- 1California Academy of Sciences, San Francisco, CA, United States
- 2Laboratório de Oceanografia Biológica, Universidade Federal da Bahia, Salvador, Brazil
- 3Southern Seas Ecology Laboratories, School of Biological Sciences and The Environment Institute, The University of Adelaide, Adelaide, SA, Australia
- 4Laboratório de Etnoconservação e Áreas Protegidas, Universidade Estadual de Santa Cruz, Ilhéus, Brazil
- 5Laboratório de Ictiologia e Conservação, Universidade Federal de Alagoas, Penedo, Brazil
- 6Reef Systems Ecology and Conservation Lab, Departamento de Biologia Marinha, Universidade Federal Fluminense, Niterói, Brazil
- 7Institute of Biology, Federal University of Bahia, Salvador, Brazil
- 8National Institute for Interdisciplinary and Transdisciplinary Studies in Ecology and Evolution (IN-TREE), Salvador, Brazil
- 9Laboratório de Ecologia de Peixes Marinhos, Universidade Federal do Espírito Santo, São Mateus, Brazil
- 10Projeto Conservação Recifal (Reef Conservation Project), Recife, Brazil
- 11Marine Ecology and Conservation Lab, Instituto do Mar, Universidade Federal de São Paulo, Santos, Brazil
- 12Instituto de Biodiversidade e Sustentabilidade, Universidade Federal do Rio de Janeiro, Macaé, Brazil
- 13The Bioinformatics and Microbial Ecology Lab – PPG Ecologia e Biomonitoramento, Universidade Federal da Bahia, Salvador, Brazil
Overfishing is notorious for triggering population collapses and disrupting marine biological functioning worldwide. To counter such a threat, policy-makers have created and implemented multiple management strategies, but most were incapable to prevent the decline of several key species. Here, we discuss a new management strategy in force since June 2019 in Brazil that aims to deter the overfishing of parrotfish species of the genera Scarus and Sparisoma. This innovative strategy, here referred to as inverted management, allows the capture of endangered parrotfish species inside management areas, such as partially protected marine areas—MPAs, but bans it elsewhere. This initiative is supposed to be built in a partnership among the government, scientists, managers, and fishers. If implemented correctly, endangered species would recover in the much larger area outside MPAs, and fishers would benefit from the conservation-value of the scarce and valued product. However, to succeed, the strategy depends on the adoption of a series of challenging management rules that are not currently being enforced along an extensive coastline. So far, few MPAs have incorporated rules for endangered species in their management plan, and those that have done so have no plans or the means to enforce them. Therefore, fishing of endangered species is currently ongoing without any management or monitoring in the entire Brazilian coast. Concerned with the challenges to develop plans to recover populations of endangered species faced by Brazilian managers, we suggest wide communication and a ban on the fisheries until management plans are implemented. Additionally, we suggest that the effectiveness of the inverted management strategy for parrotfishes should be assessed before it’s applied to other endangered species.
Introduction
Management of natural resources has always been used as a tool to mitigate consequence of human over-exploitation (Reynolds and Peres, 2005; Laffoley et al., 2019). In the past centuries, the sharp human population increase combined with industrial development intensified the exploitation of natural resources leading several marine fish stocks to depletion, which required specific management strategies to recover (Sutherland, 1999; Fryxell et al., 2014). While initially focusing on single-species models, management is increasingly becoming ecosystem-based (Long et al., 2015). For instance, marine protected areas (MPAs) are widely used to protect endangered species, natural resources and maintain ecosystems functionality and services (WWF, 2018; Williams and Graham, 2019), once they regulate extractive activities that govern mortality of wild populations. When well planned, managed, and enforced, MPAs are effective tools to achieve conservation goals due to relatively low costs to manage a delimited area compared vast exclusive economic zones (Balmford et al., 2004; Cinner et al., 2012).
A large proportion of marine biodiversity is concentrated in tropical low and lower-middle income countries, but only ∼10% of the available funding for conservation is allocated to those areas (Mittermeier et al., 2003). In fact, the lack of funds and staff capacity, associated with high dependency of coastal populations on natural resources, makes management strategies, including MPAs, challenging and often ineffective in these countries (Pattanayak et al., 2010; Gill et al., 2017). In such circumstances, innovative and quick solutions are required to meet urgent conservation needs of coastal areas. The combination of MPAs and traditional fisheries management, such as fishing bans, seasonal closures, and size and catch limits, often provide a pathway to meeting conservation and community goals. The combination of strategies has the potential to reverse stock collapses and degradation of reef ecosystems (Mcclanahan et al., 2006; Cinner et al., 2020; Waterhouse et al., 2020), but it also has its implementation and effectiveness challenges. Thus, adaptive management is important to improve coral-reef conservation and sustainability outcomes.
Parrotfishes (Labridae: Scarinae), a group that played a key role in the origin and evolution of modern coral reefs, are often the subject of marine resource management plans worldwide (Bellwood et al., 2016; Siqueira et al., 2019). While parrotfishes are responsible for essential ecological processes such as herbivory, bioerosion, sediment production and transport, predation of live coral colonies, and clearing space for coral settlement (Bonaldo et al., 2014; Lellys et al., 2019) (Figure 1), in many places they are among the main fishery-targeted fishes on reefs. Most species are protogynous hermaphrodites, with a terminal phase consisting of sexually mature males that can attain large body sizes (Bonaldo et al., 2014), which quickly disappear under high fishing pressure (Hawkins, 2003; Freitas et al., 2019; Roos et al., 2020). Consequently, parrotfishes have been reported as over-exploited throughout the world (Jackson et al., 2001; Bellwood et al., 2012; Comeros-Raynal et al., 2012), including in Brazil (Bender et al., 2014; Pereira et al., 2021).
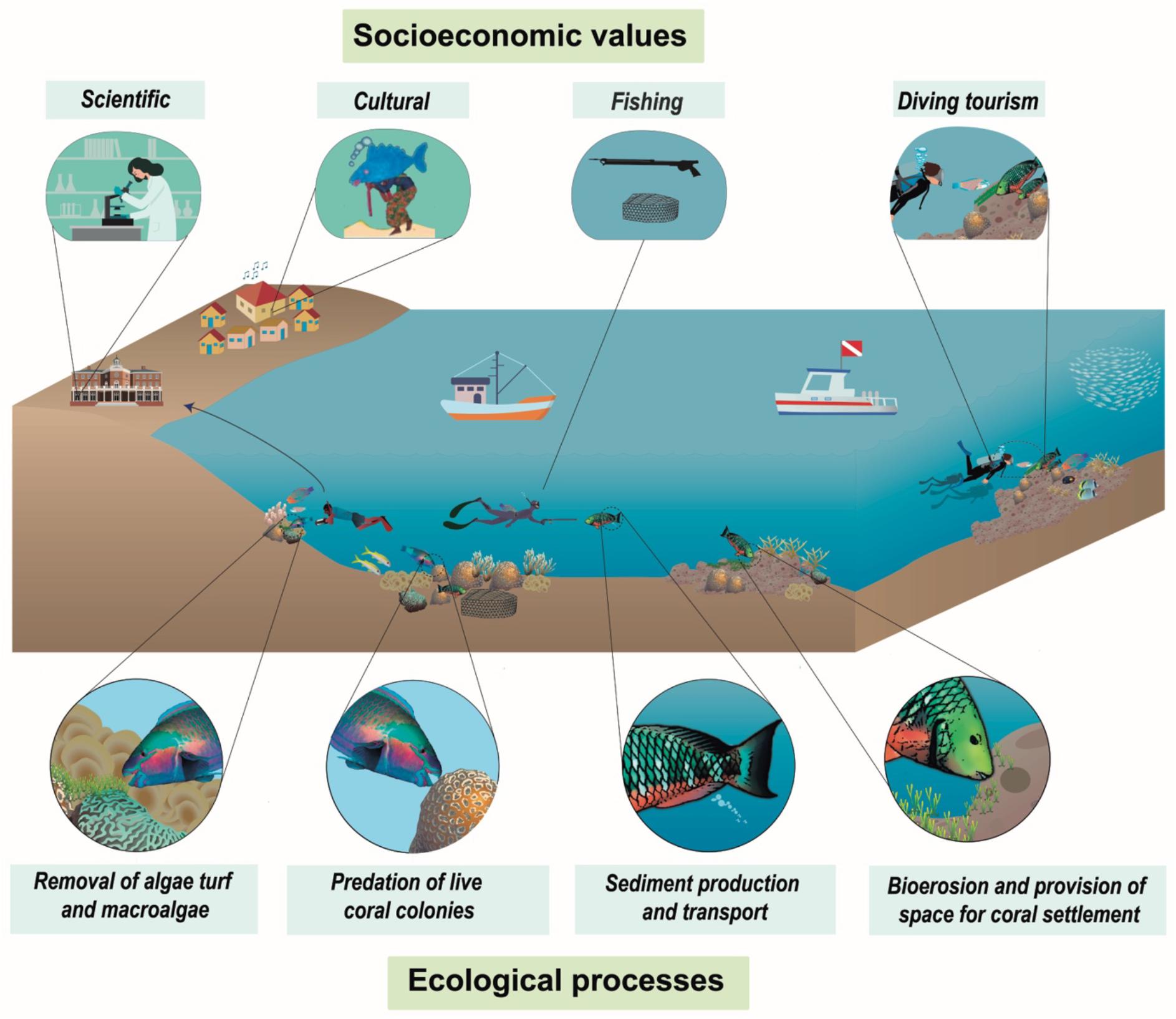
Figure 1. Socioeconomic values and ecological processes related to parrotfishes. Symbols courtesy of the Integration and Application Network (http://ian.umces.edu/symbols/).
Nations have adopted a variety of management strategies to protect parrotfishes. For example, Bermuda has banned fish traps in 1990 and does not allow spearfishing along the coastline, consequently protecting parrotfishes, what led to a rapid recovery of natural sex ratios in a previously overfished population (O’Farrell et al., 2016). Belize banned herbivorous fish harvesting in 2009, including parrotfishes, in an attempt to control the increasing macroalgal cover on much of the Mesoamerican Reef, significantly reducing parrotfish harvesting (Cox et al., 2013). Other countries sharing the Mesoamerican Reef followed the strategy. Honduras banned parrotfish fishing in the Bay Islands since 2010, Guatemala implemented a nationwide ban since 2015, and more recently, Mexico, included all parrotfish species in its endangered species list as “subject to special protection” (Cannon, 2018; SEGOB, 2019; Healthy Reefs, 2021). Such actions contribute to restoring parrotfish populations and maintain species-specific functional roles, helping conserve the integrity of their ecosystem services, and possibly increasing reef resilience. Yet, the protection of endangered coral reef species, including parrotfishes, and the functionality of coral ecosystems, are constantly threatened by political and economic pressures, particularly in low and lower-middle income countries (Edwards et al., 2014; Pinheiro et al., 2019). Even nations such as Palau, which is often cited as a shining example of conservation due to the implementation of a vast marine protected area, allow the fishing of parrotfishes (FAO, 2018; Island Times, 2018).
Here, we describe and discuss a new management strategy established in June 2019 in Brazil that aims to manage the fishing of endangered parrotfish species (Figure 2), replacing a previous fishing ban (ICMBio, 2014). This innovative management strategy represents a new concept in conservation, and it is here referred as inverted management, in which fishing is allowed inside formally instituted management areas, like partially protected MPAs [such as APA, RDS, and RESEX Brazilian categories (Brasil, 2000); IUCN Category VI] and prohibited elsewhere (Brasil, 2018a,b). Even though this new strategy has been announced—i.e., the ban is no longer in place and fishing for parrotfish within managed areas is currently allowed—it is not being enforced yet, and endangered parrotfishes are being caught without restriction everywhere. Thus, here we discuss the challenges and present caveats and recommendations for the implementation of inverted management considering the regional context (Brazil), and project its applicability to other low and lower-middle income countries.

Figure 2. Brazilian endemic parrotfishes now under inverted management: (A) Scarus trispinosus; (B) Scarus zelindae; (C) Sparisoma axillare; (D) Sparisoma frondosum. Photos: C.L.S Sampaio (A–C) and L.A. Rocha (D).
Inverted Management: The New Recovery Plan for Parrotfishes in Brazil
In Brazil, large-scale fisheries of parrotfishes started two to three decades ago, coinciding with the decrease in abundance of top predators (e.g., serranids, lutjanids, and carangids). The biggest species occurring in the Brazilian Province reaches more than 80 cm (greenback parrotfish Scarus trispinosus), and the large size makes it an obvious target. Currently, parrotfishes in Brazil are targeted by recreational and commercial fishing activities (Pinheiro et al., 2010; Nunes et al., 2012; Bender et al., 2014; Freitas et al., 2019; Guabiroba et al., 2020; Roos et al., 2020) and have traditionally been caught without any restriction and poor monitoring. However, 4 years ago, the Brazilian government published a list of endangered aquatic species, prohibiting the fishing of four endangered parrotfish species: Scarus trispinosus, Scarus zelindae, Sparisoma axillare, and Sparisoma frondosum (Figure 2). This ban was based on the best available scientific evidence, including information on severe population declines in the past 15 years (December 2014, act MMA-445; ICMBio, 2014). The publication of the Brazilian red list was followed by political turmoil (Pinheiro et al., 2015), and the government re-established the fishery of many endangered species, including parrotfishes (decree 161/2017 and decree 217/2017; ICMBio, 2017a,b). The latest decrees (63/2018; Brasil, 2018a,b), which came into force on June 1, 2019, allow the capture of parrotfishes inside formally instituted management areas, like partially protected MPAs, but only after the elaboration of a comprehensive management plan, built in partnership among the government, scientists, and fishers. Other endangered species (e.g., Mycteroperca bonaci, M. interstitialis, Lutjanus cyanopterus, Epinephelus morio, and E. marginatus) are now also allowed to be fished, but under different management rules, focused on seasonal closures and catch limits (Brasil, 2018c,d).
In Brazil, most partially protected MPAs are APAs (Environmental Protected Areas), which aims protect the biodiversity while managing human activities (Brasil, 2000). Other partially protected MPAs are established to preserve and promote the sustainability of subsistence harvesting by traditional communities that depend on natural resource extraction for their livelihoods, including RESEX (Extractive Reserve) and RDS (Sustainable Development Reserve) (Brasil, 2000). Therefore, the main goal of the inverted management strategy is to reduce the vulnerability of endangered parrotfishes while simultaneously promoting economic and social benefits to coastal and traditional communities through these partially protected MPAs.
Following the rules, endangered parrotfishes are no longer allowed to be captured elsewhere, except in the few management areas (mostly encompassing those traditional small-scale fishing grounds), based on rules that include catch size limits and quotas, closing fisheries period during spawning season, and long-term monitoring to avoid overexploitation (Brasil, 2018a,b). The RESEX of Cassurubá, located in the northeastern Brazilian coast, for instance, was the first to approve its management plan allowing parrotfish fisheries (ICMBio, 2019). In the current scenario, similar to previous nationwide bans, parrotfish catches would remain prohibited in most of the Brazilian coast (i.e., outside MPAs). Thus, once properly enforced, the ban of parrotfish fishing outside MPAs would allow the recovery of populations, and increment the sources of larvae and juveniles to replenish and help fisheries inside sustainable use management areas. Contrarily to what happens in MPAs worldwide, the density effect benefits would come from outside of the reserves (i.e., “spill in”; Figure 3), hence the term “inverted management.”
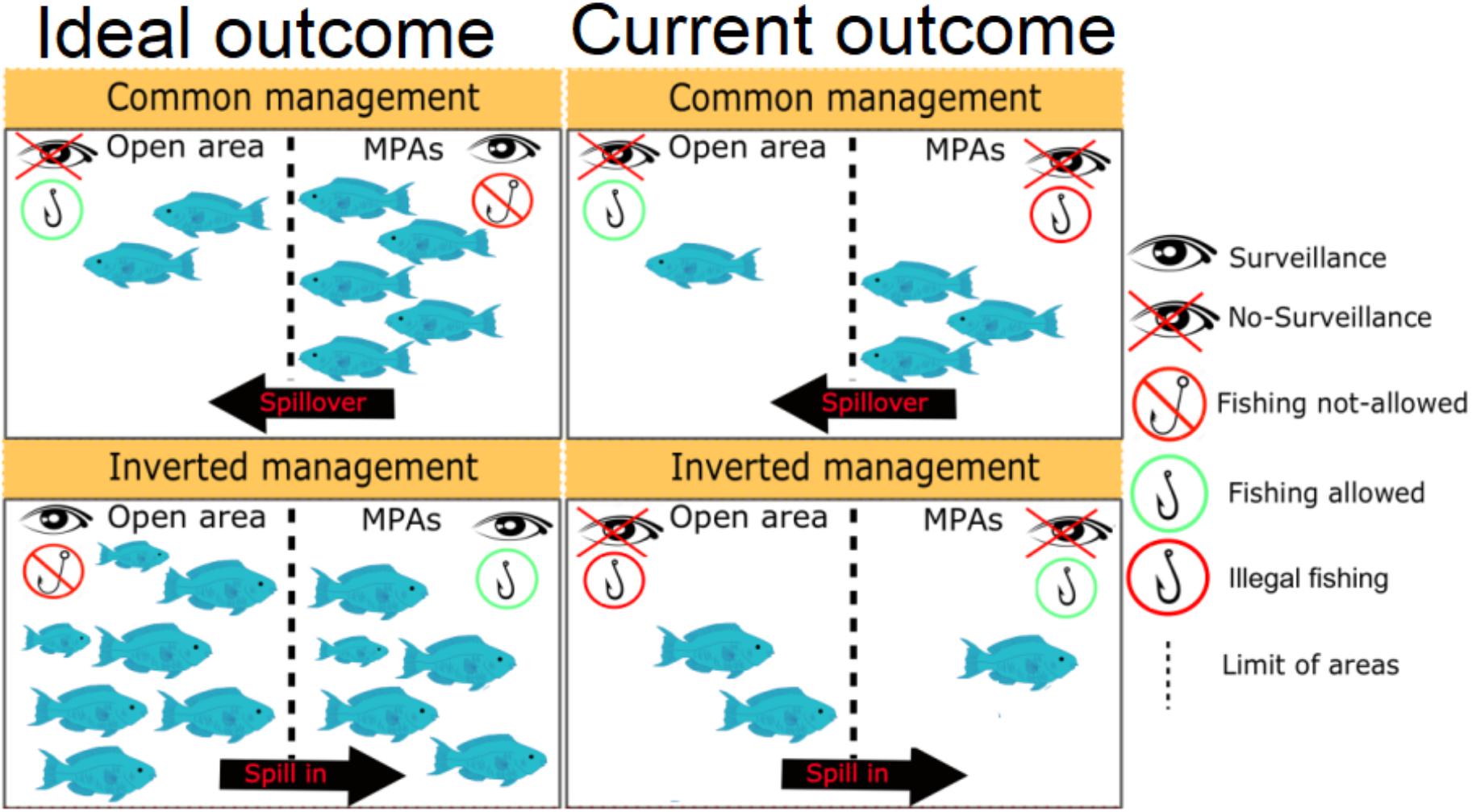
Figure 3. Scheme contrasting management strategies and showing the ideal outcomes versus the current outcomes of low- and lower-middle income countries marine protected areas.
Caveats and Recommendations
The Brazilian coastline has a large extension (∼8.000 km) and many coastal communities have depended on its resources for centuries (Victoria et al., 2005). Therefore, fish population collapses due to overfishing can have catastrophic effects to these traditional fishing communities. Nevertheless, the long-lasting lack of comprehensive national fishery statistics data leaves both scientists and managers in the dark about fish stock conditions and the effectiveness of conservation efforts.
Differently from the strategy adopted by Caribbean countries that has succeed to recover parrotfish stocks (O’Farrell et al., 2016; Healthy Reefs, 2021), the enforcement of inverted management in Brazil seems extremely difficult and currently flawed. First, the strategy depends on the quality of reef habitats outside MPAs for stocks recovery, which are usually more degraded than managed areas. For instance, although parrotfishes are distributed along the whole Brazilian coast (Roos et al., 2015), inside and outside MPAs, they show the highest biomass in isolated or protected sites (Morais et al., 2017), what reduces the “spill in” potential. Some of the Brazilian oceanic islands sustain apparently isolated populations which demands specific management (Guabiroba et al., 2020) and would not function as source for coastal populations.
Second, there are challenging management rules that are not being enforced, namely the surveillance of the catch source, and the amount and the individual length of captured fishes. Fishers that perform their activities in and out MPAs and fish at night can easily (and often do) deceive managers about the origin of the catch. Additionally, those that use non-selective methods, such as gill nets and purse seine, catch protected species everywhere. Mislabeling is also another common problem that masks illegal fishing, trade and consumption of endangered species in Brazil (Carvalho et al., 2011; Staffen et al., 2017). Thus, these fishing strategies are remarkably challenging to monitor and enforce. In addition, the endangered species are commonly targeted by recreational spearfishing, including night fisheries (Nunes et al., 2012; Pinheiro and Joyeux, 2015), what exacerbates the monitoring and enforcement due the extensive heterogeneity in space and time with multiple access points through the year.
Third, marine protected areas in Brazil receive little funding and suffer from a chronic lack of surveillance and monitoring, what inevitably leads to illegal fishing (Gerhardinger et al., 2011; Oliveira Júnior et al., 2016). Most partially protected MPAs do not receive any government funding, and the few examples that succeed are managed with strong community engagement (see Almeida et al., 2009; Nobre et al., 2017). So far, very few MPAs have incorporated rules for endangered species in their management plan, and those that have done so (e.g., ICMBio, 2019) have not yet strongly enforced it. Within this scenario, fishing activities are currently occurring outside and inside MPAs without any management (Figure 3), which is the worst possible scenario.
Therefore, to overcome these challenges, we first suggest a wide communication program focused on: (A) the prohibition, (B) severe limitations on commercialization, (C) parrotfish ecological importance, and (D) stakeholders participation on fisheries data collection and population monitoring. Fishing of endangered species needs to be broadly understood as an exception, with fishers, businesses, and consumers well aware of the prohibition. It is also essential to increase efforts to inform both commercial and recreational fishers about the ecological problems related to overfishing, how stocks increase could benefit the whole society, and the importance for them to provide fisheries data. Second, partially protected MPAs intending to manage the fishing of endangered species must explicitly state in their management plans the methods for accrediting and surveilling fishing activities, markets, and restaurants. A very limited number of small-scale fishers should be allowed to catch endangered species, and the establishment of quotas and body size range are important to preserve recruits and sexually mature individuals. Surveillance should be based on co-management and alternative livelihood options, which has been largely successful at meeting social, economic, and conservation goals (Cinner et al., 2012). Third, scientists, managers, and stakeholders should be involved, assessing endangered populations and offering feedback to fishers and the government. Nursery and spawning grounds need to be mapped and protected.
Last, although we think that the inverted management strategy can be successful if enforcement exists, many endangered species still need full protection to properly recover. Therefore, the strategy could have better results if implemented later after a fishing ban, when populations are partially recovered from the current overfishing status outside MPAs. Thus, we think that the fishery of the large-bodied and endemic greenback parrotfish Scarus trispinosus must not be part of this strategy. This species has suffered the most severe population declines in the past 20 years, leading some populations to collapse (Padovani-Ferreira et al., 2012; Bender et al., 2014; Roos et al., 2020; Pereira et al., 2021). Currently, S. trispinosus is functionally extinct in southeastern Brazil (Bender et al., 2014), and rare in most of the northeastern coast, with the only remaining large populations being restricted to a few regions and MPAs. Additionally, recent data on S. trispinosus growth rate and reproduction indicate that this species has slow growth and late maturity (Freitas et al., 2019), what makes them vulnerable to overfishing similarly to other large parrotfishes, such as the rainbow parrotfish Scarus guacamaia (its sister species) and the green humphead parrotfish Bolbometopon muricatum (Freitas et al., 2019). Since they are part of the charismatic coral reef megafauna, greenback parrotfishes may have more value alive than dead, contributing to local tourism and visitation. Colorful species like parrotfishes are a popular species and one of the photographed by recreational divers (Marconi et al., 2020). Brazil has already examples of successful projects involving the conservation of charismatic megafauna (sea turtles) through tourism and sustainable development of coastal communities (Marcovaldi and Marcovaldi, 1999).
Thus, we strongly advocate that fishing of endangered species should only start when these recommendations about the surveillance and monitoring systems are ready for implementation. Inverted management should not involve species listed as endangered before its effectiveness is demonstrated and only then it should be applied to a wider variety of other endangered and over-exploited species (such as S. trispinosus and endangered top predators).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author contribution section is mandatory and a standard statement has been inserted. Please edit as needed to accurately reflect the contribution of each author. All authors contributed equally to this study.
Funding
CMF was supported by a Young Talent fellowship through CAPES (Coordination for the Improvement of Higher Education Personnel) Brazil (grant no. 88887.194805/2018). HP and LR are supported by the Hope for Reefs initiative of the California Academy of Sciences. MH-S thanks the FAPES for Research Support Program grant (T.O: 221/2019) and CNPq for Productivity Research grant (312278/2017-9).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the researchers Roberta Bonaldo, João Paulo Krajewski, Ronaldo Francini-Filho, Francisco Barros, Guilherme Longo, Natalia Roos, Sergio Floeter, Fabiana Hackradt, Carlos Hackradt, and Diego R. Barneche for comments on early versions of this manuscript. We also appreciate the comments and suggestions of the two reviewers, which contributed significantly to improve the manuscript.
References
Almeida, O. T., Lorenzen, K., and Mcgrath, D. G. (2009). Fishing agreements in the lower Amazon: for gain and restraint. Fish. Manag. Ecol. 16, 61–67. doi: 10.1111/j.1365-2400.2008.00647.x
Balmford, A., Gravestock, P., Hockley, N., Mcclean, C. J., and Roberts, C. M. (2004). The worldwide costs of marine protected areas. Proc. Natl. Acad. Sci. U.S.A. 101, 9694–9697.
Bellwood, D. R., Goatley, C. H. R., and Bellwood, O. (2016). The evolution of fishes and corals on reefs: form, function and interdependence. Biol. Rev. 92, 878–901. doi: 10.1111/brv.12259
Bellwood, D. R., Hoey, A. S., and Hughes, T. P. (2012). Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc. R. Soc. B Biol. Sci. 279, 1621–1629. doi: 10.1098/rspb.2011.1906
Bender, M. G., Machado, G. R., Silva, P. J. A., Floeter, S. R., Monteiro-Netto, C., Luiz, O. J., et al. (2014). Local ecological knowledge and scientific data reveal overexploitation by multigear artisanal fisheries in the Southwestern Atlantic. PLoS One 9:e110332. doi: 10.1371/journal.pone.0110332
Bonaldo, R. M., Hoey, A. S., and Bellwood, D. R. (2014). The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. An Annu. Rev. 52, 81–132. doi: 10.1201/b17143-3
Brasil (2000). Lei No 9985, de 18 de Julho de 2000. Available online at: http://www.planalto.gov.br/ccivil_03/leis/L9985.htm (accessed June 02, 2020).
Brasil (2018a). Portaria No 63, de 31 de Dezembro de 2018. Available online at: http://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/57734204/do1-2019-01-03-portaria-n-63-de-31-de-dezembro-de-2018-57733751 (accessed June 02, 2020).
Brasil (2018b). Portaria Interministerial N 59-B, de 9 de Novembro de 2018. Available online at: http://www.in.gov.br/web/guest/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/50539608/do1e-2018-11-16-portaria-interministerial-n-59-b-de-9-de-novembro-de-2018-50539540 (accessed June 02, 2020).
Brasil (2018c). Portaria Interministerial No 41, de 27 de Julho de 2018. Available online at: http://www.in.gov.br/web/guest/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/34554963/do1-2018-07-30-portaria-interministerial-n-41-de-27-de-julho-de-2018-34554919 (accessed June 02, 2020).
Brasil (2018d). Portaria Interministerial No 59-C, de 9 de Novembro de 2018. Available online at: http://www.in.gov.br/web/guest/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/50539596/do1e-2018-11-16-portaria-interministerial-n-59-c-de-9-de-novembro-de-2018-50539462 (accessed June 02, 2020).
Cannon, J. C. (2018). Parrotfish, Critical to Reef Health, Now Protected Under Mexican Law. Mongabay Series Ocean. Menlo Park, CA: MONGABAY.
Carvalho, D. C., Neto, D. A. P., Brasil, B. S. A. F., and Oliveira, D. A. A. (2011). DNA barcoding unveils a high rate of mislabeling in a commercial freshwater catfish from Brazil. Mitochrondrial DNA 22, 97–105. doi: 10.3109/19401736.2011.588219
Cinner, J. E., Mcclanahan, T. R., Macneil, M. A., Graham, N. A. J., Daw, T. M., Mukminin, A., et al. (2012). Comanagement of coral reef social-ecological systems. Proc. Natl. Acad. Sci. U.S.A. 109, 5219–5222. doi: 10.1073/pnas.1121215109
Cinner, J. E., Zamborain-Mason, J., Gurney, G. G., Graham, N. A. J., Macneil, M. A., Hoey, A. S., et al. (2020). Meeting fisheries, ecosystem function, and biodiversity goals in a human-dominated world. Science 311, 307–311.
Comeros-Raynal, M. T., Choat, J. H., Polidoro, B. A., Clements, K. D., Abesamis, R., Craig, M. T., et al. (2012). The likelihood of extinction of iconic and dominant herbivores and detritivores of coral reefs: the parrotfishes and surgeonfishes. PLoS One 7:e39825. doi: 10.1371/journal.pone.0039825
Cox, C. E., Jones, C. D., Wares, J. P., Castillo, K. D., Mcfield, M. D., and Bruno, J. F. (2013). Genetic testing reveals some mislabeling but general compliance with a ban on herbivorous fish harvesting in Belize. Conserv. Lett. 6, 132–140. doi: 10.1111/j.1755-263X.2012.00286.x
Edwards, C. B., Friedlander, A. M., Green, A. G., Hardt, M. J., Sala, E., Sweatman, H. P., et al. (2014). Global assessment of the status of coral reef herbivorous fishes: evidence for fishing effects. Proc. R. Soc. B Biol. Sci. 281:20131835. doi: 10.1098/rspb.2013.1835
FAO (2018). The Republic of Palau [WWW Document]. Fishery and Aquaculture Country Profiles. Rome: FAO.
Freitas, M. O., Previero, M., Leite, J. R., Francini-Filho, R. B., Minte-Vera, C. V., and Moura, R. L. (2019). Age, growth, reproduction and management of Southwestern Atlantic’s largest and endangered herbivorous reef fish, Scarus trispinosus Valenciennes, 1840. PeerJ 7:e7459. doi: 10.7717/peerj.7459
Fryxell, J., Sinclair, A., and Caughley, G. (2014). Wildlife Ecology, Conservation, and Management. Hoboken, NJ: Wiley-Blackwell.
Gerhardinger, L. C., Godoy, E. A., Jones, P. J. S., Sales, G., and Ferreira, B. P. (2011). Marine protected dramas: the flaws of the brazilian national system of marine protected areas. Environ. Manage. 47, 630–643. doi: 10.1007/s00267-010-9554-7
Gill, D. A., Mascia, M. B., Ahmadia, G. N., Glew, L., Lester, S. E., Barnes, M., et al. (2017). Capacity shortfalls hinder the performance of marine protected areas globally. Nature 543, 665–669. doi: 10.1038/nature21708
Guabiroba, H. C., Santos, M. E. A., Pinheiro, H. T., Simon, T., Pimentel, C. R., Vilar, C. C., et al. (2020). Trends in recreational fisheries and reef fish community structure indicate decline in target species population in an isolated tropical oceanic island. Ocean Coast. Manag. 191:105194. doi: 10.1016/j.ocecoaman.2020.105194
Hawkins, J. (2003). Effects of fishing on sex-changing Caribbean parrotfishes. Biol. Conserv. 115, 213–226. doi: 10.1016/S0006-3207(03)00119-8
Healthy Reefs (2021). Healthy Reefs for Healthy People. Available online at: https://www.healthyreefs.org/cms/ (accessed January 19, 2020).
ICMBio (2014). Portaria MMA No 445, de 17 de dezembro de 2014. Available online at: https://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/00- saiba-mais/05_-_PORTARIA_MMA_Nº_445_DE_17_DE_DEZ_DE_2014.pdf (accessed June 02, 2020).
ICMBio (2017a). Portaria No 161, de 20 de Abril de 2017. Available online at: https://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/00-saiba-mais/05.3_-_PORTARIA_MMA_N°_161_DE_20_DE_ABR_DE_2017.pdf (accessed June 02, 2020).
ICMBio (2017b). Portaria no 217, de 19 de junho de 2017. Available online at: https://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/00-saiba-mais/05.5_-_RETIFICAÇÃO_MMA_DE_22_DE_JUN_DE_2017.pdf (accessed June 02, 2020).
ICMBio (2019). Resex de Cassuruba. Plano de Manejo - planejamento. Available online at: https://www.icmbio.gov.br/portal/images/stories/plano-de-manejo/plano_de_manejo_resex_de_cassuruba_planejamento_vol2.pdf (accessed June 02, 2020).
Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Laffoley, D., Baxter, J. M., Day, J. C., Wenzel, L., Bueno, P., and Katherine, Z. (2019). “Chapter 29 world seas: an environmental evaluation,” in World Seas: An Environmental Evaluation, Vol. III, ed. C. Sheppard (Cambridge, MA: Academic Press).
Lellys, N. T., Moura, R. L., Bonaldo, R. M., Francini-Filho, R. B., and Gibran, F. Z. (2019). Parrotfish functional morphology and bioerosion on SW Atlantic reefs. Mar. Ecol. Prog. Ser. 629, 149–163. doi: 10.3354/meps13102
Long, R. D., Charles, A., and Stephenson, R. L. (2015). Key principles of marine ecosystem-based management. Mar. Policy 57, 53–60. doi: 10.1016/j.marpol.2015.01.013
Marconi, M., Giglio, V. J., Pereira-Filho, G. H., and Motta, F. S. (2020). Does quality of scuba diving experience vary according to the context and management regime of marine protected areas? Ocean Coast. Manage. 194:105246. doi: 10.1016/j.ocecoaman.2020.105246
Marcovaldi, A., and Marcovaldi, G. G. (1999). Marine turtles of Brazil: the history and structure of Projeto TAMAR-IBAMA. Biol. Conserv. 91, 35–41. doi: 10.1016/s0006-3207(99)00043-9
Mcclanahan, T. R., Marnane, M. J., Cinner, J. E., and Kiene, W. E. (2006). A comparison of marine protected areas and alternative approaches to coral-reef management. Curr. Biol. 16, 1408–1413. doi: 10.1016/j.cub.2006.05.062
Mittermeier, R. A., Mittermeier, C. G., Brooks, T. M., Pilgrim, J. D., Konstant, W. R., Fonseca, G. A. B., et al. (2003). Wilderness and biodiversity conservation. Proc. Natl. Acad. Sci. U.S.A. 100, 10309–10313.
Morais, R. A., Ferreira, C. E. L., and Floeter, S. R. (2017). Spatial patterns of fish standing biomass across Brazilian reefs, Southwestern Atlantic. J. Fish Biol. 91, 1642–1667. doi: 10.1111/jfb.13482
Nobre, D. M., Alarcon, D. T., Cinti, A., and Schiavetti, A. (2017). Governance of the Cassurubá Extractive Reserve, Bahia State, Brazil: an analysis of strengths and weaknesses to inform policy. Mar. Policy 77, 44–55. doi: 10.1016/j.marpol.2016.12.008
Nunes, J. A. C. C., Medeiros, D. V., Reis-Filho, J. A., Sampaio, C. L. S., and Barros, F. (2012). Reef fishes captured by recreational spearfishing on reefs of Bahia State, northeast Brazil. Biota Neotrop. 12, 1–7.
O’Farrell, S., Luckhurst, B. E., Box, S., and Mumby, P. J. (2016). Parrotfish sex ratios recover rapidly in Bermuda following a fishing ban. Coral Reefs 35, 421–425. doi: 10.1007/s00338-015-1389-5
Oliveira Júnior, J. G. C., Ladle, R. J., Correia, R., and Batista, V. S. (2016). Measuring what matters – Identifying indicators of success for Brazilian marine protected areas. Mar. Policy 74, 91–98. doi: 10.1016/j.marpol.2016.09.018
Padovani-Ferreira, B., Floeter, S. R., Rocha, L. A., Ferreira, C. E. L., Francini-Filho, R. B., Moura, R., et al. (2012). Scarus trispinosus. The IUCN Red List of Threatened Species. 2012:e.T190748A17786694.
Pattanayak, S. K., Wunder, S., and Ferraro, P. J. (2010). Show me the money: do payments supply environmental services in developing countries? Rev. Environ. Econ. Policy 4, 254–274. doi: 10.1093/reep/req006
Pereira, P. H. C., Ternes, M. L. F., Nunes, J. A. C. C., and Giglio, V. J. (2021). Overexploitation and behavioral changes of the largest South Atlantic parrotfish (Scarus trispinosus): evidence from fishers’ knowledge. Biol. Conserv. 254:108940. doi: 10.1016/j.biocon.2020.108940
Pinheiro, H. T., Di Dario, F., Gerhardinger, L. C., Melo, M. R. S., Moura, R. L., Reis, R. E., et al. (2015). Brazilian aquatic biodiversity in peril. Science 350, 1043–1044. doi: 10.1126/science.350.6264.1043-a
Pinheiro, H. T., and Joyeux, J.-C. (2015). The role of recreational fishermen in the removal of target reef fishes. Ocean Coast. Manag. 112, 12–17. doi: 10.1016/j.ocecoaman.2015.04.015
Pinheiro, H. T., Joyeux, J.-C., and Martins, A. S. (2010). Reef fisheries and underwater surveys indicate overfishing of a brazilian Coastal Island. Nat. Conserv. 08, 151–159. doi: 10.4322/natcon.00802008
Pinheiro, H. T., Teixeira, J. B., Francini-Filho, R. B., Soares-Gomes, A., Ferreira, C. E. L., and Rocha, L. A. (2019). Hope and doubt for the world’s marine ecosystems. Perspect. Ecol. Conserv. 17, 19–25. doi: 10.1016/j.pecon.2018.11.001
Reynolds, J., and Peres, C. (2005). “Overexploitation,” in Principles of Conservation Biology, eds M. Groom, C. Meffe, and C. Carooll (Sunderland, MA: Sinauer), 253–291.
Roos, N. C., Carvalho, A. R., Lopes, P. F. M., and Pennino, M. G. (2015). Modeling sensitive parrotfish (Labridae: Scarini) habitats along the Brazilian coast. Mar. Environ. Res. 110, 92–100. doi: 10.1016/j.marenvres.2015.08.005
Roos, N. C., Taylor, B. M., Carvalho, A. R., and Longo, G. O. (2020). Demography of the largest and most endangered Brazilian parrotfish, Scarus trispinosus, reveals overfishing. Endanger. Species Res. 41, 319–327. doi: 10.3354/esr01024
SEGOB. (2019). Diario Oficial de la Federación. Available online at: https://www.dof.gob.mx/nota_detalle.php?codigo=5576689&fecha=28/10/2019 (accessed October 28, 2019)
Siqueira, A. C., Bellwood, D. R., and Cowman, P. F. (2019). The evolution of traits and functions in herbivorous coral reef fishes through space and time. Proc. R. Soc. B Biol. Sci. 286:20182672. doi: 10.1098/rspb.2018.2672
Staffen, C. F., Staffen, M. D., Becker, M. L., Löfgren, S. E., Muniz, Y. C. N., Aché, R. H., et al. (2017). DNA barcoding reveals the mislabeling of fish in a popular tourist destination in Brazil. PeerJ 5:e4006. doi: 10.7717/peerj.4006
Island Times (2018). Bill Allowing the Fishing of Napoleon Wrasse, Bumphead Parrotfish deferred. Website. https://islandtimes.org/bill-allowing-the-fishing-of-napoleon-wrasse-bumphead-parrotfish-deferred/
Victoria, I., Martins, A. S., Haimovici, M., and Andriguetto-Filho, J. M. (2005). A Pesca Marinha e Estuarina do Brasil no Início do Século XXI: Recursos, Tecnologias, Aspectos Socioeconômicos e Institucionais. Belém: Editora Universitária UFPA.
Waterhouse, L., Heppell, S. A., Pattengill-Semmens, C. V., McCoy, C., Bush, P., Johnson, B. C., et al. (2020). Recovery of critically endangered Nassau grouper (Epinephelus striatus) in the Cayman Islands following targeted conservation actions. Proc. Natl. Acad. Sci. U.S.A. 117, 1587–1595. doi: 10.1073/pnas.1917132117
Williams, G. J., and Graham, N. A. J. (2019). Rethinking coral reef functional futures. Funct. Ecol. 33, 942–947. doi: 10.1111/1365-2435.13374
WWF (2018). The Case for MPAs [WWW Document]. Available online at: http://wwf.panda.org/what_we_do/how_we_work/our_global_goals/oceans/solutions/protection/protected_areas/ (accessed February 11, 2020).
Keywords: marine protected area, fishing, parrotfish, overfishing, endangered species, socio-ecological system
Citation: Pinheiro HT, Nunes JACC, Coni EOC, Almeida ECG, Sampaio CLS, Ferreira CEL, Meirelles PM, Hostim-Silva M, Pereira PHC, Giglio VJ, Gasparini JL, Rocha LA and Ferreira CM (2021) An Inverted Management Strategy for the Fishery of Endangered Marine Species. Front. Mar. Sci. 8:604108. doi: 10.3389/fmars.2021.604108
Received: 08 September 2020; Accepted: 10 February 2021;
Published: 08 March 2021.
Edited by:
José Amorim Reis-Filho, Federal University of Pará, BrazilReviewed by:
Marco Andrello, Consiglio Nazionale delle Ricerche (CNR), ItalyJuan Carlos Villaseñor-Derbez, University of California, Santa Barbara, United States
Copyright © 2021 Pinheiro, Nunes, Coni, Almeida, Sampaio, Ferreira, Meirelles, Hostim-Silva, Pereira, Giglio, Gasparini, Rocha and Ferreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hudson T. Pinheiro, aHRwaW5oZWlyb0BnbWFpbC5jb20=
 Hudson T. Pinheiro
Hudson T. Pinheiro Jose A. C. C. Nunes
Jose A. C. C. Nunes E. O. C. Coni3
E. O. C. Coni3 E. C. G. Almeida
E. C. G. Almeida C. L. S. Sampaio
C. L. S. Sampaio Carlos E. L. Ferreira
Carlos E. L. Ferreira Pedro M. Meirelles
Pedro M. Meirelles M. Hostim-Silva
M. Hostim-Silva Pedro H. C. Pereira
Pedro H. C. Pereira Vinicius J. Giglio
Vinicius J. Giglio L. A. Rocha
L. A. Rocha