
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 21 April 2021
Sec. Marine Megafauna
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.606876
This article is part of the Research Topic Small Cetacean Conservation: Current Challenges and Opportunities View all 51 articles
Killer whale (Orcinus orca) populations specialize in both prey and prey acquisition tactics around the world and may be a primary evolutionary driver of the habits of small cetaceans. Entanglement in fishing gear is the most significant anthropogenic threat to the survival of cetaceans worldwide. Distinguishing between natural and human-caused sources of mortality and injury is a key task in marine mammal conservation and management. In British Columbia (BC), Canada, mammal-eating killer whales co-occur with Pacific white-sided dolphins (Lagenorhynchus obliquidens). Bycatch mortality rates are unknown here due to lack of systematic fisheries observer coverage. Drawing from more than three decades of first-hand observations of killer whale attacks on Pacific white-sided dolphins, we identify common themes with respect to predatory behavior of killer whales and anti-predatory responses of dolphins. With input from veterinary pathologists, we outline clues to distinguish killer whale rake marks from scars and wounds likely to be caused by fishery interactions. We examined photographs of 415 well-marked Pacific white-side dolphins for evidence of injuries and scars consistent with either killer whale attacks or fishery interactions. In this case study, healed scars from interactions with killer whale predators were ∼8× more common than scars from fishery interactions (3.9 vs. 0.5%), suggesting that predation is a much bigger threat to Pacific white-sided dolphins in the study area than anthropogenic impacts, or that dolphins are much less likely to survive a fishery interaction than a predation attempt. To advance our knowledge on poorly studied species, multiple lines of evidence will be needed.
Killer whale (Orcinus orca) populations specialize in both prey and prey acquisition tactics around the world, with prey ranging from fish and seabirds to great whales. Killer whales prey on more than 20 cetacean species (Jefferson et al., 1991). One small cetacean prey species of mammal-eating killer whales in the North Pacific Ocean is the Pacific white-sided dolphin [Lagenorhynchus obliquidens, but see Vollmer et al. (2019)]. Killer whale populations have developed an array of foraging tactics to exploit particular prey species [e.g., carousel feeding on herring (Clupea harengus) in Norway (Simila and Ugarte, 1991), intentional stranding to capture seals (Lopez and Lopez, 1985; Guinet, 1991), and “wave-washing” seals off ice floes (Visser et al., 2008; Pitman and Durban, 2012)]. These foraging tactics may be a primary evolutionary driver of habitat use and behavior of many marine small cetaceans, including their seasonal movement patterns and acoustic repertoires (Morisaka and Connor, 2007; Srinivasan and Markowitz, 2010; Kyhn et al., 2013).
Two killer whale ecotypes are seen frequently in North Pacific coastal waters: a fish-eating, “resident” ecotype that feeds primarily on large salmon (Oncorhynchus spp.); and a mammal-eating, Bigg’s (or “transient”) ecotype (Morton, 1990; Baird and Dill, 1995; Ford et al., 1998, 2000; Saulitis et al., 2000). The Bigg’s ecotype most often feeds on harbor seals (Phoca vitulina), but other pinnipeds and small cetaceans are also regular prey items (Jefferson et al., 1991; Ford et al., 1998). Pacific white-sided dolphins were rarely reported as prey of Bigg’s killer whales in diet studies conducted in the 1970s, 1980s, and early 1990s (Dahlheim and Towell, 1994; Ford et al., 1998; Ford and Ellis, 1999; Saulitis et al., 2000; Wade et al., 2007). Predation on Pacific white-sided dolphins was observed once in southeast Alaska between 1991 and 2007 (Dahlheim and White, 2010), In British Columbia (BC), Canada, only one Pacific white-sided dolphin kill was reported between 1973 and 1996, with 14 more events observed between 1996 and 2011 (Ford et al., 1998, 2013). However, few details have been published on this predator-prey relationship. Improving our knowledge of the diets of killer whale ecotypes is critical to understand the role predators play in communities, to explore and mitigate conservation conflicts among predator, prey, and fisheries, and to inform resource management programs that are robust to ecosystem considerations (Chasco et al., 2017).
The Pacific white-sided dolphin is considered a “pelagic” species, which we use in the colloquial sense of a species whose distribution extends beyond the continental shelf (Leatherwood et al., 1984). The dolphins began to seen regularly in western Canadian (BC) inshore waters around 1984 (Morton, 2000). This gradual movement toward inshore waters was concurrent with a climate-driven northward shift in distribution at the southern extent of this species range (Salvadeo et al., 2010). Occurrence in BC inshore waters appears to represent a re-colonization of this habitat rather than an unprecedented distribution shift. Evidence from First Nations’ archeological middens in the area show that Pacific white-sided dolphins have been present for thousands of years (Morton, 2000). The dolphins use this inshore habitat in part to exploit predictable prey sources (Heise, 1996), but finding herring and other fish in this complex network of narrow fjords and bays carries risk of predation, and also puts the dolphins in closer proximity to coastal gillnet fisheries that are concentrated on the continental shelf.
Pacific white-sided dolphins have few natural predators. Only one published record of a recovered carcass from the Oregon coast reports injuries consistent with an attack from an unknown shark species (Stroud and Roffe, 1979). Apart from sharks, killer whales are the only known (non-human) predator of Pacific white-sided dolphins. An increase in relative abundance of Pacific white-sided dolphins in inshore waters of BC (Heise, 1996; Morton, 2000) and Alaska (Dahlheim and Towell, 1994) was observed in the years following their initial observed reappearance in 1984 to inshore BC waters. In 2004–2005, when the first systematic line transect surveys were conducted in BC, an estimated 25,900 (95% CI: 12,900–52,100) Pacific white-sided dolphins were found in BC’s continental shelf waters during summer months (Williams and Thomas, 2007). The first record of killer whale predation on Pacific white-sided dolphins was made in Alaska in 1992 (Dahlheim and Towell, 1994). One of us (A.M.) has studied Pacific white-sided dolphins in the region since 1984 and has never observed a successful predation event, but has recorded second-hand reports (included here). Ford et al. (1998) note four “predation events” involving Pacific white-sided dolphins that were recorded as three harassment events and one kill out of 166 total observed predation events of marine mammals in BC from 1973 to 1996.
Pacific white-sided dolphins are listed as “Least Concern” by IUCN “not at risk” in Canada (Stacey and Baird, 1991), and not considered “endangered,” “threatened,” or “depleted” under the United States MMPA (Carretta et al., 2016). Although bycatch is currently not considered a major threat to Pacific white-sided dolphins, historically, it was a major problem (Carretta et al., 2016). It is estimated that the high-seas driftnet fishery for squid and salmon killed hundreds of thousands of Pacific white-sided dolphins during the 1970s and 1980s (Hobbs and Jones, 1993). A moratorium on the fishery was called for by the United Nations in 1991 and implemented in 1992. However, some illegal driftnet fishing on the high seas continues today in the North Pacific (Carretta et al., 2016). The impact of anthropogenic threats is difficult to interpret in light of uncertainty in abundance estimates and lack of information on stock structure. In BC, bycatch of Pacific white-sided dolphins in is thought to be very low, however like many pelagic dolphins, with the notable exception of those subject to bycatch in Eastern Tropical Pacific tuna fisheries, Pacific white-sided dolphins are often overlooked in monitoring programs (Taylor et al., 2007; Williams et al., 2008). Although incidental take from salmon gillnet fishing is rare (Williams et al., 2008), the likelihood of surviving an entanglement in gillnets is also low (Baird et al., 1991).
In the absence of direct efforts (e.g., estimating bycatch mortality rate during on-board fisheries observer programs), indirect evidence can be used to make inference about fisheries interactions. Photo-identification data from live animals have been used successfully to make inference about exposure of those individuals to cryptic events, such as historic entanglement in fishing gear (e.g., humpback whales; Robbins, 2007); intra-specific (male-male) aggression (Scott et al., 2005), previous ship strikes (George et al., 1994); and killer whale attacks (e.g., humpback whales; McCordic et al., 2014).
Here we report direct observations of killer whale attacks (successful and unsuccessful) on Pacific white-sided dolphins, in order to explore patterns in predatory behavior of killer whales and anti-predatory responses of dolphins. Previous analyses of cryptic sources of anthropogenic and natural mortality in marine ecosystems have investigated natural predation (George et al., 1994; Williams et al., 2004; Steiger et al., 2008), ship strikes (George et al., 1994; Knowlton and Kraus, 2001), and bycatch in unmonitored fisheries (Knowlton and Kraus, 2001; Williams et al., 2008; Moore et al., 2013). We considered two lines of evidence to assess the relationship between predatory killer whales and Pacific white-sided dolphins in BC. First, we collected details of predation attempts and events from our own field studies as well as interviews with mariners. Secondly, we examined photographs of well-marked individuals for evidence of injuries and scars that are consistent with killer whale rake marks to assess the rate at which Pacific white-sided dolphins accumulate scars from killer whale attacks.
Our own observations of killer whales attacking Pacific white-sided dolphins were compiled. In addition, local researchers, mariners, whale-watch operators, tour guides, and fisheries observers were interviewed about their observations of killer whale predation and predation attempts on Pacific white-sided dolphins in BC. Responses to a list of standard questions were gathered using online surveys, telephone interviews, and email correspondence. The goal was to compile as many opportunistic observations as possible of such predation events, and to evaluate these first-hand accounts for commonalities with respect to physical setting, group size, predatory behavior and anti-predatory tactics (behavioral responses) of the prey species. Although such observations could, in theory, provide a bare minimum estimate of the frequency of occurrence of attacks, no information was available to quantify the degree of underreporting, and therefore these observations were not intended to provide a mortality rate.
The mean proportion of observations of Bigg’s killer whale attacks involving dolphins was estimated via an intercept-only binomial GLM.
Here, as part of a long-term photo-identification study of well-marked individual dolphins, photographs were assessed for evidence of fresh wounds (“wounds”) or healed wounds (“scars”) from killer whale teeth. Other photos included evidence of scars from unknown sources that could be mistaken superficially for killer whale scars. We examined high-quality photographs of 415 marked individuals in a digital photo-identification catalog collected between 2008 and 2013 to estimate the proportion of killer whale rake mark scars in the population. This included multiple photographs, where available, of the 415 individuals in the study, because photos from multiple angles and both sides are often informative.
Recommendations have been made on the need for objective criteria for attributing an anthropogenic cause to marine mammal mortality or morbidity cases (Moore et al., 2013). We thus sought feedback from experienced marine mammal stranding coordinators and veterinary pathologists to assess whether a sample from these photographs were indeed consistent with killer whale rake marks, and to distinguish between fresh wounds and healed scars. We selected a sample of 22 high-quality photos of individual dolphins with injuries that included missing and mangled dorsal fins, parallel scars on the dorsal fin and/or body, and non-parallel or random scars on the body.
Based on feedback from the veterinary pathologists and marine mammal stranding coordinators, we developed the following criteria to inform a judgment about the likelihood that scars in the entire catalog could be attributed to killer whales. Scars were attributed to killer whale teeth when three or more parallel marks were found in close proximity (Steiger et al., 2008) that were “highly structured,” i.e., “long, thin, and parallel” (George et al., 1994), but spaced sufficiently far apart that experts felt confident that they were caused by killer whales and not conspecifics. Teeth marks from other predators were ruled out, based on the assumption that shark wounds are characterized by numerous penetrations in an oval configuration and by jagged serrations (Brodie and Beck, 1983; Riedman, 1990).
Seven scientists with expertise in marine mammal injury assessment evaluated the photographs. A survey template was used to guide responses via a web-based interview/questionnaire site, and each respondent was asked to gauge whether they considered the wounds in a given picture had been caused by killer whales, other dolphins, boat propellers, fishing nets, or another source of injury (called “injury” sample photographs). Participants were also given a control set of 14 photographs showing no injuries (called “control” sample photographs). Multiple-choice questions asked respondents to assign the injuries shown in each photograph to one of the three causes in Figure 1, but to skip any photographs they felt they could not interpret reliably. The survey response section for each photograph included space for free-form comments. The feedback from the survey on the 22 injury-sample photographs was used to inform our decision-making when examining the marked individuals in the digital photo-identification catalog.

Figure 1. Examples of the three types of photos included in the marine mammal injury expert survey: injuries thought to be caused by (1) killer whales, (2) human activities, and (3) unknown origin. Responses from experts were used to guide subsequent categorization of injuries for the well-marked Pacific white-sided dolphins in the complete digital photo-ID catalog.
Forty-five first-hand observations of Bigg’s killer whales chasing and/or attacking Pacific white-sided dolphins were documented (summarized in Table 1, all reports in Supplementary Appendix 1). The majority (69%) of the predation events took place in confined waters, either a shallow bay or a narrow passageway, with eight respondents indicating that the killer whales used the local topography to actively herd the dolphins into a place where they could be killed easily. The anti-predatory tactics used by the dolphins generally started with high-speed chases (“squall”) and, when that failed, eight respondents noted that the dolphins entered shallow waters to avoid predation, sometimes beaching themselves on shore in the process. Squalling is a term used locally (including by observers responding to our survey) to describe a sudden behavioral response to the threat of a killer whale attack. The dolphins quickly form a tight group, often in a line, and travel at very high-speed. The grouping and high-speed travel creates a great deal of turbulence on the surface of the water resembling a “squall” (Ashe, 2015). In three cases, there was evidence for incidental mortality—i.e., dolphins those died during escape, but were not consumed by the whales.
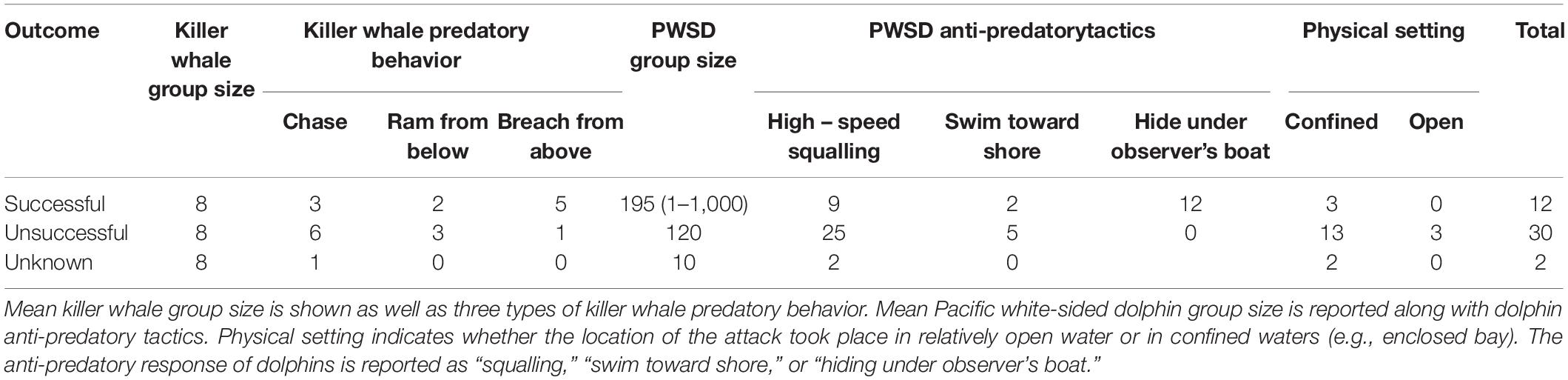
Table 1. A summary of records killer whale predation attempts on Pacific white-sided dolphins in the region.
A total of 12 mariners responded to requests for information. Based on records kept by nine of the 12 mariners, 24 attacks on dolphins were observed, collectively, out of the 252 observed attacks or presumed predation attempts witnessed by those observers on all other marine mammal species (Table 2). The mean proportion of Bigg’s killer whale attacks that they observed to include dolphins was 0.095 (95% CI: 0.061, 0.142).
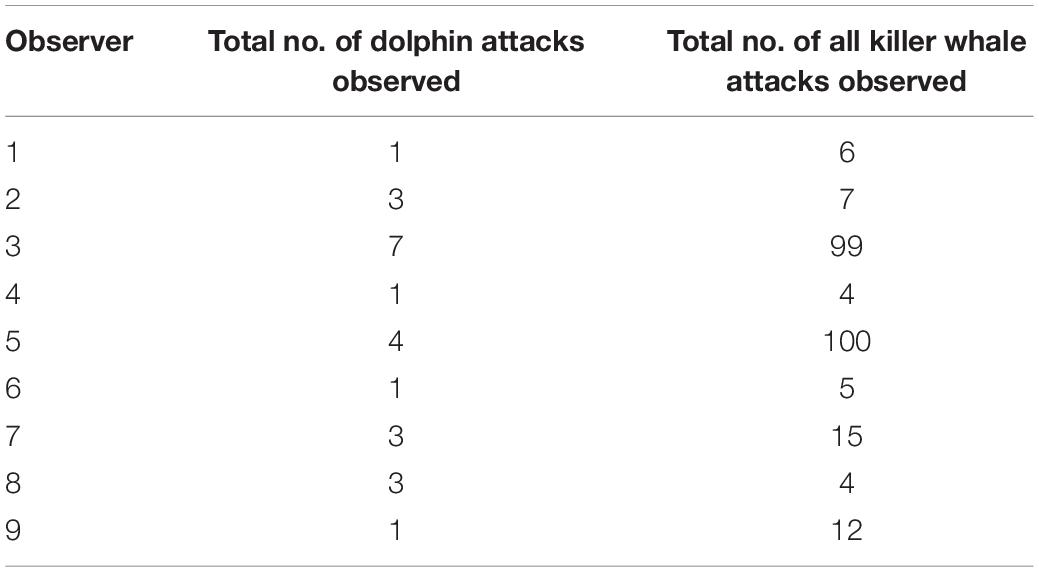
Table 2. Results from interviews from nine observers who kept records of the total number of killer whale attacks on dolphins compared to total number of killer whale attacks on all marine mammal species observed.
Several commonalities emerge in observers’ reports of killer whale and dolphin behavior. Common killer whale hunting tactics include: dividing a large group of dolphins into subsequently smaller groups; focusing on a small group; using the shoreline and the seabed as tools to help corral/herd the dolphins; and ramming the dolphins either from below the surface and/or launching the dolphin into the air and plunging down on top of the dolphin. In nearly all interviews, observers noted that they did not notice the killer whales, or that the dolphins appeared not to have noticed the killer whales, prior to the attack. However, in one report, apparently naïve dolphins approached a group of killer whales before the killer whales attacked the dolphin group. In some cases, the killer whales abandoned chase altogether or appeared to have had no intention of consumption. One mariner reported a killer whale ripping a small piece of flesh from a dolphin head and leaving the area without eating the dolphin.
There are also common patterns observed among anti-predatory responses of dolphins. The dolphins often traveled at extremely high speeds (as evidenced from the splashing water and directional, leaping behavior of the group, locally termed “squalling”) to escape. If trapped in enclosed waters, the dolphins headed to shallow waters intentionally to escape the whales or accidentally. In one case, the observer watched the dolphins attempt escape up a small creek. In some cases, dolphins beached themselves in the ensuing panic. One mariner we interviewed found 22 dolphins stranded on a beach where two killer whale attacks had occurred previously (Kent Inlet, Table 1). The mariner was convinced that the stranding must have been the result of a killer whale attack (and some dolphins possessed what looked to be fresh killer whale rake marks), but they did not witness the attack. Because no one witnessed the cause of the beaching event, we do not include this observation in our tables, but this does support our contention that maladaptive avoidance tactics on the part of the dolphins could result in incidental mortality. More recently, 16 dolphins were discovered on or near a beach in a fish trap originally constructed by Tla’amin First Nation near Powell River, BC in what appeared to be an attempt to avoid predation from mammal-eating killer whales (a group identified as the T90s). The event was filmed by several members of the public and widely reported in the media1.
In the expert evaluation, for most photos, the respondents indicated that the injuries were of unknown origin (Table 3). Two of the photos were thought to show injuries inflicted by killer whales, two from conspecific interactions, and the rest were divided among unknown, fishing, and conspecific interactions. Experts concurred with our opinion in all cases where we originally classified as killer whale injuries. Experts were uncertain of the cause of injury in 13 (59%) of 22 cases and also left no response when uncertain, 16 were found to have scars or injuries that were consistent with killer whale rake marks (e.g., top of Figure 1), and only two had marks consistent with fishing activities. This translates to 3.9% of the marked individuals in the study showing evidence of having survived a killer whale attack and 0.5% of the marked dolphins showing evidence of interaction with fishing gear at some point during their lifetime.

Table 3. Summary of responses from seven experts asked to assess 22 sample photographs of scarred Pacific white-sided dolphins.
Pacific white-sided dolphins appear to be a more commonly targeted prey item than previously thought for mammal-eating killer whales since their re-colonization of inshore, coastal waters in the late 1980s. A comprehensive review of the diet of mammal-eating killer whales from 1973–1996 reported that only 0.8% (i.e., one out of 122) of observed kills by mammal-eating killer whales involved Pacific white-sided dolphins (Ford et al., 1998). A subsequent update on that dietary study, including data from 1990 to 2011, reported that 4.6% (i.e., 15 out of 328) of observed predation events involved Pacific white-sided dolphins (Ford et al., 2013). Here we show that 3.9% of dolphins show some evidence (fresh wounds or scars) consistent with having survived a killer whale attack at some point in their lives. These conclusions are supported by direct and indirect lines of evidence. The reports from mariners are valuable not only because killer whale predation attempts on Pacific white-sided dolphins are not always reported in the literature, but also because the observations can provide additional information on the behavior of both killer whales and dolphins during an attack. Our interviews revealed that the mean proportion of Bigg’s killer whale attacks observed to include dolphins was 0.095 (95% CI: 0.061, 0.142). This proportion is roughly twice as frequent as the proportion of observed predation events 1990–2011 (Ford et al., 2013). Our estimate of 9.5% of predation events involving Pacific white-sided dolphins is roughly four times the proportion of attacks and 10 times the proportion of observed kills reported in the period 1973–1996 [i.e., four out of 166 events, 0.024 (Ford et al., 1998)] or observed kills [i.e., one out of 122 kills, or 0.008 (Ford et al., 1998)].
A proportion of scarred individuals of 3.9% is low compared to other studies of small cetaceans. In Shark Bay, Australia, 74.9% of the individual bottlenose dolphins (Tursiops truncatus) assessed for shark bites, show evidence of healed shark bite wounds (Heithaus, 2001). Similarly, Jeremy et al. (2008) found a higher proportion of animals with scars in Indo-Pacific bottlenose dolphins (Tursiops aduncus, 19%) and melon-headed whales (Peponocephala electra, 56.3%) in the Indian Ocean, but that study pooled injuries across human, conspecific, and predator interactions. There are several ways to interpret this 3.9% scar rate consistent with healed wounds caused by killer whales. Killer whales may be very efficient predators of Pacific white-sided dolphins (i.e., few dolphins survive attacks), killer whale attacks may be rare, or scars may be on parts of the body that are inaccessible to our photo-ID studies.
The proportion of scars observed raises important new questions and hypotheses about sociality in this poorly studied species that we wish to explore in future studies. The survivors of killer whale attacks may obtain information about the attacks and communicate this information to the rest of the group (Dill, 1983). Similarly, dolphins may engage in predator inspection, a somewhat counter-intuitive response to a potential predator (FitzGibbon, 1994; Fishman, 1999). Pacific white-sided dolphins are regularly observed swimming alongside salmon-eating (resident) killer whales and have been observed interacting with shark-eating (offshore) killer whales (Morton, 2004). Each of these killer whale ecotypes possesses distinct, stereotypical vocal repertoires (Ford et al., 2000). The dolphins may be able to distinguish acoustically among the ecotypes. Experiments have demonstrated that wild harbor seals respond differently when exposed to fish-eating and mammal-eating killer whale vocalizations, suggesting the seals are able to discriminate between killer whales that pose predation threat and those that pose little or no risk at all (Deecke et al., 2002). In addition, dolphins have been reported to take refuge by “hiding” among fish-eating killer whales following an attack from mammal-eating killer whales (Jared Towers, 2019). While the fish-eating killer whales do not pose a threat, inspection may allow dolphins to acquire information about the predator, such as size and agility, the energetic cost of fleeing from predators, or a risk-free opportunity to teach younger animals about predators (FitzGibbon, 1994; Fishman, 1999).
Vigilance behavior requires exclusive attention, however, and may come at the expense of other activities such as time spent feeding (Cowlishaw et al., 2004). Dolphins are constantly making decisions about where to travel, when to rest and where to forage: choosing one behavior from a set of alternative behaviors involves weighing risks and trade-offs (Dill, 1987). When mammal-eating killer whales are present, and dolphins are at risk of predation, these decisions are made in an “ecology of fear” (Brown et al., 1999; Schmitz et al., 2004). A dolphin’s ability to survive and reproduce hinges on its ability to successfully trade-off between competing needs to feed and avoid predation. Group living in highly social odontocetes is thought to have evolved to maximize the chances that a group will detect prey and minimize the chances that any one individual would be attacked by a predator (Connor, 2000). At a sub-lethal level, repeated episodes of disturbance due to predation risk may affect the dolphin’s energetic balance, which can affect its ability to survive, reproduce and feed its offspring (Lusseau, 2004; Bejder et al., 2006; Williams et al., 2006). Ultimately, predation risk can negatively impact the population as a whole. In fact, these “risk-effects” can have a greater impact than the direct effect of mortality from predation itself (Creel and Christianson, 2008; Terborgh and Estes, 2010).
This predator-prey system may be a useful one to study to better understand population consequences of disturbance of sonar and other anthropogenic activities. It has been postulated that some cetaceans respond to mid-frequency sonar signals because it may be perceived mistakenly as a killer whale call (Zimmer and Tyack, 2007; Tyack et al., 2011). Killer whale vocalizations have elicited the strongest behavioral avoidance responses ever documented among cetaceans (Cummings and Thompson, 1971; Zimmer and Tyack, 2007; Tyack et al., 2011). Consequently, killer whale vocalizations are often used as the aversive treatment in sonar behavioral response studies (Curé et al., 2013). While there is some emerging direct evidence to demonstrate that cetaceans display an aversive response to killer whale calls [e.g., sperm whales (Curé et al., 2016) and Blainville’s beaked whales (Allen et al., 2014)], observations from one of us (AM) indicated that dolphins respond to silent killer whales, not the vocal ones. Mammal-eating killer whales are typically silent when engaged in hunting and vocal following successful attacks (Barrett-Lennard et al., 1996; Deecke et al., 2005), whereas fish-eating killer whales vocalize during all activity states (Holt et al., 2011). Here we show that evasive tactics taken by cetacean prey, in response to killer whale predation attempts can lead to stranding (Barr and Barr, 1972). Killer whales may be driving the dynamics of Pacific white-sided dolphin populations, both directly and indirectly through sublethal and lethal impacts, including surplus killing (Gaydos et al., 2005).
Pacific white-sided dolphins were not observed in the study area prior to 1984, despite intense photo-identification and other studies in the region beginning in the mid-1970s (Morton, 2000). We may be observing the development of learned predation behavior in inshore waters on the part of the killer whales, and new anti-predatory tactics on the part of the Pacific white-sided dolphins. Given the evasive tactics used by the dolphins, it may take a great deal of time for killer whales to learn how to catch this species. This is supported by the lack of successful predation events observed in the early years of Pacific white-side research in the Johnstone Strait/Broughton Archipelago region. One of us (A.M.) observed Pacific white-sided dolphins in the region for at least 6 years before the first predation event was reported (Morton, 2004). Most of our records of observed predation events do not include the identities of the killer whales involved, but of the 17 events for which identities were recorded, the T055s were the only matriline known to be involved in multiple (three) predation events, only one of which resulted in a successful kill. Continued attention to matriline and individual killer whale identity could advance our understanding about the development of killer whale cultural transmission of hunting techniques (Deecke et al., 2000).
Our study opens up new lines of research questions that can improve our knowledge of both the ecology and conservation status of Pacific white-sided dolphins, and to our understanding of the many data deficient cetacean species worldwide. One priority for our future research is to quantify the relative importance of natural and anthropogenic sources of injury and mortality in these dolphins. Given the global impact of fisheries bycatch on cetacean populations (Read et al., 2006), it would not have been surprising to find that scars consistent with human-caused injuries were more common than those caused by predators. Our findings serve as a reminder that anthropogenic impacts are part of a suite of top-down and bottom-up forces that drive marine mammal population dynamics (Estes et al., 2009). As we begin to assess the conservation status of data-deficient species, low-cost and rapid assessment tools of abundance (Williams et al., 2017), bycatch (Moore et al., 2010), and threats to recovery (Ashe et al., 2021) will be needed to fill in data gaps.
Given the wealth of cetacean demographic data that can be collected using photo-identification (Hammond et al., 1990), we see value in standardizing methods used to determine causes of injury from photographs (Moore et al., 2013) but recognize that cause of injury was not determined in 59% of photographs in our study. The veterinary pathologists who participated in the survey noted that full necropsies would be required to determine cause of death, but this would require making data-poor species a higher priority for stranding response and pathological analyses than they currently are in regions that prioritize research funding based on a species’ legal listing (Raverty et al., 2020). For sub-lethal effects, estimating an activity budget in the presence and absence of killer whales may reveal behavioral responses to predation risk that can improve our understanding of energetic and ultimately population-level consequences of disturbance (King et al., 2015). Ultimately, if we want to understand the relative importance of natural and anthropogenic impacts on cetacean populations, substantial investments will need to be made in data collection across the board (Hoffmann et al., 2010).
Understanding the levels and sustainability of cetacean bycatch is particularly challenging for species, including Pacific white-sided dolphins, and fisheries that occur on the high seas or areas beyond national jurisdiction (Kaschner et al., 2012). The new seafood import provisions of the United States Marine Mammal Protection Act will require exporting and processing nations to demonstrate that their fisheries use standards for marine mammal bycatch that are comparable to those used in fisheries in waters under United States jurisdiction (Williams et al., 2016). To do so, countries will have to invest in both cetacean survey data and fisheries observer coverage in order to assess bycatch sustainability, and to implement effective mitigation in cases where levels of bycatch exceed allowable harm limits (Wade, 1998). It is our hope that these new trade rules can create a financial incentive, in the form of access to lucrative United States seafood markets, to fill in some important data gaps for poorly studied, pelagic dolphin populations.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
This animal study was authorized under a research permit issued by the Department of Fisheries and Oceans, Canada.
RW and AM assisted EA with study design, data collection, and writing. PH provided EA with advice on study design, data collection, analysis, and writing. All authors contributed to the article and approved the submitted version.
Funding for Oceans Initiative’s long-term research on Pacific white-sided dolphins has been provided from National Geographic, McLean and Marisla Foundations, and SeaDoc Society.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank the mariners and researchers who provided sightings and observations especially Jared Towers, Paul Spong, Helena Symonds, Bill McKay, Garry Henkel, Jim Borrowman, Erin Rechsteiner, Brad Hanson, Marilyn Dahlheim, Karen Hansen, Stan Hutchings, Jack Springer, Lisa Spaven, James Willson, and Don Willson. The authors would also like to thank Joe Gaydos, Michael Moore, Stephen Raverty, Frances Gulland, Ken Langelier, Antonio Fernandez, and Doug Sandilands for providing expert feedback on the dolphin photos. Marie Fournier, Christie McMillan, Melissa Boogaards, Nicole Koshure, Leila Fouda, Emily Hague, and Alyssa Rice assisted with photo processing and matching. RW and EA would like to thank the Marisla Foundation, SeaDoc Society, Sarah Haney, and individual donors for financial support to Oceans Initiative over the years for funding our Pacific white-sided dolphin study. The content of the manuscript has previously appeared in a Ph.D. thesis (EA). The authors also thank two reviewers, and the editor, JK, for careful reviews that improved the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.606876/full#supplementary-material
Supplementary Appendix 1 | Original field notes from each of the 45 eye-witness accounts of successful or unsuccessful attacks by killer whales on Pacific white-sided dolphins.
Allen, A. N., Schanze, J. J., Solow, A. R., and Tyack, P. L. (2014). Analysis of a Blainville’s beaked whale’s movement response to playback of killer whale vocalizations. Mar. Mamm. Sci. 30, 154–168. doi: 10.1111/mms.12028
Ashe, E. (2015). Ecology of Pacific White-Sided Dolphins (Lagenorhynchus Obliquidens) in the Coastal Waters of British Columbia, Canada. Ph.D. Dissertation, University of St Andrews, St Andrews.
Ashe, E., Williams, R., Clark, C., Erbe, C., Gerber, L. R., Hall, A. J., et al. (2021). Minding the data-gap trap: exploring dynamics of abundant dolphin populations under uncertainty. Front. Mar. Sci. 8:110. doi: 10.3389/fmars.2021.606932
Baird, R. W., and Dill, L. M. (1995). Occurrence and behaviour of transient killer whales: seasonal and pod-specific variability, foraging behaviour, and prey handling. Can. J. Zool. 73, 1300–1311. doi: 10.1139/z95-154
Baird, R. W., Stacey, P. J., and Langlier, K. M. (1991). Strandings and incidental mortality of cetaceans on the B.C. coast, 1990. Paper SC/43/O1 presented to the IWC Scientific Committee, (unpublished). 6.
Barr, N., and Barr, L. (1972). An observation of killer whale predation on a Dall porpoise. Can. Field Nat. 86, 170–171.
Barrett-Lennard, L., Ford, J. K. B., and Heise, K. A. (1996). The mixed blessing of echolocation: differences in sonar use by fish-eating killer whales. Anim. Behav. 51, 553–565. doi: 10.1006/anbe.1996.0059
Bejder, L., Samuels, A. M. Y., Whitehead, H. A. L., Gales, N., Mann, J., Connor, R., et al. (2006). Decline in relative abundance of bottlenose dolphins exposed to long−term disturbance. Conserv. Biol. 20, 1791–1798. doi: 10.1111/j.1523-1739.2006.00540.x
Brodie, P., and Beck, B. (1983). Predation by sharks on the grey seal (Halichoerus grypus) in eastern Canada. Can. J. Fish. Aquat. Sci. 40, 267–271. doi: 10.1139/f83-040
Brown, J. S., Laundre, J. W., and Gurung, M. (1999). The ecology of fear: optimal foraging, game theory, and trophic interactions. J. Mammal. 80, 385–399. doi: 10.2307/1383287
Carretta, J. V., Oleson, E. M., Baker, J., Weller, D. W., Lang, A. R., Forney, K. A., et al. (2016). “U.S. Pacific marine mammal stock assessments: 2015. U.S.,” in Department of Commerce, NOAA Technical Memorandum NMFS-SWFSC-561, 419.
Chasco, B. E., Kaplan, I. C., Thomas, A. C., Acevedo-Gutiérrez, A., Noren, D. P., Ford, M. J., et al. (2017). Competing tradeoffs between increasing marine mammal predation and fisheries harvest of Chinook salmon. Sci. Rep. 7:15439.
Connor, R. C. (2000). “Group living in whales and dolphins,” in Cetacean Societies: Field Studies of Whales And Dolphins, eds J. Mann, R. Connor, P. Tyack, and H. Whitehead (Chicago, IL: University of Chicago Press), 199–218.
Cowlishaw, G., Lawes, M. J., Lightbody, M., Martin, A., Pettifor, R., and Rowcliffe, J. M. (2004). A simple rule for the costs of vigilance: empirical evidence from a social forager. Proc. Biol. Sci. 271, 27–33. doi: 10.1098/rspb.2003.2522
Creel, S., and Christianson, D. (2008). Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201. doi: 10.1016/j.tree.2007.12.004
Cummings, W. C., and Thompson, P. O. (1971). Gray whales, (Eschrichtius robustus), avoid underwater sounds of killer whales, (Orcinus orca). Fish. Bull. US 69, 525–535.
Curé, C., Antunes, R., Alves, A. C., Visser, F., Kvadsheim, P. H., and Miller, P. J. (2013). Responses of male sperm whales (Physeter macrocephalus) to killer whale sounds: implications for anti-predator strategies. Sci. Rep. 3, 1–7.
Curé, C., Isojunno, S., Visser, F., Wensveen, P. J., Sivle, L. D., Kvadsheim, P. H., et al. (2016). Biological significance of sperm whale responses to sonar: comparison with anti-predator responses. Endanger. Species Res. 31, 89–102.
Dahlheim, M., and Towell, R. (1994). Occurrence and distribution of Pacific white-sided dolphins (Lagenorhynchus obliquidens) in Southeastern Alaska, with notes on an attack by killer whales (Orcinus orca). Mar. Mamm. Sci. 10, 458–464. doi: 10.1111/j.1748-7692.1994.tb00501.x
Dahlheim, M. E., and White, P. A. (2010). Ecological aspects of transient killer whales Orcinus orca as predators in southeastern Alaska. Wildlife Biol. 16, 308–322. doi: 10.2981/09-075
Deecke, V. B., Ford, J. K. B., and Spong, P. (2000). Dialect change in resident killer whales: implications for vocal learning and cultural transmission. Anim. Behav. 60, 629–638. doi: 10.1006/anbe.2000.1454
Deecke, V. B., Ford, J. K., and Slater, P. J. (2005). The vocal behaviour of mammal-eating killer whales: communicating with costly calls. Anim. Behav. 69, 395–405. doi: 10.1016/j.anbehav.2004.04.014
Deecke, V. B., Slater, P. J., and Ford, J. K. (2002). Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420, 171–173. doi: 10.1038/nature01030
Dill, L. M. (1983). Adaptive flexibility in the foraging behavior of fishes. Can. J. Fish. Aqua. Sci. 40, 398–408. doi: 10.1139/f83-058
Dill, L. M. (1987). Animal decision making and its ecological consequences: the future of aquatic ecology. Can. J. Zool. 65, 803–811. doi: 10.1139/z87-128
Estes, J. A., Doak, D. F., Springer, A. M., and Williams, T. M. (2009). Causes and consequences of marine mammal population declines in southwest Alaska: a food-web perspective. Philos. Trans. R. Soc. B Biol. Sci. 364, 1647–1658.
Ford, J. K., and Ellis, G. M. (1999). Transients: Mammal-Hunting Killer Whales of British Columbia, Washington, and southeastern Alaska. UBC Press.
Fishman, M. A. (1999). Predator inspection: closer approach as a way to improve assessment of potential threats. J. Theor. Biol. 196, 225–235. doi: 10.1006/jtbi.1998.0834
FitzGibbon, C. D. (1994). The costs and benefits of predator inspection behaviour in Thomson’s gazelles. Behav. Ecol. Sociobiol. 34, 139–148. doi: 10.1007/s002650050027
Ford, J. K. B., Ellis, G. M., and Balcomb, K. C. (2000). Killer Whales, 2nd Edn. Vancouver, BC: UBC Press.
Ford, J. K. B., Ellis, G. M., Barrett-Lennard, L. G., Morton, A. B., Palm, R. S., and Balcomb, K. C. III (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471. doi: 10.1139/z98-089
Ford, J. K. B., Stredulinsky, E. H., Towers, J.R., and Ellis, G. M. (2013). Information in Support of the Identification of Critical Habitat for Transient Killer Whales (Orcinus orca) off the West Coast of Canada DFO Can Canadian Science Advisory Secretariat Research Document 2012/155. Ottawa, Canada: Canadian Science Advisory Secretariat. iv + 46.
Gaydos, J. K., Raverty, S., Baird, R. W., Osborne, R. W., and Lewis, J. (2005). Suspected surplus killing of harbor seal pups (Phoca vitulina) by killer whales (Orcinus orca). Northwest. Nat. 86, 150–154. doi: 10.1898/1051-1733(2005)086[0150:sskohs]2.0.co;2
George, J. C., Philo, L. M., Hazard, K., Withrow, D., Carroll, G. M., and Suydam, R. (1994). Frequency of killer whale (Orcinus orca) attacks and ship collisions based on scarring on bowhead whales (Balaena mysticetus) of the Bering-Chukchi-Beaufort seas stock. Arctic 47, 247–255.
Guinet, C. (1991). Intentional stranding apprenticeship and social play in killer whales (Orcinus orca). Can. J. Zool. 69, 2712–2716. doi: 10.1139/z91-383
Hammond, P. S., Mizroch, S. A., and Donovan, G. P. (eds) (1990). “Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters,” in Proceedings of the Corporating the Proceedings of the Symposium and Workshop on Individual Recognition and the Estimation of Cetacean Population Parameters (No. 12) (Cambridge: International Whaling Commission).
Heise, K. (1996). Diet and Feeding Behaviour of Pacific White-Sided Dolphins (Lagenorhynchus Obliquidens) as Revealed Through the Collection of Prey Fragments and Stomach Contents (SC/48/SM45), Vol. 47. Cambridge: International Whaling Commission, 807–815.
Heithaus, M. R. (2001). Shark attacks on bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia: attack rate, bite scar frequencies, and attack seasonality. Mar. Mamm. Sci. 17, 526–539. doi: 10.1111/j.1748-7692.2001.tb01002.x
Hobbs, R. C., and Jones, L. L. (1993). Impacts of high seas driftnet fisheries on marine mammal populations in the North Pacific. International North Pacific Fisheries Commission Bulletin 53, 409–434.
Hoffmann, M., Hilton-Taylor, C., Angulo, A., Bohm, M., Brooks, T. M., Butchart, S. H. M., et al. (2010). The impact of conservation on the status of the world’s vertebrates. Science 330, 1503–1509. doi: 10.1126/science.1194442
Holt, M. M., Noren, D. P., and Emmons, C. K. (2011). Effects of noise levels and call types on the source levels of killer whale calls. J. Acoust. Soc. Am. 130, 3100–3106. doi: 10.1121/1.3641446
Jefferson, T. A., Stacey, P. J., and Baird, R. W. (1991). A review of killer whale interactions with other marine mammals: predation to co-existence. Mamm. Rev. 21, 151–180. doi: 10.1111/j.1365-2907.1991.tb00291.x
Jeremy, K., Pelourdeau, D., and Ridoux, V. (2008). Body scars and dorsal fin disfigurements as indicators interaction between small cetaceans and fisheries around the Mozambique Channel island of Mayotte. West. Indian Ocean J. Mar. Sci. 7:2.
Kaschner, K., Quick, N. J., Jewell, R., Williams, R., and Harris, C. M. (2012). Global coverage of cetacean line-transect surveys: status quo, data gaps and future challenges. PLoS One 7:e44075. doi: 10.1371/journal.pone.0044075
King, S. L., Schick, R. S., Donovan, C., Booth, C. G., Burgman, M., Thomas, L., et al. (2015). An interim framework for assessing the population consequences of disturbance. Methods Ecol. Evol. 6, 1150–1158. doi: 10.1111/2041-210x.12411
Knowlton, A. R., and Kraus, S. D. (2001). Mortality and serious injury of northern right whales (Eubalaena glacialis) in the western North Atlantic Ocean. J. Cetacean Res. Manag. (Special Issue) 2, 193–208. doi: 10.47536/jcrm.vi.288
Kyhn, L. A., Tougaard, J., Beedholm, K., Jensen, F. H., Ashe, E., Williams, R., et al. (2013). Clicking in a killer whale habitat: narrow-band, high-frequency biosonar clicks of harbour porpoise (Phocoena phocoena) and Dall’s porpoise (Phocoenoides dalli). PLoS One 8:e63763. doi: 10.1371/journal.pone.0063763
Leatherwood, S., Reeves, R., Bowles, A., Stewart, B., and Goodrich, K. (1984). Distribution, seasonal movements, and abundance of Pacific white-sided dolphins in the eastern North Pacific. Sci. Rep. Whales Res. Inst. 35, 129–157.
Lopez, J. C., and Lopez, D. (1985). Killer whales of Patagonia and their behavior of intentional stranding while hunting nearshore. J. Mammal. 66, 181–183. doi: 10.2307/1380981
Lusseau, D. (2004). The hidden cost of tourism: detecting long-term effects of tourism using behavioral information. Ecol. Soc. 9:14.
McCordic, J. A., Todd, S. K., and Stevick, P. T. (2014). Differential rates of killer whale attacks on humpback whales in the North Atlantic as determined by scarification. Marine Biological Association of the United Kingdom. J. Mar. Biol. Assoc. U. K. 94:1311.
Moore, J.E., Cox, T.M., Lewison, R.L., Read, A.J., Bjorkland, R., McDonald, S.L., et al. (2010). An interview-based approach to assess marine mammal and sea turtle captures in artisanal fisheries. Biol. Conserv., 143, 795-805
Moore, M. J., van der Hoop, J., Barco, S. G., Costidis, A. M., Gulland, F. M., Jepson, P. D., et al. (2013). Criteria and case definitions for serious injury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aqua. Organ. 103, 229–264. doi: 10.3354/dao02566
Morisaka, T., and Connor, R. C. (2007). Predation by killer whales (Orcinus orca) and the evolution of whistle loss and narrow-band high frequency clicks in odontocetes. J. Evol. Biol. 20, 1439–1458. doi: 10.1111/j.1420-9101.2007.01336.x
Morton, A. (2000). Occurrence, photo-identification and prey of Pacific white-sided dolphins (Lagenorhyncus obliquidens) in the Broughton Archipelago, Canada 1984-1998. Mar. Mamm. Sci. 16, 80–93. doi: 10.1111/j.1748-7692.2000.tb00905.x
Morton, A. (2004). Listening to Whales: What the Orcas Have Taught us. New York, NY: Ballantine Books.
Morton, A. B. (1990). A quantitative comparison of the behaviour of resident and transient forms of the killer whale off the central British Columbia coast. Rep. Int. Whaling Comm. Spec. 12, 245–248.
Pitman, R. L. and Durban, J. W., (2012). Cooperative hunting behavior, prey selectivity and prey handling by pack ice killer whales (Orcinus orca), type B, in Antarctic peninsula waters. Mar. Mamm. Sci. 28, 16–36. doi: 10.1111/j.1748-7692.2010.00453.x
Raverty, S., St. Leger, J., Noren, D. P., Burek Huntington, K., Rotstein, D. S., Gulland, F. M., et al. (2020). Pathology findings and correlation with body condition index in stranded killer whales (Orcinus orca) in the northeastern Pacific and Hawaii from 2004 to 2013. PLos One 15:e0242505. doi: 10.1371/journal.pone.0242505
Read, A. J., Drinker, P., and Northridge, S. (2006). Bycatch of marine mammals in US and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Riedman, M. (1990). The Pinnipeds: Seals, Sea Lions, and Walruses (Vol. 12). Univ of California Press.
Robbins, J. (2007). Structure and Dynamics of the Gulf of Maine Humpback Whale Population. Ph.D. thesis, University of St Andrews, St Andrews.
Salvadeo, C. J., Lluch-Belda, D., Gómez-Gallardo, A., Urbán-Ramírez, J., and MacLeod, C. D. (2010). Climate change and a poleward shift in the distribution of the Pacific white-sided dolphin in the northeastern Pacific. Endanger. Species Res. 11, 13–19. doi: 10.3354/esr00252
Saulitis, E., Matkin, C., Barrett-Lennard, L., Heise, K., and Ellis, G. (2000). Foraging strategies of sympatric killer whale (Orcinus orca) populations in Prince William Sound Alaska. Mar. Mamm. Sci. 16, 94–109. doi: 10.1111/j.1748-7692.2000.tb00906.x
Schmitz, O. J., Krivan, V., and Ovadia, O. (2004). Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163. doi: 10.1111/j.1461-0248.2003.00560.x
Scott, E. M., Mann, J., Watson-Capps, J. J., Sargeant, B. L., and Connor, R. C. (2005). Aggression in bottlenose dolphins: evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-rake marks and behaviour. Behaviour 142, 21–44. doi: 10.1163/1568539053627712
Simila, T., and Ugarte, F. (1991). “Killer whales Orcinus orca in Northern Norway,” in Paper Presented at the Annual Conference of the European Cetacean Society, Sandefjord.
Srinivasan, M., and Markowitz, T. M. (2010). “Predator threats and dusky dolphin survival strategies,” in The Dusky Dolphin: Master Acrobat off Different Shores, eds B. Würsig and M. Würsig (Amsterdam: Elsevier), 133–150. doi: 10.1016/b978-0-12-373723-6.00007-2
Stacey, P. J., and Baird, R. W. (1991). Status of the Pacific white-sided dolphin, (Lagenorhynchus obliquidens), in Canada. Can. Field Nat. 105, 219–232.
Steiger, G., Calambokidis, J., Straley, J., Herman, L., Cerchio, S., Salden, D., et al. (2008). Geographic variation in killer whale attacks on humpback whales in the North Pacific: implications for predation pressure. Endanger. Species Res. 4, 247–256. doi: 10.3354/esr00078
Stroud, R., and Roffe, T. (1979). Causes of death in marine mammals stranded along the Oregon coast. J. Wildl. Dis. 15, 91–97. doi: 10.7589/0090-3558-15.1.91
Taylor, B. L., Martinez, M., Gerrodette, T., Barlow, J., and Hrovat, Y. N. (2007). Lessons from monitoring trends in abundance of marine mammals. Mar. Mamm. Sci. 23, 157–175. doi: 10.1111/j.1748-7692.2006.00092.x
Terborgh, J., and Estes, J. A. (2010). Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Washington, D.C: Island Press.
Tyack, P. L., Zimmer, W. M. X., Moretti, D., Southall, B. L., Claridge, D. E., Durban, J. W., et al. (2011). Beaked whales respond to simulated and actual navy sonar. PLoS One 6:e17009. doi: 10.1371/journal.pone.0017009
Visser, I. N., Smith, T. G., Bullock, I. D., Green, G. D., Carlsson, O. G., and Imberti, S. (2008). Antarctic peninsula killer whales (Orcinus orca) hunt seals and a penguin on floating ice. Mar. Mamm. Sci. 24, 225–234. doi: 10.1111/j.1748-7692.2007.00163.x
Vollmer, N. L., Ashe, E., Brownell, R. L. Jr., Cipriano, F., Mead, J. G., Reeves, R. R., et al. (2019). Taxonomic revision of the dolphin genus Lagenorhynchus. Mar. Mamm. Sci. 35, 957–1057. doi: 10.1111/mms.12573
Wade, P. R. (1998). Calculating limits to the allowable human−caused mortality of cetaceans and pinnipeds. Mar. Mamm. Sci. 14, 1–37. doi: 10.1111/j.1748-7692.1998.tb00688.x
Wade, P. R., Burkanov, V. N., Dahlheim, M. E., Friday, N. A., Fritz, L. W., Loughlin, T. R., et al. (2007). Killer whales and marine mammal trends in the North Pacific- A re-examination of evidence for sequential megafauna collapse and the prey-switching hypothesis. Mar. Mamm. Sci. 23, 766–802. doi: 10.1111/j.1748-7692.2006.00093.x
Williams, R., and Thomas, L. (2007). Distribution and abundance of marine mammals in the coastal waters of British Columbia, Canada. J. Cetacean Res. Manage. (Special Issue) 9:14.
Williams, R., Ashe, E., Gaut, K., Gryba, R., Moore, J. E., Rexstad, E., et al. (2017). Animal Counting Toolkit: a practical guide to small-boat surveys for estimating abundance of coastal marine mammals. Endanger. Species Res. 34, 149–165. doi: 10.3354/esr00845
Williams, R., Burgess, M. G., Ashe, E., Gaines, S. D., and Reeves, R. R. (2016). US seafood import restriction presents opportunity and risk. Science 354, 1372–1374. doi: 10.1126/science.aai8222
Williams, R., Hall, A., and Winship, A. (2008). Potential limits to anthropogenic mortality of small cetaceans in coastal waters of British Columbia. Can. J. Fish. Aqua. Sci. 65, 1867–1878. doi: 10.1139/f08-098
Williams, R., Lusseau, D., and Hammond, P. S. (2006). Estimating relative energetic costs of human disturbance to killer whales (Orcinus orca). Biol. Conserv. 133, 301–311. doi: 10.1016/j.biocon.2006.06.010
Williams, T. M., Estes, J. A., Doak, D. F., and Springer, A. M. (2004). Killer appetites: assessing the role of predators in ecological communities. Ecology 85, 3373–3384. doi: 10.1890/03-0696
Keywords: bycatch, predation, data deficient, cetacean, Killer whale, conservation
Citation: Ashe E, Williams R, Morton A and Hammond PS (2021) Disentangling Natural and Anthropogenic Forms of Mortality and Serious Injury in a Poorly Studied Pelagic Dolphin. Front. Mar. Sci. 8:606876. doi: 10.3389/fmars.2021.606876
Received: 15 September 2020; Accepted: 25 March 2021;
Published: 21 April 2021.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Robin William Baird, Cascadia Research Collective, United StatesCopyright © 2021 Ashe, Williams, Morton and Hammond. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erin Ashe, ZWE4NEBzdC1hbmRyZXdzLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.