- Duke Marine Lab, Division of Marine Science and Conservation, Nicholas School of the Environment, Beaufort, NC, United States
Direct interactions with fisheries are broadly recognized as the leading conservation threat to small cetaceans. In open-ocean environments, one of the primary gear types implicated in these interactions is the pelagic longline. Unlike accidental entanglement in driftnets or deliberate entrapment by purse-seines, interactions between cetaceans and longlines are often driven by attraction of the animals to feed on bait or fish secured on the gear, a behavior known as depredation. Many small and medium-sized delphinid species have learned to exploit such opportunities, leading to economic costs to fisheries and a risk of mortality to the animals from either retaliation by fishermen or hooking or entanglement in fishing gear. Two pelagic longline fisheries in the United States experience depredation and bycatch by odontocete depredators: the Hawai‘i deep-set longline fishery, which is depredated primarily by false killer whales (Pseudorca crassidens), and the Atlantic pelagic longline fishery depredated primarily by short-finned pilot whales (Globicephala macrorhynchus). These fisheries are among the most intensively documented and managed pelagic longline fisheries in the world, with high levels of observer coverage, and bycatch mitigation measures required to reduce the mortality of seabirds, sea turtles and cetaceans. Both fisheries have active, multi-stakeholder “Take Reduction Teams,” enacted under the U.S. Marine Mammal Protection Act (MMPA), that are tasked to develop measures to reduce the bycatch of cetaceans below statutory reference points. Consequently, these two Teams represent model processes within which to address depredation and bycatch, having access to detailed, high-quality data on the nature and frequency of interactions with cetaceans, meaningful stakeholder involvement, resources to test potential solutions, and the institutional will to improve outcomes. We review how mitigation strategies have been considered, developed, and implemented by both Teams and provide a critical analysis of their effectiveness in addressing these problems. Notably, in the absence of straightforward avoidance or deterrence strategies, both Teams have developed gear and handling strategies that depend critically on comprehensive observer coverage. Lessons offered from these Teams, which have implemented consensus-driven management measures under a statutory framework, provide important insights to managers and scientists addressing other depredation problems.
Introduction
Direct interactions with fisheries are broadly recognized as the leading threat to the conservation of small cetaceans worldwide (Mitchell, 1975; Read, 2008; Brownell et al., 2019). Bycatch in gillnets is the most pressing problem (Read et al., 2006; Reeves et al., 2013), currently contributing to declines of 11 of the 13 critically endangered small-cetacean populations (Brownell et al., 2019) in freshwater, estuarine, and coastal environments. The threat is different in pelagic waters, where one of the primary gear types implicated in direct interactions with cetaceans is the pelagic longline (Lewison et al., 2014). Whereas some bycatch problems are a function of cetaceans failing to perceive gear (e.g., gillnets) or being actively entrapped by fishermen (e.g., purse seines), interactions between cetaceans and hook and line gear, such as longlines, are often driven by attraction of the animal to feed on bait or fish secured on the gear, a behavior known as depredation (Gilman et al., 2007a; Read, 2008; Hamer et al., 2012). Many odontocete species are adept at depredation and can remove large quantities of catch, which can result in substantial economic costs to fishermen (Peterson et al., 2014; Tixier et al., 2020a). Switching from energetically costly, natural foraging on free-swimming prey to consumption of high-energy, restrained prey may provide energetic benefits to depredators and it has been shown that the reproductive output of depredating whales has increased in at least two populations (Tixier et al., 2015a; Esteban et al., 2016). However, this behavior also increases the risk of hooking or entanglement in fishing gear (e.g., Garrison, 2007; Forney et al., 2011) or lethal retaliation or harassment by fishermen (Guinet et al., 2015), both of which have led to negative population consequences for depredating populations (Poncelet et al., 2010; Guinet et al., 2015; Tixier et al., 2020b).
Interactions between cetaceans and pelagic longlines have been documented as a concern for fishermen since shortly after the establishment of industrial longline operations in the 1950s (e.g., Sivasubramaniam, 1964). An increase in published reports on depredation in the past two decades suggest that depredation is an increasing problem (Tixier et al., 2020b), and there has been strong interest in characterizing patterns of interactions between cetaceans and longlines to generate mitigation strategies (Werner et al., 2015; Tixier et al., 2020b). Numerous workshops involving fishermen, scientific experts, and fishery managers have assessed available mitigation strategies and considered approaches for research, testing, and implementation. These past efforts and general syntheses of odontocete-longline interactions and research have been summarized in several previous reviews (e.g., Gilman et al., 2007a; Hamer et al., 2012; Werner et al., 2015; FAO, 2018; Hamilton and Baker, 2019; Tixier et al., 2020b).
Many of these mitigation efforts have been motivated by a public desire to ensure that seafood is ethically and sustainably sourced (e.g., Roheim et al., 2018). In the United States, for example, statutes such as the Marine Mammal Protection Act (MMPA) require fisheries to reduce incidental mortality or serious injury of marine mammals during fishing operations to “insignificant levels” [16 U.S.C. §1387]. International bodies such as the Food and Agriculture Organization of the United Nations (FAO) and regional fisheries management organizations (RFMOs), international governance bodies that manage fisheries in respective geographic regions, are increasingly addressing the bycatch of cetaceans and other vulnerable species (e.g., Clarke et al., 2014; Juan-Jordá et al., 2018; FAO, 2020). Additionally, the mitigation of cetacean depredation and bycatch is motivated by a desire to reduce the direct economic impacts to the fisheries themselves (Werner et al., 2015; Tixier et al., 2020a).
Depredation and bycatch in pelagic longline fisheries are related, but separate and unique problems that have proven exceedingly difficult to solve. There has been little success in implementing effective strategies to protect target catch and reduce the economic costs of depredation to fishermen (Hamer et al., 2012; Werner et al., 2015; Tixier et al., 2020a). Likewise, it has been challenging to reduce injuries or mortalities due to hookings or entanglements of depredating cetaceans, even when mandated by legislation (e.g., Baird, 2019). In many parts of the world, data limitations and scarce resources make it difficult to characterize the nature of these interactions and understand the scope of the problem (Hamer et al., 2012; Tixier et al., 2020b). In cases where bycatch occurs as a result of depredation, management mandates typically extend only to reducing bycatch or minimizing injury of bycaught cetaceans (e.g., the U.S. MMPA). Reducing depredation would result in a mutually positive outcome for industry and cetaceans, but in the absence of an effective strategy to reduce depredation, fishermen may be faced with costly measures to reduce bycatch that limit fishing effort, in addition to experiencing losses from depredation (Werner et al., 2015).
In this paper, we briefly outline the nature of these interactions and the primary mitigation strategies available, including recent findings relevant to mitigation and impacts on depredating odontocetes. We then explore two case studies from the United States in which attempts have been made to address the depredation and bycatch of small cetaceans. In the U.S., the MMPA requires Take Reduction Teams (TRTs) to develop methods to reduce the bycatch of marine mammals when mortality exceeds a biological reference point, known as Potential Biological Removal (PBR) [16 U.S.C. §1362 (20)]. We situate these efforts in the context of global bycatch of odontocetes, in the hope that lessons learned from these well-funded, collaborative, and statutorily-mandated attempts may offer insights to other countries and international fisheries management bodies as they grapple with these complex issues.
Overview of the Problem
Depredation
There are two distinct types of longline fishing, each susceptible to interactions with cetaceans in different ways. Demersal, or bottom, longlining is common in temperate to sub-polar ecosystems, in which gear is deployed on the sea floor to target species such as halibut (Hippoglossus spp.) or sablefish (Anoplopoma fimbria) in the Northern Hemisphere (Sigler et al., 2008; Peterson et al., 2013) and toothfish (mostly Dissostichus eleginoides) in the Southern Hemisphere (Ashford et al., 1996; Hucke-Gaete et al., 2004; Roche et al., 2007). The primary depredating odontocetes in these demersal longline fisheries in both hemispheres are killer whales (Orcinus orca) and sperm whales (Physeter macrocephalus) (Ashford et al., 1996; Hucke-Gaete et al., 2004; Roche et al., 2007; Sigler et al., 2008; Hamer et al., 2012; Peterson et al., 2013). Due to the depths in which gear is fished (500–2,000 m), depredation occurs mostly during the hauling phase (but see Richard et al., 2020). Both odontocete species have been observed hooked or entangled in demersal gear, but bycatch appears relatively rare in demersal longline fishing (e.g., Ashford et al., 1996). Negative population impacts have been tied to active killing by fishermen. For example, killer whales in the Southern Ocean are thought to have experienced population declines from the use of explosive deterrents and lethal retaliation in Illegal, Unreported, and Unregulated (IUU) fishing in the 1980s and 1990s (Poncelet et al., 2010; Guinet et al., 2015). There are other important concerns related to the interaction, for example: (1) the economic and stock consequences of target catch lost to whales (Peterson et al., 2013, 2014; Peterson and Hanselman, 2017; Hanselman et al., 2018; Tixier et al., 2020a); and (2) possible indirect effects on depredator populations and their ecological communities driven by food subsidies (Tixier et al., 2017, 2019). Two long-term collaborations among scientists, managers, and fishermen, one in the Gulf of Alaska (e.g., Straley et al., 2015) and one in the Crozet and Kerguelen Island fisheries (e.g., Guinet et al., 2015), have provided important data on the nature of these interactions.
In contrast, pelagic longlines typically target wide-ranging, pelagic species such as tunas (Thunnus spp.), swordfish (Xiphias gladius), and dolphinfish (Coryphaenus hippurus) by suspending baited hooks in the water column (Watson and Kerstetter, 2006; Ward and Hindmarsh, 2007). The gear is fished at depths that range from tens of meters for species such as swordfish, to over 400 m for deeper species such as bigeye tuna (Thunnus obesus) (Watson and Kerstetter, 2006; Ward and Hindmarsh, 2007). Pelagic longline fishing is most common in tropical and sub-tropical habitats and is one of the primary gear types to interact with many species of oceanic cetaceans (Lewison et al., 2014). At least 20 odontocete species have been observed as bycatch in pelagic longline fisheries (Werner et al., 2015; Tixier et al., 2020b), including the false killer whale (Pseudorca crassidens), short-finned pilot whale (Globicephala macrorhynchus), killer whale, and, to a lesser extent, Risso’s dolphins (Grampus griseus) (Hamer et al., 2012; Werner et al., 2015). As fishing operations and solutions to depredation vary greatly between these two fishery types, and because direct mortality of small cetaceans is currently a greater problem for pelagic than demersal longlines, we focus on depredation and bycatch mitigation in pelagic longlines in this paper.
Potential Solutions
There are three, semi-hierarchical categories of approaches generally considered for addressing odontocete depredation and bycatch (Werner et al., 2015; Hamilton and Baker, 2019): (1) reducing the spatiotemporal overlap between whales and fishing operations to minimize encounters a priori; (2) deterring whales from the gear or reducing their ability to perceive, locate, or access bait or catch, for example by disrupting the echolocation abilities of whales or deploying protective sleeves around captured fish; and (3) reducing the probability of injury and mortality despite becoming hooked or entangled, for example with weak terminal gear or hooks that allow cetaceans to break free but retain target catch (Figure 1). Many potential solutions covering these three categories have been critically evaluated by both fishermen and scientists and these are reviewed in detail elsewhere (Gilman et al., 2007a; Hamer et al., 2012; Werner et al., 2015; Hamilton and Baker, 2019; Zollett and Swimmer, 2019; Swimmer et al., 2020). Here, we briefly consider the range of options and recent findings relevant to pelagic longline fishery interactions and our two case studies.
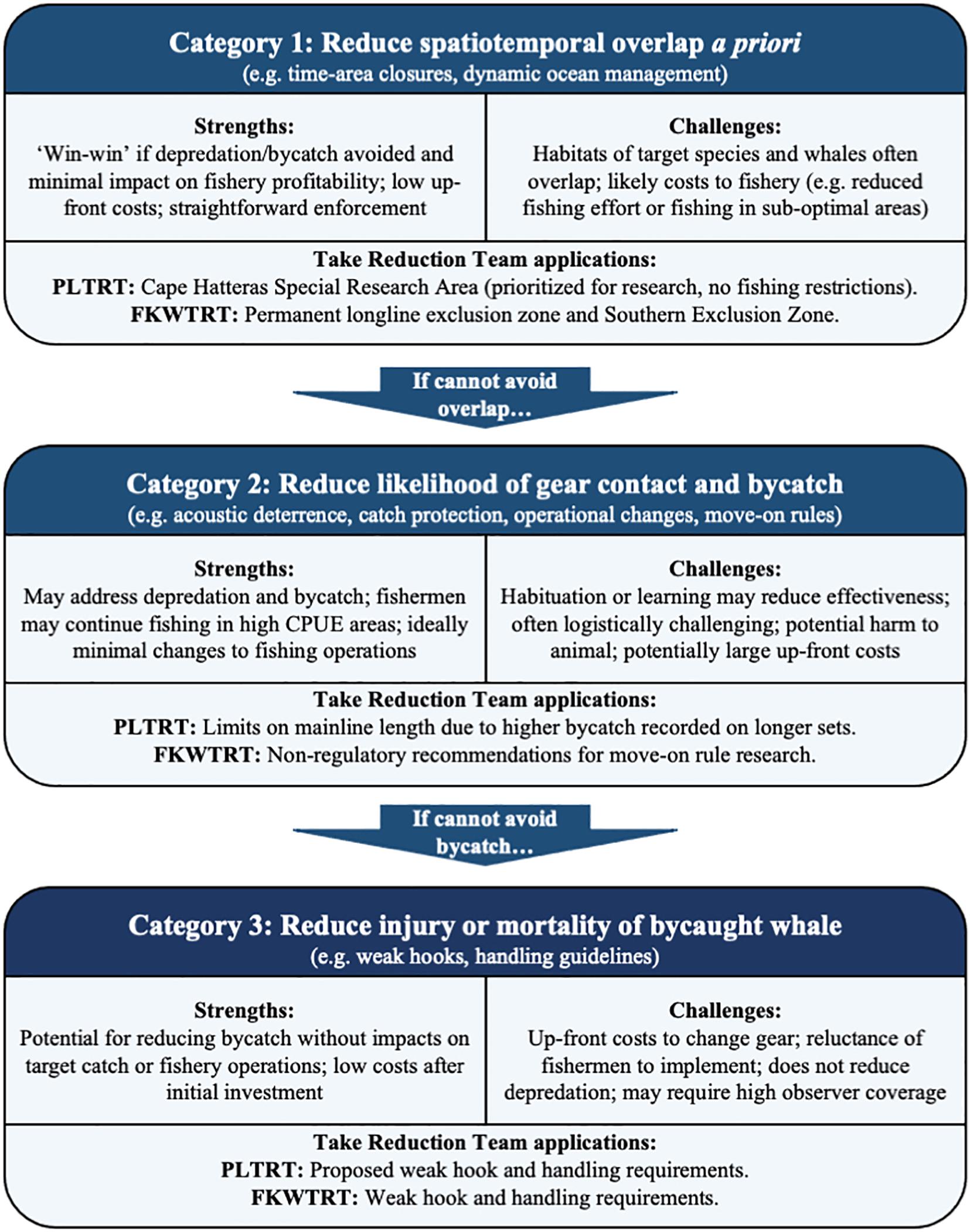
Figure 1. Outline of the hierarchical categories of bycatch mitigation solutions available to longline fisheries affected by depredation; including the strengths and weaknesses of each category and specific applications implemented by each Take Reduction Team.
Avoiding overlap between whales and longlines while maintaining target catch rates and fishery profitability (i.e., Category 1 listed above), is an ideal scenario. Many pelagic predators, including longline fishermen, range widely while tracking oceanographic conditions, and identifying the ecological drivers of co-occurrence could allow fishermen to avoid overlap and subsequent interactions. This “dynamic ocean management” (Dunn et al., 2016) has been suggested as a means of reducing negative human-wildlife interactions, such as the bycatch of sea turtles (Howell et al., 2015) and ship strikes of migrating baleen whales (Hazen et al., 2017). Indeed, constantly improving oceanographic models and animal telemetry data have allowed unprecedented insights into habitat use by marine predators (Hays et al., 2019), including odontocetes that engage in depredation (e.g., Thorne et al., 2017; Anderson et al., 2020), and such information could be used to predict their overlap with pelagic longline fisheries. However, depredating whales may target similar oceanographic features as those sought by longline vessels. For example, short-finned pilot whales use the same shelf-break habitat and sea surface temperature patterns as the pelagic longline fleet along the U.S. east coast (Garrison, 2007; Thorne et al., 2017, 2019; Stepanuk et al., 2018). Similarly, false killer whale depredation and bycatch on Hawai‘i pelagic longline vessels is likely driven by whales and fishermen targeting the same prey species (Forney et al., 2011). In such instances, the use of spatial and temporal avoidance would require fishing effort to relocate to sub-optimal areas with reduced rates of depredation and bycatch, but also lower catch rates of target species.
When broad-scale avoidance of depredators is not possible, the next logical strategy is to reduce the probability of gear contact and bycatch by deterring depredators, limiting their ability to detect or access catch, or altering fishing operations to limit contact (Category 2) (Werner et al., 2015; Swimmer et al., 2020). These strategies are all challenging due to the strong attraction that odontocetes can have toward longline gear (Werner et al., 2015), potentially driven by energetic incentives to feed on captured fish (Esteban et al., 2016). Physical harassment (e.g., explosives) and lethal retaliation have been reported in longline fisheries (e.g., Poncelet et al., 2010) but have questionable effectiveness, in addition to obvious negative conservation outcomes (Werner et al., 2015). Acoustic deterrents have garnered much interest by fishermen but have thus far proven impractical (Werner et al., 2015), as depredating whales likely quickly habituate and may even be attracted to the presence of deterrents that notify whales of the location of catch (Tixier et al., 2015b; Werner et al., 2015). Strategies to disrupt echolocation abilities or otherwise mask detection of gear can similarly be susceptible to learning and habituation (Mooney et al., 2009). Protecting target catch with sleeves or other physical barriers has shown promise in demersal longline fisheries, where they can be triggered to protect captured fish during hauling, when most depredation occurs (Moreno et al., 2008). The nature of pelagic longline gear makes this much more challenging as depredation does not occur exclusively during hauling and thus protective devices must be triggered by fish capture (Werner et al., 2015). These devices must also be cost effective and easy to store and deploy, both of which remain significant challenges; although further research may improve efficacy and feasibility (Rabearisoa et al., 2012, 2015; Hamer et al., 2015).
Catch protection devices also do not protect against odontocete depredation of bait, which has long been suspected by fishermen in some pelagic longline fisheries (Ayers and Leong, 2020). The relative rate of depredation of bait vs. catch is unknown and likely varies among fisheries, although small cetaceans are likely to engage in this behavior (Gilman et al., 2007a; Werner et al., 2015). Recent video and acoustic evidence confirmed that false killer whales depredate pelagic longline bait in the daytime using both visual and audio cues (Thode et al., 2016). Garrison (2007) also showed lower bycatch rates of Risso’s dolphins in the U.S. East Coast pelagic longline fishery when fish bait was used in place of squid bait. These results suggest the potential for bait-focused mitigation strategies (e.g., chemicals to reduce palatability of bait or using artificial bait), although these techniques are untested and potentially challenging due to unintended impacts on target and non-target catch (Werner et al., 2015; Gilman et al., 2020).
Other avoidance strategies involve operational changes to limit opportunities for interaction, such as fishermen leaving areas of known depredation, a strategy formally known as “move-on rules” (Dunn et al., 2014; Werner et al., 2015). This is a challenging strategy as odontocete depredators are highly mobile and may be able to perceive acoustic signatures from vessels over large distances. For example, sperm whales depredating demersal longlines in the Gulf of Alaska are attracted to cavitation noises of a ship’s propeller when the engine is engaged to begin hauling gear and can detect these sounds at distances of several kilometers (Thode et al., 2007). Recent findings on interactions between false killer whales and the Hawai‘i longline fishery suggest this mode of detection likely occurs with pelagic longlines as well. Passive acoustic monitoring of longline gear deployments detected false killer whales most commonly during the hauling phase, with whales potentially moving along the mainline away from the vessel as gear was being retrieved (Bayless et al., 2017). In another study, a group of satellite-tagged false killer whales was observed to show directed movements toward fishing gear during the hauling phase of some sets, although there was no apparent reaction to gear during other sets despite likely being within detection range (Anderson et al., 2020). Reducing the amount of gear set has also shown modest reductions in interaction rates in pelagic longline fisheries (Garrison, 2007), and this technique could work synergistically with move-on strategies to limit possibilities for gear detection and contact by odontocetes (Tixier et al., 2015c). Together, these findings suggest that improved reporting of depredation interactions and communication among fishing vessels could help fleets avoid acoustic detection when depredation has been observed in a particular location.
If avoidance of depredators or minimizing contact with gear is not possible, modifying the terminal gear to release hooked animals or facilitating shedding of entangled gear may be the only option to mitigate bycatch impacts (Category 3) (Werner et al., 2015; Zollett and Swimmer, 2019; Swimmer et al., 2020). In longline fisheries, this strategy generally entails guidelines to encourage fishermen to remove gear from hooked or entangled animals, or the use of hooks with a targeted bending strength, such that hooks are weak enough to straighten and release toothed whales but sufficiently strong to retain target catch (Bayse and Kerstetter, 2010; Bigelow et al., 2012). This “weak-hook” strategy has been used successfully to reduce bycatch of large, non-target bluefin tuna (Thunnus thynnus) by 46% in the U.S. Gulf of Mexico pelagic longline fishery, with no statistically significant impact on catch rates of yellowfin tuna (Thunnus albacares) (Walter, 2017). Controlled mechanical tests of bending strengths and behavior of hooks under strain in the lip tissue of dead odontocetes have helped identify candidate weak hooks for minimizing cetacean bycatch (McLellan et al., 2015). Field trials in the U.S. Atlantic large pelagics longline fishery and Hawai‘i deep-set longline fisheries have tested similar hook designs under controlled conditions (Bayse and Kerstetter, 2010; Bigelow et al., 2012). The bycatch of cetaceans was too rare to determine whether weaker hooks had a positive influence on the outcome of such events, but weaker hooks were returned straightened more often than strong hooks in each study and one pilot whale was observed released by a straightened hook in the Atlantic (Bayse and Kerstetter, 2010; Bigelow et al., 2012). Comparable rates of target catch were recorded in each study, although the Hawai‘i study was not carried out during the season when the largest tuna are caught (Bigelow et al., 2012) and the size of swordfish was slightly smaller on weak hooks in some Atlantic trials (Bayse and Kerstetter, 2010). One obstacle to implementation of such measures is the understandable reluctance of fishermen to modify their terminal tackle, particularly if such changes might reduce the catch rates of large and valuable target species (e.g., Bigelow et al., 2012; Ayers and Leong, 2020). The post-release survival rates of animals hooked or entangled in pelagic longline gear are not well understood but have obvious and important implications for understanding population-level impacts (Garrison, 2007; Werner et al., 2015).
Regulatory Frameworks to Address Odontocete-Longline Interactions in the United States
U.S. Marine Mammal Protection Act and Take Reduction Teams
The U.S. MMPA of 1972 regulates the “take” of marine mammals in commercial fisheries, with the term “take” defined as to harass, hunt, capture, or kill, or attempt to harass, hunt, capture, or kill any marine mammal [16 U.S.C. §1362 (13)]. The general prohibition on taking under the MMPA has exemptions for certain activities, including commercial fishing, and authorizes the National Marine Fisheries Service (NMFS) to enforce this prohibition for all cetacean species in U.S. jurisdictions. Amendments to the MMPA passed in 1994 provide a mandate to assess the magnitude of bycatch relative to biological reference points and to implement conservation actions when takes exceed these thresholds (16 U.S.C. §1387). The MMPA also requires assessments for all marine mammal stocks in the U.S. Exclusive Economic Zone (EEZ) to characterize, among other parameters, range and population structure, minimum population estimates, and the magnitude of bycatch in fisheries and other sources of human-induced mortality (16 U.S.C. §1386). In addition to observed mortality, entangled or hooked marine mammals are considered takes if they are “likely to die,” defined as experiencing a serious injury that presents a greater than 50% chance of death (NOAA Fisheries, 2014). Precise estimates of post-release mortality are not available for most cetacean species and types of interactions (NOAA Fisheries, 2014), but specific criteria for designating the probability of mortality to marine mammals due to fisheries interactions have been developed by NMFS in several workshops, using expert elicitation amongst marine mammal scientists and veterinarians (Angliss and Demaster, 1998; Andersen et al., 2008). These criteria have been formalized in NMFS’ Procedural Directive entitled “Process for distinguishing serious from non-serious injury of marine mammals (NOAA Fisheries, 2014),” which provides guidance for estimating mortality using the best available scientific information when follow-up on the condition of the injured animal is unavailable, as is the case in the vast majority of fishery interactions with small cetaceans, including those with pelagic longlines (NOAA Fisheries, 2014). Marine mammals experiencing either bycatch mortality or injury likely to lead to death are designated as Mortality and Serious Injury (M&SI). This parameter is then compared with a biological reference point, Potential Biological Removal (PBR) [16 U.S.C. §1362 (20)], calculated for each stock as a product of minimum population size, maximum rate of population increase, and a recovery factor (Wade, 1998). PBR represents the maximum number of individuals that can be removed while maintaining the stock at or above optimal sustainable population size [16 U.S.C. §1362 (9)], typically defined as half of carrying capacity. If human-caused M&SI for a particular stock exceeds PBR, a Take Reduction Team (TRT) [16 U.S.C. §1387 (f)] must be convened. A TRT is a stakeholder group which includes members of the fishing industry, environmental groups, academic scientists, and government managers and scientists.
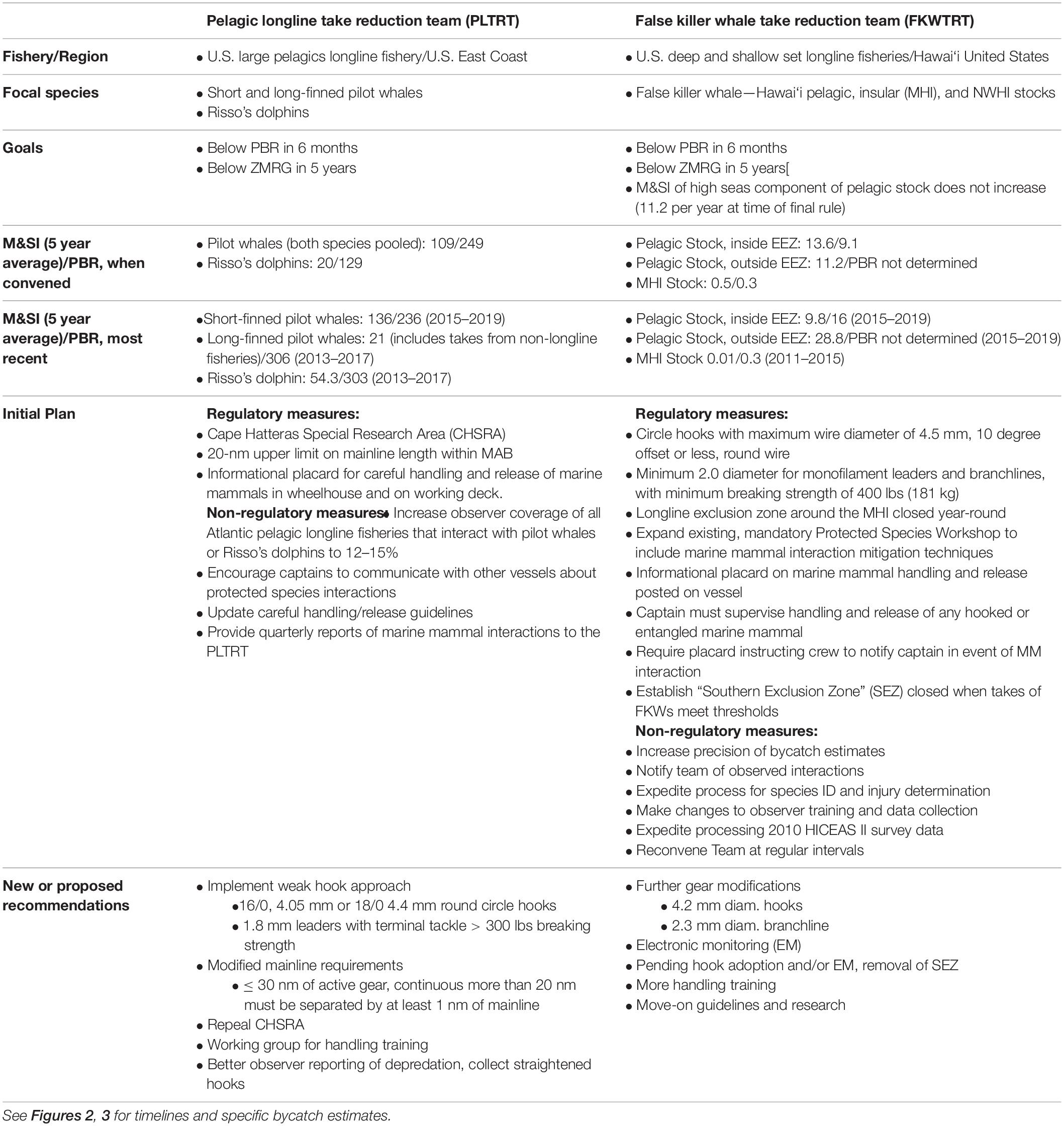
Take Reduction Teams are asked to develop a Take Reduction Plan (TRP) to reduce M&SI to below PBR within 6 months of implementation and to a level approaching zero (the Zero-Mortality Rate Goal, ZMRG, defined as < 10% of PBR) within 5 years [16 U.S.C. 1387 (f) (2)]. To reduce the effects of inter-annual variation, a 5 year average of M&SI is applied against PBR. The Team is required to agree to a plan by consensus, and the plan is typically comprised of a suite of regulatory and non-regulatory measures. In the absence of a consensus plan, NMFS must generate a plan, so the impetus is on Team members to work together to craft a more effective strategy by consensus. Seven TRTs are currently active and their successes, failures, and the strengths and weaknesses of the TRT process have been reviewed elsewhere (McDonald and Rigling-Gallagher, 2015; McDonald et al., 2016; Borggaard et al., 2017; Punt et al., 2018).
Two of these Teams directly address odontocete interactions with pelagic longlines and are reviewed here: the Pelagic Longline Take Reduction Team (PLTRT), addressing short-finned pilot whale bycatch in the Atlantic pelagic longline fishery, and the False Killer Whale Take Reduction Team (FKWTRT), addressing false killer whale bycatch in the Hawai‘i pelagic longline fishery. Below, we describe these two teams, the strategies they have developed for addressing bycatch, and assess whether these measures are helping meet the goals of the MMPA. We draw on published peer-reviewed studies, NOAA technical documents, and summary information prepared following Team meetings that are publicly available through NOAA Fisheries1,2.
Pelagic Longline Take Reduction Team
The U.S. large pelagics longline fishery targets pelagic fish species in the U.S. Atlantic and Gulf of Mexico EEZ. Both long-finned (Globicephala melas) and short-finned (G. macrorhynchus) pilot whales occur in the western North Atlantic, and both may depredate bait or catch from longlines and become hooked or entangled in gear as a result (Garrison, 2007). Concern over bycatch of pilot whales in pelagic longline fisheries emerged in the 1990s and 2000s (Waring et al., 2002), at a time when the demography and distribution of the two pilot whale species were not well understood. Initial stock assessments pooled abundance and takes of both species to calculate PBR and estimate M&SI (Waring et al., 2002). Subsequent research has identified the primary region of overlap to be along the continental shelf break between 38 and 40°N latitude, with long-finned pilot whales occurring mostly north of this area and short-finned pilot whales primarily to the south (Garrison and Rosel, 2017). Short-finned pilot whales make seasonal movements north of this area in summer months and are known to occur farther offshore into Gulf Stream waters (Garrison and Rosel, 2017; Thorne et al., 2017). Most takes occur in times and locations where long-finned pilot whales are unlikely to occur, and all bycatch is thus assigned to the short-finned pilot whale. When takes occur farther north, a logistic regression model is used to estimate the probability of species occurrence and apply the take to each species as appropriate (Garrison and Rosel, 2017; Hayes et al., 2019).
In June 2005 NMFS convened the first meeting of the PLTRT to develop a plan to reduce the bycatch of pilot whales to below ZMRG. The Team consists of approximately 20 members, including pelagic longline fishermen and other industry representatives, marine mammal and fisheries scientists, a representative from the U.S. Marine Mammal Commission, and representatives from environmental organizations and state and federal fisheries agencies. The Team held meetings every few months in 2005–2006, during which subject-matter experts provided briefings on pilot whale biology, fishery characteristics and experiences with interactions, and relevant mitigation research and efforts in other fisheries. Team members formed working groups to explore potential mitigation options in more depth and identify research priorities specific to the fishery. Discussions to clarify the goals and intended scope of the team and ensuing TRP were also conducted, e.g., whether to include Risso’s dolphins in the scope of the plan or focus only on pilot whales; ultimately both species were included, but with a greater emphasis on pilot whales3.
A draft TRP was agreed in June 2006, followed by a proposed rule open to public comment in 2008 and a final rule with regulations entering force in July 2009 (74 FR 23349) (Federal Register, 2009). The Final PLTRP comprised a series of three regulatory and four non-regulatory measures intended to significantly reduce M&SI of pilot whales and Risso’s dolphins in the Atlantic pelagic longline fishery (Table 1) (74 FR 23349) (Federal Register, 2009). The regulatory measures included the designation of a region of particularly high bycatch rates as a priority area for future research and monitoring. To fish in this “Cape Hatteras Special Research Area” (CHSRA), fishermen had to agree to carry observers who could conduct research targeted at bycatch reduction strategies (74 FR 23349) (Federal Register, 2009). The second measure was a 20 nm (37 km) upper limit on mainline length for pelagic longline sets in the Mid-Atlantic Bight (MAB), a region where bycatch is typically highest (74 FR 23349) (Federal Register, 2009). This measure was informed by analyses conducted by NMFS scientists, who identified higher bycatch rates on sets greater than 20 nm in length in the MAB (Garrison, 2007). Finally, an informational placard was required to be posted on every vessel outlining marine mammal careful handling and release guidelines (74 FR 23349) (Federal Register, 2009). This measure was also informed by the Garrison (2007) study, which suggested that approximately equal proportions of observed pilot whale bycatch interactions involved hooking vs. entanglement. In cases of entanglement, fishermen had some success in removing all trailing gear from animals using tools such as line cutters and, in such cases, entangled and released whales were typically not counted as serious injuries. Non-regulatory measures included a recommendation to increase observer coverage from ∼8 to 12–15% of all trips in the Atlantic pelagic longline fishery; encouraging vessel operators to communicate with each other regarding interactions with protected species; advising NMFS to update careful handling and release guidelines; and a requirement for more frequent (quarterly) reporting of marine mammal interactions (74 FR 23349) (Federal Register, 2009). Several additional research priorities were identified as well as an understanding that the PLTRT would regularly evaluate the success of the TRP and amend the Plan based on the results of ongoing research and monitoring (i.e., manage adaptively) (74 FR 23349) (Federal Register, 2009).
Following implementation of the Plan, the Team continued to meet regularly to assess progress toward meeting the MMPA goals, industry compliance with regulations, and outcomes of ongoing mitigation research projects. Pilot whale bycatch remained below PBR from 2009 until 2015, when the estimated 5 year average from 2010 to 2014 exceeded PBR for the first time (M&SI 192/year; PBR 159) (Hayes et al., 2017). Updated abundance estimates resulted in an increase in PBR from 159 to 236 in 2016 (Hayes et al., 2019). The most recent 5 year annual average of M&SI (2015–2019) of 136 (Garrison, personal communication) is thus below PBR, although it is still above ZMRG (Figure 2).
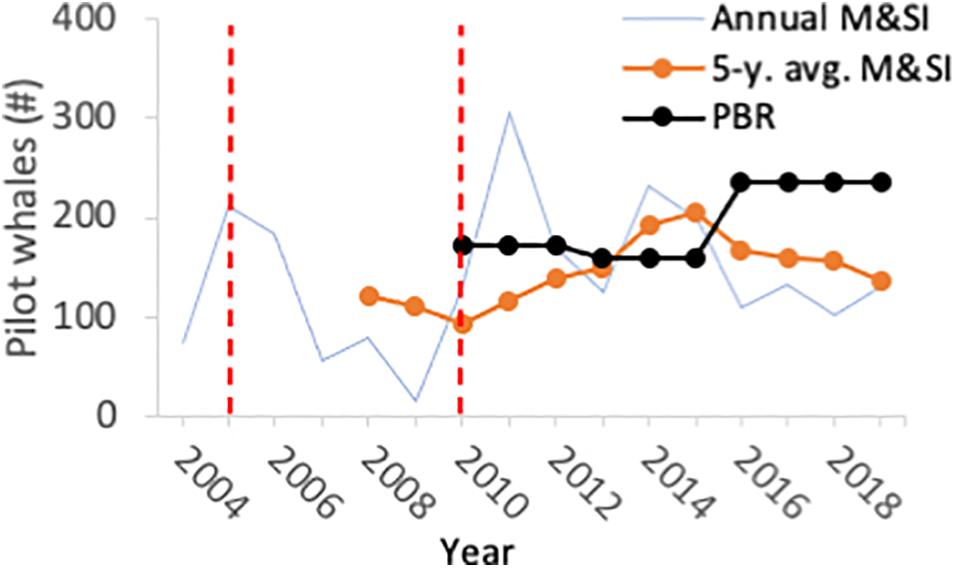
Figure 2. Annual estimated mortality and serious injury (M&SI), 5 year moving average of M&SI, and potential biological removal (PBR) for short-finned pilot whales taken in the U.S. large pelagics longline fishery off the U.S. East Coast. The dashed red lines indicate the year of Pelagic Longline Take Reduction Team establishment (left, 2005) and year of publication of the final regulatory rule implementing the Pelagic Longline Take Reduction Plan (right, 2010).
Compliance with the mainline rule was less than 50% for the first 2–3 years of Plan implementation4. Compliance improved between 2012 and 2014, as fishermen began making sequential sets in which individual mainlines were less than 20 nm in length but separated by less than one nautical mile5. Considerable discussion has been devoted to whether this fishing strategy will lead to the intended reduction in bycatch rates and possible alternative strategies to limit mainline length. A modified consensus recommendation was reached in 2016, stipulating that a vessel may set no more than 30 nm of active gear, with only one piece of gear in the water at a time, and that any mainline more than 20 nm long must include at least 1 nm of hookless line6.
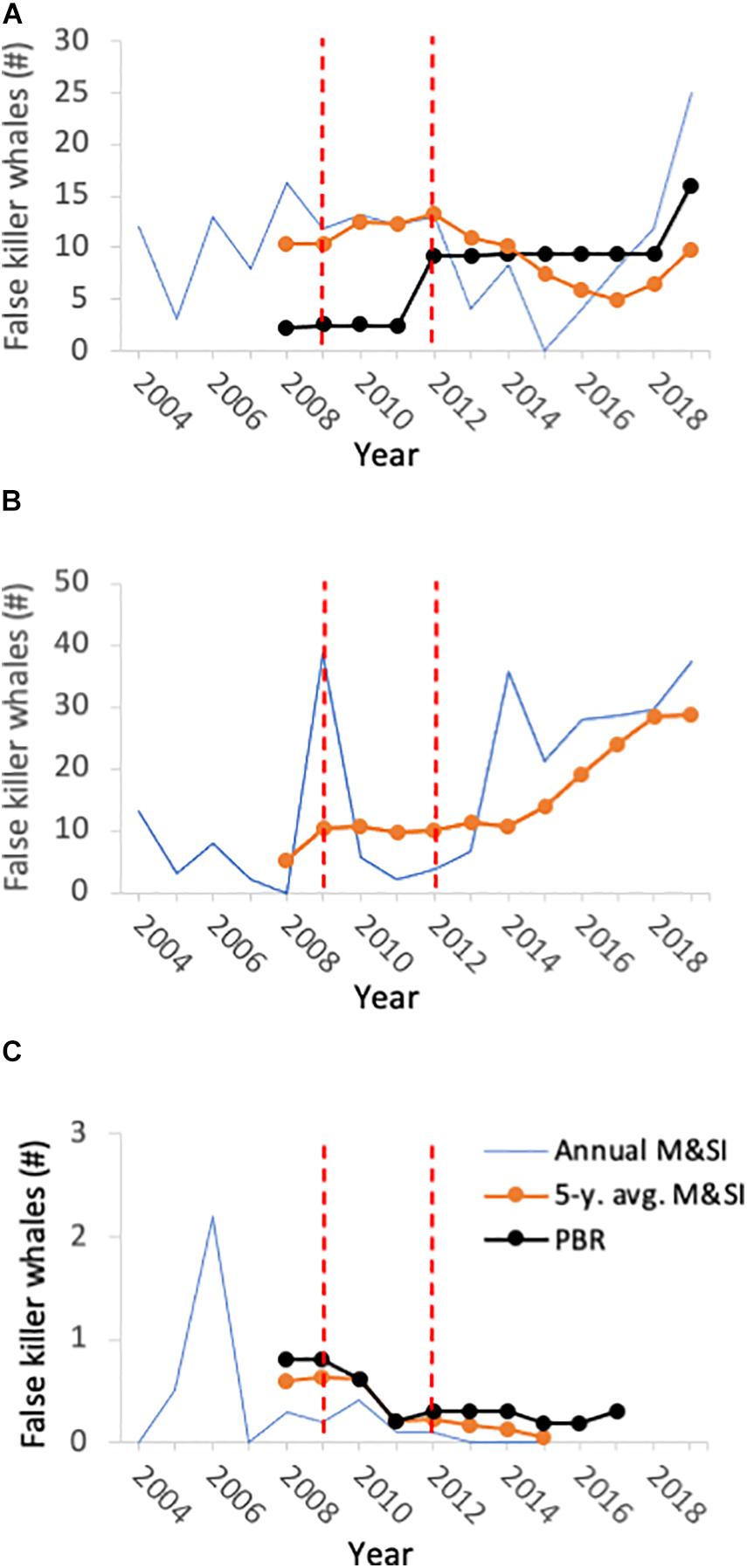
Figure 3. Annual estimated mortality and serious injury (M&SI), 5 year moving average of M&SI, and potential biological removal (PBR) for three false killer whale stocks taken in the U.S Hawai‘i pelagic longline deep-set fishery: (A) pelagic stock inside of the U.S. EEZ; (B) pelagic stock outside of the U.S. EEZ (no PBR available); and (C) insular, main-Hawaiian Islands stock. The dashed red lines indicate the year of False Killer Whale Take Reduction Team establishment (left, 2009) and year of publication of the final regulatory rule implementing the False Killer Whale Take Reduction Plan (right, 2012).
Even with full compliance, the Team has recognized the mainline rule and other measures do not appear sufficient to meet ZMRG7. Thus, the Team has also discussed implementing a weak-hook approach to further reduce mortality and serious injury. This culminated in consensus recommendations at the 2015 meeting for the adoption of weak terminal gear (1.8 mm leaders, 300 lbs breaking strength, 16/0 circle hooks with maximum 4.05 mm diameter or 18/0 circle hooks with maximum 4.4 mm diameter) and to convene a workshop to develop better handling guidelines8. The Team also decided to repeal the CHSRA requirements, which has frustrated fishermen, as they have been required to call NMFS prior to fishing in the CHSRA to facilitate increased observer coverage in this area. However, no observers have been assigned for this purpose in over 5 years since the original recommendation9. In December 2020 NMFS published a proposed rule to reflect these changes (85 FR 81168) (Federal Register, 2020).
False Killer Whale Take Reduction Team
The FKWTRT was convened in January 2010, when the 5 year average of M&SI of false killer whales in the Hawai‘i-based, pelagic longline fishery exceeded PBR (Carretta et al., 2009; Table 1). False killer whales are social, mobile, apex predators that occur in tropical and subtropical oceans worldwide (Baird, 2018). They are pursuit predators, known to feed on a range of pelagic fish species including tunas, dolphinfish, and wahoo (Acanthocybium solandri) (Baird et al., 2008), all of which are commonly captured in the Hawai‘i longline fishery. Three partially overlapping stocks of false killer whales occur around the Hawaiian Islands: an endangered, insular stock around the main Hawaiian Islands (MHIs) (Baird et al., 2008; Bradford et al., 2018), an insular stock closely associated with the Northwestern Hawaiian Islands (NWHIs) (Baird et al., 2013), and a pelagic stock that ranges broadly within and beyond the U.S. EEZ (Bradford et al., 2015; Anderson et al., 2020). Most false killer whale bycatch in the Hawai‘i longline fleet is from the pelagic stock, as vessels are currently restricted from fishing within the core range of the MHI population and not permitted to fish in the Papahkea Marine National Monument, which encompasses the NWHIs. In rare cases of takes occurring in areas of overlap, bycatches are prorated to each stock based on relative stock occurrence and fishing effort (Carretta et al., 2019).
There are two distinct, Hawai‘i-based, pelagic longline fisheries (WPRFMC, 2020). Most effort is in the “deep-set” fishery, which targets bigeye tuna year-round to the north and south of the Hawaiian Islands, both inside and outside of the U.S. EEZ. A smaller number of vessels fish with a “shallow-set” configuration, targeting swordfish mainly north of the Hawaiian Islands. Both fisheries have experienced regulatory actions due to bycatch of several protected species. High bycatches of sea turtles led to closure of shallow-set operations in 2003–2004 (Gilman et al., 2007b), and both fisheries have enacted operational and gear changes to mitigate the bycatch of sea turtles and seabirds (Gilman et al., 2007b, 2008). Odontocete depredation and bycatch involving multiple species, but primarily the false killer whale, is a more common problem for the deep-set fishery (Forney et al., 2011) and is the main focus of the FKWTRT. Detailed bycatch information on bycatch and depredation is provided by on-board, independent observers present on approximately 20% of all deep-set trips (McCracken, 2019).
NMFS convened and coordinated a series of meetings for the FWTRT, beginning with the first official in-person meeting in February 2010. At the time of Team formation, it was only possible for NMFS to calculate a PBR value for pelagic false killer whales inside the U.S. EEZ, so there was concern that fishing effort would increase outside of the EEZ to avoid punitive measures from the TRT (Federal Register, 2010, 2012). Thus, in addition to the standard MMPA goals of reducing M&SI below PBR in 6 months and below ZMRG in 5 years, the FKWTRT had a third goal that fishery M&SI for the high-seas component of the pelagic false killer whale stock (i.e., outside the U.S. EEZ) should not increase (Federal Register, 2010, 2012). As with the PLTRT, early meetings provided team members with essential background information on the fishery, false killer whale biology and ecology, and the latest research findings regarding possible depredation and bycatch mitigation options (Federal Register, 2010).
Additional research specific to the deep-set fishery was commissioned by NMFS to inform team deliberations. One analysis assessed the influence of environmental and operational covariates on the occurrence of interactions of false killer whale depredation and bycatch between 2003 and 2009 (Forney et al., 2011). This research identified few clear patterns, except a seasonal incidence of lower depredation rates in summer months when the fleet typically fishes to the north, likely beyond the core range of pelagic false killer whales, and that sets were more likely to experience odontocete depredation if the preceding set was depredated, with a slight (∼16%) decrease in risk by moving > 100 km following previous depredation. There was also some evidence that circle hooks may slightly reduce false killer whale incidental takes. A concurrent field-based, weak-hook study (Bigelow et al., 2012) determined that smaller, weaker circle hooks had no effect on bigeye tuna catch rates in the longline fleet, although fishermen on the Team disputed these results, because the trials were not conducted in spring when the biggest tuna are caught. These studies strongly influenced subsequent Team deliberations, which focused on gear changes and strategies to reduce mortality and serious injury after hooking or entanglement.
The team met in person to develop a draft TRP with the Team’s recommendations, which was ultimately published as a Final Rule in December 2012 (77 FR 71260) (Federal Register, 2012) with regulatory and non-regulatory measures and a suite of research recommendations. The primary regulatory measure aimed at reducing the M&SI of false killer whales was to make the hook the weakest part of the terminal tackle, intended to release large animals (i.e., false killer whales) with minimal trailing gear. Specifically, the fishery is required to use circle hooks with round wire and a maximum wire diameter of 4.5 mm. Monofilament leaders and branch lines must be a minimum of 2.0 mm in diameter and 400 pounds breaking strength (77 FR 71260) (Federal Register, 2012). Several additional regulatory measures were added to improve handling and release of bycaught false killer whales, such as expanding the content of existing Protected Species Workshops, requiring marine mammal handling and release informational placards to be displayed on all vessels, and requiring the captain to be notified by crew and to supervise all marine mammal bycatch interactions (77 FR 71260) (Federal Register, 2012). Finally, two space-time management measures were included: a permanent longline exclusion zone around the MHI (previously a seasonal closure area only) and a “Southern Exclusion Zone” (SEZ) to be closed when specified levels of M&SI within the U.S. Hawaiian EEZ are exceeded (77 FR 71260) (Federal Register, 2012).
The Team has continued to meet regularly to assess progress toward goals, fishery compliance, and research outcomes since Plan implementation. Annual M&SI for the pelagic stock inside the EEZ initially dropped from a pre-TRP 5 year average of 13.3 (2008–2012) (Carretta et al., 2016) to 4.92 (2013–2017) (Carretta et al., 2019; Oleson, 2020). It is currently 9.8 (2015–2019) and thus remains below PBR but above ZMRG (Table 1 and Figure 3; Oleson, 2020). M&SI outside of the EEZ has shown a different pattern. In 1 year following Plan implementation, estimated M&SI for the pelagic stock outside of the U.S. EEZ rose from 6.6 whales in 2013 to 35.8 in 2014 (Carretta et al., 2019). With some annual variation, this level has remained relatively high (and generally increasing), with the 5 year average currently at 28.8 takes per year (2015–2019) (Oleson, 2020) compared to the pre-TRP average of 10.0 (2008–2012) (Figure 3). This increase is consistent with an ongoing trend of a fleetwide shift in fishing effort to the north and east, outside of the EEZ. This shift may be due to tracking of oceanographic conditions for improved target-species catch rates, rather than a reaction to the TRP (i.e., to avoid takes inside the EEZ that would lead to SEZ closure) (Woodworth-Jefcoats et al., 2018). Nonetheless, the third goal of takes not increasing outside of the EEZ is not being met. Also problematic is that closure of the SEZ (triggered by 2 or more false killer whale takes inside the EEZ in a calendar year) occurred in two consecutive years (2018 and 2019).
There has been some progress to mitigate false killer whale bycatch, but the Team has recognized that the weak-hook and handling guidelines are not fully adequate, at least as currently executed10. The Team has been provided with detailed reports of each observed false killer whale bycatch interaction in the deep-set fleet, assessing the details of the interaction and important outcomes such as captain and crew behavior, gear performance, and the fate of the animal11. Of 49 observed false killer whale interactions in the deep-set fishery between 2013 and 2018, the line was cut by captain or crew 19 times (39%) and broke 14 times (29%)12. In only four instances did the hook straighten as intended (∼8% of interactions)13. In roughly half of the interactions the captain was not on deck, because the interaction was over before he reached the working deck or he was not notified of the interaction at all (Baird, 2019).
The final regulation for a 4.5 mm hook is not as “weak” as the initial Team consensus or hooks tested by Bigelow et al. (2012), which was 4.0 mm. This may partly explain observed hook performance, and the Team is now considering a transition to even weaker hooks and stronger branch line (4.2 mm hooks and 2.3 mm branch line), pending an additional field study to evaluate the impact of these measures on target catch rates and profitability14. There have also been concerns raised by Team members about captain and crew behavior during interactions. In particular, Baird (2019) has argued that the main bycatch reduction strategy of handling gear in a way to allow hooks to bend and release bycaught cetaceans is fundamentally flawed in the absence of full (100%) observer coverage. Even when observers were present, the line was cut in 39% of interactions and the captain was not present to supervise half of the interactions. Baird argues that appropriate handling methods or captain involvement are even less likely in the remaining 80% of trips when an observer is not present, and thus that the estimated M&SI levels are almost certainly biased low. This argument has led the scientific and conservation caucus to argue for increased electronic monitoring (EM) in the fleet15, which has been trialed successfully for a small number of Hawai‘i longline vessels (Stahl and Carnes, 2020).
At time of writing, negotiations are ongoing but have been complicated by the emergence of the novel coronavirus which has caused large disruptions in the Hawai‘i fleet and the observer program. Importantly, the fleet intended to execute a weak hook trial in 2020 but this experiment will now be delayed at least until 2021. Nonetheless, future Team discussions will likely, in some capacity, address the weak hook rules, EM, and the details of the SEZ agreement. NMFS is also working toward properly accounting for takes for the entire pelagic population (i.e., not just the portion inside the EEZ). Bradford et al. (2020) estimated, for the first time, pelagic false killer whale abundance for the entire central Pacific. A derivation of PBR for this portion of the population, as well as accounting of foreign fishery effort and potential takes in non-US fisheries, are still forthcoming. These results, when available, have the possibility to substantially alter the current PBR and M&SI situation and change the dynamics of the Team negotiations.
Discussion
Odontocete depredation and bycatch continue to be challenging management problems, despite strong interest of fishermen in avoiding depredation (Ayers and Leong, 2020) and serious conservation concerns regarding bycatch (Read, 2008). The nature of odontocete behavior and realities of longline fishing contribute to the complexity of the issue. We have summarized two attempts to reduce odontocete bycatch in U.S. pelagic longline fisheries. These two Take Reduction Teams have achieved some of their goals, but even with robust funding, political will, and collaborative stakeholder involvement, and under the authority of a robust marine mammal protective statute, neither Team has fully achieved their bycatch reduction targets, nor resolved the depredation issue. Nevertheless, we hope that our description of these collaborative processes, their common struggles, and considered solutions may provide insight to other managers and scientists working on depredation and bycatch issues around the world.
Both take reduction teams invited participation from experts in marine mammal behavior, ecology, and bycatch as well as fishermen with practical knowledge of bycatch interactions and the respective fisheries. Together, they identified a comprehensive suite of potential mitigation solutions (e.g., Werner et al., 2015) that were considered carefully along with the specific nuances of each fishery. Additional research was commissioned to inform deliberations, much of which depended on high-quality, observer data on both depredation and bycatch events. Both Teams first prioritized options in Categories 1 and 2 (Figure 1), that is, limiting overlap with depredating odontocetes or preventing their contact with gear. For example, PLTRT analyses suggested that longer mainline length led to an increased interaction risk, and so the Team implemented a physical cap on mainline length. A high-interaction region was also designated a priority area for research, although no effort limitations were included that might directly reduce takes. The FKWTRT established a permanent area closure to protect the endangered, insular Main Hawaiian Island false killer whale stock, but no robust strategies for limiting overlap and avoiding interactions with the most commonly caught pelagic stock were identified. The other space-time measure (SEZ), in which high bycatch rates trigger closure of a large area important to the fishery, was meant as an incentive to limit bycatch more broadly. Interestingly, recent false killer whale density models suggest this may actually be an important area for the pelagic false killer whale population, and thus the SEZ may take on a new significance in future negotiations (Bradford et al., 2020). In general, fishermen in both Take Reduction Teams were reluctant to agree to any measure that restricted their ability to fish in particular times and areas, considering such measures as both punitive and unfair.
Ongoing research has continued to improve understanding of the overlap and nature of interactions between these fisheries and species. Recent studies comparing the distribution of short-finned pilot whales and pelagic longline fishing effort suggest that there might be some limited possibility of using extremely fine-scale spatial measures to avoid pilot whale bycatch, although areas of high bycatch generally correspond with high target catch rates (Stepanuk et al., 2018; Thorne et al., 2019). No clear environmental patterns have been identified that would help reduce false killer whale bycatch in the Hawai‘i fleet (Forney et al., 2011). More recent research has begun to elucidate fine-scale behavior of false killer whales in the vicinity of deep-set longline gear, but thus far no clear mitigation strategies have emerged for reducing depredation or bycatch rates (Bayless et al., 2017; Anderson et al., 2020).
These research efforts have helped understand spatial and operational patterns of bycatch and suggest that avoidance strategies such as “move-on rules” could help fishermen respond more effectively when interactions occur. However, none of these mitigation measures are considered likely to reduce interactions sufficiently to meet Team goals, and without clear options for limiting odontocete contact with gear (Categories 1 and 2), both Teams have resorted to reducing the number of serious injury determinations of bycaught animals (Category 3). The PLTRT initially specified guidelines for careful handling and release of marine mammals, although it did not include gear changes in its recommendations. Now, after several years with little progress in reducing pilot whale M&SI, a weak-hook strategy has emerged as the most acceptable solution. Together with plans for developing further improved handling guidelines, this measure is currently pending under a public comment period in a new set of proposed regulations (85 FR 81168) (Federal Register, 2020).
The FKWTRT, with the advantage of learning from the PLTRT experience, adopted weak hook measures in its first round of recommendations in 2013. However, the implemented measures were not as stringent as initially agreed to by the Team (4.0 mm initially agreed on, 4.5 mm adopted in Final Rule), and are not meeting management objectives. Between 2013 and 2018, the most frequent outcome during false killer whale interactions was that the line was cut before straightening could occur. The line also broke nearly four times as often as the hook straightened. These patterns indicate that the hook is not weak enough, the captain and crew are not handling the line as intended in the regulations, or most likely, a combination of both. The Team is now exploring options for moving to even weaker hooks and improving handling guidelines.
The next few years will be important as both fisheries may implement these new or altered gear requirements. Yet, as Baird (2019) pointed out for the FKWTRT, weak-hook regulations for cetaceans depend critically on proper handling which, in the Hawai‘i longline fleet, does not seem to occur, even in the presence of an independent observer16. Concerns over crew safety and economic expediency mean that the preferred reaction to a hooked odontocete is often for a crew member to cut the line. Thus, there must be adequate incentives to ensure the necessary steps are taken, when safe and appropriate to do so, so that these interactions are resolved safely and effectively. Without comprehensive observer coverage to monitor the behavior of fishermen during interactions, this “fatal flaw” (Baird, 2019) in weak-hook and handling approaches for reducing bycatch mortality will be present in any fleet.
It is also worth noting the importance of specific statutory language and NMFS’s “Process for distinguishing serious from non-serious injury of marine mammals (NOAA Fisheries, 2014)” in shaping the resulting regulatory directives for each Team. The MMPA specifies that incidental mortality or serious injury of marine mammals occurring during commercial fishing operations must be reduced to insignificant levels (16 U.S.C. §1387). NMFS defines serious injury for marine mammals as one that is more likely than not to lead to mortality (NOAA Fisheries, 2014). However, determining the fate of hooked or entangled cetaceans released alive from gear is exceedingly difficult and likely varies with species and gear type (NOAA Fisheries, 2014). Odontocete interactions on pelagic longlines occur quickly and can be dangerous for the crew, providing little opportunity for fishermen or observers to collect identifying information (e.g., dorsal fin photos for photo-identification) or deploy location satellite-linked tags. This reduces the opportunity to collect data on survival outcomes of released whales (NOAA Fisheries, 2014), as has been done for other taxa captured accidentally on longlines such as sea turtles (Swimmer et al., 2014), billfish (Musyl et al., 2015), and sharks (Musyl and Gilman, 2019). In the absence of such empirical information, the criteria NMFS uses to categorize serious vs. non-serious injury for these fleets (NOAA Fisheries, 2014) have been developed almost entirely from expert opinion generated in a technical workshop held in 2007 (Andersen et al., 2008). The guidelines are based on scenarios that would lead directly to a determination of SI for a released marine mammal (e.g., an ingested hook) and have influenced the proposed strategies developed by each Team. For example, fishermen have been encouraged to remove hooks rather than minimizing the extent of trailing fishing line and leaving the hook in the animal. We note the importance of these 2014 guidelines for context, as they have been influential during the development of consensus recommendations by each Team. They also illustrate the challenges of understanding the population-level consequences of non-lethal bycatch which, in the case of the MMPA, provides the legal basis for fisheries management action on marine mammal bycatch. Further research on handling techniques and post-release mortality of odontocetes remains an important priority, so that the impacts of bycatch on cetacean populations can be more fully assessed (NOAA Fisheries, 2014; Zollett and Swimmer, 2019).
The examples presented here are specific to U.S. fisheries management under the auspices of the MMPA, but depredation and bycatch in pelagic longline fisheries are a global challenge. Some international instruments indirectly acknowledge the issue and, in some cases, charge fishing nations to address it. Foundationally, the United Nations Convention on the Law of the Sea obligates signatories to sustainably use and conserve marine living resources on the high seas and minimize impacts to other marine life, among other duties [UNCLOS, 1982; e.g., Articles 61, 192, 194(5)]. Other international instruments and agreements include non-binding measures and suggested guidelines to reduce marine mammal bycatch, such as, the Convention on the Conservation of Migratory Species (CMS) of Wild Animals (e.g., CMS Resolution 12.22 on Bycatch, CMS, 2018), the FAO International Guidelines on Bycatch Management and Reduction of Discards (FAO, 2011), and the draft FAO Technical Guidelines to Reduce Bycatch of Marine Mammals in Capture Fisheries (FAO, 2020). The International Whaling Commission’s recent Bycatch Mitigation Initiative is also currently working to reduce small cetacean bycatch globally. RFMOs also have authority and many have responsibilities to limit fisheries bycatch in their areas of jurisdiction. The tuna-based RFMOs have held joint meetings over the past decade, most recently in December 2019, to address bycatch in their fisheries (Joint t-RFMOs Bycatch Working Group, 2019) and some have adopted conservation and management measures with relevance to reducing cetacean bycatch (Gilman et al., 2014; Juan-Jordá et al., 2018).
Despite these existing frameworks, these case studies represent, to our knowledge, two of the most direct, regulatory attempts to mitigate bycatch of small cetaceans caused by depredation on pelagic longlines. They offer important insights as other management bodies consider implementing their own strategies to reduce depredation and bycatch. They may also be relevant to non-U.S. pelagic longline fleets and in harvesting nations that export fisheries products into U.S. markets. The 2016 MMPA Import Provisions Rule (81 FR 54389) (Federal Register, 2016) requires nations exporting fish and fish products to the U.S. to be held to comparable standards for reducing marine mammal incidental mortality and serious injury in fisheries as those stipulated by U.S. regulations. As countries work to comply with the Import Rule to continue exporting fish and fish products into the U.S., the TRT case studies presented here offer the current U.S. standard in regulatory and consensus-driven management that harvesting nations can consider in their own management.
Looking ahead: Depredation and bycatch are complex issues and will require a careful balance of monitoring, mitigation, and political will to reduce economic losses to fishermen and ameliorate population consequences for odontocetes. No mitigation measure will fully eliminate the problem, but there are a variety of mitigation and regulatory options that other fisheries can consider. We emphasize that, first and most importantly, high-quality observer programs are a crucial part of any mitigation strategy. Unbiased, independent, and representative data on fishing operations, catch, cetacean depredation, and bycatch are essential to accurately understand patterns of interactions and identify potential opportunities for mitigation. When mitigation strategies depend on gear changes and handling techniques, as in the two case studies considered here, such data are critical to ensure compliance across the fleet. It is unrealistic to expect full observer coverage across the world’s pelagic longline fleets, most of which currently operate at 5% observer coverage or lower (Ewell et al., 2020). However, rapidly improving electronic monitoring technologies can fill these gaps in highly capitalized fisheries. Addressing and acknowledging the issue of bycatch, including depredation, in fisheries regulations and incorporating a variety of stakeholder perspectives, will be a step forward for fisheries that encounter depredation. This will help the world’s pelagic longline fisheries reduce the economic cost of depredation and ameliorate the impact of bycatch on small cetaceans.
Author Contributions
JF led overall research, writing, and managing the team of authors. AR and BE contributed to policy summaries and applications. All authors reviewed, edited, and approved the final manuscript and contributed to its development and execution.
Funding
We gratefully acknowledge funding support for JF from the National Oceanic and Atmospheric Association’s (NOAA) Fisheries Bycatch Reduction Engineering Program (BREP) (Grant No. NA17NMF4720261) and the Duke University Graduate School.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We also thank all past and present Take Reduction Team members for their dedication to solving these issues and the fisheries observers who collect the data essential for understanding depredation and bycatch interactions. We thank two reviewers for their constructive feedback and suggestions on the manuscript.
Footnotes
- ^ False Killer Whale Take Reduction–Key outcomes memoranda and summaries. Accessed on 14 September 2020 at https://www.fisheries.noaa.gov/national/marine-mammal-protection/false-killer-whale-take-reduction.
- ^ Pelagic Longline Take Reduction Plan–Key outcomes memoranda and summaries. Accessed on 14 September 2020 at https://www.fisheries.noaa.gov/national/marine-mammal-protection/pelagic-longline-take-reduction-plan.
- ^ Pelagic Longline Take Reduction Team Key Outcomes Memorandum—PLTRT Meeting (January 25–27, 2006). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/70623773.
- ^ Pelagic Longline Take Reduction Team Key Outcomes Memorandum—PLTRT Meeting (August 21–23, 2012). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/70623617.
- ^ Pelagic Longline Take Reduction Team Key Outcomes Memorandum—PLTRT Webinar (June 18, 2014). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/70623614.
- ^ Pelagic Longline Take Reduction Team Key Outcomes Memorandum—PLTRT Webinar (October 31, 2016). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/70618731.
- ^ Pelagic Longline Take Reduction Team Key Outcomes Memorandum—PLTRT Meeting (December 1–3, 2015). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/70618735.
- ^ Pelagic Longline Take Reduction Team Key Outcomes Memorandum—PLTRT Meeting (December 1–3, 2015). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/70618735.
- ^ Pelagic Longline Take Reduction Team Key Outcomes Memorandum—PLTRT Meeting (December 1–3, 2015). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/70618735.
- ^ False Killer Whale Take Reduction Team Key Outcomes Memorandum—FKWTRT Meeting (June 15, 2018). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/83268595.
- ^ False Killer Whale Take Reduction Team Key Outcomes Memorandum—FKWTRT Meeting (June 15, 2018). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/83268595.
- ^ False Killer Whale Take Reduction Plan—NOAA presentation given during 2019 Marine Mammal Commission Meeting. Accessed on 05 January 2021 at. https://www.mmc.gov/wp-content/uploads/False-Killer-Whale-2-GARRETT-2019_05_21_FKWTRP_MMC_final.pdf.
- ^ False Killer Whale Take Reduction Plan—NOAA presentation given during 2019 Marine Mammal Commission Meeting. Accessed on 05 January 2021 at. https://www.mmc.gov/wp-content/uploads/False-Killer-Whale-2-GARRETT-2019_05_21_FKWTRP_MMC_final.pdf.
- ^ False Killer Whale Take Reduction Plan—NOAA presentation given during 2019 Marine Mammal Commission Meeting. Accessed on 05 January 2021 at. https://www.mmc.gov/wp-content/uploads/False-Killer-Whale-2-GARRETT-2019_05_21_FKWTRP_MMC_final.pdf.
- ^ False Killer Whale Take Reduction Team Key Outcomes Memorandum—FKWTRT Meeting (June 15, 2018). Accessed on 05 January 2021 at https://www.fisheries.noaa.gov/webdam/download/83268595.
- ^ False Killer Whale Take Reduction–Key outcomes memoranda and summaries. Accessed on 14 September 2020 at https://www.fisheries.noaa.gov/national/marine-mammal-protection/false-killer-whale-take-reduction.
References
Andersen, M. S., Forney, K. A., Cole, T. V., Eagle, T., Angliss, R., Long, K., et al. (2008). Differentiating Serious and Non-Serious Injury of Marine Mammals: Report of the Serious Injury Technical Workshop, 10-13, September 2007. NOAA Technical Memorandum NMFS-OPR-39. Seattle, WA: U.S. Department Commerce, 94.
Anderson, D., Baird Robin, W., Bradford, A. L., and Oleson, E. (2020). Is it all about the haul? Longline fishery interactions and spatial use by pelagic false killer whales in the central North Pacific. Fish. Res. 230:105665. doi: 10.1016/j.fishres.2020.105665
Angliss, R., and Demaster, D. (1998). Differentiating Serious and Non-Serious Injury of Marine Mammals Taken Incidental to Commercial Fishing Operations: Report of the Serious Injury Workshop 1-2 April 1997. NOAA Technical Memorandum NMFS-OPR-13. Silver Spring, MD: U.S. Department Commerce, 48.
Ashford, J. R., Rubilar, P. S., and Martin, A. R. (1996). Interactions between cetaceans and longline fishery operations around South Georgia. Mar. Mam. Sci. 12, 452–457. doi: 10.1111/j.1748-7692.1996.tb00598.x
Ayers, A. L., and Leong, K. (2020). Stories of Conservation Success: Results of Interviews with Hawai‘i Longline Fishers. NOAA Admin Report H-20-11. Washington, DC: NOAA, 43. doi: 10.25923/6bnn-m598
Baird, R. W. (2018). Pseudorca crassidens. The IUCN Red List of Threatened Species 2018. e.T18596A50371251. doi: 10.2305/IUCN.UK.2018-2.RLTS.T18596A50371251.en
Baird, R. W. (2019). “The perils of relying on handling techniques to reduce bycatch in a partially observed fishery: a potential fatal flaw in the false killer whale take reduction plan,” in Paper Presented at the Document PSRG-2019-14 Presented to the Pacific Scientific Review Group, March 5-7, 2019, (Olympia, WA: Protected Species Research Group).
Baird, R. W., Gorgone, A. M., McSweeney, D. J., Webster, D. L., Salden, D. R., Deakos, M. H., et al. (2008). False killer whales (Pseudorca crassidens) around the main Hawaiian Islands: long−term site fidelity, inter−island movements, and association patterns. Mar. Mam. Sci. 24, 591–612. doi: 10.1111/j.1748-7692.2008.00200.x
Baird, R. W., Oleson, E. M., Barlow, J., Ligon, A. D., Gorgone, A. M., and Mahaffy, S. D. (2013). Evidence of an Island-associated population of false killer whales (Pseudorca crassidens) in the Northwestern Hawaiian Islands. Pac. Sci. 67, 513–521. doi: 10.2984/67.4.2
Bayless, A. R., Oleson, E. M., Baumann-Pickering, S., Simonis, A. E., Marchetti, J., Martin, S., et al. (2017). Acoustically monitoring the Hawai‘i longline fishery for interactions with false killer whales. Fish. Res. 190, 122–131. doi: 10.1016/j.fishres.2017.02.006
Bayse, S. M., and Kerstetter, D. W. (2010). Assessing bycatch reduction potential of variable strength hooks for pilot whales in a western north Atlantic pelagic longline fishery. J. N. C. Acad. Sci. 126, 6–14.
Bigelow, K. A., Kerstetter, D. W., Dancho, M. G., and Marchetti, J. A. (2012). Catch rates with variable strength circle hooks in the Hawaii-based tuna longline fishery. Bull. Mar. Sci. 88, 425–447. doi: 10.5343/bms.2011.1052
Borggaard, D. L., Gouveia, D. M., Colligan, M. A., Merrick, R., Swails, K. S., Asaro, M. J., et al. (2017). Managing U.S. Atlantic large whale entanglements: four guiding principles. Mar. Policy 84, 202–212. doi: 10.1016/j.marpol.2017.06.027
Bradford, A., Oleson, E., Baird, R., Boggs, C., Forney, K., and Young, N. (2015). Revised Stock Boundaries for False Killer Whales (Pseudorca crassidens) in Hawaiian waters. NOAA Technical Memorandum NOAA-TM-NMFS-PIFSC-47. Springfield, MO: U.S. Department of Commerce, 29. doi: 10.7289/V5DF6P6J
Bradford, A. L., Baird, R. W., Mahaffy, S. D., Gorgone, A. M., McSweeney, D. J., Cullins, T., et al. (2018). Abundance estimates for management of endangered false killer whales in the main Hawaiian Islands. Endang. Species Res. 36, 297–313. doi: 10.3354/esr00903
Bradford, A. L., Becker, E. A., Oleson, E. M., Forney, K. A., Moore, J. E., and Barlow, J. (2020). Abundance Estimates of False Killer Whales in Hawaiian Waters and the Broader Central Pacific. NOAA Technical Memorandum NOAA-TM-NMFS-PIFSC-104. Washington, DC: U.S. Department of Commerce, 78. doi: 10.25923/2jjg-p807
Brownell, R. L. Jr., Reeves, R. R., Read, A. J., Smith, B. D., Thomas, P. O., Ralls, K., et al. (2019). Bycatch in gillnet fisheries threatens critically endangered small cetaceans and other aquatic megafauna. Endang. Species Res. 40, 285–296. doi: 10.3354/esr00994
Carretta, J. V., Forney, K. A., Lowry, M. S., Barlow, J., Baker, J., Johnston, D., et al. (2009). Draft US Pacific Marine Mammal Stock Assessments: 2009. NOAA Technical Memorandum NOAA-TM-NMFS-SWFSC-xxx. Washington, DC: U.S. Department of Commerce, 113.
Carretta, J. V., Forney, K. A., Oleson, E. M., Weller, D. W., Lang, A. R., Baker, J., et al. (2019). U.S. Pacific Marine Mammal Stock Assessments: 2018. NOAA Technical Memorandum NOAA-TM-NMFS-SWFSC-617. Washington, DC: U.S. Department of Commerce.
Carretta, J. V., Oleson, E. M., Baker, J. D., Weller, D. W., Lang, A. R., Forney, K. A., et al. (2016). U.S. Pacific Marine Mammal Stock Assessments, 2015. NOAA Technical Memorandum NOAA-TM-NMFS-SWFSC-561. Washington, DC: U.S. Department of Commerce, doi: 10.7289/V5/TM-SWFSC-561
Clarke, S., Sato, M., Small, C., Sullivan, B., Inoue, Y., and Ochi, D. (2014). Bycatch in Longline Fisheries for Tuna and Tuna-like Species: A Global Review of Status and Mitigation Measures. FAO Fisheries and Aquaculture Technical Paper No. 588. Rome: FAO, 199.
CMS (2018). Convention on the Conservation of Migratory Species of Wild Animals. Nairobi: United Nations Environment Programme.
Dunn, D. C., Boustany, A. M., Roberts, J. J., Brazer, E., Sanderson, M., Gardner, B., et al. (2014). Empirical move−on rules to inform fishing strategies: a New England case study. Fish Fish. 15, 359–375. doi: 10.1111/faf.12019
Dunn, D. C., Maxwell, S. M., Boustany, A. M., and Halpin, P. N. (2016). Dynamic ocean management increases the efficiency and efficacy of fisheries management. Proc. Natl. Acad. Sci. 113, 668–673. doi: 10.1073/pnas.1513626113
Esteban, R., Verborgh, P., Gauffier, P., Giménez, J., Guinet, C., and De Stephanis, R. (2016). Dynamics of killer whale, bluefin tuna and human fisheries in the Strait of Gibraltar. Biol. Conserv. 194, 31–38. doi: 10.1016/j.biocon.2015.11.031
Ewell, C., Hocevar, J., Mitchell, E., Snowden, S., and Jacquet, J. (2020). An evaluation of regional fisheries management organization at-sea compliance monitoring and observer programs. Mar. Policy 115:103842. doi: 10.1016/j.marpol.2020.103842
FAO (2011). International Guidelines on Bycatch Management and Reduction of Discards. FAO Fisheries and Aquaculture. Rome: FAO, 73.
FAO (2018). Expert Workshop on Means and Methods for Reducing Marine Mammal Morality in Fishing and Aquaculture Operations, Rome, 20-23 March 2018. FAO Fisheries and Aquaculture, Report No. 1231. Rome: FAO.
FAO (2020). Report of the Expert Meeting to Develop Technical Guidelines to Reduce Bycatch of Marine mAmmals in Capture Fisheries. Rome, Italy 17-19 September 2019. FAO Fisheries and Aquaculture, Report No. 1289. Rome: FAO, doi: 10.4060/CA7620EN
Federal Register (2009). Taking of Marine Mammals Incidental to Commercial Fishing Operations; Atlantic Pelagic Longline Take Reduction Plan. Federal Register 74 FR 23349. Washington, DC: Federal Register, 23349–23358.
Federal Register (2010). Taking of Marine Mammals Incidental to Commercial Fishing Operations; False Killer Whale Take Reduction Plan – Proposed Rule. Federal Register 76 FR 42082. Washington, DC: Federal Register, 42082–42099.
Federal Register (2012). Taking of Marine Mammals Incidental to Commercial Fishing Operations; False Killer Whale Take Reduction Plan Federal Register 77 FR 71259. Washington, DC: Federal Register, 71259–71286.
Federal Register (2016). Fish and Fish Product Import Provisions of the Marine Mammal Protection Act. Federal Register 81 FR 54389. Washington, DC: Federal Register, 54390–54419.
Federal Register (2020). Taking of Marine Mammals Incidental to Commercial Fishing Operations; Amendment to the Atlantic Pelagic Longline Take Reduction Plan. Federal Register 85 FR 81168. Washington, DC: Federal Register, 81168–81175.
Forney, K. A., Kobayashi, D. R., Johnston, D. W., Marchetti, J. A., and Marsik, M. G. (2011). What’s the catch? Patterns of cetacean bycatch and depredation in Hawaii−based pelagic longline fisheries. Mar. Ecol. 32, 380–391. doi: 10.1111/j.1439-0485.2011.00454.x
Garrison, L., and Rosel, P. (2017). Partitioning Short-Finned and Long-Finned Pilot Whale Bycatch Estimates Using Habitat and Genetic Information. Southeast Fisheries Science Center, Protected Resources and Biodiversity Division. 75. Miami.
Garrison, L. P. (2007). Interactions between marine mammals and pelagic longline fishing gear in the US Atlantic Ocean between 1992 and 2004. Fish. Bull. 105, 408–417.
Gilman, E., Brothers, N., McPherson, G., and Dalzell, P. (2007a). A review of cetacean interactions with longline gear. J. Cetacean Res. Manag. 8:215.
Gilman, E., Chaloupka, M., Bach, P., Fennell, H., Hall, M., Musyl, M., et al. (2020). Effect of pelagic longline bait type on species selectivity: a global synthesis of evidence. Rev. Fish Biol. Fish. 30, 535–551. doi: 10.1007/s11160-020-09612-0
Gilman, E., Kobayashi, D., Swenarton, T., Brothers, N., Dalzell, P., and Kinan-Kelly, I. (2007b). Reducing sea turtle interactions in the Hawaii-based longline swordfish fishery. Biol. Conserv. 139, 19–28. doi: 10.1016/j.biocon.2007.06.002
Gilman, E., Kobayashi, D. R., and Chaloupka, M. (2008). Reducing seabird bycatch in the Hawaii longline tuna fishery. Endang. Species Res. 5, 309–323. doi: 10.3354/esr00133
Gilman, E., Passfield, K., and Nakamura, K. (2014). Performance of regional fisheries management organizations: ecosystem-based governance of bycatch and discards. Fish Fish. 15, 327–351. doi: 10.1111/faf.12021
Guinet, C., Tixier, P., Gasco, N., and Duhamel, G. (2015). Long-term studies of Crozet Island killer whales are fundamental to understanding the economic and demographic consequences of their depredation behaviour on the Patagonian toothfish fishery. ICES J. Mar. Sci. 72, 1587–1597. doi: 10.1093/icesjms/fsu221
Hamer, D. J., Childerhouse, S. J., and Gales, N. J. (2012). Odontocete bycatch and depredation in longline fisheries: a review of available literature and of potential solutions. Mar. Mam. Sci. 28, E345–E374. doi: 10.1111/j.1748-7692.2011.00544.x
Hamer, D. J., Childerhouse, S. J., McKinlay, J. P., Double, M. C., and Gales, N. J. (2015). Two devices for mitigating odontocete bycatch and depredation at the hook in tropical pelagic longline fisheries. ICES J. Mar. Sci. 72, 1691–1705. doi: 10.1093/icesjms/fsv013
Hamilton, S., and Baker, G. B. (2019). Technical mitigation to reduce marine mammal bycatch and entanglement in commercial fishing gear: lessons learnt and future directions. Reviews in Fish Biology and Fisheries 29, 223–247. doi: 10.1007/s11160-019-09550-6
Hanselman, D. H., Pyper, B. J., and Peterson, M. J. (2018). Sperm whale depredation on longline surveys and implications for the assessment of Alaska sablefish. Fish. Res. 200, 75–83. doi: 10.1016/j.fishres.2017.12.017
Hayes, S. A., Josephson, E., Maze-Foley, K., and Rosel, P. E. (2017). US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments - 2016. NOAA Tech Memo NMFS NE 241. Washington, DC: NOAA.
Hayes, S. A., Josephson, E., Maze-Foley, K., and Rosel, P. E. (2019). US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments - 2018. NOAA Tech Memo NMFS NE 258. Washington, DC: NOAA.
Hays, G. C., Bailey, H., Bograd, S. J., Bowen, W. D., Campagna, C., Carmichael, R. H., et al. (2019). Translating marine animal tracking data into conservation policy and management. Trends Ecol. Evol. 34, 459–473. doi: 10.1016/j.tree.2019.01.009
Hazen, E. L., Palacios, D. M., Forney, K. A., Howell, E. A., Becker, E., Hoover, A. L., et al. (2017). WhaleWatch: a dynamic management tool for predicting blue whale density in the California Current. J. Appl. Ecol0. 54, 1415–1428. doi: 10.1111/1365-2664.12820
Howell, E. A., Hoover, A., Benson, S. R., Bailey, H., Polovina, J. J., Seminoff, J. A., et al. (2015). Enhancing the TurtleWatch product for leatherback sea turtles, a dynamic habitat model for ecosystem-based management. Fish. Oceanogr. 24, 57–68. doi: 10.1111/fog.12092
Hucke-Gaete, R., Center, B. W., Moreno, C., and Arata, J. (2004). Operational interactions of sperm whales and killer whales with the Patagonian toothfish industrial fishery off southern Chile. CCAMLR Sc. 11, 127–140.
Joint t-RFMOs Bycatch Working Group (2019). Chair’s Report of the 1st Joint Tuna RFMO Bycatch Working Group Meeting (16-18 December 2019, Porto, Portugal). Porto: Joint t-RFMOs Bycatch Working Group, 1–68.
Juan-Jordá, M. J., Murua, H., Arrizabalaga, H., Dulvy, N. K., and Restrepo, V. (2018). Report card on ecosystem-based fisheries management in tuna regional fisheries management organizations. Fish Fish. 19, 321–339. doi: 10.1111/faf.12256
Lewison, R. L., Crowder, L. B., Wallace, B. P., Moore, J. E., Cox, T., Zydelis, R., et al. (2014). Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proc. Natl. Acad. Sci. 111, 5271–5276. doi: 10.1073/pnas.1318960111
McCracken, M. (2019). Evaluation of Potential Fishing Location Bias When an Observer is Aboard a Hawaii Deep-set Longline Trip. NOAA Technical Memorandum NOAA-TM-NMFS-PIFSC-84. Washington, DC: U.S. Department of Commerce, 17. doi: 10.25923/e7qn-6x46
McDonald, S. L., Lewison, R. L., and Read, A. J. (2016). Evaluating the efficacy of environmental legislation: a case study from the US marine mammal take reduction planning process. Glob. Ecol. Conserv. 5, 1–11. doi: 10.1016/j.gecco.2015.11.009
McDonald, S. L., and Rigling-Gallagher, D. (2015). Participant perceptions of consensus-based, marine mammal take reduction planning. Mar. Policy 61, 216–226. doi: 10.1016/j.marpol.2015.08.004
McLellan, W. A., Arthur, L. H., Mallette, S. D., Thornton, S. W., McAlarney, R. J., Read, A. J., et al. (2015). Longline hook testing in the mouths of pelagic odontocetes. ICES J. Mar. Sci. 72, 1706–1713. doi: 10.1093/icesjms/fsu181
Mitchell, E. (1975). Review of biology and fisheries for smaller cetaceans. J. Fish. Res. Board Canada 32, 889–983.
Mooney, T. A., Pacini, A., and Nachtigall, P. E. (2009). False killer whale (Pseudorca crassidens) echolocation and acoustic disruption: implications for longline bycatch and depredation. Can. J. Zool. 87, 726–733. doi: 10.1139/Z09-061
Moreno, C. A., Castro, R., Mújica, L. J., and Reyes, P. (2008). Significant conservation benefits obtained from the use of a new fishing gear in the Chilean Patagonian toothfish fishery. CCAMLR Sci. 15, 79–91.
Musyl, M. K., and Gilman, E. L. (2019). Meta-analysis of post-release fishing mortality in apex predatory pelagic sharks and white marlin. Fish Fish. 20, 466–500. doi: 10.1111/faf.12358
Musyl, M. K., Moyes, C. D., Brill, R. W., Mourato, B. L., West, A., McNaughton, L. M., et al. (2015). Postrelease mortality in istiophorid billfish. Can. J. Fish. Aquat. Sci. 72, 538–556. doi: 10.1139/cjfas-2014-0323
NOAA Fisheries (2014). Process for Distinguishing Serious From Non-Serious Injury of Marine Mammals: Process for Injury Determinations. National Marine Fisheries Service Instruction 02-038-01. January 2012. 4. Available online at: https://www.fisheries.noaa.gov/webdam/download/64690368 (accessed December, 17 2020).
Oleson, E. M. (2020). Abundance, Potential Biological Removal, and Bycatch Estimates for the Hawaii pelagic Stock of False Killer Whales for 2015–2019. NOAA PIFSC Admin Rep. H-20-06. Washington, DC: NOAA, 13. doi: 10.25923/wmg3-ps37
Peterson, M. J., and Hanselman, D. (2017). Sablefish mortality associated with whale depredation in Alaska. ICES J. Mar. Sci. 74, 1382–1394. doi: 10.1093/icesjms/fsw239
Peterson, M. J., Mueter, F., Criddle, K., and Haynie, A. C. (2014). Killer whale depredation and associated costs to Alaskan sablefish, Pacific halibut and Greenland turbot longliners. PLoS One 9:e88906. doi: 10.1371/journal.pone.0088906
Peterson, M. J., Mueter, F., Hanselman, D., Lunsford, C., Matkin, C., and Fearnbach, H. (2013). Killer whale (Orcinus orca) depredation effects on catch rates of six groundfish species: implications for commercial longline fisheries in Alaska. ICES J. Mar. Sci. 70, 1220–1232. doi: 10.1093/icesjms/fst045
Poncelet, É, Barbraud, C., and Guinet, C. (2010). Population dynamics of killer whales in Crozet Archipelago, southern Indian Ocean: a mark-recapture study from 1977 to 2002. J. Cetacean Res. Manag. 11, 41–48.
Punt, A. E., Moreno, P., Brandon, J. R., and Mathews, M. A. (2018). Conserving and recovering vulnerable marine species: a comprehensive evaluation of the US approach for marine mammals. ICES J. Mar. Sci. 75, 1813–1831. doi: 10.1093/icesjms/fsy049
Rabearisoa, N., Bach, P., and Marsac, F. (2015). Assessing interactions between dolphins and small pelagic fish on branchline to design a depredation mitigation device in pelagic longline fisheries. ICES J. Mar. Sci. 72, 1682–1690. doi: 10.1093/icesjms/fsu252
Rabearisoa, N., Bach, P., Tixier, P., and Guinet, C. (2012). Pelagic longline fishing trials to shape a mitigation device of the depredation by toothed whales. J. Exp. Mar. Biol. Ecol. 432-433, 55–63. doi: 10.1016/j.jembe.2012.07.004
Read, A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mammal. 89, 541–548. doi: 10.1644/07-MAMM-S-315R1.1
Read, A. J., Drinker, P., and Northridge, S. (2006). Bycatch of marine mammals in US and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Reeves, R. R., McClellan, K., and Werner, T. B. (2013). Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endang. Species Res. 20, 71–97. doi: 10.3354/esr00481
Richard, G., Bonnel, J., Tixier, P., Arnould, J. P. Y., Janc, A., and Guinet, C. (2020). Evidence of deep-sea interactions between toothed whales and longlines. Ambio 49, 173–186. doi: 10.1007/s13280-019-01182-1
Roche, C., Guinet, C., Gasco, N., and Duhamel, G. (2007). Marine mammals and demersal longline fishery interactions in Crozet and Kerguelen exclusive economic zones: an assessment of depredation levels. CCAMLR Sci. 14, 67–82.
Roheim, C. A., Bush, S. R., Asche, F., Sanchirico, J. N., and Uchida, H. (2018). Evolution and future of the sustainable seafood market. Nat. Sustain. 1, 392–398. doi: 10.1038/s41893-018-0115-z
Sigler, M. F., Lunsford, C. R., Straley, J. M., and Liddle, J. B. (2008). Sperm whale depredation of sablefish longline gear in the northeast Pacific Ocean. Mar. Mam. Sci. 24, 16–27. doi: 10.1111/j.1748-7692.2007.00149.x
Sivasubramaniam, K. (1964). Predation of tuna longline catches in the Indian Ocean, by killer-whales and sharks. Bull. Fish. Res. Station Ceylon 17, 221–236.
Stahl, J. P., and Carnes, M. J. (2020). Detection Accuracy in the Hawai’i Longline Electronic Monitoring Program with Comparisons between Three Video Review Speeds. PIFSC Data Report DR-20-012. Washington, DC: NOAA, doi: 10.25923/n1gq-m468
Stepanuk, J. E. F., Read, A. J., Baird, R. W., Webster, D. L., and Thorne, L. H. (2018). Spatiotemporal patterns of overlap between short-finned pilot whales and the U.S. pelagic longline fishery in the Mid-Atlantic Bight: an assessment to inform the management of fisheries bycatch. Fish. Res. 208, 309–320. doi: 10.1016/j.fishres.2018.07.008
Straley, J., O’Connell, V., Liddle, J., Thode, A., Wild, L., Behnken, L., et al. (2015). Southeast Alaska sperm whale avoidance project (SEASWAP): a successful collaboration among scientists and industry to study depredation in Alaskan waters. ICES J. Mar. Sci. 72, 1598–1609. doi: 10.1093/icesjms/fsv090
Swimmer, Y., Empey Campora, C., Mcnaughton, L., Musyl, M., and Parga, M. (2014). Post-release mortality estimates of loggerhead sea turtles (Caretta caretta) caught in pelagic longline fisheries based on satellite data and hooking location. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 498–510. doi: 10.1002/aqc.2396
Swimmer, Y., Zollett, E. A., and Gutierrez, A. (2020). Bycatch mitigation of protected and threatened species in tuna purse seine and longline fisheries. Endang. Species Res. 43, 517–542. doi: 10.3354/esr01069
Thode, A., Straley, J., Tiemann, C. O., Folkert, K., and O’Connell, V. (2007). Observations of potential acoustic cues that attract sperm whales to longline fishing in the Gulf of Alaska. J. Acoust. Soc. Am. 122, 1265–1277. doi: 10.1121/1.2749450
Thode, A., Wild, L., Straley, J., Barnes, D., Bayless, A., O’Connell, V., et al. (2016). Using line acceleration to measure false killer whale (Pseudorca crassidens) click and whistle source levels during pelagic longline depredation. J. Acoust. Soc. Am. 140, 3941–3951. doi: 10.1121/1.4966625
Thorne, L. H., Baird, R. W., Webster, D. L., Stepanuk, J. E., and Read, A. J. (2019). Predicting fisheries bycatch: a case study and field test for pilot whales in a pelagic longline fishery. Divers. Distrib. 25, 909–923. doi: 10.1111/ddi.12912
Thorne, L. H., Foley, H. J., Baird, R. W., Webster, D. L., Swaim, Z. T., and Read, A. J. (2017). Movement and foraging behavior of short-finned pilot whales in the Mid-Atlantic Bight: importance of bathymetric features and implications for management. Mar. Ecol. Prog. Ser. 584, 245–257. doi: 10.3354/meps12371
Tixier, P., Authier, M., Gasco, N., and Guinet, C. (2015a). Influence of artificial food provisioning from fisheries on killer whale reproductive output. Animal Conserv. 18, 207–218. doi: 10.1111/acv.12161
Tixier, P., Barbraud, C., Pardo, D., Gasco, N., Duhamel, G., and Guinet, C. (2017). Demographic consequences of fisheries interaction within a killer whale (Orcinus orca) population. Mar. Biol. 164:170. doi: 10.1007/s00227-017-3195-9
Tixier, P., Burch, P., Massiot-Granier, F., Ziegler, P., Welsford, D., Lea, M.-A., et al. (2020a). Assessing the impact of toothed whale depredation on socio-ecosystems and fishery management in wide-ranging subantarctic fisheries. Rev. Fish Biol. Fish. 30, 203–217. doi: 10.1007/s11160-020-09597-w
Tixier, P., Gasco, N., Duhamel, G., and Guinet, C. (2015b). Habituation to an acoustic harassment device (AHD) by killer whales depredating demersal longlines. ICES J. Mar. Sci. 72, 1673–1681. doi: 10.1093/icesjms/fsu166
Tixier, P., Giménez, J., Reisinger, R. R., Méndez-Fernandez, P., Arnould, J. P. Y., Cherel, Y., et al. (2019). Importance of toothfish in the diet of generalist subantarctic killer whales: implications for fisheries interactions. Mar. Ecol. Prog. Ser. 613, 197–210. doi: 10.3354/meps12894
Tixier, P., Lea, M.-A., Hindell, M. A., Welsford, D., Mazé, C., Gourguet, S., et al. (2020b). When large marine predators feed on fisheries catches: global patterns of the depredation conflict and directions for coexistence. Fish Fish. 22, 31–53. doi: 10.1111/faf.12504
Tixier, P., Vacquie Garcia, J., Gasco, N., Duhamel, G., and Guinet, C. (2015c). Mitigating killer whale depredation on demersal longline fisheries by changing fishing practices. ICES J. Mar. Sci. 72, 1610–1620. doi: 10.1093/icesjms/fsu137
Wade, P. R. (1998). Calculating limits to the allowable human−caused mortality of cetaceans and pinnipeds. Mar. Mam. Sci. 14, 1–37. doi: 10.1111/j.1748-7692.1998.tb00688.x
Walter, J. F. (2017). Update of standardized catch rates of large bluefin tuna (Thunnus thynnus) from the U.S. pelagic longline fishery in the Gulf of Mexico 1987-2016. Collect. Vol. Sci. Pap. ICCAT 73, 2515–2527.
Ward, P., and Hindmarsh, S. (2007). An overview of historical changes in the fishing gear and practices of pelagic longliners, with particular reference to Japan’s Pacific fleet. Rev. Fish Biol. Fish. 17, 501–516. doi: 10.1007/s11160-007-9051-0
Waring, G. T., Quintal, J. M., Fairfield, C. P., Clapham, P., Cole, T., Garrison, L., et al. (2002). US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2000. NOAA Technical Memorandum NMFS-NE-162. Washington, DC: U.S. Department of Commerce.
Watson, J., and Kerstetter, D. (2006). Pelagic longline fishing gear: a brief history and review of research efforts to improve selectivity. Mar. Technol. Soc. J. 40, 6–11. doi: 10.4031/002533206787353259
Werner, T. B., Northridge, S., Press, K. M., and Young, N. (2015). Mitigating bycatch and depredation of marine mammals in longline fisheries. ICES J. Mar. Sci. 72, 1576–1586. doi: 10.1093/icesjms/fsv092
Woodworth-Jefcoats, P. A., Polovina, J. J., and Drazen, J. C. (2018). Synergy among oceanographic variability, fishery expansion, and longline catch composition in the central North Pacific Ocean. Fish. Bull. 116, 228–239. doi: 10.7755/FB.116.3.2
WPRFMC (2020). Annual Stock Assessment and Fishery Evaluation Report Pacific Island Pelagic Fishery Ecosystem Plan 2019, eds T. Remington, M. Fitchett, A. Ishizaki, and J. DeMello (Honolulu, HI: Western Pacific Regional Fishery Management Council), 372.
Keywords: bycatch, depredation, odontocetes, conservation, Take Reduction Teams, Marine Mammal Protection Act
Citation: Fader JE, Elliott BW and Read AJ (2021) The Challenges of Managing Depredation and Bycatch of Toothed Whales in Pelagic Longline Fisheries: Two U.S. Case Studies. Front. Mar. Sci. 8:618031. doi: 10.3389/fmars.2021.618031
Received: 16 October 2020; Accepted: 01 February 2021;
Published: 26 February 2021.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Yonat B. Swimmer, Pacific Islands Fisheries Science Center (NOAA), United StatesLance Garrison, National Oceanic and Atmospheric Administration (NOAA), United States
Copyright © 2021 Fader, Elliott and Read. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph E. Fader, am9lZmFkZXJAZ21haWwuY29t
 Joseph E. Fader
Joseph E. Fader Brianna W. Elliott
Brianna W. Elliott Andrew J. Read
Andrew J. Read