
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 19 March 2021
Sec. Deep-Sea Environments and Ecology
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.604879
This article is part of the Research TopicDeep-sea Sponge Ecosystems: Knowledge-based Approach Towards Sustainable Management and ConservationView all 29 articles
Cold-water coral reefs and sponge grounds are deep-sea biological hotspots, equivalent to shallow-water tropical coral reefs. In tropical ecosystems, biodiversity and productivity are maintained through efficient recycling pathways, such as the sponge loop. In this pathway, encrusting sponges recycle dissolved organic matter (DOM) into particulate detritus. Subsequently, the sponge-produced detritus serves as a food source for other organisms on the reef. Alternatively, the DOM stored in massive sponges was recently hypothesized to be transferred to higher trophic levels through predation of these sponges, instead of detritus production. However, for deep-sea sponges, the existence of all prerequisite, consecutive steps of the sponge loop have not yet been established. Here, we tested whether cold-water deep-sea sponges, similar to their tropical shallow-water counterparts, take up DOM and transfer assimilated DOM to associated fauna via either detritus production or predation. We traced the fate of 13carbon (C)- and 15nitrogen (N)-enriched DOM and particulate organic matter (POM) in time using a pulse-chase approach. During the 24-h pulse, the uptake of 13C/15N-enriched DOM and POM by two deep-sea sponge species, the massive species Geodia barretti and the encrusting species Hymedesmia sp., was assessed. During the subsequent 9-day chase in label-free seawater, we investigated the transfer of the consumed food by sponges into brittle stars via two possible scenarios: (1) the production and subsequent consumption of detrital waste or (2) direct feeding on sponge tissue. We found that particulate detritus released by both sponge species contained C from the previously consumed tracer DOM and POM, and, after 9-day exposure to the labeled sponges and detritus, enrichment of 13C and 15N was also detected in the tissue of the brittle stars. These results therefore provide the first evidence of all consecutive steps of a sponge loop pathway via deep-sea sponges. We cannot distinguish at present whether the deep-sea sponge loop is acting through a detrital or predatory pathway, but conclude that both scenarios are feasible. We conclude that sponges could play an important role in the recycling of DOM in the many deep-sea ecosystems where they are abundant, although in situ measurements are needed to confirm this hypothesis.
In the deep-sea, sponges and cold-water corals (CWC) form complex reef structures, which support rich communities of suspension-feeding fauna and play crucial roles as habitat and feeding grounds for motile taxa, including commercial fish species (Miller et al., 2012). These ecosystems are amongst the most productive deep-sea habitats and they are responsible for significant carbon (C) and nitrogen (N) cycling (van Oevelen et al., 2009; Radax et al., 2012; Kutti et al., 2013; Cathalot et al., 2015). In fact, CWC reefs and sponge grounds have been identified as benthic biodiversity hotspots, even comparable to tropical coral reefs in terms of grams organic C m–2 and kg dry weight km–2 (Polovina, 1984; van Oevelen et al., 2009; Grebmeier et al., 2015; Maldonado et al., 2017).
Paramount to the productivity of benthic ecosystems in oligotrophic waters is their capacity to efficiently retain and recycle resources. The largest organic resource in the oceans is dissolved organic matter (DOM) (Benner et al., 1992), but this complex mixture of polysaccharides, proteins, and lipids is deemed biologically unavailable to most heterotrophic organisms (Carlson, 2002). However, DOM is known to be processed by bacterioplankton and then returned to the classic food chain through planktonic grazing, a pathway termed the microbial loop (Azam et al., 1983). Essentially, by consuming DOM, bacteria remineralize nutrients that would otherwise be lost to the environment (Fenchel, 2008).
Within shallow-water tropical coral reefs, an additional DOM recycling pathway has been established: the sponge loop (de Goeij et al., 2013). In this pathway, encrusting sponges that dominate the surface of cryptic habitats (e.g., crevices, cavities), but also occupy exposed reef surfaces, assimilate DOM and produce significant amounts of particulate detritus (de Goeij et al., 2013; Alexander et al., 2014; Rix et al., 2016, 2017; Lesser et al., 2020). This detritus subsequently feeds the detrital food chain (de Goeij et al., 2013; Rix et al., 2018). Carbon fluxes through these ubiquitous, but largely hidden and thus usually “overlooked” sponges can amount to daily gross primary production rates of the entire reef (de Goeij et al., 2013, 2017). However, no detritus production was found for several sponges with a non-encrusting, but massive, emergent growth form, that generally occur on the exposed reef (McMurray et al., 2018; Wooster et al., 2019). Therefore, a complementary sponge-loop pathway was hypothesized, in which sponge-assimilated DOM is transferred to higher trophic levels via direct predation on sponge tissue (McMurray et al., 2018; Pawlik and McMurray, 2020). To date, this predatory sponge loop has not yet been confirmed. Whether via detritus production or predation, the sponge loop, together with the microbial loop, helps to explain how tropical shallow-water coral reefs maintain a high productivity and biodiversity in otherwise oligotrophic marine environments (de Goeij et al., 2013; Rix et al., 2016; Pawlik and McMurray, 2020).
The (re)cycling and transfer of DOM could be of particular importance for benthic deep-sea ecosystems as, for large parts of the year, particulate phytodetritus transported from the ocean surface cannot fulfill the carbon demands of these systems (Gooday, 2002; Duineveld et al., 2004, 2007; van Oevelen et al., 2009; Kahn et al., 2018). Recently, first evidence was found that the sponge-loop pathway may not just operate on tropical shallow-water coral reefs, but also in the deep-sea (Rix et al., 2016; Bart et al., 2020a, b; Maier et al., 2020). The capacity to take up DOM represents the first step of the sponge loop (de Goeij et al., 2013), and both encrusting and massive deep-sea sponges have been shown to utilize DOM as a food source (Rix et al., 2016; Bart et al., 2020a, b; Maier et al., 2020). The second step of the sponge loop is the assimilation of DOM into particulate organic matter (POM), leading to either the release of detritus (including pseudo faeces) or an increase in sponge biomass. Using stable isotope tracers, multiple studies have shown that deep-sea sponges are capable of assimilating DOM into biomass (Rix et al., 2016; Kazanidis et al., 2018; Bart et al., 2020a; Maier et al., 2020). Additionally, the encrusting deep-sea sponge Hymedesmia coriacea was found to convert 39% of the organic C derived from coral mucus into detritus (Rix et al., 2016), and two massive deep-sea sponge species, Geodia barretti and Mycale lingua, were found to take up DOM and produce POM, although at much lower rates (0.03 and 3%, respectively) as percentage of their biomass (Maier et al., 2020). However, the third—ecologically critical, but most difficult to experimentally identify—step of the sponge loop has not been established in the deep-sea to date: the transfer of assimilated DOM by sponges to higher trophic levels. Note also that all the aforementioned studies on DOM cycling by deep-sea sponges are based on ex situ measurements in controlled laboratory settings. The existence and ecological relevance of a deep-sea sponge loop has therefore not been established to date.
Dissolved organic carbon (DOC) may represent more than 90% of the daily carbon intake of sponges (de Goeij et al., 2017), including deep-sea species (Bart et al., 2020b), but DOM is clearly not the only food source of sponges. In fact, they are established as very efficient filter-feeders of organic particles, such as bacterio-, and phytoplankton (e.g., Reiswig, 1971; Pile et al., 1996; Leys et al., 2018). Food bacteria, as part of their POM diet, were found to be assimilated more efficiently into sponge tissue compared to DOM (Kazanidis et al., 2018; Bart et al., 2020a), implying that bacteria are a high quality and therefore crucial food source to sponges. Currently, it is unknown how the processing of DOM and POM by encrusting and massive sponges affects the subsequent steps of the sponge loop.
The present study aims to test the hypothesis that, similar to their tropical counterparts, deep-sea sponges transfer assimilated DOM and POM to associated fauna, following the prerequisite, consecutive steps of the sponge loop. Therefore, we qualitatively investigated the potential retention and subsequent transfer of C and N derived from DOM and POM (i.e., bacterioplankton) via two species of deep-sea sponges, the massive species Geodia barretti and the encrusting species Hymedesmia sp., to sponge-associated fauna (i.e., brittle stars). Brittle stars (Echinodermata: Ophiuroidea) are a predominant member of deep-sea ecosystems (Stöhr et al., 2012) and sponge infauna (Clark, 1933; Duarte and Nalesso, 1996). They are known to feed on (pseudo-)fecal droppings of bivalves (Maier et al., 2020), and, in tropical shallow-water ecosystems, on sponge detritus (Rix et al., 2018). Brittle stars may also directly feed on sponge tissue (Morison, 1979; McClintock, 1994). We traced the fate of 13C- and 15N-enriched DOM and POM in time using an ex situ pulse-chase experiment. The assimilation of 13C/15N-enriched DOM and POM into the two sponge species was assessed during the 24-h pulse. The transfer of assimilated DOM and POM by the two sponges (via either detritus or direct predation) into brittle stars was investigated during the subsequent 9-day chase, after transfer of the pulse-labeled sponges to label-free running seawater aquaria containing brittle stars.
This study investigated the nutritional relationship between two North-Atlantic deep-sea sponge species, the massive species Geodia barretti and the encrusting species Hymedesmia sp., and one deep-sea brittle star species, Ophiura sp. (Figure 1A and Supplementary Table 1).
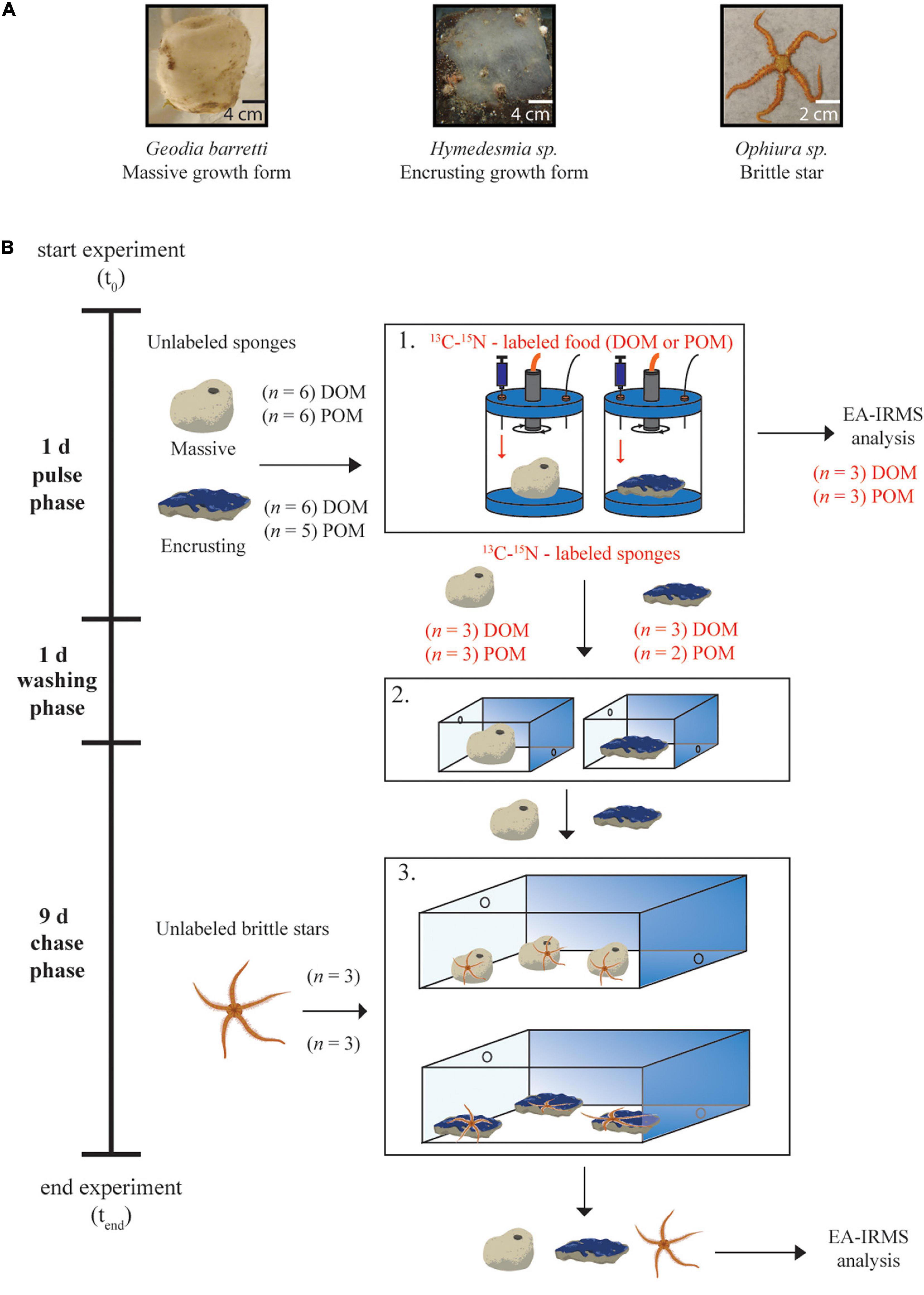
Figure 1. Experimental set up. (A) Organisms used in this study. (B) Two tracer 13C/15N-labeled food sources [dissolved or particulate organic matter (DOM or POM, respectively)] were fed to a total of 23 sponge individuals of the massive sponge Geodia barretti and the encrusting sponge Hymedesmia sp. for a total of 24 h in individual ex situ chambers (pulse phase). Three individuals of each species per food source were sacrificed for EA-IRMS-analysis after 24. The remaining three sponge individuals (or 2 for encrusting POM) were then transferred to individual 10-L running seawater flow-through aquaria for 24 h (washing phase). Lastly, the individuals were pooled per species and food source and transferred to 20-L running seawater flow-through aquaria with 3 brittle stars (Ophiura sp.) per tank for a further 9-day (chase). At the end of the experiment, tissue samples of all organisms were taken and analyzed for enrichment of 13C – 15N by EA-IRMS.
Whole, intact Geodia barretti individuals were carefully collected by ROV (Remotely Operated Vehicle), during the G.O. Sars cruise 2018108 (August 2018) at a sponge ground on the Tromsøflaket, a sea bank in the western Barents Sea (70°47′13.9″N 18°03′23.8″E). The Barents Sea is a shelf sea with an average depth of 230 m (Sundfjord et al., 2007). The benthic community at the Tromsøflaket is primarily dominated by scattered large specimens of G. barretti, which account for approximately 90% of the benthic biomass (Buhl-Mortensen et al., 2009; Jørgensen et al., 2015). In addition, Actinaria, Bryozoa, Crustacea, Echinodermata, Echiura, and Hydrozoa are part of the benthic community (Kędra et al., 2017). Hymedesmia sp. and Ophiura sp. were carefully collected attached to their rocky substrate by ROV at Stjernsund reef (70°30′N, 22°30′E), a 30 km long, 3.5 km wide, > 400 m deep glacial sound in northern Norway that connects the Altafjord to North-Atlantic waters (Rüggeberg et al., 2011; Rovelli et al., 2015). This cold-water coral reef is characterized by the presence of the reef framework-forming scleractinian coral Lophelia pertusa and reef-associated fauna such as sponges, crustaceans, and other corals (Rovelli et al., 2015).
Sponges and brittle stars were kept on board the research vessel in the dark in 20-L flow-through tanks in a climate room at 6°C. North-Atlantic seawater was pumped in from 6 m water depth at 30 L h–1. All individuals were transported without air-exposure to the laboratory facilities at the University of Bergen, Norway, where the experiments took place. In Bergen, sponges and brittle stars were kept in a dark climate room (8°C) in multiple 20-L flow-through aquaria systems. Each holding tank contained a maximum of five sponge individuals and five brittle stars. Flow originated from unfiltered water, pumped from 200 m depth from the outer fjord near Bergen at ∼ 50 L h–1 with a temperature ranging from 6 to 8°C. All sponges were acclimatized for a minimum of 1 week prior to the incubation experiments and all sponges and their attached rocky substrates were cleared from epibionts prior to incubations.
Isotopically enriched DOM was prepared by axenically culturing the marine diatom Phaeodactylum tricornutum in multiple 2 L Fernbach flasks on F/2 medium amended with 15N (80%)-NaNO3 (Eurisotop, CLM-157) and 13C (99%)-NaHCO3 (Eurisotop, CLM-441) (de Goeij et al., 2008; Bart et al., 2020a). Non-labeled axenic P. tricornutum pre-cultures were added (60 mL) to 1 L of sterile labeled F/2 medium in a flow cabinet. The diatoms were grown at 20°C on a 12:12 day:night cycle. After 10 day, diatoms were concentrated on a 0.45 μm filter (147 mm ø) and carefully flushed from the filter with sterile artificial seawater. Subsequently, the collected diatoms were centrifuged for 10 min at 750 × g, the supernatant was removed and the pellet was frozen at -20°C. To lyse the cells and release 13C/15N-labeled DOM, the frozen diatoms were lyophilized in a FD5515 Ilchin Biobase freeze-drier, after which MilliQ water was added and the solution was placed in an ultrasonic bath for 10 min. Lastly, the DOM solution was filtered over a 0.7 μm GF/F filter and subsequently over a 0.2 μm polycarbonate filter. The filtrate was collected, lyophilized, and analyzed for C and N content and isotopic composition. Before adding DOM to the incubations, aliquots of 5 mL were made by dissolving the lyophilized DOM in MilliQ to a final concentration of 80 μmol L–1 DOC or 15 μmol L–1 DON added to each incubation.
Isotopically-enriched POM was prepared by labeling ambient seawater bacterioplankton (de Goeij et al., 2008; Bart et al., 2020a). Briefly, seawater bacteria were concentrated by prefiltering natural seawater over a 0.7 μm GF/F filter and subsequent ultrafiltration (0.2 μm; Vivaflow). The inoculum was added to M63 medium (Miller, 1972), amended with thiamine (0.00001%) and MgSO4 (67 μmol L–1). Labeled bacterial cultures were amended with 13C (99%)-glucose (1 g L–1, glucose D U-13C6, Euriso-Top, CLM-1396) and 15N (99%)-NH4Cl (1.2 g L–1, Euriso-Top, NLM-467-5). The culture was grown for 48 h in the dark at 25°C and labeled bacteria were isolated by centrifuging (5 min, 10,000 × g), rinsing the pellet in 0.2 μm filtered seawater, centrifuging again, and resuspending the pellet in 0.2 μm filtered seawater before dividing in aliquots to a final concentration of 0.5 × 106 bacteria mL–1 (approximately 12 μmol L–1 bacterial carbon (BC) and 1 μmol L–1 bacterial nitrogen (BN) added to each incubation) and storing at 4°C.
A schematic of the pulse-chase experimental set-up is shown in Figure 1B and comprised three phases: a pulse, a washing, and a chase phase. From here on, for simplicity and to distinguish between the two sponge loop pathways scenarios—i.e., the hypothesis that massive sponges are predominantly cycling resources through the predatory pathway and encrusting sponges predominantly through the detrital pathway—we will refer to G. barretti as “massive,” Hymedesmia. sp. as “encrusting” and Ophiura sp. as “brittle star.”
Phase 1: pulse—During the pulse, we continuously administered two tracer 13C/15N-labeled food sources (DOM and POM) to individuals of both species using air-tight 3-L incubation flow chambers (Bart et al., 2020a, b). Six individuals of both species were fed DOM, six individuals of the massive sponge were fed POM and five individuals of the encrusting sponge were fed POM. Prior to the incubations, chambers were acid washed (0.4 mol L–1 HCl) overnight. During the experiment, the chambers were kept in a water bath to maintain a constant seawater temperature (ranging from 6 to 8°C depending on the incubation). Incubation water was replenished every 8 h (24 h total incubation time) to ensure that sponge individuals were regularly receiving fresh seawater after which new, labeled substrate was added. At the end of the incubation, sponges were removed from the chamber and rinsed in 0.2 μm filtered unlabeled seawater. This experiment was part of a larger isotope-tracer study conducted simultaneously and the uptake rates of DOM and POM by massive and encrusting sponges (n = 3 per species, per food source) were taken from already published data (Figure 3, gray marked area) (Bart et al., 2020a). To test the direct uptake rates of DOM and POM by brittle stars, separate incubations (n = 3 per food source) were performed according to the aforementioned protocol. After the 24-h incubation period, brittle stars were directly sacrificed and placed in a drying oven for 48 h at 60°C. After drying, tissue was homogenized with mortar and pestle and stored in a desiccator until further analysis by EA-IRMS.
Phase 2: washing—Post-labeling, sponge individuals were transferred to individual 10-L label-free running seawater flow through aquaria for 24 h to ensure no residual 13C- and 15N-labeled substrate remained on the (inner and outer) surface of the sponges.
Phase 3: chase—Post-washing, DOM-fed and POM-fed massive and encrusting sponges were placed in four 20-L label-free running seawater flow-through aquaria together with 3 unlabeled brittle stars per tank: one tank with three DOM-fed massive sponges plus brittle stars, one with three DOM-fed encrusting sponges plus brittle stars, one with three POM-fed massive sponge plus brittle stars, and one with two POM-fed encrusting sponges plus brittle stars. After placing the brittle stars at random positions in each aquarium, they settled themselves on the surface of the sponges (Figure 2). Detritus was collected every 3-day during the chase from each tank with a sterile plastic pipette, pooled per timepoint and frozen at -80°C for later isotopic analysis (Rix et al., 2018). After 9-day, all sponges and brittle stars were rinsed in 0.2 μm filtered, unlabeled seawater, shortly dipped in ultrapure water to remove salts, and carefully removed from their rocky substrate using a sterile scalpel blade (i.e., sponges) and oven-dried (48 h at 60°C). Then, tissue was homogenized with mortar and pestle and stored in a desiccator until further analysis for C and N content and stable isotope enrichment (13C and 15N) by EA-IRMS (see below sections for details on flux calculations). Background detritus samples and tissue samples from each species were collected prior to the pulse-chase experiment and served as non-labeled controls.

Figure 2. Geodia barretti and Ophiura sp. After placing them at random position in the aquaria, brittle stars settled themselves on the surface of the sponges.
DOM and POM substrates, labeled and non-labeled sponge tissue, brittle star, and detritus samples were analyzed for organic C and total N content on an elemental analyzer [Elementar Isotope cube (Elementar GmbH, Langenselbold, Germany)] coupled to a BioVision isotope ratio mass spectrometer (Elementar ltd, Manchester, United Kingdom) for simultaneous measurement of organic carbon and nitrogen content and 13C:12C and 15N:14N ratios. Before analysis, samples were lyophilized for 24 h in a FD5515 Ilchin Biobase freeze drier. After freeze-drying, approximately 10 mg per sample was weighed out into separate tin capsules and acidified. The peak area (from the elemental analyzer) to content ratio was calculated with respect to several replicates of a standard (acetanilide) of known C and N content. To calculate the 13C:12C and 15N:14N ratios, C and N stable isotope ratios are expressed in standard delta notation as:
where R is the ratio of 13C:12C or 15N:14N in the sample (Rsample; e.g., sponge tissue, detritus) or reference material: Vienna Pee Dee Belemnite for C (Rref = 0.01118) and atmospheric nitrogen for N (Rref = 0.00368). The above background enrichment of 13C or 15N in the samples was calculated as the excess fractional abundance of 13C or 15N in the samples compared with the background (i.e., non-enriched) values of non-labeled control samples (n = 3 for massive sponges, n = 4 for encrusting sponges, n = 2 for detritus). Unfortunately, the 15N-detritus background measurements could not be analyzed and therefore no 15N-enrichment of detritus samples was calculated.
where F is the fractional abundance of heavy isotope (13C or 15N) in the sample calculated as:
and
Total tracer incorporation was calculated by multiplying the excess fractional abundance (Esample) by the total Corg or N content (μmol) of the tissue, divided by the labeling efficiency (as atom%) of the food source. Rates were then normalized to time and tissue C or N content of the sponges or detritivores.
Both detritus (detrital pathway) and the sponge tissue itself (predatory pathway) are considered stable-isotope-enriched food sources to brittle stars during the label-free chase. We calculated brittle star tracer uptake rates for two hypothetical scenarios: in scenario 1 with detritus as food source (using the labeling efficiency of detritus), and in scenario 2 with sponge tissue as food source (using the labeling efficiency of the sponge tissue).
The C:N ratios of DOM- and POM-derived transfer to brittle stars were calculated by dividing the organic C tracer incorporation rate by the total N tracer incorporation rate.
Statistical analysis was performed in Primer V7 (Clarke and Gorley, 2015) with the add-on PERMANOVA+ (Anderson et al., 2008). Permutational multivariate analysis of variance (PERMANOVA) with Monte Carlo tests were used as this method is robust for low sample replication and non-normally distributed data. PERMANOVAs were run using Type III sum of squares and unrestricted permutation of raw data (9999 permutations); resemblance matrices were conducted using Euclidean distances. Individual one-way PERMANOVAs were conducted to test (1) the effect of treatment timepoint (24 h vs. 11-day) on stable isotope enrichment (δ13C and δ15N) of sponge tissue after incubation with isotopically labeled DOM or POM, and (2) differences between brittle star tracer incorporation rates when sponges were fed DOM or POM. These incorporation rates were calculated assuming transfer from sponge to brittle star via detritus or via direct predation. Adjustments for multiple tests were made using the Bonferroni procedure. Full statistical output is available in Supplementary Table 2.
The massive and encrusting sponge species assimilated DOM- and POM-derived 13C and 15N during the 24-h pulse (data obtained from Bart et al., 2020a). Stable isotope enrichment of sponge tissue did not significantly change between the end of the pulse and end of the chase phase (Figures 3A,B, left panels; Supplementary Tables 2, 3) for both sponge species, except for POM-fed massive sponges, where tissue isotopic enrichment decreased significantly during the chase for 13C.
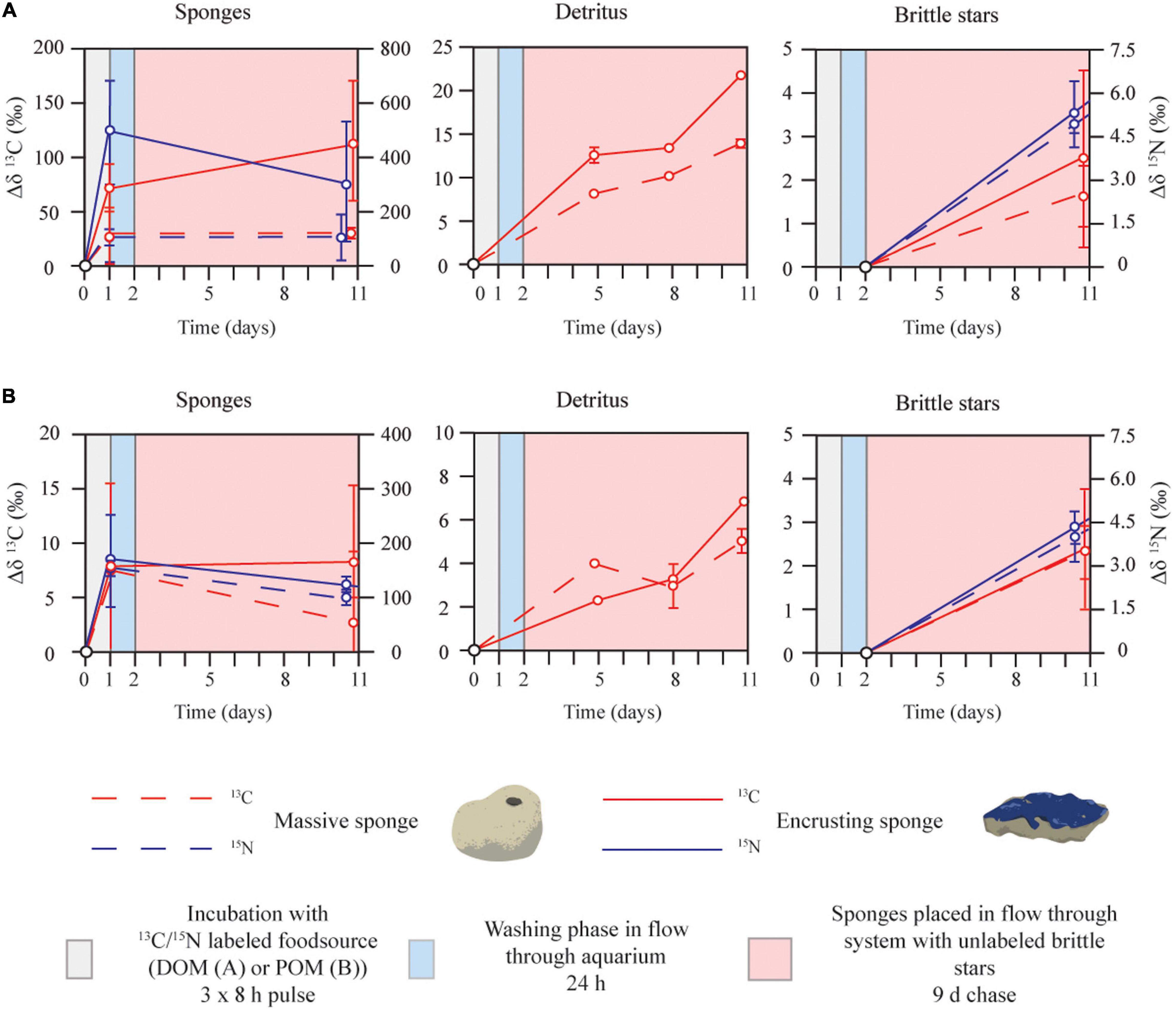
Figure 3. Sponge-driven transfer of dissolved organic matter (DOM) (A) and particulate organic matter (POM) (B), to sponge associated fauna in an ex situ aquarium set-up. The dashed and solid lines represent the fate of 13C (red line) and 15N (blue line) incorporated by massive sponges and encrusting sponges, respectively. After an initial 24-h pulse (gray shading; data obtained from Bart et al., 2020a) and a subsequent 24-h washing phase (blue shading), unlabeled brittle stars were introduced to the aquaria for a 9-day chase. Data are shown as mean ± SD above-background isotope tracer incorporation (Δδ13C‰ and Δδ15N‰) of each aquarium [(A) one aquarium with DOM-fed encrusting sponges and one with DOM-fed massive sponges and, (B) one aquarium with POM-fed encrusting sponges, and one with POM-fed massive sponges] for sponges, detritus, and detritivores. Unfortunately, 15N-detritus data were not analyzed. Note the difference in scale on the Y-axis between panels. Data is also shown in Supplementary Table 3.
Released detritus showed a continuous increase in above-background 13C-enrichment (no 15N data available) during the chase (Figures 3A,B, middle panels), demonstrating turnover of DOM and POM by sponges. After 9-day exposure to the labeled sponges and detritus, above-background enrichment of 13C and 15N was also detected in the tissue of the brittle stars (Figures 3A,B, right panels). This demonstrates transfer of sponge-assimilated DOM and POM to brittle stars.
Both detritus and the sponge tissue itself are possible sources of enrichment found in brittle stars after the label-free chase phase. Thus, transfer of DOM and POM via sponges to brittle stars is possible via detritus feeding (scenario 1) and/or by predation on sponge tissue (scenario 2).
In scenario 1 (Figure 4A and Supplementary Table 4), brittle star uptake rates of detrital-C from POM-fed massive and encrusting sponges were higher compared to DOM-fed sponges, however, these differences were not significant (Supplementary Table 2).
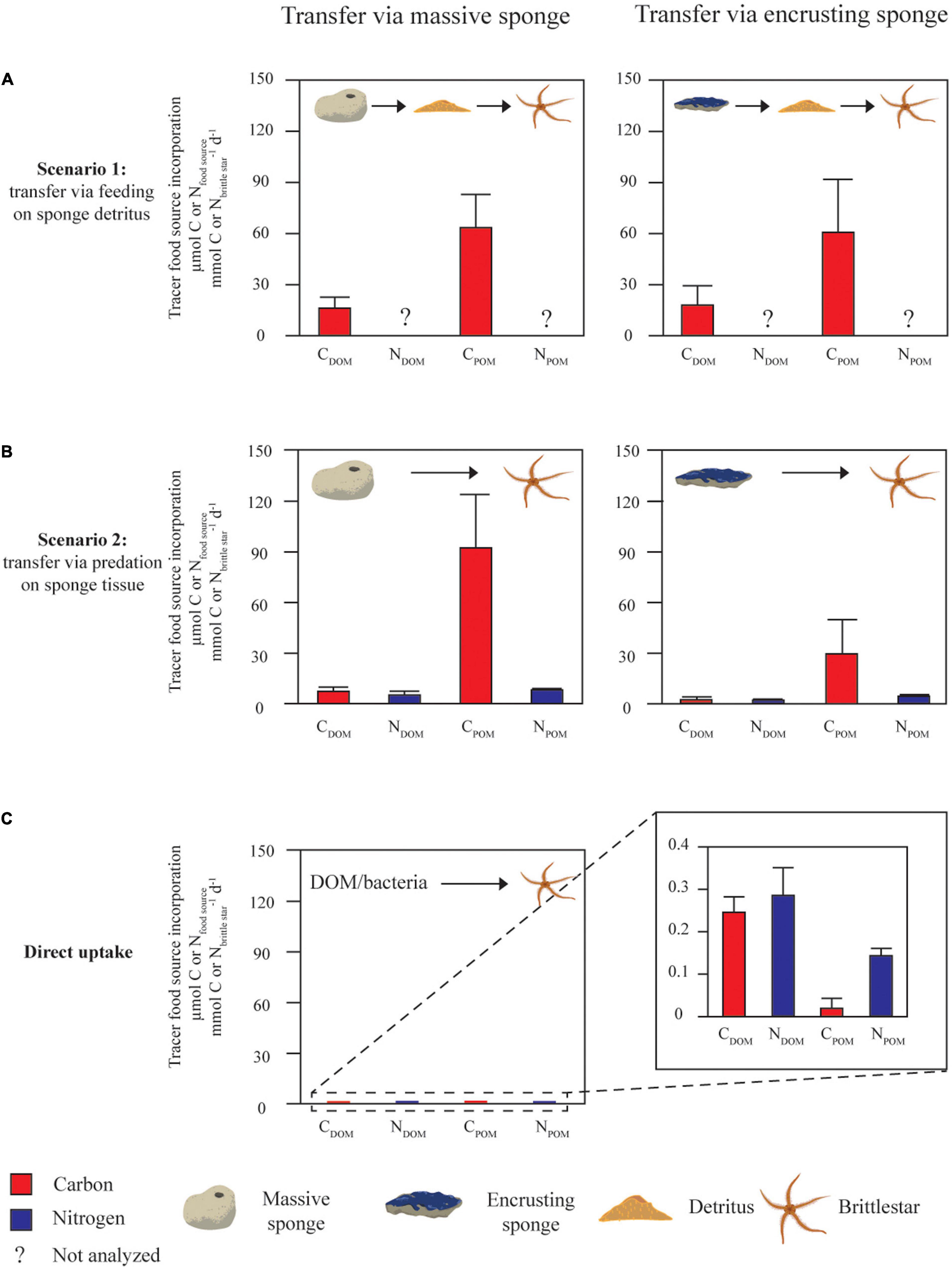
Figure 4. Uptake of carbon (C) and nitrogen (N) by brittle stars via feeding on sponge detritus (A) or predation on sponge tissue (B) after sponges were fed isotopically labeled DOM or POM, versus direct uptake of DOM or POM by brittle stars (C) (values are expressed as μmol tracer C or N per mmol brittle star C or N per day, mean ± SD). In scenario 1 (A) brittle star tracer uptake rates were quantified with enriched detritus as food source, in scenario 2 (B), uptake rates were calculated with the enriched sponge tissue as food source. The bottom panel (C) shows direct incorporation of tracer C and N by brittle stars after a 24 h pulse of isotopically labeled DOM and POM. Data are also shown in Supplementary Table 4.
In scenario 2 (Figure 4B and Supplementary Table 4), brittle star uptake rates of sponge tissue-C from POM-fed massive sponges were significantly higher than for DOM-fed massive sponges, while a similar, but non-significant trend was found between CDOM and CPOM transfer for encrusting sponges (Supplementary Table 2).
For the transfer of tissue-N via predation, no significant differences were found in brittle star uptake rates between DOM- and POM-fed sponges, for both massive and encrusting sponges (Supplementary Tables 2, 4).
The C:N ratios of uptake by brittle stars under scenario 2 (direct tissue predation) were lower for DOM-fed sponges (1.3 for massive and encrusting) than for POM-fed sponges (15 and 8 for massive and encrusting, respectively).
Direct uptake of DOM and POM by brittle stars (Figure 4C) was one to three orders of magnitude lower than transfer through scenario 1 or 2 (Supplementary Table 4).
This study provides the first evidence of all three consecutive steps of the sponge loop in deep-sea sponges. The two investigated deep-sea sponge species take up and assimilate DOM and POM, subsequently turn sponge-assimilated DOM and POM into detritus, and transfer carbon and nitrogen derived from both food sources to associated fauna. Transfer of assimilated food to associated fauna by sponges is possible via two scenarios: (1) via the production of detrital waste or (2) via direct predation on sponge tissue. The plausibility of both scenarios, and their potential ecological relevance for deep-sea ecosystems, are discussed below.
At present, DOM cycling by various types of sponges and its relevance for marine ecosystems, is a heavily debated topic (e.g., de Goeij et al., 2017; Leys et al., 2018; Pawlik and McMurray, 2020; Rix et al., 2020). The original sponge-loop hypothesis proposed that coral reef sponges recycle DOM by converting it into particulate detritus, which is then used by various detritivorous organisms and thereby re-enters the classical food chain (de Goeij et al., 2013). This pathway was tested on sponge species with mm-thin sheet to cm-thick (e.g., conulose, lobate, ficiform) encrusting growth forms—i.e. following the contours of the surface rather than growing upward—typically inhabiting crevices of the reef framework, but also appearing on the open reef. Based on encrusting sponges alone, sponge-loop carbon cycling is estimated to amount to the gross primary production rates of an entire coral reef ecosystem (de Goeij et al., 2013). Interestingly, massive upright growing sponges, living on the exposed parts of the reef, were not found to produce significant quantities of detritus (McMurray et al., 2018; Wooster et al., 2019). Massive sponges may allocate the majority of assimilated C in three-dimensional (upward) tissue growth, while (mm to cm-thin) encrusting species are restricted to space-limited, two-dimensional growth. Consequently, encrusting sponges may invest relatively more carbon in cell turnover, shedding and detritus production compared to massive species. This hypothesis was strengthened by recent work of Maier et al. (2020), who showed that deep-sea sponge detritus production rates of the lobate encrusting species Mycale lingua, exceeded detritus production rates of the globular massive species G. barretti by two orders of magnitude. McMurray et al. (2018) therefore proposed an alternative, predatory sponge-loop pathway for massive, non-space limited sponge species. Based on the limited number of replicates used in our study due to the difficulties obtaining and experimenting with live deep-sea organisms, we cannot rule out one of these scenarios, and will discuss both.
Deep-sea detrital sponge loop—Multiple studies have shown that sponge species from various deep-sea ecosystems produce particulate waste material in the form of detritus or fecal pellets (Witte and Graf, 1996; Rix et al., 2016; Kahn et al., 2018; Maier et al., 2020). Only few studies have quantified the release of detritus. The encrusting sponge Hymedesmia coriacea was found to convert 39% of ingested coral-derived DOM (i.e. coral mucus) into detritus (Rix et al., 2016), and five species of tropical shallow-water encrusting sponges released on average 12% of their biomass daily (Alexander et al., 2014). In comparison, the massive sponge G. barretti released very small amounts of detritus (0.03% of their biomass daily) (Maier et al., 2020). Yet, from an ecological perspective, detritus production by massive sponges can still have a significant effect on C turnover in marine ecosystems. The average total organic C content (Corg) of the massive G. barretti species in our experiments outweighed the organic C content of our encrusting sponges by three orders of magnitude (on average 6.5 g Corg (G. barretti) versus 0.008 g Corg (Hymedesmia sp.) per individual). Consequently, even when the relatively small G. barretti individuals used in our study release only 0.03% of its Corg as particulate detritus, detritus release rates would amount to 2 mg C d–1 per sponge individual. In contrast, a 12–39% Corg release by the much smaller encrusting sponges would mean a release of 1–3 mg C d–1 per sponge individual, which is similar to the absolute amount of detritus production by the massive sponge. Although little is known about the relative contribution of encrusting versus massive sponges in deep-sea ecosystems, the carbon standing stock of G. barretti in deep-sea ecosystems, such as the Western Barents Sea, can add up to 200 g C m–2 (Klitgaard and Tendal, 2004, converted to g C via Bart et al., 2020b). Geodia barretti can thus potentially produce 66 mg detritus per m–2 d–1, which amounts to an abundant supply of particulate organic carbon to the local deep-sea benthos. For example, Piepenburg and Schmid (1996) estimated a mineralization rate of 21.9 mg C m–2 d–1 for benthic brittle stars on the North-East Greenland shelf even though this area is extremely rich in epifauna (30–340 individuals m–2), and strongly dominated (80–98% of the total amount of organisms) by brittle stars.
Deep-sea predatory sponge loop—Spongivory is a strategy performed by various animals, including echinoderms (Randall and Hartman, 1968; Pawlik, 2011), and sponge spicules have been found in the stomachs of various brittle star species (Pearson and Gage, 1984). A study on 1165 deep-sea ophiuroid individuals of six different species by Pearson and Gage (1984) showed that deep-sea brittle stars are trophic generalists lacking dietary specialization. Feeding strategies ranged from detritivory to scavenging and even suspension feeding. Indeed, we found brittle stars to be capable of directly feeding on small amounts of our tracer DOM and POM during incubations (Figure 4C). Yet, rates of direct tracer incorporation were up to three orders of magnitude lower compared to the incorporation of sponge-derived C and N. Some massive sponge species are known to produce metabolites that deter predation (Chanas et al., 1997; Lindel et al., 2000) and thus spongivory is mostly seen on species that lack chemical defenses and have faster growth and reproduction rates (Pawlik, 2011). In contrast, chemically defended individuals that grow relatively slowly may be long lived, and therefore important for the sequestration and storage of C as biomass (McMurray et al., 2018). The massive HMA sponge used in our study, G. barretti, is known to produce various metabolites that inhibit biofouling (Sjögren et al., 2004; Hedner et al., 2008), but their effect on possible predators is unknown. Furthermore, it is difficult to make a genuine comparison with spongivory in shallow-water tropical systems, as deep-sea sponge metabolism is much slower due to lower ambient temperatures (Bart et al., 2020b), and deep-sea sponges are generally known for their longevity and C sequestration (Fallon et al., 2010; Kahn et al., 2015).
A comparison of potential deep-sea sponge-loop scenarios and comparing dissolved and particulate food sources reveals two interesting trends: when sponges feed on POM, relatively more C is transferred to associated fauna, compared to DOM. Secondly, relatively more DOM-derived C appears to be transferred via detritus compared to predation. This implies that POM (i.e. microbial plankton) is rather stored in sponge biomass, whereas DOM is mostly respired. This corresponds with the observations made by Kazanidis et al. (2018) and Bart et al. (2020a) that sponges use DOM for maintenance metabolism and turnover, while bacteria serve as an important food source for anabolic processes and are preferably incorporated into tissue. This is further reflected in the C:N ratios of uptake under the predatory sponge loop by brittle stars after sponge-feeding on DOM or POM. The drastically decreased C:N after DOM-feeding (1.3) compared to POM-feeding (8–15) does not reflect that brittle stars obtain more N from DOM, but rather that the sponges lost more C from the isotopically enriched source, likely through respiration. Deep-sea sponges are indeed found to assimilate POM at very high assimilation efficiencies (up to 97%) compared to DOM (32–77%) (Bart et al., 2020a). This strengthens our hypothesis that DOM and POM are both essential parts of deep-sea sponge diet, as source of maintenance and as building blocks, respectively. This could also have ecological consequences for the wider associated ecosystem and food web, since both C and N may transfer at different rates according to the diet of sponges and the chosen feeding strategy of associated fauna.
It is important to note that both the detrital and predatory scenario are plausible for both types of sponges (i.e. massive and encrusting) and for both food sources (i.e. DOM and POM), and we cannot distinguish between the two scenarios under the current experimental approach. Moreover, our limited replication, in both sponge species and species of associated fauna, and the effect of concentration and lability of ambient DOM in time and space available to sponges living in different deep-sea ecosystems, make it very difficult to extrapolate our data to deep-sea benthic ecosystem-wide processes. The main aim of our study was to establish whether (common) deep-sea sponges possess all the consecutive steps of a sponge loop, rather than to show its potential ecological importance. To assess the full importance of sponge-loop pathways in the deep-sea, both qualitatively and quantitatively—many questions remain to be answered. For example, the relative importance of a deep-sea sponge loop may vary with the abundance and feeding activity of other benthic suspension feeders in deep-sea benthic ecosystems, such as CWC reefs and sponge grounds, that could participate in the cycling of DOM and POM. A recent extensive isotope tracer study by Maier et al. (2020) showed a four time higher production of detritus after DOM- and POM-feeding by the bivalve Acesta excavate compared to the sponge G. barretti. This detritus was further incorporated by brittle stars, suggesting that bivalves may be the main recyclers of organic matter within the CWC reefs under study, and the sponge loop in the deep-sea may in fact be better qualified as a “suspension feeder” loop. Interestingly, this was not found for shallow water tropical coral reefs, where other suspension feeders did not take part in a DOM-metazoan-detritus-detritivore pathway, but was restricted to sponges (de Goeij et al., 2013). Note, however, that Maier et al. (2020) did not test the recycling of sponge-produced detritus to associated fauna and that sponge M. lingua was the highest producer of detritus, i.e., 25 times higher production of detritus compared to the bivalve A. excavata. To estimate each organisms’ role in the recycling of DOM and POM in deep-sea benthic ecosystems, resource fluxes need to be extrapolated to respective biomass estimates within these ecosystems. Furthermore, there is a lack of in situ studies to verify ex situ rates presented to date. In situ studies to determine uptake rates of ambient DOM and the use of stable-isotope tracer food sources on deep-sea benthic communities, including multiple sponge- and other suspension-feeding species and associated fauna, will shed light on how sponges drive food webs in the dark deep-sea.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JG and HR acquired the funding. MB and JG designed the experiments. JG, HR, and MB collected the animals. MB conducted the incubations experiments. MB, JG, and MH performed the data analyses and designed the tables and figures. MB, JG, MH, and PV wrote the manuscript. All co-authors approved the manuscript. All authors contributed to the article and approved the submitted version.
This project has received funding from the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (SponGES grant agreement no. 679849 and ERC starting grant agreement no. 715513 to JG). This document only reflects the authors’ views and the Executive Agency for Small and Medium-sized Enterprises (EASME) is not responsible for any use that may be made of the information it contains.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We dedicate this work to our EU Horizon 2020 SponGES coordinator, colleague, and friend HR who sadly passed away, too soon, on March 7 2020 before he could witness the outcomes of his project. We thank all our collaborators at the SponGES project, and all colleagues at the Department of Biological Sciences at the University of Bergen, Norway, for the use of facilities and equipment. Many thanks to the ROV crew of the ÆGIR 6000 for their careful collection of the sponges. Thanks to Erik Wurz for his help maintaining the onboard aquaria. We also thank Jorien Schoorl for her technical assistance at the University of Amsterdam. Many thanks also to Sara Campana, Titus Rombouts, and Clea van de Ven for their help with the algal batch cultures, and Angela Marulanda Gomez for her help in the field. Lastly, we thank Gina Fields for her help with the design and development of the figures.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.604879/full#supplementary-material
Alexander, B. E., Liebrand, K., Osinga, R., van der Geest, H. G., Admiraal, W., Cleutjens, J. P., et al. (2014). Cell turnover and detritus production in marine sponges from tropical and temperate benthic ecosystems. PLoS One. 9:e109486. doi: 10.1371/journal.pone.010948
Anderson, M. J., Clarke, K. R., and Gorley, R. N. (2008). PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth: PRIMER-E.
Azam, F., Fenchel, T., Field, J. G., Gray, J. S., Meyer-Reil, L. A., and Thingstad, F. (1983). The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263. doi: 10.3354/meps010257
Bart, M. C., de Kluijver, A., Hoetjes, S., Absalah, S., Mueller, B., Kenchington, E. L., et al. (2020a). Differential processing of dissolved and particulate organic matter by deep-sea sponges and their microbial symbionts. Sci. Rep. 10:17515.
Bart, M. C., Mueller, B., Rombouts, T., van de Ven, C., Tompkins, G., Osinga, R., et al. (2020b). Dissolved organic carbon (DOC) is essential to balance the metabolic demands of North-Atlantic deep-sea sponges. Limnol. Oceanogr. doi: 10.1002/lno.11652
Benner, R., Pakulski, J. D., McCarthy, M., Hedges, J. I., and Hatcher, P. G. (1992). Bulk chemical characteristics of dissolved organic matter in the ocean. Science 255, 1561–1564. doi: 10.1126/science.255.5051.1561
Buhl-Mortensen, P., Dolan, M., and Buhl-Mortensen, L. (2009). Prediction of benthic biotopes on a Norwegian offshore bank using a combination of multivariate analysis and GIS classification. ICES J. Mar. Sci. 66, 2026–2032. doi: 10.1093/icesjms/fsp200
Carlson, C. A. (2002). “Production and removal processes,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (San Diego, CA: Academic Press), 91–151. doi: 10.1016/b978-012323841-2/50006-3
Cathalot, C., Van Oevelen, D., Cox, T. J., Kutti, T., Lavaleye, M., Duineveld, G., et al. (2015). Cold-water coral reefs and adjacent sponge grounds: hotspots of benthic respiration and organic carbon cycling in the deep sea. Front. Mar. Sci. 2:37. doi: 10.3389/fmars.2015.00037
Chanas, B., Pawlik, J. R., Lindel, T., and Fenical, W. (1997). Chemical defense of the Caribbean sponge Agelas clathrodes (Schmidt). J. Exp. Mar. Biol. Ecol. 208, 185–196. doi: 10.1016/s0022-0981(96)02653-6
Clark, H. L. (1933). A Handbook of the Littoral Echinoderms of Puerto Rico and the Other West Indian Islands. Scientific Survey of Porto Rico and the Virgin Islands, Vol. 16. New York, NY: Academy of Sciences.
de Goeij, J. M., Lesser, M. P., and Pawlik, J. R. (2017). “Nutrient fluxes and ecological functions of coral reef sponges in a changing ocean,” in Climate Change, Ocean Acidification and Sponges, eds J. Carballo, and J. Bell (Switzerland, AG: Springer, Cham), 373–410. doi: 10.1007/978-3-319-59008-0_8
de Goeij, J. M., Moodley, L., Houtekamer, M., Carballeira, N. M., and Van Duyl, F. C. (2008). Tracing 13C-enriched dissolved and particulate organic carbon in the bacteria-containing coral reef sponge Halisarca caerulea: evidence for DOM-feeding. Limnol. Oceanogr. 53, 1376–1386. doi: 10.4319/lo.2008.53.4.1376
de Goeij, J. M., Van Oevelen, D., Vermeij, M. J., Osinga, R., Middelburg, J. J., De Goeij, A. F., et al. (2013). Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342, 108–110. doi: 10.1126/science.1241981
Duarte, L. F. L., and Nalesso, R. (1996). The sponge Zygomycale parishii (Bowerbank) and its endobiotic fauna. Estuar. Coast. Mar. Sci. 42, 139–151. doi: 10.1006/ecss.1996.0011
Duineveld, G. C., Lavaleye, M. S., Bergman, M. J., De Stigter, H., and Mienis, F. (2007). Trophic structure of a cold-water coral mound community (Rockall Bank, NE Atlantic) in relation to the near-bottom particle supply and current regime. bull. Mar. Sci. 81, 449–467.
Duineveld, G. C. A., Lavaleye, M. S. S., and Berghuis, E. M. (2004). Particle flux and food supply to a seamount cold-water coral community (Galicia Bank. NW Spain). Mar. Ecol. Prog. Ser. 277, 13–23. doi: 10.3354/meps277013
Fallon, S. J., James, K., Norman, R., Kelly, M., and Ellwood, M. J. (2010). A simple radiocarbon dating method for determining the age and growth rate of deep-sea sponges. Nucl. Instrum. Methods Phys. Res., B. 268, 1241–1243. doi: 10.1016/j.nimb.2009.10.143
Fenchel, T. (2008). The microbial loop–25 years later. J. Exp. Mar. Biol. Ecol. 366, 99–103. doi: 10.1016/j.jembe.2008.07.013
Gooday, A. J. (2002). Biological responses to seasonally varying fluxes of organic matter to the ocean floor: a review. J. Oceanogr. 58, 305–332.
Grebmeier, J. M., Bluhm, B. A., Cooper, L. W., Danielson, S. L., Arrigo, K. R., Blanchard, A. L., et al. (2015). Ecosystem characteristics and processes facilitating persistent macrobenthic biomass hotspots and associated benthivory in the pacific arctic. Progr. Oceanogr. 136, 92–114. doi: 10.1016/j.pocean.2015.05.006
Hedner, E., Sjögren, M., Hodzic, S., Andersson, R., Göransson, U., Jonsson, P. R., et al. (2008). Antifouling activity of a dibrominated cyclopeptide from the marine sponge Geodia barretti. J. Nat. Prod. 71, 330–333. doi: 10.1021/np0705209
Jørgensen, L. L., Ljubin, P., Skjoldal, H. R., Ingvaldsen, R. B., Anisimova, N., and Manushin, I. (2015). Distribution of benthic megafauna in the Barents Sea: baseline for an ecosystem approach to management. ICES J. Mar. Sci. 72, 595–613. doi: 10.1093/icesjms/fsu106
Kahn, A. S., Chu, J. W., and Leys, S. P. (2018). Trophic ecology of glass sponge reefs in the strait of Georgia. Br. Columbia. Sci. Rep. 8:756.
Kahn, A. S., Yahel, G., Chu, J. W., Tunnicliffe, V., and Leys, S. P. (2015). Benthic grazing and carbon sequestration by deep-water glass sponge reefs. Limnol. Oceanogr. 60, 78–88. doi: 10.1002/lno.10002
Kazanidis, G., van Oevelen, D., Veuger, B., and Witte, U. F. (2018). Unravelling the versatile feeding and metabolic strategies of the cold-water ecosystem engineer Spongosorites coralliophaga (Stephens, 1915). Deep Sea Res. Part I Oceanogr. Res. Pap. 141, 71–82. doi: 10.1016/j.dsr.2018.07.009
Kędra, M., Renaud, P. E., and Andrade, H. (2017). Epibenthic diversity and productivity on a heavily trawled Barents Sea bank (Tromsøflaket). Oceanologia 59, 93–101. doi: 10.1016/j.oceano.2016.12.001
Klitgaard, A. B., and Tendal, O. S. (2004). Distribution and species composition of mass occurrences of large-sized sponges in the northeast Atlantic. Progr. Oceanogr. 61, 57–98. doi: 10.1016/j.pocean.2004.06.002
Kutti, T., Bannister, R. J., and Fosså, J. H. (2013). Community structure and ecological function of deep-water sponge grounds in the traenadypet MPA—Northern Norwegian continental shelf. Cont. Shelf Res. 69, 21–30. doi: 10.1016/j.csr.2013.09.011
Lesser, M. P., Mueller, B., Pankey, M. S., Macartney, K. J., Slattery, M., and de Goeij, J. M. (2020). Depth-dependent detritus production in the sponge. Halisarca caerulea. Limnol. Oceanogr. 65, 1200–1216. doi: 10.1002/lno.11384
Leys, S. P., Kahn, A. S., Fang, J. K. H., Kutti, T., and Bannister, R. J. (2018). Phagocytosis of microbial symbionts balances the carbon and nitrogen budget for the deep-water boreal sponge Geodia barretti. Limnol. Oceanogr. 63, 187–202. doi: 10.1002/lno.10623
Lindel, T., Hoffmann, H., Hochgürtel, M., and Pawlik, J. R. (2000). Structure–activity relationship of inhibition of fish feeding by sponge-derived and synthetic pyrrole–imidazole alkaloids. J. Chem. Ecol. 26, 1477–1496.
Maier, S. R., Kutti, T., Bannister, R. J., Fang, J. K. H., van Breugel, P., van Rijswijk, P., et al. (2020). Recycling pathways in cold-water coral reefs: use of dissolved organic matter and bacteria by key suspension feeding taxa. Sci. Rep. 10: 9942.
Maldonado, M., Aguilar, R., Bannister, R. J., Bell, J. J., Conway, K. W., Dayton, P. K., et al. (2017). “Sponge grounds as key marine habitats: a synthetic review of types, structure, functional roles and conservation concerns,” in Marine Animal Forests: the Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas Saco del Valle (Switzerland: Springer International Publishing).
McClintock, J. B. (1994). Trophic biology of Antarctic shallow-water echinoderms. Mar. Ecol. Prog. Ser. 111, 191–202. doi: 10.3354/meps111191
McMurray, S. E., Stubler, A. D., Erwin, P. M., Finelli, C. M., and Pawlik, J. R. (2018). A test of the sponge-loop hypothesis for emergent Caribbean reef sponges. Mar. Ecol. Prog. Ser. 588, 1–14. doi: 10.3354/meps12466
Miller, J. H. (1972). Experiments in Molecular Genetics. New York, NY: Cold Spring Harbor Laboratory, Cold Spring Harbor.
Miller, R. J., Hocevar, J., Stone, R. P., and Fedorov, D. V. (2012). Structure-forming corals and sponges and their use as fish habitat in Bering Sea submarine canyons. PLoS One. 7:e33885. doi: 10.1371/journal.pone.0033885
Morison, G. W. (1979). Studies on the Ecology of the Sub-Antarctic ophiuroid Ophionotus Hexactis. [M. Phil. Thesis].[London (UK)]: University of London.
Pawlik, J. R. (2011). The chemical ecology of sponges on Caribbean reefs: natural products shape natural systems. Bioscience 61, 888–898. doi: 10.1525/bio.2011.61.11.8
Pawlik, J. R., and McMurray, S. E. (2020). The emerging ecological and biogeochemical importance of sponges on coral reefs. Ann. Rev. Mar. Sci. 12, 315–337. doi: 10.1146/annurev-marine-010419-010807
Pearson, M., and Gage, J. D. (1984). Diets of some deep-sea brittle stars in the Rockall Trough. Mar. Biol. 82, 247–258. doi: 10.1007/bf00392406
Piepenburg, D., and Schmid, M. K. (1996). Distribution, abundance, biomass, and mineralization potential of the epibenthic megafauna of the Northeast Greenland shelf. Mari. Biol. 125, 321–332. doi: 10.1007/bf00346313
Pile, A. J., Patterson, M. R., and Witman, J. D. (1996). In situ grazing on plankton < 10 μm by the boreal sponge Mycale lingua. Mar. Ecol. Prog. Ser. 141, 95–102. doi: 10.3354/meps141095
Polovina, J. J. (1984). Model of a coral reef ecosystem. Coral reefs 3, 1–11. doi: 10.1007/bf00306135
Radax, R., Hoffmann, F., Rapp, H. T., Leininger, S., and Schleper, C. (2012). Ammonia-oxidizing archaea as main drivers of nitrification in cold-water sponges. Environ. Microbiol. 14, 909–923. doi: 10.1111/j.1462-2920.2011.02661.x
Randall, J. E., and Hartman, W. D. (1968). Sponge-feeding fishes of the West Indies. Mar. Biol. 1, 216–225. doi: 10.1007/bf00347115
Reiswig, H. M. (1971). Particle feeding in natural populations of three marine demosponges. Biol. Bull. 141, 568–591. doi: 10.2307/1540270
Rix, L., de Goeij, J. M., Mueller, C. E., Struck, U., Middelburg, J. J., Van Duyl, F. C., et al. (2016). Coral mucus fuels the sponge loop in warm-and cold-water coral reef ecosystems. Sci. Rep. 6:18715.
Rix, L., de Goeij, J. M., van Oevelen, D., Struck, U., Al-Horani, F. A., Wild, C., et al. (2018). Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop. Mari. Ecol. Prog. Ser. 589, 85–96. doi: 10.3354/meps12443
Rix, L., de Goeij, J. M., van Oevelen, D., Struck, U., Al-Horani, F. A., Wild, C., et al. (2017). Differential recycling of coral and algal dissolved organic matter via the sponge loop. Funct. Ecol. 31, 778–789. doi: 10.1111/1365-2435.12758
Rix, L., Ribes, M., Coma, R., Jahn, M. T., de Goeij, J. M., van Oevelen, D., et al. (2020). Heterotrophy in the earliest gut: a single-cell view of heterotrophic carbon and nitrogen assimilation in sponge-microbe symbioses. ISME J. 14, 2554–2567. doi: 10.1038/s41396-020-0706-3
Rovelli, L., Attard, K. M., Bryant, L. D., Flögel, S., Stahl, H., Roberts, J. M., et al. (2015). Benthic O2 uptake of two cold-water coral communities estimated with the non-invasive eddy correlation technique. Mar. Ecol. Prog. Ser. 525, 97–104. doi: 10.3354/meps11211
Rüggeberg, A., Flögel, S., Dullo, W. C., Hissmann, K., and Freiwald, A. (2011). Water mass characteristics and sill dynamics in a subpolar cold-water coral reef setting at Stjernsund, northern Norway. Mar. Geol. 282, 5–12. doi: 10.1016/j.margeo.2010.05.009
Sjögren, M., Göransson, U., Johnson, A. L., Dahlström, M., Andersson, R., Bergman, J., et al. (2004). Antifouling activity of brominated cyclopeptides from the marine sponge Geodia barretti. J. Nat. Prod. 67, 368–372. doi: 10.1021/np0302403
Stöhr, S., O’Hara, T. D., and Thuy, B. (2012). Global diversity of brittle stars (Echinodermata: Ophiuroidea). PLoS One. 7:e31940. doi: 10.1371/journal.pone.0031940
Sundfjord, A., Fer, I., Kasajima, Y., and Svendsen, H. (2007). Observations of turbulent mixing and hydrography in the marginal ice zone of the Barents Sea. J. Geophys. Res. Oceans. 112:C05008.
van Oevelen, D., Duineveld, G., Lavaleye, M., Mienis, F., Soetaert, K., and Heip, C. H. (2009). The cold-water coral community as hotspot of carbon cycling on continental margins: a food-web analysis from Rockall Bank (Northeast Atlantic). Limnol. Oceanogr. 54, 1829–1844. doi: 10.4319/lo.2009.54.6.1829
Witte, U., and Graf, G. (1996). Metabolism of deep-sea sponges in the greenland-Norwegian Sea. J. Exp. Mar. Biol. Ecol. 198, 223–235. doi: 10.1016/0022-0981(96)00006-8
Keywords: deep-sea sponge loop, brittle star, carbon, nitrogen, transfer, detritus, predation, DOM
Citation: Bart MC, Hudspith M, Rapp HT, Verdonschot PFM and de Goeij JM (2021) A Deep-Sea Sponge Loop? Sponges Transfer Dissolved and Particulate Organic Carbon and Nitrogen to Associated Fauna. Front. Mar. Sci. 8:604879. doi: 10.3389/fmars.2021.604879
Received: 10 September 2020; Accepted: 22 February 2021;
Published: 19 March 2021.
Edited by:
Joana R. Xavier, University of Porto, PortugalReviewed by:
Gitai Yahel, Ruppin Academic Center, IsraelCopyright © 2021 Bart, Hudspith, Rapp, Verdonschot and de Goeij. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jasper M. de Goeij, ai5tLmRlZ29laWpAdXZhLm5s
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.