- 1Perry Institute for Marine Science, Waitsfield, VT, United States
- 2Coral Vita, Grand Bahama, Bahamas
Coral reefs of Grand Bahama and New Providence islands in The Bahamas have been surveyed several times over the past decade, and long-term monitoring indicates declines in coral cover associated with hurricanes, bleaching events, and local threats. However, the greatest declines in coral populations in The Bahamas over the past decade may be attributed to the recent introduction of stony coral tissue loss disease (SCTLD). In 2019, a comprehensive assessment of both islands was conducted using Atlantic and Gulf Rapid Reef Assessment (AGRRA) methods to characterize reefs before SCTLD was reported in The Bahamas. Following reports of SCTLD in late 2019, timed roving diver assessments of corals were conducted for Grand Bahama in March 2020 and New Providence in June 2020 to determine which species were affected by the disease and the proportion of corals that were healthy, infected with SCTLD, and those that appeared to have experienced recent mortality for the most abundant intermediate or highly susceptible species. Additional surveys were conducted for both islands in January 2021 to further assess the extent of the outbreak, and repeated assessments of several sites for each island were used to determine the impact of the disease on corals over the previous 6.5 to 10.5 months. Infection rates varied among species following patterns described for Florida and elsewhere, with higher infection rates occurring in vulnerable species for both Grand Bahama and New Providence. Pseudodiploria strigosa appears to be the most affected species with 45.6% of colonies on Grand Bahama infected and 23.1% infected on New Providence and recent mortality rates of 31.5 and 42.7%, respectively, at the time of surveys. Spatial patterns of mortality and infection rates for the most vulnerable species were greatest close to international commercial shipping ports on both islands, suggesting SCTLD has been present in those locations for a longer time, and the proportion of healthy colonies increased with distance from the port. For Grand Bahama, there was also a significant effect of depth, with shallow reefs having a higher proportion of colonies that was infected or experienced recent mortality. For New Providence, sites to the east of the port saw a sharp decline in infection and mortality rates with distance compared to sites west of the port, where nearly the entire coastline was affected by SCTLD. Temporal analyses showed an increase in recent mortality and a decrease in active infection for most species on both islands, but little change in the proportion of healthy corals, suggesting some degree of resistance to the disease. Because Freeport and Nassau are the two largest container ports in The Bahamas and are over 200 km apart with multiple islands between them where SCTLD has not yet been reported, it is probable that SCTLD arrived in The Bahamas via commercial shipping, followed by rapid spread within islands via local currents and other vectors. Results from this study stress the need for early detection and suggest that preventing the spread of the disease between islands via vessel traffic is of utmost importance.
Introduction
Coral reefs are the most biologically diverse marine ecosystem (Reaka-Kudla, 1997; Knowlton et al., 2010; Plaisance et al., 2011) and the most valuable ecosystem on a per-unit-area basis, with a value of over $352,249 per hectare per year (de Groot et al., 2012; Costanza et al., 2014). However, coral reef ecosystems are among the most threatened on the planet, suffering severe declines at an increasing rate over the past half century (Pandolfi et al., 2003; Bellwood et al., 2004; Jackson et al., 2014). This has resulted in increasing calls for improved conservation and management of reef-building corals and coral reef ecosystems (e.g., Bellwood et al., 2004).
Climate change has been identified as a leading cause of coral reef decline, primarily due to warm water bleaching events (e.g., Hoegh-Guldberg et al., 2007, 2017). Regional bleaching events have had a large effect on corals in the Caribbean region since the 1980s (e.g., Baker et al., 2008), and recent mass bleaching events on Australia’s Great Barrier Reef have reinforced the severity of this threat globally (e.g., Stuart-Smith et al., 2018). However, coral disease epidemics have also had a large impact on reef-building corals, particularly in the Caribbean (e.g., Aronson et al., 1998; Aronson and Precht, 2001; Weil et al., 2006), and their impact may be increased due to other threats such as elevated nutrient inputs (Bruno et al., 2003), predators that serve as vectors (Williams and Miller, 2005; Gignoux-Wolfsohn et al., 2012), warm sea temperatures (van Woesik and Randall, 2017; Muller et al., 2018; Gignoux-Wolfsohn et al., 2020), and other anthropogenic stressors (e.g., Kaczmarsky et al., 2005).
Stony coral tissue loss disease (SCTLD) is the latest disease to have a major impact on Caribbean reefs and may rival climate change in its impact to reef-building corals (Walton et al., 2018). The disease was first reported in Florida in 2014 (Precht et al., 2016), and the pathogen(s) causing the disease is still unknown. SCTLD is of particular concern because it infects over 20 species (Florida Coral Disease Response Research and Epidemiology Team, 2018), is waterborne, and is highly transmissible (Aeby et al., 2019), spreading within a reef and between reef areas rapidly (e.g., Precht et al., 2016; Walton et al., 2018; Aeby et al., 2019; Muller et al., 2020; Sharp et al., 2020). It is also highly virulent and can persist on reefs for multiple years, rapidly reducing colony density and living coral tissue (Walton et al., 2018). By 2019, SCTLD had spread throughout the Florida Keys (NOAA, 2018), at a rate of 0.1 km/day (Muller et al., 2020) and had reached much of the Northern Caribbean region, from Mexico (Alvarez-Filip et al., 2019; Estrada-Saldivar et al., 2020) to the Greater Antilles and Northeastern Caribbean (AGRRA, 2021).
Before late 2019, SCTLD was not reported from The Bahamas, despite The Bahamas’ proximity to Florida, where the disease first became established. From 2015 to 2019, assessments of corals at over 250 sites throughout The Bahamas have shown declines in live coral cover from bleaching events, hurricanes, and coastal development, but SCTLD was not reported, and coral disease rates averaged only 1.2% of corals surveyed (Dahlgren et al., 2020). SCTLD was first reported from The Bahamas off the island of Grand Bahama during photo monitoring of corals from November 19, 2019 to January 1, 2020 (Figures 1A–C). Photos showed the progression of SCTLD infection in several colonies of Pseudodiploria strigosa, Diploria labyrinthiformis, and Siderastrea siderea, and photos from January 2020 also showed infected colonies of Dendrogyra cylindrus, Dichocoenia stokesii, and Montastraea cavernosa. On New Providence island, initial reports of SCTLD in May 2020 came from spear fishers reporting the disease with photos and short video clips through an online reporting portal (Perry Institute for Marine Science, 2020). Here we characterize the reefs of both islands before SCTLD was reported and examine outbreaks of SCTLD using rapid assessment techniques to determine species-specific infection rates and mortality throughout the affected areas to examine spatial and temporal aspects of SCTLD outbreaks in The Bahamas.

Figure 1. Time series of a P. strigosa colony infected with stony coral tissue loss disease (SCTLD) from November 8, 2019 (A) to November 27, 2019 (B) before transitioning to recent mortality by January 16, 2020 (C). A reef with widespread mortality of P. strigosa is also shown from 2021 (D).
Materials and Methods
Study Sites
New Providence island in the central Bahamas is where Nassau, the population, tourism, and economic center of The Bahamas is located (Figure 2). Coral reefs surround the island, with shallow patch reefs scattered along the Great Bahama Bank to the south and east of the island and shallow and deep forereef habitats along the margin of the bank to the west of the island. Along the north coast, the western two thirds of the island are protected by a barrier reef that slopes into a forereef zone, and the eastern third of the island is protected by barrier islands that extend to the east of New Providence and are fringed with reefs. The main port of Nassau is located along the north coast of the island with international container shipping to the west side of the port and cruise ships, charter boats, and domestic shipping and pleasure boat marinas in the main port area. There is also a fuel shipping station on the southwest corner of the island.
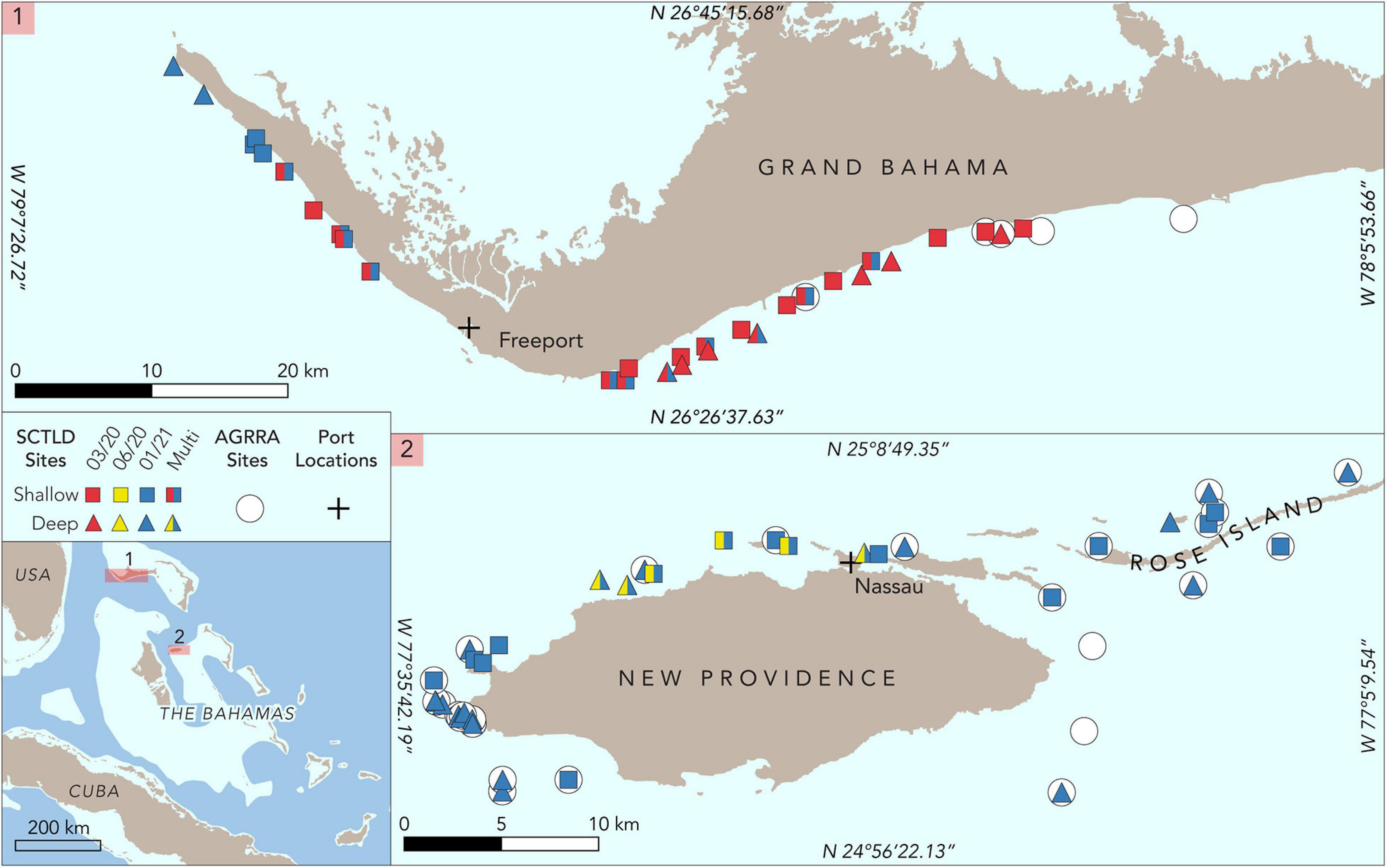
Figure 2. Map of study sites for Grand Bahama and New Providence. Sites where Atlantic and Gulf Rapid Reef Assessment (AGRRA) surveys were conducted in 2019 are denoted by circles. Roving diver assessments on shallow sites are marked with squares and deep sites with triangles. Surveys conducted in March 2020 are shown in red, June 2020 in yellow, and January 2020 in blue. Sites with two colors are those that were assessed more than once. The location of commercial shipping ports for Grand Bahama and New Providence is marked with a cross.
Grand Bahama on the Little Bahama Bank is home to The Bahamas’ second most populous city, Freeport, in the southwest part of the island. Freeport is home to the country’s busiest commercial port with one of the region’s largest container transshipment facilities and one of the largest petroleum product storage terminals in the western hemisphere (Figure 2). The island is bisected by a man-made waterway in the center and population density is low to the east of this waterway. Fringing coral reefs extend along the entire western and southern shoreline of Grand Bahama, ranging in depth from <2 to over 30 m along a shelf that typically extends 1 to 2 km from shore.
Reefs in both areas were well-studied prior to the detection of SCTLD. From 2011 to 2019, 24 reefs around New Providence and neighboring islands to the east (e.g., Rose Island) were monitored every 2 to 4 years using Atlantic and Gulf Rapid Reef Assessment (AGRRA) methods (Dahlgren et al., 2014, 2016, 2020). Eighteen reefs around Grand Bahama were also assessed two to four times using AGRRA methods from 2013 to 2019 (Dahlgren et al., 2016, 2020).
Baseline Conditions
Coral and benthic data collected using AGRRA methods as part of periodic monitoring were used to determine baseline conditions for reefs around Grand Bahama and New Providence prior to reports of SCTLD. These data were collected in 2019, between April and June for New Providence (n = 19 sites) and in October 2019 after Hurricane Dorian for Grand Bahama (n = 6 sites). Briefly, percent cover of coral was calculated based on point intercept data from multiple replicate (n = 4–6) 10-m-long transects at each site. Along each transect, divers recorded what was growing on the seafloor at 10-cm intervals for 100 points per transect. Along at least two additional 10-m-long by 1-m-wide belt transects, divers recorded all coral colonies at least partially within the belt; identified them to species; and measured each colony’s maximum length, width (perpendicular to length measurements), and height along its axis of growth. The percent of each colony that was alive and dead was recorded, with mortality divided into old mortality, where the dead portion of the colony was overgrown by macroalgae or other organisms or sediments; new mortality, where newly exposed coral skeleton was visible; and transitional mortality, where coral skeletons were beginning to be covered with turf algae and/or sediment. The percentage of the colony that was pale or bleached was also recorded, and any disease was identified and recorded for each colony. Sites were divided into shallow (<10 m) and deep (>10.1 m) reefs for analysis due to the difference in coral community composition (e.g., Geister, 1977). Percent average of living coral cover, abundance of coral species, and disease prevalence was obtained for each island in each depth range. An analysis of coral species’ relative abundance was calculated for each island and expressed as a percentage of the total number of colonies assessed for each island.
Rapid Assessments
Following reports of SCTLD, rapid assessments of coral reefs were conducted specifically to determine the spatial extent of the disease as well as species-specific infection and recent mortality rates at each site. Rapid assessments of 25 reefs off Grand Bahama were conducted in March 2020 and six reefs in June 2020 for New Providence (Figure 2). Surveys began at sites where SCTLD had been reported for each island and extended outward along the reef from those locations to assess the extent of the disease outbreak for both islands. A second set of surveys for both Grand Bahama and New Providence was conducted in January 2021 to examine changes over time at several previously surveyed reefs and at additional sites to determine the spread of the disease for each island. These last surveys were comprised of 16 sites in Grand Bahama, including 11 of the previously assessed sites, and 29 in New Providence, including all sites surveyed in June 2020 (Figure 2).
Rapid assessments were completed on snorkel for depths less than 4 m and on scuba for depths greater than 4 m. During all rapid assessments, divers swam over the reef haphazardly for 10 min, identifying all corals observed to species and tallying up to 100 colonies per species and categorizing them based on their condition—healthy (H), infected (SCTLD), or having experienced recent mortality (RM). Healthy colonies showed no signs of SCTLD. Infected colonies showed signs of SCTLD (Figures 1A,B). Colonies suffering recent mortality were completely dead, but not overgrown with macroalgae or benthic invertebrates or eroded, so that skeletal structure was clearly visible, similar to photos of corals that were monitored and known to have died from SCTLD (Figures 1C,D). Additional notes were made on whether other diseases or compromised coral conditions (e.g., pale and bleaching) were present.
Data Analysis
The total number of each coral species observed and the overall infection rates and mortality rates were calculated for each species on reefs of each island to determine the most abundant species that were affected by SCTLD for further analyses of spatial and temporal patterns of the SCTLD outbreak on each island. Spatial patterns of the disease outbreak were assessed for Grand Bahama and New Providence separately with mixed-effects generalized linear models (GLM) using SPSS software for the five most abundant taxa that were observed to be susceptible to SCTLD. Using this model, the proportion of each species that was healthy, infected with SCTLD, or had experienced recent mortality for the entire colony was compared among sites after applying a logistic transformation to proportion data and excluding data from any sites where there were less than three colonies of the species observed. Models were run separately for each species. Factors in the models included distance from the commercial shipping ports of Freeport (Grand Bahama) or Nassau (New Providence) as a continuous variable and categorical variables of direction (east or west) of the port and depth. Sites <10 m were classified as shallow, and sites >10 m were classified as deep. Distance was calculated as the shortest in-water distance between the port location and the survey site. This was computed using the ArcGIS Pro Euclidean Distance Tool with the port locations as source sites, including the outline of the land at 30-m resolution as a barrier layer and sampling the resulting distance at each of the survey points.
In addition to these variables, the interaction between depth and direction was included for New Providence only, since all sites west of Freeport, Grand Bahama, were shallow during the survey periods analyzed. Analyses were performed on March 2020 assessments of reefs around Grand Bahama and January 2021 assessments of reefs around New Providence with other times excluded due to the lower number of sites surveyed.
To examine changes in coral condition over time at sites where surveys were conducted 6.5 months apart (New Providence) or 10.5 months apart (Grand Bahama), we compared the proportion of corals that were healthy, infected with SCTLD, or suffered recent mortality for the five most abundant taxa observed with SCTLD using a G-test of goodness-of-fit model on the proportion of corals in each of the three categories for each island and applied a Williams’ correction for continuity of the data.
Results
Baseline Conditions
During 2019 baseline surveys, average live coral cover in Grand Bahama was 16.8% for shallow reefs (≤10 m) and 9.2% for deep reefs (>10 m). For New Providence, these values were 10.3 and 4.7%, respectively. Overall, the most abundant species from all surveyed reefs of both islands were Agaricia agaricites, Porites astreoides, S. siderea, Orbicella faveolata, and Orbicella annularis (Table 1). Of the corals that are considered highly susceptible to SCTLD (Florida Coral Disease Response Research and Epidemiology Team, 2018), D. labyrinthiformis and P. strigosa were the most common. Of the corals considered to be intermediately susceptible to SCTLD (e.g., Florida Coral Disease Response Research and Epidemiology Team, 2018), S. siderea, Orbicella spp., and M. cavernosa were the most common. The most common coral disease observed was dark spot disease (DS) with a prevalence between 1.9 and 0.8% of corals infected per island. No coral colonies had SCTLD in baseline surveys in 2019.
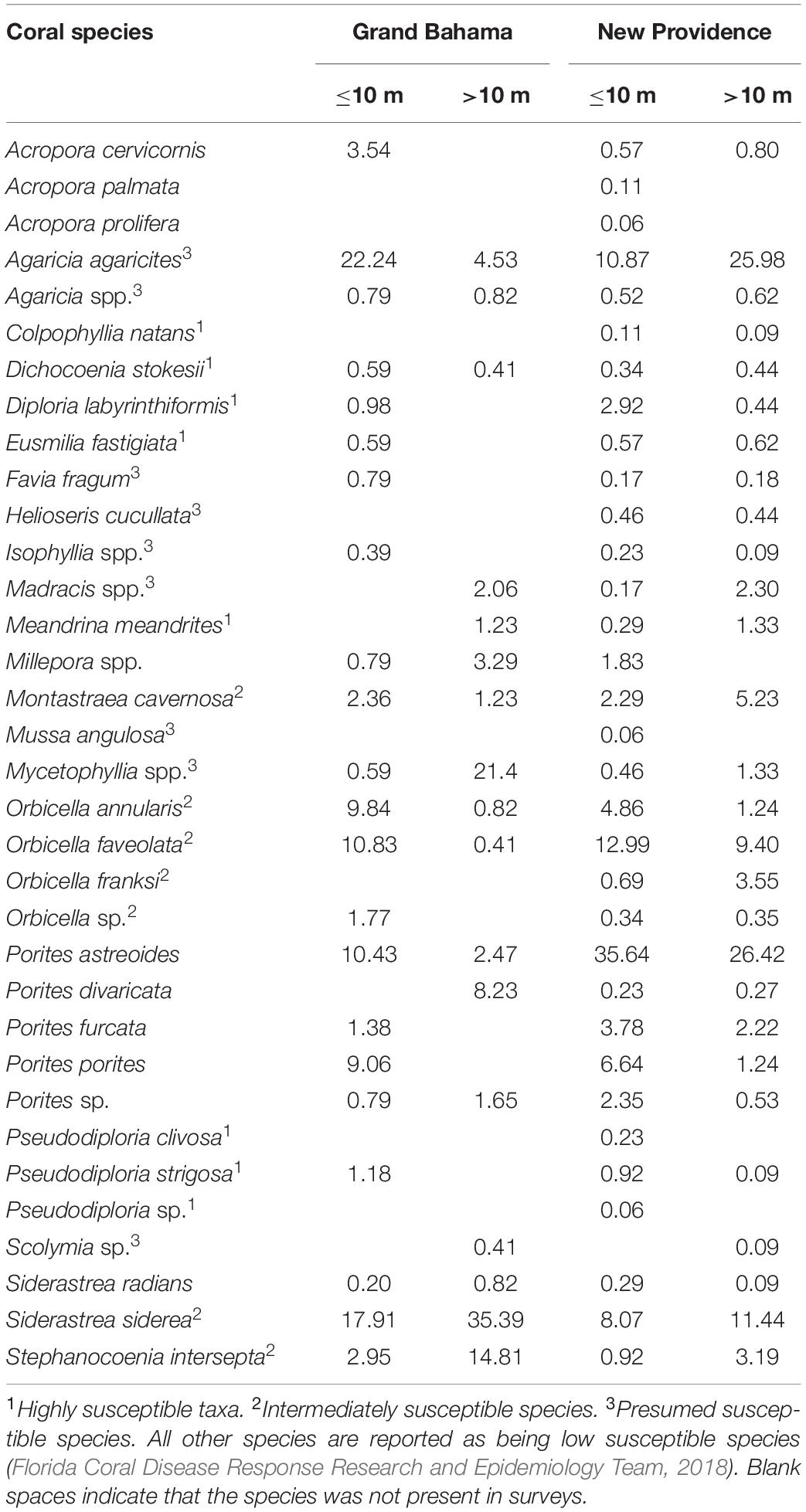
Table 1. Percent composition of coral taxa observed for Grand Bahama and New Providence (The Bahamas) from 2019 AGRRA surveys for shallow (≤10 m) to deep (>10 m) reefs.
Coral Species SCTLD Outbreak
During rapid assessments of Grand Bahama and New Providence, the condition of 13,545 corals belonging to 29 species was evaluated. Eighteen coral species were affected by SCTLD around Grand Bahama and New Providence (Table 2). Five species are considered highly or intermediately susceptible species and were frequently observed at most sites for both islands (e.g., Florida Coral Disease Response Research and Epidemiology Team, 2018): Pseudodiploria strigosa, D. labyrinthiformis, S. siderea, M. cavernosa, and star corals of the genera Orbicella, which were grouped together based on the uneven spatial distribution of the species across sites. Highly susceptible species, like P. strigosa, had SCTLD infection rates of 23.1 and 45.6% across all sites on New Providence and Grand Bahama, respectively, and recent mortality rates of up to 42.7%. For P. strigosa, SCTLD infection and recent mortality rates combined to exceed 80% at half of shallow sites where SCTLD was present. Other species that were less common also had high infection rates, including D. cylindrus, Meandrina meandrites, and D. stokesii (Table 2). Less susceptible species like S. siderea had lower SCTLD prevalence with only 3% of colonies infected on New Providence on average. However, SCTLD prevalence varied across sites and survey periods for many species, providing further insights into the SCTLD outbreak for both islands.
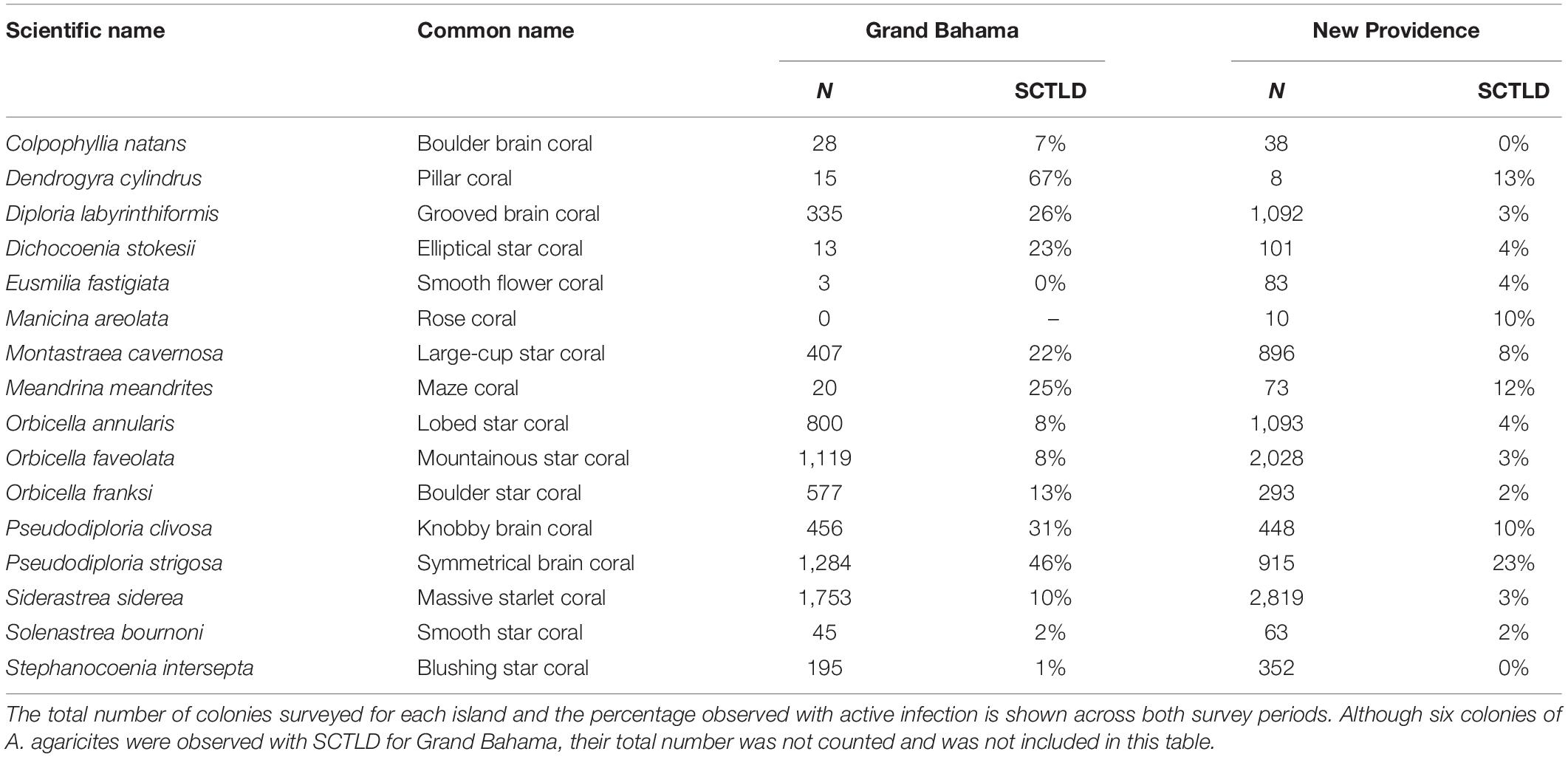
Table 2. Hard corals observed (N and %) with active stony coral tissue loss disease (SCTLD) in all reefs around Grand Bahama and New Providence.
Spatial Patterns of the SCTLD Outbreak
Spatial patterns of the SCTLD outbreak for Grand Bahama and New Providence were assessed for the five most abundant taxa vulnerable to SCTLD: P. strigosa, D. labyrinthiformis, M. cavernosa, S. siderea, and Orbicella spp., with all three Orbicella spp. grouped together since spatial distribution was variable among sites. During New Providence surveys in January 2020, no SCTLD was found for any species on reefs off Rose Island >10 km to the east of the port of Nassau (Figure 2), so these sites were omitted from analyses. The GLM analysis used to determine the relationship of distance and direction from the nearest major commercial shipping port and depth with the proportion of healthy, SCTLD infection, and recent mortality of colonies for each species is shown in Table 3. Only D. labyrinthiformis around Grand Bahama and Orbicella spp. around New Providence showed no significant relationship between distance and any of the coral conditions. In all other cases, there was a significant relationship between distance and the proportion of healthy colonies and/or recent mortality. For nearly all species, there was an increasing proportion of healthy colonies as distance from the port increased on both islands and a greater proportion of recently dead colonies closer to the port than farther away (Figures 3, 4). Figures 3, 4 show spatial patterns of coral condition for P. strigosa, M. cavernosa, and Orbicella spp. with spatial patterns of coral condition for S. siderea and D. labyrinthiformis shown in Supplementary Material, as these species generally follow patterns shown for Orbicella spp. and M. cavernosa, respectively.
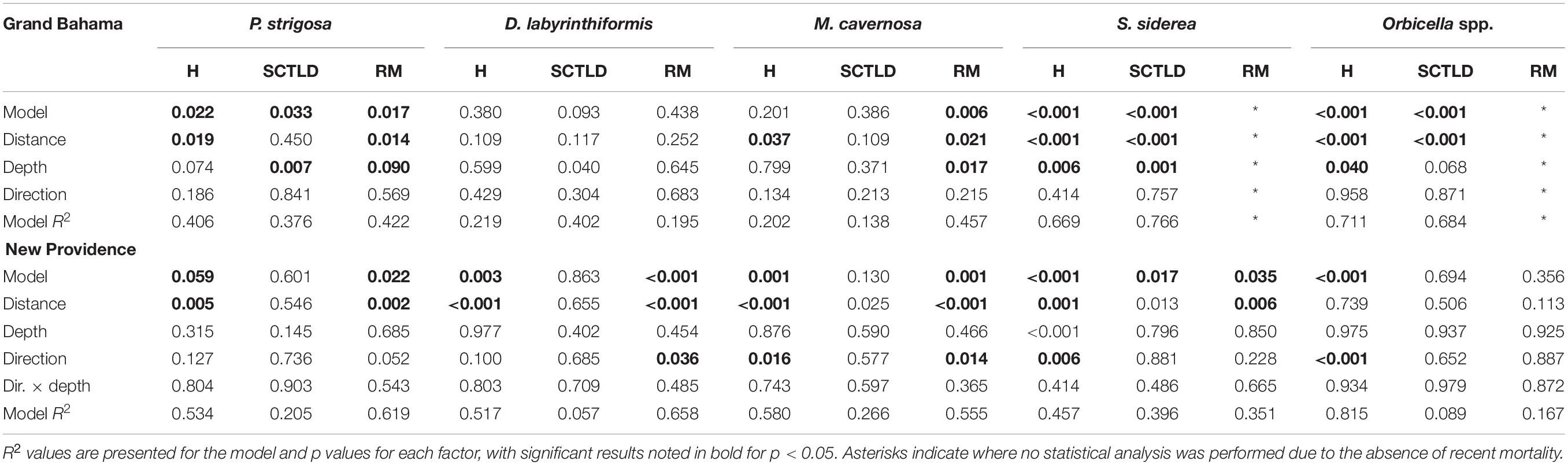
Table 3. Results of GLM model on the effect of distance and direction from port and depth of site on the proportion of healthy (H), SCTLD infected, and recent mortality (RM) for major corals on reefs of Grand Bahama and New Providence.
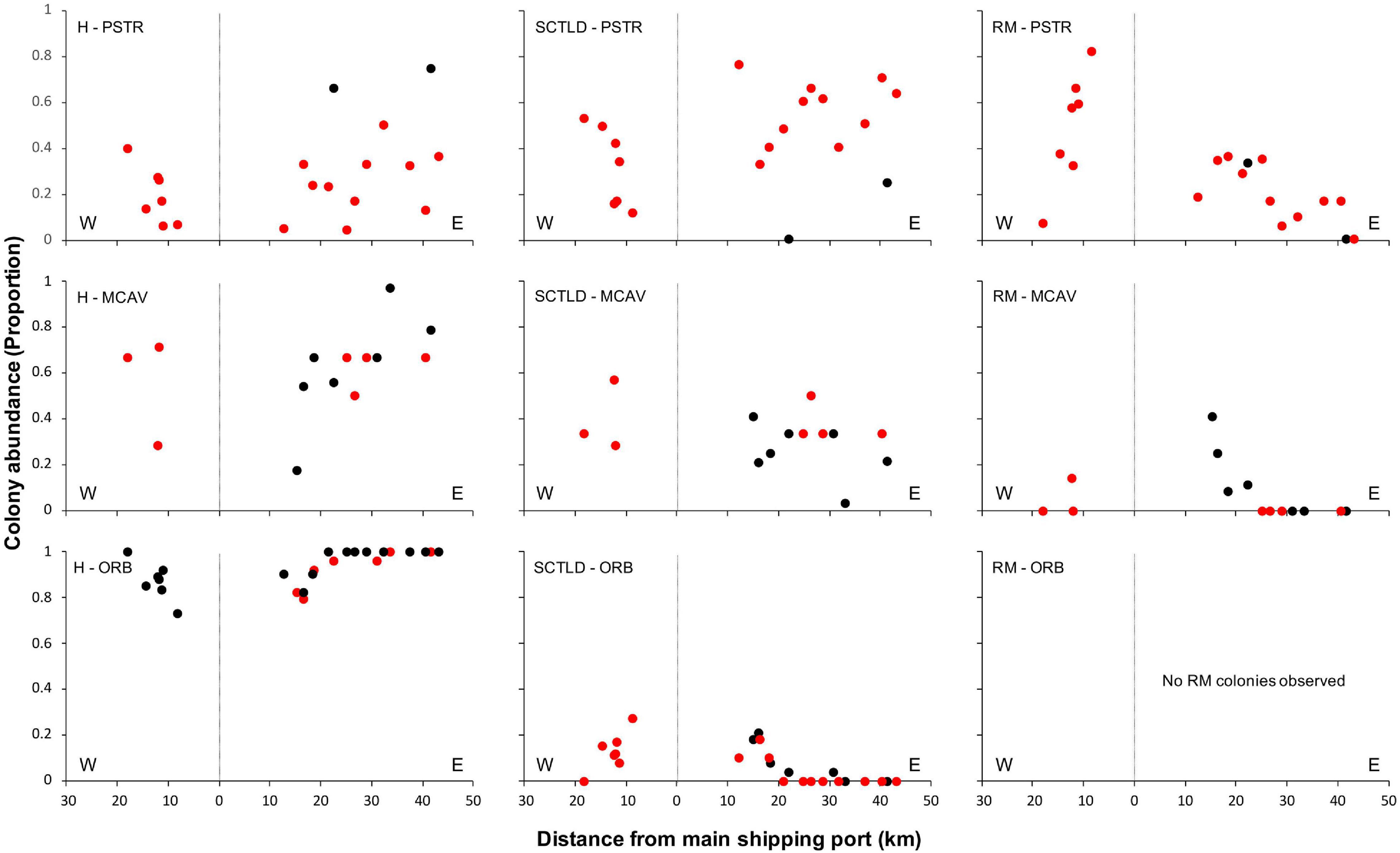
Figure 3. Proportion of colonies from Grand Bahama that were healthy (H, left), infected with SCTLD (middle), or experienced recent mortality (RM, right) for Pseudodiploria strigosa (PSTR), Montastraea cavernosa (MCAV), and Orbicella spp. (ORB), plotted against distance east and west of the port facility (distance = 0). Deep reefs (>10 m) are shown in black and shallow reefs (≤10 m) in red.
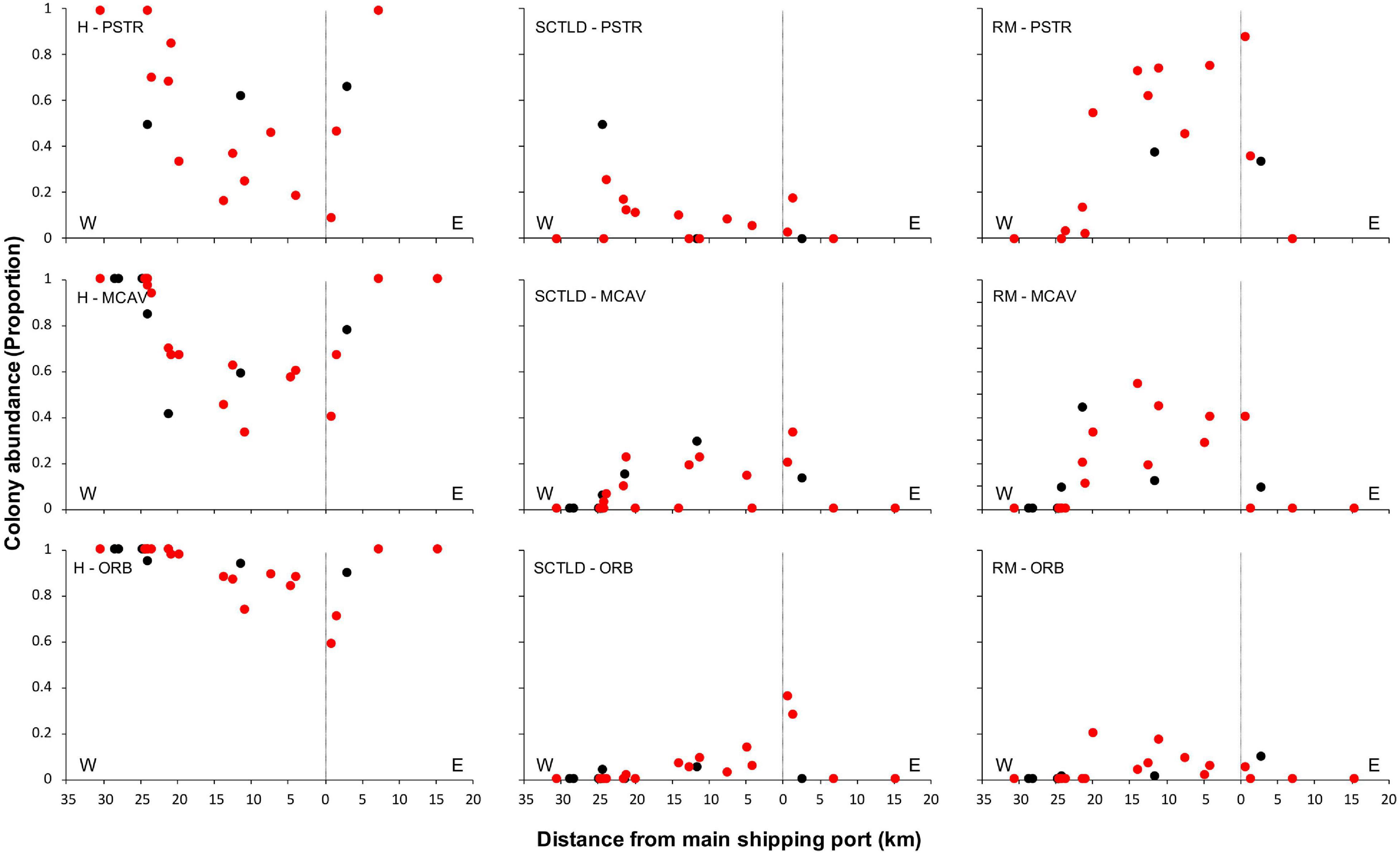
Figure 4. Proportion of colonies from New Providence that were healthy (H, left), infected with SCTLD (middle), or experienced recent mortality (RM, right) for Pseudodiploria strigosa (PSTR), Montastraea cavernosa (MCAV), and Orbicella spp. (ORB) plotted against distance east and west of the port facility (distance = 0). Deep reefs (>10 m) are shown in black and shallow reefs (≤10 m) in red.
The proportion of colonies with active SCTLD infections was significantly correlated with distance from the port for S. siderea for both islands (figure available in Supplementary Materials) and Orbicella spp. for Grand Bahama. Both S. siderea and Orbicella spp. had the lowest active SCTLD infection rates and recent mortality rates of the species examined. Species with higher infection and mortality rates did not have a significant relationship between distance and the proportion of colonies with active SCTLD infection. In addition to distance, depth was a significant factor in influencing the proportion of colonies that was healthy (S. siderea and Orbicella spp.), SCTLD infected (P. strigosa and S. siderea), and recently dead (P. strigosa and M. cavernosa) for Grand Bahama, with shallow reefs having a higher proportion of infected and recently dead colonies (Table 3 and Figure 3). For New Providence, depth was not a significant factor, but direction from the port was significant for the proportion of healthy and/or recently dead colonies for all taxa compared except S. siderea (Table 3 and Figure 4), with a steep increase in healthy colonies and a corresponding steep decline in recently dead colonies to the east of the port compared to the west where SCTLD was observed over a larger area.
Changes Over Time
The change in proportion of healthy, infected, and recently dead colonies between sampling periods was compared for the same species of corals used in spatial analyses for each island. For New Providence, analysis included six shallow sites, and for Grand Bahama, analysis included nine shallow and two deep sites (Figure 2). For each coral taxa on each island, there were statistically significant changes (G test, p < 0.05) in the proportion of colonies that were healthy, infected, and recently dead, except for Orbicella spp. on Grand Bahama, which showed no significant change in proportion among those three categories of coral condition (G test, p = 0.067). However, significant changes in coral condition do not appear to be driven by a change in the proportion of colonies that were healthy for most species. Instead, change appears to be driven by a decrease in the proportion of colonies infected with SCTLD and a corresponding increase in the proportion of recently dead corals for both islands (Figure 5). There were two exceptions to this pattern. One was Orbicella spp. off New Providence, which showed an increase in the proportion of both infected and recently dead colonies. The other unique pattern was P. strigosa off Grand Bahama, which showed a decrease in proportion of colonies infected with SCTLD and those experiencing recent mortality and a corresponding increase in the proportion of healthy colonies. It is interesting to note, however, that the total number of P. strigosa colonies at sites in the second survey period was 65% lower than the first survey period. Additionally, for some species identified as highly susceptible to SCTLD, which naturally occur in low numbers around The Bahamas (such as D. cylindrus, M. meandrites, Eusmilia fastigiata, and D. stokesii), no healthy colonies were observed during the second survey for sites where SCTLD was present in the first survey.
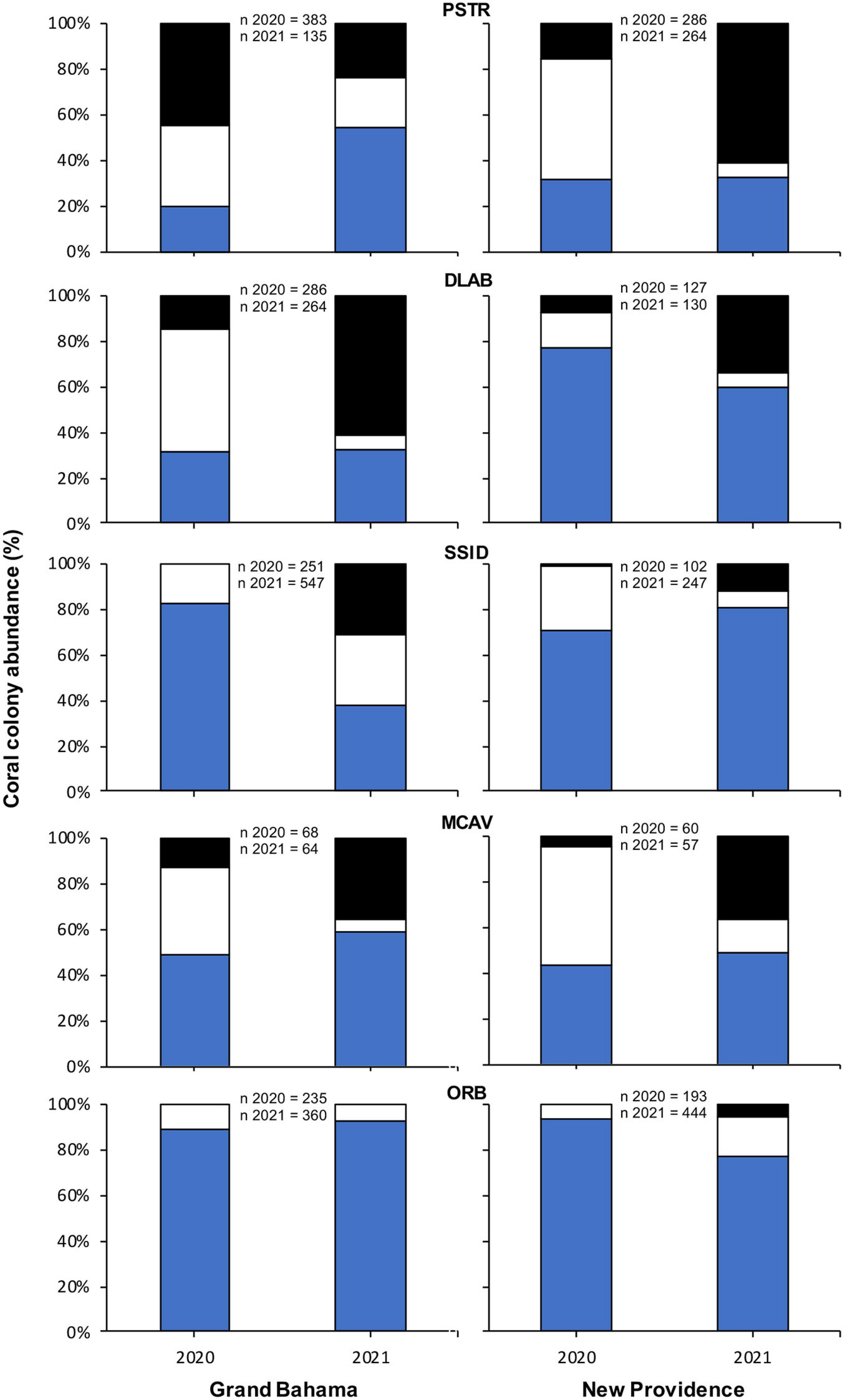
Figure 5. Change in percentage of all colonies of Pseudodiploria strigosa (PSTR), Diploria labyrinthiformis (DLAB), Siderastrea siderea (SSID), Montastraea cavernosa (MCAV), and Orbicella spp. (ORB) observed that were healthy (H, blue), infected with SCTLD (white), and recent mortality (RM, black) for sites off Grand Bahama and New Providence surveyed for SCTLD in 2020 and 2021.
Discussion
Caribbean coral reefs face a number of threats, and SCTLD adds to a large list of coral diseases that have been identified as a major threat in the region (Weil, 2004). However, the impact of SCTLD is greater than most other diseases because it affects more species and has a greater prevalence than other diseases for vulnerable coral species, potentially resulting in a rapid ecological shift and a major degradation of Caribbean reefs (Heres et al., 2021). Our study supports this assertion, suggesting that the outbreak of SCTLD in Grand Bahama and New Providence is a rapid and serious threat to populations of many coral species at local scales. Overall, SCLTD prevalence in both islands is well above the <2% disease prevalence observed before the disease was reported (Dahlgren et al., 2020), which is similar to what has been found in the Mexican Caribbean, where disease prevalence increased from 1 to 25.9% due to SCTLD in less than 2 years (Alvarez-Filip et al., 2019).
The SCTLD prevalence of susceptible species in our study was similar to that reported for Florida and Mexico, where P. strigosa, D. labyrinthiformis, S. siderea, and M. cavernosa have high disease prevalence (Precht et al., 2016; Alvarez-Filip et al., 2019). Moreover, we observed high rates of recent mortality for several of these species. In the present study, the species with the greatest infection and mortality rates from SCTLD was P. strigosa, a dominant species on shallow reefs. For this species, SCTLD infection and recent mortality proportions combined to exceed 65% across all sites for both islands and exceeded 80% at half of the shallow sites where SCTLD was present at the time of surveys. It is important to realize that the prevalence of the disease reported here, however, represents a snapshot in time across all sites surveyed. An analysis of spatial and temporal patterns in this study shows how the prevalence varies for each species across reef areas on the scale of over 60 km and temporally over a 6- to 10-month time period.
While our study did not definitively determine the cause of the recent mortality observed, we believe the vast majority of recent mortality was due to SCTLD since we did not find evidence of alternative sources of mortality including other coral disease, predation, or bleaching. For example, the only other coral disease affecting P. strigosa was black band disease, with a prevalence below 0.5%. One could argue that reefs of Grand Bahama may have been suffering from prolonged damage after Hurricane Dorian, but the spatial patterns of recent mortality reported here do not correspond with spatial patterns in damage reported in assessments immediately following Hurricane Dorian (Dahlgren et al., 2020). Furthermore, reefs of New Providence showed similar mortality patterns to Grand Bahama but did not experience severe storm conditions. Finally, species not affected by SCTLD or less susceptible to SCTLD (e.g., Florida Coral Disease Response Research and Epidemiology Team, 2018) such as Acropora spp., A. agaricites, or P. astreoides did not show signs of recent mortality similar to highly and intermediately susceptible species as one would expect if other causes of widespread mortality were common. Thus, we believe that our assessment of recent mortality largely reflects the death of colonies resulting from SCTLD.
Temporal changes in coral condition for P. strigosa on Grand Bahama differed from other species and locations, by showing a decrease in colonies with SCTLD and those that had experienced recent mortality over time, accompanied by an increase in the proportion of healthy colonies between survey periods. This result is likely due to high mortality rates from SCTLD for P. strigosa on Grand Bahama between survey periods. During the second surveys of Grand Bahama, there was a 65% decrease in the total number of P. strigosa colonies that could be assessed and classified as healthy, infected with SCTLD, or experiencing recent mortality. Because this species was observed to experience high infection and mortality rates at the time of the first survey (Figure 3), many of these colonies were likely to have been overgrown by macroalgae or other organisms, preventing these colonies from being included in the second surveys (Box and Mumby, 2007). Time series photos that prompted our assessments (e.g., Figures 1A–C) indicated that corals could be classified as experiencing recent mortality for up to a month or two after their death, but many of these colonies are likely to have been overgrown in the 10.5 months between surveys (Figure 1D). Thus, in January 2021, colonies showing a degree of resistance and still unaffected by SCTLD comprised a greater proportion of colonies surveyed.
It was not just the common species that suffered mortality from SCTLD. Bahamian reefs could experience local extinctions of some species that are already rare such as D. cylindrus, M. meandrites, and E. fastigiata. It has been suggested that D. cylindrus could become extinct due to this disease outbreak (Neely et al., 2021), and our data indicates that the majority of colonies encountered on Grand Bahama were affected by SCTLD (Table 2). Our data suggest a rapid loss of M. meandrites and E. fastigiata, particularly for New Providence where six sites that had multiple healthy colonies of both species in 2020 had none in 2021. These results imply that Bahamian coral reefs could suffer a major change in coral community composition, thus impacting the ecological functionality of coral reefs.
However, not all species surveyed in this study responded in the same way to SCTLD. A temporal analysis for Orbicella spp. suggests that these species are either less vulnerable to the disease than P. strigosa or the effects of SCTLD operate on timescales longer than our study. This is similar to patterns reported from Florida (Florida Coral Disease Response Research and Epidemiology Team, 2018), where lower incidences of infection and mortality rates over time were found for Orbicella spp. Changes over time in the proportion of healthy, infected, and recent mortality for D. labyrinthiformis, M. cavernosa, and S. siderea showed a stable proportion of healthy colonies but a decrease in the proportion of infected corals and an increase in recent mortality. This suggests that 40% or more of the population of these species may be somewhat resistant to SCTLD infection within the first year of infection at the site. Monitoring the fate of these individuals over time to see if they become infected and examining the characteristics of these corals and their associated microbiome will be important to understand resistance to the disease.
Spatial and temporal patterns observed in this study have significant implications for the management of SCTLD in The Bahamas. The rate of spread of SCTLD is a great concern and challenge for the management of reefs. The absence of SCTLD from surveys of Grand Bahama and New Providence in 2019 but a widespread occurrence across at least two thirds of the reef area in 2020 for both islands indicate a rapid spread of the disease. In the case of Grand Bahama, SCTLD was not detected in AGRRA surveys in October 2019 but was widespread at several of the same sites 5 months later and was reported in photo monitoring at nearby sites as early as November 2019, only a month after AGRRA assessments. Dive operators later recalled seeing corals dying off Freeport during the summer of 2019, but this was unreported. This is consistent with spatial patterns observed in this study where reefs closest to the port of Freeport had the greatest proportion of infected colonies or recent mortality and greater proportions of healthy colonies farther away.
The same spatial pattern with respect to reefs close to commercial shipping ports was seen for New Providence and suggests that shipping may be the vector responsible for introducing SCTLD to The Bahamas. During March 2020 surveys off Grand Bahama and a subsequent visit in April 2021, large tanker ships off Freeport were observed to be pumping out large quantities of water (C. Dahlgren, personal observation), suggesting that the practice of releasing ballast water on site may be common. If ballast water was taken on board in a port area where SCTLD was present and not exchanged in open water away from reefs as required in The Bahamas, ballast water may have been the means by which SCTLD reached the Grand Bahama area some time in 2019. This hypothesis is further supported by the fact that no SCTLD has been reported in The Bahamas from reefs closer to Florida, which have been affected by SCTLD for 5 years or more (e.g., Bimini). Even in Grand Bahama, which is only 100 km from Florida, the reefs to the west that were closest to Florida had lower infection or mortality rates than those closer to the port of Freeport. An alternative hypothesis that may explain this pattern is that SCTLD may not have originated close to the port on either island, but corals there may have been more vulnerable to the disease due to pollution, nutrients, or other environmental conditions (e.g., Dougan et al., 2020). We believe that this is a less likely scenario, however, as previous studies have documented declines in reef condition associated with pollution in both the east and west of New Providence (Sealey, 2004; Dahlgren et al., 2020) and the area of eastern Grand Bahama where Hurricane Dorian significantly impacted reefs (Dahlgren et al., 2020), but all of these areas showed lower effects of SCTLD than more centrally located reefs close to the ports in this study.
Once reaching The Bahamas, the rapid spread of the disease within reefs at a scale of meters to hundreds of meters may be due to direct contact (Aeby et al., 2019) or contact with particles that pathogens may adhere to Dobbelaere et al. (2020), water movement (Sharp et al., 2020), and biotic vectors such as foureye butterflyfish and other organisms observed to feed on colonies infected with SCTLD (Noonan and Childress, 2020). Between-reef spread over kilometers is likely due to local current patterns (Muller et al., 2020) that varied among islands. In New Providence, there was a dramatic decline in infection and mortality rates with increasing distance away from the port to the east (Figure 4). This is consistent with mean surface current patterns predicted by hydrographic models of the area, although these currents can vary (Cherubin, 2014). The spread of SCTLD between reefs on Grand Bahama covered a larger area than New Providence, suggesting an earlier introduction period and/or more rapid transmission of the disease. Alternatively, the spread of SCTLD may have been influenced by Hurricane Dorian for Grand Bahama, either by making corals more vulnerable to the disease, thus allowing SCTLD to become established on new reefs more rapidly and have a greater effect on colonies, or through changes in local currents with the passage of the storm that increased spread of SCTLD between reefs. Hurricane Dorian may have played a significant role in transporting SCTLD to the east of Freeport, Grand Bahama, which is typically upstream from the port area (Cherubin, 2014). Hurricane Dorian had a large impact on reefs to the east of Freeport on Grand Bahama, dislodging corals, spreading debris from land, and altering reef structure (Dahlgren et al., 2020), and the passage of the storm caused increased water flow eastward as the storm stalled over Grand Bahama (C. Paris, U. Miami, personal communication). Smaller vessels including commercial and recreational fishing boats, yachts, and inter-island mailboats may have also contributed to the spread of SCTLD within each island and to other parts of The Bahamas, like north Eleuthera Island where SCTLD was confirmed in December 2020.
Conclusion
Our study is the first assessment of SCTLD for The Bahamas. The rapid assessments were intended to provide marine resource managers with information on where the disease was occurring and what species were being affected in a short time period. Future studies in The Bahamas should also include AGRRA surveys of sites where previous data exist to better quantify coral loss and changes to benthic communities and fish assemblages at each site. These surveys will also help to identify which coral species could suffer local extinctions and provide recommendations to marine resource managers for the treatment of corals with antibiotics (Aeby et al., 2019; Neely et al., 2020) and the adoption of other measures such as coral rescue facilities (Zoccola et al., 2020; Florida Department of Environmental Protection, 2021), to prevent loss of these species locally. Furthermore, this study highlights the need for early detection and rapid response to disease outbreaks (Precht, 2021). In less than 5 months between AGRRA surveys of Grand Bahama in October 2019 and rapid assessments of reefs in March 2020, SCTLD had not only become established in the study area but had also spread over reefs along at least 75 km of the southern shoreline of Grand Bahama. Finally, prevention of the spread of the disease through effective regulation of ballast water transfer and implementing regulations to limit potential spread of waterborne pathogens in bilge water (e.g., on site pumping of bilge water and disinfection) and other human spread is essential.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
CD designed the initial study following reports of potential SCTLD from JO. CD obtained permits and responsible for project budgeting. CD, KS, and VP collected field data used in analyses. JO provided the photographs. WG provided spatial analysis and map in Figure 2. CD and VP organized and analyzed the data and wrote the first manuscript draft. All authors contributed to final draft edits before first submission.
Funding
Financial support was provided by the Devereux Ocean Foundation, Disney Conservation Fund, and Bahamas Ministry of Tourism (CD grants), and in-kind support was provided by The Bahamas National Trust and Coral Vita. The funding bodies did not participate in the data analysis, interpretation, or in the writing of this manuscript.
Conflict of Interest
JO was employed by Coral Vita, a commercial company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special thanks go out to Pachancia Knowles, Sam Teicher, Gator Halpern, Shelley Cant-Woodside, Olethea Gardiner, Bradley Watson, Anwar Godet, and Hayley-Jo Carr for their participation in field assessments. The support of The Bahamas Stony Coral Tissue Loss Disease Task Force is also acknowledged. Comments from two reviewers improved the manuscript. Research was conducted under permit numbers MAMR/FIS/17 from The Bahamas Department of Marine Resources and SRBS-016-2020-CD from the Bahamas Environment Science and Technology Commission.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.682114/full#supplementary-material
Supplementary Figure 1 | Proportion of colonies from Grand Bahama (GB; top two rows) and New Providence (NP; bottom two rows) that were healthy (H, left), infected with SCTLD (middle) or experienced recent mortality (RM, right) for Diploria labyrinthiformis (DLAB) and Siderastrea siderea (SSID) plotted against distance east and west of the port facility (Distance = 0). Deep reefs (>10 m) are shown in black and shallow reefs (≤10 m) in red.
References
Aeby, G. S., Ushijima, B., Campbell, J. E., Jones, S., Williams, G. J., Meyer, J. L., et al. (2019). Pathogenesis of a tissue loss disease affecting multiple species of corals along the Florida Reef Tract. Front. Mar. Sci. 6:678. doi: 10.3389/fmars.2019.00678
AGRRA (2021). Coral Disease Outbreak. Available online at: https://www.agrra.org/coral-disease-outbreak/ (accessed February 26, 2021)
Alvarez-Filip, L., Estrada-Saldívar, N., Pérez-Cervantes, E., Molina-Hernández, A., and González-Barrios, F. J. (2019). A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ 7:8069. doi: 10.7717/peerk.8069
Aronson, R. B., and Precht, W. F. (2001). “White-band disease and the changing face of Caribbean coral reefs,” in The Ecology and Etiology of Newly Emerging Marine Diseases, ed. J. W. Porter (Dordrecht: Springer), 25–38. doi: 10.1007/978-94-017-3284-0-2
Aronson, R. B., Precht, W. F., and Macintyre, I. G. (1998). Extrinsic control of species replacement on a Holocene reef in Belize: the role of coral disease. Coral Reefs 17, 223–230. doi: 10.1007/s003380050122
Baker, A. C., Glynn, P. W., and Riegl, B. (2008). Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471. doi: 10.1016/j.ecss.2008.09.003
Bellwood, D. R., Hughes, T. P., Folke, C., and Nyström, M. (2004). Confronting the coral reef crisis. Nature 429, 827–833. doi: 10.1038/nature02691
Box, S. J., and Mumby, P. J. (2007). Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser. 342, 139–149. doi: 10.3354/meps342139
Bruno, J. F., Petes, L. E., Drew Harvell, C., and Hettinger, A. (2003). Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 6, 1056–1061. doi: 10.1046/j.1461-0248.2003.00544x
Cherubin, L. M. (2014). High-resolution simulation of the circulation in the Bahamas and Turks and Caicos Archipelagos. Prog. Oceanogr. 12, 21–46. doi: 10.1016/j.pocean.2014.05.006
Costanza, R., de Groot, R., Sutton, P., Van der Ploeg, S., Anderson, S. J., Kubiszewski, I., et al. (2014). Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158. doi: 10.1016/gloenvcha.2014.04.002
Dahlgren, C., Kramer, P. R., Lang, J., and Sherman, K. (2014). New Providence and Rose Island, Bahamas 2014 Coral Reef Report Card. Vermont: Perry Institute for Marine Science.
Dahlgren, C., Sherman, K., Haines, L., Knowles, L., and Callwood, K. (2020). Bahamas Coral Reef Report Card Volume 2: 2015-2020. Vermont: Perry Institute for Marine Science.
Dahlgren, C., Sherman, K., Lang, J., and Kramer, P. R. (2016). Bahamas Coral Reef Report Card Volume 1: 2011-2013. Vermont: Perry Institute for Marine Science.
de Groot, R., Brander, L., van der Ploeg, S., Costanza, R., Bernard, F., Braat, L., et al. (2012). Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 1, 50–61. doi: 10.1016/j.ecoser.2012.07.005
Dobbelaere, T., Muller, E. M., Gramer, L. J., Holstein, D. M., and Hanert, E. (2020). Coupled epidemio-hydrodynamic modeling to understand the spread of a deadly coral disease in Florida. Front. Mar. Sci. 7:1016. doi: 10.3389/fmars.2020.591881
Dougan, K. E., Ladd, M. C., Fuchs, C., Vega Thurber, R., Burkepile, D. E., and Rodriguez-Lanetty, M. (2020). Nutrient pollution and predation differentially affect innate immune pathways in the coral Porites. Front. Mar. Sci. 7:563865. doi: 10.3389/fmars.2020.563865
Estrada-Saldivar, N., Molina-Hernández, A., Pérez-Cervantes, E., Medellín-Maldonado, F., González-Barrios, F. J., and Alvarez-Filip, L. (2020). Reef-scale impacts of the stony coral tissue loss disease outbreak. Coral Reefs 39, 861–866. doi: 10.1007/s00338-020-01949-z
Florida Coral Disease Response Research and Epidemiology Team (2018). SCTLD Case Definition. Available online at: https://floridadep.gov/sites/default/files/Copy%20of%20StonyCoralTissueLossDisease_CaseDefinition%20final%2010022018.pdf (accessed February 5, 2021).
Florida Department of Environmental Protection (2021). Stony Coral Tissue Loss Disease Response. Available online at: https://floridadep.gov/rcp/coral/content/stony-coral-tissue-loss-disease-response (accessed March 4, 2021)
Geister, J. (1977). “The influence of wave exposure on the ecological zonation of Caribbean coral reefs,” in Proceedings of Third International Coral Reef Symposium, Vol. 2, ed. D. L. Taylor (Miami, FL: Rosenstiel School of Marine and Atmospheric Science), 23–29.
Gignoux-Wolfsohn, S. A., Marks, C. J., and Vollmer, S. V. (2012). White band disease transmission in the threatened coral, Acropora cervicornis. Sci. Rep. 2:804. doi: 10.1038/srep00804
Gignoux-Wolfsohn, S. A., Precht, W. F., Peters, E. C., Gintert, B. E., and Kaufman, L. S. (2020). Ecology, histopathology, and microbial ecology of a white-band disease outbreak in the threatened staghorn coral Acropora cervicornis. Dis. Aquat. Org. 137, 217–237. doi: 10.3354/dao03441
Heres, M. H., Farmer, B. H., Elmer, F., and Hertler, H. (2021). Ecological consequences of stony coral tissue loss disease in the Turks and Caicos Islands. Coral Reefs 40, 1–16. doi: 10.1007/00338-021-02071-4
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Hoegh-Guldberg, O., Poloczanska, E. S., Skirving, W., and Dove, S. (2017). Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci. 4:158. doi: 10.3389/fmars/2017.00158
Jackson, J. B. C., Donovan, M. K., Cramer, K. L., and Lam, V. V. (2014). Status and Trends of Caribbean Coral Reefs: 1970-2012. Gland: Global Coral Reef Monitoring Network, IUCN.
Kaczmarsky, L. T., Draud, M., and Williams, E. H. (2005). Is there a relationship between proximity to sewage effluent and the prevalence of coral disease. Caribb. J. Sci. 41, 124–137.
Knowlton, N., Brainard, R. E., Fisher, R., Moews, M., Plaisance, L., and Caley, M. J. (2010). “Coral reef biodiversity,” in Life in the World’s Oceans: Diversity Distribution and Abundance, ed. A. D. McIntyre (Oxford: Blackwell Publishing Ltd), 65–74.
Muller, E. M., Bartels, E., and Baums, I. B. (2018). Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. eLife 7:e35066. doi: 10.7554/eLife.35066.001
Muller, E. M., Sartor, C., Alcaraz, N. I., and van Woesik, R. (2020). Spatial epidemiology of the stony-coral-tissue-loss disease in Florida. Front. Mar. Sci. 7:163. doi: 10.3389/fmars.2020.00163
Neely, K. L., Lewis, C. L., Lunz, K. S., and Kabay, L. (2021). Rapid population decline of the pillar coral Dendrogyra cylindrus along the Florida Reef Tract. Front. Mar. Sci. 8:434. doi: 10.3389/fmars.2021.656515
Neely, K. L., Macaulay, K. A., Hower, E. K., and Dobler, M. A. (2020). Effectiveness of topical antibiotics in treating corals affected by stony coral tissue loss disease. PeerJ 8:e9289. doi: 10.7717/peerj.9289
NOAA (2018). Florida’s Coral Reef Disease Outbreak. Available online at: https://floridakeys.noaa.gov/coral-disease/ (accessed March 5, 2021)
Noonan, K. R., and Childress, M. J. (2020). Association of butterflyfishes and stony coral tissue loss disease in the Florida Keys. Coral Reefs 39, 1581–1590. doi: 10.1007/s00338-020-01986-8
Pandolfi, J. M., Bradbury, R. H., Sala, E., Hughes, T. P., Bjorndal, K. A., Cooke, R. G., et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958. doi: 10.1126/science.1085706
Perry Institute for Marine Science (2020). Report Stony Coral Tissue Loss Disease (SCTLD). Available online at: http://www.perryinstitute.org/reportsctld/ (accessed March 5, 2021)
Plaisance, L., Caley, M. J., Brainard, R. E., and Knowlton, N. (2011). The diversity of coral reefs: what are we missing? PLoS One 6:e25026. doi: 10.1371/journal.pone.0025026
Precht, W. (2021). Failure to respond to a coral disease epizootic in Florida: causes and consequences. Rethink. Ecol. 6, 1–47.
Precht, W. F., Ginert, B. E., Robbart, M. L., Fura, R., and van Woesik, R. (2016). Unprecedented disease-related coral mortality in Southeastern Florida. Sci. Rep. 6:31374. doi: 10.1038/srep31374
Reaka-Kudla, M. L. (1997). “The global biodiversity of coral reefs: a comparison with rain forest,” in Biodiversity II: Understanding and Protecting Our Biological Resources, eds M. L. Reaka-Kudla, D. E. Wilson, and E. O. Wilson (Washington, DC: Joseph Henry Press), 83–108.
Sealey, K. S. (2004). Large-scale ecological impacts of development on tropical islands systems: comparison of developed and undeveloped islands in the central Bahamas. Bull. Mar. Sci. 75, 295–320.
Sharp, W. C., Shea, C. P., Maxwell, K. E., Muller, E. M., and Hunt, J. H. (2020). Evaluating the small-scale epidemiology of the stony-coral-tissue-loss-disease in the middle Florida Keys. PLoS One 15:e0241871. doi: 10.1371/journal.pone.0241871
Stuart-Smith, R. D., Brown, C. J., Ceccarelli, D. M., and Edgar, G. J. (2018). Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560, 92–96. doi: 10.1038/s41586-018-0359-9
van Woesik, R., and Randall, C. J. (2017). Coral disease hotspots in the Caribbean. Ecosphere 8:e01814. doi: 10.1002/ecs2.1814
Walton, C. J., Hayes, N. K., and Gilliam, D. S. (2018). Impacts of a regional, multi-year, multi-species coral disease outbreak in Southeast Florida. Front. Mar. Sci. 5:323. doi: 10.3389/fmars.2018.00323
Weil, E. (2004). “Coral reef diseases in the wider Caribbean,” in Coral Health and Disease, eds R. Rosenberg and Y. Loya (Berlin: Springer), 35–68.
Weil, E., Smith, G., and Gil-Agudelo, D. L. (2006). Status and progress in coral reef disease research. Dis. Aquat. Org. 69, 1–7. doi: 10.3354/dao069001
Williams, D. E., and Miller, M. W. (2005). Coral disease outbreak: pattern, prevalence and transmission in Acropora cervicornis. Mar. Ecol. Prog. Ser. 301, 119–128.
Keywords: SCTLD, coral disease, coral community, Caribbean, reef monitoring, Bahamas
Citation: Dahlgren C, Pizarro V, Sherman K, Greene W and Oliver J (2021) Spatial and Temporal Patterns of Stony Coral Tissue Loss Disease Outbreaks in The Bahamas. Front. Mar. Sci. 8:682114. doi: 10.3389/fmars.2021.682114
Received: 17 March 2021; Accepted: 31 May 2021;
Published: 12 July 2021.
Edited by:
William F. Precht, Dial Cordy and Associates, Inc., United StatesReviewed by:
Karen Lynn Neely, Nova Southeastern University, United StatesMauricio Rodriguez-Lanetty, Florida International University, United States
Copyright © 2021 Dahlgren, Pizarro, Sherman, Greene and Oliver. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Craig Dahlgren, Y2RhaGxncmVuQHBlcnJ5aW5zdGl0dXRlLm9yZw==
 Craig Dahlgren
Craig Dahlgren Valeria Pizarro1
Valeria Pizarro1 Krista Sherman
Krista Sherman William Greene
William Greene