- 1School of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel
- 2The Steinhardt Museum of Natural History, Israel National Center for Biodiversity Studies, Tel Aviv University, Tel Aviv, Israel
Polychaetes are among the most common marine organisms, and in many habitats they dominate both in species richness and abundance. They are often found in association with other organisms. Specifically, sponges with their complex three-dimensional internal architecture, are known to host a great diversity of polychaetes. Due to the fact that a large number of sponge-associated polychaete species are known to be common on other substrates, most studies agree that they represent an opportunistic part of the sponge-inhabiting assemblage, without any selection of species tightly associated with sponges. The current study aimed to shed light on polychaetes affinity to sponges. We applied Clarke and Warwick’s taxonomic distinctness indices and test for random assembly, to a dataset compiled of 13 publications that provided polychaete species lists from massive-sponges across the Mediterranean Sea, and compared them with benthic-polychaete species lists from all of the Mediterranean and its zoogeographical regions. These indices are considered to be independent of sampling efforts and setting, and can be applied on presence/absence data, making it possible to compare data from multiple studies. We further compared the trophic structure of sponge-associated polychaetes between the Mediterranean’s zoogeographical regions. Our results show that the trophic structure of the sponge-associated polychaete community, was found to be stable across the entire Mediterranean Sea. Moreover, randomization tests showed that at almost all observational scales (e.g., study, region, Mediterranean) the phylogenetic diversity is not assembled at random, and that sponge-associated polychaete communities are composed of taxonomically related species. These results support the statement that polychaete assemblages inhabiting sponges are not a transient facultative assembly of species, but rather stable, diverse and specialized communities which are well adapted for life in this habitat.
Introduction
Sponges have a great ecological and economic value, in part due to their three-dimensional complex structure, which supports rich metazoans communities, by providing micro-habitats and ideal shelters (Gerovasileiou et al., 2016). A major component of these communities are polychaetes (phylum: Annelida), often the dominant group in terms of abundance, richness, or biomass (Pansini, 1970; Rützler, 1976; Amoureux et al., 1980; Pansini and Daglio, 1980; Alos et al., 1981; Peattie and Hoare, 1981; Westinga and Hoetjes, 1981; Dauer, 1984; Koukouras et al., 1985, 1992, 1996; Voultsiadou-Koukoura et al., 1987; Cinar and Ergen, 1998; Çinar et al., 2019). Sponge-associated fauna, and specifically sponge-associated polychaetes, have been studied extensively around the world, from the tropics to the poles (Klitgaard, 1995; Ribeiro et al., 2003; Abdo, 2007; Fiore and Jutte, 2010; Schejter et al., 2012; Kersken et al., 2014; Gerovasileiou et al., 2016). Among polychaetes, only a few species may be considered truly obligatory to sponges, presenting adaptations to life within a sponge, e.g., parasitic forms such as Dorvillea sociabilis, Branchiosyllis exilis, Branchiosyllis oculata, Haplosyllis spongicola, Exogone spp., or commensal forms such as Potamilla symbiotica, Hydroides spongicola, and Sphinther arcticus (Martin and Britayev, 1998). However, in most cases the nature of the relationships between the polychaetes and their sponge hosts remains unknown. Moreover, most studies agree that polychaetes represent an opportunistic part of the sponge-inhabiting assemblage, without any selection of species tightly associated with sponges (Long, 1968; Frith, 1976; Alos et al., 1981; Koukouras et al., 1985, 1996; Gherardi et al., 2001). The main reason for the latter statement is due to the fact that a large number of sponge-associate polychaete species are known to be common on other substrates, therefore, their composition is related to the polychaete fauna of the surrounding benthos, rather than to the sponge itself (Long, 1968; Alos et al., 1981; Peattie and Hoare, 1981; Koukouras et al., 1985, 1996). By contrast, Westinga and Hoetjes (1981) and Koukouras et al. (1985) have stated that sponge-associated fauna constitutes a real “ecological community” characterized by a constant composition.
Based on the above statement and on our own observations, we hypothesize that sponge-associated polychaetes communities are not a random assembly. The current study examined this apparent discrepancy and aimed to shed light on polychaetes affinity to (massive) sponges. Massive sponges are defined in the “Thesaurus of sponge morphology” (Boury-Esnault and Rützler, 1997) as large compact sponges without definable shape. Most of the studies investigating sponge-inhabitants deals with massive sponges, as they usually contain large oscula and inner spaces, that can support greater diversity of organisms. By using data mined from the literature and applying Clarke and Warwick’s randomization test (1998), we wished to determine the extent to which sponge-associated polychaete species lists represent random subset from the larger benthic polychaete species pool (Clarke and Warwick, 1998; Warwick et al., 2002; Somerfield et al., 2009). We further aimed to find whether polychaetes constitute a constant and stable community inside sponges, across several zoogeographic regions.
Materials and Methods
Faunistic Data Collection and Taxonomic Updating
In order to obtain the entire available information on sponge-inhabiting polychaete assemblages reported from the Mediterranean Sea, literature describing sponge-associated macroinvertebrates communities was examined. In total 13 publications provided polychaete species lists from massive-sponges along the Mediterranean covering a time span of 95 years (Santucci, 1922; Pansini and Daglio, 1980; Alos et al., 1981; Koukouras et al., 1985, 1996; Voultsiadou-Koukoura et al., 1987; Cinar and Ergen, 1998; Gherardi et al., 2001; Gerovasileiou et al., 2016; Pavloudi et al., 2016; Papatheodoulou et al., 2019; Çinar et al., 2019; Goren et al., 2021). In accordance with previous studies, the Mediterranean Sea was divided to five zoogeographic zones (Figure 1; Arvanitidis et al., 2002; Voultsiadou, 2009; Chatzigeorgiou et al., 2017). Literature data were incorporated into a Microsoft Excel database along with zoogeographic (study area, region, and entire Mediterranean Sea), ecological (feeding guilds), and taxonomical information.
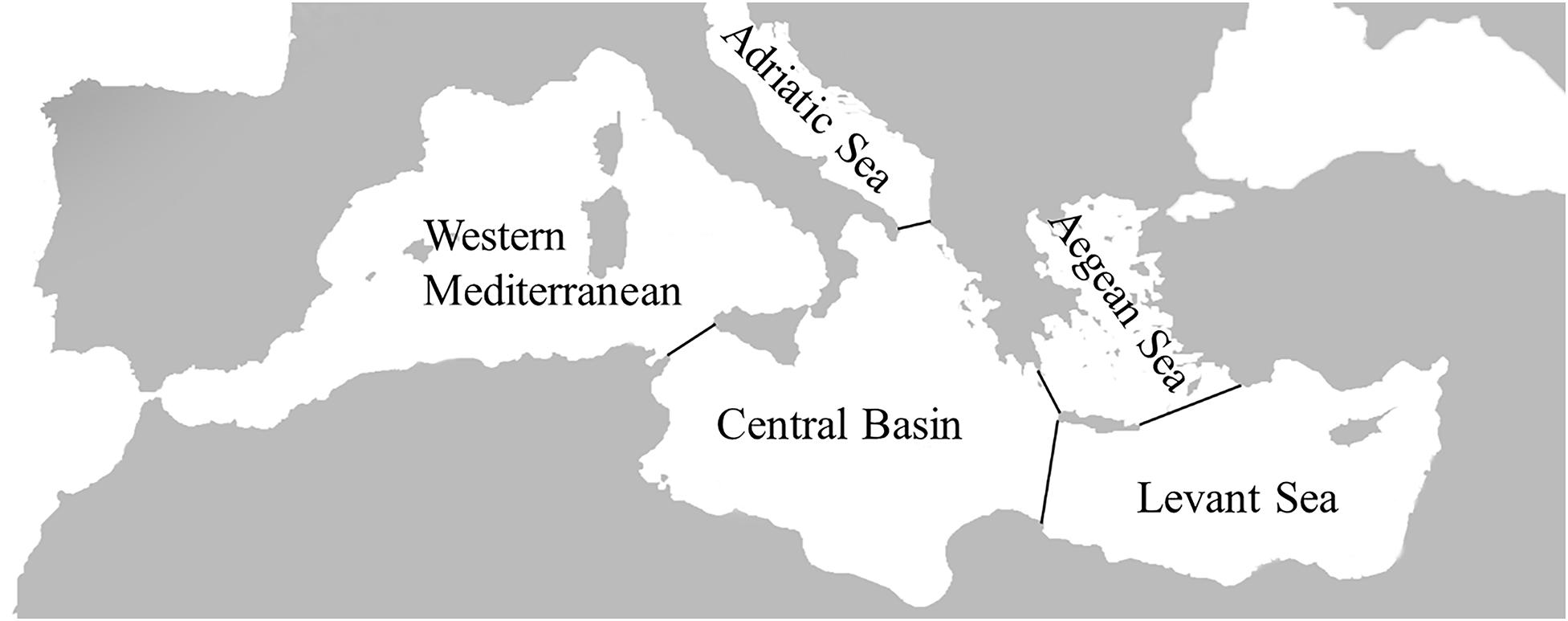
Figure 1. Map of the Mediterranean Sea and the Zoogeographic zones within it considered in this study.
Species distribution data in the Mediterranean Sea were compiled from the literature (Campoy, 1979; Ben-Eliahu and Boudouresque, 1995; Arvanitidis, 2000; Bellan, 2001; Arvanitidis et al., 2002; Çinar, 2005; Castelli et al., 2008; Coll et al., 2010; Gil, 2011; Çinar et al., 2014; Mikac, 2015; Faulwetter et al., 2017), to construct the master list of benthic polychaeta and subsequent regional lists, including lists of only sponge-associated polychaetes. Species known to be exclusively pelagic have been excluded from this inventory. The corresponding higher taxonomic classification was based on what is proposed in the World Register of Marine Species (WoRMS). Polychaete species were assigned to feeding guilds categories according to Fauchald and Jumars (1979), Jumars et al. (2015) and the PolyTraits website (Faulwetter et al., 2014). Five categories of trophic guilds were used: carnivores, herbivores, omnivores, deposit feeders and filter feeders.
Statistical Analysis
Pearson correlation was used to test the relationship between the number of studies conducted in a region and the number of sponge-associated polychaete species found in it. It was also used to test if there is a correlation between the number of polychaete species and the number of host sponge species studied in each region. An initial binary matrix was constructed for species’ presence/absence in the Mediterranean areas. Bray Curtis dissimilarity coefficients were utilized to create a similarity matrix (Legendre and Legendre, 1998), that was used for both cluster analysis (group average linkage) and non-metric multidimensional scaling (nMDS). Analyses were performed using R version 3.6.1 (R Core Team, 2019), RStudio (Rstudio Team, 2020), and the Vegan package (Oksanen et al., 2013).
Taxonomic Relatedness and Test for Random Assembly
In order to test whether sponge-associated polychaete species lists are randomly assembled, from those of the benthic polychaete species pools in the respective and larger geographic regions, we employed a hierarchical approach. Three successive levels of comparison in the analysis have been defined: (a) study, (b) region (Levant Sea, Aegean Sea, Central Basin, Adriatic Sea, and Western Mediterranean), and (c) master (Mediterranean species pool; Figure 2).
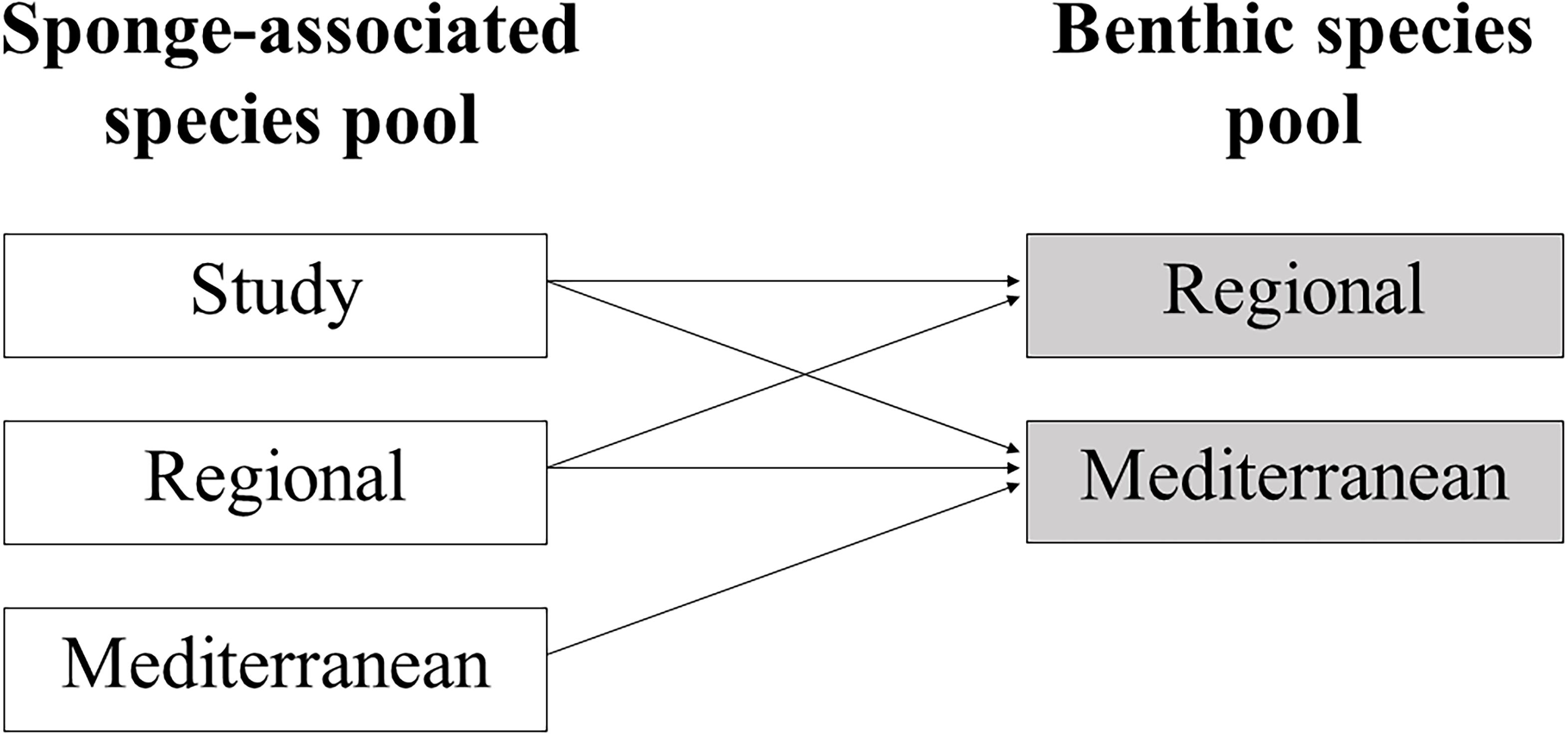
Figure 2. Diagram representing the different levels of comparisons in the taxonomic relatedness analysis.
We used the two indices of taxonomic relatedness, the average taxonomic distinctness (Δ+) and the variation in taxonomic distinctness (Λ+) (Clarke and Warwick, 1998, 2001). The indices were calculated for each of the locations from the 13 published studies. These indices were selected because they have been shown to be insensitive to sample-size and sample-effort (Clarke and Warwick, 2001). Both indices use the presence/absence data: Δ+ calculates the average path length between every pair of species using the information on their higher taxonomic classification within a sample; the Λ+ index assesses how evenly the species are distributed, in higher taxonomic categories. The 95% confidence limits of the expected distribution of both indices’ values were calculated by simulations derived from the randomly constructed subsets of species from the regional (Western Mediterranean, Central Basin, Adriatic, Aegean, and Levant Basin) and the Mediterranean benthic polychaete species pools. The Δ+/Λ+ values calculated for the local (each study), regional and Mediterranean sponge-associated polychaete species-pools, were then superimposed on these funnel-shaped confidence limits. Data points which fall outside the relevant 95% contour imply a statistically significant departure from expectation (Clarke and Warwick, 2001), and indicate that species are more or less (above or below the funnel, respectively) closely related to each other than would be expected if they were assembled at random.
Four taxonomic levels were considered; species, genus, family, and order. Branch lengths between the taxonomic classes were defined following Clarke and Warwick (2001). We assumed equal step lengths between each successive taxonomic level, setting the path length ω to 100 for two species connected at the highest possible level (taxonomically coarsest). So, the weights are ω = 25 (same genus, but different species), ω = 50 (same family but different genera), ω = 75 (same order but different families) and ω = 100 (different orders).
We further modified the methodology suggested by Somerfield et al. (2009), we used a randomization test in which the criterion set was that if species inhabiting sponges are assembled randomly from the larger, local, regional, and Mediterranean benthic pools, than on average, 95% of the calculated indices values, should fall within the 95% probability limit set for these tests. All analyses were performed using the PRIMER v.7 (Clarke and Warwick, 1998).
Results
Literature study showed that in total 210 polychaete species from 30 families were recorded (Supplementary Table 1) in 11 species of host sponges (Supplementary Table 2). The sponge-associated polychaete species represent ∼19% of the benthic polychaete fauna of the Mediterranean and about 43% of the families. The number of species reported from sponges in the Mediterranean and the number of studies considered in this research are shown in Figure 3. No clear pattern of an increase or a decrease in richness was found according to geographic gradients. The sponge-associated polychaete fauna of the Aegean was found to be the richest, but it is also the best studied area (seven studies), while the Adriatic and the Central Basin are by far the most impoverished and least studied (with one study each area). There is a strong positive correlation between the number of species in a region and the number of studies conducted in it (Pearson correlation, df = 3, Rho = 0.91, p = 0.03). A similar positive correlation was found between the number of polychaete species and the number of host sponge species studied in each region (Pearson correlation, df = 3, Rho = 0.97, p = 0.004; Figure 4).
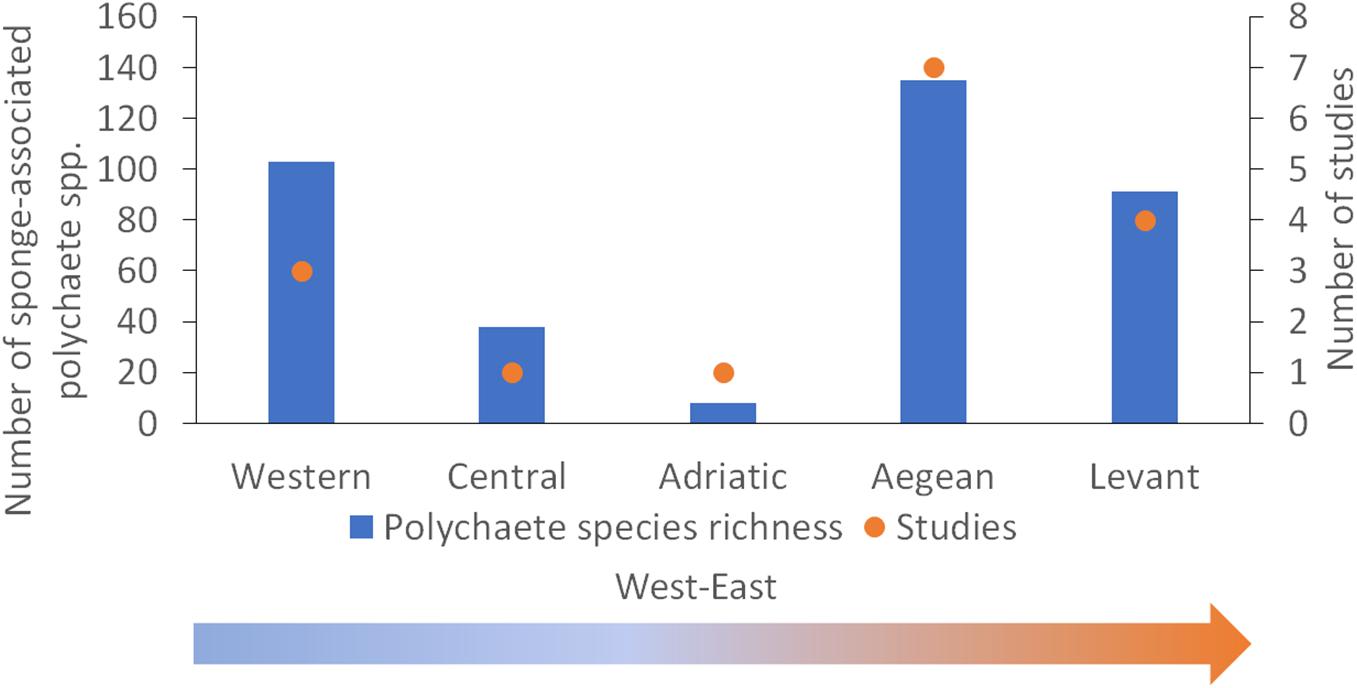
Figure 3. The number of polychaete species reported from sponges in the Mediterranean Sea and the number of studies considered in this research.
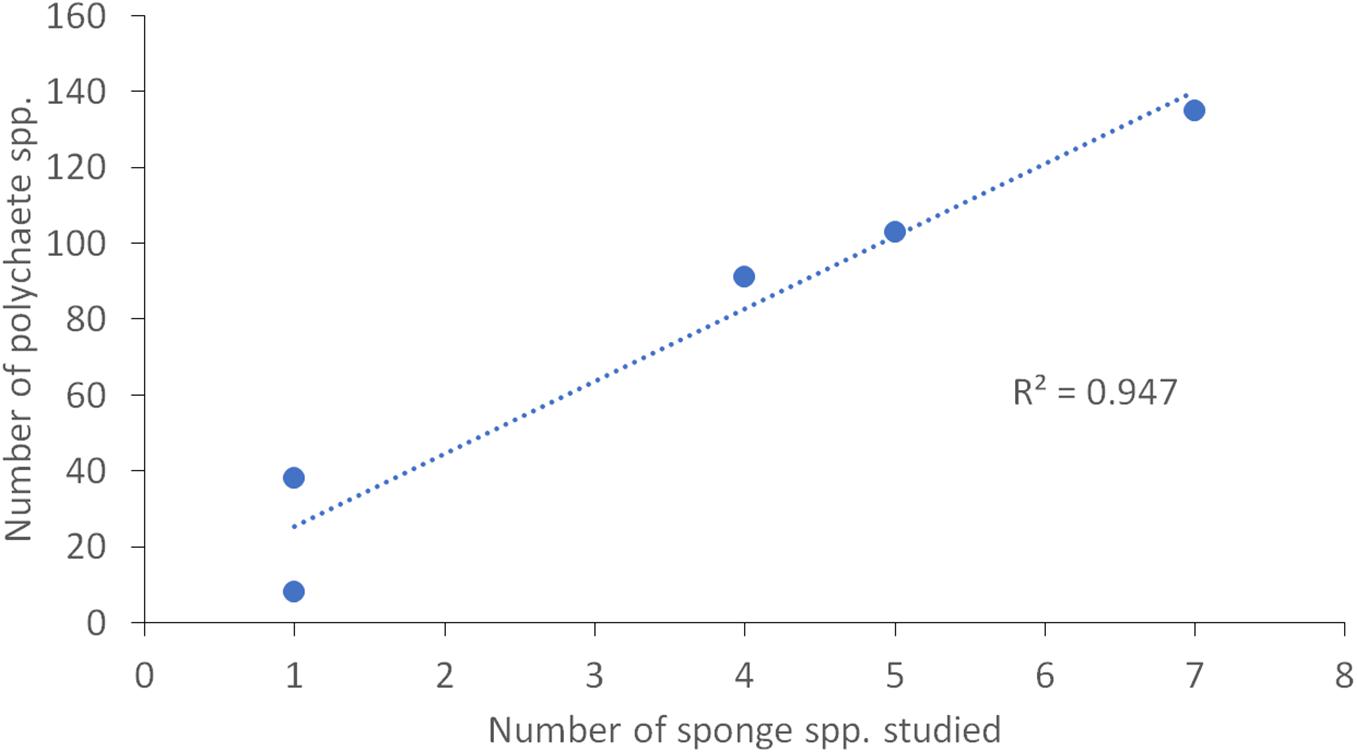
Figure 4. Correlation between the number of sponge-associated species and the number of host sponge species studied in each zoogeographic region in the Mediterranean Sea.
Estimation of resemblance among the polychaete species of the five Mediterranean regions by group-average-linkage cluster analysis, revealed a similarity of 40% between the Western Mediterranean and the Aegean and Levant Seas. The first two regions had a 50% similarity between them. These results are visualized in an nMDS ordination (Figure 5).
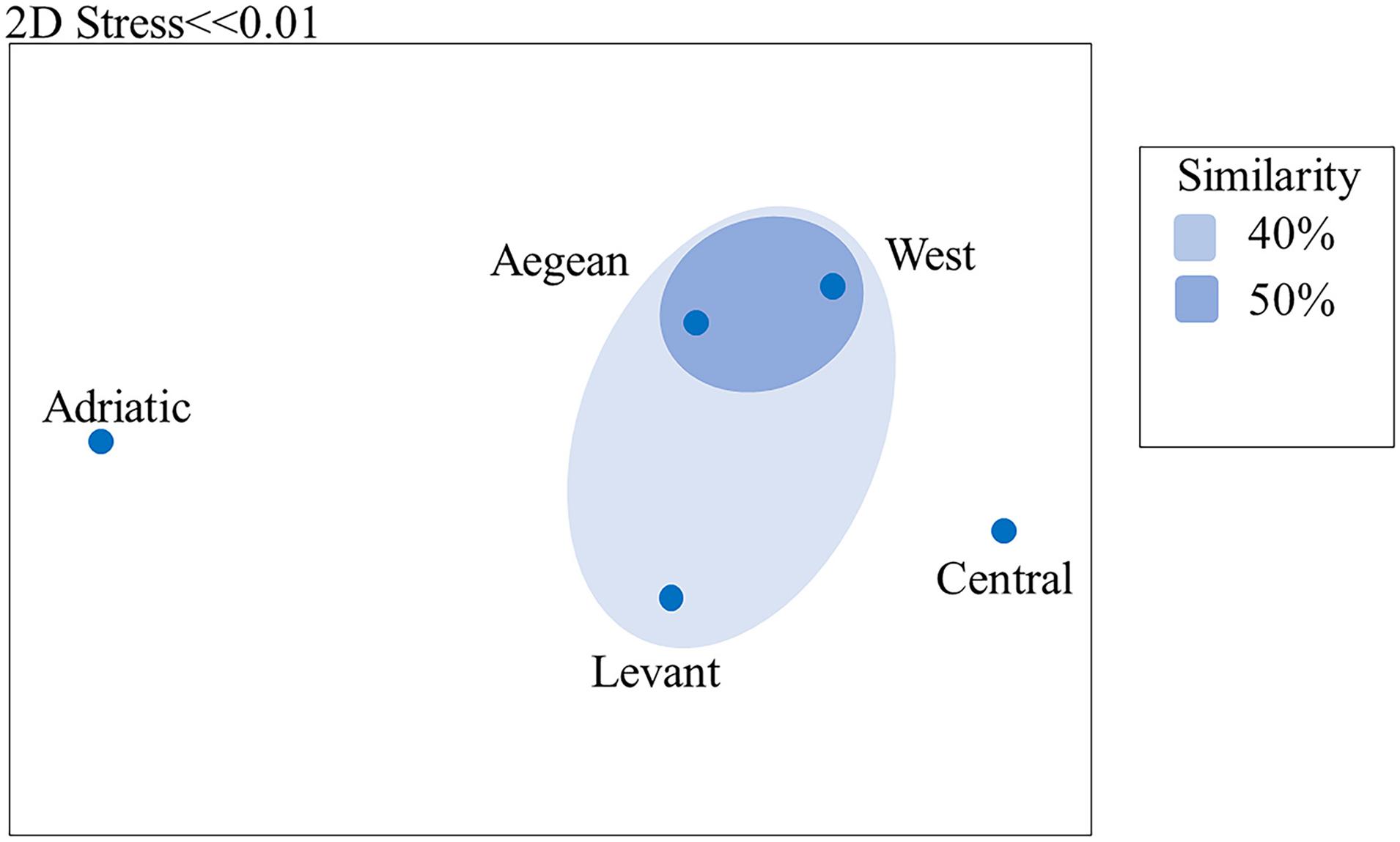
Figure 5. Resemblance of sponge-associated polychaete assemblages in the Mediterranean regions demonstrated in nMDS plot. Similarity was calculated by group-average-linkage cluster-analysis.
An estimation of the resemblance among the polychaete families (not shown) of the five Mediterranean regions yielded the same pattern but with 60% similarity between the Aegean, Levant and Western Mediterranean, and 70% similarity between the Aegean Sea and the Western Mediterranean.
Five feeding guilds were characterized in the list of the sponge-associated polychaetes in the Mediterranean Sea (Figure 6): carnivores (72 spp.), deposit feeders (54 spp.), omnivores (40 spp.), filter feeders (39 spp.), and herbivores (10 spp.). Carnivores were the richest group in the Western Mediterranean, the Aegean and the Levant Seas, followed by omnivores. In the Adriatic Sea, both carnivores and omnivores were the most speciose groups, and in the Central Mediterranean, omnivores were the richest group followed by carnivores. The trophic composition, did not differ between the regions. As the Adriatic Sea had only eight species, it was excluded from this analysis.
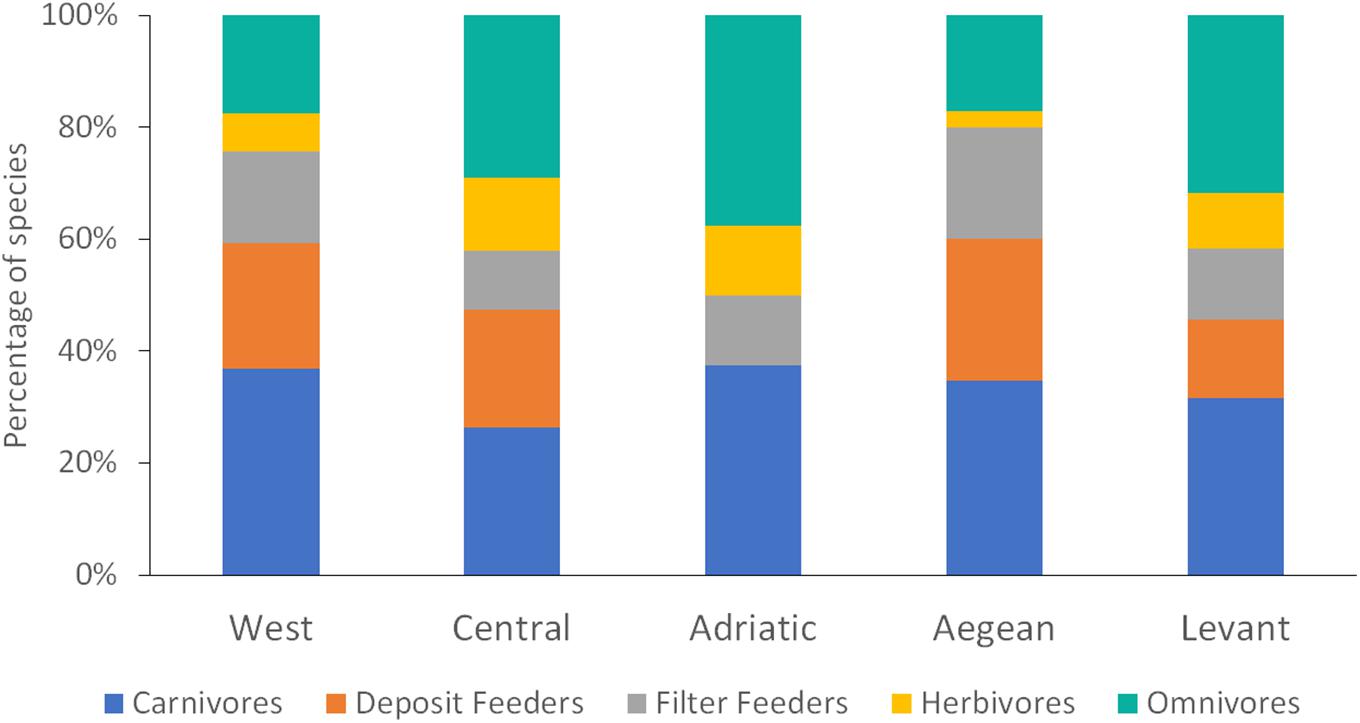
Figure 6. The trophic composition of sponge-associated polychaetes found in five regions of the Mediterranean Sea.
Taxonomic Distinctness and Test of Random Assembly
The taxonomic relatedness indices for the Mediterranean regions are presented in Figure 7. The values of Δ+ and Λ+ calculated for the regional sponge-associated polychaete species-pools, are superimposed on the simulated 95% confidence limits funnel of the expected distribution derived from the Mediterranean benthic-polychaete species-pool. Only the Adriatic Sea fell within the 95% confidence limits of the Δ+ funnel, and the Λ+ funnel. This indicates a skewed aggregation of species into higher taxa for the other Mediterranean regions, thus rejecting the hypothesis of random assembly. The same result was obtained for almost all comparisons of study vs. region, and region vs. region. These results are summarized in Figure 8, which specifies the percentage of samples falling within the 95% confidence limits of the funnel in each group of comparisons. In all scales of observation, less than 95% of the tests can be considered to be assembled at random.
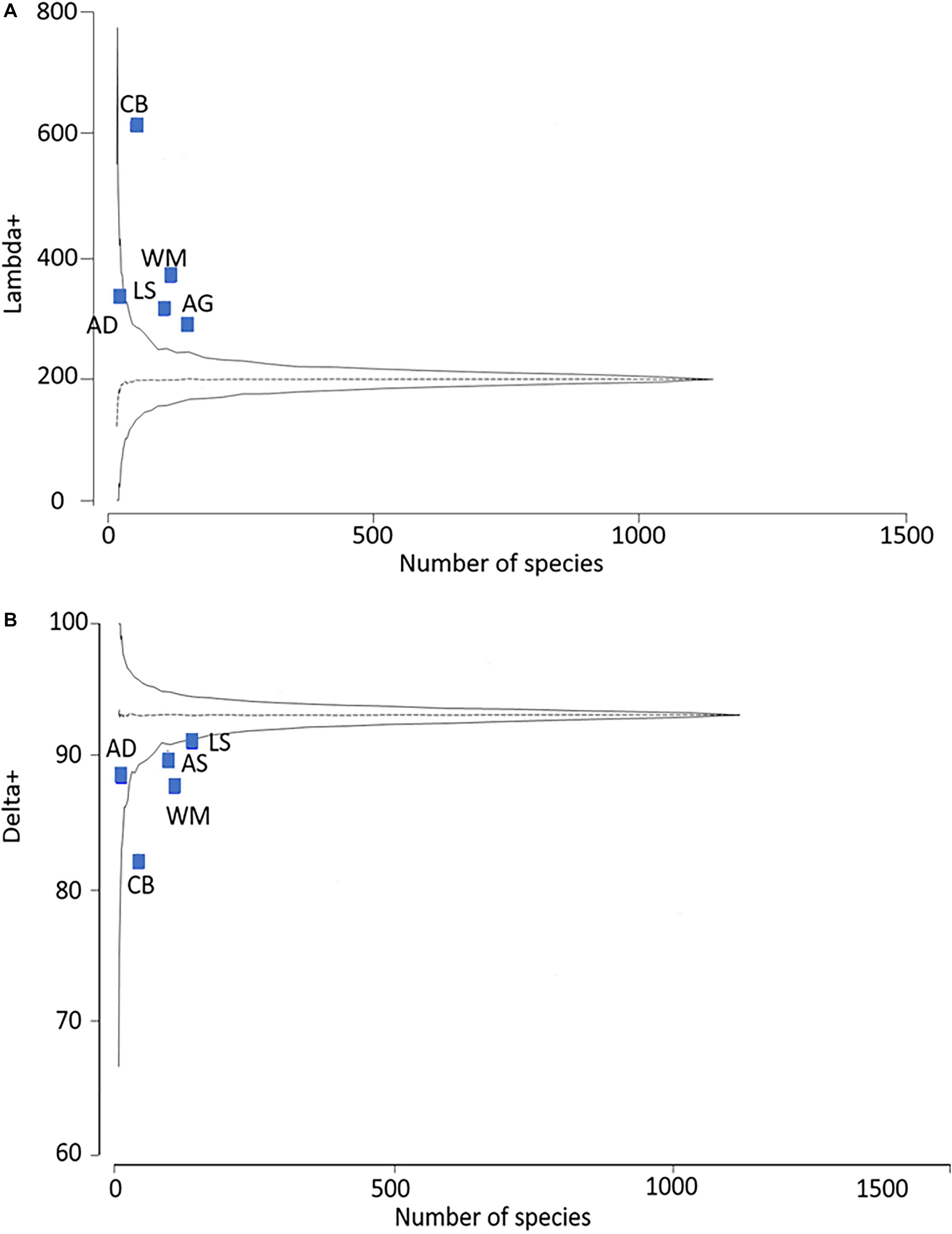
Figure 7. (A) Average taxonomic distinctness (AvTD) and (B) Variation in taxonomic distinctness (VarTD) for the Mediterranean regions superimposed on the 95% confidence limits funnel curve for the expected values of the Mediterranean benthic polychaetes master list. The central line represents the mean value. AD, Adriatic Sea; AS, Aegean Sea; CB, Central Basin; LS, Levant Sea; WM, Western Mediterranean.
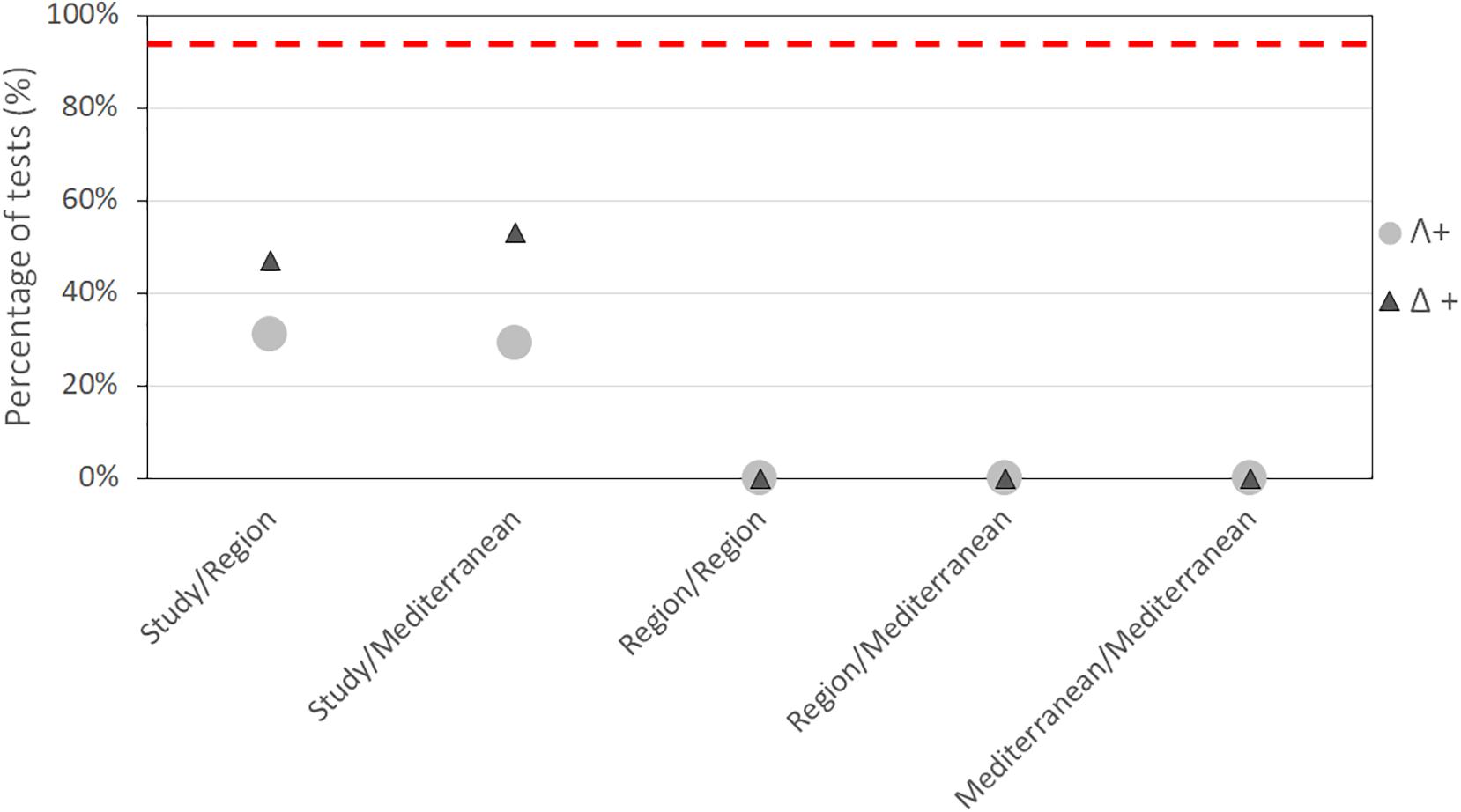
Figure 8. Summary of randomization tests at all spatial scales. Values on the y-axis show the percentage of species lists for which the calculated taxonomic relatedness indices values (Δ+ and Λ+) fall within the 95% confidence limits of the simulated funnels. The dashed line represents the 95% threshold for which it might be reasonable to assume that the hypothesis of assembly at random cannot be rejected.
Discussion
The relationship between sponges and their associated macrofaunal communities has been studied in the Mediterranean Sea more intensively than anywhere else in the world. However, this study is the first to consider sponge-associated polychaete communities on a large biogeographic scale. The sponge-associated polychaetes are usually considered to be facultative transient part of the community, whose composition is probably governed mostly by the zoogeographic region (Pavloudi et al., 2016) and nearby habitats (Voultsiadou-Koukoura et al., 1987; Çinar et al., 2002; Ávila and Ortega-Bastida, 2015). Our results, suggest that it is a specialized community, and that the species in it tend to be more closely related to each other than would be expected if species were assembled at random from the regional or Mediterranean species pool.
Only at the very local scale (study), some of the communities could be considered as a random assembly of the regional pool. These cases probably represent the effect of specific sponge species and individuals’ morphology as well as sponge internal architecture, on the diversity and community composition of the polychaetes. The sponge general morphology (Koukouras et al., 1996); diameter and volume of canals (Koukouras et al., 1996; Ávila and Ortega-Bastida, 2015); sponge volume (Cinar and Ergen, 1998; Ávila and Ortega-Bastida, 2015); the presence of an ectosome (Gherardi et al., 2001); oxygen saturation within sponge areas; and the dynamics of water flow within them (Leys et al., 2011). All of which create many potential sub-habitats within the sponge, that probably have a profound effect on the composition of their inhabitants’ communities.
Greater taxonomic similarity of the sponge-associated polychaetes shown by the low Δ+ and high Λ+, indicate that environmental conditions, rather than inter-specific interactions, are probably the dominant force in shaping their communities (Elton, 1946; Webb, 2000; Somerfield et al., 2009). This suggests that in general, habitat conditions (i.e., sponge conditions), play a greater role in shaping its inhabitants’ community than historical evolutionary processes. A decrease in Δ+ and an increase in Λ+ are considered to be a feature of degraded environments (Clarke and Warwick, 2001; Warwick et al., 2002), but since the richness of sponge-associated polychaete communities is rather high compared with polychaete communities from other habitats in the Mediterranean (Box et al., 2010; Chatzigeorgiou et al., 2017), it should not be considered as an indication of habitat degradation. Rather it indicates that the polychaete communities inhabiting sponges share similar ecological adaptations to survive in this specific habitat, i.e., they are a specialized community.
The trophic structure of the sponge-associated polychaete community, remains stable across the entire Mediterranean Sea. No meaningful differences between the ratios of feeding guilds in each region (except for the Adriatic Sea which suffered from lack of data) were found. The main guild was carnivores, as it is often the case in other areas of the world (Frith, 1976; Westinga and Hoetjes, 1981; Dauer, 1984). This finding further undermines the notion of random assembly of sponge-associated polychaete communities.
No relationship was found between the sponge-associated polychaete community composition with major zoogeographic patterns that affect the entire benthic fauna, such as the North-West to South-East gradient of decreasing diversity that was found in benthic polychaetes and other organisms in the Mediterranean, which relates to differences in salinity and temperatures (Arvanitidis et al., 2002; Voultsiadou, 2009). This finding may be linked to the stability of polychaete communities inside of sponges. However, this latter result can also be attributed to the uneven research effort across the Mediterranean Sea, as areas such as the Aegean Sea, with five studies considered in this work, had greater richness than the Adriatic or the Central Basin, with a single study in each. Both cluster analysis and nMDS support this notion, and show that regions with higher richness, were more similar to each other.
Taken together, the results of this study show the importance of sponges as ecosystem engineers in temperate marine environment as they provide shelter and habitat to diverse assemblages of invertebrates. The results further emphasize that polychaete assemblages inhabiting sponges are not a transient facultative assembly of species, but rather stable, diverse and specialized communities which are well adapted for life in this habitat. However, in order to better understand the engineering function of the hosting sponges and their relationship with their surroundings and associated fauna, further research on the role of sponge morphology and zoogeographical gradients on sponge-associated fauna is necessary, especially in the Mediterranean Sea.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
LG organized the database and wrote the first draft of the manuscript. LG and TI performed the statistical analysis. All authors contributed to the conception and design of the study, manuscript revision, and read and approved the submitted version.
Funding
LG was supported by a VATAT fellowship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge and thank Christos Arvanitidis from the Hellenic Centre for Marine Research (HCMR) in Crete, Greece, for advice regarding taxonomic relatedness. We also thank Vasilis Gerovasileiou and Christina Pavloudi from HCMR who kindly shared their databases of sponge-associated macrofauna. We are grateful to Yoni Belmaker from Tel-Aviv University, for advice in the initiation of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.695163/full#supplementary-material
References
Abdo, D. A. (2007). Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from southwest Australia. Mar. Biol. 152, 845–854. doi: 10.1007/s00227-007-0736-7
Alos, C., Campoy, A., and Pereira, F. (1981). “Contribución al estudio de los anélidos poliquetos endobiontes de esponjas. Actas do II Simposio Iberico de Estudos do Benthos Marino,” in Actas del II Simposio Iberico Estudios del Bentos Marino, eds J. Ros and F. X. Niell (Barcelona: Universidad de Barcelona), 139–157.
Amoureux, L., Josef, G., and O’Connor, B. (1980). Annélides Polychètes de l’éponge Fasciospongia cavernosa Schmidt. Cah. Biol. Mar. 21, 387–392.
Arvanitidis, C. (2000). Polychaete fauna of the Aegean Sea: Inventory and new information. Bull. Mar. Sci. 66, 73–96.
Arvanitidis, C., Bellan, G., Drakopoulos, P., Valavanis, V., Dounas, C., Koukouras, A., et al. (2002). Seascape biodiversity patterns along the Mediterranean and the Black Sea: Lessons from the biogeography of benthic polychaetes. Mar. Ecol. Prog. Ser. 244, 139–152. doi: 10.3354/meps244139
Ávila, E., and Ortega-Bastida, A. L. (2015). Influence of habitat and host morphology on macrofaunal assemblages associated with the sponge Halichondria melanadocia in an estuarine system of the southern Gulf of Mexico. Mar. Ecol. 36, 1345–1353. doi: 10.1111/maec.12233
Bellan, G. (2001). “Polychaeta,” in European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification, eds M. J. Costello, C. Emblow, and R. J. White (Paris: Muséum national d’Histoire naturelle), 214–231.
Ben-Eliahu, M. N., and Boudouresque, C. F. (1995). A list of Polychaeta along the Levant coast. Haasiana 1, 78–93.
Boury-Esnault, N., and Rützler, K. (1997). Thesaurus of sponge morphology. Smithsonian Contribut. Zool. 1997:596.
Box, A., Martin, D., and Deudero, S. (2010). Cambios en la comunidad de poliquetos asociados a la invasión de Caulerpa racemosa var. cylindracea (Chlorophyta: Caulerpales): Estructura de comunidad, estrategias tróficas y distinción taxonómica. Sci. Mar. 74, 317–329. doi: 10.3989/scimar.2010.74n2317
Campoy, A. (1979). Lista de especies de Anélidos Poliquetos conocidas de las costas de la Península Ibérica. Investig. Pesq. 42, 727–766.
Castelli, A., Bianchi, C. N., Canantone, G., Çinar, M. E., Gambi, M. C., Giangrande, A., et al. (2008). “Annelida: Polychaeta,” in Checklist della Flora e della Fauna dei mari italiani (parte I), ed. G. Relini (Italy: Ministero dell’ambiente e della tutela del territorio e del mare), 323–373.
Chatzigeorgiou, G., Keklikoglou, K., Faulwetter, S., Badalamenti, F., Kitsos, M. S., and Arvanitidis, C. (2017). Midlittoral polychaete communities in the Eastern Mediterranean Sea: new information from the implementation of the Natural Geography in Shore Areas (NaGISA) protocol and comparisons at local and regional scales. Mar. Ecol. 38, 1–15. doi: 10.1111/maec.12339
Çinar, M. E. (2005). Polychaetes from the coast of northern Cyprus (eastern Mediterranean Sea), with two new records for the Mediterranean Sea. Cah. Biol. Mar. 46, 143–159.
Çinar, M. E., Bakir, K., Doðan, A., Açik, S., Kurt, G., Kataðan, T., et al. (2019). Macro-benthic invertebrates associated with the black sponge Sarcotragus foetidus (Porifera) in the Levantine and Aegean Seas, with special emphasis on alien species. Estuar. Coast. Shelf Sci. 227:106306. doi: 10.1016/j.ecss.2019.106306
Çinar, M. E., Daðli, E., and Şahin, G. K. (2014). Checklist of Annelida from the coasts of Turkey. Turk. J. Zool. 38, 734–764. doi: 10.3906/zoo-1405-72
Cinar, M. E., and Ergen, Z. (1998). Polychaetes associated with the sponge sarcotragus muscarum schmidt, 1864 from the turkish aegean coast. Ophelia 48, 167–183. doi: 10.1080/00785236.1998.10426964
Çinar, M. E., Katagan, T., Ergen, Z., and Sezgin, M. (2002). Zoobenthos-inhabiting Sarcotragus muscarum (Porifera: Demospongiae) from the Aegean Sea. Hydrobiologia 482, 107–117. doi: 10.1023/A:1021260314414
Clarke, K. R., and Warwick, R. M. (1998). A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 35, 523–531.
Clarke, K. R., and Warwick, R. M. (2001). A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 216, 265–278. doi: 10.3354/meps216265
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Lasram, F. B. R., Aguzzi, J., et al. (2010). The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS One 5:e11842. doi: 10.1371/journal.pone.0011842
Dauer, D. M. (1984). The use of polychaete feeding guilds as biological variables. Mar. Pollut. Bull. 15, 301–305. doi: 10.1016/0025-326X(84)90199-1
Elton, C. (1946). Competition and the Structure of Ecological Communities. J. Anim. Ecol. 15, 54–68. doi: 10.2307/1625
Fauchald, K., and Jumars, P. A. (1979). The diet of worms: a study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 17, 193–284. doi: 10.12691/marine-1-1-6
Faulwetter, S., Markantonatou, V., Pavloudi, C., Papageorgiou, N., Keklikoglou, K., Chatzinikolaou, E., et al. (2014). Polytraits: A database on biological traits of marine polychaetes. Biodivers. Data J. 2014:e1024. doi: 10.3897/BDJ.2.e1024
Faulwetter, S., Simboura, N., Katsiaras, N., Chatzigeorgiou, G., and Arvanitidis, C. (2017). Polychaetes of Greece: An updated and annotated checklist. Biodivers. Data J. 5:e20997. doi: 10.3897/BDJ.5.e20997
Fiore, C. L., and Jutte, P. C. (2010). Characterization of macrofaunal assemblages associated with sponges and tunicates collected off the southeastern United States. Invertebr. Biol. 129, 105–120. doi: 10.1111/j.1744-7410.2010.00184.x
Frith, D. W. (1976). Animals associated with sponges at North Hayling, Hampshire. Zool. J. Linn. Soc. 58, 353–362. doi: 10.1111/j.1096-3642.1976.tb01005.x
Gerovasileiou, V., Chintiroglou, C. C., Konstantinou, D., and Voultsiadou, E. (2016). Sponges as “living hotels” in Mediterranean marine caves. Sci. Mar. 80, 279–289. doi: 10.3989/scimar.04403.14B
Gherardi, M., Giangrande, A., and Corriero, G. (2001). Epibiontic and endobiontic polychaetes of Geodia cydonium (Porifera, Demospongiae) from the Mediterranean Sea. Hydrobiologia 443, 87–101. doi: 10.1023/A:1017500321330
Goren, L., Idan, T., Shefer, S., and Ilan, M. (2021). Macrofauna inhabiting massive demosponges from shallow and mesophotic habitats along the Israeli Mediterranean coast. Front. Mar. Sci. 7:612779. doi: 10.3389/fmars.2020.612779
Jumars, P. A., Dorgan, K. M., and Lindsay, S. M. (2015). Diet of worms emended: An update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 7, 497–520. doi: 10.1146/annurev-marine-010814-020007
Kersken, D., Göcke, C., Brandt, A., Lejzerowicz, F., Schwabe, E., Anna Seefeldt, M., et al. (2014). The infauna of three widely distributed sponge species (Hexactinellida and Demospongiae) from the deep Ekström Shelf in the Weddell Sea, Antarctica. Deep. Res. Part II Top. Stud. Oceanogr. 2014, 101–112. doi: 10.1016/j.dsr2.2014.06.005
Klitgaard, A. B. (1995). The fauna associated with outer shelf and upper slope sponges (porifera, demospongiae) at the faroe islands, northeastern Atlantic. Sarsia 80, 1–22. doi: 10.1080/00364827.1995.10413574
Koukouras, A., Russo, A., Voultsiadou-Koukoura, E., Arvanitidis, C., and Stefanidou, D. (1996). Macrofauna associated with sponge species of different morphology. Mar. Ecol. 17, 569–582. doi: 10.1111/j.1439-0485.1996.tb00418.x
Koukouras, A., Russo, A., Voultsiadou−Koukoura, E., Dounas, C., and Chintiroglou, C. (1992). Relationship of Sponge Macrofauna with the Morphology of their Hosts in the North Aegean Sea. Int. Rev. Gesamten Hydrobiol. Hydrogr. 77, 609–619. doi: 10.1002/iroh.19920770406
Koukouras, A., Voultsiadou-Koukoura, E., Chintiroglou, H., and Dounas, C. (1985). Benthic bionomy of the North Aegean sea. III: A comparison of the macrobenthic animal assemblages associated with seven sponge species. Cah. Biol. Mar. 26, 300–319.
Legendre, P., and Legendre, L. (1998). Numerical ecology: developments in environmental modellin. Amsterdam: Elsevier Ltd.
Leys, S. P., Yahel, G., Reidenbach, M. A., Tunnicliffe, V., Shavit, U., and Reiswig, H. M. (2011). The sponge pump: the role of current induced flow in the design of the sponge body plan. PLoS One 6:e27787. doi: 10.1371/journal.pone.0027787
Long, E. R. (1968). The associates of four species of marine sponges of Oregon and Washington. Pacific Sci. 22, 347–351.
Martin, D., and Britayev, T. A. (1998). Symbiotic polychaetes: review of known species. Oceanogr. Mar. Biol. Annu. Rev. 36, 217–340.
Mikac, B. (2015). A sea of worms: polychaete checklist of the Adriatic Sea. Zootaxa 3943, 1–172. doi: 10.11646/zootaxa.3943.1.1
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., and O’Hara, R. B. (2013). Package vegan. R Packag ver. Vienna: R Core Team.
Pansini, M. (1970). Inquilinismo in Spongia officinalis, Ircinia fasciculata e Petrosia ficiformis della Riviera Ligure di Levante. Boll. Musei Degli Ist. Biol. Dell’univer. Genova 38, 5–17.
Pansini, M., and Daglio, S. (1980). Aspetti dell’inquilinismo di Policheti in alcune Demospongie del litorale ligure. Mem. Biol. Mar. Oceanogr. 10, 427–428.
Papatheodoulou, M., Jimenez, C., Petrou, A., and Thasitis, I. (2019). Endobiotic communities of Marine Sponges in Cyprus (Levantine Sea). Heliyon 5:e01392. doi: 10.1016/j.heliyon.2019.e01392
Pavloudi, C., Christodoulou, M., and Mavidis, M. (2016). Macrofaunal assemblages associated with the sponge Sarcotragus foetidus Schmidt, 1862 (Porifera: Demospongiae) at the coasts of Cyprus and Greece. Biodivers. Data J. 4:e8210. doi: 10.3897/BDJ.4.e8210
Peattie, M. E., and Hoare, R. (1981). The sublittoral ecology of the Menai Strait. II. The sponge Halichondria panicea (Pallas) and its associated fauna. Estuar. Coast. Shelf Sci. 13, 621–635. doi: 10.1016/S0302-3524(81)80044-8
Ribeiro, S. M., Omena, E. P., and Muricy, G. (2003). Macrofauna associated to Mycale microsigmatosa (Porifera, Demospongiae) in Rio de Janeiro State, SE Brazil. Estuar. Coast. Shelf Sci. 57, 951–959. doi: 10.1016/S0272-7714(02)00425-0
Santucci, R. (1922). La Geodia cydonium come centro di associazione biologica. R. Com. Thalass. ltaliano 103, 5–19.
Schejter, L., Chiesa, I. L., Doti, B. L., and Bremec, C. (2012). Mycale (aegogropila) Magellanica (Porifera: Demospongiae) en el Atlántico suroeste: Fauna endobiótica y nuevos datos de su distribución. Sci. Mar. 76, 753–761. doi: 10.3989/scimar.03490.21A
Somerfield, P. J., Arvanitidis, C., Faulwetter, S., Chatzigeorgiou, G., Vasileiadou, A., Amouroux, J. M., et al. (2009). Assessing evidence for random assembly of marine benthic communities from regional species pools. Mar. Ecol. Prog. Ser. 382, 279–286. doi: 10.3354/meps07934
R Core Team (2019). R: A language and environment for statistical computing. Vienna: R Core Team, doi: 10.1108/eb003648
Rstudio Team (2020). RStudio: Integrated Development for R. Boston: Rstudio Team, doi: 10.1145/3132847.3132886
Voultsiadou-Koukoura, H. E., Koukouras, A., and Eleftheriou, A. (1987). Macrofauna associated with the sponge Verongia aerophoba in the north Aegean Sea. Estuar. Coast. Shelf Sci. 24, 265–278. doi: 10.1016/0272-7714(87)90069-2
Voultsiadou, E. (2009). Reevaluating sponge diversity and distribution in the Mediterranean Sea. Hydrobiologia 628, 1–12. doi: 10.1007/s10750-009-9725-9
Warwick, R. M., Ashman, C. M., Brown, A. R., Clarke, K. R., Dowell, B., Hart, B., et al. (2002). Inter-annual changes in the biodiversity and community structure of the macrobenthos in Tees Bay and the Tees estuary, UK, associated with local and regional environmental events. Mar. Ecol. Prog. Ser. 234, 1–13. doi: 10.3354/meps234001
Webb, C. O. (2000). Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 156, 145–155. doi: 10.1086/303378
Keywords: taxonomic distinctness, symbiosis, community ecology, biodiversity, Porifera, Annelida, Mediterranean Sea
Citation: Goren L, Idan T, Shefer S and Ilan M (2021) Sponge-Associated Polychaetes: Not a Random Assemblage. Front. Mar. Sci. 8:695163. doi: 10.3389/fmars.2021.695163
Received: 14 April 2021; Accepted: 06 May 2021;
Published: 31 May 2021.
Edited by:
Christian Marcelo Ibáñez, Andres Bello University, ChileReviewed by:
Matthew Lee, Universidad de Los Lagos, ChileEnrique Ávila, National Autonomous University of Mexico, Mexico
Copyright © 2021 Goren, Idan, Shefer and Ilan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liron Goren, Z29yZW4ubGlyb25AZ21haWwuY29t
 Liron Goren
Liron Goren Tal Idan
Tal Idan Sigal Shefer
Sigal Shefer Micha Ilan
Micha Ilan