- CSIR - National Institute of Oceanography, Regional Centre, Mumbai, India
Tropical ecosystems sustain higher biodiversity and face faster species extinction. However, baseline information of these areas is either inadequate or scattered due to various reasons. The 2,360 km long coast of North West India (NWI), is a heavily industrialized and urbanized zone. This coast with unique biogeographical and climatic features with two notified marine protected areas also supports rich biodiversity. This review was motivated by a need to construct a synoptic view on marine benthic ecology and functioning by consolidating available information of macrobenthos. Two thousand seventy-eight macrobenthic taxa belonging to 14 phyla were compiled from 147 references and were composed mostly by Polychaeta (n = 617), Gastropoda (n = 602), and Bivalvia (n = 216). Habitat wise, intertidal and subtidal zones were more intensely studied and contributed most to the diversity records. Sediment texture and salinity were the major drivers of macrobenthic community structure in the subtidal areas and estuaries, respectively. In the intertidal zones, zonation patterns related to the tidal levels and time of exposure were distinct with the high water zones being sparsely populated and lower intertidal zones sustaining higher species and functional diversities. All zones of NWI coast were distinctly impacted to various extent by anthropogenic activities affecting the resident macrobenthos. Decline in species richness and species substitution due to pollution were reported in urbanized zones. Non-monsoonal months favored a more conducive environment for the macrobenthic diversity and functionality. Hypoxia tolerant polychaete species mainly belonging to Spionidae and Cossuridae dominated during the low oxygen conditions of upwelling and OMZ zones of NWI. Inadequate identification and inconsistency of sampling methods were major deterrents for concluding trends of distributions. Suggestions for future macrobenthic research include focusing on lesser studied groups and areas, seasonal as well anthropogenic hypoxic zones and well planned long-term monitoring studies. Major data lacunae were identified in the taxonomy, molecular, functional aspects, and bioinvasive studies of macrobenthos in this geographical zone despite clear evidence of high diversity of extant macrofauna. This compendium should help prioritize research areas and objectives aimed at enhancing our understanding of macrobenthos and improve predictive capabilities of community shifts that may occur due to global climate change scenarios.
Introduction
Coastal ecosystems host some of the most diverse, extensive, and complex floral and faunal assemblages and the sustainability of these systems is maintained by the myriad functional roles of the constituent biota. Biodiversity plays an important role in maintaining ecosystem services and possible losses of biodiversity can certainly interrupt key ecological processes (Harrison et al., 2014). Ecological consequences of biodiversity loss on the efficient functioning of ecosystem are now firmly established (Loreau et al., 2001; Danovaro et al., 2008) and this reaffirms the need to quantify and evaluate available information of various facets of biodiversity.
The species diversity of marine systems are majorly constituted by sediment dwelling flora and fauna (Radulovici et al., 2010) accounting for 98% of all known marine species. Marine benthos includes living members of the 29 non-symbiont animal phyla described thus far, except one (Porifera, Placozoa, Cnidaria, Ctenophora, Platyhelminthes, Gnathostomulida, Nemertea, Kinoryhncha, Nematoda, Loricifera, Rotifera, Priapula, Gastrotricha, Pogonophora, Tardigrada, Echiura, Mollusca, Chaetognatha, Kamptozoa, Phoronida, Sipuncula, Brachiopoda, Annelida, Echinodermata, Arthropoda, Hemichordata, Bryozoa, and Chordata) (Ray and Grassle, 1991). The dominant component of marine benthic assemblages is macrobenthos (larger than 500 μm) which includes some of the most speciose groups i.e., annelids, crustaceans, molluscs, etc. (Snelgrove, 1999). Macrobenthic investigations have garnered most scientific attention among the benthic groups due to its distinctive community characteristics and complex functional role at the water–sediment interface. They are key components in the benthic functioning of coastal ecosystems and are a vital link in global carbon, nitrogen, and sulfur cycling (Snelgrove, 1998). They are major food sources for varied demersal predators occupying the same ecosystem (Griffiths et al., 2017). Macrobenthos also are potential biological indicators due to their sedentary nature, long-life spans, environmental plasticity, ability to integrate the environmental quality status, and effectively reflect the system condition at spatio-temporal scales (Fitch and Crowe, 2010). Also, they have diverse species composition and show marked responses to several stressors such as hypoxia and anthropogenic disturbances (Dauvin et al., 2012; Peng et al., 2014).
Benthic faunal diversity is a stepping stone for exploring the relationships between biodiversity and ecosystem functioning. Understanding the biodiversity in marine sediments and its linkages to various ecosystem processes is important for maintaining ecological services and to estimate the species being lost or extinct due to various pressures. A decrease in benthic biodiversity may have a concomitant negative impact on key ecosystem functions and biogeochemical cycling particularly in shelf regions that are known to contribute to the bulk of benthic productivity (Field et al., 1998). Unknown diversity exceeds much more than known information (Venkataraman and Wafar, 2005). Global trends in macrobenthic diversity distributions and its causes can be successfully evolved only if sufficient and reliable data from lesser studied areas are made available within a cogent framework. Efficient evaluation of biodiversity and functioning losses of nearshore habitats is dependent on the availability of adequate data banks, which is largely lacking in many geographical regions owing to a host of reasons (Konar et al., 2010). The lacunae are all the more conspicuous in case of tropical coastlines, despite these zones constituting one-third of the world shelf area (Muniz et al., 2005) and supporting high biodiversity (Olsgard et al., 1998). Coastal systems are more vulnerable than deep sea systems due to the dual impacts of anthropogenic pressures and global warming. Projected catastrophic ecosystem responses of climate change are mostly expected to be individualistic (i.e., at species level) and may include modification of species assemblages, alteration of species interactions, species shifts, or even extinctions (Gayton, 2008). This necessitates planning and implementation of sound ecosystem management procedures focusing on areas most vulnerable to climatic vagaries.
The Arabian Sea, located in the northeast Indian Ocean, is a geochemically active area along the western boundary of India. The coast of North West India (NWI) (15–24°N) is one of the most productive zone with unique characteristics. NWI has two of the biggest, highly industrialized and urbanized coastal states, Maharashtra and Gujarat (Figure 1). The NWI is about 2,320 km which is nearly 29% of the nation’s total coastal length and has the most extensive shelf and slope areas that translate into one of the richest fishing grounds of India. The marine fish production of NWI for 2019 was 3.56 million tones accounting for 26.6% of the total marine fish production from the country (CMFRI, 2019). The coast presents a variegated coastal physiography and geomorphology that provides diversity of habitats like mangroves abounding estuary mouths and creeks, coral reefs, mudflats, vast sandy, and rocky intertidal areas. There are two notified Marine Protected Areas (MPAs) with coral formations i.e., Malvan along Maharashtra coast and the Gulf of Kachchh Marine National Park and Sanctuary (GoK MNPS) in the state of Gujarat.
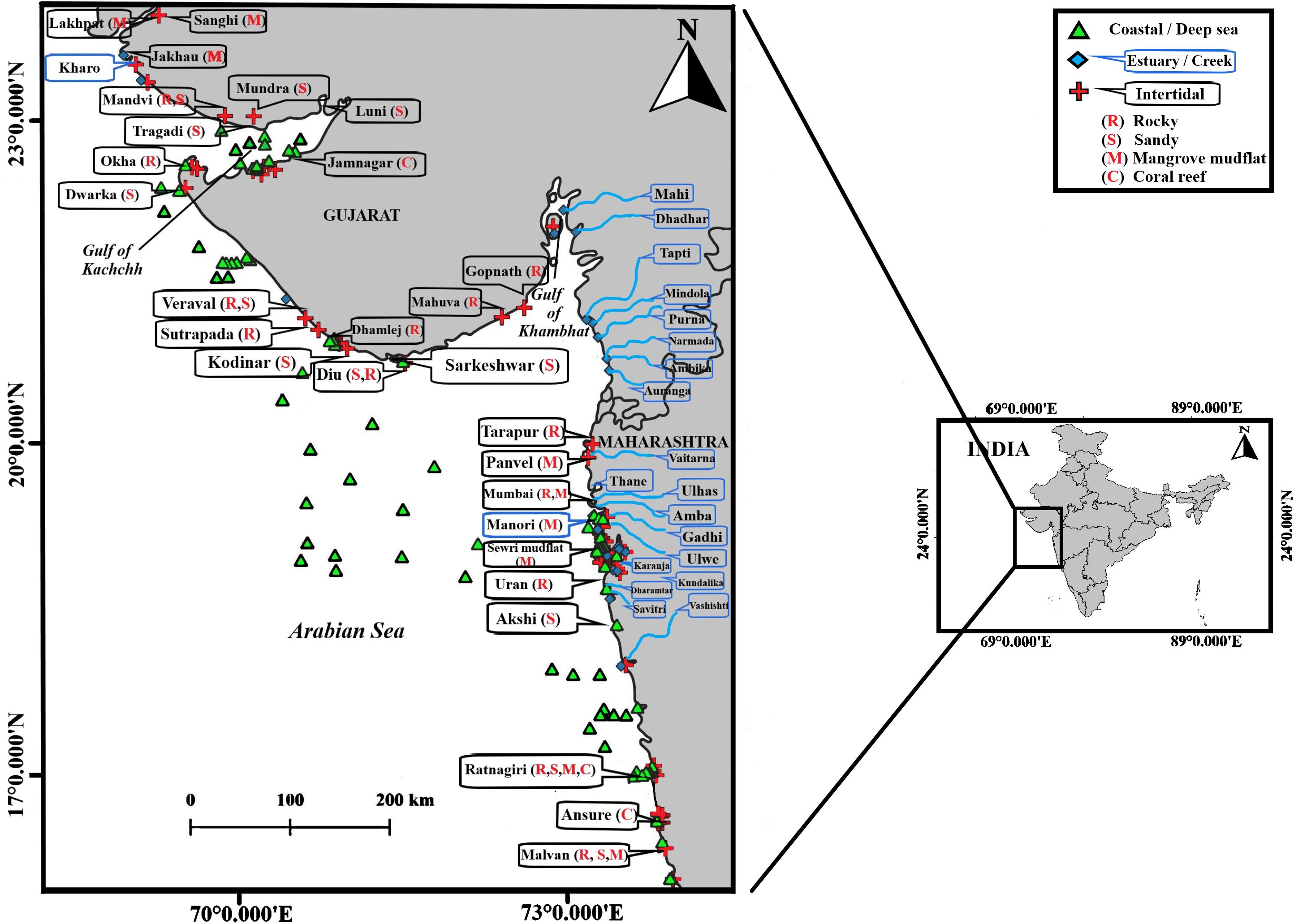
Figure 1. Map of the study locations of macrobenthic investigations along North West coast of India.
The western Indian continental shelf is one of the five major upwelling zones of the world (Benguela, Humboldt, California, Canary, and Somali) (Naqvi et al., 2009). The characteristic upwelling process during the southwest monsoon leads to the development of coastal hypoxia along the west coast, forming one of the world’s largest natural oxygen-deficient systems (Naqvi et al., 2000). This has implications on the coastal productivity and influences benthic biological processes as well (Jaleel, 2012). During the last few decades, this coastal region has been experiencing rapid industrial developments and burgeoning urbanization (Sachs et al., 2002) while sustaining high commercial fish landings. Some of the major problems faced are related to rapid industrialization and associated urbanization resulting in coastal erosion, siltation, habitat loss, and deterioration of water quality in some zones.
The Arabian Sea is experiencing a secular warming since 1995 as a response to regional climate change which has manifested in the form of disruption of seasonal cycles and increased frequency of extreme events in the adjacent Indian mainland in the last decade (Prasanna et al., 2009). Continued warming in sea surface temperatures will have long term implications on the coastal biodiversity. In a scenario wherein the coastal systems are increasingly facing the detrimental effects of climate change and exponential anthropogenic pressures, it is vital to co-ordinate global monitoring efforts, gather the baseline data of extant biodiversity and analyze them proactively. Gaps in present monitoring and reporting systems require to be identified and rectified to strengthen existing programs (Mawdsley et al., 2009). In this review, we have attempted to collate all available information pertaining to the vital aspects of macrobenthic assemblages of the NWI, encompassing varied habitats such as the continental shelf, creeks, estuaries, intertidal zones including coralline. Furthermore, we have summarized the studies carried out thus far, inventoried the recorded species, deduced general trends, examined environmental factors determining macrobenthic community structure and endeavored to identify data lacunae, and areas that merit further investigations. While several government sanctioned macrobenthic surveys have been executed, the data acquired have been largely scattered, random, and in disparate forms. This makes evolving of an overarching holistic view of macrobenthic research largely unsuccessful, thus weakening efforts to plug knowledge voids. The review would benefit managers of the coastal zone in drawing up conservation plans and expend resources toward areas identified as diversity hotspots or least studied.
Data Collection and Taxa Inventory
The meta data for this review was compiled from published research papers on the various aspects of macrobenthos. Totally, 185 scientific articles were gathered from various sources, the oldest references dating to the 1940s (Figure 2A). The articles were categorized based on the type of zone. The comprehensive checklist of macrobenthic taxa identified at least till the generic level along with the corresponding references is presented in Supplementary Table 1. All the names were verified with the WoRMS (World Register of Marine Species) database. Around 105 species names were not found on any of the verified online databases (tagged with an asterisk). Thirty-nine species could not be traced on WoRMS, but were mentioned on other Google databases. Thirteen species were tagged as nomen dubium (a name of uncertain taxonomic significance) on WoRMS. Totally, 2,078 macrobenthic taxa belonging to 14 phyla have been identified from various realms of the NW coast of India. More number of species was identified from the intertidal (1,113) and coastal (1,063) zones as compared to estuaries (Figure 2B). Also, comparatively lower number of species were reported from estuaries (336). The inventory was detailed in the case of polychaetes (617 species), gastropods (602 species), bivalves (216 species), brachyurans (109 species), and amphipods (107 species) (Figure 3 and Supplementary Table 1).
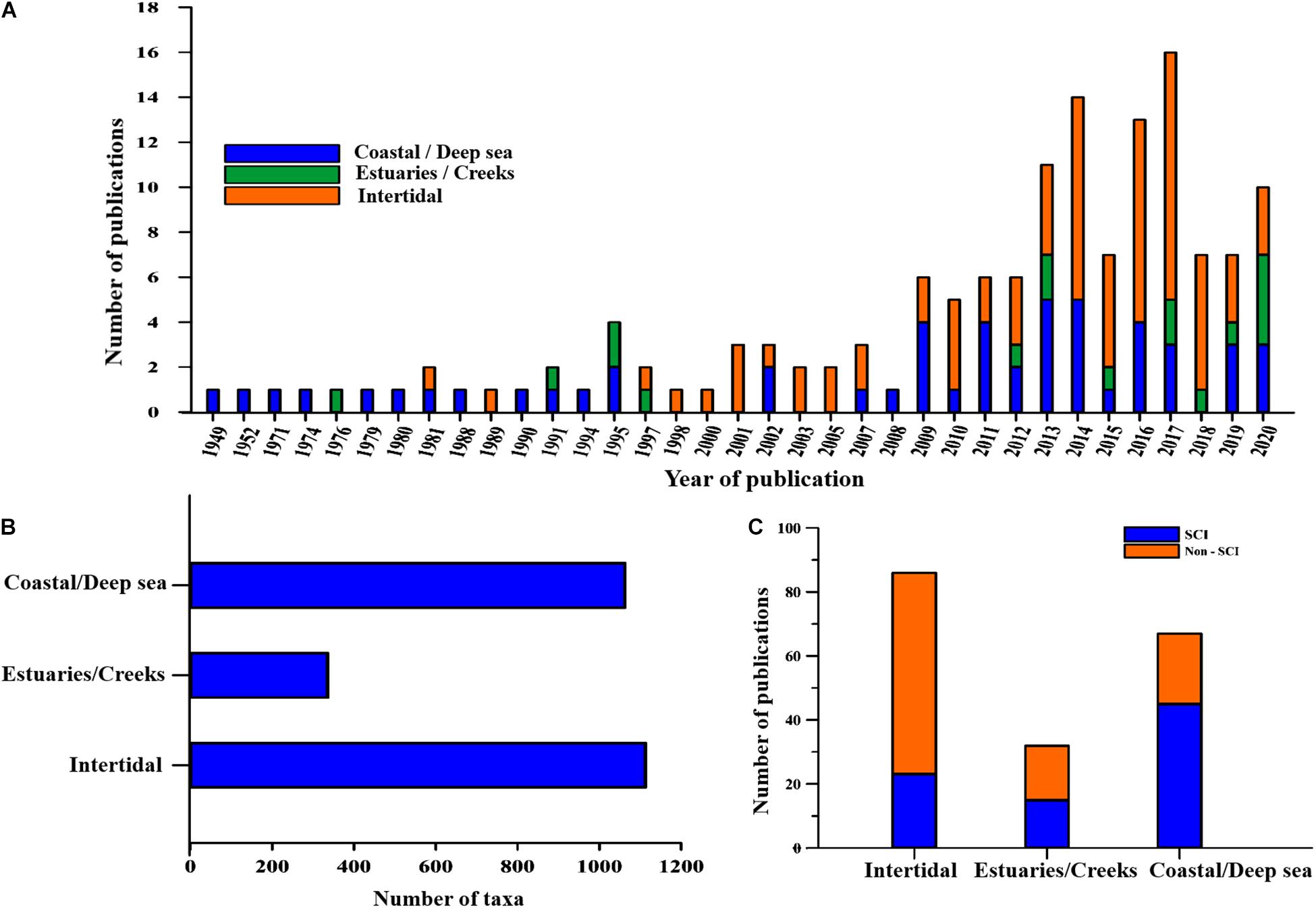
Figure 2. (A) Annual number of publications indicating zonal categorization (1949–2020). (B) Zonal categorization of macrobenthic taxa along North West coast of India. (C) SCI and non-SCI literature of macrobenthic studies along North West coast of India presented zone-wise.
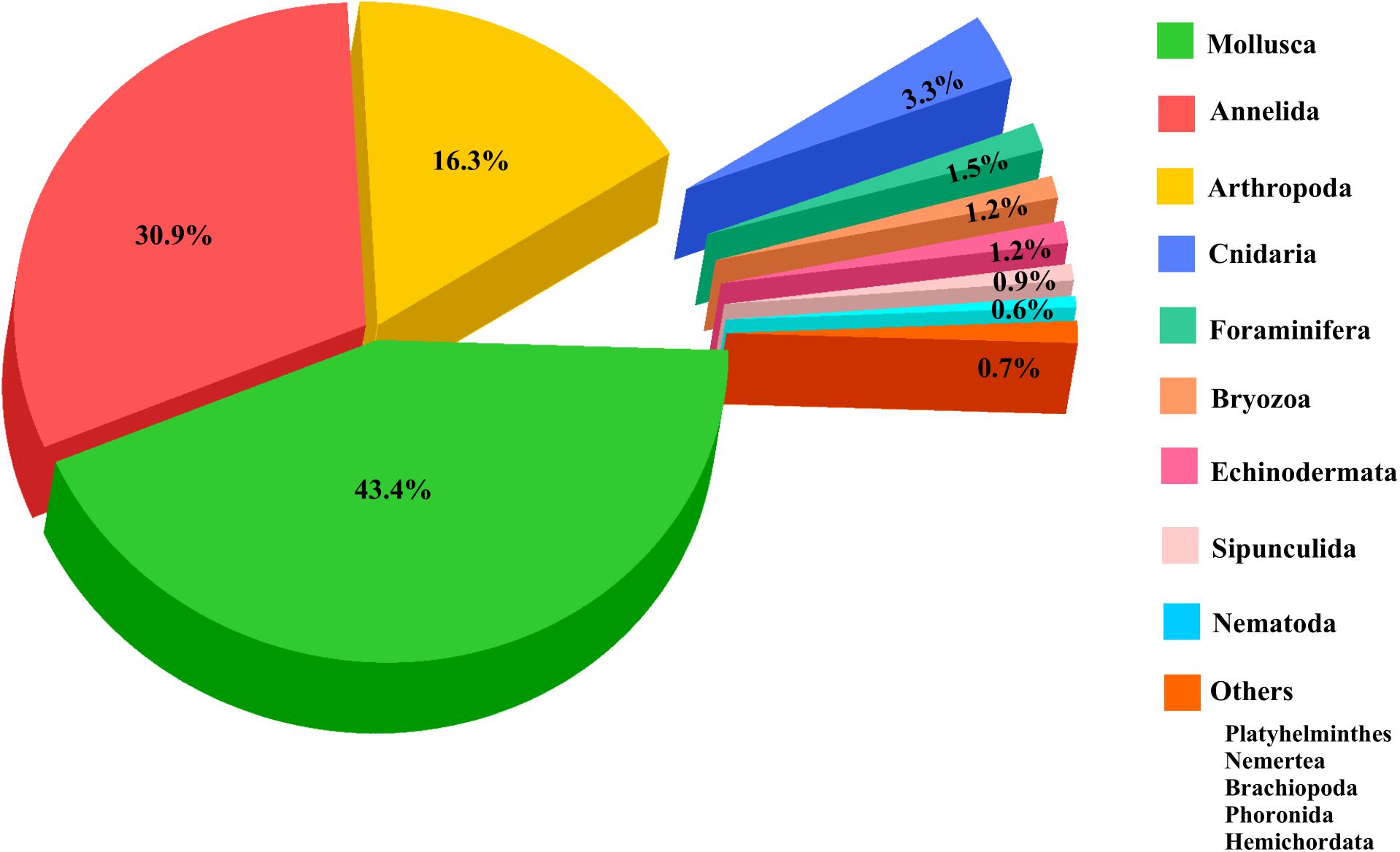
Figure 3. Phylum level composition percentage of macrobenthic taxa recorded and listed in Supplementary Table 1.
The Intertidal Zone
Among the intertidal categories of NWI, the dominant types are tidal mudflats and mangroves. The Maharashtra coast is dotted by sandy and rocky beaches flanked by promontories. Intertidal mudflats abound along the estuarine mouth and creeks which sustain substantial mangrove patches. While the Maharashtra coast is mainly linear, the Gujarat coast has two major indentations, the Gulf of Kachchh and the Gulf of Khambhat, each having its distinct physico-chemical and hydrodynamic characteristics. The Gujarat coastline has varied geomorphology having a network of tidal creeks with abundant mangroves, rocky shores, vast mudflats, coral reefs, sandy beaches, calcium carbonate cliffs, and sand dunes. The following account summarizes the macrobenthic studies of intertidal locations by categorizing data into four major habitat types. The various locations mentioned in this compilation are marked in Figure 1.
Rocky Intertidal
The Malvan coast of Maharashtra has exceptionally high biodiversity due to its multifarious habitats which has earned it the MPA status. Therefore, this intertidal ecology has been the subject of several investigations related particularly to sea anemones (Parulekar, 1966, 1969, 1971a,b), polychaetes (Parulekar, 1973), and molluscs (Joshi, 1969). Gaikwad and Ranade (1979) provided a checklist of 72 polychaete fauna collected from Ratnagiri, of which 19 were new records from the west coast as well as Indian waters, respectively. Around 92 and 56 species of intertidal fauna were identified from the rocky pool and rocky shore environments of Malvan coast, respectively (Parulekar, 1981). From the Ratnagiri coast, Wadkar (1989) reported five species of polychaetes. Eight hydroid species from eight rocky intertidal locations of the Konkan coast were described by Nagale and Apte (2013a). In a comprehensive study on the vertical diversity, density, distribution, and ecological aspects of Gastropoda at 14 predominantly rocky intertidal sites along the Ratnagiri coast, 127 species belonging to 20 families were recorded (Kurhe et al., 2014). Pioneering taxonomic studies on crabs of the Maharashtra coast were conducted by Chhapgar (1957) after which very little was discovered on this group. The crabs, Xenophthalmus wolfii, Xenophthalmus garthii, and Ozius tuberculosus were reported from both rocky and sandy beaches of Maharashtra (Khot et al., 2016b, a, Khot et al., 2019).
Very high biodiversity was reported from different types of intertidal zones of Mumbai megacity located in Maharashtra, as early as 1959 (260 species; Bhatt, 1959). Earlier accounts on the molluscan diversity along the then Bombay Presidency were given by Abercrombie (1892), Melvill and Abercrombie (1893), Melvill and Standen (1901), and Rai (1931). Hornell (1949) identified 225 gastropod species and 199 bivalve species. Bhatt (1959) had reported 58 live gastropod and 35 bivalve species. During a survey along the Mumbai coast line, 38 and 51 molluscan species were identified from two different rocky beaches (Jaiswar and Kulkarni, 2005). The rich biodiversity of the rocky intertidal zone of Mumbai, despite the anthropogenic stressors prevalent, have been accounted by Datta et al. (2010). From three rocky beaches, around 50 macrobenthic species were reported which included 41 gastropods and 5 pelecypods. In a later study, 70 macrobenthic taxa, were identified in the rocky pools of the intertidal area of Colaba, Mumbai (Sukumaran et al., 2014). Around 30 and 34 species of macrobenthos dominated by molluscs were identified from the Haji Ali and Marine Drive intertidal zones (Balasaheb et al., 2017).
From the rocky intertidal zones near an effluent outfall at Uran bordering the eastern side of the Thane Creek, Ram et al. (1998) reported nine gastropod species though the major groups were amphipods, barnacles, and polychaetes. Macrofaunal density and diversity were higher in rocky intertidal zones as compared to nearby sandy shores and the subtidal areas. From the Uran coast of Maharashtra, about 60 species belonging to 25 families of Gastropoda was listed by Pawar and Al-Tawaha (2017a), mostly collected from rocky stretches. Further studies by Pawar and Al-Tawaha (2017c) reported 170 species belonging to 83 families dominated by gastropods, brachyurans, pelecypods, etc. from the same intertidal areas at Uran, though the species checklist was not provided. Twenty-five macrobenthic groups including 16 polychaete families were recorded from the sand interspersed rocky intertidal zones of Tarapur coast around an atomic power station, Maharashtra (Kubal et al., 2016).
The Gulf of Khambhat at Gujarat has very high turbidity owing to high tidal amplitude and the heavy load of suspended sediments channelized through the perennial rivers emptying into the Gulf. From the littoral sites of Mahuva, Gopnath (bordering the Gulf), and Veraval (away from the Gulf), 32 macrobenthic species belonging to 24 families were recorded (Raghunathan et al., 2003). In the rocky zones of Veraval, Sutrapada, Okha, and Dwarka, molluscs (28 species), arthropods (14 species), and annelids (3 species) were observed to be associates of zoanthids (Pandya et al., 2014). Similarly, Trivedi et al. (2014) studied the macrobenthos associated with the zoanthid, Palythoa mutuki at four different sites (Sutrapada, Dhamlej, Kodinar, and Veraval) along the Saurashtra coast of Gujarat. Sixty-seven species belonging to Arthropoda, Mollusca, Cnidaria, Annelida, etc. were identified. Seven species of opisthobranch were reported from intertidal habitats of south Saurashtra by Poriya et al. (2015) among which five species were first records from the coast. Vakani et al. (2016) recorded 38 molluscan species from rocky intertidal region of Veraval. A comparative study undertaken at Tragadi (muddy substratum), Nana Layja (rocky), and Luni (sandy) along the northern coast of the GoK recorded 15 species with gastropods forming the dominant group (Naz et al., 2016). The highest species diversity was recorded at the rocky shore. Twenty-four species belonging to 18 families of bryozoans were identified from the rocky intertidal zones of Gujarat and Maharashtra by Venkatraman et al. (2018) and 23 species of limpets were reported from the same coastal stretch by Vakani and Kundu (2018) and Vakani et al. (2020). Vadher et al. (2020) provided a checklist of 95 sea slug species from the intertidal region of Gujarat coast.
Sandy Intertidal
From the sandy shores of Malvan, 57 macrobenthic species were reported by Parulekar (1981) during surveys spanning different seasons during several years. Forty-seven molluscan species were identified from a predominantly sandy intertidal zone interspersed with rocky patches along the Akshi coastline of Maharashtra by Kurve and Kurve (2010). Fifty-six macrobenthic taxa were reported from a sandy beach at Ratnagiri where the intertidal macrofauna were dominated by crustaceans, gastropods and polychaetes (Sivadas et al., 2012). Shannon Wiener index varied from 0.094 to 0.92 indicating low diversity in the polluted sandy beaches of Versova, Mumbai (Anandan et al., 2003). Major macrobenthic groups were polychaetes, amphipods followed by isopods, crabs, hermit crabs, decapods, pelecypods, and gastropods. Seven macrobenthic species were reported from the sandy Girgaon intertidal zone (Balasaheb et al., 2017). The molluscan species, Cellana radians (Gastropoda), Chiton (Amphineura), Trochus intercostalis (Gastropoda) were the most abundant species reported from the sandy intertidal zones at Veraval and Diu (Misra and Kundu, 2005; Agravat and Raval, 2019). The distribution pattern, community structure of the sabellariid worm, Neosabellaria clandestinus from the biogenic reef of Gulf of Khambhat, Gujarat was studied by Chaudhari et al. (2016). The Mandvi in the northern GoK coastline is wide, sandy with various landforms and distinct geomorphological features. 18 species of crustaceans, 15 species of polychaetes, one nemertea were investigated for their bioturbatory activities and the resultant micro-environment (Patel and Desai, 2009). From the sandy rocky intertidal zones of Mandvi, 25 gastropod species were reported by Kardani et al. (2014).
Mangrove Mudflats
The mangrove mudflats of Malvan had comparatively lesser species diversity (29 species) as compared to other intertidal habitat types (Parulekar, 1981). In the intertidal zones of Ansure creek at Ratnagiri, 17 benthic species were recorded dominated by the mollusc, Cerithideopsilla cingulata (Apte et al., 2014). A recent study on the polychaete diversity and seasonal variation in the mangrove covered intertidal area of Shirgaon, Ratnagiri revealed 6 species (Karhale et al., 2018). From the mangrove sites around Mumbai coast, 46 genera of macrobenthos belonging to Mollusca, Polychaeta, Brachyura, etc. were identified (Takar et al., 2020). Eighteen macrobenthic species including molluscs and brachyurans were reported from the mudflats of Gorai creek having extensive mangroves (Balasaheb et al., 2017). Kantharajan et al. (2017) identified 61 molluscan species from eight mangrove ecosystems along Mumbai coast. This included 46 species of Gastropoda, 14 species of Bivalvia, and 1 Polyplacophora. Higher presence of gastropods was attributed to the superior tolerance of this group to adverse conditions in mangroves. Only 9 polychaete species were identified from the vast intertidal mudflats of Thane creek that borders the Mumbai metropolis (Quadros et al., 2009). Expectedly the diversity indices were poor indicating low diversity and evenness.
Previously, Awati and Karandikar (1948) were the earliest to record three Onchidium species from the Mumbai coast. Three species of Onchidium were recorded along the Uran intertidal zone, Onchidium verruculatum and Platevindex sp. were abundant in marshy loamy substratum and Onchidium peronni was mostly associated with mangroves (Patil and Kulkarni, 2013). From the mangrove mudflats at Uran, Pawar et al. (2019) identified 86 macrobenthic species belonging to 45 families. At two intertidal locations along the Karanja creek in Maharashtra, a two year study reported high densities of the polychaete, Perinereis cultrifera along with other species of polychaetes, gastropods, crustaceans, pelecypods, etc. and it was concluded that the area supported good diversity and abundance of macrobenthos (Pawar and Kulkarni, 2009). Pawar et al. (2019) assessed the macrobenthic species composition and abundance from the intertidal zones of the Panvel creek.
Pandya (2013) investigated the biogenic structures of macrobenthos due to their bioturbatory activities in the tidal mudflats of Mahi estuary and coastal Gulf of Khambhat. The author suggested that the ichnological evidences can be used as indicators of animals even in their absence, which can be useful for biodiversity, ecological, and functional studies. Marine gastropod fauna from a large ship breaking yard of Alang was studied by Baxi et al. (2017) with prevailing environmental parameters. The intertidal region, despite being industrially influenced, sustained a good molluscan diversity with 77 species. Around 62 species of mangrove associated macrofauna consisting of crustaceans, gastropods, bivalves, polychaetes were recorded from three locations in the GoK (Saravanakumar et al., 2007). A study on the macrobenthos in mangrove zones of the Jakhau coast at the mouth of the GoK identified 27 macrobenthic species (Rohit et al., 2016). Another survey on the brachyuran diversity at 8 different locations in this area yielded 19 species belonging to eight families (Trivedi et al., 2012) where the crabs preferred open mud flats as compared to other habitats. Three crab species all belonging to the family Campantriidae from the intertidal mangrove areas of Jakhau and Lakhpat were described by Trivedi et al. (2017). From the mangrove rich intertidal zones of Mundra and Sanghi in the northern GoK, 13 and 17 gastropod species respectively were reported by Kardani et al. (2014). Dominant species were Cerithidea cingulata, Cerithidea fluviatilis, Umbonium vestarium, and Assiminea sp. Further north, three brachyuran crab species from the intertidal mangrove mudflats of Lakhpat and Jakhau located in the GoK mouth, were new records for Indian waters (Trivedi et al., 2017). Kachhiya et al. (2017) described seven species of hermit crab belonging to genus Clibanarius from intertidal zones of Gujarat of which six were first records from Indian west coast.
Coral Reefs
The Marine National Park and Sanctuary (MNPS) at Jamnagar, GoK has highly heterogeneous intertidal habitats that support multitude of species including corals. Sedimentation is a major natural threat in this area that adversely affects the coral species and its associated organisms. Several reef associated species have been listed by many workers (Kundu, 2001; Vaghela et al., 2010; Ramamoorthy et al., 2012; Adhavan et al., 2014). A new species of echiurid Acanthobonellia pirotanensis was described by Jose (1964) from Pirotan islands of the MNPS. Records of some rare species like Bonnelia (Echiuroidea), Coeloplana (Ctenophora), Convoluta and Leptoplana (Platyhelminthes), and Lineaus (Nemertea) were also made (Kundu, 2001). A checklist of opisthobranch (mollusca) fauna of GoK by Apte et al. (2010) reported 33 species belonging to 19 families of which 21 were new records to Gujarat and 13 were new records for the Indian coast. Hydroid diversity records (17 species) from the intertidal zones of Gujarat were earlier contributed by Thornely (1916) whereas Nagale and Apte (2013b, 2014) added new records of hydroids from the intertidal zones of GoK. Kamboj (2014) reported the occurrence of about 30 crab species and more than 200 molluscan species from the shallow intertidal areas and deeper waters of the MNPS. Five species of hermit crabs from the area were described by Jhala et al. (2017). Three species of sea anemone and one species of crab belonging to the rare category were reported by Mirza et al. (2017) from the GoK. Four sea anemones, one crab, and one polychaete species were described by Mirza et al. (2019) from another survey in the GoK. The detailed description and taxonomical details of two minute gastropods (Pseudoliotia henjamensis and Cyclostrema ocrinium) were reported from the MNPS by Mukhopadhyay et al. (2019). New taxon records for the GoK were contributed by Padate et al. (2018). Seventy-one species of intertidal amphipods belonging to 23 families were identified from the coralline zones of the MNPS (Srinivas et al., 2020). Five functional traits of these amphipods were also studied to understand the spatial patterns in amphipod functioning.
The Estuaries and Creeks
The estuaries along the NW India are vulnerable to various anthropogenic activities and the resultant deteriorating ecology has raised concerns in the local administration and general public. They are also subject to pronounced natural variations caused by the distinct monsoon season resulting in significant freshwater inflow and flushing (Rao et al., 2019). Indian estuaries receive heavy freshwater discharge during the seasonal rainfall period that often exceeds the total capacity of the estuaries resulting in fresh water domination (Sarma et al., 2011). The strong flushing during monsoon alters the abiotic variables such as water temperature, pH, DO, and salinity causing natural stress in the estuarine environment (Feebarani et al., 2016). Significant freshwater inputs during monsoon cause reduction in the macrobenthic fauna of the estuary due to abrupt salinity fluctuations and increased turbidity (Alongi, 1990). The mortality and recruitment of macroinvertebrates during different seasons result in continuous seasonal variation in their abundance and composition.
The macrobenthos of Vashishti estuary in Maharashtra, mainly constituted by Polychaeta, Mollusca and Crustacea, was lower than that of the nearshore coastal system off the estuary (Nair et al., 1998). Benthic foraminifera were used to assess the ecological status of Gadhi and Ulwe estuaries by Kale (2017), wherein 23 live species were identified. The diversity and distribution of macrobenthos of Ulhas estuary and Thane creek were studied by Govindan et al. (1976), Athalye (1988), Athalye and Gokhale (1991), Gokhale and Athalye (1995), Mathew and Govindan (1995) and Athalye et al. (2003). In the abovementioned studies, Polychaeta, being the dominant macrobenthic taxon, was the focus group. A comprehensive account on the polychaete diversity of Indian estuaries was provided by Khan and Murugesan (2005). The first comprehensive seasonal study of the amphipod community structure and their spatio-temporal distribution in four estuarine (Ulhas, Amba, Savitri, and Kundalika) sediments of Maharashtra was provided by Srinivas et al. (2019b). The study investigated the species composition, diversity and distribution, functional traits of amphipods, and the influence of hydrosedimentological variables on community structure. Altogether, 57 amphipod taxa were recorded from these estuaries and majority of the taxa belonged to the functional group that was characterized by detritivory and domiculous behavior. Overall, 189 macrobenthic taxa were identified from the Ulhas, Amba, and Savitri estuaries (Mulik et al., 2020c) during the three seasons, wherein Polychaeta was the most dominant group.
Benthic studies along south Gujarat estuaries (Purna, Auranga, Ambika, Purna, Mindola, and Narmada) were undertaken (Varshney et al., 1981; Govindan et al., 1983, Dange, 2018). A study from Kharo creek, Kachchh (Gujarat) (Nigam and Chaturvedi, 2000) revealed 47 foraminiferal species of which 44 were benthic (only seven were live). The brachyuran crab diversity and distribution was studied out by Pandya and Vachhrajani (2013), Shukla et al. (2013), Gadhavi (2015) and Gadhavi and Vachhrajani (2015) in Mahi estuary, Gulf of Khambhat. Two species i.e., Dotilla intermedia and Macrophthalmus brevis, were recorded for the first time from this region. Meetei et al. (2020) investigated the macrobenthic community structure of Tapi estuary and recorded 49 taxa from 13 sites of this river wherein Mollusca was the dominant group.
The Coastal Zone
The Indian west coast, especially, contributes to >70% of the marine capture fishery of the country (Ingole et al., 2009). In the Indian subcontinent, the integral relationship of the demersal fishery with benthos was well established by Harkantra et al. (1980) and Parulekar et al. (1982). These studies established a direct relation between benthic fauna and the distribution of exploited demersal fisheries, especially the shrimps and proposed that the quantitative data on benthos can be used as an efficient tool for the assessment of demersal fishery resources. Subtidal macrobenthic fauna off Dabhol, Maharashtra was studied by Ingole et al. (2002) who concluded that the coastal environment was favorable for feeding and breeding of commercially important prawn and crab species. Joydas and Damodaran (2009), investigated the NWI shelf and established that the benthic community played a key role in dictating the demersal fishery production of the vast western Indian shelf. Investigations in the inner shelf of central west Indian coast by Parulekar (1973) and Ansari et al. (1977) indicated presence of rich macrobenthic density near the coasts (dominated by polychaetes) that progressively decreased with depth. Kasinathan et al. (1974) studied macrofauna off Dwarka and Okha (GoK) that were characterized by different substrate types. A study in the subtidal region off Versova, Mumbai by Varshney et al. (1988) suggested that the macrobenthic species diversity was poor in nearshore stations due to the coastal pollution and higher in the unpolluted offshore region. A comprehensive work by Nair (2002) provided the status of flora and fauna of GoK including macrobenthic communities.
“Taxonomy” forms the “root” of any ecological study. Several workers have contributed toward taxonomic analysis of different macrobenthic groups along the NWI. Rao and Soota (1981) provided a list of 37 polychaete species collected from intertidal and subtidal habitats of various parts of Gujarat. Most of the collected species formed the first locality records from the respective areas while two species i.e., Ceratonereis costae and Cirriformia tentaculata were recorded for the first time from Indian waters. A new polychaete species, Serpula indica and six new records of Serpula sp. associated with variety of flora and fauna of Ratnagiri coast were reported by Parab and Gaikwad (1989). Kurhe et al. (2009) recorded 75 species of marine gastropods from Ratnagiri coast. Pati et al. (2015) provided an updated list of all the valid polychaete species with their distribution along the Maharashtra coast. A bi-operculate form of fouling polychaete, Hydroides operculatus was recorded from Diu by Kubal et al. (2012). Yokoyama and Sukumaran (2012) reported three polychaete species of family Spionidae i.e., Paraprionospio cordifolia, Paraprionospio cristata, and Paraprionospio patiens from NWI coast. The species were previously misidentified as Paraprionospio pinnata. Saiz et al. (2015) described a new subspecies of the sipunculid, Phascolion pacificum denticulatum from shallow waters off Malvan and Ratnagiri that represented the first record of any Phascolion species from the Indian coast. A new polychaete species, Heterospio indica was described from the NWI coast by Parapar et al. (2016). Mirza et al. (2017) reported three sea anemone and one crab species from the southern GoK. Recently Sivadas and Carvalho (2020) provided an updated checklist of annelid fauna from India comprising 727 species belonging to 334 genera and 72 families. The study also provided an overview of species with “erroneous” or “cosmopolitan” status. Nerurkar et al. (2020) provided species inventory of the gastropod family Nassariidae of India.
Limited macrobenthic studies were conducted along the NWI coast that provided information on the functional aspects. Sivadas et al. (2013) studied the functional diversity of macrofauna of Kalbadevi Bay, Ratnagiri, using three traits like feeding, mobility, habitat with temporal variations. Ingole et al. (2014) studied the feeding behavior of the macrobenthic fauna off Ratnagiri and Mumbai and observed that surface deposit feeders, carnivores and interface feeding spionids dominated the sediments during interfall monsoon. Sukumaran et al. (2016) also studied the feeding strategy of polychaetes and registered dominance of microphagous feeders, post the disturbance of cyclone Phyan, however, recovery was observed by re-establishment of multiple feeding guilds later. Vijapure et al. (2019a) studied five functional traits like feeding mode, motility, body size, bioturbation mode, and habitat of macrobenthic polychaete species. This is the only extensive study that employed “Fuzzy coding” procedure to understand the functioning of polychaete species of a large geographical zone.
Extensive Ecological Surveys
In the past decade, many extensive macrobenthic studies were undertaken in broad geographic areas along the western continental shelves (Jayaraj et al., 2007, 2008; Joydas and Damodaran, 2009, 2014; Joydas et al., 2009; Anilkumar, 2017). These workers documented the macrobenthic diversity, distribution, abundance, and community structure in relation to the environmental influences on them. Parulekar and Wagh (1975) reported occurrence of 9 macrobenthic groups, dominated by Polychaetes from the NWI coast in the depth range of 20–140 m. Harkantra et al. (1980) studied the distribution, abundance of benthos, and sedimentological properties in depth range of 10–70 m from western Indian shelf (covering a decent length of NW coastline). The study revealed that the benthic diversity was rich in nearshore habitats while it decreased toward deeper waters. Joydas and Damodaran (2009) sampled a vast area of Indian west shelf, covering varying depths (30–1,000 m), along as many as 17 transects, covering the NWI shelf. The investigation recorded 165 species of polychaetes belonging to 33 families from the entire west Indian shelf while 106 were identified from the NW region exclusively.
Studies by Jayaraj et al. (2007, 2008) and Anilkumar (2017) were confined to the NWI shelf from Dwarka to Mumbai at a depth of 30–200 m. In the same area, Jayaraj et al. (2007, 2008) reported the presence of 133 polychaete species, belonging to 39 families. An extensive temporal sampling effort by Sukumaran et al. (2016) demonstrated the impact of tropical cyclone Phyan on the polychaete community of the Malvan MPA, followed by a complete recovery. Rengaiyan et al. (2017) carried out a study in the coastal waters of the south-eastern Arabian Sea, including Ratnagiri and Mumbai transects from the NWI coast. The study determined spatial variations in the polychaete assemblage that consisted of 16 families and 71 species, mainly governed by substrate composition. The diversity and distribution patterns of polychaete species along the NWI coastline were surveyed during the three major seasons and overall 140 species belonging to 42 families were identified by Vijapure et al. (2019a).
Molecular Studies and Phylogeny
The molecular approach not only assigns appropriate molecular tags to named or unnamed entities but also helps to reveal cryptic species and understand the role of cryptics in ecological and evolutionary processes. With the help of molecular lens, a number of studies worldwide have delineated cryptic species diversity of wide range of macroinvertebrate phyla (Kawauchi and Giribet, 2014; Nygren et al., 2018; Fauvelot et al., 2020) that were hidden under the umbrella of single morphospecies. Few molecular studies have been carried on the macrobenthos along the NWI coastal region. Magesh et al. (2014) redescribed a nereid, Namalycastis glasbyi from the Gorai creek, Mumbai with COI sequences. An interesting work by Joseph et al. (2014) revealed the presence of three cryptic species of limpet Cellana karachiensis from Veraval with different shell color banding pattern and indicated a close molecular resemblance to Oman species populations using the COI gene. The research was extended further by Joseph et al. (2016b) in terms of coverage of study area (five sites along Gujarat coast) and genetic markers (COI and 16S). The study demonstrated the existence of six haplotypes of C. karachiensis with phenotypic plasticity in the study region, suggesting initiation of the speciation. Phylogenetic analysis by Joseph et al. (2016a) proved efficacy of COI gene in distinguishing six zoanthid species from the Saurashtra coast. Detailed taxonomic investigation of a new polychaete species Heterospio indica from NWI coast by Parapar et al. (2016) was complemented with molecular analysis where 18S and COI genes were deposited in GenBank and represented the first sequencing of the species of Heterospio belonging to monogeneric family Longosomatidae. Rengaiyan and Ingole (2018) in the eastern Arabian Sea, sequenced 18S rDNA gene from 54 polychaetes species. Barcodes of five markers COI, 18S, 28S, cytb, ITS2 were generated from Hydroides inornata by Sun et al. (2017) from Ratnagiri for the phylogenetic analysis of Hydroides operculata-complex. Vijapure et al. (2019b) generated a comprehensive reference library of 58 COI barcodes of thirty-one polychaete species from NWI coast. The effectiveness of molecular characterization and phylogenetics in distinguishing the polychaete species, including invasive, pollution indicator Streblospio sp. was proven by this investigation. The genetic scrutiny facilitated the identification of a prospective new Streblospio species by segregating them from the closely related congeners. Trivedi et al. (2019) suggested a modified protocol for genomic DNA extraction and amplification of foraminifera Ammonia sp. from intertidal region of Nana Layja, GoK. In order to resolve the masked phenotypic differences of invaders and early detection of bioinvasion, DNA barcoding (Miralles et al., 2016; Sun et al., 2017), and metabarcoding (Rey et al., 2020; Westfall et al., 2020) efforts have proved effective worldwide. However, these advanced molecular methods will work effectively only if species are taxonomically identified and corresponding sequences are deposited in public reference library (Brasier et al., 2016) with constant updates of the repository (Curry et al., 2018).
Application of Macrobenthos in Environmental Monitoring
Expectedly, most studies pertaining to the application of macrobenthos in environmental monitoring along the NWI coast have been concentrated in and around heavily urbanized and industrialized coastal zones like Mumbai, estuaries that are the hub of a plethora of anthropogenic activities or protected zones like the GoK MNPS. Further on, the various studies are discussed under broad anthropogenic categories for ease of comprehension.
Univariate and Benthic Biotic Indices
Univariate indices were applied more frequently in environmental studies, probably due to the ease of application (Quadros et al., 2009; Kulkarni et al., 2010, 2011; Sukumaran et al., 2013, 2014, 2016; Naz et al., 2016; Dias et al., 2018; Takar et al., 2020). Several diversity indices (Shannon-Wiener, Index of frequency, Rarity index, Dominance and Evenness Indices, Margalef Richness Index) were applied to the macrobenthic density in various studies (Varshney and Govindan, 1995; Pawar and Kulkarni, 2007; Pawar et al., 2019). A lot of interest has been evinced in the usage of Benthic Biotic Indices (BBIs) during the last two decades in evaluating the ecological quality status of marine and estuarine waters. The unawareness of the taxonomic characteristics of macrobenthos has impeded the application of benthic indices in assessing the ecological quality status of the tropical environment (Bigot et al., 2008). Studies investigating the efficacy of BBIs in determining the estuarine ecological status along the NWI India have gathered momentum in the past decade. AZTI’s indices (AMBI/M-AMBI) were successful in discerning an appropriate ecological status of most of the salinity zones across four estuaries (Ulhas, Amba, Savitri, and Kundalika) (Mulik et al., 2017, 2020a,b; Dias et al., 2018) and coastal zones (Nandgaon coastal waters, adjacent to the atomic power station in Maharashtra, Sivaraj et al., 2014; Mumbai and Ratnagiri coastal waters; Sivadas et al., 2016). The exclusion of monsoon data and averaging the status of the non-monsoonal seasons for the efficient application of benthic indices was recommended. Data transformations were observed to have no influence on the efficiency of AMBI (Dias et al., 2018; Mulik et al., 2020b; Mulik et al., 2020a). The Benthic Opportunistic Polychaeta Amphipoda (BOPA) index was applied to tidal pool communities of a tropical shore to evaluate the impact of an oil spill on intertidal ecology (Sukumaran et al., 2014).
Habitat Modification and Loss of Species
A comparison of the polychaete diversity of the intertidal flats of the anthropogenically stressed Thane creek with past available data indicated the deteriorating environmental conditions of the creek had resulted in species substitution. The changing sediment texture and increase in hypoxic conditions and nutrients resulted in resilient polychaetes like Namalycastis senegalensis and Polydora tentaculata being replaced by even more pollution tolerant types like Ceratonereis burmensis (Quadros et al., 2009). Khot et al. (2016a) have remarked that many species which were earlier recorded from Maharashtra coast during the nineties were not observed in recent times possibly due to increasing anthropogenic stress. Vaghela et al. (2010) compared the intertidal chemistry and macrobenthic assemblages of the industrialized zone of Sikka, GoK with previous data and concluded that while the abiotic parameters remained unchanged, the macrofaunal diversity indices were significantly lower than that reported previously. This decline was attributed to the habitat modification brought about by the anthropogenic pressures in that area. An example of diminishing diversity due to anthropogenic pollution was provided by Jaiswar and Kulkarni (2005) from the heavily polluted Mahim intertidal zone where gastropod species were absent. Previously, from the same area, 90 species of gastropods were reported (Subrahmanyam et al., 1952). The absence of the previously reported Aplysia benedicti, from most of the coastal zones of Mumbai was noted by the authors who attributed this species loss to the severity of pollution in Mumbai.
Oil Pollution
North West India coast is especially vulnerable to oil spills on account of the heavy ship traffic, presence of oil terminals and Single Point Moorings (SPMs). An intertidal study conducted in the vicinity of the waste water discharge of an oil terminal at Uran revealed a localized marginal impact on intertidal organisms characterized by low biodiversity and dominance of stress tolerant macrobenthic species (Ram et al., 1998). Gajbhiye et al. (1995) studied the impact of oil spill on the biological characteristics and also reported complete recovery of marine environment off Murud, Maharashtra after a year from oil spill. An oil spill that occurred in the mouth region of Mumbai Harbor affected the rocky intertidal region of Colaba. A 15 month long study using macrobenthic diversity concluded that the area was only temporarily affected (Sukumaran et al., 2014). Another study that evaluated the impact of an oil spill on intertidal communities in the rocky beach of Uran, reported large scale mortality of brachyurans, decapods and large polychaetes at the high water line (Vijapure et al., 2016). However, the low water line had live macrobenthic groups like tanaidaceans, gastropods, amphipods, isopods, etc.
Discharge of Pollutants
While examining the health of the coastal marine environment around the Mumbai region, Zingde and Govindan (2000) observed increased macrobenthic abundance and reduced diversity. However, the impact of anthropogenic stress decreased from coast to offshore region and was minimal beyond 5 km from the coastline. Thane creek is amongst the major water bodies in an enclosed area in India and it disconnects Mumbai from mainland (Vijay et al., 2015). Various studies were carried out on the biotic and abiotic components of Thane creek (Virkar et al., 2004; Poojary et al., 2012; Quadros and Athalye, 2012; Athalye, 2013). It was reported that the water and sediment quality of Thane creek had deteriorated due to the discharges of pollutants from industrial and domestic sources. The shells of Paphia textile were observed to have been blackened by deposition of hydrogen sulfide due to domestic waste discharge at Girgaon along the Mumbai coast (Balasaheb et al., 2017). An assessment of the macrobenthic communities in the intertidal zones around an atomic power plant functional for more than four decades at Tarapur, Maharashtra concluded that the impact of discharges from the power plant was minimal (Kubal et al., 2016).
Harbors and Ports
Harbors and ports form the “hearts” of the coasts that aid in building a large share of the economy for the country. However, they are also areas where industrial and domestic effluents dominate the spectrum of anthropogenic inputs. A number of macrobenthic studies were undertaken in the harbor and port waters along NWI coastline (Ingole et al., 2009; Sukumaran and Saraladevi, 2009; Swami and Udhayakumar, 2010). Sukumaran and Saraladevi (2009) suggested spionids as a biological indicator for cost-effective and rapid environmental assessment of highly disturbed environments like ports. Ingole et al. (2009) examined the health status of three ports of central west India including Ratnagiri harbor from NWI coast. The stressed conditions at these areas were reflected by a number of biotic indices like Polychaete: Amphipod ratio, ABC curve, and geometric class abundance. Reduced gastropod diversities in areas subject to human intervention apropos to those away from such influences were observed at coastal areas of Uran which has a large marine container terminal (Pawar and Al-Tawaha, 2017a). Sukumaran et al. (2013) provided a valuable baseline data of macrobenthos and polychaetes (family level) along with prevailing environmental conditions in and around oil terminals situated in the GoK MNPS. The investigation concluded that although pockets of higher stress to benthic fauna could occasionally occur, the impact of oil and gas activities on macrobenthic communities in this eco-sensitive region was probably low.
Increased maritime transportation has in turn posed a risk to the environment from both pollution and bioinvasions (Seebens et al., 2013). Introductions of alien biota could pose a threat to local coastal and marine ecosystems with both economic losses and reduction in native biodiversity. Some biofouling studies have been undertaken along the maritime states of Maharashtra and Gujarat that have commercially important harbors and ports. Few studies have investigated biofouling by deploying different types of test panels/coupons at harbors of Vijaydurg (Srinivas et al., 1992, Mumbai (Swami and Chhapgar, 2002; Swami and Udhayakumar, 2010), and also at Bombay High (Venugopalan and Wagh, 1990). The busiest and commercially important Mumbai and Jawaharlal Nehru ports of Maharashtra were rigorously sampled by Gaonkar et al. (2010a) and 29 benthic species of hard substratum fauna and 14 associated fauna were recorded. Protula tubularia, a tube dwelling polychaete species, was a new introduction in the region. Invasive species of bivalve Mytilopsis sallei was also recorded from the enclosed habitats of the port. Another work in the Mumbai harbor, reported the introduction of 14 polychaete species (Gaonkar et al., 2010b). The study provided an account of bioinvasion and highlighted the risks of ship-mediated introductions and proliferation of tolerant invasive species in near future. Biofouling by the oyster, Saccostrea cucullata in mangrove forests of the Vashishti estuary was reported by Panchang (2014). Srinivas et al. (2019a) reported the first occurrence of a possible alien caprellid, Phtisica marina from the coastal waters off Vadinar and Sikka in Indian waters.
Overview and General Trends
Some common trends of the macrobenthic assemblages of NWI that have emerged from the above compiled literature are (a) Polychaeta was the dominant macrobenthic group; (b) anthropogenic impact on macrobenthos was reported across all zones; (c) sediment texture and salinity were the major drivers of macrobenthic community structure in the coastal waters and estuaries, respectively; (d) typically, the mesohaline and oligohaline zones of all estuaries were anthropogenically impacted, hence subject to the “Estuarine Quality Paradox”; (e) distribution of tolerant species was largely influenced by salinity as they inhabited specific salinity zones; and (f) sharper distributional trends were visualized in more polluted zones vis-a-vis moderately disturbed types.
In the intertidal studies conducted along the NWI, Mollusca has received more academic interest than other groups like Polychaeta and Amphipoda. This phylum contributed majorly to the intertidal species diversity of the NWI coast. Molluscs were the major taxonomic group of macrobenthos observed at different intertidal areas elsewhere too (Barnes and Barnes, 2012; Petracco et al., 2012). Habitat preferences were evident with more gastropod species being reported from rocky shores than sandy and muddy areas. Alongi (1989) had opined that tropical sandy intertidal communities were dominated by brachyurans and bivalves and mudflats were inhabited by mainly polychaetes and microcrustaceans citing superior behavioral escape mechanisms to avoid environmental extremes. The present review indicated that this generalization may not work for this geographical zone. Sandy zones had abundance of polychaetes and crustaceans while mangrove mudflats had molluscs and gastropods.
Alongi (1989) compared tropical macrobenthic literature with temperate macrobenthic ecosystems and conceptualized that (a) tropical species diversities were not essentially greater in tropics on account of greater environmental stress and (b) infaunal communities were mostly comprised of opportunistic species. The species list compiled herein (Supplementary Table 1) of only macrobenthic invertebrates (which automatically excludes six meiobenthic phyla) included 2,078 taxa that belonged to 14 of the 28 known non-symbiont animal phyla that comprises all known marine benthic taxa (Widdicombe and Spicer, 2008), thus re-iterating that tropical ecosystems supported high biodiversity (Gray, 1997; Hillebrand, 2004). This also raises the possibility of novel species yet to be described from this biogeographical zone. From Supplementary Table 1, it was also evident that infaunal communities of NWI included more sensitive species (62.2%) than opportunistic (37.8%), thus diverging from the view held by Alongi (1989). The comparison in Table 1 is indicative and may be viewed with the caveat that the studies have employed different sampling scales, sizes, and techniques. However, from the compilation of studies conducted in other geographical zones (Table 1), it appears that the first concept may not hold firm in the case of intertidal zones. The species diversities of intertidal zones presented in this study were certainly not lesser than that reported from temperate areas. In the case of estuaries, macrobenthic diversity along NWI was comparable with values reported elsewhere with Polychaeta being the dominant group in most of the studies (Table 1). Since the extensive macrobenthic studies in the coastal waters of NWI primarily focused on Polychaeta, the relative evaluation was based on this taxon. Table 1 suggested that the diversity of Indian polychaete fauna was comparable or relatively lower than other regions. This could be due to the bias in sampling methodology, gear, stations surveyed. Spionidae was one of the common speciose families, in the majority of the studies. Spionidae is known to be one of the most ubiquitous benthic taxa (Fauchald, 1977; Blake, 1996) with cosmopolitan species assemblage (Dix et al., 2004).

Table 1. Comparison of the dominant macrobenthic taxa and major factors exerting influence on the macrobenthic community structure from some indicative studies reported elsewhere with investigations included in this review (indicated by the shaded region).
In the intertidal zones of NWI, zonation patterns with relation to tidal levels and time of exposure were distinct with the high water zones being sparsely populated due to high degree of exposure and desiccation (Table 1). Higher species and functional diversities were observed at the lower intertidal zones of the GoK MNPS which had structurally complex habitats. The species distributional patterns in the intertidal zone were net effect of various physiological needs, limits and adaptations at different tidal levels (Srinivas et al., 2020). The type of substratum, shorter durations of exposure and moderate wave action of the intertidal zone contributed toward higher distribution of species rather than abiotic parameters like water temperature, pH and salinity (Misra and Kundu, 2005). Similarly, Karhale et al. (2018) concluded that the intertidal polychaete abundances were not dictated by water quality parameters. However, others (Anandan et al., 2003; Rohit et al., 2016; Pawar and Al-Tawaha, 2017c) have reported that intertidal macrobenthic biomass and abundances were influenced by water and sediment quality parameters. Other reported driving factors influencing the diversity, distribution and abundance of intertidal organisms were food availability, organic carbon, pheophytin, and sediment texture (Table 1). In general, the spatial patterns of intertidal assemblages in all the studies have by and large followed the general model described by Menge and Sutherland (1987) who postulated that in stressful environments, organisms were directly regulated by environmental stress and less impacted by competition-predation activities.
While a panoply of factors have been ascribed importance in influencing macrobenthic community structure of intertidal zones, all studies agreed on the primacy of salinity and sediment structure as determinants of macrobenthic assemblages of estuaries along NWI (Table 1). The significance of the salinity and sedimentary texture in influencing macrobenthic community structure has been well documented in the estuaries of north Queensland, Australia (Inglis and Kross, 2000), estuaries of South Africa (Teske and Wooldridge, 2003), Schelde estuary, NW Europe (Ysebaert et al., 2003; Hampel et al., 2009), Nueces estuary, Texas (Ritter et al., 2005), Amazon estuary, Brazil (Silva et al., 2011) and Yangtze estuary, China (Yan et al., 2017). This was collectively attributed to the drought and flood events that change salinity and sediment properties and thus impacting macrobenthic communities and the functioning of the estuarine ecosystems. This suggests that the salinity gradient is the most influential parameter of estuaries belonging to different biogeographical regions, climatic conditions and diverse macrobenthic species composition. Majority of estuaries along the NWI coast were distinctly impacted to various extent by anthropogenic activities affecting the resident macrobenthos (Govindan et al., 1983; Shukla et al., 2013; Kale, 2017; Dias et al., 2018; Mulik et al., 2020c). It was also discerned from various studies that anthropogenic factors influenced macrobenthic assemblages mostly in the poly-mesohaline and oligohaline zones where the salinity was lower leading to what experts have termed as “Estuarine Paradox” (Elliott and Quintino, 2007). The role of both the natural and anthropogenic factors in determining the estuarine macrobenthic community structure have been documented in other tropical and temperate estuaries as well [Douro estuary, Spain, Mucha et al. (2003); Lima estuary, Portugal, Sousa et al. (2006); Odiel-Tinto estuary, Spain, Sánchez-Moyano et al. (2010); Cachoeira River estuary, Brazil, Ourives et al. (2011); Santos estuary, Brazil, Abessa et al. (2019)]. Sediment texture appeared to be the overwhelming consensual determinant parameter of macrobenthos structure in the subtidal zones along the NWI coast which resonated well with studies in other geographical zones too (Table 1). Sediment characteristics were found to govern the distribution pattern of polychaete taxon in different habitats, followed by some hydrological parameters. It was thus evident from Table 1 that both sedimentary and hydrodynamic parameters (with different subset of variables) have significant role in determining the diversity and distribution of polychaete community structure in different geographic locations.
Extinction rates are higher in tropical species as they are more susceptible for disturbance which is referred to as the “tropical biodiversity crisis” (Vamosi and Vamosi, 2008). Documenting species loss due to various stressors and comprehending the ramifications are daunting tasks by itself; the difficulty compounds in the absence of inventories of extant species. Declining biodiversity around urbanized zones like Mumbai was clearly evidenced by decline in gastropod species from 1893 (227 species) to 1996–1998 (67 species). Similarly, pelecypod species reduced from 199 species to 30 species during the same duration (Jaiswar and Kulkarni, 2005). Datta et al. (2010) reported that fewer species than those reported previously were observed in the rocky intertidal zones of Mumbai and attributed this loss partly to the anthropogenic activities. In the Mahim creek and Bay area, where previously 90 species of gastropods were recorded by Subrahmanyam et al. (1952), gastropods had completely disappeared as the areas became polluted due to indiscriminate disposal of industrial and domestic effluents leading sometimes to the development of hydrogen sulfide (Jaiswar and Kulkarni, 2005). Similar decline of species richness due to increasing pollution have been have been reported by Simboura et al. (1995). Such biodiversity losses may lead to loss of genetic diversity, functioning and sustainability of ecosystems in the long run (Battaglia et al., 1980; Danovaro et al., 2008).
The NWI coast has a marked monsoonal season which influences the abiotic parameters of the coastal waters and underlying sediments thereby influencing the macrobenthos (Alongi, 1989). Different distribution studies of macrobenthic fauna reviewed in this compendium have unequivocally stated that non-monsoonal months favored a more conducive environment for the macrobenthic diversity and functionality as compared to the monsoon season. Higher values of macrobenthic abundance, diversity and biomass were obtained during the non-monsoonal seasons in many studies (Pawar and Kulkarni, 2009; Sukumaran et al., 2014; Pawar et al., 2019; Mulik et al., 2020c, Takar et al., 2020). The pre and post-monsoon periods present a more stable environment that is suitable for macrobenthic organisms, already stressed from the natural variations in the environment. An exception to this general trend was visualized from the mangrove, rocky, sandy intertidal zones in the northern Gulf of Kachchh. Here the gastropod densities were higher during monsoon, which was contradictory to the general trend of lower macrobenthic standing stock during monsoon observed for the west coast of India. One reason could be the less precipitation occurring in the arid Kachchh area resulting in little or no fluctuation in salinity (Kardani et al., 2014). However, Vijapure et al. (2019a) have also observed lower macrobenthic abundances during monsoon in the coastal waters of GoK even though signatures of upwelling were not recorded here.
The extended work by Joydas and Damodaran (2014) reported Oxygen Minimum Zones (OMZ) at 200 m depth of entire western shelf of India that was more pronounced in the northern continental shelf edge. Decline in macrobenthic density, biomass, polychaete species richness and diversity with the dominance of species belonging to polychaete families like Spionidae, Cirratulidae, and Paraonidae were reported in the OMZ. Levin (2003) extensively studied macrobenthos from OMZ around the world that included Indian western shelf enclosing NWI coast. The investigation stated that the species residing in OMZ could survive in hypoxic environments through morphological and physiological adaptations for improved oxygen uptake. Besides the OMZ, seasonal hypoxic events have become a fact of concern worldwide (Briggs et al., 2017) that alter both the structure and function of benthic communities. The episodic event generally involves proliferation of soft bodied annelids, especially opportunistic polychaetes that can tolerate the extreme environmental conditions (Levin et al., 2009). Vijapure et al. (2019a) reported that the monsoonal coastal hypoxia had altered taxonomic and functional composition of polychaete assemblages along NWI coast. It was evident from the comparison presented in Table 2 that Polychaeta was the dominant macrobenthic group in the hypoxic settings described globally. The overview suggested that hypoxia tolerant polychaete species mainly belonged to the genera (Family) Paraprionospio (Spionidae) and Cossura (Cossuridae) that are morphologically and physiologically adapted to sustain low oxygen levels (Lamont and Gage, 2000; Levin, 2003).
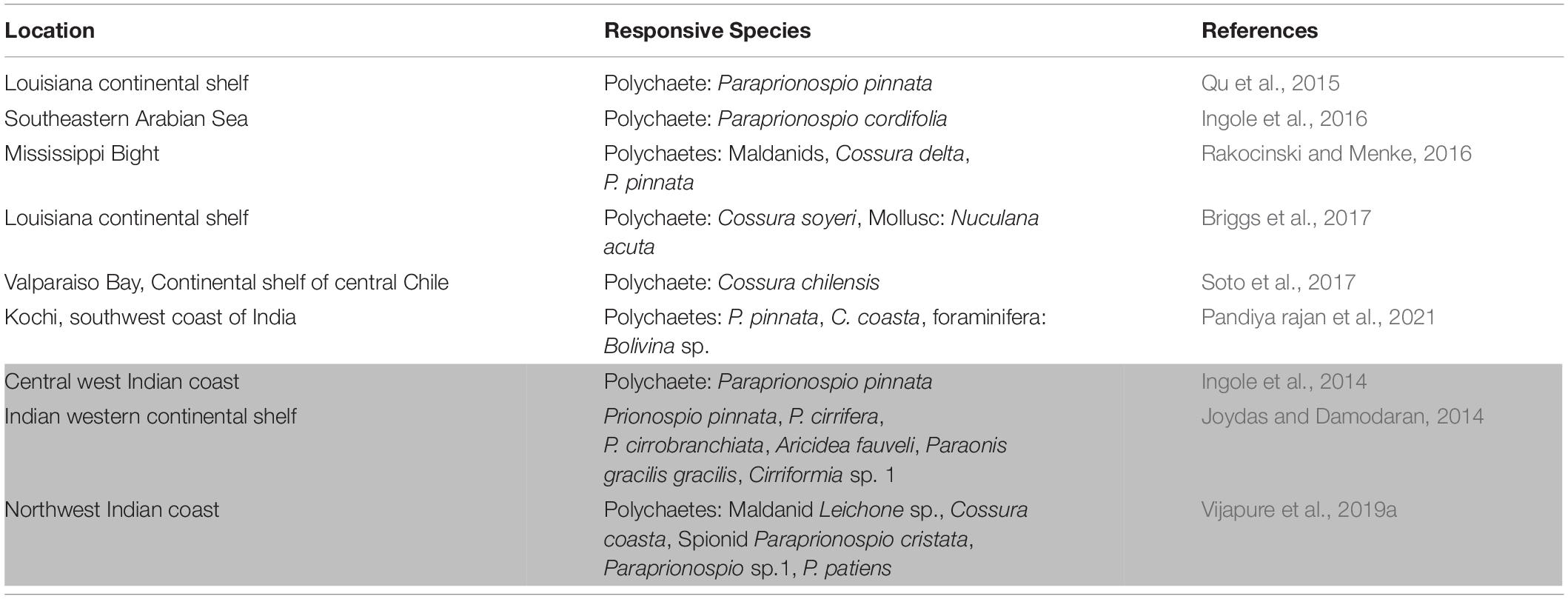
Table 2. Responsive species to hypoxia in different geographical settings (Shaded region indicates studies from NWI).
Several studies conducted in temperate zones have recommended the application of multiple indices to get a better assessment of the ecological status of estuaries considering that the macrobenthic communities are complex and geographically unlike (Salas et al., 2006; Kröncke and Reiss, 2010; Wetzel et al., 2012) and that indices were originally developed for particular stressors (Afli et al., 2008). However, the NWI studies as well as a number of other tropical estuaries have successfully applied AMBI for ecological quality status assessment (Borja and Tunberg, 2011; Valença and Santos, 2012; Brauko et al., 2015; Mulik et al., 2017, 2020a,b). The ecological assignments made available by European databases for the application of AMBI may not work in a tropical setting. Therefore, some of the Indian studies required the re-assignation of species to appropriate ecological groups based on expert judgment and knowledge of study area as recommended by Teixeira et al., 2010. In the Ulhas estuary, the opportunistic species, Streblospio gynobranchiata was classified to EG V from EG IV (Mulik et al., 2017, 2020a,b). In another study from the Mandovi and Zuari estuaries, Magelona sp. (II), Protodorvillea sp.(II), and Staurocephalus sp. (IV) were considered as EG III (Sivadas et al., 2016) so as to improve the sensitivity of AMBI. The tropical estuaries along the NWI are generally affected by heavy urbanization and industrialization (Dias et al., 2018; Mulik et al., 2020c). Hence, it becomes challenging to derive reference conditions for these estuaries because of heterogeneous sediment unavailability of sites with minimal impact, lack of historical data and absence of numerical models (Feebarani et al., 2016; Sivadas et al., 2016). Considering the above mentioned criteria, in the estuaries of NWI, the reference conditions are set by increasing the highest diversity and richness to 15% for each salinity zone as a preventive approach for the reliable use of the M-AMBI index (Borja et al., 2012).
Gaps and Limitations
It is well known that intertidal habitats provide the most heterogeneous habitats than any other realm and thus supports high species diversity. Investigations into the intertidal macrobenthic diversity were plentiful and more intensive than other habitats possibly due to the ease of access to the study areas, higher species diversity and also lower costs involved in sampling. Of the 1,113 species reported from the intertidal zones, 438 belonged to Gastropoda, 140 to Polychaeta, 105 to Bivalvia, whereas no species records were available for groups like tanaidaceans, isopods, etc. Thus it was concluded that while the expertise of macrobenthic taxonomy was limited to some major groups, groups like nematodes, cumaceans, sergestids, mysids, tanaidaceans, and sipunculids were largely ignored. This could be due to the paucity of taxonomical experts and available taxonomical keys. Also, it is possible that groups like gastropods and bivalves were more abundant, visibly sized and therefore easier to collect. Many of the molluscan species were identified from empty shells too.
It was also observed that the intensity and type of sampling and the availability of taxonomical keys could significantly increase the species diversity statistics. For example, in the comparatively unpolluted rocky beach at Colaba in Mumbai, Sukumaran et al. (2014) identified 70 macrobenthic taxa. The previous study by Datta et al. (2010) reported 48 species, however in this account only the epifauna were accounted for and the identification of many groups were not resolved upto species. These kinds of disparities make any logical long term comparison and evaluation of macrobenthic diversity redundant.
Contrary to the easily accessible intertidal regions, assessment of subtidal regions along the coast and offshore habitats requires specialized logistical support thus making it an expensive task (Fitch and Crowe, 2010). Majority of the efforts were made by the Council of Scientific and Industrial Research-National Institute of Oceanography (CSIR-NIO) as a leading organization to undertake this challenge along with few other regional research/educational institutes. Due to the requirement of extensive and exhaustive sampling efforts, many of these studies have relied on one time sampling. However, this has lead to data lacunae of seasonal fluctuations of benthic structural and functional diversity patterns. As the NWI region is subject to upwelling related hypoxia during monsoon, it is important to capture the macrobenthic response during this characteristic phenomenon.
Many of the studies investigating macrobenthos in the NWI coast have been concentrated around certain regions, whereas large tracts remain to be investigated. Few places than others have attracted more studies i.e., Malvan, Ratnagiri Mumbai, Uran, Sikka-Vadinar etc. It appears that areas subject to severe pollution on account of increasing urbanization like Mumbai and areas of high biodiversity like the Malvan Marine Sanctuary and the GoK Marine National Park have been studied more frequently than other locations. Also, the proximity of a coastal zone to biological research/educational institutions probably encouraged more studies. For example, Mumbai and Uran studies were mostly undertaken by scientific personnel belonging to research/educational institutions present in the city. The macrobenthic ecology of coastal zones between Malvan and Ratnagiri, Ratnagiri and Uran, Mumbai to Saurashtra, Gulf of Khambhat, inner and northern shores of Gulf of Kachchh remain mostly unknown.
The estuarine macrobenthos are very much under surveyed, which is surprising considering that the estuaries of the NWI zone have industrial and urban clusters along its banks and receives most of the disposals of these establishments. Some sort of information on macrobenthos is available from 17 estuaries out of 43 major estuaries along the NWI. However, detailed macrobenthic species level data are available only for four major estuaries (Ulhas, Amba, Savitri, and Kundalika) along the Maharashtra coast. In tropical ecosystems, there is still an urgent need to identify consistent spatio-temporal patterns and influencing parameters of estuarine benthic assemblages in order to understand the ecological processes which in itself is a major challenge in ecology. It was also observed that the studies dealing with macrobenthic assemblages in estuarine systems often sample pockets from the entire estuary, making it difficult to understand the broad general patterns. Estuarine organisms are frequently confined to distinct zones of salinity gradients, resulting in clear distribution patterns (Wolff, 1983). Hence, it is of utmost importance to know the distribution pattern of macrobenthos along the entire salinity gradient of the estuary to understand the general functioning of these ecosystems. Only few studies on the complex interaction between chemical parameters, sediment dynamics and hydrodynamics in controlling the estuarine macrobenthic distribution patterns of NWI have been undertaken thus far.
Despite the Indian coast being rich in macrobenthic diversity, only a handful of molecular studies have been carried so far. The contribution of sequence depositions from multiple loci of Indian macrobenthic species at GenBank is meager. Thus, there exists a pressing need than ever before, to widen the taxonomic coverage and capture the information in the genetic database. It is important to add molecular tools like DNA barcoding and metabarcoding techniques along with traditional microscopy for improving accuracy and speed of species analyses. Toward this, a well- equipped library of DNA barcodes needs to be constructed for the NWI coast. The literature from NWI region suggests that DNA metabarcoding approach has not been implemented along this region so far. Hence, the sensitivity and breadth of the biodiversity assessment should be revitalized with advanced, sensitive and cost effective, high throughput DNA sequencing technique like metabarcoding in future. From the literature, it was also evident that very few biofouling studies have been undertaken in the past in the area. Ignoring problems caused by biofoulers and invasive introductions could pose a threat to harbors and ports with potentially unending tide of invaders. Hence, these studies need to be undertaken in future with adaptation of advanced molecular techniques as timely detection of biofoulers will allow us to apply preventive measures to stop the aggregation of macrofoulers.
From the literature, it was apparent that many macrofaunal groups from the NWI coast still remain understudied and therefore the marine biodiversity of India is presently under-reported (Venkataraman and Wafar, 2005). In order to improve this state, awareness for taxonomic studies in India requires to be cultivated in the research communities. Building taxonomic capacity at national, regional and sub-regional levels with relevant faunal identification guides and manuals in both printed as well as electronic versions is also a crucial measure. While putting efforts in compiling the database for the present study, we also faced problems in obtaining printed manuals that were not available readily online. These manuals are not only important for researchers focusing on a particular faunal group of interest but also for cultivating the taxonomy interest in contemporary generations of researchers. Unfortunately, these valuable literature are often conserved in institutional custody and unavailable to readers in the present era of digitization. Hence, it is important to facilitate the online availability of valuable old literature/manuals/identification keys/thesis to improve the ease of taxonomic research. While this account has tried to compile all topic related information available via the digital media, efforts were taken to obtain the hard copies of those manuscripts that were unavailable. A perusal of the literature review of many of the older papers made one conclude that a considerable amount of data was available in the form of Ph.D. thesis, majorly which were not available in a digital format. Therefore, such data which was not in a published form were left out in this compilation which may underestimate the number of species reported.
Tropics with warmer temperatures are known to boost early larval developments and hence shorter pelagic larval durations than the temperate regions (Álvarez-Noriega et al., 2020). However, the century is facing a rapid climate change where issues like global warming is not only altering the reproduction patterns, spawning time of benthic marine invertebrates (Bronstein et al., 2016) but also affecting their sensitive planktonic life stages (Nguyen et al., 2012). Hence, compilations such as this one will help researchers and ecologists to undertake studies on reproductive traits of already known marine invertebrate taxa of the ecoregion and recognize the alterations in the life history patterns and trace the changes in the future, if any, due to climatic events. Oceanographic processes also have a huge impact on larval dispersal including upwelling phenomenon (Bashevkin et al., 2020). It would be interesting to understand benthic larval ecology in upwelling regions along NWI coast that is not undertaken thus far.
A noteworthy lacunae would be the absence of long term consistent times series seasonal data which could be used to derive long term changes and trends in ecology due to the climate change and anthropogenic interventions. Even in areas like Mumbai, Malvan, GoK, etc., which have been studied more intensively, the sampling size and methodology, analytical, and statistical tools applied were varied in different studies making long term comparisons cumbersome and difficult. The most commonly used diversity index was (H’). However, some studies employed the base as log2 whereas others applied log10. Additionally, Shannon index have been calculated on various data formats i.e., species, genus, family abundances or even a mixture of various taxonomical levels resulting in problematic comparisons. The limitations imposed by discrete studies, such as those reviewed here, will have to be overcome by undertaking carefully planned improved observational studies at known biodiversity hotspots and also at areas previously not studied. In conjunction with observations on the macrobenthos, it is equally important to measure similar abiotic parameters using same standard methodologies so as to derive meaningful conclusions. Long -term monitoring studies are a must for evaluating changes in the ecosystem and ascribe reasons for these changes (anthropogenic vs climate change), test the efficacy of present biodiversity regulations, assign “biodiversity hot spot” to a region and to measure recovery processes from disturbances (Crowe et al., 2000; Hawkins et al., 2002).
Extensive monitoring studies with a broad geographic coverage require enormous resources like project funding, sampling effort and several man-hours to generate the species level data. Economic constraints have taken a toll on biomonitoring programs in several countries, despite their importance (Borja and Elliott, 2013). Likewise, there has been a lack of funding (that is likely to happen more than before due to the pandemic situation) from the Indian government to undertake large-scale environmental monitoring programs. This may limit the number of transects surveyed and hamper the sampling periodicity that will ultimately end up in data lacunae. A possible solution to this is to apply the Taxonomic Sufficiency (TS) approach that can effectively reduce the analytical man-hours and resultant monetary costs for species identification. However, extreme caution needs to be taken to avoid loss of information while employing TS (Fiori and Soares-Gomes, 2002; Bevilacqua et al., 2009). Dissimilar response of the constituents within higher taxa to environmental changes may also limit the applicability of TS (Bertasi et al., 2009). Despite the practical applicability of this approach, only two studies have employed TS approach in the Indian waters using macrobenthic polychaetes thus far (Joydas and Damodaran, 2013; Vijapure and Sukumaran, 2019). Both the TS studies indicated that generic as well as family level data could reliably detect the distribution patterns analogous to that of species, in spite of differences in sampling areas/depths, periodicity and influential data transformation types. Despite apprehensions of loss of information on biodiversity by several studies (Terlizzi et al., 2003; Giangrande et al., 2005), the TS worked successfully in this region due to consistent and sizable proportion of monotypic polychaete taxon and uniformity in responses of the constituents of higher polychaeta.
Of late, multivariate indices like AMBI and M-AMBI are considered to be more effective in evolving the ecological status of estuaries and coasts (Sivaraj et al., 2014; Sivadas et al., 2016, Mulik et al., 2017, 2020a; Dias et al., 2018) and are more reliable than the application of H’. Few studies have applied any BBI other than the Shannon Diversity index. For accurate environmental assessment it is important to apply a BBI which is impervious to seasonal perturbations, especially in coastal zones of NWI, where many authors have emphasized the deleterious effects of the monsoon on benthic species diversity and abundances. As taxonomical records keep updating, some species may be merged under a common species name. For example, Quadros et al. (2009) reported a decline in the abundance of polychaete species, Nereis glandicincta and Ceratonereis burmensis in the intertidal zones of the heavily polluted Thane creek. At present both species have been merged into a single species, Neanthes glandicincta on WoRMS. Further there are considerable alterations and updations to other species as well. Almost all studies referred in this review have dealt with either species/group diversity/species descriptions/distribution with some giving species checklists. Studies dealing with the molecular taxonomy, phylogenetics, benthic food web dynamics of macrobenthos are completely missing. Hence it would be beneficial if the next level of benthic ecological studies involved these omitted aspects of benthic research.
A good proportion of information for this review was extracted from non-Science Citation Index (SCI) journals particularly those pertaining to the intertidal zones (Figure 2C). Publishing in SCI journals may need to be encouraged so that the entire information is peer-reviewed in the best possible manner that can provide reliable baseline for future studies. Some of the studies considered for this review, while reporting high number of species have not included the checklist in their reports making it difficult for this compilation to add to the cumulative species list. Some authors have also included groups like seaweeds in their macrofaunal list. And finally, it is quite possible that the macrobenthic species of NWI may be vastly under reported due to lack of well planned surveys. The NWI coast, particularly has potential for new species occurrences (Vijapure et al., 2019a). Hence, there exists a scope for exploring the undescribed diversity with taxonomic as well as molecular efforts that will aid the future ecological studies effectively. As evident from the above compilation of literature, great effort needs to be put forward for improving taxonomic research in India.
Author Contributions
SS, TV, and JM contributed to the conception, design, collation and analyses of data, and preparation of the manuscript. HR contributed to the collation of data, data analysis, and preparation of table and figures. SS wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to the director, CSIR-NIO for facilitating the study. TV and JM are grateful to CSIR for awarding a Senior Research Fellowship that gave them the opportunity to carry out the present study. This is CSIR-NIO contribution no. 6776.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.671245/full#supplementary-material
References
Abercrombie, A. (1892). The common marine shells of Bombay shore. J. Bombay Nat. Hist. Soc. 8, 212–222.
Abessa, D. M. S., Rachid, B. R. F., Zaroni, L. P., Gasparro, M. R., and Pinto, Y. A. (2019). Natural factors and chemical contamination control the structure of macrobenthic communities in the Santos Estuarine System (SP. Brazil). Comm. Ecol. 20, 121–137. doi: 10.1556/168.2019.20.2.3
Adhavan, D., Kamboj, R. D., Chavdaand, D. V., and Bhalodi, M. M. (2014). Status of intertidal biodiversity of narara reef marine national park. Gulf of Kachchh, Gujarat. J. Mar. Biol Oceanogr. 3:2. doi: 10.4172/2324-8661.1000132
Afli, A., Ayari, R., and Zaabi, S. (2008). Ecological quality of some Tunisian coast and lagoon locations, by using benthic community parameters and biotic indices. Estuar. Coast. Shelf. Sci. 80, 269–280. doi: 10.1016/j.ecss.2008.08.010
Agravat, P. A., and Raval, J. V. (2019). Diversity of marine gastropod at four selected Saurashtra coast and its distribution along Veraval coast of Arabian Sea. Int. J. Sci. Basic Appl. Res. 9, 48–59. doi: 10.13140/RG.2.2.21856.51206
Alongi, D. M. (1989). Ecology of tropical soft-bottom benthos: a review with emphasis on emerging concepts. Revista de Biol. Trop. 37, 85–100.
Alongi, D. M. (1990). The ecology of tropical soft-bottom benthic ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 28, 381–496.
Álvarez-Noriega, M., Burgess, S. C., Byers, J. E., Pringle, J. M., and Wares, J. P. (2020). Global biogeography of marine dispersal potential. Nat. Ecol. Evol. 4, 1196–1203. doi: 10.1038/s41559-020-1238-y
Anandan, K., Varshney, P. K., Jaiswar, A. K., and Prakash, C. (2003). Macrobenthos of intertidal zone of Versova along the coast of Mumbai. J. Indian. Fish. Assoc. 30, 9–17.
Anilkumar, P. R. (2017). Macrobenthos of the North Western Continental Margin (200-1000m) of India with Special Reference to Polychaetes. Doctoral dissertation, Kochi: Cochin University of Science and Technology.
Ansari, Z. A., Parulekar, A. H., Harkantra, S. N., and Nair, A. (1977). Shallow water macrobenthos along the central west coast of India. Mahasagar 10, 123–127.
Apte, D., Bhave, V., and Parasharya, D. (2010). An annotated and illustrated checklist of the opisthobranch fauna of Gulf of Kutch, Gujarat, India with 21 new records for Gujarat and 13 new records for India: Part 1. J. Bombay Nat. Hist. Soc. 107, 14–23.
Apte, D., Bhave, V., Pitale, R., Nagale, P., Bhave, A., and Prabhu, S. (2014). Rapid Biodiversity Assessment of Ansure Creek, Rajapur, Ratnagiri, Maharashtra. CMPA Technical Series No. 47. New Delhi: Indo-German Biodiversity Programme, GIZ-India.
Athalye, R. P. (1988). Status of Macrobenthos in Detritus Food Chain of Thane Creek Near Thane City. Ph.D. thesis, Mumbai: University of Bombay, 197.
Athalye, R. P., and Gokhale, K. S. (1991). Heavy metals in the polychaete Lycastis ouanaryensis from Thane creek. India. Mar. Pollut. Bull. 22, 233–236. doi: 10.1016/0025326X(91)90916-G
Athalye, R. P., Patil, N. N., Borka, U., Quadros, G., and Somani, V. U. (2003). Study of Flora, Intertidal Macrobenthic Fauna and Fishery of Ulhas River Estuary and Thane Creek to Assess the Pollution Status and Decide Mitigative Strategy. Maharashtra: BN Bandodkar College of Science.
Athalye, R. P. (2013). “Biodiversity of Thane Creek,” in National Conference on Biodiversity: Status and Challenges in Conservation, (FAVEO: Karnataka), 9–14.
Awati, P. R., and Karandikar, K. R. (1948). Onchidium Verruculatum (Anatomy, Embryology and Bionomics). Zoological Memories. Bombay: University of Bombay, 1–53.
Balasaheb, K., Atul, B., Ashok, J., and Rahul, K. (2017). Present status of intertidal biodiversity in and around Mumbai (West Coast of India). Trans. Rev. Systematical Ecol. Res. 19, 61–70. doi: 10.1515/trser-2017-0006
Barnes, R. S. K., and Barnes, M. K. S. (2012). Shore height and differentials between macrobenthic assemblages in vegetated and unvegetated areas of an intertidal sandflat. Estuar. Coast. Shelf. Sci. 106, 112–120. doi: 10.1016/j.ecss.2012.05.011
Barros, F., de Carvalho, G. C., Costa, Y., and Hatje, V. (2012). Subtidal benthic macroinfaunalassemblages in tropical estuaries: generality amongst highly variable gradients. Mar. Environ. Res. 81, 43–52. doi: 10.1016/j.marenvres.2012.08.006
Bashevkin, S. M., Dibble, C. D., Dunn, R. P., Hollarsmith, J. A., Ng, G., and Satterthwaite, E. V. (2020). Larval dispersal in a changing ocean with an emphasis on upwelling regions. Ecosphere 11:e03015. doi: 10.1002/ecs2.3015
Battaglia, B., Bisol, P. M., and Rodino‘, E. (1980). Experimental studies on the genetic effects of the marine pollution. Helgola¨Nder Meeresunters 33, 587–595. doi: 10.1007/bf02414782
Baxi, K. D., Kundu, R. S., Beleem, I. B., Poriya, P. U., and Gohil, B. M. (2017). Diversity and distribution of marine gastropods (Mollusca) along the intertidal zone of ship breaking yard - Alang, Gujarat, India. Adv. Biores. 8, 51–59. doi: 10.15515/abr.0976-4585.8.4.5159
Bertasi, F., Colangelo, M. A., Colosio, F., Gregorio, G., and Abbiati, M. (2009). Comparing efficacy of different taxonomic resolutions and surrogates in detecting changes in soft bottom assemblages due to coastal defence structures. Mar. Pollut. Bull. 58, 686–694. doi: 10.1016/j.marpolbul.2009.01.003
Bevilacqua, S., Fraschetti, S., Musco, L., and Terlizzi, A. (2009). Taxonomic sufficiency in the detection of natural and human-induced changes in marine assemblages: a comparison of habitats and taxonomic groups. Mar. Pollut. Bull. 58, 1850–1859. doi: 10.1016/j.marpolbul.2009.07.018
Bhatt, Y. M. (1959). A Study of Intertidal Organisms of Bombay. PhD thesis, Bombay: University of Bombay.
Bigot, L., Grémare, A., Amouroux, J. M., Frouin, P., Maire, O., and Gaertner, J. C. (2008). Assessment of the ecological quality status of soft-bottoms in Reunion Island (tropical Southwest Indian Ocean) using AZTI marine biotic indices. Mar. Pollut. Bull. 56, 704–722. doi: 10.1016/j.marpolbul.2007.12.020
Blake, J. A. (1996). “Chapter 4. family spionidae grube, 1850,” in Taxonomic Atlas of the Santa Maria Basin and Western Santa Barbara Channel, eds J. A. Blake, B. Hilbig, and P. H. Scott, 81–223.
Borja, A., Basset, A., Bricker, S., Dauvin, J. C., Elliot, M., and Harrison, T. (2012). Classifying ecological quality and integrity of estuaries. Treatise Estuarine Coastal Sci. 2012, 125–162. doi: 10.1016/B978-0-12-374711-2.00109-1
Borja, A., and Elliott, M. (2013). Marine monitoring during an economic crisis: the cure is worse than the disease. Mar. Pollut. Bull. 68, 1–3. doi: 10.1016/j.marpolbul.2013.01.041
Borja, A., and Tunberg, B. G. (2011). Assessing benthic health in stressed subtropical estuaries, eastern Florida, USA using AMBI and M-AMBI. Ecol. Indic. 11, 295–303. doi: 10.1016/j.ecolind.2010.05.007
Brasier, M. J., Wiklund, H., Neal, L., Jeffreys, R., and Linse, K. (2016). DNA barcoding uncovers cryptic diversity in 50% of deep-sea Antarctic polychaetes. R. Soc. Open Sci. 3:160432. doi: 10.1098/rsos.160432
Brauko, K. M., Souza, F. M., Muniz, P., Camargo, M. G., and Lana, P. C. (2015). Spatial variability of three benthic indices for marine quality assessment in a subtropical estuary ofSouthern Brazil. Mar. Pollut. Bull. 91, 454–460. doi: 10.1016/j.marpolbul.2014.10.025
Briggs, K. B., Craig, J. K., Shivarudrappa, S., and Richards, T. M. (2017). Macrobenthos and megabenthos responses to long-term, large-scale hypoxia on the Louisiana continental shelf. Mar. Environ. Res. 123, 38–52. doi: 10.1016/j.marenvres.2016.11.008
Bronstein, O., Kroh, A., and Loya, Y. (2016). Reproduction of the long-spined sea urchin Diademasetosum in the Gulf of Aqaba-implications for the use of gonad-indexes. Sci. Rep. 6:29569. doi: 10.1038/srep29569
Chapman, M. G., and Tolhurst, T. J. (2004). The relationship between invertebrate assemblages and bio-dependant properties of sediment in urbanized temperate mangrove forests. J. Exp. Mar. Biol. Ecol. 304, 51–73. doi: 10.1016/j.jembe.2003.11.019
Chaudhari, B., Poriya, P., and Kundu, R. (2016). Community structure of the honeycomb worm neosabellaria clandestinus from a biogenic reef of Gujarat Coast. India. Int. J. Ecol. Environ. Sci. 42, 333–340.
Çinar, M. E., and Dagli, E. (2013). Polychaetes (Annelida: Polychaeta) from the Aegean and Levantine coasts of Turkey, with descriptions of two new species. J. Nat. Hist. 47, 911–947. doi: 10.1080/00222933.2012.752543
Crowe, T. P., Thompson, R. C., Bray, S., and Hawkins, S. J. (2000). Impacts of anthropogenic stress on rocky intertidal communities. J. Aquatic Ecosystem Stress Recovery 7, 273–297.
Curry, C. J., Gibson, J. F., Shokralla, S., Hajibabaei, M., and Baird, D. J. (2018). Identifying North American freshwater invertebrates using DNA barcodes: are existing COI sequence libraries fit for purpose? Freshw. Sci. 37, 178–189. doi: 10.1086/696613
Dandan, R. T., and Diocton, R. C. (2019). The quality of intertidal sediments and biodiversity of macrobenthic invertebrates. Aloha Int. J. Multidisciplinary Adv. (AIJMU) 1, 76–81.
Dange, S. P. (2018). Studies on the Benthic Communities of Riverine and Estuarine Zone of Purna River, Navsari Gujarat. Ph. D. thesis, Veer Narmad South Gujarat University.
Danovaro, R., Gambi, C., Dell’Anno, A., Corinaldesi, C., Fraschetti, S., Vanreusel, A., et al. (2008). Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr. Biol. 18, 1–8. doi: 10.1016/j.cub.2007.11.056
Datta, S. N., Chakraborty, S. K., Jaiswar, A. K., and Ziauddin, G. (2010). A comparative study on intertidal faunal biodiversity of selected beaches of Mumbai coast. J. Environ. Biol. 31, 981–986.
Dauvin, J. C., Alizier, S., Rolet, C., Bakalem, A., and Bellan, G. (2012). Response of different benthic indicesto diverse human pressures. Ecol. Indic. 12, 143–153. doi: 10.1016/j.ecolind.2011.03.019
Davidson, I. C., Crook, A. C., and Barnes, D. K. A. (2004). Macrobenthic migration and its influence on the intertidal diversity dynamics of a meso-tidal system. Mar. Biol. 145, 833–842. doi: 10.1007/s00227-004-1373-z
Degraer, S., Mouton, I., De Neve, L., and Vincx, M. (1999). Community structure and intertidal zonation of the macrobenthos on a macrotidal, ultra-dissipative sandy beach: summer-winter comparsion. Estuaries 22, 742–752. doi: 10.2307/1353107
Dias, H. Q., Sukumaran, S., Srinivas, T., and Mulik, J. (2018). Ecological quality status evaluation of a monsoonal tropical estuary using benthic indices: comparison via a seasonal approach. Environ. Sci. Pollut. 25, 22672–22688. doi: 10.1007/s11356-018-2344-0
Dix, T. L., Karlen, D. J., Grabe, S. A., Goetting, B. K., Holden, C. M., and Markham, S. E. (2004). “Spionid polychaetes as environmental indicators: an example from Tampa Bay, Florida,” in Estuarine Indicators, ed. S. A. Bortone (Boca Raton, FL: CRC Press), 299–318.
Dong, Y. W., Huang, X. W., Wang, W., Li, Y., and Wang, J. (2016). The marine ‘great wall’of China: local-and broad-scale ecological impacts of coastal infrastructure on intertidal macrobenthic communities. Divers. Distrib. 22, 731–744. doi: 10.1111/ddi.12443
Elliott, M., and Quintino, V. (2007). The Estuarine quality Paradox, environmental homeostasisand the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 54, 640–645. doi: 10.1016/j.marpolbul.2007.02.003
Fauchald, K. (1977). The polychaete worms, definitions and keys to the orders, families and genera. Nat. History Museum Los Angeles County Sci. Series 28, 1–190. doi: 10.2307/3239967
Fauvelot, C., Zuccon, D., Borsa, P., Grulois, D., Magalon, H., and Riquet, F. (2020). Phylogeographical patterns and a cryptic species provide new insights into Western Indian Ocean giant clams phylogenetic relationships and colonization history. J. Biogeogr. 47, 1086–1105. doi: 10.1111/jbi.13797
Feebarani, J., Joydas, T. V., Damodaran, R., and Borja, A. (2016). Benthic quality assessment in a naturally-and human-stressed tropical estuary. Ecol. Indic. 67, 380–390. doi: 10.1016/j.ecolind.2016.03.005
Field, C. B., Behrenfeld, M. J., Randerson, J. T., and Falkowski, P. (1998). Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. doi: 10.1126/science.281.5374.237
Fiori, C. D. S., and Soares-Gomes, A. (2002). “Taxonomic sufficiency for a monitoring program in a tropical continental shelf, Rio de Janeiro, Brazil,” in Oil and Hydrocarbon Spills III. Modelling, Analysis and Control, ed. C. A. Brebbia (London: Wessex Institute of Technology).
Fitch, J. E., and Crowe, T. P. (2010). Effective methods for assessing ecological quality in intertidal soft-sediment habitats. Mar. Pollut. Bull. 60:10. doi: 10.1016/j.marpolbul.2010.06.027
Gadhavi, M. K. (2015). Studies on Estuarine Brachyuran Crabs: Behavioural Ecology and Ecotoxicology. Doctoral dissertation, Gujarat: MS University of Baroda.
Gadhavi, M. K., and Vachhrajani, K. D. (2015). Macrobenthos interactions and their distribution on lower macrobenthos interactions and their distribution on lower estuarine mudflats of Mahi river. Gujarat, India. J. Mar. Biol. Oceanogr. 6:3. doi: 10.4172/2324-8661.1000180
Gaikwad, U. D., and Ranade, M. R. (1979). Study of the marine fauna of Ratnagiri (West coast, India). II- Polychaeta (Annelida). Bull. Fish. Konkan Agric. Univ. Indi. 1, 45–51.
Gajbhiye, S. N., Mustafa, S., Mehta, P., and Nair, V. R. (1995). Assessment of biological characteristics on coastal environment of Murud (Maharashtra) during the oil spill (17 May 1993). Indian J. Mar. Sci. 24, 196–202.
Gaonkar, C. A., Sawant, S. S., Anil, A. C., Krishnamurthy, V., and Harkantra, S. N. (2010a). Changes in the occurrance of hard substratum fauna: a case study from Mumbai harbour. India. Indian J. Mar. Sci. 39, 74–84.
Gaonkar, C. A., Sawant, S. S., Anil, A. C., Venkat, K., and Harkantra, S. N. (2010b). Mumbai harbour, India: gateway for introduction of marine organisms. Environ. Monit. Assess. 163, 583–589. doi: 10.1007/s10661-009-0860-6
Gayton, D. V. (2008). Impacts of climate change on British Columbia’s biodiversity: a literature review. Extended Abstract. BCJ. Ecosyst. Manag. 9, 26–30.
Giangrande, A., Licciano, M., and Musco, L. (2005). Polychaetes as environmental indicators revisited. Mar. Pollut. Bull. 50, 1153–1162. doi: 10.1016/j.marpolbul.2005.08.003
Gokhale, K. S., and Athalye, R. P. (1995). Study of Impact of Pollution on Mangrove Fauna of Thane Creek Near Thane City. New Delhi: Report submitted to the Ministry of Environment and Forests, 40.
Govindan, K., Varshney, P. K., and Desai, B. N. (1983). Benthic studies in South Gujarat estuaries. Mahasagar 16, 349–356.
Govindan, K., Kasinathan, R., and Desai, B. N. (1976). Qualitative studies on macrobenthic fauna in the polluted Thana creek and Bombay harbour. J. Indian Fish Assoc. 6, 127–139
Gray, J. S. (1997). Marine biodiversity: patterns, threats and conservation needs. Biodivers. Conserv. 6, 153–175.
Griffiths, J. R., Kadin, M., Nascimento, F. J. A., Tamelander, T., Törnroos, A., and Bonaglia, S. (2017). The importance of benthic-pelagic coupling for marine ecosystem functioning in a changing world. Glob. Chang. Biol. 23, 2179–2196. doi: 10.1111/gcb.13642
Hampel, H., Elliott, M., and Cattrijsse, A. (2009). Macrofaunal communities in the habitats ofintertidal marshes along the salinity gradient of the Schelde estuary. Estuar. Coast. Shelf Sci. 84, 45–53. doi: 10.1016/j.ecss.2009.05.029
Harkantra, S. N., Nair, A., Ansari, Z. A., and Parulekar, A. H. (1980). Benthos of the shelf region along the west coast of India. Indian J. Mar. Sci. 9, 106–110.
Harrison, P. A., Berry, P. M., Simpson, G., Haslett, J. R., Blicharska, M., and Bucur, M. (2014). Linkages between biodiversity attributes and ecosystem services: a systematic review. Ecosyst. Serv. 9, 191–203. doi: 10.1016/j.ecoser.2014.05.006
Hawkins, S. J., Gibbs, P. E., Pope, N. D., Burt, G. R., Chesman, B. S., Bray, S., et al. (2002). Recovery of polluted ecosystems: the case for long-term studies. Mar. Environ. Res. 54, 215–222. doi: 10.1016/S0141-1136(02)00117-4
Hernández-Alcántara, P., Cuéllar-Mercado, D. M., Barbosa-López, A., and Solís-Weiss, V. (2017). Spatial patterns of species richness and taxonomic composition of polychaetes along the Baja California Peninsula. Eastern Pacific. J. Mar. Biolog. Assoc. U.K 97, 1037–1049. doi: 10.1017/s0025315417000893
Hillebrand, H. (2004). Strength, slope and variability of marine latitudinal gradients. Mar. Ecol. Prog. Ser. 273, 251–267. doi: 10.3354/meps273251
Hornell, J. (1949). The study of Indian molluscs. J. Bombay Nat. Hist. Soc. 48, 1–34. doi: 10.1002/9781119045212.ch1
Ibanez-Erquiaga, B., Pacheco, A. S., Rivadeneira, M. M., and Tejada, C. L. (2018). Biogeographical zonation of rocky intertidal communities along the coast of Peru (3.5–13.5 S Southeast Pacific). PLoS One 13:e0208244. doi: 10.1371/journal.pone.0208244
Inglis, G. J., and Kross, J. E. (2000). Evidence for systemic changes in the benthic fauna of tropical estuaries as a result of urbanization. Mar. Pollut. Bull. 41, 367–376. doi: 10.1016/S0025-326X(00)00093-X
Ingole, B., Rodrigues, N., and Ali Ansari, Z. (2002). Macrobenthic communities of the coastal waters of Dabhol, west coast of India. Indian J. Mar. Sci. 31, 93–99.
Ingole, B., Sivadas, S., Nanajkar, M., Sautya, S., and Nag, A. (2009). A comparative study of macrobenthic community from harbours along the central west coast of India. Environ. Monit. Assess. 154, 135–146. doi: 10.1007/s10661-008-0384-5
Ingole, B. S., Gaonkar, U. V., Deshmukh, A., Mukherjee, I., Sanitha, K., and Gophane, A. (2014). Macrobenthic community structure of coastal Arabian Sea during the Fall intermonsoon. Indian J. Mar. Sci. 43, 2223–2232.
Ingole, B. S., Rengaiyan, P., and De, K. (2016). Macrobenthic community structure response to coastal hypoxia off Southeastern Arabian Sea. J. Coast. Zone Manag. 19:436. doi: 10.4172/2473-3350.1000436
Jaiswar, A. K., and Kulkarni, B. G. (2005). Conservation of molluscan biodiversity from intertidal area of Mumbai coast. J. Nat. Conserv. 17, 93–105.
Jaleel, K. A. (2012). Macrobenthos of the Continental Margin (200-1000m) of South Eastern Arabian Sea with Special Reference to Polychaetes. Ph.D thesis, Kerala: Cochin University of Science and Technology.
Jayaraj, K. A., Jayalakshmi, K. V., and Saraladevi, K. (2007). Influence of environmental properties on macrobenthos in the northwest Indian shelf. Environ. Monit. Assess. 127, 459–475. doi: 10.1007/s10661-006-9295-9295
Jayaraj, K. A., Josia, J., and Kumar, P. K. (2008). Infaunal macrobenthic community of soft bottom sediment in a tropical shelf. J. Coast. Res. 24, 708–718. doi: 10.2112/06-0790.1
Jhala, D., Munjpara, S. B., Joshi, J., and Joshi, K. (2017). Spatial distribution of intertidal hermit crab (Decapoda: Anomura) together with gastropod shell availability and utilization pattern on the Southern Coast of Gulf of Kachchh. Gujarat. India. Indian J. Life Sci. 6, 77–85.
Jose, K. V. (1964). The morphology of Acanthobonellia pirotanensis n. sp., a bonellid from the Gulf of Kutch, India. J. Morphol. 115, 53–68. doi: 10.1002/jmor.1051150105
Joseph, S., Poriya, P., and Kundu, R. (2016a). Probing the phylogenetic relationships of a few newly recorded intertidal zoanthids of Gujarat coast (India) with mt DNA COI sequences. Mitochondrial DNA Part A 27, 3858–3864. doi: 10.3109/19401736.2014.971239
Joseph, S., Vakani, B., and Kundu, R. (2016b). Molecular phylogenetic study on few morphotypes of a patellogastropod Cellana karachiensis from northern Arabian Sea reveals unexpected genetic diversity. Mitochondrial DNA Part A: DNA Mapping, Sequencing Anal. 29, 181–191. doi: 10.1080/24701394.2016.1261854
Joseph, S., Poriya, P., Vakani, B., Singh, S. P., and Kundu, R. (2014). Identification of a group of cryptic marine limpet species, Cellana karachiensis (Mollusca: Patellogastropoda) off Veraval coast, India, using mtDNA COI sequencing. Mitochondrial DNA 27, 1328–1331. doi: 10.3109/19401736.2014.945577
Joydas, T. V., and Damodaran, R. (2009). Infaunal macrobenthos along the shelf waters of the west coast of India. Arabian Sea. Indian J. Mar. Sci. 38, 191–204.
Joydas, T. V., and Damodaran, R. (2013). Testing depth-related multivariate patterns of macrofauna on the Indian continental shelf using reduced taxonomic resolution and data transformation. J. Mar. Biolog. Assoc. U.K 93, 37–45. doi: 10.1017/S0025315411001858
Joydas, T. V., and Damodaran, R. (2014). Infaunal macrobenthos of the oxygen minimum zone on the Indian western continental shelf. Mar. Ecol. 35, 22–35. doi: 10.1111/maec.12052
Joydas, T. V., Jayalakshmy, K. V., and Damodaran, R. (2009). Polychaete community structure of Indian west coast shelf, Arabian Sea. Curr. Sci. 97, 634–636.
Kachhiya, P., Raval, J., Poriya, P., and Kundu, R. (2017). Diversity and new records of intertidal hermit crabs of the genus Clibanarius (Crustacea: Decapoda: Diogenidae) from Gujarat coast off the northern Arabian Sea, with two new records for the mainland Indian coastline. J. Threatened Taxa 9, 10334–10339. doi: 10.11609/jott.2268.9.6.10334-10339
Kale, P. G. (2017). Application of benthic foraminifera to determine ecological status of Panvel Creek and adjoining River Estuaries. Int. J. Life Sci. 5, 269–276.
Kamboj, R. D. (2014). Conservation initiatives for coral reef ecosystem in Marine National Park, Gulf of Kachchh, Gujarat, India. Tigerpaper 41, 1–11. doi: 10.15436/2381-0750.15.006
Kantharajan, G., Pandey, P. K., Krishnan, P., Deepak Samuel, V., Bharti, V. S., and Purvaja, R. (2017). Molluscan diversity in the mangrove ecosystem of Mumbai, west coast of India. Reg. Stud. Mar. Sci. 14, 102–111. doi: 10.1016/j.rsma.2017.06.002
Kardani, H. K., Mankodi, P. C., and Thivakaran, G. A. (2014). Diversity and distribution of gastropods of intertidal region of northern gulf of Kachchh, Gujarat, India. Ecol. Environ. Conserv. 20, 105–110.
Karhale, S. M., Adsul, A. D., Indulkar, S. T., and Pai, R. (2018). Seasonal variation in polychaetes of intertidal mangrove area of Shirgaon, Ratnagiri, Maharashtra, India. Int. J. Life Sci. 6, 156–160.
Kasinathan, R., Govindan, K., and Desai, B. N. (1974). Distribution of Benthic fauna in the Gulf of Kachchh. J. Indian. Fish. Assoc. 3, 68–79
Kawauchi, G. Y., and Giribet, G. (2014). Sipunculus nudus Linnaeus, 1766 (Sipuncula): cosmopolitan or a group of pseudo-cryptic species? An integrated molecular and morphological approach. Mar. Ecol. 35, 478–491. doi: 10.1111/maec.12104
Kelehar, B. P., Underwood, A. J., and Chapman, M. G. (1998). Effect of boardwalks on the semaphore crab Heloecius cordiformis in temperate urban mangrove forests. J. Exp. Mar. Biol. Ecol. 227, 281–300. doi: 10.1016/S0022-0981(97)00276-1
Khan, S. A., and Murugesan, P. (2005). Polychaete diversity in Indian estuaries. J. Mar. Sci. 34, 114–119.
Khot, M., Jaiswar, A. K., and Ng, P. K. L. (2019). The taxonomy of two species of xenophthalmidae from maharashtra, india, and the generic placement of xenophthalmus garthii sankarankutty, 1969 (Decapoda, Brachyura). Crustaceana 92, 1337–1348. doi: 10.1163/15685403-00003947
Khot, M., Jaiswar, A. K., and Chakraborty, S. K. (2016a). “Biodiversity of brachyurans around Mumbai and Jaitapur coast,” in Proceedings of the International Conference On Aquatic Resources & Sustainable Management. Maharashtra
Khot, M., Jaiswar, A. K., and Pawase, A. (2016b). First record of ozius tuberculosus h. milne edwards (Crustacea: Decapoda: Brachyura) from maharashtra, West coast of india, with notes on taxonomy. Biosystematica 10, 33–37.
Konar, B., Iken, K., Pohle, G., Miloslavich, P., Cruz-Motta, J. J., Benedetti-Cecchi, et al. (2010). Surveying Nearshore Biodiversity. Chichester: Wiley-Blackwell, 27–41. doi: 10.1002/9781444325508.ch2
Kröncke, I., and Reiss, H. (2010). Influence of macrofauna long-term natural variability on benthic indices used in ecological quality assessment. Mar. Pollut. Bull. 60, 58–68. doi: 10.1016/j.marpolbul.2009.09.001
Kubal, P., Sukumaran, S., and Vijapure, T. (2012). Record of bi-operculate fouling serpulid polychaete, Hydroides operculatus (Treadwell, 1929) from Diu, west coast of India. Indian J. Mar. Sci. 41, 381–384.
Kubal, P. V., Ambekara, A. A., Prakasha, C., and Sawanta, P. B. (2016). Spatial and seasonal variation of macrobenthos diversity around Nuclear Power Plant site, Tarapur, Maharashtra. J. Bio. Env. Sci. 8, 77–87.
Kulkarni, V. A., Jagtap, T. G., Mhalsekar, N. M., and Naik, A. N. (2010). Biological and environmental characteristics of mangrove habitats from Manori creek, West Coast, India. Environ. Monit. Assess 168, 587–596. doi: 10.1007/s10661-009-1136-x
Kulkarni, V. A., Naidu, V. S., and Jagtap, T. G. (2011). Marine ecological habitat: a case study on projected thermal power plant around Dharamtar creek, India. J. Environ. Biol. 32, 213–219.
Kundu, R. (2001). Ecological status survey of the intertidal macrofauna of the Sikka and Vadinar coast off Gulf of Kachchh, India. Otsuchi Mar. Sci. J. 26, 66–71.
Kurhe, A. R., Mane, U. H., and Pawar, B. A. (2009). Studies on marine gastropod molluscs from ratnagiri coast Maharashtra, India. J. Exp. Zool. 12, 409–414.
Kurhe, A. R., Rodrìguez, M. A., and Suryawanshi, G. (2014). Vertical distribution and diversity of gastrpods molluscs from intertidal habitats of the Ratnagiri Coast Maharashtra, India. Int. Res. J. Nat. Appl. Sci. 1, 1–13.
Kurve, P., and Kurve, N. K. (2010). Occurrence of macrofauna from intertidal zone along the coastline of village Akshi, District Raigad, Maharashtra. Ecoscan 4, 2–3.
Lamont, P. A., and Gage, J. D. (2000). Morphological responses of macrobenthic polychaetes to low oxygen on the Oman continental slope, NW Arabian Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 47, 9–24. doi: 10.1016/s0967-0645(99)00102-2
Levin, L. A. (2003). Oxygen minimum zone benthos: adaptation and community response to hypoxia. Oceanogr. Marine Biol. Annu. Rev. 41, 1–45.
Levin, L. A., Ekau, W., Gooday, A. J., Jorissen, F., Middelburg, J. J., and Naqvi, S. W. A. (2009). Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6, 2063–2098. doi: 10.5194/bg-6-2063-2009
Liuzzi, M. G., and Gappa, J. L. (2008). Macrofaunal assemblages associated with coralline turf: species turnover and changes in structure at different spatial scales. Mar. Ecol. Prog. Ser. 363, 147–156. doi: 10.3354/meps07449
Loreau, M., Naeem, S., Inchausti, P., Bengtsson, J., Grime, J. P., and Hector, A. (2001). Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. doi: 10.1126/science.1064088
Lui, T. H., Lee, S. Y., and Sadovy, Y. (2002). Macrobenthos of a tidal impoundment at the Mai Po marshes nature reserve, Hong Kong. Hydrobiologia 468, 193–212. doi: 10.1023/A:1015281114542
Magesh, M., Glasby, C. J., and Kvist, S. (2014). Redescription of namalycastis glasbyi fernando and Rajasekaran, 2007 (Annelida, Nereididae, Namanereidinae) from India. Proc. Biol. Soc. Wash. 127, 455–465. doi: 10.2988/0006-324X-127.3.455
Martins, R., Sampaio, L., Rodrigues, A. M., and Quintino, V. (2013). Softbottom Portuguese continental shelf polychaetes: diversity and distribution. J Mar Syst. 12, 41–54. doi: 10.1016/j.jmarsys.2013.04.008
Mathew, A., and Govindan, K. (1995). Macrobenthos in the near shore coastal system of Bombay. Proc. Natl. Acad. Sci. India A 65, 411–430.
Mawdsley, J. R., O’Malley, R., and Ojima, D. S. (2009). A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv. Biol. 23, 1080–1089. doi: 10.1111/j.1523-1739.2009.01264.x
Meetei, W. A., Bhakta, D., Kamble, S. P., Chanu, T. N., Koushlesh, S. K., Gogoi, P., et al. (2020). Macrobenthic invertebrates community structure of river Tapti, a westerly flowing river in peninsular India. J. Inland Fish. Soc. India 52:060. doi: 10.47780/jifsi.52.1.2020.106545
Melvill, J. C., and Abercrombie, A. (1893). The marine mollusca of Bombay. Mem. and Proc. Manchester. Let. Phil. Soc. Ser. 7, 17–51.
Melvill, J. C., and Standen, R. (1901). The mollusca of the persian gulf, Gulf of oman and arabian sea as evidenced mainly through the collections of F. W. Townsend, 1893-1900, with description of new species. Proc. Zool. Soc. 71, 327–460. doi: 10.1111/j.1469-7998.1901.tb08181.x
Menge, B. A., and Sutherland, J. P. (1987). Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am. Nat. 130, 730–757. doi: 10.1086/284741
Miralles, L., Ardura, A., Arias, A., Borrell, Y. J., and Clusa, L. (2016). Barcodes of marine invertebrates from north Iberian ports: native diversity and resistance to biological invasions. Mar. Pollut. Bull. 112, 183–188. doi: 10.1016/j.marpolbul.2016.08.022
Mirza, R., Viradiya, A., Salunke, S., and Padate, G. (2017). Report of four imperative marine macrobenthic fauna from the Gulf of Kachchh, Gujarat, India. J. Mar. Biol Oceanogr. 6:3.
Mirza, R., Viradiya, A., Salunke, S., Padate, G., and Ruzbeh Mirza, C. (2019). Some important sightings of marine fauna in Southern Gulf of Kachchh, Gujarat, India. J. Entomol. Zool. 7, 410–416.
Misra, S., and Kundu, R. (2005). Seasonal variations in population dynamics of key intertidal molluscs at two contrasting locations. Aquat. Ecol. 39, 315–324. doi: 10.1007/s10452-0051779-9
Mucha, A. P., Vasconcelos, M. T. S. D., and Bordalo, A. A. (2003). Macrobenthic community in the Douro estuary: relations with trace metals and natural sediment characteristics. Environ. Pollut. 121, 169–180. doi: 10.1016/S0269-7491(02)00229-4
Mukhopadhyay, A., Tripathy, B., Sajan, S., and Ghosh, A. (2019). Two new records of minute marine gastropods from Marine National Park, Gulf of Kachchh in India. Strombus 25, 5–9.
Mulik, J., Sukumaran, S., and Dias, H. Q. (2020a). Can the ecological status of three differentially impacted monsoonal tropical estuaries in NW India, be adequately assessed by a common estuarine benthic index? Ecol. Indic. 119:106807. doi: 10.1016/j.ecolind.2020.106807
Mulik, J., Sukumaran, S., and Dias, H. Q. (2020b). Is the benthic index AMBI impervious to seasonality and data transformations while evaluating the ecological status of an anthropized monsoonal estuary? Ocean Coast Manag. 186:105080. doi: 10.1016/j.ocecoaman.2019.105080
Mulik, J., Sukumaran, S., and Srinivas, T. (2020c). Factors structuring spatio-temporal dynamics of macrobenthic communities of three differently modified tropical estuaries. Mar. Pollut. Bull. 150:110767. doi: 10.1016/j.marpolbul.2019.110767
Mulik, J., Sukumaran, S., Srinivas, T., and Vijapure, T. (2017). Comparative efficacy of benthic biotic indices in assessing the Ecological Quality Status (EcoQS) of the stressed Ulhas estuary, India. Mar. Pollut. Bull. 120, 192–202. doi: 10.1016/j.marpolbul.2017.05.014
Muniz, P., Venturini, N., Pires-Vanin, A. M., Tommasi, L. R., and Borja, Á (2005). Testing the applicability of a Marine Biotic Index (AMBI) to assessing the ecological quality of soft-bottom benthic communities, in the South America Atlantic region. Mar. Pollut. Bull. 50, 624–637. doi: 10.1016/j.marpolbul.2005.01.006
Mutlu, E., Çinar, M. E., and Ergev, M. B. (2010). Distribution of soft-bottom polychaetes of the Levantine coast of Turkey, eastern Mediterranean Sea. J. Mar. Syst. 79, 23–35. doi: 10.1016/j.jmarsys.2009.06.003
Nagale, P., and Apte, D. (2013a). First record of the hydroid Eudendrium carneum (Cnidaria: Hydro-Aoa: Hydroidolina) in Indian waters. Mar. Biodivers. Rec. 6, 3–6. doi: 10.1017/S1755267213000547
Nagale, P., and Apte, D. (2013b). Some hydroids (Cnidaria: Hydrozoa: Hydroidolina) from the Konkan coast, Maharashtra, India. J. Threat. Taxa. 5, 4814–4818. doi: 10.11609/jott.o3484.4814-8
Nagale, P., and Apte, D. (2014). Intertidal hydroids (Cnidaria: Hydrozoa: Hydroidolina) from the Gulf of Kutch, Gujarat, India. Mar. Biodivers. Rec. 7, 1–9. doi: 10.11646/zootaxa.3171.1.1
Nair, V. R., Mustafa, S., Mehta, P., Govindan, K., Ram, M. J., and Gajbhiye, S. N. (1998). Biological characteristics of the Vashishti estuary, Maharashtra (west coast of India). Indian J. Mar. Sci. 27, 310–316.
Nair, V. (2002). Status of The Flora and Fauna of Gulf of Kachchh, India. Goa: National Institute of Oceanography Dona Paula.
Naqvi, S. W. A., Jayakumar, D. A., Narvekar, P. V., Naik, H., and Sarma, V. V. (2000). Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature 408, 346–349. doi: 10.1038/35042551
Naqvi, S. W. A., Naik, H., Jayakumar, A., Pratihary, A. K., Narvenkar, G., Kurian, S., et al. (2009). Seasonal anoxia over the western Indian continental shelf. Indian Ocean Biogeochem. Proc. Ecol. Variabil. Geophys. Monograph Ser. 185, 333–345. doi: 10.1029/2008gm000745
Naz, R. A., Thivakaran, G. A., George, J., Devi, V., Lal, N., and Suriyanarayanan, S. (2016). Comparative studies on the intertidal marine biodiversity of Macrobenthos of Kachchh coastal region. Gujarat, India. Bull. Environ. Contam. Toxicol. 5, 11–15.
Nerurkar, S., Galindo, L. A., and Apte, D. (2020). Current status of taxonomic knowledge on family Nassariidae Iredale, 1916 (1835) (Mollusca, Gastropoda, Buccinoidea) from India. J. Bombay Nat. Hist. Soc. 117, 3–38. doi: 10.17087/jbnhs/2020/v117/143923
Nguyen, H. D., Doo, S. S., Soars, N. A., and Byrne, M. (2012). Noncalcifying larvae in a changing ocean: warming, not acidification/hypercapnia, is the dominant stressor on development of the sea star Meridiastra calcar. Glob. Change Biol. 18, 2466–2476. doi: 10.1111/j.1365-2486.2012.02714.x
Nigam, R., and Chaturvedi, S. K. (2000). Foraminiferal study from Kharo creek, Kachchh (Gujarat), north west coast of India. Indian J. Mar. Sci. 29, 133–138.
Nygren, A., Parapar, J., Pons, J., Meibner, K., and Bakken, T. (2018). A mega-cryptic species complex hidden among one of the most common annelids in the North East Atlantic. PLoS One 13:e0198356. doi: 10.1371/journal.pone.0198356
Olsgard, F., Somerfield, P. J., and Carr, M. R. (1998). Relationships between taxonomic resolution, macrobenthic community patterns and disturbance. Mar. Ecol. Prog. Ser. 172, 25–36. doi: 10.3354/meps172025
Ourives, T. M., Rizzo, A. E., and Boehs, G. (2011). Composition and spatial distribution of thebenthic macrofauna in the Cachoeira River estuary, Ilhéus, Bahia, Brazil. Revi de Biol. Mar. Oceanog. 46, 17–25. doi: 10.4067/s0718-19572011000100003
Padate, G., Viradiya, A., Salunke, S., and Mirza, R. (2018). Additional records of macrobenthic fauna in and around Marine National Park and Sanctuary, Gujarat, India. J. Mar. Biol. Oceanogr. 7:2.
Panchang, R. (2014). Biofouling by Saccostrea cucullata - a major threat to mangroves of Vasishthi Estuary, Maharashtra. Indian J. Mar. Sci. 44, 1155–1161.
Pandiya rajan, R., Jyothibabu, R., Arunpandi, N., Jagadeesan, L., Krishnan, S. S., and Parthasarathi, S. (2021). Macrobenthos community response to the seasonal hypoxia associated with coastal upwelling off Kochi, along the Southwest coast of India. Cont. Shelf Res. 224:104450. doi: 10.1016/j.csr.2021.104450
Pandya, K. M., Jyoti, S. K., Kumari, M., Upadhyay, K., and Mankodi, P. C. (2014). Benthic associates of zoanthids on intertidal zone of Saurashtra coast, Gujarat. Electron. J. Environ. Sci. 7, 13–18. doi: 10.32381/jpm.2019.36.01.2
Pandya, P. (2013). Use of bioturbation structures in coastal wetlands as an important tool in benthic faunal studies. Jalaplavit 74:3.
Pandya, P. J., and Vachhrajani, K. D. (2013). Brachyuran crab diversity of lower estuarine mud flats of Mahi River with new record of two species from Gujarat. India. Arthropods 2, 242–250.
Parab, P. P., and Gaikwad, U. D. (1989). Occurrence and ecology of Serpula indica sp. nov.(Serpulidae- Polychaeta) from Ratnagiri coast. J. Ecobiol. 1, 223–232.
Parapar, J., Vijapure, T., Moreira, J., and Sukumaran, S. (2016). A new species of Heterospio (Annelida, Longosomatidae) from the Indian Ocean. Eur. J. Taxon. 220, 1–17.
Parulekar, A. H. (1969). Neoaiptasia commensali gen. et. sp. nov. : an actinarian commensal of hermit crab. J. Bombay Nat. Hist. Soc. 66, 57–62.
Parulekar, A. H. (1971a). A new sea anemone, Cribrinopsis robertii (Endomyaria: Actiniidae) from Maharashtra and Goa coast. J. Bombay Nat. Hist. Soc. 68, 726–749.
Parulekar, A. H. (1971b). Polychaetes from Maharashtra and Goa, of J. Bombay Nat. Hist. Soc. 68, 726–749.
Parulekar, A. H. (1973). Quantitative distribution of benthic fauna on the inner shelf of central west coast of India. Indian J. Mar. Sci. 2, 113–115.
Parulekar, A. H., Harkantra, S. N., and Ansari, Z. A. (1982). Benthic production and assessment of demersal fishery resources of the Indian seas. Indian J. Mar. Sci. 2, 107–114.
Parulekar, A. H., and Wagh, A. B. (1975). Quantitative studies on benthic macrofauna of north-eastern Arabian Sea shelf. Indian J. Mar. Sci. 4, 174–176.
Patel, S. J., and Desai, B. G. (2009). Animal-sediment relationship of the crustaceans and polychaetes in the intertidal zone around Mandvi, Gulf of Kachchh, Western India. J. Geol. Soc. India 74, 233–259. doi: 10.1007/s12594-009-0125-6
Pati, S. K., Swain, D., Sahu, K. C., and Sharma, R. M. (2015). “Diversity and distribution of polychaetes (Annelida: Polychaeta) along Maharashtra coast, India,” in Aquatic Ecosystem: Biodiversity, Ecology and Conservation, eds M. Rawat, S. Dookia, and C. Sivaperuman (Berlin: Springer).
Patil, P., and Kulkarni, B. G. (2013). The Onchidium (Gastropoda: Pulmonata: Onchidiidae: Genus: Onchidium) of the Uran, West Coast of India. Int. J. Zool. Res. 3, 23–30.
Pawar, P. R., and Al-Tawaha, A. (2017a). Biodiversity of marine gastropods along the Uran coast, Navi Mumbai, west coast of India. Am.-Eurasian J. Sustain. Agric. 11, 18–30.
Pawar, P. R., and Al-Tawaha, A. (2017b). Community structure of macrobenthos with reference to biomass to study effects of Jawaharlal Nehru Port on coastal biodiversity along Uran coast, Navi Mumbai. Adv. Environ. Biol. 11, 20–33.
Pawar, P. R., and Al-Tawaha, A. (2017c). Population density of marine macrobenthos: a tool for monitoring pollution-induced disturbance along Uran coast, Navi Mumbai. Adv. Environ. Biol. 10, 32–52.
Pawar, P. R., and Kulkarni, B. G. (2007). Diversity Indices of Selected Macrobenthos in Karanja Creek (District-Raigad), Maharashtra, west coast of India. J. Indian Fish Assoc. 34, 1–9. doi: 10.9734/jsrr/2017/33916
Pawar, P. R., and Kulkarni, B. G. (2009). Population density and biomass of selected macrobenthos in Karanja creek (Dist.–Raigad), Maharashtra, west coast of India. Ecologia 9, 79–86.
Pawar, P. R., Meshram, L. N., Udawant, S. M., and Inamdar, R. F. (2019). Assessment of coastal pollution using faunal composition of macrobenthos from Panvel Creek, Navi Mumbai, West Coast of India. Res. Chronicler 7, 28–38.
Peng, S., Li, X., Wang, H., and Zhang, B. (2014). Macrobenthic community structure and species composition in the Yellow Sea and East China Sea in jellyfish bloom. Chin. J. Oceanol. Limnol. 32, 576–594. doi: 10.1007/s00343-014-3068-8
Petracco, M., Cardoso, R. S., Corbisier, T. N., and Turra, A. (2012). Brazilian sandy beach macrofauna production: a review. Braz. J. Oceanography 60, 473–484. doi: 10.1590/s1679-87592012000400006
Poojary, D., Joshi, G. M., and Kale, P. G. (2012). Study of the effect of sand dredging on macrobenthos of Thane creek. Patrons 34–36.
Poriya, P., Vakani, B., Chaudhari, B., Kachhiya, P., and Kundu, R. (2015). Diversity and first record of heterobranch gastropods (opisthobranchs) from the Saurashtra coast of Kathiawar Peninsula, India. Mar. Biodivers. Rec. 8, 1–7.
Prasanna, K., Roshin, R. P., Narvekar, J., Kumar, P. D., and Vivekanandan, E. (2009). Response of the Arabian Sea to global warming and associated regional climate shift. Mar. Environ. Res. 68, 217–222. doi: 10.1016/j.marenvres.2009.06.010
Pridmore, R. D., Thrush, S. F., Hewitt, J. E., and Roper, D. S. (1990). Macrobenthic community composition of six intertidal sandflats in Manukau Harbour, New Zealand. N. Z. J. Mar. Freshwater Res. 24, 81–96. doi: 10.1080/00288330.1990.9516404
Qu, F., Nunnally, C., and Rowe, G. T. (2015). Polychaete annelid biomass size spectra: the effects of hypoxia stress. J. Mar. Biol. 2015, 1–9. doi: 10.1155/2015/983521
Quadros, G., and Athalye, R. P. (2012). Decline of fish diversity in the anthropogenically polluted Thane creek along the Central West Coast of India. Int. Res. J. Biol. Sci. 4, 17–21.
Quadros, G., Sukumaran, S., and Athalye, R. P. (2009). Impact of the changing ecology on intertidal polychaetes in an anthropogenically stressed tropical creek. India. Aquat. Ecol. 43, 977–985. doi: 10.1007/s10452-009-9229-8
Radulovici, A. E., Archambault, P., and Dufresne, F. (2010). DNA barcodes for marine biodiversity: moving fast forward? Diversity 4, 450–472. doi: 10.3390/d2040450
Raghunathan, C., Tewari, A., Joshi, H. V., Kumar, V. G. S., Trivedi, R. H., and Khambhaty, Y. (2003). Impact of turbidity on intertidal macrofauna at Gopnath, Mahuva and Veraval coasts (west coast of India). Indian J. Mar. Sci. 32, 214–221.
Rai, H. S. (1931). The shell fisheries of the Bombay Presidency. Rep. Bombay Nat. History Soc. Surv. 35, 826–847.
Rakocinski, C. F., and Menke, D. P. (2016). Seasonal hypoxia regulates macrobenthic function and structure in the Mississippi Bight. Mar. Pollut. Bull. 105, 299–309. doi: 10.1016/j.marpolbul.2016.02.006
Ram, M. J., Mehta, P., and Govindan, K. (1998). Phytoplankton pigments and macrobenthos in nearshore waters off an oil terminal at Uran (Maharashtra), west coast of India. Indian J. Mar. Sci. 27, 317–322.
Ramamoorthy, K., Sankar, G., and Sakkaravarthi, K. (2012). Assessment of reef associated biota in the Pirotan Island, Gulf of Kachchh, Gujarat, India. Eur. J. Exp. Biol. 2, 551–561.
Rao, C., and Soota, T. (1981). On some polychaetes from Gujarat Coast. Rec. Zool. Surv. India 79, 73–82.
Rao, M. N., Ram, A., Pradhan, U. K., and Siddaiah, V. (2019). Factors controlling organic matter composition and trophic state in seven tropical estuaries along the west coast of India. Environ. Geochem. Health 41, 545–562. doi: 10.1007/s10653-018-0150-8
Ray, G. C., and Grassle, J. (1991). Marine biological diversity: a scientific program to help conserve Marine biological diversity is urgently required. Mar. Biol. Divers. 41, 453–457.
Rengaiyan, P., De, K., and Ingole, B. (2017). Polychaete assemblage driven by substrate composition along the coastal waters of the South-eastern Arabian Sea. Reg. Stud. Mar. Sci. 16, 208–215. doi: 10.1016/j.rsma.2017.09.004
Rengaiyan, P., and Ingole, B. (2018). 18S rDNA sequencing data of benthic polychaetes from the Eastern Arabian Sea. Data Brief 20, 1749–1752. doi: 10.1016/j.dib.2018.09.015
Rey, A., Basurko, O. C., and Rodriguez-Ezpeleta, N. (2020). Considerations for metabarcoding-based port biological baseline surveys aimed at marine nonindigenous species monitoring and risk assessments. Ecol. Evol. 10, 2452–2465. doi: 10.1002/ece3.6071
Ritter, C., Montagna, P. A., and Applebaum. (2005). Short-term succession dynamics of macrobenthos in a salinity-stressed estuary. J. Exp. Mar. Biol. Ecol. 323, 57–69. doi: 10.1016/j.jembe.2005.02.018
Rodríguez-Villanueva, V., Martínez-Lara, R., and Zamora, V. M. (2003). Polychaete community structure of the northwestern coast of Mexico: patterns of abundance and distribution. Hydrobiologia 496, 385–399. doi: 10.1023/a:1026138108252
Rohit, P., Nikunj, G., and Nishit, D. (2016). Impact of water quality on macrofauna abundance in mangrove ecosystem of Gulf of Kachchh. J. Wetl Biodivers. 6, 61–71.
Sachs, J. D., Bajpai, N., and Ramiah, A. (2002). Understanding Regional Economic Growth in India. Cambridge, MA: Centre for International Development, Harvard University.
Saiz, J. I., Bustamante, M., Tajadura, J., Vijapure, T., and Sukumaran, S. (2015). A new subspecies of Phascolion Théel, 1875 (Sipuncula: Golfingiidae) from Indian waters. Zootaxa 3931, 433–437. doi: 10.11646/zootaxa.3931.3.7
Salas, F., Marcos, C., Neto, J. M., Patrício, J., and Pérez-Ruzafa, A. (2006). User friendly guide for using benthic ecological indicators in coastal and marine quality assessment. Ocean Coast. Manage. 49, 308–331. doi: 10.1016/j.ocecoaman.2006.03.001
Sánchez-Moyano, J. E., García-Asencio, I., and García-Gómez, J. C. (2010). Spatial and temporal variation of the benthic macrofauna in a grossly polluted estuary from southwestern Spain. Helgol. Mar. Res. 64, 155–168. doi: 10.1007/s10152-009-0175-6
Saravanakumar, A., Serebiah, J. S., Thivakaran, G. A., and Rajkumar, M. (2007). Benthic macrofaunal assemblage in the arid zone mangroves of gulf of Kachchh - Gujarat. J. Ocean Univ. China 6, 303–309. doi: 10.1007/s11802-007-0303-3
Sarma, V. V., Kumar, N. A., Prasad, V. R., Venkataramana, V., Appalanaidu, S., Sridevi, B., et al. (2011). High CO2 emissions from the tropical Godavari estuary (India) associated with monsoon river discharges. Geophys. Res. Lett. 38:L08601.
Sasekumar, A. (1974). Distribution of macrofauna on a Malayan mangrove shore. J. Anim. Ecol. 43, 51–69. doi: 10.2307/3157
Seebens, H., Gastner, M. T., Blasius, B., and Courchamp, F. (2013). The risk of marine bioinvasion caused by global shipping. Ecol. Lett. 16, 782–790. doi: 10.1111/ele.12111
Shukla, M., Patel, B., Trivedi, J., and Vachhrajani, K. (2013). Brachyuran crabs diversity of mahi and dhadhar estuaries, Gujarat, India. Res. J. Mar. Sci. 1, 2321–1296.
Silva, R. F., Rosa Filho, J. S., Souza, S. R., and Souza-Filho, P. W. (2011). Spatial and temporal changes in the structure of soft-bottom benthic communities in an Amazon estuary (Caeté estuary, Brazil). J. Coast. Res. 64, 440–444.
Simboura, N., Zenetos, A., Panayotidis, P., and Makra, A. (1995). Changes in benthic community structure along an environmental pollution gradient. Mar. Pollut. Bull. 30, 470–474. doi: 10.1016/0025-326x(95)00237-h
Sivadas, S. K., and Carvalho, R. (2020). Marine annelida of India: Taxonomy and status evaluation and an updated checklist. J. Threat. Taxa 12, 16647–16714.
Sivadas, S. K., Ingole, B., Ganesan, P., Sautya, S., and Nanajkar, M. (2012). Role of environmental heterogeneity in structuring the macrobenthic community in a tropical sandy beach, west coast of India. J. Oceanogr. 68, 295–305. doi: 10.1007/s10872-011-0099-z
Sivadas, S. K., Ingole, B. S., and Fernandes, C. E. G. (2013). Environmental gradient favours functionally diverse macrobenthic community in a placer rich tropical bay. Sci. World J. 2013:750580.
Sivadas, S. K., Nagesh, R., Gupta, G. V. M., Gaonkar, U., Mukherjee, I., Ramteke, D., et al. (2016). Testing the efficiency of temperate benthic biotic indices in assessing the ecological status of a tropical ecosystem. Mar. Pollut. Bull. 106, 62–76. doi: 10.1016/j.marpolbul.2016.03.026
Sivaraj, S., Murugesan, P., Muthuvelu, S., Vivekanandan, K. E., and Vijayalakshmi, S. (2014). AMBI and M-AMBI indices as a robust tool for assessing the effluent stressed ecosystem in Nandgaon Coastal waters, Maharashtra, India. Estuar. Coast. Shelf Sci. 146, 60–67. doi: 10.1016/j.ecss.2014.05.024
Snelgrove, P. (1999). Getting to the bottom of marine biodiversity: Sedimentary habitats: ocean bottoms are the most widespread habitat on earth and support high biodiversity and key ecosystem services. BioScience 49, 129–130. doi: 10.2307/1313538
Snelgrove, P. V. (1998). The biodiversity of macrofaunal organisms in marine sediments. Biodivers. Conserv. 7, 1123–1132. doi: 10.1023/a:1008867313340
Soto, E., Quiroga, E., Ganga, B., and Alarcón, G. (2017). Influence of organic matter inputs and grain size on soft-bottom macrobenthic biodiversity in the upwelling ecosystem of central Chile. Mar. Biodivers 47, 433–450. doi: 10.1007/s12526-016-0479-0
Sousa, R., Dias, S., and Antunes, J. C. (2006). Spatial subtidal macrobenthic distribution in relation to abiotic conditions in the Lima estuary. NW of Portugal. Hydrobiologia 559, 135–148. doi: 10.1007/s10750-005-1371-2
Srinivas, D., Sawant, S. S., and Wagh, A. B. (1992). Biofoulers on aluminum and stainless steel panels at Vijaydurg harbor, central west coast of India. Indian J. Mar. Sci. 21, 143–145.
Srinivas, T., Sukumaran, S., and Dias, H. Q. (2019a). Extended distribution of Phtisica marina Slabber, 1769 (Crustacea: Amphipoda): first observation of alien Caprellid in the coastal waters of Indian subcontinent. Bioinvasions Rec. 8, 96–107. doi: 10.3391/bir.2019.8.1.10
Srinivas, T., Sukumaran, S., Mulik, J., and Dias, H. Q. (2019b). Community structure of benthic amphipods in four estuaries of northwest India. Reg. Stud. Mar. Sci. 27:100532. doi: 10.1016/j.rsma.2019.100532
Srinivas, T., Sukumaran, S., Neetu, S., and Ramesh Babu, K. (2020). Diversity and functional patterns of benthic amphipods in the coralline intertidal zones of a Marine National Park. India. Front. Mar. Sci. 7:589195. doi: 10.3389/fmars.2020.589195
Subrahmanyam, T. V., Karandikar, K. R., and Murti, N. N. (1952). Marine gastropoda of Bombay. J. Univ. Bombay B 20, 21–34.
Sukumaran, S., Mulik, J., Rokade, M. A., and Kamble, A. (2014). Impact of ‘Chitra’ oil spill on tidal pool macrobenthic communities of a tropical rocky shore (Mumbai, India). Estuaries Coast. 37, 1415–1431. doi: 10.1007/s12237-014-9791-8
Sukumaran, S., and Saraladevi, K. S. (2009). Polychaete diversity and its relevance in the rapid environmental assessment of Mumbai Port. Curr. Sci. 97:25.
Sukumaran, S., Vijapure, T., Kubal, P., Mulik, J., Rokade, M. A., Salvi, S., et al. (2016). Polychaete community of a marine protected area along the West Coast of India-prior and post the tropical cyclone. Phyan. PLoS One 11:e0159368. doi: 10.1371/journal.pone.0159368
Sukumaran, S., Vijapure, T., Mulik, J. A., Rokade, M., and Gajbhiye, S. N. (2013). Macrobenthos in anthropogenically influenced zones of a coralline marine protected area in the Gulf of Kachchh, India. J. Sea Res. 76, 39–49. doi: 10.1016/j.seares.2012.11.001
Sun, Y., Al-Kandari, M., Kubal, P., Walmiki, N., and Kupriyanova, E. K. (2017). Cutting a gordian knot of tubeworms with DNA data: the story of the hydroides operculata-complex (Annelida, Serpulidae). Zootaxa 4323, 39–48. doi: 10.11646/zootaxa.4323.1.3
Surugiu, V., Revkov, N., Todorova, V., Papageorgiou, N., Valavanis, V., and Arvanitidis, C. (2010). Spatial patterns of biodiversity in the Black Sea: an assessment using benthic polychaetes. Estuar. Coast. Shelf Sci. 88, 165–174. doi: 10.1016/j.ecss.2010.03.012
Swami, B. S., and Chhapgar, B. F. (2002). Settlement pattern of ascidians in harbour waters of Mumbai, west coast of India. Indian J. Mar. Sci. 31, 207–212.
Swami, B. S., and Udhayakumar, M. (2010). Seasonal influence on settlement, distribution and diversity of fouling organisms at Mumbai harbour. Indian J. Mar. Sci. 39, 57–67.
Takar, S., Gurjar, U. R., Saroj, J., Raveendar, B., Singh, J., and Deshmukhe, G. (2020). Benthic organisms in mumbai mangroves: diversity and distribution in relation to environmental parameters. J. Exp. Zool. India 23, 599–605.
Tavanayan, S., Sharifian, S., Kamrani, E., Mortazavi, M. S., and Behzadi, S. (2021). Influence of environmental factors on the characteristics of macrobenthic communities in soft bottoms around coral reefs of Larak Island (Persian Gulf). Hydroécol. Appl. 21, 93–113. doi: 10.1051/hydro/2021002
Teixeira, H., Borja, T., Weisberg, S. B., Ananda Ranasinghe, J., and Cadien, D. B. (2010). Assessing coastal benthic macrofauna community condition using best professional judgment – developing consensus across North America and Europe. Mar. Pollut. Bull. 60, 589–600. doi: 10.1016/j.marpolbul.2009.11.005
Terlizzi, A., Bevilacqua, S., Fraschetti, S., and Boero, F. (2003). Taxonomic sufficiency and the increasing insufficiency of taxonomic expertise. Mar. Pollut. Bull. 46, 556–561. doi: 10.1016/s0025-326x(03)00066-3
Teske, P. R., and Wooldridge, T. H. (2003). What limits the distribution of subtidal macrobenthos in permanently open and temporarily open/closed South African estuaries? Salinity vs. sediment particle size. Estuar. Coast. Shelf Sci. 57, 225–238. doi: 10.1016/s0272-7714(02)00347-5
Thornely, L. R. (1916). “Report on the hydroida collected by Mr. James Hornell at Okhamandal in Kattiawar in 1905–6,” in Report to the Government of Baroda on the marine zoology of Okhamandal in Kattiawar, Part II, ed. J. Hornell (London: Williams and Northgate), 147–150.
Trivedi, J. N., Arya, S., and Vachhrajani, K. D. (2014). Study of the macro faunal associates of the littoral zoanthid palythoa mutuki (Cnidaria, Anthozoa) from Saurashtra Coast, Gujarat, India. Int. J. Mar. Sci. 4, 1–9.
Trivedi, J. N., Gadhavi, M. K., and Vachhrajani, K. D. (2012). Diversity and habitat preference of brachyuran crabs in Gulf of Kutch, Gujarat, India. Arthropods 1, 13–23.
Trivedi, J. N., Trivedi, D. J., and Vachhrajani, K. D. (2017). Range extension of brachyuran crabs of the family camptandriidae stimpson, 1858 (Crustacea: Decapoda: Brachyura) in Indian waters. Check List 13, 9–13.
Trivedi, M. H., Bhatt, K. A., Pandit, A. S., Shukla, N. C., Ayachit, G. N., Joshi, M. N., et al. (2019). Evaluation and Optimization of DNA Extraction Methods for Benthic Foraminifera from the Gulf of Kachchh, Gujarat, India. New Delhi: NISCAIR-CSIR.
Vadher, P., Kardani, H., and Beleem, I. (2020). An annotated checklist of sea slug fauna of Gujarat coast, India. J. Threatened Taxa 12, 15835–15851. doi: 10.11609/jott.5278.12.8.15835-15851
Vaghela, A., Bhadja, P., Ramoliya, J., Patel, N., and Kundu, R. (2010). Seasonal variations in the water quality, diversity and population ecology of intertidal macrofauna at an industrially influenced coast. Water Sci. Technol. 61, 1505–1514. doi: 10.2166/wst.2010.503
Vakani, B., Poriya, P., and Kundu, R. (2016). Spatio-temporal variations in the distribution pattern of key molluscs in a rocky intertidal habitat along South Saurashtra coastline of Gujarat. Int. J. Ecol. Environ. Sci. 42, 341–348.
Vakani, B., and Kundu, R. (2018). Habitat Characterization of Gastropod Limpets in the Intertidal Region of the Mainland Indian Coastline. Sustainable Management of Aquatic Resources, Part-1. Delhi: Narendra Publishing House, 231–245.
Vakani, B., Nakano, T., and Kundu, R. (2020). Diversity and taxonomy of the intertidal patellogastropod limpets of the mainland Indian coastline. Zootaxa 4728, 211–226. doi: 10.11646/zootaxa.4728.2.3
Valença, A. P. M. C., and Santos, P. J. P. (2012). Macrobenthic community for assessment of estuarine health in tropical areas (Northeast, Brazil): review of macrofauna classification in ecological groups and application of AZTI Marine Biotic Index. Mar. Pollut. Bull. 64, 1809–1820. doi: 10.1016/j.marpolbul.2012.06.003
Vamosi, J. C., and Vamosi, S. M. (2008). Extinction risk escalates in the tropics. PLoS one 3:3886. doi: 10.1371/journal.pone.0003886
Varshney, P. K., and Govindan, K. (1995). Macrobenthos off Mahim (Bombay), west coast of India in relation to coastal pollution and aquaculture. J. Indian. Fish. Assoc. 25, 47–56.
Varshney, P. K., Govindan, K., and Desai, B. N. (1981). Benthos of the Narmada estuary. Mahasagar 14, 141–148.
Varshney, P. K., Govindan, K., Gaikwad, U. D., and Desai, B. N. (1988). Macrobenthos off Versova (Bombay), west coast of India, in relation to environmental conditions. Indian J. Mar. Sci. 17, 222–227.
Venkataraman, K., and Wafar, M. (2005). Coastal and marine biodiversity of India. Indian J. Mar. Sci. 34, 57–75.
Venkatraman, C., Padmanaban, P., Louis, S., and Shrinivaasu, S. (2018). Marine bryozoans of Gujarat and Maharashtra. Rec. Zool. Surv. India 118, 389–404.
Venugopalan, V., and Wagh, A. (1990). Biofouling of an offshore oil platform: faunal composition and biomass. Indian J. Mar. Sci. 19, 53–56.
Vijapure, T., and Sukumaran, S. (2019). Optimization of the taxonomic resolution of an indicator taxon for cost-effective ecological monitoring: perspectives from a heterogeneous tropical coastline. J. Environ. Manage 247, 474–483. doi: 10.1016/j.jenvman.2019.05.154
Vijapure, T., Sukumaran, S., and Manohar, C. S. (2019b). Molecular characterization and phylogenetics of Indian polychaete fauna: scope for implementation in ecological monitoring. Aquat. Ecol. 53, 665–677. doi: 10.1007/s10452-019-09717-0
Vijapure, T., Sukumaran, S., Neetu, S., and Chandel, K. (2019a). Macrobenthos at marine hotspots along the northwest Indian inner shelf: patterns and drivers. Mar. Environ. Res. 144, 111–124. doi: 10.1016/j.marenvres.2018.12.007
Vijapure, T., Sukumaran, S., Mulik, J., and Nageswar Rao, M. (2016). Mass mortality of macrobenthos in a biodiverse rocky beach- Impact of a minor oil spill. Indian J. Mar. Sci. 45, 1098–1100.
Vijay, R., Khobragade, P. J., Dhage, S. S., Gupta, A., and Wate, S. R. (2015). Tidal and seasonal variations in water quality of Thane creek, Mumbai, India: a statistical analysis. Indian J. Mar. Sci. 44, 808–817.
Virkar, P., Athalye, R. P., Kurve, P. N., Borkar, M. U., and Quadros, G. (2004). Comparative Study of the abiotic and biotic components of an aquaculture pond and its adjacent Thane Creek Area, Maharashtra, India. J. Aqua. Biol. 2004, 73–78.
Wadkar, S. H. (1989). Studies on Nereidae (Polychaeta -Annelida) of Ratnagiri coast. thesis, Dapoli: Dr. Balasaheb Sawnt Konkan Krishi Vidyapeeth, 193.
Wang, X., Li, X., Li, B., and Wang, H. (2009). Summertime community structure of intertidal macrobenthos in Changdao Archipelago, Shandong Province, China. Chin. J. Oceanol. Limnol. 27:425. doi: 10.1007/s00343-009-9140-0
Westfall, K. M., Therriault, T. W., and Abbott, C. L. (2020). A new approach to molecular biosurveillance of invasive species using DNA metabarcoding. Glob. Change Biol. 26, 1012–1022. doi: 10.1111/gcb.14886
Wetzel, M. A., Peter, C., Manz, W., Koop, J. H., and Wahrendorf, D. S. (2012). The ecological quality status of the Elbe estuary. A comparative approach on different benthic biotic indices applied to a highly modified estuary. Ecol. Indic. 19, 118–129. doi: 10.1016/j.ecolind.2011.08.007
Whitlatch, R. B. (1977). Seasonal changes in the community structure of the macrobenthos inhabiting the intertidal sand and mud flats of Barnstable Harbor, Massachusetts. Biol. Bull. 152, 275–294. doi: 10.2307/1540565
Widdicombe, S., and Spicer, J. I. (2008). Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J. Exp. Mar. Biol. Ecol. 366, 187–197. doi: 10.1016/j.jembe.2008.07.024
Wolff, W. F. (1983). “Estuarine benthos” in Ecosystems of the World, Estuaries and Enclosed Seas, ed. B. H. Ketchum (New York, NY: Elsevier) 337–374.
Yan, J., Xu, Y., Sui, J., Li, X., and Wang, H. (2017). Long-term variation of the macrobenthic community and its relationship with environmental factors in the Yangtze River estuary and its adjacent area. Mar. Pollut. Bull. 123, 339–348. doi: 10.1016/j.marpolbul.2017.09.023
Yokoyama, H., and Sukumaran, S. (2012). First records of three Paraprionospio species (Polychaeta: Spionidae) from Indian waters. Cah. Biol. Mar. 53, 279–287.
Ysebaert, T., Herman, P. M. J., Meire, P., Craeymeersch, J., et al. (2003). Large-scale spatial patterns in estuaries: estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar. Coast. Shelf Sci. 57, 335–355. doi: 10.1016/s0272-7714(02)00359-1
Zhang, A., Yuan, X., Yang, X., Shao, S., Li, J., and Ding, D. (2016). Temporal and spatial distributions of intertidal macrobenthos in the sand flats of the Shuangtaizi Estuary, Bohai Sea in China. Acta Ecol. Sinica 36, 172–179. doi: 10.1016/j.chnaes.2016.04.003
Keywords: review, macrobenthos, biodiversity, functioning, India
Citation: Sukumaran S, Vijapure T, Mulik J and Ridha H (2021) Marine Macrobenthos of NorthWest India-Reviewing the Known and Unknown. Front. Mar. Sci. 8:671245. doi: 10.3389/fmars.2021.671245
Received: 23 February 2021; Accepted: 28 June 2021;
Published: 27 July 2021.
Edited by:
Neloy Khare, Ministry of Earth Sciences, IndiaReviewed by:
Xiaoshou Liu, Ocean University of China, ChinaRahul Kundu, Saurashtra University, India
Copyright © 2021 Sukumaran, Vijapure, Mulik and Ridha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soniya Sukumaran, c29uaXlhQG5pby5vcmc=
 Soniya Sukumaran
Soniya Sukumaran Tejal Vijapure
Tejal Vijapure Jyoti Mulik
Jyoti Mulik Hurmine Ridha
Hurmine Ridha