
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 26 July 2021
Sec. Aquatic Physiology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.702101
This article is part of the Research Topic Omics Approaches in Aquatic Nutritional Physiology View all 7 articles
The peanut worm (Sipunculus nudus) is an economically important fishery resource in China. To determine how dietary carbohydrate and protein levels affect the growth performance of juvenile S. nudus and identify the mechanisms underlying observed patterns, five isoenergetic and isolipidic diets with different levels of carbohydrate and protein were formulated and fed to juvenile S. nudus; the experimental groups were referred to as EG1, EG2, EG3, EG4, and EG5, respectively. After 90 days of feeding, S. nudus had significantly lower survival rates when fed D5 compared with other diets (P < 0.05), and the highest survival rate was observed in EG2 individuals. The weight gain rate and specific growth rate were significantly higher in EG2 compared with the other groups (P < 0.05). Metabolomic profiling using liquid chromatography–mass spectrometry revealed 83 significantly differential metabolites (POS: 59; NEG: 24), which were identified via an in-house MS2 database. Pathway analysis indicated that the significantly different metabolites were involved in 22 metabolic pathways (POS: 9; NEG: 13), including tyrosine, phenylalanine, and tryptophan biosynthesis; phenylalanine metabolism; D-glutamate and D-glutamine metabolism; proline and arginine metabolism; aspartate, alanine, and glutamate metabolism; and aminoacyl-tRNA biosynthesis. These analyses implied that the biosynthetic capabilities of juvenile S. nudus were greater in the EG2. The results of this research enhance our understanding of the effects of dietary carbohydrate and protein levels on the growth performance of juvenile S. nudus.
Peanut worm (Sipunculus nudus) is an important resource in China and is often referred to as the “cordyceps of the sea” because of its high medicinal and nutritional value (Shen et al., 2004; Luo et al., 2016; Zhang et al., 2018). It is cultured along beaches and obtains nutrients from surface sediments (Adrianov and Maiorova, 2010). S. nudus is widely distributed along the coast of southern China, including the Zhanjiang, Guangdong and Beihai, Guangxi (Du et al., 2008, 2009). Wild-collected seeds are commonly used for the culture of S. nudus; however, excessive harvesting of the seeds can rapidly deplete wild seed resources (Du et al., 2009). Artificial breeding can be used to mitigate the exploitation of wild resources. Many studies have been conducted on the population genetics (Du et al., 2008, 2009) and reproductive biology (Wang et al., 2005; Cao et al., 2020; Yang et al., 2020; Zhang J. W. et al., 2021) of S. nudus. Given that growth performance is greatly affected by diet quality, studies of the nutritional requirements, and artificial feed of juvenile S. nudus could aid its artificial breeding, enhance its aquaculture, and promote the restoration of wild populations. S. nudus has a simple body structure, and it possesses the digestive tract and the posterior renal tube that is bathed in coelomic fluid but has no liver and pancreas. Coelomic fluid is a complex internal environment system, where many physiological and biochemical processes are completed (Xian and Huang, 2011). And coelomic fluid was mainly involved in catalytic activity and metabolic processes, most of which were associated with carbohydrate and protein metabolism (Cao et al., 2021).
The metabolic profiles of animals vary with diet quality. Metabolomics provides a “snapshot” of the metabolites and is particularly suitable for metabolic studies (Cappello et al., 2017, 2018). It is commonly used to quantify low-molecular-weight metabolites, such as sugars, lipids, and amino acids, through various tools such as gas chromatography–mass spectrometry, nuclear magnetic resonance, and liquid chromatography–mass spectrometry (LC–MS; Venter et al., 2018; Yang et al., 2021; Zhang J. B. et al., 2021). Several studies have used this approach to assess the effects of nutrient levels (Jin et al., 2015; Yang et al., 2019a, b), food shortages (Tuffnail et al., 2009; Baumgarner and Cooper, 2012), nutrient supplementation (Wagner et al., 2014; Andersen et al., 2015), and the substitution of nutrients (Cheng et al., 2016; Yang et al., 2018b) in aquatic animals.
Incorporation of the optimal level of carbohydrate and protein in diets helps improve feed efficiency and growth rate in aquatic animals. The aim of this study was to determine the optimum balance of dietary carbohydrates and proteins for juvenile S. nudus and compare its metabolomic responses when fed two diets differing in dietary carbohydrate and protein levels using LC–MS-based metabolomics. The results of this study aid our understanding of the metabolomic responses of juvenile S. nudus to different diets and provide new information on its nutritional requirements.
Five isoenergetic and isolipidic diets (D1, D2, D3, D4, and D5) were formulated with different levels of carbohydrate and protein based on previous studies (Zhang et al., 2011, 2012; Xu et al., 2013). Proteins were obtained from fish meal, Spirulina platensis powder, yeast powder, and soybean meal; carbohydrates were obtained from starch and kelp powder; and lipids were obtained from fish oils, average crude lipid level was 8.71%. The formulated diets were ground into desirable particle sizes (180 mesh). The ingredients and proximate composition of the formulated diets are shown in Supplementary Table 1. The amino acid profiles of the formulated diets are shown in Supplementary Table 2. D1–D5 were fed to animals (mean total weight: 1.55 ± 0.25 g) in five groups: EG1, EG2, EG3, EG4, and EG5, respectively; each experimental group had three replicates, and each replicate (1 m2) had 120 animals. The experiment was run for 90 days. During the experiment, the juvenile peanut worms were fed twice at 8:00 and 20:00 per day, and up to 3% of their total biomass each time. About 20% of the water volume was renewed daily. The concentration of dissolved oxygen in the water was 5.00 mg/L, the water salinity was 30, and the temperature ranged from 24.1–29.7°C, ammonia-N was <0.5 mg/L, and pH was around 7.4–7.9.
The protocols for measuring crude protein, crude lipid, ash, and amino acid of the formulated diets were detailed by Wang et al. (2016), carbohydrate values were calculated following the methods of Xia et al. (2015), gross energy values were calculated following the methods of Xie et al. (2017).
The total number and total weight of juvenile S. nudus in each group were determined at the beginning and end of the experiment. Total weight was obtained using an electronic balance. Survival rate, weight gain rate (WGR), and specific growth rate (SGR) were calculated following the methods of Xia et al. (2015).
After the 90-day culture period, eight S. nudus from each group were randomly selected and coelomic fluid from juvenile S. nudus was extracted following the methods of Yang et al. (2020), immediately placed in liquid nitrogen, and stored at –80°C.
A 100-μL sample was pipetted into an Eppendorf tube, and 400 μL of extract solution (acetonitrile: methanol = 1: 1) containing an isotopically labeled internal standard mixture was added. Metabolite extraction followed the methods of Yang et al. (2021).
A UHPLC system (Vanquish, Thermo Fisher Scientific) with a UPLC BEH Amide column (2.1 mm × 100 mm, 1.7 μm) and a Q Exactive HFX mass spectrometer (Orbitrap MS, Thermo) was used for LC–MS/MS analyses. The analysis was carried out with an elution gradient as described by Yang et al. (2021). The flow rate was 0.5 mL/min; the auto-sampler temperature was 4°C; the column temperature was 30°C; and the injection volume was 2 μL.
MS/MS spectra were acquired using a QE HFX mass spectrometer through the information-dependent acquisition mode in Xcalibur software (Thermo Fisher Scientific). The software continuously evaluates the full-scan MS spectrum in this mode. The sheath gas flow rate was 30 Arb; the Aux gas flow rate was 25 Arb; the capillary temperature was 350°C; the full MS resolution was 60,000; the MS/MS resolution was 7,500; the collision energy was 10/30/60 in NCE mode; and the spray voltage was 3,600 V (positive, POS) or –3,200 V (negative, NEG).
ProteoWizard was used to convert the raw data to mzXML format, followed by processing with an in-house program. This program was used for extraction, peak detection, alignment, and integration and was developed using R. The cutoff for annotation was 0.3. The metabolite identification was performed via an in-house MS2 database, including metlin, pubchem, and self-built database of Biotree Biotech Co., Ltd. (Shanghai, China).
The final dataset contained information such as the sample name, peak number, and normalized peak area and was imported to SIMCA16.0.2 (Sartorius Stedim Data Analytics AB, Umea, Sweden) for orthogonal projections to latent structures discriminant analysis (OPLS-DA) and principal component analysis (PCA). Data were scaled and logarithmically transformed to minimize the effect of noise and high variance among the variables. Data were analyzed as previously described (Yang et al., 2021). In the OPLS-DA analysis, the variable importance in projection (VIP) of the first principal component in OPLS-DA analysis was obtained. Metabolites with P < 0.1 (Student’s t-test) and VIP > 1 were significantly different metabolites (SDMs). In addition, the KEGG1 and MetaboAnalyst2 databases were utilized for pathway enrichment analysis.
Survival rate, WGR, and SGR values were determined using one-way analysis of variance, followed by Duncan’s multiple comparisons test. The data were presented as mean ± SEM; significant differences (P < 0.05) between variables.
The survival rates of juvenile S. nudus ranged from 70.37 to 82.59%. EG5 S. nudus had significantly lower survival rates compared with S. nudus in other groups (P < 0.05, Table 1). Although no significant differences were observed among EG1, EG2, EG3, and EG4, the highest survival rate was observed in EG2 (P > 0.05, Table 1). Juvenile S. nudus in group EG2 had significantly higher WGR and SGR compared with the other groups (Table 1, P < 0.05).
The peak area and peak retention time of the total ion chromatograms from all QC samples showed extensive overlap, indicating the stability of the analytical system (Supplementary Figure 1). A total of 4,160 positive (POS) and 3,227 negative (NEG) peaks were obtained for the coelomic fluid from juvenile S. nudus. These valid peaks were matched to 207 and 103 metabolites in POS and NEG, respectively, via an in-house MS2 database.
In the PCA score scatter plot, similar datasets were clustered more closely, whereas different datasets were placed further apart (Figures 1A,B). The values for R2X of the PCA model between EG2 and EG5 were 0.572 (POS) and 0.574 (NEG), respectively. OPLS–DA was performed to characterize the different metabolic patterns of coelomic fluid from juvenile S. nudus (Figures 1C,D). The values for R2X, R2Y, and Q2 in the OPLS–DA model of POS between EG2 and EG5 were 0.229, 0.977, and 0.609, respectively. The values for R2X, R2Y, and Q2 in the OPLS–DA model of NEG between EG2 and EG5 were 0.348, 0.957, and 0.520, respectively. A permutation test was used to avoid the transition fit in the OPLS–DA mode (Figures 1E,F). The R2Y and Q2 intercepts were 0.97 and –0.46 between EG2 and EG5 (POS) and 0.91 and –0.52 between EG2 and EG5 (NEG), respectively. Thus, the OPLS–DA model was not overfit and showed high stability; it was thus suitable for use in subsequent analyses.
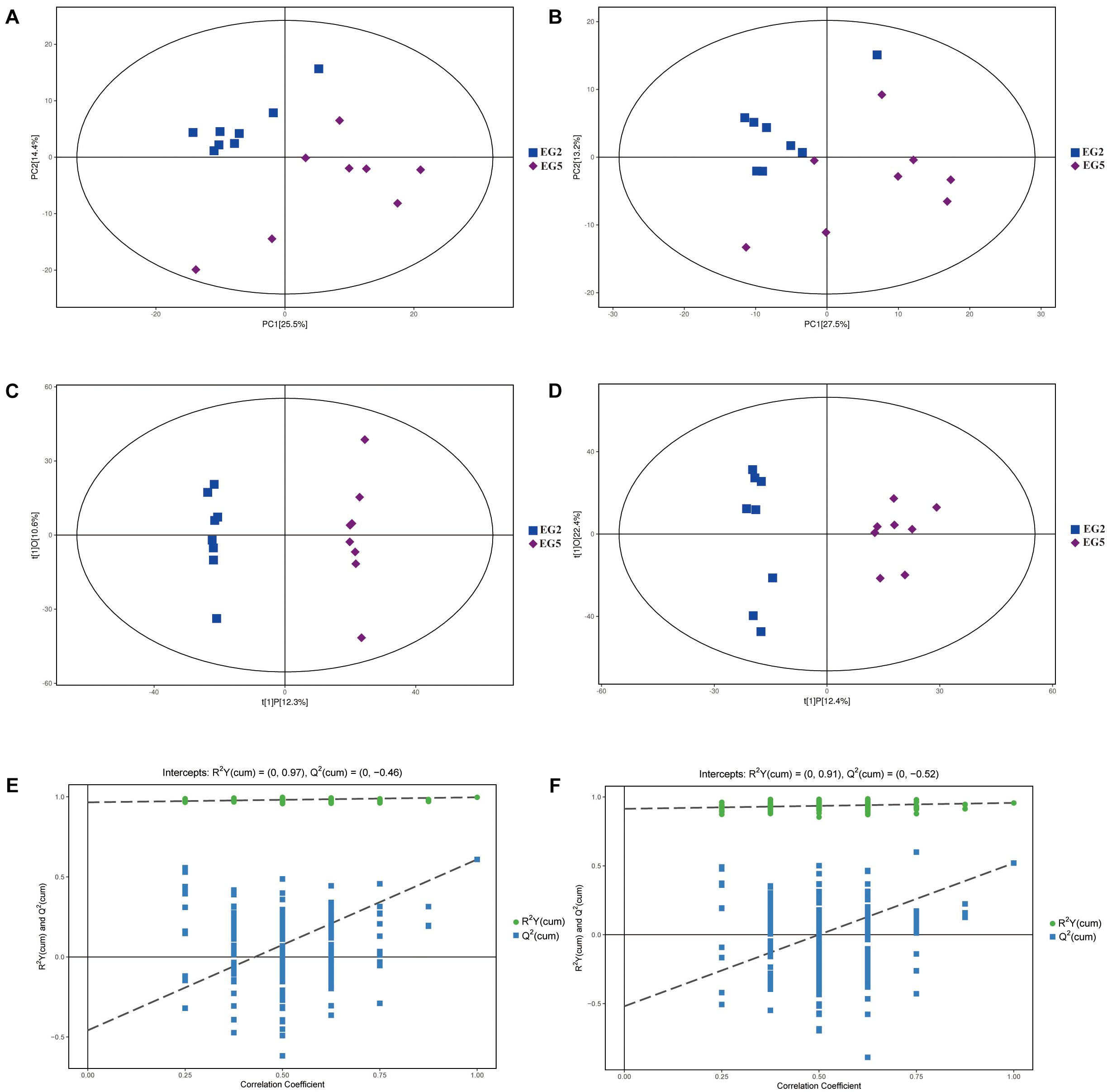
Figure 1. PCA model score scatter plot, OPLS–DA model, and permutation test for groups EG2 and EG5. The PCA model (A,B), OPLS–DA model (C,D), and permutation test of the OPLS–DA model (E,F) were derived from the UHPLC–QTOF/MS metabolomics profiles. (A,C,E) were derived from the POS ion mode, and (B,D,F) were derived from the NEG ion mode.
Volcano plots were used to visualize the SDMs; the plots revealed several significant SDMs (Figures 2A,B). In total, 83 SDMs between EG2 and EG5 (POS: 59; NEG: 24) were identified via an in-house MS2 database (Figures 3, 4 and Supplementary Table 3). Compared with EG5, 37 SDMs had higher concentrations in EG2 (POS: 25; NEG: 12; Figures 3, 4 and Supplementary Table 3).
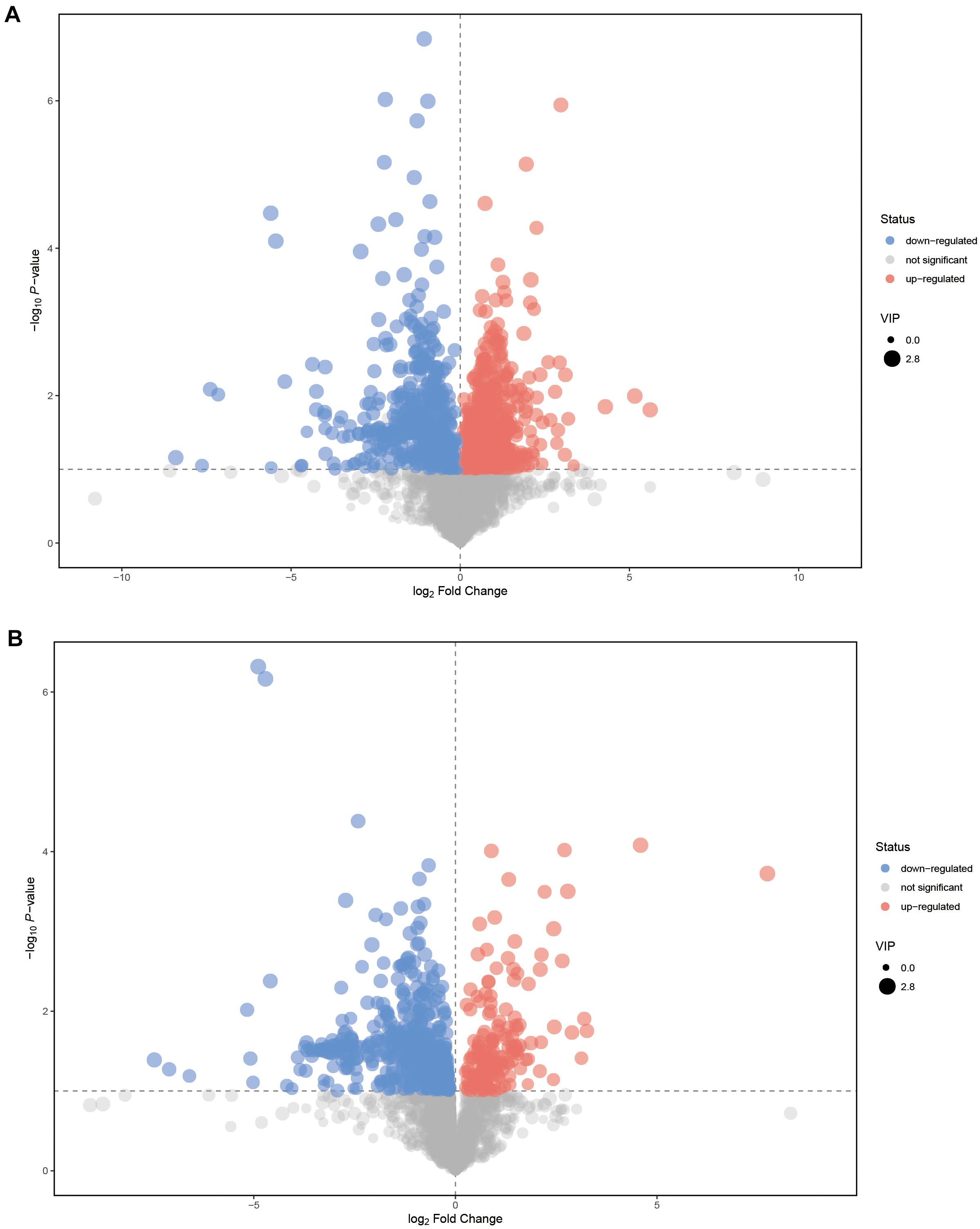
Figure 2. Volcano plots for groups EG2 and EG5. (A,B) were derived from POS and NEG, respectively. In the volcano plot, each point represents a metabolite, and the point size represents the VIP value of this metabolite in the OPLS–DA model. Red, blue, and gray indicate a significantly up-regulated metabolite in EG2, the opposite, and no significant difference between the two groups, respectively.
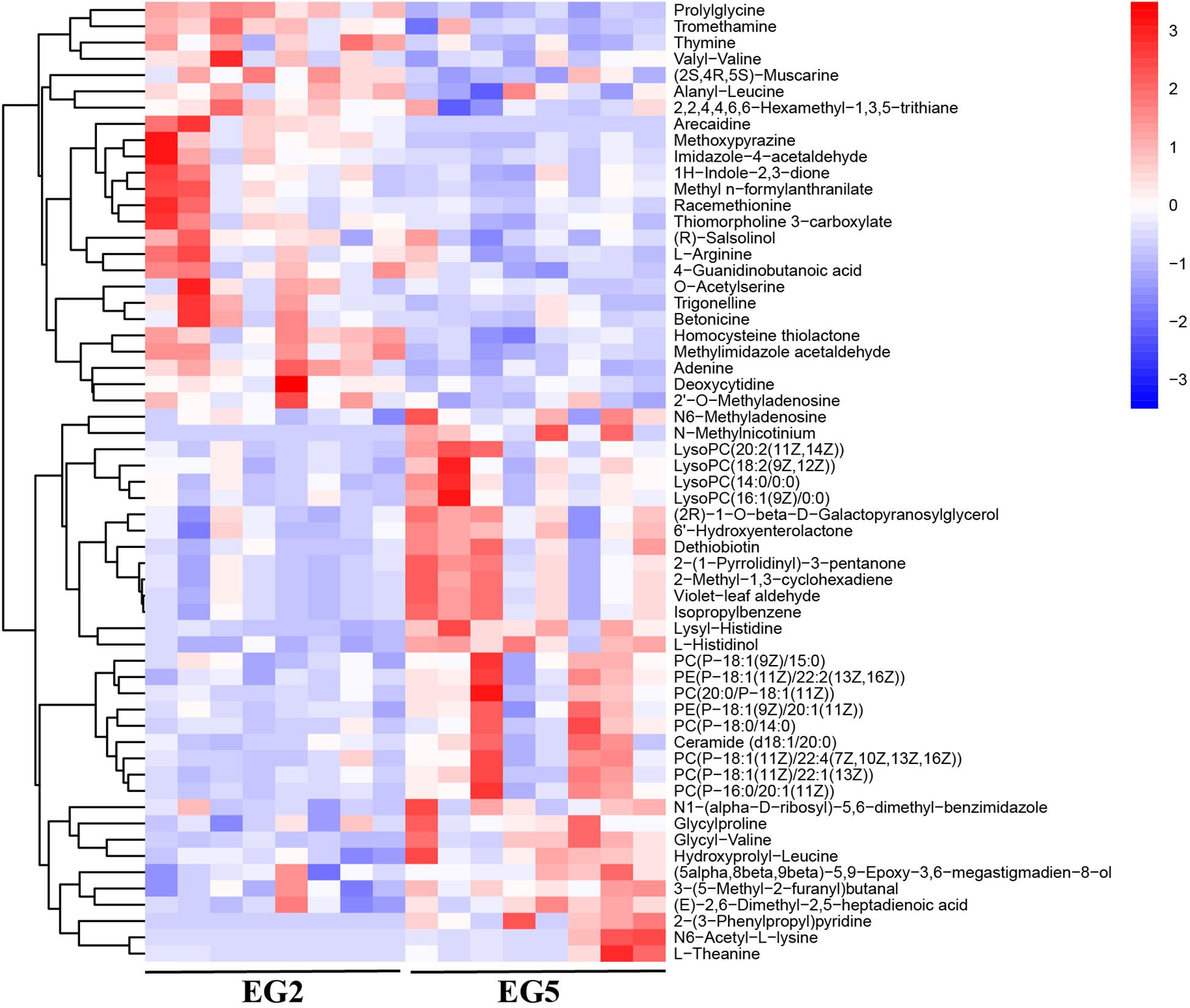
Figure 3. Hierarchical clustering analysis on SDMs from POS. The relative metabolite level is depicted according to the color scale. Red and blue indicate upregulation and downregulation, respectively.
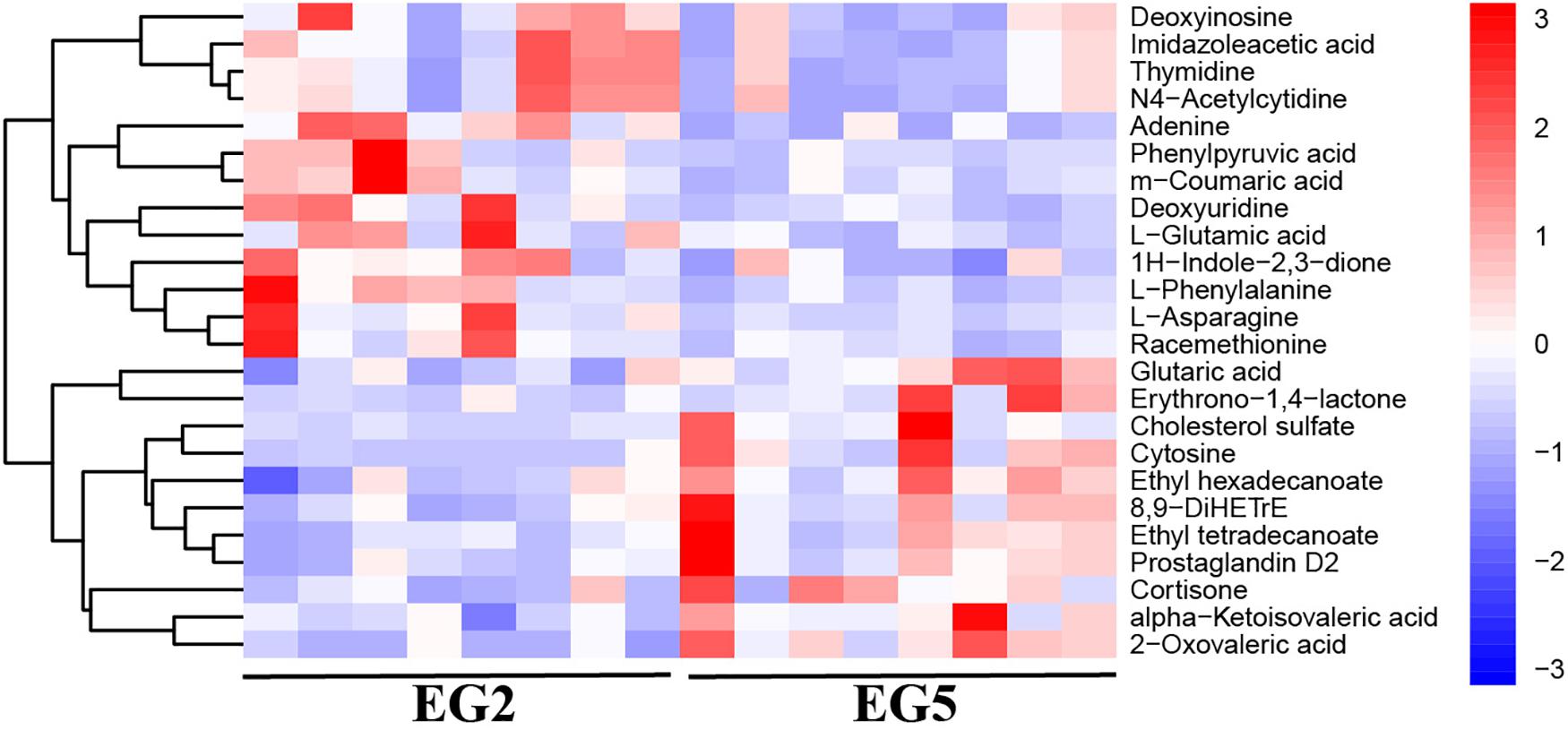
Figure 4. Hierarchical clustering analysis on SDMs from NEG. The relative metabolite level is depicted according to the color scale. Red and blue indicate upregulation and downregulation, respectively.
To explore potential metabolic pathways, the SDMs were imported into MetaboAnalyst 4.0. 22 metabolic pathways were identified between EG2 and EG5 (POS: 9; NEG: 13; Figure 5 and Supplementary Table 4). The metabolic pathways in POS between EG2 and EG5 identified were histidine metabolism, sulfur metabolism, riboflavin metabolism, selenoamino acid metabolism, cysteine and methionine metabolism, proline and arginine metabolism, pyrimidine metabolism, purine metabolism, and aminoacyl-tRNA biosynthesis. The relevant metabolic pathways in NEG between EG2 and EG5 identified were tyrosine, phenylalanine, and tryptophan biosynthesis; phenylalanine metabolism; D-glutamate and D-glutamine metabolism; aspartate, alanine, and glutamate metabolism; proline and arginine metabolism; and aminoacyl-tRNA biosynthesis.
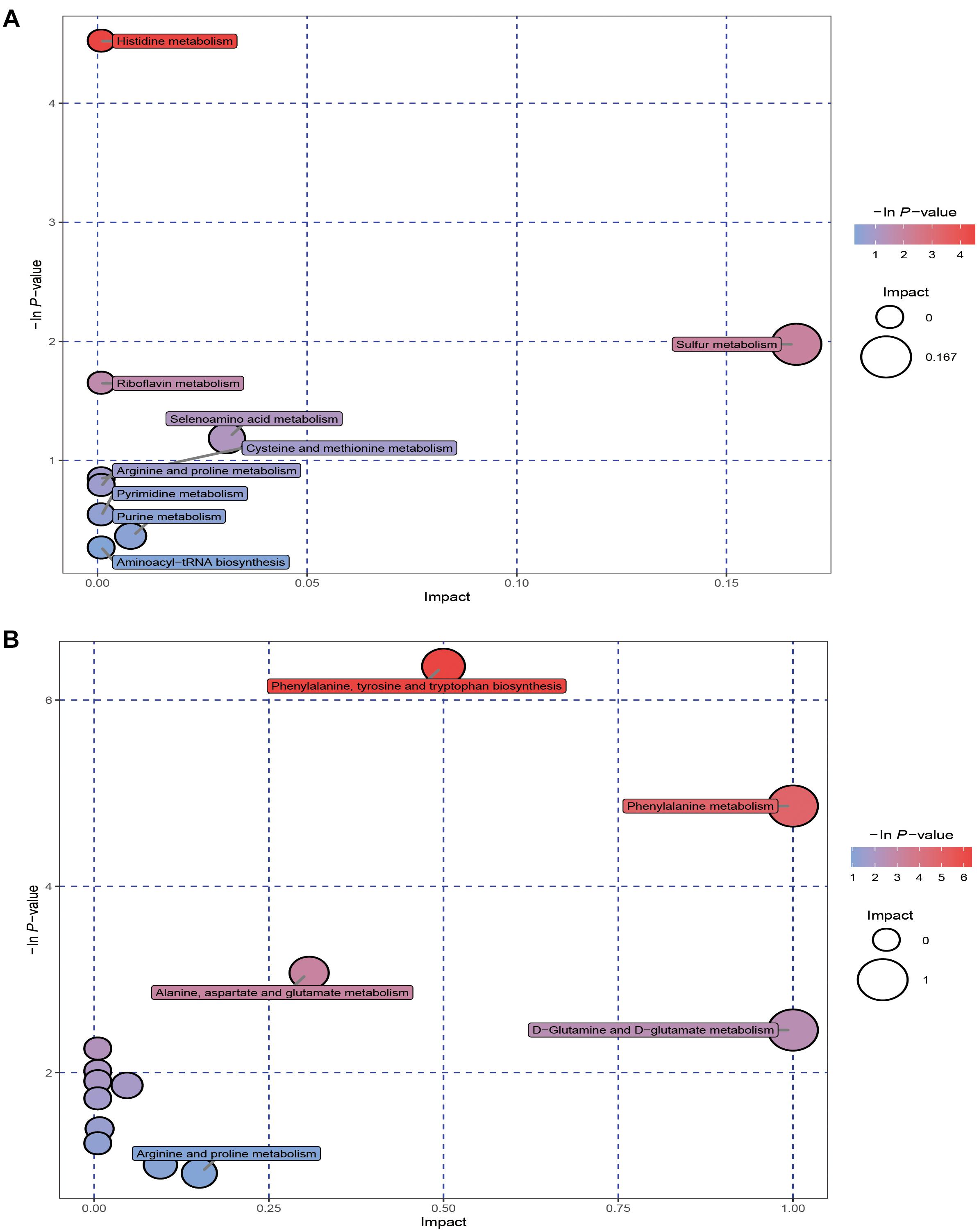
Figure 5. Metabolome view map of significant metabolic pathways characterized in the coelomic fluid of S. nudus for groups EG2 and EG5. (A,B) are derived from POS and NEG, respectively. Significantly changed pathways based on enrichment and topology analysis are shown. The x- and y-axes represent pathway enrichment and pathway impact, respectively. Large sizes and dark colors represent major pathway enrichment and high pathway impact values, respectively.
The WGR and SGR of juvenile S. nudus increased as the level of dietary carbohydrates increased from 21.50 to 34.97% and decreasing dietary protein level from 34.07 to 24.02%. The WGR and SGR decreased with further elevation in carbohydrate level from 34.97 to 39.46% and reducing dietary protein level from 24.02 to 20.67%. Suboptimal amounts of dietary carbohydrate and protein result in the poor growth of aquatic animals (Xia et al., 2015; Guy et al., 2018; Surintorn et al., 2018; Yang et al., 2019b). Moreover, juvenile peanut worm fed with D2 (34.97% carbohydrates, 24.02% protein) got the highest survival rate. Thus, D2 was considered the most optimal diet.
Characterizing the effects of different levels of carbohydrates in the diet and exploring the mechanism underlying variation in the responses of organisms to differences in diets is essential for optimizing the diets and growth rates of aquacultural species. Metabolomics approaches are useful for providing an organism-scale overview of relevant pathways, as the metabolic pathways identified through the SDMs are those most sensitive to dietary or medical interventions (Yang et al., 2018a). Nutritional metabolomics research is a relatively new field in the context of aquaculture. LC–MS-based metabolomics analysis was performed to characterize differences in the metabolomic responses of juvenile S. nudus fed D2 and D5. Differences in the metabolites and changes in the coelomic fluid of S. nudus were also compared between these two groups. The key metabolic pathways were identified based on the common key metabolic pathways and pathways altered in the coelomic fluid.
Biosynthesis-related pathways, including tyrosine, phenylalanine, and tryptophan biosynthesis and aminoacyl-tRNA biosynthesis, were enriched. The content of amino acids involved in aminoacyl-tRNA biosynthesis, such as L-asparagine, L-phenylalanine, L-glutamic acid, and L-arginine, was higher in juvenile S. nudus fed D2. Amino acids can be transferred to ribosomal synthetic proteins through aminoacyl-tRNA biosynthesis (Smith and Hartman, 2015). This suggested that juvenile S. nudus got higher synthesis protein ability when fed D2 than when fed D5. Yang et al. (2020) also surmised that S. nudus exhibited higher synthesis ability with higher aminoacyl-tRNA biosynthesis ability in the breeding season. Altogether, arginine is an important amino acid that is involved in a variety of biological functions like protein synthesis, urea production, metabolism of glutamic acid and proline, and synthesis of creatine and polyamines in aquatic animals (Mai, 2011; Jin et al., 2016; Qi et al., 2021). It is a precursor for creatine and nitric oxide synthesis and serves as a potent stimulant of growth hormone suggestion that it may play an important role in anabolic processes (Zhou et al., 2012; Lin et al., 2015; Wei et al., 2021). EG2 individuals had higher arginine acid content compared with EG5 individuals, which implied that the biosynthetic capabilities of juvenile S. nudus were greater in the EG2. Therefore, the superior synthesis ability of EG2 relative to EG5 might explain the greater growth performance of juvenile S. nudus in EG2.
The oxidation of cytoplasmically produced nicotinamide adenine dinucleotide can be affected by glutamic acid. In addition, glutamic acid has an anaplerotic function in the tricarboxylic acid cycle and acts as a signaling agent between the immune system and nervous system. Glutamic acid is an essential transamination partner for glutathione synthesis, which mitigates oxidative stress (Larsson et al., 2014). The glutamic acid content was substantially higher in juvenile S. nudus when fed D2 than when fed D5, which may reflect the superior growth, immunity, and antioxidant status of juvenile S. nudus when fed D2. Glutamic acid has also been observed to be elevated in fast-growing farmed Haliotis midae (Venter et al., 2018). Based on these findings, we speculate that changes in glutamic acid may affect D-glutamate and D-glutamine metabolism, as well as aspartate, alanine, and glutamate metabolism and thus explain the poorer growth performance of juvenile S. nudus in EG5 compared with EG2. This finding was also observed in the pearl oyster Pinctada fucata martensii, which was fed diets varying in carbohydrate and protein levels (Yang et al., 2019b).
Phenylalanine is an indispensable aromatic amino acid that is the sole precursor of tyrosine and is required for normal growth and metabolic processes (Zehra and Khan, 2014). When phenylalanine enters organisms, it may follow one of three pathways: conversion to tyrosine, conversion to phenylpyruvic acid, or incorporation into cellular proteins (Vockley et al., 2014). Thus, phenylalanine is an essential amino acid for aquatic organisms that enhances growth performance and plays a role in various physiological functions (Jin et al., 2015). In this study, the phenylalanine and phenylpyruvic acid content of S. nudus was higher when fed D2 than when fed D5, as D2 had a lower phenylalanine content compared with D5 (Supplementary Table 2). Hence, the dysfunctional phenylalanine metabolism in EG5 may in part explain the poorer growth performance of juvenile S. nudus fed D5 relative to EG2 individuals, which were fed D2. However, the specific mechanism underlying these patterns requires further study.
In this study, juvenile S. nudus fed D2 (34.97% carbohydrates, 24.02% protein) had the highest survival rate, WGR, and SGR. Comparison of the metabolic status of juvenile S. nudus fed D2 and D5 using an in-house MS2 database revealed a total of 83 SDMs (POS: 59; NEG: 24) between group EG2 and EG5, which were involved in 22 metabolic pathways (POS: 9; NEG: 13), including tyrosine, phenylalanine, and tryptophan biosynthesis; phenylalanine metabolism; D-glutamate and D-glutamine metabolism; aspartate, alanine, and glutamate metabolism; proline and arginine metabolism; and aminoacyl-tRNA biosynthesis. The results of this research enhance our understanding of the effects of dietary carbohydrate levels on the growth of juvenile S. nudus.
The data presented in the study are deposited in the MetaboLights repository, accession number MTBLS2887.
CY, QW, and YD designed the research. JH, YL, and RZ conducted the research. JH, CY, and QW analyzed the data. JH, RZ, CY, QW, YL, and YD contributed to the final writing of the manuscript. All authors have read and approved the final manuscript.
This work was supported by the Science and Technology Department, Guangdong Province (Grant Numbers: 2020A04009, 163-2019-XMZC-0009-02-0059, and 2016A02 0209010) and the Guangdong Ocean University Student’s Plan for Innovation and Entrepreneurship (Grant Number: 201810566049).
Data preprocessing and annotation of metabolomics analysis were assisted by Biotree Biotech Co., Ltd. (Shanghai, China).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Metabolomics analysis was assisted by Biotree Biotech Co., Ltd. (Shanghai, China). The authors would like to thank TopEdit (www.topeditsci.com) for linguistic assistance during preparation of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.702101/full#supplementary-material
Adrianov, A. V., and Maiorova, A. S. (2010). Reproduction and development of common species of peanut worms (Sipuncula) from the sea of Japan. Russ. J. Mar. Biol. 36, 1–15. doi: 10.1134/s1063074010010013
Andersen, S. M., Assaad, H. I., Lin, G., Wang, J., Wu, G., and Aksnes, A. (2015). Metabolomic analysis of plasma and liver from surplus arginine fed Atlantic salmon. Front. Biosci. (Elit. Ed). 7, 77–89. doi: 10.2741/E718
Baumgarner, B. L., and Cooper, B. R. (2012). Evaluation of a tandem gas chromatography/time-of-flight mass spectrometry metabolomics platform as a single method to investigate the effect of starvation on whole-animal metabolism in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 215, 1627–1632. doi: 10.1242/jeb.059873
Cao, F. J., Zhong, R. Z., Yang, C. Y., Hao, R. J., Wang, Q. H., Liao, Y. S., et al. (2020). Transcriptomic analysis of differentially expressed genes in the larval settlement and metamorphosis of peanut worm Sipunculus nudus. Aquac. Rep. 18:100475. doi: 10.1016/j.aqrep.2020.100475
Cao, Y. P., Lu, X. L., Dai, Y. P., Li, Y. H., Liu, F. H., Zhou, W., et al. (2021). Proteomic analysis of body wall and coelomic fluid in Sipunculus nudus. Fish Shellfish Immunol. 111, 16–24. doi: 10.1016/j.fsi.2021.01.004
Cappello, T., Giannetto, A., Parrino, V., Maisano, M., Oliva, S., Marcoet, G. D., et al. (2018). Baseline levels of metabolites in different tissues of mussel Mytilus galloprovincialis (Bivalvia: mytilidae). Comp. Biochem. Physiol. Part D Genomics Proteomics 26, 32–39. doi: 10.1016/j.cbd.2018.03.005
Cappello, T., Maisano, M., Mauceri, A., and Fasulo, S. (2017). 1H NMR-based metabolomics investigation on the effects of petrochemical contamination in posterior adductor muscles of caged mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 142, 417–422. doi: 10.1016/j.ecoenv.2017.04.040
Cheng, K., Wagner, L., Moazzami, A. A., Gómez-Requeni, P., Schiller Vestergren, A., Brännäs, E., et al. (2016). Decontaminated fishmeal and fish oil from the Baltic Sea are promising feed sources for Arctic char (Salvelinus alpinus L.)-studies of flesh lipid quality and metabolic profile. Eur. J. Lipid Sci. Technol. 118, 862–873. doi: 10.1002/ejlt.201500247
Du, X. D., Chen, Z. A., Deng, Y. W., and Wang, Q. H. (2009). Comparative analysis of genetic diversityand population structure of Sipunculus nudusas revealed by mitochondrial COI sequences. Biochem. Genet. 47, 884–891. doi: 10.1007/s10528-009-9291-x
Du, X. D., Chen, Z. A., Deng, Y. W., Wang, Q. H., and Huang, R. L. (2008). Genetic diversity and population structure of the peanut worm (Sipunculus nudus) in southern China as inferred from mitochondrial 16S rRNA sequences. Isr. J. Aquacu. Bamid. 60, 237–242.
Guy, E. L., Li, M. H., and Allen, P. J. (2018). Effects of dietary protein levels on growth and body composition of juvenile (age-1) Black Buffalo Ictiobus niger. Aquaculture 492, 67–72. doi: 10.1016/j.aquaculture.2018.04.002
Jin, M., Zhou, Q. C., Wang, M. Q., Huo, Y. W., Huang, W. W., and Mai, K. S. (2016). Dietary arginine requirement of juvenile swimming crab, Portunus trituberculatus. Aquac. Nutr. 22, 1174–1184. doi: 10.1111/anu.12350
Jin, Y., Tian, L. X., Xie, S. W., Guo, D. Q., Yang, H. J., Liang, G. Y., et al. (2015). Interactions between dietary protein levels, growth performance, feed utilization, gene expression and metabolic products in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 437, 75–83. doi: 10.1016/j.aquaculture.2014.11.031
Larsson, T., Koppang, E. O., Espe, M., Terjesen, B. F., Krasnov, A., Moreno, H. M., et al. (2014). Fillet quality and health of atlantic salmon (Salmo salar L.) fed a diet supplemented with glutamate. Aquaculture 42, 288–295. doi: 10.1016/j.aquaculture.2014.01.034
Lin, H. Z., Tan, X. H., Zhou, C. P., Niu, J., Xia, D. M., Huang, Z., et al. (2015). Effect of dietary arginine levels on the growth performance, feed utilization, non-specific immune response and disease resistance of juvenile golden pompano Trachinotus ovatus. Aquaculture 437, 382–389. doi: 10.1016/j.aquaculture.2014.12.025
Luo, S. J., Yang, C. Y., Wang, Q. H., and Chen, Z. G. (2016). Nutrition Components Analysis and Evaluation on Four Wild Populations of Peanut Worm Sipunculus nudus. J. Guangdong Ocean Univ. 36, 25–30.
Qi, C. L., Wang, X. D., Han, F. L., Chen, X. F., Li, E. C., Zhang, M. L., et al. (2021). Dietary arginine alleviates the oxidative stress, inflammation and immunosuppression of juvenile Chinese mitten crab Eriocheir sinensis under high pH stress. Aquac. Rep. 19:100619. doi: 10.1016/j.aqrep.2021.100619
Shen, X. R., Jiang, D. W., Jia, F. X., and Chen, M. H. (2004). Study on anti-senescence effect of Sipunculus nudus preparation. Chin. J. Mar. Drugs 1, 30–32.
Smith, T. F., and Hartman, H. (2015). The evolution of Class II Aminoacyl-tRNA synthetases and the first code. FEBS Lett. 589, 3499–3507. doi: 10.1016/j.febslet.2015.10.006
Surintorn, B., Araya, J., Suksan, K., Elisabeth, P. J., Vincent, V., Christine, B., et al. (2018). Adaptation of nile tilapia (Oreochromis niloticus) to different levels of dietary carbohydrates: new insights from a long term nutritional study. Aquaculture 496, 58–65. doi: 10.1016/j.aquaculture.2018.07.011
Tuffnail, W., Mills, G. A., Cary, P., and Greenwood, R. (2009). An environmental, 1H NMR metabolomic study of the exposure of the marine mussel, Mytilus edulis, to atrazine, lindane, hypoxia and starvation. Metabolomics 5, 33–43. doi: 10.1007/s11306-008-0143-1
Venter, L., Vosloo, A., Loots, D. T., Mienie, L. J., Rensburg, P. J. J. V., and Lindeque, J. Z. (2018). Characterising the metabolic differences related to growth variation in farmed Haliotis midae. Aquaculture 493, 144–152. doi: 10.1016/j.aquaculture.2018.04.052
Vockley, J., Andersson, H. C., Antshel, K. M., Braverman, N. E., Burton, B. K., Frazier, D. M., et al. (2014). Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. 16, 188–200. doi: 10.1038/gim.2013.157
Wagner, L., Trattner, S., Pickova, J., Gómez-Requeni, P., and Moazzami, A. A. (2014). 1H NMR-based metabolomics studies on the effect of sesamin in Atlantic salmon (Salmo salar). Food Chem. 147, 98–105. doi: 10.1016/j.foodchem.2013.09.128
Wang, Q. H., Du, X. D., Huang, H. Y., and Qin, H. G. (2005). Development of germ cells and reproductive cycle of Sipunculus nudus L in Zhanjiang. J. Zhanjiang Ocean Univ. 25, 5–9.
Wang, Q. H., Yang, C. Y., Du, X. D., Liu, X. W., Sun, R. J., and Deng, Y. W. (2016). Growth performance and biochemical composition of juvenile pearl oyster Pinctada martensii fed on artificial diets. Aquac. Int. 24, 995–1005. doi: 10.1007/s10499-015-9966-8
Wei, Y. L., Zhang, Q. G., Jia, L. L., Xu, H. G., and Liang, M. Q. (2021). Effects of dietary arginine levels on growth, intestinal peptide and amino acid transporters, and gene expressions of the TOR signaling pathway in tiger puffer, Takifugu rubripes. Aquaculture 532:736086. doi: 10.1016/j.aquaculture.2020.736086
Xia, B., Gao, Q. F., Wang, J., Li, P., Zhang, L., and Zhang, Z. (2015). Effects of dietary carbohydrate level on growth, biochemical composition and glucose metabolism of juvenile sea cucumber Apostichopus japonicus (Selenka). Aquaculture 448, 63–70. doi: 10.1016/j.aquaculture.2015.05.038
Xian, X. J., and Huang, R. L. (2011). Electropherograms analysis and determination of soluble proteins in the coelomic fluid of Sipunculus nudus. Guangdong Agricu. Sci. 1, 144–145.
Xie, D. Z., Yang, L. P., Yu, R. M., Chen, F., Lu, R. H., Qin, C. B., et al. (2017). Effects of dietary carbohydrate and lipid levels on growth and hepatic lipid deposition of juvenile tilapia, Oreochromis niloticus. Aquaculture 479, 696–703. doi: 10.1016/j.aquaculture.2017.07.013
Xu, M. Z., Zhang, Q., Tong, W. P., Dong, L. F., Tong, T., Cheng, G. P., et al. (2013). Effects of dietary carbohydrate level on growth, body composition and digestive enzymes activities of juvenile peanut worm, Sipunculus nudus. Chin. J. Anim. Nutr. 25, 534–542.
Yang, C. Y., Du, X. D., Hao, R. J., Wang, Q. H., Deng, Y. W., and Sun, R. J. (2019a). Effect of vitamin D3 on immunity and antioxidant capacity of pearl oyster Pinctada fucata martensii after transplantation: insights from LC–MS-based metabolomics analysis. Fish. Shellfish Immunol. 94, 271–279. doi: 10.1016/j.fsi.2019.09.017
Yang, C. Y., Hao, R. J., Du, X. D., Wang, Q. H., Deng, Y. W., and Sun, R. J. (2019b). Response to different dietary carbohydrate and protein levels of pearl oysters (Pinctada fucata martensii) as revealed by GC-TOF/MS-based metabolomics. Sci. Total Environ. 650, 2614–2623. doi: 10.1016/j.scitotenv.2018.10.023
Yang, C. Y., Hao, R. J., Du, X. D., Wang, Q. H., Deng, Y. W., Sun, R. J., et al. (2018b). GC–TOF/MS-based metabolomics studies on the effect of protein sources in formulated diet for pearl oyster Pinctada fucata martensii. Aquaculture 486, 139–147. doi: 10.1016/j.aquaculture.2017.12.020
Yang, C. Y., Hao, R. J., Du, X. D., Deng, Y. W., Sun, R. J., and Wang, Q. H. (2018a). Metabolomics responses of pearl oysters (Pinctada fucata martensii) fed a formulated diet indoors and cultured with natural diet outdoors. Front. Physiol. 9:944. doi: 10.3389/fphys.2018.00944
Yang, C. Y., Zeng, Y. T., Liao, Y. S., Deng, Y. W., Du, X. D., and Wang, Q. H. (2021). Integrated GC–MS- and LC–MS-Based Untargeted Metabolomics Studies of the Effect of Vitamin D3 on Pearl Production Traits in Pearl Oyster Pinctada fucata martensii. Front. Mol. Biosci. 8:614404. doi: 10.3389/fmolb.2021.614404
Yang, C. Y., Zhang, J. W., Zhong, R. Z., Guo, Z. C., Wang, Q. H., and Zheng, Z. (2020). Characterization of metabolomic differences in peanut worm Sipunculus nudus between breeding and nonbreeding seasons. Aquac. Rep. 16:100271. doi: 10.1016/j.aqrep.2019.100271
Zehra, S., and Khan, M. A. (2014). Dietary phenylalanine requirement and tyrosine replacement value for phenylalanine for fingerling Catla catla (hamilton). Aquaculture 433, 256–265. doi: 10.1016/j.aquaculture.2014.06.023
Zhang, J. B., Xiong, X. W., Deng, Y. W., Zheng, Z., and Du, X. D. (2021). Integrated application of transcriptomics and metabolomics provides insights into the larval metamorphosis of pearl oyster (Pinctada fucata martensii). Aquaculture 532:736067. doi: 10.1016/j.aquaculture.2020.736067
Zhang, J. W., Su, Y. L., Zhong, R. Z., Wang, Q. H., Jiao, Y., and Yang, C. Y. (2021). Transcriptome analysis reveals key molecular events of germinal vesicle breakdown in Sipunculus nudus. Aquac. Res. 52, 1757–1766. doi: 10.1111/are.15031
Zhang, J. W., Yang, C. Y., Wang, Q. H., and Guo, Y. S. (2018). Analysis of Fatty Acid and Food Composition in Four Populations of Sipunculus nudus. J. Guangdong Ocean Univ. 38, 1–7.
Zhang, Q., Tong, W. P., Dong, L. F., Jiang, Y., and Tong, T. (2011). Effects of dietary lipid levels on growth performance, body composition and digestive enzyme activities of juvenile peanut worm, Sipunculus nudus Linnaeus. Progress Fish. Sci. 32, 99–106.
Zhang, Q., Tong, W. P., Dong, L. F., Jiang, Y., and Tong, T. (2012). Effects of dietary protein level on growth performance and body composition of juvenile peanut worm, Sipunculus nudus Linnaeus. Progress Fish. Sci. 33, 86–92.
Keywords: Sipunculus nudus, growth performance, metabolomics, LC–MS, carbohydrate, protein
Citation: Huang J, Zhong R, Yang C, Wang Q, Liao Y and Deng Y (2021) Dietary Carbohydrate and Protein Levels Affect the Growth Performance of Juvenile Peanut Worm (Sipunculus nudus): An LC–MS-Based Metabolomics Study. Front. Mar. Sci. 8:702101. doi: 10.3389/fmars.2021.702101
Received: 29 April 2021; Accepted: 21 June 2021;
Published: 26 July 2021.
Edited by:
Xiaotong Wang, Ludong University, ChinaReviewed by:
Chenglong Ji, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences (CAS), ChinaCopyright © 2021 Huang, Zhong, Yang, Wang, Liao and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuangye Yang, eWFuZ2N5QGdkb3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.