- 1Environmental Science and Policy Department, George Mason University, Fairfax, VA, United States
- 2Naval Research Laboratory, Hancock county, MS, United States
- 3Division of Coastal Sciences, School of Ocean Science and Technology, University of Southern Mississippi, Ocean Springs, MS, United States
The release of hydrocarbons and chemical dispersant in marine environments may disrupt benthic ecosystems, including artificial reefs, formed by historic steel shipwrecks, and their associated organisms. Experiments were performed to determine the impacts of crude oil, dispersed crude oil, and dispersant on the community structure and function of microorganisms in seawater (SW) and biofilms formed on carbon steel, a common ship hull construction material. Steel corrosion was also monitored to illustrate how oil spills may impact preservation of steel shipwrecks. Microcosms were filled with seawater (SW) and incubated at 4°C. Carbon steel disks (CSDs) were placed in each tank, and tanks were amended with crude oil and/or dispersant or no treatment. SW and CSD biofilms were sampled biweekly for genetic analysis using Illumina sequencing of 16S ribosomal RNA gene amplicons. Predicted and sequenced bacterial metagenomes were analyzed to examine impacts of oil and dispersant on metabolic function. Gammaproteobacteria, Alphaproteobacteria, and Flavobacteriia dominated SW and biofilms. Bacterial community structure differed significantly between treatments for SW and biofilms. OTUs affiliated with known (Pseudomonas) and potential (Marinomonas) hydrocarbon-degraders were roughly twice as abundant in biofilms treated with oil and dispersed oil, and steel corrosion of CSDs in these treatments was higher compared to control and dispersant treatments. OTUs affiliated with the Rhodobacteraceae family (biofilm formers and potential oil degraders) were less abundant in the dispersant treatment compared to other treatments in biofilm and SW samples, but OTUs affiliated with the Pseudoalteromonas genus (biofilm formers and proposed hydrocarbon degraders) were more abundant in dispersant-treated biofilms. Overall, functional gene analyses revealed a decrease in genes (predicted using PICRUSt and observed in sequenced metagenomes) associated with hydrocarbon degradation in dispersant-treated biofilms. This study indicates that exposure to oil and dispersant could disrupt the composition and metabolic function of biofilms colonizing metal hulls, as well as corrosion processes, potentially compromising shipwrecks as ecological and historical resources.
Introduction
The drilling rig Deepwater Horizon (DWH) exploded on April 20, 2010 and sank 2 days later. The spill that followed resulted in ~5 million barrels of oil discharged into the Gulf of Mexico (Valentine et al., 2014). Approximately 47 thousand barrels of the chemical dispersant Corexit 9500 was used in an attempt to mitigate the impact of the spill (Joye et al., 2016). These events culminated in the largest and deepest oil spill in history in U.S. waters. The spill at the Macondo well, found at 1,500 m depth, resulted in a deep-water plume of hydrocarbons (Socolofsky et al., 2011). Corexit 9500, containing the anionic surfactant dioctyl sodium sulfosuccinate, was injected at the Macondo well to disperse oil into neutrally buoyant droplets (swollen micelles) that would not coalesce or rise to the surface (Campo et al., 2013). Application of dispersant to surface oil caused oil to break into smaller droplets that more readily mixed with seawater. Hydrocarbons from the DWH spill were deposited across 2,280 km2 of seafloor (Stout et al., 2017) due to transport of both surface oil and oil entrained in the deep-water plume (Socolofsky et al., 2011; Passow et al., 2012; Reddy et al., 2012; Valentine et al., 2014; Chanton et al., 2015; Stout et al., 2017).
The DWH footprint deleteriously impacted benthic infauna and corals in deep-sea habitats in the northern Gulf of Mexico (Montagna et al., 2013; Fisher et al., 2014). Given the extent of hydrocarbons on the seafloor, it is reasonable to anticipate that any ecosystem within the DWH footprint was, or remains, at risk for spill-associated effects on organismal viability, abundance, and metabolic function. This may be especially the case for ecosystems located within the “acute footprint” of the spill, located 16 km or less to the southwest of the Macondo well, where hopane (C30H52) concentrations exceeded 800 ng g−1 in soft bottom sediments (Valentine et al., 2014; Stout et al., 2017).
The area within the DWH acute footprint hosts artificial reef ecosystems formed by two steel-hulled shipwrecks, the German U-boat U-166 and the passenger ship Robert E. Lee, both resting in ~1,400 m of water ~10 km southwest of the Macondo well (Church et al., 2007). Proximity to the well, and location within the DWH acute footprint, raises concerns about spill impact the physical integrity of the wrecks. Impacts to shipwreck hull structure place artificial reef ecosystems and cultural artifacts at risk. In addition, twentieth century shipwrecks often contain crude oil and fuel, presenting a potential environmental hazard if structural breaches occur (Jimenez et al., 2017).
While efforts are ongoing to understand the effects of the spill on deep-sea sediment and water column microorganisms (Joye et al., 2014; Yergeau et al., 2015; Arnosti et al., 2016; Kleindienst et al., 2016; Yang et al., 2016; Ziervogel et al., 2016), the impacts of the DWH oil footprint on deep-water shipwrecks and associated microorganisms are largely unknown. Few studies have explored microorganisms associated with shipwrecks, with prior works generally focused on morphology and not composition of microbial communities. Only recently have studies been published that use molecular approaches to characterize microbial colonization and biofilm formation (biofouling) on shipwreck hulls and surfaces (Palacios et al., 2009; Fagervold et al., 2012; Leary et al., 2014). Marine biofilms play important roles in establishing settlement conditions, including adhesive properties, and recruitment cues for macro-organisms (Svane and Petersen, 2001; Huggett et al., 2006). Disturbances can impact community assembly and diversity of biofilm microorganisms (Milferstedt et al., 2013), and sequentially, the settlement of macro-organisms comprising artificial reef ecosystems (Lau et al., 2005; Chiu et al., 2007).
Limited efforts have characterized marine microbially-mediated decay processes on carbon steel shipwreck hulls (Blanchette, 2000; Cullimore and Johnston, 2008; Gjelstrup Björdal, 2012)—especially with regard to hydrocarbon exposure. However, microbiologically influenced corrosion (MIC) of carbon steel coupled to microbial degradation of hydrocarbons is of significant concern in the oil and gas industry and has been investigated (Neria-González et al., 2006). The DWH spill raised immediate questions about changes to communities of macro-organisms and microorganisms on artificial reefs associated with shipwrecks in the Gulf of Mexico and the potential for increased corrosion of carbon steel hulls. In response to those concerns, an experiment was designed to evaluate the effects of crude oil, dispersed crude oil, and dispersant on the diversity of biofilm communities formed on carbon steel and impacts on metal corrosion. Null hypotheses were: exposure to crude oil and/or dispersant will not impact (1) biodiversity of biofilm-forming microorganisms, or (2) carbon steel corrosion relative to controls over a time course experiment. This study represents the first step in assessing potential environmental impacts of oil spills on the preservation of metal, including shipwreck materials, in the marine environment.
Materials and Methods
Microcosm Experimental Design and Sample Collection
An experiment was carried out over a period of 16 weeks in four microcosms constructed using 25-L cylindrical, opaque plastic tanks. Carbon steel disks (CSDs) (1.59 cm diameter × 0.16 cm thick) were fabricated from alloy UNS C10200 (wt%: C, 0.17–0.24; Mn, 0.25–0.60; P max, 0.04, S max, 0.05; Fe, bal.) plate by Metal Samples (Munford, AL). CSDs were mounted in EpoThin™ 2 epoxy (Buehler, Lake Bluff, IL) with one face (2 cm2) of the CSD epoxy-free with the as-mill finish intact. PVC plastic towers housing replicate epoxy mounted CSDs (n = 32) were placed in each tank (Figure S1). Unfiltered coastal seawater (SW) (38 PSU) was collected in January 2015 from the Naval Research Laboratory—Key West pier (Key West, Florida, USA), shipped overnight to George Mason University, and stored in a 4°C cold room for 1 week prior to filling experimental tanks. Each tank was filled with 16 L of seawater, placed on a stir plate, and incubated at 4°C (±0.4). Seawater in each tank was continuously circulated with a magnetic stir bar. Tanks were covered with lids and only opened during weekly seawater measurements and bi-weekly sampling, respectively. Initial (T = 0) temperature, salinity, and dissolved oxygen concentrations were measured for each tank, and monitored weekly for the duration of the experiment (Table S1). Replicate SW samples (250 mL, n = 2 per tank) were collected, filtered onto 0.22 micron Millipore® Sterivex™ filters (Millipore, Billerica, MA, USA) using a peristaltic pump to collect bacterioplankton, and stored frozen at −80°C until analysis.
Following a 2-week tank acclimation period, microcosms were amended with crude oil (5 mg/L Louisiana sweet crude), Corexit EC9500 dispersant (0.05 mg/L), a mixture of crude oil (5 mg/L) and dispersant (0.05 mg/L), or no treatment (unamended control). Concentrations of oil and dispersant expected during the spill were calculated using published data (Table S2) and data from the NOAA Office of Response and Restoration's Environmental Response Management Application database (ERMA—gomex.erma.noaa.gov). ERMA-derived estimates were calculated by querying hopane concentrations from deep-water samples (900–1,550 m) within 1 km from the Macondo well and using a conversion factor of 58 ± μg hopane/g oil (Valentine et al., 2014).
SW and CSD biofilm samples were collected for bacterial genetic analysis every 2 weeks over the 16-week experiment. Samples at the 2-week time point (T = 2) were taken before addition of oil and/or dispersant. CSD samples were removed aseptically, placed upright in sterile plastic containers, and either stored frozen at −80°C for later genetic analysis (n = 1 per time point) or air dried for 2 days under a sterile glass beaker at room temperature and shipped to the U.S. Naval Research Laboratory (Stennis Space Center, Mississippi, USA) for analysis (n = 1 per time point).
DNA Extraction and Sequencing of 16s rRNA Gene Amplicons and Bacterial Metagenomes
Surface material, including biofilms and corrosion products (88 ± 45 mg) were removed from CSD (n = 32) using a flame-sterilized scoopula and placed in Lysing matrix E tubes containing lysis solutions from the FastDNA™ Spin Kit for Soil (MP Biomedicals LLC, Santa Ana, CA, USA). Bovine serum albumin (1 μg BSA) was added to samples prior to extraction to prevent competitive binding of DNA by iron. Genomic DNA was extracted using a modified version of the FastDNA™ protocol described elsewhere (Hamdan et al., 2013). The same protocol, excluding the addition of BSA, was used to extract genomic DNA from SW samples (n = 72 total) after filter cartridges were cracked and filters removed using flame-sterilized scalpels and tweezers. Total genomic DNA (ng/mL) extracted from each sample was quantified using the Qubit® 2.0 Fluorometric Quantitation system (Invitrogen, Carlsbad, CA, USA) and quality checked using a Nanodrop 2000 (Thermo Scientific) spectrophotometer. Spectral A260/280 and A260/230 ratios for metagenome samples were 1.8 and 2.0, respectively, or greater.
Genomic DNA template (~2 ng/μL per sample) was sent to the Integrated Microbiome Resource (IMR) facility at Dalhousie University (Halifax, Nova Scotia, Canada) for 16S rRNA gene amplification and sequencing. PCR amplifications were carried out in 50-μL volumes as described in Comeau et al. (2011), using fusion primer sets B969F/BA1406 and A956F/A1401R to target the V6-V8 variable regions of the 16S rRNA gene for bacteria and archaea, respectively. PCR reactions were performed in duplicate for each sample with: 1X HF buffer (New England Biolabs Inc., Ipswich, MA, USA), 200 μM of each dNTP (Feldan Bio, Quebec, Canada), 0.4 mg/mL BSA (Thermo Fisher Scientific, Waltham, MA, USA), 0.2 μM of each primer (Invitrogen), 1 U of Phusion® High-Fidelity DNA polymerase (New England Biolabs Inc.), and 1–3 μL of DNA template. Thermocycler run conditions were: 1 cycle of 30 s at 98°C; 30 cycles of 10 s at 98°C, 30 s at 55°C, and 30 s at 72°C; 1 cycle of 5 min at 72°C. PCR products were screened with an Invitrogen 96-well E-gel (Invitrogen). PCR duplicates were pooled into a single plate, cleaned, and normalized using the Invitrogen SequalPrep 96-well Plate Kit. Samples were pooled into a library, quantified, and run on an Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA), generating 300 bp, paired-end sequences. No archaeal data are presented, as few samples had enough high-quality sequences to assemble amplicons. These samples were included on a sequencing run with environmental samples of the same type from another study that yielded sufficient archaeal sequences to describe communities. Therefore, the paucity of archaeal sequences in this data set does not appear to be a methodological error.
Genomic DNA template from CSD biofilms sampled at t = 16 wks (~2 ng/μL per sample) was sent to IMR for sequencing of microbial community metagenomes. At IMR, DNA libraries were prepared with the Nextera XT DNA Library Preparation Kit (Illumina Inc.) with a modification: the Agencourt® AMPure® XP clean-up and Illumina bead normalization was replaced with the Just-a-Plate™ 96 PCR Purification and Normalization Kit (Charm Biotech, San Diego, CA, USA). Sequencing was performed with an Illumina NextSeq 550, with a high-output flow cell generating 150 bp, paired-end sequences.
Bioinformatic Analysis
Bioinformatic analysis of raw partial 16S rRNA sequences was carried out using pipelines generated with USEARCH (v8.1) and UPARSE (Edgar, 2013) and Quantitative Insights into Microbial Ecology (QIIME, MacQIIME version 1.9.1) (Caporaso et al., 2010). UPARSE was used to merge 6.7 million (M) paired-end sequences, with an allowable minimum merge length of 200 base pairs (bps) and a maximum difference of 32 bps in the overlap region (allowing for a 20% error rate). Merged sequences (2.2 M) were quality filtered, discarding reads with a maximum number of expected errors >0.5 (based on Phred quality scores) (Edgar and Flyvbjerg, 2015). A total of 2M sequences, with an average sequence length of 434 base pairs, were obtained after quality filtering. Sequences were de-replicated, with unique sequences (n = 719,308) serving as representative operational taxonomic units (OTUs), and remaining sequences assigned to OTUs based on greedy clustering and ≥97% sequence similarity. Chimeric sequences (23%) were removed (de novo) and singletons (n = 597,179) were included. A table containing the relative abundance of OTUs in each sample was generated. A representative sequence from each OTU was used for taxonomic identification and alignment using UCLUST and the GreenGenes reference database (v 13.8) (DeSantis et al., 2006). OTUs with taxonomic identification are herein also referred to as “bacterial phylotypes.”
CSD biofilm and SW bacterioplankton 16S rRNA sequence data were processed through the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt 1.1.0) pipeline to predict the metagenome functional content of identified bacterial taxa across all microcosm samples (Langille et al., 2013; picrust.github.com). Sequences were initially processed through the UPARSE pipeline up to the de-replication step, as described previously. Sequence data were run in parallel through the “pick closed reference” pipeline in QIIME to pick OTUs, assign taxonomy, and create an OTU table. In PICRUSt, the OTU table was normalized by predicted 16S rRNA copy numbers and run through the remainder of the pipeline to obtain predicted functional trait abundances based on KEGG orthologs. KEGG ortholog predictions were examined individually and also collapsed into KEGG pathways (K3 level). The weighted nearest sequenced taxon index (weighted NSTI) was calculated for each sample, which indicates the accuracy of metagenome prediction by providing a score that summarizes to what extent the microorganisms in a given sample are related to previously sequenced genomes. Weighted NSTI is calculated as the average branch length separating each OTU in a sample from a reference genome, weighted by the abundance of the OTU in the sample. The metagenome contributions script was used to determine which OTUs were contributing to specific functions.
The Microbiome Helper workflow (Comeau et al., 2017) was used for initial quality control of raw microbial metagenome sequence data. Briefly, multiple lanes of sequences were concatenated, paired-end reads were stitched together using PEAR (v0.9.10) (Zhang et al., 2014), contaminant sequences were screened and removed with Bowtie2 (v2.2.7), and sequences were trimmed using Trimmomatic (v0.36) (Bolger et al., 2014). Functional analysis of metagenome sequences was carried out using the The HMP Unified Metabolic Analysis Network 2 (v0.9.9), or HUMAnN2, pipeline (Abubucker et al., 2012). HUMAnN2 quantifies the presence/absence and relative abundance of gene families and pathways within a microbial community to infer the functional and metabolic potential of the community and its constituents. The legacy KEGG gene annotation database (v56) from HUMAnN (v0.99) was used to determine KEGG pathway gene abundances.
Accession Numbers
Sequences have been deposited to NCBI's Sequence Read Archive (SRA) and are available under BioProject number PRJNA382210.
Statistical Analyses
PRIMER v. 6.1.13 with PERMANOVA+ v. 1.0.3 (PRIMER-E Ltd., Plymouth, UK) software was used for statistical analysis of bacterial community structure (Clarke and Warwick, 2001; Clarke and Gorley, 2006; Anderson et al., 2008). Bray–Curtis dissimilarities were calculated from square-root transformed sequence abundance data. Non-metric multidimensional scaling (NMDS) ordination of Bray-Curtis dissimilarities was performed to yield a “best fit” 2-dimensional graphical representation of similarities in bacterial community structure among samples. Hierarchical clustering analysis (CLUSTER) was used to generate similarity dendrograms and the similarity profile permutation test (SIMPROF) was used to identify significant groupings among samples. CLUSTER analyses were based on group average linkage and SIMPROF tests were performed at the 0.05 significance level. Analysis of similarity (ANOSIM) and permutational multiple analysis of variance (PERMANOVA) tests (Marti J. Anderson, 2001) were used to identify differences in bacterial community structure between sample groups (e.g., treatment and time). PERMANOVA analyses were run with: 9999 permutations, permutation of residuals under a reduced model, type III (partial) sum of squares, Monte Carlo tests, and fixed effects sum to zero. The “similarity percentages” analysis (SIMPER) was run with a 90% cutoff and used to rank the percent contribution of individual bacterial phylotypes to within group similarity or between group differences (Clarke and Warwick, 2001; Clarke and Gorley, 2006).
SigmaPlot Version 11.0 was used to run a two-way analysis of variance (ANOVA) to test for changes in diversity indices between treatments and over time for both sample types. Analysis of PICRUSt predicted metagenome data were carried out using the Statistical Analysis of Metagenomic Profiles (STAMP v2.1.3) open-source software program (Parks et al., 2014). ANOVA with a Tukey-Kramer post-hoc test and Benjamini–Hochberg correction were used for multiple-group analysis.
Evaluation of CSD Corrosion
Corrosion morphology and corrosion product elemental composition were characterized with ElectroScan® environmental scanning electron microscopy (ESEM) and energy dispersive spectroscopy (EDS), respectively (Ray and Little, 2003). Coupons were removed from epoxy mounts, acid cleaned to remove corrosion products according to ASTM Standard G1-03 (2003), and re-examined with ESEM. Acid-cleaned coupons were scanned using a Nanovea (Irvine, CA) non-contact optical profiler (model PS50) with a 400 μm optical laser pen to reconstruct high contrast 3-D digital images. Pit depths were measured from the reconstructed images. Corrosion rates (mm/y) were calculated based on maximum pits depths following ASTM Standard G1-03 (2003). Two-way ANOVAs were run to test for changes in CSD metal loss between treatments and over time.
Results
Bacterial Composition and Community Succession
Sequence averages, Shannon's diversity indices, and average OTUs for CSD biofilms and SW are summarized in Table 1. All diversity indices were calculated using total OTUs found in samples. Species richness and Shannon's diversity increased over time in all treatments (ANOVA P ≤ 0.001), while dominance increased initially and then leveled off over time (ANOVA P ≤ 0.001), indicating the recruitment and establishment of biofilms on CSDs (Figure 1 and Figure S2). Averaged across treatments, biofilms contained 25 ± 8 bacterial phylotypes at t = 2 weeks, increasing to 71 ± 4 phylotypes at t = 16 weeks. Post treatment addition (≥ t = 4 weeks), average species richness was lower in oil-treated biofilms relative to the control and other treatments (ANOVA P = 0.007). Average Shannon's diversity was lower in dispersant-treated biofilms relative to the control and oil-dispersant treatment (ANOVA P = 0.014). No significant differences were observed in dominance.
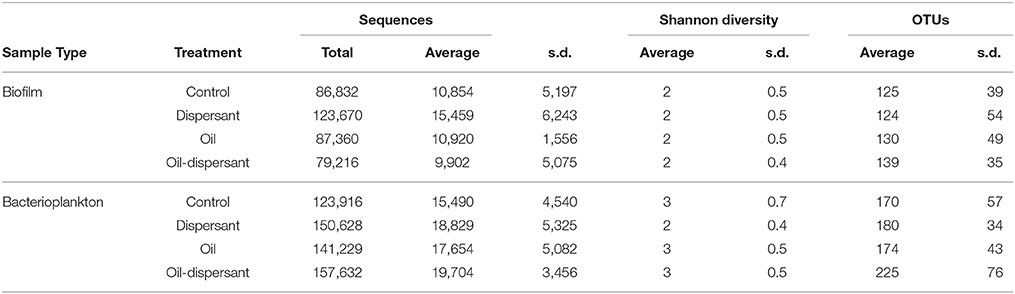
Table 1. Total and average number of sequences, average Shannon's diversity index, and average number of OTUs for microcosm carbon steel disk biofilm samples (n = 8 per treatment) and bacterioplankton samples (n = 16 per treatment).
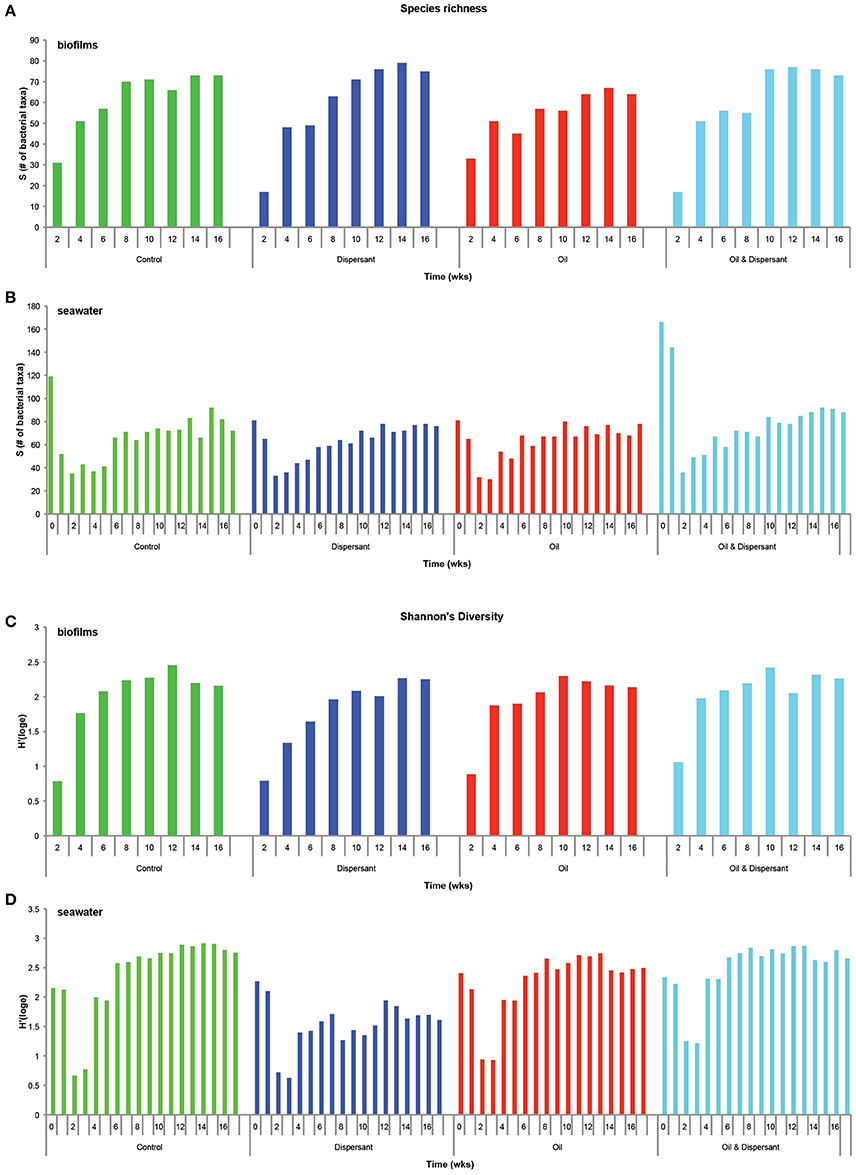
Figure 1. Biodiversity indices for carbon steel disk (CSD) biofilms and seawater bacterioplankton based on total OTUs present in samples. For seawater, each time point has two replicates. (A,B) Species richness (total # of “species”) for biofilms and seawater, respectively; (C,D) Shannon's diversity index (abundance of individuals and taxa: community with 1 taxon has a value of 0) for biofilms and seawater, respectively.
A shift in SW species richness was observed during the acclimation period, with average number of bacterial phylotypes decreasing from 97 ± 41 at the initial sampling point (t = 0 weeks) to 37 ±6 (t = 2 weeks) across all treatments (Figure 1 and Figure S2). Average number of phylotypes increased to 79 ± 8 at t = 16 weeks. Post-treatment addition (≥ t = 4 weeks), average species richness was higher in oil-dispersant and dispersant-treated SW relative to the control and oil treatment (ANOVA P = 0.001). Average Shannon's diversity was lower in oil-treated SW relative to the control and other treatments (ANOVA P < 0.001). Dominance was also higher in oil-dispersant and dispersant-treated SW relative to the control and oil treatment (ANOVA P < 0.001).
CSD biofilms were dominated by Gammaproteobacteria, comprising 58 ± 19% of sequences on average and decreasing over time across treatments (Figure 2A). Alphaproteobacteria and Flavobacteriia were the next two most abundant groups, constituting 34 ± 16 and 6 ± 4% of the biofilm community, respectively, and increasing over time across treatments. SW was also dominated by Gammaproteobacteria (62 ± 23%), Alphaproteobacteria (23 ± 18%), and Flavobacteria (12 ± 8%); but despite major shifts during the acclimation period, no clear increasing/decreasing trends over time were observed (Figure 2B).
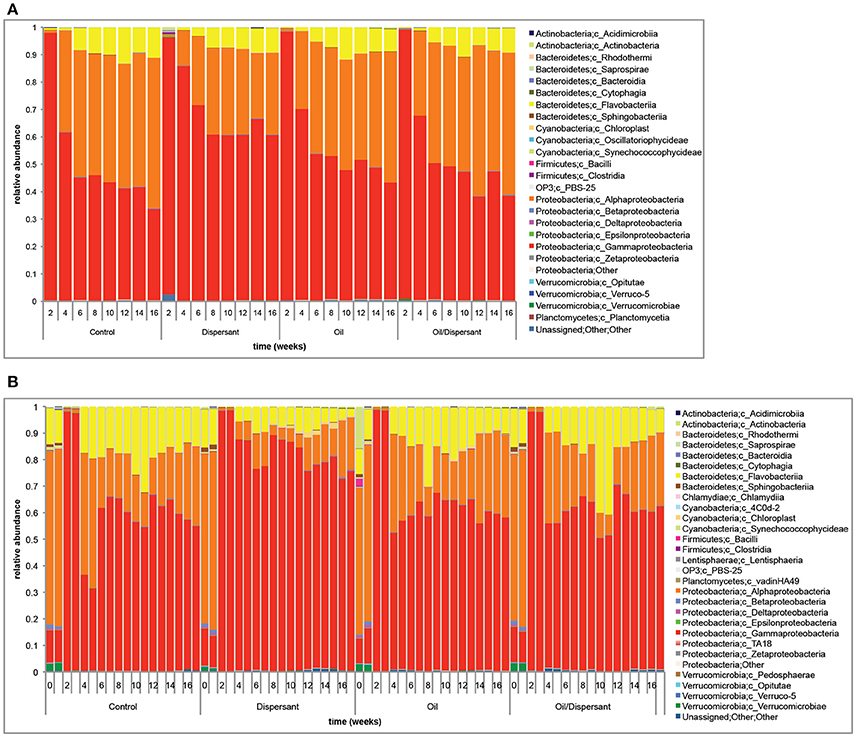
Figure 2. (A) Bacterial community composition of carbon steel disk (CSD) biofilms from the four experimental treatments over time. Bacteria are identified to the Class taxonomic level. CSD biofilm samples were collected every 2 weeks during the 16-week experiment. (B) Bacterial community composition of SW bacterioplankton samples from the four experimental treatments over time. Bacteria are identified to the Class taxonomic level. Duplicate seawater samples were collected for SW bacterioplankton every 2 weeks during the 16-week experiment.
Community Structure Response
Biofilms on CSDs changed significantly over time for all treatments (Global R = 0.478, P = 0.001; Pseudo-F = 18.204, P = 0.0001), with a marked shift between t = 2 and t = 4 weeks (R = 0.938, P = 0.029) (Figure 3A). Biofilm composition differed significantly between treatment groups after oil and/or dispersant additions at t = 2 weeks (Global R = 0.526, P = 0.001; Pseudo-F = 20.891, P = 0.0001) and changed over the time course of the experiment (Global R = 0.367, P = 0.001; Pseudo-F = 11.450, P = 0.0001).
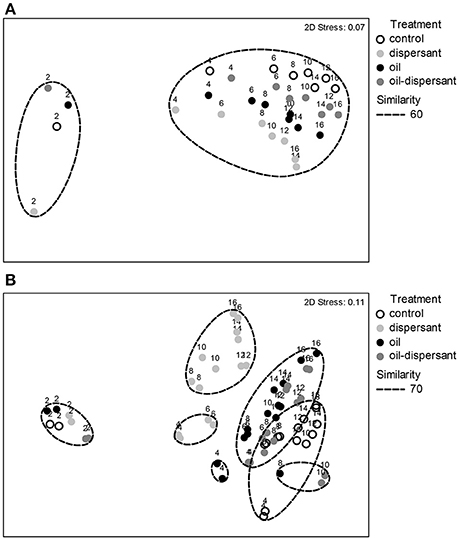
Figure 3. (A) Non-metric multidimensional scaling ordination of square-root-transformed Bray-Curtis dissimilarities calculated for carbon steel disk (CSD) biofilm samples (n = 32). Each point represents a discrete sample. Superscripts denote time (weeks) sampled. The dashed line delineates the 60% Bray-Curtis similarity threshold. (B) Non-metric multidimensional scaling ordination of square-root-transformed Bray-Curtis dissimilarities calculated for SW bacterioplankton samples, with 2 replicates per time point (n = 72). Superscripts denote time (wks) sampled; t = 0 data excluded. The dashed line delineates the 70% Bray-Curtis similarity threshold.
SIMPER analysis of biofilms revealed that within-group similarities (post 2 weeks) were >77% for all treatment groups, with OTUs affiliated with the Rhodobacteraceae family and the Pseudoalteromonas genus ranking as the top two bacterial phylotypes contributing to within-group similarity. Between-group dissimilarity ranged from 24 to 31% with 10 OTUs affiliated with families Rhodobacteraceae, Vibrionaceae, Shewanellaceae, and Pseudoalteromonadaceae, and genera Pseudoalteromonas, Bizonia, Pseudomonas, Marinomonas, Psychrobacter, and Methylophaga, ranking among the top contributing OTUs (~25%) to observed differences between treatment groups.
SW communities changed significantly over the course of the 16-week experiment for all treatments (Global R = 0.681, P = 0.001; Pseudo-F = 115.32, P = 0.0001) (Figure 3B). Marked shifts occurred during the acclimation period, between t = 0 and t = 2 weeks (Global R = 1, P = 0.001), and after treatments, between t = 2 and t = 4 weeks (Global R = 0.964, P = 0.001). Seawater communities differed significantly between treatment groups after oil and/or dispersant addition at t = 2 weeks (Global R = 0.577, P = 0.001; Pseudo-F = 99.584, P = 0.0001) and changed over the time of the experiment (Global R = 0.375, P = 0.001; Pseudo-F = 44.813, P = 0.0001).
SIMPER analysis of SW revealed that within-group similarities (post 2 weeks) were >72% for all treatment groups and, similar to biofilms, OTUs affiliated with the Rhodobacteraceae and Pseudoalteromonas genus were the top two phylotypes contributing to within-group similarity. Between-group dissimilarity ranged from 32 to 39% with 11 OTUs affiliated with families Vibrionaceae, Rhodobacteraceae, Shewanellaceae, Cryomorphaceae, and Oceanospirillaceae, and genera Pseudoalteromonas, Bizonia, Fluviicola, Marinomonas, Pseudomonas, and Methylophaga, ranking among the top contributing OTUs (~25%) to observed differences between treatment groups.
Generally, in both biofilms and SW samples, there was enrichment in OTUs affiliated with the Rhodobacteraceae family relative to OTUs affiliated with the Pseudoalteromonas genus, with the exception of the dispersant treated samples. For example, Pseudoalteromonas-affiliated OTUs in biofilms from the dispersant treatment accounted for 66% of OTUs compared to 40 to 49% in all other treatments. The average abundance of OTUs affiliated with the Rhodobacteraceae in biofilms from the dispersant treatment was 50% compared to 62–66% in other treatments. These differences were more distinct in SW samples: OTUs affiliated with the Pseudoalteromonas in the dispersant treatment were 81 vs. 43 to 49% in other treatments. The average abundance of OTUs affiliated with the Rhodobacteraceae in the SW dispersant treatment was 28% compared to 45–47% in the remaining treatments.
SIMPER analysis indicated that biofilm and SW community composition in controls was significantly different from all other treatments due to an increased average abundance of OTUs affiliated with the Vibrionaceae. Vibrionaceae in biofilm and SW control samples were 20 and 27% respectively, vs. 3–8 and 4–11%, respectively in all other treatments.
OTUs affiliated with the genus Pseudomonas (known oil-degraders) in oil and oil-dispersant treatments were twice as abundant (~20 vs. ~10%) as found in control and dispersant treatments for both biofilms and SW. OTUs affiliated with the genus Marinomonas (potential oil degraders; Brakstad et al., 2008) were enriched in oil (~20%) vs. control and other treatments (8–14%) for both sample types. OTUs affiliated with the Rhodobacteraceae family, which includes biofilm formers and potential oil degraders (Dang and Lovell, 2000; Kostka et al., 2011), were less abundant in the dispersant treatment compared to other treatments in biofilm and SW samples. However, OTUs affiliated with the Pseudoalteromonas genus (biofilm formers and proposed hydrocarbon degraders) were more abundant in dispersant-treated biofilms.
Presence of Microorganisms Involved in Sulfur Metabolism
OTUs affiliated with Paracoccus and Sulfurimonas, both capable of sulfur oxidization, were present, but minimally abundant, in SW and biofilm samples (Table S3). Paracoccus was present in 15% of SW samples with an average abundance of 0.01%. It was not present in any of the control or oil-dispersant SW samples, was present in 2 of 16 dispersant-treated SW samples, and 11 of 16 oil-treated SW samples (0.05% average abundance and not present before 6-weeks). Paracoccus was present in 19% of biofilm samples with an average abundance of 0.04%. Paracoccus was not present in any of the control, oil-dispersant, or dispersant-treated SW samples, but was present in 6 of 8 oil-treated biofilm samples (0.15% average abundance). Sulfurimonas was present in 20% of SW samples with an average abundance of 0.01%; however, there was no clear trend across samples or treatments. In biofilm samples, Sulfurimonas was present in one dispersant-treated sample.
With the exception of Pseudomonas, OTUs affiliated with bacteria capable of sulfur-reduction were minimally present in SW and biofilm samples (Table S3). Desulfobacterales and Desulfovibrionales were both present in 3 of 64 SW samples, with no clear trends across samples or treatments, and completely absent in biofilm samples.
Functional Response
To understand treatment effects on metabolic function in biofilms, PICRUSt was used to predict metagenome functional content of identified bacterial taxa over the course of the 16-week experiment. The average weighted nearest sequenced taxon index (weighted NSTI) score was 0.05 ± 0.01 s.d. for biofilm samples. A larger number of functional genes than can be reported here significantly increased or decreased in abundance in dispersant-treated biofilms compared to other treatments, and are reported in the Supplementary Material. Here, focus was placed on genes involved in bacterial motility, MIC, and hydrocarbon degradation. Notably absent in the KEGG database are pathways involved in iron metabolism, which may be relevant to MIC.
Based on PICRUSt predictions, functional genes involved in bacterial motility (ANOVA P = 9.72e-4), flagellar assembly (ANOVA P = 1.30e-3), bacterial chemotaxis (ANOVA P = 1.41e-3), sulfur metabolism (ANOVA P = 1.20e-5), and sulfur relay system pathways (ANOVA P = 1.41e-3) were more abundant in the dispersant treatment relative to all other treatments. In contrast, genes associated with polycyclic aromatic hydrocarbon (PAH) degradation (ANOVA P = 4.81e-3), chlorocyclohexane and chlorobenzene degradation (ANOVA P = 1.95e-3), chloroalkane and chloroalkene degradation (ANOVA P = 3.79e-3), naphthalene degradation (ANOVA P = 3.94e-5), and styrene degradation pathways (ANOVA P = 7.55e-5) were less abundant in the dispersant treatment compared to all other treatments.
Metagenomes from t = 16 samples from all four treatments were analyzed to provide further insight into functional capabilities of the biofilm community. In general, HUMANn2 metagenome analysis from t = 16 weeks mirrored PICRUSt predicted metagenome results for 16S rRNA samples at t = 16 weeks. HUMANn2 analysis revealed that the relative abundance of genes involved in pathways for bacterial chemotaxis, flagellar assembly, sulfur metabolism, and the sulfur relay system were more abundant in the dispersant treatment relative to all other treatments (Figure S3). Genes involved in PAH degradation were absent in both the dispersant treatment and control, while elevated in both oil and oil-dispersant treatments. The metagenome data partially disagreed with PICRUSt results in regard to genes associated with chlorocyclohexane and chlorobenzene degradation, where they were more abundant in both the oil and dispersant treatments. Bacterial motility, chloroalkane and chloroalkene degradation, and naphthalene degradation pathways were absent in the HUMANn2 metagenome results and, in contrast to PICRUSt analysis, styrene degradation pathway genes were more abundant in the t = 16 dispersant treatment relative to other treatments at that time point.
PICRUSt-predicted kegg orthologs (KOs) of particular relevance to this study were examined individually in biofilms to be able to comment on individual phylotypes associated with specific functions. K00392 (sulfite reductase; ferredoxin) was higher in oil and oil-dispersant treatments vs. control and dispersant treatments (ANOVA P = 1.14e-7) (Figure 4A). From the PICRUSt predicted metagenomes and metagenome contributions analyses, the increase in K00392 in oil and oil-dispersant samples is attributed to OTUs associated with the Pseudomonadaceae family, which contain polycyclic aromatic hydrocarbon degraders. K01561 (haloacetate dehalogenase), involved in chlorocyclohexane, chlorobenzene, chloroalkane, and chloroalkene degradation pathways, was lower in the dispersant treatment relative to the control and other treatments (ANOVA P = 8.96e-6). The decrease in K01561 in the dispersant-treated samples was attributed to OTUs associated with the Rhodobacteraceae family (47 ± 21% contribution), which were less abundant in the dispersant treatment (Figure 4B). KOs involved in chemotaxis (K13486, K13488, and K13489) were elevated in all treatments relative to the control (ANOVA P = 4.32e-6, 4.35e-6, and 4.37e-6, respectively).
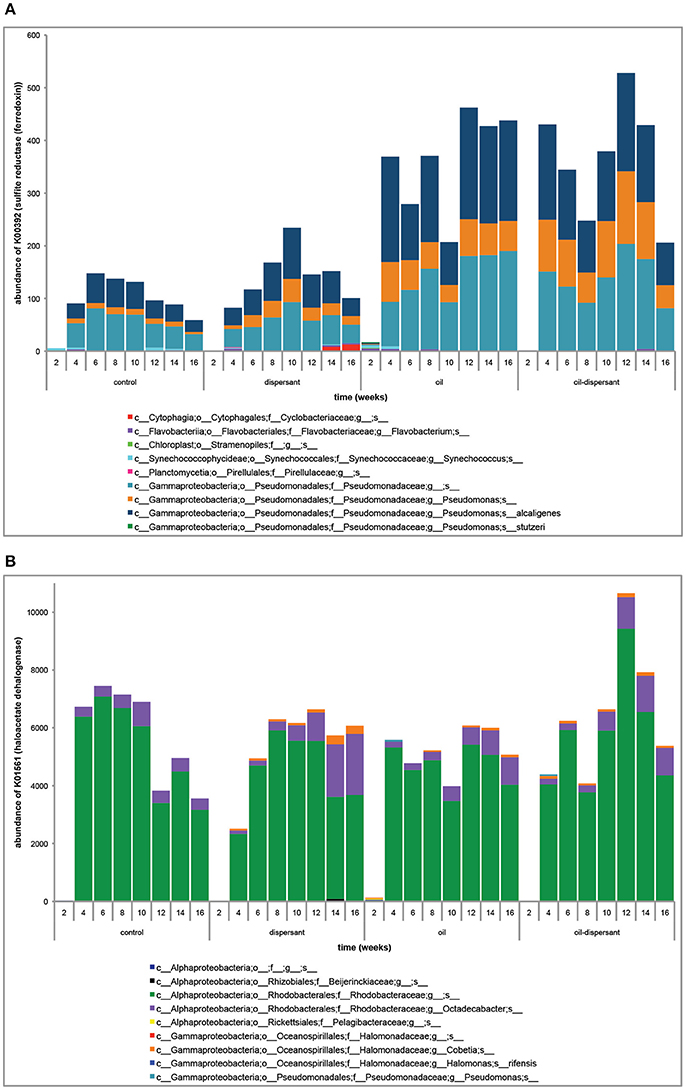
Figure 4. (A) The abundance of K00392 [sulfite reductase (ferredoxin)] and predicted metagenome contributions of individual bacterial phylotypes (>1% of total) across treatments and over time. (B) The abundance of K01561 (haloacetate dehalogenase) and predicted metagenome contributions of individual bacterial phylotypes (>1% of total) across treatments and over time.
Post-Exposure Analysis of CSDs
All CSDs were covered with iron corrosion products. Corrosion products were predominantly iron with lesser amounts of sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chloride, potassium and calcium as identified in EDS spectra. Sulfur concentrations increased from ~1 to 5 wt% in corrosion formed in the presence of oil. Total CSD pit depth increased with time in all treatments (ANOVA P ≤ 0.001) (Figure 5A). At 16 weeks, biofilms from oil and oil-dispersant treatments experienced the deepest pits (164 and 133 μm) compared to control and dispersant treatments (pit depth 81 μm for both). Corrosion rates, based on maximum pit depth, were also greater in the oil and oil-dispersant treatments compared to control and dispersant only treatments (Figure 5B). The estimated corrosion rate for the oil treatment was 0.53 mm/y, compared to 0.43 for oil-dispersant treatment, and 0.26 mm/y for both the control and dispersant treatment.
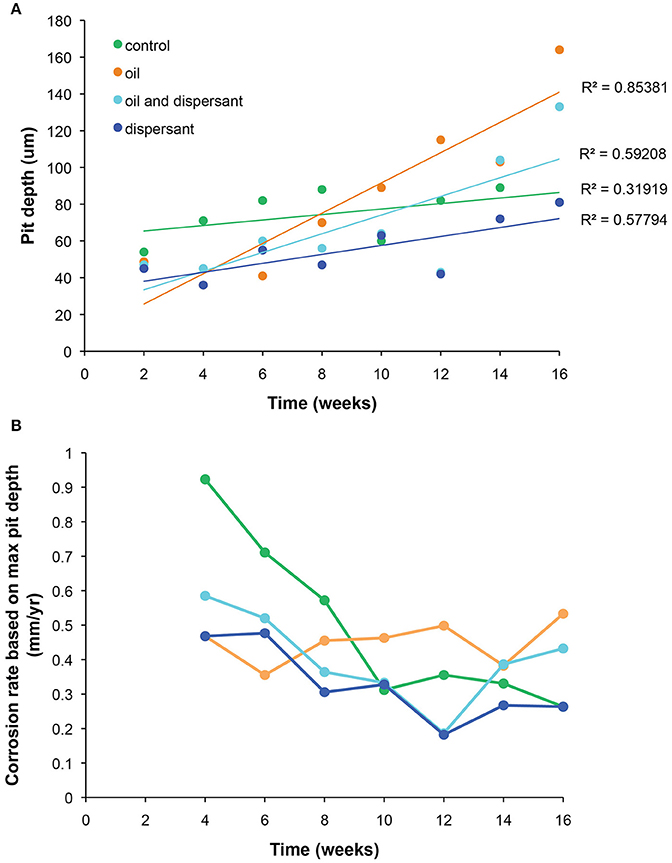
Figure 5. Surface profilometry analysis of carbon steel disks (CSDs). (A) Maximum pitting depth (μm) resulting from abiotic and biotic processes is plotted against time (16 weeks) for all treatments. (B) Carbon steel disk (CSD) corrosion rates based on maximum pitting depth, resulting from abiotic and biotic processes, plotted against time for all treatments.
Discussion
Biofilm Formation and Succession of Bacterial Taxa
Marked succession of bacterial taxa in CSD biofilms and SW samples was observed over the course of the experiment. The assumption that bacteria comprising biofilms on CSDs were recruited from SW, and not an alternate source or from contamination, was supported by the observation that both sample types were dominated by the same OTUs. Changes in the relative abundance of dominant OTUs over time are what distinguished biofilm and SW communities from each other. Notably, the dominant OTUs in biofilms and SW were affiliated with the Rhodobacteraceae family and the Pseudoalteromonas genus; both play important roles in marine biofilm formation (Dang and Lovell, 2000, 2002, 2016; Dang et al., 2008; Iijima et al., 2009; Bernbom et al., 2013; Elifantz et al., 2013).
The initial decrease in SW species richness, diversity, dominance (Figure 1) and community structure from t = 0 to t = 2 implies a bacterial community response to being moved from collection containers to microcosm tanks with circulating (via stir bars) seawater. However, species richness did not remain depressed, but continued to increase to near initial values and then leveled off, despite treatment additions. With the exception of the dispersant treatment, which fluctuated over time, similar trends were observed for species diversity and dominance. For all treatments, Alphaproteobacteria in SW samples increased in abundance after t = 2, to ratios more similar to initial SW bacterial community composition (Figure 2B). These observations provide important information for using closed microcosm experiments to test hypotheses pertaining to microbial ecology.
Impacts of Oil and Dispersant on Community Structure and Function
Treatments had a significant effect on community structure in both CSD biofilms and SW relative to controls. The main drivers of observed differences included OTUs affiliated with bacterial phylotypes that are known, or proposed, to metabolize hydrocarbons (genera Pseudomonas and Marinomonas) (Kostka et al., 2011; Lamendella et al., 2014). In treatments containing oil (oil and oil-dispersant), significant increases in the abundance of these OTUs were observed, suggesting an ability of these microorganisms to take advantage of the presence of oil and outcompete other microorganisms. In the dispersant-only treatments, an increase in the abundance of Pseudoalteromonas-affiliated OTUs was seen, concomitant with a decrease in OTUs affiliated with the Rhodobacteraceae family. In addition to playing significant roles in biofilm formation, phylotypes from these two groups have been implicated in hydrocarbon degradation (Dang et al., 2008; Gutierrez, 2011; Kostka et al., 2011; Kleindienst et al., 2015; Hazen et al., 2016). Pseudoalteromonas are also capable of producing compounds with antimicrobial, anti-fouling, algicidal, and other toxic properties that enable them to outcompete other organisms (Bowman, 2007). Although the specific mechanism remains to be identified, it is possible that these, or similar adaptations, imparted an advantage for Pseudoalteromonas to persist in an environment with heightened levels of chemicals unique to dispersant. Dispersant addition may, in some cases, facilitate hydrocarbon degradation by particular bacterial taxa, but it also has the potential to decrease the effectiveness of hydrocarbon metabolism by impairing or selecting against dispersant-sensitive hydrocarbon degraders and/or by selecting for microorganisms that preferentially use dispersant-derived compounds for growth (Hamdan and Fulmer, 2011; Kleindienst et al., 2015).
Exposure to dispersant in the absence of crude oil significantly impacted bacterial community structure and function across the course of the experiment. The presence of functional genes involved in the degradation of hydrocarbons was predicted to be significantly depressed compared to the control and other treatments by the PICRUSt analysis. This was confirmed by the metagenome analysis for t = 16 weeks samples. A similar response was documented by Kleindienst et al. (2015) in which addition of dispersant to microcosms and oiled marine surface water did not enhance, and inhibited, hydrocarbon oxidation rates, respectively.
For the most part, PICRUSt and metagenome data agreed; however, in certain instances, results did not align. Both methods have individual biases that need to be considered. PICRUSt is used to estimate bacterial and archaeal genes present in a microbial community genome using 16S rRNA data, yet a metagenome is merely a subset of the underlying biological metagenome (Langille et al., 2013). Primer bias and the availability of complete, sequenced genomes in the reference database can skew PICRUSt results. However, our results yielded relatively low weighted NSTI values, indicating that microbes in our samples had good reference genome coverage. Our primer sets (B969F/BA1406 and A956F/A1401R) targeted the V6-V8 variable regions of the 16S rRNA gene for bacteria and archaea and could be a potential source of bias in PICRUSt results (Tremblay et al., 2015). Metagenome sequencing, and to a lesser extent 16S rRNA gene sequencing, depth can also impact the apparent accuracy of PICRUSt predictions, and could be playing a role in our dataset (Langille et al., 2013). These biases are important to keep in mind when using metagenomic tools to interpret microbial community function.
Although Oceanospiralles, Colwellia, and Cycloclasticus-affiliated sequences did not dominate communities in our microcosms as seen in previous Gulf of Mexico deep water plume oil spill studies (Valentine et al., 2010; Redmond and Valentine, 2012), with the exception of Cycloclasticus, they were present in biofilm and seawater samples. Other bacterial phylotypes with demonstrable hydrocarbon-degrading qualities (e.g., Pseudoalteromonas, Marinobacter, Alcanivorax) which were present in sequence reads from deepwater plume samples during the active phase of the spill (Gutierrez et al., 2013), were also present in biofilm and seawater samples incubated in surface seawater. Kleindienst et al. (2015) found that dispersant exposure inhibited hydrocarbon turnover in both oil-contaminated surface seawater samples and deepwater microcosms, suggesting that similar metabolic processes may be at play in both surface and deepwater despite potential differences in bacterial taxonomic composition between the two habitats.
Impacts of Treatments on Corrosion
After 16 weeks, maximum pit depths, and corrosion rates based on maximum pit depths, were highest in CSDs from the oil and oil-dispersant treatments (Figure 5). EDS spectra of CSD surfaces indicated small differences in the elemental composition of surface corrosion products, specifically enrichment in sulfur in oil-containing treatments. Previous studies have proposed the importance of sulfur-oxidizing and sulfate-reducing bacteria (SRB) in carbon steel corrosion (Dang et al., 2011). Based on PICRUSt predicted metagenomes, the KO for sulfite reductase was enriched in the oil and oil-dispersant treatments (Figure 4A). Sulfite reductase catalyzes the reduction of sulfite to hydrogen sulfide—a compound that is corrosive to some metals including carbon steel. The increase in this KO was attributed to OTUs related to Pseudomonas alcaligenes, which is often used in bioremediation of oil spills due to its ability to degrade PAHs (O'Mahony et al., 2006). Bacteria from the Pseudomonas genus can also produce hydrogen sulfide (Lapin and Koburger, 1974; Friedrich et al., 2001). The findings of this study indicate that oil-containing treatments selected for specific hydrocarbon-degrading microbes that may also play a role in hydrogen sulfide production.
Other microorganisms that have been cited as causing MIC of carbon steel in marine environments and shipwreck corrosion include iron-oxidizing and iron-reducing bacteria. This study did not identify an abundance of iron-oxidizing bacteria in CSD biofilms or SW. OTUs affiliated with the marine iron-oxidizing chemolithotrophic Zetaproteobacteria, were found in a single control (t = 2 weeks) biofilm sample in low abundance (0.06% OTUs affiliated with genus Mariprofundus). Although, originally isolated from, and most commonly found at, iron-rich hydrothermal vents, Zetaproteobacteria, have also been identified in shallow coastal environments (Emerson et al., 2007; Dang et al., 2011; McAllister et al., 2011). Zetaproteobacteria at hydrothermal vents are typically found in flocculent microbial mats on the seafloor, whereas in shallow environments, they reportedly inhabit sediment in relatively low abundance (McBeth et al., 2011). These microcosms contained coastal SW, but were devoid of sediment, and therefore, likely lacked a significant source of Zetaproteobacteria.
Conclusions and Potential Impacts on Shipwrecks and Associated Fauna
This study demonstrated that the addition of oil, dispersant, and dispersed oil significantly affected bacterial community structure and function in SW and biofilms. Responses to treatments were immediate and persistent for a 14-week exposure. The addition of oil led to an increase in OTUs attributed to oil-degrading microorganisms. This shift was accompanied by an increase in corrosion of CSDs that had been previously exposed to unamended SW for 2 weeks. Notably, the addition of dispersant had the greatest immediate impact on SW and biofilm communities, with community structure unable to recover over the 14-week exposure and many metabolic and functional pathways potentially depressed.
The main objectives of this study were to assess the potential environmental impact of oil spills on metal-colonizing biofilms and shipwreck corrosion. Based on the results of this experiment, exposure of shipwrecks to oil and chemical dispersant could immediately impact the biodiversity and metabolic function of microbial biofilms colonizing metal-hulled shipwrecks. Changes in these biofilms could have downstream effects on corrosion rates of metal hulls, potentially impacting their longevity in the marine environment. Biofilm disruption could also impact the recruitment of invertebrate and vertebrate organisms to these artificial reefs. Further study is needed to quantify the long-term impacts of oil and dispersant exposure on metal-colonizing microbial biofilms, to understand the implications of imprecisely applying chemical dispersants in the environment, and to identify unforeseen consequences of the DWH spill on shipwrecks as historical and ecological resources.
Author Contributions
LH designed the study and secured funding. JS carried out the microcosm experiment, including setup, sample processing, and data analysis. LH and JS performed bioinformatic analyses and wrote the manuscript. BL and JL designed and performed corrosion analysis and contributed to writing the manuscript.
Funding
Funding was provided by the U.S. Department of the Interior, Bureau of Ocean Energy Management, Environmental Studies Program under Cooperative Agreement Number M13AC00015 and Interagency Agreement Number M13PG00020.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ricky Ray for providing microcosm tanks, Melanie Damour (BOEM) for input and oversight on this study, and Christine Figan, Zeima Kassahun, and Matthew Johnson for laboratory assistance. Study collaboration was provided by the U.S. Department of the Interior, Bureau of Ocean Energy Management, Environmental Studies Program.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00196/full#supplementary-material
References
Abubucker, S., Segata, N., Goll, J., Schubert, A. M., Izard, J., Cantarel, B. L., et al. (2012). Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput. Biol. 8:e1002358. doi: 10.1371/journal.pcbi.1002358
Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth, UK: PRIMER-E.
Arnosti, C., Ziervogel, K., Yang, T., and Teske, A. (2016). Oil-derived marine aggregates – hot spots of polysaccharide degradation by specialized bacterial communities. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 179–186. doi: 10.1016/j.dsr2.2014.12.008
ASTM Standard G1-03 (2003). “Standard practice for preparing, cleaning, and evaluating corrosion test specimens,” in ASTM Handbook 3.02 Corrosion of Metals; Wear and Erosion (West Conshohocken, PA: ASTM International), 17–25.
Bernbom, N., Ng, Y. Y., Olsen, S. M., and Gram, L. (2013). Pseudoalteromonas spp. serve as initial bacterial attractants in mesocosms of coastal waters but have subsequent antifouling capacity in mesocosms and when embedded in paint. Appl. Environ. Microbiol. 79, 6885–6893. doi: 10.1128/AEM.01987-13
Blanchette, R. A. (2000). A review of microbial deterioration found in archaeological wood from diierent environments. Int. Biodeterior. Biodegrad. 46, 189–204. doi: 10.1016/S0964-8305(00)00077-9
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Bowman, J. P. (2007). Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 5, 220–241. doi: 10.3390/md504220
Brakstad, O. G., Nonstad, I., Faksness, L.-G., and Brandvik, P. J. (2008). Responses of microbial communities in arctic sea ice after contamination by crude petroleum oil. Microb. Ecol. 55, 540–552. doi: 10.1007/s00248-007-9299-x
Campo, P., Venosa, A. D., and Suidan, M. T. (2013). Biodegradability of corexit 9500 and dispersed south louisiana crude oil at 5 and 25 °C. Environ. Sci. Technol. 47, 1960–1967. doi: 10.1021/es303881h
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chanton, J., Zhao, T., Rosenheim, B. E., Joye, S., Bosman, S., Brunner, C., et al. (2015). Using natural abundance radiocarbon to trace the flux of petrocarbon to the seafloor following the deepwater horizon oil spill. Environ. Sci. Technol. 49, 847–854. doi: 10.1021/es5046524
Chiu, J. M.-Y., Thiyagarajan, V., Pechenik, J. A., Hung, O.-S., and Qian, P.-Y. (2007). Influence of bacteria and diatoms in biofilms on metamorphosis of the marine slipper limpet Crepidula onyx. Mar. Biol. 151, 1417–1431. doi: 10.1007/s00227-006-0580-1
Church, R., Warren, D., Cullimore, R., Johnston, L., Schroeder, W., Patterson, W., et al. (2007). Archaeological and Biological Analysis of World War II Shipwrecks in the Gulf of Mexico: Artificial Reef Effect in Deep Water. OCS Study MMS, 387. Available online at: https://www.boem.gov/ESPIS/4/4239.pdf (Accessed October 15, 2017).
Clarke, K., and Gorley, R. (2006). PRIMER v6: User Manual/Tutorialpers. 192. Available online at: http://www.primer-e.com/Primary_papers.htm
Clarke, K. R., and Warwick, R. M. (2001). Changes in Marine Communities: an Approach to Statistical Analysis and Interpretation. Available online at: http://www.vliz.be/imis/imis.php?refid=117939
Comeau, A. M., Douglas, G. M., and Langille, M. G. I. (2017). Microbiome Helper: a Custom and Streamlined Workflow for Microbiome Research. mSystems 2. Available online at: http://msystems.asm.org/content/2/1/e00127-16 (Accessed August 17, 2017).
Comeau, A. M., Li, W. K. W., Tremblay, J. É., Carmack, E. C., and Lovejoy, C. (2011). Arctic ocean microbial community structure before and after the 2007 record sea ice minimum. PLoS ONE 6:e27492. doi: 10.1371/journal.pone.0027492
Cullimore, D. R., and Johnston, L. A. (2008). Microbiology of concretions, sediments and mechanisms influencing the preservation of submerged archaeological artifacts. Int. J. Hist. Archaeol. 12, 120–132. doi: 10.1007/s10761-008-0045-y
Dang, H., Chen, R., Wang, L., Shao, S., Dai, L., Ye, Y., et al. (2011). Molecular characterization of putative biocorroding microbiota with a novel niche detection of Epsilon- and Zetaproteobacteria in Pacific Ocean coastal seawaters. Environ. Microbiol. 13, 3059–3074. doi: 10.1111/j.1462-2920.2011.02583.x
Dang, H., Li, T., Chen, M., and Huang, G. (2008). Cross-ocean distribution of Rhodobacterales bacteria as primary surface colonizers in temperate coastal marine waters. Appl. Environ. Microbiol. 74, 52–60. doi: 10.1128/AEM.01400-07
Dang, H., and Lovell, C. R. (2000). Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl. Environ. Microbiol. 66, 467–75. doi: 10.1128/AEM.66.2.467-475.2000
Dang, H., and Lovell, C. R. (2002). Numerical dominance and phylotype diversity of marine Rhodobacter species during early colonization of submerged surfaces in coastal marine waters as determined by 16S ribosomal DNA sequence analysis and fluorescence in situ hybridization. Appl. Environ. Microbiol. 68, 496–504. doi: 10.1128/AEM.68.2.496-504.2002
Dang, H., and Lovell, C. R. (2016). Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 80, 91–138. doi: 10.1128/MMBR.00037-15
DeSantis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072. doi: 10.1128/AEM.03006-05
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C., and Flyvbjerg, H. (2015). Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 31, 3476–3482. doi: 10.1093/bioinformatics/btv401
Elifantz, H., Horn, G., Ayon, M., Cohen, Y., and Minz, D. (2013). Rhodobacteraceae are the key members of the microbial community of the initial biofilm formed in Eastern Mediterranean coastal seawater. FEMS Microbiol. Ecol. 85, 348–357. doi: 10.1111/1574-6941.12122
Emerson, D., Rentz, J. A., Lilburn, T. G., Davis, R. E., Aldrich, H., Chan, C., et al. (2007). A novel lineage of proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE 2:e667. doi: 10.1371/journal.pone.0000667
Fagervold, S. K., Galand, P. E., Zbinden, M., Gaill, F., Lebaron, P., and Palacios, C. (2012). Sunken woods on the ocean floor provide diverse specialized habitats for microorganisms. FEMS Microbiol. Ecol. 82, 616–628. doi: 10.1111/j.1574-6941.2012.01432.x
Fisher, C. R., Hsing, P.-Y., Kaiser, C. L., Yoerger, D. R., Roberts, H. H., Shedd, W. W., et al. (2014). Footprint of deepwater horizon blowout impact to deep-water coral communities. Proc. Natl. Acad. Sci. U.S.A. 111, 11744–11749. doi: 10.1073/pnas.1403492111
Friedrich, C. G., Rother, D., Bardischewsky, F., Quentmeier, A., and Fischer, J. (2001). Oxidation of reduced inorganic sulfur compounds by bacteria: emergence of a common mechanism? Appl. Environ. Microbiol. 67, 2873–2882. doi: 10.1128/AEM.67.7.2873-2882.2001
Gjelstrup Björdal, C. (2012). Microbial degradation of waterlogged archaeological wood. J. Cult. Herit. 13, S118–S122. doi: 10.1016/j.culher.2012.02.003
Gutierrez, T. (2011). Identifying polycyclic aromatic hydrocarbon-degrading bacteria in oil-contaminated surface waters at Deepwater Horizon by cultivation, stable isotope probing and pyrosequencing. Rev. Environ. Sci. Biotechnol. 10, 301–305. doi: 10.1007/s11157-011-9252-9
Gutierrez, T., Singleton, D. R., Berry, D., Yang, T., Aitken, M. D., and Teske, A. (2013). Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 7, 2091–2104. doi: 10.1038/ismej.2013.98
Hamdan, L. J., Coffin, R. B., Sikaroodi, M., Greinert, J., Treude, T., and Gillevet, P. M. (2013). Ocean currents shape the microbiome of Arctic marine sediments. ISME J. 7, 685–696. doi: 10.1038/ismej.2012.143
Hamdan, L. J., and Fulmer, P. A. (2011). Effects of COREXIT® EC9500A on bacteria from a beach oiled by the Deepwater Horizon spill. Aquat. Microb. Ecol. 63, 101–109. doi: 10.3354/ame01482
Hazen, T. C., Prince, R. C., and Mahmoudi, N. (2016). Marine oil biodegradation. Environ. Sci. Technol. 50, 2121–2129. doi: 10.1021/acs.est.5b03333
Huggett, M. J., Williamson, J. E., de Nys, R., Kjelleberg, S., and Steinberg, P. D. (2006). Larval settlement of the common Australian sea urchin Heliocidaris erythrogramma in response to bacteria from the surface of coralline algae. Oecologia 149, 604–619. doi: 10.1007/s00442-006-0470-8
Iijima, S., Washio, K., Okahara, R., and Morikawa, M. (2009). Biofilm formation and proteolytic activities of Pseudoalteromonas bacteria that were isolated from fish farm sediments. Microb. Biotechnol. 2, 361–369. doi: 10.1111/j.1751-7915.2009.00097.x
Jimenez, C., Andreou, V., Evriviadou, M., Munkes, B., Hadjioannou, L., Petrou, A., et al. (2017). Epibenthic communities associated with unintentional artificial reefs (modern shipwrecks) under contrasting regimes of nutrients in the Levantine Sea (Cyprus and Lebanon). PLoS ONE 12:e0182486. doi: 10.1371/journal.pone.0182486
Joye, S. B., Bracco, A., Özgökmen, T. M., Chanton, J. P., Grosell, M., MacDonald, I. R., et al. (2016). The Gulf of Mexico ecosystem, six years after the Macondo oil well blowout. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 4–19. doi: 10.1016/j.dsr2.2016.04.018
Joye, S. B., Teske, A. P., and Kostka, J. E. (2014). Microbial dynamics following the macondo oil well blowout across gulf of mexico environments. Bioscience 64, 766–777. doi: 10.1093/biosci/biu121
Kleindienst, S., Grim, S., Sogin, M., Bracco, A., Crespo-Medina, M., and Joye, S. B. (2016). Diverse, rare microbial taxa responded to the Deepwater Horizon deep-sea hydrocarbon plume. ISME J. 10, 400–415. doi: 10.1038/ismej.2015.121
Kleindienst, S., Seidel, M., Ziervogel, K., Grim, S., Loftis, K., Harrison, S., et al. (2015). Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. Proc. Natl. Acad. Sci. U.S.A. 112, 14900–14905. doi: 10.1073/pnas.1507380112
Kostka, J. E., Prakash, O., Overholt, W. A., Green, S. J., Freyer, G., Canion, A., et al. (2011). Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the deepwater horizon oil spill. Appl. Environ. Microbiol. 77, 7962–7974. doi: 10.1128/AEM.05402-11
Lamendella, R., Strutt, S., Borglin, S., Chakraborty, R., Tas, N., Mason, O. U., et al. (2014). Assessment of the Deepwater Horizon oil spill impact on Gulf coast microbial communities. Front. Microbiol. 5:130. doi: 10.3389/fmicb.2014.00130
Langille, M. G. I., Zaneveld, J., Caporaso, J. G., McDonald, D., Knights, D., Reyes, J. A., et al. (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821. doi: 10.1038/nbt.2676
Lapin, R. M., and Koburger, J. A. (1974). Hydrogen sulfide production by Pseudomonas putrefaciens in shrimp experimentally packed in nitrogen. Appl. Microbiol. 27, 666–70.
Lau, S., Thiyagarajan, V., Cheung, S., and Qian, P. (2005). Roles of bacterial community composition in biofilms as a mediator for larval settlement of three marine invertebrates. Aquat. Microb. Ecol. 38, 41–51. doi: 10.3354/ame038041
Leary, D. H., Li, R. W., Hamdan, L. J., Hervey, W. J., Lebedev, N., Wang, Z., et al. (2014). Integrated metagenomic and metaproteomic analyses of marine biofilm communities. Biofouling 30, 1211–1223. doi: 10.1080/08927014.2014.977267
Marti, J., and Anderson (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
McAllister, S. M., Davis, R. E., McBeth, J. M., Tebo, B. M., Emerson, D., and Moyer, C. L. (2011). Biodiversity and emerging biogeography of the neutrophilic iron-oxidizing Zetaproteobacteria. Appl. Environ. Microbiol. 77, 5445–5457. doi: 10.1128/AEM.00533-11
McBeth, J. M., Little, B. J., Ray, R. I., Farrar, K. M., and Emerson, D. (2011). Neutrophilic iron-oxidizing "zetaproteobacteria" and mild steel corrosion in nearshore marine environments. Appl. Environ. Microbiol. 77, 1405–1412. doi: 10.1128/AEM.02095-10
Milferstedt, K., Santa-Catalina, G., Godon, J.-J., Escudié, R., Bernet, N., and Szewzyk, U. (2013). Disturbance frequency determines morphology and community development in multi-species biofilm at the landscape scale. PLoS ONE 8:e80692. doi: 10.1371/journal.pone.0080692
Montagna, P. A., Baguley, J. G., Cooksey, C., Hartwell, I., Hyde, L. J., Hyland, J. L., et al. (2013). Deep-sea benthic footprint of the deepwater horizon blowout. PLoS ONE 8:e70540. doi: 10.1371/journal.pone.0070540
Neria-González, I., Wang, E. T., Ramírez, F., Romero, J. M., and Hernández-Rodríguez, C. (2006). Characterization of bacterial community associated to biofilms of corroded oil pipelines from the southeast of Mexico. Anaerobe 12, 122–133. doi: 10.1016/j.anaerobe.2006.02.001
O'Mahony, M. M., Dobson, A. D., Barnes, J. D., and Singleton, I. (2006). The use of ozone in the remediation of polycyclic aromatic hydrocarbon contaminated soil. Chemosphere 63, 307–314. doi: 10.1016/j.chemosphere.2005.07.018
Palacios, C., Zbinden, M., Pailleret, M., Gaill, F., and Lebaron, P. (2009). Highly similar prokaryotic communities of sunken wood at shallow and deep-sea sites across the oceans. Microb. Ecol. 58, 737–752. doi: 10.1007/s00248-009-9538-4
Parks, D. H., Tyson, G. W., Hugenholtz, P., and Beiko, R. G. (2014). STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. doi: 10.1093/bioinformatics/btu494
Passow, U., Ziervogel, K., Asper, V., and Diercks, A. (2012). Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ. Res. Lett. 7:35301. doi: 10.1088/1748-9326/7/3/035301
Ray, R., and Little, B. (2003). “Environmental electron microscopy applied to biofilms,” in Biofilms in Medicine, Industry and Environmental Biotechnology, ed P. Lens (London: IWA), 331–351.
Reddy, C. M., Arey, J. S., Seewald, J. S., Sylva, S. P., Lemkau, K. L., Nelson, R. K., et al. (2012). Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. U.S.A. 109, 20229–20234. doi: 10.1073/pnas.1101242108
Redmond, M. C., and Valentine, D. L. (2012). Natural gas and temperature structured a microbial community response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. U.S.A. 109, 20292–20297. doi: 10.1073/pnas.1108756108
Socolofsky, S. A., Adams, E. E., and Sherwood, C. R. (2011). Formation dynamics of subsurface hydrocarbon intrusions following the Deepwater Horizon blowout. Geophys. Res. Lett. 38, 1–6. doi: 10.1029/2011GL047174
Stout, S. A., Rouhani, S., Liu, B., Oehrig, J., Ricker, R. W., Baker, G., et al. (2017). Assessing the footprint and volume of oil deposited in deep-sea sediments following the Deepwater Horizon oil spill. Mar. Pollut. Bull. 114, 327–342. doi: 10.1016/j.marpolbul.2016.09.046
Svane, I., and Petersen, J. K. (2001). On the problems of epibioses, fouling and artificial reefs, a review. Mar. Ecol. 22, 169–188. doi: 10.1046/j.1439-0485.2001.01729.x
Tremblay, J., Singh, K., Fern, A., Kirton, E. S., He, S., Woyke, T., et al. (2015). Primer and platform effects on 16S rRNA tag sequencing. Front. Microbiol. 6:771. doi: 10.3389/fmicb.2015.00771
Valentine, D. L., Fisher, G. B., Bagby, S. C., Nelson, R. K., Reddy, C. M., Sylva, S. P., et al. (2014). Fallout plume of submerged oil from Deepwater Horizon. Proc. Natl. Acad. Sci. U.S.A. 111, 15906–15911. doi: 10.1073/pnas.1414873111
Valentine, D. L., Kessler, J. D., Redmond, M. C., Mendes, S. D., Heintz, M. B., Farwell, C., et al. (2010). Propane respiration jump-starts microbial response to a deep oil spill. Science 330, 208–211. doi: 10.1126/science.1196830
Yang, T., Speare, K., McKay, L., MacGregor, B. J., Joye, S. B., and Teske, A. (2016). Distinct bacterial communities in surficial seafloor sediments following the 2010 deepwater horizon blowout. Front. Microbiol. 7:1384. doi: 10.3389/fmicb.2016.01384
Yergeau, E., Maynard, C., Sanschagrin, S., Champagne, J., Juck, D., Lee, K., et al. (2015). Microbial community composition, functions, and activities in the gulf of mexico 1 year after the deepwater horizon accident. Appl. Environ. Microbiol. 81, 5855–5866. doi: 10.1128/AEM.01470-15
Zhang, J., Kobert, K., Flouri, T., and Stamatakis, A. (2014). PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620. doi: 10.1093/bioinformatics/btt593
Keywords: deepwater horizon oil spill, dispersant, crude oil, metagenomics, microcosm experiment, microbial corrosion, marine microbes, marine biofilms
Citation: Salerno JL, Little B, Lee J and Hamdan LJ (2018) Exposure to Crude Oil and Chemical Dispersant May Impact Marine Microbial Biofilm Composition and Steel Corrosion. Front. Mar. Sci. 5:196. doi: 10.3389/fmars.2018.00196
Received: 09 December 2017; Accepted: 17 May 2018;
Published: 06 June 2018.
Edited by:
Alison Buchan, University of Tennessee, Knoxville, United StatesReviewed by:
Matthew Saxton, University of Georgia, United StatesIrene Wagner-Doebler, Helmholtz-Zentrum für Infektionsforschung (HZI), Germany
Copyright © 2018 Salerno, Little, Lee and Hamdan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leila J. Hamdan, bGVpbGEuaGFtZGFuQHVzbS5lZHU=
 Jennifer L. Salerno
Jennifer L. Salerno Brenda Little2
Brenda Little2 Leila J. Hamdan
Leila J. Hamdan