- 1Lyell Centre for Earth and Marine Science and Technology, Edinburgh, United Kingdom
- 2School of Energy, Geoscience, Infrastructure and Society, Heriot-Watt University, Edinburgh, United Kingdom
One fifth of Mediterranean waters can be classified as shelf—much higher than the global average. Consequently, the shelf/coastal zone plays a proportionally greater biogeochemical role than in the major oceans, including the support of a wide range of range of endemic or culturally important species and ecosystems. However, despite their known importance in regulating ecosystem function and the marine sulfur cycle, our understanding of the dynamics of dimethylated sulfur compounds such as dimethylsulphide (DMS) and dimethylsulphoniopropionate (DMSP) in Mediterranean benthic habitats is limited. Here, a community-level approach was adopted to quantify DMS and DMSP dynamics in Mediterranean ecosystems including seagrass (Posidonia oceanica) meadows, coralligène (an algal carbonate reef found along the Mediterranean shelf) and macroalgal stands. It was found that P. oceanica and coralligène are likely to act as significant benthic stocks of DMSP in the coastal/shelf environment. “Hotspots” of water column DMS and DMSP processing were observed where net benthic production was high (e.g., P. oceanica meadows), demonstrating that benthic communities are able to modify DMS biogeochemistry in the overlying water column. High variability between, and within, habitat types illustrates the importance of ecosystem structure and light availability in determining benthic DMS and DMSP accumulation, and highlights a previously under-appreciated complexity in benthic dimethylated sulfur dynamics.
Introduction
The Mediterranean Sea is the largest and deepest semi-enclosed sea in the world, providing coastline to 21 states and serving as a tourist destination for 200 million people every year (Aquarone et al., 2010). The Mediterranean's varied geological history, including full isolation from primary oceans and subsequent abrupt flooding (Garcia-Castellanos et al., 2009), has shaped its current characteristics: one fifth of Mediterranean waters can be classified as shelf—much higher than the 7% global average (Pinardi et al., 2006). This has resulted in high cultural and biological diversity (Coll et al., 2010). Whilst comprising only 0.32% of the global ocean volume, the Mediterranean Sea harbors 4–18% of all known marine macroscopic species (Bianchi and Morri, 2000; Coll et al., 2010), including a high percentage of endemic and charismatic species (Coll et al., 2010). Thus, shelf waters and their associated benthic habitats play a proportionally greater biological and biogeochemical role in Mediterranean ecosystem function than elsewhere. However, rates of biogeochemical processing remains a grand challenge in marine science (Achterberg, 2014), and deficiencies in observational data and multidisciplinary research strategies in the Mediterranean limits our modeling capacity for the region (De Madron et al., 2011). Acute anthropogenic pressures that threaten the long-term survival of Mediterranean coastal and shelf benthic ecosystems (Coll et al., 2010, 2012) further call for a detailed understanding of coastal / shelf biogeochemistry (Doney, 2010). The impact of long-term projected climate change on these ecosystems is likely to be complex and interactive (Lejeusne et al., 2010)—some species may be “winners” (e.g., seagrasses, fleshy macroalgae), whilst others may be “losers” (e.g., calcifying macro- and micro-organisms) (Kroeker et al., 2013). The subsequent impact on benthic-pelagic coupling will almost certainly affect ecosystem structure and function, but these processes are not yet adequately understood for the present day (Griffiths et al., 2017), limiting the accuracy of future projections.
Benthic production of dimethylated sulfur—particularly dimethylsulphide (DMS) and its precursor dimethylsulphoniopropionate (DMSP)—is known to be significant for a range of benthic ecosystems, including coral reefs (e.g., Van Alstyne et al., 2006; Fischer and Jones, 2012), saltmarshes (e.g., Steudler and Peterson, 1984) and macroalgal assemblages (e.g., Burdett et al., 2015). Dimethylated sulfur is a major component of the marine sulfur cycle, but has also been linked with key ecosystem functions including organismal tolerance to environmental variability (e.g., Burdett et al., 2013) and community-level trophic interactions via infochemical signaling (Wolfe et al., 1997; De Bose et al., 2008; Seymour et al., 2010; Savoca and Nevitt, 2014). However, the role of Mediterranean shelf and coastal ecosystems in dimethylated sulfur production is poorly researched, despite the prevalence of shelf waters and the known role as a source of atmospheric DMS (Simo et al., 1997; Besiktepe et al., 2004) and algal-derived sulfate aerosols (Ganor et al., 2000). Posidonia oceanica—a seagrass species endemic to the Mediterranean—and coralligenous frameworks (locally known as “coralligéne”)—unique reef-like structures composed of encrusting red coralline algae—are widespread throughout the Mediterranean (Martin et al., 2014; Telesca et al., 2015) and support highly diverse ecosystems (Hemminga and Duarte, 2000; Ballesteros, 2006). Red coralline algae and P. oceanica are also known to be significant producers of dimethylsulphoniopropionate (DMSP) (Borges and Champenois, 2015; Burdett et al., 2015). The Mediterranean has been noted to be an important source of atmospheric (DMS, a breakdown product of DMSP), but we have little information on the role of Mediterranean benthic communities (as fully integrated systems) in the production of dimethylated sulfur compounds.
Community-level investigations of biogeochemical dynamics provide a more accurate representation of natural ecosystem responses (Riebesell and Gattuso, 2015), but this approach remains poorly adopted, in part due to greater logistical complexities (Riebesell and Gattuso, 2015). The aim of this study was to investigate the Mediterranean benthos in terms of DMSP stock and community-level benthic-pelagic exchange of DMS and DMSP. This will provide new insight into the role of Mediterranean benthic communities in the cycling of these ecologically and biogeochemically important compounds.
Materials and Methods
Study Site and Sample Collection
Sampling took place at Banyuls-sur-Mer, south-west France along a depth gradient in August 2014; benthic DMSP concentrations (Borges and Champenois, 2015; Burdett et al., 2015) and DMS production rates (Vila-Costa et al., 2008) are known to be highest in the summer months. There were four main sampling depths, at which dominant substrate types were sampled:
• 2 m: Padina sp. gardens, dead seagrass mats, sand patches (top 1 cm sampled); collected via snorkeling
• 3 m: Posedonia oceanica meadow (leaves sampled); collected via snorkeling
• 15 m: coralligène (Mesophyllum alternans) and sand patches (top 1 cm of sand sampled); collected via SCUBA
• 20 m: coralligène (Mesophyllum alternans) and sand patches (top 1 cm of sand sampled); collected via SCUBA
At each depth, the dominant substrate types were sampled for intracellular DMSP concentrations (n = 10 samples per substrate type). Immediately on collection, intracellular DMSP samples were returned to the laboratory, where they were patted dry and their mass recorded and stored in 10 M NaOH in 5 ml crimp-top vials fitted with Pharma-Fix septa (Fisher Scientific).
Net community benthic flux of dissolved DMSP (DMSPd) and DMS were determined for each substrate type (n = 5 per substrate type) using unstirred in situ benthic chambers that were fixed to the seabed (270 ml volume, 95 cm2 surface area, transparent to incoming photosynthetically active radiation). Larger chambers (3,650 ml volume, 180 cm2 surface area) were used on the seagrass bed because of the height of the plants. Initial water samples (T0) were taken with glass syringes fitted with a one-way valve and transported back to the laboratory for processing (<20 min travel time). Chambers were then closed and the incubation started. Incubations lasted for ~4 h (exact time noted for each incubation), following which a second water sample (T1) was taken from each chamber using a glass syringe and transported back to the laboratory. On return to the laboratory, water samples were immediately processed following the small volume gravity filtration method (Kiene and Slezak, 2006), allowing for the determination of DMSPd, particulate DMSP (DMSPp) and DMS. Storage in NaOH converts all DMSP in samples to DMS for quantification via gas chromatography (GC, instrumental details below).
Water samples were also taken at 3–5 depths through the water column depending on the overall depth (n = 5 per depth), including one depth close to the seabed. As with the benthic samples, one batch of water samples were immediately processed on return to the laboratory (T0), whilst a second batch of water samples were taken from gas-tight chambers maintained at ambient conditions for the same duration as the benthic chamber incubations (T1), allowing for complementary determination of net changes in water column concentrations at the same time as the benthic flux measurements (T1 – T0, i.e., positive change = net increase in concentration). Net water column concentration changes near the sea bed were subtracted from the associated benthic incubation measurements to differentiate between net concentration changes as a result of water column or benthic drivers (i.e., acting as a control).
Additional water samples (concentrations only, no flux calculations) over deeper coralligène substrate were taken at four additional sites, in water depths of 25, 35 m (3, 19, and 34.8 m), 45 m (3, 24, and 44.8 m) and 65 m (3, 34, and 64.8 m) water depths by deploying Niskin bottles to the specified depths (n = 5 per depth per sample site, DMS and DMSPd concentrations only).
All sampling took place during the day (incubations conducted ~10 a.m.−2 p.m.; intracellular DMSP samples taken ~midday). A subset of the full sample collection was also taken during the night (~10 p.m.−2 a.m.) to provide complementary night-time measurements.
Dimethylated Sulfur Analysis
All water samples were quantified for DMS via GC-FPD using the purge-cryotrap method previously described (Turner et al., 1990; Kiene and Slezak, 2006). Intracellular DMSP samples were analyzed by direct injection of the vial headspace into the GC injector port. A SRI 8610C chromatograph was used for all analyses, fitted with a 15 m capillary column set at 45°C and a nitrogen carrier gas (8 psi). The FPD detector was at 150°C for all analyses (air @ 2 psi, H2 @ 27 psi). A DMSP standard was used to calibrate the analyses, obtained from Research Plus Inc. The limit of detection for intracellular samples was 1 μg S (headspace injection method) and 0.18 ng S for water samples (cryotrap method); standard and sample precision was within 1%.
Statistical Analyses
ANOVAs were used to compare intracellular DMSP concentrations and dimethylated sulfur production between benthic types. Correlation analyses were used to investigate the relationship between water column dimethylated sulfur concentrations and depth. Statistical analyses were conducted in R v3.2.0 and Minitab v14.1. For all parametric tests, the data met assumptions for normality and homogeneity of variance. Cross-sectional plots of water column concentrations and change in concentrations over time were plotted using Ocean Data View V4.7.8 using weighted average gridding interpolation.
Results
Intracellular/Associated DMSP Concentrations
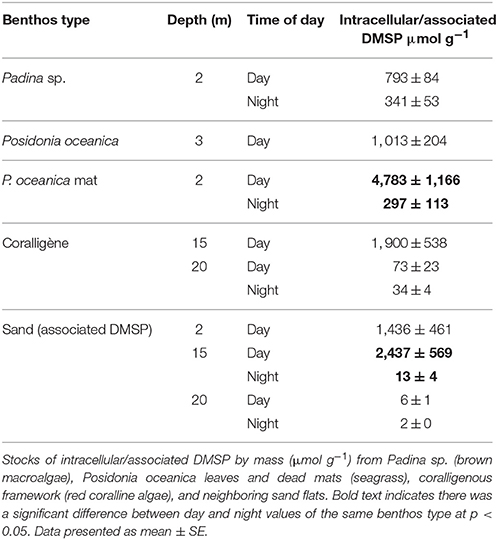
There was a significant difference in intracellular/associated DMSP concentrations between the different benthos types sampled, spanning three orders of magnitude [F(12, 130) = 13.03, p < 0.001; Table 1]. The highest observed intracellular concentrations were for the dead P. oceanica mat during the day, followed by the sand at 15 m (associated DMSP) and M. alternans that make up the coralligène framework (Table 1). DMSP concentrations were consistently higher during the day, but this was only significant for the dead P. oceanica mats and the sand at 15 m (associated DMSP) (Table 1).
Community Level Production of Dimethylated Sulfur
There was a significant difference in the benthic production of DMS [F(8, 27 = 90.86, p < 0.001] and DMSPd [F(8, 34) = 23.51, p < 0.001] across the macrophytic benthic types (Figure 1; coralligène from different depths considered separately). No significant correlation was observed between the rates of change in dimethylated sulfur and intracellular DMSP of the primary benthic component. During the night, DMS and DMSPd production by the P. oceanica meadow community was significantly higher than the other benthic communities. During the day, DMSPd, but not DMS, production were significantly higher in the P. oceanica meadow compared to other benthic types. High DMS production was also recorded from the dead P. oceanica mat. During the night, significant DMS uptake by the 15 m depth coralligène community was observed. Production / uptake rates by other benthic communities were lower and statistically similar. In contrast to intracellular DMSP, no consistent trend between day/night comparisons was observed. Measured concentrations for T0 and T1 incubation timepoints are provided in Figure S1 in the Supplementary Information.
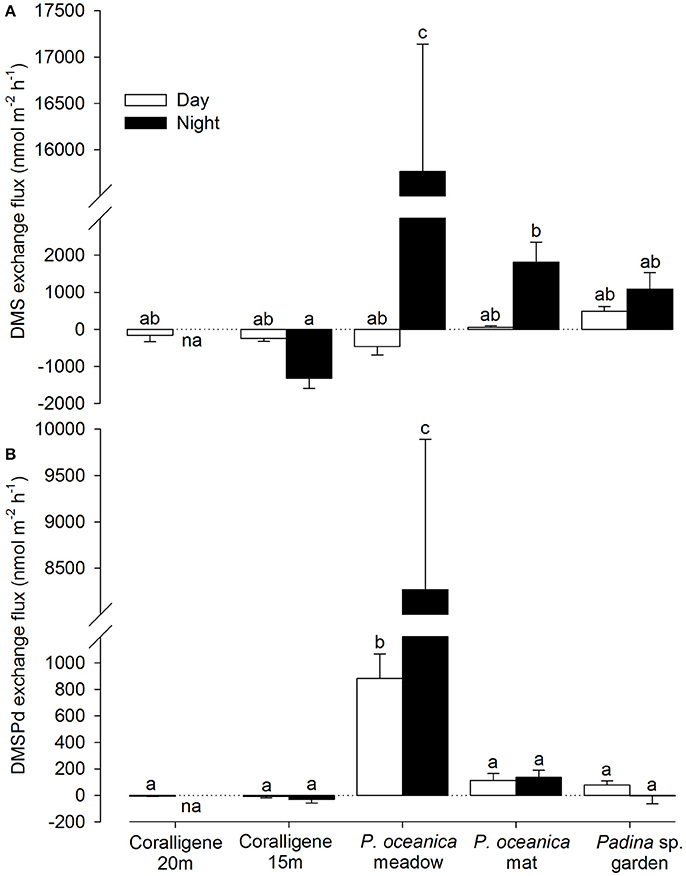
Figure 1. Community-level exchange flux of dimethylated sulfur from key Mediterranean benthos. Net exchange flux in (A) DMS and (B) dissolved DMSPd (DMSPd) from coralligene, Posidonia oceanica meadows and dead mats and Padina sp. garden communities during the day (white bars) and night (black bars). Data presented as mean ± SE. Bars with different letters a-c are statistically different in net concentration change at p < 0.05. na = data not available. Positive flux indicates a net release from the benthos.
Water Column Concentrations and Net Change over Time
A trend toward lower DMSPp (9.93 ± 1.92 nM, mean ± SD) concentrations compared to DMS (12.37 ± 1.37 nM) and DMSPd (13.32 ± 1.02 nM) was observed when all water column samples were treated as one dataset (i.e., regardless of depth/location), although this difference was not significant [F(2, 63) = 1.41, p = 0.253, Figure 2]. Similarly, neither water column DMS, DMSPd nor DMSPp were significantly correlated with the depth at which the sample was taken (Figure 2).
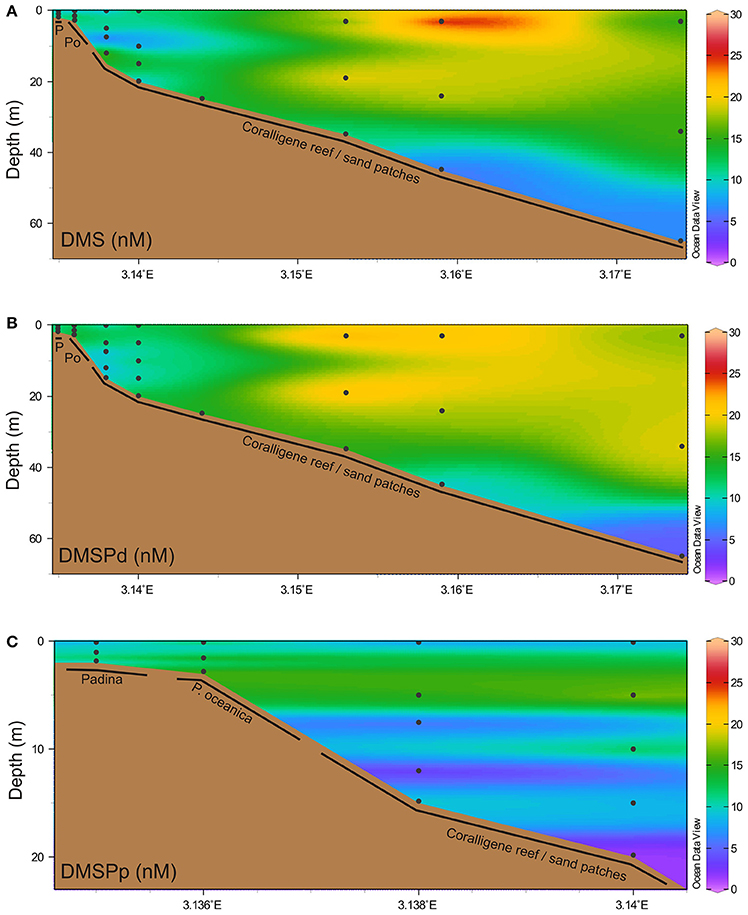
Figure 2. Water column concentrations of dimethylated sulfur. Water column concentrations (nM) of (A) DMS, (B) dissolved DMSP (DMSPd), and (C) particulate DMSP (DMSPp). Black dots indicate discrete sampling locations. P, Padina; Po, Posidonia oceanica.
Net change in DMS and DMSPd concentration was highest in the water column overlying the seagrass beds (Figure 3), where a net DMS accumulation rate of >10 nM h−1 contrasted with a net DMSPd utilization rate of >10 nM h−1. Shallower and deeper than this, net change in DMSPd was <1 nM h−1. DMS water column production rates in deeper waters was generally higher than DMSPd rates, up to 5 nM h−1. Measured concentrations for T0 and T1 incubation timepoints are provided in Figure S1 in the Supplementary Information.
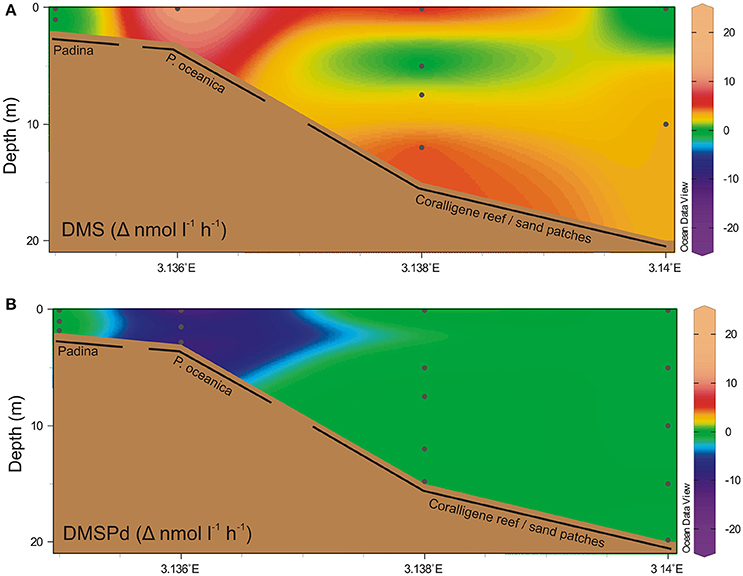
Figure 3. Temporal net change in water column dimethylated sulfur. Net change in water column concentrations over time (Δ nmol l−1 h−1) of (A) DMS and (B) dissolved DMSP. Black dots indicate discrete sampling locations. Positive change indicates a net increase in concentration.
Discussion
This work has provided new information on the role of Mediterranean benthic macrophytic communities in the production and exchange dynamics of ecologically important dimethylated sulfur compounds. Coral reefs are the most widely studied ecosystem with regards to the benthic production of dimethylated sulfur compounds. Algal intracellular DMSP concentrations measured here are comparable, if not somewhat higher, than scleractinian corals, but remain less than the extremely high DMSP concentrations found in mucus ropes (Van Alstyne et al., 2006). Intracellular DMSP concentrations of Spartina alterniflora, one of the few DMSP-producing higher plants, are typically lower than those measured here (McFarlin and Alber, 2013), as are previous estimates for seagrasses (e.g., Zostera marina: 139 nmol g−1 wet weight, P. oceanica: 5 μmol g−1 dry weight) (Jonkers et al., 2000; Borges and Champenois, 2015). However, in these studies, leaves were cleaned of epiphytes prior to analysis. Here, the intracellular DMSP concentrations reflect the seagrass “holobiont” (i.e., including all associated epiphytes and microorganisms). Whilst this likely contributed to the observed high variability between samples due to heterogeneity in epiphyte composition, it means the concentrations presented here are more representative of the natural community found in situ, and are more comparable to other higher plants (e.g., Spartina alterniflora), where extracellular accumulation of DMSP is known to occur (Dean and Kiene, 1992).
The combined high intracellular DMSP concentrations and widespread distribution in the Mediterranean (Martin et al., 2014; Telesca et al., 2015) means that both P. oceanica meadows and coralligène reefs act as significant benthic stocks of DMSP. Since coralligène can be found at greater depths than other macrophytic ecosystems (up to at least 120 m; Ballesteros, 2006), this increases the potential depth range of benthic DMSP stocks into the mesophotic zone—an area of the ocean whose biogeochemical importance is only now being realized (Giering et al., 2014). Further investigations are required to determine the relationship between coralligène intracellular DMSP and depth, to establish the importance of mesophotic coralligène communities as a benthic DMSP stock. Rapid declines in intracellular DMSP concentrations with depth have been previously observed in corals, attributed to a combination of factors including thermal boundaries and declines in irradiance and holobiont community interactions (Yost et al., 2012). It is proposed that similar factors play a role in the observed attenuation of coralligène intracellular DMSP concentrations with depth. While Padina sp. intracellular DMSP concentrations were also high, its spatially limited distribution means that this macrophyte (and associated community) is unlikely to represent a major, regional-scale, stock of DMSP.
Due to varying methodologies adopted by different studies to quantify the flux of dimethylated sulfur from benthic habitats, it is difficult to make robust comparisons with other systems, particularly since this study focused on community-level responses rather than isolated individuals. Average net production of DMS and DMSPd from the P. oceanica community was high during the night, perhaps reflecting an overflow mechanism for removing DMSP from cells during a day-time accumulation (Stefels, 2000), or an increase in night-time grazing activity that can facilitate intracellular DMSP release (Van Alstyne, 2009). Dimethylated sulfur release from re-processing of this organic matter by faunal and microbiological activity at the sediment surface may explain why DMS exchange rates were higher than previous individual organism studies (which cannot integrate processes happening at the sediment surface). DMS production is also known to be higher in anoxic seagrass-based sediments (Jonkers et al., 2000). The coralligène communities, which have an intermediate level of structural complexity, were characterized by only small net exchanges in DMS and DMSPd. In contrast to all other habitat types, a significant net uptake of DMS by the coralligène community during the night at 15 m depth was observed. This may reflect a unique microbial community that favors DMS utilization rather than production, which could be significantly impacting dimethylated sulfur cycling in deeper coastal/shelf waters of the Mediterranean (where coralligène formations are found). Microbial activity is a critical for the processing of dimethylated sulfur compounds (Moran et al., 2012; Curson et al., 2017), and the microbial community of crustose coralline algae is known to be highly specific (Sneed et al., 2015). Further investigation into the specific microbial communities associated with each benthic type (and their functionality) is thus required. Additionally, invertebrate accumulation of dimethylated sulfur (Van Alstyne and Puglisi, 2007) may be elevated during the night due to increased grazing activity.
A consistent daytime increase in intracellular DMSP across all benthos types was observed, supporting the proposed antioxidative role for DMSP (and its breakdown products) in marine macrophytes (Rix et al., 2012; Burdett et al., 2014). However, day/night trends of dimethylated sulfur benthic flux across habitat types were less consistent, reflecting the increase in community complexity as one moves through ecological scales—intracellular DMSP concentrations were determined from the holobiont of individual organisms, whilst benthic exchange fluxes were determined at the ecosystem community level, i.e., integrating the interactive effect of multiple organisms and holobionts across multiple trophic levels. This underlines the importance of in situ measurements conducted at the community level, rather than on isolated individuals in controlled laboratory environments, for appreciating the true biological and biogeochemical complexity of natural marine systems (Gattuso et al., 2014).
Despite the apparently large benthic release of DMS from P. oceanica meadows, sea-air flux of DMS in the Mediterranean is relatively modest (Simo et al., 1997; Simó and Grimalt, 1998; Besiktepe et al., 2004). Borges and Champenois (2015) previously suggested that DMSP released by seagrass plants might accumulate within the seagrass canopy. This study supports that hypothesis and extends it to also include DMS, but also demonstrates that seagrass community dynamics can influence water column dynamics meters above the canopy, creating a biogeochemical “hotspot” of DMS production and DMSPd utilization. Interestingly, another DMS “hotspot” was also observed around the 15 m coralligène community, despite small net uptake of DMS/DMSP, suggesting that the presence of high-DMSP containing organisms (such as coralline algae) can influence overlying water column concentrations without significant benthic-pelagic exchange. It is therefore suggested that high-DMSP producing benthic communities influence microbial/phytoplankton communities and biogeochemical functionality overlying the habitat, perhaps through the provision of other nutrients not measured here (e.g., organic matter substrate, nitrogen, phosphorus, etc.). Benthic influences of water column concentrations of dimethylated sulfur also helps to explain the lack of correlation between water column DMS/DMSPd/DMSPp concentrations and sample depth.
Conclusions
This work highlights the role of the Mediterranean coastal/shelf ecosystems as a significant stock of DMSP, but also that their role as sources or sinks of dimethylated sulfur is system-specific and related to structural complexity, highlighting the complex nature of dimethylated sulfur cycling in the shelf and coastal zone. Biogeochemical processing was especially evident for P. oceanica meadows—a DMSP stock which has faced rapid decline since the late 1800s (Waycott et al., 2009), but one which was able to modify dimethylated sulfur dynamics in the overlying water column and drive localized biogeochemical “hotspots.” Although smaller in their intracellular concentrations, this study suggests that coralligène communities may affect dimethylated sulfur dynamics at the regional scale because of this ecosystem's widespread distribution throughout the Mediterranean. However, the sensitivity of coralline algae to projected environmental change (Burdett et al., 2012, 2015) threatens the long-term survival of the coralligène ecosystem. Distinct diel patterns provide further evidence for a link between dimethylated sulfur and photosynthetic processes. Importantly, this study took a community-level approach, thus providing a relevant baseline from which to move forward when monitoring future changes to marine communities from an ecological and biogeochemical perspective.
Author Contributions
HB designed the study, collected and analyzed the samples, analyzed the data and wrote the paper.
Funding
Fieldwork and sample analyses were conducted whilst HB was in receipt of a Research Fellowship from the Marine Alliance for Science and Technology for Scotland (MASTS). MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions. Fieldwork was supported by the European Union FP7 Assemble Marine program (grant # 227799), awarded to HB.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Jean-Claude Roca and Bruno Hesse at the Observatoire Océanologique de Banyuls for diving support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2017.00431/full#supplementary-material
References
Achterberg, E. P. (2014). Grand challenges in marine biogeochemistry. Front. Mar. Sci. 1:7. doi: 10.3389/fmars.2014.00007
Aquarone, M., Adams, S., and Mifsud, P. (2010). IV-7 Mediterranean Sea: LME #26 [Online]. Available online at: http://lme.edc.uri.edu/index.php?option=com_content&view=article&id=46:mediterranean-sea-lme-26&catid=16&Itemid=114 (Accessed 18th January 2017).
Ballesteros, E. (2006). Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr. Mar. Biol. Ann. Rev. 44, 123–195. doi: 10.1201/9781420006391.ch4
Besiktepe, S., Tang, K. W., Vila, M., and Simó, R. (2004). Dimethylated sulfur compounds in seawater, seston and mesozooplankton in the seas around Turkey. Deep Sea Res. Part I Oceanogr. Res. Papers 51, 1179–1197. doi: 10.1016/j.dsr.2004.05.008
Bianchi, C. N., and Morri, C. (2000). Marine biodiversity of the Mediterranean Sea: situation, problems and prospects for future research. Mar. Pollut. Bull. 40, 367–376. doi: 10.1016/S0025-326X(00)00027-8
Borges, A. V., and Champenois, W. (2015). Seasonal and spatial variability of dimethylsulfoniopropionate (DMSP) in the Mediterranean seagrass Posidonia oceanica. Aquat. Bot. 125, 72–79. doi: 10.1016/j.aquabot.2015.05.008
Burdett, H. L., Aloisio, E., Calosi, P., Findlay, H. S., Widdicombe, S., Hatton, A. D., et al. (2012). The effect of chronic and acute low pH on the intracellular DMSP production and epithelial cell morphology of red coralline algae. Mar. Biol. Res. 8, 756–763. doi: 10.1080/17451000.2012.676189
Burdett, H. L., Donohue, P. J., Hatton, A. D., Alwany, M. A., and Kamenos, N. A. (2013). Spatiotemporal variability of dimethylsulphoniopropionate on a fringing coral reef: the role of reefal carbonate chemistry and environmental variability. PLoS ONE 8:e64651. doi: 10.1371/journal.pone.0064651
Burdett, H. L., Hatton, A. D., and Kamenos, N. A. (2015). Coralline algae as a globally significant pool of marine dimethylated sulfur. Glob. Biogeochem. Cycles 29, 1845–1853. doi: 10.1002/2015GB005274
Burdett, H. L., Keddie, V., Macarthur, N., McDowall, L., McLeish, J., Spielvogel, E., et al. (2014). Dynamic photoinhibition exhibited by red coralline algae in the Red Sea. BMC Plant Biol. 14:139. doi: 10.1186/1471-2229-14-139
Coll, M., Piroddi, C., Albouy, C., Ben Rais Lasram, F., Cheung, W. W., Christensen, V., et al. (2012). The Mediterranean Sea under siege: spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 21, 465–480. doi: 10.1111/j.1466-8238.2011.00697.x
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Ben Rais Lasram, F., Aguzzi, J., et al. (2010). The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE 5:e11842. doi: 10.1371/journal.pone.0011842
Curson, A. R., Liu, J., Bermejo Martínez, A., Green, R. T., Chan, Y., Carrión, O., et al. (2017). Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nat. Microbiol. 2:17009. doi: 10.1038/nmicrobiol.2017.9
Dean, J., and Kiene, R. (1992). Foliar release of dimethylsulfonioproprionate from Spartina alterniflora. Mar. Ecol. Prog. Ser. 81, 277–287. doi: 10.3354/meps081277
De Bose, J. L., Lema, S. C., and Nevitt, G. A. (2008). Dimethylsulfoniopropionate as a foraging cue for reef fishes. Science 319:1356. doi: 10.1126/science.1151109
De Madron, X. D., Guieu, C., Sempéré, R., Conan, P., Cossa, D., D'Ortenzio, F., et al. (2011). Marine ecosystems' responses to climatic and anthropogenic forcings in the Mediterranean. Prog. Oceanogr. 91, 97–166. doi: 10.1016/j.pocean.2011.02.003
Doney, S. C. (2010). The growing human footprint on coastal and open-ocean biogeochemistry. Science 328, 1512–1516. doi: 10.1126/science.1185198
Fischer, E., and Jones, G. (2012). Atmospheric dimethysulphide production from corals in the Great Barrier Reef and links to solar radiation, climate and coral bleaching. Biogeochemistry 110, 31–46. doi: 10.1007/s10533-012-9719-y
Ganor, E., Foner, H., Bingemer, H., Udisti, R., and Setter, I. (2000). Biogenic sulphate generation in the Mediterranean Sea and its contribution to the sulphate anomaly in the aerosol over Israel and the Eastern Mediterranean. Atmos. Environ. 34, 3453–3462. doi: 10.1016/S1352-2310(00)00077-7
Garcia-Castellanos, D., Estrada, F., Jiménez-Munt, I., Gorini, C., Fernández, M., Vergés, J., et al. (2009). Catastrophic flood of the Mediterranean after the Messinian salinity crisis. Nature 462, 778–781. doi: 10.1038/nature08555
Gattuso, J. P., Kirkwood, W., Barry, J. P., Cox, E., Gazeau, F., Hansson, L., et al. (2014). Free-ocean CO2 enrichment (FOCE) systems: present status and future developments. Biogeosciences 11, 4057–4075. doi: 10.5194/bg-11-4057-2014
Giering, S. L. C., Sanders, R., Lampitt, R. S., Anderson, T. R., Tamburini, C., Boutrif, M., et al. (2014). Reconciliation of the carbon budget in the ocean's twilight zone. Nature 507, 480–483. doi: 10.1038/nature13123
Griffiths, J. R., Kadin, M., Nascimento, F. J. A., Tamelander, T., Törnroos, A., Bonaglia, S., et al. (2017). The importance of benthic-pelagic coupling for marine ecosystem functioning in a changing world. Glob. Chang. Biol. 23, 2179–2196. doi: 10.1111/gcb.13642
Hemminga, M. A., and Duarte, C. M. (2000). Seagrass Ecology. Cambridge, UK: Cambridge University Press.
Jonkers, H. M., Van Bergeijk, S. A., and Van Gemerden, H. (2000). Microbial production and consumption of dimethyl sulfide (DMS) in a sea grass (Zostera noltii)-dominated marine intertidal sediment ecosystem (Bassin d'Arcachon, France). FEMS Microbiol. Ecol. 31, 163–172. doi: 10.1111/j.1574-6941.2000.tb00681.x
Kiene, R. P., and Slezak, D. (2006). Low dissolved DMSP concentrations in seawater revealed by small-volume gravity filtration and dialysis sampling. Limnol. Oceanogr. Methods 4, 80–95. doi: 10.4319/lom.2006.4.80
Kroeker, K. J., Micheli, F., and Gambi, M. C. (2013). Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Clim. Change 3, 156–159. doi: 10.1038/nclimate1680
Lejeusne, C., Chevaldonné, P., Pergent-Martini, C., Boudouresque, C. F., and Pérez, T. (2010). Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. (Amst). 25, 250–260. doi: 10.1016/j.tree.2009.10.009
Martin, C. S., Giannoulaki, M., De Leo, F., Scardi, M., Salomidi, M., Knitweiss, L., et al. (2014). Coralligenous and maërl habitats: predictive modelling to identify their spatial distributions across the Mediterranean Sea. Sci. Rep. 4:5073. doi: 10.1038/srep05073
McFarlin, C., and Alber, M. (2013). Foliar DMSO:DMSP ratio and metal content as indicators of stress in Spartina alterniflora. Mar. Ecol. Prog. Ser. 474, 1–13. doi: 10.3354/meps10184
Moran, M. A., Reisch, C. R., Kiene, R. P., and Whitman, W. B. (2012). Genomic Insights into Bacterial DMSP Transformations. Ann. Rev. Mar. Sci. 4, 523–542. doi: 10.1146/annurev-marine-120710-100827
Pinardi, N., Arneri, E., Crise, A., Ravaioli, M., and Zavatarelli, M. (2006). “The physical, sedimentary and ecological structure and variability of shelf areas in the Mediterranean sea,” in The Sea, eds A. Robinson and K. Brink (Cambridge, MA: Harvard University Press), 1245–1331.
Riebesell, U., and Gattuso, J.-P. (2015). Lessons learned from ocean acidification research. Nat. Clim. Change 5, 12–14. doi: 10.1038/nclimate2456
Rix, L. N., Burdett, H. L., and Kamenos, N. A. (2012). Irradiance-mediated dimethylsulphoniopropionate (DMSP) responses of red coralline algae. Estuar. Coast. Shelf Sci. 96, 268–272. doi: 10.1016/j.ecss.2011.11.022
Savoca, M. S., and Nevitt, G. A. (2014). Evidence that dimethyl sulfide facilitates a tritrophic mutualism between marine primary producers and top predators. Proc. Natl. Acad. Sci. U.S.A. 111, 4157–4161. doi: 10.1073/pnas.1317120111
Seymour, J. R., Simó, R., Ahmed, T., and Stocker, R. (2010). Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science 329, 342–345. doi: 10.1126/science.1188418
Simó, R., and Grimalt, J. O. (1998). Spring–summer emission of dimethyl sulphide from the North-western Mediterranean Sea. Estuar. Coast. Shelf Sci. 47, 671–677. doi: 10.1006/ecss.1998.0386
Simo, R., Grimalt, J. O., and Albaigés, J. (1997). Dissolved dimethylsulphide, dimethylsulphoniopropionate and dimethylsulphoxide in western Mediterranean waters. Deep Sea Res. Part II: Top. Stud. Oceanogr. 44, 929–950. doi: 10.1016/S0967-0645(96)00099-9
Sneed, J. M., Ritson-Williams, R., and Paul, V. J. (2015). Crustose coralline algal species host distinct bacterial assemblages on their surfaces. ISME J. 9:2527. doi: 10.1038/ismej.2015.67
Stefels, J. (2000). Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J. Sea Res. 43, 183–197. doi: 10.1016/S1385-1101(00)00030-7
Steudler, P. A., and Peterson, B. J. (1984). Contribution of gaseous sulphur from salt marshes to the global sulphur cycle. Nature 311, 455–457. doi: 10.1038/311455a0
Telesca, L., Belluscio, A., Criscoli, A., Ardizzone, G., Apostolaki, E. T., Fraschetti, S., et al. (2015). Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 5:12505. doi: 10.1038/srep12505
Turner, S. M., Malin, G., Bagander, L. E., and Leck, C. (1990). Interlaboratory calibration and sample analysis of dimethyl sulfide in water. Mar. Chem. 29, 47–62. doi: 10.1016/0304-4203(90)90005-W
Van Alstyne, K. (2009). “Ecological and physiological roles of dimethylsulphoniopropionate and its products in marine macroalgae,” in Algal Chemical Ecology, ed C. D. Amsler (Heidelberg: Springer), 173–194.
Van Alstyne, K., and Puglisi, M. (2007). DMSP in marine macroalgae and macroinvertebrates: distribution, function, and ecological impacts. Aquat. Sci. 69, 394–402. doi: 10.1007/s00027-007-0888-z
Van Alstyne, K., Schupp, P., and Slattery, M. (2006). The distribution of dimethylsulfoniopropionate in tropical Pacific coral reef invertebrates. Coral Reefs 25, 321–327. doi: 10.1007/s00338-006-0114-9
Vila-Costa, M., Kiene, R. P., and Simo, R. (2008). Seasonal variability of the dynamics of dimethylated sulfur compounds in a coastal northwest Mediterranean site. Limnol. Oceanogr. 53, 198–211. doi: 10.4319/lo.2008.53.1.0198
Waycott, M., Duarte, C. M., Carruthers, T. J., Orth, R. J., Dennison, W. C., Olyarnik, S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Wolfe, G. V., Steinke, M., and Kirst, G. O. (1997). Grazing-activated chemical defence in a unicellular marine alga. Nature 387, 894–897. doi: 10.1038/43168
Keywords: dimethylsulphide (DMS), dimethylsulphoniopropionate (DMSP), community, ecosystem, seagrass, crustose coralline algae (CCA), macroalgae
Citation: Burdett HL (2017) Exchange Dynamics Reveal Significant Accumulation of Dimethylated Sulfur by Mediterranean Benthic Communities. Front. Mar. Sci. 4:431. doi: 10.3389/fmars.2017.00431
Received: 04 May 2017; Accepted: 14 December 2017;
Published: 22 December 2017.
Edited by:
Toshi Nagata, The University of Tokyo, JapanReviewed by:
Nick Kamenos, University of Glasgow, United KingdomChristophe Rabouille, UMR8212 Laboratoire des Sciences du Climat et de l'Environnement (LSCE), France
Copyright © 2017 Burdett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi L. Burdett, aC5idXJkZXR0QGh3LmFjLnVr
 Heidi L. Burdett
Heidi L. Burdett