
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 07 March 2019
Sec. Marine Ecosystem Ecology
Volume 6 - 2019 | https://doi.org/10.3389/fmars.2019.00052
Some of the major challenges in seagrass restoration on exposed open coasts are the choice of transplant design that is optimal for coastlines periodically exposed to high water motion, and understanding the survival and dynamics of the transplanted areas on a long time-scale over many years. To contribute to a better understanding of these challenges, we describe here part of a large-scale seagrass restoration program conducted in a Marine Park in Portugal. The goal of this study was to infer if it was possible to recover seagrass habitat in this region, in order to restore its ecosystem functions. To infer which methods would produce better long term persistence to recover seagrass habitat, three factors were assessed: donor seagrass species, transplant season, source location. Monitoring was done three times a year for 8 years, in which areas and densities of the planted units were measured, to assess survival and growth. The best results were obtained with the species Zostera marina transplanted during spring and summer as compared to Zostera noltii and Cymodocea nodosa. Long-term persistence of established (well rooted) transplants was mainly affected by extreme winter storms but there was evidence of fish grazing effects also. Our results indicate that persistence assessments should be done in the long term, as all transplants were successful (survived and grew initially) in the short term, but were not resistant in the long term after a winter with exceptionally strong storms. The interesting observation that only the largest (11 m2) transplanted plot of Z. marina persisted over a long time, increasing to 103 m2 in 8 years, overcoming storms and grazing, raised the hypothesis that for a successful shift to a vegetated state it might be necessary to overpass a minimum critical size or tipping point. This hypothesis was therefore tested with replicates from two donor populations and results showed effects of size and donor population, as only the larger planting units (PUs) from one donor population persisted and expanded. It is recommended that in future habitat restoration efforts large PUs are considered.
Seagrass restoration has been conducted for nearly 70 years, since the middle of the last century (e.g., Addy, 1947). However, the vast majority of seagrass projects have been of limited extent (<0.5 ha), often experimental and almost exclusively in sheltered estuarine waters. Restoration attempts in more wave exposed coasts are still few (Bull et al., 2004; van Katwijk et al., 2009). To our knowledge, open coast large-scale, non-experimental restoration has only been attempted in Western Australia (Fonseca et al., 1998a; Paling et al., 2003, 2007).
Restoration of seagrasses on exposed open coasts poses special challenges, both logistical and environmental (Paling et al., 2003), and the operation of divers and safety issues in open ocean settings raise costs significantly (Calumpong and Fonseca, 2001). In addition, high wave energy, whether stochastic or periodic, limits operations and can quickly erode planted areas or mobilize sediments that bury seagrasses, particularly for young meadows that have not yet reached sufficient abundance to reach an equilibrium with the disturbance regime (Den Hartog, 1971; Patriquin, 1975; Fonseca et al., 1983; Marbà et al., 1994; Fonseca and Bell, 1998; Turner et al., 1999; Bryars, 2008).
Restoration of seagrasses represents an attempt to induce a change in ecological state, from a condition of low structural complexity (typically unvegetated seafloor) to a more complex form (vegetated). Theory on alternative stable states predicts that ecosystems can exist in a given location under multiple combinations of physical, chemical and biological conditions or “states” (Lewontin, 1969; Sutherland, 1974; May, 1977; Maxwell et al., 2015). These states are considered to be stable because they persist for relatively long periods of time and only shift from one state to another when key components are disturbed beyond a threshold characterizing their resistance to change (sensu Holling, 1973). Introduction of seagrass transplants qualifies as just such a perturbation. Once a state shift is achieved, the system is also characterized by resistance to revert back to the original state, even when the pressures that lead to the regime shift are released. However, factors governing stable states of seagrass beds are not well-known. Although some studies of seagrass ecosystems quantitatively link disturbance regimes with seagrass responses on a landscape-scale, the link may be location dependent (Duarte and Sand-Jensen, 1990; Kirkman and Kuo, 1990; Fonseca and Bell, 1998; Kendrick et al., 1999; Turner et al., 1999). Several authors clearly define environmental thresholds that may drive seagrass coverage from one state to another (Carr et al., 2010; Maxwell et al., 2015, 2016; Moksnes et al., 2018). Therefore, if seagrass restoration is viewed as an effort to catalyze a state shift (from an unvegetated to a vegetated state), the factors limiting that transition must be understood as a basis for setting realistic restoration goals and expectations. For restoration projects in physically dynamic environments, the challenge is to create resilient seagrass habitat before a perturbation could cross a critical environmental threshold and revert the system back to the bare sand condition; i.e., failure of the restoration effort.
Here, we describe what became an adaptive process in an attempt to restore an open coast (Atlantic) seagrass habitat in Portugal. What was once an extensive seagrass bed of approximately 30 ha, had disappeared completely by 2007, leaving unvegetated sandy seafloor (Cunha et al., 2012). Various scenarios were considered for how this transition came about. From historical photographic evidence (Figures 1a,b,d) and anecdotal information obtained from local residents, it appeared that both mooring and anchor scarring from the seasonal influx of recreational vessels, and clam dredging prior to designation of Marine Protected Area (MPA) status, likely combined to directly eliminate a significant part of the seagrass cover (Cunha et al., 2012). Simultaneously it drove the system to a fragmented state (as seen in aerial photographs) that may have been susceptible to collapse (sensu Scheffer et al., 2012), because fragmented rhizome mats are less resistant to perturbations. This is a potential “threshold-like state response” (Andersen et al., 2009) where the driver mooring damage continues to increase while the ecosystem state (seagrass cover) collapses below some critical threshold of cover needed to persist in this open coastline setting (sensu Fonseca and Bell, 1998). The bivalve trawling practices would fit Andersen et al.’s (2009) “driver-state hysteresis” model because trawling pressure would likely drop as the resource was exhausted, but leaving the ecosystem unable to recover to its previous state. These state change scenarios are increasingly recognized in seagrass restoration (Maxwell et al., 2016), but difficult to consider in the restoration planning because the drivers of previous state changes are, as seen here, often conjectural. However, consideration of these scenarios ultimately provided the basis upon which an adaptive decision was made to increase the initial size of the planting plot.

Figure 1. (a) Restoration site before plant disappearance, with clear evidence of mooring impact over the seagrass community (the bare patches around each mooring); (b) same geographic area as previous image, in 2007 when restoration started, in which no seagrass cover is observed; (c) diver transplanting seagrass in sods, from a tray to the restoration site; (d) anchor found impacting directly over a recent transplant; (e) evidence of herbivory by Sarpa salpa; (f) invasive seaweed Asparagopsis armata covering part of a transplant, also evident are bite marks on the leaves; (g) transplant with initial size bigger than 1 m2; (h) transplant with initial size smaller than 1 m2. Image (a) reproduced with permission from Turismo de Portugal, https://www.visitportugal.com/en/NR/ exeres/891DBFC4-995B-4F61-99AB-33B8D93D95A5; image (b) to co-author Emanuel Gonçalves; images (c–h) to Diogo Paulo.
At the time the restoration project was planned, an on-site sampling survey revealed one remaining patch of Zostera marina covering approximately 60 m2, in which only four distinct plants (i.e., genotypes) were found (Diekmann and Serrao, 2012). However, this last meadow did not survive the winter of 2006/2007 (Cunha et al., 2013), just before the start of the restoration project. The causes for this winter loss are unknown, but storms and associated floods loading the coast with sediments are hypotheses that have been raised for the disappearance of other seagrass habitats along the coast of Portugal during that same winter (Cunha et al., 2013).
The goal of the transplants in this study was to bring seagrass habitat to the Marine Park again, to restore the essential ecosystem functions provided by seagrass habitat. Due to the exploratory nature of seagrass restoration on an exposed open costal area, we designed transplant areas to answer the following research questions: (1) which seagrass species (among the three native species Zostera noltii, Z. marina and Cymodocea nodosa)? (2) which time of the year is best for transplanting? (3) which donor populations provide higher restoration success? and (4) which initial transplant size? This paper reports on how addressing these questions shaped our understanding of the factors controlling the unvegetated state of the area, allowing us to adaptively overcome stressors that limited seagrass recolonization and eventually led this restoration project to achieve persistent seagrass establishment.
The initial seagrass restoration goal followed traditional approaches aimed to create as many patches as possible, spaced over the past seagrass area, to achieve patches over a large area that could eventually coalesce in the future. The initial approach consisted in spreading small, individual planting units (PUs), with the intent that plant survival, rhizome spreading and meadow coalescence (with associated increasing stability) could occur to form a stable rhizome net before a major storm or disturbance event. Monitoring occurred over a 9-year period, representing a remarkably very long time series, as is rarely seen in such restoration efforts (van Katwijk et al., 2016).
Several seagrass species were used in the transplanting efforts because Z. marina, the species that used to occupy the area (Palminha, 1958), was not initially (at the beginning of the project in 2007) very abundant in any of the possible donor populations, in contrast with the species Z. noltii and C. nodosa. Testing different species for the transplants in the Marine Park was discussed with the Portuguese Nature Conservation Institute. The last remaining seagrass patch in 2006 had been identified as Z. marina. However, the objective was to restore seagrass habitat and not the species in itself. All species occur in the area nearby, at the Sado estuary. There, Z. marina is near its southern distribution whereas C. nodosa is at its northern limit in the Atlantic. Considering global warming, it is possible that in the near future increased water temperature may negatively affect Z. marina and favor C. nodosa. Therefore, to assess the optimal strategy to recolonize the area with seagrasses to bring back their ecosystem functions, all three species were tested. All legal standards and safety procedures were followed.
The Marine Park Professor Luiz Saldanha is a MPA that is part of the Arrábida Natural Park, which covers 52 km2 and stretches over a 38 km coastline (Figure 2). The coastline is rocky with high cliffs, punctuated by occasional sandy bays. These bays, with low organic content in the sediment, include the previous seagrass area (Cunha et al., 2013) where the transplanting was performed (Portinho da Arrábida and Galapos Bay, Figure 2).
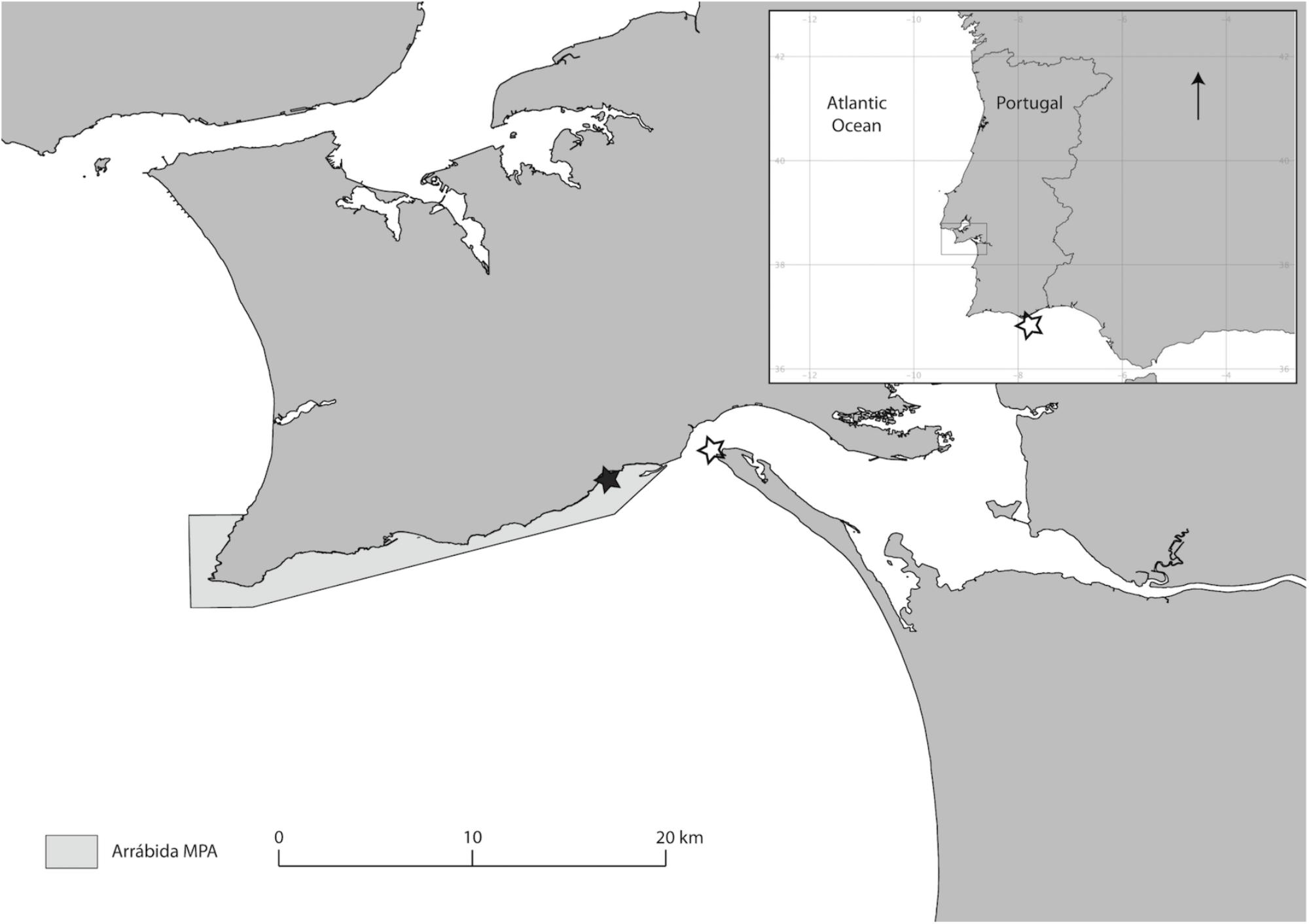
Figure 2. Coast of Portugal showing the geographical location of the project site. The continuous line identifies the limits of the marine protected area of the Arrábida Natural Park. Black star indicates the restoration area. Open stars indicate donor populations: Ria Formosa (250 km south) and Sado Estuary (ca. 5 km eastwards).
In 2006, the restoration site was evaluated to meet seven key criteria for restoration (sensu Fonseca et al., 1998b; Calumpong and Fonseca, 2001): (1) Had the same water depths as nearby (Sado Estuary) natural seagrass meadows; (2) Had a history of having seagrasses; (3) Was not naturally recolonizing; (4) Had sufficient area to accommodate the desired transplant size; (5) Was not regularly disturbed; (6) Could support similar quality (seagrass) habitat; (7) Was no longer experiencing human impacts. The transplant site clearly met points 1–4 and presumably point 6. Points 5 and 7 are later revisited to evaluate their contribution to restoration performance in a qualitative retrospective analysis.
Two donor populations were selected for the transplant operation, due to their species composition, abundance and to a lesser degree, accessibility: (1) Sado Estuary and (2) Ria Formosa (Figure 2). The Sado Estuary was the closest population, located only 5–10 km east of the transplant areas. In contrast, Ria Formosa is a coastal lagoon located 250 km south from the transplant area and was the only other location that had large amounts of seagrass coverage for all three species (Cunha et al., 2009).
Three different methods were tested in an initial optimization phase: sediment-free seagrass fixation with (1) staples or (2) mesh frames and (3) sods containing seagrass in their natural sediment. These were utilized in small-scale test plots during 2007 and the sod method was selected as the best one for this environment and thereafter used for all transplants. For the sod method, seagrasses were collected with their original sediment, in sections of approximately 20 × 20 × 5 (sediment depth) cm. Sods were harvested from water depths ranging from 1 to 5 m (depending on location and tide, Figure 1c) by SCUBA divers using a small shovel and were placed in non-buoyant plastic trays (1 m × 0.5 m). Harvest sites were spread across ∼100 m of each donor area.
From Sado Estuary, sods were transported by boat for ∼30 min before being submersed at the Arrábida MPA planting site until planted (within 24–36 h). Plants collected at Ria Formosa were similarly exposed to 30 min boat drive after harvest but were then loaded on trays with seawater covering the sods for a 3 h automobile drive until being submersed at the Arrábida site prior to planting (also within 24–36 h of arrival).
Because of the high failure rate of sediment-free methods (typically 100% within 1–3 months), here we report only on sod methods. Sods of seagrasses were planted as individual PUs at 1 m from each other to create discrete test Plots. Fifty-five sod transplants were done between 2008 and 2017, in spring, summer and autumn (including 10 placements of sods conterminously in large patches; see below). However, as this work was integrated in a habitat restoration project, many transplant areas were aimed to increase effort over the whole area, and not replicated enough to create statistically testable data. Therefore, here we only report the planting efforts which followed a statistical design aimed to test hypotheses, related to key questions: what species, from which donor population, in which season, how large, would produce better long term persistence of the restored plots (Table 1).
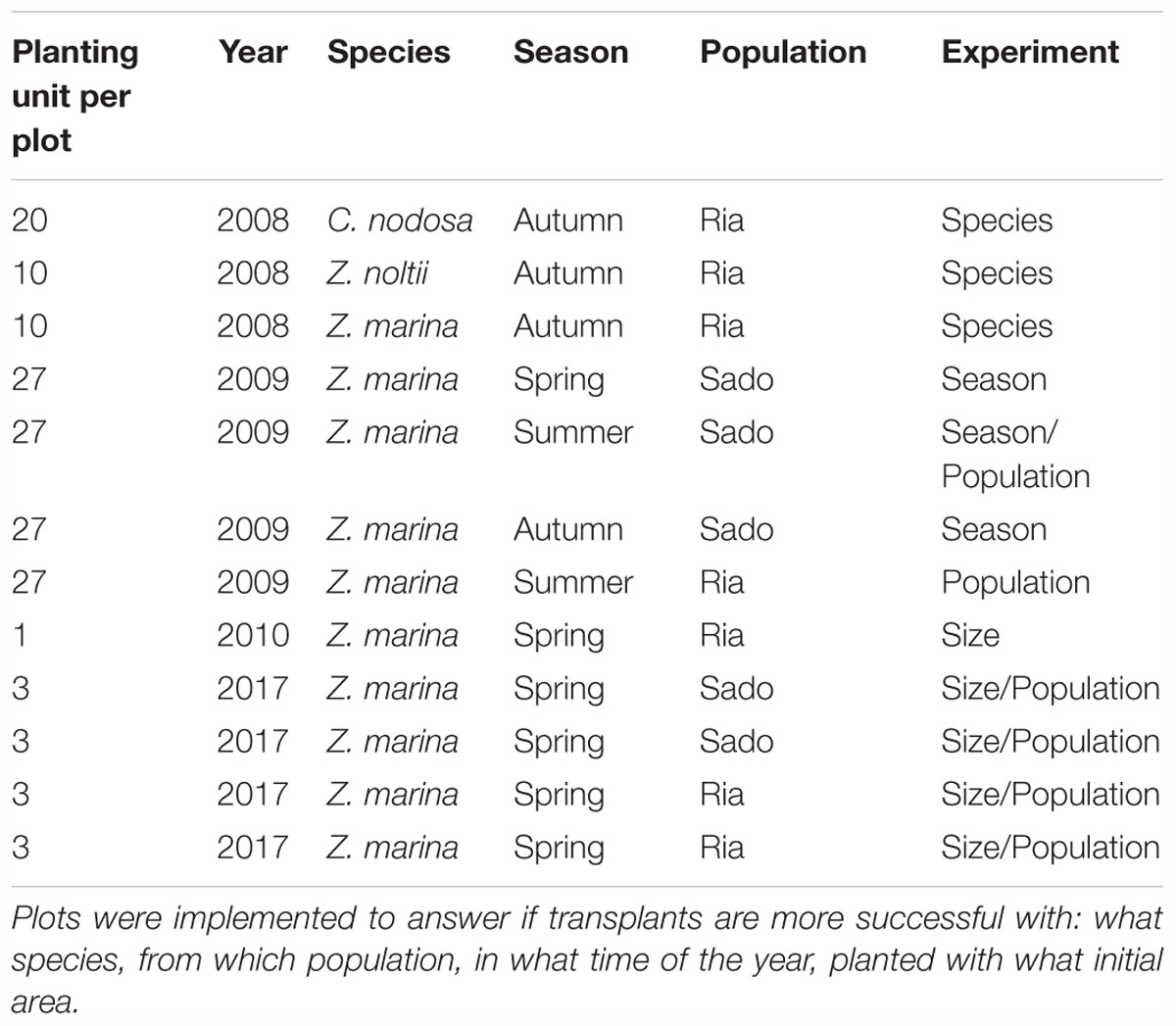
Table 1. Number of planting units per plot (Planting unit per plot), of a given species (Species), in a year (Year) and season (Season), which were collected from a source population (Population): Ria Formosa (Ria) or Sado estuary (Sado).
At the transplant site, to minimize plant damage, sods were removed from the transport trays underwater by hand and placed into a depression in the sand created by the diver. The depth of the sod in the sediment was carefully aligned to match its natural vertical relationship to the sediment surface. After transplanting, the sediment removed to create the depression was gently redistributed amongst the sod and the adjacent seafloor to create a homogeneous elevation of the planting plot.
Monitoring was done in winter, spring, summer and autumn over the period 2008–2016 or until no biomass was observed. Plots transplanted after 2017 were monitored periodically in spring, summer, autumn, winter 2017 and spring 2018. The individual PU area (m2) within a test plot was computed by measuring the two longest perpendicular axes of all individual PUs per plot. PU shoot density (shoots m-2) was computed by counting shoots within a quadrat (25 cm × 25 cm) centered on the PU. An individual PU was considered a success if shoot density and/or area increased after planting. Density was determined in the 2008–2009 plots by counting number of shoots per sod (25 cm × 25 cm). In the 2010–2017 plots, density was determined by using three 25 cm × 25 cm quadrats in the PU. Canopy height (cm) was only determined for the 2017 plots at each monitoring time by measuring 10 shoots in a sod from the sediment until the end of the longest leave. For the 2017 plots, a non-parametric Wilcoxon test was performed to compare differences between the canopy height and density along the monitoring times (R Core Team, 2015).
To determine the most successful plot settings, three variables were analyzed and compared between transplant attempts: net change in PU area (m2); net change in PU shoot density (shoots m-2); and Growth, a binomial response based on the positive or negative output of area and or density.
To deal with the repeated measures nature of our sampling we used linear mixed models (LMMs) in package nlme (Linear and non-linear mixed effects models) from R (R Core Team, 2015) to test differences in area (LMM 1) and density (LMM 2) as a function of the factors: species composition (three levels), source population (two levels) and time of the year (three levels); including in the model a term for repeated measures for each plot.
The binomial (0/1) factor “growth” was considered to be positive (1) when either net area or net density had a positive result. However, by testing a binomial factor, the use of a LMM model was not adequate. To test differences in growth a Generalized Linear Mixed Model (GLM) was built using function glm (generalized linear mixed model) in package glm2 from R (R Core Team, 2015). This model differs from the previous used LMM in that it allows for the choice of a binomial data distribution.
Because sources of seagrass for this project were obtained from two geographic areas at very different distances, the effect of relocation on the photosynthetic status of the plants was examined on the first series of transplants (2008–2009). Plants taken from the nearby Sado Estuary were less than 10 km distant and were quickly moved by boat. However, plants from Ria Formosa were approximately 250 km distant and had to be transferred to a surface vehicle and transported overland. If a substantial depression of photosynthetic efficiency was detected as a consequences of relocation, this could then affect the comparative analysis of source stock. For short distances, plants were placed in tubs and covered with wet towels. For the long-distance transport, plants are kept out of the water in plastic trays with seawater for periods of maximum 4 h.
Maximum quantum yield of photosynthesis can be used as an indicator of plant recovery from adverse conditions (Hanelt, 1992; Beer and Björk, 2000; Malta et al., 2006), such as transplants. To calculate the maximum quantum yield of photosynthesis, an underwater pulse amplitude modulate fluorometer was used (Diving PAM, Walz). To maintain a constant distance between the PAM sensor and the plant in order to dark acclimate the tissue, leaf clips were attached at 0.5 cm above the base of the second youngest leaf of all plants. A far-red weak pulse was applied for 5 s (Hanelt, 1998) to oxidate the electron transport chain, after which the shutters of the dark leaf clips were manually closed and plants acclimated for 10 min. Base fluorescence of chlorophyll a (F0) was obtained by turning on the PAM measuring light and opening the shutter. A saturating light pulse (≈5,000 μmol photons m-2 s-1) was applied for 0.6 s to measure maximum fluorescence (Fm), therefore variable fluorescence (Fv = Fm-F0) and maximum quantum yield (Fv/Fm) were calculated.
Maximum quantum yield was measured in 10 plants previously to harvest, after transport, immediately after transplant, 10, 15 and 30 days after the transplant in Z. marina and Z. noltii from Ria Formosa and Sado Estuary, and in C. nodosa from Ria Formosa monitoring finished at the end of 15 days. Previously to transplant Fv/Fm values were used as reference to be compared to the subsequent monitor timings. Differences between Fv/Fm in the harvest moment and along time were tested using One-way ANOVA test in R version 2.15.2 (The R Foundation for Statistical Computing, 2012).
To address the questions (1) which species in (2) what time of the year, from (3) which donor population, provides better restoration results we transplanted in autumn 2008 three seagrass species plots: Z. marina (PU = 10), Z. noltii (PU = 10) and C. nodosa (PU = 20). Due to natural donor population areas and densities we have opted to do this test with plants form Ria Formosa Coastal Lagoon. After knowing which species had better transplant results, we chose to transplant in 2009 Z. marina plots from Sado Estuary during autumn (PU = 27), spring (PU = 27) and summer (PU = 27), to test the best season. Also in 2009, donor population source was tested with Z. marina plots collected in Ria Formosa Coastal Lagoon (PU = 27) and Sado Estuary (PU = 27) during summer (Table 1).
In spring 2010, at the end of the restoration plan, we attempted to overcome the 2009 winter storms impacts by creating two mega plots of Z. marina with a PU each of 11 m2 initial transplant area. The donor populations were Sado Estuary and Ria Formosa and they were transplanted in Praia dos Coelhos. Because this yielded interesting results, suggesting an effect of patch size and donor population (see below), in spring 2017 a new experiment was set to test if there was an effect of a minimum patch size. This used four Z. marina plots: two plots with three PUs of 6 m2 initial size; and two plots with three PUs of 0.04 m2 initial size. Half of the plants (three big and three small PUs) came from Ria Formosa and the other half from Sado Estuary. The plots were all transplanted in the vicinity of the pilot test transplanted in 2010.
The maximum quantum yield, measured as a photosynthetic efficiency indicator at different stages of transplant operations, showed for Z. marina plants collected in Ria Formosa a significant increase (ANOVA P < 0.01) between harvest and 30 days after transplant. The maximum quantum yield of Z. marina collected in Sado Estuary was significantly lower after transport (ANOVA P < 0.01), increasing until 15 days after transplant from which it stabilized in to values equal to the initial harvest moment. Z. noltii collected in Ria Formosa had no significant difference in maximum quantum yield during the experiment (ANOVA P > 0.05). Z. noltii from Sado Estuary had a significant decrease in Fv/Fm between harvest and the end of the transport (ANOVA P < 0.01). After transplant Fv/Fm increased to values not different from those observed at harvest time (ANOVA P > 0.98) until the last monitoring 30 days after planted. C. nodosa maximum quantum yield increased significantly 1 and 15 days after transplant (ANOVA P < 0.01) (Figure 6).
From the three transplanted plots in autumn 2008, Z. noltii persisted 100 days not surpassing 2008 winter; Z. marina persisted 500 days; and C. nodosa 400 days. None of the transplants survived the 2009 winter storms. The species that had highest growth was Z. marina (GLM P < 0.01 between the other two species), followed by C. nodosa which had higher growth than Z. noltii (GLM P < 0.03). Net area variation was different between all species (LME P < 0.01 between Z. marina and the other two species; and LME P < 0.01 between C. nodosa and Z. noltii). The species Z. marina had the biggest net area increase (Figure 3). The lowest net density was measured in Z. noltii (LME P < 0.01), there were no differences between Z. marina and C. nodosa net densities (LME P > 0.90) (Figure 3).
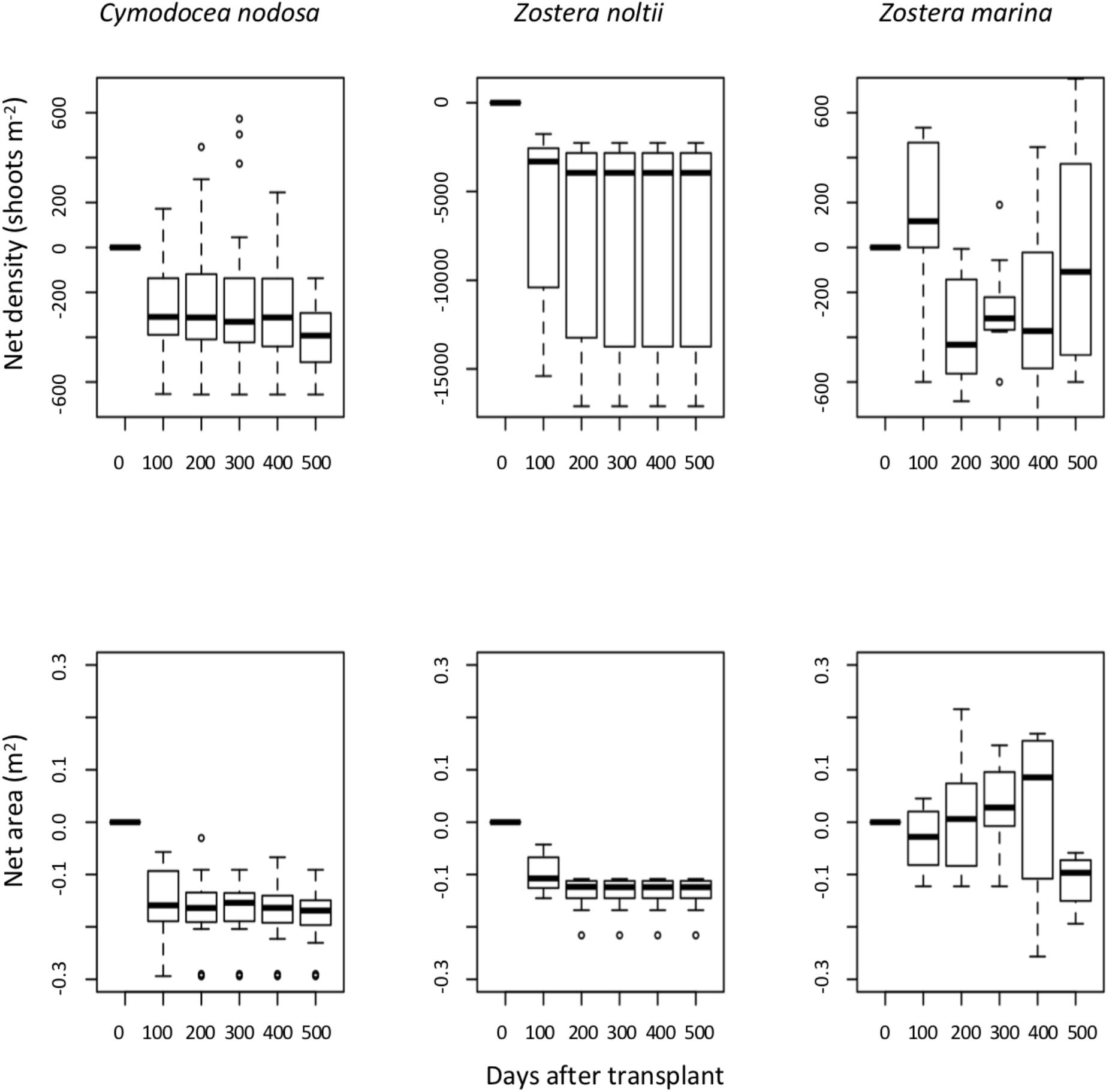
Figure 3. Net planting unit area (m2) and net planting unit density (shoots m-2) estimated along time for the seagrass species Z. marina, Z. noltii and C. nodosa, transplanted in autumn 2008 from Ria Formosa coastal lagoon to Praia dos Coelhos.
Based on the 2008 transplant results (that compared species), the species chosen to test transplant season was Z. marina. Due to logistic considerations (less distant), a nearby (∼10 km) Sado Estuary meadow was used as donor population for testing season. The spring transplant persisted 290 days, the summer one 260 days and the autumn transplant 170 days. Again, none of the transplants resisted the 2009 winter, but before that there was positive growth in all. In the first 150 days after transplant there was no difference in growth between treatments. The autumn transplant had lower growth than the spring and summer ones (GLM P < 0.01). No growth differences were found between summer and spring transplants (GLM P > 0.86). Spring transplants generated bigger areas than summer and autumn ones (LME P < 0.01). No net area differences were found between summer and autumn (LME P > 0.78) (Figure 5). Net density variation was similar for transplants done in summer and spring (LME P > 0.80). Autumn transplants had a lower net density increase compared with summer and spring (LME P < 0.01) (Figure 4).
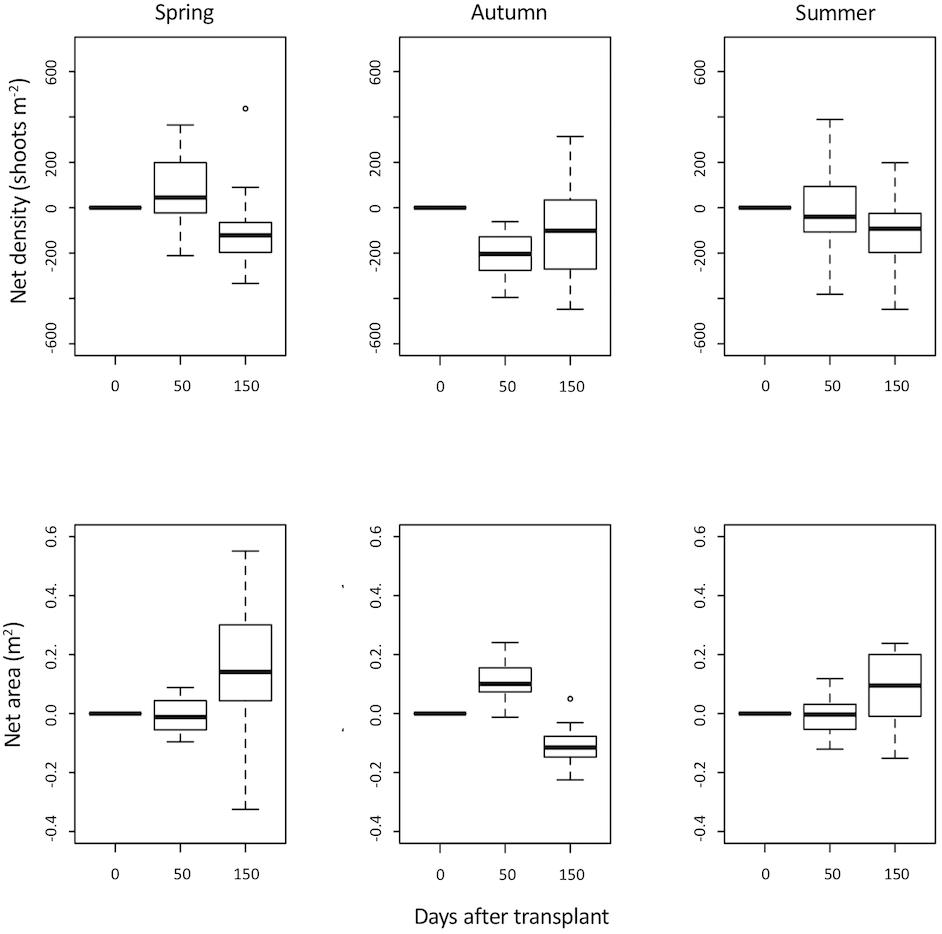
Figure 4. Zostera marina net area (m2) and net density (shoots m-2) estimated after 0, 50 and 150 days of transplant. Plants were collected in Sado Estuary in 2009 and were transplanted to Praia dos Coelhos, in different seasons: autumn, spring, summer.
Both transplants persisted 250 days, but similarly to the other tests, plants did not survive the 2009 winter storms. Growth was significantly higher in Sado Z. marina transplants than Ria Formosa ones (GLM P < 0.01). However, net area and density variations were not significantly different between different source populations (LME P > 0.05; LME P > 0.062) (Figure 5).
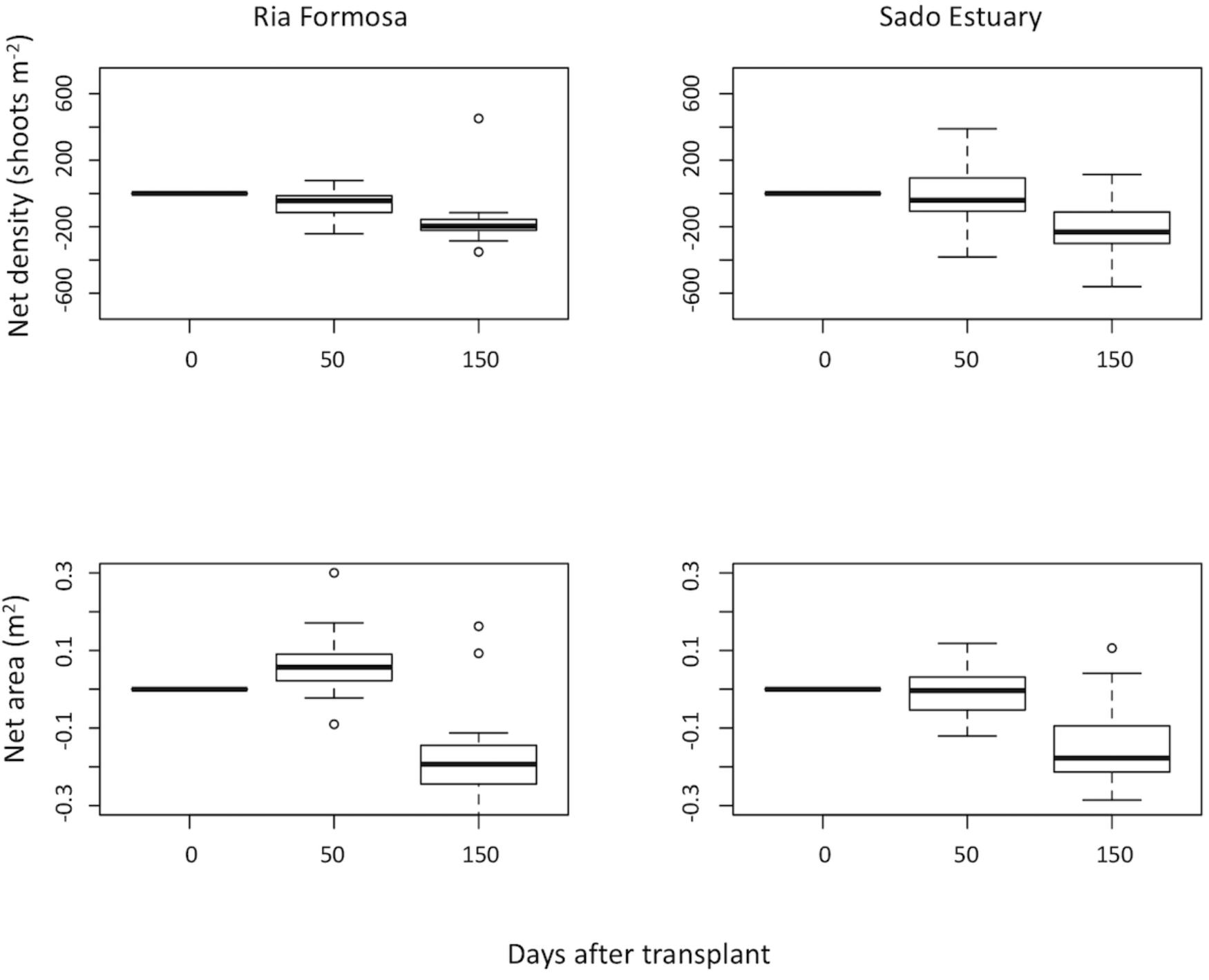
Figure 5. Zostera marina net density (shoots m-2) and net area (m2) estimated along time (0, 50 and 150 days) after being transplanted from different donor population sites: Ria Formosa Coastal Lagoon and Sado Estuary into Praia dos Coelhos, in the summer of 2009.
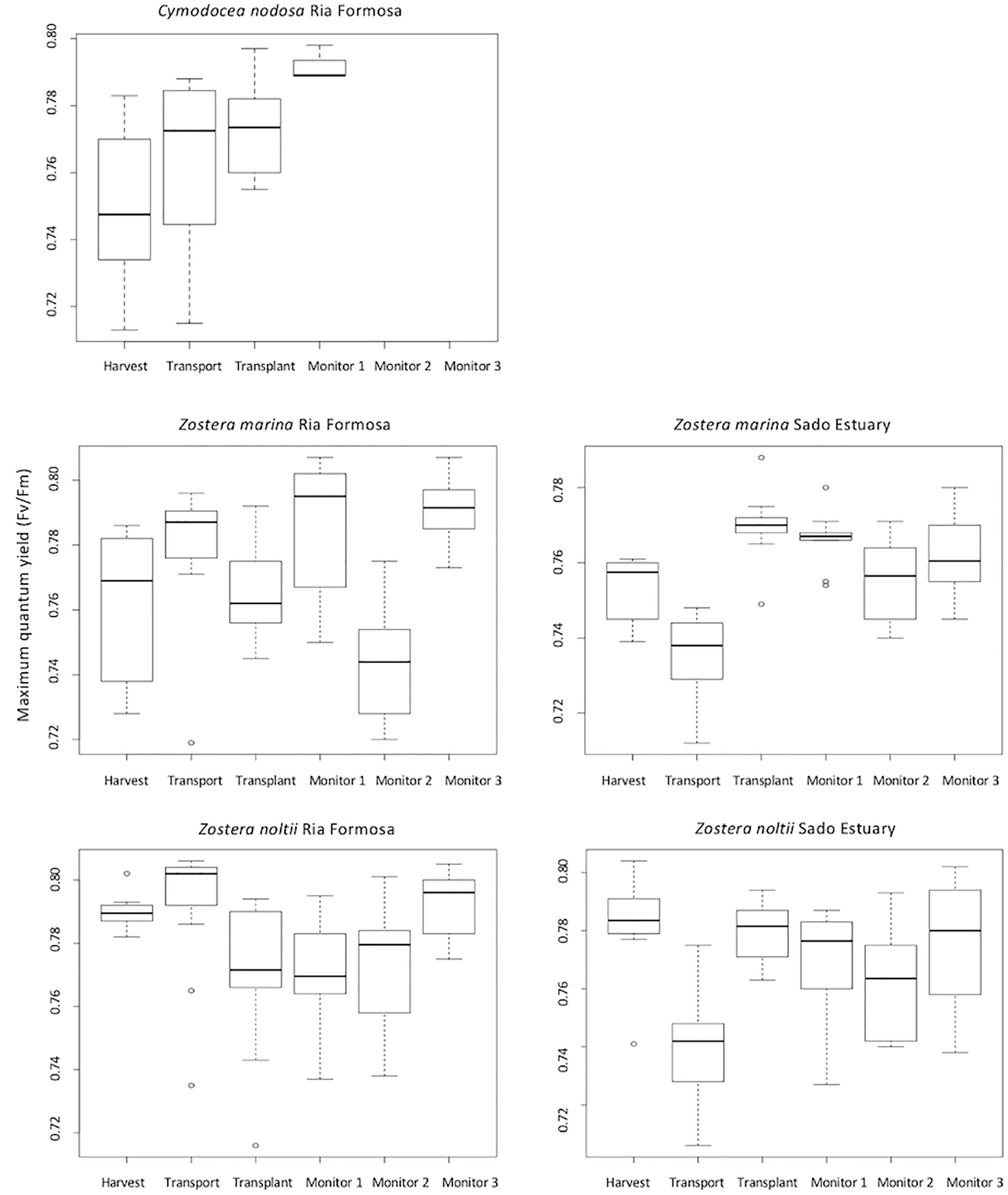
Figure 6. Maximum quantum yield (Fv/Fm) calculated for Zostera marina and Z. noltii harvested in Ria Formosa and Sado Estuary; and Cymodocea nodosa harvested in Sado Estuary. Fluorescence measures were taken during harvest, at the end of transport, after transplant, and 10, 15 and 30 days after transplant (labeled as monitor 1, 2, 3, respectively), for all treatments with the exception of C. nodosa in which the monitoring ended at the end of 15 days.
Only the largest Z. marina plot, with 11 m2 initial PU size, from Ria Formosa, mitigated the many impacts that were eventually identified as limiting seagrass colonization in the bay (Figures 1g,h). This plot, set in spring 2010 as a pilot test, increased almost 10 times its initial area from 11 m2 in 2010 to 103 m2 in 2018 (Figure 7).
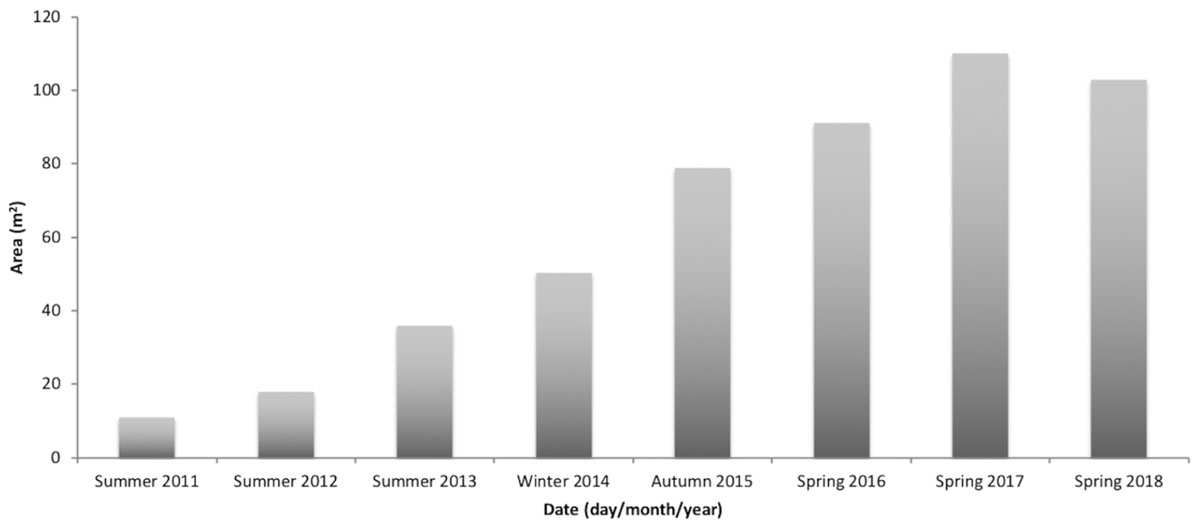
Figure 7. Area (m2) increase along time of a Zostera marina plot, transplanted in spring 2010 from Ria Formosa donor population with an initial size of 11 m2, which persists until the end of the monitoring program (June 2018).
In the complete experimental setup, the small plots (0.04 m2) transplanted in spring 2017 from Ria Formosa and Sado Estuary were absent in summer 2017, the first monitoring time. As expected, all the bigger plots persisted for a longer period, but with different outcomes depending on the donor populations.
The transplants from the closest donor population (Sado Estuary) decreased area from spring 2017 to autumn 2017 and did not survive winter 2017. Leaf length and plant density decreased consistently along the monitoring time. In autumn 2017, average canopy height was 3.4 cm (SE 1.9 cm) (Figure 8) revealing the intense grazing activity, which was also observed visually in the leaves.
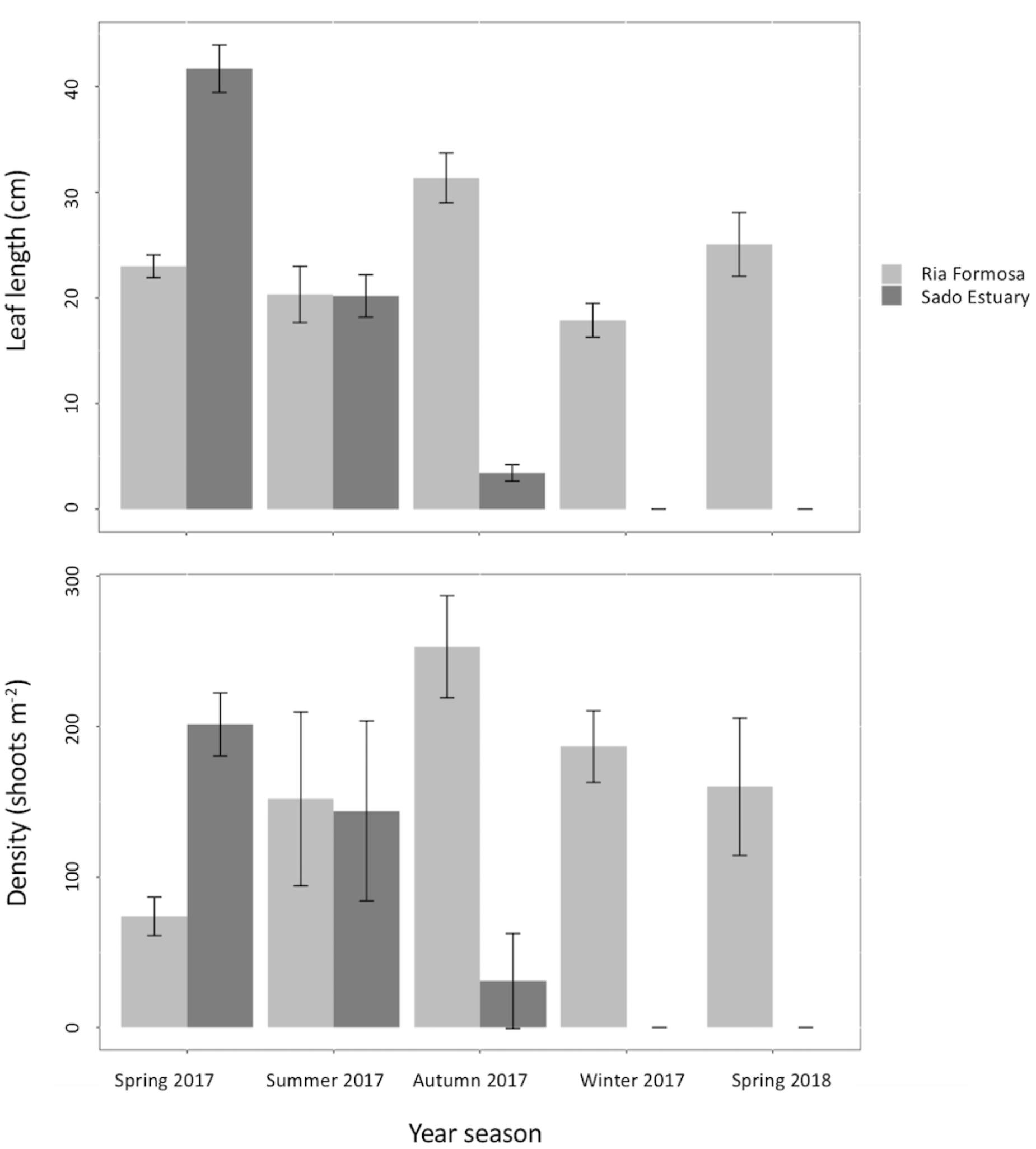
Figure 8. Seasonal density (shoots m-2) and canopy height (cm) of Z. marina along 1 year. Two plots were collected in Ria Formosa and two in Sado Estuary, in spring 2019, and were transplanted into Praia dos Coelhos. Each plot was built out of three 6 m2 planting units.
The three big transplants from Ria Formosa were more resilient than the ones from Sado Estuary. Although one PU from Ria Formosa did not survive the winter 2017–2018, the two remaining PUs increased from the initial (spring 2017) vegetated area of 6 m2 each to 8 and 9 m2 in spring 2018. Canopy height and plant density from the Ria Formosa PUs changed along the monitoring time in the surviving plots (Figure 8). Plant density in the Ria Formosa plots was equal between summer 2017–winter 2017, summer 2017–spring 2018, and winter 2017–spring 2018 (Wilcox test P-value >0.05). Plant density was minimum in spring 2017 and maximum in autumn 2017. Canopy heights from the Ria Formosa plots were equal in between spring 2017–2018 and spring 2017–summer 2017 (Wilcox test P-value >0.05). Minimum canopy height was detected in winter (17.88 cm SE 0.78 cm) 2017 and maximum (31.37 cm SE 1.12 cm) in autumn 2017 (Figure 8).
In our case study, all seagrass transplants presented growth, both in the form of area and or density increase. Seagrasses acclimated rapidly to local conditions as indicated by maximum quantum yield as a proxy for good physiological state. The results demonstrate the general suitability of the local conditions for the seagrasses transplanted since they all survived and grew. However, this initial success was not verified in the very long term (8 years, which encompassed strong winter storms), highlighting how persistence assessments can have distinct results depending on the time scale of the evaluation conducted. The results also showed that species, donor populations and transplant season possibility influenced the colonization success of new unvegetated areas with the same level of performance. The most successful case coincided with using the seagrass species previously found at the site (Z. marina), from one particular donor population (Ria Formosa), transplanted during spring/summer, which is the season that maximizes growth and minimizes impacts of water motion.
Although, we have registered growth in transplants from both source populations, plants from Ria Formosa lagoon had higher growth than those Sado Estuary. The lack of an impact to the photosynthetic capacity of the plants demonstrated not only that there was no apparent bias in our evaluation of transplant performance as the result of different handling but also revealed that seagrasses can be successfully transplanted over very long distances.
Almost all seagrass restoration projects face the problem of shifting the state of the seafloor from an unvegetated to a vegetated state (Fonseca, 2011; van Katwijk et al., 2016), achieving such changes under wave exposed conditions is particularly daunting given that extreme wave energy acts as an external driver holding the site in a persistent unvegetated state (sensu Den Hartog, 1971; Calumpong and Fonseca, 2001). In this case, however, the historical presence of seagrass in these open coast settings and the success of seagrass transplanting in even higher wave energy regimes (Paling et al., 2003) suggested the possibility of successful restoration.
Numerous human impacts in the transplant sites were identified during the 8 years of monitoring. As the study unfolded, we concluded that two key initial site selection criteria had not been fully met: was not regularly disturbed (criterion #5) and was no longer experiencing human impact (criterion #7). The site was observed to be disturbed by unpredictable large storms in winter and was still experiencing illegal human impacts such as boat moorings, despite existing regulations and our own project measures against those. Establishing a pilot study that could effectively exclude human impacts prior to the development of future seagrass restoration project may help to forecast such effects (Cunha et al., 2012).
There is no evidence that grazing had been a factor influencing the loss of the seagrass meadow but it might have contributed to prevent recovery. Through observation (Figure 1e) and periodic underwater video deployments (not shown) heavy grazing impacts by the sparid fish Sarpa salpa were observed in the 2008–2009 transplants. Grazing by S. salpa has been identified to have a top down control on seagrass meadows modifying habitat structure (Pagès et al., 2012) and has been shown to limit seagrass in MPA settings in the Mediterranean (Ferrari et al., 2008). We presume that in large seagrass areas, such as the one previously existing at this site, grazing pressure would not be a key factor hindering seagrass persistence because grazing pressure would have been widely distributed. However, in a colonizing situation with seagrass as small patches as in this study, grazing by S. salpa was observed frequently, with both plant leaves and sheaths grazed down to within centimeters of the sediment surface, often within 24 h of planting. Additionally, heavy fouling of grazing exclusion cages (not part of the plot responses described here) by the invasive rhodophyta Asparagopsis armata (Bonnemaisoniaceae) (Figure 1f) appeared to cause the cages to become dislodged which prevented their usefulness (data not shown).
Storms have been identified as a major threat to restoration of seagrass ecosystems (Calumpong and Fonseca, 2001). During winter months increased wave action, particularly those associated with a large storm in 2009 resulted in the relocation of sediments which in some cases covered plants completely, reverting vegetated to non-vegetated seafloor. Similar effects were observed in the source population, a naturally occurring Z. marina meadow in the nearby Sado Estuary (Figure 2) during the same 2009 storm event, resulting in the loss of approximately 3 ha of seagrass (pers. obs.). The 2009 winter storm moved sediments and decreased water clarity for many weeks, which might have been a major factor causing plant disappearance in both transplanted sites and donor population, as turbidity limits photosynthesis, a key factor for plant survival (Carr et al., 2010). When affected by extreme weather events, in low seagrass density the sparse rhizome network is unable to stabilize the sediment (Suykerbuyk et al., 2016). After 2010, no other extreme weather event was observed in the area, and the nearby donor population at the Sado estuary also fully recovered from impact (unpublished data). Seagrass meadows were known to exist in our study site, covering 10 ha of seabed, indicating that even with seasonal storms the meadows were able to recover naturally. Thus, it is likely that a combination of several stressors led to the extinction of the meadows in this area, as the anthropogenic disturbances fragmented the meadow and the resulting smaller patches might have become more vulnerable to critical events. In an attempt to restore this habitat, we hypothesized that the larger the initial transplant area, the larger the chances of long term survival and expansion. Our results support this hypothesis, it is likely that the 6 m2 initial size transplants have overcome the threshold of un-stability. Although it remains unknown what would be the effect of another large-scale storm on the transplant survival, and therefore the future of the three surviving plots (one from 2010 and two from 2017) remains uncertain, it is noteworthy that the plots have survived a major storm in March 2018, that destroyed restaurants and car parks on the restoration site like no previous storm had done (Appendix and Supplementary Figures S1, S2).
Our results suggest that under specific conditions (initial creation of large patch sizes), Z. marina transplanted from Ria Formosa may surpass a threshold that shifts unvegetated areas to stable states of vegetated seafloor. The initial patch size hypothesis was tested with two initial sizes (0.04 and 6 m2) and our research confirmed that the larger initial size transplants were more resistant in the long term. This finding brings important information for future efforts, suggesting that it may be better to concentrate efforts in achieving larger initial sizes rather than spreading the effort in scattered smaller PUs over a larger area. Therefore, future studies should be conducted to determine the minimum size that could bring success to seagrass restoration efforts.
The causes of the differences on success between donor populations are unknown, but hypotheses can be raised for future studies to test. Direct observations suggest that Z. marina from the nearby Sado Estuary were more grazed than the ones from the distant donor site Ria Formosa. All 2017 transplants PU showed grazing evidence, as leaves were bitten at the extremities, but plants from Sado Estuary were either more grazed or did not recover from this impact as fast as the ones from Ria Formosa. In the 2008–2009 transplants, grazing was also a problem but no differences in donor population preferences were noted, as all plants were similarly grazed. The causes for the differences in resistance or resilience to grazing observed in 2017 are unknown, future research on the leaf growth and grazing preferences is needed. It is documented that some seagrass populations can lead to better transplant results than others (Meinesz et al., 1993; van Katwijk et al., 1998). Recent studies have linked higher transplant success to the close distance of the donor populations (van Katwijk et al., 2016), contrasting with studies that did not found any relation (Novak et al., 2017), or that even found the opposite, such as Piazzi et al. (1998) that found evidence of bigger transplant success when using Posidonia oceanica plants from distant populations rather than closer. Seagrass morphology has been suggested as a factor that could increase transplant success (Novak et al., 2017), but similarly to the donor population proximity, there is no academic consensus, with studies finding that donor population seagrass morphology does not need to be equal to the population to be restored (Eriander et al., 2016). Future research should be focused on testing hypotheses to understand the difference between the contrasting transplant success of different donor populations, in terms of long term plant persistence.
This study reports a case in which we attempted to initiate a state shift from bare sediment to seagrass cover, which was successfully achieved, but not in the majority of the attempts. Seagrass restoration regime shifts can take more than 10 years to occur, even in areas of great suitability (McGlathery et al., 2012). We raise the hypothesis that these results might represent case examples of regime shifts between different ecosystem states. In this location, seagrass previously formed homogenous and stable seafloor coverage that was resistant to the natural levels of grazing and storms. Past human-induced disturbance that fragmented the seagrass meadows may have pushed the system past a tipping point that is now very difficult to reverse. Secondary factors such as storms and grazing, that used to be present also in the previous ecosystem state now might be acting against the possibility of seagrass recovery from very small patches. Alone, these may not have been able to influence the seagrass meadow in the previous stable state. As the ecosystem was altered, it is likely that a combination of different factors are acting as antagonistic feedback mechanisms preventing its recovery and restoration (Moksnes et al., 2018). Given that seagrass transplants survived well initially and were robust to transport, and because the successful restoration was achieved only in the cases which used the largest PU size, it appears this open coast area is receptive to seagrass recolonization and that the largest hurdle to overcome is post-planting disturbance from storms and grazing. We suggest that future restoration attempts consider the use of large initial transplants (in this case over several square meters in size) increasing the likelihood to resist the current disturbance regime.
The seagrass transplantation initiative and planning was done by DP, AC, ES, EG, and MF. Field work was done by DP, JB, and MF. Data analyses was done by DP, ES, and MF. The manuscript was written by DP, ES, and MF. All co-authors edited and approved the manuscript.
This research was supported by Project BIOMARES (LIFE06 NAT/P/192), funded by the European Union LIFE Program and by the cement company SECIL, Companhia de Cal e Cimentos S.A., Portugal. DP was supported by a doctoral grant (FCT SFRH/BD/81086/2011) from Fundação para a Ciência e a Tecnologia. ES thanks a Pew Marine Fellowship. The help provided by the staff of the Natural Park of Arrábida is much appreciated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all the BIOMARES partners, the numerous staff, students and volunteers for general support and help in the field and Dr. Jorge Assis and Dra. Barbara Costa for productive discussions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00052/full#supplementary-material
FIGURE S1 | Monthly precipitation in mm since February 2009 until June 2018. Data were downloaded from Montijo meteorological station, located 40 km away from the study site.
FIGURE S2 | Monthly wind direction in degrees since February 2009 until June 2018. Data were downloaded from Montijo meteorological station, located 40 km away from the study site.
Andersen, T., Carstensen, J., Hernandez-Garcia, E., and Duarte, C. M. (2009). Ecological thresholds and regime shifts: approaches to identification. Trends Ecol. Evol. 24, 49–57. doi: 10.1016/j.tree.2008.07.014
Beer, S., and Björk, M. (2000). Measuring rates of photosynthesis of two tropical seagrasses by pulse amplitude modulated (PAM) fluorometry. Aquat. Bot. 66, 69–76. doi: 10.1016/S0304-3770(99)00020-0
Bryars, S. (2008). Restoration of Coastal Seagrass Ecosystems: Amphibolis antarctica in Gulf St Vincent, South Australia. Adelaide: South Australian Research and Development Institute.
Bull, J. S., Reed, D. C., and Holbrook, S. J. (2004). An experimental evaluation of different methods of restoring Phyllospadix torreyi (surfgrass). Restor. Ecol. 12, 70–79. doi: 10.1111/j.1061-2971.2004.00258.x
Calumpong, H. P., and Fonseca, M. S. (2001). Seagrass transplantation and other seagrass. Global Seagrass Res. Methods 33:425. doi: 10.1016/B978-044450891-1/50023-2
Carr, J., D’Odorico, P., McGlathery, K., and Wiberg, P. L. (2010). Stability and bistability of seagrass ecosystems in shallow coastal lagoons: role of feedbacks with sediment resuspension and light attenuation. J. Geophys. Res. Biogeosci. 115:G03011. doi: 10.1029/2009JG001103
Cunha, A. H., Assis, J., and Serrão, E. A. (2009). Estimation of available seagrass meadow area in Portugal for transplanting purposes. J. Coast. Res. 56, 1100–1104.
Cunha, A. H., Assis, J. F., and Serrão, E. A. (2013). Seagrasses in Portugal: a most endangered marine habitat. Aquat. Bot. 104, 193–203. doi: 10.1016/j.aquabot.2011.08.007
Cunha, A. H., Marbá, N. N., van Katwijk, M. M., Pickerell, C., Henriques, M., Bernard, G., et al. (2012). Changing paradigms in seagrass restoration. Restor. Ecol. 20, 427–430. doi: 10.1111/j.1526-100X.2012.00878.x
Den Hartog, C. (1971). The dynamic aspect in the ecology of seagrass communities. Thalassia Jugoslavica 7, 1101–1127.
Diekmann, O. E., and Serrao, E. A. (2012). Range-edge genetic diversity: locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Mol. Ecol. 21, 1647–1657. doi: 10.1111/j.1365-294X.2012.05500.x
Duarte, C. M., and Sand-Jensen, K. (1990). Seagrass colonization: patch formation and patch growth in Cymodocea nodosa. Mar. Ecol. Prog. Ser. 65, 193–200. doi: 10.3389/fpls.2017.01067
Eriander, L., Infantes, E., Olofsson, M., Olsen, J. L., and Moksnes, P. O. (2016). Assessing methods for restoration of eelgrass (Zostera marina L.) in a cold temperate region. J. Exp. Mar. Biol. Ecol. 479, 76–88. doi: 10.1016/j.jembe.2016.03.005
Ferrari, B., Raventos, N., and Planes, S. (2008). Assessing effects of fishing prohibition on Posidonia oceanica seagrass meadows in the marine natural reserve of cerbere-banyuls. Aquat. Bot. 88, 295–302.
Fonseca, M. S. (2011). Addy revisited: what has changed with seagrass restoration in 64 years? Ecol. Restor. 29, 73–81. doi: 10.1016/j.aquabot.2007.12.002
Fonseca, M. S., and Bell, S. S. (1998). Influence of physical setting on seagrass landscapes near Beaufort, North Carolina, USA. Mar. Ecol. Progr. Ser. 171, 109–121. doi: 10.3368/er.29.1-2.73
Fonseca, M. S., Kenworthy, W. J., and Paling, E. (1998a). Restoring Seagrass Ecosystems in High Disturbance Environments. In Ocean Community Conference. Washington, DC: Marine Technology Society. doi: 10.3354/meps171109
Fonseca, M. S., Kenworthy, W. J., and Thayer, G. W. (1998b). Guidelines for the Conservation and Restoration of Seagrasses in the United States and Adjacent Waters. Silver Spring, MD: NOAA/National Centers for Coastal Ocean Science.
Fonseca, M. S., Zieman, J. C., Thayer, G. W., and Fisher, J. S. (1983). The role of current velocity in structuring eelgrass (Zostera marina L.) meadows. Estuar. Coastal Shelf Sci. 17, 367–380. doi: 10.1016/0272-7714(83)90123-3
Hanelt, D. (1992). Photoinhibition of photosynthesis in marine macrophytes of the South China Sea. Mar. Ecol. Prog. Ser. 82, 199–206. doi: 10.3354/meps082199
Hanelt, D. (1998). Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution. Mar. Biol. 131, 361–369. doi: 10.1007/s002270050329
Holling, C. S. (1973). Resilience and stability of ecological systems. Ann. Rev. Ecol. Syst. 4, 1–23. doi: 10.1146/annurev.es.04.110173.000245
Kendrick, G. A., Eckersley, J., and Walker, D. I. (1999). Landscape-scale changes in seagrass distribution over time: a case study from success bank, Western Australia. Aquat. Bot. 65, 293–309. doi: 10.1016/S0304-3770(99)00047-9
Kirkman, H., and Kuo, J. (1990). Pattern and process in southern Western Australian seagrasses. Aquat. Bot. 37, 367–382. doi: 10.1016/0304-3770(90)90022-D
Malta, E. J., Brun, F. G., Vergara, J. J., Hernández, I., and Pérez-Lloréns, J. L. (2006). Recovery of Cymodocea nodosa (Ucria) Ascherson photosynthesis after a four-month dark period. Sci. Mar. 70, 413–422. doi: 10.3989/scimar.2006.70n3413
Marbà, N., Cebrián, J., Enríquez, S., and Duarte, C. M. (1994). Migration of large-scale subaqueous bedforms measured with seagrasses (Cymodocea nodosa) as tracers. Limnol. Oceanogr. 39, 126–133. doi: 10.4319/lo.1994.39.1.0126
Maxwell, P. S., Eklöf, J. S., van Katwijk, M. M., O’brien, K. R., de la Torre-Castro, M., Boström, C., et al. (2016). The fundamental role of ecological feedback mechanisms for the adaptive management of seagrass ecosystems–a review. Biol. Rev. 92, 1521–1538. doi: 10.1111/brv.12294
Maxwell, P. S., Pitt, K. A., Olds, A. D., Rissik, D., and Connolly, R. M. (2015). Identifying habitats at risk: simple models can reveal complex ecosystem dynamics. Ecol. Appl. 25, 573–587. doi: 10.1890/14-0395.1
May, R. M. (1977). Thresholds and breakpoints in ecosystems with a multiplicity of stable states. Nature 269, 471–477. doi: 10.1038/269471a0
McGlathery, K. J., Reynolds, L. K., Cole, L. W., Orth, R. J., Marion, S. R., and Schwarzschild, A. (2012). Recovery trajectories during state change from bare sediment to eelgrass dominance. Mar. Ecol. Prog. Ser. 448, 209–221. doi: 10.3354/meps09574
Meinesz, A., Caye, G., Loques, F., and Molenaar, H. (1993). Polymorphism and development of Posidonia oceanica transplanted from different parts of the mediterranean into the National Park of Port-Cros. Bot. Mar. 36, 209–216. doi: 10.1515/botm.1993.36.3.209
Moksnes, P. O., Eriander, L., Infantes, E., and Holmer, M. (2018). Local regime shifts prevent natural recovery and restoration of lost eelgrass beds along the Swedish West Coast. Estuar. Coasts 41, 1712–1731. doi: 10.1007/s12237-018-0382-y
Novak, A. B., Plaisted, H. K., Hays, C. G., and Hughes, R. A. (2017). Limited effects of source population identity and number on seagrass transplant performance. PeerJ 5:e2972. doi: 10.7717/peerj.2972
Pagès, J. F., Farina, S., Gera, A., Arthur, R., Romero, J., and Alcoverro, T. (2012). Indirect interactions in seagrasses: fish herbivores increase predation risk to sea urchins by modifying plant traits. Funct. Ecol. 26, 1015–1023. doi: 10.1111/j.1365-2435.2012.02038.x
Paling, E. I., Van Keulen, M., and Tunbridge, D. J. (2007). Seagrass transplanting in cockburn sound, Western Australia: a comparison of manual transplantation methodology using Posidonia sinuosa Cambridge et Kuo. Restor. Ecol. 15, 240–249. doi: 10.1111/j.1526-100X.2007.00207.x
Paling, E. I., Van Keulen, M., Wheeler, K. D., Phillips, J., and Dyhrberg, R. (2003). Influence of spacing on mechanically transplanted seagrass survival in a high wave energy regime. Restor. Ecol. 11, 56–61. doi: 10.1046/j.1526-100X.2003.00072.x
Palminha, F. (1958). As Algas Marinhas de Zona Costeira da Arrábida e sua Protecção (Contribuição Para o Estudo Ecológico), A Serra da Arrábida e a Protecção à Natureza. Lisboa: Publicações da Liga para a Protecç ão XIV, 5–23.
Patriquin, D. G. (1975). “Migration” of blowouts in seagrass beds at Barbados and Carriacou, West Indies, and its ecological and geological implications. Aquat. Bot. 1, 163–189. doi: 10.1016/0304-3770(75)90021-2
Piazzi, L., Balestri, E., Magri, M., and Cinelli, F. (1998). Experimental transplanting of Posidonia oceanica (L.) Delile into a disturbed habitat in the Mediterranean Sea. Bot. Mar. 41, 593–602. doi: 10.1515/botm.1998.41.1-6.593
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available at: https://www.r-project.org/
Scheffer, M., Carpenter, S. R., Lenton, T. M., Bascompte, J., Brock, W., Dakos, V., et al. (2012). Anticipating critical transitions. Science 338, 344–348. doi: 10.1126/science.1225244
Sutherland, J. P. (1974). Multiple stable points in natural communities. Am. Nat. 108, 859–873. doi: 10.1086/282961
Suykerbuyk, W., Bouma, T. J., Govers, L. L., Giesen, K., de Jong, D. J., Herman, P., et al. (2016). Surviving in changing seascapes: sediment dynamics as bottleneck for long-term seagrass presence. Ecosystems 19, 296–310. doi: 10.1007/s10021-015-9932-3
Turner, S. J., Hewitt, J. E., Wilkinson, M. R., Morrisey, D. J., Thrush, S. F., Cummings, V. J., et al. (1999). Seagrass patches and landscapes: the influence of wind-wave dynamics and hierarchical arrangements of spatial structure on macrofaunal seagrass communities. Estuar. Coasts 22, 1016–1032. doi: 10.2307/1353080
van Katwijk, M. M., Bos, A. R., De Jonge, V. N., Hanssen, L. S. A. M., Hermus, D. C. R., and De Jong, D. J. (2009). Guidelines for seagrass restoration: importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Mar. Poll. Bull. 58, 179–188. doi: 10.1016/j.marpolbul.2008.09.028
van Katwijk, M. M., Schmitz, G. H., Hanssen, L. S., and den Hartog, C. (1998). Suitability of Zostera marina populations for transplantation to the Wadden Sea as determined by a mesocosm shading experiment. Aquat. Bot. 60, 283–305. doi: 10.1016/S0304-3770(98)00053-9
van Katwijk, M. M., Thorhaug, A., Marbà, N., Orth, R. J., Duarte, C. M., Kendrick, G. A., et al. (2016). Global analysis of seagrass restoration: the importance of large-scale planting. J. Appl. Ecol. 53, 567–578. doi: 10.1111/1365-2664.12562
Biotic data at the transplant site.
In spring 2018, a visual census was performed inside and outside the Zostera marina transplanted plots in Praia dos Coelhos to assess demersal macrofauna and seaweeds. Three band transects of 30 m × 2 m were done in the sand 100 m away from the seagrass patches at the same depth as the plots. In the seagrass plots, transects were as long as the plots, which resulted in various lengths (from 10 to 4 m). Quadrats of 50 by 50 cm were used every 5 m along the transects for benthic macrofaunal and flora identification. All transects were performed in the same day.
A total of 19 species were found in the seagrass plots and 4 in the sand (Table A1). As expected, there were more species inside the seagrass patches than in the sand, confirming that seagrass habitats are important biodiversity shelters.
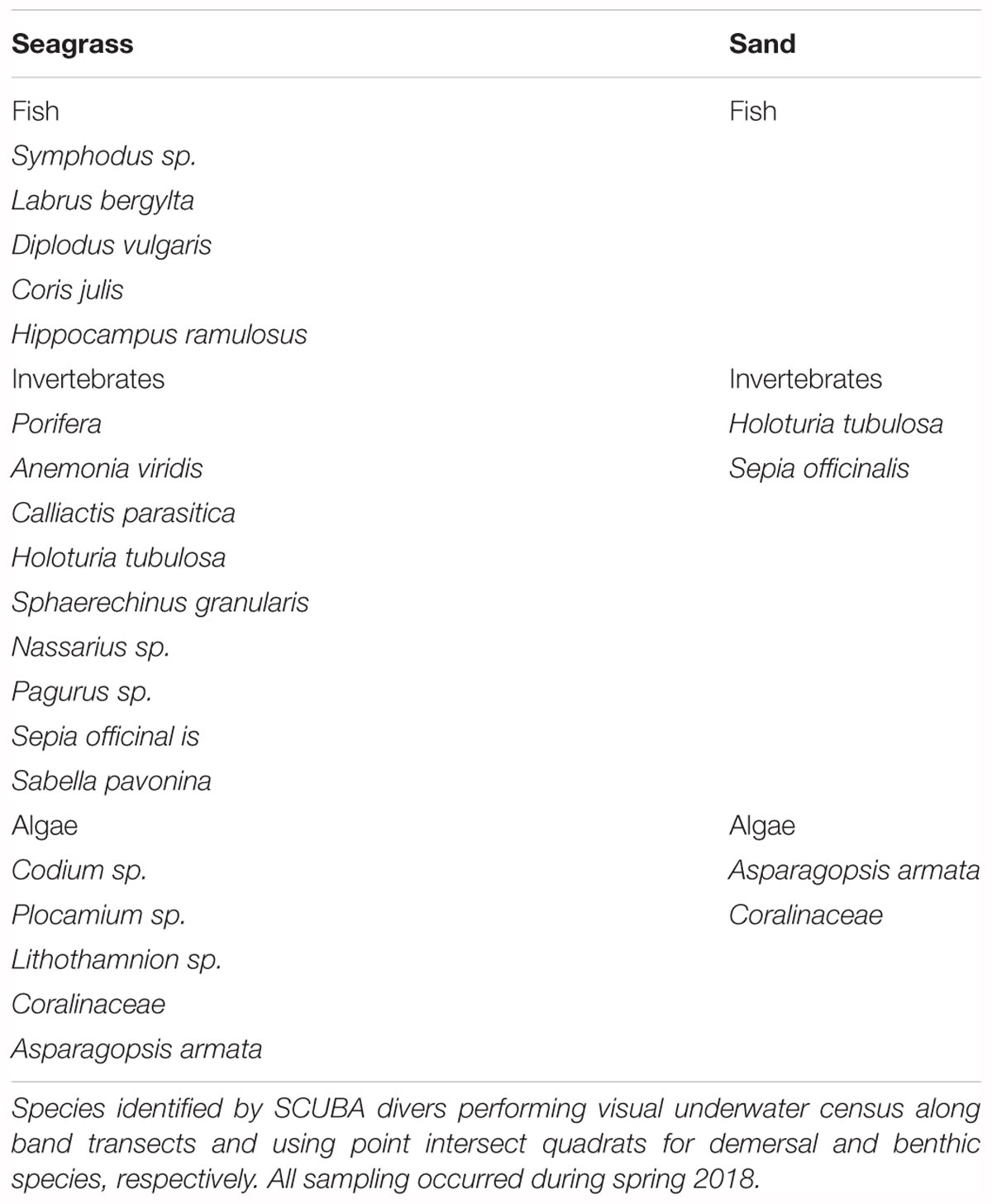
Table A1. Species found inside the transplanted seagrass plots in Praia dos Coelhos and in the nearby sand area.
Abiotic data at the transplant site.
Predominant wind regime in Portugal is from the North, and the study area is facing South, protecting the transplants from the most frequent storms. All the winds coming from 180° to 270° are potentially harmful for the transplants, as they produce swell that impacts the shallow shores were the transplants were set. The study area is in the proximity of the Sado river Estuary, therefore when precipitation is high the water turbidity increases and it is expected that the photosynthetic available radiation is limited. Precipitation and wind direction data where analyzed from February 2009 until June 2018 in the Figures S2.1 and S2.2. The data was uploaded from the meteorological station in Montijo, 40 km from our site.
The water conditions at the transplant site might have been highly variable from year to year since some years had much more precipitation than others. In the winter of 2010/2011 there was extreme precipitation. The winters of 2013/2014 and 2014/2015 presented above average levels of precipitation for the studied period. All the winters from 2009 until 2017 had predominant south – south west wind regimes with the exception of the winter 2013/2014. High precipitation values and south wind regimes are likely to be a combination that can limit photosynthesis, physically disturb sediments and the seagrass meadows, which can potentially represent an impact level capable of transforming seagrass dominated ecosystems into bare sand. Such a combination was observed in the winters of 2010/2011 and 2014/2015. There are likely many other variables that contribute for the impact of storms in the area, such as wave period, height, wing velocity and the number of consecutive impact days. Future meteorological studies are needed to better understand and predict such events. Anecdotal description from mainstream media have shown images in national television of the impact created by a south storm in March 2018. The local population described the storm as the biggest and most destructive ever observed. Local restaurants and car parking lots were destroyed by the waves. Looking in to the data from the Montijo meteorological station this storm is not detected, as the precipitation and wind are “diluted” into many days of observation in that month. This leads us to conclude that to better understand this phenomenon more detailed data (daily and not monthly) must be gathered. The plots transplanted in Spring 2010 and 2017 survived the impact of the March 2018 storm, increasing the evidence that larger initial size plots are likely to be able to resist in the long term.
Keywords: stable states, bi-stability, marine population transplanting, long term habitat monitoring, resilient minimum critical size
Citation: Paulo D, Cunha AH, Boavida J, Serrão EA, Gonçalves EJ and Fonseca M (2019) Open Coast Seagrass Restoration. Can We Do It? Large Scale Seagrass Transplants. Front. Mar. Sci. 6:52. doi: 10.3389/fmars.2019.00052
Received: 18 August 2018; Accepted: 30 January 2019;
Published: 07 March 2019.
Edited by:
Stelios Katsanevakis, University of the Aegean, GreeceReviewed by:
Marieke M. van Katwijk, Radboud University Nijmegen, NetherlandsCopyright © 2019 Paulo, Cunha, Boavida, Serrão, Gonçalves and Fonseca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diogo Paulo, ZGZwYXVsb0B1YWxnLnB0 Ester A. Serrão, ZXNlcnJhb0B1YWxnLnB0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.