- DiSTAV, Department of Earth, Environment and Life Sciences, University of Genoa, Genoa, Italy
Two severe heat waves triggered coral bleaching and mass mortality in the Maldives in 1998 and 2016. Analysis of live coral cover data from 1997 to 2019 in shallow (5 m depth) reefs of the Maldives showed that the 1998 heat wave caused more than 90% of coral mortality leaving only 6.8 ± 0.3% of survived corals in all the shallow reefs investigated. No significant difference in coral mortality was observed among atolls with different levels of human pressure. Maldivian reefs needed 16 years to recover to the pre-bleaching hard coral cover values. The 2016 heat wave affected all reefs investigated, but reefs in atolls with higher human pressure showed greater coral mortality than reefs in atolls with lower human pressure. Additionally, exposed (ocean) reefs showed lower coral mortality than those in sheltered (lagoon) reefs. The reduced coral mortality in 2016 as compared to 1998 may provide some support to the Adaptive Bleaching Hypothesis (ABH) in shallow Maldivian reefs, but intensity and duration of the two heat waves were different. Analysis of coral cover data collected along depth profiles on the ocean sides of atolls, from 10 to 50 m, allowed the comparison of coral mortality at different depths to discuss the Deep Refuge Hypothesis (DRH). In the upper mesophotic zone (i.e., between 30 and 50 m), coral mortality after bleaching was negligible. However, live coral cover did not exceed 15%, a value lower than coral survival in shallow reefs. Low cover values of corals surviving in the mesophotic reefs suggest that their role as refuge or seed banks for the future recovery of some species in shallow-water reefs of the Maldives may be small. The repeatedly high coral mortality after bleaching events and the long recovery period, especially in sites with human pressure, suggest that the foreseen increased frequency of bleaching events would jeopardize the future of Maldivian reefs, and ask for reducing local pressures to improve their resilience.
Introduction
Coral reefs throughout the world are facing the consequences of changing Earth’s climate. The ENSO phenomenon, which is a natural periodic fluctuation in sea surface temperature (El Niño) and air pressure of the overlying atmosphere (Southern Oscillation) across the equatorial Pacific Ocean (Dijkstra, 2006), has important consequences for the climate around the globe. Ocean warming worldwide and particularly intense ENSO episodes are leading to an increase in the severity, duration and frequency of coral bleaching events, which threatens the long-term stability of coral reefs, hampering their resilience to local human pressure (Hughes et al., 2018a). Reefs in the world have recently been affected by three major global bleaching events, in 1998, 2010, and 2016. The U.S. National Oceanic and Atmospheric Administration (NOAA) declared that the last bleaching event can be considered as the longest, the most widespread, and probably the most damaging on record (Hughes et al., 2017). More than 70% of coral reefs around the world bleached and experienced catastrophic levels of coral mortality (Eakin et al., 2016).
Corals suffer thermal stress when sea surface temperatures exceed the seasonal means and protract for extended periods to cause the disruption of the mutualistic symbiosis between corals and their dinoflagellate endosymbionts (zooxanthellae), resulting in the expulsion of zooxanthellae and consequent coral mortality (LaJeunesse et al., 2018). The relationship among heat exposure, bleaching, and consequent mortality of different taxa is still not well understood (Hughes et al., 2018b). However, a protracted value of 1°C above the maximum summer mean has been often described as high enough to cause coral bleaching (Kayanne, 2017), depending also upon sea and whether conditions. Current projections foresee the collapse of coral reefs by the end of this century due to the increased frequency and severity of coral bleaching events worldwide (Hoegh-Guldberg, 1999; Hoegh-Guldberg et al., 2007; Veron et al., 2009; Bay et al., 2017; Heron et al., 2017). However, these projections do not consider the potential of corals to survive a changing climate by adapting and/or developing responses apt to increase their resilience to climate-driven stressors. In this regard, two hypotheses have been proposed: (i) the adaptive bleaching hypothesis (hereafter ABH); and (ii) the deep refuge hypothesis (hereafter DRH).
Although coral reefs are confined to stable habitats and narrow environmental conditions, the ABH postulates that the loss of one or more zooxanthellae strains, rapidly replaced by different ones with the formation of a new symbiotic consortium, has the potential to create new and more tolerant organisms, capable of surviving a variety of extreme conditions (Buddemeier and Fautin, 1993). Capacity to adapt ensured coral reef communities to persist over geological time scales through significant climate and sea-level fluctuations (Buddemeier et al., 2004); however, oceans are presently warming at a faster rate with respect to the geological past and decadal evolutionary responses to warming may drive the selection of the most adequate species able to adapt rapidly to environmental change (Padfield et al., 2015). Genetic adaptation to sea warming has been predicted (Matz et al., 2018), and reduced bleaching and mortality by some corals exposed to previous bleaching events has been often reported (Coles et al., 2018, and references therein). Adaptive bleaching mechanisms are not common in all corals (McClanahan and Muthiga, 2014; McClanahan, 2017), and shifts in species composition and decline in coral diversity are predicted to occur as temperature increases (Bahr et al., 2016; Richardson et al., 2018).
According to the DRH, marine organisms in deeper habitats are buffered from high surface temperatures and increased temperature variability (Bongaerts et al., 2010; Morri et al., 2017; Bianchi et al., 2019, and references therein). Mesophotic coral ecosystems are light-dependent communities developing at tropical and subtropical latitudes that extend from 30 m depth to as deep as about 165 m (Kahng et al., 2010), at the lower limit of the photic zone (Hinderstein et al., 2010; Loya et al., 2016; Laverick et al., 2017). Although working at mesophotic depths is constrained by traditional scuba limits, investigations on mesophotic coral reefs have grown exponentially in recent years due to advances in technology and because of the increasing recognition of their potential role as refuge for shallow-water reefs (Hoegh-Guldberg and Bruno, 2010; Loya et al., 2016; Muir et al., 2017, 2018). Corals show bleaching and mortality especially in shallow waters (Jokiel, 2004) whilst the occurrence of bleaching events and consequent mortality is normally lower at greater depths (Baird et al., 2018). DRH therefore suggests that mesophotic coral ecosystems may provide refuge and a source of recruits to facilitate recovery of shallow-water reefs damaged after a disturbance (Glynn, 1996; Riegl and Piller, 2003; Holstein et al., 2015; Thomas et al., 2015; Prasetia et al., 2017; Baird et al., 2018). Mesophotic reefs may display an important degree of overlap in species composition, and maintain connectivity with shallow reefs (Lesser et al., 2018; Muir et al., 2018), thus contributing to the local persistence of heat sensitive species despite periods of elevated seawater temperatures during strong ENSO episodes (Gatti et al., 2017; Shlesinger et al., 2018). Most families of scleractinian corals have been shown to be represented in the mesophotic zone of the Great Barrier Reef, with many also extending to greater depths, thus giving each evolutionary lineage some potential for being safeguarded in the case of shallow-reef degradation (Muir et al., 2018).
Repeated heat waves in the Maldives during the last decades provided the opportunity to better understand the impact of bleaching and mass mortality on coral communities. The general trend of coral recovery after the severe bleaching event of 1998 has already been investigated in previous papers (Bianchi et al., 2003, 2006, 2017; Lasagna et al., 2008), while the bleaching event of 2010 was comparatively milder and did not cause widespread mortality (Morri et al., 2015). The consequences of the further severe bleaching event of 2016 have been analyzed by Montefalcone et al. (2018a), especially with respect to the constructional potential of Maldivian reefs. The availability of a twenty-three year series of data on coral cover in the Maldives, encompassing the two most important bleaching events of 1998 and 2016 allowed comparing their impact and exploring the following hypotheses: (1) coral mortality after the 2016 bleaching event should be lower than after the 1998 event, according to the ABH; (2) coral mortality in deep waters should be lower than in shallow waters, meaning that mesophotic reefs can offer refuge to thermal stress as postulated by the DRH. Clearly, we did not analyze the ecophysiological mechanisms of ABH and DRH but simply concentrated on their manifest consequence: increased coral survival.
This study aims at: (i) reconstructing the thermal regime in the Maldives to identify the heat waves that caused severe bleaching and mass coral mortality in the last two decades; (ii) reconstructing the trend of human pressure (tourism and resident population) in the Maldives to be compared with coral mortality rates in coincidence with the bleaching events; (iii) evaluating the trajectories of change and recovery patterns in shallow reef communities after the two mass mortality events; (iv) investigating geographical patterns of hard coral survival during the last bleaching event according to location (i.e., atolls with high or low human pressure) and reef exposure (i.e., lagoon- or ocean-facing reefs); (v) discussing the ABH using our long-term series of coral cover data collected on shallow reefs at about 5 m depth; (vi) discussing the DRH by analyzing coral cover reduction and mortality along depth profiles, from 10 to 50 m, surveyed in years with and without coral mortality.
Materials and Methods
Study Area and Field Activities
The Maldives Archipelago consists of 27 atolls with ca. 1190 small coral islands stretched over an area of 860 km long from about 7°070 N to 0°400 S in latitude and 72°330 E to 73°450 E in longitude, with more than 99% of its territory covered by water. Coral reefs of the Maldives are considered as the seventh largest coral reef system on Earth, representing 3.14% of the World’s reef area (Dhunya et al., 2017).
Scientific cruises took place annually in late April – early May between 1997 and 2019. Every year, eight to eleven sites were chosen randomly and surveyed across the atolls of Ari, Felidhoo, Gaafu Alifu (Suvadiva), North Malé, South Malé, Rasdhoo, and Thoddoo (Supplementary Table S1). Based on the occurrence of inhabited islands and infrastructures, the atolls of North Malé and South Malé were considered to be subjected to higher human pressure with respect to the remaining atolls (Godfrey, 2006). Data (see below for detail) were collected by scuba diving at reef sites located either on the ocean-exposed reefs or in lagoon sites (lagoon-facing sides of the atoll rim or patch reefs).
To evaluate the overall reef status of the Maldives (with a main focus on the central atolls because of logistic constraints), our sampling design implied a completely randomly selection of the study sites each year, to assure data independency. Monitoring the history of a specific reef site was indeed not within the scope of this long series of cruises. Each year an equal number of ocean reefs and lagoon reefs has been sampled, always distinguishing between atolls with high or low human pressure. Although the cruise route differed from year to year, some reefs (31%) were casually revisited in different years (Supplementary Table S1). In total, 168 sites were surveyed; their geographical position was recorded using a portable GPS.
Climate and Local Human Pressure Regimes
Sea surface temperature data was used as a proxy for climate change (Montefalcone et al., 2018b). A twenty-three year trend (1997–2019) of monthly maximum sea surface temperature (SST) was plotted from data provided by the U.S. National Oceanic and Atmosphere Administration (NOAA) (available at http://coralreefwatch.noaa.gov/vs/gauges/maldives.php) for the area of the Maldives Archipelago. Satellite data were calibrated by the usual process of linear regression with discontinuous field data on sea surface temperature from our own archives, collected contemporaneously with the biological data. Maximum SSTs were compared to the two regional bleaching thresholds defined for the Maldives, corresponding to: (i) 30.9°C for severe bleaching events that are likely to cause widespread coral mortality and live coral cover reduction (NOAA, 2016); and (ii) 30.5°C for moderate bleaching events that usually have no wide-scale impacts on Maldivian coral reefs (Montefalcone et al., 2018a).
Since both the intensity and the duration of thermal stress are key factors in bleaching response, the Coral Bleaching Degree Heating Week (DHW) has been developed by NOAA (Kayanne, 2017) as an index of thermal stress, which measures the amount of heat stress accumulated in an area over the past 12 weeks. Intensity and duration of thermal stress are combined into the DHW single number, which is thus expressed as “degree C-weeks”. DHW values >4.0 are likely to induce some bleaching, whilst DHW values >8.0 result in widespread bleaching and mass coral mortality (Kayanne, 2017). To estimate DHW for the Maldives we counted the number of weeks per year when the monthly mean SST was higher than the temperatures triggering moderate (30.5°C) or severe (30.9°C) bleaching events in the Maldives, and we added up all temperatures exceeding the regional bleaching thresholds during that time period. The estimated values of DHW for the Maldives were then compared with DHW values available from the NOAA coral reef watch database1 considering the region of the “northern-eastern hemisphere centre” (i.e., the central Indian Ocean) over an area of 60 degrees × 40 degrees. A linear regression was performed on a total of 19 observations to test relationships between estimated values of DHW for the Maldives and DHW values available from NOAA coral reef watch.
As regards human pressure, the twenty-three year trends (1997–2019) of the resident population (number of inhabitants) and of the number of tourist arrivals in the Maldives were plotted from data provided by the United Nations (Department of Economic and Social Affairs Population Division), by the National Bureau of Statistics in the Maldives (Department of National Registration), and by the Ministry of Tourism data (compiled from annual reports, available at https://www.tourism.gov.mv/downloads/stats). For the four main central atolls investigated in this study (North and South Malé, Felidhoo, and Ari), the resident population (number of inhabitants) obtained from the national census carried out in 1995, 2000, 2006, and 2014 (available at http://statisticsmaldives.gov.mv/yearbook/statisticalarchive) and the twenty-three year trends (1997–2019) of the number of beds in the resorts were also provided.
Data Collection
Shallow reefs at 5±1m depth were surveyed each year. Composition and status of reef communities were described using live hard coral, bleached (but still alive) coral, and recently dead coral. The latter has been defined as coral deprived of living polyps but with the whole colony still in place and with a well-defined shape of the corallites (meaning that the main erosive processes have not started yet, i.e., usually within 1 year from coral death). The percent substratum cover for each of the three categories was visually estimated by the plain view technique of Wilson et al. (2007). Divers hovered 1–2 m above the bottom observing an area of 20 m2, in three replicate spots (tens of meters apart) at each reef site and at each sampling time. To reduce bias in visual estimations, the same observers collected data during the surveys from 1997 to 2013, while new observers trained by the former estimated cover values from 2014 to 2019.
In a limited number of years (i.e., 1997, 1998, 1999, 2007, 2012, 2013, 2015, 2016, 2017, 2018, and 2019) the same surveying method (making sure to maintain a constant depth during each data collection) has also been used to visually estimate the percent substratum cover of live hard coral, bleached coral, and recently dead coral down depth profiles in each of the surveyed reefs (Supplementary Table S1), at depths corresponding to the lower photic reef (i.e., 10 and 20 m) and at depths corresponding to the upper mesophotic reef (i.e., 30, 40, and 50 m) (Loya et al., 2016, and references therein).
Data Management and Analysis
Twenty-three year (1997–2019) trends of the mean (± standard error) percent cover of live hard coral and recently dead coral in shallow reefs were obtained. While the severe bleaching event of 1998 had been already described in detail in previous papers (Bianchi et al., 2003, 2006, 2017; Lasagna et al., 2008; Morri et al., 2015), here we analyzed with greater detail the bleaching event of 2016, exploring patterns of live hard coral cover in the pre-bleaching year (2015), during the event (2016), in the post-bleaching year (2017), and in the following early recovery years (i.e., 2018 and 2019), contrasting atolls with high or low human pressure.
To compare the two bleaching events of 1998 and 2016, two subsets were extrapolated from the whole dataset (Montefalcone et al., 2018a): the pre-bleaching years (1997 vs. 2015), the years of the bleaching (1998 vs. 2016), and the 2 years after (1999 and 2000 vs. 2017 and 2018).
The mean (± standard error) coral mortality (in %) was computed as the ratio of recently dead coral to live coral in the two subsets (i.e., 1997–2000 and 2015–2018), using the following formula:
where RDC is the cover of recently dead coral, HC the cover of live hard coral, and BC the cover of bleached coral.
The mean (± standard error) of the coral mortality and of the percent cover of HC and RDC was computed down the depth profile (10 m to 50 m) in two periods: i) years without coral mortality (i.e., 1997, 1998, 2012, 2013, 2015, and 2019), and ii) years with coral mortality during or short after either the moderate or severe bleaching events (i.e., 1999, 2007, 2016, 2017, and 2018).
Non-parametric permutational analysis of variance (one-way PERMANOVA, PRIMER6 + PERMANOVA; Anderson, 2001), run on untransformed data using the Euclidean distance, was used to test for differences in:
(i) live hard coral cover in shallow reefs (5 m depth) between atolls with high human pressure (North Malé and South Malé) and atolls with low local human pressure (2 levels) after the bleaching event of 2016;
(ii) live hard coral cover in shallow reefs (5 m depth) between lagoon reefs and ocean reefs (2 levels) after the bleaching event of 2016;
(iii) coral mortality in shallow reefs (5 m depth) among the years when coral mortality occurred (4 levels: 1999, 2016, 2017, and 2018);
(iv) coral mortality across depths (5 levels: 10, 20, 30, 40, and 50 m) when coral mortality occurred (pooling data from 1999, 2007, 2016, 2017, and 2018).
To test for differences in the percent cover of live hard corals, a two-way PERMANOVA was also performed with the factor “Mortality occurrence” (2 fixed levels: years without coral mortality and years with coral mortality during or short after bleaching events) and the factor Depth (5 levels: 10, 20, 30, 40, and 50 m) fixed and orthogonal.
The pair-wise test was used to discriminate among levels of significant factors.
Results
Climate and Human Pressure Regimes in the Maldives
Peaks in maximum SST exceeded the moderate regional bleaching threshold in various years of the investigated period, but only the heat waves of 1998 and 2016 surpassed the severe regional bleaching threshold of 30.9°C (Figure 1). The heat wave of 1998 lasted from April to June and reached an estimated DHW value of 11.2, distinctly higher than the value of 8.2 reported in the NOAA database (Table 1). In 2016, we estimated a DHW value of 8.8, comparable to that of 8.9 provided by NOAA (Table 1), which was reached between mid-April and mid-June 2016. Notwithstanding occasional discrepancies due to the different size of the cells investigated, the DHW values we estimated for the Maldives proper were positively correlated (r = 0.96, n = 19) with those contained in the NOAA database for the whole central Indian Ocean.
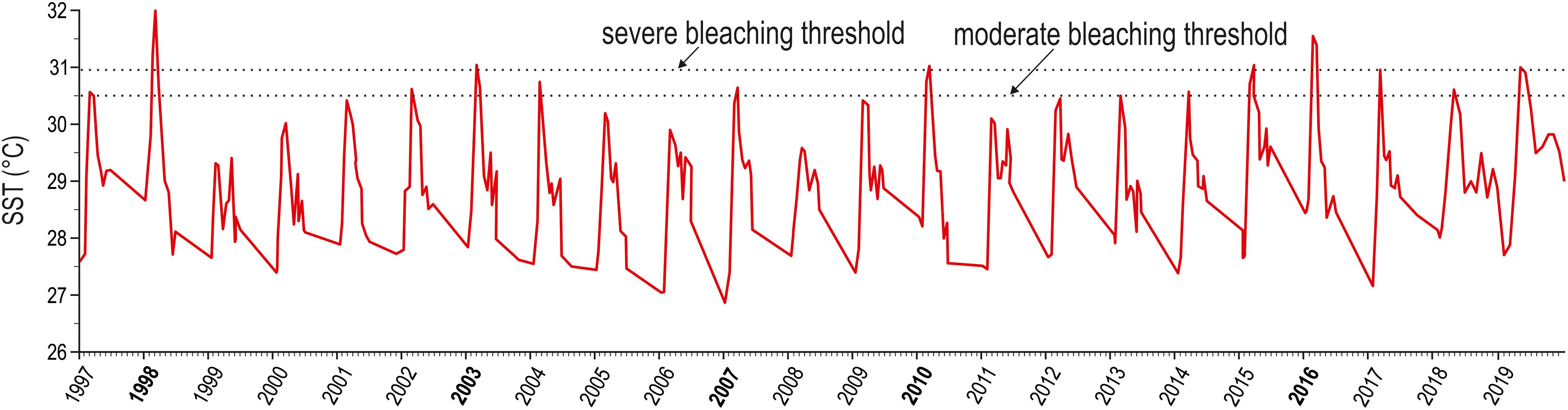
Figure 1. Twenty-three year trend (1997–2019) of maximum monthly sea surface temperature (SST, red solid line). Bold values on the x axis correspond to years when bleaching events have been reported for the Maldives (from Morri et al., 2015, and references therein; NOAA, 2016). The threshold temperatures triggering moderate and severe bleaching events are also indicated (according to NOAA, 2016 and Montefalcone et al., 2018a).
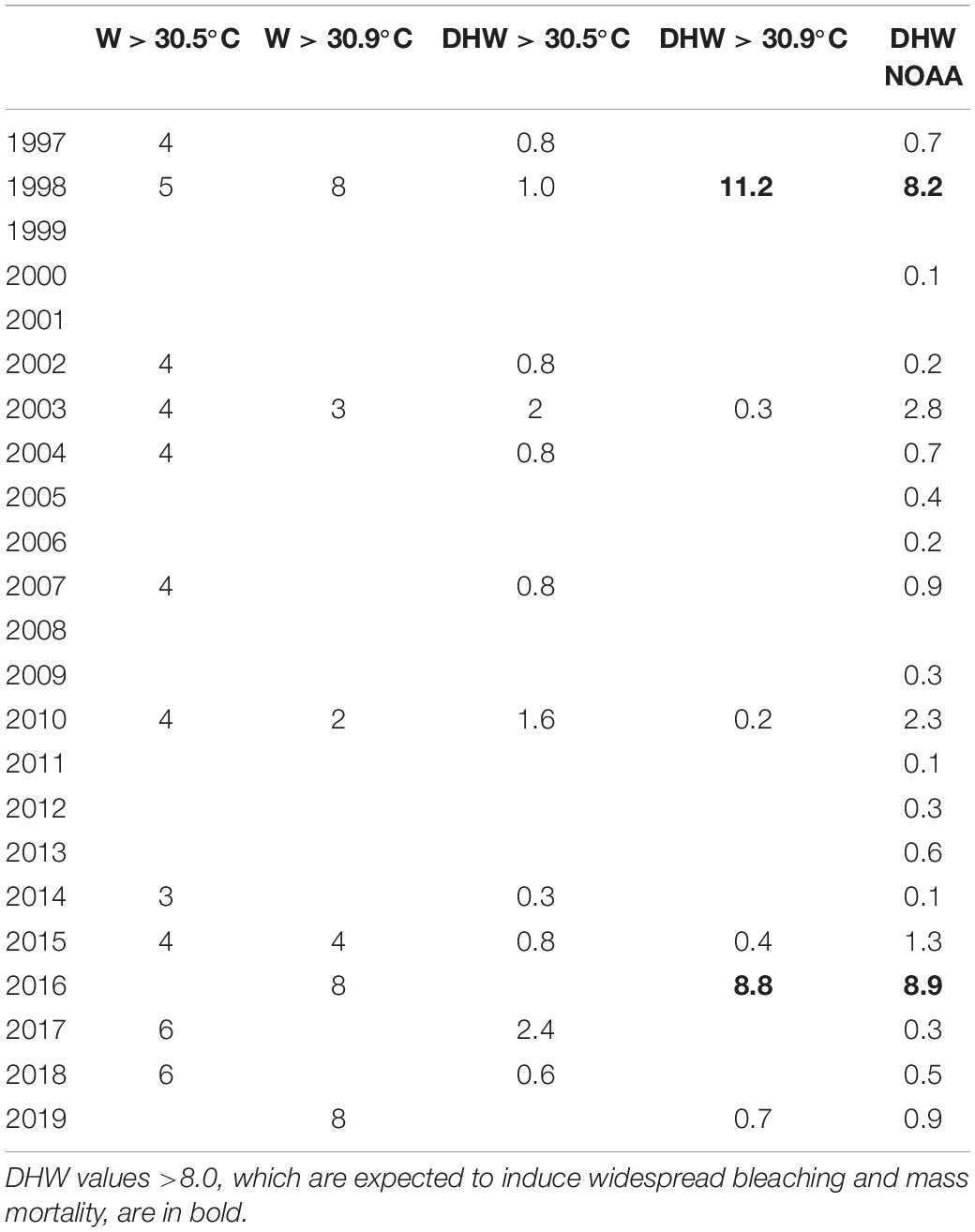
Table 1. Number of weeks (W) per year when the monthly mean sea surface temperature (SST) was higher than the temperatures triggering moderate (30.5°C) and severe (30.9°C) bleaching; degree heating weeks (DHW) above the moderate (30.5°C) and severe (30.9°C) bleaching thresholds per year as estimated from the monthly SST for the Maldives and DHW values reported in the NOAA coral reef watch database from the region “northern-eastern hemisphere centre” (i.e., central Indian Ocean) over an area of 60 degrees × 40 degrees (available at https://coralreefwatch.noaa.gov/product/5km/index_5km_composite.php).
Resident population in the Maldives increased slowly but steadily in the last decades (Figure 2A), passing from 244,814 people in the census of the year 1995 to 298,968 in 2006 and to 344,023 in 2014; the trend has been linear, leading to an estimated growth rate of 1.8% yearly. The same constant increase of the resident population was observed for the atolls of North and South Malé (Figure 2B), which have seen their population doubled in the last 20 years with an estimated growth rate of 4.2% yearly. On the contrary, the atoll of Ari passed from 11,955 people in the census of the year 1995 to 14,050 in 2014 and that of Felidhoo reduced from 1,678 people to 1,601 in the same period.
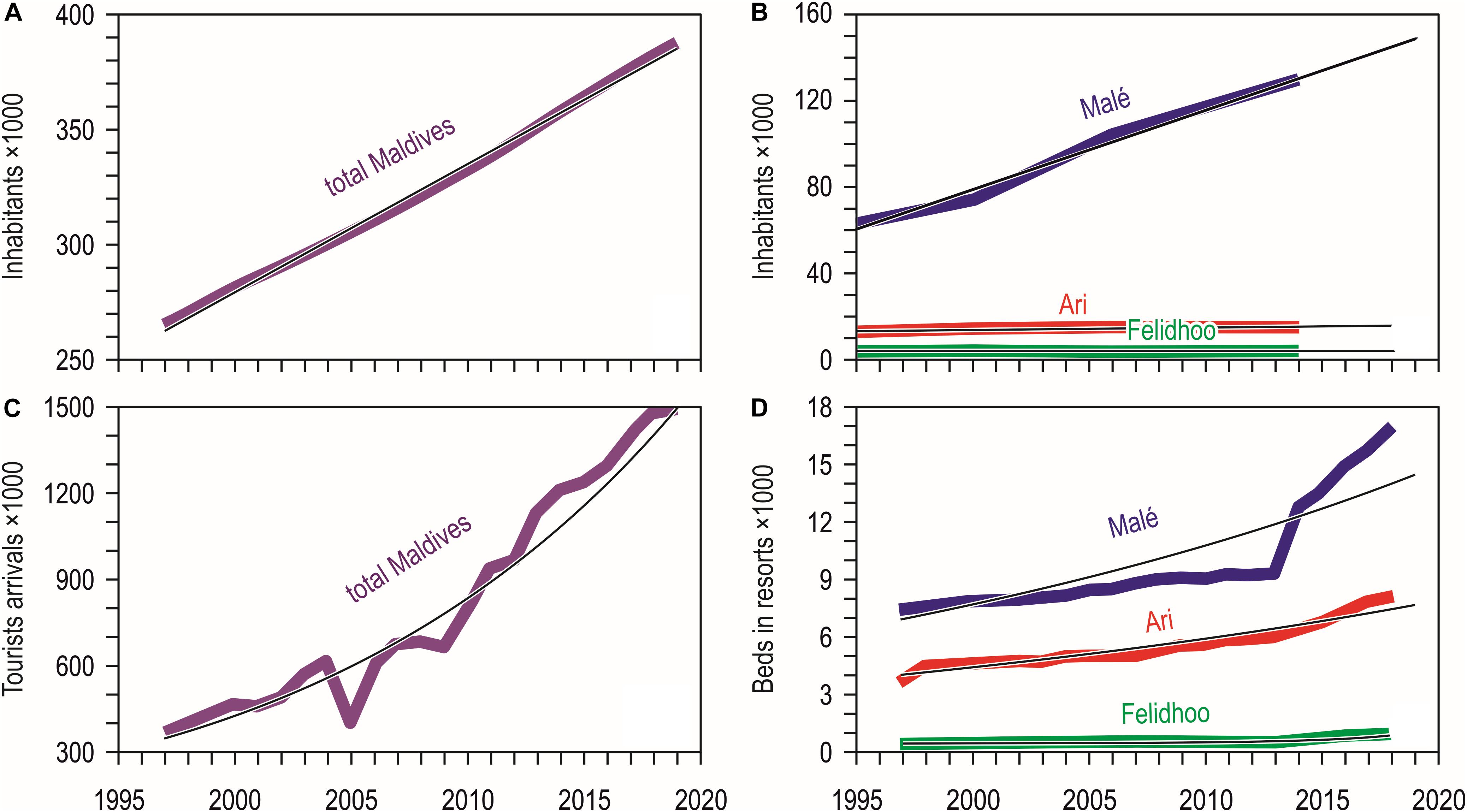
Figure 2. (A) Twenty-three year trend (1997–2019) of the total number of inhabitants of the Maldives (number of inhabitants = 5.584 year-10889; R2 = 0.997) (from United Nations, Department of Economic and Social Affairs, Population Division; National Bureau of Statistics in Maldives, Department of National Registration); (B) Number of inhabitants according to the national census of 1995, 2000, 2006, and 2014 (available at http://statisticsmaldives.gov.mv/yearbook/statisticalarchive) for the four main atolls compared in this study: North and South Malé (number of inhabitants = 3.659 year-7240; R2 = 0.987), Ari (number of inhabitants = 0.106 year-199.2; R2 = 0.733), and Felidhoo (number of inhabitants = –0.006 year-1396; R2 = 0.492); (C) Twenty-three year trend (1997–2019) of tourists arrivals in the Maldives (tourists arrivals = 2E-56⋅e0.067⋅year; R2 = 0.943) (from Ministry of Tourism data, compiled from annual reports and available at https://www.tourism.gov.mv/downloads/stats); (D) Twenty-three year trends (1997–2019) of the number of beds in the resorts of the four main atolls compared in this study: North and South Malé (number of beds = 9E-27⋅e0.034⋅year; R2 = 0.779), Ari (number of beds = 3E-22⋅e0.029⋅year; R2 = 0.920), and Felidhoo (number of beds = 3E-36⋅e0.043⋅year; R2 = 0.821) (from Ministry of Tourism data, compiled from annual reports and available at https://www.tourism.gov.mv/downloads/stats).
Tourist arrivals in the country grew exponentially, passing from around 366 thousands in 1997 to one million and half in 2019, with an increase rate of over 400%; a reduction in 2005 - possibly a consequence of the December 2004 tsunami – was rapidly recovered in 2006 (Figure 2C). The number of beds in the resorts increased exponentially in the atolls of Ari and Felidhoo, and more than exponentially in North and South Malé, where an impressive increase occurred especially after 2013 (Figure 2D); the number of beds in North and South Malé was twice than in Ari and one order of magnitude higher than that in Felidhoo.
Impact of Bleaching on Shallow Reefs
Live hard coral cover on shallow reefs of the Maldives dropped from values around 70% to a value lower than 8% after the 1998 bleaching event, and gradually returned to a value comparable to the pre-bleaching one only by 2014 (Figure 3). In 2015, recovery was apparently complete, with live hard coral cover reaching over 70% in several reefs. However, in reefs of North Malé atoll subjected to high human pressure live hard coral cover hardly exceeded 40% (Figure 4 and Supplementary Table S2). In 2016, many hard corals died following the new mass bleaching and dropped to a mean live cover value of around 20% (Figure 3). Bleaching affected indistinctly all sites (Supplementary Table S2) in both lagoon and ocean reefs. However, coral mortality after the bleaching event showed high spatial variability (Figure 4 and Supplementary Table S2), with reefs in atolls affected by comparatively lower human pressure exhibiting higher values of live hard coral cover compared to reefs in the two atolls of North Malé and South Malé, where human pressure is high on average (Table 2). Reefs started to recover already in 2017, and reached a mean live hard coral cover value of over 30% (Figure 3). Recovery, however, was distinctly lower in the atolls of North and South Malé (Figure 4) than in atolls with lower human pressure. Pattern of recovery was also different according to reef type: ocean reefs had suffered lower mortality with respect to lagoon reefs (Figure 5), and were therefore able to show live hard coral cover values higher than lagoon reefs in the immediate aftermath of the bleaching event (Table 3). Between 2018 and 2019 live hard coral in reefs subjected to low human pressure exhibited cover values up to 50–70%, whilst most reefs in atolls with high human pressure hardly reached 40% (Figure 4 and Supplementary Table S2).
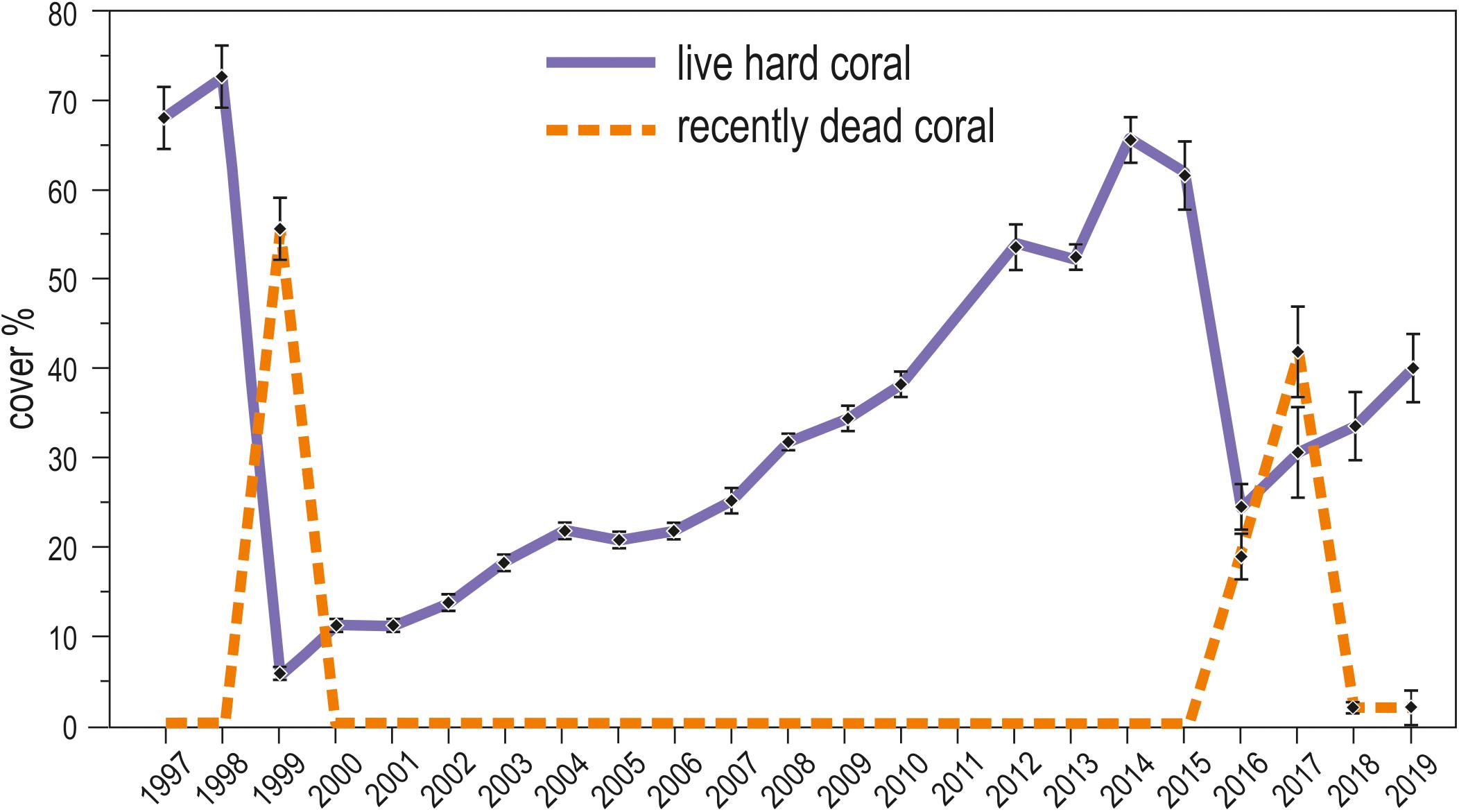
Figure 3. Twenty-three year trends (1997–2019) of the mean (± standard error) percent cover of live hard coral (violet solid line) and recently dead coral (orange dashed line) in shallow reefs (about 5 m depth) of the Maldives.
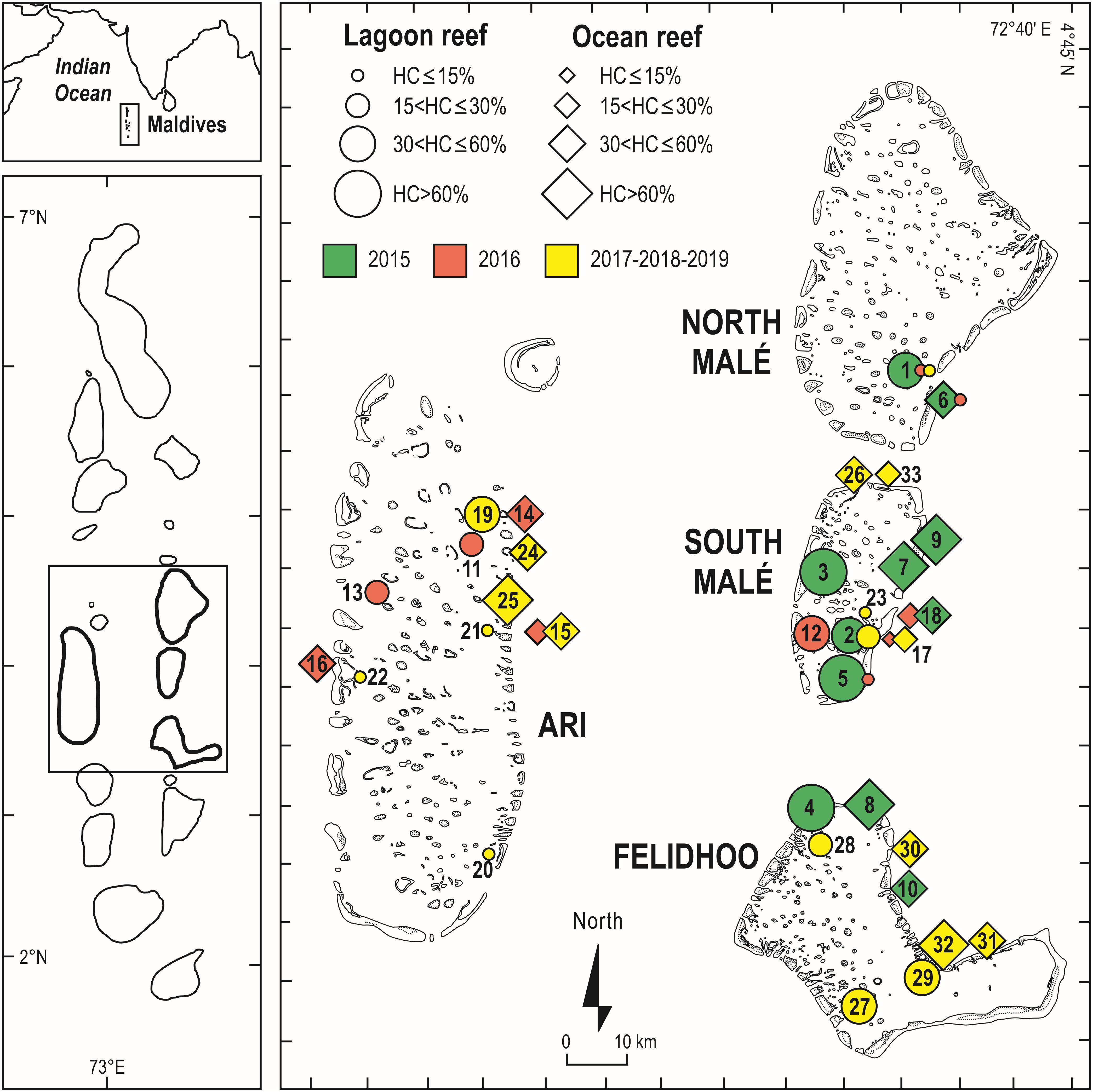
Figure 4. Geographical setting of the Maldives Archipelago and of the central atolls surveyed in recent years, with the mean percent cover of live hard corals at shallow reefs (5 m depth) in the 51 reefs surveyed during the 2015, 2016, 2017, 2018, and 2019 scientific cruises. Each reef is labeled according to the codes reported in Supplementary Table S2. In green are the reefs surveyed before the 2016 mass bleaching event (i.e., the pre-bleaching year in 2015), in red are the reefs surveyed during the bleaching event (in 2016), and in yellow are the sites surveyed after the bleaching event. For a safe of clarity the post-bleaching year (in 2017) and the years of early recovery (2018 and 2019) are grouped in this figure. North Malé and South Malé are atolls affected by high human pressure, whilst Ari and Felidhoo atolls by low human pressure.
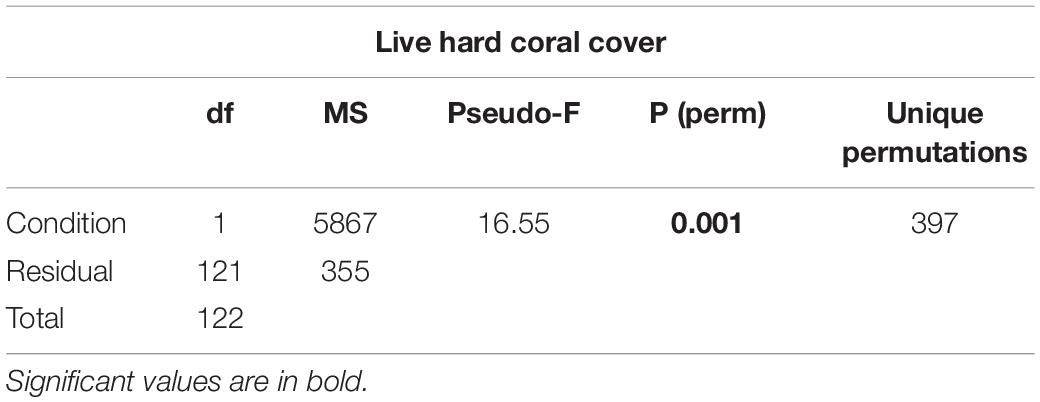
Table 2. Results of one-way PERMANOVA on live hard coral cover in shallow reefs (5 m depth) between atolls with high human pressure (North Malé and South Malé) and atolls with low local human pressure after the bleaching event of 2016.
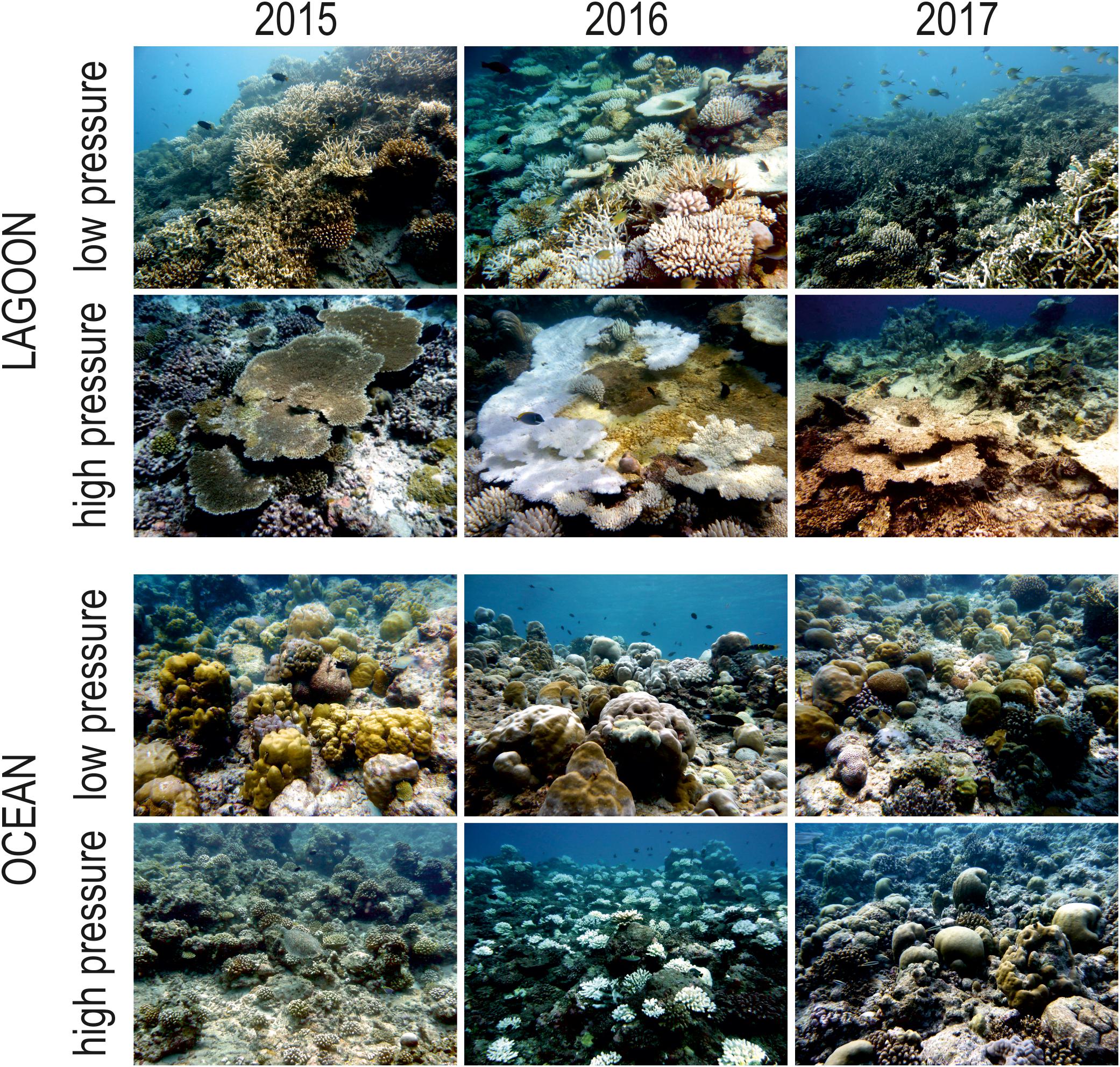
Figure 5. Representative pictures of Maldivian lagoon and ocean reefs subjected to low and high human pressure in 2015, 2016, and 2017 (i.e., before, during, and after the 2016 mass bleaching event, respectively) at 5 m depth.
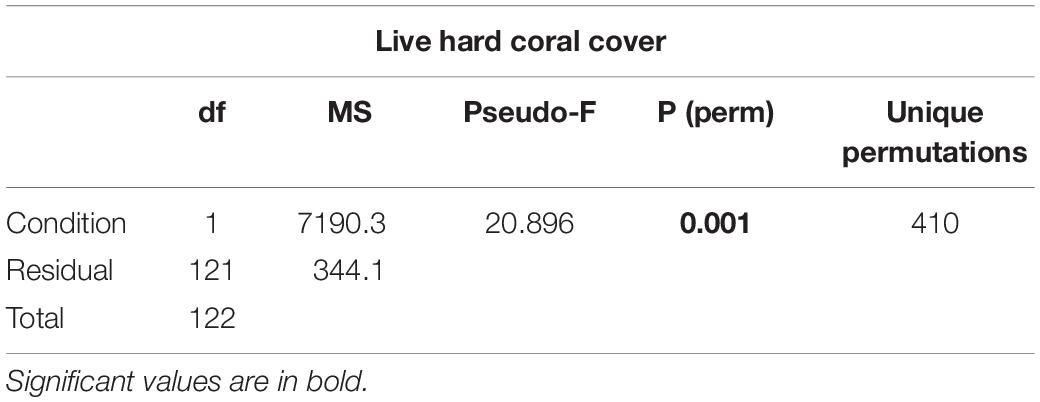
Table 3. Results of one-way PERMANOVA on live hard coral cover in shallow reefs (5 m depth) between lagoon reefs and ocean reefs after the bleaching event of 2016.
After the two bleaching events of 1998 and 2016, at 5 m depth, cover of recently dead corals was higher in 1999 than in 2017 (Figure 3). Accordingly, coral mortality was significantly higher in 1999 than in the remaining years with mortality (Figure 6 and Table 4). However, differently from the 1998 bleaching event, bleached and recently dead corals were observed also in the 3 years following the 2016 bleaching event (Figure 3 and Supplementary Table S2). Mortality was always higher in high human pressure atolls than in low pressure atolls (Figure 6).
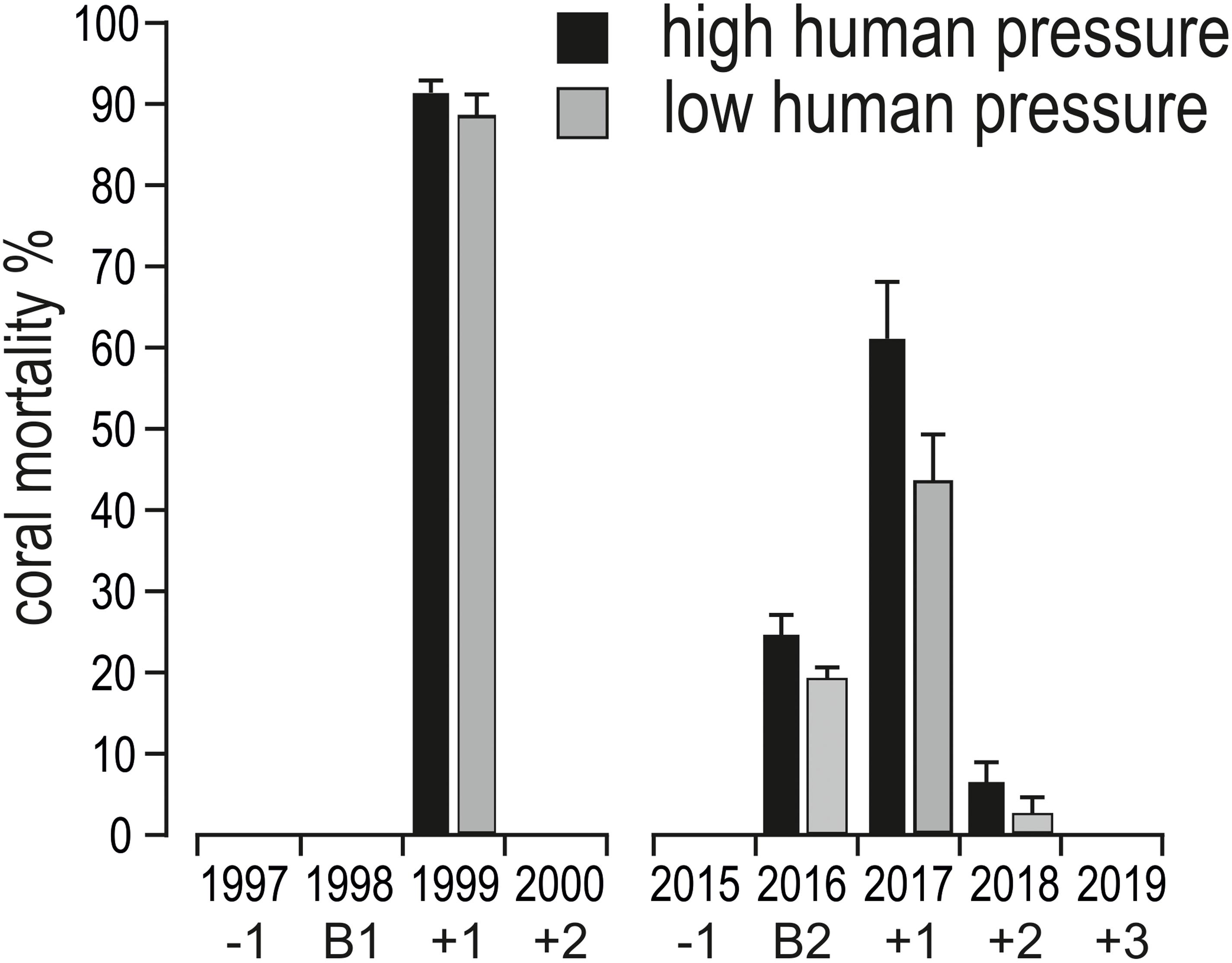
Figure 6. Mean (+ standard error) coral mortality (in %) in shallow reefs (5 m depth) of the Maldives in the years before (–1, i.e., 1997, 2015), during (B1, i.e., 1998, and B2, i.e., 2016), in the 2 years after (+1 and +2, i.e., 1999 and 2000) the mass bleaching event of 1998, and in the 3 years after (+1, +2, and +3, i.e., 2017, 2018, and 2019) the mass bleaching event of 2016.
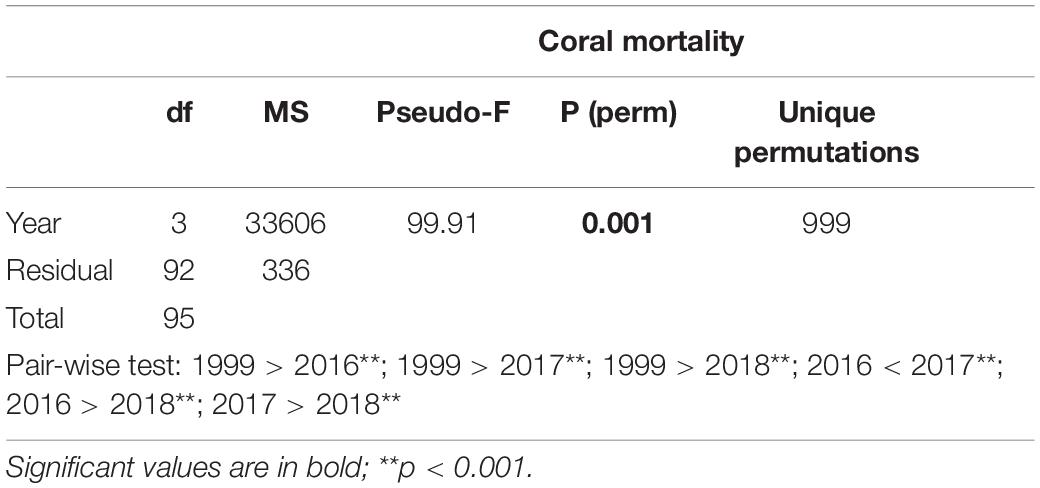
Table 4. Results of one-way PERMANOVA on coral mortality in shallow reefs (5 m depth) among the years when coral mortality occurred (i.e., 1999, 2016, 2017, and 2018).
Impact of Bleaching With Depth
Hard corals bleached from shallow water down to about 30 m depth. Consistently, bleaching-induced coral mortality was high at 10 m and 20 m, low at 30 m and negligible at 40 m and 50 m depth (Figure 7A and Table 5). The cover of live hard corals decreased gradually with depth (Figure 7B). At the lower photic depths (10 and 20 m), live coral cover decreased after the mass mortality episodes, whilst at the upper mesophotic depths (i.e., 30, 40, and 50 m) live hard coral cover showed no significant decline (Figure 7B). Only at lower photic depths (10 and 20 m) differences in live hard coral cover between years with or without mortality were significant (Table 6).
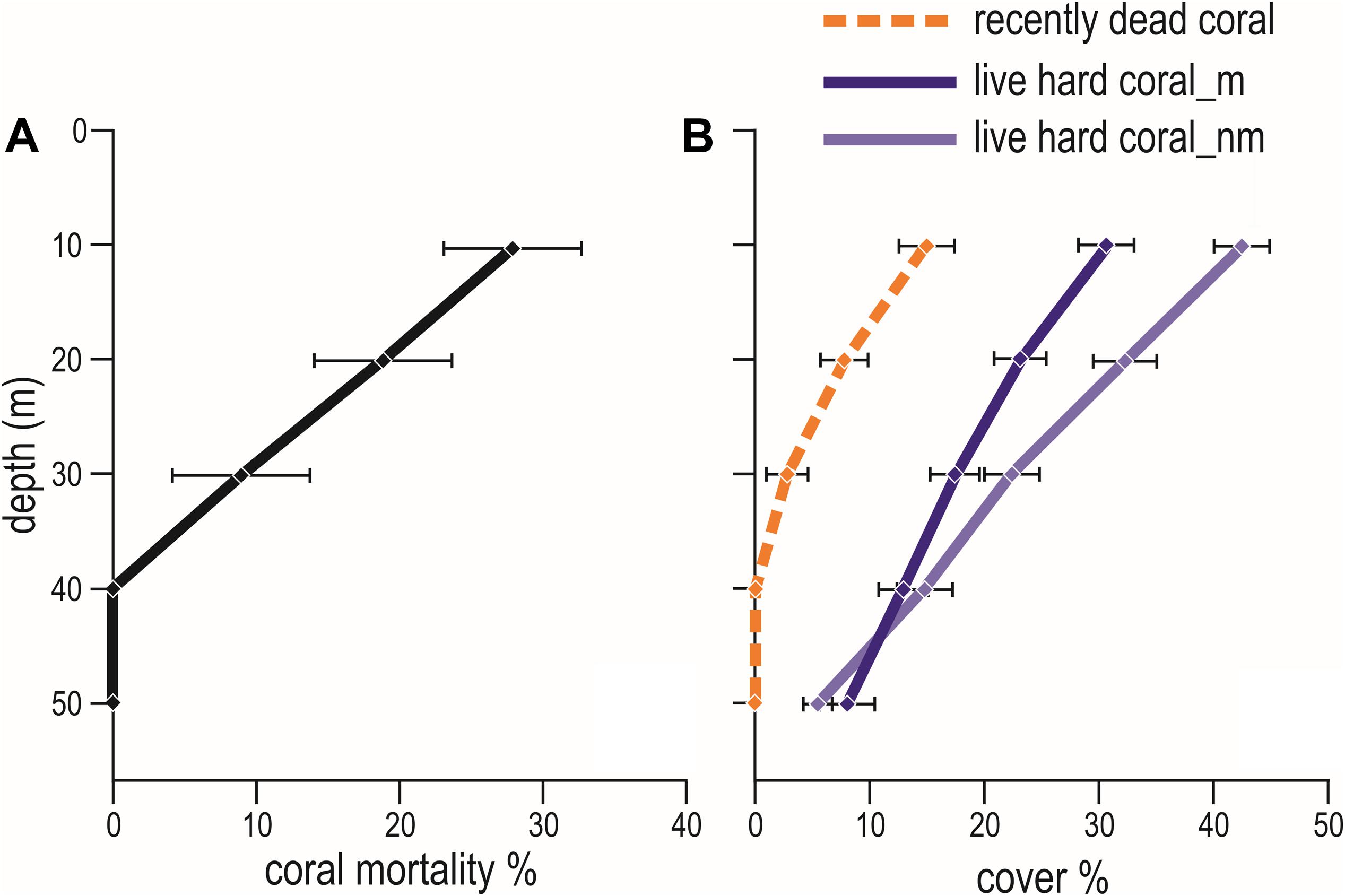
Figure 7. Mean (± standard error) values of: (A) coral mortality down a depth profile (from 10 m to 50 m); and (B) percent cover of live hard coral in years with coral mortality (i.e., 1999, 2007, 2016, 2017, and 2018) (live coral_m, dark violet solid line) and in years without coral mortality (i.e., 1997, 1998, 2012, 2013, 2015, and 2019) (live coral_nm, violet solid line), and of recently dead coral (orange dashed line) in the Maldives.
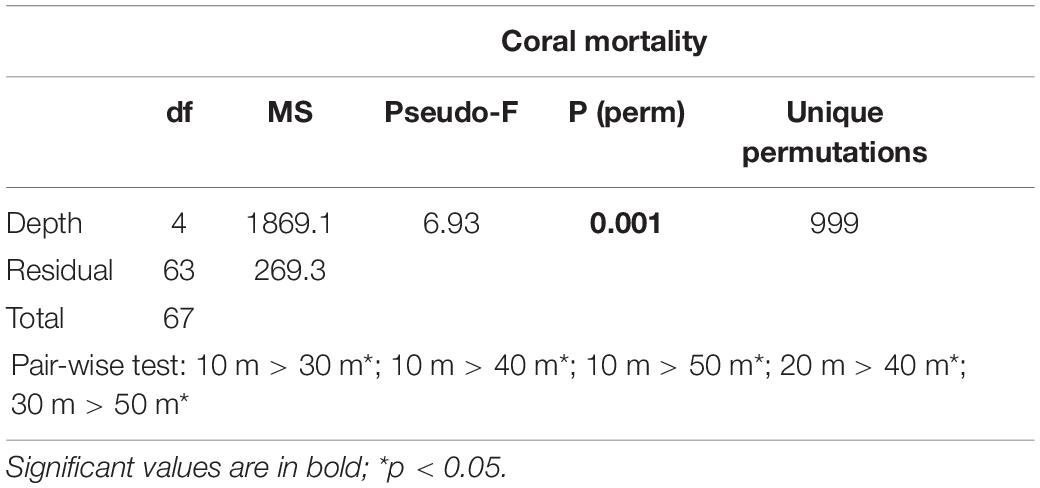
Table 5. Results of one-way PERMANOVA on coral mortality across depths (10, 20, 30, 40, and 50 m) when coral mortality occurred in the Maldives (pooling data from 1999, 2007, 2016, 2017, and 2018).
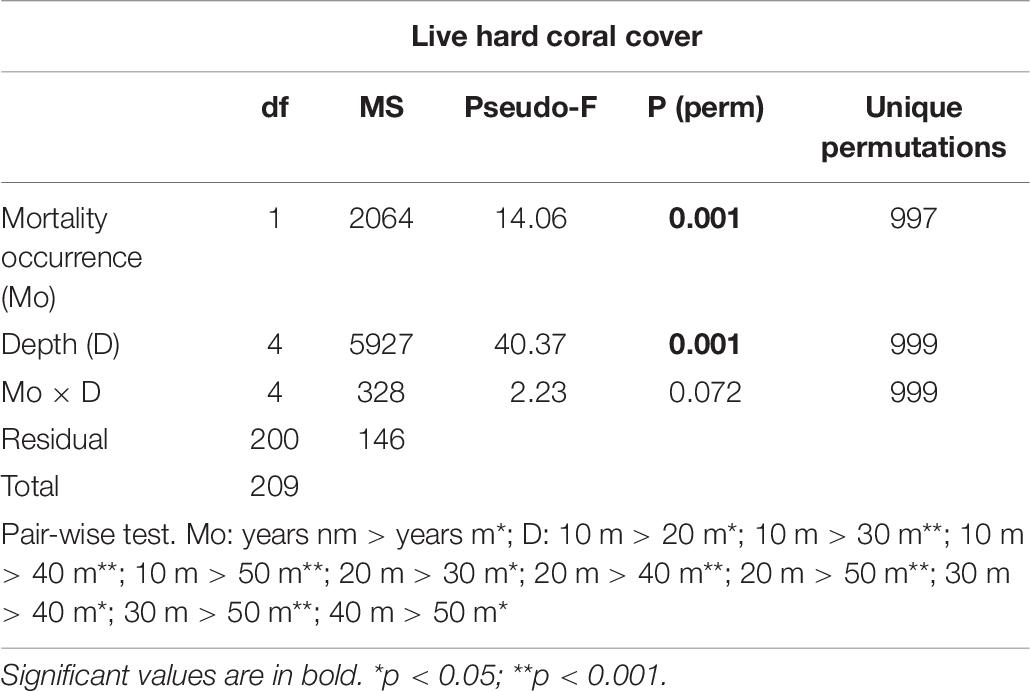
Table 6. Results of two-way PERMANOVA on the percentage cover (in %) of live hard coral in the years without coral mortality (years nm) and years with coral mortality (years m), across a depth profile (10, 20, 30, 40, and 50 m) in the Maldives.
Discussion
Maldivian coral reefs experienced two severe bleaching events in 1998 and in 2016. After the 1998 bleaching event, more than 90% of hard corals died (Bianchi et al., 2003, 2006), and it took 16 years for reefs to recover the pre-bleaching values of live hard coral cover (Morri et al., 2015). Such a recovery was consistent with that observed in the eastern tropical Pacific Ocean (Romero-Torres et al., 2020), but was surprisingly slow as compared to the fast coral cover recovery observed in the neighboring Chagos Archipelago (Sheppard et al., 2008) and in other remote Indian Ocean locations with similar oceanographic conditions (Gilmour et al., 2013). All these latter studies concerned nearly uninhabited islands; on the contrary, the Maldives are experiencing a still moderate but nevertheless continuously increasing level of human pressure because of population increase, coastal development, and tourism intensification (Jaleel, 2013; Nepote et al., 2016). In addition, starting from 2016, the atolls of North and South Malé have been subject to important land reclamation engineering works with consequent increase of sediments in the lagoons (Pancrazi et al., 2020).
Visual estimation of substratum cover is among the most universally used metric to quantify sessile benthic organisms (Bianchi et al., 2004), but might be an insufficient descriptor of recovery when considered alone (Johns et al., 2014). In the Maldives, coral species richness was recovered after 4 years (Benzoni and Pichon, 2007) and Acropora colonies density and size after 6 years (Lasagna et al., 2010b). However, recover of all descriptors at ecosystem level (community structure, seascape, trophic organization, and structural complexity) took 14 to more than 16 years and was rarely complete (Bianchi et al., 2017). While previous papers already investigated the general trend of coral recovery in the Maldives after the mass bleaching event in 1998 (Lasagna et al., 2008; Morri et al., 2015; Bianchi et al., 2017), this study showed that during the 2016 bleaching event, 60% of hard corals in the Maldives bleached and died when the accumulated heat exposure exceeded the critical regional bleaching threshold of degree heating weeks (DHW = 8.0) for 2 months. Hard corals bleached in almost all reefs surveyed during 2016, although differences in coral mortality were observed among the reefs investigated in the subsequent years. Local ecological and environmental differences influenced the severity of coral bleaching in the various areas (McClanahan et al., 2018). Reefs in remote areas or affected by a comparatively lower degree of human pressure, such as in Felidhoo and Ari atolls, kept a higher value of live hard coral cover than reefs in more developed and urbanized areas, such as in North and South Malé atolls (Dhunya et al., 2017; Stevens and Froman, 2019). Reefs affected by local human pressure have already been shown to be more vulnerable to climate disturbance (Montefalcone et al., 2011; Nepote et al., 2016).
Corals in ocean reefs proved more resistant to the 2016 bleaching event than those in lagoon reefs. Ocean reefs are close to deep cooler waters, have higher water movement and waves that may attenuate light intensity and ensure mixing of water, and are generally unaffected by nutrients coming from land: all factors that can potentially reduce bleaching and mortality (Muir et al., 2017). Differences between ocean and lagoon reefs are expressed not only in morphology, topography and exposure (Lasagna et al., 2008, 2010a; Rovere et al., 2018), but also in the species composition of the coral communities. Maldivian lagoon reefs are typically dominated by tabular and branching Acropora corals (Bianchi et al., 1997; Lasagna et al., 2010b). Susceptibility to thermal stress of Acropora corals is known to be widely variable (Muir et al., 2018), but in the Maldives they suffered a catastrophic die-off in both 1998 (Tkachenko, 2014) and 2016 (Pisapia et al., 2019), as already reported by studies in other localities (Loya et al., 2001; Pratchett et al., 2013). On the contrary, ocean reefs are dominated by massive corals (Bianchi et al., 1997), which experienced only partial colony mortality after the two bleaching events (Bianchi et al., 2006; Perry and Morgan, 2017; Pisapia et al., 2019). Higher resistance of ocean reefs is also likely linked to higher propagule exchanges due to stronger currents and water movements in comparison to lagoon reefs (Kinlan and Gaines, 2003).
Although the 2016 heat wave has been declared as the most damaging on record (Eakin et al., 2016; Hughes et al., 2017), corals in the Maldives survived to a larger extent with respect to 1998. According to our calculation for the Maldives, the thermal anomaly of 1998 was distinctly higher than that of 2016 in terms of DHW, but the two were comparable according to the NOAA coral reef watch database (which, however, considered an area wider than the Maldives). In any case, the 1998 heat wave was a relatively longer event, whilst the 2016 heat wave was less prolonged. The lower coral mortality in 2016 might give some support for the ABH in some species of shallow Maldivian coral reefs, as already hypothesized by McClanahan and Muthiga (2014). However, differences in duration and intensity between the two heat waves hamper a definite answer. Coral adaptability might be not the only explanation for a lower coral mortality in 2016: processes of acclimatization, ecological reorganization, and an effective coral heterotrophy (Houlbrèque and Ferrier-Pagès, 2009; Grottoli et al., 2014; McClanahan, 2017; Coles et al., 2018) have also been evoked.
In the Maldives (as elsewhere in the world) shallow reefs in the upper photic zone, within 10 m depth, are usually the target of most coral-reef monitoring programs (Jimenez et al., 2012; Tkachenko, 2012). Due to scuba diving constrains, comparatively fewer explorations have been conducted in the lower photic zone, below 10 m depth, where coral reefs can still be constructional (Morri et al., 1995; Bianchi et al., 1997) and even exhibit high coral cover in some cases (Sheppard, 1980). Although mesophotic reefs are rarely included in reef assessments (Pyle et al., 2016; Garavelli et al., 2018; Studivan and Voss, 2018a), there is a growing interest to investigate the upper mesophotic coral-reef ecosystems, 30 m to 50 m depth, for their potential to serve as thermal refuge (Semmler et al., 2016). Nevertheless, ecological information on mesophotic reefs in the Maldives is poor compared to that on shallow reefs (Morri et al., 1995, 2010; Bianchi et al., 1997).
Our results showed significantly reduced bleaching in the upper mesophotic reefs and a negligible coral mortality after the two bleaching events at depths between 30 and 50 m, thus providing some support to DRH. In many coral reefs of the world, the upper mesophotic reef community represents an extension of the shallow-water community (Pyle et al., 2016; Lesser et al., 2018), as many coral species are shared across these depths (Kahng et al., 2017; Studivan and Voss, 2018b; Zlatarski, 2018). However, our results also indicated that coral cover reduced drastically with depth from shallow to deep waters, with cover values always <20% in the latter. These values are lower than that of the survivors on shallow reefs. Even assuming a great fecundity for mesophotic coral species, there is no reason to think that their potential for contributing to reef recovery after severe events surpasses that of the survivors in photic reefs.
Although widely used as indicator of reef health (Montefalcone et al., 2018a), coral cover does not account for differences in susceptibility between species. Susceptibility of the different species to bleaching over depth has never been widely quantified, and available information on deep reefs during severe bleaching events is limited (Muir et al., 2017; Morais and Santos, 2018). Except for the ultrasensitive fire corals of the genus Millepora (Smith et al., 2014; Morri et al., 2017), some recent studies suggest that the applicability of the DRH may be only site- and species-specific (Semmler et al., 2016), and should not be considered as a general phenomenon (Smith et al., 2016; Bongaerts et al., 2017). Some controversy occurs regarding the hypothesis that deep reefs can provide a refuge from bleaching. An important role of mesophotic habitats has been demonstrated in the Indo-Pacific Ocean due to the high species richness of scleractinian corals, particularly at 30–45 m depths, able to preserve evolutionary lineages (Muir et al., 2018). During the 2016 bleaching event, 73% of the Maldivian coral species at 24–30 m depth have been not affected by bleaching (Muir et al., 2017). Deep areas of the ocean-side reefs on the Great Barrier Reef provided a refuge from the 2016 bleaching event for some colonies of the most abundant and ecologically important coral genera, e.g., Acropora, Pocillopora, and Porites (Baird et al., 2018). The survivors at depth may well be species that have been severely depleted in shallow reefs, thus making their contribution potentially critical. Shallow-reef assemblages in the Maldives share species with reefs at 30 m depth, but there are species that are not depth generalists (Benzoni and Pichon, 2007; Bigot and Amir, 2012): contrasting results might reflect local variations in both species composition and depth distribution of individual species down the reef slope (Laverick et al., 2018; Rocha et al., 2018). More data on recovery through time and at different depths would be necessary to further test this hypothesis and to understand to which extent mesophotic Maldivian reefs may act as refuge to safeguard shallow reefs.
Similarly, to investigate the ABH, estimates on coral cover can only provide a first picture on the effects of thermal stress. Further investigations on community taxonomic composition and on the relative sensitivity of species to thermal stress should be envisaged to prove both the ABH and the DRH in the Maldivian coral reefs. Ecophysiology, molecular biology, and genetics would be fundamental approaches to prove resistance and adaptability of corals to ocean warming.
Periods of unusually high sea surface temperatures have already become long-lasting and frequent (Frölicher et al., 2018), and this trend is predicted to accelerate under future global warming scenarios (Hughes et al., 2018a). Coral tolerance limits are expected to be frequently exceeded due to the incessant increase in sea surface temperature (Heron et al., 2017) and predictions indicate that the majority of coral reefs will not survive the most pessimistic scenarios of global warming (Perry et al., 2018). Even trusting in coral adaptation to ocean warming, this process would probably be slower than the rate at which sea temperatures are currently rising - therefore it will not prevent the extinction of many stenothermal coral species (Frieler et al., 2013; Bay et al., 2017). Up to 16 years had been necessary for Maldivian coral reefs to recover from the severe bleaching event of 1998 (Morri et al., 2015; Pisapia et al., 2016; Perry and Morgan, 2017), and the predicted frequency of two severe bleaching events per decade (Hughes et al., 2018a) would thus jeopardize future recovery of Maldivian reefs (Montefalcone et al., 2018a). In addition to climatic effects, the increase in local human pressure in the last decades is cause of concern (Nepote et al., 2016; Pancrazi et al., 2020). Resident population in the Maldives, and especially in the highly anthropized atolls (such as those of Malé), is continuously growing, but this is not the case for the most remote and less developed atolls (such as Ari and Felidhoo). Tourism, in particular, has become the main component of Maldivian economy (Bertaud, 2002) and may be expected to keep growing exponentially in the near future. The only flexion in tourism growth has been in 2005, as a consequence of the tsunami of December 2004 (Morri et al., 2015), whilst in the atolls of Malé tourism has been showing a huge rise since 2013. Our study showed that the impact of 2016 bleaching and mortality was significantly greater on coral communities in atolls with higher human pressure. The observed 16 years required to Maldivian coral reefs to recovery from a severe bleaching event are likely to be not enough for recovering from the last mass bleaching event of 2016, especially for those reefs where the disturbance regime has growth exponentially. While containing climate stress requires international actions, regional management practices may prove successful in reducing local human pressure (Lasagna et al., 2014; Dhunya et al., 2017), thus making Maldivian coral reef ecosystems more resilient to climate change (Brown et al., 2013; Shaver et al., 2018). Keeping local impacts under control may represent a better conservation strategy than relying upon coral adaptive responses and depth refuge efficacy.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
Part of this work received economic support from University of Genoa internal funds (FRA, Fondi Ricerca d’Ateneo).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Albatros Top Boat (Verbania, Milan and Malé) organized our scientific cruises in the Maldives: we especially thank Donatella ‘Dodi’ Telli, Massimo Sandrini, and Herbert Fontana for their support, and all the staff of Conte Max and Duke of York boats for assistance during field work. We also thank Save the Beach Maldives, especially Beybe (Assan Hamed), Sara Montagnani (University of Genoa), and all participants in field activities, who helped collecting data. The advice of Luigi Piazzi (University of Sassari) on statistical analyses is greatly acknowledged.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00587/full#supplementary-material
TABLE S1 | Sites surveyed during scientific cruises at the Maldives from 1997 to 2019.
TABLE S2 | Percentage cover (in %, mean ± s.e.) of live hard coral (HC), bleached coral (B), and recently dead coral (RDC) in the shallow reefs (5 m depth) surveyed in the Maldives during 2015, 2016, 2017, 2018, and 2019.
Footnotes
References
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Bahr, K. D., Jokiel, P. L., and Rodgers, K. S. (2016). Relative sensitivity of five Hawaiian coral species to high temperature under high-pCO2 conditions. Coral Reefs 2, 1–10.
Baird, A. H., Madin, J. S., Álvarez-Noriega, M., Fontoura, L., Kerry, J. T., Kuo, C.-Y., et al. (2018). A decline in bleaching suggests that depth can provide a refuge from global warming in most coral taxa. Mar. Ecol. Progr. Ser. 603, 257–264. doi: 10.3354/meps12732
Bay, R. A., Rose, N. H., Logan, C. A., and Palumbi, S. R. (2017). Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Sci. Adv. 3:e1701413. doi: 10.1126/sciadv.1701413
Benzoni, F., and Pichon, M. (2007). Taxonomic re-appraisal of zooxanthellate scleractinian corals in the Maldive Archipelago. Zootaxa 1441, 21–33. doi: 10.11646/zootaxa.1441.1.2
Bertaud, A. (2002). A Rare Case of Land Scarcity: The Issue of Urban Land in the Maldives. New York, NY: Mimeo, 1–9.
Bianchi, C. N., Azzola, A., Bertolino, M., Betti, F., Bo, M., Cattaneo-Vietti, R., et al. (2019). Consequences of the marine climate and ecosystem shift of the 1980-90s on the Ligurian Sea biodiversity (NW Mediterranean). Eur. Zool. J. 86, 458–487. doi: 10.1080/24750263.2019.1687765
Bianchi, C. N., Colantoni, P., Geister, J., and Morri, C. (1997). “Reef geomorphology, sediments and ecological zonation at Felidu Atoll, Maldive Islands (Indian Ocean),” in Proceedings of the 8th international coral reef symposium, eds H. A. Lessios and I. G. MacIntyre (Panamá: Smithsonian Tropical Research Institute), 431–436.
Bianchi, C. N., Morri, C., Lasagna, R., Montefalcone, M., Gatti, G., Parravicini, V., et al. (2017). “Resilience of the marine animal forest: lessons from Maldivian coral reefs after the mass mortality of 1998,” in Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International), 1241–1269. doi: 10.1007/978-3-319-21012-4_35
Bianchi, C. N., Morri, C., Pichon, M., Benzoni, F., Colantoni, P., Baldelli, G., et al. (2006). “Dynamics and pattern of coral recolonization following the 1998 bleaching event in the reefs of the Maldives,” in Proceedings of the 10th International Coral Reef Symposium, eds Y. Suzuki, T. Nakamori, M. Hidaka, H. Kayanne, B. E. Casareto, K. Nadaoka, et al. (Tokyo: Japanese Coral Reef Society), 30–37.
Bianchi, C. N., Pichon, M., Morri, C., Colantoni, P., Benzoni, F., Baldelli, G., et al. (2003). Le suivi du blanchissement des coraux aux Maldives: leçons à tirer et nouvelles hypothèses. Océanis 29, 325–354.
Bianchi, C. N., Pronzato, R., Cattaneo-Vietti, R., Benedetti-Cecchi, L., Morri, C., Pansini, M., et al. (2004). Hard bottoms. Biol. Mar. Medit. 11(Suppl. 1), 185–215.
Bigot, L., and Amir, H. (2012). Scleractinia corals of Baa Atoll (Maldives): first checklist and overview of stony corals community structure. Atoll Res. Bull. 590, 67–83.
Bongaerts, P., Ridgway, T., Sampayo, E., and Hoegh-Guldberg, O. (2010). Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Bongaerts, P., Riginos, C., Brunner, R., Englebert, N., Smith, S. R., and Hoegh-Guldberg, O. (2017). Deep reefs are not universal refuges: reseeding potential varies among coral species. Sci. Adv. 3:e1602373. doi: 10.1126/sciadv.1602373
Brown, C. J., Sauders, M. I., Possingham, H. P., and Richardson, A. J. (2013). Managing for interactions between local and global stressors of ecosystems. PLoS One 8:e65765. doi: 10.1371/journal.pone.0065765
Buddemeier, R. W., Baker, A. C., Fautin, D. G., and Jacobs, J. R. (2004). “The adaptive hypothesis of bleaching,” in Coral Health and Disease, eds E. Rosenberg and Y. Loya (Berlin: Springer), 427–444. doi: 10.1007/978-3-662-06414-6_24
Buddemeier, R. W., and Fautin, D. G. (1993). Coral bleaching as an adaptive mechanism. A testable hypothesis. BioScience 43, 320–326. doi: 10.2307/1312064
Coles, S. L., Bahr, K. D., Rodgers, K. S., May, S. L., McGowan, A. E., Tsang, A., et al. (2018). Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ 6:e5347. doi: 10.7717/peerj.5347
Dhunya, A., Huang, Q., and Aslam, A. (2017). Coastal habitats of Maldives: status, trends, threats, and potential conservation strategies. Int. J. Sci. Engineer. Res. 8, 47–62.
Dijkstra, H. A. (2006). The ENSO phenomenon: theory and mechanisms. Adv. Geosci. 6, 3–15. doi: 10.5194/adgeo-6-3-2006
Eakin, M., Liu, G., Gomez, A., De la Cour, J., Heron, S., and Skirving, W. (2016). Global coral bleaching 2014-2017: status and an appeal for observations. Reef Encount. 31, 20–26.
Frieler, K., Meinshausen, M., Golly, A., Mengel, M., Lebek, K., Donner, S. D., et al. (2013). Limiting global warming to 2°C is unlikely to save most coral reefs. Nat. Clim. Change 3, 165–170. doi: 10.1038/nclimate1674
Frölicher, T. L., Fischer, E. M., and Gruber, N. (2018). Marine heatwaves under global warming. Nature 560, 360–366.
Garavelli, L., Studivan, M. S., Voss, J. D., Kuba, A., Figueiredo, J., and Chérubin, L. M. (2018). Assessment of mesophotic coral ecosystem connectivity for proposed expansion of a marine sanctuary in the northwest Gulf of Mexico: larval dynamics. Front. Mar. Sci. 5:174.
Gatti, G., Bianchi, C. N., Montefalcone, M., Venturini, S., Diviacco, G., and Morri, C. (2017). Observational information on a temperate reef community helps understanding the marine climate and ecosystem shift of the 1980-90s. Mar. Poll. Bull. 114, 528–538. doi: 10.1016/j.marpolbul.2016.10.022
Gilmour, J. P., Smith, L. D., Heyward, A. J., Baird, A. H., and Pratchett, M. S. (2013). Recovery of an isolated coral reef system following severe disturbance. Science 340, 69–71. doi: 10.1126/science.1232310
Glynn, P. W. (1996). Coral reef bleaching: facts, hypotheses and implications. Glob. Change Biol. 2, 495–509. doi: 10.1111/j.1365-2486.1996.tb00063.x
Godfrey, T. (2006). Dive Maldives: A Guide to the Maldives Archipelago, 3rd Edn. Washington, DC: Atoll Press.
Grottoli, A. G., Warner, M. E., Levas, S. J., Aschaffenburg, M. D., Schoepf, V., McGinley, M., et al. (2014). The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Change Biol. 20, 3823–3833. doi: 10.1111/gcb.12658
Heron, S. F., Eakin, C. M., and Douvere, F. (2017). Impacts of Climate Change on World Heritage Coral Reefs: A First Global Scientific Assessment. Paris: UNESCO World Heritage Centre.
Hinderstein, L. M., Marr, J. C. A., Martinez, F. A., Dowgiallo, M. J., Puglise, K. A., Pyle, R. L., et al. (2010). Mesophotic coral ecosystems: characterization, ecology, and management. Cor. Reefs 29, 247–251. doi: 10.1007/s00338-010-0614-5
Hoegh-Guldberg, O. (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Fresh. Res. 50, 839–866.
Hoegh-Guldberg, O., and Bruno, J. F. (2010). The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528. doi: 10.1126/science.1189930
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742.
Holstein, D. M., Smith, T. B., Gyory, J., and Paris, C. B. (2015). Fertile fathoms: deep reproductive refugia for threatened shallow corals. Sci. Rep. 5:12407.
Houlbrèque, F., and Ferrier-Pagès, C. (2009). Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17. doi: 10.1111/j.1469-185x.2008.00058.x
Hughes, T. P., Kerry, J. T., Álvarez Noriega, M., Álvarez Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377.
Hughes, T. P., Kerry, J. T., Baird, A. H., Sean, R., Connolly, S. R., Dietzel, A., et al. (2018a). Global warming transforms coral reef assemblages. Nature 556, 492–506.
Hughes, T. P., Anderson, K. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., et al. (2018b). Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. doi: 10.1126/science.aan8048
Jaleel, A. (2013). The status of the coral reefs and the management approaches: the case of the Maldives. Ocean Coast. Manag. 82, 104–118. doi: 10.1016/j.ocecoaman.2013.05.009
Jimenez, H., Bigot, L., Bourmaud, C., Chabanet, P., Gravier-Bonnet, N., Hamel, M. A., et al. (2012). Multi-taxa coral reef community structure in relation to habitats in the Baa Atoll Man and Biosphere UNESCO Reserve (Maldives), and implications for its conservation. J. Sea Res. 72, 77–86. doi: 10.1016/j.seares.2012.04.011
Johns, K. A., Osborne, K. O., and Logan, M. (2014). Contrasting rates of coral recovery and reassembly in coral communities on the Great Barrier Reef. Cor. Reefs 33, 553–563. doi: 10.1007/s00338-014-1148-z
Jokiel, P. L. (2004). “Temperature stress and coral bleaching,” in Coral Health and Disease, eds E. Rosenberg and Y. Loya (Berlin: Springer), 401–425. doi: 10.1007/978-3-662-06414-6_23
Kahng, S. E., Copus, J. M., and Wagner, D. (2017). “Mesophotic coral ecosystems,” in Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International), 185–206. doi: 10.1007/978-3-319-21012-4_4
Kahng, S. E., Garcia-Sais, J. R., Spalding, H. L., Brokovich, E., Wagner, D., Weil, E., et al. (2010). Community ecology of mesophotic coral reef ecosystems. Cor. Reefs 29, 255–275. doi: 10.1007/s00338-010-0593-6
Kayanne, H. (2017). Validation of degree heating weeks as a coral bleaching index in the northwestern Pacific. Cor. Reefs 36, 63–70. doi: 10.1007/s00338-016-1524-y
Kinlan, B. P., and Gaines, S. D. (2003). Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84, 2007–2020. doi: 10.1890/01-0622
LaJeunesse, T. C., Parkinson, J. E., Gabrielson, P. W., Jeong, H. J., Reimer, J. D., Voolstra, C. R., et al. (2018). Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Cur. Biol. 28, 2570–2580.
Lasagna, R., Albertelli, G., Colantoni, P., Morri, C., and Bianchi, C. N. (2010a). Ecological stages of Maldivian reefs after the coral mass mortality of 1998. Facies 56, 1–11. doi: 10.1007/s10347-009-0193-5
Lasagna, R., Albertelli, G., Morri, C., and Bianchi, C. N. (2010b). Acropora abundance and size in the Maldives six years after the 1998 mass mortality: patterns across reef typologies and depths. J. Mar. Biol. Ass. U. K. 90, 919–922. doi: 10.1017/s0025315410000020
Lasagna, R., Albertelli, G., Giovannetti, E., Grondona, M., Milani, A., Morri, C., et al. (2008). Status of Maldivian reefs eight years after the 1998 coral mass mortality. Chem. Ecol. 24(Suppl. 1), 155–160.
Lasagna, R., Gnone, G., Taruffi, M., Morri, C., Bianchi, C. N., Parravicini, V., et al. (2014). A new synthetic index to evaluate reef coral condition. Ecol. Ind. 40, 1–9. doi: 10.1016/j.ecolind.2013.12.020
Laverick, J. H., Andradi-Brown, D. A., and Rogers, A. D. (2017). Using light-dependent Scleractinia to define the upper boundary of mesophotic coral ecosystems on the reefs of Utila, Honduras. PLoS One 12:e0183075. doi: 10.1371/journal.pone.0183075
Laverick, J. H., Piango, S., Andradi-Brown, D. A., Exton, D. A., Bongaerts, P., Bridge, T. C. L., et al. (2018). To what extent do mesophotic coral ecosystems and shallow reefs share species of conservation interest? A systematic review. Environ. Evid. 7:15.
Lesser, M. P., Slattery, M., Laverick, J. H., Macartney, K. J., and Bridge, T. C. (2018). Global community breaks at 60 m on mesophotic coral reefs. Glob. Ecol. Biogeogr. 28, 1403–1416. doi: 10.1111/geb.12940
Loya, Y., Eyal, G., Treibitz, T., Lesser, M. P., and Appeldoorn, R. (2016). Theme section on mesophotic coral ecosystems: advances in knowledge and future perspectives. Cor. Reefs 35, 1–9. doi: 10.1007/s00338-016-1410-7
Loya, Y., Sakai, K., Yamazato, K., Nakano, Y., Sambali, H., and Van Woesik, R. (2001). Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131. doi: 10.1046/j.1461-0248.2001.00203.x
Matz, M. V., Treml, E. A., Agayamova, G. V., and Bay, L. K. (2018). Potential and limits for rapid genetic adaptation to warming a Great Barrier Reef coral. PLoS Gen. 14:e1007220. doi: 10.1371/journal.pgen.1007220
McClanahan, T. R. (2017). Changes in coral sensitivity to thermal anomalies. Mar. Ecol. Progr. Ser. 70, 71–85. doi: 10.3354/meps12150
McClanahan, T. R., and Muthiga, N. A. (2014). Community change and evidence for variable warm-water temperature adaptation of corals in Northern Male Atoll, Maldives. Mar. Poll. Bull. 80, 107–113. doi: 10.1016/j.marpolbul.2014.01.035
McClanahan, T. R., Weil, E., and Baird, A. H. (2018). “Consequences of coral bleaching,” in Ecological Studies. Coral Bleaching: Patterns, Processes, Causes and Consequences, eds M. J. H. van Oppen and J. M. Lough (Cham: Springer International), 231–263. doi: 10.1007/978-3-319-75393-5_10
Montefalcone, M., Morri, C., and Bianchi, C. N. (2018a). Long term change in bioconstruction potential of Maldivian coral reefs following extreme climate anomalies. Glob. Change Biol. 24, 5629–5641. doi: 10.1111/gcb.14439
Montefalcone, M., De Falco, G., Nepote, E., Canessa, M., Bertolino, M., Bavestrello, G., et al. (2018b). Thirty year ecosystem trajectories in a submerged marine cave under changing pressure regime. Mar. Environ. Res. 137, 98–110. doi: 10.1016/j.marenvres.2018.02.022
Montefalcone, M., Parravicini, V., and Bianchi, C. N. (2011). “Quantification of coastal ecosystem resilience,” in Treatise on Estuarine and Coastal Science, eds E. Wolanski and D. S. McLusky (Waltham, MA: Academic Press), 49–70. doi: 10.1016/b978-0-12-374711-2.01003-2
Morais, J., and Santos, B. A. (2018). Limited potential of deep reefs to serve as refuges for tropical Southwestern Atlantic corals. Ecosphere 9:e02281. doi: 10.1002/ecs2.2281
Morri, C., Aliani, S., and Bianchi, C. N. (2010). Reef status in the Rasfari region (North Malé Atoll, Maldives) five years before the mass mortality event of 1998. Est. Coast. Shelf Sci. 86, 258–264. doi: 10.1016/j.ecss.2009.11.021
Morri, C., Bianchi, C. N., and Aliani, S. (1995). Coral reefs at Gangehi (North Ari Atoll, Maldive Islands). Publ. Serv. Géol. Luxembourg 29, 3–12.
Morri, C., Bianchi, C. N., Di Camillo, C. G., Ducarme, F., Allison, W. R., and Bavestrello, G. (2017). Global climate change and regional biotic responses: two hydrozoan tales. Mar. Biol. Res. 13, 573–586. doi: 10.1080/17451000.2017.1283419
Morri, C., Montefalcone, M., Lasagna, R., Gatti, G., Rovere, A., Parravicini, V., et al. (2015). Through bleaching and tsunami: coral reef recovery in the Maldives. Mar. Poll. Bull. 98, 188–200. doi: 10.1016/j.marpolbul.2015.06.050
Muir, P. R., Marshall, P. A., Abdulla, A., and Aguirre, J. D. (2017). Species identity and depth predict bleaching severity in reef-building corals: shall the deep inherit the reef? Proc. Royal Soc. B 284:20171551. doi: 10.1098/rspb.2017.1551
Muir, P. R., Wallace, C. C., Pichon, M., and Bongaerts, P. (2018). High species richness and lineage diversity of reef corals in the mesophotic zone. Proc. Royal Soc. B 285:20181987. doi: 10.1098/rspb.2018.1987
Nepote, E., Bianchi, C. N., Chiantore, M., Morri, C., and Montefalcone, M. (2016). Pattern and intensity of human impact on coral reefs depend on depth along the reef profile and on the descriptor adopted. Est. Coast. Shelf Sci. 178, 86–91. doi: 10.1016/j.ecss.2016.05.021
NOAA (2016). Data From: Coral Reef Watch, updated daily. NOAA Coral Reef Watch Daily Global 5-km Satellite Virtual Station Time Series. Data for the Maldives, 1/1/16 to 1/4/17. College Park, MA: NOAA.
Padfield, D., Yvon-Durocher, G., Buckling, A., Jennings, S., and Yvon-Durocher, G. (2015). Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol. Lett. 19, 133–142. doi: 10.1111/ele.12545
Pancrazi, I., Ahmed, H., Cerrano, C., and Montefalcone, M. (2020). Synergic effect of global thermal anomalies and local dredging activities on coral reefs of the Maldives. Mar. Pollut. Bull. (in press).
Perry, C. T., Alvarez-Filip, L., Graham, N. A. J., Mumby, P. J., Wilson, S. K., Kench, P. S., et al. (2018). Loss of coral reef growth capacity to track future increases in sea level. Nature 558, 396–400. doi: 10.1038/s41586-018-0194-z
Perry, C. T., and Morgan, K. M. (2017). Post-bleaching coral community change on southern Maldivian reefs: is there potential for rapid recovery? Cor. Reefs 36, 1189–1194. doi: 10.1007/s00338-017-1610-9
Pisapia, C., Burn, D., and Pratchett, M. S. (2019). Changes in the population and community structure of corals during recent disturbances (February 2016-October 2017) on Maldivian coral reefs. Sci. Rep. 9:8402.
Pisapia, C., Burn, D., Yoosuf, R., Najeeb, A., Anderson, K. D., and Pratchett, M. S. (2016). Coral recovery in the central Maldives archipelago since the last major mass-bleaching in 1998. Sci. Rep. 6:34720.
Prasetia, R., Sinniger, F., Hashizume, K., and Harii, S. (2017). Reproductive biology of the deep brooding coral Seriatopora hystrix: implications for shallow reef recovery. PLoS One 12:e0177034. doi: 10.1371/journal.pone.0177034
Pratchett, M. S., McCowan, D., Maynard, J. A., and Heron, S. F. (2013). Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS One 8:e70443. doi: 10.1371/journal.pone.0070443
Pyle, R. L., Boland, R., Bolick, H., Bowen, B. W., Bradley, C. J., Kane, C., et al. (2016). A comprehensive investigation of mesophotic coral ecosystems in the Hawaiian Archipelago. PeerJ 4:e2475. doi: 10.7717/peerj.2475
Richardson, L. E., Graham, N. A. J., Pratchett, M. S., Eurich, J. G., and Hoey, A. S. (2018). Mass coral bleaching causes biotic homogenization of reef fish assemblages. Glob. Change Biol. 24, 3117–3129. doi: 10.1111/gcb.14119
Riegl, B., and Piller, W. E. (2003). Possible refugia for reefs in times of environmental stress. Int. J. Earth Sci. 92, 520–531. doi: 10.1007/s00531-003-0328-9
Rocha, L. A., Pinheiro, H. T., Shepherd, B., Papastamatiou, Y. P., Luiz, O. J., Pyle, R. L., et al. (2018). Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361, 281–284. doi: 10.1126/science.aaq1614
Romero-Torres, M., Acosta, A., Palacio-Castro, A. M., Trem, E. A., Zapata, F. A., Paz-García, D. A., et al. (2020). Coral reef resilience to thermal stress in the eastern tropical Pacific. Glob. Change Biol. 26, 3880–3890. doi: 10.1111/GCB.15126
Rovere, A., Khanna, P., Bianchi, C. N., Droxler, A. W., Morri, C., and Naar, D. F. (2018). Submerged reef terraces in the Maldivian Archipelago (Indian Ocean). Geomorphology 317, 218–232. doi: 10.1016/j.geomorph.2018.05.026
Semmler, R. F., Hoot, W. C., and Reaka, M. L. (2016). Are mesophotic coral ecosystems distinct communities and can they serve as refugia for shallow reefs? Cor. Reefs 27, 1–12.
Shaver, E. C., Burkepile, D. E., and Silliman, B. R. (2018). Local management actions can increase coral resilience to thermally-induced bleaching. Nat. Ecol. Evol. 2:1075. doi: 10.1038/s41559-018-0589-0
Sheppard, C. R. C. (1980). Coral cover, zonation and diversity on reef slopes of Chagos atolls, and population structures of the major species. Mar. Ecol. Progr. Ser. 2, 193–205. doi: 10.3354/meps002193
Sheppard, C. R. C., Harris, A., and Sheppard, S. L. A. (2008). Archipelago-wide coral recovery patterns since 1998 in the Chagos Archipelago, central Indian Ocean. Mar. Ecol. Progr. Ser. 362, 109–117. doi: 10.3354/meps07436
Shlesinger, T., Grinblat, M., Rapuano, H., Amit, T., and Loya, Y. (2018). Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology 99, 421–437. doi: 10.1002/ecy.2098
Smith, T. B., Glynn, P. W., Maté, J. L., Toth, L. T., and Gyory, J. (2014). A depth refugium from catastrophic coral bleaching prevents regional extinction. Ecology 95, 1663–1673. doi: 10.1890/13-0468.1
Smith, T. B., Gyory, J., Brandt, M. E., Miller, W. J., Jossart, J., and Nemeth, R. S. (2016). Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Glob. Change Biol. 22, 2756–2765. doi: 10.1111/gcb.13175
Stevens, G. M. W., and Froman, N. (2019). “The Maldives archipelago,” in World Seas: An Environmental Evaluation, ed. C. Sheppard (Cambridge, MA: Academic Press), 211–236. doi: 10.1016/b978-0-08-100853-9.00010-5
Studivan, M. S., and Voss, J. D. (2018a). Assessment of mesophotic coral ecosystem connectivity for proposed expansion of a marine sanctuary in the northwest Gulf of Mexico: population genetics. Front. Mar. Sci. 5:152.
Studivan, M. S., and Voss, J. D. (2018b). Population connectivity among shallow and mesophotic Montastraea cavernosa corals in the Gulf of Mexico identifies potential for refugia. Cor. Reefs 37, 1183–1196. doi: 10.1007/s00338-018-1733-7
Thomas, C. J., Bridge, T. C. L., Figueiredo, J., Deleersnijder, E., and Hanert, E. (2015). Connectivity between submerged and near-sea-surface coral reefs: can submerged reef populations act as refuges? Div. Distrib. 21, 1254–1266. doi: 10.1111/ddi.12360
Tkachenko, K. S. (2012). The northernmost coral frontier of the Maldives: the coral reefs of Ihavandippolu Atoll under long-term environmental change. Mar. Environ. Res. 82, 40–48. doi: 10.1016/j.marenvres.2012.09.004
Tkachenko, K. S. (2014). The influence of repetitive thermal stresses on the dominance of reef-building Acropora spp. (Scleractinia) on coral reefs of the Maldive Islands. Russ. J. Mar. Biol. 40, 286–294. doi: 10.1134/s1063074014040105
Veron, J. E., Hoegh-Guldberg, O., Lenton, T., Lough, J. M., Obura, D. O., Pearce-Kelly, P., et al. (2009). The coral reef crisis: the critical importance of < 350 ppm CO2. Mar. Poll. Bull. 58, 1428–1436.
Wilson, S. K., Graham, N. A. J., and Polunin, N. V. C. (2007). Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 15, 1069–1076. doi: 10.1007/s00227-006-0538-3
Keywords: coral reefs, local human pressure, sea water warming, adaptive bleaching hypothesis, mesophotic reefs, deep refuge hypothesis, Maldives
Citation: Montefalcone M, Morri C and Bianchi CN (2020) Influence of Local Pressures on Maldivian Coral Reef Resilience Following Repeated Bleaching Events, and Recovery Perspectives. Front. Mar. Sci. 7:587. doi: 10.3389/fmars.2020.00587
Received: 03 March 2020; Accepted: 25 June 2020;
Published: 24 July 2020.
Edited by:
Massimo Ponti, University of Bologna, ItalyReviewed by:
Paolo Galli, University of Milano-Bicocca, ItalyLorenzo Bramanti, UMR8222 Laboratoire d’Ecogéochimie des Environnements Benthiques (LECOB), France
Copyright © 2020 Montefalcone, Morri and Bianchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica Montefalcone, bW9uaWNhLm1vbnRlZmFsY29uZUB1bmlnZS5pdA==
 Monica Montefalcone
Monica Montefalcone Carla Morri
Carla Morri Carlo Nike Bianchi
Carlo Nike Bianchi