
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 24 April 2020
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00198
This article is part of the Research Topic Fish Nutrition, Metabolism and Physiology View all 20 articles
 Jing Zheng1,2
Jing Zheng1,2 Pei Yang1,2
Pei Yang1,2 Jihong Dai1,2
Jihong Dai1,2 Guijuan Yu1,2
Guijuan Yu1,2 Weihao Ou1,2
Weihao Ou1,2 Weiqi Xu1,2
Weiqi Xu1,2 Kangsen Mai1,2,3
Kangsen Mai1,2,3 Yanjiao Zhang1,2,3*
Yanjiao Zhang1,2,3*This study was conducted to investigate the dynamic characterization of enteritis of juvenile turbot (Scophthalmus maximus L.) during the development of enteritis induced by dietary β-conglycinin (7S) and the recovery by fish meal (FM). Two isonitrogenous and isolipidic experimental diets were formulated, FM diet and FM supplemented with 8% 7S diet. The feeding trial consists of three groups, turbot fed FM and 7S diet for 10 weeks, respectively, and turbot fed 7S diet for 3 weeks and then fed FM diet for another 7 weeks (7S&FM). The distal intestines and serum of turbot in the 7S&FM group were sampled at the zeroth day, third day, first week, second week, third week, sixth week, and tenth week. At the end of the feeding trial, the growth performance and feed utilization of turbot in the FM and 7S&FM group were significantly higher than that of turbot in the 7S group. According to the observation of intestinal histology, the typical symptoms of enteritis were observed at the third week and then mitigated by FM gradually until the tenth week. The activities of diamine oxidase (DAO) and content of D-lactate in serum showed a similar trend in 10 weeks. The gene expression of pro-inflammatory cytokines IL-1β, IL-8, IL-22, and IFN-γ was significantly increased by 7S in the first 3 weeks and then significantly decreased by FM since the sixth week, as well as the expression of NF-κB p65. The opposite trend of gene expression was observed in anti-inflammatory cytokine TGF-β. The gene expression of tight junction proteins claudin-3, claudin-4, claudin-like, occludin, and ZO-1 was significantly decreased during the first 3 weeks and then the gene expression of claudin-4 and occludin was significantly increased by dietary FM since the sixth week. Meanwhile, the gene expression of myosin light chain kinase (MLCK) increased significantly during the first 3 weeks and then significantly declined at the tenth week. Collectively, dietary 8% 7S could induce the serious enteritis of turbot in 3 weeks and the mitigation progress could be observed when turbot are fed with FM diet. The intestinal inflammatory cytokines and tight junction proteins showed different responses during the development and recovery of enteritis.
The intestine of animals is not only the organ for digestion and absorption of nutrients but also an important place to defend against foreign invaders. The intestinal mucosal barriers, mainly including the immune barrier and physical barrier, are the most important defensive barriers of gut health in animals (Oshima and Miwa, 2016). The intestinal immune barrier is of vital importance in response to enteritis, which consists of a variety of cytokines. Cytokines are key mediators of cellular interactions in the intestine in both physiology and pathophysiology and could be mainly divided into pro-inflammatory cytokines and anti-inflammatory cytokines (Friedrich et al., 2019). During enteritis, the activation of intestinal immune cell may proceed unchecked and lots of cytokines may be released to promote a persistent enteritis (Quintans et al., 2019). The intestinal physical barrier is mainly composed of intestinal epithelial cells and tight junction. Tight junction forms a complex protein structure maintained by transmembrane proteins (e.g., claudins and occludin) and cytoplasmic proteins (e.g., ZO-1) between adjacent epithelial cells (Balda and Karl, 2008). The decreased gene expression of tight junction proteins usually represents the weakened intestinal physical barrier function, which facilitates more pathogen invasion in intestine (Takiishi et al., 2017). The secretion of inflammatory cytokines and the reassembly of tight junction structure are thought to be important in the initiation and/or development of enteritis (Hee, 2015). Evidences in grass carp (Ctenopharyngodon idella) (Luo et al., 2014; Chen K. et al., 2018; Wu et al., 2018), Jian carp (Cyprinus carpio) (Jiang et al., 2015), and turbot (Scophthalmus maximus) (Chen Z. et al., 2018; Liu et al., 2018) have shown that the up-regulated gene expression of pro-inflammatory cytokines occurs along with the down-regulated gene expression of tight junction proteins in enteritis. However, the nature of inflammatory cytokines and tight junction dynamics during enteritis remains poorly understood.
During rapid growth of aquaculture, soybean meal has been widely used in fish feed due to the limited supply and high price of fish meal. However, application of soybean meal in feeds for marine fish generally induced enteritis (Li et al., 2017; Chen Z. et al., 2018; Liu et al., 2018). 7S is known to be a main allergenic protein in soybean that favors the development of mammal enteritis (Lamia and Boye, 2007; Krishnan et al., 2009). In mice and piglets, the supplementation of 7S in diet inhibited the growth performance and impaired the histological structure and mucosal barrier of intestine (Guo et al., 2007; Liu et al., 2008; Hao et al., 2009; Xu et al., 2010; Sun et al., 2013; Zhao et al., 2014). However, very few studies are available regarding the effects of 7S on intestinal health of fish despite the widely reported studies with mammals. In fish, it has been reported that soy preparations with high levels of 7S in diet caused inferior growth performance in rainbow trout (Oncorhynchus mykiss) (Rumsey et al., 1994). Zhang et al. (2013) reported that 8% dietary 7S (purity, 80%) induced intestinal oxidation lesions and dysfunction of intestinal digestion and absorption of Jian carp and finally suppressed fish growth performance. Li et al. (2017) reported that turbot fed a diet contained 8% 7S (purity, ∼78%) showed obvious intestinal mucosal lesions and inferior feed utilization. However, the studies on the effect of dietary 7S on intestinal health usually followed a long period of feeding trial. The action mechanism of 7S on intestinal tract during the development of enteritis is urgently in need of clearer survey.
Turbot has been farmed as a valuable marine fish worldwide. The present study was aimed to further elucidate the dynamics of intestinal inflammatory cytokines and tight junction proteins during the development and recovery of enteritis of turbot induced by dietary 7S. The current results are beneficial to find targets in mitigation of 7S-induced enteritis of fish.
All sampling protocols, as well as fish-rearing practices, were reviewed and approved by the Animal Care and Use Committee of Ocean University of China.
Purified 7S was offered by Prof. Shuntang Guo at China Agricultural University (the purified protein was made by fractional salting-out method with sodium and potassium salts of different pH, Patent No. 200410029589.4, China). The crude protein content of 7S was 96.0% (determined by the Kjeldahl method) and the protein profile of 7S fraction was revealed by the SDS-PAGE analysis (Figure 1). Quantitative analysis of the gel image was performed using Quantity One (Bio-Rad, Hercules, CA, United States), which shows that the purity of 7S fraction is 81.3% 7S, the rest being 5.8% glycinin and 12.9% non-allergenic proteins. The immunologically active 7S in the diet was determined by a commercial kit (National Feed Engineering Technology Research Center, Beijing, China) and it was 5.91%.
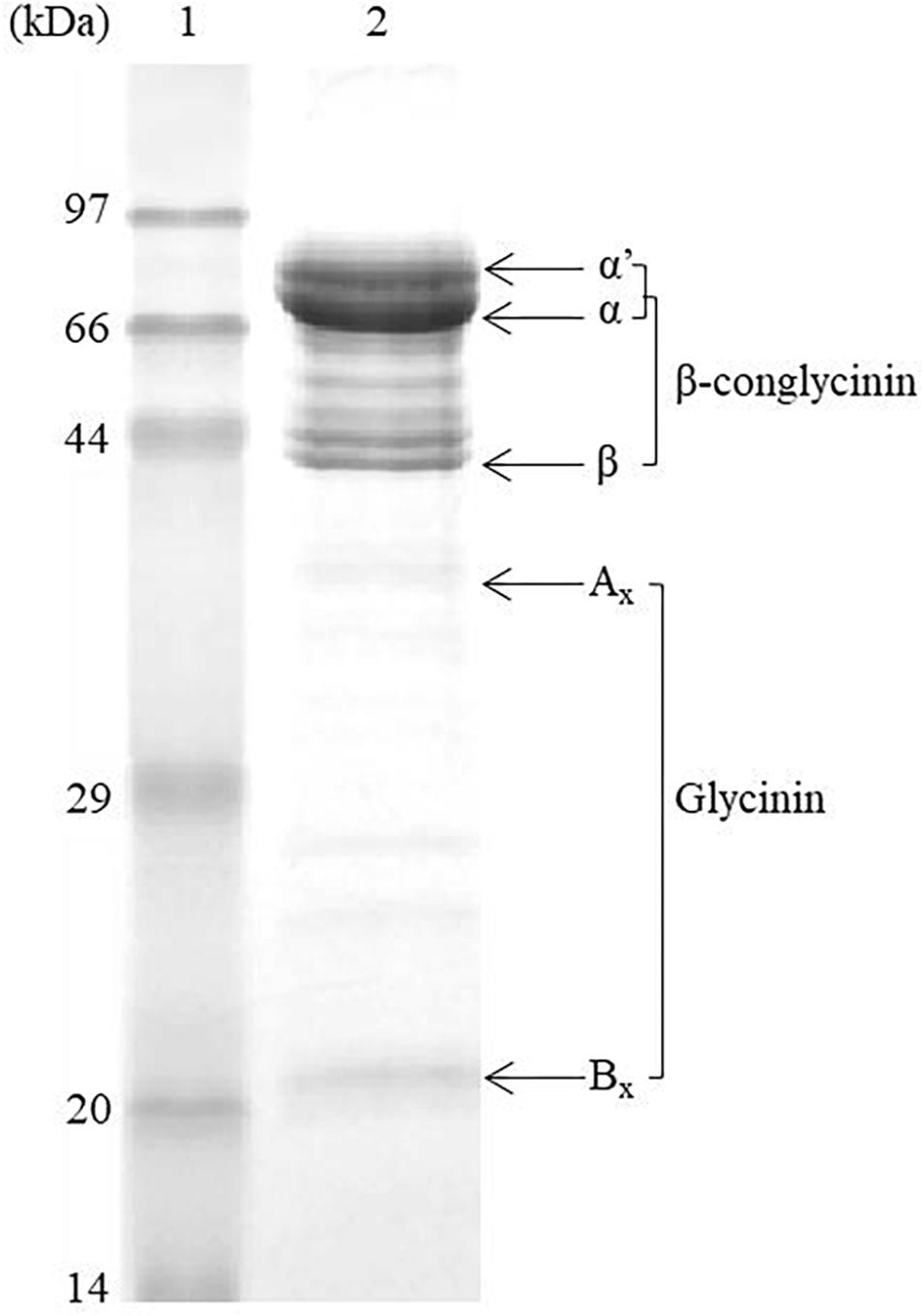
Figure 1. The SDS-PAGE profile of purified β-conglycinin fraction. Lane 1: protein marker; Lane 2: β-conglycinin fraction. α′, α, and β are subunits of β-conglycinin, and Ax and Bx are acidic and basic subunits of glycinin (see Supplementary Figure S2 for the original blots).
The results of our previous research in turbot (Li et al., 2017) showed that diet with 4–8% 7S (purity, ∼78%) can cause the typical enteritis of turbot after a 12-week feeding trial. In addition, another study in turbot showed that diet with 6% 7S (purity, ∼75.87%) could damage the intestinal histology after a 4-week feeding trial (Gu et al., 2016). Also, enteritis was observed after a 12-week feeding trial in turbot fed diet with 37.9% soybean meal (about 6% 7S) (Chen Z. et al., 2018; Liu et al., 2018). Based on these researches in turbot and the purpose of our study, a level of 8% 7S (purity, 81.3%) in diet was chosen to ensure that the 7S-induced enteritis can occur in turbot in a short time.
Two isonitrogenous and isolipidic diets were formulated to contain 52% crude protein and 11% crude lipid (Table 1). The basal diet used fish meal, casein and gelatin as the main protein sources (FM). 7S was included into the basal diet to replace 8% casein and gelatin (7S). Crystalline amino acids were supplemented to obtain the same essential amino acid profile across all the diets. Dietary ingredients were ground into fine powder through 320-μm mesh. All ingredients were thoroughly mixed with fish oil, and then water was added to produce stiff dough. The dough was then pelleted with an experimental single-screw feed mill. After being pelleted, the feeds were dried in a ventilated oven at 45°C for about 12 h and then stored in a freezer at −20°C.
Disease-free and healthy juvenile turbots were obtained from a commercial fish farm in Penglai, Shandong Province, China. They were fed FM diet for 2 weeks to acclimate to the experimental conditions before the start of feeding trial. Then, turbots with similar sizes (initial mean weight, 8.29 ± 0.13 g) were randomly distributed to 12 fiberglass tanks (300 L) with 40 per tank; fish in three tanks were fed FM and 7S diet, respectively, and fish in six tanks were fed 7S diet for the first 3 weeks and FM diet for another 7 weeks (7S&FM). They were carefully hand-fed until apparent satiation twice daily (08:00 and 17:00). Uneaten feed was collected from the tank outlets and dried for the calculation of FI. Each aquarium was supplied with free-flowing water. Seawater was pumped from the adjacent coast to the experiment base; passed through sand filters, froth separator, and biofilter in turn; and finally flowed into each tank at a rate of 2 L min–1. The system water was exchanged at 50% each day with new water. Continuous aeration was supplied with a single air-stone connected to a central air blower. During the feeding trial, the water temperature ranged from 15 to 17°C; salinity was about 35‰; dissolved oxygen was higher than 6 mg L–1.
According to the relevant references and our previous study on soybean-induced enteritis of fish, 3 weeks of the development of enteritis and 3 weeks of the recovery of enteritis can be seen clearly when 33% soybean meal was added in the diet of Atlantic salmon (Baeverfjord and Krogdahl, 1996). There is also a similar research investigating the histological evaluation of the distal intestine at the first day, second day, third day, fifth day, and seventh day when 20% soybean meal was used in the diet of Atlantic salmon, and the first signs of enteritis were revealed at the fifth day (Sahlmann et al., 2013). In turbot, Liu et al. (2018) found that the expression of intestinal tight junction proteins was suppressed by soybean meal since the second week. Indeed, we sampled the intestines of turbot fed diet with 7S at the zeroth day, first day, third day, fifth day, first week, second week, third week, sixth week, eighth week, and 10th week. Then, the representative time points, according to the results in the present study, including the zeroth day, first day, third day, first week, second week, third week, sixth week, and 10th week, were used in this manuscript to give a clearer change.
The serum and distal intestines were taken at the zeroth day, third day, first week, second week, third week, sixth week, and 10th week after anesthetizing with eugenol (1:10,000) (purity 99%, Shanghai Reagent Corp., Shanghai, China) from three of six tanks fed 7S&FM. At the termination of the experiment, fish were fasted for 24 h before harvest. Total number and body weight of turbot in the FM group (three tanks), the 7S group (three tanks), and the other three tanks in the 7S&FM group were counted and measured. Six distal intestinal tissue samples from six fish were taken and fixed at neutral formalin solution (10%) for histological evaluation and another nine distal intestinal tissue samples from nine fish were taken and frozen in liquid nitrogen immediately and stored at −80°C for gene expression analysis. All distal intestinal tissue samples were dissected from the middle part of distal intestines (about 1 cm in length). Blood was withdrawn using a syringe from the caudal vein of fish and stored at 4°C until it clotted. Serum was collected after centrifugation (4000 g, 10 min, 4°C) and stored at −80°C as separate aliquots until analysis.
Distal intestine was fixed routinely in the neutral formalin solution (10%) for 24 h and then transferred to 70% ethanol for better long-term preservation. According to standard histology procedures, distal intestine tissues were dehydrated in ethanol, equilibrated in xylene, and embedded in paraffin wax. Tissue sections with approximate thickness of 5 μm were cut and stained with H&E. In addition, the slides were examined under a light microscope (DP72; Olympus, Tokyo, Japan) equipped with a camera (E 600, Nikon, Tokyo, Japan) and an image acquisition software (CellSens Standard, Olympus, Tokyo, Japan) for the presence of degenerative changes of epithelial cells. Intestinal histology was evaluated mainly according to the following criteria: (1) the widening of the central LP within the intestinal folds, with increased amounts of connective tissue; (2) the infiltration of a mixed leukocyte population in the LP; (3) the infiltration of a mixed leukocyte population in the submucosa layer; (4) the disorder arrangement of MV; and (5) the increase of the number of GCs. The degree of change of the distal intestine histological features was graded into normal structure; slight enteritis; moderate enteritis; and severe enteritis for more concise statistics of the state of enteritis in all samples (Baeverfjord and Krogdahl, 1996; Krogdahl et al., 2003; Silva et al., 2015; Gu et al., 2016).
According to the manufacturer’s instructions, plasma DAO activity was measured by DAO assay kit (A088-1, Nanjing Jiancheng Bioengineering Institute, China). A total of 80 μL of plasma was added to the test tube, mixed with 800 μL of the reaction mixture, and incubated at 37°C for 10 min. The optical density was measured at 340 nm. The results were expressed as unit per liter. The level of D-lactate in plasma was measured by D-lactate ELISA kit (H263, Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. The test samples were prepared with assay buffer in a 96-well plate and then mixed with the reaction mixture for 30 min at room temperature. The optical density was measured at 450 nm. A standard curve was generated by serial dilution of a D-lactate standard solution, and the concentrations of the samples were calculated. The results were expressed as nanomole per milliliter.
To extract and purify the total RNA, distal intestine tissues were ground to powder in liquid nitrogen and added to RNAiso Plus (9109; Takara Biotech, Dalian, China). The integrity of RNA was evaluated by electrophoresis using 1.2% denatured agarose gel and then followed by concentration determination with Nano-Drop®ND1000 (Nano-Drop Technologies, Wilmington, DE, United States). The 260/280 nm absorbance ratios of all samples were close 2.0, and ratios were similar and stable, indicating a satisfactory purity of the RNA samples. Then, RNA was reversed transcribed to cDNA by PrimeScript®RT reagent Kit with gDNA Eraser (Perfect Real Time, Takara, Japan) following the manufacturer’s instructions (Lu et al., 2017; Xu et al., 2017).
The gene expression stability of reference gene GAPDH was assessed, whose mRNA levels in the distal intestine are stable among all the samples in all the time points of the 7S&FM group. Specific primers for target genes and housekeeping genes (Table 2) were synthesized by Qingke (Shandong, China) and then assessed to determine the application efficiency. Real-time PCR was conducted in a quantitative thermal cycler (Mastercyclerep realplex, Eppendorf, Germany) in a final volume of 25 μL containing 12.5 μL of 2 × SYBR Green Real-time PCR Master Mix [SYBR® Premix Ex TaqTM (Tli RNaseH Plus)] (TaKaRa, Japan), 1 μL (10 μM) of each forward and reverse primer, 1 μL of 200 ng/μL complementary DNA template, and 9.5 μL of dH2O. PCR conditions began with 2 min at 95°C, followed by 35 cycles of 15 s at 95°C, 15 s at 58°C, and 20 s at 72°C. A melting curve analysis (1.85°C increment/min from 58 to 95°C) was performed after each amplification phase to confirm amplification of one product only. The gene expression levels were calculated using the 2–ΔΔCT method (Livak and Schmittgen, 2001).
Survival (%) = 100 × final amount of fish/initial amount of fish
SGR (% day–1) = 100 × (lnWt − lnW0)/t
FI (% day–1) = 100 × feed consumed × 2/(W0 + Wt)/t
FER = (W0 − Wt)/feed consumed
Where Wt and W0 are final and initial fish weight, respectively; t is the duration of experimental days; feed and protein consumed are calculated on a dry matter basis.
For data not analyzed in the previous section, one-way ANOVA was performed using SPSS 17.0 for Windows, followed by the Tukey’s multiple-range test. Tank means were used as the statistical unit in the analyses. Differences were regarded as significant when P < 0.05 and the results are presented as means ± standard error.
The survival of experimental fish was greater than 99% in each group. The SGR, FER, and final weight in the FM and 7S&FM group were significantly higher than that in the 7S group (P < 0.05); however, the FI in the 7S group was the highest (P < 0.05). There is no significant difference of final weight, SGR, FER, and FI between the FM group and the 7S&FM group (P > 0.05) (Table 3).
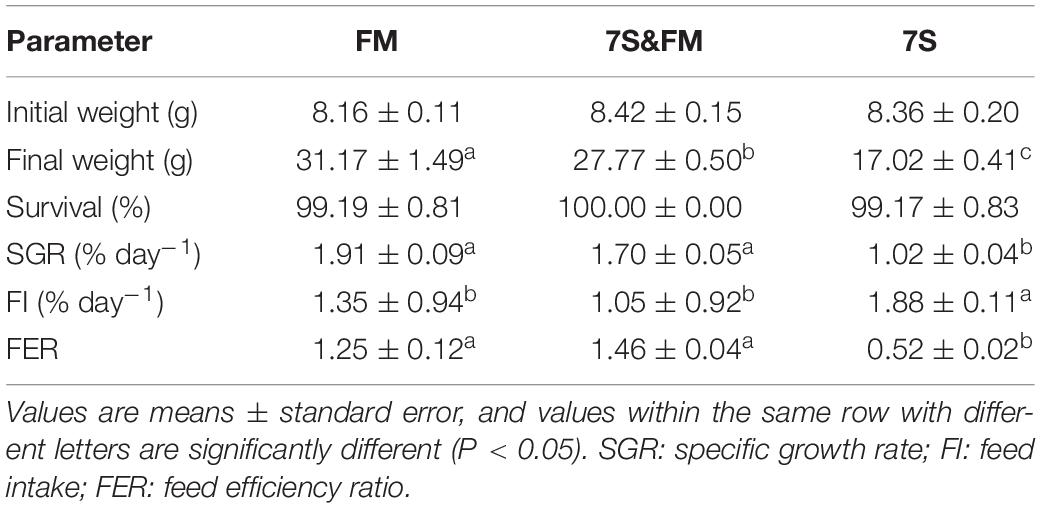
Table 3. Effects of growth performance and feed utilization of the FM group, 7S&FM group, and 7S group.
The first signs of enteritis are visible at the third day, which are the slight widening of LP and the disordered arrangement of MV in one of six fish. All fish showed the widening of LP, the disordered arrangement of MV, and the increased number of GCs at the first and second week. At the third week, all sampled fish showed profound inflammatory cell infiltration of LP. At the sixth week, the symptoms of enteritis were the widening of LP in two of six fish and the slight widening of LP in four of six fish; meanwhile, the disordered arrangement of MV was obviously improved. At the 10th week, only the slight widening of LP was observed (Figure 2, Supplementary Figure S1, and Table 4).
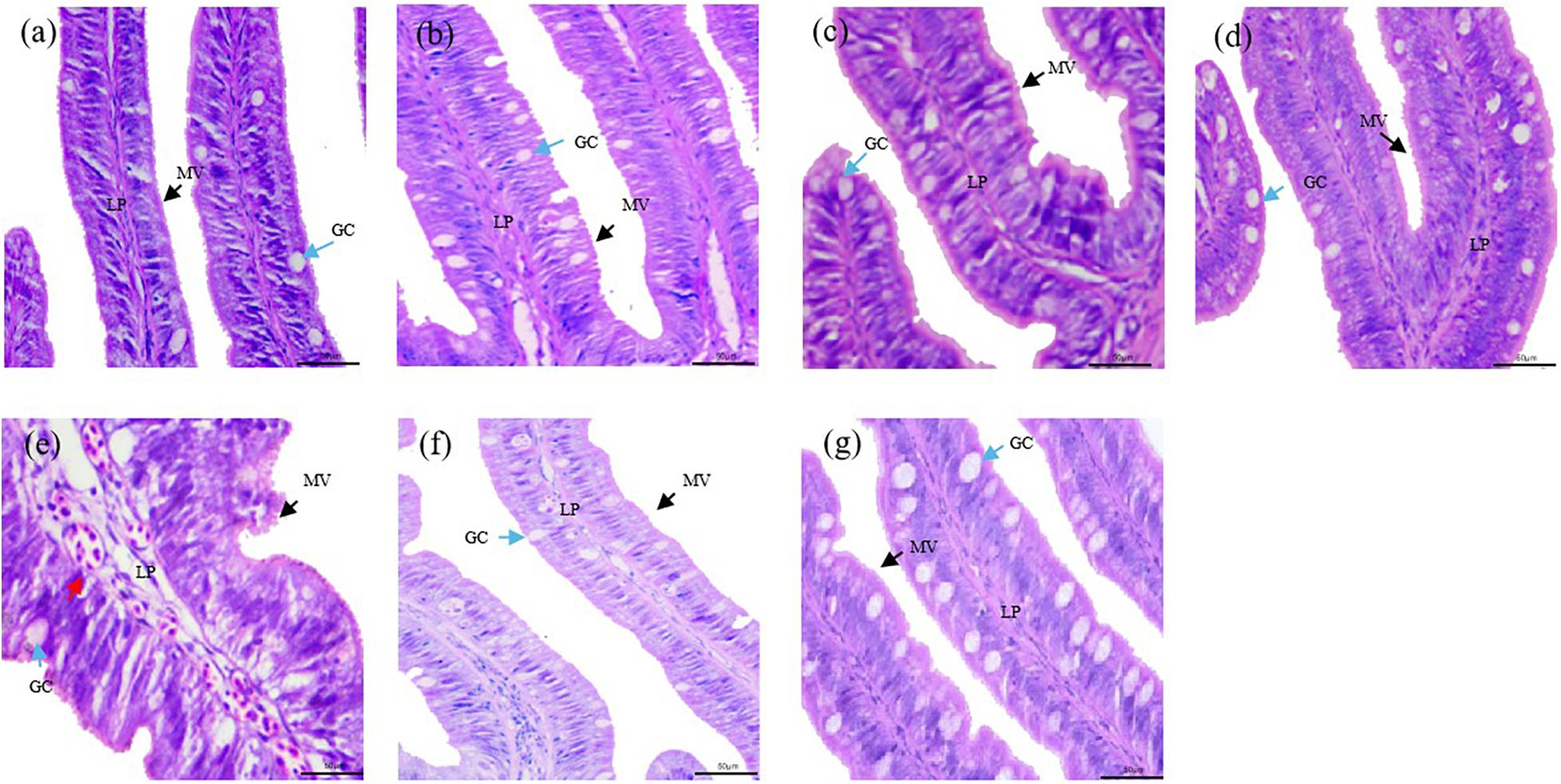
Figure 2. Histological changes of the distal intestine section from turbot in the 7S&FM group at the zeroth day (a), the third day (b), the first week (c), the second week (d), the third week (e), the sixth week (f), and the 10th week (g). MV, microvilli (black arrows); GC, goblet cells (blue arrows); infiltration of mixed leukocytes (red arrows); LP, lamina propria. Scale bar, 50 μm. Staining: H&E.
The activity of serum DAO and the content of D-lactate significantly increased from the zeroth day to the third week and then showed continuous declination till the 10th week (P < 0.05) (Figure 3).
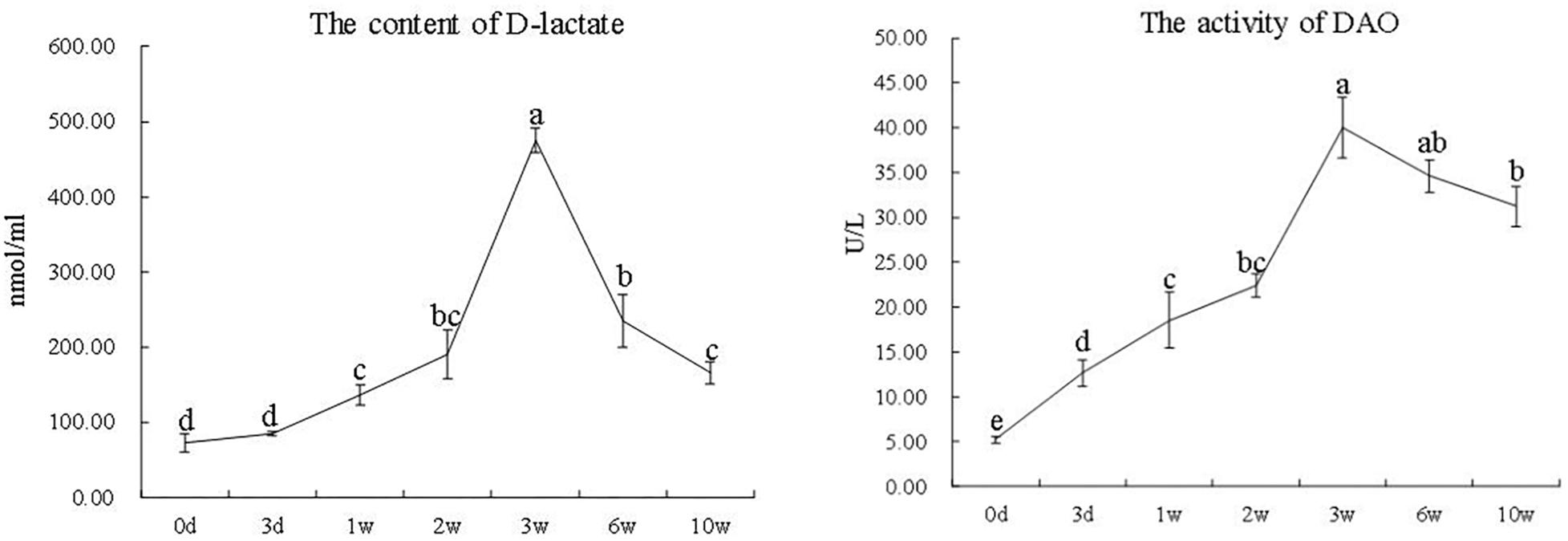
Figure 3. The activity of DAO and the content of D-lactate in serum at different time points in the 7S&FM group. Results are expressed as means ± standard error. Different letters above the broken lines denote significant (P < 0.05) differences at different time points.
During the first 3 weeks, the gene expression of IL-1β, IL-8, IL-22, and NF-κB p65 was significantly increased (P < 0.05), whereas the gene expression of TGF-β was significantly decreased (P < 0.05). The gene expression of IFN-γ was significantly increased from the second week to the sixth week (P < 0.05). Compared with the zeroth day, the gene expression of IL-1β, IL-8, IL-22, and IFN-γ, as well as NF-κB p65, all showed no difference at the 10th week (P > 0.05) (Figure 4).
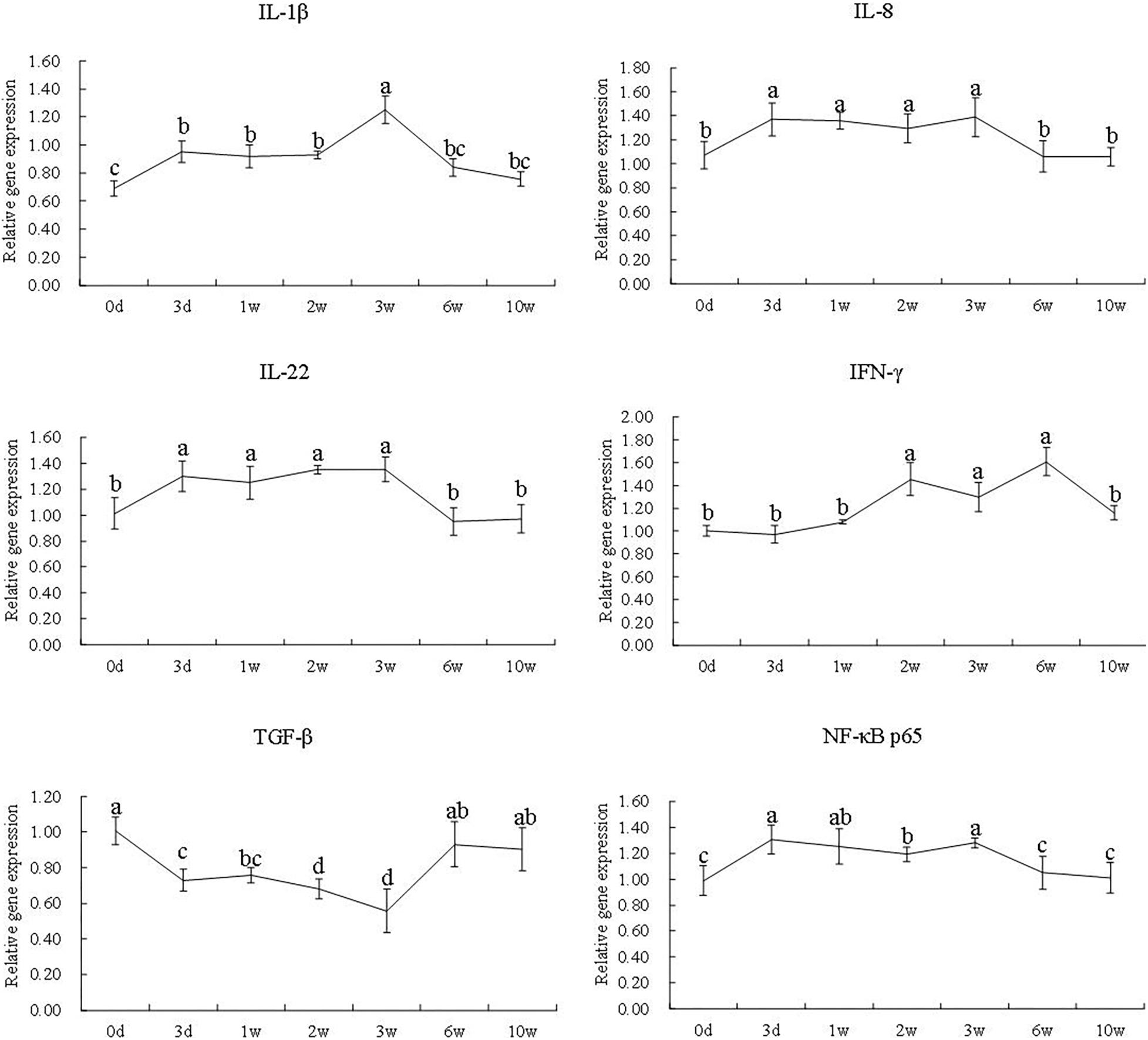
Figure 4. The gene expression of IL-1β, IL-8, IL-22, IFN-γ, TGF-β, and NF-κB p65 at different time points in the 7S&FM group. Results are expressed as means ± standard error. Different letters above the broken lines denote significant (P < 0.05) differences at different time points.
The gene expression of occludin, claudin-like, and claudin-4 significantly declined during the first 3 weeks (P < 0.05). The gene expression of claudin-3 was significantly down-regulated from the third week to the 10th week (P < 0.05). At the second and third week, the gene expression of ZO-1 was significantly decreased (P < 0.05) and the gene expression of MLCK was significantly increased (P < 0.05). Then, at the 10th week, the gene expression of occludin, claudin-like, claudin-4, claudin-3, and ZO-1 was still significantly lower than that of the zeroth day except for MLCK (P < 0.05) (Figure 5).
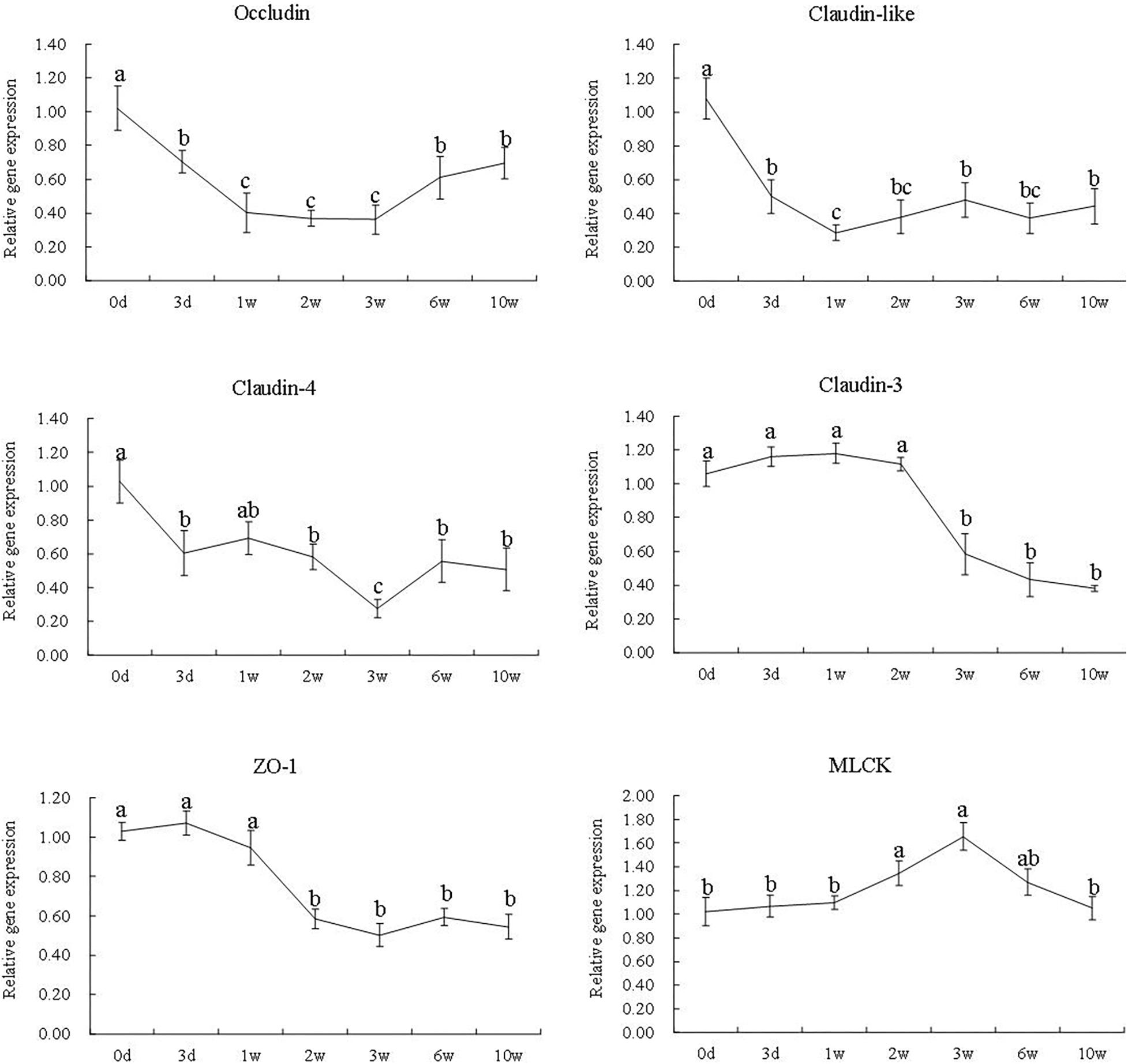
Figure 5. The gene expression of occludin, claudin-like, claudin-4, claudin-3, ZO-1, and MLCK at different time points in the 7S&FM group. Results are expressed as means ± standard error. Different letters above the broken lines denote significant (P < 0.05) differences at different time points.
In this study, the turbot got enteritis when fish was fed 7S diet in the first 3 weeks. The mild symptoms of enteritis were observed in the distal intestines at the first week and all samples of distal intestine exhibited typical symptoms of enteritis at the third week. The relief of enteritis was observed after feeding FM diet in the next 7 weeks. Similar research in Atlantic salmon showed that diet with 33% soybean meal induced severe enteritis of distal intestine within the first week and the symptoms continued to be worse until the third week; however, enteritis was alleviated after converting to FM diet for another 3 weeks (Baeverfjord and Krogdahl, 1996). The faster enteritis development and recovery in Atlantic salmon than that in turbot might be because of the difference in fish species and the use of soybean meal or 7S in diet. In carp, when 20% soybean meal was added in diet, the symptoms of enteritis got worse from the first week to the fourth week but the symptoms got better spontaneously at the fifth week (Urán et al., 2008). The higher adaptability of plant ingredients of omnivorous fish may be the main reason for their recovery from enteritis spontaneously. Results above showed a clear process of the development and recovery of enteritis in turbot induced by 7S.
Diamine oxidase is an intracellular enzyme that is mainly found in the intestinal epithelial cell, and D-lactate is a metabolic product of bacteria that is mainly found in the intestinal mucosa (Luk et al., 1980; Nielsen et al., 2011). The intestine provides an intact mucosal barrier function to prevent DAO and D-lactate infiltrating the portal blood; therefore, they could be used as the symbols of intestinal mucosal injury (Luk et al., 1980; Wu et al., 2016). In the current study, the activity of DAO and the content of D-lactate in serum remarkably increased in the first 3 weeks and then significantly decreased till the 10th week. The previous research showed that the activity of DAO and the content of D-lactate in serum of piglet fed with diet 7S gradually increased from the third week to the fifth week (Wu et al., 2016). Collectively, the results of the histological structure of distal intestine as well as the activity of DAO and the content of D-lactate in serum showed the accordance tendency during the 10 weeks.
Cytokines are a group of biologically active molecules, which act as modulators of the immune responses (Tarakanov et al., 2005; Rauta et al., 2012). In general, the pro-inflammatory cytokines, such as IL-1β, IL-8, IL-22, and IFN-γ, and the anti-inflammatory cytokines, such as TGF-β, are all involved in both the initiation and amplification of enteritis (Opal and Depalo, 2000; Al-Sadi et al., 2012; Wang J. et al., 2017). NF-κB is considered to play an important role in regulating inflammatory response (Peng et al., 2018). In mice and piglets, the secretion of IFN-γ and IL-8 as well as the activation of NF-κB were remarkably induced by dietary 7S (Hao et al., 2009; Sun et al., 2013; Peng et al., 2018). Similar results were observed in the present study; the excessive expression of IL-1β, IL-8, IL-22, IFN-γ, and NF-κB p65 of turbot was induced by dietary 8% 7S in the first 3 weeks. Meanwhile, the gene expression of TGF-β was remarkably reduced. Li et al. (2017) also showed the excessive expression of IL-1β and the decreased expression of TGF-β in the distal intestine of turbot fed with the diet containing 4% 7S for 12 weeks. Consistent with the results of intestinal histology, the gene expression of IL-1β, IL-8, IL-22, IFN-γ, and NF-κB p65 was significantly decreased after feeding FM diet, and the opposite trend was observed in the gene expression of TGF-β. Consequently, the secretion of cytokines induced by 7S was mitigated effectively by FM. However, the results of intestinal tight junction proteins were different.
Generally, tight junction serves as an important physical barrier to protect the intestine, and the damaged structure of the tight junction could facilitate the invasion of pathogen and pathogenic bacteria from intestinal lumen (Le et al., 2008; Turner, 2009; Sturgeon and Fasano, 2016; Takiishi et al., 2017). Claudins are the major barrier-forming proteins of tight junction structure (Wang Y. et al., 2017). Occludin is essential for some mechanisms of tight junction regulation and responsible for part of the structural function of tight junction (Buckley and Turner, 2017). ZO-1 is a scaffolding protein that anchors the tight junction transmembrane proteins to the actin cytoskeleton to regulate the recruitment and assembly of tight junction (Fanning et al., 2002; Umeda et al., 2004; Odenwald et al., 2018). It has been reported that the gene expression of occludin and ZO-1 was decreased by dietary 7S in piglet (Zhao et al., 2014). In the present study, dietary 7S remarkably suppressed the gene expression of claudins, occludin, and ZO-1. Meanwhile, the results showed that the response of occludin and claudins is faster than that of ZO-1. The difference is largely unknown at present; however, claudins are outermost proteins of tight junction that might be easier disrupted than other tight junction proteins in the pathological complex intercellular environment. For instance, research on human gastrointestine showed that some claudins might be functionally incapacitated by binding to pathogens through extracellular receptors (Shrestha and Mcclane, 2013). MLCK regulates the contraction of the perijunctional actin ring by causing phosphorylation of MLC, which could induce disruption of tight junction (Zolotarevsky et al., 2002; Clayburgh et al., 2004; Shen and Turner, 2005). It has been confirmed that the reduction of the expression of ZO-1 is related to the activation of MLCK (Shen et al., 2006), and the recovery of ZO-1 following the inhibition of MLCK activity has been reported as well (Yu et al., 2010). Interestingly, at the sixth and 10th week, the gene expression of MLCK had declined remarkably, while the gene expression of ZO-1 still showed no increase. The reason is largely unknown presently; furthermore, there is a research showing that the damage of tight junction was not correctable by the inhibition of MLCK, which might be due to intestinal epithelial apoptosis (Zolotarevsky et al., 2002). The research in piglets showed that apoptosis was a probable way of the disruption of intestinal tight junction caused by 7S both in vivo and in vitro (Chen et al., 2011). In this study, the expression of intestinal tight junction proteins did not increase significantly after feeding FM diet. Besides, research on the dynamic regulation of tight junction is inadequate, and more researches are needed to investigate the impaired and recovery mechanism of tight junction.
In summary, the present results first showed a clear process of enteritis induced by dietary 8% 7S and the recovery by FM in turbot. During the process, the dynamic responses of intestinal inflammatory cytokines and tight junction proteins were quite different, especially the responses of different tight junction proteins. Besides, both NF-κB p65 and MLCK were involved in enteritis. The present results provided new insight into the mitigation methods of 7S-induced enteritis in fish.
All datasets analyzed for this study are included in the article/Supplementary Material.
The animal study was reviewed and approved by the Animal Care Committee of Ocean University of China.
YZ, KM, and JZ designed the study. JZ, PY, and JD performed the experiments. JZ, GY, WO, and WX performed the analyses. JZ and PY analyzed the data. JZ and YZ wrote the article. All authors read and approved the final version of the manuscript.
This work was financially supported by the National Natural Science Foundation of China (Nos. 31872577 and 41576137), the Youth Talent Program supported by the Laboratory for Marine Fisheries Science and Food Production Processes, the Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018-MFS-T10), and China Agriculture Researches System (Grant No. CARS 47-G10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are very grateful to Prof. Qinghui Ai and Prof. Wenbing Zhang for their help in writing the article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00198/full#supplementary-material
FIGURE S1 | Histological changes of the distal intestine section from turbot in 7S&FM group at the zero day (A), the third day (B), the first week (C), the second week (D), the third week (E), the sixth week (F) and the tenth week (G). MF: mucosal fold; MM: muscularis mucosa; SML: submucous layer; Scale bar, 500 μm. Staining: H & E.
FIGURE S2 | The original blots of SDS-PAGE. The lines from left to right are 7S, 11S, protein marker, 7S, 11S, protein marker, 11S and 11S. The part in the frame was used in the article.
7S, β-conglycinin; DAO, diamine oxidase; FER, feed efficiency ratio; FI, feed intake; FM, fish meal; GAPDH, glyceraldehyde-3-phosphatedehydrogenase; GC, goblet cell; H&E, hematoxylin and eosin; IFN- γ, interferon- γ; IL-1 β, interleukin-1 β; IL-8, interleukin-8; IL-22, interleukin-22; LP, lamina propria; MF, mucosal fold; MLC, myosin II regulatory light chain; MLCK, myosin light chain kinase; MM, muscularis mucosa; MV, microvilli; NF- κ B, nuclear factor-kappa B; SGR, specific growth rate; SML, submucous layer; TGF- β, transforming growth factor- β; TNF- α, tumor necrosis factor- α; ZO-1, zonula occludens-1.
Al-Sadi, R., Guo, S., Dokladny, K., Smith, M. A., Ye, D., Kaza, A., et al. (2012). Mechanism of interleukin-1β induced-increase in mouse intestinal permeability, in vivo. J. Interf. Cytokine Res. 32, 474–484. doi: 10.1089/jir.2012.0031
Baeverfjord, G. T., and Krogdahl, A. (1996). Development and regression of soybean meal induced enteritis in atlantic salmon, Salmo salar L. Distal Intestine: a comparison with the intestines of fasted fish. J. Fish Dis. 19, 375–387. doi: 10.1046/j.1365-2761.1996.d01-92.x
Balda, M. S., and Karl, M. (2008). Tight junctions at a glance. J. Cell Sci. 121, 3677–3682. doi: 10.1242/jcs.023887
Buckley, A., and Turner, J. R. (2017). Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 10:a029314. doi: 10.1101/cshperspect.a029314
Chen, F., Hao, Y., Piao, X. S., Ma, X., and Wang, J. J. (2011). Soybean-derived beta-conglycinin affects proteome expression in pig intestinal cells in vivo and in vitro. J. Anim. Sci. 89, 743–753. doi: 10.2527/jas.2010-3146
Chen, K., Zhou, X. Q., Jiang, W. D., Wu, P., Liu, Y., Jiang, J., et al. (2018). Impaired intestinal immune barrier and physical barrier function by phosphorus deficiency: regulation of tor, NF-κB, MLCK, JNK and Nrf2 signalling in grass carp (Ctenopharyngodon idella) after infection with, aeromonas hydrophila. Fish Shellfish Immunol. 74, 175–189. doi: 10.1016/j.fsi.2017.12.060
Chen, Z., Liu, Y., Li, Y., Yang, P., Hu, H., Yu, G., et al. (2018). Dietary arginine supplementation mitigates the soybean meal induced enteropathy in juvenile turbot, Scophthalmus maximus L. Aquacult. Res. 49, 1535–1545. doi: 10.1111/are.13608
Clayburgh, D. R., Rosen, S., Witkowski, E. D., Wang, F., Blair, S., Dudek, S., et al. (2004). A differentiation-dependent splice variant of myosin light chain kinase, mlck1, regulates epithelial tight junction permeability. J. Biol. Chem. 279, 55506–55513. doi: 10.1074/jbc.M408822200
Fanning, A. S., Ma, T. Y., and Anderson, J. M. (2002). Isolation and functional characterization of the actin-binding region in the tight junction protein zo-1. FASEB J. 16, 1835–1837. doi: 10.1096/fj.02-0121fje
Friedrich, M., Pohin, M., and Powrie, F. (2019). Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50, 992–1006. doi: 10.1016/j.immuni.2019.03.017
Gu, M., Bai, N., Zhang, Y., and Krogdahl, A. (2016). Soybean meal induces enteritis in turbot Scophthalmus maximus at high supplementation levels. Aquaculture 464, 286–295. doi: 10.1016/j.aquaculture.2016.06.035
Guo, P., Piao, X., Ou, D., Li, D., and Hao, Y. (2007). Characterization of the antigenic specificity of soybean protein beta-conglycinin and its effects on growth and immune function in rats. Arch. Anim. Nutr. 61, 189–200. doi: 10.1080/17450390701318358
Hao, Y., Zhan, Z. F., Guo, P. F., Piao, X., and Li, D. (2009). Soybean β-conglycinin-induced gut hypersensitivity reaction in a piglet model. Arch. Anim. Nutr. 63, 188–202. doi: 10.1080/17450390902860026
Hee, L. S. (2015). Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest. Res. 13, 11–18. doi: 10.5217/ir.2015.13.1.11
Jiang, W. D., Hu, K., Zhang, J. X., Liu, Y., Jiang, J., Wu, P., et al. (2015). Soyabean glycinin depresses intestinal growth and function in juvenile jian carp (Cyprinus carpio var jian): protective effects of glutamine. Br. J. Nutr. 114, 1569–1583. doi: 10.1017/S0007114515003219
Krishnan, H. B., Kim, W. S., Jang, S., and Kerley, M. S. (2009). All three subunits of soybean β-conglycinin are potential food allergens. J. Agric. Food Chem. 57, 938–943. doi: 10.1021/jf802451g
Krogdahl, A., BakkecmcKellep, A. M., and Baeverfjord, G. (2003). Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquacult. Nutr. 9, 361–371. doi: 10.1046/j.1365-2095.2003.00264.x
Lamia, L., and Boye, J. I. (2007). Allergenicity of soybean: new developments in identification of allergenic proteins, cross-reactivities and hypoallergenization technologies. Crit. Rev. Food Sci. Nutr 47, 127–143. doi: 10.1080/10408390600626487
Le, S., Weber, C. R., and Turner, J. R. (2008). The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 181, 683–695. doi: 10.1083/jcb.200711165
Li, Y., Hu, H., Liu, J., Yang, P., Zhang, Y., Ai, Q., et al. (2017). Dietary soya allergen β-conglycinin induces intestinal inflammatory reactions, serum-specific antibody response and growth reduction in a carnivorous fish species, turbot, Scophthalmus maximus L. Aquacult. Res. 48, 4022–4037. doi: 10.1111/are.13224
Liu, X., Feng, J., Xu, Z. R., Wang, Y. Z., and Liu, J. X. (2008). Oral allergy syndrome and anaphylactic reactions in balb/c mice caused by soybean glycinin and β-conglycinin. Clin. Exp. Allergy 38, 350–356. doi: 10.1111/j.1365-2222.2007.02893.x
Liu, Y., Chen, Z., Dai, J., Yang, P., Hu, H., Ai, Q., et al. (2018). The protective role of glutamine on enteropathy induced by high dose of soybean meal in turbot. Scophthalmus maximus, L. Aquaculture 497, 510–519. doi: 10.1016/j.aquaculture.2018.08.021
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001
Lu, K. L., Wang, L. N., Zhang, D. D., Liu, W. B., and Xu, W. N. (2017). Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Physiol. Biochem. 43, 65–76. doi: 10.1007/s10695-016-0268-5
Luk, G. D., Bayless, T. M., and Baylin, S. B. (1980). Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J. Clin. Invest. 66, 66–70. doi: 10.1172/JCI109836
Luo, J. B., Feng, L., Jiang, W. D., Liu, Y., Wu, P., Jiang, J., et al. (2014). The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol. 40, 197–207. doi: 10.1016/j.fsi.2014.07.003
Nielsen, C., Lindholt, J. S., Erlandsen, E. J., and Mortensen, F. V. (2011). D-lactate as a marker of venous-induced intestinal ischemia: an experimental study in pigs. Int. J. Surg. 9, 428–432. doi: 10.1016/j.ijsu.2011.04.004
Odenwald, M. A., Choi, W., Kuo, W. T., Singh, G., Sailer, A., Wang, Y., et al. (2018). The scaffolding protein zo-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J. Biol. Chem. 293, 17317–17335. doi: 10.1074/jbc.RA118.003908
Opal, S. M., and Depalo, V. A. (2000). Anti-inflammatory cytokines. Chest 117, 1162–1172. doi: 10.1378/chest.117.4.1162
Oshima, T., and Miwa, H. (2016). Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 51, 768–778. doi: 10.1007/s00535-016-1207-z
Peng, C., Cao, C., He, M., Shu, Y., Tang, Y., Wang, Y., et al. (2018). Soybean glycinin and β-Conglycinin-induced intestinal damage in piglets via the p38/JNK/NF-κB signaling pathway. J. Agric. Food Chem. 66, 9534–9954. doi: 10.1021/acs.jafc.8b03641
Quintans, J. S. S., Saravanan, S., Heimfarth, L., Araújo Adriano Antunes, S., Almeida, J. R. G. D. S., Picot, L., et al. (2019). Monoterpenes modulating cytokines - a review. Food Chem. Toxicol. 123, 233–257. doi: 10.1016/j.fct.2018.10.058
Rauta, P. R., Nayak, B., and Das, S. (2012). Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms. Immunol. Lett. 148, 23–33. doi: 10.1016/j.imlet.2012.08.003
Rumsey, G. L., Siwicki, A. K., Anderson, D. P., and Bowser, P. R. (1994). Effect of soybean protein on serological response, non-specific defense mechanisms, growth, and protein utilization in rainbow trout. Vet. Immunol. Immunopathol. 41, 323–339. doi: 10.1016/0165-2427(94)90105-8
Sahlmann, C., Sutherland, B. J. G., Kortner, T. M., Koop, B., Krogdahl, A., and Bakke, A. M. (2013). Early response of gene expression in the distal intestine of Atlantic salmon (Salmo salar L.) during the development of soybean meal induced enteritis. Fish Shellfish Immunol. 34, 599–609. doi: 10.1016/j.fsi.2012.11.031
Shen, L., Black, E. D., Witkowski, E. D., Lencer, W. I., Guerriero, V., and Schneeberger, E. E. (2006). Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 119, 2095–2106. doi: 10.1242/jcs.02915
Shen, L., and Turner, J. R. (2005). Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell 16, 3919–3936. doi: 10.1091/mbc.E04-12-1089
Shrestha, A., and Mcclane, B. A. (2013). Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of clostridium perfringens enterotoxin. mBio 4:e00594-12. doi: 10.1128/mBio.00594-12
Silva, P. F., Mcgurk, C., Knudsen, D. L., Adams, A., Thompson, K. D., and Bron, J. E. (2015). Histological evaluation of soya bean-induced enteritis in Atlantic salmon (Salmo salar L.): Quantitative image analysis vs. semi-quantitative visual scoring. Aquaculture 44, 542–556. doi: 10.1016/j.aquaculture.2015.04.002
Sturgeon, C., and Fasano, A. (2016). Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 4:e1251384. doi: 10.1080/21688370.2016.1251384
Sun, H., Liu, X., Wang, Y. Z., Liu, J. X., and Feng, J. (2013). Allergen-specific immunoglobulin, histamine and T-cell responses induced by soybean glycinin and b-conglycinin in balb/c mice of oral sensitisation. Food Agricult. Immunol. 24, 489–501. doi: 10.1080/09540105.2012.730501
Takiishi, T., Fenero, C. I. M., and Câmara, N. O. S. (2017). Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers 5:e1373208. doi: 10.1080/21688370.2017.1373208
Tarakanov, A. O., Goncharova, L. B., and Tarakanov, O. A. (2005). A Cytokine Formal Immune Network. European Conference on Artificial Life. Berlin: Springer, doi: 10.1007/11553090_52
Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. doi: 10.1038/nri2653
Umeda, K., Matsui, T., Nakayama, M., Furuse, K., Sasaki, H., Furuse, M., et al. (2004). Establishment and characterization of cultured epithelial cells lacking expression of zo-1. J. Biol. Chem. 279, 44785–44794. doi: 10.1074/jbc.M406563200
Urán, P. A., Gonçalves, A. A., Taverne-Thiele, J. J., Schrama, J. W., Verreth, J. A. J., and Rombout, J. H. W. M. (2008). Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 25, 0–760. doi: 10.1016/j.fsi.2008.02.013
Wang, J., Kong, M., Zhou, Z., Yan, D., Yu, X., Cheng, X., et al. (2017). Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydr. Polym. 157, 596–602. doi: 10.1016/j.carbpol.2016.10.021
Wang, Y., Mumm, J. B., Herbst, R., Kolbeck, R., and Wang, Y. (2017). IL-22 increases permeability of intestinal epithelial tight junctions by enhancing claudin-2 expression. J. Immunol. 199, 3316–3325. doi: 10.4049/jimmunol.1700152
Wu, J. J., Cao, C. M., Ren, D. D., Zhang, Y., Kou, Y. N., Ma, L. Y., et al. (2016). Effects of soybean antigen proteins on intestinal permeability, 5-hydroxytryptamine levels and secretory IgA distribution in the intestine of weaned piglets. Italian J. Anim. Sci. 15, 174–180. doi: 10.1080/1828051X.2016.1148559
Wu, P., Tian, L., Zhou, X. Q., Jiang, W. D., Liu, Y., Jiang, J., et al. (2018). Sodium butyrate enhanced physical barrier function referring to Nrf2, JNK and MLCK signaling pathways in the intestine of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 73, 121–132. doi: 10.1016/j.fsi.2017.12.009
Xu, H., Cao, L., Zhang, Y., Johnson, R. B., Wei, Y., Zheng, K., et al. (2017). Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis), depending on fish gender and maturation stage. Aquaculture 468, 378–385. doi: 10.1016/j.aquaculture.2016.11.002
Xu, J., Zhou, A. G., Wang, Z. S., and Ai, D. (2010) Effects of glycinin and β-conglycinin on integrity and immune responses of mouse intestinal epithelium cells. J. Anim. Plant Sci. 20, 170–174.
Yu, D., Marchiando, A. M., Weber, C. R., Raleigh, D. R., Wang, Y., Shen, L., et al. (2010). Mlck-dependent exchange and actin binding region-dependent anchoring of zo-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. U.S.A. 107, 8237–8241. doi: 10.1073/pnas.0908869107
Zhang, J.-X., Guo, L.-Y., Feng, L., Jiang, W.-D., Kuang, S.-Y., Liu, Y., et al.. (2013). Soybean β-conglycinin induces inflammation and oxidation and causes dysfunction of intestinal digestion and absorption in fish. PLoS One 8:e58115. doi: 10.1371/journal.pone.0058115
Zhao, Y., Qin, G., Han, R., Wang, J., Zhang, X., and Liu, D. (2014). β-conglycinin reduces the tight junction occludin and zo-1 expression in IPEC-J2. Int. J. Mol. Sci. 15, 1915–1926. doi: 10.3390/ijms15021915
Keywords: inflammatory cytokines, tight junction proteins, β-conglycinin, enteritis, turbot
Citation: Zheng J, Yang P, Dai J, Yu G, Ou W, Xu W, Mai K and Zhang Y (2020) Dynamics of Intestinal Inflammatory Cytokines and Tight Junction Proteins of Turbot (Scophthalmus maximus L.) During the Development and Recovery of Enteritis Induced by Dietary β-Conglycinin. Front. Mar. Sci. 7:198. doi: 10.3389/fmars.2020.00198
Received: 30 December 2019; Accepted: 13 March 2020;
Published: 24 April 2020.
Edited by:
Chunnuan Zhang, Henan University of Science and Technology, ChinaReviewed by:
Qi Cun Zhou, Ningbo University, ChinaCopyright © 2020 Zheng, Yang, Dai, Yu, Ou, Xu, Mai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjiao Zhang, eWFuamlhb3poYW5nQG91Yy5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.