Corrigendum: Use of Synthetic Salmon GnRH and Domperidone (Ovaprim®) in Sharks: Preparation for ex situ Conservation
- 1College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, South Korea
- 2Hanwha Marine Biology Research Center, Hanwha Hotels & Resorts Co., Ltd., Seoul, South Korea
- 3Department of Marine Industry and Maritime Police, College of Ocean Science, Jeju National University, Jeju, South Korea
Shark populations are constantly decreasing owing to environmental destruction and overfishing; thus, sharks are now at a risk of extinction, with 27.9% of shark species classified as endangered on the International Union for Conservation of Nature’s Red List. Sharks are apex predators and a keystone species in balancing the marine food chain; their extinction will create an imbalance of the entire marine ecosystem. Assisted reproductive technology is the last resort for protecting animals facing severe extinction. Here, as a proactive effort toward building a hormone-induced artificial insemination protocol for endangered wild sharks, we identified the possibility of germ cell maturation by administration of Ovaprim®, a commercially produced synthetic salmon gonadotropin-releasing hormone, and calculated its optimum dosage and injection timing. The experiment was conducted on two shark species—Triakis scyllium and Triaenodon obesus. We found that intramuscular injections of 0.2 mL/kg of Ovaprim® for male T. scyllium and T. obesus, 0.2 mL/kg + 0.5 mL/kg at a 24 h interval for female T. scyllium, and 0.2 mL/kg + 0.2 mL/kg or 0.2 mL/kg + 0.3 mL/kg at a 24 h interval for female T. obesus were optimal dose protocols. These doses effectively induced the maturation and ovulation of oocytes and the release of semen. Our results confirm that Ovaprim® is a suitable tool for shark hormone-induced artificial insemination and indicate that this method may enable the conservation of the endangered shark species.
Introduction
Shark populations have been severely threatened by indiscriminate hunting over the last 40–50 years (Lack and Sant, 2011). Of the species identified on the International Union for Conservation of Nature’s (IUCN) Red List, 27.9% of shark species are listed as “vulnerable,” “endangered,” or “critically endangered1.” Sharks are apex predators and keystone species of the ocean food chain. If their populations are excessively reduced or become extinct, there will be a rapid imbalance in the marine ecosystem (Ferretti et al., 2010) and the quantity of marine food sources will also drop sharply. Protecting endangered shark species is imperative for humanity and for nature (Myers et al., 2007).
However, sharks have very slow reproduction rate owing to their long gestation period, slow sexual maturation, and small litter size (Ferretti et al., 2010). Introduction of assisted reproductive technology is necessary for securing genetic diversity and to accelerate breeding rates (Comizzoli et al., 2000). Artificial insemination, one of the most important techniques of assisted reproductive technology, is a method of mixing spermatozoa and oocytes by artificial means. In animals that undergo internal fertilization, it entails direct injection of spermatozoa into the vagina/uterus at the time of ovulation. Artificial insemination has been conducted on various endangered species, ranging from terrestrial animals such as whooping crane (Grus americana), Przewalski’s horses (Equus ferus), Asian elephants (Elephas maximus), and northern white rhinoceros (Ceratotherium simum cottoni) to aquatic animals including lake sturgeon (Acipenser fulvescens), Mahseer, Osteobrama belangeri, Padba (Ompok pabo), Tangra (Mystus guillo), etc. (Ogale, 1997; Mijkherjee et al., 2002; Ogale, 2002; Hermes et al., 2007; Hildebrandt et al., 2007; Blanco et al., 2009; Devi et al., 2009; Thongtip et al., 2009).
Thus far, only two papers have been published on shark artificial insemination in the white-spotted bamboo shark (Chiloscyllium plagiosum) and cloudy catshark (Scyliorhinus torazame) (Masuda et al., 2003, 2005). These sharks are mesopredators of the marine food web as they regulate populations of mollusks, crustaceans, and bony fishes and serve as suitable prey for larger elasmobranchs meeting their metabolic requirements (Taniuchi, 1988; Ferretti et al., 2010; Tambling et al., 2018). As these species have small body sizes and can store spermatozoa in the female reproductive tracts, their artificial insemination strategies can be simplistic (Masuda et al., 2003, 2005). Some artificial insemination studies, which have not been officially reported in the academic literature, have been performed in aquariums using large apex predator sharks. They used the shark’s natural hormonal cycle, for which blood is collected and the hormone concentration changes are tracked periodically for at least 1 year. The biggest drawback of this strategy is that it can only be carried out on shark species that are safe and accessible to humans. Most endangered sharks in the wild may have very limited access to human beings either owing to their numbers or temper and applying the existing method to them is simply not possible.
Hormone-induced artificial insemination is a method of increasing the number of germ cells sampled by inducing gamete maturation and controlling the timing of fertilization through artificial hormone administration. The biggest advantage of hormone-induced artificial insemination is that the timing of reproduction can be artificially adjusted; it has been commonly used in teleost owing to its commercial efficiency as it effectively increases the productivity of a fish farm (Hill et al., 2009; Karami et al., 2011; Hoga et al., 2018). If the hormone-induced artificial insemination method can be established in sharks, it would minimize shark-human contact by inducing reproduction at the desired timepoint. This advantage could significantly increase the chances of artificial insemination success in endangered shark species.
For hormone-induced artificial insemination, we used the hypothalamus-pituitary-gonadal axis that has been used in Osteichthyes (Rottmann et al., 1991; Cardinaletti et al., 2010; Genz et al., 2014; Yom-Din et al., 2016; Shanthanagouda and Khairnar, 2018). The hypothalamus secretes gonadotropin-releasing hormone (GnRH), the pituitary secretes gonadotropic hormones, and the gonads secrete gonadal steroids. This results in the maturation and release of germ cells in both males and females. Ovaprim® is one of the most popular agents that induces reproduction using the hypothalamus-pituitary-gonadal axis in fish. It has been used successfully for various species, proving its effectiveness and safety on fish, which are important criteria in hormone-induced artificial insemination (Yanong et al., 2009).
Ovaprim® is composed of a salmon gonadotropin-releasing hormone analog (20 μg/mL) and domperidone (10 mg/mL)2. The salmon gonadotropin-releasing hormone analog elicits the release of gonadotropins from the pituitary and domperidone acts as a Dopamine D2 receptor antagonist, negating other mechanisms of GnRH release inhibition (Yanong et al., 2009). Ovaprim® facilitates gonadotropin release and eventually induces maturation and release of germ cells, which is conducive to artificial insemination. However, since there is no official record of Ovaprim® application to elasmobranchs yet, it is not known whether Ovaprim® will work on sharks. To confirm whether it works, experiments using two shark species: the banded houndshark (Triakis scyllium) and the whitetip reef shark (Triaenodon obesus) were performed.
Triakis scyllium and T. obesus belong to the order Carcharhiniformes (the former to the family Triakidae and the latter, to Carcharhinidae). Triakis scyllium inhabits the Northwest Pacific Ocean and shows aplacental viviparity with internal fertilization (Compagno, 1984). They are relatively easier to handle by aquarists when bred in aquariums, thanks to their small body size (less than 1.5 m). Triakis scyllium is classified into the group of “least concern” by the IUCN Red List3 and is one of the most accessible shark species in the Republic of Korea. However, T. obesus lives in the warm waters of the Indo-Pacific Ocean. This species also fertilizes its gametes internally but shows placental viviparity. Although the IUCN Red List classifies this species into the “critically endangered” group4 and the species is actually facing extinction, it is also a species commonly seen in various aquariums where indoor breeding methods are well set-up. We chose these two species owing to their accessibility, abundance in the aquarium, and their smaller size in which both are thought to be sexually mature if the body length is longer than ∼1 m. Another important reason is that both show a synchronous reproduction strategy, which makes it meaningful to induce reproduction at the desired time point. A group of sharks showing reproductive synchrony have same stage of the reproduction cycle at a time, manifesting seasonal breeding (Castro, 2009).
Herein, to the best of our knowledge, we administered Ovaprim® for the first time to T. scyllium and T. obesus, confirming its effect on oocyte maturation, ovulation, and semen production. Based on this, a platform where artificial insemination in endangered sharks becomes practically possible, was constructed.
Materials and Methods
Sharks for the Experiment
As a result of selecting sharks with a total body length of approximately 90 centimeters or more, a total of five male and seven female T. scyllium, and three male and three female T. obesus individuals were collected from Hanwha Aqua Planet Jeju, Jeju-do, Republic of Korea. We searched for more adult sharks in large aquariums and fish markets in Korea for a sufficiently large experimental population. There were many immature sharks, but fully grown ones were not available. In other words, the sharks used in this experiment were virtually the best cohort available in the country. Triakis scyllium males were named M001–M005 and T. obesus WM001–WM003. Females were named F001–F007 and WF001–WF003, respectively. For identification, punch biopsy (Kai Medical, Japan) was done at the tip of the left pectoral fin of the sharks using a binary numbering system.
Body length and body weight were measured in all sharks and clasper calcification, elongation, bending, and rhipidion formation were checked in male sharks to judge sexual maturity and suitability for experimentation (Clark and Von Schmidt, 1965; Fujinami and Tanaka, 2013; Supplementary Table S1). As all males met the standards, all were utilized for experimentation.
Abdominal ultrasonography of the uterus and ovaries was performed on all female sharks to judge sexual maturity and for pregnancy detection using an Aloka ProSound 2 (Hitachi-Aloka Medical Ltd., Tokyo, Japan) with a convex probe set to a frequency of 26 MHz (Madigan et al., 2015; Swider et al., 2017; Anderson et al., 2018). Only F003 among the female T. scyllium displayed an ovarian follicle during ultrasonography and F001 and F002 were confirmed as pregnant. Blood sampling was carried out to check the hormone level baseline of the pregnant sharks, and they were excluded from the rest of the experiments. Ultrasounds conducted for over a month confirmed that the other individuals were not pregnant and showed differences in their blood hormone levels compared to those that were obviously pregnant (F001 and F002) (Table 1). Since the other females (F004–F007) did not show any follicle in their ovaries, it was highly likely that they were sexually immature. Despite this, we wanted to confirm the possibility of an Ovaprim® administration triggering the first ovarian cycle in the sharks since they were approaching the body length of sexually matured specimens. So, every non-pregnant female T. scyllium (F003–F007) was used as an experimental candidate.

Table 1. Hormone concentrations of Triakis scyllium and Triaenodon obesus prior to Ovaprim® administration.
The sharks’ breeding history was examined and according to it the T. scyllium have shown irregular pregnancy regardless of the season, and the T. obesus did not show any pregnancy at all throughout the exhibition. Since there was little change in water temperature and circadian rhythm throughout the year in the facility, their loss of seasonality is explainable (Schaller, 2006; George et al., 2017).
A physical examination, including swimming status, food response, and respiratory rate, was done to screen overall health status after more than a month of the acclimatization period. Blood examinations including hematology, blood chemistry tests, and blood gas analysis were performed. Packed cell volume was determined by centrifugation of whole blood. Plasma was separated from whole blood in a lithium heparin tube for testing Na+, K+, Cl–, Ca+, P, Mg2+, blood urea nitrogen, creatinine, uric acid, total protein, albumin, glucose, total bilirubin, direct bilirubin, total cholesterol, glutamic-pyruvate transaminase, glutamic-oxaloacetic transaminase, gamma-glutamyl transferase, lactate dehydrogenase, creatine phosphokinase, alkaline phosphatase, amylase, lipase, triglyceride, and NH3 using a Fuji Dri-Chem 4000i analyzer (Fujifilm, Tokyo, Japan). Blood gas analysis was also performed to determine TCO2, pCO2, pO2, sO2, pH, glucose, HCO3–, base excess, Na+, K+ and ionized Ca2+ using whole blood without anticoagulants with a CG8 + cartridge and VetScan i-STAT (Abaxis, CA, United States).
No anomalies were detected in complete blood count, blood chemistry, and blood gas analysis of the experimental candidates (Supplementary Table S2). All sharks were judged to have a body condition score of 3/5, and no abnormalities were identified by ultrasonography. All sharks showed normal swimming, good vitality, and good appetite throughout the experiment. Based on all these data, veterinarians (WHH and SWK) judged that all sharks were healthy and available for experimentation. Water quality of the shark tanks was maintained consistently throughout the experiment (Supplementary Table S3).
Water Quality and Environmental Management
Male and female sharks were contained separately in two sea water tanks in the Hanwha Aqua Planet Jeju. Both tanks were supplied with filtered water siphoned directly from the coastal sea around Jeju Island. Concentrations of dissolved oxygen (DO), nitrite (NO2–), nitrate (NO3–), ammonia (NH3), water temperature, salinity, gravity, and pH were maintained constant and checked at least once a week in both tanks. The sharks were target-fed mackerel, whiteleg shrimp, and squid thrice a week, and Mazuri Vita-Zu® Shark/Ray II Tablet (Purina Mills LCC, United States) once a week.
Reproductive Hormone Levels Before Ovaprim® Administration
Since only minimally invasive procedures were used in this study, sharks were sedated for every step. We used tricaine methanesulfonate (MS-222) in 50–60 ppm concentration to induce sedation for the experiments. To determine the hormone level before Ovaprim® (Syndel Laboratories, Vancouver, Canada) injection, 3 mL of blood was collected from all 16 sharks from their tail vein and placed in BD Vacutainer® SSTTM II Advance (BD, NJ, United States) tubes for serum separation. Serum estradiol, progesterone, and testosterone concentrations were measured by electrochemiluminescence immunoassay (ECLIA) using Elecsys® progesterone III assay, Elecsys® estradiol III assay, and Elecsys® testosterone II assay on the Cobas 8000 modular analyzer series (Roche Diagnostics Corp., Indianapolis, IN, United States; Neodin Veterinary Laboratory, Seoul, Republic of Korea). Blood collection and blood hormone analysis were carried out in the same manner for all subsequent experiments. The hormone concentration analyses were repeated four times per a shark over a one-month period. These data were used as the basis values for later experiments. In order to establish an accurate control group, it was necessary to administer a biologically ineffective fluid instead of Ovaprim® for each experiment, but due to a lack of population size, using this basis value was chosen as an alternative.
Dose Optimization of Ovaprim® and Its Effects in Male T. scyllium
The final purpose of this study was to determine if Ovaprim® could draw biological reactions through the shark’s hypothalamus-pituitary-gonadal axis, and if confirmed, to find out the optimum dose for performing artificial insemination in the sharks. In the case of male sharks, the criterion of judgment was quantity and quality of collected semen. For females, the administered dose should induce follicular maturation and ovulation but should not result in egg dropping. This is described schematically in Figure 1, and the doses falling under the shaded area indicate the optimum range for female sharks. Too high a dose will result in egg dropping, and too little cannot induce follicular maturation. Throughout this study, diverse doses were tested and according to their biological reactions in the tested sharks, the experiments were marked on the schematic graph (Figure 1). Optimized doses were assessed through this process for T. scyllium and T. obesus.
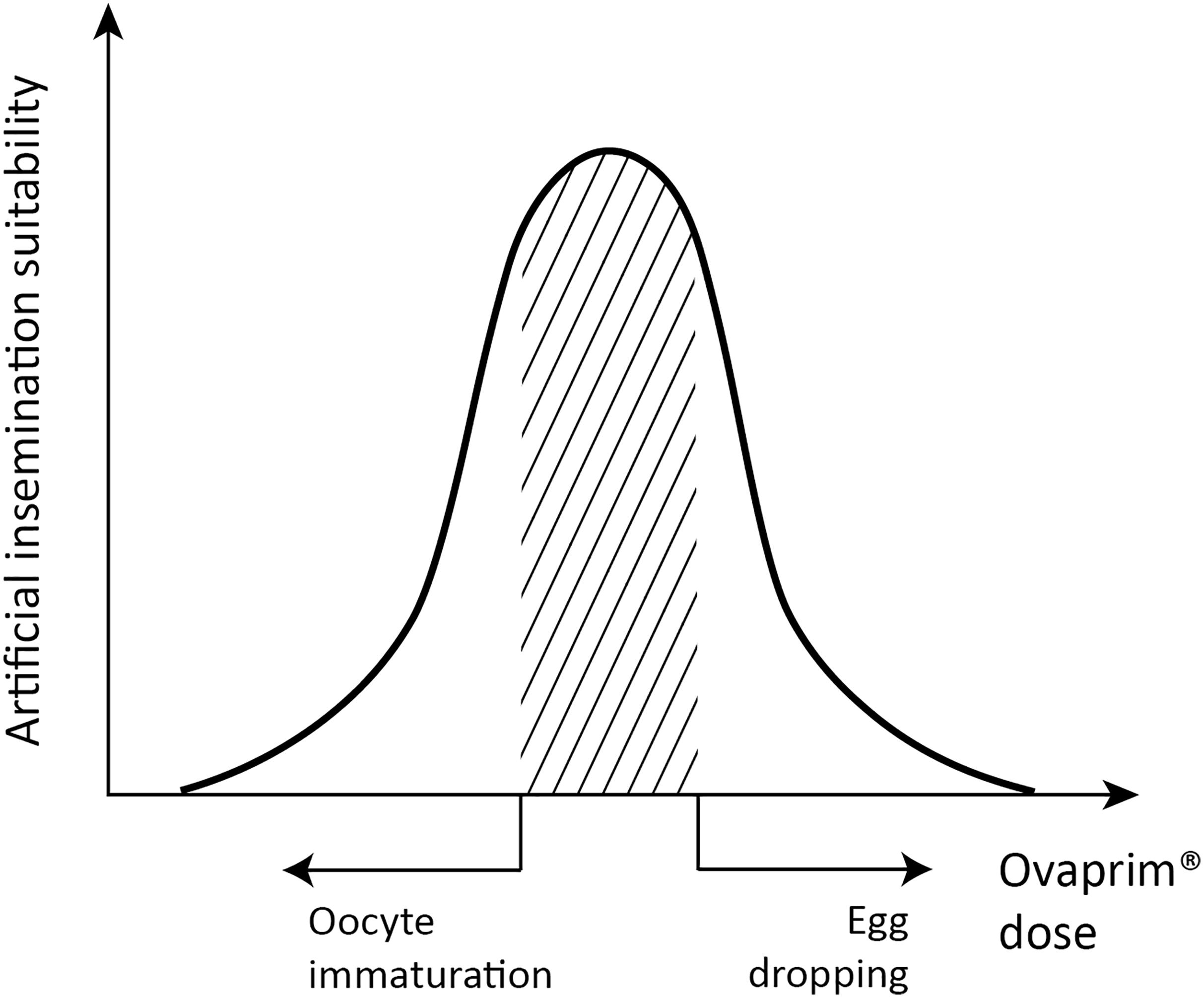
Figure 1. Schematic graph of dose optimization in female sharks. A conceptual graph showing the correlation between biological phenomena (x-axis) and artificial insemination suitability (y-axis) that change depending on the total dose of Ovaprim® administered. The shaded area denotes mature oocytes after follicle growth but prior to egg dropping, which is the targeted area for females in this study.
After testing the basic hormone level of the sharks, dose optimization experiments were performed using T. scyllium, which had a larger population size than T. obesus. The experiment was first performed on male sharks. Female doses were tested in another experiment, based on the determined male dose.
As there was no reported information about Ovaprim® dosage in sharks, we set experimental doses based on the amounts previously administered to Osteichthyes (Nandeesha et al., 1990; Khan et al., 2006; Genz et al., 2014; Paul and Chanda, 2014). We used male sharks for this experiment and divided them into three groups as follows: Group A: M004; Group B: M001 and M002; Group C: M003 and M005. Although Group A contained only one shark, it was still designated as a “Group” for ease of understanding. The sharks were grouped in this manner because we wanted to distribute both small and large specimens evenly in each group. We injected 0.1, 0.2, and 0.4 mL/kg of Ovaprim® intramuscularly (IM) to the Groups A, B, and C, respectively, into the epaxial muscle (Yanong et al., 2009). The change in the concentration of blood estradiol, progesterone, and testosterone was monitored, and semen collection trials were performed at 10 min pre-injection and at 1, 2, and 6 h post-injection. Semen was taken from the urogenital papilla of each shark using a syringe lacking the needle while the abdomen was massaged gently. The first transparent part of the ejaculated semen was discarded and only the subsequent cloudy part was taken, thereby excluding urine and maximizing the concentration of spermatozoa. After collection, semen volume was measured and stored at 21°C for subsequent experiments. Semen was diluted with activating solution and checked using an optical microscope for motile spermatozoa. Through this step, we confirmed whether Ovaprim® affected the hormonal system in sharks and determined the effective dosage for males.
After dose optimization, injection tests were performed using the optimized dose (0.2 mL/kg) to observe the timing for semen collection and blood hormone level changes up to 48 h post-injection. In total, three experiments were performed in a row with more than a month interval between experiments to allow the hormone concentrations to return to baseline levels. Semen collection was performed at 1, 12, and 36 h post-injection in each experiment. The blood-concentration of estradiol, progesterone, and testosterone were checked at 10 min pre-injection and at 1, 12, 24, 36, and 48 h post-injection in all three experiments.
Dose Optimization of Ovaprim® and Its Effects in Female T. scyllium
In females, Ovaprim® was administered twice in every experiment. The primary injection was to trigger follicular maturation and the second was for induction of ovulation. This strategy is commonly used in bony fish (Nandeesha et al., 1990; Khan et al., 2006; Genz et al., 2014; Paul and Chanda, 2014).
For dose optimization in females, four different dosages were tried. The first trial dose was deduced from the optimum dose in males based on Osteichthyes protocols (Nandeesha et al., 1990; Khan et al., 2006; Genz et al., 2014; Paul and Chanda, 2014). Based on the biological reactions following the injection, optimization was performed in serial, controlling the dose in the experiments. As the optimum dose for males was concluded to be 0.2 mL/kg IM in the epaxial muscle in the previous experiment, doses used in each of the four experiment were as follows: First: 0.6 mL/kg + 0.6 mL/kg; Second: 0.2 mL/kg + 0.4 mL/kg; Third: 0.2 mL/kg + 0.6 mL/kg; and Fourth: 0.2 mL/kg + 0.5 mL/kg. Blood estradiol, progesterone, and testosterone concentration analysis, follicular size change and ovulation checks with ultrasonography, and egg dropping checks were carried out at 10 min pre-injection and at 12, 24, and 36 h post-injection. Axes lengths and area of the follicle’s largest cross section at every time points were measured using the embedded tool from the ultrasonography device. Eccentricity was calculated manually with the long and short axis values. The time gap between the primary and the secondary injections in the first trial was set to 6 h, following protocols commonly used in other species (Nandeesha et al., 1990; Paul and Chanda, 2014). As the hormone level graph and biological reactions from each experiment were being monitored, the timings for the second injection were adjusted and applied throughout the experiments as follows: First: 6 h; Second: 12 h; Third: 24 h; and Fourth: 24 h. To perform the following experiment after the hormone concentration returned to baseline, a time interval of more than 1 month was set between each experiment.
Dose Optimization of Ovaprim® and Its Effects in T. obesus
Based on the experimental outcome in T. scyllium, 0.2 mL/kg Ovaprim® was administered to T. obesus males. As the biological reaction to the injection was thought to be appropriate, three replicate experiments were performed without further dose optimization for males. The concentration of estradiol, progesterone, and testosterone was assessed in blood drawn 10 min pre-injection and 12, 24, 36, and 48 h post-injection. Semen collection was performed at 15 min, and 1, 12, 24, 36, and 48 h post-injection.
In females, 0.2 mL/kg Ovaprim® was administered twice at a 24 h interval for the first experiment, extrapolating the results of female T. scyllium and male T. obesus. Two experimental doses were administered in the female group with more than a month interval between the trials. Administered doses were as follows: First: 0.2 mL/kg + 0.2 mL/kg; Second: 0.2 mL/kg + 0.3 mL/kg at a 24 h interval. Blood collection for hormone analyses, ovary ultrasonography for follicular growth measurement, and egg drop checking were carried out 10 min pre-injection and at 12, 24, 36, and 48 h post-injection, using the same methodologies that were applied to T. scyllium.
Results
Reproductive Hormone Levels Before Ovaprim® Administration
Results of blood hormone levels are shown in Table 1. In females, it was possible to distinguish between pregnant and non-pregnant individuals based on their estradiol concentration. An average estradiol level of 9159.80 pg/mL for the pregnant group, and 1018.68 pg/mL for the non-pregnant group was recorded, showing more than a ninefold difference between the two (Table 1). There were evident interspecific differences, especially in estradiol and testosterone concentrations in females and males, respectively. Male T. obesus showed a more than six times higher testosterone level than that in male T. scyllium. Female T. scyllium, however, showed a more than 400 times higher estradiol concentration than that in female T. obesus. This suggests that the baseline data must be established separately for each species. As there is no previously published information on hormone levels for both T. scyllium and T. obesus, the data presented in Table 1 merits academic use in other studies and by clinicians.
Dose Optimization of Ovaprim® and Its Biological Effects in Male T. scyllium
The results from the hormone level analysis of male T. scyllium are shown in Figure 2. We found that all three Ovaprim® concentrations administered to Groups A, B, and C induced hormonal reactions in sharks. This was the first time that Ovaprim® has been confirmed to be effective physiologically in the shark. Among the three hormones tested, testosterone level fluctuations were the most obvious, and thus, it was chosen as one of the bases for monitoring the biological reaction of the male sharks. The initial fluctuation was greatest in group B at 59.6 ng/mL, whereas the fluctuation of testosterone was 34.3 ng/mL in group A and 13.0 ng/mL in group C. The results from group B and C confirmed that a 0.2 mL/kg dose was effective to elicit in vivo reactions, as the fluctuation was larger in group B, even though similar baseline concentrations at 0 h (Group B: 94.8 ng/mL; Group C: 98 ng/mL) were evident.
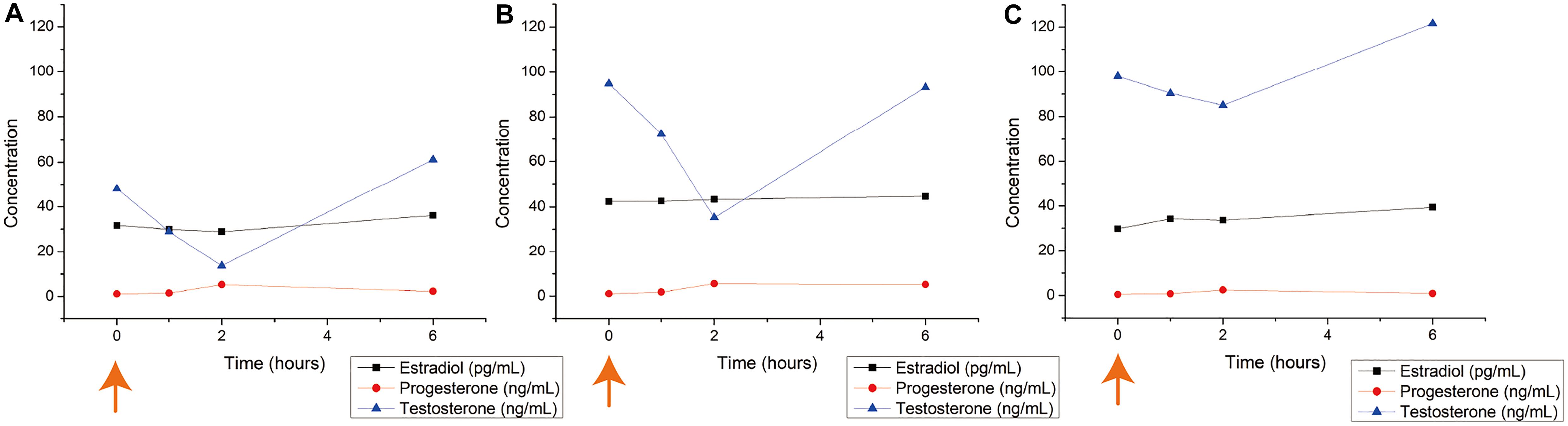
Figure 2. Blood hormone level changes after Ovaprim® injection in male Triakis scyllium. Ovaprim® was injected intramuscularly into the epaxial muscle. (A) Average hormone concentration changes of Group A (0.1 mL/kg). (B) Average hormone concentration changes of Group B (0.2 mL/kg). (C) Average hormone concentration changes of Group C (0.4 mL/kg). The orange arrows indicate the time points of Ovaprim® injection.
Semen from all sharks was sampled. It was most easily sampled at 1 h post-injection. The average volume of semen sampled at 1 h post-injection was 1.9 mL for Group A; 7.7 mL for Group B; and 2.8 mL for Group C. No semen could be sampled from any of the experiments in any group at 6 h post-injection and at 10 min pre-injection. Thus, all three doses triggered semen emissions, of which the dosage of 0.2 mL/kg resulted in the largest volume of ejaculate. It also confirmed that 1 h post-injection was an appropriate interval at which to procure semen samples. Based on these observations, we concluded that the optimum Ovaprim® dose in male T. scyllium was 0.2 mL/kg.
To check the biological influence of this optimum dose in T. scyllium, 0.2 mL/kg Ovaprim® was injected into all five male sharks. This experiment was performed in triplicate and the resulting hormone level data are shown in Figure 3A. Up to 48 h of observation showed that testosterone levels decreased in the beginning, recovered, and then increased, reaching peak values at 36 h. In addition, we attempted semen sampling at 1, 12, and 36 h post-injection. The average volume of the five semen samples was 4.6 mL at 1 h; 2.0 mL at 12 h; and 0 mL at 36 h post-injection. We were able to confirm that 1 h post-injection was the most optimum time for semen sampling in T. scyllium.
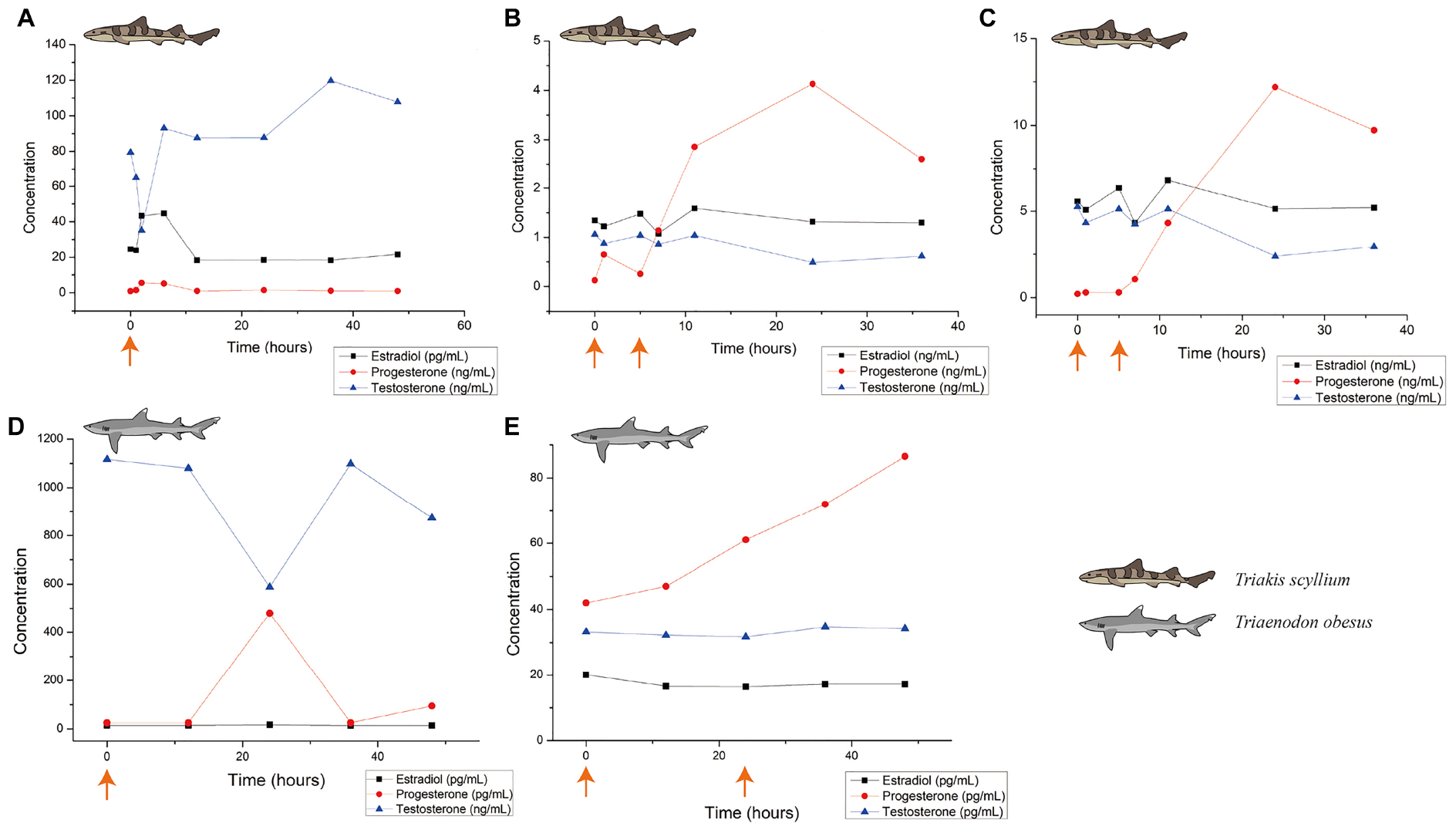
Figure 3. Blood hormone level changes after Ovaprim® injection in Triakis scyllium and Triaenodon obesus. (A) Average hormone concentration changes in male T. scyllium. Ovaprim® was injected at 0.2 mL/kg dose at the orange arrowed time point. (B) Average hormone concentration changes of female T. scyllium. Ovaprim® was injected at 0.6 mL/kg dose at the orange arrowed time points. (C) Average hormone concentration changes of T. scyllium (Female #3; F003). Ovaprim® was injected at 0.6 mL/kg dose at the orange arrowed time point. (D) Average hormone concentration changes of male T. obesus. Ovaprim® was injected at 0.2 mL/kg dose at the orange arrowed time point. (E) Average hormone concentration changes in female T. obesus. Ovaprim® was injected at 0.2 mL/kg and 0.3 mL/kg doses in serial at the orange arrowed time points.
Dose Optimization of Ovaprim® and Its Effects in Female T. scyllium
In females, dose optimization was performed in four steps, which resulted in the blood hormone concentration graph in Figure 3B. Of the three hormones, progesterone showed the clearest form of fluctuation, reaching its peak at 24 h. Optimum dosage was judged based on biological reactions. As the purpose of Ovaprim® administration in females is to successfully induce follicular maturation and artificial insemination-performable conditions, the goal was to isolate safe zones that would avoid the two undesirable phenomena for artificial insemination: immature follicles and egg dropping (Figure 3). The aim of this experiment was to find the doses that would coincide with the shaded area. It was determined that it would only be possible to perform artificial insemination when the experimental conditions fell into this area.
In the first experiment, Ovaprim® was administered to all female T. Scyllium sharks (F003–F007). As a result, the average hormone concentration change graph was as shown in Figure 3B. However, data showed a large gap between the hormonal levels in F003 and the rest of the female sharks. The hormone concentration graph for F003 is shown in Figure 3C. In F003, the basic concentration of hormones itself was higher than in the other sharks. In addition, the change in progesterone concentration itself was significantly greater in F003 than in the other sharks (F003: 12.0 ng/mL; F004: 3.1 ng/mL; F005: 2.3 ng/mL; F006: 3.4 ng/mL; F007: 1.7 ng/L).
There was another clue differentiating F003 from the other females: the changes in the ovary follicle size before and after Ovaprim® administration using ultrasonography. In F003, it was possible to identify the presence of ovarian follicles both before and after administration, and the observed size change of the follicle was apparent. However, for F004–F007, no visible follicles were observed on ultrasonography, both before and after hormone administration. Comparing the ultrasound reports of F003 before and after Ovaprim® administration, the increase in follicle size could be clearly discerned not only through measured figures (Figure 4A), but also with the naked eye (Figures 4B,C).
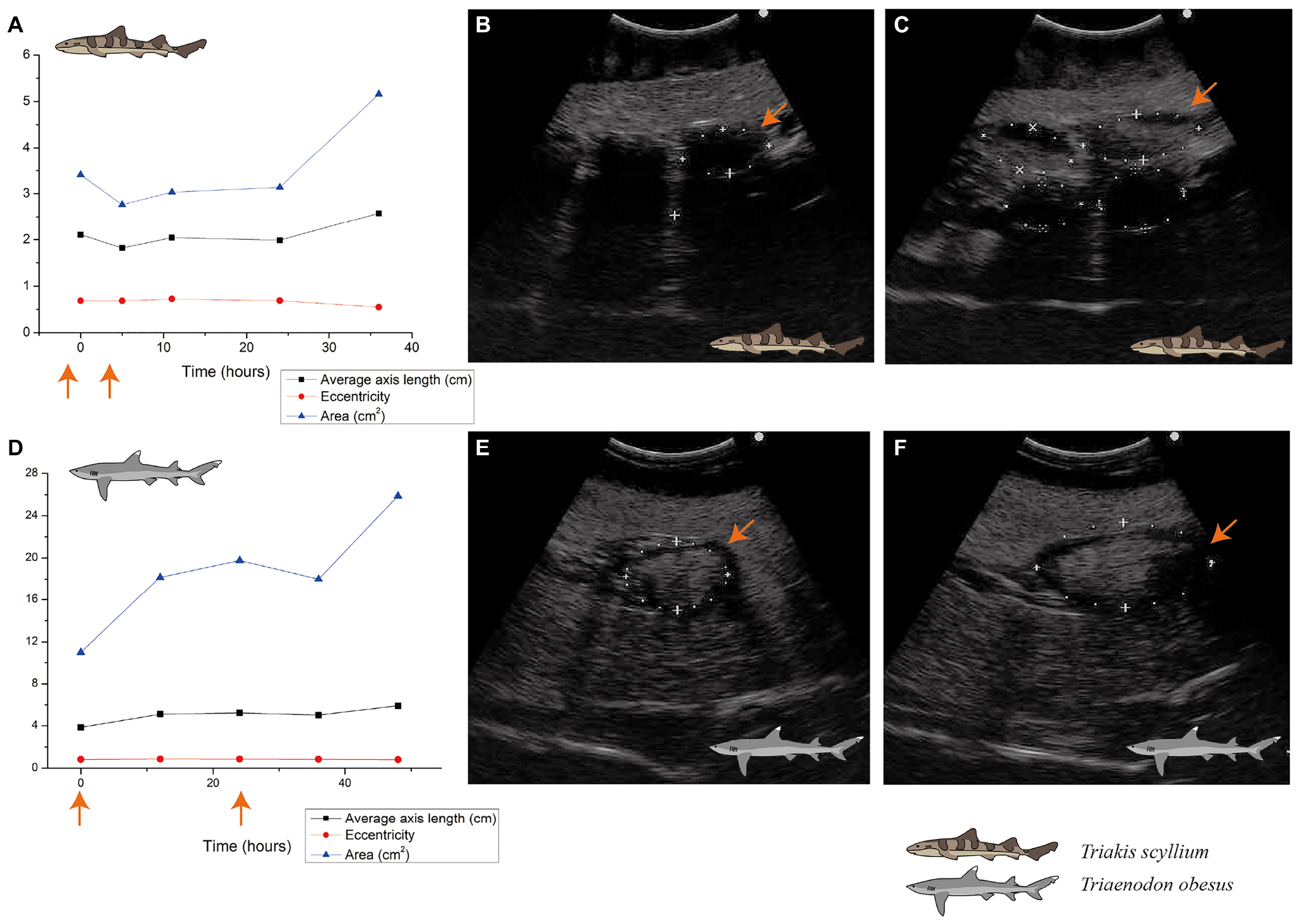
Figure 4. Ovarian follicle size change after Ovaprim® injection in Triakis scyllium and Triaenodon obesus. (A) Ovarian follicle size changes after Ovaprim® injection in female T. scyllium. Axis length and area values were measured directly on ultrasonography. Eccentricity was calculated with the long and short axis values. The orange arrows indicate the time points of Ovaprim® administration. (B) Ultrasonography of left ovary before Ovaprim® injection in T. scyllium (F003). The orange arrows indicate the same follicle in (C) at different time point. Size of the follicle with the orange arrow was 3.7 cm × 1.9 cm (5.45 cm2). (C) Ultrasonography of left ovary after Ovaprim® injection in T. scyllium (F003). Follicles showed distinct size increase. The orange arrows indicate the same follicle in (B) at different time point. Size of the follicle with the orange arrow was 4.9 cm × 2.0 cm (7.60 cm2). (D) Ovarian follicle size changes after Ovaprim® injection in female T. obesus. Axis length and area values were measured directly on ultrasonography. Eccentricity was calculated with the long and short axis values. The orange arrows indicate the time points of Ovaprim® injection. (E) Ultrasonography of left ovary before Ovaprim® injection in WF002 (T. obesus). The orange arrows indicate the same follicle in (F) at different time point. Size change of the follicles can be recognized by ultrasonography. Size of the follicle with the orange arrow was 4.4 cm × 3.0 cm (10.27 cm2). (F) Ultrasonography of left ovary after Ovaprim® injection in WF002 (T. obesus). The orange arrows indicate the same follicle in (E) at different time point. Size of the follicle with the orange arrow was 7.4 cm × 3.7 cm (21.77 cm2).
Collectively, we concluded F003 was the most and the only mature shark, and thus, further examinations were done with F003 only. Therefore, three more dose optimization experiments were conducted only on F003. The optimum time interval between the first and second injections in each experiment had to be decided, and the first experiment using the time gap of 6 h resulted in peak values of hormone concentration change (Figure 3B) and in increased follicle size (Figure 4A) at 24 h post-injection. Based on these observations, the time interval between injections was set at 24 h for the third and fourth experiments.
The biological reactions in the experiments on F003 are shown in Figure 5A: first experiment (0.6 mL/kg + 0.6 mL/kg): both follicle maturation and egg dropping occurred; second experiment (0.2 mL/kg + 0.4 mL/kg): neither follicle maturation nor egg dropping occurred; third experiment (0.2 mL/kg + 0.6 mL/kg): both follicle maturation and egg dropping occurred; fourth experiment (0.2 mL/kg + 0.5 mL/kg): follicle maturation occurred but egg dropping did not. As T. scyllium is an aplacental viviparous species, or ovoviviparous species, the fact that the eggs were dropped means these were not conducive conditions for artificial insemination. Around 15–20 eggs were found on the bottom of the female tank during the first and third experiments. As a result of all four experiments, the optimum protocol for female T. scyllium was concluded to be two injections of 0.2 and 0.5 mL/kg of Ovaprim® at a 24 h interval.
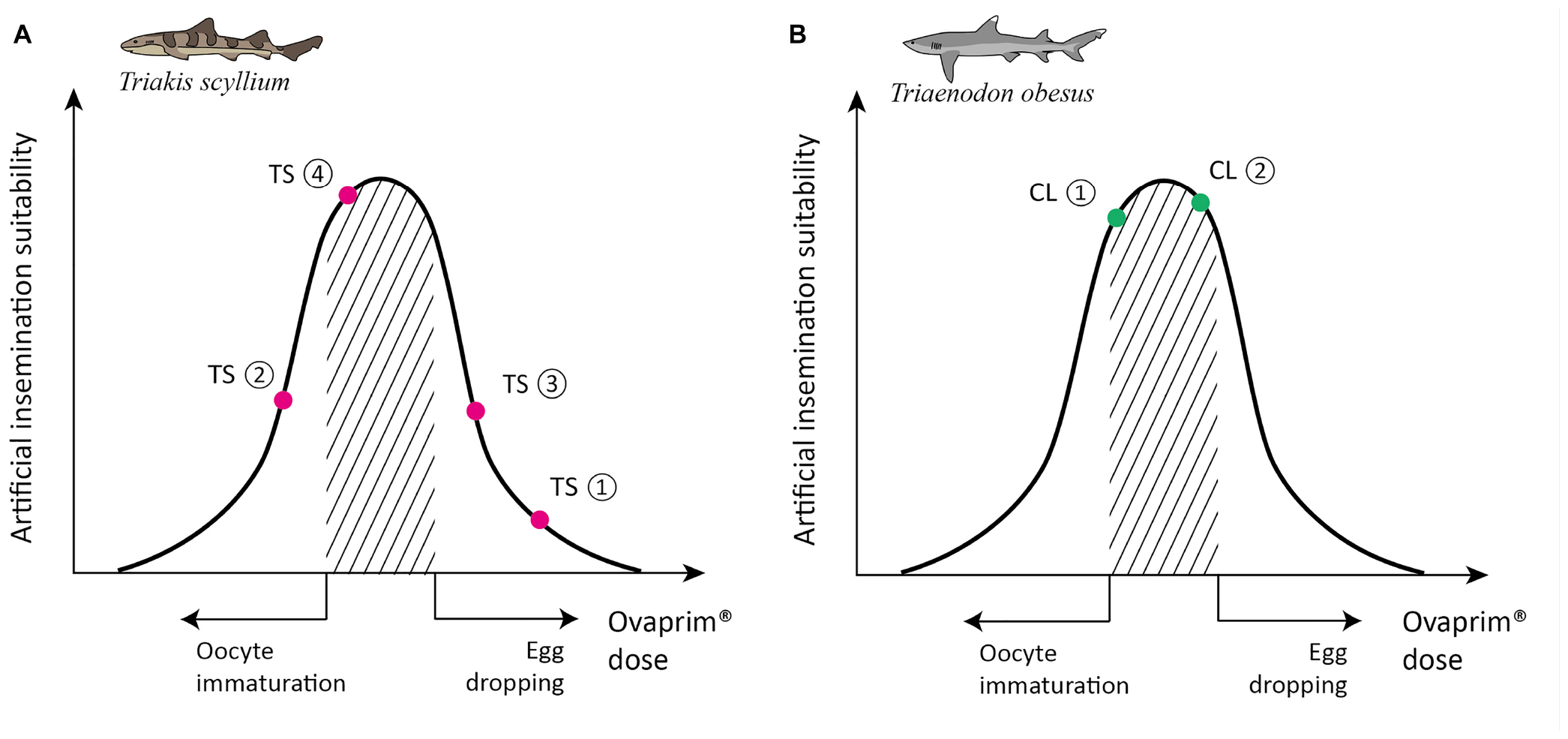
Figure 5. Schematic graph of dose optimization in Triakis scyllium and Triaenodon obesus. A conceptual graph showing the correlation between biological phenomena (x-axis) and artificial insemination suitability (y-axis) that change depending on the total dose of Ovaprim® administered. The shaded area denotes mature oocytes after follicle growth but prior to egg dropping, which is the targeted area for females in this study. (A) Ovaprim® experimental doses for female T. scyllium. TS①: 0.6 mL/kg + 0.6 mL/kg, 6 h time gap; TS②: 0.2 mL/kg + 0.4 mL/kg, 12 h time gap; TS③: 0.2 mL/kg + 0.6 mL/kg, 24 h time gap; TS④: 0.2 mL/kg + 0.5 mL/kg, 24 h time gap. (B) Ovaprim® experimental doses for female T. obesus. CL①: 0.2 mL/kg + 0.2 mL/kg, 24 h time gap; CL②: 0.2 mL/kg + 0.3 mL/kg, 24 h time gap.
Dose Optimization of Ovaprim® and Its Effects in T. obesus
Changes in blood hormone levels after administration of 0.2 mL/kg Ovaprim® in males are as shown in Figure 3D. Overall, testosterone levels after salmon gonadotropin-releasing hormone analog administration were significantly higher in T. obesus than those in T. scyllium, although these decreased at 24 h post-injection. Progesterone, however, showed peak concentrations at 24 h compared to at 12 h in T. scyllium, thus showing different increments between species. Estradiol remained constant at very low levels. Unlike T. scyllium, semen was not sampled at both 1 h and 12 h post-injection. The sampling time point was further reduced to 15 min post-injection and as a result, sufficient amounts of semen could be collected with an average volume of 6.5 mL. Semen sampling was found to be effective if carried out almost immediately after Ovaprim® administration in T. obesus. Semen sampling at later stages could result in the loss of semen to the surrounding environment.
In females, both the first (0.2 mL/kg + 0.2 mL/kg) and second (0.2 mL/kg + 0.3 mL/kg) experiments showed follicular maturation (Figures 4D–F) and no egg dropping (Figure 5B). Follicular size change could be recognized clearly both by the naked eyes in ultrasonography and through measurements in Figure 4D. No egg dropping suggested that the sharks were in a suitable state for artificial insemination. Overall, hormone levels after Ovaprim® administration were lower than those in female T. scyllium (Figure 3E). Estradiol and testosterone levels remained constant, whereas progesterone concentrations showed a steady increase. As there was no peak within 48 h, it was thought that the progesterone level would increase after 48 h.
Discussion
Biological Reactions Following Ovaprim® Administration in Sharks
For seasonally breeding sharks, natural ovarian follicular maturation occurs gradually throughout the year concomitant with a gradual increase in follicular diameter (Sulikowski et al., 2007; George et al., 2017). Therefore, the follicular maturation that we observed over 1 or 2 days in the current study is not a natural phenomenon. We found that follicular diameters increased by more than 1.5–2.0 cm within only 48 h in response to the administration of Ovaprim®, thereby confirming that this salmon gonadotropin-releasing hormone can effectively induce follicular maturation in both T. scyllium and T. obesus.
The observation that sharks lost their seasonality during the captive period also supports the argument that the follicular maturation was due to Ovaprim® administration (Schaller, 2006; George et al., 2017). The loss of seasonality, as a consequence of a reduction in water temperature and circadian rhythm changes over seasons, has been observed in sharks maintained in some aquaria, and indeed, maintaining constant water temperatures year-round is suggested as a method for reducing reproductive activity (Schaller, 2006; Henningsen et al., 2008; George et al., 2017). Consequently, taking into consideration the fact that the sharks lose seasonality and thereby undergo a reduction in reproductive activity, we assume that their ovarian follicular growth observed in the present study is more likely to have been a response to the administration of Ovaprim®, rather than a manifestation of the natural reproductive cycle.
Given that reproduction is the most important aspect of conservation efforts, the loss of seasonality phenomenon in captive-bred sharks can represent a major hurdle in ex situ conservation programs. Accordingly, our observations that Ovaprim® can induce follicular maturation in sharks that have lost their seasonality highlight the potential utility of this agent in overcoming the reproductive hurdle. In this regard, numerous previous studies on bony fish have demonstrated the successful promotion of reproduction via hormone induction, even during periods other than the natural breeding seasons of these fish. For example, in carp, in which the administration of Ovaprim® is a commonly used treatment, breeding can be induced more than twice annually during non-breeding periods5. Thus, if Ovaprim® can be used to overcome natural patterns in fish reproductive cycles, it could become a potentially useful tool in the ex situ conservation of a range of endangered species.
In this study, Ovaprim® could induce biological reactions only when the sharks matured sexually to some extent—confirmed by both hormone concentration analyses and Ovaprim® injection experiments using sharks F003–F007. We found that the basal hormonal concentrations in shark F003 were higher than those observed in the six other sharks. Given the significant differences in physique between sharks F004–F007 and F003 (Supplementary Table S1), our findings, with respect to differences in hormonal concentrations, are consistent with those reported in previous studies showing that the levels of gonadal hormones increase as an animal matures (Lutton et al., 2005). The other four sharks (F004–F007) did not have active follicles prior to the administration of Ovaprim®, indicating that these sharks were not sufficiently mature. As observed previously in bony fish, the lack of response to Ovaprim® administration in the sharks confirms that the gamete developmental stage is an important factor influencing the response to exogenous hormonal stimulation (Anderson et al., 2013).
Accordingly, it is important to determine sexual maturity accurately in sharks prior to using Ovaprim®. In this study, especially in the case of females, it was the ultrasonography images of the ovarian follicles that played a decisive role in determining sexual maturity—only the sharks that had follicles before administration of Ovaprim® were mature enough to be used in the experiment. Data on the baseline concentrations of sex hormones has not been established for most shark species; thus, it is very important to check the existence, size change, and ovulation of follicles using ultrasonography. In various piscine species, ultrasound is commonly used to determine sexual maturity, maximizing the efficiency of AI (Petochi et al., 2011; Du et al., 2017; Kujawa et al., 2019). As artificial intervention is much more difficult for sharks than other fish, accurate ultrasonography use is important for successful conservational attempt.
Based on measured gonadal hormone concentrations, our findings indicate that both female and male sharks showed responses to the administration of Ovaprim®. These observations indicate that the hypothalamus-pituitary-gonadal axis, which has previously been identified in bony fishes, including sturgeons, might also exist in sharks (Yom-Din et al., 2016). Although the roles of salmon gonadotropin-releasing hormone analog and gonadal steroids have been identified, we did not directly determine the pituitary levels of hormones, including gonadotropic hormones, in the present study. However, as the basic role of gonadotropic hormones is to induce fluctuations in gonadal steroid levels in each sex and to induce the maturation and release of germ cells, their roles were being carried out effectively in the sharks examined in the present study.
Finally, our observations that salmon gonadotropin-releasing hormone analog functions in two species from different genera, i.e., species showing different types of reproductive strategies based on the presence or absence of the placenta, indicate that Ovaprim® could also induce similar biological reactions in other shark species. These findings are consistent with those of Lovejoy et al. (1992), who identified molecules similar to salmon GnRH and chicken GnRH in sharks. Further studies are required to ascertain whether these two different forms of GnRH act similarly.
Necessity and Possibility of Ovaprim® Usage for Hormone-Induced Artificial Insemination in Wild Shark Conservation
Populations of a diverse range of piscine species are being threatened by human activities, and accordingly, efforts are being made to conserve some of these species (Halpern et al., 2008; Arthington et al., 2016). Induced reproduction is one of the important tools for the ex situ conservation of endangered Osteichthyes species (Ogale, 1997; Mijkherjee et al., 2002). For example, Ogale (2002) and Devi et al. (2009) have reported the breeding and conservation of Mahseer and Osteobrama belangeri, respectively, and hormone-induced artificial reproduction has been conducted using sturgeon (Anderson et al., 2013). Furthermore, in India, the West Bengal Government is making active use of induced breeding techniques and shares the culture methods used for endangered species with local farmers (Mijkherjee et al., 2002). They also have professed their plans to study and share induced breeding techniques in other endangered species.
Among the different classes of fish, the Chondrichthyes are characterized as having the highest proportion of threatened species (30.39%)6. Although the risk of extinction among these cartilaginous fish is more serious than that of Actinopterygii (16.77%), Chondrichthyes conservation tends to rank low on the international conservation agenda (Dulvy et al., 2008; Jacques, 2010), which presumably reflects the fact that few Chondrichthyes species are of direct commercial importance. Accordingly, the importance of their conservation is recognized to a greater extent from an ecological perspective rather than from an economic imperative (Jacques, 2010). However, marked declines in the populations of apex predators can potentially precipitate the collapse of marine food chains, and thus in many instances may result in a reduction of food resources for humans (Myers et al., 2007; Ferretti et al., 2010). Consequently, although Chondrichthyes conservation may not seem to be of direct economic benefit, the opposite may in fact be true.
The conservation of Chondrichthyes species includes both in situ and ex situ initiatives (Friedrich et al., 2014; Fox et al., 2018), the former of which includes establishing marine reserves or regulating fishing, and thereby maintaining populations of animals in their native habitats, whereas the latter preserves animals using facilities external to their native ranges (Arthington et al., 2016; Fox et al., 2018). Although most of the conservation efforts for Chondrichthyes are in situ, some ex situ-managed breeding and monitoring programs are being conducted by several aquaria (Fox et al., 2018). However, to the best of our knowledge, none of these programs have employed hormone-induced breeding for the purposes of Chondrichthyes conservation.
In the case of wild sharks, aquaria could potentially serve as sites for ex situ conservation. However, if water temperatures and circadian rhythms at the facilities are not precisely controlled, there is a high probability that reproductive activity would be reduced, as indicated by the findings of the present study (Henningsen et al., 2008). Furthermore, when targeting large wild sharks, it is generally difficult to accurately determine the timing of the ovulation of mature eggs, as conventional artificial insemination methods involve frequent ultrasonography, blood collection, and hormone concentration analysis. Given these aforementioned factors, hormonal triggering is deemed to be among the essential tools required for the ex situ conservation of sharks.
Hormonal inducers such as Ovaprim® can be used for a number of different purposes, from inducing natural courtship behaviors to facilitating artificial reproduction (Viveiros et al., 2002; Zarski et al., 2009; Montchowui et al., 2011). Successful induction of reproduction in an internal fertilizer requires conditions such as the stable ovulation of mature eggs, acquisition of spermatozoa with good fertility and quantity, and artificial insemination at the appropriate time, and by manipulating the timing and number of reproduction events using hormonal inducers, the efficacy of reproduction can be maximized7. As a hormonal inducer, Ovaprim® has commonly been used to induce spawning for successful fertilization and hatching in vulnerable wild species, and is also used in the establishment of ovulation protocols for introducing new species to the aquaculture industry (Sarkar et al., 2004; Zadmajid et al., 2017). These uses of Ovaprim® have been based on one common feature, namely, the successful induction of reproduction in wild fish. However, prior to the present study, it had not been established whether this product could be used to induce biological reactions in Chondrichthyes.
As a proactive step toward the development of a hormone-induced artificial insemination protocol for endangered wild sharks, we used Ovaprim® to induce germ cell maturation and established suitable dosages and an injection protocol. We found that a single 0.2 mL/kg Ovaprim® dose induced semen release in the males of both study species and that the administration of two 0.2 mL/kg + 0.5 mL/kg doses (at a 24 h interval) in T. scyllium, and 0.2 mL/kg + 0.2 mL/kg or 0.3 mL/kg (at a 24 h interval) in T. obesus females induced follicular maturation without egg dropping. Accordingly, given that the basic conditions for hormone-induced artificial insemination can be established using Ovaprim®, we can reasonably expect that hormone-induced reproduction can be achieved in sharks. Compared with the development of hormone-induced artificial insemination technology in other fish species, the research reported herein is in its infancy; however, the findings of the present study, which to the best of our knowledge, is the first to have demonstrated successful hormone-induced reproduction in sharks, can serve to guide the direction of further related studies (Nandeesha et al., 1990; Khan et al., 2006; Brzoska and Adamek, 2008; Olumuji and Mustapha, 2012; Dhara and Saha, 2013). Considering the importance of hormone-induced reproduction techniques in the ex situ conservation of wild sharks, we believe that the findings of the present study will make a potentially important contribution to the conservation of endangered shark species.
Study Limitations and Future Perspectives
Studies on the populations of wild animals are often constrained by sample scarcity, limited study periods, and insufficient funding, inter alia (Morrison et al., 2008). Sharks as species representative of marine wildlife are notably difficult targets for sampling, and the small sample sizes typically obtained invariably impose certain experimental limitations: only simple comparison between groups was possible, and different conditions were tested on an animal with temporal intervals of more than a month between experiments. Therefore, further studies designed to augment population size are inevitable.
Nevertheless, even given such limitations, we believe that the findings of the present study provide a clear direction for further research on shark hormone-induced artificial insemination. Ovaprim® was found to induce biological reactions in the shark, and as expected, the induced reactions were confirmed to be related to germ cell maturation and release. We were able to establish a general dose range and determined an appropriate administration protocol, and although fewer experiments were carried out for T. obesus than for T. scyllium, the desired biological responses could be induced, thereby indicating that the protocol developed in this study has potentially broad applicability in sharks.
Although Ovaprim® was found to induce ovulation and semen release, we examined only the maturity or quantity of the germ cells, and consequently, further studies will be necessary to verify whether there are any negative effects of synthetic salmon gonadotropin-releasing hormone analog administration on the quality of these germ cells. Aside from volume, other metrics of semen quality—morphology (normal spermatozoa), motility (progressive spermatozoa), viability, and concentration—should be evaluated in further studies to confirm the time to collect quality samples. Furthermore, given that we did not undertake an actual artificial insemination experiment in the present study, we were unable to determine the fertilization rate of the artificially maturated germ cells. Moreover, we did not perform analyses of the pituitary hormones, and thus, further studies are required to verify the presence, changes in concentration, and interactions of pituitary-level hormones in the hypothalamus-pituitary-gonadal axis of sharks.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved by Seoul National University Institutional Animal Care and Use Committee (approval number: SNU-181218-2).
Author Contributions
SWK conceived the study, designed, prepared, and performed the experiments, analyzed the data, wrote the manuscript, and produced the figures and tables. WHH adjusted field scheduling, prepared and performed the experiments, analyzed the data, and revised the manuscript. SJH, JK, SBL, and HK helped in performing the experiments. SSG and SGK revised the manuscript. BYK and DWK facilitated the experiments on Jeju Island. GJ and BCL commented on the experiment design and preparations and revised the manuscript. SCP supervised the entire project. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1A02086128). This research was also supported by the ‘Korea Research Fellowship Program’ of the National Research Foundation of Korea (NRF), Ministry of Science and ICT (KRF: 2016H1D3A1909005).
Conflict of Interest
WHH and DWK were employed by the company Hanwha Hotels & Resorts Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the help provided by the staff of Hanwha Aqua Planet Jeju. We also express gratitude to Won Min Han and Young Man Park of Hanwha Aqua Planet, who helped conduct the experiments in their facilities.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.571741/full#supplementary-material
Footnotes
- ^ IUCN Red List of Threatened Species 2019. http://www.iucnredlist.org (accessed September 10, 2020).
- ^ U.S.FDA. FOI Summary Ovaprim. https://www.fda.gov/animal-veterinary/minor-useminor-species/foi-summary-ovaprim (Accessed September 9, 2020).
- ^ IUCN Red List of Threatened Species 2019. Banded houndshark (Triakis scyllium). https://www.iucnredlist.org/species/161395/5413845 (Accessed March 16, 2020).
- ^ IUCN Red List of Threatened Species 2019. Oceanic whitetip reef shark (Triaenodon obesus). https://www.iucnredlist.org/species/39374/2911619 (Accessed March 16, 2020).
- ^ Sukumaran, K.K. (1985). Seed production and hatchery management. http://www.fao.org/3/ac229e/AC229E03.htm (Accessed September 16, 2020).
- ^ IUCN Red List of Threatened Species 2019. Summary Statistics. https://www.iucnredlist.org/resources/summary-statistics (Accessed September 10, 2020).
- ^ Sukumaran, K.K. (1985). Seed production and hatchery management. http://www.fao.org/3/ac229e/AC229E03.htm (Accessed September 16, 2020).
References
Anderson, B., Belcher, C., Slack, J., and Gelsleichter, J. (2018). Evaluation of the use of portable ultrasonography to determine pregnancy status and fecundity in bonnethead shark Sphyrna tiburo. J. Fish Biol. 93, 1163–1170. doi: 10.1111/jfb.13831
Anderson, W. G., Genz, J., and McDougall, C. (2013). Using OvaprimTM as a Conservation Tool for Lake Sturgeon, Acipenser fulvescens: The Short and Long Term Effects of Endocrine Manipulation During the Reproductive Cycle. Winnipeg: University of Manitoba.
Arthington, A. H., Dulvy, N. K., Gladstone, W., and Winfield, I. J. (2016). Fish conservation in freshwater and marine realms: status, threats and management. Aquatic Conserv. 26, 838–857. doi: 10.1002/aqc.2712
Blanco, J. M., Wildt, D. E., Hofle, U., Voelker, W., and Donoghue, A. M. (2009). Implementing artificial insemination as an effective tool for ex situ conservation of endangered avian species. Theriogenology 71, 200–213. doi: 10.1016/j.theriogenology.2008.09.019
Brzoska, E., and Adamek, J. (2008). Artificial spawning of European catfish, Silurus glanis L.: stimulation of ovulation using LHRH-a, Ovaprim and carp pituitary extract. Aquac. Res. 30, 59–64. doi: 10.1046/j.1365-2109.1999.00301.x
Cardinaletti, G., Franzoni, M. F., Palermo, F. A., Cottone, E., Mosconi, G., Guastalla, A., et al. (2010). “Environmental and neuroendocrine control of fish reproduction,” in Recent Advances in Fish Reproduction Biology, eds A. G. Ayala, J. M. Penalver, and E. C. Pozo (New York, NY: Research Signpost), 67–87.
Castro, J. I. (2009). Observations on the reproductive cycles of some viviparous North American sharks. Aqua, Int. J. Ichthyol. 15, 4–15.
Clark, E., and Von Schmidt, K. (1965). Sharks of the central gulf coast of Florida. Bull. Mar. Sci. 15, 13–83.
Comizzoli, P., Mermillod, P., and Mauget, R. (2000). Reproductive biotechnologies for endangered mammalian species. Reprod. Nutr. Dev. 40, 493–504. doi: 10.1051/rnd:2000113
Compagno, L. J. V. (1984). Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date, Vol. 4, FAO Species Catalogue. Rome: FAO, 432.
Devi, G. A., Devi, G. S., Singh, O. B., Munilkumar, S., and Reddy, A. K. (2009). Induced spawning and hatching of Osteobrama belangeri (Valenciennes) using Ovatid, an ovulating agent. Asian Fish. Sci. 22, 1107–1115.
Dhara, K., and Saha, N. C. (2013). Controlled breeding of Asian catfish Clarias batrachus using pituitary gland extracts and Ovaprim at different temperatures, latency periods and their early development. J. Aquac. Res. Dev. 4:186. doi: 10.4172/2155-9546.1000186
Du, H., Zhang, X., Leng, X., Zhang, S., Luo, J., Liu, Z., et al. (2017). Gender and gonadal maturity stage identification of captive Chinese sturgeon, Acipenser sinensis, using ultrasound imagery and sex steroids. Gen. Comp. Endocrinol. 245, 36–43. doi: 10.1016/j.ygcen.2016.08.004
Dulvy, N. K., Baum, J. K., Clarke, S., Compagno, L. J. V., Cortés, E., Domingo, A., et al. (2008). You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshwater. Ecosyst. 18, 459–482. doi: 10.1002/aqc.975
Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R., and Lotze, H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x
Fox, G., Darolti, I., Hibbitt, J., Preziosi, R. F., Fitzpatrick, J. L., and Rowntree, J. K. (2018). Bespoke markers for ex-situ conservation: application, analysis and challenges in the assessment of a population of endangered undulate rays. J. Zoo Aquar. Res. 6, 50–56. doi: 10.19227/jzar.v6i2.299
Friedrich, L. A., Jefferson, R., and Glegg, G. (2014). Public perceptions of sharks: gathering support for shark conservation. Mar. Policy 47, 1–7. doi: 10.1016/j.marpol.2014.02.003
Fujinami, Y., and Tanaka, S. (2013). Age, growth and reproduction of the banded houndshark Triakis scyllium around the tip of the Izu Peninsula. Japan. Nippon Suisan Gakk. 79, 968–976. doi: 10.2331/suisan.79.968
Genz, J., McDougall, C. A., Burnett, D., Arcinas, L., Khetoo, S., and Anderson, W. G. (2014). Induced spawning of wild-caught adult lake sturgeon: assessment of hormonal and stress response, gamete quality, and survival. J. Appl. Ichthyol. 30, 1565–1577. doi: 10.1111/jai.12548
George, R. H., Steeil, J., and Baine, K. (2017). “Diagnosis and treatment of common reproductive problems in elasmobranchs,” in The Elsmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives, eds M. Smith, D. Warmolts, D. Thoney, R. Hueter, M. Murray, and J. Ezcurra (Columbus, OH: The Ohio State University Printing Servicies), 363–374.
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, C. V., Micheli, F., D’Agrosa, C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. doi: 10.1126/science.1149345
Henningsen, A. D., Murru, F. L., Rasmussen, L. E. L., Whitaker, B. R., and Violetta, G. C. (2008). Serum levels of reproductive steroid hormones in captive sand tiger sharks, Carcharias taurus (Rafinesque), and comments on their relation to sexual conflicts. Fish Physiol. Biochem. 34, 437–446. doi: 10.1007/s10695-008-9202-9
Hermes, R., Göritz, F., Streich, W. J., and Hildebrandt, T. B. (2007). Assisted reproduction in female rhinoceros and elephants - current status and future perspective. Reprod. Domest. Anim. 42, 33–44. doi: 10.1111/j.1439-0531.2007.00924.x
Hildebrandt, T. B., Hermes, R., Walzer, C., Sós, E., Molnar, V., Mezösi, L., et al. (2007). Artificial insemination in the anoestrous and the postpartum white rhinoceros using GnRH analogue to induce ovulation. Theriogenology 67, 1473–1484. doi: 10.1016/j.theriogenology.2007.03.005
Hill, J. E., Kilgore, K. H., Pouder, D. B., Powell, J. F. F., Watson, C. A., and Yanong, R. P. E. (2009). Survey of Ovaprim use as a spawning aid in ornamental fishes in the United States as administered through the University of Florida tropical aquaculture laboratory. N. Am. J. Aquacult. 71, 206–209. doi: 10.1577/A08-020.1
Hoga, C. A., Almeida, F. L., and Reyes, F. G. R. (2018). A review on the use of hormones in fish farming: analytical methods to determine their residues. CyTA J. Food. 16, 679–691. doi: 10.1080/19476337.2018.1475423
Jacques, P. J. (2010). The social oceanography of top oceanic predators and the decline of sharks: a call for a new field. Prog. Oceanogr. 86, 192–203. doi: 10.1016/j.pocean.2010.04.001
Karami, A., Christianus, A., Zokaeifar, H., Saad, K. Z., Imraan, F. T. J., Shakibazadeh, S., et al. (2011). Ovaprim treatment promotes oocyte development and milt fertilization rate in diploid and triploid African catfish (Clarias gariepinus). Aquac. Int. 19, 1025–1034. doi: 10.1007/s10499-011-9419-y
Khan, A. M., Shakir, H. A., Ashraf, M., and Ahmad, Z. (2006). Induced spawning of Labeo rohita using synthetic hormones. Punjab Univ. J. Zool. 21, 67–72.
Kujawa, R., Nowosad, J., Biegaj, M., Cejko, B. I., and Kucharczyk, D. (2019). Use of ultrasonography to determine sex in sexually immature European river lamprey Lampetra fluviatilis (L.). Anim. Reprod. Sci. 204, 95–100. doi: 10.1016/j.anireprosci.2019.03.009
Lack, M., and Sant, G. (2011). The Future of Sharks: A Review of Action and Inaction. TRAFFIC Report. Cambridge: TRAFFIC International and the Pew Environment Group, 44.
Lovejoy, D. A., Ashmead, M. B., Coe, I. R., and Sherwood, N. M. (1992). Presence of gonadotropin-releasing hormone immunoreactivity in dogfish and skate brains. J. Exp. Zool. 263, 272–283. doi: 10.1002/jez.1402630307
Lutton, B. V., George, J., Murrin, C. R., Fileti, L. A., and Callard, I. P. (2005). “The Elasmobranch Ovary,” in Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids, and Chimaeras, ed. W. C. Hamlett (Luton: Science Publishers), 237–282.
Madigan, D. J., Brooks, E. J., Bond, M. E., Gelsleichter, J., Howey, L. A., Abercrombie, D. L., et al. (2015). Diet shift and site-fidelity of oceanic whitetip sharks Carcharhinus longimanus along the Great Bahama Bank. Mar. Ecol. Prog. Ser. 529, 185–197. doi: 10.3354/meps11302
Masuda, M., Izawa, Y., Kametsuta, S., Ikuta, H., and Isogai, T. (2003). Artificial insemination of the cloudy catshark. J. Japan. Assoc. Zoo. Aquar. 44, 39–43.
Masuda, M., Izawa, Y., Kametsuta, S., Ikuta, H., and Isogai, T. (2005). Artificial insemination of the white-spotted bamboo shark. Chiloscyllium plagiosum. J. Jpn. Assoc. Zoo. Aquar. 46, 91–96.
Mijkherjee, M., Praharaj, A., and Das, S. (2002). Conservation of endangered fish stocks through artificial propagation and larval rearing technique in West Bengal. India. Aquaculture Asia 7, 8–11.
Montchowui, E., Laleye, P., Philippart, J. C., and Poncin, P. (2011). Reproductive behaviour in captive African carp, Labeo parvus Boulenger, 1902 (Pisces: Cyprinidae). J. Fish. Int. 6, 6–12. doi: 10.3923/jfish.2011.6.12
Morrison, M. L., Block, W. M., Strickland, M. D., Collier, B. A., and Peterson, M. J. (2008). Wildlife Study Design. New York, NY: Springer-Verlag.
Myers, R. A., Baum, J. K., Shepherd, T. D., Powers, S. P., and Peterson, C. H. (2007). Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850. doi: 10.1126/science.1138657
Nandeesha, M. C., Rao, K. G., Jayanna, R. N., Parker, N. C., Varghese, T. J., Keshavanath, P., et al. (1990). “Induced spawning of Indian major carps through single application of Ovaprim-C,” in The Second Asian Fisheries Forum, eds R. Hirano and I. Hanvu (Manila: Asian Fisheries Society), 581–585.
Ogale, S. N. (2002). Mahseer Breeding and Conservation and Possibilities of Commercial Culture. The Indian Experience. FAO Fisheries Technical Paper. 431. Rome: FAO, 193–212.
Ogale, S. N. (1997). Induced spawning and hatching of golden mahseer Tor putitora (Hamilton) at Lonavla, Pune District (Maharashtra) in Western Ghats. Fishing Chimes 1997, 27–29.
Olumuji, O. K., and Mustapha, M. K. (2012). Induced breeding of African mud catfish, Clarias gariepinus (Burchell 1822), using different doses of normal saline diluted Ovaprim. J. Aquac. Res. Dev. 3:133. doi: 10.4172/2155-9546.1000133
Petochi, T., Di Marco, P., Donadelli, V., Longobardi, A., Corsalini, D., Bertotto, D., et al. (2011). Sex and reproductive stage identification of sturgeon hybrids (Acipenser naccarii × Acipenser baerii) using different tools: ultrasounds, histology and sex steroids. J. Appl. Ichth. 27, 637–642. doi: 10.1111/j.1439-0426.2011.01715.x
Rottmann, R. W., Shireman, J. V., and Chapman, F. A. (1991). Introduction to Hormone-Induced Spawning of Fish. SRAC publication No. 421. Stoneville, MS: SRAC.
Sarkar, U. K., Negi, R. S., Deepak, P. K., Singh, S. P., Srivastava, S. M., and Roy, D. (2004). Captive breeding of vulnerable Indian carp Cirrhinus reba with Ovaprim for conservation of wild populations. Aquac. Asia 9, 5–7.
Schaller, P. (2006). Husbandry and reproduction of Whitetip reef sharks at Steinhart Aquarium. San Francisco. Int. Zoo Yearb 40, 232–240. doi: 10.1111/j.1748-1090.2006.00232.x
Shanthanagouda, A. H., and Khairnar, S. O. (2018). Breeding and spawning of fishes: role of endocrine gland. Int. J. Fish. Aquat. Stud. 6, 472–478.
Sulikowski, J. A., Driggers, W. B. III, Ford, T. S., Boonstra, R. K., and Carlson, J. K. (2007). Reproductive cycle of the blacknose shark Carcharhinus acronotus in the Gulf of Mexico. J. Fish Biol. 70, 428–440. doi: 10.1111/j.1095-8649.2007.01314.x
Swider, D. A., Corwin, A. L., Kamerman, T. Y., Zimmerman, S. L., Violetta, G. C., Davis, J., et al. (2017). “Reproduction of spotted eagle rays, Aetobatus narinari, in aquaria,” in Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives, eds M. Smith, D. Warmolts, D. Thoney, R. Hueter, M. Murray, and J. Ezcurra (Columbus, OH: The Ohio State University Printing Servicies), 433–442.
Tambling, C. J., Avenant, N. L., Drouilly, M., and Melville, H. I. (2018). “The role of Mesopredators in ecosystems: potential effects of managing their populations on ecosystem processes and biodiversity,” in The Livestock Predation and its Management in South Africa: a Scientific Assessment, eds G. I. H. Kerley, S. L. Wioson, and D. Balfour (Port Elizabeth: Nelson Mandela University), 205–227.
Taniuchi, T. (1988). Aspects of reproduction and food habits of the japanese swellshark Cephaloscyllium umbratile from Choshi. Japan. Nippon Suisan Gakk 54, 627–633. doi: 10.2331/suisan.54.627
Thongtip, N., Mahasawangkul, S., Thitaram, C., Pongsopavijitr, P., Kornkaewrat, K., Pinyopummin, A., et al. (2009). Successful artificial insemination in the Asian elephant (Elephas maximus) using chilled and frozen-thawed semen. Reprod. Biol. Endocrin. 7:75. doi: 10.1186/1477-7827-7-75
Viveiros, A. T. M., Fessehaye, Y., Veld, M., Schulz, R. W., and Komen, J. (2002). Hand-stripping of semen and semen quality after maturational hormone treatments, in African catfish Clarias gariepinus. Aquaculture 213, 373–386. doi: 10.1016/S0044-8486(02)00036-4
Yanong, R. P. E., Martinez, C., and Watson, C. A. (2009). Use of Ovaprim in Ornamental Fish Aquaculture. Gainesville, FL: University of Florida IFAS Extension, FA161.
Yom-Din, S., Hollander-Cohen, L., Aizen, J., Boehm, B., Shpilman, M., Golan, M., et al. (2016). Gonadotropins in the russian sturgeon: their role in steroid secretion and the effect of hormonal treatment on their secretion. PLoS One 11:e0162344. doi: 10.1371/journal.pone.0162344
Zadmajid, V., Mirzaee, R., Hoseinpour, H., Vahedi, N., and Butts, I. A. E. (2017). Hormonal induction of ovulation using OvaprimTM [(D-Arg6, Pro9NEt)-sGnRH + domperidone] and its impact on embryonic development of wild-caught Longspine scraper, Capoeta trutta (Heckel, 1843). Anim. Reprod. Sci. 187, 79–90. doi: 10.1016/j.anireprosci.2017.10.009
Keywords: Triakis scyllium, Triaenodon obesus, synthetic salmon GnRH, Ovaprim, hormone-induced artificial insemination
Citation: Kim SW, Hong WH, Han SJ, Kwon J, Ko H, Lee SB, Giri SS, Kim SG, Kim BY, Jang G, Lee BC, Kim DW and Park SC (2020) Use of Synthetic Salmon GnRH and Domperidone (Ovaprim®) in Sharks: Preparation for ex situ Conservation. Front. Mar. Sci. 7:571741. doi: 10.3389/fmars.2020.571741
Received: 11 June 2020; Accepted: 28 October 2020;
Published: 19 November 2020.
Edited by:
Bahram Falahatkar, University of Guilan, IranReviewed by:
Kit Yue Kwan, Beibu Gulf University, ChinaDariusz Kucharczyk, University of Warmia and Mazury in Olsztyn, Poland
Copyright © 2020 Kim, Hong, Han, Kwon, Ko, Lee, Giri, Kim, Kim, Jang, Lee, Kim and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Se Chang Park, cGFya3NlY0BzbnUuYWMua3I=
†These authors have contributed equally to this work
 Sang Wha Kim1†
Sang Wha Kim1† Sib Sankar Giri
Sib Sankar Giri Se Chang Park
Se Chang Park