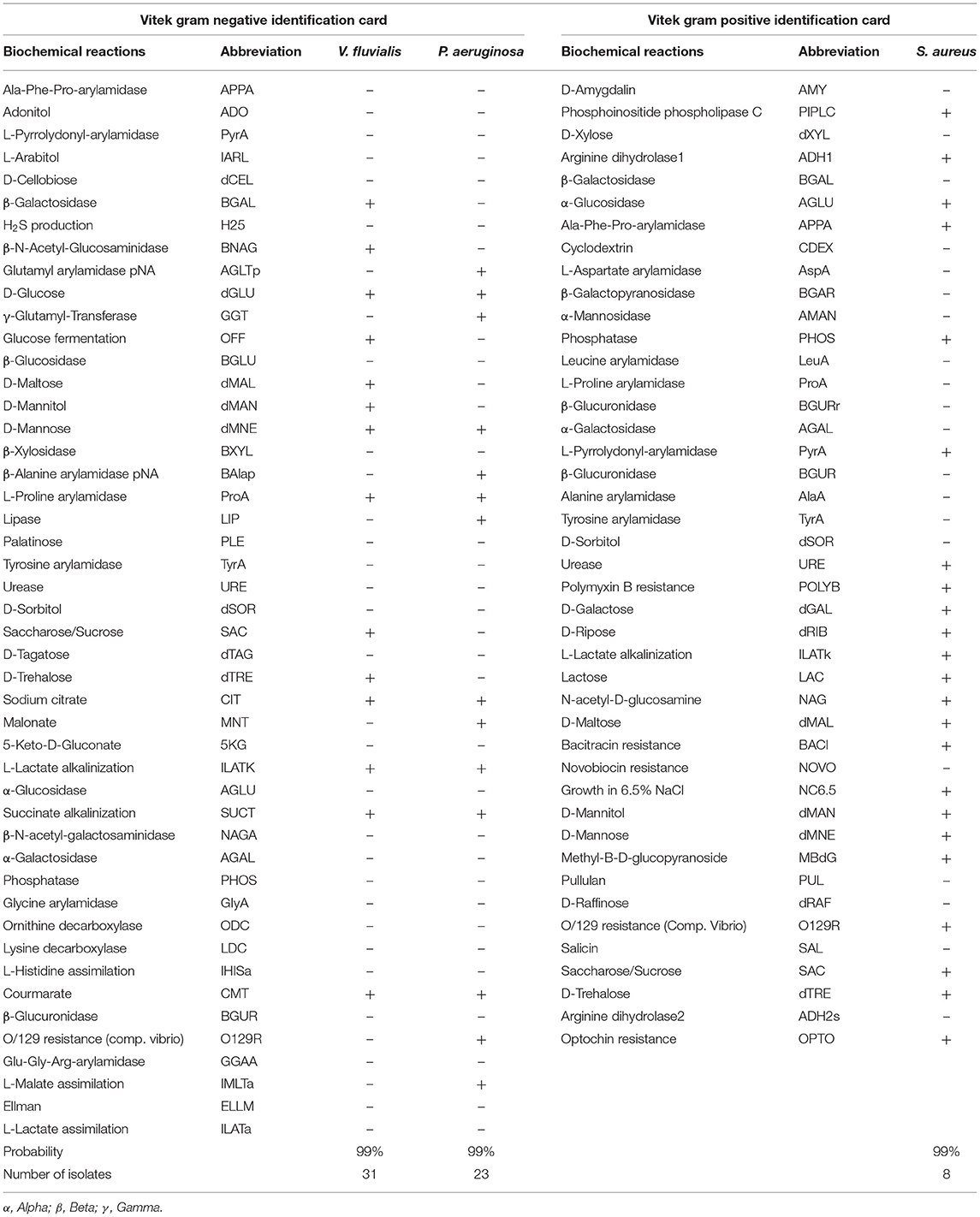
Table 2. The biochemical profile of Vibrio fluvialis, Pseudomonas aeruginosa and Staphylococcus aureus isolates.
Thirty-one V. fluvialis isolates were grown on TCBS as yellow, medium-size colonies about 2–3 mm in diameter. All the 23 P. aeruginosa isolate appeared as yellowish-green colonies on pseudomonas selective agar. On mannitol salt agar, eight isolates appeared as small yellowish colonies characteristic for S. aureus confirming the automated biochemical identification results (Figure 1).
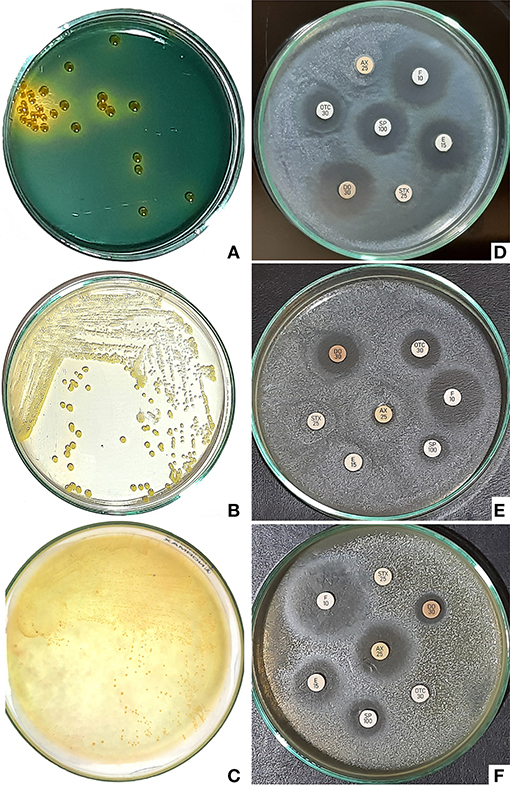
Figure 1. (A) Yellow colonies of Vibrio fluvialis on TCBS about 2–3 mm in diameter. (B) Yellowish green colonies of Pseudomonas aeruginosa on pseudomonas selective agar. (C) Small yellow colonies characteristic for Staphylococcus aureus on mannitol salt agar. (D) Antibiogram of Vibrio fluvialis indicated susceptibility to doxycycline, florfenicol and erythromycin. (E) Antibiogram of Pseudomonas aeruginosa showing high susceptibility to doxycycline and florfenicol. (F) Antibiogram of Staphylococcus aureus revealed good susceptibility to florfenicol.
The cumulative mortalities during the pathogenicity test are presented in Table 3. P. aeruginosa was the most virulent pathogen, followed by S. aureus then V. fluvialis with calculated LD50 equals 2.37 × 106, 1.38 × 107 and 4.67 × 107 CFU/fish, respectively.
Challenge test results indicated a direct correlation between water temperature and the mortality rate induced by V. fluvialis infection; the mortality rate dramatically increased when water temperature exceeds 30°C, as observed in Table 4.
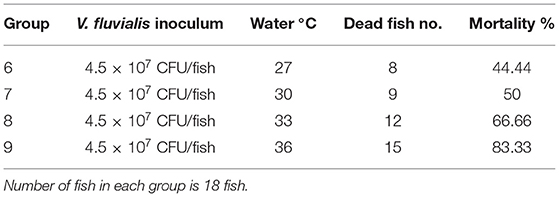
Table 4. Effect of water temperature on the cumulative mortality induced by experimental infection with Vibrio fluvialis.
Plasma cortisol values significantly increased when the water temperature exceeded 30°C, as represented in Table 5.
Superoxide dismutase and catalase values were significantly increased when the water temperature exceeded 30°C. Malondialdehyde significantly increased when the water temperature exceeds 33°C, as presented in Table 5.
The first clinical sign observed on naturally and experimentally infected fish was decreased feed intake followed by complete cessation. In the affected farms, fish accumulated at the water surface for air gasping, particularly in the afternoon due to low dissolved oxygen concentration. Diseased fish showed haemorrhagic ulcerations on the external body surface, eroded haemorrhagic pectoral, pelvic and tail fin. Internally the diseased fish showed congested and haemorrhagic internal organs mainly, the liver and posterior kidney, while the stomach and intestine were mostly empty. All the experimentally infected fish with V. fluvialis, P. aeruginosa and S. aureus showed the same clinical signs.
V. fluvialis was susceptible to doxycycline, florfenicol, and erythromycin; it was also intermediately sensitive to spiramycin, but resisted oxytetracycline, sulfamethoxazole-trimethoprim, and amoxicillin. P. aeruginosa was susceptible to doxycycline and florfenicol, intermediately sensitive to oxytetracycline, but resisted sulfamethoxazole-trimethoprim, amoxicillin, spiramycin and erythromycin. S. aureus was susceptible only to florfenicol, intermediately sensitive to amoxicillin, spiramycin and erythromycin, but resisted doxycycline, oxytetracycline and sulfamethoxazole-trimethoprim. Antimicrobial susceptibility test results are represented in Table 6.
Histopathological examination of D. labrax fingerlings experimentally infected with V. fluvialis showed massive degenerative changes in all studied tissues, as presented in Figure 2. Hepatopancreas showing diffused hepatic cell vacuolation and congestion in the pancreatic blood vessels. Inflammation, necrosis, mononuclear cell infiltrations and nuclear pyknosis were also dominant in the hepatic tissues of diseased fish. The posterior kidney showed diffuse mononuclear cell infiltration, abundant melanomacrophage centers activation, glomerular hypertrophy with the absence of bowman's space and degenerated renal tubules. The normal gill tissue architecture was absent due to severe hyperplasia, diffusion and curling of the secondary gill lamellae. Epithelial lifting with the detachment of some secondary gill lamellae was also prominent.
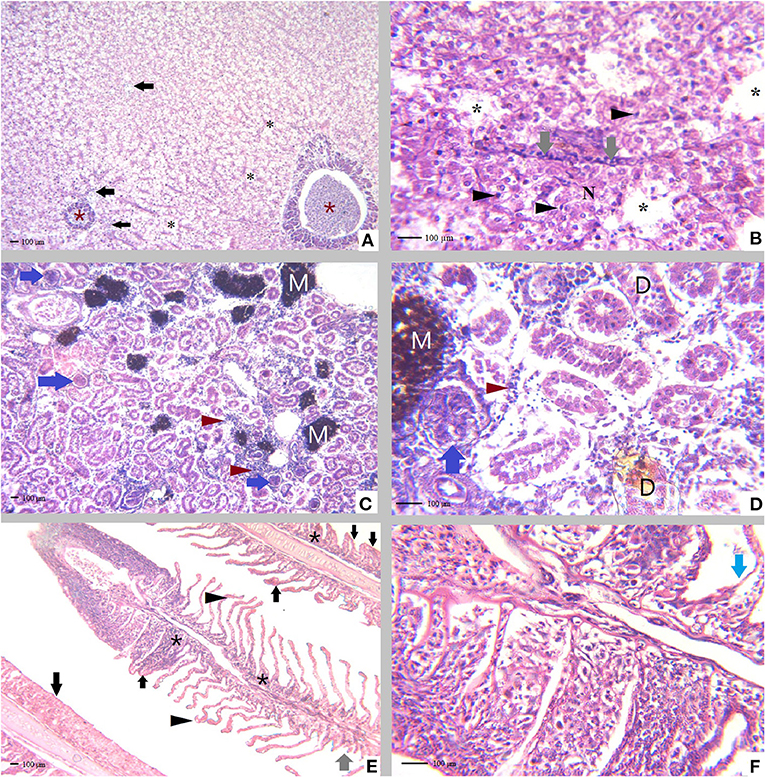
Figure 2. Tissue sections of Dicentrarchus labrax fingerlings experimentally infected with Vibrio fluvialis, (A) Hepatopancreas showing diffused hepatic cell vacuolation (black asterisk), congested engorged pancreatic blood vessels (brown asterisk) with the presence of leucocytic infiltration (black arrow), H&E, X = 100. (B) Hepatopancreas showing necrosis (N), mononuclear cell infiltration (gray arrow) and pyknotic hepatic cell nuclei (black arrowhead), H&E, X = 400. (C) Posterior kidney with diffused mononuclear cell infiltration (brown arrow head), abundant melanomacrophage centers (M) activation glomerular hypertrophy (blue arrow), H&E, X = 100. (D) Posterior kidney with diffused mononuclear cell infiltration (brown arrowhead), melanomacrophage centers activation (M) glomerular hypertrophy with a complete absence of bowman's space (blue arrow), degenerated renal tubules (D), H&E, X = 400. (E) Gill tissues demonstrating curling of the secondary gill lamellae (black arrowhead) hyperplasia of the secondary gill lamellar epithelium with the absence of normal tissue architecture due to severe diffused hyperplasia (black arrow), area of detached secondary gill lamellae (gray arrow) and presence of abundant inflammatory cells (black asterisk), H&E, X = 100. (F) Epithelial lifting (blue arrow), complete lamellar fusion with marked epithelial hyperplasia.
Summer mortality syndrome is considered the most serious challenge for the Egyptian freshwater and mariculture sector (Jansen et al., 2019; Ali et al., 2020; Adeleke et al., 2021). The mortality percent in affected farms ranged between 30 and 100%, with estimated annual financial losses exceeding 1 billion and 25 million USD in freshwater and mariculture farms, respectively.
Climate change represents the most dangerous environmental challenges for fish farming (Olsvik et al., 2013); global warming is recognized as the most significant environmental problem affecting the earth in the last decade. It has been implicated in mass mortalities of several aquatic species, including plants, fish, corals and mammals (Handisyde et al., 2006; Mohanty et al., 2010; Barange et al., 2018).
Egypt is considered a potential hot-spot of climate warming; the frequency of exposure to extreme temperatures became more familiar in Egypt (Mostafa et al., 2019). Saleh et al. (2017) reported that the maximum air temperature was ranged between 37 and 41°C during July and August (2005–2015) at the Nile Delta (the main center of aquaculture in Egypt).
Dissolved oxygen in all studied farms was markedly lower than the comfortable level for fish culture (5 mg L−1); fish suffered from signs of hypoxia and accumulation at the water surface for air gasping. Oxygen solubility is reversely proportional to water temperature, so dissolved oxygen significantly decreased with increased water temperature (Quinn et al., 2011). Water temperature fundamentally impacts fish physiology and biochemistry by activating many enzymatic systems at higher water temperatures (Somero, 2004). Hypoxia directly affects fish immune responses and increases diseases susceptibility (Abdel-Tawwab et al., 2019).
Toxic ammonia was 2–3 folds higher than the acceptable value in all of the studied seabass farms, indicating exposure of fish to chronic ammonia toxicity. Tested farms fed on trash fish directly elevated the toxic ammonia value; moreover, decreased dissolved oxygen enhanced ammonia toxicity. Chronic ammonia toxicity directly affects the survival rate, inducing skin, fin and tail erosions also acts as a direct cause of immune suppression and high mortality (Li et al., 2014).
Thirty-one V. fluvialis, 23 P. aeruginosa and 8 S. aureus isolates were identified from diseased fish. This work is considered the first recorded for the recovered bacterial pathogens from cultured seabass in Egypt. Vibrio was the most abundant bacterial pathogen affecting marine fishes; this result was also mentioned by Muniesa et al. (2019). Ayaz and Karatas (2008) and Kapetanovic et al. (2019) have also isolated V. fluvialis from farmed diseased D. labrax. P. aeruginosa induced a severe septicaemic disease in cultured S. aurata (Khalifa et al., 2016), S. aureus was responsible for a severe infection affecting farmed D. labrax in Turkey (Canak and Timur, 2020).
P. aeruginosa was the most virulent pathogen affecting D. labrax with the lowest LD50. P. aeruginosa possesses a wide range of virulence factors responsible for pathogenicity including, lipopolysaccharide, flagellum and Pili, and these virulence factors have a role in attachment and adhesion to the different cell types. P. aeruginosa also has type III and VI secretion system, exotoxin A and proteases, which have a role in local tissue damage and invasion. Quorum sensing and biofilm formation are considered among the most important virulence factors of P. aeruginosa; they protect against phagocytosis and promote host tissue invasion. Finally, pyocyanin pigment is responsible for tissue damage during disease progression (Hossain, 2014; Wu et al., 2015; Rocha et al., 2019).
S. aureus was highly pathogenic for D. labrax; it has two main mechanisms of virulence, the first is avoiding phagocytic killing, and the other is tissue invasion and destruction. Cheung et al. (2021) reported that S. aureus evades the phagocytic killing mechanism of the host through aggregation, inhibiting the opsonization and biofilm formation. Shettigar and Murali's (2020) recorded that S. aureus has an excellent ability for tissue invasion colonization and destruction by the aid of several exoproteins (enterotoxins). The epidermal cell differentiation factor produced by S. aureus favoring bacterial dissemination and arginine catabolic mobile provides bacteria with the ability to colonies the skin. Extracellular adherence protein and biofilm formation also play a role in tissue attachment and invasion.
V. fluvialis was the most abundant type of isolated bacterial pathogen, but it was the least in virulence, as V. fluvialis has the highest LD50 (4.67 × 107 CFU/fish). Liu et al. (2021) identified two quorum-sensing systems and type VI secretion systems from V. fluvialis; quorum sensing systems are responsible for producing extracellular enzymes, pigments, toxins and expression of virulence genes. The VI secretion systems play a critical role in bacterial virulence by injecting toxic effector proteins into the target cells. Type VI secretion systems activated under warm temperatures and high osmolarity conditions explain why V. fluvialis the most dominant type between the isolated pathogens affecting cultured D. labrax in the studied farms. Confirming this hypothesis, Ramamurthy et al. (2014) reported that a rise in seawater temperature had increased V. fluvialis identification rate considerably by 29%. The potential pathogenicity of V. fluvialis could be attributed to expression of many putative virulence factors, including cytolysin, heat-labile cytotoxin, cytotonic, hemolysin and mucinase; also, V. fluvialis has excellent capacity for cell adherence and inducing cell vacuolation.
The mortality rate of D. labrax fingerlings challenged with V. fluvialis was proportionally related to water temperature. The group reared at 33 and 36°C are showed the highest mortality rate compared to other groups maintained at 27 and 30°C; the former findings can explain this difference. Exposure to thermal stress has resulted in a series of biochemical and physiological changes, including high plasma cortisol and oxidative stress, which subsequently resulted in immune suppression (both cellular and humeral) and increased susceptibility to bacterial pathogens as V. fluvialis.
Thermal stress associated with heatwaves is the leading cause of summer mortality syndrome affecting cultured D. labrax. High plasma cortisol and oxidative stress markers indicated the direct effect of thermal stress on fish physiology and biochemistry as a body compensation mechanism to alleviate thermal stress's deleterious effect and reach homeostasis. High plasma cortisol and oxidative stress markers resulted in decreasing the immune response of fish increasing disease susceptibility. The high temperature also induced high toxic ammonia and decreased dissolved oxygen level makes fish conditions more worth.
Oxidative stress resulted from the imbalance between the production of oxidant and antioxidants; antioxidant enzymes are the first line of defense against the oxidative damage of cells as they are a part of the enzymatic mechanisms involved in the detoxification of the reactive oxidative species (ROS). Superoxide dismutase converts the superoxide radicals to oxygen and hydrogen peroxide; after that catalase converts hydrogen peroxide to oxygen and water (Howcroft et al., 2009). SOD and CAT provide the first line of defense against oxidative damage in animals and fish. The present research results showed that SOD and CAT significantly increased at 33 and 36°C in comparison with fish maintained at 27°C, which indicated that high water temperature exceeding 30°C accompanied with oxidative stress in D. labrax, in harmony with the present results. Nakano et al. (2014) reported increased SOD of salmon subjected to thermal stress.
Malondialdehyde (MDA) significantly increased in the fish group subjected to 33°C and sharply increased at 36°C, elucidating peroxidation of the plasma membrane in repose to thermal stress. The present findings coincided with Nakano et al. (2014) they recorded increased MDA after short term thermal stress in salmon. MDA is a final product of the lipid peroxidation process; it is considered one of the best indicators of cell membrane injury due to oxidative stress (Del Rio et al., 2005; Ayala et al., 2014).
Increased oxidative stress markers are considered a defiance mechanism against oxidative stress associated with thermal stress; through the oxidative stress response, the body tries to maintain internal homeostasis and restore body cells' normal physiological function. The present results showed that water temperature above 30°C induces oxidative stress in D. labrax accompanied by ROS production. This finding is supported by the research conducted by Kamyab et al. (2017) and Do et al. (2019).
Biller and Takahashi (2018) reported that oxidative stress limits the immune response by impairing the defense mechanism associated with ROS production to decrease ROS production. High oxidative stress markers are accompanied by reducing the immune response and so promote disease susceptibility in fish.
The present work has proved the harmful effect of thermal stress (increased water temperature) on both healthy and experimentally infected D. labrax fingerlings. Plasma cortisol is the major stress indicator in fish (Schreck and Tort, 2016). Plasma cortisol level was significantly increased in response to water temperature exceeding 30°C; moreover, the plasma cortisol level was duplicated by more than seven-folds when the water temperature reached 36°C, which confirms the subjection of fish to severe stress. Person-Le Ruyet et al. (2004) recorded that the suitable farming temperature for D. labrax is between 22 and 26°C. High cortisol level negatively affects the tissue inflammatory response through the inhibitory effects on cytokine production, lysozyme and complement activities decreasing the immune response increased susceptibility to different pathogens (Aluru and Vijayan, 2009; Cortes et al., 2013). Cortisol also suppresses the cellular immune response in fish through decreasing phagocytic responses, lymphocyte numbers and antibody production (Nardocci et al., 2014).
Diseased fish were accumulated on the water surface of the affected farm to compensate decreased dissolved oxygen concentration. Signs of septicemia observed on infected fish were associated with expressing the different virulence factors of the invading bacterial pathogens. In harmony with the present results; Austin (2019) reported that signs of septicemia are the most prominent indicator of bacterial fish diseases.
Antibiogram indicated that all the recovered bacterial isolates were highly susceptible to florfenicol. Florfenicol is considered the most effective antibiotic that must use in controlling such infection. FDA approved florfenicol to treat the susceptible bacterial diseases in aquaculture (Zeng et al., 2019). Many studies indicated the efficacy of florfenicol in treating bacterial fish diseases (Gaunt et al., 2010; Aboyadak et al., 2016; Abdelhamed et al., 2019). Florfenicol is one of the most recent antibiotics currently used in veterinary medicine, so bacterial resistance is unfamiliar.
To our knowledge, this is the first work proving the pathogenicity of V. fluvialis for D. labrax. Histopathological examination indicated the presence of many degenerative changes affecting the hepatopancreas, kidney and gills of infected fish as inflammation, congestion and necrosis. These findings confirm the virulence of V. fluvialis for D. labrax. The recorded gross and microscopic pathology could be attributed to the invasion of V. fluvialis to diseased fish tissues during septicemia and the expression of different virulence-associated factors produced by this pathogen.
Multi stress factors are responsible for summer mortality syndrome affecting cultured D. labrax in Egypt; thermal stress associated with heatwaves is the main predisposing factor. Thermal stress initiates a cascade of subsequent stress responses, including increasing plasma cortisol, oxidative stress, decreased dissolved oxygen level, and elevated toxic ammonia levels. Under this multifactor stress, fish were severely suffering, turned immune-compromised and subsequently highly susceptible to infectious diseases. On the other hand, high water temperature enhanced bacterial bacteria growth, multiplication and expression of their virulence factors, so the commensals and environmental bacteria turned pathogenic.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by NIOF Committee for Ethical Care of Marine Organisms and Experimental Animals.
NA: designed the study, performed clinical, postmortem examination, bacterial isolation and identification, pathogenicity and challenge test, and wrote the manuscript. IA: analysis of water parameters, antimicrobial susceptibility, assayed cortisol and oxidative stress biomarkers, perform statistical analysis, and helped in manuscript writing and preparations. AE-N: fish sampling, rearing, and observation. MG: performed the histopathological examination and written histopathological results. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelhamed, H., Ozdemir, O., Waldbieser, G., Perkins, A. D., Lawrence, M. L., and Karsi, A. (2019). Effects of florfenicol feeding on diversity and composition of the intestinal microbiota of channel catfish (Ictalurus punctatus). Aquacult. Res. 50, 3663–3672. doi: 10.1111/are.14325
Abdel-Tawwab, M., Monier, M. N., Hoseinifar, S. H., and Faggio, C. (2019). Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 45, 997–1013. doi: 10.1007/s10695-019-00614-9
Aboyadak, I. M., and Ali, N. G. (2021). Validation of a New Method for Determining LD50 of Microbial Pathogens in Aquatic and Other Animals.
Aboyadak, I. M., Ali, N. G., Abdel-Aziz, M. M., Gado, M. S., and El-Shazly, K. A. (2016). Role of some antibacterial drugs in control Streptococcus iniae infection in Oreochromis niloticus. J. Pharmacol. Clin. Res. 1:555573. doi: 10.19080/JPCR.2016.01.555573
Adeleke, B., Robertson-Andersson, D., Moodley, G., and Taylor, S. (2021). Aquaculture in Africa: a comparative review of Egypt, Nigeria, and Uganda vis-a-vis South Africa. Rev. Fish. Sci. Aquacult. 29, 167–197. doi: 10.1080/23308249.2020.1795615
Alfonso, S., Gesto, M., and Sadoul, B. (2020). Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2020, 1–13. doi: 10.1111/jfb.14599
Ali, N. G., and Aboyadak, I. M. (2021). Summer Mortality Syndrome Affecting Egyptian Mariculture Sector and its Control Strategies, First Edn. Cairo: Egyptian National Library and Archives, 1–14.
Ali, N. G., Aboyadak, I. M., and Gouda, M. Y. (2018). Rapid detection and control of Gram-negative bacterial pathogens isolated from summer mortality outbreak affecting tilapia farms. J. Biol. Sci. 19, 24–33. doi: 10.3923/jbs.2019.24.33
Ali, N. G. M., Aboyadak, I. M., and El-Sayed, H. S. (2019). Chemotherapeutic control of Gram-positive infection in white sea bream (Diplodus sargus, Linnaeus 1758) broodstock. Vet. World 12, 316–324. doi: 10.14202/vetworld.2019.316-324
Ali, S. E., Jansen, M. D., Mohan, C. V., Delamare-Deboutteville, J., and Charo-Karisa, H. (2020). Key risk factors, farming practices and economic losses associated with tilapia mortality in Egypt. Aquaculture 527:735438. doi: 10.1016/j.aquaculture.2020.735438
Alleman, J. E. (1998). Online Free Ammonia-Nitrogen Calculator. Available online at: http://home.eng.iastate.edu/~jea/w3-research/free-ammonia/nh3.html (accessed August 12, 2020).
Aluru, N., and Vijayan, M. M. (2009). Stress transcriptomics in fish: a role for genomic cortisol signaling. Gen. Comp. Endocrinol. 164, 142–150. doi: 10.1016/j.ygcen.2009.03.020
APHA (2018). Standard Methods for Examination of Water and Wastewater, 23nd Edn. Washington, DC: American Public Health Association.
Austin, B. (2019). Methods for the diagnosis of bacterial fish diseases. Mar. Life Sci. Technol. 1, 41–49. doi: 10.1007/s42995-019-00002-5
Austin, B., and Austin, D. A. (2016). Bacterial Fish Pathogens: Disease of Farmed and Wild Fish, 6th Edn. Switzerland: Springer International Publishing.
Ayala, A., Munoz, M. F., and Arguelles, S. (2014). Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Long. 2014:360438. doi: 10.1155/2014/360438
Ayaz, A., and Karatas, S. (2008). Dominant aerobic bacterial community of sea bass (Dicentrarchus labrax L. 1758) larvae during weaning from Artemia to dry feed in culture conditions. Turk. J. Vet. Anim. Sci. 34, 501–6. doi: 10.3906/vet-0805-24
Barange, M., Bahri, T., Beveridge, M. C. M., Cochrane, K. L., Funge-Smith, S., and Poulain, F. (2018). Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options. FAO Fisheries and Aquaculture Technical Paper No. 627. Rome: FAO.
Barnabe, G. (1990). “Rearing bass and gilthead bream,” in Aquaculture, Vol. 2, ed G. Barnabè (Sussex: Ellis Horwood), 647–686.
Biller, J. D., and Takahashi, L. S. (2018). Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 90, 3403–3414. doi: 10.1590/0001-3765201820170730
Black, J. G., and Black, L. J. (2015). Microbiology: Principles and Explorations, ninth Edition. Hoboken: Wiley.
Bosworth, B. G., and Small, B. C. (2004). Effects of transport water temperature, aerator type, and oxygen level on Channel Catfish Ictalurus punctatus fillet quality. J. World Aquacult. Soc. 35, 412–419. doi: 10.1111/j.1749-7345.2004.tb00105.x
Canak, O., and Timur, G. (2020). An initial survey on the occurrence of staphylococcal infections in Turkish marine aquaculture (2013–2014). J. Appl. Ichthyol. 36, 932–941. doi: 10.1111/jai.14141
CCAC (2005). Guidelines on: the care and use of fish in research, teaching and testing. Ottawa: Canadian Council on Animal Care.
Cheng, C.-H., Yang, F.-F., Ling, R.-Z., Liao, S.-A., Miao, Y.-T., Ye, C.-X., et al. (2015). Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 164, 61–71. doi: 10.1016/j.aquatox.2015.04.004
Cheung, G. Y. C., Bae, J. S., and Otto, M. (2021). Pathogenicity and virulence of Staphylococcus aureus. Virulence 12, 547–569. doi: 10.1080/21505594.2021.1878688
Clinical and Laboratory Standards Institute (CLSI) (2016). Document M45-A. Methods for antimicrobial dilution and disk susceptibility of infrequently isolated or fastidious bacteria; approved guideline. Pennsylvania: CLSI.
Cortes, R., Teles, M., Tridico, R., Acerete, L., and Tort, L. (2013). Effects of cortisol administered through solw-release implants on innate immune responses in Rainbow Trout (Oncorhynchus mykiss). Int. J. Genomics 2013:619714. doi: 10.1155/2013/619714
Currie, S., and Schulte, P. (2014). “The physiology of fishes,” in CRC Marine Biology Series, 4th Edn, chapter 8, eds D. H. Evans, J. B. Claiborne, and S. Currie (Routledge: Taylor and Francis Group).
Del Rio, D., Stewart, A. J., and Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15, 316–328. doi: 10.1016/j.numecd.2005.05.003
Do, T. D., Mai, T. N., Khoa, T. N. D., Abol-Munafi, A. B., Liew, H. J., Kim, C. B., et al. (2019). Molecular characterization and gene expression of glutathione peroxidase 1 in Tor tambroides exposed to temperature stress. Evol. Bioinform. 15, 1–8. doi: 10.1177/1176934319853580
Eissa, A. E., Abou-Okada, M., Alkurdi, A. R. M., El Zlitne, R. A., Prince, A., Abdelsalam, M., et al. (2021). Catastrophic mass mortalities caused by Photobacterium damselae affecting farmed marine fish from Deeba Triangle, Egypt. Aquac. Res. 52, 4455–4466. doi: 10.1111/are.15284
El-Bahar, H. M., Ali, N. G., Aboyadak, I. M., Khalil, S. A., and Ibrahim, M. S. (2019). Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus. Int. Microbiol. 22, 479–490. doi: 10.1007/s10123-019-00075-3
Fathi, M., Dickson, C., Dickson, M., Leschen, W., Baily, J., Muir, F., et al. (2017). Identification of Tilapia Lake Virus in Egypt in Nile tilapia affected by ‘summer mortality’ syndrome. Aquaculture 473, 430–432. doi: 10.1016/j.aquaculture.2017.03.014
Fatira, E., Papandroulakis, N., and Pavlidis, M. (2014). Diel changes in plasma cortisol and effects of size and stress duration on the cortisol response in European sea bass (Dicentrarchus labrax). Fish Physiol. Biochem. 40, 911–919. doi: 10.1007/s10695-013-9896-1
GAFRD (2020). Fish Statistics Yearbook 2018. Egypt: General Authority for Fish Resources, Ministry of Agriculture Publications
Gaunt, P. S., Gao, D., Sun, F., and Endris, R. (2010). Efficacy of florfenicol for control of mortality caused by Flavobacterium columnare infection in channel catfish. J. Aquat. Anim. Health 22, 115–122. doi: 10.1577/H09-057.1
Ghneim, H. K., Al-Sheikh, Y. A., Alshebly, M. M., and Aboul-Soud, M. A. (2016). Superoxide dismutase activity and gene expression levels in Saudi women with recurrent miscarriage. Mol. Med. Rep. 13, 2606–2612. doi: 10.3892/mmr.2016.4807
Hadwan, M. H. (2018). Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 19:7. doi: 10.1186/s12858-018-0097-5
Handisyde, N. T., Ross, L. G., Badjeck, M.-C., and Allison, E. H. (2006). The Effects of Climate Change on World Aquaculture: A Global Perspective. Final Technical Report produced by the Institute of Aquaculture, Stirling, U.K. and sponsored by the Department for International Development, DFID.
Hossain, Z. (2014). “Bacteria: Pseudomonas,” in Encyclopedia of Food Safety, eds Y. Motarjemi (New York, NY: Academic Press), 490–500.
Howcroft, C. F., Amorim, M. J. B., Gravato, C., Guilhermino, L., and Soares, A. M. V. M. (2009). Effects of natural and chemical stressors on Enchytraeus albidus: Can oxidative stress parameters be used as fast screening tools for the assessment of different stress impacts in soils? Environ. Int. 35, 318–324. doi: 10.1016/j.envint.2008.08.004
Jansen, M. D., Dong, H. T., and Mohan, C. V. (2019). Tilapia lake virus: a threat to the global tilapia industry? Rev. Aquacult. 11, 725–739. doi: 10.1111/raq.12254
Kaleem, O., and Sabi, A. B. S. (2021). Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquacult. Fish. 6, 535–547. doi: 10.1016/j.aaf.2020.07.017
Kamyab, E., Kuhnhold, H., Novais, S. C., Alves, L. M., Indriana, L., Kunzmann, A., et al. (2017). Effects of thermal stress on the immune and oxidative stress responses of juvenile sea cucumber Holothuria scabra. J. Comp. Physiol. B 187, 51–61. doi: 10.1007/s00360-016-1015-z
Kapetanovic, D., Gavrilovic, A., Jug-Dujakovic, J., Vardic Smrzlic, I., Kazazic, S., Bojanic-Rasovic, M., et al. (2019). Assessment of microbial sea water quality and health status of farmed European seabass (Dicentrarchus labrax) in Eastern Adriatic Sea (Montenegro and Croatia). Stud. Mar. 32:3584222. doi: 10.5281/zenodo.3584222
Khalifa, E., Khallaf, M., and Hashem, M. (2016). Molecular study on some virulence and fluoroquinolone resistance genes of Pseudomonas aeruginosa isolated from naturally infected cultured sea bream fish (Sparus aurata) in Egypt. J. Infect. Dis. Prevent. Med. 4:2. doi: 10.4172/2329-8731.1000136
Lanari, D., D'Agaro, E., and Ballestrazzi, R. (2002). Growth parameters in European sea bass (Dicentrarchus labrax L.): effects of live weight and water temperature. Ital. J. Anim. Sci. 1: 181–185.
Leon, J. A. D., and Borges, C. R. (2020). Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 159:e61122. doi: 10.3791/61122.PMID:32478759
Levitus, S., Antonov, I., Boyer, T. P., Baranova, O. K., Garcia, H. E., Locarnini, R. A., et al. (2012). World Ocean heat content and thermosteric sea level change (0–2000 m), 1955–2010. Geophys. Res. Lett. 39, L10603. doi: 10.1029/2012GL051106
Li, M., Yu, N., Qin, J. G., Li, E., Du, Z., and Chen, L. (2014). Effects of ammonia stress, dietary linseed oil and Edwardsiella ictaluri challenge on juvenile darkbarbel catfish Pelteobagrus vachelli. Fish Shellfish Immunol. 38, 158–165. doi: 10.1016/j.fsi.2014.03.015
Liu, X., Pan, J., Gao, H., Han, Y., Zhang, A., Huang, Y., et al. (2021). CqsA/LuxS-HapR Quorum sensing circuit modulates type VI secretion system VflT6SS2 in Vibrio fluvialis. Emerg. Microb. Infect. 10, 589–601. doi: 10.1080/22221751.2021.1902244
Martinez-Porchas, M., Martinez-Cordova, L. R., and Ramos-Enriquez, R. (2009). Cortisol and glucose: reliable indicators of fish stress? Panam. J. Aquat. Sci. 4, 158–178. Available online at: https://panamjas.org/pdf_artigos/PANAMJAS_4(2)_158-178.pdf
McNally, W. H., and Mehta, A. J. (2004). “Sediment transport and deposition in estuaries,” in Encyclopedia of life support systems (EOLSS): Coastal Zones and Estuaries (Paris: UNSECO). Available online at: http://www.eolss.net/sample-chapters/c09/E2-06-01-04.pdf (accessed March 17, 2021).
Mohanty, B. P., Mohanty, S., Sahoo, J. K., and Sharma, A. P. (2010). Climate change: impacts on fisheries and aquaculture, Chapter 7, climate change and variability. Zagreb; Rijeka: InTech. doi: 10.5772/9805
Mostafa, A. N., Wheida, A., El Nazer, M., Adel, M., El Leithy, L., Siour, G., et al. (2019). Past (1950–2017) and future (−2100) temperature and precipitation trends in Egypt. Weather Clim Extrem 26:100225. doi: 10.1016/j.wace.2019.100225
Muniesa, A., Basurco, B., Aguilera, C., Furones, D., Reverte, C., Sanjuan-Vilaplana, A., et al. (2019). Mapping the knowledge of the main diseases affecting sea bass and sea bream in Mediterranean. Transbound. Emerg. Dis. 67:1089–1100. doi: 10.1111/tbed.13482
NACLAR (2004). National Advisory Committee for Laboratory Animals Research. Available online at: http://research.ntu.edu.sg/guides/Documents/Ethics/NACLAR-guide%20Lines.pdf (accessed July 30, 2020).
Nakano, T., Kameda, M., Shoji, Y., Hayashi, S., Yamaguchi, T., and Sato, M. (2014). Effect of severe environmental thermal stress on redox state in salmon. Redox Biol. 2, 772–776. doi: 10.1016/j.redox.2014.05.007
Nardocci, G., Navarro, C., Cortes, P. P., Imarai, M., Montoya, M., Valenzuela, B., et al. (2014). Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol. 40, 531–538. doi: 10.1016/j.fsi.2014.08.001
Nicholson, P., Fathi, M. A., Fischer, A., Mohan, C., Schieck, E., Mishra, N., et al. (2017). Detection of Tilapia Lake Virus in Egyptian fish farms experiencing high mortalities in 2015. J. Fish Dis. 40, 1925–1928. doi: 10.1111/jfd.12650
Olsvik, P. A., Vikesa, V., Lie, K. K., and Hevroy, E. M. (2013). Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology. BMC Genomics 14:817. doi: 10.1186/1471-2164-14-817
Person-Le Ruyet, J., Mahe, K., Le Bayon, N., and Le Delliou, H. (2004). Effects of temperature on growth and metabolism in a Mediterranean population of European sea bass, Dicentrarchus labrax. Aquaculture 237, 269–280. doi: 10.1016/j.aquaculture.2004.04.021
Quinn, N. L., McGowan, C. R., Cooper, G. A., Koop, B. F., and Davidson, W. S. (2011). Identification of genes associated with heat tolerance in Arctic charr exposed to acute thermal stress. Physiol. Genomics 43, 685–696. doi: 10.1152/physiolgenomics.00008.2011
Ramamurthy, T., Chowdhury, G., Pazhani, G. P., and Shinoda, S. (2014). Vibrio fluvialis: an emerging human pathogen. Front. Microbiol. 5:91. doi: 10.3389/fmicb.2014.00091
Requena, A., Fernnndez-Borras, L., and Planas, J. (1997). The effects of a temperature rise on oxygen consumption and energy budget in gilthead sea beam. Aquacult. Int. 5, 415–426.
Rocha, A. J., Barsottini, M. R., Rocha, R. R., Laurindo, M. V., de Moraes, F. L., and da Rocha, S. L. (2019). Pseudomonas Aeruginosa: virulence factors and antibiotic resistance genes. Braz. Arch. Biol. Technol. 62:e19180503. doi: 10.1590/1678-4324-2019180503
Saleh, N. E., Helal, M., Ali, N. G., Abbas, E., and Abdel-Tawwab, M. (2021). Effects of using vital wheat gluten in practical diets on growth, intestinal histopathology, proinflammation related gene expression, and resistance of white seabream (Diplodus sargus) to Staphylococcus epidermidis infection. Aquaculture 537:736508. doi: 10.1016/j.aquaculture.2021.736508
Saleh, S. M., Heggi, M. A. M., Abdrabbo, M. A. A., and Farag, A. A. (2017). Heat waves investigation during last decades in some climate regions in Egypt. Egypt. J. Agric. Res. 95, 863–889.
Schreck, C. B., and Tort, L. (2016). “The concept of stress in fish,” in Biology of Stress in Fish, eds C. B. Schreck, L. Tort, A. P. Farrell, and C. J. Brauner (London: Academic Press, Elsevier).
Shaalan, M., El-Mahdy, M., Saleh, M., and El-Matbouli, M. (2018). Aquaculture in Egypt: insights on the current trends and future perspectives for sustainable development. Rev. Fish. Sci. Aquacult. 26, 99–110. doi: 10.1080/23308249.2017.1358696
Shettigar, K., and Murali, T. S. (2020). Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection. Eur. J. Clin. Microbiol. Infect. Dis. 39:2235–2246. doi: 10.1007/s10096-020-03984-8
Simon, D. (2009). Analysis of Variance Test for Normality Online Version. Available online at: http://sdittami.altervista.org/shapirotest/ShapiroTest.html (accessed January 15, 2020).
Snedecor, G. W., and Cochran, W. G. (1989). Statistical Methods, 8th Edn. Ames: Iowa State University Press.
Soliman, N. F., and Yacout, D. M. M. (2016). Aquaculture in Egypt: status, constraints and potentials. Aquacult. Int. 24, 1201–1227. doi: 10.1007/s10499-016-9989-9
Somero, G. N. (2004). Adaptation of enzymes to temperature: searching for basic strategies. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 139, 321–333. doi: 10.1016/j.cbpc.2004.05.003
Suvarna, S. K., Layton, C., and Bancroft, J. D. (2018). Bancroft's theory and practice of histological techniques, 8th Edition. Amsterdam: Elsevier.
Uzun, E., and Ogut, H. (2015). The isolation frequency of bacterial pathogens from sea bass (Dicentrarchus labrax) in the Southeastern Black Sea. Aquaculture 437, 30–37. doi: 10.1016/j.aquaculture.2014.11.017
Vandeputte, M., Gagnaire, P.-A., and Allal, F. (2019). The European Sea bass: a key marine fish model in the wild and in aquaculture. Anim. Genet. 50, 195–206. doi: 10.1111/age.12779
Wang, W., Dong, H., Sun, Y., Cao, M., Duan, Y., Li, H., et al. (2019). The efficacy of eugenol and tricaine methanesulphonate as anaesthetics for juvenile Chinese sea bass (Lateolabrax maculatus) during simulated transport. J. Appl. Ichthyol. 35, 551–557. doi: 10.1111/jai.13844
Wu, W., Jin, Y., Bai, F., and Jin, S. (2015). “Chapter 41—Pseudomonas aeruginosa,” in Molecular Medical Microbiology (Second Edition), eds Y. Tang, M. Sussman, D. Liu, I. Poxton and J. Schwartzman. New York, NY: Academic Press, Pages 753–767
Keywords: Dicentrarchus labrax, cortisol, antioxidant, pathogenicity, histopathology, Vibrio fluvialis, Pseudomonas aeruginosa, Staphylococcus aureus
Citation: Ali NG, El-Nokrashy AM, Gouda MY and Aboyadak IM (2021) Summer Mortality Syndrome Affecting Cultured European Seabass at Kafrelsheikh Province, Egypt. Front. Mar. Sci. 8:717360. doi: 10.3389/fmars.2021.717360
Received: 30 May 2021; Accepted: 31 October 2021;
Published: 06 December 2021.
Edited by:
Vengatesen Thiyagarajan, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Marina Machado, University of Porto, PortugalCopyright © 2021 Ali, El-Nokrashy, Gouda and Aboyadak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadia Gabr Ali, bmFkaWFnYWJyYWxpQGdtYWlsLmNvbQ==; orcid.org/0000-0003-0970-0014
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.