- 1Department of Ocean Integrated Science, Chonnam National University, Yeosu, South Korea
- 2Department of Environmental Oceanography, Chonnam National University, Yeosu, South Korea
- 3Ballast Water Research Center, Korea Institute of Ocean Science and Technology, Geoje, South Korea
The calanoid copepod Acartia ohtsukai predominates the estuarine and coastal waters of East Asia during summer. Its occurrence characteristics confer it with good potential as live prey for fish larvae through mass culture. To investigate the effect of temperature and salinity combinations on its egg production rate (EPR), hatching success (HS), and mortality rate, experiments were undertaken and repeated three times for combinations of five temperatures (10, 15, 20, 25, and 30°C) and seven salinities (10, 15, 20, 25, 27, 30, and 33 psu). EPR and HS were highest at temperatures of 25 and 30°C, respectively, with a salinity of 27 psu. Mortality rate was highest at 10°C in almost all salinity gradients, whereas it was lower at water temperature and salinity ranges of 20–30°C and 20–30 psu, respectively. These findings indicate that A. ohtsukai can inhabit wide ranges of water temperatures and salinities, and that the optimized condition for mass culture is a combination of water temperature of 25°C and salinity of 27 psu.
Introduction
In the aquaculture industry, Artemia spp. and rotifers have widely been used as prey for fish larvae (Hoff and Snell, 1999; Chesney, 2005), although fish larvae prefer copepod nauplii to comprise more than 50% of the stomach contents (Støttrup, 2000). However, a lack of variation in sizes and nutritional contents has led to the search for alternative prey sources. Copepods, which connect primary producers such as phytoplankton and higher consumers in the marine food web, have been considered as excellent live feeds owing to the size variation in their developmental stages as well as nutritional sufficiency in terms of fatty acids, free amino acids, and other essential micronutrients (Sargent and Falk-Petersen, 1988; McEvoy et al., 1998; Støttrup, 2000, 2003; van der Meeren et al., 2008). In particular, since copepods mainly contain essential n–3 highly unsaturated fatty acids (HUFA), docosahexaenoic acid (22: 6n–3; DHA), eicosapentaenoic acid (20: 5n–3; EPA), and phospholipid n–6 HUFA arachidonic acid (20: 4n–6; ARA), it is critical for marine fish larvae to feed on them to ensure adequate growth and development (Gapasin and Duray, 2001; Bell et al., 2003). Additionally, marine fish larvae fed with copepods instead of Artemia spp. and rotifers have advantages such as higher survival, better pigmentation, and more robust growth (Naess et al., 1995; Støttrup and Norsker, 1997; Wilcox et al., 2006).
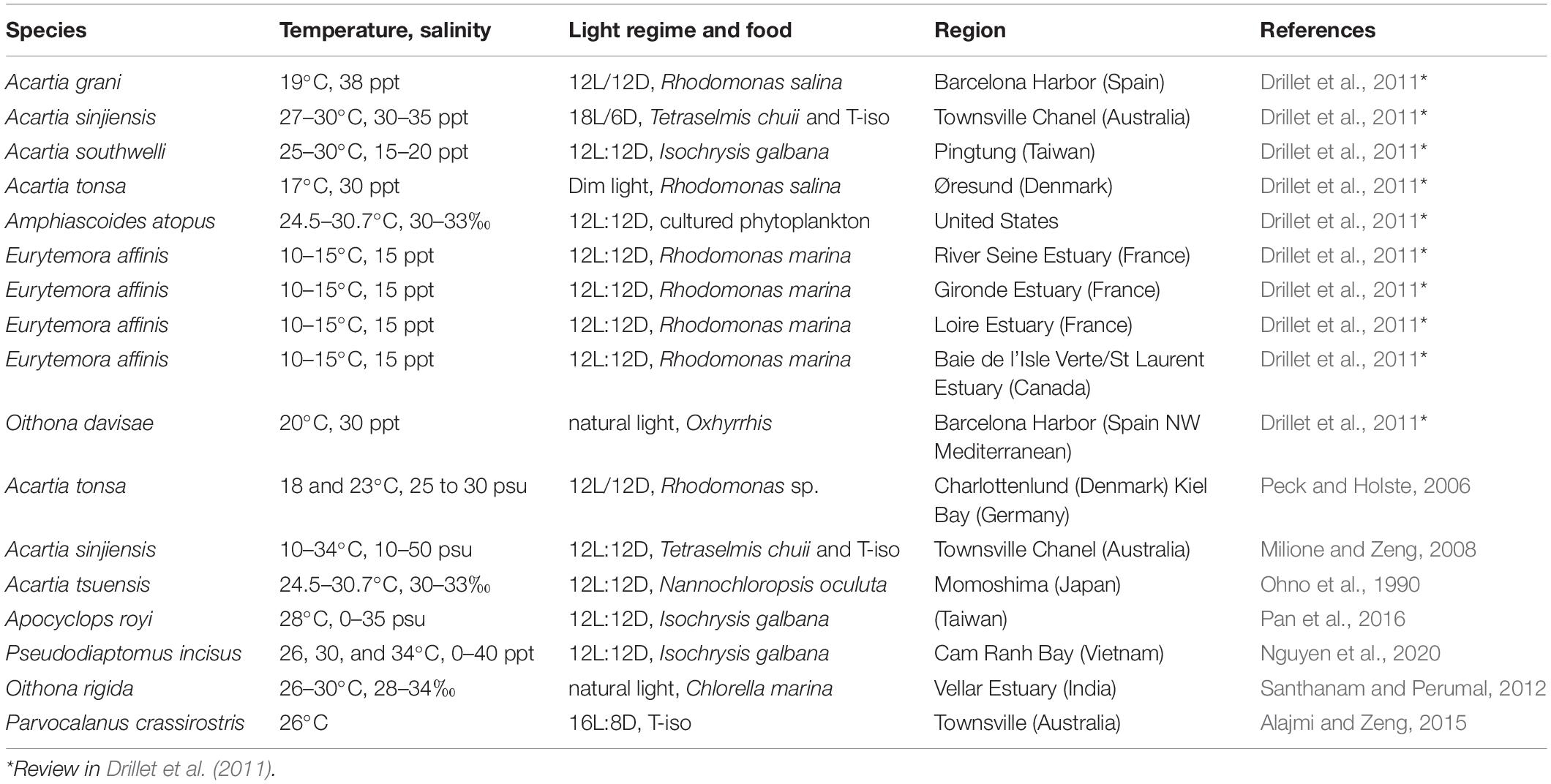
Several studies on the mass cultivation of copepods have been undertaken to investigate their commercial use as live prey for fish larvae (Table 1), but only a few species have successfully been reared for mass cultivation for aquaculture because there is very little information available on the physiological processes and population dynamics of the best candidates (van der Meeren and Naas, 1997; Støttrup, 2000, 2003). Of the calanoid copepods, Acartia tsuensis, Acartia tonsa, and Eurytemora affinis have been considered for use in mass cultivation (Ban, 1994; Takahashi and Ohno, 1996; Peck and Holste, 2006). The egg production rate (EPR), hatching rate, survival/mortality, and growth of nauplii and copepodites of these species are affected by various environmental factors such as water temperature, salinity, pH, and quantity and quality of food (Chinnery and Williams, 2004; Holste and Peck, 2005; Peck and Holste, 2006).
In particular, temperature and salinity are the two most important environmental variables that affect the growth and egg production of marine copepods (Miller and Marcus, 1994; Peck and Holste, 2006). Rhyne et al. (2009) found that temperature had a significant effect on nauplii production and survival in Pseudodiaptomus pelagicus. Ban (1994) showed that temperature altered egg production and adult size in E. affinis. Previous studies on Acartia species indicated that salinity was not completely responsible for the hatching success (HS) of eggs (Chinnery and Williams, 2004; Dutz and Christensen, 2018; Wilson et al., 2021). Dutz and Christensen (2018) showed that there were no significant differences in the HS of eggs in the brackish water species Acartia longiremis at different salinities (4, 5, 6, 7.7, and 16 psu). The netric species Acartia fancetti, which inhabits hypersaline areas, showed no significant differences in the HS of eggs at different salinities (30, 40, and 50 psu), except at 60 psu. However, Holste and Peck (2005) showed that the HS of eggs produced by the copepod A. tonsa significantly decreased under a salinity of less than 15 psu. Variations in salinity were found to lead to different rates of population growth and egg hatching in the tropical copepod Acartia sinjiensis (Milione and Zeng, 2008). Notably, Chinnery and Williams (2004) reported that salinity treatment altered egg hatching rates and nauplii survival in four Acartia species. Several studies have also indicated an interactive effect of salinity and temperature (Bradley, 1986; Nagaraj, 1988). Such discrepancies in results indicate high levels of phenotypic plasticity among copepod species in terms of the salinity effect (Wilson et al., 2021). Thus, assessing the effects of temperature and salinity on calanoid copepods can provide information for their utilization as live prey in aquaculture.
Acartia ohtsukai, which occurs in the estuarine and coastal waters of Korea, China, Vietnam, and Japan (Razouls et al., 2005-2020), is also an excellent candidate live prey species for aquaculture, because it is found in high densities owing to its adaptability to rapid changes in temperature and salinity (Choi et al., 2019; Lee et al., 2020). A. ohtsukai dominates in summer and autumn in Yeoja Bay, comprising more than 60% of the zooplankton community in the inner bays in September (Lee, 2019). The aim of the present study was to understand the combined effect of temperature and salinity on egg production, HS, and mortality in A. ohtsukai, with the ultimate aim of assessing its use in mass cultivation.
Materials and Methods
Sampling and Rearing for the Experiments
The present experiments used A. ohtsukai from a single population. A. ohtsukai individuals were collected using a conical net (mesh size 200 μm, mouth opening size 45 cm) from Sangjin Port (34°48′43′′ N, 127°24′22′′ E) in the northern part of Yeoja Bay, South Korea on August 9, 2019. At the sampling site, the water temperature was 29°C and the salinity was 26 psu. The stock culture was stored in 20 L carboys filled with 1 μm filtered seawater at a salinity of 25 ± 1 psu and a temperature of 30 ± 1°C, and gentle aeration was provided through a 1 ml serological pipette at the bottom of the carboy. Water quality was maintained by replacing 100% of the culture water once every 5 days. The photoperiod was maintained at 12L–12D (12 h light:12 h dark) and a light intensity of 120 lux. Temperature and salinity were measured daily during the experiments (WM-32EP, DDK-TOA CO., Japan). For culture maintenance and experiments, daily rations of marine microalgae Isochrysis galbana (4.5 μm diameter) and Tetraselmis suecica (8 μm diameter) were provided, and a mixed diet of 40,000 cells/ml and 3,000 cells/ml (Milione and Zeng, 2007), respectively, was fed. Microalgae I. galbana (Haptophyceae) and T. suecica have been used in previous diet experiments of the temperate copepod A. tonsa (Støttrup et al., 1986; Feinberg and Dam, 1998). Microalgae cultures were grown in a 2 L roller bottle under a fluorescent light regime of 12L–12D with an intensity of 2500 lux. Microalgae I. galbana and T. suecica were grown in f/2 medium (Guillard and Ryther, 1962). The culture conditions were kept stable until the end of the experiments.
Egg Production Rate
Experiments were conducted to investigate the EPR of A. ohtsukai in relation to a wide range of culture temperatures and salinities. The daily EPR of A. ohtsukai was quantified at seven salinities (10, 15, 20, 25, 27, 30, and 33 ± 1 psu) and five temperatures (10, 15, 20, 25, and 30 ± 1°C). In previous studies, the critical points of water temperature and salinity of A. ohtsukai have been reported to be 10–30°C and 4–30 psu, respectively (Youn and Choi, 2008; Park et al., 2015). Since eggs incubated for 48 h at low salinity could burst and be erroneously identified as hatching eggs, the low salinity treatment was not used (Holste et al., 2004). Copepods used in the experiments were gradually adapted to various water temperatures and salinities via increments of 5°C and 2 psu every 6 h until the required water temperature and salinity level were reached. Three replicates were set up for each treatment for egg production experiments. Twenty-one replicate sets per temperature were used, with a total of 105 sets of A. ohtsukai adults and 200 ml beakers with 2 females and 2 males, respectively. A U-shaped 100 μm mesh was attached to the bottom of each of the 200 ml beakers to prevent egg cannibalism and facilitate egg collection. After approximately 24 h of incubation, all the contents of the replicate bottles were drained into a 40 μm mesh and the numbers of eggs that remained on the mesh were determined and recorded. All samples were counted and recorded under a stereo microscope at ×20–40 magnification (Nikon SMZ 745; Nikon, Japan).
Hatching Success
To investigate the effect of temperature and salinity on HS, the eggs spawned at the bottom of the beaker during the EPR experiments were collected by siphoning. After these eggs were rinsed with filtered seawater and randomly distributed with 20 eggs per well in six-well cell culture plates, HS was observed at 12 h intervals for 48 h. Each of the six-well cell culture plates used parafilm to minimize contaminants and evaporation. The HS (%) was calculated by dividing the number of eggs hatched after 48 h by the number of original eggs. The water temperature and salinity gradients for measuring HS were the same as those used for EPR.
Mortality Rate
To investigate the effect of temperature and salinity on mortality rate, healthy adult A. ohtsukai females (n = 10) were placed in 200 ml beakers with seven salinities (10, 15, 20, 25, 27, 30, and 33 ± 1 psu), and the beakers were incubated in a Multi-Room Incubator (WIM-RL4, DAIHAN Scientific Co., Wonju, South Korea) set to five temperatures (10, 15, 20, 25, and 30 ± 1°C). Females were observed three to five times (5–8 h intervals) a day under a stereo microscope (Nikon SMZ 745; Nikon, Japan) for 10 days or until death. A. ohtsukai individuals were transferred from a 200 ml beaker with water prepared once every 3 days to a beaker with the same conditions to minimize manipulation stress. Other experimental conditions were maintained according to the stock culture protocol. The mortality rate of the A. ohtsukai females was calculated as follows:
Statistical Analysis
Data from all experiments were analyzed by two-way analysis of variance (ANOVA) without replication. When a significant difference (p < 0.05) was found, it was tested using the Tukey’s multiple comparison test. All statistical analysis was performed using the SPSS program version 20.0 (SPSS Inc., Chicago, IL, United States). Standard errors for EPR, HS, and mortality are presented to show the variation within each treatment.
Results
Egg Production Rate
Egg production rates of A. ohtsukai were significantly different at various water temperature and salinity combinations (Table 2). The EPR of A. ohtsukai ranged from 1.90 ± 0.40 to 11.10 ± 1.20 eggs f–1 d–1 (eggs per female per day) depending on the water temperature and was highest at 25°C and lowest at 10°C (Figure 1). An exceptionally high EPR of more than 11 eggs f–1 d–1 was observed at 20–30°C. However, the EPR at 10 and 15°C was lower than 5 eggs f–1 d–1. In addition, egg productivity differed for different water temperature and salinity combinations (Figures 2A–E). For the 10 and 15°C treatments, eggs were not laid at specific salinities of 10, 15, and 33, and at 10 and 33 psu, respectively (Figures 2A,B). For the 20–30°C range, eggs were produced in all salinity ranges (Figures 2C–E). In particular, at 25 and 30°C, a high EPR was observed at 25–30 and 15–27 psu, respectively. There was also a clear decline in EPR as salinity increased above 27 psu at water temperatures of 10 and 15°C.
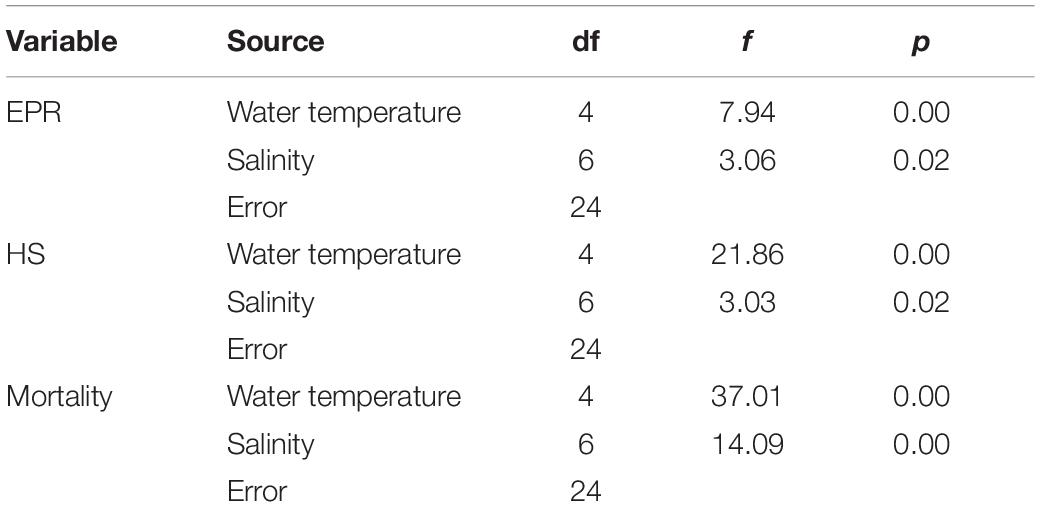
Table 2. Two-way ANOVA without replication to test the effect of egg production rate (EPR), hatching success (HS), and mortality on water temperature and salinity.
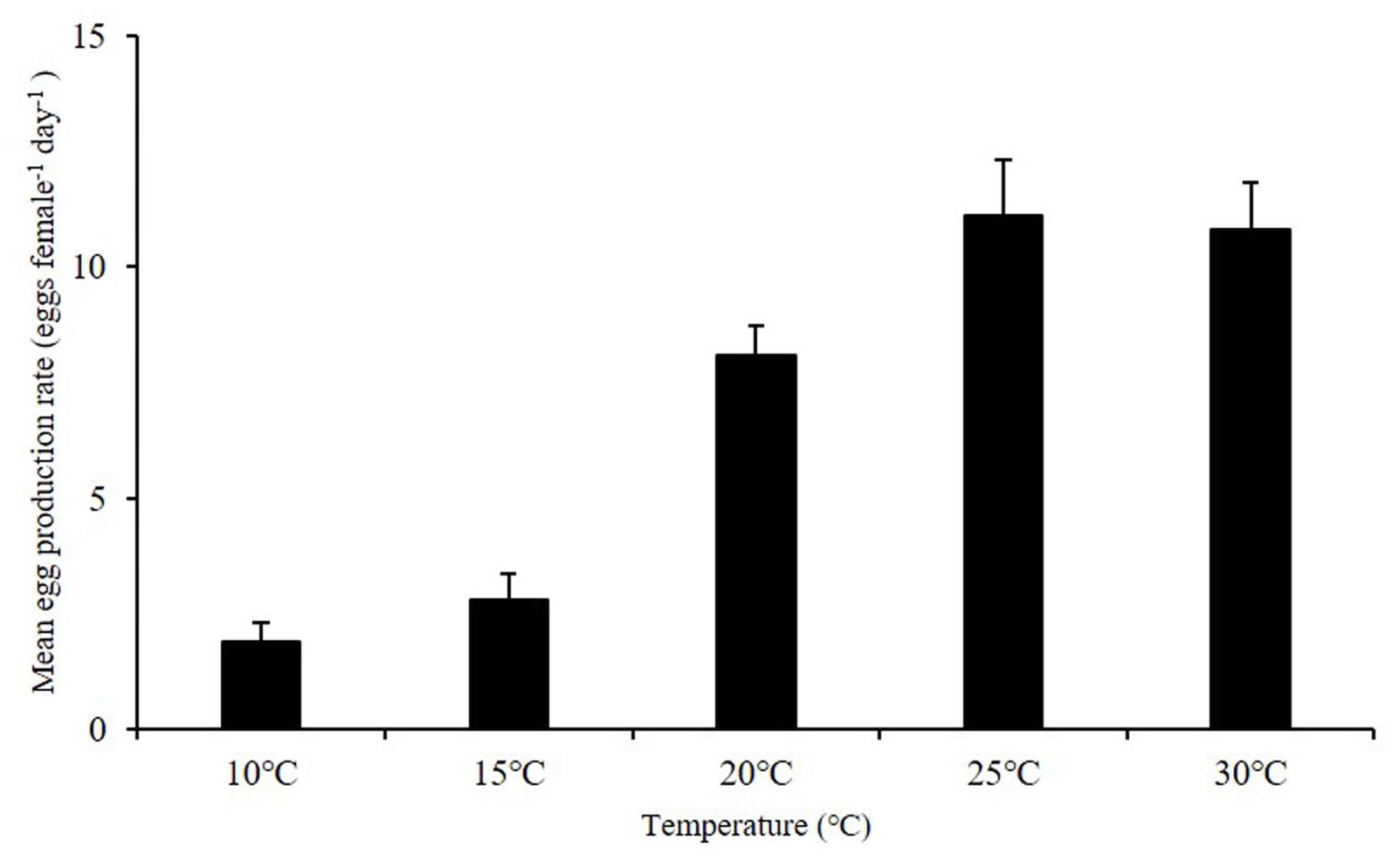
Figure 1. Egg production rate (EPR) of adult female Acartia ohtsukai at each temperature. For each treatment, the mean 24-h EPR at seven salinities (10, 15, 20, 25, 27, 30, and 33 psu) is shown. Data are presented as mean ± standard error (SE). Different letters above the bars indicate significant differences (p < 0.05).
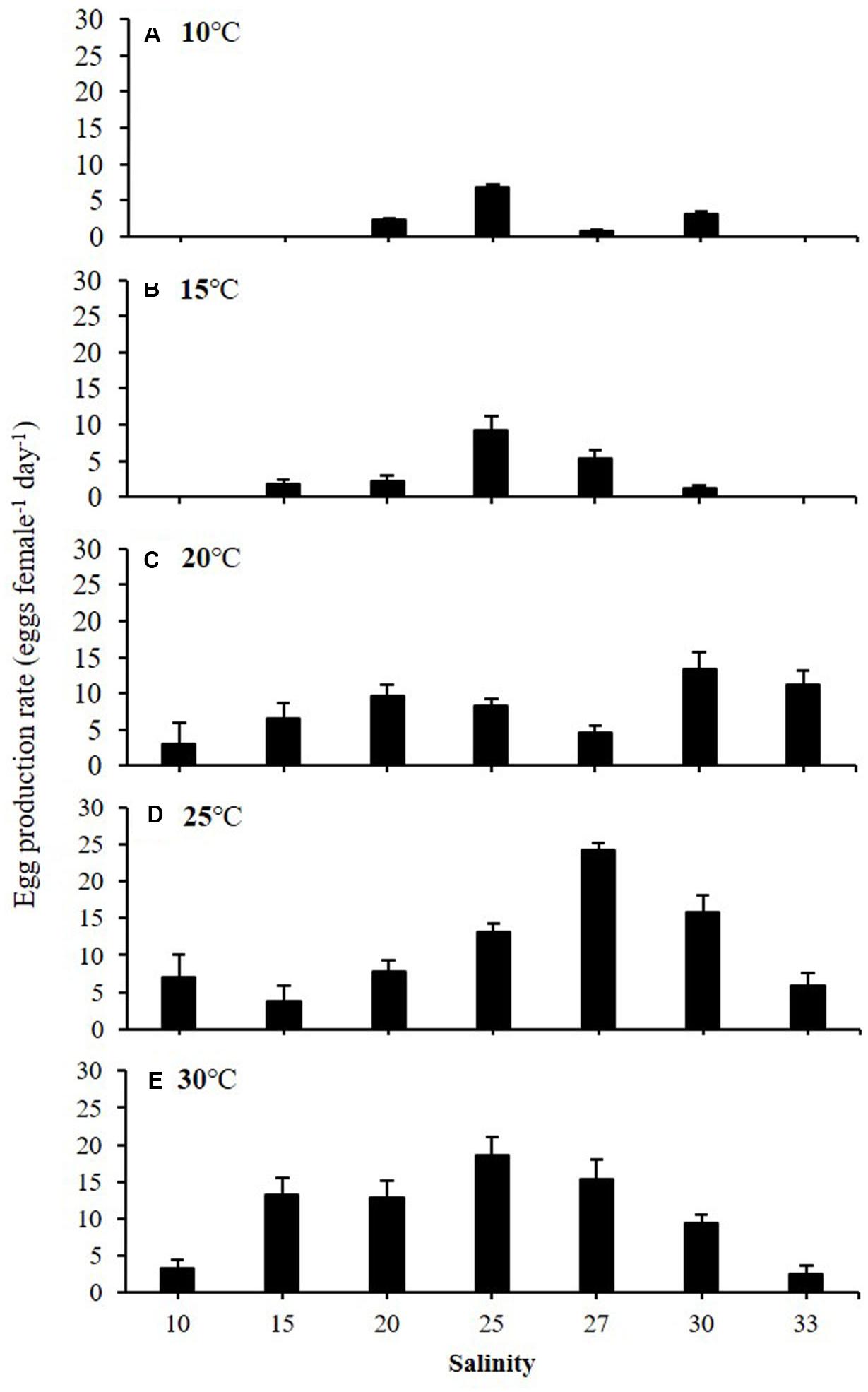
Figure 2. Egg production rate (EPR) of adult female Acartia ohtsukai at each temperature and salinity. (A) 10°C, (B) 15°C, (C) 20°C, (D) 25°C, and (E) 30°C. Data are presented as mean ± standard error (SE). Different letters above the bars indicate significant differences (p < 0.05).
Hatching Success
The average HS (%) of A. ohtsukai increased as the water temperature increased (p < 0.01) (Table 2 and Figure 3A). The average HS was lowest at 10°C (0.2 ± 0.4%) and highest at 30°C (42.1 ± 8.7%). In terms of the water temperature and salinity, the HS was 1.1 ± 0.1% at 10°C and 25 psu, and in other salinity conditions, hatching was not observed (Figure 3B). At 15°C, the HS was highest at 20 psu (10.7 ± 0.8%), and no hatching was observed at 10, 15, 27, and 33 psu. At 20°C, the HS was highest at 27.3 ± 1.2% at 10 psu. At 25°C, the HS was highest (37.2 ± 2.0%) at 10 psu, and 0% at 33 psu. At 30°C, the HS was about 37% or more between 10 and 30 psu, and no hatching was observed at 33 psu.
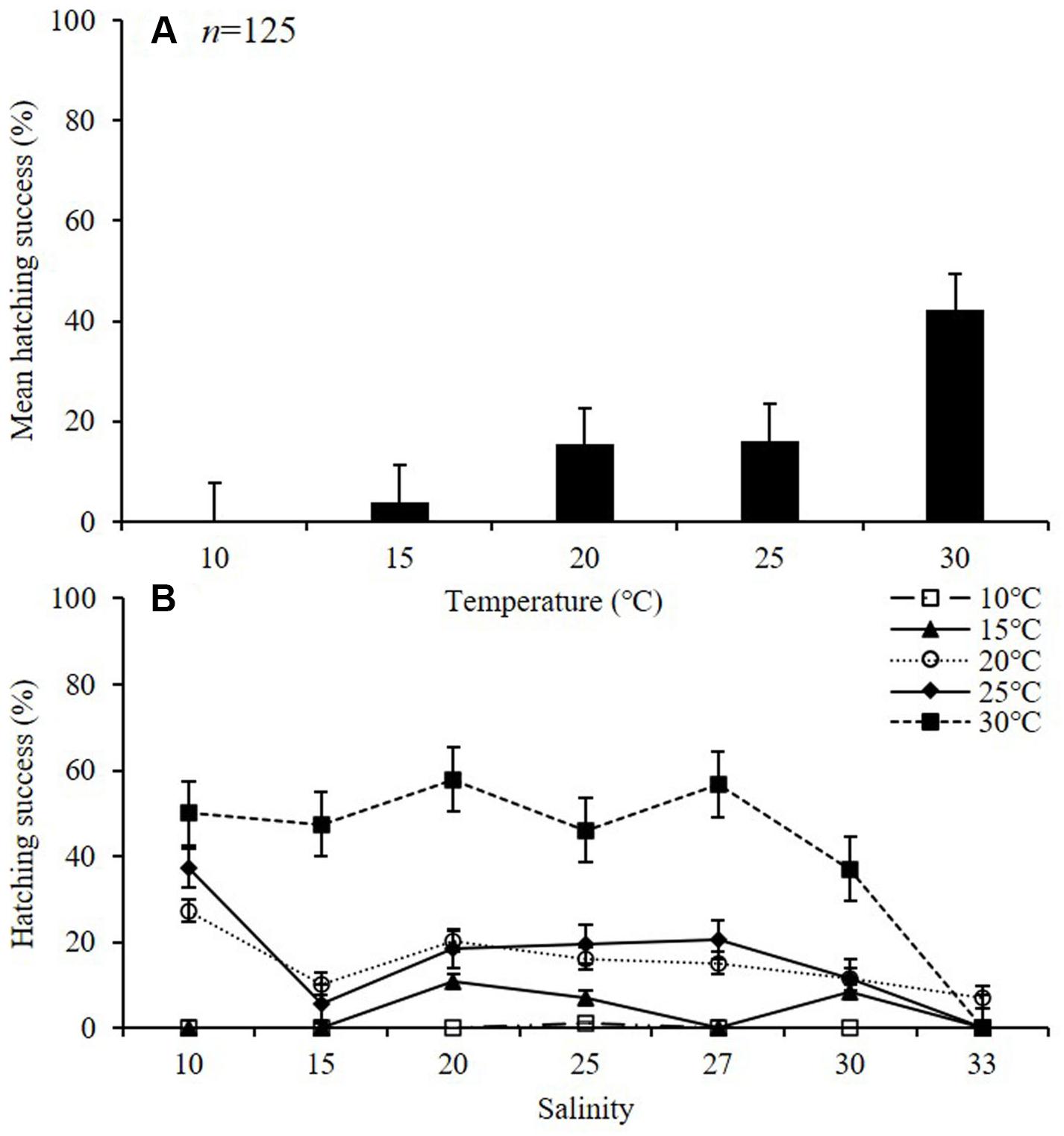
Figure 3. Hatching success (HS) of eggs of adult female Acartia ohtsukai. Mean HS at each temperature (A) and HS at each temperature and salinity (B) are shown. Data are presented as mean ± standard error (SE). Different letters above the bars indicate significant differences (p < 0.05).
Mortality
During the experiment, the mortality of adult females was about 18% at temperatures above 20°C, and above 39% at lower temperatures (10 and 15°C) (p < 0.01) (Table 2 and Figure 4A). The average mortality was lowest at 25°C (16.6 ± 11.4%) and highest at 10°C (42.6 ± 8.4%). The mortality was over 42% at 10°C, and the lowest mortality was observed at 27 psu (32 ± 1.4%) (Figure 4B). In the 15°C treatment, the mortality was highest at 15 psu, with a value of 45.0 ± 0.8%, whereas in the 20°C treatment, the mortality was highest at 10 psu, with a value of 35 ± 2.1%. In the 25°C treatment, the mortality was highest at 10 psu, with a value of 34 ± 1.9%, whereas in the 30°C treatment, the mortality was highest at 33 psu, with a value of 34 ± 1.9%.
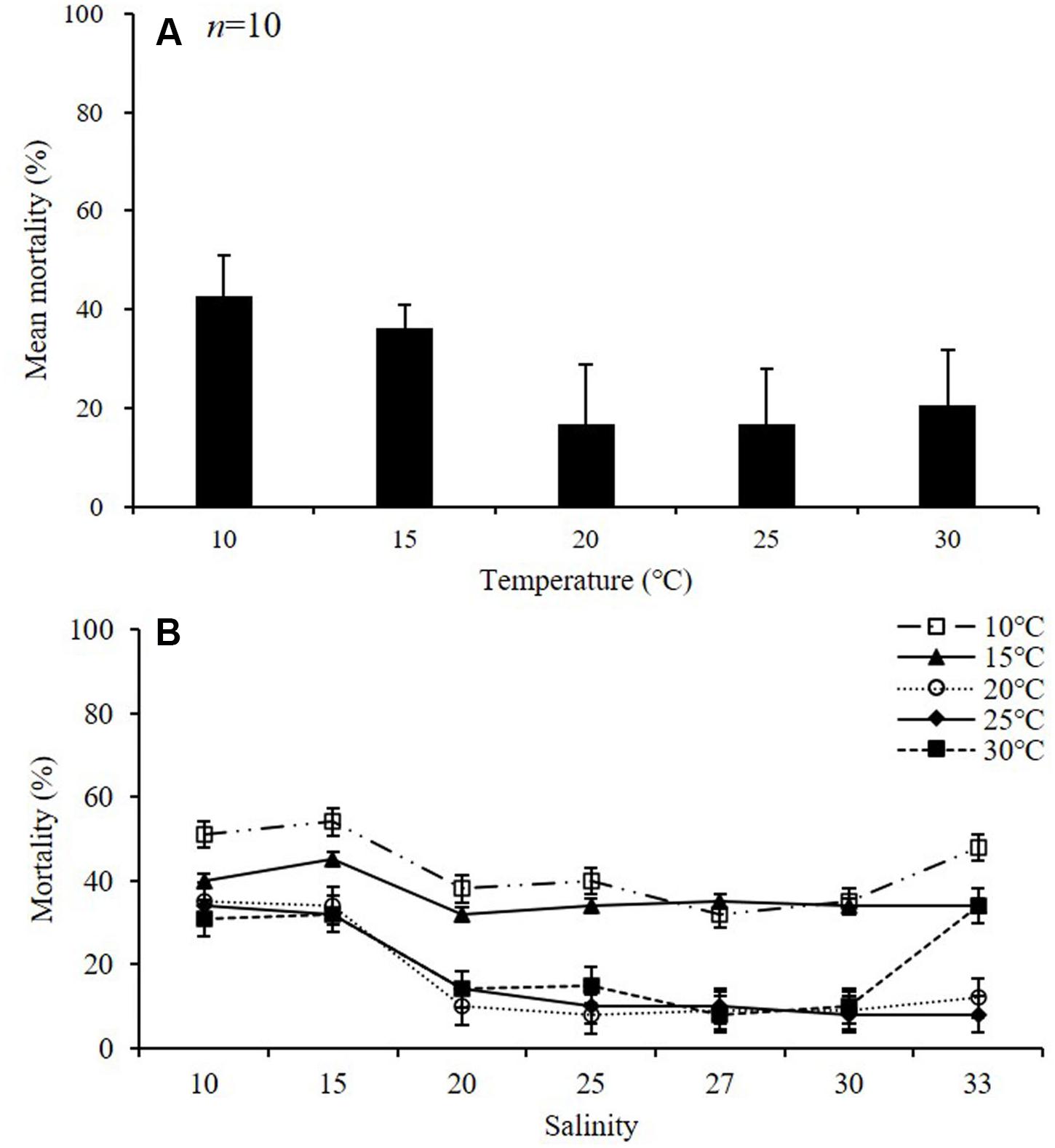
Figure 4. Mortality of adult female Acartia ohtsukai. Mean mortality at each temperature (A) and mortality at each temperature and salinity (B) are shown. Data are presented as mean ± standard error (SE). Different letters above the bars indicate significant differences (p < 0.05).
Discussion
Copepods egg production, hatching rate, survival are affected by several factors, including water temperature, salinity, food quantity and quality, and photoperiod (Peterson et al., 1991; Miralto et al., 2003; Shin et al., 2003). In particular, water temperature and salinity are important factors affecting not only the temporal and spatial distribution of copepods, but also egg production, HS, and mortality rates (Miller and Marcus, 1994; Peck et al., 2015). However, these depend on the different physiological responses of each species at various temperatures and salinities. Chinnery and Williams (2004) showed that the HS of Acartia clausi and A. tonsa was highest at 20°C and 33.3 psu, and at 20°C and 15.5 psu, respectively, whereas that of Acartia bifilosa was significantly higher at 10°C than at 20°C, with no significant influence of salinity. Further, they showed that the HS of Acartia discaudata did not differ significantly with temperature (5, 10, and 20°C) and salinity (15.5, 20.6, 25.1, and 33.3 psu). They also reported that nauplii of four Acartia species (A. discaudata, A. clausi, A. tonsa, and A. bifilosa) had improved survival and showed faster development as temperature increased from 5 to 20°C. Ohs et al. (2010) reported that the optimal salinity range for achieving maximum nauplii production in P. pelagicus was 15–25 g/L, and the percentage of ovigerous females peaked at 20 g/L and decreased in proportion of ovigerous females at salinities above or below this value. Milione and Zeng (2008) found that A. sinjiensis had the highest HS at 34°C, but this did not differ significantly when cultured at 25, 30, and 34°C. In addition, the hatching rate was highest at 30 psu, but there were no significant differences at other salinity concentrations. Further, they suggested that when the temperature rises above a certain level, the positive effect on egg production and hatching rates decreases (Milione and Zeng, 2008). Although A. tsuensis can develop normally from egg to adult within the temperature range of 17.5–30°C, optimal growth and minimum mortality were confirmed to occur at about 25°C (Takahashi and Ohno, 1996).
The results of this study on the effect of temperature on egg production and egg HS in A. ohtsukai are consistent with those of previous studies. With regard to the effect of temperature, the EPR of A. ohtsukai increased at 20°C in comparison to that at 25°C, but it slightly decreased at 30°C, while it was lower at 10 and 15°C. In particular, the EPR at 30°C was three times higher than that at 10–15°C (Figure 1). On the other hand, the combined effect of temperature and salinity was highest at 25°C and 27 psu (Figure 2). The HS increased as the water temperature increased and was highest at 30°C in all salinity gradients except at 33 psu (Figure 3). The present study shows that A. ohtsukai had different ranges of EPR and HS under various water temperature and salinity conditions. The egg production at water temperatures of 10 and 15°C occurred only in a specific salinity range (15–30 psu). When the water temperature reached 20°C or higher, the EPR was expanded to a wider range, ranging from 10 to 33 salinity. The species showed very low HS at 10 and 15°C, and relatively high hatching rates above 20°C in a wide salinity range. The highest HS was shown at 30°C, and hatching was not observed at a salinity of 33 psu. In other words, at temperatures below 20°C, A. ohtsukai showed low EPR and HS in a narrow salinity range, whereas above 20°C, it showed high EPR and HS in a wide salinity range. Therefore, the EPR and HS of A. ohtsukai can be expected to vary depending on the salinity range based on the water temperature of 20°C. The interaction between water temperature and salinity is particularly important in determining the range of physiological tolerance of copepods (Holste and Peck, 2005; Peck et al., 2015).
The results of this study show that as the temperature decreases, the HS (%) markedly decreases, indicating that more than 90% of the eggs do not hatch within 48 h at temperatures of 10 and 15°C. It is speculated that this may be due to differences in the proportions of the different types of eggs that are produced based on different factors (water temperature, salinity, and food quality and quantity). In many species of the family Acartiidae, each single female can simultaneously produce subitaneous and diapause eggs (Onoue et al., 2004), and eggs could be morphologically distinguished depending on the species (Belmonte, 1992, 1998). The previous studies conducted in Gamak Bay have shown that A. ohtsukai produces normal, subitaneous, and resting eggs (Choi et al., 2019). Normal and subitaneous eggs hatch rapidly within a few days after spawning, whereas diapause egg hatch after a certain period (the refractory phase) of time (Uye, 1980; Marcus, 1996). The observed HS (%) during 48 h used in this study may not be sufficient time for resting eggs (Marcus, 1996; Peck and Holste, 2006). No difference was identified in the shape of hatching eggs and non-hatching eggs in this study (magnification ×400, Nikon ECLIPSE 80i; Nikon, Japan). Recent studies have shown that it could be difficult to recognize differences in egg types in the family Acartiidae (Belmonte and Rubino, 2019; Choi et al., 2021).
Mortality rates provide basic information on the tolerance of organisms to environmental conditions (Pörtner and Peck, 2010). In this study, A. ohtsukai showed high mortality rates in all salinities at 10 and 15°C, whereas at 20–30°C it showed a low mortality rate in the range of salinity of 20–33 psu. On the other hand, Støttrup et al. (1986) reported that the daily copepod mortality rate of A. tonsa was constant at 5% in the optimal salinity gradient, but it increased by about 50% or more in high salinity with values above 25 psu (Medina and Barata, 2004). It is not practical to accommodate more than 50% mortality of live prey in mass production (Jepsen et al., 2015). The mortality of A. ohtsukai observed for 10 days showed a low rate of less than 25% on average at all water temperatures and salinities except at salinity values of 10 and 15 psu at a water temperature of 10°C. Further, extensive salinity changes, except for a high salinity value of 33 psu, did not significantly affect HS in A. ohtsukai over 48 h. However, in the low temperature (10 and 15°C) experiment, no eggs were produced at salinities of 10, 15, and 33 psu. These results showed that the productivity was affected by salinity in the experiment at low temperatures, while it was affected by temperature when the values were above 20°C. Therefore, the optimal range for the survival of A. ohtsukai based on the combined effect of water temperature and salinity is estimated to be approximately 20–30°C temperature and 20–33 psu salinity. The physiological properties of A. ohtsukai suggest that the species can be an excellent candidate for mass cultivation as a prey of fish larvae, and that it may persist in the water column under various water temperature and salinity conditions.
Some recent studies suggest an unclear role of salinity on biological traits of the genus Acartia. Castro-Longoria (2003) found that the EPR of Acartia species (Acartia margalefi, A. discaudata, A. clausi, and A. tonsa) were not significantly affected by a salinity range of 15–33 psu. Figure 2 shows that the EPR decreased as the salinity increased above 27 psu at water temperatures of 10 and 15°C. However, in contrast to water temperature, the effect of salinity on EPR in A. ohtsukai was less consistent in the present study. For example, Peck and Holste (2006) found that A. tonsa had the highest EPR at a salinity value of 15 psu among a salinity range of 5–30 psu, whereas EPR gradually decreased in salinity above 20 psu. In addition, an optimal EPR of Acartia japonica was observed at a salinity value of 15 psu and a tendency of decrease in EPR at salinity values above 20 psu among a salinity range of 5–30 psu was shown (Wilson et al., 2021).
Several calanoid species living in estuarine waters have been reported to be able to withstand a wide range of salinity conditions (Uye, 1982). Støttrup (2000) showed that although salinity can affect productivity, these species can survive in less than suboptimal conditions. Although adults can withstand a relatively wide range of water temperature and salinity, the nauplius stage that is used as live feed for larvae of several fish species, including red snapper (Lutjanus campechanus), mangrove jack (Lutjanus argentimaculatus), and grouper (Epinephelus coioides) (Schipp et al., 1999; Lee et al., 2010), is sensitive in terms of salinity tolerance (Lance, 1964; Chinnery and Williams, 2004). In particular, in the case of the nauplius stage, exposure to adverse environments at this sensitive stage leads to developmental delay, ecdysis, or death (Tester and Turner, 1991; Devreker et al., 2004). Due to the difference in mortality rates according to developmental stages, it was difficult to estimate the mortality rate of nauplii according to water temperature and salinity in this study. Therefore, it is necessary to understand the effects on the population of A. ohtsukai according to environmental changes by investigating the mortality rate in future developmental stages. Although this study has not been undertaken on nauplii and copepodites according to their developmental stages, we have provided information on the optimum water temperature and salinity conditions of A. ohtsukai that can be applied to mass culture.
Conclusion
Acartia ohtsukai could survive in a relatively wide range of temperature and salinity conditions, but it was confirmed that temperature significantly affected EPR and HS. Salinity is considered to also have a significant effect on EPR and HS of A. ohtsukai, but to affect mortality rate to a lesser degree. To optimize the intensive culture of A. ohtsukai, we recommend the following: in order to produce and cultivate a high rate of eggs, the culture should be maintained at a temperature of 25°C and salinity of 27 psu. The immediate use as live feed for fish larvae is considered to be efficient when hatching occurs at 30°C. This demonstrates that the plasticity of A. ohtsukai enables it to adapt to changes in water temperature and salinity and that this species can be used as an efficient live feed in the cultivation of larvae of various fishes.
Data Availability Statement
These data generated from this study are available on request to the corresponding author.
Author Contributions
SYC conceived and designed the experiments, performed the experiments, analyzed the data, prepared the figures and tables, authored, reviewed drafts of the manuscript, and approved the final draft. EHL analyzed the data, authored, reviewed drafts of the manuscript, and approved the final draft. HYS and M-CJ conceived and designed the experiments, performed the experiments, analyzed the data, authored, reviewed drafts of the manuscript, and approved the final draft. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Marine Biodiversity Institute of Korea (2021M01100) and the Korea Institute of Ocean Science and Technology (PE99912).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank researcher SeokJu Lee of the Marine Biological Resource Center for assistance in sample collection. We also thank Editage for the English editing services.
References
Alajmi, F., and Zeng, C. (2015). Evaluation of microalgal diets for the intensive cultivation of the tropical calanoid copepod, Parvocalanus crassirostris. Aquac. Res. 46, 1025–1038. doi: 10.1111/are.12254
Ban, S. (1994). Effect of temperature and food concentration on post-embryonic development, egg production and adult body size of calanoid copepod Eurytemora affinis. J. Plankton Res. 16, 721–735. doi: 10.1093/plankt/16.6.721
Bell, J. G., McEvoy, L. A., Estevez, A., Shields, R. J., and Sargent, J. R. (2003). Optimising lipid nutrition in first-feeding flatfish larvae. Aquaculture 227, 211–220. doi: 10.1016/S0044-8486(03)00504-0
Belmonte, G. (1992). Diapause egg production in Acartia (Paracartia) latisetosa (Crustacea, Copepoda, Calanoida). Ital. J. Zool. 59, 363–366. doi: 10.1080/11250009209386694
Belmonte, G. (1998). The egg morphology of 7 Acartiidae species: a preliminary survey of the ootaxonomy of calanoids. J. Mar. Syst. 15, 35–39. doi: 10.1016/S0924-7963(97)00047-X
Belmonte, G., and Rubino, F. (2019). Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. Annu. Rev. 57, 1–88. doi: 10.1201/9780429026379-1
Bradley, B. P. (1986). Genetic expression of temperature tolerance in the copepod Eurytemora affinis in different salinity and temperature environments. Mar. Biol. 91, 561–565. doi: 10.1007/bf00392608
Castro-Longoria, E. (2003). Egg production and hatching success of four Acartia species under different temperature and salinity regimes. J. Crustac. Biol. 23, 289–299. doi: 10.1163/20021975-99990339
Chesney, E. J. (2005). “Copepods as live prey: a review of factors that influence the feeding success of marine fish larvae,” in Copepods in Aquaculture, eds C.-S. Lee, P. J. O’Bryen, and N. H. Marcus (Melbourne, VIC: Blackwell Scientific Publication Ltd), 133–150. doi: 10.1002/9780470277522.ch11
Chinnery, F. E., and Williams, J. A. (2004). The influence of temperature and salinity on Acartia (Copepoda: Calanoida) nauplii survival. Mar. Biol. 145, 733–738. doi: 10.1007/s00227-004-1354-2
Choi, S. Y., Jang, M. C., Youn, S. H., Seo, M. H., and Soh, H. Y. (2021). Egg production and hatching patterns of Acartia erythraea (Copepoda, Calanoida), with a note on its two egg types, in a eutrophic bay in Korea. J. Plankton Res. 43, 428–441. doi: 10.1093/plankt/fbab030
Choi, S. Y., Seo, M. H., Shin, K., Jang, M., and Soh, H. Y. (2019). Spatial distribution of Acartia (Copepoda, Calanoida) species in the southern coastal waters of Korea during summer. Korean J. Environ. Biol. 37, 299–308. doi: 10.11626/KJEB.2019.37.3.299
Devreker, D., Souissi, S., and Seuront, L. (2004). Development and mortality of the first naupliar stages of Eurytemora affinis (Copepoda, Calanoida) under different conditions of salinity and temperature. J. Exp. Mar. Biol. Ecol. 303, 31–46. doi: 10.1016/j.jembe.2003.11.002
Drillet, G., Frouël, S., Sichlau, M. H., Jepsen, P. M., Højgaard, J. K., Joarder, A. K., et al. (2011). Status and recommendations on marine copepod cultivation for use as live feed. Aquaculture 315, 155–166. doi: 10.1016/j.aquaculture.2011.02.027
Dutz, J., and Christensen, A. M. (2018). Broad plasticity in the salinity tolerance of a marine copepod species, Acartia longiremis, in the Baltic Sea. J. Plankton Res. 40, 342–355. doi: 10.1093/plankt/fby013
Feinberg, L. R., and Dam, H. (1998). Effects of diets on dimensions, density and sinking rates of fecal pellets of the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 175, 87–96. doi: 10.1016/j.jembe.2013.04.018
Gapasin, R., and Duray, M. (2001). Effects of DHA-enriched live food on growth, survival and incidence of opercular deformities in milkfish (Chanos chanos). Aquaculture 193, 49–63. doi: 10.1016/S0044-8486(00)00469-5
Guillard, R. R. L., and Ryther, J. H. (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 8, 229–239. doi: 10.1139/m62-029
Hoff, F. H., and Snell, T. W. (1999). Plankton Culture Manual, 5th Edn. Dade City, FL: Florida Aqua Farms Inc., 160.
Holste, L. (2004). The Influence of Temperature, Salinity and Feeding History on Population Characteristics of Baltic Acartia tonsa: Egg Production, Hatching Success and Cohort Development. Master’s thesis. Hamburg: University of Hamburg, 79.
Holste, L., and Peck, M. A. (2005). The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): a laboratory investigation. Mar. Biol. 148, 1061–1070. doi: 10.1007/s00227-005-0132-0
Jepsen, P. M., Andersen, C. V., Schjelde, J., and Hansen, B. W. (2015). Tolerance of un-ionized ammonia in live feed cultures of the calanoid copepod Acartia tonsa Dana. Aquac. Res. 46, 420–431. doi: 10.1111/are.12190
Lance, J. (1964). The salinity tolerances of some estuarine planktonic crustaceans. Biol. Bull. 127, 108–118. doi: 10.2307/1539348
Lee, C. H., Dahms, H. U., Cheng, S. H., Souissi, S., Schmitt, F. G., Kumar, R., et al. (2010). Predation of Pseudodiaptomus annandalei (Copepoda: Calanoida) by the grouper fish fry Epinephelus coioides under different hydrodynamic conditions. J. Exp. Mar. Biol. Ecol. 393, 17–22. doi: 10.1016/j.jembe.2010.06.005
Lee, E. H. (2019). Occurrence Pattern of Zooplankton and Physiological Charaterstics of Dominant Copepod Acartia ohtsukai Ueda and Bucklin in Northwestern area Yeoja Bay. MA thesis. Yeosu: Chonnam National University, 39.
Lee, E. H., Choi, S. Y., Seo, M. H., Lee, S. J., and Soh, H. Y. (2020). Effects of temperature and pH on the egg production and hatching success of a common Korean Copepod. Diversity 12:372. doi: 10.3390/d12100372
Marcus, N. H. (1996). Ecological and evolutionary significance of resting eggs in marine copepods: past, present, and future studies. Hydrobiologia 320, 141–152. doi: 10.1007/bf00016815
McEvoy, L. A., Naess, T., Bell, J. G., and Lie, Ø (1998). Lipid and fatty acid composition of normal and malpigmented Atlantic halibut (Hippoglossus hippoglossus) fed enriched Artemia: a comparison with fry fed wild copepods. Aquaculture 163, 237–250. doi: 10.1016/S0044-8486(98)00237-3
Medina, M., and Barata, C. (2004). Static-renewal culture of Acartia tonsa (Copepoda: Calanoida) for ecotoxicological testing. Aquaculture 229, 203–213. doi: 10.1016/S0044-8486(03)00389-2
Milione, M., and Zeng, C. (2007). The effects of algal diets on population growth and egg hatching success of the tropical calanoid copepod, Acartia sinjiensis. Aquaculture 273, 656–664. doi: 10.1016/j.aquaculture.2007.07.014
Milione, M., and Zeng, C. (2008). The effects of temperature and salinity on population growth and egg hatching success of the tropical calanoid copepod, Acartia sinjiensis. Aquaculture 275, 116–123. doi: 10.1016/j.aquaculture.2007.12.010
Miller, D. D., and Marcus, N. H. (1994). The effects of salinity and temperature on the density and sinking velocity of eggs of the calanoid copepod Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 179, 235–252. doi: 10.1016/0022-0981(94)90117-1
Miralto, A., Guglielmo, L., Zagami, G., Buttino, I., Granata, A., and Ianora, A. (2003). Inhibition of population growth in the copepods Acartia clausi and Calanus helgolandicus during diatom blooms. Mar. Ecol. Prog. Ser. 254, 253–268. doi: 10.3354/meps254253
Naess, T., Germain-Henry, M., and Naas, K. E. (1995). First feeding of Atlantic halibut (Hippoglossus hippoglossus) using different combinations of Artemia and wild zooplankton. Aquaculture 130, 235–250. doi: 10.1016/0044-8486(94)00323
Nagaraj, M. (1988). Combined effects of temperature and salinity on the complete development of Eurytemora velox (Crustacea: Calanoidea). Mar. Biol. 99, 353–358. doi: 10.1007/BF02112127
Nguyen, T. T., Le, M. H., Doan, N. X., Nguyen, S. T., Truong, T. S., Vu, M. T., et al. (2020). Salinity and temperature effects on productivity of a tropical calanoid copepod Pseudodiaptomus incisus. Aquac. Res. 51, 3768–3779. doi: 10.1111/are.14727
Ohno, A., Takahashi, T., and Taki, Y. (1990). Dynamics of exploited populations of the calanoid copepod, Acartia tsuensis. Aquaculture 84, 27–39. doi: 10.1016/0044-8486(90)90297-Z
Ohs, C. L., Rhyne, A. L., Grabe, S. W., DiMaggio, M. A., and Stenn, E. (2010). Effects of salinity on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture 307, 219–224. doi: 10.1016/j.aquaculture.2010.07.017
Onoue, Y., Toda, T., and Ban, S. (2004). Morphological features and hatching patterns of eggs in Acartia steueri (Crustacea, Copepoda) from Sagami Bay, Japan. Hydrobiologia 511, 17–25. doi: 10.1023/b:hydr.0000014013.37891.46
Pan, Y. J., Souissi, A., Souissi, S., and Hwang, J. S. (2016). Effects of salinity on the reproductive performance of Apocyclops royi (Copepoda, Cyclopoida). J. Exp. Mar. Biol. Ecol. 475, 108–113. doi: 10.1016/j.jembe.2015.11.011
Park, E. O., Suh, H. L., and Soh, H. Y. (2015). Spatio-temporal distribution of Acartia (Copepoda: Calanoida) species along a salinity gradient in the Seomjin River estuary, South Korea. J. Nat. Hist. 49, 2799–2812. doi: 10.1080/00222933.2015.1022619
Peck, M. A., and Holste, L. (2006). Effects of salinity, photoperiod and adult stocking density on egg production and egg hatching success in Acartia tonsa (Calanoida: Copepoda): optimizing intensive cultures. Aquaculture 255, 341–350. doi: 10.1016/j.aquaculture.2005.11.055
Peck, N., Peters, J., Diekmann, R., Laakmann, S., and Renz, J. (2015). Interactive effects of temperature and salinity on population dynamics of the calanoid copepod Acartia tonsa. J. Plankton Res. 37, 197–210. doi: 10.1093/plankt/fbu093
Peterson, W. T., Tiselius, P., and Kiørboe, T. (1991). Copepod egg production, moulting and growth rates, and secondary production, in the Skagerrak in August 1988. J. Plankton Res. 13, 131–154. doi: 10.1093/plankt/13.1.131
Pörtner, H. O., and Peck, M. A. (2010). Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J. Fish Biol. 77, 1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x
Razouls, C., de Bovée, F., Kouwenberg, J., and Desreumaux, N. (2005-2020). Diversity and Geographic Distribution of Marine Planktonic Copepods. Sorbonne University, CNRS. Available online at http://copepodes.obs-banyuls.fr/en (accessed May 02, 2021).
Rhyne, A. L., Ohs, C. L., and Stenn, E. (2009). Effects of temperature on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture 292, 53–59. doi: 10.1016/j.aquaculture.2009.03.041
Santhanam, P., and Perumal, P. (2012). Evaluation of the marine copepod Oithona rigida Giesbrecht as live feed for larviculture of Asian seabass Lates calcarifer bloch with special reference to nutritional value. Ind. J. Fish. 59, 127–134.
Sargent, J. R., and Falk-Petersen, S. (1988). The lipid biochemistry of calanoid copepods. Hydrobiologia 167–168, 101–114. doi: 10.1007/978-94-009-3103-9_9
Schipp, G. R., Bosmans, J. M., and Marshall, A. J. (1999). A method for hatchery culture of tropical calanoid copepods, Acartia spp. Aquaculture 174, 81–88. doi: 10.1016/S0044-8486(98)00508-0
Shin, K., Jang, M. C., Jang, P. K., Ju, S. J., Lee, T. K., and Chang, M. (2003). Influence of food quality on egg production and viability of the marine planktonic copepod Acartia omorii. Prog. Oceanogr. 57, 265–277. doi: 10.1016/S0079-6611(03)00101-0
Støttrup, J. G. (2000). The elusive copepods: their production and suitability in marine aquaculture. Aquac. Res. 31, 703–711. doi: 10.1046/j.1365-2109.2000.318488.x
Støttrup, J. G. (2003). “Production and nutritional value of copepods,” in Live Feeds in Marine Aquaculture, eds J. G. Støttrup and L. A. McEvoy (Oxford: Blackwell Science), 145–205. doi: 10.1002/9780470995143.ch5
Støttrup, J. G., and Norsker, N. H. (1997). Production and use of copepods in marine fish larviculture. Aquaculture 155, 231–247. doi: 10.1016/S0044-8486(97)00120-8
Støttrup, J. G., Richardson, K., Kirkegaard, E., and Pihl, N. J. (1986). The cultivation of Acartia tonsa Dana for use as a live food source for marine fish larvae. Aquaculture 52, 87–96. doi: 10.1016/0044-8486(86)90028-1
Takahashi, T., and Ohno, A. (1996). The temperature effect on the development of calanoid copepod, Acartia tsuensis, with some comments to morphogenesis. J. Oceanogr. 52, 125–137. doi: 10.1007/BF02236536
Tester, P. A., and Turner, J. T. (1991). “Why is Acartia tonsa restricted to estuarine habitats?” in Proceedings of the 4th International Conference on Copepoda (Karuizawa: Bulletin of the Plankton Society of Japan), 603–611.
Uye, S. (1980). Development of neritic copepods Acartia clausi and A. steueri. II. Isochronal larval development at various temperatures. Bull. Plankton Soc. Japan 27, 11–18.
Uye, S. (1982). Population dynamics and production of Acartia clausi Giesbrecht (Copepoda: Calanoida) in inlet waters. J. Exp. Mar. Biol. Ecol. 57, 55–83. doi: 10.1016/0022-0981(82)90144-7
van der Meeren, T., and Naas, K. E. (1997). Development of rearing techniques using large enclosed ecosystems in the mass production of marine fish fry. Rev. Fish. Sci. 5, 367–390. doi: 10.1080/10641269709388606
van der Meeren, T., Olsen, R. E., Hamre, K., and Fyhn, H. J. (2008). Biochemical composition of copepods for evaluation of feed quality in production of juvenile marine fish. Aquaculture 274, 375–397. doi: 10.1016/j.aquaculture.2007.11.041
Wilcox, J. A., Tracy, P. L., and Marcus, N. H. (2006). Improving live feeds: effect of a mixed diet of copepod nauplii (Acartia tonsa) and rotifers on the survival and growth first-feeding larvae of the Southern Flounder, Paralichthys lethostigma. J. World Aquac. Soc. 37, 113–120. doi: 10.1111/j.1749-7345.2006.00014.x
Wilson, J. M., Ignatius, B., Sawant, P. B., Santhosh, B., and Chadha, N. K. (2021). Productivity of the calanoid copepod Acartia tropica in response to different salinities and multigenerational acclimatization. Aquaculture 531:735818. doi: 10.1016/j.aquaculture.2020.735818
Keywords: Acartiidae, intensive culture, reproduction, Yeoja Bay, egg production rates
Citation: Choi SY, Lee EH, Soh HY and Jang M-C (2021) Effects of Temperature and Salinity on Egg Production, Hatching, and Mortality Rates in Acartia ohtsukai (Copepoda, Calanoida). Front. Mar. Sci. 8:704479. doi: 10.3389/fmars.2021.704479
Received: 03 May 2021; Accepted: 07 June 2021;
Published: 12 July 2021.
Edited by:
Sami Souissi, Lille University of Science and Technology, FranceReviewed by:
Vladimir G. Dvoretsky, Murmansk Marine Biological Institute, RussiaGenuario Belmonte, University of Salento, Italy
Chaoshu Zeng, James Cook University, Australia
James J. Pierson, University of Maryland Center for Environmental Science, United States
Copyright © 2021 Choi, Lee, Soh and Jang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ho Young Soh, aHlzb2hAY2hvbm5hbS5hYy5rcg==; Min-Chul Jang, bWNqYW5nQGtpb3N0LmFjLmty
 Seo Yeol Choi
Seo Yeol Choi Eun Hye Lee
Eun Hye Lee Ho Young Soh1,2*
Ho Young Soh1,2* Min-Chul Jang
Min-Chul Jang