- 1Normandie Univ., UNICAEN, Université de Caen Normandie, CNRS, Laboratoire Morphodynamique Continentale et Côtière, UMR 6143 M2C, Caen, France
- 2Centre for Environmental and Marine Studies (CESAM), Department of Biology, University of Aveiro, Aveiro, Portugal
- 3CIIMAR Interdisciplinary Centre of Marine and Environmental Research, University of Porto, Matosinhos, Portugal
- 4Department of Biology, Faculty of Sciences, University of Porto, Porto, Portugal
The Ampeliscidae Kröyer, 1842 is amongst the most diverse amphipod families; it comprises four genera, Ampelisca being the richest with more than 200 species. The Ampelisca genus presents high morphological homogeneity and the identification of the species by ecologists remains difficult. Ampelisca are also characterized by a high degree of sympatry, a rare situation in amphipods, and in this study we report up to nine species coexisting at the same site. Recent benthic sampling and publications, namely on the Portuguese continental shelf and the English Channel, permit to revisit the available data on the taxonomy and propose an updated species identification key, as well as the distribution and ecology of the 40-recorded Ampelisca species along the North Eastern Atlantic coast, from the Strait of Gibraltar, in the South, to the Strait of Dover, in the North. The data allow discussing on the sympatry and syntopy of such diverse amphipod family with the co-occurrence of several species at various scales of observations, from the wider regional area, to the narrower local habitat. Two Ampelisca species were recorded exclusively on hard bottom, while the other tend to inhabit specific types of soft bottom, ranging from deep mud to shallow coarse sand and gravel, with a preference for continental shelf muddy and sandy habitats. A future sea water temperature increase scenario could modify the species geographical distribution and reproductive cycle, in this temperate North-eastern Atlantic province.
Introduction
The Ampeliscidae Kröyer, 1842 is one of the most diverse amphipod families, together with the Gammaridae Leach, 1814 and the Lysianassidae Lana, 1849. Ampeliscidae is composed of four genera comprising more than 300 species, with 9 species of Byblisoides, 27 species of Haploops, 75 species of Byblis and 203 species of Ampelisca recorded on the World Register of Marine Species [http://www.marinespecies.org/; accessed on 16th October 2020). The Ampeliscidae family accounts nowadays for 314 species, representing 3.0% of the described amphipod species, 10,320 in total (Horton et al., 2021)]. New species for this family continue to be annually described. Between 2000 and 2020, 3 Byblisoides, 14 Byblis, 9 Haploops, and 32 Ampelisca new to science were described, corresponding to 18% of the known species of this family.
Numerous data and reviews on the Ampeliscidae (see Bellan-Santini, 1982, 1983; Dauvin and Bellan-Santini, 1985, 1986, 1988, 1996, 2000, 2002; Bellan-Santini and Dauvin, 1988a,b, 1989, 1997; Dauvin, 1996) permitted to differentiate the general distribution patterns of the genera, Byblisoides, Byblis and Haploops being mainly deep- and cold-water genera and Ampelisca more tropical and sub-tropical and shallow water genus. Nevertheless, dense Haploops populations have been reported in South Brittany shallow waters, Northern part of the Bay of Biscay (Rigolet et al., 2012, 2014), while dense Ampelisca populations occurred in North Brittany at the entrance of the English Channel (Dauvin, 1988a,b,c,d, 1989).
Given the Ampelisca high morphological homogeneity, the taxonomic identification of specimen to the species level remains difficult to ecologists, in spite of the existence of illustrated keys (Dauvin and Bellan-Santini, 1988). As a consequence, most ecological studies tend to report only the presence of Ampelisca sp. or Ampelisca spp., without detailing the species. This difficulty arises in part from poorly detailed descriptions accompanying the reporting of new Ampelisca species, most being known only from the type locality and represented by very few specimens. Other difficulties include the existence of cryptic species, such as Ampelisca brevicornis sensu lato and the discovery of several species for this large cosmopolitan species, i.e., A. cavicoxa Reid, 1951 and A. pectenata Reid, 1951 for the North-Eastern Atlantic (Kaim-Malka, 2000) or more recently the description of A. troncosoi Tato et al. (2012) from Galicia, Spain, previously miss-confused with A. heterodactylta (Schellenberg, 1925; Tato et al., 2012). Additionally, the high level of taxonomic expertise and the long time required to identify the Ampelisca to species level, especially for large sample collections, may not always be compatible with numerous ecological work. Apart from the high diversity of the Ampelisca, another particularity of this genus is to present a high degree of sympatry, not only at the regional and local scales, but also at the scale of the grab replicate sample (Dauvin et al., 1993). The Ampelisca species tend to inhabit distinct types of substratum. The genus is mostly present on soft bottom, from deep mud to shallow coarse sand and gravel (Bellan-Santini and Dauvin, 1988a,b; Bellan-Santini and Dauvin, 1989) and only very few species are found on hard bottom (i.e., A. rubella A. Costa, 1864 or A. lusitanica Bellan-Santini and Marques, 1986).
Recent benthic sampling campaigns on the Portuguese continental shelf (Martins et al., 2012, 2013a,b, 2014; Sampaio et al., 2016) and in the English Channel, i.e., in the Rade de Cherbourg (Baux et al., 2017; Andres et al., 2020) and in the Bay of Seine (Alizier, 2011), permit to revisit the available data on the taxonomy, distribution and ecology of the Ampelisca species along the North Eastern Atlantic, from the Strait of Gibraltar, in the South to the Strait of Dover, in the North.
Materials and Methods
Sampling Sites and Recently Available Data
Portuguese Continental Shelf
A total of 326 sites were visited during sampling campaigns with a 0.1 m2 Smith McIntyre grab (one grab per site) conducted on the entire Portuguese continental shelf from Caminha on the Northwest, to Vila Real de Santo António on the Southeast (Martins et al., 2012, 2013a,b, 2014; Sampaio et al., 2016). The shallow and mid depth north-western shelf and areas located close to the major submarine canyons are characterized by coarser sediments with low fines and organic matter content, whereas the south-western and the deep north-western shelf are dominated by fine sands with moderate fines and organic matter content. The western part of the southern shelf is very heterogeneous while muds predominate off the major Portuguese rivers, the Tagus (Lisbon) and the Douro (Porto) on the west and the Guadiana (Vila Real Santo de António), on the south coast (see namely Cardoso et al., 2019).
English Channel
New data was obtained mainly from the Rade the Cherbourg and the Bay of Seine. The Rade de Cherbourg is Europe's largest roadstead, extending over a total area of 15 km2, with a maximum depth ~20 m, and a mean depth of ~13 m. The macrofauna was sampled with a 0.1 m2 Van Veen grab (three replicates per site) for different studies from 2012 to 2018 on the four sediment facies of the Rade and in surrounding bays in the North Cotentin for a total of 61 sites (Baux et al., 2017; Andres et al., 2020). The eastern part of the Bay of Seine (eastern part of the English Channel) and the lower part of the Seine estuary cover an area of ~400 km2, with a maximum depth of ~20 m. Macrofauna was sampled in 2008–2009 (Alizier, 2011) and in 2016–2017 (Baux, 2018) for a total of about 100 sites with a 0.1 m2 Van Veen grab (three to five replicates per site).
Geographical Distribution
The coast along the North Eastern Atlantic coast, from the Strait of Gibraltar, in the South, to the Strait of Dover, in the North, was divided in eight zones corresponding to the available data on the Ampelisca distribution, i.e., South Spain (Bellan-Santini and Dauvin, 1988b); Portugal (Bellan-Santini and Dauvin, 1988b; Sampaio et al., 2016); Galicia (Bellan-Santini and Dauvin, 1988b); South-Eastern Bay of Biscay (Bachelet et al., 2003); South Brittany (Bellan-Santini and Dauvin, 1988b); Iroise Sea (Dauvin and Toulemont, 1988); Western part of the English Channel (Bellan-Santini and Dauvin, 1988b, 1989; Dauvin, 1999; Le Mao, 2006) and Eastern part of the English Channel North Cotentin (Dauvin, 1999; Alizier, 2011; Baux et al., 2017; Andres et al., 2020).
According to their occurrences, the species were classified in three categories, (1) rare, corresponding to species recorded at up to 10 sites, (2) common, corresponding to species recorded in numerous soft-bottom sites mainly from muddy to sandy sediment, and (3) very common, for species recorded in most of the sampled soft-bottom communities from the Strait of Gibraltar to the Dover Strait and at a large range of sediment types, from muddy to gravely sediment.
A Jaccard similarity matrix among the samples was obtained and exploited by cluster analysis using the average clustering algorithm and by ordination analysis, using non-metric multidimensional scaling (nMDS). The Jaccard Similarity Coefficient is most appropriate to analyze our data (presence of species in geographical areas, Table 1), because it is precisely devoted to study the similarity between samples solely on the presence-absence of the species. Other presence-absence similarity coefficients could be used, but the Jaccard Coefficient is a classic choice, possibly one of the most used in Ecology. Moreover, the Jaccard similarity between two samples also has a very straightforward interpretation, representing the proportion of the common species to the two samples. An ordination analysis was also performed using principal components analysis (PCA), following a Hellinger transformation, this analysis allowing the joint plot of the samples (the geographical locations) and the associated species. All multivariate analysis were performed with PRIMER v.6 (Clarke and Gorley, 2006).
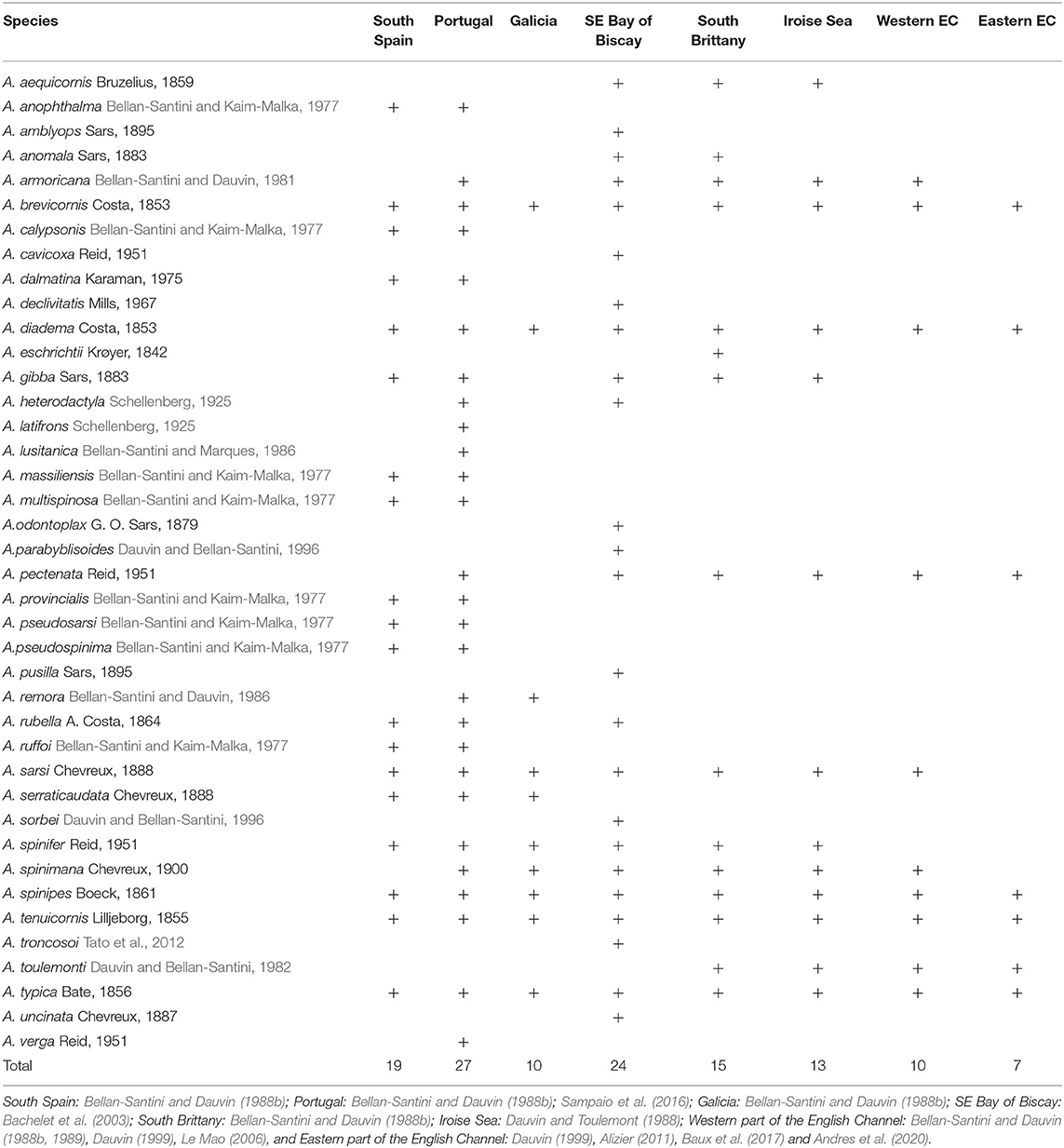
Table 1. Latitudinal distribution of Ampelisca from the Strait of Gibraltar (south Atlantic coast of Spain) to the Strait of Dover.
Results
Taxonomy
A total of 40 species were recorded along the North-eastern Atlantic coast from the Strait of Gibraltar to the Strait of Dover (Appendix A in Supplementary Material). Since the publication of the Ampelisca taxonomic key from the north-eastern Atlantic by Dauvin and Bellan-Santini (1988), the number of species known to this area has increased. Two species, A. declivitatis and A. macrocephala, were added to the list of recorded species in this area (see namely Dauvin and Bellan-Santini, 1996) and four new species were described for science (Ampelisca cavicoxa, A. parabyblisoides, A. sorbei, and A. troncosoi). The following taxonomic key includes these additions. A1 corresponds to the first pair of antennae, A2 to the second pairs and P7 to the pereiopod 7. The main morphological characters which served to the identification of Ampelisca species and used in the following key are indicated in Figure 1.
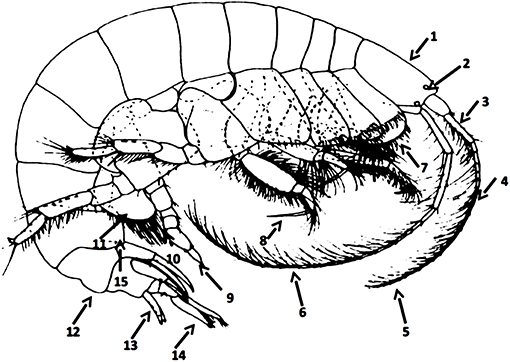
Figure 1. Main morphological characters used in the female Ampelisca key. 1. Head shape. 2. Corneal lenses. 3. Length of the peduncle of the first pairs of antennae. 4. Length of the first pairs of antennae vs. length of the peduncle of the second pairs of antennae. 5. Length of the first pair of antennae. 6. Length of the second pairs of antennae. 7. Shape of the coxal plates. 8. Length of the pereiopod 3–4 dactylus. 9. Shape and length of the pereiopod 7 dactylus. 10. Posterior lobe on merus. 11. Shape of the basal of the pereiopod 7 and presence/absence of spines. 12. Form of the carina of the urosome 1. 13. Form of the telson and absence of presence of dorsal spines and setae. 14. Length of the rami of the uropod 3. 15. Postero distal form of the epimeral 2 and 3.
1. Dorsal sucker-like structure on pleon segment 1 ………………………………………………….A. remora
1. Without dorsal sucker-like structure on pleon segment 1…………….……………………………………2
2. Without corneal lenses ………………………………………….……………………………………….3
2. With corneal lenses 11
3. P7, with a large posterior lobe on merus ………………………………………………….A uncinata
3. P7 without large posterior lobe on merus…………………………………………………….4
4. Epimera l plate 3 with a tooth……………………….5
4. Epimera l plate 3 without tooth…………………………………………………………………………………….7
5. Uropod 2 rami with a long subtermina l spine …………………….……….……………… A. odontoplax
5. Uropod 2 rami without long subterminal spine……………………………………………………….6
6. Uropod 2, rami with few short spines, telson dorsal surface with spines A. amblyops
7. P7 basis, margin distally excavate A. heterodactyla
7. P7 basis, margin rounded.………………………………8
8. A1 length > head + 3 anterior segments of pereon…………………………………………………….9
8. A1 length < head + 3 anterior segments of pereon.……………………………………………………10
9. A1, first two articles of peduncle equal in length; first segment of urosome with a slight carina.……………………………A. declivitatis
9. Al, article 2 of peduncle > article 1; first segment of urosome virtually without carina.………………………………………A. anophthalma
10. Al = A2.……………………………………A. pusilla
10. Al < ped A2.……………………………………………………A. parabyblisoides
11. Only one pair of corneal lenses, head with a rostrum, antennae short A. troncosoi
11. Two pairs of corneal lenses 12
12. P7, basis outer surface with numerous spines, urosome 1 with a peak-ended keel……………………………………………. A. spinifer
12. P7, basis outer surface without spines, urosome 1 different 13
13. Blots of black pigment behind corneal lenses; P7, ischial to dactylus cylindrical…………………………………………………………………………….A. . rubella
13. Head without blots of black pigment, P7 different 14
14. P7 merus with a large posterior lobe 15
14. P7 merus without large posterior lobe 18
15. Head with anterior margins not parallel, Urosome seg. 1 with a pronounced angular keel A. gibba
15. Head with antero-superior and antero-inferior margins parallel 16
16. Epimeral plate 3, posterior margin bisinuous, postero-distal angle with a large tooth A. brevicornis
16. Epimeral plate 3, posterior margin sinuous, posterior distal angle with a small or moderate tooth 17
17. Urosome seg. 1 with a cockscomb dorsal keel……………………………………………. A. pectenata
17. Urosome seg. 1 with a small convex carina A. cavicoxa
18. Head, anterior half narrow 19
20. Head different 20
19. A 2 shorter than body length; P3-4, dactylus = carpus + propodus A. sarsi
19. A2 more longer than body length; P3-4, dactylus > carpus + propodus A. pseudosarsi
20. Head broad, anterior edge truncate 21
20 Head different 22
21. A2 < body length; without distinguished A. latifrons
21. A2= body length; carina high and rounded A. provincialis
22. Uropode 3, inner ramus denticulate or serrulate 23
22. Uropode 3, inner ramus tapered not denticulate or serrulate……………………………………………….25
23. P7, merus not prolonged anteriorly in peg-shape ………. A. serraticaudata
23. P7, merus prolonged anteriorly in large peg-shape 24
24 A1 subegal to A2 A. unidentata
24. A1 shorter than A2 A. lusitanica
25 A2 > body. .…………………………………… A. sorbei
25. A2 < body. .……………………………………….26
26. Uropode 2 bearing long spine(s) 27
26. Uropode 2 bearing only short spines 28
27. Uropode 2, outer ramus with long marginal spines increasing in length distally; P7, carpus anterior margin notched A. eschrichtii
27. Uropode 2, outer ramus with single long subterminal spine; P7, carpus anterior margin rounded A. macrocephala
28. Uropode 2 fringed with numerous small spines 29
28. Uropode 2 with few small spines 35
29. A2 longer than body length 30
29. A2 shorter than body length 32
30. Urosome 1 with high carina dorsally bisinuate. Uropode 2 rami fringed regularly on both sides by rows of small spines A. multispinosa
30. Urosome 1 with small rounded carina. Uropode 2 rami not regularly fringed with small spines 31
31. A1 shorter than A2 peduncle A. ruffoi
31. A1 slightly longer than A2 peduncle A. pseudospinimana
32. A1 shorter than A2 peduncle. Head with antero-distal margin broadly round A. tenuicornis
32. A1 longer than A 2 peduncle ……………………….33
33. Al slightly longer than A2 peduncle. Epimeral plate 3 rounded A. diadema
33. A1 longer than A2 peduncle and equal to half length of A2. Epimeral plate 3 quadrate 34
34. A2 shorter than half length of body. Epimeral 2 postero-distal angle with a small tooth A. armoricana
34. A2 longer than half length of body. Epimeral 2 postero-distal angle rounded A. spinipes
35. P3-4 dactylus shorter than carpus + propodus 36
35. P3-4 dactylus longer than carpus + propodus 37
36. Epimeral plate 2 postero-distal angle with a distinct tooth A. verga
36. Epimeral plate 2 postero-distal angle rounded A. aequicornis
37. Gnathopode 1 with large spines on palm A. spinimana
37. Gnathopode 1 without spine on palm .……………38
38. Urosome seg. 1 with prominent carina 39
38. Urosome seg. 1 with moderate carina 41
39. A1 longer than half A2. Telson dorsal surface inermous. Epimera l plate 2 rounded A. anomala
39. A1 shorter than half A2. Telson dorsal surface with spines. Epimera l plate 2, posterodistal corner angle a small tooth 40
40. Urosome seg. 1 with pronounced angular carina. A1 shorter than A2 peduncle A. typica
40. Urosome seg. 1 with a raiser high dorsal carina, posterior edge overflowing. A1 slightly longer than A2 peduncle A. toulemonti
41. A1 equal to A2 length 42
41. A1 shorter than A2, Urosome seg. 1 with a high rounded carina A. massiliensis
42. A1 and A2 longer than body length. P7 merus prolonged anteriorly in peg-shape covering a part of carpus A. calypsonis
42. A1 and A2 nearly equal to body length. P7 merus without lobe A. dalmatina.
Distribution
A total of 40 Ampelisca species are currently known from the study area, comprehending the North-eastern Atlantic coast between the Strait of Gibraltar, in Southern Spain, to the Strait of Dover, in the Eastern part of the English Channel (Figure 2). The presence of these 40 species were reported to eight geographical locations, indicated in Table 1, following the studies consulted and mentioned in Appendix A in Supplementary Material.
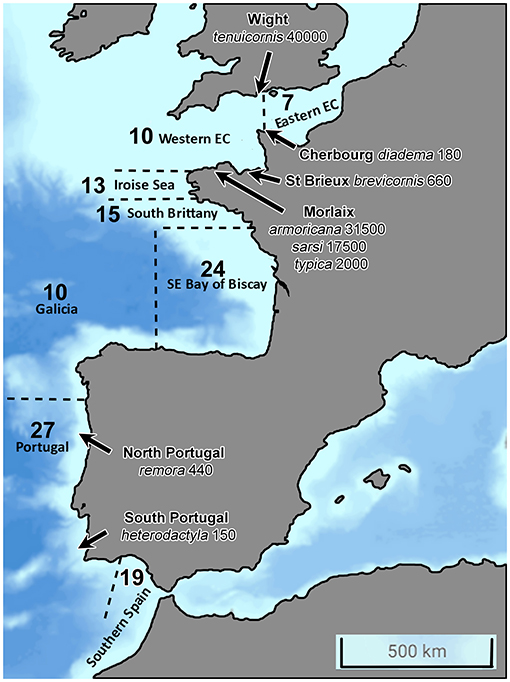
Figure 2. North-eastern Atlantic Coast from the Strait of Gibraltar, South, to the Strait of Dover, North, showing the eight geographical locations under study for the distribution of Ampelisca species. The bold number correspond to the number of Ampelisca species found in the eight areas; the species name and numbers correspond to the maximal abundances per m2 in different sites.
The number of species varied from a maximum of 27 along the Portuguese coast, to a minimum of 7, in the eastern part of the English Channel. The second richest area was the southern part of the Bay of Biscay with 24 species, including eight deep-water species. There was a clear reduction of the number of species from the south to the north and undoubtedly a lack of data for two areas: the southern Atlantic coast of Spain and Galicia.
Five species were present in all areas: A. brevicornis, A. diadema, A. spinipes, A. tenuicornis, and A. typica. A. pectenata showed also a large distribution but was absent in Galicia and Southern Spain. Twelve species were recorded only in the southern part of the study area; most were Mediterranean and recorded in the Atlantic up to the southern coast of Portugal, in Algarve (Marques and Bellan-Santini, 1991, 1993; Sampaio et al., 2016): A. anophthalma, A. calypsonis, A. dalmatina, A. latifrons, A. lusitanica, A. massiliensis, A. multispinosa, A. provincialis, A. pseudosarsi, A; pseudospinima, A. ruffoi and A. verga.
Ampelisca aequicornis was present only in the Bay of Biscay.
The Southern species were distributed in the northern areas up to Galicia such as A. serraticaudata or the southern part of the Bay of Biscay such as A. heterodactyla, A. rubella and A. serraticaudata. Ampelisca gibba was absent in the English Channel probably due to the absence of a mud habitat in this megatidal sea due to high hydrodynamics and dominance of coarse sediments. Three species, A. armoricana, A. sarsi, A. spinimana were absent in the eastern part of the English Channel. Five species showed a limited spatial distribution, possibly due to lack of data or because they were recently described: A. caxicoxa, A. remora, A. sorbei, A. troncosoi and A. toulemonti.
Eight deep-water species were only reported in the bathyal part of the Bay of Biscay, possibly because of the deeper sampling solely performed in this study location: A. amblyops, A. anomala, A; declivitatis, A. eschrichtii, A. parabyblisoides, A. odontoplax, A. pusilla and A. uncinata.
Among the new observations in the study area, Ampelisca verga, a West African species originally described as a variety of A. aequicornis by Reid (1951, in Dauvin and Bellan-Santini, 1985) off Dakar, Senegal, was recorded in the Algarve coast, southern Portugal, setting a new northern distribution limit for this species (Sampaio et al., 2016). Ampelisca toulemonti, described from one female coming from the Iroise Sea (Dauvin and Bellan-Santini, 1982), was recorded in the eastern part of the Rade de Cherbourg in the North Cotentin, setting the eastern most location of this species in the English Channel (Andres et al., 2020).
Among the 40 species (Appendix A in Supplementary Material), 17 were found only on the Continental Shelf, 16 were recorded both on the Continental Shelf and the Continental slope at depths up to 510 m. Seven species were strictly bathyal with a maximum sampling depth of 1,097 m.
A data matrix representing Table 1, 40 species ×8 locations, was analyzed to study the faunal resemblance among the samples representing the geographical locations.
The dendogram (Figure 3) showed high similarity of the fauna between the Iroise Sea and South Brittany, and between Southern Spain and Portugal. This cluster analysis showed two main groups, which were split at a similarity level of 35%, the southern group, including Southern Spain and Portugal, from the northern group, with the other locations. Within this group, at a level of 40% the South Eastern Bay of Biscay was separated, and at 50% Galicia was also separated from the other northern locations.

Figure 3. Cluster analysis (dendrogram) and ordination analysis (nMDS) of the Jaccard similarity matrix among the Ampelisca samples representing the geographical areas depicted in Figure 2.
The nMDS (Figure 2) showed the opposition, along the horizontal axis, of the most northern and southern groups. The central locations (Bay of Biscay and Galicia) appeared in a transition position along this axis, between the most southern and northern. The vertical axis isolated the Bay of Biscay. A similar ordination solution was shown by the PCA analysis (Figure 4), with the succession along the horizontal axis, from left to right, of the most northern to the most southern locations, with the separation on the positive pole of axis 2 of the samples from Bay of Biscay. This analysis also depicted the species, as vectors. The southern group (South Spain and Portugal) was characterized by 11 species, most of them having a Mediterranean distribution. The Western and the Eastern English Channel, the Iroise Sea and South Brittany areas were characterized by seven species, largely distributed along the south/north gradient and present in the northern areas. The south eastern Bay of Biscay was characterized by 17 species, among which the deep-water Ampelisca species.
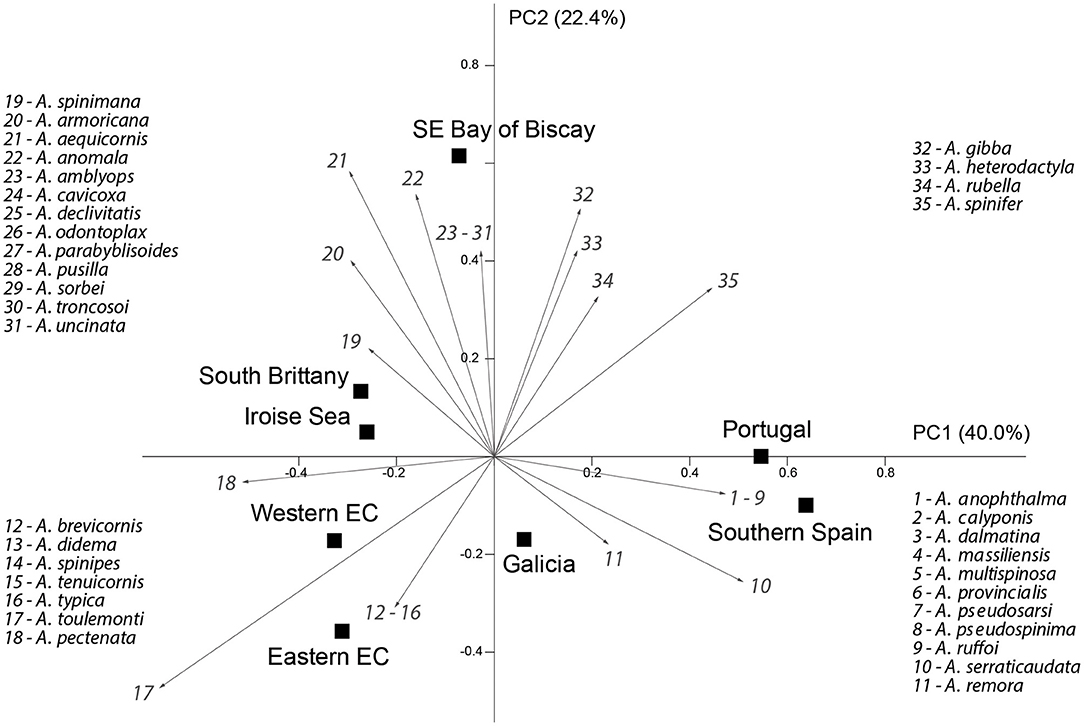
Figure 4. Principal components analysis of the Ampelisca data from Table 1. The analysis was performed with the full set of species but the figure excluded five species with eigenvectors bellow 0.1 (A. eschrichtii, A. latifrons, A. lusitanica, A. sarsi and A. verga). The vectors representing the species were amplified (x4) to clarify their position in relation to the samples.
Ecology
Abundance
The denser populations of Ampelisca were registered in the northern part of the study area (Table 2). Most of the species occurred rarely and were found in very low numbers of individuals, which, for some species, corresponded only to the specimens used for their description. With the exception of A. lusitanica and A. rubella, which occurred on hard-bottom, Ampelisca were mostly found on soft-bottom sediments, ranging from mud to gravel (Bellan-Santini and Dauvin, 1988b, 1989). Most of the species recorded in the study area inhabited mainly muddy sand and sandy mud habitats and were abundant only in shallow muddy fine sand sediment (Bellan-Santini and Dauvin, 1988a, 1989; Sampaio et al., 2016).
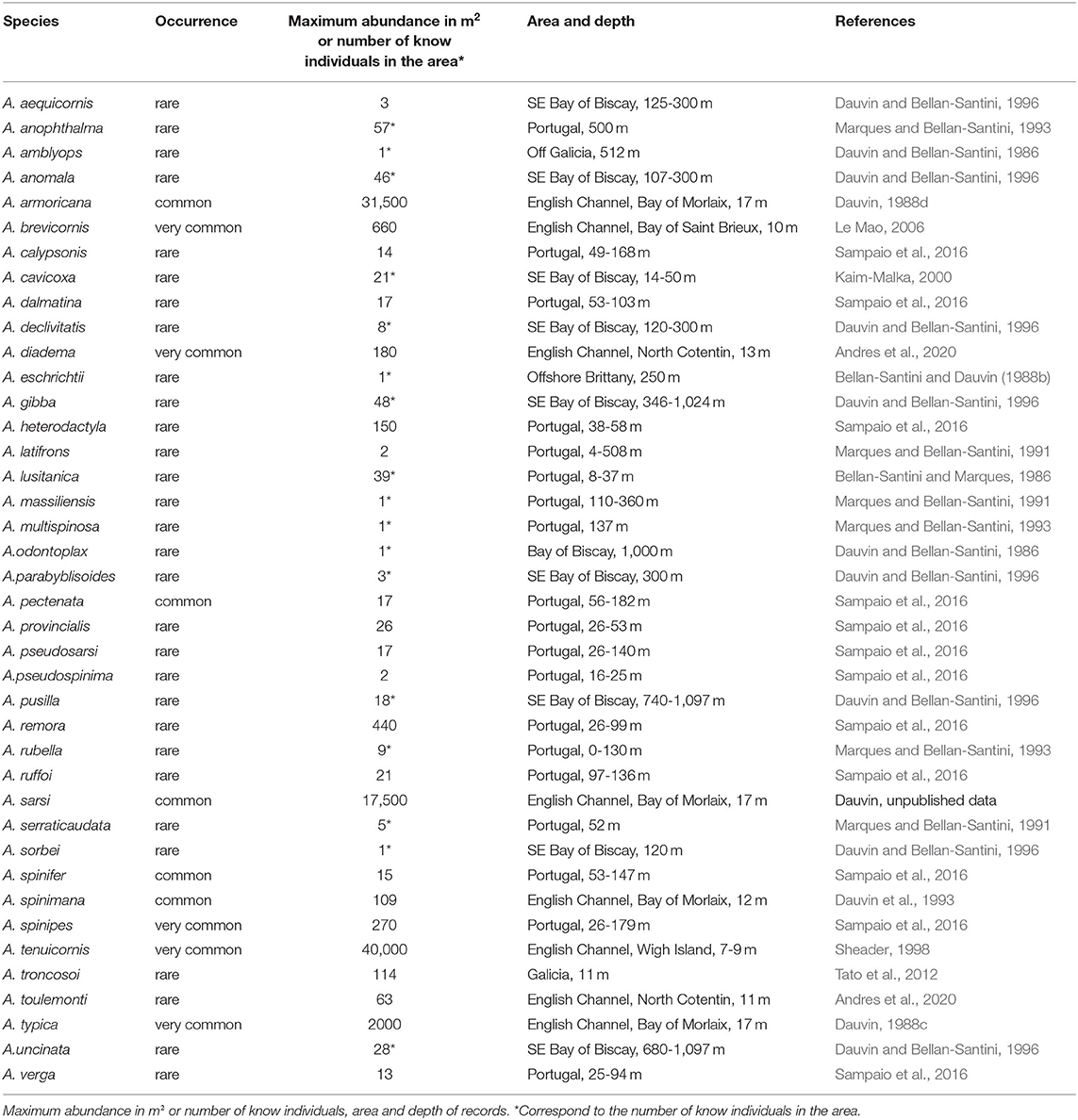
Table 2. Ampelisca occurrence of the species classified in three categories: rare, few records; common: numeroux records, and very common species in most of the shallow soft-bottom communities.
The common species formed abundant populations in the English Channel. Ampelisca brevicornis reached 500 ind.m−2 in October 1982 in the Bay of Morlaix Bay, 403 ind.m2 in July 1978 and 370 ind.m2 in October 1979 in the Rance (Dauvin, 1988b). An abundant population was also reported in the subtidal fine sand of the Bay of Saint Brieux with 660 ind.m−2 (Le Mao, 2006).
Ampelisca tenuicornis formed very high abundance in the Rance, with 6,020 ind.m−2 in summer 1978, 3,870 ind.m−2 in summer 1979 and 2,830 ind.m−2 in summer 1980 (Dauvin, 1988a), and showed a peak of abundance at the end of September 1996 with 25,000 ind.m−2 (Desroy, 1998). In the Bay of Morlaix, its abundance reached 4,000 ind.m−2 in October 1977 (Dauvin, 1988a) and 17,500 ind.m−2 in October 1997 (Dauvin, unpublished data). Long-term study at a fine muddy-sand site to the east of the Isle of Wight on the south coast of England (depth 7–9 m) its abundance reached a maximum of about 40,000 ind.m−2 in late summer (Sheader, 1998). In the North Cotentin, the population reached 9,000 ind.m−2 in the North Cotentin (Andres et al., 2020), while its underpassed 1,000 ind.m−2 in the eastern part of the Bay of Seine (Alizier, 2011).
In the Bay of Morlaix, at the Pierre Noire Station located on an Abra alba fine sand community, the Ampelisca populations showed very high abundances, with 31,500 ind.m−2 for A. armoricana in October 1977 (Dauvin, 1988d), 17,500 ind.m−2 in October 1997 for A. tenuicornis in October 1997 (Dauvin, unpublished data), 6,640 ind.m−2 in October 1987 (Dauvin, 1989), and 17,500 ind.m2 in October 1994 (unpublished data) for A. sarsi, and 2,000 ind.m2 in October 1987 for A. typica (Dauvin, 1988c).
Along the Portugal coast, Ampelisca armoricana populations reached 1,050 ind.m−2; while those of A. brevicornis showed an abundance of 640 ind.m−2 and those of A. remora 440 ind.m−2 (Tables 2, 3).
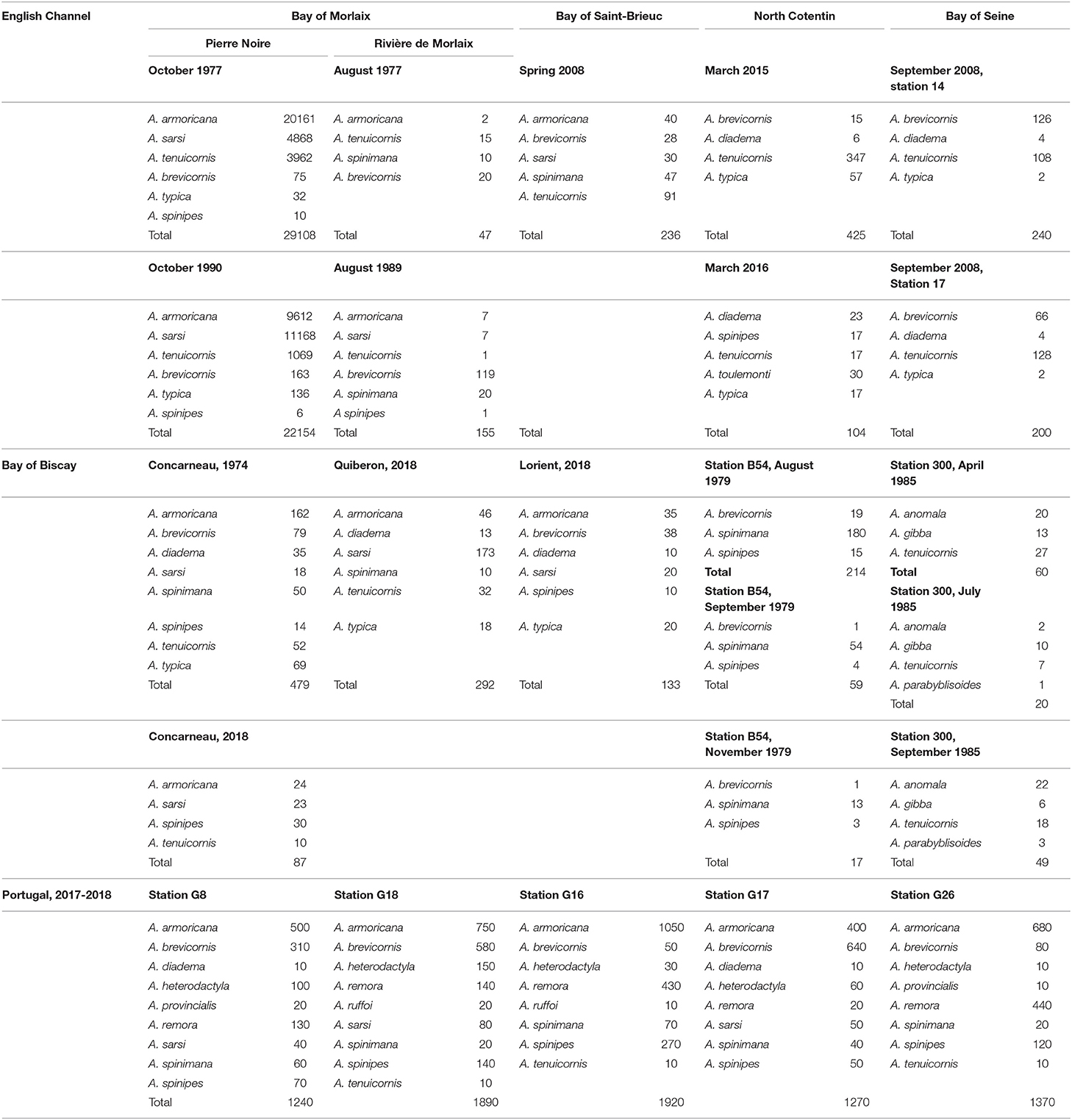
Table 3. Abundance of Ampelisca species in number of ind. m2 in some stations where the species were in sympatry from the English Channel, Bay of Biscay and Portugal coasts.
Sympatry in Ampelisca
Ampelisca populations often occur together, so that their distribution areas overlap or coincide. Several species can occur together in the same habitat and it is possible to identify up to eight species in 1974 in the same station in the Bay of Concarneau, South Brittany (Mesneguen, 1980) and nine species in two stations off the Portugal coast in 2007–2008 (Table 3).
In the western English Channel, Bay of Morlaix, nine species were recorded: A. armoricana, A. brevicornis, A. diadema, A. sarsi, A. spinipes, A. spinimana, A. pectenata, A. tenuicornis and A. typica (Dauvin et al., 1993). In 1977, on a subtidal fine sand community of this bay, three species were dominant A. armoricana, A. sarsi et A. tenuicornis (several thousands of individuals per m2) representing 90% of the abundance, 38% of the biomass and 50% of the secondary production of the community (Dauvin et al., 1993). The Ampelisca diversity (eight recorded species) and abundance were lower (<100 ind.m−2, cf. Table 3) in a muddy sand community of the Bay of Morlaix at the same period. The Ampelisca were shown to be very sensitive to hydrocarbon pollution and as a consequence to the Amoco Cadiz oil spill, which occurred in March 1978 in North Brittany (western English Channel), the species disappeared in the fine sand community (Dauvin, 1988a,b,c,d; Dauvin, 1989). Similar population collapse was also observed following the Aegean oil spill in the North of Galicia, Spain (Gomez Gesteira and Dauvin, 2005). In 1990, 12 years after the Amoco Cadiz oil spill, the abundances of the Ampelisca were at the same order of magnitude of those observed before the incident (Table 3). Ampelisca sarsi dominated the community, replacing A. armoricana which became the second most abundant species, while A. tenuicornis remained the third most abundant; the three other species showed lower abundances before and after the spill. The long-term survey of the colonization of Ampelisca illustrated the high resilience of these holobenthic species, without pelagic larvae, but with high capacity to reconstitute their populations (Dauvin et al., 1993).
In other northern sites, the Ampelisca also showed sympatric distribution; nevertheless, the abundances of the populations never surpassed 500 ind.m−2 (Table 3).
On the 326 stations off the Portuguese continental shelf, Ampelisca were found in 221 stations (68% of the sampled stations) for a total of 19 species and the dominance of five species A. armoricana, A. brevicornis, A. spinimana, A. spinipes and A. tenuicornis. From two to nine species were found in 60% of the stations, while only one species was recorded in 40% of the stations. Nine stations accounted five species, nine other six species, four seven species, four eight species and finally nine species had been found in two stations (Table 3). The total abundances of such sympatric populations were high and included between 1,240 and 1,920 ind. m−2 (Table 3).
Discussion
Taxonomy and Distribution
The North-eastern Atlantic Coast between the Strait of Gibraltar and the Strait of Dover registers 40 Ampelisca species, which is more than the total number of Ampelisca recorded for the Mediterranean Sea, 28 species (Bellan-Santini and Ruffo, 2003). The number of species reached was on the same order of magnitude along the Portuguese coast with 27 species where there is a mixture of Mediterranean, Atlantic and African faunas. The second richest area was the southern part of the Bay of Biscay with 24 species, including eight deep-water species, and 16 species habiting the continental shelf, number which remained lower than those of the Portuguese continental shelf. The biodiversity of Ampelisca showed a clear decrease from the south to the north in the studied area.
The Ampelisca taxonomy is well-established for this North-Atlantic area, and almost 50% of the species (17) had already been described by the end of the nineteenth century, with an extra 19 species during the second part of the twentieth century, while a single species, Ampelisca troncosoi Tato et al. (2012) was recently described.
Presently, new species of Ampelisca at the scale of the world are mainly described in the tropical zone of the Pacific Ocean and in the Indian Ocean (World Register of Marine Species, consulted on November 1st, 2020). In the early years of the twentieth century, the amphipod fauna of the European part of the North-Atlantic Ocean was amongst the better known worldwide, including the Ampelisca mainly due to the large number of new species descriptions by G.O. Sars, and E. Chevreux at the end of the nineteenth and the beginning of the twentieth century. Numerous new species were then described at the end of the twentieth century by D. Bellan-Santini and her co-authors, mainly for the Mediterranean Sea and surrounding areas (North Africa, Portugal and Spain).
Therefore, the discovery of new Ampelisca species for science in the study area is improbable. Nevertheless, genetic studies could be used to elucidate the existence of species hidden in “complex” species with a large geographical distribution such as Ampelisca brevicornis, for which sub-species could be morphologically distinguished (Kaim-Malka, 2000).
Changes in the Species Biology and Distribution in Relation to Climatic Changes
Climatic changes with an increase in sea water temperature along the North-eastern Atlantic coasts could affect both the distribution and the biology of the Ampelisca species.
Concerning the distribution, species tend to extend north their geographical reach, as observed for A. armoricana, A. sarsi, A. spinimana and A. toulemonti in the eastern part of the English Channel, and A. aequicornis and A. spinifer in the English Channel. Southern species presently known to occur along the North Atlantic coast of Africa could progress north and reach the south of Spain and the Portuguese coast, such as A. bidentata, A. ctenopus, A. hupferi, A. monoculata, A. palmata and A. senegalensis (Dauvin and Bellan-Santini, 1988). In both areas, the south of the Iberian Peninsula and the English Channel, encouragement should be given to identify the Ampelisca up to species level, in order to fully grasp such geographical changes in target species.
Concerning the biology, two reproductive cycles are known in the Ampelisca species (Bellan-Santini and Dauvin, 1988b). Univoltine cycles occurs namely in A. armoricana (Dauvin, 1988d) and A. sarsi (Dauvin, 1989). Females are ovigerous at the end of spring and release their young in summer, which reproduce only 1 year later, leading to a single generation and class per reproductive year. Other species present a bivoltine cycle such as A. tenuicornis (Dauvin, 1988a) and A. typica (Dauvin, 1988c). In these, the females are ovigerous at the end of winter-beginning of spring and release their young in spring, which reproduce at the end of summer, beginning of autumn, leading to two generations and one class per reproductive year. Some species are known to show both cycles, depending on the environmental conditions. This is the case of A. brevicornis, which showed both reproductive cycles in the English Channel depending on the sea water temperature, with a bivoltine cycle in years with warmer spring (Dauvin, 1988b), while being solely bivoltine in the Mediterranean Sea (Kaim-Malka, 1969). Sea water increase notably in spring could favor a bivoltine reproductive cycle in the future, which will change the secondary production of such amphipods.
Sympatry and Syntopy in Ampelisca
Rivas (1964) defined sympatric and syntopic species, which corresponded, respectively to “the reference to two or more related species which have the same or overlapping geographic distributions, regardless of whether or not they occupy the same macrohabitats (whether or not the species occurred together the same locality,” and “in reference to two or more related species which occupy the same macrohabitat. These species occur together in the same locality, are observably in close proximity, and could possibly interbreed.” The large geographical and local distributions of Ampelisca species illustrate plainly these both concepts. With a high species richness at the scale of a region, such as along the Portuguese coast with the overlapping of 27 species, and the number of species occurring in the same benthic habitat, such as on Brittany and Portuguese soft-bottom habitats, with up to eight species in the same habitat in South Brittany and nine species in two stations from the Portuguese continental shelf (Table 3).
Co-occurrence, sympatry and syntopy of amphipods were not very common and mainly described to species living in the intertidal zone or very shallow waters such as the Haustoriidae, where five species cohabited on the New Hampshire intertidal zone (Croker, 1967), the Gammaridae with the case of five species of Gammarus coexisting in Danish brackish waters (Kolding and Fenchel, 1979), Pontoporeiidae with two Pontoporeia co-occurring in the Swedish Baltic Sea (Hill and Elmgren, 1987), Hyalidae where six phytal amphipod species of the genus Hyale occurring on the intertidal rocky shores of Coquirnbo, Chile (Lancelotti and Trucco, 1993), Talidridae with Talorchestia brito and of two age classes of Talitrus saltator co-existing along the French Atlantic coast (Fallaci et al., 1999) while eight species were collected in some Tunisian lagoons (Jelassi et al., 2015), and Ischyroceridae with three species of the genus Jassa co-occurring on a wide range of hard substrates in the Helgoland Island in the south of the North Sea (Jelassi et al., 2015).
In the Bay of Belfast, Parker (1984) examined the distribution of Ampelisca brevicornis, A. tenuicornis and A. typica. He showed that these species could live in the same biotope and had no sedimentary preferences in this region, contrary to Sheader (1977) who showed marked sedimentary preferences for two species A. brevicornis and A. tenuicornis in the sandy-muddy bottoms of north-eastern England, in the North Sea.
Few studies, such as that of Buhl-Jensen (1986) on the coasts of Norway had showed the coexistence in the same samples of two to four species of Ampelisca although one species, A. gibba presented high abundances in three of the 21 sampling stations. In the Bay of Concarneau (southern Brittany), Mesneguen (1980) recorded until eight species per station in 1974, but the abundance of the species remained lower than those observed in the Bay of Morlaix in the western entrance of the English Channel in 1977 (Table 3). The Ampelisca Portuguese continental shelf fauna appeared particularly rich with 19 species recorded in the sampling campaigns in 2007–2008 covered the entire Portugal coast with a more dense number of stations in the North (Sampaio et al., 2016). This was in this location that nine Ampelisca species in a single grab (0.1 m2) were sampled, while between seven to nine species were found in 10 stations. This co-occurrence of Ampelisca species in a single grab per station was at our knowledge a record and was incomparable.
Along the North-America Pacific coast from the Baja California to the Bering Sea, large Ampelisca populations coexisted but only a single or a couple of species dominate the soft-bottom communities: A. macrocephala and A. eschriichti in the Bering Sea, A. agassizi and A. careyi off Vancouver Island (Canada), while in the Baja California only A. agassizi was present (Oliver et al., 1983).
Schaffner and Boesch (1982) studied the spatial distribution of A. agassizi and A. vadorum in the palaeo-dunes of the continental shelf off New Jersey (USA), which reach 6 m high. The simultaneous sampling of these two species was strongly linked to the exact position of the sample on the dunes: the deep depressions were almost exclusively populated by A. agassizi, reaching in this habitat abundances of 10,000 individuals per m2. The shallower depressions and the sides of the dunes were populated by both species. The authors suggested that A. agassizi could be more capable to use nutrient resources.
In the end, if the coexistence of Ampelisca species seems to exist most often, the coexistence of large populations such as those observed in Morlaix Bay seems exceptional.
Perspectives
In conclusion, the following research recommendations for the European Ampelisca can be put forward:
• Ampelisca species with a wide geographical distribution should be further studied using molecular tools in order to elucidate their taxonomy. Such studies could focus in the species A. brevicornis, A. diadema, A. tenuicornis, A. typica and A. spinipes;
• Encourage ecologists to identify Ampelisca to species level in order to increase the overall knowledge on their distribution, including the geographical ranges;
• Study in detail, and possibly experimentally, the reasons behind the important sympatry in Ampelisca and how it relates namely to resources competition;
• Verify if trophic guilds, namely suspension feeders and surface deposit feeders, present distinct morphological characteristics, such as length of antennae, presence of setae, and number of setae, and if such adaptations could account for the coexistence of several species without trophic competition;
• Conduct population biology studies at historical sites where the species were previously studied in order to examine the effects of climate change, namely increased temperature in coastal waters, on the life history traits of the Ampelisca.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
J-CD and VQ designed research. J-CD, LS, AR, and VQ performed research and analyzed data. J-CD and VQ wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This study received funding from Normandy Region and the Groupement d'Intérêt Public Seine-Aval for the Bay of Seine sampling, and the Direction Régionale de l'Environnement, de l'Aménagement et du Logement de Normandie for the North-Cotentin sampling. The sampling along the Portuguese coast was supported by the research projects ACOSHELF (POCI/MAR/56441/2004-PPCDT/MAR/56441/2004), MeshAtlantic, with the support of the European Union ERDF-Atlantic Area Program 2009-1/110, and Monitorização Ambiental do Emissário Submarino e da ETAR da Guia do Sistema de Saneamento da Costa do Estoril, funded by SANEST, S.A. The authors declare that this study received funding from SANEST, S.A. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors acknowledge Normandy Region and the Groupement d'Intérêt Public Seine-Aval, the Direction Régionale de l'Environnement, de l'Aménagement et du Logement de Normandie and FCT/MCTES for the financial support to CESAM (UIDP/50017/2020+UIDB/50017/2020), through national funds. The authors thank the two reviewers of the first version of the paper for their very useful remarks and suggestions, and Lucie Millet for the Figures 1, 2.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.643078/full#supplementary-material
References
Alizier, S. (2011). Echelles spatio-temporelles d'observation des relations macrobenthos/sédiments: organisation et changements à long-terme (1988–2009) des communautés benthiques subtidales de la partie orientale de la baie de Seine (PhD Thesis). France: Université de Lille 1.
Andres, S., Pezy, J. P., Martinez, M., Baux, N., Baffreau, A., Méar, Y., et al. (2020). Soft bottom macrobenthic communities in sandy enclaves from the North Cotentin Peninsula (Central English Channel). J. Mar. Biol. Oceanogr. 9:1. doi: 10.4172/2324-8661.1000210
Bachelet, G., Dauvin, J. C., and Sorbe, J. C. (2003). An updated checklist of marine and brackish water amphipoda (crustacea: peracarida) of the southern Bay of Biscay (NE Atlantic). Cah. Biol. Mar. 44, 121–151.
Baux, N. (2018). Dynamique des habitats benthiques sous-contraintes anthropiques: le cas du site de dépôt de dragage d'Octeville (PhD Thesis). France: Université de Caen Normandie.
Baux, N., Pezy, J. P., Bachelet, Q., Baffreau, A., Méar, Y., Poizot, E., et al. (2017). An exceptional rich soft-bottom macrobenthic habitat in a semi-enclosed bay bordering the english channel: rade de cherbourg. Reg. Stud. Mar. Sci. 9, 106–116. doi: 10.1016/j.rsma.2016.11.010
Bellan-Santini, D. (1982). Family ampeliscidae. the amphipoda of the mediteranean. Part 1. gammaridea (acanthonotozomatidae to gammaridea). Mém. Inst. Océanogr. 13, 19–69.
Bellan-Santini, D. (1983). Distribution des Ampelisca (Crustacea, Amphipoda) de Méditerranée. Selected papers on Crustacea. Prof. N. K. Pillai Felicitation Volume. Trivandrum: The Aquarium. p. 155–161.
Bellan-Santini, D., and Dauvin, J. C. (1981). Description d'une nouvelle espèce d'Ampelisca des côtes françaises (Amphipoda). Crustaceana 40, 242–252. doi: 10.1163/156854081X00714
Bellan-Santini, D., and Dauvin, J. C. (1986). Ampelisca remora (Amphipoda): nouvelle espèce des côtes de galice (Atlantique Nord-Est). Crustaceana 51, 38–48. doi: 10.1163/156854086X00043
Bellan-Santini, D., and Dauvin, J. C. (1988a). Actualisation des Données sur L'écologie, la Biogéographie et la Phylogénie des Ampeliscidae (Crustacés - Amphipodes) Atlantiques Après la Révision des Collections d'E. Chevreux. Aspects Récents de la Biologie des Crustacés. 10ème Réunion des Carcinologistes de Langue Française, Concarneau, 6-9 juin 1987. Plouzané: IFREMER. p. 207–215.
Bellan-Santini, D., and Kaim-Malka, R. A. (1977). Ampelisca nouvelles de Méditerranée (Crustacea, Amphipoda). Boll. Mus. Civ. Stor. Nat. Verona 4, 479–523.
Bellan-Santini, D., and Dauvin, J. C. (1988b). Eléments de synthèse sur les Ampelisca du Nord-Est Atlantique. Crustaceana 13, 20–60.
Bellan-Santini, D., and Dauvin, J. C. (1989). Distribution verticale et répartition biogéographique de crustacés holobenthiques filtreurs: exemple des amphipodes du genre Ampelisca, groupe zoologique à forte spéciation. Bull. Soc. Géol. France 3, 561–568.
Bellan-Santini, D., and Dauvin, J. C. (1997). Ampeliscidae (Amphipoda) from Iceland with a description of a new species (Contribution to the BIOICE Research Program. J. Nat. Hist. 31, 1157–1173. doi: 10.1080/00222939700770621
Bellan-Santini, D., and Marques, J. C. (1986). Une nouvelle espèce d'Ampelisca (Crustacea, Amphipoda) des Côtes du Portugal (Atlantique Nord-Est): Ampelisca lusitanica n.sp. Cah. Biol. Mar. 27, 153–162.
Bellan-Santini, D., and Ruffo, S. (2003). Biogeography of benthic marine Amphipoda in Mediterranean Sea. Biogeographia 24, 273–292. doi: 10.21426/B6110176
Buhl-Jensen, L. (1986). The benthic Amphipod fauna of the west-Norwegian continental shelf compared with the fauna of five adjacent fiords. Sarsia 71, 193–208. doi: 10.1080/00364827.1986.10419690
Cardoso, F. G., Dolbeth, M., Sousa, R., Relvas, P., Santos, R., Silva, A., et al. (2019). “The Portuguese coast,” in World Seas: An Environmental Evaluation, Second Edition, Volume I: Europe, The Americas and West Africa, ed C. Sheppard (London: Academic Press, Elsevier), 189–208. doi: 10.1016/B978-0-12-805068-2.00009-7
Clarke, K. R., and Gorley, R. N. (2006). PRIMER v6: User Manual/Tutorial. Plymouth: PRIMER-E, 190 pp.
Croker, R. A. (1967). Niche diversity in five sympatric species of intertidal amphipods (Crustacea: Haustoriidae). Ecol. Monogr. 37, 173–200. doi: 10.2307/1948437
Dauvin, J. C. (1988a). Biologie, dynamique et production de populations de Crustacés Amphipodes de la Manche occidentale. 1. Ampelisca tenuicornis Liljeborg. J. Exp. Mar. Biol. Ecol., 118, 55–84. doi: 10.1016/0022-0981(88)90122-0
Dauvin, J. C. (1988b). Biologie, dynamique et production de populations de Crustacés Amphipodes de la Manche occidentale. 2. Ampelisca brevicornis (Costa). J. Exp. Mar. Biol. Ecol., 119, 213–233. doi: 10.1016/0022-0981(88)90194-3
Dauvin, J. C. (1988c). Biologie, dynamique et production de populations de Crustacés Amphipodes de la Manche occidentale. 3. Ampelisca typica (Bate). J. Exp. Mar. Biol. Ecol. 121, 1-22. doi: 10.1016/0022-0981(88)90020-2
Dauvin, J. C. (1988d). Life cycle, dynamics and productivity of crustacea-amphipoda from the western part of the English Channel. 4. Ampelisca armoricana Bellan-Santini and Dauvin. J. Exp. Mar. Biol. Ecol. 123, 235–252. doi: 10.1016/0022-0981(88)90045-7
Dauvin, J. C. (1989). Life cycle, dynamics and productivity of Crustacea-Amphipoda from the western part of the English Channel. 5. Ampelisca sarsi Chevreux. J. Exp. Mar. Biol. Ecol. 128, 31–56. doi: 10.1016/0022-0981(89)90091-9
Dauvin, J. C. (1996). Ampeliscidae from the Faroe Islands. Contribution to the Biofar programme. Boll. Mus. Civ. Stor. Nat. Verona 20, 47–60.
Dauvin, J. C. (1999). Mise à jour de la liste des espèces d'Amphipodes (Crustacea: Peracarida) présents en Manche. Cah. Biol. Mar. 40, 165–183.
Dauvin, J. C., and Bellan-Santini, D. (1982). Description de deux nouvelles espèces d'Ampelisca des côtes Françaises Atlantiques (Crustacea, Amphipoda): Ampelisca toulemonti n.sp. et Ampelisca spooneri n.sp. Cah. Biol. Mar. 23, 253–268.
Dauvin, J. C., and Bellan-Santini, D. (1985). Collection des Ampeliscides d'Edouard Chevreux du Muséum national d'Histoire naturelle: description d'Ampelisca melitae et d'A. monoculata n.sp. et redescription d'A. verga Reid. Bull. Mus. Nat. Hist. Nat. 4,7A3, 659–675.
Dauvin, J. C., and Bellan-Santini, D. (1986). Révision de la collection des Ampeliscidés (Crustacean, Amphipoda) d'Edouard Chevreux au Muséum national d'Histoire naturelle. Bull. Mus. Nat. Hist. Nat. 4, 867–891.
Dauvin, J. C., and Bellan-Santini, D. (1988). Illustrated key to Ampelisca species from the North-Eastern Atlantic. J. Mar. Biol. Assoc. 68, 659–676. doi: 10.1017/S0025315400028782
Dauvin, J. C., and Bellan-Santini, D. (1996). Ampeliscidae (Amphipoda) from the Bay of Biscay. J. Crust. Biol. 16, 149–168. doi: 10.2307/1548938
Dauvin, J. C., and Bellan-Santini, D. (2000). Biodiversity and the biogeographic relationships of the Amphipoda Gammaridea along the French coastline. J. Mar. Biol. Assoc. 84, 621–628. doi: 10.1017/S0025315404009658h
Dauvin, J. C., and Bellan-Santini, D. (2002). Les Crustacés Amphipodes Gammaridea benthiques des côtes françaises métropolitaines: bilan des connaissances. Crustaceana 75, 299–340. doi: 10.1163/156854002760095408
Dauvin, J. C., Bellan-Santini, D., and Bellan, G. (1993). Les genres Ophelia et Ampelisca de la région de Roscoff: exemples d'allotopie et de syntopie dans les communautés marines de substrat meuble. Cah. Biol. Mar. 34, 1–15.
Dauvin, J. C., and Toulemont, A. (1988). Données Préliminaires sur les Amphipodes de l'Iroise et de ses Abords, Leurs Affinités Biogéographiques. Aspects Récents de la Biologie des Crustacés. 10ème Réunion des Carcinologistes de Langue Française, CONCARNEAU, 6-9 Juin 1987. Plouzané: IFREMER. p. 217–222.
Desroy, N. (1998). Les peuplements benthiques de substrats meubles du bassin maritime de la Rance. Evolution de la biodiversité et effets de l'activité prédatrice de Nephthys hombergii (Annélide polychète) sur le recrutement (PhD thesis). France: University of Rennes 1. p. 206.
Fallaci, M., Aloia, A., Audoglio, M., Colombini, I., Scapini, F., and Chelazzi, L. (1999). Differences in behavioural strategies between two sympatric talitrids (Amphipoda) inhabiting an exposed sandy beach of the French Atlantic coast. Estuar. Coast. Shelf Sci. 48, 469–482. doi: 10.1006/ecss.1998.0437
Gomez Gesteira, J. L., and Dauvin, J. C. (2005). Impact of the Aegean sea oil spill on the Ría of Ares and Betanzos fine sand community (Northwest Spain). Mar. Envir. Res. 60, 289–316. doi: 10.1016/j.marenvres.2004.11.001
Hill, C., and Elmgren, R. (1987). Vertical distribution in the sediment in the co-occurring benthic amphipods Pontoporeia affinis and P. femorata. Oikos 49, 221–229. doi: 10.2307/3566029
Horton, T., Lowry, J., De Broyer, C., Bellan-Santini, D., Coleman, C. O., Daneliya, M., et al. (2021). World Amphipoda Database. Available online at: http://www.marinespecies.org/amphipoda (accessed January 1, 2021).
Jelassi, R., Khemaissia, H., Zimmer, M., Garbe-Schönberg, D., and Nasri-Ammar, K. (2015). Biodiversity of Talitridae (Crustacea, Amphipoda) in some Tunisian coastal lagoons. Zool. Studi. 54:17. doi: 10.1186/s40555-014-0096-1
Kaim-Malka, R. A. (1969). Biologie et écologie de quelques Ampelisca de la région de Marseille. Téthys 1, 977–1022.
Kaim-Malka, R. A. (2000). Elevation of two eastern Atlantic varieties of Ampelisca brevicornis (Costa, 1853) (Crustacea, Amphipoda) to full specific rank with redescription of the species. J. Nat. Hist. 34, 1939–1966. doi: 10.1080/00222930050144792
Kolding, S., and Fenchel, T. M. (1979). Coexistence and life cycle characteristics of five species of the amphipod genus Gammarus. Oikos 33, 323–327. doi: 10.2307/3544009
Lancelotti, D. A., and Trucco, R. G. (1993). Distribution patterns and coexistence of six species of the amphipod genus Hyale. Mar. Ecol. Progr. Ser. 93, 131–141. doi: 10.3354/meps093131
Le Mao, P. (2006). Inventaire de la Biodiversité Marine du Golfe Normano-Breton. Les Crustacés Malacostracés. Amphipodes. Rapport RST/DOP.LER/SM 06–13. Saint-Malo: Ifremer.
Marques, J. C., and Bellan-Santini, D. (1991). Gammaridea and Caprellidea (Crustacea-Amphipoda) of the Portuguese south-western continental shelf: taxonomy and distributional ecology. Bijdr. Dierk. 61, 65–87. doi: 10.1163/26660644-06102001
Marques, J. C., and Bellan-Santini, D. (1993). Biodiversity in the ecosystem of the Portuguese continental shelf: distributional ecology and the role of benthic amphipods. Mar. Biol. 115, 555–564. doi: 10.1007/BF00349362
Martins, R., Azevedo, M. R., Mamede, R., Sousa, B., Freitas, R., Rocha, F., et al. (2012). Sedimentary and geochemical characterization and provenance of the Portuguese continental shelf soft-bottom sediments. J. Mar. Syst. 91, 41–52. doi: 10.1016/j.jmarsys.2011.09.011
Martins, R., Quintino, V., and Rodrigues, A. M. (2013a). Diversity and spatial distribution patterns of the soft-bottom macrofauna communities on the Portuguese continental shelf. J. Sea Res. 83, 173–186. doi: 10.1016/j.seares.2013.03.001
Martins, R., Sampaio, L., Quintino, V., and Rodrigues, A. M. (2014). Diversity, distribution and ecology of benthic molluscan communities on the Portuguese continental shelf. J. Sea Res. 93, 75–89. doi: 10.1016/j.seares.2013.11.006
Martins, R., Sampaio, L., Rodrigues, A. M., and Quintino, V. (2013b). Soft-bottom Portuguese continental shelf polychaetes: diversity and distribution. J. Mar. Syst. 123–124, 41–54. doi: 10.1016/j.jmarsys.2013.04.008
Mesneguen, A. (1980). La macrofaune benthique de la baie de Concarneau: peuplements, dynamique de populations, prédation exercée par les poissons (PhD thesis). France: Université de Bretagne Occidentale. 127pp.
Oliver, J. S., Slaitery, P. N., Silberstein, M. A., and O'Connor, E. F. (1983). A comparison of gray whale, Eschrichtius robustus, feeding in the Bering Sea and Baja California. Fish. Bull. 81, 513–522.
Parker, J. C. (1984). The distribution of the subtidal Amphipoda in Belfast Lough in relation ta sediment types. Ophelia 23, 119–140. doi: 10.1080/00785326.1984.10426608
Rigolet, C., Dubois, S. F., Droual, G., Caisey, X., and Thiébaut, E. (2012). Life history and secondary production of the amphipod Haploops nirae (Kaim-Malka, 1976) in the Bay of Concarneau (South Brittany). Estuar. Coast. Shelf Sci. 113, 259–271. doi: 10.1016/j.ecss.2012.08.014
Rigolet, C., Dufois, S. F., and Thiébaut, E. (2014). Benthic control freaks: effects of the tubiculous amphipod Haploops nirae on the specific diversity and functional structure of benthic communities. J. Sea Res. 85, 413–427. doi: 10.1016/j.seares.2013.07.013
Rivas, L. R. (1964). A reinterpretation of the concepts “Sympatric” and “Allopatric” with proposal of the additional terms “Syntopic” and “Allotopic”. Syst. Zool. 13, 42–43. doi: 10.2307/2411436
Sampaio, L., Mamede, R., Ricardo, F., Magalhães, L., Rocha, H., Martins, R., et al. (2016). Soft-sediment crustacean diversity and distribution along the Portuguese continental shelf. J. Mar. Syst. 163, 43–60. doi: 10.1016/j.jmarsys.2016.06.011
Schaffner, L. C., and Boesch, D. F. (1982). Spatial and temporal ressource use by dominant benthic Amphipoda (Ampeliscidae and Corophiidae) on the Middle Atlantic Bight outer continental shelf. Mar. Ecol. Prog. Ser. 9, 231–243. doi: 10.3354/meps009231
Schellenberg, A. (1925). Crustacea VIII. Amphipoda. In Michaelsen Beitrage zu Kennt. Meeresfauna West Africas III, 13–204.
Sheader, M. (1977). Production and population dynamics of Ampelisca tenuicornis (Amphipoda) with notes on the biology of its parasite Sphaeronella longipes (Copepoda). J. Mar. Biol. Ass. 57, 955–968. doi: 10.1017/S0025315400026047
Sheader, M. (1998). Grazing predation on a population of Ampelisca tenuicornis (Gammaridae: Amphipoda) off the south coast of England. Mar. Ecol. Progr. Ser. 164, 253–262. doi: 10.3354/meps164253
Keywords: North-Eastern Atlantic, Ampeliscidae, key of species, distribution, abundance, bio-geographical gradient, co-occurrence
Citation: Dauvin J-C, Sampaio L, Rodrigues AM and Quintino V (2021) Taxonomy and Ecology of Sympatric Ampelisca Species (Crustacea, Amphipoda) From the Strait of Gibraltar to the Strait of Dover, North-Eastern Atlantic. Front. Mar. Sci. 8:643078. doi: 10.3389/fmars.2021.643078
Received: 17 December 2020; Accepted: 09 February 2021;
Published: 15 March 2021.
Edited by:
Juan Moreira Da Rocha, Autonomous University of Madrid, SpainReviewed by:
Ignacio Winfield, National Autonomous University of Mexico, MexicoXiaoshou Liu, Ocean University of China, China
Copyright © 2021 Dauvin, Sampaio, Rodrigues and Quintino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Claude Dauvin, amVhbi1jbGF1ZGUuZGF1dmluQHVuaWNhZW4uZnI=
 Jean-Claude Dauvin
Jean-Claude Dauvin Leandro Sampaio
Leandro Sampaio Ana Maria Rodrigues
Ana Maria Rodrigues Victor Quintino
Victor Quintino