
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 01 May 2019
Sec. Marine Megafauna
Volume 6 - 2019 | https://doi.org/10.3389/fmars.2019.00215
 Elitza S. Germanov1,2,3*
Elitza S. Germanov1,2,3* Lars Bejder3,4
Lars Bejder3,4 Delphine B. H. Chabanne1,3
Delphine B. H. Chabanne1,3 Dharmadi Dharmadi5
Dharmadi Dharmadi5 I. Gede Hendrawan6
I. Gede Hendrawan6 Andrea D. Marshall2
Andrea D. Marshall2 Simon J. Pierce2
Simon J. Pierce2 Mike van Keulen1,3
Mike van Keulen1,3 Neil R. Loneragan1,3,7
Neil R. Loneragan1,3,7Manta rays (Mobula spp.) are highly valued in nature-based tourism globally. In Indonesia, although manta rays are protected, critical information is lacking on their habitat use, population dynamics and movements. We investigate the population structure and residency patterns of reef manta rays (Mobula alfredi) in the Nusa Penida Marine Protected Area (MPA). From photo-identification data logged by citizen scientists and trained observers (mantamatcher.org), we identified 624 reef manta rays from 5,913 sightings (January 2012–April 2018) based on their unique ventral coloration patterns. Year-round records were collected from two shallow (<20 m) reefs – Manta Bay (MB; n = 3,029 sightings) and Manta Point (MP; n = 3,058) – that are used frequently by tourism operators. Maximum likelihood techniques and a Markov movement analysis were used to model residency patterns and movement between these sites within the MPA. Manta rays at MB were predominantly male (64%, n = 261 individuals), with immature males (14%, n = 59) being sighted most frequently (39%, n = 1,170). In contrast, few immature individuals were sighted at MP (6%, n = 28), and they were sighted on few occasions (2%, n = 45), while mature female manta rays comprised 26% (n = 127) of the MP community and were the most frequently sighted (48%, n = 1,413). Lagged identification rates indicated high site fidelity at each location. However, 44% (n = 278) of individuals moved between the two sites and cumulative discovery curves showed a continued recruitment of individuals over the 6 years of the study. In addition, the behaviors displayed by the manta rays differed markedly between the two sites: MB appears to be a foraging ground, especially for juveniles, and potentially a nursery, while MP is used mainly for cleaning and courtship, indicating a social and reproductive site. Reproductive behavior coincided with the peak annual sightings in May. To prevent disturbance to this threatened species by tourism, regulations for the number of boats and interactions, especially during key reproductive times should be considered. Further, strict fishing regulation in the area is recommended as fishing gear entanglement was identified as a threat to this population.
Many marine megafauna species, such as sea turtles (Bowen and Karl, 2007), cetaceans (Baird et al., 2008), large teleost fish (Robichaud and Rose, 2001; Rooker et al., 2008), and elasmobranchs (Hueter et al., 2005), range widely, but within these large areas show high fidelity to defined areas for reproduction and feeding (Chapman et al., 2015). Further, a specific demographic within a population might depend on limited habitats during different stages in their development (e.g., coastal nursery areas versus open ocean foraging grounds) (Hueter et al., 2005; Chapman et al., 2015). While Marine Protected Areas (MPAs) are often created to protect critical habitats of threatened species, these areas are sometimes designated without in-depth knowledge of how the habitats are used by the target species. An understanding of habitat use is needed to achieve conservation goals, which can also require that tourism and other threats are managed to avoid disruption to the biology and behavior of the threatened species in question (e.g., Hueter et al., 2005).
Manta rays are large, pelagic, filter-feeding mobulid rays (Mobula spp.) found in equatorial and tropical waters (e.g., Couturier et al., 2012), that are threatened with extinction. Several known aggregation areas exist within the Coral Triangle region, particularly within Indonesia (Marshall et al., 2009; Couturier et al., 2012; Germanov and Marshall, 2014). Manta rays are long-lived, slow to mature, and exhibit low fecundity (Marshall and Bennett, 2010; Stevens, 2016; Rambahiniarison et al., 2018). Owing in part to these conservative life history traits and to overfishing across their range (Dulvy et al., 2014), both Mobula alfredi (Krefft, 1868) and the larger M. birostris (Walbaum, 1792) are currently listed as vulnerable to extinction on the IUCN Red List of Endangered Species (Marshall et al., 2011). Concerns over the population status of manta rays led to their listing on the Convention on the International Trade of Endangered Species (CITES) and the Convention on the Conservation of Migratory Species (CMS), which aim to curb their consumptive over-exploitation by fisheries (Ward-Paige et al., 2013). Until recently, Indonesia was ranked third for the highest annual mobulid catches, including manta rays (Heinrichs et al., 2011). In 2014, Indonesia protected both manta ray species within their entire exclusive economic zone (an area of over 6 million km2) through the Ministerial Decree of Marine Affairs and Fisheries No. 4/2014 (Ministry of Marine Affairs and Fisheries, 2014), in the hopes of slowing population declines. Currently, limited information is available for Indonesian manta ray populations to assist in tracking the current status of these slow-growing species.
Manta ray protection within Indonesia came largely in response to the growth of the manta ray tourism industry. Indonesia is ranked second in the world for manta ray tourism, with an estimated value of USD $15 million per year (O’Malley et al., 2013). Prior to their national protection, manta rays were first protected within three sanctuaries east of Bali: The Nusa Penida MPA (200 km2), West Manggarai including Komodo National Park (7,000 km2), and Raja Ampat (11,655 km2). Together, these sites encompass the bulk of the manta ray tourism industry in Indonesia (O’Malley et al., 2013). In addition to CITES and CMS regulations in Indonesia, evidence-based conservation strategies are needed to protect the remaining manta ray populations from the potential threats associated with unregulated tourism (reviewed by Tyne et al., 2014; Trave et al., 2017; Stewart et al., 2018a). Globally, as awareness grows of the chronic stresses to manta rays associated with an increase of tourism, measures aimed at curbing these impacts are underway or planned in some popular manta ray dive sites (e.g., Raja Ampat, Indonesia, Kasmidi and Gunadharma, 2017; and West Hawaii, United States, Division of Boating and Ocean Recreation, 2016). Other hazards to manta rays (reviewed by Stewart et al., 2018a) include entanglement in fishing lines (Croll et al., 2016) and boat mooring lines.
The Nusa Penida MPA, located 18 km south-east of Bali Island (Figure 1), encompasses the habitats for M. alfredi (hereafter manta rays). The waters of the Nusa Penida MPA, established in 2010 (District Fisheries and Marine Agency, 2010), are very productive (Ayers et al., 2014), with complex oceanographic conditions owing to their proximity to a major channel of the Indonesian Through Flow (ITF) current (Murray and Arief, 1988; Nyegaard, 2018). The islands of Ceningan, Lembongan, and Penida, located within the MPA, are home to 48,000 people and have been a tourist destination since the 1990s. In 2015, the MPA received an estimated 200,000 tourists per year, with an annual estimated increase of 7.7% (Barr et al., 2017). Manta ray watching is a major drawcard for tourist visits to the MPA, with annual estimates ranging from 4,200 (Barr et al., 2017) to 10,440 people (Aquatic Alliance, personal communication) or ∼ 63,500 manta dives and ∼ 15,000 manta swims (O’Malley et al., 2013). As manta rays are present in the MPA year-round (Germanov and Marshall, 2014), this manta ray population is subjected to chronic pressure from tourism. Currently, the number of boats allowed to enter manta ray habitats is unregulated, as is general boat traffic in the area (Barr et al., 2017). Further, codes of conduct for manta ray interactions are voluntary and are not strictly enforced, leading to concerns over low compliance (Barr et al., 2017). Moreover, basic information about the local manta ray population structure, habitat use, and movement patterns is lacking in the region, yet is required to refine spatial management within the MPA and promote ecologically sustainable tourism (Trave et al., 2017).
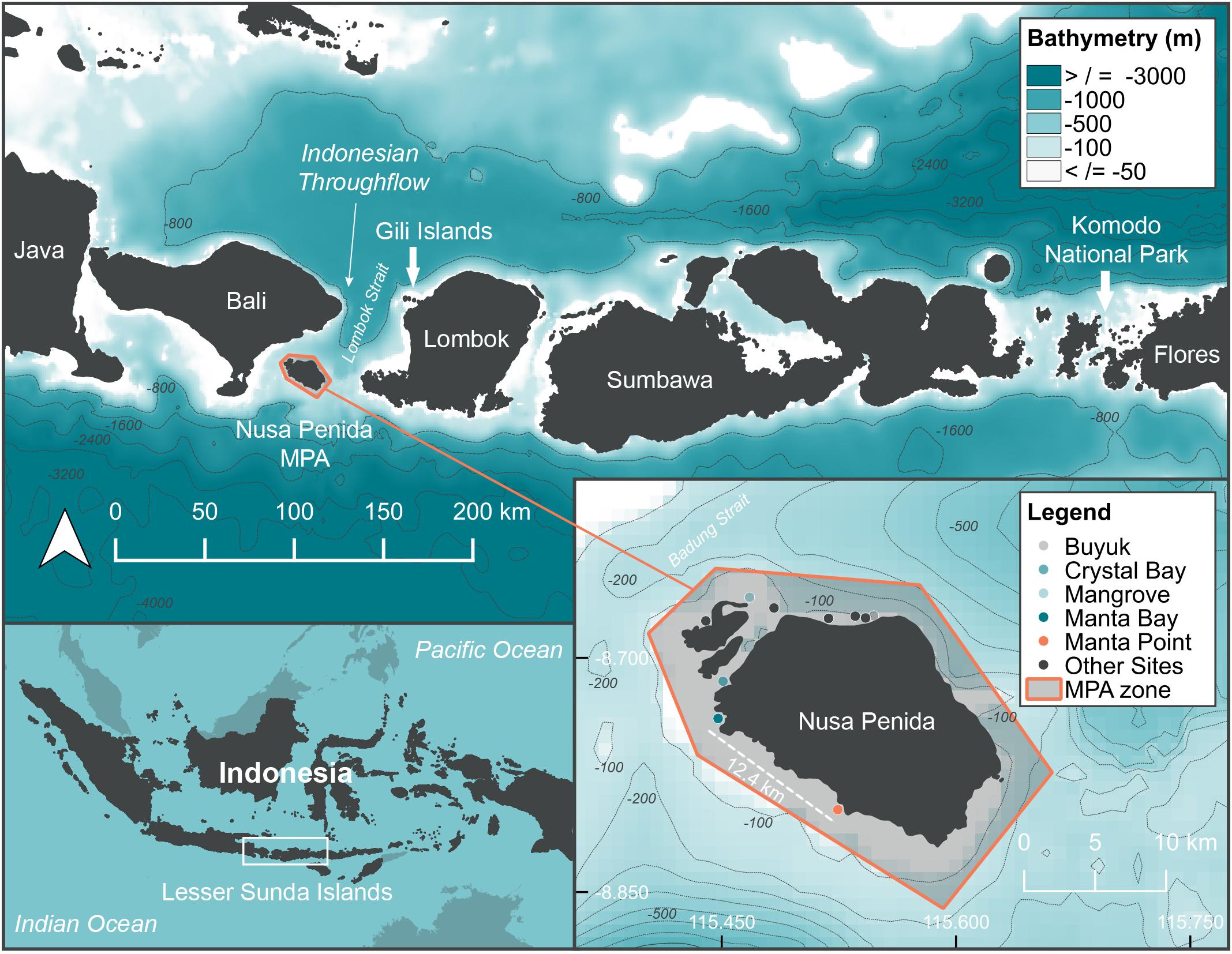
Figure 1. Location of study sites in the Indonesian archipelago along the west coastline of Nusa Penida, Manta Bay (MB), and Manta Point (MP) shown in relation to Bali and the Lesser Sunda Islands, Indonesia. Popular SCUBA diving locations around the Nusa Penida MPA with infrequent manta ray sightings are also marked as described in the legend. Sites marked as “other” are ‘Pontoon,’ ‘Toyapakeh,’ ‘Sekolah Desa,’ ‘Pura Ped,’ ‘Sental,’ and ‘Pura Mas Gading.’ Bathymetry information was available from: GEBCO_2014 Grid, version 20150318; www.gebco.net.
Here, we used a citizen science approach accessing over six years of publically contributed photographs and individual-based sighting records of manta rays within the Nusa Penida MPA, which were logged on the online database ‘Manta Matcher’ Wildbook1 (Marshall and Holmberg, 2018), to investigate population dynamics and habitat use. The aim of this work is to inform evidence-based management plans to ensure the long-term sustainability of manta ray tourism within the Nusa Penida MPA for both operators and manta rays. This was done by comparing the habitat use and population dynamics at two, high-intensity tourism sites, located only 12 km apart, to better understand the fine-scale habitat use of the manta population in the region. Given the proximity of the two sites, we predicted that they would share common aspects of population demography and behavior. With this study, we provide in-depth information on: (1) population structure, (2) injury rates, (3) fine scale habitat use, (4) local movement, and (5) behavior, and provide specific recommendations for effective manta ray management within the Nusa Penida MPA.
The Nusa Penida MPA is located in a unique oceanographic region, with a shallow (200 m) continental shelf adjacent to deep water basins of up to 4,200 m depth. The three islands located within the Nusa Penida MPA (Figure 1, created using QGIS Development Team, 2016, v 2.18) are in the direct path of the ITF current due to their proximity to the Lombok Strait (Murray and Arief, 1988). This major current separates two significant marine and terrestrial biogeographical realms (i.e., the ‘Wallace Line,’ Barber et al., 2000). The current flows largely from north to south between the larger islands of Bali and Lombok, with its strength varying seasonally (i.e., the north-west vs. south-east windward seasons, Mayer and Damm, 2012). The ITF brings warmer water (Tillinger, 2011) and nutrients (Ayers et al., 2014) from the Pacific to the Indian Ocean. Deep water upwelling (Ningsih et al., 2013) south of Bali further contributes nutrient rich waters to the region, creating productive foraging grounds within the Nusa Penida MPA for sunfish (Thys et al., 2016; Tito and Susilo, 2017; Nyegaard, 2018), and potentially other megafauna like manta rays.
Although 11 sites are dived frequently (number of days > 20 per year) in the Nusa Penida MPA, the majority of manta ray sightings (∼ 93%), based on year-round dive logs for 2016 (n = 809) and 2017 (n = 753), by one dive operator (Supplementary Figure 1), were at two sites, Manta Bay (MB) and Manta Point (MP) (Figure 1). Thus, these two sites were selected for detailed investigation. Both are shallow bays (7–25 m depth) fringed by steep rocky cliffs and deep water, separated by a straight-line distance of approximately 12.4 km. A range of habitats used by manta rays, including cleaning stations, foraging aggregation areas, and reproductive grounds are found at the two sites (Germanov and Marshall, 2014).
The online database ‘Manta Matcher’ Wildbook (see text footnote 1) (Marshall and Holmberg, 2018) was used to collate data (date, time, location, and identifying photographs of manta rays) from the public and researchers (Germanov and Marshall, 2014) (Supplementary Figure 2). Citizen science was an important part of this process and has been demonstrated to provide basic information when data are limited (e.g., Jaine et al., 2012; Rohner et al., 2013; Couturier et al., 2014; Germanov and Marshall, 2014; Robinson et al., 2016; Norman et al., 2017; Pierce et al., 2018) and engage local communities in marine stewardship (Miller-Rushing et al., 2012). Approximately 40 observers were trained to take photos of manta ray ventral spot patterns, record sex and maturity data, estimate size, and to note relevant behaviors (discussed below), as well as the maximum number of boats present at the sites. Tourists were regularly informed about ‘Manta Matcher’ through dive operator briefings and weekly educational presentations starting in 2012, resulting submissions from 276 different emails. A more traditional sampling approach with structured surveys (e.g., Deakos et al., 2011; Couturier et al., 2014) is required for a traditional mark-recapture approach to population analyses to estimate abundance and survival.
Photographs of the ventral surfaces of individual manta rays (Deakos et al., 2011; Kitchen-Wheeler et al., 2011; Marshall et al., 2011; Couturier et al., 2014) were logged year-round by visiting tourists to the Nusa Penida MPA and members of the local dive community (i.e., residents and dive professionals). The earliest available photographs and sighting records date back to June 2004, while year-round survey effort by trained observers started in 2012 (Supplementary Table 1). Only records collected between January 2012 and April 2018, when greater than 400 sightings were logged in any 1 year, were used for the analyses. Further, data from 2012, 2015, and 2018 were excluded from seasonal analyses as year-round survey effort was lower, or not available, during these years. For the purposes of this study, we assign a survey day as the unit of survey effort, as the number of dives per day and hours of diving were not recorded on a regular basis.
All sighting records were validated manually for accuracy, completeness, and adequate photo quality by trained observers. Sightings lacking information on location or date and those with an indistinct ventral image of the manta ray were excluded. Photographs were manually matched to an ID catalog with the assistance of built-in (Town et al., 2013; see Germanov and Marshall, 2014) and/or external software (‘MantaUtil,’ Winstanley, 2016) by trained observers and error-checked by a regional research manager, who assigned new individuals if no match was found. The sex and maturity status of manta rays were assessed based on the absence (female) or presence (male) of claspers, clasper size and/or clasper scarring for males, and pregnancy bulge and/or presence of pectoral fin mating scars for females (Marshall and Bennett, 2010; Marshall et al., 2011). Immature females were not assigned throughout the study because of the difficulty in definitively classifying females lacking maturity indicators in the absence of accurate size measurements. Manta ray sizes, based on disk width, were estimated relative to an entity of known size (i.e., swimmer or dive equipment) and placed into 0.25 m size classes. Only size estimates recorded by two observers that spanned the entire study period were considered (n = 260) in an effort to reduce observer bias. Injuries to the manta rays, including cephalic and pectoral fin truncations or disfigurement, and fishing line cuts and entanglements, were also noted (Supplementary Figure 3).
Manta ray behavior was assessed from sighting data based on mouth and cephalic fin positioning, the presence and absence of cleaner fish, and records of social interactions, including mating trains as described by Marshall and Bennett (2010), Deakos (2012), Jaine et al. (2012), and Stevens et al. (2018). Based on these previous studies, behavior was categorized into four mutually exclusive categories: foraging, cleaning, cruising, and courtship (Supplementary Figure 4). Two additional non-exclusive behavior categories were included – foraging/cleaning and courtship/cleaning to account for instances when an individual was observed performing two behaviors within the same encounter (maximum 60-min dive time). ‘Foraging’ manta rays were defined as individuals with open mouths and unrolled cephalic fins near the surface of the water column, or those who were completing full vertical rotations (‘barrel-rolling’). ‘Cleaning’ manta rays were defined as individuals hovering over reef patches (‘cleaning stations’) with cleaner fish species (Chaetodon kleinii, Thalassoma lunare, Labroides bicolor, and Labroides dimidiatus) in close proximity. ‘Cruising’ manta rays were those sighted with closed mouths and rolled up cephalic fins. ‘Courtship’ behavior was recorded when several male manta rays pursue a mature female (in the absence of foraging behavior), or when two or more individuals gave acrobatic displays as described by Stevens et al. (2018).
We use the term ‘site fidelity’ to denote the return of an individual to a site of previous residency after a periodic absence greater or equal to the residency period, following Chapman et al. (2015). However, a caveat of solely using this terminology is that some individuals might regularly use both study areas within the Nusa Penida MPA, moving frequently between the two. Further, periods between sightings varied greatly between individuals, precluding us from assigning a standard residency and absence period. In these uncertain scenarios, where individuals could be moving between areas across the large home range, we use the term ‘site affinity’ to describe our results (Couturier et al., 2011; Jaine et al., 2014).
A modified maximum likelihood approach was used to compare manta ray re-sighting data against residency models, implemented in the program SOCPROG 2.8 (Whitehead, 2009). These statistical models were previously used for manta rays in Hawaii by Deakos et al. (2011) and in several more recent studies on whale sharks Rhincodon typus (Fox et al., 2013; Robinson et al., 2016; McCoy et al., 2018) and cetaceans (e.g., Chabanne et al., 2017). This approach determines the spatial and temporal distribution of sampling effort using the re-identification data itself, making this approach suitable for opportunistic and presence-only sighting data (Fox et al., 2013). Residency patterns for individuals within the study areas were investigated by using the ‘Movement Analyses’ module of SOCPROG 2.8 to calculate the lagged identification rate (LIR), the probability of re-sighting an individual manta ray after a variable lag time (Whitehead, 2009). These empirical LIR data, based on a per day time lag, were then compared to a series of model scenarios encompassing both closed and open populations. Open population models were developed by incorporating varying situations with emigration, immigration, re-immigration, and/or mortality (Table 1). The lowest value from the quasi-Akaike information criterion (QAIC), used to account for over-dispersion of the data (Whitehead, 2007), was used to select which model best approximated the residency characteristics of manta rays at each study site. The sole exception was for female manta rays at MP, where AIC was used to select a best-fit model, as the data were not over-dispersed. The QAIC difference (or ΔQAIC) between the best-fit model and any other indicates how well the data support the less favored model as follows: ΔQAIC < 2 = substantial support; ΔQAIC 4–7 = considerably less support; and ΔQAIC > 10 = essentially no support (Whitehead, 2007). The LIR analyses were extended to a ‘within/between’ analysis to estimate the probability that an individual manta ray, identified first within one site, will be re-sighted at the other site after a specified time lag (in days). This analysis effectively provides a significance test for population-level mixing between sites. Model fits were bootstrapped 1,000 times to generate standard errors (SE).
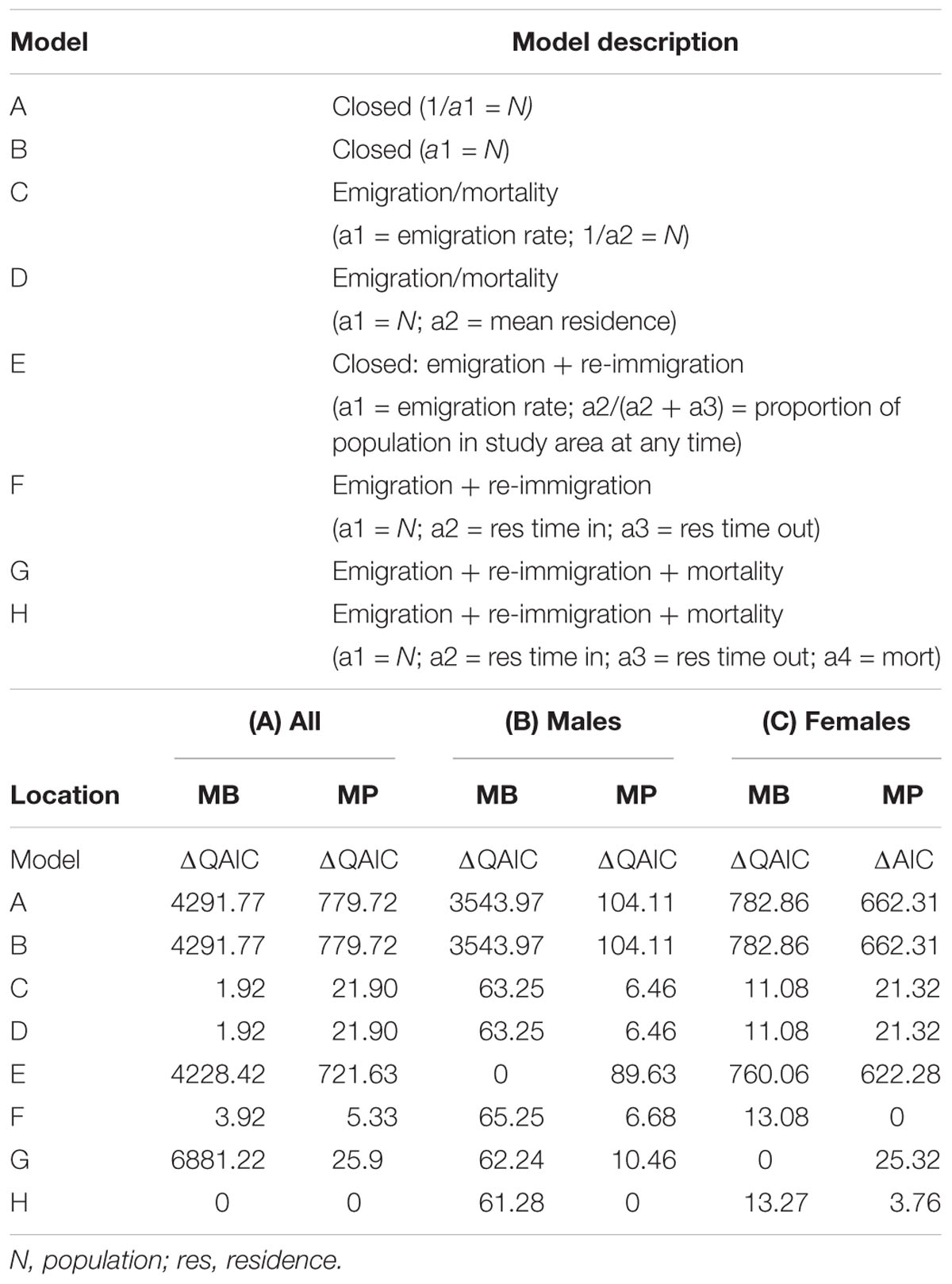
Table 1. Model parameters, fits (ΔQAIC) and comparison for the Lagged Identification Rate (LIR) of (A) all manta rays, (B) males and (C) females at Manta Bay (MB) and Manta Point (MP) in the Nusa Penida MPA, Indonesia.
The transition probabilities between sites were calculated using a parameterized Markov movement model (Whitehead, 2009), in which an individual has a certain probability of moving from one area to another at the specified time lag (1 year; Table 2). This model includes movements to and from both MB and MP and also accounted for movements to a third, hypothetical area (i.e., an area ‘outside’ of MB or MP). Optimized values of transition probabilities were bootstrapped 1,000 times to generate SEs and the maximum number of evaluations was set to 100,000. Mortality, including permanent emigration from all areas within the MPA, was considered in the model.
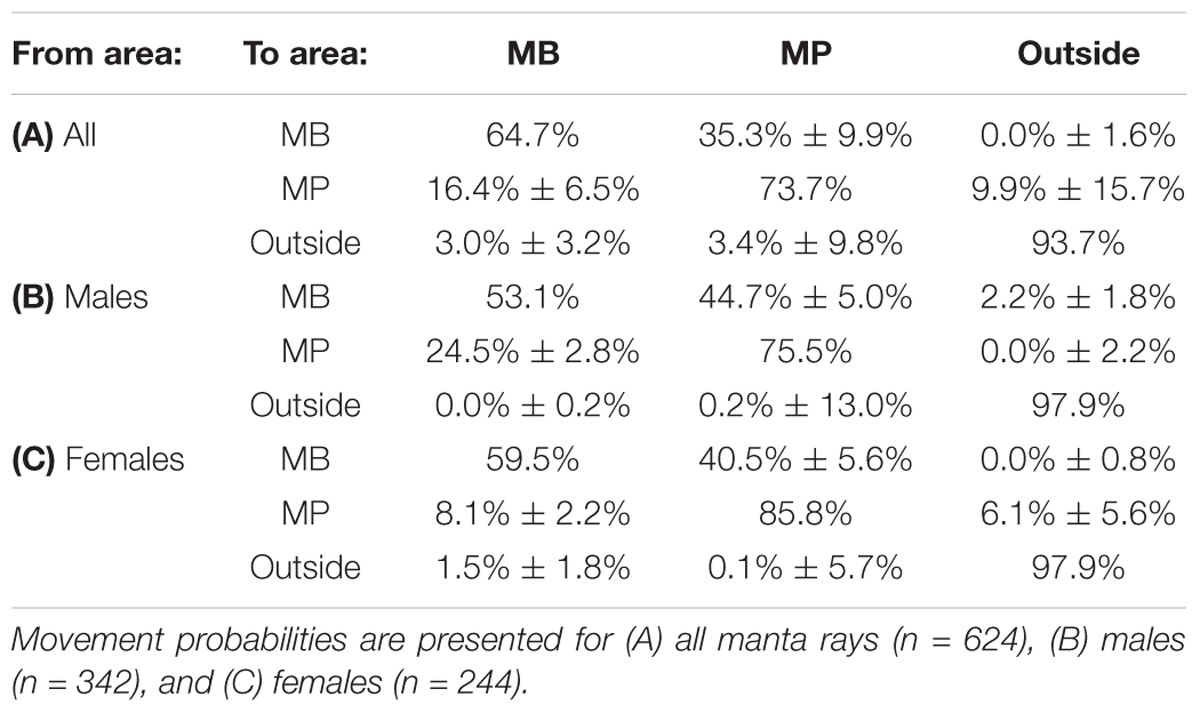
Table 2. The estimated probability (±1 SE) of an individual manta ray originally identified from one area being re-sighted within the same or different area.
The correlation between the annual number of survey days and sightings was estimated using Pearson’s product-moment correlation, cor.test function of the R statistical software (R Core Team, 2018). Differences in the number of individuals and sightings between the sexes at MB and MP were tested using chisq.test function of the R statistical software. Chi-squared (χ2) goodness of fit tests (one-dimensional contingency table) were used to compare sex ratio data for each site, while a two-dimensional contingency table was used to compare sex ratios and behavior frequencies between the two sites. Behavior data were collapsed into four categories to facilitate χ2 testing where counts were less than five in either of the site categories (i.e., foraging/cleaning was reclassified to foraging; and courtship/cleaning was reclassified to courtship). The Yates’ continuity correction was applied to tests where there was one degree of freedom.
Data from 6,087 sightings (excluding daily duplicate data for the same individuals; n = 163) of manta rays were collected within the Nusa Penida MPA from 25 June 2004 to 9 April 2018. Sightings came from 965 unique dates, with ∼ 97% of the records (5,913 sightings, excluding daily duplicates) logged between January 2012 and April 2018 and from our two main focal sites of MB and MP (Supplementary Table 1). Data prior to 2012 allowed 136 individual manta rays to be identified from 174 sightings within the MPA. After 2012, as education about manta rays amongst tour operators in the area improved, the number of manta ray identification photos and sighting information available increased (see Supplementary Figure 2). The average annual sighting rate from 2012 until 2017 (i.e., the full survey years) was 917 sightings/year ± 125 (± 1 SE), while 412 sightings were recorded for the first quarter of 2018.
The number of annual manta ray sightings varied across the study period (Figure 2A and Supplementary Table 1), with sightings being positively correlated with survey effort (r = 0.92, p < 0.01) for all study years. As the days where no manta rays were sighted (i.e., absence data) are not logged with ‘Manta Matcher,’ we used survey dives logged by trained observers as a proxy for survey effort (Supplementary Figure 2). The number of annual manta ray sightings submitted to ‘Manta Matcher’ varied between MB and MP. In the earlier study years (i.e., 2012–2014), more sightings were recorded (daily mean and total) at MB than MP, while there were more sightings at MP in the later years (i.e., 2015–2017) (Figure 2A and Supplementary Table 1). These observations reflect the gradual increase in reported surveys at MP and the steep decrease of reported surveys at MB after 2014, influenced by a shift in site usage by tourism operators (i.e., more dive operators using MP and more snorkel operators using MB; EG pers. obs.).
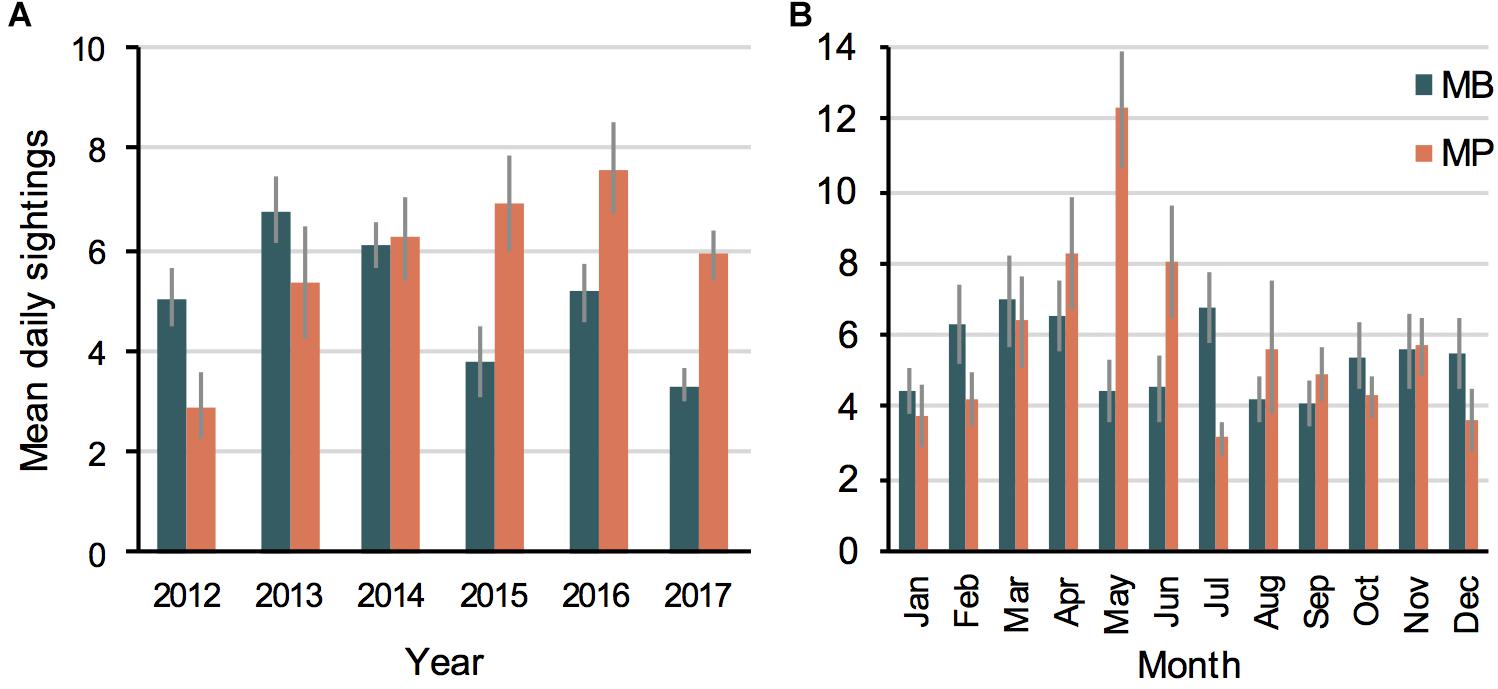
Figure 2. The average annual (A) and monthly (B) manta ray sightings per unit effort (day) recorded between 2012 and 2017 in the Nusa Penida MPA at Manta Bay (MB) and Manta Point (MP) presented as the daily means (±1 SE). Data from 2012 and 2015 is excluded from (B) due to lower year-round effort. (A) Sightings (MB = 2,995, MP = 2,918), days (MB = 581, MP = 511). (B) Sightings (MB = 2,295, MP = 2,137), days (MB = 420, MP = 336).
Logged presence/absence data indicated variable survey effort by trained observers between years and months (Supplementary Figure 5), with a lack of complete year-round effort in 2012 and 2015. Further, annual weather patterns, such as the north-west monsoon season (December–January), that result in unfavorable weather conditions and limit access to the study sites, regularly contributed to a seasonal reduction in survey effort. Nevertheless, there was an approximately twofold increase in manta ray sightings for MP in the month of May (Figure 2B). In contrast, sightings at MB were only marginally higher from February to April and in July than other months of the year.
A total of 624 individual manta rays were identified from sightings within the Nusa Penida MPA between 2012 and 2018. Of these individuals, 407 were sighted at MB and 494 were sighted at MP, with 277 sighted at both sites. A discovery curve shows a steep rise in newly identified manta rays within the Nusa Penida MPA until approximately 400 individuals, but the curve kept increasing to 624 individuals, though at a slower rate, until 2,260 days, with no evidence of an asymptote (Supplementary Figure 6). When treated separately, neither of the discovery curves for the two study sites show signs of approaching an asymptote. However, a brief period of slower discovery (i.e., a plateau) of new individuals was observed at MB after the identification of approximately 280 individuals at 800 days, until 1,600 days or between April 2014 and December 2015 (i.e., years 2 and 3 of data collection), after which the numbers of individuals identified increased to 407 after 2,260 days.
Overall, more male (n = 344; 55%) than female (n = 243; 39%) manta rays were identified in the Nusa Penida MPA (χ21 = 17.378, p < 0.001; Figure 3), while the sex was unknown for 37 individuals (6%). There was a significant association between sex and site for individuals (χ21 = 13.864, p < 0.001), and between sex and site for sightings (χ21 = 1080.2, p < 0.001). Of the entire population, at least 53% (n = 129) of the females were considered to be sexually mature, based on their pregnant appearance or the presence of mating scars. Among the males, 81% (n = 277) were considered sexually mature based on the size and shape of their claspers, with the remaining 19% (n = 66) being immature. In addition, 123 manta rays (∼ 20% of the total identified) were classed as immature at some point throughout the study, with 57 known to reach maturity. The population structure of individuals and the demographics of sightings differed between MB and MP (Figure 3). At MB, males made up 64% (n = 261) of identified individuals, whereas only 29% (n = 120) were female (χ21 = 52.18, p < 0.001). Males were also more frequently encountered (n = 2,388; 80%) than females (n = 564; 19%) at MB (χ21 = 1127, p < 0.001) and immature males had the highest proportion of the total sightings (n = 1,170; 39%). At MB, three males and one female were estimated to be 1.5 m in width on their first recording and another thirteen individuals were estimated to be ≤ 2 m, while 40 individuals were estimated to be ≥ 3 m. At MP, the majority of the individuals were mature males (n = 240; 49%) and females (n = 127; 26%), with overall more males (n = 268; 54%) than females (n = 212; 43%; χ21 = 6.53, p = 0.011). However, females were more frequently encountered (n = 1,759; 60%) than males (n = 1,142; 39%; χ21 = 131.23, p ≤ 0.001). Immature males were rarely encountered at MP, comprising just 2% of sightings (n = 45), a nearly 20-fold difference to MB. Five manta rays were estimated to be ≤ 2 m in width on their first recording with the smallest being 1.75 m, while 130 were estimated to be ≥ 3 m. The maturity status could not be determined (unknown) for 66 and 85 females (on 16 and 17% of total sightings) at MB and MP, respectively.
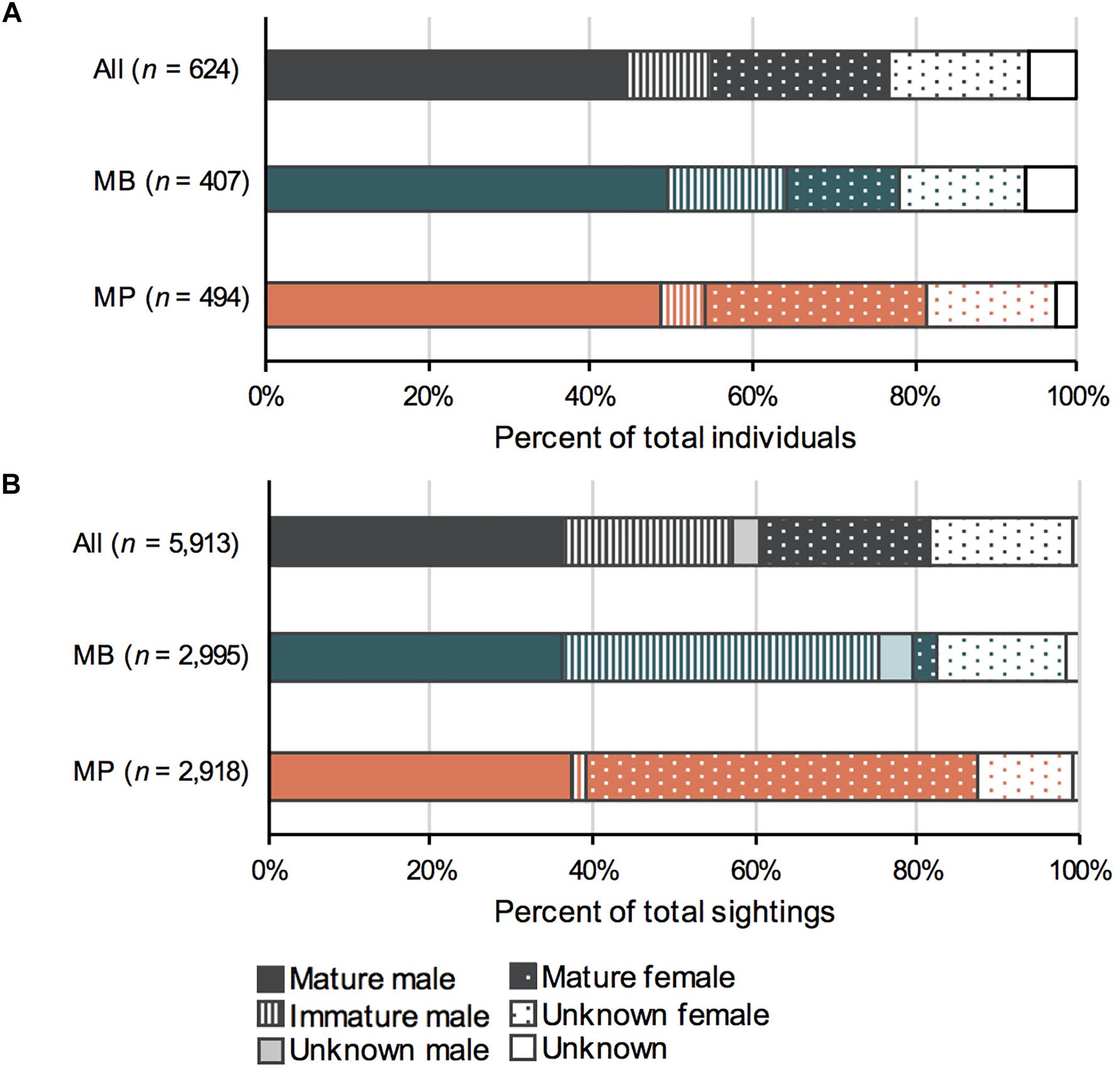
Figure 3. Population structure of manta rays in the Nusa Penida MPA, with (A) the number of individuals and (B) the number of sightings for Nusa Penida (All) and the two sites Manta Bay (MB) and Manta Point (MP). Based on sightings from January 2012 to April 2018. Percentages were calculated from the total number of individuals (A) or sightings (B) for each site.
Notably, 87 (∼ 14%) of identified manta rays had either cephalic fin, pectoral fin, and/or fishing line injuries, including 11 that were observed to be pregnant during the study period (three of the individuals were sighted pregnant two times with a span of at least 1 year in between). A total of 48 manta rays (7.7%) were observed entangled in fishing line or with fishing line marks (Supplementary Figure 3) and 49 (7.9%) had permanent injuries, either cephalic (n = 29; 4.6%) and/or pectoral fin (n = 21; 3.3%) truncations or disfigurements.
Of the 624 uniquely identified manta rays, the majority were encountered more than once (82%), with 181 individuals (29%) sighted more than 10 times, and 30 individuals (5%) sighted 31–99 times (Figure 4). The mean number of re-sightings per individual was 9.5 (± 0.46). The majority (n = 514; 82%) of these individuals were re-sighted across multiple years and 18% (n = 110) were sighted in only 1 year. The longest time between re-sightings of an individual was 13.8 years (including pre-2012 data). Overall, of the ten most re-sighted individuals, nine were male and one was a female. The most re-sighted individual was a male that was seen 99 times, but at MB only, between February 2012 and October 2017. This individual was classified as a juvenile at the first encounter, in February 2012, but matured over the monitoring period and was reclassified as an adult in July 2016. Similarly, the other nine most re-sighted individuals at MB were all male, with six out of 10 classified as juveniles upon first encounter (Supplementary Figure 7A). The most re-sighted female was seen 56 times (54 times at MP and 2 at MB) between June 2015 and April 2018. Her maturity status was unknown until March 2017, at which point she was classified as mature as she appeared to be pregnant. Similarly, the other nine most re-sighted individuals at MP were females, and all were seen pregnant at least once during the study period (Supplementary Figure 7B). The average period between re-sightings across all individuals (i.e., lag) was 118 days (± 3), ranging from 1 day to 5.3 years. For the most sighted male and female, greater than 90% of re-sightings had a lag period of less than 2 months, with the longest lag periods of these individuals being 234 and 239 days, respectively.
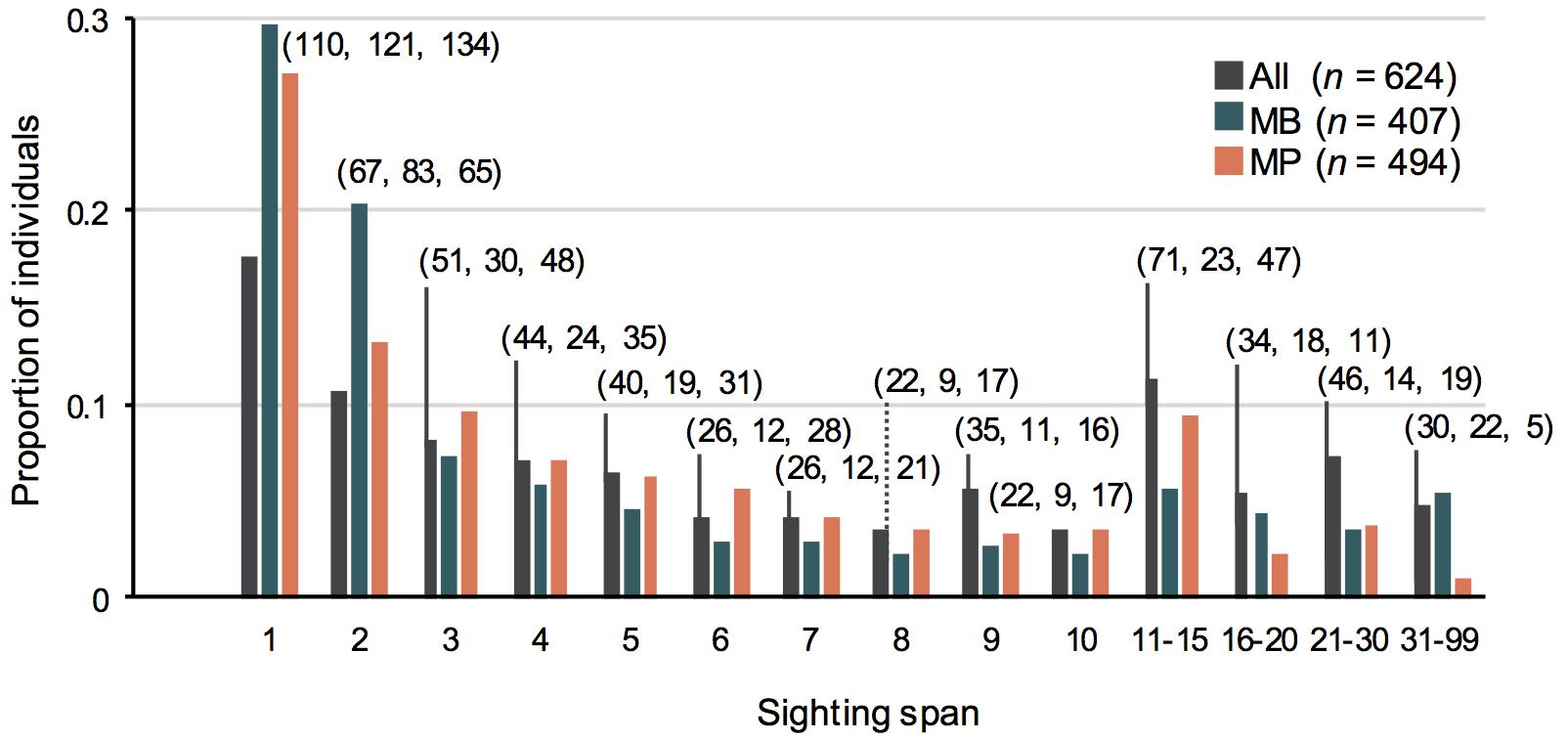
Figure 4. The proportion of manta rays identified in the Nusa Penida MPA (All), and study sites Manta Bay (MB) and Manta Point (MP) plotted against the number of times individuals were sighted between January 2012 and April 2018. Total number of individuals for All, MB, MP, are shown for each number of sightings.
We found that 277 (44%) individuals moved between the two study sites, and tested whether the two sites were fully mixed (a single population) using LIR analysis (Figure 5A). The two LIR curves did not converge during the study period, indicating that, although there is interchange between MB and MP, most individuals have a high affinity for one site over the other (i.e., communities were not mixed), at least when considering time lags of 1,500 days (∼ 4.1 years) or less. This notion is supported by the Markov movement model, which indicated high site affinity rates for MB (64.7%) and MP (73.7%) (Table 2A). A higher percentage of individuals moved from MB to MP (35.3 ± 9.9%) than vice versa (16.4 ± 6.5%) (Table 2A). Further, a higher percentage of individuals move away from MP (9.9 ± 15.7%) to ‘outside’ areas than from MB (0.0 ± 1.6%), indicating that the MP community is more mobile.
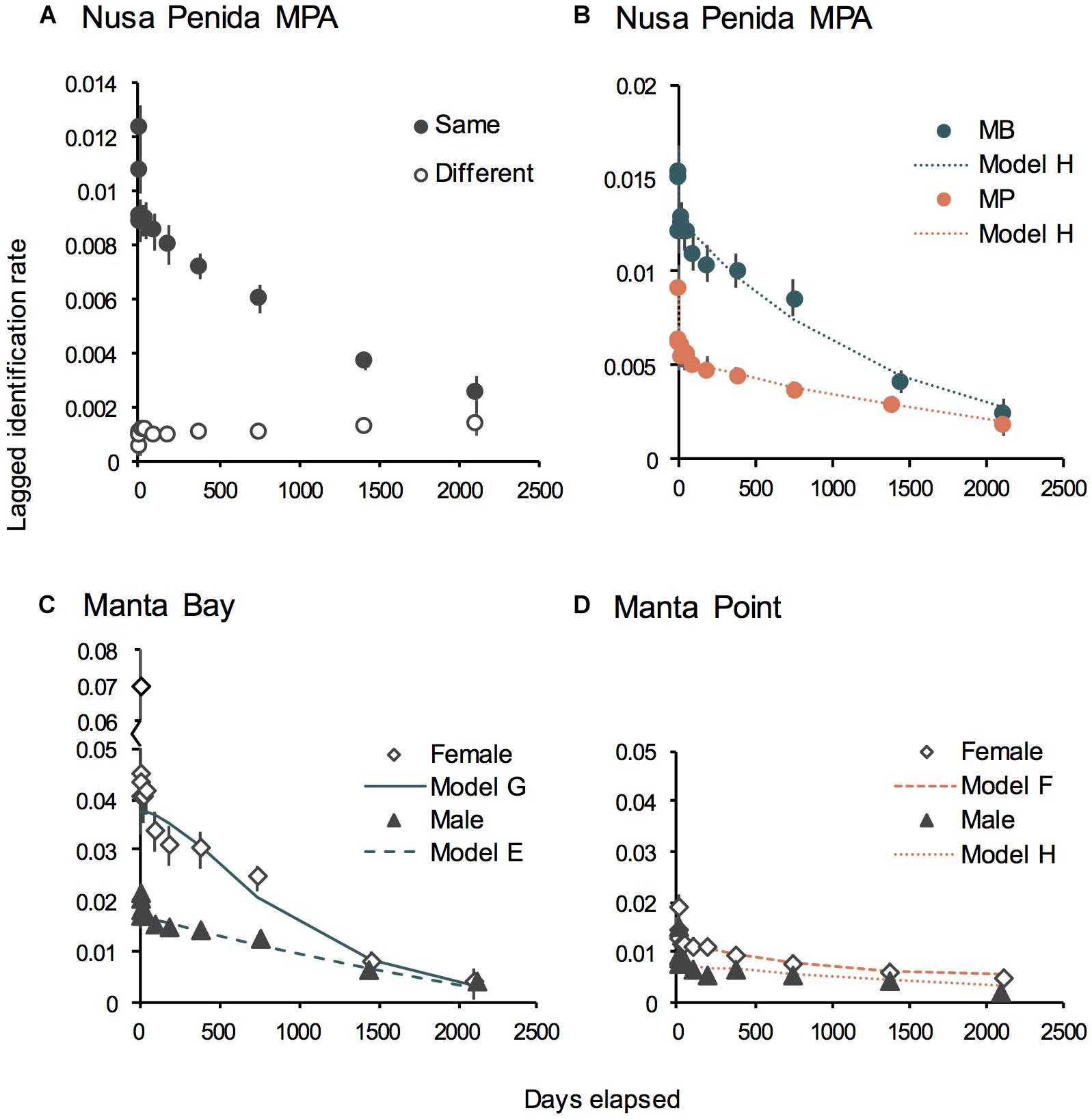
Figure 5. Lagged Identification Rates (LIR ± SE) of manta rays within the two study areas, Manta Bay (MB) and Manta Point (MP), Nusa Penida MPA, Indonesia. The mean probability of re-identifying an individual manta ray, from the time of first identification until approximately 6 years later within the same or different area in the Nusa Penida MPA (A), MB and MP (B); and broken down by sex within the sites MB (C); and MP (D). The predicted LIRs for models of best fit (Table 1) are shown for each group. Records from January 2012 to April 2018.
Results of the ‘within/between’ analysis showed that the sites should be treated separately for detailed analysis. Based on the ΔQAIC (Table 1A), the best fitting model for both MB and MP was Model H, which described a pattern of emigration, re-immigration and mortality. Models C and D, where emigration and mortality are included, were also supported (to a lesser degree) for MB (based on ΔQAIC < 2). Although a similar number of sightings were recorded for both areas, re-sighting rates are initially (up to two-year time lag) much higher at MB than MP, indicating that manta rays tended to reside longer in MB than MP (Figure 5B). Model H scenarios provide information on the proportion of time individuals spend within (residence time in) and out (residence time out) of an area, as well as mortality. Individual manta rays stayed approximately twice as long (2.0 days ± 187.7) at MB than at MP (0.9 days ± 0.2). Further, individuals sighted at MP spent an average of 2.9 (± 0.6) days outside the site, being absent nearly twice as long as those initially sighted at MB (1.6 ± 3.1 days). However, estimations of residence time within and out of MB were variable, suggesting that sex and/or demographic-specific (i.e., males vs. females, immature individuals vs. adults) or high individual differences in residence patterns exist. Mortality (which includes permanent emigration) was negligible for both sites (∼ 0).
To further explore sex specific differences in LIRs, we ran separate analyses for each site and sex (Tables 1B,C). Based on the ΔQAIC (except for females at MP, which was based on ΔAIC), the best fitting models were Models E (closed population with emigration and re-immigration) and H (emigration, re-immigration and mortality) for males and Model G (emigration, re-immigration and mortality) and F (emigration and re-immigration) for females at MB and MP, respectively (Tables 1B,C). We further explored maturity influenced differences in LIR for males (immature females were not assigned) at MB (Supplementary Table 2) using sighting records (immature = 487; mature = 707) of individuals (immature = 59; mature = 127) whose maturity status remained the same throughout the duration of the study (January 2012 to April 2018). The best fitting models (based on ΔQAIC) were Models C and D (emigration/mortality) for immature males and Model H for mature males. Site and sex-specific LIR indicated that, on average, female manta rays that use MB, and to a lesser extent MP, are two times more likely to be re-sighted at the site of first sighting during the first two years after their initial identification than males (Figures 5C,D and Tables 2B,C). However, immature males were twice as likely to be re-sighted at MB than mature males (Supplementary Figure 8). The Model H scenario indicates the residence time in for mature males was 32.3 days (± 47.0), while Model D estimated the mean residence time for immature males to be ∼ 23-fold longer (745.3 days ± 169.9). Markov movement models indicated differential movement patterns with males, moving from MP to MB (24.5 ± 2.8% SE) at a higher rate than females (8.1 ± 2.2% SE). Further, a modestly higher percentage of females are staying within MP than males (85.8% vs. 75.5%). Notably, the model maximum number of iterations was exceeded, suggesting that the estimated SEs are inaccurate and larger than expected (H. Whitehead, Dalhousie University, personal communication). Nevertheless, taken together these results show a lower probability of movement between the study sites (i.e., higher residence) for female manta rays than mature males; although, immature males display high residency to MB.
A range of manta ray behaviors (Supplementary Figure 4) were observed (n = 3,692) at both study sites, including foraging, cleaning, courtship, cruising and a mix of cleaning and courtship, and cleaning and foraging (Figure 6). However, the frequency of observed behaviors differed between the two sites, and a significant association between behavior and site was present (χ23 = 2303.7, p < 0.001). From the 1,211 sightings with recorded behavior at MB, manta rays were foraging in 79% of sightings (surface ram feeding and near surface barrel-rolling), with cleaning and courtship behaviors observed in 7% and < 1.5% of sightings, respectively. In contrast, at MP (2,481 sightings with recorded behavior), manta rays were foraging in < 4% of sightings. The principal observed behaviors at MP were cleaning (59%), courtship (7%), and courtship combined with cleaning (8%) during single encounter sessions. Courtship behavior was observed year-round at MP but peaked in April–May (Figure 6B). More cruising behavior was observed at MP (22%) than MB (13%).
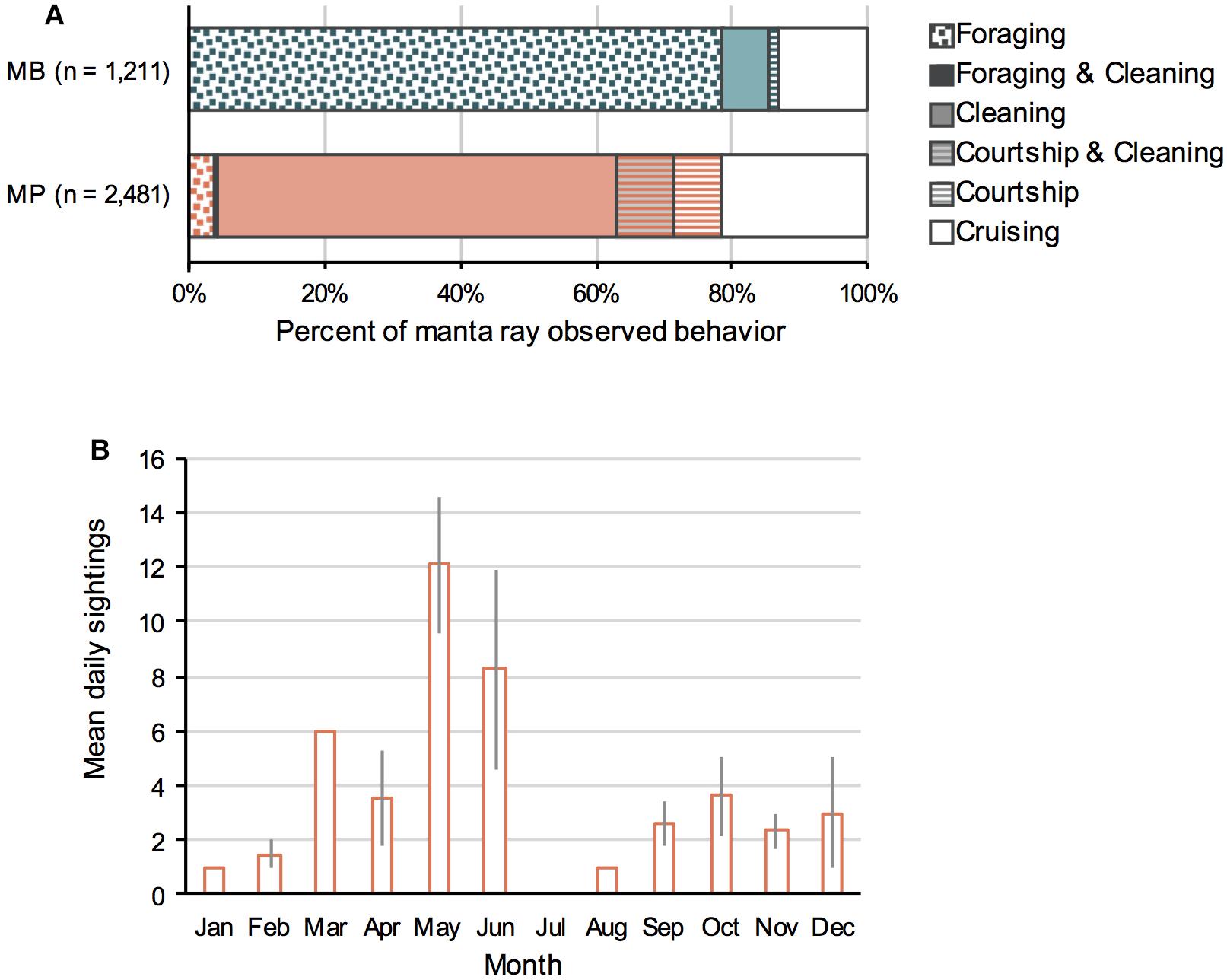
Figure 6. (A) The percentage of observed manta ray behaviors determined from sighting logs and photographs from Manta Bay (MB, n = 1,211) and Manta Point (MP, n = 2,481) from January 2012 to April 2018. Foraging and cleaning, as well as courtship and cleaning behaviors by an individual manta ray were on occasion both witnessed during the same sampling event and identified as such. (B) The average monthly sightings per unit effort (day) of manta rays engaging in courtship behavior. Data recorded between 2013 and 2017 (excluding 2015 due to lower year-round effort) in MP and presented as the daily means (± 1 SE).
The average annual number of boats (7.2) present at the two manta ray sites increased by 60% (Supplementary Figure 9) from 2012 to 2017. This increase was greater at MP than at MB, with the average annual number of boats increasing from 5.3 in 2012 to 13.4 in 2017 at MP (153% increase) compared with virtually no increase at MB (5%, annual average number of boats = 6.2, range = 5.3–7.3) (Supplementary Figure 9A). The monthly average number boats at MP over all years was higher from April–October (10.9–14.0), than the other months (< 8.3), with peaks in May and September (Supplementary Figure 9B). In contrast, the average number of boats at MB was relatively constant throughout the year (5.4–7.9), peaking in August.
We found the Nusa Penida MPA was an important habitat for a substantial number of manta rays (624 identifications in approximately 6 years), with manta rays present year-round and showing high site fidelity to two sites, ∼ 12 km apart. Individual re-sighting rates reported in the Nusa Penida MPA are higher than those reported for any other M. alfredi population in the world (Table 3), with 82% of the individuals re-sighted more than once. The manta rays display high site fidelity to the west coast of Penida Island and are rarely reported from other parts of the MPA. Our analyses highlighted that the two main study sites, MB and MP, are used for different purposes by different life stages of manta rays. MB is a foraging ground, used primarily by immature individuals, whereas MP is an important site for adult social and reproductive activity. Female and male manta rays also used the sites differently, with more males frequenting MB than MP. As a whole, the sex bias found in the Nusa Penida MPA (males: females = 1.4:1) is the largest reported bias toward males worldwide (Table 3). On the other hand, the proportion of females confirmed as sexually mature (53%) is greater than anywhere else in the world (Table 3). This clear segregation in habitat use by different manta ray demographics at almost adjacent sites is noteworthy for marine spatial planners and conservationists globally.
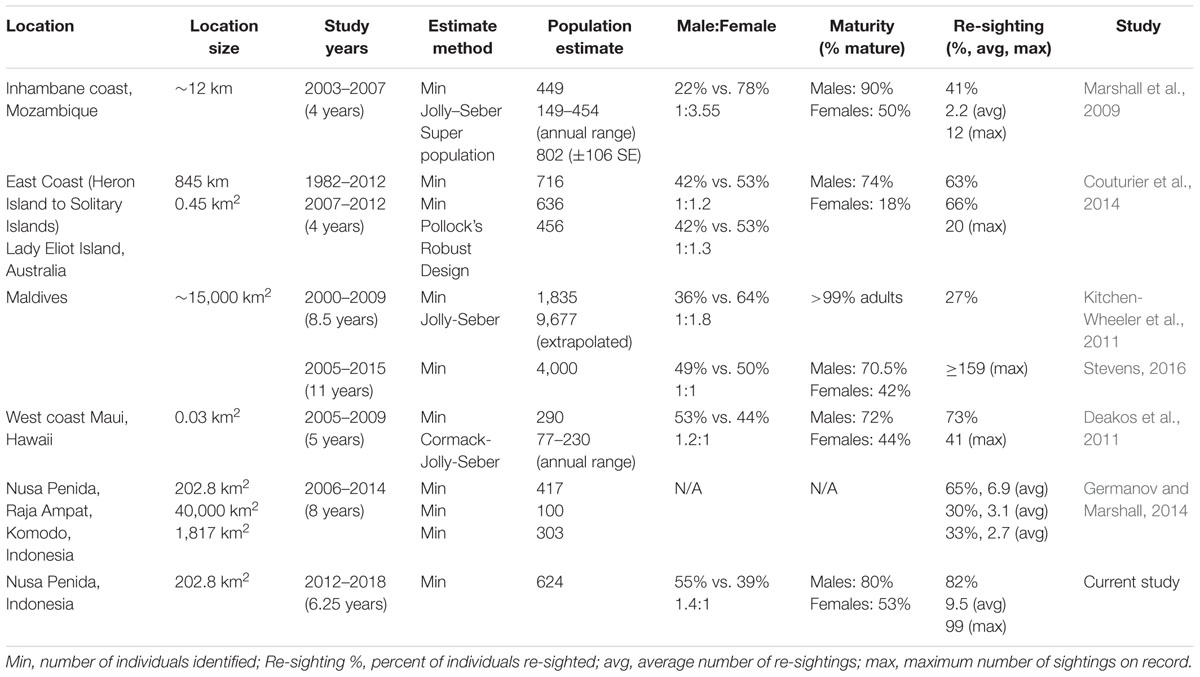
Table 3. A comparison of manta ray (M. alfredi) population studies and demographics from the available literature.
Unlike other manta ray aggregation sites studied to date, MB was used mostly by males with a few resident females. The largest percentage of sightings at MB (38%) were of 59 immature males (one sighted 99 times during the study period). This is particularly noteworthy as few studies report such frequent sightings of juvenile manta rays (Stevens, 2016). Thus, the steady accumulation of new individuals being identified at this site may reflect recruitment of juveniles to an important foraging ground, or potentially a nursery, following birth. The smallest manta rays recorded in the duration of the study were approximately 1.5 m in disk width, which is within the size range of newborn M. alfredi (1.3–1.6 m; Stewart et al., 2018a). Future research looking into the size of manta rays using standardized measurement techniques (Costa et al., 2006; Deakos, 2010; Couturier et al., 2014) would further our understanding of how different age classes use these habitats. Continued recruitment of new, immature, individuals to MB suggests that females give birth nearby, which is a topic worthy of future research.
The location of birthing grounds (i.e., pupping grounds) and (or existence of) nursery grounds of manta rays and other mobulids are major outstanding questions in mobulid ecology (Stewart et al., 2018a). The first nursery habitat described for M. birostris in Flower Garden Banks National Marine Sanctuary in the northwestern Gulf of Mexico (Stewart et al., 2018b), followed the most recent re-description of the term ‘nursery’ for elasmobranchs, proposed by Heupel et al. (2007), and hinges on: (1) more immature individuals are found in this area relative to others nearby; (2) immature individuals reside for extended periods in this area relative to others nearby; and (3) immature individuals show long-term habitat use of this area relative to others. Thus, nursery areas are expected to be areas that provide beneficial opportunities to the young, such as increased food availability (Heupel et al., 2007; McCauley et al., 2014), protection from predation, or areas for thermoregulation via basking behavior (Stevens, 2016; Stewart et al., 2018b). The high re-sighting rates of immature manta rays, the observation of foraging as the dominant behavior, and meeting the criteria proposed by Heupel et al. (2007) and others for a nursery area (McCauley et al., 2014; Stevens, 2016; Stewart et al., 2018b), provide strong evidence that MB forms part of a nursery habitat for the local M. alfredi population. The proposed nursery criteria are aligned with our study in that (1) more immature individuals are found at MB than the nearby MP; (2) much longer residency of immature individuals is observed at MB than at MP; and (3) manta rays identified as immature on first sighting show long-term habitat use of MB relative to MP.
In oligotrophic environments, immature and smaller sized manta rays (more often male because females reach a larger maximum size) possibly prefer to aggregate in shallow coastal areas that offer protection from predators (McCauley et al., 2014; Stevens, 2016). In the Nusa Penida MPA, smaller individuals might use MB as a prominent foraging ground because of reliable food availability. The semi-enclosed nature of the bay, located at the base of the ITF through the Badung Strait and neighboring channel between Ceningan and Penida Islands, and proximity to deep water to the south, could entrain plankton and thereby provide consistent shallow water foraging opportunities for these immature rays.
The manta rays sighted at MP were typically larger than those at MB, with mature females making up 40% of sightings. Thus, the recruitment of new individuals to MP is probably due mainly to immigration, not births. Notably, we found the highest percentage of mature females at this site ever reported (63%) globally (Table 3). The most frequent behavior observed at MP was cleaning (67%). The removal of parasites and other external fouling and the promotion of wound healing is likely an important service provided by cleaner fish (Marshall, 2008; Stevens, 2016). Cleaning activity appears to be a daily ritual, with individuals sometimes spending hours at cleaning stations during the day (Dewar et al., 2008; Marshall, 2008; O’Shea et al., 2010).
Visits to reefs serving as cleaning stations may also provide an opportunity for social interactions in elasmobranchs, including mobulids (O’Shea et al., 2010; Oliver et al., 2011; Murie and Marshall, 2016; Stevens, 2016). Both cleaning and courtship behaviors were observed during 8% of sightings at MP. Courtship behavior is clearly initiated at predictable aggregation sites for mature individuals, with cleaning stations potentially acting as lekking sites (Stevens, 2016). Individuals might visit these aggregation sites during the breeding season in search of mates, rather than for cleaning (Deakos et al., 2011; Stevens, 2016). These suggestions are consistent with the peak in reported sightings at MP during the main reproductive months of April–May. The seasonal pattern of manta ray courtship behavior varies throughout the world (Marshall and Bennett, 2010; Deakos et al., 2011; Couturier et al., 2014; Stevens, 2016), with no single driver for initiating courtship identified to date. Reproductive behavior is likely to be linked to seasonal productivity in the water column, as fecundity has been linked to food availability (Stevens, 2016).
Manta rays are very mobile species and range widely in both archipelago environments (Germanov and Marshall, 2014; Conservation International Indonesia, 2016) and along continental coastlines (Couturier et al., 2014; Jaine et al., 2014). However, recent studies (Couturier et al., 2018; Setyawan et al., 2018) show that they do not use the extent of their home range uniformly, and that individuals concentrate the majority of their activities (foraging, cleaning, and courtship) within specific critical habitats. While our study focused on sightings in two specific areas, it is evident that habitat usage within the Nusa Penida MPA is not uniform.
The year-round sightings of manta rays and their large population size in the Nusa Penida MPA are likely to be linked to the sustained productivity in the region (Surinati, 2009; Ayers et al., 2014; Tito and Susilo, 2017; Nyegaard, 2018). Food availability and sustained foraging opportunities are thought to be responsible for dictating the size of manta ray populations aggregating in a particular area (Deakos et al., 2011) and the frequency of their pregnancies (Stevens, 2016). In most of the manta ray populations studied worldwide, seasonal increases in manta ray abundance coincide with increased oceanic productivity (Dewar et al., 2008; Jaine et al., 2012) and in prey density (Armstrong et al., 2016; Rohner et al., 2017). For example, the largest estimated manta ray population globally is found in the Maldives (Kitchen-Wheeler et al., 2011; Stevens, 2016), where alternating monsoons result in year-round productivity (Anderson et al., 2011). The fine-scale oceanography of the Nusa Penida area is poorly known at present, though large differences in water temperature, indicating regional upwelling and the potential for nutrient mixing and increased nutrient availability to support primary productivity, were recorded over distances of about 30 km from the northern coast to the south-western coast during research on sunfish populations in the region (Tito and Susilo, 2017; Nyegaard, 2018). Studies that evaluate both the presence/movements of filter feeders and the concurrent biological and physical oceanographic characteristics (Jaine et al., 2012; Rohner et al., 2013) would enhance the understanding of manta ray ecology and the drivers of differential site use, increased seasonal abundance and reproductive behavior in this complex area.
Although the manta rays of Nusa Penida display some site preference, emigration and interchange between MB and MP does occur. Almost half of the individual manta rays recorded (n = 277; 44%) visited both locations during the study period. However, the interchange between the two sites was not symmetric and differed between the sexes. Higher transition probabilities for movement were observed from MB to MP (∼ 35%) than for the reverse movement (∼ 16%), raising the possibility that a longer-term study may demonstrate that the two sites, taken together, constitute a single population. The higher percentage of movement from MB to MP could be individuals leaving their nursery ground as they grow larger, to forage offshore and spend more time interacting with other mature individuals (McCauley et al., 2014; Stevens, 2016).
Further, the sex-based differences in habitat use for manta rays might be mainly linked to reproductive behavior (Deakos et al., 2011; Stevens, 2016). Similar to other pelagic species of marine megafauna including sharks, sea turtles and cetaceans (Hueter et al., 2005; Lee et al., 2007; Engelhaupt et al., 2009), female manta rays, as the sex with the greater parental investment (i.e., the ‘limiting sex’), gain a greater choice of mates by residing in a popular aggregation area, assuming that ample foraging opportunities are available nearby. Males, in contrast, benefit from moving between aggregation areas in search of mates. Consistent with this hypothesis, female manta rays from the Nusa Penida MPA are likely to reside in either of the sites for a longer time than males.
Moreover, the Nusa Penida MPA manta rays occasionally move to areas ‘outside’ MB and MP. Previous research (Germanov and Marshall, 2014; Conservation International Indonesia, 2016) shows that manta rays move long distances from the Nusa Penida MPA to locations such as the Gili Islands (80 km straight-line distance north-east), Sumbawa, and the Komodo National Park (up to 450 km straight-line distance east).
The Nusa Penida MPA serves as year-round critical habitat for a substantial local manta ray population making it a high priority area for conservation and management. MB is used year-round for foraging, particularly by juvenile manta rays, while MP is an important area for cleaning and reproductive behaviors that peak in May each year. This has implications for MPA management, zoning and the types of activities to be allowed in these areas during distinct times of year. Management actions should address the following threats to the local manta ray populations: (1) disruption of manta ray behavior through habitat crowding and human disturbance from excessive tourism (Venables et al., 2016; Barr et al., 2017; Trave et al., 2017) and (2) entanglement in fishing gear and injury from continuing fisheries in the area (present study, Aquatic Alliance, personal communication).
While tourism to the area is increasing, there are currently no regulations in place for manta ray interactions (Barr et al., 2017). A 2016 survey (Barr et al., 2017) directed at divers and snorkelers participating in manta ray watching activities, within the Nusa Penida MPA, reported participant conduct that is considered disruptive to the manta rays (e.g., closely following and touching manta rays was reported by 10% and 3.5% of participants, respectively). Disturbance stimuli to animals, such as loud boat engine noise, fast approaches by boats, in water chasing, touching, and crowding behavior by tourists (Norman, 1999; Bejder et al., 2006a,b; Anderson et al., 2010; Higham et al., 2016; Venables et al., 2016; Barr et al., 2017), is argued to be analogous to a predation risk (reviewed by Frid and Dill, 2002). Thus, tourism pressure at MB might disrupt foraging behaviors, reducing growth rates of immature manta rays and the fitness of mature individuals (Stewart et al., 2018a). Further, higher boat numbers at MP in May (Supplementary Figure 9) coincide with the seasonal increase in sightings at MP and in reproductive behavior. With the Nusa Penida MPA serving as an important reproductive ground, disruption of these important social behaviors is a concern. Based on the criteria to evaluate marine wildlife tourism practices outlined by Trave et al. (2017), we recommend that: (1) science based carrying capacity calculations of tourism operations be carried out to estimate the acceptable number of tour boats and diver interactions for the area (Ríos-Jara et al., 2013; Zelenka and Kacetl, 2014) potentially limiting the number of boats/divers/swimmers allowed at one time (Division of Boating and Ocean Recreation, 2016; Kasmidi and Gunadharma, 2017); (2) codes of conduct for diving and snorkeling with manta rays become mandatory (see Garrud, 2016; Venables et al., 2016), akin to regulations for whale shark interactions in Ningaloo Reef, Australia (Mau, 2008; Catlin and Jones, 2010); (3) a licensing system for tour operators with penalties for breaches be implemented (Mau, 2008; Division of Boating and Ocean Recreation, 2016); and (4) area-time closures be considered as a management option (Tyne et al., 2014; Setyawan et al., 2018) to protect the manta rays from disturbance during the peak time of mating, especially at MP.
Small-scale fishing often takes place in MB and nearby coastlines (EG, pers. obs., Supplementary Figure 10). Over the course of the study, ∼ 14% (n = 87) of manta rays were observed either trailing hooks and lines or with cephalic and pectoral fin injuries and/or amputations, which occur when fishing lines or nets cut through the skin and cartilage skeleton. The true scale of the issue is under-represented as only animals that have survived the entanglement event are counted. Currently, the long-term impacts of these effects on individuals that survive entanglement are not known, nor whether foraging and swimming efficiency is impaired and if there are any impacts on fecundity. However, observations of pregnant individuals within the Nusa Penida MPA, with single cephalic fin amputations, suggest that manta rays retain their reproductive fitness even with these sub-lethal injuries. Further, the proportion of pregnant manta rays from the Nusa Penida MPA with injuries (0.11) was not significantly lower than the overall proportion of females with injuries (0.12; χ21 = 0.676, p > 0.5), suggesting that injured manta rays are not substantially impaired reproductively. Nevertheless, it is unclear whether these injuries have an initial impact on the success of their pregnancies, as many related species (e.g., batoids) will abort their fetuses if their individual survival is threatened (entanglement, landing, predation; reviewed by Adams et al., 2018). Further, biomechanical modeling of manta ray pectoral fin movements indicate that the major thrust force comes from the distal portion of the pectoral fins (Liu et al., 2015), thus pectoral fin truncations could significantly impact manta ray swimming efficiency, energy consumption and ability to evade predation. While all fishing activities are officially prohibited in both MB and MP, increased enforcement of this regulation is necessary. As a precautionary measure, management could prohibit all fishing activities along the west coast of Nusa Penida, which would require strong compliance if it was to be effective in contributing to the recovery of manta rays in the Nusa Penida MPA.
Understanding localized habitat use, identifying nursery areas, as well as key times of year for reproduction, provides enhanced information for developing effective management plans for manta rays in this region related to specific manta ray behaviors. Context-dependent and adaptive management solutions are important for manta ray populations as well as for people, as manta ray watching tourism supports many livelihoods through activities that rely on healthy manta ray populations (i.e., snorkeling and SCUBA diving). With their 2014 protection in Indonesia, manta rays have entered the spotlight for conservation initiatives and serve as ideal flagship species to refocus future research on the overarching challenges and opportunities facing ocean health in the Coral Triangle (see also Germanov et al., 2018), the world’s premier marine biodiversity hotspot.
This research was completed under Indonesian Research permit #458/FRP/E5/Dit. KI/XI/2015, #11K1/XI/2016; #86/KI/XI/2017. This study was carried out in accordance with the recommendations of the Animal Ethics Committee, Murdoch University. The protocol was approved (R2781/15) by the Animal Ethics Committee, Murdoch University. Public-contributed photographic data were collected opportunistically by recreational divers and deposited by them into a public online repository (mantamatcher.org), which was developed explicitly to facilitate citizen science contributions.
EG and AM conceived the study. EG analyzed the data with LB, DC, and SP advising on the analysis. EG wrote the manuscript with contribution to drafting, critical review, and editorial input from LB, DC, DD, IH, NL, AM, SP, and MvK. All authors critically reviewed the manuscript with final proofs.
While no specific funding was received for this research, EG is supported by an Australian Postgraduate Award and Murdoch International Top Up (32608315), which supports her doctoral studies; Ocean Park Conservation Foundation (FH04_1516), which has funded a large portion of fieldwork and laboratory work for her doctoral studies; Foundation Fortuna, which supports the field work for her doctoral studies; Idea Wild, which has donated field sampling equipment; Mantahari Oceancare, which has funded manta ray research capacity building programs related to her doctoral studies; PADI Foundation (14668), which supports the field work for her doctoral studies; and private donors, which support the field work for her doctoral studies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JDS declared a past co-authorship with several of the authors, EG and AM, to the handling Editor.
This study would not have been possible without the generous support of Lembongan Marine Association, the local dive community and many citizen scientists to whom were are indebted. Further, we thank the Aquatic Alliance for spearheading this project and Marine Megafauna Foundation staff and volunteers for carrying it forward. Notably, we thank, H. Mitchell, P. Bassett, and L. Ellevog for their unwavering dedication to Indonesia’s manta rays and enabling this study. Further, we appreciate the data processing assistance provided by S. Ecob, L. Auditore, R. Cooper, and E. Sinderson. We thank G. Winstanley who provided the use of ‘MantaUtil’ for streamlined data processing and facilitated ‘batch’ lodging of legacy data to ‘MantaMatcher.’ We thank H. Whitehead for the guidance he provided on the use of Markov movement models, M. Calver and R. Admiraal for statistical analysis assistance, and S. Venables for plotting using R. Bathymetry information used in the creating Figure 1 was available from: GEBCO_2014 Grid, version 20150318; www.gebco.net. We thank members of Faculty of Marine Sciences and Fisheries, Udayana University, Bali, Indonesia and C. O. Lafuente for assisting in creating Figure 1. We are grateful for the study permit from the Indonesian Ministry of Research (Ristek Dikti). This manuscript represents HIMB and SOEST contribution numbers 1755 and 10681, respectively. We are grateful for the reviewers’ input, which much improved the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00215/full#supplementary-material
Adams, K. R., Fetterplace, L. C., Davis, A. R., Taylor, M. D., and Knott, N. A. (2018). Sharks, rays and abortion: the prevalence of capture-induced parturition in elasmobranchs. Biol. Conserv. 217, 11–27. doi: 10.1016/j.biocon.2017.10.010
Anderson, R. C., Adam, M. S., and Goes, J. I. (2011). From monsoons to mantas: seasonal distribution of Manta alfredi in the Maldives. Fish. Oceanogr. 202, 104–113. doi: 10.1111/j.1365-2419.2011.00571.x
Anderson, R. C., Adam, M. S., Kitchen-Wheeler, A. M., and Stevens, G. (2010). Extent and economic value of manta ray watching in Maldives. Tour. Mar. Environ. 7, 15–27. doi: 10.3727/154427310X12826772784793
Armstrong, A. O., Armstrong, A. J., Jaine, F. R. A., Couturier, L. I. E., Fiora, K., Uribe-Palomino, J., et al. (2016). Prey density threshold and tidal influence on reef manta ray foraging at an aggregation site on the great barrier reef. PLoS One 11:e0153393. doi: 10.1371/journal.pone.0153393
Ayers, J. M., Strutton, P. G., Coles, V. J., Hood, R. R., and Matear, R. J. (2014). Indonesian throughflow nutrient fluxes and their potential impact on Indian Ocean productivity. Geophys. Res. Lett. 4114, 5060–5067. doi: 10.1002/2014GL060593
Baird, R. W., Gorgone, A. M., McSweeney, D. J., Webster, D. L., Salden, D. R., Deakos, M. H., et al. (2008). False killer whales (Pseudorca crassidens) around the main Hawaiian Islands: long-term site fidelity, inter-island movements, and association patterns. Mar. Mamm. Sci. 243, 591–612. doi: 10.1111/j.1748-7692.2008.00200.x
Barber, P. H., Palumbi, S. R., Erdmann, M. V., and Moosa, M. K. (2000). Biogeography: a marine Wallace’s line? Nature 406:692.
Barr, E., Yudiarso, P., Welly, M., and Pratama, C. D. (2017). Improving Manta protection in Nusa Penida Marine Protected Area: A willingness-to-pay exploration of potential permit scheme. Bali: Conservation Strategy Fund. Available at: https://www.conservation-strategy.org/en/publication/improving-manta-protection-nusa-penida-marine-protected-area-willingness-pay-exploration#.XCxa8M8zbL8 (accessed January 2, 2019).
Bejder, L., Samuels, A., Whitehead, H., and Gales, N. (2006a). Interpreting short-term behavioural responses to disturbance within a longitudinal perspective. Anim. Behav. 725, 1149–1158. doi: 10.1016/j.anbehav.2006.04.003
Bejder, L., Samuels, A., Whitehead, H., Gales, N., Mann, J., Connor, R., et al. (2006b). Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conserv. Biol. 20, 1791–1798. doi: 10.1111/j.1523-1739.2006.00540.x
Bowen, B. W., and Karl, S. (2007). Population genetics and phylogeography of sea turtles. Mol. Ecol. 1623, 4886–4907. doi: 10.1111/j.1365-294X.2007.03542.x
Catlin, J., and Jones, R. (2010). Whale shark tourism at Ningaloo Marine Park: a longitudinal study of wildlife tourism. Tourism Manage. 313, 386–394. doi: 10.1016/j.tourman.2009.04.004
Chabanne, D. B., Finn, H., and Bejder, L. (2017). Identifying the relevant local population for environmental impact assessments of mobile marine fauna. Front. Mar. Sci. 4:148. doi: 10.3389/fmars.2017.00148
Chapman, D. D., Feldheim, K. A., Papastamatiou, Y. P., and Hueter, R. E. (2015). There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Ann. Rev. Mar. Sci. 7, 547–570. doi: 10.1146/annurev-marine-010814-015730
Conservation International Indonesia. (2016). Laporan Program Kerjasama Tagging Satelit Pari Manta. Jakarta: Conservation International Indonesia.
Costa, C., Loy, A., Cataudella, S., Davis, D., and Scardi, M. (2006). Extracting fish size using dual underwater cameras. Aquacult. Eng. 35, 218–227. doi: 10.1016/j.aquaeng.2006.02.003
Couturier, L. I. E., Dudgeon, C. L., Pollock, K. H., Jaine, F. R. A., Bennett, M. B., Townsend, K., et al. (2014). Population dynamics of the reef manta ray Manta alfredi in eastern Australia. Coral Reefs 33, 329–342. doi: 10.1007/s00338-014-1126-5
Couturier, L. I. E., Jaine, F. R. A., Townsend, K. A., Weeks, S. J., Richardson, A. J., and Bennett, M. B. (2011). Distribution, site affinity and regional movements of the manta ray, Manta alfredi (Krefft, 1868), along the east coast of Australia. Mar. Freshw. Res. 62, 628–637. doi: 10.1071/MF10148
Couturier, L. I. E., Marshall, A. D., Jaine, F. R. A., Kashiwagi, T., Pierce, S. J., Townsend, K. A., et al. (2012). Biology, ecology and conservation of the Mobulidae. J. Fish Biol. 80, 1075–1119. doi: 10.1111/j.1095-8649.2012.03264.x
Couturier, L. I. E., Newman, P., Jaine, F. R. A., Bennett, M. B., Venables, W. N., Cagua, E. F., et al. (2018). Variation in occupancy and habitat use of Mobula alfredi at a major aggregation site. Mar. Ecol. Prog. Series. 599, 125–145. doi: 10.3354/meps12610
Croll, D. A., Dewar, H., Dulvy, N. K., Fernando, D., Francis, M. P., Galván-Magaña, F., et al. (2016). Vulnerabilities and fisheries impacts: the uncertain future of manta and devil rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 562–575. doi: 10.1002/aqc.2591
Deakos, M. H. (2010). Paired-laser photogrammetry as a simple and accurate system for measuring the body size of free-ranging manta rays Manta alfredi. Aquat. Biol. 10, 1–10. doi: 10.3354/ab00258
Deakos, M. H. (2012). The reproductive ecology of resident manta rays (Manta alfredi) off Maui, Hawaii, with an emphasis on body size. Environ. Biol. Fishes 94, 443–456. doi: 10.1007/s10641-011-9953-5
Deakos, M. H., Baker, J. D., and Bejder, L. (2011). Characteristics of a manta ray Manta alfredi population off Maui, Hawaii, and implications for management. Mar. Ecol. Prog. Ser. 429, 245–260. doi: 10.3354/meps09085
Dewar, H., Mous, P., Domeier, M., Muljadi, A., Pet, J., and Whitty, J. (2008). Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar. Biol. 155, 121–133. doi: 10.1007/s00227-008-0988-x
District Fisheries and Marine Agency. (2010). Peraturan Bupati Klungkung No/12/2010 tentang Kawasan Konservasi Perairan Daerah. Klungkung. Bali: District Fisheries and Marine Agency. Available at: http://kkji.kp3k.kkp.go.id/index.php/en/marine-protected-area-data/details/7/84 (accessed January 2, 2019).
Division of Boating, and Ocean Recreation (2016). Manta Viewing within the West Hawaii Ocean Recreation Management Area: Introduction of Proposed Administrative Rules. Kona: Department of Land and Natural resources. Available at: https://dlnr.hawaii.gov/dobor/files/2013/08/MantaDiveSitesManagementPlan-9.9.16.pdf (accessed January 2, 2019).
Dulvy, N. K., Pardo, S. A., Simpfendorfer, C. A., and Carlson, J. K. (2014). Diagnosing the dangerous demography of manta rays using life history theory. PeerJ 2:e400. doi: 10.7717/peerj.400
Engelhaupt, D., Rus Hoelzel, A., Nicholson, C., Frantzis, A., Mesnick, S., Gero, S., et al. (2009). Female philopatry in coastal basins and male dispersion across the North Atlantic in a highly mobile marine species, the sperm whale (Physeter macrocephalus). Mol. Ecol. 1820, 4193–4205. doi: 10.1111/j.1365-294X.2009.04355.x
Fox, S., Foisy, I., De La Parra, Venegas, R., Galván Pastoriza, B., Graham, R., et al. (2013). Population structure and residency of whale sharks Rhincodon typus at Utila, Bay Islands, Honduras. J. Fish Biol. 833, 574–587. doi: 10.1111/jfb.12195
Frid, A., and Dill, L. (2002). Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 61:11.
Garrud, E. (2016). Does Tourist Behaviour Affect Reef Manta Ray Feeding Behaviour? An Analysis of Human and Manta alfredi Interactions in Baa Atoll, the Maldives. York: University of York.
Germanov, E. S., and Marshall, A. D. (2014). Running the gauntlet: regional movement patterns of Manta alfredi through a complex of parks and fisheries. PLoS One 9:e110071. doi: 10.1371/journal.pone.0110071
Germanov, E. S., Marshall, A. D., Bejder, L., Fossi, M. C., and Loneragan, N. R. (2018). Microplastics: no small problem for filter-feeding megafauna. Trends Ecol. Evol. 33, 227–232. doi: 10.1016/j.tree.2018.01.005
Heinrichs, S., O’Malley, M., Medd, H., and Hilton, P. (2011). Manta Ray of Hope 2011 Report: The Global Threat to Manta and Mobula Rays. San Francisco: WildAid. Available at: https://wildaid.org/wp-content/uploads/2017/09/The-Global-Threat-to-Manta-and-Mobula-Rays-WEB.pdf (accessed January 1, 2012).
Heupel, M. R., Carlson, J. K., and Simpfendorfer, C. A. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Higham, J. E., Bejder, L., Allen, S. J., Corkeron, P. J., and Lusseau, D. (2016). Managing whale-watching as a non-lethal consumptive activity. J. Sustain. Tour. 241, 73–90. doi: 10.1080/09669582.2015.1062020
Hueter, R., Heupel, M., Heist, E., and Keeney, D. (2005). Evidence of philopatry in sharks and implications for the management of shark fisheries. J. Northwest Atl. Fish. Sci. 35, 239–247. doi: 10.2960/J.v35.m493
Jaine, F. R. A., Couturier, L. I. E., Weeks, S. J., Townsend, K. A., Bennett, M. B., Fiora, K., et al. (2012). When giants turn up: sighting trends, environmental influences and habitat use of the manta ray Manta alfredi at a coral reef. PLoS One 7:e46170. doi: 10.1371/journal.pone
Jaine, F. R. A., Rohner, C. A., Weeks, S. J., Couturier, L. I. E., Bennett, M. B., Townsend, K. A., et al. (2014). Movements and habitat use of reef manta rays off eastern Australia: offshore excursions, deep diving and eddy affinity revealed by satellite telemetry. Mar. Ecol. Prog. Ser. 510, 73–86. doi: 10.3354/meps10910
Kasmidi, M., and Gunadharma, A. (2017). Diving in Manta Sandy? Here’s What You Need to Know! Bird’s Head Seascape. Available at: http://birdsheadseascape.com/diving/diving-manta-sandy-heres-need-know-meidiarti-kasmidi-nikka-amandra-gunadharma/ (accessed January 2, 2019).
Kitchen-Wheeler, A. M., Ari, C., and Edwards, A. J. (2011). Population estimates of Alfred mantas (Manta alfredi) in central maldives atolls: north male, ari and baa. Environ. Biol. Fishes 93, 557–575. doi: 10.1007/s10641-011-9950-8
Lee, P. L., Luschi, P., and Hays, G. C. (2007). Detecting female precise natal philopatry in green turtles using assignment methods. Mol. Ecol. 161, 61–74. doi: 10.1111/j.1365-294X.2006.03115.x
Liu, G., Ren, Y., Li, C., Dong, H., Bart-Smith, H., and Fish, F. (2015). “Video: fin flexion and flow modulation in Manta’s forward swimming,” in Proceedings of the 68th Annual Meeting of the APS Division of Fluid Dynamics, Boston, MA, 22–24. doi: 10.1103/APS.DFD.2015.GFM.V0089
Marshall, A. D. (2008). Biology and Population Ecology of Manta birostris in Southern Mozambique. Brisbane, QLD: University of Queensland.
Marshall, A. D., and Bennett, M. B. (2010). Reproductive ecology of the reef manta ray Manta alfredi in southern Mozambique. J. Fish Biol. 77, 169–190. doi: 10.1111/j.1095-8649.2010.02669.x
Marshall, A. D., Compagno, L. J. V., and Bennett, M. B. (2009). Redescription of the genus Manta with resurrection of Manta alfredi. Zootaxa 28, 1–28.
Marshall, A. D., Dudgeon, C. L., and Bennett, M. B. (2011). Size and structure of a photographically identified population of manta rays Manta alfredi in southern Mozambique. Mar. Biol. 158, 1111–1124. doi: 10.1007/s00227-011-1634-6
Marshall, A. D., and Holmberg, J. (2018). MantaMatcher Photo-identification Library. Available at: http://www.mantamatcher.org/ (accessed May 16, 2018).
Mau, R. (2008). Managing for conservation and recreation: the Ningaloo whale shark experience. J. Ecotour. 7, 213–225. doi: 10.1080/14724040802140550
Mayer, B., and Damm, P. (2012). The makassar strait throughflow and its jet. J. Geophys. Res. Oceans 117:C07020. doi: 10.1029/2011JC007809
McCauley, D. J., DeSalles, P. A., Young, H. S., Papastamatiou, Y. P., Caselle, J. E., Deakos, M. H., et al. (2014). Reliance of mobile species on sensitive habitats: a case study of manta rays (Manta alfredi) and lagoons. Mar. Biol. 161, 1987–1998. doi: 10.1007/s00227-014-2478-7
McCoy, E., Burce, R., David, D., Aca, E. Q., Hardy, J., Labaja, J., et al. (2018). Long-term photo-identification reveals the population dynamics and strong site fidelity of adult whale sharks to the coastal waters of Donsol, Philippines. Front. Mar. Sci. 5:271. doi: 10.3389/fmars.2018.00271
Miller-Rushing, A., Primack, R., and Bonney, R. (2012). The history of public participation in ecological research. Front. Ecol. Environ. 10:285. doi: 10.1890/110278
Ministry of Marine Affairs and Fisheries (2014). Keputusan Menteri Kelautan dan Perikanan Republik Indonesia No4/Kepmen-KP/2014 tentang Penetapan Status Perindungan Penuh Ikan Pari Manta. Jakarta: Ministry of Marine Affairs and Fisheries. Available at: http://kkji.kp3k.kkp.go.id/index.php/dokumen/regulasi-hukum/keputusan-menteri/finish/14-keputusan-menteri/516-kepmen-kp-no-4-tahun-2014-tentang-penetapan-status-perlindungan-penuh-ikan-pari-manta (accessed January 2, 2019).
Murie, C. J., and Marshall, A. D. (2016). Mobula kuhlii cleaning station identified at an inshore reef in southern Mozambique. PeerJ PrePrints 4:e1724v1. doi: 10.7287/peerj.preprints.1724v1
Murray, S. P., and Arief, D. (1988). Throughflow into the indian ocean through the lombok strait, January 1985–January 1986. Nature 3336172:444.
Ningsih, N. S., Rakhmaputeri, N., and Harto, A. B. (2013). Upwelling variability along the southern coast of Bali and in Nusa Tenggara waters. Ocean Sci. J. 481, 49–57. doi: 10.1007/s12601-013-0004-3
Norman, B. M. (1999). Aspects of the Biology and Ecotourism Industry of the Whale Shark Rhincodon typus in North-Western Australia. Perth, WA: Murdoch University.
Norman, B. M., Holmberg, J. A., Arzoumanian, Z., Reynolds, S. D., Wilson, R. P., Rob, D., et al. (2017). Undersea constellations: the global biology of an endangered marine megavertebrate further informed through citizen science. Bioscience 6712, 1029–1043. doi: 10.1093/biosci/bix127
Nyegaard, M. (2018). There be Giants! The Importance of Taxonomic Clarity of the Large Ocean Sunfishes (genus Mola, Family Molidae) for Assessing Sunfish Vulnerability to Anthropogenic Pressures. Perth, MA: Murdoch University.
Oliver, S. P., Hussey, N. E., Turner, J. R., and Beckett, A. J. (2011). Oceanic sharks clean at coastal seamount. PLoS One 63:e14755. doi: 10.1371/journal.pone.0014755
O’Malley, M. P., Lee-Brooks, K., and Medd, H. B. (2013). The global economic impact of manta ray watching tourism. PLoS One 8:e65051. doi: 10.1371/journal.pone.0065051
O’Shea, O. R., Kingsford, M. J., and Seymour, J. (2010). Tide-related periodicity of manta rays and sharks to cleaning stations on a coral reef. Mar. Freshw. Res. 611, 65–73. doi: 10.1071/MF08301
Pierce, S. J., Holmberg, J., Kock, A. A., and Marshall, A. D. (2018). Photographic Identification of Sharks. Boca Raton, FL: CRC Press.
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available at: https://www.R-project.org/ (accessed April 1, 2018).
Rambahiniarison, J. M., Lamoste, M. J., Rohner, C. A., Murray, R., Snow, S., Labaja, J., et al. (2018). Life history, growth, and reproductive biology of four mobulid species in the bohol sea, philippines. Front. Mar. Sci. 5:269. doi: 10.3389/fmars.2018.00269
Ríos-Jara, E., Galván-Villa, C. M., Rodríguez-Zaragoza, F. A., López-Uriarte, E., and Munoz-Fernández, V. T. (2013). The tourism carrying capacity of underwater trails in Isabel Island National Park, Mexico. Environ. Manage. 522, 335–347. doi: 10.1007/s00267-013-0047-3
Robichaud, D., and Rose, G. (2001). Multiyear homing of Atlantic cod to a spawning ground. Can. J. Fish. Aquat. Sci. 5812, 2325–2329. doi: 10.1139/f01-190
Robinson, D. P., Jaidah, M. Y., Bach, S., Lee, K., Jabado, R. W., Rohner, C. A., et al. (2016). Population structure, abundance and movement of whale sharks in the Arabian Gulf and the Gulf of Oman. PLoS One 116:e0158593. doi: 10.1371/journal.pone.0158593
Rohner, C. A., Burgess, K. B., Rambahiniarison, J. M., Stewart, J. D., Ponzo, A., and Richardson, A. J. (2017). Mobulid rays feed on euphausiids in the Bohol Sea. R. Soc. Open Sci. 4:161060. doi: 10.1098/rsos.161060
Rohner, C. A., Pierce, S. J., Marshall, A. D., Weeks, S. J., Bennett, M. B., and Richardson, A. J. (2013). Trends in sightings and environmental influences on a coastal aggregation of manta rays and whale sharks. Mar. Ecol. Prog. Ser. 482, 153–168. doi: 10.3354/meps10290
Rooker, J. R., Secor, D. H., De Metrio, G., Schloesser, R., Block, B. A., and Neilson, J. D. (2008). Natal homing and connectivity in Atlantic bluefin tuna populations. Science 3225902, 742–744. doi: 10.1126/science.1161473
Setyawan, E., Sianipar, A. B., Erdmann, M. V., Fischer, A. M., Haddy, J. A., Beale, C. S., et al. (2018). Site fidelity and movement patterns of reef manta rays (Mobula alfredi): mobulidae using passive acoustic telemetry in northern Raja Ampat, Indonesia. Nat. Conserv. Res. 34, 1–15. doi: 10.24189/ncr.2018.043
Stevens, G. M. W. (2016). Conservation and Population Ecology of Manta Rays in the Maldives. York: University of York.
Stevens, G. M. W., Hawkins, J. P., and Roberts, C. M. (2018). Courtship and mating behaviour of manta rays Mobula alfredi and M. birostris in the Maldives. J. Fish Biol. 93, 344–359. doi: 10.1111/jfb.13768
Stewart, J. D., Jaine, F. R., Armstrong, A. J., Armstrong, A. O., Bennett, M. B., Burgess, K. B., et al. (2018a). Research priorities to support effective manta and devil ray conservation Front. Mar. Sci. 5:314. doi: 10.3389/fmars.2018.00314
Stewart, J. D., Nuttall, M., Hickerson, E. L., and Johnston, M. A. (2018b). Important juvenile manta ray habitat at flower garden banks national marine sanctuary in the northwestern Gulf of Mexico. Mar. Biol. 165:111. doi: 10.1007/s00227-018-3364-5
Thys, T., Ryan, J. P., Weng, K. C., Erdmann, M., and Tresnati, J. (2016). Tracking a marine ecotourism star: movements of the short ocean sunfish Mola ramsayi in Nusa Penida, Bali, Indonesia. J. Mar. Biol. 2016:8750193. doi: 10.1155/2016/8750193
Tillinger, D. (2011). Physical oceanography of the present day Indonesian Throughflow. Geol. Soc. Lon Spec. Pub. 3551, 267–281. doi: 10.1144/SP355.13
Tito, C. K., and Susilo, E. (2017). The Correlation of upwelling phenomena and ocean sunfish occurrences in Nusa Penida, Bali. IOP Conf. Ser. Earth Environ. Sci. 55:012031. doi: 10.1088/1755-1315/55/1/012031
Town, C., Marshall, A., and Sethasathien, N. (2013). Manta Matcher: automated photographic identification of manta rays using keypoint features. Ecol. Evol. 37, 1902–1914. doi: 10.1002/ece3.587
Trave, C., Brunnschweiler, J., Sheaves, M., Diedrich, A., and Barnett, A. (2017). Are we killing them with kindness? Evaluation of sustainable marine wildlife tourism. Biol. Conserv. 209, 211–222. doi: 10.1016/j.biocon.2017.02.020
Tyne, J., Loneragan, N., and Bejder, L. (2014). The use of area-time closures as a tool to manage cetacean-watch tourism. Whale-watching: sustainable. Tour. Ecol. Manage. 27, 242–260.
Venables, S., McGregor, F., Brain, L., and van Keulen, M. (2016). Manta ray tourism management, precautionary strategies for a growing industry: a case study from the Ningaloo Marine Park, Western Australia. Pacific Conserv. Biol. 22, 295–300. doi: 10.1071/PC16003
Ward-Paige, C. A., Davis, B., and Worm, B. (2013). Global population trends and human use patterns of Manta and Mobula rays. PLoS One 8:e74835. doi: 10.1371/journal.pone.0074835
Whitehead, H. (2007). Selection of models of lagged identification rates and lagged association rates using AIC and QAIC. Commun. Stat. Simul. C 36, 1233–1246. doi: 10.1080/03610910701569531
Whitehead, H. (2009). SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. doi: 10.1007/s00265-008-0697-y
Winstanley, G. W. (2016). MantaUtil: Manta Database Utility. Available at: http://www.pelagicon.com/software/mantautil/ (accessed March 24, 2017).
Keywords: Mobula alfredi, citizen science, photo-identification, population structure, animal behavior, site fidelity, nursery, tourism management
Citation: Germanov ES, Bejder L, Chabanne DBH, Dharmadi D, Hendrawan IG, Marshall AD, Pierce SJ, van Keulen M and Loneragan NR (2019) Contrasting Habitat Use and Population Dynamics of Reef Manta Rays Within the Nusa Penida Marine Protected Area, Indonesia. Front. Mar. Sci. 6:215. doi: 10.3389/fmars.2019.00215
Received: 22 January 2019; Accepted: 05 April 2019;
Published: 01 May 2019.
Edited by:
Mark Meekan, Australian Institute of Marine Science (AIMS), AustraliaReviewed by:
Gail Schofield, Queen Mary University of London, United KingdomCopyright © 2019 Germanov, Bejder, Chabanne, Dharmadi, Hendrawan, Marshall, Pierce, van Keulen and Loneragan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elitza S. Germanov, ZWxpdHphQG1hcmluZW1lZ2FmYXVuYS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.