- 1Smithsonian Marine Station, Fort Pierce, FL, United States
- 2Department of Biology and Marine Biology, University of North Carolina Wilmington, Wilmington, NC, United States
- 3Mote Marine Laboratory, Elizabeth Moore International Center for Coral Reef Research and Restoration, Summerland Key, FL, United States
Stony coral tissue loss disease (SCTLD) is affecting corals across the Western Atlantic and displays species-specific and regional differences in prevalence, incidence, degree of mortality, and lesion morphology. We examined two Florida sites with different temporal histories of disease emergence; Fort Lauderdale where SCTLD is endemic and the Lower Florida Keys where SCTLD has recently emerged. Our objectives were to (1) assess the potential impact of SCTLD on overall reef condition by surveying reefs in each region, (2) in a single common species, Montastraea cavernosa, examine differences in SCTLD prevalence, colony mortality, and lesion morphology in each region, and (3) look for differences in contagion by conducting transmission experiments using lesions from each region. Reef surveys found sites in both regions had low coral cover, high algae cover, and similar coral species composition. SCTLD prevalence was higher in the Lower Keys than at Fort Lauderdale and two of the common species, M. cavernosa and S. siderea at Fort Lauderdale were dominated by smaller colonies (<5 cm) whereas larger colonies occurred in the Lower Keys. Tagged M. cavernosa SCTLD-affected colonies were followed for 2 years at one site in each region. In both years, Fort Lauderdale colonies showed declining disease prevalence, low colony mortality, and disease lesions were mainly bleached spots lacking tissue loss. In contrast, Lower Keys colonies tagged in the first year maintained 100% disease prevalence with high mortality, and disease lesions were predominantly tissue loss with no bleached edges. However, SCTLD dynamics changed, with year two tagged colonies showing declining disease prevalence, low mortality, and lesion morphology switched to a mixture of bleached polyps and tissue loss with or without bleached edges. Lesion morphology on colonies was a significant predictor of amount of tissue loss. Aquaria studies found the rate of SCTLD transmission using lesions from the different zones (emergent and endemic) were similar. Our study highlights that differences in coral mortality from SCTLD are not necessarily linked to host species, lesion morphology is reflective of subsequent rate of mortality, and disease dynamics change through time on reefs where the disease has newly emerged.
Introduction
The Florida Reef Tract (FRT) is currently experiencing the most widespread, virulent, and longest running coral disease outbreak in recent history. The disease, termed stony coral tissue loss disease (SCTLD), affects over 24 species of hard corals and has resulted in extensive colony mortality (Precht et al., 2016; Walton et al., 2018; Aeby et al., 2019; Sharp et al., 2020). A case definition of the disease has been developed describing the visual appearance and histology of SCTLD (Landsberg et al., 2020). Lesions are described as focal or multifocal areas of acute or subacute progressive tissue loss. Similar lesions have been found on corals throughout the world and are usually termed “white syndromes” (Bourne et al., 2014). However, SCTLD differs at the cellular level from other tissue loss diseases in that lesions consistently have lytic necrosis originating in the gastrodermis of the basal body wall (Landsberg et al., 2020). This points to the fact that most coral diseases cannot be accurately diagnosed in the field and supports the utility of describing coral disease histologically (Work and Meteyer, 2014). The disease was first reported off the coast of Miami-Dade County in southeast Florida in 2014 (Precht et al., 2016) and has spread throughout the FRT spreading to reefs beyond Key West (Walton et al., 2018; Aeby et al., 2019; Muller et al., 2020; Sharp et al., 2020). Unfortunately, the disease has also spread to the reefs of the Caribbean including Mexico, Jamaica, Belize, the Dominican Republic, St. Maarten, the United States Virgin Islands, the Turks and Caicos Islands, and Sint Eustatius (Alvarez-Filip et al., 2019; Kramer et al., 2019; Meiling et al., 2020; Heres et al., 2021; Thome et al., 2021).
The etiology of SCTLD is still unknown but the infectious nature of the disease has been observed in field studies (Muller et al., 2020; Sharp et al., 2020) as well as documented in aquaria studies showing the disease is transmissible though direct contact as well as being waterborne (Aeby et al., 2019; Meiling et al., 2020). Epidemiological models show that progression of SCTLD along the FRT was also consistent with waterborne transmission and was positively associated with high coral diversity (Muller et al., 2020). Application of antibiotics to affected coral colonies slows or stops disease progression both in the field (Neely et al., 2020; Shilling et al., 2021; Walker et al., 2021) and in the laboratory (Aeby et al., 2019) implicating the involvement of bacterial pathogens in the disease process.
Stony coral tissue loss disease affects many coral species and there are pronounced species-specific as well as regional differences in disease prevalence, incidence and degree of mortality (Precht et al., 2016; Aeby et al., 2019; Estrada-Saldivar et al., 2020; Meiling et al., 2020; Muller et al., 2020; Sharp et al., 2020; Heres et al., 2021). Disease signs also vary within and among affected coral species with differences in rates of tissue loss (acute and subacute) and lesion morphology (with and without bleached borders). Aeby et al. (2019) reported high mortality in corals with SCTLD on a reef in the middle Keys but low mortality on corals on a reef off the coast of Fort Lauderdale. Lesion morphology also differed with acute tissue loss lesions in the middle Keys but subacute tissue loss lesions with a bleached border in Fort Lauderdale. However, there was no overlap among coral species between the two regions and so they suggested that differences in disease signs could be due to variability in host response, different stages of the disease or differences in environmental conditions between regions that might be affecting host-pathogen dynamics. Sharp et al. (2020) followed the emergence of SCTLD on reefs in the middle Keys and also initially reported high mortality but found mortality decreased through time. It could be that there are species, regional and/or temporal differences that are impacting the dynamics of SCTLD.
Baseline disease surveys in many regions, including Florida, show that coral populations on reefs commonly host a number of endemic diseases that usually occur at comparatively low prevalence (Weil et al., 2000; Santavy et al., 2001, 2005; Aeby, 2006; Aeby et al., 2011). However, endemic diseases can occur at outbreak levels or novel pathogens can enter the system following a disruption of the host-pathogen relationship with environmental degradation from human impacts suggested as a major factor (Daszak et al., 2000; Dobson and Foufopoulos, 2001; Harvell et al., 2007). SCTLD was first reported in 2014 during two concurrent stresses: a summer bleaching event that occurred across the FRT in 2014 and 2015 (Manzello, 2015; Walton et al., 2018) and dredging operations between 2013 and 2015 in the channel at Port of Miami, FL, that resulted in massive sedimentation near the initial site of the outbreak (Miller et al., 2016). Usually disease outbreaks are localized occurring on a limited number of reefs or regions and are ephemeral in nature. For example, Aeby et al. (2016) documented the emergence of a novel tissue loss disease (acute Montipora white syndrome) on the reefs of Kaneohe Bay, Oahu during the winter but noted that the disease had subsided within a number of months. Similarly, outbreaks of tissue loss disease have occurred numerous times on Caribbean reefs but were spatially limited and ephemeral in nature (Bruckner, 2016). In contrast, SCTLD has been spreading from its epicenter for the past 7 years from 2014 to date and continues to persist on reefs even after the initial outbreak has subsided (Walton et al., 2018; Aeby et al., 2019; Heres et al., 2021). This creates the opportunity to examine SCTLD dynamics on reefs where SCTLD has already swept through the coral populations, usually with significant mortality on susceptible coral species (Precht et al., 2016; Sharp et al., 2020; Williams et al., 2021), but remains at endemic levels as compared to areas where SCTLD is just emerging.
We examined two sites with different temporal histories of disease emergence; Fort Lauderdale where SCTLD emerged in 2014–2015 and is still present (endemic zone) and in the Lower Florida Keys where SCTLD recently emerged in 2018 (emergent zone). Our objectives were to (1) assess the potential impact of SCTLD on overall reef condition by surveying reefs in each zone, (2) in a single species, Montastraea cavernosa, examine differences in SCTLD prevalence, colony mortality, and lesion morphology in each zone over 2 years, and (3) look for differences in contagion by conducting transmission experiments using lesions from each zone.
Materials and Methods
Study Sites
The Fort Lauderdale site was located in southeast Florida, offshore of Broward County. The reef system in this region is a northern continuation of the FRT. The site (26.14859°N, 80.09592°W) is located approximated 0.5 km from shore at approximately 8 m in depth. The Fort Lauderdale site was used for previous studies on tagged colonies with SCTLD (Aeby et al., 2019). The other site was in the lower Florida Keys located within the Looe Key Existing Management Area1. The study site (24.54599°N, 81.40400°W) was approximately 13 km from Summerland Key at approximately 7 m in depth. Looe Key is a popular dive site within the Florida Keys National Marine Sanctuary which is visited by hundreds of thousands of snorkelers and divers each year2. The sites (Fort Lauderdale, Lower Florida Keys) are approximately 217 km from each other.
Reef Surveys
To compare coral reef conditions in each zone, surveys were conducted in November 2019 at the same sites as our tagged colonies. A 30 m tape measure was laid on the reef and all colonies within 1 m on each side of the tape were identified to species, enumerated and assigned to a size class (<5 cm, 5–10 cm, 11–20 cm, 21–40 cm, 41–80 cm, and >80 cm). These are standard size classes that have been used successfully in numerous other studies (Kenyon et al., 2008, 2010). Benthic cover [hard coral, soft coral, algae (turf and macroalgae), crustose coralline algae (CCA)] was documented via point-intercept whereby the substrate underlying the tape measure was recorded at 50 cm intervals. Belt transects covered 60 m2 (30 m × 2 m) in Fort Lauderdale and 35 m2 (17.5 m × 2 m) in the Lower Keys. Looe Key is an offshore reef with spur and groove formation on the shallow fore reef and the spur we surveyed only extended 17.5 m along its axis. Disease surveys were conducted along the same transect lines but with wider belts with 105 m2 (17.5 m × 6 m) surveyed in the Lower Keys and 240 m2 (30 m × 8 m) surveyed in Fort Lauderdale. Belt width for disease surveys was dependent on colony densities with lower density sites requiring a wider belt. All colonies with signs of disease were described and photographed.
Disease Virulence and Prevalence of Tagged Colonies Through Time on Reefs in the Ft Lauderdale Region vs. the Lower Keys
To determine rates of in situ tissue loss, we tagged and tracked individual M. cavernosa colonies displaying signs of SCTLD on a reef in Fort Lauderdale and a reef in the Lower Keys. Colonies in the Fort Lauderdale region were first tagged and mapped in 2017 or 2018 and any tagged colonies with signs of SCTLD in November 2018 were chosen for inclusion in this study (n = 23). Seventeen M. cavernosa colonies with SCTLD were tagged and mapped in the Lower Keys region on November 7, 2018. The first year of the study was conducted between November 2018 and August 2019. The second year of the study ran from November 2019 to July 2020. In the second year, an additional 14 M. cavernosa with SCTLD were tagged in the Lower Keys due to high mortality within the first set of tagged colonies.
Colonies were resurveyed and photographed approximately every other month. The three-dimensional structure of the coral colonies and the presence of widespread multi-focal lesions complicated the use of digital image analysis for calculating the rates of tissue loss. Instead, a semi-quantitative estimate of tissue loss was used whereby visual estimates of colony health (proportion of colony healthy, old dead, or with recent tissue loss attributed to disease) and lesion morphology (bleached polyps, tissue loss bordered by bleached polyps, and tissue loss with no obvious bleached border) were assessed from photo review. Lesions were considered “recent tissue loss” based on the presence of bare, white skeleton and “old dead” were tissue loss areas covered in sediment, algae or other organisms. The rate of tissue loss was determined by visually estimating the proportion of overall loss for each colony between each survey time period. Degree of colony morbidity (proportion of tissue loss) and disease prevalence (number of tagged colonies with lesions/total number live tagged colonies) were followed through time, and case fatality rate (number colonies with complete tissue loss/total number colonies) was calculated for the study periods. Rates of tissue loss (% tissue loss/day) were compared between years within regions, and between regions for each of the 2 years of the study. Data were not normally distributed and sample sizes were unequal between regions, so a non-parametric Wilcoxon two-sample test was used to examine differences in rates of tissue loss.
Lesion morphologies were recorded at the beginning of the study for each tagged colony (tissue loss with or without bleached edges or bleached polyps or edges). The proportion of total tagged colonies displaying distinct lesion morphologies was examined between regions using a two-sided Fisher Exact Test. The association between initial lesion type and disease pathogenesis (outcome) by the end of the study was examined using a multinomial regression analysis. Disease outcome (remained diseased or died, lost disease lesions and remained lesion-free, or lesions waxed and waned) was the response variable and region (Fort Lauderdale, Lower Keys) and initial lesion morphology were the predictors. Rates of tissue loss (% tissue loss/day) were compared among lesion types using a non-parametric Wilcoxon two-sample test. All statistics were completed using JMP® Pro 15.2.
Transmission Rate of SCTLD Using Disease Lesions From Fort Lauderdale vs. Lower Keys
We hypothesized that lesions collected from the emergent zone (Lower Keys) would transmit disease more quickly compared to lesions from the endemic zone (Fort Lauderdale). To test this, aquaria studies were set up to determine whether the rate of SCTLD transmission differed when using diseased M. cavernosa from Fort Lauderdale vs. the Lower Keys. Experiments were conducted May 10–25, 2019. Individual 5 L aquaria were placed in larger temperature-controlled water tables with circulating freshwater adjusted with a cooling and heating system to maintain water temperatures at summer ambient levels (28–29°C). Aquaria were filled with seawater filtered through a 0.22 μm pore filter (FSW) and a bubbler placed to create water motion. Experiments were conducted using a block design using three aquaria (2 experimental and 1 control) per block (Figure 1). Eleven blocks were tested. For the two experimental aquaria, one contained a fragment of M. cavernosa with SCTLD collected from the Fort Lauderdale and the other contained a fragment of M. cavernosa with SCTLD collected from the Lower Keys. In the experimental tanks, an infected fragment with a distinct tissue loss lesion was placed in direct contact with a healthy fragment of M. cavernosa (direct transmission). The same set up was used in control aquaria, except the diseased fragment was replaced with a healthy fragment of M. cavernosa to control for lesions created by coral-to-coral aggressive interactions. The apparently healthy fragments used in transmission studies, within each block, were genetically identical (a larger fragment was cut into three approximately equal size pieces). Partial water changes (∼2 L) were conducted daily to maintain water quality and coral fragments were photographed and examined for development of new tissue loss lesions. Fragments remained in contact until the end of the study (maximum 14 days) or until development of a lesion. To discriminate between lesions caused by aggression vs. a transmissible disease, any corals that developed lesions in the experimental or control tanks were removed from contact but remained in the tank, and observed for signs of lesion progression or recovery until the end of the experiment. Lesions that progressed following removal from contact were considered indicative of disease transmission. Lesions that failed to progress or healed were considered indicative of coral-to-coral aggression. Experiments were conducted outdoors under natural sunlight with shade cloth covers that reduced ambient light by ∼50%. A Mantel-Cox test was used to examine regional differences in lesion contagion in the transmission trials. A Mantel-Cox test is a non-parametric test comparing the survival distributions of two groups and examines the outcome of the trial (transmission or no transmission) and the time to the outcome.
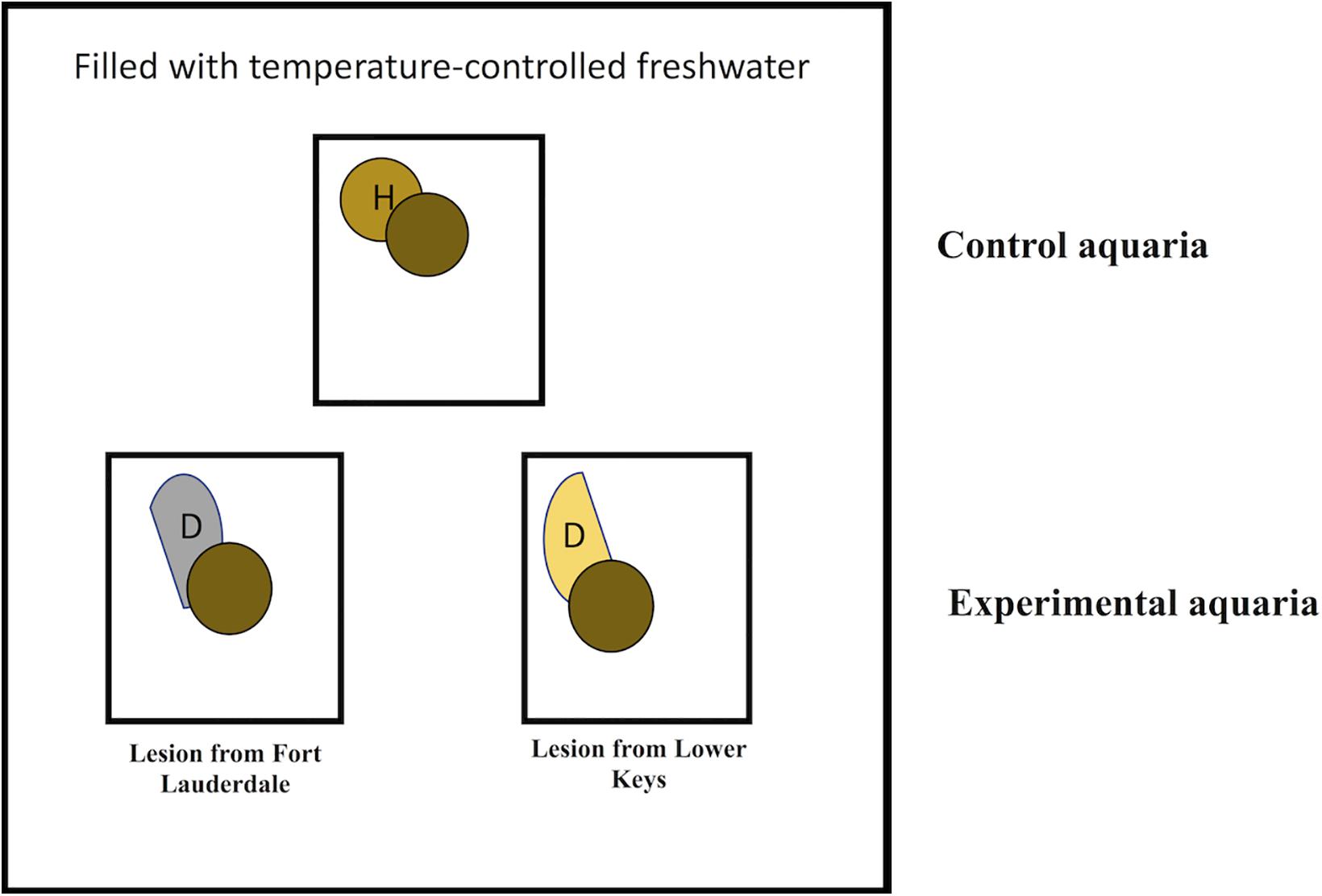
Figure 1. Schematic showing experimental design for transmission studies in aquaria contained within a larger temperature controlled water table. Circles with the same color indicate coral fragments collected from the same colony.
Results
Coral Reef Condition on Reefs in Endemic vs. Emergent Zones
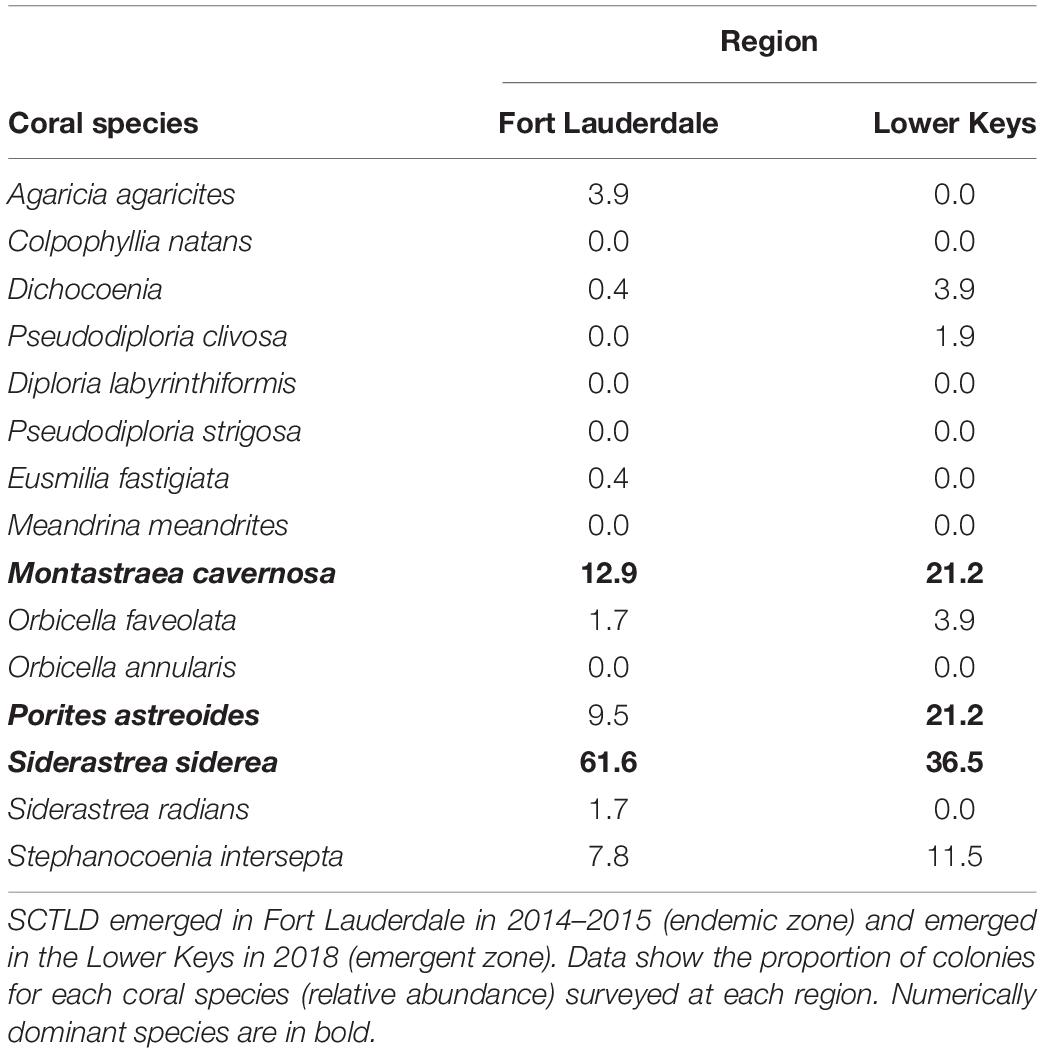
Hard coral cover was low and algae cover high at both sites. Hard coral cover was 2% and macroalgae cover 60% at Fort Lauderdale and hard coral cover was 2.9% and algae cover 65.7% in the Lower Keys. There was more soft coral cover (18%) and CCA cover (12%) at Fort Lauderdale as compared to the Lower Keys site (5.7% soft coral and 0% CCA cover). There were 232 colonies (3.9 colonies/m2) within the belt transect at Fort Lauderdale and 52 colonies (1.5 colonies/m2) in the Lower Keys. The coral community was similar between the two regions with both sites numerically dominated by M. cavernosa and Siderastrea siderea although Porites astreoides was also common at the Lower Keys site (Table 1). At Fort Lauderdale, M. cavernosa and S. siderea colonies were dominated by smaller size classes, with the majority of colonies found within transects less than 5 cm in diameter (Figure 2). Species highly susceptible to SCTLD were uncommon at both sites.
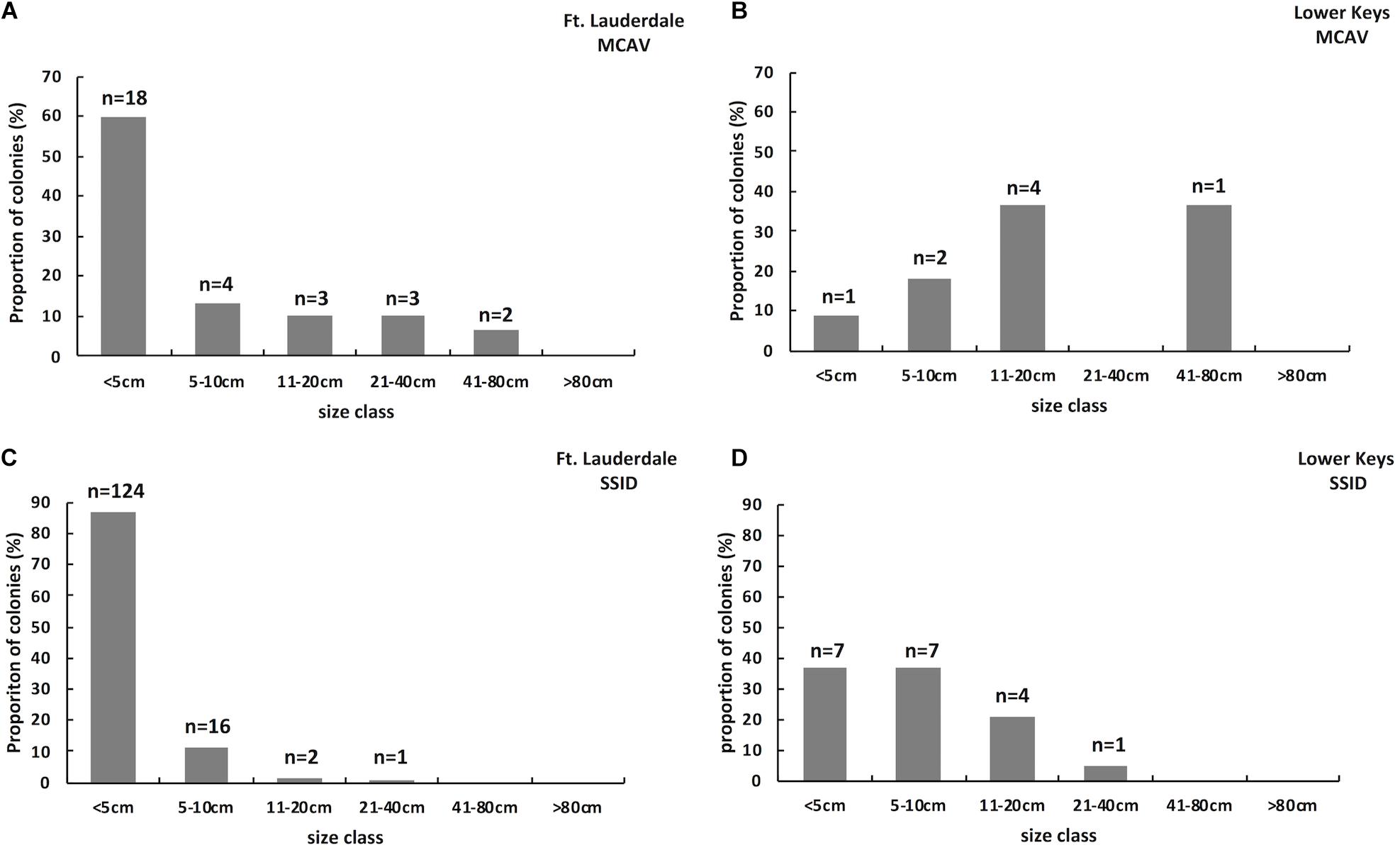
Figure 2. Size class structure of M. cavernosa on a reef (Fort Lauderdale) in an endemic SCTLD zone (n = 30; A) and on a reef (Lower Keys) in a zone where SCTLD just emerged (n = 11; B). Size class structure of S. siderea on a reef (Fort Lauderdale) in an endemic SCTLD zone (n = 143; C) and on a reef (Lower Keys) in a zone where SCTLD just emerged (n = 19; D).
At Fort Lauderdale, lesions consistent with disease were found on four coral species, M. cavernosa, S. siderea, Orbicella faveolata, and Colpophyllia natans and overall disease prevalence (all diseases) was 1.6%. All lesions were consistent with SCTLD (subacute to acute tissue loss with or without a bleached lesion) with the exception of some S. siderea colonies with bleached areas or stripes not consistent with SCTLD (Figure 3). At the Lower Keys site, two coral species had disease lesions, M. cavernosa and S. siderea, and overall disease prevalence was 9.0%. All M. cavernosa lesions were consistent with SCTLD and lesions on S. siderea were dark pigmentation with tissue loss or multi-focal chronic tissue loss which may or may not be SCTLD (Figure 4). Disease prevalence on the two dominant species, M. cavernosa and S. siderea, was also lower at Fort Lauderdale, 5 and 1.2%, respectively, as compared to 27.3 and 8.8%, respectively, in the Lower Keys.

Figure 4. Examples of lesions found on S. siderea from the Lower Keys. Lesions may or may not be indicative of SCTLD.
Disease Virulence (Harm to Host) and Prevalence of Tagged Colonies Through Time on Reefs in Fort Lauderdale vs. the Lower Keys
Disease Prevalence on Tagged Montastraea cavernosa Colonies
At the start of the study all tagged colonies in both regions had signs of disease (100% prevalence). At the Fort Lauderdale site, disease prevalence declined to 30% by August 2019 (year one; Figure 5A) and in year two, disease prevalence remained below 30% except for a spike to 57.1% in October 2020 (Figure 5B). In contrast, at the Lower Keys site disease prevalence remained 100% for the duration of year one (Figure 5C) but dropped to 0% at the end of year two (Figure 5D).
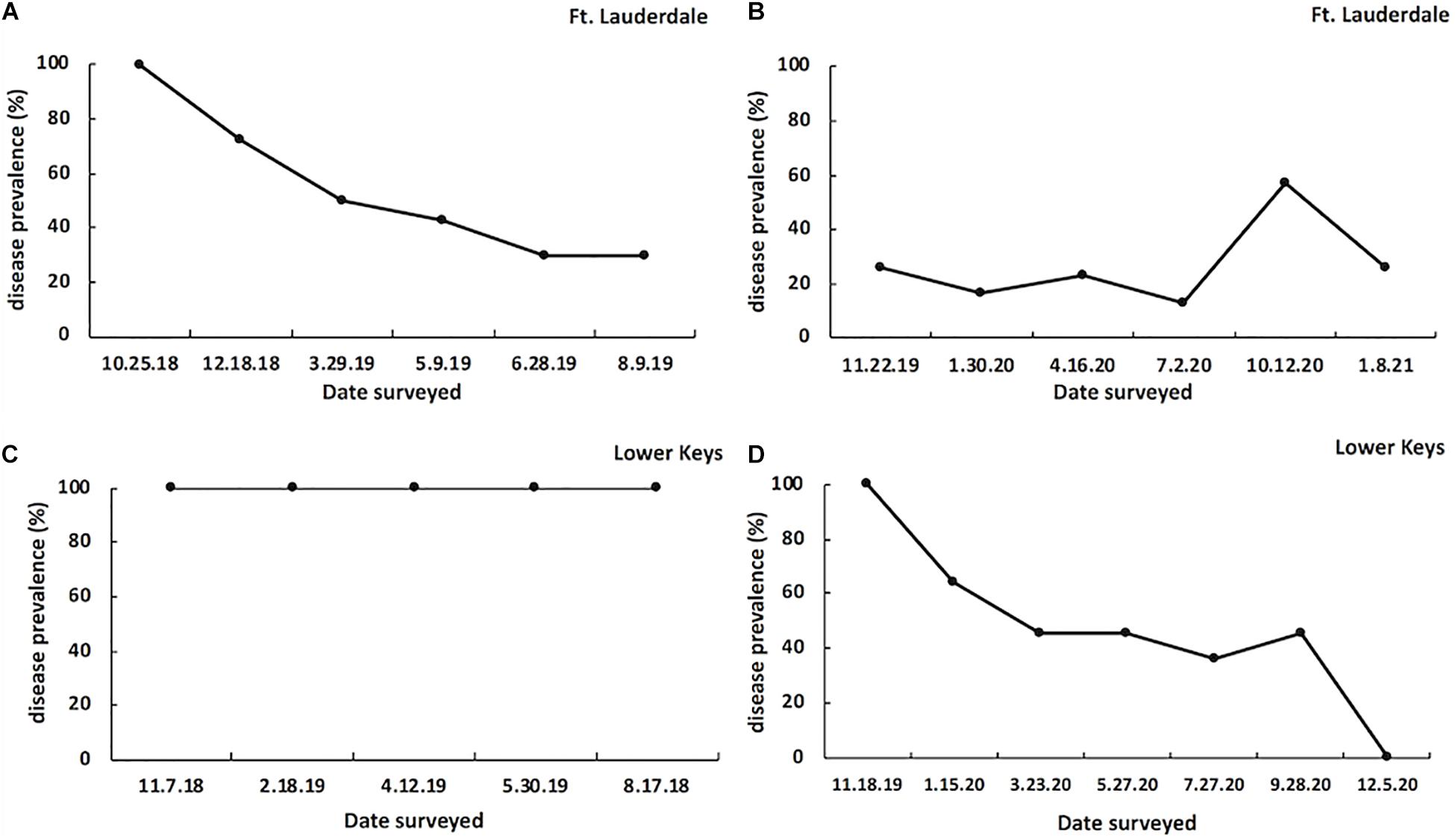
Figure 5. SCTLD prevalence on tagged M. cavernosa colonies in Fort Lauderdale during year one (n = 23; A) and year two (n = 20; B) of the study, and on tagged M. cavernosa colonies in the Lower Keys during year one (n = 17; C) and year two (n = 14; D) of the study.
Rate of Tissue Loss
At the Fort Lauderdale site, the average rate of tissue loss of tagged M. cavernosa colonies in year one was 0.14% (SE ±0.08%) per day (∼4.34%/month; n = 23) which was similar to year two with 0.03% (SE ±0.05%) per day (∼0.9%/month; n = 20; Wilcoxon two-sample test, Z = 1.25, df = 1, and P = 0.21). At the Lower Keys site, average rate of tissue loss of colonies in year one was 0.63% (SE ±0.07%) per day (∼19%/month; n = 17) which was significantly higher than in year two which averaged 0.18% (SE ±0.08%) per day (∼5.4%/month; n = 14; Wilcoxon two-sample test, Z = 3.42, df = 1, and P = 0.0006). In year one, the rate of tissue loss of tagged M. cavernosa at the Lower Keys site was significantly higher than the colonies at Fort Lauderdale in year one (Wilcoxon two-sample test, Z = −4.7, df = 1, and P = 0.0001) but in year two that regional difference was no longer significant (Wilcoxon two-sample test, Z = 0.38, df = 1, and P = 0.70).
Mortality of Tagged Colonies
Colony mortality from disease was low for both years in Fort Lauderdale with 3 of the 23 colonies dying the first year (case fatality rate = 13%) and one out of the remaining 20 colonies dead in the second year (case fatality rate = 5%). In contrast, at the Lower Keys site, 15 out of the 17 tagged colonies died the first year (case fatality rate = 88.2%) but only 3 out of 14 died the second year (case fatality rate = 21.4%).
Lesion Morphology and Disease Outcome
Three lesion morphologies were documented during the study including bleached polyps or edges (Figure 6A), chronic or subacute tissue loss bordered by bleached polyps (Figure 6B), and subacute to acute tissue loss with no distinct bleached borders (Figure 6C). We arbitrarily define chronic vs. subacute vs. acute tissue loss based on the amount of bare white skeleton along the lesion edge: chronic tissue loss (<1 cm), subacute (1–5 cm), and acute (>5 cm). Lesion morphologies within the tagged colonies differed between regions at the start of the study (Fisher exact test, P < 0.0001) with colonies at Fort Lauderdale dominated by bleached polyps or edges whereas Lower Keys colonies were dominated by tissue loss lesions with no distinct bleaching (Figure 7). In the second year of the study, the Fort Lauderdale colonies remained dominated by bleached polyps but the new colonies in the Lower Keys differed from the colonies in year one with their lesions now dominated by bleached polyps or edges and no longer differed from lesion morphologies of Fort Lauderdale colonies (Fisher exact test, P < 0.83; Figure 7).

Figure 6. Examples of the three types of lesion morphologies associated with SCTLD. (A) bleached polyps, (B) tissue loss bordered by bleached polyps, and (C) tissue loss without distinct bleached border.
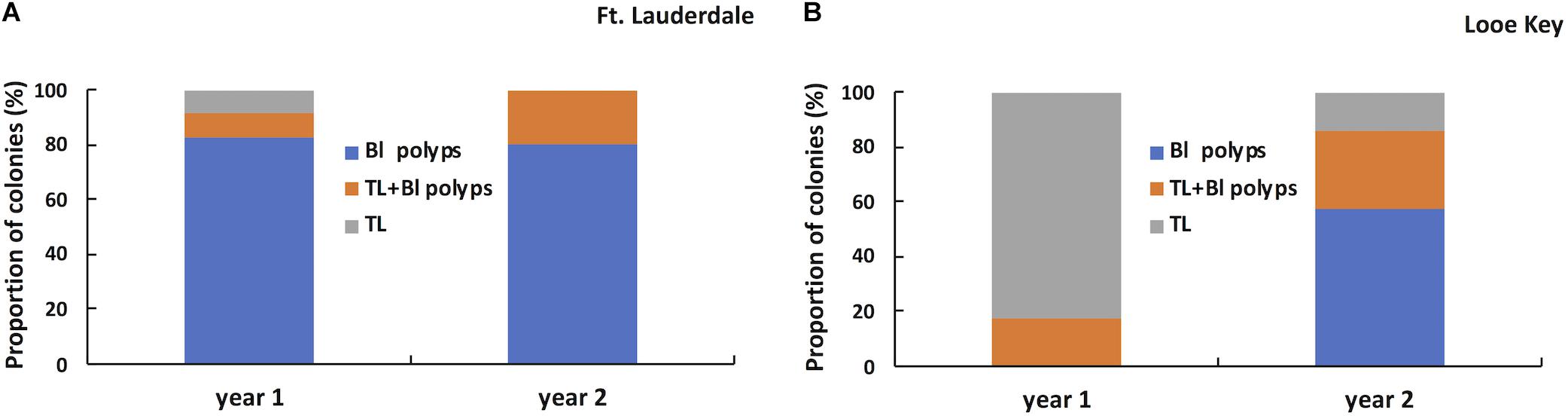
Figure 7. Proportion of tagged M. cavernosa displaying different types of lesion morphologies in year one (n = 23) and year two (n = 20) in Fort Lauderdale (A) and in year one (n = 17) and year two (n = 14) in the Lower Keys (B).
The lesion morphology (bleached polyps, tissue loss + bleached polyps, and tissue loss) of each tagged colony at the start of the study was compared to the pathogenesis of disease during the 2-year study and to the subsequent amount of tissue loss per day. Tagged M. cavernosa at Fort Lauderdale were followed for the entire 2 years of the study but the tagged M. cavernosa in the Lower Keys was split up into year one colonies (most of which died) and the newly tagged year two colonies. Each cohort of tagged colonies in the Lower Keys were followed for a year and the data combined for analysis. SCTLD pathogenesis for each tagged colony was scored as (a) colonies remained diseased throughout the study or died, (b) colonies lost disease lesions and remained lesion-free, or (c) lesions on colonies were lost but reoccurred during the study. Initial lesion morphology was a significant predictor of disease outcome (X2 = 38.85, df = 4, and P = 0.0001) and outcome was consistent between regions (X2 = 0.033, df = 2, and P = 0.98; Supplementary Table 1). Tagged M. cavernosa that had an initial lesion morphology of tissue loss with bleached border or tissue loss alone, ended up remaining diseased for the duration of the study or dying. In contrast, colonies with an initial lesion morphology of bleached spots or edges had one of three outcomes with a small percentage of the colonies remaining diseased but the most common outcomes were colonies becoming lesion-free or lesion progression waxed and waned (Figure 8). The average percent tissue loss for colonies with initial tissue loss lesions with or without bleached borders was 0.49% (SE ±0.07%) per day which was significantly higher than for colonies with bleached polyps as the initial lesion which had an average of 0.05% (SE ±0.03) per day (Wilcoxon two-sample test, Z = −5.55, df = 1, and P = 0.0001).
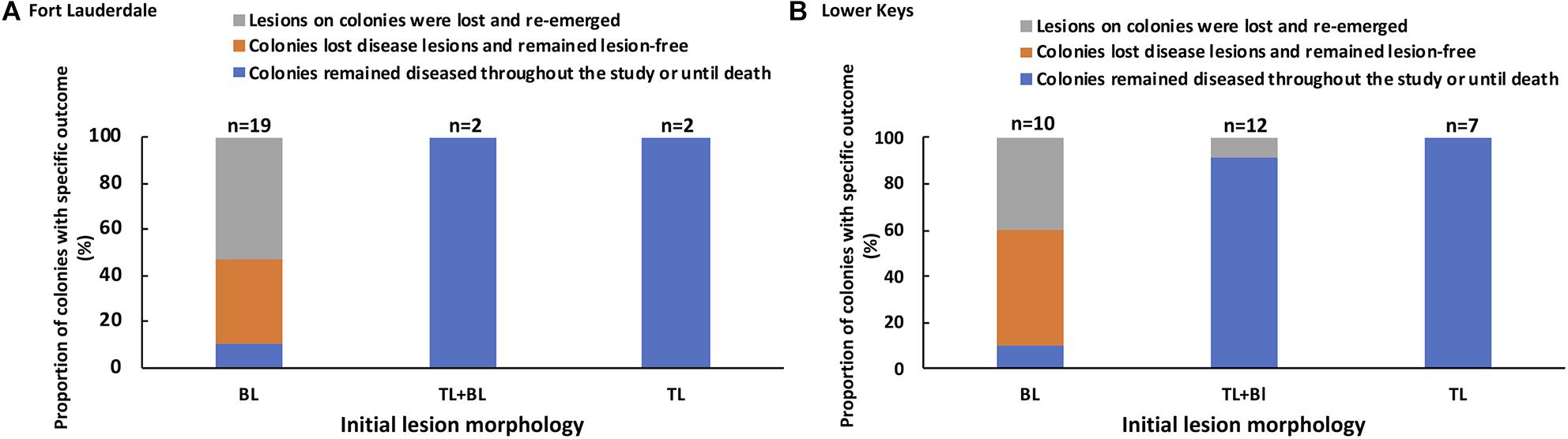
Figure 8. Association between initial lesion morphology and subsequent disease outcome for tagged M. cavernosa at study sites in Fort Lauderdale (A) and in the Lower Keys (B). Colonies at Fort Lauderdale were followed for a little over 2 years (Nov. 2018 – Jan. 2021) and two sets of colonies in the Lower Keys were each followed for a year. The Lower Keys year one tagged colonies were followed Nov. 2018 to Aug. 2019 and year two tagged colonies were followed Nov. 2019 to Dec. 2020. The number of colonies displaying each type of lesion is displayed above each bar.
SCTLD Rate of Transmission in Lesions Collected From Fort Lauderdale vs. the Lower Keys
The diseased M. cavernosa fragments from the Fort Lauderdale reefs had a 45.5% transmission success after an average of 3.8 days (range = 2–7 days; n = 11). Three additional coral fragments developed a bleached zone at the point of contact but did not develop into tissue loss during the experiment (maximum duration of 14 days). Diseased M. cavernosa fragments from the Lower Keys reefs had a 27.3% transmission success after an average of 6 days (range = 3–9 days; n = 11; Figure 9). They also had an additional three coral fragments develop a bleached zone at the point of contact that did not develop into tissue loss during the experiment (maximum duration of 14 days). There were no significant differences in transmission rate using coral lesions from Fort Lauderdale vs. the Lower Keys (Log-Rank test, X2 = 0.9997, df = 1, and p = 0.32). None of the control corals developed lesions.
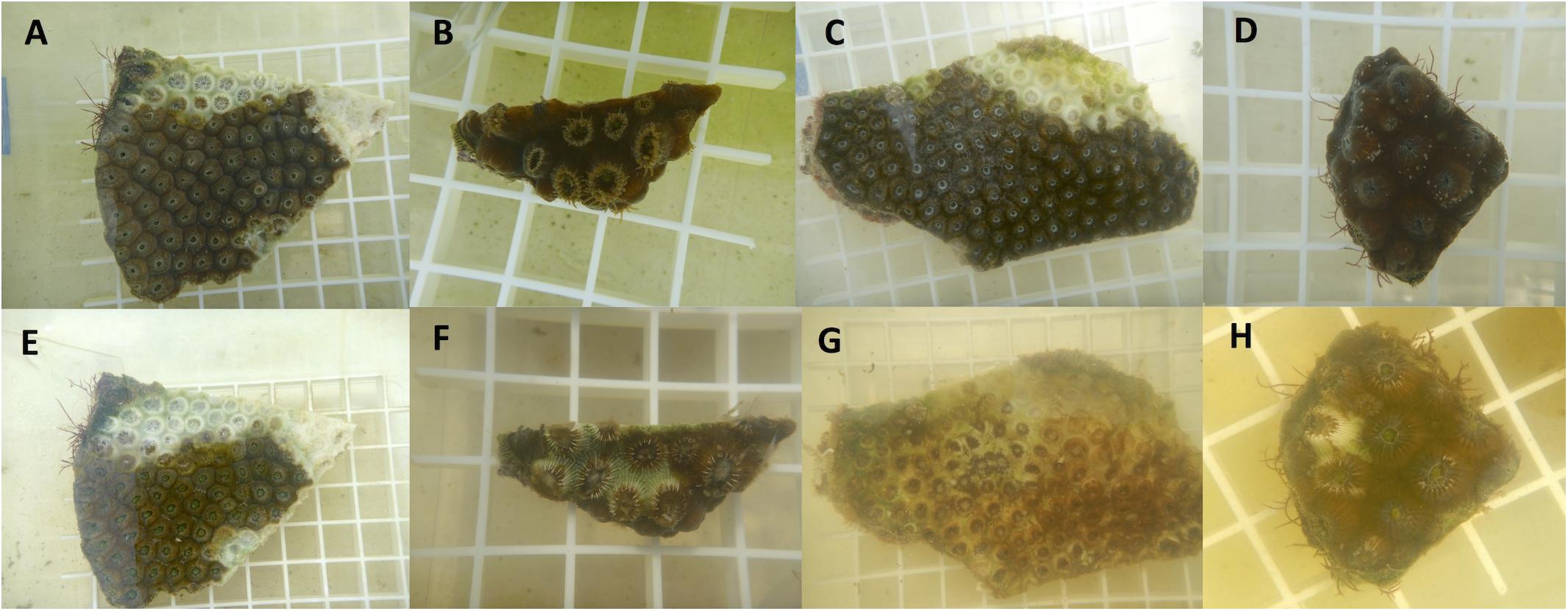
Figure 9. Examples of disease transmission from aquaria studies. Top row are fragments on day 0 (A–D) and bottom row are following disease transmission after 2–3 days exposure (E–H). Fragment (A) is the diseased fragment from Fort Lauderdale and fragment (B) was the healthy test piece touching the lesion. Fragment (C) is the diseased fragment from the Lower Keys and fragment (D) is the healthy test piece. Fragments (B), (D) are genets.
Discussion
We examined SCTLD in an endemic zone (Fort Lauderdale) compared to an emergent zone (Lower Keys). This study found that M. cavernosa colonies that first developed SCTLD at the site in the emergent zone suffered high mortality whereas colonies that developed SCTLD in the 2nd year of the study, at the same site, had significantly less mortality. In comparison, at the endemic site colonies had low morbidity and mortality for both years of the study. Aquaria studies showed transmission rates did not differ between lesions collected in the emergent vs. endemic zones despite the differences in SCTLD virulence observed on diseased M. cavernosa in the field in these two zones. We also showed that initial lesion morphology was predictive of disease outcome regardless of zone. Finally, we found that reef conditions were poor at both sites and although SCTLD prevalence was higher at the emergent site it was still present in the endemic site years after the initial appearance of the disease.
Coral reef conditions on the Fort Lauderdale reef in the endemic zone and the Lower Keys in the emergent zone were grossly similar with low coral cover, high algae cover and a comparable coral species composition. These poor reef conditions probably reflect the overall degradation the FRT has experienced the past several decades from densely populated coastlines, high visitor numbers, polluted terrestrial run-off, disease outbreaks, bleaching events, and overfishing (Gardner et al., 2003; Jackson et al., 2014). One difference that did occur was in the size class distributions of two coral species common in both regions, M. cavernosa and S. siderea. On the study reef at Fort Lauderdale, most of the colonies within transects, of both species, were less than 5 cm in diameter whereas there were proportionally more large colonies at the site in the Lower Keys. SCTLD has been killing coral colonies at Fort Lauderdale since 2014–2015 and Walton et al. (2018) and Sharp et al. (2020) found the risks of contracting SCTLD are higher for larger corals. Hence, the low number of large colonies found at Fort Lauderdale may be indicative of loss of the larger colonies due to disease. We have no baseline data at our study site from before the disease outbreak so it could be that for some other reason, this site had already lost most of its larger corals of those two species. However, at nearby sites decreases in coral cover and colony densities, including M. cavernosa and S. siderea, had already been documented and attributed to the SCTLD outbreak (Walton et al., 2018). The continued spread of SCTLD at our sites with low colony densities is consistent with a waterborne or vector-borne mode of transmission as has been suggested elsewhere (Aeby et al., 2019; Muller et al., 2020; Noonan and Childress, 2020). However, the large number of M. cavernosa and S. siderea recruits found within the Fort Lauderdale study site is an encouraging sign of reef resilience.
Not surprising, overall disease prevalence was higher in the Lower Keys than at Fort Lauderdale as SCTLD had just emerged at the site in the Lower Keys and was rapidly spreading through the coral populations. At Fort Lauderdale, endemic disease prevalence (1.6%) was lower than the average disease levels reported on Florida reefs before the outbreak (∼6%; Santavy et al., 2005). Disease prevalence is often linked with host density (Willis et al., 2004; Aeby et al., 2010; Caldwell et al., 2020) and since coral cover and thus host density has declined precipitously on Florida’s reefs since the 1970s (Gardner et al., 2003; Jackson et al., 2014) it is not surprising that this might translate into lower endemic disease levels.
An interesting observation at our Fort Lauderdale site was that even though SCTLD was first recorded in the region in 2014–2015 (Walton et al., 2018), it remains in the coral populations to date, with newly infected colonies continuing to emerge through time. The site also contained numerous healthy C. natans colonies, considered a highly SCTLD susceptible species, of which one was observed with SCTLD in 2019 and subsequently died. The pathogen appears to still be in the environment which is an important consideration as plans for restoration proceed. It also indicates that colonies that are initially disease-free following the emergence of SCTLD on a reef, but contracted the disease in subsequent years, were not immune to the disease. Such colonies were either not initially exposed to the pathogen(s) or to a high enough pathogen load during earlier years of the outbreak (random chance), or were exposed to the pathogen(s) at the same levels as their conspecifics that did develop SCTLD but did not develop signs of disease (differences in capacity to respond to infection).
A pattern is also emerging whereby colonies of SCTLD-susceptible species that are first infected on a reef experience high mortality regardless of species (Precht et al., 2016; Aeby et al., 2019; Meiling et al., 2020; Sharp et al., 2020; Thome et al., 2021). This study also documented high mortality in M. cavernosa in year one at an emergent zone. However, there also seems to be a temporal component with M. cavernosa infected in year two of the study, different colonies but located on the same reef, able to withstand the infection with reduced mortality and lesions waxing and waning or colonies becoming lesion-free by the end of the study. Tagged colonies at the Fort Lauderdale site (endemic zone) displayed a similar pathogenesis with lesions waxing and waning or colonies becoming lesion-free, similar to what has been reported elsewhere for M. cavernosa (Aeby et al., 2019). SCTLD has been reported as slowing down (reduced mortality) through time for other species as well (Meiling et al., 2020; Sharp et al., 2020). The environment could also play a role in a colony’s ability to resist or recover from disease as found by Meiling et al. (2020) who reported that signs of SCTLD disappeared in O. faveolata following a bleaching event. We observed no obvious differences in environmental conditions at our Lower Keys site between year one and two, nor signs of bleaching, which could explain the observed shift in disease virulence. In addition, Meiling et al. (2020) noted that M. cavernosa at their sites did not lose signs of disease following bleaching. The interplay between pathogen, host and environment is complicated and for SCTLD the environment appears to be of importance but differs among species.
Our study does not address why there were differences in SCTLD mortality in tagged M. cavernosa at the Lower Keys site in year one vs. year two of the study. The change in SCTLD dynamics through time might be explained by pathogen evolution, intrinsic differences among colonies in response to SCTLD, a combination of these factors or other co-factors not yet understood. The extended duration of the current disease event on Florida’s reefs opens up the possibility that the pathogen(s) could be evolving reduced virulence. Especially when one considers how rapidly microbial pathogens can evolve (Holden et al., 2009; Croll and McDonald, 2012). Alternatively, corals have genotypic differences in disease resistance (Vollmer and Kline, 2008; Muller et al., 2018) and employ diverse immune strategies to protect themselves from potential pathogens and types and levels of defense vary inter- and intra-specifically (Mullen et al., 2004; Gochfeld and Aeby, 2008; Shore-Maggio et al., 2015). These differences might enable particular species, or genotypes to have an advantage over others in resisting or overcoming invasion by pathogens.
We also examined the transmission dynamics of M. cavernosa lesions from the two regions. We hypothesized that lesions collected from the emergent zone (Lower Keys) where colony mortality from SCTLD was higher would transmit disease more quickly compared to lesions from the endemic zone (Fort Lauderdale). However, we found no differences in rate of disease transmission in our aquaria studies when comparing test lesions collected from the emergent zone vs. endemic zones. Although SCTLD virulence diminishes through time on reefs, this did not translate into differences in transmissibility. This suggests that SCTLD contagion is not necessarily linked to the subsequent virulence expressed on a coral host. This is contrast to what Aeby et al. (2019) found in aquaria studies, with 100% transmission in M. cavernosa when exposed to acute lesions on C. natans, collected from an emergent zone, compared to 30% transmission, when exposed to subacute lesions on M. cavernosa from an endemic zone. However, their study examined transmission of acute lesions between different coral species so perhaps aggression between C. natans and M. cavernosa facilitated higher transmission. Yet, they also found a higher transmission rate for fragments exposed to waterborne transmission (10 vs. 60%). It must also be noted that a pathogen has not been identified for SCTLD and its possible that multiple pathogens are involved. The factors initiating a SCTLD infection could also differ from factors facilitating subsequent disease progression. Ushijima et al. (2020) found that the known coral pathogen, Vibrio coralliilyticus, was detected in approximately 20% of M. cavernosa and O. faveolata displaying signs of SCTLD. In aquaria studies, they found that coral fragments that were positive for a toxic protein specific to V. coralliilyticus, lost tissue at a significantly higher rate than fragments that scored negative for the pathogen. They suggest that V. coralliilyticus may be an opportunistic pathogen causing coinfections within SCTLD lesions, that are contributing to the differences in observed tissue loss. Interestingly, we had one set of diseased fragments used in our transmission experiments (shown in Figure 6) opportunistically tested for V. coralliilyticus infections. The fragment from the Lower Keys tested positive whereas the fragment from Fort Lauderdale did not, and although this was just one set of fragments, the rate of tissue loss on the Lower Keys coral was much faster than the Fort Lauderdale coral consistent with the findings of Ushijima et al. (2020).
We identified three distinct lesion morphologies associated with SCTLD on M. cavernosa and report a link between lesion morphologies and disease outcome. Most colonies that initially had tissue loss lesions with or without a distinct bleached border maintained disease lesions throughout the study or until complete colony mortality. In contrast, most colonies that initially had bleached polyps or bleached edges without tissue loss had lesions that resolved and reoccurred or resolved, and the colonies remained lesion-free to the end of the study, although there were a number of bleached lesions that did progress onto tissue loss. Differences in disease pathogenesis, including duration on colonies, translated into variation in rates of tissue loss with colonies possessing bleached polyps suffering significantly less tissue loss. Similarly, Aeby et al. (2019) found that duration of the infection on M. cavernosa was the best predictor of degree of morbidity from SCTLD. As such, lesion morphology on M. cavernosa should be taken into consideration when choosing colonies for disease treatment or when evaluating success of disease treatments. We also found that the bleached lesions without tissue loss were the dominant lesion on tagged colonies at our Fort Lauderdale site in an endemic zone. In contrast, at our Lower Keys site in an emergent zone, tissue loss lesions were dominant on tagged colonies in year one of the study but year two colonies switched to a much higher proportion of colonies with bleached lesions similar to colonies in an endemic zone. This information may be useful to managers as they continue to monitor coral reefs for possible emergent or past SCTLD infections.
The bleached polyps associated with SCTLD have been found in other coral diseases such as yellow band disease found in the Caribbean (Cervino et al., 2004; Bruckner and Bruckner, 2006; Weil et al., 2009), Porites white patch syndrome found on the Great Barrier Reef (Roff et al., 2008) and Porites bleaching with tissue loss found in Hawaii (Sudek et al., 2012a,b). Similar to SCTLD, colonies with yellow band disease and Porites bleaching with tissue loss usually progress from bleached areas to tissue loss but lesions can also resolve (lose signs of disease) through time (Bruckner and Bruckner, 2006). Porites white patch syndrome and Porites bleaching with tissue loss are associated with viral infections (Lawrence et al., 2014a,b) and yellow band disease is thought to be a bacterial infection (Cervino et al., 2008). It has been suggested that the pathogens associated with Porites white patch syndrome (Kenkel et al., 2020) and yellow band disease (Cervino et al., 2004, 2008) primarily affect the symbiotic zooxanthellae within the coral rather than the coral host itself. Similarly, Landsberg et al. (2020) suggest that SCTLD is caused by a disruption of the host-zooxanthellae symbiosis. However, none of the other three diseases have shown the spatial spread or virulence associated with SCTLD. It must be noted that this study examined a single coral species within a single site in each zone (endemic, emergent) and so SCTLD dynamics may be different for different coral species or other regions within these different disease zones. While there are still many unknowns associated with SCTLD, our study adds to the growing body of knowledge by highlighting that differences in coral mortality from SCTLD are not necessarily linked to host species, lesion morphology is reflective of subsequent rate of mortality, and disease dynamics change through time on reefs where the disease has newly emerged.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GA conceived the study. GA, VP, SJ, EB, JK, and CW conducted the fieldwork. GA and BU conducted the transmission experiments. All authors were involved in writing the manuscript.
Funding
This research was funded by the Office of Resilience and Coastal Protection in the Florida Department of Environmental Protection to VP and GA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Woody Lee and Jay Houk for help setting up and maintaining the water tables. Thanks to the Indian River State College students Shelby Roberts, Diego Garcia, and Myron Clemons whose enthusiastic help made the aquaria studies possible. Thanks also to Broward County personnel, Ken Banks, Angel Rivera, Kirk Kilfoyle, and Pat Quinn for logistical support in the field. Field collections were authorized under Florida Fish and Wildlife Conservation Commission Special Activity Licenses SAL-18-1702A-SRP and SAL-19-1702-SRP and Florida Keys National Marine Sanctuary permit # FKNMS-2017-128-A2. This is contribution #1165 from the Smithsonian Marine Station.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.699075/full#supplementary-material
Footnotes
References
Aeby, G. S. (2006). Baseline levels of coral disease in the Northwestern Hawaiian Islands. Atoll. Res. Bull. 543, 471–488.
Aeby, G. S., Callahan, S., Cox, E. F., Runyon, C., Smith, A., Stanton, F. G., et al. (2016). Emerging coral diseases in Kāne‘ohe Bay, O‘ahu, Hawai‘i: two major disease outbreaks of acute Montipora white syndrome. Dis. Aquat. Org. 119, 189–198. doi: 10.3354/dao02996
Aeby, G. S., Ross, M., Williams, G. J., Lewis, T. D., and Work, T. M. (2010). Disease dynamics of Montipora white syndrome within Kaneohe Bay, Oahu, Hawaii: distribution, seasonality, virulence, and transmissibility. Dis. Aquat. Org. 91, 1–8. doi: 10.3354/dao02247
Aeby, G. S., Ushijima, B., Campbell, J. E., Jones, S., Williams, G. J., Meyer, J. L., et al. (2019). Pathogenesis of a tissue loss disease affecting multiple species of corals along the Florida Reef Tract. Front. Mar. Sci. 6:678. doi: 10.3389/fmars.2019.00678
Aeby, G. S., Williams, G. J., Franklin, E. C., Kenyon, J., Cox, E. F., Coles, S., et al. (2011). Patterns of coral disease across the Hawaiian archipelago: relating disease to environment. PLoS One 6:e20370. doi: 10.1371/journal.pone.0020370
Alvarez-Filip, L., Estrada-Saldívar, N., Pérez-Cervantes, E., Molina-Hernández, A., and González-Barrios, F. J. (2019). A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ. 7:e8069. doi: 10.7717/peerj.8069
Bourne, D. G., Ainsworth, T. D., Pollock, F. J., and Willis, B. L. (2014). Towards a better understanding of white syndromes and their causes on Indo-Pacific coral reefs. Coral Reefs. 34, 233–242. doi: 10.1007/s00338-014-1239-x
Bruckner, A. (2016). “White syndromes of western Atlantic reef-building corals” in Diseases of Coral. eds C. Woodley, C. Downs, A. Bruckner, and J. Porter Galloway (New Jersey: Wiley Blackwell). 316–332. doi: 10.1002/9781118828502.ch22
Bruckner, A., and Bruckner, R. (2006). Consequences of yellow band disease (YBD) on Montastraea annularis (species complex) populations on remote reefs off Mona Island. Puerto. Rico. Dis. Aquat. Org. 69, 67–73. doi: 10.3354/dao069067
Caldwell, J. M., Aeby, G., Heron, S. F., and Donahue, M. J. (2020). Case-control design identifies ecological drivers of endemic coral diseases. Sci. Rep. 10:2831. doi: 10.1038/s41598-020-59688-8
Cervino, J. M., Hayes, R., Goreau, T. J., and Smith, G. W. (2004). Zooxanthellae regulation in yellow blotch/band and other coral diseases contrasted with temperature related bleaching: in situ destruction vs expulsion. Symbiosis 37, 63–85.
Cervino, J. M., Thompson, F. L., Gomez-Gil, B., Lorence, E. A., Goreau, T. J., Hayes, R. L., et al. (2008). The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. J. Appl. Microbiol. 105, 1658–1671. doi: 10.1111/j.1365-2672.2008.03871.x
Croll, D., and McDonald, B. A. (2012). The accessory genome as a cradle for adaptive evolution in pathogens. PLoS Pathog. 8:e1002608. doi: 10.1371/journal.ppat.1002608
Daszak, P., Cunningham, A. A., and Hyatt, A. D. (2000). Emerging Infectious Diseases of Wildlife– Threats to Biodiversity and Human Health. Science 287, 443–449. doi: 10.1126/science.287.5452.443
Dobson, A., and Foufopoulos, J. (2001). Emerging infectious pathogens of wildlife. Phil. Trans. R. Soc. Ser. Biol. Sci. 356, 1001–1012. doi: 10.1098/rstb.2001.0900
Estrada-Saldivar, N., Molina-Hernandez, A., Perez-Cervantes, E., Medellin-Maldon, F., Gonzalez-Barrios, F., and Alvarez-Filip, L. (2020). Reef-scale impacts of the stony coral tissue loss disease outbreak. Coral Reefs. 39, 861–866. doi: 10.1007/s00338-020-01949-z
Gardner, T. A., Cote, I. M., Gill, J. A., Grant, A., and Watkinson, A. R. (2003). Long-term region-wide declines in Caribbean corals. Science 301, 958–960. doi: 10.1126/science.1086050
Gochfeld, D., and Aeby, G. (2008). Antibacterial chemical defenses in Hawaiian corals provide possible protection from disease. Mar. Ecol. Progr. Ser. 362, 119–128. doi: 10.3354/meps07418
Harvell, C. D., Jordan-Dahlgren, E., Merkel, S., Rosenberg, E., Raymundo, L., Smith, G., et al. (2007). Coral disease, environmental drivers and the balance between coral and microbial associates. Oceanography 20, 172–195. doi: 10.5670/oceanog.2007.91
Heres, M., Farmer, B., Elmer, F., and Hertler, H. (2021). Ecological consequences of Stony Coral Tissue Loss Disease in the Turks and Caicos Islands. Coral Reefs 40, 609–624. doi: 10.1007/s00338-021-02071-4
Holden, M. T. G., Hauser, H., Sanders, M., Ngo, T. H., Cherevach, I., Cronin, A., et al. (2009). Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One 4:17. doi: 10.1371/journal.pone.0006072
Jackson, E. J., Donovan, M., Cramer, K., and Lam, V. (2014). Status and Trends of Caribbean Coral Reefs: 1970-2012. Switzerland: IUCN.
Kenkel, C. D., Mocellin, V. J. L., and Bay, L. K. (2020). Global gene expression patterns in Porites white patch syndrome: disentangling symbiont loss from the thermal stress response in reef-building coral. Mol. Ecol. 29, 3907–3920. doi: 10.1111/mec.15608
Kenyon, J., Wilkinson, C., and Aeby, G. (2008). Community structure of hermatypic corals at Maro reef in the Northwestern Hawaiian Islands: a unique open atoll. Atoll. Res. Bull. 558, 1–22. doi: 10.5479/si.00775630.558.1
Kenyon, K., Wilkinson, C., and Aeby, G. (2010). Community structure of hermatypic corals at Midway Atoll in the Northwestern Hawaiian Islands: a legacy of human disturbance. Atoll Res. Bull. 581, 1–6. doi: 10.5479/si.00775630.581.24
Kramer, P. R., Roth, L., and Lang, J. (2019). Map of stony coral tissue loss disease outbreak in the Caribbean. Available online at: www.agrra.org
Landsberg, J. H., Kiryu, Y., Peters, E. C., Wilson, P. W., Perry, N., Waters, Y., et al. (2020). Stony Coral Tissue Loss Disease in Florida is associated with disruption of host–zooxanthellae physiology. Front Mar Sci. 7:576013. doi: 10.3389/fmars.2020.576013
Lawrence, S. A., Davy, J. E., Aeby, G. S., Wilson, W. H., and Davy, S. K. (2014a). Quantification of virus-like particles suggests viral infection in corals affected by Porites tissue loss. Coral Reefs 33, 687–691. doi: 10.1007/s00338-014-1168-8
Lawrence, S. A., Davy, J. E., Wilson, W. H., Hoegh-Guldberg, O., and Davy, S. K. (2014b). Porites white patch syndrome: associated viruses and disease physiology. Coral Reefs 34, 249–257. doi: 10.1007/s00338-014-1218-2
Manzello, D. (2015). Rapid recent warming of coral reefs in the Florida Keys. Sci. Rep. 5, 1–10. doi: 10.1038/srep16762
Meiling, S., Muller, E. M., Smith, T. B., and Brandt, M. E. (2020). 3D Photogrammetry reveals dynamics of Stony Coral Tissue Loss Disease (SCTLD) lesion progression across a thermal stress event. Front. Mar. Sci. 7:597643. doi: 10.3389/fmars.2020.597643
Miller, M., Karazsia, J., Groves, C., Griffin, S., Moore, T., Pace, W., et al. (2016). Detecting sedimentation impacts to coral reefs resulting from dredging the Port of Miami. Florida USA. PeerJ. 4:e2711. doi: 10.7717/peerj.2711
Mullen, K. M., Peters, E. C., and Harvell, C. D. (2004). “Coral Resistance to Disease” in Coral Health and Disease. eds E. Rosenberg and Y. Loya (Berlin: Springer). 377–399. doi: 10.1007/978-3-662-06414-6_22
Muller, E. M., Bartels, E., and Baums, I. B. (2018). Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. Elife 7:e35066. doi: 10.7554/eLife.35066.028
Muller, E. M., Sartor, C., Alcaraz, N., and van Woesik, R. (2020). Spatial epidemiology of the Stony Coral Tissue Loss Disease in Florida. Front. Mar. Sci. 7:163. doi: 10.3389/fmars.2020.00163
Neely, K. L., Macaulay, K. A., Hower, E. K., and Dobler, M. A. (2020). Effectiveness of topical antibiotics in treating corals affected by Stony Coral Tissue Loss Disease. PeerJ. 8:e9289. doi: 10.7717/peerj.9289
Noonan, K. R., and Childress, M. J. (2020). Association of butterflyfishes and stony coral tissue loss disease in the Florida Keys. Coral Reefs 39, 1581–1590. doi: 10.1007/s00338-020-01986-8
Precht, W. F., Gintert, B. E., Robbart, M. L., Fura, R., and van Woesik, R. (2016). Unprecedented disease-related coral mortality in Southeastern Florida. Sci. Rep. 6:31374. doi: 10.1038/srep31374
Roff, G., Ulstrup, K. E., Fine, M., Ralph, P. J., and Hoegh-Guldberg, O. (2008). Spatial heterogeneity of photosynthetic activity within diseased corals from the Great Barrier Reef. J. Phycol. 44, 526–538. doi: 10.1111/j.1529-8817.2008.00480.x
Santavy, D. L., Mueller, E., Peters, E. C., MacLaughlin, L., Porter, J. W., Patterson, K. L., et al. (2001). “Quantitative assessment of coral diseases in the Florida Keys: strategy and methodology” in The Ecology and Etiology of Newly Emerging Marine Diseases Dordrecht. ed. J. W. Porter (Netherlands: Springer). 39–52. doi: 10.1007/978-94-017-3284-0_3
Santavy, D., Summers, J., Engle, V., and Harwell, L. (2005). The condition of coral reefs in South Florida (2000) using coral disease and bleaching as indicators. Environ. Mon. Assess. 100, 129–152. doi: 10.1007/s10661-005-4767-6
Sharp, W. C., Shea, C. P., Maxwell, K. E., Muller, E. M., and Hunt, J. H. (2020). Evaluating the small-scale epidemiology of the stony-coral -tissue-loss-disease in the middle Florida Keys. PLoS One 15:e0241871. doi: 10.1371/journal.pone.0241871
Shilling, E., Combs, I., and Voss, J. (2021). Assessing the effectiveness of two intervention methods for stony coral tissue loss disease on Montastraea cavernosa. Sci. Rep. 11:8566. doi: 10.1038/s41598-021-86926-4
Shore-Maggio, A., Runyon, C. M., Ushijima, B., Aeby, G. S., and Callahan, S. M. (2015). Differences in bacterial community structure in two color morphs of the Hawaiian reef coral Montipora capitata. Appl. Environ. Microbiol. 81, 7312–7318. doi: 10.1128/AEM.01935-15
Sudek, M., Aeby, G. S., and Davy, S. K. (2012a). Localized bleaching in Hawaii causes tissue loss and a reduction in the number of gametes in Porites compressa. Coral Reefs 31, 351–355. doi: 10.1007/s00338-011-0844-1
Sudek, M., Work, T. M., Aeby, G. S., and Davy, S. K. (2012b). Histological observations in the Hawaiian reef coral, Porites compressa, affected by Porites bleaching with tissue loss. J. Invertebr. Pathol. 111, 121–125. doi: 10.1016/j.jip.2012.07.004
Thome, P. E., Rivera-Ortega, J., Rodríguez-Villalobos, J. C., Cerqueda-García, D., Guzmán-Urieta, E. O., García-Maldonado, J. Q., et al. (2021). Local dynamics of a white syndrome outbreak and changes in the microbial community associated with colonies of the scleractinian brain coral Pseudodiploria strigosa. PeerJ. 9:e10695. doi: 10.7717/peerj.10695
Ushijima, B., Meyer, J. L., Thompson, S., Pitts, K., Marusich, M. F., Tittl, J., et al. (2020). Disease diagnostics and potential coinfections by Vibrio coralliilyticus during an ongoing coral disease outbreak in Florida. Front. Microbiol. 11:569354. doi: 10.3389/fmicb.2020.569354
Vollmer, S. V., and Kline, D. I. (2008). Natural disease resistance in threatened staghorn corals. PLoS One 3:e3718. doi: 10.1371/journal.pone.0003718
Walker, B., Turner, N., Noren, H., Buckley, S., and Pitts, K. (2021). Optimizing stony coral tissue loss disease (SCTLD) intervention treatments on Montastraea cavernosa in an endemic zone. Front. Mar. Sci. 8:666224. doi: 10.3389/fmars.2021.666224
Walton, C. J., Hayes, N. K., and Gilliam, D. S. (2018). Impacts of a regional, multi-year, multi-species coral disease outbreak in Southeast Florida. Front. Mar. Sci. 5:323. doi: 10.3389/fmars.2018.00323
Weil, E., Croquer, A., and Urreiztieta, I. (2009). Yellow band disease compromises the reproductive output of the Caribbean reef-building coral Montastraea (Anthozoa. Scleractinia). Dis. Aquat. Org. 87, 45–55. doi: 10.3354/dao02103
Weil, E., Urreiztieta, I., and Garzon-Ferreira, J. (2000). Geographic variability in the incidence of coral and octocoral diseases in the wider Caribbean. Proc. 9th Int. Coral Reef Symp. 2, 1231–1238.
Williams, S., Walter, C., and Muller, E. (2021). Fine scale temporal and spatial dynamics of the stony coral tissue loss disease outbreak within the lower Florida Keys. Front. Mar. Sci. 8:631776. doi: 10.3389/fmars.2021.631776
Willis, B. L., Page, C. A., and Dinsdale, E. A. (2004). “Coral Disease on the Great Barrier Reef” in Coral Health and Disease. eds E. Rosenberg and Y. Loya (Berlin: Springer). 69–104. doi: 10.1007/978-3-662-06414-6_3
Keywords: Florida reef tract, tissue loss disease, virulence, transmission, lesion morphology, disease pathogenesis
Citation: Aeby G, Ushijima B, Bartels E, Walter C, Kuehl J, Jones S and Paul VJ (2021) Changing Stony Coral Tissue Loss Disease Dynamics Through Time in Montastraea cavernosa. Front. Mar. Sci. 8:699075. doi: 10.3389/fmars.2021.699075
Received: 22 April 2021; Accepted: 27 August 2021;
Published: 16 September 2021.
Edited by:
Les Kaufman, Boston University, United StatesReviewed by:
Francoise Cavada-Blanco, Zoological Society of London, United KingdomGuillermo Horta-Puga, Universidad Nacional Autónoma de México, Mexico
Copyright © 2021 Aeby, Ushijima, Bartels, Walter, Kuehl, Jones and Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Greta Aeby, Z3JldGFAaGF3YWlpLmVkdQ==
 Greta Aeby
Greta Aeby Blake Ushijima
Blake Ushijima Erich Bartels
Erich Bartels Cory Walter
Cory Walter Joseph Kuehl
Joseph Kuehl Scott Jones
Scott Jones Valerie J. Paul
Valerie J. Paul