- 1NORCE Environment, Norwegian Research Centre (NORCE), Bergen, Norway
- 2Department of Biological Sciences, University of Bergen, Bergen, Norway
- 3Harbor Branch Oceanographic Institute, Florida Atlantic University, Fort Pierce, FL, United States
Carnivorous sponges belonging to family Cladorhizidae (Porifera, Demospongiae, Poecilosclerida) are unique within phylum Porifera due to their ability to capture and envelop small prey. While other sponges use an aquiferous system to filter water, the aquiferous system of the cladorhizids is partially reduced or completely absent. Carnivorous sponges can be found worldwide at all depths, but are more common in the deep sea, where oligotrophic conditions containing less suspended particles may favor a carnivorous feeding strategy. Here, we provide formal descriptions of eight cladorhizid sponge species from 1229 to 5813 m depth collected as part of the NOAA 2016 Deepwater Exploration of the Marianas with the NOAA ship Okeanos Explorer (EX1605) during the investigation of the bathyal and abyssal seafloor off the Northern Mariana Islands and Guam: the harp sponge Chondrocladia (Symmetrocladia) lyra, Chondrocladia (Chondrocladia) coronata sp. nov., Lycopodina subtile sp. nov., Abyssocladia fryerae sp. nov., Abyssocladia kellyae sp. nov., Abyssocladia marianensis sp. nov., Abyssocladia stegosaurensis sp. nov., and Abyssocladia villosa sp. nov. We also provide a phylogenetic analysis showing the systematic position of the described species. Our results show that the deep-sea seafloor off the Mariana Islands contains a diverse and previously virtually unknown cladorhizid fauna. The examined species display similarities with species from other parts of the Pacific, and add considerably to the available information for the genus Abyssocladia. The high number of new species is consistent with other recent publications from the Pacific and Indian Ocean, and shows that despite recent sampling efforts, the cladorhizid fauna of these areas remains very poorly investigated. Additional sampling effort will in all probability yield both additional species and a better understanding of the biogeographical distribution of already known cladorhizids.
Life Science Identifiers (LSIDs):
Abyssocladia fryerae urn:lsid:zoobank.org:act:BD7C52A3-146E-48CC-917F-57D7081245BD.
Abyssocladia kellyae urn:lsid:zoobank.org:act:7174B51E-5145-4907-AA63-D218C66DF5E7.
Abyssocladia marianensis urn:lsid:zoobank.org:act:9761A542-C7F7-4449-BADD-E1F988454969.
Abyssocladia stegosaurensis urn:lsid:zoobank.org:act:02F01ACE-2AC1-406E-8C50-65A5EC97F8C5.
Abyssocladia villosa urn:lsid:zoobank.org:act:DAAA1ED2-5D36-40A3-9A78-55994 CDE9702.
Chondrocladia coronata urn:lsid:zoobank.org:act:ED85F7AD-84F8-47EC-88E6-87753 AD931C4.
Lycopodium subtile urn:lsid:zoobank.org:act:5F73AA9E-3182-49DD-8DD5-EDE501 C33E51.
Introduction
Carnivorous sponges represent a novel innovation within phylum Porifera. These sponges lack or have a reduced aquiferous system, and instead feed by passively capturing prey items such as larvae or small crustaceans (Vacelet, 2007). Carnivorous sponges are typically erect, with stalked or branching bodies and a large number of thin filaments. Prey items become entangled in the filaments, and are subsequently gradually enveloped and digested by sponge cells migrating to the area of contact (Vacelet and Duport, 2004). Carnivory is typically assumed to be an adaptation to oligotrophic conditions in the deep sea, where traditional filter feeding is less effective (Vacelet, 2007). While these sponges are reasonably common in shallow waters, they constitute a larger and more conspicuous part of the total sponge fauna at greater depths (Hestetun et al., 2017b).
All currently reported carnivorous sponges belong to Cladorhizidae Dendy, 1922, a large poecilosclerid family with nine genera containing more than 175 currently recognized species (Van Soest et al., 2019). The majority of early species descriptions are from the North Atlantic (e.g., Carter, 1874, 1876; Topsent, 1892; Lambe, 1900; Lundbeck, 1905; Topsent, 1909), though a smaller number of older species descriptions also exist for the Pacific (e.g., Ridley and Dendy, 1886; Lévi, 1964; Koltun, 1970).
Over the last 25 years, a number of studies have contributed to significantly expanding the number of described species and the known morphological variation within the family (e.g., Cristobo et al., 2005; Vacelet, 2007; Vacelet and Kelly, 2008; Vacelet et al., 2009; Kelly and Vacelet, 2011; Lopes et al., 2011; Dressler-Allame et al., 2016; Goodwin et al., 2017; Hestetun et al., 2017b; Lundsten et al., 2017). The high number of new species described in these articles highlights that the number and distribution of carnivorous sponges are still poorly known.
The Mariana Islands are a chain of islands, part of the Izu–Bonin–Mariana arc system, a convergent plate boundary formed by the subduction of the western Pacific plate. Parts of the system include the Mariana Trench, the Mariana Arc, and the Mariana Trough, a complex and geologically active area including seafloor at various depths and substrates, vent systems, and the volcanic islands of the Mariana Islands themselves (Stern et al., 2004).
Here, we describe carnivorous sponges collected during the first and third legs of the 2016 Deepwater Exploration of the Marianas (EX1605), a sampling effort in the area around the U.S. territories of Guam and the Commonwealth of the Northern Mariana Islands with the National Oceanic and Atmospheric Administration (NOAA) ship Okeanos Explorer. The present study provides descriptions of eight carnivorous sponges, including seven previously undescribed species, and expands the current knowledge of carnivorous sponges in the West Pacific in general, and the genus Abyssocladia Lévi, 1964, in particular.
Materials and Methods
Collection
While the sampling effort of the EX1605 cruise was conducted throughout the deep-sea areas on both sides of the Mariana Islands, most of the cladorhizid samples were recovered from the Mariana Trench part of the system on the eastern side of the islands during the third leg of the cruise (Figure 1). In all, nine carnivorous sponge specimens were collected at seven collection localities. Except for the western Mariana Trough specimen (1229 m), all species were recovered from depths below 3000 m (Table 1). Samples were collected with the ROV Deep Discoverer. Dives were continuously recorded, meaning HD video was available for in situ observation of specimen morphology in all but one species. Expedition data, including ROV video, are available from the NOAA Ocean Exploration and Research (OER) Digital Atlas Portal (National Oceanic and Atmospheric Administration [NOAA], 2019).
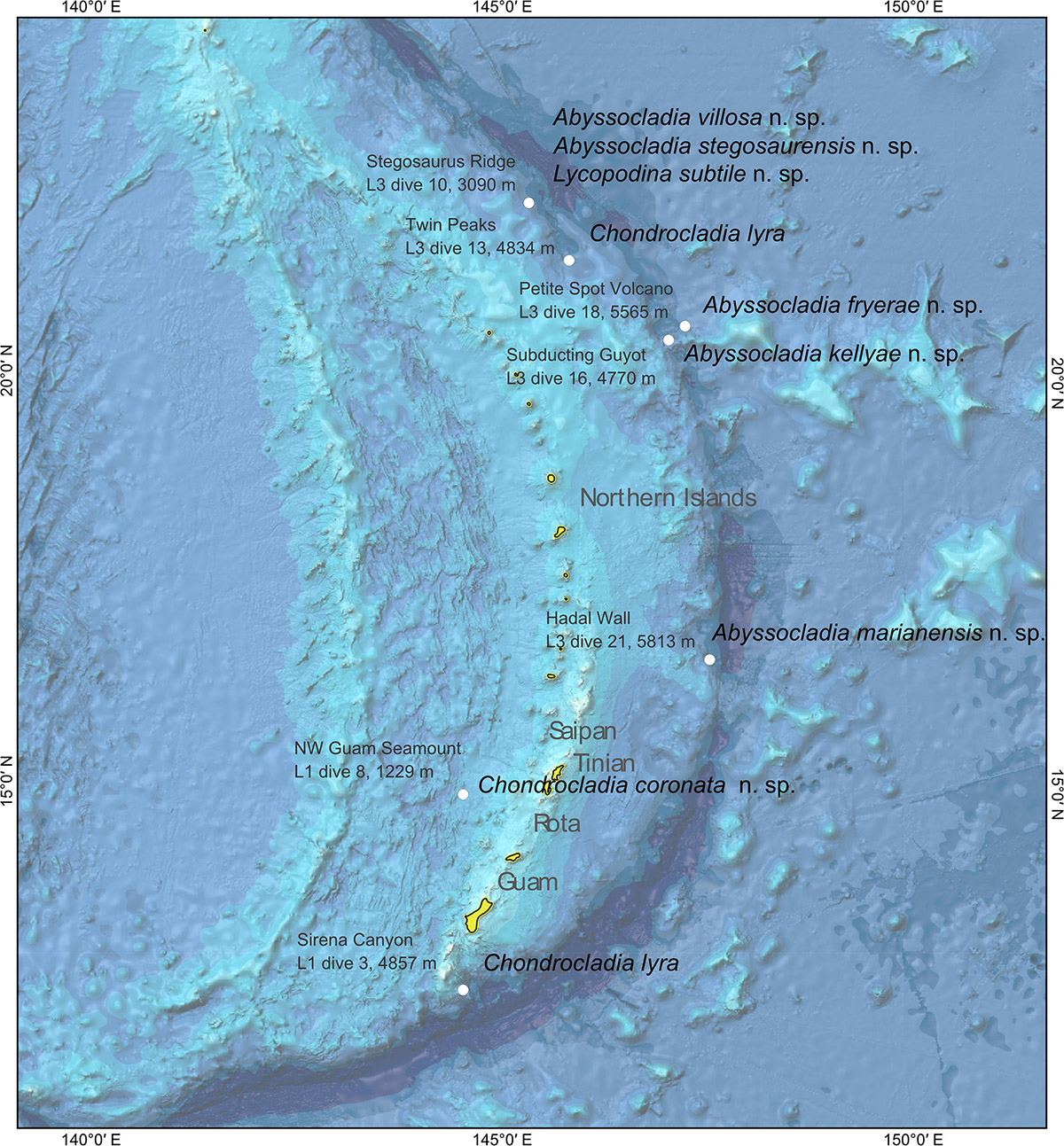
Figure 1. Bathymetric map of the sea floor around the Mariana Islands with collection localities for the species in this study.
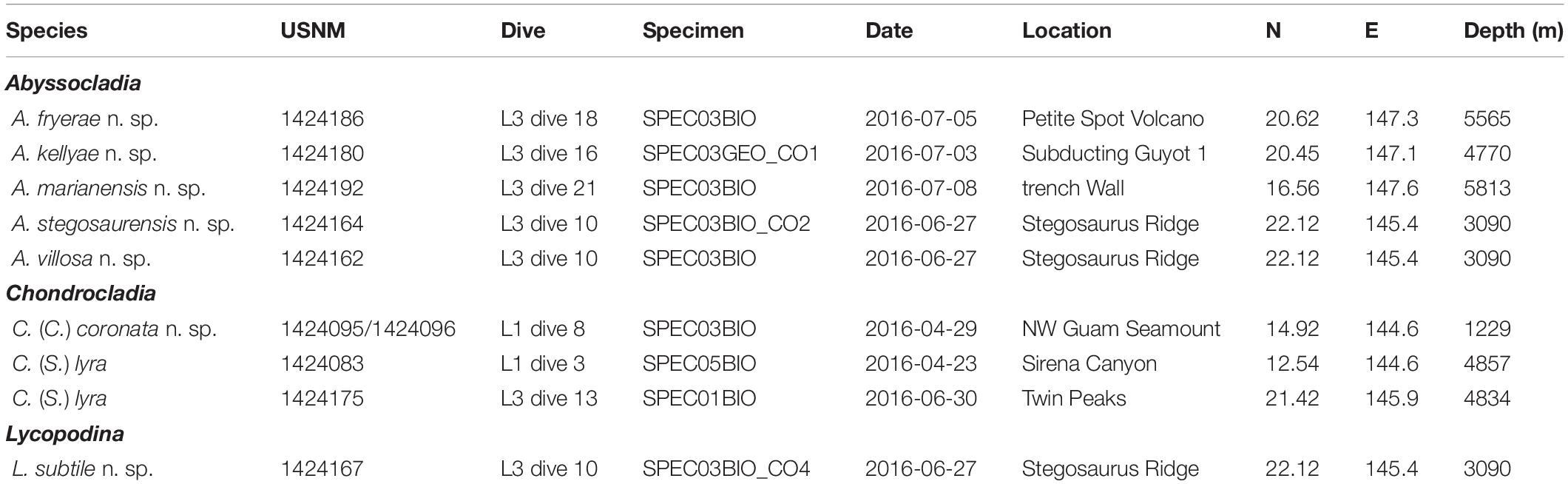
Table 1. Collection and voucher information for carnivorous sponge specimens examined in this study.
Most specimens were preserved in 96% ethanol only, or as several subsamples with different fixation media. Collected specimens were deposited in the U.S. Smithsonian Institution Museum of Natural History (voucher IDs in Table 1). Subsamples of some of the specimens were also sent to the Ocean Genome Legacy Center for possible future sequencing; however, molecular work here is based on the Smithsonian samples.
Morphological Examination
Examination of specimens was done at the biodiversity labs of the Department of Biosciences at the University of Bergen, Norway. Specimens were photographed on arrival in Bergen, and subsamples were taken for morphological and molecular analysis.
Spicule slides and scanning electron microscopy (SEM) preparations were made from sponge subsamples according to the method described in Boury-Esnault and Rützler (1997), either directly or by recovering the spicules during DNA extraction. A minimum of 30 measurements were made of each spicule type. In the case of diactine megascleres, both length and width (at widest point excluding tyles) were recorded. Measurements in the article are given as minimum and maximum, with average in parenthesis. Spicule terminology follows Boury-Esnault and Rützler (1997), with some additional conventions: fusiform styles are described as mycalostyles sensu Hajdu et al. (1994); chelae are described as arcuate or palmate sensu Hajdu et al. (1994); the term cleistochela is used for arcuate isochelae with opposite frontal teeth touching or almost touching, while the term abyssochela is used in a more restrictive sense for laterally expanded cleistochelae sensu Lopes et al. (2011).
Molecular Methods
Extraction and PCR protocols are identical to Hestetun et al. (2016b): The E.Z.N.A.® Mollusc DNA Kit (Omega Bio-tek) was used, and spicules were removed after initial lysis before adding ethanol. Three partitions were sequenced: partial 28S rRNA, ALG11, and the Folmer and Erpenbeck COI partitions. These partitions were chosen to enable comparison with sequences currently available in GenBank from previous studies, and have been shown to work well for identifying phylogenetic relationships within the Cladorhizidae (e.g., Hestetun et al., 2016b).
The analysis included 102 COI, 75 ALG11, and 91 28S sequences. See Supplementary Table S1 for accession numbers of all sequences included in the analysis. Accession numbers for new sequences are also listed for each species below. Alignment and concatenated phylogenetic analysis was performed using the same pipeline as in Hestetun et al. (2016b), including Bayesian inference with MrBayes 3.2.2 (GTR+G, 8 chains, 25*106 generations, 5*106 generations burn-in) and maximum likelihood (ML) with RAxML 8.2.10 (GTRGAMMA, 2000 rapid bootstraps).
Results
Taxonomic Index
Phylum Porifera Grant, 1836
Class Demospongiae Sollas, 1885
Subclass Heteroscleromorpha Cárdenas et al., 2012
Order Poecilosclerida Topsent, 1928
Family Cladorhizidae Dendy, 1922
Genus Abyssocladia Lévi, 1964
Abyssocladia fryerae n. sp.
Abyssocladia kellyae n. sp.
Abyssocladia marianensis n. sp.
Abyssocladia stegosaurensis n. sp.
Abyssocladia villosa n. sp.
Genus Chondrocladia Thomson, 1873
Subgenus Chondrocladia Thomson, 1873
Chondrocladia (Chondrocladia) coronata n. sp.
Subgenus Symmetrocladia Lee et al., 2012
Chondrocladia (Symmetrocladia) lyra Lee et al., 2012
Genus Lycopodina Lundbeck, 1905
Lycopodina subtile n. sp.
Species Description
Genus Abyssocladia Lévi, 1964
Type species
Abyssocladia bruuni Lévi, 1964 (type by monotypy).
Diagnosis
Cladorhizidae most often pedunculate, carrying a disciform or flabelliform body with a radial architecture, in other cases pinnate or branching. Microscleres are a combination of abyssochelae, cleistochelae, arcuate chelae, and/or sigmancistras, but not placochelae (from Hestetun et al., 2016b).
Remarks
The genus Abyssocladia contains 26 species in addition to the new species described here (Van Soest et al., 2019). Most species are recorded from the abyssal and deep bathyal Pacific, but some species have also been recorded from shallower areas and from the Indian and Atlantic Oceans.
Abyssocladia fryerae sp. nov.
Type material
R/V Okeanos Explorer EX1605L3, ROV Deep Discoverer. Holotype: USNM 1424186, dive 18, SPEC03BIO, 2016-07-05, Petite Spot Volcano, 20.62°N, 147.3°E, 5565 m.
Diagnosis
Pedunculate sponge composed of a short stem and a disc-shaped, slightly droplet-formed body with filaments radiating outward in a single plane from the disc margin. Megascleres are subtylostyles 550–1150 μm. Microscleres are arcuate isochelae 75–110 μm and sigmancistras 17–23 μm.
Description
The holotype (Figures 2A,B) is a damaged disc-shaped body with a radial skeletal organization, a pair of remaining damaged filaments, and a short piece of the upper stem. ROV images show that the live sponge is stalked and disc-shaped, with filaments radiating from the disc margin at regular intervals, and is connected to the substrate with a short stem. The holotype is 25 mm tall, but the top part of the disc is missing (Figure 2C). The stem is 15 mm long and 0.7–0.9 mm wide, and the disc is 12 mm wide. Based on ROV video, intact filaments are approximately 2 cm long. The color of the sponge is white to translucent gray in both live specimen and in ethanol.
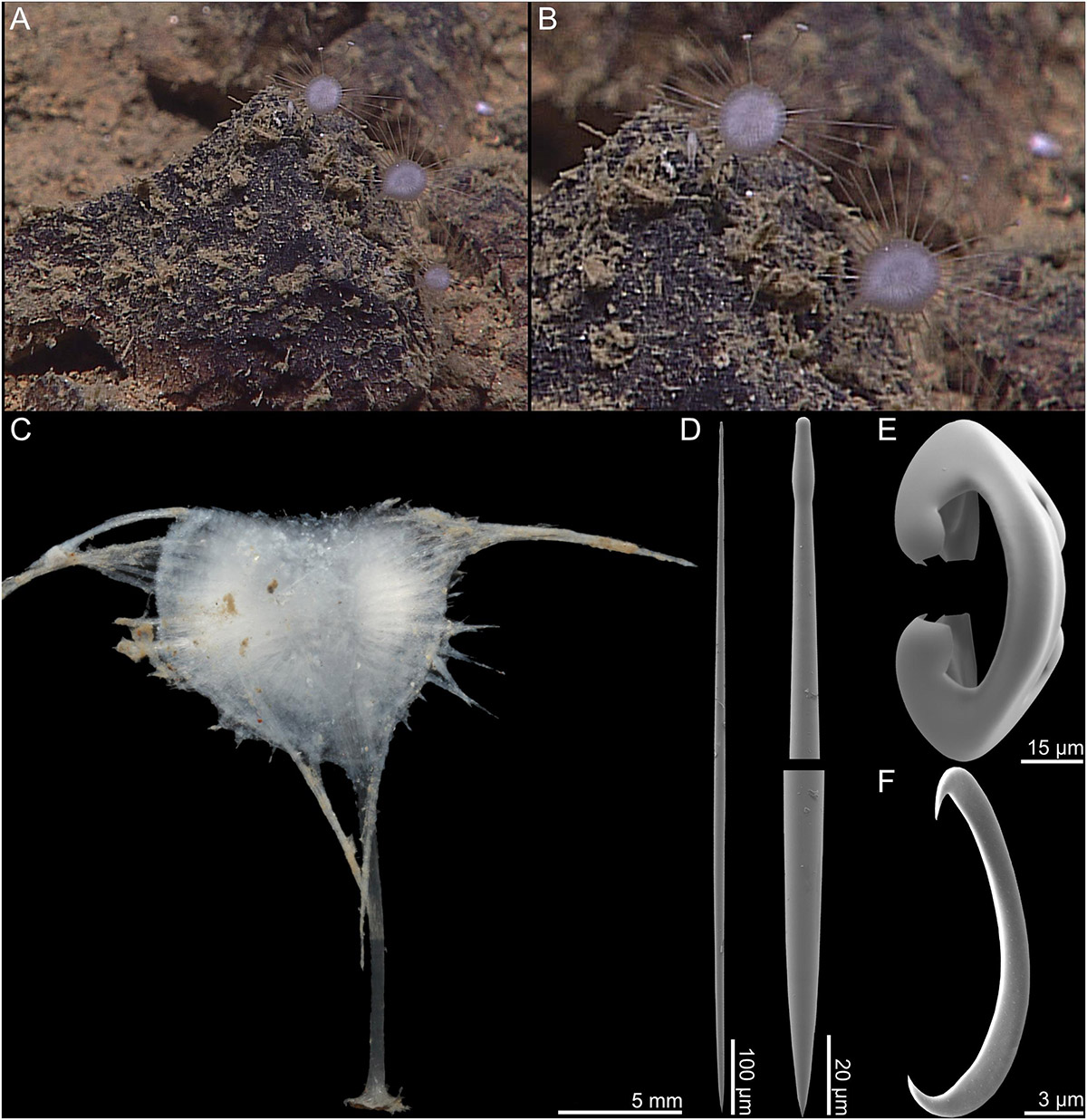
Figure 2. Abyssocladia fryerae n. sp. (A,B) In situ images from ROV video, (C) complete recovered specimen, (D) subtylostyle with elongated tyle, (E) arcuate isochela, and (F) sigmancistra.
Skeleton
The main structural skeleton of the stem is made up of tightly packed subtylostyles and enters the center of the disc-shaped body. The subtylostyles also make up the skeleton of the filaments and the structural skeleton of the soft tissue of the disc.
Spicules
1. Subtylostyles, straight and fusiform, with elongated tyles, 582–1130 (806) μm long and 3.8–23.5 (11.3) μm wide (Figure 2D).
2. Arcuate isochelae, with strongly curved shaft and frontal tooth and lateral alae of equal length, around 45% of total length 77.9–110.3 (91.6) μm (Figure 2E).
3. Sigmancistras, thick, 17.9–22.9 (20.5) μm (Figure 2F).
GenBank accession numbers
COI, MK922984.
Distribution and habitat
This species is only known from its type locality off the Mariana Islands at the Petite Spot Volcano locality (5565 m) from hard/rocky substrate situated on a small rock.
Etymology
The species is named after Dr. Patricia Fryer, Hawai'i Institute of Geophysics and Planetology, University of Hawai'i at Mānoa, in recognition of >30 years of exploration, discovery, and research on the geology and tectonic evolution of the Mariana forearc. Dr. Fryer was co-science lead on leg 3 of EX1605 and shared in the excitement of discovery and collection of carnivorous sponges from the Mariana Islands.
Remarks
A disc-shaped Abyssocladia species closely related to similar Pacific species such as A. kellyae n. sp., A. dominalba Vacelet, 2006, and A. lakwollii Vacelet and Kelly, 2014 (see remarks for A. kellyae n. sp., phylogenetic results and Table 2). The stem is shorter and the disc larger relative to A. kellyae n. sp.
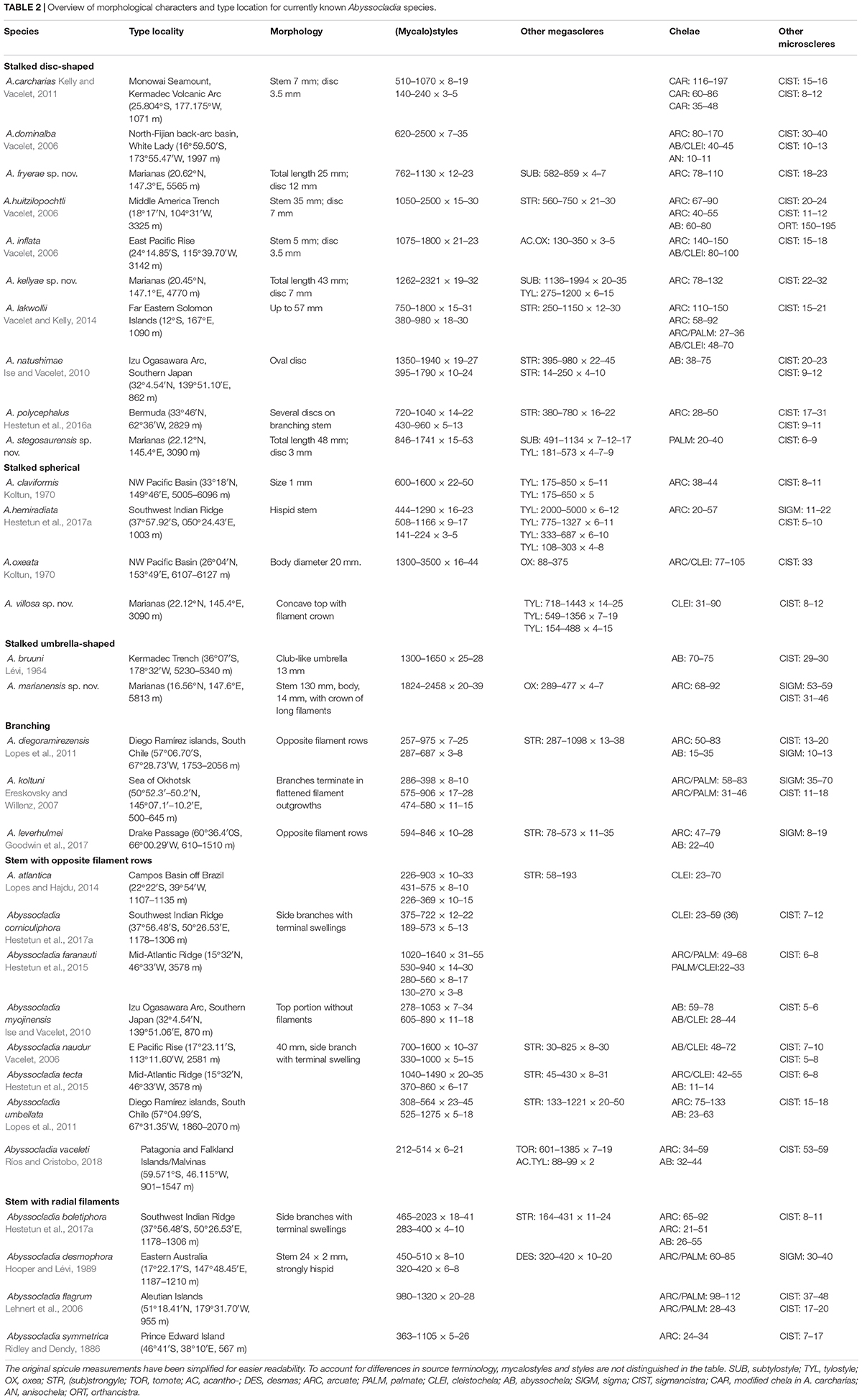
Table 2. Overview of morphological characters and type location for currently known Abyssocladia species.
Abyssocladia kellyae sp. nov.
Type material
R/V Okeanos Explorer EX1605L3, ROV Deep Discoverer. Holotype: USNM 1424180, dive 16, SPEC03GEO_CO1, 2016-07-03, Subducting Guyot 1, 20.45°N, 147.1°E, 4770 m.
Diagnosis
Stalked sponge with a disc-shaped body and filaments radiating in a single plane from the disc margin. Megascleres are mycalostyles 1250–2400 μm, subtylostyles 1000–2000 μm, and tylostyles 270–600 μm. Microscleres are arcuate isochelae 75–135 μm and sigmancistras 21–32 μm.
Description
The holotype (Figures 3A,B) is a single stalked sponge with a vertical, flat, disc-shaped body and a solid, flexible stem. The edge of the disc is set with evenly spaced filaments radiating outward in a single plane. The lower stem is widened into a basal plate connected to hard substrate. The holotype is 43 mm tall. The disc is 7 mm in diameter excluding filaments. The stem is 35 mm long and 0.5–0.7 mm wide. The color of the sponge is white to slightly gray in ethanol. No in situ video was available for this specimen.
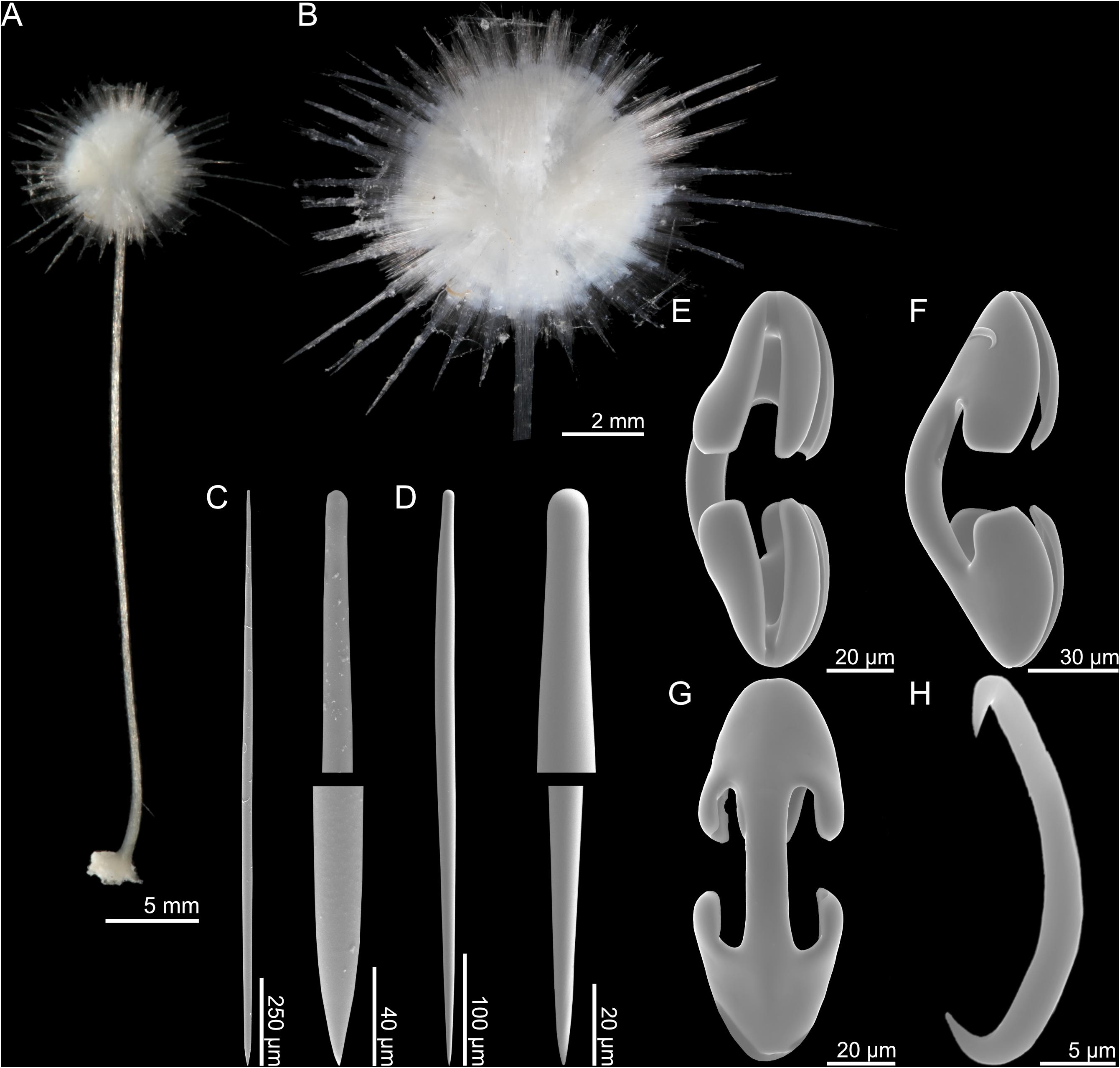
Figure 3. Abyssocladia kellyae n. sp. (A) whole specimen, (B) detail, (C) mycalostyle, (D) subtylostyle, (E–G) arcuate anisochela front, side and back view, and (H) sigmancistra.
Skeleton
The main structural skeleton is composed of tightly arranged longitudinal mycalostyles. The stem is solid and bare, with no visible surface layer. The mycalostyles of the stem reach into the middle of the sponge body. Radiating filaments are also composed of mycalostyles, while subtylostyles and tylostyles are found within the sponge body itself.
Spicules
4. Mycalostyles, straight, fusiform, in the stem, 1262–2321 (1668) μm long, and 18.7–32.5 (25.7) μm wide (Figure 3C).
5. Subtylostyles, straight, fusiform, 1018–1994 (1638) μm long, and 20.0–35.2 (27.2) μm wide (Figure 3D).
6. Tylostyles, straight, 275–592 (485) μm long, and 6.3–15.1 (9.3) μm wide.
7. Arcuate isochelae, with frontal tooth and lateral alae of equal length, around 45% of total length, and strongly curved shaft 78.3–132.3 (118.3) μm (Figures 3E–G).
8. Sigmancistras, thick, 21.6–31.7 (27.7) μm (Figure 3H).
GenBank accession numbers
28S rRNA, MK935688; ALG11, MK922989.
Distribution and habitat
This species is only known from its type locality off the Mariana Islands at the Subducting Guyot 1 locality (4770 m). The specimen was attached by its basal plate to a geological sample.
Etymology
This species is named after Dr. Michelle Kelly at the New Zealand National Institute of Water and Atmospheric Research (NIWA), for her numerous contributions to sponge science, and her work on deep-sea carnivorous and non-carnivorous sponges from the SW Pacific in particular.
Remarks
This species shares similarities with other disc-shaped Abyssocladia species. It is a close relative to A. fryerae n. sp. and has roughly similar chela and sigmancistra sizes, but has significantly longer subtylostyles and tylostyles are present (lacking in A. fryerae n. sp.) (Table 2). The phylogenetic results show a close relationship between this species, A. marianensis n. sp. and A. fryerae n. sp., together with the other Pacific disc-shaped species A. dominalba and A. lakwollii.
Abyssocladia marianensis sp. nov.
Type material
R/V Okeanos Explorer EX1605L3, ROV Deep Discoverer. Holotype: USNM 1424192, dive 21, SPEC03BIO, 2016-07-08, trench wall, 16.56°N, 147.6°E, 5813 m.
Diagnosis
Stalked sponge composed of a long stem connected to hard substrate with a small basal plate and ending in an umbrella-shaped body with a pointed apex containing numerous embryos and a horizontally arranged crown of approximately 50 filaments emerging from the sponge margin initially turning slightly downward, then bending slightly upward. Megascleres are mycalostyles, 1800–2500 μm, and oxeas 250–500 μm. Microscleres are arcuate isochelae 65–95 and 24–45 μm, and sigmancistras 50–65 and 30–50 μm.
Description
The holotype (Figures 4A,C) consists of a long, rigid, but flexible stem, and an apical, slightly pointed umbrella-shaped body with a single, horizontal filament crown of around 50 filaments emerging from the body margin. Proximal parts of filaments turn slightly downward, and then gently upward at distal end. The sponge is connected to hard substrate with a small basal plate. Numerous embryos are clearly visible through the outer layer of the apical point of the body (Figure 4D). Only the proximal parts of the filaments were recovered in the holotype specimen. The holotype is 13 cm tall, and the body 14 mm tall and 20 mm wide excluding filaments. The color of the body is white-to-light beige, while the stem is darker beige in both live specimen and in ethanol.
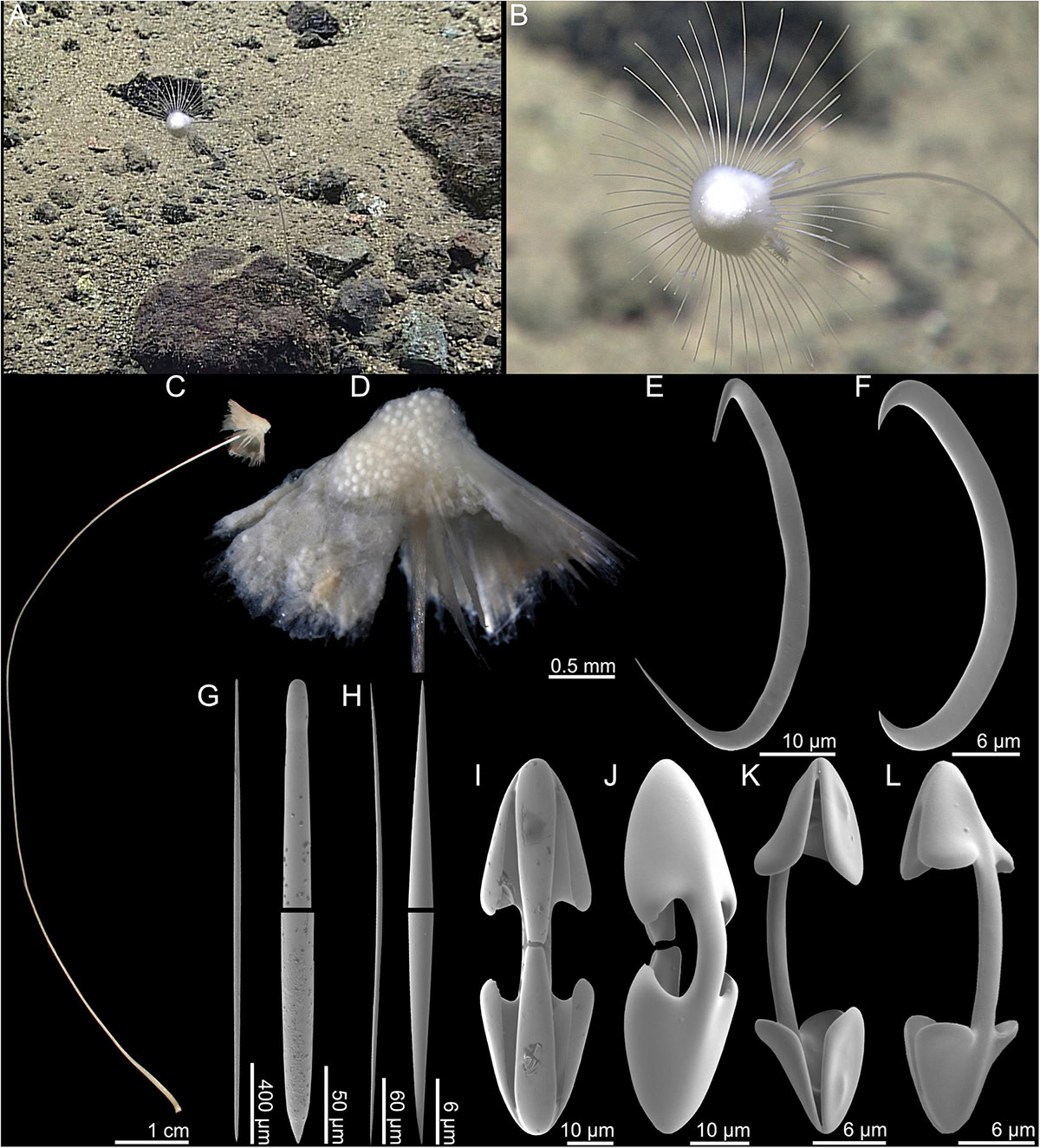
Figure 4. Abyssocladia marianensis n. sp. (A,B) In situ images from ROV feed, (C) whole specimen, (D) detail of body, (E) sigmancistra 1, (F) sigmancistra 2, (G) mycalostyle with faint tyle, (H) oxea, (I) cleistochela front view, (J) cleistochela back view, (K) palmate chela front view, and (L) palmate chela back view.
Skeleton
The skeleton of the stem is composed of tightly packed mycalostyles which are slightly twisted in a counter-clockwise direction. The umbrella is made up of radiating bundles of mycalostyles creating the skeleton of the filaments with soft tissue in between. Oxeas are associated with the fleshy top part containing embryos.
Spicules
9. Mycalostyles, straight, fusiform, very slightly tylote, 1824–2458 (2178) μm long, and 19.7–39.0 (31.3) μm wide (Figure 4G).
10. Oxeas, straight, fusiform 289–477 (413) μm long, and 4.1–7.2 (5.7) μm wide (Figure 4H).
11. Palmate chelae/cleistochelae, palmate forms more common, with frontal tooth and lateral alae of equal length, around 40% of total length, and strongly curved shaft 67.9–91.7 (81.8) μm (Figures 4I,J).
12. Palmate chelae/cleistochelae, palmate forms more common, with slightly curved shaft and alae size slightly less than one-third of total chela size, 26.4–42.4 (36.1) μm (Figures 4K,L).
13. Sigmancistras 1, 50.5–64.9 (57.3) μm (Figure 4E).
14. Sigmancistras 2, 30.7–45.5 (35.8) μm (Figure 4F).
GenBank accession numbers
COI, MK922985; 28S rRNA, MK935689; ALG11, MK922990.
Distribution and habitat
This species is only known from its type locality on a trench wall off the Mariana Islands at the (5813 m) on mixed sandy and rocky substrate situated on a small rock.
Etymology
This species is named after its collection locality on a vertical wall of the Mariana Trench off the Mariana Islands.
Remarks
The long stem and umbrella-shaped morphology of this species is unusual for the genus: The only other Abyssocladia species described as umbrella-like is A. bruuni Lévi, 1964, but the only illustration of this species is in Koltun (1970) which shows a clavate species. Furthermore, Lévi (1964) gives the size of A. bruuni as 10 mm, which is much smaller than the present specimen.
Morphologically, it is more reminiscent of a group of deep-sea Cladorhiza species such as C. mirabilis Ridley and Dendy, 1886 (S Pacific, 4115 m), C. longipinna Ridley and Dendy, 1886 (Hawai'i, 5486 m), C. similis Ridley and Dendy, 1886 (mid-Pacific, 4362 m), C. kensmithi Lundsten et al., 2017, C. mexicana Lundsten et al., 2017, and C. hubbsi Lundsten et al., 2017. No molecular data are available for Cladorhiza species with stalked rather than branching morphology, which makes it difficult to further investigate evolutionary relationships at this time. Molecular data for the present species show a close relationship with the group of Pacific disc-shaped Abyssocladia species also including A. kellyae n. sp. and A. fryerae n. sp.
Abyssocladia stegosaurensis sp. nov.
Type material
R/V Okeanos Explorer EX1605L3, ROV Deep Discoverer. Holotype: USNM 1424164, dive 10, SPEC03BIO_CO2, 2016-06-27, Stegosaurus Ridge, 22.12°N, 145.4°E, 3090 m.
Diagnosis
Stalked sponge with a disc-shaped body and filaments radiating in a single plane from the disc margin. Megascleres are mycalostyles 840–1800 μm, subtylostyles 490–1140 μm, and tylostyles 180–580 μm. Microscleres are palmate isochelae, 20–40 μm, and sigmancistras, 5–10 μm.
Description
The holotype (Figures 5A,B) is a stalked sponge consisting of a vertical, disc-shaped body on top of a rigid, but flexible stem. Filaments emerge in a radial pattern from the disc edge (Figure 5C). The holotype is 48 mm tall. The body excluding filaments is 3 mm in diameter, with filaments up to 6 mm in length. The stem is solid and compact, 45 mm long, and 0.8–1 mm in diameter. The color of the sponge is translucent white to slightly gray in both the live specimen and in ethanol.
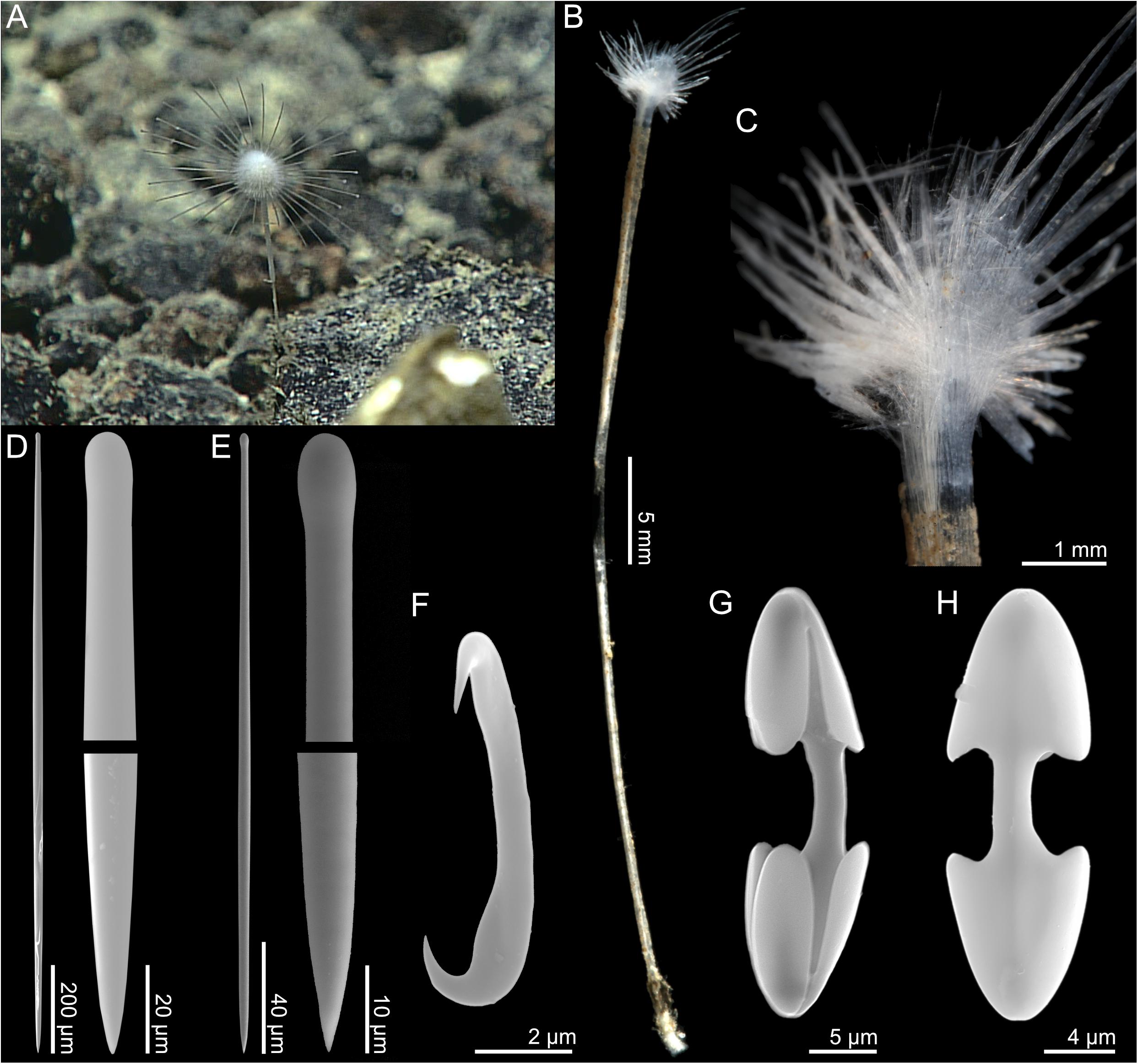
Figure 5. Abyssocladia stegosaurensis n. sp. (A) In situ image from ROV feed, (B) whole specimen, (C) detail, (D) mycalostyle, (E) tylostyle, (F) sigmancistra, (G) palmate chela front view, and (H) palmate chela back view.
Skeleton
The main structural skeleton consists of a core axis made up of longitudinal, tightly arranged mycalostyles. The stem is bare, with little to no soft tissue. Subtylostyles and tylostyles are found in the fleshy parts of the radial sponge body.
Spicules
1. Mycalostyles, very slightly tylote, large, straight, fusiform, 846–1741 (1478) μm long, and 14.8–52.9 (14.8–52.9(28.8)) μm wide (Figure 5D).
2. Subtylostyles, straight, fusiform, 491–1134 (736) μm long, and 7.3–16.7 (11.7) μm wide (Figure 5E).
3. Tylostyles, straight, not fusiform, 181–573 (423) μm long, and 4.5–9.4 (7.0) μm wide.
4. Palmate isochelae, with frontal tooth and lateral alae of equal length, around 40% of total length, 20.4–39.7 (31.1) μm (Figures 5H,I).
5. Sigmancistras, thick, 5.8–9.3 (7.4) μm (Figure 5G).
GenBank accession numbers
28S rRNA, MK935690; ALG11, MK922991.
Distribution
While there were several possible observations of the species on the video feed, this species was only collected from the type locality off the Mariana Islands on the Stegosaurus Ridge (3090 m) from hard/rocky substrate situated on a small rock.
Etymology
This sponge is named after the collection locality on the Stegosaurus Ridge off the Northern Mariana Islands.
Remarks
This species shares similarities with other disc-shaped Abyssocladia species from the Pacific. Morphologically it is close to A. kellyae n. sp. and A. fryerae n. sp. in megasclere types and sizes as well as lack of either abyssochelae or cleistochelae. Chelae are small compared to other disc-shaped species in the genus (Table 2). The phylogenetic results here imply a more distant relationship, however, placing this species in a basal position relative to other Abyssocladia species described in this article.
Abyssocladia villosa sp. nov.
Type material
R/V Okeanos Explorer EX1605L3, ROV Deep Discoverer. Holotype: USNM 1424162, dive 10, SPEC03BIO, 2016-06-27, Stegosaurus Ridge, 22.12°N, 145.4°E, 3090 m.
Comparative material examined
Abyssocladia hemiradiata Hestetun et al. (2017a), holotype (BMNH 2016.1.13.3).
Diagnosis
Stalked, hispid sponge with a cup-shaped body and horizontal, radial, slightly apically oriented filament crown close to the top margin. Upper stem widens in a conical fashion close to the body. Megascleres are fusiform tylostyles with the broadest part close to the tip, 865–1443 μm, two size categories of straight non-fusiform tylostyles 549–1356 and 154–457 μm long. Microscleres are cleistochelae, 38.2–87.5 μm, and sigmancistras, 7.6–10.0 μm.
Description
The holotype (Figures 6A,B) is a single specimen of a stalked sponge consisting of a cup-shaped body with filament crown close to the apical margin, radiating outward and slightly upward, and a hispid stem connected to hard substrate with a small basal plate. The holotype is 5 cm long from the base to the top of the body. The body is 5.5 mm tall and 7.5 mm in diameter. The stem is 45 mm long and 0.5–2 mm in diameter, widening toward the top (Figures 6C,D). The color of the body is white to white gray, while the stem is light brown in both live specimen and in ethanol.
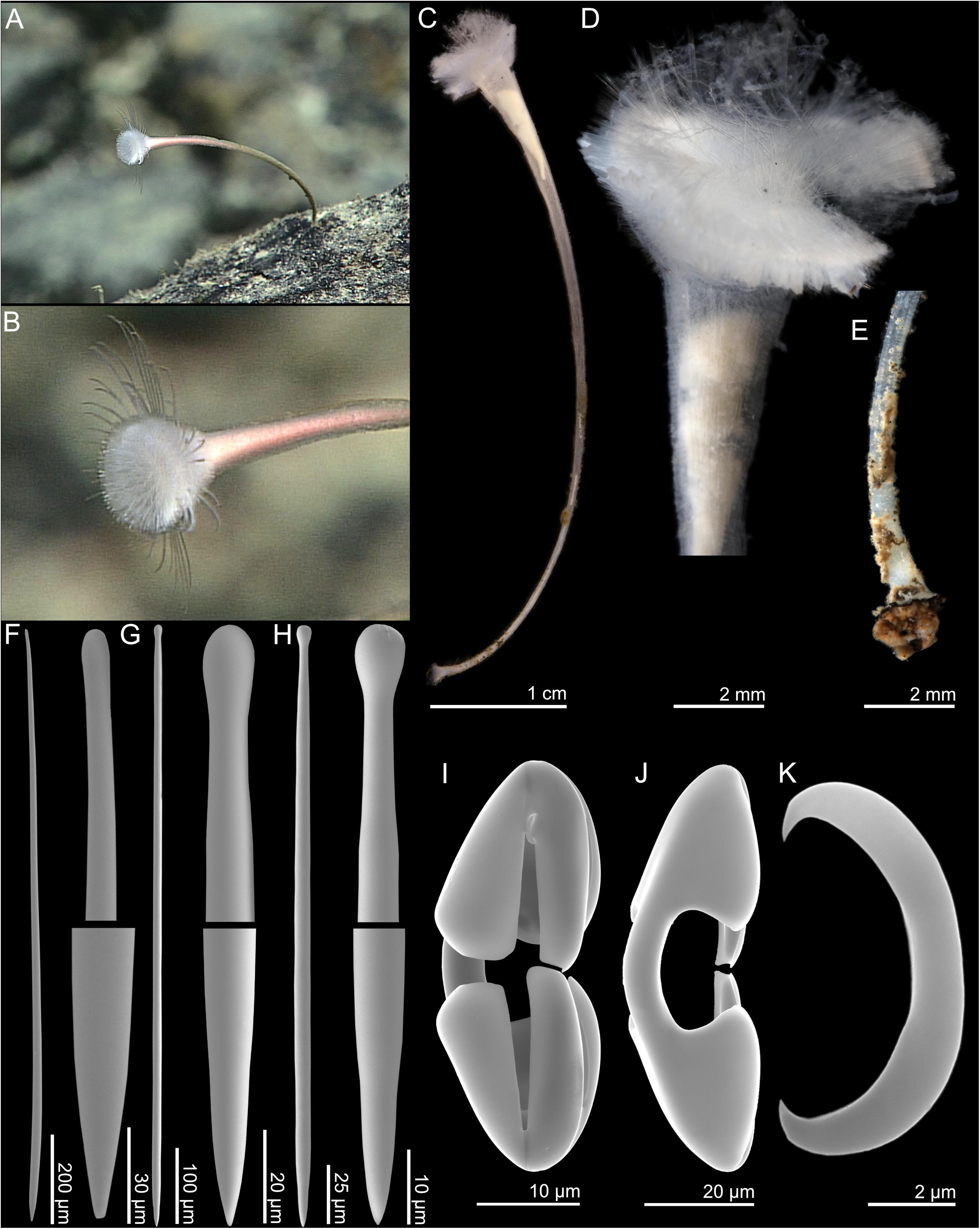
Figure 6. Abyssocladia villosa n. sp. (A,B) In situ images from ROV feed, (C) whole specimen, (D) detail of body, (E) detail of basal plate, (F) subtylostyle, (G) tylostyle I, (H) tylostyle II, (I) cleistochela front view, (J) cleistochela back view, and (K) sigmancistra.
Skeleton
The skeleton is composed of a solid core of tightly packed, large longitudinal subtylostyles. The core skeleton of the stem is covered with a thin surface layer of soft tissue, which contains numerous plumose brushes of smaller tylostyles that project outward and give the specimen a hispid surface. The structural skeleton widens toward the top body, which contains a radiating skeleton of the larger tylostyles (Figures 6D,E).
Spicules
1. Subtylostyles, straight, fusiform, with the thickest part closest to the tip, and gradually tapering toward the base, 718–1443 (1080) μm long, and 13.8–24.5 (20.8) μm wide (Figure 6F).
2. Tylostyles I, straight, not fusiform, 549–1356 (923) μm long, and 7.1–18.7 (12.1) μm wide (Figure 6G).
3. Tylostyles II, straight, not fusiform, making up the plumose brushes of the stem, 154–488 (282) μm long, and 4.3–14.8 (7.3) μm wide (Figure 6H).
4. Cleistochelae, arcuate isochelae with frontal tooth touching or nearly touching, 30.5–90.3 (58.5) μm (Figures 6I,J). This spicule has a large size range, but there is a continuous size distribution, so it is presented as a single category here.
5. Sigmancistras, thick, 7.6–12.4 (9.4) μm (Figure 6K).
GenBank accession numbers
COI, MK922986; 28S rRNA, MK935691; ALG11, MK922992.
Distribution and habitat
This species is only known from its type locality off the Mariana Islands on the Stegosaurus Ridge (3090 m) from hard/rocky substrate situated on a large rock.
Etymology
From Latin “villosa” meaning shaggy or hairy. The sponge is named after the hispid surface of the stem and apical body.
Remarks
Other Abyssocladia species with a spherical pedunculate body include A. claviformis Koltun, 1970 and A. oxeata Koltun, 1970. These were both collected from the NW Pacific basin, and are possible close relatives to A. villosa n. sp.
This species has a hispid surface due to the presence of plumose brushes of the shorter category of tylostyle projecting through the outer layer of the stem. A similar arrangement is found in a small number of other species in the genus, including A. claviformis Koltun, 1970, A. desmophora (Hooper and Lévi, 1989), and A. hemiradiata Hestetun et al., 2017a, and the phylogenetic results confirm a close relationship between this species and A. hemiradiata.
Genus Chondrocladia Thomson, 1873
Type species
Chondrocladia (Chondrocladia) virgata Thomson, 1873 (type by monotypy).
Diagnosis
Cladorhizidae with anchorate isochelae (from Lee et al., 2012).
Remarks
Genus Chondrocladia contains 37 species in three subgenera (Van Soest et al., 2019). While species in the genus usually have defined, regional distributions, the genus as a whole is cosmopolitan. Certain species have a shallower distribution of 500–1000 m, while others have been reported from lower bathyal and abyssal depths.
Subgenus Chondrocladia Thomson, 1873
Type species
Chondrocladia (Chondrocladia) virgata Thomson, 1873 (type by monotypy).
Diagnosis
Chondrocladia without a layer of special spicules (subtrochirhabds or trochirhabds), lacking special rostriform (snout-like) subtylostyles in filaments or terminal balls, and without planar vanes formed of evenly spaced upright branches (from Lee et al., 2012).
Remarks
Subgenus Chondrocladia contains 30 of the 37 species in genus Chondrocladia (Van Soest et al., 2019). It is defined as Chondrocladia species lacking any of the diagnostic criteria for the other two subgenera, Meliiderma and Symmetrocladia.
Chondrocladia (Chondrocladia) coronata sp. nov.
Type material
R/V Okeanos Explorer EX1605L3, ROV Deep Discoverer. Holotype: USNM 1424095-1424096, dive 8, SPEC03BIO, 2016-04-29, Northwest Guam Seamount, 14.92°N, 144.6°E, 1229 m (subsample: Ocean Genomic Legacy S24102).
Diagnosis
Erect sponge consisting of a single stem with filaments emerging in a radial pattern along its whole length. Filaments become longer toward the top of the sponge. The top of the main stem widens into a funnel shape with a ring-like margin and a secondary crown of smaller filaments at a 45° angle pointing apically. Megascleres are straight, fusiform mycalostyles, 1430–2100 μm, and straight, fusiform subtylostyles 475–950 μm. Microscleres are three categories of anchorate isochelae, 40–60, 20–32, and 15–20 μm, and sigmas, 30–50 μm.
Description
The holotype is composed of two fragments of an erect sponge composed of a single stem with numerous side filaments; the larger fragment (USNM 1424096) is a 38 mm piece from the top of the sponge including part of the enlarged apical body (Figure 7C), while the smaller fragment (USNM 1424095) is an 8 mm segment of the stem (Figure 7D). Both fragments are damaged, and it is only possible to get a complete idea of the morphology of the sponge by examination of the ROV video (Figures 7A,B). Based on the video data and recovered fragments, the complete sponge is likely around 10–15 cm tall. The image of the live sponge shows around 150 filaments, emerging in all directions from the stem across its whole length, with filaments getting gradually longer closer to the top of the sponge. The top of the sponge contains a funnel-shaped enlargement ending in a fleshy, horizontal ring with a shallow central depression. A secondary, smaller set of around 30 filaments emerge from this ring at a 45° upward angle. Embryos are present in the main stem. The sponge is attached to a rock with a small base, which was not recovered. The color of the live sponge varies from light beige in the main stem, to light gray and white in filaments and top part.

Figure 7. Chondrocladia coronata n. sp. (A,B) In situ images from ROV feed, (C) whole specimen, (D) stem detail, (E) style, (F) subtylostyle, (G) anchorate chela 1, (H) anchorate chela 2, (I) anchorate chela 3, and (J) sigma.
Skeleton
The main structural skeleton of the stem and filaments is made up of a core of longitudinal, tightly packed mycalostyles. Subtylostyles are associated with the fleshy outer surface of the stem and top part, where they are arranged to support the associated tissue. All anchorate chelae size classes are found throughout the sponge. Sigmas are found in the apical crown part of the sponge.
Spicules
1. Mycalostyles, straight, fusiform, 1430–2104 (1734) μm long, and 26.9–52.0 (39.4) μm wide (Figure 7E).
2. Subtylostyles, straight, fusiform, 488–948 (642) μm long, and 10.2–27.6 (17.0) μm wide (Figure 7F).
3. Anchorate isochelae 1, 40.9–59.8 (52.5) μm, with three teeth of equal length, each around 20% of total length and curved shaft (Figure 7G).
4. Anchorate isochelae 2, 21.1–31.5 (26.2) μm, with three teeth of equal length, each around 30% of total length and curved shaft (Figure 7H).
5. Anchorate isochelae 3, 14.9–19.6 (17.4) μm, with three teeth of equal length, each around 30% of total length and curved shaft (Figure 7I).
6. Sigmas, 33.6–48.7 (39.6) μm (Figure 7J)
GenBank accession numbers
COI, MK922987; 28S rRNA, MK935692; ALG11, MK922993.
Distribution and habitat
This species is only known from its type locality off the Mariana Islands at the Guam Seamount (1229 m) from mixed sandy and rocky substrate situated on exposed rock.
Etymology
From Latin, “coronata,” meaning crowned. Named after the filament crown of the apical body of the sponge.
Remarks
It is difficult to identify close relatives to this species given its unique general morphology within the genus: Chondrocladia species are usually either pedunculate with a top body containing filaments, or elongated and club-shaped with fistules emerging from the stem, while this species has a stem with numerous smaller filaments as well as a well-developed top part.
The molecular data suggest a closer affinity to C. (S.) lyra and stalked species such as C. vaceleti and C. fatimae rather than large, club-shaped species such as C. grandis and C. concrescens. The current subgenus classification of Chondrocladia is likely not monophyletic (Hestetun et al., 2016b). As it is lacking diagnostic characters for the subgenera Meliiderma and Symmetrocladia, we have placed C. coronata n. sp. in subgenus Chondrocladia.
Subgenus Symmetrocladia Lee et al., 2012
Type species
Chondrocladia (Symmetrocladia) lyra Lee et al., 2012.
Diagnosis
Chondrocladia with biradial, triradial, tetraradial, or pentaradial symmetry of right triangles (vanes) formed of vertically aligned branches arising unilaterally from basal stolons. Without a stalk. Spicules are styles, rostriform (snout-like) subtylostyles in the filaments and on the terminal balls, and unguiferous anchorate isochelae and sigmas. Trochirhabds and forceps are absent (from Lee et al., 2012).
Chondrocladia (Symmetrocladia) lyra Lee et al., 2012
Examined material
R/V Okeanos Explorer, ROV Deep Discoverer. USNM 1424083, EX1605L1, dive 3, SPEC05BIO, 2016-04-23, Sirena Canyon, 12.54°N, 144.6°E, 4857 m (subsample: Ocean Genomic Legacy S24096). USNM 1424175, EX1605L3, dive 13, SPEC01BIO, 2016-06-30, Twin Peaks, 21.42°N, 145.89°E, 4834 m.
Comparative material examined
Chondrocladia (Symmetrocladia) lyra (subsample from holotype), CASIZ18877, South Escanaba Ridge, CA, 2005-08-30, dive T-891-A2, 40.98°N, 127.49°W, 3318 m.
Diagnosis
Chondrocladia with biradial, triradial, tetraradial, or pentaradial symmetry of right triangles (vanes) formed of vertically aligned branches arising unilaterally from basal stolons. Without a stalk. Spicules are styles, rostriform (snout-like) subtylostyles in the filaments and on the terminal balls, and unguiferous anchorate isochelae and sigmas. Trochirhabds and forceps are absent (from Lee et al., 2012).
Description
Two specimens were recovered, one from Sirena Canyon (4857 m) and one from Twin Peaks (4834 m). Specimen USNM 1424083 (Sirena Canyon) is composed of a short stem and two opposite vanes with 10 and 11 vertical branches (Figures 8A,B). The recovered specimen is around 13 cm from end to end, but lacks a couple of branches on each side, meaning the complete animal is larger in situ. Branches are around 4–5 cm in length. Both stolon and branches feature a large number of filaments 2–5 mm in length. Stolon or branch swellings are not apparent. ROV footage shows ophiuroids clinging to stolon and branches (Figure 8B).
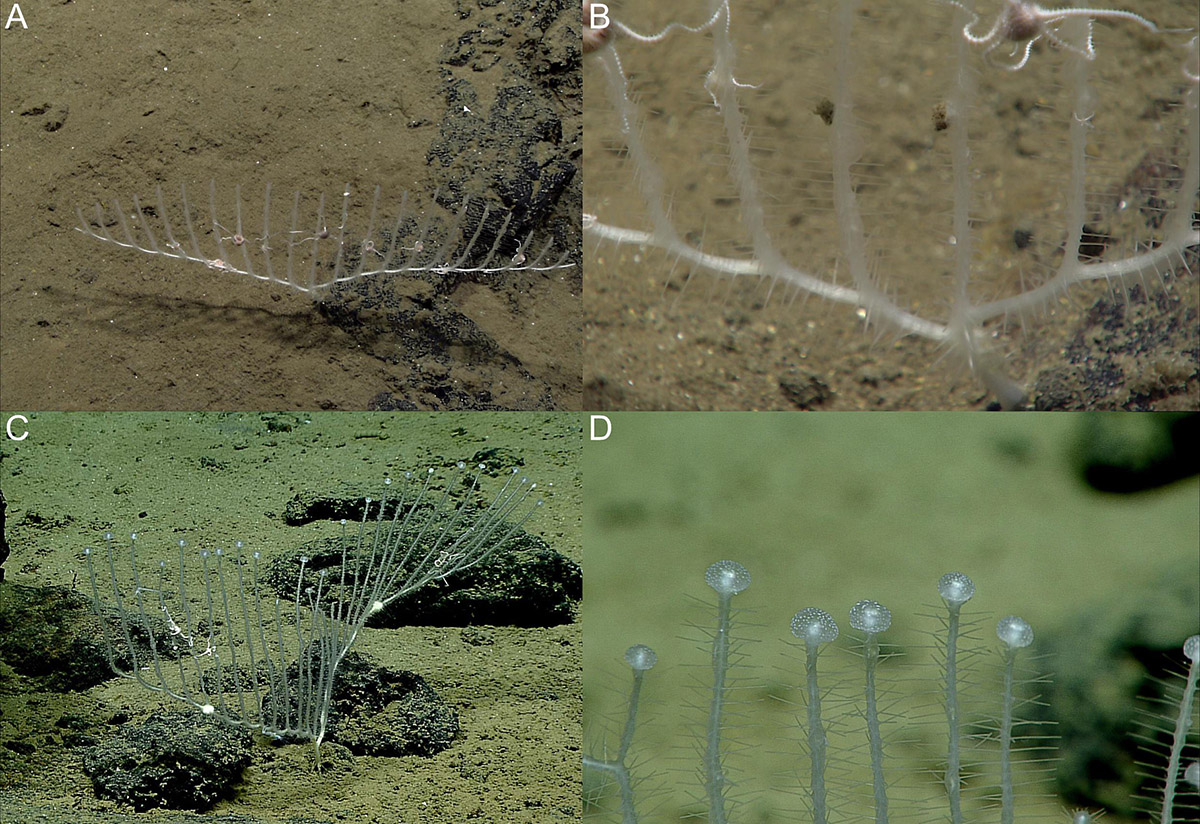
Figure 8. Chondrocladia (Symmetrocladia) lyra in situ images from ROV feed. (A,B) Specimen USNM 1424083 and (C,D) specimen USNM 1424173.
Specimen USNM 1424175 (Twin Peaks) also has two opposite vanes, but the recovered specimen is fragmented. Stolons carry 13 vertical branches each. Branches are longer than in the preceding specimen, and end with terminal swellings (Figures 8C,D, 9C), but no complete branches were recovered, so they could not be measured. The stolons also feature a couple of solid enlargements. Stolon and branches feature a large number of filaments in all directions from the stem.
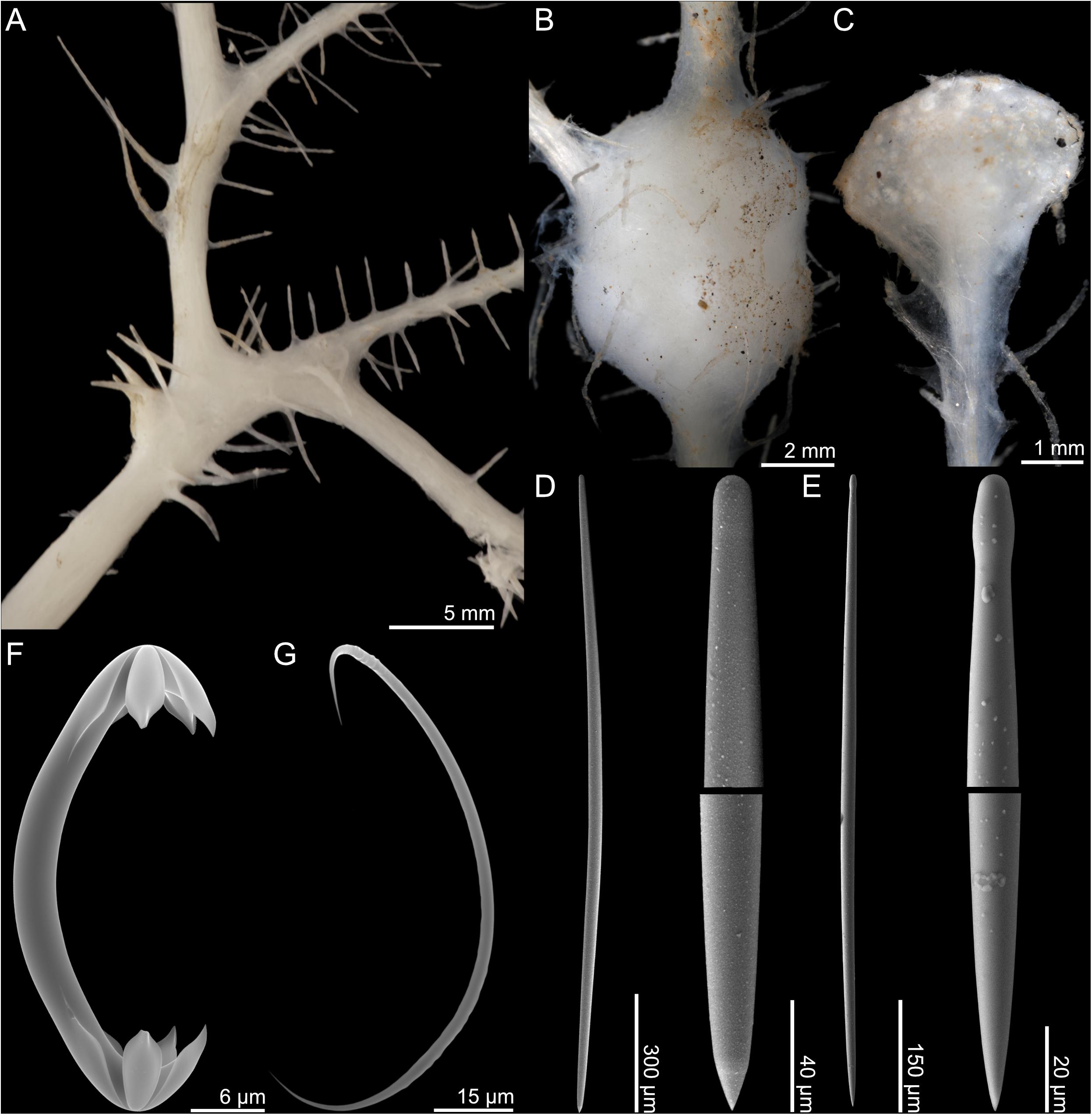
Figure 9. Chondrocladia (Symmetrocladia) lyra. (A) Branch detail from USNM 1424083, (B) stolon swelling from USNM 1424173, (C) terminal swelling from USNM 1424173, (D) style, (E) subtylostyle, (F) anchorate chela, and (G) sigma.
Both specimens were recovered on mixed sandy and rocky bottom, and are anchored into the sandy sediment with rhizoids.
Skeleton
The mycalostyles make up the main structural skeleton of the stolons and branches, while megascleres found in the filaments and swellings are subtylostyles. Isochelae are found in all parts of the sponge. Large sigmas are common in filaments, while small sigmas are confined to the swellings and were not found in specimen USNM 1424083.
Spicules
1. Mycalostyles, straight, fusiform, stolons and branch stems, 1425–1959 (1726) μm long, and 23.1–41.2 (35.4) μm wide (Figure 9D).
2. Subtylostyles, straight, fusiform, filaments, 740–1156 (858) μm long, and 12.6–22.0 (17.2) μm wide (Figure 9E).
3. Anchorate isochelae, 41.5–53.1 (47.1) μm, with three teeth of equal length, each around one-sixth of total length, and curved shaft (Figure 9F).
4. Sigmas, 78.7–117.7 (98.4) μm (Figure 9G). Associated mainly with filaments.
5. Sigmas, 45.9–64.2 (55.5) μm. Associated with terminal swellings.
GenBank accession numbers
USNM 1424083: COI, MK922988; 28S rRNA, MK935693; ALG11, MK922994. USNM 1424175: COI, MK922996.
Distribution and habitat
The type location for C. (S.) lyra is off California at the South Escanaba Ridge with additional reported sightings at the Monterey Canyon and a depth range of 3317–3503 m (Lee et al., 2012). The original source mentions that there are also unpublished records that states that distribution range is probably much larger than specimens specifically described in that article, and gives distribution as NE Pacific (Lee et al., 2012). The distance between the type locality and the Marianas confirms that the species is present on both sides of the Pacific, and most likely has a very wide distribution at bathyal to abyssal depths (∼3000–5000 m).
Remarks
This species is well described in the original article by Lee et al. (2012). Molecular data from the type material are included in the phylogeny of Hestetun et al. (2016b), and the molecular data here confirm that the present material belongs to the same species. Some of the type specimens are larger, with more than two vanes, but the present material is within the variation of the originally published set of specimens.
Because of its unique morphology, Lee et al. (2012) erected a new, monotypic subgenus, Symmetrocladia, for C. (S.) lyra. Molecular results in Hestetun et al. (2016b) suggest a basal position to smaller, pedunculate Chondrocladia species sometimes informally referred to as “Crinorhiza” type in older literature (e.g., Topsent, 1930; Lévi, 1993).
Genus Lycopodina Lundbeck, 1905
Type species
Lycopodina lycopodium (Levinsen, 1887) (type by subsequent designation).
Diagnosis
Pedunculate Cladorhizidae with body either in the form of an erect stem or sphere with filaments in all directions, or cup-shaped. Megascleres are mycalostyles and commonly shorter (tylo)styles. Microscleres are one type of arcuate or palmate anisochela where the smaller end is in the shape of a central plate and two rudimentary, flat, lateral teeth, all with serrated edges toward the middle. To this, forceps spicules are often added, but may be rare or absent in particular species or specimens of a single species. Never sigmas or sigmancistras (from Hestetun et al., 2016b).
Remarks
The genus contains 29 species in addition to the species described here (Van Soest et al., 2019). Originally erected as a subgenus by Lundbeck (1905), it was re-erected as a genus based on results from Hestetun et al. (2016b). The genus is cosmopolitan, with species known from all major oceans, and from low tide down to hadal depths.
Lycopodina subtile sp. nov.
Type material
R/V Okeanos Explorer EX1605L3, ROV Deep Discoverer. Holotype: USNM 1424167, dive 10, SPEC03BIO_CO4, 2016-06-27, Stegosaurus Ridge, 22.12°N, 145.4°E, 3090 m.
Comparative material examined
L. lycopodium (Levinsen, 1887) (ZMBN 103465).
Diagnosis
Sponge composed of a slender stem with a large number of fine filaments emerging in all directions outward from the stem. Spicules are mycalostyles around 400–1500 μm long and palmate anisochelae around 7–11 μm long.
Description
An erect sponge consisting of a thin, vertical stalk with a short basal stem. A large number of filaments are arranged in a radial fashion projecting outward in all directions from the central stalk. The holotype is 34 mm long and 0.5 mm in diameter, with filaments up to 3 mm long. The color of the sponge is translucent white to slightly gray in both live specimen and in ethanol.
Skeleton
The main structural skeleton consists of a core axis made up of longitudinal, tightly arranged mycalostyles. The filaments are composed of mycalostyles perpendicularly inserted into the main stem, radiating at their base. The stem core is covered by a thin surface layer of soft tissue. Palmate anisochelae are found at the surface of the stem and filaments.
Spicules
1. Mycalostyles, very slightly tylote, large, straight, fusiform, 393–1438 (804) μm long, and 6.3–32.1 (18.5) μm wide (Figures 10D,E).
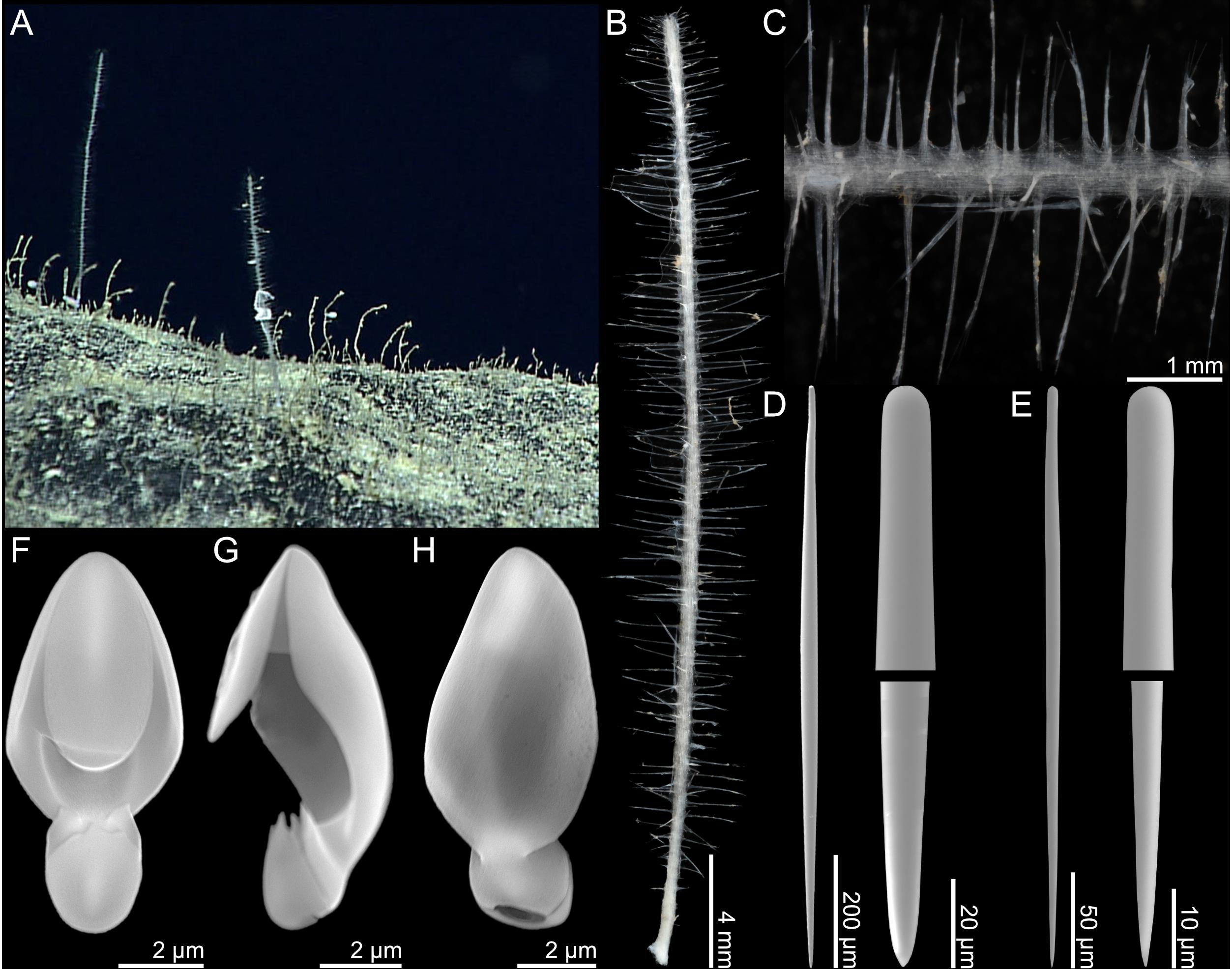
Figure 10. Lycopodina subtile n. sp. (A) In situ image from ROV video, (B) holotype USNM 1424167, (C) stem detail, (D) large style, (E) small style, (F–H) palmate anisochela front, side and back view.
2. Palmate anisochelae, with frontal tooth and lateral alae of equal length, around 40% of total length, 7.1–10.7 (9.1) μm (Figures 10F,H).
GenBank accession numbers
28S rRNA, MK935694; ALG11, MK922995.
Distribution
This species is only known from its type locality off the Mariana Islands at the Stegosaurus Ridge (3090 m) on a large rock outcropping.
Etymology
From Latin, “subtile,” meaning fine, slender, or delicate. Named after the small size and slender stem of the sponge.
Remarks
The anisochelae of L. subtile n. sp. are small compared to most species of the genus, and are similar in size to the smaller category of chela in Asbestopluma. However, the morphology of the spicule and the phylogenetic results show that it is clearly Lycopodina.
Species identification within Lycopodina can be challenging since spicules like smaller categories of megascleres and forceps spicules seem to be associated with specific parts of the sponge or reproductive structures such as embryos or spermatic cysts (Riesgo et al., 2007). In many cases, larger megascleres and anisochelae are the only spicules that can be reliably found, such as for L. subtile n. sp. here.
A stalked morphology with a radial arrangement of filaments is common within the genus, and given intraspecific spicule size variation, exact species boundaries are uncertain for species such as L. lycopodium, L. occidentalis, L. lebedi, and L. gracilis (e.g., Koltun, 1962, 1970; Van Soest, 2016; Hestetun et al., 2017b).
Only a few stalked Lycopodina species have been reported from lower bathyal depths and below: Lycopodina wolffi Lévi, 1964 and Lycopodina hadalis Lévi, 1964 from the Kermadec and Kuril–Kamchatka Trenches (∼4350–8840) (Lévi, 1964; Koltun, 1970). While Koltun (1970) argued that L. hadalis is a synonym of L. occidentalis, this is not considered valid here. The phylogenetic results recover L. subtile n. sp. with L. cupressiformis, though it is likely that it is more closely related to other Lycopodina species where molecular data are missing.
Phylogenetic Analysis
The ML best tree from the phylogenetic analysis of the concatenated partial 28S rRNA, COI, and ALG11 dataset is presented here with Bayesian posterior probabilities and ML bootstrap values indicated for nodes recovered in both analyses (Figure 11). The analysis includes available cladorhizid sequences on GenBank (see the section “Materials and Methods” for references containing accession numbers) as well as new sequences from the specimens examined in this article (see species descriptions for accession numbers).
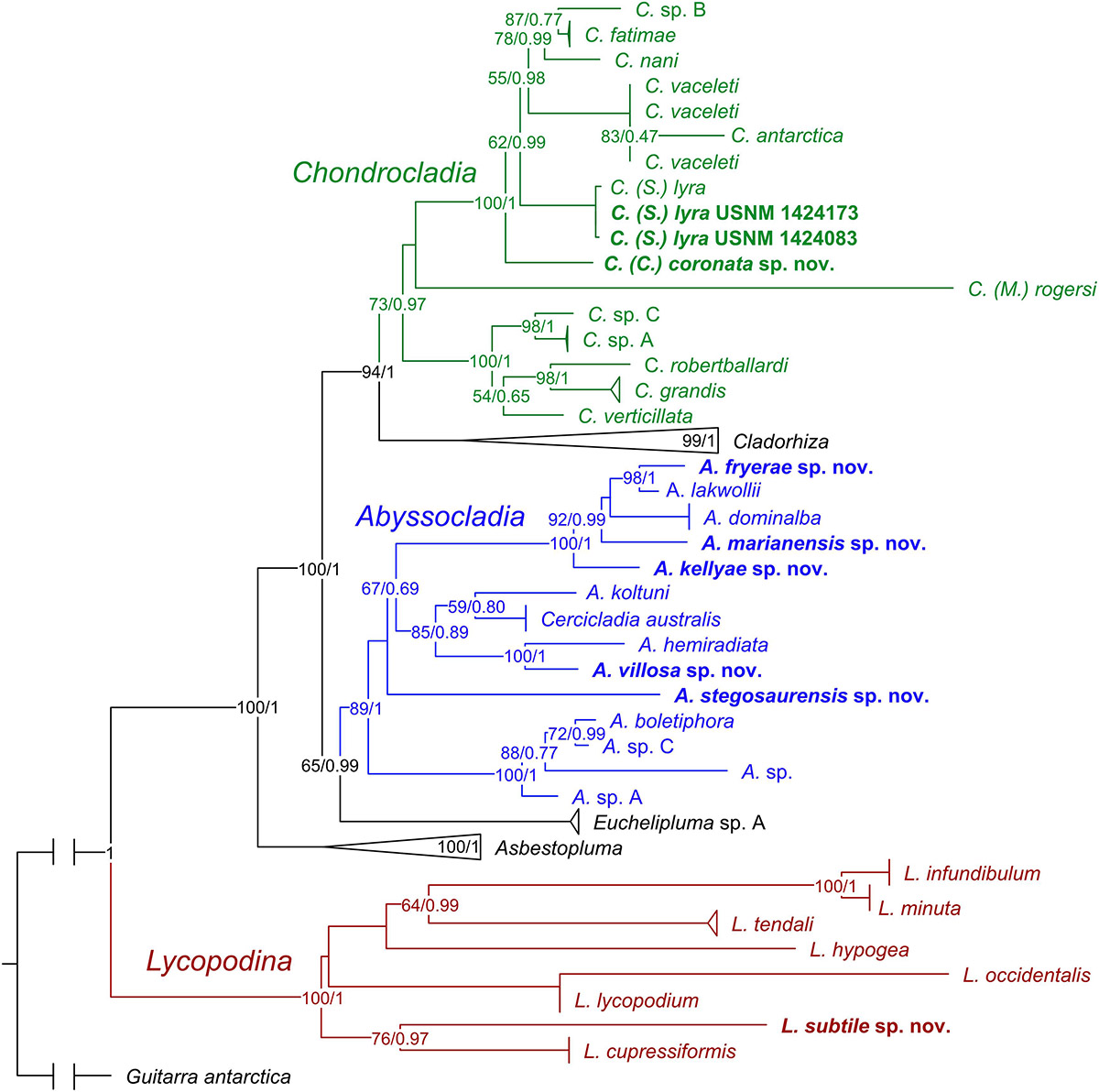
Figure 11. ML consensus tree of a concatenated phylogeny of the Cladorhizidae based on partial 28S rRNA, COI, and ALG11. The analysis includes available cladorhizid sequences from GenBank as well as an outgroup taxon (Guitarra antarctica). Sequences for specimens described in this article are indicated in bold. Bayesian posterior probabilities (0–1) and ML bootstrap values (1–100) are indicated for nodes that were recovered in both analyses. Genera including species described in this article are color coded green for Chondrocladia, blue for Abyssocladia, and red for Lycopodina. The genera Cladorhiza and Asbestopluma and species with sequences from several specimens have been collapsed to aid readability.
In general, genus and species relationships are similar to previous cladorhizid phylogenies in e.g., Hestetun et al. (2016b): Lycopodina forms a basal clade followed by Asbestopluma, Euchelipluma/Abyssocladia, and finally Cladorhiza and Chondrocladia. The species sequenced for this article are recovered in positions consistent with morphological characters: The new Chondrocladia (S.) lyra specimens are virtually identical to the previously sequenced specimen from the type material. C. coronata n. sp. is recovered in a basal position relative to C. (S.) lyra and other Chondrocladia species with a Crinorhiza-type morphology (cf. discussion of the genus in Hestetun et al., 2016b) (Figure 11).
The new Abyssocladia species are found in three main positions within the genus: A. stegosaurensis n. sp. was recovered basal to other species described here. A. villosa n. sp. was recovered as a sister taxon to A. hemiradiata Hestetun et al., 2017a. The last three species, A. kellyae n. sp., A. fryerae n. sp., and A. marianensis n. sp., were recovered together with the Pacific disc-shaped species A. dominalba Vacelet, 2006 and A. lakwollii Vacelet and Kelly, 2014 (Figure 11).
Discussion
Abyssocladia Diversity and Distribution
In the 2001 publication of the Systema Porifera, the cladorhizid genus Abyssocladia Lévi, 1964 was considered a synonym to Phelloderma Ridley and Dendy, 1886 (Van Soest and Hajdu, 2002). Re-erected in 2006 based on a re-examination of chela characters and newly discovered species, Abyssocladia at that time contained seven abyssal Pacific species with a short diagnosis that included a pedunculate, disc-shaped body, and abyssochela spicules (Vacelet, 2006). Including the species in the present article, it has over a period of 10 years grown into a genus containing 31 species with considerable morphological and spicule variation, and is now also known from the North and South Atlantic as well as seamounts on the Southwest Indian Ridge (see Table 2 for references).
Body shapes within the genus now include stalked forms with disc-shaped, spherical- or umbrella-shaped bodies, pinnate forms with opposite rows of filaments or filaments radiating in all directions from the stem, as well as branching forms. Megascleres commonly include mycalostyles, styles, (sub)tylostyles, oxeas, (sub)strongyles, in a couple of cases acantho(tylo)styles or acanthostrongyles, and in one case desmas. Microscleres include arcuate or palmate isochelae, cleistochelae, and abyssochelae, in some cases with specific morphology or modification, and finally sigmas and sigmancistras. As many species only feature arcuate isochelae rather than the previously diagnostic abyssochelae, it is difficult to establish a clear synapomorphy for the genus (Table 2).
While the genus can be roughly divided into different body shapes, it is difficult to relate this to the variation in spicule complement and morphology. Sequence data for the genus are limited, and there is yet no clear pattern between morphological characters and phylogenetic position (Figure 11). Phylogenetic results indicate that Euchelipluma Topsent, 1909 is a sister group to Abyssocladia, but the systematic relationship between Abyssocladia and the cladorhizid genera Cercicladia Ríos et al., 2011, Koltunicladia Hestetun et al., 2016b, and Lollipocladia Vacelet, 2008 is unclear (Hestetun et al., 2016b).
Most described Abyssocladia species are based on only one specimen or specimens from a single expedition. It is thus likely that the increase in the number of known species will continue, which will provide additional morphological context and fill the gaps between current species of the genus. Cladorhizid specimens are fragile and easily distort or break as a result of preservation. ROV in situ video footage, ideally together with high-quality photos during collection prior to preservation, is thus critical to get a complete understanding of the morphology and function of a given cladorhizid species. Increasing the amount of sequence data available for phylogenetic analysis of the carnivorous sponges will allow further exploration of the evolutionary relationships within Abyssocladia and its sister taxa. New morphological and molecular data thus both serve to increase the knowledge of the diversity and evolution of the genus and cladorhizids in general.
Pacific Cladorhizid Diversity and the Mariana Islands
Before the present study, no cladorhizid species have been reported for the deep sea around the Mariana Islands. Other records from the West Pacific show that cladorhizids are reasonably common, however, with several recently described species (e.g., Vacelet, 2006; Vacelet et al., 2009; Ise and Vacelet, 2010; Kelly and Vacelet, 2011; Vacelet and Kelly, 2014).
Results from this article demonstrate that the deep-sea seafloor around the Mariana Islands contains a rich and previously unknown cladorhizid fauna. Somewhat surprisingly, only one of the present species, the harp sponge Chondrocladia (Symmetrocladia) lyra, is previously described.
While the species here are almost all new to science, they are clearly related to the general cladorhizid fauna of the Pacific, with a high proportion of Abyssocladia and extending the known distribution of C. (S.) lyra. The generally poor sampling coverage makes it difficult to assess whether the fauna is unique to the area, or is part of a larger, shared Pacific fauna.
While some cladorhizid species use rhizoid structures to attach to soft bottom substrate, many species are found on hard or mixed bottom types, where they are fastened to rock patches or outcroppings. Their fragile morphology and small size makes it easy to overlook cladorhizids in both video transects and using traditional sampling gear. Still, as demonstrated by the material described in the current study, field personnel with appropriate expertise and a thorough sampling design facilitate preliminary identification and collection of this group.
The results here are also congruent with past studies in previously understudied areas in the Southwest Pacific and elsewhere, in that recovered cladorhizid specimens have a high chance of belonging to undescribed species. It is clear that the deep Pacific is home to a diverse and still very poorly known carnivorous sponge fauna with many unique characters compared to the somewhat better known Atlantic cladorhizids, and that additional sampling is needed to get a better picture of the total diversity of carnivorous sponges in the area.
Data Availability
The datasets generated for this study can be found in GenBank: MK922984, MK922985, MK922986, MK922987, MK922988, MK922989, MK922990, MK922991, MK922992, MK922993, MK922994, MK922995, MK922996, MK935688, MK935689, MK935690, MK935691, MK935692, MK935693, and MK935694.
Author Contributions
JTH planned the morphological and molecular analyses, examined the specimens, and wrote the main draft of the article. HTR facilitated the molecular sequencing and morphological lab work, provided input on specimen data and species descriptions, and worked on the text of the manuscript. SP collected the specimens, provided collection data and footage, and worked on the text of the manuscript.
Funding
This work was supported by the NOAA/OER, award number NA14OAR4320260 to the Cooperative Institute for Ocean Exploration, Research, and Technology (to SP) and by the Bergen Research Foundation (BFS) (to HTR).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to the U.S. National Oceanic and Atmospheric Administration (NOAA), Office of OER for graciously allowing us to use ROV video footage from the EX1605 expedition for the purposes of documenting the species described in this article. We also thank the captain and crew of the NOAA ship Okeanos Explorer and the crew from the Global Foundation for Ocean Exploration, whose expertise in ROV operations enabled the collection of these fragile sponges. We thank the CAPSTONE chief scientist Dr. Christopher Kelley (University of Hawai‘i at Mānoa), mission coordinator Kasey Cantwell (NOAA/OER), and leg 3 geology science lead Dr. Patricia Fryer (University of Hawai‘i at Mānoa). Furthermore, we would like to thank David John Rees at the Department of Biology at the University of Bergen for sequencing the specimens in this article. Finally, we would like to thank Allen Collins at the National Museum of Natural History for facilitating the loan of the specimens from the USNM.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00371/full#supplementary-material
TABLE S1 | List of sequences used for the phylogenetic analysis.
References
Boury-Esnault, N., and Rützler, K. (1997). Thesaurus of Sponge Morphology. Washington, D.C: Smithsonian Institution Press.
Cárdenas, P., Pérez, T., and Boury-Esnault, N. (2012). Sponge systematics facing new challenges. Adv. Mar. Biol. 61, 79–209. doi: 10.1016/B978-0-12-387787-1.00010-6
Carter, H. J. (1874). Descriptions and figures of deep-sea sponges and their spicules from the Atlantic Ocean, dredged up on board H.M.S. Porcupine, chiefly in 1869; with figures and descriptions of some remarkable spicules from the Agulhas shoal and Colon, Panama. Ann. Magaz. Nat. Hist. 14, 245–257. doi: 10.1080/00222937408680964
Carter, H. J. (1876). Descriptions and figures of deep-sea sponges and their spicules, from the Atlantic Ocean, dredged up on board H.M.S. Porcupine, chiefly in 1869 (concluded). Ann. Magaz. Nat. Hist. 18, 226–240. doi: 10.1080/00222937608682035
Cristobo, F. J., Urgorri, V., and Ríos, P. (2005). Three new species of carnivorous deep-sea sponges from the DIVA-1 expedition in the Angola Basin (South Atlantic). Org. Divers. Evol. 5, 203–213. doi: 10.1016/j.ode.2004.11.004
Dendy, A. (1922). Report on the sigmatotetraxonida collected by H.M.S.‘Sealark’ in the Indian Ocean. Trans. Linn. Soc. Lond. 18, 1–164, pls 1–18.
Dressler-Allame, M., Göcke, C., Kersken, D., Plotkin, A., and Janussen, D. (2016). Carnivorous sponges (Cladorhizidae) of the deep Weddell Sea, with descriptions of two new species. Deep Sea Res. Part II 137, 190–206. doi: 10.1016/j.dsr2.2016.08.006
Ereskovsky, A. V., and Willenz, P. (2007). Esperiopsis koltuni sp. nov. (Demospongiae: Poecilosclerida: Esperiopsidae), a carnivorous sponge from deep water of the Sea of Okhotsk (North Pacific). J. Mar. Biol. Assoc. U. K. 87, 1379–1386. doi: 10.1017/s0025315407058109
Goodwin, C., Berman, J., Downey, R., and Hendry, K. (2017). Carnivorous sponges (Porifera, Demospongiae, Poecilosclerida, Cladorhizidae) from the Drake Passage (Southern Ocean) with a description of eight new species and a review of the family Cladorhizidae in the Southern Ocean. Invertebr. Syst. 31, 37–64.
Grant, R. E. (1836). “Animal kingdom,” in The Cyclopaedia of Anatomy and Physiology, Vol. 1, ed. R. B. Todd (London: Sherwood, Gilbert, and Piper), 1–813.
Hajdu, E., Van Soest, R. W. M., and Hooper, J. N. A. (1994). “Proposal for a phylogenetic subordinal classification of poecilosclerid sponges,” in Sponges in Time and Space, eds R. W. Van Soest, T. M. Van Kempen, and J.-C. Braekman (Rotterdam: Balkema), 123–139.
Hestetun, J., Fourt, M., Vacelet, J., Boury-Esnault, N., and Rapp, H. (2015). Cladorhizidae (Porifera, Demospongiae, Poecilosclerida) of the deep Atlantic collected during Ifremer cruises, with a biogeographic overview of the Atlantic species. J. Mar. Biol. Assoc. U. K. 95, 1311–1342. doi: 10.1017/s0025315413001100
Hestetun, J. T., Pomponi, S., and Rapp, H. T. (2016a). The cladorhizid fauna of the Caribbean and adjacent areas. Zootaxa 4175, 521–538. doi: 10.11646/zootaxa.4175.6.2
Hestetun, J. T., Vacelet, J., Boury-Esnault, N., Borchiellini, C., Kelly, M., Ríos, P., et al. (2016b). The systematics of carnivorous sponges. Mol. Phylogenet. Evol. 94, 327–345. doi: 10.1016/j.ympev.2015.08.022
Hestetun, J. T., Rapp, H. T., and Xavier, J. (2017a). Carnivorous sponges (Porifera, Cladorhizidae) from the Southwest Indian Ocean Ridge seamounts. Deep Sea Res. Part II 137, 166–189. doi: 10.1016/j.dsr2.2016.03.004
Hestetun, J. T., Tompkins-Macdonald, G., and Rapp, H. T. (2017b). A review of carnivorous sponges (Porifera: Cladorhizidae) from the boreal North Atlantic and Arctic. Zool. J. Linn. Soc. 181, 1–69. doi: 10.1093/zoolinnean/zlw022
Hooper, J. N. A., and Lévi, C. (1989). Esperiopsis desmophora n. sp. (Porifera: Demospongiae): a desma-bearing Poecilosclerida. Mem. Queensl. Mus. 27, 437–441.
Ise, Y., and Vacelet, J. (2010). New carnivorous sponges of the genus Abyssocladia (Demospongiae, Poecilosclerida, Cladorhizidae) from Myojin Knoll, Izu-Ogasawara Arc, southern Japan. Zool. Sci. 27, 888–894. doi: 10.2108/zsj.27.888
Kelly, M., and Vacelet, J. (2011). Three new remarkable carnivorous sponges (Porifera, Cladorhizidae) from deep New Zealand and Australian (Macquarie Island) waters. Zootaxa 2976, 55–68.
Koltun, V. M. (1962). Four rayed and siliceous horny sponges from the Pacific shallow waters of Paramushir and Shumshu Islands. Issledovaniya dal’nevostochnykh morei SSSR 8, 181–199.
Koltun, V. M. (1970). “Sponge fauna of the northwestern Pacific from the shallows to the hadal depths,” in Fauna of the Kurile-Kamchatka Trench and its environment. Institute of Oceanology of the Academy of Sciences of the U.S.S.R. [English translation, 1972], ed. V. Bogorov (Jerusalem: Israel Program for Scientific Translations Ltd.), 1–372, pls I–VIII.
Lambe, L. M. (1900). Sponges from the coasts of northeastern Canada and Greenland. Trans. R. Soc. Can. 6, 19–49.
Lee, W. L., Reiswig, H. M., Austin, W. C., and Lundsten, L. (2012). An extraordinary new carnivorous sponge, Chondrocladia lyra, in the new subgenus Symmetrocladia (Demospongiae, Cladorhizidae), from off of northern California, USA. Invertebr. Biol. 131, 259–284. doi: 10.1111/ivb.12001
Lehnert, H., Stone, R., and Heimler, W. (2006). New species of deep-sea demosponges (Porifera) from the Aleutian Islands (Alaska, USA). Zootaxa 1250, 1–35.
Lévi, C. (1964). Spongiaires des zones bathyale, abyssale et hadale. Galath. Rep. Sci. Results Danish Deep Sea Exped. Round World 7, 63–112, pls II–XI.
Lévi, C. (1993). Porifera demospongiae: spongiaires bathyaux de Nouvelle-Calédonie, récoltés par le «Jean Charcot» Campagne BIOCAL, 1985. Mém. Mus. Natl. Hist. Nat. 158, 9–87.
Levinsen, G. M. R. (1887). Kara-havets svampe. Dijmphna-Togtets zoologisk-botaniske Udbytte 1, 339–372, pls XXIX–XXXI.
Lopes, D. A., Bravo, A., and Hajdu, E. (2011). New carnivorous sponges (Cladorhizidae: Poecilosclerida: Demospongiae) from off Diego Ramírez Archipelago (south Chile), with comments on taxonomy and biogeography of the family. Invertebr. Syst. 25, 407–443.
Lopes, D. A., and Hajdu, E. (2014). Carnivorous sponges from deep-sea coral mounds in the Campos Basin (SW Atlantic), with the description of six new species (Cladorhizidae, Poecilosclerida, Demospongiae). Mar. Biol. Res. 10, 329–356. doi: 10.1080/17451000.2013.797587
Lundsten, L., Reiswig, H. M., and Austin, W. C. (2017). Three new species of Cladorhiza (Demospongiae, Poecilosclerida, Cladorhizidae) from the Northeast Pacific Ocean. Zootaxa 4317, 247–260. doi: 10.11646/zootaxa.3786.2.1
National Oceanic and Atmospheric Administration [NOAA] (2019). Ocean Exploration and Research (OER) Digital Atlas Portal. Available at: https://www.ncddc.noaa.gov/website/google_maps/OE/mapsOE.htm (accessed May 30, 2019).
Ridley, S. O., and Dendy, A. (1886). Preliminary report on the monaxonida collected by HMS ‘Challenger’. Ann. Magaz. Nat. Hist. 5, 470–493. doi: 10.1080/00222938609459998
Riesgo, A., Taylor, C., and Leys, S. P. (2007). Reproduction in a carnivorous sponge: the significance of the absence of an aquiferous system to the sponge body plan. Evol. Dev. 9, 618–631. doi: 10.1111/j.1525-142x.2007.00200.x
Ríos, P., and Cristobo, J. (2018). Abyssocladia vaceleti (Porifera, Cladorhizidae): a new deep-sea carnivorous sponge from Patagonia. Zootaxa 4466, 164–173. doi: 10.11646/zootaxa.4466.1.13
Ríos, P., Kelly, M., and Vacelet, J. (2011). Cercicladia australis, a new carnivorous sponge with novel chelae from the tasman basin and the argentine patagonian margin (Porifera, Cladorhizidae). Zootaxa 3131, 52–62.
Sollas, W. J. (1885). A classification of the sponges. Ann. Magaz. Nat. Hist. 16, 395. doi: 10.1080/00222938509459901
Stern, R. J., Fouch, M. J., and Klemperer, S. L. (2004). An overview of the Izu-Bonin-Mariana subduction factory. Geophys. Monogr. 138, 175–222. doi: 10.1029/138gm10
Topsent, E. (1892). Contribution à l’étude des Spongiaires de l’Atlantique Nord (Golfe de Gascogne, Terre-Neuve, Açores). Résultats des Campagnes Scientifiques Accomplies sur son Yacht Albert 1er Prince Souverain de Monaco 2, 1–165, pls I–IX.
Topsent, E. (1909). Etude sur quelques Cladorhiza et sur Euchelipluma pristina n. g. et n. sp. Bull. l’Instit. Océanogr. Monaco 151, 1–23.
Topsent, E. (1928). Spongiaires de l’Atlantique et de la Méditerranée provenant des croisières du Prince Albert ler de Monaco. Résultats des Campagnes Scientifiques Accomplies par le Prince Albert I. Monaco, 74, 1–376, pls I–XI. Available at: http://www.marinespecies.org/porifera/porifera.php?p=sourcedetails&id=8393Google Scholar
Topsent, E. (1930). Chondrocladia yatsui n. sp., de la Baie de Sagami. Annot. Zool. Jpn 1930, 421–432.
Vacelet, J. (2006). New carnivorous sponges (Porifera, Poecilosclerida) collected from manned submersibles in the deep Pacific. Zool. J. Linn. Soc. 148, 553–584. doi: 10.1111/j.1096-3642.2006.00234.x
Vacelet, J. (2007). Diversity and evolution of deep-sea carnivorous sponges. Porifera Res. 28, 107–115.
Vacelet, J. (2008). A new genus of carnivorous sponges (Porifera: Poecilosclerida, Cladorhizidae) from the deep N-E Pacific, and remarks on the genus Neocladia. Zootaxa 1752, 57–65. doi: 10.5281/zenodo.181741
Vacelet, J., and Duport, E. (2004). Prey capture and digestion in the carnivorous sponge Asbestopluma hypogea (Porifera: Demospongiae). Zoomorphology 123, 179–190. doi: 10.1242/jeb.072371
Vacelet, J., and Kelly, M. (2008). New species from the deep Pacific suggest that carnivorous sponges date back to the Early Jurassic. Nat. Preced.
Vacelet, J., and Kelly, M. (2014). A new species of Abyssocladia (Porifera, Demospongiae, Poecilosclerida, Cladorhizidae) and other carnivorous sponges from the far eastern Solomon Islands. Zootaxa 3815, 386–396. doi: 10.11646/zootaxa.3815.3.4
Vacelet, J., Kelly, M., and Schlacher-Hoenlinger, M. (2009). Two new species of Chondrocladia (Demospongiae: Cladorhizidae) with a new spicule type from the deep south Pacific, and a discussion of the genus Meliiderma. Zootaxa 2073, 57–68.
Van Soest, R. W. M. (2016). Sponge-collecting from a drifting ice floe: the Porifera obtained in the Kara Sea by the Dutch Polar Expedition 1882-83. Contrib. Zool. 85, 311–336. doi: 10.1163/18759866-08503004
Van Soest, R. W. M., Boury-Esnault, N., Hooper, J. N. A., Rützler, K., De Voogd, N. J., Alvarez, B. D. G., et al. (2019). World Porifera Database. Available at: http://www.marinespecies.org/porifera (accessed May 30, 2019).
Keywords: carnivorous sponges, Cladorhizidae, taxonomy, deep sea, Marianas, West Pacific, abyssal, Abyssocladia
Citation: Hestetun JT, Rapp HT and Pomponi S (2019) Deep-Sea Carnivorous Sponges From the Mariana Islands. Front. Mar. Sci. 6:371. doi: 10.3389/fmars.2019.00371
Received: 29 March 2019; Accepted: 14 June 2019;
Published: 10 July 2019.
Edited by:
Diva Amon, Natural History Museum, United KingdomReviewed by:
Dorte Janussen, Senckenberg Museum, GermanyTina Molodtsova, P.P. Shirshov Institute of Oceanology (RAS), Russia
Copyright © 2019 Hestetun, Rapp and Pomponi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jon Thomassen Hestetun, amhlc0Bub3JjZXJlc2VhcmNoLm5v; am9uLmhlc3RldHVuQGdtYWlsLmNvbQ==
 Jon Thomassen Hestetun
Jon Thomassen Hestetun Hans Tore Rapp
Hans Tore Rapp Shirley Pomponi3
Shirley Pomponi3