- 1Marine Mammal and Marine Bioacoustics Laboratory, Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences, Sanya, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3School of Biological Sciences, University of Aberdeen, Aberdeen, United Kingdom
- 4National Institute of Aquatic Resources, Technical University of Denmark, Lyngby, Denmark
- 5Tropical Marine Science Institute, National University of Singapore, Singapore, Singapore
Group size is a key social trait influencing population dynamics of group-living animals. The Indo-Pacific humpback dolphins (IPHDs), Sousa chinensis, a shallow water delphinid species, display a fission-fusion social system. Yet little is known about how social organization of this species vary with temporal scales and behavioral state. In this study, we sampled group size estimates from the world’s second largest population of humpback dolphins (Sousa spp.), which inhabit the eastern waters of Zhanjiang, China. IPHD group sizes changed seasonally and inter-annually, but not with tidal phases. Group sizes also changed with behavioral state of IPHD groups and with number of mother-calf pairs present. IPHDs formed larger groups in the autumn than in other seasons, which might be related to seasonal changes in food availability and reproductive cycle. Of the groups observed, we recorded the presence of mother-calf pair in 85 groups (i.e., nursery groups: 47 ones with one pair, 25 ones with two pairs, and others with three pairs). Notably, nursery groups were about 2–4 times larger than non-nursery groups. In addition, group sizes greatly increased with the number of mother-calf pairs. Living in relatively large groups, more protection, food, and resources might be available for IPHD mothers and calves, and such social strategy provide higher reproduction efficiency and survival success for this species. During our observations, feeding (45.5%) and traveling (25.2%) represented the majority of IPHD’s behavioral budget, while socializing (8.4%) and resting/milling (6.8%) were not frequently observed. Resting/milling groups were approximately 50% smaller than feeding, traveling, or socializing groups, while the latter three types had a similar mean group size. Large groups when IPHDs foraged, traveled, or socialized, might provide more added group benefits. For the first time, our findings clearly revealed intra-population variability in IPHD group sizes across different behavioral and temporal variables, and provided a better understanding of IPHDs’ adaptations to various biological processes and ecological constraints.
Introduction
For social animals, group-living is an important behavioral strategy, and their social interactions are usually variable and dynamic (Silk, 2007). Living in a group, social relationships of group members are generally considered a product of trade-offs between energetic costs and benefits (Parrish and Edelstein-Keshet, 1999; Lusseau, 2003). In dolphin societies, the energetic trade-offs are typically associated with food (Heithaus and Dill, 2002), safety (Lima and Dill, 1990), reproduction (Mann et al., 2000), and resources. Consequently, group-living strategy of dolphin species offers a foundation to build more complex social relationships, such as cooperation or competition that have the scope to increase survival and reproduction (Benoit-Bird and Au, 2003; Orbach et al., 2014), and therefore, ultimately affect population dynamics (Lusseau and Newman, 2004).
Almost all dolphins were described with fission-fusion societies (Kent et al., 2008), but there is large intra- and inter-specific variability in social organization depending on ecological landscape in which the dolphin species reside (Gygax, 2002a; Lusseau et al., 2003; Gowans et al., 2007). Group size is among the main characteristics of social organization of dolphin populations (Lusseau et al., 2006; Cantor et al., 2012; Kappeler, 2019). Changes in group size over time and space can reflect fission-fusion dynamics of dolphins, thus are essential to represent the variability of social interactions (Connor, 2000; Gygax, 2002b; Lusseau et al., 2003). Dolphins can vary their group sizes at spatial scales (Bouveroux et al., 2018; Liu et al., 2020b), and at temporal scales (e.g., year, season, month, and day; Koper et al., 2016; Wang et al., 2016; Sarabia et al., 2018).
The Indo-Pacific humpback dolphin (Sousa chinensis Osbeck, 1765), hereafter referred as IPHD, is a shallow water delphinid species (Jefferson and Curry, 2015; Jefferson and Smith, 2016). Its habitat preference of shallow and near-shore waters has been widely documented in most of the known IPHD populations, as well as in the other three recognized relative species of Sousa spp. (Jefferson and Rosenbaum, 2014). The IPHD was assessed “Vulnerable” by the IUCN Red List of Threatened Species (Jefferson et al., 2017). Our socio-behavioral knowledge on humpback dolphins (Sousa spp.) mainly came from studies on IPHDs in the Chinese waters (Chen et al., 2011; Dungan et al., 2012, 2016; Wang et al., 2015), Australian humpback dolphins (S. sahulensis) in the Australian waters (Parra et al., 2011; Hunt et al., 2019; Hawkins et al., 2020), and Indian Ocean humpback dolphins (S. plumbea) in the South Africa waters (Karczmarski, 1999; Koper et al., 2016; Bouveroux et al., 2019). Some studied populations were documented to display fission-fusion dynamics with some long-lasting social relationships. Typically, the IPHDs live in groups of less than 10 individuals (Parsons, 2004; Chen et al., 2011; Würsig et al., 2016), and their societies include both stable (i.e., preferred companionships) and fluid (i.e., casual acquaintances) social interactions (Dungan et al., 2012, 2016; Wang et al., 2015).
Intra-specific variability in IPHD group sizes is not fully investigated, although some previous studies have basically described social characteristics of humpback dolphins. Previous studies suggested dolphin group sizes and composition may be associated with species characteristics, habitat structure, and social-environmental aspects of populations (Baird and Dill, 1996; Gibson and Mann, 2008b; Degrati et al., 2019). Changes in humpback dolphin group size, such as annual (Koper et al., 2016), seasonal (Chen et al., 2011; Wang et al., 2016), and behavioral variations (Würsig et al., 2016), are often habitat-specific and affected by a series of environmental variables at a regional scale. For instance, the mean group size observed for the Indian Ocean humpback dolphins in the Algoa Bay, South Africa, decreased from 7 individuals in 1990s to only 3 in 2010s (Karczmarski, 1999; Koper et al., 2016), whilst such a sharp decline has not been observed in other regions. In several known humpback dolphin populations, it has been reported that feeding groups, especially those groups following fishing trawlers (Parsons, 2004; Würsig et al., 2016), and breeding groups (Baldwin et al., 2004; Liu et al., 2020b), were much larger than those groups engaged in other behaviors, indicating a potential influence of behavioral states on group size.
A few studies have reported group size variations in the IPHDs. For example, the IPHDs in the Xiamen Bay, China, showed seasonal variations in their group sizes, with larger groups formed during the winter and spring when compared to summer and autumn (Wang et al., 2016). In the eastern Taiwan Strait, Dungan et al. (2016) revealed that IPHD groups were larger when contained calves, suggesting the importance of nursery behavior on IPHDs’ sociality. However, we still lack an understanding of temporal and behavioral factors associated with intra-population variability in the IPHD group sizes. Thus, we know little about how the IPHDs vary their group sizes to adapt to various habitats.
In this study, we showed the variability in IPHD group sizes recorded in the eastern waters of Zhanjiang, China. We assessed whether IPHD group sizes varied at three temporal scales (year, season, and tide) and across two behavioral domains (number of mother-calf pairs, and behavioral state). We expect that IPHD group sizes vary with some of the above factors. This study aims to provide a better understanding of social characteristics of IPHDs at a population level, and to reveal potential factors important for the social dynamics of this population specifically, and IPHDs more generally.
Materials and Methods
Study Area
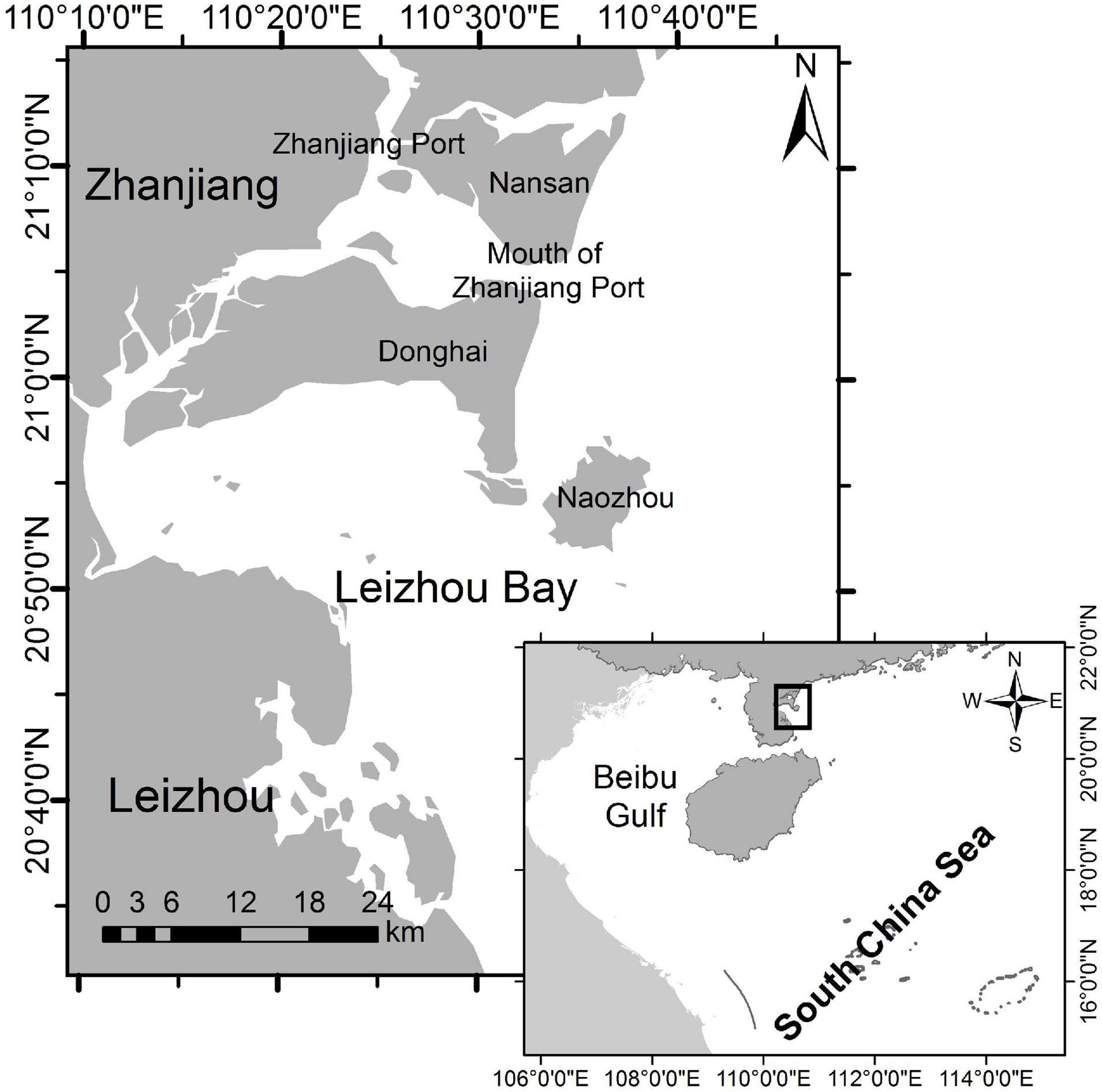
Our survey area is the near-shore, eastern waters of Zhanjiang, China (Figure 1), covering an area of approximately 1,000 km2. This area is a shallow-water embayment (water depth range: 2–40 m) with a sandy/muddy seafloor (Zhou et al., 2007; Xu et al., 2015; Liu et al., 2017a). The population of IPHDs residing in this area was first reported by Zhou et al. (2007). To provide protection for this population, the local Zhanjiang government established a protected area i.e., Zhanjiang Leizhou Bay Municipal Humpback Dolphin Nature Reserve (110° 26′−110° 29′ E, 20° 44′−20° 46′ N; Area: 21-km2; Figure1) in 2007 (Xu et al., 2015; Liu et al., 2020a). Based on local rainfalls and climate characteristics, we defined four season phases for the study area: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February) (Liu et al., 2017b). We divided tidal condition of a day into four consecutive phases: high, ebb, low, and flood (Liu et al., 2021).
Data Collection
To conduct field surveys, we used either a 12-m-length wooden fishing boat (60 HP outboard engine) or a 7-m-length fiberglass speed boat (75 HP outboard engine). We carried out surveys during October-November of 2013, and quarterly from January 2015 to May 2018. During our surveys, at least two experienced observers scanned the front 180° of sea surface, and searched IPHDs with the naked eyes and/or 7 × 50 binoculars (Li et al., 2016; Liu et al., 2020a). We performed the surveys only during the daytime and good visual conditions without rain or fog, and only under satisfied sea states of Beaufort scale ≤ 3.
In this study, we used the term “group” to define one or more dolphins observed with spatial co-occurrence (each member within 200 m of any other members) or social associations (all individuals within a unit in a similar behavioral state) (Wang et al., 2016; Liu et al., 2020b, 2021). Once a IPHD group was sighted, we approached and observed the group with 10−50 m between our boat and the group, unless the group actively approach us. During each observation, we recorded date, time, GPS location, group size, group composition based on age classes, number of mother-calf pairs (absence as 0), and primary behavioral state.
We used a hand-held Garmin 78 s GPS receiver (Garmin, Taiwan, China) to obtain information on date, time, and GPS location. For each group, we used the method of multiple-counts (minimum/best/maximum) to generate observer-based group size estimates (Gerrodette et al., 2002). We determined the group composition based on IPHD coloration patterns along with age classes (Jefferson et al., 2012). We determined the presence/absence of mother-calf pair and number of mother-calf pairs by observing and counting how many individuals are poorly marked, obviously dark-gray, small (i.e., ∼1m length, less than half of adult body length), and at consistent echelon positions with an adult. We determined the behaviors of IPHDs using five recognizable behavioral states: feeding, traveling, socializing, resting, and milling (Parsons, 2004; Stockin et al., 2009; Würsig et al., 2016). See behavioral definitions in Table 1. Raw group size data (observer-based best estimates) were later verified with photographs taken during each sighting: if the observer-based best count was smaller than the number of individuals photographically identified for a group, the group size was modified as the latter (López et al., 2018).

Table 1. Summary of behavioral definitions and observations of Indo-Pacific humpback dolphins in the eastern waters of Zhanjiang, China.
Data Analysis
To test whether variability in IPHD group sizes was associated with temporal and/or behavioral variables, we constructed univariate generalized linear models (GLMs) with multivariate analysis of variance. We included five factors into our models, including three temporal factors i.e., year (2013, 2015, 2016, 2017, and 2018), season (spring, summer, autumn, and winter), and tide (high, ebb, low, and flood), and two behavioral factors i.e., number of mother-calf pairs (0, 1, 2, and 3) and behavioral state (feeding, traveling, socializing, and resting/milling) (Table 2). Not only resting and milling represented a small percentage in the behavioral budget of the dolphins, but these behavioral states are also similar in low activity rate (Table 1). Thus, we integrated resting and milling into one single behavioral state for analysis.
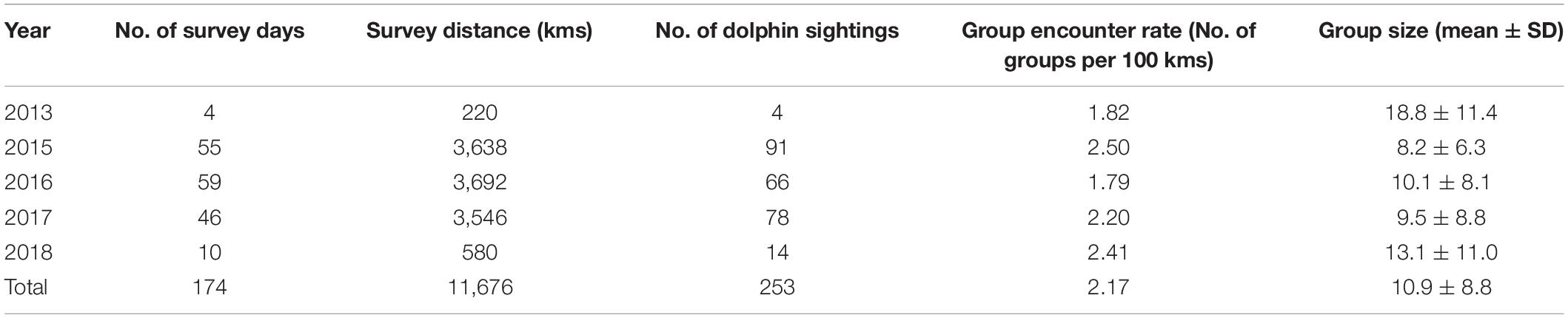
Table 2. Annual survey effort, sighting information, and group size of Indo-Pacific humpback dolphins in the eastern waters of Zhanjiang, China, in 2013, and 2015–2018.
In total, we built five main effects and ten pairwise interaction terms into the GLMs. Our null hypothesis was that there was no difference in the IPHD group sizes across different years, seasons, tidal phases, number of mother-calf pairs, and behavioral states. Once a significant effect was found for either main factor, we performed the Kruskal-Wallis tests to make post hoc pair-wise multiple comparisons using Tukey’s HSD (equal variances, p > 0.05) or Tamhane’s T2 method (unequal variances, p < 0.05). We also built and pruned a classification and regression tree (CART), in order to determine which variable is predominant in affecting the IPHD group sizes (De’ath and Fabricius, 2000; Liu et al., 2019). Results on IPHD group sizes are reported as mean ± standard deviation (SD) unless otherwise stated. We conducted all statistical analyses in the IBM SPSS 19.0 (SPSS Inc., Chicago, Illinois), and defined a significance level of P < 0.05.
Results
Over 5 years (2013 and 2015–2018), we carried out 174-day boat-based surveys in the study area (Table 2). In total, we achieved 11,676 km survey effort (Figure 2A) and sighted 253 IPHD groups (Figure 2B). Throughout the survey period, group encounter rate was 2.17 sightings per 100 km (Table 2). Of the 253 groups, we sampled 229 (90.5%) with available group size estimates, generating a mean group size of 10.9 ± 8.8 individuals (range: 1–48). Of the 229 sampled groups, 227 (99.1%), 225 (98.3%), 195 (85.2%), and 134 (58.5%) were comprised of less than 40, 30, 20, and 10 individuals, respectively (Figure 3). In addition, 24 groups (9.2%) consisted of single individual, and 17 (7.4%) were observed in a pair of individuals.
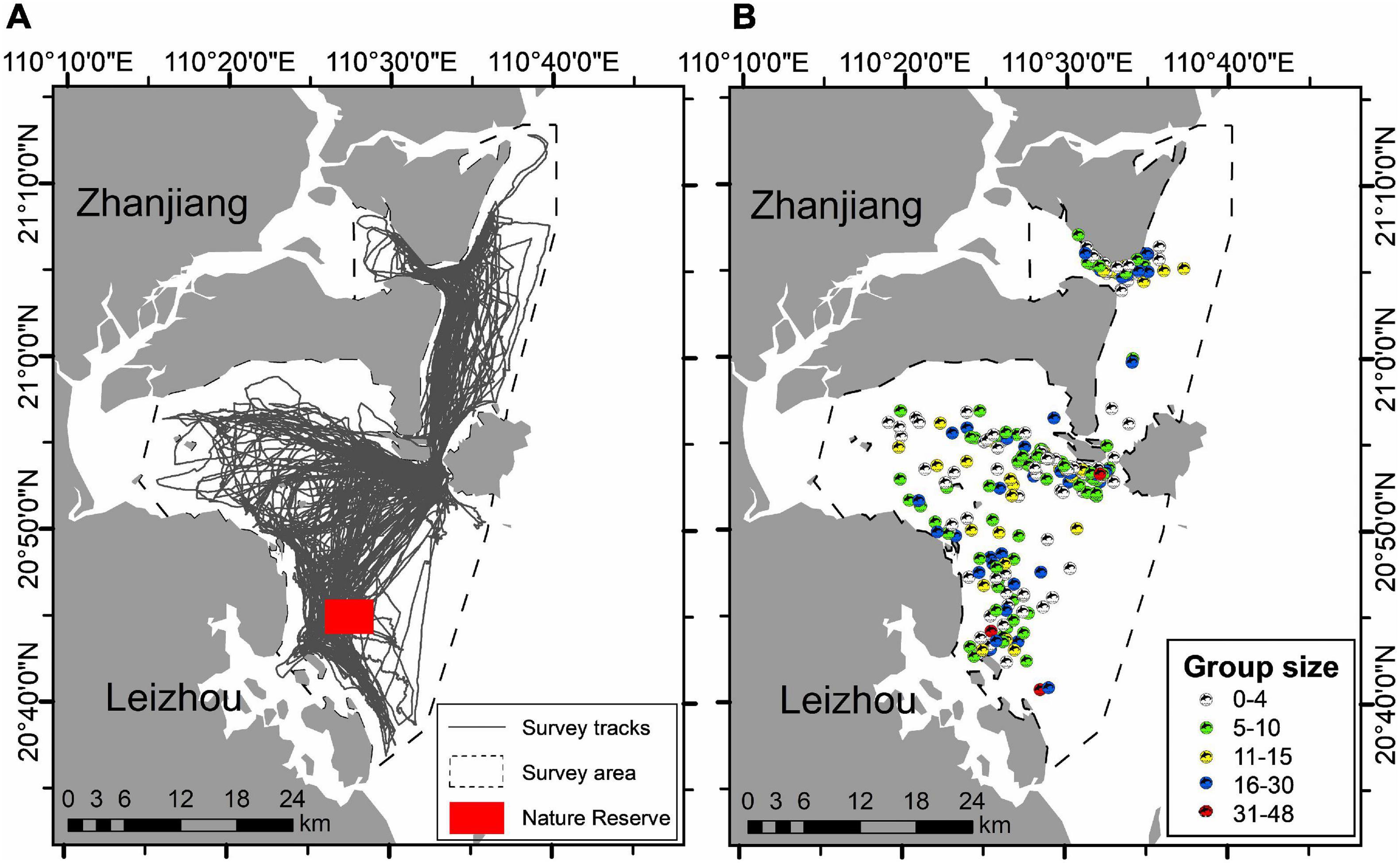
Figure 2. (A) Survey routes and (B) 253 sightings of Indo-Pacific humpback dolphins achieved in the eastern waters of Zhanjiang, China in 2013, and 2015–2018.
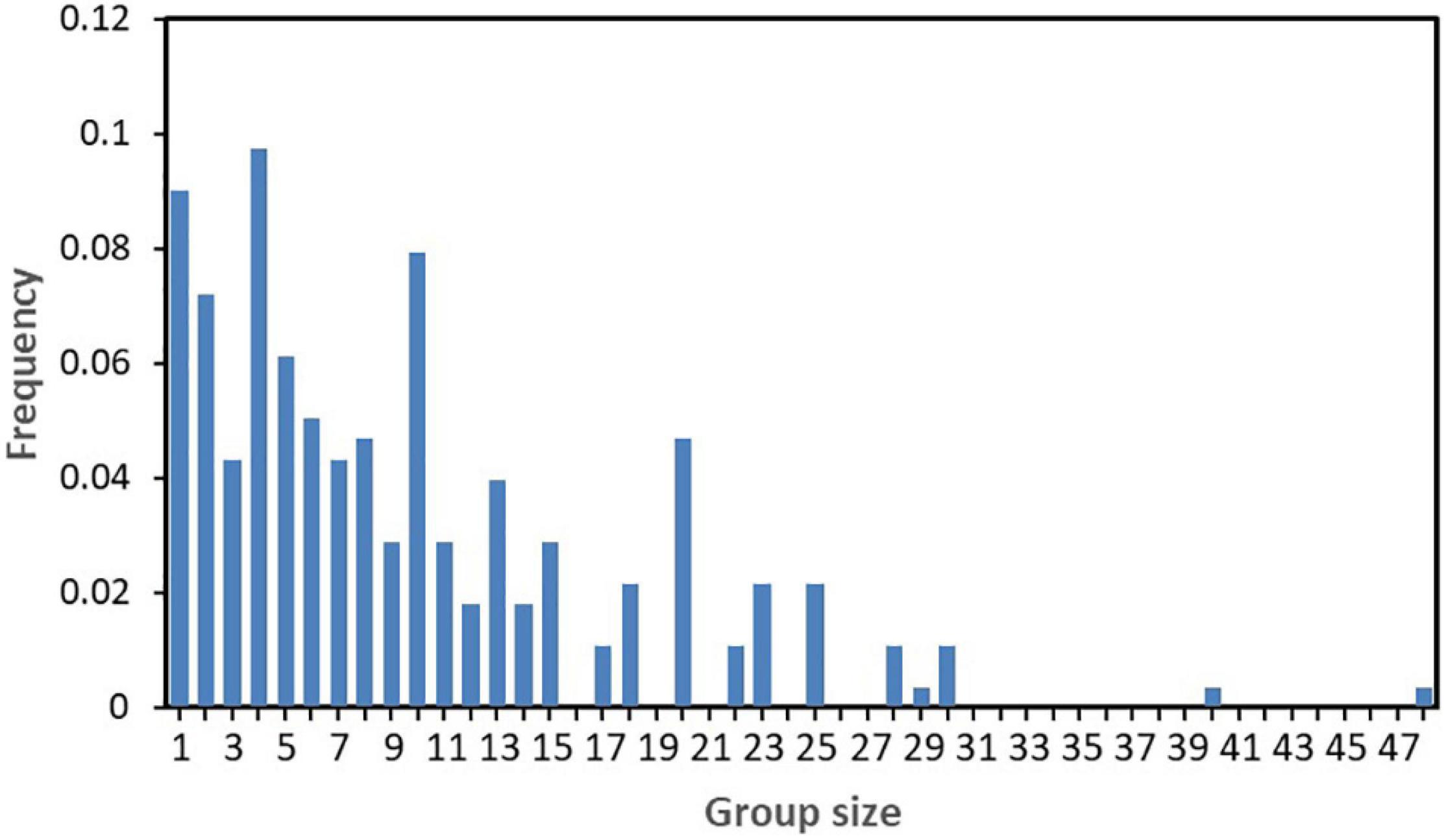
Figure 3. Frequency histogram of 229 group size estimates of Indo-Pacific humpback dolphins in the eastern waters of Zhanjiang, China.
We recorded the presence of mother-calf pair in 85 groups (33.6%), where 47 had one pair of mother-calf (18.6%), 25 had two pairs (9.9%), and the other 13 had three pairs (5.1%) (Figure 4A). Feeding (30.0%) and traveling (31.2%) represented the great majority of behavioral states recorded in the study area (Figure 4B). Furthermore, we recorded 38 socializing groups (8.4%), 8 resting groups (3.2%), and 9 milling groups (3.6%), while 43 groups (17.0%) could not be determined with identifiable behavioral state (Table 1). IPHD group size was highest in 2013 (18.8 ± 11.4) and lowest in 2015 (8.2 ± 6.3) (Table 2). Although mean values of group sizes varied across years, there was no variation in group sizes among different years (Kruskal-Wallis test, χ2 = 8.8, P = 0.168). Our GLMs showed that variations in group sizes were associated with the season (F = 1.0, df = 3, P = 0.002), number of mother-calf pairs (F = 9.0, df = 3, p < < 0.001), behavioral state (F = 0.9, df = 3, P = 0.033), year × season (F = 4.9, df = 12, P = 0.04), year × number of mother-calf pairs (F = 5.1, df = 12, P = 0.001), year × behavioral state (F = 3.3, df = 12, P = 0.014), and season × behavioral state (F = 2.8, df = 9, P = 0.031), but were not associated with other factors or interaction terms (Table 3).
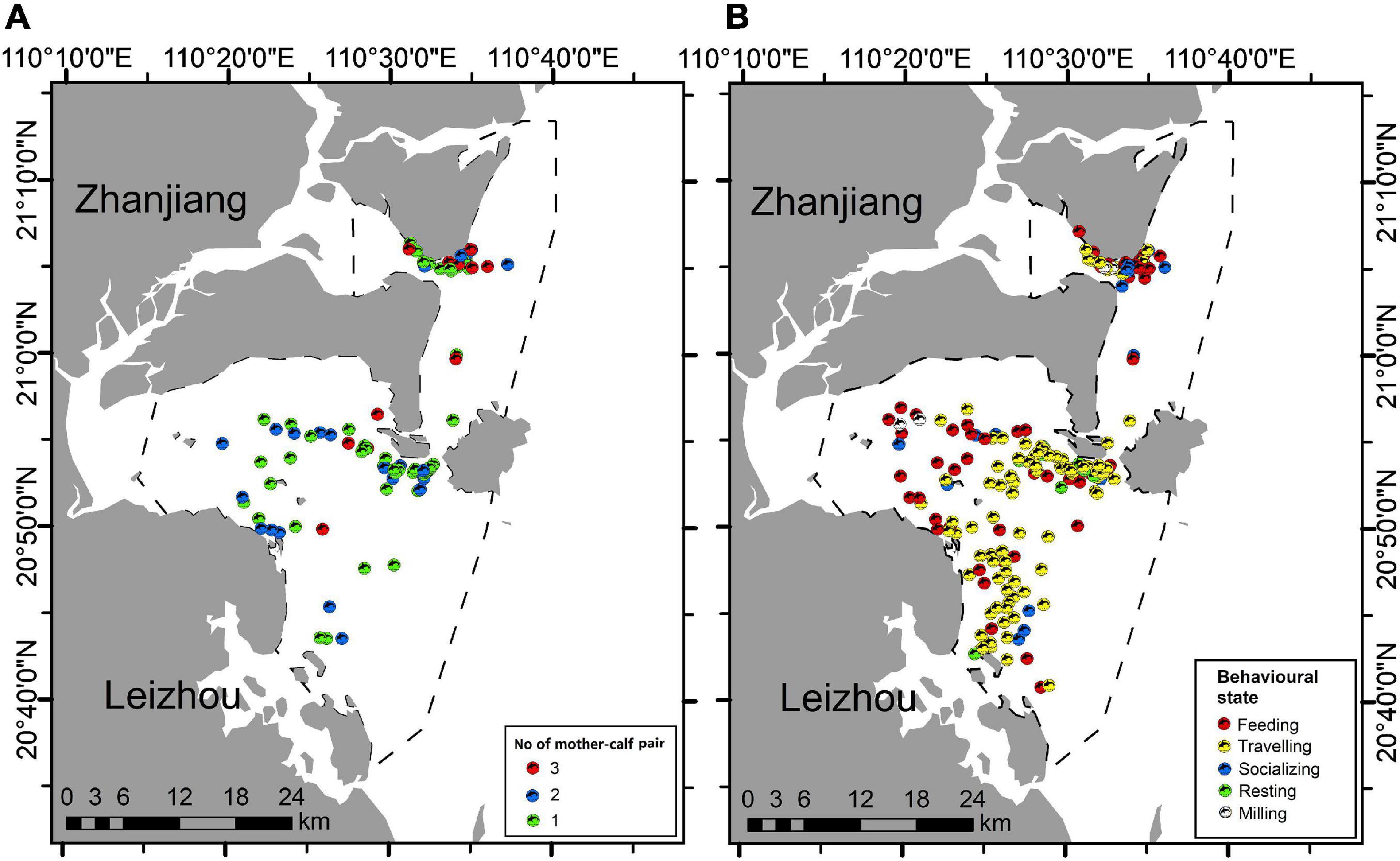
Figure 4. Sighting locations of Indo-Pacific humpback dolphins in the eastern waters of Zhanjiang, China: (A) nursery groups (number of mother-calf pairs: 1, 2, and 3), and (B) groups engaged in various behavioral states (feeding, traveling, socializing, resting, and milling).
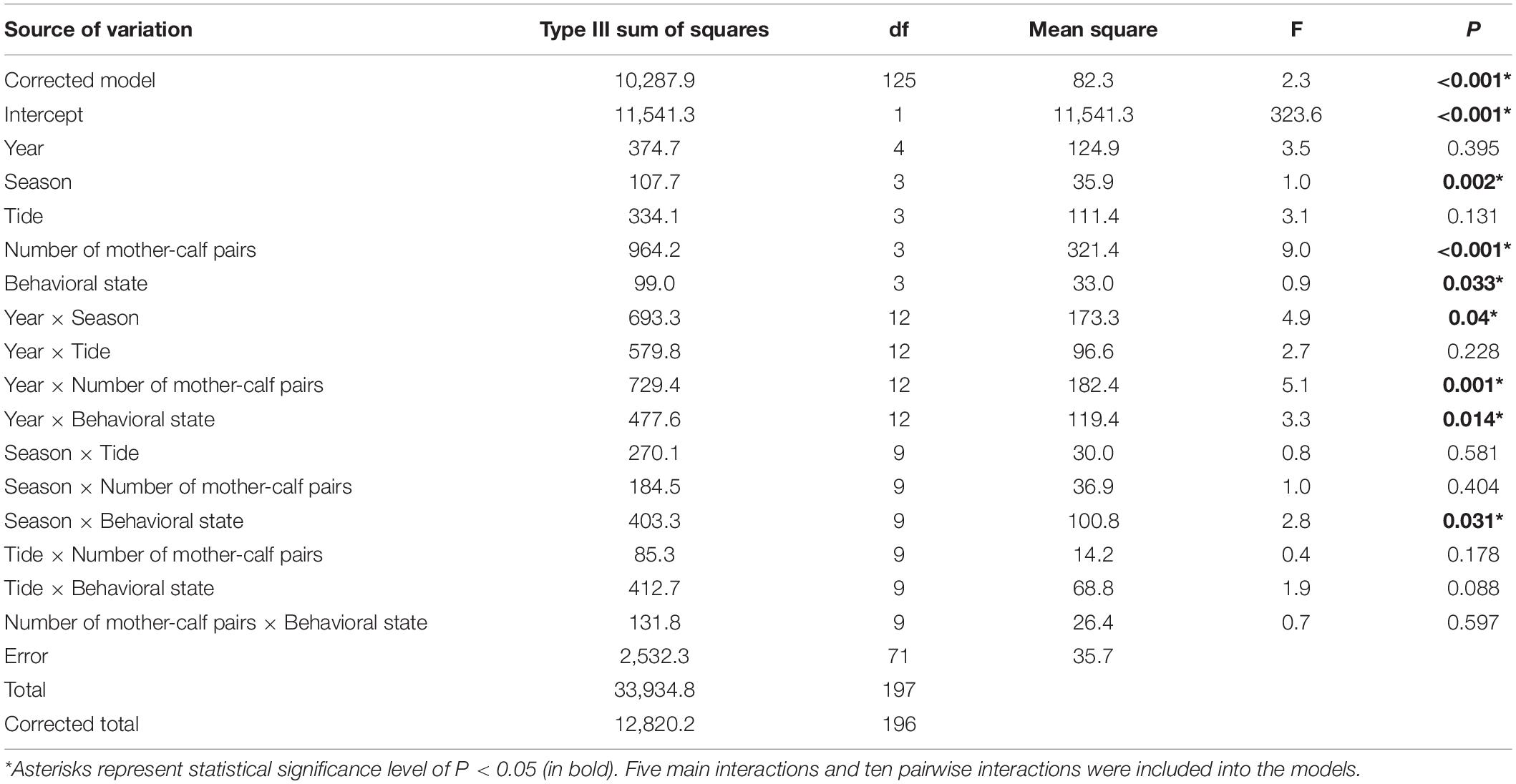
Table 3. Generalized linear models (GLMs) built to determine potential temporal (year, season, and tide) and/or behavioral effects (number of mother-calf pairs, and behavioral state) on group sizes of Indo-Pacific humpback dolphins in the eastern waters of Zhanjiang, China.
IPHD group sizes varied among seasons (Kruskal-Wallis test, χ2 = 2.6, P = 0.045). Group size in the autumn (14.1 ± 9.4) was larger than those in the spring (9.0 ± 6.9), summer (9.0 ± 6.6) and winter (7.8 ± 6.0) (Tukey’s HSD tests, Pautumn vs. spring = 0.022, Pautumn vs. summer = 0.018, and Pautumn vs. winter = 0.007), while there was no variation in group sizes across spring, summer, and winter (Figure 5A). In addition, there was no variation in group sizes with tidal phases (Kruskal-Wallis test, χ2 = 1.343, P = 0.719) (Figure 5B).
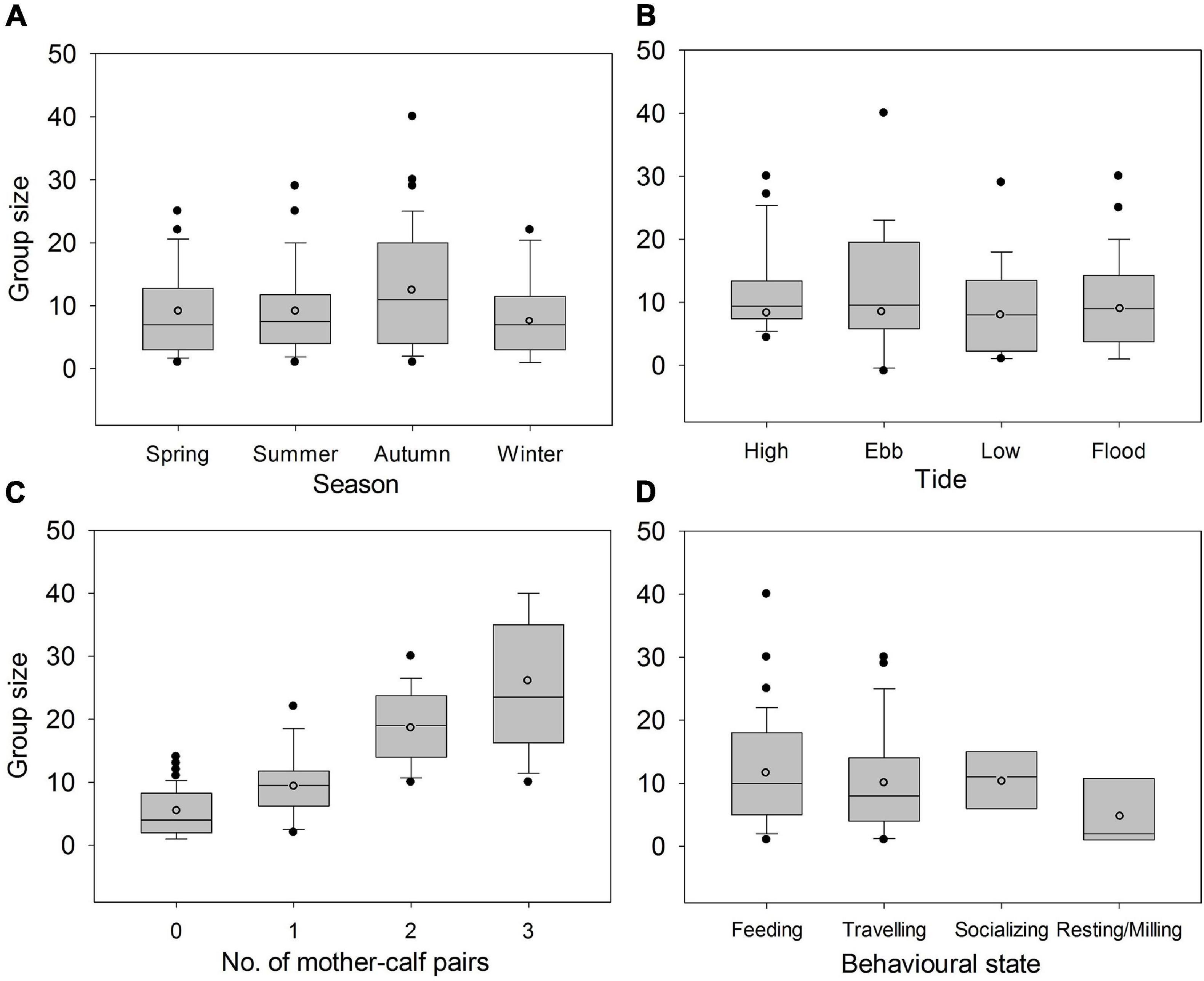
Figure 5. Boxplots of group sizes of Indo-Pacific humpback dolphins in the eastern waters of Zhanjiang, China, categorized by (A) season, (B) tide, (C) number of mother-calf pairs, and (D) behavioral state. Mean values (open circles), median values (black horizontal line), lower (25%) and upper (75%) quartiles, and outlier values (black dots) were illustrated.
IPHD group sizes varied with the presence of mother-calf pair, as nursery groups (16.8 ± 5.2) were about 2–4 times larger than non-nursery groups (i.e., groups without mother-calf pair, 6.8 ± 6.3). Group sizes also varied with the number of mother-calf pair (Kruskal-Wallis test, χ2 = 76.417, P < 0.001). We found a positive influence of the number of mother-calf pairs on IPHD group sizes: group sizes with one pair of mother-calf, two pairs, and three pairs were 10.2 ± 6.2, 17.4 ± 5.4, and 24.9 ± 9.6, respectively (Figure 5C).
We detected variation in IPHD group sizes across different behavioral states (Kruskal-Wallis test, χ2 = 14.1, P = 0.003). Resting/milling group size (5.5 ± 3.9) was smaller than feeding (12.1 ± 8.7), traveling (10.1 ± 8.1), and socializing group size (12.3 ± 6.5) (Pfeeding vs. resting/milling = 0.002, P traveling vs. resting/milling = 0.012, and Psocializing vs. resting/milling = 0.003; Figure 5D). However, group size was similar among feeding, traveling, and socializing behaviors (Figure 5D).
We built a CART with six leaves (Figure 6), including only three final explanatory variables i.e., number of mother-calf pairs, season, and behavioral state. We excluded the other two variables, i.e., year and tide, because they were insignificant in our GLMs (Table 3). In total, 68.1% of the variances in IPHD group sizes could be explained by the CART. The first split of CART was based on the number of mother-calf pairs, with ≤ 1 in the left branch [group size ≤ 10) and > 1 in the right branch (group size > 10)]. Then, these two branches were continuously divided into autumn in the left (group size > 8 or 15), spring, summer, and winter in the right (group size ≤ 8 or 15). The final splitting process was repeated for the two right seasonal branches, separating behavioral states into resting/milling in the next left (group size ≤ 5 or 10), and feeding, traveling, and socializing in the next right (group size > 5 or 10).
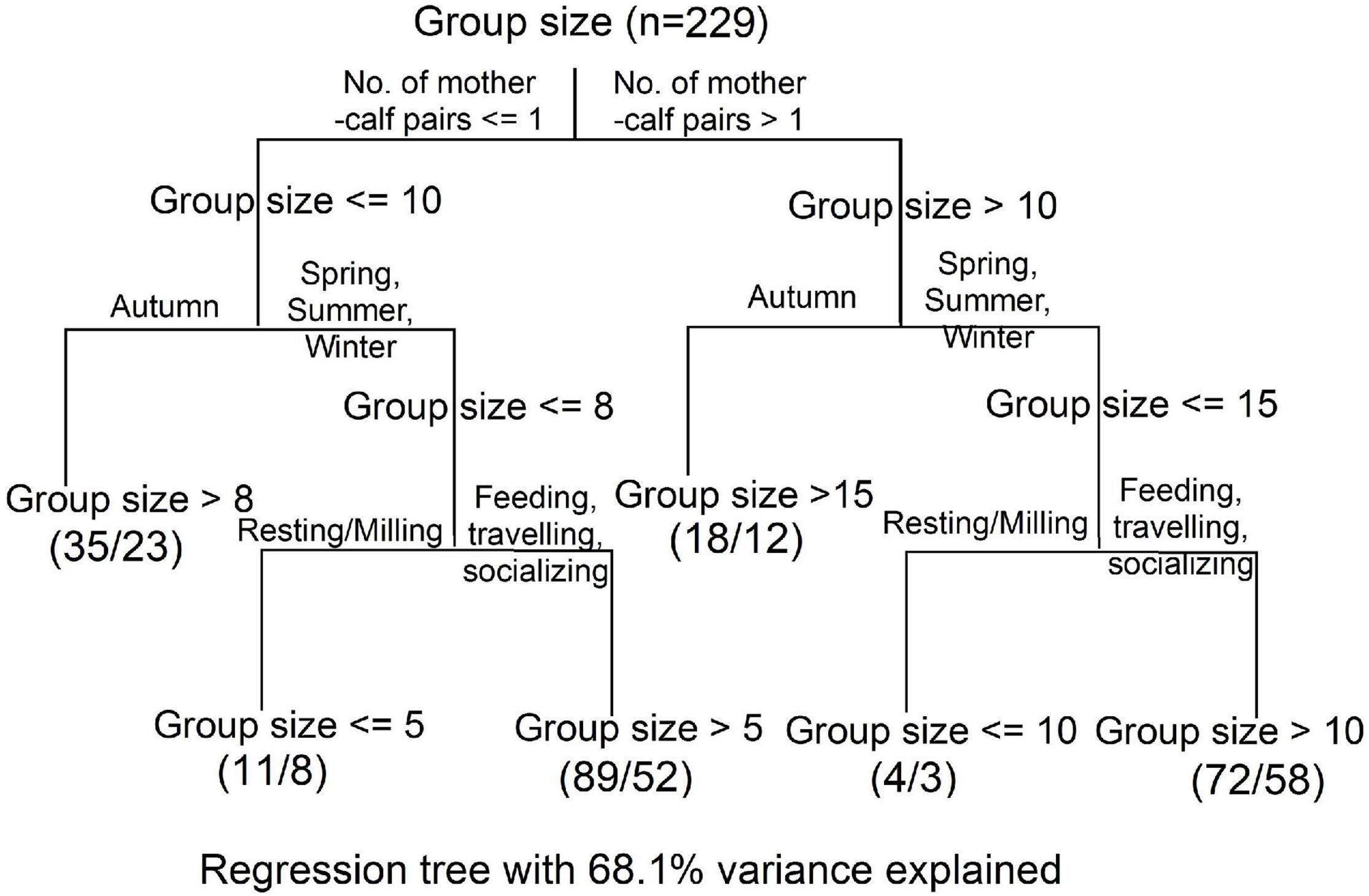
Figure 6. A classification and regression tree (CART) to explain group size variability of Indo-Pacific humpback dolphins in the eastern waters of Zhanjiang, China. Explanatory variables included number of mother-calf pairs (0, 1, 2, and 3), season (spring, summer, autumn, and winter) and behavioral state (feeding, traveling, socializing, and resting/milling). Each split was labeled with a threshold of group size that determined the split. For each of six final leaves, successfully observed values and failure ones were shown, respectively. Each terminal node was labeled with a final threshold of group size.
Discussion
Our study yielded several critical findings. First, we demonstrated that IPHD group sizes in the Zhanjiang waters were influenced by the season, number of mother-calf pairs, behavioral state, and the interaction between these factors. Second, our results showed that IPHD group size was larger in the autumn (September-November) compared to the other seasons. Third, we observed a positive relation of nursery behavior in IPHD group sizes, as nursery groups were 2−4 times larger than those non-nursery groups, and group sizes increased with the number of mother-calf pairs. Lastly, we displayed variations in IPHD group sizes across various behavioral states: resting/milling groups were approximately 50% smaller than feeding, traveling, or socializing groups, but the latter three had a similar size.
We observed a mean group size larger for the IPHDs in the Zhanjiang waters when compared to other estimates reported in this are by previous studies, including 8 (median) documented by Zhou et al. (2007), 7.5 ± 5.45 by Xu et al. (2012), and 8.12 ± 5.85 by Xu et al. (2015). These differences among studies can be related to different methodologies, as the previous studies only applied photo-identification technique to estimate the group size, while we used observer-based best counts complemented with photo-identification estimates. Photo-identification approach often generates an underestimated group size for each IPHD group (Liu et al., 2020b), which is based on the natural markings of each identifiable dolphin. However, some individuals within one group might be not photographically captured, and some young individuals and especially claves, are poorly marked or unmarked, both of which would lead to an underestimation of IPHD group size (Gerrodette et al., 2002; López et al., 2018). Thus, IPHD group size data used in this study are more methodologically credible.
We observed annual fluctuations of the IPHD group sizes, while there was no statistical difference in group sizes across different years. The inter-annual fluctuations of group sizes were obvious, with the largest value in 2013 (18.8 ± 11.4) and the smallest in 2015 (8.2 ± 6.3), which might be due to the small sample size in 2013 (n = 4). Additionally, we observed that IPHD group sizes were relatively stable with a mean of ∼8–9 individuals across different tidal phases, indicating scant tidal fluctuations of group sizes.
Within a certain population, temporal and behavioral variations in social characteristics were generally attributed to environmental adaptations of dolphins to various biological requirements and ecological constraints, such as food availability (Heithaus and Dill, 2002), mating opportunities (Orbach et al., 2014), predation risk (Kelley et al., 2011), or nurturing offspring (Mann et al., 2000). Compared to oceanic dolphin species, most IPHD populations are subject to relatively low predation risk from sharks or killer whales (Gowans et al., 2007; Würsig et al., 2016). Therefore, the intra-population variability in IPHD group sizes illustrated by our GLMs and CART might be primarily explained by food availability and reproductive processes, which were considered to vary temporally and by behaviors (Wang et al., 2016; Liu et al., 2020b).
Since 1998, the Chinese government designated a mandatory summer-fishing-ban-season (from May 1 to August 16 per year) in the territorial waters of South China Sea, aiming to preserve fisheries resources especially those reproduction-driven fish aggregations. Consequently, fisheries resources during and after the fishing ban season could be more abundant than before the season. Such seasonal variations in IPHD food resources might be a main driver leading to larger feeding groups in the autumn. Besides, previous studies in the study area indicated that the newborn IPHD calves peaked at the period between July and October (Zhou et al., 2007; Xu et al., 2012, 2015). Consequently, seasonal variations in IPHD group sizes can be related to their tendency to form larger breeding/mating aggregation during the autumn to improve mating opportunities and reproductive success (Baldwin et al., 2004; Orbach et al., 2014). However, seasonal variations in IPHD group sizes in the study area was different from that observed in the Xiamen Bay, China, where the mean group size of IPHDs during the winter-spring (7.2 individuals) were larger than those during the summer-autumn (mean: 4.4 individuals) (Wang et al., 2016). Such regional difference suggested that the variability in IPHD group sizes might vary across various habitats, as an adaptation to different ecological constraints in different geographical regions (Liu et al., 2021).
As demonstrated by our data, more mother-calf pairs were recorded in IPHD groups, the group size would be larger. More importantly, our CART clearly indicated that IPHD group sizes were primarily determined by the number of mother-calf pairs. Such positive impact of nursery behavior on enlarging group size has not only been reported for the IPHDs in the eastern Taiwan Strait (Dungan et al., 2016), and also for other dolphin species such as bottlenose dolphins (Tursiops spp.) (Gibson and Mann, 2008b), dusky dolphins (Lagenorhynchus obscurus Gray, 1828) (Degrati et al., 2019), and Guiana dolphins (Sotalia guianensis) (Azevedo et al., 2005; Santos and Rosso, 2007; Emin-Lima et al., 2010). This social strategy i.e., dolphin group became larger when calves were present, could bring a variety of added benefits such as enhanced calf-assistance, cooperative calf-caring, reduced maternal investments, and increased calf-protection (against predators or intraspecific aggression) (Mann et al., 2000; Gibson and Mann, 2008a; Kent et al., 2008).
Our data indicated that IPHD groups were mainly engaged in feeding and traveling behaviors, while socializing and resting/milling were less frequently observed. Such a behavioral budget was consistent with the patterns documented for humpback dolphins in the Hong Kong waters (Parsons, 2004; Würsig et al., 2016), in the Algoa Bay, South Africa (Karczmarski, 1999), and in the Cleveland Bay, Australia (Parra et al., 2011). Our results showed that resting/milling IPHD groups were smaller than feeding, traveling, or socializing groups, while the latter three group types had a similar group size. This increase in feeding, traveling, or socializing group size has been reported for the bottlenose dolphins (Heithaus and Dill, 2002), common dolphins (Delphinus spp.) (Neumann, 2001; Stockin et al., 2009), and dusky dolphins (Degrati et al., 2019). IPHDs tended to form large, temporary, and functional gathering of different social units when they were not resting or milling (Würsig et al., 2016), which might help strengthen group added benefits (Baird and Dill, 1996; Neumann, 2001; Yeater et al., 2013).
To conclude, our data are essential to show temporal and behavioral variations in IPHD group sizes in the Zhanjiang waters. Our findings suggested that the intra-population variability of IPHD group sizes was potentially associated with some environmental cycles and behavioral changes, and could be influenced by the food availability and reproductive process of IPHDs. To better protect the IPHDs in the Zhanjiang waters, we highlight the importance of protecting nursery groups/activities of IPHDs. According to our findings, we empathize that breeding season is an important period in the annual cycle of IPHDs, and in the study area, particular conservation effort is required during the autumn. The IPHDs in the study area also tended to form larger groups when they were engaged in feeding behavior and when food resources are more abundant (e.g., summer-fishing-ban-season), which indicated that social dynamics of IPHDs could be greatly influenced food availability. Therefore, protecting food resources from overfishing should be one of the most important actions to maintain social dynamics of IPHDs and to conserve this species. Compared to previous studies, we found that the intra-population variations in IPHD group sizes might vary among different habitats. Therefore, more data on IPHD mating strategies, reproductive fitness, prey resources, fisheries-dolphin conflicts, and how these factors may influence social dynamics of IPHDs are interesting venues of future research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by IDSSE-SYLL-MMMBL-01.
Author Contributions
MLiu and MLin: data collection. MLiu: formal analysis and writing—original draft. SL and MLin: funding acquisition. MLiu, MLin, DL, and SL: methodology. MLin, DL, and SL: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was financially supported by the National Natural Science Foundation of China (41406182, 41306169, and 41422604), the Biodiversity Investigation, Observation and Assessment Program (2019-2023) of Ministry of Ecology and Environment of China, and the Ocean Park Conservation Foundation of Hong Kong (MM02-1516, AW02-1920). The writing of this paper was supported in part by the China-United Kingdom Newton Fund Ph.D. Placement from China Scholarship Council and British Council.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our big thanks to all the colleagues and students at the Marine Mammal and Marine Bioacoustics Laboratory for their logistical support in field work. We thank Peijun Zhang, Lijun Dong, Mingzhong Liu, Kuan Li, and Francesco Caruso for their assistance in field work.
References
Azevedo, A. F., Viana, S. C., Oliveira, A. M., and Van Sluys, M. (2005). Group characteristics of marine tucuxis (Sotalia fluviatilis) (Cetacea: Delphinidae) in Guanabara Bay, south-eastern Brazil. J. Mar. Biol. Assoc. U.K. 85, 209–212. doi: 10.1017/s0025315405011082h
Baird, R. W., and Dill, L. M. (1996). Ecological and social determinants of group size in transient killer whales. Behav. Ecol. 7, 408–416. doi: 10.1093/beheco/7.4.408
Baldwin, R. M., Collins, M., Van Waerebeek, K., and Minton, G. (2004). The indo-pacific humpback dolphin of the Arabian region: a status review. Aquat. Mamm. 30, 111–124. doi: 10.1578/am.30.1.2004.111
Benoit-Bird, K., and Au, W. (2003). Hawaiian spinner dolphins aggregate midwater food resources through cooperative foraging. J. Acoust. Soc. Am. 114, 2300–2300. doi: 10.1121/1.4780872
Bouveroux, T. N., Caputo, M., Froneman, P. W., and Plön, S. (2018). Largest reported groups for the indo-Pacific bottlenose dolphin (Tursiops aduncus) found in Algoa Bay, South Africa: trends and potential drivers. Mar. Mamm. Sci. 34, 645–665. doi: 10.1111/mms.12471
Bouveroux, T., Kirkman, S. P., Conry, D., Vargas-Fonseca, O. A., and Pistorius, P. A. (2019). The first assessment of social organisation of the indian ocean humpback dolphin (Sousa plumbea) along the south coast of South Africa. Can. J. Zool. 97, 855–865. doi: 10.1139/cjz-2018-0244
Cantor, M., Wedekin, L. L., Guimaraes, P. R., Daura-Jorge, F. G., Rossi-Santos, M. R., and Simoes-Lopes, P. C. (2012). Disentangling social networks from spatiotemporal dynamics: the temporal structure of a dolphin society. Anim. Behav. 84, 641–651. doi: 10.1016/j.anbehav.2012.06.019
Chen, T., Qiu, Y., Jia, X., Hung, S. K., and Liu, W. (2011). Distribution and group dynamics of indo-Pacific humpback dolphins (Sousa chinensis) in the western Pearl River Estuary, China. Mamm. Biol. 76, 93–96. doi: 10.1016/j.mambio.2010.01.001
Connor, R. C. (2000). “Group living in whales and dolphins,” in Cetacean Societies: Field Studies of Dolphins and Whales, eds J. Mann, R. C. Connor, P. L. Tyack, and H. Whitehead (Chicago: University of Chicago Press), 199–218.
De’ath, G., and Fabricius, K. E. (2000). Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81, 3178–3192. doi: 10.1890/0012-9658(2000)081[3178:cartap]2.0.co;2
Degrati, M., Coscarella, M. A., Crespo, E. A., and Dans, S. L. (2019). Dusky dolphin group dynamics and association patterns in Península Valdés, Argentina. Mar. Mamm. Sci. 35, 416–433. doi: 10.1111/mms.12536
Dungan, S. Z., Hung, S. K., Wang, J. Y., and White, B. N. (2012). Two social communities in the Pearl River Estuary population of Indo-Pacific humpback dolphins (Sousa chinensis). Can. J. Zool. 90, 1031–1043. doi: 10.1139/z2012-071
Dungan, S. Z., Wang, J. Y., Araújo, C. C., Yang, S. C., and White, B. N. (2016). Social structure in a critically endangered Indo-Pacific humpback dolphin (Sousa chinensis) population. Aquat. Conserv. 26, 517–529. doi: 10.1002/aqc.2562
Emin-Lima, R., Moura, L. N., Rodrigues, A. F., and Silva, M. L. (2010). Note on the group size and behavior of Guiana dolphins (Sotalia guianensis) (Cetacea: Delphinidae) in Marapanim Bay, Pará, Brazil. Lat. Am. J. Aquat. Mamm. 8, 167–170.
Gerrodette, T., Perryman, W., and Barlow, J. (2002). Calibrating Group Size Estimates of Dolphins in the Eastern Tropical Pacific Ocean. Administrative Report LJ-02-08. La Jolla, CA: Southwest Fisheries Science Center, 1–20.
Gibson, Q. A., and Mann, J. (2008a). Early social development in wild bottlenose dolphins: sex differences, individual variation and maternal influence. Anim. Behav. 76, 375–387. doi: 10.1016/j.anbehav.2008.01.021
Gibson, Q. A., and Mann, J. (2008b). The size, composition and function of wild bottlenose dolphin (Tursiops sp.) mother-calf groups in Shark Bay, Australia. Anim. Behav. 76, 389–405. doi: 10.1016/j.anbehav.2008.01.022
Gowans, S., Würsig, B., and Karczmarski, L. (2007). The social structure and strategies of delphinids: predictions based on an ecological framework. Adv. Mar. Biol. 53, 195–294. doi: 10.1016/s0065-2881(07)53003-8
Gygax, L. (2002a). Evolution of group size in the dolphins and porpoises: interspecific consistency of intraspecific patterns. Behav. Ecol. 13, 583–590. doi: 10.1093/beheco/13.5.583
Gygax, L. (2002b). Evolution of group size in the superfamily Delphinoidea (Delphinidae, Phocoenidae and Monodontidae): a quantitative comparative analysis. Mamm. Rev. 32, 295–314. doi: 10.1046/j.1365-2907.2002.00114.x
Hawkins, E. R., Pogson-Manning, L., Jaehnichen, C., and Meager, J. J. (2020). Social dynamics and sexual segregation of Australian humpback dolphins (Sousa sahulensis) in Moreton Bay, Queensland. Mar. Mamm. Sci. 36, 500–521. doi: 10.1111/mms.12657
Heithaus, M. R., and Dill, L. M. (2002). Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83, 480–491. doi: 10.1890/0012-9658(2002)083[0480:faatsp]2.0.co;2
Hunt, T. N., Allen, S. J., Bejder, L., and Parra, G. J. (2019). Assortative interactions revealed in a fission-fusion society of Australian humpback dolphins. Behav. Ecol. 30, 914–927. doi: 10.1093/beheco/arz029
Jefferson, T. A., and Curry, B. E. (2015). Humpback dolphins: a brief introduction to the genus Sousa. Adv. Mar. Biol. 72, 1–16. doi: 10.1016/bs.amb.2015.04.001
Jefferson, T. A., and Rosenbaum, H. C. (2014). Taxonomic revision of the humpback dolphins (Sousa spp.), and description of a new species from Australia. Mar. Mamm. Sci. 30, 1494–1541. doi: 10.1111/mms.12152
Jefferson, T. A., and Smith, B. D. (2016). Re-assessment of the conservation status of the Indo-Pacific humpback dolphin (Sousa chinensis) using the IUCN Red List criteria. Adv. Mar. Biol. 73, 1–26. doi: 10.1016/bs.amb.2015.04.002
Jefferson, T. A., Hung, S. K., Robertson, K. M., and Archer, F. I. (2012). Life history of the Indo-Pacific humpback dolphin in the Pearl River Estuary, southern China. Mar. Mamm. Sci. 28, 84–104. doi: 10.1111/j.1748-7692.2010.00462.x
Jefferson, T. A., Smith, B. D., Braulik, G. T., and Perrin, W. (2017). Sousa chinensis, the IUCN red list of threatened species 2017: e.T82031425A50372332. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T82031425A50372332.en (Accessed on 2 March 2019)
Karczmarski, L. (1999). Group dynamics of humpback dolphins (Sousa chinensis) in the Algoa Bay region, South Africa. J. Zool. 249, 283–293. doi: 10.1111/j.1469-7998.1999.tb00765.x
Kelley, J. L., Morrell, L. J., Inskip, C., Krause, J., and Croft, D. P. (2011). Predation risk shapes social networks in fission-fusion populations. PLoS One 6:e24280. doi: 10.1371/journal.pone.0024280
Kent, E. E., Mazzoil, M., McCulloch, S. D., and Defran, R. H. (2008). Group characteristics and social affiliation patterns of bottlenose dolphins (Tursiops truncatus) in the Indian River Lagoon, Florida. Florida Sci. 71, 149–168.
Koper, R. P., Karczmarski, L., du Preez, D., and Plön, S. (2016). Sixteen years later: occurrence, group size, and habitat use of humpback dolphins (Sousa plumbea) in Algoa Bay, South Africa. Mar. Mamm. Sci. 32, 490–507. doi: 10.1111/mms.12279
Li, S., Lin, M., Xu, X., Xing, L., Zhang, P., Gozlan, R. E., et al. (2016). First record of the Indo-Pacific humpback dolphins (Sousa chinensis) southwest of Hainan Island, China. Mar. Biodivers. Rec. 9:3.
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Liu, M., Bejder, L., Lin, M., Zhang, P., Dong, L., and Li, S. (2020a). Determining spatial use of the world’s second largest humpback dolphin population: implications for place-based conservation and management. Aquat. Conserv. 30, 364–374. doi: 10.1002/aqc.3253
Liu, M., Dong, L., Lin, M., and Li, S. (2017a). Broadband ship noise and its potential impacts on Indo-Pacific humpback dolphins: implications for conservation and management. J. Acoust. Soc. Am. 142, 2766–2775. doi: 10.1121/1.5009444
Liu, M., Lin, M., Dong, L., Xue, T., Zhang, P., Tang, X., et al. (2020b). Group sizes of Indo-Pacific humpback dolphins in waters southwest of Hainan Island, China: insights into rare records of large groups. Aquat. Mamm. 46, 259–265. doi: 10.1578/am.46.3.2020.259
Liu, M., Lin, M., Dong, L., Zhang, P., and Li, S. (2021). Spatiotemporal variations in fine-scale habitat use of the world’s second largest population of humpback dolphins. J. Mamm. gyab001. doi: 10.1093/jmammal/gyab001 [Epub ahead of print].
Liu, M., Lin, M., Turvey, S. T., and Li, S. (2017b). Fishers’ knowledge as an information source to investigate bycatch of marine mammals in the South China Sea. Anim. Conserv. 20, 182–192. doi: 10.1111/acv.12304
Liu, M., Lin, M., Turvey, S. T., and Li, S. (2019). Fishers’ experiences and perceptions of marine mammals in the South China Sea: insights for improving community-based conservation. Aquat. Conserv. 29, 809–819. doi: 10.1002/aqc.3073
López, B. D., Grandcourt, E., Methion, S., Das, H., Bugla, I., Al Hameli, M., et al. (2018). The distribution, abundance and group dynamics of Indian Ocean humpback dolphins (Sousa plumbea) in the Emirate of Abu Dhabi (UAE). J. Mar. Biol. Assoc. U.K. 98, 1119–1127. doi: 10.1017/s0025315417001205
Lusseau, D. (2003). The emergent properties of a dolphin social network. Proc. R. Soc. Lond. B Biol. Sci. 270, S186–S188.
Lusseau, D., and Newman, M. E. (2004). Identifying the role that animals play in their social networks. Proc. R. Soc. Lond. B Biol. Sci. 271, S477–S481.
Lusseau, D., Schneider, K., Boisseau, O. J., Haase, P., Slooten, E., and Dawson, S. M. (2003). The bottlenose dolphin community of doubtful Sound features a large proportion of long-lasting associations. Behav. Ecol. Sociobiol. 54, 396–405. doi: 10.1007/s00265-003-0651-y
Lusseau, D., Wilson, B. E. N., Hammond, P. S., Grellier, K., Durban, J. W., Parsons, K. M., et al. (2006). Quantifying the influence of sociality on population structure in bottlenose dolphins. J. Anim. Ecol. 75, 14–24. doi: 10.1111/j.1365-2656.2005.01013.x
Mann, J., Connor, R. C., Barre, L. M., and Heithaus, M. R. (2000). Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behav. Ecol. 11, 210–219. doi: 10.1093/beheco/11.2.210
Neumann, D. R. (2001). Activity budget of free-ranging common dolphins (Delphinus delphis) in the northwestern Bay of Plenty, New Zealand. Aquat. Mamm. 27, 121–136.
Orbach, D. N., Packard, J. M., and Würsig, B. (2014). Mating group size in dusky dolphins (Lagenorhynchus obscurus): costs and benefits of scramble competition. Ethology 120, 804–815. doi: 10.1111/eth.12253
Parra, G. J., Corkeron, P. J., and Arnold, P. (2011). Grouping and fission-fusion dynamics in Australian snubfin and Indo-Pacific humpback dolphins. Anim. Behav. 82, 1423–1433. doi: 10.1016/j.anbehav.2011.09.027
Parrish, J. K., and Edelstein-Keshet, L. (1999). Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101. doi: 10.1126/science.284.5411.99
Parsons, E. C. M. (2004). The behavior and ecology of the Indo-Pacific humpback dolphin (Sousa chinensis). Aquat. Mamm. 30, 38–55. doi: 10.1578/am.30.1.2004.38
Santos, M. D. O., and Rosso, S. (2007). Ecological aspects of marine tucuxi dolphins (Sotalia guianensis) based on group size and composition in the Cananéia estuary, southeastern Brazil. Lat. Am. J. Aquat. Mamm. 6, 71–82.
Sarabia, R. E., Heithaus, M. R., and Kiszka, J. J. (2018). Spatial and temporal variation in abundance, group size and behaviour of bottlenose dolphins in the Florida coastal Everglades. J. Mar. Biol. Assoc. U. K. 98, 1097–1107. doi: 10.1017/s002531541700090x
Silk, J. B. (2007). The adaptive value of sociality in mammalian groups. Philos. Trans. R. Soc. B Biol. Sci. 362, 539–559. doi: 10.1098/rstb.2006.1994
Stockin, K. A., Binedell, V., Wiseman, N., Brunton, D. H., and Orams, M. B. (2009). Behavior of free-ranging common dolphins (Delphinus sp.) in the Hauraki Gulf, New Zealand. Mar. Mamm. Sci. 25, 283–301. doi: 10.1111/j.1748-7692.2008.00262.x
Wang, X., Wu, F., Turvey, S. T., Rosso, M., and Zhu, Q. (2016). Seasonal group characteristics and occurrence patterns of Indo-Pacific humpback dolphins (Sousa chinensis) in Xiamen Bay, Fujian Province, China. J. Mamm. 97, 1026–1032. doi: 10.1093/jmammal/gyw002
Wang, X., Wu, F., Turvey, S. T., Rosso, M., Tao, C., Ding, X., et al. (2015). Social organization and distribution patterns inform conservation management of a threatened Indo-Pacific humpback dolphin population. J. Mamm. 96, 964–971. doi: 10.1093/jmammal/gyv097
Würsig, B., Parsons, E. C. M., Piwetz, S., and Porter, L. (2016). The behavioural ecology of Indo-Pacific humpback dolphins in Hong Kong. Adv. Mar. Biol. 73, 65–90. doi: 10.1016/bs.amb.2015.08.008
Xu, X., Song, J., Zhang, Z., Li, P., Yang, G., and Zhou, K. (2015). The world’s second largest population of humpback dolphins in the waters of Zhanjiang deserves the highest conservation priority. Sci. Rep. 5:8147.
Xu, X., Zhang, Z., Ma, L., Li, P., Yang, G., and Zhou, K. (2012). Site fidelity and association patterns of Indo-Pacific humpback dolphins off the east coast of Zhanjiang, China. Acta Theriol. 57, 99–109. doi: 10.1007/s13364-011-0058-5
Yeater, D. B., Miller, L. E., Caffery, K. A., and Kuczaj, S. A. II (2013). Effects of an increase in group size on the social behavior of a group of rough-toothed dolphins (Steno bredanensis). Aquat. Mamm. 39, 344–355. doi: 10.1578/am.39.4.2013.344
Keywords: sociality, humpback dolphin, group size, social dynamics, season, mother-calf pairs, behavior
Citation: Liu M, Lin M, Lusseau D and Li S (2021) Intra-Population Variability in Group Size of Indo-Pacific Humpback Dolphins (Sousa chinensis). Front. Mar. Sci. 8:671568. doi: 10.3389/fmars.2021.671568
Received: 24 February 2021; Accepted: 20 May 2021;
Published: 14 June 2021.
Edited by:
Darren Croft, University of Exeter, United KingdomReviewed by:
Clarissa Teixeira, Instituto de Pesquisas Cananeia, BrazilKerki Jog, James Cook University, Australia
Copyright © 2021 Liu, Lin, Lusseau and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songhai Li, bGlzaEBpZHNzZS5hYy5jbg==
†ORCID: Mingming Liu, orcid.org/0000-0003-3802-1002 Mingli Lin, orcid.org/0000-0002-9182-0519 David Lusseau, orcid.org/0000-0003-1245-3747 Songhai Li, orcid.org/0000-0003-4977-1722
 Mingming Liu
Mingming Liu Mingli Lin1†
Mingli Lin1† Songhai Li
Songhai Li