- Oceanography Section, Istituto Nazionale di Oceanografia e di Geofisica Sperimentale (OGS), Trieste, Italy
This study is a rare example of “the ecosystem approach to management” that has been carried out for the purpose of providing practical support to decision-makers in managing a Site of National Interest (SIN) where activities such as fishing, aquaculture and swimming are restricted. Benthic ecosystem functioning was assessed to verify whether it would be possible to exclude the less contaminated part from the SIN and its legislative constraints. At five macrosites subjected to diversified industrialization and anthropization, we evaluated the structural characteristics of the sediments, both heterotrophic and phototrophic communities, and the main processes of production, transformation and consumption of organic matter at seven stations, plus a reference site. Along the north-eastern boundary of the bay, the port, shipbuilding and iron foundry areas, characterized by high levels of contaminants, low macrozoobenthic diversity, major organic contents (up to 51.1 mgC g−1) and higher numbers of hydrocarbon degrading bacteria (up to 5,464 MPN gdry−1), differed significantly (RANOSIM = 0.463, p = 2.9%) from the other areas (stations). Oxygen consumption (−15.22 ± 1.59 mgC m−2) prevailed over primary production and the trophic state was net heterotrophic. In contrast, on the other side of the harbor (residential area/center bay), contamination levels were below the legal limits and both the microalgal and macrobenthic communities displayed higher biodiversity. Higher macrofaunal abundances (up to 753 ± 174.7 ind.m−2), primary production rates (up to 58.60 ± 8.41 mgC m−2) and exoenzymatic activities were estimated. nMDS and SIMPROF analyses performed on benthic communities significantly separated the most contaminated stations from the other ones. Overall, by applying this holistic approach, a better environmental situation was highlighted along the southern boundary of the bay and according to these results this part of the bay could be excluded from the SIN. However, further sampling is required along a finer sampling grid in the less contaminated side of the port in order to confirm these first results. Our work is one of the first case studies where such an ecosystem approach has been applied to a port area, in order to provide practical support to decision-makers involved in the spatial planning of harbor zones.
Introduction
Increasing pressure on the marine realm calls for a well-planned approach to the management of marine space. Fisheries and aquaculture, the gas and oil industry, shipping, tourism and, on the other hand, the need for marine conservation, all compete for the same valuable space. All these activities influence the structure and functioning of marine ecosystems and the use of coastal zones, calling for a robust approach to future spatial planning. Marine areas have been traditionally managed on a case-by-case, sector-by-sector basis, ignoring the interdependent nature of ecosystem components (Katsanevakis et al., 2011). Although informative, the assessment of the environmental status of a given area based solely on the commonly used parameters such as contaminants in the sediments and abundance/composition of macrofauna, could lead to insufficient understanding of the whole ecosystem functioning, overlooking other environmental aspects that should be considered when addressing suitable corrective actions. There is, therefore, an urgent need for a fundamental shift in the way we manage our coast toward the development of a holistic approach that considers all the main components of ecosystem functioning and its integration in management (Katsanevakis et al., 2011).
The study of ecosystem functioning is a complex issue, which implies the simultaneous investigation of both structural and functional parameters and their consequent integration in order to depict an overall view of the C flow and cycling through the system. An ecosystem may be defined as an open dynamic system, composed of “subunits” that are interlinked by a complex web of “connections.” In benthic ecosystems, the soft bottoms are inhabited by several communities of organisms arbitrarily classified according to their body size into: pico-, microphyto-, meio-, and macrobenthos. From an ecological point of view, organisms can be grouped into three main categories: producers (autotrophs), consumers (heterotrophs) and decomposers (prokaryotes) that are interlinked by a dense web of connections (Odum, 1983). Primary producers represent a food source for herbivores that graze on algae and are, in turn, food for carnivores along what is commonly considered as the classical grazing benthic food web. However, both meio- and macrofaunal detritivores do not graze exclusively on microalgae but ingest large amounts of sediment obtaining energy from the organic matter present in them (Franzo et al., 2015; Nasi et al., 2017; and references therein). Therefore, the grazing food web is integrated with the detrital food web, i.e., the flow of energy from the (non-living) detrital organic matter toward the higher trophic levels such as meio- and macrobenthos. The biotic and abiotic components of the system are further interlinked by processes of organic matter production (primary production), its transformation (mineralization) and energy dissipation (respiration) (Boero and Bonsdorff, 2007). The system is extremely flexible and responds to physical, chemical and biological factors of both natural and anthropogenic origin. Therefore, modifications of the ecosystem structure and C flow indicate the presence of a stressor. Aquatic ecosystems are able to adapt and respond to pollution by adopting strategies at different levels (organism, community and system) (Cibic et al., 2012b). Only by combining biological processes with the structure of benthic communities, following a holistic approach, is it possible to gain a better understanding of the functioning of an ecosystem and its response to contamination.
The idea behind “the ecosystem approach to management” is that the management of human activities is based on the limits within which ecosystem structure, functioning, productivity and biological diversity can be maintained. The concept has been developed and incorporated into a number of international agreements over the past 10–15 years (Ottersen et al., 2011). But there are very few examples of its actual implementation, mainly due to the difficulties in coupling environmental safety and sustainable use of resources with the needs and expectations of stakeholders. There are still major scientific and knowledge gaps in applying the ecosystem approach to management, related to our limited understanding of the dynamics and resilience of ecosystems, the cumulative impacts of human uses on the marine environment and the effectiveness of management and governance systems (Katsanevakis et al., 2011, and references therein). Moreover, the details outlined in the scientific literature are often only loosely incorporated into management plans and actions (Ottersen et al., 2011).
Port terminals and harbor areas play important roles in the economy worldwide through the transport and storage of traded goods. However, they have several negative environmental impacts on the coastal zone, such as pollution due to the discharge of contaminants, e.g., wastewater, petroleum and its derivatives. Port activities are often associated with aquatic pollution and the spreading of contaminants through different compartments, such as water, sediments and biota. Special attention must be given to sediments, which frequently present higher concentrations of contaminants compared to the water column, and may constitute not only a sink but also a secondary source of contaminants for the water column and biota (Buruaem et al., 2012). Benthic organisms, in particular, due to their limited mobility, are exposed to accumulated contaminants and respond to stress conditions both at individual and community level through the selection of taxa, the elimination of sensitive ones and abundance changes (Solis-Weiss et al., 2004). Many studies report on changes in abundance or diversity of micro- or macrobenthic communities in chronically polluted marine environments. Other fundamental aspects, such as how pollution affect respiration rates, primary production (Sundbäck et al., 2004; Forster et al., 2006; Rubino et al., 2016), prokaryotic heterotrophic production or other microbial processes (Manini et al., 2004; Pusceddu et al., 2014; Sweetman et al., 2014; Franzo et al., 2016a) have seldom been analyzed in chemically polluted environments. Studies on benthic ecosystem functioning, based on actual estimates of biological processes integrated with the qualitative and quantitative composition of communities at different trophic levels, are even rarer (Schaffner et al., 2008; Cibic et al., 2012b; Franzo et al., 2016b).
The port of Trieste (northern Adriatic Sea) is located within a Site of National Interest (SIN). These sites are defined by the Italian State as very large contaminated areas, in need of soil, surface water and groundwater remediation. SIN are identified in relation to the characteristics of the site, the quantity and hazardous nature of pollutants, the importance of the impact on the surrounding environment, in terms of health and ecology, as well as damage to cultural and environmental heritage. The SIN of Trieste was founded by Decree of the Italian Ministry of the Environment in 2003 and covers a marine coastal area of about 12,000,000 m2 in the eastern part of the Gulf of Trieste. It is divided into 5 macrosites on the basis of the main activities located there and their consequent anthropogenic pressures: (1) a port area, (2) a shipbuilding area, (3) an iron foundry area with a steel plant, (4) a petroleum area where petroleum products are handled, stored and processed, and (5) a residential area/center bay, the largest among the 5 areas. Since activities such as fishing, aquaculture, swimming and urban development are restricted within the SIN, the local authorities are interested in assessing whether the entire area should continue to form part of the SIN. Updated and focused environmental assessments could allow the detection of subareas, if present, characterized by a less deteriorated environmental situation than those expected for a SIN (in terms of contamination levels and ecosystem functioning). In this way, it would be possible to exclude part of the marine space from one or more of its current legislative constraints. This can lead, subsequently, to a management scheme focused on the establishment of productive activities that could contribute to the economic development of the area. The Port Authority of Trieste, which is the managing body of the SIN, adopted a strategy based on the integrated ecosystem approach in order to implement the new Environmental Characterization Plan of the SIN, as required by the most recent Italian law. We have been entrusted by the Port Authority of Trieste to assess the benthic ecosystem functioning in the harbor area in order to verify whether it would be possible to exclude certain specific areas (the strip of sea off Muggia and part of the center of the bay) from the SIN. For this purpose, we evaluated how biodiversity and benthic ecosystem functioning vary among the five port macrosites, subjected to diversified industrialization and anthropization. We followed a holistic approach by considering the structural characteristics of the sediments, both heterotrophic and phototrophic communities, together with the main processes of production, transformation and consumption of organic matter.
Assessment of benthic ecosystem functioning was carried out only once to address the following questions: (1) How does benthic ecosystem functioning vary at the investigated macrosites of the port subjected to diversified industrialization and anthropization? (2) Can the assessment of benthic ecosystem functioning support decision-makers in the management of marine space within the SIN?
Materials and Methods
Study Site
The Gulf of Trieste, located at the north-western end of the Adriatic Sea, is a shallow embayment of about 500 km2 with a coastline of about 100 km. It is almost completely surrounded by land; its southwest limit is the line connecting Punta Tagliamento in Italy with Punta Salvore in Croatia. It is isolated from the rest of the Adriatic by a sill, from Grado to Salvore peninsula (Ogorelec et al., 1991); 10% of its area is <10 m deep and maximum depth is about 25 m. Average salinities range from 33 to 38 psu at the surface and from 36 to 38.5 psu at the bottom. Annual temperatures fluctuate from 8°C to ≥ 24°C at the surface and from 8°C to ≥ 20°C at the bottom. Tidal amplitude is about 1.5 m, which is the highest in the Mediterranean Sea (Cardin and Celio, 1997). Water enters the Gulf from the southeast and circulation at the surface is predominantly from southeast to northwest. Sedimentation is controlled mainly by river input rather than marine currents (Brambati and Catani, 1988). Winds and water column stratification are the major factors influencing the characteristics of the composition, evolution and persistence of marine life in the Gulf of Trieste (Solis-Weiss et al., 2001).
Within the Gulf of Trieste, the Bay of Muggia is a shallow embayment (8-20 m) about 7 km long and 4 km wide and oriented NW- SE (Figure 1; Ghirardelli and Pignatti, 1968). Sedimentation is controlled by the low hydrodynamism (Solis-Weiss et al., 2004; and references therein) and fluvial inputs: two streams enter the Bay, Rosandra and Ospo that may discharge large amounts of fine sediment containing chemical fertilizers (www.porto.trieste.it1). The Bay of Muggia houses the port and industrial area of Trieste and its morphology makes it prone to the accumulation of contaminants, since it is sheltered from currents and characterized by an elongated shape and low hydrodynamism.
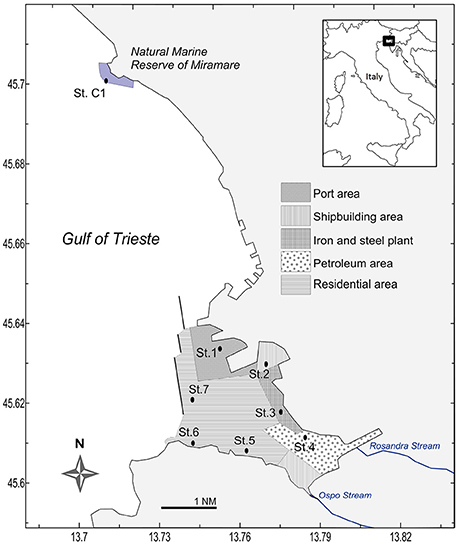
Figure 1. Location of the seven sampling stations within the five macrosites of the Site of National Interest (SIN) of Trieste, and the reference station C1.
The development of the port dates back to the early 1900s with the construction of three external dams (1904-1909), the creation of large industrial structures in the Gaslini area, and the establishment of an iron and steel manufacturing industrial complex. In subsequent decades, other industrial structures were built, such as the industrial channel (completed in the 50s), the navigation channel (1966) as well as the construction of the Trieste - Monaco of Bavaria (SIOT) (1967) pipeline terminal (Solis-Weiss et al., 2004 and references therein), the most important pipeline that serves central Europe (about 36 · 106 tons of crude oil discharged in 2001) (www.porto.trieste.it) and finally, the expansion of the commercial docks.
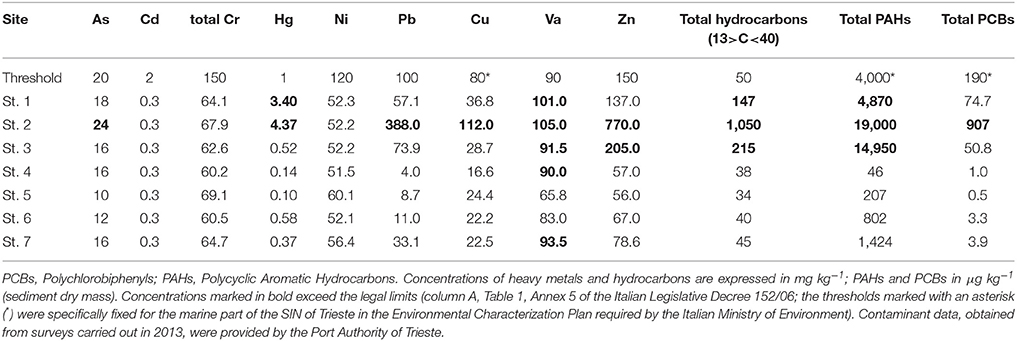
Since 2003, the SIN of Trieste has been intensely monitored to assess the chemical quality status of the sediments and the water column, as required under the current legislation. As required by the latest Environmental Characterization Plan, in 2013 contaminants in the surface sediments were evaluated by the Port Authority of Trieste at more than 40 sampling stations. Among those, we chose the ones closest to our 7 sampling stations so as to have an indication of the concentrations of the major contaminants (Table 1).
Sampling
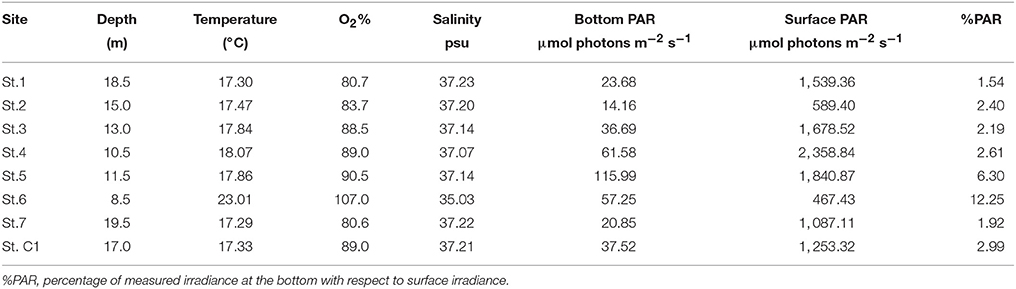
In June 2013, seven stations were sampled within the SIN of the Port of Trieste (Figure 1). Station C1, located in the Marine Reserve of Miramare, approximately 5.4 nm (about 10 km) from the port (45° 42.050′ N, 13° 42.600′ E) was chosen as reference site. This site was declared a marine protected area in 1979 and represents the only completely protected area in the Italian part of the Gulf of Trieste, sheltered from all direct human activities and boat traffic. Except for the shallowest station (St. 6), the depth of St. C1 (17.0 m) fell within the range of the other stations (10.5–19.5 m) of this study. In addition, the grain-size of St. C1 was similar to that of the port stations. At each site, 6 virtually undisturbed sediment cores were collected by replicate hauls using an automatic KC Haps bottom corer (KC-Denmark) and polycarbonate sample tubes (12.7 cm i.d. with a sample area of 127 cm2); 1 sediment core was used for meiofauna sampling, 1 for oxygen microprofiling while, from the 4 remaining cores, the uppermost layer (0–1 cm) was sampled, pooled and homogenized for the following analyses: grain-size, total organic and biopolymeric C, pigments, bacterial groups, microphytobenthos, primary and prokaryotic C production, degradative bacterial activities (referred to later on as “homogenized sediment”). Since photoautotrophs and their photosynthetic activity are confined to the uppermost layer, the analytic effort of this study was focused on the top sediment layer to allow a comparison with the other sampled variables. Macrozoobenthos was sampled in three replicates per station using a stainless steel van Veen grab (sampling area 0.1 m2). Macroalgae were absent at all sampling stations. Bottom water samples were collected using a 2-L horizontal Niskin bottle. At the time of sampling, Photosynthetically Available Radiation (PAR) was recorded in situ by a Profiling Natural Fluorometer PNF-300 (Bioshperical instruments Inc., San Diego, CA, USA). PAR at the bottom was expressed as the percentage of measured irradiance with respect to surface irradiance (%PAR). Bottom sea temperature, dissolved oxygen and salinity were measured by a 19plus SEACAT profiler CTD (Sea-Bird Electronics, Inc., Bellevue, Washington, USA).
Granulometry, Total Organic Carbon (TOC) and Pigments
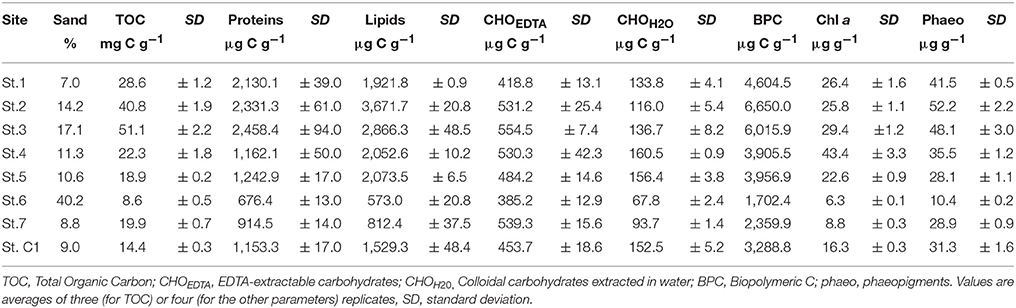
For each station, an aliquot (10–15 g) of homogenized sediment was collected for grain-size analysis and processed as described by Franzo et al. (2016b). The analyses were performed using a Malvern Multisizer 2000S. Data are expressed as a percentage of sand, silt and clay. For TOC analyses, triplicate subsamples of homogenized sediment (<250 μm) were weighed directly in a capsule (5 × 9 mm), treated with increasing concentrations of HCl (0.1N and 1N) to remove carbonates (Nieuwenhuize et al., 1994) and determined according to the methods of Pella and Colombo (1973) and Sharp (1974). Pigments were extracted overnight (4°C, 90% acetone) from 0.7 to 0.9 g of wet sediment (in four replicates) and analyzed spectrofluorometrically following the procedures described by Lorenzen and Jeffrey (1980).
Biopolymeric Carbon (BPC)
Subsamples of homogenized sediment were freeze-dried and processed for the determination of carbohydrates, lipids and proteins. Colloidal and EDTA extractable carbohydrates (CHO) were analyzed following the method described by Blasutto et al. (2005). Lipids were analyzed following the method proposed by Bligh and Dyer (1959) and modified for sediments. Proteins were extracted in NaOH (0.5 M) for 4 h and determined according to Hartree (1972). All analyses were carried out in four replicates. Carbohydrate, lipid and protein concentrations were converted to carbon equivalents (Fichez, 1991). The sum of carbohydrates, lipids and proteins was referred to as Biopolymeric Carbon (BPC).
Enumeration of Alkane- and Petroleum-Degrading Bacterial Groups
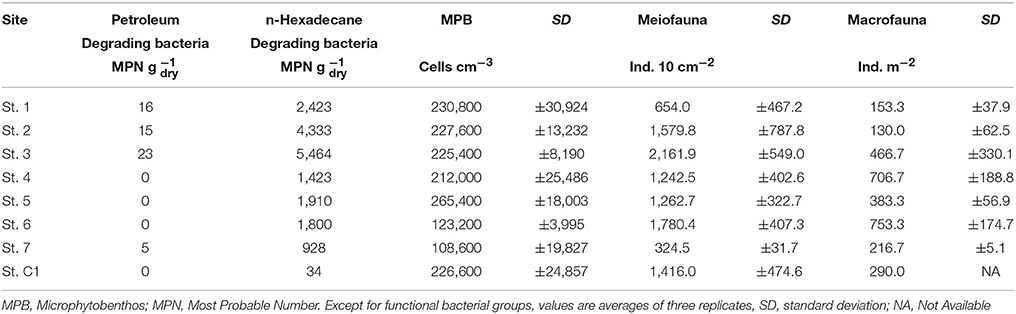
The standard five-tube most-probable-number (MPN) method (Alexander, 1965) was adopted to estimate the abundances of specific bacterial groups. Subsamples of homogenized sediment (~5 gwet) were placed in sterile tubes with 45 mL of pre-filtered bottom water (0.2 μm filters), stirred, sonicated and centrifuged at 956 × g for 1 min to remove sediment particles. Supernatant and 5 serial 10-fold diluted inoculums were added to the wells containing specific medium (10% v/v) and incubated at 20°C. Alkane- and petroleum-degrading bacteria were enumerated by adding n-Hexadecane (C16) (3.4% v/v) and unleaded petrol (3.4% v/v) to BH medium (Bushnell and Haas, 1941). Hydrocarbon degradation was detected after 15 days by adding a solution of 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium (INT, 0.6 g L−1 final concentration) and considering as positive reaction the red-violet dye turning (Johnsen et al., 2002).
Microphytobenthos (MPB)
For the microphytobenthic analyses, aliquots of 2 cm3 of homogenized sediment were withdrawn using a syringe and directly fixed with 10 mL of formaldehyde (4% final concentration) buffered solution CaMg(CO3)2 in pre-filtered bottom seawater (0.2 μm filters). After manual stirring, 20-μL aliquots of the sediment suspension were drawn off from the sediment suspension in triplicate and placed into 2.5 mL counting chambers. Only cells containing pigments and not empty frustules were counted under a Leitz inverted light microscope (Leica Microsystems AG, Wetzlar, Germany) using a ×32 objective (×320 final magnification). Samples were counted following the protocol described by Cibic et al. (2007a, 2012a). At least 100 cells were counted for each replicate. The qualitative identification was carried out using the floras cited therein. The microalgal taxonomy was based on Round et al. (1992).
Meiofauna
From a virtually undisturbed sediment core, three replicates were gently taken using cut-off plastic syringes (2.7 cm i.d., length 11.4 cm) and immediately frozen at −20°C (Higgins and Thiel, 1988). Directly after thawing, the top 10 cm of the sediment core were preserved in buffered 4% formaldehyde solution using pre-filtered seawater and stained with Rose Bengal (0.5 g L−1). Sediment samples were sieved through 1,000 and 38 μm mesh net and the extraction of organisms (from the sediment retained on the 38 μm sieve) was performed by triple centrifugation (1,932 × g, 10 min) with Ludox HS-40 (density 1.15–1.18 g cm−3) as described by Danovaro et al. (2004). All meiobenthic organisms were counted and taxonomically classified into the main groups, according to Higgins and Thiel (1988), under a stereomicroscope (Olympus SZX12; final magnification of 40 or 80X). Abundance was expressed as individuals per 10 cm2.
Macrofauna
Macrofauna was sampled with a van Veen grab (sample area: 0.1 m2, volume: 18 L); three replicates per station were taken, sieved through a 1,000 μm sieve and immediately frozen at −20°C. After defrosting and sorting, animals were counted and identified to the lowest possible taxonomic level using a stereomicroscope (Olympus SZX12) at 7-40X magnification (Rees et al., 1990). For the identification of organisms, the taxonomic keys listed in Morri et al. (2004) were used. The abundance was expressed as individuals per m−2. In order to investigate the functional structure of the macrozoobenthic community in relation with the other benthic communities (i.e., microphytobenthos and meiofauna), the macrofaunal feeding habits were considered. Six different feeding habits were assigned to all individuals: surface deposit feeders (SDF), subsurface deposit feeders (SSDF), suspension feeders (SF), predators (P), omnivores (OMN), and grazers (G) using the following criteria: morphology of the feeding apparatus, feeding mode, nature and origin of the food. Traits for each taxon were derived from Roth and Wilson (1998) and Jumars et al. (2015). Taxonomic resolution was kept at species level whenever possible but adjusted to genus or family when the information on traits was only available at higher taxonomic level.
Gross Primary Production (GPP), Oxygen Consumption, and Benthic Trophic State
GPP was estimated in the laboratory from 14C-incubation of slurries (Cibic et al., 2008). At each station, 10 cm3 of homogenized surface sediment was sucked up with a syringe, resuspended in 190 mL of overlying filtered seawater (0.2 μm filter) and inoculated with 20 μCi (0.74 MBq) of NaH14CO3 (DHI, Denmark) (Steemann-Nielsen, 1952). After stirring, the slurry was transferred to 9 mL glass vials and incubated in a thermostatic chamber at in situ temperature under a gradient of light intensities (20–50–100–200–300 μmol photons m−2 s−1). After 45 min, carbon incorporation was stopped by adding 200 μL of HCl 5N (final HCl concentration 0.11N) (Cibic and Virgilio, 2010). Subsequently, samples were treated as described in detail by Cibic et al. (2008). At each station, the highest GPP value obtained from the light gradient was used. O2 consumption rates were obtained from oxygen microprofiling carried out on intact sediment cores in the dark. For technical reasons, oxygen consumption rates are not available for St.4. Steady-state O2 microprofiles were measured using Clark-type O2 microelectrodes with a guard cathode (Revsbech, 1989) having external tip diameter <100 μm, stirring sensitivity <2%, and 90% response time <8 s. The sensor current was measured using a Unisense PA2000 picoammeter; data was recorded using the Unisense Profix software version 3.10 (Unisense, Aarhus, Denmark). A step size of 100 μm was used. For the interpretation of the measured O2 concentration profiles, the PROFILE version 1.0 software package was used (Berg et al., 1998). Areal rates of oxygen respiration were calculated as described by Cibic et al. (2007b). As the microsensors measure diffusive oxygen uptake, the method potentially underestimates faunal-mediated oxygen consumption.
Applying a Respiratory Quotient of 1, oxygen data were converted to mg C m−2 h−1 to allow an estimation of the trophic status (autotrophy/heterotrophy) of the investigated sites. Gross primary production (GPP) represents the sum of net primary production (NPP) and community respiration (CR). The 14C technique measures something between GPP and NPP, depending on the incubation time: shorter incubation times are closer to GPP whereas incubation times ≥ 6 h are closer to NPP (Gazeau et al., 2004). In our study, the incubation time was about 45 min; therefore, a GPP rate was measured. Subtracting CR, assessed as the oxygen consumption in the dark, we attempted to estimate NPP.
Degradative Extracellular Bacterial Activities
Extracellular enzymatic activities were assayed using fluorogenic substrate analogs (Hoppe, 1993) derived from 7-amino-4-methyl-coumarin (AMC) and 4-methyl-umbelliferone (MUF). Protease activity (leucine aminopeptidase activity—AMA) was assayed as the hydrolysis rate of leucine-AMC, while β-glucosidase (β-GLU), lipase (LIP) and chitinase (CHIT) were assayed using MUF-β-D-glucoside, MUF-oleate and MUF- β-D-glucosamide (Sigma-Aldrich), respectively. Enzyme activities were expressed in terms of the rate of MUF or AMC production. Sediment slurries were prepared by adding 6 mL of 0.2 μm-filtered bottom water to 0.5 g of wet sediment withdrawn from the homogenized pool. Hydrolysis rates were measured by incubating slurries in the dark for 1 h at in situ temperature and after the addition of (final concentration) 800-μM MUF-β-D-glucoside, 400-μM leucine-AMC and 200-μM MUF-N-acetyl-β-D-glucosaminide and MUF-oleate (Sigma). These concentrations were chosen after the evaluation of substrate saturation (tested concentration: 50, 100, 200, 400, 800 μM), performed on surface sediments from the Gulf of Trieste. Before spectrofluorometric measurement, each sample was centrifuged for 2 min at 3,000 rpm. Fluorescence increase due to MUF and AMC hydrolysed from the model substrates was measured using a Jasco FP 6500 spectrofluorometer (MUF = 365-nm excitation and 455-nm emission; AMC = 380-nm excitation and 440-nm emission). Standard solutions of MUF and AMC were used to produce calibration curves with 0.2 μm filtered bottom water. Triplicate blanks without fluorogenic substrate were used to determine the natural fluorescence increase in the samples not attributable to the tested enzymes. Hydrolytic activities were converted into C mobilization using the conversion factor 72 for glucose and leucine and 216 for oleic acid.
Prokaryotic Carbon Production (PCP)
PCP in sediment samples was carried out following the method of van Duyl and Kop (1994), as detailed by Manini et al. (2004). Each sample (0.2 mL of 1:1 vol/vol slurry prepared using the homogenized sediment pool) was added to 6 μCi of 3H-leucine and incubated in the dark for 1 h at in situ temperature. After incubation, radiotracer incorporation was stopped by adding 80% ethanol (1.7 mL). After two washes of the samples with ethanol (80%) by mixing, centrifuging and decanting the supernatant, the sediment was transferred with ethanol (80%) onto a polycarbonate filter (0.2 μm mesh size). Subsequently, the filters were washed twice with 5% trichloroacetic acid. Samples were heated in 2M NaOH for 2 h in a water bath at 100°C. One mL of supernatant was transferred to scintillation vials and 10 mL of Hionic Fluor scintillation fluid was added. For each sample, three replicates and two ethanol-treated blanks were analyzed. Activity in the samples was determined by a β-counter (TRI-CARB 2900 TR Liquid Scintillation Analyser).
Statistical Analyses
Descriptive statistics were obtained for microphytobenthos, meiofauna and macrofauna in order to calculate the Relative Abundance (RA) of the main taxa. Univariate diversity analysis was applied to the abundances of both benthic diatoms and macrofaunal taxa considering richness (d, Margalef, 1986), equitability (J', Pielou, 1966), diversity (H', Shannon and Weaver, 1949) and dominance (λ, Simpson, 1949).
Prior to analysis, the microphytobenthic and macrozoobenthic abundance data from the eight stations (seven from the port area and the reference site) were square-root transformed to reduce the influence of dominant species. The transformed data were then analyzed using cluster analysis (performed with the complete linkage clustering algorithm) in conjunction with the similarity profiles (SIMPROF) routine, performed on the same Bray-Curtis similarity matrices (Clarke et al., 2014). This analysis was applied to highlight possible significant differences (p < 0.05) in microphytobenthic and macrofaunal communities among sites. The groups of stations significantly gathered by the SIMPROF test were overlaid on the non-metric multidimensional scaling (nMDS) ordination plots. These tests were not applied to meiofauna because the taxonomic level of identification was too low (phylum to order).
To test for the variability of abiotic variables among stations (sand fraction, TOC, BPC, pigments) and those contaminants that were above the legal limits (As, Hg, Pb, Cu, Va, Zn, PAHs, and PCBs, see Table 1), the analysis of similarity (ANOSIM) was applied. The ANOSIM statistic R is based on the difference of mean ranks between groups and within groups. The statistical significance of observed R is assessed by permuting the grouping vector to obtain the empirical distribution of R under null-model. ANOSIM tests a priori defined groups (in our case, stations at which contaminants were above or below the legal limits) against random groups in ordinate space. Zero (R = 0) indicates that there is no difference among groups, while one (R = 1) indicates that all the samples within groups are more similar to one another than any samples from different groups (Clarke et al., 2014). All univariate and multivariate analyses were performed using PRIMER 7 (PRIMER-E Ltd., Plymouth, UK) software.
The main chemical and biological structural data were checked for significant differences between the three contaminated stations (St. 1, 2, and 3) and the other ones, by applying the Mann-Whitney test (STATISTICA 7).
Results
Physico-Chemical Data
Temperature ranged between 17.29°C at the deepest St.7 and 23.01°C at the shallowest St. 6. At the latter station, the bottom layer was supersaturated in O2 whereas at the other stations O2 ranged from 80.6 to 90.5%. The lowest salinity was registered at St. 6 while it did not vary much among the other stations (range: 37.07–37.23 psu). PAR at the bottom ranged from 14.16 μmol photons m−2 s−1 at St. 2 to 115.99 μmol photons m−2 s−1 at St. 5. At St. 6, the %PAR, i.e., the percentage of measured irradiance at the bottom with respect to surface irradiance, reached the highest value due to the limited depth (12.25) whereas, with the exception of St. 5, it varied between 1.54 and 2.61 at the other stations (Table 2).
According to Shepard's (1954) classification, the sediment was clayey silt at all stations except for St. 6 where it was sandy silt. The percentage of sand at St. 6 was in fact > 40% while it ranged between 7.0% (St. 1) and 17.1% (St. 3) at the other sites (Table 3). Mean TOC content reached 51.1 ± 2.2 mg g−1 at St. 3 (in the proximity of the coal-fired steel plant), three times the value recorded at the reference station. At St. 2 and 3, much higher values of BPC were detected compared to the other investigated sites. The lipid content represented the main fraction of the labile organic matter pool at St. 2, 3, 4 and C1, while at St. 1, 6, and 7, the protein content dominated, varying from 29.8 to 46.3% of total BPC. EDTA-extractable carbohydrates did not exceed 22.9% of BPC and their content was higher at St. 6 and 7, while the contribution of colloidal carbohydrates was negligible (Table 3). The highest Chl a value was measured at St. 4 (43.4 ± 3.3 μg g−1) while major phaeopigment contents were observed at St. 2 and 3 compared to the other sites. TOC, BPC and phaeopigment contents were significantly higher at St. 1, 2, and 3 compared to the other sites (Mann-Withney test, z = 2.12, p < 0.05).
The analysis of similarity performed on a matrix based on abiotic variables and contaminants further confirmed that the group of contaminated stations (St. 1, 2, and 3) was significantly different from that of all the other stations (RANOSIM = 0.463, p = 2.9%).
Benthic Communities
Petroleum degrading bacteria were isolated from the surface sediments of St. 3, 1, 2, and 7 (up to 23 MPN g−1), whereas they were not observed at the other stations. Similarly, the presence of bacteria able to degrade n-Hexadecane was higher at St. 3 (5,464 MPN g−1), St. 2 and 1, i.e., stations more contaminated by hydrocarbons. Lower values were obtained offshore Muggia and in the center of the bay even if they were one order of magnitude higher than those at the reference site (Table 4). Both bacterial groups were significantly higher at St. 1, 2, and 3 compared to the other sites (Mann-Withney test, z = 2.12, p < 0.05).
Total microphytobenthic abundances ranged from 108,600 cells cm−3 at St.7 to 265,400 cells cm−3 at St. 5 (Table 4). The microalgal community was dominated by diatoms (>99% at all the investigated sites). Among them, Nitzschia was the most abundant genus, with a relative abundance (RA) varying from 26.6% at St. 7 to 44.1% at St. 3, followed by Navicula (11.5% < RA < 27.9%), at all stations except for St. 5 where Gyrosigma prevailed. Cylindrotheca was observed with a higher RA at the deeper stations (St. 7, C1, and 1) (Table 5). Nitzschia cfr. commutata was the dominant species at St. 2, 3, and 4, with an RA of 22.6, 14.2, and 11.0%, respectively. In contrast, at the reference site and in the center of the bay, its percentage did not exceed 1.5%. The univariate diversity indices applied to the diatom community revealed that the highest richness and diversity were observed at St. 5 (d = 3.24; H' = 3.03), whereas the lowest diversity (H' = 2.51) as well as major dominance were obtained at St. 2 (ʎ = 2.63) due to the high abundance of Nitzschia cfr. commutata at this site. At St. 7, the diatom community was more evenly distributed (J' = 0.84) compared to the other stations.
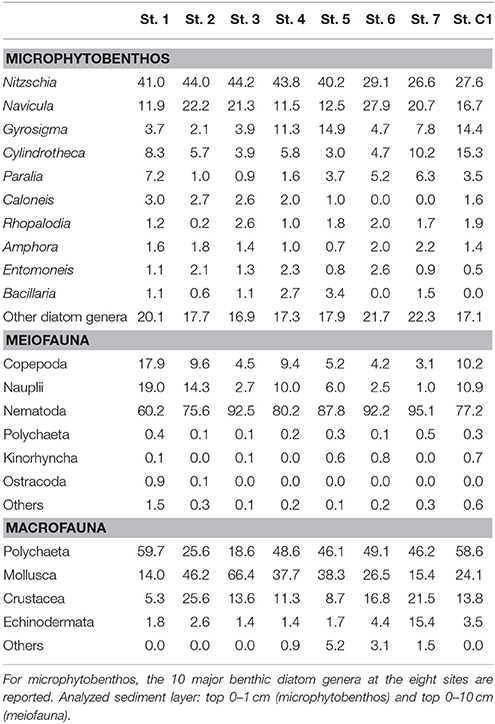
Table 5. Relative abundance, expressed as a percentage, of the major groups constituting the benthic communities.
The nMDS analysis based on the microphytobenthic species highlighted clear spatial differences among stations (Figure 2). Three significant groups of samples were identified using the SIMPROF test: those stations where the level of contamination was above the legal limits (St.1, St. 2, and St.3) were gathered in the same group. The other stations were split into two different groups (St. 4, St. 5, and St. 6, St. 7) while St. C1 was completely separated from the port stations.
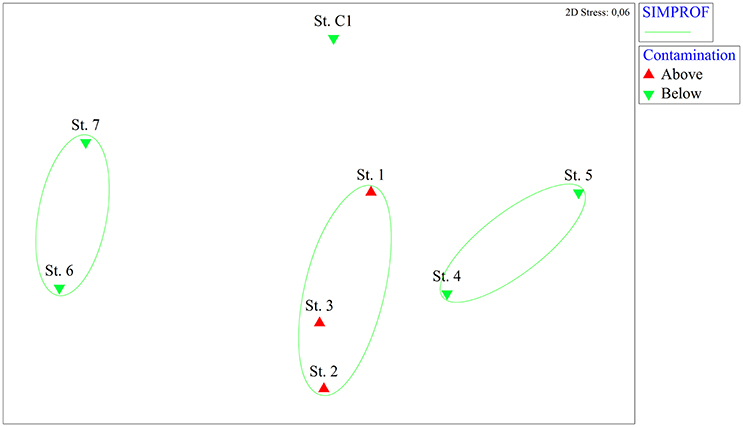
Figure 2. Non-metric multidimensional scaling (nMDS) analysis based on microphytobenthic abundance data from the eight stations. On the nMDS ordination plot, groups of stations significantly gathered by the SIMPROF test are superimposed. Red triangles: stations where contaminants are above the legal limits, green inverted triangles: stations where contaminants are below the legal limits.
Meiofaunal abundances ranged between 324.5 ± 31.7 ind. 10 cm−2 and 2,167.9 ± 549.0 ind. 10 cm−2 at St. 7 and St. 3, respectively (Table 4). A total of 15 taxa were detected: 9 belonged to permanent meiofauna (Nematoda, Copepoda Harpacticoida and their naupliar stages, Kinorhyncha, Ostracoda, Tardigrada, Turbellaria, Gatrotricha, Acarina) and 6 to temporary meiofauna (i.e., juvenile macrofauna: Polychaeta, Bivalvia, Gastropoda, Amphipoda, Decapoda and Tanaidacea). Nematoda represented the dominant group at all the studied stations. Their RA, in fact, reached more than 60.2% (St. 1) of the whole community, with a maximum of 95.1% at St. 7. Copepoda Harpacticoida, together with their naupliar stages, represented the second most abundant taxon, with RA ranging from 4.1 to 36.9% at St. 7 and St. 1, respectively (Table 5). The other observed taxa showed very low RA (<1%) at all sites.
Macrofaunal abundances ranged from 130.0 ± 62.5 ind. m−2 at St. 2 to 753.3 ± 174.7 ind. m−2 at St. 6 (Table 4). The community was dominated by Polychaeta (>40% at all the investigated sites), except for St. 2 and St. 3, where Mollusca accounted for 46.1% and 66.4% of total abundance, respectively. Overall, Crustacea were less abundant, although with slightly higher numbers at St. 2, 6, and 7 (25.6, 30.6, and 21.5%, respectively). Echinodermata represented more than 10% of the community only at St. 7 (15.4%) (Table 5). The univariate diversity indices applied to macrofauna revealed that among the eight sites the highest richness and diversity were observed at St. 4 (d = 10.78 and H' = 5.10), whereas the lowest diversity (H' = 3.22) as well as a lower value of evenness were obtained at St. 3 (J = 0.66) due to the prevalence of Corbula gibba (45%). Overall, the community feeding habits were well expressed at each sampling site. Suspension feeders were observed in high numbers, particularly at St. 6 and St. 3 (306.6 and 283.6 ind m−2, respectively). Deposit feeders (both SDF and SSDF) reached a high density at St. 1, St.7, and St. C1, mainly due to SDF that reached high numbers at St. C1 and St. 7 (83.3 and 73.3 ind. m−2, respectively) and SSDF at St. 1 (63.3 ind. m−2). The number of predators ranged from 13.3 to 133.3 ind. m−2 at St. 2 and St. 4, respectively (Figure 3).
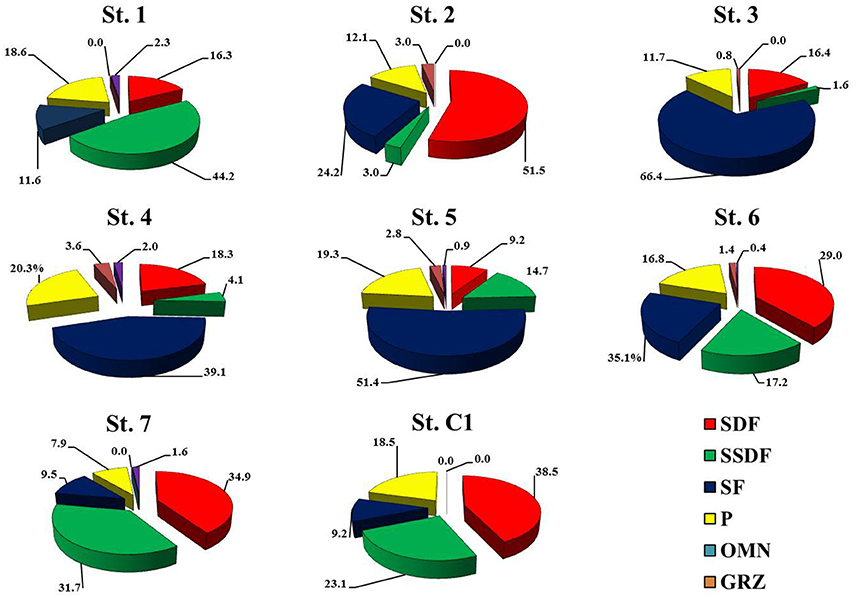
Figure 3. Relative abundance, expressed as a percentage, of the macrofaunal feeding habits at the eight sites. SDF, surface-deposit feeders; SSDF, subsurface-deposit feeders; SF suspension feeders; P, predators; O, omnivores; G, grazers.
The nMDS analysis based on the macrozoobenthic species abundances revealed spatial differences among stations, though less pronounced than those based on the microphytobenthos (Figure 4). The SIMPROF analysis gathered St. 2 and St. 3 in the same group, while the other contaminated site (St. 1) was grouped with St. 7. Stations 4 and 5 showed a high level of macrofaunal similarity whereas the reference site was separated from the other stations.
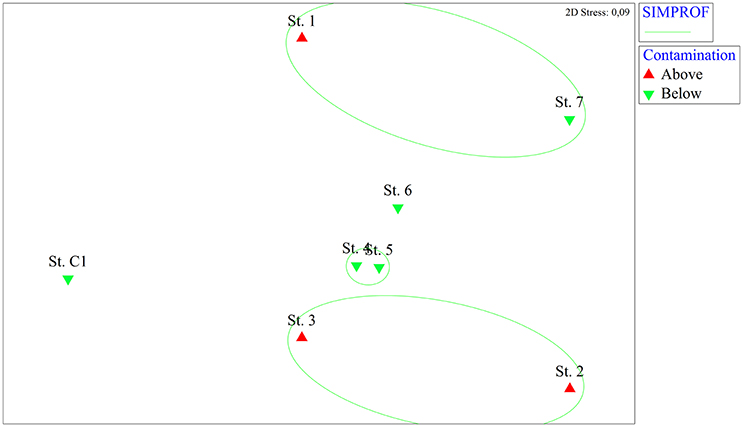
Figure 4. Non-metric multidimensional scaling (nMDS) analysis based on macrozoobenthic abundance data from the eight stations. On the nMDS ordination plot, groups of stations significantly gathered by the SIMPROF test are superimposed. Red triangles: stations where contaminants are above the legal limits, green triangles: stations where contaminants are below the legal limits.
Gross Primary Production (GPP), Oxygen Consumption, and Benthic Trophic State
The highest GPP rates were reached at the given light intensity of 300 μE m−2 s−1 at all stations except for the shallowest St. 6, where the maximum was estimated at 200 μE m−2 s−1. At the deepest St. 7 (19.5 m), the GPP rate did not exceed 3.23 ± 0.57 mg C m−2 h−1 whereas the absolute maximum was estimated at the shallowest St.6 (58.60 ± 8.41 mg C m−2 h−1). Maximum oxygen consumption, converted to C equivalents, was observed at St. 3 (−15.22 ± 1.59 mg C m−2), followed by St. 2 (−11.90 ± 2.15 mg C m−2) whereas at the other stations more modest rates were obtained and were comparable to the oxygen consumption calculated at the reference site (−5.66 ± 0.77 mg C m−2) (Figure 5). Offshore Muggia, the benthic system resulted net autotrophic, with an estimated value of 3.99 mg C m−2 h−1 at St. 5 while at St. 6 it was highly autotrophic (52.81 mg C m−2 h−1). At the reference site, estimated Net Production was barely positive (0.52 mg C m−2 h−1) whereas at all the other stations the benthic system was net heterotrophic (Figure 5).
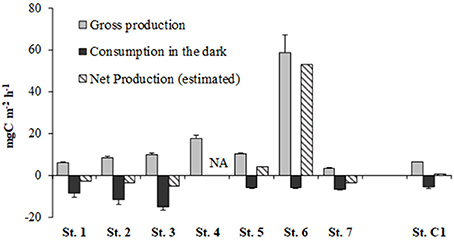
Figure 5. Rates of Gross Production (from 14C data), Consumption estimated in the dark and at in situ temperature (converted from O2 consumption data) and Net Production (estimated) at the eight stations. NA, data not available.
Prokaryotic Carbon Production (PCP) and Extracellular Enzymatic Activities
Higher PCP values were measured at St. 1, 2, 3, and 4, with a mean rate of 0.98 ± 0.02 μg C cm−3 h−1. The stations within the residential area/center of the bay were characterized by slightly lower PCP rates that ranged between 0.46 ± 0.06 μg C cm−3 h−1 (St. 7) and 0.77 ± 0.11 μg C cm−3 h−1 at St. 6 (Figure 6). The degradation rates of proteins, polysaccharides, lipids and chitin are reported in Figure 7. Protease activities showed the highest hydrolysis rates in the study area. The highest value was measured at St. 4 (320.69 ± 9.31 nmol cm−3 h−1) and the lowest at St. 6 (160.15 ± 17.22 nmol cm−3 h−1) (Figure 7A).The other stations showed an average protease activity of 222.2 ± 16.7 nmol cm−3 h−1. β-glucosidase rates did not vary substantially at the eight stations and values ranged from 6.67 ± 0.22 nmol cm−3 h−1 (St. C1) to 12.09 ± 0.44 nmol cm−3 h−1 (St. 3) (Figure 7B). Lipolytic activities were low and quite homogeneous at all stations, with the exception of St. 5, where remarkably higher rates were measured (14.31 ± 1.05 nmol cm−3 h−1) (Figure 7C). Chitinase showed a mean value of 7.3 ± 0.4 nmol cm−3 h−1 and did not vary among stations, although slightly higher hydrolytic rates were observed at St. 7 (14.51 ± 0.50 nmol cm−3 h−1) (Figure 7D).
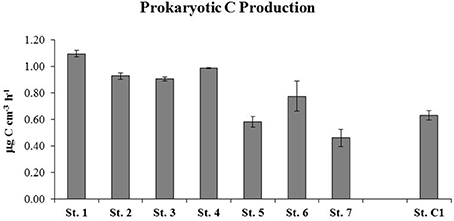
Figure 6. Prokaryotic C production (PCP) estimated at the seven stations within the Site of National Interest and at the reference site (St. C1).
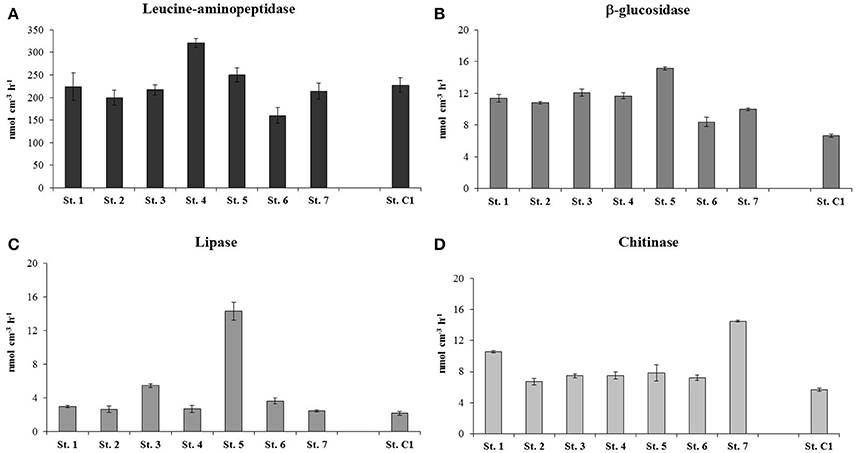
Figure 7. Extracellular enzymatic activities estimated at the eight stations: (A) Leucine aminopeptidase; (B) ß-glucosidase; (C) Lipase; (D) Chitinase. For Leucine aminopeptidase a different scale on the y-axis is used.
Discussion
Benthic Trophic Web in the Port Area
In the benthic trophic web, the primary producers (microphytobenthos), consumers (meio- and macrofauna) and decomposers (bacteria) are interlinked by a dense web of connections. On soft bottoms of shallow areas, where macroalgae are lacking, benthic microalgae represent the main photoautotrophs of the system. The high abundance of benthic diatoms in the port area and at the three contaminated sites suggests that the latter are not negatively affected by high concentrations of heavy metals and hydrocarbons. Benthic diatom mats are rich in extracellular polymeric substances (EPS), which may function as a protective barrier against toxic compounds, as well as enhance the uptake of favorable ones. This feature could decrease the vulnerability of microbial mats to toxic compounds (Sundbäck et al., 2007 and reference therein). Overall, microphytobenthic abundance in the port area was of the same order of magnitude as that previously estimated at a site in the middle of the Gulf of Trieste (Cibic et al., 2012a) but higher than previously reported from the coastal St. C1 (Franzo et al., 2016b). Among the investigated stations, a lower microalgal density was observed at St. 7, approximately half of that obtained at the first four stations, probably because of the greater depth (19.5 m) and lower irradiance at the bottom to support a thriving microbial phototrophic community. In contrast, the lower microphytobenthic abundance at St. 6 was likely due to the different grain-size composition, i.e., the higher percentage of sand, but also the high hydrodynamism that hinders the attachment of diatom cells to the sea bottom. This is in accordance with the findings of Cibic et al. (2016b) who reported lower epipelic (living forms that inhabit muddy sediments) diatom numbers under high hydrodynamic conditions. Overall, community structure at the investigated sites was quite diverse, as confirmed by the nMDS and SIMPROF analysis that separated the contaminated St. 1, 2, and 3 from the others. At St. 2 and 3, in particular, some diatom species that are tolerant to pollution were observed. For instance, Nitzschia cfr. commutata was the most abundant species both in the shipbuilding and the iron foundry area, with relative abundances (RA) of 25.9 and 14.4%, respectively. In contrast, this diatom was observed in very low RA at St. 7, while it was completely absent at St. C1. According to Rogelja et al. (2017), the increase of N. commutata in harbor sediments could be linked to contamination. However, even at the two most impacted sites of the port of Trieste, the microalgal community seemed to be affected by contamination to a much lesser extent than the one thriving in the surface sediments of the Mar Piccolo of Taranto (Ionian Sea, Italy), where only few diatom specimens were encountered at the most contaminated site (Rubino et al., 2016). The authors reported that only those diatoms that thrive just above the surface sediments, i.e., Paralia sulcata and Bacillaria paxillifera, rather than within the sediments, survived the extremely high concentrations of PCBs and heavy metals, whereas diatoms with other life strategies likely died. Similarly, the paucity of benthic diatoms compared to their planktonic counterparts was recently highlighted by Potapova et al. (2016) in lagoonal sediments contaminated by heavy metals and PAHs.
From a trophic point of view, microalgal proliferation in the Gulf of Trieste is known to support the meiofaunal community. Specifically, in the summer, meiofauna appears to respond to pulsed inputs of fresh organic matter, which are ascribable to higher abundances of benthic diatoms that represent an important food source for these heterotrophs by increasing the nutritional value of sediment (Franzo et al., 2016b). In the SIN of Trieste, meiofaunal numbers showed the same order of magnitude reported previously at the reference site St. C1 over a 2-year study (Franzo et al., 2016b). When comparing similar sampling periods (i.e., June), abundances were even more alike, suggesting that sediment contamination, especially at St. 2 and 3, did not seem to severely affect total meiofaunal numbers. The community, dominated by nematodes and copepods as in most coastal areas of the Adriatic Sea (Balsamo et al., 2010), reflected the composition already reported for the Gulf of Trieste (Cibic et al., 2009). Compared to the stations inside the port area, the highest number of meiofaunal groups (n = 10) was reported at St. C1, suggesting the presence of a more structured community at the reference station. Accounting for more than 60% of the community, nematodes were the dominant meiofaunal group at all stations. These organisms are known to survive under severe contamination. Some taxa, such as Theristus, Terschellingia and Desmodora, were reported to tolerate extremely high concentrations of heavy metals, PAHs and PCBs. In fact, nematodes were reported as almost the only representatives of meiofauna in the sediments of the Mar Piccolo of Taranto, where the levels of Hg and PCBs were higher than those of the SIN of Trieste (Cibic et al., 2016a; Franzo et al., 2016a). The resistance of nematodes to such inhospitable environments allows meiofauna to keep its ecological role within the trophic web, i.e., to convey C and energy from the detritus to the higher trophic levels. Although the meiofaunal community in the SIN was likely not well-structured, our results indicate, to some extent, the presence of an active community at the middle level of the trophic web and responsible for the transfer of C to macrofauna.
Macrofaunal development in the port area was influenced by bathymetry and sediment grain size. Indeed, among the physical variables, grain size is considered one of the most important factors influencing the colonization of sediments by benthic organisms at different trophic levels, from microphytobenthos (Round et al., 1992) to meiofauna (Balsamo et al., 2010) and macrofauna (Solis-Weiss et al., 2004). Within the port area, St. 6 differed from the other stations primarily by its reduced depth (8.5 m) and a higher percentage of sand (about 40%) that favored high macrofaunal abundances. Besides these physical features, the different levels of contamination among stations affected the species distributional patterns also, as revealed by the nMDS and SIMPROF analysis that separated the most contaminated St. 2 and St. 3 from the other stations. Indeed, moving from the external boundaries of the SIN toward St. 2 and St. 3 (i.e., stations closer to the main sources of pollution), the macrozoobenthic community responded to the increasing stress conditions with lower biodiversity and the presence of stress-tolerant species, such as the polychaetes Marphysa sanguinea, Lumbrineris latreilli and the bivalves Corbula gibba, Abra alba and Tellina distorta. Simboura and Zenetos (2002) related the presence of these particular invertebrates to an opportunistic behavior. These results are in accordance with previous studies carried out in the industrial area of the port of Trieste (Ghirardelli et al., 1973; Solis-Weiss et al., 2004). In contrast, the sandier site (St. 6) and the shallower ones (St. 4 and St. 5) were characterized by the highest abundances and diversity. The high biodiversity observed at St.4 (petroleum macroarea) could be related to the spatial complexity of sediments, resulting in additional ecological niches, in accordance with the findings of Ergen et al. (2007). Indeed, due to the occurrence of pebbles at this site, both muddy-bottom species (the polychaete Vermiliopsis striaticeps) and typically hard-substrate species (e.g., the bivalve Hiatella arctica and the polychaete Serpula vermicularis) were found. Furthermore, at St. C1 and at St. 6, two indicator species of environmental stability were observed: the echinoidea Echinocardium cordatum and Ova canaliferus (Clarke et al., 2014).
High numbers of deposit feeders were observed particularly at St. 1 and St. 2 that could be related to the high microphytobenthic densities at these stations. According to Checon et al. (2016), diatoms are an important component of benthic food webs due to their high assimilation and low gut residence. Benthic diatoms, in particular, are the most palatable food for deposit feeder polychaetes. In the guts of most polychaetes, the authors observed, among others, diatom specimens belonging to Navicula and Nitzschia that were also the dominant genera in our study, particularly at St. 2 and 3. The amount of lipids (e.g., triglycerides and long fatty acids chains) in the microalgal cells renders them very palatable to deposit feeder invertebrates (Goedkoop et al., 2011). This indicates the importance of local benthic primary production in the trophic web at these stations. Among predators, high abundances of Hilbigneris gracilis and Eunice vittata were observed at St. 4 and St. 6, in correspondence with high meiofaunal densities. Predators generally prey on small invertebrates and a potential top-down control on meiofauna is well-known (Van Colen et al., 2015). The polychaetes observed at these two stations belong to the families Lumbrineridae and Eunicidae, respectively, which have paired mandibles and complex sets of maxillae in a strongly muscular and eversible pharynx and are able crawlers and borrowers in muddy sediments (Jumars et al., 2015). These features render them very effective meiofauna predators. The low number of predators observed at the most contaminated sites of the port, i.e., St. 2 and St. 3, are in contrast with the findings of Franzo et al. (2016a). These authors reported high numbers of predators at the severely polluted site in the Mar Piccolo of Taranto, where mobile organisms (as predators) likely survived due to their ability to avoid hotspots of contaminants through their active movements (Ward et al., 2013). Overall, despite the presence of macrofaunal stress-tolerant species (e.g., A. alba, C. gibba, and L. latreilli), environmental contamination within the SIN of Trieste does not seem to be so severe as to affect the macrofaunal feeding structure.
As regards decomposer representatives, in this study we focused on the bacterial community capable of exploiting specific C sources, i.e., n-Hexadecane and petroleum. The presence of bacteria capable of degrading n-Hexadecane and petroleum, especially at St. 2 and 3, suggests that contaminated sediments harbor consortia of bacteria that are highly specialized in exploiting non-readily-available sources of C in the form of hydrocarbons. These bacteria were also detected off Muggia (St. 5 and 6), although in lower numbers, indicating that the ability of the bacterial community to degrade hydrocarbons was widespread in the entire area. Our findings are consistent with Cibic et al. (2012b), who reported the presence of a specialized bacterial community inhabiting sediments in a channel characterized by industrial contamination, such as the SIN of Trieste. The higher abundances of both petroleum and n-Hexadecane degrading bacteria at the most contaminated stations of the current study and those observed during a case study carried out by Cibic et al. (2012b) indicate that, overall, benthic ecosystems can adapt to pollution by developing stress-resistant communities able to occupy new ecological niches. The freshly produced C is conveyed, via bacterial biomass, to higher trophic levels, establishing highly specialized food webs at such contaminated sites.
Benthic Processes and Ecosystem Functioning
Ecosystem functioning is a general concept that refers, in essence, to the overall performance of ecosystems. Describing or measuring ecosystem functioning is difficult as it encompasses a number of phenomena; the overall functioning of an ecosystem is complex and involves many factors related to the chemical, physical and biological components of the system. No single parameter can be used to describe the functioning of entire ecosystems. Thus, consideration of multiple variables may be the most appropriate way to shed light on the concept (Bremner, 2008 and references therein). Studies on benthic ecosystem functioning that consider both the main autotrophic and heterotrophic pathways are still very rare in the literature (Cibic et al., 2012b, 2016a; Franzo et al., 2016b). Most of them, in fact, investigate the autotrophic side of ecosystem functioning or its heterotrophic counterpart. In this study, the synoptic investigation of both pathways allowed to elucidate their reciprocal interactions and how their combination resulted in the observed ecosystem functioning.
Ecosystem functioning encompasses several processes such as organic matter production, decomposition, consumption and C transfer to higher trophic levels. Regarding the process of phototrophic C fixation, the main driver of the productivity of the microalgal community inhabiting surface sediments is light availability at the bottom. In fact, GPP rates were lower at the deeper stations (7, 1, C1) and higher at the shallowest sites. Indeed, at St. 6, the microalgal community was extremely active, as also indicated by oxygen oversaturation at the bottom layer (107.1%). Overall, GPP rates did not seem to be affected by contamination levels since they were comparable to those estimated at the reference site. This further indicates that benthic microalgae were able to adapt to stress induced by high levels of chemical compounds accumulated in these sediments. In contrast, Rubino et al. (2016) estimated extremely low primary production rates (close to zero) in a shallow area of the Ionian Sea severely contaminated by PCBs and heavy metals. The authors infer that the synergistic effect of the contaminants was such as to interfere with the proper functioning of certain benthic processes, i.e., microalgal photosynthetic capability. Interestingly, in another contaminated area located in a riverine-lagoonal system, Cibic et al. (2012b) reported GPP values that were almost three times higher than those found in our port area. In the latter study, the high contamination levels seemed to strongly affect the meiofaunal and macrofaunal numbers rather than microalgal density and, due to the consequent low grazing pressure, a very abundant and highly productive microalgal community was found.
The trophic state of the system varied noticeably according to the site: it was net autotrophic offshore Muggia (St. 5 and 6) and net heterotrophic at the other sites, where oxygen consumption prevailed over primary production. Again, the trophic state was primarily influenced by depth, since positive values were observed only at the shallower stations (depth< 11.5 m) where the microalgal assemblages were photosynthetically active. At the other sites of the SIN, the values ranged between −2.76 mg C m−2 h−1 at St.1 and −5.37 mg C m−2 h−1 at St. 3. In the iron foundry area, in particular, the high oxygen uptake was probably due to the degradation of the accumulated synthetic organic substances. However, the minimum (as absolute number) observed in the iron foundry area was still two times lower than that previously reported for a riverine-lagoonal system that was strongly heterotrophic (−12.00 mg C m−2 h−1) (Cibic et al., 2012b), where the major oxygen demand was due to degradation of the high organic load. We further compared our values to those reported from the severely contaminated Mar Piccolo of Taranto, where the trophic state of the benthic system was net heterotrophic at all sites and during both seasons (April and June), with values ranging from −3.62 mg Cm−2 h−1 to −8.17 mg C m−2 h−1 (Rubino et al., 2016).
Prokaryotic C conversion efficiency is the ratio between the Prokaryotic C Production (PCP) and the amount of C degraded enzymatically, expressed as a percentage (Danovaro and Pusceddu, 2007). Since it combines, in a direct way, two key aspects of benthic ecosystem functioning (i.e., the organic matter re-cycling and its incorporation into new living biomass at the lower level of the trophic web), it can be considered as a proxy of ecosystem functioning, at least for these pivotal aspects. In the SIN of Trieste, higher efficiencies were calculated at St. 1, 2, and 3, with a maximum of 5.96% in the port area, while the sites within the residential area/center of the bay and St. C1 showing efficiencies of <4%. St. 6 (5.76%), represented an exception due to limited depth and coarser sediments (Figure 8). Our results are comparable with the findings of a study carried out in a riverine-lagoonal system severely contaminated by PAHs and Hg (Cibic et al., 2012b). The authors reported a more efficient microbial community at the most contaminated site, the Banduzzi channel, with a prokaryotic C conversion efficiency of 6.21%. Our results from the SIN of Trieste confirm that contaminated sediments can harbor a very active microbial community. Such organisms can be autotrophic, as indicated by high abundances of microalgae, and heterotrophic, as pointed out by the presence of specialized consortia of bacteria that are able to use and tolerate toxic compounds. The fresh organic material derived from microalgal proliferation fuels prokaryotic processes of mineralization and C incorporation, and represents a food source for meio- and macrofauna. At another site of the Gulf of Trieste, a shift of the benthic ecosystem from a “source system” during summer to a “detritus sink system” during winter was documented (Franzo et al., 2016b). The variable proliferation of microalgae was identified as one of the main drivers responsible for such change. Our results, obtained in early summer, confirmed the pivotal role of these organisms in the ecosystem functioning of the SIN. The active microbial community observed even at the most contaminated sites increases the nutritional value of the sediments masking, consequently, the potential negative effects of contamination on meio- and macrofauna. Thanks to the presence of tolerant taxa, such as nematodes, the system appeared to be adapted to the anthropogenic stressors persisting in the area. There is a flow of C and energy both within a consolidated microbial loop and, although not well-structured, even toward the higher trophic levels. This was confirmed by the well-balanced structure of macrofaunal feeding habits.
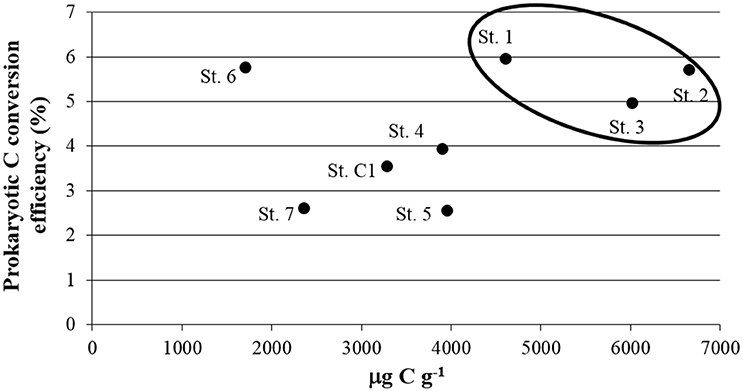
Figure 8. Relationship between Biopolymeric C and Prokaryotic C conversion efficiency, determined as the ratio of Prokaryotic C Production and the amount of C degraded enzymatically and expressed as a percentage (as proposed by Danovaro and Pusceddu, 2007).
This study is an example of “the ecosystem approach to management” and has been carried out in order to provide practical support to the decision-makers responsible for managing the port area and assessing whether or not to exclude a part of the bay from the SIN of Trieste. Benthic ecosystem functioning appeared to vary among sites characterized by diversified industrialization and anthropization levels. Our results indicate that along the north-eastern boundary of the SIN (St. 1-port area; St. 2-shipbuilding area; St. 3-iron foundry area) the sediments are characterized by high levels of contaminants, low macrozoobenthic diversity, major TOC and BPC contents. Overall, at these stations, oxygen consumption prevailed over primary production, and the trophic state was net heterotrophic. Therefore, according to our results, they are rightly included in the SIN of Trieste. Nevertheless, the higher numbers of hydrocarbon degrading bacteria and major Prokaryotic C conversion efficiency suggest that the sediments are colonized by an active and specialized microbial community potentially capable of exploiting other sources of C, such as different hydrocarbons that have accumulated at these sites. Moreover, despite the presence of macrofaunal stress-tolerant species (e.g., Abra alba, Corbula gibba, and Lumbrineris latreilli), overall environmental contamination of these three macrosites does not appear to be severe enough to affect the macrofaunal feeding structure and the total meiofaunal and microphytobenthos numbers. In contrast, at the other side of the harbor (St. 5, 6, and 7-residential area/center bay), contamination levels were below the legal limits and both the microalgal and macrobenthic communities were more biodiverse and/or abundant. Primary production and exoenzymatic activities showed higher rates and an overall better environmental status. Therefore, according to our results, this part of the SIN does not necessarily have to be included in the SIN.
However, before providing guidance to stakeholders in this direction, further sampling is required along a finer sampling grid in the less contaminated side of the port in order to confirm these initial results. Moreover, the same approach should be temporally replicated with further sampling to detect seasonal and interannual variability, which will provide a baseline for the assessment of the benthic ecosystem over time for the entire SIN. Although some of the considered variables did not appear to be clearly affected by contamination (i.e., GPP or the macrofaunal feeding structure), they should still be included in future sampling design, since these parameters proved to be sensitive to pollution at other severely contaminated sites.
The “ecosystem approach to management” applied in this study is quite expensive and requires specific skills and highly qualified staff; it is not suitable for routine sampling, i.e., traditional monitoring. However, we argue that it is a useful and essential tool for the management of marine space or for planning environmental interventions that have societal consequences in highly urbanized areas. This ecosystem approach can be exported and applied to other port areas and contaminated sites to support decision-makers. We believe that this novel approach could be included in the management techniques used for the zoning of harbors, and contaminated areas in general, an environmental issue of widespread importance.
Conclusions
The assessment of benthic ecosystem functioning performed in this study provided useful information to support decision-makers in the management of marine space within the SIN. The identification of the main drivers that influence the functioning of the system and an understanding of how such drivers act, not only helped the Port Authority to carry out the environmental assessment but it could also prove valuable in addressing future management of the marine space. Although our results derive from a single harbor and, therefore, are necessarily case-specific, our work could offer insights to scientists and stakeholders as regards the management of harbors and contaminated areas in general.
Author Contributions
TC: Project coordinator, manuscript preparation, data production and interpretation, statistical analysis. AF: Manuscript preparation, data production and interpretation, statistical analysis. FN: Field sampling, manuscript preparation, data production and interpretation. RA: data production and interpretation, statistical analysis, manuscript preparation. PD: Project leader, data interpretation, manuscript preparation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The activities described in this publication were funded by the Project Bandiera RITMARE—La Ricerca Italiana per il Mare coordinated by the National Research Council and funded by the Ministry for Education, University and Research within the National Research Programme 2011-2013. We are very grateful to C. Comici for grain-size and TOC analyses, C. De Vittor for BPC and pigment data, C. Fabbro for functional bacterial group estimates and M. Rogelja for the map of the port area. We also wish to thank F. Varisco for contaminant data.
Footnotes
1. ^http://www.porto.trieste.it. Website of the Port Authority of Trieste. Visited October 2016.
References
Alexander, M. (1965). “Most-probable-number method for microbial populations,” in Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, ed A. G. Norman (Madison, WI: American Society of Agronomy; Soil Science Society of America), 1467–1472.
Balsamo, M., Albertelli, G., Ceccherelli, V. U., Coccioni, R., Colangelo, M. A., Curini-Galletti, M., et al. (2010). Meiofauna of the Adriatic Sea: present knowledge and future perspectives. Chem. Ecol. 26, 45–63. doi: 10.1080/02757541003705492
Berg, P., Risgaard-Petersen, N., and Rysgaard, S. (1998). Interpretation of measured concentration profiles in sediment pore water. Limnol. Oceanogr. 43, 1500–1510. doi: 10.4319/lo.1998.43.7.1500
Blasutto, O., Cibic, T., De Vittor, C., and Fonda Umani, S. (2005). Microphytobenthic primary production and sedimentary carbohydrates along salinity gradients in the lagoons of grado and marano (Northern Adriatic Sea). Hydrobiologia 550, 47–55. doi: 10.1007/s10750-005-4361-5
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/o59-099
Boero, F., and Bonsdorff, E. (2007). A conceptual framework for marine biodiversity and ecosystem functioning. Mar. Ecol. 28, 134–145. doi: 10.1111/j.1439-0485.2007.00171.x
Brambati, A., and Catani, G. (1988). Le coste e i fondali del Golfo di Trieste dall'Isonzo a Punta Sottile: aspetti geologici, geomorfologici, sedimentologici e geotecnici. Hydrores Information 5, 13–28.
Bremner, J. (2008). Species' traits and ecological functioning in marine conservation and management. J. Exp. Mar. Biol. Ecol. 366, 37–47. doi: 10.1016/j.jembe.2008.07.007
Buruaem, L. M., Hortellani, M. A., Sarkis, J. E., Costa-Lotufo, L. V., and Abessa, D. M. S. (2012). Contamination of port zone sediments by metals from Large Marine Ecosystems of Brazil. Mar. Pollut. Bull. 64, 479–488. doi: 10.1016/j.marpolbul.2012.01.017
Bushnell, L. D., and Haas, H. F. (1941). The utilization of certain hydrocarbons by microorganisms. J. Bacteriol. 41:653.
Cardin, V., and Celio, M. (1997). Cluster analysis as a statistical method for identification of the water bodies present in the Gulf of Trieste (Northern Adriatic Sea). Bollettino di Geofisica Teorica ed Applicata 38, 119–135.
Checon, H. H., Pardo, E. V., and Zacagnini Amaral, A. C. (2016). Breadth and composition of polychaete diets and the importance of diatoms to species and trophic guilds. Helgol. Mar. Res. 70:19. doi: 10.1186/s10152-016-0469-4. [Epub ahead of print].
Cibic, T., Blasutto, O., and Bettoso, N. (2009). Microalgal-meiofaunal interactions in a sublittoral site of the Gulf of Trieste (northern Adriatic Sea, Italy): a three-year study. J. Exp. Mar. Biol. Ecol. 370, 144–154. doi: 10.1016/j.jembe.2008.12.006
Cibic, T., Blasutto, O., Burba, N., and Fonda Umani, S. (2008). Microphytobenthic primary production as 14C uptake in sublittoral sediments of the Gulf of Trieste (northern Adriatic Sea): methodological aspects and data analyses. Estuar. Coast. Shelf S. 77, 113–122. doi: 10.1016/j.ecss.2007.09.005
Cibic, T., Blasutto, O., and Fonda Umani, S. (2007a). Biodiversity of settled material in a sediment trap in the Gulf of Trieste (northern Adriatic Sea). Hydrobiologia 580, 57–75. doi: 10.1007/s10750-006-0465-9
Cibic, T., Blasutto, O., Hancke, K., and Johnsen, G. (2007b). Microphytobenthic species composition, pigment concentration, and primary production in sublittoral sediments of the Trondheimsfjord. J. Phycol. 43, 1126–1137. doi: 10.1111/j.1529-8817.2007.00405.x
Cibic, T., Bongiorni, L., Borfecchia, F., Di Leo, A., Franzo, A., Giandomenico, S., et al. (2016a). Ecosystem functioning approach applied to a large contaminated coastal site: the study case of the Mar Piccolo of Taranto (Ionian Sea). Environ. Sci. Pollut. Res. 23, 12739–12754. doi: 10.1007/s11356-015-4997-2
Cibic, T., Comici, C., Bussani, A., and Del Negro, P. (2012a). Benthic diatom response to changing environmental conditions. Estuar. Coast. Shelf S. 115, 158–169. doi: 10.1016/j.ecss.2012.03.033
Cibic, T., Franzo, A., Celussi, M., Fabbro, C., and Del Negro, P. (2012b). Benthic ecosystem functioning in hydrocarbon and heavy-metal contaminated sediments of an Adriatic lagoon. Mar. Ecol. Prog. Ser. 458, 69–87. doi: 10.3354/meps09741
Cibic, T., Rogelja, M., Querin, S., Segarich, M., and Del Negro, P. (2016b). Microphytobenthic community development under different hydrodynamic conditions nearby the rocky outcrops of the northern Adriatic Sea. Biol. Mar. Mediterr. 23, 174–177.
Cibic, T., and Virgilio, D. (2010). Different fixatives and chloridric acid concentrations in microphytobenthic primary production estimates using radiolabeled carbon: their use and misuse. Limnol. Oceanogr. Meth. 8, 453–461. doi: 10.4319/lom.2010.8.453
Clarke, K. R., Gorley, R. N., Somerfield, P. J., and Warwick, R. M. (2014). Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. Plymouth, UK: PRIMER-E Ltd.
Danovaro, R., Gambi, C., Mirto, S., Sandulli, R., and Cecchierelli, V. (2004). “Meiofauna,” in Mediterranean Marine Benthos: A Manual of Methods for its Samplings and Study, eds M. C. Gambi and M. Dappiano (Genova), 55–97.
Danovaro, R., and Pusceddu, A. (2007). Biodiversity and ecosystem functioning in coastal lagoons: does microbial diversity play any role? Estuar. Coast. Shelf S. 75, 4–12. doi: 10.1016/j.ecss.2007.02.030
Ergen, Z., Çinar, M. E., Dagli, E., and Kurt, G. (2007). “Seasonal dynamics of soft-bottom polychaetes in Izimir Bay (Aegean Sea, eastern Meditterranean),” in Scientific Advances in Polychaete Research, eds R. Sarda, G. San Martin, E. Lopez, D. Martin, and D. George (Barcelona), 197–207.
Fichez, R. (1991). Composition and fate of organic-matter in submarine cave sediments-implications for the biogeochemical cycle of organic-carbon. Oceanol. Acta 14, 369–377.
Forster, R. M., Créach, V., Sabbe, K., Vyverman, W., and Stal, L. J. (2006). Biodiversity-ecosystem function relationship in microphytobenthic diatoms of the Westerschelde estuary. Mar. Ecol. Prog. Ser. 311, 191–201. doi: 10.3354/meps311191
Franzo, A., Auriemma, R., Nasi, F., Vojvoda, J., Pallavicini, A., Cibic, T., et al. (2016a). Benthic ecosystem functioning in the severely contaminated Mar Piccolo of Taranto (Ionian Sea, Italy): focus on heterotrophic pathways. Environ. Sci. Pollut. Res. 23, 12645–12661. doi: 10.1007/s11356-015-5339-0
Franzo, A., Cibic, T., and Del Negro, P. (2016b). Integrated approach for the assessment of the benthic ecosystem functioning at a coastal site in the northern Adriatic Sea. Cont. Shelf Res. 121, 35–47. doi: 10.1016/j.csr.2015.12.005
Franzo, A., Cibic, T., Del Negro, P., and De Vittor, C. (2015). Spatial distribution of microphytobenthos, meiofauna and macrofauna in the north-western Adriatic Sea: a synoptic study. Adv. Oceanogr. Limnol. 6, 58–75. doi: 10.4081/aiol.2015.5470
Gazeau, F., Smith, S. V., Gentili, B., Frankignoulle, M., and Gattuso, J. P. (2004). The European coastal zone: characterization and first assessment of ecosystem metabolism. Estuar. Coast. Shelf Sci. 60, 673–694. doi: 10.1016/j.ecss.2004.03.007
Ghirardelli, E., Orel, G., and Giaccone, G. (1973). L'inquinamento del Golfo di Trieste. Atti Mus. Civ. Stor. Nat. 28, 431–450.
Ghirardelli, E., and Pignatti, S. (1968). Conséquences de la pollution sur le peuplements du ‘Vallone de Muggia’ pre's de Trieste. Rev. Intern. Oceanogr. Med. 10, 111–122.
Goedkoop, W., Sonesten, L., Ahlgren, G., and Boberg, M. (2011). Fatty acids in profundal benthic invertebrates and their major food resources in Lake Erken, Sweden: seasonal variation and trophic indications. Can. J. Fish. Aquatic Sci. 57, 2267–2279. doi: 10.1139/f00-201
Hartree, E. F. (1972). Determination of protein: a modification of the lowry method that gives a linear photometric response. Anal. Biochem. 48, 422–427. doi: 10.1016/0003-2697(72)90094-2
Higgins, R. P., and Thiel, H. (1988). Introduction to the Study of Meiofauna. London: Smithsonian Institution Press.
Hoppe, H.-G. (1993). “Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria,” in Current Methods in Aquatic Microbial Ecology, eds P. F. Kemp, B. F. Sherr, E. B. Sherr, J. J. Cole (Boca Raton, FL: CRC Press), 423–431.
Johnsen, A. R., Bendixen, K., and Karlson, U. (2002). Detection of microbial growth on polycyclic aromatic hydrocarbons in microtiter plates by using the respiration indicator WST-1. Appl. Environ. Microbiol. 68, 2683–2689. doi: 10.1128/AEM.68.6.2683-2689.2002
Jumars, P. A., Dorgan, K. M., and Lindsay, S. M. (2015). Diet of worms emended: an update of polychaete feeding guilds. Annu. Rev. Mar. Sci. 7, 497–520. doi: 10.1146/annurev-marine-010814-020007
Katsanevakis, S., Stelzenmüller, V., South, A., Sørensen, T. K., Jones, P. J. S., Kerr, S., et al. (2011). Ecosystem-based marine spatial management: review of concepts, policies, tools, and critical issues. Ocean Coast. Manage. 54, 807–820. doi: 10.1016/j.ocecoaman.2011.09.002
Lorenzen, C., and Jeffrey, S. (1980). Determination of chlorophyll in seawater. Unesco Tech. Pap. Mar. Sci 35, 1–20.
Manini, E., Luna, G. M., and Danovaro, R. (2004). Benthic bacterial response to variable estuarine water inputs. FEMS Microbiol. Ecol. 50, 185–194. doi: 10.1016/j.femsec.2004.06.011
Morri, C., Bellan-Santini, D., Giaccone, G., and Bianchi, C. (2004). Principles of bionomy: definition of assemblages and use of taxonomic descriptors (macrobenthos). Biol. Mar. Medit. 11, 573–600.
Nasi, F., Auriemma, R., Bonsdorff, E., Cibic, T., Aleffi, I. F., Bettoso, N., et al. (2017). Biodiversity, feeding habits and reproductive strategies of benthic macrofauna in a protected area of the northern Adriatic Sea: a three-year study. Med. Mar. Sci. 18, 292–309. doi: 10.12681/mms.1897
Nieuwenhuize, J., Maas, Y. E. M., and Middelburg, J. J. (1994). Rapid analysis of organic carbon and nitrogen in particulate materials. Mar. Chem. 45, 217–224. doi: 10.1016/0304-4203(94)90005-1
Ogorelec, B., Mišič, M., and Faganeli, J. (1991). Marine geology of the Gulf of Trieste (northern Adriatic): sedimentological aspects. Mar. Geol. 99, 79–92. doi: 10.1016/0025-3227(91)90084-H
Ottersen, G., Olsen, E., van der Meeren, G. I., Dommasnes, A., and Loeng, H. (2011). The Norwegian plan for integrated ecosystem-based management of the marine environment in the Norwegian Sea. Mar. Policy 35, 389–398. doi: 10.1016/j.marpol.2010.10.017
Pella, E., and Colombo, B. (1973). Study of carbon, hydrogen and nitrogen determination by combustion-gas chromatography. Microchim. Acta 61, 697–719. doi: 10.1007/BF01218130
Pielou, E. (1966). Shannon's formula as a measure of specific diversity: its use and misuse. Am. Nat. 118, 463–465. doi: 10.1086/282439
Potapova, M., Desianti, N., and Enache, M. (2016). Potential effects of sediment contaminants on diatom assemblages in coastal lagoons of New Jersey and New York States. Mar. Pollut. Bull. 107, 453–458 doi: 10.1016/j.marpolbul.2016.01.028
Pusceddu, A., Gambi, C., Corinaldesi, C., Scopa, M., and Danovaro, R. (2014). Relationships between meiofaunal biodiversity and prokaryotic heterotrophic production in different tropical habitats and oceanic regions. PLoS ONE 9:e91056. doi: 10.1371/journal.pone.0091056
Rees, H. L., Moore, D. C., Pearson, T. H., Elliot, M., Service, M., Pomfret, J., et al. (1990). Procedures for the Monitoring of Marine Benthic Communities at UK Sewage Sludge Disposal Sites. Edinburgh: Department of Agriculture and Fisheries for Scotland. Scottish Fisheries Information Pamphlet No.18, 79.
Revsbech, N. P. (1989). An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 34, 474–478. doi: 10.4319/lo.1989.34.2.0474
Rogelja, M., Cibic, T., Rubino, F., Belmonte, M., and Del Negro, P. (2017). Active and resting microbenthos in differently contaminated marine coastal areas: insights from the Gulf of Trieste (northern Adriatic, Mediterranean Sea). Hydrobiologia. doi: 10.1007/s10750-017-3366-1
Roth, S., and Wilson, J. G. (1998). Functional analysis by trophic guilds of macrobenthic community structure in Dublin Bay, Ireland. J. Exp. Mar. Biol. Ecol. 222, 195–217. doi: 10.1016/S0022-0981(97)00145-7
Round, F. E., Crawford, R. M., and Mann, D. G. (1992). The Diatoms. Cambridge: Cambridge University Press.
Rubino, F., Cibic, T., Belmonte, M., and Rogelja, M. (2016). Microbenthic community structure and trophic status of sediments in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea). Environ. Sci. Pollut. Res. 23, 12624–12644. doi: 10.1007/s11356-015-5526-z
Schaffner, L. C., Anderson, I. C., Stanhope, J. W., Gillett, D. J., Metcalfe, W. J., and Brylawski, A. M. Z. (2008). An Integrated Approach to Understand Relationships Between Shallow Water Benthic Community Structure and Ecosystem Function. Department of Defense Strategic Environmental Research and Development Program SERDP Project SI-1335.
Shannon, C. E., and Weaver, W. (1949). The Mathematical Theory of Communication. Urbana, IL: University of Illinois Press IL.
Sharp, J. H. (1974). Improved analysis for particulate organic carbon and nitrogen from seawater. Limnol. Oceanogr. 19, 984–989. doi: 10.4319/lo.1974.19.6.0984
Simboura, N., and Zenetos, A. (2002). Benthic indicators to use in Ecological Quality classification of Mediterranean soft bottom marine ecosystems, including a new Biotic Index. Medit. Mar. Sci. 3/2, 77–111. doi: 10.12681/mms.249
Solis-Weiss, V., Aleffi, F., Bettoso, N., Rossin, P., Orel, G., and Fonda-Umani, S. (2004). Effects of industrial and urban pollution on the benthic macrofauna in the Bay of Muggia (industrial port of Trieste, Italy). Sci. Total Environ. 328, 247–263. doi: 10.1016/j.scitotenv.2004.01.027
Solis-Weiss, V., Rossin, P., Aleffi, F., Bettoso, N., Orel, G., and Vrišer, B. (2001). “Gulf of Trieste: sensitivity areas using benthos and GIS techniques” in Proceedings of the Fifth International Conference on the Mediterranean Coastal Environment, MEDCOAST 01, ed E. Özhan (Ankara), 1567–1578.
Steemann-Nielsen, E. (1952). The use of radioactive carbon (14C) for measuring organic production in the sea. J. Conserv. Int. Explor. Merid. 16, 117–140. doi: 10.1093/icesjms/18.2.117
Sundbäck, K., Linares, F., Larson, F., and Wulff, A. (2004). Benthic nitrogen fluxes along a depth gradient in a microtidal fjord: the role of denitrification and microphytobenthos. Limnol. Oceanogr. 49, 1095–1107. doi: 10.4319/lo.2004.49.4.1095
Sundbäck, K., Petersen, D. G., Dahllöf, I., and Larson, F. (2007). Combined nutrient-toxicant effects on a shallow-water marine sediment system: sensitivity and resilience of ecosystem functions. Mar. Ecol. Prog. Ser. 330, 13–30. doi: 10.3354/meps330013
Sweetman, A. K., Norling, K., Gunderstad, C., Haugland, B. T., and Dale, T. (2014). Benthic ecosystem functioning beneath fish farms in different hydrodynamic environments. Limnol. Oceanogr. 59, 1139–1151. doi: 10.4319/lo.2014.59.4.1139
Van Colen, C., Thrush, S. F., Parkes, S., Harris, R., Woodin, S. A., et al. (2015). Bottom–up and top–down mechanisms indirectly mediate interactions between benthic biotic ecosystem components. J. Sea Res. 98, 42–48. doi: 10.1016/j.seares.2014.10.016
van Duyl, F. C., and Kop, A. J. (1994). Bacterial production in North Sea sediments: clues to seasonal and spatial variations. Mar. Biol. 120, 323–337. doi: 10.1007/BF00349694
Keywords: benthic ecosystem functioning, microphytobenthos, meiofauna, macrozoobenthos, benthic primary production, prokaryotic heterotrophic production, extracellular enzymatic activities, trophic state
Citation: Cibic T, Franzo A, Nasi F, Auriemma R and Del Negro P (2017) The Port of Trieste (Northern Adriatic Sea)—A Case Study of the “Ecosystem Approach to Management”. Front. Mar. Sci. 4:336. doi: 10.3389/fmars.2017.00336
Received: 29 November 2016; Accepted: 12 October 2017;
Published: 27 October 2017.
Edited by:
Bernardo Duarte, Centro de Ciências do Mar e do Ambiente (MARE), PortugalReviewed by:
J. German Rodriguez, AZTI-Tecnalia, SpainFelix Janssen, Alfred Wegener Institut Helmholtz Zentrum für Polar und Meeresforschung (AWI), Germany
Copyright © 2017 Cibic, Franzo, Nasi, Auriemma and Del Negro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamara Cibic, dGNpYmljQGlub2dzLml0
 Tamara Cibic
Tamara Cibic Annalisa Franzo
Annalisa Franzo Paola Del Negro
Paola Del Negro