
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 10 July 2020
Sec. Coral Reef Research
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00487
The degree to which biotic communities are regionally enriched or locally saturated, and roles of key structuring processes, remain enduring ecological questions. Prior studies of reef-building corals of the Indo-West Pacific (IWP) found consistent evidence of regional enrichment, a finding subsequently questioned on methodological grounds. Here we revisit this relation and associated relations between richness and abundance (as “effective number of species”), and coral cover, used as a proxy for disturbance and competition. From 1994 to 2017, we sampled > 2,900 sites on shallow (typically < 8–10 m depth below reef crest) and deeper reef slopes in 26 coral ecoregions, from Arabia to the Coral Triangle, Eastern Australia, Micronesia and Fiji, for a total pool of 672 species. Sampling intensity varied among ecoregions but always approached asymptotic richness. Local coral communities on both shallow and deep reef slopes were, on average, comprised of 25% of regional pools, ranging from 12 to 43% for individual ecoregions. The richest individual shallow and deep sites, averaged across all ecoregions, comprised 42 and 40% of regional pools, ranging from 30 to 60%, the highest in environmentally marginal ecoregions. Analyses using log-ratio regression indicated that IWP coral communities on deeper reef slopes were intermediate between regionally enriched and locally saturated. Communities on shallow reef slopes showed more evidence of regional enrichment, consistent with these being most susceptible to disturbance. Unimodal curvilinear relations between local richness and coral cover provide support for disturbance mediation and competitive exclusion. IWP coral communities are clearly dynamic, shaped by biological, ecological, and oceanographic processes and disturbance regimes that influence reproduction, dispersal, recruitment, and survival. Yet there is also evidence for a degree of local saturation, consistent with a niche-neutral model of community assembly. The richest sites hosted > 200 species, > 40% of regional pools and > 25% of the IWP total. These places may represent the asymptote of local richness in reef-building corals, rare examples of the ecological complexity for which these increasingly endangered communities are justly renowned.
Over the past half-century, much practical and theoretical research has examined patterns and processes structuring ecological communities (e.g., MacArthur and Wilson, 1967; Hurlbert, 1971; Whittaker, 1972; Grime, 1973; Hill, 1973; Pielou, 1975; Connell, 1978, 1980; Huston, 1979, 1985; Menge and Sutherland, 1987; Roughgarden et al., 1989; Bengtsson et al., 1994; Chesson, 2000; Hubbell, 2001; Hall et al., 2012 among many others). This work, in generating a variety of hypotheses, has shown that levels of species richness and abundance are scale dependent, spatially and temporally.
For reef-building corals, richness responds to biological, ecological, and oceanographic processes and disturbance regimes that influence reproduction, dispersal, recruitment, maintenance of space and survival. Over “deep time” and across regional spatial scales, richness responds to legacies of past events, including plate tectonics, ocean circulation and dispersal, evolution, and extinction (Veron, 1995). In “shallow time” and on local spatial scales, richness also responds to variations in habitat, histories of disturbance, and species interactions, including competition, predation, and mutualism (Connell, 1978, 1980; Done, 1982; Sheppard, 1982; Rogers, 1993; Aronson and Precht, 1995; Veron, 1995; Cornell and Karlson, 1996, 2000; Hubbell, 1997, 2001; Karlson and Cornell, 1999, 2002; Nekola and White, 1999; van Woesik, 2002; Karlson et al., 2004, 2007; Done et al., 2015).
Within this conceptual overview, the degree to which local communities are regionally enriched or locally saturated, and roles of key structuring processes, remain enduring ecological questions. In addressing the relative importance of local versus regional processes, two models were initially developed (Terborgh and Faaborg, 1980; Ricklefs, 1987). The first proposed a linear relationship between local and regional richness (named Type I), and the second a saturation effect (Type II). In the latter, ecological niches are compressed to a threshold size, beyond which incoming new species are balanced by local extinction events. In assessing the two models, Ricklefs (1987) concluded that local diversity depends on regional diversity, that regional and historical processes, as well as unique events and circumstances, profoundly influence local community structure.
Others have also argued for dynamism, rather than saturation, in ecological communities (Cornell and Harrison, 2014; Harmon and Harrison, 2015). In these models, competitive equilibria and full occupation of niche space are prevented by disturbances, keystone predation, resource pulses, or other habitat changes, along with limited dispersal enhanced by large spatial scales and natural barriers. Reviewing empirical studies of communities containing many rare, transient, and/or weakly interacting species, Harmon and Harrison (2015) found little evidence for saturation, such as negative correlations between diversity and abundance or between diversity and niche breadth.
In assessing the local–regional richness relation for reef-building corals, prior studies have combined multiple, disparate datasets, mostly at small spatial scales (e.g., Cornell and Karlson, 1996; Karlson and Cornell, 1999) or one larger-scale dataset that sampled the biodiversity gradient from Indonesia to the Society Islands (Karlson et al., 2004, 2007; Cornell et al., 2008). The latter studies demonstrated consistent relations between local (SL) and regional species richness (SR), in general accord with Ricklefs (1987) Type I model of regional enrichment. Using traditional linear regression of SL against SR, Karlson et al. (2004) found that local coral assemblages were comprised consistently of 27% of regional species pools, with no evidence of saturation. Reviewing these studies, Harmon and Harrison (2015) concluded that even on the most diverse coral reefs, local coral assemblages are profoundly affected by regional-scale processes.
However, conclusions derived from the traditional statistical approach, of regression of untransformed SL against SR, have been criticized on technical and conceptual grounds, notably statistical issues, choice of appropriate spatial scales, and effects of different forms of local interactions (see e.g., Loreau, 2000; Hillebrand, 2005; Szava-Kovats et al., 2012, 2013). Here we address these criticisms with a broad-scale, Indo-West Pacific (IWP) wide, corals dataset, providing insight into the local–regional richness relation, and roles of key structuring processes, for reef-building corals.
Local interactions: Corals, being sessile, or in a few species sedentary, as adults, possess a variety of morphological and physiological mechanisms to defend and gain space (Lang, 1973; Sheppard, 1979; Lang and Chornesky, 1990). Local interactions are most important when corals are crowded, growing in close proximity (i.e., when coral cover is high). This is an important consideration in interpretation of results, including disturbance-mediation and saturation effects on richness.
Intermediate disturbance theory postulates that, in the absence of disturbance, intense competitive interactions can eliminate species, promoting dominance by one or a few, with an associated reduction in diversity (Connell, 1978; Huston, 1985; Aronson and Precht, 1995). Thus, factors that prevent or slow competitive exclusion can result in higher diversity. Where competition is related to growth, as with sessile corals, highest diversity may be expected at relatively low growth rates that are still adequate to allow survival, and when periodic mortality prevents domination of space by a few species (Huston, 1985). Notably, space occupancy is also contingent on factors other than lack of disturbance and can be restored in less than one decade by fast-growing corals in suitable habitats (Tomascik et al., 1996; Done et al., 2007, 2010). Another important consideration is that space on reefs is also occupied by a diverse array of other sessile benthos, including other groups of anthozoans, sponges, ascidians, bryozoans and algae, many of which also have evolved strategies for maintaining and gaining space (Jackson and Buss, 1976).
Statistical issues: Responding to Hillebrand (2005), Cornell et al. (2008) claimed that inferences gained via traditional regression on the importance of regional enrichment need not be weak if they are based in an appropriate theoretical background and/or analysis of multiple local and regional influences. However, Szava-Kovats et al. (2012, 2013) concluded that regression of untransformed SL by SR violates the assumption of variable independence and induces spurious correlation. This is because SL is always less than or equal to SR, and both variables are always positive values. Addressing these flaws, Szava-Kovats et al. (2012, 2013) developed the log-ratio model, with additional categories for the SL:SR relation, namely intermediate and indeterminate, the former falling between regionally enriched and saturated, the latter uninterpretable because of issues of spatial scale and lack of data.
In the developing statistical approach to assessing richness, two key points continue to engage ecologists (Jost, 2010; Chao et al., 2014). First, that observed species richness is highly sensitive to sample size (the sampling problem, see below), and second, that species richness does not incorporate any information about the relative abundance of species (the abundance problem). By counting all species equally, species richness weights rare species identically to common ones. If two assemblages have identical species richness, the accepted concept of “diversity” is that it will be higher in the assemblage with more-equal abundances among all constituent species and lower in the assemblage that is dominated by one or a few common species (Pielou, 1975). This is not to discount the importance of rare species, which may play key roles in ecosystem function and are often of greatest conservation and management concern.
In light of these considerations, numerous diversity indices and rarefaction techniques have been developed, with a broad range in statistical properties and efficacy (Hurlbert, 1971; Hill, 1973; Lande, 1996; Jost, 2007, 2010; Chao et al., 2014). In an attempt to minimize limitations of other indices, Hill (1973) and others proposed use of “effective number of species” (ENS). This is the calculated number of equally abundant species needed to obtain the same mean proportional species abundance of a community with typical, uneven abundances (Hill, 1973; Jost, 2007, 2010, but also see Cao and Hawkins, 2019, for a different view on efficacy). Because our dataset includes an estimate of local abundance of each species in each site, we also estimated ENS to examine its influence on the local–regional richness relation.
Scale: Regarding arbitrary choice, and appropriateness, of spatial scale, sizes of local and regional sampling area can introduce systematic bias in the SL:SR relationship. In sampling the local community, choice of scale can influence the observed extent of interactions between populations and their environment (Caley and Schluter, 1997; Huston, 1999; Chesson, 2000; Loreau, 2000). Furthermore, linear relationships thought to indicate unsaturated trends tend to become more likely as the ratio of local to regional area increases (Szava-Kovats et al., 2013). Setting the division between local and regional scales is challenging, particularly given that these form part of a nested hierarchy characterized by different patterns and processes, yet intergrade along a continuum and differ among taxonomic groups (Whittaker, 1977; Loreau, 2000). For corals, these scales range across patch (101–102 m2), community (102–105 m2), reef (101–102 km2), reefscape (103–104 km2), ecoregion (ER) (ca. 104–105 km2), biogeographic province (ca. 106 km2), and ocean basin (e.g., Pacific Ocean ca. 107 km2).
Of the various different bioregionalizations for corals and marine biota, more generally (Spalding et al., 2007; Briggs and Bowen, 2012; Veron et al., 2015) we chose previously delineated “ecoregions” (ERs, Figure 1; see Veron et al., 2015, 2020 for details) to represent the regions. This choice of scale is consistent with considerations of dispersal, whereby propagules of all species should be available to all localities over ecological time scales (Hugueny et al., 1997). Corals are capable of dispersing, via their pelagic larval phase, from < 1 km to 103 km, although it is reasonable to assume that most settle within 102 km or less of their natal reef (Harrison and Wallace, 1990; Miller and Mundy, 2003; Jones et al., 2009), a scale consistent with our ERs. At local scale, our focus was the “coral community,” comprising areas of homogenous species composition typically covering several hectares to hundreds of hectares of reef slope in similar environmental and topographic conditions, within which we sampled areas of < 5,000 m2 (0.5 ha).
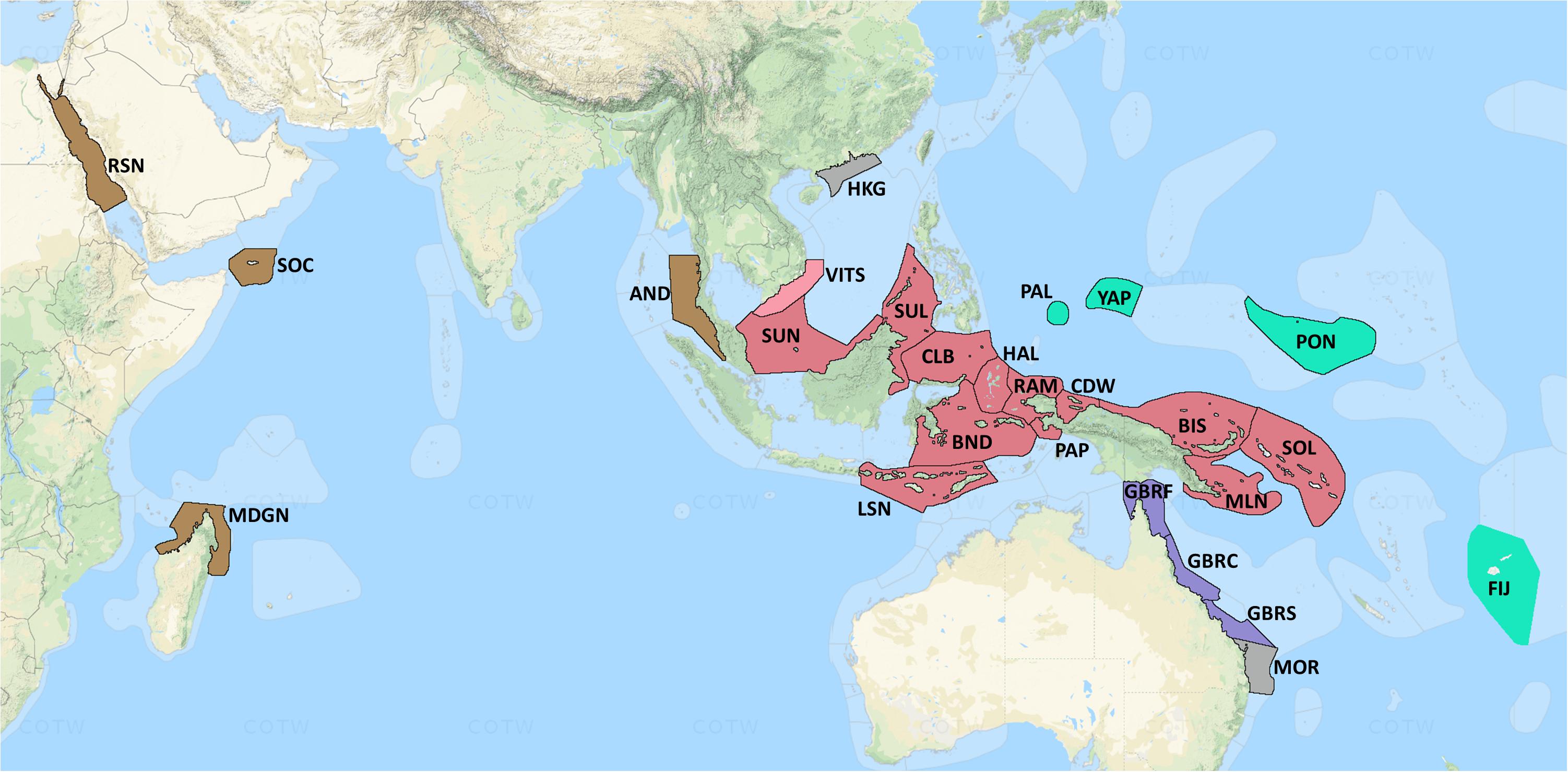
Figure 1. Map of location of 26 coral ecoregions across the IWP region. ER names are provided in Table 1. Map courtesy of COTW (Veron et al., 2020). Color shadings reflect broad biogeographic affinities, and for Moreton Bay and Hong Kong ERs, environmental marginality. These colors and name codes are used to aid interpretation in subsequent figures.
All methods of sampling and analyzing species richness have their advantages and disadvantages (Gotelli and Colwell, 2001). For underwater survey in remote locations, logistic constraints can be as critical as research aims. Here we employed a consistent “semi-quantitative” technique of field survey (DeVantier et al., 1998) to address these constraints and aims, as described below. This contribution follows that of DeVantier and Turak (2017), using a subset of the data presented there.
The full dataset includes 31 ERs, ranging from the Red Sea and Arabia in the west, the Andaman Sea, across the Coral Triangle to Eastern Australia, Fiji, and Micronesia in the east. Of these, five ERs were excluded from the present analyses because of sampling inadequacy, explained later. Of the 26 remaining (Table 1), Hong Kong had no deep sites, and another two, Moreton Bay and Great Barrier Reef (GBR) South, had insufficient sites on their deep and shallow slopes, respectively. With these exemptions, 2,931 sites were included in the present analysis, of which 1,853 were shallow and 1,078 were deep. Details of the boundaries, topography, environments, and disturbance regimes of each ER are provided on the open-access website www.coralsoftheworld.org.
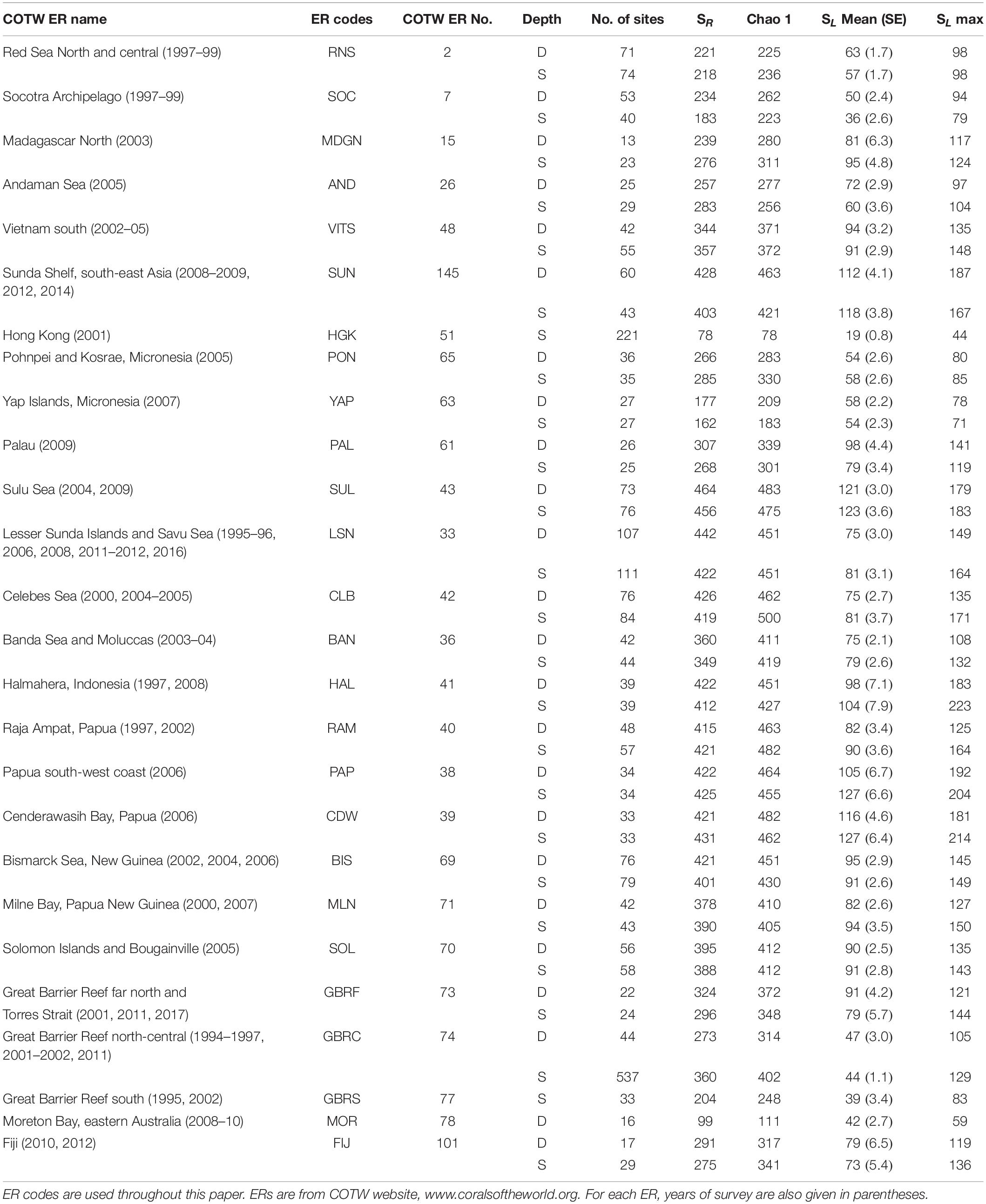
Table 1. Summary of ER coral richness estimates for deep (D) and shallow (S) sites, including regional species richness (SR), the Chao1 estimate, mean local species richness (SL Mean) with standard error (SE), and maximum local species richness (SL max).
Surveys encompassed a broad range of reef habitats within each ER, as the “semi-quantitative” methodology (DeVantier et al., 1998) was employed originally to inform design and management of marine protected areas. In this respect, this study is less ordered than the highly-structured design of some previous studies (Karlson et al., 2004, 2007; Cornell et al., 2008), which employed a hierarchical, quantitative approach of replicated transects within habitats within island groups within regions across a coral diversity gradient. Our dataset does, however, provide a much broader picture of the variability in richness of reef corals at local scale within and among ERs, and the IWP more generally. This is because we set out to sample the full range of reef habitats available, from highly sheltered turbid nearshore reefs to exposed, wave-washed, clear-water, open-ocean reefs.
Precise locations of survey stations in each ER were selected in consultation with local scientists, using oceanographic charts, aerial photographs, and satellite imagery, within the logistic constraints of a particular assessment. Each survey station was recorded with a portable geographic positioning system tool. Wherever the reef slope had sufficient depth, two vertically adjacent, non-overlapping sites were sampled at each station using SCUBA, on the deeper and shallower slopes, respectively. Although the vast majority of reef-building coral species have broad depth distributions (DeVantier and Turak, 2017), most show preferences for particular depth ranges (Sheppard, 1982; Huston, 1985; Turak and DeVantier, 2019), creating characteristic communities on shallow and deep reef slopes (Huston, 1985). Every reef site is unique, and so designations of sampling depths were determined on-site, rather than a priori. The division between neighboring deep (D) and shallow (S) sites was based on the typically well-defined shift in coral community structure at around 8–10 m depth below reef crest, but varying among sites in relation to local topographic and physicochemical conditions, notably attenuation in illumination and wave energy. We recorded visual estimates of some parameters, assimilated over the course of the survey swim (DeVantier et al., 1998). These included water clarity (as underwater visibility to the nearest 5 m, other than in very murky conditions), angle of reef slope (to the nearest 10 degrees to the horizontal), substrate type (into one of seven broad categories), and exposure to waves (into one of four broad categories). Depths of separation between deep and shallow sites at each station were set according to these considerations. Where permitted by relevant dive regulations, maximum survey depths extended to ca 40 m depth (deepest 62 m), or the base of the reef slope, if shallower. Because these were “one-off” surveys, effectively a “snapshot” of each site at a point in time, the study was not designed to examine the long-term effects of environmental conditions on biological processes that may influence coral growth, competition, and survival.
Each site covered an area of no greater than 5,000 m2 (typically ∼50 m across-slope × 100 m along-slope). Within this area, precise survey distances and times were not standardized, rather the local coral community was surveyed until no new taxa had been encountered for a period of approximately 5 min. Prior analysis of relations between species richness and duration of survey have demonstrated a very weak relationship (DeVantier et al., 1998). This is because some local communities are comprised of few species and can be effectively sampled quickly, while others are rich and require more time to census. In respect of the scaling considerations introduced above, our sites were much larger than the 200–300 m2 recommended for sampling local species richness of reef corals (Cornell et al., 2008). In our experience, such small areas do not adequately represent local coral communities, their richness estimates falling well below asymptotes of local richness sampled using our method.
At each site, a “roving diver” method of rapid ecological assessment was used to survey the local reef-building coral fauna. Within the survey area, we searched for all coral species present. Active searching for new species across each site, rather than recording only those intersected by a set of transect lines, routinely returns double or greater estimates of local richness than transects conducted concurrently (DeVantier et al., 2004).
Corals were identified visually and recorded on waterproof datasheets. It is not possible to identify all corals to species-level underwater, including many juvenile corals < 5 cm diameter and a group of massive corals in the genus Porites. For the latter, species-level estimates of occurrence and abundance were calculated (see DeVantier and Turak, 2017 for details). For all other taxa whose identity was uncertain, photographs and/or small specimens were taken to aid identification, the latter where permitted by management authorities. These reside in their countries of origin or as voucher specimens at the Museum of Tropical Queensland, Townsville, Australia.
Taxonomy underpinning our identifications evolved over the duration of the study, and we have updated species records as far as practicable, in accord with the taxonomic framework of Veron et al. (2015, 2020) and recent taxonomic publications.
Visual estimates of the local relative abundance of each species were compiled on completion of each survey dive, on a five-point scale, where 1 = locally rare, 2 = uncommon, 3 = common, 4 = abundant, and 5 = dominant. These ordinal ranked categories approximate a log 4 scale (Table 2) and provide a rough estimate of the numbers of coral colonies (or individuals for solitary taxa) in each taxon.
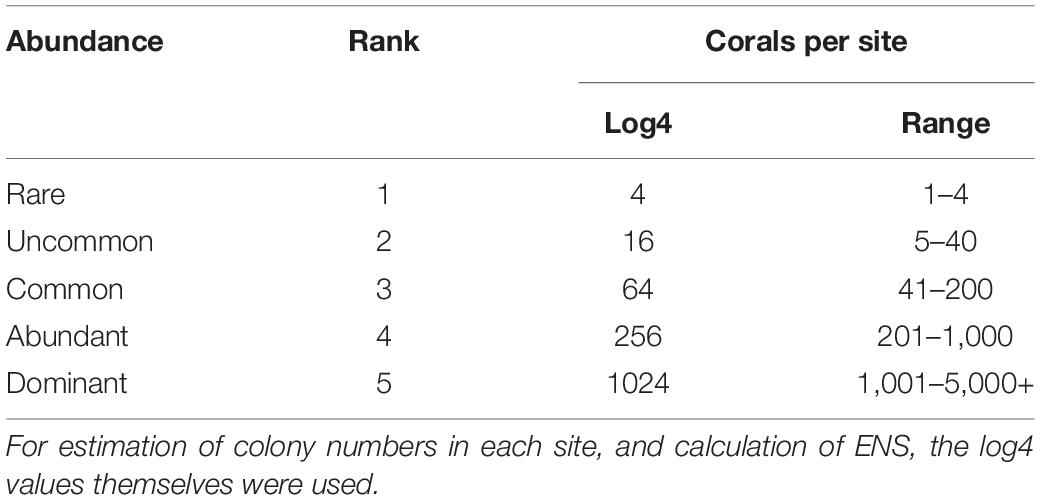
Table 2. Local relative abundance ranks and equivalent log 4 conversions and approximate ranges in colony numbers.
Benthic cover of living corals, as a percentage of the total substrate, was also estimated visually at each site (Miller and De’ath, 1996; DeVantier et al., 1998; Miller and Muller, 1999). Cover was typically estimated to the nearest 5%, other than for very low percentages. Scores for the different attributes were recorded during and immediately following each survey dive. Field methods, with full taxonomic references, are explained in detail elsewhere (DeVantier et al., 1998; DeVantier and Turak, 2017; Turak and DeVantier, 2019).
The dataset used in the analyses is provided in Supplementary Table 1. Species accumulation and rarefaction curves and associated statistics were generated using the Estimates statistical software package (Colwell and Elsensohn, 2014; Colwell, 2019) from the species abundance scores from each site for the two depth ranges in each ER. Abundance ranks were converted to the log 4 values (Table 2), other than for rank scores of 1, which represented locally rare species. In our log 4 range, this rank could include species with local colony numbers of one to four. However, these were retained as score 1 in the EstimateS analyses, to accommodate the possibility of “singleton” species in the dataset, and hence to be as conservative as possible in the estimation of Chao1 value, which uses singletons and doubletons in estimating total richness (Colwell and Elsensohn, 2014).
Of the 31 ERs, we excluded Red Sea south (D,S sites), Gulf of Aden (D,S), Lakshadweep (D,S), Philippines north (D,S), GBR southeast (D,S), Moreton Bay (S only), and GBR south (D only) from further analyses because of small sample sizes, failure of the accumulation curves to reach asymptotes, and/or differences of > 20% between the sample total richness and Chao1 mean estimates. Thus, a total of 49 ER × depth samples (25 from shallow reef slopes and 24 from deep reef slopes) were included. Species accumulation curves for these all approached an asymptote and were within 20% of the Chao1 estimator of total richness (Table 1). In all, 2,931 sites were included, of which 1,078 sites were deep and 1,853 sites were shallow.
Most prior assessments of the local–regional richness relation have focused only on mean local richness. Here we also examine the relation for the richest individual sites in each ER. Mean local richness is affected by various factors, including, during the course of this study, impacts from a wide array of anthropogenic and natural disturbances, many of which have escalated over recent decades at local, regional, and global scales. Our findings need to be considered in light of this novel disturbance regime. Hence, we also selected examples of maximum local richness from each ER to assess “best case” circumstances in the local–regional richness relation over the period of this study (also see Harrison et al., 1992).
To examine the relation between local (SL) and regional (SR) richness, both the traditional (Ricklefs, 1987; Karlson et al., 2004) and log-ratio methods of regression (Szava-Kovats et al., 2012, 2013) were used. Despite its documented flaws, we used the traditional method to enable comparison of our results with those of Karlson et al. (2004), the other broad-scale analysis conducted to date for reef-building corals.
Mean and maximum values of SL were transformed into an unbounded log-ratio, ln[SL/(SR–SL)], and regressed against ln(SR). In this model, Type I relations produce a slope whose confidence interval includes 0, and Type II relations produce a slope whose confidence interval includes –1. Unlike untransformed models, the Type II relation is accurately defined by the log-ratio model, and the distinction between Type I and Type II relations is maintained independent of the value of SR (Szava-Kovats et al., 2013).
Linear regression of the log-ratio transformed data was conducted in R (R Core Team, 2019) to determine the slope and corresponding 95% confidence intervals. Relations between SL and SR were then designated into one of four classes based on the range of confidence intervals with respect to the model continuum. These were Type I, with a slope whose confidence interval includes the upper limits of the continuum; Type II, with a slope whose confidence interval includes the lower limit of the continuum; intermediate, with a slope whose confidence interval lies entirely within the continuum; and indeterminate, with a slope whose confidence interval spans the entire continuum. For more details of the method (see Szava-Kovats et al., 2012, 2013).
Analyses were conducted for shallow and deep reef slopes separately, to investigate depth-related similarities and differences in the local–regional richness relation. This also facilitated comparison with earlier analyses (Karlson et al., 2004; Cornell et al., 2008) of data from shallow reef slopes. Analyses were conducted for both mean and maximum values of SL in each ER. For mean values, weighted linear regression was used to minimize issues of variance heteroscedasticity. For the maximum SL value, for deep and shallow slopes, we used unweighted linear regression, to accommodate the lack of variance for weighting.
A further set of analyses of local–regional richness relations used ENS, based on the Simpson Index instead of species richness counts, to examine the effect of species abundance on the relationship. For these analyses, the ranked abundance scores were converted to their log 4 equivalents (Table 2), to obtain rough estimates of colony numbers at each site. In this analysis ENS = 1/Σp2, where p is the assigned abundance of each species as per Table 2.
In this study, we used the simple metric of coral cover as a rough proxy of disturbance (following Connell, 1978). Relations between SL and coral cover, as for example where SL may stabilize or decline via competition when coral cover is high, were explored for each ER and depth, using polynomial regression. Coral cover at each site was Logit transformed for analysis, to avoid statistical issues of plotting untransformed or arcsine transformed data (Warton and Hui, 2011).
The local–regional richness ratio, plotted for both mean and maximum values of SL:SR, ranged widely among ERs (Figures 2A–D). Highest mean SL:SR ratios were found at Moreton Bay (deep: 43%), Madagascar north (deep and shallow: 34%), and Yap (deep and shallow: 33%). Conversely, the GBR north-central ER had the lowest values for mean SL:SR on both deep (17%) and shallow (12%) reef slopes. Over all ERs, the mean of mean SL:SR was approximately 25% on both deep and shallow reef slopes. For all sites pooled, irrespective of ER, mean SL:SR was 24% on deep slopes, and 21% on shallow slopes, the latter’s greater reduction due to the effect of the large number of locally depauperate sites in the GBR north-central ER.
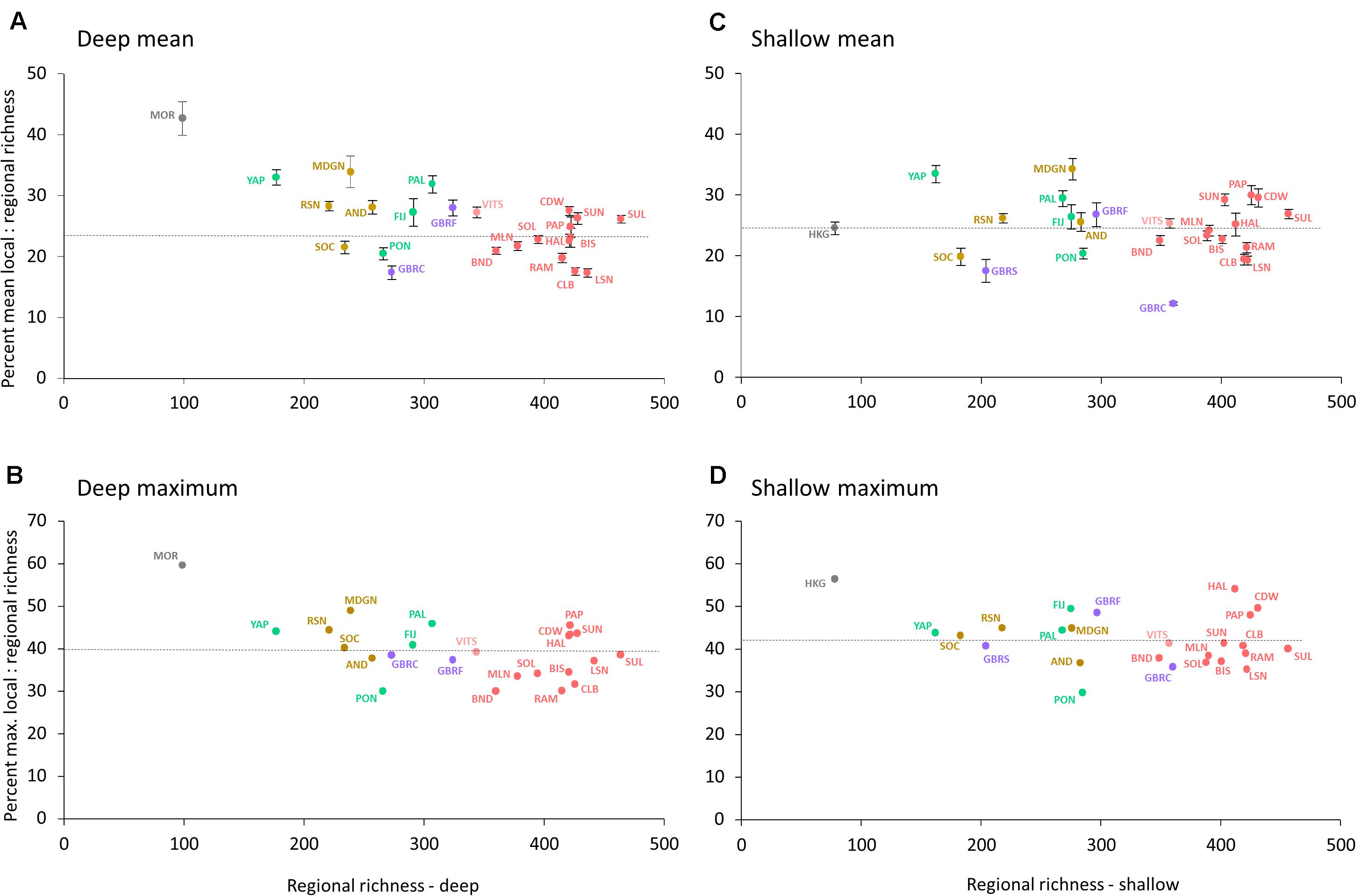
Figure 2. Scatter plots of relations between mean and maximum scores of the local:regional ratio (SL:SR) on deep and shallow reef slopes on all ERs. Error bars are standard error of the means: (A) deep slope mean scores, (B) deep slope maximum scores, (C) shallow slope mean scores, and (D) shallow slope maximum scores. Horizontal lines represent overall mean scores across all ecoregions.
Individual ERs also ranged widely in richest site values of SL:SR, from 30 to 60% on deep reef slopes and from 30 to 56% on shallow slopes. Sites in marginal ERs of Moreton Bay (D) and Hong Kong (S) had the highest scores. The means across all ERs for richest site values of SL:SR on deep and shallow slopes were 40 and 42%, respectively.
The highest individual SL tally, 223 species (54% of SR), was recorded on a shallow reef slope in the Halmahera (Indonesia) ER. Several other sites in the Coral Triangle had SL scores exceeding 200 species, their communities comprising more than 40% of SR and approximately 25% of IWP and 20% of global species pools.
Typical of complex communities, IWP coral assemblages were comprised predominantly by species that were locally uncommon or rare, representing more than 90% of all species on deep and shallow reef slopes (Figure 3, and see DeVantier and Turak, 2017). Locally common species comprised most of the remainder, with very few species attaining high local abundance (ranks 4 and 5, Table 2). Surprisingly, the richest sites hosted, on average, fewer rare than uncommon species, particularly on shallow reef slopes (Figure 3B).
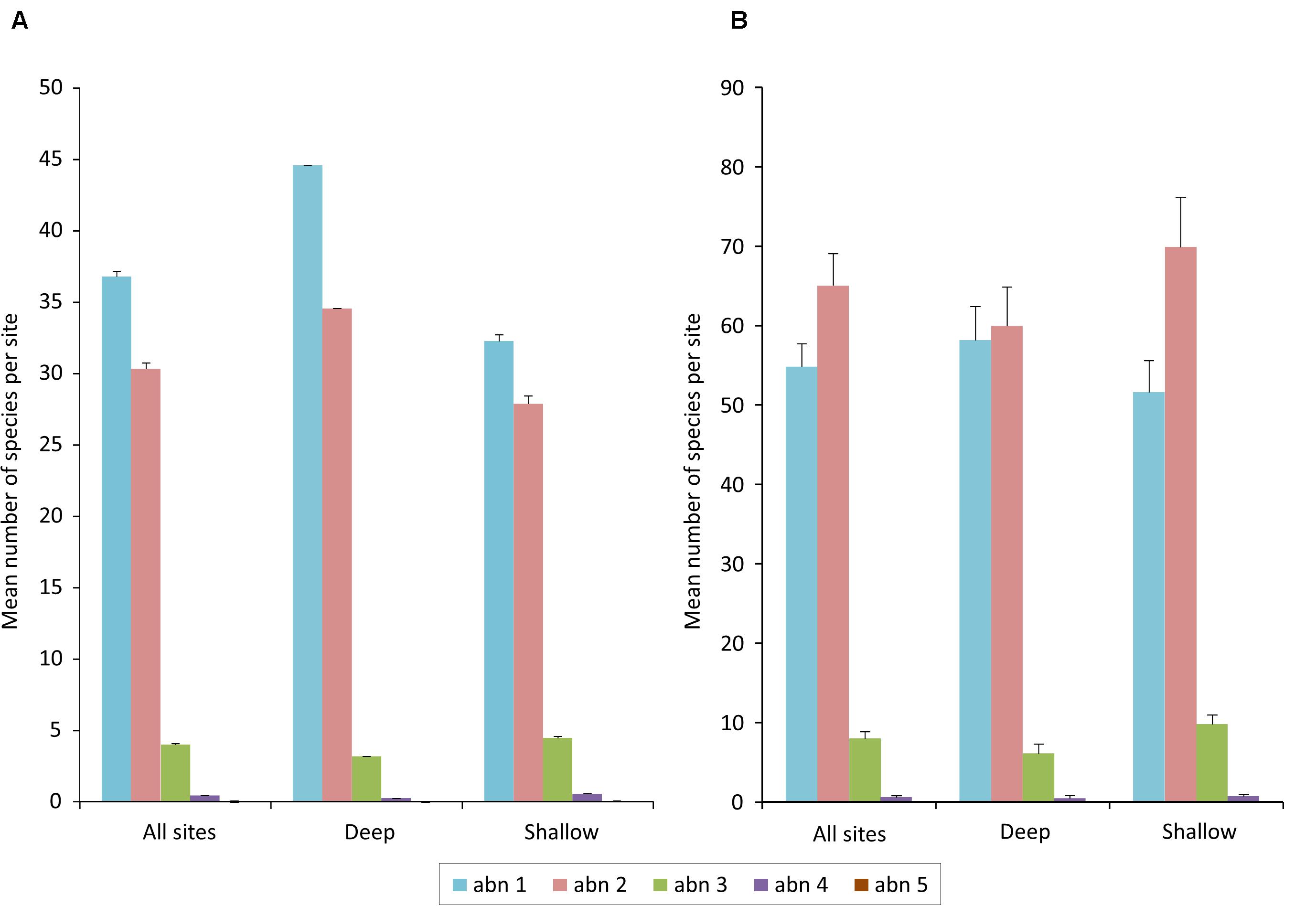
Figure 3. Bar graphs of distribution of species abundances as mean numbers of species per site in each of five ranked abundance categories, with standard error: (A) for all sites, and (B) for richest sites.
The locally common, abundant, and dominant species, despite their small contribution to local richness, can play significant roles in creating coral cover and topographic complexity, and in community structure. The relatively depauperate coral communities of Hong Kong, developed in marginal conditions, showed largest discrepancies, having a higher proportion of locally common and abundant species.
The traditional untransformed linear regressions for all analyses (Supplementary Figures 1, 2) were in general accord with Ricklefs (1987) model and the results of Karlson et al. (2004) and Cornell et al. (2008). However, the log-ratio regressions revealed negative slopes to the trend lines for both deep and shallow reef slopes, for both mean and maximum values of SL and for ENS, consistent with varying degrees of local saturation (Figure 4).
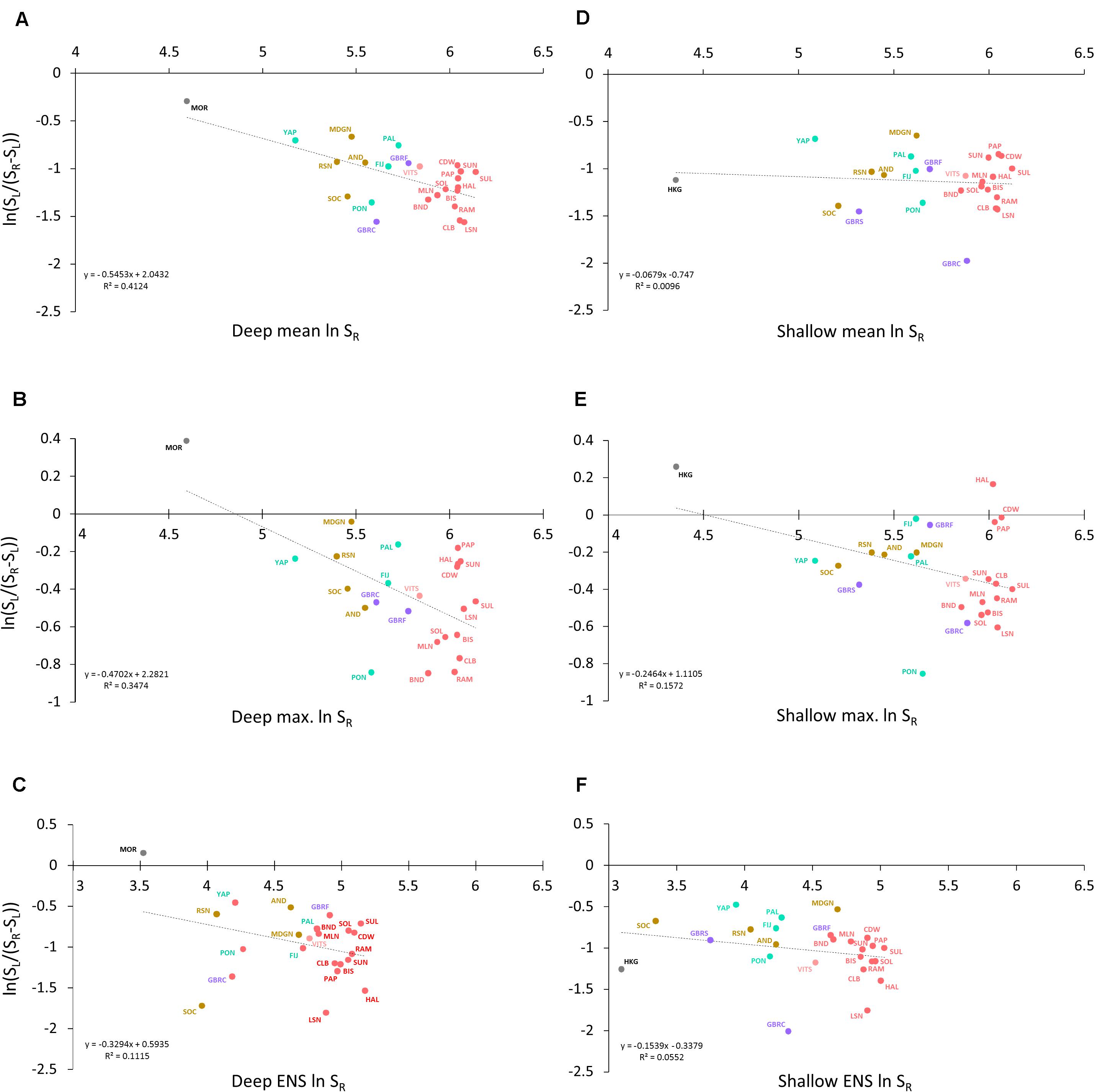
Figure 4. Log ratio regressions of local–regional richness relations for (A) deep reef slope means, (B) deep slope maximums, (C) deep slope ENS, (D) shallow slope means, (E) shallow slope maximums, and (F) shallow slope ENS. Abbreviated ER names are those in Table 1 and Figure 1.
Placing our findings in the Types I–II schema (Figure 5), deep reef slope communities for the 24 ERs were intermediate between regionally enriched and locally saturated for both mean and maximum values of SL and for ENS. Shallow slope communities were either regionally enriched (Type I for mean SL and ENS) or intermediate (max. SL). Hence, incorporating estimates of local abundance (ENS) into the SL:SR relation did not alter the results appreciably.
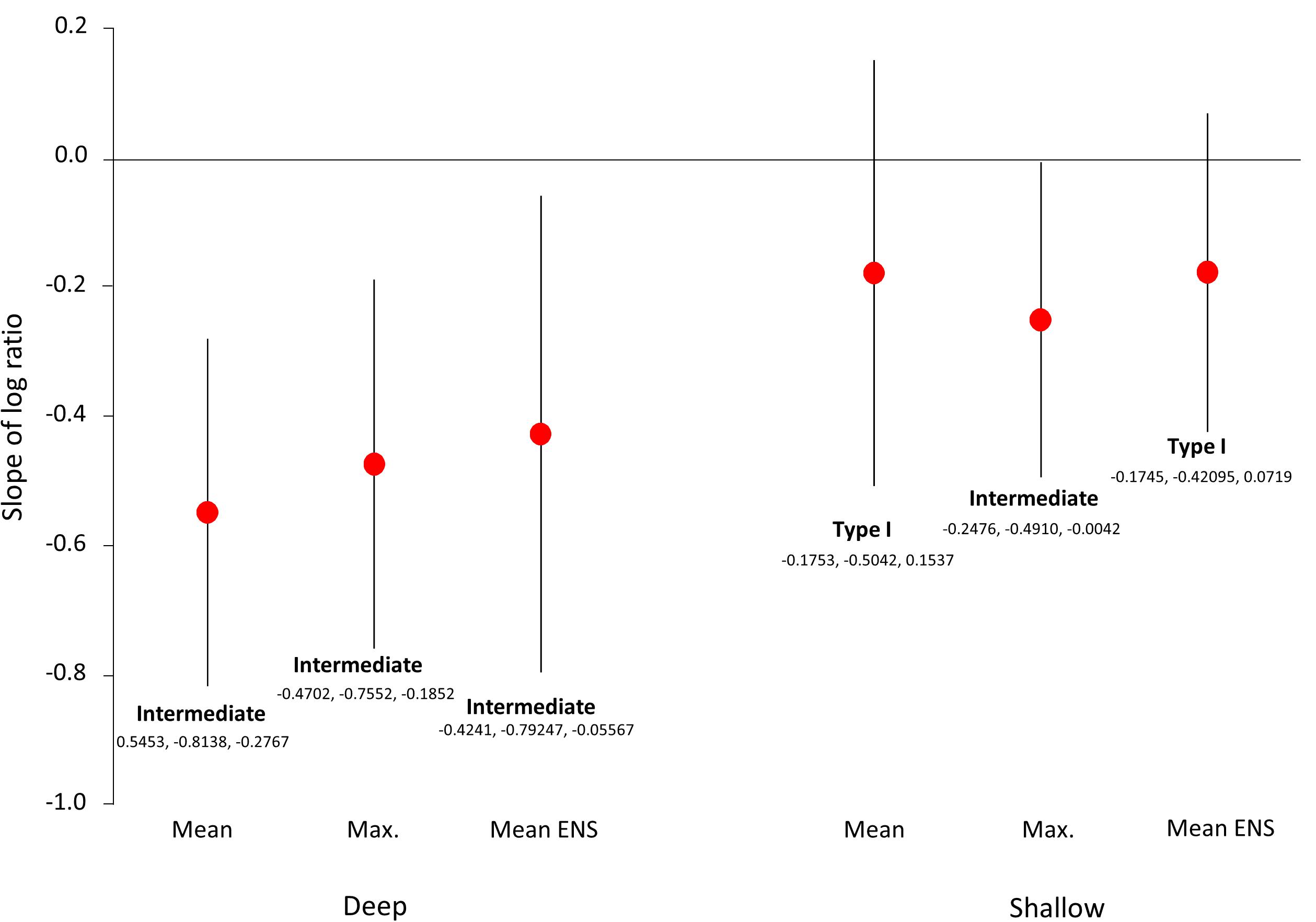
Figure 5. Positions of log ratio regressions with respect to the Type I–Type II continuum for deep and shallow mean and maximum local richness and for ENS. Values of regression slopes (red dots) and 95% confidence intervals (error bars) are listed for each analysis.
Cover of living reef-building corals ranged widely, from <5% to >90%, and was higher overall on shallow reef slopes (HC: 31%, S.E. 0.5) than deep slopes (HC: 23%, S.E. 0.5). No estimates of 100% cover of reef-building corals were recorded, a result of the large local spatial scale of our site surveys.
Relations between local richness and coral cover for each ER were mostly unimodal (Figure 6). On deep and shallow reef slopes, two-thirds of ERs showed “humped-back model” relations (concave –), where SL peaked at intermediate levels of cover. Only one ER on both deep (Red Sea north-central) and shallow (GBR far north) reef slopes showed the reverse relation (concave +), where SL troughed at intermediate levels of cover. In the few remaining ERs, SL tended to stabilize or increase with coral cover.
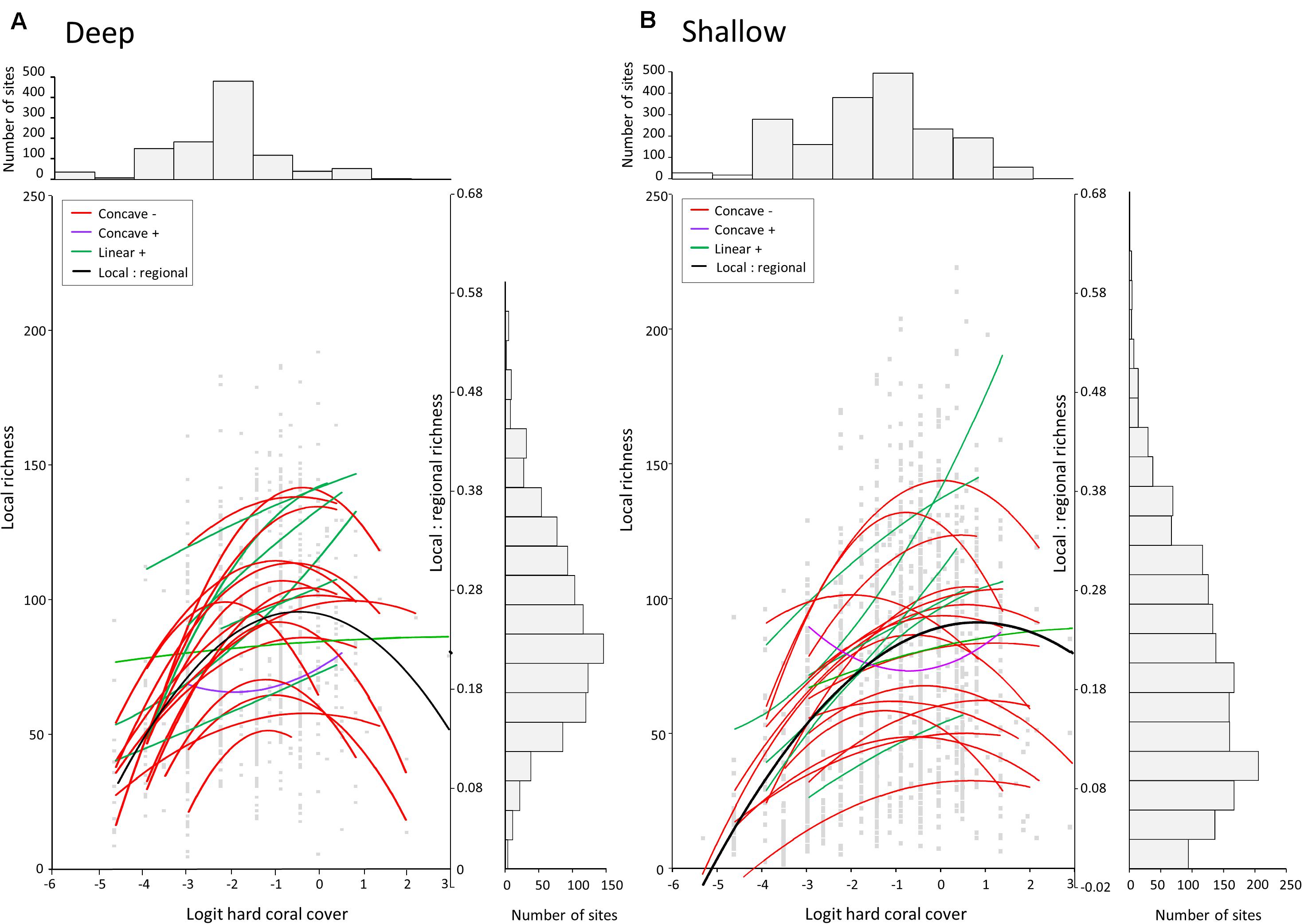
Figure 6. Polynomial regressions of local richness (SL) with cover of living hard corals (Logit transformed, see Methods) for each ER and overall (solid black line). (A) Deep reef slope and (B) shallow reef slope. Color codes for curves illustrate different forms of the relation. For the overall relation (black line), SL:SR for each site in each ER was plotted against logit cover. Bar graphs illustrate the frequency distributions of SL and coral cover in all sites. Logit cover of 0 is equivalent to 50% cover, negative values are less than 50%, and positive values greater than 50%. For the shallow reef slope, the apparent skewed proportion of sites with low richness (B), vertical bar graph, is mainly attributable to the large number of sites surveyed on the north-central GBR.
The unimodal trend was strongest on deeper reef slopes, with relatively low richness for both low and high coral cover (Figure 6A). Shallow slopes also had low richness with low hard coral cover, likely attributable to disturbance. However, some shallow sites also retained high diversity with high cover (Figure 6B). Notably, among the 10 richest shallow sites, with SL ranging from 189 to 223 species, six sites had coral cover of 60% or higher. Conversely, of the 10 richest deep sites, with SL ranging from 169 to 192 species, only two sites had coral cover of 50% or greater. Reasons include site-specific assemblage composition and related capabilities for space maintenance, and the interactive effects of depth, disturbance, and growth rates on coral cover, discussed later.
Relations between local coral abundances, as estimated numbers of corals from the log4 rank abundance scores in each site (Table 2) and SL were also unimodal (not shown). This result may be expected, indeed axiomatic, given that coral cover and abundance are correlated, although qualifiers on strength of the correlation include site-specific differences in sizes, and levels of injury, of coral colonies, both affecting amount of living cover.
Choice of spatial scales, sampling and analytical methods can strongly influence interpretation of richness relationships. Despite such differences with prior studies, our overall mean values for the percentages of regional richness represented locally on deep and shallow reef slopes across all ERs of approximately 25% (Figure 2) were similar. For comparison, Karlson et al. (2004) found local richness in shallow reef habitats consistently represented 27% of regional values. Our results were, however, much more variable, with mean local richness ranging from 12 to 43% of regional pools of different ERs. Richest individual sites exceeded 50% of their regional pools (Figure 2), and in the Coral Triangle, with > 200 species, represented > 25% of Indo-Pacific and > 20% of global richness (ca. 831 species, Veron et al., 2020). Excluding recent introductions, no species occur naturally in both the Indo-Pacific and relatively depauperate Atlantic (73 species). Local richness in the exceptional sites may well indicate the asymptote for reef-building corals, albeit reliant on regionally (indeed globally) high regional richness. For deep and shallow sites combined, the highest local tally reached 280 species ha–1.
Reasons for the variability in our dataset may include the less structured sampling regime and “roving diver” sampling strategy, broader range of habitats and of ERs spread from the global center of diversity in the Coral Triangle to its margins. These differences resulted in our much higher scores when compared, for similar locations, to those surveyed previously (Karlson et al., 2004; Cornell et al., 2008; Supplementary Figure 3). Our estimate for overall regional richness of 507 species for shallow sites in those similar locations is approximately one and a half times greater than the 333 species of the prior study, consistent with occurrence of many locally uncommon or rare species (Figure 3 and also see Figure 9 in DeVantier and Turak, 2017). Such species are best sampled by active searching. At site level, Cornell et al. (2008) recorded maximum local richness of < 80 species (their Figure 1), compared with maxima from our shallow sites of > 200 species (Supplementary Figures 2, 3). Nevertheless, taxonomic difficulties of identifying juvenile corals, among other sampling challenges, mean that SL was always underestimated.
At regional scales, confidence that our method provides reliable estimates of richness is bolstered by the Chao 1 estimates(Tables 1, 3), and comparison with the recent global compilation in “corals of the world” (COTW)1 (Veron et al., 2020), which includes numerous peer-reviewed publications additional to our own. Over the 26 ERs sampled in this study, COTW lists 724 species of confirmed and highly predicted taxa. Of these, the 672 species recorded here represents 93% (Table 3).
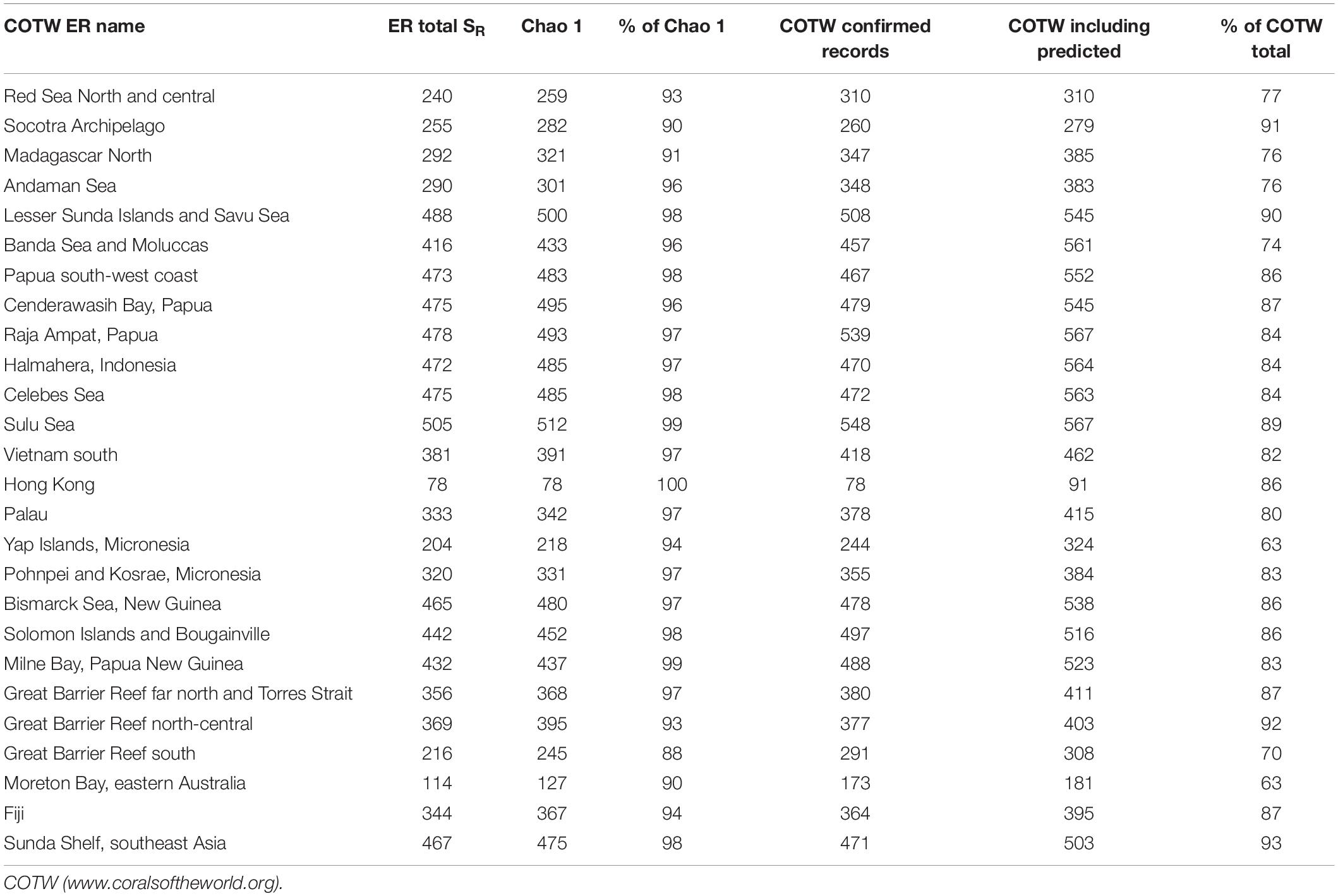
Table 3. Comparisons between various estimates of regional richness for IWP reef-building corals, for the two depth ranges combined.
Our results, and much prior work by colleagues, do not support the recent conclusion that highest coral richness resides in the Western Indian Ocean, centered on Madagascar (Chao et al., 2020; Kusumoto et al., 2020). That conclusion, based on analysis of two global datasets (Global Biodiversity Information Facility and the Ocean Biogeographic Information System), did not consider relevant studies that have assessed coral richness in the region (e.g., Obura, 2012), and its relation to the IWP more generally (Veron et al., 2015, 2020; DeVantier and Turak, 2017). Indeed, Obura (2012) surveyed more than 230 reef sites across the Western Indian Ocean, and corals of Madagascar have been the focus of multiple dedicated surveys since the 1970s, including by one of the present authors (Veron and Turak, 2005; Obura, 2009; and see Obura, 2012 for references). Although an Indian Ocean biodiversity hotspot, the region does not possess the reef area or habitat complexity of the Coral Triangle, nor its complex geo-tectonic and marine evolutionary history. These, and other factors, account for the more than 250 additional coral species present in the Coral Triangle (Table 3, and see Veron et al., 2015). Clearly, those global datasets require significant updating.
At the spatial scales examined, strongest evidence for regional enrichment occurred on shallow reef slopes (Figure 5), more prone to disturbance, with faster rates of species turnover more reliant on dispersal and recruitment than their deeper counterparts. Karlson et al. (2004) acknowledged the role of disturbance, among several potential mechanisms, for their findings of apparently strong and consistent regional enrichment. These also included oceanic transport, location relative to biodiversity hotspots, and evolutionary processes generating variation in the size of regional species pools.
On deeper reef slopes, IWP coral assemblages displayed “intermediate” patterns of richness (Figure 5), neither fully Type I nor Type II (Szava-Kovats et al., 2013), rather forming part of the continuum (also see Karlson and Cornell, 1999). In the latter study, the importance of integrating dispersal and niche attributes in finding “middle ground” was emphasized, a proposal explored briefly below. In subsequent studies, however, the middle ground was abandoned in favor of regional enrichment (Karlson et al., 2004; Cornell et al., 2008). Given that the latter studies focused exclusively on shallow reef slopes, the change in conclusion is understandable and supported by the present study for mean local richness and ENS (Figure 5).
Deep reef slope communities show more evidence of saturation, for both mean and maximum values of local richness and for ENS. Traditionally, evidence for saturation required SL to attain a maximum limit, depending on ecological and/or areal constraints, when the environmental carrying capacity of the community was reached, producing a curvilinear SL:SR relation. However, complete independence of local from regional richness can only occur where all habitats within a region are strongly constrained by competition at the same time. Patch occupancy models indicate that even limited disturbance influences the relation (Hillebrand, 2005).
One extreme interpretation of saturation would require every individual in a community to represent a different species. Several sites in the Coral Triangle, and elsewhere, did approach this extreme, the former hosting exceptionally rich communities of 200 species or more, of which most species (>85–98%) were locally uncommon or rare (Figure 3), and representing greater than 50% of SR (Figure 2).
The concept of environmental carrying capacity implies a steady-state level of richness specific to a particular site, set by resource availability and other local conditions, and maintained despite changes in species composition (Brown et al., 2001; Mateo et al., 2017). For sessile species, where space is occupied as discrete units at a scale, the absolute number of units may set an upper limit to local richness at that scale (Loreau, 2000). Sessile, or in a few cases sedentary, as adults and requiring light for their endosymbionts, reef-building corals have evolved a variety of mechanisms for maintaining, gaining and creating space, and hence increasing local carrying capacity.
Corals, as foundation species, provide much of the three-dimensional, multi-layered reef framework. This form of ecological facilitation (Stachowicz, 2001; Bruno et al., 2003) creates habitat and can thereby foster ongoing recruitment, from local or regional sources. Where dispersal is strong, the contribution of regional to local richness can thus be enhanced in species-rich “sink communities” (Mouquet and Loreau, 2003; Michalet et al., 2015), such as many coral reefs. Notably, other forms of facilitation, including via chemical cues, exist in corals and other reef biota (Heyward and Negri, 1999; Price, 2010; Dixson et al., 2014).
Facilitation mainly occurs at the intermediate position along gradients of increasing environmental severity (Bruno et al., 2003; Michalet et al., 2015), enhancing local richness in opposition to disturbance stress at one end of the gradient, and competition at the other. Our findings of unimodal relations between local richness and cover (Figure 6, concave–) and abundance (not shown) are consistent with roles for facilitation, disturbance-mediation and/or competition (Grime, 1973; Connell, 1978; Huston, 1985; Rogers, 1993; Aronson and Precht, 1995), at sites in different positions along the gradient.
Using coral cover as a rough proxy of disturbance (following Connell, 1978), we assumed that high cover reflects a lack of disturbance and contributes to increased levels of competition, as adjacent corals interact. At the spatial scale of our local surveys (<0.5 ha), cover of living reef-building corals per se never attained 100%, and hence sites were never totally “saturated,” at least not by corals. These caveats notwithstanding, we considered that in sites with high coral cover, free space was limited and competition could occur.
Relations of local richness with coral cover ranged among ERs, but most were unimodal (Figure 6), exhibiting the “humped-back model,” peaking at intermediate levels of cover and declining at the highest levels, particularly on deep reef slopes. These results are consistent with Connell (1978) intermediate disturbance hypothesis (see Connell, 1978, Figure 2; and Huston, 1985; Aronson and Precht, 1995). That local richness declined where live cover was exceptionally high, albeit with some important exceptions, also provides limited support for competitive exclusion. Those exceptions, of exceedingly rich sites with coral cover exceeding 60%, may “prove the rule” and offer evidence of corals’ capability for space maintenance and niche partitioning (Figure 7 and see later).
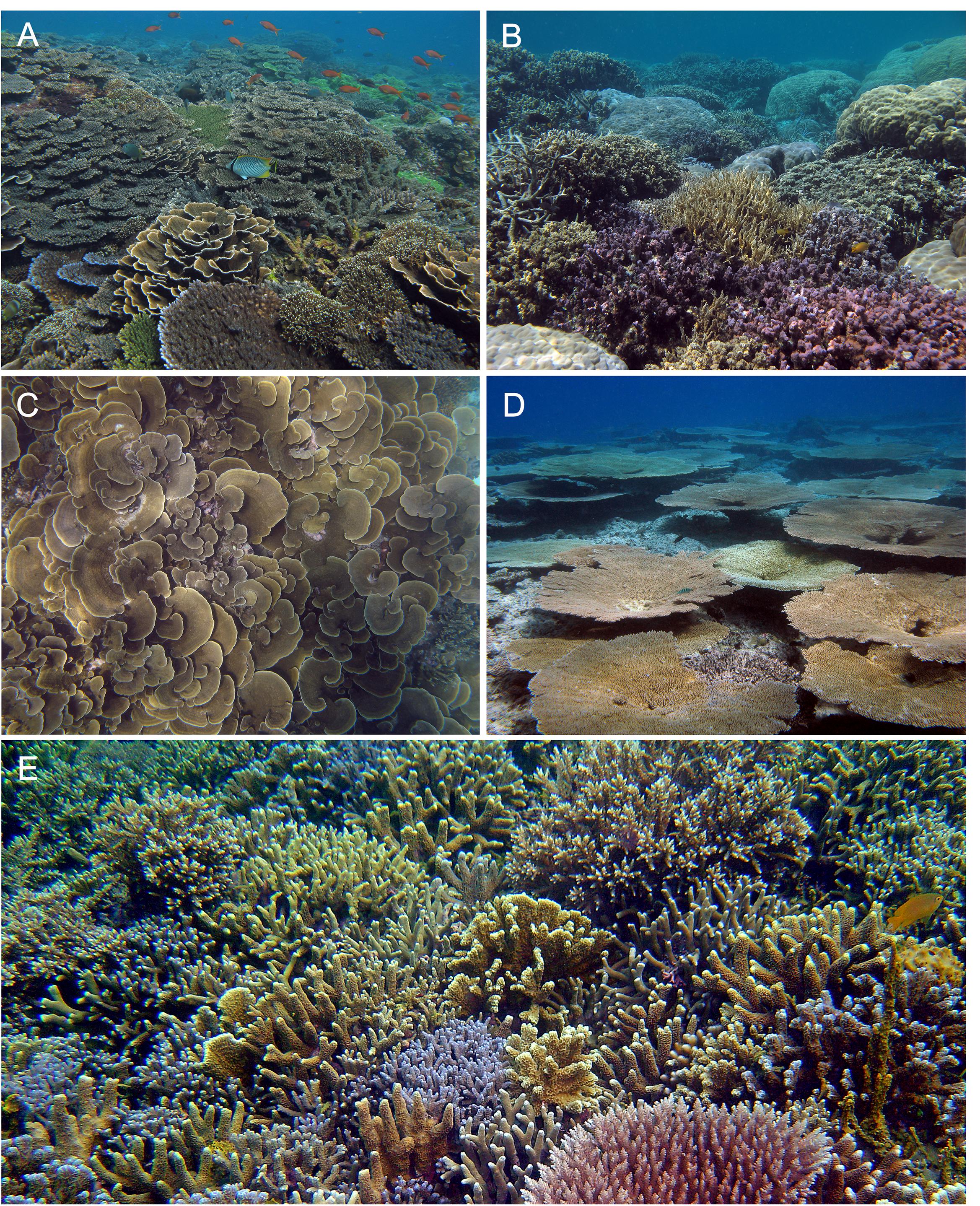
Figure 7. Photos illustrating various forms of the relation between local species richness and high coral cover: (A) moderate richness of Acropora and Montipora spp. growing in close proximity, Bali, Indonesia, 2008; (B) moderate richness of massive and submassive species of Porites, including P. rus (foreground), Pachyseris rugosa, and Echinopora horrida, Anambas Islands, Indonesia, 2012; (C) monospecific stands of Montipora aequituberculata, Palawan, Philippines, 2009; (D) monospecific tables of Acropora cytherea, fostering local recovery a decade following mass bleaching, Palau, Micronesia, 2009. In the absence of further disturbance, the tables would occupy most remaining space within several years, overtopping each other in the process; and (E) rich assemblage of Porites, Acropora, and Montipora spp. growing in close proximity, and with Acropora microclados (bottom right) overtopping neighboring Porites spp., Cenderawasih Bay, Indonesia, 2006.
Our unimodal findings are broadly consistent with those from the world’s grassland ecosystem, which exhibits humped-back relations between richness and biomass (Fraser et al., 2015), but less so with the conclusions of Harmon and Harrison (2015), who found little evidence of negative correlations between diversity and abundance, arguing strongly for dynamism.
Predominantly unimodal relations between local richness and coral cover, and intermediate relations between local and regional richness, argue for a nuanced understanding. There is no question that IWP coral communities are dynamic and influenced by regional processes, yet there is also evidence for a degree of local saturation. In these respects, a similar dataset from the Eastern Pacific or Caribbean Sea, where both local and regional richness are relatively low but cover and local abundance can be high, may reveal different patterns (Karlson and Cornell, 1999).
Over the more than two decadal course of this study, sites exhibiting very high coral cover and/or abundance formed only small proportions of the totals on both deep and shallow reef slopes (Figure 6 bar graphs). Despite their low frequencies, these have provided a degree of “spatial insurance” (Loreau et al., 2003) for those that were recently disturbed or in a state of recovery.
Today, coral reefs in many regions of the IWP are highly disturbed, levels of chronic and acute disturbance having grown rapidly in recent decades (Glynn, 1993; Ginsburg and Smith, 1995; Brown, 1997; Jackson, 1997; Wilkinson, 1999; Kleypas et al., 2001; Hoegh-Guldberg et al., 2007; and many subsequent papers). Our surveys included many sites that had been disturbed in the recent past by one or more impacts (see e.g., DeVantier et al., 1998, 2004, 2006), undoubtedly affecting our results, and emblematic of Hillebrand (2005) point regarding lack of complete independence of local from regional richness. Indeed, the stronger level of regional enrichment on shallow reef slopes compared with their deeper counterparts is consistent with depth-related differences in levels of disturbance, including from bleaching (Glynn, 1996; Muir et al., 2017), storms and floods (van Woesik et al., 1995; Fabricius et al., 2008), and destructive fishing (pers. obs.).
It is axiomatic that disturbance can influence species richness in many ways, depending on its form, intensity, duration, and frequency (Connell, 1978; Huston, 1985; Ricklefs, 1987; Done, 1999; van Woesik, 2002; Hall et al., 2012). In selecting against less-tolerant species, disturbance can denude communities, reducing local richness. It can also create space for settlement and recruitment, and by increasing environmental heterogeneity and interrupting the return of systems to some form of equilibrium, as with very high coral cover, disturbance may promote local richness by limiting competitive exclusion. Conversely, if of sufficient frequency and/or intensity, disturbances can continually set back recovery, select for a tolerant subset of species, depleting richness (DeVantier et al., 2006), or change the state of a community to an alternative one, as for example where algae, sponges, or other biota replace corals (Knowlton, 1992; Done, 1999; van Woesik, 2002; Aronson et al., 2004).
In this study, the strongest evidence of a regionwide reduction in local richness was on shallow reef slopes of the central and northern GBR, which showed anomalously low SL:SR relations (Figure 2). Corals on many reefs there had been heavily impacted by repeated outbreaks of predatory crown-of-thorns seastars, mass bleaching events, episodic cyclonic storms, and river flooding carrying sediments, nutrients, and pesticides in plumes (van Woesik et al., 1995; Berkelmans and Oliver, 1999; Done, 1999; Fabricius, 2005; Fabricius et al., 2008; Done et al., 2010; Brodie et al., 2012; Brodie and Landos, 2019), collectively depressing local richness over a large area while regional richness remained high (DeVantier et al., 2006).
In the worst-impacted area, the disturbance regime had selected for a tolerant subset of species, notably massive corals in the genus Porites. In those areas of low local richness, post-recruitment effects – the disturbance regime – were at least as important to community assembly as “supply-side ecology” (Gaines and Roughgarden, 1985), governed by the availability of propagules, planulae in the corals’ case.
Today, massive species of Porites are the most-abundant corals on IWP reefs (DeVantier and Turak, 2017; also see Potts et al., 1985). These typically survive disturbances that kill most others in their vicinity, albeit with injury and death to parts of colonies (Done, 1988; Done and Potts, 1992). Their pre-eminence may be linked to the rising disturbance regime. We thus question whether surveys conducted at an earlier time period, prior to the rapid rise in disturbances (Sheppard, 1995; Jackson, 1997; Dietl et al., 2019), would have returned similar results, albeit at risk of invoking the “ghost of stability past.” Yet even for these hardy species, the prognosis is far from assured in a climate-changed future (Cacciapaglia and van Woesik, 2017; van Woesik et al., 2018).
Contrary to the predominantly unimodal relations between local richness and coral cover (Figure 6), some of the richest sites, particularly on shallow reef slopes, had high coral cover (>60%). In such weakly disturbed or undisturbed habitats, where living benthic cover is high, co-occurring corals and other sessile taxa compete – gain, lose, or maintain space (“stand-offs”) – using a variety of biological mechanisms (see Lang and Chornesky, 1990 for review). Yet in the exceptional sites, competitive interactions had not caused major apparent loss of richness. Such sites each reflect a locally unique set of ecological “circumstances” at a specific point in time. Those circumstances include species composition, nearest neighbors and growth rates, colony age, and size distributions, among other factors (Figure 7). Fundamentally, such richness relies on the continuing supply of propagules from a diverse array of species, enabling ongoing recruitment.
In those exceptional sites, our results suggest that interspecific interactions fostered maintenance of space rather than competitive exclusion. Corals, mostly sessile as adults, have evolved a variety of mechanisms that aid maintenance of space and competition more generally. These include use of mesenterial filaments and tentacles (Lang, 1973; Sheppard, 1979; Chornesky, 1989; Abelson and Loya, 1999; Lapid and Chadwick, 2006), elaboration of allelochemicals (Jackson and Buss, 1976; Gunthorpe and Cameron, 1990; Fearon and Cameron, 1996), and via a diverse array of colony morphologies and variable capacities for phenotypic plasticity, among other life history attributes (Supplementary Table 2). In many instances of intra- and interspecific aggression, competitors are not killed outright, although interacting colony margins may be (Chornesky, 1989, 1991; Connell et al., 2004; Idjadi and Karlson, 2007; also see Bengtsson et al., 1994).
These various mechanisms foster access to resources, notably light, crucial for autotrophy by corals’ endo-symbiont zooxanthellae. Nocturnally and in low light conditions, corals become increasingly mixo- to heterotrophic, the well-named “wall of mouths.” Levels of autotrophy, mixotrophy, and heterotrophy vary intra- and interspecifically (Conti-Jerpe et al., 2020) in different environmental settings, including with increasing depth (Muscatine et al., 1989) and different sediment regimes (Anthony and Fabricius, 2000). The capacity to regulate changes in trophic mode to sustain a positive energy balance (Anthony and Fabricius, 2000) – a form of trophic “plasticity” – aids expansion of the physiological niche (Hoogenboom and Connolly, 2009; Cooper et al., 2011). For example, shifts between phototrophic and heterotrophic dependence during periods of stronger illumination enable rapid rates of photoacclimation and replenishment of energy reserves (Anthony and Larcombe, 2000).
These capacities for resource partitioning do not restrict the vast majority of IWP coral species to particular habitats. Rather, most have broad spatial niches, extending from shallow reef flats into the upper mesophotic zone (Turak and DeVantier, 2019). Of the 672 species in our dataset, more than 90% occurred on both shallow and deep slopes, a result at first sight more consistent with neutral theory (Hubbell, 2001) than niche partitioning.
However, most IWP coral species do show depth-related preferences, occurring more commonly, albeit not exclusively, on shallow or deep slopes (Sheppard, 1982; Huston, 1985; Turak and DeVantier, 2019). Of the species pool sampled in the last study, 3% were considered mesophotic zone specialists, 18% preferred lower reef slopes, 51% preferred midreef slopes, 11% preferred shallow slopes, and 17% were depth generalists. Furthermore, a small suite of species appear restricted to the very shallow reef slope, with 25 spp. identified to date (DeVantier and Turak, 2017). A similar number of species appear restricted to deeper slopes, of which a subset is considered a mesophotic specialist (Pochon et al., 2015). These preferences create the more-or-less well-defined structure of IWP coral communities (e.g., GBR: Done, 1982; van Woesik and Done, 1997; DeVantier et al., 1998, 2006).
As with regional enrichment and local saturation, neutral and niche-based states of species co-existence are not mutually exclusive, rather forming part of a continuum from stochastic to competitive exclusion (Gravel et al., 2006) and differing with taxonomic composition. For example, many species in the most speciose IWP genus, Acropora, share similar biology, and may thus be considered as having a very similar “multi-species” niche (van Woesik, 2002). Other corals have different biology and habitat preferences, providing evidence for significant niche differentiation, or “high niche dimensionality” (Clark et al., 2007), with species co-occurring via partitioning across multiple niche dimensions (Supplementary Table 2). Today, with coral cover declining globally (Bruno and Selig, 2007), frequencies of local interactions and potential for competitive exclusion are also declining, consistent with a niche-neutral model such as “emergent neutrality” (Scheffer and Van Nes, 2006; Vergnon et al., 2009; Scheffer et al., 2018).
The Scleractinia have been extant for more than 200 million years, with numerous genera and some species present since the Eocene, having slow rates of reticulate evolution (Veron, 1995). During that period, corals have evolved an array of morphological, physiological, and metabolic traits to facilitate access to, and partitioning of, space and other key resources (Supplementary Table 2; and see Porter, 1976; Muscatine et al., 1989; Lang and Chornesky, 1990; Stafford-Smith and Ormond, 1992; Anthony and Fabricius, 2000; Anthony and Larcombe, 2000; Anthony and Connolly, 2004). Over evolutionary time, with increased packing and elaboration of axes of niche hyperspace, there is no evident intrinsic limit on the increase in species number (Whittaker, 1972).
This rich diversity of traits may also suggest some form of co-evolutionary divergence – invoking another ghost, that of competition past (Connell, 1980). Yet competing species that are not directly reliant on each other, as is the case for reef-building corals, need not consistently co-occur or co-evolve. Increased diversity, by reducing the consistency of co-occurrence, may also reduce the chance of co-evolution (Connell, 1980; but also see Scheffer and Van Nes, 2006). Today’s reef-building corals exhibit a high diversity of life histories (Supplementary Table 2). At which stages in their long history the divergences that engendered this diversity occurred, and their drivers, remain salient paleontological and evolutionary questions.
Over the more than two-decadal span of this study, the relative importance of regional enrichment and local saturation on species richness of IWP corals differed consistently on shallow and deep reef slopes. These differences may be attributed to greater levels of disturbance, and turnover, on shallow slopes. At this time of rapid environmental change, IWP coral communities are clearly dynamic and influenced by regional processes. Yet there was also evidence for a degree of local saturation in richest sites of both shallow and deep reef slopes, and on deeper reef slopes more generally. Unimodal relations between local richness and coral cover provide support for disturbance mediation and competitive exclusion. Additionally, however, the co-occurrence of exceptional local richness and high coral cover provide evidence for strong capabilities for space maintenance, consistent with multi-dimensional niche partitioning. Together these various mechanisms foster the exceptional local and regional richness of earth’s most diverse marine ecosystem. With differential coral mortality increasing globally, maintaining even a semblance of such richness will prove a major challenge as we enter the Anthropocene “bottleneck” (Guinotte et al., 2003; Carpenter et al., 2008; Veron et al., 2009; Foden et al., 2013; Sheppard et al., 2020; Trisos et al., 2020).
All datasets generated for this study are included in the article/Supplementary Material.
LD and ET designed and conducted the surveys that form the basis of this study, analyzed the data, with significant input from RS-K. All authors wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The field work for this study was funded by various government and non-government organizations, acknowledged in detail in DeVantier and Turak (2017). Preparation of this article was undertaken without financial support.
Spanning more than two decades, the field work for this study benefited from the assistance of many people and institutions in tropical nations across the Indo-West Pacific, acknowledged in detail in DeVantier and Turak (2017). The manuscript benefited greatly from the constructive insights of four reviewers (two of whom inexplicably did not complete the process), the editor and valued colleagues Richard Aronson, Terry Done, Robert van Woesik and Charlie Veron.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00487/full#supplementary-material
Abelson, A., and Loya, Y. (1999). Interspecific aggression among stony corals in Eilat, Red Sea: a hierarchy of aggression ability and related parameters. Bull. Mar. Sci. 65, 851–860.
Anthony, K., and Larcombe, P. (2000). “Coral reefs in turbid waters: sediment-induced stresses in corals and likely mechanisms of adaptation,” in Proceedings of the 9th International Coral Reef Symposium I, Bali, 239–244.
Anthony, K. R., and Connolly, S. R. (2004). Environmental limits to growth: physiological niche boundaries of corals along turbidity–light gradients. Oecologia 141, 373–384. doi: 10.1007/s00442-004-1647-7
Anthony, K. R. N., and Fabricius, K. E. (2000). Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 252, 221–253. doi: 10.1016/s0022-0981(00)00237-9
Aronson, R. B., Macintyre, I. G., Wapnick, C. M., and O’Neill, M. W. (2004). Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology 85, 1876–1891.
Aronson, R. B., and Precht, W. F. (1995). Landscape patterns of reef coral diversity: a test of the intermediate disturbance hypothesis. J. Exp. Mar. Biol. Ecol. 192, 1–14. doi: 10.1016/0022-0981(95)00052-S
Bengtsson, J., Fagerstrom, T., and Rydin, H. (1994). Competition and coexistence in plant communities. Trends Ecol. Evol. 9, 246–250. doi: 10.1016/0169-5347(94)90289-5
Berkelmans, R., and Oliver, J. (1999). Large-scale bleaching of corals on the Great Barrier Reef. Coral Reefs 18, 55–60. doi: 10.1007/s003380050154
Briggs, J. C., and Bowen, B. W. (2012). A realignment of marine biogeographic provinces with particular reference to fish distributions. J. Biogeogr. 39, 12–30. doi: 10.1111/j.1365-2699.2011.02613
Brodie, J., and Landos, M. (2019). Pesticides in Queensland and Great Barrier Reef waterways - potential impacts on aquatic ecosystems and the failure of national management. Estuar. Coast. Shelf Sci. 230:106447. doi: 10.1016/j.ecss.2019.106447
Brodie, J. E., Kroon, F. J., Schaffelke, B., Wolanski, E. C., Lewis, S. E., Devlin, M. J., et al. (2012). Terrestrial pollutant runoff to the Great Barrier Reef: an update of issues, priorities and management responses. Mar. Pollut. Bull. 65, 81–100. doi: 10.1016/j.marpolbul.2011.12.012
Brown, B. E. (1997). Coral bleaching: causes and consequences. Coral Reefs 16, S129–S138. doi: 10.1007/s003380050249
Brown, J. H., Morgan Ernest, S. K., Parody, J. M., and Haskell, J. P. (2001). Regulation of diversity: maintenance of species richness in changing environments. Oecologia 126, 321–332. doi: 10.1007/s004420000536
Bruno, J. F., and Selig, E. R. (2007). Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One 2:e711. doi: 10.1371/journal.pone.0000711
Bruno, J. F., Stachowicz, J. J., and Bertness, M. D. (2003). Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125. doi: 10.1016/S0169-5347(02)00045-9
Cacciapaglia, C., and van Woesik, R. (2017). Marine species distribution modelling and the effects of genetic isolation under climate change. J. Biogeog. 45, 154–163. doi: 10.1111/jbi.13115
Caley, M. J., and Schluter, D. (1997). The relationship between local and regional diversity. Ecology 78, 70–80.
Cao, Y., and Hawkins, C. P. (2019). Weighting effective number of species measures by abundance weakens detection of diversity responses. J. Appl. Ecol. 56, 1200–1209. doi: 10.1111/1365-2664.13345
Carpenter, K. E., Abrar, M., Aeby, G., Aronson, R. B., Banks, S., Bruckner, A., et al. (2008). One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. doi: 10.1126/science.1159196
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Ma, K. H., Colwell, R. K., et al. (2014). Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi: 10.1890/13-0133.1
Chao, A., Kubota, Y., Zelený, D., Chiu, C.-H., Li, C.-F., Kusumoto, B., et al. (2020). Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 35, 292–314. doi: 10.1111/1440-1703.12102
Chesson, P. (2000). Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Evol. Syst. 31, 343–366. doi: 10.1080/00131881.2019.1600376
Chornesky, E. A. (1989). Repeated reversals during spatial competition between corals. Ecology 70, 843–855. doi: 10.2307/1941353
Chornesky, E. A. (1991). The ties that bind: inter-clonal cooperation may help a fragile coral dominate shallow high-energy reefs. Mar. Biol. 109, 41–51. doi: 10.1007/BF01320230
Clark, J. S., Dietze, M., Chakraborty, S., Agarwal, P. K., Ibanez, I., LaDeau, S., et al. (2007). Resolving the biodiversity paradox. Ecol. Lett. 13, 647–659. doi: 10.1111/j.1461-0248.2007.01041.x
Colwell, R. K. (2019). EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Available online at: http://purl.oclc.org/estimates (accessed November 2019).
Colwell, R. K., and Elsensohn, J. E. (2014). EstimateS turns 20: statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 37, 609–613. doi: 10.1111/ecog.00814
Connell, J. H. (1980). Diversity and the Coevolution of Competitors, or the Ghost of Competition Past. Oikos 35, 131–138. doi: 10.2307/3565721
Connell, J. H., Hughes, T. P., Wallace, C. C., Tanner, J. E., Harms, K. E., and Kerr, A. M. (2004). A long-term study of competition and diversity of corals. Ecol. Monogr. 74, 179–210.
Conti-Jerpe, I. E., Thompson, P. D., Wong, C. W. M., Oliveira, N. L., Duprey, N. N., Moynihan, M. A., et al. (2020). Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 6:eaaz5443. doi: 10.1126/sciadv.aaz5443
Cooper, T. F., Ulstrup, K. E., Dandan, S. S., Heyward, A. J., Kühl, M., Muirhead, A., et al. (2011). Niche specialization of reef-building corals in the mesophotic zone: metabolic trade-offs between divergent Symbiodinium types. Proc. R. Soc. B 278, 1840–1850. doi: 10.1098/rspb.2010.2321
Cornell, H. V., and Harrison, S. P. (2014). What are species pools and when are they important? Annu. Rev. Ecol. Evol. Syst. 45, 45–67. doi: 10.1146/annurev-ecolsys-120213-091759
Cornell, H. V., and Karlson, R. H. (1996). Species richness of reef building corals determined by local and regional processes. J. Anim. Ecol. 62, 233–241.
Cornell, H. V., and Karlson, R. H. (2000). Coral species richness: ecological versus biogeographical influences. Coral Reefs 19, 37–49. doi: 10.1007/s003380050224
Cornell, H. V., Karlson, R. H., and Hughes, T. P. (2008). Local-regional species richness relationships are linear at very small to large scales in west-central Pacific corals. Coral Reefs 27, 145–151. doi: 10.1007/s00338-007-0303-1
DeVantier, L., and Turak, E. (2017). Species richness and relative abundance of reef-building corals in the Indo-West Pacific. Diversity 9, 1–30. doi: 10.3390/d9030025
DeVantier, L. M., De’Ath, G., Done, T. J., and Turak, E. (1998). Ecological assessment of a complex natural system: a case study from the Great Barrier Reef. Ecol. Appl. 8, 480–496.
DeVantier, L. M., De’ath, G., Klaus, R., Al-Moghrabi, S., Abdal-Aziz, M., Reinicke, G. B., et al. (2004). Reef-building corals and coral communities of the Socotra Islands, Yemen: a zoogeographic ‘crossroads’ in the Arabian Sea. Fauna Arabia 20, 117–168.
DeVantier, L. M., De’ath, G., Turak, E., Done, T. J., and Fabricius, K. (2006). Species richness and community structure of reef-building corals on the nearshore Great Barrier Reef. Coral Reefs 25, 329–340. doi: 10.1007/s00338-006-0115-8
Dietl, G. P., Smith, J. A., and Durham, S. A. (2019). Discounting the past: the undervaluing of paleontological data in conservation science. Front. Ecol. Evol. 7:108. doi: 10.3389/fevo.2019.00108
Dixson, D. L., Abrego, D., and Hay, M. E. (2014). Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345, 892–897. doi: 10.1126/science.1255057
Done, T., Gilmour, J., and Fisher, R. (2015). Distance decay among coral assemblages during a cycle of disturbance and recovery. Coral Reefs 34, 727–738. doi: 10.1007/s00338-015-1302-2
Done, T. J. (1982). Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1, 95–107. doi: 10.1007/BF00301691
Done, T. J. (1988). Simulation of recovery of pre-disturbance size structure in populations of Porites spp. damaged by the crown of thorns starfish Acanthaster planci. Mar. Biol. 100, 51–61. doi: 10.1007/BF00392954
Done, T. J. (1999). Coral community adaptability to environmental change at the scales of regions, reefs and reef zones. Am. Zool. 39, 66–79. doi: 10.1093/icb/39.1.66
Done, T. J., DeVantier, L. M., Turak, E., Fisk, D. A., Wakeford, M., and van Woesik, R. (2010). Coral growth on three reefs: development of recovery benchmarks using a space for time approach. Coral Reefs 29, 815–833. doi: 10.1007/s00338-010-0637-y
Done, T. J., and Potts, D. C. (1992). Influences of habitat and natural disturbances on contributions of massive Porites corals to reef communities. Mar. Biol. 114, 479–493. doi: 10.1007/BF00350040
Done, T. J., Turak, E., Wakeford, M., DeVantier, L., McDonald, A., and Fisk, D. (2007). Turbid water coral communities at Pandora Reef, Great Barrier Reef: loss of resilience or too soon to tell? Coral Reefs 26, 785–805. doi: 10.1007/s00338-007-0265-3
Fabricius, K. E. (2005). Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 50, 125–146. doi: 10.1016/j.marpolbul.2004.11.028
Fabricius, K. E., De’ath, G., Poutinen, M. L., Done, T., Cooper, T. F., and Burgess, S. C. (2008). Disturbance gradients on inshore and offshore coral reefs caused by a severe tropical cyclone. Limnol. Oceanogr. 53, 690–704. doi: 10.4319/lo.2008.53.2.0690
Fearon, R. J., and Cameron, A. M. (1996). Larvotoxic extracts of the hard coral Goniopora tenuidens: allelochemicals that limit settlement of potential competitors? Toxicon 34, 361–367. doi: 10.1016/0041-0101(95)00137-9
Foden, W. B., Butchart, S. H. M., Stuart, S. N., Vié, J.-C., Akçakaya, H. R., Angulo, A., et al. (2013). Identifying the world’s most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS One 8:e65427. doi: 10.1371/journal.pone.0065427
Fraser, L. H., Pither, J., Jentsch, A., Sternberg, M., Zobel, M., Askarizadeh, D., et al. (2015). Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science 349, 302–305. doi: 10.1126/science.aab3916
Gaines, S., and Roughgarden, J. (1985). Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proc. Natl. Acad. Sci. U.S.A. 82, 3707–3711. doi: 10.1073/pnas.82.11.3707
Ginsburg, R. N., and Smith, F. G. W. (1995). Proceedings of the colloquium on global aspects of coral reefs: health, hazards, and history, June 1993. Oceanogr. Lit. Rev. 42:673.
Glynn, P. W. (1993). Coral reef bleaching: ecological perspectives. Coral Reefs 12, 1–17. doi: 10.1007/BF00303779
Glynn, P. W. (1996). Coral reef bleaching: facts, hypotheses and implications. Glob. Change Biol. 2, 495–509. doi: 10.1111/j.1365-2486.1996.tb00063.x
Gotelli, N. J., and Colwell, R. K. (2001). Quantifying biodiversity: procedure and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391. doi: 10.1046/j.1461-0248.2001.00230.x
Gravel, D., Canham, C. D., Beaudet, M., and Messier, C. (2006). Reconciling niche and neutrality: the continuum hypothesis. Ecol. Lett. 9, 399–409. doi: 10.1111/j.1461-0248.2006.00884.x
Grime, J. P. (1973). Competitive exclusion in herbaceous vegetation. Nature 242, 344–347. doi: 10.1038/242344a0
Guinotte, J. M., Buddemeier, R. W., and Kleypas, J. A. (2003). Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin. Coral Reefs 22, 551–558. doi: 10.1007/s00338-003-0331-4
Gunthorpe, L., and Cameron, A. M. (1990). Widespread but variable toxicity in scleractinian corals. Toxicon 28, 1199–1219. doi: 10.1016/0041-0101(90)90120-V
Hall, A. R., Miller, A. D., Leggett, H. C., Roxburgh, S. H., Buckling, A., and Shea, K. (2012). Diversity-disturbance relationships: Frequency and intensity interact. Biol. Lett. 8, 768–771. doi: 10.1098/rsbl.2012.0282
Harmon, J., and Harrison, S. (2015). Species diversity is dynamic and unbounded at local and continental scales. Am. Nat. 185, 584–593. doi: 10.1086/680859
Harrison, P. L., and Wallace, C. C. (1990). “Reproduction, dispersal and recruitment of scleractinian corals,” in Coral Reef Ecosystems, ed. Z. Dubinsky (Amsterdam: Elsevier), 133–207.
Harrison, S., Ross, S. J., and Lawton, J. H. (1992). Beta diversity on geographic gradients in Britain. J. Anim. Ecol. 61, 151–158. doi: 10.2307/5518
Heyward, A. J., and Negri, A. P. (1999). Natural inducers for coral larval metamorphosis. Coral Reefs 18, 273–279. doi: 10.1007/s003380050193
Hill, M. O. (1973). Diversity and Evenness: a unifying notation and its consequences. Ecology 54, 427–432. doi: 10.2307/1934352
Hillebrand, H. (2005). Regressions of local on regional diversity do not reflect the importance of local interactions or saturation of local diversity. Oikos 110, 195–198. doi: 10.1111/j.0030-1299.2005.14008.x
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Hoogenboom, M. O., and Connolly, S. R. (2009). Defining fundamental niche dimensions of corals: synergistic effects of colony size, light, and flow. Ecology 90, 767–780.
Hubbell, S. (2001). The Unified Neutral Theory of Biodiversity and Biogeography. Princeton Monographs in Population Biology. Princeton, NJ: Princeton University Press, 375.
Hubbell, S. P. (1997). A unified theory of biogeography and relative species abundance and its application to tropical rain forests and coral reefs. Coral Reefs 16, S9–S21. doi: 10.1007/s003380050237
Hugueny, B., Tito de Morais, L., Mérigoux, S., de Mérona, B., and Ponton, D. (1997). The relationship between local and regional species richness: comparing biotas with different evolutionary histories. Oikos 80, 583–587. doi: 10.2307/3546633
Hurlbert, S. H. (1971). The nonconcept of species diversity: a critique and alternative parameters. Ecology 52, 577–586. doi: 10.2307/1934145
Huston, M. A. (1985). Patterns of species diversity of coral reefs. Annu. Rev. Ecol. Syst. 16, 149–177.
Huston, M. A. (1999). Local processes and regional patterns: appropriate scales for understanding variation in the diversity of plants and animals. Oikos 86, 393–401. doi: 10.2307/3546645
Idjadi, J. A., and Karlson, R. H. (2007). Spatial arrangement of competitors influences coexistence of reef-building corals. Ecology 88, 2449–2454.
Jackson, J. B. C., and Buss, L. (1976). Allelopathy and spatial competition among coral-reef invertebrates. Proc. Natl. Acad. Sci. U.S.A. 72, 5160–5163. doi: 10.1073/pnas.72.12.5160
Jones, G. P., Almany, G. R., Russ, G. R., Sale, P. F., Steneck, R. S., van Oppen, M. J. H., et al. (2009). Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–325. doi: 10.1007/s00338-009-0469-9
Jost, L. (2007). Partitioning diversity into independent alpha and beta components. Ecology 88, 2427–2439. doi: 10.1890/06-1736.1
Jost, L. (2010). The relation between evenness and diversity. Diversity 2, 207–232. doi: 10.3390/d2020207
Karlson, R. H., and Cornell, H. V. (1999). Integration of local and regional perspectives on the species richness of coral assemblages. Am. Zool. 39, 104–112.
Karlson, R. H., and Cornell, H. V. (2002). Species richness of coral assemblages: Detecting regional influences at local spatial scale. Ecology 83, 452–463.
Karlson, R. H., Cornell, H. V., and Hughes, T. P. (2004). Coral communities are regionally enriched along an oceanic biodiversity gradient. Nature 429, 867–870. doi: 10.1038/nature02685
Karlson, R. H., Cornell, H. V., and Hughes, T. P. (2007). Aggregation influences coral species richness at multiple spatial scales. Ecology 88, 170–177.
Kleypas, J. A., Buddemeier, R. W., and Gattuso, J.-P. (2001). The future of coral reefs in an age of global change. Int. J. Earth Sci. 90, 426–437. doi: 10.1007/s005310000125
Knowlton, N. (1992). Thresholds and multiple stable states in coral reef community dynamics. Am. Zool. 32, 674–682. doi: 10.1093/icb/32.6.674
Kusumoto, B., Costello, M. J., Kubota, Y., Shiono, T., Wei, C.-L., Yasuhara, M., et al. (2020). Global distribution of coral diversity: biodiversity knowledge gradients related to spatial resolution. Ecol. Res. 2020, 1–12. doi: 10.1111/1440-1703.12096
Lande, R. (1996). Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76, 5–13. doi: 10.2307/3545743
Lang, J. (1973). Interspecific aggression by scleractinian corals II. Why the race is not only to the swift. Bull. Mar. Sci. 23, 260–279.
Lang, J. C., and Chornesky, E. A. (1990). “Competition between scleractinian reef corals – a review of mechanisms and effects,” in Ecosystems of the World: Coral Reefs, ed. Z. Dubinsky (Amsterdam: Elsevier), 209–252.
Lapid, E. D., and Chadwick, N. E. (2006). Long-term effects of competition on coral growth and sweeper tentacle development. Mar. Ecol. Prog. Ser. 313, 115–123. doi: 10.3354/meps313115
Loreau, M. (2000). Are communities saturated? On the relationship between α, β and γ diversity. Ecol. Lett. 3, 73–76. doi: 10.1046/j.1461-0248.2000.00127.x
Loreau, M., Mouquet, N., and Gonzalez, A. (2003). Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl. Acad. Sci. U.S.A. 100, 12765–12770. doi: 10.1073/pnas.2235465100
MacArthur, R. H., and Wilson, E. O. (1967). The Theory of Island Biogeography. Princeton, NJ: Princeton University Press, 203.
Mateo, R. G., Mokany, K., and Guisan, A. (2017). Biodiversity models: What if unsaturation is the rule? Trends Ecol. Evol. 32, 556–566. doi: 10.1016/j.tree.2017.05.003
Menge, B. A., and Sutherland, J. P. (1987). Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am. Nat. 130, 730–757. doi: 10.1086/284741
Michalet, R., Maalouf, J.-P., Choler, P., Clément, B., Rosebery, D., Royer, J.-M., et al. (2015). Competition, facilitation and environmental severity shape the relationship between local and regional species richness in plant communities. Ecography 38, 335–345. doi: 10.1111/ecog.01106
Miller, I. R., and De’ath, G. (1996). Effects of training on observer performance in assessing benthic cover by means of the manta tow technique. Mar. Freshw. Res. 47, 19–26. doi: 10.1071/MF9960019
Miller, I. R., and Muller, R. (1999). Validity and reproducibility of benthic cover estimates made during broadscale surveys of coral reefs by manta tow. Coral Reefs 18, 353–356. doi: 10.1007/s003380050212
Miller, K., and Mundy, C. (2003). Rapid settlement in broadcast spawning corals: implications for larval dispersal. Coral Reefs 22, 99–106. doi: 10.1007/s00338-003-0290-9
Mouquet, N., and Loreau, M. (2003). Community patterns and source-sink metacommunities. Am. Nat. 162, 544–567. doi: 10.1086/378857
Muir, P. R., Marshall, P. A., Abdulla, A., and Aguirre, J. D. (2017). Species identity and depth predict bleaching severity in reef-building corals: shall the deep inherit the reef? Proc. R. Soc. B 284:20171551. doi: 10.1098/rspb.2017.1551
Muscatine, L., Porter, J. W., and Kaplan, I. R. (1989). Resource partitioning by reef corals as determined from stable isotope composition I. δ13C of zooxanthellae and animal tissue vs depth. Mar. Biol. 100, 185–193. doi: 10.1007/bf00391957
Nekola, J. C., and White, P. S. (1999). The distance decay of similarity in biogeography and ecology. J. Biogeogr. 26, 867–878. doi: 10.1046/j.1365-2699.1999.00305.x
Obura, D. (2009). “Corals of Northeast Madagascar,” in A Rapid Marine Biodiversity Assessment of the Coral Reefs of Northeast Madagascar., Vol. 61, eds D. Obura, G. Di Carlo, A. Rabearisoa, and T. Oliver (Arlington County, VA: Conservation International), 17–24. doi: 10.1896/054.061.0102
Obura, D. (2012). The diversity and biogeography of Western Indian Ocean reef-building corals. PLoS One 7:e45013. doi: 10.1371/journal.pone.0045013
Pochon, X., Forsman, Z. H., Spalding, H. L., Padilla-Gamiño, J. L., Smith, C. M., and Gates, R. D. (2015). Depth specialization in mesophotic corals (Leptoseris spp.) and associated algal symbionts in Hawai’i. R. Soc. Open Sci. 2:140351. doi: 10.1098/rsos.140351
Porter, J. W. (1976). Autotrophy, heterotrophy, and resource partitioning in Caribbean reef-building corals. Am. Nat. 110, 731–742. doi: 10.1086/283100
Potts, D. C., Done, T. J., Isdale, O. P., and Fisk, D. (1985). Dominance of a coral community by the genus Porites (Scleractinia). Mar. Ecol. Prog. Ser. 23, 79–84. doi: 10.3354/meps023079
Price, N. (2010). Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163, 747–758. doi: 10.1007/s00442-010-1578-4
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ricklefs, R. E. (1987). Community diversity: relative roles of local and regional processes. Science 235, 167–171. doi: 10.1126/science.235.4785.167
Rogers, C. S. (1993). Hurricanes and coral reefs: the intermediate disturbance hypothesis revisited. Coral Reefs 12, 127–137. doi: 10.1007/BF00334471
Roughgarden, J., May, R. M., and Levin, S. A. (eds) (1989). Perspectives in Ecological Theory. Princeton, NJ: Princeton University Press, 404.
Scheffer, M., and Van Nes, E. H. (2006). Self-organized similarity, the evolutionary emergence of groups of similar species. Proc. Natl. Acad. Sci. U.S.A. 103, 6230–6235. doi: 10.1073/pnas.0508024103
Scheffer, M., van Nes, E. H., and Vergnon, R. (2018). Toward a unifying theory of biodiversity. Proc. Natl. Acad. Sci. U.S.A. 115, 639–641. doi: 10.1073/pnas.1721114115
Sheppard, C. (1995). The shifting baseline syndrome (editorial). Mar. Pollut. Bull. 30, 766–767. doi: 10.1016/0025-326X(95)90079-Q
Sheppard, C., Sheppard, A., and Fenner, D. (2020). Coral mass mortalities in the Chagos Archipelago over 40 years: regional species and assemblage extinctions and indications of positive feedbacks. Mar. Pollut. Bull. 154, 1–12. doi: 10.1016/j.marpolbul.2020.111075
Sheppard, C. R. (1979). Interspecific aggression between reef corals with reference to their distribution. Mar. Ecol. Prog. Ser. 1, 237–247.
Sheppard, C. R. (1982). Coral populations on reef slopes and their major controls. Mar. Ecol. Prog. Ser. 7, 83–115.
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdaña, Z. A., Finlayson, M., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57, 573–583. doi: 10.1641/B570707
Stachowicz, J. J. (2001). Mutualism, facilitation, and the structure of ecological communities. Bioscience 51, 235–246.
Stafford-Smith, M., and Ormond, R. F. G. (1992). Sediment-rejection mechanisms of 42 species of Australian scleractinian corals. Aust. J. Mar. Freshw. Res. 43, 683–705. doi: 10.1071/MF9920683
Szava-Kovats, R. C., Ronk, A., and Pärtel, M. (2013). Pattern without bias: local-regional richness relationship revisited. Ecology 94, 1986–1992.
Szava-Kovats, R. C., Zobel, M., and Pärtel, M. (2012). The local-regional species richness relationship: new perspectives on the null-hypothesis. Oikos 121, 321–326. doi: 10.1111/j.1600-0706.2011.19603.x
Terborgh, J. W., and Faaborg, J. (1980). Saturation of bird communities in the West Indies. Am. Nat. 116, 178–195.
Tomascik, T., van Woesik, R., and Mah, A. J. (1996). Rapid coral colonization of a recent lava flow following a volcanic eruption, Banda Islands, Indonesia. Coral Reefs 15, 169–175. doi: 10.1007/BF01145887
Trisos, C. H., Merow, C., and Pigot, A. L. (2020). The projected timing of abrupt ecological disruption from climate change. Nature 580, 496–501. doi: 10.1038/s41586-020-2189-9
Turak, E., and DeVantier, L. (2019). “Reef-Building Corals of the Upper Mesophotic Zone of the Central Indo-West Pacific,” in Mesophotic Coral Ecosystems, Coral Reefs of the World, Vol. 12, eds Y. Loya et al. 621–651. doi: 10.1007/978-3-319-92735-0_34
van Woesik, R. (2002). Processes regulating coral communities. Comments Theor. Biol. 7, 201–214. doi: 10.1080/08948550214054
van Woesik, R., De Vantier, L. M., and Glazebrook, J. S. (1995). Effects of Cyclone ‘Joy’ on nearshore coral communities of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 128, 261–270. doi: 10.3354/meps128261
van Woesik, R., and Done, T. J. (1997). Coral communities and reef growth in the southern Great Barrier Reef. Coral Reefs 16, 103–115. doi: 10.1007/s003380050064
van Woesik, R., Köksal, S., Ünal, A., Cacciapaglia, C. W., and Randall, C. J. (2018). Predicting coral dynamics through climate change. Sci. Rep. 8:17997. doi: 10.1038/s41598-018-36169-7
Vergnon, R., Dulvy, N. K., and Freckleton, R. P. (2009). Niches versus neutrality: uncovering the drivers of diversity in a species-rich community. Ecol. Lett. 12, 1079–1090. doi: 10.1111/j.1461-0248.2009.01364.x
Veron, J. E. N. (1995). Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. New York, NY: Cornell University Press, 321.
Veron, J. E. N., Hoegh-Guldberg, O., Lenton, T. M., Lough, J. M., Obura, D. O., Pearce-Kelly, P., et al. (2009). The coral reef crisis: the critical importance of< 350 ppm CO2. Mar. Pollut. Bull. 10, 1428–1436. doi: 10.1016/j.marpolbul.2009.09.009
Veron, J. E. N., Stafford-Smith, M. G., DeVantier, L. M., and Turak, E. (2015). Overview of distribution patterns of zooxanthellate Scleractinia. Front. Mar. Sci. 1:81. doi: 10.3389/fmars.2014.00081
Veron, J. E. N., Stafford-Smith, M. G., Turak, E., and DeVantier, L. M. (2020). Corals of the World; Version 0.01 Beta. Available online at: http://www.coralsoftheworld.org (accessed April 14, 2020).
Veron, J. E. N., and Turak, E. (2005). “Reef corals of northwest Madagascar,” in A Rapid Marine Biodiversity Assessment of the Coral Reefs of Northwest Madagascar. RAP Bulletin of Biological Assessment 31, eds S. McKenna, G. R. Allen, and H. Randrianasolo (Washington, DC: Conservation International), 23–30.
Warton, D. I., and Hui, F. K. C. (2011). The arcsine is asinine: the analysis of proportions in ecology. Ecology 92, 3–10. doi: 10.1890/10-0340.1
Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon 21, 213–251. doi: 10.2307/1218190
Whittaker, R. H. (1977). “Evolution of species diversity in land communities,” in Evolutionary Biology, Vol. 10, eds M. K. Hecht and B. W. N. C. Steere (New York, NY: Plenum Press), 1–67.
Keywords: species richness, regional enrichment, saturation, reef-building corals, niche-breadth, intermediate disturbance, competition, effective number of species
Citation: DeVantier L, Turak E and Szava-Kovats R (2020) Species Richness and Abundance of Reef-Building Corals in the Indo-West Pacific: The Local–Regional Relation Revisited. Front. Mar. Sci. 7:487. doi: 10.3389/fmars.2020.00487
Received: 13 December 2019; Accepted: 02 June 2020;
Published: 10 July 2020.
Edited by:
Carla Zilberberg, Federal University of Rio de Janeiro, BrazilReviewed by:
Ronaldo Bastos Francini-Filho, Federal University of Paraíba, BrazilCopyright © 2020 DeVantier, Turak and Szava-Kovats. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lyndon DeVantier, TGRldmFudGllckBhb2wuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.