- 1College of Life Science, Hebei University, Baoding, China
- 2Institute of Life Science and Green Development, Hebei University, Baoding, China
- 3Ecology Group, Technische Universität Kaiserslautern, Kaiserslautern, Germany
- 4Department of Molecular Ecology, Technische Universität Kaiserslautern, Kaiserslautern, Germany
- 5Key Laboratory of Mariculture, Ministry of Education, Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao, China
We isolated a population of Oxytricha granulifera granulifera Foissner and Adam (1983) from a hot spring in Iceland. The pure culture of this isolate was established at room temperature in the laboratory. This allowed for a detailed investigation, informed by integrated approaches, of the isolate’s morphology and morphogenesis, as well as molecular phylogeny. Results showed that the morphological and morphogenetic characteristics of the Iceland population are consistent with those of other populations. During the 3-year long period of laboratory cultivation, some abnormal individuals appeared repeatedly in the culture system. Interestingly, the morphological characteristics of these abnormal cells were rather stable, and were as follows: 1) body slender and elliptical-shaped; 2) remarkably shortened adoral zone and significantly reduced number of adoral membranelles; and 3) loss of undulating membranes. Resting cysts, binary fission and conjugate reproduction were not found in abnormal specimens. Although the morphology of abnormal individuals changed significantly, the sequences of the SSU rDNA of the normal and abnormal morphotypes were the same. Phylogenetic analyses showed that the two morphotypes clustered in a clade with other populations of O. granulifera granulifera.
Introduction
Ciliates are a large and diverse group of protozoa, which are distributed in a variety of habitats, including extreme environments (Foissner, 2016; Chen et al., 2019; Li et al., 2019; Luo et al., 2019; Shao et al., 2019, 2020; Bai et al., 2020; Wu et al., 2020). Among ciliates, Oxytricha is one of the most diverse and species-rich genera, with approximately 40 nominal species having been reported so far (Berger, 1999; Song et al., 2009; Shao et al., 2012, 2015; Bai et al., 2018; Kim and Min, 2019; Kaur et al., 2019; Song et al., 2019). Oxytricha granulifera Foissner and Adam (1983), type species of the genus, was first discovered from soil in Austria (Foissner and Adam, 1983). Thereafter, more populations were recorded elsewhere, for example in Australia (soil; Blatterer and Foissner, 1988); China (soil; Shao et al., 2014); Costa Rica (bark of Acacia trees; Foissner, 1994, 1997); Japan (soil; Berger, 1999); Marion Island (soil; Foissner, 1996); Namibia (soil; Foissner et al., 2002); Peru (soil; Foissner, 1997); and South Korea (freshwater; Kwon and Shin, 2013).
Recently, Méndez-Sánchez et al. (2018) subdivided Oxytricha granulifera into three subspecies, namely Oxytricha granulifera granulifera (Foissner and Adam, 1983), O. granulifera quadricirrata (Blatterer and Foissner, 1988), and O. granulifera chiapasensis (Méndez-Sánchez et al., 2018). This subdivision was mainly based on morphological characteristics, i.e., the patterns of undulating membrane, the numbers of dorsal kineties and transverse cirri. Diagnosis of O. granulifera granulifera was as follows: cells 70–127 × 28–75 μm in vivo; adoral zone with 29–32 membranelles; paroral and endoral membranes in Oxytricha-pattern; the right marginal cirri row starting at the same level of the rightmost frontal cirrus; constantly five transverse cirri; rarely a sixth dorsal kinety; confined to terrestrial habitats (Foissner and Adam, 1983; Méndez-Sánchez et al., 2018).
During a protistan fauna investigation, one encysted ciliate was isolated from a geothermal field (hot spring) in Iceland. This isolate was identified as O. granulifera granulifera Foissner and Adam (1983). Its pure culture was established at room temperature (20–25°C) in Petri dishes, with rice grains to enrich the bacterial food. During the cultivation, some abnormal individuals appeared repeatedly in the system. Although the abnormal individuals appeared at different times, their morphological characteristics remained consistent, which made it easier to mistakenly deduct that a “new” species had been discovered. Oxytricha granulifera has not been recorded from hot springs, especially the abnormal forms in long-term indoor culture system have not been reported, therefore, the morphology and SSU rDNA sequences of the normal and abnormal forms are described in this article, and the morphogenesis of the normal form is also reported.
Materials and Methods
Sampling and Cultivation
The sludge sample was collected from a microbial mat in a geothermal field in Iceland near the Hellisheiðarvirkjun geothermal power station (64°01′11.5′′N, 21°23′50.5′′W), as described in Qu et al. (2018). The temperature was approximately 75°C at the sampling site. The surface sediments were transferred to Petri dishes in water from the sample site and maintained as a raw culture in the laboratory for several days at room temperature (about 25°C). The salinity (0‰) of the hot spring water was measured on site with a portable refractometer. The pH level (7.3) was measured using portable pH sensors. In the laboratory, the pure cultures of O. granulifera granulifera were established and maintained using volvic water and sterilized wheat grains. Wheat grains enriched bacteria as a food source for the ciliate. Clonal cultures were obtained by gradually transferring single ciliates from the enrichment culture to artificially distilled freshwater (Qu et al., 2020). One to two wheat grains per 20 mL cell culture were added to support the growth of bacteria. To maintain this population, approximately once every 2 weeks, several cells were picked up and transferred into a new Petri dish containing new water and rice grains to establish a new pure cultivation. The attempt to establish pure cultures using abnormal individuals solely failed, because the abnormal individuals gradually disappeared over the course of about one week. The pH level of the culture systems was 6.0–7.0.
Morphological and Ontogenetic Investigations
Live observations were carried out using a bright field microscope (Olympus BX53) equipped with differential interference contrast (DIC). The protargol silver staining method of Wilbert (1975) was used to reveal the ciliature and the nuclear apparatus. Protargol was synthesized mainly according to Pan et al. (2013). Drawings of stained specimens were conducted at a magnification of 1,000× with the aid of a camera lucida. Measurements were made with an ocular micrometer. To illustrate the changes that occur during ontogenetic processes, ciliary structures of parental cells were depicted by contours, whereas those of the daughter cells were shaded black. The terminology used herein follows Berger (1999) and Song and Shao (2017).
DNA Extraction, PCR Amplification, and Sequencing
Total genomic DNA of the normal and abnormal forms of O. granulifera granulifera were extracted from single cells in pure cultures, using the DNeasy Blood and Tissue Kit (Qiagen, Gmbh, Germany) according to the manufacturer’s instructions. Each cell was washed three times with distilled water and two times in ultra-pure water in order to remove contaminants and then was immediately transferred to 1.5-mL microfuge tubes with an ATL buffer (Jin et al., 2020). PCR amplifications of SSU rDNA were performed with universal eukaryotic primers 18S-F (5′-AAC CTG GTT GAT CCT GCC AGT-3′) and 18S-R (5′-TGA TCC TTC TGC AGG TTC ACC TAC-3′) (Medlin et al., 1988). The amplification conditions were as follows: a pre-run of 30 s at 98°C followed by 35 cycles consisting of a denaturation at 98°C for 30 s, an annealing at 60°C for 20 s, and an extension at 72°C for 1 min. After 35 cycles, the final extension step was run at 72°C for 2 min. The PCR products were sequenced by Beijing Genomics Institute in Shenzhen, China. Three internal primers, 900S-F (5′-CGA TCA GAT ACC GTC CTA GT-3′), 900S-R (5′-ACT AGG ACG GTA TCT GAT CG-3′) and B (5′-AAY CTG GTT GAT YYT GCC AG-3′) were used in sequencing.
Phylogenetic Analyses
Apart from the newly sequenced SSU rDNA sequences of the normal and abnormal forms of O. granulifera granulifera in this article, all of the sequences used in the phylogenetic analyses were obtained from the GenBank database (accession numbers see Figure 4). Novistrombidium orientale, Parastrobidinopsis minima, Strombidinopsis acuminata, and Strombidium apolatum were selected as outgroups since they were used to construct the phylogenies recently reported by Méndez-Sánchez et al. (2018) and Liao et al. (2020). Sequences were aligned using the MUSCLE package on the European Bioinformatics Institute web page1. Both ends of the alignment were then trimmed manually using BioEdit 7.0.9.1, resulting in a final matrix of 1,579 nucleotide positions. A maximum-likelihood (ML) tree was constructed using RAxML-HPC2 on XSEDE v.8.2.10 (Stamatakis, 2014) on the CIPRES Science Gateway (Miller et al., 2010). The model GTR + I + G was used. Support for the best ML tree came from 1,000 bootstrap replicates (Jin et al., 2020). Bayesian inference (BI) analysis was carried out on CIPRES Science Gateway using the MrBayes 3.2.6 on XSEDE (Ronquist and Huelsenbeck, 2003). TreeView v1.6.6 (Page, 1996) and MEGA 7.0 (Kumar et al., 2016) were used to visualize tree topologies.
Results
Morphology of Normal Forms
Size 90–130 × 30–45 μm (average 110 × 35 μm, n = 10) in vivo, and 79–135 × 28–61 μm (average 102 × 42 μm) in protargol preparations. Body flexible and slightly contractile, elliptical in shape with a ratio of length: width approximately 3.5:1, right margin usually straight and left margin more or less convex. Cell dorsoventrally flattened with a width: thickness ratio of approximately 2:1, ventral side flat and dorsal slightly convex (degree depending of nutrition status). Constantly two globular to ellipsoid macronuclear nodules, on average 16.1 × 9.2 μm in protargol preparations, located in anterior and posterior third of cell. One to four globular micronuclei, on average 2.2 μm across, attached to macronuclear nodules (Figures 1C, 3A,B). Contractile vacuole (about 8 μm across) in mid-body, close to left cell margin; pulsing at intervals of about 8 s (Figures 1A, 2B,D). Cytoplasm colorless, usually containing food vacuoles (about 5 μm), lipid droplets (about 3 μm) and minute crystals (Figures 1A, 2A,B). Colorless cortical granules (about 1 μm across), arranged in longitudinal rows on dorsal side and sparsely arranged in irregular short rows on ventral side (Figures 2C,D). Locomotion by swimming in water or crawling on substrate rapidly.

Figure 1. Morphology of normal (A–C) and abnormal (D–F) forms of Oxytricha granulifera granulifera in vivo (A,D) and after protargol staining (B,C,E,F). (A) Ventral view of a representative normal individual. (B,C) Ventral (B) and dorsal (C) views of a typical specimen to show ciliature and nuclear apparatus, arrow in (B) marks the buccal cirrus. (D) Ventral view of a representative abnormal cell. (E,F) Ventral (E) and dorsal (F) views of an abnormal cell, arrow in (F) marks the buccal cirrus. AZM, adoral zone of membranelles; CC, caudal cirri; CV, contractile vacuole; E, endoral; FC, frontal cirri; LMR, left marginal row; FVC, frontoventral cirri (III/2, IV/3, VI/3, and VI/4); Ma, macronuclear nodules; Mi, micronuclei; P, paroral; PTVC, pretransverse ventral cirri; PVC, postoral ventral cirri (IV/2, V/3, and V/4); RMC, right marginal cirri row; TC, transverse cirri. 1–5, dorsal kineties 1–5. Scale bars = 50 μm.
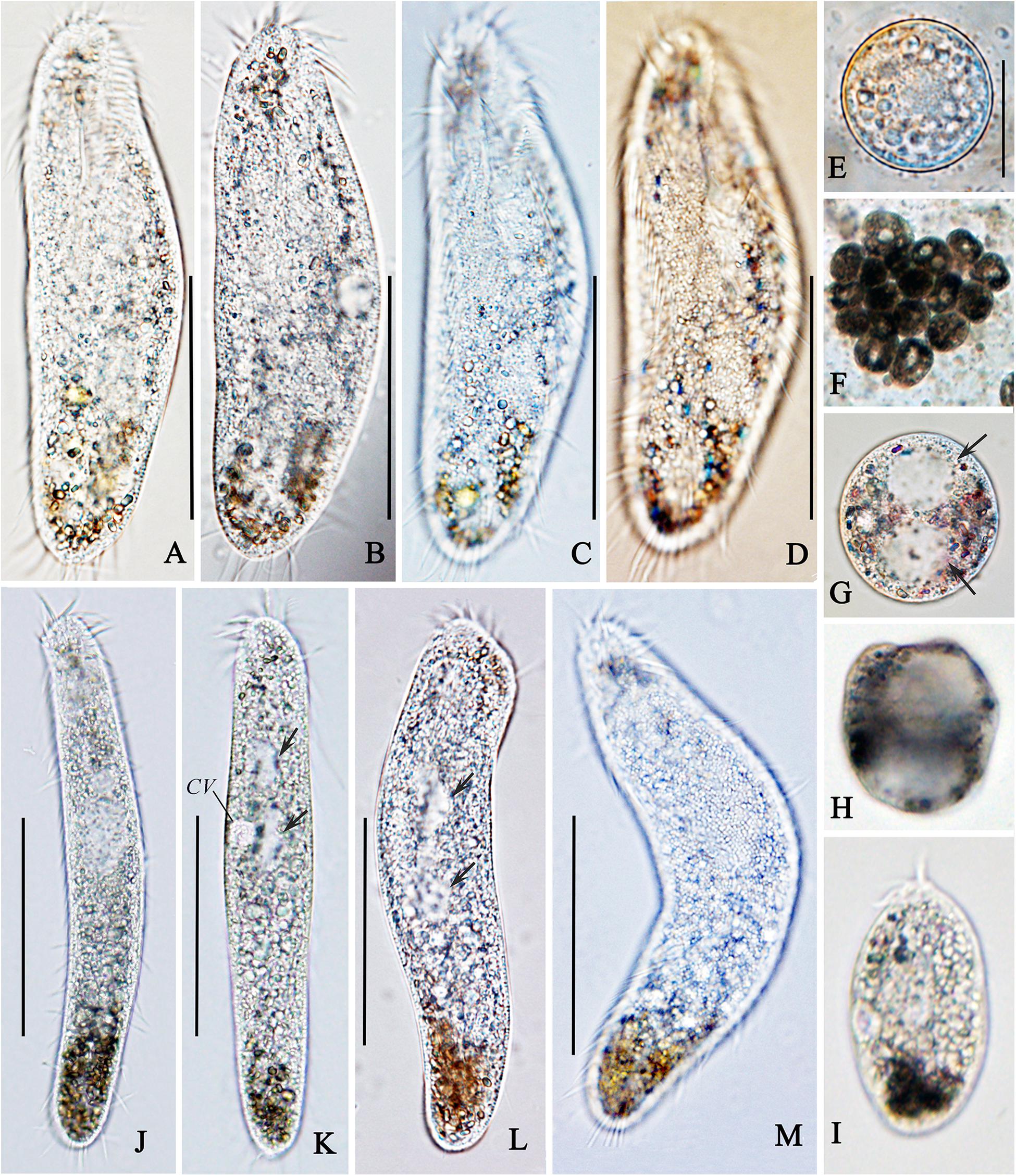
Figure 2. Photomicrographs of normal (A–E) and abnormal forms (F–M) of Oxytricha granulifera granulifera in vivo. (A–D) Typical body shapes of the normal cells. (E) A normal cyst. (F–H) Abnormal cysts; each cyst with two macronuclear nodules (arrows in G). (I) A newly excysted abnormal cell. (J–L) Ventral view of abnormal forms, showing the changes of body shape, arrows in (K,L) indicating the macronuclear nodules. (M) Cortical granules of an abnormal cell from the dorsal side. CV, contractile vacuole. Scale bars = 50 μm.

Figure 3. Ciliature of normal (A,B) and abnormal (C–E) forms, and the morphogenesis (F–L) of Oxytricha granulifera granulifera after protargol staining. (A,B) Ventral and dorsal views of a normal specimen. (C–E) Ventral (C,E) and dorsal (D) views of abnormal specimens. (F) Ventral view of a very early stage to show the newly formed oral primordium. (G) Partial view of an early divider showing the six streaks (anlagen I–VI) of the opisthe. (H) Ventral view of a middle divider, to show the development of marginal cirri anlagen and the fused macronucleus. (I) Dorsal view of a divider showing the newly formed dorsal kineties 1–4. (J,K) Ventral (J) and dorsal views (K) of a late divider, to show the newly formed cirri and the origin of dorsal kinety 4. (L) Dorsal view of a very late divider which is about to divide. AZM, adoral zone of membranelles; BC, buccal cirri; E, endoral; FC, frontal cirri; LMR, left marginal cirri row; Ma, macronuclear nodules; Mi, micronuclei; OP, oral primordium; P, paroral; PTVC, pretransverse ventral cirri; RMR, right marginal cirri row; TC, transverse cirri. 1–4, dorsal kineties; 5, dorsal marginal kinety. Scale bars = 50 μm.
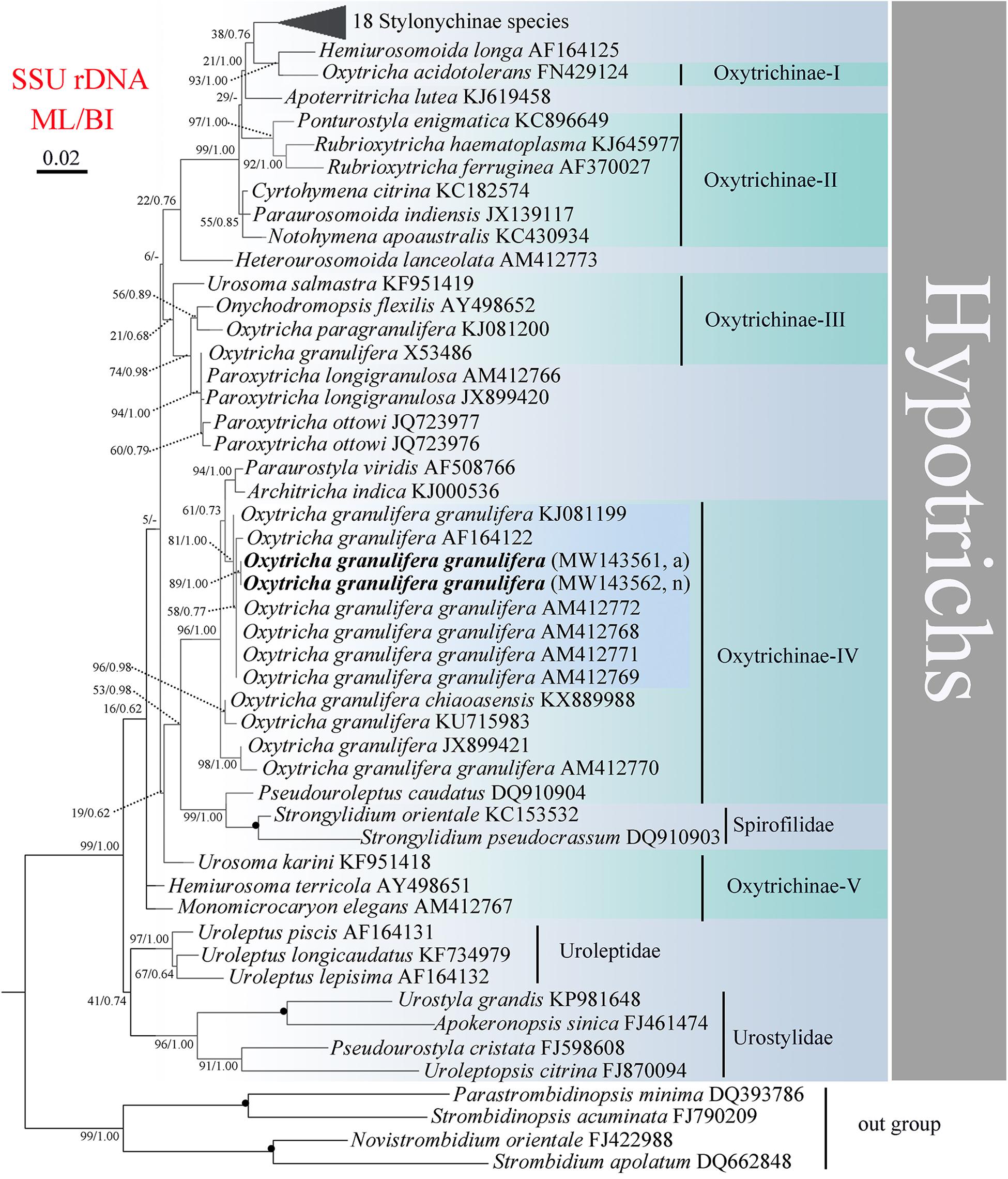
Figure 4. Maximum likelihood (ML) phylogenetic tree inferred from SSU rDNA sequences of 67 hypotrichs. Phylogenetic positions of normal and abnormal forms of Oxytricha granulifera granulifera are indicated in bold. Black circles indicate full bootstrap values from both ML and BI (100/1.00). – indicates the topology disagreement between ML and BI, and only the bootstrap values of ML are shown. The scale bar corresponds to two substitutions per 100 nucleotide positions (0.02). a, abnormal form; n, normal form.
Adoral zone 33–43 μm long after protargol staining, occupying ca. 36% of body length, composed of 26–32 (on average 30) membranelles, with cilia about 12 μm long (Table 1 and Figures 1A,B, 3A,B). Undulating membranes in Oxytricha-pattern (Figures 1B, 3A). Three slightly enlarged frontal cirri arranged at the anterior end of the cell, one buccal cirrus located near anterior end of paroral membrane, invariably four frontoventral cirri positioned between the anterior portion of the right marginal row and the paroral, cirrus III/2 arranged anterior to the level of cirrus VI/3 (Figure 1B), three postoral ventral cirri behind the proximal end of the adoral zone, cirrus IV/2 located more anteriorly than V/4 (Figure 1B), two pretransverse cirri arranged in front of the transverse cirri, five transverse cirri in a hook-shaped row, inconspicuously projecting above posterior body margin. One right and one left marginal row comprised of 24–36 and 21–34 cirri (cilia about 10 μm long), respectively. Marginal rows not confluent posteriorly (Figures 1B, 3A). All of the cirri about 12 μm long in vivo.
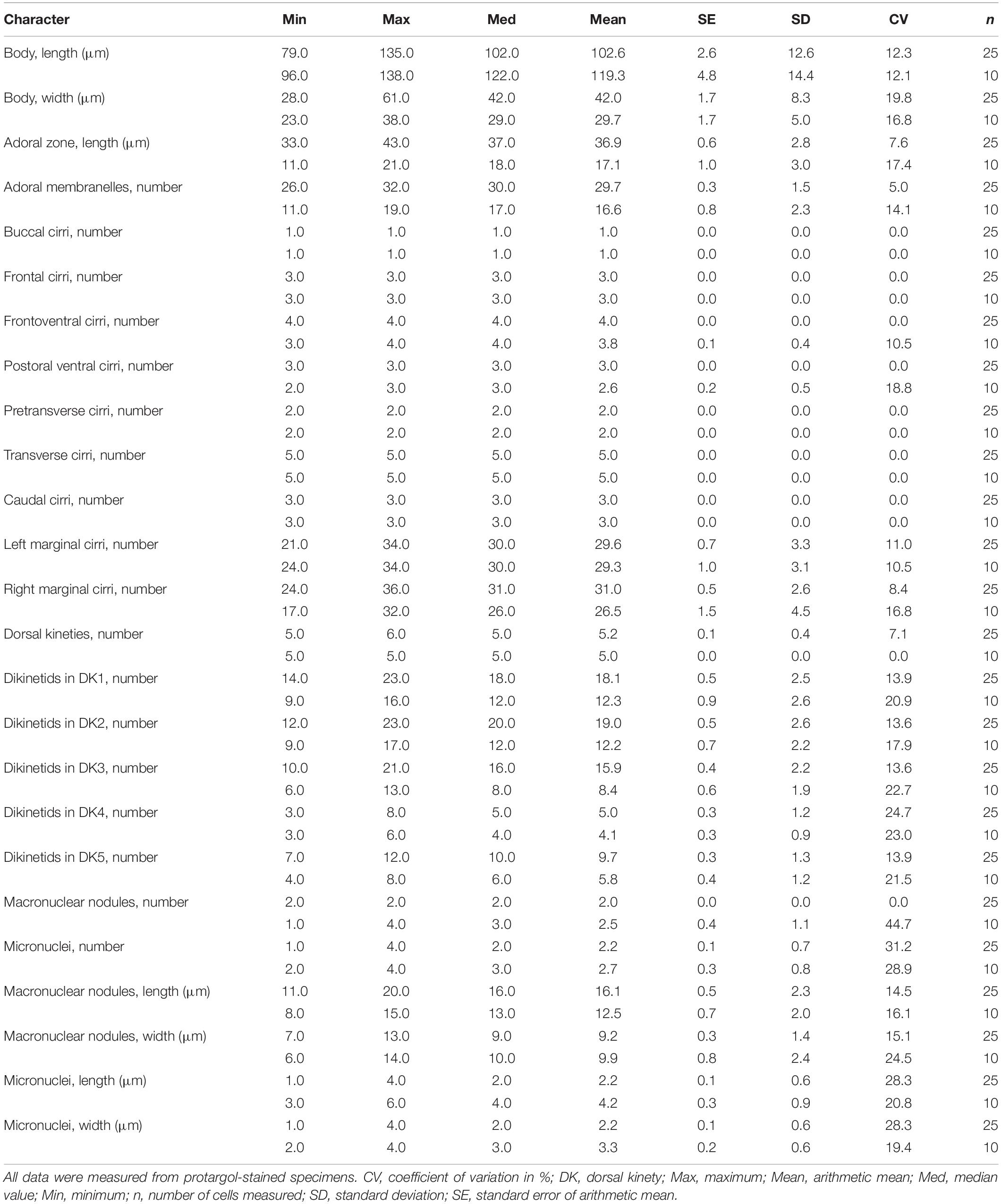
Table 1. Morphometric data of normal (upper lines) and abnormal (lower lines) specimens of Oxytricha granulifera granulifera in the present work.
Five rows of dorsal bristles in typical Oxytricha pattern. Two bipolar rows left of midline of body, each associated with a caudal cirrus at its rear end; row 3 ends at posterior third; row 4 starts below mid-body and also produces a caudal cirrus at its posterior end; row 5 terminates ahead of mid-body (Figures 1C, 3B). Dorsal bristles about 2 μm long in vivo.
Resting cyst globular, smooth and colorless, with an average diameter of 35 μm (Figure 2E). Abnormal resting cyst usually ellipsoidal, gray, even black, in life about 53 × 36 μm, usually with two hyaline vacuole-like structures (Figures 2F–H).
Ontogenesis
The ontogenetic process of O. granulifera granulifera is very similar to that of the genus. For further details see Figures 3F–L. The main morphogenetic features are: 1) the oral primordium of the opisthe originates de novo and the parental adoral zone of membranelles remains unchanged and inherited by the proter; 2) six streaks of frontal-ventral-transverse cirral anlagen are formed; 3) the marginal rows and the nuclear apparatus develop in the usual manner; 4) dorsal kineties 1–3 develop within the parental kineties, dorsal kinety 4 originates from kinety 3 to kinety 5 originates dorsomarginally, that is, from an anlage near the right marginal row (Figures 3K,L). Ontogenesis confirms there is only one dorsomarginal kinety.
Detection of Abnormal Forms in Culture System
The present population of O. granulifera granulifera was isolated in the summer of 2017 and has been cultured at room temperature in the laboratory since then. However, since November 2018 many abnormal individuals have been found in some cultures. Usually, the course of these abnormal individuals can be described as follows: to begin with, a large number of normal populations bloomed in Petri dishes, and then a lot of abnormal resting cysts (Figures 2F–H) were quickly formed, and a certain proportion (about 10–15%) of these cysts then excysted to form abnormal individuals (Figures 2I–M). This phenomenon occurred repeatedly in the culture system.
Morphology of Abnormal Forms
Size in vivo 115–140 × 20–25 μm, and 96–138 × 23–38 μm in protargol preparations. Body slim with both ends rounded, length: width ratio about 6.5:1, slightly dorsoventrally flattened (Figures 1D,E, 2J–M). Cell highly flexible and slightly contractile. One, two, three or four ellipsoidal macronuclear nodules (percentages are 30, 10, 40, and 20%, respectively), positioned around mid-body, 8–15 × 6–14 μm in size. Two to four micronuclei, size 3–6 × 2–4 μm in protargol preparations (Figures 3C–E). Contractile vacuole about 6 μm in diameter located at about 40% of body length near the left body margin (Figures 1D, 2K). Cortical granules colorless, about 1 μm across (Figure 2M). Cytoplasm packed with numerous granules (Figures 1D, 2J–L).
Adoral zone occupying about 14% of body length, composed of 11–19 (average 17) membranelles. Buccal cavity short and narrow, undulating membranes absent (Figures 1E, 3C,E). Consistently three frontal, one buccal, and two pretransverse and five transverse cirri; other cirral groups unstable in number, for example three to four frontoventral, two to three postoral ventral cirri (Figures 1D–F, 3C,D). One left and one right marginal row, composed of 24–34 and 17–32 cirri, respectively (Figures 1E, 3C).
Five rows of dorsal bristles, row 1 and row 2 bipolar, composed of 9–16 and 9–17 bristles; row 3, row 4, and row 5 short, composed of 6–13, 3–6, and 4–8 bristles, respectively (Figures 1F, 3D). Three caudal cirri, associated with row 1, row 2, and row 4, respectively. Movement by crawling slowly on the substrate.
Abnormal individuals have never been found to be able to form resting cysts, or carry on dividing and conjugate reproduction. The attempt to cultivate these abnormal individuals failed and the population gradually disappeared within one week.
Sequence Similarity and Phylogenetic Analyses
The partial SSU rDNA sequences of the normal and abnormal forms of O. granulifera granulifera were deposited into the GenBank database with the accession numbers MW143562 and MW143561, with a length of 1,677 and 1,631 bp, respectively. The similarity of the common 1,631 bp covered by both forms is 100%.
The topology of ML and BI trees are similar, and, therefore, only the ML tree is shown (Figure 4). Subfamily Oxytrichinae was polyphyletic, and five subgroups were recognized (Oxytrichinae-I–V). All strains of Oxytricha granulifera except X53486 were exclusively located within Oxytrichinae-IV, while O. granulifera X53486 clustered in Oxytrichinae-III. The normal and abnormal forms of O. granulifera granulifera from the present research clustered together without full support (ML/BI, 89, 1.00). These two sequences were close to four other strains of O. granulifera granulifera (AM412768, AM412769, AM412771, and AM412772) and a strain of O. granulifera (AF164122). This branch then grouped with other O. granulifera strains.
Discussion
Identification of Iceland Population as O. granulifera granulifera
Oxytricha granulifera was first reported by Foissner and Adam (1983). So far, three subspecies of Oxytricha granulifera have been recognized, namely O. granulifera granulifera (Foissner and Adam, 1983), O. granulifera quadricirrata (Blatterer and Foissner, 1988), and O. granulifera chiapasensis (Foissner and Adam, 1983; Blatterer and Foissner, 1988; Méndez-Sánchez et al., 2018). Based on the general morphological features, the Iceland population conforms with the subspecies O. granulifera granulifera (Table 2). Thus, we identify our isolates as O. granulifera granulifera. This classification is also supported by the phylogenetic analyses based on the SSU rDNA sequence (Figure 4).
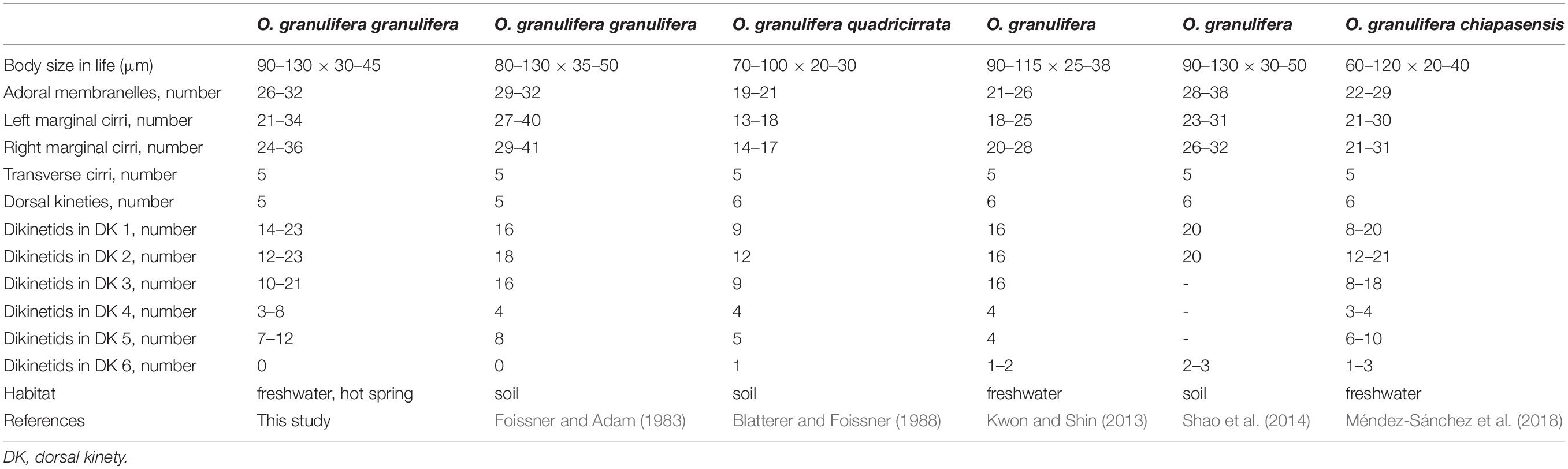
Table 2. Morphological comparison of Iceland population of Oxytricha granulifera granulifera with other Oxytricha populations.
Abnormal Form Could Easily Be Deduced as a “New” Species
The general infraciliature and arrangement of cortical granules of the normal and abnormal individuals are consistent. However, some morphological characteristics of abnormal individuals are quite different from those of normal individuals, and are mainly as follows: 1) the body size: in life 90–128 × 31–43 μm in normal forms vs 115–140 × 21–24 μm in abnormal forms; 2) the body shape: very narrowly elliptical in abnormal forma vs elliptical in normal forms; 3) the length of the adoral zone (14% of the body length in abnormal forms vs 36% of the body length in normal forms) and the number of membranelles (on average in abnormal forms 17 vs on average 30 membranes in normal forms); 4) the undulating membranes (absence in abnormal forms vs presence in normal forms); 5) number of macronuclei (1 to 4 in abnormal forms vs invariably 2 in normal forms); 6) mortality (abnormal individuals slow vs fast in normal individuals). However, because the abnormal individuals appear spontaneously, repeatedly and have stable morphological characteristics in the culture system, it could be easy to regard them as a “new” species without continuous and comprehensive investigation.
Occurrence of Abnormal Resting Cysts and Individuals
The population of O. granulifera granulifera Foissner and Adam (1983) was isolated from terrestrial environment, and no abnormal cysts or form were reported. While our population was isolated from sludge in a hot spring, and abnormal cysts and individuals occurred spontaneously in the culture system. Some researchers have reported abnormal cysts from artificial inducing in some ciliate species. Hashimoto (1962, 1964) induced abnormal cysts of Oxytricha fallax by embedding cells in agar, exposed to high temperatures, starved, or changing the composition of the culture medium. Another research article reported that abnormal cysts were formed in Euplotes taylori due to pressure of the coverslip (Garnjobst, 1928). As for our case, we speculate that the lack of food (necessary nutrients) and space after the outbreak of the high population density from single source of food (wheat grains) might trigger the potential polymorphism, then the abnormal cysts and forms appeared. The simpler characteristics enabled the abnormal forms to consume less energy than the formal forms which served as a strategy for the ciliate to endure the deteriorative environment. This speculation should be tested by transcriptomics in future work.
Conclusion
O. granulifera granulifera was detected in a hot spring in Iceland for the first time. So far, this species has been recorded in all of the main biogeographic regions, indicating its worldwide distribution. Currently, laboratory cultivation is necessary for the study of morphology, ontogenesis and molecular biology of ciliates (Foissner, 1996; Lian et al., 2020; Wang et al., 2020; Zhang et al., 2020). In this study, owing to stable morphological features of abnormal individuals of this species, the possibility that it may be misidentified as a “new” species has been discussed above. Attention to this possibility must be paid in future research. Likewise, the causes of deformities also need to be investigated further.
Protargol Stained Specimens
The protargol slides containing the normal (ZR20181112A–G) and the abnormal specimens (ZR20181129A–F) have been deposited in the Laboratory of Hydrobiology, Hebei University, China.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
SF and TS sampled the material. RZ and QZ conducted indoor culture of ciliate population and silver staining, generated the molecular data, and wrote the manuscript. XH, FL, ZQ, SF, and TS revised and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (Project Numbers: 31872206 and 41976086) and the Scientific Research Foundation of Hebei Province for returned scholars (C201801).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Bai, Y., Li, S., Li, Y., Miao, M., and Hu, X. (2018). Morphogenesis and molecular characterization of a little known soil ciliate, Oxytricha nauplia Berger et Foissner, 1987 (Ciliophora, Sporadotrichida). Acta Protozool. 57, 79–94. doi: 10.4467/16890027AP.18.008.8982
Bai, Y., Wang, R., Song, W., Toshikazu, S., and Hu, X. (2020). Redescription of five tintinnine ciliates (Alveolata: Ciliophora: Oligotrichea) from coastal waters of Qingdao, China. Mar. Life Sci. Technol. 2, 209–221. doi: 10.1007/s42995-020-00034-2
Berger, H. (1999). Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monogr. Biol. 78, 1–1080. doi: 10.1007/978-94-011-4637-1_1
Blatterer, H., and Foissner, W. (1988). Beitrag zur terricolen Ciliatenfauna (Protozoa: Ciliophora) von Australiens und Afrika. Stapfia 17, 1–84.
Chen, X., Jiang, Y., Gao, F., Zheng, W., Krock, T. J., Stover, N. A., et al. (2019). Genome analyses of the new model protist Euplotes vannus focusing on genome rearrangement and resistance to environmental stressors. Mol. Ecol. Resour. 19, 1292–1308. doi: 10.1111/1755-0998.13023
Foissner, W. (1994). Pentahymena corticicola nov. gen., nov. spec., a new colpodid ciliate. (Protozoa, Ciliophora) from bark of Acacia trees in Costa Rica. Arch. Protistenk. 144, 289–295. doi: 10.1016/s0003-9365(11)80140-5
Foissner, W. (1996). Terrestrial ciliates (protozoa, Ciliophora) from two islands (Gough, Marion) in the southern oceans, with description of two new species, Arcuospathidium cooperi and Oxytricha ollowi. Biol. Fertil. Soils 23, 282–291. doi: 10.1007/bf00335956
Foissner, W. (1997). Soil ciliates (Protozoa: Ciliophora) from evergreen rain forests of Australia, South America and Costa Rica: diversity and description of new species. Biol. Fertil. Soils 25, 317–339. doi: 10.1007/s003740050322
Foissner, W. (2016). Terrestrial and semiterrestrial ciliates (Protozoa, Ciliophora) from Venezuela and Galapagos. Denisia 35, 1–912.
Foissner, W., Agatha, S., and Berger, H. (2002). Soil ciliates (Protozoa, Ciliophora) from Namibia. (Southwest Africa), with emphasis on two contrasting environments, the Ethosha region and the Namibia desert. Denisia 5, 1–1459.
Foissner, W., and Adam, H. (1983). Morphologie und Morphogenese des Bondenciliaten Oxytricha granulifera sp. n. (Ciliophora, Oxytrichidae). Zool. Scr. 12, 1–11. doi: 10.1111/j.1463-6409.1983.tb00543.x
Garnjobst, L. (1928). Induced encystment and excystment in Euplotes taylori, sp. nov. Physiol. Zool. 1, 561–575. doi: 10.1086/physzool.1.4.30151345
Hashimoto, K. (1962). Relationships between feeding organelles and encystment in Oxytricha fallax Stein. J. Protozool. 9, 161–169. doi: 10.1111/j.1550-7408.1962.tb02601.x
Hashimoto, K. (1964). Localization of ciliary primordia in induced abnormal cysts of Oxytricha fallax. J. Protozool. 11, 75–84. doi: 10.1111/j.1550-7408.1964.tb01722.x
Jin, D., Qu, Z., Wei, B., Montagnes, D. J., Fan, X., and Chen, X. (2020). Two parasitic ciliates (Protozoa: Ciliophora: Phyllopharyngea) isolated from respiratory-mucus of an unhealthy beluga whale: characterization, phylogeny and an assessment of morphological adaptations. Zool. J. Linn. Soc. (in press). doi: 10.1093/zoolinnean/zlaa086/5900937
Kaur, H., Negi, S. R. K., and Kamra, K. (2019). Morphological and molecular characterization of. Neogastrostyla aqua nov. gen., nov. spec. (Ciliophora, Hypotrichia) from River Yamuna, Delhi; comparison with Gastrostyla-like genera. Eur. J. Protistol. 68, 68–79. doi: 10.1016/j.ejop.2019.01.002
Kim, K. S., and Min, G. S. (2019). Morphology and molecular phylogeny of Oxytricha seokmoensis sp. nov. (Hypotrichia: Oxytrichidae), with notes on its morphogenesis. Eur. J. Protistol. 71, 125–641. doi: 10.1016/j.ejop.2019.125641
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics. analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Kwon, C. B., and Shin, M. K. (2013). Two oxytrichid ciliates, Cyrtohymena primicirrata and Oxytricha granulifera (Ciliophora: Sporadotrichida: Oxytrichidae) unknown from Korea. Anim. Syst. Evol. Divers. 29, 23–30. doi: 10.5635/ASED.2013.29.1.23
Li, F., Qu, Z., Luo, D., Filker, S., Hu, X., and Stoeck, T. (2019). Morphology, morphogenesis and molecular phylogeny of a new obligate halophile ciliate, Schmidtiella ultrahalophila gen. nov., spec. nov. (Ciliophora, Hypotrichia) isolated from a volcanic crater on Sal (Cape Verde Islands). J. Eukaryot. Microbiol. 66, 694–706. doi: 10.1111/jeu.12714
Lian, C., Luo, X., Warren, A., Zhao, Y., and Jiang, J. (2020). Morphology and. phylogeny of four. marine or brackish water spirotrich ciliates from China, with descriptions of two new species. Eur. J. Protistol. 72:125663. doi: 10.1016/j.ejop.2019.125663
Liao, W., Gong, Z., Ni, B., Fan, X., and Petroni, G. (2020). Documentation of a new hypotrich species in the family Amphisiellidae, Lamtostyla gui n. sp. (Protista, Ciliophora) using a multidisciplinary approach. Sci. Rep. 10:3763. doi: 10.1038/s41598-020-60327-5
Luo, X., Huang, J. A., Li, L., Song, W., and Bourland, W. A. (2019). Phylogeny of the ciliate family Psilotrichidae (Protista, Ciliophora), a curious and poorly-known taxon, with notes on two algae-bearing psilotrichids from Guam, USA. BMC Evol. Biol. 19:125. doi: 10.1186/s12862-019-1450-z
Medlin, L., Elwood, H. J., Stickel, S., and Sogin, M. L. (1988). The characterization of enzymatically amplified eukaryotic 16S-likerRNA-coding regions. Gene 71, 491–499. doi: 10.1016/0378-1119(88)90066-2
Méndez-Sánchez, D., Mayén-Estrada, R., Luo, X., and Hu, X. (2018). A new subspecies of Oxytricha granulifera (Hypotrichia: Oxytrichidae) from Mexico, with notes on its morphogenesis and phylogenetic position. J. Eukaryot. Microbiol. 65, 357–371. doi: 10.1111/jeu.12479
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). “Creating the CIPRES science gateway for inference of large phylogenetic trees,” in Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA. 1–8.
Page, R. D. M. (1996). Tree View: an application to display phylogenetic tree on personal computers. Comput. Appl. Biosci. 12, 357–358. doi: 10.1093/bioinformatics/12.4.357
Pan, X., Bourland, W. A., and Song, W. (2013). Protargol synthesis: an in-house protocol. J. Eukaryot. Microbiol. 60, 609–614. doi: 10.1111/jeu.12067
Qu, Z., Groben, R., Marteinsson, V., Agatha, S., Filker, S., and Stoeck, T. (2018). Redescription of Dexiotricha colpidiopsis (Kahl, 1926) Jankowski, 1964 (Ciliophora, Oligohymenophorea) from a hot spring in Iceland with identification key for Dexiotricha species. Acta Protozool. 57, 95–106. doi: 10.4467/16890027AP.18.009.8983
Qu, Z., Weinisch, L., Fan, X., Katzenmeiera, S., Stoeck, T., and Filker, S. (2020). Morphological, Phylogenetic and ecophysiological characterization of a new ciliate, Platynematum rossellomorai n. sp. (Oligohymenophorea, Scuticociliatia), detected in a hypersaline pond on Mallorca, Spain. Protist 171:125751. doi: 10.1016/j.protis.2020.125751
Ronquist, F., and Huelsenbeck, J. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Shao, C., Hu, C., Fan, Y., Warren, A., and Lin, X. (2019). Morphology, morphogenesis and molecular phylogeny of a freshwater ciliate, Monomicrocaryon euglenivorum euglenivorum (Ciliophora, Oxytrichidae). Eur. J. Protistol. 68, 25–36. doi: 10.1016/j.ejop.2019.01.001
Shao, C., Lu, X., and Ma, H. (2015). A general overview of the typical 18 frontal-ventral-transverse cirri Oxytrichidae s. l. genera (Ciliophora, Hypotrichia). J. Ocean Univ. China 14, 522–532. doi: 10.1007/s11802-015-2482-7
Shao, C., Lv, Z., Pan, Y., Al-Rasheid, K., and Yi, Z. (2014). Morphology and phylogenetic. analysis of two oxytrichid soil ciliates from China, Oxytricha paragranulifera n. sp. and Oxytricha granulifera Foissner and Adam, 1983 (Protista, Ciliophora, Hypotrichia). Int. J. Syst. Evol. Microbiol. 64, 3016–3027. doi: 10.1099/ijs.0.062281-0
Shao, C., Song, W., Al-Rasheid, K. A. S., and Berger, H. (2012). Redefinition and. reassignment of the 18-cirri genera Hemigastrostyla, Oxytricha, Urosomoida, and Actinotricha (Ciliophora, Hypotricha), and description of one new genus and two new species. Acta Protozool. 50, 263–287. doi: 10.4467/16890027AP.11.025.0062
Song, W., Warren, A., and Hu, X. (2009). Free-living Ciliates in the Bohai and Yellow Seas. Beijing: Science Press.
Song, Y., Liu, Y., Pan, B., Luo, X., Song, W., and Warren, A. (2019). Morphological studies on four brackish water ciliates of the class Spirotrichea (Protista, Ciliophora). J. Ocean Univ. China 18, 663–674. doi: 10.1007/s11802-019-4096-y
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis. of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Wang, J., Li, J., and Shao, C. (2020). Morphology, morphogenesis, and molecular phylogeny of a novel saline soil ciliate, Heterourosomoida sinica n. sp. (Ciliophora, Hypotrichia). Eur. J. Protistol. 73:25666. doi: 10.1016/j.ejop.2016.07.005
Wilbert, N. (1975). Eine verbesserte Technik der Protargo-limpragnation für Ciliaten. Mikrokosmos 64, 171–179.
Wu, T., Li, Y., Lu, B., Shen, Z., Song, W., and Warren, A. (2020). Morphology, taxonomy and molecular phylogeny of three marine peritrich ciliates, including two new species: Zoothamnium apoarbuscula n. sp. and Z. apohentscheli n. sp. (Protozoa, Ciliophora, Peritrichia). Mar. Life Sci. Technol. 2, 334–348. doi: 10.1007/s42995-020-00046-y
Keywords: abnormal form, Oxytricha, hot spring, Iceland, morphology, phylogeny
Citation: Zhu R, Qu Z, Zhang Q, Filker S, Stoeck T, Li F and Hu X (2021) A New Record of Oxytricha granulifera granulifera Foissner and Adam, 1983 (Protozoa, Ciliophora, Oxytrichidae) From a Hot Spring in Iceland, With Notes on Its Abnormal Form During Cultivation. Front. Mar. Sci. 8:621349. doi: 10.3389/fmars.2021.621349
Received: 26 October 2020; Accepted: 14 January 2021;
Published: 04 February 2021.
Edited by:
Thomas Wilke, University of Giessen, GermanyReviewed by:
Xiangrui CHEN, Ningbo University, ChinaWenzhe Xu, Tianjin University of Science and Technology, China
Copyright © 2021 Zhu, Qu, Zhang, Filker, Stoeck, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengchao Li, bGlmZW5nY2hhbzIwMDBAMTI2LmNvbQ==
 Rong Zhu1,2
Rong Zhu1,2 Zhishuai Qu
Zhishuai Qu Thorsten Stoeck
Thorsten Stoeck Fengchao Li
Fengchao Li