- 1Department of Ocean and Mechanical Engineering, Florida Atlantic University, Boca Raton, FL, United States
- 2Harbor Branch Oceanographic Institute, Florida Atlantic University, Fort Pierce, FL, United States
The characterization of particle and plankton populations, as well as microscale biophysical interactions, is critical to several important research areas in oceanography and limnology. A growing number of aquatic researchers are turning to holography as a tool of choice to quantify particle fields in diverse environments, including but not limited to, studies on particle orientation, thin layers, phytoplankton blooms, and zooplankton distributions and behavior. Holography provides a non-intrusive, free-stream approach to imaging and characterizing aquatic particles, organisms, and behavior in situ at high resolution through a 3-D sampling volume. Compared to other imaging techniques, e.g., flow cytometry, much larger volumes of water can be processed over the same duration, resolving particle sizes ranging from a few microns to a few centimeters. Modern holographic imaging systems are compact enough to be deployed through various modes, including profiling/towed platforms, buoys, gliders, long-term observatories, or benthic landers. Limitations of the technique include the data-intensive hologram acquisition process, computationally expensive image reconstruction, and coherent noise associated with the holograms that can make post-processing challenging. However, continued processing refinements, rapid advancements in computing power, and development of powerful machine learning algorithms for particle/organism classification are paving the way for holography to be used ubiquitously across different disciplines in the aquatic sciences. This review aims to provide a comprehensive overview of holography in the context of aquatic studies, including historical developments, prior research applications, as well as advantages and limitations of the technique. Ongoing technological developments that can facilitate larger employment of this technique toward in situ measurements in the future, as well as potential applications in emerging research areas in the aquatic sciences are also discussed.
Introduction
Since the introduction of holography to the scientific community in a pair of seminal papers more than seven decades ago (Gabor, 1948, 1949), it has become an indispensable technique used across a range of disciplines, including but not limited to, environmental and applied fluid mechanics, interferometric studies, metrology, and medical imaging. While the utility of holography in oceanographic and limnological field research has been evident since the 1970s, the last decade and a half has brought significant technological advances and an increase in off-the-shelf availability of holographic imaging systems, leading to a surge in its use by the aquatic sciences community.
This review endeavors to provide a brief background on the history of in situ holographic instrumentation in the aquatic sciences, predominantly focusing on the advances made since the advent of digital holography at the turn of the millennium. While a brief introduction to the technique and processing methods is provided, an exhaustive discussion is beyond the scope of this review and can be found elsewhere (Schnars and Jüptner, 2002, 2005; Katz and Sheng, 2010). An overview of the diverse applications of in situ holography that demonstrate its versatility is provided. Finally, existing limitations, scope for further development of the technique to enable widespread utility in the aquatic sciences community, and future avenues of applications in emerging research areas are discussed. For additional information about the development of holography, the readers are referred to other publications (Watson, 2005, 2011), where an overview of the technique, with a focus on different systems developed at the University of Aberdeen over the decades is provided. While these reviews highlight developments in holography for aquatic applications prior to 2011, their main focus is on the system design and processing approaches. Furthermore, marked improvements in the technology and the development of several commercial holographic systems have led to its increased use in the last decade for a variety of scientific applications, which will be covered here. Lab-based studies (e.g., Sheng et al., 2007; Hong et al., 2012) as well as flow through based approaches (e.g., Yourassowsky and Dubois, 2014; Zetsche et al., 2014) characterizing aquatic particles/plankton find a fleeting mention but are generally out of the purview of this article. Hologrammetry used as a tool for underwater inspections of offshore installations (e.g., Foster and Watson, 1997) is also not covered here. Historically speaking, holography in the aquatic sciences has been predominantly used for marine applications, and this fact is reflected in the material covered in the review. While all these applications are easily transferable to limnological research, prior field studies in lake environments have been scarce and are only touched upon briefly.
Aquatic Particles and in situ Measurement Techniques
A diverse range of planktonic species, marine snow, detrital matter, sediments, microplastics, and fecal pellets constitute the particle population in the world’s water bodies (Lal, 1977; Turner, 2015). Together, these particles and their interactions with the local environment, affect a range of processes across vast spatial and temporal scales, with consequence to marine ecology, human health, climate change, coastal engineering, ocean optics, acoustics, and remote sensing. For example, healthy phytoplankton and zooplankton populations are critical to sustaining aquatic ecosystems (Tett et al., 2008); conversely, abnormal and drastic increases in plankton abundance can lead to massive harmful algal blooms (HABs), which can severely impact aquatic ecosystems, human health and local economies (Anderson et al., 2002; Carmichael and Boyer, 2016; Glibert et al., 2018). Under favorable conditions, plankton can accumulate in enhanced concentrations to form “thin layers,” which can enhance foraging success rates for fish and other species (Sullivan et al., 2010a). The presence (or absence) of particles, and their composition, concentration and orientation, can alter optical and acoustic propagation through the ocean (Sullivan et al., 2005; Holliday et al., 2009; Basterretxea et al., 2020). Sinking particles are major pathways of carbon transport into the ocean’s interior and important to understanding the ocean carbon budget, and consequently, climate change research (Turner, 2015; Briggs et al., 2020). Sediment suspension and transport influence key coastal processes (Conley et al., 2012). Thus, developing and utilizing methods and instrumentation to quantify particle composition, distribution and particle-flow interactions have been an important focus of the aquatic sciences community for decades.
Among these methods, in situ imaging provides a direct way to record particle characteristics in their natural environment. A more comprehensive review on the field of underwater optical imaging can be found elsewhere (Dahms and Hwang, 2010; Jaffe, 2014). To date, different imaging techniques used by aquatic scientists include, but are not limited to, lidar imaging (Churnside and Wilson, 2004; Busck, 2005; McKenzie et al., 2020), bulk and planar laser-induced fluorescence (Prairie et al., 2011; Jaffe et al., 2013), laser sheet reflective imaging, e.g., Laser Optical Plankton Recorder (Herman et al., 2004; Checkley et al., 2008), underwater light microscopy (Mullen et al., 2016), imaging-in-flow cytometry (Olson and Sosik, 2007), silhouette photography (Milligan, 1996), and holography (Katz et al., 1999; Watson, 2011). Among these, holography can provide 3-D spatial distributions of particles in the micron to centimeter range, within a freestream sample volume, thus enabling mapping of particle characteristics at high resolution without fragmenting them. Furthermore, as opposed to methods like flow cytometry, much larger volumes can be sampled.
Broadly speaking, the applications of holography toward particle characterization can provide the following information: (a) Particle counts and size distributions; (b) Particle shape metrics, including cross-sectional area, major and minor axis lengths, and aspect ratio; (c) 3-D spatial structure of the particle field, e.g., nearest neighbor distances (NNDs) and particle orientation; (d) Particle identification and classification; (e) Particle interactions, including aggregation, settling speed, predator-prey behavior, and organism swimming trajectories; and (f) 3-D velocity distributions by integrating particle image velocimetry with holography (HPIV).
Holography
Briefly, holography involves the recording of an interference pattern (hologram) of the diffracted light field from particles in a given volume illuminated by a coherent light source (e.g., laser) and the reference beam (Schnars and Jüptner, 2002; Katz and Sheng, 2010). The hologram encapsulates the information about the phase and intensity of the diffracted light field. Direct visual observations of a recorded hologram would typically not produce any useful information, unless a particle is very close to the hologram plane; rather, optical or numerical reconstruction techniques are required to elicit information about in-focus particles in different 2-D cross-sections within the 3-D sample volume. Based on the geometric configuration of the object and reference beams, holography can be further classified as inline and off-axis holography. In the inline configuration, the reference and object beams are parallel to each other. Here, the sample volume is illuminated by a coherent beam of light, and the interference pattern recorded is caused by the portion of the beam scattered by the particles and the undisturbed part of the same beam. In the off-axis configuration, the object and reference beams are at an angle to each other. Inline geometric configurations are relatively simple, especially in the context of field instrumentation design; thus, most (but not all) in situ holographic systems have opted to use the inline setup. Readers are referred to other review articles or texts on holography for a more detailed explanation on different geometrical configurations (Vikram, 1992; Murata and Yasuda, 2000; Pan and Meng, 2003; Katz and Sheng, 2010; Picart and Montresor, 2020).
History of Development of Holography for in situ Aquatic Applications
The history of holographic development and in situ applications for marine sciences can be broadly divided into the pre-digital (e.g., recording on photographic plates) and the digital eras (Figure 1). Two related technological developments (lasers and digital cameras) have led to separate inflection points four decades apart, each leading to a subsequent surge in diversifying scientific applications of holography. While holography was introduced in 1948, it was only in the 1960s, that the emergence of lasers as a source of coherent light led to significant progress and expansion of the scope of holography. To the best of our knowledge, the potential of holography toward oceanographic applications was first demonstrated by Knox (1966), wherein a lab-based inline setup was used to image planktonic organisms (also see Beers et al., 1970). This was later extended to recording high-speed holographic movies of plankton (70 frames per second), where all particles >10 μm were resolved (Knox and Brooks, 1969). Stewart et al. (1973) developed and deployed the first known submersible in situ holographic imaging system, which weighed ∼1000 kg and could sample approximately 100 L of water per hologram. Further modifications to this system followed, with an off-axis configuration developed by Heflinger et al. (1978). Over the next decade and a half, several groups led other early efforts toward developing holographic imaging systems for diverse applications, including plankton characterization, studying cavitation nuclei and particle size, and settling rates in the ocean (Carder, 1979, Carder et al., 1982; Katz et al., 1984; O’Hern et al., 1988; Costello et al., 1989). A remotely operable inline submersible holocamera with an adaptable optical configuration (i.e., capability to switch between in-line and off-axis modes) was developed by Katz et al. (1999), with multiple deployments of the same instrument reported in other studies (Malkiel et al., 1999, 2006). Submersible holocameras which recorded both inline and off-axis holograms simultaneously were reported by Watson et al. (2001) and Hobson and Watson (2002). The readers are also referred to Watson (2005) and Hobson and Watson (2002) for further details on historical film-based holographic imaging systems. While film-based holographic systems used powerful, pulsed lasers that could sample very large volumes of water at high resolution, the systems themselves were bulky, physically unwieldy and challenging to deploy (e.g., Katz et al., 1999). Also, hologram processing was done manually, which is exceedingly cumbersome; for reference, processing one hologram could take several days, an unimaginable scenario in today’s big data world. Malkiel et al. (2004) reported improvements in processing methods with the development of an automated approach; however, this still required 5 h to process a 500 mL sample volume over one hologram. Furthermore, film-based approaches severely constrained the number of holograms that could be recorded, with these data limitations oftentimes leading to a paucity of data to draw conclusions from. For example, Katz et al. (1999) reported the recording of 300 holograms during each deployment of the submersible system. To put this in context of typical sampling rates of 15 Hz in digital holographic systems today, 300 holograms correspond to data recorded across a 20 s time interval. An instrument can record 9,000 holograms over a 10 min vertical profile, albeit with much smaller sample volumes for each hologram as opposed to film-based systems (between 1–10%).
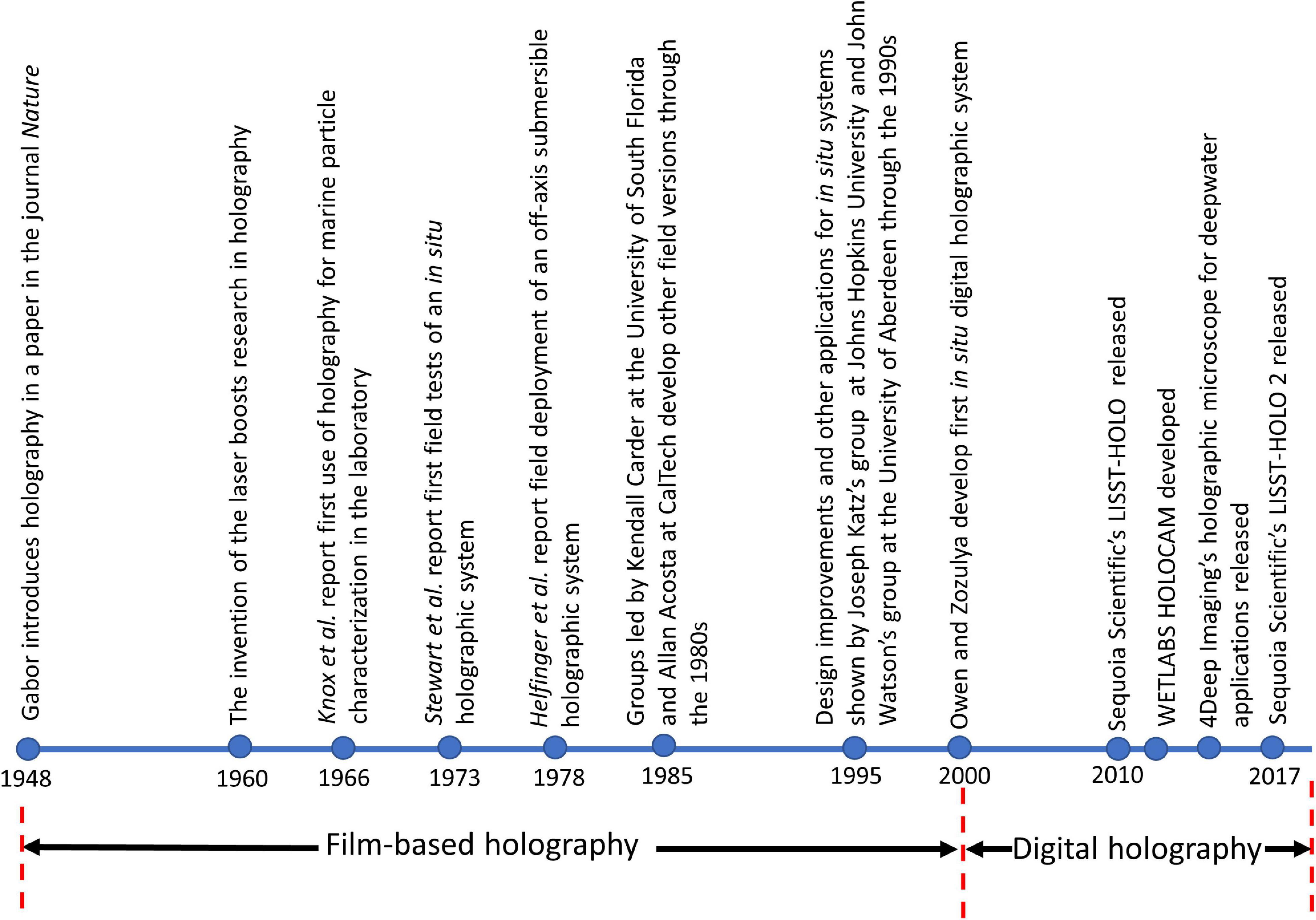
Figure 1. A timeline of the historical developments in holography in the context of in situ aquatic applications.
Around the turn of the century, with the development of digital cameras as well as increasing miniaturization of lasers, the second burst in advancement in underwater holographic imaging systems occurred. The first known digital in situ holographic system was developed by Owen and Zozulya (2000), where they employed a 10 mW diode laser to image over a ∼25 cm deep depth of field, resolving particulate sizes down to 5 μm. Jericho et al. (2006) developed a holographic microscope that used point source illumination to record marine organisms in situ. Bochdansky et al. (2013) developed the first deep-sea holographic microscope rated to 6000 m water depth. Other successfully deployed in situ holographic sensors with different optical setups and varying sampling parameters include those by Pfitsch et al. (2005, 2007), Sun et al. (2008), Graham et al. (2012), Talapatra et al. (2012, 2013), Dyomin et al. (2019, 2020), and Nayak et al. (2020). Table 1 provides further details on some selected systems. The last decade has also seen the development of a handful of commercial holographic imaging systems, including Sequoia Scientific’s LISST-HOLO (Davies and Nepstad, 2017; Ouillon, 2018), 4Deep Imaging’s holographic microscope (Rotermund et al., 2016) and the WET Labs HOLOCAM (Moore et al., 2017, 2019; Nayak et al., 2018a, b). These developments leave the scientific community poised to take further advantage of the holographic technique in the near future.
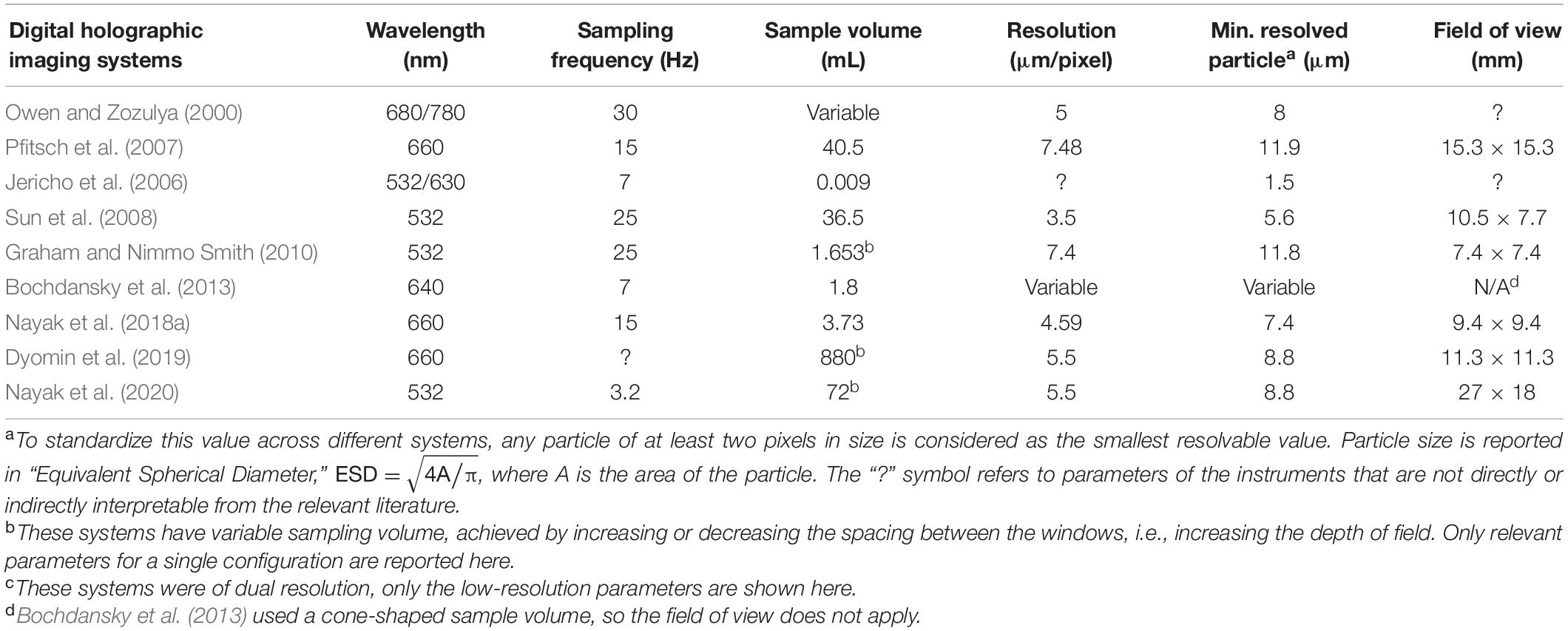
Table 1. Sampling characteristics of selected free-stream, digital holographic imaging systems reported in literature since 2000.
Holographic Data Processing
Digital holographic data processing can be a computationally expensive process, which is a function of several parameters. Briefly, the image processing steps for each hologram can be sub-divided as follows: (a) Image pre-processing; (b) Hologram reconstruction; (c) 3-D segmentation and/or image plane consolidation; and (d) Particle feature extraction. Pre-processing of raw holograms is required to eliminate any nonuniformities associated with uneven laser beam illumination and dust or other unwanted particles on the windows. Typically, this is achieved by computing the average background intensity calculated from a sequence of holograms and subtracting this from each single one (Talapatra et al., 2013; Nayak et al., 2018a). Alternately, a reference background hologram can be recorded just prior to data acquisition and then subtracted from each of the holograms to generate a “contrast hologram” (Bochdansky et al., 2013). A further pre-processing step could include grayscale histogram equalization (Toloui and Hong, 2015). After suitable pre-processing, the hologram reconstruction is carried out using numerical techniques, usually the Kirchoff-Fresnel transform, which is mathematically described elsewhere (Owen and Zozulya, 2000; Katz and Sheng, 2010). Reconstruction at different depths (or planes) brings all particles in each particular plane in focus over the entire 3-D sample volume. Typically, hologram reconstruction is the most memory intensive step in the entire processing routine. After reconstruction, the task is to isolate and label in-focus particles. 3-D segmentation can be directly implemented on the reconstructed stack to achieve this, providing in-focus locations of particles (Katz and Sheng, 2010). In cases where the particle field is sparse and the objects are compact, the stack can first be consolidated into a single composite image, because the in-focus images do not overlap one another, thus expediting processing. Further operations could include median filtering, image thresholding and segmentation and defining regions of interest (Jericho et al., 2006; Graham and Nimmo Smith, 2010; Bochdansky et al., 2013; Talapatra et al., 2013; Nayak et al., 2018a). All these steps are then repeated for each hologram in the dataset to generate a distribution of particles for further analysis. Figure 2 shows the steps involved in the holographic processing routine. Figure 3 shows a collage of planktonic images from processed holograms collected in diverse environments using the WET Labs HOLOCAM.
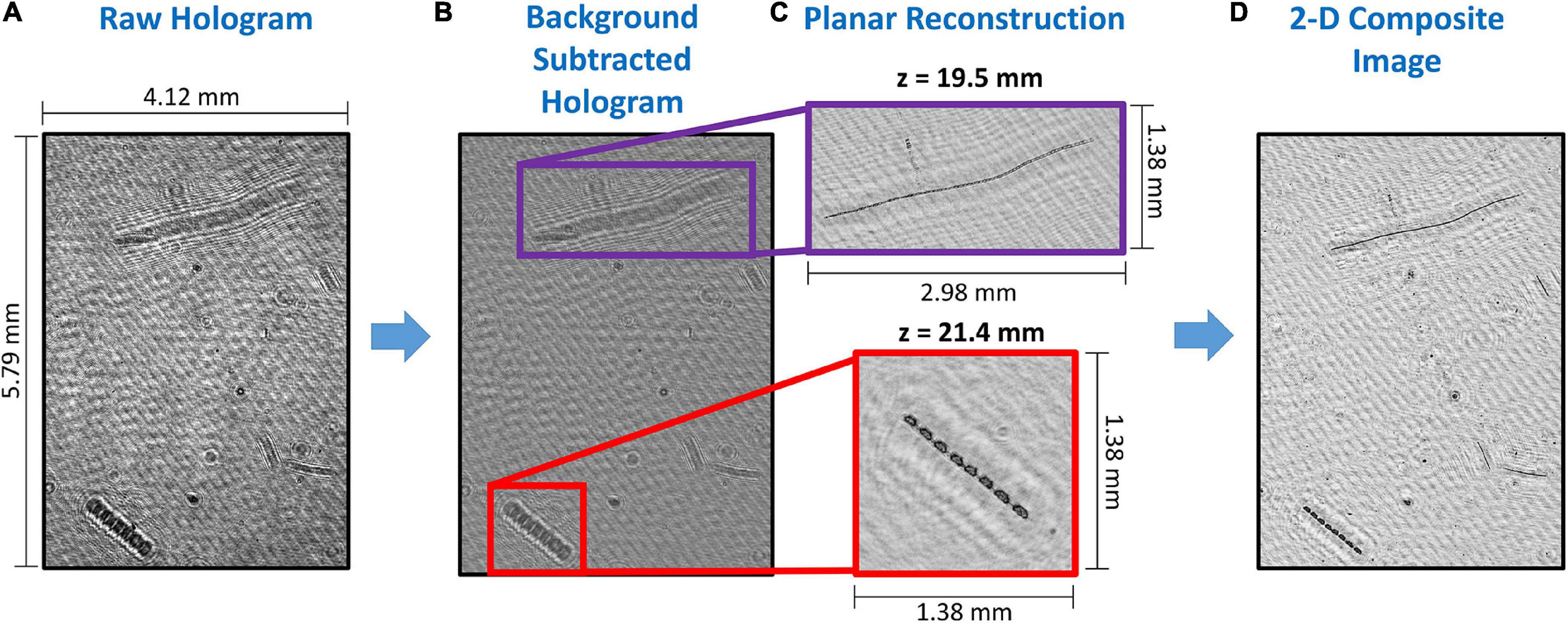
Figure 2. An illustrative example showing the different steps in holographic data processing: (A) A sample raw unprocessed hologram; (B) The same image after background subtraction, with regions of interest highlighted, and showing only interference patterns; (C) The in-focus diatom chains at different reconstruction depths (z) within the sample volume in the highlighted region; and (D) The 2-D composite image, where all in-focus particles are collapsed onto the same plane for further analysis.
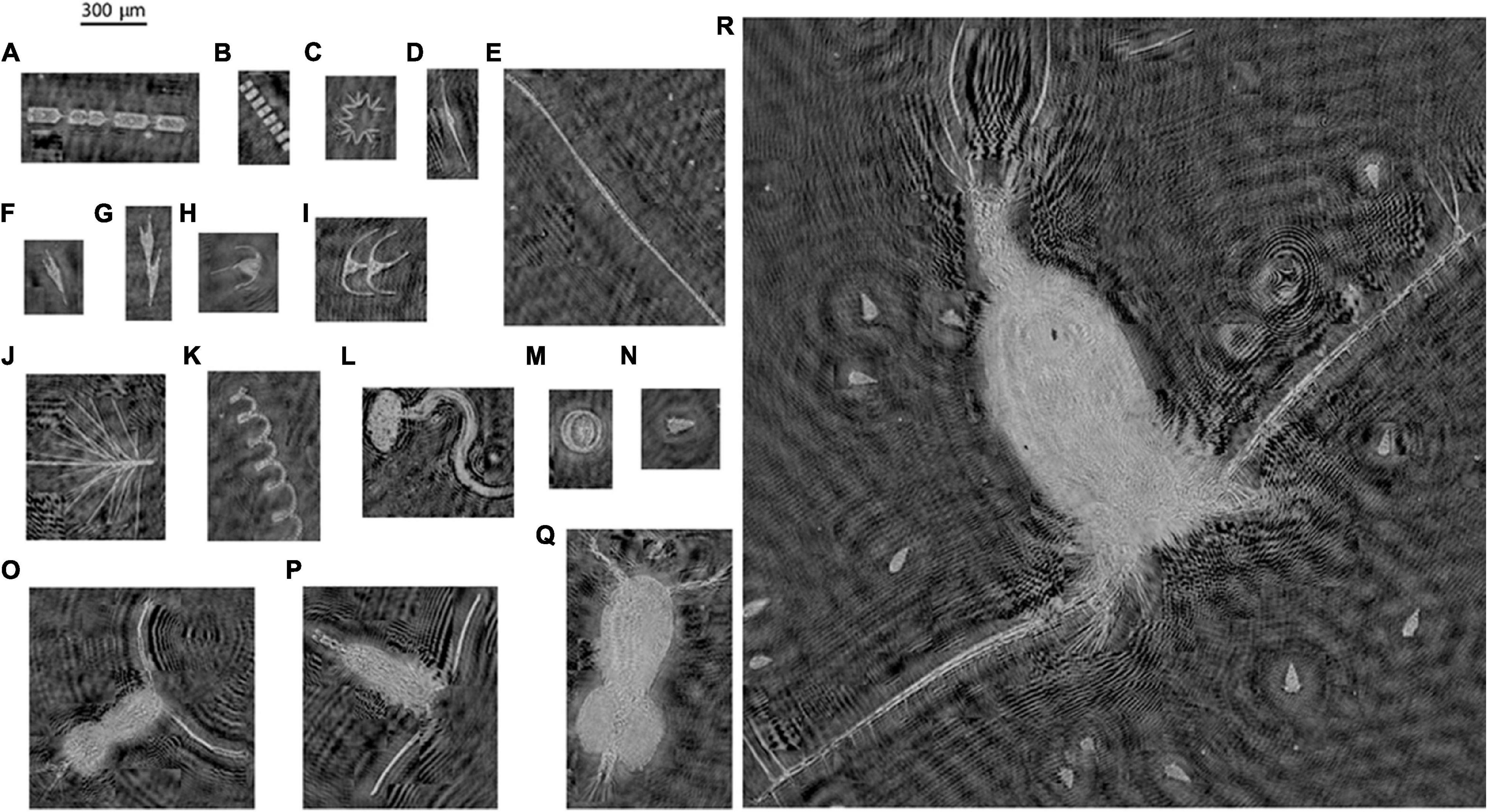
Figure 3. A collage of different plankton recorded from diverse environments on field cruises in the Atlantic Ocean (Delaware shelf), the Gulf of Mexico and East Sound, Washington, a coastal fjord in the Pacific Northwest. (A) Ditylum brightwelli; (B) Thalassiosira sp.; (C)Thalassionema sp.; (D) Tripos cf. fusus; (E) Unidentified colony; (F) Tripos cf. furca; (G) Tripos cf. furca, dividing cell; (H) Tripos muelleri; (I) Tripos sp. dividing; (J) Chaetoceros sp.; (K) Chaetoceros cf. debilis; (L) Appendicularian; (M) Coscinodiscus sp.; (N) cf. Strombidium sp.; (O) Calanoid copepod with egg sac; (P) Calanoid copepod; (Q) Cyclopoid copepod with paired egg sacs; and (R) Unidentified large copepod, potentially feeding on cf. Strombidium sp.
Sample Applications of Holography in Aquatic Research
Aquatic Particle Characterization
Particle Size Distributions
The characterization of spatial and temporal particle size distributions (PSDs) is vital to many oceanographic research topics. For example, PSDs are an important parameter in sediment transport studies (Mikkelsen and Pejrup, 2001; Conley et al., 2012). Physical processes such as molecular diffusion, turbulence, and settling, lead to aggregation and fragmentation of particles which are reflected in variations in the PSDs; this is important in the quantification of particle fluxes in the ocean (Jackson et al., 1997; Sullivan et al., 2005). PSDs can also affect light absorption and scattering in the ocean (Ulloa et al., 1994; Jonasz and Fournier, 2007). Phytoplankton functional type variability, of relevance to global biogeochemical models, are assessed using modeled PSDs (Kostadinov et al., 2010; Stemmann and Boss, 2012). While some of the seminal work on oceanic PSDs have employed an in situ imaging system (Underwater Video Profiler), sometimes in conjunction with a shipboard particle counter, to resolve a broad size spectrum (Stemmann et al., 2000, 2002, 2008), PSDs are usually estimated from direct samples collected and processed in the laboratory using instruments such as Coulter counters and flow cytometers (Jackson et al., 1997; Boss et al., 2001; McFarland et al., 2015). Inherent biases exist in inferring PSDs from discrete samples, primarily because particles get fragmented during the collection, sampling, and analysis process, potentially leading to an enhanced concentration of smaller particles. Indirect methods focus on employing models to invert acoustic or optical scattering to infer PSDs (e.g., Sequoia Scientific’s LISST series of instruments), and may require some a priori knowledge of particle shape and scattering properties. Thus, validation by direct imaging is recommended to ensure fidelity in measurements (Graham et al., 2012).
Aquatic PSDs are most commonly described using a simple power law, also referred to as a “Junge type” distribution, because such a relationship is generally representative in diverse environments for smaller particles <100 μm that are important for aquatic optical properties (Kitchen et al., 1982; Buonassissi and Dierssen, 2010). Here, the PSD shape is embodied in a single parameter, i.e., the exponent, which is convenient for modeling and inversions (Fournier and Forand, 1994; Boss et al., 2001; Twardowski et al., 2001). While particles <100 μm control optical properties such as scattering and absorption, larger particles can affect diffraction at very near-forward angles. More complex models such as the two-component gamma (Risović, 1993) have also been developed to describe oceanic PSDs. The wide particle size range that can be mapped by holography (few microns to cm) makes it an attractive technique for direct in situ PSD measurements.
A few studies have compared PSDs obtained from holographic systems and other instruments. For example, O’Hern et al. (1988) evaluated PSDs using holographic and Coulter counter measurements. It was reported that the Coulter counter consistently under-estimated the PSDs, with the authors concluding that this most likely occurred due to assumptions inherent in the Coulter counter calibration procedures. Kumar et al. (2020) compared PSDs estimated using a laser diffraction based approach and holography, with a lab-based setup to characterize spray droplets. Here, it was found that the PSDs were under-estimated in the laser diffraction based approach for droplet sizes higher than 1 mm. Graham et al. (2012) co-deployed a LISST-100X alongside a holographic system, where they reported that in the overlapping size ranges, the LISST-100X showed higher particle concentrations, especially in smaller size bins. They suggest that in particle fields dominated by non-spherical particles, the LISST-100X might report a single large particle as several individual particles, thus skewing the PSD. While the above studies use holography as a standard to compare other instrumentation, a study by Walcutt et al. (2020) is also pertinent to this discussion. Here, a 4Deep holographic microscope was simultaneously used along with a FlowCam, Imaging Flow Cytobot (IFCB), and standard microscope, to compare PSDs. Ocean water samples, lab-grown cultures and microspheres provided diverse datasets to characterize PSDs across these methods. After appropriate post processing corrections, it was found that holographic measurements under-estimated the PSD by about 3–10%. However, the slopes of the distributions remained comparable. The authors list several potential factors, e.g., nonuniform illumination of the sample volume, which might possibly need to be accounted for to gain higher statistical confidence in inferring particle concentrations and distributions from holographic data.
Bochdansky et al. (2016) reported the particle distributions in bathy- and abyssopelagic waters (up to 5,500 m depth) at several stations in the North Atlantic Ocean, using a digital inline holographic microscope. A majority of the particles seen in the holograms consisted of marine snow, fecal pellets, phytoplankton, and other detrital matter. When all other particles excluding marine snow were considered, they fit a typical oceanic PSD, with a systematic decrease in particle counts in the larger size bins. However, when only marine snow was considered, the particle counts across the size bins did not exhibit any specific trends, such as a skew toward the smaller sizes. For all particles, including marine snow, the PSD slope deviated significantly from an expected Junge distribution above a certain size threshold (∼380 μm). The authors termed particles that were significantly more frequent than expected from the frequency distribution of smaller particles as “dragon kings.” Aggregation is an important process that can skew PSDs and cause deviations from a simple power law (Boss et al., 2009; Hill et al., 2011).
Similar trends in deviations of the PSDs from expected profiles have been reported by other studies in diverse aquatic environments. Nayak et al. (2018a) analyzed the small-scale PSD variations with depth in a coastal fjord, where a holographic system was slowly profiled through a ∼25 m deep water column. Here, the particle field was dominated by diatoms, and size was represented by considering the equivalent size diameter (, where A represents the measured area of the particle). At shallow depths, a broad peak in the PSD was observed, centered around 300 μm, which corresponded to an enhanced abundance in diatom chains in that size range (Figure 4A). Across stations and depths, PSD slopes presented two linear ranges, with a shallower slope (−1.7 to −1.9) fitting the 50–250 μm range. For particles above 250 μm, slopes were much steeper (−5.7 to −6.1). Moore et al. (2017) reported the deployment of a holographic system to characterize PSDs across different stations in western Lake Erie during a cyanobacterial bloom. In locations dominated by detrital particles, the mean PSD slopes were ∼−3.45 across depths. At stations where the particle field was dominated by the cyanobacteria Microcystis aeruginosa which forms amorphous colonies, the PSDs show a pronounced bump, broadly between 200 and 500 μm, with overall mean slope ∼−2.3 (Figure 4B). Modeling studies characterizing colony size and associated vertical migrations have previously shown that a colony ∼250 μm in size corresponded to the highest migration ranges (Visser et al., 1997). Finally, at stations dominated by a second, morphologically distinct (elongate) cyanobacteria, Planktothrix aghardii, similar PSD slopes to the M. aeruginosa dominant waters were observed, albeit with the absence of the bumps (Figure 4C). These different studies in diverse conditions demonstrate the feasibility of using holography to characterize fine-scale PSDs in the water column and highlight trends which other methods might miss. It should be noted that while oceanic bubbles do not fall under the general ambit of particles being discussed in most of this paper, there is a substantial community focusing research efforts in this direction. Acoustics and optics remain the preferred method of characterization for large and small bubbles, respectively (Czerski et al., 2011; Twardowski et al., 2012). Holography has been successfully demonstrated to be applicable to in situ bubble research by at least two previous studies (O’Hern et al., 1988; Talapatra et al., 2012).
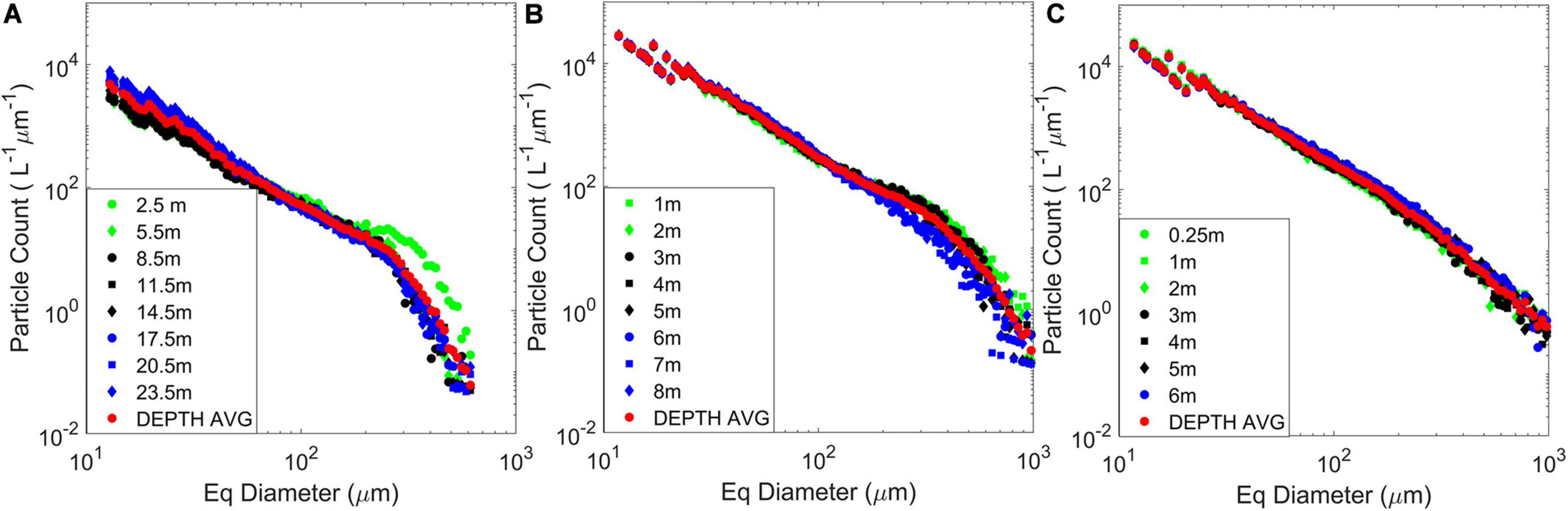
Figure 4. Depth-binned and depth-averaged particle size distributions (PSDs) obtained from vertical profiles of a holographic imaging system during two field experiments at East Sound, Washington, in September 2015 and at Lake Erie in August 2014. Figure adapted from data presented in Moore et al. (2017) and Nayak et al. (2018a; 2018b). (A) The East Sound profile was recorded during the presence of a “thin layer” of diatoms, dominated by Ditylum brightwelli; (B) Data recorded during a Microcystis aeruginosa bloom in western Lake Erie; and (C) PSDs at Lake Erie in a water mass dominated by the cyanobacteria Planktothrix aghardii.
Particle Settling Velocities
Particle size and density directly affects sinking rates and transport in the water column (McDonnell and Buesseler, 2010; Bach et al., 2012). In the open ocean, particle sinking is an important pathway for carbon sequestration to the ocean’s interior (Trull et al., 2008; Fischer and Karakas, 2009; Nayak and Twardowski, 2020), while settling rates are critical to inform coastal studies involving sediment suspension and transport (Agrawal and Pottsmith, 2000). Carder et al. (1982) integrated a holographic setup into a free-floating sediment trap to quantify settling rates of individual particles in the water column, with holograms recorded at discrete intervals. The processed data provided size distributions of particles >15 μm. Some of the faster sinking particles were segregated to compute attributes: observed particle densities ranged from 1370–5100 kg/m3, with associated settling velocities corresponding to daily excursions of 16–199 m across the particle spectrum. This was the first demonstration of holography for oceanic particle settling analysis, with results corroborated from microscopic analysis of the particles collected in the sediment trap. Graham and Nimmo Smith (2010) demonstrated the applicability of holography to study suspended sediment size distributions and settling velocities in the coastal ocean. Automated tracking software, incorporating parameters such as particle area and major and minor axis lengths was used to identify a particle across different frames, thus facilitating computation of trajectories and consequently velocities. Cross et al. (2013) studied particle resuspension in the context of the relation between turbulence and particle size. Holographic data was used to identify suspended particle matter and characterize variations in their size distribution over different tidal cycles. These data were compared to turbulence measurements obtained from a microstructure profiler; based on their observations, the authors postulated that the growth and size of flocs are dependent not only on turbulence, but also biological controls.
Particle Orientation
In atmospheric studies, preferential (or non-random) orientation of particles and ice crystals in clouds has been relatively well-documented (Ono, 1969; Sassen and Takano, 2000; Noel and Chepfer, 2004). Only fairly recently has this area of research gained traction in oceanography. The consequences of particle orientation are significant to optical oceanographers as most models assume that particles are randomly oriented in the ocean (Bohren and Huffman, 1983; Jonasz and Fournier, 2007). In aquatic ecology, preferential horizontal orientation of phytoplankton chains could potentially increase light capture and thus enhance primary productivity (Sun et al., 2016; McFarland et al., 2020). Several laboratory and modeling studies have shown that particle and plankton orientation is a function of the small-scale flow physics (Karp-Boss and Jumars, 1998; Karp-Boss et al., 2000; Marcos et al., 2011) and influences light propagation (Marcos et al., 2011; McFarland et al., 2020). Basterretxea et al. (2020) provide a useful overview of phytoplankton orientation in the ocean, laying it in the context of microscale plankton-flow interactions. While recent innovative approaches at inferring in situ particle orientation from indirect scattering measurements have been reported (Font-Muñoz et al., 2019, 2020), direct imaging still provides the best means to do so.
Most reports to date on particle orientation in aquatic environments have used holographic systems. To the best of our knowledge, the first, albeit limited, direct observations on non-random particle orientation in the ocean were reported by Malkiel et al. (1999). From a sample of 300 holograms recorded during a vertical scan of the water column, the majority of diatom chains present (Chaetoceros spp. and Ditylum sp.) at a certain depth (15.3 m) were found to be preferentially oriented within ±15° to the horizontal. Talapatra et al. (2013) documented the occurrence of enhanced preferential horizontal orientation of colonial diatom species (primarily Chaetoceros debilis and Chaetoceros radicans) at several distinct depths in two vertical profiles. A pulsed laser acting as the illumination source allowed for particles to be tracked over successive frames, enabling co-located small-scale flow and turbulence measurements, which indicated that regions of enhanced preferential particle concentrations coincided with regions of low turbulent kinetic energy (TKE) dissipation. A comprehensive set of measurements of oceanic particle orientation were later provided by Nayak et al. (2018a). A bio-optical profiling package consisting of a holographic system, an acoustic Doppler velocimeter, and a CTD, among other instruments, was gently profiled through the water column at several stations to simultaneously quantify particle orientation, small-scale shear, and TKE dissipation rates with depth. Across all stations, preferential horizontal orientation was observed at several depths, each time corresponding to regions of low shear and TKE dissipation rates. Further analysis highlighted the relation between instantaneous orientation of a particle and its aspect ratio (ratio of the major axis length to the minor axis length of a particle). In simple 2-D laminar shear flow, Jeffrey (1922) derived a set of relations for spheroidal particles, showing that they exhibit periodic flipping motions, where higher aspect ratio particles spend more time oriented in the horizontal direction; in situ data presented in Nayak et al. (2018a) were in reasonable agreement with Jeffrey’s theory.
Plankton Distributions and Patchiness
In situ observations of plankton distributions, behavior and interactions with the natural environment provide valuable insights into the functioning of aquatic ecosystems. It has been well-established that plankton distributions in aquatic systems tend to be incredibly “patchy,” i.e., non-homogeneous (Durham and Stocker, 2012; McManus and Woodson, 2012; Prairie et al., 2012; Graff and Menden-Deuer, 2016). There exists a plethora of possible mechanisms for the formation of these patches across different spatial scales, including small-scale shear, horizontal and vertical mixing, and enhanced nutrient availability (Powell and Okubo, 1994; Abraham, 1998; Sullivan et al., 2010a; Breier et al., 2018). Examples of plankton patchiness include algal blooms (harmful or otherwise) and phytoplankton and zooplankton “thin layers,” which are discussed further below.
Phytoplankton Blooms and Vertical Distributions
Large phytoplankton blooms covering several hundreds of kilometers occur annually in different gyres, sometimes tied with the formation and transport of mesoscale eddies (Guidi et al., 2012). Anderson et al. (2018) deployed Sequoia Scientific’s LISST-HOLO on a neutrally buoyant towed body from an autonomous vehicle to characterize a summer phytoplankton bloom in the North Pacific subtropical gyre, which was dominated by several species of colonial diatoms. Holographic images were processed to obtain cell and aggregate counts of Hemiaulus and Rhizosolenia sp. over the course of the campaign. These observations showed that the overall bloom was comprized of two distinct phases, where either species dominated; simultaneous large abundances of both taxa seldom occurred. Similarly, Bochdansky et al. (2017) reported the particle composition in the Ross Sea in Antarctica, where massive phytoplankton blooms occur seasonally, connecting the distribution of important phytoplankton species to carbon export. In particular, large scale sinking of Phaeocystis antarctica colonies from the surface to the deep layer below the pycnocline was noted. These observations were made during the weakening of the pycnocline, thus establishing the significant contribution of Phaeocystis colonies to total carbon export, even during the non-bloom season.
In a limnological study, Moore et al. (2017, 2019) used holography to characterize phytoplankton community composition during a HAB event in western Lake Erie, where the particle field was dominated by two cyanobacterial species, M. aeruginosa and P. aghardii. The profiling system enabled characterization of vertical distributions of colony and cell counts of either species. A majority of Microcystis colonies were found to be located near the surface, while Planktothrix tended to be located in larger numbers deeper in the water column, and the vertical variability was correlated to the light availability and tolerance of either species. All these studies further highlight the ability of in situ holography (and direct imaging in general) to tease out small-scale patterns and variations between distinct particle and plankton populations which could help scientists to better understand ecosystem dynamics as well as inform robust modeling efforts.
Thin Layers and Phytoplankton in Turbulence
The term “thin layers” refers to vertically limited (few cm to meters) dense aggregations of plankton which are temporally coherent, i.e., last for several hours to days (Sullivan et al., 2010a; Durham and Stocker, 2012). Manual sample collections at discrete depths oftentimes miss these finescale structures in the water column; high frequency in situ observations provide the best means to characterize them (Donaghay et al., 1992; Twardowski et al., 1999; Rines et al., 2002). Previous field studies have shown that thin layers are frequently present in coastal waters, with their vertical location controlled by the physical structure and/or vertical migration of the organisms (Sullivan et al., 2010a). For a comprehensive study of thin layers, the readers are directed to Sullivan et al. (2010b) and articles within that special issue.
In a constrained coastal fjord at East Sound, Washington, where thin layers have been previously well-documented (Dekshenieks et al., 2001; McManus et al., 2003), Talapatra et al. (2013) deployed a holographic system to characterize the small-scale biophysical interactions and particle populations. A thin layer comprising of small, non-motile particles as well as larger colonies of the diatom Chaetoceros socialis were observed. Associated CTD profiles indicated that this layer was present at the base of a strong pycnocline. Zooplankton distributions showed that they seemed to avoid the thin layer. At the same geographical location, other studies reported the presence of a strong thin layer, predominantly containing the diatom Ditylum brightwellii, again coincident with the location of a near-surface pycnocline (Figure 5; Nayak et al., 2018a, b; McFarland et al., 2020). In all these reports, the water column was stably stratified, and the presence of the thin layer was associated with regions of low shear and TKE dissipation.
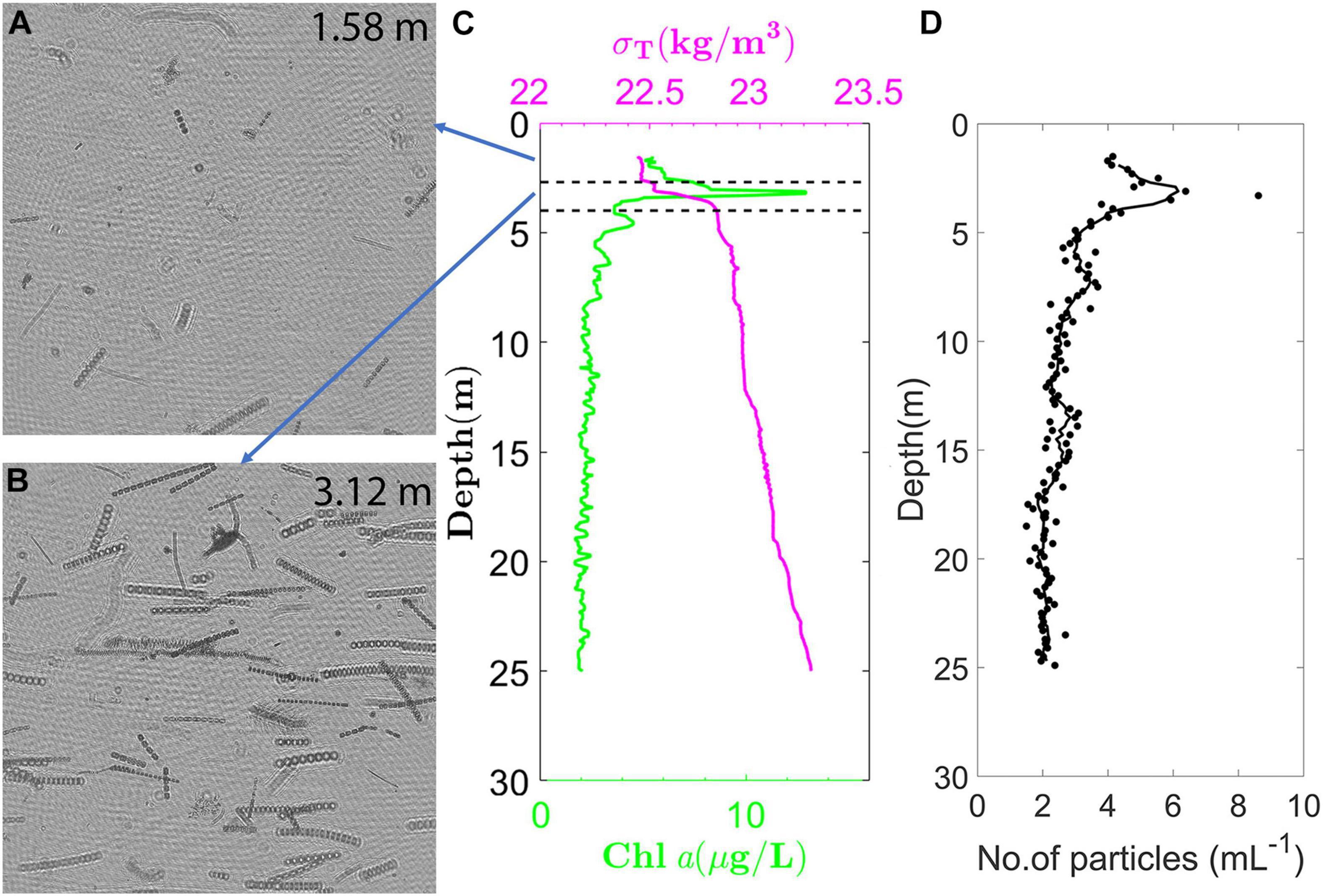
Figure 5. Holographic data acquired on September 22, 2015 during a vertical profile through a diatom thin layer at East Sound, Washington. Background subtracted holograms showing the representative particle fields (A) above the thin layer at 1.58 m depth; and (B) within the thin layer at 3.12 m depth; (C) Chl a and density profiles acquired during the same profile, showing the thin layer occurred in a stably stratified region within the pycnocline; and (D) Particle distribution with depth, where only particles >200 μm in length and aspect ratio >5 are considered, consisting of different elongate diatom species, and dominated primarily by Ditylum brightwelli. Figure generated from a dataset presented in Nayak et al. (2018a; 2018b).
Turbulence has long been known to play an important role in predator-prey encounters, enhancing phytoplankton nutrient uptake, as well as affecting growth rates (Karp-Boss et al., 1996; Sullivan and Swift, 2003; Durham et al., 2013); thus, there is a lot of interest in simultaneously quantifying plankton populations and turbulence in situ. Cross et al. (2014) used integrated holographic imaging and microstructure turbulence profiler measurements to study the effect of episodic and enhanced turbulent mixing on vertical distributions of colonial diatoms. Although limited to a single set of observations over a tidal cycle, enhanced mixing seemed to correlate with shorter chain lengths, with diatoms being transported to lower depths, where they were found to aggregate with other suspended matter. Nayak et al. (2018a; 2018b), albeit qualitatively, also report similar observations with chain lengths, demonstrating the importance of slowly profiling the water column with large instrument packages used in particle characterization studies. Vigorous mixing during fast profiles can lead to bias in observations, with enhanced concentrations of particles at lower sizes, possibly due to breakage of chains due to enhanced turbulence.
Nearest Neighbor Distances
Yet another interesting application of holography is the ability to characterize instantaneous 3-D spatial distributions across each individual hologram in a time series, thus allowing computation of vital statistics such as NNDs. These parameters allow for quantification of predator-prey interactions and population dynamics in the local community, which is of great interest to biological oceanographers and ecological modelers.
Malkiel et al. (1999, 2006) computed NNDs between particles detected in holograms by employing two-tailed hypothesis testing (Freedman et al., 2007). Qualitatively, differences were represented by looking at the histogram of NNDs of the sample and a random distribution. Quantitatively, a “z-score” was computed as where and N are the mean NND and the size of the sample, respectively, while and σR represent the mean and standard deviation of the NND obtained by a random distribution. If |z| > 2, the sample data are deemed different from a random distribution. Using this method, statistics were computed to study NNDs between individual particles (or plankton) within the same class, as well as different classes, to come up with the following observations: (a) Detrital particles exhibited clustering at certain depths below the pycnocline, while appearing to be randomly distributed elsewhere; (b) No significant clustering was found among dinoflagellates; and (c) No significant correlation between the location of copepods with respect to small particle clusters were detected. To the best of our knowledge, these are the only in situ holographic studies characterizing NNDs to date; further observations in different environments to better understand population dynamics are warranted.
Bacterial and Zooplankton Behavioral Studies
Holographic cinematography provides a way to visualize 3-D trajectories of multiple motile organisms, including zooplankton, simultaneously, as well as observe their natural behavior in situ. While this ability has been leveraged by several laboratory studies, far too many to list here in detail (e.g., Sheng et al., 2007; Hong et al., 2012), very few studies report this in the field. During deployment in a Lagrangian, drifting mode, Pfitsch et al. (2005) observed the behavior of swimming medusa jellyfish in situ, in the Ria de Pontevedra in coastal Spain. The same authors also recorded the swimming behavior of an appendicularian (Pfitsch et al., 2007). Appendicularians (or larvaceans) are filter feeders that generate a mucous “house” around them to trap particles. The long tail, flapping at a particular frequency, helps pump water through the house and facilitates their feeding (Alldredge, 1981; Selander and Tiselius, 2003). In this instance, the flapping frequency of the tail was found to be in the range of those observed in controlled laboratory measurements (Pfitsch et al., 2007). Other observations with the same instrument included interactions between copepods and dinoflagellates, which can provide pertinent data to modelers such as perception distances by predator/prey species in the turbulent environment. These observations include instances of copepods initiating jumps and orienting their feeding currents after prey and dinoflagellate behavioral changes (jumps and quick spiraling) to avoid a cyclopoid copepod that was cruising through their neighborhood (Malkiel et al., 2007). Jericho et al. (2006) also reported observations of the trajectory of organisms during a field deployment; however, the organisms were not clearly identifiable as they were at the edge of the detection limit of the instrument.
While not a free stream approach, Lindensmith et al. (2016) developed a flow-through based off-axis submersible holographic microscope, geared toward studying microbial motility in extreme, icy environments. During a deployment in Greenland over winter, brine samples were collected and inserted into the sampling chamber of the microscope, to characterize microbial motility. Analyzed samples recorded both prokaryotes and eukaryotes; motility was observed in only a few instances in either case. Swimming trajectories clearly recorded the motion of organisms, at swimming rates varying from 5–50 μm/s between different individuals. This highlights the ability of holographic microscopy for use in extreme environments, including in potential future extraterrestrial missions (Bedrossian et al., 2017).
Discussion
The previous sections have shown the applicability of holography toward diverse research areas in the aquatic sciences. Here, existing limitations, future potential applications and advancements of holographic technology in emergent aquatic research areas are outlined.
Limitations
The main limitation associated with holography is the depth-of-focus (DOF) problem, which refers to the depth over which a particle in the image tends to appear in focus in the axial direction (Vikram, 1992; Katz et al., 1999; Katz and Sheng, 2010). The DOF can be approximated based on the smallest particle diameter (d) resolvable and the wavelength of light used (λ), being proportional to d2/λ (Yang et al., 2005; Katz and Sheng, 2010). For example, an optical system capable of resolving particles sized 10 and 50 μm and above, will have a depth of focus of ∼0.16 and 4.17 mm, respectively, assuming the illumination source is red light (660 nm). Thus, while the particle location in the image plane can be detected fairly accurately, there is a certain degree of uncertainty associated with detecting the axial location (Gao et al., 2013).
The quality of recorded holograms (as with other optical methods) also depends, to a certain extent, on the turbidity and particle size and abundance within the water column. In-line systems in particular are susceptible to loss of image quality for particle concentrations beyond certain thresholds (Katz et al., 1999). Furthermore, the presence of a large number of sub-micron particles below the resolution limit of typical holographic systems can lead to loss of coherency in the reference laser beam, thus leading to bad quality recordings. A related and important problem in holography is the inherent coherent noise, which includes (a) parasitic interference fringes, caused by multiple reflections or scattering (Pan et al., 2017); and (b) speckle noise, resulting from scattering by particles larger than the wavelength of the laser in dense particle fields (Meng et al., 1993; Garcia-Sucerquia, 2013). This can lead to serious degradation in image quality and holographic reconstructions. Typically, these noise reduction techniques involve engineering the coherent source (Garcia-Sucerquia, 2013; Pan et al., 2017), or optimizing numerical reconstruction schemes (e.g., Kosmeier et al., 2012; Leo et al., 2014). While a detailed discussion is beyond the scope, readers are also referred to Bianco et al. (2018), where different approaches to tackle the speckle noise problem in digital holography are reviewed.
The data-intensive nature of hologram acquisition and the extensive processing routines involved have so far limited the use of in situ holographic systems in long-term and/or real-time monitoring networks. For near real-time observations, data transmission is one of the main bottlenecks due to the large file sizes; rapid onboard processing to provide simplified data (e.g., organism presence and counts) in a compressed format, instead of transmitting entire images could help alleviate this problem. For this to work, is it key to develop not only fast and efficient automated classification algorithms, but also those feasible to be developed in a light-weight architecture (Guo et al., in press). Previous and ongoing efforts toward automated classification of detected particles using various machine learning techniques, including convolutional neural networks, still need holograms to be reconstructed and processed (Davies et al., 2015; Bianco et al., 2020). Recent work has focused on the application of deep learning techniques to extract features from the interference patterns recorded on the raw holograms (Guo et al., in press; Shao et al., 2020). Successful translation to in situ applications would help revolutionize the field, as this would enable skipping the holographic reconstruction step, greatly reducing processing times and facilitating near real-time data dissemination. To continue improving these algorithms, building enormous labeled datasets of hundreds of different particle and plankton types and morphologies from in situ deployments is critical.
Current Research and Future Frontiers
Holographic Particle Image Velocimetry (HPIV)
Different methods of particle tracking as well as 2-D and stereo PIV have been used to characterize small-scale 2-D and 3-D velocity distributions in aquatic environments (Nimmo Smith, 2008; Steinbuck et al., 2010; Nayak et al., 2015). Talapatra et al. (2013) used holographic data from in-focus particles at a particular plane to perform PIV and calculate the 2-D velocity fields (not 3-D measurements). The ability to simultaneously quantify particles and flow distributions within the same volume holds enormous appeal and would greatly help elucidate particle-flow interaction studies. In laboratory studies, HPIV has been successfully established as a valuable tool to characterize three-dimensional velocity measurements within a sample volume (e.g., Barnhart et al., 1994; Hinsch, 2002; Katz and Sheng, 2010). Translating this to field applications, while challenging, would be the next step.
Other potential directions include integrating holography with other methodologies to develop new techniques that could significantly enhance particle or plankton characterization capabilities. For example, the ability to quantify the refractive index (RI) of individual particles is valuable to optical oceanographers. Typically, the particle of interest is immersed in fluids of varying RIs and studied under a microscope (Hodgson and Newkirk, 1975). When the RI of the particle is the same as the fluid in which it is immersed, the particle becomes nearly invisible (or least opaque). This is a highly tedious process; thus, previous efforts have estimated the bulk RI of a water sample from scattering measurements (Carder et al., 1972; Twardowski et al., 2001). Holotomography has recently emerged as a tool to map high-resolution 3-D refractive indices of cells, including diatoms, in laboratory measurements (Charrière et al., 2006; Merola et al., 2017; Umemura et al., 2020), and has high potential to be transformed to in situ aquatic applications (Poulin and Zhang, 2020).
Microplastics
Microplastics have emerged as a primary source of pollutants in aquatic systems in recent times, leading to an increasing number of studies focusing on their presence, fate and transport in aquatic environments, and effects on aquatic ecosystems (Cózar et al., 2014; Zhao et al., 2018; Granek et al., 2020). Early estimates have shown that only a fraction of the known anthropogenic microplastic inputs into the oceans have been accounted for, leading to questions about the fate of the remaining portion. Consequently, improvements in monitoring and detection techniques of microplastics have become crucial to understanding this issue (Garaba and Dierssen, 2018; Mai et al., 2018). Recent laboratory studies have shown that holography can be a valuable tool in the detection of microplastics in the ocean (Merola et al., 2018; Bianco et al., 2020). Future efforts geared toward testing these in field environments are needed.
Long-Term and Real-Time Observation Networks
The democratization of oceanographic data access has received significant attention in the past decade; consequently, data collected from networks of floats, gliders, buoys, and other observation and monitoring platforms are freely available to scientists and the public. Examples include National Oceanic and Atmospheric Administration’s Integrated Ocean Observing System (IOOS) and Marine Biodiversity Observation Networks (MBONs), National Science Foundation’s Ocean Observatories Initiative (OOI), the Argo global network of profiling floats, etc. Other in situ imaging systems, such as the IFCB, are now being routinely used for real-time monitoring of plankton community composition as well as HAB events (Kudela et al., 2015). The versatile nature of holography allows for enough flexibility to be used in a variety of purposes, with appropriate modifications or trade-offs in resolution and image quality. For example, efforts to develop holographic imaging systems for long-term aquatic deployments with a wide particle resolution range (Nayak et al., 2020) can be complemented with low-cost robotic holographic samplers for environmental monitoring (Mallery et al., 2019). Future work should be geared toward integrating holographic systems into aquatic monitoring networks, e.g., MBONs and/or Argo floats. Continuing improvements in image processing methods, technological improvements in data transmission and storage, and an increasing focus on machine learning for automated particle and plankton classification are promising indicators of the aquatic sciences community being poised to embrace holographic systems on a large scale in the near future.
Author Contributions
AN conceived the manuscript and compiled the literature survey. AN, EM, MM, MT, and JS prepared the manuscript. All authors contributed to the article and approved the submitted version.
Funding
AN, MM, and JS were primarily supported through United States National Science Foundation awards OCE-1634053 and OCE-1657332. AN and MT were partially supported by the Harbor Branch Oceanographic Institute Foundation (HBOIF). AN was also supported by an Early Career Research Fellowship from the Gulf Research Program of the National Academies of Sciences, Engineering, and Medicine.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Gulf Research Program of the National Academies of Sciences, Engineering, and Medicine.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Lisa Nyman for her help in generating some of the figures used in this manuscript and Ranjoy Barua for useful inputs.
References
Abraham, E. R. (1998). The generation of plankton patchiness by turbulent stirring. Nature 391, 577–580. doi: 10.1038/35361
Agrawal, Y., and Pottsmith, H. (2000). Instruments for particle size and settling velocity observations in sediment transport. Mar. Geol. 168, 89–114. doi: 10.1016/s0025-3227(00)00044-x
Alldredge, A. L. (1981). The impact of appendicularian grazing on natural food concentrations in situ. Limnol. and Oceanogr. 26, 247–257. doi: 10.4319/lo.1981.26.2.0247
Anderson, D. M., Glibert, P. M., and Burkholder, J. M. (2002). Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25, 704–726. doi: 10.1007/bf02804901
Anderson, E. E., Wilson, C., Knap, A. H., and Villareal, T. A. (2018). Summer diatom blooms in the eastern north pacific gyre investigated with a long-endurance autonomous surface vehicle. PeerJ 6:e5387. doi: 10.7717/peerj.5387
Bach, L. T., Riebesell, U., Sett, S., Febiri, S., Rzepka, P., and Schulz, K. G. (2012). An approach for particle sinking velocity measurements in the 3–400 μm size range and considerations on the effect of temperature on sinking rates. Mar. Biol. 159, 1853–1864. doi: 10.1007/s00227-012-1945-2
Barnhart, D. H., Adrian, R. J., and Papen, G. C. (1994). Phase-conjugate holographic system for high-resolution particle-image velocimetry. Appl. Opt. 33, 7159–7170. doi: 10.1364/ao.33.007159
Basterretxea, G., Font-Munoz, J. S., and Tuval, I. (2020). Phytoplankton orientation in a turbulent ocean: a microscale perspective. Front. Mar. Sci. 7:185. doi: 10.3389/fmars.2020.00185
Bedrossian, M., Lindensmith, C., and Nadeau, J. L. (2017). Digital holographic microscopy, a method for detection of microorganisms in plume samples from enceladus and other icy worlds. Astrobio 17, 913–925. doi: 10.1089/ast.2016.1616
Beers, J. R., Knox, C., and Strickland, J. D. (1970). A permanent record of plankton samples using holography. Limnol. Oceanogr. 15, 967–970. doi: 10.4319/lo.1970.15.6.0967
Bianco, V., Memmolo, P., Carcagnì, P., Merola, F., Paturzo, M., and Distante, C. (2020). Microplastic identification via holographic imaging and machine learning. Adv. Int. Sys. 2:1900153. doi: 10.1002/aisy.201900153
Bianco, V., Memmolo, P., Leo, M., Montresor, S., Distante, C., and Paturzo, M. (2018). Strategies for reducing speckle noise in digital holography. Light Sci. Appl. 7, 1–16.
Bochdansky, A. B., Clouse, M. A., and Hansell, D. A. (2017). Mesoscale and high-frequency variability of macroscopic particles (> 100 μm) in the Ross Sea and its relevance for late-season particulate carbon export. J. Mar. Syst. 166, 120–131. doi: 10.1016/j.jmarsys.2016.08.010
Bochdansky, A. B., Clouse, M. A., and Herndl, G. J. (2016). Dragon kings of the deep sea: marine particles deviate markedly from the common number-size spectrum. Sci. Rep. 6, 1–7.
Bochdansky, A. B., Jericho, M. H., and Herndl, G. J. (2013). Development and deployment of a point-source digital inline holographic microscope for the study of plankton and particles to a depth of 6000 m. Limnol. Oceanogr. Methods 11, 28–40. doi: 10.4319/lom.2013.11.28
Bohren, C., and Huffman, D. (1983). Light Scattering and Absorption by Small Particles. New York, NY: Wiley.
Boss, E., Slade, W., and Hill, P. (2009). Effect of particulate aggregation in aquatic environments on the beam attenuation and its utility as a proxy for particulate mass. Opt. Express 17, 9408–9420. doi: 10.1364/oe.17.009408
Boss, E., Twardowski, M. S., and Herring, S. (2001). Shape of the particulate beam attenuation spectrum and its inversion to obtain the shape of the particulate size distribution. Appl. Opt. 40, 4885–4893. doi: 10.1364/ao.40.004885
Breier, R. E., Lalescu, C. C., Waas, D., Wilczek, M., and Mazza, M. G. (2018). Emergence of phytoplankton patchiness at small scales in mild turbulence. Proc. Nat. Acad. Sci. U.S.A. 115, 12112–12117. doi: 10.1073/pnas.1808711115
Briggs, N., Dall’Olmo, G., and Claustre, H. (2020). Major role of particle fragmentation in regulating biological sequestration of co2 by the oceans. Science 367, 791–793. doi: 10.1126/science.aay1790
Buonassissi, C., and Dierssen, H. (2010). A regional comparison of particle size distributions and the power law approximation in oceanic and estuarine surface waters. J. Geophys. Res. Oceans 115:C10028.
Busck, J. (2005). Underwater 3-d optical imaging with a gated viewing laser radar. Opt. Eng. 44:116001. doi: 10.1117/1.2127895
Carder, K. L. (1979). Holographic microvelocimeter for use in studying ocean particle dynamics. Opt. Eng. 18:185524.
Carder, K. L., Steward, R. G., and Betzer, P. R. (1982). In situ holographic measurements of the sizes and settling rates of oceanic particulates. J. Geophys. Res. Oceans 87, 5681–5685. doi: 10.1029/jc087ic08p05681
Carder, K. L., Tomlinson, R. D., and Beardsley, G. F. Jr. (1972). A technique for the estimation of indices of refraction of marine phytoplankters. Limnol. Oceanogr. 17, 833–839. doi: 10.4319/lo.1972.17.6.0833
Carmichael, W. W., and Boyer, G. L. (2016). Health impacts from cyanobacteria harmful algae blooms: implications for the north american great lakes. Harmful Algae 54, 194–212. doi: 10.1016/j.hal.2016.02.002
Charrière, F., Marian, A., Montfort, F., Kuehn, J., Colomb, T., and Cuche, E. (2006). Cell refractive index tomography by digital holographic microscopy. Opt. Lett. 31, 178–180. doi: 10.1364/ol.31.000178
Checkley, D. Jr., Davis, R., Herman, A., Jackson, G., Beanlands, B., and Regier, L. (2008). Assessing plankton and other particles in situ with the solopc. Limnol. Oceanogr. 53(5, Pt 2), 2123–2136. doi: 10.4319/lo.2008.53.5_part_2.2123
Churnside, J. H., and Wilson, J. J. (2004). Airborne lidar imaging of salmon. Appl. Opt. 43, 1416–1424. doi: 10.1364/ao.43.001416
Conley, D., Buscombe, D., and Nimmo Smith, A. (2012). Use of digital holographic cameras to examine the measurement and understanding of sediment suspension in the nearshore. Coast. Eng. Proc. 1, 73. doi: 10.9753/icce.v33.sediment.73
Costello, D., Carder, K., Betzer, P., and Young, R. (1989). In situ holographic imaging of settling particles: applications for individual particle dynamics and oceanic flux measurements. Deep Sea Res. Part A 36, 1595–1605. doi: 10.1016/0198-0149(89)90060-5
Cózar, A., Echevarría, F., González-Gordillo, J. I., Irigoien, X., Úbeda, B., and Hernández-León, S. (2014). Plastic debris in the open ocean. Proc. Nat. Acad. Sci. U.S.A. 111, 10239–10244.
Cross, J., Nimmo Smith, W. A. M., Hosegood, P. J., and Torres, R. (2014). The dispersal of phytoplankton populations by enhanced turbulent mixing in a shallow coastal sea. J. Mar. Syst. 136, 55–64. doi: 10.1016/j.jmarsys.2014.03.009
Cross, J., Nimmo Smith, W. A. M., Torres, R., and Hosegood, P. J. (2013). Biological controls on resuspension and the relationship between particle size and the kolmogorov length scale in a shallow coastal sea. Mar. Geol. 343, 29–38. doi: 10.1016/j.margeo.2013.06.014
Czerski, H., Vagle, S., Farmer, D. M., and Hall-Patch, N. (2011). Improvements to the methods used to measure bubble attenuation using an underwater acoustical resonator. J. Acoust. Soc. Am. 130, 3421–3430. doi: 10.1121/1.3569723
Dahms, H.-U., and Hwang, J.-S. (2010). Perspectives of underwater optics in biological oceanography and plankton ecology studies. J. Mar. Sci. Technol. 18, 112–121.
Davies, E. J., Buscombe, D., Graham, G. W., and Nimmo Smith, W. A. M. (2015). Evaluating unsupervised methods to size and classify suspended particles using digital in-line holography. J. Atmos. Oceanic Technol. 32, 1241–1256. doi: 10.1175/jtech-d-14-00157.1
Davies, E. J., and Nepstad, R. (2017). In situ characterisation of complex suspended particulates surrounding an active submarine tailings placement site in a norwegian fjord. Reg. Stud. Mar. Sci. 16, 198–207. doi: 10.1016/j.rsma.2017.09.008
Dekshenieks, M. M., Donaghay, P. L., Sullivan, J. M., Rines, J. E., Osborn, T. R., and Twardowski, M. S. (2001). Temporal and spatial occurrence of thin phytoplankton layers in relation to physical processes. Mar. Ecol. Prog. Ser. 223, 61–71. doi: 10.3354/meps223061
Donaghay, P., Rines, H. M., and Sieburth, J. M. (1992). Simultaneous sampling of fine-scale biological, chemical, and physical structure in stratified waters. Arch. Hydrobiol. 36, 1–14.
Durham, W. M., Climent, E., Barry, M., De Lillo, F., Boffetta, G., and Cencini, M. (2013). Turbulence drives microscale patches of motile phytoplankton. Nat. Commun. 4, 1–7.
Durham, W. M., and Stocker, R. (2012). Thin phytoplankton layers: characteristics, mechanisms, and consequences. Annu. Rev. Mar. Sci. 4, 177–207. doi: 10.1146/annurev-marine-120710-100957
Dyomin, V., Davydova, A., Morgalev, S., Kirillov, N., Olshukov, A., and Polovtsev, I. (2020). Monitoring of plankton spatial and temporal characteristics with the use of a submersible digital holographic camera. Front. Mar. Sci. 7:653. doi: 10.3389/fmars.2020.00653
Dyomin, V., Gribenyukov, A., Davydova, A., Zinoviev, M., Olshukov, A., and Podzyvalov, S. (2019). Holography of particles for diagnostics tasks. Appl. Opt. 58, G300–G310.
Fischer, G., and Karakas, G. (2009). Sinking rates and ballast composition of particles in the atlantic ocean: implications for the organic carbon fluxes to the deep ocean. Biogeosciences 6, 85–102. doi: 10.5194/bg-6-85-2009
Font-Muñoz, J. S., Jeanneret, R., Arrieta, J., Anglès, S., Jordi, A., and Tuval, I. (2019). Collective sinking promotes. selective cell pairing in planktonic pennate diatoms. Proc. Nat. Acad. Sci. U.S.A. 116, 15997–16002
Font-Muñoz, J. S., Jeanneret, R., Tuval, I., and Basterretxea, G. (2020). Method for the determination of preferential orientation of marine particles from laser diffraction measurements. Opt. Express 28, 14085–14099.
Foster, E., and Watson, J. (1997). Holography for underwater inspection and measurement: an overview of current work. Opt. Laser Technol. 29, 17–23.
Fournier, G. R., and Forand, J. L. (1994). “Analytic phase function for ocean water,” in Ocean Optics XII, ed. J. S. Jaffe (Bellingham, WA: International Society for Optics and Photonics), 194–201.
Freedman, D., Pisani, R., and Purves, R. (2007). Statistics. New York, NY: W.W. Norton & Company, Inc.
Gabor, D. (1949). Microscopy by reconstructed wave-fronts. Proc. R. Soc. London, Ser. A 197, 454–487.
Gao, J., Guildenbecher, D. R., Reu, P. L., and Chen, J. (2013). Uncertainty characterization of particle depth measurement using digital in-line holography and the hybrid method. Opt. Express 21, 26432–26449.
Garaba, S. P., and Dierssen, H. M. (2018). An airborne remote sensing case study of synthetic hydrocarbon detection using short wave infrared absorption features identified from marine-harvested macro-and microplastics. Remote Sens. Environ. 205, 224–235.
Garcia-Sucerquia, J. (2013). Noise reduction in digital lensless holographic microscopy by engineering the light from a light-emitting diode. Appl. Opt. 52, A232–A239.
Glibert, P. M., Berdalet, E., Burford, M. A., Pitcher, G. C., and Zhou, M. (2018). Global Ecology and Oceanography of Harmful Algal Blooms. Berlin: Springer.
Graff, J. R., and Menden-Deuer, S. (2016). Physical and optical properties of phytoplankton-rich layers in a coastal fjord: a step toward prediction and strategic sampling of plankton patchiness. Mar. Ecol. Prog. Ser. 544, 1–14.
Graham, G., Davies, E., Nimmo Smith, W., Bowers, D., and Braithwaite, K. (2012). Interpreting LISST-100X measurements of particles with complex shape using digital in-line holography. J. Geophys. Res. Oceans 117:C05034.
Graham, G. W., and Nimmo Smith, W. A. M. (2010). The application of holography to the analysis of size and settling velocity of suspended cohesive sediments. Limnol. Oceanogr. Methods 8, 1–15.
Granek, E. F., Brander, S., and Holland, E. (2020). Microplastics in aquatic organisms: improving understanding and identifying research directions for the next decade. Limnol. Oceanogr. Lett. 5, 1–4.
Guidi, L., Calil, P. H., Duhamel, S., Björkman, K. M., Doney, S. C., and Jackson, G. A. (2012). Does eddy-eddy interaction control surface phytoplankton distribution and carbon export in the north pacific subtropical gyre? J. Geophys. Res. Biogeosci. 117:G02024.
Guo, B., Nyman, L., Nayak, A. R., Milmore, D., McFarland, M., Twardowski, M., et al. (in press). Automated plankton classification from holographic imagery with deep convolutional neural networks. Limnol. Oceanogr. Methods 19, 21–36. doi: 10.1002/lom3.10402
Heflinger, L., Stewart, G., and Booth, C. (1978). Holographic motion pictures of microscopic plankton. Appl. Opt. 17, 951–954.
Herman, A., Beanlands, B., and Phillips, E. (2004). The next generation of optical plankton counter: the laser-opc. J. Plankton Res. 26, 1135–1145.
Hill, P., Boss, E., Newgard, J., Law, B., and Milligan, T. (2011). Observations of the sensitivity of beam attenuation to particle size in a coastal bottom boundary layer. J. Geophys. Res. Oceans 116:C02023.
Hobson, P. R., and Watson, J. (2002). The principles and practice of holographic recording of plankton. J. Opt. A Pure Appl. Opt. 4:S34.
Hodgson, R. T., and Newkirk, D. D. (1975). “Pyridine immersion: a technique for measuring the refractive index of marine particles,” in Ocean Optics IV, (Bellingham, WA: International Society for Optics and Photonics), 62–65.
Holliday, D., Donaghay, P., Greenlaw, C., Napp, J., and Sullivan, J. (2009). High-frequency acoustics and bio-optics in ecosystems research. ICES J. Mar. Sci. 66, 974–980.
Hong, J., Talapatra, S., Katz, J., Tester, P. A., Waggett, R. J., and Place, A. R. (2012). Algal toxins alter copepod feeding behavior. PLoS One 7:e36845. doi: 10.1371/journal.pone.0036845
Jackson, G., Maffione, R., Costello, D., Alldredge, A. L. B., and Dam, H. (1997). Particle size spectra between 1 um and 1 cm at monterey bay determined using multiple instruments. Deep Sea Res. Part I 44, 1739–1767.
Jaffe, J., Franks, P., Briseño-Avena, C., Roberts, P., and Laxton, B. (2013). “Advances in underwater fluorometry: from bulk fluorescence to planar laser imaging,” in Subsea Optics and Imaging, eds J. Watson and O. Zielinski (Amsterdam: Elsevier), 536–549.
Jaffe, J. S. (2014). Underwater optical imaging: the past, the present, and the prospects. IEEE J. Oceanic Eng. 40, 683–700.
Jeffrey, G. (1922). The motion of ellipsoidal particles immersed in a viscous fluid. Proc. R. Soc. London, Ser. A 102, 161–179.
Jericho, S., Garcia-Sucerquia, J., Xu, W., Jericho, M., and Kreuzer, H. (2006). Submersible digital in-line holographic microscope. Rev. Sci. Instrum. 77:043706.
Jonasz, M., and Fournier, G. (2007). Light Scattering by Particles in Water: Theoretical and Experimental Constraints. Cambridge, MA: Academic Press.
Karp-Boss, L., Boss, E., and Jumars, P. (1996). Nutrient fluxes to planktonic osmotrophs in the presence of fluid motion. Oceanogr. Mar. Biol. 34, 71–108.
Karp-Boss, L., Boss, E., and Jumars, P. A. (2000). Motion of dinoflagellates in a simple shear flow. Limnol. Oceanogr. 45, 1594–1602. doi: 10.4319/lo.2000.45.7.1594
Karp-Boss, L., and Jumars, P. A. (1998). Motion of diatom chains in steady shear flow. Limnol. Oceanogr. 43, 1767–1773. doi: 10.4319/lo.1998.43.8.1767
Katz, J., Donaghay, P., Zhang, J., King, S., and Russell, K. (1999). Submersible holocamera for detection of particle characteristics and motions in the ocean. Deep Sea Res. Part A 46, 1455–1481. doi: 10.1016/S0967-0637(99)00011-4
Katz, J., O’Hern, T., and Acosta, A. (1984). “An underwater holographic camera system for detection of microparticulates,” in Proceedings of the ASME Cavitation and Multiphase Flow Forum, New Orleans.
Katz, J., and Sheng, J. (2010). Applications of holography in fluid mechanics and particle dynamics. Annu. Rev. Flu. Mech. 42, 531–555. doi: 10.1146/annurev-fluid-121108-145508
Kitchen, J., Zaneveldan, J., and Pak, H. (1982). Effect of particle size distribution and chlorophyll content on beam attenuation spectra. Appl. Opt. 21, 3913–3918. doi: 10.1364/AO.21.003913
Knox, C. (1966). Holographic microscopy as a technique for recording dynamic microscopic subjects. Science 153, 989–990. doi: 10.1126/science.153.3739.989
Knox, C., and Brooks, R. (1969). Holographic motion picture microscopy. Proc. R. Soc. London, Ser. B 174, 115–121. doi: 10.1098/rspb.1969.0083
Kosmeier, S., Langehanenberg, P., Von Bally, G., and Kemper, B. (2012). Reduction of parasitic interferences in digital holographic microscopy by numerically decreased coherence length. Appl. Phys. B 106, 107–115. doi: 10.1007/s00340-011-4667-0
Kostadinov, T., Siegel, D., and Maritorena, S. (2010). Global variability of phytoplankton functional types from space: assessment via the particle size distribution. Biogeosci. Discuss. 7, 4295–4340. doi: 10.5194/bgd-7-4295-2010
Kudela, R. M., Bickel, A., Carter, M. L., Howard, M. D., and Rosenfeld, L. (2015). “The monitoring of harmful algal blooms through ocean observing: the development of the california harmful algal bloom monitoring and alert program,” in Coastal Ocean Observing Systems, eds Y. Liu, H. Kerkering, and R. Weisberg (Amsterdam: Elsevier), 58–75. Available online at: https://www.elsevier.com/books/coastal-ocean-observing-systems/liu/978-0-12-802022-7
Kumar, S. S., He, Z., Hogan, C. J., Fredericks, S. A., and Hong, J. (2020). Evaluation of laser diffraction-based particle size measurements using digital inline holography. Meas. Sci. Technol. 31:125201. doi: 10.1088/1361-6501/aba78b
Lal, D. (1977). The oceanic microcosm of particles. Science 198, 997–1009. doi: 10.1126/science.198.4321.997
Leo, M., Piccolo, R., Distante, C., Memmolo, P., Paturzo, M., and Ferraro, P. (2014). Multilevel bidimensional empirical mode decomposition: a new speckle reduction method in digital holography. Opt. Eng. 53:112314. doi: 10.1117/1.OE.53.11.112314
Lindensmith, C. A., Rider, S., Bedrossian, M., Wallace, J. K., Serabyn, E., and Showalter, G. M. (2016). A submersible, off-axis holographic microscope for detection of microbial motility and morphology in aqueous and icy environments. PLoS One 11:e0147700. doi: 10.1371/journal.pone.0147700
Mai, L., Bao, L.-J., Shi, L., Wong, C. S., and Zeng, E. Y. (2018). A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. 25, 11319–11332. doi: 10.1007/s11356-018-1692-0
Malkiel, E., Abras, J., Widder, E., and Katz, J. (2006). On the spatial distribution and nearest neighbor distance between particles in the water column determined from in situ holographic measurements. J. Plankton Res. 28, 149–170. doi: 10.1093/plankt/fbi107
Malkiel, E., Abras, J. N., and Katz, J. (2004). Automated scanning and measurements of particle distributions within a holographic reconstructed volume. Meas. Sci. Technol. 15:601. doi: 10.1088/0957-0233/15/4/001
Malkiel, E., Alquaddoomi, O., and Katz, J. (1999). Measurements of plankton distribution in the ocean using submersible holography. Meas. Sci. Technol. 10:1142. doi: 10.1088/0957-0233/10/12/305
Malkiel, E., Pfitsch, D. W., Sheng, J., and Katz, J. (2007). “Observing the 3-dimensional zooplankton world with holography,” in ASLO Aquatic Sciences Meeting, Santa Fe, MX.
Mallery, K., Canelon, D., Hong, J., and Papanikolopoulos, N. (2019). Design and experiments with a robot-driven underwater holographic microscope for low-cost in situ particle measurements. arXiv [preprint], Avaliable at: https://arxiv.org/abs/1911.10231 (accessed May 15, 2020).
Marcos, J. R., Seymour, J. R., Luhar, M., Durham, W. M., Mitchell, J. G., and Macke, A. (2011). Microbial alignment in flow changes ocean light climate. Proc. Nat. Acad. Sci. U.S.A. 108, 3860–3864. doi: 10.1073/pnas.1014576108
McDonnell, A. M., and Buesseler, K. O. (2010). Variability in the average sinking velocity of marine particles. Limnol. Oceanogr. 55, 2085–2096. doi: 10.4319/lo.2010.55.5.2085
McFarland, M., Nayak, A. R., Stockley, N., Twardowski, M., and Sullivan, J. (2020). Enhanced light absorption by horizontally oriented diatom colonies. Front. Mar. Sci. 7:494. doi: 10.3389/fmars.2020.00494
McFarland, M. N., Rines, J., Sullivan, J., and Donaghay, P. (2015). Impact of phytoplankton size and physiology on particulate optical properties determined with scanning flow cytometry. Mar. Ecol. Prog. Ser. 531, 43–61. doi: 10.3354/meps11325
McKenzie, T. L., Twardowski, M., Briggs, N., Nayak, A. R., Boswell, K. M., and Dalgleish, F. (2020). Three-dimensional imaging lidar for characterizing particle fields and organisms in the mesopelagic zone. Front. Mar. Sci. 7:1021.
McManus, M., Alldredge, A., Barnard, A., Boss, E., Case, J., and Cowles, T. (2003). Characteristics, distribution and persistence of thin layers over a 48 hour period. Mar. Ecol. Prog. Ser. 261, 1–19. doi: 10.3354/meps261001
McManus, M. A., and Woodson, C. B. (2012). Plankton distribution and ocean dispersal. J. Exp. Biol. 215, 1008–1016. doi: 10.1242/jeb.059014
Meng, H., Anderson, W., Hussain, F., and Liu, D. D. (1993). Intrinsic speckle noise in in-line particle holography. J. Opt. Soc. Am. A 10, 2046–2058. doi: 10.1364/JOSAA.10.002046
Merola, F., Memmolo, P., Bianco, V., Paturzo, M., Mazzocchi, M., and Ferraro, P. (2018). Searching and identifying microplastics in marine environment by digital holography⋆. Eur. Phys. J. Plus 133:350. doi: 10.1140/epjp/i2018-12190-y
Merola, F., Memmolo, P., Miccio, L., Savoia, R., Mugnano, M., and Fontana, A. (2017). Tomographic flow cytometry by digital holography. Light Sci. Appl. 6:e16241. doi: 10.1038/lsa.2016.241
Mikkelsen, O., and Pejrup, M. (2001). The use of a LISST-100 laser particle sizer for in-situ estimates of floc size, density and settling velocity. Geo Mar. Lett. 20, 187–195. doi: 10.1007/s003670100064
Milligan, T. (1996). In situ particle (floc) size measurements with the benthos 373 plankton silhouette camera. J. Sea Res. 36, 93–100. doi: 10.1016/S1385-1101(96)90777-7
Moore, T. S., Churnside, J. H., Sullivan, J. M., Twardowski, M. S., Nayak, A. R., and McFarland, M. N. (2019). Vertical distributions of blooming cyanobacteria populations in a freshwater lake from lidar observations. Remote Sens. Environ. 225, 347–367. doi: 10.1016/j.rse.2019.02.025
Moore, T. S., Mouw, C. B., Sullivan, J. M., Twardowski, M. S., Burtner, A. M., and Ciochetto, A. B. (2017). Bio-optical properties of cyanobacteria blooms in western lake erie. Front. Mar. Sci. 4:300. doi: 10.3389/fmars.2017.00300
Mullen, A. D., Treibitz, T., Roberts, P. L., Kelly, E. L., Horwitz, R., and Smith, J. E. (2016). Underwater microscopy for in situ studies of benthic ecosystems. Nat. Commun. 7, 1–9. doi: 10.1038/ncomms12093
Murata, S., and Yasuda, N. (2000). Potential of digital holography in particle measurement. Opt. Laser Technol. 32, 567–574. doi: 10.1016/S0030-3992(00)00088-8
Nayak, A. R., Li, C., Kiani, B. T., and Katz, J. (2015). On the wave and current interaction with a rippled seabed in the coastal ocean bottom boundary layer. J. Geophys. Res. Oceans 120, 4595–4624. doi: 10.1002/2014JC010606
Nayak, A. R., McFarland, M., Sullivan, J. M., Dalgleish, F., and Garavelli, L. (2020). “AUTOHOLO: A novel, in situ, autonomous holographic imaging system for long-term particle and plankton characterization studies in diverse marine environments,” in Ocean Sciences Meeting 2020, San Diego, CA.
Nayak, A. R., McFarland, M. N., Sullivan, J. M., and Twardowski, M. S. (2018a). Evidence for ubiquitous preferential particle orientation in representative oceanic shear flows. Limnol. Oceanogr. 63, 122–143. doi: 10.1002/lno.10618
Nayak, A. R., McFarland, M. N., Twardowski, M. S., and Sullivan, J. M. (2018b). “On plankton distributions and biophysical interactions in diverse coastal and limnological environments,” in Ocean Sensing and Monitoring X, Bellingham, WA: International Society for Optics and Photonics, 106310. doi: 10.1117/12.2309798
Nayak, A. R., and Twardowski, M. S. (2020). “Breaking” news for the ocean’s carbon budget. Science 367, 738–739. doi: 10.1126/science.aba7109
Nimmo Smith, W. A. (2008). A submersible three-dimensional particle tracking velocimetry system for flow visualization in the coastal ocean. Limnol. Oceanogr. Methods 6, 96–104. doi: 10.4319/lom.2008.6.96
Noel, V., and Chepfer, H. (2004). Study of ice crystal orientation in cirrus clouds based on satellite polarized radiance measurements. J. Atmos. Sci. 61, 2073–2081. doi: 10.1175/1520-0469(2004)061<2073:SOICOI>2.0.CO;2
O’Hern, T., d’Agostino, L., and Acosta, A. (1988). Comparison of holographic and coulter counter measurements of cavitation nuclei in the ocean. J. Fluids Eng. 110, 200–207. doi: 10.1115/1.3243535
Olson, R. J., and Sosik, H. M. (2007). A submersible imaging-in-flow instrument to analyze nano-and microplankton: imaging flowcytobot. Limnol. Oceanogr. Methods 5, 195–203. doi: 10.4319/lom.2007.5.195
Ono, A. (1969). The shape and riming properties of ice crystals in natural clouds. J. Atmos. Sci. 26, 138–147. doi: 10.1175/1520-0469(1969)026<0138:TSARPO>2.0.CO;2
Ouillon, S. (2018). Why and how do we study sediment transport? Focus on coastal zones and ongoing methods. Water 10:390. doi: 10.3390/w10040390
Owen, R. B., and Zozulya, A. A. (2000). In-line digital holographic sensor for monitoring and characterizing marine particulates. Opt. Eng. 39, 2187–2197. doi: 10.1117/1.1305542
Pan, F., Yang, L., and Xiao, W. (2017). Coherent noise reduction in digital holographic microscopy by averaging multiple holograms recorded with a multimode laser. Optics Express 25, 21815–21825. doi: 10.1364/OE.25.021815
Pan, G., and Meng, H. (2003). Digital holography of particle fields: reconstruction by use of complex amplitude. Appl. Opt. 42, 827–833. doi: 10.1364/AO.42.000827
Pfitsch, D., Malkiel, E., Ronzhes, Y., King, S., Sheng, J., and Katz, J. (2005). “Development of a free-drifting submersible digital holographic imaging system,” in Proceedings of OCEANS 2005 MTS/IEEE, Washington, DC, 690–696. doi: 10.1109/OCEANS.2005.1639833
Pfitsch, D., Malkiel, E., Takagi, M., Ronzhes, Y., King, S., and Sheng, J. (2007). Analysis of in-situ microscopic organism behavior in data acquired using a free-drifting submersible holographic imaging system. OCEANS 2007, 1–8. doi: 10.1109/OCEANS.2007.4449197
Picart, P., and Montresor, S. (2020). “Digital holography,” in Optical Holography-Materials, Theory and Applications, ed. P.-A. Blanche (Amsterdam: Elsevier), 83–120. doi: 10.1016/B978-0-12-815467-0.00005-0
Poulin, C., and Zhang, X. (2020). “Evaluating holotomography as a new way to measure the refractive index of phytoplankton,” in Ocean Sciences Meeting 2020, San Diego, CA.
Powell, T. M., and Okubo, A. (1994). Turbulence, diffusion and patchiness in the sea. Phil. Trans. R. Soc. London. Ser. B 343, 11–18. doi: 10.1098/rstb.1994.0002
Prairie, J. C., Franks, P. J., Jaffe, J. S., Doubell, M. J., and Yamazaki, H. (2011). Physical and biological controls of vertical gradients in phytoplankton. Limnol. Oceanogr. Fluids Environ. 1, 75–90. doi: 10.1215/21573698-1267403
Prairie, J. C., Sutherland, K. R., Nickols, K. J., and Kaltenberg, A. M. (2012). Biophysical interactions in the plankton: a cross-scale review. Limnol. Oceanogr. Fluids Environ. 2, 121–145. doi: 10.1215/21573689-1964713
Rines, J., Donaghay, P. L., Dekshenieks, M., Sullivan, J., and Twardowski, M. (2002). Thin layers and camouflage: hidden Pseudo-nitzschia spp.(Bacillariophyceae) populations in a fjord in the San Juan Islands. Washington, USA. Mar. Ecol. Prog. Ser. 225, 123–137. doi: 10.3354/meps225123
Risović, D. (1993). Two-component model of sea particle size distribution. Deep Sea Res. Part I 40, 1459–1473. doi: 10.1016/0967-0637(93)90123-K
Rotermund, L., Samson, J., and Kreuzer, H. (2016). A submersible holographic microscope for 4-d in-situ studies of micro-organisms in the ocean with intensity and quantitative phase imaging. J. Mar. Sci. Res. Dev. 6:181. doi: 10.4172/2155-9910.1000181
Sassen, K., and Takano, Y. (2000). Parry arc: a polarization lidar, ray-tracing, and aircraft case study. Appl. Opt. 39, 6738–6745. doi: 10.1364/AO.39.006738
Schnars, U., and Jüptner, W. P. (2002). Digital recording and numerical reconstruction of holograms. Meas. Sci. Technol. 13:R85. doi: 10.1088/0957-0233/13/9/201
Selander, E., and Tiselius, P. (2003). Effects of food concentration on the behaviour of oikopleuradioica. Mar. Biol. 142, 263–270. doi: 10.1007/s00227-002-0949-8
Shao, S., Mallery, K., Kumar, S. S., and Hong, J. (2020). Machine learning holography for 3D particle field imaging. Opt. Express 28, 2987–2999. doi: 10.1364/OE.379480
Sheng, J., Malkiel, E., Katz, J., Adolf, J., Belas, R., and Place, A. R. (2007). Digital holographic microscopy reveals prey-induced changes in swimming behavior of predatory dinoflagellates. Proc. Nat. Acad. Sci. U.S.A. 104, 17512–17517. doi: 10.1073/pnas.0704658104
Steinbuck, J. V., Roberts, P. L., Troy, C. D., Horner-Devine, A. R., Simonet, F., and Uhlman, A. H. (2010). An autonomous open-ocean stereoscopic PIV profiler. J. Atmos. Oceanic Technol. 27, 1362–1380. doi: 10.1175/2010JTECHO694.1
Stemmann, L., and Boss, E. (2012). Plankton and particle size and packaging: from determining optical properties to driving the biological pump. Annu. Rev. Mar. Sci. 4, 263–290. doi: 10.1146/annurev-marine-120710-100853
Stemmann, L., Eloire, D., Sciandra, A., Jackson, G., Guidi, L., and Picheral, M. (2008). Volume distribution for particles between 3.5 to 2000 μm in the upper 200 m region of the south pacific gyre. Biogeosciences 5, 299–310. doi: 10.5194/bg-5-299-2008
Stemmann, L., Gorsky, G., Marty, J.-C., Picheral, M., and Miquel, J.-C. (2002). Four-year study of large-particle vertical distribution (0–1000 m) in the NW Mediterranean in relation to hydrology, phytoplankton, and vertical flux. Deep Sea Res. Part II 49, 2143–2162. doi: 10.1016/S0967-0645(02)00032-2
Stemmann, L., Picheral, M., and Gorsky, G. (2000). Diel variation in the vertical distribution of particulate matter (> 0.15 mm) in the nw mediterranean sea investigated with the underwater video profiler. Deep Sea Res Part I 47, 505–531. doi: 10.1016/S0967-0637(99)00100-4
Stewart, G. L., Beers, J. R., and Knox, C. (1973). “Application of holographic techniques to the study of marine plankton in the field and in the laboratory,” in Developments in Laser Technology II, (Bellingham, WA: International Society for Optics and Photonics), 183–188. doi: 10.1117/12.953852
Sullivan, J. M., Donaghay, P. L., and Rines, J. E. (2010a). Coastal thin layer dynamics: consequences to biology and optics. Cont. Shelf Res. 30, 50–65. doi: 10.1016/j.csr.2009.07.009
Sullivan, J. M., Van Holliday, D., McFarland, M., McManus, M. A., Cheriton, O. M., and Benoit-Bird, K. J. (2010b). Layered organization in the coastal ocean: an introduction to planktonic thin layers and the loco project. Cont. Shelf Res. 30, 50–65. doi: 10.1016/j.csr.2009.09.001
Sullivan, J. M., and Swift, E. (2003). Effects of small-scale turbulence on net growth rate and size of ten species of marine dinoflagellates 1. J. Phycol. 39, 83–94. doi: 10.1046/j.1529-8817.2003.02094.x
Sullivan, J. M., Twardowski, M. S., Donaghay, P. L., and Freeman, S. A. (2005). Use of optical scattering to discriminate particle types in coastal waters. Appl. Opt. 44, 1667–1680. doi: 10.1364/AO.44.001667
Sun, B., Kattawar, G. W., Yang, P., Twardowski, M. S., and Sullivan, J. M. (2016). Simulation of the scattering properties of a chain-forming triangular prism oceanic diatom. J. Quant. Spec. Rad. Trans. 178, 390–399. doi: 10.1016/j.jqsrt.2016.02.035
Sun, H., Benzie, P. W., Burns, N., Hendry, D. C., Player, M. A., and Watson, J. (2008). Underwater digital holography for studies of marine plankton. Phil. Trans. R. Soc. A 366, 1789–1806. doi: 10.1098/rsta.2007.2187
Talapatra, S., Hong, J., McFarland, M., Nayak, A. R., Zhang, C., and Katz, J. (2013). Characterization of biophysical interactions in the water column using in situ digital holography. Mar. Ecol. Prog. Ser. 473, 29–51. doi: 10.3354/meps10049
Talapatra, S., Sullivan, J., Katz, J., Twardowski, M., Czerski, H., and Donaghay, P. (2012). “Application of in-situ digital holography in the study of particles, organisms and bubbles within their natural environment,” in Ocean Sensing and Monitoring IV, (Bellingham, WA: International Society for Optics and Photonics), 837205. doi: 10.1117/12.920570
Tett, P., Carreira, C., Mills, D., Van Leeuwen, S., Foden, J., and Bresnan, E. (2008). Use of a phytoplankton community index to assess the health of coastal waters. ICES J. Mar. Sci. 65, 1475–1482. doi: 10.1093/icesjms/fsn161
Toloui, M., and Hong, J. (2015). High fidelity digital inline holographic method for 3d flow measurements. Opt. Express 23, 27159–27173. doi: 10.1364/OE.23.027159
Trull, T., Bray, S., Buesseler, K., Lamborg, C., Manganini, S., Moy, C., et al. (2008). In situ measurement of mesopelagic particle sinking rates and the control of carbon transfer to the ocean interior during the vertical flux in the global ocean (vertigo) voyages in the north pacific. Deep Sea Res. Part II 55, 1684–1695. doi: 10.1016/j.dsr2.2008.04.021
Turner, J. T. (2015). Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog. Oceanogr. 130, 205–248. doi: 10.1016/j.pocean.2014.08.005
Twardowski, M., Zhang, X., Vagle, S., Sullivan, J., Freeman, S., and Czerski, H. (2012). The optical volume scattering function in a surf zone inverted to derive sediment and bubble particle subpopulations. J. Geophys. Res. Oceans 117:C00H17. doi: 10.1029/2011JC007347
Twardowski, M. S., Boss, E., Macdonald, J. B., Pegau, W. S., Barnard, A. H., and Zaneveld, J. R. V. (2001). A model for estimating bulk refractive index from the optical backscattering ratio and the implications for understanding particle composition in Case I and Case II waters. J. Geophys. Res. Oceans 106, 14129–14142. doi: 10.1029/2000JC000404
Twardowski, M. S., Sullivan, J. M., Donaghay, P. L., and Zaneveld, J. R. V. (1999). Microscale quantification of the absorption by dissolved and particulate material in coastal waters with an ac-9. J. Atmos. Oceanic Technol. 16, 691–707. doi: 10.1175/1520-0426(1999)016<0691:MQOTAB>2.0.CO;2
Ulloa, O., Sathyendranath, S., and Platt, T. (1994). Effect of the particle-size distribution on the backscattering ratio in seawater. Appl. Opt. 33, 7070–7077. doi: 10.1364/AO.33.007070
Umemura, K., Matsukawa, Y., Ide, Y., and Mayama, S. (2020). Corrigendum to “label-free imaging and analysis of subcellular parts of a living diatom cylindrotheca sp. using optical diffraction tomography” [methodsx: 7 (2020) 100889]. MethodsX 7:100923. doi: 10.1016/j.mex.2020.100889
Vikram, C. (1992). Particle Field Holography, 1st Edit Edn. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511524196
Visser, P. M., Passarge, J., and Mur, L. R. (1997). Modelling vertical migration of the cyanobacterium Microcystis. Hydrobiologia 349, 99–109. doi: 10.1023/A:1003001713560
Walcutt, N. L., Knörlein, B., Cetinić, I., Ljubesic, Z., Bosak, S., and Sgouros, T. (2020). Assessment of holographic microscopy for quantifying marine particle size and concentration. Limnol. Oceanogr. Methods 18, 516–530. doi: 10.1002/lom3.10379
Watson, J. (2005). “Underwater holography: past and future,” in Holography 2005: International Conference on Holography, Optical Recording, and Processing of Information, (Bellingham, WA: International Society for Optics and Photonics), 62521T.
Watson, J. (2011). Submersible digital holographic cameras and their application to marine science. Opt. Eng. 50:091313. doi: 10.1117/1.3605678
Watson, J., Alexander, S., Craig, G., Hendry, D. C., Hobson, P., and Lampitt, R. (2001). Simultaneous in-line and off-axis subsea holographic recording of plankton and other marine particles. Meas. Sci. Technol. 12:L9. doi: 10.1088/0957-0233/12/8/101
Yang, W., Kostinski, A. B., and Shaw, R. A. (2005). Depth-of-focus reduction for digital in-line holography of particle fields. Opt. Lett. 30, 1303–1305. doi: 10.1364/OL.30.001303
Yourassowsky, C., and Dubois, F. (2014). High throughput holographic imaging-in-flow for the analysis of a wide plankton size range. Opt. Express 22, 6661–6673. doi: 10.1364/OE.22.006661
Zetsche, E. M., El Mallahi, A., Dubois, F., Yourassowsky, C., Kromkamp, J. C., and Meysman, F. J. (2014). Imaging-in-flow: digital holographic microscopy as a novel tool to detect and classify nanoplanktonic organisms. Limnol. Oceanogr. Methods 12, 757–775. doi: 10.4319/lom.2014.12.757
Keywords: holography, underwater imaging, particle interactions, plankton distributions, plankton imaging, biophysical interactions, particle patchiness
Citation: Nayak AR, Malkiel E, McFarland MN, Twardowski MS and Sullivan JM (2021) A Review of Holography in the Aquatic Sciences: In situ Characterization of Particles, Plankton, and Small Scale Biophysical Interactions. Front. Mar. Sci. 7:572147. doi: 10.3389/fmars.2020.572147
Received: 12 June 2020; Accepted: 30 December 2020;
Published: 22 January 2021.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
Pietro Ferraro, National Research Council (CNR), ItalyAlexander B. Bochdansky, Old Dominion University, United States
Copyright © 2021 Nayak, Malkiel, McFarland, Twardowski and Sullivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aditya R. Nayak, YW5heWFrQGZhdS5lZHU=
 Aditya R. Nayak
Aditya R. Nayak Ed Malkiel
Ed Malkiel Malcolm N. McFarland
Malcolm N. McFarland Michael S. Twardowski
Michael S. Twardowski James M. Sullivan
James M. Sullivan