
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 13 February 2018
Sec. Marine Ecosystem Ecology
Volume 5 - 2018 | https://doi.org/10.3389/fmars.2018.00047
This article is part of the Research Topic The Importance of Behavior in the Recruitment of Marine Fauna and Flora View all 10 articles
 Jeffrey M. Leis1,2*
Jeffrey M. Leis1,2*Most marine ecologists have in the past 25 years changed from supporting a passive-dispersal paradigm for larval marine fishes to supporting a biophysical-dispersal paradigm wherein the behaviour of larvae plays a central role. Research shows larvae of demersal perciform fishes have considerable swimming and orientation abilities over a major portion of their pelagic larval duration. These abilities depend on sensory function, and some recent research has indicated anthropogenic acidification of the oceans will by the end of the century result in sensory dysfunction. This could strongly alter the ability of fish larvae to orientate in the pelagic environment, to locate suitable settlement habitat, to bet-hedge, and to colonize new locations. This paper evaluates the available publications on the effects of acidification on senses and behaviours relevant to dispersal of fish early life-history stages. A large majority of studies tested CO2 values predicted for the middle to end of the century. Larvae of fourteen families—all but two perciform—were studied. However, half of studies used Damselfishes (Pomacentridae), and except for swimming, most studies used settlement-stage larvae or later stages. In spite of these taxonomic and ontogenetic restrictions, all but two studies on sensory function (chemosensation, hearing, vision, detection of estuarine cues) found deleterious effects from acidification. The four studies on lateralization and settlement timing all found deleterious effects from acidification. No clear effect of acidification on swimming ability was found. If fish larvae cannot orientate due to sensory dysfunction, their dispersal will, in effect, conform to the passive dispersal paradigm. Modelling incorporating larval behaviour derived from empirical studies indicates that relative to active larvae, passive larvae will have less self-recruitment, higher median and mean dispersal distances, and lower settlement rates: further, bet hedging and colonization of new locations will decrease. The biophysical dispersal paradigm will be lost in theory and in fact, which is predicted to result in lower recruitment and less bet hedging for demersal, perciform fishes. More research is required to determine if the larvae of other Orders will be effected in the same way, or if warm- and cold-water fish faunas will be similarly effected.
Most marine bony fishes have a pelagic larval stage that differs morphologically from the adult (Moser, 1981) and that is, at least potentially, dispersive. Especially for demersal fish species in the tropics, adults are often relatively site attached, and dispersal by the larvae determines the spatial scale of population connectivity (Sale, 1980, 1991). Although there are reasons to expect larval dispersal to differ between low and high latitudes (Hunt von Herbing, 2002), there is little evidence that it does. Further, making latitudinal comparisons in dispersal metrics and the factors that determine dispersal outcomes is difficult due to a variety of factors including very large differences in the taxonomic composition of marine fish communities with latitude (Leis et al., 2013). Marine fish larvae spend weeks to months in pelagic open waters away from demersal adult habitat (Luiz et al., 2013) before they must find suitable habit into which to settle. Settlement is often, but not always, associated with abrupt metamorphosis, but regardless of morphological changes, an animal that has experienced nothing but a pelagic environment must quickly adapt to a very different demersal habitat (Leis, 1991).
Traditionally, marine biologists assumed that dispersal of fish larvae is essentially a passive process, with movement of the presumed weakly-swimming larvae totally determined by currents (Roberts, 1997). More recently, swimming, orientation, behavioural, and sensory capabilities of larval marine perciform fishes have been shown to be remarkably strong for much of the pelagic larval duration, or PLD (Leis, 2006, 2010). Larvae can swim in an orientated way at speeds that are often comparable to the currents of the waters in which they live, thus directly influencing their dispersal. Larvae can detect and respond to a range of olfactory, auditory, and visual cues from settlement habitat, from within the pelagic environment (Leis et al., 2011b) and even to celestial (Mouritsen et al., 2013; Leis et al., 2014) and magnetic (Bottesch et al., 2016; O'Connor and Muheim, 2017) cues. By controlling their vertical distribution, larvae may indirectly influence their dispersal because current velocities often vary with depth. The proper functioning of the sense organs of fish larvae is essential in all these processes. As a result of these findings, the passive dispersal paradigm in larval marine fishes has been widely rejected in favour of a biophysical dispersal paradigm that recognizes the strong influence of biological factors, including behaviour of larvae, on dispersal outcomes (Leis, 2015).
As part of this paradigm shift our view of the spatial scale of larval dispersal and population connectivity has altered. Previously, marine ecologists considered populations of demersal marine fishes to be genetically and demographically open (Roberts, 1997)—that is, the new recruits to a given area did not result from propagules produced there, but from larvae that drifted passively with the currents from elsewhere. We now know that most demersal marine fish populations are closed to a greater or lesser extent (Jones et al., 2009), and that this “self-recruitment” is the result of biophysical processes, including behaviour of the larvae themselves (Leis, 2015). Nevertheless, a proportion of the larvae produced in any area are also exported to other areas. These conclusions are supported by modelling that takes into account empirically demonstrated larval-fish behaviour, e.g., Wolanski and Kingsford (2014).
The effects of lower pH as human-produced CO2 is absorbed by the ocean have received increasing attention, and will perhaps be more profound and less spatially variable than those of the predicted increase in temperature. Acidification can affect dispersal of fish larvae in two ways. It can cause developmental errors that lead to morphological deformities, and damage to organs, including sense organs that may impair their function. Acidification also results in neurotransmitter dysfunction (Nilsson et al., 2012), and as shown in a number of studies, this interferes with the ability of fish larvae to use the sensory systems that are vital to orientation in the ocean. Without orientation ability, swimming by larvae will have greatly diminished net influence on dispersal. It is likely depth selection ability of larvae will also be diminished, resulting in less structured vertical distributions. This has important implications for dispersal outcomes. It is also possible acidification may affect other behaviours, such as swimming ability, that influence dispersal outcomes (Nagelkerken and Munday, 2016).
Studies on fish-larvae behaviour, particularly in situ studies, routinely find statistically significant relationships between, for example, swimming speed or depth and size of the larvae but there is always a high level of variability associated with the relationships (Figures 1A,B, see Leis, 2006, 2010). Some individual larvae have a more meandering swimming path through the ocean than others and will therefore pass over different demersal habitat, and have a slower start-to-finish net swim speed than more directional swimmers (Figure 1C). Similarly, fish larvae often have significant among-individual (within-species) swimming directionality, but there are invariably many individuals that swim in directions other than the overall mean (Figure 1D). These variations mean that a greater or lesser proportion of individuals will have different dispersal outcomes than the majority. This minority with different dispersal outcomes due to differences in behaviour can be regarded as contributing to bet-hedging and to colonizing new localities as, at the end of their PLD, they will be at different locations than the majority, either in direction or distance from their point of origin. Theoretical considerations commonly cite bet hedging and colonization of new localities as reasons for the evolution of a bipartite life history with a pelagic larval dispersal stage.
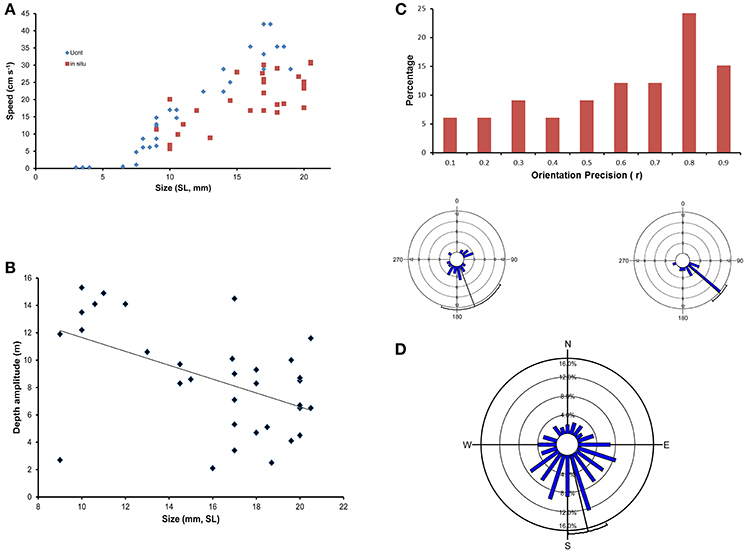
Figure 1. (A) Ontogenetic change in swimming speed as measured in a laboratory raceway (critical speed [Ucrit], Diamonds), and in the ocean (in situ speed, Squares) of a serranid, Epinephelus coioides. Note the wide range in speeds at any size, particularly in the ocean. Although there is a significant increase in speed with size for both measures, the laboratory measure relationship explains 92% of the variation over the common size range whereas the in situ relationship explains only 54%. Data from Leis et al. (2007, 2009). (B) Ontogenetic change in in situ depth amplitude (difference between greatest and least depth) in larvae of a serranid, Epinephelus coioides. Note the wide range in depth amplitude at any size. The significant ontogenetic decrease in depth amplitude explains only 25% of the variation. Size of larvae is standard length (SL). Data from Leis et al. (2009). (C) Frequency distribution of in situ orientation precision (r, length of the mean vector) of 33 individual larvae of a serranid, Epinephelus coioides. The circular insets show the distribution of swimming bearings of two individuals measured every 30 s over a 10 min observation period. Although both individuals have significantly directional swimming to the South-East, the one on the left (r = 0.43, p = 0.02) has a more meandering swim path than the one on the right (r = 0.89, p << 0.0001). Data from Leis et al. (2009). The thin black radius is the mean direction, and the arc at its end is the 95% confidence interval. (D) In situ directional swimming by larvae of a pomacentrid, Chromis atripectoralis off the west side of Lizard Island, Great Barrier Reef, showing the frequency distribution of mean swimming bearings of 205 individually measured larvae with significant within-individual swimming directionality. The among-individual swimming direction was to the South-South-East (mean = 166.7 ± 12.8°), and highly significant (p << 0.0001, Rayleigh Test), but 22% of larvae swam in a direction that differed more than 90° from the mean. Data from Leis et al. (2014).
The present paper evaluates the evidence relating to adverse effects of acidification on behaviour of fish larvae and the function of their sensory systems relevant to orientation and dispersal, and discusses implications for larval dispersal and the implications of this for populations of demersal marine fishes, especially those of lower latitudes.
Relatively few published studies address the effects of ocean acidification on sensory systems or behaviour of fully pelagic larvae, although the numbers of such publications are increasing (Nagelkerken and Munday, 2016). Much of the experimental work on the effects of ocean acidification has been done on fish that are in transition between the pelagic dispersal stage and the relatively site-attached demersal stage. How far the young fishes are through this transition varies, as does the nomenclature used for them by researchers. A study may use settlement-stage larvae captured in light traps as they approach a reef from open water, but then held in a low-pH environment for several days (usually four) before experiments are undertaken. Or, it may use larvae reared in captivity (usually in low pH conditions) and then tested at their normal age of settlement. Larvae undergoing the major ecological transition from being a pelagic animal to being a demersal animal are also undergoing major physiological, morphological and behavioural transitions. For example, the “very high swimming speeds of pre-settlement larval reef fishes are accompanied by the highest rates of oxygen uptake found in ectothermic vertebrates, but once the larvae settle, their capacity for rapid oxygen uptake falls, and the ability for high-affinity oxygen uptake at low oxygen levels increases”: they become hypoxia tolerant (Nilsson et al., 2007). Similarly, swimming abilities of larvae abruptly decrease upon settlement (Leis et al., 2011a). In fact, the transitions in physiology and morphology can begin some time before the larvae reach their settlement habitat (Holzer et al., 2017). Therefore, caution is required in assuming results based on larvae in transition apply to fully pelagic individuals. Publications on studies that hold wild settlement-stage larvae in low pH conditions for several days before testing seldom describe the tanks in which they are held. The pelagic-to-demersal transition can be slowed by holding the larvae in relatively featureless conditions (McCormick, 1999; Holzer et al., 2017). This approach means larvae undergo minimal transition to their demersal stage before testing, and if it is used, should be mentioned in methods sections of publications. Even more caution is required when assessing results based on young juveniles that have been settled for weeks or longer. Very few demersal bony fishes avoid the pelagic life history stage entirely (Leis, 1991), but the damselfish Acanthochromis polyacanthus is one. This species hatches on the reef well-developed at a relatively large size (Kavanagh, 2000), and is frequently used in experimental work. However, the use of A. polyacanthus young as proxies for pelagic larvae of other species is questionable, as is emphasized by the fact that it's recently hatched young do not undergo the transition from high oxygen consumption to hypoxia tolerance, being well-adapted to hypoxia and having relatively low maximum rates of oxygen uptake from hatching (Nilsson et al., 2007). Therefore, it is important to take into account not only the species that has been tested, but also its life-history stage.
The present evaluation is confined to marine early-life history stages of fishes. A recent paper (Ashur et al., 2017) summarizes the effects of acidification on chemosensation, hearing, and vision in aquatic invertebrates and fishes. The results of that review relevant to the present paper will be drawn upon, plus a few additional publications. Behaviours of relevance to dispersal will also be considered: swimming, lateralization, settlement timing. The early life-history nomenclature used in Ashur et al. (2017) is that of the publications they reviewed, but no standard nomenclature was used by those studies: e.g., settlement-stage larvae held in laboratory tanks for 4 days are called settlement-stage larvae or juveniles in different studies. The nomenclature used here (defined in Table 1) is based on examination of the methods sections of the original publications, regardless of nomenclature used therein.
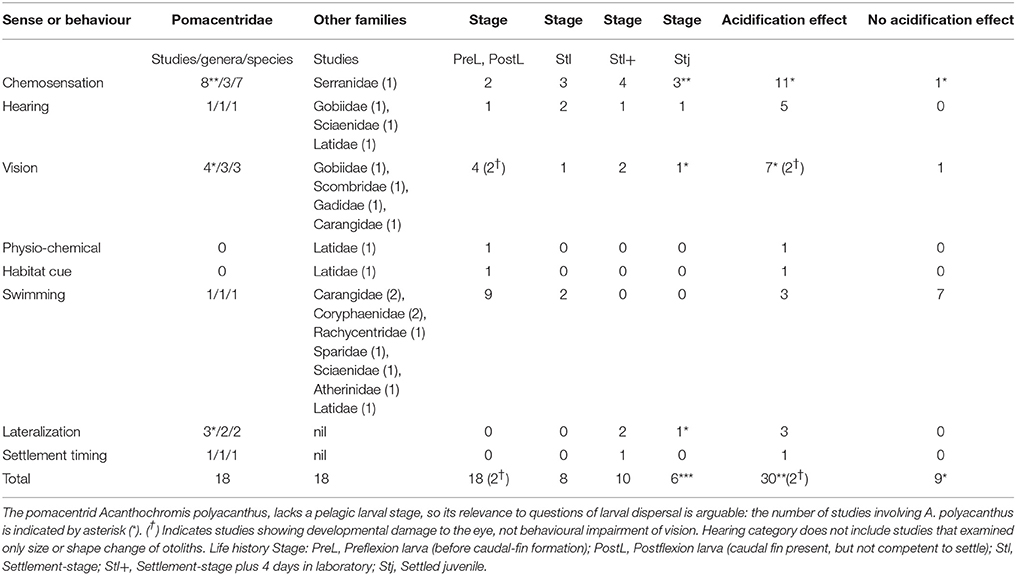
Table 1. Studies of effects of acidification on sensory function or behaviour relevant to dispersal of marine fish larvae.
Adding Sundin et al. (2017) to the relevant studies listed by Ashur et al. (2017) gives nine studies of chemosensation covering eight species of two families (Table 1). Seven species are Damselfishes (Pomacentridae), one of which is A. polyacanthus, and the eighth, a Grouper (Serranidae). Three study-by-species combinations are on reared, settlement-stage larvae; four are settlement-stage larvae from light traps held in the laboratory for 4 days; and three are settled juveniles (two of which are A. polyacanthus, and the third, a reared Grouper). Only two are on fully pelagic larvae: reared Damselfish (Amphiprion percula). Eight studies detected an acidification-induced change in behaviour: either a reversal or loss of normal behaviour, or a decrease in it's prevalence. Only a study on the reaction to predator chemical cues of 3-month old A. polyacanthus juveniles did not detect an effect of acidification (Sundin et al., 2017). So, for chemosensation, acidification has the consistent effect of altering normal behaviour in pelagic larvae, and those in transition during settlement.
Adding Castro et al. (2017) to the relevant hearing studies listed by Ashur et al. (2017) gives four studies covering four species of four families (Table 1). One species is a Damselfish, plus one each Goby (Gobiidae), Croaker (Sciaenidae), and Barramundi (Latidae). Two studies are on settlement-stage larvae, one on both presettlement and settlement-stage larvae, and one on recently settled Damselfish, all reared. In all studies, acidification caused a change in behaviour in relation to a habitat sound. So, for hearing, the results are consistent, and apply to a range of families and early life-history stages, although with only four studies, more research on hearing in fish larvae is needed.
In addition, Ashur et al. (2017) list 10 studies on the effects of acidification on otolith size or shape in marine fish early-life history stages. Six record an increase in size or change in shape, four found no change. In none of these studies was hearing investigated, even though there are theoretical reasons to expect changed otoliths will change hearing (Lychakov and Rebane, 2005). The otoliths of fishes are involved in both hearing and balance, and otolith size may be more influential on balance and swimming than on hearing (Popper et al., 2005). So it is difficult to predict how changes in otolith shape or size would influence hearing. The mixed results of the effects of acidification on otolith size and shape imply that there will be no simple answer. Therefore, no conclusion is attempted here about the sensory implications of changes in otoliths.
Ashur et al. (2017) list four relevant vision studies to which can be added Allan et al. (2014) and Munday et al. (2016). These cover six species of three families (Table 1): four are on Damselfish (one is A. polyacanthus), and one each on a Goby and Yellowtail (Carangidae). Three are on reared larvae, either early preflexion or settlement-stage (Allan et al., 2014). Two are on settlement-stage larvae from light traps held in laboratory tanks for 4 days, and one on 55–80 mm, settled A. polyacanthus juveniles. The last found a reduced retina maximal flicker frequency, which could impair the fish's capacity to react to fast events. Acidification caused changes in all three vision-related anti-predator behaviour studies. Newly hatched goby larvae had a change in phototactic response (Forsgren et al., 2013). Yellowtail preflexion larvae had no change in startle response or phototaxis (Munday et al., 2016).
In addition, two studies document developmental damage to the eyes of either preflexion or post-flexion reared larvae of Cod (Gadidae) and Tuna (Scombridae) (Frommel et al., 2012, 2016) (Table 1). This sort of damage could impair vision, with a variety of deleterious effects on the larvae. It is noteworthy that studies reporting morphological damage to organs of fish larvae do not always mention the full range of organs examined, so there may be unreported results of no acidification-related eye damage.
Although, all but one study report an effect of acidification on vision, none directly tested whether acidification would adversely effect vision in a way that could influence orientation. This might include, for example, using a celestial cue for orientation or using light intensity for adjustment of vertical distribution.
A single study on reared post-flexion larvae of catadromous Barramundi (Latidae) reports acidification changes to temperature and salinity preferences (Pistevos et al., 2017), and also reports a change from avoidance to preference for estuarine water in preflexion larvae (Table 1). The latter may be a response to either physio-chemical or olfactory cues.
Nine studies concern aspects of swimming, eight with reared larvae of various stages, and one with wild, post-flexion Silverside (Atherinidae) larvae (Table 1). The Damselfish and Barramundi studies involved settlement-stage larvae. Mean routine or critical swimming speed, both laboratory measures, decreased in Carangidae (Munday et al., 2016, 2017a), Coryphaenidae (Bignami et al., 2014), and Latidae (Rossi et al., 2015), but did not change in Atherinidae (Silva et al., 2016), Coryphaenidae (Pimentel et al., 2014), Pomacentridae (Munday et al., 2009a), or Rachycentridae (Bignami et al., 2013). Other aspects of swimming behaviour had no or minor changes in Gadidae (Maneja et al., 2013), Sciaenidae, and Sparidae (Pimentel et al., 2016). The measures of swimming most relevant to dispersal—in situ speed and endurance—were not tested. The mixed results with swimming speeds are in contrast to the much more consistent results with sensory function. There is little evidence for a widespread effect of acidification on swimming.
Three studies on Damselfishes tested acidification effects on lateralization. Two used wild settlement-stage Neopomacentrus azysron larvae from light traps held 4 days in laboratory tanks (Domenici et al., 2011; Nilsson et al., 2012), and the third used month-old juveniles of A. polyacanthus (Welch et al., 2014). The first two studies report a loss of lateralization, and the third a decrease in lateralization.
A single study on wild Damselfish Pomacentrus chrysurus settlement-stage larvae held for 4 days in the laboratory in low pH conditions found that peak settlement shifted from new to full moon (Devine et al., 2012).
The levels of acidity used varies amongst studies. The large majority (32) of studies on senses used atmospheric CO2 levels predicted for middle and/or end of this century (550–1020 μatm): all but two of these found detrimental changes to sensory performance. One vision and three hearing studies with lowest tested CO2 values above end of century values (1368–1675 μatm) all found an effect. Amongst otolith-only studies, five used mid-to-end of century values, and five used more acidic conditions (1050–1800 μatm), and both low and high CO2 groups had the same result: three studies with a change in otolith size or shape, and two with no change. For swimming, six studies used CO2 values for the middle to the end of the century (two had an effect, four did not), whereas four used values of 1400–1800 μatm (one had an effect, three did not). In some cases, higher CO2 levels were selected based on regional differences in ocean pH such as upwelling (e.g., Castro et al., 2017), but in others, it was not clear upon what basis more acidic conditions were chosen. However, it does not appear that acidity very much higher than expected by 2100 is required for detrimental sensory performance or behaviours to be likely. Most acidification studies use CO2 levels for the middle to end of this century, but for some marine environments temperature may have already reached very damaging levels before this. Coral reefs, for example, are already experiencing repeated serious bleaching, and may be so degraded by mid-century (Hughes et al., 2017) that questions about acidification effects on larval-fish dispersal may be secondary to questions about whether adult habitat will be able to support reef-dependent fishes.
Several conclusions arise from this evaluation of studies on the effects of acidification on senses and behaviours of fishes during their marine early life history. First, the effects on sensory function are much more consistent than those on behaviours such as swimming speed: sensory functionality is deleteriously impacted, either reversed, lost or decreased by acidification. In only two cases was an adverse effect on sensory function not found: chemosensation in 55–80 mm A. polyacanthus, and vision in preflexion Yellowtail carangid. Second, chemosensation is the most studied sense (n = 9), followed by vision and swimming (n = 8 each), and hearing (n = 4). Third, studies on senses—particularly chemosensation and vision—overwhelmingly used Damselfishes and larvae at the end of their pelagic dispersal stage (settlement-stage and beyond). In fact, about half of all studies are on Damselfishes, and if swimming is excluded, nearly two-thirds of studies are on Damselfishes. Only a narrow range of 12 families has been studied thus far: 7 are coastal demersal (1 catadromous), 1 coastal epipelagic, and 4 oceanic or neritic. Most studies used tropical or warm temperate species, and there is so little overlap in the studied senses or behaviours that no conclusions can be reached about whether there are latitudinal differences in acidification effects. This situation applies to the general question of whether larval-fish dispersal differs between high and low latitudes (Leis et al., 2013). No clear conclusions can be reached because of the very large differences in taxa (usually at the Order level) between low and high latitudes, and because there are relatively few studies on aspects of larval behaviour or sensory abilities relevant to dispersal from high latitudes (Leis et al., 2013). Fourth, there is little, if any, indication that life-history stage influences whether acidification will have an effect, but this has to be qualified by noting that younger, less developed larvae are understudied, except for swimming. In the context of orientation of swimming, the lack of work on preflexion larvae is less important than for post-flexion larvae, because swimming ability of preflexion larvae is much less than that of older larvae (Fisher and Leis, 2009). In contrast, larvae control their vertical distribution for nearly all of their PLD. In one of three cases young A. polyacanthus had results that differed from earlier life history stages of other Damselfishes, emphasising the need for caution in the use of this species as a proxy for the pelagic stages of other species. Of course, it would be preferable to have studies across a wider range of taxa, and with more emphasis on larvae before they start their transition to the demersal environment. However, the consistency of the results to date provide reason to expect that acidification will have deleterious effects on the sensory systems and some behaviours of marine fish larvae of a range of species. Regardless, we need to spread our taxonomic and ontogenetic nets wider to include more families and more presettlement-stage larvae, to obtain a firmer basis of knowledge.
The loss or diminution of sensory function due to acidification has serious implications for the orientation ability of fish larvae in the pelagic environment, and for the ability of settlement-stage larvae to locate suitable settlement habitat. This has been mentioned before numerous times, but without much detail: e.g., Munday et al. (2009b). The studies evaluated here provide consistent evidence that orientation depending on olfaction, hearing, vision, and lateralization will be disrupted. The effect of acidification on magnetic and celestial senses has not been examined, but given the nature of the source of this sensory dysfunction (Nilsson et al., 2012; Nilsson and Lefevre, 2016), it seems likely that they, too, will be disrupted. In contrast, it does not seem that swimming itself is influenced very much, if at all, by acidification.
The single study showing a change in settlement timing is concerning, as one would expect that the relatively precise settlement timing normally found in many species has evolved to maximize arrival at settlement habitat and minimize mortality during the settlement transition. If true, then settlement at non-optimal times would result in lower recruitment.
An inherent part of the biophysical dispersal paradigm is that fish larvae influence their dispersal, but without the ability to orientate their swimming or control vertical position or to react appropriately to habitat cues, their ability to influence dispersal either directly or indirectly will be limited. In short, the larvae will disperse in a more passive manner, and the biophysical dispersal paradigm will, in theory and in fact, be lost and replaced by a passive dispersal paradigm. Modelling indicates that dispersal by passive larvae results in a smaller proportion of larvae reaching settlement habitat, a lower level of self-recruitment, and increased mean and median dispersal distances: we can expect the same for real fish larvae in a low pH ocean. This means the shape and height of dispersal kernels which have evolved in the ocean over long periods of time will be altered by human generation of atmospheric CO2 over a few decades. In addition, the behaviours that lead to bet-hedging and locating new locations will decrease or be lost due to decrease or loss in orientation ability.
We are only now beginning to develop a clear understanding of the biophysical dispersal paradigm, and if it is overturned by ocean acidification, it is ironic that it will be replaced by the traditional passive dispersal paradigm. This may make the job of dispersal modellers simpler by eliminating the need for understanding of and integration into models, of a range of behaviours and abilities that change with ontogeny of larvae. But the cost will be high to aquatic ecosystems and the demersal fisheries from which humans derive a great deal of their protein. We don't know to what extent all of this will apply to higher latitude, cold water demersal fishes and their larvae, just as we don't know if larval dispersal in general differs with latitude (Leis et al., 2013). More research on the effects of acidification on sensory systems of larvae of high-latitude species and on non-perciform species generally, should be a priority.
Ocean acidification may also adversely effect other aspects of larval-fish biology and behaviour such as predator avoidance (Wang et al., 2017), growth rates, and feeding ability, among other things (Nagelkerken and Munday, 2016). Any of these could lead to increased mortality and reduced recruitment, but the results of studies of acidification effects on them have not been consistent. They have not been reviewed here because in an acidified ocean, these aspects would apply equally to larvae that are passively dispersed as to those that behaviourally participate in and influence their dispersal. That is, they would be expected to have a similar effect on the passive dispersal paradigm as on the biophysical dispersal paradigm.
A best-case scenario is that marine fish species may be able to acclimate or adapt through natural selection so their larvae can deal with the low-pH, high temperature conditions of the near future pelagic ocean in which larvae live, develop, grow, and disperse. The exact physiological and biochemical mechanisms involved in sensory disruption in fish larvae are not entirely understood, so prediction is difficult, but there are reasons to expect within-generation acclimation will not be possible (Nilsson and Lefevre, 2016), and research so far supports this expectation (Munday et al., 2013b, 2014).
Research on the possibility of transgenerational acclimation to elevated CO2 has returned mixed results. Some report improved growth and survival in offspring (Miller et al., 2012; Murray et al., 2014), while others report no or limited capacity for transgenerational acclimation of olfactory responses or predator escape (Allan et al., 2014; Welch et al., 2014). This led to speculation that metabolic traits may have greater potential than behavioural traits for transgenerational acclimation to elevated CO2 (Welch et al., 2014).
Most studies that test the effects of acidification on senses and behaviours of fish larvae show variation in sensitivity among individuals. This has led to conclusions that there is clear “potential for natural variation in sensitivity among individuals to lead to genetic adaptation in marine fishes” (Leduc et al., 2013). However, this is true only if the variation has a genetic basis and is heritable: a recent study on A. polyacanthus has found this for temperature (Munday et al., 2017b). But, thus far, there is no evidence of this for acidification, as has been noted by several—(e.g., Munday et al., 2013a). In larval fishes, ontogenetic changes in many aspects of physiology, behaviour, and morphology are large and ongoing, and variation in sensitivity can have a number of non-genetic bases, among which are variation in size or age of larvae, in how far metamorphosis has progressed, or in nutritional or physiological condition. An example is (Munday et al., 2013a), a study cited by Leduc et al. (2013). In the publication, studied fish were sequentially called larvae upon capture and CO2 treatment, larvae and juveniles during olfaction tests, and juveniles during observations of behaviour and mortality rates (Munday et al., 2013a): the fish were metamorphosing during the experiments, and individuals varied in developmental stage at any time. Larvae of the study species also varied in age and size at capture: 16–21 days (Munday et al., 2013a), and 15.2–18.0 mm total length (Fisher et al., 2005). These variations might contribute to variation among individuals in sensitivity to acidification at any time. Therefore, although variation among individual larvae in sensitivity to acidification may indicate heritable “raw material” upon which selection can act, other possibilities exist. As noted by Munday et al. (2013a) “quantitative genetic analyses, such as comparisons of parent-offspring or half-sib variation will be required to estimate heritability.”
There are two ways of approaching the question of adaptation: measuring standing genetic variation in pH-sensitive traits, and conducting evolution experiments in real time (Sunday et al., 2014). Both approaches have advantages and disadvantages, and the latter especially apply to species such as marine fishes with relatively long life cycles and complex life histories. I was unable to find any published studies using genetic variation to study pH-sensitive traits relevant to larval dispersal in marine fishes. An approach that is used for species with short generation times such as plankton is laboratory rearing over multiple generations in high CO2 conditions to determine if adaptation takes place (Munday et al., 2013c). This would be extremely challenging in marine demersal fishes because the conditions and challenges that larvae face in the pelagic environment while dispersing are so different than those faced by the more site-attached, bottom-associated adult. So, perhaps it is not surprising that there are apparently no published attempts to do this with marine, demersal fishes. At present, adaptation to acidification in marine fishes is an undemonstrated possibility. It is noteworthy that where adaptation has been demonstrated in marine species with very short generation times, adaptation has taken several hundred generations to occur (Sunday et al., 2014), and CO2 levels expected before or by the end of this century will already be causing sensory dysfunction in marine fish larvae. In short, even if adaptation to elevated CO2 levels is possible there is “considerable risk that the present rate of CO2 increase is too high to allow adaptation through natural selection” (Regan et al., 2016). Further, the physiologist who discovered the mechanism behind elevated CO2 sensory dysfunction in fish larvae has written: “since atmospheric CO2 levels have probably remained below 500 μatm for the last 30 million years, there is a great risk that genes needed for coping with a sustained elevation of CO2 are no longer functional” (Nilsson and Lefevre, 2016).
Behaviour and sensory systems of larval marine fish species may or may not acclimate or adapt to elevated (and rising) CO2 levels, but there is little relevant research on the question, in spite of calls for it. Assuming that acclimation or adaptation will happen quickly enough to overcome the rapid rate at which CO2 is rising is optimistic given our current level of understanding. A more certain result can be achieved by strongly reducing anthropogenic CO2 production so ocean pH does not decrease as it will under a “business as usual” scenario.
Research to date consistently shows that ocean acidification will have a negative effect on the sensory abilities of larval, marine perciform fishes. This will adversely impact on the ability of the larvae to orientate in the ocean, to find settlement habitat and to adjust their vertical distribution, and thus influence their dispersal. In short, the biophysical larval dispersal paradigm will no longer apply. Acidification will also decrease the bet-hedging and colonization of new locations that behavioural variability enables. Dispersal modelling predicts that, as a result, recuitment of demersal fishes will decrease, as will local recruitment while mean and median dispersal distances will increase.
JL conceived, drafted, and is accountable for all aspects of this work, interpreted the data, and gave final approval for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
My thanks to: Claire Paris and Eric Wolanski for inviting me to contribute this paper to their collection, Phil Munday for discussions about effects of acidification on the ocean and its fishes, Suzanne Leis for editorial assistance and the reviewers for constructive input.
Allan, B. J., Miller, G. M., McCormick, M. I., Domenici, P., and Munday, P. L. (2014). Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc. R. Soc. B 281:20132179. doi: 10.1098/rspb.2013.2179
Ashur, M. M., Johnston, N. K., and Dixson, D. L. (2017). Impacts of ocean acidification on sensory function in marine organisms. Integr. Comp. Biol. 57, 63–80. doi: 10.1093/icb/icx010
Bignami, S., Sponaugle, S., and Cowen, R. K. (2013). Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Glob. Chang. Biol. 19, 996–1006. doi: 10.1111/gcb.12133
Bignami, S., Sponaugle, S., and Cowen, R. K. (2014). Effects of ocean acidification on the larvae of a high-value pelagic fisheries species, mahi-mahi Coryphaena hippurus. Aquat. Biol. 21, 249–260. doi: 10.3354/ab00598
Bottesch, M., Gerlach, G., Halbach, M., Bally, A., Kingsford, M. J., and Mouritsen, H. (2016). A magnetic compass that might help coral reef fish larvae return to their natal reef. Curr. Biol. 26, R1266–R1267. doi: 10.1016/j.cub.2016.10.051
Castro, J. M., Amorim, M. C.P., Oliveira, A. P., Gonçalves, E. J., Munday, P. L., Simpson, S. D., et al. (2017). Painted goby larvae under high-CO2 fail to recognize reef sounds. PLoS ONE 12:e0170838. doi: 10.1371/journal.pone.0170838
Devine, B. M., Munday, P. L., and Jones, G. P. (2012). Rising CO2 concentrations affect settlement behaviour of larval damselfishes. Coral Reefs 31, 229–238. doi: 10.1007/s00338-011-0837-0
Domenici, P., Allan, B., McCormick, M. I., and Munday, P. L. (2011). Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol. Lett. 8, 1–3. doi: 10.1098/rsbl.2011.0591
Fisher, R., and Leis, J. M. (2009). “Swimming performance in larval fishes: from escaping predators to the potential for long distance migration,” in Fish Locomotion: An Etho-Ecological Approach, eds P. Domenici and B. G. Kapoor (Enfield, NH: Science Publishers), 333–373.
Fisher, R., Leis, J. M., Clark, D. L., and Wilson, S. K. (2005). Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Mar. Biol. 147, 1201–1212. doi: 10.1007/s00227-005-0001-x
Forsgren, E., Dupont, S., Jutfelt, F., and Amundsen, T. (2013). Elevated CO2 affects embryonic development and larval phototaxis in a temperate marine fish. Ecol. Evol. 3, 3637–3646. doi: 10.1002/ece3.709
Frommel, A. Y., Maneja, R., Lowe, D. M., Malzahn, A. M., Geffen, A. J., Folkvord, A., et al. (2012). Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat. Clim. Chang. 2, 42–46. doi: 10.1038/nclimate1324
Frommel, A. Y., Margulies, D., Wexler, J. B., Stein, M. S., Scholey, V. P., Williamson, J. E., et al. (2016). Ocean acidification has lethal and sub-lethal effects on larval development of yellowfin tuna, Thunnus albacares. J. Exp. Mar. Biol. Ecol. 482, 18–24. doi: 10.1016/j.jembe.2016.04.008
Holzer, G., Besson, M., Loïc, A. L., Barth, F. P., Gillet, B., Hughes, S., et al. (2017). Fish larval recruitment to reefs is a thyroid hormone-mediated metamorphosis sensitive to the pesticide chlorpyrifos. Elife 6:e27595. doi: 10.7554/eLife.27595
Hughes, T. P., Barnes, M. L., Bellwood, D. R., Cinner, J. E., Cumming, G. S., Jackson, J. B. C., et al. (2017). Coral reefs in the anthropocene. Nature 546, 82–90. doi: 10.1038/nature22901
Hunt von Herbing, I. (2002). Effects of temperature on larval fish swimming performance: the importance of physics to physiology. J. Fish Biol. 61, 865–876. doi: 10.1111/j.1095-8649.2002.tb01848.x
Jones, G. P., Almany, G. R., Russ, G. R., Sale, P. F., Steneck, R. S., van Oppen, M. J. H., et al. (2009). Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–326. doi: 10.1007/s00338-009-0469-9
Kavanagh, K. D. (2000). Larval brooding in the marine damselfish Acanthochromis polyacanthus (Pomacentridae) is correlated with highly divergent morphology, ontogeny and life-history traits. Bull. Mar. Sci. 66, 321–337.
Leduc, A. O., Munday, P. L., Brown, G. E., and Ferrari, M. C. (2013). Effects of acidification on olfactory-mediated behaviour in freshwater and marine ecosystems: a synthesis. Philos. Trans. R. Soc. B Biol. Sci. 368:20120447. doi: 10.1098/rstb.2012.0447
Leis, J. M. (1991). “The pelagic phase of coral reef fishes:larval biology of coral reef fishes,” in The Ecology of Fishes On Coral Reefs, ed P. F. Sale (San Diego, CA: Academic Press), 183–230.
Leis, J. M. (2006). Are larvae of demersal fishes plankton or nekton? Adv. Mar. Biol. 51, 59–141. doi: 10.1016/S0065-2881(06)51002-8
Leis, J. M. (2010). Ontogeny of behaviour in larvae of marine demersal fishes. Ichthyol. Res. 57, 325–342. doi: 10.1007/s10228-010-0177-z
Leis, J. M. (2015). “Is dispersal of larval reef fishes passive?,” in Ecology of Fishes On Coral Reefs, ed C. Mora (Cambridge: Cambridge University Press), 223–226.
Leis, J. M., Caselle, J. E., Bradbury, I. R., Kristiansen, T., Llopiz, J. K., Miller, M. J., et al. (2013). Does fish larval dispersal differ between high and low latitudes?. Proc. R. Soc. B Biol. Sci. 280, 1–9. doi: 10.1098/rspb.2013.0327
Leis, J. M., Hay, A. C., and Gaither, M. R. (2011a). Swimming ability and its rapid decrease at settlement in wrasse larvae (Teleostei: Labridae). Mar. Biol. 158, 1239–1246. doi: 10.1007/s00227-011-1644-4
Leis, J. M., Hay, A. C., and Howarth, G. J. (2009). Ontogeny of in situ behaviours relevant to dispersal and connectivity in larvae of coral-reef fishes Mar. Ecol. Prog. Ser. 379, 163–179. doi: 10.3354/meps07904
Leis, J. M., Hay, A. C., Lockett, M. M., Chen, J.-P., and Fang, L. S. (2007). Ontogeny of swimming speed in larvae of pelagic-spawning, tropical, marine fishes. Mar. Ecol. Prog. Ser. 349, 257–269. doi: 10.3354/meps07107
Leis, J. M., Paris, C. B., Irisson, J. O., Yerman, M. N., and Siebeck, U. E. (2014). Orientation of fish larvae in situ is consistent among locations, years and methods, but varies with time of day. Mar. Ecol. Prog. Ser. 505, 193–208. doi: 10.3354/meps10792
Leis, J. M., Siebeck, U., and Dixson, D. L. (2011b). How Nemo finds home: the neuroecology of dispersal and of population connectivity in larvae of marine fishes. Integr. Comp. Biol. 51, 826–843. doi: 10.1093/icb/icr004
Luiz, O. J., Allen, A. P., Robertson, D. R., Floeter, S. R., Kulbicki, M., Vigliola, L., et al. (2013). Adult and larval traits as determinants of geographic range size among tropical reef fishes. Proc. Natl. Acad. Sci. U.S.A. 110, 16498–16502. doi: 10.1073/pnas.1304074110
Lychakov, D. V., and Rebane, Y. T. (2005). Fish otolith mass asymmetry: morphometry and influence on acoustic functionality. Hear. Res. 201, 55–69. doi: 10.1016/j.heares.2004.08.017
Maneja, R. H., Frommel, A. Y., Browman, H. I., Clemmesen, C., Geffen, A. J., Folkvord, A., et al. (2013). The swimming kinematics of larval Atlantic cod, Gadus morhua L., are resilient to elevated seawater pCO2. Mar. Biol. 160, 1963–1972. doi: 10.1007/s00227-012-2054-y
McCormick, M. I. (1999). Delayed metamorphosis of a tropical reef fish (Acanthurus triostegus): a field experiment. Mar. Ecol. Prog. Ser. 176, 25–38. doi: 10.3354/meps176025
Miller, G. M., Watson, S.-A., Donelson, J. M., McCormick, M. I., and Munday, P. L. (2012). Parental environment mediates impacts of increased carbon dioxide on coral reef fish. Nat. Clim. Chang. 2, 858–861. doi: 10.1038/nclimate1599
Moser, H. G. (1981). “Morphological and functional aspects of marine fish larvae,” in Marine Fish Larvae: Morphology, Ecology and Relation to Fisheries, ed R. Lasker (Seattle, WA: University of Washington Press), 90–130.
Mouritsen, H., Atema, J., Kingsford, M. J., and Gerlach, G. (2013). Sun compass orientation helps coral reef fish larvae return to their natal reef. PLoS ONE 8:e66039. doi: 10.1371/journal.pone.0066039
Munday, P., Allan, B., Mcqueen, D., Nicol, S., Parsons, D., Pether, S., et al. (2017a). “Warming has a greater effect than ocean acidication on the early life history, development and swimming performance of a coastal pelagic fish, Seriola lalandi,” in 10th Indo-Pacific Fish Conference (Papeete).
Munday, P. L., Cheal, A. J., Dixson, D. L., Rummer, J. L., and Fabricius, K. E. (2014). Behavioural impariment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Chang. 4, 487–492. doi: 10.1038/nclimate2195
Munday, P. L., Donelson, J. M., Dixson, D. L., and Endo, G. G., (2009a). Effects of ocean acidification on the early life history of a tropical marine fish. Proc. R. Soc. B 276, 3275–3283. doi: 10.1098/rspb.2009.0784
Munday, P. L., Donelson, J. M., and Domingos, J. A. (2017b). Potential for adaptation to climate change in a coral reef fish. Glob. Change Biol. 23, 307–317. doi: 10.1111/gcb.13419
Munday, P. L., Leis, J. M., Lough, J. M., Paris, C. B., Kingsford, M. J., Berumen, M. L., et al. (2009b). Climate change and coral reef connectivity. Coral Reefs 28, 379–395. doi: 10.1007/s00338-008-0461-9
Munday, P. L., McCormick, M. I., Meekan, M., Dixson, D. L., Watson, S. A., Chivers, D. P., et al. (2013a). Selective mortality associated with variation in CO2 tolerance in a marine fish. Ocean Acidif. 1, 1–5. doi: 10.2478/oac-2012-0001
Munday, P. L., Pratchett, M. S., Dixson, D. L., Donelson, J. M., Endo, G. G. K., Reynolds, A. D., et al. (2013b). Elevated CO2 affects the behavior of an ecologically and economically important coral reef fish. Mar. Biol. 160, 2137–2144. doi: 10.1007/s00227-012-2111-6
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M., and Marshall, D. J. (2013c). Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500. doi: 10.1111/ele.12185
Munday, P. L., Watson, S. A., Parsons, D. M., King, A., Barr, N. G., Mcleod, I. M., et al. (2016). Effects of elevated CO2 on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. ICES J. Mar. Sci. 73, 641–649. doi: 10.1093/icesjms/fsv210
Murray, C. S., Malvezzi, A., Gobler, C. J., and Baumann, H. (2014). Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Mar. Ecol. Prog. Ser. 504, 1–11. doi: 10.3354/meps10791
Nagelkerken, I., and Munday, P. L. (2016). Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob. Chang. Biol. 22, 974–989. doi: 10.1111/gcb.13167
Nilsson, G. E., Dixson, D. L., Domenici, P., McCormick, M. I., Sørensen, C., Watson, S. A., et al. (2012). Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2, 201–204. doi: 10.1038/nclimate1352
Nilsson, G. E., and Lefevre, S. (2016). Physiological challenges to fishes in a warmer and acidified future. Physiology 31, 409–417. doi: 10.1152/physiol.00055.2015
Nilsson, G. E., Ostlund-Nilsson, S., Penfold, R., and Grutter, A. S. (2007). From record performance to hypoxia tolerance: respiratory transition in damselfish larvae settling on a coral reef. Proc. R. Soc. B 274, 79–85. doi: 10.1098/rspb.2006.3706
O'Connor, J., and Muheim, R. (2017). Pre-settlement coral-reef fish larvae respond to magnetic field changes during the day. J. Exp. Biol. 220, 2874–2877. doi: 10.1242/jeb.159491
Pimentel, M., Pegado, M., Repolho, T., and Rosa, R. (2014). Impact of ocean acidification in the metabolism and swimming behavior of the dolphinfish (Coryphaena hippurus) early larvae. Mar. Biol. 161, 725–729. doi: 10.1007/s00227-013-2365-7
Pimentel, M. S., Faleiro, F., Marques, T., Bispo, R., Dionísio, G., Faria, A. M., et al. (2016). Foraging behaviour, swimming performance and malformations of early stages of commercially important fishes under ocean acidification and warming. Clim. Change 137, 495–509. doi: 10.1007/s10584-016-1682-5
Pistevos, J. C., Nagelkerken, I., Rossi, T., and Connell, S. D. (2017). Ocean acidification alters temperature and salinity preferences in larval fish. Oecologia 183, 545–553. doi: 10.1007/s00442-016-3778-z
Popper, A. N., Ramcharitar, J., and Campana, S. E. (2005). Why otoliths? Insights from inner ear physiology and fishery biology. Mar. Freshw. Res. 56, 497–504. doi: 10.1071/MF04267
Regan, M. D., Turko, A. J., Heras, J., Andersen, M. K., Lefevre, S., Wang, T., et al. (2016). Ambient CO2, fish behaviour and altered GABAergic neurotransmission: exploring the mechanism of CO2-altered behaviour by taking a hypercapnia dweller down to low CO2 levels. J. Exp. Biol. 219, 109–118. doi: 10.1242/jeb.131375
Roberts, C. M. (1997). Connectivity and management of Caribbean coral reefs. Science 278, 1454–1456. doi: 10.1126/science.278.5342.1454
Rossi, T., Nagelkerken, I., Simpson, S. D., Pistevos, J. C., Watson, S. A., Merillet, L., et al. (2015). Ocean acidification boosts larval fish development but reduces the window of opportunity for successful settlement. Proc. R. Soc. B 282:20151954. doi: 10.1098/rspb.2015.1954
Sale, P. F. (1991). “Reef fish communities: open nonequilibrial systems,” in The Ecology of Fishes on Coral Reefs, ed P.F. Sale (San Diego, CA: Academic Press), 564–598.
Silva, C. S., Novais, S. C., Lemos, M. F., Mendes, S., Oliveira, A. P., Gonçalves, E. J., et al. (2016). Effects of ocean acidification on the swimming ability, development and biochemical responses of sand smelt larvae. Sci. Tot. Environ. 563–564, 89–98, doi: 10.1016/j.scitotenv.2016.04.091
Sunday, J. M., Calosi, P., Dupont, S., Munday, P. L., Stillman, J. H., and Ruesch, T. B. (2014). Evolution in an acidifying ocean. Trends in Ecol. Evol. 29, 117–125. doi: 10.1016/j.tree.2013.11.001
Sundin, J., Amcoff, M., Mateos-González, F., Raby, G. D., Jutfelt, F., and Clark, T. D. (2017). Long-term exposure to elevated carbon dioxide does not alter activity levels of a coral reef fish in response to predator chemical cues. Behav. Ecol. Sociobiol. 71, 1–12. doi: 10.1007/s00265-017-2337-x
Wang, X., Song, L., Chen, Y., Ran, H., and Song, J. (2017). Impact of ocean acidification on the early development and escape behavior of marine medaka (Oryzias melastigma). Mar. Environ. Res. 131(Suppl. C), 10–18. doi: 10.1016/j.marenvres.2017.09.001
Welch, M. J., Watson, S. A., Welsh, J. Q., McCormick, M. I., and Munday, P. L. (2014). Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat. Clim. Change 4, 1086–1089. doi: 10.1038/nclimate2400
Keywords: fish, larva, dispersal, sensory ability, CO2, acidification, behaviour, connectivity
Citation: Leis JM (2018) Paradigm Lost: Ocean Acidification Will Overturn the Concept of Larval-Fish Biophysical Dispersal. Front. Mar. Sci. 5:47. doi: 10.3389/fmars.2018.00047
Received: 29 November 2017; Accepted: 31 January 2018;
Published: 13 February 2018.
Edited by:
Eric Wolanski, James Cook University, AustraliaReviewed by:
Rafael Perez-Dominguez, Office for National Statistics, United KingdomCopyright © 2018 Leis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey M. Leis, amVmZnJleS5sZWlzQHV0YXMuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.