- Department of Plant Biology and Biotechnology, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
This investigation is aimed at providing a baseline survey of the current status of the occurrence and spatio-temporal distribution of amnesic shellfish poisoning (ASP) and paralytic shellfish poisoning (PSP) in the Nigerian coast, Gulf of Guinea. The study applied the Jellett Rapid Test technique to algal samples collected from 8 states of South-south (SS) and South-west (SW) zones of coastal Nigeria, spanning the Bight of Bonny to the Bight of Benin, in the Gulf of Guinea, during the rainy and dry seasons, to screen for the presence of the human syndromes of ASP and PSP produced by domoic acid and saxitoxin, respectively. Classified as low, medium, high and highest, various levels of these syndromes were detected across the length of the Nigerian coast. Comparatively, the SW region had more syndromes (PSP and ASP) (64%) than the SS (36%) region of Nigerian coast. The prevalence of PSP (68%) was more than ASP (31%) in both zones with rainy season also recording higher (27%) ASP and PSP for SW than SS (12%) zone. Seasonal consideration revealed that more syndromes (ASP and PSP) were recorded in the rainy season compared to the dry season. With the confirmed presence, spatial and temporal distribution of ASP and PSP in the coastal waters of Nigeria, the need for regular monitoring of algal syndromes and toxins screening is advocated.
Introduction
A small percentage of algae produce toxins. These toxins cause harm to human, culminating in what is known as human health syndromes. Among the toxins synthesized are domoic acid (DA) and saxitoxin (Sxt) responsible for human events that form groups of undesirable signs, patterns and symptoms indicative of a specific disease or disorder termed syndromes. Domoic acid and saxitoxin induce algal syndromes, respectively, known as amnesic shellfish poisoning (ASP) and paralytic shellfish poisoning (PSP) Zaccaroni and Scaravelli (2008). Of all algal syndromes, ASP, PSP, occur world-wide (Gerssen et al., 2010).
Amnesic shellfish poisoning is symptomatic of a pathological loss of short-term memory, dizziness, loss of balance, headache, disorientation, nausea, and vomiting (Lucas et al., 2005) while sensational vibration of the perioral area, respiratory distress, frequent and excessive bowel movements, nausea, slow and continued increase in grave paralysis, spiky responsiveness of the fingertips, headache, dizziness, fever, ataxia, protem blindness and eventual death via respiratory paralysis are signs of PSP (Van Dolah, 2000; Kadiri, 2011; Trainer and Hardy, 2015; Ajani et al., 2017). Domoic acid which is responsible for ASP, is classified as tricarboxylic amino acid on the basis of chemical structure, while saxitoxin which causes PSP, is a purine-derived heterocyclic guanidine compound Zaccaroni and Scaravelli (2008). They are both hydrophilic neurotoxic compounds affecting the exchange of information between the brain and the tendons (Arapov, 2013). Some effects of these toxins range from loss of short term memory to gastrointestinal disorders, diarrhea, vomiting, headache, abdominal cramps, loss of balance, nausea, dizziness, incomprehension and paralysis (Lucas et al., 2005; Ajani et al., 2017).
In the in-land and coastal Nigerian waters, just like other coastal areas in the world, variation in climate, pollution and eutrophication resulting from environmental changes can cause an upsurge in toxin amounts by indirect initiation of massive bloom of algae Ajani et al. (2017), though Trainer and Hardy (2015) opined that they can also occur in pristine areas with little or no influence of nutrients input from anthropogenic activities. Shellfish transplantation and transportation of ballast water at the seaports can facilitate the in-flow of harmful/toxic, exotic species in and out of a country (Hallegraeff, 1998; Zhang and Dickman, 1999). Filter feeding shellfish ASP vectors, Mytilus edulis (blue mussels), M. galloprovincialis (black mussel) Ajani et al. (2017) and PSP vector, Saxidomus giganteus (clams) Lucas et al. (2005), zooplankton and herbivorous fishes ingest these algae and act as vectors to humans either directly (e.g., shellfish) or through further food web transfer of sequestered toxin to higher trophic level. Consumption of sea food contaminated with algal toxins result in sea food poisoning syndromes. Other associated human syndromes are diarrhetic shellfish poisoning (DSP), caused by a group of toxins represented by okadaic acid, neurotoxic shellfish poisoning (NSP), caused by brevetoxin and ciguatera fish poisoning (CFP) caused by ciguatoxin, azaspiracid shell fish poisoning (AZP) and clupeotoxin fish poisoning (CLP) (Wang, 2008; Ajani et al., 2017).
Though the consequential effects of algal toxin in coastal water and the ecosystems are of global concern, nothing is known about ASP and PSP status and their monitoring in the coast of Nigeria. Previous studies in Nigeria specifically focus on algal taxonomy in specific water bodies within Nigerian coastline (Nwankwo, 1991; Ajuzie and Houvenaghel, 2009; Kadiri, 2011). This work represents the first comprehensive and extensive study covering the Nigerian coastline as well as the pioneer study on algal syndromes in Nigeria. The aim of the study is to investigate the occurrence and spatio-temporal distribution of ASP and PSP in the coastal areas (South-south and South-west) of Nigeria. The coastal communities eat a lot of shellfish and these shellfish are filter feeders, feeding on the toxic phytoplankton algae when present. The study therefore will examine the presence of the syndromes consequent upon the feeding on the prevalent phytoplankton toxic algae.
Materials and Methods
Study Area
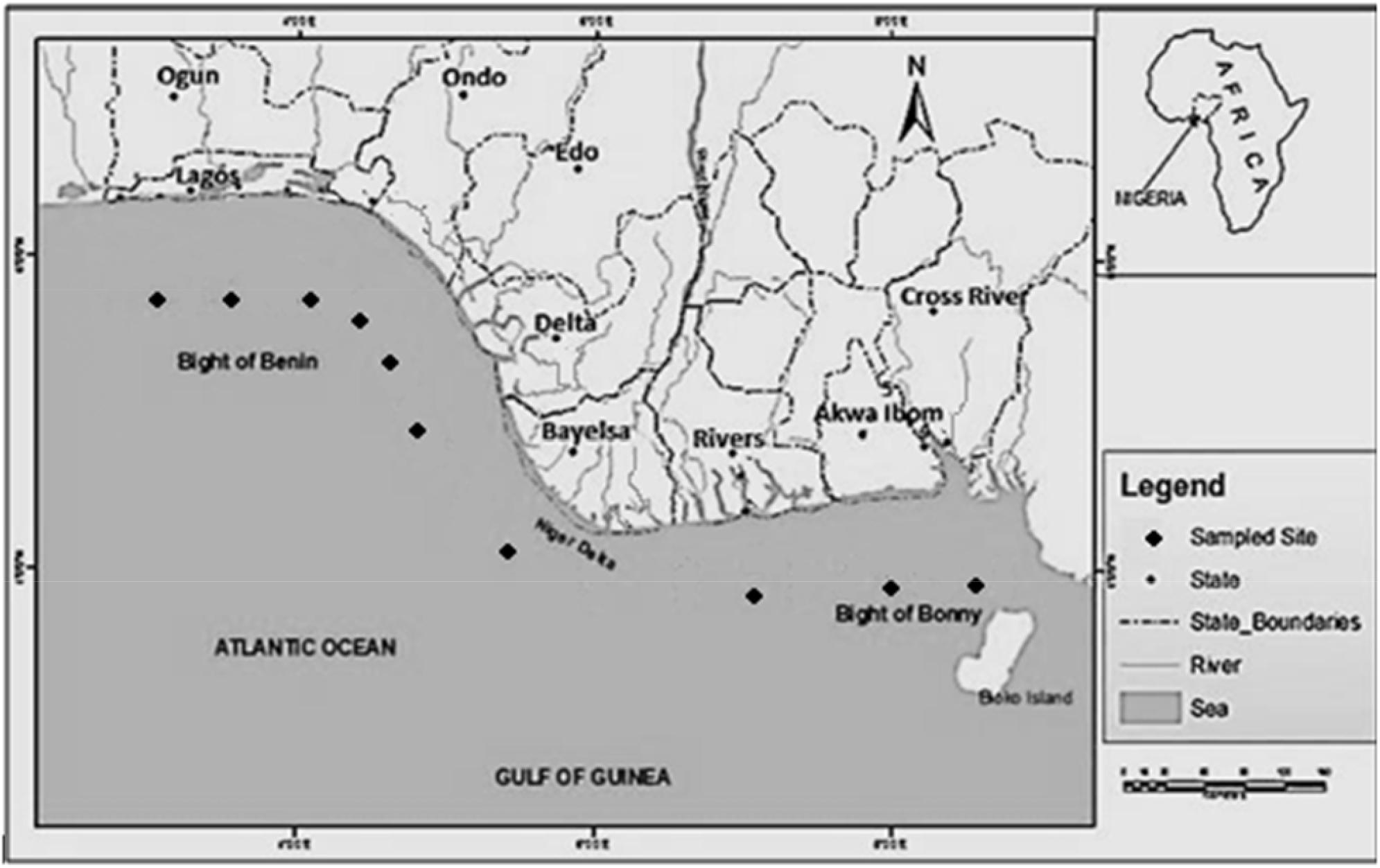
The study was carried out in the Atlantic Ocean in Gulf of Guinea, in the Bight of Bonny to the east and Bight of Benin to the west.
The study area covers 20 stations selected across 10 locations to cover the entire coast (Cross River, Akwa Ibom, Rivers, Bayelsa, Delta, Ondo, Ogun, Lagos) Lekki, Bar beach and Badagry (Figure 1) of Nigeria (Kadiri and Isagba, 2016) lying between longitudes 3°24′′ and 8°19′′ E and latitude 4°58′′ and 6°24′′ N along the Nigerian coastline (Kadiri, 2002; Ajuzie and Houvenaghel, 2009). The stations Cross River, Akwa Ibom, Bayelsa and Delta are located in the South-south of Nigeria while Ondo, Ogun, Lagos- Lekki, Bar beach and Badagry are located in the South-west of Nigeria. Climatically, there are two main seasons in the area namely the rainy (wet) season spanning from May to October and dry season from November to April. The coastal area is humid with a mean average temperature of 24–32°C and an average annual rainfall ranging between 1,500 and 4,000 m (Kuruk, 2004).
Sample Collection
From March 2014 to February 2015, at 3 months intervals (March, June, October 2014 and February 2015), phytoplankton algal samples were collected from the surface with horizontal tows of 10μm mesh size plankton net tied to a moving boat for about 10 min and content transferred to clean sample containers. At each location, one sample was collected each from the ocean and adjoining water body.
Algal Syndrome Screening
The Jellett ASP/PSP rapid testing, an in vitro qualitative lateral flow screen diagnostic test to detect the presence or absence of ASP/PSP toxins in phytoplankton was applied following the manufacturer’s (Jellett Rapid Testing Ltd., 4654 Route #3, Chester Basin, Nova Scotia, Canada BOJ 1K0) instructional manual (Batch 40000-18 Feb 14-512). Phytoplankton concentrates were obtained by filtering 10 L of sea water through a plankton net of 10 micrometers. From these concentrated phytoplankton cells, 0.5 ml of 0.1 M acetic acid was added to 0.5 ml of phytoplankton cells in a clean vial, tightly capped and shaken 6 to 8 times. 0.4 ml of buffer was placed into this vial, and then 0.1 ml of phytoplankton cells was added and mixed. The mixture was placed into the sample well and the result read between 35 min and 1 h.
In order to check for differences between seasons and location, the data were subjected to statistical analysis, using Chi square test using SPSS software.
Results
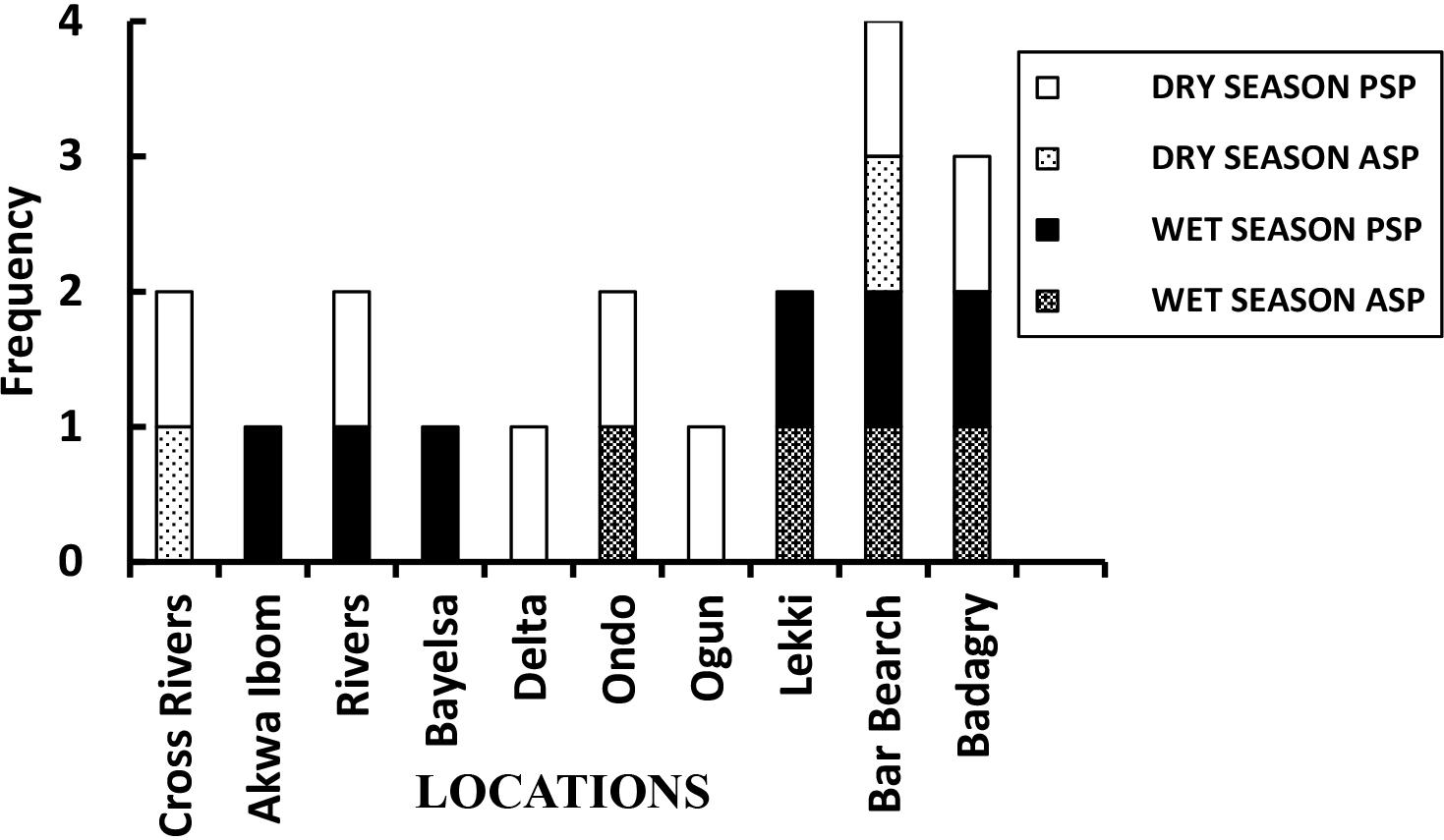
From this study, results showed the presence of the human syndromes of ASP and PSP. Syndrome detection was grouped and rated as low (25%) where only 1 syndrome was detected. Where 2 different syndromes either in same season or same syndrome type in different seasons were detected was rated medium (50%). A high (75%) syndrome detection rating was ascribed to situations where 3 syndromes (including 2 same syndromes at different seasons) were observed. The highest (100%) toxin detection rating corresponds to where 4 syndromes (including 2 of the same syndromes at different seasons) were found. Spatially, all of the locations across the coast recorded at least one detectable presence of a particular syndrome (Figure 2) from the South-south (SS) to the South-west (SW) coast of Nigeria. Low (25%) occurrence of syndromes were detected at 4 of the total of 10 locations, 3 of which were located at the SS (Akwa Ibom, Bayelsa, and Delta) and 1 at the SW (Ogun).
Seasonal consideration revealed that Akwa Ibom and Bayelsa had PSP in the wet season while Delta and Ogun had PSP in the dry season. Similarly, 4 of the 10 locations had medium (50%) detectable syndromes-records of 2 syndromes (including 2 same syndromes at different seasons) each. Two of these locations were observed in the SS (Cross Rivers, Rivers) and 2 in the SW (Ondo and Lekki). Cross Rivers location had both PSP and ASP detected in the dry season. Rivers location had PSP detected both in the dry and wet seasons. Ondo location had PSP in the dry and ASP in the wet seasons. Lekki location had both syndromes (PSP and ASP) in the wet season. A high (75%) syndrome detection of 3 detectable syndromes record (including 2 same syndromes at different seasons) was observed in the SW Badagry location. Here, PSP and ASP were detected in the wet season and PSP reoccurred in the dry season. Also in the SW, the highest (100%) detection of 4 detectable syndromes record (both syndromes at both seasons) was observed only at the Bar Beach location.
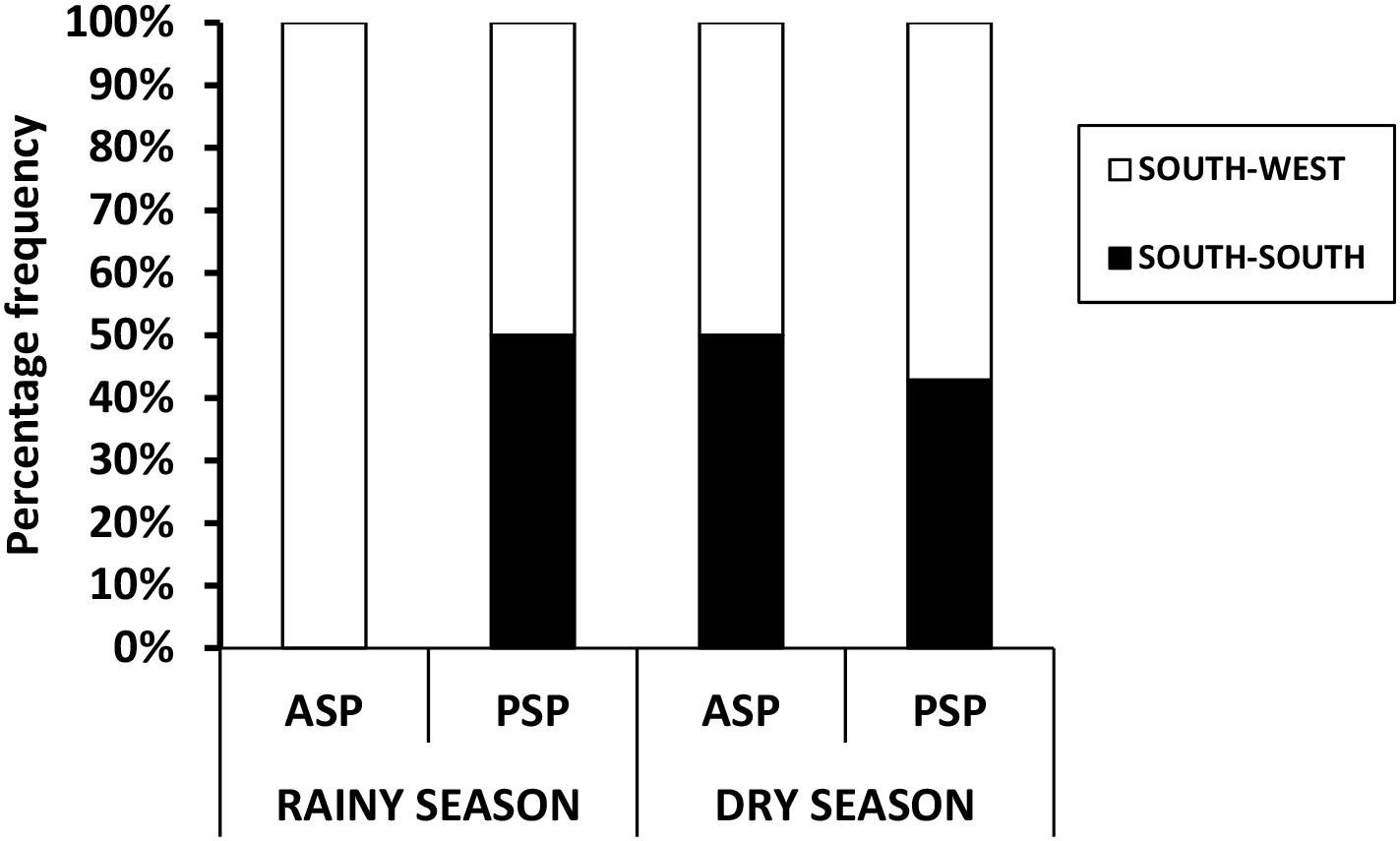
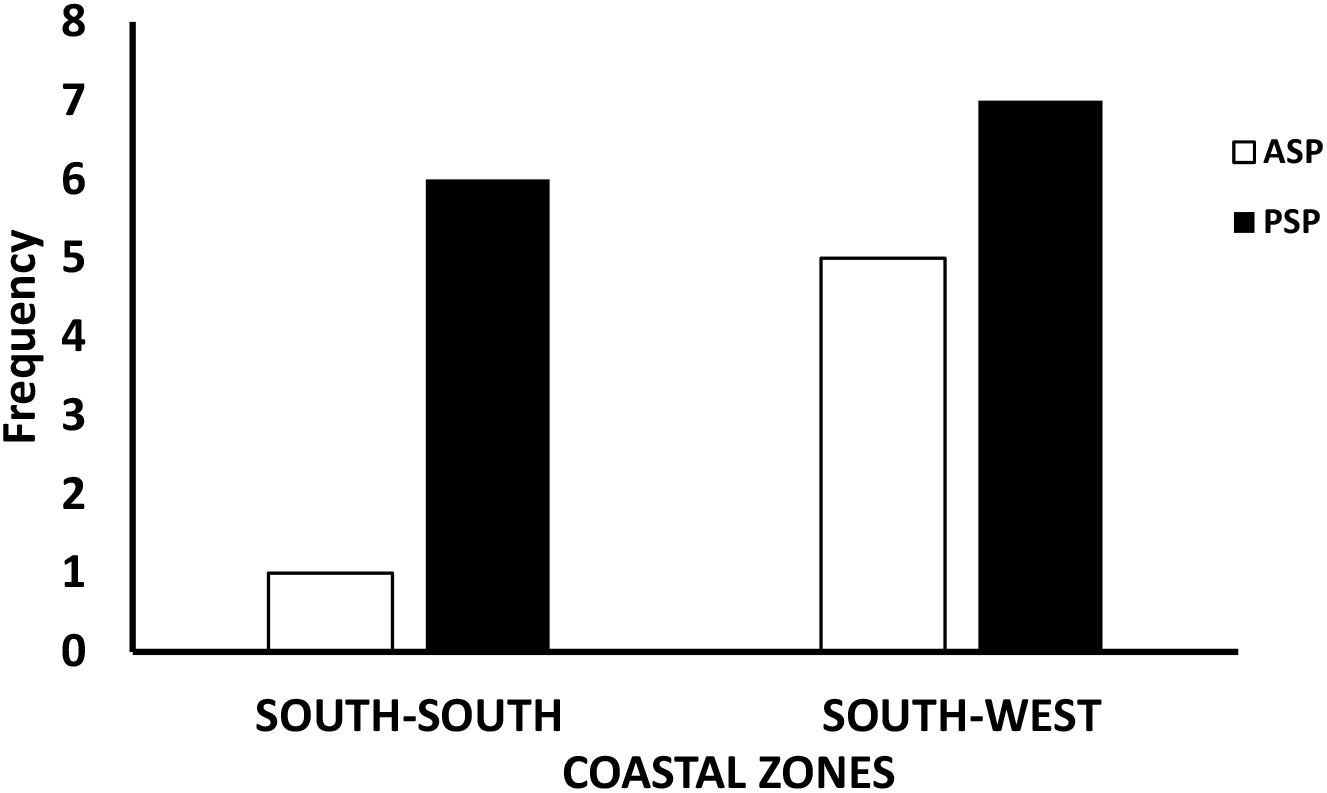
Figure 3 shows the seasonal percentage syndrome distribution across the zones, reflecting the frequency of ASP and PSP prevalence in both SS and SW zones. The SW region had higher (64%) preponderance of total (ASP + PSP) syndromes detected in both seasons compared to the SS region with a total of 36%. Within the SW, rainy (wet) season alone, 37% (of the 64% total) syndrome was recorded while the other 27% was recorded for dry season SW. Conversely, lower values were recorded in the SS rainy season with 12% (of the 36% total) syndrome detected and 24% for dry season SS. Figure 3 also reveals that rainy season has 100% ASP in the SW and 0% in SS and 50% PSP each for SW and SS. Also, dry season had 50% ASP each for SW and SS but a bit higher 53% PSP for SW and 47% for SS. Figure 4 shows the profile of ASP and PSP distribution between the SS and SW regions. From the results, PSP had higher profile than ASP in both the SS and SW regions. The PSP values were concurrently higher in the SW than the SS. Overall, PSP had 68% incidence (37% in the SW and 31% in SS) while ASP had 31% (26% in the SW and 5% in SS). In summary, the SW region contained more ASP and PSP syndromes than SS region. PSP generally has higher occurrence than ASP in both zones. Rainy season has higher ASP and PSP for SW than SS.
Statistically, for PSP, no significant relationship was found between seasons and locations where samples were collected, X2 (1, N = 200) = 2.020, p = 0.155, whereas in the case of ASP, a significant relationship was found between seasons and locations where samples were collected, X2 (1, N = 200) = 66.667, p < 0.001.
Discussion
From the SS to the SW, all sampled locations/zones had records of at least one syndrome of ASP or PSP (Figures 2, 3). The SW locations recorded more ASP and PSP syndromes than the SS locations in this study with the Bar beach and Badagry locations with highest occurrences. This may not be unconnected with the higher prevalence of toxin-producing species generally observed in the SW in contrast to the SS locations (Kadiri et al., 2016). Earlier study by Zendong et al. (2016) recorded substantial quantities of specific marine algal biotoxins in SW Lekki and Bar beach relative to SS Rivers and Akwa Ibom. The SW coastal Nigeria is reckoned to be of more elevated salinity than the SS (Zendong et al., 2016) with greater preponderance for toxic dinoflagellates to survive (Delmas et al., 1992; Zendong et al., 2016) whereas the salinity of SS is diluted by the Niger delta inflows. The high salinity of Bar beach and Lekki was also stressed by Ajuzie and Houvenaghel (2009). The result obtained in this study is in consonance with (Lucas et al., 2005) who reported ASP and PSP profiles with clear variations between regions, with the toxin content in the sample material obtained north of Aberdeen, the Scottish east coast, being lower than that in the remaining area under their investigation. The toxin profile obtained on the Scottish east coast was ascribed to the presence of different species or strains of toxic algae.
Major routes through the seaports by which ballast waters with toxic species open in and out to other countries have been recognized as sources of toxin contamination (Zhang and Dickman, 1999; Doblin et al., 2004). It is interesting to note that the traffic of shipping activities in Nigeria is considerably a lot higher in the SW than in the SS region. In the northern and central California (2002 to 2007) and many other areas worldwide, ASP and PSP have been detected (Rositano et al., 2001). The presence of domoic acid, an ASP producer, was reported also in north and western European coastal zones (Lundholm et al., 1995; Dizer et al., 2001). In the Australian coast, Ajani et al. (2017) identified amidst other syndromes, PSP and ASP, describing them as major causes of worry, culminating in a huge loss of approximately AUD$23M in Tasmania in 2012.
The higher prevalence of PSP in comparison to ASP across locations and zones could be explained by the fact that only one algal genus, Pseudonitzschia within the diatom group, is responsible for ASP while quite an assortment of different genera/species of the dinoflagellate group and blue-green algae are responsible for PSP. Pseudonitzschia delicatissima, P. multiseries, P. cuspidata, P. pungens, and P. australis are diatom species generally implicated in the biosynthesis of DA responsible for ASP (Ajani et al., 2017). Ever since the first report of PSP event (illness and deaths) near San Francisco, CA, United States, caused by Alexandrium catenella, members of three dinoflagellate genera namely Alexandrium, Gymnodinium, and Pyrodinium have been reported to be the major sources of PSP toxins (Shumway, 1990). Gymnodinium catenatum, Alexandrium catenella, A. acatenella, A. fundyense, A. minutum, A. tamarense, A. ostenfeldii, Pyrodinium bahamense (Ajani et al., 2017), Cylindrospermopsis raciborskii, Aphanizomenon flos-aquae, Lyngbya spp., Anabaena circinalis (Lucas et al., 2005; Bittencourt-Oliveira et al., 2015) all synthesize saxitoxin responsible for PSP in both marine and fresh water. Most of these species have also been identified from the Nigerian coast (Kadiri et al., 2016).
The seasonal variation as observed in higher PSP/ASP in the rainy season relative to the dry season may have resulted from favorable conditions enhancing eutrophication, higher influx and proliferation of toxin producers brought by the rains in both SS and SW zones. The linkage of harmful algae to eutrophication has been documented and corroborated by other workers (Anderson et al., 2002). Coastal eutrophication or nutrient enrichment is invoked by high inorganic nutrients from river discharges (Hodgkiss and Lu, 2004) and this culminates in HABs (Wang et al., 2003; Imai et al., 2006). Nutrient enrichment in coastal areas arises from high-inorganic nutrients in freshwater runoff, sewage discharge, agricultural fertilizers, and nearby high-density coastal aquaculture (Qian and Liang, 1999). The phosphate load in the aquatic ecosystems could also be attributed to farming activities whereby farmers apply phosphate fertilizers in their farms, hence surface run-off from the farms could increase phosphate load in the river water.
The syndromes ASP and PSP are characterized by several human health hazards. ASP is caused by Domoic acid, a water-soluble tricarboxylic amino acid and a potent glutamate receptor agonist. The symptomatic effects of ASP include gastrointestinal effects (e.g., nausea, vomiting, and diarrhea) and neurologic effects such as brain lesions, dizziness, disorientation, lethargy, seizures, short-term memory loss (amnesia), coma, and death (Quilliam and Wright, 1989; Hiolski et al., 2014). Hiolski et al. (2014) found at asymptomatic exposure, significant alteration of transcription genes for neurological function and development, as well as impairment of mitochondrial function.
Paralytic shellfish poisoning is the oldest known intoxication and one of the most dangerous for humans, with a high rate of mortality. It is a worldwide distributed poisoning, with cases reported for North and South America, Europe, Africa and Asia (Zaccaroni and Scaravelli, 2008). It is reported that worldwide that 1,600 cases of PSP occur yearly (Rodrigue et al., 1990). Though PSP was more prevalent in temperate countries, it is now increasing in tropical regions (Rodrigue et al., 1990). Report of Rodrigue et al. (1990) citing epidemic occurrence of PSP, indicated that the symptoms-persistent headaches, memory loss and fatigue occurred for weeks in some instances in Guatemala.
Paralytic shellfish poisoning is characterized by gastrointestinal and neurological symptoms, with nausea, vomiting, diarrhea, tingling or numbness around lips, gradual and more and more severe paralysis, respiratory difficulty, death in humans through respiratory paralysis (Kodama, 2000; Zaccaroni and Scaravelli, 2008). PSP syndrome is caused by a suite of heterocyclic guanidines collectively called saxitoxins (STXs) which are heat-or thermo-stable and water-soluble non-proteinaceous toxins. Saxitoxin (STX) is one of the few toxins which are produced by both marine and fresh water (cyanobacteria) algae. Saxitoxins are responsible for about 2000 human cases/year, with a mortality rate ranging from 15 to 50% (Van Dolah, 2000; Marcaillou-Le Baut et al., 2001).
Trainer and Hardy (2015) substantiated the high risk to human health following the detection of these toxin-causing syndromes in diets, water, stomach contents and ecological samples. Similarly, Repavich et al. (1990) reported the demise of cattle and other mammals from acute and chronic effects of these toxins. Pulido (2016) also found bloom of toxic Pseudo-nitzschia from the west coast of North America causing ASP syndrome when crab and clams were contaminated, leading to the shutting down of many harvesting centers and caution issued by regulatory agencies to end users.
Conclusion
The algal syndromes ASP and PSP have been recorded at substantial levels in coastal areas of Nigeria. These syndromes have various symptomatic effects on humans. It is evident from this study that Nigeria, which currently has no established harmful algal bloom/toxic algal monitoring program, has dangerous harmful algal syndromes throughout the entire coast. Beyond this pioneer qualitative study which has established unequivocally, the presence of these syndromes and perhaps others yet to be investigated within the SW and SS Nigeria, there is absolute need for further research to quantify the actual toxin concentration in our freshwater and marine ecosystems. The need for urgent regular monitoring/monitoring programs of Nigerian coastal waters is therefore emphasized.
Author Contributions
MK was the principal investigator, conceptualized and executed the project. SI assisted in the work.
Funding
Tertiary Education Trust Fund was gratefully acknowledged for the National Research Fund (TETF/NRF 2009) grant provided for this study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Denise Mukoro, Jeffrey Ogbegbor, Osasere Omoruyi and Timothy Unusiotame are also gratefully acknowledged in their assistance at sample collection.
References
Ajani, P., Harwoood, D. T., and Murray, S. A. (2017). Recent trends in marine phycotoxins from Australian coastal waters. Mar. Drugs 15, 33–53. doi: 10.3390/md15020033
Ajuzie, C., and Houvenaghel, G. (2009). Preliminary survey of potentially harmful dinoflagellates in Nigeria’s coastal waters. Fottea 9, 107–120. doi: 10.5507/fot.2009.010
Anderson, D. M., Glibert, P. M., and Burkholder, J. M. (2002). Harmfulalgal blooms and eutrophication: nutrient sources, composition and consequences. Estuaries 25, 562–584. doi: 10.1007/BF02804901
Arapov, J. A. (2013). Review of shellfish phycotoxin profile and toxic phytoplankton species along Croatian coast of the Adriatic Sea. Acta Adriat. 54, 283–298.
Bittencourt-Oliveira, M. C., Chia, A. M., de Oliveira, H. S. B., Cordeiro-Araújo, M. K., Molica, R. J. R., and Dias, C. T. S. (2015). Allelopathic interactions between microcystin- producing and non-microcystin-producing cyanobacteria and green microalgae: implications for microcystins production. J. Appl. Phycol. 27, 275–284. doi: 10.1007/s10811-014-0326-2
Delmas, D., Herbland, A., and Maestrini, S. Y. (1992). Environmental conditions which lead to increase in cell density of the toxic dinoflagellates Dinophysis spp in nutrient rich and nutrient-poor waters of the French Atlantic coast. Mar. Ecol. Prog. Ser. 89, 53–61. doi: 10.3354/meps089053
Dizer, H., Fischer, B., Harabawy, A. S. A., Hennion, M. C., and Hansen, P. D. (2001). Toxicity of domoic acid in the marine mussel Mytilus edulis. Aquat. Toxicol. 55, 149–156. doi: 10.1016/S0166-445X(01)00178-3
Doblin, M. A., Popels, L. C., Coyne, K. J., and Hutchins, D. A. (2004). Transport of the harmful bloom alga Aureococcus anophagefferens by ocean-going ships and coastal boats. Appl. Environ. Microbiol. 70, 6495–6500. doi: 10.1128/AEM.70.11.6495-6500.2004
Gerssen, A., Pol-Hofstad, I. E., Poelman, M., Mulder, P. P. J., van den Top, H. J., and de Boer, J. (2010). Marine toxins: chemistry, toxicity, occurrence and detection, with special reference to the Dutch situation. Toxins 2, 878–904. doi: 10.3390/toxins2040878
Hallegraeff, G. M. (1998). Transport of toxic dinoflagellates via ships’ ballast water: bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar. Ecol. Prog. Ser. 168, 297–309. doi: 10.3354/meps168297
Hiolski, E. M., Preston, S. K., Frame, E. R., Myers, M. S., Bammler, T. K., Beyer, R. P., et al. (2014). Chronic low-level domoic acid exposure alters gene transcription and impairs mitochondrial function in the CNS. Aquat. Toxicol. 155, 151–159. doi: 10.1016/j.aquatox.2014.06.006
Hodgkiss, I. J., and Lu, S. H. (2004). The effect of nutrients and their ratios on phytoplankton abundance in Junk Bay. Hong Kong. Hydrobiologia 512, 215–229. doi: 10.1023/B:HYDR.0000020330.37366.e5
Imai, I., Yamaguchi, M., and Hori, M. (2006). Eutrophication and occurrences of harmful algalblooms in the Seto inland Sea. Japan. Plankton Benthos Res. 1, 71–84. doi: 10.3800/pbr.1.71
Kadiri, M. O. (2002). A spectrum of phytoplankton flora along salinity gradient in the Eastern Niger Delta area of Nigeria. Acta Bot. Hung. 44, 75–83. doi: 10.1556/ABot.44.2002.1-2.6
Kadiri, M. O. (2011). Notes on harmful algae from Nigerian coastal waters. Acta Bot. Hung. 53, 137–143. doi: 10.1556/ABot.53.2011.1-2.12
Kadiri, M. O., and Isagba, S. (2016). “PCR and enzyme-linked immunosorbent assay of microcystin in the bights of bonny and Benin, Nigeria,” in Proceedings of the 2nd University of Benin Annual Research Day (UBARD) Conference, Benin, 449–452.
Kadiri, M. O., Ogbebor, J. U., and Omoruyi, O. A. (2016). “Spatial distribution of some potentially harmful algae in coastal waters of Nigeria,” in Proceedings of the 2nd University of Benin Annual Research Day (UBARD) Conference, Benin, 363–366.
Kodama, M. (2000). “Ecology, classification, and origin,” in Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, eds L. Botana and M. Dekker (New York, NY: Taylor & Francis), 125–150.
Kuruk, P. (2004). Customary Water Laws and Practices: Nigeria. Available at: http://www.fao.org/legal/advserv/FOA/UCNCS.Nigeria.pdf
Lucas, B., Dahlmann, J., Erler, K., Gerdts, G., Wasmund, N., and Hummert, C. (2005). Overview of key phytoplankton toxins and their recent occurrence in the North and Baltic Sea. Environ. Toxicol. 20, 1–17. doi: 10.1002/tox.20072
Lundholm, N., Skov, J., Pocklington, R., and Moestrup, O. (1995). Domoic acid, the toxic amino acid responsible for amnesic shellfish poisoning, now in Pseudonitzschia seriata (Bacillariophyceae) in Europe. Phycologia 33, 475–478. doi: 10.2216/i0031-8884-33-6-475.1
Marcaillou-Le Baut, C., Krys, S., and Bourdeau, P. (2001). “Syndromes observés et données épidémiologiques,” in Toxines D’algues Dans L’alimentation, eds J. M. Frémy and P. Lassus (Issy-les-Moulineaux: Ifremer), 371–399.
Nwankwo, D. I. (1991). A survey of the dinoflagellates of Nigeria. armoured dinoflagellates of Lagos lagoon and associated tidal creeks. Niger. J. Bot. 4, 49–60.
Pulido, O. M. (2016). Phycotoxins by harmful algal blooms (HABS) and human poisoning: an overview. Int. Clin. Pathol. J. 2:00062. doi: 10.15406/icpjl.2016.02.00062
Qian, H. L., and Liang, S. (1999). Study on the red tide in the Pearl River estuary and its near waters. Mar. Environ. Sci. 18, 69–74.
Quilliam, M., and Wright, J. (1989). The amnesic shellfish poisoning mystery. Anal. Chem. 61, 105–106. doi: 10.1021/ac00193a745
Repavich, W. M., Sonzoggni, W. C., Standridge, J. H., and Wedepohl, R. E. (1990). Cyanobacteria (blue-green algae) in Wisconsin waters: acute and chronic toxicity. Water Res. 24, 225–231. doi: 10.1016/0043-1354(90)90107-H
Rodrigue, D. C., Etzel, R. A., Hall, S., de Porras, E., Velasquez, O. H., Tauxe, R. V., et al. (1990). Lethal paralytic shellfish poisoning in Guatemala. Am. J. Trop. Med. Hyg. 42, 267–271. doi: 10.4269/ajtmh.1990.42.267
Rositano, J., Newcombe, G., Nicholson, B., and Sztajnbok, P. (2001). Ozonation of algal toxins in four treated waters. Water Res. 35, 23–32. doi: 10.1016/S0043-1354(00)00252-9
Shumway, S. E. (1990). A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquac. Soc. 21, 65–104. doi: 10.1111/j.1749-7345.1990.tb00529.x
Trainer, V. L., and Hardy, F. J. (2015). Integrative monitoring of marine and freshwater harmful algae in washington state for public health protection. Toxins 7, 1206–1234. doi: 10.3390/toxins7041206
Van Dolah, F. M. (2000). Marine algal toxins: origins, health effects, and their increased occurrence. Environ. Health Perspect. 108, 133–141. doi: 10.1289/ehp.00108s1133
Wang, D. (2008). Neurotoxins from marine dinoflagellates: a brief review. Mar. Drugs 6, 349–371. doi: 10.3390/md20080016
Wang, H. K., Huang, L. M., Huang, X. P., Song, X. Y., Wang, H. J., Wu, N. J., et al. (2003). A red tide caused by Gyrodinium instriatum and its environmental characters in Zhujiang Riverestuary. J. Trop. Oceanogr. 22, 55–62.
Zaccaroni, A., and Scaravelli, D. (2008). “Toxicity of sea algal toxins to humans and animals,” in Algal Toxins: Nature, Occurrence, Effect and Detection, eds V. Evangelista, L. Barsanti, A. M. Frassanito, V. Passarelli, and P. Gualtieri (Berlin: Springer Science + Business Media), 91–157. doi: 10.1007/978-1-4020-8480-5_4
Zendong, Z., Kadiri, M., Herrenknecht, C., Nezan, E., Mazzeo, A., and Hess, P. (2016). Algal toxin profiles in Nigerian coastal waters (Gulf of Guinea) using passive sampling and liquid chromatography coupled to mass spectrometry. Toxicon 114, 16–27. doi: 10.1016/j.toxicon.2016.02.011
Keywords: harmful algae syndrome, amnesic shellfish poisoning, paralytic shellfish poisoning, domoic, saxitoxin
Citation: Kadiri MO and Isagba S (2018) Amnesic Shellfish Poisoning (ASP) and Paralytic Shellfish Poisoning (PSP) in Nigerian Coast, Gulf of Guinea. Front. Mar. Sci. 5:481. doi: 10.3389/fmars.2018.00481
Received: 11 February 2018; Accepted: 29 November 2018;
Published: 21 December 2018.
Edited by:
Rathinam Arthur James, Bharathidasan University, IndiaReviewed by:
Yelda Aktan, Istanbul University, TurkeyChidambaram Sabarathinam, Annamalai University, India
Copyright © 2018 Kadiri and Isagba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Medina Omo Kadiri, bW9rYWRpcmlAdW5pYmVuLmVkdQ==; bW9rYWRpcmlAaG90bWFpbC5jb20=
 Medina Omo Kadiri
Medina Omo Kadiri Solomon Isagba
Solomon Isagba