- Central Marine Fisheries Research Institute, Kochi, India
The recent fluctuations in abundance of the Indian oil sardine Sardinella longiceps, a tropical small pelagic clupeid fish, was investigated in the light of overfishing and variations in its habitat ecology in southeastern Arabian Sea. In 2012, its landings peaked to an all-time record making it the fifth largest sardine fishery in the world, and within 3 years the catches were reduced to nearly a tenth of that level. This study examined the fishery dependant factors such as effort, catch rates and expansion of fishing area; the biological variations in fish size, maturity and recruitment; and tried to relate this to the environmental variations in the sardine habitat and food availability. The 2012 mega harvest was a result of a 2-time increase in gear size and engine capacity of fishing crafts and a 3.7-time increase in fishing effort. The female maturation process was strongly influenced primarily by rainfall and then by upwelling and the resulting influx of cold nutrient-rich water in the habitat from April much before the start of the monsoon in June. After 2013, the weak monsoons and the 2015 El Nino Southern Oscillation resulted in a warmer (by an average of 1.1°C) period which negatively impacted the maturation process. The abundance of jellyfishes which are larval and young fish predators in the habitat negatively affected recruitment after 2013. The mismatch in timing of phytoplankton productivity and sardine larvae in the habitat also affected the recruitment success. These environmental divergences coupled with the excessive capture (beyond maximum sustainable yields) of spawning stock and juveniles from 2010 has resulted in this biological catastrophe which has affected the livelihood of thousands of small-scale fishers. A more responsive fisheries administration with timely restriction on fishing effort and protection of spawning stocks by way of fishery closure would have helped minimize the impacts.
Introduction
The Indian oil sardine, Sardinella longiceps, is a small pelagic clupeid fish with distribution limited to tropical waters of northern and western Indian Ocean: Gulf of Aden, Gulf of Oman, but apparently not Red Sea or the Persian Gulf, and eastward to India, including the Andaman Islands. This forage species comes under the group ‘herring, anchovies and sardine, (HAS) which contributes to 18.6% (15.3 million tons) of the global marine capture fishery production (FAO, 2016). Among the five nations which harvest oil sardine (0.572 million tons) from their coastal waters, India’s average production is the highest with 65% (376,189 t) followed by Oman, 63,439 t, Yemen, 58,839 t, Iran, 45,800 t, and Pakistan, 27,372 t (FAO, 2016).
Globally, the sardine and anchovy fisheries have shown wide fluctuations and the reasons for these have been extensively investigated (Lluch-Belda et al., 1992a,b; Schwartzlose et al., 1999). Typically collapses are characterized by a reduction in catch of less than 10% of the maximum and by a long recovery time after reaching a biomass minimum (Mullon et al., 2005; Worm et al., 2009; Petitgas et al., 2010).
During the period 1950–2014, India has been the major contributor of oil sardine catch with 66–96% (average 80%) of the global oil sardine catch. Among the Indian maritime states, Kerala has always been the major producer of oil sardine (Hornell, 1937; Nair and Chidambaram, 1951; Pillai et al., 2003) as it is one of the most productive upwelling zones (Banse, 1959) along the southeastern Arabian Sea. With average annual landing of 0.22 million tons during 2001 to 2010, the Kerala State’s landing exceeded the catch from other nations like Oman, Yemen, Iran and Pakistan (FAO, 2016).
The Indian oil sardine supports the main non-motorized and motorized fisheries sectors of Kerala which are operated along 590 km coastline and spread across 222 villages. About 145,396 fishermen are actively involved in this small-scale coastal fisheries (CMFRI, 2012). The importance of the sardine fishery can be judged from the fact that this species has singly contributed on an average to 37% of the total marine fish landings of Kerala (0.58 million tons) during the period 2001–2010. At the national level the total oil sardine catch from all states contributed to 15% of the all India marine fish production of 3.7 million tons during 2011–2015.
This fishery has declined and collapsed several times during 19th and 20th centuries and several fishery based reasons have been cited along with effects of environmental factors especially the southwest monsoon and coastal upwelling (Raja, 1969; Longhurst and Wooster, 1990; Madhupratap et al., 1994; Jayaprakash, 2002; Krishnakumar et al., 2008; Xu and Boyce, 2009). However, reasons for fluctuations in oil sardine fishery linking the fishery dependent and habitat changes on the biology of sardine and the resultant density changes has not been attempted so far.
In the present century, oil sardine catches of Kerala did not decline drastically till 2012. The recent sardine crisis started in 2013, with the total landing declining to 0.21 million tons from an all-time peak of 0.39 million tons in 2012, indicating a decline of 46%. It further declined to 0.15 million tons in 2014, and continued to decline in 2015 when only 0.068 million tons were landed indicating a decline of 82% from that of 2012.
The biology of the fish has been a subject of detailed investigations. Studies have been made on fecundity, spawning seasons, size at maturity, gonadal development, sex ratio and diets (Jayaprakash and Pillai, 2000). It has been observed that there is differential growth between the early and late recruits arising from the same spawning stock in a year and that the fish attains 9.5–11.0 and 11.0–12.5 cm in the 2nd and 3rd month itself (Raja, 1973; Yohannan et al., 1998). Oil sardine grows rapidly, matures early and a few continue to survive beyond 18 months (Longhurst and Wooster, 1990). Radhakrishnan (1965) observed that the minimum size at first maturity is 12.0–13.9 cm. Majority of investigations indicate that the size at first maturity is 15.0 cm and that the spawning period is from May to August coinciding with the southwest monsoon (Jayaprakash and Pillai, 2000). It has also been observed that the fecundity varies with the age of the fish and size of the ovary and generally ranges between 37,000 to 80,000 (Jayaprakash and Pillai, 2000).
The reason for this decline was investigated by analyzing the fishery related and fishery independent factors including the environmental and biological variations and the biotic pressures on the oil sardine population along Kerala. This study investigated the change in pattern of fishing oil sardines and the resultant impacts. Further, we studied the environmental parameters controlling maturity, spawning and recruitment of sardines and analyzed the influence of major ocean atmospheric processes such as El Niño.
The investigation used various sets of real-time and satellite based datasets for the period 2010–2015 which were collected as a part of investigations of projects supported by the Ministry of Agriculture and the Ministry of Earth Sciences, India.
Materials and Methods
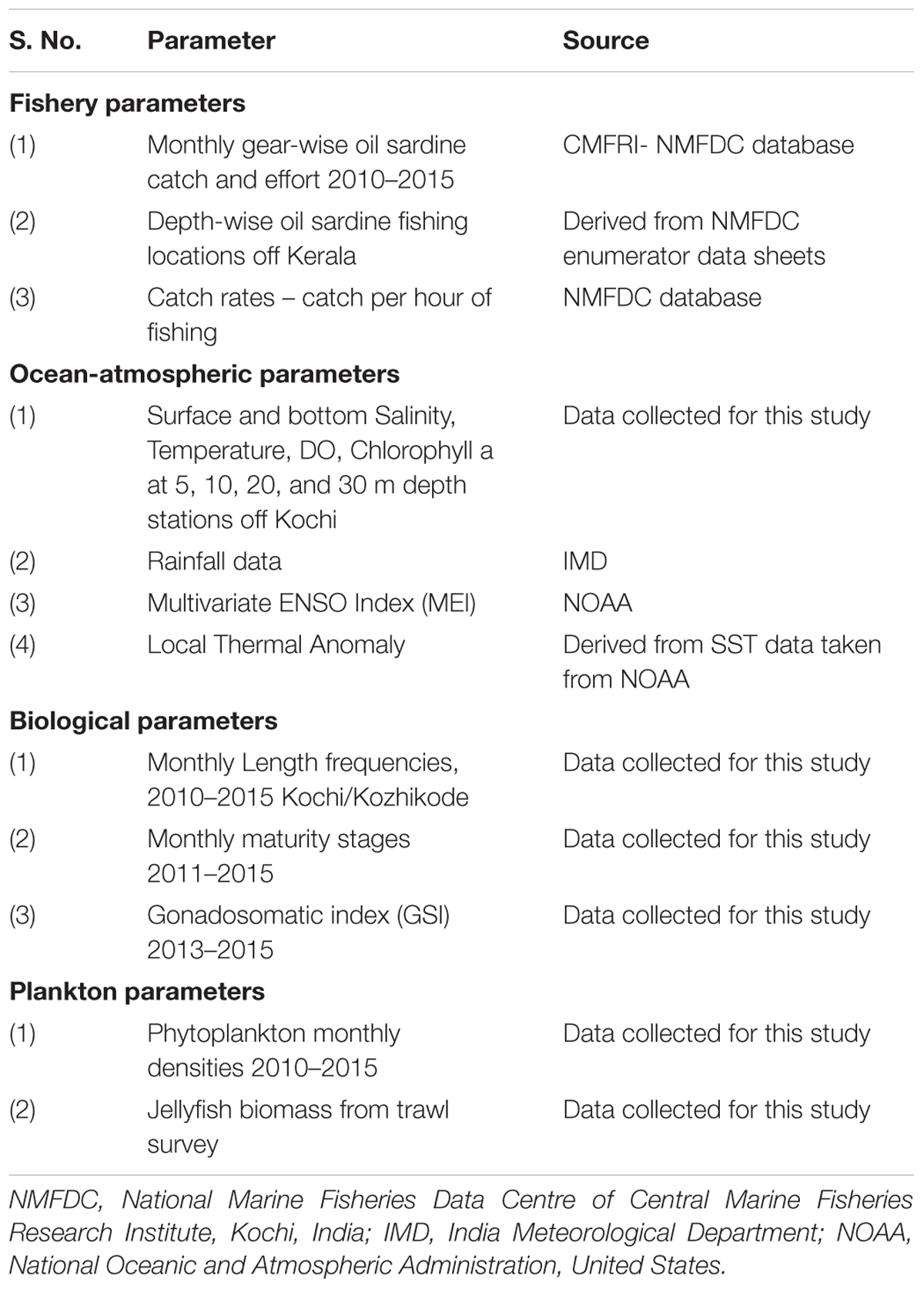
Details of datasets pertaining to sardine fishery, ocean-atmospheric parameters of the fishing grounds, fish biology and phytoplankton densities are given in Table 1 and Supplementary Table S1. The biological details of the fish from Kochi and Kozhikode, two major oil sardine landing centers (Figure 1) were used in the analysis. Hydrological sampling was done off Kochi (Figure 1). Oil sardines are distributed (Figure 1) as small and large shoals up to about 50–100 m depth, but are most common up to 30 m depth (Pillai et al., 2003).
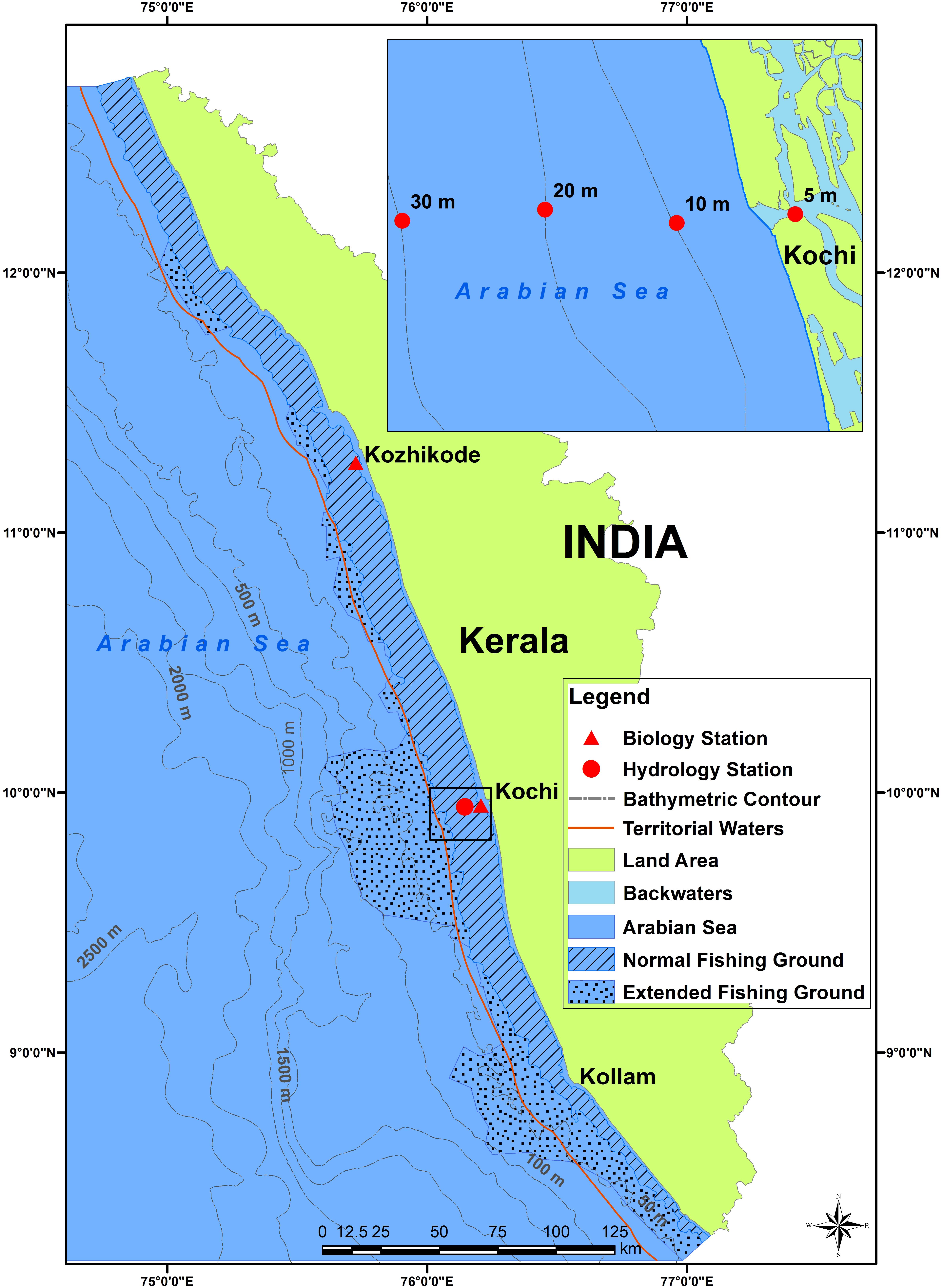
FIGURE 1. Map showing southeastern Arabian Sea off Kerala State with bathymetric contours. The hydrology and biology sampling stations are indicated. Inset shows detail of hydrology sampling stations. Shaded areas indicate the traditional oil sardine fishing grounds and the stippled area indicates the extended fishing grounds.
Oil Sardine Landing Data
Oil sardines are fished by different craft-gear combinations like, fishing boats with outboard engines using ring seines (OBRS), mechanized units using ring seines (MRS), outboard units using gill nets (OBGN), mechanized trawlers (MTN), outboard units using boat seines (OBBS), other motorized units (MOTRS). Outboard units are those traditional crafts fitted with outboard engines, while mechanized units have inboard engines. Estimation of catch and effort were made by using a sampling method, the multistage stratified random sampling design (Srinath et al., 2005). The method involves recording of catch and effort at each landing center by experienced observers for a 24 h period on a sampling day. Normally, 16–18 days in a month are selected at random for observation. The daily catch and effort from at least 10% of vessels were multiplied with total number of vessels on the observation day to arrive at daily estimates for 187 landing centers. The fishing effort is expressed by the number of unit operations by a craft-gear combination (unit), the fishing hours expended by the unit during the month, the man-hours expended by the units during the month. This daily estimate from different sampling days in a month were pooled and multiplied with number of fishing days to arrive at monthly estimates of catch and effort.
Catch data from 1960 to 2015 and catch per unit effort (CPUE) of sardines in various gears for the period 2007 to 2015 were used for the analysis. CPUE was used as an index of abundance in the absence of biomass data. Details on the gear used and the engine horsepower were collected through direct enquiry at the landing centers and fishing villages. Apart from this, the annual sardine catch data for the period 1960–2015 were used to categorize the stock into abundant, less abundant, declining and collapsed based on catch-based rapid stock status method (Mohamed et al., 2010) which is modified version of the method developed by Froese and Kesner-Reyes (2002).
Oil Sardine Biology
Weekly random samples of sardines (N = 100) were collected from the ring seine catches from the landing at Kochi and Kozhikode for biological parameters during 2010–2015. Sardines were measured (N = 31,200) to the nearest mm using a scale and weighed in an electronic balance (nearest 0.1 g) after blotting the water from the surface of the fish. The length frequency data, were grouped into three viz., less than 10 cm, 10.1–14 cm, and above 14 cm, assuming that sardine less than 10 cm are immature juveniles, 10–14 are maturing sardines and above 14 cm are adult mature sardines based on previous reports on the size at first maturity (Jayaprakash and Pillai, 2000; Nair et al., 2016). The length data for each month were pooled from both the centers and the percentage contribution for each group was estimated and then monthly sardine length frequencies were weighted to the catch. These were used to derive monthly mean, minimum and maximum lengths.
Maturity was estimated by the macro and microscopic observation of 6104 male and female gonads. The female gonads were staged based on a modified ICES scale proposed by Raja (1969) and Zaki et al. (2012) as immature (stage 1), developing virgin (stage IIa), maturing (stages III and IV), mature or gravid (stages V and VI), partially spent and spent (stages VIIa and b) and spent resting (stage IIb) (Table 2). For analysis the data were grouped into mature (Stages III to IV); partially spent (VIIa); spent (VIIb) and spent resting (IIb). The gonadosomatic index (GSI) for females was calculated using the formula: GSI = (weight of gonad × 100)/weight of fish) every month and mean values were plotted.
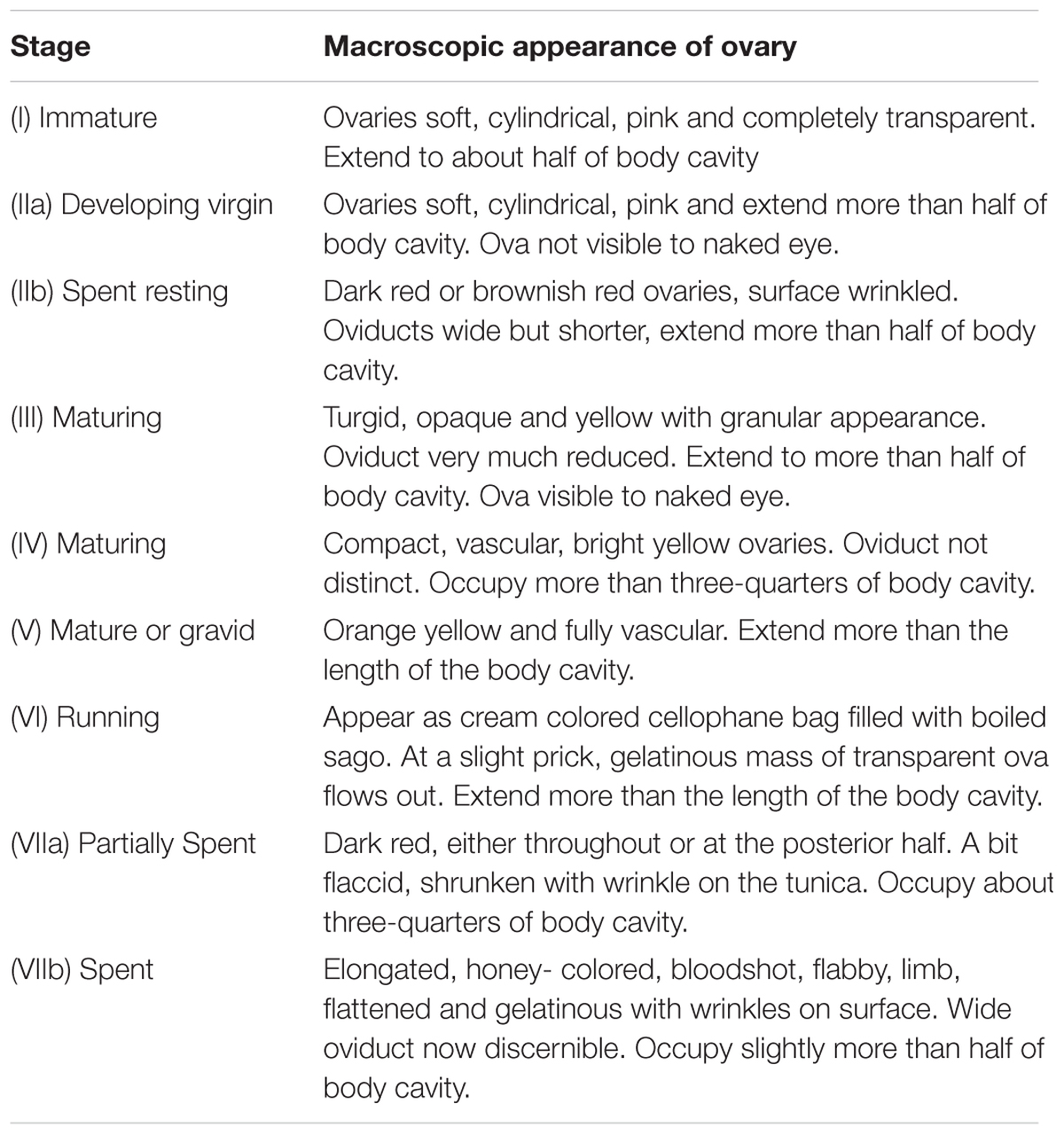
TABLE 2. Maturity staging of female Sardinella longiceps by macroscopic analysis used in the study following Raja (1966).
Hydrography and Climatology of Oil Sardine Habitat
Water samples from sardine fishing area off Kochi were collected from four stations (surface and bottom at 5, 10, 20, and 30 m depth zones) at monthly intervals (Figure 1). This transect was considered as representative of the sardine fishing area off Kerala coast. Sea water temperature and salinity at different depths were recorded using a CTD probe (YSI Incorporated, United States). The water samples were fixed and analyzed as per standard methods for dissolved oxygen, chlorophyll-a (CHL) and nitrate (Strickland and Parsons, 1968; APHA, 1998). From the same stations phytoplankton (PHY) samples were collected using a 20 μm Indian Ocean Standard net with digital flow meter (KC Denmark, Denmark), fixed in buffered formalin and cells were counted in the laboratory after identifying the major species.
To analyze the variations of parameters that are closely related to upwelling viz., temperature, salinity (PSU), dissolved oxygen (DOX) and nitrate content, two dimensional surfaces of these parameters were generated with time (month) on x-axis and depth on y-axis for the observation station. The observations from 30 m depth station were about 25 km from the shore. Since in 2013–2014 the sardine fishing grounds were abound with coelenterate medusa, experimental trawling was done at these stations and the Hydrozoan and Scyphozoan medusa collected were identified and biomass estimated based on the swept area method (Pauly, 1983).
Monthly rainfall data (RNF) for Kerala state were obtained from the website of Indian Meteorological Department. Upwelling regions are known to have cooler waters and the difference in temperature between two locations along the same latitude has been considered as an indicator of upwelling (Jayaram et al., 2010). Based on this principle, Local Thermal Anomalies (LTA) have been used extensively to study the variation in upwelling all along the Indian west coast (Smitha et al., 2008; Shah et al., 2015). Using the equation LTA = Toff - Tcoast where Toff is the temperature of offshore grid (Longitude: 75, 76; Latitude: 10, 11) and Tcoast is that of a grid (Longitude: 72, 73: Latitude: 10, 11) nearer to the coast. Positive LTA values suggest coastal upwelling processes.
Multivariate ENSO Index (MEI) was estimated using six major observed variables over the tropical Pacific which were obtained from the NOAA web site1 (Wolter and Timlin, 1993). MEI gives the variations in El Niño/Southern Oscillation (ENSO) which is considered as the most important coupled ocean-atmosphere phenomenon causing global climate variability on inter-annual time scales.
Statistical Analysis
In order to relate oil sardine abundance and life history events with physical, chemical and climatological parameters, the data on fishery, ecology and biological characteristics were subjected to Pearson’s correlation and statistically significant correlations were identified. Principal component analysis (PCA) was applied to define the relationships between catch rate (catch per hour [CPH] in MRS and OBRS), recruitment (less than 10 cm fish, LT10), mature fish (greater than 14 cm fish, GT14) and environmental variables. All biological parameters were fourth root transformed and scaled before the analysis. The Pearson correlation matrices were created utilizing R (R Core Team, 2014) ‘Performance Analytics’ package and PCA was done using ‘factoextra’ package. Rows in the data frame with null values in any of the fields were discarded while carrying out the PCA.
To assess relative contribution of different parameters (RNF, MEI, LTA, PSU, DOX, CHL, PHY, GT14, and LT10) to CPUE, two multiple linear regression equations were fitted with CPHOBRS and CPHMRS as response variables and RNF, MEI, LTA, PSU, DOX, CHL, PHY, GT14, and LT10 as regressors. The relative importance of regressors, i.e., individual regressor’s contribution to the multiple regression model, were assessed using the Lindeman, Merenda and Gold method applied in the ‘relaimpo’ package in R (Lindeman et al., 1980; Ulrike, 2006).
Results
Fishery Dependent Factors
During the period 1961–2015, the sardine catch fluctuated between a low of 1554 tons in 1994 to an all-time peak of 0.39 million tons in 2012 (Figure 2). As per the rapid stock status method, the collapsed status was reached only once (1994); however, the stock status was depleted during 1986 and almost reached the depleted status twice in 1963 and 2015 (Figure 2). One significant observation was the abundant status of the stock during 2010–2012. In comparison to 2010, the landings increased by 24.2 and 54.1% in 2011 and 2012, respectively. The catch was significantly (P < 0.001) and negatively related to MEI, phytoplankton density (P < 0.01), chlorophyll (P < 0.05), and rainfall (P < 0.05) (Table 3).
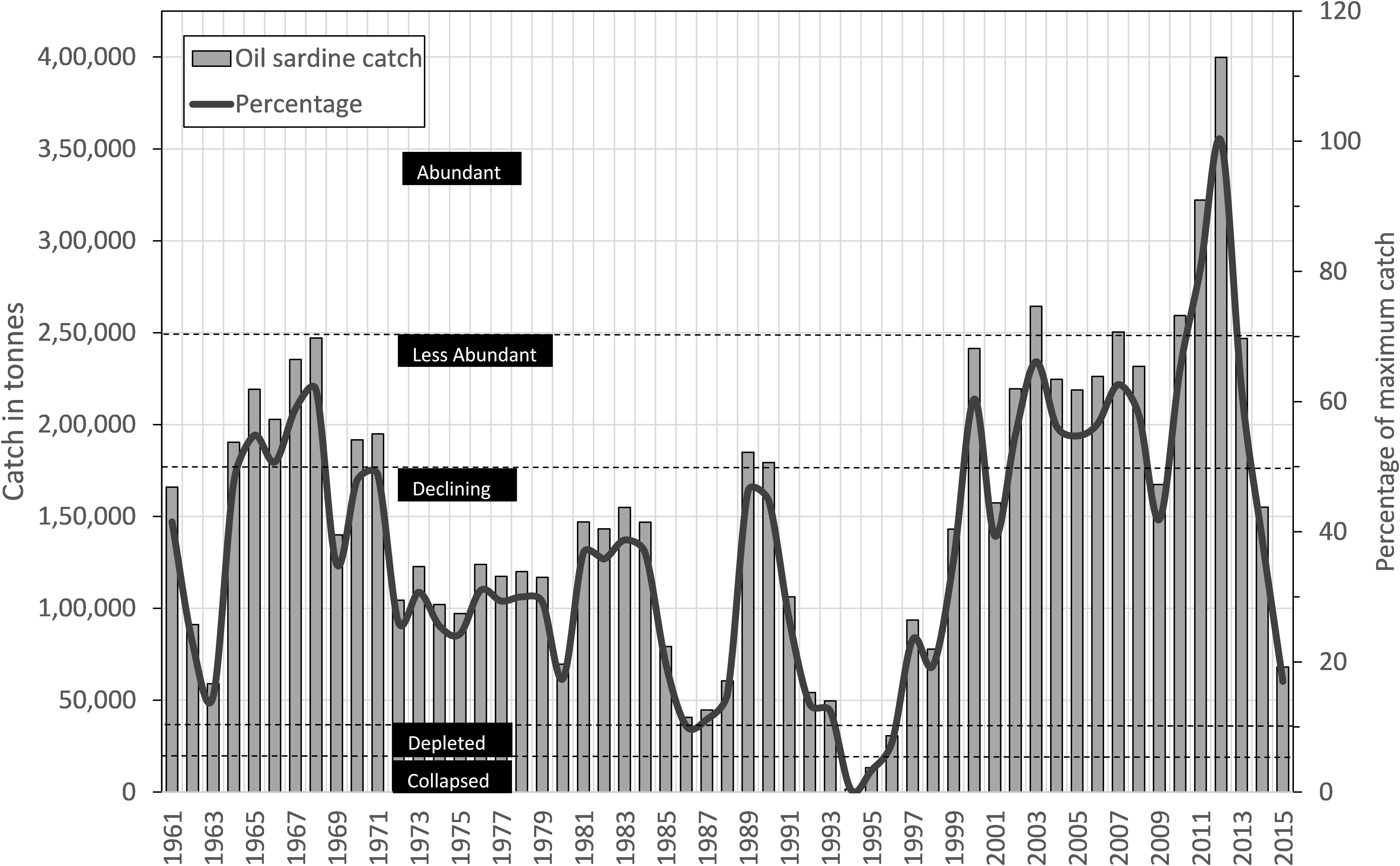
FIGURE 2. Time series of estimated oil sardine catch (bars) from Kerala State with percentage of historical maximum (line) and classification of stock-status based on the percentage.
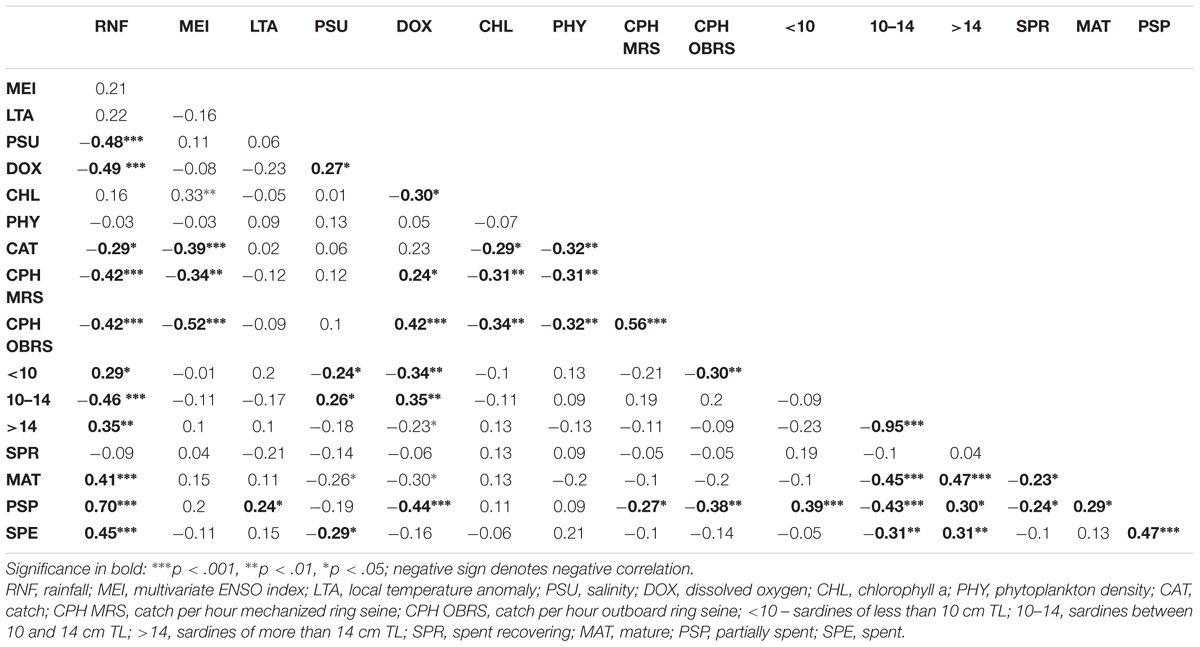
TABLE 3. Pearson correlation matrix (r-values and significance) between oil sardine catch, catch rates, size groups, maturity stages and environmental factors.
Increase in Effort and Catch Per Unit Effort
The oil sardines were exploited by a number of crafts including mechanized, motorized and non-motorized. The two principal gears exploiting oil sardines were MRS and OBRS and their percentage contribution to the total catch was inversely proportional (Figure 3). Mechanized pelagic trawl nets also caught considerable oil sardine during peak abundance period of 2012 and 2013. The percentage of oil sardine in non-mechanized decreased from 16% in 2007 to 6% in 2015.
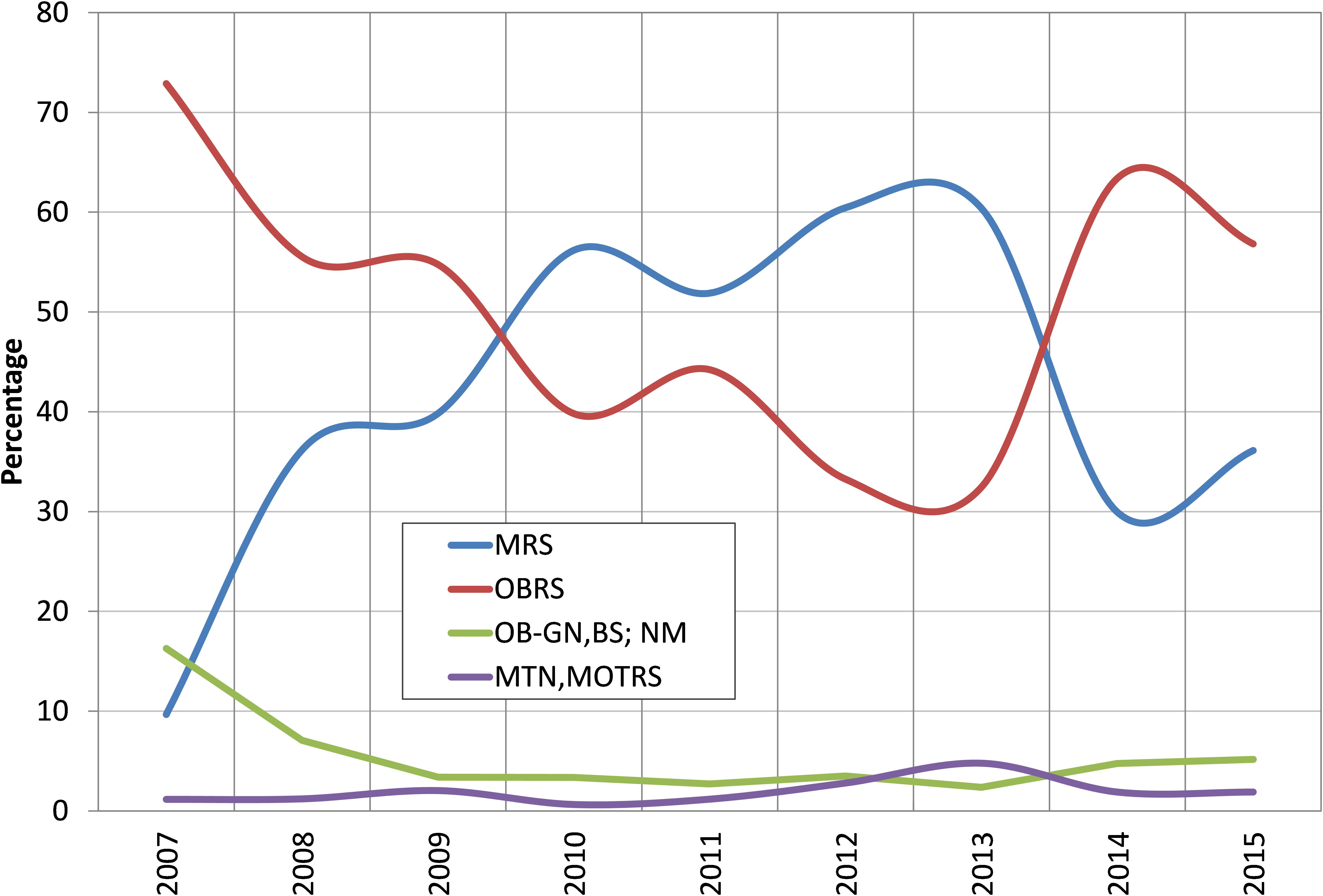
FIGURE 3. Percent contribution of different gears to oil sardine catches during 2007–2015. MRS, mechanized ring seines; OBRS, outboard ring seines; OB-GN, outboard gillnetters; BS, beach seines; NM, non-mechanized; MTN, mechanized trawl nets; MOTRS, mechanized other gears.
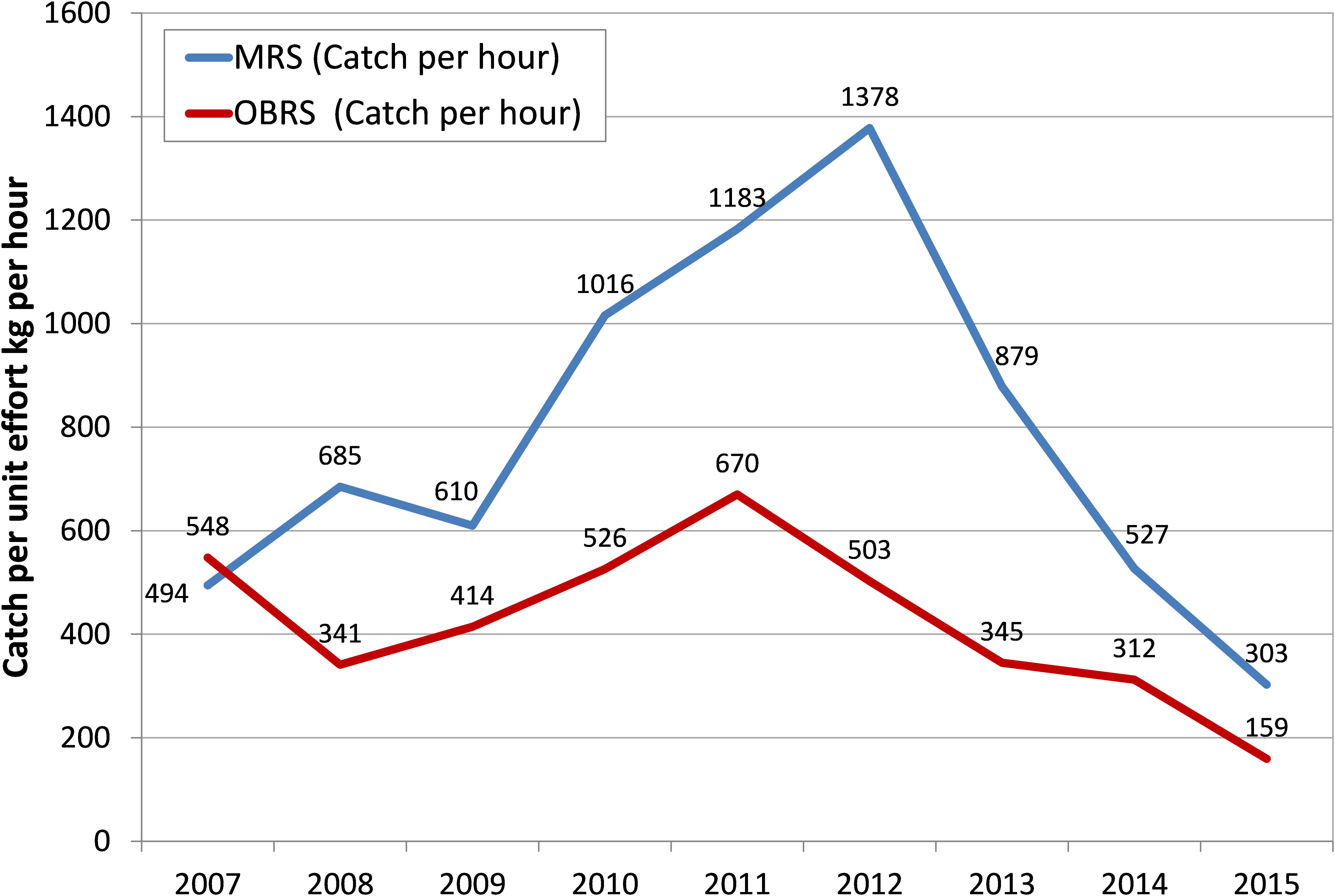
The effort in terms of number of MRS units increased from 20,152 in 2007 to 74,416 units in 2012 – 3.7 times increase over that of 2007 and the catch per hour of these units increased from an average of 494 kg h-1 in 2007 to 1378 kg h-1in 2012 and then declined to 303 kg h-1 in 2015 (Figure 4). The increase in catch during 2012 was primarily due to the increase in effort by these units. Though the OBRS unit operations increased during 2008, their numbers decreased subsequently. However, their unit operations increased marginally during 2012. The catch rate of OBRS units was high, 548 kg h-1 in 2007 which decreased to 341 kg h-1 in 2008. From 2009 onwards, the catch rate showed an increasing trend and reached 670 kg h-1 during 2011 and thereafter decreased steadily to 159 kg h-1 in 2015. During the period 2013–2015, the effort (not shown) as well as the catch rate of both MRS and OBRS units decreased considerably.
The catch rates in MRS and OBRS were negatively related to rainfall (P < 0.001), MEI (P < 0.001); chlorophyll a (P < 0.01) and phytoplankton density (P < 0.01). The catch rates in both MRS and OBRS were positively correlated with dissolved oxygen (P < 0.05; P < 0.00, respectively) (Table 3).
Fishing Beyond Conventional Fishing Grounds
Until 2013, the main fishing area for sardines was between the 5–30 m depth zones Figure 1. However, the MRS with high powered engines have fished in areas beyond the conventional sardine fishing zones mainly during March, May, and June which is the period when sardines mature and become ripe for spawning. Such fishing activities were mainly from the two main fishing ports of Kochi and Kollam.
Biological Variations
Length Based Analysis
The monthly length frequency analysis of the sardine catch indicated there was excessive harvesting of juveniles (less than 10 cm) size group during the period 2011–2013 (Figure 5). About 16,040 tons of juveniles (less than 10 cm) forming 4% of the total catch were harvested in 2012 and about 4802 tons in 2013. The 10–14 cm size group which is 0-year class forms a major component of sardine catches. The 1 year class (>14 cm) formed the maximum percentage in 2010, 2011, 2012, and 2014 (Figure 5).
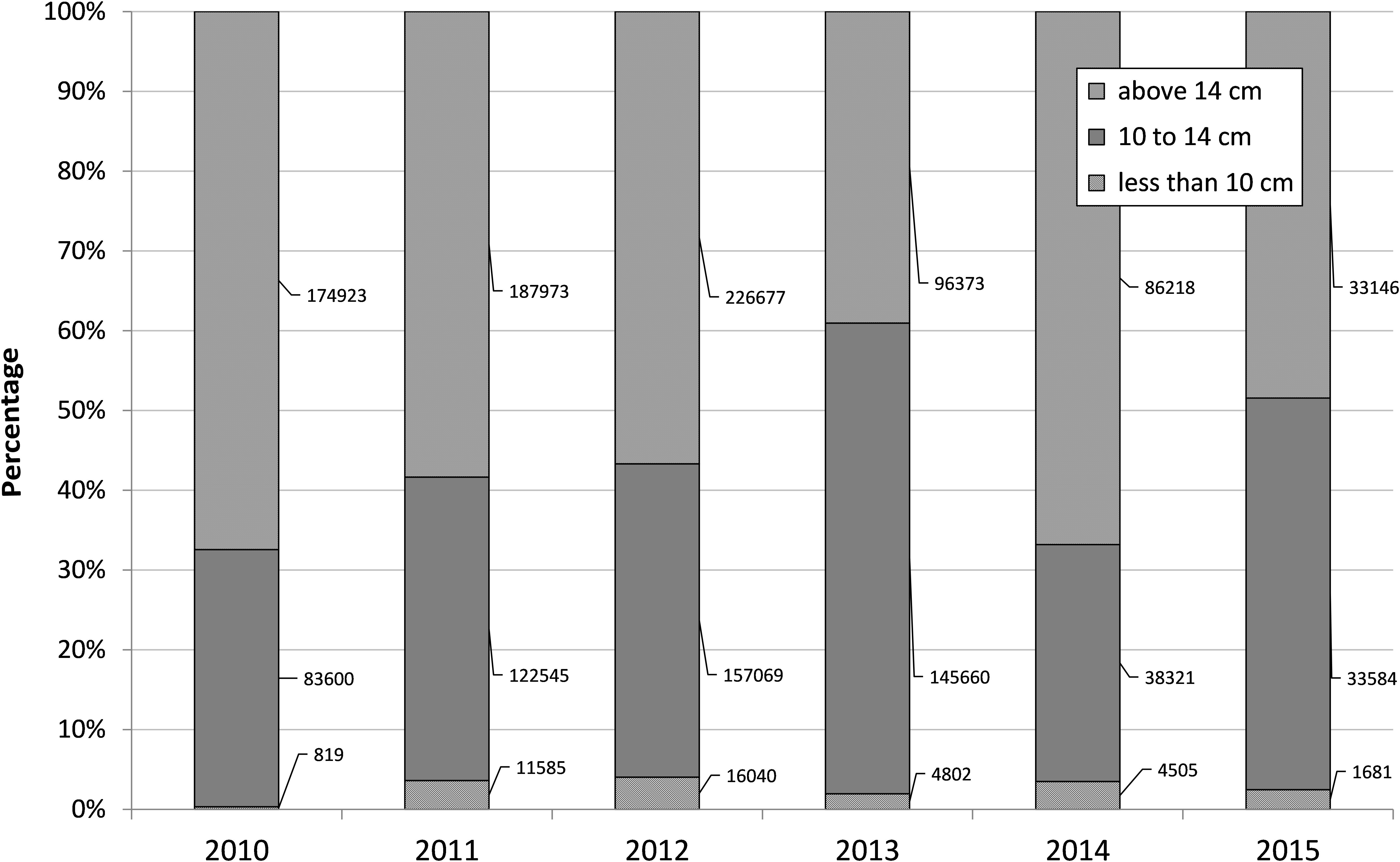
FIGURE 5. Percent contribution of the three size groups of oil sardine to annual catch during 2010–2015. Figures indicate size group wise tonnage.
Juvenile sardine (<10 months) and 1+ year group sardine catch were significantly and positively correlated with rainfall (P < 0.05) and negatively correlated with salinity (P < 0.001) and dissolved oxygen (P < 0.01). While the 10–14 cm TL group catches were negatively correlated to rainfall (P < 0.001) and positively correlated to salinity (P < 0.05) and dissolved oxygen (P < 0.01). The 10–14 and above 14 cm groups were inversely correlated (P < 0.001) (Table 2).
The monthly mean, maximum and minimum lengths during 2010–2015 are shown in Figure 6. The mean lengths fluctuated between 10.0 and 17.5 cm. The decrease in the mean lengths coincided with peak recruitment in those months. The variations in minimum lengths indicated that peak recruitment took place only once a year invariably during July to September. In 2011, two peak recruitments were noticed, one in February and another in July. The maximum lengths varied between 12 and 22 cm but were mostly above 15 cm.
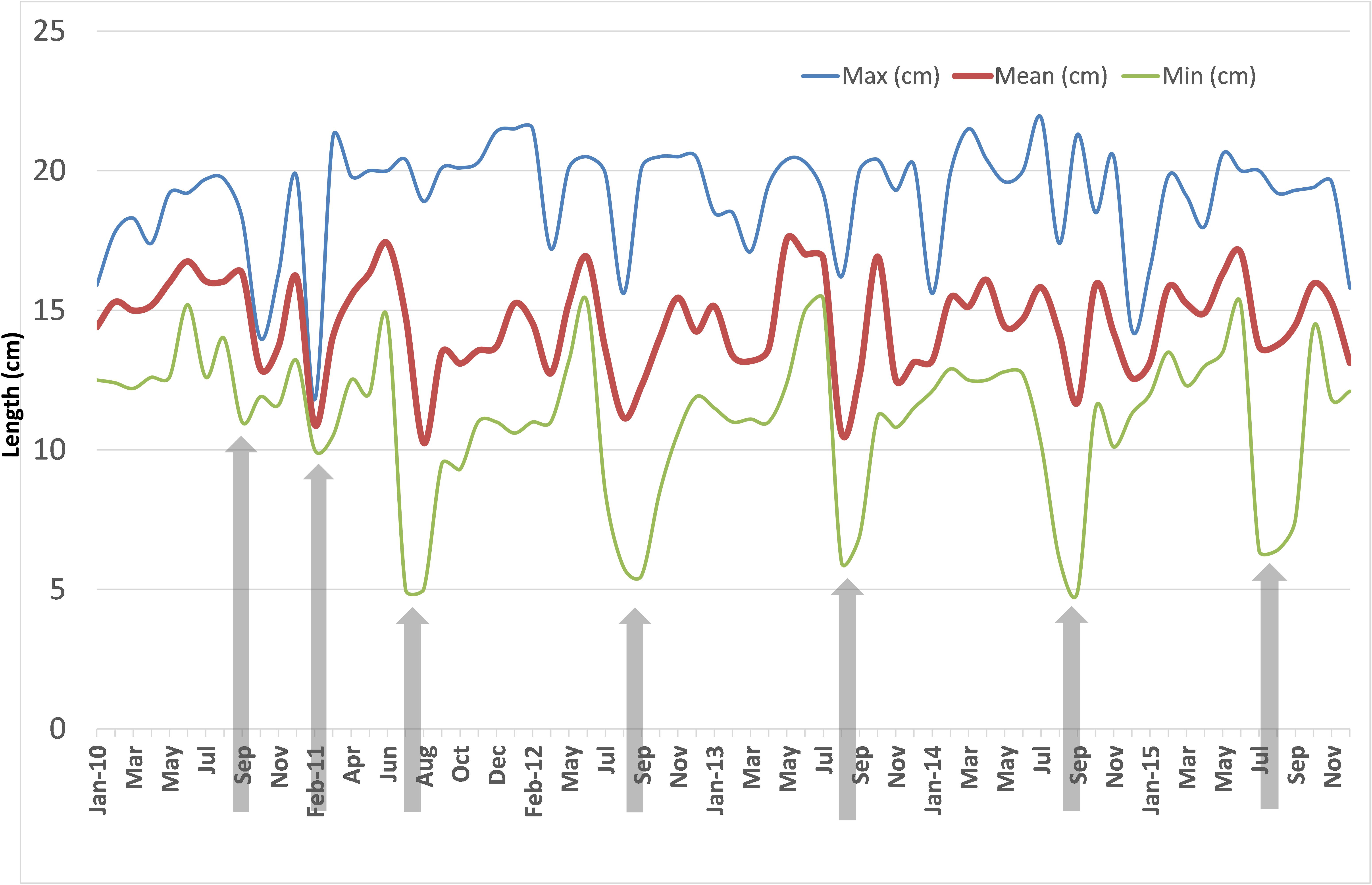
FIGURE 6. Monthly mean, maximum and minimum size of oil sardine in fishery catches from January 2010 to December 2015. Arrowheads indicate period of peak recruitment as judged from minimum sizes.
Sardine Reproduction – Maturity, Spawning and Recruitment
Sardines were found to mature and spawn from May/June to August/September in most years although there were wide inter-annual variations in initiation of maturation and peak spawning activity. The percentage of different maturity stages in the sardine population is given in Figures 7a–f. The GSI during 2013 to 2015 is presented in Figure 8.
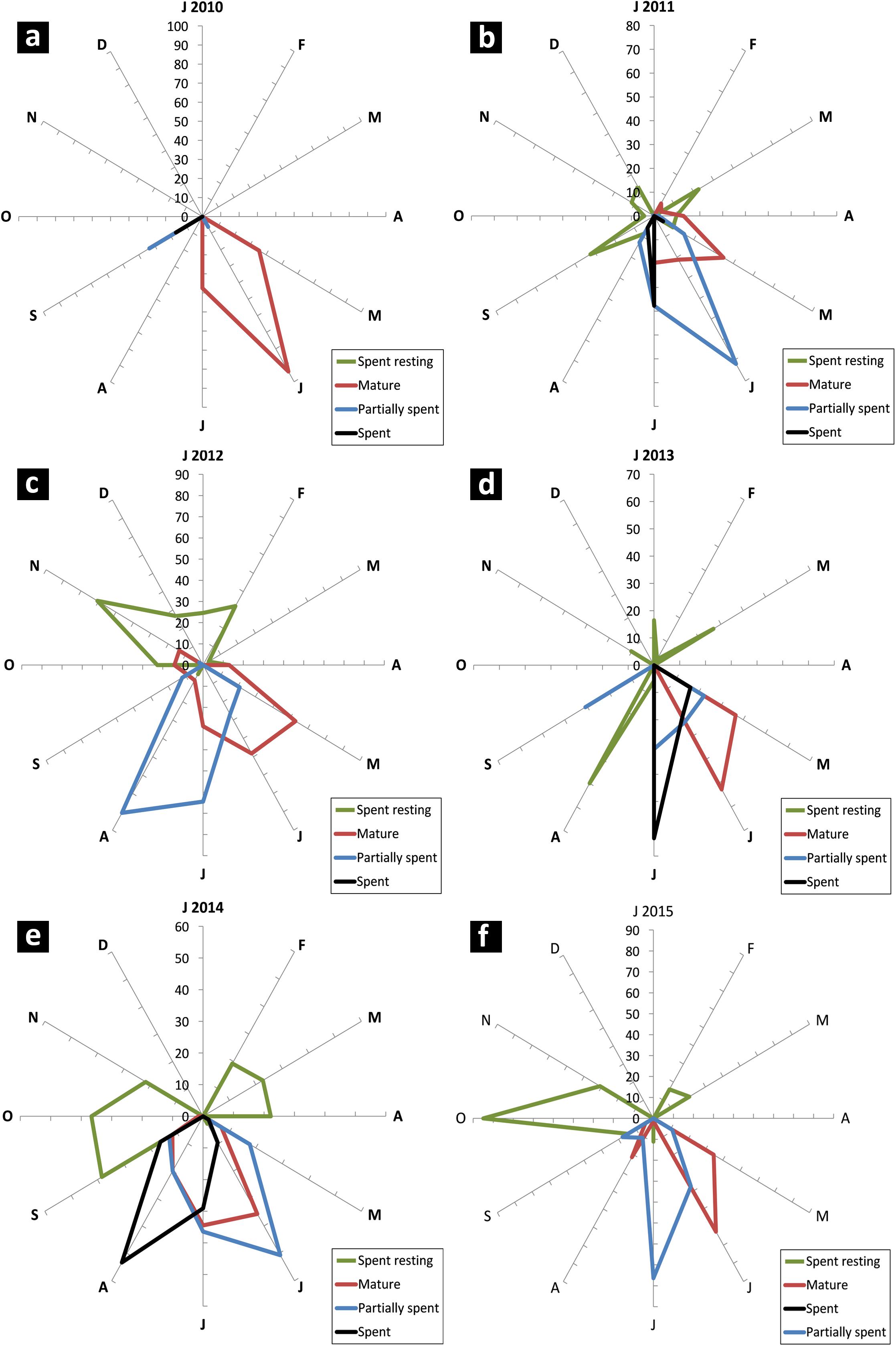
FIGURE 7. Radar chart of monthly female maturity stages (in percent) of oil sardine from 2010 to 2015 (a–f). Maturity stages important for indicating peak breeding and spawning are shown. Due to overlap, some stages are not visible.
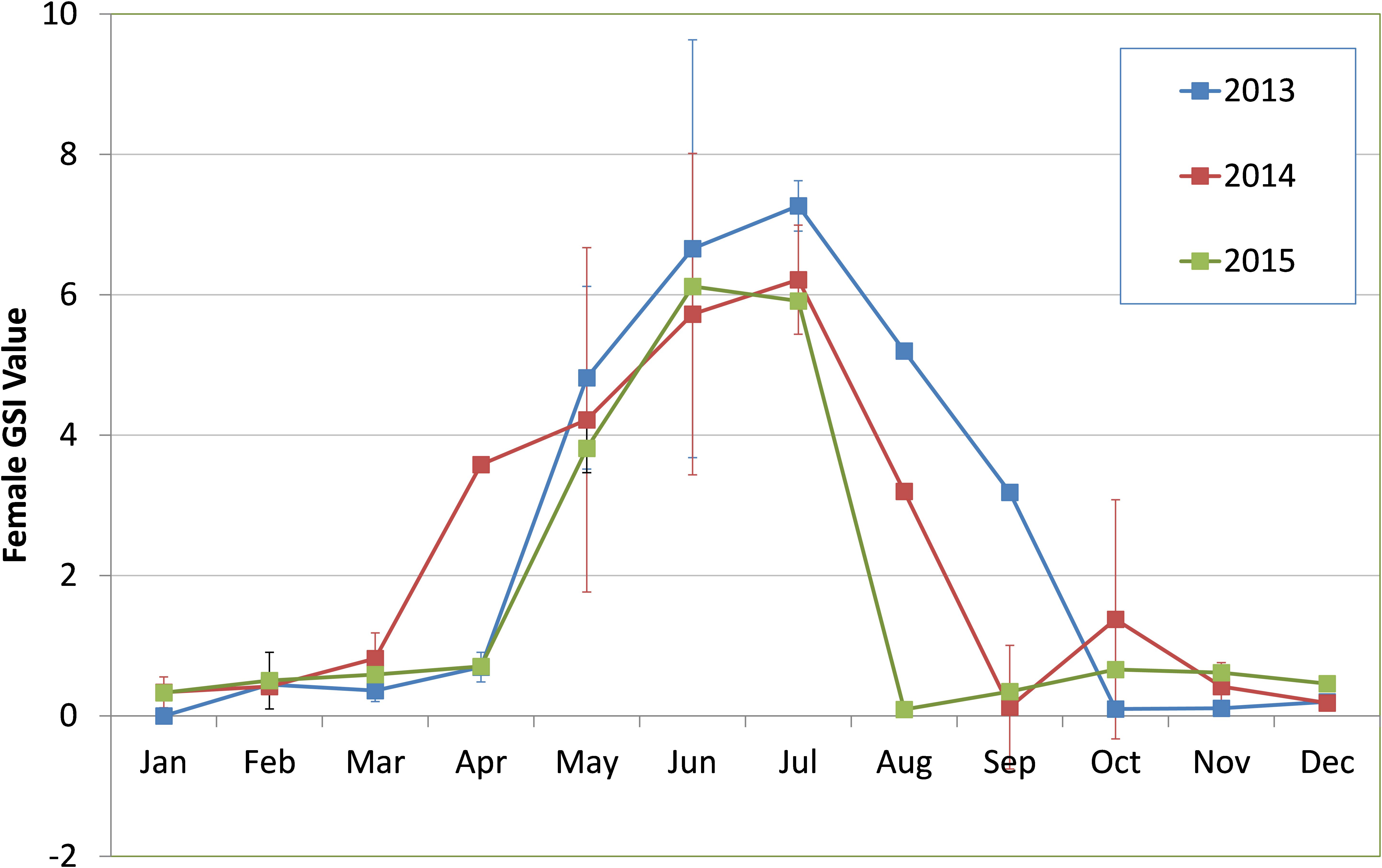
FIGURE 8. Monthly trend in mean gonadosomatic index (GSI) values of female oil sardines during 2013–2015. Vertical lines indicate standard deviation from the mean.
In years in which the fishery was a success (2010–2013), the female maturation process was broad starting from May and strong (more than 50% of population). In a departure from this pattern, in 2014 and 2015, the maturation process was narrow and erratic with two pulses in 2015.
In 2014, though there was maturation from May onwards, the percentage mature did not exceed 40% of the population. Spawning may have taken place during 3rd week (17th and 18th of June) as inferred from the high GSI values which fell to 3.1 by end of June. However, within a month, the GSI increased and remained high throughout July as indicated by more number of partially spent sardines during the spawning period. Though recruitment was observed in July, peak was observed to be slightly delayed, shifting to August and September as inferred from the low mean lengths (Figure 6).
During 2015, though the gonads matured by May/June, the gonadal maturation was not complete as indicated by the low GSI values (Figure 8). In 2013 and 2014, the GSI values were very high indicating good maturation, but in 2015, the highest GSI value, 6.12 was observed during end of June (Figure 8). This low GSI decreased further to below 1, indicating that maturation was poor and incomplete. Another significant observation was the large percentage of spent resting sardines in 2015. Completely spent females did not contribute to a significant percentage.
The mature, partially spent and spent females were positively correlated (P < 0.001) to rainfall (Table 2). They were also negatively correlated to 10–14 cm TL size groups. The PSP were positively correlated to LTA (P < 0.001) and negatively correlated to dissolved oxygen. The PSP were also negatively correlated to catch rates of MRS (P < 0.05) and OBRS (P < 0.01).
Environmental Variations and Impacts of Ocean-Atmospheric Phenomena on Sardine Habitat
Variations in Hydrography
The major sardine habitat in the coastal waters with depth from 5 to 30 m is characterized by wide environmental fluctuations. This region is influenced by upwelling and also by the river runoff due to monsoon. The variations in temperature, salinity, dissolved oxygen, and chlorophyll-a content of the sardine habitat are shown in Figure 9.
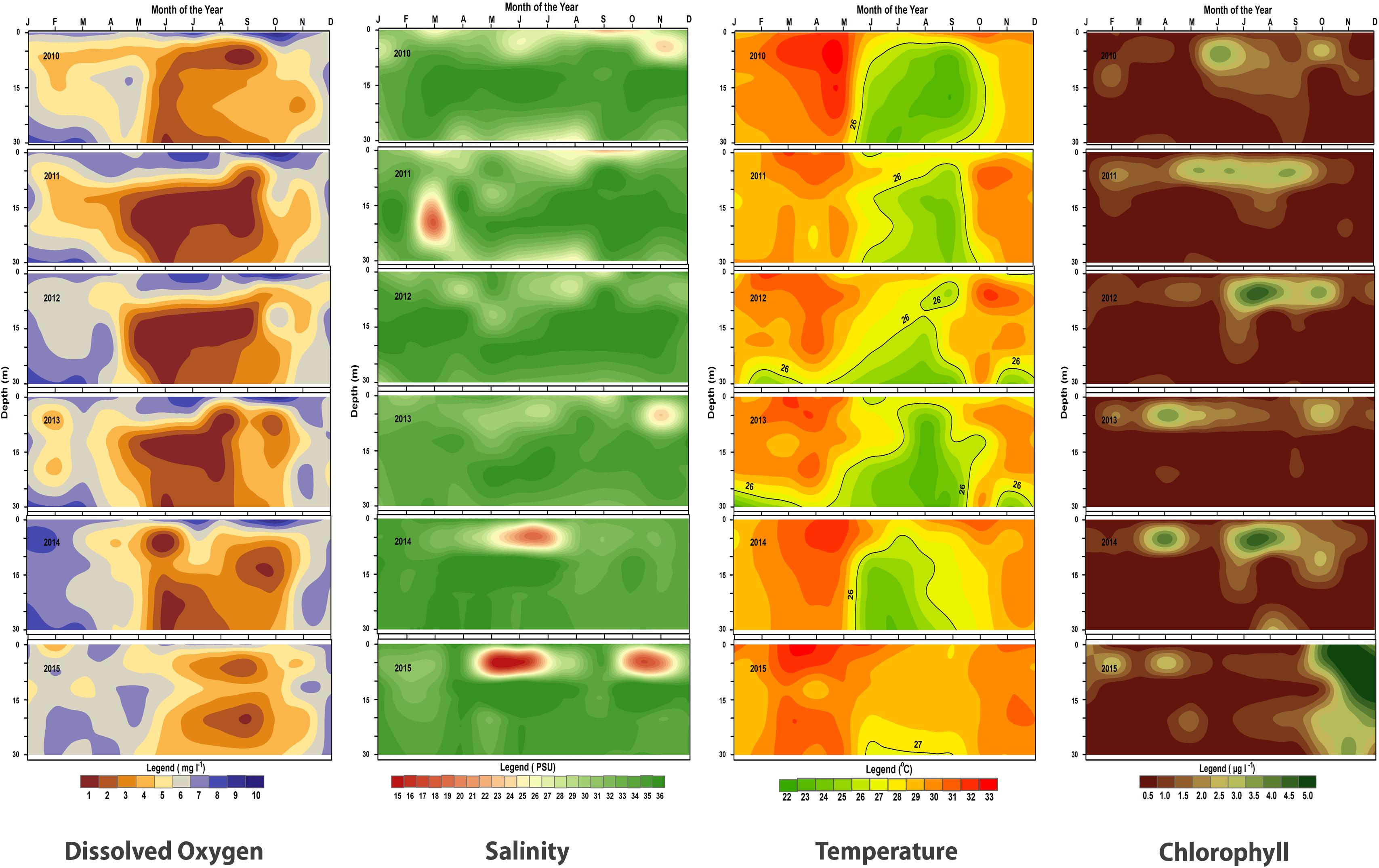
FIGURE 9. Contour plots of monthly values of dissolved oxygen, salinity, temperature and chlorophyll-a at different depths (0–30 m) during 2010–2015 in the oil sardine fishing grounds off Kochi, southeastern Arabian Sea.
The upwelling strength was highly variable in all years with strong and sustained upwelling as indicated by low temperature and low dissolved oxygen values observed during 2010–2012 (Figures 9, 10). The temperature difference between the surface and bottom waters (deltaT) was high during 2012 and there seemed to be fairly cool conditions for an extended period which is reflected in the estimated LTA values. The duration and intensity of upwelling was also high during 2012 compared to that of subsequent years. During the subsequent period (2013–2015), the upwelling strength was greatly reduced.
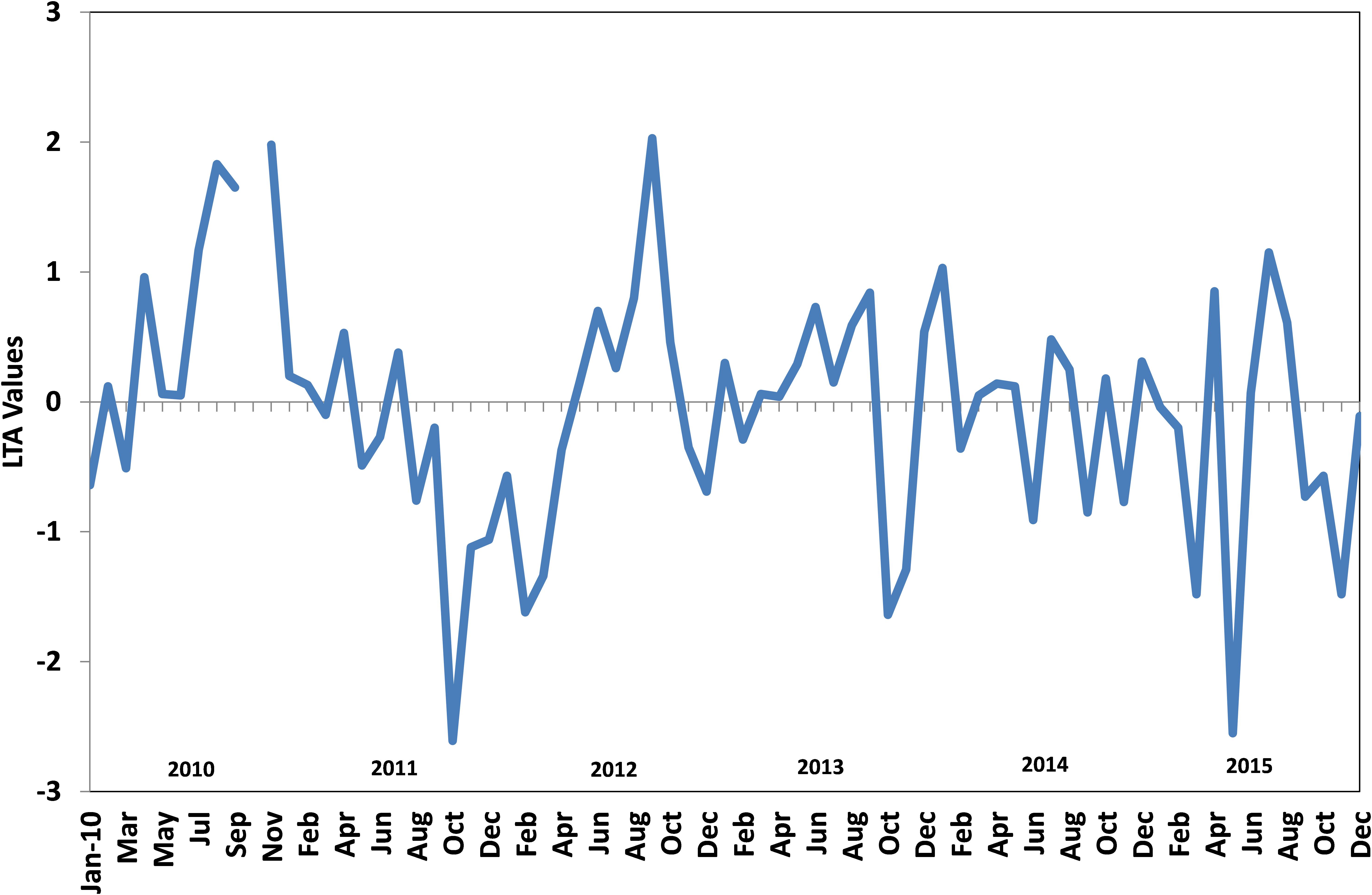
FIGURE 10. Monthly Local Thermal Anomaly (LTA) values from 2010 to 2015 in the oil sardine fishing grounds off Kochi, southeastern Arabian Sea.
In September, 2013 the surface waters were low saline while the bottom waters were high saline, which would have led to salinity stratification. In 2014, upwelling was weak throughout the year. The in situ observations show that the temperature of bottom water were below 25°C during July–August. By June, the dissolved oxygen values decreased in the 10–30 m area and the salinity remained high indicating limited upwelling in the shelf area. However, at 5 m depth the salinity had dropped due to rainfall runoff.
Upwelling was generally weak in 2015 and not observed in 5, 10, and 20 m depth zones. At 30 m depth slight lowering of temperature (26.6°C) was observed in July and as per LTA data there was upwelling in July. However, the extent and the intensity was low. Low oxygen conditions were observed but were not severe as compared to previous years (Figure 9). The temperature during this period was above 27°C from the surface to 20 m depth all through the year. Moreover, in 2015, ΔT was low (max = 4.4°C) indicating poor upwelling. The average temperature in the sardine fishing grounds during 2015 were nearly 1.1°C higher than that observed during the period 2010–2014.
Variations in Monsoon
The variation in monsoon and departures from normal during June to September was found to be different during all years particularly in 2013 and 2014. The rainfall during June and July of 2013 was 60 and 14% more than the normal. Monsoon was deficient during June/July of 2014 but 74 and 22% more during August and September.
The total rainfall showed a negative deviation from normal during 2012 (-24%) and 2015 (-26%) and was above normal in 2013 (+26%) and near normal in 2011 and 2014. When the deviation trend in monthly rainfall during monsoon (June to September) was plotted it showed wide fluctuations (-50% to +74%). The widest deviation from normal was observed in July, August, and June rainfall. After 2012, the deviation in September rainfall was moderate and was mostly positive.
Changes in El Niño/Southern Oscillation – ENSO
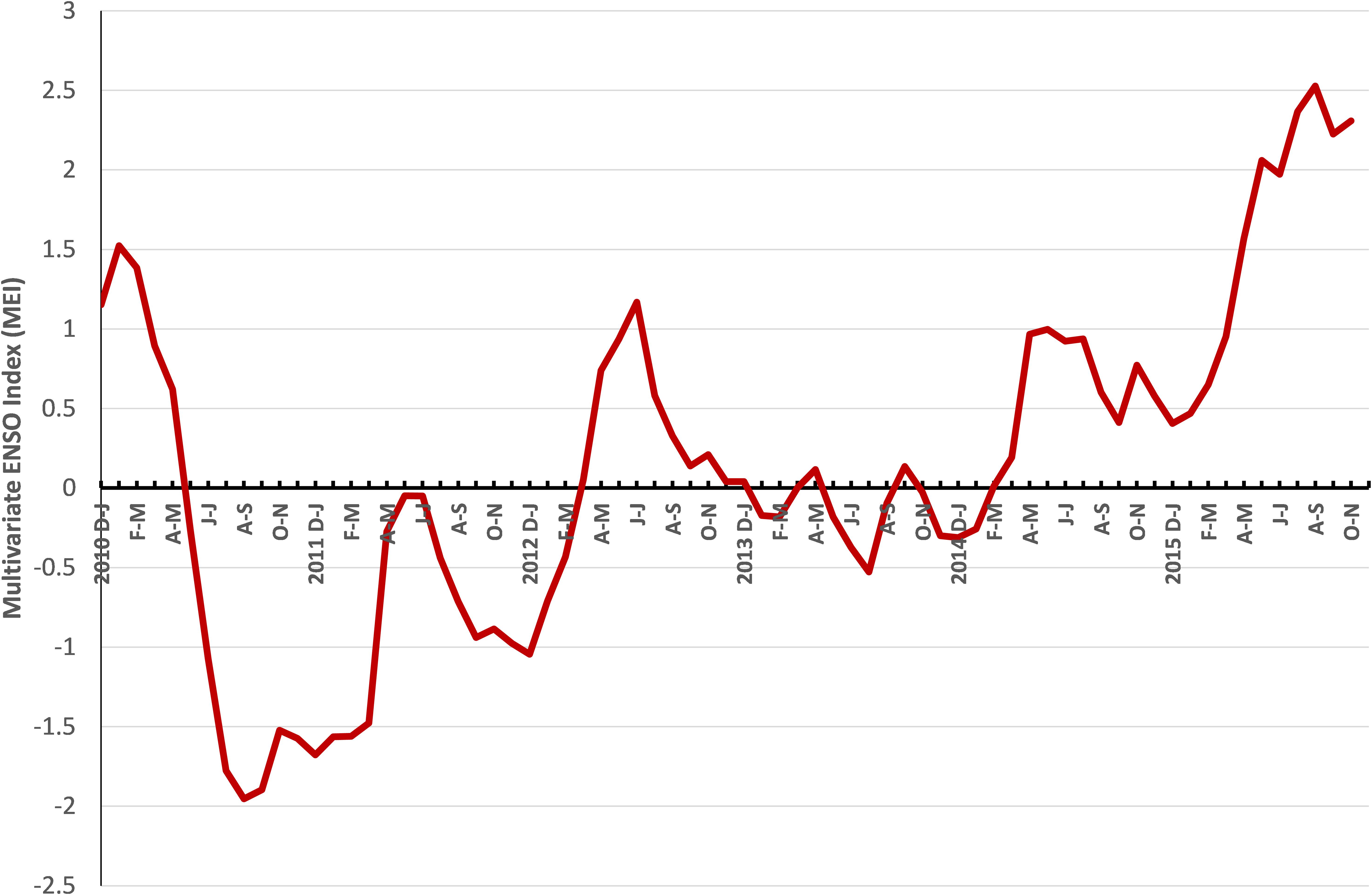
ENSO is the most important coupled ocean-atmosphere phenomenon that causes global climate variability on inter-annual time scales. The multivariate ENSO index (MEI) plotted from 2010 to 2015 showed that the index which was positive during the early part of 2010 declined and remained negative from June–July 2010 to April–May 2012 (Figure 11). Subsequently the MEI fluctuated without much deviation until February–March 2014, and then increased to high levels in August–September 2015.
Phytoplankton Densities
The phytoplankton cell densities in sardine fishing areas fluctuated between 1.57 × 104 cells l-1 in April 2013 and 3.1 × 106 cells l-1 in March 2014. The average values were high in 2011 and 2012, declined in April 2013 and then reached very high densities from June 2013 to December 2014. These high values were primarily due to phytoplankton blooms of diatoms species such as Skeletonema, Chaetoceros, Bacteriastrum, and Fragilaria, besides harmful dinoflagellate blooms of Trichodesmium (April 2014) and Notiluca (September 2013). In 2010, 2011, and 2012 there were phytoplankton density peaks during monsoon.
Larval Predators - Jellyfishes
During June 2013 and Aug–September 2014, in the inshore surface and column waters of the main sardine fishing area from 5 to 30 m depth zone, jellyfish blooms were observed. The Hydrozoan jellyfish (Aequorea pensilis), Scyphozoan jellyfish (Lychnorhiza malayensis) and crown jellyfish (Netrostoma coerulescens) were observed in the sardine habitat at biomass of 1483, 918, and 4625 kg.nm-2 during June 2013, August and September 2014, respectively. Predation by these macro-plankters would also have affected oil sardine recruitment.
Principal Component Analysis and Relative Importance Model
Principal component analysis results (Figure 12) indicated that the abundance of sardines in both MRS and OBRS showed similar relations. Sardine abundance, recruitment and female maturity were more influenced by rainfall, upwelling (LTA), chlorophyll, MEI and salinity. The influence of dissolved oxygen and phytoplankton density were relatively low. The first 3 axes explains the majority (72.8%) of the variance in the data as indicated by the Eigenvalues (Table 4).
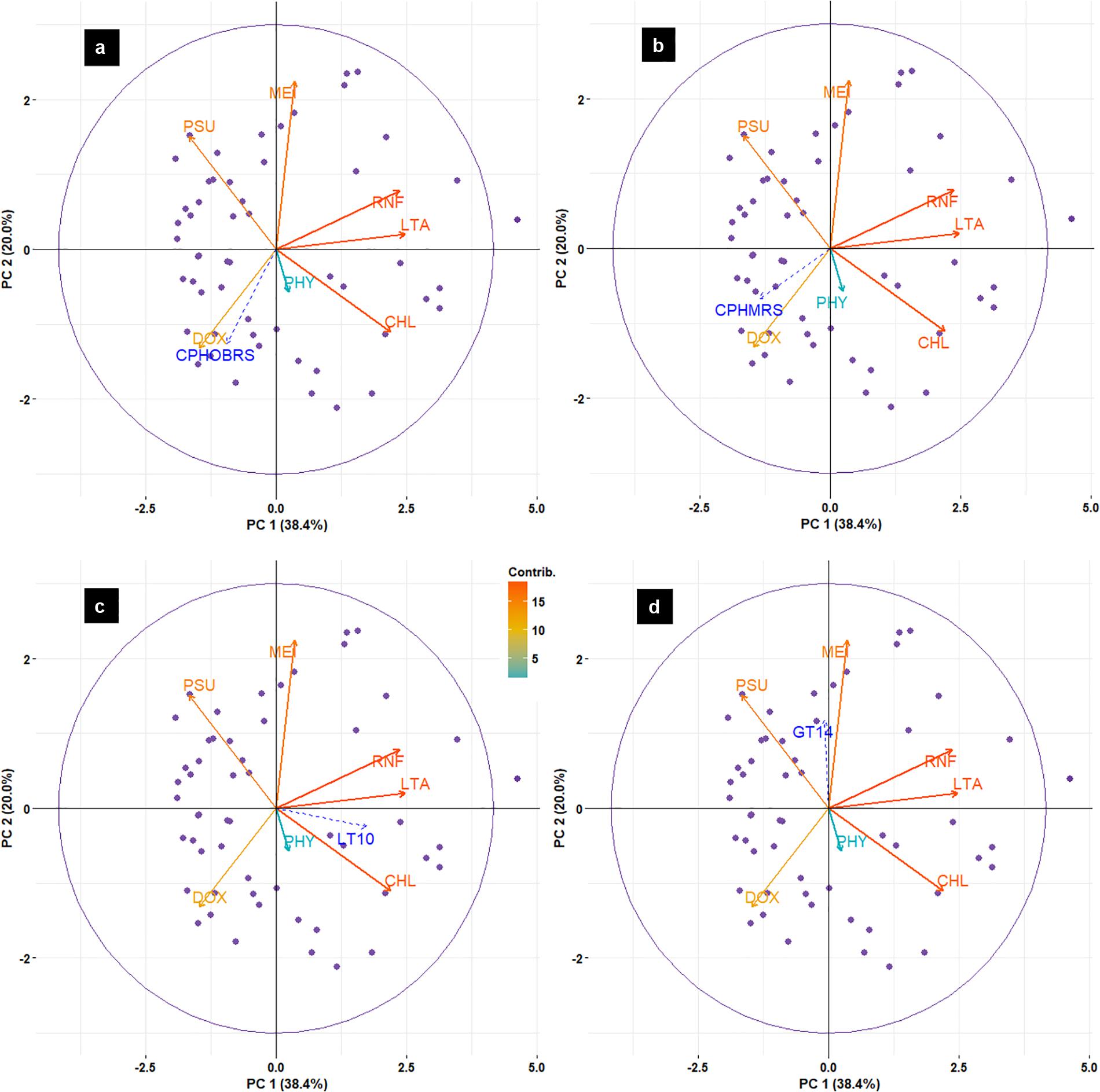
FIGURE 12. Biplot of principal component analysis (PCA) for environmental data and oil sardine abundance [CPHOBRS (a) and CPHMRS (b)], recruitment [LT10 (c)] and maturity [GT14 (d)] in southeastern Arabian Sea.
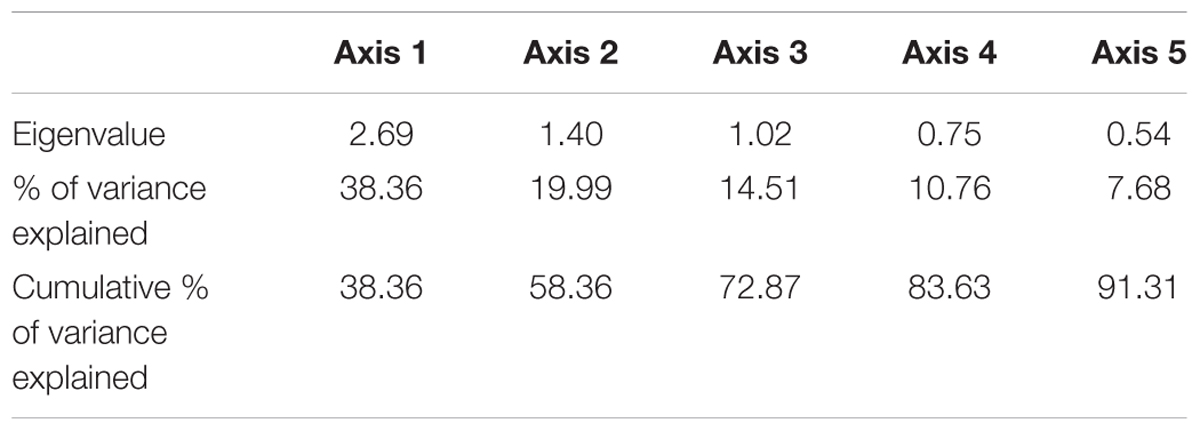
TABLE 4. Eigenvalues and the explained variance and cumulative variances of the principal components limited to 5 axes for oil sardine environmental data in southeastern Arabian Sea.
The assessment of relative contributions of different parameters/regressors to the fitted multiple linear regression model indicated that the CPUE in OBRS was influenced by MEI, PHY, LT10, DOX, and LTA in decreasing order (Table 5). Other parameters showed minimal influence. In MRS, the major regressors which influenced the CPUE were MEI, PHY, RNF, LTA, and DOX in decreasing order (Table 5). The proportion of variance explained by the model for CPHOBRS was 59.7% and for CPHMRS was 47.9%.
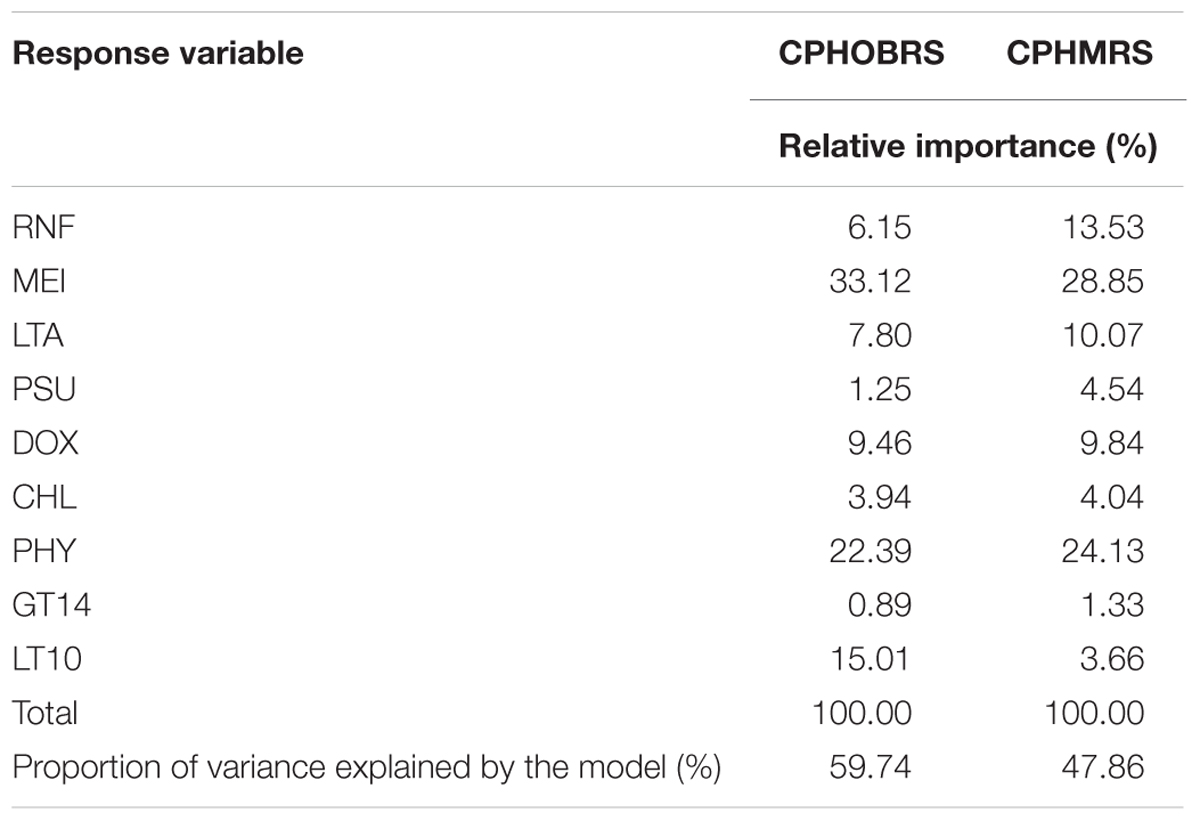
TABLE 5. Percentage of variance in CPUE (CPHOBRS and CPHMRS) explained by the parameters rainfall (RNF), multivariate ENSO index (MEI), local thermal anomaly (LTA), salinity (PSU), dissolved oxygen (DOX), chlorophyll a (CHL), phytoplankton (PHY), greater than 14 mm fish (GT14) and less than 10 mm fish (LT10) and the percent contribution of each variable to the fitted regression model.
Discussion
Fishery Dependent Factors
Change in Gear Length/Depth and Engine Power
An assessment of the ring seine fishery of Kerala using time series data on catch and effort during the period 1984–2004 through surplus production model had indicated that the fishing was close to the state of equilibrium in 2007 (Balan and Sathianandan, 2007). However, even after 2008, more units were operated and more intense fishing was carried out. The MSY estimated was 0.23 million tons (Balan and Sathianandan, 2007) and the landings exceeded these figures during 2010 and 2011 before peaking to 0.39 million tons in 2012. Earlier MSY estimates of oil sardine for the entire west coast were much lower indicating the fluctuating nature of the sardine biomass (Sekharan, 1974; Annigeri et al., 1992).
In the present investigation, overfishing is identified as one of the reasons for oil sardine fishery decline and the modifications made in the gear, increase in effort and area of operation have contributed to overfishing. In India, since last one and half centuries, sardines have been fished from coastal waters mainly using seines (ICAR, 1971; Pillai et al., 2003) but recently these have been enlarged in size.
In the recent past, the gear dimensions were increased. The length and width of these seines increased from about 42 m × 5.2 m in 1960s to 620 m × 100 m in 1990 and to about 900 m × 90 m in 2004 (Edwin et al., 2010). The engine capacities were increased and the number of units also exceeded the number recommended by fisheries institutes (Edwin et al., 2010; Bhoopendranath and Hameed, 2012). Our observations indicate that the engine capacity of the motorized country crafts were more than doubled to 65 hp while the inboard engine crafts were nearly doubled to 190 hp. The increased catch during 2012 can be attributed to the increased efficiency in fishing gear leading to increased fishing effort and extension of fishing grounds.
The increase in gear length would have led to increase in efficiency and more volume of the shoal would have been trapped by the encircling gear. The size of sardine shoal has been estimated to range from 2 to 20 m in length and depth (Balan, 1961) and their speed as 5 km hr-1. Smaller shoals are also known to make rippling sounds and join to form larger shoals and larger gears can completely encircle the shoal.
Increase in Effort and Catch Per Unit Effort
During the period 2013–2015, the effort as well as the CPUE of both MRS and OBRS units decreased considerably which forced the units to abstain from fishing in 2015. In fact, the increase in effort during 2012 should have resulted in low CPUE if the sardine biomass was low. However, the CPUE was not affected in 2011 and 2012 mainly because there was high abundance of sardines in the coastal waters. The reason maybe the favorable environmental conditions that led to successful recruitment which led to high biomass. The sardine biomass declined in 2013, 2014, and 2015 which is indicated by the low CPUE in spite of reduction in effort and this is clearly evident in the catch per hour obtained during the period by MRS and OBRS units. Similar decline in catch rates have been recorded in forage fishes and it has been observed that such population collapses shared a set of common and unique characteristics such as high fishing pressure for several years before collapse and a sharp drop in natural population productivity (Essington et al., 2015).
Fishing Beyond Conventional Fishing Grounds
Before modernization, the sardine fishing was restricted to about 16 km from the shore (Nair and Chidambaram, 1951). Subsequently, fishing was expanded to about 25 m depth zone along the coastline. In all the documents on sardine fishery during the 19th century to this century, it has been indicated that sardines are not available in near shore areas after March and they are presumed to migrate to inshore waters during June (ICAR, 1971). The present study indicates that sardines are available in areas beyond 30 m depth during the pre-monsoon season and exploitation of this hitherto unexploited portion of the stock would have affected stock regeneration.
Biological Variations
Length Frequency
During 2012, excessive exploitation of juvenile were observed and this would have affected the sardine population in the subsequent years. In recent years there was a decline in smaller size groups and the modal length increased indicating low recruitment which can also be due to unfavorable environmental conditions. Studies (Chidambaram, 1950) on the length frequency of oil sardine during 1936 to 1943, coinciding with high catch in 1930s followed by collapse of fishery in 1943, indicated that when immature sardines were caught in large quantities, it resulted in proportionate reduction in the number of spawners in the fishery in succeeding years. Thus it is inferred that the excessive capture of juveniles affected the stock to a considerable extent.
The fisheries-induced changes in age structure may impact the dynamics of the stocks in various ways (Rouyer et al., 2011) and in the present situation the exploitation of juveniles caused an imbalance in the age structure to a population that has already been impacted by the low productivity and high abiotic stress in the preferred habitat. All size classes of oil sardine were significantly influenced by environmental variables such as rainfall, salinity and dissolved oxygen.
Sardine Reproduction – Maturity, Spawning and Recruitment
The successful recruitment during 2012 can be attributed to the good maturation and successful spawning for a prolonged period (May–November). This can also be related to the comparatively low temperature in the sardine habitat during this period. Similar instances have been observed in Monterey Bay sardine Sardinops caerulea a temperature dependent species which forms a fishery on the west coast of North America. Upwelling and cold water have been correlated with higher catches and catch per unit of effort (CPUE) for the Monterey Bay sardine, whereas warmer water produced lower catch and CPUE, especially during an El Nino event (Lluch-Belda et al., 1986). It was evident that the favorable environment led to prolonged spawning and this was reflected in the year classes. Conversely, when warm waters spread the coastal areas as during an El Nino event, the Californian sardines restrict their movement and the catch rates decline. Several commonalities with the Californian sardines and Japanese sardines (Kawasaki and Omori, 1988) exist along the southwest coast of India. Earlier fluctuations in oil sardine fishery have been related to the surface temperature, specific gravity of seawater, availability of food, spawning and survival of the young ones (Nair and Chidambaram, 1951).
Our evidences show that in the oil sardine all reproductive activities were heightened during the comparatively cooler period and were very poor in 2015. Based on this, we conclude that Indian oil sardines are affected by El Nino as indicated by high and positive MEI values and the high negative correlation of catch rates with MEI. Though this is a tropical habitat, within the low range of temperature fluctuations, oil sardines prefer the cooler period than the warmer period. This preference for lower temperature may be the reason for sardine catches in deeper waters during March when the surface waters are comparatively warm. This can also be the reason for the disappearance of oil sardine from coastal surface waters during the period. They become partly demersal during the warm period and maybe that is the reason for their availability in mid-water trawls during the summer months.
The oil sardine fishery along the southeastern Arabian Sea is mainly by the 0-year class and this makes the fishery vulnerable to factors which control recruitment. The oil sardine fishery along Oman coast also depends on the current year’s recruitment (Al-Jufaili et al., 2006; Zaki et al., 2012). After a detailed analysis of the length frequency of sardine landed along Kerala coast during the period 1962–1966 it was concluded that the juvenile broods born during June to August support a good fishery while those born later in the year September/October fail to establish themselves due to weaker number (Raja, 1969). In 2013 and 2014 the oil sardine spawning was erratic and was extended to periods beyond August. The resultant broods would have been weak due to the dinoflagellate blooms in 2013 and the early chlorophyll maxima in 2014. The recruitment success was not strong as that of 2010, 2011, and 2012 when spawning and recruitment were early.
From the evidences gathered in the present study it can be is concluded that maturation in April/May, spawning in May/June/July and recruitment by July/August would support a good population of 10–14 cm size group which would lead to good spawning population in the ensuing year; provided, food is not limiting and there are no environmental stressors. Recruitment was mostly during July/August in almost all years. However, during 2013 and 2014, more recruitment was during August/September extending even up to October. Because of delayed spawning the food availability for the recruits was affected. This supports the match-mismatch hypothesis proposed by Cushing (1990) and later expanded by Ji et al. (2010), Asch (2015), and Checkley et al. (2017).
The environmental stressors especially salinity stratification, hypoxic conditions, predator pressures and the competition for food during the period with other ichthyoplankton would have led to larval mortality and/or low rates of larval survival. The maturation process was strongly and positively influenced by rainfall and spawning by LTA. Low recruitment led to decline of the fishery during 2013–2015. In 2015, the impacts of poor maturation during May and occurrence of large percentage of spent resting oil sardines (due to gonad atrophy) indicate poor spawning strength. The 2015 maturation process was also weak and much narrower as indicated by low GSI values. In earlier studies, spent resting stages were not observed during a larger part of the year (Longhurst and Wooster, 1990). This also would have led to poor recruitment. Availability of appropriate food is a perquisite for gonad maturation (Hunter and Leong, 1981). During 2015, phytoplankton density was low and the chlorophyll maxima occurred very late in the year which are indicative of low food availability. Moreover, the poor upwelling would have prevented nutrient enrichment. Changes in sardine reproductive traits in the northern Atlantic and the western Mediterranean have been found to be related to environmentally driven changes in food availability (Silva et al., 2006).
Environmental Variations and Impacts of Ocean-Atmospheric Phenomena on Sardine Habitat
The major coastal and ocean atmospheric events controlling the Malabar upwelling region which is the major habitat of Indian oil sardine have been described in detail (Madhupratap et al., 1994). A diagrammatic calendar on the biology of oil sardine and the major environmental events controlling these changes have also been presented (Longhurst and Wooster, 1990). Though no correlation was found between rainfall and landings, it was agreed that the major period of oil sardine abundance occurred only during periods when monsoon onset was trending toward earlier rather than later dates (Longhurst and Wooster, 1990). In the present study also rainfall was found to be positively correlated to juvenile abundance and maturity stages and PCA confirmed maximum influence of rainfall. However, high rainfall, very high positive departure from normal monsoon (during JJAS) was also found to negatively affect recruitment. Here, the theory of optimum environmental window becomes significant (Cury and Roy, 1989).
Longhurst and Wooster (1990) have argued that events taking place during the early part of the year, especially March/April, have more influence on success of sardine fishery. We also agree with this observation since we have found that the GSI starts increasing from these months clearly indicating the relationship between, nutrient enrichment from upwelling followed by diatom bloom leading to maturation. Lack of food during this period can lead to gonad atrophy (Raja, 1964) or even poor maturation as was observed during 2015. Thus, we also argue that the events during the pre-monsoon period is important. A good monsoon by itself cannot guarantee successful recruitment, if good gonad maturation has not taken place during pre-monsoon period. Hence we conclude that initiation of upwelling during pre-monsoon (for maturation); followed by normal monsoon (for spawning); which increases the food availability in the near shore waters that is essential for growth of juveniles (for recruitment) are key to the overall success of the oil sardine fishery.
Low Oxygen
One of the reasons which affected oil sardine recruitment in 2013 is the low oxygen during August in the inshore waters. Such oxygen deficient upwelled waters in the Arabian Sea have been observed earlier also (Muraleedharan and Prasannakumar, 1996; Gupta et al., 2016). It has been indicated by Longhurst and Wooster (1990) that if such low oxygen water spreads the shelf before the spawners enter the coastal waters, then they may be prevented from reaching the near shore waters and this can lead to a poor fishery. Such an instance has been observed in 1994 (Kripa et al., 2015) when very low oxygen values were reported in the near shore waters. In this investigation, oxygen deficient waters were found to affect the early life stages of the sardine and the spawners. It has been observed earlier that low oxygen waters is not a near bottom feature, rather it rises to within 10 m of surface off Kochi toward the end of monsoon and this favors the oil sardine fisheries as indicated by the positive correlation of oxygen with catch rates. However, the low oxygen condition during intense upwelling toward end of monsoon can affect recruitment of sardine as in 2014 and 2015. Both juveniles and adult sardines are affected by low oxygen values as indicated by the correlation analysis and the relative importance model.
Phytoplankton Variation - Low Food Availability
The oil sardine has been identified mainly as a filter feeder, and its main food consists of diatoms, dinoflagellates and small zooplankters (Remya et al., 2013). However, it has been observed that the intensity of grazing is high even in 10–20 m depth and that very rich feeding grounds exist at these depths. A common characteristic of forage fish collapse is the high fishing pressure for several years before collapse combined with a sharp drop in natural productivity (Essington et al., 2015). In the present study these two factors are clearly evident. Remarkably, phytoplankton densities were inversely correlated with oil sardine catch and catch rate and the relative importance model showed that food was a major factor affecting abundance. This may be due to high levels of grazing during periods of high abundance and catches. A similar inverse relationship between phytoplankton abundance and pelagic fish biomass in the Mediterranean Sea has been observed (Patti et al., 2011).
Upwelling
The LTA index which indicates the strength of the upwelling was positively correlated with maturity of oil sardine particularly partially spent females. Processes related to upwelling along the Kerala coast is usually initiated by early April much before the monsoon (Longhurst and Wooster, 1990). This correlation gives a clue that the initiation of maturation in oil sardine is trigged by the initiation of upwelling. However, this needs to be investigated in greater detail.
Upwelling is a major oceanographic phenomenon happening along the west coast of India with high inter-annual variations in its intensity. Most pelagic fishes take advantage of the upwelling and the resulting higher primary productivity during the monsoon season by timing their breeding and spawning to coincide with this period (Madhupratap et al., 1994). The upwelling index along Kerala coast showed an increasing trend from 1998 to 2007 which resulted in higher catches of small pelagics (Manjusha et al., 2013). The LTA values in the present study were generally positive up to 2012, and thereafter, showed a declining trend. Furthermore, climate change is expected to affect sardine and anchovy populations through ocean warming, change in nutrient supply and changes in food webs (Checkley et al., 2017). Therefore, the environmental events and their timing is of great importance in the maturation process, spawning and recruitment success of oil sardines in the southeastern Arabian Sea.
Impact of El Niño/Southern Oscillation - ENSO
Globally, 2015 has been considered as a warm year with high temperature and low food in the oceans (Diamond and Schreck, 2016). The average seawater temperature in sardine habitat was 29.8°C during 2015, which is nearly 1.1°C higher than the average observed (28.6°C) for the last 5 years. Positive SST anomalies exceeding 0.6°C dominated in the tropical Indian Ocean. There was a substantial warming in the tropical Indian Ocean, partially due to influences of the 2015 El Niño. The mean SST in the tropical Indian Ocean increased by 0.13–0.2°C in 2015, becoming the warmest year since 1950 (Xue et al., 2016). Explicably, the oil sardine catch and catch rates were negatively correlated with MEI and the relative importance model showed that MEI was the most important parameter explaining the variance (33%), thus indicating that the El Niño had negatively affected the sardine stock and its fisheries. In the round herring (Etrumeus teres) fishery in Ecuador, landings were related to the MEI in a non-linear analysis by up to 80% (Ormaza-González et al., 2016). Among the different factors affecting spawning and recruitment of anchoveta populations, El Niño has been reported as the most striking (Checkley et al., 2009).
Biotic Pressures
Intense blooms of four species of jellyfishes were observed in the sardine habitat especially in the shallower regions during the peak recruitment period. It is presumed that this would have contributed partly to the recruitment failure since they predate on zooplankton and fish larvae and are known to affect recruitment in fish populations (Lynam et al., 2005). They occupied the same niches preferred by young oil sardines. When they form blooms, jellyfishes can disrupt pelagic ecosystems (Mills, 2001). In the recent years, jellyfishes have been considered to prey on the young stages of small pelagics and compete for their food (Roux et al., 2013).
The oil sardine along the coast of Oman has also shown inter-annual changes and has exhibited a declining trend from 2001 to 2011. The major reasons cited are rising sea temperature, thermal stratification of the water column and the trophic pressure imposed on sardine populations by large pelagic predators (Piontkovski et al., 2011).
Sardine Fisheries Management and Conclusions
The Indian oil sardine plays a major role in coastal economy of Kerala and is nicknamed as “family provider.” During 1895 the demand from the oil and fertilizer industries prompted fishermen to use small meshed nets along the Malabar (part of Kerala) Coast which led to harvesting of fishes less than 10cm. However, it is documented that elderly fishermen who had the foresight to see the danger behind use of these nets requested the local village authorities to ban use of these nets which they believed would destroy the stock of their preferred fish (Nair and Chidambaram, 1951). Maybe this move by fishermen about 120 years back is the first record of fishermen themselves coming forward with a request to impose ban on small meshed nets for protection of sardine fishery resources in the Indian sub-continent.
In 1943, when the sardine fishery declined and collapsed, the then Government of Madras, which had the control of fisheries of Kerala State restricted fishing of oil sardine in Malabar which was later extended to another 2 years from 1945. This rule prohibited use of small meshed nets for immature sardine all through the year and also controlled juvenile fishing by prohibiting landing of oil sardine below 15 cm. The legislation lapsed in 1947 due to practical difficulties encountered in enforcement.
In 2014, when the sardine fishery reached the declined status, the scientists recommended to the Government to ban fishing and landing of oil sardines below 10 cm (Mohamed et al., 2014). In the same year Government of Kerala promulgated the ban on juvenile fishing. The fishermen also understood the need for protecting juveniles and abstained from juvenile fishery. In similar instances, in other sardine fisheries, short term regulatory mechanism have been introduced to protect the stock. It has been indicated that in 1985 when more juveniles of Monterrey Bay sardine were present in the surface waters the fishery was declared as closed to prevent growth overfishing (Lluch-Belda et al., 1986).
The age and size structure of exploited fish stocks is one of the criteria for Good Environmental Status of commercial fish (Brunel and Piet, 2013). The stock collapse of sardine and anchovies in California was largely unavoidable regardless of exploitation level (Lindegren et al., 2013). Although lower catch ratios would not eliminate the probability of collapse, reducing exploitation would markedly affect the rate of decline i.e., delay the stock collapse and accelerate its subsequent recovery.
The MSY for oil sardines in Kerala has been estimated as 250,000 tons (Balan and Sathianandan, 2007) and from 2010 till 2013 (4 years), the catch has consistently exceeded this target. This over exploitation combined with unfavorable environmental conditions, have put the stock under pressure leading to decline in abundance. Based on historic catch records it has been estimated that oil sardine takes an average 8 years to recover after historic depletions (Mohamed and Veena, 2016). It has been inferred that the Indian oil sardine makes short inshore migrations for spawning along the Kerala coast. Just before spawning the adult sardines move toward the coast and then after spawning, the juveniles are more abundant in the near shore waters. The dependence of these life stages on each of these coastal niches can play significant role in the stock recovery process and the physical as well as biotic changes within the habitats can affect their survival. In order to make more meaningful spatial management of these stocks, better understanding of the stock migration is necessary.
A large number of fishers are dependent on the Indian oil sardine for their livelihood, and the current stock depletion has affected their economic well-being. Evidence gathered in the present study indicate that the key factor influencing oil sardine biomass in the Arabian Sea off Kerala is MEI and the resultant changes in food availability, rainfall, upwelling and dissolved oxygen. The unbridled increase in fishing effort and capacity is also partly responsible for the present crisis. Maintaining more abundant populations is a way to increase the species’s capacity to adapt to environmental change (Sumaila et al., 2011). Although the stock productivity and recruitment are affected by various ocean-atmosphere phenomena listed above, in order to give a reasonable stability to the stock population density, it is necessary that the fisheries administration of the Kerala State urgently execute effort control and introduce a fishery closure during the peak breeding and/or spawning period.
Data Availability
The raw data supporting the conclusions of this manuscript have been uploaded as Supplementary Information.
Author Contributions
VK and KM contributed to conception and design of the study. VK organized the database. SP, TA, and SK performed the statistical analysis. VK and KM wrote the first draft of the manuscript. KK, RJ, DP, PA, PN, AD, KA, JB, ND, AS, and PV collected and analyzed the samples. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the Director of Central Marine Fisheries Research Institute for facilities and encouragement. The authors thank the Skipper and Crew of FV Silver Pompano for all cooperation during the sampling cruises. They are also grateful to many fishers for wide ranging discussions on oil sardine behavior.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00443/full#supplementary-material
Footnotes
References
Al-Jufaili, S. M., Al-Azri, A. R., and Al-Shuaily, S. S. (2006). A preliminary investigation on the Omani sardines and anchovies stock fluctuation; Recommendations for future studies. Pak. J. Biol. Sci. 9, 1073–1082. doi: 10.3923/pjbs.2006.1073.1082
Annigeri, G. G., Kurup, K. N., Kumaran, M., Mohan, M., Luther, G., Nair, P. R., et al. (1992). Stock assessment of oil sardine (Sardinella longiceps) Val., off west coast of India. Indian J. Fish. 39, 125–135.
APHA (1998). Standard Methods for the Examination of Water and Wastewater, 20th Edn, eds A. D. Eaton., L. S. Clesceri, and A. E. Greenberg. Washington, DC: America Public Health Association, 1220.
Asch, R. G. (2015). Climate change and decadal shifts in the phenology of larval fishes in the California Current ecosystem. Proc. Nat. Acad. Sci. U.S.A. 112, E4065–E4074. doi: 10.1073/pnas.1421946112
Balan, K., and Sathianandan, T. V. (2007). An assessment of ring seine fishery in Kerala through surplus production model. Indian J. Fish. 54, 135–140. http://eprints.cmfri.org.in/5856/
Balan, V. (1961). Some observations on the shoaling behaviour of the oil-sardine Sardinella longiceps Val. Indian J. Fish. 8, 207–221.
Banse, K. (1959). On upwelling and bottom-trawling off the southwest coast of India. J. Mar. Biol. Assoc. India 1, 33–49.
Bhoopendranath, M., and Hameed, M. S. (2012). Energy analysis of the ring seine operations off Cochin, Kerala. Fish. Tech. 49, 141–146.
Brunel, T., and Piet, G. J. (2013). Is age structure a relevant criterion for the health of fish stocks? ICES J. Mar. Sci. 70, 270–283. doi: 10.1093/icesjms/fss184
Checkley, D., Alheit, J., Oozeki, Y., and Roy, C. (2009). Climate Change and Small Pelagic Fish. New York, NY: Cambridge University Press. doi: 10.1017/CBO9780511596681
Checkley, D. M. Jr., Rebecca, G. A., and Ryan, R. R. (2017). Climate, anchovy, and sardine. Annu. Rev. Mar. Sci. 9, 469–493. doi: 10.1146/annurev-marine-122414-033819
Chidambaram, K. (1950). Studies on the length frequency of oil sardine (Sardinella longiceps) and on certain factors influencing their appearance on the Calicut coast of the Madras Presidency. Proc. Indian Acad. Sci. 31, 252–328.
CMFRI (2012). Marine Fisheries Census 2010, Part 1I 6. Kochi: Ministry of Agriculture and Central Marine Fisheries Research Institute, 115. Available at: http://eprints.cmfri.org.in/9004/
Cury, P., and Roy, C. (1989). Optimal environmental window and pelagic fish recruitment success in upwelling areas. Can. J. Fish. Aquat. Sci. 46, 670–680. doi: 10.1139/f89-086
Cushing, D. H. (1990). Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv. Mar. Biol. 26, 249–293. doi: 10.1016/S0065-2881(08)60202-3
Diamond, H. J., and Schreck, C. J. (2016). “Tropics overview,” in State of the Climate in 2015, eds J. Blunden and D. S. Arndt (Boston, MA: American Meteorological Society), 93–94.
Edwin, L., Nasser, M., Hakkim, V. I., Jinoy, V. G., Dhijudas, P. H., and Boopendranath, M. R. (2010). “Ring Seine for the small pelagic fishery,” in Coastal Fishery Resources of India: Conservation and Sustainable Utilisation, eds B. Meenakumari, M. R. Boopendranath, L. Edwin, T. V. Sankar, N. Gopal, and G. Ninan (Kochi: Society of Fisheries Technologists, India), 305–313.
Essington, T. E., Pamela, E. M., Halley, E. F., Emma, E. H., Laura, E. K., Kiva, L. O., et al. (2015). Fishing amplifies forage fish population collapses. Proc. Natl. Acad. Sci. U.S.A. 112, 6648–6652. doi: 10.1073/pnas.1422020112
FAO (2016). Fishery and Aquaculture Statistics Yearbook 2014. Rome: Food and Agriculture Organization, 105.
Froese, R., and Kesner-Reyes, K. (2002). Impact of fishing on the abundance of marine species. ICES CM. 2002/L 12:15.
Gupta, G. V. M., Sudheesh, V., Sudharma, K. V., Saravanane, N., Dhanya, V., Dhanya, K. R., et al. (2016). Evolution to decay of upwelling and associated biogeochemistry over the southeastern Arabian Sea shelf. J. Geophys. Res. Biogeosci. 121, 159–175. doi: 10.1002/2015JG003163
Hornell, J. (1937). The fishing methods of Madras Presidency, Part II. Malabar Coast. Madras Fish. Bull. 27, 1–69.
Hunter, J. R., and Leong, R. (1981). The spawning energetics of female northern anchovy, Engraulis mordax. Fish Bull. 79, 215–230.
ICAR (1971). Report of the Working Party on Sardine and Mackerel Resources, India, eds J. Jain and R. B. Hoe. New Delhi: Indian Council of Agricultural Research, 84.
Jayaprakash, A. A. (2002). Long term trends in rainfall, sea level and solar periodicity: a case study for forecast of Malabar sole and oil sardine fishery. J. Mar. Biol. Assoc. India 44, 163–175.
Jayaprakash, A. A., and Pillai, N. G. K. (2000). “The indian oil sardine,” in Marine Fisheries Research and Management, eds V. N Pillai and N. G. Menon (Kochi: Central Marine Fisheries Research Institute), 259–281.
Jayaram, C., Neethu, C., Ajith, J. K., and Balchand, A. N. (2010). Interannual variability of upwelling indices in the southeastern Arabian Sea: a satellite based study. Ocean Sci. J. 45, 27–40. doi: 10.1007/s12601-010-0003-6
Ji, R., Edwards, M., Mackas, D. L., Runge, J. A., and Thomas, A. C. (2010). Marine plankton phenology and life history in a changing climate: current research and future directions. J. Plankton Res. 32, 1355–1368. doi: 10.1093/plankt/fbq062
Kawasaki, T., and Omori, M. (1988). “Fluctuations in the three major sardine stocks in the Pacific and the global trend in mean temperature”, in Long Term Changes in Marine Fish Populations, eds T. Wyatt and M. G. Larraneta (Vigo: Institute for Marine Research), 273–290.
Kripa, V., Prema, D., Jeyabaskaran, R., Khambadkar, L. R., Nandakumar, A., Anilkumar, P. S., et al. (2015). Inter-annual variations of selected oceanographic parameters and its relation to fishery of small pelagics off Kochi, southwest coast of India. J. Mar. Biol. Assoc. India 57, 52–57.
Krishnakumar, P. K., Mohamed, K. S., Asokan, P. K., Sathianandan, T. V., Zacharia, P. U., Abdurahiman, K. P., et al. (2008). How environmental parameters influenced fluctuations in oil sardine and mackerel fishery during 1926-2005 along the southwest coast of India. Mar. Fish. Infor. Serv. T & E Ser. 198, 1–5.
Lindegren, M., Checkley, D. M. Jr., Rouyer, T., MacCall, A. D., and Stenseth, N. C. (2013). Climate, fishing, and fluctuations of sardine and anchovy in the California current. Proc. Natl. Acad. Sci. U.S.A. 110, 13672–13677. doi: 10.1073/pnas.1305733110
Lindeman R. H., Merenda P. F., and Gold, R. Z. (1980). Introduction to Bivariate and Multivariate Analysis. Glenview, IL: Scott, Foresman.
Lluch-Belda, D., Hernandez-Vazquez, S., Lluch-Cota, D. B., Salinas-Zavala, C. A., and Schwartzlose, R. A. (1992a). The recovery of the Californian sardine as related to global change. CalCOFI Report 33, 50–59.
Lluch-Belda, D., Schwartzlose, R. A., Serra, R., Parrish, R. H., Kawasaki, T., Hedgecock, D., et al. (1992b). Sardine and anchovy regime fluctuations of abundance in four regions of the world oceans: a workshop report. Fish. Oceanogr. 1, 339–347. doi: 10.1111/j.1365-2419.1992.tb00006.x
Lluch-Belda, D., Magallon, B. F. J., and Schwartzlose, R. A. (1986). Large fluctuations in the sardine fishery in the Gulf of California: possible causes. CalCOFI Report 27, 136–140.
Longhurst, A. R., and Wooster, W. S. (1990). Abundance of oil sardine (Sardinella longiceps) and upwelling in the southwest coast of India. Can. J. Fish. Aquat. Sci. 47, 2407–2419. doi: 10.1139/f90-268
Lynam, C. P., Stephen, J. H., and Andrew, S. B. (2005). Jellyfish abundance and climatic variation: contrasting responses in oceanographically distinct regions of the North Sea, and possible implications for fisheries. J. Mar. Biol. Assoc. UK 85, 435–450. doi: 10.1017/S0025315405011380
Madhupratap, M., Shetye, S. R., Nair, K. V., and Sreekumaran, N. R. (1994). Oil sardine and Indian mackerel: their fishery problems and coastal oceanography. Curr. Sci. 66, 340–348.
Manjusha, U., Jayasankar, J., Remya, R., Ambrose, T. V., and Vivekanandan, E. (2013). Influence of coastal upwelling on the fishery of small pelagics off Kerala, south-west coast of India. Indian J. Fish. 60, 37–42.
Mills, C. E. (2001). Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451, 55–68. doi: 10.1023/A:1011888006302
Mohamed, K. S., and Veena, S. (2016). How long does it take for tropical marine fish stocks to recover after declines? Case studies from the southwest coast of India. Curr. Sci. 110, 584–594. doi: 10.18520/cs/v110/i4/584-594
Mohamed, K. S., Zacharia, P. U., Maheswarudu, G., Sathianandan, T. V., Abdussamad, E. M., Ganga, U., et al. (2014). Minimum legal size (MLS) of capture to avoid growth overfishing of commercially exploited fish and shellfish species of Kerala. Mar. Fish. Infor. Serv. T & E Ser. 220, 3–7.
Mohamed, K. S., Sathianandan, T. V., Zacharia, P. U., Asokan, P. K., Krishnakumar, P. K., Abdurahiman, K. P., et al. (2010). “Depleted and collapsed marine fish stocks along southwest coast of India – A simple criterion to assess the status,” in Coastal Fishery Resources of India; Conservation and Sustainable Utilisation, eds B. Meenakumari, M. R. Boopendranath, L. Edwin, T. V. Sankar, N. Gopal, and G. Ninan (Kochi: Society of Fisheries Technologists), 67–76.
Mullon, C., Freon, P., and Cury, P. (2005). The dynamics of collapse in world fisheries. Fish Fish. 6, 111–120. doi: 10.1111/j.1467-2979.2005.00181.x
Muraleedharan, P. M., and Prasannakumar, S. (1996). Arabian Sea upwelling- a comparison between coastal and open ocean regions. Curr. Sci. 71, 842–846.
Nair, P. G., Joseph, S., Kripa, V., Remya, R., and Pillai, V. N. (2016). Growth and maturity of Indian oil sardine Sardinella longiceps (Valenciennes, 1847) along southwest coast of India. J. Mar. Biol. Assoc. India 58, 64–68. doi: 10.6024/jmbai.2016.58.1.1899-07
Nair, R. V., and Chidambaram, K. (1951). A review of the Indian oil sardine fishery. Proc. Nat. Inst. Sci. India 17, 71–85.
Ormaza-González, F. I., Mora-Cervetto, A., Bermúdez-Martínez, R. M., Hurtado-Domínguez, M. A., Peralta-Bravo, M. R., and Jurado-Maldonado, V. M. (2016). Can small pelagic fish landings be used as predictors of high-frequency oceanographic fluctuations in the 1–2 El Niño region? Adv. Geosci. 42, 61–72. doi: 10.5194/adgeo-42-61-2016
Patti, B. A., Bonanno, M., D’Elia, E. Q., Giacalone, G. I., Fontana, S., Aronica, G., et al. (2011). Daytime pelagic schooling behaviour and relationships with plankton patch distribution in the Sicily Strait (Mediterranean Sea). Adv. Oceanogr. Limnol. 14, 79–92. doi: 10.4081/aiol.2011.5318
Pauly, D. (1983). Some simple methods for the assessment of tropical fish stocks. FAO Fish. Tech. Pap. 234:52.
Petitgas, P., Secor, D. H., McQuinn, I., Huse, G., and Lo, N. (2010). Stock collapses and their recovery: mechanisms that establish and maintain lifecycle closure in space and time. ICES J. Mar. Sci. 67, 1841–1848. doi: 10.1093/icesjms/fsq082
Pillai, N. G. K., Ganga, U., and Jayaprakash, A. A. (2003). “Indian oil sardine,” in Status of exploited marine fishery resources of India, eds M. J. Modayil and J. J. Jayaprakash (Kochi: Central Marine Fisheries Research Institute), 8–24.
Piontkovski, S. A., Al-Oufi, H. S., and Al-Jufaili, S. (2011). Seasonal and interannual changes of Indian oil Sardine (Sardinella longiceps) landings in the the Sea of Oman. Mar. Fish. Rev. 76, 50–59. doi: 10.7755/MFR.76.3.3
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Raja, A. B. T. (1964). Some aspects of spawning biology of Indian oil sardine (Sardinella longiceps). Indian J. Fish. 11, 45–120.
Raja, A. B. T. (1966). On the maturity stages of Indian oil sardine (Sardinella longiceps) with notes on incidence of atretic follicles in advanced ovaries. Indian J. Fish. 13, 27–47.
Raja, A. B. T. (1969). The Indian Oil Sardine. Kochi: Central Marine Fisheries Research Institute: Bulletin No.16, 151.
Raja, A. B. T. (1973). The Indian oil-sardine fishery: problems in perspective. J. Mar. Biol. Assoc. India 15, 735–749.
Remya, R., Vivekanandan, E., Manjusha, U., and Nair, P. G. (2013). Seasonal variations in the diet of the Indian oil sardine, Sardinella longiceps Valenciennes off Cochin, Kerala. Indian J. Fish. 60, 55–59.
Roux, J.-P. Van der Lingen, C. D., Gibbons, M. J., Moroff, N. E., Shannon, L. J., Smith, A. D. M., et al. (2013). Jellyfication of marine ecosystems as a likely consequence of overfishing small pelagic fish: lessons from the Benguela. Bull. Mar. Sci. 89, 249–284. doi: 10.5343/bms.2011.1145
Rouyer, T., Ottersen, G., Durant, J. M., Hidalgo, M., Hjermann, D., Persson, J., et al. (2011). Shifting dynamic forces in fish stock fluctuations triggered by age truncation? Glob. Change Biol. 17, 3046–3057. doi: 10.1111/j.1365-2486.2011.02443.x
Schwartzlose, R. A., Alheit, J., Bakun, A., Baumgartner, T. R., Cloete, R., Crawford, R. J. M., et al. (1999). Worldwide large-scale fluctuations of sardine and anchovy populations. S. Afr. J. Mar. Sci. 21, 289–347. doi: 10.2989/025776199784125962
Sekharan, K. V. (1974). Estimates of the stocks of oil sardine and mackerel in the present fishing grounds off the west coast of India. Indian J. Fish. 21, 177–182.
Shah, P., Sajeev, R., and Gopika, N. (2015). Study of upwelling along the west coast of India – A climatological approach. J. Coast. Res. 31, 1151–1158. doi: 10.2112/JCOASTRES-D-13-00094.1
Silva, A., Santos, M. B., Caneco, B., Pestana, G., Porteiro, C., Carrera, P., et al. (2006). Temporal and geographic variability of sardine maturity at length in the northeastern Atlantic and the western Mediterranean. ICES J. Mar. Sci. 63, 663–676. doi: 10.1016/j.icesjms.2006.01.005
Smitha, B. R., Sanjeevan, V. N., Vimalkumar, K. G., and Revichandran, C. (2008). On the upwelling off the southern tip and along the west coast of India. J. Coast. Res. 24, 95–102. doi: 10.2112/06-0779.1
Srinath, M., Kuriakose, S., and Mini, K. G. (2005). Methodology for the Estimation of Marine Fish Landings in India. Kochi: Central Marine Fisheries Research Institute, 57.
Strickland, J. D., and Parsons, T. R. (1968). Practical Handbook of Seawater Analyses. Ottawa: Fisheries Research Board of Canada, 311.
Sumaila, U. R., Cheung, W. L., Lam, V. W. Y., Pauly, D., and Herrick, S. (2011). Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Change 1, 449–456. doi: 10.1038/NCLIMATE1301
Ulrike, G. (2006). Relative importance for linear regression in r: the package relaimpo, J. Stat. Soft. 17, 1–27.
Wolter, K., and Timlin, M. S. (1993). “Monitoring ENSO in COADS with a seasonally adjusted principal component index,” in Proceedings of the 17th Climate Diagnostics Workshop Norman OK. NOAA/NMC/CAC, NSSL, Oklahoma Clim. Survey, CIMMS and the School of Meteor (Norman, OK: University of Oklahoma), 52–57.
Worm, B., Hilborn, R., Baum, J. K., Branch, T. A., Collie, J. S., Costello, C., et al. (2009). Rebuilding global fisheries. Science 325, 578–585. doi: 10.1126/science.1173146
Xu, C., and Boyce, M. S. (2009). Oil sardine (Sardinella longiceps) off the malabar coast: density dependence and environmental effects. Fish. Oceanogr. 18, 359–370. doi: 10.1111/j.1365-2419.2009.00518.x
Xue, Y., Hu, Z. Z., Kumar, A., Banzon, V., Huang, B., and Kennedy, J. (2016). “Sea surface temperature,” in State of the Climate in 2015, eds J. Blunden and D. S. Arndt (Boston, MA: American Meteorological Society), 192–193.
Yohannan, T. M., Balasubramanian, K. K., and Janaki, V. K. (1998). Comparison of the growth patterns of Indian mackerel and oil sardine. J. Mar. Biol. Assoc. India 40, 205–208.
Keywords: Indian oil sardine, overfishing, biology and recruitment, environmental drivers, food availability, timing mismatch
Citation: Kripa V, Mohamed KS, Koya KPS, Jeyabaskaran R, Prema D, Padua S, Kuriakose S, Anilkumar PS, Nair PG, Ambrose TV, Dhanya AM, Abhilash KS, Bose J, Divya ND, Shara AS and Vishnu PG (2018) Overfishing and Climate Drives Changes in Biology and Recruitment of the Indian Oil Sardine Sardinella longiceps in Southeastern Arabian Sea. Front. Mar. Sci. 5:443. doi: 10.3389/fmars.2018.00443
Received: 08 August 2018; Accepted: 05 November 2018;
Published: 23 November 2018.
Edited by:
Nazli Demirel, Istanbul University, TurkeyReviewed by:
Athanassios C. Tsikliras, Aristotle University of Thessaloniki, GreeceM. Cristina Mangano, Bangor University, United Kingdom
Copyright © 2018 Kripa, Mohamed, Koya, Jeyabaskaran, Prema, Padua, Kuriakose, Anilkumar, Nair, Ambrose, Dhanya, Abhilash, Bose, Divya, Shara and Vishnu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kolliyil S. Mohamed, a3Ntb2hhbWVkQGdtYWlsLmNvbQ==
 Vasant Kripa
Vasant Kripa Kolliyil S. Mohamed
Kolliyil S. Mohamed K. P. Said Koya
K. P. Said Koya R. Jeyabaskaran
R. Jeyabaskaran Shelton Padua
Shelton Padua T. V. Ambrose
T. V. Ambrose John Bose
John Bose