
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 14 January 2021
Sec. Global Change and the Future Ocean
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.615214
This article is part of the Research Topic Marine Ecosystem Assessment for the Southern Ocean: Meeting the Challenge for Conserving Earth Ecosystems in the Long Term View all 25 articles
A correction has been applied to this article in:
Corrigendum: Future Risk for Southern Ocean Ecosystem Services Under Climate Change
 Rachel D. Cavanagh1*
Rachel D. Cavanagh1* Jess Melbourne-Thomas2,3
Jess Melbourne-Thomas2,3 Susie M. Grant1
Susie M. Grant1 David K. A. Barnes1
David K. A. Barnes1 Kevin A. Hughes1
Kevin A. Hughes1 Svenja Halfter4
Svenja Halfter4 Michael P. Meredith1
Michael P. Meredith1 Eugene J. Murphy1
Eugene J. Murphy1 Rowan Trebilco2,3
Rowan Trebilco2,3 Simeon L. Hill1
Simeon L. Hill1The Southern Ocean supports ecosystem services that are important on a global scale. Climate change and human activities (tourism, fishing, and research) will affect both the demand for, and the provision of, these services into the future. Here we synthesize recent assessments of the current status and expected future climate-driven changes in Southern Ocean ecosystems and evaluate the potential consequences of these changes for the provision of ecosystem services. We explore in detail three key services (the ‘blue carbon’ pathway, the Antarctic krill fishery, and Antarctic tourism), tracing the consequences of climate change from physical drivers through biological impacts to the benefits to humans. We consider potential non-climatic drivers of change, current and future demands for the services, and the main global and regional policy frameworks that could be used to manage risks to the provision of these services in a changing climate. We also develop a formal representation of the network of interactions between the suite of potential drivers and the suite of services, providing a framework to capture the complexity of this network and its embedded feedback loops. Increased consideration of the linkages and feedbacks between drivers and ecosystem services will be required to underpin robust management responses into the future.
Ecosystem services are the benefits that people obtain from ecosystems (Costanza et al., 1997; Millennium Ecosystem Assessment [MEA], 2005). Despite ongoing debate about the concept, this area of research continues to grow, with the recognition that incorporating measures of ecosystem services into assessments of change provides a useful means of integrating environmental, economic and social factors, and the development of comprehensive frameworks to help achieve this (Costanza et al., 1997; UNEP, 2010; United Nations et al., 20141; Guerry et al., 2015; Seddon et al., 2016; Townsend et al., 2018; IPBES, 2019; Bateman and Mace, 2020). The extent to which people benefit from ecosystem services depends on both the ecosystem’s capacity to supply them and society’s demand for, and use of, them. The capacity to supply ecosystem services is a function of both ecosystem structure and process (Figure 1). For example, the capacity to supply harvestable fish depends on the existence and availability of those fish (structure), and the production of new biomass (process). Both the capacity to supply ecosystem services and the demand for them may be influenced by a variety of drivers, with climate-driven environmental change potentially a key driver of both. Information on how supply and demand may change in future is thus a critical requirement for decision-making in relation to the conservation of ecosystems, the management of human activities that affect these ecosystems and for planning the response to climate-driven change. However, despite an increasing body of research on the impacts of climate change on ecosystem services (Mooney et al., 2009; Montoya and Raffaelli, 2010; Pedrono et al., 2016), quantitative syntheses are limited (Runting et al., 2017).
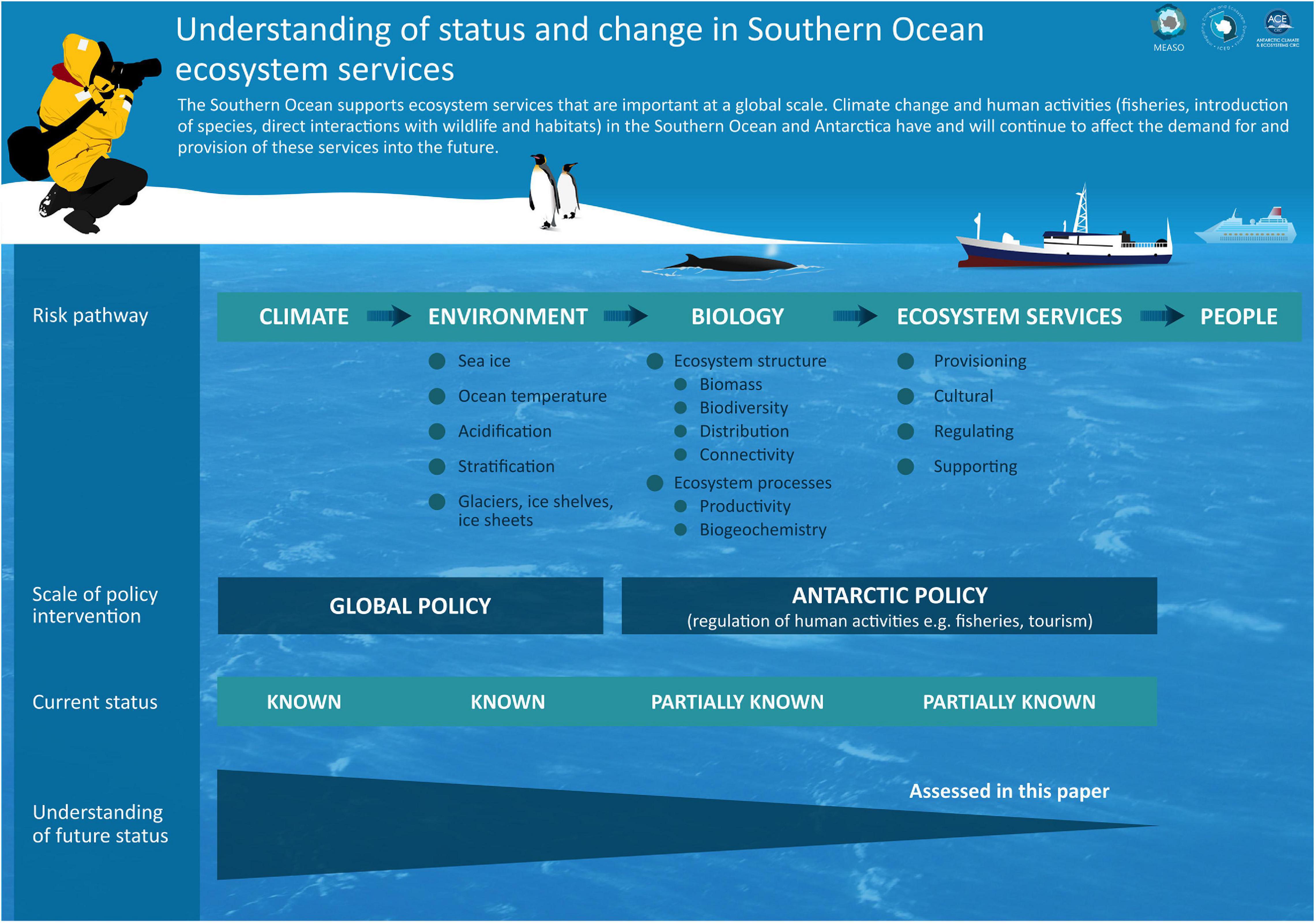
Figure 1. Key risk pathways affecting Southern Ocean ecosystem services, from climate change impacts on physical drivers through to biological impacts and on to the benefits obtained by humans. The variables listed under ‘Environment’ and ‘Biology’ are examples of the wider suite of variables that could be affected.
The focus of this study is on that part of the Southern Ocean overseen by the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) (2hereafter we refer to this as the Southern Ocean for simplicity). The Southern Ocean is amongst the most rapidly changing oceans of the world, with consequences for global-scale storage and cycling of heat, carbon and other climatically- and ecologically-important properties. For example, the circulation and properties of Southern Ocean water masses are changing due to atmospheric effects including the loss of stratospheric ozone and increasing greenhouse gas concentrations, and are, in turn, affecting the physical and biological carbon pumps. Ocean temperatures are increasing; sea ice duration and extent is changing; and ocean acidification is advancing markedly in polar waters (Mayewski et al., 2009; Turner et al., 2009, 2014; Constable et al., 2014; Meredith et al., 2019; Morley et al., 2020). Such changes exert influences on biota and thence ecosystem services (Figure 1). The instruments of the Antarctic Treaty System (ATS) recognise the importance of Antarctic ecosystems to people in terms of their living resources, their value to scientific research, and their “wilderness and aesthetic value.” The entire area south of 60°S is protected under the Antarctic Treaty3, while the Convention on the Conservation of Antarctic Marine Living Resources extends further north to the Polar Front (Grant et al., in preparation), and underpins the management of fishing activities in the Southern Ocean. The Convention entered into force in 1982, and established CCAMLR4 as its decision-making body. Information on how Southern Ocean ecosystems are changing, and what this means for their conservation and management, is becoming increasingly important for CCAMLR and the wider ATS (Grant and Penhale, 2016; Hughes et al., 2018).
Over the last decade extensive research has been undertaken to understand the effects of change on Southern Ocean ecosystems, with increasing recognition of the need for interdisciplinary approaches to integrate traditionally separate scientific disciplines such as climate science and ecology (Smetacek and Nicol, 2005; Murphy et al., 2008). In addition to national research programmes, there are a number of initiatives to coordinate international observation and research efforts, for example Scientific Committee on Antarctic Research (SCAR) programmes and initiatives such as the Southern Ocean Observing System (SOOS); the Integrated Climate and Ecosystem Dynamics in the Southern Ocean (ICED) Programme [co-sponsored by SCAR and the Integrated Marine Biosphere Research (IMBeR) project], which includes the MEASO (Marine Ecosystem Assessment for the Southern Ocean); and the United Nations Decade of the Ocean which includes a Southern Ocean contribution (Murphy et al., 2008; Meredith et al., 2013; Hofmann et al., 2020; Constable et al., in preparation). Key research questions include how climate change affects critical environmental variables, and how these changes impact Southern Ocean biota (e.g. see: Constable et al., 2014; Gutt et al., 2015; Rogers et al., 2020). Much of this work is synthesised and assessed in the recently published United Nations’ Intergovernmental Panel on Climate Change (IPCC) Special Report on the Ocean and Cryosphere in a Changing Climate (SROCC) (IPCC, 2019).
While various exercises have assessed ecosystem services in most regions of the globe (Millennium Ecosystem Assessment [MEA], 2005; Guerry et al., 2015; Dunford et al., 2018), similar comprehensive assessments are lacking for the Southern Ocean and Antarctica (Grant et al., 2013b; Pertierra and Hughes, 2019). A number of studies have, however, considered Southern Ocean ecosystem services using a range of approaches. These include an attempt to catalogue the main services at the circumpolar scale (Grant et al., 2013b), regional assessments (Deininger et al., 2016; Neumann et al., 2019) and studies considering the potential effects of change (Murphy et al., 2016; Rogers et al., 2020; Trebilco et al., 2020). However, at present, although it is evident that the Southern Ocean provides globally important ecosystem services, information is sparse on their current status, and how they may change into the future. Such information would improve understanding of the wider consequences of change in Southern Ocean ecosystems (Cavanagh et al., 2016a), and help to ensure that the range of services are adequately recognised in decision-making at regional and global scales (Hill and Grant, 2013).
While the SROCC assessment (and the studies it draws upon) provides a central source of information on the effects of climate change on the Antarctic marine environment, the potential impacts of these changes on Southern Ocean biota, and the level of agreement about, or confidence in, these impacts, it includes only limited information on the implications for ecosystem services. Here, we use the SROCC as our primary source of information on the key drivers of change, and how they are projected to affect the biological components that underpin the suite of Southern Ocean ecosystem services, to undertake a risk assessment regarding the impacts of climate change on the capacity of the ecosystem to deliver the services in the future. Our assessment of risk translates qualitative projected change in drivers to qualitative consequences for ecosystem elements underpinning ecosystem services. This qualitative approach is pragmatic in the absence of specific information on the magnitude of impact to ecosystem services. We acknowledge non-climatic influences, including the large-scale historical perturbation of the Southern Ocean ecosystem as a result of sealing and whaling (discussed in detail in Grant et al., in preparation), and the difficulties of disentangling these impacts and their associated recoveries from the effects of environmental change, particularly in establishing baselines against which future change and risk can be assessed (Smetacek and Nicol, 2005). Our risk assessment also implicitly assumes that other influential factors, including the scale and distribution of ongoing human activities (e.g. fishing, tourism) in the Southern Ocean, remain unchanged, and does not account for regional differences in these factors (e.g. accessibility). We note, however, that the effects of these other drivers, as well as future changes to their scale or geographic reach, could be as important as those of climate change, particularly at local or regional scales (Grant et al., 2013b; Klein et al., 2018; Morley et al., 2020; Grant et al., in preparation). We therefore explore in more detail the potential effects of combined drivers for three case studies focused on key ecosystem services: the ‘blue carbon’ pathway (i.e., the process of carbon capture and fixation by marine organisms, through storage in organism bodies to sequestration), the Antarctic krill fishery, and tourism. We identify key areas of demand and use of these services where relevant, noting that there are considerable regional differences (identified in more detail in Grant et al., in preparation). We also consider future demand for services, and identify the main global and regional policy frameworks that could be used to manage risks to the provision of these services in a changing climate. We use the information in our assessment to develop a formal representation of the network of relationships between this suite of ecosystem services and their drivers. This provides an accessible framework for understanding the complexity of this network, as well as a basis for future modelling to underpin robust management responses into the future.
We extracted information from the SROCC (IPCC, 2019), predominantly Chapter 3 (Meredith et al., 2019) regarding drivers of change that have a clear connection to Southern Ocean ecosystem services. This includes information about future changes in these drivers; the level of confidence in this information (see Abram et al., 2019); the biological significance of this information (and level of confidence if stated); and the ecosystem services to which it relates (Supplementary Table S1). We then used this information to assess understanding of how future climate change might impact the ecosystem services. Following Grant et al. (2013b), we described each ecosystem service, categorised as either provisioning, regulating, supporting and cultural (Millennium Ecosystem Assessment [MEA], 2005). Our study focuses on provisioning, regulating and supporting services, and one cultural service, namely tourism (Supplementary Table S2a), but we did not evaluate other cultural services nor those services that existed either in the past (e.g., the harvesting of the now overfished marbled rockcod Notothenia rossii) and those that might become important in the future (e.g., harvesting of mesopelagic fish). These additional services are listed in Supplementary Table S2b. Note that we did not include the ecosystem services of water or air quality regulation., i.e., the uptake of chemicals and pollutants from the ocean and atmosphere, or waste treatment, i.e., decomposition of organic wastes by bacteria and microorganisms. These are underpinned by a complex network of interacting ecosystem processes and merit separate studies. We also briefly described the biological components that underpin each of the services. We then summarised the projected impacts of climate-related drivers of change on these biological components, acknowledging other drivers, anticipated changes in demand for the service (i.e., the societal need for the service, ranging from the demand for fish as a food source to the reliance on the role of the blue carbon pathway in climate regulation), and whether the service (and/or its biological components) are considered in theory and/or practice by instruments of the ATS (Supplementary Table S2). Our assessment focuses on the circumpolar scale, with regional-scale differences, including the distribution of human activities, discussed in the case studies.
We used this information to undertake a risk assessment regarding the impacts of climate change on the capacity of the ecosystem to deliver each service (in Supplementary Table S2a) in the future. Specifically, we assessed the risk of negative climate-driven impacts on ecosystem services given the conditions in the 2100 forecast for the IPCC Representative Concentration Pathway (RCP) 4.5 scenario (Abram et al., 2019).
The level of risk was determined based on the likelihood of change in each driver (derived from the level of confidence for directional change in these drivers), and the potential consequences for each ecosystem service (derived from the nature of the impact of each driver on biological components underpinning ecosystem services). Negative impacts of climate change are assumed to carry higher risk for ecosystem services. We then assigned confidence to these risk ratings based on the level of confidence regarding the impact of drivers on biological components (as stated in the SROCC, Table 1). A combined assessment of risk across all climate-related drivers for each ecosystem service is presented in the results (Table 2). In determining an overall risk rating for the capacity of the ecosystem to deliver each service in the future we assumed that the drivers do not exacerbate each other, such that the maximum risk for any driver is the overall risk. This assumption is unlikely to be true in all cases, but information regarding the relationship between impacts of drivers (i.e., whether they are antagonistic, neutral or synergistic) is not currently available. Confidence levels are presented for each individual risk pathway (i.e., for each driver-ecosystem service combination).
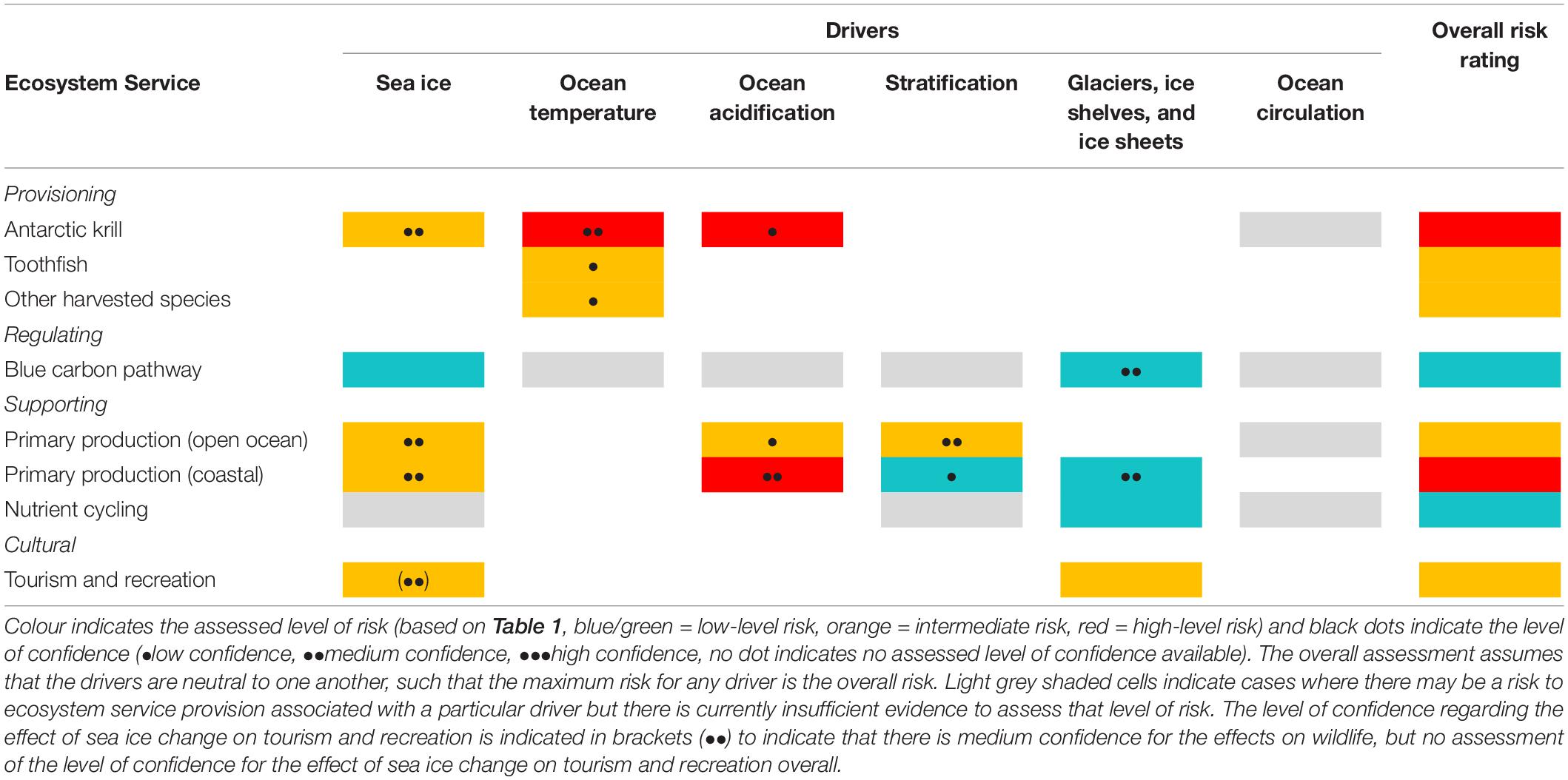
Table 2. Summary risk assessment for ecosystem services (only those in Supplementary Table S2a have been assessed).
We have focused in detail on three key services: the blue carbon pathway, the Antarctic krill fishery, and Antarctic tourism. These were chosen based on their global as well as regional importance (Grant et al., 2013b; Deininger et al., 2016; Murphy et al., in preparation), and because they are clearly distinct from each other, differing in terms of the ecological underpinning, benefits they provide to humans, and how they are managed. Each case study provides a description of the ecosystem service, including its importance to people and the biological components essential to provision of the service. Through the case studies we traced the consequences of climate change from physical drivers through to biological impacts and subsequently to the benefits obtained by people. We relate these to current and future demand for the services, consider other relevant (non-climatic) drivers, and identify the main global and regional policy frameworks that could be used to manage risks to them in the future.
Based on the connections between services, biota and drivers identified in our assessment, we developed a formal network representation of these interactions to better elucidate how the capacity of the ecosystem as a whole to deliver each service may be affected (Figure 2). We used the established symbology for signed digraphs that is used in qualitative network modelling (Melbourne-Thomas et al., 2012; see also Trebilco et al., 2020). Connections between ecosystem services and drivers are identified as positive, negative, positive and/or negative (i.e., direction variable or dependent on context) and weak or uncertain (where the current state of knowledge is such that the strength of these links is unclear). In the context of this paper, the network representation provides a method for tracing risk pathways for particular case studies (see below) and for visualising the connections and feedbacks between different services and drivers. Direct and indirect effects can be identified, providing further context for the risk assessment (where we consider direct effects of drivers only). Biodiversity (which, in this case, also includes ecosystem structure and processes but is labelled simply as ‘biodiversity’ because of space constraints in the network figure) is represented as underpinning ecosystem services (Seddon et al., 2016). It should be noted that the network representation is not intended to be a precise representation of all factors detailed in our assessment and case studies, but instead as a complementary approach for viewing pathways of change.
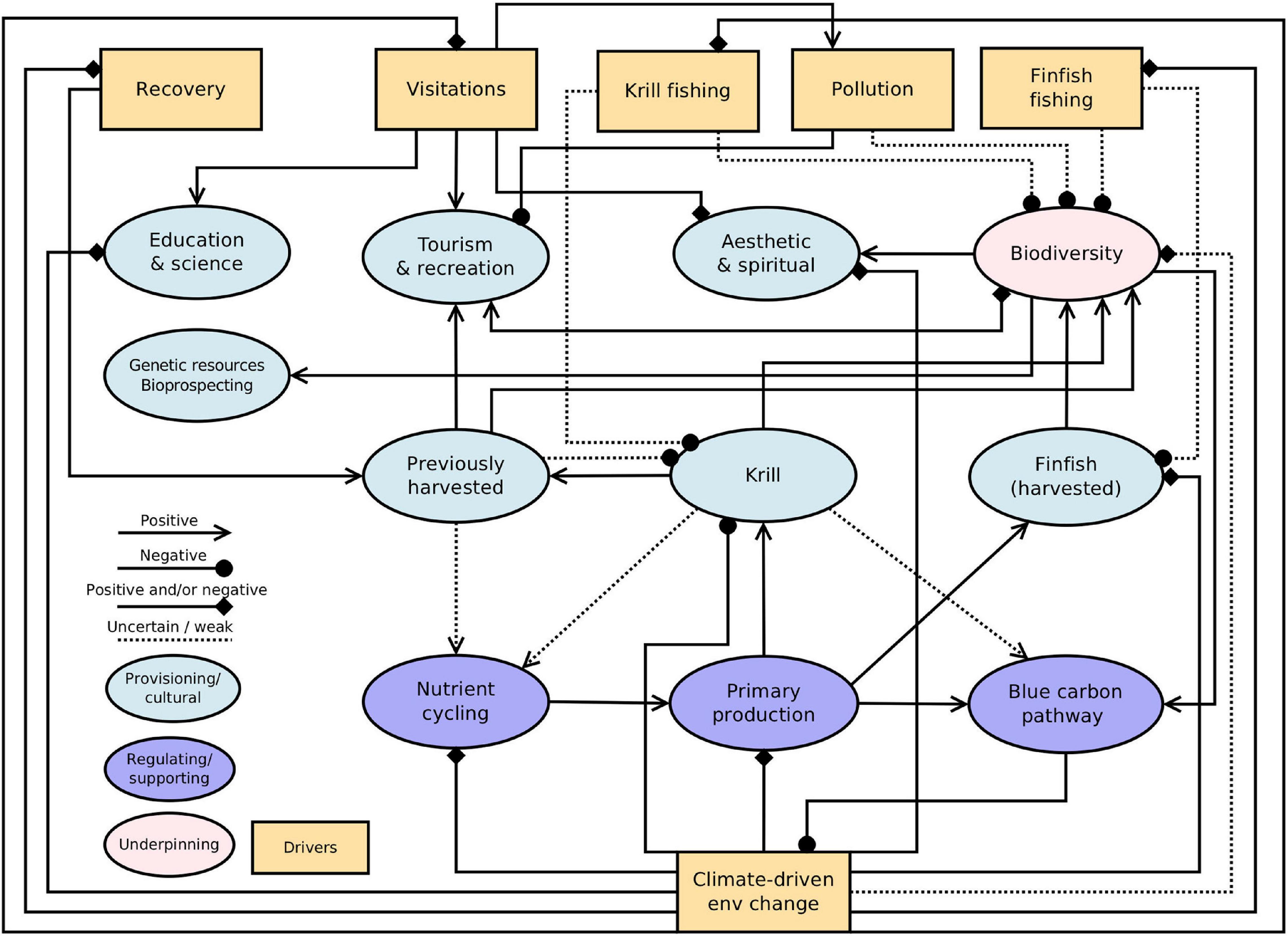
Figure 2. Network representation of the links and feedbacks between ecosystem services and drivers. Connections between components indicate the direction of effects from one node to another, as indicated in the legend. Diamonds indicate cases where the direction of the effect is not known or unclear (i.e., it could be positive and/or negative), and dashed lines represent uncertain or weak linkages. The effect of fishing on associated ecosystem services (krill and finfish) is represented as weak, because we assume that current management is effective in minimising the impact of fishing on the capacity of stocks to continue to deliver ecosystem services. Services and key drivers included in the network are summarised from Supplementary Table S2 and grouped as provisioning and cultural or regulating and supporting. Biodiversity is represented as underpinning ecosystem services (and also includes ecosystem structure and processes) but can be directly affected by drivers, with consequent effects for services. Bioprospecting refers collectively to biochemicals, medicines, and pharmaceuticals. Changes to drivers have cascading effects to ecosystem services, including feedbacks across other linked drivers.
It is evident that many climate-related drivers of change, including sea ice, ocean temperature, ocean acidification, stratification (including mixed layer depth), retreating glaciers, ice sheets and ice shelf loss, and ocean circulation, have clear connections with the delivery of key Southern Ocean ecosystem services through their influence on ecosystem structure and processes (Supplementary Table S1). Our stepwise consideration of the effects of climate-driven change on ecosystem services, from physical drivers (and the nature of projected change in these drivers) through to biological impacts and on to the benefits obtained by people (Figure 1), provides a structured approach to (i) consider the complexity of climate-driven change on ecosystem service delivery and (ii) track uncertainty from drivers to impacts. For example, changes in sea ice habitats are known to have high biological importance, with cascading effects on ecosystem structure and processes that underpin a number of ecosystem services (Supplementary Table S1). Despite low confidence in future projections of Antarctic sea ice, future declines in extent are indicated over the course of the 21st century (Meredith et al., 2019, Supplementary Table S1), with negative associated impacts for Antarctic krill (Euphausia superba) populations (medium confidence), mixed effects on phytoplankton (medium confidence) and on marine mammals and penguins (high confidence), and potentially positive effects on carbon uptake and storage by benthic communities (Meredith et al., 2019, Supplementary Table S2). These impacts will in turn affect the ecosystem’s capacity to deliver provisioning (fishery products), supporting (primary production), regulating (climate regulation), and cultural services (e.g. tourism) (see also Case Studies 2 and 3 below).
Our risk assessment was based on each of the stages in the stepwise approach. Our findings suggest that overall, Southern Ocean provisioning services face an intermediate (finfish) to high-level risk (Antarctic krill) from climate change, with supporting services low-level through to high-level risk, with cultural services and regulation services facing intermediate and low-level risks respectively (Table 2). The information we used in our assessment is summarised in Supplementary Tables S1, S2a. For example, for the Antarctic krill fishery, the key drivers are sea ice, ocean temperature and acidification. The levels of confidence for directional change in these drivers are low, medium, and high respectively, all assumed to have overall negative effects on the biological components of this ecosystem service, which we assign as intermediate, high-level, and high-level risk respectively, as per Table 1, with confidence levels medium, medium, and low respectively. The overall risk to this ecosystem service from climate-driven change is therefore high-level (Table 2). In summary, our assessment indicates that climate change related risk is highest for the Antarctic krill fishery and for coastal primary production (supporting service), that the risk level is intermediate for most services, but that it is currently low-level for the blue carbon pathway and for nutrient cycling (Table 2). Confidence levels underpinning these assessments are generally low-to-medium level.
The network analysis revealed a complex suite of interdependencies, including feedbacks across other linked drivers, such as fishing (Figure 2). Our network representation highlights that not only are there connections and feedbacks between Southern Ocean ecosystem services, but that there is also a high degree of connectivity between drivers. Indeed, climate-driven environmental change is connected to all other human drivers shown in Figure 2 (fishing, visitations, and recovery of previously exploited species), except for pollution. These connections mean that, for example, climate-driven environmental change can affect the capacity of Southern Ocean ecosystems to support tourism and other cultural services, both via direct effects on biodiversity, and indirectly through the effects of enhanced visitations (as areas become more accessible due to reduced sea ice; see Case Study 3 on Antarctic tourism below). Supplementary Table S2 includes brief information on other key drivers (see also Grant et al., in preparation), future demand (see also Chown and Brooks, 2019; Rogers et al., 2020), and conservation and management measures within the ATS. We consider these factors in more detail in the case studies below.
The blue carbon pathway is the process of carbon capture and fixation by marine organisms, through storage in the bodies of organisms (for up to 100 years) to sequestration (which is removal of carbon from the carbon cycle for 100 + years). Southern Ocean continental shelves are seasonally highly productive through carbon capture and fixation by phytoplankton. Some of this carbon sinks to the seabed and is buried, some is stored in the bodies of consumers (zooplankton and benthos) and some is recycled by bacteria. Burial at the seabed can lead to sequestration.
The reduction of atmospheric carbon is a major societal goal (United Nations Framework Convention on Climate Change (UNFCCC) Paris Agreement5 and with the so-called ‘shadow cost of carbon’ (i.e. of industrial carbon capture) reaching ∼US$38-78 tCO2, the storage and sequestration of blue carbon is recognised as a powerful and valuable ecosystem service. Coastal marine habitats are very efficient at carbon pathways but are decreasing in area almost everywhere because of habitat destruction, land reclaim, pollution etc; the exception is the polar regions, where they are increasing due to declines in sea ice, glacier retreat and ice shelf collapse (Barnes et al., 2018; Gogarty et al., 2020). In recent years it has become apparent that biodiversity within polar oceans hold some important ‘natural capital’ in stored carbon. Carbon storage by marine organisms is dwarfed by physical storage in the water, however, it is important to note that biological capture and storage of carbon is increased by ocean warming (negative feedback on global warming) whereas physical storage is decreased (positive feedback), so they respond in different ways to climate change (Barnes et al., 2018).
There are essentially four components to this ES. These are: (1) phytoplankton capture carbon from dissolved CO2; (2) zooplankton and benthos consume the phytoplankton and store this carbon; (3) on death some phytoplankton and animals are buried at the seabed sequestering carbon; but (4) most carbon is recycled in the microbial loop by bacterial breakdown of phytoplankton and animals. Some species are disproportionately important to carbon storage, particularly those with high biomass (e.g., krill) and those which are longer lived (e.g., barrel sponges, corals, and baleen whales). Their impact on genuine carbon sequestration may also be higher but to date remains unclear.
Polar continental shelves have extreme light climate, sea ice (reducing light penetration), and low temperature (slow enzyme work rate). Carbon capture is predominantly in phytoplankton blooms, which differ to those in warmer water by being very brief and dominated by diatoms. These have hard silica tests that are difficult to assimilate without physically rupturing cell walls. Such blooms are limited by the low availability of micronutrients, especially iron, throughout much of the Southern Ocean (Moore and Abbott, 2000). It has been argued however that recovery of baleen whale populations could result in greater nutrient recycling in the upper water column and therefore more intense or widespread blooms (Lavery et al., 2014; Ratnarajah et al., 2015). Carbon capture rates from Antarctic primary production approximate to 200 gC m2yr–1 (Ducklow et al., 2007) but vary with year and region. Phytoplankton are a crucial part of the provision of the ecosystem service, but unlike trees on land, they cannot store carbon because their lives are brief.
The long lived components of polar marine foodwebs are animals, so most carbon storage occurs in these (see Henley et al., 2020). Below regular ice scour depths, Southern Ocean animals develop and grow slowly and live long lives. Antarctica’s continental shelves are deep, wide and little disturbed, all of which allows considerable accumulation of biomass and stored carbon (Arntz et al., 1994). However, the immediate fate of about half of Southern Ocean primary production is breakdown in the water or seabed microbial loop, and so it is recycled (Azam, 1998; Henley et al., 2020). Transfer of carbon to primary consumers in the foodweb is efficient; about 30% amongst pelagic and benthic suspension feeders (Hill et al., 2012; Barnes, 2017, respectively). Rate of carbon accumulation decreases offshore and with depth, seemingly due to reduced exposure to phytoplanktonic food (Barnes, 2017). Of the carbon that is stored in Southern Ocean pelagic and benthic animals, most of this too will be broken down on death through the microbial loop, but some is buried upon death.
Sequestration of blue carbon happens through various paths, the simplest being direct sinking (∼1%) of primary production to the seabed (typically < 4 gC m2 yr–1, Ducklow et al., 2007). Some is consumed by zooplankton which can potentially sequester through faecal pellets and/or carcasses raining to the seabed and (a small proportion) escaping microbial breakdown in the water column and seabed (see Barnes and Tarling, 2017). The rate of such pelagos burial below oxygenated surface sediments (below zones of bioturbation and bio-irrigation) is yet to be quantified. Most Southern Ocean species are benthic, and the primary consumers amongst them include suspension, deposit and grazing feeders, some with estimated lifespans making them amongst the oldest animals on Earth (some live for 100+ years). This is a shorter route to sequestration (no exposure to water column microbial loop) and many have organic carbon sandwiched within thick, external skeletons (e.g., some corals, bryozoans, and sponges).
In terms of carbon storage and cycling, the contribution of biology in the polar oceans is relatively small, e.g., with respect to forests and low latitude coastal habitats. However, the wide, deep and mainly undisturbed polar continental shelves may be very efficient at burial and thus sequestration (at which forests are poor). Most importantly polar continental shelf carbon capture and storage increases with marine ice losses and mild warming, so acts as a negative feedback on climate change.
Threats to the ecosystem service are complex, poorly understood and difficult to evaluate, but have some alignment with general threats to Southern Ocean biodiversity (e.g., see Gutt et al., 2015; Morley et al., 2019). These include climate-driven change, disruption by non-indigenous species establishments, pollution, harvesting (e.g., longline bycatch), and habitat destruction from increased ice scour. The quantification of the status of carbon capture, storage, and sequestration by polar organisms is in its infancy. However, it has been shown that biological stores of carbon on the seabed increase with declines in sea ice extent (Barnes, 2015) and vice versa (Pineda-Metz et al., 2020), due to links between sea ice and phytoplankton timing, duration and bloom size (Arrigo et al., 2008). The uncertainty in sea ice projections (IPCC, 2014, 2019; Cavanagh et al., 2017; Turner and Comiso, 2017) means that future predictions for biological carbon pathways to sequestration also have considerably uncertainty (Rogers et al., 2020). Such uncertainty is further bolstered by the possibility that seabed biological carbon may not be linked to sea ice extent in the Arctic (at least in the Barents Sea, see Souster et al., 2020).
Whilst forests, mangroves, salt marshes and other non-polar carbon sinks are shrinking, polar blue carbon is increasing considerably, at least around West Antarctica. Polar carbon sinks are rare examples of negative feedbacks on climate change (Barnes et al., 2018; Pineda-Metz et al., 2020). Phytoplankton blooms have increased (Arrigo et al., 2008) and zoobenthic blue carbon storage has doubled around West Antarctica (Barnes, 2015) in response to sea ice losses over the last 25 years. Giant icebergs formed from ice shelf collapses also generate considerable increases in onshore capture (Peck et al., 2010) and offshore capture (DuPrat et al., 2016). Primary consumers convert this to storage of blue carbon, to the order of about a million tonnes per giant iceberg (Barnes et al., 2018). Furthermore small (∼1°C) temperature rises may further increase zoobenthic growth and carbon storage (Ashton et al., 2017). This powerful negative feedback is not without its complexities though, with changing species composition of phytoplankton and zooplankton (Rogers et al., 2020).
Sea ice around the Antarctic had been increasing in extent since the 1970s, but more recently it decreased to record low levels, with the greatest decline in the Weddell Sea (Turner and Comiso, 2017; Turner et al., 2020). There is considerable regional variability, but if such reductions continue there could be major gains in biologically stored carbon, measurable within a decade, although gauging the extent to which this is converted to sequestered carbon may take longer. This will lead to redistribution of hotspots of blue carbon. Areas that were gaining sea ice and losing blue carbon (Pineda-Metz et al., 2020) may now start gaining blue carbon in response to new sea ice losses (Turner and Comiso, 2017; Barnes et al., 2018).
Overall, the responses of this ecosystem service to climate forcing may be parabolic; blue carbon increases with phytoplankton availability, which in turn increases with sea ice, glacier and ice shelf losses (Barnes et al., 2018) and independently also increase with moderate warming (Ashton et al., 2017). However sea ice, glacier, and ice shelf losses are finite and losses of sea ice during winter darkness will yield little enhancement to phytoplankton. Furthermore temperature increases beyond 1°C might start to threaten temperature survival envelopes of species (Ashton et al., 2017; Peck, 2018). End of century onwards decreases may be increasingly driven by strengthening ocean acidification making storage slower and sequestration more difficult (IPCC, 2019). Figure 3 provides a network representation of connections and feedbacks between the blue carbon pathway and other Southern Ocean ecosystem services, together with some key drivers of change.
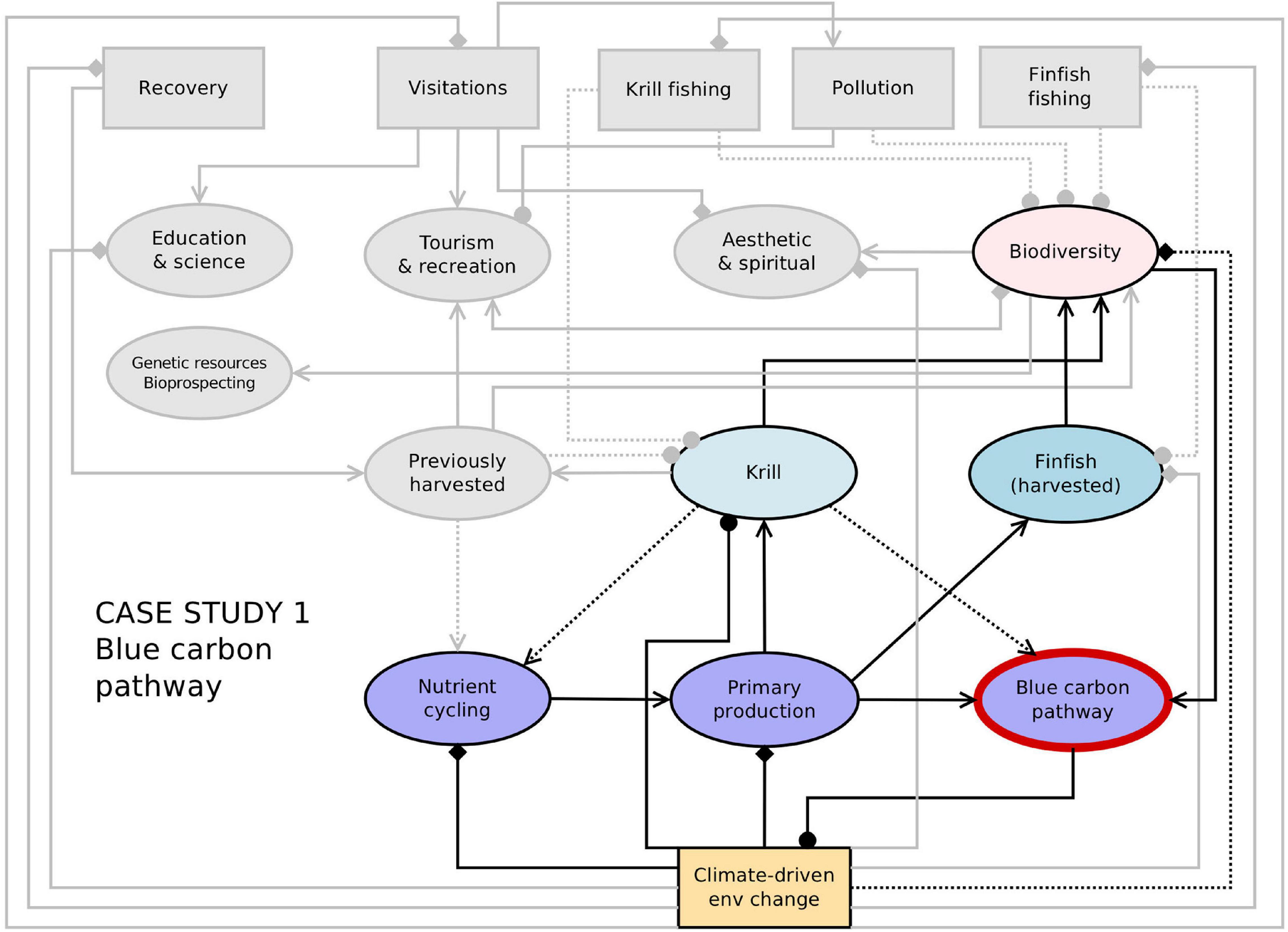
Figure 3. Network representation of the links and feedbacks between ecosystem services and drivers (from Figure 2) with key elements for the blue carbon pathway highlighted. Connections between components indicate the direction of effects from one node to another, as indicated in the legend. Diamonds indicate cases where the direction of the effect is not known or unclear (i.e., it could be positive and/or negative), and dashed lines represent uncertain or weak linkages.
The increasingly urgent need to meet climate and carbon agreements means that increased efforts to protect coastal habitat, and the ecosystem service services conferred therein, are likely to be sustained. However, the level of most Antarctic biological carbon storage is unmeasured in the majority of areas, so large scale change is currently unquantified. Even without directly-comparable metrics it is clear that, relative to absorbtion into oceans or rainforests, the stored carbon budget is small, and when considered alongside mangroves or seagrasses the efficiency of sequestration is poor.
There is currently no specific management or policy for this ecosystem service in the Southern Ocean. However, there are policies and active management on fishing, pollution and prevention of non-indigenous species transport, as well as those to protect or reduce impact on benthic habitats and Vulnerable Marine Ecosystems (VMEs) (see Supplementary Table S2). All these actions have the potential to influence biological carbon storage and its likelihood of sequestration, despite not being specifically designed to meet this objective.
Current management policies within the ATS have little impact on the global origin of threats to the ES, such as climate change and pollution. Recent fishing impacts have been mostly minimised by CCAMLR regulations and practices (e.g., Trathan et al., 2014), and there has been progress on limiting ballast water exchange but none on hull fouling by non-indigenous marine species (Hughes et al., 2020). However, current management and policy is not sufficiently agile to respond to sea ice losses (e.g. by implementing protection for newly-opened areas), so uncertainty remains in relation to safeguarding important recent gains in biological carbon stores (Gogarty et al., 2020).
The carbon pathway in terrestrial ecosystems forms part of the consideration and merit of protected area status elsewhere in the world. An important area of progress could be to similarly consider the blue carbon pathway as part of the decision-making process on the establishment of marine protected area systems. Intuitively it is hard to envisage a more cost-effective pathway to maintain carbon sequestration than protection of large areas of remote continental shelf that are currently little utilised, especially when they are a rare ecosystem service which works as a negative feedback on climate change. However, measurement of this ecosystem service has not been possible by Earth observation remote sensing, and thus is expensive and time-consuming, requiring multidisciplinary scientists on research vessels working far from research stations, and over limited areas. Development of new approaches for larger-scale analyses would therefore be beneficial in further quantifying and mapping areas that are important for the blue carbon pathway.
Antarctic krill (Euphausia superba) is a characteristic pelagic invertebrate of the Southern Ocean, which is noted for its relatively large size (maximum length c. 60 mm), high biomass, and highly aggregated spatial distribution (Atkinson et al., 2008, 2009; Siegel and Watkins, 2016; Tarling et al., 2016, 2018). These characteristics underpin its importance to various ecosystem services, including as a key food source for an array of vertebrate predators (Trathan and Hill, 2016) that attract tourism and as a contributor to biogeochemical cycles (Belcher et al., 2019; Cavan et al., 2019). They also make it an attractive resource for the fishing industry, which currently operates in the Scotia Sea region, specifically the waters of the North Antarctic Peninsula and around the islands of the Scotia Arc (Grant et al., 2013a; Kawaguchi and Nicol, 2020).
In the 2017/18 fishing season, 11 vessels from five nations participated in the fishery, catching 312,989 tonnes (6CCAMLR Krill Fishery Report 2018). This accounts for 95% of the annual catch in the Southern Ocean but <0.5% of the global annual marine fisheries catch (GAMFC). The main products are fishmeal, which is used as aquaculture feed, and omega 3 rich oils sold as premium priced diet supplements, while very little of the catch is used as a direct food source for people (Hill, 2013b; Nicol and Foster, 2016). Nonetheless, the fishery is one of a handful that are theoretically capable of supporting significantly higher catches and may therefore become important future food resources (Garcia and Grainger, 2005). Catch limits established by CCAMLR suggest that the ecosystem could sustain krill catches of >8 million t yr–1, equivalent to >10% of GAMFC (Nicol and Foster, 2016) and enough to supply 1.5% of the human population with 1 g day–1 of omega 3 (Hill, 2013b). The financial value of krill catches is unclear and depends on the relative production of meal and higher value krill oil. Using the oil:meal ratio of a major krill oil producer, Grant et al. (2013b) estimated that the catch value for 2011 (181,000 tonnes) could be up to $241 million, whereas a study on behalf of CCAMLR found that value at the point of landing averaged $69 million yr–1 in the period 2011–2015 when catches averaged 216,000 tonne year–1 (CCAMLR Commission Report 2016). These lower values probably reflect a much lower oil:meal ratio than that (18:82) assumed by Grant et al. (2013b). However, this ratio is likely to change over time as the krill oil market grows.
The productivity of the krill stock is the main factor affecting the ecosystem’s capacity to support the fishery. No stock-recruit relationship has been identified for Antarctic krill but it is likely that low population levels would impact productivity (Siegel and Nicol, 2000; Hill, 2013a). Productivity also relies on the availability of food and appropriate habitats for processes such as spawning, hatching, larval overwintering and growth (Atkinson et al., 2006, 2008; Murphy et al., 2017; Perry et al., 2019). Each of these habitat types is patchily distributed, so connectivity between them is also important (Hofmann and Murphy, 2004; Piñones et al., 2013; Perry et al., 2019; Thorpe and Tarling, 2019). The distribution of fishing effort is highly aggregated at the circumpolar and regional scales (Grant et al., 2013a; Nicol and Foster, 2016; Kawaguchi and Nicol, 2020). In the Scotia Sea region, all catches have been concentrated in less than a quarter of the sea area open to fishing (Grant et al., 2013a; Kawaguchi and Nicol, 2020). The fishery relies on exploitable concentrations of krill in operationally suitable areas, which are generally close to land (Hill et al., 2009) and in the upper few hundred metres of the water column which is accessible to fishing gear. The majority of Antarctic krill biomass is not located in these fishing grounds but in the open ocean, and in lower concentrations that might not be possible for the fishery to efficiently exploit (Atkinson et al., 2008; Hill et al., 2009). Furthermore the animals are capable of occupying a variety of habitats including the bathypelagic zone (Schmidt et al., 2011). Thus, the current provision of the service relies on the specific behaviour of a fraction of the krill population.
The projected effects of climate change are expected to cause contraction of krill habitat and reductions in productivity as a result of direct physiological effects on krill, loss of critical habitat and changes in the community of primary producers (Flores et al., 2012; Hill et al., 2013; Kawaguchi et al., 2013; Constable et al., 2014; Piñones and Fedorov, 2016; Klein et al., 2018; Meredith et al., 2019; Tulloch et al., 2019; Veytia et al., 2020). Key potential drivers include warming and loss of sea ice. Krill are adapted to a narrow range of temperatures with those > 3°C impeding both embroyonic development and adult growth (Atkinson et al., 2006; Perry et al., 2020). Sea ice is an important overwintering habitat for larval krill and reductions in its extent and duration could negatively impact recruitment to the adult population (Meyer et al., 2017; Schaafsma et al., 2017). Laboratory experiments have confirmed that low pHs impact embryonic development (Kawaguchi et al., 2013) although the effects in the field are not well understood. Changes in the timing of events such as ice break out and phytoplankton blooms might also have negative impacts as the krill life cycle is tightly synchronised to the current seasonal cycle (Quetin et al., 2007; Veytia et al., 2020). As the Southern Annular Mode (SAM; the leading extratropical mode of climate variability in the Southern Hemisphere, and a first-order indicator of circumpolar wind strength) is one of the strongest correlates of past changes in krill productivity in the Scotia Sea region (Atkinson et al., 2019), any future changes in advection and eddy activity could also affect krill distribution and abundance. Projections suggest that changes to the distribution and abundance of both krill and its predators are likely to be apparent by the end of the current century (Hill et al., 2013; Piñones and Fedorov, 2016; Klein et al., 2018; Tulloch et al., 2019; Veytia et al., 2020). While there is some debate over the magnitude of recent changes in krill numerical density in the Scotia Sea region (Atkinson et al., 2004, 2019; Cox et al., 2018; Cox et al., 2019; Hill et al., 2019), evidence of climate-driven changes in productivity and distribution (Huang et al., 2011; Forcada and Hoffman, 2014; Atkinson et al., 2019; McMahon et al., 2019; Yang et al., 2020), suggest that change has already occurred.
Some studies suggest that climate change may bring limited benefits to some life stages. For example modelling studies suggest that habitat availability for some larval krill stages might increase as sea ice becomes less stable (Melbourne-Thomas et al., 2016) and that adult growth rates might increase in some areas (Veytia et al., 2020). The productivity of the krill stock might also be affected by factors such as the recovery of depleted whale populations and the extent to which the krill stocks are impacted by exploitation.
The prognosis is that climate change will reduce the extent of productive krill habitat and the productivity of the krill stock, at least in the Scotia Sea region. In projection studies, the severity of these impacts increases with the severity of climate change (i.e., the RCP scenario). Paradoxically, demand for krill products could increase as the krill stock declines, especially if ecosystem degradation diminishes the capacity of ecosystems elsewhere to supply the food needs of a growing human population (Garcia and Grainger, 2005). Equally, demand could be fuelled by increasing demand for omega 3 (Hill, 2013b). Parts of the fishing industry have invested heavily in krill fishing over the last two decades, suggesting that demand for krill products is stable or increasing. However, the industry has not indicated any desire to increase catches beyond current limits (Cavanagh et al., 2016b). Simulation studies suggest that fishing at currently permitted levels could exacerbate climate-driven population declines in some krill predators in areas open to fishing (Klein et al., 2018). Thus one of the main factors that will affect the supply of krill products into the future is how CCAMLR’s management of the fishery responds to change. Figure 4 provides a network representation of connections and feedbacks between the krill fishery and other Southern Ocean ecosystem services, together with some key drivers of change.
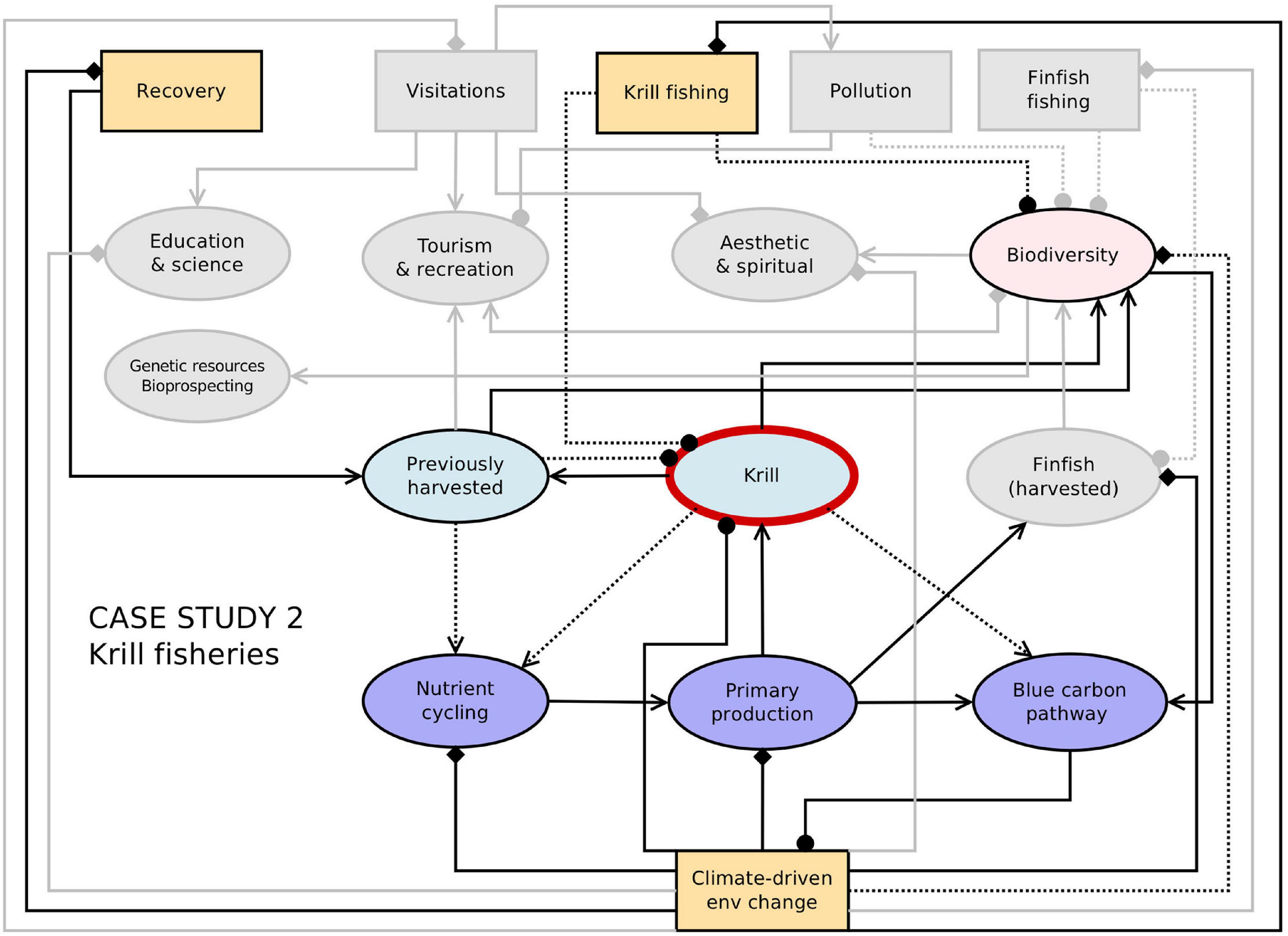
Figure 4. Network representation of the links and feedbacks between ecosystem services and drivers (from Figure 2) with key elements for the Antarctic krill fishery highlighted. Connections between components indicate the direction of effects from one node to another, as indicated in the legend. Diamonds indicate cases where the direction of the effect is not known or unclear (i.e., it could be positive and/or negative), and dashed lines represent uncertain or weak linkages.
Current management allows the fishing industry to increase catches and to adapt to changes in the krill population by relocating to more productive fishing grounds either within the Scotia Sea region or at the circumpolar scale. Reduced winter sea ice around the Antarctic Peninsula has allowed the fishery to become more concentrated in winter and move southwards in the last two decades (Kawaguchi and Nicol, 2020; Meyer et al., 2020). While climate change might open up new fishing grounds as ice retreats, it could also disrupt fishing if wave intensity increases (Collins et al., 2019) or the number and size of icebergs increase with ice shelf collapse. Parts of the Southern Ocean other than the Scotia Sea region may become increasingly attractive to the industry as markets and supply chains change.
Antarctic krill contributes <1% of global production of fishmeal and omega 3, and it is not currently an important human food source. The companies responsible for most of the krill catch are relative newcomers to the fishery (joining in the last 15 years) and their business plans might already account for the risks and opportunities associated with climate change. Thus the key implications of future change degrading the ecosystem’s capacity to support the krill fishery are a reduction in the availability of a potential but currently minor food source, and the potential for the combined effects of climate change and fishing to impact the other ecosystem services that krill has a role in supporting, including climate regulation and tourism.
Current management of the krill fishery is based on a series of interim measures pending the development of an approach that appropriately protects the wider ecosystem including krill predators (Hill et al., 2016, 2020; Nicol and Foster, 2016). The fishery is managed through the application of fixed catch limits applied to large spatial units (>0.6 million km2) without reference to the current status of the stock in these units (Hill et al., 2020).
The krill fishery is characterised by extremely low exploitation rates (catch per unit biomass) relative to other similar fisheries, and industry compliance with regulations (Cullis-Suzuki and Pauly, 2010; Hill et al., 2016, 2020; Nicol and Foster, 2016). In addition current circumpolar catches remain below 70% of the combined catch limit for the Scotia Sea region (Meyer et al., 2020). However, a recent study concludes that this is not sufficient to protect krill predators from the combined effects of fishing and climate change (Watters et al., 2020). Krill fishery management lags behind that of other similar fisheries in that catch limits do not respond to changes in stock size (Hill et al., 2020). Nor can fixed catch limits respond to changes in distribution. Without finer-scale management CCAMLR will be unable to prevent the localised impacts on krill and its predators that might result from the combined effects of fishing and climate change, or from changes in fishing patterns (Klein et al., 2018; Watters et al., 2020; Meyer et al., 2020). While CCAMLR’s scientific working groups have been working for three decades to improve krill fishery management, their focus has been on the Scotia Sea regions which means that CCAMLR is less well equipped to manage the fishery if it expands into other regions.
One of CCAMLR’s current regulations identifies potential mechanisms “to further improve future management of krill” and recognises that “advances are urgently needed.” CCAMLR also has a resolution on climate change which “urges increased consideration of climate change impacts in the Southern Ocean to better inform CCAMLR management decisions” (CCAMLR Resolution 30/XXVIII). However in the decade since passing that resolution, CCAMLR has not altered the catch limits for krill or clarified how consideration of climate change impacts will inform future decisions. Thus the challenge for CCAMLR is to progress from aspiration to implementation. A test of its ability to do so will come in 2021 with the expiry of a key current regulation which has successfully limited catches in the North Antarctic Peninsula seas to 155,000 tonne yr–1.
Management of the krill fishery in a changing climate requires an agility that has eluded CCAMLR to date. The single advance that would most help to manage the fishery through future change is to make regular monitoring of krill stock size at an appropriate scale for management a precondition for any catch limit. Such monitoring is necessary to ensure that exploitation rates do not exceed safe limits (Hill and Cannon, 2013; Hill et al., 2016, 2020). Given CCAMLR’s obligations to protect the wider ecosystem, there is a need for mechanisms that minimise the risk of localised impacts on critical krill life stages and on krill predators (Meyer et al., 2020; Watters et al., 2020). Better prognostic information would also be useful, including forecasts of future demand for krill products and of impacts on the krill stock from climate change and from predator populations recovering from past overexploitation. The former may already exist within the krill fishing industry while the latter requires a coordinated and properly resourced effort by the scientific community. Arguably the unresolved debate about past change in krill populations is an impediment to progress which could be resolved through a more complete articulation of uncertainties (Hill et al., 2019; Meyer et al., 2020).
Antarctica provides cultural ecosystem services through tourism and recreation. Benefits include visitor enjoyment of nature and spectacular landscapes of high wilderness and aesthetic value, as well as the provision of income opportunities for nature tourism service providers.
Most Antarctic tourists (>95%) visit the Antarctica Peninsula on cruise vessels, with many calling at coastal sites for brief shore-based activities. Around 50 yachts travel to Antarctica each year, with either independent travellers or fee-paying passengers (United Kingdom et al., 2019). Land-based tourism provides opportunities to visit ‘deep field’ sites that are to some extent driven by recreational adventure tourism centred on mountaineering (for example Mount Vinson or Ulvetanna).
Antarctic tourism is one of the most significant human activities undertaken in the region, beginning in the 1950s followed by a long period of expansion dating from the late 1980s. The duration of the Antarctic tourism season has expanded to >150 days (November to April). During the 2019/20 season, 74,401 tourists visited Antarctica, including cruise-only visitors. Assuming a ship-based visit to Antarctica to cost around US$8000, the Antarctic tourism industry may have had a turnover of >US$600 million in 2019/20. COVID-19 may greatly reduce tourist visitor numbers, at least in the short term, as demand drops, nations with gateway ports limit visitor access and Antarctic Treaty governments take steps to prevent the virus reaching Antarctica (Hughes and Convey, 2020).
Many of the activities, such as birdwatching (including penguin colony visits), whale watching, diving, use of submersibles and photography, are dependent on the local marine wildlife and habitats (Burger and Gochfeld, 2007).
Given the expansion of the tourism industry during the past 30 years, the predicted increase in global demand for Antarctic visits, and increasing diversity of activities, the ecosystems attracting Antarctic tourism may be vulnerable to over exploitation (Eijgelaar et al., 2010; Liggett et al., 2017; Pertierra et al., 2017).
Ecosystem impacts caused by increasing visitation and the potential establishment of permanent infrastructure (noting that these are not exclusive to tourism) could include (a) a loss of wilderness and aesthetic values (Summerson and Tin, 2018), (b) increased risk of non-native species introduction (Chown et al., 2012), (c) increased risk of pollution events at both small (e.g., dropping of litter) and large scale (e.g., fuel spill from a ship; Aronson et al., 2011) resulting in impacts on biota, (d) trampling of vegetation or disturbance of wildlife (Burger and Gochfeld, 2007; Tejedo et al., 2016), (e) increased atmospheric emissions from vessels, aircrafts, and land vehicles (Amelung and Lamers, 2007; Eijgelaar et al., 2010) and (f) increasing cumulative impacts at ice-free locations, including where scientific, logistic and tourism activities coincide (e.g., Deception Island).
The Antarctic Peninsula region has exhibited the greatest levels of climate change in the continent in the past 60 years, as well as being the area most visited by tourists (between 95 and 99% of tourists visit the Peninsula compared to the rest of Antarctica). Climate models predict substantial climate change across the whole continent in the coming century, resulting in increases in ice-free ground through glacier and ice sheet retreat, reductions in sea ice extent, shifts in the distribution range of wildlife and increases in distribution and spatial coverage of terrestrial species (both native and non-native) (Chown et al., 2012; Turner et al., 2014; Lee et al., 2017; Siegert et al., 2019). Climate change impacts may increase tourism industry interactions with Antarctic ecosystems and wildlife by potentially facilitating access to new locations, but may also affect the biota present at established landing sites. Figure 5 provides a network representation of connections and feedbacks between Antarctic tourism and other Southern Ocean ecosystem services, together with some key drivers of change.
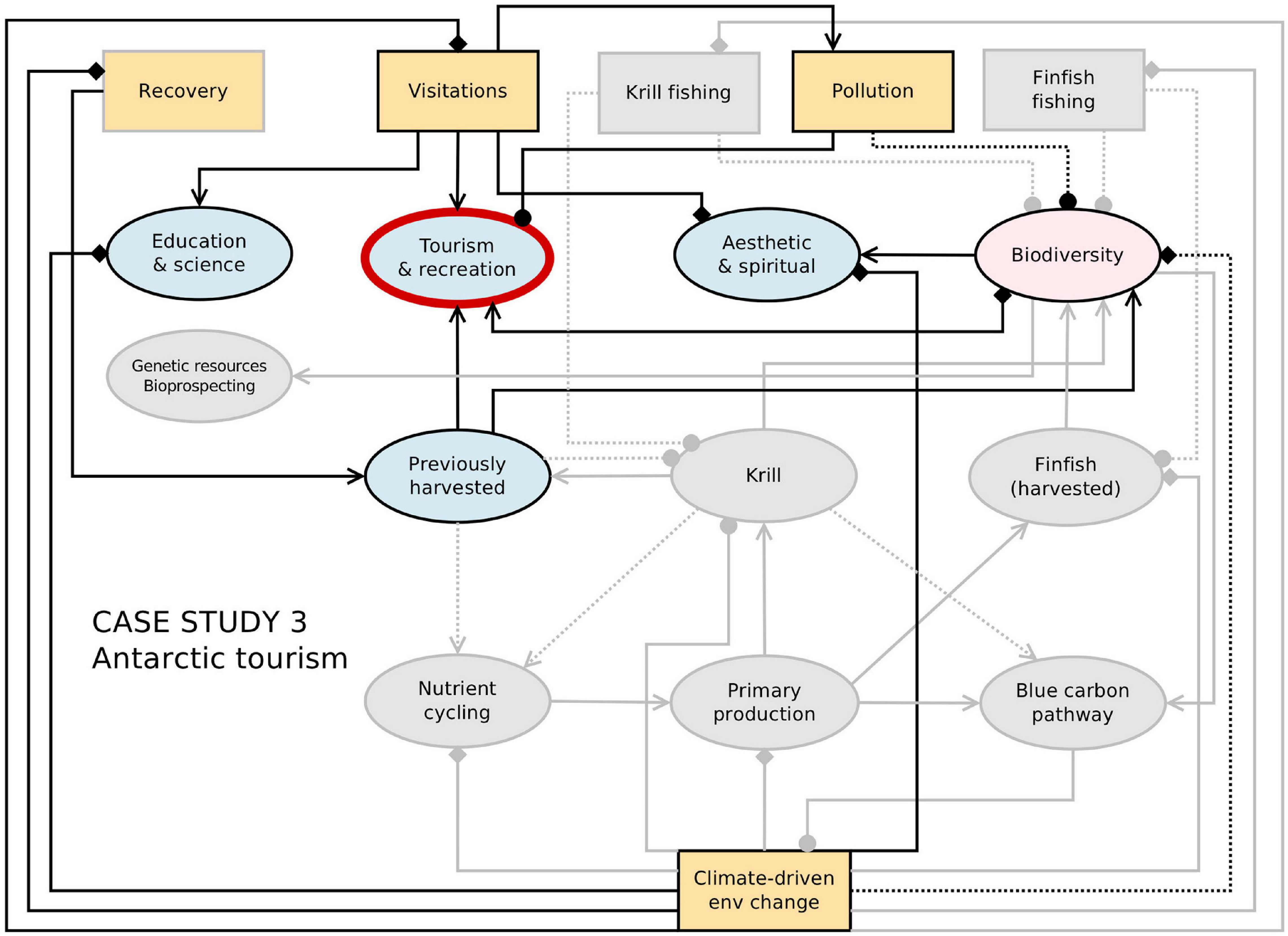
Figure 5. Network representation of the links and feedbacks between ecosystem services and drivers (from Figure 2) with key elements for Antarctic tourism highlighted. Connections between components indicate the direction of effects from one node to another, as indicated in the legend. Diamonds indicate cases where the direction of the effect is not known or unclear (i.e., it could be positive and/or negative), and dashed lines represent uncertain or weak linkages.
While visual tourism impacts appear to be minimal, the recent dramatic rise (and predicted longer-term increase) in tourist visitation could result in rapid change being detectable in the near future.
The industry imposes strict control of vessel movement between landing sites to maintain an impression of remoteness for visitors; as such, an increase in vessel traffic may degrade this experience for the visitor. Consequently, as some sites reach their carrying capacity, there may be increasing pressure to access previously little visited locations, with associated anthropogenic impact upon resident biota.
Positive benefits from increased tourism may include increased revenue receipt and an increase in individuals with greater appreciation of the beauty and fragility of Antarctic environments (often termed ‘Antarctic Ambassadors’ by the industry’s member organisation, the International Association of Antarctica Tour Operators, IAATO), who may be prepared to promote Antarctic conservation messages in their home nations (Eijgelaar et al., 2010; Lamers et al., 2011).
The non-linear relationship between environmental degradation and attraction to tourism should also be considered. Meredith et al. (2019) cite the increase of ‘last chance tourism’ whereby tourists explicitly seek to experience vanishing landscapes or seascapes, endangered wildlife, and natural and social heritage, before they disappear (Lemelin et al., 2010; Lamers et al., 2013; Meredith et al., 2019).
The Antarctic Treaty Consultative Meeting (ATCM) has responsibility for managing tourism activities in Antarctica, in accordance with the objectives of the ATS, and has put in place international agreements to regulate Antarctic tourism (Liggett et al., 2011; Bastmeijer, 2013; Jabour, 2014). All tourism operators undertaking activities under the jurisdiction of nations that are signatories to the Protocol on Environmental Protection to the Antarctic Treaty must also comply with the Protocol. Since the Protocol entered into force in 1998, two Measures and several non-binding Resolutions have been adopted, including the ‘General Principles of Antarctic Tourism’ [Resolution 7 (2009)]. The ATCM has established Visitor Site Guidelines, currently for 42 locations, to direct tourist and national governmental operator activity in order to minimise human impact.
Tourist operators originating in nations that are not signatories to the Antarctic Treaty may not be bound by its agreements, including on tourism activities.
IAATO co-ordinates most tourism activities in Antarctica and represents the industry at the ATCM.
Antarctic Treaty Parties have expressed concerns regarding the environmental impacts of tourism, impacts on wilderness values, difficulties in regulation and monitoring of the industry and effective sharing of tourism expertise and co-operation by Parties. However, little comprehensive ecological assessment and monitoring of tourist visitor locations has occurred, resulting in little evidence to support policy decision-making. Furthermore, Treaty Parties have been reluctant to agree more general limits on levels of tourism visitations.
Antarctic Treaty Parties have noted that to date, ‘regulation of Antarctic tourism via the ATS has been largely reactive and that a more proactive, strategic vision for Antarctic tourism development and regulation is warranted’ (Netherland and New Zealand., 2019).
The future of tourism expansion and regulation remains in doubt (Bastmeijer et al., 2008; Liggett et al., 2017) but development of a strategic vision that can be practically implemented will be necessary to limit potential impacts on Antarctica.
On the sub-Antarctic island of South Georgia (located just to the north of the Antarctic Treaty area), tourists pay a substantial landing fee for access to the island, with the income going to the Government of South Georgia and the South Sandwich Islands and used to fund environmental protection and management. A system whereby the tourism industry pays for access to Antarctic ecosystem services, as successfully used in many other parts of the world, previously did not gain agreement (see 7ATCMXXXIX Final Report, para 245, Secretariat of the Antarctic Treaty, 2016), but reconsideration of this suggestion may be timely (Verbitsky, 2018).
The absence of a permanent human population, together with the remote and hostile nature of the Southern Ocean, constrains direct use of its ecosystem services (Grant et al., 2013b). Nevertheless, these services are of increasing global importance to remote beneficiaries, as is clear from the three case studies described above: the blue carbon pathway, the Antarctic krill fishery, and Antarctic tourism. That climate change has the potential to affect these important services is highlighted by Rogers et al. (2020) who concluded that “ecosystem services in the Southern Ocean will likely increase” by 2100. Our findings suggest that climate change will decrease the overall capacity of the Southern Ocean to supply ecosystem services even if there is an increase in the capacity to supply some services. We expect this reduction in provision to be accompanied by an increase in demand for most services.
We assert that in order to fully understand the consequences of climate change on the delivery of ecosystem services, it is necessary to factor in each stage of the risk pathway from climate to people, illustrated in Figure 1. Our stepwise approach tracks the effects of climate change from observed and projected habitat change (with associated uncertainty) through to impacts on ecosystem structure and processes, and the consequences for the capacity of ecosystems to support ecosystem service delivery. For example, the seasonal presence and extent of Antarctic sea ice will affect and be affected by future ocean heating. Disruptions to this important habitat for Southern Ocean biota will have multiple direct and indirect effects on ecosystem structure (e.g., distribution and connectivity) and processes (e.g., productivity), affecting a range of services, from supporting (e.g., primary production) and regulating (e.g., blue carbon pathway) to provisioning (e.g., Antarctic krill fishery) and cultural (e.g., Antarctic tourism). These services might also be impacted by other effects of ocean heating (e.g., degradation of habitat quality at lower latitudes) and those of other climate-related drivers. Increased ocean temperatures may be detrimental to krill and to Antarctic toothfish, but could also create the potential to target new species due to poleward range shifts (as is being observed in the Arctic; Frainer et al., 2017; also see Pinsky et al., 2018).
By combining information on the drivers of change and how they might affect the biological components that underpin each service, our assessment indicates that for the majority of Southern Ocean ecosystem services (with the exception of the blue carbon pathway and nutrient cycling) the risk from climate change ranges from intermediate to high-level. Although detailed quantitative and region-specific information is currently lacking on, for example, the magnitude of change in the different drivers and their impact on ecosystem structure and processes, we suggest that conveying climate change impacts in terms of overall risk to ecosystem services is useful for decision-makers. The information on the components contributing to the risk assessment (including levels of confidence) (Tables 1, 2), can be used to inform decisions and to highlight gaps in knowledge, uncertainty and priorities for future work to further our understanding of change (see Box 1). The inclusion of the case studies has allowed us to explore the risk pathways of three of the services in more detail.
Box 1. Research gaps and priorities.
A key priority is to conduct a comprehensive, spatially-resolved ecosystem services assessment of the Southern Ocean (Grant et al., 2013b; Hill and Grant, 2013) to provide a baseline against which projected changes can be evaluated. Fundamental to this is improved understanding of the mechanisms that link physical variables to ecological processes, their interactive effects, and connections to ecosystem services. We recommend that this work is undertaken within a framework that facilitates consideration of environmental, economic and social factors, enabling the wider implications of decisions to be evaluated (United Nations et al., 2014; Cavanagh et al., 2016a; IPBES, 2019; Bateman and Mace, 2020).
Our analysis identified key knowledge gaps which currently limit assessment of the future potential of Southern Ocean ecosystems to support ecosystem services. These relate primarily to (i) uncertainties in model projections of key habitat variables (particularly sea ice), and (ii) understanding of the interactive effects of projected changes in multiple habitat variables and how these will affect ecosystem services. Projections of Antarctic sea ice in CMIP6 (Coupled Model Intercomparison Project) models that are informing the IPCC’s sixth assessment report are considered to be significantly improved compared with the previous set (CMIP5), but there is still a large intermodel spread. In particular, these updated models project sea ice loss over the 21st century in all scenarios, but confidence in the rate of loss is limited (Roach et al., 2020). There is a mismatch between the temporal scale of climate projections and those of ecological processes and policy responses. On the decadal timescales required for ecosystem-based management, future change will be dominated by natural variability of the climate system which is difficult to predict with current climate models (Cavanagh et al., 2017). However, given that directional ecosystem change is already being observed and that there are inherent lags in the capacity of management to respond to these changes, there is a need to consider and implement adaptive management responses now or in the near future. Our risk assessment approach should help in this regard, although future refinement will be useful to consider different scenarios at finer spatial and temporal scales.
Ecosystem models provide a tool to consider how changing habitat variables and multiple drivers might interact to affect particular species or networks of interacting species (e.g., Klein et al., 2018; Tulloch et al., 2019), and over what timeframes. But these models are yet to be directly applied in a systematic way to assess and project change in ecosystem service delivery. Ecosystem models might also assist in addressing uncertainty about how ecosystem structure and function respond to change, such as shifts in dominance of energy pathways, tipping points and emergence of new stable states. Significant changes in the configuration of ecological networks in Southern Ocean systems have the potential to cause non-linear changes in the capacity of these systems to support ecosystem services.
Improved forecasts of future demand for services are required. As indicated in the main text, changes in both the demand for and provision of ecosystem services vary between different regions of the Southern Ocean, with some, such as the south west Atlantic under particular pressure from tourism and fishing, and also experiencing rapid rates of climate-driven environmental change. The network models developed here could be used to directly simulate the effects of interacting drivers on the direction of change in ecosystem service provision at regional scales (e.g. at the scale of the MEASO assessment sectors; Constable et al., in preparation).
A further challenge for end-to-end ecosystem modelling (and bioeconomic modelling) with respect to assessment and prediction of provisioning ecosystem services (particularly fisheries) is to resolve whether the emergence of new target species due to climate-driven poleward range shifts or even targeting mesopelagic fish (as indicated in Rogers et al., 2020) might have the capacity to bolster resilience of Southern Ocean fisheries and support delivery of provisioning ecosystem services into the future. This uncertainty does not obviate the need to factor climate change into management of current target species, given the key ecological roles of both krill and toothfish.
The blue carbon pathway case study reflects our assessment of low-level risk for this ecosystem service through the detail it provides on the positive effects of aspects of climate-driven change, and how they influence biological components crucial to the functioning of the pathway. These positive effects arise primarily from moderate warming and the loss of ice (sea ice, glaciers, and ice shelves). However, the case study cautions that if climate-driven changes continue to increase, some benefits may be lost. Temperature increases beyond 1°C might start to threaten the survival of important species, and increasing ocean acidification may mean that carbon storage is slower and sequestration more difficult. In addition, negative effects such as benthic habitat destruction from increased ice scour need to be factored in, albeit on more localised scales. In the case of the Antarctic krill fishery we assess the risk to be high-level based on the predominantly negative impacts of changes to sea ice, ocean warming and ocean acidification. The case study discusses the complexity of such changes on the biological components that support the fishery, with further contraction of krill habitat and declines in productivity of the krill stock expected. The uncertainty regarding the effects of acidification on krill highlights the need to carefully consider the information underlying the risk assessment, although the overall risk of climate change to the fishery would still be considered high-level with or without this driver. The importance of ocean circulation is also discussed in that changes in mean flow and eddy variability have the potential to affect krill distribution, thus highlighting the need for further consideration of the “grey areas” (Table 2) in the risk assessment. Moreover, climate change may bring limited benefits to some krill life stages.
The case studies also demonstrate that the effects of climate change need to be considered alongside other factors that may affect ecosystem services, and that regional-scale differences in the impacts of change, as well as in the demand and use of services, must be taken into account. It is noteworthy that the strongest effects of climate change occur in the main region in which the krill fishery operates. As described in the tourism case study, the Antarctic Peninsula region has not only experienced dramatic climate-driven changes, it is also the area most visited by tourists. Climate change impacts such as loss of sea ice may increase tourist interactions with Antarctic ecosystems and wildlife by potentially facilitating access to new locations and affecting the range of wildlife present at established landing sites. The demand for, and global importance of, Southern Ocean ecosystem services seems set to increase during the 21st century (Chown and Brooks, 2019; Rogers et al., 2020). This may increase the use of some services (e.g. fisheries and tourism), depending on how demand is managed. Indeed, as discussed in Case Study 2, demand for krill products could increase as the krill stock declines, especially if ecosystem degradation diminishes the capacity of ecosystems elsewhere to supply the food needs of a growing human population. Case Study 3 mentions the emergence of ‘last chance tourism’ whereby tourists seek to experience vanishing landscapes or threatened wildlife species.
The combined effects of increased demand and climate change impacts on supply poses a significant challenge for the conservation of Southern Ocean ecosystems and management of activities that affect them. These activities, including fishing and visitation (tourism, education, and research), are part of a group of potential drivers of ecosystem change that also includes the recovery of previously harvested species such as whales (see Morley et al., 2020 and Grant et al., in preparation) (Figure 2). The majority of these drivers are influenced by global issues external to the region, including socio-economic drivers (Murphy et al., in preparation). The case studies discuss some of these, including non-indigenous species establishments and pollution in the context of the blue carbon pathway. In addition to climate change, the productivity of the krill stock might also be affected by factors such as the recovery of depleted whale populations and the extent to which the krill stocks are impacted by fishing. Our representation of the network of connections between ecosystem services, ecosystem structure and processes, climate change, and other important drivers provides a basis for understanding the complexity of interactions. For instance, krill fishing has the potential to impact every other service in the network through its interaction with the krill population (Figure 2), e.g., increased krill fishing could impact the blue carbon pathway through disrupting carbon flux to deep water via krill faecal pellets or moulted exoskeletons (Cavan et al., 2019; Manno et al., 2020). Furthermore, the effects of climate change on supporting services, such as the projected overall increase in primary production, will also have implications for provisioning services. Policy interventions that target critical interactions might be necessary to maximise resilience. A next step is to develop models based on our network representation to identify critical interactions and pathways (Box 1).
While demand for Southern Ocean ecosystem services may be increasing, our assessment of their future state assumes the maintenance of existing measures within the ATS to manage the impacts of human activities now and into the future. For example, we assume maintenance of CCAMLR’s current operational fisheries management approach and no significant increase in fishing effort. At present the area under CCAMLR management constitutes 10% of the Earth’s sea areas but has the lowest annual fisheries catch of any ocean; equivalent to c. 0.5% of global marine capture fishery production. Existing conservation and management actions through the ATS regulate fishing, tourism, scientific research and other human activities, with the overarching aim of minimising impacts on Antarctic ecosystems, and thus the services they provide (although the maintenance of ecosystem services is not stated as an explicit aim). Management actions may in some cases help to protect several ecosystem services simultaneously. For example the prohibition of all bottom trawling activities in the CCAMLR Convention Area reduces impacts on benthic ecosystems and thus contributes to the protection of demersal fish stocks and the blue carbon pathway. Large scale conservation actions, such as the 1.55 million km2 no-take zone of the Ross Sea region Marine Protected Area, may also help to protect multiple ecosystem services at the regional scale. However, management is often limited to local scales, for example, limits on krill fishing in nearshore areas, protection of VMEs, or restrictions on numbers of tourists at landing sites. At all scales, management actions implemented under the instruments of the ATS may help to limit the potential effects of other human activities that might otherwise exacerbate the impacts of climate change. Ecosystems that are protected from these additive effects might be more resilient than those that are not. The recommendations from the case studies in this paper provide a compelling case to explicitly consider a wider range of ecosystem services within the ATS, the key being to ensure that precautionary measures are in place to minimise impacts on them. However, none of these measures alone can prevent climate-driven changes to Southern Ocean ecosystems. Given the risks presented by climate-driven change, the provision of Southern Ocean ecosystem services into the future is therefore heavily reliant on global policy and action on climate change, particularly the UNFCCC Paris Agreement, which must be implemented in addition to local and regional management measures in order to protect ecosystem health in the longer term.
Despite their global importance, there is limited recognition of the wide range of benefits people receive from polar ecosystems (Meredith et al., 2019; Pertierra and Hughes, 2019). Beyond the more obvious direct-use benefits there is an array of non-use values, such as bequest (knowledge of benefits being used by future generations) and existence (knowledge that species and ecosystems continue to exist) values (Grant et al., 2013b). These present some persuasive reasons for conservation (Chan et al., 2012). Indeed CCAMLR’s precautionary approach to management encompasses non-use values such as conserving resources for future use and the avoidance of irreversible decisions (Supplementary Table S2); see Roberts et al. (in preparation) and Solomonsz et al. (in preparation) for more detailed consideration of cultural values and connections. Conducting a comprehensive ecosystem services assessment of the Southern Ocean, including consideration of the relative value of each service, as recommended by Hill and Grant (2013), remains a key priority. In the meantime, our assessment of potential change should be regarded as relative to an uncertain baseline. An important challenge in evaluating potential change to Southern Ocean ecosystem services is that the current capacity of the ecosystem to support these services is poorly understood. Our structured approach and risk assessment provides an important step toward comprehensive, spatially-resolved assessments of Southern Ocean ecosystem services, and highlights the potential benefits of this approach in conservation and management decision-making, particularly in terms of considering risk and trade-offs. Increased consideration of the linkages and feedbacks between these services may assist in developing robust management responses into the future.
(1) The demand for, and global importance of, Southern Ocean ecosystem services is set to increase over the 21st century.
(2) Climate change effects on Southern Ocean habitats, foodwebs and ecosystems will affect the provision of Southern Ocean ecosystem services.
(3) In general, climate change poses an intermediate to high-level risk for Southern Ocean ecosystem services, but there are some cases where climate change might increase the capacity of Southern Ocean ecosystems to support ecosystem services, at least in the medium-term.
(4) Increased consideration of the linkages and feedbacks between these services may assist in developing robust management responses into the future.
(1) Southern Ocean ecosystem services are globally important. Examples include the three case studies in this paper (blue carbon pathway; Antarctic krill fishery; Antarctic tourism).
(2) These ecosystem services are generally at intermediate to high-level risk due to climate change drivers.
(3) Existing management actions within the Antarctic region are focused on the regulation of fishing, tourism, and other human activities.
(4) There are trade-offs between the extent to which the benefits of different ecosystem services can be realised, due to the complexity of interactions between different services and the drivers that affect them.
(5) Despite their global importance, understanding of Southern Ocean ecosystem services lags behind the understanding of ecosystem services in the rest of the world.
(6) A spatially-resolved baseline assessment is therefore lacking and urgently needed.
The data analysed in this study is the information compiled in the IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (IPCC, 2019) which can be found here: https://www.ipcc.ch/srocc/.
RC, SG, and SHi conceived the study. RC led the study and writing of the manuscript. JM-T, SHi, and SG contributed substantially to developing the manuscript. DB, SHi, and KH led the writing of the case studies. SHa, MM, EM, and RT contributed to writing the manuscript. SHi, RC, JM-T, and SG designed Figure 1 with assistance from the MEASO Support Group. JM-T produced Figures 2–5.
The BAS authors (DB, RC, SG, SHi, KH, MM, and EM) are supported by the Natural Environment Research Council (NERC) core funding to BAS. SHa was supported by the Australian Research Council through a Laureate awarded to Philip Wallace Boyd, IMAS, UTAS, Australia (FL160100131).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work is a core contribution to the first Marine Ecosystem Assessment for the Southern Ocean (MEASO), part of the Integrated Marine Biosphere Research project’s (IMBeR) Integrating Climate and Ecosystem Dynamics in the Southern Ocean (ICED) programme. We thank the MEASO Support Group and Steering Committee for assisting with figures, coordination and editing of the text. We also thank Andrew Constable for constructive discussions on the development of the manuscript and on the risk assessment. This manuscript contributes to the British Antarctic Survey (BAS) Polar Science for Planet Earth “Ecosystems” programme, the BAS Environment Office Long Term Monitoring and Survey project (EO-LTMS), and the “State of the Antarctic Ecosystem” research programme (AntEco) of the Scientific Committee on Antarctic Research (SCAR).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.615214/full#supplementary-material
Abram, N., Gattuso, J.-P., Prakash, A., Cheng, L., Chidichimo, M. P., and Crate, S., et al. (eds) (2019). “Framing and context of the report,” in IPCC Special Report on the Oceans and Cryosphere in a Changing Climate, eds H.-O. Pörtner, D. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, et al. (Geneva: Intergovernmental Panel on Climate Change).
Amelung, B., and Lamers, M. (2007). Estimating the greenhouse gas emissions from Antarctic tourism. Tour. ism Mar. Environ. 4, 121–133. doi: 10.3727/154427307784772020
Arntz, W., Brey, T., and Gallardo, V. A. (1994). Anarctic zoobenthos. Oceanogr. Mar. Biol.32, 241–304.
Aronson, R. B., Thatje, S., McClintock, J. B., and Hughes, K. A. (2011). Anthropogenic impacts on marine ecosystems in Antarctica. Ann. N. Y, Acad. Sci. 1223, 82–107. doi: 10.1111/j.1749-6632.2010.05926.x
Arrigo, K. R., van Dijken, G. L., and Bushinsky, S. (2008). Primary production in the Southern Ocean, 1997–2006. J. Geophys. Res. 113:C08004. doi: 10.1029/2007JC004551
Ashton, G. V., Morley, S. A., Barnes, D. K. A., Clark, M. S., and Peck, L. S. (2017). Warming by 1°C drives species and assemblage level responses in Antarctica’s marine shallows. Curr. Biol. 27, 2698–2705. doi: 10.1016/j.cub.2017.07.048
Atkinson, A., Hill, S. L., Pakhomov, E. A., Siegel, V., Reiss, C. S., Loeb, V. J., et al. (2019). Krill (Euphausia superba) distribution contracts southward during rapid regional warming’. Nat. Clim. Change 9, 142–147. doi: 10.1038/s41558-018-0370-z
Atkinson, A., Shreeve, R. S., Hirst, A. G., Rothery, P., Tarling, G. A., and Pond, D. W. (2006). Natural growth rates in Antarctic krill (Euphausia superba): II. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnol. Oceanogr. 51, 973–987. doi: 10.4319/lo.2006.51.2.0973
Atkinson, A., Siegel, V., Pakhomov, E., and Rothery, P. (2004). Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100–103. doi: 10.1038/nature02996
Atkinson, A., Siegel, V., Pakhomov, E., Rothery, P., Loeb, V., and Ross, R. (2008). Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 362, 1–23. doi: 10.3354/meps07498
Atkinson, A., Siegel, V., Pakhomov, E. A., Jessopp, M. J., and Loeb, V. (2009). A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 727–740. doi: 10.1016/j.dsr.2008.12.007
Azam, F. (1998). Microbial control of oceanic carbon flux: the plot thickens. Science 280, 694–696. doi: 10.1126/science.280.5364.694
Barnes, D. K. A. (2015). Antarctic sea ice losses drive gains in benthic carbon drawdown. Curr. Biol. 25, R789–R790. doi: 10.1016/j.cub.2015.07.042
Barnes, D. K. A. (2017). Iceberg killing fields limit huge potential for benthic blue carbon in Antarctic shallows. Glob. Change Biol. 23, 2649–2659. doi: 10.1111/gcb.13523
Barnes, D. K. A., Fleming, A., Sands, C. J., Quartino, M. L., and Deregibus, D. (2018). Icebergs, sea ice, blue carbon and Antarctic climate feedbacks. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 376:20170176. doi: 10.1098/rsta.2017.0176
Barnes, D. K. A., and Tarling, G. A. (2017). Polar oceans in a changing climate. Curr. Biol. 27, R454–R460. doi: 10.1016/j.cub.2017.01.045
Bastmeijer, K. (2013). “Report of the informal contact group on the increasing diversity of tourism and other non-governmental activities in antarctica’,” in Working Paper (WP47) for the XXXVIth, Antarctic Treaty Consultative Meeting (ATCM), Brussels.
Bastmeijer, K., Lamers, M., and Harcha, J. (2008). Permanent land-based facilities for tourism in antarctica: the need for regulation. Rev. Eur. Comm. Int. Environ. Law 17, 84–99. doi: 10.1111/j.1467-9388.2008.00585.x
Bateman, I. J., and Mace, G. M. (2020). The natural capital framework for sustainably efficient and equitable decision making. Nat. Sust. 3, 776–783. doi: 10.1038/s41893-020-0552-3
Belcher, A., Henson, S. A., Manno, C., Hill, S. L., Atkinson, A., Thorpe, S. E., et al. (2019). Krill faecal pellets drive hidden pulses of particulate organic carbon in the marginal ice zone. Nat. Commun. 10, 1–8. doi: 10.1038/s41467-019-08847-1
Burger, J., and Gochfeld, M. (2007). Responses of Emperor Penguins (Aptenodytes forsteri) to encounters with ecotourists while commuting to and from their breeding colony. Polar Biol. 30, 1303–1313. doi: 10.1007/s00300-007-0291-1
Cavan, E. L., Belcher, A., Atkinson, A., Hill, S. L., Kawaguchi, S., McCormack, S., et al. (2019). The importance of Antarctic krill in biogeochemical cycles. Nat. Commun. 10, 1–13. doi: 10.1038/s41467-019-12668-7
Cavanagh, R. D., Broszeit, S., Pilling, G. M., Grant, S. M., Murphy, E. J., and Austen, M. C. (2016a). Valuing biodiversity and ecosystem services: a useful way to manage and conserve marine resources? Proc. R. Soc. B Biol. Sci. 283:20161635. doi: 10.1098/rspb.2016.1635
Cavanagh, R. D., Hill, S. L., Knowland, C. A., and Grant, S. M. (2016b). Stakeholder perspectives on ecosystem-based management of the Antarctic krill fishery. Mar. Policy 68, 205–211. doi: 10.1016/j.marpol.2016.03.006
Cavanagh, R. D., Murphy, E. J., Bracegirdle, T. J., Turner, J., Knowland, C. A., Corney, S. P., et al. (2017). A Synergistic approach for evaluating climate model output for ecological applications. Front. Mar. Sci. 4:308. doi: 10.3389/fmars.2017.00308
Chan, K. M. A., Satterfield, T., and Goldstein, J. (2012). Rethinking ecosystem services to better address and navigate cultural values. Ecol. Econ. 74, 8–18. doi: 10.1016/j.ecolecon.2011.11.011
Chown, S. L., and Brooks, C. M. (2019). The state and future of antarctic environments in a global context. Annu. Rev. Environ. Resour. 44, 1–30. doi: 10.1146/annurev-environ-101718-033236
Chown, S. L., Huiskes, A. H. L., Gremmen, N. J. M., Lee, J. E., Terauds, A., Crosbie, K., et al. (2012). Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proc. Natl. Acad. Sci. U.S.A. 109, 4938–4943. doi: 10.1073/pnas.1119787109
Collins, M., Sutherland, M., Bouwer, L., Cheong, S.-M., Frölicher, T., and Jacot Des Combes, H., et al. (eds) (2019). “Extremes, abrupt changes and managing risks,” in IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, eds H.-O. Pörtner, D. C. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, et al. (Geneva: Intergovernmental Panel on Climate Change.).
Constable, A. J., Melbourne−Thomas, J., Corney, S. P., Arrigo, K. R., Barbraud, C., Barnes, D. K. A., et al. (2014). Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Glob. Change Biol. 20, 3004–3025. doi: 10.1111/gcb.12623
Costanza, R., d’Arge, R., de Groot, R., Farber, S., Grasso, M., Hannon, B., et al. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260. doi: 10.1038/387253a0
Cox, M. J., Candy, S., de la Mare, W. K., Nicol, S., Kawaguchi, S., and Gales, N. (2018). No evidence for a decline in the density of Antarctic krill Euphausia superba Dana, 1850, in the Southwest Atlantic sector between 1976 and 2016. J. Crustac. Biol. 38, 656–661. doi: 10.1093/jcbiol/ruy072
Cox, M. J., Candy, S., De la Mare, W. K., Nicol, S., Kawaguchi, S., and Gales, N. (2019). Clarifying trends in the density of Antarctic krill Euphausia superba Dana, 1850 in the South Atlantic. A response to Hill et al. J. Crustac. Biol. 39, 323–327. doi: 10.1093/jcbiol/ruz010
Cullis-Suzuki, S., and Pauly, D. (2010). Failing the high seas: a global evaluation of regional fisheries management organizations. Mar. Policy 34, 1036–1042. doi: 10.1016/j.marpol.2010.03.002
Deininger, M., Koellner, T., Brey, T., and Teschke, K. (2016). Towards mapping and assessing Antarctic marine ecosystem services–the Weddell Sea case study. Ecosyst. Serv. 22, 174–192. doi: 10.1016/j.ecoser.2016.11.001
Ducklow, H. W., Baker, K., Martinson, D. G., Quetin, L. B., Ross, R. M., Smith, R. C., et al. (2007). Marine pelagic ecosystems: the west Antarctic Peninsula. Philos. Trans. R. Soc. B Biol. Sci. 362, 67–94. doi: 10.1098/rstb.2006.1955
Dunford, R., Harrison, P., Smith, A., Dick, J., Barton, D. N., Martin-Lopez, B., et al. (2018). Integrating methods for ecosystem service assessment: experiences from real world situations. Ecosyst. Serv. 29, 499–514. doi: 10.1016/j.ecoser.2017.10.014
DuPrat, L. P. A. M., Bigg, G. R., and Wilton, D. J. (2016). Enhanced Southern Ocean marine productivity due to fertilization by giant icebergs. Nat. Geosci. 9, 219–221. doi: 10.1038/ngeo2633
Eijgelaar, E., Thaper, C., and Peeters, P. (2010). Antarctic cruise tourism: the paradoxes of ambassadorship,“last chance tourism” and greenhouse gas emissions. J. Sust. Tour. 18, 337–354. doi: 10.1080/09669581003653534
Flores, H., Atkinson, A., Kawaguchi, S., Krafft, B. A., Milinevsky, G., Nicol, S., et al. (2012). Impact of climate change on Antarctic krill. Mar. Ecol. Prog. Ser. 458, 1–19. doi: 10.3354/meps09831
Forcada, J., and Hoffman, J. I. (2014). Climate change selects for heterozygosity in a declining fur seal population. Nature 511, 462–465. doi: 10.1038/nature13542
Frainer, A., Primicerio, R., Kortsch, S., Aune, M., Dolgov, A. V., Fossheim, M., et al. (2017). Climate-driven changes in functional biogeography of Arctic marine fish communities. Proc. Natl. Acad. Sci. U.S.A. 114, 12202–12207. doi: 10.1073/pnas.1706080114
Garcia, S. M., and Grainger, R. J. R. (2005). Gloom and doom? The future of marine capture fisheries. Philos. Trans. R. Soc. B Biol. Sci. 360, 21–46. doi: 10.1098/rstb.2004.1580
Gogarty, B., McGee, J., Barnes, D. K. A., Sands, C. J., Bax, N., Haward, M., et al. (2020). Protecting Antarctic blue carbon: as marine ice retreats can the law fill the gap? Clim. Policy 20, 149–162. doi: 10.1080/14693062.2019.1694482
Grant, S. M., Hill, S. L., and Fretwell, P. T. (2013a). Spatial distribution of management measures, Antarctic krill catch and Southern Ocean bioregions: implications for conservation planning. CCAMLR Sci. 20, 119–137.
Grant, S. M., Hill, S. L., Trathan, P. N., and Murphy, E. J. (2013b). Ecosystem services of the Southern Ocean: trade-offs in decision-making. Antarct. Sci. 25, 603–617. doi: 10.1017/S0954102013000308
Grant, S. M., and Penhale, P. A. (2016). “Conveners’ report of the joint CEP/SC-CAMLR workshop on climate change and monitoring,” in Paper SC-CAMLR-XXXV. SC-CAMLR XXXV, Hobart.
Guerry, A. D., Polasky, S., Lubchenco, J., Chaplin-Kramer, R., Daily, G. C., Griffin, R., et al. (2015). Natural capital and ecosystem services informing decisions: from promise to practice. Proc. Natl. Acad. Sci. U.S.A. 112, 7348–7355. doi: 10.1073/pnas.1503751112
Gutt, J., Bertler, N., Bracegirdle, T. J., Buschmann, A., Comiso, J., Hosie, G., et al. (2015). The Southern Ocean ecosystem under multiple climate change stresses−an integrated circumpolar assessment. Glob. Change Biol. 21, 1434–1453. doi: 10.1111/gcb.12794
Henley, S. F., Cavan, E. L., Fawcett, S. E., Kerr, R., Monteiro, T., Sherrell, R. M., et al. (2020). Changing biogeochemistry of the Southern Ocean and its ecosystem implications. Front. Mar. Sci. 7:581. doi: 10.3389/fmars.2020.00581
Hill, S., and Grant, S. (2013). Could ecosystem assessment improve the protection of antarctic ecosystems? Antarct. Sci. 25:601. doi: 10.1017/S0954102013000709
Hill, S. L. (2013a). From strategic ambiguity to technical reference points in the Antarctic krill fishery: the worst journey in the world? Environ. Conserv. 40, 394–405. doi: 10.1017/S0376892913000088
Hill, S. L. (2013b). “Prospects for a sustainable increase in the availability of long chain omega 3s: lessons from the Antarctic Krill fishery,” in Omega-6/3 Fatty Acids. Nutrition and Health, eds F. De Meester, R. Watson, and S. Zibadi, (Totowa, NJ: Humana Press), doi: 10.1007/978-1-62703-215-5_14
Hill, S. L., Atkinson, A., Darby, C., and Fielding, S. (2016). Is current management of the Antarctic krill fishery in the Atlantic sector of the Southern Ocean precautionary? CCAMLR Sci. 23, 17–30.
Hill, S. L., Atkinson, A., Pakhomov, E. A., and Siegel, V. (2019). Evidence for a decline in the population density of Antarctic krill Euphausia superba still stands. A comment on Cox et al. J. Crustac. Biol. 39, 316–322. doi: 10.1093/jcbiol/ruz004
Hill, S. L., and Cannon, M. (2013). A potential feedback approach to ecosystem-based management: model predictive control of the Antarctic krill fishery. CCAMLR Sci. 20, 119–137.
Hill, S. L., Hinke, J., Bertrand, S., Fritz, L., Furness, R. W., Ianelli, J. N., et al. (2020). Reference points for predators will progress ecosystem−based management of fisheries. Fish Fish. 21, 368–378. doi: 10.1111/faf.12434
Hill, S. L., Keeble, K., Atkinson, A., and Murphy, E. J. (2012). A foodweb model to explore uncertainties in the South Georgia shelf pelagic ecosystem. Deep Sea Res. Part II Top. Stud. Oceanogr. 59, 237–252. doi: 10.1016/j.dsr2.2011.09.001
Hill, S. L., Phillips, T., and Atkinson, A. (2013). Potential climate change effects on the habitat of Antarctic krill in the Weddell quadrant of the Southern Ocean. PLoS One 8:e72246. doi: 10.1371/journal.pone.0072246
Hill, S. L., Trathan, P. N., and Agnew, D. J. (2009). The risk to fishery performance associated with spatially resolved management of Antarctic krill (Euphausia superba) harvesting. ICES J. Mar. Sci. 66, 2148–2154. doi: 10.1093/icesjms/fsp172
Hofmann, E., Biddle, L., de Bruin, T., Brooks, C., Corney, S., Haumann, A., et al. (2020). 1st Southern Ocean Regional Workshop for the UN Decade of Ocean Science for Sustainable Development Report. Geneva: Zenodo, doi: 10.5281/ZENODO.3973745
Hofmann, E. E., and Murphy, E. J. (2004). Advection, krill, and Antarctic marine ecosystems. Antarct. Sci. 16, 487–499. doi: 10.1017/S0954102004002275
Huang, T., Sun, L., Stark, J., Wang, Y., Cheng, Z., Yang, Q., et al. (2011). Relative Changes in Krill Abundance Inferred from Antarctic Fur Seal. PLoS One 6:e27331. doi: 10.1371/journal.pone.0027331
Hughes, K., and Convey, P. (2020). Implications of the COVID-19 pandemic for Antarctica. Antarct. Sci. 32, 426–439. doi: 10.1017/S095410202000053X
Hughes, K. A., Constable, A., Frenot, Y., López-Martínez, J., McIvor, E., Njåstad, B., et al. (2018). Antarctic environmental protection: strengthening the links between science and governance. Environ. Sci. Policy 83, 86–95. doi: 10.1016/j.envsci.2018.02.006
Hughes, K. A., Pescott, O. L., Peyton, J., Adriaens, T., Cottier−Cook, E. J., Key, G., et al. (2020). Invasive non−native species likely to threaten biodiversity and ecosystems in the Antarctic Peninsula region. Glob. Change Biol. 26, 2702–2716. doi: 10.1111/gcb.14938
IPBES, (2019). Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, eds S., Díaz J., Settele E. S., Brondízio H. T., Ngo M., Guèze J., Agardet al. Bonn: IPBES Secretariat, 56. doi: 10.5281/zenodo.3553579
IPCC, (2014). “Climate Change 2014: synthesis report,” in Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Core Writing Team Pachauri, R. K., and Meyer, L. A., (Geneva: IPCC), 151.
IPCC, (2019). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, eds H.-O. Pörtner, D. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, et al. (Geneva: IPCC).
Jabour, J. (2014). “Strategic management and regulation of Antarctic tourism,” in Antarctic Futures, eds T. Tin, D. Liggett, P. Maher, and M. Lamers, (Berlin: Springer), 273–286. doi: 10.1007/978-94-007-6582-5_12
Kawaguchi, S., Ishida, A., King, R., Raymond, B., Waller, N., Constable, A., et al. (2013). Risk maps for Antarctic krill under projected Southern Ocean acidification. Nat. Clim. Change 3, 843–847. doi: 10.1038/nclimate1937
Klein, E. S., Hill, S. L., Hinke, J. T., Phillips, T., and Watters, G. M. (2018). Impacts of rising sea temperature on krill increase risks for predators in the Scotia Sea. PLoS One 13:e0191011. doi: 10.1371/journal.pone.0191011
Lamers, M., Eijgelaar, E., and Amelung, B. (2011). “Last-chance tourism in Antarctica - Cruising for change?,” in Last-Chance Tourism: Adapting Tourism Opportunities in a Changing World, eds H. Lemelin, J. Dawson, and E. Stewart, (London: Routledge), 2011.
Lamers, M., Eijgelaar, E., and Amelung, B. (2013). “Last chance tourism in Antarctica,” in Last Chance Tourism: Adapting Tourism Opportunities in a Changing World, eds H. Lemelin, J. Dawson, and E. J. Stewart, (London: Routledge), 25–41.
Lavery, T., Roudnew, B., Seymour, J., Mitchell, J., Smetacek, V., and Nicol, S. (2014). Whales sustain fisheries: blue whales stimulate primary production in the Southern Ocean. Mar. Mamm. Sci. 30, 888–904. doi: 10.1111/mms.12108
Lee, J. R., Raymond, B., Bracegirdle, T. J., Chadès, I., Fuller, R. A., Shaw, J. D., et al. (2017). Climate change drives expansion of Antarctic ice-free habitat. Nature 547, 49–54. doi: 10.1038/nature22996
Lemelin, H., Dawson, J., Stewart, E. J., Maher, P., and Lueck, M. (2010). Last-chance tourism: the boom, doom, and gloom of visiting vanishing destinations. Curr. Issues Tour. 13, 477–493. doi: 10.1080/13683500903406367
Liggett, D., Frame, B., Gilbert, N., and Morgan, F. (2017). Is it all going south? Four future scenarios for Antarctica. Polar Record 53, 459–478. doi: 10.1017/S0032247417000390
Liggett, D., McIntosh, A., Thompson, A., Gilbert, N., and Storey, B. (2011). From frozen continent to tourism hotspot? Five decades of Antarctic tourism development and management, and a glimpse into the future. Tour. Manag. 32, 357–366. doi: 10.1016/j.tourman.2010.03.005
Manno, C., Fielding, S., Stowasser, G., Murphy, E. J., Thorpe, S. E., and Tarling, G. A. (2020). Continuous moulting by Antarctic krill drives major pulses of carbon export in the north Scotia Sea, Southern Ocean. Nat. Commun. 11:6051. doi: 10.1038/s41467-020-19956-7
Mayewski, P. A., Meredith, M. P., Summerhayes, C. P., Turner, J., Worby, A., Barrett, P. J., et al. (2009). State of the Antarctic and Southern Ocean climate system. Rev. Geophys. 47:RG1003. doi: 10.1029/2007RG000231
McMahon, K. W., Michelson, C. I., Hart, T., McCarthy, M. D., Patterson, W. P., and Polito, M. J. (2019). Divergent trophic responses of sympatric penguin species to historic anthropogenic exploitation and recent climate change. Proc. Natl. Acad. Sci. U.S.A. 116, 25721–25727. doi: 10.1073/pnas.1913093116
Melbourne-Thomas, J., Corney, S. P., Trebilco, R., Meiners, K. M., Stevens, R. P., Kawaguchi, S., et al. (2016). Under ice habitats for Antarctic krill larvae: could less mean more under climate warming? Geophys. Rese. Lett. 43, 10,310–322,327. doi: 10.1002/2016GL070846
Melbourne-Thomas, J., Wotherspoon, S., Raymond, B., and Constable, A. (2012). Comprehensive evaluation of model uncertainty in qualitative network analyses. Ecol. Monogr. 82, 505–519. doi: 10.1890/12-0207.1
Meredith, M., Sommerkorn, M., Cassotta, S., Derksen, C., Ekaykin, A., and Hollowed, A., et al. (eds) (2019). IPCC Special Report on the Oceans and Cryosphere in a changing climate. Geneva: Intergovernmental Panel on Climate Change.
Meredith, M. P., Schofield, O., Newman, L., Urban, E., and Sparrow, M. (2013). The vision for a Southern Ocean Observing System. Curr. Opin. Environ. Sust. 5, 306–313. doi: 10.1016/j.cosust.2013.03.002
Meyer, B., Freier, U., Grimm, V., Groeneveld, J., Hunt, B. P. V., Kerwath, S., et al. (2017). The winter pack-ice zone provides a sheltered but food-poor habitat for larval Antarctic krill. Nat. Ecol. Evol. 1, 1853–1861. doi: 10.1038/s41559-017-0368-3
Meyer, B., Atkinson, A., Bernard, K. S., Brierley, A. S., Driscoll, R., Hill, S. L., et al. (2020). Successful ecosystem-based management of Antarctic krill should address uncertainties in krill recruitment, behaviour and ecological adaptation. Commun. Earth Environ. 1. doi: 10.1038/s43247-020-00026-1
Millennium Ecosystem Assessment [MEA], (2005). Ecosystems and Human Well-Being: Synthesis. Washington, DC: Island Press.
Montoya, J. M., and Raffaelli, D. (2010). Climate change, biotic interactions and ecosystem services. Philos. Trans. R. Soc. B Biol. Sci. 365, 2013–2018. doi: 10.1098/rstb.2010.0114
Mooney, H., Larigauderie, A., Cesario, M., Elmquist, T., Hoegh-Guldberg, O., Lavorel, S., et al. (2009). Biodiversity, climate change, and ecosystem services. Curr. Opin. Environ. Sust. 1, 46–54. doi: 10.1016/j.cosust.2009.07.006
Moore, K., and Abbott, M. R. (2000). Phytoplankton chlorophyll distributions and primary production in the Southern Ocean. J. Geophys. Res. 105, 709–728. doi: 10.1029/1999JC000043
Morley, S. A., Abele, D., Barnes, D. K. A., Cárdenas, C. A., Cotté, C., Gutt, J., et al. (2020). Global drivers on southern ocean ecosystems: changing physical environments and anthropogenic pressures in an Earth system. Front. Mar. Sci. 7:547188. doi: 10.3389/fmars.2020.547188
Morley, S. A., Barnes, D. K. A., and Dunn, M. J. (2019). Predicting which species succeed in climate-forced Polar seas. Front. Mar. Sci. 5:507. doi: 10.3389/fmars.2018.00507
Murphy, E. J., Cavanagh, R. D., Drinkwater, K. F., Grant, S. M., Heymans, J. J., Hofmann, E. E., et al. (2016). Understanding the structure and functioning of polar pelagic ecosystems to predict the impacts of change. Proc. R. Soc. B Biol. Sci. 283:20161646. doi: 10.1098/rspb.2016.1646
Murphy, E. J., Cavanagh, R. D., Johnston, N. M., Reid, K., and Hofmann, E. E. (2008). Integrating Climate and Ecosystem Dynamics (ICED): Science Plan and Implementation Strategy. GLOBEC Report no. 25. See. Available at: http://www.iced.ac.uk/ (accessed December 2020).
Murphy, E. J., Thorpe, S. E., Tarling, G. A., Watkins, J. L., Fielding, S., and Underwood, P. (2017). Restricted regions of enhanced growth of Antarctic krill in the circumpolar Southern Ocean. Sci. Rep. 7:6963. doi: 10.1038/s41598-017-07205-9
Netherland, and New Zealand. (2019). “Proactive management of Antarctic tourism: time for a fresh approach. Information Paper 26,” in Antarctic Treaty Consultative Meeting XLII, Prague.
Neumann, B., Mikoleit, A., Bowman, J. S., Ducklow, H. W., and Müller, F. (2019). Ecosystem service supply in the antarctic peninsula region: evaluating an expert-based assessment approach and a novel seascape data model. Front. Environ. Sci. 7:157. doi: 10.3389/fenvs.2019.00157
Nicol, S., and Foster, J. (2016). “The fishery for Antarctic krill: Its current status and management regime,” in Biology and Ecology of Antarctic Krill, ed. V. Siegel, (Berlin: Springer), 387–421. doi: 10.1007/978-3-319-29279-3_11
Peck, L. S. (2018). “Antarctic marine biodiversity: adaptations, environments and responses to change,” in Oceanography and Marine Biology, eds S. J. Hawkins, A. J. Evans, A. C. Dale, L. B. Firth, and I. P. Smith, (Milton Park: Taylor & Francis).
Peck, L. S., Barnes, D. K. A., Cook, A. J., Fleming, A. H., and Clarke, A. (2010). Negative feedback in the cold: ice retreat produces new carbon sinks in Antarctica. Glob. Change Biol. 16, 2614–2623. doi: 10.1111/j.1365-2486.2009.02071.x
Pedrono, M., Locatelli, B., Ezzine-de-Blas, D., Pesche, D., Morand, S., and Binot, A. (2016). “Impact of climate change on ecosystem services,” in Climate Change and Agriculture Worldwide, ed. E. Torquebiau, (Berlin: Springer), 251–261.
Perry, F. A., Atkinson, A., Sailley, S. F., Tarling, G. A., Hill, S. L., Lucas, C. H., et al. (2019). Habitat partitioning in Antarctic krill: spawning hotspots and nursery areas. PLoS One 14:e0219325. doi: 10.1371/journal.pone.0219325
Perry, F. A., Kawaguchi, S., Atkinson, A., Sailley, S. F., Tarling, G. A., Mayor, D. J., et al. (2020). Temperature–induced hatch failure and nauplii malformation in Antarctic Krill. Front. Mar. Sci. 7:501. doi: 10.3389/fmars.2020.00501
Pertierra, L. R., and Hughes, K. A. (2019). Evaluating ecosystem services in Antarctica - why are we falling behind? Antarct. Sci. 31, 229–230. doi: 10.1017/S0954102019000312
Pertierra, L. R., Hughes, K. A., Vega, G. C., and Olalla-Tárraga, M. Á (2017). High resolution spatial mapping of human footprint across Antarctica and its implications for the strategic conservation of avifauna. PLoS One 12:e0168280. doi: 10.1371/journal.pone.0168280
Pineda-Metz, S. E. A., Gerdes, D., and Richter, C. (2020). Benthic fauna declined on a whitening Antarctic continental shelf. Nat. Commun. 11:2226. doi: 10.1038/s41467-020-16093-z
Piñones, A., and Fedorov, A. V. (2016). Projected changes of Antarctic krill habitat by the end of the 21st century. Geophys. Res. Lett. 43, 8580–8589. doi: 10.1002/2016GL069656
Piñones, A., Hofmann, E. E., Daly, K. L., Dinniman, M. S., and Klinck, J. M. (2013). Modeling the remote and local connectivity of Antarctic krill populations along the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 481, 69–92. doi: 10.3354/meps10256
Pinsky, M. L., Reygondeau, G., Caddell, R., Palacios-Abrantes, J., Spijkers, J., and Cheung, W. W. L. (2018). Preparing ocean governance for species on the move. Science 360, 1189–1191. doi: 10.1126/science.aat2360
Quetin, L. B., Ross, R. M., Fritsen, C. H., and Vernet, M. (2007). Ecological responses of Antarctic krill to environmental variability: can we predict the future? Antarct. Sci. 19, 253–266. doi: 10.1017/S0954102007000363
Ratnarajah, L., Bowie, A. R., Lannuzel, D., Meiners, K. M., and Nicol, S. (2015). Correction: the biogeochemical role of baleen whales and krill in southern ocean nutrient cycling. PLoS One 10:e0125134. doi: 10.1371/journal.pone.0125134
Roach, L. A., Dörr, J., Holmes, C. R., Massonnet, F., Blockley, E. W., Notz, D., et al. (2020). Antarctic Sea Ice Area in CMIP6. Geophys. Res. Lett. 47:e2019GL086729. doi: 10.1029/2019GL086729
Rogers, A. D., Frinault, B. A. V., Barnes, D. K. A., Bindoff, N. L., Downie, R., Ducklow, H. W., et al. (2020). Antarctic futures: an assessment of climate-driven changes in ecosystem structure, function, and service provisioning in the Southern Ocean. Annu. Rev. Mar. Sci. 12, 87–120. doi: 10.1146/annurev-marine-010419-011028
Runting, R. K., Bryan, B. A., Dee, L. E., Maseyk, F. J. F., Mandle, L., Hamel, P., et al. (2017). Incorporating climate change into ecosystem service assessments and decisions: a review. Glob. Change Biol. 23, 28–41. doi: 10.1111/gcb.13457
Schaafsma, F. L., Kohlbach, D., David, C., Lange, B. A., Graeve, M., Flores, H., et al. (2017). Spatio-temporal variability in the winter diet of larval and juvenile Antarctic krill. Euphausia superba, in ice-covered waters. Mar. Ecol. Prog. Ser. 580, 101–115. doi: 10.3354/meps12309
Schmidt, K., Atkinson, A., Steigenberger, S., Fielding, S., Lindsay, M. C., Pond, D. W., et al. (2011). Seabed foraging by Antarctic krill: implications for stock assessment, bentho−pelagic coupling, and the vertical transfer of iron. Limnol. Oceanogr. 56, 1411–1428. doi: 10.4319/lo.2011.56.4.1411
Secretariat of the Antarctic Treaty, (2016). Final Report of the Thirty-Ninth Antarctic Treaty Consultative Meeting. 23 May – 1 June 2016. Santiago: Buenos Aires, 408. Available at: https://documents.ats.aq/ATCM39/fr/ATCM39_fr001_e.pdf (accessed December 2020).
Seddon, N., Mace, G. M., Naeem, S., Tobias, J. A., Pigot, A. L., Cavanagh, R., et al. (2016). Biodiversity in the Anthropocene: prospects and policy. Proc. R. Soc. B Biol. Sci. 283:20162094. doi: 10.1098/rspb.2016.2094
Siegel, V., and Nicol, S. (2000). “Population parameters,” in Krill : Biology, Ecology and Fisheries, ed. I. Everson, (Oxford: Blackwell Science), 103–149. doi: 10.1002/9780470999493.ch5
Siegel, V., and Watkins, J. L. (2016). “Distribution, biomass and demography of Antarctic krill, Euphausia superba,” in Biology and Ecology of Antarctic krill, ed. V. Siegel, (Berlin: Springer), 21–100. doi: 10.1007/978-3-319-29279-3_2
Siegert, M. J., Rumble, J., Atkinson, A., Rogelj, J., Edwards, T., Davies, B. J., et al. (2019). The Antarctic Peninsula under a 1.5 C global warming scenario. Front. Environ. Sci. 7:102. doi: 10.3389/fenvs.2019.00102
Smetacek, V., and Nicol, S. (2005). Polar ocean ecosystems in a changing world. Nature 437, 362–368. doi: 10.1038/nature04161
Souster, T. A., Barnes, D. K. A., and Hopkins, J. (2020). Variation in zoobenthic blue carbon in the Arctic’s Barents Sea shelf sediments. Philos. Trans. R. Soc. A Math., Phys. Eng. Sci. 378:20190362. doi: 10.1098/rsta.2019.0362
Summerson, R., and Tin, T. (2018). Twenty years of protection of wilderness values in Antarctica. Polar J. 8, 265–288. doi: 10.1080/2154896X.2018.1541548
Tarling, G. A., Hill, S., Peat, H., Fielding, S., Reiss, C., and Atkinson, A. (2016). Growth and shrinkage in Antarctic krill Euphausia superba is sex-dependent. Mar. Ecol. Prog. Ser. 547, 61–78. doi: 10.3354/meps11634
Tarling, G. A., Thorpe, S. E., Fielding, S., Klevjer, T., Ryabov, A., and Somerfield, P. J. (2018). Varying depth and swarm dimensions of open-ocean Antarctic krill Euphausia superba Dana, 1850 (Euphausiacea) over diel cycles. J. Crustac. Biol. 38, 716–727. doi: 10.1093/jcbiol/ruy040
Tejedo, P., Benayas, J., Cajiao, D., Albertos, B., Lara, F., Pertierra, L. R., et al. (2016). Assessing environmental conditions of Antarctic footpaths to support management decisions. J. Environ. Manag. 177, 320–330. doi: 10.1016/j.jenvman.2016.04.032
Thorpe, S., and Tarling, G. (2019). Circumpolar patterns in Antarctic krill larval recruitment: an environmentally driven model. Mar. Ecol. Prog. Ser. 613, 77–96. doi: 10.3354/meps12887
Townsend, M., Davies, K., Hanley, N., Hewitt, J. E., Lundquist, C. J., and Lohrer, A. M. (2018). The challenge of implementing the marine ecosystem service concept. Front. Mar. Sci. 5:359. doi: 10.3389/fmars.2018.00359
Trathan, P. N., Collins, M. A., Grant, S. M., Belchier, M., Barnes, D. K. A., Brown, J., et al. (2014). The South Georgia and the South Sandwich Islands MPA: protecting a biodiverse oceanic island chain situated in the flow of the Antarctic Circumpolar Current. Adv. Mar. Biol. 69, 15–78.
Trathan, P. N., and Hill, S. L. (2016). “The importance of krill predation in the Southern Ocean,” in Biology and Ecology of Antarctic krill, ed. V. Siegel, (Berlin: Springer), 321–350. doi: 10.1007/978-3-319-29279-3_9
Trebilco, R., Melbourne-Thomas, J., and Constable, A. J. (2020). The policy relevance of Southern Ocean food web structure: implications of food web change for fisheries, conservation and carbon sequestration. Mar. Policy 115:103832. doi: 10.1016/j.marpol.2020.103832
Tulloch, V. J., Plagányi, ÉE., Brown, C., Richardson, A. J., and Matear, R. (2019). Future recovery of baleen whales is imperiled by climate change. Glob. Change Biol. 25, 1263–1281. doi: 10.1111/gcb.14573
Turner, J., Barrand, N. E., Bracegirdle, T. J., Convey, P., Hodgson, D. A., Jarvis, M., et al. (2014). Antarctic climate change and the environment: an update. Polar Record 50, 237–259. doi: 10.1017/S0032247413000296
Turner, J., Bindschadler, R., Convey, P., di Prisco, G., Fahrbach, E., Gutt, J., et al. (2009). Antarctic Climate Change and the Environment. Cambridge: Scientific Committee on Antarctic Research Scott Polar Research Institute.
Turner, J., and Comiso, J. (2017). Solve Antarctica’s sea-ice puzzle. Nature 547:275. doi: 10.1038/547275a
Turner, J., Guarino, M. V., Arnatt, J., Jena, B., Marshall, G. J., Phillips, T., et al. (2020). Recent decrease of summer sea ice in the Weddell Sea, Antarctica. Geophys. Res. Lett. 47. doi: 10.1029/2020GL087127
UNEP, (2010). “Strategic plan for biodiversity 2011–2020 and the Aichi targets,” in Report of the Tenth Meeting of the Conference of the Parties to the Convention on Biological Diversity. Avaliable at: https://www.cbd.int/doc/meetings/cop/cop-10/official/cop-10-27-en.pdf (accessed December 2020).
United Kingdom, Argentina, Chile, and IAATO, (2019). “Data collection and reporting on yachting activitiy in Antarctica in 2018-19,” in Information Paper 107 rev.1. Antarctic Treaty Consultative meeting XLII, Prague.
United Nations, European Commission, Food and Agricultural Organization of the United Nations, Organisation for Economic Co-operation and Development, and The World Bank. (2014). System of Environmental-Economic Accounting 2012 – Experimental Ecosystem Accounting. New York, NY: United Nations.
Verbitsky, J. (2018). Ecosystem services and Antarctica: the time has come? Ecosyst. Serv. 29, 381–394. doi: 10.1016/j.ecoser.2017.10.015
Veytia, D., Corney, S., Meiners, K. M., Kawaguchi, S., Murphy, E. J., and Bestley, S. (2020). Circumpolar projections of Antarctic krill growth potential. Nat. Clim. Change 10, 568–575. doi: 10.1038/s41558-020-0758-4
Watters, G. M., Hinke, J. T., and Reiss, C. S. (2020). Long-term observations from Antarctica demonstrate that mismatched scales of fisheries management and predator-prey interaction lead to erroneous conclusions about precaution. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-59223-9
Keywords: Southern Ocean, Antarctic krill, blue carbon, ecosystem services, climate change, Antarctic tourism, Antarctic Treaty System, IPCC (Intergovernmental Panel on Climate Change)
Citation: Cavanagh RD, Melbourne-Thomas J, Grant SM, Barnes DKA, Hughes KA, Halfter S, Meredith MP, Murphy EJ, Trebilco R and Hill SL (2021) Future Risk for Southern Ocean Ecosystem Services Under Climate Change. Front. Mar. Sci. 7:615214. doi: 10.3389/fmars.2020.615214
Received: 08 October 2020; Accepted: 18 December 2020;
Published: 14 January 2021.
Edited by:
Jacob Carstensen, Aarhus University, DenmarkReviewed by:
Matthew Savoca, Stanford University, United StatesCopyright © 2021 Cavanagh, Melbourne-Thomas, Grant, Barnes, Hughes, Halfter, Meredith, Murphy, Trebilco and Hill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel D. Cavanagh, cmNhdkBiYXMuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.