- 1Oceans and Atmosphere, Commonwealth Scientific and Industrial Research Organisation, St Lucia, QLD, Australia
- 2Van Oord Dredging and Marine Contractors B.V., Rotterdam, Netherlands
- 3Ports and Waterways, Delft University of Technology, Delft, Netherlands
Accelerating the recovery of marine coastal ecosystems is a global challenge that has been attempted on many systems around the world. Restoration efforts have shown varying levels of success at localized-scales, but developing techniques for large-scale application are still in their nascent stage for many systems. For seagrass meadows and marsh plants, large-scale successes have been realized by distributing seeds from moving boats or planes, respectively. Similarly for coral reefs, the harvesting, culturing and releasing of wild coral-spawn slicks to targeted reefs is anticipated to achieve cost-efficient, large-scale restoration of coral communities with low-impact technology. Yet, operational protocols for full-scale application still require development by practitioners. In this study we conducted a field trial to evaluate the actual feasibility of harvesting wild coral-spawn slicks for large-scale restoration activities, incorporating technologies used in oil spill remediation, dredging operations, and land-based aquaculture. Testing the potential for scalability to commercial vessels, our trial focused on concentrating and collecting wild coral-spawn slicks for culturing until settlement competency using an experimental 50,000 L aquaculture facility built on a tugboat. Five objectives were set and all were achieved successfully, with only one requiring further optimization. Overall, this restoration approach allows for long-distance translocation of genetically diverse coral assemblages, and may be combined with other larval conditioning techniques that are being developed to increase the resistance to stress and survival of coral recruits. Most importantly, it is fully scalable to produce billions of coral larvae for delivery to target reefs, with negligible impact to source populations.
Introduction
Habitat degradation is one of the most pervasive global impacts of environmental degradation and threats to biodiversity loss in terrestrial, freshwater and marine ecosystems (Millennium Ecosystem Assessment, 2005; Butchart et al., 2010; Mazor et al., 2018). Habitat degradation occurs through direct human impacts such as land clearing (Foley et al., 2005), coastal development (Lotze et al., 2006), and bottom trawling (Thrush and Dayton, 2002), as well as anthropogenic mediated climate change drivers (Intergovernmental Panel on Climate Change, 2014) that are typically larger in scale than direct human impacts. Globally, all coastal marine habitat providing organisms such as seagrasses, macroalgae, corals and other sessile invertebrates have been impacted by recent extreme warming events (Smale et al., 2019). Given the pervasiveness of habitat degradation, there is increasing recognition that active restoration interventions are necessary in order to ensure the viability of natural ecosystems and their services into the medium-term future (Bullock et al., 2011; Menz et al., 2013). The urgency of the situation is such that the United Nations recently declared the “UN Decade of Ecosystem Restoration 2021-30” that aims to massively scale-up the restoration of degraded and destroyed ecosystems (United Nations Environment Programme, 2019).
It remains unclear whether the current application of traditional coral restoration approaches can be operationalized to achieve effective ecosystem-scale restoration. Recent successes on coral reefs destroyed from dynamite fishing have shown the effective transplantation of fragments onto stable substrata made from steel rods to restore approximately 7,000 m2 (Williams et al., 2019), and increases from 0 to 44% coral cover over 16 years when stable substrata from quarried rocks was provided in rubble fields in an area of reef with abundant larval supply from nearby reefs (Fox et al., 2019). Yet such ‘gardening’ or substrate stabilization approaches aren’t viable options when donor colonies or natural larval supply are limited. In such circumstances, strategies of mass seeding have proven an effective large-scale approach in other coastal marine ecosystems. For example, the distribution of 38 million Zostera seeds from boats throughout a coastal bay system over a period of 11 years restored 1,700 hectares of seagrass habitat (Orth et al., 2012). In coastal saltmarshes, reseeding trials of Spartina using delivery from aeroplanes have also proven effective, with planting occurring at rates of 16 s per hectare (Utomo et al., 2016). Transportation of seeds or larvae derived from nurseries or distant habitats therefore provides a means of supplying degraded habitats with new propagules to aid recovery.
The direct release of vagile larvae from vessels to areas of reef that lack a sufficient supply of larvae provides a ‘reseeding’ approach for coral reefs equivalent to those applied with seeds in seagrass meadows and salt marshes. Recent modeling of industrial-scale harvesting, development, and release of wild coral-spawn slicks onto reef indicates that the approach has the potential to achieve large-scale restoration of coral communities with low impact technology at low cost per colony (Doropoulos et al., 2019). Yet while approaches for the collecting and culturing of coral-spawn slicks for use in coral reef restoration have been trialed using a variety of techniques (Heyward et al., 2002; Omori et al., 2007; Guest et al., 2014; Edwards et al., 2015; de la Cruz and Harrison, 2017), the scales at which they have been applied and their potential for large-scale applications and transportability are limited. Moreover, results from empirical studies using larval saturation techniques in highly localized areas of coral reefs have been mixed, providing nominal (Edwards et al., 2015) and positive (de la Cruz and Harrison, 2017) outcomes for coral recovery. Thus, the methods required to achieve restoration of reefs by harvesting wild spawn at an industrial-scale remain a critical information gap.
Scaling-up environmental restoration efforts to ecologically meaningful scales requires partnerships between scientists, engineers, and industry practitioners (De Vriend and Van Koningsveld, 2012; Gillies et al., 2015). For example, oil spill remediation routinely contains surface oil slicks with large booms that can also be used to contain wild coral-spawn slicks prior to collection (Doropoulos et al., 2019). Dredging operations use large vessels such as trialing suction hoppers dredgers that pump, contain, transport, and deposit fine particles at quantities >20 ton (Laboyrie et al., 2018), which can be adapted for the industrial-scale collection of densely concentrated coral-spawn slicks. Land-based aquaculture industries culture billions of marine bivalve larvae in single facilities (Lucas et al., 2019) that can be applied to abundant coral-spawn slicks. Here, we incorporate these principles in a method to operationalize and test techniques required for the industrial-scale harvesting of coral-spawn slicks and culturing to settlement competency, enabling long distance transportation and release onto targeted reefs. To do so, we spotted coral-spawn slicks from a helicopter, contained them using an oil boom, and transferred them into a 50,000 L aquaculture facility built on the deck of a tugboat (Figure 1) using industrial scale pumps. We addressed the questions below that include 5 objectives to test the feasibility of the approach for further upscaling:

Figure 1. Testing the feasibility of operationalizing the spotting, containing, collecting, and culturing of coral-spawn slicks at industrial-scales. (A) View of a spawn slicks in the open ocean from a helicopter. (B) Containing coral-spawn slick in an oil boom for (C) collection onto the tugboat. (D) Overview of the back deck of the tugboat with the 4,500 L steel (n = 6) and plastic (n = 6) tanks.
1. Concentration – can coral-spawn slicks be encountered and contained (obj. 1) in high enough concentrations to make collection viable?
2. Collection – can coral-spawn be pumped at high abundances (obj. 2) and survival rates (obj. 3) for rapid transfer?
3. Culturing and transportation – can larval culturing at large-scales in transportable containers using materials commonly found on commercial vessels occur at high survival rates (obj. 4) to competency (obj. 5)?
Materials and Methods
Overall Study Approach
Prior to the field-testing component of the study, theoretical pump selection and initial testing and optimization of the pumping system were conducted in a controlled environment. Coral fecundity was then confirmed at Heron Reef in late-October 2019, and the field execution of the trial occurred in late-November 2019. Mass coral-spawning occurred on November 29 and 30, in the wild and by colonies housed on board the vessel. The concentration of live embryos was estimated from wild-slick coral-spawn slicks (n = 11). Four hand collected samples from wild spawn and four from colonies housed on board were pumped and survival estimated. An additional two samples of wild coral-spawn were skimmed and pumped directly from the water surface. All the pumped samples were cultured for up to 102 h in the aquaculture facility. During culturing, larval concentration and survival were estimated every 24 h. At 72 h following spawning, settlement tiles were placed in each 4,500 L culturing tank, and coral settlement was estimated 48 h later. A methodological flow-diagram (Figure 2) presents the overall approach of the study, time-points, and replication at the different stages.
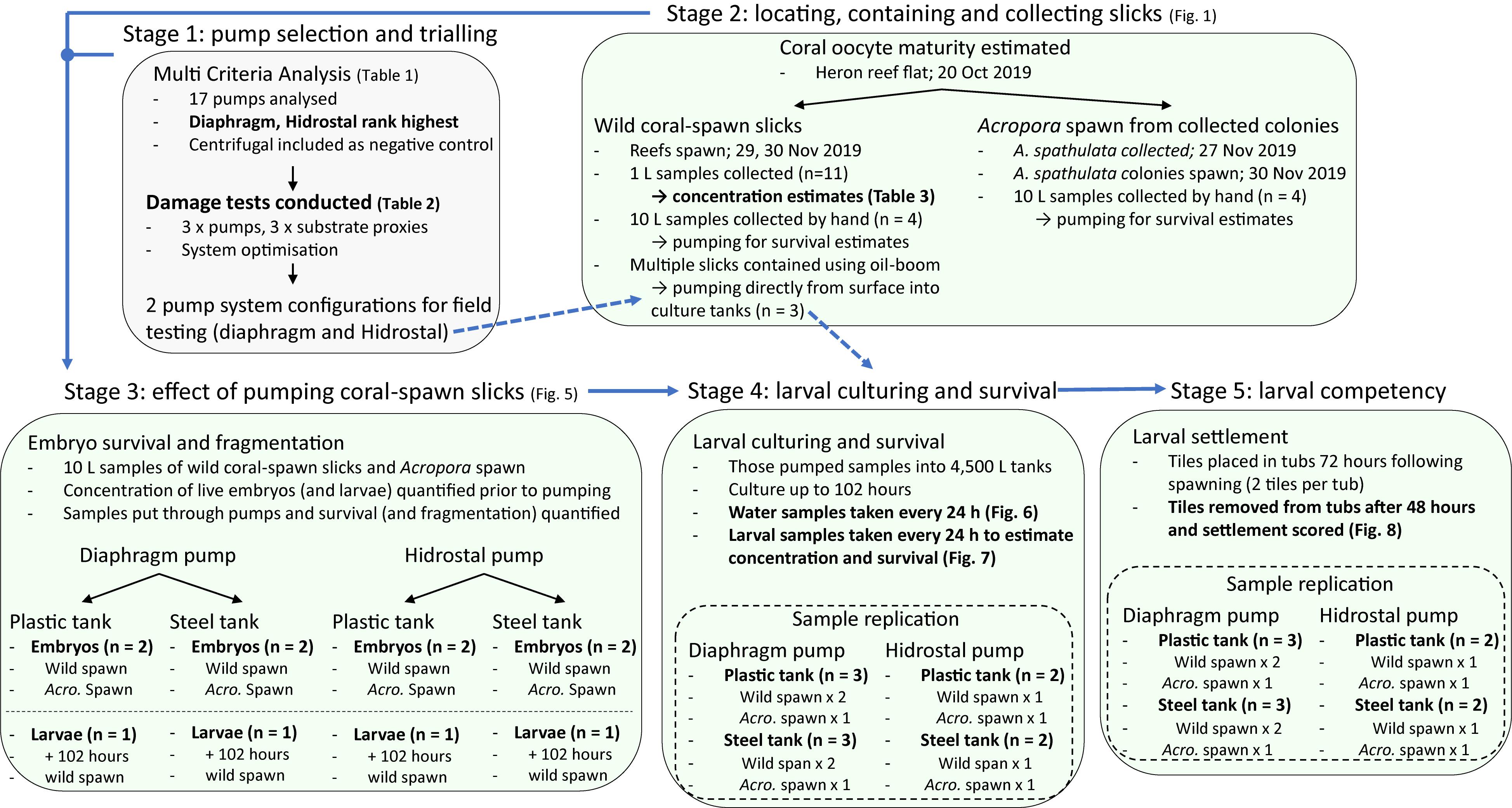
Figure 2. Workflow diagram presenting the overall approach of the study, the five major stages, time-points of observations, and replication for each experiment.
Pump Selection and Trialing
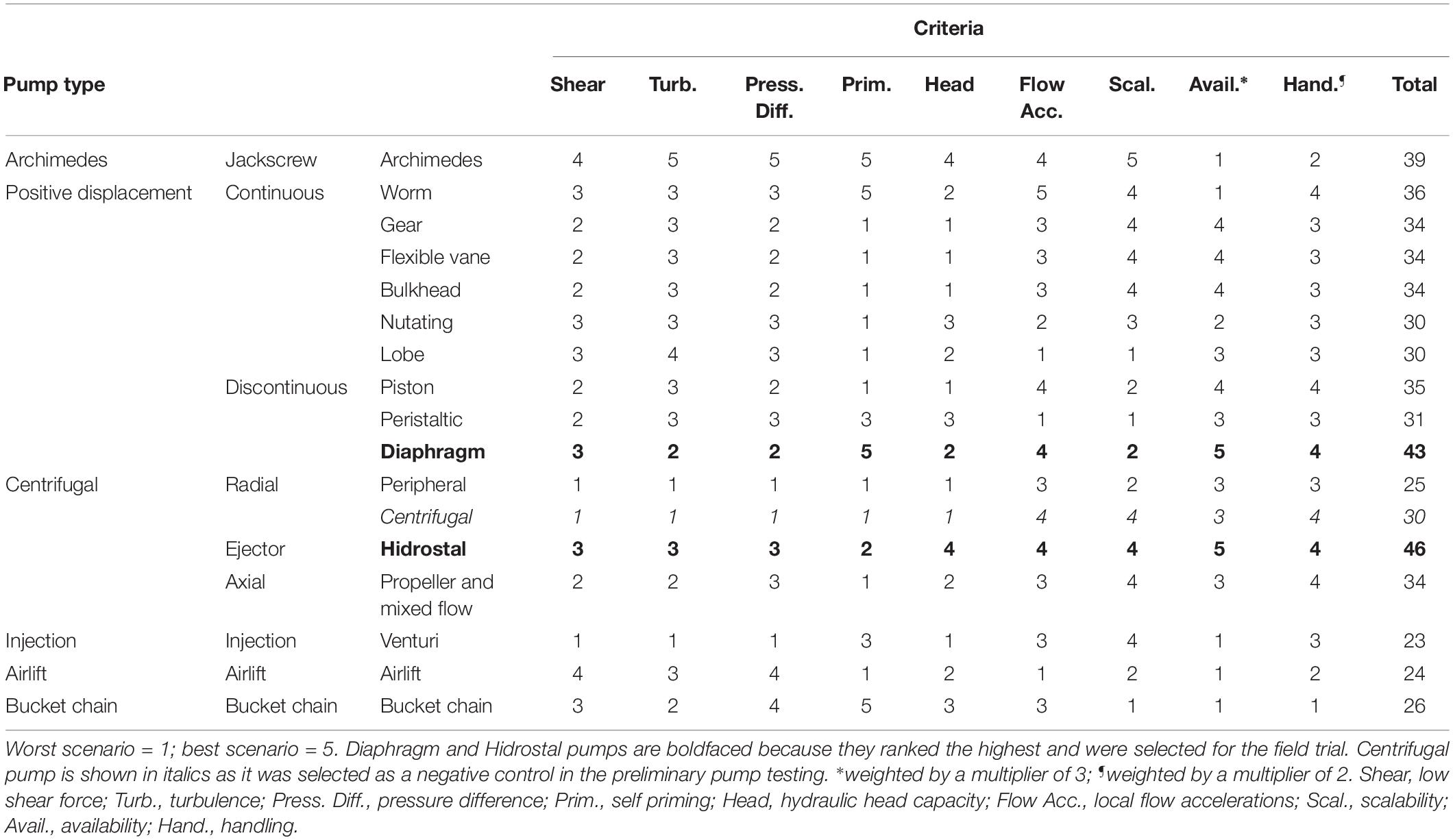
Different pumping principles and capabilities were initially compared to evaluate the potential of differing pump designs for safely and effectively pumping coral slicks. A short list of 17 potentially suitable pumps were ranked using a Multi Criteria Analysis (Statnikov and Matusov, 2002) by pump and dredging experts from Van Oord and Delft University of Technology. Theoretical criteria associated with shear stress, flow accelerations and pressure changes were included following Ulanowicz (1976). Four practical criteria were selected and included pump priming, availability, handling, and scalability. Following the Multi Criteria Analysis (Table 1), diaphragm and Hidrostal pumps ranked the highest and were selected for initial testing and optimization. Centrifugal pumps were included as a negative control.
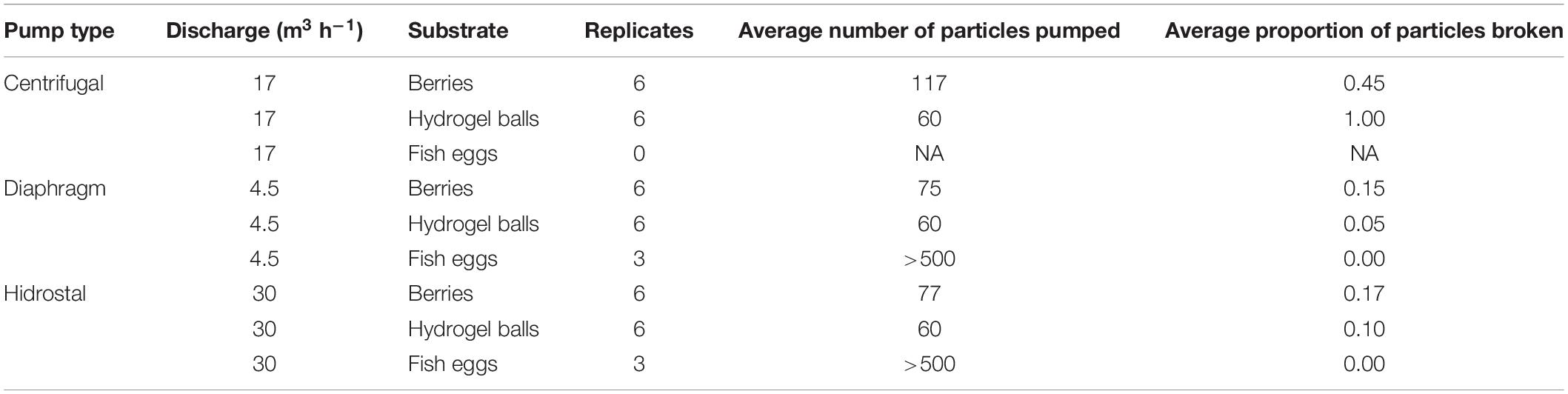
Initial tests were conducted within a controlled setting to assess the damage on different particles used as proxies for coral embryos, including hydrogel balls, berries, and fish eggs. Trials indicated that increasing hose diameter and free passages (both of which decrease surface contact), as well as lowering flow velocity, all reduced particle damage. With these configuration principles incorporated in an optimized configuration of the pumping system, the diaphragm and Hidrostal pumps both incurred little damage on the particles, especially in comparison to the centrifugal pump (Table 2), so both were selected for testing on coral-spawn slicks during the field operation. For the field operation the two pumps with 100 mm intake diameter were capable of pumping >1 m3 min–1, with the diaphragm pump driven by air while the Hidrostal pump was electric and submersible.
Field Study Location and Timing
The field testing was timed to occur during the mass coral-spawning event after the full moon of November 23, 2018. Based on ecological monitoring data, we decided to base our work in the southern Great Barrier Reef where coral cover is presently the highest (Australian Institute of Marine Sciences, 2018). Other parts of the reef have recently been affected by crown-of-thorns starfish, cyclones, and bleaching mortality that have reduced overall coral cover, so higher coral cover in the southern region provided the best chance of the formation of coral-spawn slicks that could be harvested using pumps. While mass spawning of corals has been found to peak 4-5 nights after full moon on the central Great Barrier Reef (Harrison et al., 1984; Babcock et al., 1986), examination of records of spawning at Heron Island in the southern Great Barrier Reef indicated that the main night of spawning is slightly later, most commonly between 6 and 8 nights after the full moon (Hock et al., 2019).
Locating, Containing and Collecting Slicks
Naturally occurring coral-spawn slicks were located using a hierarchy of temporal and spatial approaches. Colony fecundity and oocyte maturity was estimated at Heron Reef in late-October, and the majority of Acropora colonies were likely to spawn after the November 23rd full moon (pers. comm. Dr. Selina Ward).
A contingency plan was incorporated into the study so that pump testing and larval culturing could still be trialed in the event that wild coral-spawn slicks could not be located. Fecund colonies of Acropora spathulata were located on the reef and transferred to 4 basins on the deck of the tugboat. Each basin was 1 m3 and contained up to 12 mature A. spathulata colonies that were supplied with constant water flow and aeration until they spawned. Colonies spawned in the basins from 22:00 to 22:30 on November 30, 2018. All egg-sperm bundles were stirred and agitated, left for 2 h to allow sufficient time for cross-fertilization, and used in trials thereafter.
Scientists working at Heron Island Research Station confirmed spawning by Porites cylindrica in aquaria on the night of November 27, with further spawning of some Acropora spp. in tanks on the night of the 28th. Weather on the night of the 28th was windy and no slicks were seen from the vessel on the water’s surface during that night. However, signs of slicks were evident around Heron Island on the morning of November 29.
Further spawning occurred in tanks at Heron Island on the night of the 29th, and spawn slicks were also observed on the surface in Heron Island Channel at calm sea state conditions. Samples of these slicks were collected using 20 L buckets for use in pumping trials on board the vessel (see below “Pump selection and trialing”). From 06:45 to 07:30 am on November 30, aerial observations were made from a helicopter of multiple slicks along the south and east of Heron Reef (Figure 1A). GPS points were recorded and communicated so that the tugboat and support vessel could move to the slicks for sampling. On the evening of November 30, wild spawn slicks were evident on the sea surface, as well as on the following morning December 1. Wild slicks located on the sea-surface in daylight were contained with a 90 m long × 45 cm oil boom for direct pumping into the aquaculture tanks (Figures 1B,C).
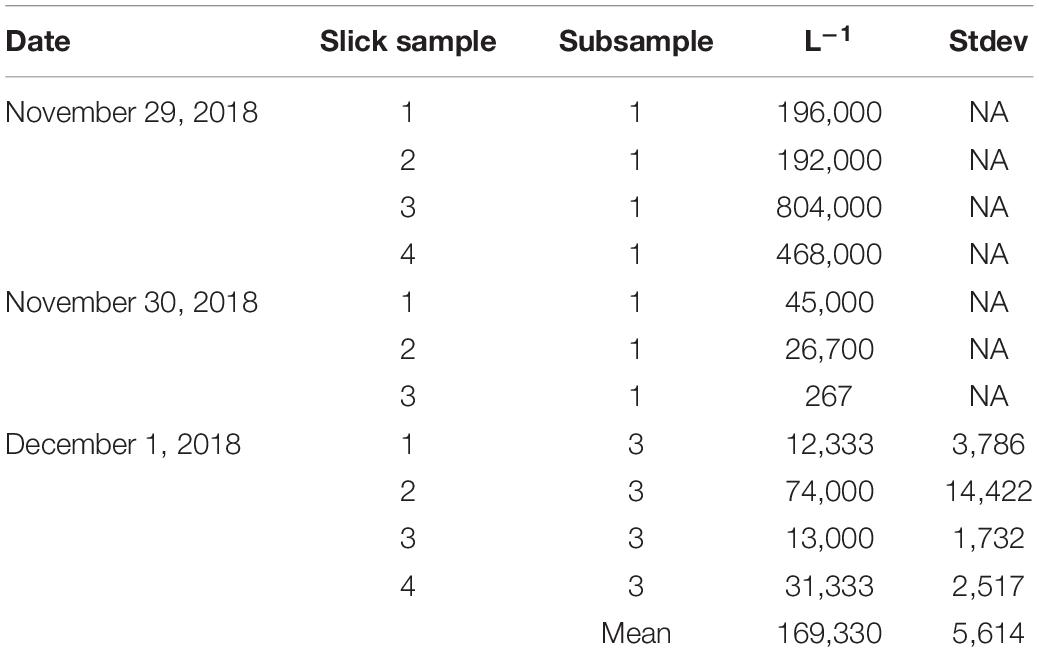
To compare the concentrations of slicks to the only previous published account by Oliver and Willis (1987), 1 L samples were taken when slicks were encountered and concentrations of live embryos found in wild coral-spawn slicks estimated. Samples of slicks were conducted from the surface and in their center. A total of 11 samples were taken from November 29 to December 1. From each bulk sample, 1–3 × 1 mL subsamples were taken and the number of live embryos counted under a dissecting microscope. Bulk samples were well mixed prior to sampling.
Effect of Pumping Coral-Spawn Slicks
The level of impact of pumping coral-spawn slicks with the diaphragm and Hidrostal pumps was evaluated by comparing the total abundance of live embryos immediately before and after pumping. Total abundances of live embryos were estimated by sampling 50 mL of the harvested or pumped coral-spawn, immediately counting 1–3 × 1 mL subsamples for the number of live embryos under a dissecting microscope, and multiplying embryo density by the absolute volume. Bulk samples were well mixed prior to sampling.
Harvested coral-spawn was poured into a spare culturing tank on the tugboat, positioning the hose inflow inside the tank close to the water surface, and then pumped into a receiving tank. The total volume of actual coral-spawn poured through the pumps was 10.1 L per tank on average (min = 8.14 L, max = 12.0 L), with ca. 1 min of pumping per trial required to capture the full volume of gametes. Average discharge rates with the diaphragm pump were 38 m3 h–1 (min = 16; max = 68) and with the Hidrostal pump 94 m3 h–1 (min = 58; max = 128). An average of 4.8 million live embryos (min = 2,304,000; max = 8,884,400) were pumped per trial with the wild slicks from 00:30 to 01:30 on November 30, whereas an average of 660,000 live embryos (min = 456,064; max = 830,490) were pumped per trial using A. spathulata from 00:00 to 01:00 on December 1.
Four trials were conducted using the coral embryos derived from the wild coral-spawn slicks and four trials were conducted using the coral embryos derived from the A. spathulata spawn. Four additional trials were conducted using settlement competent larvae originating from the harvested wild coral-spawn slicks, at 11:00 on December 4, 4.5 days (108 h) following original collection. Trials were equally split between Diaphragm and Hidrostal pumps, resulting in 4 replicates per pump type for coral embryos, and 2 replicates per pump type for competent coral larvae.
Aquaculture Tank Construction and Water Quality Control
As a first-step of scaling-up prior to full-scale trials, we aimed to rear the wild coral-spawn slicks using a transportable 50,000 L system consisting of 12 tanks in total (6 × steel, 6 × plastic; Figure 1D). Previous studies have scaled the in situ rearing of wild coral-spawn slicks in floating ponds up to a total of 22,000 L (7 × 3,200 L, Omori et al., 2007), and in laboratory rearing up to 13,000 L (1 × 1,000 L fiberglass tank and 3 × 4,000 L inflatable pools, Edwards et al., 2015). We used 6 × 4,500 L steel rainwater tanks, as scaled equivalents of hoppers commonly used in commercial vessels, to investigate critical factors for coral larval survival. The insides of the steel tanks were sandblasted to remove the protective zincalume coating and mimic a raw steel surface equivalent to that in a dredger. In addition, 6 × 5,000 L capacity polyethylene (food grade) rainwater tanks were used as an inert control. Steel tanks measured 1900 mm diameter x 1500 mm height, while polyethylene tanks measured 1800 mm diameter x 2200 mm height. Tank replicates were randomly positioned and secured on the back deck of the tugboat (Figure 1D).
Water quality in the tanks was maintained by filtration (50 and 5 μm cartridge filters) and UV treatment (80Watt Emperor Aquatics UV) of seawater supplied to the tanks using a constant flow-through system (Figure 3). Cartridge filtration removes particles such as sediment, phytoplankton and zooplankton from the seawater, while UV filtration sterilizes the water from bacteria and viruses. No cleaning of the tanks was conducted throughout the entire culturing period, but water quality relied on the constant flow of filtered seawater.
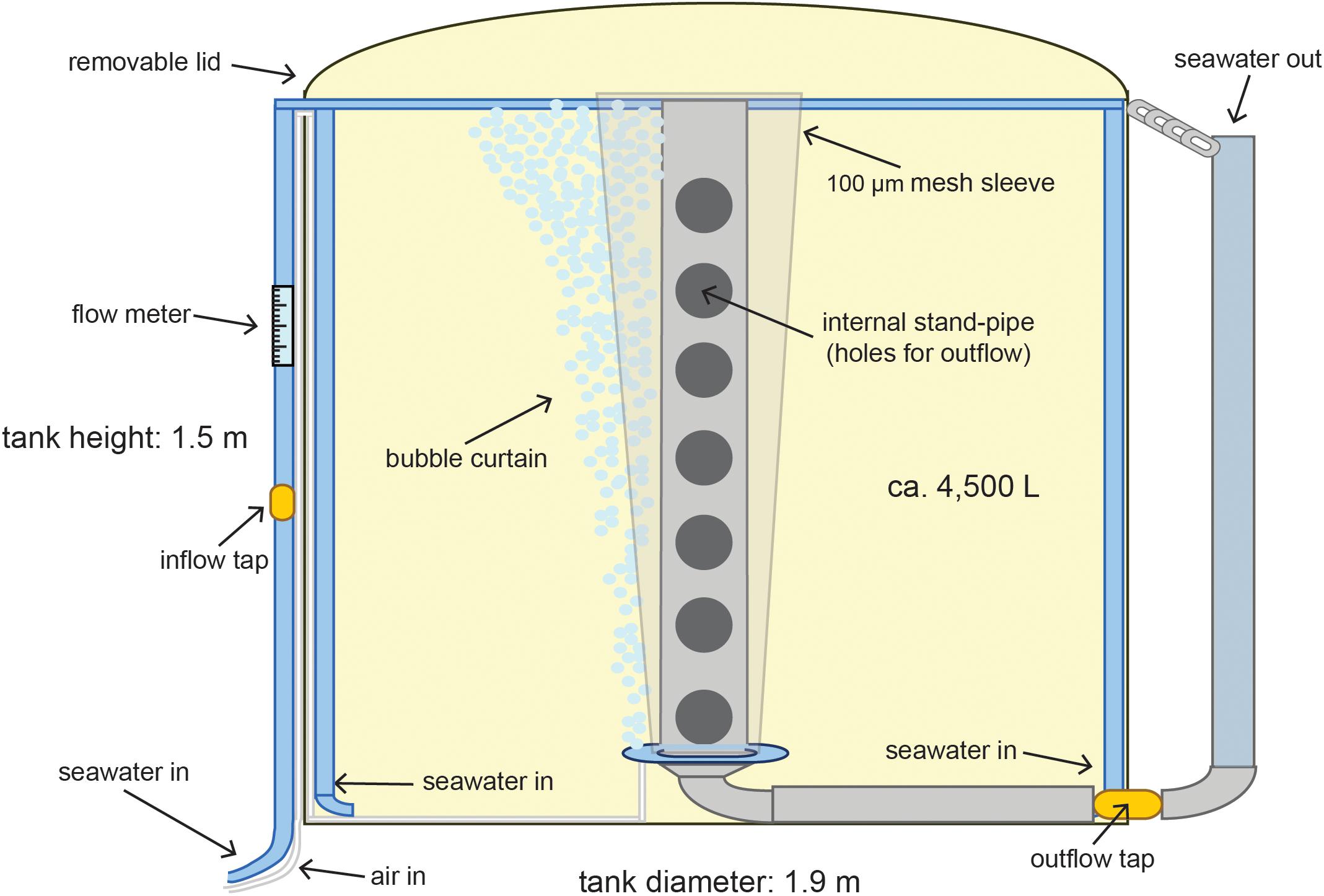
Figure 3. Detail of tank design for flow-through rearing of the coral larvae. The water is circulated by the two opposing inflows and mixed from the flow of bubbles. The cone shaped mesh provides a large surface area that prevents larvae being lost to the outflow holes on the internal standpipe while the bubble curtain prevents them sticking to the mesh.
Trace metal and physico-chemical properties of the seawater were characterized for the duration of the culturing period in the tanks. For total recoverable metals, three replicate steel and three replicate plastic tanks were sampled at 11:00 am on November 30th, December 2nd and 4th. At each time point, 150 mL of seawater was sampled, preserved with Nitric acid, and frozen. Seawater samples were sent to the Australian Government National Measurement Institute for analysis of total copper, iron, manganese, and nickel concentrations. Standard measurements of water quality including temperature, dissolved oxygen, salinity and pH were monitored using a YSI EXO1 Multiparameter Sonde. Water quality measurements were taken at 11:00 am, on November 30th, and December 2–4. Measurements were taken from the top and bottom of each tank, but no differences between depths was observed.
Larval Culturing and Survival
Pumped coral-spawn slicks were cultured in the steel and plastic rainwater tanks until competency was confirmed. Two approaches of collection were used to collect the coral-spawn slicks to test survival during culturing to competent larvae. Firstly, as described in “Pump selection and trialing”, collections by hand of wild slicks on November 30 and from the A. spathulata colonies housed on the tugboat on December 1 were poured through the pumps to estimate survival. Three additional samples were collected using a secondary approach by directly pumping wild slicks that had been concentrated and contained in oil booms from the sea-surface using the diaphragm pump (Figures 1B,C). Hence, a total of 7 replicates of competent larvae were pumped through the diaphragm pump – with 4 cultured in steel tanks and 3 cultured in plastic tanks; and a total of 4 replicates of competent larvae were pumped with the Hidrostal pump – with 2 cultured in steel tanks and 2 cultured in plastic tanks. All collected slicks were reared through to competency, with the concentrations of live embryos estimated every 24 h to test for differences in survival in the different tank types and following collection with the different pump types. Estimates of larval concentrations were conducted as described previously.
Larval Settlement Competency
To test the competency of larvae reared in the aquaculture facility, artificial tiles were placed in each rainwater tank at 72 h following slick collection. Ceramic tiles measured 5 × 5 cm, had an outer surface with fine-manufactured rugosity, with a hole drilled in the center for attachment (for further details see Doropoulos et al., 2014). The tiles were placed on the reef crest of Heron Island and preconditioned for 3 weeks. Upon collection, tiles were brushed to remove any fleshy algae and sediment, and placed in the rearing tanks. Two tiles were placed in each tank, with one tile laid flat on the bottom of the tank and the other tile left hanging at 10 cm above the bottom of the tank. The tiles were scored for larval settlement (i.e., attachment and metamorphosis) under a dissecting microscope 48 h after being placed into each tank.
Data Analyses
The effect of pumping stress was tested on the instantaneous survival of coral embryos and larvae. The proportional change of fertilized gametes was assessed by slick source (wild, Acropora), life phase (embryo, larvae), pump type (diaphragm, Hidrostal), and tank type (steel, plastic) using a linear model with Gaussian variance structure. Interactions among pump type, tank type, and slick source were specified in the model.
The concentration and survival of live gametes, from the embryo to larval phases, were both tested using generalized additive models with Gaussian variance structures for each model. Temporal trends of gamete concentration and survival were smoothed using natural splines (knots = 4), with pump type and tank type, and their interaction, incorporated as categorical predictors.
The abundance of settled larvae were tested using a generalized linear model using a quasi-Poisson variance structure due to overdispersion of the model residuals (Crawley, 2013). Settlement abundance was tested according to pump type, tank type, tile orientation (upwards, downwards), depth (flat, hanging), and their interactions.
All analyses were conducted in R (R Development Core Team, 2018), using the ‘lme4’ (Bates et al., 2015), ‘gamm4’ (Wood and Scheipl, 2013), ‘lsmeans’ (Lenth, 2016) libraries. All models were assessed by visualizing residual fits and normal distribution of the raw data. Statistical significance was based on comparison between the full model with reduced models using likelihood ratio test χ2 P values for each term.
Results
Very high concentrations of live embryos were consistently encountered in the naturally occurring coral-spawn slicks. Average densities revealed in situ concentrations of 169,330 live embryos L–1 (Table 3). These samples were collected by hand from a small boat and were composed of a variety of taxa based on sizes, color, and presence or absence of symbiotic zooxanthellae (Figure 4).
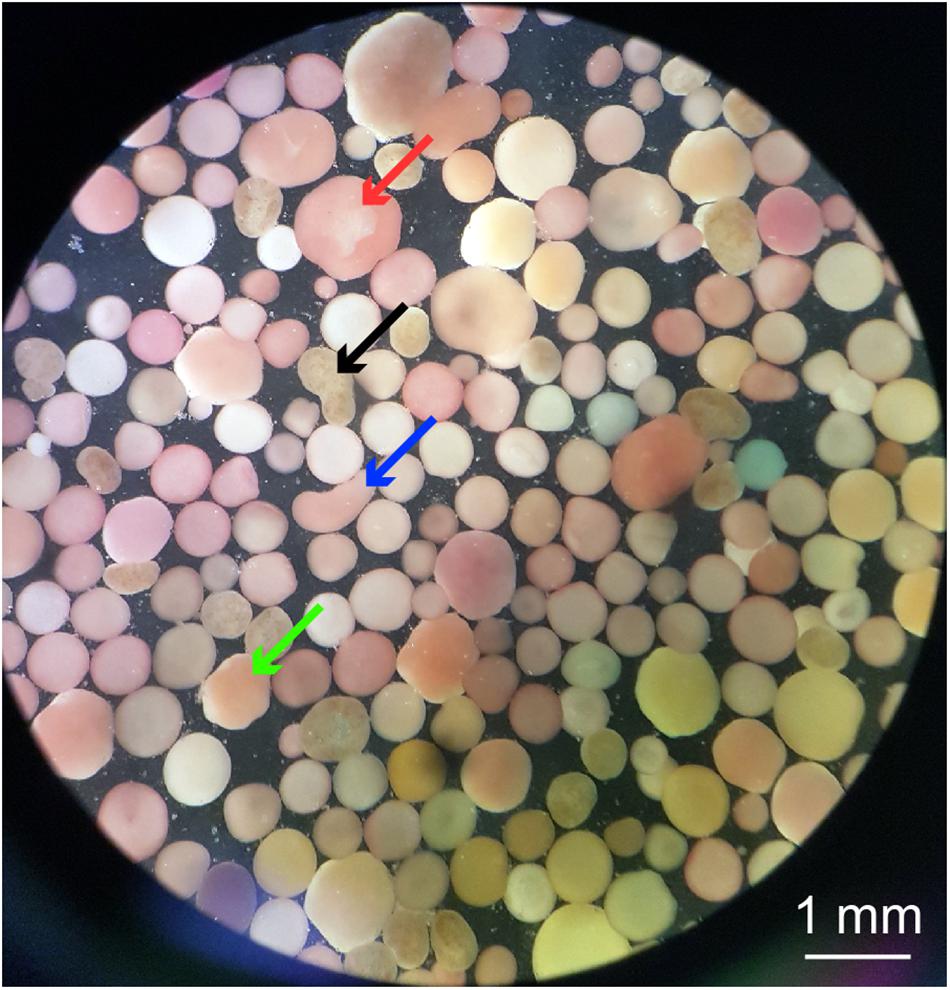
Figure 4. Microscope image of a wild coral-spawn slick sample taken from one of the culture tanks approximately 36 h following spawning. The sample indicates a variety of coral species found in a wild coral-spawn slick, recognized through the range of sizes, colors, and those that have zooxanthellae already present (e.g., black arrow) or absent (e.g., red, blue and green arrows). Observed are a range of developmental stages of intact embryos to larvae, including: morula (e.g., green arrow), prawn chip (e.g., red arrow), and elongated planulae (e.g., black and blue arrows) (terms following Jones et al., 2015).
The boom was deployed multiple times to successfully surround coral-spawn slicks (Figures 1B,C), but attempts to move the slick met with mixed success. Occasionally, small wave action caused overtopping of the contained slick. Also, tidal flows in the area were substantial, meaning that even very low tow speeds resulted in loss of spawn-slick material below the weighted 45 cm depth of the boom skirt. Concentrations of live embryos collected by pumping these slicks from the sea surface were far lower than collection conducted by hand, averaging 44 live embryos L–1 (n = 3).
The total number of live coral embryos pumped was approximately 29 million – i.e., the amount in recipient tanks directly after pumping regardless of the collection or pumping method. “Survival” rates were often recorded to be greater than 100% following pumping, which includes embryos that fragmented and remained alive. Pumping live coral embryos <3 h following collection from a tank on deck to another tank on deck occurred with very high levels of survival (Figure 5A), averaging 131 ± 51% (stdev; n = 8). Occasional formation of vortices above the suction mouth did not appear reduce survival. It was also possible to pump 5-day old competent larvae (Figure 5B), with survival averaging 112 ± 88% (n = 4).
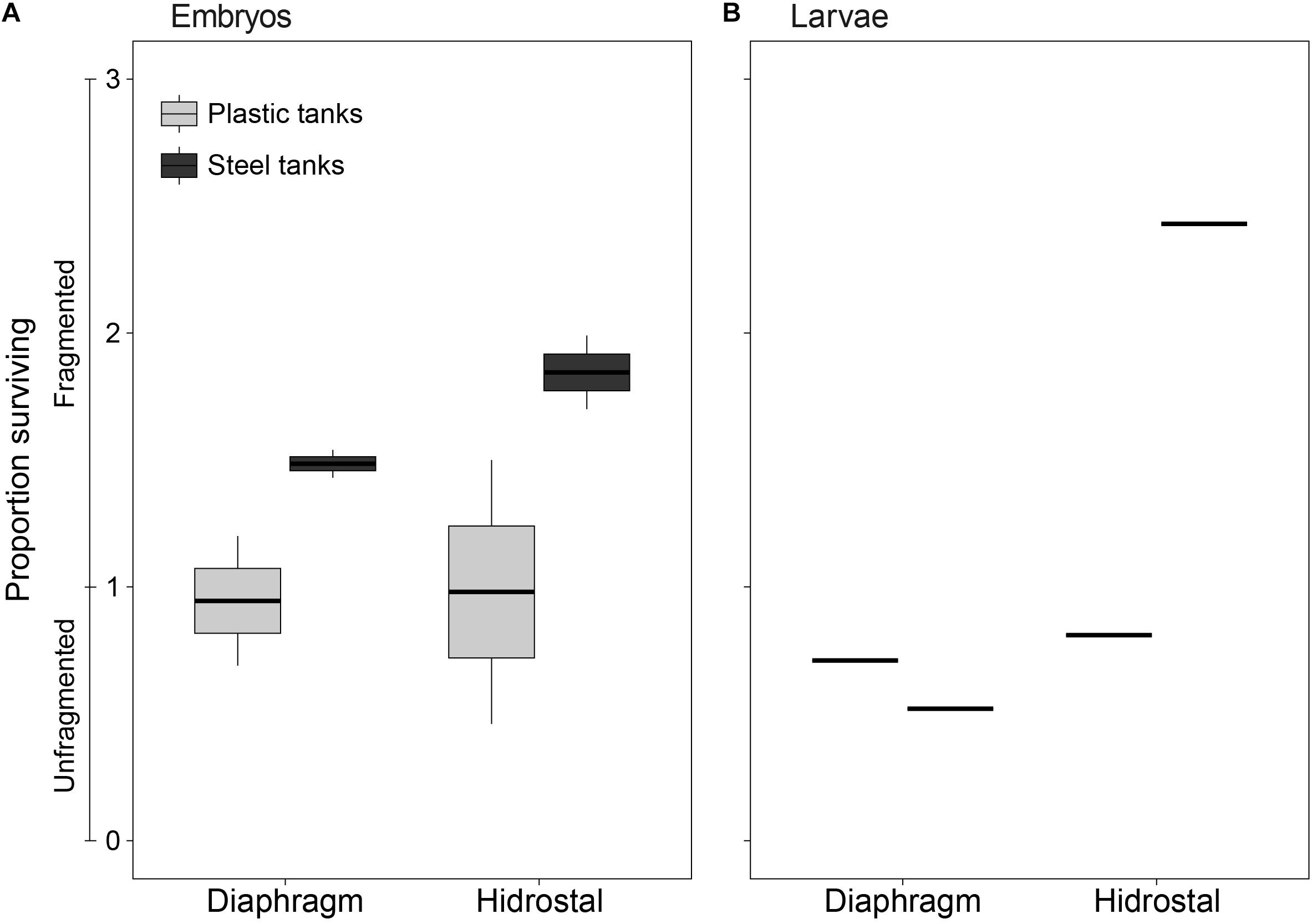
Figure 5. Survival of coral (A) embryos and (B) larvae immediately following pumping by diaphragm and Hidrostal pumps in the plastic and steel tanks. Values ranging from 0 to 1 indicate unfragmented embryo survival, whereas values ranging 1–3 indicate the proportion of fragmented and surviving embryos. The middle line of each boxplot indicates the median value, upper and lower hinges indicate the 75 and 25% quantiles, upper and lower whiskers represent the maximum and minimum observations +1.5 × the inter-quartile range, and individual dots represent outliers (n = 2 per tank type × pump type for embryos; n = 1 per tank type × pump type for larvae).
An interaction between pump type and tank type (p = 0.029) showed that the survival of embryos and larvae was higher when pumped with the Hidrostal pump into steel tanks compared to plastic tanks (steel 204% vs. plastic 92%, pair-wise p = 0.0027), whereas when embryos were pumped with the diaphragm pump there was no statistical difference between steel tanks compared to plastic tanks (steel 116% vs plastic 87%, pair-wise p = 0.426). An interaction was also evident between pump type and slick source (p = 0.035), due to higher rates of survival when wild slicks were pumped with the Hidrostal pump compared to the diaphragm pump (161% vs 87%, pair-wise p = 0.021).
Flow rates in the aquaculture tanks were set to provide at least two full water changes per day within each tank, maintaining a high standard of water quality throughout the larval culturing period (Figure 6). Concentrations of trace metals were similar between steel and plastic tanks, were always below 1 μg L–1 for nickel and manganese, were slightly higher for copper at 1.5 μg L–1, and averaged 11.7 μg L–1 for iron. Minor variations in salinity and water temperature were found between steel and plastic tanks. Seawater temperature averaged 26.8°C throughout the culturing period (min = 25.5°C, max = 28.8°C), with steel tanks on average 0.5°C warmer than plastic tanks. Oxygen saturation was always over 100%, and pH appeared to increase steadily over the period that larvae were cultured, rising from 8.24 to 8.30.
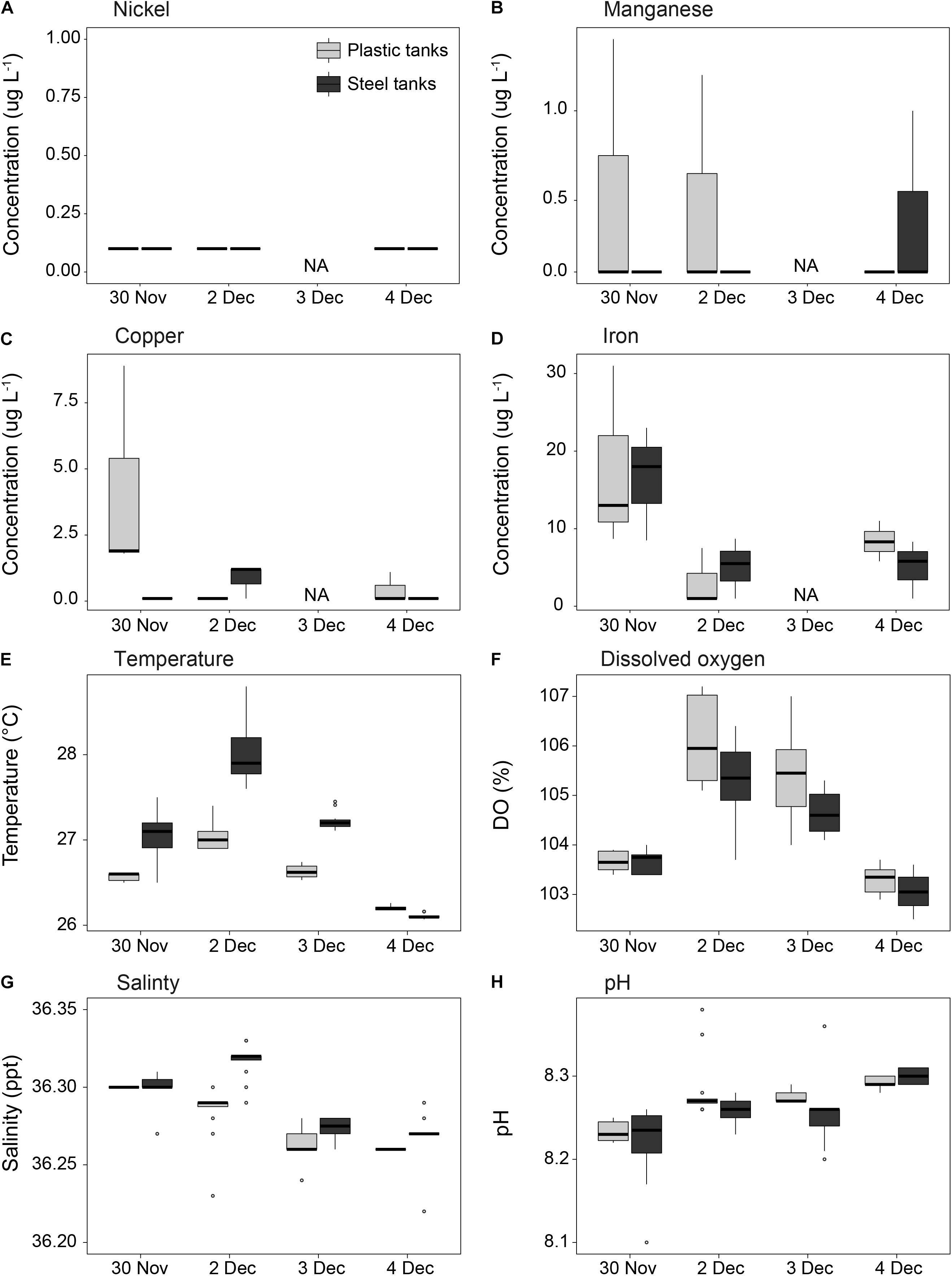
Figure 6. Measurements of (A–D) dissolved metal concentrations and (E–H) water quality in the culture tanks throughout the duration of the coral larvae culturing period. The first batch of spawn slicks were pumped into the culture tanks at 00:30 am on Nov 30. Light gray = plastic tanks; dark gray = steel tanks (n = 3 per tank type for A–D; n = 6 per tank type for E–H; NA = no samples for A–D).
On collection of the coral-spawn slicks, initial stocking densities averaged 1272 live embryos L–1, ranging from 14 to 8,000 L–1. Differences in larval concentrations (Figures 7A,B) or survival rates (Figures 7C,D) throughout the culturing period were not affected by either pump type or tank type. The only significant factor influencing concentration and survival was time since collection, with overall concentrations (p = 0.038) and survival (p < 0.001) decreasing with larval age. By 4.5 days following spawning, coral-spawn pumped and reared in the aquaculture facility on the deck of the tugboat were fully competent, with a total of 5.6 million larvae remaining. Average survival of the initial stock from the coral-spawn slicks to larval competency was 14 ± 8% (stdev; n = 11).
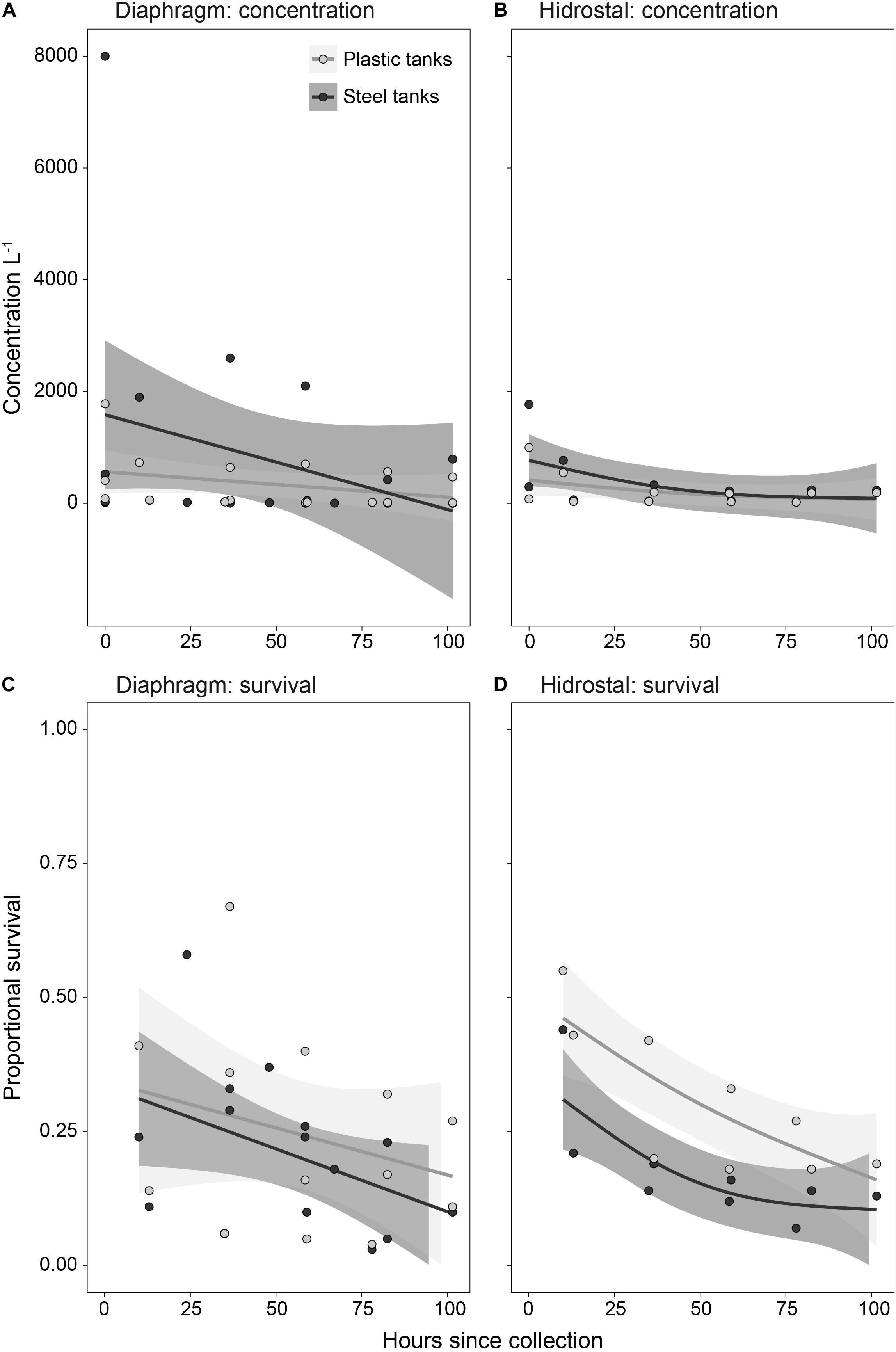
Figure 7. Temporal trends in larval (A,B) concentration and (C,D) proportion survival following pumping by diaphragm and Hidrostal pumps in the plastic and steel tanks. Solid circles represent individual data points, solid lines represent mean of the generalized additive model fits, and shading is the 95% confidence interval of model fits (n = 3 per tank type for the diaphragm pump; n = 2 per tank type for the Hidrostal pump).
High densities of settled coral larvae were found on conditioned tiles from both steel and plastic tanks, with an average of 7 settlers cm–2 on tile topsides (Figure 8A) and 15 settlers cm–2 on tile undersides (Figure 8B; settlement orientation p = 0.014). An interaction between pump type and tank type (p = 0.007) showed much lower settlement on tiles in steel tanks following pumping by the diaphragm pump (1.6 settlers cm–2) compared to all other combinations (16.1 settlers cm–2).
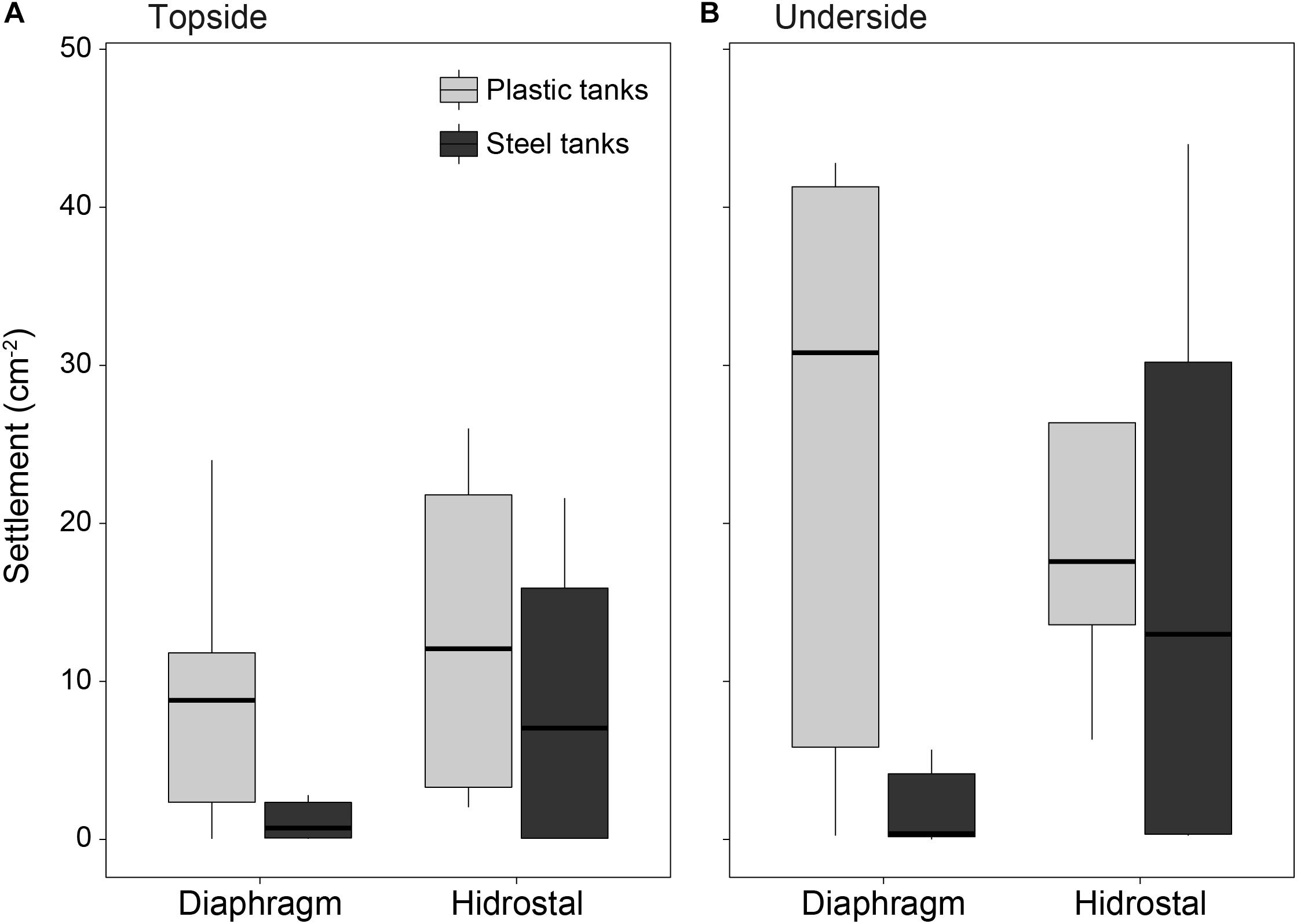
Figure 8. Coral settlement density (cm– 2) on tile (A) topsides and (B) undersides placed in the plastic and steel culture tanks. Two 5 × 5 cm settlement tiles were placed in each tank and scored for settlement at 4.5 days following the introduction of embryos into the tanks (n = 6 per tank type × tile orientation for the diaphragm pump; n = 4 per tank type × tile orientation for the Hidrostal pump).
Discussion
Industrial-scale collection, culturing, and transport of coral propagules may provide an important tool for the restoration of coral reefs. Prior to full scale application of our suggested approach (Doropoulos et al., 2019), considerations relating to containment, collection, and culturing needed empirical trialing. This field trial has demonstrated that harvesting and culturing of wild coral-spawn slicks for delivery to targeted reefs is achievable using a transportable 50,000 L aquaculture system built on-board a tugboat. To our knowledge, our study is the first to (1) harvest and culture coral-spawn at such a large volume, (2) use an aquaculture facility that can transport larval cultures 1000’s of km’s – distances relevant to the world’s largest coral reef ecosystems, and (3) show that live coral embryos and larvae can be pumped for rapid collection and distribution, respectively, surviving the shear forces and turbulence generated by pumping. These outcomes suggest that further upscaling of coral-spawn harvesting and culturing to millions of liters for release onto reefs is feasible.
Mass coral spawning events occur on many reefs at predictable times of the year (e.g., Great Barrier Reef - Harrison et al., 1984; Babcock et al., 1986; Penland et al., 2003; north-western Australia - Gilmour et al., 2016), so, weather dependent, encountering coral-spawn slicks for harvest provides a reliable source of live embryos from a diverse mix of species. Given this periodicity of a known source of gametes, it is surprising that there was only one prior published account of the concentration of live embryos found in coral spawn slicks – i.e., 230 L–1 from the central Great Barrier Reef (Oliver and Willis, 1987). Following sampling from 11 slicks in our present study, the average concentration of live embryos found in the coral-spawn slicks was orders of magnitude higher than the concentration previously published. This information, in addition to previous in situ culturing of wild coral-spawn slicks (Heyward et al., 2002; Omori et al., 2007), show that wild coral-spawn slicks provide a highly abundant source of material that can be utilized for large-scale restoration efforts.
Pumping coral-spawn slicks provides a means of collection for scaling-up coral reef restoration. Prior to conducting this field study, the potential for coral embryo survival during pumping was unknown and proportional survival estimated at 0.7–0.8 for modeling purposes (Doropoulos et al., 2019). Previous work has found size-dependent survival decreases as a linear function of increasing shear stress for eggs to juveniles of five fish taxa (Killgore et al., 2001), whereas our preliminary tests provided promising results with little to no damage on fish eggs and other proxies using the diaphragm and Hidrostal pumping systems (Table 2). When we applied these pumps to coral embryos in our field study, we discovered that coral embryos at two distinct developmental phases, i.e., fertilized eggs and competent larvae, could be pumped with high levels of survival that included fragmentation. That is, the overall average level of survival following pumping was >120%, indicating that fragmentation had occurred on the coral embryos. These smaller, fragmented embryos remained alive and continued to develop to fully competent larvae, a phenomena that has previously been demonstrated and hypothesized as a tool for clonal reproduction in corals (Heyward and Negri, 2012).
Survival of the pumped coral-spawn slicks to competent larvae following 4.5 days of culturing averaged 14% of the initial stock. Storage and culturing in 4,500 L steel and plastic rainwater tanks did not result in differences in survival. Steel tanks did have slightly higher temperatures than plastic tanks on average, a factor that may be related to lower rates of settlement. Overall however, the use of steel basins does not appear to pose a limitation to larval culturing in scaling to larger hoppers that are typically found in commercial vessels hoppers that typically use raw steel, such as those found in hopper dredgers of commercial vessels.
The larval-culturing survival rate of 14% using a 50,000 L facility with an average stocking density of 1,272 L–1 (range 18–8,000) in our study is among the highest found in the literature, demonstrating that the filtering system and flow rates provided water quality that is sufficient for larval culturing. In comparison, culturing of wild coral-spawn slicks using in situ ponds with a constant exchange of ambient seawater have found averages of ca. 17% survival using a 22,000 L capacity with initial stocking densities ca. 840 L–1 (Omori et al., 2007), and 5% survival using a 6,000 L capacity with initial stocking densities ca. 5,000 L–1 (Heyward et al., 2002). Such high initial stocking densities of ca. 5,000 L–1 in Heyward et al. (2002) likely resulted in such low proportional survival. Similarly in our study, while differences in larval concentrations between steel and plastic tank types were not statistically different, trends indicate that the initial reductions in concentration were steeper in the steel tanks than plastic tanks, caused by two steel tanks with much higher initial concentrations (Figures 7A,B). Work by Pollock et al. (2017) shows that density-dependent survival occurs when initial stocking densities are ≥500 larvae L–1, and Edwards et al. (2015) suggested initial stocking densities of no more than 300 larvae L–1. Thus, adjusting larval concentrations to 300–500 L–1 and increasing flow rates for steel tanks to keep water temperatures at ambient levels may help improve larval survival and competency.
While encountering, collecting, pumping, and culturing the coral-spawn slicks through to competency were realized with great success during this study, containing the slicks in the oil booms and skimming the coral-spawn slicks directly from the surface of the seawater with the used pumping configuration was met with mixed outcomes that require further development. Additional tests are needed to improve the skimmer head design, such as configuring the skimmer to pass spawn slicks into the pump intake at depth. Optimizing this step of the collection phase will provide capacity for full-scale collection of wild coral-spawn slicks by minimizing any requirement of containment and manual handling. If containment of coral-spawn slicks on the sea surface is necessary, temporary collection into floating ponds could be an option prior to collection into the primary aquaculture facility on-board a vessel.
The type of pumping system for future large-scale implementation also requires consideration. While the Hidrostal pump has optimal flow control, pumps of this type require priming to enable a continuous water flow which can be time consuming and is likely to be problematic in rougher conditions since air may enter the pump resulting in partial or total loss of effectiveness. In contrast, diaphragm pumps do not require priming and are therefore easier to handle and quicker to deploy. Ultimately the choice of pump may need to be made taking into consideration the final design of the skimmer head, since diaphragm pumps will be more difficult to scale up further in terms of capacity, while this is more feasible with the Hidrostal design if priming and air intake issues can be overcome.
Of the upmost importance is that if harvesting is to be up-scaled and used as a common practice, initial characterizations of where excess material is located must be conducted to remove any possibility of over-harvesting that could reduce natural meta-population recovery (Doropoulos and Babcock, 2018). For example, based on demographic modeling from Doropoulos et al. (2019), only <0.0001% of the gametes spawned from Heron Reef slope alone were used in this current study. Moreover, coral-spawn harvesting should only occur where larval abundances are not degraded compared to historical levels, as has recently been found in the central to northern Great Barrier Reef (Hughes et al., 2019).
The potential for scalability of our approach is one of the key attributes that makes it an ideal candidate for use in addressing the challenge of coral restoration efforts at ecologically relevant scales. The number of embryos that could be harvested using our technique with a medium-sized trialing suction hopper dredger – volume 14,000 m3 – was initially estimated at 1.6 billion, translating to >500 million competent larvae, and 11 million newly settled coral recruits onto reefs (Doropoulos et al., 2019). Opportunities using other vessels with the capacity to hold large volumes of water while maintaining high levels of water quality can also be utilized. Developing and moving this number of larvae is unprecedented and practically impossible using conventional approaches. However, following some optimization to the collection component of our approach, full-scale application of this restoration tool appears fully feasible. We are now at the stage to scale further and pump competent larvae onto a degraded reef in an unconstrained manner to test whether this restoration approach is viable for routine and large-scale application.
Data Availability Statement
The data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
CD, JE, FV, RH, MK, and RB contributed to the conception and design of the study. CD, JE, FV, RH, KS, MK, and RB conducted the study. CD organized the database, performed the analyses, and wrote the first draft of the manuscript. FV and RB wrote sections of the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Funding
Funding was provided by an Advanced Queensland Small Business Innovation Research Challenge (TF6.7.2 RECRUIT – Recovery of Reefs Using Industrial Techniques) to RB, CD, JE, RH, and MK. The authors declare that this study also received internal funding from CSIRO Oceans and Atmosphere Coastal Development and Management program and by Van Oord Dredging and Marine Contractors B.V., neither of which had any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
FV, JE, RH, and MK was employed by company Van Oord Dredging and Marine Contractors B.V.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank A. Chai, F. Cowman, S. Dalton, N. Gutierrez-Isaza, C. Sims, M. Tonks, and S. Ward for field support, B. Murphy and G. Coman for aquaculture advice, Pacific Tugs for vessel and logistical support, and Hidrostal Australia for pump support. A video of the pump configuration development can be found at https://vimeo.com/314794547 and of the field trial at https://vimeo.com/312848060.
References
Australian Institute of Marine Sciences (2018). Long-Term Reef Monitoring Program - Annual Summary Report on Coral Reef Condition for 2017/18. Townsville: Australian Institute of Marine Sciences.
Babcock, R. C., Bull, G. D., Harrison, P. L., Heyward, A. J., Oliver, J. K., Wallace, C. C., et al. (1986). Synchronous spawnings of 105 scleractinian coral species on the great barrier reef. Mar. Biol. 90, 379–394. doi: 10.1007/bf00428562
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48.
Bullock, J. M., Aronson, J., Newton, A. C., Pywell, R. F., and Rey-Benayas, J. M. (2011). Restoration of ecosystem services and biodiversity: conflicts and opportunities. Trends Ecol. Evol. 26, 541–549. doi: 10.1016/j.tree.2011.06.011
Butchart, S. H. M., Walpole, M., Collen, B., van Strien, A., Scharlemann, J. P. W., Almond, R. E. A., et al. (2010). Global biodiversity: indicators of recent declines. Science 328, 1164–1168. doi: 10.1126/science.1187512
de la Cruz, D. W., and Harrison, P. L. (2017). Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Sci. Rep. 7:13985. doi: 10.1038/s41598-017-14546-y
De Vriend, H., and Van Koningsveld, M. (2012). Building With Nature: Thinking, Acting and Interacting Differently. Dordrecht: Ecoshape.
Doropoulos, C., and Babcock, R. C. (2018). Harnessing connectivity to facilitate coral restoration. Front. Ecol. Environ. 16, 558–559. doi: 10.1002/fee.1975
Doropoulos, C., Elzinga, J., ter Hofstede, R., van Koningsveld, M., and Babcock, R. C. (2019). Optimizing industrial-scale coral reef restoration: comparing harvesting wild coral spawn slicks and transplanting gravid adult colonies. Restor. Ecol. 27, 758–767. doi: 10.1111/rec.12918
Doropoulos, C., Roff, G., Zupan, M., Nestor, V., Isechal, A. L., and Mumby, P. J. (2014). Reef-scale failure of coral settlement following typhoon disturbance and macroalgal bloom in Palau, Western Pacific. Coral Reefs 33, 613–623. doi: 10.1007/s00338-014-1149-y
Edwards, A. J., Guest, J. R., Heyward, A. J., Villanueva, R. D., Baria, M. V., Bollozos, I. S., et al. (2015). Direct seeding of mass-cultured coral larvae is not an effective option for reef rehabilitation. Mar. Ecol. Prog. Ser. 525, 105–116. doi: 10.3354/meps11171
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., et al. (2005). Global consequences of land use. Science 309, 570–574. doi: 10.1126/science.1111772
Fox, H. E., Harris, J. L., Darling, E. S., Ahmadia, G. N., Estradivari, L., and Razak, T. B. (2019). Rebuilding coral reefs: success (and failure) 16 years after low-cost, low-tech restoration. Restor. Ecol. 27, 862–869.
Gillies, C. L., Fitzsimons, J. A., Branigan, S., Hale, L., Hancock, B., Creighton, C., et al. (2015). Scaling-up marine restoration efforts in Australia. Ecol. Manag. Restor. 16, 84–85. doi: 10.1111/emr.12159
Gilmour, J., Speed, C. W., and Babcock, R. (2016). Coral reproduction in Western Australia. PeerJ 4:e2010. doi: 10.7717/peerj.2010
Guest, J., Baria, M., Gomez, E., Heyward, A., and Edwards, A. (2014). Closing the circle: is it feasible to rehabilitate reefs with sexually propagated corals? Coral Reefs 33, 45–55. doi: 10.1007/s00338-013-1114-1
Harrison, P. L., Babcock, R. C., Bull, G. D., Oliver, J. K., Wallace, C. C., and Willis, B. L. (1984). Mass spawning in tropical reef corals. Science 223, 1186–1189. doi: 10.1126/science.223.4641.1186
Heyward, A., and Negri, A. (2012). Turbulence, cleavage, and the naked embryo: a case for coral clones. Science 335, 1064–1064. doi: 10.1126/science.1216055
Heyward, A. J., Smith, L. D., Rees, M., and Field, S. N. (2002). Enhancement of coral recruitment by in situ mass culture of coral larvae. Mar. Ecol. Prog. Ser. 230, 113–118. doi: 10.3354/meps230113
Hock, K., Doropoulos, C., Gorton, R., Condie, S. A., and Mumby, P. J. (2019). Split spawning increases robustness of coral larval supply and inter-reef connectivity. Nat. Commun. 10:3463. doi: 10.1038/s41467-019-11367-7
Hughes, T. P., Kerry, J. T., Baird, A. H., Connolly, S. R., Chase, T. J., Dietzel, A., et al. (2019). Global warming impairs stock–recruitment dynamics of corals. Nature 568, 387–390. doi: 10.1038/s41586-019-1081-y
Intergovernmental Panel on Climate Change (2014). “Summary for policymakers,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, et al. (New York, NY: Intergovernmental Panel on Climate Change), 1–32.
Jones, R., Ricardo, G. F., and Negri, A. P. (2015). Effects of sediments on the reproductive cycle of corals. Mar. Pollut. Bull. 100, 13–33. doi: 10.1016/j.marpolbul.2015.08.021
Killgore, K. J., Maynord, S. T., Chan, M. D., and Morgan, R. P. (2001). Evaluation of propeller-induced mortality on early life stages of selected fish species. N. Am. J. Fish. Manag. 21, 947–955. doi: 10.1577/1548-8675(2001)021<0947:eopimo>2.0.co;2
Laboyrie, H. P., Van Koningsveld, M., Aarninkhof, S. G. J., Van Parys, M., Lee, M., Jensen, A., et al. (2018). “Dredging for sustainable infrastructure,” in CEDA/IADC (The Hague: HR Wallingford).
Lotze, H. K., Lenihan, H. S., Bourque, B. J., Bradbury, R. H., Cooke, R. G., Kay, M. C., et al. (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809. doi: 10.1126/science.1128035
Lucas, J. S., Southgate, P. C., and Tucker, C. S. (2019). Aquaculture: Farming Aquatic Animals and Plants. Hoboken, NJ: Wiley-Blackwell.
Mazor, T., Doropoulos, C., Schwarzmueller, F., Gladish, D. W., Kumaran, N., Merkel, K., et al. (2018). Global mismatch of policy and research on drivers of biodiversity loss. Nat. Ecol. Evol. 2, 1071–1074. doi: 10.1038/s41559-018-0563-x
Menz, M. H. M., Dixon, K. W., and Hobbs, R. J. (2013). Hurdles and opportunities for landscape-scale restoration. Science 339, 526–527. doi: 10.1126/science.1228334
Millennium Ecosystem Assessment, (2005). Ecosystems and Human Well-being: Biodiversity Synthesis. Washington, DC: World Resources Institute.
Oliver, J. K., and Willis, B. L. (1987). Coral-spawn slicks in the great barrier reef - preliminary observations. Mar. Biol. 94, 521–529. doi: 10.1007/bf00431398
Omori, M., Shibata, S., Yokokawa, M., Aota, T., and Iwao, K. (2007). Survivorship and vertical distribution of coral embryos and planula larvae in floating rearing ponds. Galaxea 8, 77–81. doi: 10.3755/jcrs.8.77
Orth, R. J., Moore, K. A., Marion, S. R., Wilcox, D. J., and Parrish, D. B. (2012). Seed addition facilitates eelgrass recovery in a coastal bay system. Mar. Ecol. Prog. Ser. 448, 177–195. doi: 10.3354/meps09522
Penland, L., Kloulechad, J., and Idip, D. (2003). Timing of Coral Spawning in Palau. Koror: Palau International Coral Reef Center.
Pollock, F. J., Katz, S. M., van de Water, J. A., Davies, S. W., Hein, M., Torda, G., et al. (2017). Coral larvae for restoration and research: a large-scale method for rearing Acropora millepora larvae, inducing settlement, and establishing symbiosis. PeerJ 5:e3732. doi: 10.7717/peerj.3732
R Development Core Team (2018). R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. R Foundation for Statistical Computing. Vienna: R Development Core Team.
Smale, D. A., Wernberg, T., Oliver, E. C. J., Thomsen, M., Harvey, B. P., Straub, S. C., et al. (2019). Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 9, 306–312. doi: 10.1038/s41558-019-0412-1
Statnikov, R. B., and Matusov, J. B. (2002). Multicriteria Analysis in Engineering. Berlin: Springer.
Thrush, S. F., and Dayton, P. K. (2002). Disturbance to marine benthic habitats by trawling and dredging: implications for marine biodiversity. Ann. Rev. Ecol. Syst. 33, 449–473. doi: 10.1146/annurev.ecolsys.33.010802.150515
Ulanowicz, R. E. (1976). “The mechanical effects of water flow on fish eggs and larvae,” in Fisheries and Energy Production: a Symposium, ed. S. B. Saila (Lexington, MA: D.C. Heath), 77–87.
United Nations Environment Programme (2019). New UN Decade on Ecosystem Restoration to Inspire Bold UN Environment Assembly Decisions. Nairobi: United Nation Environment Programme.
Utomo, H. S., Wenefrida, I., and Linscombe, S. D. (2016). Use of an airplane and simulated aerial planting to evaluate seed coating, seed pelletization, and seeding rate on smooth cordgrass vegetation. Ecol. Restor. 34, 10–14. doi: 10.3368/er.34.1.10
Williams, S. L., Sur, C., Janetski, N., Hollarsmith, J. A., Rapi, S., Barron, L., et al. (2019). Large-scale coral reef rehabilitation after blast fishing in Indonesia. Restor. Ecol. 27, 447–456. doi: 10.1111/rec.12866
Keywords: aquaculture, coral reef restoration, coral-spawn slick, eco-engineering, Great Barrier Reef, harvest, marine invertebrate larvae, reseeding
Citation: Doropoulos C, Vons F, Elzinga J, ter Hofstede R, Salee K, van Koningsveld M and Babcock RC (2019) Testing Industrial-Scale Coral Restoration Techniques: Harvesting and Culturing Wild Coral-Spawn Slicks. Front. Mar. Sci. 6:658. doi: 10.3389/fmars.2019.00658
Received: 01 July 2019; Accepted: 09 October 2019;
Published: 25 October 2019.
Edited by:
Avigdor Abelson, Tel Aviv University, IsraelReviewed by:
Carolyn J. Lundquist, National Institute of Water and Atmospheric Research (NIWA), New ZealandSusana Perera-Valderrama, National Commission for the Knowledge and Use of Biodiversity (CONABIO), Mexico
Copyright © 2019 Doropoulos, Vons, Elzinga, ter Hofstede, Salee, van Koningsveld and Babcock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Doropoulos, Q2hyaXN0b3BoZXIuRG9yb3BvdWxvc0Bjc2lyby5hdQ==; Russell C. Babcock, UnVzcy5CYWJjb2NrQGNzaXJvLmF1
 Christopher Doropoulos
Christopher Doropoulos Focco Vons2,3
Focco Vons2,3 Remment ter Hofstede
Remment ter Hofstede Mark van Koningsveld
Mark van Koningsveld Russell C. Babcock
Russell C. Babcock