
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 05 December 2017
Sec. Marine Evolutionary Biology, Biogeography and Species Diversity
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00392
The Longqi hydrothermal vent field is the first deep-sea active vent field to be explored on the ultra-slow spreading Southwest Indian Ridge. Although a number of larger taxa has been described or characterised, many smaller and less conspicuous animals remain undescribed. Here, two small (<7 mm) coiled gastropods belonging to the vent-endemic family Peltospiridae are characterised and formally named from Longqi. Lirapex politus n. sp. is characterised by its entirely smooth shell lacking in axial sculpture, which distinguishes it from the three described congeners from East Pacific Rise and Mid-Atlantic Ridge. Dracogyra subfuscus n. gen., n. sp. is conchologically most similar to Depressigyra globulus from the northeastern Pacific, differing in having an almost closed umbilicus and lacking a basal notch in the outer lip. Radula characteristics clearly distinguish the two, however, with Dracogyra n. gen. having a much wider, shorter, sturdier central tooth and stronger laterals. Molecular phylogeny reconstruction using the cytochrome c oxidase I (COI) barcoding fragment indicate that Dracogyra n. gen. is in fact most closely related to Gigantopelta and Lirapex is sister to Pachydermia. The pairwise distance in COI between Dracogyra n. gen. and other peltospirid genera (14.4%~26.6%, mean 21.3%) are sufficient to justify separate genera. Both new species were found around diffuse flow venting areas in association with giant holobiont peltospirid snails Chrysomallon and Gigantopelta. The addition of these two new species increases the total macrofauna species known from Longqi field to 23.
ZooBank article registration: urn:lsid:zoobank. org:pub:697D89DE-0532-4934-9E52-9BA4C69FD4D1
The Southwest Indian Ridge is an ultra-slow spreading ridge running from the Rodrigues Triple Junction in southwest Indian Ocean to the Bouvet Triple Junction in southeast Atlantic Ocean (Dick et al., 2003). The Longqi hydrothermal vent field (Figure 1A), the first active vent visually confirmed on the ridge, was initially discovered in 2007 using an autonomous underwater vehicle and a camera-grab system (Tao et al., 2012, 2014). The earliest exploration of the site with a submersible was carried out on-board RRS James Cook expedition JC67 in 2011 using ROV Kiel6000 (Copley et al., 2016). Subsequently, the Chinese manned submersible HOV Jiaolong also visited the Longqi field on-board R/V Xiangyanghong 9 on several occasions (Zhou et al., 2017).
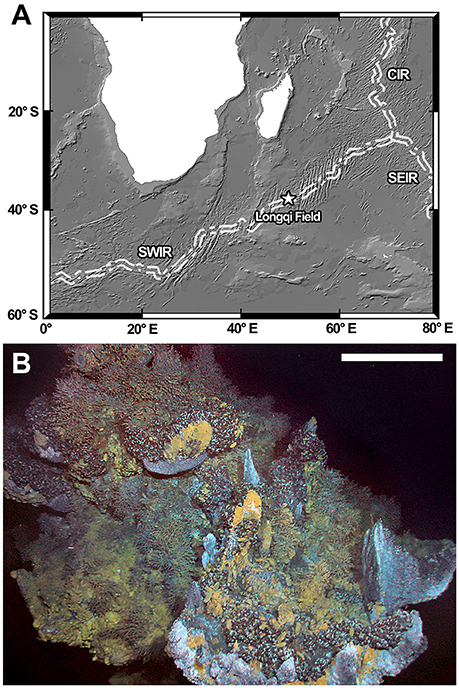
Figure 1. (A) Location of the Longqi vent field on the Southwest Indian Ridge. (B) The “Tiamat” Chimney where the two small peltospirids were first collected. Scale bar = approximately 1 m on the foreground. Abbreviations: CIR, Central Indian Ridge; SEIR, Southeast Indian Ridge; SWIR, Southwest Indian Ridge.
Up to this point, 21 megafauna species have been reported from the Longqi field (Copley et al., 2016). Among the dominant fauna of the Longqi field are two large snails in Peltospiridae, the scaly-foot gastropod Chrysomallon squamiferum Chen et al., 2015 and Gigantopelta aegis Chen et al., 2015 which often co-occur and form large aggregations side-by-side on diffuse flow venting areas (Chen et al., 2015a; Copley et al., 2016). Peltospiridae is one of three families in the gastropod clade Neomphalina, the other two being Melanodrymiidae and Neomphalidae (Heß et al., 2008). The entire clade appears to be restricted to chemosynthetic ecosystems and Peltospiridae is so far only known from hot vents (Chen et al., 2015b). Of the 11 currently recognised peltospirid genera, Chrysomallon and Gigantopelta are the only two that definitively rely on endosymbiotic bacteria for nutrition as adults (Chen et al., 2017; although Hirtopelta has also been reported to have endosymbionts in gill filaments; Beck, 2002), while most others are known to be deposit feeders and/or grazers (Fretter, 1989; Warén and Bouchet, 1989, 2001). Two other gastropods are reported from the Longqi field, including a Lepetodrilus species (Vetigastropoda: Lepetodrilidae) which is likely a grazer or filter-feeder and a species of Phymorhynchus (Neogastropoda: Raphitomidae) which is a carnivore/scavenger (Copley et al., 2016).
The species richness of small-sized marine invertebrates is still largely unexplored globally (e.g., Bouchet et al., 2002; Albano et al., 2011), and it is therefore not surprising that careful sorting of materials targeting small species may result in new discoveries. After expedition JC67, sorting of washings from C. squamiferum and G. aegis fixed and preserved in 99% ethanol on shore revealed two further distinct morphotypes of small (<7 mm) coiled peltospirid snails. The same two peltospirids were also recovered from materials collected by HOV Jiaolong in 2015 during the expedition DY35 of China. The two small peltospirids were associated with the two large peltospirids (C. squamiferum and G. aegis), living together and sometimes found on their body surface, but their small sizes indicate that they are unlikely to be holobionts. Many small peltospirids such as species in genera Rhynchopelta, Peltospira, Nodopelta, Lirapex, and Depressigyra are known to live on the surface of tubeworm bushes or Bathymodiolus deep-sea holobiont mussels where they feed (Warén et al., 2006), and the two small peltospirids from the Longqi field may be associated with Chrysomallon and Gigantopelta in a similar way. The purpose of the present study is to characterise these two snails and to ascertain their taxonomic and systematic affinity, as well as their ecological roles.
Small peltospirid snails examined herein were chiefly collected using a suction sampler from the Longqi vent field, Southwest Indian Ridge by ROV Kiel6000 (Dive 142) during RRS James Cook expedition JC67. All specimens were recovered from washings fixed and stored in 99% ethanol. Additional specimens were collected in the same field by HOV Jiaolong during dives DV94 and DV100 on-board R/V Xiangyanghong 9 expedition DY35, fixed and preserved in 95% ethanol. Specifically, the peltospirids were found on chimney structures named “Tiamat” (Figure 1B; HOV Jiaolong Marker DFF11) and “Jabberwocky” (HOV Jiaolong Marker DFF1). Detailed location maps of each chimney within the Longqi vent field can be found in Copley et al. (2016).
For anatomical investigations, shells were dissolved by submerging the specimens in 1 mol/L hydrochloric acid solution for several hours. The periostracum was then carefully peeled using fine tweezers. Since all specimens available for morphological investigation were fixed and preserved in 99% ethanol, the extracted soft parts were then subjected to rehydration in MilliQ water for 48 h before further dissection and observation using a stereo dissecting microscope (Carl Zeiss SteREO Discovery V.12). This also meant that no specimens were available for histological studies. Photographs of both intact specimens and soft parts were taken using a Nikon D5000 DSLR camera mounted to the microscope trinocular. Shell measurements were taken using a digital calliper (rounded up to one decimal point).
Prior to scanning electron microscopy (SEM) radula sacs were dissected out and tissues dissolved for a couple of minutes in diluted commercial bleach. As all specimens had apex covered by mineral deposits and no very young individuals were available, attempts to reveal the protoconch was carried out by softening the deposits using diluted commercial bleach and then carefully peeling with a fine needle. For investigation of shell microstructure small fragments of shells were broken off the aperture and also washed in bleach. Following bleach treatments specimens were washed in MilliQ water twice and 99% ethanol twice before being mounted on SEM stubs using carbon tapes. For examination of digestive tract contents, materials inside the stomach and the intestine were dissected out from one specimen of each species and directly mounted on SEM stubs using carbon tapes, and pre-examined with a dissection microscope (as above) before SEM examination. These were then air-dried and subjected to uncoated SEM imaging with a Hitachi TM-3000 SEM at 15 kV.
Barcoding sequences of the cytochrome c oxidase subunit I (COI) gene of the small peltospirids were sequenced using the primer pair LCO1490 and HCO2198 (Folmer et al., 1994) and compared with other neomphaline data available on GenBank (taxa, accession number, and voucher information of sequences used are listed in Table 1). A COI sequence of Lirapex costellatus Warén & Bouchet, 2001 (392bp; collection data: Tour Eiffel, Lucky Strike, Mid-Atlantic Ridge; 37°17′N, 32°16′W; 1693 m deep; Momareto 2005, sta. PL251) was kindly supplied by Dr. Yasunori Kano (the University of Tokyo) and Dr. Anders Warén (Swedish Museum for Natural History). Detailed procedures for DNA extraction, amplification, purification, and sequencing are as detailed in Chen et al. (2015a). Pairwise distance of COI sequences were calculated using MEGA7 (Kumar et al., 2016) with the Tamura-Nei (Tamura and Nei, 1993) model estimated by the composite likelihood method (Tamura et al., 2004). The alignment of COI gene used for phylogenetic reconstruction was 569 bp in length. The most suitable substitution model for each codon was selected using the programme PartitionFinder v.2.1.1 (Lanfear et al., 2017), using scores for the Bayesian information criterion. The models selected were GTR+I+G for all codons. MrBayes v.3.2.6 (Ronquist et al., 2012) was used for phylogenetic reconstruction using Bayesian inference. Metropolis-coupled Monte Carlo Markov Chains were run for one million generations, with topologies sampled every 100 generations. To ensure convergence Tracer v.1.6 (Rambaut et al., 2013) was used to visualise traces and calculate adequate burn-in values (2500 in this case). Newly generated sequences in this study are deposited in GenBank under the accession numbers MF977760- MF977763.
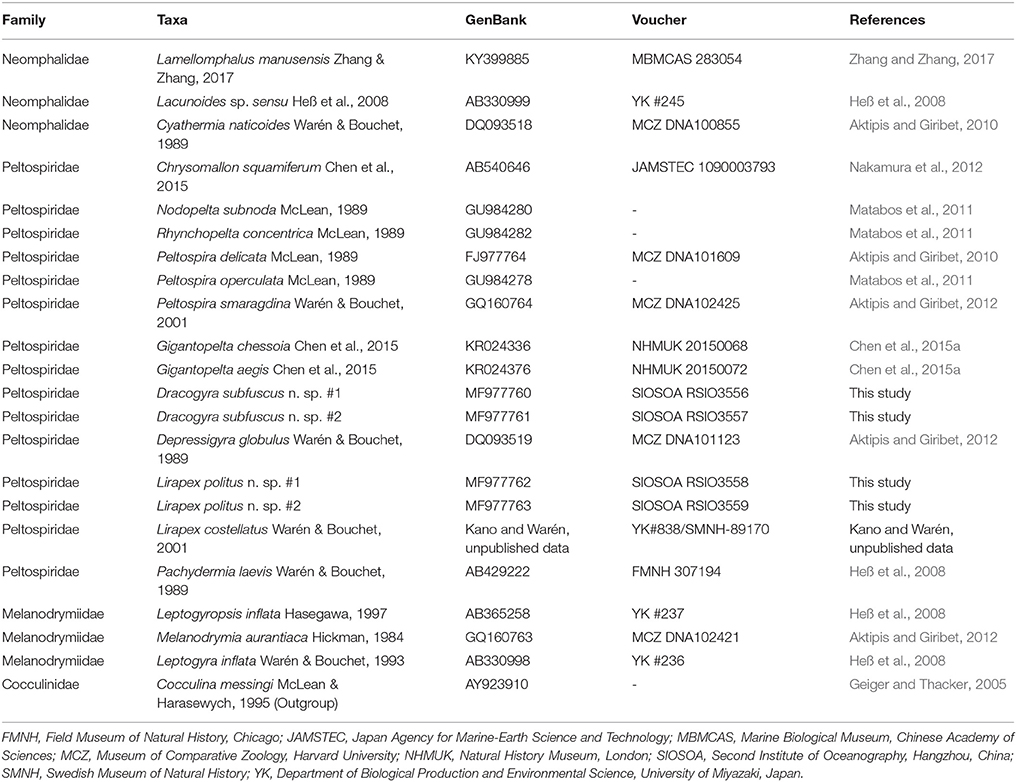
Table 1. List of taxa used for phylogenetic analysis using COI gene, with GenBank accession number, voucher number (where available), and original references shown.
Type specimens are deposited at the Natural History Museum, London (NHMUK), the Swedish Museum of Natural History (SMNH), the University Museum, the University of Tokyo (UMUT), and the Second Institute of Oceanography, State Oceanic Administration, Hangzhou, China (SIOSOA).
Type species: Lirapex humatus Warén & Bouchet, 1989 (by original designation).
Diagnosis: Coiled, skeneimorph peltospirids, small to medium sized (<5 mm) for the family. A film-like operculum always present. Shell usually with distinct axial sculpture that is strongest at shoulder and umbilicus. Protoconch initially with distinct spiral ridges which disappear toward the last half. Radula with hook-like marginals. Sexes separate. Snout even in breadth, tentacles smooth and not modified into copulatory organ (Warén and Bouchet, 1989, 2001).
ZooBank registration: urn:lsid:zoobank.org:act:C5BB998D-34B1-4B4F-B095-F3E5974D5F76
Diagnosis: A Lirapex with a totally smooth shell surface lacking in significant axial sculpture and a less disjunct final whorl in adults when compared with other known species.
Type locality: Longqi vent field, Southwest Indian Ridge, 37°47.03′ S, 49°38.97′E (“Tiamat” Chimney/DFF11), 2785 m deep, RRS James Cook expedition JC67, ROV Kiel6000 Dive 142, 2011/xi/29.
Type material: Holotype, Figures 2A–D, NHMUK 20170383. Paratype 1, one specimen, Figures 2E–F, SMNH Type-8948. Paratype 2, one specimen, Figures 2G–J, UMUT RM32664. Paratype 3, one specimen, Figures 2K–L, SIOSOA RSIO35614. Paratype 4, one specimen, NHMUK 20170384. Paratype 5, two specimens with shell dissolved (one intact, one dissected), Figures 4A–D, UMUT RM32665. Paratype 6, a lot of 20 specimens, NHMUK 20170385. Paratype 7, a lot of 20 specimens, SMNH Type-8949. Paratype 8, a lot of 20 specimens, UMUT RM32666. Paratype 9, a lot of 20 specimens, SIOSOA RSIO35615. All types from a single collecting event at the type locality as indicated above; fixed and stored in 99% ethanol.
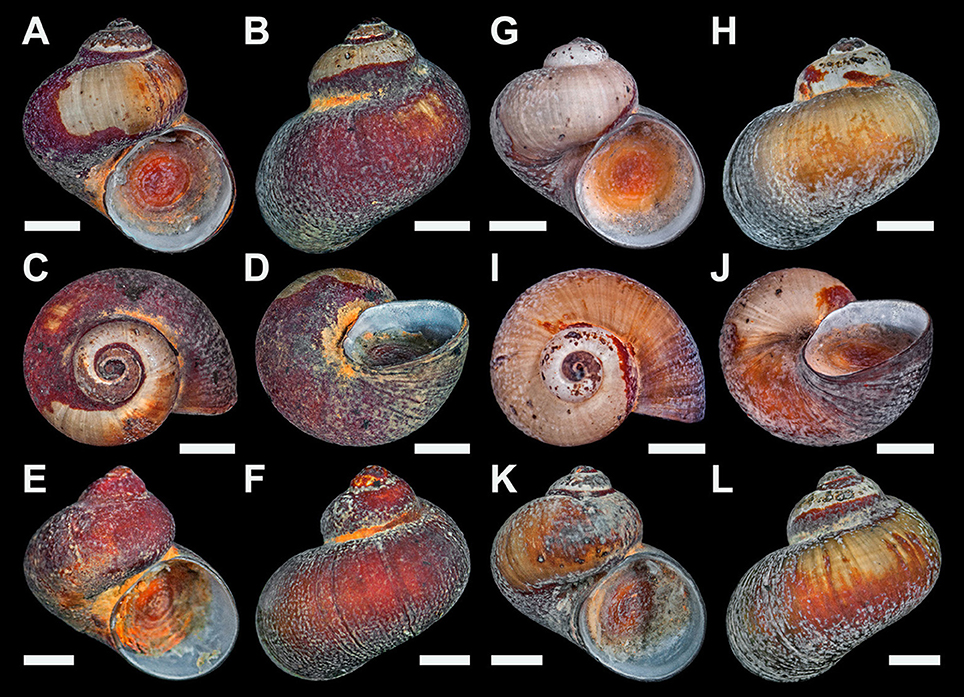
Figure 2. Lirapex politus n. sp. type specimens. (A–D) Holotype (NHMUK 20170383). (E–F) Paratype 1 (SMNH Type-8948). (G–J) Paratype 2 (UMUT RM32664). (K–L) Paratype 3 (SIOSOA RSIO35614). All scale bars = 1 mm.
Additional Materials Examined:
Approximately 50 specimens, same data as type locality.
Fourty specimens fixed and stored in 95% ethanol, Longqi vent field, Southwest Indian Ridge, 37° 47.03′ S/49° 39.01′ E (“Tiamat” Chimney/DFF11), 2761 m deep, R/V Xiangyanghong 9 expedition DY35, HOV Jiaolong Dive 94, 2015/i/11.
Fifteen specimens fixed and stored in 95% ethanol, Longqi vent field, Southwest Indian Ridge, 37° 47.00′ S/49° 39.01′ E (“Jabberwocky” Chimney/DFF1), 2736 m deep, R/V Xiangyanghong 9 expedition DY35, HOV Jiaolong Dive 100, 2015/ii/02.
Shell (Figure 2) solid, skeneimorph, usually at least partly encrusted by mineral deposits. Tightly coiled, loosening in the final 0.25 whorls but does not become obviously disjunct. Medium-sized for the group, as tall as wide, the largest specimen had a shell height of 4.4 mm and shell width of 4.5 mm. For more measurements see Table 2. Cross-section almost round. Covered by a layer of semi-transparent greyish green periostracum that is usually covered in turn by mineral deposit. Protoconch (Figure 3A) just less than half a whorl (diameter 220 μm), initially sculptured with five distinct spiral ridges and finer wrinkles, disappearing before reaching the centre part of the protoconch. Distal part of the protoconch remains smooth without any significant sculpture. Teleoconch consists of 3–3.5 whorls, white, entirely smooth and lacking in sculpture (except fine growth lines; Figure 3B), without nacreous layer. Aperture weakly opisthocline, nearly circular, not significantly thickened in adults. Umbilicus narrow. Shell microstructure (Figure 3C) comprises of two distinct layers, a thick cross-lamellar layer on the inside and a thin granular layer on the outside. Shell pores opening to the interior of the teleoconch present, gradually tapering toward the granular layer but does not reach it.
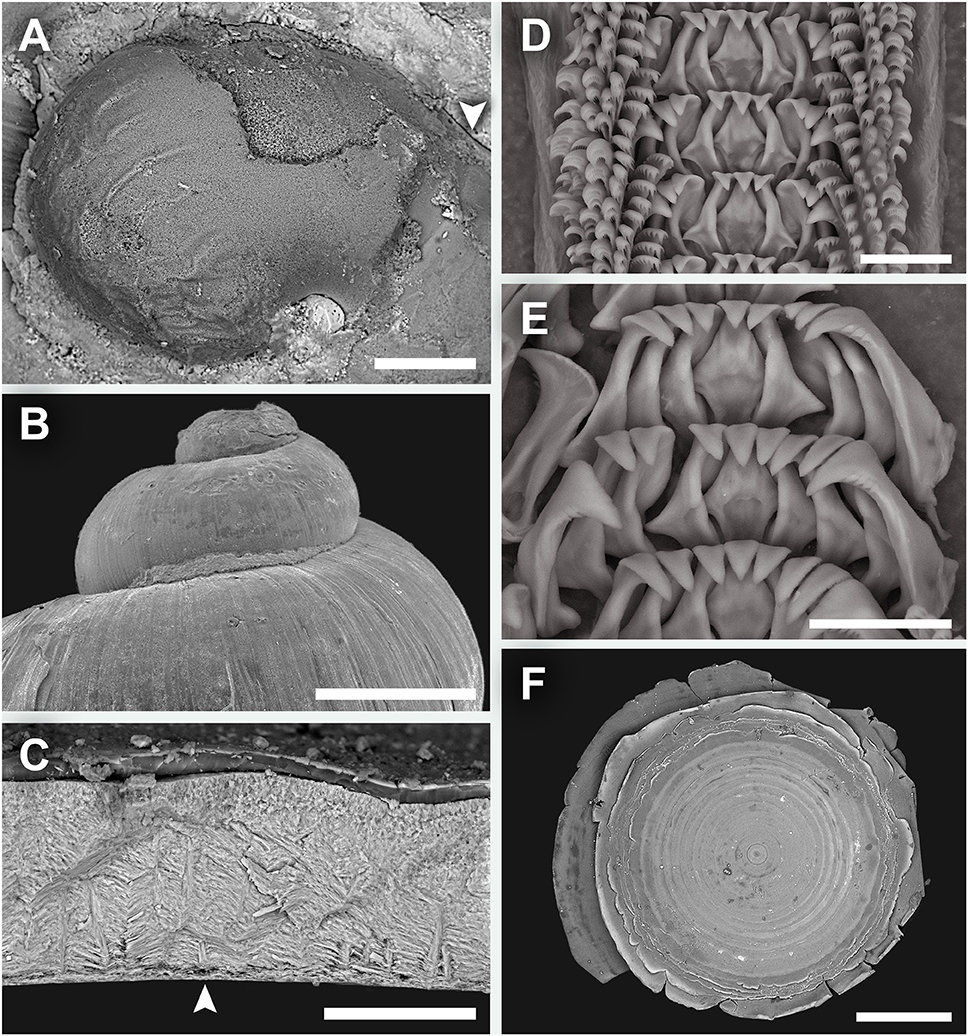
Figure 3. Lirapex politus n. sp. scanning electron micrographs. (A) Protoconch, (B) Shell surface of the upper half of the shell, as seen from the abapertural view, (C) Shell microstructure, (D) Radula, (E) Close-up of central and lateral teeth, (F) Operculum. Scale bars: (A) 50 μm, (B) 1 mm, (C) 50 μm, (D–E) 25 μm, (F) 0.5 mm.
Radula (Figures 3D,E) rhipidoglossate, formula ~25 + 4 + 1 + 4 + ~25. Central and laterals rigid, well-reinforced. Central teeth triangular, with a smooth, triangular overhanging cusp. The three inner laterals are similar in shape with bifurcating reinforcement but while the innermost lateral has smooth cusp the other two tend to have minor serrations. The outer lateral is much broader and have conspicuous serrations. The laterals all have a minor protuberance near the base. Marginal teeth long and thin, tapering distally. The innermost two have wider, triangular cusps that are serrated into about 10 denticles. The outer marginals have hook-like cusps that are serrated into very fine denticles. Size of outer marginals decreases outwards.
Operculum (Figure 3F) multispiral, with over 20 volutions in adults, film-like and transparent. In later volutions the edge of the previous volution extends freely to form an obvious fringe over the next.
Soft parts (Figure 4). Head large, eyes lacking, with no apparent cephalic copulation appendages. Snout short, flattened and even in breadth, with a ventral mouth. Cephalic tentacles smooth, elongated, about twice as long as the snout when contracted, conical and gradually decrease in size distally. Neck-lobe and cephalic lappets lacking. Foot rather well developed with a distinct transverse furrow separating propodium and mesopodium. Epipodial tentacles present between the operculum and foot, arranged in a semi-circular series surrounding posterior two-thirds of the opercular attachment and numbering 9–11 on each side (18–22 in total). Size of epipodial tentacles decrease slightly anterodorsally. Sexes separate, head-foot of both sexes identical in external anatomy. Pallial edge smooth and lacks appendages. Columellar muscle shallow, reaches about 0.3 whorls behind pallial edge, with a large attachment area on the right side of body connected to a smaller attachment area on the left side by a rather thick band of ventral muscular tissue.
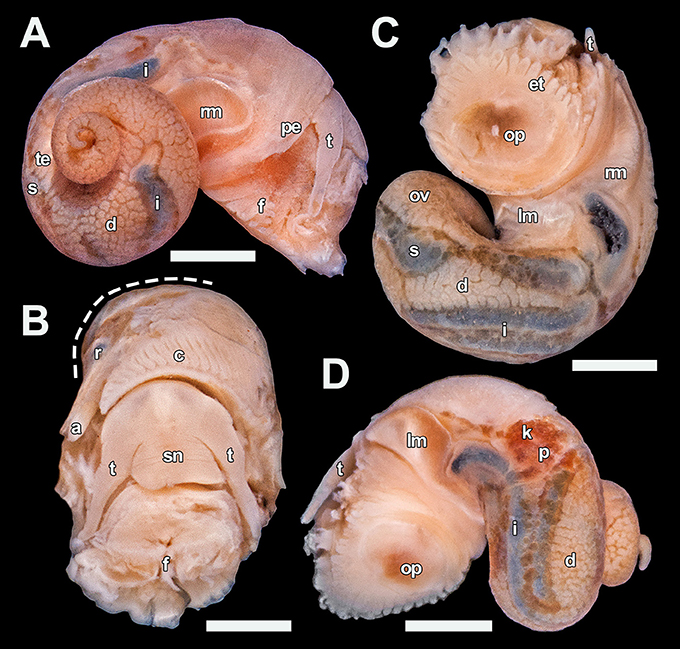
Figure 4. Lirapex politus n. sp. soft parts, paratype 5 (UMUT RM32665). (A) Right view, (B) Anterior view with part of the mantle roof removed to expose the ctenidium (visceral mass cut away at dashed line), (C) Ventral view, (D) Left view. Scale bars: (A), (C,D) 1 mm, (B) 0.5 mm. a, anus; c, ctenidium; d, digestive gland; et, epipodial tentacle; f, foot; i, intestine; k, right kidney; lm, left columellar muscle; ov, ovary; op, opercular attachment; p, pericardium; pe, pallial edge; r, rectum; rm, right columellar muscle; s, stomach; sn, snout; t, cephalic tentacle; te, testis.
Mantle cavity moderate in size, extending 0.4 whorls posterior of pallial edge. Ctenidium sizeable, bipectinate with approximately 40 pairs of leaflets. Digestive tract easily visible due to shiny, dark mineral deposits in the tract contents. Stomach quite large and significantly thickened compared to the intestine, situated one whorl posterior of the pallial edge. Rectum curves only slightly after entering the mantle cavity. It is attached to mantle ceiling and only becomes detached just posterior of the anus. Intestine long, looping twice between the left kidney and the stomach, partly concealed by the overlaying digestive gland. Pericardium not penetrated by the intestine. Heart monotocardian with a posteroventral ventricle and an anterodorsal auricle. Other than the large stomach and intestine the visceral mass is occupied dorsally by digestive gland up to the apex and ventrally by the gonad. Gonopore simple, opens just behind the anus.
Stomach and intestine filled by organic material, mixed with numerous conspicuous shiny mineral particles which are dark in colouration. Outline of mid- and hindgut highly conspicuous from exterior due to these dark mineral particles.
Distribution: Only known from Longqi hydrothermal vent field, Southwest Indian Ridge. Found on active chimneys among assemblages dominated by the two giant peltospirids C. squamiferum and Gigantopelta aegis. It is frequently found underneath the two species which often form dense aggregations in the same location (see Chen et al., 2015a), and also sometimes seen on body surface of the two giant peltospirids.
Etymology: “Politus” (Latin), meaning polished or smoothed. This refers to the smooth shell surface lacking in significant sculpture.
ZooBank registration: urn:lsid:zoobank.org:act:C5BB998D-34B1-4B4F-B095-F3E5974D5F76
Remarks: Three species are currently placed in Lirapex other than the present new species: L. humatus and L. granularis Warén & Bouchet, 1989 from East Pacific Rise (21°N) and L. costellatus Warén & Bouchet, 2001 from Mid-Atlantic Ridge (Lucky Strike and Eiffel Tower) (Warén and Bouchet, 1993, 2001). The protoconch and radula morphology as well as the shell shape of L. politus n. sp. does not deviate significantly from other members of Lirapex and unambiguously places this new species in that genus. Lirapex politus n. sp. is easily distinguished from the three congeners by a lack of any significant axial teleoconch or periostracal sculpture in all specimens examined (n > 100). Although other Lirapex species are usually quite variable in surface sculpture within a species, they always have axial sculpture present at least on the shoulder and around the umbilicus (Warén and Bouchet, 2001). In L. humatus and L. costellatus the sculpture is on the teleoconch itself. In the case of Lirapex granularis apparently the sculpture is only on the periostracum and not the shell itself (Warén and Bouchet, 2001), but that species is much smaller (shell height to 1 mm in adult) that no confusion with the present new species is possible. The smooth cusps of the central tooth, as well as the narrower umbilicus, helps separating the new species from L. humatus. Furthermore, the extent of whorl detachment in the final stage of growth in L. politus n. sp. is less than that of other Lirapex species.
ZooBank registration: urn:lsid:zoobank.org:act:F3A5936A-34EC-46EA-A074-CFC2CEFEDD26
Type species: Dracogyra subfuscus n. sp. (by original designation).
Diagnosis: Coiled, depressed globular peltospirids of medium size for the family (<7 mm shell diameter). Protoconch indistinctly coiled, approximately 0.5 whorls (sculpture unknown). Teleoconch smooth, without any distinct sculpture. Aperture opisthocline, without basal notch. Periostracum thick, leathery. A rather thick, opaque operculum always present. Gonochoristic. Snout equal in breadth, cephalic tentacles simple and exhibiting no sexual dimorphism. Central radular tooth very compressed, wide and rigid, nearly equilateral triangular in shape. The shafts of lateral teeth significantly longer than that of the central teeth, also rigid and well-supported.
Description: See that of D. subfuscus n. sp. below.
Etymology: “Draco” (Latin), dragon; “gyrus” (Latin), to circle or coil. Named for the Longqi vent field; “Longqi” means “dragon flag” in Chinese and “Dragon vent field” has been used as an alternative name (Roterman et al., 2013; Copley et al., 2016).
ZooBank registration: urn:lsid:zoobank.org:act:F3A5936A-34EC-46EA-A074-CFC2CEFEDD26
ZooBank registration: urn:lsid:zoobank.org:act:DE688815-288B-45EF-802A-0EC470D567AC
Diagnosis: See that of Dracogyra n. gen. above.
Type locality: Longqi vent field, Southwest Indian Ridge, 37°47.03′ S, 49°38.97′E (“Tiamat” Chimney), 2785 m deep, RRS James Cook expedition JC67, ROV Kiel6000 Dive 142, 2011/xi/29.
Type material: Holotype, Figures 5A–D, NHMUK 20170386. Paratype 1, one specimen, Figures 5E,F, SMNH Type-8950. Paratype 2, one specimen, Figures 5G,H, UMUT RM32667. Paratype 3, one specimen, Figures 5I,J, SIOSOA RSIO35616. Paratype 4, one specimen, NHMUK 20170387. Paratype 5, two specimens with shell dissolved (one intact, one dissected), Figures 7A–D, UMUT RM32668. Paratype 6, two empty shells, NHMUK 20170388. Paratype 7, one empty shell, SMNH Type-8951. Paratype 8, one empty shell, UMUT RM32669. Paratype 9, one empty shell, SIOSOA RSIO35617. All types from a single collecting event at the type locality as indicated above; fixed and stored in 99% ethanol.
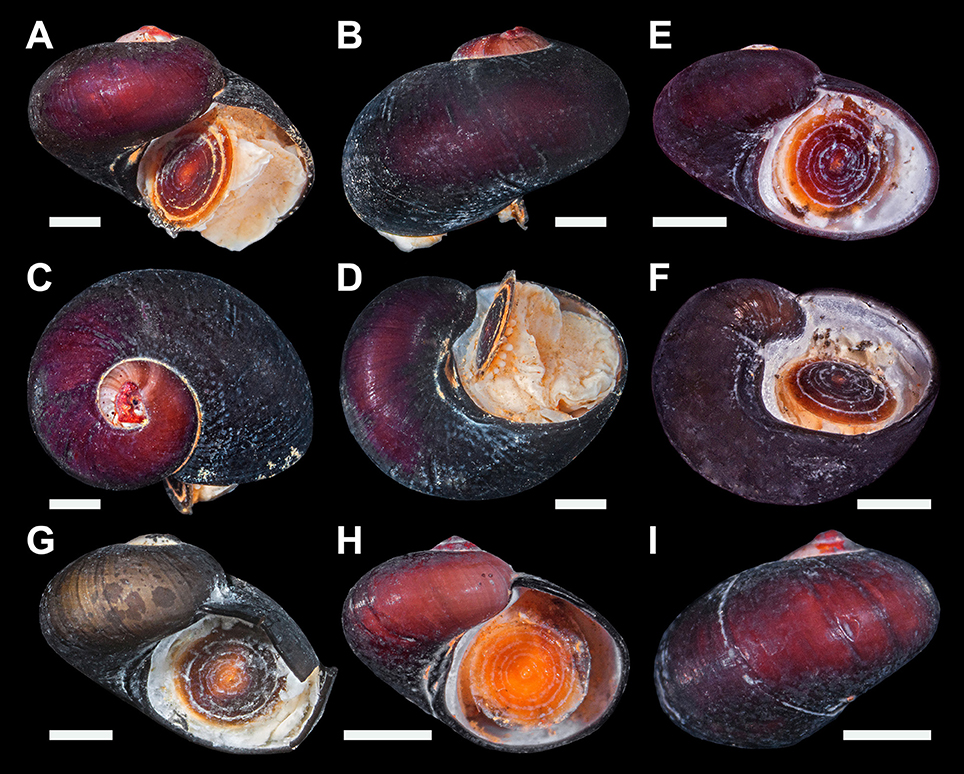
Figure 5. Dracogyra subfuscus n. gen. n. sp., type specimens. (A–D) Holotype (NHMUK 20170386). (E–F) Paratype 1 (SMNH Type-8950). (G) Paratype 2 (UMUT RM32667). (H–I) Paratype 3 (SIOSOA RSIO35616). All scale bars = 1 mm.
Additional materials examined:
Five dead shells with calcareous layer dissolved and only periostracum remaining, same data as in type locality above.
About 100 specimens fixed and stored in 95% ethanol, Longqi vent field, Southwest Indian Ridge, 37° 47.03′ S/49° 39.01′ E (“Tiamat” Chimney/DFF11), 2761 m deep, R/V Xiangyanghong 9 expedition DY35, HOV Jiaolong Dive 94, 2015/i/11.
Twelve specimens fixed and stored in 95% ethanol, Longqi vent field, Southwest Indian Ridge, 37° 47.00′ S/49° 39.01′ E (“Jabberwocky” Chimney/DFF1), 2736 m deep, R/V Xiangyanghong 9 expedition DY35, HOV Jiaolong Dive 100, 2015/ii/02.
Shell (Figure 5) tightly coiled, strongly depressed with a low spire, shape between skeneiform and neritiform. Medium-sized for the family, the largest specimen available (a dead shell) measured 4.6 mm in shell height and 6.3 mm in shell width. More measurements shown in Table 2. Protoconch (Figure 6A) indistinctly coiled, 200 μm in diameter. Specimen with protoconch sculpture preserved was not found in materials available. Teleoconch white and lacks nacreous layer, moderately thick, consist of about 2.7 whorls, depressed oval in cross section. Perfectly smooth except fine growth striations (Figure 6B). Aperture opisthocline to the coiling axis, oval in shape, lacking any significant notch. Outer lip simple, not thickened in adults. Inner lip with a small section of columellar callus extending toward the umbilical area. Umbilicus extremely narrow, sometimes obscured by callus. Microstructure (Figure 6C) of a thin, granular outer layer and a thick, cross-lamellar inner layer. The inner layer bears shell pores which open to the interior of the teleoconch, pores do not reach the outer layer. Periostracum thick, leathery. Initially light reddish brown, gradually darkening to near-black in last 0.5 whorls of adults. Lacks significant sculpture or pattern except fine growth lines. Outer edge extends over the outer lip, enveloping it. Never seen covered by thick mineral deposits in specimens available, although scattered deposits and evidence of corrosion is commonplace.
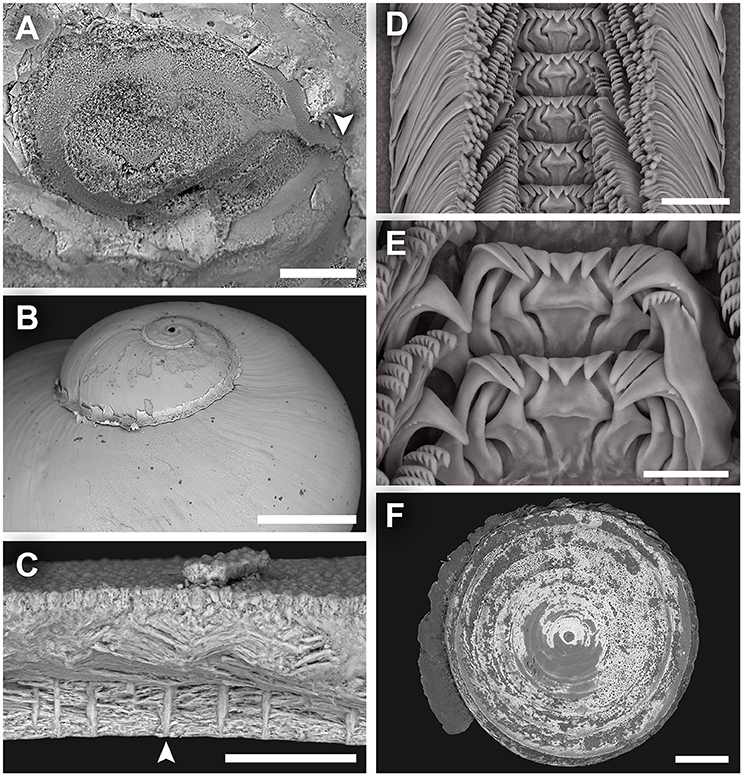
Figure 6. Dracogyra subfuscus n. gen., n. sp. scanning electron micrographs. (A) Protoconch, (B) Shell surface of the upper half of the shell, as seen from the abapertural view, (C) Shell microstructure, (D) Radula, (E) Close-up of central and lateral teeth, (F) Operculum. Scale bars: (A) 50 μm, (B, F) 0.5 mm, (C) 30 μm, (D–E) 25 μm.
Radula (Figure 6D,E) rhipidoglossate, formula ~35 + 4 + 1 + 4 + ~35. Central tooth solid with a smooth triangular cusp and very robust antero-lateral supports. The shaft is unusually horizontally compressed in impression as it is rather short and rapidly broadening basally to become almost equilateral triangle in shape. Lateral teeth have sturdy shafts that are interlocking, and becomes taller laterally. The innermost lateral has a significantly stronger, more oblique lateral supporting ridge compared to the second and third laterals which are essentially the same. All three inner laterals have elongate, triangular cusps, cutting edge smooth without serration. The outermost lateral is much broader than the rest and carry a few coarse serrations on the outer edge of its cusp. Marginal teeth truncated at the distal end with a rather flattened cusp which is serrated into 8–15 denticles. Outer marginals smaller than inner marginals, with somewhat finer serration.
Operculum (Figure 6F) multispiral, about 8 volutions, slightly convex. Brown, thick and opaque near centre (in adults) and becoming semi-transparent toward the edge, often covered by rusty mineral deposit. The outermost volutions often has a thin fringe projecting freely outwards and over the next.
Soft parts (Figure 7) occupy 2.5 whorls from the apex to the pallial margin. Head large with a short snout of equal breadth and a subventral mouth, eyes lacking. Cephalic tentacles very broad and stout, slightly tapering and approximately 1.5 times as long as the snout when contracted. The animal is gonochoristic but lacks sexual dimorphism on the head-foot, cephalic tentacles are not modified into copulation appendages. Propodium and mesopodium separated by a furrow. Epipodial tentacles numbering about 20 (10 on each side), arranged like a fringe around the posterior 2/3 of the opercular attachment. The sizes of the tentacles gradually decrease anterodorsally. Pallial margin simple without tentacles or other appendages. Columellar muscle horse-shoe shaped, with two attachment areas on each side of the body connected by a thin band of ventral musculature, the right attachment area is much larger than the left.
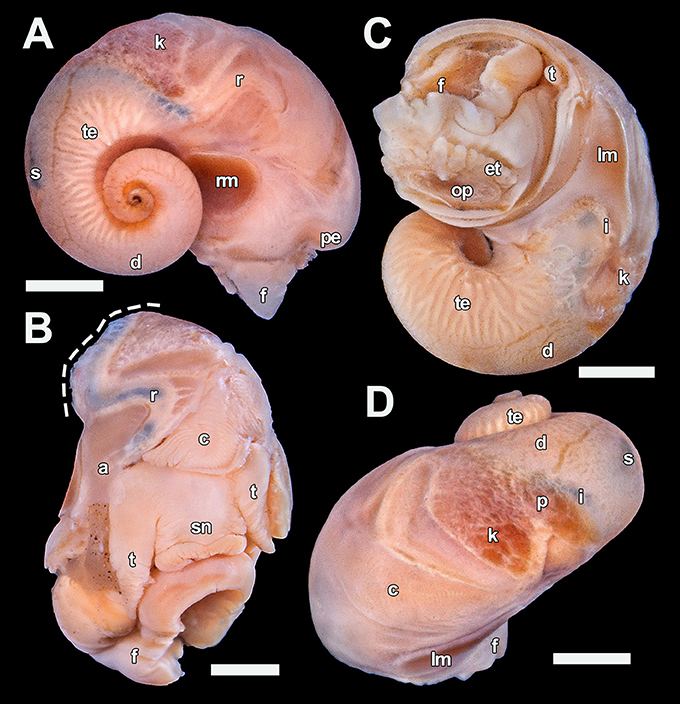
Figure 7. Dracogyra subfuscus n. gen., n. sp. soft parts, paratype 5 (UMUT RM32668). (A) Right view, (B) Anterior view with part of the mantle roof removed to expose the ctenidium (visceral mass cut away at dashed line), (C) Ventral view, (D) Dorsal view. Scale bars: (A), (C–D) = 1 mm, (B) 0.5 mm. a, anus; c, ctenidium; d, digestive gland; et, epipodial tentacle; f, foot; i, intestine; k, right kidney; lm, left columellar muscle; op, opercular attachment; p, pericardium; pe, pallial edge; r, rectum; rm, right columellar muscle; s, stomach; sn, snout; t, cephalic tentacle; te, testis.
Mantle cavity extends to approximately 0.3 whorls behind the pallial margin, largely occupied by a rather large bipectinate ctenidium with about 50 rows of leaflets. Only a small section of the intestine is visible between the left kidney and the stomach, as much of it is hidden under the mass of digestive gland. Stomach positioned half a whorl posterior of the pallial margin, clearly visible from outside as a dark patch. Pericardium not penetrated by the intestine, heart monotocardian with the ventricle positioned posteroventral to the single auricle. The visceral mass, other than the stomach and the intestine loop, is occupied by an extensive digestive gland on the dorsal half (filling the apex) and a large mass of gonad on the ventral half. The rectum turns half a loop clockwise after entering mantle cavity (right side), it remains attached to the mantle wall except a very short stretch just before the anus.
Stomach and intestine contents consist of mostly fine organic material, mixed with a small amount of very fine mineral particles.
Distribution: Only known from Longqi hydrothermal vent field, Southwest Indian Ridge. Found on diffuse flow chimneys in association with C. squamiferum and Gigantopelta aegis.
Etymology: From Latin “subfuscus” (“sub-” + “fuscus,” black), meaning brownish and darkish. Refers to the colouration of the periostracum which is initially brownish but gradually darkens to black.
ZooBank registration: urn:lsid:zoobank.org:act:DE688815-288B-45EF-802A-0EC470D567AC
Remarks: Dracogyra subfuscus n. sp. is most similar conchologically to Depressigyra globulus Warén & Bouchet, 1989, the only species currently assigned to Depressigyra (Warén and Bouchet, 2001). The shells of the two are of a similar size and shape, although the shell of D. subfuscus n. sp. tends to be more depressed with a narrower umbilicus and the aperture lacks a basal notch present in Depressigyra. Radular characters, however, unambiguously separates the two genera. The central and lateral teeth of D. globulus is unusually narrow, which is starkly different from that of D. subfuscus n. sp. which has very rigid central and laterals. The central tooth of D. subfuscus n. sp. especially, is very wide and compressed compared to the laterals. The distinctive shape of the central teeth is also useful in distinguishing the new species from juvenile G. aegis (also with central and laterals being similar in length) which may be confused due to the co-occurrence of the two species. Dracogyra subfuscus n. sp. is also discernable from G. aegis of a similar size conchologically by its much lower spire and more depressed shell form. As the protoconch sculpture could not be examined for Dracogyra n. gen. based on the existing material, differences in protoconch characters cannot be discussed at this point.
The Bayesian consensus tree generated based on the COI barcoding fragment is shown in Figure 8. Neomphalina was recovered as a fully supported clade (Posterior Probability, PP = 1.00). The placement of both new species within Neomphalina is confirmed, with L. politus n. sp. falling sister to L. costellatus and the genus Lirapex in turn falling sister to Pachydermia (although the support was low with PP < 0.70 in both relationships) and Dracogyra n. gen. sister to Gigantopelta with full support (PP = 1.00). These relationships support the placement of the two new species in family Peltospiridae, and does not contradict with placing L. politus n. sp. in genus Lirapex. Since it has been well discussed that the barcoding fragment of COI alone is not sufficient to resolve familial relationships among Neomphalina due to saturation (Heß et al., 2008; Chen et al., 2017) and many branches in the present tree is not well supported, it is difficult to discuss familial assignment of other genera from this tree. This is likely the reason why Peltospiridae appears to be paraphyletic in the present tree, which is not the case in a recent five gene phylogenetic reconstruction (see Chen et al., 2017).
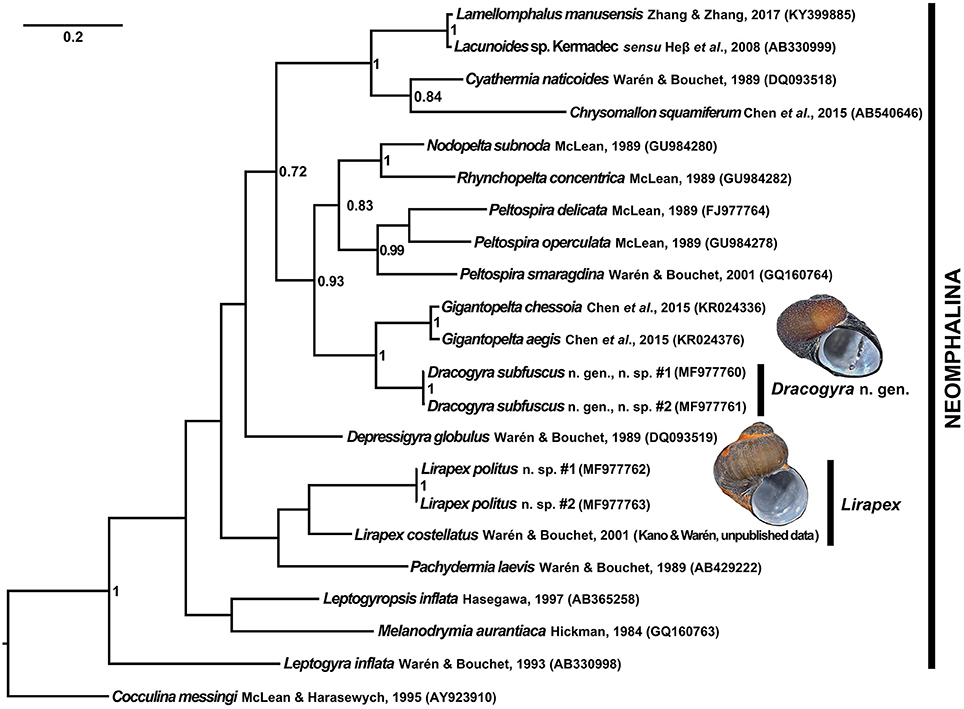
Figure 8. Bayesian inference consensus tree generated from a 569 bp alignment of the COI barcoding fragment. GenBank accession numbers are shown in brackets. Node values are Bayesian posterior probabilities, only those above 0.70 are shown. Individuals of the two new species sequenced were from the type locality (“Tiamat” Chimney/DFF11, Longqi field), images shown are shells of the sequenced individual #1 for each species.
Existing museum materials of the other two described species of Lirapex (i.e., L. humatus and L. granularis) have been fixed in formalin (Philippe Bouchet, pers. comm.) and they are only known from East Pacific Rise vents where the authors have no plans to visit in the near future, they could not be included in the present phylogeny to compare with L. politus n. sp. The genus Lirapex is defined by a combination of synapomorphic characters shared by all three described species (after Warén and Bouchet, 1989, 2001), detailed as follows: (1) Coiled, often sculptured, skeneimorph teleoconch with the adults' coiling loosening in the last 0.25 to 0.5 whorls; (2) Radula with formula n + 4 + 1 + 4 + n and outer lateral broader than the inner ones, importantly the outer marginals possess hook-like, denticulated cusps; and (3) Anterior part of the intestine clearly visible as a double-coil between stomach and the left nephridium. As described above L. politus n. sp. possesses: (1) Skeneimorph shell with the coiling loosening toward the final 0.25 whorls (similar to L. granularis, does not become obviously disjunct from the previous whorl like in L. humatus or L. costellatus; Figure 2); (2) Formula of the radula is ~25 + 4 + 1 + 4 + ~25 with the outer lateral much broader than the inner three, and outer marginals possess denticulated hook-like cusps (Figures 3D,E); and (3) Two coils of the intestine are clearly visible (Figure 4D). Two further important characteristics, although not unique to Lirapex within Peltospiridae, are shared by L. humatus, L. granularis, and L. costellatus and are important for classification in this genus when combined with the abovementioned synapomorphic characters, namely a protoconch with distinct spiral ridges on first half that disappears toward the protoconch-teleoconch boundary and epipodial tentacles being only present surrounding the opercular attachment. L. politus n. sp. possesses a protoconch with five distinct spiral ridges that disappear posteriorly (Figure 3A) and the only epipodial tentacles present are the 18-22 tentacles surrounding opercular attachment (Figure 4C). The placement of L. politus n. sp. in genus Lirapex is therefore also robustly supported by all available morphological characteristics, in addition to its sister relationship with L. costellatus in the present phylogeny.
A maximum-likelihood distance matrix of all nine peltospirid genera with available COI sequences was generated using a 579 bp alignment and shown in Table 3. The average genetic distance (in pairwise difference) between Dracogyra n. gen. and other eight genera averaged 21.3% (range 14.4~26.6%), which is similar to distances among those peltospirid genera other than Dracogyra n. gen. which averaged at 22.4% (range 12.8~28.6%). This is in support of the generic status of Dracogyra n. gen. within Peltospiridae. The genetic distance between the two individuals used in phylogenetic reconstruction over the same COI alignment was 0.2% for L. politus n. sp. and 0.4% for Dracogyra subfusca n. sp. It should be noted that the genetic distances in COI among the three Peltospira species included ranged between 13.0 and 16.5%, which fit within the range among peltospirid genera specified above. The relationship among the three Peltospira spp. may require reexamination in future studies.
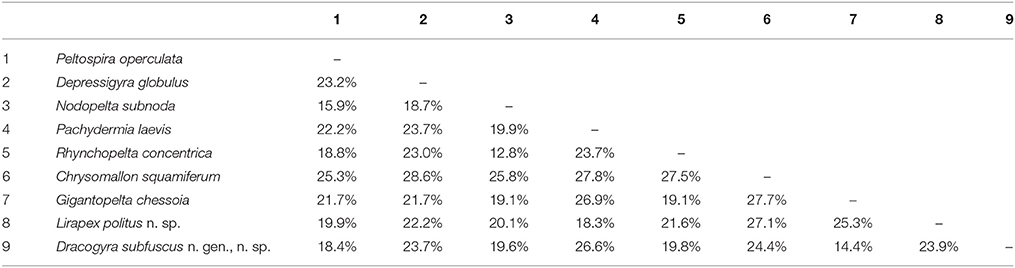
Table 3. Maximum-likelihood distance matrix of nine genera in Peltospiridae, including Lirapex politus n. sp. representing Lirapex and Dracogyra subfuscus n. sp. representing Dracogyra n. gen.
Lirapex politus n. sp. is the first species of its genus to be discovered from Indian Ocean, meaning the distribution of Lirapex now spans Pacific, Indian, and Atlantic oceans; a first for the clade Neomphalina as a whole. Although Peltospira also spans Pacific (three species in East Pacific Rise) and Atlantic (one species in Mid-Atlantic Ridge) oceans, it is not known from Indian Ocean (Warén and Bouchet, 2001). Unfortunately, due to lack of available material, the phylogenetic and biogeographic relationship within genus Lirapex (i.e., among L. politus n. sp. and its three congeners) could not be examined and remains a topic for future research.
The fact that Dracogyra n. gen. appears to be the most closely related peltospirid genus to Gigantopelta (although only with COI data at this point) serves to strengthen the lines of reasoning that Gigantopelta and Chrysomallon independently and convergently evolved the peculiar lifestyle of housing endosymbionts in a “trophosome”-like oesophageal gland (Chen et al., 2017).
During genetic analyses it came to light that Lamellomphalus manusensis Zhang & Zhang, 2017, a neomphalid species recently described from Manus Back-Arc Basin, was genetically very close to Lacunoides sp. sensu Heß et al., 2008 from a vent 1336 m deep in Brothers Caldera, Kermadec Arc, New Zealand (Heß et al., 2008). The pairwise distance between the two was merely 1.4%. The authors of L. manusensis only included described species in their phylogenetic tree and thus Lacunoides sp. sensu Heß et al., 2008 was excluded (Zhang and Zhang, 2017). Since the interspecific pairwise differences in COI of marine gastropods is usually above 3–4% (Meyer and Paulay, 2005) this suggests that Lacunoides sp. Kermadec is likely in fact L. manusensis and the distribution of L. manusensis (or at least the genus Lamellomphalus) probably extend to Kermadec Arc. This issue warrents more careful investigation using more genetic markers in the future.
Stomach contents suggest both L. politus n. sp. and Dracogyra subfuscus n. sp. are grazers or deposit feeders like most peltospirids, and some radula wear was seen in both species which is also in support of this. The digestive tract of L. politus n. sp. is filled by organic material mixed with a large proportion of conspicuous shiny particles of mineral deposit, making the outline of intestines and stomach easily visible from outside. This is similar to the case in other Lirapex species (Warén and Bouchet, 1989, 2001). In Dracogyra subfuscus n. sp. the digestive tract contents contain a higher proportion of organic material mixed with only very fine mineral particles, perhaps indicative of difference in food preference compared to L. politus n. sp. The lack of hypertrophied oesophageal gland as in Chrysomallon and Gigantopelta (Chen et al., 2017) or very enlarged ctenidium as in Hirtopelta adult individuals (Fretter, 1989; Beck, 2002) further imply that neither new species rely on endosymbiont bacteria for nutrition. A number of small peltospirid genera such as Peltospira, Rhynchopelta, Nodopelta are associated with siboglinid tubeworms such as Riftia and Alvinella, these peltospirids live on the surface of tubeworms and feed there (Warén and Bouchet, 1993, 2001; Warén et al., 2006). Other species of Lirapex are also known to be associated in a similar way with Bathymodiolus mussels (Warén et al., 2006). The fact that the two new small peltospirid species live underneath Chrysomallon and Gigantopelta or even on their body surface implies a similar association. The two giant peltospirids might aid in providing nutrition to them in some way, for example the two small new species may feed on epibionts of Chrysomallon or Gigantopelta (for epibionts of Chrysomallon see Goffredi et al., 2004).
The two new species described herein live amongst dense aggregations of C. squamiferum and G. aegis meaning the four genera of peltospirids live side-by-side. The situation is similar to Western Pacific vents of North Fiji, Manus, and Lau where two holobiont provannid genera Alviniconcha and Ifremeria co-occur with two deposit feeding provannid genera Provanna and Desbruyeresia (Warén and Bouchet, 1993; Johnson et al., 2010). This is interesting as Provannidae is the only gastropod family other than Peltospiridae known to house chemosynthetic symbionts intracellularly (Sasaki et al., 2010), and indicate that both families likely succeeded in chemosynthetic ecosystems by diversifying into both symbiotic and non-symbiotic feeding niches. Although neomphalines are highly diverse and more than 10 genera across all three families Neomphalidae, Peltospiridae, and Melanodrymiidae co-occur in East Pacific Rise and nearby areas (McLean, 1989; Warén and Bouchet, 2001; Heß et al., 2008), the present work presents the first record of giant holobiont peltospirids (Chrysomallon and Gigantopelta) co-occurring with other, non-chemosymbiotic peltospirids. Prior to the present study, the two holobiont genera were the only neomphalines reported from East Scotia Ridge and Indian Ocean vents where they occur (Rogers et al., 2012; Chen et al., 2015a,c; Copley et al., 2016). The discovery of L. politus n. sp. and Dracogyra subfuscus n. sp. increases the number of macro- and megafauna species known from the Longqi field and Southwest Indian Ridge as a whole to 23 species, of which seven are molluscs (Copley et al., 2016).
Study species were gastropod molluscs collected in International Waters and permission for sampling was not necessary. Animals were preserved in ethanol.
CC and YZ conceived the study and participated in the design of the study. CC collected and analysed the morphology data and performed all microscopy; YZ and CC collected and analysed the genetics data. All authors took part in the collection of samples and field data used in this study. CC drafted the manuscript which was critically revised and improved by YZ, CW, and JC. All authors gave final approval for submission and publication.
RRS James Cook expedition JC67 was funded by a UK Natural Environment Research Council (NERC) Small Research Grant NE/H012087/1 to JC. This study was further supported by the National Key Research and Development Program of China (no. 2017YFC0306603) and the foundation of China Ocean Mineral Resources R & D Association (No. DYHC-125-35). At the time of writing CC was supported by an International Postdoctoral Fellowship from JAMSTEC. The funders had no role in research design, data analysis, publication decisions, or manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the Master and crew of RRS James Cook and R/V Xiangyanghong 9, as well as the pilots and the technical team of ROV Kiel6000 and HOV Jiaolong, during expeditions JC67 and DY35, respectively, for their great support of scientific activity. All scientists on-board the expedition are gratefully acknowledged for their tireless work. Gratitude goes to staff of the UK National Marine Facilities at the National Oceanography Centre for providing logistics and shipboard support. The three reviewers are thanked for providing valuable comments that greatly improved the paper. Dr. Yasunori Kano (the University of Tokyo) and Dr. Anders Warén (SMNH) are thanked for their kindness in sharing the unpublished COI sequence of Lirapex costellatus for use in the present study, and Dr. Philippe Bouchet (Muséum National d'Histoire Naturelle, Paris) is thanked for helpful discussions regarding museum specimens of Lirapex. In addition, the authors are grateful to Ms. Yukiko Nagai (JAMSTEC) and Dr. Takashi Toyofuku (JAMSTEC) for their assistance in microscopy.
Aktipis, S. W., and Giribet, G. (2010). A phylogeny of Vetigastropoda and other “archaeogastropods”: re-organizing old gastropod clades. Inverteb. Biol. 129, 220–240. doi: 10.1111/j.1744-7410.2010.00198.x
Aktipis, S. W., and Giribet, G. (2012). Testing relationships among the vetigastropod taxa: a molecular approach. J. Molluscan Stud. 78, 12–27. doi: 10.1093/mollus/eyr023
Albano, P. G., Sabelli, B., and Bouchet, P. (2011). The challenge of small and rare species in marine biodiversity surveys: microgastropod diversity in a complex tropical coastal environment. Biodivers. Conserv. 20, 3223–3237. doi: 10.1007/s10531-011-0117-x
Beck, L. A. (2002). Hirtopelta tufari sp. n., a new gastropod species from hot vents at the East Pacific Rise (21°S) harbouring endocytosymbiotic bacteria in its gill (Gastropoda: Rhipidoglossa: Peltospiridae). Archiv für Molluskenkunde 130, 249–257.
Bouchet, P., Lozouet, P., Maestrati, P., and Heros, V. (2002). Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 75, 421–436. doi: 10.1046/j.1095-8312.2002.00052.x
Chen, C., Copley, J. T., Linse, K., Rogers, A. D., and Sigwart, J. (2015a). The heart of a dragon: 3D anatomical reconstruction of the “scaly-foot gastropod” (Mollusca: Gastropoda: Neomphalina) reveals its extraordinary circulatory system. Front. Zool. 12, 13. doi: 10.1186/s12983-015-0105-1
Chen, C., Linse, K., Copley, J. T., and Rogers, A. D. (2015b). The ‘scaly-foot gastropod’: a new genus and species of hydrothermal vent-endemic gastropod (Neomphalina: Peltospiridae) from the Indian Ocean. J. Molluscan Stud. 81, 322–334. doi: 10.1093/mollus/eyv013
Chen, C., Linse, K., Roterman, C. N., Copley, J. T., and Rogers, A. D. (2015c). A new genus of large hydrothermal vent-endemic gastropod (Neomphalina: Peltospiridae). Zool. J. Linn. Soc. 175, 319–335. doi: 10.1111/zoj.12279
Chen, C., Uematsu, K., Linse, K., and Sigwart, J. D. (2017). By more ways than one: rapid convergence at hydrothermal vents shown by 3D anatomical reconstruction of Gigantopelta (Mollusca: Neomphalina). BMC Evol. Biol. 17, 62. doi: 10.1186/s12862-017-0917-z
Copley, J. T., Marsh, L., Glover, A. G., Hühnerbach, V., Nye, V. E., Reid, W. D. K., et al. (2016). Ecology and biogeography of megafauna and macrofauna at the first known deep-sea hydrothermal vents on the ultraslow-spreading Southwest Indian Ridge. Sci. Rep. 6:39158. doi: 10.1038/srep39158
Dick, H. J. B., Lin, J., and Schouten, H. (2003). An ultraslow-spreading class of ocean ridge. Nature 426, 405–412. doi: 10.1038/nature02128
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Fretter, V. (1989). The anatomy of some new archaeogastropod limpets (Superfamily Peltospiracea) from hydrothermal vents. J. Zool. 218, 123–169. doi: 10.1111/j.1469-7998.1989.tb02530.x
Geiger, D. L., and Thacker, C. E. (2005). Molecular phylogeny of Vetigastropoda reveals non-monophyletic Scissurellidae, Trochoidea, and Fissurelloidea. Molluscan Res. 25, 47–55.
Goffredi, S. K., Warén, A., Orphan, V. J., Van Dover, C. L., and Vrijenhoek, R. C. (2004). Novel forms of structural integration between microbes and a hydrothermal vent gastropod from the Indian Ocean. Appl. Environ. Microbiol. 70, 3082–3090. doi: 10.1128/AEM.70.5.3082-3090.2004
Heß, M., Beck, F., Gensler, H., Kano, Y., Kiel, S., and Haszprunar, G. (2008). Microanatomy, shell structure and molecular phylogeny of Leptogyra, Xyleptogyra and Leptogyropsis (Gastropoda: Neomphalida: Melanodrymiidae) from sunken wood. J. Molluscan Stud. 74, 383–401. doi: 10.1093/mollus/eyn030
Johnson, S., Warén, A., Lee, R., Kano, Y., Kaim, A., Davis, A., et al. (2010). Rubyspira, new genus and two new species of bone-eating deep-sea snails with ancient habits. Biol. Bull. 219, 166–177. doi: 10.1086/BBLv219n2p166
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., and Calcott, B. (2017). Partitionfinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34, 772–773. doi: 10.1093/molbev/msw260
Matabos, M., Plouviez, S., Hourdez, S., Desbruyères, D., Legendre, P., Warén, A., et al. (2011). Faunal changes and geographic crypticism indicate the occurrence of a biogeographic transition zone along the southern East Pacific Rise. J. Biogeogr. 38, 575–594. doi: 10.1111/j.1365-2699.2010.02418.x
McLean, J. H. (1989). New archaeogastropod limpets from hydrothermal vents: new family Peltospiridae, new superfamily Peltospiracea. Zool. Scripta 18, 49–66. doi: 10.1111/j.1463-6409.1989.tb00123.x
Meyer, C. P., and Paulay, G. (2005). DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 3:e422. doi: 10.1371/journal.pbio.0030422
Nakamura, K., Watanabe, H., Miyazaki, J., Takai, K., Kawagucci, S., Noguchi, T., et al. (2012). Discovery of new hydrothermal activity and chemosynthetic fauna on the central Indian ridge at 18°-20°S. PLoS ONE 7:e32965. doi: 10.1371/journal.pone.0032965
Rambaut, A., Suchard, M., and Drummond, A. (2013). Tracer v1.6. Available online at: from http://tree.bio.ed.ac.uk/software/tracer/
Rogers, A. D., Tyler, P. A., Connelly, D. P., Copley, J. T., James, R., Larter, R. D., et al. (2012). The discovery of new deep-sea hydrothermal vent communities in the Southern Ocean and implications for biogeography. PLoS Biol. 10:e1001234. doi: 10.1371/journal.pbio.1001234
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Roterman, C. N., Copley, J. T., Linse, K. T., Tyler, P. A., and Rogers, A. D. (2013). The biogeography of the yeti crabs (Kiwaidae) with notes on the phylogeny of the Chirostyloidea (Decapoda: Anomura). Proc. Biol. Sci. 280:20130718. doi: 10.1098/rspb.2013.0718
Sasaki, T., Warén, A., Kano, Y., Okutani, T., Fujikura, K., and Kiel, S. (2010). “Gastropods from recent hot vents and cold seeps: systematics, diversity and life strategies,” in The Vent and Seep Biota, ed S. Kiel (Netherlands: Springer), 169–254.
Tamura, K., and Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526.
Tamura, K., Nei, M., and Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U.S.A. 101, 11030–11035. doi: 10.1073/pnas.0404206101
Tao, C., Li, H., Jin, X., Zhou, J., Wu, T., He, Y., et al. (2014). Seafloor hydrothermal activity and polymetallic sulfide exploration on the southwest Indian ridge. Chin. Sci. Bull. 59, 2266–2276. doi: 10.1007/s11434-014-0182-0
Tao, C., Lin, J., Guo, S., Chen, Y. J., Wu, G., Han, X., et al. (2012). First active hydrothermal vents on an ultraslow-spreading center: Southwest Indian Ridge. Geology 40, 47–50. doi: 10.1130/G32389.1
Warén, A., and Bouchet, P. (1989). New gastropods from East Pacific hydrothermal vents. Zool. Scr. 18, 67–102. doi: 10.1111/j.1463-6409.1989.tb00124.x
Warén, A., and Bouchet, P. (1993). New records, species, genera, and a new family of gastropods from hydrothermal vents and hydrocarbon seeps. Zool. Scr. 22, 1–90. doi: 10.1111/j.1463-6409.1993.tb00342.x
Warén, A., and Bouchet, P. (2001). Gastropoda and Monoplacophora from hydrothermal vents and seeps; new taxa and records. Veliger 44, 116–231.
Warén, A., Bouchet, P., and von Cosel, R. (2006). “Gastropoda: Neomphalina,” in Handbook of Deep-Sea Hydrothermal Vent Fauna (Second completely revised edition.) Denisia #18, eds D. Desbruyères, M. Segonzac, and M. Bright (Linz: Oberösterreichisches Landesmuseum) 104–120.
Zhang, S., and Zhang, S. (2017). A new genus and species of Neomphalidae from a hydrothermal vent of the Manus Back-Arc Basin, western Pacific (Gastropoda: Neomphalina). Nautilus 131, 76–86.
Keywords: deep-sea, hydrothermal vent, Indian Ocean, Mollusca, new species, Peltospiridae
Citation: Chen C, Zhou Y, Wang C and Copley JT (2017) Two New Hot-Vent Peltospirid Snails (Gastropoda: Neomphalina) from Longqi Hydrothermal Field, Southwest Indian Ridge. Front. Mar. Sci. 4:392. doi: 10.3389/fmars.2017.00392
Received: 19 September 2017; Accepted: 20 November 2017;
Published: 05 December 2017.
Edited by:
Wei-Jen Chen, National Taiwan University, TaiwanReviewed by:
David R. Lindberg, University of California, Berkeley, United StatesCopyright © 2017 Chen, Zhou, Wang and Copley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong Chen, Y2NoZW5AamFtc3RlYy5nby5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.