
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 24 January 2022
Sec. Marine Ecosystem Ecology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.635756
This article is part of the Research Topic Oceanography and Benthic Ecology of Patagonian fjords - 500 years from the Discovery of the Strait Magellan View all 16 articles
 Madeleine Hamame*
Madeleine Hamame* Paula Ortiz
Paula OrtizThe Aysén region of Chile (North Patagonia), has had limited studies on the effectiveness of management and exploitation areas of benthic resources, and performance relative to open access areas in this region has never been evaluated. We evaluated seven management areas (MAs) and five open access areas (OAAs) between 43.9°S and 45.2°S for exploitation intensity of three commercial species (Concholepas concholepas, Loxechinus albus, and Ameghinomya antiqua) together with characterization of the benthic community. Indicators based on size, density and weight were used to evaluate exploitation intensity of commercial species. Associated benthic communities were evaluated considering density, species composition, and community structure. We found a high species richness and a community structure with low variability between MAs and OAAs. Low densities and small sizes classes of C. concholepas in most of the areas indicated high exploitation intensity in both MAs and OAAs. In this context, a permanent ban to harvest C. concholepas within OAAs may need to be reevaluated since with no enforcement and monitoring, the exploitation status of this species remains unclear in these areas. L. albus in most areas were absent in the harvestable sizes, which could be indicating high exploitation intensity in both regimes. High densities and small sizes of C. concholepas and L. albus in some MAs, indicated a potential recruitment zones which bears further investigation. A. antiqua, showed better conditions than other commercial species evaluated, with no significant differences in densities and size-based indicators when comparing OAAs and MAs. Benthic communities were dominated numerically by the Echinoidea class in both MAs and OAAs, with L. albus, Arbacia dufresnii and Pseudechinus magellanicus being the dominant species. High densities of sea urchins co-occurring with low coverage of macroalgae found in MAs-Gala could indicate that a sea urchin barren was dominant during the study period. On the other hand, high densities of Cosmasterias lurida, a predatory sea star, in conjunction with low densities of C. concholepas in most of the studied areas suggested that a shift in predator roles is occurring. No differences were estimated in terms of fisheries indicators and benthic community structure across the two management regimes, suggesting the poor performance of MAs in Aysén region. Our data also support the need to improve monitoring of MAs especially with respect to associated benthic community incorporating a broader spatial scale.
Management and exploitation areas of benthic resources (MAs) were established along the Chilean coast to contribute to the ecological sustainability of benthic resources; their establishment was driven by the biological collapse in the 80s of the muricid mollusk Concholepas concholepas, a previously profitable benthic fishery (Castilla, 1994; Stotz, 1997; Castilla et al., 1998; Gonzalez et al., 2006). This administrative system gives territorial user rights (TURFs) to artisanal fisheries organizations that are legally constituted for the co-management (with the state) of benthic resources within a limited geographic area (Zuñiga et al., 2008; Gelcich et al., 2010). After two decades of MAs functioning (Stotz, 1997; Gonzalez et al., 2006; Gelcich et al., 2016), the ecological effects of this management approach have been somewhat diverse. In Central Chile, higher densities, larger sizes, and lower mortality rates of commercial species (C. concholepas, Loxechinus albus, and Fissurella spp.) were found in MAs compared to Open-Access Areas (OAAs, i.e., no access restriction) (Castilla and Fernandez, 1998; Gelcich et al., 2010; Defeo et al., 2014; Andreu-Cazenave et al., 2017). Positive effects were also reported in the associated macroinvertebrate communities where higher species richness, biomass and densities were observed within MAs compared to OAAs (Gelcich et al., 2008, 2012, Biggs et al., 2016). In contrast, performances of MAs in northern Chile were classified as poorly sustainable, with few stocks showing stability in densities, sizes, and catches over time (Arias and Stotz, 2020). Moreover, to maintain productivity, studies showed that at least half of the catch was extracted from areas outside MAs (Gonzalez et al., 2006). The performance of MAs has been heterogeneous and appears to betoo complex to generalize for a country such as Chile, with an extensive coast that spans ∼38° of latitude, where a variety of environmental, social, geographical, and economical factors combine to produce different outcomes. In this context local research is needed to integrate site-specific particularity into the global analyses of benthic management.
The Aysén region of Chile (43°38′–49°16′S) harbors many small coves (fishers’ villages) throughout islands, fjords and channels in which the subsistence primarily relies on artisanal fisheries. Their fishing grounds are situated within a marine ecosystem considered not only pristine compared to northern regions (Godoy et al., 2010; Navarrete et al., 2010), but also characterized by a high species richness (Fernandez et al., 2000; Häussermann and Försterra, 2009; Försterra et al., 2016; Betti et al., 2017; Bertolino et al., 2020). The presence of diverse and unique habitats not only support species richness but also host high abundances and biomass of important commercial species (Flores et al., 2020; Pardo et al., 2020). These highly productive ecosystems have been the target of illegal fishing, and regional fisheries authorities have needed to co-operate with local fishers to address the constant influx of fishers from neighboring regions. In this context, the introduction of MAs provided the potential for exclusive privileges in accessing benthic resources within a limited geographic area, and the interest and cooperation of local fishers has therefore increased in recent years. Currently in Aysén, there are 75 established MAs (data obtained from the Chile’s Undersecretariat of Fisheries and aquaculture, Subpesca, 2020a), with 56% (43 total MAs) having resource management plans approved and 18 of which have extracted the estimated catch quota over the last years (2016–2019). Prior to this (2010–2015), only 7 MAs contributed to landings, showing that there is an increased interest from fishers to harvest under this management system. Moreover, there are now 210 MAs pending approval in Aysén. The remoteness and intricate geography of Aysén has limited studies on the effectiveness of MAs (Moreno and Revenga, 2014; Romero et al., 2019), and no studies have yet evaluated their performance relative to OAAs in this region. On the other hand, follow up studies that considered an annual stock assessment in the management area provide data on catch quotas for commercial species but neither explicitly evaluated the intensity of exploitation, nor assessed the ecological effects on the associated benthic community. Even though, several studies have indicated the potential degrading impact of harvests upon habitats and biodiversity (Perez-Matus et al., 2017; De Juan et al., 2018; Contreras et al., 2019), and therefore on resilience of communities and populations in continuing to provide an ecosystem service. The responses of communities to harvest can be highly variable and complex since many factors are involved, on one hand there is the magnitude, timing, and areal extent of fishing (often can be well documented), on the other, the productivity of the ecosystems and the biological, and ecological characteristics of harvestable species (often with a poor level of understanding) which determine different effects on ecosystem processes and functions (Levin et al., 2009; De Juan et al., 2015). The effects of harvest on the benthic community structure can be both direct and indirect (Jennings and Kaiser, 1998; Pinnegar et al., 2000). Decrease in the abundances and changes in size structures of commercial species had been described as direct effects while some examples of indirect effects are benthic habitat degradation and changes on trophic interactions (Pinnegar et al., 2000). The monitoring of these cascade-type effects, such as a decrease in macroalgae biomass due to grazing effects by strict herbivores (e.g., L. albus) (Dayton, 1985; Buschmann et al., 2004; Wright et al., 2005; Contreras et al., 2019) or the detection of changes in the roles of top predators (e.g., C. concholepas) and their concomitant effect on benthic communities structure (Gaymer and Himmelman, 2008; Navarrete et al., 2010), are crucial to increase the knowledge related to responses of the ecosystem associated to impact of fishing.
The main objective of the present study was to evaluate MAs in the Aysén region by comparing their performance with OAAs in relation to: (i) exploitation intensity of three commercial species (Concholepas concholepas, Loxechinus albus, and Ameghinomya antiqua) and (ii) characterization of the overall benthic community structure associated with these commercial species. These results are discussed in the context of management of fisheries and benthic communities in Patagonian coastal waters.
The study was carried out in Northern Patagonia, in an area located between 43.9–45.2°S and 73.5–73.7°W (Figure 1), situated between southern Corcovado Gulf (Guaitecas Islands) and southern Moraleda Channel (Meninea constriction, 45.2°S–73.6°W). The Moraleda Channel is 90 miles long, has an approximate average depth of 250 m, and is the main channel that separates the archipelago to the west from the continental coast to the east.
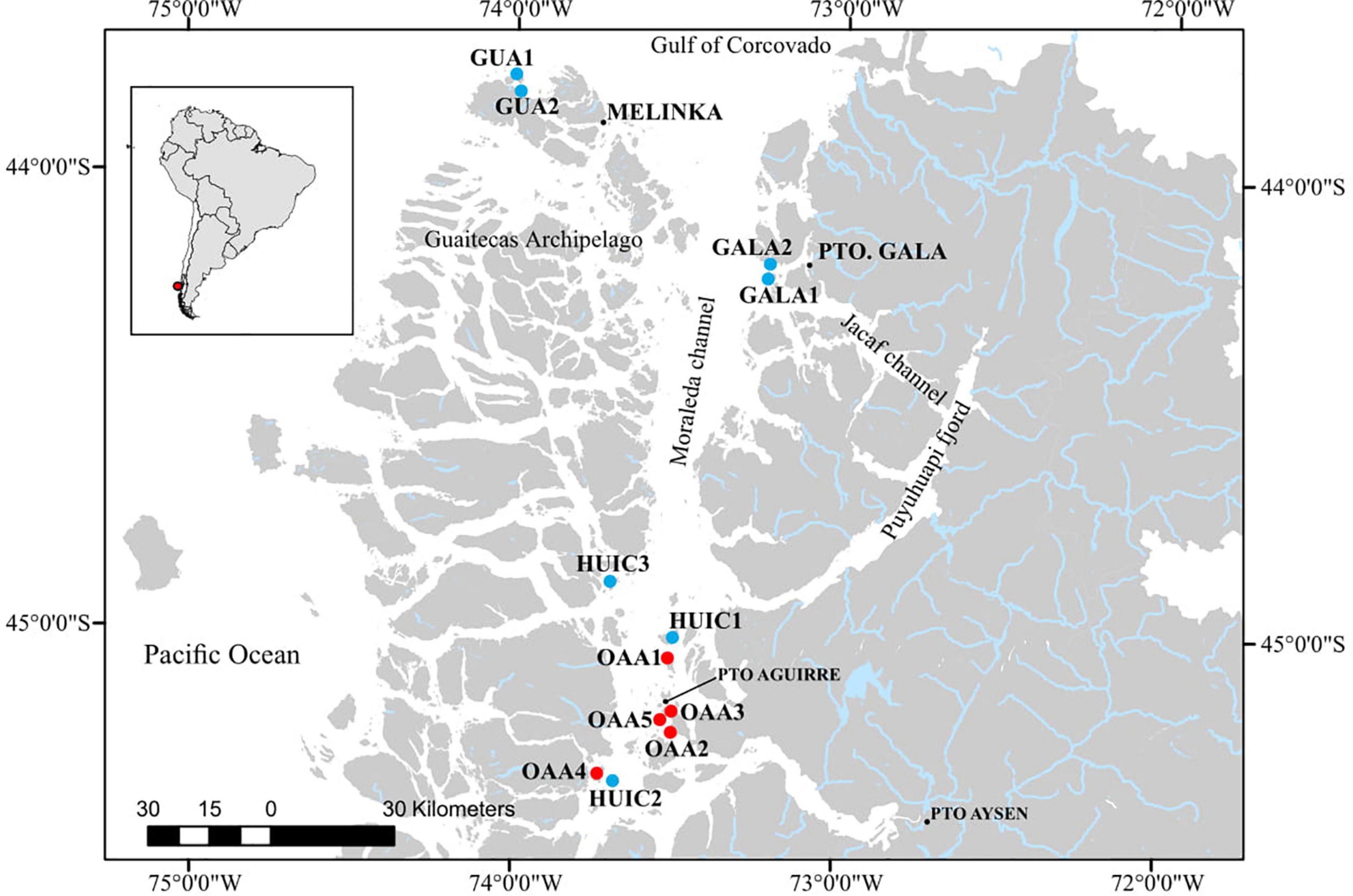
Figure 1. Map of study area in the Aysén Region showing the MAs (blue circles) and OAAs (red circles) sampled between January and April 2016.
Water masses and circulation in this region (Sievers and Silva, 2008) indicate that in the northern zone, Sub-Antarctic Water [SAAW: up to 150 m depth; Salinity between 32.5 and 34 psu; 1.2–1.6 μM PO4–3; 12–20 μM NO3–; 4–6 ml L–1 Dissolved Oxygen (DO)] flow on the surface through Boca del Guafo (Guaitecas Islands) mixing progressively toward the south with Estuarine Water (EW; 0–20 m depth, salinity between 2 and 25 psu; 1–8 μM NO3–; 0.1–0.8 μM PO4–3; 6–8 ml L–1 DO). As a result of this mixture, SAAW is modified creating a water mass with an intermediate salinity (MSAAW; ∼31 psu; 1.2–1.6 μM PO4–3; 12–16 μM NO3–; 5–6 ml L–1 DO), that flows toward the interior of the Moraleda Channel up to the Meninea constriction-sill (30–60 m depth) (Silva and Vargas, 2014). The vertical water characteristics creates a two-layer structure; a superficial layer that is warmer, more oxygenated, less saline and with lower nutrient concentrations that have greater seasonal variability (MASAAW or EW) compared to the deep layer (Silva and Guzman, 2006; Silva and Palma, 2008; Schneider et al., 2014; Silva and Vargas, 2014).
In the study area, seven MAs and five OAAs were sampled; two MAs (GUA1 and GUA2) localized northwest of Las Guaitecas archipelago near Melinka Cove (∼43.9°S) with more oceanic influence (i.e., SAAW) and with greater exposure to wave action compared to the other studied areas (Sievers and Silva, 2008). The main substrates (>80%) of these areas were hard bottom (i.e., boulder and rock slabs) in GUA1 and mixed bottom (hard and soft bottom) in GUA2 (i.e., sand, shells cover, boulder, and rock, Table 1). The other areas were localized in the so-called “inland sea” in the central-south zone of the Moraleda Channel where there is a greater influence of fresh waters (main water masses are represented by EW and MSAAW). In the central study zone, two MAs (GALA1 and GALA2) were located outside the Jacaf Channel near Puerto Gala Cove (∼44.2°S). Jacaf is a narrow and deep channel (>400 m) that connects with the Puyuhuapi Fjord, from which it receives freshwater influence (Schneider et al., 2014). The main substrate in these areas was hard bottom (rock slabs and rock) in Gala2 and soft bottom (sand) in Gala1. In the southern zone (Huichas), three MAs (HUIC1, HUIC2, and HUIC3) and five OAAs (OAAs1 to OAAs5) were located mainly in small, protected bays along the Moraleda Channel, close to Puerto Aguirre Cove (∼45.2°S). The main substrate in these MAs (HUIC1, 2, 3) was hard bottom (rock, rock slabs, and boulder). In most of OAAs (OAA3, 4, and 5), substrate was dominated by mixed (sand, gravel, rock slabs, rock, and boulder), being soft bottom (sand) the main substrate only in OAA2, and hard bottom in OAA1. In MAs, substrate type was obtained from baseline studies that considered characterization of the total surface of each area (Table 1). In the case of OAAs area, substrate type was characterized and quantified in each area. MAs were grouped considering their closeness to their coves since each of them has its socio-economic particularities that could influence their management, in this sense three zones were established: (1) Melinka (MAs-Guaitecas), (2) Gala (MAs-Gala), and (3) Aguirre (MAs-Huichas).
Open access areas were selected according to historical fishing grounds declared by local fishers of commercial species evaluated in the present study (C. concholepas, L. albus, and A. antiqua). In these areas, fishers who have registered commercial resources in the National Fishery Service can harvest them with the only restriction of following national regulations (e.g., minimum legal size of extraction, reproductive closure). Although only OAAs were studied around Huichas due to logistic problems, it is expected that these OAAs would allow for comparison among management regimes since these areas have a similar suitable substrate for the commercial species evaluated in MAs and its associated benthic communities.
Commercial species were selected in MAs according to baseline studies (Subpesca, 2016) that described the species that constitutes the object of the management and exploitation plan according to their direct quantification (Subpesca, 1995). In this context, three species were considered; the red sea urchin Loxechinus albus (Molina, 1782) that principally inhabits kelp beds, rocky and mixed substrates (rock and sand), the gastropod mollusk Concholepas concholepas (Bruguiere, 1789) that is present in mixed/rocky habitats, and the bivalve mollusk Ameghinomya antiqua (King, 1832) which is mainly an inhabitant of soft substrates. All three species have a wide distribution along the Chilean coast (18°–56°S). These same species were evaluated in OAAs. Sampling was carried out during summer 2016 (9th of January till 14th of February) in most of the areas, except for three OAAs that were evaluated at the beginning of autumn 2016 (9th till 12th of April, Table 2).
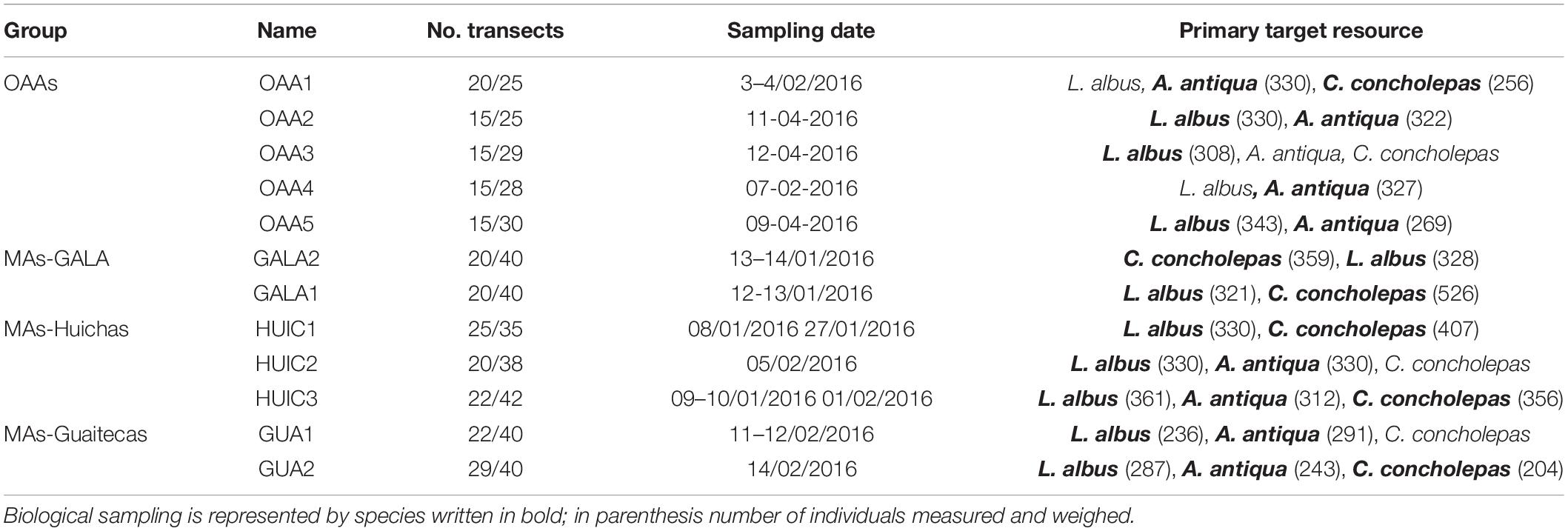
Table 2. Primary target resource evaluated per area, number of transects for benthic community/commercial species and sampling dates.
In each area (MAs and OAAs), density of commercial species and mega-invertebrates were sampled by scuba diving surveys conducted from fishing boats. Five quadrats (0.25 m2) were randomly placed within each transect (10 × 2 m) which were arranged parallel to the coast, at depths of up to 20 m (Supplementary Table S1). The total number of transects per area was established according to their surface area, fluctuating between 25 and 42 for the selected commercial species (C. concholepas, L. albus, and A. antiqua), and between 15 and 29 for analysis of benthic community structure (Table 2). On soft bottom benthic habitat, only the commercial species were evaluated with no analysis undertaken for the macroinfauna community. A total of 238 community transects were sampled, in which 19,818 individuals were identified to their lowest possible taxonomic level (Forcelli, 2000; Häussermann and Försterra, 2009); with the exception of a few taxa that could only be identified to higher taxonomic levels (e.g., Demospongiae, Actinaria, and Holothuroidea).
Biological sampling (measured and weighed) of primary target resources (C. concholepas, L. albus and A. antiqua) was performed in all areas, except in the ones with low densities and scarce presence of individuals along transects (<20% of the evaluated transects). Between 269 and 526 individuals per each primary target resource per area were collected by scuba diving, immediately after they were measured and weighed on shore (Table 2). For size of individuals test diameter was taken for L. albus and shell length for C. concholepas (peristome length: maximum length from the siphonal notch to the posterior edge of the shell) and A. antiqua (valvar length along the maximum axis). Size was measured using a Vernier caliper with a precision of 0.1 mm, and body wet weight (including the shell in mollusks) was measured using an electronic balance with a precision of 0.1 g.
Performances of MAs relative to OAAs were evaluated using three indicators as a proxy for exploitation intensity of commercial species (C. concholepas, L. albus, and A. antiqua). Fishing regulations have established a legal minimum size of extraction (MLS) and fishers clearly target larger individuals; therefore, the absence of the largest harvestable individuals can be assumed as a proxy for exploitation intensity of a given species (Blanchard et al., 2005; Miethe et al., 2016). In this context, two fisheries indicators were chosen based on size. The first indicator considered the average size of the largest 5% of the sample (Lmax5) and was selected as a measure that is less affected by environmental effects and recruitment variability (Miethe et al., 2016). The second indicator relates the number of individuals in a specific size class to the total number of individuals collected [proportional stock density (PSD)]. The limit sizes adopted were the MLS established by Subpesca by the time the studied was carried out (C. concholepas = 100 mm; L. albus = 70 mm, and A. antiqua = 55 mm), and PSD therefore estimates the percentage of individuals whose size exceeds these MLS. A third indicator was not based on size, but instead considered average density of commercial species, estimated from the average number of individuals counted within each quadrat, divided by surface area (0.25 m2). Average density of individuals for each area (ind.m–2) was computed from replicate transects. As a measure of the nutritional condition of each species, a fourth indicator was estimated, the Medium Condition Factor (MCF; Arana, 2006). This indicator relates the average weight of the individuals in a specific size range in one area to the average weight of all individuals in that specific size range in all the areas. To compare the weight of a similar number of individuals between areas, size range was chosen considering the class intervals that had the highest but even number of individuals between areas. Values exceeding one, indicate that individuals in one area have a better nutritional condition (i.e., suggests adequate food supply) than in other areas. This indicator had been used previously to assess gonadal development in L. albus over a time series (Arana, 2006). Since our study examined only spatial variability, sampling was carried out trying to study the areas during the same period to find individuals in similar reproductive conditions. According to reproductive cycle of L. albus, C. concholepas, and A. antiqua, during our sampling, post-spawning individuals in a reproductive rest state were collected and therefore it would be reasonable to assume that the MCF indicators are comparable reflecting relative nutritional condition. In the case of L. albus reproductive cycle in the northern neighboring region (41°45′) and northern part of Aysén Region (Melinka, 43°53′) indicated that a higher proportion of mature individuals can be found between spring and beginning of summer (Bay-Schmith et al., 1981; Arias et al., 1995, Molinet et al., 2016) which coincides with the reproductive seasonal closure established between 15th of October till 15th of January (D. Ext. N°439, Subpesca, 2000). Studies of reproductive cycle of C. concholepas (41.8°S; Manriquez et al., 2009) indicated maximum gonadal development in December. Seasonal closure of this species is established between the 1st of September and 31th of January (D. Ext. 697, Subpesca, 2011). A. antiqua, has no seasonal closure; maximum reproductive months had been observed between July and August in northern part of Aysén Region (43.8°S), with spawning period occurring between spring and early summer (Canales et al., 2019).
The 12 areas studied were divided, according to their closeness to their coves and management regimes, into four groups: (1) MAs-Huichas (3 areas), (2) MAs-Gala (2 areas), (3) MAs-Guaitecas (2 areas), and (4) Open Access Areas (5 areas).
Since data was not normally distributed (Kolmogorov–Smirnov, p < 0.01) and data presented no homogeneity of variance (Levene’s test p < 0.05), Kruskal–Wallis non-parametric test (Statistica software 7.0) was used to independently compare the different indicators between groups.
Benthic communities were characterized, considering the four sampling sites described previously, relative to: (i) average density (number of individuals in each quadrat divided by its surface area of 0.25 m2 with data standardized to ind.m–2) and (ii) dominance (% D, percentage of individuals of one species relative to the total number of individuals considering all species). Since density data was not normally distributed (Kolmogorov–Smirnov, p < 0.01), the Kruskal–Wallis non-parametric statistical test (Statistica software 7.0) was used to independently compare between the four groups. Additional measures of community structure were calculated, including total number of species (S), Shannon-Wiener diversity index (H′) and Evenness index (J′) (Pielou, 1977). The rarefaction method (ESn) was used to compare taxa richness in samples of unequal size for benthic macrofauna (Hurlbert, 1971). These measures were calculated using the software package PAST V4.03 (Hammer et al., 2001).
To determine which taxa contributed to the dissimilarity of the faunal assemblages between groups, an analysis of similarity was conducted (SIMPER, Clarke, 1993). Non-Metric Multidimensional Scaling (nMDS) and an analysis of similarity test (one-way ANOSIM) were used to evaluate similarities between groups, and for these analyses, species densities were transformed to the fourth root and the Bray-Curtis distance index was applied to estimate the degree of similarity between groups/species (Clarke, 1993). Transects were treated as replicates to provide an average density for each area. Differences between groups were evaluated using a one-way permutational multivariate variance analysis (PERMANOVA; Anderson, 2001). Since all OAAs were localized in Huichas, a more detailed statistical analyze was performed to compare them with MAs geographically closed. For this, nMDs and one-way ANOSIM analyses were performed using transects as replicas. SIMPER, nMDS, and ANOSIM analyses were completed using the software package PRIMER Version 6 (Clarke and Gorley, 2006).
Concholepas concholepas was evaluated in all MAs studied since it is a primary target resource in all management plans. Suitable substrate in OAAs, indicated that four of the five areas studied should have had this resource, but it was present only in two of them. Mean densities varied between 0.4 and 3.8 ind.m–2 (Figure 2A) with significant differences (Kruskal–Wallis test, p < 0.05) in MAs (2.3 ± 5.6 ind.m–2) compared to OAAs (0.4 ± 1.3 ind.m–2). These differences were due to higher densities in MAs-Gala (2.6 ind.m–2) and MAs-Huichas (2.6 ind.m–2) compared to OAAs (Kruskal–Wallis test, p < 0.05). Within MAs-Huichas, the absence of this resource in MA-HUIC2 contrast with higher densities estimated in the other nearby areas (HUIC3 and HUIC1). In MAs-Gala high density was represented by small sized individuals (average 61 ± 11 mm) that resulted in Lmax5 of 84 (±15 mm) in comparison with MAs-Huichas where high densities were dominated by larger individuals (Lmax5 = 111 ± 11 mm) (Figure 3A). Densities between both groups of MAs (Huichas and Gala) were not significantly different (Kruskal–Wallis test, p > 0.05) but when comparing Lmax5, significant difference were estimated (Kruskal–Wallis test, p < 0.05). Due to low densities in most of the areas, fisheries indicators (Lmax and PSD) could only be estimated in one OAAs preventing its statistical comparison with MAs. The percentage of individuals exceeding MLS (PSD) was 45% in MA-HUIC3, whereas in all other areas, PSD was <8%. MA-Gala1 represented an extreme example where none of the individuals sampled exceeded 100 mm (average size was 58 ± 9 mm). MCF, calculated over the size range of 70–89 mm, showed that individuals in MAs-Gala were also in poor nutritional condition compared to other areas (Table 3).
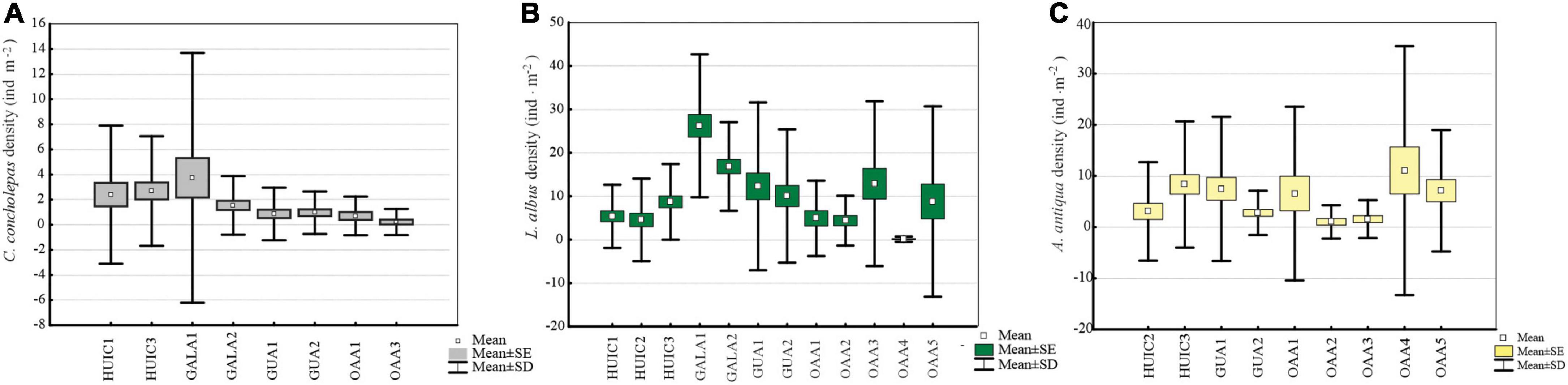
Figure 2. Average densities (ind.m– 2) and standard deviation (SD) of commercial species in MAs and OAAs; (A) C. concholepas, (B) L. albus, and (C) A. antiqua.
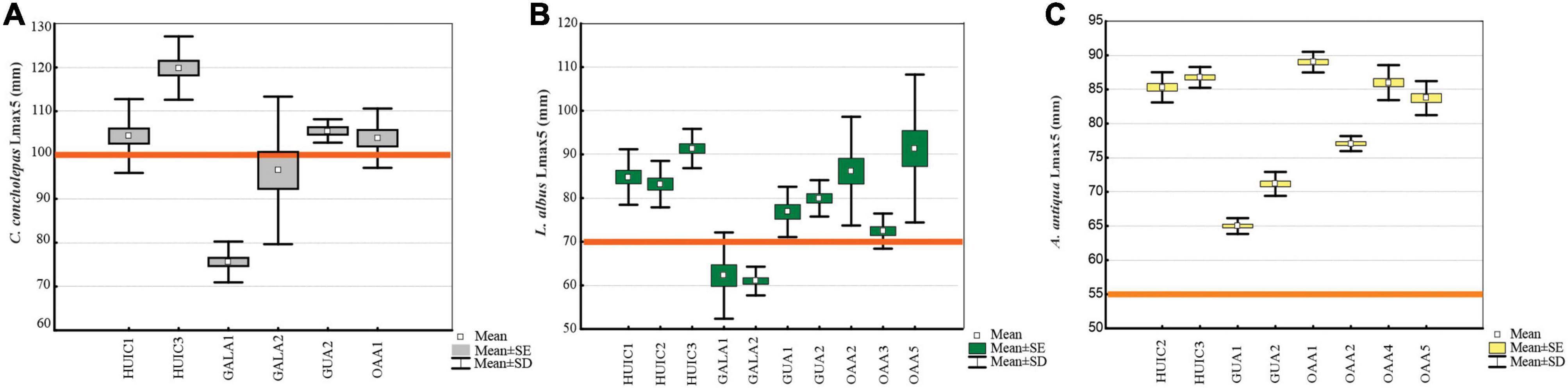
Figure 3. Average size of the largest 5% (Lmax5, mm) and standard deviation (SD) of commercial species in MAs and OAAs; (A) C. concholepas, (B) L. albus, and (C) A. antiqua.

Table 3. Proportional stock density (PSD) and medium condition factor (MCF) calculated for the commercial species within MAs and OAAs.
Loxechinus albus was the only commercial species present in almost all studied areas. Estimated densities varied between 4 and 26 ind.m–2, significant differences (Kruskal–Wallis test, p < 0.05) were found for MAs (12 ± 8 ind.m–2) compared to OAAs (6 ± 5 ind.m–2). This difference resulted from MAs-Gala, where densities were significantly higher than the other areas (Kruskal–Wallis test, p < 0.05), with an average of 22 ± 13 ind.m–2 (Figure 2B). Other MAs (Huichas and Guaitecas) did not present significant differences in densities when comparing with OAAs (Kruskal–Wallis test, p > 0.05). High density in MAs-Gala was represented by small sized sea urchins (39 ± 10 mm) corresponding with Lmax5 of 62 mm (Figure 3B). Lmax5 varied between this minimum and a maximum estimated in MA-HUIC3 (Lmax5 = 91 mm). No significant differences (Kruskal–Wallis test, p > 0.05) were found for Lmax5 between MAs (77 ± 13 mm) and OAAs (84 ± 15 mm). When comparing Lmax5 between MAs closed to OAAs, i.e., MAs-Huichas with OAAs, significant difference where estimated (Kruskal–Wallis test, p < 0.05). Lmax5 was higher in MAs (86 ± 4 mm) compared to OAAs (83 ± 10 mm).
Proportional stock density which represented the percentage of individuals over the MLS (≥70 mm) showed high variability between areas (MAs; average 17 ± 15% and OAAs; average 10 ± 14%). In MAs, this indicator fluctuated between 0.3 and 55.7% (Table 2), with maximum values estimated in MA-HUIC3 and a minimum in MA-Gala2 (0.3%). In OAAs, PSD varied between 4.2 and 21.6%, with no significant differences between OAAs and MAs (Kruskal–Wallis test, p > 0.05). The Medium condition factor (MCF) considered size range between 40 and 59 mm showed that sea urchins in MAs-Gala had the lowest nutritional condition (MCF < 1, Table 2). No significant differences in MCF were found between MAs and OAAs (Kruskal–Wallis test, p > 0.05).
Ameghinomya antiqua was present in all OAAs and in four MAs (Table 2), with densities that varied between 1 and 11 ind.m–2 (Figure 2C). No significant differences in densities were found between OAAs (5 ± 4 ind.m–2) and MAs (4 ± 3 ind.m–2) (Kruskal–Wallis test, p > 0.05). Lmax5, was significantly higher (Kruskal–Wallis test, p < 0.05) in OAAs (84 ± 5 mm) compared to MAs (77 ± 10 mm). Within MAs, the lowest Lmax5 was estimated in Guaitecas (68 ± 3 mm) (Figure 3C) and was significantly different from areas localized in Huichas (MAs and OAAs, Kruskal–Wallis test, p < 0.05). No significant differences in Lmax5 were found between MAs-Huichas and OAAs (Kruskal–Wallis test, p > 0.05). PSD representing the percentage of individuals above 55 mm (MLS), fluctuated between 84% and 97% in OAAs, and between 44% and 92% in MAs, with no significant difference between both management regimes (Kruskal–Wallis test, p > 0.05). The MCF indicator was estimated considering the weight of individuals between sizes of 60 and 69 mm. Relatively lower values (MCF < 1) were found in MA-GUA1 which was consistent with the data of the other indicators (PSD and Lmax5). No significant differences were found between MAs and OAAs (Kruskal–Wallis test, p > 0.05).
A total of 102 taxa were recorded in the 12 areas studied, 84 taxa/species in MAs and 71 taxa/species in OAAs. The most significant Phyla in terms of species richness were Mollusca (MAs n = 30; OAAs n = 24), Echinodermata (MAs n = 21; OAAs n = 16) and Arthropoda (MAs n = 12; OAAs n = 10). Of the total taxa registered in the survey, 21 corresponded to species of commercial interest, and most of these were mollusks (Figure 4A and Supplementary Table S2).
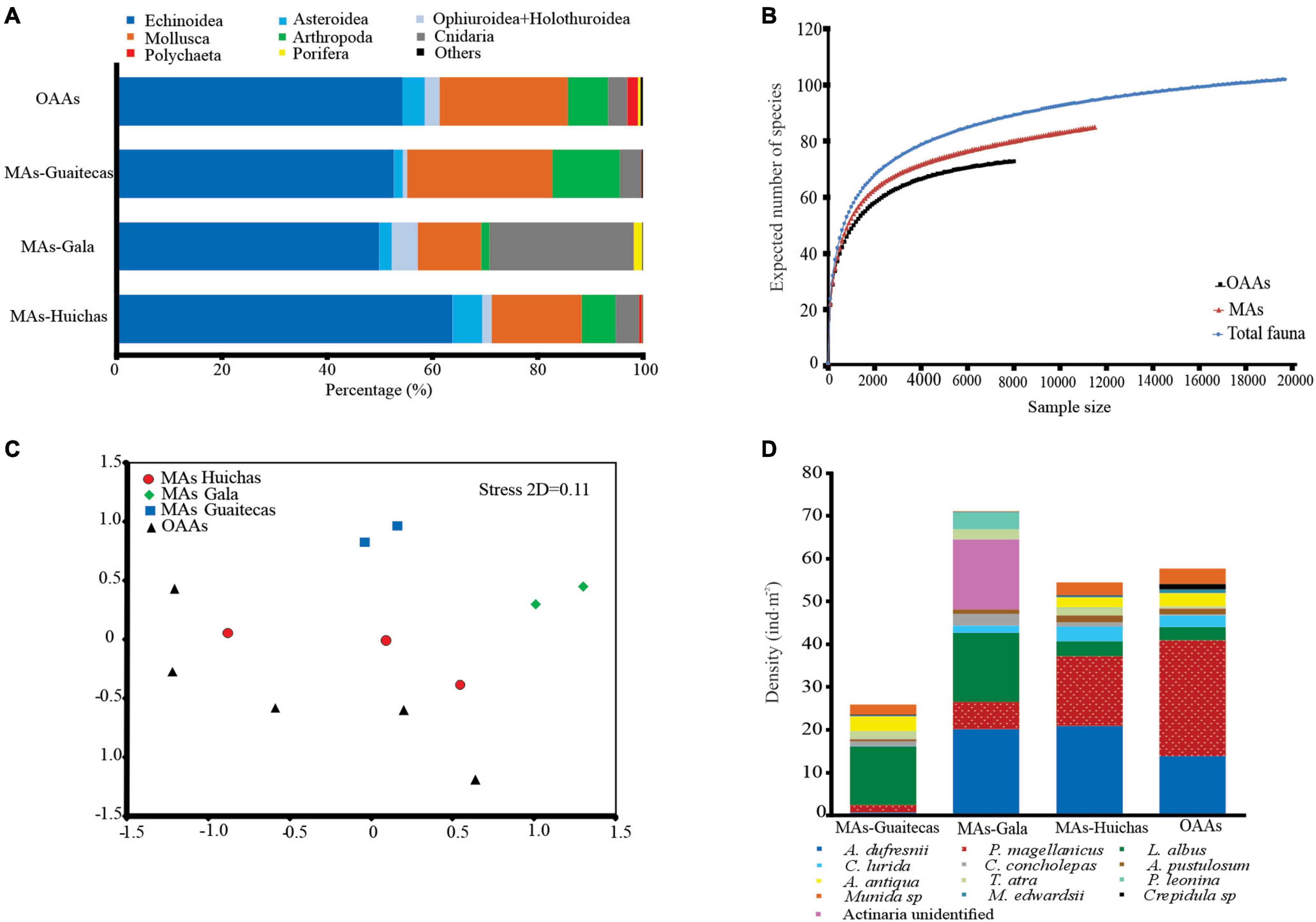
Figure 4. (A) Benthic fauna contribution in terms of density in percentage (%) for main taxonomic groups. Others: Ascidiacea, Nemertea, Echiura, and Platyhelminthes. (B) rarefaction (ES) plot for the benthic fauna enumerated in MAs, OAAs and for total benthic fauna (MAs + OAAs). (C) Non-metric multidimensional scaling analysis (nMDS), showing OAAs (black triangles), MAs-Huichas (red circles), MAs-Guaitecas (blue squares), MAs-Gala (green diamonds). (D) Densities of the principal species contributing to dissimilarity between areas. The list is based on the species that contribute to >50% cumulative of the dissimilarity according to SIMPER analysis.
The Echinoidea class represented mainly by Loxechinus albus, Arbacia dufresnii, and Pseudechinus magellanicus tended to dominate numerically (50–64% of total fauna density) in both MAs and OAAs. The bivalves Ameghinomya antiqua (11% of total fauna density) and Aulacomya atra (11% of total fauna density) also represented important numerical contributions to the overall density in MAs-Guaitecas and in OAAs, respectively (Figure 4A and Supplementary Table S2).
Average densities of benthic species (considering all the community) showed significant differences in MAs-Gala (Kruskal–Wallis test, p < 0.05) in comparison with other areas (Table 4). The Shannon-Wiener diversity index ranged between 2.09 and 2.33, indicating low variability between areas. Evenness index (J′) was ∼0.6 in all areas, indicating that the community was dominated by few species; no significant differences were estimated in either of the above indexes between the four groups (Table 4; Kruskal–Wallis test, p > 0.05). Rarefaction curves indicate equal values of ES(100) = 22 both in MAs and OAAs (Figure 4B), although a trend of higher species richness was observed in MAs, with ES(2000) = 63 compared to OAAs with ES(2000) = 58. The shape of the curve for total-fauna, which considers all 102 taxa/species observed in MAs and OAAs, indicates that species richness was well represented by sampling effort in the studied areas (Figure 4B).
Analysis of data using nMDS highlighted three main groups: (i) MAs-Gala, (ii) MAs-Guaitecas, and (iii) another cluster grouping all OAAs with MAs-Huichas (Figure 4C). The one-way ANOSIM showed separation between groups (Global R = 0.32, p < 0.05) but only significant differences were found between MAs-Gala and OAAs (PERMANOVA test, p < 0.05). Results associated to the evaluation of the similarity between areas geographically closed (MAs-Huichas and OAAs) did not showed defined groups, associated to management regimes, in relation to the community composition and their abundances (one-way ANOSIM, Global R = 0.33, p < 0.05).
SIMPER similarity analysis was used to determine the species that contributed to the similarity (or dissimilarity) of the faunal assemblages within and between areas; high similarities within the areas (47–65%) were obtained, with group MAs-Gala having the highest percentage (65%). Dissimilarity between MAs and OAAs, showed MAs-Huichas as having lower dissimilarity (49%) compared to OAAs and MAs-Gala as having greater dissimilarity (63%) compared to OAAs. The main species that contributed to these dissimilarities (MAs-Gala and OAAs) were Arbacia dufresnii, Loxechinus albus, Concholepas concholepas, Pentactella leonina, Tegula atra, Cosmasterias lurida, Argobuccinum pustulosum, Pseudechinus magellanicus, Ameghinomya antiqua, Actinaria unidentified, Aulacomya atra, and Metacarcinus edwardsii (Figure 4D). However, only the first five of the above species showed significantly higher densities (Kruskal–Wallis test, p < 0.05) in MAs-Gala than in OAAs. In contrast to the other species, the sea star Cosmasterias lurida, showed significantly lower densities in MAs-Gala compared to MAs-Huichas and OAAs (Kruskal–Wallis test, p < 0.05).
Concholepas concholepas is a species for which extraction has been banned from OAAs since 2002 (San Martin et al., 2010). Low densities in OAAs hinder the statistical comparison of fisheries indicators for C. concholepas between this regime and MAs. In most MAs, low densities combined with low values of the fisheries indicators could be indicative of a high exploitation intensity. To contribute to the discussion of these results, total allowable catch (TAC) for this resource and the others considered in this study, was estimated using the same methodology for MAs and OAAs (details of the methodology and quotas are given in Supplementary Table S3). In this context, only two areas obtained a quota for C. concholepas and only in one (MA-HUIC3), was high enough to be worth the extraction effort, suggesting that the exploitation intensity for this resource was high in most of the areas, despite their management regimes. Historical TACs for this resource in the studied MAs (obtained from Subpesca, 2020b) sustained this hypothesis since they had been decreasing since their first baseline study, except in HUIC3. Considering all MAs in Aysén, landings of C. concholepas have been decreasing since 2010, from 65,447 kg during the period 2010–2015, to 9,641 kg during the period 2016–2019, and in 2020 (up to October) only 1,180 kg has been harvested (data obtained from Servicio Nacional de Pesca, Sernapesca, 2020). OAAs were selected according to historical fishing grounds of the primary target resources, but no quotas were obtained suggesting illegal fishing activities. Data for illegal fishing shows that in the last 5 years, a total of 3,000 kg of C. concholepas has been confiscated (Sernapesca, 2020); although this is likely to be an underestimation of the true illegal harvest. In central Chile, a study of illegal extraction of this species concluded that fishers harvest illegally not only in OAAs, but also within their local MAs and within MAs of other organizations (Oyanedel et al., 2017). The same study estimated that official landings account for only 14–30% of the total C. concholepas catch. The massive extraction of this species within OAAs is not only of concern regarding total biomass, but also in the context of MLS; estimates suggest that up to 48% of this illegal catch is composed of undersized individuals, compared to only 8% in MAs (Fernandez et al., 2020). Our data therefore support the need to examine the effectiveness of the permanent ban on harvest of C. concholepas within OAAs (Bandin and Quiñones, 2014; Andreu-Cazenave et al., 2017).
In contrast to the C. concholepas fishery, L. albus can be extracted from OAAs, but is managed through an annual TAC. Higher densities estimated in MAs compared to OAAs were mainly driven by small sizes individuals in MAs-Gala. Low values of the fisheries indicators in both MAs and OAAs, could be indicating that harvest had been intense in most of the areas, except in some MAs in Huichas. Estimated TACs supports these findings, since on average, low quotas were estimated for MAs and OAAs (11 ton and ≤3 ton in MAs and OAAs, respectively). In Aysén, increased exploitation of this resource in MAs had been registered since 2010 (Sernapesca, 2020). Indeed, from 2016, this species has contributed to 99.5% of landings within MAs. It appears that MAs present an opportunity for fishers to extract and commercialize this resource at a higher price in MAs (USD 0.7 ± 0.2 in OAAs and USD 1.5 ± 0.1 in MAs; A. Lafon, personal communication, December 12, 2020), at a time when the regional TAC had been already harvested in OAAs.
MAs in Gala presented high densities of C. concholepas and L. albus but of small sizes individuals which could be indicating that this zone could be important in terms of recruitment. For both resources, the average sizes were in the lower limit of the first size at maturity; estimated between 70 and 90 mm for C. concholepas (41.8°S; Manriquez et al., 2009) and between 41 and 44 mm for L. albus (Arias et al., 1995). A regular sampling (at least annually) that includes habitat characteristics (e.g., food supply, water exchange) will allow to determine if this situation is permanent and the area has become important in terms of recruitment or small sizes are the result of the intense exploitation of harvestable individuals.
Ameghinomya antiqua is a species restricted to habitats with sandy-muddy bottoms. This clam has only been targeted as a primary resource in four MAs (Huichas and Guaitecas) and was present in all OAAs. Unlike other commercial species evaluated in the present study, there are no restrictions for fishers to extract this species in OAAs, since no TACs had been implemented. Our results showed no significant differences in densities between management regimes. In both MAs and OAAs, size-frequency distribution was skewed toward larger sizes showing that exploitation intensity was low both in OAAs and MAs. Since suitable substrate for this species was generally restricted within these areas, estimated quotas were not high (average TACs: MAs = 26 ton; OAAs = 13 ton). MAs-Guaitecas were the only areas with historical TACs. In both MAs, these first studies estimated higher quotas (∼ 113 ton) that the ones estimated in our study. After that, stable quotas of around 30–50 ton (Subpesca, 2020b) could be indicating and supporting our findings of low exploitation intensity.
The benthic community was represented by a high number of species (102 taxa/species), dominated by Echinoidea (Arbacia dufresnii, Pseudechinus magellanicus, and Loxechinus albus). Diversity and evenness indexes did not show differences between areas with different management regimes. Only one group of MAs (Gala) showed significant differences with OAAs regarding the densities of commercial species (L. albus and C. concholepas) and other secondary species (Arbacia dufresnii, Pentactella leonina, and Tegula atra).
High densities of L. albus (10 ± 15 ind.m–2) were comparable with those reported in northern Patagonia (11 ind.m–2, Molinet et al., 2016; 29 ind.m–2, Contreras et al., 2019). L. albus is a strict herbivore (Vasquez et al., 1984; Gonzalez et al., 2008) and therefore could have a controlling effect on macroalgae biomass and in surrounding communities, since macroalgae are three dimensional structures that favor settlement and serve as refuges for juveniles’ stages (Dayton, 1985; Buschmann et al., 2004; Wright et al., 2005; Contreras et al., 2019). In MAs-Gala, high densities of sea urchins were observed, co-incident with low macroalgae abundance (<5%, Supplementary Table S4). These types of habitats dominated mainly by sea urchins and devoid of macroalgae, resemble the sea urchin barrens (Fillbee-Dexter and Schebling, 2014) that have been described in regions that support kelp beds as in Patagonia (Dayton, 1985; Fernandez et al., 2000). Sea urchin barrens can shift to a stage where kelp beds can recuperate or could maintain a stable state associated to long-term persistence of the parameters that drive and sustain high abundances of sea urchins (e.g., urchin grazing rate, kelp growth rate, recruitment rates, per capita predation rates) (Dayton, 1985; Fillbee-Dexter and Schebling, 2014). Threshold densities of sea urchins could determine these shifts: in fjords of the northern hemisphere, urchin densities of 45–75 ind.m–2 and 10–16 ind.m–2, have been reported for barrens, and kelp beds, respectively (Fillbee-Dexter and Schebling, 2014). In northern and central Chile, barrens have been shown to alternate with kelps patches (Vasquez and Buschmann, 1997) and persistence of barrens has been associated with unregulated extraction of macroalgae (Vasquez, 2008). In the fjords and channels of Patagonia (45°–54°S) barrens dominated by L. albus have been reported (with densities up to 100 ind.m–2) (Dayton, 1985). In contrast, low densities of L. albus (<2 ind.m–2) have been reported in dense kelp forest in parts of the Magellan region (Friedlander et al., 2018). In MAs-Gala, high densities of L. albus (22 ± 14 ind.m–2, with a maximum of 78 ind.m–2) and A. dufresnii (20 ± 14 ind.m–2 with a maximum of 51 ind.m–2) coincided with relatively low coverage of macroalgae which could be indicating a sea urchin barren in these areas. This contention is supported by juveniles of L. albus (39 ± 9 mm) and C. concholepas (61 ± 11 mm) dominating the community in these areas. High settlement in barrens has been reported, probably due to the lower structural complexity, which could limit available habitats for predators (Fillbee-Dexter and Schebling, 2014). Further research is needed to evaluate the persistence of this sea urchin barren state in this zone.
An unexpected finding of the present study was related to Cosmasterias lurida, a predatory sea star that inhabits the subtidal environment. This species was present in high frequency of occurrence (64% of transects) and higher densities (2 ± 3 ind.m–2) in the sampling sites compared to other studies in Patagonia (Vasquez and Castilla, 1984; Garrido, 2012). Recognized top predator such as C. concholepas (Navarrete et al., 2010) occurred infrequently and in low densities in our study, indicating that C. lurida would have no competitors being the highest-order predator. The intense harvesting of C. concholepas in the study area could be contributing to shifts on roles of predators that could have cascading effects on the structure of benthic communities (Fernandez et al., 2000; Pinnegar et al., 2000; Gaymer and Himmelman, 2008; Perez-Matus et al., 2017), and further evaluation of its effects is now a high priority.
Our results indicate that MAs in the Aysén region are not fulfilling the objectives of sustainability regarding exploitation of benthic resources. Few of the MAs that were studied showed significantly lower exploitation intensities than OAAs, with most showing similar exploitation intensity. In both MAs and OAAs, benthic communities showed signs of disturbance characterized by the absence of the largest harvestable individuals (L. albus and C. concholepas). High densities of echinoderms (sea urchins and sea stars) were also indicative of changes in the community structure (e.g., trophic roles, competition, and dominances).
Currently, information provided by follow up studies is deficient in the sense that it does not permit evaluation of changes in benthic community structure associated to harvest. However, efforts have been made by the national fisheries institute (Spanish acronyms, IFOP) to evaluate MAs performance mainly in the northern regions. These results indicate that the community associated to C. concholepas presented two groups (north-central zone 28°–33°S and south zone 39°–41°S), with latitudinal differences in their abundances and composition of their functional groups. However, community indicators (e.g., density, diversity of functional groups, diversity, uniformity, and dominance indexes) resulted highly variable spatially and temporally (Ariz et al., 2017, 2018; Romero et al., 2019). Due to the high heterogeneity of these indicators, the use of a regional scale was recommended (Romero et al., 2019). A regional spatial scale will allow through environmental, biological and socioeconomic monitoring to identify more defined spatial patterns, and apply local management measures and policies (e.g., minimum extraction sizes). This monitoring should include integral spatial management since all commercial species considered in MAs management plans have long dispersive larval stages hence settlement, recruitment and growth are processes that probably greatly exceed the boundaries of MAs (Romero et al., 2019). In short, the productivity of one area is dependent on processes and management in other OAAs and MAs (Molinet et al., 2010; Arias and Stotz, 2020). It should be highlighted that our results are the first attempt to evaluate MAs’ performance in comparison with OAAs regime on a regional scale with a community approach in Aysén (43.9°–45.2°S).
Illegal fishing has been recognized as a major impediment to marine conservation, and although MAs were designed to provide a disincentive, our data combined with regional statistics, highlight deficiencies in the existing system. The surveillance of fishers within MAs in the Aysén region has been almost impossible to implement because of long travel distances from fishing villages to their MAs (distances between coves and MAs in this study varied between ∼9 and ∼32 km and up to 180 km when considering all regional MAs). It should be highlighted that most fishers own small boats which resulted in 1 or 2 h sailing from their coves to their MAs. In this context, the only area (HUIC3) showing improved fisheries indicators for commercial species was one with year-round surveillance provided by the proximity of local fishers. In this case, fishermen asked for a land concession next to the area to diminish cost associated to surveillance. This surveillance system worked well because of social cohesion within their union, which highlighted the social capital as a key factor in the successful functioning of MAs (Marin and Gelcich, 2012). MAs-Gala were the only other management areas close to coves (∼9 km apart) but due to low social capital no vigilance has been implemented. Social capital is especially important in Aysén region because fishing coves are not only remote from MAs but also from markets. Clearly, the establishments of collaborative networks should be considered as a priority in the strengthening of this benthic management regime.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MH: conceptualization, methodology, writing – reviewing and editing, supervision, and visualization. PO: conceptualization, methodology, and writing – reviewing and editing. Both authors contributed to the article and approved the submitted version.
This study was financially supported by the Subpesca through grant CUI 2015-7-FAP “Asistencia para el desarrollo de Estudio de Situación Base de Área y Seguimiento en Áreas de Manejo de la Región de Aysén, Etapa 1”. Regional Program ANID R17A10002 and R20F0002 partial funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Ramiro Contreras for the discussion of the results and Subpesca for data handling. The authors would also like to thank Aldo Hernandez y Carlos Leal for methodology field support and estimation of catch quota, Eduardo Palma for diving during field trips, and David Crawford and Brian Reid for the English revision of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.635756/full#supplementary-material
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Andreu-Cazenave, M., Subida, M. D., and Fernandez, M. (2017). Exploitation rates of two benthic resources across management regimes in central Chile: evidence of illegal fishing in artisanal fisheries operating in open access areas. PLoS One 12:e0180012. doi: 10.1371/journal.pone0180012
Arana, P. (2006). Demography and fishery of the sea urchin Loxechinus albus (Echinodermata: Echinidae) in south-austral Chile region. Int. J. Trop. Biol. 53, 367–382.
Arias, E., Barahona, E., Lozada, E., and Jerez, G. (1995). Monitoreo Del Recurso Erizo En La X y XI Región. Informe Final FIP 93-13. Valparaíso: Instituto de Fomento Pesquero.
Arias, N., and Stotz, W. (2020). Sustainability analysis of the benthic fisheries managed in the TURF system in Chile. Int. J. Commons 14, 344–365. doi: 10.5334/ijc.1011
Ariz, L., Arenas, G., Clavijo, L., Díaz, L., Figueroa, L., García, A., et al. (2017a). Convenio De Desempeño IFOP – Subsecretaría De Economía y Empresa De Menor Tamaño. Informe Final. Valparaíso: Instituto de Fomento Pesquero (IFOP), 192. + Anexos.
Ariz, L., Arenas, G., Clavijo, L., Díaz, L., Figueroa, L., García, A., et al. (2018). Programa de Seguimiento Pesquerías Bajo Régimen Áreas de Manejo, 2017. Convenio de Desempeño IFOP - Subsecretaría de Economía y Empresa de Menor Tamaño. Informe Final. Valparaíso: Instituto de Fomento Pesquero (IFOP), 192.
Ariz, L., Clavijo, L., Carrasco, S., Díaz, L., Figueroa, L., Galleguillos, F., et al. (2017). Programa De Seguimiento Pesquerías Bajo Régimen Áreas de Manejo, 2016. Convenio de Desempeño IFOP – Subsecretaría de Economía y Empresa de Menor Tamaño. Informe Final. Valparaíso: Instituto de Fomento Pesquero (IFOP), 308 + Anexos.
Bandin, R. M., and Quiñones, R. A. (2014). Impacto de la captura ilegal en pesquerías artesanales bentónicas bajo el régimen de co-manejo: el caso de Isla Mocha, Chile. Latin Am. J. Aquat. Res. 42, 547–579. doi: 10.3856/vol42-issue3-fulltext-14
Bay-Schmith, E., Werlinger, C., and Silva, J. (1981). Ciclo Anual de Reproducción Del Recurso Erizo Loxechinusalbus Entre la X y XII Región. Concepción: Universidad de Concepción.
Bertolino, M., Costa, G., Bavestrello, G., Pansini, M., and Daneri, G. (2020). New sponge species from Seno Magdalena, Puyuhuapi Fjord and Jacaf Canal (Chile). Eur. J. Taxon. 715, 1–49.
Betti, F., Bavestrello, G., Bo, M., Enrichetti, F., Loi, A., Wanderlingh, A., et al. (2017). Benthic biodiversity and ecological gradients in the Seno Magdalena (Puyuhuapi Fjord, Chile). Estuar. Coast. Shelf Sci. 198, 269–278. doi: 10.1016/j.ecss.2017.09.018
Biggs, D., Amar, F., Valdebenito, A., and Gelcich, S. (2016). Potential synergies between nature-based tourism and sustanaible use of marine resources: insights from dive tourism in territorial user rights for fisheries in Chile. PLoS One 11:e0148862. doi: 10.1371/journal.pone.0148862
Blanchard, J. L., Dulvy, N. K., Jennings, S., Ellis, J. R., Pinnegar, J. K., Tidd, A., et al. (2005). Do climate and fishing influence size-based indicators of Celtic Sea fish community structure? ICES J. Mar. Sci. 62, 405–411. doi: 10.1016/j.icesjms.2005.01.006
Bruguiere, J. G. (1789). Encycloped. Méth. (loe. tipo: Costas del Perú, por Designación Original) (Descripción Original). 252.
Buschmann, A. H., Garcia, C., Espinoza, R., Filun, L., and Vasquez, J. A. (2004). “Sea urchin (Loxechinus albus) and kelp (Macrocystis pyrifera) in protected areas in southern Chile,” in Sea Urchin Biology, eds J. Lawrence and O. Guzmán (Harrisburg, PA: DEStech Publications), 120–130.
Canales, C., Adasme, N., Sanchez, N., Curiel, J., Mardones, M., Barahona, N., et al. (2019). Estrategias de Manejo de la Pesquería de la Almeja Venus Antiqua en la Región de Los Lagos y la Región de Aysén Del General Carlos Ibáñez del Campo”. Proyecto FIPA 2018-32. 244. Anexos. Subpesca, Valparaíso, Chile.
Castilla, J. C. (1994). The Chilean small-scale benthic shell fisheries and the institutionalization of new management practices. Ecol. Inter. Bull. 21, 47–63.
Castilla, J. C., and Fernandez, M. (1998). Small-scale benthic fisheries in Chile: on co-management and sustainable use of benthic invertebrates. Ecol. Appl. 8(Suppl.) S124–S132.
Castilla, J. C., Manriquez, P., Alvarado, J., Rosson, A., Pino, C., and Espoz, C. (1998). Artisanal “Caletas” as units of production and co-managers of benthic invertebrates in Chile. Can. Spec. Publ. Fish. Aquat. Sci. 125, 407–413.
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Clarke, K. R., and Gorley, R. N. (2006). PRIMER v5: User Manual/Tutorial. PRIMER-E. Plymouth: Plymouth Marine Laboratory.
Contreras, C., Niklitschek, N., Molinet, C., Díaz, P., and Díaz, M. (2019). Fishery-induced reductions in density and size truncation of sea urchin Loxechinus albus affects diversity and species composition in benthic communities. Estuar. Coast. Shelf Sci. 219, 409–419. doi: 10.1016/j.ecss.2019.02.030
Dayton, P. K. (1985). The structure and regulation of some South American kelp communities. Ecol. Monogr. 55, 447–468. doi: 10.2307/2937131
De Juan, S., Hewitt, J. E., Thrush, S. F., and Freeman, D. (2015). Standardizing the assessment of functional integrity in benthic ecosystems. J. Sea Res. 98, 33–41. doi: 10.1016/j.seares.2014.06.001
De Juan, S., Subida, M. D., Gelcich, S., and Fernandez, M. (2018). Ecosystem health in coastal areas targeted by small-scale artisanal fisheries: insights on monitoring and assessment. Ecol. Indic. 88, 361–371. doi: 10.1016/j.ecolind.2018.01.054
Defeo, O., Castrejon, M., Perez-Castaneda, R., Castilla, J. C., Gutierrez, N., Essington, T., et al. (2014). Co-management in Latin American small-scale shellfisheries: assessment from long-term case studies. Fish Fish. 17, 176–192. doi: 10.1111/faf.12101
Fernandez, M., Jaramillo, E., Marquet, P., Moreno, C., Navarrete, S., Ojeda, P., et al. (2000). Diversity, dynamics and biogeography of Chilean benthic nearshore ecosystems: an overview and guidelines for conservation. Rev. Chil. Hist. Nat. 73, 797–830. doi: 10.4067/S0716-078X2000000400021
Fernandez, M., Kriegl, M., Garmendia, V., Aguilar, A., and Subida, M. D. (2020). Evidence of illegal catch in the benthic artisanal fisheries of central Chile: patterns across species and management regimes. Latin Am. J. Aquat. Res. 48, 287–303. doi: 10.3856/vol48-issue2-fulltext-2475
Fillbee-Dexter, K., and Schebling, R. (2014). Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 495, 1–25. doi: 10.3354/meps10573
Flores, E., Parada, C., Castro, L., Narvaez, D., and Sepulveda, H. (2020). Connectivity in early life stages of the southern hake, Merluccius australis, in northern Chilean Patagonia. J. Mar. Sci. 212:103452. doi: 10.1016/j.jmarsys.2020.103452
Forcelli, D. (2000). Moluscos Magallánicos: Guía de los Moluscos de la Patagonia y Del Sur de Chile. Buenos Aires: Vásquez Mazzini Editores.
Försterra, G., Häussermann, V., and Laudien, J. (2016). “Animal forests in the Chilean fjords: discoveries, perspectives and threats in shallow and deep waters,” in Marine Animal Forests, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (New York, NY: Springer).
Friedlander, A. M., Ballesteros, E., Bell, T. W., Giddens, J., Henning, B., Hüne, M., et al. (2018). Marine biodiversity at the end of the world: cape Horn and Diego Ramirez islands. PLoS One 13:e0189930. doi: 10.1371/journal.pone.0189930
Garrido, I. (2012). Ecologíatrófica del Asteroideocosmasteriaslurida (Phillipi, 1858) enelSeno del Reloncaví (Sur de Chile): Distribución, Abundancia, Alimentación y Movimiento. Tesis de Grado Biológía Marina. Valdivia: Universidad Austral de Chile, 86.
Gaymer, C., and Himmelman, J. (2008). A keystone predatory sea star in the intertidal zone is controlled by a higher-order predatory sea star in the subtidal zone. Mar. Ecol. Prog. Ser. 370, 143–153. doi: 10.3354/meps07663
Gelcich, S., Cinner, J., Donlan, C. J., Tapia-Lewin, S., Godoy, N., and Castilla, J. C. (2016). Fishers’ perceptions on the Chilean coastal TURF system after two decades: problems, benefits, and emerging needs. Bull. Mar. Sci. 93, 53–67. doi: 10.5343/bms.2015.1082
Gelcich, S., Fernández, M., Godoy, N., Canepa, A., Prado, L., and Castilla, J. C. (2012). Territorial user rights for fisheries as ancillary instruments for marine coastal conservation in Chile. Conserv. Biol. 26, 1005–1015. doi: 10.1111/j.1523-1739.2012.01928.x
Gelcich, S., Godoy, N., Prado, L., and Castilla, J. C. (2008). Add-on conservation benefits of marine territorial user rights fishery policies in central Chile. Ecol. Appl. 18, 273–281. doi: 10.1890/06-1896.1
Gelcich, S., Hughes, T. P., Olsson, P., Folke, C., Defeo, O., Fernández, M., et al. (2010). Navigating transformations in governance of Chilean marine coastal resources. Proc. Natl. Acad. Sci. U.S.A. 107, 16794–16799. doi: 10.1073/pnas.1012021107
Godoy, N., Gelcich, S., Vasquez, J., and Castilla, J. C. (2010). Spearfishing to depletion: evidence from temperate reef fishes in Chile. Ecol. Appl. 20, 1504–1511. doi: 10.1890/09-1806.1
Gonzalez, J., Stotz, W., Garrido, J., Orensanz, J. M., Parma, A. M., Tapia, C., et al. (2006). The Chilean TURF system: how is it performing in the case of the loco fishery? Bull. Mar. Sci. 78, 499–527.
Gonzalez, S. J., Caceres, C. W., and Ojeda, F. P. (2008). Feeding and nutritional ecology of the edible sea urchin Loxechinus albus in the northern Chilean coast. Rev. Chil. Hist. Nat. 81, 575–584.
Hammer, O., Harper, D., and Ryan, P. (2001). PAST: Palaeontological Statistics Software Package for Education and Data Analysis, Version 4.03. Palaeontol Electron 4: 9. Available online at: www.nhm.uio.no/english/research/infrastructure/past/ (accessed March 1, 2021).
Häussermann, V., and Försterra, G. (2009). Marine Benthic Fauna of Chilean Patagonia. Santiago: Nature in Focus, 1000.
Hurlbert, S. H. (1971). The nonconcept of species diversity: a critique and alternative parameters. Ecology 52, 577–586. doi: 10.2307/1934145
Jennings, S., and Kaiser, J. (1998). The effects of fishing on marine ecosystems. Adv. Mar. Biol. 34, 201–352. doi: 10.1016/S0065-2881(08)60212-6
King, P. P. (1832). Description of the Cirrhipeda, Conchifera and Mollusca, in a collection formed by the officers of H.M.S. Adventure and Beagle employed between the years 1826 and 1830 in surveying the southern coasts of South America, including the Straits of Magalhaens and the coast of Tierra del Fuego. Zool. J. 5, 332–349.
Levin, P. S., Murawski, S. A., and Fogarty, M. J. (2009). Integrated ecosystem assessments: developing the scientific basis for ecosystem-based management of the ocean. PLoS Biol. 7:e14. doi: 10.1371/journal.pbio.1000014
Manriquez, P., Alvarado, J., Huaquin, L., Carrillo, H., Rosson, A., Romero, C., et al. (2009). Comportamiento y Parametros Reproductivos de C. Concholepas en la VIII y X Regiones. Proyecto FIP N° 2006-24. 181. Anexos. Subpesca, Valparaíso, Chile.
Marin, A., and Gelcich, S. (2012). Gobernanza y capital social en el comanejo de recursos bentónicos en Chile: aportes del análisis de redes al estudio de la pesca artesanal de pequeña escala. Cult. Hombre Soc. 22, 131–153. doi: 10.7770/cuhso-V22N1-art428
Miethe, T., Dobby, H., and McLay, A. (2016). The Use of Indicators for Shellfish Stocks and Fisheries: A Literature Review. Pitlochry: Scottish Marine and Freshwater Science.
Molina, J. I. (1782). Saggiosullastorianaturale de Chili. Disponible en Biblioteca Digital – Real Jardín Botánico – CSIC (en Italiano). Boloña: Stamperia di S. Tomasod’Aquino, 367.
Molinet, C., Barahona, N., Díaz, M., Díaz, P. A., Millanao, M. O., Araya, P., et al. (2016). Using drift video transects and maximum likelihood geostatistics for quantifying and monitoring exploited subpopulations of Loxechinus albus at a mesoscale. Mar. Coast. Fish. 8, 70–80. doi: 10.1080/19425120.2015.1121939
Molinet, C., Niklitschek, E., Arevalo, A., and Matamala, M. (2010). Patch structure of benthic resources exploited in Chilean management areas: the shellfish bed concept under a territorial use rights for fisheries framework. Bull. Mar. Sci. 86, 555–569.
Moreno, A., and Revenga, C. (2014). The System of Territorial Use Rights in Fisheries in Chile. Arlington, TX: The Nature Conservancy.
Navarrete, S., Gelcich, S., and Castilla, J. C. (2010). Long-term monitoring of coastal ecosystems at Las Cruces, Chile: defining baselines to build ecological literacy in a world of change. Rev. Chil. Hist. Nat. 83, 143–157.
Oyanedel, R., Keim, A., Castilla, J. C., and Gelcich, S. (2017). Illegal fishing and territorial user rights in Chile. Conserv. Biol. 32, 619–627. doi: 10.1111/cobi.13048
Pardo, L. M., Rubilar, P., and Fuentes, J. P. (2020). North patagonian estuaries appear to function as nursery habitats for marble crab (Metacarcinusedwardsii). Reg. Stud. Mar. Sci. 36:101315. doi: 10.1016/j.rsma.2020.101315
Perez-Matus, A., Ospina-Alvarez, A., Camus, P. A., Carrasco, S. A., Fernandez, M., Gelcich, S., et al. (2017). Temperate rocky subtidal reef community reveals human impacts across the entire foodweb. MEPS 567, 1–16. doi: 10.3354/meps12057
Pinnegar, J. K., Polunin, N. V. C., Francour, P., Badalamenti, F., Chemello, R., Harmelin-Vivien, M. L., et al. (2000). Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ. Conserv. 27, 179–200. doi: 10.1017/s0376892900000205
Romero, P., Arenas, G., Velasco, E., Ariz, L., González, C., Manquehual, G., et al. (2019). Programa de Seguimiento Pesquerías Bajo Régimen Áreas de Manejo, 2018-2019. Convenio de Desempeño IFOP – Subsecretaría de Economía y Empresa de Menor Tamaño. Informe Final. Valparaíso: Instituto de Fomento Pesquero (IFOP), 347. pp + Anexos.
San Martin, G., Parma, A. M., and Orensanz, J. M. (2010). “The Chilean experience with territorial use rights in fisheries,” in Handbook of Marine Fisheries Conservation and Management, eds R. Grafton, D. Squires, M. Tait, and M. Williams (New York, NY: Oxford University Press).
Schneider, W., Perez-Santos, I., Ross, L., Bravo, L., Seguel, R., and Hernandez, F. (2014). On the hydrography of puyuhuapi channel, Chilean Patagonia. Prog. Oceanogr. 128, 8–18. doi: 10.1016/j.pocean.2014.03.007
Sernapesca. (2020). Anuario Estadístico de Pesca y Acuicultura. Desembarque Desde Áreas de Manejo Por Especie y Mes. Subsector pesquero Artesanal. Anuarios Estadísticos de Pesca y Acuicultura. Servicio Nacional de Pesca y Acuicultura. Available online at: www.sernapesca.cl (accessed October 8, 2020).
Sievers, A. H., and Silva, N. (2008). “Water masses and circulation in austral Chilean channels and fjords,” in Progress in the Oceanographic Knowledge of Chilean Inner Waters, from Puerto Montt to Cape Horn, eds N. Silva and S. Palma (Valparaíso: Pontificia Universidad Católica de Valparaíso), 53–58.
Silva, N., and Guzman, D. (2006). Condiciones oceanográficas fisicas y quimicas, entre la boca del Guafo y fiordo Aysén (crucero CIMAR 7 Fiordos). Cienc. Technol. Mar. 29, 25–44.
Silva, N., and Palma, S. (2008). Progress in the Oceanographic Knowledge of Chilean Interior Waters, From Puerto Montt to Cap Horn. Valparaíso: Comité Oceanográfico Nacional Pontificia Universidad Católica de Valparaíso, 161.
Silva, N., and Vargas, C. (2014). Hypoxia in Chilean patagonian fjords. Prog. Oceanogr. 129 (part A), 62–74. doi: 10.1016/j.pocean.2014.05.016
Stotz, W. (1997). Las areas de manejo en la Ley de Pesca y Acuicultura: primeras experiencias y evaluación de la utilidad de esta herramienta para el recurso loco. Estud. Oceanol. 16, 67–86.
Subpesca (1995). D.S. N° 355-1995 Reglamento sobre Áreas de Manejo y Explotación de Recursos Bentónicos. Available online at: http://www.subpesca.cl/portal/615/w3-article-11086.html (accessed February 5, 2020).
Subpesca (2000). Establece Veda Biológica del Recurso Erizo en Área y Periodo que Indica. Decreto Exento N°439. 27 de Diciembre 2000. Ministerio de Economía Fomento y Reconstrucción, Valparaíso, Chile.
Subpesca (2011). D.EX. N° 697-2011, Mod. D.EX.N° 409-03 Veda Biológica VII-XI Reg. (F.D.O. 01/08/2011). Valparaíso: Ministerio de Economía, Fomento y Turismo.
Subpesca (2016). XI Región - SUBPESCA Normativa. Available online at: www.subpesca.cl (accessed February 5, 2020).
Subpesca. (2020a). Aplicación de Visualización de Mapas de la Subsecretaría de Pesca y Acuicultura. Visualizador de Mapas. Santiago.
Subpesca (2020b). XI Región. Available online at: https://www.subpesca.cl/portal/615/w3-propertyvalue-849.html (accessed December 10, 2020).
Vasquez, J. A. (2008). Production, use and fate of Chilean brown seaweeds: resources for a sustainable fishery. J. Appl. Phycol. 20, 457–467.
Vasquez, J. A., and Buschmann, A. H. (1997). Herbivore-kelp interactions in Chilean subtidal. Rev. Chil. Hist. Nat. 70, 41–52.
Vasquez, J. A., and Castilla, J. C. (1984). Some aspects of the biology and trophic range of Cosmasteriaslurida (ASTEROIDEA, ASTERIINAE) in belts of Macrocystis pyrifera at Puerto Toro, Chile. Ambient. Acuat. 7, 47–51.
Vasquez, J. A., Castilla, J. C., and Santelices, B. (1984). Distributional patterns and diet of four species of sea urchin giant kelp forest (Macrocystis pyrifera) of Puerto Toro, Navarino Island, Chile. Mar. Ecol. Prog. Ser. 19, 55–63. doi: 10.3354/meps019055
Wright, J. T., Dworjanyn, S. A., Rogers, C. N., Steinberg, P. D., Williamson, J. E., and Poore, A. G. B. (2005). Density-dependent sea urchin grazing: differential removal of species, changes in community composition and alternative community states. Mar. Ecol. Prog. Ser. 298, 143–156. doi: 10.3354/meps298143
Keywords: North Patagonia, benthic community, exploitation intensity, management areas, C. concholepas, L. albus
Citation: Hamame M and Ortiz P (2022) Assessment of Exploitation Intensity of Commercial Species and Associated Benthic Communities, in Chilean Marine Management Areas of North Patagonia. Front. Mar. Sci. 8:635756. doi: 10.3389/fmars.2021.635756
Received: 30 November 2020; Accepted: 13 December 2021;
Published: 24 January 2022.
Edited by:
Eduardo Joel Quiroga Jamett, Pontificia Universidad Católica de Valparaíso, ChileReviewed by:
Carlos Rios, University of Magallanes, ChileCopyright © 2022 Hamame and Ortiz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madeleine Hamame, bWhhbWFtZUBjaWVwLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.