
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 17 August 2021
Sec. Marine Ecosystem Ecology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.641137
This article is part of the Research Topic Integrated Marine Biosphere Research: Ocean Sustainability, Under Global Change, for the Benefit of Society View all 31 articles
 Kaixuan Cui1,2,3†
Kaixuan Cui1,2,3† Yi Dong1,2,4*†
Yi Dong1,2,4*† Xiaoxia Sun3,5
Xiaoxia Sun3,5 Li Zhao1,2,4
Li Zhao1,2,4 Haijian Du1
Haijian Du1 Jia Liu1
Jia Liu1 Chaofeng Wang1,2,4
Chaofeng Wang1,2,4 Chen Liang1
Chen Liang1 Yicong Zhao1,2,3
Yicong Zhao1,2,3 Si Chen1,2,3
Si Chen1,2,3 Jun Xuan1,2,3
Jun Xuan1,2,3 Suheng Li1,2,3
Suheng Li1,2,3 Yuan Zhao1,2,4
Yuan Zhao1,2,4 Tian Xiao1,2,4*
Tian Xiao1,2,4*Coliform bacteria (CB) can be used as an indicator of seawater quality. Long-term monitoring of seawater quality based on CB abundance is lacking in Jiaozhou Bay. In this study, CB abundance in surface seawater of 12 different stations in Jiaozhou Bay was investigated by culturing method. The results showed that: (1) the abundance of CB showed a decreasing tendency during the investigation. During 2004–2007, 2008–2013, and 2014–2017, the average CB abundance decreased significantly, forming a “three stages phenomenon”; (2) the average CB abundance in the first half of the year was lower than that in the second half; (3) the CB abundance in Jiaozhou Bay was spatially heterogeneous. The maximum average CB abundance was observed in the estuary area, and followed by the bay mouth area, the outer bay area, and the inner bay area. The highest abundance may be associated with sewage discharge related to human activities; (4) the abundance of CB was most positively correlated with the concentration of ammonium salt and nitrate, while most negatively correlated with salinity; (5) the years 2007 (2008) and 2013 (2014) were time points of the “three stages phenomenon.” These time points coincide with environmental governance actions, indicating that the actions have played a prominent role in improving seawater quality. Long-term survey of CB can not only serve as an indicator of seawater quality, but also provide a basis for the development of environmental governance strategies and pollution control.
Coliform bacteria (CB) are a group of bacteria found in many environments, but are primarily associated with human and animal intestines and wastes. They are commonly used as an indicator of water quality, and have a long history of being used for assessing seafood safety (Tennant and Reid, 1961; Hunt et al., 1981). For example, over 8 years (from 1987 to 1994), Schiff and Kinney (2001) used total coliforms as an indicator of bacterial contamination of stormwater discharging into Mission Bay (an enclosed water body) in California, United States. They found that, based on more than 7000 samples from 20 stations, total coliform numbers were always higher during wet conditions than dry conditions. Bharathi et al. (2018) also used total coliforms to investigate seasonal pollution of Ennore coastal waters, India in 2011. Similar to the study of Schiff and Kinney, they found that the abundance of CB was higher in wet conditions.
Jiaozhou Bay (35°58′–36°18′N, 120°04′–120°23′E) is a typical shallow coastal semi-enclosed bay located in the western Yellow Sea, southeast of Shandong Peninsula (Xing et al., 2017b). It is most widely known as the sailing venue for the 29th Olympic Games in 2008. The bay has a water area of 370 km2 and an average depth of 7 m (Xing et al., 2017a), is surrounded on three sides by the city of Qingdao, Jiaozhou and the west coast new area of Qingdao. The Yellow Sea and Jiaozhou Bay are connected by a narrow bay mouth of approximately 2.5 km width (Shen, 2001). Seasonal freshwater inflow, a half-day tidal exchange with the open sea (Feng et al., 2018), the East Asian monsoon, and the Yellow Sea water mass are some of the natural factors affecting the bay, but it is also affected by anthropogenic factors including rapid economic development, population expansion, and pollution from land-based source. Because of the interactions between natural changes and human activities, Jiaozhou Bay is an ideal study area for ecological investigations (Feng et al., 2018). Many marine ecological investigations have been carried out in the bay, including studies of nutrients, microorganisms, phytoplankton, zooplankton, and the macrobenthos. For example, Liu et al. (2005) studied the relationship between nutrient composition and phytoplankton composition, trophic interactions, and the sustainability of living resources. Hu et al. (2018) studied the morphological and phylogenetic characterization of an isolate of the dinoflagellate Margalefidinium fulvescens. Ma et al. (2015) used stable isotopes to explore the trophic spectrum of the food web in the bay in spring and fall, and Wang et al. (2017) reported that macrofaunal assemblages in the bay were significantly negatively correlated with the abundance of Manila clams.
With respect to microorganisms, Dang et al. (2010) found that continental inputs may influence the composition and abundance of ammonia-oxidizing Betaproteobacteria in Jiaozhou Bay sediment. Zhou et al. (2018) investigated the role of bacteria in the transport and conversion of organic matters in the bay, and found that the average bacterial contribution to organic carbon in the sediments was higher in the western inner bay than in the outer bay, while the average activity of extracellular enzymes showed the opposite trend. The microbial community in Jiaozhou Bay has also been a focus of our research. For example, we investigated bacterial communities during Ulva blooms using denaturing gradient gel electrophoresis and 16S rDNA clone libraries, and showed that the microbial community changed during blooms and differed between the seawater and the surface of Ulva prolifera (Liu et al., 2011).
Depending on the different application functions and protection objectives of the sea area, the National Seawater Quality Standard of People’s Republic of China (NSQS, GB 3097-1997) divides seawater quality into four classes. From Class I to Class IV, the seawater quality is declining. The CB abundance limit is set as 1.00 × 103 MPN/100 ml for Class I to III and Class IV (Supplementary Table 1). The abundance of CB in Jiaozhou Bay has been monitored for a long time. Zhao (2007) focused on seasonal changes, and found that in summer and autumn from 2002 to 2004 the abundance of CB greatly exceeded Class IV of the NSQS. Dong (2013) found the abundance of CB was significantly correlated with salinity and the concentration of total nitrogen and dissolved organic carbon in Jiaozhou Bay seawater, and showed distinct geographical distributions (highest in the estuary area and lowest in the inner bay area) during the period 2004 to 2010.
Economic development occurred very rapidly in recent years. As Jiaozhou Bay is semi-enclosed, it has been significantly affected by human activities, so long-term research in this area could help explain the environmental response mechanisms. In this regard, the biological communities in Jiaozhou Bay have been investigated at various time scales. For instance, Sun et al. (2011b) reported meteorological and hydrological factors, the phytoplankton community structure (Sun et al., 2011c) and the zooplankton community (Sun et al., 2011a) at various time scales in the bay. They found that: temperature has fluctuated with a trend of increase over the past 100 years; the abundance of phytoplankton and the zooplankton biomass has been increasing for approximately 30 years; and the composition of dominant phytoplankton species has changed. However, no long-term research (>10 years) on CB has been reported.
With rapid economic development, the pressure to control seawater quality is also increasing. To ensure an ecologically sustainable and livable city, the government has implemented a series of environmental protection laws, such as the “Plan for Prevention and Control of Pollution in Coastal Waters” (The Ministry of Environmental Protection of the People’s Republic of China, 2017) and the “Protection Regulations of Qingdao Jiaozhou Bay” (The Ocean and Fishery Administration of Qingdao, 2014). Two important time periods in the study are noteworthy, one in 2007 (2008) and the other in 2013 (2014). To hold successful sailing competitions during the 2008 Olympic Games, the water quality in Jiaozhou Bay was strictly and successfully controlled in 2007 and 2008. In 2012, the Eighteenth National Congress of the Communist Party of China was held. Under the guidance of the spirit of the congress, the construction of ecological civilization has received more and more attention. The “Protection Regulations of Qingdao Jiaozhou Bay,” adopted in 2014, aims to promote the construction of ecological civilization. As a practical area, Jiaozhou Bay has been affected by the policies and regulations and received extensive attention. These policies and regulations focused on the environment and resource protection of Jiaozhou Bay, such as pollution control, ecological restoration, supervision, ecological protection, etc. Although pollution discharges have increased year by year with the rapid economic development, total emissions have decreased thanks to the improvement of sewage treatment capacity (Yue et al., 2016). The Jiaozhou Bay seawater quality has improved over this period. As CB are environmental indicator bacteria, in this study we investigated whether their abundance is declining over time and whether it is affected by environmental conditions.
Surface seawater samples were taken monthly by the Jiaozhou Bay National Marine Ecosystem Research Station from January 2004 to December 2017. The twelve sampling stations (Sts.; Figure 1 and Table 1) were distributed in four areas, including the estuary area (Sts. A5 and C4, near the Licun and Haibo rivers, respectively), the inner bay area (Sts. A3, B2, C1, C3, and D1), the bay mouth area (Sts. D3 and D5) and the outer bay area (Sts. D6, D7, and D8).
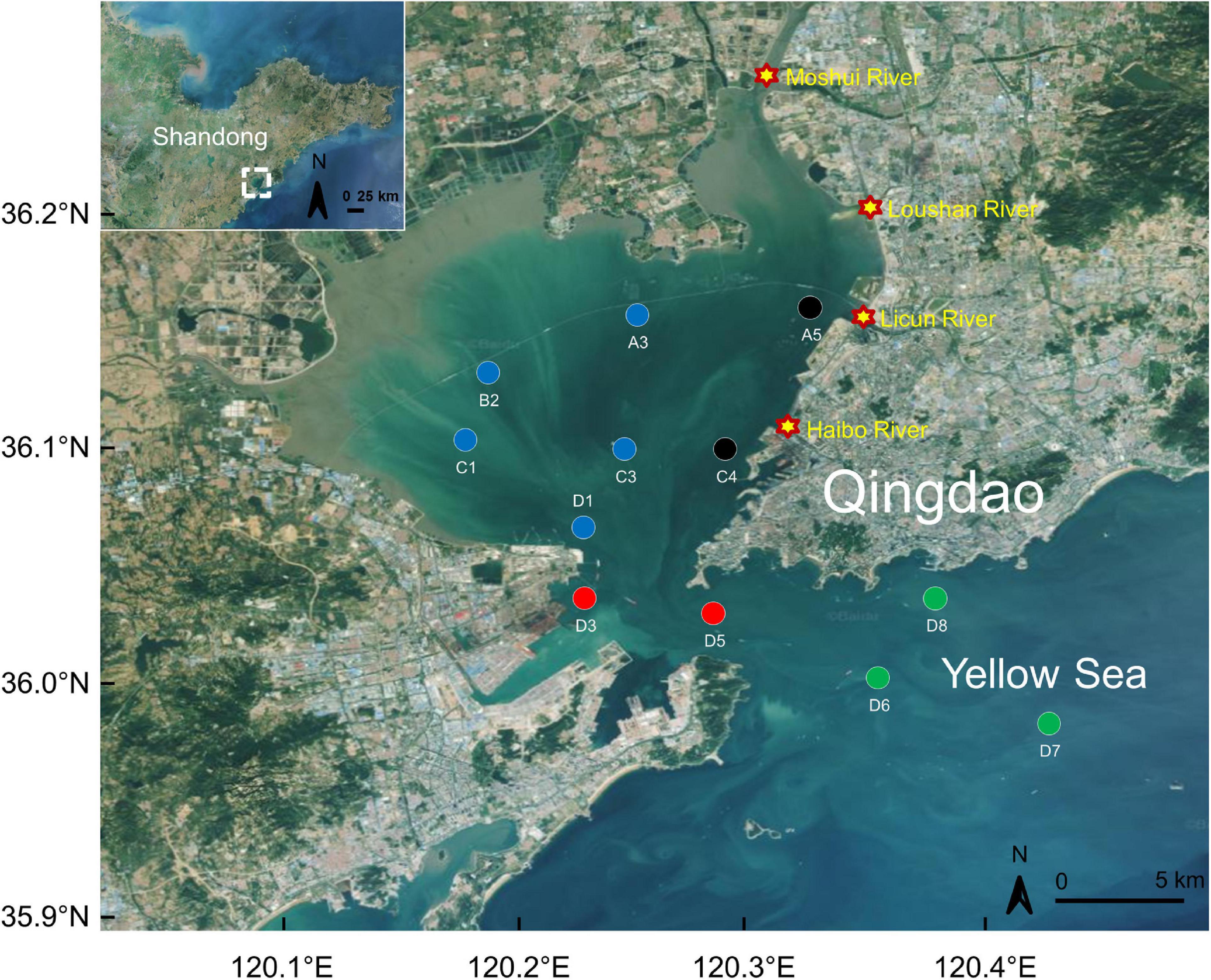
Figure 1. Sampling stations (circles) within the study areas. Black, estuary area; blue, inner bay area; red, bay mouth area; green, outer bay area. Stars indicate river mouths. Map data were obtained from © Baidu and downloaded at https://map.baidu.com/.
In order to cultivate CB and obtain their abundance, about 8 ml surface seawater was collected using a Niskin hydrophore sampler and stored in 10 ml centrifuge tubes in an ice box on the R/V Chuangxin. All samples were returned to the laboratory for cultivation within 6 h.
For heterotrophic bacteria (HB) abundance determination, seawater samples were also collected by Niskin hydrophore sampler and then fixed with paraformaldehyde (final concentration 1%) immediately. The samples were then stored in liquid nitrogen until analyzed.
During the study, the data of temperature (T) and salinity (S) of seawater were measured during sampling by conductivity temperature depth (CTD).
The concentrations of dissolved inorganic silicate (DISi), dissolved inorganic phosphate (DIP), nitrate (), ammonium salt (), and chlorophyll a (Chl a) were measured according to National Standard of the Specification for Marine Monitoring of People’s Republic of China (GB 17378-2007). Specifically, the concentration of DISi was measured by silico-molybdenum blue method, DIP was measured by phosphorus molybdenum blue method, was measured by cadmium-copper column reduction method, was measured by indophenol blue method, and Chl a was measured by fluorescence spectrophotometry. According to National Standard of the Specification for Oceanographic Survey of People’s Republic of China (GB 12763-2007), the concentration of nitrite () was measured by diazo-coupling method. The abundance of HB was analyzed using flow cytometry (Zhao et al., 2016).
The rainfall data were obtained from the Qingdao Meteorological Bureau.
Cold-stored samples returned to the laboratory were processed in a clean bench. Three dilutions (original, 10-fold, and 100-fold) of each seawater sample were prepared using sterilized seawater. For each dilution, 1 ml of sample was added to a tube containing 9 ml sterile lactose peptone broth medium; this process was repeated in triplicate. The tubes were incubated at 37°C for 48 ± 2 h. After incubation, those tubes showing color change and/or gas production were counted. Based on these data the abundance of CB was determined using most probable number (MPN) table (National Standards of the People’s Republic of China, GB 4789.3–2016).
Monthly average abundances were calculated as the average of the total combined abundance for the various sampling stations. The data were summed up and then average the summation. The monthly average abundances in specific months were used to calculate the 14-year average abundances for those months. The annual average abundance for each year was calculated from the average monthly abundances in those years. The average abundance at each station was calculated from the abundance at each station in different years.
The statistical significance of differences of CB abundances was all assessed by the PERMANOVA analysis using the Vegan package in R (version 4.0.5), and the pair-wise test was performed to assess the differences further. Probabilities were corrected by the Benjamini method. The analyses focused on the data of the whole Jiaozhou Bay were performed to determine whether there was a significant difference in CB abundances considering the factors: (1) different stages of the study (temporal factor), 12 sampling stations (spatial factor), and their interactions; (2) different periods in the annual study (temporal factor), 12 sampling stations (spatial factor), and their interactions; and (3) the 14 years of the investigation (temporal factor), different areas of the sampling stations (spatial factor), and their interactions, respectively.
In order to analyze the correlations between CB abundance and environmental factors, and to identify the most important factors affecting the variation of CB abundance in Jiaozhou Bay, the Spearman’s rho correlation analysis and principal component analysis (PCA) among the CB, environmental parameters and HB were calculated using the Psych and Factoextra package in R (version 4.0.5), respectively. Analysis using PCA is useful for clustering and reducing the dimensionality of data (Choi et al., 2004), and can also preserve most of the variations.
The average depths of 12 stations varied from 3.0 to 29.3 m (Table 1). But due to the tidal, the depth of each sampling station was slightly different from the average depth.
During the study period, the average temperature (T), salinity (S), and concentrations of DISi, DIP, (), , , and Chl a were 14.56°C, 30.70, 6.24 mg/L, 0.76 mg/L, 1.61 mg/L, 9.16 mg/L, 8.13 mg/L, and 1.51 mg/L, respectively (data provided by the Jiaozhou Bay National Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences) (Supplementary Table 2).
The abundance of HB in surface seawater during the investigation changed from 7.96 × 106 cells/L to 1.04 × 1010 cells/L (Supplementary Table 2).
The rainfall data varied from 0.00 to 482.20 mm in 168 months (Supplementary Table 2). In annual variation, the maximum data occurred in July (174.33 mm) while the minimum value occurred in January (6.74 mm).
A total of 1997 samples were collected from 2004 to 2017 for measurement of the abundance of CB. Over those 14 years, 168 monthly average CB abundance were calculated (Figure 2). The maximum abundance was in September 2005 (6.13 ± 5.21 × 103 MPN/100 ml), while the minimum was in March 2015 (0.03 ± 0.03 × 103 MPN/100 ml). The difference between the maximum and minimum was more than 200-fold.
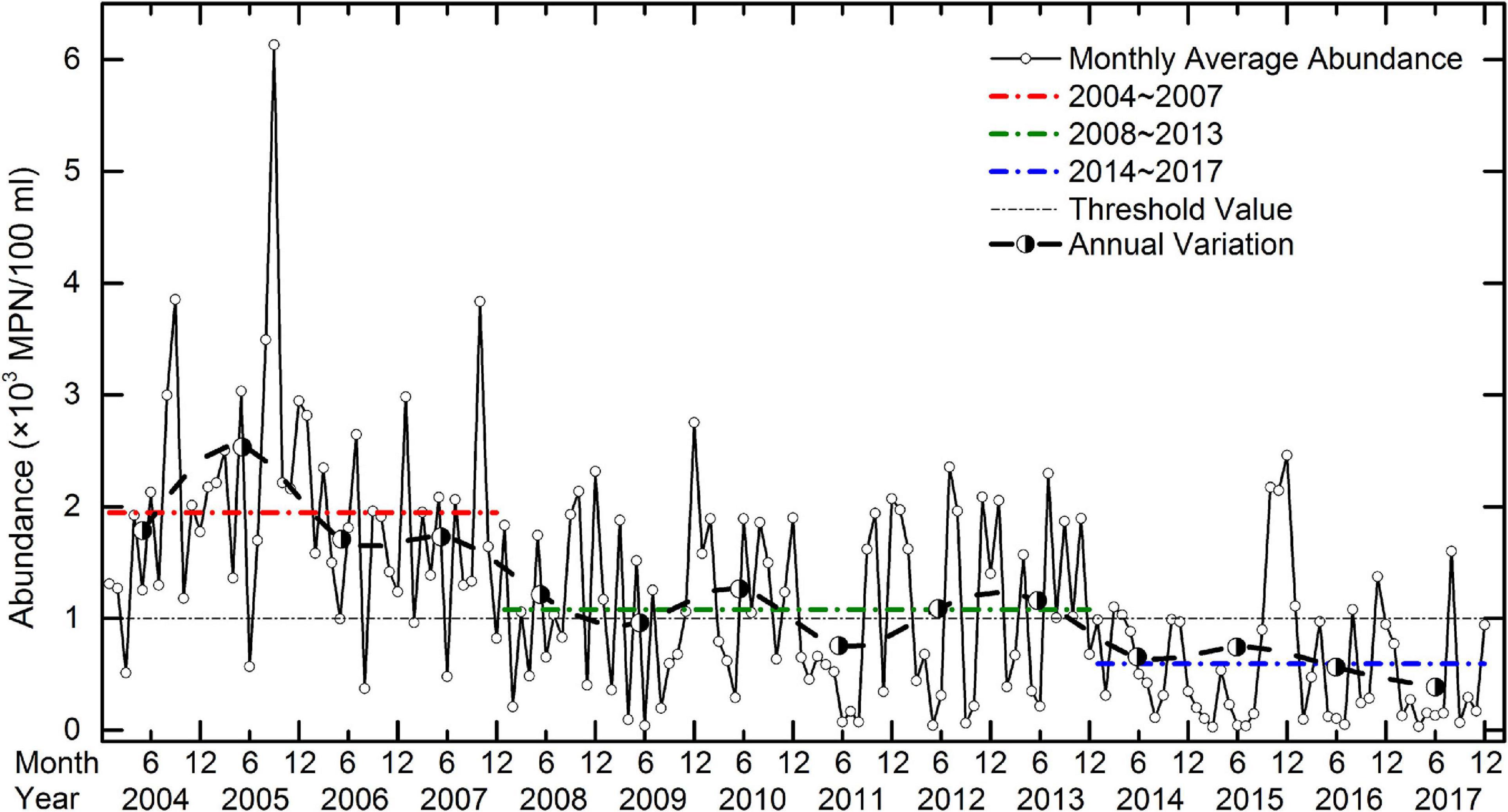
Figure 2. Monthly average abundance (× 103 MPN/100 ml) of CB in Jiaozhou Bay. The red, green, and blue lines show the average abundances from 2004 to 2007, 2008 to 2013, and 2014 to 2017, respectively. The dotted line (threshold value) represents the Class IV standard of CB abundance specified by the NSQS. The bold broken line shows the annual average abundance of CB.
During the study, the annual abundance of CB in Jiaozhou Bay tended to decrease (Figure 2) suggesting that the environmental conditions in Jiaozhou Bay improved. The maximum annual average abundance occurred in 2005 (2.54 ± 1.37 × 103 MPN/100 ml) while the minimum occurred in 2017 (0.39 ± 0.47 × 103 MPN/100 ml). The difference between the maximum and minimum was more than sixfold.
In the 14 years of the study investigation, the CB abundance showed a stepwise decline involving three clear steps termed the “three stages phenomenon.” From 2004 to 2007, the average abundance (1.95 ± 1.04 × 103 MPN/100 ml) was almost a factor of two higher than the Class IV standard of the NSQS. From 2008 to 2013, the average abundance (1.08 ± 0.74 × 103 MPN/100 ml) was close to the Class IV standard. And, from 2014 to 2017, the average abundance (0.60 ± 0.60 × 103 MPN/100 ml) was much lower than the Class IV standard. The average abundances in the three stages were significantly different (p < 0.05) (Supplementary Table 3). The occurrence of this phenomenon indicated that the seawater quality in Jiaozhou Bay progressively improved over the study period.
During the investigation, the annual variation of CB abundance showed a fluctuating trend (Figure 3A). The maximum abundance occurred in December (1.61 ± 0.82 × 103 MPN/100 ml) while the minimum occurred in June (0.64 ± 0.74 × 103 MPN/100 ml), representing a more than twofold difference. The annual variation in the abundance of CB showed that the average for the first half of the year (0.99 ± 0.79 × 103 MPN/100 ml) was lower than that in the second half (1.39 ± 1.06 × 103 MPN/100 ml) significantly (p < 0.05) (Supplementary Table 4). In the first half of the year, the maximum abundance occurred in January (1.54 ± 0.81 × 103 MPN/100 ml), while in the second half, there were two peaks in abundance, one in September (1.60 ± 1.66 × 103 MPN/100 ml) and the other in December (1.61 ± 0.82 × 103 MPN/100 ml). The average abundance in the first half of the year was very close to the Class IV standard of the NSQS, but in the second half of the year, it was a factor of approximately 1.5 higher than the standard.
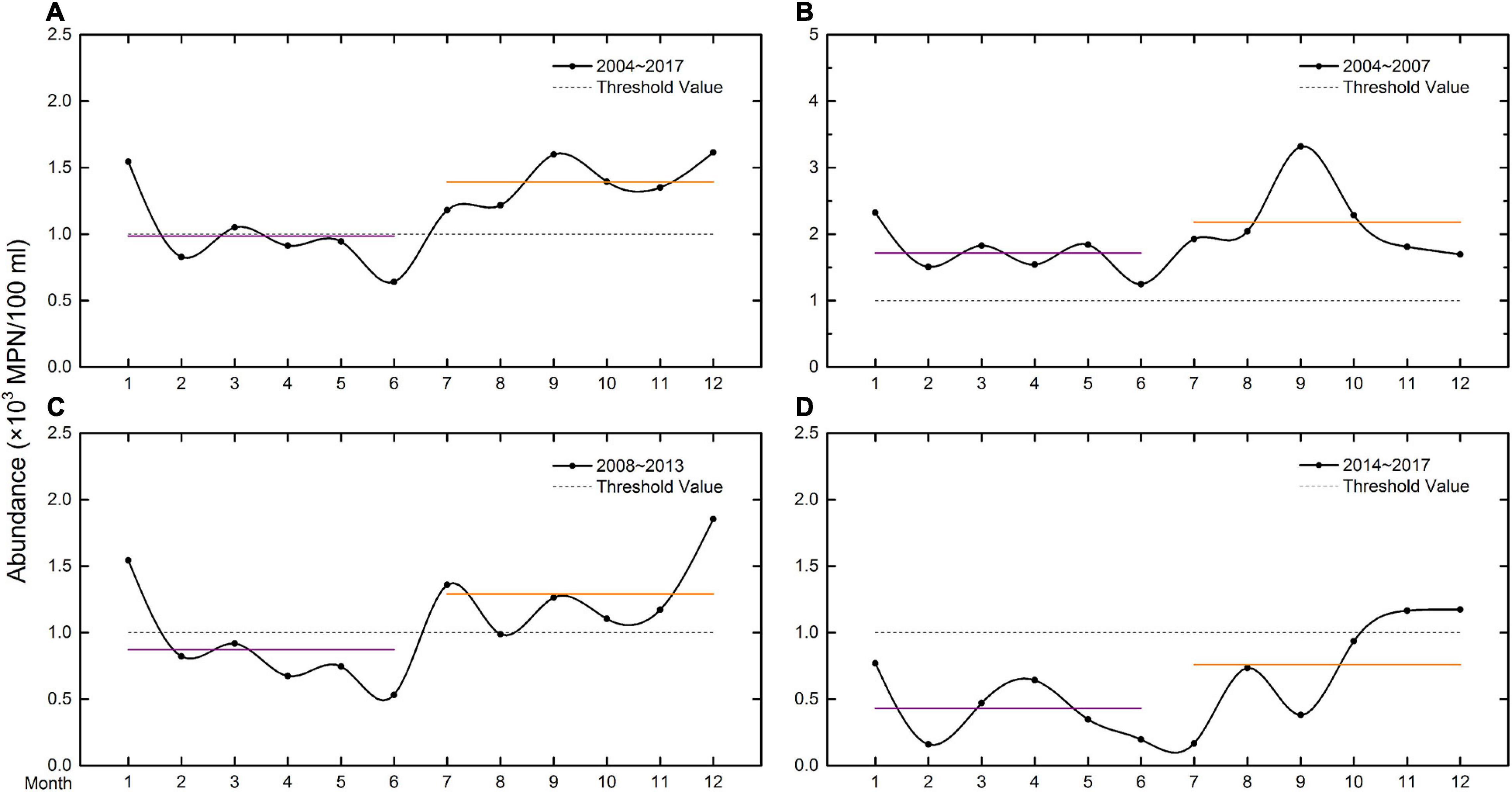
Figure 3. Annual variation in the average abundance of CB (×103 MPN/100 ml) in Jiaozhou Bay: (A) from 2004 to 2017; (B) from 2004 to 2007; (C) from 2008 to 2013; and (D) from 2014 to 2017. The purple and orange lines represent average abundances in the first and second halves of the year, respectively. The dotted line (threshold value) represents the Class IV standard for CB abundance specified by the NSQS.
The annual variation in CB abundance was analyzed with respect to the “three stages phenomenon” (Figures 3B-D). Although the average abundance in the first half of the year in all years of the study was lower than in the second half, the three stages showed different characteristics. In the first stage (Figure 3B), both the average abundances in the first and second halves of the year (1.71 ± 0.74 × 103 MPN/100 ml and 2.18 ± 1.23 × 103 MPN/100 ml, respectively; difference = 0.47 × 103 MPN/100 ml, p < 0.05) exceeded the Class IV standard of the NSQS. In the second stage (Figure 3C), the average abundance in the first half of the year (0.87 ± 0.66 × 103 MPN/100 ml) was lower than the Class IV standard, while in the second half (1.29 ± 0.77 × 103 MPN/100 ml), it was higher than the standard (difference = 0.42 × 103 MPN/100 ml, p < 0.05). In the last stage (Figure 3D), the average abundances in the first and second halves of the year (0.43 ± 0.39 × 103 MPN/100 ml and 0.76 ± 0.73 × 103 MPN/100 ml, respectively; difference = 0.33 × 103 MPN/100 ml, p < 0.05) were all lower than the Class IV standard. Thus, over the 14 years of the study, the differences between the first and second halves of the year became progressively smaller. And the variations in the first and second half-year average abundances also indicated that the seawater quality in Jiaozhou Bay improved.
In the first stage, the average abundances of CB in each of the 12 months of the year were higher than the Class IV standard. In the second stage, the abundances were higher than the standard in 6 months in a year. While in the third stage, the standard was exceeded in only 2 months. These data also suggested that the seawater quality gradually improved.
Coliform bacteria abundance varied in different areas and generated obvious spatial distribution characteristics (Figure 4 and Supplementary Table 5). The highest abundance was in the estuary area (4.94 ± 2.39 × 103 MPN/100 ml), followed by the bay mouth area (0.72 ± 0.72 × 103 MPN/100 ml), and the outer bay area (0.46 ± 0.48 × 103 MPN/100 ml), while the lowest abundance occurred in the inner bay area (0.33 ± 0.42 × 103 MPN/100 ml). The abundance in the estuary area was approximately 15-fold higher than in the inner bay area. Station A5 had the highest abundance (5.83 ± 4.68 × 103 MPN/100 ml), while station A3 (0.24 ± 0.96 × 103 MPN/100 ml) had the lowest. The highest value was more than 24-fold higher than the lowest value.
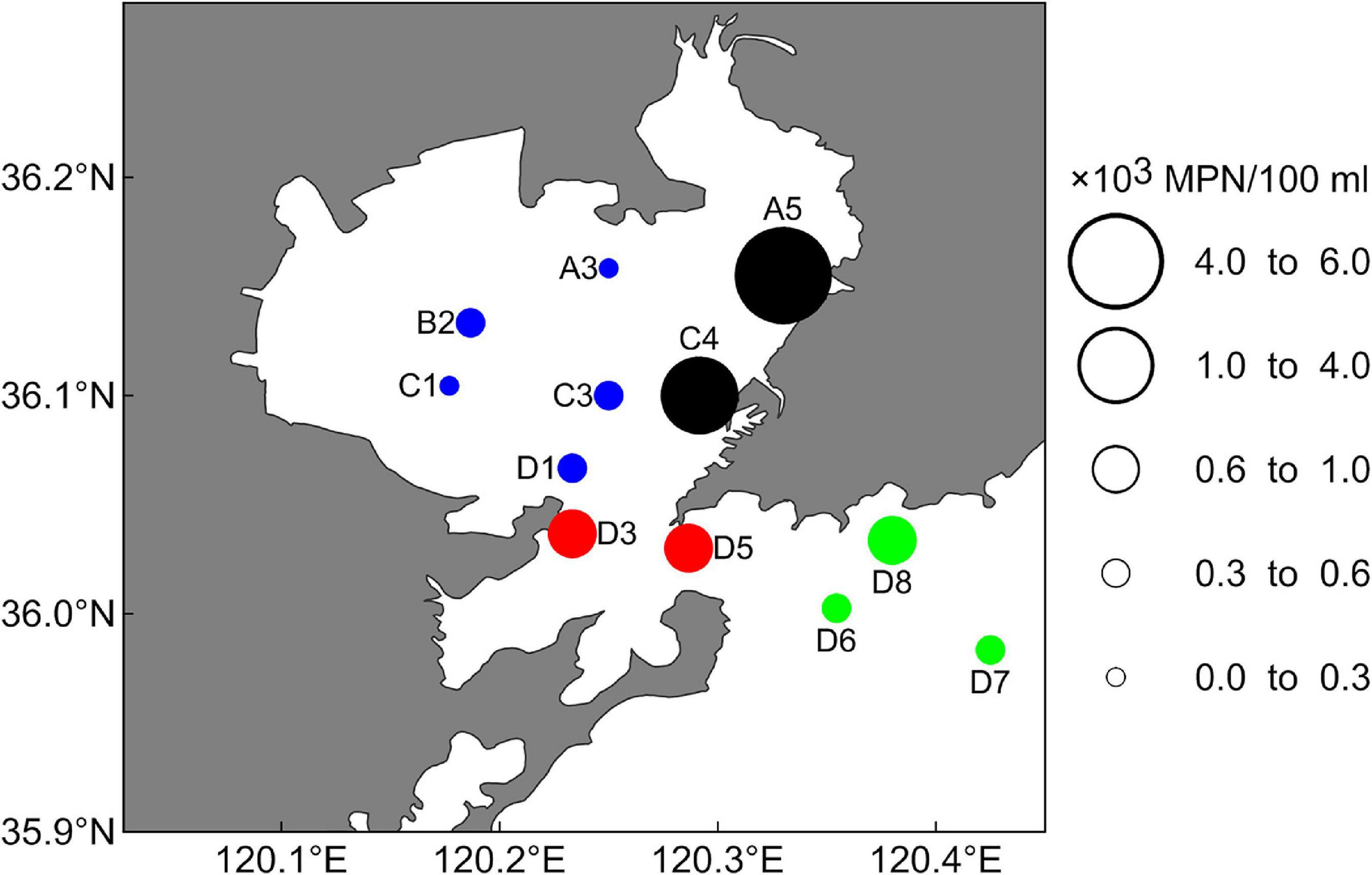
Figure 4. Spatial distribution of the average abundances in 12 stations. The sizes of the colored circles indicate relative average abundances of CB. The black, blue, red, and green circles indicate sampling stations in the estuary area, the inner bay area, the bay mouth area, and the outer bay area, respectively.
The annual variation in CB abundance in Jiaozhou Bay was spatially heterogeneous (Figure 5). The estuary, inner bay and outer bay areas showed clear differences between the first and the second halves of the year, with the average abundance in the first half of the year being lower than in the second half. The differences in the estuary area (1.45 × 103 MPN/100 ml) were greatest, followed by the inner bay area (0.30 × 103 MPN/100 ml) and the outer bay area (0.12 × 103 MPN/100 ml). In the bay mouth area, there was little difference (0.01 × 103 MPN/100 ml) between the average abundances in the first and second halves of the year. The ratio of the average abundance in the second half of the year to the first half of the year ranged from 1.00 in the bay mouth area to 2.67 in the inner bay area. The average abundances in the first and second halves of the years were lower than the Class IV standard of the NSQS, except the estuary area.
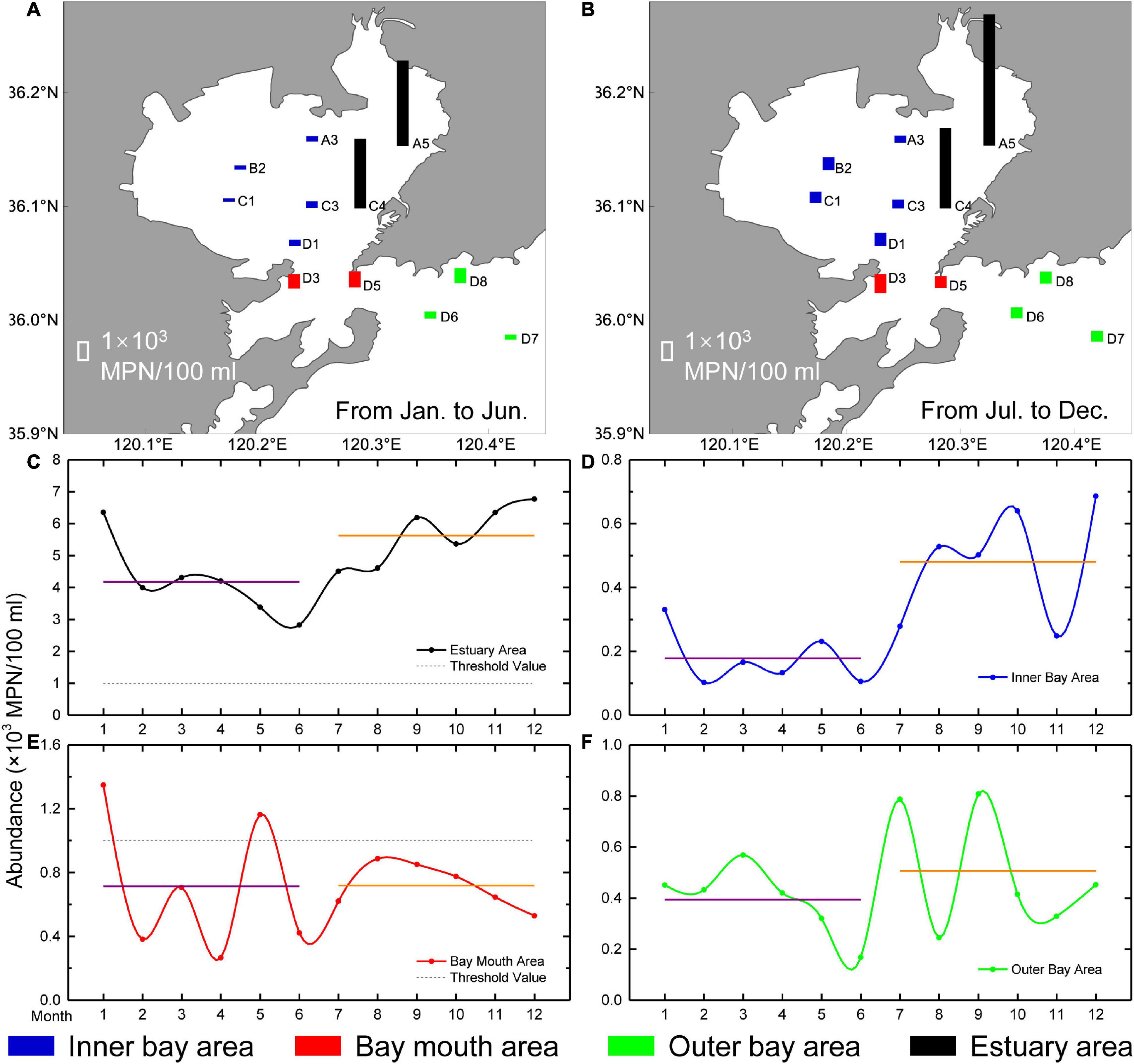
Figure 5. Annual variation of CB abundance (× 103 MPN/100 ml) in Jiaozhou Bay. The columns with various lengths in different sampling stations indicate the average abundance of CB during the investigating period: (A) in the first half of the year; and (B) in the second half of the year. Variations in the annual abundance of CB in four areas in Jiaozhou Bay: (C) the estuary area; (D) the inner bay area; (E) the bay mouth area; and (F) the outer bay area. The purple and orange lines represent the average abundances in the first and second halves of the year, respectively. The black dotted line (threshold value) represents the Class IV standard for CB abundance specified by the NSQS.
The changes in CB abundance in the four areas of Jiaozhou Bay also reflected the “three stages phenomenon” (Figure 6). Average abundance declined from the first stage (2004–2007) to the third stage (2014–2017) in all four areas, but the characteristics of change varied. For example, in the estuary area, the average abundance dropped from the first stage (7.52 ± 3.20 × 103 MPN/100 ml) to the third stage (2.42 ± 2.71 × 103 MPN/100 ml), the reduced value was more than 5 × 103 MPN/100 ml (the highest in all four areas), and the abundance decreased by almost 70%. In this area, the lowest abundance stage still exceeded the Class IV standard. In the bay mouth area, the average abundance also decreased from the first stage (1.43 ± 1.93 × 103 MPN/100 ml) to the third stage (0.27 ± 0.51 × 103 MPN/100 ml), the decline in average abundance was more than 80%, and it was the greatest decline among the four areas. Unlike the estuary and bay mouth areas (where the CB abundance of more than one stage was higher than the Class IV standard), in the inner and outer bay areas, the CB abundance was lower than the Class IV standard during the three stages. In these two areas, the abundances in the last two stages were similar. The abundance in the inner bay area fell by 70%, while in the outer bay area it declined by 64%.
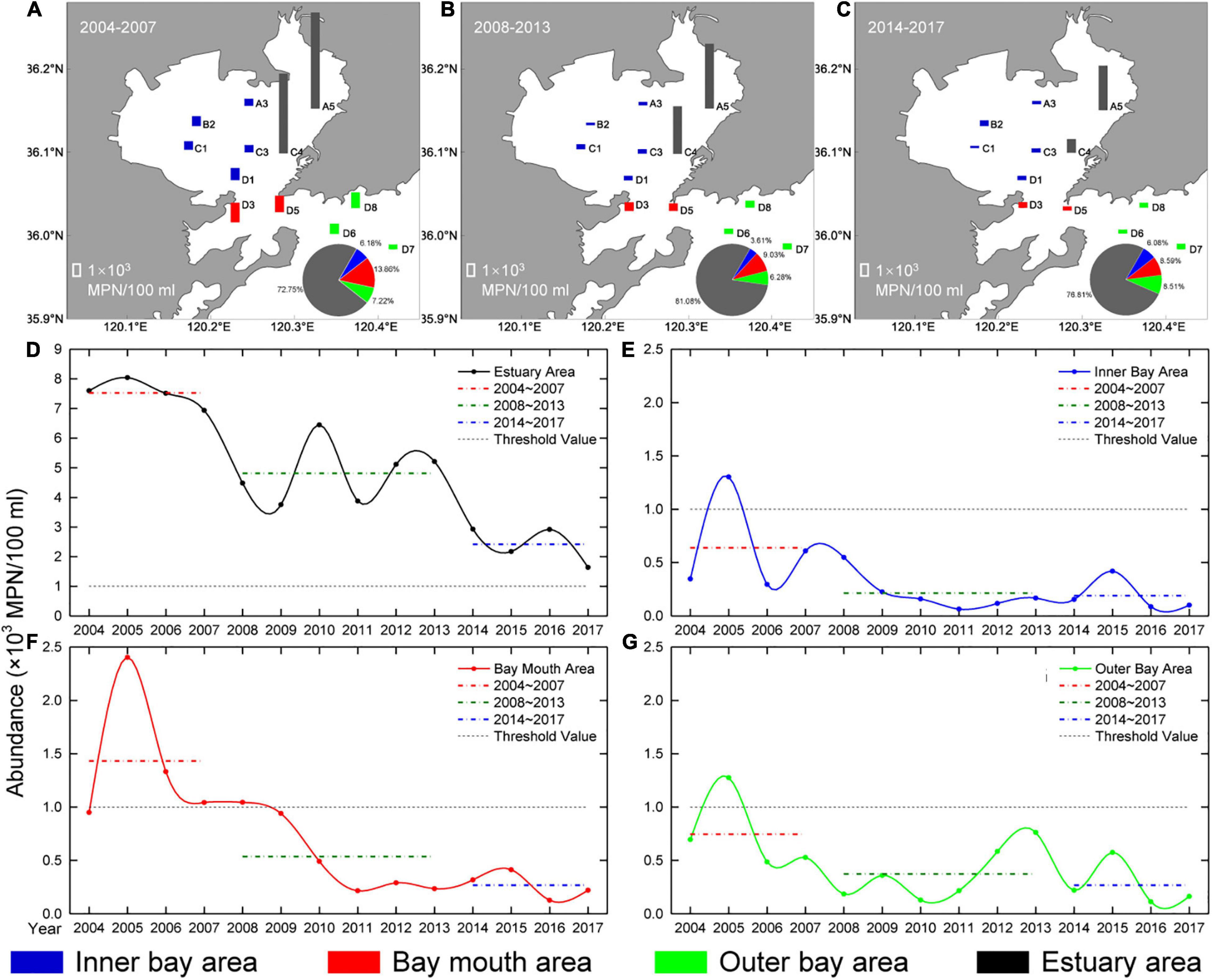
Figure 6. Interannual variation of CB abundance (× 103 MPN/100 ml) in Jiaozhou Bay. Columns with different lengths in 12 stations show the average abundance of CB in: (A) the first stage; (B) the second stage; and (C) the third stage. The pie charts on the maps represent the proportion of the average abundance of CB in different areas. Interannual variation in CB abundance of four areas in Jiaozhou Bay: (D) the estuary area; (E) the inner bay area; (F) the bay mouth area; and (G) the outer bay area. The red, green, and blue dotted lines represent the average abundances in the first stage (2004–2007), the second stage (2008–2013), and the third stage (2014–2017), respectively. The black dotted line (threshold value) represents the Class IV standard for CB abundance specified by the NSQS.
The results of the correlation analysis between CB abundance and environmental factors were shown in Table 2. To ensure data integrity, a total of 1843 sets of data were selected for analysis. All the correlations were corrected by the Bonferroni method.
The results showed that the CB abundance was significantly positively correlated with the concentrations of DISi (r = 0.20, p < 0.01), DIP (r = 0.20, p < 0.01), nitrogen salts (nitrate, nitrite, and ammonium) (0.19 ≤ r ≤ 0.30, p < 0.01), and significantly negatively correlated with salinity (r = −0.11, p < 0.01). Among the positively correlated factors, the abundance of CB showed the strongest correlation with the ammonium concentration (r = 0.30, p < 0.01).
Factoextra package in R language was used in PCA. The analysis studied 1840 sets of data and was performed to access the relationships between environmental factors and CB abundance (Figure 7). The result of the PCA paralleled those of the correlation analysis, and showed that the first two principal components explained 51.1% of the variation. The CB abundance was most positively affected by the concentrations of , , and , while salinity had the negative impact.
Long-term monitoring (14 years) of the abundance of CB in Jiaozhou Bay showed that there has been a trend of decline at interannual, annual, and spatial scales, and that the decrease has occurred associated with three stages (2004–2007, 2008–2013, 2014–2017). Around the year 2008, seawater quality was strictly regulated to build a qualified water sports venue for the Olympic Games. Since 2013, a series of environmental protection work has been carried out under the guidance of the Eighteenth National Congress of the Communist Party of China. In recent years, the concept of “lucid waters and lush mountains are invaluable assets” has been put into practice. This was consistent with the significant decrease in CB abundance decreased at these two time points. Among the “three stages phenomenon,” the percentage of months with average abundance below the NSQS Class IV standard was less than 15% during 2004–2007, approximately 50% during 2008–2013, and more than two third during 2014–2017. These findings were in accordance with the described improvement of seawater quality of Jiaozhou Bay by the Report on Marine Environmental Quality of Qingdao1, and implied that the abundance of CB could be employed as an indicator to reflect the impact of environmental treatment accurately.
Along with a decrease in the abundance of CB over the three stages, there was a decline in the concentrations of DISi, DIP, and (Supplementary Table 2). This suggested that the environmental quality in Jiaozhou Bay has gradually improved. In the correlation analysis, the abundance of CB was most positively correlated with the concentration of nitrogen salts in all three stages. In the four study areas, the abundance of CB showed various relationships with nutrient concentrations. For example, in the estuary and inner bay areas, the abundance of CB was associated with the concentration of nitrogen salts. Water in the estuary area probably contains more nutrient residues (Cross and Rebordinos, 2003) and also receives untreated groundwater. Groundwater is an important land-based source of nutrients entering coastal ecosystems (Hernández-Terrones et al., 2010), and human activities may influence their concentrations (Hernández-Terrones et al., 2015). In previous studies it has been shown that nutrients enhance CB growth and reproduction, particularly inorganic nitrogen (Prasad et al., 2015). This could account for the correlation of nutrients in Jiaozhou Bay with the abundance of CB.
The abundance of CB was negatively correlated with salinity in Jiaozhou Bay. Similar correlations were also reported by Rozen and Belkin (2001) and Anderson et al. (2005). We also found a pattern of annual abundance of CB in Jiaozhou Bay that was not seasonal. This involved a marked increase from July and the maintenance of a high average abundance until January the following year. The similar phenomenon was found by Xue et al. (2018). To investigate possible explanations for this phenomenon, we compared the annual change in CB abundance with temperature, salinity, and rainfall. Temperature showed an obvious seasonal change that was different from that of changes in CB abundance. Salinity and rainfall both varied in the first and the second halves of the year. The average salinity was high in the first half of the year and low in the second half of the year, while the pattern was opposite for rainfall (Supplementary Figure 1). Correlation analysis of the surface seawater salinity and rainfall showed that these two indices were negatively correlated. Previous studies have reported that pollutants and bacterial cells can be carried to coastal bays through estuarine systems (Ridgway and Shimmield, 2002; Prasad et al., 2015), and rainfall can carry pollutants into estuarine areas in surface runoff and groundwater. Consequently, we infer that the abundance of CB may have been influenced by land-sourced pollutants carried into Jiaozhou Bay by rainfall. The hypothesis had some resemblance to Procopio et al. (2017) and Tabanelli et al. (2017).
Station A5 (near the mouth of Licun River), A3, C4 (near the mouth of Haibo River), C1, and C3 were at similar latitudes, but the differences in CB abundance at these stations varied by a factor of 10. This also suggests that the abundance of CB in Jiaozhou Bay was mainly influenced by land-sourced pollutants. Schiff and Kinney (2001) reported that high bacterial densities detected in discharges were not the result of point-source pollution. Therefore, the abundance of CB may have been influenced by sewage carried by nearby rivers. The abundance of CB was correlated with salinity and the concentration of nitrogen salts and silicates in the estuary and inner bay areas, but not in the bay mouth or outer bay area. Rivers may carry pollutants to the estuary area of Jiaozhou Bay in rainfall, and in the inner bay area, the pollutions may diffuse by the seawater flowed. Some studies have reported that bacterial abundance was higher in aquacultural organisms than in seawater (Lucena et al., 1994; Kolm and Absher, 2008), as the inner bay is an aquaculture area, this may explain why the abundance of CB was lower in the inner bay area. Furthermore, bacteria can travel and be transported some distance (Jeng et al., 2005), which could be another reason for the phenomena in the estuary and inner bay areas. In the bay mouth and outer bay areas, the exchange of seawater between Jiaozhou Bay and the open sea is greater, so pollutions may be diluted to very low concentrations, and have less influence on CB abundances as highlighted in the research of Farrapeira et al. (2010). Seawater could purify itself may be another possible explanation for the spatial distribution of CB in Jiaozhou Bay. For station D8 (Supplementary Figure 2D), which is inside the 2008 Olympic Games sailing venue area, the average abundance of CB gradually decreased from the first to the third stage. The average abundance exceeded the Class IV standard of the NSQS in the first stage but was below it in the other two stages. Compared with the other two stations in the outer bay area, the abundance of CB at D8 station fluctuated the most, and most frequently exceeded the Class IV standard. This may have been influenced by tourism and water sports, and highlighting the need for the water environment to be consistently monitored and regulated.
We compared the abundance of CB in Jiaozhou Bay with other areas worldwide (Supplementary Table 6). Farrapeira et al. (2010) found that, based on MPN analysis, total coliforms in an estuary area in Brazil were more than 2.4 × 103 MPN/100 ml, while the abundance was 0.9 × 10 MPN/100 ml at a port station in May, 2007. Also in Brazil, Mignani et al. (2013) reported that in 2007–2008, the mean abundance of CB in a cultivation area and a contaminated area was 18 and 156 MPN/100 ml, respectively. Locations and pollution levels can influence the abundance of CB, as occurs in Jiaozhou Bay. The average abundance of CB in Jiaozhou Bay during the last two stages was lower than that in seawater of the intertidal zone in the Persian Gulf in Iran in 2014 (more than 1.2 × 103 MPN/100ml) (Karbasdehi et al., 2017), suggesting that the seawater quality of Jiaozhou Bay is better than that of the Persian Gulf. The abundance of CB in a Xiamen (China) intertidal shellfish aquaculture area in 2005 and 2006 (Zhong et al., 2012) was higher than we found in the inner bay area of Jiaozhou Bay, suggesting that the aquaculture environment in Jiaozhou Bay is better. Xia et al. (2011) studied the abundance of CB in surface seawater of an aquaculture area was 72 MPN/100ml (in March) and 23 MPN/100ml (in May) in Jiangsu Province (China) in 2010, indicated that the seawater quality was better in Jiangsu Province than in Jiaozhou Bay at that time. Shen et al. (2006) reported that the highest abundance of CB occurred near a sewage outlet, which was similar to our results. We also found that the abundance of CB in Jiaozhou Bay was four orders of magnitude lower than that in the Wanan catchment in China (Xue et al., 2018). This catchment is a freshwater environment, and could be more highly influenced by human activities (e.g., agricultural and urban activities) than Jiaozhou Bay, because it is an endorheic environment.
We analyzed the variations in abundance of CB at various stations in the four areas (Supplementary Figure 2). From an overall viewpoint, the abundance of CB in Jiaozhou Bay should be treated regionally. In the estuary area, where the decline in abundance of CB was greatest, the seawater quality still exceeded the Class I to Class III standards of the NSQS. For the inner bay, outer bay, and bay mouth areas, the average abundance of CB fluctuated in 2015, and the average abundance in the outer bay also fluctuated in 2007, 2009, 2012, and 2013. Possible explanations include increased use for anchorage, increased human activity in the open seawater area (e.g., tourist ships and sailing), and mariculture developments. Based on our study, we have formulated strategies for future seawater environmental management. These include, for example: the regulation of pollution discharge in key areas and in differing periods of the year, to maintain the present status and strengthen management and control; to strictly monitor, regulate, and manage various water activities; and to standardize aquaculture activities.
There was a deficiency in our study that we did not pay attention to the specific composition of CB. In the following monitoring, we will pay more attention to the species which had specific roles such like pathogenicity (Dey et al., 2017) and drug resistance (Al-Badaii and Shuhaimi-Othman, 2014). These may make the indicative function more accurate of CB. And, in further study, we will try to distinguish the factors that influence CB abundance whether from human activity or animals’ excretion. That will be more accurate to analyze the impact of the environmental factors.
In this study we analyzed the abundance of CB in Jiaozhou Bay during the period 2004–2017. Surface seawater samples were collected monthly at 12 stations, and the abundance of CB and a range of environmental factors were measured. The long-term investigation showed that the abundance of CB reflected a “three stages phenomenon”, involving critical time points that coincided with increased environmental governance. The abundance of CB showed obvious annual and spatial variations, although their abundance declined during the study. Based on the National Seawater Quality Standard, the abundance of CB at individual stations in individual months still exceeded the Class III standard. As the abundance was correlated with human activities, changes in abundance can be used as a guide to the development and implementation of environmental governance strategies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YD and TX supervised the entire project. KC and YD integrated the all data and wrote the manuscript. XS, LZ, and YZ provided the data of environmental factors and heterotrophic bacteria, respectively. HD, JL, CW, CL, YCZ, SC, JX, and SL finished the experiment and accumulated the experimental data. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 41806178 and 42076139), the International Partnership Program of Chinese Academy of Sciences (Grant No. 133137KYSB20200002), and the National Key Research and Development Program of China (Grant No. 2017YFA0603204).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Jiaozhou Bay National Marine Ecosystem Research Station, Qingdao Meteorological Bureau, and captain and crew of the R/V Chuangxin for their assistance during the research. We also acknowledge Yongfang Zhao, Rui Zhang, Xue Chen, Cong Xu, Zhaojie Teng, and Yanchu Zhao for their support toward this work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.641137/full#supplementary-material
Al-Badaii, F., and Shuhaimi-Othman, M. (2014). Water pollution and its impact on the prevalence of antibiotic-resistant E. coli and total coliform bacteria: a study of the Semenyih river, Peninsular Malaysia. Water Qual. Expo. Health. 7, 319–330. doi: 10.1007/s12403-014-0151-5
Anderson, K. L., Whitlock, J. E., and Harwood, V. J. (2005). Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71, 3041–3048. doi: 10.1128/aem.71.6.3041-3048.2005
Bharathi, M. D., Sundaramoorthy, S., Patra, S., Madeswaran, P., and Sundaramanickam, A. (2018). Seasonal and spatial distribution of heterotrophic bacteria in relation to physico-chemical properties along Ennore coastal waters. Indian J. Geomarine Sci. 47, 587–597.
Choi, H.-K., Choi, Y. H., Verberne, M., Lefeber, A. W. M., Erkelens, C., and Verpoorte, R. (2004). Metabolic fingerprinting of wild type and transgenic tobacco plants by 1H NMR and multivariate analysis technique. Phytochemistry 65, 857–864. doi: 10.1016/j.phytochem.2004.01.019
Cross, I., and Rebordinos, L. (2003). Effect of marine contamination on the genetic population structure of the bivalve Crassostrea angulata. Cienc. Mar. 29, 239–250. doi: 10.7773/cm.v29i2.142
Dang, H., Li, J., Chen, R., Wang, L., Guo, L., Zhang, Z., et al. (2010). Diversity, abundance, and spatial distribution of sediment ammonia-oxidizing betaproteobacteria in response to environmental gradients and coastal eutrophication in Jiaozhou Bay, China. Appl. Environ. Microbiol. 76, 4691–4702. doi: 10.1128/aem.02563-09
Dey, S., Uddin, M., and Manchur, M. (2017). Physicochemical and bacteriological assessment of surface water quality of the Karnaphuli river in Bangladesh. J. Pure Appl. Microbiol. 11, 1721–1728. doi: 10.22207/jpam.11.4.10
Dong, Y. (2013). Microbial Community Structure and Its Relationship With Environmental Variations in Typical Areas of the Yellow Sea and East China Sea. Doctoral thesis. Beijing: University of Chinese Academy Sciences.
Farrapeira, C., Mendes, E., Dourado, J., and Guimarães, J. (2010). Coliform accumulation in Amphibalanus amphitrite (Darwin, 1854) (Cirripedia) and its use as an organic pollution bioindicator in the estuarine area of Recife, Pernambuco, Brazil. Braz. J. Biol. 70, 301–309. doi: 10.1590/s1519-69842010000200011
Feng, M., Wang, C., Zhang, W., Zhang, G., Xu, H., Zhao, Y., et al. (2018). Annual variation of species richness and lorica oral diameter characteristics of tintinnids in a semi-enclosed bay of western Pacific. Estuar. Coast. Shelf Sci. 207, 164–174. doi: 10.1016/j.ecss.2018.04.003
Hernández-Terrones, L., Rebolledo-Vieyra, M., Merino-Ibarra, M., Soto, M., Le-Cossec, A., and Monroy-Ríos, E. (2010). Groundwater pollution in a karstic region (NE Yucatan): baseline nutrient content and flux to coastal ecosystems. Water Air Soil Pollut. 218, 517–528. doi: 10.1007/s11270-010-0664-x
Hernández-Terrones, L. M., Null, K. A., Ortega-Camacho, D., and Paytan, A. (2015). Water quality assessment in the Mexican Caribbean: impacts on the coastal ecosystem. Cont. Shelf Res. 102, 62–72. doi: 10.1016/j.csr.2015.04.015
Hu, Z., Deng, Y., Li, Y., and Tang, Y. Z. (2018). The morphological and phylogenetic characterization for the dinoflagellate Margalefidinium fulvescens (=Cochlodinium fulvescens) isolated from the Jiaozhou Bay, China. Acta Oceanol. Sin. 37, 11–17. doi: 10.1007/s13131-018-1295-0
Hunt, D. A., Lucas, J. P., McClure, F. D., Springer, J., and Newell, R. (1981). Comparison of modified A-1 method with standard EC test for recovery of fecal coliform bacteria for shellfish. J. Assoc. Off. Anal. Chem. 64, 607–610. doi: 10.1093/jaoac/64.3.607
Jeng, H. A. C., Englande, A. J., Bakeer, R. M., and Bradford, H. B. (2005). Impact of urban stormwater runoff on estuarine environmental quality. Estuar. Coast. Shelf Sci. 63, 513–526. doi: 10.1016/j.ecss.2004.11.024
Karbasdehi, V. N., Sina, D., Nabipour, I., Ostovar, A., Arfaeinia, H., Vazirizadeh, A., et al. (2017). Indicator bacteria community in seawater and coastal sediment: the Persian Gulf as a case. J. Environ. Health Sci. Eng. 15:6. doi: 10.1186/s40201-017-0266-2
Kolm, H. E., and Absher, T. M. (2008). Bacterial density and coliform organisms in waters and oysters of Paranaguá Estuarine complex, Paraná, Brazil. Bol. Inst. Pesca. 34, 49–59.
Liu, M., Dong, Y., Zhao, Y., Zhang, G., Zhang, W., and Xiao, T. (2011). Structures of bacterial communities on the surface of Ulva prolifera and in seawaters in an Ulva blooming region in Jiaozhou Bay, China. World J. Microbiol. Biotechnol. 27, 1703–1712. doi: 10.1007/s11274-010-0627-9
Liu, S. M., Zhang, J., Chen, H. T., and Zhang, G. S. (2005). Factors influencing nutrient dynamics in the eutrophic Jiaozhou Bay, North China. Prog. Oceanogr. 66, 66–85. doi: 10.1016/j.pocean.2005.03.009
Lucena, F., Lasobras, J., McIntosh, D., Forcadell, M., and Jofre, J. (1994). Effect of distance from the polluting focus on relative concentrations of Bacteroides fragilis phages and coliphages in mussels. Appl. Environ. Microbiol. 60, 2272–2277. doi: 10.1128/aem.60.7.2272-2277.1994
Ma, Q., Han, D., Liu, H., Xue, Y., Ji, Y., and Ren, Y. (2015). Construction of a continuous trophic spectrum for the food web in Jiaozhou Bay using stable isotope analyses. Acta Ecol. Sin. 35, 7207–7218.
Mignani, L., Barbieri, E., Marques, H.L.d.A, and Oliveira, A.J.F.C.d (2013). Coliform density in oyster culture waters and its relationship with environmental factors. Pesqui. Agropecu. Bras. 48, 833–840. doi: 10.1590/s0100-204x2013000800004
The Ministry of Environmental Protection of the People’s Republic of China (2017). Plan for Prevention and Control of Pollution in Coastal Waters (in Chinese).
The Ocean and Fishery Administration of Qingdao (2014). Protection Regulations of Qingdao Jiaozhou Bay (in Chinese).
Prasad, V. R., Srinivas, T. N. R., and Sarma, V. V. S. S. (2015). Influence of river discharge on abundance and dissemination of heterotrophic, indicator and pathogenic bacteria along the east coast of India. Mar. Pollut. Bull. 95, 115–125. doi: 10.1016/j.marpolbul.2015.04.032
Procopio, N. A., Atherholt, T. B., Goodrow, S. M., and Lester, L. A. (2017). The likelihood of coliform bacteria in NJ domestic wells based on precipitation and other factors. Groundwater 55, 722–735. doi: 10.1111/gwat.12518
Ridgway, J., and Shimmield, G. (2002). Estuaries as repositories of historical contamination and their impact on shelf seas. Estuar. Coast. Shelf Sci. 55, 903–928. doi: 10.1006/ecss.2002.1035
Rozen, Y., and Belkin, S. (2001). Survival of enteric bacteria in seawater. FEMS Microbiol. Rev. 25, 513–529. doi: 10.1111/j.1574-6976.2001.tb00589.x
Schiff, K., and Kinney, P. (2001). Tracking sources of bacterial contamination in stormwater discharges to Mission Bay, California. Water Environ. Res. 73, 534–542. doi: 10.2175/106143001x139605
Shen, X., Chen, Y., and Chen, Q. (2006). Distribution characteristic of coliform and heterotrophic bacteria in Fengxian of Hangzhou Bay in summer. Mar. Environ. Sci. 25, 20–23.
Shen, Z.-L. (2001). Historical changes in nutrient structure and its influences on phytoplantkon composition in Jiaozhou Bay. Estuar. Coast. Shelf Sci. 52, 211–224. doi: 10.1006/ecss.2000.0736
Sun, S., Li, C., Zhang, G., Sun, X., and Yang, B. (2011a). Long-term changes in the zooplankton community in the Jiaozhou Bay. Ocean. Limnol. Sin. 42, 625–631.
Sun, S., Sun, X., Zhang, G., Tang, H., Liu, Q., and Li, G. (2011b). Long-term changes in major meteorological and hydrological factors in the Jiaozhou Bay. Ocean. Limnol. Sin. 42, 632–638.
Sun, X., Sun, S., Wu, Y., Zhang, Y., and Zheng, S. (2011c). Long-term changes of phytoplankton community structure in the Jiaozhou Bay. Ocean. Limnol. Sin. 42, 639–646.
Tabanelli, G., Montanari, C., Gardini, A., Maffei, M., Prioli, C., and Gardini, F. (2017). Environmental factors affecting Escherichia coli concentrations in striped venus clam (Chamelea gallina L.) harvested in the North Adriatic Sea. J. Food Prot. 80, 1429–1435. doi: 10.4315/0362-028X.JFP-17-058
Tennant, A. D., and Reid, J. E. (1961). Coliform bacteria in sea water and shellfish: i. lactose fermentation at 35.5° and 44°C. Can. J. Microbiol. 7, 725–731. doi: 10.1139/m61-086
Wang, L., Fan, Y., Yan, C., Gao, C., Xu, Z., and Liu, X. (2017). Assessing benthic ecological impacts of bottom aquaculture using macrofaunal assemblages. Mar. Pollut. Bull. 114, 258–268. doi: 10.1016/j.marpolbul.2016.09.032
Xia, P., Shen, X., Yuan, Q., and Jiang, M. (2011). Distribution of bacteria and coliform bacteria in the Meretrix meretrix culture environment on Rudong beach of Jiangsu Province. Mar. Environ. Sci. 30, 57–60,71.
Xing, J., Song, J., Yuan, H., Li, X., Li, N., Duan, L., et al. (2017a). Fluxes, seasonal patterns and sources of various nutrient species (nitrogen, phosphorus and silicon) in atmospheric wet deposition and their ecological effects on Jiaozhou Bay, North China. Sci. Total Environ. 576, 617–627. doi: 10.1016/j.scitotenv.2016.10.134
Xing, J., Song, J., Yuan, H., Wang, Q., Li, X., Li, N., et al. (2017b). Atmospheric wet deposition of dissolved trace elements to Jiaozhou Bay, North China: fluxes, sources and potential effects on aquatic environments. Chemosphere 174, 428–436. doi: 10.1016/j.chemosphere.2017.02.004
Xue, F., Tang, J., Dong, Z., Shen, D., Liu, H., Zhang, X., et al. (2018). Tempo-spatial controls of total coliform and E. coli contamination in a subtropical hilly agricultural catchment. Agric. Water Manag. 200, 10–18. doi: 10.1016/j.agwat.2017.12.034
Yue, L., Gao, H., Liu, M., Zou, T., and Chen, X. (2016). The relationships between environment and economy of Qingdao city by using water quality parameters in Jiaozhou Bay. Mar. Environ. Sci. 35, 106–112.
Zhao, L., Zhao, Y., Xu, J., Zhang, W., Huang, L., Jiang, Z., et al. (2016). Distribution and seasonal variation of picoplankton in Sanggou Bay, China. Aquac. Environ. Interact. 8, 261–271. doi: 10.3354/aei00168
Zhao, S.-J. (2007). The Ecological Study of the Picoplankton in the East China Sea and Yellow Sea. Doctoral thesis. Beijing: Graduate School of Chinese Academy Sciences.
Zhong, S., Huang, W., and Chen, Y. (2012). Coliforms and heterotrophic bacteria in the environment of shellfish culture in Xiamen sea area. Adv. Mar. Sci. 30, 357–368.
Keywords: coliform bacteria, average abundance, environmental governance, correlation analysis, seawater quality
Citation: Cui K, Dong Y, Sun X, Zhao L, Du H, Liu J, Wang C, Liang C, Zhao Y, Chen S, Xuan J, Li S, Zhao Y and Xiao T (2021) Long-Term Temporal and Spatial Distribution of Coliform Bacteria in Jiaozhou Bay Associated With Human Activities and Environmental Governance. Front. Mar. Sci. 8:641137. doi: 10.3389/fmars.2021.641137
Received: 14 December 2020; Accepted: 22 July 2021;
Published: 17 August 2021.
Edited by:
Carol Robinson, University of East Anglia, United KingdomReviewed by:
Punyasloke Bhadury, Indian Institute of Science Education and Research Kolkata, IndiaCopyright © 2021 Cui, Dong, Sun, Zhao, Du, Liu, Wang, Liang, Zhao, Chen, Xuan, Li, Zhao and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Dong, eWlkb25nQHFkaW8uYWMuY24=; Tian Xiao, dHhpYW9AcWRpby5hYy5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.