
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 12 December 2017
Sec. Marine Biotechnology and Bioproducts
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00399
Four new sporochartines B–E were isolated from the marine fungus Hypoxylon monticulosum CLL-205, isolated from a sponge belonging to the Sphaerocladina order and collected in Tahiti coast. Sporochartine A (1), the first representative of this family was previously isolated from the same fungus. The structures of sporochartines B–E were elucidated using 1D and 2D NMR, HRMS and IR data. Their configurations were established according to ROE correlations and comparison with the absolute configuration of sporochartine A (1) previously obtained from X-ray analysis. Sporochartines A–D (2–4) may be derived from endo Diels-Alderase type catalysis and sporochartine E (5) from an exo Diels-Alderase catalysis. The spatial conformation of sporochartines drastically influences the results of the cytotoxic bioassay against HCT-116, PC-3, and MCF-7 human cancer cell lines.
The fungal Xylariaceae family includes more than 16 genera and 130 species (Sánchez-Ballesteros et al., 2000) and has been extensively investigated for the chemo diversity and biological activity of their metabolites (Stadler et al., 2006, 2008). Among the 16 genera reported, Hypoxylon with 14 species is largely distributed in various marine and terrestrial habitats, and producing a large variety of bioactive compounds among which cohaerins (Quang et al., 2005a; Surup et al., 2013), daldinins and daldinones (Quang et al., 2004; Gu et al., 2007), cytochalasin (Espada et al., 1997), fragiformin (Stadler et al., 2006), mitorubrinols (Quang et al., 2005b), hypoxylonols (Fukai et al., 2012), hypoxylans (Kuhnert et al., 2015a), hypoxyvermelhotins (Kuhnert et al., 2014), rickenyls (Kuhnert et al., 2015b), rutilins (Quang et al., 2005b), carneic acids (Quang et al., 2006), hymatoxins (Bodo et al., 1987; Borgschulte et al., 1991), malettinins (Angawi et al., 2005), hypoxysordarin (Daferner et al., 1999), lenormandins (Kuhnert et al., 2015c), nodulisporic acids (Bills et al., 2012), schweinitzin A (Linh et al., 2014), truncatones (Sudarman et al., 2016), macrocyclic polyesters 15G256 family (Schlingmann et al., 2002), and sporothriolide (Krohn et al., 1994; Surup et al., 2014; Cao et al., 2016).
Sporothriolide belongs to the furofurandione family of natural compounds first published in 1994 (Krohn et al., 1994). This compound exhibits antifungal activity and benefits from substantial synthetic efforts (Sharma and Krishnnudu, 1995; Yu et al., 2001; Fernandes and Ingle, 2009; Ishihara et al., 2014). The name sporothriolide is related to Sporothrix sp. Hektoen and Perkins (strain 700), from which this compound was first isolated. Sporothrix genus belongs to a different ascomycete family, ophiostomataceae. The first report on sporothriolide in 1994 detailed both the structure and bioactivity of this product (Krohn et al., 1994). It shows that Sporothrix produces sporothriolide, dihydrosporothriolide, as well as various sporothriolide analogs with different side-chain length (canadensolide, discosiolide, avenaciolide, ethiosolide). The authors reported also the antifungal/herbicidal activities of these compounds (Krohn et al., 1994) (Figure 1).
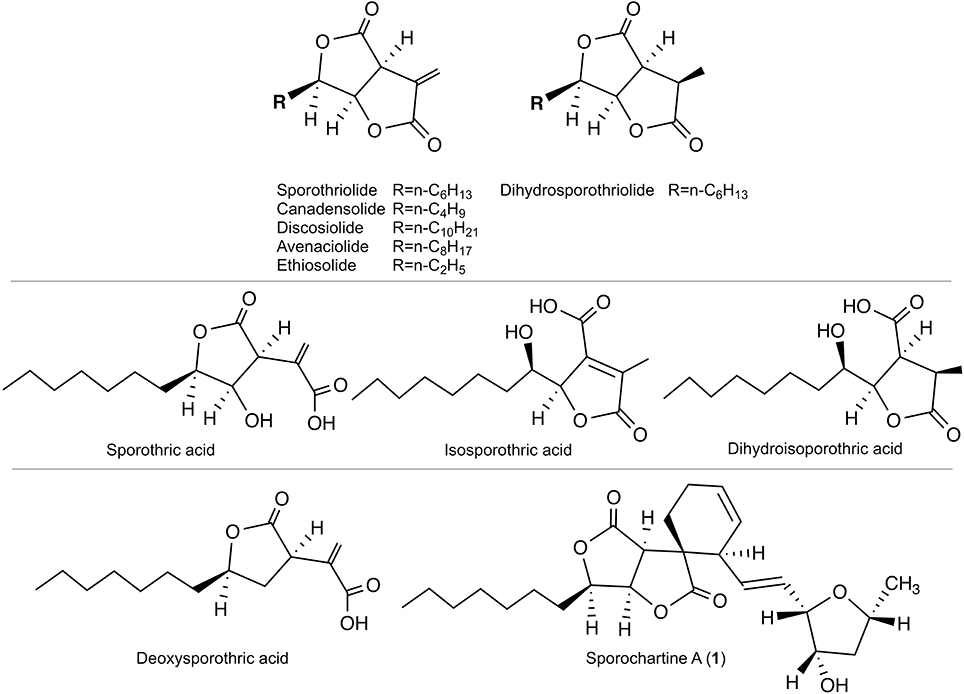
Figure 1. Top: Sporothriolide and analogs reported in 1994 (Krohn et al., 1994). Middle: Monocyclic precursors reported in 2014 (Surup et al., 2014). Bottom: Deoxysporothric acid and sporochartine A reported in our previous work (Leman-Loubière et al., 2017).
Twenty years later, sporothriolide and dihydrosporothriolide were isolated from Hypoxylon monticulosum together with three monocyclic acid precursors: sporothric acid, isosporothric acid and dihydroisosporothric acid (Figure 1) (Surup et al., 2014). More recently, sporothriolide was isolated from Nodulisporium sp., an anamorph of Hypoxylon, and the herbicidal activity was confirmed (Cao et al., 2016).
In our previous contribution, we added to the scarce sporothriolide family two new compounds, deoxysporothric acid and a new complex architecture sporochartine A, combining sporothriolide and trienylfuranol A moieties. The trienylfuranol A was recently isolated from a different Hypoxylon submoniticulosum (Burgess et al., 2017).
In the present work we report four new sporochartines B to E. Their structures were elucidated using 1D and 2D NMR, HRMS, IR and comparison with sporochartine A data, for which the absolute configuration was previously established by X-Ray analysis. A Diels-Alderase type reaction is probably involved in the biosynthesis of the five isolated sporochartines, as discussed below.
The human cancer cell-lines cytotoxicity bioassay shows that the conformation of sporochartines has an impact on the biological activity.
According to our previous work, sporochartine A (1) was obtained after 5 days cultivation of H. monticulosum CLL-205 in PDB broth (Leman-Loubière et al., 2017). By extending the cultivation of the same microorganism by a further 4 days, the ethyl actetate extract gives the HPLC chromatogram presented in Figure 2.
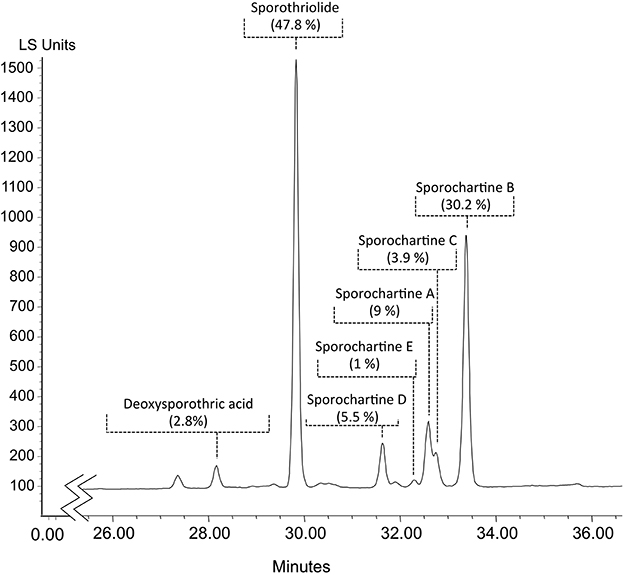
Figure 2. HPLC analysis of the ethyl acetate extract of Hypoxylon monticulosum CLL-205 cultivated in PBD for 9 days. Percentages were deduced from peak integration based on Light Scattering Detection chromatogram (LSD).
Sporochartines B–D were isolated as white powders by flash chromatography followed by semi-preparative HPLC. They had similar [M+H]+ HRESIMS molecular weights, molecular formula C24H34O6 and IR spectra compared with sporochartine A (1) (Table 2) (Leman-Loubière et al., 2017). The 1H and 13C NMR spectra of sporochartines B–D were similar to those of compound 1 (Tables 1, 2). Optical rotations , IR bands and HRESIMS are reported in Table 2.
COSY and HMBC spectra, confirmed that sporochartines A–D had the same connectivities supporting similar planar scaffold (Figure 3). In addition, the common coupling constant of 15.4–15.6 Hz between H-18 and H-19 confirmed that the double bond C-18/C-19 is in E configuration.
Based on the previously reported absolute configuration of sporochartine A (1) and ROE correlations, we deduced the absolute configuration of sporochartines B–D (2–4) (Figure 4).
The common ROE correlations between H-2 and H-5 and between H-5 and H-6 requiring a cis orientation of these three protons was found in the sporochartine A–D. Therefore, the stereochemistry of the sporothriolide moiety was identical. Moreover, based on ROE correlations between H-20 and H-21 and between H-21 and H-23, the strerochemistry of the tetrahydrofurane moiety is also a common feature in sporochartines A–D.
For sporochartine B (2) (Figure 4), we did not observe ROE correlations between H-17 and H-2 and between H-17 and H-14b as in sporochartine A (1), while a new correlation is observed between H-17 and H-13. This data suggests that the carbon C-17 have opposite stereochemistry compared to 1 supporting a 3S,17R configuration of 2 (instead of 3S,17S in 1).
For sporochartine C (3) (Figure 4), the H-17/H-2 and H-17/H-14b correlations observed in sporochartine A (1) are absent. In addition, we observed a correlation between H-17 and H-13 and H-2 and H-14a in compound 3. Based on this data, we suggest that compound 3 has a 3R,17S configuration.
Sporochartine D (4) (Figure 4) conserved the correlations between H-17 and H-2 and between H-17 and H-14b reported for sporochartine A (1). Furthermore, the correlation between H-17 and H-13 is absent in both 4 and 1. In 4 we have an additional correlation between H-13 and H-2, absent in 1. These observations support the conclusion that 4 is the 3R,17R isomer of 1.
A new compound referred as sporochartine E (5) was also isolated as a white powder. Compound 5 has the same molecular formula C24H34O6 as compound 1, deduced from HRESIMS m/z [M+H]+ 419.2433. Here again we have eight degrees of unsaturation accounting for two γ-lactones, two double bonds, one six-membered cycle moiety and one tetrahydrofurane moiety.
Compound 5 has a terminal methylene group (at δC 119.6, δH 5.27 and δH 5.21) while the tetrahydrofuran moiety connected to C-19 in 1 is connected to C-14 in 5.
Based on COSY correlations (Figure 5), the sporothriolide moiety was the same in compound 5 as in 1. Moreover, COSY correlations from H-13 to H-19 through H-14 (δH 3.25), H-15 (δH 6.16), H-16 (δH 5.56), H-17 (δH 2.80) and H-18 (δH 5.65) together with HMBC correlation between H-13 and C-3 and H-17 and C-3 formed a cyclohexane fragment like in 1. Finally, by using the COSY correlations from H-24 (δH 1.34) to H-20 (δH 3.22), through H-23 (δH 3.96), H-22 (δH 1.53 and 2.39) and H-21 (δH 4.28) we deduce the tetrahydrofuran moiety. The HMBC correlations between H-20 and C-14 and C-15 allowed us to connect this tetrahydrofurane moiety to the sp3 methine C-14.
The absolute configuration of sporochartine E (5) was suggested using ROE correlations compared to the absolute configuration of sporochartine A (1) (Figure 6).
ROE correlations between H-2 and H-5/H-6 in the sporothriolide moiety and the ROE correlation between H-20 and H-21 and between H-23 and H-21 in the tetrahydrofuran moiety indicated a similar to that in 1.
Sporochartine E (5), showed a correlation between H-17 and H-2, like in compounds 1 and 4. H-2 also exhibited a correlation with H-13b but not with H-14. This suggests that C-3 and C-17 has the same relative configuration than 4. For C-14, we observed ROE correlations between H-14 and H-13a, H-13a, and H-17 and H-14 and H-24, suggesting a 3R, 14S, 17S configuration for compound 5.
Based on the structure of sporothriolide and the recently reported trienylfuranol A isolated from H. submoniticulosum, we suggested a hypothetic biosynthetic pathway of sporochartines, involving a “spiro” Diels-Alderase reaction as shown in Figure 7 (Klas et al., 2015; Byrne et al., 2016). The possibility of a non-enzymatic catalysis was excluded as reported previously (Leman-Loubière et al., 2017).
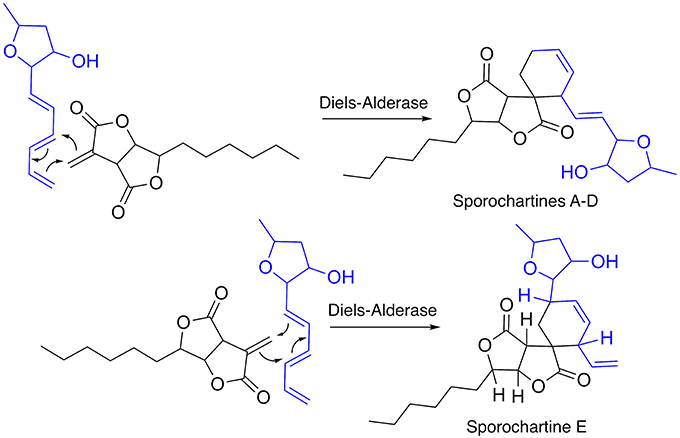
Figure 7. Hypothetic biosynthesis of sporochartines through Diels-Alderase type reaction between sporothtriolide and trienylfuranol A.
The cytotxicity of sporochartines was evaluated on three human cancer cell lines, HCT-116 (human colon carcinoma), PC-3 (prostate cancer cell lines) and MCF-7 (breast cancer cell line). The results presented in Table 3 are highly contrasting but nevertheless clearly indicate the impact of sporochartine conformation on the bioassay results.
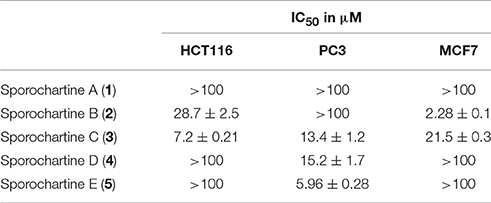
Table 3. IC50 values recorded for sporochartines A–E against three cancer cell lines, HCT-116 (human colon carcinoma), PC-3 (human prostate cancer cell lines), and MCF-7 (human breast cancer cell line).
Thus, sporochartine C (3) is toxic against the three cell lines with IC50 ranging from 7.2 to 21.5 μM. In contast, sporochartine A (1) is totally inactive at concentrations higher than 100 μM. This may be due to the substantial difference in the spatial conformation of compounds 1 and 3 (Figure 4).
The lower IC50 values were recorded for different sporochartines and against different cell lines, sporochartine B (2) for MCF-7 (2.28 μM), sporochartine C (3) for HCT-116 (7.2 μM) and sporochartine E (5) for PC-3 (5.96 μM).
Our future efforts will focus on the cytotoxic profile, biosynthesis and synthesis of sporochartines. The cytotoxicity profile reveals a non-cytotoxic sporochartine A (1), a large spectrum cytotoxic sporochartine C (3) and more cell line specific sporochartines B (2), D (4), and E (5). This finding merits future investigation on the mechanisms of action of these new scaffolds of cytotoxic compounds.
The biosynthesis of sporochartines, and the biosynthesis of its two moieties, sporothriolide and trienylfuranol A are still unknown. This opens new and promising opportunities for the discovery of novel biosynthetic microbial clusters.
Finally, having in hand hundreds of milligrams of sporothriolide, the hemi-synthesis of sporochartines is currently in progress based on a final Diels-Alder connection. The selectivity of the chemical catalysis and the proportion of different isomers will be compared to the microbial counterpart. According to our expertise in biocatalysis-based chemodiversification of natural compounds (Adelin et al., 2011; Martins et al., 2015), sporothriolide will be submitted to a panel of microorganisms in order to pursue the enrichment of sporothriolide related compounds.
Optical rotations [α]D were measured using an Anton Paar MCP-300 polarimeter. IR spectra were obtained using a Perkin Elmer BX FT-IR spectrometer. NMR experiments were performed using a Bruker Avance 500 MHz in CDCl3 at room temperature. High-resolution mass spectra were obtained on a Waters LCT Premier XE spectrometer equipped with an ESI-TOF (electrospray-time of flight) by direct infusion of the purified compounds. Preparative HPLC was performed using Waters modules consisting of an autosampler 717, a pump 600, a photodiode array detector 2996 and an evaporative light-scattering detector, ELSD 2420. Prepacked silica gel Redisep columns were used for flash chromatography using a Combiflash-Companion chromatogram (Serlabo, France). All other chemicals and solvents were purchased from SDS (France).
The Sphaerocladina sponge was collected on 17 December 2015 from the coast of Tahiti (9°45.421′S–139°08.275′W) at 20 m depth (Leman-Loubière et al., 2017).
H. monticulosum CLL205 was isolated from the sponge Sphaerocladina and grown at 28°C on a PDB medium (Potatoes Dextrose Broth, DIFCO). The ITS rDNA gene amplification and sequencing were performed, and submitted to NCBI/BLAST database (GenBank). The primers used for PCR amplification were ITS1 F: CTT GGT CAT TTA GAG GAA GTA A (Tm: 55°C) and ITS4: TCC TCC GCT TAT TGA TATGC (Tm: 53°C). The GenBank accession number for H. monticulosum CLL205 sequence is SUB2477083 25758633.seq KY744359. H. monticulosum CLL205 was cultivated in a 2 L Erlenmeyer containing 1L of PDB medium (DIFCO) in a rotary shaker at 28°C and 130 rpm.
The culture broth was extracted with ethyl acetate (3 × 500 mL). The solvent was concentrated to dryness in vacuo to afford 430 mg of crude extract. 300 mg were submitted to flash chromatography on a Combiflash Companion using a Redisep 12 g silica column, eluted with a heptane-ethyl acetate mixture. After concentration in vacuo, we obtained sporothriolide (30 mg), compound 1 (9 mg), 2 (14 mg), 3 (4 mg), 4 (3 mg), 5 (1 mg).
A tetrazolium dye [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide; MTT]-based colorimetric assay was used to measure the inhibition on the proliferation of various human tumor cell lines HCT-116 (human colon carcinoma), PC-3 (prostate cancer cell lines) and MCF-7 (breast cancer cell line). The tested compounds were formulated in DMSO and added to the cells such that the final DMSO concentration ranged from 1 to 3%. Cells were grown in D-MEM medium supplemented with 10% fetal calf serum (Invitrogen), in the presence of penicillin, streptomycin, and fungizone, and plated in 96-well microplates. After 24 h of growth, cells were treated with target compounds from 100 μM to 10 nM. After 72 h, MTS reagent (Promega) was added, and the absorbance was monitored (490 nm) to measure the inhibition of cell proliferation compared to untreated cells. IC50 determination experiments were performed in separate duplicate experiments.
Sporochartine A (1)33: white needles, M.p. 86.5–87.9°C; −57 (c 0.5, CHCl3). See Tables 1, 2 for complete 1H, 13C NMR and IR data. HRESIMS m/z 419.2433 [M + H]+ (calcd for C24H35O6, 419.2433).
Sporochartine B (2): white powder; +72 (c 1.0, CHCl3). See Tables 1, 2 for complete 1H, 13C NMR and IR data. HRESIMS m/z 419.2419 [M + H]+ (calcd for C24H35O6, 419.2433).
Sporochartine C (3): white powder (4 mg); +93 (c 0.27, CHCl3). See Tables 1, 2 for complete 1H, 13C NMR and IR data. HRESIMS m/z 419.2433 [M + H]+ (calcd for C24H35O6, 419.2433).
Sporochartine D (4): white powder; −152 (c 0.27, CHCl3). See Tables 1, 2 for complete 1H, 13C NMR and IR data. HRESIMS m/z 419.2431 [M + H]+ (calcd for C24H35O6, 419.2433).
Sporochartine E (5): white powder; +51 (c 0.3, CHCl3). See Tables 1, 2 for complete 1H, 13C NMR and IR data. HRESIMS m/z 419.2425 [M + H]+ (calcd for C24H35O6, 419.2434).
Structural elucidation data are reported in the Supplementary Materials.
Detailed 1D and 2DNMR, MS and IR spectra of sporochartines are available free of charge via the Internet at http://pubs.acs.org.
CL-L: microbiologie chemistry; GL: chemistry; CD: invetebrate investigation; JO: head of the team and science manager.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by TASCMAR project funded by the European Union's Horizon 2020 research and innovation program under grant agreement n° 634674, and the Ph.D. support program of ICSN.
The authors are grateful to the CIBI Plateforme of CNRS-ICSN for cytotoxicity essays (Jérôme Bignon & Hélène Levaique).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2017.00399/full#supplementary-material
Adelin, E., Servy, C., Cortial, S., Lévaique, H., Gallard, J.-F., Martin, M.-T., et al. (2011). Biotransformation of natural compounds. Oxido-reduction of Sch-642305 by Aspergillus ochraceus ATCC 1009. Bioorg. Med. Chem. Lett. 21, 2456–2459. doi: 10.1016/j.bmcl.2011.02.063
Angawi, R. F., Swenson, D. C., Gloer, J. B., and Wicklow, D. T. (2005). Malettinins B–D: new polyketide metabolites from an unidentified fungal colonist of hypoxylon stromata (NRRL 29110). J. Nat. Prod. 68, 212–216. doi: 10.1021/np049625r
Bills, G. F., González-Menéndez, V., Martin, J., Platas, G., and Fournier, J. (2012). Hypoxylon pulicicidum sp. nov. (Ascomycota, Xylariales), a pantropical insecticide-producing endophyte. PLoS ONE 7:e46687. doi: 10.1371/journal.pone.0046687
Bodo, B. D., Davoust, D., Lecommandeur, D., Rebuffat, S., Genetet, I., and Pinon, J. (1987). Hymatoxin A, a diterpene sulfate phytotoxin of Hypoxylon mammatum, parasite of aspen. Tetrahedron Lett. 28, 2355–2358. doi: 10.1016/S0040-4039(00)96123-9
Borgschulte, K., Rebuffat, S., Trowitzsch-Kienast, W., Schomburg, D., Pinon, J., and Bodo, B. (1991). Isolation and structure elucidation of hymatoxins B–E and other phytotoxins from Hypoxylon mammatum fungal pathogen of leuce poplars. Tetrahedron 47, 8351–8360. doi: 10.1016/S0040-4020(01)96176-9
Burgess, K. M. N., Ashraf Ibrahim, A., Sørensen, D., and Sumarah, M. W. (2017). Trienylfuranol A and trienylfuranone A-B: metabolites isolated from an endophytic fungus, Hypoxylon submoniticulosum, in the raspberry Rubus idaeus. J. Antibiot. 70, 721–725. doi: 10.1038/ja.2017.18
Byrne, M. J., Lees, N. R., Han, L.-C., Van der Kamp, M. W., Mulholland, A. J., Stach, J. E. M., et al. (2016). The catalytic mechanism of a natural diels–alderase revealed in molecular detail. J. Am. Chem. Soc. 138, 6095–6098. doi: 10.1021/jacs.6b00232
Cao, L.-L., Zhang, Y.-Y., Liu, Y.-J., Yang, T.-T., Zhang, J.-L., Zhang, Z.-G., et al. (2016). Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium sp. A21 in Ginkgo biloba. Pestic. Biochem. Physiol. 129, 7–13. doi: 10.1016/j.pestbp.2015.10.002
Daferner, M., Mensch, S., Anke, T., and Sternerb, O. (1999). Hypoxysordarin, a New Sordarin Derivative from Hypoxylon croceum. Z. Naturforsch. 54c, 474–480. doi: 10.1515/znc-1999-7-803
Espada, A., Rivera-Sagredo, A., De la Fuente, J. M., Hueso-Rodriguez, J. A., and Elson, S. W. (1997). New cytochalasins from the fungus Xylaria hypoxylon. Tetrahedron 53, 6485–6492. doi: 10.1016/S0040-4020(97)00305-0
Fernandes, R. A., and Ingle, A. B. (2009). Chiral vicinal diols as platforms for separable diastereomers in Johnson–Claisen rearrangement: a new short route to (–)-nor-canadensolide, (–)-canadensolide and (–)-sporothriolide. Tetrahedron Lett. 50, 1122–1124. doi: 10.1016/j.tetlet.2008.12.084
Fukai, M., Tsukada, M., Miki, K., Suzuki, T., Sugita, T., Kinoshita, K., et al. (2012). Hypoxylonols C–F, Benzo[j]fluoranthenes from Hypoxylon truncatum. J. Nat. Prod. 75, 22–25. doi: 10.1021/np2004193
Gu, W., Ge, H. M., Song, Y. C., Ding, H., Zhu, H. L., Zhao, X. A., et al. (2007). Cytotoxic Benzo[j]fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J. Nat. Prod. 70, 114–117. doi: 10.1021/np0604127
Ishihara, J., Tsuru, H., and Hatakeyama, S. (2014). Total synthesis of (–)-dihydrosporothriolide utilizing an indium-mediated reformatsky–claisen rearrangement. J. Org. Chem. 79, 5908–5913. doi: 10.1021/jo5008948
Klas, K., Tsukamoto, S., Sherman, D. H., and Williams, R. M. (2015). Natural diels–alderases: elusive and irresistable. J. Org. Chem. 80, 11672–11685. doi: 10.1021/acs.joc.5b01951
Krohn, K., Ludewig, K., Aust, H.-J., Draeger, S., and Schulz, B. (1994). Biologically active metabolites from fungi. 3. sporothriolide, discosiolide, and 4-epi-ethisolide–new furofurandiones from Sporothrix sp., Discosia sp., and Pezicula livida. J. Antibiot. 47, 113–118. doi: 10.7164/antibiotics.47.113
Kuhnert, E., Heitkämper, S., Fournier, J., Surup, F., and Stadler, M. (2014). Hypoxyvermelhotins A–C, new pigments from Hypoxylon lechatii sp. nov. Fungal Biol. 118, 242–252. doi: 10.1016/j.funbio.2013.12.003
Kuhnert, E., Surup, F., Herrmann, J., Huch, V., Müller, R., and Stadler, M. (2015a). Rickenyls A–E, antioxidative terphenyls from the fungus Hypoxylon rickii (Xylariaceae, Ascomycota). Phytochemistry 118, 68–73. doi: 10.1016/j.phytochem.2015.08.004
Kuhnert, E., Surup, F., Sir, E. B., Lambert, C., Hyde, K. D., Hladki, A. I., et al. (2015b). Lenormandins A–G, new azaphilones from Hypoxylon lenormandii and Hypoxylon jaklitschii sp. nov., recognised by chemotaxonomic data. Fungal Divers. 71, 165–184. doi: 10.1007/s13225-014-0318-1
Kuhnert, E., Surup, F., Wiebach, V., Bernecker, S., and Stadler, M. (2015c). Botryane, noreudesmane and abietane terpenoids from the ascomycete Hypoxylon rickii. Phytochemistry 117, 116–122. doi: 10.1016/j.phytochem.2015.06.002
Leman-Loubière, C., Le Goff, G., Retailleau, P., Debitus, C., and Ouazzani, J. (2017). Sporothriolide-related compounds from the fungus Hypoxylon monticulosum CLL-205 isolated from a sphaerocladina sponge from the tahiti coast. J. Nat. Prod. 80, 2850–2854. doi: 10.1021/acs.jnatprod.7b00714
Linh, D. T. P., Hien, B. T. T., Que, D. D., Lam, D. M., Arnold, N., Schmidt, J., et al. (2014). Cytotoxic constituents from the vietnamese fungus xylaria schweinitzii. Nat. Prod. Commun. 9, 659–660.
Martins, M. P., Ouazzani, J., Arcile, G., Jeller, A. H., de Lima, J. P. F., Seleghim, M. H. R., et al. (2015). Biohydroxylation of (–)-Ambrox®, (–)-Sclareol, and (+)-Sclareolide by whole cells of Brazilian marine-derived fungi. Mar. Biotechnol. 17, 211–218. doi: 10.1007/s10126-015-9610-7
Quang, D. N., Hashimoto, T., Nomura, Y., Wollweber, H., Hellwig, V., Fournier, J., et al. (2005a). Cohaerins A and B, azaphilones from the fungus Hypoxylon cohaerens, and comparison of HPLC-based metabolite profiles in Hypoxylon sect. Annulata. Phytochemistry 66, 797–809. doi: 10.1016/j.phytochem.2005.02.006
Quang, D. N., Hashimoto, T., Stadler, M., and Asakawa, Y. (2005b). Dimeric azaphilones from the xylariaceous ascomycete Hypoxylon rutilum. Tetrahedron 61, 8451–8455. doi: 10.1016/j.tet.2005.06.077
Quang, D. N., Hashimoto, T., Tanaka, M., Stadler, M., and Asakawa, Y. (2004). Cyclic azaphilones daldinins E and F from the ascomycete fungus Hypoxylon fuscum (Xylariaceae). Phytochemistry 65, 469–473. doi: 10.1016/j.phytochem.2003.09.022
Quang, D. N., Stadler, M., Fournier, J., and Asakawa, Y. (2006). Carneic Acids A and B, chemotaxonomically significant antimicrobial agents from the xylariaceous ascomycete Hypoxylon carneum. J. Nat. Prod. 69, 1198–1202. doi: 10.1021/np0602057
Sánchez-Ballesteros, J., González, V., Salazar, O., Acero, J., Portal, M. A., Julián, M., et al. (2000). Phylogenetic study of Hypoxylon and related genera based on ribosomal ITS sequences. Mycologia 92, 964–977. doi: 10.2307/3761591
Schlingmann, G., Milne, L., and Carter, G. T. (2002). Isolation and identification of antifungal polyesters from the marine fungus Hypoxylon oceanicum LL-15G256. Tetrahedron 58, 6825–6835. doi: 10.1016/S0040-4020(02)00746-9
Sharma, G. V. M., and Krishnnudu, K. (1995). Radical reactions on furanoside acetals: first synthesis of sporothriolide and 4-epi- ethisolide from 'diacetone glucose'. Tetrahedron Lett. 36, 2661–2664. doi: 10.1016/0040-4039(95)00327-9
Stadler, M., Fournier, J., Laessøe, T., Lechat, C., Tichy, H., and Piepenbring, M. (2008). Recognition of hypoxyloid and xylarioid Entonaema species and allied Xylaria species from a comparison of holomorphic morphology, HPLC profiles, and ribosomal DNA sequences. Mycol. Prog. 7, 53–73. doi: 10.1007/s11557-008-0553-5
Stadler, M., Quang, D. N., Tomita, A., Hashimoto, T., and Asakawa, Y. (2006). Changes in secondary metabolism during stromatal ontogeny of Hypoxylon fragiforme. Mycol. Res. 110, 811–820. doi: 10.1016/j.mycres.2006.03.013
Sudarman, E., Kuhnert, E., Hyde, K. D., Sir, E. B., Surup, F., and Marc Stadler, M. (2016). Truncatones A–D, benzo[j]fluoranthenes from Annulohypoxylon species (Xylariaceae, Ascomycota). Tetrahedron 72, 6450–6454. doi: 10.1016/j.tet.2016.08.054
Surup, F., Kuhnert, E., Lehmann, E., Heitkämper, S., Hyde, K. D., Fournier, J., et al. (2014). Sporothriolide derivatives as chemotaxonomic markers for Hypoxylon monticulosum. Mycology 5, 110–119. doi: 10.1080/21501203.2014.929600
Surup, F., Mohr, K. I., Jansen, R., and Stadler, M. (2013). Cohaerins G–K, azaphilone pigments from Annulohypoxylon cohaerens and absolute stereochemistry of cohaerins C–K. Phytochemistry 95, 252–258. doi: 10.1016/j.phytochem.2013.07.027
Keywords: Hypoxylon, Sphaerocladina, sporothriolide, sporochartines, cytotoxic compounds
Citation: Leman-Loubière C, Le Goff G, Debitus C and Ouazzani J (2017) Sporochartines A–E, A New Family of Natural Products from the Marine Fungus Hypoxylon monticulosum Isolated from a Sphaerocladina Sponge. Front. Mar. Sci. 4:399. doi: 10.3389/fmars.2017.00399
Received: 17 October 2017; Accepted: 24 November 2017;
Published: 12 December 2017.
Edited by:
Antonio Trincone, Istituto di Chimica Biomolecolare (CNR), ItalyReviewed by:
Johannes F. Imhoff, GEOMAR Helmholtz Centre for Ocean Research Kiel (HZ), GermanyCopyright © 2017 Leman-Loubière, Le Goff, Debitus and Ouazzani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jamal Ouazzani, amFtYWwub3VhenphbmlAY25ycy5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.