- 1Harvard Forest, Harvard University, Petersham, MA, United States
- 2Connecticut Institute for Resilience and Climate Adaptation, University of Connecticut, Groton, CT, United States
- 3Faculty of Architecture, Building and Planning, The University of Melbourne, Melbourne, VIC, Australia
- 4Department of Geography, National University of Singapore, Singapore, Singapore
Rehabilitated and restored mangrove ecosystems have important ecological, economic, and social values for coastal communities. Although a sine qua non of successful mangrove rehabilitation or restoration projects is accurate attention to local hydrology and basic biology of mangrove trees and their associated fauna, their long-term success depends on far more axes, each with their own challenges. Rehabilitation projects: are planned, designed, executed, and managed by people with diverse backgrounds and different scientific and socio-political agendas; need to be responsive to these multiple stakeholders and agents who hold different values; are often influenced by laws and treaties spanning local to international scales; and must be able to adapt and evolve both geomorphologically and socioeconomically over decades-to-centuries in the context of a rapidly changing climate. We view these challenges as opportunities for innovative approaches to rehabilitation and restoration that engage new and larger constituencies. Restored mangrove ecosystems can be deliberately designed and engineered to provide valuable ecosystem services, be adaptable to climatic changes, and to develop platforms for educating nonspecialists about both the successes and failures of restored mangrove ecosystems. When mangrove rehabilitation or restoration projects are developed as experiments, they can be used as case-studies and more general models to inform policy- and decision-makers and guide future restoration efforts. Achieving this vision will require new investment and dedication to research and adaptive management practices. These ideas are illustrated with examples from mangrove restoration and rehabilitation projects in the Indo-West Pacific and Caribbean regions, the two hotspots of mangrove biodiversity and its ongoing loss and degradation.
1. Introduction
We are living in the era of ecosystem rehabilitation and restoration (Wilson, 1992). Restoration ecology has progressed rapidly from its initial, unrealistic “ecocentric” goal of eliminating or compensating for human influences on ecosystems (Jordan and Lubick, 2011) to its current “meliorative” framework of creating and maintaining sustainable socio-ecological systems (e.g., Ostrom, 2009; Kibler et al., 2018; Krievins et al., 2018). Methods and approaches for rehabilitating and restoring coastal and marine ecosystems have progressed especially rapidly. It is now realistic to envision that, with concerted effort and careful attention to climatic change, many coastal and marine ecosystems could be “substantially to completely” rebuilt by the middle of this century (Duarte et al., 2020). In this review, we discuss approaches to rehabilitation and restoration of mangroves that integrate ecocentric and meliorative approaches. We use two contrasting case studies to show how mangrove rehabilitation and restoration can be seen as an adaptive management tool for mangroves considered as socio-ecological systems.
Throughout this review, we use the contemporary definition of “ecological restoration” as any activity with the goal of achieving substantial ecosystem recovery relative to an appropriate reference model, regardless of the time required to achieve recovery (Gann et al., 2019, emphasis in original). In this context, “restoration” is distinguished from “rehabilitation” in that the former aspires to substantial recovery of the native biota and ecosystem functions (Gann et al., 2019, emphasis in original), whereas the latter strives not to recover an entire ecosystem formed only of native species but only to reinstate a level of ecosystem functioning sufficient to provide ongoing, defined ecosystem services. In this sense, a rehabilitated ecosystem may include nonnative components (see also Miller and Bestelmeyer, 2016; Zimmer, 2018). Although Elliott et al. (2007) suggested that “restoration” be used to describe any activity (including restoration, rehabilitation, and reclamation) aimed at promoting any type of ecosystem recovery in coastal and estuarine environments (including mangroves), we follow Field (1998), Abelson et al. (2016), and Gann et al. (2019) in distinguishing rehabilitation from restoration in mangrove ecosystems. Rehabilitation and restoration also are at one end of the spectrum of management interventions that support recovery of ecosystems from damaged, degraded, or destroyed states (Ounanian et al., 2018). Stages preceding rehabilitation and restoration include protection, remediation, and recreation (Abelson et al., 2016; Ounanian et al., 2018).
2. Background
2.1. What Are Mangroves?
Mangroves are a taxonomically diverse group of ±70 tree, shrub, and fern species (in at least 25 genera and 19 families) that grow in anoxic and saline peaty soils on sheltered, tropical coasts. Mangroves share a suite of genetic, morphological, physiological, and functional traits that provide one of the most convincing cases for convergent evolution among diverse taxa in response to similar environmental constraints (Polidoro et al., 2010; Tomlinson, 2016; Xu et al., 2017). Mangroves can be found throughout the tropics, with representatives of the major mangrove genera Rhizophora and Avicennia present in both the Indo-West Pacific (IWP) and the Atlantic, Caribbean, and Eastern Pacific (ACEP) realms (Ellison et al., 1999; Tomlinson, 2016). Mangrove species diversity is much lower in the ACEP, where it reaches a maximum of 8–9 species at any given site, than in the IWP, where 30 or more species from the regional pool of at least 46 can co-occur (Ellison et al., 1999). At least 16% of mangrove species worldwide are currently considered to be of conservation concern (Polidoro et al., 2010).
Where they grow, mangroves can form dense, often monospecific stands whose species composition is determined in large part by tidal elevation (Ellison and Farnsworth, 2001). Mangroves are thought to be one of the few good examples of foundation tree species in the tropics (Ellison et al., 2005; Ellison, 2019). They create habitats for many terrestrial, intertidal, and marine species, stabilize shorelines, and modulate nutrient cycling and energy flow through the forests they define (Ellison and Farnsworth, 2001). Mangrove forests have some of the highest reported net primary productivity of any ecosystem on the planet, and their loss or deliberate removal leads to rapid build-up of acid sulfides in the soil, increased shoreline erosion and sedimentation onto offshore coral reefs, and collapse of intertidal food webs and inshore fisheries (Ellison and Farnsworth, 2001).
A recent fine-scale analysis of global mangrove forest cover yielded an estimate of ≈ 84, 000km2 spread across 105 countries (including special administrative areas and French overseas provinces: Hamilton and Casey, 2016). In the latter decades of the twentieth century, FAO (2007) and Spalding et al. (2010) estimated mangrove deforestation rates approaching 1% · yr−1, but during the first dozen years of the twenty-first century, only ≈ 2% of global mangroves were lost, corresponding to a much lower rate of ≈ 0.16% · yr−1 (Hamilton and Casey, 2016). Thus, there is a strong imperative to rehabilitate or restore mangroves to offset continued losses of mangroves around the world, and the number of mangrove restoration and rehabilitation projects worldwide has nearly tripled in the last 20 years (Duarte et al., 2020). The majority of these projects have been in Southeast Asia and Brazil (Duarte et al., 2020).
2.2. Ecosystem Services and Human Uses of Mangroves
Mangroves provide a wide range of benefits—a.k.a. ecosystem services (sensu Millenium Ecosystem Assessment, 2005)—to human populations (e.g., Ellison, 2008; Barbier et al., 2011). Coastal communities have long relied on the provisioning services of mangroves, such as the extraction of construction materials and fuel wood (Dahdouh-Guebas et al., 2000; Chow, 2018), and the capture of food sources such as shellfish and finfish (Ellison, 2008; Carrasquilla-Henao et al., 2019). Coastal communities also derive cultural ecosystem services from mangroves, including tangible services such as recreation and intangible services such as aesthetic appeal and spiritual values (e.g., James et al., 2013; Thiagarajah et al., 2015; Spalding and Parrett, 2019). Mangroves also provide a range of regulating services, including coastal protection (Horchard et al., 2019; Ranjan, 2019), pollutant assimilation (Tam and Wong, 1995), and macroclimate regulation and mitigation of global climatic change through carbon (C) storage and sequestration (Adame et al., 2018). Some regulating services (e.g., coastal protection) accrue directly to co-located coastal communities, whereas others (e.g., regulation of macroclimate) benefit the global commonwealth.
2.3. A Brief History of Mangrove Rehabilitation and Restoration
Rehabilitation and restoration of mangroves has been practiced for decades (salient reviews are provided by Lewis, 1982, 2005, 2009; Field, 1998; Ellison, 2000; Lewis et al., 2019). The rationales for rehabilitating or restoring mangroves reflect specific ecosystem services, including creation or maintenance of forest stands for “sustainable” high yields, coastal protection, landscaping, conservation of biodiversity, or because laws require it (e.g., local regulations mandating “No Net Loss” of wetlands following development projects). Broad classes of rehabilitation and restoration methods include: (1) incorporation of mangroves into engineered hard coastal defense structures (Cheong S.-M. et al., 2013; Lai et al., 2015; Mayer-Pinto et al., 2017; Morris et al., 2018, 2019); (2) monoculture plantations (e.g., Chan, 1996; Field, 1998; Ellison and Farnsworth, 2001; Matsui et al., 2012; Chow, 2018); and (3) “ecological mangrove restoration” (EMR) approaches, in which the intertidal zone is manipulated (e.g., regraded, dredged, filled) so that biophysical conditions (particularly inundation) are within tolerable limits for mangrove establishment, growth, and reproduction (e.g., Lewis, 2005; Lee et al., 2019; Lewis et al., 2019; Suman, 2019). Zimmer (2018) has proposed an additional method, (4) “mangrove ecosystem design,” which foregrounds people and their needs, and then uses those needs to define the set of ecosystem services to be included in the project. Subsequent rehabilitation or restoration activities are then focused on meeting those needs and services, given biophysical constraints. Mangrove ecosystem design is described in more detail in section 2.6.
For example, in terms of regulating ecosystem services, growth rates and biomass accumulation tend to be greater in young plantations than in older ones, but recruitment of saplings may increase with plantation age or be completely absent (Bosire et al., 2008). Rehabilitated mangroves sequester more C than the land-use cover-types they replace (Sasmito et al., 2019). Successful rehabilitation has led to rapid accumulation of biomass C stocks, and over longer time scales can increase soil carbon stocks by 83 (Matsui et al., 2012) to 96 Mg C/ha (Cameron et al., 2019a). Rehabilitated mangroves on previously abandoned and exposed aquaculture ponds emit substantially less CO2 from their soils than do the abandoned, exposed ponds themselves (Cameron et al., 2019a). In parallel, rates of peat accumulation in constructed mangrove forests can exceed that of natural stands (Osland et al., 2020). Carbon is a traded commodity, and the selling of C credits provides financial incentives for mangrove rehabilitation through Payments for Ecosystem Services (PES). PES has been promoted for its potential to offset greenhouse gas emissions while providing livelihood opportunities to local communities (Locatelli et al., 2014). The high rates of C accumulation and positive impact on baseline soil C fluxes in mangrove ecosystems means that they provide more cost-effective PES than most terrestrial ecosystems (Cameron et al., 2019b).
Rehabilitated mangroves also provide provisioning ecosystem services that local communities benefit from and appreciate. These include construction materials and fuel wood, non-timber products such as natural dyes, and nursery grounds for molluscs collected for food (Rönnback et al., 2007; Ellison, 2008). Following mangrove rehabilitation, fish catches by artisanal fishers often increase and positive influences on offshore commercial fish catches also have been observed (Das, 2017).
2.4. The Socioecology of Mangrove Rehabilitation and Restoration
Mangrove rehabilitation and restoration projects almost always are conceived and executed as “one-off” projects with surprisingly little attention paid to transference of valuable information about previous successes, failures, or technical knowledge that could guide successful projects (Field, 1998; Ellison, 2000; Lewis, 2005, 2009; Lewis et al., 2019). Unsurprisingly, the failure rate of mangrove restoration and rehabilitation projects remains unacceptably high (Brown, 2017; Kodikara et al., 2017; Lee et al., 2019).
Technically, rehabilitation or restoration of mangroves can be surprisingly easy: [T]he single most important factor in designing a successful mangrove restoration project is determining the normal hydrology (depth, duration and frequency, and of tidal flooding) of existing natural mangrove plant communities ([i.e.,] a reference site) in the area in which you wish to do restoration (Lewis, 2005, p. 409). Actual planting of mangrove propagules is often used but rarely needed (Field, 1998), except perhaps when the goal is a monoculture or forest plantation, or when stem-density targets need to be achieved more quickly than natural regeneration would allow (Field, 1998; Ellison, 2000; Lewis, 2005).
However, rehabilitation and restoration projects do not succeed on technical grounds alone (Gann et al., 2019; Lovelock and Brown, 2019), and, as noted above, most mangrove restoration or rehabilitation projects have failed. Follow-up monitoring has been sporadic and, at best, short-term. Most failures result from the lack of community involvement, appropriate governance structures, and alignment of objectives and goals of external agents (including scientists) and local stakeholders (Field, 1998; Mazón et al., 2019). Cormier-Salem (1999) argued that interacting dynamics of natural and social systems was a sine qua non of effective long-term management of mangroves, but that social scientists had not been included in mangrove restoration projects. Similarly, Walters (1997, 2000) found that socio-economic factors including peoples' traditional knowledge about trees and tree planting; patterns of land use and ownership; perceived economic costs and benefits; and community social organization interacted were far more important than ecological factors in determining success of mangrove reforestation in the Philippines. Unfortunately, the advice and insights of these authors have been notably absent from subsequent major reviews of mangrove rehabilitation or restoration (Lewis, 2005, 2009; Bosire et al., 2008; Lewis et al., 2019); Dale et al. (2014) is a useful counter-example.
The last few years has seen a resurgence in interest in bringing ideas and theories about socioecological systems to bear on restoration and rehabilitation of mangroves (e.g., Biswas et al., 2009; Brown, 2017; Ranjan, 2019). Ounanian et al. (2018) identified four “restoration discourses” applicable to marine (including mangrove) ecosystem restoration that are based on the degree of human intervention (the how of restoration) and the motivation for action (the why of restoration) (Figure 1). The goal of mangrove rehabilitation (rarely restoration) projects prior to the early 1980s—afforestation for silviculture (Ellison, 2000)—reflected the idea that mangroves should support people (lower left quadrant of Figure 1). Lewis (1982) and Field (1998) moved the discourse up the ecocentric axis of Figure 1, “Putting Nature first” and bringing biological diversity, habitat creation, and food sources for near-shore and pelagic food webs into the discussion. EMR approaches moved the needle toward the upper-right, “Bringing Nature back” quadrant of Figure 1, but most ecocentric approaches continue to place people outside of “nature” and squarely within the classic ecocentric framing of ecological restoration (Jordan and Lubick, 2011). In contrast, Community Based Ecological Mangrove Rehabilitation (CBEMR) has adapted EMR to include local communities and bring people back into nature (Brown et al., 2014; Mantrove Action Project, 2019).
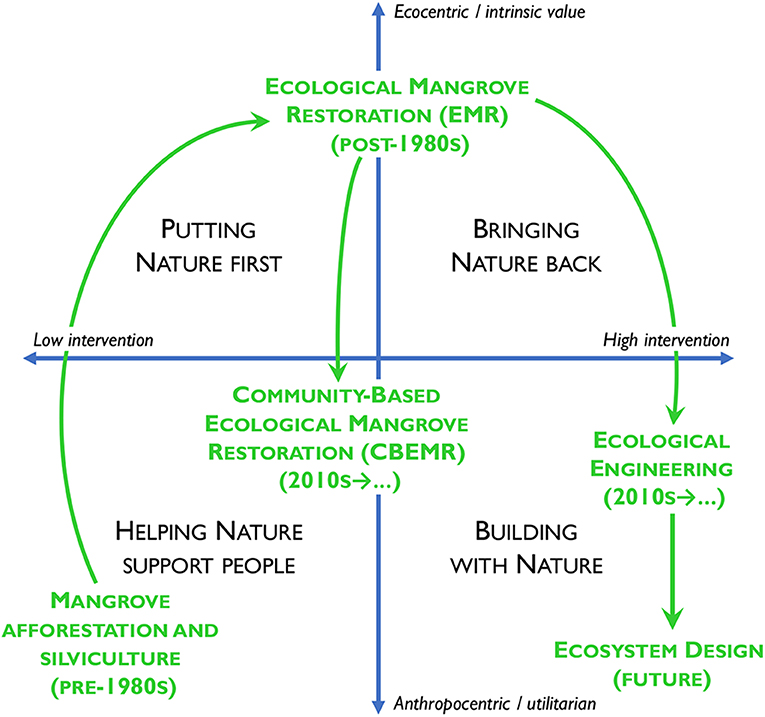
Figure 1. Four discourses for marine ecosystem restoration, after Ounanian et al. (2018), with placement of major types of mangrove rehabilitation and restoration overlain in appropriate quadrants.
For example, rehabilitation and restoration activities are linking ecological integrity (“Bringing Nature Back”) with actions to mitigate negative effects of climatic change (“Helping Nature Support Humans”) while building local capacity in island ocean states (Wilson and Forsyth, 2018). At the same time, methods to better monitor and assess social and ecological status of coastal habitats are being developed (Cáardenas et al., 2017; Wongbusarakum et al., 2019). In any project, participants and stakeholders are unequal and weak or asymmetric relationships among them—differences in capacity, power, or ideologies—can lead to gaps in policies, project design, and implementation (Vaughn, 2017; Thompson, 2018). These asymmetries can be overcome through long-term commitments to funding and monitoring, stronger collaborations between the funders and individuals carrying out the restoration projects, and resolution of conflicts between bottom-up (local) environmental initiatives and top-down (governmental) legislation (Sa'at and Lin, 2018; Thompson, 2018). Clarification of ownership and title to mangrove-covered areas can limit deforestation, encourage environmental stewardship, and maintain rehabilitated or restored areas (Lovelock and Brown, 2019; Suman, 2019).
2.5. The Potential of and for Adaptive Management of Rehabilitated and Restored Mangroves
Adaptive management, a structured, iterative process of “learning-by-doing” and decision-making in the face either of continuous change (environmental, social, cultural, or political) or uncertainty (Holling, 1978), can and should be the standard approach for any ecological restoration project, irrespective of how well-resourced that project may be (Gann et al., 2019, p. S16). Not only does adaptive management require regular monitoring of key indicators to determine if the objectives and goals of a rehabilitation or restoration project are being met, it also requires clear triggers or decision-points for appropriate intervention and action if the objectives or goals are not being met (Gann et al., 2019). For mangrove rehabilitation and restoration projects, long-term monitoring is uncommon (e.g., Mazón et al., 2019), and adaptive management is rarely applied. However, Eriksson et al. (2016) showed clearly that adaptive management of an “ecosystem approach” improved outcomes associated with managing mangroves for small-scale fisheries in Indonesia (Lombok), the Philippines, Solomon Islands, and Tanzania. They used the Participatory Diagnosis and Adaptive Management framework (Andrew et al., 2007) with participants who represented the interests of natural ecosystems, the livelihood of people (economic drivers), institutions and governance, and external drivers including macroeconomic instability, climatic change, and environmental uncertainty. Their goal was to determine stakeholder priorities and identify key interventions to support the transition from purely exploitative to more sustainable fisheries. One key conclusion of Eriksson et al. (2016) was that strengthening governance was as important as mangrove rehabilitation, economic improvement, and other technical and data-driven aspects of management. This conclusion is not restricted to fisheries management (Eriksson et al., 2016), but applies more broadly to any mangrove protection, conservation, rehabilitation, or restoration project (Lovelock and Brown, 2019; Suman, 2019).
2.6. Experimentation Plus Deliberate Design as an Integrating Framework for Mangrove Rehabilitation and Restoration
Observational monitoring of key indicators is necessary to evaluate success of goals and objectives of rehabilitation or restoration projects and to guide adaptive management and decision-making. Formal experiments, either within an observational before-after-control-impact (BACI) framework (e.g., Stewart-Oaten et al., 1986) or using manipulations with appropriate controls and adequate sample sizes, could improve causal interpretation of observed patterns and identify key processes (Gann et al., 2019). Restoration and rehabilitation projects provide ideal opportunities and sites for “real-world”-scale experiments to determine whether, for example, particular engineering solutions, planting patterns, or facilitative (positive) interactions among species could improve restoration success (Halpern et al., 2007; Gedan and Silliman, 2009), but these have yet to be integrated into any mangrove restoration or rehabilitation project (Renzi et al., 2019).
At the same time, intentional design of ecosystems with functional characteristics to provide particular services has been proposed as an alternative to rehabilitation or restoration projects with high costs or low likelihoods of success; to take advantage of nonnative species with equivalent functionality; or to be effective in rapidly changing environmental conditions (Hobbs et al., 2006; Morse et al., 2014; Miller and Bestelmeyer, 2016). Such designed ecosystems foreground people, societies, and the ecosystem services that support them, and use engineering principles and technical knowledge to assemble a group of taxa into appropriate environments (Zimmer, 2018). More deliberately designed or engineered mangrove systems have been tried in Bangladesh and Singapore (Cheong S.-M. et al., 2013). A more complete designed mangrove landscape that provides stormwater management, flood protection, and recreational opportunities, was constructed in the Fengxinglong Ecological Park at the junction of the Sanya and Linchun Rivers in China's southern Hainan Province (Nengshi et al., 2018).
Ecological, socioeconomic, and governance issues come together in discourses that define rehabilitation and restoration of ecosystems. Adaptive management, including monitoring and triggers for intervention and modification of management require participation of individuals with technical expertise and those who can make culturally-informed decisions. Including manipulative experiments with appropriate attention to sample size and scope of inference could permit more rapid conclusions about how a system is actually working, and provide additional guidance for adaptive management. All of these threads come together in designed ecosystems that foreground needed ecosystem services, specify them as project goals or deliverables, and assemble groups of species that can provide said services in a given environmental context that is treated as a long-term experiment in rehabilitation and restoration.
In the next section, we use case studies of mangrove rehabilitation and restoration projects in Singapore and Belize to illustrate these principles (Figure 2). We chose case studies from these two countries because Singapore and Belize are in different mangrove realms and have different sociopolitical and economic histories and contexts, but they also face similar challenges in rehabilitating, restoring, and managing mangroves. Belize is 30-fold larger than Singapore and has more a 1,000-fold greater mangrove cover but only 1/300th of Singapore's GDP (The World Bank, 2018). Conservation and preservation of large areas of extant mangroves is still possible in Belize, but not in Singapore. People and the governments in both countries recognize the value of the ecosystem services that mangroves provide. At the same time, coastal zones in both countries are being engineered, and their mangrove rehabilitation projects are mostly small-scale and driven primarily by ecocentric goals.
Figure 2. World map showing locations of Singapore (in magenta inset) and Belize (in blue inset). Additional details about each country are given in each of the case studies; the extent of mangroves in each country is illustrated in Figures 3, 6.
3. Case Study 1: Singapore
Singapore (1.290270 °N, 103.851959 °E) is a 721-km2 city-state in Southeast Asia. Its equatorial climate and biophysical environment is well-suited to supporting minerogenic mangrove systems. Singapore is located close to the epicenter of mangrove species diversity and diversification (Ellison et al., 1999); 35 of the ≈ 46 mangrove species found in the Southeast Asian region of the IWP mangrove realm, including the critically endangered Bruguiera hainesii, have been recorded from Singapore (Yang et al., 2011).
Since it became an independent state in 1965, Singapore has lost > 90% of its mangrove forest extent as land has been reclaimed for industrial development and aquaculture, freshwater reservoirs have been constructed in previously mangrove-fringed estuaries, and the shoreline has eroded and been increasingly polluted (Lai et al., 2015; Friess, 2017). Mangrove coverage in Singapore was estimated at only 0.81 km2 in 2018 (Figure 3; Gaw et al., 2019).
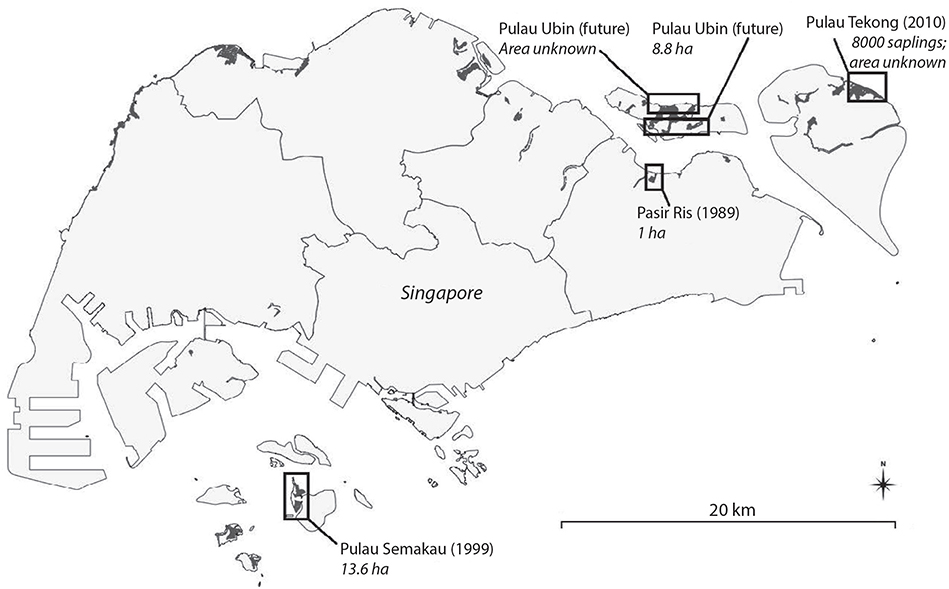
Figure 3. Distribution of mangrove forests (black) in Singapore in 2014 with locations of major rehabilitation projects. Redrawn from Friess (2017).
3.1. Socioeconomic and Sociopolitical Context
Although Singapore's historical economic and sociopolitical drivers provided large incentives to destroy mangrove forests, the present-day sociopolitical context has opened up possibilities for mangrove rehabilitation or restoration. Some mangrove areas now are protected as Nature Parks or Nature Reserves, and protected area coverage continues to increase in the country (Tay, 2018). In 2019, the Singapore Government announced that preparing for and dealing with the impacts of climatic change are key government priorities; measures to protect the country and adapt to sea-level rise are estimated to cost at least US $74 billion over the next century (Prime Minister's Office, 2019); US $5 billion is allocated in next year's budget. The government has highlighted nature-based solutions, especially mangrove rehabilitation, as key adaptive responses to sea-level rise (Tan and Fogarty, 2019).
3.2. Mangrove Rehabilitation and Restoration Activities in Singapore
3.2.1. Bringing Nature Back: Recreating Biophysical Conditions
Because much of Singapore's highly urbanized coastline is on reclaimed land in the intertidal zone, large spaces within appropriate biophysical bounds for successful mangrove establishment and growth do not exist. Thus, the first step for mangrove rehabilitation projects in Singapore has been to “Bring Nature Back” (upper right quadrant of Figure 1) by recreating the necessary biophysical conditions to support mangroves and their associated biota.
3.2.2. Types of Mangrove Rehabilitation Efforts in Singapore
Previous and current rehabilitation efforts in Singapore can be classified into three broad tiers that correspond to the three standard methods of mangrove rehabilitation and restoration: ecological engineering, plantations, and EMR approaches (Figure 4; Friess, 2017). All of these efforts have been opportunistic, and have occurred in response to individual development projects or specific management concerns. Most fall into the “Bringing Nature Back” or “Building with Nature” paradigms of Figure 1.
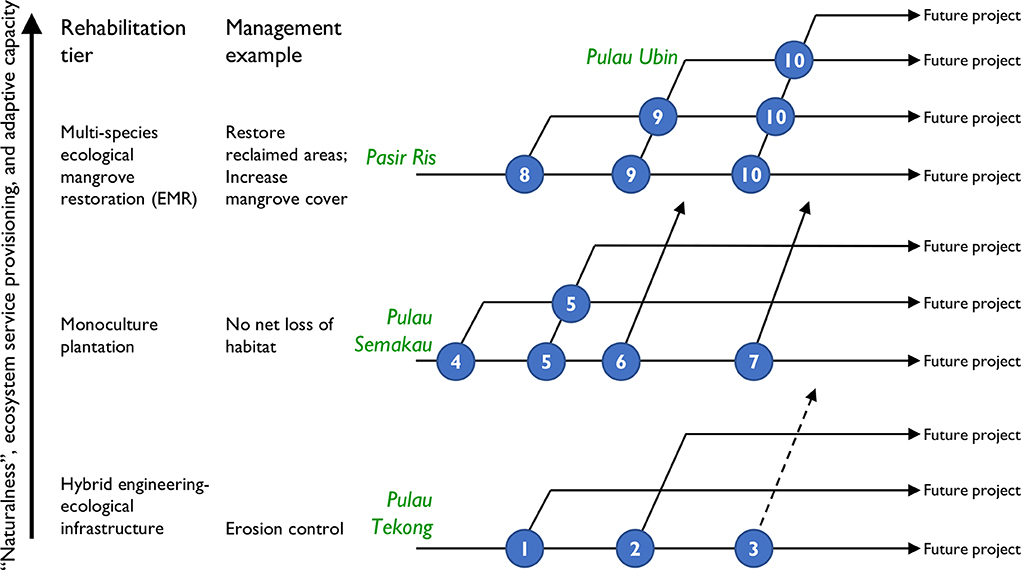
Figure 4. Conceptual mangrove rehabilitation trajectories and key decision points (white numbers in blue circles) where each trajectory can be changed to improve project outcomes. The decision points are: (1) Improve success with better selection of species; (2) Shift from gardening to greater inclusion of mangrove ecosystem services; (3) Improve biophysical conditions (unlikely without extensively engineered works or ongoing maintenance); (4) Plant at correct intertidal elevation; (5) Plant multiple species; (6) Improve pre-rehabilitation planning of all biophysical parameters with an eye toward future ecological mangrove restoration (EMR); (7) Identify new areas for rehabilitation (to improve beyond no net loss); (8) Extensive site and community assessments prior to initiating rehabilitation; (9) Ensure participation of local communities and stakeholders; (10) Scale up size of pilot projects and full projects. The projects at Pulau Tekong, Pulau Semakau, Pasir Ris, and Pulau Ubin are all discussed in text (section 3.2.2).
An example of the first (smallest) tier is on Pulau Tekong, an island off Singapore's northeast coast, where mangrove saplings have been planted within new and existing hard coastal defense structures. This mangrove area was experiencing substantial shoreline erosion from storms, ship wakes, and changing hydrodynamic conditions resulting from nearby land reclamation (Cheong K. H. et al., 2013; Cheong S.-M. et al., 2013). Because the intertidal zone was eroding, simple planting would have been insufficient to rehabilitate a mangrove forest. Instead, an artificial rock wall was built, sediment was introduced into the system in biodegradable bags, and multi-species plantings were done inside 8,000 plastic planting tubes placed between the rocks (Yang et al., 2011; Cheong S.-M. et al., 2013).
The second-tier rehabilitation approach of planting monocultures has been done on Pulau Semakau. At this site, 13.6 ha of mangrove forest were cleared during the construction of a landfill. After construction, a rehabilitation project was initiated to ensure no net loss of habitat. Reclaimed land was overlain with a layer of mangrove mud at an elevation of 1.8–2.2 m above chart datum low water, and as many as 400,000 propagules of Rhizophora apiculata and R. mucronata were successively planted across the site at a density of 1,900 propagules per hectare. This density was required to account for the ≈ 94% mortality rate of the planted propagules (Tatani et al., 2001; Yang et al., 2011; Friess, 2017). Such high mortality is uncommon in long-term, multiple-rotation mangrove forestry plantations (Chan, 1996) but common in monoculture rehabilitation projects more broadly (Kodikara et al., 2017).
Two rehabilitation sites in Singapore have implemented principles of EMR (Tier 3). The first, at Pasir Ris, was designed to allow mangrove seedlings to naturally colonize a 1-ha area that had been reclaimed previously; the goal here was to make connections with other mangrove patches along this coastline (Figure 5; Lee et al., 1996). The reclaimed land was regraded to a lower elevation that allowed flooding up to 50 times per month. Once tidal exchange had been re-established, the site was rapidly colonized by Avicennia alba and Sonneratia alba, two key mangrove pioneer species that can survive in the lower intertidal zone. After 20 years, a high diversity of mangrove species, molluscs, crustacea, and snakes had established at Pasir Ris (Lee et al., 1996; Karns et al., 2002). Fish diversity, but not abundance, has been higher at Pasir Ris than in adjacent constructed shorelines (Jaafar et al., 2004; Benzeev et al., 2017). Pasir Ris also provides valuable cultural ecosystem services, including spiritual/religious (“sense of peace”), inspirational (“connecting with nature”), and recreation/tourism (“recreation” and “enjoying time with family”) (Thiagarajah et al., 2015).

Figure 5. Restored mangroves at Pasir Ris. (A) Channels are maintained to allow for tidal exchange; (B) A boardwalk was constructed through the mangrove to facilitate access while minimizing impacts to the forest; (C) Fringing mangrove provides habitat for juvenile fish and birds; (D) Interpretive and exhortative signs for education, engagement, and outreach are widespread along the boardwalk. White numbers in blue circles correspond to restoration and rehabilitation decision points illustrated in Figure 4. Photographs by Aaron M. Ellison.
The second, and most recent EMR project is ongoing within 8.8 ha of abandoned aquaculture ponds on Pulau Ubin. This project differs from other mangrove rehabilitation projects previously undertaken in Singapore in that it has been a community-based initiative, organized by local NGOs, community groups, and academics, and strongly supported by government agencies responsible for nature conservation (Friess, 2017; RUM, 2017). The first phase of this project has required extensive mapping of biophysical conditions across the site and neighboring natural mangroves, particularly tidal flows and elevations relative to tidal conditions, to ensure that subsequent construction works modify physical site conditions to approximate as closely as possible those within surrounding “baseline” mangroves. The second phase will begin the reconstruction of appropriate hydrological conditions and sediment infilling to support natural or augmented regeneration.
Data as yet are unavailable to assess the long-term success of Singapore's two EMR projects because reference stands have not been systematically monitored; natural regeneration at Pasir Ris cannot be compared to the observed self-thinning of planted stands; and the ongoing mangrove restoration at Pulau Ubin is still in its early stages. However, EMR projects in other countries generally have been more successful in terms of seedling survival than planted monocultures (Djamaluddin, 2007). There is, however, some evidence that EMR has been ecologically more successful in Singapore than lower-tier rehabilitation approaches. The multi-species, natural regeneration at Pasir Ris has led to faster tree growth and biomass accumulation than at other mangrove rehabilitation sites in Singapore (Lee et al., 1996; Friess, 2017).
3.3. Lessons to Inform Adaptive Management
Mangrove rehabilitation in Singapore has yielded key lessons in how to enhance ecological diversity and ecosystem services in an urbanized coastal setting. The Pulau Tekong hybrid engineering project illustrated how to incorporate mangrove vegetation into traditional coastal defense structures and has built competency in large-scale coastal ecological engineering (Friess, 2017). This project also highlighted the importance of planting multiple species and matching species traits to prevailing environmental conditions (Lewis, 1982; Field, 1998). To achieve target tree densities in the monoculture plantation on Pulau Semakau, successive replantings of up to 400,000 Rhizophora propagules were required because of high seedling mortality rates resulting from suboptimal biophysical conditions at the site for the planted species. The ongoing rehabilitation project on Pulau Ubin also has highlighted the importance of increasing project scope to include community engagement and involvement, which secured community support and buy-in for the restoration works (see also Damastuti and de Groot, 2017; Sa'at and Lin, 2018; Powell et al., 2019; Ranjan, 2019).
Rehabilitation projects at all sites have shown the importance of recreating the correct biophysical conditions to allow mangrove seedlings to grow, including creating artificial structures to protect seedlings from hydrodynamic energy or using EMR to create site elevations suitable for mangrove establishment (see also Lewis, 1982, 2005; Ellison, 2000). All projects also have highlighted the challenges needed to scale them up to larger areas. Thus far, mangrove rehabilitation sites in Singapore have ranged from < 1 to just under 14 ha in size. These small sizes reflect a legacy of executing rehabilitation projects along urban shorelines with severe space constraints and for which there are conflicting coastal management priorities. However, larger rehabilitation sites may be able to support higher levels of biodiversity and provide a greater number of ecosystem services.
Analysis of mangrove rehabilitation projects in Singapore has identified ten key decision points within an adaptive management framework that provide opportunities to improve restoration trajectories in this coastal setting (Figure 4). These decision points can be categorized broadly as: diversifying target species for rehabilitation (decision points 1 and 5); stronger incorporation of key biophysical thresholds that determine mangrove survivorship (decision points 4, 6, and 8); and increasing the scope and scale of rehabilitation (decision points 2, 7, 9, and 10). In some instances it may be possible to jump between rehabilitation tiers at these decision points. For example, planted monocultures could incorporate aspects of EMR such as identification and mapping of biophysical constraints on mangrove establishment that may be used to encourage natural recruitment of mangrove propagules. This could be done at existing sites (decision point 6) or along an entire coastline to identify suitable areas for future rehabilitation (decision point 7). In other situations, it may not be possible to jump to a higher rehabilitation tier. For example, hybrid engineering approaches are used in eroding areas where mangrove planting or natural recruitment would never be successful because of biophysical constraints.
Although the small number of restoration sites in Singapore has limited the opportunities to take advantage of these lessons or use the proposed adaptive management framework (Figure 4), possibilities abound for the future. More than 63% of Singapore's 319 km coastline is armored (Lai et al., 2015) and new coastal rehabilitation projects could take advantage of lessons learned in hybrid engineering at Pulau Tekong. Ecological enhancement of sea walls for corals and associated fauna has been a strong, focused area of basic (Loke and Todd, 2016) and applied research (Loke et al., 2019; Morris et al., 2019). Mangrove rehabilitation on sea walls would benefit from a similar research focus. An additional 75 ha of previously abandoned aquaculture ponds in Singapore potentially are available for restoration to their original mangrove cover (Friess, 2017). If these areas were restored successfully, the mangrove extent in Singapore would increase by up to 10%, with appreciable, positive gains in provisioning of ecosystem services by Singapore's mangroves.
4. Case Study 2: Belize
Belize (17.49952 °N, −88.19756 °W) is small country (land area = 22,963 km2) on the eastern side of Central America, bordered by Guatemala to the west, Honduras to the south, Mexico to the north, and the Caribbean Sea to the east. The varied topography and geology of Belize include two physiographic regions: the Maya Mountains in the south and west and the northern lowlands in the north and east. The latter form broad coastal plains with sandy soils underlain by limestone bedrock. Vegetation types of the northern lowlands include semi-deciduous forests and savannas, and extensive wetlands, swamps, and coastal lagoons, mangroves, and seagrass meadows (Figure 6; Hartshorn et al., 1984; Ellison, 2004; Cherrington et al., 2010a). Offshore, the 300-km Belize barrier reef is the largest continuous section of the 900-km Mesoamerican reef system (Rützler and Macintyre, 1982) and a designated UNESCO World Heritage site (UNESCO, 1996). Belize still has substantial intact mangrove habitat covering ≈ 747 km2 along its 386 km of marshy coastline and many of its ≈ 300 coral cayes (islands). The four most common mangrove species in the ACEP mangrove realm—Rhizophora mangle, Avicennia germinans, Laguncularia racemosa, and Conocarpus erectus—grow in Belize (Murray et al., 2003; Neal et al., 2008).
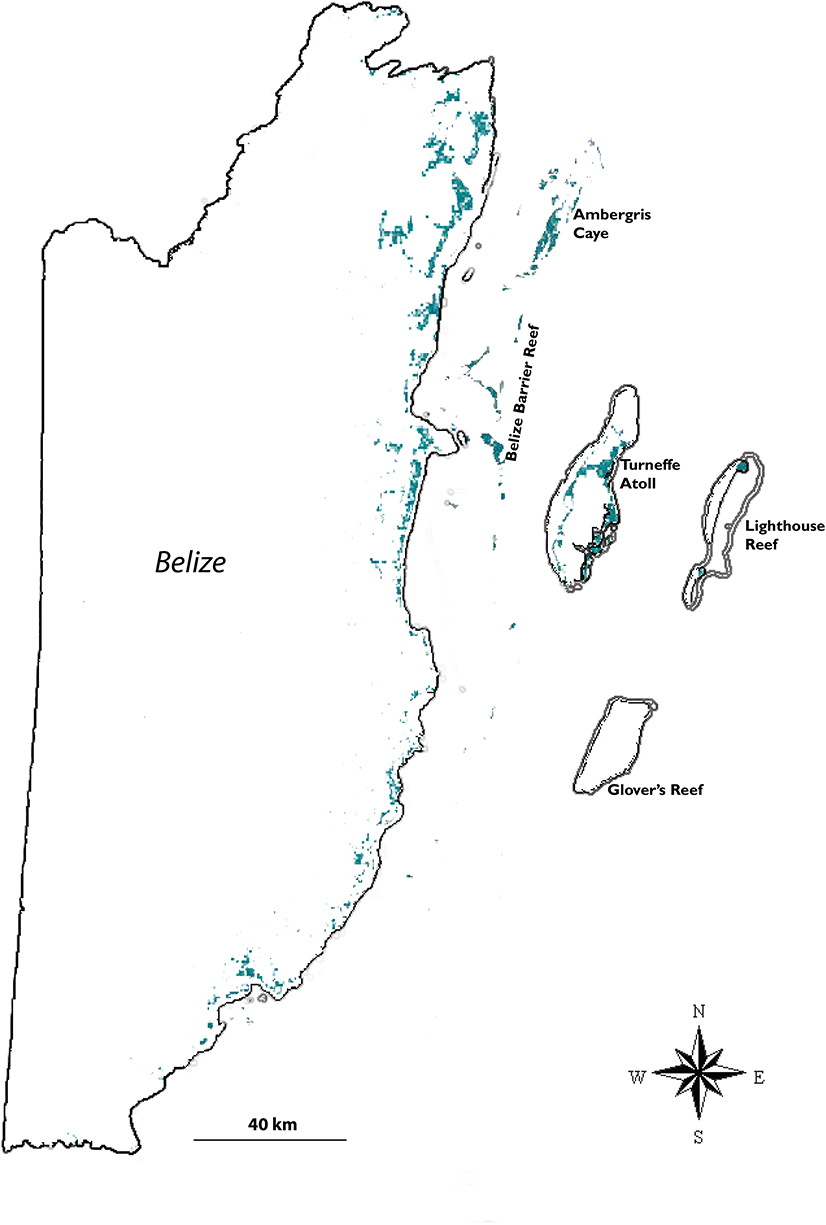
Figure 6. Map of Belize, showing areas of mangrove cover in teal. The offshore mangrove forests include dozens of mangrove-covered cays along the Belizean barrier reef complex, and on two of the three atolls further east. Occasional mangrove seedlings have been seen at Glover's Reef, but they have failed to survive. Location of mangroves based on map in Meerman and Sabido (2001).
4.1. Socioeconomic and Sociopolitical Context
Mangroves are legally protected under Belize's Forests Act (Government of Belize, 2003) and remain mostly intact in Belize. At least 60% of the population of Belize depends directly or indirectly on ecosystem services from coastal and marine habitats (Belize Tourism Industry Association, 2010), but foreign ownership and recent trends in land-use change, development, wastewater management, and tourism (especially a rapid increase in arriving cruise ships) all put pressures on mangroves and other coastal habitats. Approximately 70% of Belize's coastline is privately owned, mostly by foreign entities, and mangrove cover has declined by ≈ 2% since 1980 (Brooksmith Consulting, 2011). Burgeoning aquaculture operations for rearing prawns and tilapia along the coast are constructed in mangrove estuaries. Following deforestation for their construction, increased nutrient loading from their operations into adjacent wetlands and waterways drives additional mangrove loss (Government of Belize, 2002).
The Belizean barrier reef complex, with its cayes, mangroves, seagrasses, and coral reefs, is an important component of Belize's tourism economy. Mangroves alone contribute ≈ US $174–249 million each year to the country's economy (Cooper et al., 2009). Ongoing climatic change negatively impacting coastal ecosystems across the Caribbean will lead to an estimated reduction in tourism revenue by 2,100 of > 25% of total GDP across all countries in the Caribbean Basin (Bueno et al., 2008) and ≈ US $28 million in Belize alone (Richardson, 2009). Ironically, the expanded tourism and fisheries that drive the economy of Belize also threaten the ecosystems that support these activities. Trends in expansion of tourism and fisheries coupled with climatic change raise serious concerns for future conservation and management of mangroves in Belize (Ellison and Farnsworth, 1996; Farnsworth and Ellison, 1997; Clarke et al., 2012).
Recent legislation has continued to reform and amend mangrove clearance laws in Belize, and includes higher fines and stronger regulations (Government of Belize, 2018). However, developers routinely work around these restrictions. Mangrove clearance often occurs without proper permits and enforcement of mangrove regulations is rare. Although recent losses of mangroves in Belize overall have averaged 0.9% · yr−1 (Hamilton and Casey, 2016), in urban and urbanizing areas (e.g., in and around the capital, Belize City), mangrove cover has declined by as much as > 3% · yr−1 as stands have been cleared for housing, industry, farming, and septic and wastewater systems (Furley and Ratter, 1992). In these areas, conservation or management of mangroves is rare, and occurs mostly on small scales. Mangroves also are used in urban areas to extend septic systems and treat wastewater; the main sewage treatment system in Belize City is a constructed lagoon. More rural areas also are developing rapidly and along similar trajectories. For example, the 30-km Placencia Peninsula in southeast Belize has extensive mangroves along the western “lagoon” side and white-sand beaches with housing and substantial resort development on the eastern side facing the Caribbean Sea. While much of the lagoon's intertidal zone remains mangrove-covered, the mangrove stands are being degraded by coastal development for real estate and tourism, aquaculture and agriculture, sedimentation from upland deforestation and mangrove removal, and diverse impacts of dredging and armoring the coast with bulkheads and other hard infrastructure (Ellison and Farnsworth, 1996; Murray et al., 2003; Coastal Zone Management Authority & Institute, 2014; Dale et al., 2014).
4.2. Mangrove Rehabilitation and Restoration Activities in Belize
4.2.1. Putting Nature First: Preserving Extant Mangroves Comes First
Because extensive and intact stands of mangroves still remain throughout Belize, their preservation, protection, and sustainable management or explicit inclusion in development projects will be the most effective and efficient means of maintaining mangrove ecosystem functions in Belize. To foster mangrove preservation, the Mangrove Challenge contest was established in 2010–2011 (Brooksmith Consulting, 2011). The Mangrove Challenge not only helped to identify individuals involved in effective mangrove conservation and restoration across Belize, but also fostered creation of networks among them by awarding small cash prizes (US $250–500). Awards were made to: conservation organizations that were maintaining mangrove reserves; building projects that were maintaining substantial mangroves in place; formal landscaping and design proposals for resorts or homes that incorporated mangrove hedges and aesthetic trimming; educational opportunities created by boardwalks through mangrove forests city parks with mangroves; and docks built along mangroves rather than removing mangrove shorelines. The Mangrove Challenge also highlighted locations where substantial contiguous habitat patches existed or had been protected. A positive outcome of the Mangrove Challenge has been the expanded recognition of mangroves and various spin-off projects from the original competition (Brooksmith Consulting, 2011).
4.2.2. Mangrove Protection in Belize Involves Governmental and Non-governmental Organizations
Addressing issues contributing to mangrove degradation and loss in Belize are being addressed through both bottom-up (grass-roots) and top-down (governmental) approaches. For example, the Government of Belize recently has enacted new laws to support of mangrove conservation (Government of Belize, 2018). Instituting effective zoning policies guiding development and enforcing regulations need to be communicated better and more effectively, especially to foreign property-owners (Flomenhoft et al., 2007). The Belize Coastal Zone Management Authority & Institute (CZMAI) has recognized that protection and preservation of existing extant stands of mangroves will continue to be a high priority. CZMAI is working to increase awareness and oversight of existing mangroves to maintain their spatial and functional integrity, and seeking to develop institutional stability for organizations that create and monitor mangrove reserves. For example, an action item in the Belize Integrated Coastal Zone Management Plan of 2016 is to establish a national water quality monitoring program and a long-term national strategy for monitoring the health of reef, seagrass, mangroves, and other coastline habitats (Cherrington et al., 2010b; Coastal Zone Management Authority & Institute, 2016). They proposed to develop an annual State of the Coast Report and to develop a centralized data repository that will include baseline ecological data and information on coastal uses. Most stakeholders now recognize that legal support from real-estate lawyers to address issues around mangrove removal, encroachment, and ownership disputes, and methods for enforcing the current regulations around mangrove conservation is essential. Crafting a system of incentives to back this legal support, funded, and implemented by the Government of Belize is one way forward.
In parallel, the Belize Association of Private Protected Areas (BAPPA) and the Belizean Mangrove Conservation Network encourage land owners to work toward conservation and restoration of mangroves and other natural areas. Another local NGO, Friends of Placencia Lagoon, has documented impacts of nearby shrimp-farm effluent disposal on the local water quality. In general, active participation in mangrove management and conservation by individuals and experts who use coastal resources on a day-to-day basis will increase understanding and awareness of the ecosystem services provided by Belize's mangroves and coastal waters.
4.3. Lessons to Inform Adaptive Management
At ≈ 6 m above sea level, Belize City and its population are extremely vulnerable to coastal effects of ongoing climatic change: rising seas, increases in flooding frequency and duration, sediment deposition, and erosion. Because the pace and scope of climatic change continues to vary, adaptive management solutions can address immediate concerns while creating flexibility to respond to longer-term dynamics (Holling, 1978). A key aspect of adaptive management is the revision of management actions in response to new observations or environmental conditions. But adaptive management responses can be accelerated if additional information is available from designed experiments focused on outcomes of management interventions.
4.3.1. Observations Driving Adaptive Management in Belize
Crucial ecosystem services that mangroves provide for Belize include water filtration and treatment and coastal defense (Barbier et al., 2011; Horchard et al., 2019) and mangrove rehabilitation and restoration projects in and around Belize City may mitigate some effects of climatic change (Figure 7). Working with CZMAI and co-author Alex Felson, Coryelle Pondy (unpublished data) gathered and geospatially analyzed data on drainage and flood risk associated with storm events, current regulations and practices, predicted sea-level rise, water quality and wastewater infrastructure, and current projects and future plans for Belize City. She studied existing infrastructure and critical needs of Belize City (Figure 7), focusing on adaptions to sea-level rise and stormwater discharge into and from wastewater and septic systems, drainage networks, and roadways.
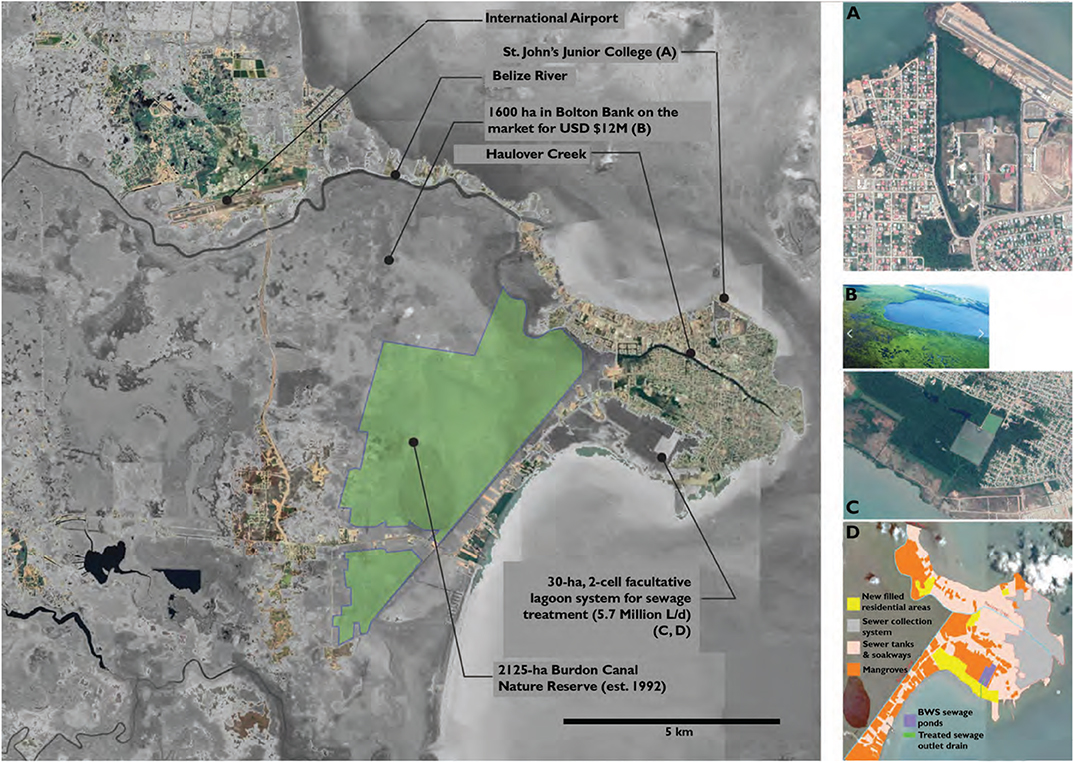
Figure 7. Belize City (large aerial photograph) is located just south of the mouth of the Belize River on a delta surrounded by mangrove forests and the Caribbean Sea. The four insets illustrate key sites discussed in the case study of mangroves impacted by past development and proposed for rehabilitation These sites include (A) Saint John's Junior College (see also Figures 8, 9); (B) the Bolton Bank land for sale; (C) the sewage lagoon system managed by the Belize Water Services; and (D) the overall sewered and mangrove areas of Belize City.
Opportunities for both preservation and rehabilitation of mangroves could take advantage of these valuable ecosystem services. For example, the 1,620 ha Bolton Bank parcel on the western side of Belize City is currently on the market for US $12M (Figure 7B). Mangrove preservation coupled with “smart” development in this area could provide significant civic benefits with extensive flood water storage capacity and habitat value.
The many canals in Belize City provide critical drainage but they overflow during heavy rains and floods, sending contaminated water into the city. Dredging and clearing trash from the canals is essential to optimize their flood management function. A recent International Development Bank project will dredge and install a pump and sluice gates to the canal outlets to manage flow and siltation (Interview with Belize Water Services 2017-06-16; see also Grau et al., 2013).
The Belize Water Services (BWS) runs the lagoon sewage processing facility, which is located adjacent to a low-income neighborhood on the south side of Belize City (Figures 7C,D). The sewage system drains ≈ 18% of the city and treats the ≈ 5.5 million L of sewage per day generated by ≈ 65% of the city's population (Silva, 2013); the rest of the city's inhabitants and those in neighboring communities either have private septic systems or discharge wastewater directly into adjacent mangroves. A facultative lagoon system (Figure 7C) operating as cells in series treats the sewage; each cell provides 10 days of hydraulic retention time. The sewer ponds are divided into three zones with the outfall being discharged through two pipes and into an excavated drain that connects to the Caribbean Sea. The collection systems are interconnected with Zone 1 flowing into Zone 2 and Zone 2 into Zone 3. On occasions, one of these zones fails and the wastewater is directly discharged into a canal, the river, or the sea through a series of outfalls designed as a fail-safe mechanism to deal with overflows, malfunctions, or power outages (Silva, 2013). Otherwise, treated effluent flows through canals cut through a mangrove wetland and is discharged into the Caribbean Sea (Belize Water Services, 2013). BWS engineers have raised concerns about salt intrusion while sea-level rise is pressuring them to raise the elevation of the berms around the lagoons (Interview with Belize Water Services 2017-06-16). There are additional conflicts between nearby low-income populations and the BWS, which wants to expand the lagoon system.
4.3.2. Designing Experiments With Management in Mind
With mangrove restoration and conservation as goals, we identified an additional critical parcel of mangroves that has undergone substantial land-use changes (Figures 7A, 8). This parcel now presents opportunities for mangrove rehabilitation that could provide ecosystem services including natural habitat, storm surge and wave attenuation, drainage, and erosion control (i.e., EMR with decision points 8–10 in Figure 4). Because the parcel is located on land owned by St. John's Junior College, it also provides additional opportunities for education, outreach, and community engagement. In addition to buildings, the site includes mangroves and land connecting freshwater runoff to the sea; other than the BWS sewage lagoon and Bolton Bank, the “St. John's” parcel is the most valuable extant mangrove within Belize City to target for conservation and rehabilitation.

Figure 8. Land-use changes at St John's Junior College illustrate (left) preserved mangroves and existing structures built from when the campus was located there in 1952 until 2006 and new changes resulting from the 2012 to 2016 extension of the Belize City Municipal Airstrip (center, right). The series of panels illustrates conditions before (2006), during (2014), and after airstrip construction was completed (2019). This US $8.5M extension required clearing of ≈ 8.5 ha of intact mangroves.
Based on a preliminary assessment of St. John's campus, and through discussions with CZMAI and a review of Belize's Integrated Coastal Zone Management Plan (Coastal Zone Management Authority & Institute, 2016), we have developed a concept-design proposal to combine nature-based infrastructure development with mangrove ecosystem management for Belize City. The project also proposes experimental research and monitoring (Figure 9) in an educational setting to work with students and faculty interested in learning about ecosystem functions and services of natural, rehabilitated, and restored mangroves, and best management practices to sustain them.
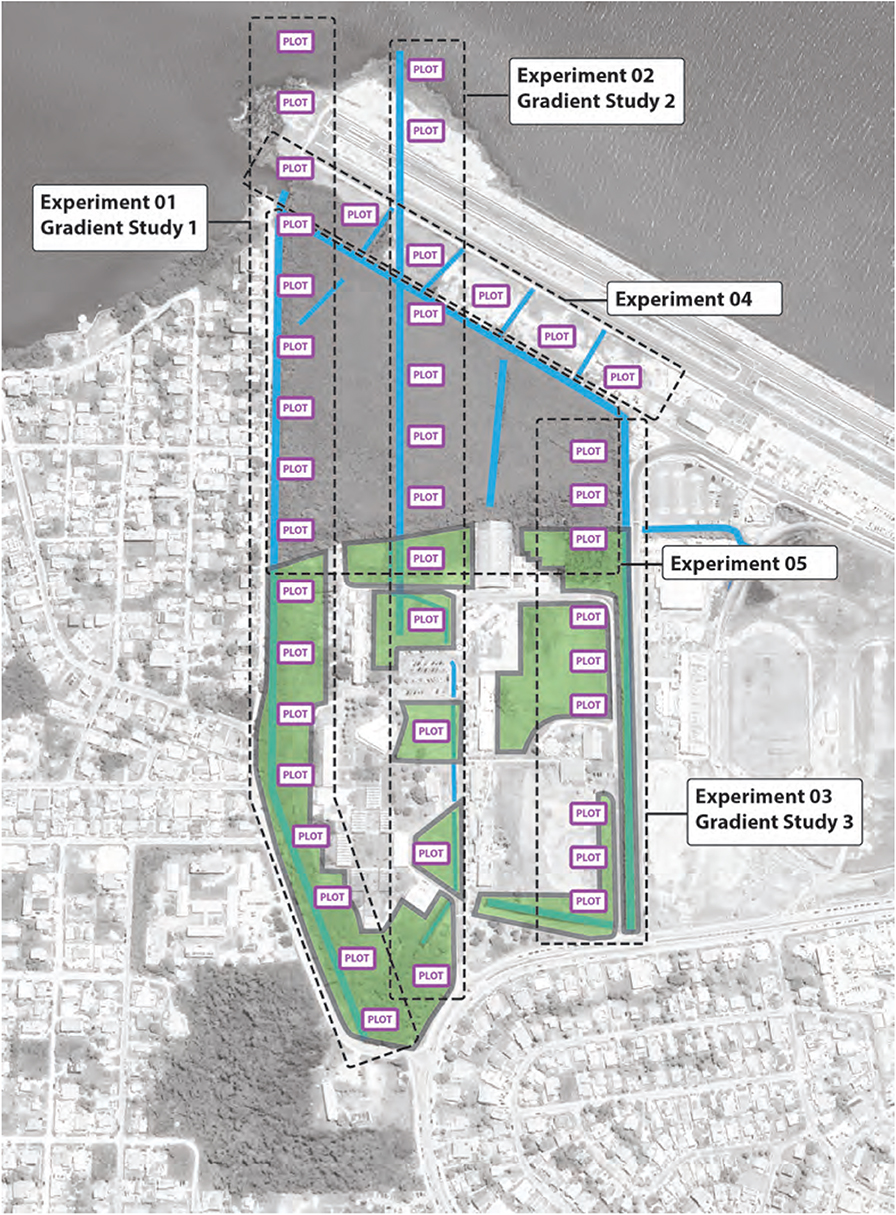
Figure 9. Saint John's Junior College is hydrologically interconnected with channels and low-lying wet areas and includes multiple existing (degraded) habitats. Our proposed mangrove rehabilitation and restoration plan for the campus will establish five designed experiments (identified on the figure and described in detail in section 4.3.2) to develop research and encourage education-based restoration to improve the campus identity and inform smart land-use decisions and conservation practices.
As with any EMR-based project, the St. John's project will start with gathering baseline biophysical data. Given its location in a human-dominated landscape, these biophysical data will be linked climate-change scenarios and ongoing development plans. To effectively develop these strategies, we defined a set of experimental zones (Figure 9) in which we will use observations and manipulative experiments to understand relationships between reconstructed tidal hydrology, connections within the existing mangrove systems, natural regeneration, and deliberate plantings (Felson and Pickett, 2005).
Proposed experiments take advantage of several environmental gradients at the site. Experiments 01 and 02 (Figure 9) would include plots sited on fill above highest high water adjacent to the airstrip, and within an existing mangrove stand, coastal uplands, and inland of the latter. These experiments would evaluate plant physiological responses and seedling or sapling growth and population dynamics as a function of inundation rate and frequency, water quality, and hydrological changes imposed by airstrip construction. Plots within Experiment 03 (Figure 9) would be used to study responses of seedlings and saplings to disturbance along a gradient from existing, intact mangroves to parts of the campus that are mown and fertilized.
Plots within Experiment 04 (Figure 9) would be used as test plots to develop nursery stock of appropriate species for restoration and rehabilitation efforts onsite and elsewhere in Belize. The primary driver variables here would be species identity and, to a lesser extent, water quality and soil nutrients that differ with distance from the existing mangrove stand onsite. Finally, Experiment 05 would be sited within the existing tidal wetland. This area would be used to explore diversity of mangrove-associated flora and fauna across a tidal range and associated impacts of the adjacent airstrip.
We also propose to construct a boardwalk in this mangrove stand to provide access to the restoration project and create opportunities for education and outreach (cf. Figure 5). Embedding the rehabilitated and restored stands on campus also will establish a stronger research-based identity for the campus landscape designed as an experiment while securing the remaining natural mangrove stands and improving the public understanding of the benefits of mangrove restoration (Felson et al., 2013a; Felson, 2016). The ultimate goal of this project is to change public awareness of the coastal management areas of Belize City and shift perceptions so that everyone recognizes the responsibility of inhabitants and institutions to share management and governance of Belize City's environmental assets.
5. Discussion
Mangroves are socio-ecological systems whose functions provide a wide range of ecosystem services (e.g., Barbier et al., 2011, and section 2.2). Although mangroves, like other wetlands, have been undervalued, cut over, and converted to other uses for millenia, their anthropocentric and ecocentric values increasingly are appreciated. Technical needs for successful mangrove rehabilitation and restoration have been understood for at least fifty years (Lewis, 1982; Ellison, 2000, and section 2.3), but knowledge transference between mangrove restoration projects remains the exception (Lewis, 2009). At the same time, long-term success of any rehabilitation or restoration project must use bring together ecology, sociology, economics, and governance through community involvement to define, measure, monitor, and update project objectives and goals (e.g., Ounanian et al., 2018; Gann et al., 2019, section 2.4 and Figure 4). The case studies of mangrove rehabilitation and restoration in Singapore and Belize that we presented above illustrate these ideas and provide directions for future work.
In countries such as Belize that still have extensive, intact mangrove forests, large-scale experiments (e.g., Figure 9) can be designed and implemented to yield general results. Benchmarks for, and adaptive management of, rehabilitation and restoration projects can be guided by experimental results and observations of co-located “reference” stands. Management authorities in urban areas can partner with NGOs focused on conservation and preservation in rural areas to build ecological functions (e.g., biodiversity, habitat structure) and ecosystem services into rehabilitation and restoration efforts in city-based projects. Urban rehabilitation efforts, such as those proposed for the St. John's College campus will be visible to large audiences and can create new constituencies interested in cooperative governance of the broader environment. In contrast, in countries such as Singapore where little intact mangroves remain, rehabilitation and restoration projects will be opportunistic and constrained by local conditions and constituencies. Although large-scale ecological experiments are unlikely in these localities, social dynamics and key decision points (e.g., Figure 4) will define project objectives and goals while informing or accelerating adaptive management.
As climatic change continues to accelerate, key biophysical characteristics determining mangrove survival, growth, and reproduction—notably local sea-level, salinity, and temperature—will change in tandem. Thus, biophysical optima for specific mangroves in particular locations will change, altering patterns of local species diversity (Record et al., 2013). Intentionally designed and engineered ecosystems that include novel combinations of species (Hobbs et al., 2006; Miller and Bestelmeyer, 2016) may be more resilient to ongoing and future climatic changes while providing a broad suite of desirable ecosystem services (Cheong S.-M. et al., 2013; Zimmer, 2018). Such designed mangroves should be guided by proven EMR and CBEMR approaches in Singapore (Figures 4, 5) and elsewhere (Brown et al., 2014; Lewis et al., 2019), deliberate experimental infrastructure in educational settings being developed in Belize (Figure 9), and integration of participatory adaptive management frameworks used throughout the world (Felson et al., 2013b; Eriksson et al., 2016; Brown, 2017).
Author Contributions
AE was invited by the editors of this special issue to organize this article, He defined its conceptual framework. AE, AF, and DF together wrote and edited the article.
Funding
AE's contribution to this work has been supported by the Harvard Forest. AF's contribution benefited from Yale University's support of a senior undergraduate engineering student.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
For invaluable discussions and assistance with the Belize work presented here, AF thanks members of the Urban Ecology and Design Laboratory at Yale University; and the CZMAI Team, including: Arlene Young and Chantalle Clarke-Samuels; Carla Patnett from the City Council, Planning and Works Department; Catherine Cumberbatch, Chief Officer, and Tennielle Williams, Principal Hydrologist, both in Belize Government's Hydrology Department; Phillip Willoughby, City Council-CEMO for Security, Flood Mitigation, & Climate Change; Sharon Lindo, Caribbean Community Climate Change Center; Sandra Bartels from the Inter-American Development Bank. Special thanks to Coryelle Pondy who worked on the critical needs assessment as her senior project at Yale, and to Gaboury Benoit (Yale) who co-advised that project. DF acknowledges members of the Restore Ubin Mangroves (RUM) Initiative and the National Parks Board, and Ben Brown (Charles Darwin University).
References
Abelson, A., Halpern, B. S., Reed, D. C., Orth, R. J., Kendrick, G. A., Beck, M. W., et al. (2016). Upgrading marine ecosystem restoration using ecological-social concepts. Bioscience 66, 156–163. doi: 10.1093/biosci/biv171
Adame, M. F. E N, Lovelock, C. E., and Brown, C. J. (2018). Avoided emissions and conservation of scrub mangroves: potential for a Blue Carbon project in the Gulf of California, Mexico. Biol. Lett. 14:20180400. doi: 10.1098/rsbl.2018.0400
Andrew, N. L., Béné, C., Hall, S. J., Allison, E. H., Heck, S., and Ratner, B. D. (2007). Diagnosis and management of small-scale fisheries in developing countries. Fish Fish. 8, 227–240. doi: 10.1111/j.1467-2679.2007.00252.x
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. doi: 10.1890/10-1510.1
Belize Tourism Industry Association (2010). 2010 Census-Placencia. Available online at: https://www.placencia.com/population/ (accessed October 2, 2020).
Belize Water Services (2013). Waste Water Treatment. Available online at: https://www.bws.bz/our-operations/waste-water-treatment/ (accessed October 2, 2020).
Benzeev, R., Hutchinson, N., and Friess, D. A. (2017). Quantifying fisheries ecosystem services of mangroves and tropical artificial urban shorelines. Hydrobiologia 803, 225–237. doi: 10.1007/s10750-017-3299-8
Biswas, S. R., Mallik, A. U., Choudhury, J. K., and Nishat, A. (2009). A unified framework for the restoration of Southeast Asian mangroves-bridging ecology, society and economics. Wetl. Ecol. Manage. 17, 365–383. doi: 10.1007/s11273-008-9113-7
Bosire, J. O., Dahdouh-Guebas, F., Walton, M., Crona, B. I., Lewis, R. R., Field, C., et al. (2008). Functionality of restored mangroves: a review. Aquat. Bot. 89, 251–259. doi: 10.1016/j.aquabot.2008.03.010
Brooksmith Consulting (2011). Mangrove Conservation as Climate Change Adaptation in Belize, Central America. Belmopan: World Wildlife Fund.
Brown, B. (2017). “Ecological rehabilitation in mangrove systems: the evolution of the practice and the need for strategic reform of policy and planning,” in Routledge Handbook of Ecological and Environmental Restoration, eds S. K. Allison and S. D. Murphy (London: Routledge), 295–311. doi: 10.4324/9781315685977-20
Brown, B., Fadillah, R., Nurdin, Y., Soulsby, I., and Ahmad, R. (2014). Case study: Community based ecological mangrove rehabilitation (CBEMR) in Indonesia. SAPIENS 7. Availabe online at http://journals.openedition.org/sapiens/1589
Bueno, R., Herzfeld, C., Stanton, E. A., and Ackerman, F. (2008). The Caribbean and Climate Change: The Cost of Inaction. Medford, MA: Stockholm Environment Institute-US Center; Global Development and Environment Institute; Tufts University.
Cáardenas, N. Y., Joyce, K. E., and Maier, S. W. (2017). Monitoring mangrove forests: are we taking full advantage of technology? Int. J. Appl. Earth Observ. Geoinform. 63, 1–14. doi: 10.1016/j.jag.2017.07.004
Cameron, C., Hutley, L. B., Friess, D. A., and Brown, B. (2019a). Community structure dynamics and carbon stock change of rehabilitated mangrove forests in Sulawesi, Indonesia. Ecol. Appl. 29:e01810. doi: 10.1002/eap.1810
Cameron, C., Hutley, L. B., Friess, D. A., and Brown, B. (2019b). High greenhouse gas emissions mitigation benefits from mangrove rehabilitation in Sulawesi, Indonesia. Ecosyst. Serv. 40:101035. doi: 10.1016/j.ecoser.2019.101035
Carrasquilla-Henao, M., Ban, N., Rueda, M., and Juanes, F. (2019). The mangrove-fishery relationship: A local ecological knowledge perspective. Mar. Policy 108:103656. doi: 10.1016/j.marpol.2019.103656
Chan, H. T. (1996). “Mangrove reforestation in Peninsular Malaysia: a case study of Matang,” in Restoration of Mangrove Ecosystems, ed C. Field (Okinawa: International Tropical Timber Organization and International Society for Mangrove Ecosystems), 65–76.
Cheong, K. H., Koh, T., and Yee, L. (2013). Malaysia and Singapore: The Land Reclamation Case. Singapore: Straits Times Press.
Cheong, S.-M., Silliman, B., Wong, P. P., van Wesenbeeck, B., Kim, C.-K., and Guannel, G. (2013). Coastal adaptation with ecological engineering. Nat. Clim. Change 3, 787–791. doi: 10.1038/nclimate1854
Cherrington, E. A., Ek, E., Cho, P., Howell, B. F., Hernandez, B. E., Anderson, E. R., et al. (2010a). Forest Cover and Deforestation in Belize: 1980-2010. Panama City: Water Center for the Humid Tropics of Latin America and the Caribbean.
Cherrington, E. A., Hernández Sandoval, B. E., Trejos, N. A., Smith, O. A., Anderson, E. R., Flores, A. I., et al. (2010b). Identification of Threatened and Resilient Mangroves in the Belize Barrier Reef System. Panama City: Water Center for the Humid Tropics of Latin America and the Caribbean.
Chow, J. (2018). Determinants of household fuelwood collection from mangrove plantations in coastal Bangladesh. For. Policy Econ. 96, 83–92. doi: 10.1016/j.forpol.2018.08.007
Clarke, C., Rosado, S., Rosenthal, A., Arkema, K., Canto, M., Gillett, I., et al. (2012). Coastal Zone Planning for Belize. Washington, DC: Belize Coastal Zone Management Authority & Institute; Natural Capital Project; World Wildlife Fund.
Coastal Zone Management Authority & Institute (2014). State of the Belize Coastal Zone Report 2003-2013. Belize City: CZMAI.
Coastal Zone Management Authority & Institute (2016). Belize Integrated Coastal Zone Management Plan. Belize City: CZMAI.
Cooper, E., Burke, L., and Bood, N. (2009). Coastal Capital Belize: The Economic Contribution of Belize's Coral Reefs and Mangroves. Washington, DC: World Resources Institute.
Cormier-Salem, M. C. (1999). The mangrove: an area to be cleared…for social scientists. Hydrobiologia 413, 135–142. doi: 10.1023/A:1003847011720
Dahdouh-Guebas, F., Mathenge, C., Kairo, J. G., and Koedam, N. (2000). Utilization of mangrove wood products around Mida Creek (Kenya) amongst subsistence and commercial users. Econ. Bot. 54, 513–527. doi: 10.1007/BF02866549
Dale, P. E. R., Knight, J. M., and Dwyer, P. G. (2014). Mangrove rehabilitation: a review focusing on ecological and institutional issues. Wetl. Ecol. Manage. 22, 587–604. doi: 10.1007/s11273-014-9383-1
Damastuti, E., and de Groot, R. (2017). Effectiveness of community-based mangrove management for sustainable resource use and livelihood support: a case study of four villages in Central Java, Indonesia. J. Environ. Manage. 203, 510–521. doi: 10.1016/j.jenvman.2017.07.025
Das, S. (2017). Ecological restoration and livelihood: contribution of planted mangroves as nursery and habitat for artisanal and commercial fishery. World Dev. 94, 492–502. doi: 10.1016/j.worlddev.2017.02.010
Djamaluddin, R. (2007). Cost-effective Mangrove Rehabilitation Focusing on the Restoration of Hydrology. Rufford Small Grant Report. Rufford Foundation. Available online at: https://www.rufford.org/files/2-10.02.06%20Detailed%20Final%20Report.pdf
Duarte, C. M., Agusti, S., Barbier, E., Britten, G. L., Castilla, J. C., Gattuso, J.-P., et al. (2020). Rebuilding marine life. Nature 580, 39–51. doi: 10.1038/s41586-020-2146-7
Elliott, M., Burdon, D., Hemingway, K. L., and Apitz, S. E. (2007). Estuarine, coastal and marine ecosystem restoration: confusing management and science-a revision of concepts. Estuar. Coast. Shelf Sci. 74, 349–366. doi: 10.1016/j.ecss.2007.05.034
Ellison, A. M. (2000). Mangrove restoration: do we know enough? Restor. Ecol. 8, 219–229. doi: 10.1046/j.1526-100x.2000.80033.x
Ellison, A. M. (2004). Wetlands of Central America. Wetl. Ecol. Manage. 12, 3–55. doi: 10.1023/B:WETL.0000016809.95746.b1
Ellison, A. M. (2008). Managing mangroves with benthic biodiversity in mind: moving beyond roving banditry. J. Sea Res. 59, 2–15. doi: 10.1016/j.seares.2007.05.003
Ellison, A. M. (2019). Foundation species, non-trophic interactions, and the value of being common. iScience 13, 254–268. doi: 10.1016/j.isci.2019.02.020
Ellison, A. M., Bank, M. S., Clinton, B. D., Colburn, E. A., Elliott, K., Ford, C. R., et al. (2005). Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 3, 479–486. doi: 10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2
Ellison, A. M., and Farnsworth, E. J. (1996). Anthropogenic disturbance of Caribbean mangrove ecosystems: past impacts, present trends, and future predictions. Biotropica 28, 549–565. doi: 10.2307/2389096
Ellison, A. M., and Farnsworth, E. J. (2001). “Mangrove communities,” in Marine Community Ecology, 1st Edn, eds M. D. Bertness, S. Gaines, and M. E. Hay (Sunderland, MA: Sinauer Associates), 423–442.
Ellison, A. M., Farnsworth, E. J., and Merkt, R. E. (1999). Origins of mangrove ecosystems and the mangrove biodiversity anomaly. Glob. Ecol. Biogeogr. 8, 95–115. doi: 10.1046/j.1466-822X.1999.00126.x
Eriksson, H., Adhuri, D. S., Adrianto, L., Andrew, N. L., Apriliani, T., Daw, T., et al. (2016). An ecosystem approach to small-scale fisheries through participatory diagnosis in four tropical countries. Glob. Environ. Change Hum. Policy Dimens. 36, 56–66. doi: 10.1016/j.gloenvcha.2015.11.005
Farnsworth, E. J., and Ellison, A. M. (1997). The global conservation status of mangroves. Ambio 26, 328–334.
Felson, A. (2016). Designed experiments for transformational learning: forging new opportunities through the integration of ecological research into design. Designed Experiments. Available online at: https://dusp.mit.edu/sites/dusp.mit.edu/files/attachments/project/Projections%2011_MIT%20DUSP_0215web.pdf
Felson, A. J., Bradford, M. A., and Oldfield, E. (2013a). Involving ecologists in shaping large-scale green infrastructure projects. BioScience 63, 881–890. doi: 10.1525/bio.2013.63.11.7
Felson, A. J., Bradford, M. A., and Terway, T. M. (2013b). Promoting earth stewardship through designed experiments. Front. Ecol. Environ. 11, 362–367. doi: 10.1890/130061
Felson, A. J., and Pickett, S. T. A. (2005). Designed experiments: new approaches to understanding urban ecosystems. Front. Ecol. Environ. 3, 549–556. doi: 10.1890/1540-9295(2005)003[0549:DENATS]2.0.CO;2
Field, C. D. (1998). Rehabilitation of mangrove ecosystems: an overview. Mar. Pollut. Bull. 37, 383–392. doi: 10.1016/S0025-326X(99)00106-X
Flomenhoft, G., Cayetano, M., and Young, C. (2007). Black gold, white gold and the gentrification of Belize. Belizean Stud. 29, 4–19.
Friess, D. A. (2017). Mangrove rehabilitation along urban coastlines: a Singapore case study. Region. Stud. Mar. Sci. 16, 279–289. doi: 10.1016/j.rsma.2017.09.013
Furley, P., and Ratter, J. (1992). Mangrove Distribution, Vulnerability and Management in Central America. London: ODA-OFI Forestry Research Programme.
Gann, G. D., McDonald, T., Walder, B., Aronson, J., Nelson, C. R., Jonson, J., et al. (2019). International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 27, S1–S46. doi: 10.1111/rec.13035
Gaw, L. Y.-F., Yee, A. T. K., and Richards, D. R. (2019). A high-resolution map of Singapore's terrestrial ecosystems. Data 4:116. doi: 10.3390/data4030116
Gedan, K. B., and Silliman, B. R. (2009). Using facilitation theory to enhance mangrove restoration. Ambio 38:109. doi: 10.1579/0044-7447-38.2.109
Government of Belize (2002). First National Communication to the Conference of the Parties of the United Nations Framework. Convention on Climate Change. Belmopan: Government Printer.
Government of Belize (2003). Substantive Laws of Belize. Revised Edition 2003: Chapter 2013, Forests Act Subsidiary Laws. Belmopan: Government Printer.
Government of Belize (2018). Forests (Protection of Mangroves) Regulations 2018. Belmopan: Government Printer.
Grau, J., del Rosario Navia, M., Rihm, A., Ducci, J., Martin, D., and Kuratomi, T. (2013). Water and Sanitation in Belize. Technical note No. IDB-TN-609. Washington, DC: Inter-American Development Bank.
Halpern, B. S., Silliman, B. R., Olden, J., Bruno, J., and Berness, M. D. (2007). Incorporating positive interactions in aquatic restoration and conservation. Front. Ecol. Environ. 5, 153–160. doi: 10.1890/1540-9295(2007)5[153:IPIIAR]2.0.CO;2
Hamilton, S. E., and Casey, D. (2016). Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 25, 729–738. doi: 10.1111/geb.12449
Hartshorn, G., Nicolait, L., Hartshorn, L., Bevier, G., Brightman, R., Cal, J., et al. (1984). Belize: Country Environmental Profile: A Field Study. Belize City: Robert Nicolait & Associates Ltd.
Hobbs, R. J., Arico, S., Aronson, J., Baron, J. S., Bridgewater, P., Cramer, V. A., et al. (2006). Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 15, 1–7. doi: 10.1111/j.1466-822X.2006.00212.x
Holling, C. S. (1978). Adaptive Environmental Assessment and Management. New York, NY: John Wiley & Sons.
Horchard, J. P., Hamilton, S., and Barbier, E. B. (2019). Mangroves shelter coastal economic activity from cyclones. Proc. Natl. Acad. Sci. U.S.A. 116, 12232–12237. doi: 10.1073/pnas.1820067116
Jaafar, Z., Hajisamae, S., Chou, L. M., and Yatiman, Y. (2004). Community structure of coastal fishes in relation to heavily impacted human modified habitats. Hydrobiologia 511, 113–123. doi: 10.1023/B:HYDR.0000014034.27109.20
James, G. K., Adegoke, J. O., Osagie, S., Ekechukwu, S., Nwilo, P., and Akinyede, J. (2013). Social valuation of mangroves in the Niger Delta region of Nigeria. Int. J. Biodivers. Sci. Ecosyst. Services Manage. 9, 311–323. doi: 10.1080/21513732.2013.842611
Jordan, W. R. I., and Lubick, G. M. (2011). Making Nature Whole: A History of Ecological Restoration. Washington, DC: Island Press. doi: 10.5822/978-1-61091-042-2
Karns, D. R., Voris, H. K., and Goodwin, T. G. (2002). Ecology of Oriental-Australian rear-fanged water snakes (Colubridae: Homalopsinae) in the Pasir Ris Park Mangrove Forest, Singapore. Raffles Bull. Zool. 50, 487–498. Available online at: https://lkcnhm.nus.edu.sg/app/uploads/2017/06/50rbz487-498.pdf
Kibler, K. M., Cook, G. S., Chambers, L. G., Donnelly, M., Hawthorne, T. L., Rivera, F. I., and Walters, L. (2018). Integrating sense of place into ecosystem restoration: a novel approach to achieve synergistic social-ecological impact. Ecol. Soc. 23:25. doi: 10.5751/ES-10542-230425
Kodikara, K. A. S., Mukherjee, N., Jayatissa, L. P., Dahdouh-Guebas, F., and Koedam, N. (2017). Have mangrove restoration projects worked? An in-depth study in Sri Lanka. Restor. Ecol. 25, 705–716. doi: 10.1111/rec.12492
Krievins, K., Plummer, R., and Baird, J. (2018). Building resilience in ecological restoration processes: a social-ecological perspective. Ecol. Restor. 36, 195–207. doi: 10.3368/er.36.3.195
Lai, S., Loke, L. H., Hilton, M. J., Bouma, T. J., and Todd, P. A. (2015). The effects of urbanisation on coastal habitats and the potential for ecological engineering: a Singapore case study. Ocean Coast. Manage. 103, 78–85. doi: 10.1016/j.ocecoaman.2014.11.006
Lee, S. K., Tan, W. H., and Havanond, S. (1996). Regeneration and colonisation of mangrove on clay-filled reclaimed land in Singapore. Hydrobiologia 319, 23–35. doi: 10.1007/BF00020968
Lee, S. Y., Hamilton, S., Barbier, E. B., Primavera, J., and Lewis, R. R. (2019). Better restoration policies are needed to conserve mangrove ecosystems. Nat. Ecol. Evol. 3, 870–872. doi: 10.1038/s41559-019-0861-y
Lewis, R. R. (1982). “Mangrove forests,” in Creation and Restoration of Coastal Plant Communities, ed R. R. Lewis (Boca Raton, FL: CRC Press), 153–171.
Lewis, R. R. (2005). Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 24, 403–418. doi: 10.1016/j.ecoleng.2004.10.003
Lewis, R. R. (2009). Knowledge overload, wisdom underload. Ecol. Eng. 35, 341–342. doi: 10.1016/j.ecoleng.2008.10.006
Lewis, R. R., Brown, B. M., and Flynn, L. L. (2019). “Methods and criteria for successful mangrove forest rehabilitation,” in Coastal Wetlands: An Integrated Ecosystem Approach, 2nd Edn, eds G. M. E. Perillo, E. Wolanski, D. R. Cahoon, and C. S. Hopkinson (Amsterdam: Elsevier), 863–887. doi: 10.1016/B978-0-444-63893-9.00024-1
Locatelli, T., Binet, T., Kairo, J. G., King, L., Madden, S., Patenaude, G., et al. (2014). Turning the tide: how blue carbon and payments for ecosystem services (pes) might help save mangrove forests. Ambio 43, 981–995. doi: 10.1007/s13280-014-0530-y
Loke, L., Chisholm, R. A., and Todd, P. A. (2019). Effects of habitat area and spatial configuration on biodiversity in an experimental intertidal community. Ecology 100:e02757. doi: 10.1002/ecy.2757
Loke, L. H., and Todd, P. A. (2016). Structural complexity and component type increase intertidal biodiversity independently of area. Ecology 97, 383–393. doi: 10.1890/15-0257.1
Lovelock, C. E., and Brown, B. M. (2019). Land tenure considerations are key to successful mangrove restoration. Nat. Ecol. Evol. 3:1135. doi: 10.1038/s41559-019-0942-y
Mantrove Action Project (2019). ”CBEMR” Mangrove Restoration. Available online at: https://mangroveactionproject.org/mangrove-restoration/ (accessed February 19, 2020).
Matsui, N., Morimune, K., Meepol, W., and Chukwamdee, J. (2012). Ten year evaluation of carbon stock in mangrove plantation reforested from an abandoned shrimp pond. Forests 3, 431–444. doi: 10.3390/f3020431
Mayer-Pinto, M., Johnston, E. L., Bugnot, A. B., Glasby, T. M., Airoldi, L., Mitchell, A., et al. (2017). Building 'blue': an eco-engineering framework for foreshore development. J. Environ. Manage. 189, 109–114. doi: 10.1016/j.jenvman.2016.12.039
Mazón, M., Aguirre, N., Echeverría, C., and Aronson, J. (2019). Monitoring attributes for ecological restoration in Latin America and the Caribbean region. Restor. Ecol. 27, 992–999. doi: 10.1111/rec.12986
Meerman, J. C., and Sabido, W. (2001). Central American Ecosystems: Belize. Belize City: Programme for Belize.
Millenium Ecosystem Assessment (2005). Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press.
Miller, J. A., and Bestelmeyer, B. T. (2016). What's wrong with novel ecosystems, really? Restor. Ecol. 24, 577–582. doi: 10.1111/rec.12378
Morris, R. L., Heery, E. C., Loke, L. H. L., Lau, E., Strain, E. M. A., Airoldi, L., et al. (2019). Design options, implementation issues and evaluating success of ecologically engineered shorelines. Oceanogr. Mar. Biol. 57, 169–228. doi: 10.1201/9780429026379-4
Morris, R. L., Konlechner, T. M., Ghisalberti, M., and Swearer, S. E. (2018). From grey to green: Efficacy of eco-engineering solutions for nature-based coastal defence. Glob. Change Biol. 24, 1827–1842. doi: 10.1111/gcb.14063
Morse, N. B., Pellissier, P. A., Cianciola, E. N., Brereton, R. L., Sullivan, M. M., Shonka, N. K., et al. (2014). Novel ecosystems in the Anthropocene: a revision of the novel ecosystem concept for pragmatic applications. Ecol. Soc. 19, 12–21. doi: 10.5751/ES-06192-190212
Murray, M. R., Zisman, S. A., Furley, P. A., Munro, D. M., Gibson, J., Ratter, J., et al. (2003). The mangroves of Belize. Part 1. Distribution, composition and classification. For. Ecol. Manage. 174, 265–279. doi: 10.1016/S0378-1127(02)00036-1
Neal, D., Ariola, E., and Muschamp, W. (2008). Vulnerability Assessment of Belize Coastal Zone-2007: Enabling Activities for the Preparation of Belize's Second National Communication (SNC) to the United Nations Framework Convention on Climate Change (UNFCCC) Project. Belmopan: UNDP/GEF Climate Change Project.
Nengshi, Z., Haoran, M., Chiyan, P., Yuan, L., Grotewal, R., and Klaus, U. (2018). Resilient ecology and landscape systems of the Fengxinglong Ecological Park, Sanya. Landsc. Architect. Front. 6, 32–41. doi: 10.15302/J-LAF-20180403
Osland, M. J., Feher, L. C., Spivak, A. C., Nestlerode, J. A., Almario, A. E., Cormier, N., et al. (2020). Rapid peat development beneath created, maturing mangrove forests: ecosystem changes across 25-year chronosequence. Ecol. Appl. 28:e02085. doi: 10.1002/eap.2085
Ostrom, E. (2009). A general framework for analyzing sustainability of social-ecological systems. Science 325, 419–422. doi: 10.1126/science.1172133
Ounanian, K., Carballo-Cárdenas, E., van Tatenhove, J. P. M., Delaney, A., Papadopoulou, K. N., and Smith, C. J. (2018). Governing marine ecosystem restoration: the role of discourses and uncertainties. Mar. Policy 96, 136–144. doi: 10.1016/j.marpol.2018.08.014
Polidoro, B. A., Carpenter, K. E., Collins, L., Duke, N. C., Ellison, A. M., Ellison, J. C., et al. (2010). The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS ONE 5:e10095. doi: 10.1371/journal.pone.0010095
Powell, E. J., Tyrrell, M. C., Milliken, A., Tirpak, J. M., and Staudinger, M. D. (2019). A review of coastal management approaches to support the integration of ecological and human community planning for climate change. J. Coast. Conserv. 23, 1–18. doi: 10.1007/s11852-018-0632-y
Prime Minister's Office (2019). National Day Rally 2019. Government of Singapore. Available online at: https://pmo.gov.sg/Newsroom/National-Day-Rally-2019
Ranjan, R. (2019). Optimal mangrove restoration through community engagement on coastal lands facing climatic risks: the case of Sundarbans region in India. Land Use Policy 81, 736–749. doi: 10.1016/j.landusepol.2018.11.047
Record, S., Charney, N. D., Zakaria, R. M., and Ellison, A. M. (2013). Projecting global mangrove species and community distributions under climate change. Ecosphere 4:34. doi: 10.1890/ES12-00296.1
Renzi, J. J., He, Q., and Silliman, B. R. (2019). Harnessing positive species interactions to enhance coastal wetland restoration. Front. Ecol. Evol. 7:131. doi: 10.3389/fevo.2019.00131
Richardson, R. B. (2009). Belize and Climate Change: The Cost of Inaction. Belmopan: United Nations Development Programme.
Rönnback, P., and Crona, B. L.I K. (2007). The return of ecosystem goods and services in replanted mangrove forests: perspectives from local communities in Kenya. Environ. Conserv. 34, 313–324. doi: 10.1017/S0376892907004225
RUM (2017). Restore Ubin Mangroves (RUM) Initiative. Singapore: RUM. Available online at: https://rum-initiative.blogspot.com/
Rützler, K., and Macintyre, I. G. (1982). The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize, 1: Structure and Communities, Vol. 539 of Smithsonian Contributions to the Marine Sciences. Washington, DC: Smithsonian Institution Press. doi: 10.5479/si.01960768.12.539
Sa'at, N. S., and Lin, P.-S. S. (2018). Janus-Faced linkages: understanding external actors in community-based natural resource management in southern Thailand. Soc. Nat. Resour. 31, 773–789. doi: 10.1080/08941920.2017.1423433
Sasmito, S. D., Taillardat, T., Clendenning, J. N., Cameron, C., Friess, D. A., Murdiyarso, D., et al. (2019). Effect of land-use and land-cover change on mangrove blue carbon: a systematic review. Glob. Change Biol. 25, 4291–4302. doi: 10.1111/gcb.14774
Silva, H. (2013). Baseline Assessment Study on Wastewater Management. Study for the GEF Caribbean Regional Fund for Wastewater Management for Belize and Inter-American Development Bank, Annex 04-Existing Issues of Water Supply and Sewerage in Belize City, 2011. Washington, DC: Inter-American Development Bank.
Spalding, M., Kainuma, M., and Collins, L. (2010). World Atlas of Mangroves. London: Earthscan Publishers Ltd. doi: 10.4324/9781849776608
Spalding, M., and Parrett, C. L. (2019). Global patterns in mangrove recreation and tourism. Mar. Policy 110:103540. doi: 10.1016/j.marpol.2019.103540
Stewart-Oaten, A., Murdoch, W., and Parker, K. R. (1986). Environmental impact assessment: “pseudoreplication” in time? Ecology 67, 929–940. doi: 10.2307/1939815
Suman, D. O. (2019). “Mangrove management: challenges and guidelines,” in Coastal Wetlands: An Integrated Ecosystem Approach, 2nd Edn, eds G. M. E. Perillo, E. Wolanski, D. R. Cahoon, and C. S. Hopkinson (Amsterdam: Elsevier), 1055–1079.
Tam, N. F., and Wong, Y. S. (1995). Mangrove soils as sinks for wastewater-borne pollutants. Hydrobiologia 295, 231–241. doi: 10.1007/BF00029130
Tan, A., and Fogarty, D. (2019). Singapore Will Use Nature-Based Solutions to Deal With Sea Level Rise: Masagos. The Straits Times.
Tatani, M., Tanaka, Y., Arita, K., and Yauchi, E. (2001). “Mangrove replanting and the growth process as a mitigation project,” in Proceedings of the 29th Meeting of the International Association of Hydraulic Research, Theme B (Beijing: IAHR), 390–395.
The World Bank (2018). GDP (current US$). Available online at: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD (accessed February 2, 2020).
Thiagarajah, J., Wong, S. K. M., Richards, D. R., and Friess, D. A. (2015). Historical and contemporary cultural ecosystem service values in the rapidly urbanizing city state of Singapore. Ambio 44, 666–677. doi: 10.1007/s13280-015-0647-7
Thompson, B. S. (2018). The political ecology of mangrove forest restoration in Thailand: institutional arrangements and power dynamics. Land Use Policy 78, 503–514. doi: 10.1016/j.landusepol.2018.07.016
Tomlinson, P. B. (2016). The Botany of Mangroves, 2nd Edn. Cambridge, UK: Cambridge University Press. doi: 10.1017/CBO9781139946575
UNESCO (1996). Belize Barrier Reef Reserve System. Available online at: https://whc.unesco.org/en/list/764/ (accessed February 2, 2020).
Vaughn, S. E. (2017). Disappearing mangroves: the epistemic politics of climate adaptation in Guyana. Cult. Anthropol. 32, 242–268. doi: 10.14506/ca32.2.07
Walters, B. B. (1997). Human ecological questions for tropical restoration: experiences from planting native upland trees and mangroves in the Philippines. For. Ecol. Manage. 99, 275–290. doi: 10.1016/S0378-1127(97)00211-9
Walters, B. B. (2000). Local mangrove planting in the Philippines: are fisherfolk and fishpond owners effective restorationists? Restor. Ecol. 8, 237–246. doi: 10.1046/j.1526-100x.2000.80035.x
Wilson, A. M. W., and Forsyth, C. (2018). Restoring near-shore marine ecosystems to enhance climate security for island ocean states: aligning international processes and local practices. Mar. Policy 93, 284–294. doi: 10.1016/j.marpol.2018.01.018
Wongbusarakum, S., Brown, V., Loerzel, A., Gorstein, M., Kleiber, D., Quinata, M., et al. (2019). Achieving social and ecological goals of coastal management through integrated monitoring. J. Appl. Ecol. 56, 2400–2409. doi: 10.1111/1365-2664.13494
Xu, S., He, Z., Guo, Z., Zhang, Z., Wyckoff, G. J., Greenberg, A., et al. (2017). Genome-wide convergence during evolution of mangroves from woody plants. Mol. Biol. Evol. 34, 1008–1015. doi: 10.1093/molbev/msw277
Yang, S., Lim, R. L., Sheue, C.-R., and Yong, J. W. (2011). “The current status of mangrove forests in Singapore,” in Proceedings of Nature Society, Singapore's Conference on 'Nature Conservation for a Sustainable Singapore'-16th October 2011, eds L. T. Ming and H. H. Chew (Singapore: The Nature Society), 99–120.
Zimmer, M. (2018). “Ecosystem design: when mangrove ecology meets human needs,” in Threats to Mangrove Forests: Hazards, Vulnerability, and Management, Vol. 25 of Coastal Research Library, eds C. Makowski and C. W. Finkl (Cham: Springer International Publishing, AG), 367–376. doi: 10.1007/978-3-319-73016-5_16
Keywords: Belize, designed ecosystems, ecological mangrove restoration (EMR), ecosystem services, landscape architecture, Singapore, socio-ecological systems (SES)
Citation: Ellison AM, Felson AJ and Friess DA (2020) Mangrove Rehabilitation and Restoration as Experimental Adaptive Management. Front. Mar. Sci. 7:327. doi: 10.3389/fmars.2020.00327
Received: 19 February 2020; Accepted: 21 April 2020;
Published: 15 May 2020.
Edited by:
Brian Silliman, Duke University, United StatesReviewed by:
Kevin Alexander Hovel, San Diego State University, United StatesMarc Hensel, University of Massachusetts Boston, United States
Copyright © 2020 Ellison, Felson and Friess. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aaron M. Ellison, YWVsbGlzb25AZmFzLmhhcnZhcmQuZWR1
 Aaron M. Ellison
Aaron M. Ellison Alexander J. Felson
Alexander J. Felson Daniel A. Friess
Daniel A. Friess