
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 20 August 2021
Sec. Marine Conservation and Sustainability
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.692068
 Cataldo Pierri1
Cataldo Pierri1 Frine Cardone2
Frine Cardone2 Giuseppe Corriero1
Giuseppe Corriero1 Tamara Lazic1*
Tamara Lazic1* Federico Quattrocchi3
Federico Quattrocchi3 Giorgio Alabiso4
Giorgio Alabiso4 Michele Gristina5
Michele Gristina5Illegal wildlife trade is considered one of the most serious threats to biodiversity worldwide, along with habitat loss/degradation and overfishing of wild stocks. Seahorses are considered at high risk as these fish represent an important component of traditional Chinese medicine but are also sold as curios and ornamental fish. On a worldwide level, illegal trade is controlled by numerous laws and regulations, but it seems to continue by assuming more dynamic routes. In the Mediterranean Sea, Hippocampus guttulatus formed one of the largest populations at Mar Piccolo di Taranto in South-Eastern Italy. During the routine monitoring of this population in 2016, a dramatic density decrease was observed. By using questionnaires and long-term datasets, the present study determined possible causes of this decline by investigating habitat changes, temperature trends and the existence of seahorse trafficking while also examining abundance trends during the last decade. The results indicated a sharp density decline starting from 2015, co-occurring with the period of high temperatures, while habitats remained almost constant. However, interviews with main stakeholders described both illegal and legal fishing activities as the main drivers for the declining seahorse density. Indeed, at one of the studied sites, which was under strict military control, seahorse abundance started to decline only after the intensification of fishing pressure in the basin. The study suggests that Mar Piccolo di Taranto could be one of the sources for international seahorse trade, thus highlighting the need for more intense and effective actions to prevent and combat illegal poaching, while threatened populations are requiring continuous and close monitoring. Due to unfavorable socio-economic conditions, a viable and thriving seahorse population at Mar Piccolo di Taranto could contribute to the revitalization of the coastal economy and the development of environmental awareness.
Illegal, Unreported and Unregulated (IUU) fishing and Illegal Wildlife Trade (IWT) are considered to be among the most severe threats for sustainable fisheries and aquatic animals’ conservation on a worldwide level [Food and Agriculture Organization (FAO)., 2016; Margulies et al., 2019]. The illegal wildlife trade has expanded dramatically in the last decades due to the globalization of the world’s economy with the reduction of borders (Haken, 2011) and the outbreak of unregulated e-commerce platforms (Yu and Jia, 2015; Harrison et al., 2016; Xu et al., 2020). Despite the existence of international regulations (such as the Convention on International Trade in Endangered Species of Wild Fauna and Flora—CITES) and national and regional laws, illegal wildlife trade is still a rising concern driven by numerous factors such as economic hardship, alternative medicine, accessories, and the pet trade (Phelps et al., 2016; Pires and Moreto, 2016).
Marine species are considered the largest group traded for value (Engler, 2008), with approximately 30 millionfish and 1, 5 million coral colonies traded per year. Since most of these species are not targeted by commercial fishing, their main source is IUU fishing (Agnew et al., 2009). Seahorses (47 species of the genus Hippocampus) are considered particularly threatened by IWT and were the first marine bony fishes subjected to the trade regulation globally (Foster et al., 2019). Despite the potential risk to the sustainability of populations (Vincent et al., 2011), approximately 37 million seahorses are estimated to be caught each year, mostly as bycatch from non-selective fisheries (Lawson et al., 2017). Seahorses are sold mainly as dried traditional Chinese medicine (TCM) components, and to a lesser extent, as curios, while live specimens are traded as aquarium fishes (Foster et al., 2019). The existence of a seahorse trade indeed confirms widespread wildlife trafficking flows from biodiversity-rich developing to the developed countries (Roe, 2002). The majority of wild seahorses are exported from Southeast Asia or West Africa and imported by China, Hong Kong SAR and Taiwan (McPherson and Vincent, 2004; Foster et al., 2016). Wildlife trafficking, as described by Kuo and Vincent (2018), is a complex and dynamic network in which the smuggling routes used for natural resources of great commercial value (e.g., oil and timber) are usually favored over conventional ones (Bosshard, 2008). For many years, Europe has been one of the largest and most diversified markets for wildlife and wildlife products, serving as both an end-market and transit hub1. Over the last decade, however, the European countries have begun exporting several marine species to economically booming China (World Wildlife Crime Report). Animals with little or no commercial value in the European market (e.g., Holothuroidea) have, therefore, reached competitive prices due to their widespread usage in TCM (Anderson et al., 2011). Among them, it seems that the European seahorses have also recently become targets of illegal trade (Correia, 2018).
Like other congeneric species, the European long-snouted seahorse, Hippocampus guttulatus Cuvier, 1829, is listed on Appendix II of CITES and the IUCN Red List where is classified as “Near Threatened” (NT) in both the Mediterranean Sea (IUCN., 2020) and along the Italian coast (Relini et al., 2018). Along the Italian coast, patchily distributed seahorse populations are frequently having low densities or could be concentrated in restricted and easily accessible areas, such as the case of Mar Piccolo di Taranto. Increased concerns about IUU fishing and its possible effects led to the ban on seahorse capture by Italian law (n.175, 27/05/99).
Mar Piccolo di Taranto (South-Eastern Italy; hereafter called MPT) until recently hosted one of the largest populations of this species in the Mediterranean Sea (Gristina et al., 2015). At this site, a specific combination of several factors (e.g., tourist-discouraging large industries, extensive mussel farms preventing towed gears impacts, high eutrophication ensuring a high trophic level and thus large crustacean populations) created an ideal environment for feeding, hiding and reproduction of seahorses (Gristina et al., 2015, 2017; Lazic et al., 2018; Ape et al., 2019). However, in spring 2016, during the routine monitoring of local populations, a sharp decline in the seahorse abundance was recorded. By analyzing long-term data, the present study sought to investigate the causes assuming two possible factors that led to the observed decline: (i) environmental drivers, mainly temperature, which is decisive for the survival and nutrition of juveniles through affecting resource availability (e.g., Planas et al., 2012); or (ii) the existence of trafficking of seahorses as reported by Manna (2019) in February 20192. Regarding the latter, and due to the difficulties related to the illegal and criminal nature of trafficking, the present study carried out interviews with the principal stakeholders to obtain information and fill knowledge gaps. Moreover, abundance trends of the long-snouted seahorse population at MPT during the last decade were assessed and discussed given the new and unexpected threat of illegal trade.
The long-snouted seahorse H. guttulatus can be found from Eastern Atlantic to the Western Mediterranean Sea, usually inhabiting seagrass meadows and algal beds (Lourie et al., 2004). The species is characterized by sedentary behavior, high site fidelity and small home ranges (Curtis and Vincent, 2005; Caldwell and Vincent, 2013; Gristina et al., 2015, 2017; Pierri et al., 2020). Like other members of the family Syngnathidae, H. guttulatus is mainly threatened by degradation of coastal habitats (Olden et al., 2007), incidental capture in fishing gears (bycatch) (Vincent, 1996), use in traditional medicines and aquarium trade (Salin et al., 2005).
Mar Piccolo di Taranto (MPT) (40°28′N, 17°16′W) is located at the northern end of the Gulf of Taranto (Ionian Sea). It has a total surface area of approximately 20.63 km2 and is characterized by low water turnover and movement. The water circulation takes place mainly through two channels, supplied by tidal currents and powerful pumps utilized for industrial purposes. Water temperature varies from 11.06 ± 1.08 (SD)°C (January) to 29.81 ± 0.77 (SD)°C (August). The average salinity is around 36 psu (Caroppo and Cardellicchio, 1995). Dissolved oxygen shows wide seasonal variability. From May to October, the oxygen deficit in the deep-water layers (7–10 m of depth) of the inner part of the basin can reach marked levels, leading to dystrophic events (Alabiso et al., 1997; Gaino et al., 2010). Soft bottoms, varying from muds to mixed sands, are dominant and sparsely covered with patches of Cymodocea nodosa (Cecere and Petrocelli, 2009), Caulerpa prolifera and, in the deeper areas (between 6 and 9 m) by Cladophora sp. Where vegetation is missing, many sabellids, mollusks and ceriantharians have colonized soft bottoms. Hard substrata are abundant and diversified, consisting mainly of concrete and debris of human origin, ropes and material abandoned by mussel farmers and fishermen. Besides, there are several kilometers of stonewalls along the coastline (for further details see Gristina et al., 2015; Gristina et al., 2017). Despite high levels of degradation and pollution (Petronio et al., 2012), this site hosts one of the largest populations of H. guttulatus in the entire Mediterranean Sea (Gristina et al., 2015, 2017; Lazic et al., 2018; Ape et al., 2019).
The present study was conducted at two sites within MPT (Figure 1). Site 1 (in local language “muretto”), surveyed from 2011, is located far from the town and is accessible from both land and sea. The site is characterized by a concrete wall dominated by the algal cover, sabellidae, ascidians and mollusks (see Supplementary Table 1 for further description of habitats). The sea bottom under the wall is sandy with small, scattered rocks and numerous sabellidae tubes (mainly genus Megalomma). Sandy bottom forms a continuous 50 m strip along the concrete wall at a depth of 0.5 to 3 m. There are also patches of seagrass (Cymodocea nodosa), algal beds (Caulerpa prolifera) and rhodolite bottoms. At the site edge and in the most distant points from the coast, numerous iron poles can be found, evidencing traditional methods of mussel breeding in the basin. Poles are embedded in the bottom and are covered by a dense fouling community, representing a suitable habitat for seahorses (Gristina et al., 2015). The site is only occasionally patrolled by supervisory bodies such as the Italian Coast Guard. Site 2 is located within the Italian Air Force base, where the surveys started only in spring 2017 because of the difficulties in obtaining permissions. Like Site 1, this site is also characterized by a concrete perimeter wall covered with a dense macrobenthic community (see Supplementary Table 1 for further description of habitats). Sandy bottom under the wall develops in a 10-meter-wide band at 0.5 to 2 m of depth and is characterized by the presence of large and numerous blocks. The bottom is rarely uncovered, and Cymodocea nodosa forms a uniform and compact bed. There are also numerous sabellidae tubes, algae (Cystoseira spp.) and bivalve mollusks. The site is accessible only by the sea, and although its coastline is strictly controlled, small fishing boats with gill nets can approach.
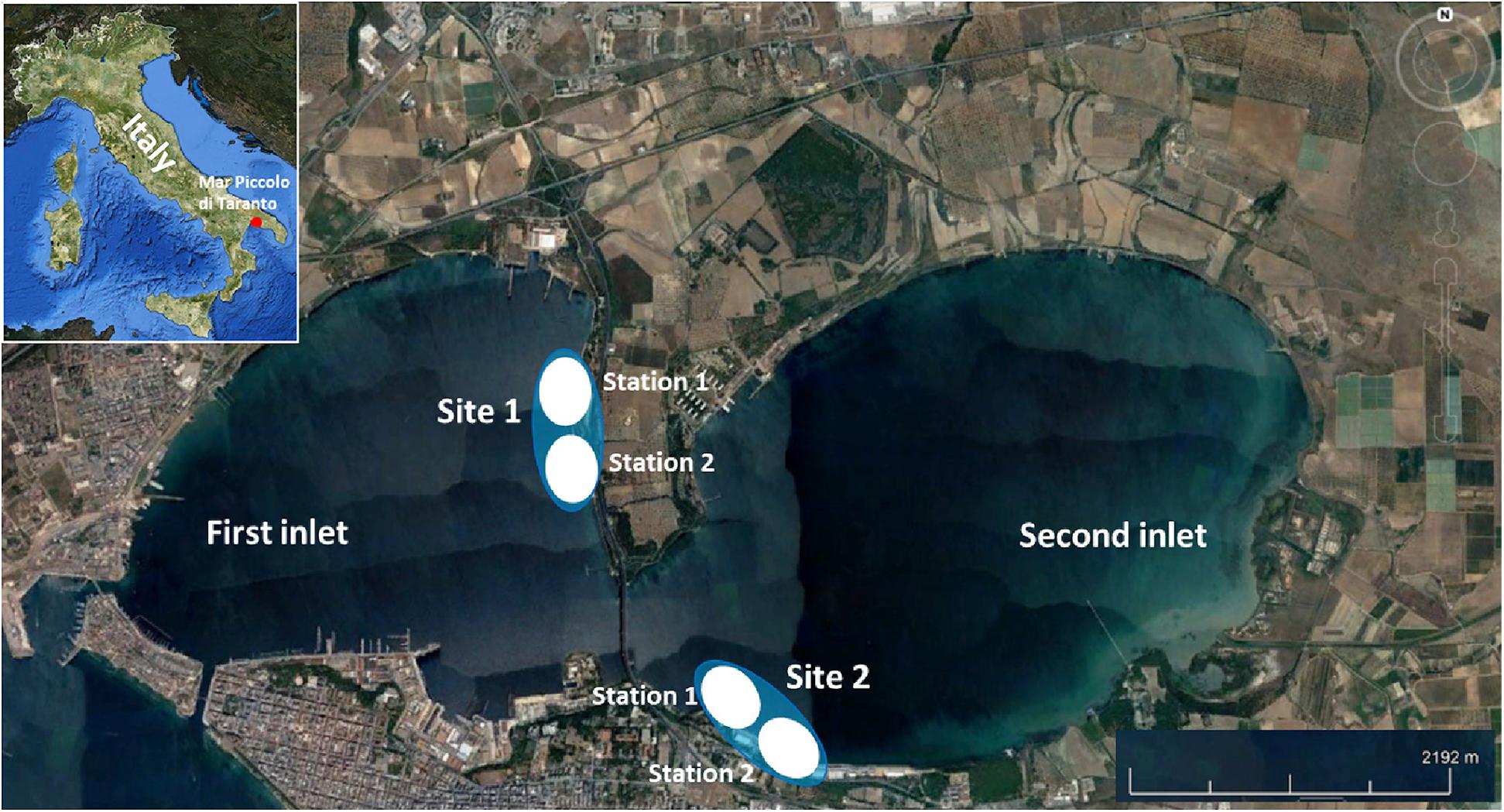
Figure 1. Study area. Site 1, The sampling area only occasionally patrolled by supervisory bodies. Site 2, The coastline is strictly patrolled because of the presence of military structures.
At each site, two stations (Figure 1) were established and surveyed twice a year (spring and autumn) using standard Underwater Visual Census (UVC) along three 2 m × 150 m linear transects (Samoilys, 1997). Transects, placed > 50 m apart, were perpendicular to the coastline and located at a depth varying from 0.5 to 5 m. All UVC surveys were performed in the morning and were carried out by two divers. For each sighted H. guttulatus, size, sex, holdfast, and habitat were recorded. In both sampling sites, the extension of seahorse habitats was measured using a metric rope to monitor possible changes in size and spatial distribution, and the total coverage of each habitat was calculated.
Online and in-person questionnaires on environmental and anthropogenic stressors affecting H. guttulatus population were used to assess the perception and Local Ecological Knowledge (LEK) of stakeholders operating at MPT. From February to April 2020, an 18-item questionnaire (Supplementary Data Sheet 1) was distributed among the main categories of people operating (for both professional and recreational purposes) at MPT. Interviews were carried out with research institutes (University and National Research Council), environmental associations (WWF, Legambiente, Marevivo), press agencies, military and governmental institutions, artisanal fishermen and mussel farmers. Governmental and local institutions and administrations were firstly contacted by telephone to identify the person with expertise and responsibility regarding marine conservation and\or management of endangered habitats and species. Researchers met fishermen and mussel farmers directly on the port dock or during their activities.
The questionnaire sought information on four key topics: (1) General information about respondents (i.e., employment, the activity carried out at MPT, number of diving hours per year, the average number of seahorse sighted per hour); (2) Perceived temporal trend of seahorse population (i.e., increasing, decreasing, year of onset); (3) Main perceived causes of the changes in population density (i.e., fishing activities, natural causes, pollution); (4) Existence of fishing activities specifically targeting seahorses, and ancillary information (i.e., the number of vessels, number of operators, commercial value). Self-contradictory responses were not considered for further analysis.
In this study, air temperature data were used because of the missing values for water temperatures throughout the survey period (January 2011–December 2019). The air temperature dataset (°C) was downloaded from the Environmental Protection Agency of Apulia website (ARPA Puglia; available at http://old.arpa.puglia.it/web/guest/serviziometeo). The significant correlation between air and seawater temperature was a priori tested (see Supplementary Table 2).
Hippocampus guttulatus density and temperature series were analyzed together to assess, identify, and compare trends and the presence of breakpoints. Correspondences of the long-term patterns and breaks between series could indicate the temperature influence on seahorse density. Generalized Least Square regressions (GLS) were used to assess a trend in density and temperature series. GLS are commonly used in time series regressions because offering the possibility to deal with errors due to temporal correlation (independence assumption; e.g., Zuur et al., 2009). Specifically, an Auto-Regressive model of order 1 (AR1) for the residual structure was employed for both series.
To assess the presence of structural changes during the investigated period, breakpoint analysis (R package strucchange, Zeileis et al., 2013) was performed on both series. This procedure assesses the deviation from stability in linear regression, hence evaluating if there are breakpoints where the coefficients shift from one stable relationship to a different one and consequently if there will be different segments in which the regression coefficients are constant. The breaks are estimated automatically by fitting ordinary least squares (OLS) on a sequence of breakpoints and by comparing the residuals sum of squares (RSS) with those of the unsegmented regression (Zeileis et al., 2003, 2013). This comparison, allowing the choice of the optimal number of breaks, is performed by combining both minimization of RSS and Bayesian Information Criterion (BIC, Schwarz, 1978). Two series were analyzed separately and in the case of coincidence of breakpoints, the aforementioned influences of the environment as a source of variability cannot be excluded. All analyses were performed using R studio software. To evaluate the significance of perception of decline in H. guttulatus and presence of target fishery, we used a robust two-sample test on median differences determining p-values through bootstrap t-method (Mair and Wilcox, 2020). For each respondent’s category, positive and negative responses were divided for the number of responses and then, based on bootstrap, differences in median values were tested.
Hippocampus guttulatus density (n° ind/m2) at Site 1 (“Muretto”) was stable from Autumn 2011 to Autumn 2015 and ranged from 0.116 ± 0.03 (average ± SD) in Autumn 2013 to 0.169 ± 0.04 in Autumn 2015 (Figure 2). Starting from Spring 2016, the data indicated a sharp decline in the seahorse density, with the values ranging from 0.03 ± 0.006 in Autumn 2016 to 0.01 ± 0.003 in Autumn 2019 (Figure 2). According to the GLS time-series regressions, there were no temperature changes during this period (Figure 3A and Table 1), while a significant decreasing trend in the seahorse density was found (Figure 3B and Table 1). Breakpoint analysis detected a clear discontinuity in the seahorse density (Figure 4 and Table 2) and indicated the breakpoint occurring in 2016. Before and after 2016, the average density changed from 0.136 to 0.017 n° ind/m2, respectively.
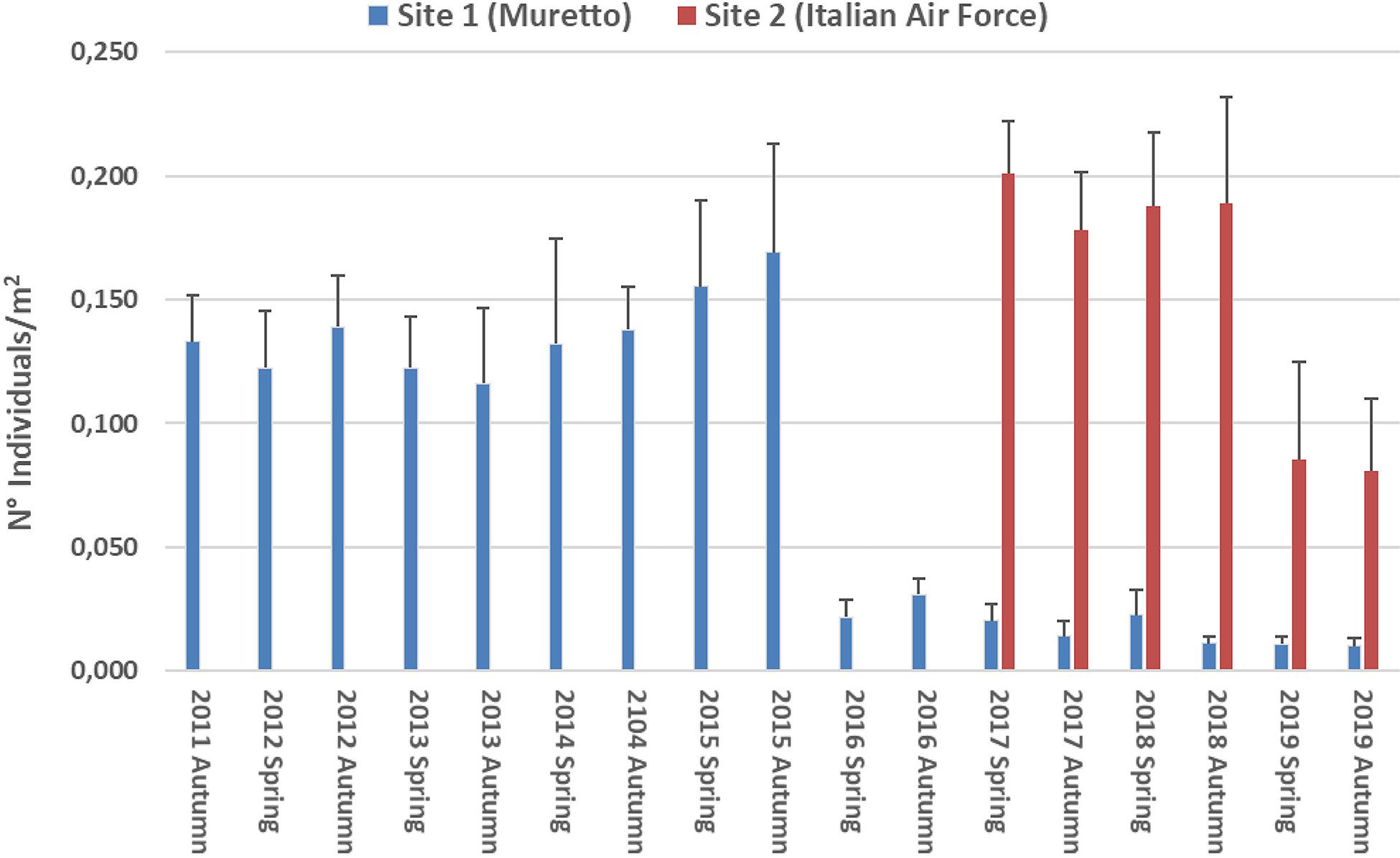
Figure 2. Average density values (±s.d.) of H. guttulatus from Autumn 2011 to Autumn 2019. In blue, surveys carried out at Site 1 (No control). In red, surveys carried out at Site 2 (Light control).
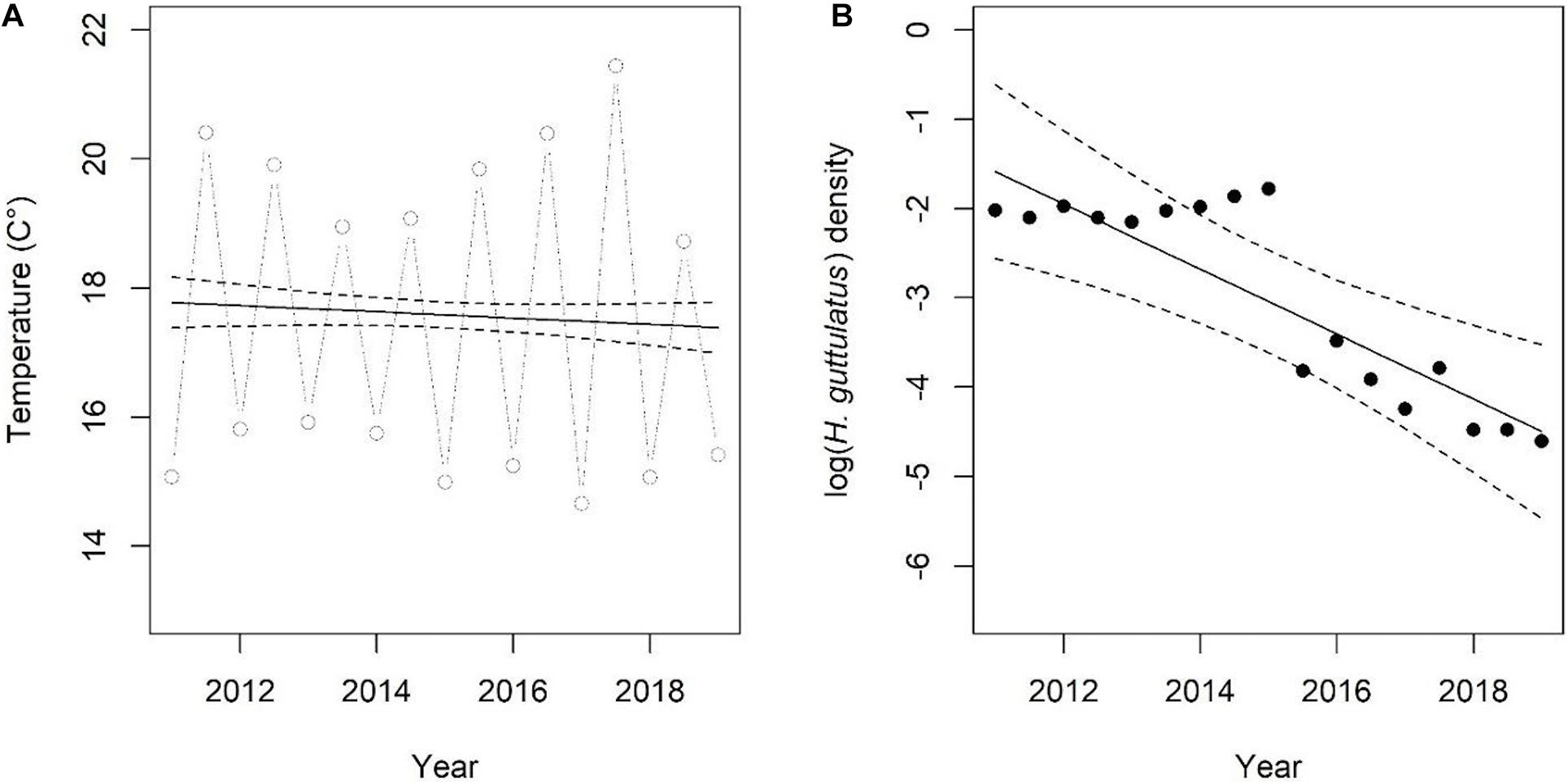
Figure 3. (A,B) Time-series of temperature and H. guttulatus density. Lines indicate fitted values obtained using GLS models. Model details are reported in Table 1.
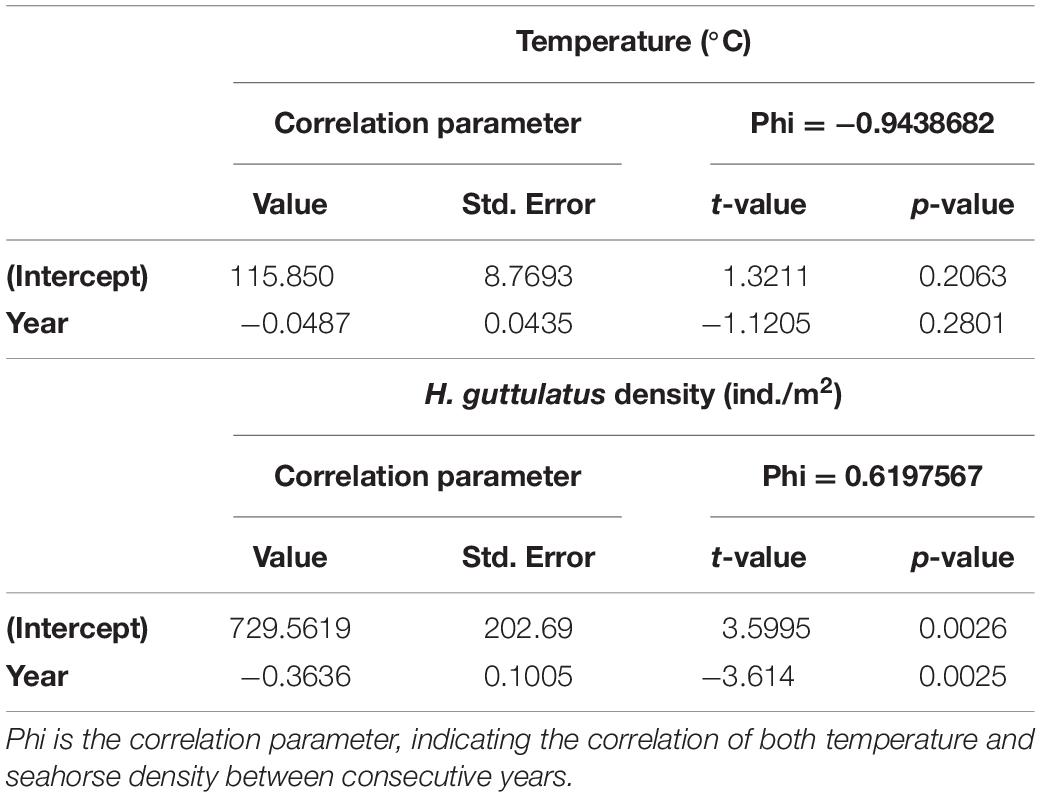
Table 1. Generalized Least Square regression (GLS) results showing no significant trend for temperature and a significant decrease in H. guttulatus density.
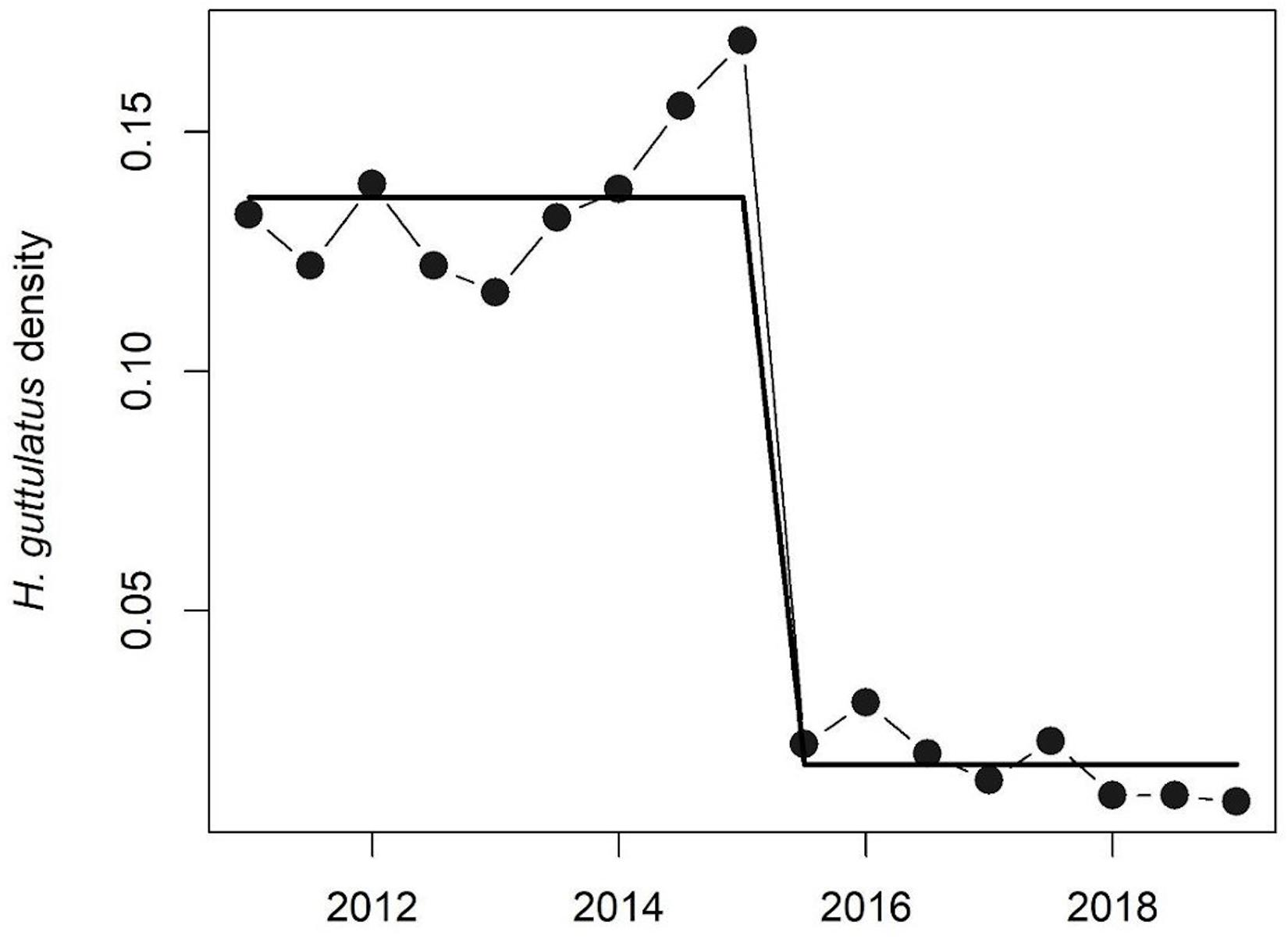
Figure 4. Breakpoint analysis of H. guttulatus density. Solid lines indicate fitted values from breakpoint analysis. Model details are reported in Table 2.
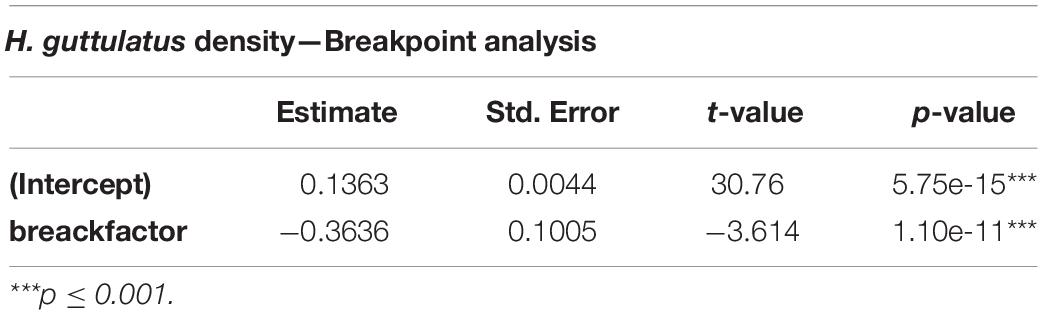
Table 2. Breakpoint regression analysis results indicating significant differences in the seahorse density before and after an estimated break in 2016.
Comparable to the values observed at Site 1 until 2015, the densities of H. guttulatus at Site 2 (Italian Air Force base) were almost constant until Autumn 2018 (ranging from 0.01 ± 0.02 to 0.178 ± 0.023 n° ind/m2). In Spring and Autumn 2019, a noticeable decrease (0.085 ± 0.01 and 0.081 ± 0.01 n° ind/m2, respectively) was observed at this site (Figure 2).
The spatial distribution of preferential seahorse habitats (Gristina et al., 2015, 2017) remained almost constant at both sampling sites throughout the investigated period (Supplementary Table 1). Seasonal variations, compatible with life cycles, have been observed only for Cymodocea nodosa and Cystoseira spp. Characterized by the presence of small stones, artificial hard substrates and until 2015, colonized by sabellids and solitary ascidians, sandy/muddy bottoms have shown an impoverishment of the megabenthos over time (sabellids mean density ranged from 4.3 ± 1.3 in 2015 to 0.04 ± 0.01 n° ind/m2 in 2019; ascidians from 0.8 ± 0.3 in 2015 to 0.02 ± 0.005 n° ind/m2 in 2019).
A total of 59 questionnaires were filled out. Six categories of stakeholders were consulted, although the contribution of categories potentially involved in the activities that could threaten the seahorse population at MPT (artisanal fishermen, mussel farmers) was rare and scanty (Table 3). Eight questionnaires, which showed self-contradictory responses, were not considered for further analysis.
The number of respondents declaring a decline in population density of H. guttulatus (n = 40; 78.4%) was statistically higher than those who reported a constant seahorse presence over 3 years (2017–2019) (n = 8; 15.6%) (median Yes = 0.81; No = 0.29, Test statistic: 0.5231, p-value = 0.01169) (Figure 5 and see Supplementary Figure 4 for details on responses of each category). 37.5% of respondents have perceived a decline in seahorse density since 2019, 15% since 2018 and 25% claimed the decline since more than 3 years (before 2017). According to the respondents (61.5%), the main threat to the seahorse population was fishing activity (Figure 6 and see Supplementary Figure 5 for details on each category responses for each threat). 28.2% of respondents claimed that the density decline was caused by natural events, whereas 7.7% of respondents considered pollution as the principal cause of the long-snouted seahorse population collapse.
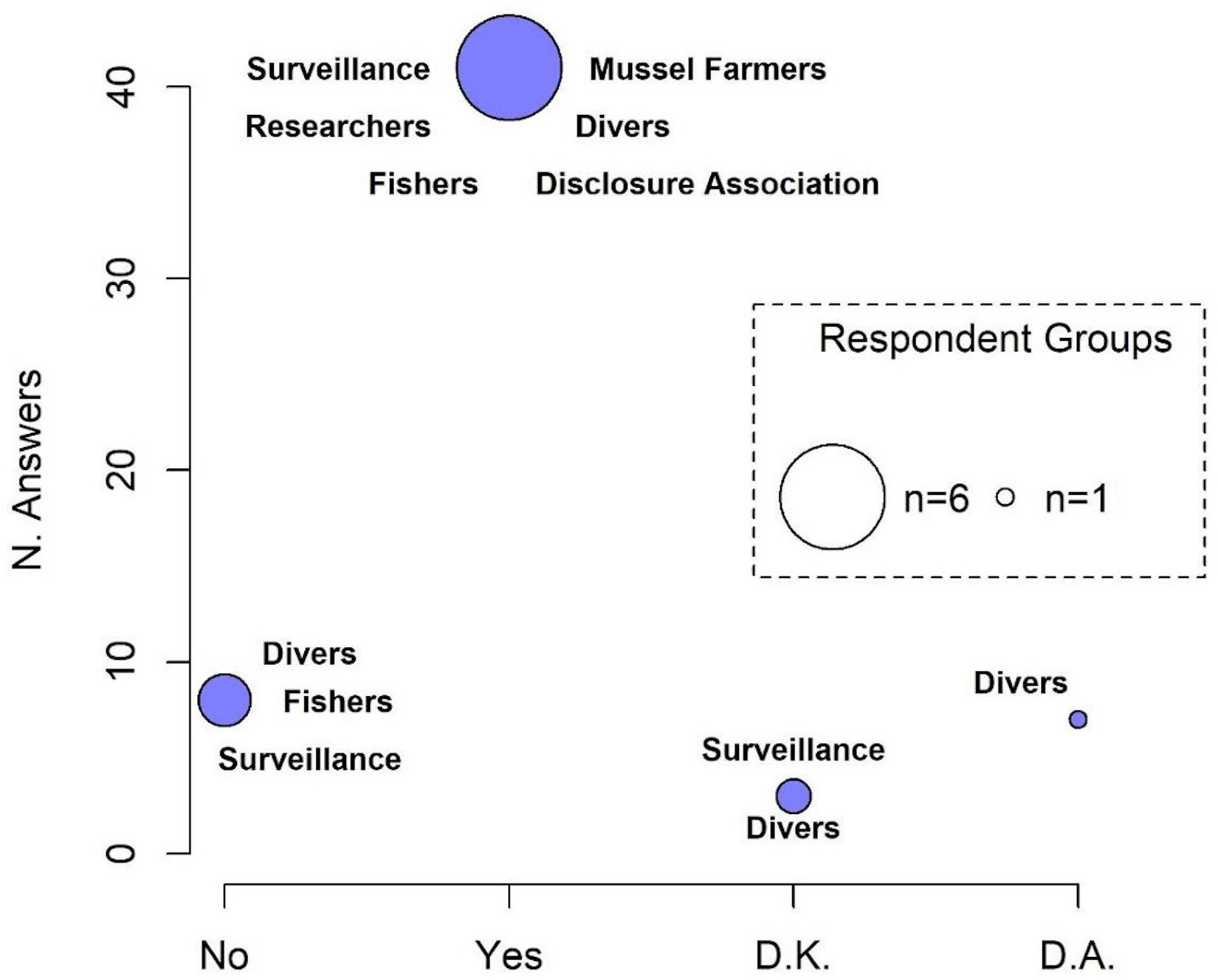
Figure 5. Perception of population density decline in H. guttulatus. The size of the circle is proportional to the number of responding categories. D.K., do not know; D.A., do not answer.
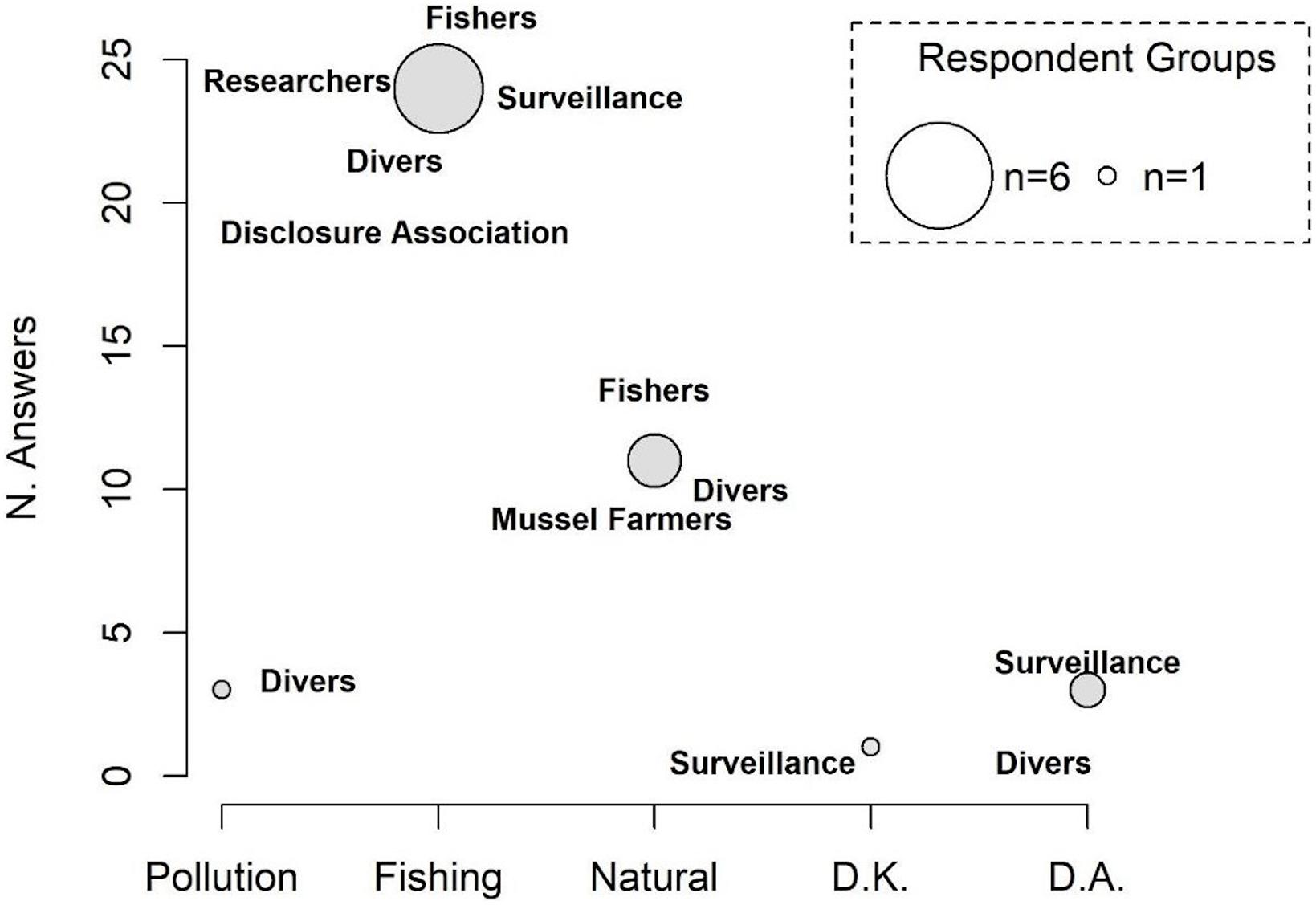
Figure 6. Main causes of the decline in H. guttulatus population density. The size of the circle is proportional to the number of responding categories. D.K., do not know; D.A., do not answer.
According to the respondents, fisheries are conducted using different traditional techniques (trammel nets, small purse seine, small skid gears called “firrchjar,” hand-collection of Sepia spp. juveniles) that incidentally capture seahorses, although 19,4% (Divers 44%, Academics 22% and D. associations 22%) of respondents highlighted that seahorse specimens caught by professional fishermen are retained as curios or sold in the illegal market. However, no significant differences (median presence of target fishery Yes = 1; No = 0.84, Test statistic: 0.1591, p-value = 0.35) were found when the test was performed to evaluate whether the target fishery occurred at MPT.
Data obtained through questionnaires only partially highlighted the existence of fisheries targeting seahorses (Figure 7 and see Supplementary Figure 6 for details on responses of each category). Among respondents that answered, approximately 36.1% have never directly witnessed, while 46.8% of respondents confirmed that this activity is commonly practiced at MPT. Among the latter, 35% stated that the fleet is composed of 3–5 boats, 20% of 2 and 10% of 5–8 boats. Only 9 out of 51 questionnaires provided information on the commercial value of illegally caught seahorses. However, indicated values were highly variable and not related to personal experiences and were therefore not considered in this work.
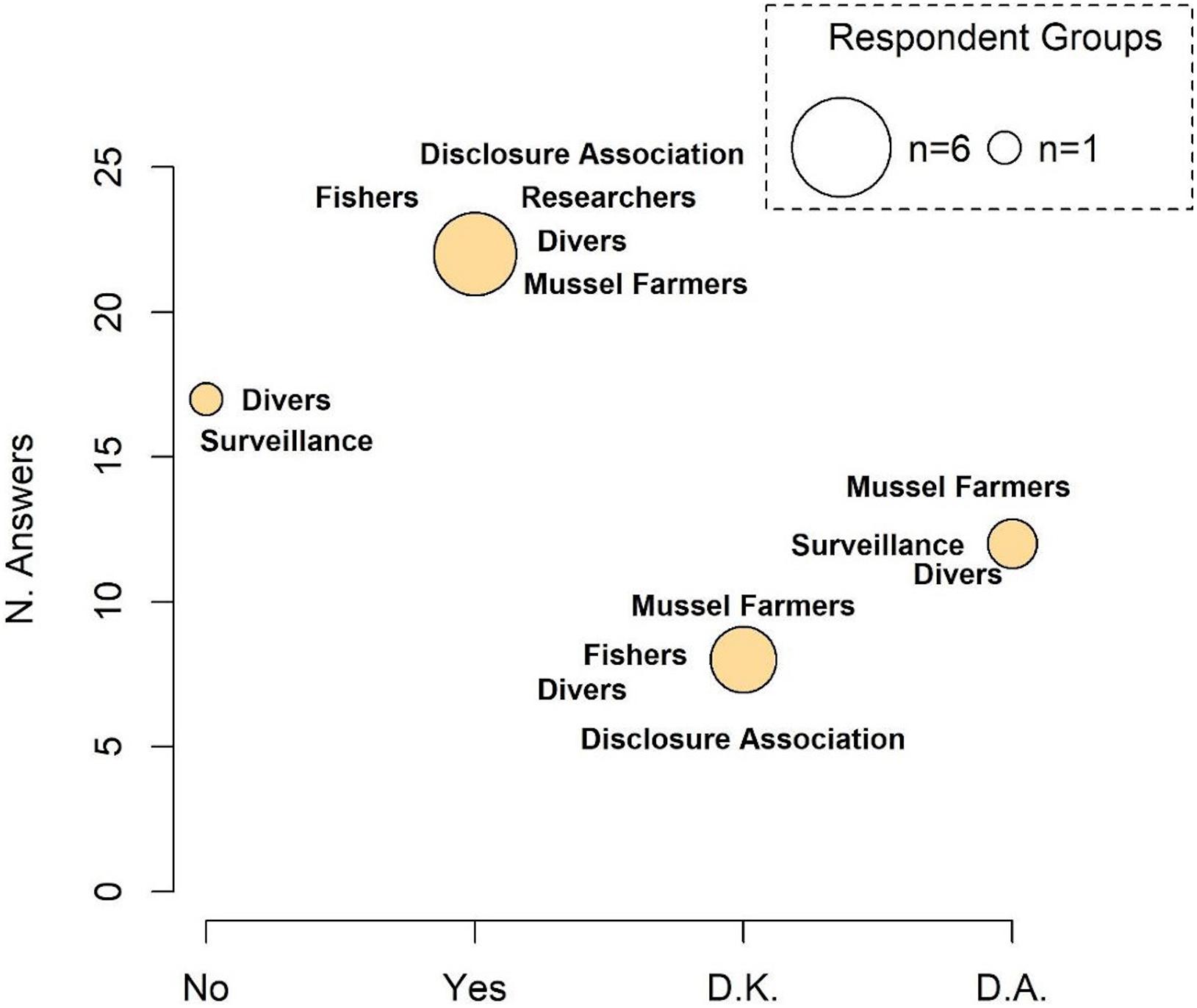
Figure 7. Presence of a target fishery. The size of the circle is proportional to the number of responding categories. D.K., do not know; D.A., do not answer.
In the Mediterranean Sea, the population of long-snouted seahorses at Mar Piccolo di Taranto (MPT) has been evaluated as one of the largest in the entire Mediterranean and European waters (Gristina et al., 2015). In this semi-enclosed marine area, the abundance of H. guttulatus was comparable to that recorded at Ria Formosa (Portugal) and Mar Menor (Spain)3 where wide fluctuations in H. guttulatus density have already been described and were positively correlated with habitat changes, available holdfast coverage (Correia et al., 2015) and eutrophication processes (see text footnote 3). At MPT, the seahorse population showed slight fluctuations over time, but no major changes in its abundance were ever recorded since the first scientific report appeared in 1938 (Cerruti, 1938). Although constant monitoring of seahorses began only 10 years ago, no evidence of past significant declines has ever been found, and indeed local fishermen testify to frequent occurrences of seahorses in nets and consistent stranding after severe storms. First studies on this population reported a density of up to 0.4 individuals per square meter (Gristina et al., 2015). The results of the present study, however, indicated a sudden and sharp seahorse decline starting in 2015. At Site 1, it occurred between autumn 2015 and spring 2016 and affected approximately 90% of the population. This strong reduction was significantly highlighted by GLS time-series regressions (see Figure 3B and Table 1) and Breakpoint Analysis (see Figure 4 and Table 2). The observations indicated no macro-changes in the extent of H. guttulatus preferential habitats at two sampling sites (artificial hard substrates, soft bottoms with scattered bioconcretions, poles, with soft bottom Cymodocea nodosa and Cladophora prolifera beds; for further details see Gristina et al., 2017). However, at Site 1, sabellids and ascidians, which were characteristic organisms of the muddy/sandy soft bottoms until 2015, have almost completely disappeared together with the reference grid points of monitoring stations. This might suggest effects of anthropogenic disturbances, such as bivalve collection by hand- or boat- rakes and nets. Sampling Site 2 maintained density levels comparable to those observed at Site 1 until 2016 and no evidence of anthropogenic impact was found, probably because of the strict military surveillance and the small extent of the soft bottom (compatible with the use of hand- or boat- rakes and nets). Both studied sites have similar characteristics in terms of confinement degree, salinity, and temperature. GLS time-series regressions on the temperature dataset did not demonstrate changes during the investigated period (2011–2019). Although Correia et al. (2018) indicated a negative correlation between H. guttulatus density and temperature in the Ria Formosa lagoon, heat waves, prolonged temperature peaks, and other thermal anomalies could not be used to explain the sudden collapse of the H. guttulatus population at MPT. At least until Autumn 2018, high-density values of seahorses recorded at 2,500 m-far Site 2, indeed, seem to confirm this statement.
The present study did not consider the potential effects of pollutants, such as trace metals, hydrocarbons, and pesticides (Petronio et al., 2012), on the health and survival of H. guttulatus. Although great anthropogenic influences date back to the establishment of a military arsenal in the late nineteenth century and an industrial pole in the mid-twentieth, the seahorse population never showed great signs of suffering until the last decade. According to the Italian Ministry of the Environment, MPT is considered a Site of National Interest (SNI) due to the high levels of contaminants, and since 2015 has been under rigorous monitoring of the competent authorities (DL 5 January 2015, n. 1). Therefore, it is reasonable to assume that the environmental conditions at MPT have remained constant during the study.
The interviews conducted with the main stakeholders at MPT permitted the acquisition of useful information on activities that may have impacted the H. guttulatus population. Most respondents observed a decrease in the seahorse sightings (Figure 5) and claimed that the principal cause was fishing activity (both legal and illegal) (Figure 6). During traditional and legal fisheries, however, most respondents stated that seahorses are only a bycatch and are usually released back into the sea. In such a case, combined effects of fishing gears on the seabed, and displacement and subsequent release of individuals in different locations and habitats, could have had substantial impacts on the seahorse population. As suggested by Caldwell and Vincent (2012), the ability of seahorses to return to the capture site is weak and greatly influenced by conditions in the new environment. The interviews also indicated the presence of illegal fisheries that specifically target seahorses (Figure 7), following the online report on illegal fishing and trafficking of seahorses from MPT to the Far East (China) (Manna, 2019; see text footnote 2).
Until recently, fishing along Italian coasts was considered a negligible threat to seahorse populations, which were rather threatened by degradation and fragmentation of coastal habitats (Olden et al., 2007). The data of the present study, however, indicate the possible onset of a new threat reflected in a renewed demand for seahorses on the TCM market. Indeed, illegal wildlife trafficking of marine organisms is not a new phenomenon in the European seas. The Spanish Nature Protection Service recently seized more than 2,000 dead seahorses headed to China [United Nations Office on Drugs and Crime (UNODC)., 2016], while approximately 40 tons of sea cucumbers from 2016 onward have been seized in several Italian coastal regions. On a worldwide level, seahorse trade is a widely known phenomenon that has recently reached impressive dimensions. It has been estimated that approximately 37 million seahorses are caught each year as a bycatch, predominantly in developing countries (Lawson et al., 2017; Foster et al., 2019). Seahorses are usually collected in South America (Perù, Mexico) (Baum and Vincent, 2005; Rosa et al., 2011), Africa (Senegal), Southeast Asia (Malaysia, Philippine, Viet Nam, Cambodia) (Lawson et al., 2017), India (Vaidyanathan et al., 2020) and are illegally smuggled to China that seems to represent the foremost trading hub for the trafficking of dried seahorses. According to Kuo and Vincent, 2018, routes of wildlife trade have a dynamic network. Indeed, it seems that the case study of long-snouted seahorses at MPT highlights this dynamism in which the Mediterranean acts as a source and Asia as a final market. Until a few years ago, Europe was considered as an end market and transit hub for wildlife trafficking, with European seas and airports commonly used for smuggling from Africa to Asia (see text footnote 1).
The present study helps in turning the spotlight on the trade of Mediterranean marine organisms (e.g., seahorses, Holothurians) (Dominguez-Godino et al., 2016) for use in Traditional Chinese Medicine. Although not confirmed by objective data, the phenomenon should not be underestimated, given that the consequences of such activities on seahorses in lagoons might be great. Indeed, such an unexpected and unpredictable condition would represent a new and fearful threat to the Mediterranean seahorses and could potentially lead populations to the brink of extinction. Due to peculiar and critical socio-economic conditions at MPT, the viable and thriving seahorse population could be important for the revitalization of the coastal economy and the development of environmental awareness. In this context, the establishment of Mar Piccolo Regional Park (D.L. n° 88—July 16, 2020) and its imminent activation could represent a useful tool to help protect and recover threatened species. Although marine protected areas do not appear to have significant effects on the recovery of seahorse populations (Yasuè et al., 2012; Harasti et al., 2014), the establishment of Mar Piccolo Regional Park can attract public interest and raise environmental awareness. Together with more effective controls on artisanal fisheries and illegal wildlife trade, Mar Piccolo Regional Park can provide economic and conservation benefits (i.e., by raising awareness and conservation ethics) and could prove to be crucial for the status of Mediterranean seahorses in future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the animal study because the animals were only observed during the dives.
CP, GC, and MG conceived the ideas and designed the methodology. CP, FC, GC, TL, FQ, GA, and MG collected the data. FQ and MG analyzed the data. CP and MG led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank all respondents who participated in the research.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.692068/full#supplementary-material
Agnew, D. J., Pearce, J., Pramod, G., Peatman, T., Watson, R., Beddington, J. R., et al. (2009). Estimating the Worldwide Extent of Illegal Fishing. PLoS One 4:e4570. doi: 10.1371/journal.pone.0004570
Alabiso, G., Cannalire, M., Ghionda, D., Milillo, M., Leone, G., and Caciorgna, O. (1997). Particulate matter and chemical-physical conditions of an inner sea: the Mar Piccolo in Taranto. A new statistical approach. Mar. Chem. 58, 373–338. doi: 10.1016/s0304-4203(97)00063-7
Anderson, S., Flemmings, J. M., Watson, R., and Lotze, H. K. (2011). Serial exploitation of global sea cucumber fisheries. Fish Fish. 12, 317–339. doi: 10.1111/j.1467-2979.2010.00397.x
Ape, F., Corriero, G., Mirto, S., Pierri, C., Lazic, T., and Gristina, M. (2019). Trophic flexibility and prey selection of the wild long-snouted seahorse Hippocampus guttulatus Cuvier, 1829 in three coastal habitats. Estuar. Coast. Shelf Sci. 224, 1–10. doi: 10.1016/j.ecss.2019.04.034
Baum, J. K., and Vincent, A. C. J. (2005). Magnitude and inferred impacts of the seahorse trade in Latin America. Environ. Conserv. 32, 305–319. doi: 10.1017/s0376892905002481
Caldwell, I. R., and Vincent, A. C. J. (2013). A sedentary fish on the move: effects of displacement on long-snouted seahorse (Hippocampus guttulatus Cuvier) movement and habitat use. Environ. Biol. Fish. 96, 67–75. doi: 10.1007/s10641-012-0023-4
Caldwell, J. R., and Vincent, A. C. J. (2012). Revisiting two sympatric European seahorse species: apparent decline in the absence of exploitation. Aquat. Conserv. 22, 427–435. doi: 10.1002/aqc.2238
Caroppo, C., and Cardellicchio, N. (1995). Phytoplankton of Mar Piccolo of Taranto (Jonian sea). Oebalia 21, 61–76.
Cecere, E., and Petrocelli, A. (2009). “The Mar Piccolo of Taranto,” in Flora and Vegetation of the Italian Transitional Water Systems, eds E. Cecere, A. Petrocelli, A. Sfriso, and G. Izzo (Venezia: Stampa Multigraf), 195–227.
Cerruti, A. (1938). Le condizioni oceanografiche e biologiche del Mar Piccolo di Taranto durante l’agosto del 1938. Roma: Bollettino Piscicoltura Idrobiologia, 711–751.
Correia, M. (2018). Seahorses in the Ria Formosa Lagoon. Final Report - Campaign “Salvar os cavalos-marinhos da Ria Formosa”. Portugal: Fundação Oceano Azul/Oceanário de Lisboa.
Correia, M., Caldwell, I. R., Koldewey, H. J., Andrade, J. P., and Palma, J. (2015). Seahorse (Hippocampinae) population fluctuations in the Ria Formosa lagoon, South Portugal. J. Fish. Biol. 87, 679–690. doi: 10.1111/jfb.12748
Correia, M., Koldewey, H. J., Andrade, J. P., Esteves, E., and Palma, J. (2018). Identifying key environmental variables of two seahorse species (Hippocampus guttulatus and Hippocampus hippocampus) in the Ria Formosa lagoon. South Portugal. Environm. Biol. Fish. 101, 1357–1368. doi: 10.1007/s10641-018-0782-7
Curtis, J. M. R., and Vincent, A. C. J. (2005). Distribution of sympatric seahorse species along a gradient of habitat complexity in a seagrass – dominated community. Mar. Ecol. Prog. Ser. 291, 81–91. doi: 10.3354/meps291081
Dominguez-Godino, J. A., Aydin, M., and Wanguemert, M. (2016). “Profitability of the new target sea cucumber species from Mediterranean and NE Atlantic: Holothuria polii, H. mammata, H. tubulosa and H. arguinensis,”in Front. Mar. Sci. Conference Abstract: International Meeting on Marine Research (Portugal: IMMR).
Food and Agriculture Organization (FAO). (2016). The State of World Fisheries and Aquaculture. Rome: Food and Agriculture Organization.
Foster, S. J., Kuo, T. C., Wan, A. K. Y., and Vincent, A. C. J. (2019). Global seahorse trade defies export bans under CITES action and national legislation. Mar. Policy 103, 33–41. doi: 10.1016/j.marpol.2019.01.014
Foster, S. J., Wiswedel, S., and Vincent, A. C. J. (2016). Opportunities and challenges for analysis of wildlife trade using CITES data – seahorses as a case study. Aquat. Conserv. 26, 154–172. doi: 10.1002/aqc.2493
Gaino, E., Cardone, F., and Corriero, G. (2010). Reproduction of the intertidal sponge Hymeniacidon perlevis (Montagu) along a bathymetric gradient. Open Mar. Biol. J. 4, 47–56. doi: 10.2174/1874450801004010047
Gristina, M., Cardone, F., Carlucci, R., Castellano, L., Passarelli, S., and Corriero, G. (2015). Abundance, distribution and habitat preference of Hippocampus guttulatus and Hippocampus hippocampus in a semi-enclosed central Mediterranean marine area. Mar. Ecol. 36, 57–66. doi: 10.1111/maec.12116
Gristina, M., Cardone, F., Desiderato, A., Mucciolo, S., Lazic, T., and Corriero, G. (2017). Habitat use in juvenile and adult life stages of the sedentary fish Hippocampus guttulatus. Hydrobiologia 784, 9–19. doi: 10.1007/s10750-016-2818-3
Haken, J. (2011). Transnational Crime in the Developing World. Washington: Global Financial Integrity.
Harasti, D., Martin-Smith, M., and Gladstone, W. (2014). Does a No-Take Marine Protected Area Benefit Seahorses? PLoS One 9:e105462. doi: 10.1371/journal.pone.0105462
Harrison, J. R., Roberts, D. L., and Hernandez-Castro, J. (2016). Assessing the extent and nature of wildlife trade on the dark web. J. Conserv. Biol. 30, 900–904. doi: 10.1111/cobi.12707
IUCN. (2020). The IUCN Red List of Threatened Species. Switzerland: International Union for Conservation of Nature.
Kuo, T. C., and Vincent, A. (2018). Assessing the changes in international trade of marine fishes under CITES regulations – A case study of seahorses. Mar. Policy 88, 48–57. doi: 10.1016/j.marpol.2017.10.031
Lawson, J. M., Foster, S. J., Amanda, C. J., and Vincent, A. C. J. (2017). Low Bycatch Rates Add Up to Big Numbers for a Genus of Small Fishes. Fisheries 42, 19–33. doi: 10.1080/03632415.2017.1259944
Lazic, T., Pierri, C., Gristina, M., Carlucci, R., Cardone, F., Colangelo, P., et al. (2018). Distribution and habitat preferences of Hippocampus species along the Apulian coast. Aquat. Conserv. Mar. Freshw. Ecosyst. 28, 1317–1328. doi: 10.1002/aqc.2949
Lourie, S. A., Foster, S. J., Cooper, E. W. T., and Vincent, A. C. J. (2004). A Guide to the Identification of Seahorses. Washington: University of British Columbia and World Wildlife Fund, 1–114.
Mair, P., and Wilcox, R. (2020). Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 52, 464–488. doi: 10.3758/s13428-019-01246-w
Manna, L. (2019). Cavallucci marini e oloturie: L’illecito China Export che parte dai mari di Taranto. VeraLeaks. Available online at: https://veraleaks.org (accessed April 30, 2021).
Margulies, J. D., Bullough, L. A., Hinsley, A., Ingram, D., Cowell, C., Goettsch, B., et al. (2019). Illegal wildlife trade and the persistence of ‘plant blindness’. Plants People Planet 1, 173–182. doi: 10.1002/ppp3.10053
McPherson, J. M., and Vincent, A. C. J. (2004). Assessing East African trade in seahorse species as a basis for conservation under international controls. Aquat. Conserv. Mar. Freshw. Ecosyst. 14, 521–538. doi: 10.1002/aqc.629
Olden, J. D., Hogan, Z. S., and Vander Zanden, M. J. (2007). Small fish, big fish, red fish, blue fish: size biased extinction risk of the world’s freshwater and marine fishes. Glob. Ecol. Biogeogr. 16, 694–701. doi: 10.1111/j.1466-8238.2007.00337.x
Petronio, B., Cardellicchio, N., Calace, N., Pietroletti, M., Pietrantonio, M., and Caliandro, L. (2012). Spatial and temporal heavy metal concentration (Cu, Pb, Zn, Hg, Fe, Mn, Hg) in sediments of the Mar Piccolo in Taranto (Ionian Sea, Italy). Water Air Soil Pollut. 223, 863–875. doi: 10.1007/s11270-011-0908-4
Phelps, J., Biggs, D., and Webb, E. L. (2016). Tools and terms for understanding illegal wildlife trade. Front. Ecol. Environ. 14, 479–489. doi: 10.1002/fee.1325
Pierri, C., Lazic, T., Corriero, G., Cardone, F., Onen Tarantini, S., Desiderato, A., et al. (2020). Site fidelity of Hippocampus guttulatus Cuvier, 1829 at Mar Piccolo of Taranto (Southern Italy; Ionian Sea). Environ. Biol. Fish 103, 1105–1118. doi: 10.1007/s10641-020-01008-0
Pires, S. F., and Moreto, W. D. (2016). The Illegal Wildlife Trade. Oxford: Oxford Handbooks Online.
Planas, M., Blanco, A., Chamorro, A., Valladares, S., and Pintado, J. (2012). Temperature-induced changes of growth and survival in the early development of the seahorse Hippocampus guttulatus. J. Exp. Mar. Biol. Ecol. 438, 154–162. doi: 10.1016/j.jembe.2012.10.003
Relini, G., Tunesi, L., Vacchi, M., Andaloro, F., D’Onghia, G., Fiorentino, F., et al. (2018). Lista Rossa IUCN dei Pesci ossei marini Italiani. Roma: Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare.
Roe, D. (2002). Making a killing or making a living: Wildlife trade, trade controls, and rural livelihoods (No. 6). London: IIED.
Rosa, I. L., Oliveira, T. P. R., Osorio, F. M., Moraes, L. E., Castro, A. L. C., Barros, G. M. L., et al. (2011). Fisheries and trade of seahorses in Brazil: historical perspective, current trends, and future directions. Biodivers. Conserv. 20, 1951–1971. doi: 10.1007/s10531-011-0068-2
Salin, K. R., Yohannan, T. M., and Mohanakumaran, M. (2005). Fisheries and trade of seahorses. Hippocampus spp, in southern India. Fish. Manage. Ecol. 12, 269–273. doi: 10.1111/j.1365-2400.2005.00450.x
Samoilys, M. A. (1997). Manual for Assessing Fish Stocks on Pacific Coral Reefs. Brisbane: Queensland Department of Primary Industries.
United Nations Office on Drugs and Crime (UNODC). (2016). World Wildlife Crime Report: Trafficking in protected species. Vienna: United Nations Office on Drugs and Crime.
Vaidyanathan, T., Zhang, X., Balakrishnan, R., and Vincent, A. C. J. (2020). Catch and trade bans for seahorses can be negated by non-selective fisheries. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 43–59. doi: 10.1002/aqc.3419
Vincent, A. C. J. (1996). The International Trade in Seahorses. Cambridge: TRAFFIC International, 163.
Vincent, A. C. J., Foster, S. J., and Koldewey, H. J. (2011). Conservation and management of seahorses and other Syngnathidae. J. Fish Biol. 78, 1681–1724. doi: 10.1111/j.1095-8649.2011.03003.x
Xu, Q., Cai, M., and Mackey, T. K. (2020). The illegal wildlife digital market: analysis of Chinese wildlife marketing and sale on Facebook. Environ. Conserv. 47, 206–212. doi: 10.1017/S0376892920000235
Yasuè, M., Nellas, A., and Vincent, A. C. J. (2012). Seahorses helped drive creation of marine protected areas, so what did these protected areas do for the seahorses? Environ. Conserv. 39, 183–193. doi: 10.1017/s0376892911000622
Yu, X., and Jia, W. (2015). Moving Targets: Tracking Online Sales of Illegal Wildlife. Cambridge: TRAFFIC.
Zeileis, A., Kleiber, C., Krämer, W., and Hornik, K. (2003). Testing and dating of structural changes in practice. Comput. Stat. Data Anal. 44, 109–123. doi: 10.1016/s0167-9473(03)00030-6
Zeileis, A., Leisch, F., Hornik, K., Kleiber, C., and Hansen, B. (2013). Struc-change: Testing, Monitoring, and Dating Structural Changes. Available online at: http://cran.r-project.org/web/packages/strucchange/index.html (Accessed February 25, 2015)
Keywords: Hippocampus guttulatus, bycatch, CITES, conservation, illegal fishing, illegal trade
Citation: Pierri C, Cardone F, Corriero G, Lazic T, Quattrocchi F, Alabiso G and Gristina M (2021) Density Decline in a Mediterranean Seahorse Population: Natural Fluctuations or New Emerging Threats? Front. Mar. Sci. 8:692068. doi: 10.3389/fmars.2021.692068
Received: 07 April 2021; Accepted: 30 July 2021;
Published: 20 August 2021.
Edited by:
Annette Breckwoldt, Leibniz Centre for Tropical Marine Research (LG), GermanyReviewed by:
José Pedro Andrade, University of Algarve, PortugalCopyright © 2021 Pierri, Cardone, Corriero, Lazic, Quattrocchi, Alabiso and Gristina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamara Lazic, dGFtYXJhLmxhemljQHVuaWJhLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.