- 1Department of Biology, Seaweed and Seagrass Research Unit, Division of Biological Science, Faculty of Science, Prince of Songkla University, Hat Yai, Thailand
- 2Institute of Environmental and Marine Sciences, Silliman University, Dumaguete, Philippines
- 3School of Marine Biosciences, Kitasato University, Sagamihara, Japan
- 4Institute of Marine Science, Burapha University, Saen suk, Thailand
- 5Excellence Centre for Biodiversity of Peninsular Thailand, Faculty of Science, Prince of Songkla Univerisity, Hatyai, Thailand
In the tropical ecosystem, sea cucumbers are associated with seagrass meadows in various ways, often forming a network of ecological interactions. From this myriad of interactions, the trophic relationship between the seagrasses and sea cucumbers has received recent attention with the advent of analytical techniques. However, little is understood about the exact mechanism by which seagrasses are sustaining the sea cucumber populations in the food chain, considering the high number of refractory components in seagrasses and the lack of digestive enzymes among sea cucumbers. This manuscript aims to review existing concepts in ecology concerning the association between tropical seagrasses and sea cucumbers to provide directions for research and management of this vital resource. We searched literature from electronic databases and identified key concepts concerning sea cucumber and seagrass communities based on geographic distribution, nutrient compositions, seagrass decomposition process, and trophic enrichments in the food chain. A conceptual model was then developed detailing the factors influencing the association between the seagrass meadows and sea cucumbers. Despite the limited published information on the seagrass–sea cucumber association, a synthesis of the current understanding of this topic is provided to address the declining sea cucumber populations in the tropical seagrass meadows. We suggest that the successful restoration of sea cucumber fisheries requires a thorough understanding of the seagrass decomposition process, which is vital to the diet of sea cucumbers.
Introduction
Tropical seagrass meadows are among the most productive ecosystems in the coastal zones. They have an important role in stabilizing bottom sediments (Den Hartog, 1970), reducing the water current and thereby promoting sedimentation and settlement of planktonic larvae (Agawin and Duarte, 2002; Panyawai et al., 2019; Mercier et al., 2000), providing habitat for resident and transient fauna (Den Hartog, 1979; Heck et al., 2008; Ralph et al., 2013; Nishihama and Tanita, 2021) sustaining high primary production (Estacion and Fortes, 1988; Calumpong and Meñez, 1994; Rattanachot and Prathep, 2011), promoting carbon sequestration from autochthonous and allochthonous sources (Duarte et al., 2004; Kon et al., 2015; Trevathan-Tackett et al., 2017; Stankovic et al., 2021), providing protection from predators as a function of habitat complexity (Orth et al., 1984; Hair et al., 2020), supporting direct grazing (Thayer et al., 1984; Cebrián and Duarte, 1998), and assimilating seagrass particulate organic matter (Ricart et al., 2015; Domínguez-Godino et al., 2019; Floren et al., 2021). In return, seagrasses benefit from the burrowing activities of faunal communities, such as sea cucumbers, resulting in a considerable increase in the growth rates of seagrass leaves (Arnull et al., 2021). Additionally, sea cucumbers play a vital role as bioturbators or ecosystem engineers in the sediments that promote recycling of nutrients within the seagrass meadows (Uthicke and Klumpp, 1998; Costa et al., 2014; Lee, 2016). Despite the ecological and economic functions of seagrass meadows and associated fauna, this ecosystem suffers due to coastal developments and regular typhoons that affect its proper functioning.
Perhaps one of the most valuable faunas associated with the tropical seagrass meadows is the sea cucumber. The Holothuridae and Stichopodidae families form an important part of the fishery industry that has been in existence in the Indo-Pacific for over 1000 years (Bruckner et al., 2003). This association of sea cucumbers with the seagrasses received considerable attention in recent times due to the intense fishing pressure on this vital resource in the wild (e.g., Mercier et al., 2000; Hamel et al., 2001; Domínguez-Godino et al., 2019), prompting authorities to seek for solutions aimed at restoring depleted stocks. Despite the restoration initiatives being undertaken to mitigate the depleting sea cucumber populations, managing the wild sea cucumber fisheries has been proven largely unsuccessful (e.g., Choo, 2008; Vincent and Morrison-Saunders, 2013; Robinson and Lovatelli, 2015). One of the causes for this failure is the general lack of understanding of the underlying mechanisms that characterize this trophic relationship.
In this review, we examine the factors that influence seagrass–sea cucumber association based on their geographical distribution, nutrient composition, and seagrass decomposition process in order to elucidate the roles of each in the maintenance of sea cucumber populations. Apart from the studies done in the tropical region, some studies conducted in temperate areas were included to augment the paucity of information on this topic.
This study is guided by the research question: what characteristics of seagrasses are important to the survival of sea cucumbers? This question came about with the recent findings indicating that seagrasses are the primary diet for some deposit-feeding holothurians (Liu et al., 2013; Costa et al., 2014; Ricart et al., 2015; Domínguez-Godino et al., 2019; Floren et al., 2021). This review manuscript aims to provide a synthesis of information that may help direct future research and address the declining stocks of sea cucumber populations brought about by overexploitation and weak management system.
Methods
The primary sources of published literature were obtained from the authors’ personal library supplemented with online literature searches using the keywords “seagrass∗,” “sea cucumber∗,” “holothurian∗ or holothuroid∗,” and “sea cucumber∗ and seagrass interaction∗” in the Google, Google Scholar, Science Direct, Scopus, and Web of Science electronic databases, published from 1970 to 2021. Additional sources of information were also obtained from the section “References” of each article. Research articles relevant to the global seagrass distribution patterns described by Short et al. (2007) were used as criteria for whether a potential association between the sea cucumbers and seagrasses exists. Briefly, there are six seagrass bioregions in the world, consisting of four Temperate bioregions and two Tropical bioregions. The latter is more diverse in terms of species composition and support for mega-herbivore grazers and numerous faunas with great ecological and economic values.
Review articles on the decomposition of terrestrial plants were also included in the discussion to provide insights on decomposition processes occurring in the seagrass ecosystems. The themes that arose from the literature search included a discussion on the important role of seagrass meadows as habitat and food source for the sea cucumbers. This was followed by linking cucumber distributions with the distribution of seagrasses. Another important theme that arose from the literature search is the linking of the seagrasses with the diet sources of sea cucumbers, given the seagrass decomposition process and corresponding by-products. Finally, a conceptual model of the factors influencing the seagrass–sea cucumber association was developed to emphasize the important role of the seagrass decomposition process and the corresponding environmental variables that control it.
Results
A total of 28 articles were selected from the search that dealt with seagrass–sea cucumber associations, corresponding to a 0.54 publication per year for the last 51 years. This frequency of publication is in reference to the first landmark paper published in 1970 and authored by Den Hartog (1970) wherein the seagrasses of the world were categorized according to the geographical distribution. These research publications are presented and summarized in Supplementary Table 1. About 65% of the research articles on the seagrass–sea cucumber associations were conducted in the tropical seagrass meadows, while 35% were done in the temperate seagrass meadows. Despite the majority of research being undertaken in tropical seagrass meadows, there is not enough to understand the trophic association between sea cucumbers and the seagrass meadows and the ecological and economic benefits of this highly productive ecosystem. In terms of the sea cucumber fishery alone, about 78,000 t were harvested from the South Pacific and Southeast Asian region, while those derived from the temperate oceans accounted for only 12,000 t (Bruckner et al., 2003). Despite the increasing production of sea cucumbers from marine culture, there is still not enough to meet the continuously rising demand (Bruckner et al., 2003; Choo, 2008; Kinch et al., 2008).
Results indicate that research studies were either conducted in the laboratory condition (LS) or in the field condition (FS), such that the interpretation of their findings is conditional to the environmental variables (i.e., Explanatory and Response) present in the laboratory, field, and environment. A conceptual model of the interaction between sea cucumbers and the biotic and abiotic factors also showed the complex interplay of these variables while the trophic interaction between the sea cucumbers and the seagrass forming the backbone among the interactions.
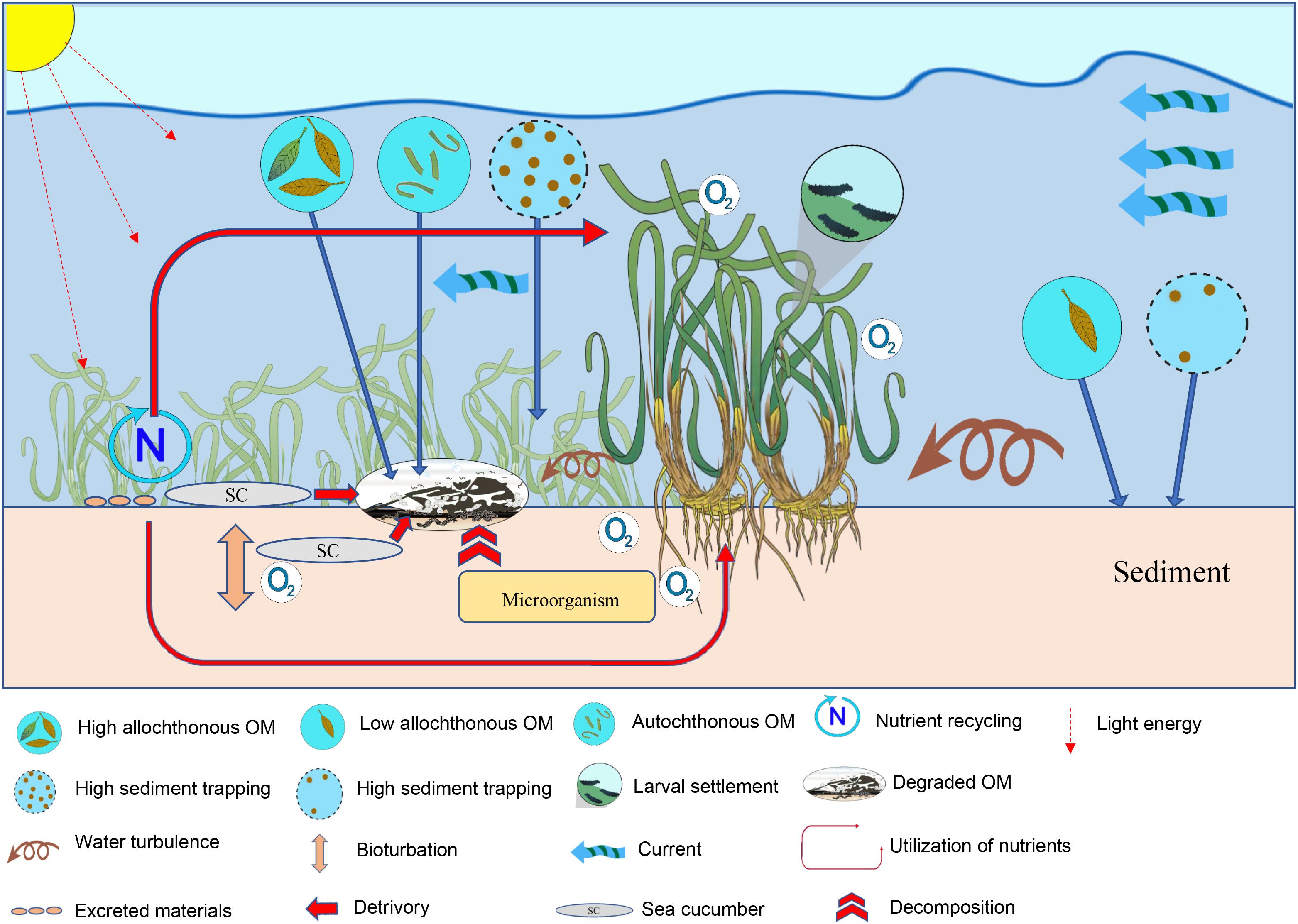
Overall, there appears to be only a handful of research manuscripts being undertaken to understand the seagrass–sea cucumber association, although a synthesis of concepts illustrated through a conceptual model was developed in order to explain the factors influencing the association between the seagrass meadows and sea cucumbers (Figure 1).
Discussion
Linking Sea Cucumber Distribution to Seagrasses
The distribution of sea cucumbers relative to the seagrasses may be influenced by several factors operating at various spatial scales. For example, the sea cucumber Holothuria scabra Jaeger, 1833 or “Sand fish” occurs from the Red Sea and East coast of Africa, throughout the Indo-Pacific, to Japan and Cook Islands (Massin et al., 2000). This tropical region is characterized by having abundant sunlight and warm temperatures (>27°C), thereby enhancing plant diversity and trophic interactions among the seagrass meadows and sea cucumber species (Kinch et al., 2008). In fact, the most productive ecosystems (such as the mangroves, seagrasses, and coral reefs) are found in this area and provide suitable habitats and ecosystem services to associated fauna. In addition, this region is also characterized by shallow intertidal zones, thereby allowing submerged aquatic plants and benthic microalgae to photosynthesize and contribute to the primary production in the benthic environment. Interestingly, the distribution of H. scabra coincides with the distribution of the seagrasses, Enhalus acoroides and T. hemprichii, which are only found in the Tropical Indo-Pacific bioregion (see Short et al., 2007). Both species form a meadow that persists for a long period of time and is also referred to as “enduring” meadows (Kilminster et al., 2015), thus, enabling the sea cucumbers to thrive. The overlapping distributions of sea cucumbers and seagrasses in the specific bioregions may have resulted from the evolutionary processes that similarly occurred among terrestrial plants and detritivores (Gessner et al., 2010).
Influence of Microhabitat on Sea Cucumber Distribution
The distribution of sea cucumber species in coastal habitats often varies with the location and on the hydrodynamic conditions of the area. In general, habitat preferences have been observed for certain sea cucumber species. For example, the sea cucumber H. scabra is typically found in the sheltered portions of the coral reefs, lagoons, and seagrass beds (Kinch et al., 2008; Hamel et al., 2013). However, this species is also capable of burrowing in the soft substrates under harsh weather conditions or when threatened by predators (Mercier et al., 2000). In Kogu Veke, Solomon Islands, H. scabra avoided substrates with the finest silt or coral pebbles and sediments with high (≥30%) organic matter content (Mercier et al., 2000).
In comparison to H. scabra, populations of Holothuria atra were found in more diverse habitats, such as in the inner and outer reef flats, back reefs, coastal lagoons, seagrass beds, and on sandy to muddy grounds with rubble or coral patches (Conand et al., 2013). The distribution of H. atra in the coastal habitats may also be distinguished from that of H. scabra since the former is typically found closely associated with the calcareous algae, Halimeda sp., as found by Purcell et al. (2012) in Mauritius. In the coastal waters of Sri Lanka, the shallow (< 10 m) intertidal seagrass habitats with sediments characterized by 2–3.5% organic content, 15–25% of gravel, and coarse sand were the most preferred habitats of H. atra (Dissanayake and Stefansson, 2012). In Ishigaki Island, Japan, where seagrass beds are sparse, the sea cucumber Stichopus japonicus (original description) was mostly found on the rocky beach of the lagoons while most of the Holothuria leucospilota were found hiding under the rocks and crevices (Tanita and Yamada, 2019), although the latter was also common in the seagrass meadows of Sri Lanka (Dissanayake and Stefansson, 2012).
Among the coastal habitats, seagrass beds near the mangrove forest appear to be the preferred habitat for H. scabra (Hamel et al., 2013). Cultured juvenile H. scabra were also found to survive better in seagrass–mangrove sites than in coral reef sites (Dance et al., 2003). Finally, the leaves of E. acoroides and T. hemprichii are also the preferred substrate for the settlement of the juveniles of H. scabra in the Solomon Islands (Mercier et al., 2000), indicating the importance of seagrasses in the early life stage development of this sea cucumber. No other sea cucumber species preferentially settling on seagrass blades were found in the literature, and this aspect may require further investigation.
Hence, the distribution of sea cucumbers in coastal habitats may be influenced by climatic and/or localized conditions such as sediment properties, water depth, sheltering, and habitat types. In terms of the microhabitat preference, seagrass beds appear to provide the sea cucumbers, H. scabra, with a suitable substrate for larval settlement and burying activities, as well as protection from water turbulence. As Massin and Duomen (1986) pointed out, coastal processes, such as hydrodynamics that influence sediment granulometry, also play an essential part in regulating the distribution of sea cucumbers and are important in defining the niches of holothurians. Thus, it is likely that sea cucumbers prefer a more complex habitat like the seagrass meadows, which have different microhabitats across small areas (Domínguez-Godino et al., 2019).
Linking Seagrass Detritus to Sea Cucumber Diets
Decomposition Process of Seagrasses
The decomposition of seagrass leaves is a complex process that may be influenced by several factors, including the quality and nutrient contents of the detritus (e.g., Peduzzi and Herndl, 1991; Pollard and Moriarty, 1991; Lavelle et al., 1993; Harmon et al., 2009). For example, the decomposition rate in anoxic conditions is 10–30% lower than in oxic conditions, indicating the importance of oxygen in the decomposition process. Generally, seagrasses have two major phases of decomposition: (1) soluble substances are rapidly leached out from the leaves, where roughly 50% of the original weight is lost in 7–8 months, providing nutrients for the growth of microorganisms in the water medium (Robertson et al., 1982; Peduzzi and Herndl, 1991), and (2) detritus compounds are broken down into particulate organic matter (POM). The second phase of the decomposition is largely dependent on the C:N ratio of the detritus, with higher ratios having a slower rate of decomposition (e.g., Lavelle et al., 1993; Moore and Jung, 2001; Fourqurean and Schrlau, 2003). Additionally, seagrass leaves have low lignin which acts as a physical barrier to microbial enzymes (Moore and Jung, 2001). However, only about 40% of the decomposition corresponds to microbial metabolic consumption, while the rest are lost in the form of fine organic debris which can be mineralized, exported, or stored in the sediment (Mateo and Romero, 1996). This suggests that seagrasses are not completely decomposed by the microorganisms, leaving the rest of the detritus available for further decomposition or consumption by the detritivores. Nonetheless, seagrass decomposition by anaerobic bacteria is necessary to prevent the buildup of toxic sulfide and excess organic matter in the sediments (de Fouw et al., 2018), although the seagrass roots are the main drivers of oxygenation in the sediments (Rattanachot and Prathep, 2015).
Generally, hemicellulases are favored by non-ruminant animals (including sea cucumbers) with limited capacity for digesting a high-fiber diet. These compounds are easily hydrolyzed by dilute acid (Wang et al., 2013) and various enzymes (see Ward, 2014 for review). The decomposition of seagrass into simpler compounds compliments the lack of cellulase activity in the digestive glands of Holothuria monacaria and S. japonicus (original description) (see Yokoe and Yasumasu, 1964). Similarly, H. scabra has low levels of lyase, cellulase, mannanase, agarase, and xylanase in their intestines (Zarate et al., 2009), preventing it from digesting complex compounds, such as lignin and cellulose.
Interactions of Factors
The factors affecting the seagrass–sea cucumbers associations are summarized in Figure 1 and Supplementary Table 1. Although seagrasses are providing habitat and food sources for the sea cucumbers, only a handful of studies have dealt with the interaction of sea cucumbers and seagrasses. One of these few studies, which was done in Southeast Queensland, Australia, found that the biomass and growth rate of Cymodocea serrulata were significantly reduced when sea cucumber H. scabra was excluded in the treatment (Wolkenhauer et al., 2010). However, increasingly more studies deal with the trophic interaction between seagrasses and sea cucumbers with the advent of modern analytical techniques (see Fry, 2006).
Direct grazing on seagrass leaves appears uncommon in sea cucumbers since they lack many enzymes that break down complex carbohydrates (Yokoe and Yasumasu, 1964; Zarate et al., 2009). Alternatively, seagrasses that have undergone decomposition may be utilized by sea cucumbers as diet sources. For example, a study in Shandong, northern China, revealed that a mixture of 40% Zostera marina detritus and sediment materials provided the best growth rates of S. japonicus (original description) (Liu et al., 2013). In contrast, the study done in northwest Algeria, found that the contribution of Posidonia oceanica detritus to the diet of four sea cucumber species was considered minimal (see Belbachir et al., 2019). In addition, an experimental study in Northwest Philippines did not improve the growth rates of Stichopus cf. horrens juveniles despite varying levels of decaying macroalgal and seagrass detritus in the sediments (Palomar-Abesamis et al., 2018). However, the authors stressed that this could also be due to the buildup of organic matter causing an anoxic condition in the culture tanks over a period of time.
There was also no relationship between sea cucumber densities and sediment organic matter content or grain size in the coral reef–seagrass sites of Ishigaki Island, Japan (Tanita and Yamada, 2019). However, using stable isotopes and simulated feeding experiments, Floren et al. (2021) found that 61–70% of the diet of H. scabra and Holothuria atra-leucospilota in the tropical Andaman Sea was derived from the seagrasses E. acoroides, T. hemprichii, and Halophila ovalis. A similar result was found in temperate Mediterranean seagrass meadow wherein the diets of Holothuria poli and Holothuria tubulosa were derived from the seagrasses Cymodocea nodosa and P. oceanica at 63–74% (Boncagni et al., 2019). These findings were supported by an earlier study done in a temperate Northwest Mediterranean seagrass meadow wherein the diet of H. poli and Holothuria tubulosa-mamatta complex was derived mainly from the seagrass P. oceanica (Ricart et al., 2015). These studies provide empirical evidence of the importance of seagrasses as a food source and habitat for the sea cucumbers, although the condition may vary still with the location.
The idea that microorganisms are the primary diet of sea cucumbers (e.g., Plotieau et al., 2014) appears less likely to occur among the detritus-feeding species considering the relatively neutral acidity (6.4–7.7 pH) inside the gut of sea cucumbers (Yokoe and Yasumasu, 1964) and the relatively short residence time of food in the gut of non-ruminants, which are not ideal for digesting bacteria and fibrous plant materials (see Demment and Van Soest, 1985). Although some studies indicate significant changes in the density and composition of the microbial communities transiting through the gut of H. scabra (e.g., Plotieau et al., 2013), this does not necessarily imply the assimilation of microbial biomass by the sea cucumbers. Similarly, although it was demonstrated in hatchery conditions that H. scabra juveniles assimilated 15N-labeled bacterial cell wall from sediments, the concentrations used in the experiment were much higher than those that typically occur in the seagrass meadows, resulting in the rapid assimilation of the radioactive tracer by more than 2000‰ (see Plotieau et al., 2014). In fact, in the gut of the related species, H. atra, the bacterial microflora was not likely digested by the host animal as evidenced by a higher bacterial population in the hindgut than in the foregut (see Ward-Rainey et al., 1996). Overall, microorganisms are crucial to the decomposition of organic matter originating from plants but are insufficient to meet the caloric demands of most deposit feeders, especially when the latter are in high abundance (Lopez and Levinton, 1987; Moriarty et al., 1990). Thus, the idea that microorganisms attached to sediment and detritus particles constitute the major food source for deposit feeders is being replaced by more complex models that incorporate interactions between animals and the food sources in the sedimentary matrix (Lopez and Levinton, 1987).
Synthesis of Concepts
The concepts discussed in the preceding sections are synthesized in a conceptual model illustrating the interaction between sea cucumbers and the biotic and abiotic factors (Figure 1). This model is based on the idea that ecology is about jointly undertaking interactions among organisms and between organisms and their abiotic environment (Olff et al., 2009). The sea seagrass ecosystem has four main components comprised of (1) the primary producers as represented by the seagrasses, (2) the detritivores as represented by the sea cucumbers, (3) the microbial communities, and (4) the abiotic factors, such as environmental conditions, nutrient composition, and sediment grain size. The seagrass–sea cucumber interaction may also form spatial interactions with the adjacent ecosystems through the immigration, export, or harvesting of fisheries. Initially, seagrasses derive their energy from the sun while simultaneously obtaining nutrients from the sediments. As seagrass matures, it undergoes a series of decomposition processes with the aid of the microbial communities whose activity is also dependent on the environmental conditions and the type of substrates (e.g., seagrass species) as an energy source. The detritus formed during seagrass decomposition are consumed by the sea cucumbers or mineralized to become part of the sediments or dissolved substances with the aid of the microorganisms. Excreted materials from the sea cucumbers are then utilized by the seagrasses and microorganisms, forming networks of interactions with the rest of the member compartments. The energy lost from the seagrass ecosystem may also be represented by the harvesting of sea cucumbers, so that the depletion of this resource in the food chain may affect the entire ecosystem.
While there are a growing set of possible important ecological interactions, it was emphasized by Olff et al. (2009) that the consumer and the resource form the backbone of the food web in which consumers interact with their resources. For example, it was concluded by Olff et al. (2009) that the decomposition of plants was primarily regulated by soil macrofauna rather than by climatic and edaphic (abiotic) factors. The rest of the factors affecting the interactions between the biotic and abiotic conditions are extensively discussed elsewhere (e.g., Harrison, 1989; Mazzella et al., 1992; Jernakoff and Nielsen, 1998; Nakaoka, 2005; Bostrom et al., 2006; Heck et al., 2008; Vizzini, 2009; Ralph et al., 2013) and does not require further elaboration.
The conceptual model developed in this review (Figure 1) provides a general framework for the seagrass-holothurian-environment interactions typically occurring in the tropical seagrass ecosystem. This model emphasizes the importance of the seagrass species as a vital component in sustaining the caloric and habitat requirements of the sea cucumbers. In fact, Olff et al. (2009) suggested that food webs should be structured within two main axes of organization: a vertical axis representing trophic position and a new horizontal “ecological stoichiometry” axis representing the decreasing quality of detritus and slower turnover rates (Olff et al., 2009). This new axis had found meaning in the tropical seagrass meadow of Libong Island, Thailand, where the seagrass E. acoroides has been seen as the primary diet source of sea cucumbers, H. scabra and H. atra-leucospilota complex compared to other sources with lower quality (Floren et al., 2021).
A strong point of food web ecology is its promise for generality: it holds the potential to be useful in comparing different ecosystems and hence produce general conclusions on the organizational forces and principles at work (Olff et al., 2009). There is little doubt that seagrass detritus is important to the diet of sea cucumbers in both temperate and tropical seagrass ecosystems, although this contribution to the sea cucumbers may still vary depending on the location and on the species of sea cucumbers and seagrasses. To illustrate, Ricart et al. (2015) found that off the coast of Northeastern Spain, P. oceanica leaves and epiphytes constituted the majority of the diet (up to 63%) of the sea cucumbers, H. poli or H. tubulosa-mamatta complex. An earlier study using a stable carbon isotope conducted in northern Sicily also provided evidence of seagrass detritus (P. oceanica) being assimilated by H. tubulosa (Costa et al., 2014). Additionally, in the tropical seagrass meadow off the coast of Southwest Thailand, the seagrass E. acoroides provided about 61–70% of the diet of H. scabra and H. atra-leucospilota but with less contributions from T. hemprichii and H. ovalis (Floren et al., 2021). In contrast, the contribution of P. oceanica detritus to the diet of four sea cucumber species (Holothuria. sanctori, H. tubulosa, and Holothuria forskali) off the coast of Northwest Algeria was found minimal, although the result could have been different had the mixing model for diet and appropriate trophic enrichments been incorporated in the analyses (see Belbachir et al., 2019). Although there is still limited information on the assimilation of seagrass detritus by the sea cucumbers in the tropical seagrass meadow, the association between tropical seagrasses and sea cucumbers through detritivory is now gaining popularity with the advent of modern analytical approaches, such as the use of stable isotopes.
Factors influencing seagrass–sea cucumber association in tropical seagrass meadows are complex, involving the interaction of climatic, abiotic, biotic, and anthropogenic (i.e., harvesting) factors. Despite the great economic importance of tropical sea cucumbers (e.g., Choo, 2008; Eggertsen et al., 2020), only a few studies were undertaken to understand the complexity of the interactions among the member components in seagrass ecosystems. Such information is vital to the rehabilitation or restoration of sea cucumber populations in the wild, which are now threatened by depletion due to ineffective management schemes. Nevertheless, the advent of modern stable isotope techniques has provided some advances toward understanding the basic consumer–resource interactions which constitute the backbone of the seagrass food web. This review only provides a synthesis of the current information about this topic to provide directions for future research and immediate rehabilitation of existing sea cucumber fisheries. One important aspect of managing sea cucumber resources is the further investigation of the plant decomposition process and the specific metabolic pathways by which products are subsequently assimilated by the sea cucumbers.
In managing sea cucumber fisheries, we advocate for the protection of the seagrass meadows, the habitat and food source for the sea cucumbers. Conversely, the sea cucumber population has to be protected as well, considering its important role in the recycling of nutrients needed by the seagrasses. Additionally, a seasonal closure of fisheries when the sea cucumbers are exhibiting enhanced spawning activities would probably allow the sea cucumber population to recover and replenish the depleted stocks. Alternatively, a minimum size or weight requirement could be implemented to allow the sea cucumbers to reproduce before being harvested. Conservation of sea cucumbers should be biased toward those species with important ecological functions through their bioturbation activities to promote the proper ecosystem functioning of the seagrass meadow. By contrast, some sea cucumber species, such as the H. leucospilota, could be harvested since they have the broadest geographic distributions of all the holothurian species. It is also different from the H. scabra and H. atra (Purcell et al., 2012) because this species can survive in the absence of a seagrass meadow and is unlikely to become depleted.
Author Contributions
AF conceived and wrote the manuscript. PT provided visualizations of the conceptual model. K-IH, SP, and AP provided valuable information and insights on writing this review manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Higher Education Research Promotion and Thailand’s Education Hub for Southern Region of ASEAN countries Project Office of the Higher Education Commission. The support provided by the SSRU, Graduate School of Prince of Songkla University, and Silliman University is also gratefully acknowledged. AP acknowledged the sea cucumber project grant: FDA-CO2561-7938-TH. This work was partially supported by JSPS Core-to-Core Program CREPSUM JPJSCCB20200009.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Special thanks to CE Nuevo for manuscript comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.696134/full#supplementary-material
References
Agawin, N. S. R., and Duarte, C. M. (2002). Evidence of direct particle trapping by a tropical seagrass meadow. Estuaries 25, 1205–1209. doi: 10.1007/BF02692217
Arnull, J., Wilson, A. M. W., Brayne, K., Dexter, K., Donah, A. G., Gough, C. L. A., et al. (2021). Ecological co-benefits from sea cucumber farming: Holothuria scabra increases growth rate of seagrass. Aquat. Environ. Interact. 13, 301–310. doi: 10.3354/aei00409
Belbachir, N. E., Lepoint, G., and Mezali, K. (2019). Comparison of isotopic niches of four sea cucumbers species (Holothuroidea: Echinodermata) inhabiting two seagrass meadows in the southwestern Mediterranean Sea (Mostaganem, Algeria). Belgian J. Zool. 149, 95–106. doi: 10.26496/bjz.2019.32
Bellchambers, L. M., Meeuwig, J. J., Evans, S. N., and Legendre, P. (2011). Modelling habitat associations of 14 species of holothurians from an unfished coral atoll: implications for fisheries management. Aquat. Biol. 14, 57–66. doi: 10.3354/ab00381
Boncagni, P., Rakaj, A., Fianchini, A., and Vizzini, S. (2019). Preferential assimilation of seagrass detritus by two coexisting Mideteranean sea cucumbers: Holothuria polii and Holothuria tubulosa. Estuar. Coast Shelf Sci. 231:106464. doi: 10.1016/j.ecss.2019.106464
Bostrom, C., Jackson, E. L., and Simenstad, C. A. (2006). Seagrass landscapes and their effects on associated fauna: a review. Estuar. Coast Shelf Sci. 68, 383–403. doi: 10.1016/j.ecss.2006.01.026
Bruckner, A. W., Johnson, K. A., and Field, J. D. (2003). Conservation for sea cucumbers: Can a CITES APPENDIX II listing promote sustanable international trade? Bechedemer Inf. Bull. 18, 24–33.
Calumpong, H. P., and Meñez, E. G. (1994). Seagrass beds in the Philippines. Ann. Trop. Re. 16, 1–15.
Cebrián, J., and Duarte, C. M. (1998). Patterns in leaf herbivory on seagrasses. Aquat. Bot. 60, 67–82.
Ceccarelli, D. M., Logan, M., and Purcell, S. W. (2018). Analysis of optimal habitat for captive release of the sea cucumber Holothuria scabra. Mar. Ecol. Prog. Ser. 588, 85–100. doi: 10.3354/meps12444
Choo, P. (2008). Population Status, Fisheries and Trade of Sea Cucumbers in Asia: Sea cucumbers A Glob Rev Fish trade FAO Fish Aquac Tech Pap No 516 81–118, eds V. Toral-Granda, A. Lovatelli, and M. Vascon (Rome: FAO),
Conand, C., Gamboa, R., and Purcell, S. W. (2013). Holothuria atra, Lollyfish. IUCN Red List Threat. Species 2013:8235.
Costa, V., Mazzola, A., and Vizzini, S. (2014). Holothuria tubulosa Gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J. Exp. Mar. Biol. Ecol. 461, 226–232. doi: 10.1016/j.jembe.2014.08.008
Dance, S. K., Lane, I., and Bell, J. D. (2003). Variation in short-term survival of cultured sandfish (Holothuria scabra) released in mangrove-seagrass and coral reef flat habitats in Solomon Islands. Aquaculture 220, 495–505. doi: 10.1016/S0044-8486(02)00623-3
de Fouw, J., van der Heide, T., van Belzen, J., Govers, L. L., Sidi Cheikh, M. A., Olff, H., et al. (2018). A facultative mutualistic feedback enhances the stability of tropical intertidal seagrass beds. Sci. Rep. 8:12988. doi: 10.1038/s41598-018-31060-x
Demment, M., and Van Soest, P. (1985). A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am. Soc. Nat. 125, 641–672. doi: 10.1086/284369
Den Hartog, C. (1970). The Seagrasses of the World. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, AFD. Natuurkunde. Tweede Reeks, Deel 59, No. 1. Amsterdam: Northholland Publishing Company.
Den Hartog, C. (1979). Seagrasses and seagrass ecosystems, an appraisal of the research approach. Aquat. Bot. 7, 105–117. doi: 10.1016/0304-3770(79)90015-9
Dissanayake, D. C. T., and Stefansson, G. (2012). Habitat preference of sea cucumbers: Holothuria atra and Holothuria edulis in the coastal waters of Sri Lanka. J. Mar. Biol. Assoc. U. K. 92, 581–590. doi: 10.1017/s0025315411000051
Domínguez-Godino, J. A., and González-Wangüemert, M. (2020). Habitat associations and seasonal abundance patterns of the sea cucumber Holothuria arguinensis at Ria Formosa coastal lagoon (South Portugal). Aquat. Ecol. 54, 337–354. doi: 10.1007/s10452-020-09746-0
Domínguez-Godino, J. A., Santos, T. F., Pereira, H., Custódio, L., and González-Wangüemert, M. (2019). Seagrass debris as potential food source to enhance Holothuria arguinensis’ growth in aquaculture. Aquac Res. 51, 1487–1499. doi: 10.1111/are.14495
Duarte, C. M., Middeburg, J. J., and Caraco, N. (2004). Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2:2005. doi: 10.5194/bg-2-1-2005
Eggertsen, M., Eriksson, H., Slater, M. J., Raymond, C., and de la Torre-Castro, M. (2020). Economic value of small-scale sea cucumber fisheries under two contrasting management regimes. Ecol. Soc. 25:20. doi: 10.5751/ES-11436-250220
Estacion, J., and Fortes, M. D. (1988). Growth rates and primary production of Enhalus acoroides (L . f .) royle from Lag-it, North Bais Bay, the Philippines. Aquat. Bot. 29, 347–356. doi: 10.1016/0304-3770(88)90078-2
Floren, A., Hayashizaki, K., Tuntiprapas, P., and Prathep, A. (2021). Contributions of seagrasses and other sources to sea cucumber diets in a tropical seagrass ecosystem. Chiang Mai J. Sci. 48, 1259–1270.
Fourqurean, J., and Schrlau, J. (2003). Changes in nutrient content and stable isotope ratios of C and N during decomposition of seagrasses and mangrove leaves along a nutrient availability gradient in Florida Bay, USA. Chem. Ecol. 19, 373–390. doi: 10.1080/02757540310001609370
Gessner, M. O., Swan, C. M., Mckie, B. G., Dang, C. K., Bardgett, R. D., Wall, D. H., et al. (2010). Diversity meets decomposition. Trends Ecol. Evol. 25, 372–380. doi: 10.1016/j.tree.2010.01.010
Hair, C., Militz, T., Daniels, N., and Southgate, P. C. (2020). Comparison of survival, growth and burrying behavior of cultured and wild sandfish (Holothuria scabra) juveniles: Implications for ocean mariculture. Aquaculture 526:735355. doi: 10.1016/j.aquaculture.2020.735355
Hair, C., Mills, D. J., McIntyre, R., and Southgate, P. C. (2016). Optimising methods for community-based sea cucumber ranching: experimental releases of cultured juvenile Holothuria scabra into seagrass meadows in Papua New Guinea. Aquat. Rep. 3, 198–208. doi: 10.1016/j.aqrep.2016.03.004
Hamel, J. F., Conand, C., Pawson, D. L., and Mercier, A. (2001). The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): its biology and exploitation as Beche-de-mer. Adv. Mar. Biol. 41, 129–223. doi: 10.1016/S0065-2881(01)41003-0
Hamel, J.-F., Mercier, A., Conand, C., Purcell, S., Toral-Granda, V., and Gamboa, R. (2013). Holothuria scabra, golden sandfish. IUCN Red List Threat. Species 2013:8235. doi: 10.2305/IUCN.UK.2013-1.RLTS.T180257A1606648.en
Harmon, M. E., Silver, W. L., Fasth, B., Chen, H., Burke, I. C., Partons, W. J., et al. (2009). Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Glob. Chang. Biol. 15, 1320–1338. doi: 10.1111/j.1365-2486.2008.01837.x
Harrison, P. (1989). Detrital proessing in seagrass systems: a review of factors affecting decay rates, remineralization and detritivory. Aquat. Bot. 23, 263–288. doi: 10.1016/0304-3770(89)90002-8
Heck, K. L., Carruthers, T. J. B., Duarte, C. M., Hughes, A. R., Kendrick, G., Orth, R. J., et al. (2008). Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 11, 1198–1210. doi: 10.1007/s10021-008-9155-y
Indriana, L., Wahyudi, A., and Kunzmann, A. (2018). Assimilation dynamics of different diet sources by the sea cucumber Holothuria scabra, with evidence from stable isotope signature. Annu. Res. Rev. Biol. 28, 1–10. doi: 10.9734/arrb/2018/42591
Jernakoff, P., and Nielsen, J. (1998). Plant – animal associations in two species of seagrasses in Western Australia. Aquat. Bot. 60, 359–376.
Kilminster, K., Mcmahon, K., Waycott, M., Kendrick, G. A., Scanes, P., McKenzie, L., et al. (2015). Unravelling complexity in seagrass systems for management: Australia as a microcosm. Sci. Total Environ. 534, 97–109. doi: 10.1016/j.scitotenv.2015.04.061
Kinch, J., Purcell, S., Uthicke, S., and Friedman, K. (2008). Population Status, Fisheries and Trade of Sea Cucumbers in the Western Central Pacific. In V. Toral-Granda, A. Lovatelli and M. Vasconcellos. Sea cucumbers. A Global Review of Fisheries and Trade. FAO Fisheries and Aquaculture Technical Paper. No. 516. Rome: FAO, 7–55.
Kon, K., Tongnunui, P., and Kurokura, H. (2015). Do allochthonous inputs represent an important food resource for benthic macrofaunal communities in tropical estuarine mudflats? Food Webs 2, 10–17. doi: 10.1016/J.FOOWEB.2015.03.001
Lavelle, P., Blanchart, E., Martin, A., Martin, S., and Spain, A. (1993). A hierarchical model for decomposition in terrestrial ecosystems: application to soils of the humid tropics. Biotropica 25, 130–150.
Lee, S. D. A. (2016). Sedimentary Response to Sea Cucumber (Holothuria scabra) Removal. MS Thesis. Bremen: University of Bremen, 71.
Lepoint, G., Nyssen, F., Gobert, S., Dauby, P., and Bouquegneau, J. M. (2000). Relative impact of a seagrass bed and its adjacent epilithic algal community in consumer diets. Mar. Biol. 136, 513–518. doi: 10.1007/s002270050711
Liu, X., Zhou, Y., Yang, H., and Ru, S. (2013). Eelgrass detritus as a food source for the sea cucumber Apostichopus japonicus Selenka (Echinidermata: Holothuroidea) in Coastal Waters of North China: an experimental study in flow-through systems. PLoS One 8:e58293. doi: 10.1371/journal.pone.0058293
Lopez, G. R., and Levinton, J. S. (1987). Ecology of deposit-feeding animals in marne sediments. Q. Revies. Biol. 62, 235–260. doi: 10.1086/415511
Massin, C., and Duomen, C. (1986). Distribution and feeding of epibenthic holothuroids on the reef flat of Laing Island (Papua New Guinea). Mar. Ecol. Prog. Ser. 31, 185–195.
Massin, C., and Jangoux, M. (1976). Obervations Écologiues sur Holothuria tubulosa, H. Poli et H. forskali (Echinodermata-Holothuroidea) et comportement alimentaire de H. tubulosa. Cah. Biol. Mar. 17, 45–59.
Massin, C., Mercier, A., and Hamel, J. F. (2000). Ossicle change in Holothuria scabra with a discussion of ossicle evolution within the Holothuriidae (Echinodermata). Acta Zool. 81, 77–91. doi: 10.1046/j.1463-6395.2000.00039.x
Mateo, M. A., and Romero, J. (1996). Evaluating seagrass leaf litter decomposition: an experimental comparison between litter-bag and oxygen-uptake methods. J. Exp. Mar. Biol. Ecol. 202, 97–106. doi: 10.1016/0022-0981(96)00019-6
Mazzella, L., Buia, M. C., Gambi, M., Lorenti, M., Russo, G. F., Scipione, M. B., et al. (1992). “Plant-animal trophic relationships in the Posidonia oceanica ecosystem of the Mediterranean sea: a review,” in Plant-Animal Interactions in the Marine Benthos, eds D. M. John, S. J. Hawkins, and J. H. Price (Oxford: Clarendon Press), 165–187.
Mercier, A., Battaglene, S. C., and Hamel, J. F. (2000). Settlement preferences and early migration of the tropical sea cucumber Holothuria scabra. J. Exp. Mar. Biol. Ecol. 249, 89–110. doi: 10.1016/S0022-0981(00)00187-8
Mezali, K., and Soualili, D. L. (2013). Capacité de Sélection des Particules Sédimentaires et de la Matière Organique chez les Holothuries. La Beche-de-Mer, Bull la CPS No33. Noumea: Section information halietique CPS, 38–43.
Moriarty, D. I. W., Roberts, D. G., and Pollard, P. C. (1990). Primary and bacterial productivity of tropical seagrass communities in the Gulf of Carpentaria, Australia. Mar. Ecol. Prog. Ser. 61, 145–157. doi: 10.3354/meps061145
Mosher, C. (1980). Distribution of Holothuria arenicola Semper in the Bahamas with observations on habitat, behavior, and feeding acitvity (Echinodermata: Holothuroidea). Bull. Mar. Sci. 30, 1–12.
Nakaoka, M. (2005). Plant-animal interactions in seagrass beds: Ongoing and future challenges for understanding population and community dynamics. Pop. Ecol. 47, 167–177. doi: 10.1007/s10144-005-0226-z
Nishihama, S., and Tanita, I. (2021). Holothurian assemblages before the harvest-boom era in inner reefs of Ishigaki Island, focusing on population dynamics of lollyfish Holothuria atra Jäger, 1883. Plankton Benthos Res. 16, 165–178. doi: 10.3800/pbr.16.165
Olff, H., Alonso, D., Berg, M. P., Eriksson, B. K., Loreau, M., Piersma, T., et al. (2009). Parallel ecological networks in ecosystems. Philos Trans. R. Soc. B Biol. Sci. 364, 1755–1779. doi: 10.1098/rstb.2008.0222
Orth, R. J., Heck, K. L., and vans Montefrans, J. (1984). Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator-prey relationships. Esturies. 7, 339–350. doi: 10.2307/1351618
Palomar-Abesamis, N., Abesamis, R. A., and Juinio-Meñez, M. A. (2017). Distribution and microhabitat associations of the juveniles of a high-value sea cucumber, Stichopus cf. horrens, in northern Philippines. Aquat. Ecol. 51, 17–31. doi: 10.1007/s10452-016-9591-2
Palomar-Abesamis, N., Juinio-Meñez, M. A., and Slater, M. J. (2018). Macrophyte detritus as nursery diets for juveniles sea cucumber Stichopus cf. horrens. Aquat. Res. 49, 3614–3623. doi: 10.1111/are.13829
Panyawai, J., Tuntiprapas, P., and Prathep, A. (2019). High macrophyte canopy complexity enhances sediment retention and carbon storage in coastal vegetative meadows at Tangkhen Bay, Phuket, Southern Thailand. Ecol. Res. 34, 210–212. doi: 10.1111/1440-1703.1066
Peduzzi, P., and Herndl, G. J. (1991). Decomposition and significance of seagrass leaf litter (Cymodocea nodosa) for the microbial food web in coastal waters (Gulf of Trieste, northern Adriatic Sea). Mar. Ecol. Prog. Ser. 71, 163–174. doi: 10.3354/meps071163
Plotieau, T., Lavitra, T., Gillan, D. C., and Eeckhaut, I. (2013). Bacterial diversity of the sediments transiting through the gut of Holothuria scabra (Holothuroidea?; Echinodermata). Mar. Biol. 160, 3087–3101. doi: 10.1007/s00227-013-2297-2
Plotieau, T., Lepoint, G., Lavitra, T., and Eeckhaut, I. (2014). Isotopic tracing of sediment components assimilated by epibiontic juveniles of Holothuria scabra (Holothuroidea). J. Mar. Biol. Assoc. U. K. 94, 1–6. doi: 10.1017/S0025315414000502
Pollard, P. C., and Moriarty, D. J. W. (1991). Organic carbon decomposition, primary and bacterial productivity, and sulphate reduction, in tropical seagrass beds of the Gulf of Carpentaria, Australia. Mar. Ecol. Prog. Ser. 69, 149–159. doi: 10.3354/meps069149
Purcell, S. W., and Simutoga, M. (2008). Spatio-temporal and size-dependent variation in the success of releasing cultured sea cucumbers in the wild. Rev. Fish. Sci. 16, 204–214. doi: 10.1080/10641260701686895
Purcell, S. W., Samyn, Y., and Conand, C. (2012). Commercially Important Sea Cucumbers of the World, FAO Species Catalogue for Fishery Purposes No. 6. Rome: Food and Agriculture Organization of the United Nations.
Ralph, G. M., Seitz, R. D., Orth, R. J., Knick, K. E., and Lipcius, R. M. (2013). Broad-scale assocaition between sagrass cover and juvenile blue crab density in Chesapeake Bay. Mar. Ecol. Prog. Ser. 488, 51–63.
Rattanachot, E., and Prathep, A. (2011). Temporal variation in growth and reproduction of Enhalus acoroides (L . f .) Royle in a monospecific meadow in Haad Chao Mai National Park, Trang Province, Thailand. Bot. Mar. 54, 201–207. doi: 10.1515/bot.2011.018
Rattanachot, E., and Prathep, A. (2015). Species specific effects of three morphologically different belowground seagrasses on sediment properties. Estuar. Coast. Shelf Sci. 167, 427–435. doi: 10.1016/j.ecss.2015.10.019
Ricart, A. M., Dalmau, A., Perez, M., and Romero, J. (2015). Effects of landscape configuration on the exchange of materials in seagrass ecosystems. Mar. Ecol. Prog. Ser. 532, 89–100. doi: 10.3354/meps11384
Robertson, M. L., Mills, A. L., and Zieman, J. C. (1982). Microbial synthesis of detritus-like particulates from dissolved organic carbon released by tropical seagrasses. Mar. Ecol. Prog. Ser. 7, 279–285.
Robinson, G., and Lovatelli, A. (2015). Global sea cucumber fisheries and aquaculture FAO’s inputs over the past few years. FAO Aquac Newsl 53, 55–57.
Rogers, A., Hamel, J. F., and Mercier, A. (2018). Population structure and reproductive cycle of the commercial sea cucumber Holothuria mexicana (Echinodermata: Holothuroidea) in Belize. Rev. Biol. Trop. 66, 1624–1648. doi: 10.15517/rbt.v66i4.32551
Short, F., Carruthers, T., Dennison, W., and Waycott, M. (2007). Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Biol. Ecol. 350, 3–20. doi: 10.1016/j.jembe.2007.06.012
Long, B. G., Skewes, T. D., Dennis, D. M., Poiner, I. R., Pitcher, C. R., Taranto, T. J., et al. (1996). Distribution and abundance of beche-de-mer on Torres Strait reefs on Torres Strait reefs. Beche demer Inf. Bull. 9, 17–27.
Stankovic, M., Hayashizaki, K. I., Tuntiprapas, P., Rattanachot, E., and Prathep, A. (2021). Two decades of seagrass area change: organic carbon sources and stock. Mar. Pollut. Bull. 163:111913. doi: 10.1016/j.marpolbul.2020.111913
Tanita, I., and Yamada, H. (2019). Distribution of sea cucumbers in relation to sediment characteristics in coral reef lagoons and adjacent waters around Ishigaki Island, southern Japan. Mar. Ecol. 40:e12564. doi: 10.1111/maec.12564
Thayer, G. W., Bjorndal, K. A., Ogden, J. C., Williams, S. L., and Zieman, J. C. (1984). Role of herbivores in seagrass communities. Estuarine 7, 351–376. doi: 10.2307/1351619
Trevathan-Tackett, S. M., Macreadie, P. I., Sanderman, J., Ralph, P. J., Howes, J. M., and Ralph, P. J. (2017). A Global assessment of the chemical recalcitrance of seagrass tissues: implications for long-term carbon sequestration. Front. Plant Sci. 8:925. doi: 10.3389/fpls.2017.00925
Uthicke, S., and Klumpp, D. W. (1998). Microphytobenthos community production at a near-shore coral reef: seasonal variation and repsonse to ammonium recycled by holothurians. Mar. Ecol. Prog. Ser. 169, 1–11.
Vincent, I. V., and Morrison-Saunders, A. (2013). Applying sustainability assessment thinking to a community-governed development: a sea cucumber farm in Madagascar. Impact Asses. Proj. Apprais. 31, 208–213. doi: 10.1080/14615517.2013.773720
Vizzini, S. (2009). Analysis of the trophic role of Mediterranean seagrasses in marine coastal ecosystems: a review. Bot. Mar. 52, 383–393. doi: 10.1515/BOT.2009.056
Wang, W., Yuan, T., Cui, B., and Dai, Y. (2013). Bioresource technology investigating lignin and hemicellulose in white rot fungus-pretreated wood that affect enzymatic hydrolysis. Bioresour. Technol. 134, 381–385. doi: 10.1016/j.biortech.2013.02.042
Ward, B. (2014). “Bacterial energy metabolism,” in Molecular Medical Microbiology, eds Y. W. Tang, M. Sussman, D. Liu, I. Poxton, and J. Schwartzman (Amsterdam: Elsevier Ltd), 201–233. doi: 10.1016/B978-0-12-397169-2.00011-1
Ward-Rainey, N., Rainey, F. A., and Stackebrandt, E. (1996). A study of the bacterial flora associated with Holothuria atra. J. Exp. Mar. Biol. Ecol. 203, 11–26. doi: 10.1016/0022-0981(96)02566-X
Wolkenhauer, S., Uthicke, S., Burridge, C., Skewes, T., and Pitcher, R. (2010). The ecological role of Holothuria scabra (Echinodermata: Holothuroidea) within subtropical seagrass beds. J. Mar. Biol. Assoc. U. K. 90, 215–223. doi: 10.1017/S0025315409990518
Yokoe, Y., and Yasumasu, I. (1964). The distribution of cellulase in invertebrates. Comp. Biochem. Physiol. 13, 323–338. doi: 10.1016/0010-406x(64)90027-1
Keywords: trophic interaction, seagrasses, holothurians, habitat, management
Citation: Floren AS, Hayashizaki K, Putchakarn S, Tuntiprapas P and Prathep A (2021) A Review of Factors Influencing the Seagrass-Sea Cucumber Association in Tropical Seagrass Meadows. Front. Mar. Sci. 8:696134. doi: 10.3389/fmars.2021.696134
Received: 16 April 2021; Accepted: 21 October 2021;
Published: 15 November 2021.
Edited by:
Rochelle Diane Seitz, William & Mary’s Virginia Institute of Marine Science, College of William & Mary, United StatesReviewed by:
Heather D. Penney, Memorial University of Newfoundland, CanadaTim Smith, James Cook University, Australia
Copyright © 2021 Floren, Hayashizaki, Putchakarn, Tuntiprapas and Prathep. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adonis S. Floren, ZG5zZmxvcmVuQGdtYWlsLmNvbQ==
 Adonis S. Floren
Adonis S. Floren Ken-ichi Hayashizaki
Ken-ichi Hayashizaki Sumaitt Putchakarn
Sumaitt Putchakarn Piyalap Tuntiprapas
Piyalap Tuntiprapas Anchana Prathep
Anchana Prathep