- 1MARE – Centro de Ciências do Mar e do Ambiente, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal
- 2Laboratório de Fitoplâncton e Microorganismos Marinhos, Instituto de Oceanografia, Universidade Federal do Rio Grande, Rio Grande, Brazil
- 3Laboratório de Estudos dos Oceanos e Clima, Instituto de Oceanografia, Universidade Federal do Rio Grande, Rio Grande, Brazil
- 4Departamento de Biologia Vegetal, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal
- 5Laboratório de Ecologia e Conservação da Megafauna Marinha, Instituto de Oceanografia, Universidade Federal do Rio Grande, Rio Grande, Brazil
The Northern Antarctic Peninsula (NAP), located in West Antarctica, is amongst the most impacted regions by recent warming events. Its vulnerability to climate change has already led to an accumulation of severe changes along its ecosystems. This work reviews the current findings on impacts observed in phytoplankton communities occurring in the NAP, with a focus on its causes, consequences, and the potential research priorities toward an integrated comprehension of the physical–biological coupling and climate perspective. Evident changes in phytoplankton biomass, community composition and size structure, as well as potential bottom-up impacts to the ecosystem are discussed. Surface wind, sea ice and meltwater dynamics, as key drivers of the upper layer structure, are identified as the leading factors shaping phytoplankton. Short- and long-term scenarios are suggested for phytoplankton communities in the NAP, both indicating a future increase of the importance of small flagellates at the expense of diatoms, with potential devastating impacts for the ecosystem. Five main research gaps in the current understanding of the phytoplankton response to climate change in the region are identified: (i) anthropogenic signal has yet to be disentangled from natural climate variability; (ii) the influence of small-scale ocean circulation processes on phytoplankton is poorly understood; (iii) the potential consequences to regional food webs must be clarified; (iv) the magnitude and risk of potential changes in phytoplankton composition is relatively unknown; and (v) a better understanding of phytoplankton physiological responses to changes in the environmental conditions is required. Future research directions, along with specific suggestions on how to follow them, are equally suggested. Overall, while the current knowledge has shed light on the response of phytoplankton to climate change, in order to truly comprehend and predict changes in phytoplankton communities, there must be a robust collaboration effort integrating both Antarctic research programs and the whole scientific community under a common research framework.
Introduction
The Southern Ocean is instrumental in the functioning of the global ocean. It is responsible for over a third of the global CO2 ocean sequestration, playing a key role in driving global biogeochemistry (Gruber et al., 2009). While covering only 30% of the global ocean’s surface, it is predicted to account up to 75% of the heat uptake (Frölicher et al., 2015), underlining its importance under the reported and predicted impacts of anthropogenic-driven climate change. Thus, a better understanding of Southern Ocean processes on a circumpolar and regional scales and their vulnerability is urgent, especially considering that climate-driven changes over its biogeochemistry likely to impact from primary producers to changes on the ecosystem functioning (Henley et al., 2020).
Current knowledge shows that impacts of climate change in the Southern Ocean vary spatially. The Antarctic Peninsula, located in West Antarctica, is amongst the most rapidly warming regions worldwide, particularly its western (WAP) and northern (NAP) sectors (Clarke et al., 2007; Ducklow et al., 2012; Kerr et al., 2018b; Moffat and Meredith, 2018; Henley et al., 2019). Spatially, the NAP (Figure 1) roughly stretches from the Anvers Island to the oceanic waters surrounding the northern tip of the Antarctic Peninsula, including the Bransfield Strait, the southernmost Drake Passage and the NW Weddell Sea (Kerr et al., 2018b). Therefore, it is a diverse region, encompassing a range of oceanic and coastal ecosystems with complex hydrographic and biogeochemistry dynamics.
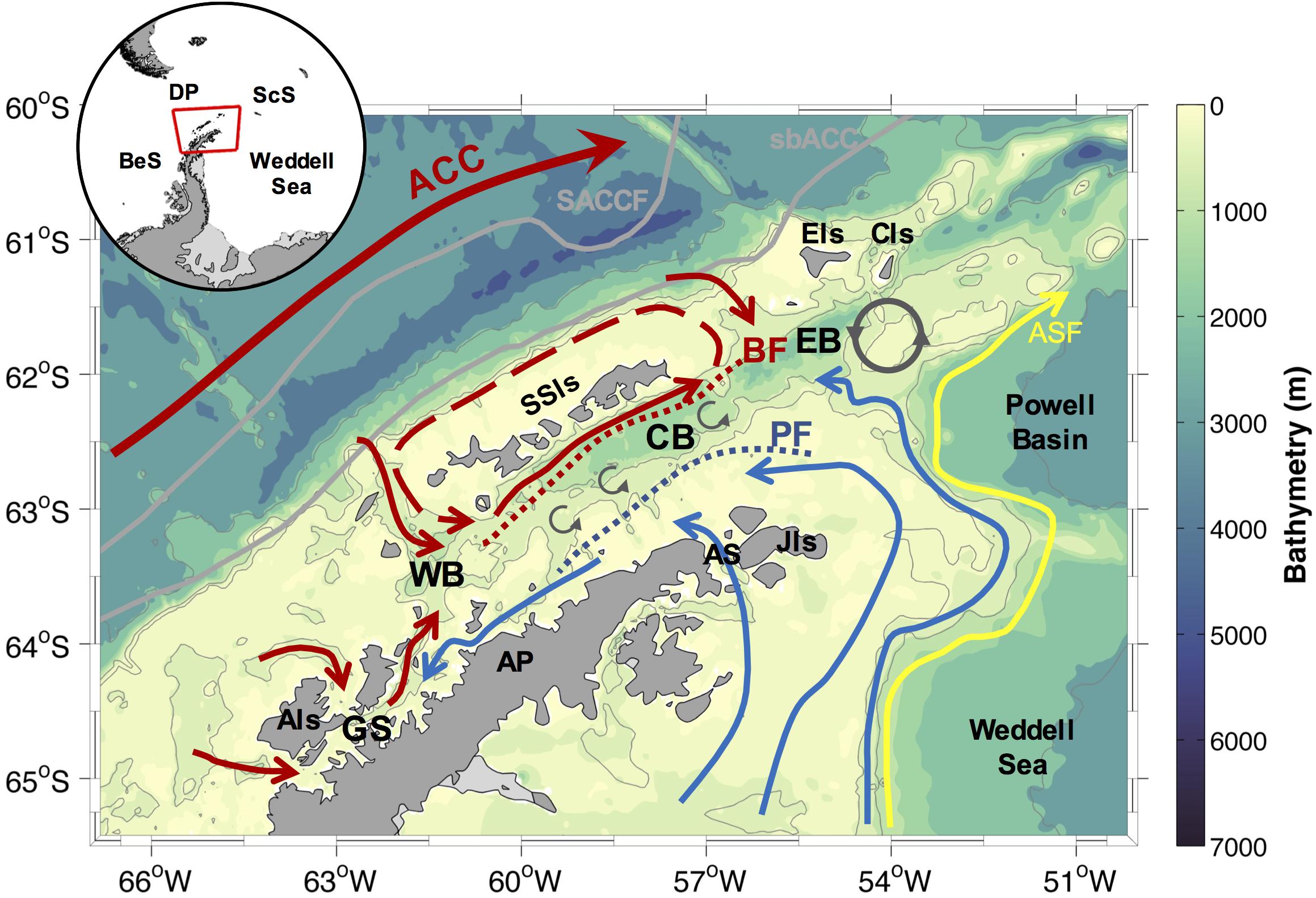
Figure 1. Schematic of the ocean circulation patterns and mesoscale features in the Northern Antarctic Peninsula (NAP), following Smith et al. (1999); Garcia et al. (2002), Zhou et al. (2002); Savidge and Amft (2009), and Sangrà et al. (2017). The red arrows depict intrusion of warm waters, whereas the blue arrows depict the cold regime by Weddell Sea shelf waters advection into the NAP. The blue and red dotted lines show the surface Peninsula Front (PF) and the subsurface Bransfield Front (BF), respectively. The red dashed arrow depicts the recirculation around the South Shetland Islands (SSIs). The yellow arrow represents the location of the Antarctic Slope Front (ASF) and the flow associated to the Antarctic Slope Current, following Heywood et al. (2004). A stationary anticyclonic eddy south of Clarence Island (CIs), and the submesoscale eddies along the Bransfield Strait, are shown by dark gray arrows. The inset shows the location of the NAP, and the main areas surrounding the study region. Antarctic Circumpolar Current (ACC), Antarctic Peninsula (AP), Antarctic Sound (AS), Bellingshausen Sea (BeS), Drake Passage (DP), Scotia Sea (ScS), Anvers Island (AIs), Gerlache Strait (GS), Western basin (WB), Central basin (CB), Eastern basin (EB), Joinville Island (JIs), Elephant Island (EIs). The gray lines depict the Southern ACC Front (SACCF) and the southern boundary of the ACC (sbACC) following Orsi et al. (1995).
The NAP is highly relevant for the Southern Ocean dynamics, acting as a transition zone between sub-Antarctic and Antarctic waters. On the NAP’s eastern boundary, the NW Weddell Sea acts as a critical site of formation and exportation of deep waters to the global ocean (Franco et al., 2007; de Carvalho Ferreira and Kerr, 2017; Kerr et al., 2018a). One of the source of this deep water is the relatively salty shelf water with temperatures near the freezing point (Huhn et al., 2008; Kerr et al., 2009; van Caspel et al., 2015; Kerr et al., 2018a), which is spread westward across the NAP environments by the coastal currents (Von Gyldenfeldt et al., 2002; Collares et al., 2018). On its western boundary, the Antarctic Circumpolar Current (ACC) transports relatively warm, salty, deoxygenated and nutrient- and carbon rich intermediate waters from the Bellingshausen Sea into the NAP, mainly influencing the shelf ecosystems along the west of the Antarctic Peninsula (Niller et al., 1991; Barlett et al., 2018). In-between, the Bransfield Strait is influenced by both Weddell and Bellingshausen-sourced waters, promoting a unique and varying blend that lead to changes in physical and chemical seawater conditions along the NAP (Dotto et al., 2016; Huneke et al., 2016).
Atmospheric warming in the northernmost region of the Peninsula has already exceeded 1.5°C since 1950 (Turner et al., 2005). The vulnerability of the NAP to climate change has led to an accumulation of severe changes along its ecosystems. In the western sector of NAP, sea surface temperatures are significantly rising (Vaughan et al., 2003; Meredith and King, 2005; Smith and Polvani, 2017), seasonal sea ice extent and duration are decreasing (Parkinson and Cavalieri, 2012), and glaciers are becoming thinner (Cook et al., 2016). Region-wise, the intrusion of anthropogenic carbon below 100 m in the Gerlache Strait (Kerr et al., 2018c) and deep-water cooling, freshening, and lightening in the Bransfield Strait have been reported (Azaneu et al., 2013; Dotto et al., 2016). Furthermore, tourism-related vessel traffic is intensifying (Bender et al., 2016) and marine debris concentrations are rising along the Antarctic Peninsula western coast (Waller et al., 2017; Lacerda et al., 2019). Marine debris are associated with the release of chemicals, which may be toxic, as well as with promoting the invasion of non-indigenous species (Oberbeckmann et al., 2015). Moreover, the microbial community associated with plastics – the plastisphere (Zettler et al., 2013) – includes microalgae and may alter the sinking rates of the surrounding waters phytoplankton (Long et al., 2015).
In the eastern sector of the NAP, studies have observed the disintegration and thinning of ice shelves (Pritchard et al., 2012; Paolo et al., 2015), as well as the freshening of shelf waters (Hellmer et al., 2011; Schmidtko et al., 2014). However, an increase of sea ice extent in the Weddell Sea has also been identified (Parkinson and Cavalieri, 2012). This recent reversal in sea ice decline, together with a plateau in atmospheric warming rates since the late 1990s along the region (Turner et al., 2016), have been associated with a short-term internal variability, due to periods of more neutral to negative Southern Annular Mode (SAM) frequencies or positive SAM offset by El Niño, which have currently superimposed on longer-term positive SAM trends (Henley et al., 2019). As a result, there has been occurring a large spatial-temporal variability of physical and biogeochemical changes between southern and northern regions along the NAP (Oliva et al., 2016; Monteiro et al., 2020a), as well as in other WAP coastal regions (Brown et al., 2019), although overall atmospheric warming and sea ice losses are still persistent and statistically significant (Henley et al., 2019).
While it is still uncertain how these stressors will shape biological communities along the NAP, several responses have already been observed across multiple trophic levels. At lower levels, repercussions include changes in phytoplankton biomass, composition and size (Montes-Hugo et al., 2009; Mendes et al., 2013; Schofield et al., 2017), as well as declines in Antarctic krill (Euphausia superba) biomass in favor of gelatinous zooplankton taxa (Atkinson et al., 2004; Steinberg et al., 2015). Higher trophic levels have also been impacted: pack-ice seals habitats have reduced (Forcada et al., 2012) and ice-dependent penguin species are declining (Trivelpiece et al., 2011; Clucas et al., 2014). Tackling climate change in the NAP requires a deep understanding and constant monitoring of its biological communities.
Phytoplankton communities are expected to change across the Southern Ocean (Deppeler and Davidson, 2017) and monitoring their response is critical to predict the cascading effects of climate change within the NAP ecosystem. Despite top–down control having an important role in regulating the phytoplankton community composition and biomass during the austral summers (e.g., Mendes et al., 2012; Pillai et al., 2018), the bottom–up control effects have been more pronounced in both WAP (Venables et al., 2013; Saba et al., 2014) and NAP (Costa et al., 2020). This bottom–up control has been associated with sea ice cover season, which prevents wind mixing during winter and, along with glacial melting, provides meltwater to stabilize the upper ocean during summer, supporting phytoplankton growth (Venables et al., 2013). In addition, meltwater inputs further supply micronutrients to the upper ocean, such as iron, which may also be regulating biological production during summer along the Antarctic coastal waters (Boyd et al., 2007; Annett et al., 2015). Therefore, given the substantial physical changes over the past warming decades along the NAP, phytoplankton have long been regarded as a good indicator of change due to their sensitivity to effects of large scale climate oscillations, which drive sea ice season and glacial melting dynamics (Rozema et al., 2017; Brown et al., 2019).
For the past 20 years, the Brazilian High Latitude Oceanography Group (GOAL) has intensively studied the NAP (Kerr et al., 2018b; Mata et al., 2018). The work promoted by the GOAL has been instrumental to understand the evolving changes in phytoplankton communities in this region (Mendes et al., 2012, 2013, 2018a,b; Detoni et al., 2015; Gonçalves-Araújo et al., 2015; Costa et al., 2020), as studies have sought to disentangle the occurring high spatial variability within the region. While its southernmost sector, overlapping with the WAP, is relatively well studied for a Southern Ocean region (e.g., Ducklow et al., 2012), there are still many aspects of phytoplankton communities in the NAP that warrant future research. Moreover, the spatial variability inherent to the region must be considered as climate change impacts may also vary spatially. Finally, while several works have been performed over the past decades, this knowledge is scattered for most areas outside the WAP.
Over the next sections, this study reviews the regional ocean circulation and current findings on changes observed in phytoplankton communities occurring in the NAP. The main existing knowledge gaps are subsequently identified and addressed. For each gap, its main challenges are discussed, and suitable future research directions are proposed with the main goal of advancing our understanding in the NAP under the context of climate change.
Ocean Circulation in the NAP
The main pattern of the ocean circulation in the NAP (Figure 1) is formed by a complex current system governed by (i) the cyclonic gyre and the presence of a surface and a subsurface thermal fronts in the Bransfield Strait (i.e., the Bransfield and Peninsula fronts), (ii) the intrusions of relatively warm, salty and deoxygenated waters derived mainly from the Circumpolar Deep Water (CDW), which flow within the ACC, (iii) the advection of shelf waters by the Antarctic Coastal Current from the NW Weddell Sea continental shelf surrounding the Joinville Island and through the Antarctic Sound, (vi) the southward flow along the west coast of the Antarctic Peninsula toward the Gerlache Strait, and (v) the northward surface waters advection from the Gerlache Strait toward the Bransfield Strait (Smith et al., 1999; Garcia et al., 2002; Von Gyldenfeldt et al., 2002; Zhou et al., 2002, 2010; Heywood et al., 2004; Zhou et al., 2006; Savidge and Amft, 2009; Sangrà et al., 2011; Dotto et al., 2016; Huneke et al., 2016; Sangrà et al., 2017). In addition, a stationary eddy south of Clarence Island and other mesoscales features sourced by displacements of the ACC system and the Bransfield and Peninsula fronts, together with continental input of glacial meltwater, add complexity to the hydrography and ocean mixture along the NAP (Thompson et al., 2009; Azaneu et al., 2017; Moffat and Meredith, 2018).
In this context, the surface water masses characteristics are highly variable and likely essential in providing optimal conditions to the growth of specific phytoplankton blooms in the NAP (Mendes et al., 2012; Detoni et al., 2015; Costa et al., 2020). In general, the surface waters along the NAP zones can be split into cold and warm variety (Holm-Hansen et al., 1997). The former is derived mainly from the Weddell Sea shelf waters and it is located east of the Peninsula Front and southeast of the Elephant and Clarence islands (Holm-Hansen et al., 1997). This cold regime is sourced by waters with generally lower concentrations of macronutrients (a difference of at least ∼10 μM of nitrate and silicate than found in the Bransfield Strait; Kang et al., 2001), providing conditions less favorable for phytoplankton growth than that on the warm regime (Gonçalves-Araújo et al., 2015; Russo et al., 2018). The warm variety is mainly derived from the ACC surface waters (modified by CDW-sourced incursions from the southern region of the NAP), being distributed northwards along the NAP western shelves and the west of the Bransfield Front (Barlett et al., 2018). The warm conditions west of the Peninsula Front are enriched of macronutrients in subsurface waters as warm, nutrient- and carbon-rich CDW crosses the western Antarctic Peninsula shelves (Henley et al., 2017, 2018). This provides favorable conditions to a more productive environment (Smith et al., 1996; Prézelin et al., 2000; Mendes et al., 2012, 2013). Additionally, a northward surface water flow from the Gerlache Strait to the warmer side of the Bransfield Strait (Zhou et al., 2002) is responsible for advecting waters from a highly productive coastal zone to a more open region north of the NAP (Kerr et al., 2018b; Costa et al., 2020; Monteiro et al., 2020b).
The surface water properties of the sheltered zone of the Bransfield Strait (between the Bransfield and Peninsula fronts) are regulated by the strengthening of the mixing and volume of source waters (both cold and warm varieties). These waters intrude into the region, which is modulated by the dominant climate-driven mode of variability (Dotto et al., 2016; Barlett et al., 2018; van Caspel et al., 2018), modifying the location of the offshore ACC fronts (Loeb et al., 2010). During periods of El Niño conditions and negative SAM, the region receives higher amounts of cold waters derived from the Weddell Sea, while during La Niña conditions and positive SAM the region is flooded by the warm variety derived from the CDW (Dotto et al., 2016; Barlett et al., 2018). In addition, during positive SAM/La Niña conditions, warmer and stronger northerly winds blow across the NAP, resulting in a shortened sea ice season, while the opposite occurs during negative SAM/El Niño conditions (Stammerjohn et al., 2008; Henley et al., 2019). Nevertheless, the effects of positive SAM may also be offset by El Niño conditions, leading to colder winds and increased sea ice extent (Henley et al., 2019).
The main patterns and mechanisms controlling the ocean circulation in the NAP are well known (Figure 1). Nevertheless, some of the regional flows and physical processes that impact the water mass mixing, hydrography, and the advection of surface, intermediate and shelf waters in the region are still undetermined. For example, new information is needed for a better understanding of (i) intrusions of Warm Deep Water (a Weddell Sea local water mass sourced by CDW) at intermediate levels from the Powell Basin toward the Bransfield Strait (e.g., Azaneu et al., 2017); (ii) the periodicity and frequency of CDW intrusions along the western continental shelf of the NAP (e.g., Moffat et al., 2009; Couto et al., 2017; McKee et al., 2019); (iii) the rate of water masses mixing along the deep Bransfield basins (e.g., Brearley et al., 2017); and (iv) the rate of Weddell Sea shelf waters advection and renewal from the east (e.g., Renner et al., 2012; Dotto et al., 2016). All these unknown mechanisms directly impact the vertical stratification of the upper water column, consequently, impacting the development and distribution of the phytoplankton groups due the local and regional changes of the physical and chemical properties.
Changes in the Phytoplankton Communities in the NAP
Current Knowledge
Most living organisms in the NAP are influenced by the advance and retreat timing of sea ice cover (Montes-Hugo et al., 2009; Trivelpiece et al., 2011). As sunlight increases and sea ice melts during spring, the mixed layer begins to shoal as a result of both thermal and freshwater stratification. Consequently, phytoplankton growth rates increase, particularly in the marginal sea ice zones (Arrigo et al., 2017; Schofield et al., 2018). Recent continuous high-resolution measurements (e.g., using autonomous floats) have also confirmed the importance of seasonal ice retreat for the timing and intensity of phytoplankton blooms (von Berg et al., 2020). Therefore, the beginning of sea ice melting in spring (September–November) linked to the increase of daylight length (Vernet et al., 2012), is the main factor that triggers phytoplankton blooms in the NAP (Varela et al., 2002; Garibotti et al., 2005b).
The variability of this overall bottom–up control (Saba et al., 2014; Petrou et al., 2016), associated with large-scale climate oscillations, is related to shifts in phytoplankton communities (Montes-Hugo et al., 2009; Schofield et al., 2010; Mendes et al., 2013, 2018a). Due to the north-south orientation of the Peninsula, changes in phytoplankton have exhibited different patterns according to latitude (Montes-Hugo et al., 2009). In the NAP, contrary to southernmost WAP, chlorophyll a has declined along with an increase (decrease) in the importance of nanophytoplankton (diatom) cells, particularly in areas of glacial ice melt (Montes-Hugo et al., 2009; Mendes et al., 2013, 2018b). These changes, verified using both in situ and satellite data, have been associated with an increase in cloudy days, shortened sea ice cover, glacier retreat, stronger winds and deeper summer mixed layer depths (MLDs) (Montes-Hugo et al., 2009).
The ongoing loss of winter sea-ice, associated to the recent rapid regional climate warming, has also been identified as an important factor modulating the dynamics of phytoplankton blooms in the region (Venables et al., 2013; Saba et al., 2014). Interannual differences in stratification have been shown to alter the intensity of phytoplankton blooms, with relatively low biomass concentrations generally following winters with low sea ice cover (Ducklow et al., 2013; Rozema et al., 2017). These results have been associated with light limitation, most severe following winters with low sea ice cover, due to weak stratification and anomalously deep mixed layers during spring/summer, resulting from higher exposure to winter winds (Venables et al., 2013; Arrigo et al., 2017). Therefore, due to the current downward trend in the NAP sea ice extent (Montes-Hugo et al., 2009; Venables et al., 2013; Saba et al., 2014; Schofield et al., 2018; Henley et al., 2019), mixed layers have been deeper and less stable, leading to overall lower phytoplankton biomass across the region (Montes-Hugo et al., 2009; Mendes et al., 2013, 2018b; Gonçalves-Araújo et al., 2015).
The NAP hosts high concentrations of Antarctic krill, mainly surrounding the South Shetland Islands (Atkinson et al., 2019), making it a highly productive marine ecosystem characterized as a key-feeding area for whales (Dalla Rosa et al., 2008; Secchi et al., 2011; Seyboth et al., 2018) and other krill-predators (e.g., Trivelpiece et al., 2011; Southwell et al., 2012). This reflects the NAP’s high primary production potential, despite the substantial decline in phytoplankton biomass over the past warming decades (Holm-Hansen and Mitchell, 1991; Varela et al., 2002; Montes-Hugo et al., 2009; Russo et al., 2018; Costa et al., 2020).
Prior to the 1990s, the NAP austral summer, typically associated with high sea ice cover and negative SAM trend (Stammerjohn et al., 2008), exhibited high primary productivity linked to massive blooms and greater abundance of diatoms (Holm-Hansen and Mitchell, 1991 and references therein). From the 1990s to the early 2000s, studies also reported an abundant phytoplankton biomass (e.g., Castro et al., 2002; Rodriguez et al., 2002; Varela et al., 2002) and a community dominated by large diatoms, Phaeocystis antarctica and large flagellate Pyramimonas (a genus of unicellular green flagellates). However, this decade showed a greater positive SAM trend associated with higher sea ice retreat (Stammerjohn et al., 2008). In addition, cryptophytes were already observed in the southernmost regions of the NAP, particularly in the Gerlache Strait and adjacent waters (Castro et al., 2002; Rodriguez et al., 2002; Varela et al., 2002; Garibotti et al., 2005a). From the early 2000s onward, remote-sensing-derived phytoplankton biomass along the NAP during the austral summer has significantly decreased (Montes-Hugo et al., 2009). Corroborating this trend, in situ measurements have shown a sharp decline in diatom biomass coincident with an increase in nanophytoplankton abundance, such as cryptophytes (Mendes et al., 2013, 2018b). The dominance of cryptophytes over diatoms in the NAP is associated with low salinity and warm stratified waters (Moline and Prézelin, 1996; Moline et al., 2004; Mendes et al., 2018a). This marked shift from large to small phytoplankton cells has impacted high biomass development during the austral summers, overall decreasing primary productivity in the NAP (Montes-Hugo et al., 2009; Mendes et al., 2013, 2018a).
This shift in phytoplankton composition goes hand in hand with longer-term warming and deglaciation processes, which have been linked with anthropogenic CO2 influence and coupled with the ongoing increase in positive SAM anomalies (Petrou et al., 2016). However, short-term natural variability must also be considered (Turner et al., 2016). Therefore, and contrary to the decreasing trend in phytoplankton biomass along the NAP, a recent study reported an intense diatom bloom (reaching > 45 mg m–3) spanning a vast area of the NAP during a late summer oceanographic survey conducted in February 2016 (Costa et al., 2020). This bloom was mainly composed by a large centric diatom (Odontella weissflogii) and was linked to a significant local ocean carbon uptake (>60 mmol m–2 d–1). This was likely associated with an atypical lag period in sea ice retreat caused by the first extreme El Niño of the 21st century (Santoso et al., 2017). Nevertheless, in the same austral summer, but during different sampling periods (December 2015 and April 2016), another work found an absence of diatom blooms in a NAP fjord (Andvord Bay, Gerlache Strait), despite abundant macronutrient and iron concentrations (Pan et al., 2020). On the contrary, a dominance of cryptophytes was reported in December, linked to glacial melting process and shallow mixed layers (Pan et al., 2020).
Although cryptophytes exhibit preference for growing in association with surface glacial melting waters, diatom blooms associated with the presence of freshwater plumes originating from glacier-melt outflow have also been registered (Mendes et al., 2012; Detoni et al., 2015; Höfer et al., 2019). This suggests that the shift from diatoms to cryptophytes in the NAP is not caused by physiological stress at low salinity and/or iron scarcity, as glacial ice melting is one of the main sources of both freshwater and iron input to surface waters around the Antarctic Peninsula (Dierssen et al., 2002; Annett et al., 2015). It should, however, be considered that early sea ice and glaciers retreat, together with the increase in sea surface temperature, may lead to the establishment of a shallow water column stratification, confining marine planktonic organisms near the surface and exposing them to high irradiance (Moreau et al., 2010). The recurrent growth of cryptophytes in the NAP could be attributed to their unique abilities to thrive under extremely high light levels normally found in confined stratified upper layers (Mendes et al., 2018a,b). Such conditions are becoming more frequent and intense in the NAP coastal waters and will probably have significant implications for the regional food web and biodiversity patterns (Henley et al., 2019). Recently, several studies have linked the decrease of Antarctic krill to the dominance of smaller phytoplankton cells in regional food webs, which favors salp-dominance (Atkinson et al., 2004, 2019; Moline et al., 2004; Montes-Hugo et al., 2009). Moreover, the dominant phytoplankton groups may have an influence on the local net sea-air CO2 fluxes (Kerr et al., 2018c), given that diatoms achieve significantly higher biomass and oceanic CO2 uptake than cryptophytes and other phytoplankton (Brown et al., 2019). For instance, observations in the WAP show an intensification of local CO2 uptake during the austral summers driven by biological production (Brown et al., 2019; Monteiro et al., 2020b).
While ship-based surveys have shown that cryptophytes across the Antarctic Peninsula can occupy a range of distinct hydrographic niches (Henley et al., 2019), it is evident that these nanoflagellates are well-adapted to stable and shallow MLDs (preferentially < 20 m) under highly illuminated conditions (e.g., Mendes et al., 2018a,b). Nevertheless, despite the observed competition or niche segregation between diatoms and cryptophytes (Moline et al., 2004; Mendes et al., 2013, 2018a,b; Rozema et al., 2017; Schofield et al., 2017; Pan et al., 2020), the underlining critical factors and physiological evidences remain weakly understood.
Short- and Long-Term Changes Under A Climate Changing Scenario
The NAP coastal waters have been depicted as a significant source of dissolved iron (Annett et al., 2015, 2017; Sherrell et al., 2018), unlike most offshore waters in the Southern Ocean (Petrou et al., 2016). Consequently, light availability, as a function of water column structure, is usually the main driver of phytoplankton growth during the austral summer (Costa et al., 2020). As the MLD is controlled by wind, sea ice and meltwater dynamics, which in turn are driven by large scale climate oscillations (Stammerjohn et al., 2008; Venables et al., 2013; Saba et al., 2014; Brown et al., 2019), climatic changes at both short- and long-term scales shape phytoplankton communities and primary productivity through their influence on the physical compartments.
Anthropogenic greenhouse gas emission are predicted to continue to climb over the years (Santoso et al., 2017). Models also predict that the positive SAM frequency will increase for the next 50 years, contributing to persistent strong westerly winds and rising temperatures along the NAP. This will result in further glacial retreat and sea ice decline (Gillett and Fyfe, 2013; Turner et al., 2014). Due to increasing processes of deglaciation, in short-term, it is believed that the MLD will become shallower, leading to a strengthening of the already described increase in the integrated daily irradiance available to phytoplankton in surface waters (Marinov et al., 2010; Petrou et al., 2016). Thus, phytoplankton cells are expected to be more exposed to light stress in the upper shallower layers, which would present a fundamental niche favorable to the development of cryptophytes (Mendes et al., 2013, 2018a,b; Figure 2) and potentially lead to an increase in the overall abundance of these nanoflagellates.
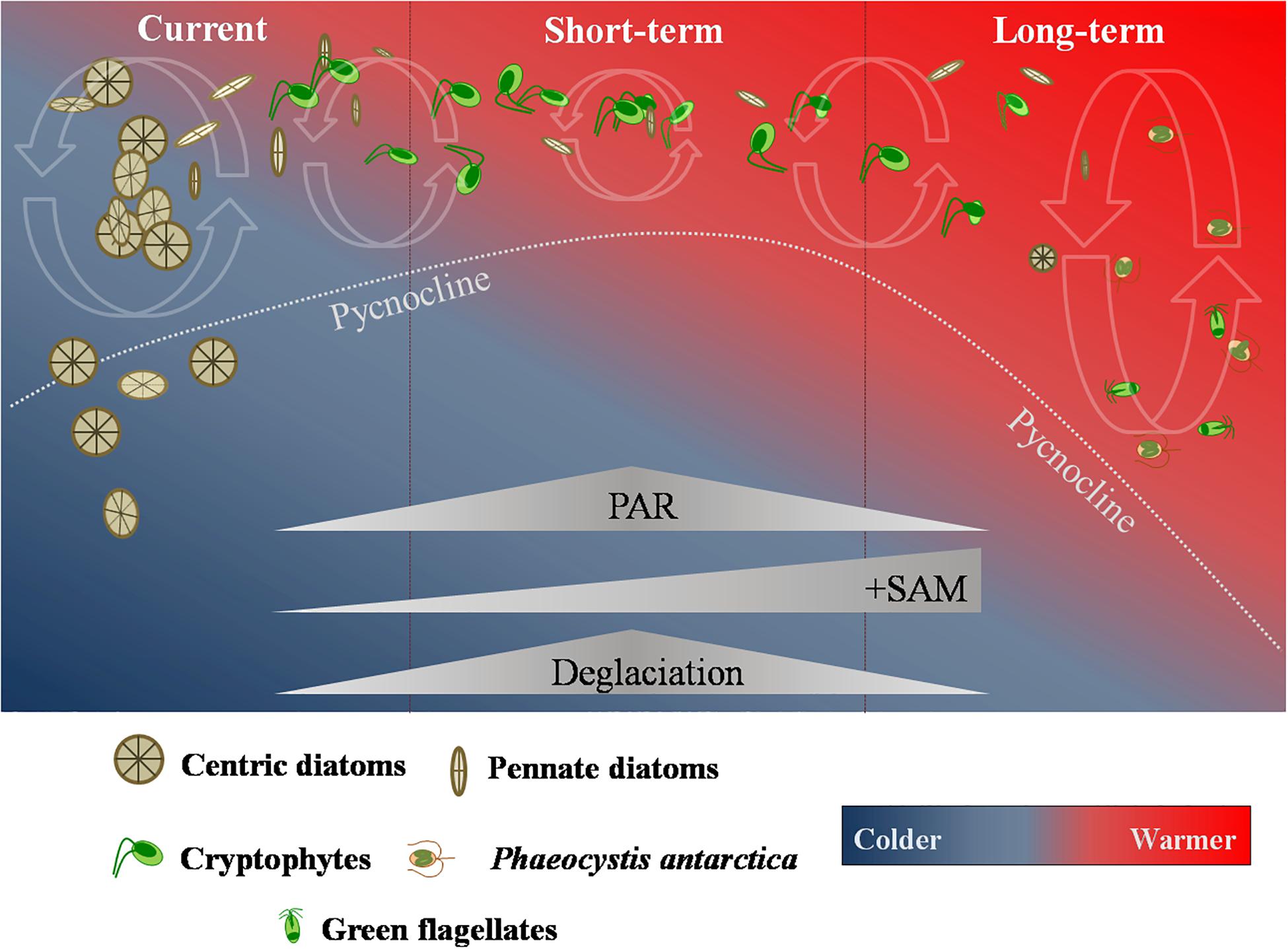
Figure 2. Current and future short- and long-term scenarios of phytoplankton response to projected climate change impacts in the Northern Antarctic Peninsula suggested in this work. The coupled influence of the predicted changes in the mixed-layer available photosynthetic active radiation (PAR), the Southern Annular Mode (SAM) and deglaciation processes in the Northern Antarctic Peninsula are expected to significantly alter the phytoplankton community composition and size.
In the long term (Figure 2), climate change effects in the NAP are expected to intensify (Montes-Hugo et al., 2009; Atkinson et al., 2019; Brown et al., 2019). Due to the projected stronger and persistent westerly winds and greater losses in the sea ice cover, the MLD will eventually deepen as the influence of sea ice in Antarctic coastal waters reduces. This could lead to a shift in the phytoplankton community to smaller cells adapted to low light conditions and deeper MLDs, such as Phaeocystis antarctica (Petrou et al., 2016). Although the glacial melting could offset the MLD deepening, this effect would likely be nearshore localized and still favor small cryptophytes (Mendes et al., 2013, 2018a; Schofield et al., 2017). Therefore, it is possible that phytoplankton communities, both in the short- and long-term, become nanoflagellates-dominated. Since high biomass is normally associated with a dominance of (large) diatoms, this would lead to a decrease in overall biomass and primary productivity, with myriad consequences in several compartments along the NAP ecosystem.
Ultimately, while the synergistic effects between natural internal variability and anthropogenic climate change on phytoplankton composition dynamics remain uncertain and/or poorly understood (Turner et al., 2016; Henley et al., 2019), impacts on phytoplankton communities in the NAP are ongoing and may follow one or more pathways in the future, likely following the short- and long-term scenarios suggested here.
Main Knowledge Gaps and Future Research Directions
While the current knowledge gathered in the NAP has shed light on the response of phytoplankton to climate change, it should be noted that it is merely a glimpse of what may happen. A coordinated effort is needed to comprehensively understand and predict changes in the phytoplankton communities in the NAP.
The main knowledge gap in the NAP is the intrinsic elusive relationship between anthropogenic climate change and natural climate variability. The assessment of the impact of climate change requires disentangling natural internal variability from long-term alterations (Deser et al., 2012). This task becomes harder when facing shorter temporal or spatial scales, as the ratio of anthropogenic signal to noise is much lower (Stott and Tett, 1998). For instance, even at a global scale, a time series with a minimum length of 17 years or even longer is required for detecting human effects on mean air temperature (Santer et al., 2011; McKinley et al., 2016; Henson et al., 2018). In the Antarctic Peninsula, however, air temperature exhibits significant internal variability and it has been suggested that trends such as the rapid regional warming since the 1950s (Meredith and King, 2005) and the subsequent cooling since 1998 (Turner et al., 2016) could both be within the Peninsula’s natural decadal variability (Turner et al., 2016; Smith and Polvani, 2017). A recent study for the Arctic, however, suggested that a similar cooling from 1998 onward may have been an artifact of missing data (Huang et al., 2017). Moreover, tracking biological changes has been proven to be particularly challenging (Hughes, 2000).
In the Antarctic Peninsula, continuous phytoplankton measurements only began in the second half of the 20th century and have been mostly focused in the WAP (Boyce et al., 2010). Despite spanning four decades, the sampling effort in the NAP is mainly limited to opportunistic summer oceanographic cruises. As a result, most studies capture a snapshot of the local phytoplankton communities (e.g., Rodriguez et al., 2002; Garibotti et al., 2003a,b; Mendes et al., 2012; Hernando et al., 2015; Costa et al., 2020) compared to the multidecadal-long studies in the WAP (e.g., Saba et al., 2014; Arrigo et al., 2017; Schofield et al., 2017, 2018). The yearly summer cruises led by GOAL have been important to begin understanding phytoplankton processes in the NAP, yet they only span 12 years (2008–2020) and are mainly limited to February. There is a need for continuous measurements that can help capture the biological and biogeochemical seasonality of this region (e.g., Monteiro et al., 2020a), particularly during late spring and the early summer period. Furthermore, decadal timeseries are insufficient to extract climate change-driven trends in phytoplankton (Henson et al., 2010), as studies suggest 30–60 years of data are required (Bopp et al., 2001; Boyd et al., 2008; Henson et al., 2010). Thus, the current uncertainty in the NAP must be considered when analyzing biological changes if the goal is to understand the impact of climate change in the Antarctic ecosystem.
To fill this gap, several research directions need to be followed (Figure 3). First and foremost, the current sampling effort in the NAP must be expanded. It is of paramount importance that current West Antarctic research programs collaborate, increasing the amount of data available and facilitating its dissemination among the scientific community (Schofield et al., 2010; Newman et al., 2019). Since many Antarctic research stations are in the NAP and research vessels are frequent during the summer, it would be possible to accurately cover the region’s summer biological succession under a multi-national cooperation framework. Kim et al. (2016, 2018) have shown how phytoplankton and nutrient data from stations spaced throughout the NAP can help unravel the importance of local-scale forcing on phytoplankton dynamics.
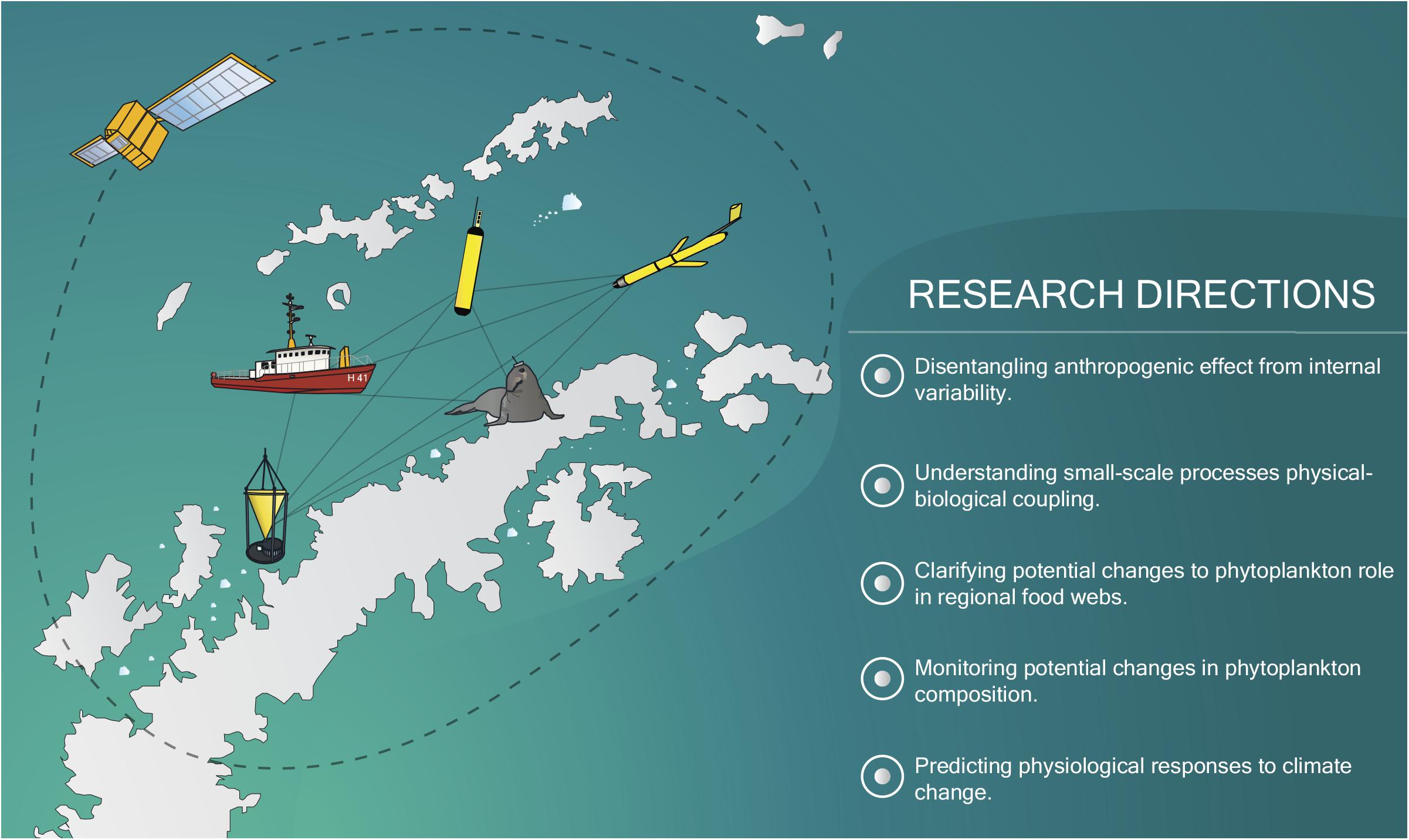
Figure 3. Summary of the future main research directions required to accurately understand and predict the response of phytoplankton under climate change outlined in this work. Several in situ and remote-sensing tools, which will be essential toward this goal (e.g., animal-attached sensors, ocean color satellites, biogeochemical floats, underwater gliders), are represented.
The projected increase in summer tourism along the NAP (Bender et al., 2016), despite its potential environmental impacts, may also be used as a platform to further monitor biological changes, whether through sensors attached to ships or through citizen science (Brosnan et al., 2015). Moreover, autonomous in situ data sources, such as underwater gliders, floats, drifters or animal-attached instruments, have already shown their potential to further increase the in situ data volume in the Southern Ocean (e.g., Meredith et al., 2013; Roquet et al., 2014; Haëntjens et al., 2017; Thomalla et al., 2017; Hindell et al., 2020). Autonomous data sources allow for the monitoring of the detailed seasonality of phytoplankton biomass and physical and chemical factors (Eriksen et al., 2018). While such sources do require extensive validation, they also offer very high spatial and temporal resolution. In some cases (e.g., floats, underwaters gliders, animal-attached instruments), they even allow for data along the water column. Autonomous in situ measurements will also allow for in-depth studies of the dynamics between phytoplankton and sea ice. While a few studies using floats already exist in marginal ice zones (e.g., Moreau et al., 2020; von Berg et al., 2020), further works would help reveal how phytoplankton changes with sea ice retreat at finer scales.
Ocean color remote sensing, i.e., satellite-based measurements of visible light reflected off the upper ocean, will be essential toward studying the NAP ecosystem. Benefiting from 20+ years of continuous, high-resolution data, ocean color remote sensing will complement in situ data, allowing for increased temporal and spatial coverage. Apart from its direct use to assess and monitor the impact and adaptation of anthropogenic climate change in phytoplankton communities, ocean color remote sensing has the potential to contribute to several study areas, including the validation and improvement of biogeochemical models, the global carbon cycle and ocean acidification (Groom et al., 2019).
Nevertheless, satellite data requires extensive validation with in situ data, particularly in polar regions, where cloud cover is ubiquitous and performance is typically poor (Dierssen and Smith, 2000; Cota et al., 2003). For instance, satellite chlorophyll a global algorithms have been seen to frequently underestimate in situ chlorophyll a in the Southern Ocean, which could be related to specific optical properties of the water in this region (e.g., Dierssen and Smith, 2000; Johnson et al., 2013). Despite efforts to create regional algorithms, these have either relied on relatively small in situ datasets (e.g., Garcia et al., 2005; Jena, 2017; Pereira and Garcia, 2018) or focused on large, heterogeneous regions (Johnson et al., 2013), limiting their applicability in Antarctic waters. An increase in in situ chlorophyll a measurements would be key in validating satellite data in the NAP, enabling the development of regionally tuned algorithms to produce accurate, multi-decadal phytoplankton data to monitor the region and quantify related uncertainties. For instance, in the Arctic, where in situ data is more abundant, Kahru et al. (2011) have estimated that phytoplankton blooms had advanced 50 days, within the period 1997–2009, attributing this shift to earlier ice melting. Moreover, this could pave the way to the use of satellite data to assess phytoplankton functional types, which would help assess changes through time in phytoplankton composition and structure.
Another important way to understand how phytoplankton communities in the NAP respond to climate change is through phytoplankton fossil records (Crampton et al., 2016). While limited to taxa with hard shells or coverings (e.g., diatoms, coccolithophores, dinoflagellate cysts), changes in their response to past warming conditions can provide an analog for present climate change (Wilson et al., 2018). Such studies could be essential to assess the natural variability of the NAP, as highlighted in recent studies (Houben et al., 2013; Crampton et al., 2016).
While solving the large-scale spatial and temporal variability should be the focus of future research in the NAP, there are other knowledge gaps which must be addressed. First, while studies have shown that ocean circulation within the NAP influence phytoplankton communities (e.g., Mendes et al., 2012, 2013, 2018a; Gonçalves-Araújo et al., 2015; Costa et al., 2020), a better understanding of the physical-biological coupling in the NAP is required. As mentioned in this work, several regional mesoscales processes (i.e., meanders, eddies, ocean fronts) still remain understudied, some of which have already been suggested to shape phytoplankton abundance and distributions, such as the advection processes from the Gerlache Strait into the Bransfield Basin, and the development of the Peninsula Front (Mendes et al., 2012, 2013; Russo et al., 2018; Costa et al., 2020). Therefore, a better understanding on how such processes change on both interannual and seasonal scales, and contribute to shape phytoplankton communities could be essential, particularly as climate change threatens to change regional circulation (Moffat and Meredith, 2018). Higher-resolution tools such as underwater gliders or animal-attached instruments may be extremely useful to this end.
Second, the role of phytoplankton in the food web and biological pump and carbon sequestration in the NAP must also be better understood. Climate change can threaten the future structure of regional food webs by leading to shifts in phytoplankton and zooplankton composition (e.g., Atkinson et al., 2004; Mendes et al., 2013; Steinberg et al., 2015). More fundamental studies on the structure and connectivity of the trophic web in the NAP are needed, building on past works (e.g., Cornejo-Donoso and Antezana, 2008; Seyboth et al., 2018). Only then it will be possible to accurately assess and predict how climate-derived changes in phytoplankton composition may shape the upper levels of the food web, as done for the WAP (Saba et al., 2014). Additionally, given that Antarctic krill biomass has been contracted in southward areas (Atkinson et al., 2019), it would also be possible to understand how this key species may be modulating the phytoplankton community composition and biomass in the NAP (top–down effects).
Furthermore, the predicted changes in phytoplankton composition toward smaller phytoplankton cells will likely affect the biological pump in the Antarctic Peninsula. It is of key importance to assess how a possible shift in phytoplankton composition may impact carbon uptake and further water column export, since smaller flagellates are more likely to be consumed, increasing remineralization, and ultimately decreasing carbon export due to their lower CO2 uptake efficiency and sinking rates, contrasting large diatom cells (Brown et al., 2019; Trimborn et al., 2019). Thus, placing sediment traps throughout the NAP during the austral summer should also be a priority to allow for the estimate of regional organic carbon fluxes, along with monitoring diatom contribution over short-term scales (e.g., Ebersbach et al., 2011; Rembauville et al., 2015). This alternative approach would not only help to assess the local diatoms carbon export but also their variability in abundance and intraspecific contribution over time.
Third, one of the main impacts associated with climate change is the observed geographical shift of several marine taxa toward higher latitudes (Pinsky et al., 2013). Several studies have now documented southward expansion of coccolithophores and dinoflagellates in the Southern Ocean (McLeod et al., 2012; Winter et al., 2014). For instance, in recent years there has been a marked increase in the frequency and biomass of Gymnodinioid dinoflagellates along the NAP, apparently occupying sites/conditions less favorable to cryptophytes, i.e., with an adaptation/preference to deeper well-stratified mixed layers (e.g., Mendes et al., 2018b). The occurrence of dinoflagellates is of particular interest because they include toxic species adapted to disperse in coastal currents and frontal systems (Smayda, 2002). Although autotrophic dinoflagellates (mainly small Gymnodiniales < 20 μm) have already been reported as important contributors to total biomass in some well-stratified Antarctic waters (Savidge et al., 1995; Kang et al., 2001; Mendes et al., 2012, 2013, 2018b), an ecological approach to explain the distribution patterns of this group in Antarctic environments has not yet been explicitly addressed. In the coccolithophores case, while rare south of the ACC, there is reason to believe that such poleward expansion may be facilitated by regional increases in temperature and stratification, promoting coccolithophore density in the sector of the NAP coinciding with the southern Drake Passage (Charalampopoulou et al., 2016). Furthermore, the introduction of non-native phytoplankton species into the NAP, whether from non-Antarctic ecosystems or from different parts of Antarctica, should also be considered (Frenot et al., 2005). While the current Antarctica-specific environmental protocols should reduce the risk of invasion, several studies have already highlighted potential introduction pathways as a result of increased anthropogenic presence in Antarctica (Lewis et al., 2003; Frenot et al., 2005). Since the current knowledge is not enough to estimate the risk of introduction or assess its potential impact, it remains unclear how the expansion or introduction of species could alter the phytoplankton community in the NAP. Nevertheless, in situ monitoring and specific actions focused on potential introduction pathways, such as ballast waters (Frenot et al., 2005) and marine plastic debris (Lacerda et al., 2019, 2020), should be introduced in the future.
Finally, understanding regional phytoplankton responses to climate change will be impossible without a better knowledge of phytoplankton physiology in the NAP. Experimental studies at a species level are key to predict how phytoplankton will modulate their internal processes under a changing climate (Petrou et al., 2016). While species-specific responses to warming and acidification have already been identified in the Southern Ocean (Petrou et al., 2016), similar works in the NAP are still scarce and typically test the response to individual stressors (e.g., Buck et al., 2010; Hernando et al., 2015; Trimborn et al., 2015). To truly understand and predict the physiological response of species to climate change, multi-stressors studies, focused on major phytoplankton species’ response to factors such as Fe, CO2, macronutrients, light, pH, salinity are required for the NAP (e.g., Boyd et al., 2016; Andrew et al., 2019; Boyd, 2019). Boyd et al. (2016) highlighted Fe and temperature as key factors modulating subantarctic phytoplankton growth, while CO2, macronutrients and light were seen to be less important. Moreover, an emphasis should be placed on the potentially severe consequences of changes in phytoplankton composition to photosynthetic physiology due to its pivotal role in the ecosystem (Takao et al., 2014; Trimborn et al., 2015; Petrou et al., 2016; Brown et al., 2019).
Concluding Remarks
The NAP is one of the most vulnerable regions to anthropogenic climate change. As its effects on phytoplankton are becoming evident, the complex regional ocean circulation (Figure 1) may contribute to shape phytoplankton communities differently along the transition environments surrounding the NAP. While the response of regional phytoplankton communities is expected to differ at short- and long-term, an overall trend toward smaller flagellates is expected, with potential devastating impacts for the ecosystem (Figure 2). Five main research gaps in the current understanding on phytoplankton response to climate change in the NAP are identified. Future research directions (Figure 3), along with specific suggestions on how to tackle them, are suggested: (i) disentangling anthropogenic effect from internal variability; (ii) understanding the small-scale processes on the physical-biological coupling; (iii) clarifying potential changes to the role of phytoplankton in regional food webs; (iv) monitoring potential changes in phytoplankton composition; and (v) predicting physiological responses to climate change at species and community level. Achieving these goals will only be possible with the inter-collaboration of Antarctic research programs and scientific community under a common research framework.
Author Contributions
AF, RC, and CM contributed to the conception and design of this work. AF, RC, TD, RK, and CM wrote the first draft of the manuscript. AF, RC, and TD designed the figures. VT, AB, VB, and ES contributed to the organization and writing of the final version of the manuscript. All the authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 810139. Financial support was also provided by National Council for Research and Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). This study was conducted within the activities of the PROVOCCAR and ECOPELAGOS projects (CNPq grant nos. 442628/2018-8 and 442637/2018-7, respectively), and is within the scope of two Projects of the Institutional Internationalization Program (CAPES PrInt-FURG – Call no. 41/2017). It also received support from the Fundação para a Ciência e a Tecnologia (FCT; grant no. UID/MAR/04292/2020 and Portuguese Polar Program - PROPOLAR). AF received a Ph.D. grant (SFRH/BD/144586/2019) and AB was supported by the Scientific Employment Stimulus Programme (CEECIND/00095/2017), both funded by FCT. TD acknowledges financial support from the CNPq-Brazil PDJ scholarship no. 151248/2019-2. RC, CM (PQ 306899/2018-3), RK (PQ 304937/2018-5), and ES (PQ 310597/2018-8) are granted with researcher fellowships from CNPq. CAPES also provided free access to many relevant journals through the portal “Periódicos CAPES.”
Acknowledgments
This is a multidisciplinary study as part of the Brazilian High Latitude Oceanography Group (GOAL) activities in the Brazilian Antarctic Program (PROANTAR). This research is also framed within the College on Polar and Extreme Environments (Polar2E) of the University of Lisbon. The authors are deeply indebted to Carolina Sá for her critical feedback and thoughtful remarks during discussion of the manuscript. The authors are also grateful to the two reviewers and editor whose comments/suggestions helped significantly improve the manuscript.
References
Andrew, S. M., Morell, H. T., Strzepek, R. F., Boyd, P. W., and Ellwood, M. J. (2019). Iron availability influences the tolerance of Southern Ocean phytoplankton to warming and elevated irradiance. Front. Mar. Sci. 6:681. doi: 10.3389/fmars.2019.00681
Annett, A. L., Fitzsimmons, J. N., Séguret, M. J., Lagerström, M., Meredith, M. P., Schofield, O., et al. (2017). Controls on dissolved and particulate iron distributions in surface waters of the Western Antarctic Peninsula shelf. Mar. Chem. 196, 81–97. doi: 10.1016/j.marchem.2017.06.004
Annett, A. L., Skiba, M., Henley, S. F., Venables, H. J., Meredith, M. P., Statham, P. J., et al. (2015). Comparative roles of upwelling and glacial iron sources in Ryder Bay, coastal western Antarctic Peninsula. Mar. Chem. 176, 21–33. doi: 10.1016/j.marchem.2015.06.017
Arrigo, K. R., van Dijken, G. L., Alderkamp, A. C., Erickson, Z. K., Lewis, K. M., Lowry, K. E., et al. (2017). Early spring phytoplankton dynamics in the Western Antarctic Peninsula. J. Geophys. Res. Oceans. 122, 9350–9369. doi: 10.1002/2017JC013281
Atkinson, A., Hill, S. L., Pakhomov, E. A., Siegel, V., Reiss, C. S., Loeb, V. J., et al. (2019). Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Change 9, 142–147. doi: 10.1038/s41558-018-0370-z
Atkinson, A., Siegel, V., Pakhomov, E., and Rothery, P. (2004). Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 432, 100–103. doi: 10.1038/nature02996
Azaneu, M., Heywood, K. J., Queste, B. Y., and Thompson, A. F. (2017). Variability of the Antarctic slope current system in the Northwestern Weddell sea. J. Phys. Oceanogr. 47, 2977–2997. doi: 10.1175/JPO-D-17-0030.1
Azaneu, M., Kerr, R., Mata, M. M., and Garcia, C. A. (2013). Trends in the deep Southern Ocean (1958–2010): Implications for Antarctic Bottom Water properties and volume export. J. Geophys. Res. Oceans 118, 4213–4227. doi: 10.1002/jgrc.20303
Barlett, E. M. R., Tosonotto, G. V., Piola, A. R., Sierra, M. E., and Mata, M. M. (2018). On the temporal variability of intermediate and deep waters in the Western Basin of the Bransfield Strait. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 31–46. doi: 10.1016/j.dsr2.2017.12.010
Bender, N. A., Crosbie, K., and Lynch, H. J. (2016). Patterns of tourism in the Antarctic Peninsula region: a 20-year analysis. Antarct. Sci. 28, 194–203
Bopp, L., Monfray, P., Aumont, O., Dufresne, J. L., Le Treut, H., Madec, G., et al. (2001). Potential impact of climate change on marine export production. Global Biogeochem. Cy. 15, 81–99. doi: 10.1029/1999GB001256
Boyce, D. G., Lewis, M. R., and Worm, B. (2010). Global phytoplankton decline over the past century. Nature 466, 591–596. doi: 10.1038/nature09268
Boyd, P. W. (2019). Physiology and iron modulate diverse responses of diatoms to a warming Southern Ocean. Nat. Clim. Change 9, 148–152. doi: 10.1038/s41558-018-0389-1
Boyd, P. W., Dillingham, P. W., McGraw, C. M., Armstrong, E. A., Cornwall, C. E., Feng, Y. Y., et al. (2016). Physiological responses of a Southern Ocean diatom to complex future ocean conditions. Nat. Clim. Change 6, 207–213. doi: 10.1038/nclimate2811
Boyd, P. W., Doney, S. C., Strzepek, R., Dusenberry, J., Lindsay, K., and Fung, I. (2008). Climate-mediated changes to mixed-layer properties in the Southern Ocean: assessing the phytoplankton response. Biogeosciences 5, 847–864. doi: 10.5194/bg-5-847-2008
Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., and Coale, K. H., (2007). Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 315, 612–617. doi: 10.1126/science.1131669
Brearley, J. A., Meredith, M. P., Garabato, A. C. N., Venables, H. J., and Inall, M. E. (2017). Controls on turbulent mixing on the West Antarctic Peninsula shelf. Deep Sea Res. Part II Top. Stud. Oceanogr. 139, 18–30. doi: 10.1016/j.dsr2.2017.02.011
Brosnan, T., Filep, S., and Rock, J. (2015). Exploring synergies: Hopeful tourism and citizen science. Ann. Tour. Res. 53, 96–98. doi: 10.1016/j.annals.2015.05.002
Brown, M. S., Munro, D. R., Feehan, C. J., Sweeney, C., Ducklow, H. W., and Schofield, O. M. (2019). Enhanced oceanic CO2 uptake along the rapidly changing West Antarctic Peninsula. Nat. Clim. Change 9, 678–683. doi: 10.1038/s41558-019-0552-3
Buck, K. N., Selph, K. E., and Barbeau, K. A. (2010). Iron-binding ligand production and copper speciation in an incubation experiment of Antarctic Peninsula shelf waters from the Bransfield Strait. Southern Ocean. Mar. Chem. 122, 148–159. doi: 10.1016/j.marchem.2010.06.002
Castro, C. G., Ríos, A. F., Doval, M. D., and Perez, F. F. (2002). Nutrient utilization and chlorophyll distribution in the Atlantic sector of the Southern Ocean during Austral summer 1995–96. Deep Sea Res. Part II Top. Stud. Oceanogr. 49, 623–641. doi: 10.1016/S0967-0645(01)00115-1
Charalampopoulou, A., Poulton, A. J., Bakker, D. C., Lucas, M. I., Stinchcombe, M. C., and Tyrrell, T. (2016). Environmental drivers of coccolithophore abundance and calcification across Drake Passage (Southern Ocean). Biogeosciences. 13, 5917–5935. doi: 10.5194/bg-13-5917-2016
Clarke, A., Murphy, E. J., Meredith, M. P., King, J. C., Peck, L. S., Barnes, D. K., et al. (2007). Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 149–166. doi: 10.1098/rstb.2006.1958
Clucas, G. V., Dunn, M. J., Dyke, G., Emslie, S. D., Levy, H., Naveen, R., et al. (2014). A reversal of fortunes: climate change ‘winners’ and ‘losers’ in Antarctic Peninsula penguins. Sci. Rep. 4:5024. doi: 10.1038/srep05024
Collares, L. L., Mata, M. M., Kerr, R., Arigony-Neto, J., and Barbat, M. M. (2018). Iceberg drift and ocean circulation in the northwestern Weddell Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 10–24. doi: 10.1016/j.dsr2.2018.02.014
Cook, A. J., Holland, P. R., Meredith, M. P., Murray, T., Luckman, A., and Vaughan, D. G. (2016). Ocean forcing of glacier retreat in the western Antarctic Peninsula. Science. 353, 283–286. doi: 10.1126/science.aae0017
Cornejo-Donoso, J., and Antezana, T. (2008). Preliminary trophic model of the Antarctic Peninsula Ecosystem (Sub-area CCAMLR 48.1). Ecol. Modell. 218, 1–17. doi: 10.1016/j.ecolmodel.2008.06.011
Costa, R. R., Mendes, C. R. B., Tavano, V. M., Dotto, T. S., Kerr, R., Monteiro, T., et al. (2020). Dynamics of an intense diatom bloom in the Northern Antarctic Peninsula. February 2016. Limnol. Oceanogr. 65, 2056–2075. doi: 10.1002/lno.11437
Cota, G. F., Harrison, W. G., Platt, T., Sathyendranath, S., and Stuart, V. (2003). Bio−optical properties of the Labrador Sea. J. Geophys. Res. Oceans 108:3228. doi: 10.1029/2000JC000597
Couto, N., Martinson, D. G., Kohut, J., and Schofield, O. (2017). Distribution of Upper Circumpolar Deep Water on the warming continental shelf of the West Antarctic Peninsula. J. Geophys. Res. Oceans. 122, 5306–5315. doi: 10.1002/2017JC012840
Crampton, J. S., Cody, R. D., Levy, R., Harwood, D., McKay, R., and Naish, T. R. (2016). Southern Ocean phytoplankton turnover in response to stepwise Antarctic cooling over the past 15 million years. PNAS. 113, 6868–6873. doi: 10.1073/pnas.1600318113
Dalla Rosa, L., Secchi, E. R., Maia, Y. G., Zerbini, A. N., and Heide-Jørgensen, M. P. (2008). Movements of satellite-monitored humpback whales on their feeding ground along the Antarctic Peninsula. Polar Biol. 31, 771–781. doi: 10.1007/s00300-008-0415-2
de Carvalho Ferreira, M. L., and Kerr, R. (2017). Source water distribution and quantification of North Atlantic deep water and Antarctic bottom water in the Atlantic Ocean. Prog. Oceanogr. 153, 66–83. doi: 10.1016/j.pocean.2017.04.003
Deppeler, S. L., and Davidson, A. T. (2017). Southern Ocean phytoplankton in a changing climate. Front. Mar. Sci. 4:40. doi: 10.3389/fmars.2017.00040
Deser, C., Phillips, A., Bourdette, V., and Teng, H. (2012). Uncertainty in climate change projections: the role of internal variability. Clim. Dyn. 38, 527–546. doi: 10.1007/s00382-010-0977-x
Detoni, A. M., de Souza, M. S., Garcia, C. A., Tavano, V. M., and Mata, M. M. (2015). Environmental conditions during phytoplankton blooms in the vicinity of James Ross Island, east of the Antarctic Peninsula. Polar Biol. 38, 1111–1127. doi: 10.1007/s00300-015-1670-7
Dierssen, H. M., and Smith, R. C. (2000). Bio−optical properties and remote sensing ocean color algorithms for Antarctic Peninsula waters. J. Geophys. Res. Oceans. 105, 26301–26312. doi: 10.1029/1999JC000296
Dierssen, H. M., Smith, R. C., and Vernet, M. (2002). Glacial meltwater dynamics in coastal waters west of the Antarctic peninsula. PNAS. 99, 1790–1795. doi: 10.1073/pnas.032206999
Dotto, T. S., Kerr, R., Mata, M. M., and Garcia, C. A. (2016). Multidecadal freshening and lightening in the deep waters of the Bransfield Strait. Antarctica. J. Geophys. Res. Oceans. 121, 3741–3756. doi: 10.1002/2015JC011228
Ducklow, H. W., Fraser, W. R., Meredith, M. P., Stammerjohn, S. E., Doney, S. C., Martinson, D. G., et al. (2013). West Antarctic Peninsula: an ice-dependent coastal marine ecosystem in transition. Oceanography. 26, 190–203. doi: 10.2307/24862081
Ducklow, H., Clarke, A., Dickhut, R., Doney, S. C., Geisz, H., Huang, K., et al. (2012). “The marine system of the Western Antarctic Peninsula,” in Antarctic ecosystems: an extreme environment in a changing world, eds A. D. Rogers, N. M. Johnston, E. J. Murphy, and A. Clarke (John Wiley and Sons), 121–159.
Ebersbach, F., Trull, T. W., Davies, D. M., and Bray, S. G. (2011). Controls on mesopelagic particle fluxes in the Sub-Antarctic and Polar Frontal Zones in the Southern Ocean south of Australia in summer—Perspectives from free-drifting sediment traps. Deep Sea Res. Part II Top. Stud. Oceanogr. 58, 2260–2276.
Eriksen, R., Trull, T. W., Davies, D., Jansen, P., Davidson, A. T., Westwood, K., et al. (2018). Seasonal succession of phytoplankton community structure from autonomous sampling at the Australian Southern Ocean Time Series (SOTS) observatory. Mar. Ecol. Prog. Ser. 589, 13–31. doi: 10.3354/meps12420
Forcada, J., Trathan, P. N., Boveng, P. L., Boyd, I. L., Burns, J. M., Costa, D. P., et al. (2012). Responses of Antarctic pack-ice seals to environmental change and increasing krill fishing. Biol. Conserv. 149, 40–50. doi: 10.1016/j.biocon.2012.02.002
Franco, B. C., Mata, M. M., Piola, A. R., and Garcia, C. A. (2007). Northwestern Weddell Sea deep outflow into the Scotia Sea during the austral summers of 2000 and 2001 estimated by inverse methods. Deep Sea Res. Part I Oceanogr. Res. Pap. 54, 1815–1840. doi: 10.1016/j.dsr.2007.06.003
Frenot, Y., Chown, S. L., Whinam, J., Selkirk, P. M., Convey, P., Skotnicki, M., et al. (2005). Biological invasions in the Antarctic: extent, impacts and implications. Biol. Rev. 80, 45–72. doi: 10.1017/S1464793104006542
Frölicher, T. L., Sarmiento, J. L., Paynter, D. J., Dunne, J. P., Krasting, J. P., and Winton, M. (2015). Dominance of the Southern Ocean in anthropogenic carbon and heat uptake in CMIP5 models. J. Clim. 28, 862–886. doi: 10.1175/JCLI-D-14-00117.1
Garcia, C. A. E., Garcia, V. M. T., and McClain, C. R. (2005). Evaluation of SeaWiFS chlorophyll algorithms in the Southwestern Atlantic and Southern Oceans. Remote Sens. Environ. 95, 125–137. doi: 10.1016/j.rse.2004.12.006
Garcia, M. A., Castro, C. G., Rıos, A. F., Doval, M. D., Rosón, G., Gomis, D., et al. (2002). Water masses and distribution of physico-chemical properties in the Western Bransfield Strait and Gerlache Strait during Austral summer 1995/96. Deep Sea Res. Part II Top. Stud. Oceanogr. 49, 585–602. doi: 10.1016/S0967-0645(01)00113-8
Garibotti, I. A., Vernet, M., and Ferrario, M. E. (2005a). Annually recurrent phytoplanktonic assemblages during summer in the seasonal ice zone west of the Antarctic Peninsula (Southern Ocean). Deep Sea Res. Part I: Oceanogr. Res. Papers 52, 1823–1841. doi: 10.1016/j.dsr.2005.05.003
Garibotti, I. A., Vernet, M., Ferrario, M. E., Smith, R. C., Ross, R. M., and Quetin, L. B. (2003a). Phytoplankton spatial distribution patterns along the western Antarctic Peninsula (Southern Ocean). Mar. Ecol. Prog. Ser. 261, 21–39. doi: 10.3354/meps261021
Garibotti, I. A., Vernet, M., Kozlowski, W. A., and Ferrario, M. E. (2003b). Composition and biomass of phytoplankton assemblages in coastal Antarctic waters: a comparison of chemotaxonomic and microscopic analyses. Mar. Ecol. Prog. Ser. 247, 27–42. doi: 10.3354/meps247027
Garibotti, I. A., Vernet, M., Smith, R. C., and Ferrario, M. E. (2005b). Interannual variability in the distribution of the phytoplankton standing stock across the seasonal sea-ice zone west of the Antarctic Peninsula. J. Plankton Res. 27, 825–843. doi: 10.1093/plankt/fbi056
Gillett, N. P., and Fyfe, J. C. (2013). Annular mode changes in the CMIP5 simulations. Geophys. Res. Lett. 40, 1189–1193. doi: 10.1002/grl.50249
Gonçalves-Araújo, R., de Souza, M. S., Tavano, V. M., and Garcia, C. A. E. (2015). Influence of oceanographic features on spatial and interannual variability of phytoplankton in the Bransfield Strait. Antarctica. J. Mar. Syst. 142, 1–15. doi: 10.1016/j.jmarsys.2014.09.007
Groom, S. B., Sathyendranath, S., Ban, Y., Bernard, S., Brewin, B., Brotas, V., et al. (2019). Satellite ocean colour: current status and future perspective. Front. Mar. Sci. 6:485. doi: 10.3389/fmars.2019.00485
Gruber, N., Gloor, M., Mikaloff Fletcher, S. E., Doney, S. C., Dutkiewicz, S., Follows, M. J., et al. (2009). Oceanic sources, sinks, and transport of atmospheric CO2. Global Biogeochem. Cy. 23, doi: 10.1029/2008GB003349
Haëntjens, N., Boss, E., and Talley, L. D. (2017). Revisiting Ocean Color algorithms for chlorophyll a and particulate organic carbon in the Southern Ocean using biogeochemical floats. J. Geophys. Res. Oceans. 122, 6583–6593. doi: 10.1002/2017JC012844
Hellmer, H. H., Huhn, O., Gomis, D., and Timmermann, R. (2011). On the freshening of the northwestern Weddell Sea continental shelf. Ocean Sci 7, 305–316. doi: 10.5194/os-7-305-2011
Henley, S. F., Cavan, E. L., Fawcett, S. E., Kerr, R., Monteiro, T., Sherrell, R. M., et al. (2020). Changing biogeochemistry of the Southern Ocean and its ecosystem implications. Front. Mar. Sci. 7:581. doi: 10.3389/fmars.2020.00581
Henley, S. F., Jones, E. M., Venables, H. J., Meredith, M. P., Firing, Y. L., Dittrich, R., et al. (2018). Macronutrient and carbon supply, uptake and cycling across the Antarctic Peninsula shelf during summer. Philos. Trans. A Math. Phys. Eng. Sci. 376:20170168. doi: 10.1098/rsta.2017.0168
Henley, S. F., Schofield, O. M., Hendry, K. R., Schloss, I. R., Steinberg, D. K., Moffat, C., et al. (2019). Variability and change in the west Antarctic Peninsula marine system: Research priorities and opportunities. Prog. Oceanogr. 173, 208–237. doi: 10.1016/j.pocean.2019.03.003
Henley, S. F., Tuerena, R. E., Annett, A. L., Fallick, A. E., Meredith, M. P., Venables, H. J., et al. (2017). Macronutrient supply, uptake and recycling in the coastal ocean of the west Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 139, 58–76. doi: 10.1016/j.dsr2.2016.10.003
Henson, S. A., Cole, H. S., Hopkins, J., Martin, A. P., and Yool, A. (2018). Detection of climate change−driven trends in phytoplankton phenology. Glob. Chang. Biol. 24, e101–e111. doi: 10.1111/gcb.13886
Henson, S. A., Sarmiento, J. L., Dunne, J. P., Bopp, L., Lima, I., Doney, S. C., et al. (2010). Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity. Biogeosciences. 7, 621–640. doi: 10.5194/bg-7-621-2010
Hernando, M., Schloss, I. R., Malanga, G., Almandoz, G. O., Ferreyra, G. A., Aguiar, M. B., et al. (2015). Effects of salinity changes on coastal Antarctic phytoplankton physiology and assemblage composition. J. Exp. Mar. Biol. Ecol. 466, 110–119. doi: 10.1016/j.jembe.2015.02.012
Heywood, K. J., Naveira Garabato, A. C., Stevens, D. P., and Muench, R. D. (2004). On the fate of the Antarctic Slope Front and the origin of the Weddell Front. J. Geophys. Res. Oceans 109, C06021. doi: 10.1029/2003JC002053
Hindell, M. A., Reisinger, R. R., Ropert-Coudert, Y., Hückstädt, L. A., Trathan, P. N., Bornemann, H., et al. (2020). Tracking of marine predators to protect Southern Ocean ecosystems. Nature. 580, 87–92. doi: 10.1038/s41586-020-2126-y
Höfer, J., Giesecke, R., Hopwood, M. J., Carrera, V., Alarcón, E., and González, H. E. (2019). The role of water column stability and wind mixing in the production/export dynamics of two bays in the Western Antarctic Peninsula. Prog. Oceanogr. 174, 105–116. doi: 10.1016/j.pocean.2019.01.005
Holm-Hansen, O., and Mitchell, B. G. (1991). Bio-optical properties of Antarctic Peninsula waters: Differentiation from temperate ocean models. Deep Sea Res. Part I Oceanogr. Res. Pap. 38, 1009–1028. doi: 10.1016/0198-0149(91)90094-V
Holm-Hansen, O., Hewes, C. D., Villafane, V. E., Helbling, E. W., Silva, N., and Amos, T. (1997). Distribution of phytoplankton and nutrients in relation to different water masses in the area around Elephant Island. Antarctica. Polar Biol. 18, 145–153. doi: 10.1007/s003000050169
Houben, A. J., Bijl, P. K., Pross, J., Bohaty, S. M., Passchier, S., Stickley, C. E., et al. (2013). Reorganization of Southern Ocean plankton ecosystem at the onset of Antarctic glaciation. Science. 340, 341–344. doi: 10.1126/science.1223646
Huang, J., Zhang, X., Zhang, Q., Lin, Y., Hao, M., Luo, Y., et al. (2017). Recently amplified arctic warming has contributed to a continual global warming trend. Nat Clim. Change 7, 875–879. doi: 10.1038/s41558-017-0009-5
Hughes, L. (2000). Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 15, 56–61. doi: 10.1016/S0169-5347(99)01764-4
Huhn, O., Hellmer, H. H., Rhein, M., Rodehacke, C., Roether, W., Schodlok, M. P., et al. (2008). Evidence of deep-and bottom-water formation in the western Weddell Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 1098–1116. doi: 10.1016/j.dsr2.2007.12.015
Huneke, W. G. C., Huhn, O., and Schröeder, M. (2016). Water masses in the Bransfield Strait and adjacent seas, austral summer 2013. Polar Biol. 39, 789–798. doi: 10.1007/s00300-016-1936-8
Jena, B. (2017). The effect of phytoplankton pigment composition and packaging on the retrieval of chlorophyll-a concentration from satellite observations in the Southern Ocean. Int. J. Remote Sens. 38, 3763–3784. doi: 10.1080/01431161.2017.1308034
Johnson, R., Strutton, P. G., Wright, S. W., McMinn, A., and Meiners, K. M. (2013). Three improved satellite chlorophyll algorithms for the Southern Ocean. J. Geophys. Res. Oceans. 118, 3694–3703. doi: 10.1002/jgrc.20270
Kahru, M., Brotas, V., Manzano-Sarabia, M., and Mitchell, B. G. (2011). Are phytoplankton blooms occurring earlier in the Arctic? Glob. Chang. Biol. 17, 1733–1739. doi: 10.1111/j.1365-2486.2010.02312.x
Kang, S. H., Kang, J. S., Lee, S., Chung, K. H., Kim, D., and Park, M. G. (2001). Antarctic phytoplankton assemblages in the marginal ice zone of the northwestern Weddell Sea. J. Plankton Res. 23, 333–352. doi: 10.1093/plankt/23.4.333
Kerr, R., Goyet, C., da Cunha, L. C., Orselli, I. B., Lencina-Avila, J. M., Mendes, C. R. B., et al. (2018a). Carbonate system properties in the Gerlache Strait. Northern Antarctic Peninsula (February 2015): II. Anthropogenic CO2 and seawater acidification. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 182–192. doi: 10.1016/j.dsr2.2017.07.007
Kerr, R., Mata, M. M., and Garcia, C. A. (2009). On the temporal variability of the Weddell Sea Deep Water masses. Antarct. Sci. 21:383. doi: 10.1017/S0954102009001990
Kerr, R., Mata, M. M., Mendes, C. R. B., and Secchi, E. R. (2018b). Northern Antarctic Peninsula: a marine climate hotspot of rapid changes on ecosystems and ocean dynamics. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 4–9. doi: 10.1016/j.dsr2.2018.05.006
Kerr, R., Mata, M. M., Mendes, C. R. B., and Secchi, E. R. (2018c). Northern antarctic peninsula: a marine climate hotspot of rapid changes on ecosystems and ocean dynamics. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 4–9. doi: 10.1016/j.dsr2.2018.05.006
Kim, H., Doney, S. C., Iannuzzi, R. A., Meredith, M. P., Martinson, D. G., and Ducklow, H. W. (2016). Climate forcing for dynamics of dissolved inorganic nutrients at Palmer Station. Antarctica: an interdecadal (1993–2013) analysis. J. Geophys. Res.: Biogeosci. 121, 2369–2389. doi: 10.1002/2015JG003311
Kim, H., Ducklow, H. W., Abele, D., Ruiz Barlett, E. M., Buma, A. G., Meredith, M. P., et al. (2018). Inter-decadal variability of phytoplankton biomass along the coastal West Antarctic Peninsula. Philos. Trans. Math., Phys. Eng. Sci. 376:20170174. doi: 10.1098/rsta.2017.0174
Lacerda, A. L. D. F., Proietti, M. C., Secchi, E. R., and Taylor, J. D. (2020). Diverse groups of fungi are associated with plastics in the surface waters of the Western South Atlantic and the Antarctic Peninsula. Mol. Ecol. 29, 1903–1918. doi: 10.1111/mec.15444
Lacerda, A. L. D. F., Rodrigues, L. D. S., Van Sebille, E., Rodrigues, F. L., Ribeiro, L., Secchi, E. R., et al. (2019). Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-019-40311-4
Lewis, P. N., Hewitt, C. L., Riddle, M., and McMinn, A. (2003). Marine introductions in the Southern Ocean: an unrecognised hazard to biodiversity. Mar. Pollut. Bull. 46, 213–223. doi: 10.1016/S0025-326X(02)00364-8
Loeb, V., Hofmann, E. E., Klinck, J. M., and Holm-Hansen, O. (2010). Hydrographic control of the marine ecosystem in the South Shetland-Elephant Island and Bransfield Strait region. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 519–542. doi: 10.1016/j.dsr2.2009.10.004
Long, M., Moriceau, B., Gallinari, M., Lambert, C., Huvet, A., Raffray, J., et al. (2015). Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar. Chem. 175, 39–46. doi: 10.1016/j.marchem.2015.04.003
Marinov, I., Doney, S. C., and Lima, I. D. (2010). Response of ocean phytoplankton community structure to climate change over the 21st century: partitioning the effects of nutrients, temperature and light. Biogeosciences. 7:3941. doi: 10.5194/bg-7-3941-2010
Mata, M. M., Tavano, V. M., and Garcia, C. A. (2018). 15 years sailing with the Brazilian High Latitude Oceanography Group (GOAL). Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 1–3. doi: 10.1016/j.dsr2.2018.05.007
McKee, D. C., Martinson, D. G., and Schofield, O. (2019). Origin and attenuation of mesoscale structure in circumpolar deep water intrusions to an Antarctic shelf. J. Phys. Oceanogr. 49, 1293–1318. doi: 10.1175/JPO-D-18-0133.1
McKinley, G. A., Pilcher, D. J., Fay, A. R., Lindsay, K., Long, M. C., and Lovenduski, N. S. (2016). Timescales for detection of trends in the ocean carbon sink. Nature 530, 469–472. doi: 10.1038/nature16958
McLeod, D. J., Hallegraeff, G. M., Hosie, G. W., and Richardson, A. J. (2012). Climate-driven range expansion of the red-tide dinoflagellate Noctiluca scintillans into the Southern Ocean. J. Plankton. Res. 34, 332–337. doi: 10.1093/plankt/fbr112
Mendes, C. R. B., de Souza, M. S., Garcia, V. M. T., Leal, M. C., Brotas, V., and Garcia, C. A. E. (2012). Dynamics of phytoplankton communities during late summer around the tip of the Antarctic Peninsula. Deep Sea Res. Part I Oceanogr. Res. Pap. 65, 1–14. doi: 10.1016/j.dsr.2012.03.002
Mendes, C. R. B., Tavano, V. M., Dotto, T. S., Kerr, R., De Souza, M. S., Garcia, C. A. E., et al. (2018a). New insights on the dominance of cryptophytes in Antarctic coastal waters: a case study in Gerlache Strait. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 161–170. doi: 10.1016/j.dsr2.2017.02.010
Mendes, C. R. B., Tavano, V. M., Kerr, R., Dotto, T. S., Maximiano, T., and Secchi, E. R. (2018b). Impact of sea ice on the structure of phytoplankton communities in the northern Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 111–123. doi: 10.1016/j.dsr2.2017.12.003
Mendes, C. R. B., Tavano, V. M., Leal, M. C., de Souza, M. S., Brotas, V., and Garcia, C. A. E. (2013). Shifts in the dominance between diatoms and cryptophytes during three late summers in the Bransfield Strait (Antarctic Peninsula). Polar Biol. 36, 537–547. doi: 10.1007/s00300-012-1282-4
Meredith, M. P., and King, J. C. (2005). Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett 32:L19604 doi: 10.1029/2005GL024042
Meredith, M. P., Schofield, O., Newman, L., Urban, E., and Sparrow, M. (2013). The vision for a southern ocean observing system. Curr. Opin. Environ. Sustain. 5, 306–313. doi: 10.1016/j.cosust.2013.03.002
Moffat, C., and Meredith, M. (2018). Shelf–ocean exchange and hydrography west of the Antarctic Peninsula: a review. Philos. Trans. A Math. Phys. Eng. Sci. 376:20170164. doi: 10.1098/rsta.2017.0164
Moffat, C., Owens, B., and Beardsley, R. C. (2009). On the characteristics of Circumpolar Deep Water intrusions to the west Antarctic Peninsula continental shelf. J. Geophys. Res. Oceans 114:C05017, doi: 10.1029/2008JC004955
Moline, M. A., and Prézelin, B. B. (1996). Long-term monitoring and analyses of physical factors regulating variability in coastal Antarctic phytoplankton biomass, in situ productivity and taxonomic composition over subseasonal, seasonal and interannual time scales. Mar. Ecol. Prog. Ser. 145, 143–160. doi: 10.3354/meps145143
Moline, M. A., Claustre, H., Frazer, T. K., Schofield, O., and Vernet, M. (2004). Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Glob. Chang. Biol. 10, 1973–1980. doi: 10.1111/j.1365-2486.2004.00825.x
Monteiro, T., Kerr, R., and Machado, E. C. (2020a). Seasonal variability of net sea-air CO2 fluxes in a coastal region of the northern Antarctic Peninsula. Sci. Rep. 10:14875. doi: 10.1038/s41598-020-71814-0
Monteiro, T., Kerr, R., Orselli, I. B., and Lencina-Avila, J. M. (2020b). Towards an intensified summer CO2 sink behaviour in the Southern Ocean coastal regions. Prog. Oceanogr. 183:102267. doi: 10.1016/j.pocean.2020.102267
Montes-Hugo, M., Doney, S. C., Ducklow, H. W., Fraser, W., Martinson, D., Stammerjohn, S. E., et al. (2009). Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science. 323, 1470–1473. doi: 10.1126/science.1164533
Moreau, S., Boyd, P. W., and Strutton, P. G. (2020). Remote assessment of the fate of phytoplankton in the Southern Ocean sea-ice zone. Nat. Comm. 11, 1–9. doi: 10.1038/s41467-020-16931-0
Moreau, S., Ferreyra, G. A., Mercier, B., Lemarchand, K., Lionard, M., Roy, S., et al. (2010). Variability of the microbial community in the western Antarctic Peninsula from late fall to spring during a low ice cover year. Polar Biol. 33, 1599–1614. doi: 10.1007/s00300-010-0806-z
Newman, L., Heil, P., Trebilco, R., Katsumata, K., Constable, A., van Wijk, E., et al. (2019). Delivering sustained, coordinated, and integrated observations of the Southern Ocean for global impact. Front. Mar. Sci. 6:433. doi: 10.3389/fmars.2019.00433
Niller, P. P., Amos, A., and Hu, J. H. (1991). Water masses and 200 m relative geostrophic circulation in the western Bransfield Strait region. Deep Sea Res. Part I Oceanogr. Res. Pap. 38, 943–959. doi: 10.1016/0198-0149(91)90091-S
Oberbeckmann, S., Löder, M. G., and Labrenz, M. (2015). Marine microplastic-associated biofilms–a review. Environ. Chem. 12, 551–562. doi: 10.1071/EN15069
Oliva, M., Navarro, F., Hrbáèek, F., Hernández, A., Nıvlt, D., Pereira, P., et al. (2016). Recent regional climate cooling on the Antarctic Peninsula and associated impacts on the cryosphere. Sci. Tot. Environ. 580, 210–223. doi: 10.1016/j.scitotenv.2016.12.030
Orsi, A. H., Whitworth, T. III, and Nowlin, W. D. Jr. (1995). On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Res. Part I Oceanogr. Res. Pap. 42, 641–673. doi: 10.1016/0967-0637(95)00021-W
Pan, B. J., Vernet, M., Manck, L., Forsch, K., Ekern, L., Mascioni, M., et al. (2020). Environmental drivers of phytoplankton taxonomic composition in an Antarctic fjord. Prog. Oceanogr. 183:102295. doi: 10.1016/j.pocean.2020.102295
Paolo, F. S., Fricker, H. A., and Padman, L. (2015). Volume loss from Antarctic ice shelves is accelerating. Science. 348, 327–331. doi: 10.1126/science.aaa0940
Parkinson, C. L., and Cavalieri, D. J. (2012). Antarctic sea ice variability and trends, 1979–2010. Cryosphere. 6, 871–880. doi: 10.5194/tc-6-871-2012
Pereira, E. S., and Garcia, C. A. (2018). Evaluation of satellite-derived MODIS chlorophyll algorithms in the northern Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 124–137. doi: 10.1016/j.dsr2.2017.12.018
Petrou, K., Kranz, S. A., Trimborn, S., Hassler, C. S., Ameijeiras, S. B., Sackett, O., et al. (2016). Southern Ocean phytoplankton physiology in a changing climate. J. Plant. Physiol. 203, 135–150. doi: 10.1016/j.jplph.2016.05.004
Pillai, H. U. K., Anilkumar, N., Achuthankutty, C. T., Mendes, C. R., Sabu, P., Jayalakshmi, K. V., et al. (2018). Planktonic food web structure at SSTF and PF in the Indian sector of the Southern Ocean during austral summer 2011. Polar Res. 37:1495545. doi: 10.1080/17518369.2018.1495545
Pinsky, M. L., Worm, B., Fogarty, M. J., Sarmiento, J. L., and Levin, S. A. (2013). Marine taxa track local climate velocities. Science. 341, 1239–1242. doi: 10.1126/science.1239352
Prézelin, B. B., Hofmann, E. E., Mengelt, C., and Klinck, J. M. (2000). The linkage between Upper Circumpolar Deep Water (UCDW) and phytoplankton assemblages on the west Antarctic Peninsula continental shelf. J. Mar. Res. 58, 165–202. doi: 10.1357/002224000321511133
Pritchard, H., Ligtenberg, S. R., Fricker, H. A., Vaughan, D. G., van den Broeke, M. R., and Padman, L. (2012). Antarctic ice-sheet loss driven by basal melting of ice shelves. Nature. 484, 502–505. doi: 10.1038/nature10968
Rembauville, M., Salter, I., Leblond, N., Gueneugues, A., and Blain, S. (2015). Export fluxes in a naturally iron-fertilized area of the Southern Ocean–Part 1: Seasonal dynamics of particulate organic carbon export from a moored sediment trap. Biogeosciences 12, 3153–3170. doi: 10.5194/bg-12-3153-2015
Renner, A. H., Thorpe, S. E., Heywood, K. J., Murphy, E. J., Watkins, J. L., and Meredith, M. P. (2012). Advective pathways near the tip of the Antarctic Peninsula: Trends, variability and ecosystem implications. Deep Sea Res. Part I Oceanogr. Res. Pap. 63, 91–101. doi: 10.1016/j.dsr.2012.01.009
Rodriguez, F., Varela, M., and Zapata, M. (2002). Phytoplankton assemblages in the Gerlache and Bransfield Straits (Antarctic Peninsula) determined by light microscopy and CHEMTAX analysis of HPLC pigment data. Deep Sea Res. Part II Top. Stud. Oceanogr. 49, 723–747. doi: 10.1016/S0967-0645(01)00121-7
Roquet, F., Williams, G., Hindell, M. A., Harcourt, R., McMahon, C., Guinet, C., et al. (2014). A Southern Indian Ocean database of hydrographic profiles obtained with instrumented elephant seals. Sci. Data. 1:140028. doi: 10.1038/sdata.2014.28
Rozema, P. D., Venables, H. J., Van De Poll, W. H., Clarke, A., Meredith, M. P., and Buma, A. G. J. (2017). Interannual variability in phytoplankton biomass and species composition in northern Marguerite Bay (West Antarctic Peninsula) is governed by both winter sea ice cover and summer stratification. Limnol. Oceanogr. 62, 235–252. doi: 10.1002/lno.10391
Russo, A. D. P. G., de Souza, M. S., Mendes, C. R. B., Tavano, V. M., and Garcia, C. A. E. (2018). Spatial variability of photophysiology and primary production rates of the phytoplankton communities across the western Antarctic Peninsula in late summer 2013. Deep Sea Research Part II: Topical Studies in Oceanography 149, 99–110. doi: 10.1016/j.dsr2.2017.12.010
Saba, G. K., Fraser, W. R., Saba, V. S., Iannuzzi, R. A., Coleman, K. E., Doney, S. C., et al. (2014). Winter and spring controls on the summer food web of the coastal West Antarctic Peninsula. Nat. Commun. 5, 1–8. doi: 10.1038/ncomms5318
Sangrà, P., Gordo, C., Hernández-Arencibia, M., Marrero-Díaz, A., Rodríguez-Santana, A., Stegner, A., et al. (2011). The Bransfield current system. Deep Sea Res. Part I Oceanogr. Res. Pap. 58, 390–402. doi: 10.1016/j.dsr.2011.01.011
Sangrà, P., Stegner, A., Hernández-Arencibia, M., Marrero-Díaz, Á, Salinas, C., Aguiar-González, B., et al. (2017). The Bransfield gravity current. Deep Sea Res. Part I Oceanogr. Res. Pap 119, 1–15. doi: 10.1016/j.dsr.2016.11.003
Santer, B. D., Mears, C., Doutriaux, C., Caldwell, P., Gleckler, P. J., Wigley, T. M. L., et al. (2011). Separating signal and noise in atmospheric temperature changes: The importance of timescale. J. Geophys. Res. Atmos 116, 1–19 doi: 10.1029/2011JD016263
Santoso, A., Mcphaden, M. J., and Cai, W. (2017). The defining characteristics of ENSO extremes and the strong 2015/2016 El Niño. ıRev. Geophys. 55, 1079–1129. doi: 10.1002/2017RG000560
Savidge, D. K., and Amft, J. A. (2009). Circulation on the West Antarctic Peninsula derived from 6 years of shipboard ADCP transects. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 1633–1655. doi: 10.1016/j.dsr.2009.05.011
Savidge, G., Harbour, D., Gilpin, L. C., and Boyd, P. W. (1995). Phytoplankton distributions and production in the Bellingshausen Sea. Austral spring 1992. Deep Sea Res. Part II Top. Stud. Oceanogr. 42, 1201–1224. doi: 10.1016/0967-0645(95)00062-U
Schmidtko, S., Heywood, K. J., Thompson, A. F., and Aoki, S. (2014). Multidecadal warming of Antarctic waters. Science. 346, 1227–1231. doi: 10.1126/science.1256117
Schofield, O., Brown, M., Kohut, J., Nardelli, S., Saba, G., Waite, N., et al. (2018). Changes in the upper ocean mixed layer and phytoplankton productivity along the West Antarctic Peninsula. Philos. Trans. A Math. Phys. Eng. Sci. 376, 20170173. doi: 10.1098/rsta.2017.0173
Schofield, O., Ducklow, H. W., Martinson, D. G., Meredith, M. P., Moline, M. A., and Fraser, W. R. (2010). How do polar marine ecosystems respond to rapid climate change? Science. 328, 1520–1523. doi: 10.1126/science.1185779
Schofield, O., Saba, G., Coleman, K., Carvalho, F., Couto, N., Ducklow, H., et al. (2017). Decadal variability in coastal phytoplankton community composition in a changing West Antarctic Peninsula. Deep Sea Res. Part I Oceanogr. Res. Pap. 124, 42–54. doi: 10.1016/j.dsr.2017.04.014
Secchi, E., Luciano, D.-R., Kinas, P. G., Nicolette, R. F., Rufino, A. M., and Azevedo, A. F. (2011). Encounter rates and abundance of humpback whales (Megaptera novaeangliae) in Gerlache and Bransfield Straits, Antarctic Peninsula. J. Cetacean. Res. Manag 3, 107–111.
Seyboth, E., Botta, S., Mendes, C. R. B., Negrete, J., Dalla Rosa, L., and Secchi, E. R. (2018). Isotopic evidence of the effect of warming on the northern Antarctic Peninsula ecosystem. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 218–228. doi: 10.1016/j.dsr2.2017.12.020
Sherrell, R. M., Annet, L. A., Fitzsimmons, J. N., Roccanova, V. J., and Meredith, M. P. (2018). A ‘shallow bathtub ring’of local sedimentary iron input maintains the Palmer Deep biological hotspot on the West Antarctic Peninsula shelf. Philos. Trans. A Math. Phys. Eng. Sci. 376:20170171. doi: 10.1098/rsta.2017.0171
Smayda, T. J. (2002). Turbulence, watermass stratification and harmful algal blooms: an alternative view and frontal zones as “pelagic seed banks”. Harmful Algae 1, 95–112. doi: 10.1016/S1568-9883(02)00010-0
Smith, D. A., Hofmann, E. E., Klinck, J. M., and Lascara, C. M. (1999). Hydrography and circulation of the west Antarctic Peninsula continental shelf. Deep Sea Res. Part I Oceanogr. Res. Pap. 46, 925–949. doi: 10.1016/S0967-0637(98)00103-4
Smith, K. L., and Polvani, L. M. (2017). Spatial patterns of recent Antarctic surface temperature trends and the importance of natural variability: lessons from multiple reconstructions and the CMIP5 models. Clim. Dyn. 48, 2653–2670. doi: 10.1007/s00382-016-3230-4
Smith, R. C., Dierssen, H. M., and Vernet, M. (1996). Phytoplankton biomass and productivity in the western Antarctic Peninsula region. Antarct. Res. Ser. 70, 333–356. doi: 10.1029/AR070p0333
Southwell, C., Bengtson, J., and Bester, M. (2012). A review of data on abundance, trends in abundance, habitat use and diet of ice-breeding seals in the Southern Ocean. CCAMLR Sci. 19, 49–74.
Stammerjohn, S. E., Martinson, D. G., Smith, R. C., Yuan, X., and Rind, D. (2008). Trends in Antarctic annual sea ice retreat and advance and their relation to El Niño–Southern Oscillation and Southern Annular Mode variability. J. Geophys. Res. Oceans 113:C03S90 doi: 10.1029/2007JC004269
Steinberg, D. K., Ruck, K. E., Gleiber, M. R., Garzio, L. M., Cope, J. S., Bernard, K. S., et al. (2015). Long-term (1993–2013) changes in macrozooplankton off the Western Antarctic Peninsula. Deep Sea Res. Part I Oceanogr. Res. Pap. 101, 54–70. doi: 10.1016/j.dsr.2015.02.009
Stott, P. A., and Tett, S. F. (1998). Scale-dependent detection of climate change J. Clim 11, 3282–3294. doi: 10.1175/1520-04421998011
Takao, S., Hirawake, T., Hashida, G., Sasaki, H., Hattori, H., and Suzuki, K. (2014). Phytoplankton community composition and photosynthetic physiology in the Australian sector of the Southern Ocean during the austral summer of 2010/2011. Polar Biol. 37, 1563–1578. doi: 10.1007/s00300-014-1542-6
Thomalla, S. J., Ogunkoya, A. G., Vichi, M., and Swart, S. (2017). Using optical sensors on gliders to estimate phytoplankton carbon concentrations and chlorophyll-to-carbon ratios in the Southern Ocean. Front. Mar. Sci. 4:34. doi: 10.3389/fmars.2017.00034
Thompson, A. F., Heywood, K. J., Thorpe, S. E., Renner, A. H., and Trasviña, A. (2009). Surface circulation at the tip of the Antarctic Peninsula from drifters. J. Phys. Oceanogr. 39, 3–26. doi: 10.1175/2008JPO3995.1
Trimborn, S., Hoppe, C. J., Taylor, B. B., Bracher, A., and Hassler, C. (2015). Physiological characteristics of open ocean and coastal phytoplankton communities of Western Antarctic Peninsula and Drake Passage waters. Deep Sea Res. Part I Oceanogr. Res. Pap. 98, 115–124. doi: 10.1016/j.dsr.2014.12.010
Trimborn, S., Thoms, S., Bischof, K., and Beszteri, S. (2019). Susceptibility of two Southern Ocean phytoplankton key species to iron limitation and high light. Front. Mar. Sci. 6:167. doi: 10.3389/fmars.2019.00167
Trivelpiece, W. Z., Hinke, J. T., Miller, A. K., Reiss, C. S., Trivelpiece, S. G., and Watters, G. M. (2011). Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. PNAS. 108, 7625–7628. doi: 10.1073/pnas.1016560108
Turner, J., Barrand, N. E., Bracegirdle, T. J., Convey, P., Hodgson, D. A., Jarvis, M., et al. (2014). Antarctic climate change and the environment: an update. Polar Rec. 50, 237–259. doi: 10.1017/S0032247413000296
Turner, J., Colwell, S. R., Marshall, G. J., Lachlan−Cope, T. A., Carleton, A. M., Jones, P. D., et al. (2005). Antarctic climate change during the last 50 years. Int. J. Climatol 25, 279–294. doi: 10.1002/joc.1130
Turner, J., Lu, H., White, I., King, J. C., Phillips, T., Hosking, J. S., et al. (2016). Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535, 411–415. doi: 10.1038/nature18645
van Caspel, M., Hellmer, H. H., and Mata, M. M. (2018). On the ventilation of Bransfield Strait deep basins. Deep Sea Res. Part II Top. Stud. Oceanogr. 149, 25–30. doi: 10.1016/j.dsr2.2017.09.006
van Caspel, M., Schröder, M., Huhn, O., and Hellmer, H. H. (2015). Precursors of Antarctic Bottom Water formed on the continental shelf off Larsen Ice Shelf. Deep Sea Res. Part I Oceanogr. Res. Pap. 99, 1–9. doi: 10.1016/j.dsr.2015.01.004
Varela, M., Fernandez, E., and Serret, P. (2002). Size-fractionated phytoplankton biomass and primary production in the Gerlache and south Bransfield Straits (Antarctic Peninsula) in Austral summer 1995–1996. Deep Sea Res. Part II Top. Stud. Oceanogr. 49, 749–768. doi: 10.1016/S0967-0645(01)00122-9
Vaughan, D. G., Marshall, G. J., Connolley, W. M., Parkinson, C., Mulvaney, R., Hodgson, D. A., et al. (2003). Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Change 60, 243–274. doi: 10.1023/A:1026021217991
Venables, H. J., Clarke, A., and Meredith, M. P. (2013). Wintertime controls on summer stratification and productivity at the western Antarctic Peninsula. Limnol. Oceanogr. 58, 1035–1047. doi: 10.4319/lo.2013.58.3.1035
Vernet, M., Kozlowski, W. A., Yarmey, L. R., Lowe, A. T., Ross, R. M., Quetin, L. B., et al. (2012). Primary production throughout austral fall, during a time of decreasing daylength in the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 452, 45–61. doi: 10.3354/meps09704
von Berg, L., Prend, C. J., Campbell, E. C., Mazloff, M. R., Talley, L. D., and Gille, S. T. (2020). Weddell Sea phytoplankton blooms modulated by sea ice variability and polynya formation. Geophys. Res. Lett. 47:e2020GL087954. doi: 10.1029/2020GL087954
Von Gyldenfeldt, A. B., Fahrbach, E., García, M. A., and Schröder, M. (2002). Flow variability at the tip of the Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr 49, 4743–4766. doi: 10.1016/S0967-0645(02)00157-1
Waller, C. L., Griffiths, H. J., Waluda, C. M., Thorpe, S. E., Loaiza, I., Moreno, B., et al. (2017). Microplastics in the Antarctic marine system: an emerging area of research. Sci. Total Environ. 598, 220–227. doi: 10.1016/j.scitotenv.2017.03.283
Wilson, J. D., Monteiro, F. M., Schmidt, D. N., Ward, B. A., and Ridgwell, A. (2018). Linking marine plankton ecosystems and climate: a new modeling approach to the warm early Eocene climate. Paleoceanogr. Paleoclimatol. 33, 1439–1452. doi: 10.1029/2018PA003374
Winter, A., Henderiks, J., Beaufort, L., Rickaby, R. E., and Brown, C. W. (2014). Poleward expansion of the coccolithophore Emiliania huxleyi. J. Plankton Res. 36, 316–325. doi: 10.1093/plankt/fbt110
Zettler, E. R., Mincer, T. J., and Amaral-Zettler, L. A. (2013). Life in the “plastisphere”: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7146. doi: 10.1021/es401288x
Zhou, M., Niiler, P. P., and Hu, J. H. (2002). Surface currents in the Bransfield and Gerlache straits. Antarctica. Deep Sea Res. Part I Oceanogr. Res. Pap. 49, 267–280. doi: 10.1016/S0967-0637(01)00062-0
Zhou, M., Niiler, P. P., Zhu, Y., and Dorland, R. D. (2006). The western boundary current in the Bransfield Strait. Antarctica. Deep Sea Res. Part I Oceanogr. Res. Pap. 53, 1244–1252. doi: 10.1016/j.dsr.2006.04.003
Keywords: West Antarctica, phytoplankton response, climate change, bottom–up impacts to the ecosystem, research gaps and directions
Citation: Ferreira A, Costa RR, Dotto TS, Kerr R, Tavano VM, Brito AC, Brotas V, Secchi ER and Mendes CRB (2020) Changes in Phytoplankton Communities Along the Northern Antarctic Peninsula: Causes, Impacts and Research Priorities. Front. Mar. Sci. 7:576254. doi: 10.3389/fmars.2020.576254
Received: 25 June 2020; Accepted: 18 September 2020;
Published: 28 October 2020.
Edited by:
Elif Eker-Develi, Mersin University, TurkeyReviewed by:
Hugh Ducklow, Columbia University, United StatesWillem Hendrik Van De Poll, University of Groningen, Netherlands
Copyright © 2020 Ferreira, Costa, Dotto, Kerr, Tavano, Brito, Brotas, Secchi and Mendes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Afonso Ferreira, YW1iZmVycmVpcmFAZmMudWwucHQ=
 Afonso Ferreira
Afonso Ferreira Raul R. Costa
Raul R. Costa Tiago S. Dotto3
Tiago S. Dotto3 Rodrigo Kerr
Rodrigo Kerr Ana C. Brito
Ana C. Brito Vanda Brotas
Vanda Brotas Eduardo R. Secchi
Eduardo R. Secchi Carlos R. B. Mendes
Carlos R. B. Mendes