
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 20 September 2017
Sec. Coral Reef Research
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00305
This article is part of the Research Topic The Future of Coral Reefs Subject to Rapid Climate Change: Lessons from Natural Extreme Environments View all 16 articles
Larval supply is a principal factor determining the establishment, structure, and diversity of sessile benthic assemblages on coral reefs. Benthic reef communities in north-eastern Arabia have been subject to recurrent disturbances in recent years, and subsequent recovery will be, in part, driven by variation in the supply of available colonists. Using settlement tiles deployed seasonally over 1 year at eight sites encompassing three environmentally divergent regions (southern Arabian Gulf, the Musandam Peninsula in the Strait of Hormuz, and the Sea of Oman) we assessed spatial and seasonal variability in settlement of benthic reef organisms. There was strong spatial variation in composition of new colonists among regions, mainly driven by the high abundance of coralline algae in the Arabian Gulf, colonial ascidians on the Musandam Peninsula and barnacles in the Sea of Oman. Seasonal differences in composition of new colonists were less important than regional differences, with seasonal variation in settlement not consistent among regions. The number of corals settling to the tiles was low compared to those reported for other regions, with mean densities ranging from 0 corals m−2 year−1 in the Sea of Oman to 30 (± 0.6 SE) and 38 (± 0.5 SE) in Musandam and the Arabian Gulf, respectively. Peak coral settlement abundance in the Gulf occurred in summer and autumn and in Musandam in spring (averaging 82 and 70 settlers m−2 year−1, respectively, during the peak settlement season). This work provides the first record of large-scale spatial and seasonal patterns of settlement in north-eastern Arabia and provides valuable information on the supply of settlers available to recolonize heavily disturbed reefs in this region. The extremely low rates of coral settlement suggest that these marginal reefs are likely to be extremely slow to recover from on-going and future disturbances.
Coral reefs provide a variety of significant socio-economic benefits to coastal communities (Ferrario et al., 2014; Guannel et al., 2016), however, climate change and increasing local pressures are jeopardizing the future of reefs (Ateweberhan et al., 2013; Bruno, 2013; Spalding and Brown, 2015). Over the past several decades, reefs have been increasingly degraded by overfishing, pollution, sedimentation, disease and coral predator outbreaks (Maina et al., 2013; Riegl et al., 2013; Pollock et al., 2014; Wear and Thurber, 2015; Mumby, 2016). These stressors have resulted in an estimated loss of up to 50% of coral cover from many reefs in the past several decades (De'ath et al., 2012; Hughes et al., 2017), with a third of reef-building coral species now considered at risk of extinction (Carpenter et al., 2008).
Disturbances such as bleaching, cyclones and crown of thorns outbreaks that cause large-scale coral mortality are becoming increasingly common (Spalding and Brown, 2015). Whether reefs can recover from these disturbances will depend, in part, on the supply of colonists available to settle to newly opened space that appears on reefs following a disturbance (Gilmour et al., 2013). Both stochastic processes which affect the supply of potential colonists and deterministic processes such as habitat availability, larval preferences, and interactions with existing community members, can influence settlement patterns (Lillis et al., 2016). Post-settlement processes then further shape subsequent community development (Caley et al., 1996). As a result, the trajectory of community recovery following disturbance is highly dynamic, with some studies reporting a relatively rapid return to pre-disturbance assemblage structure (Halford et al., 2004; Adjeroud et al., 2009), while others have observed dramatic and long-term shifts (Roff et al., 2015; Guest et al., 2016).
Historically, studies of the role of supply in recovery dynamics have focused on coral recruitment, and have largely overlooked settlement by other members of the benthic community (Zhang et al., 2014). However, algae, sponges, ascidians, and various other sessile members of the benthos are also abundant and integral members of reef communities that support diverse functional roles (Mallela, 2007; Bell, 2008; Glynn and Enochs, 2011; De Goeij et al., 2013; Enochs and Glynn, 2017), although the role that these organisms play in recovery dynamics is not well understood. Non-coral benthos are typically far more abundant than coral spat in early settlement communities (Dunstan and Johnson, 1998; Díaz-Castañeda and Almeda-Jauregui, 1999; Glassom et al., 2004; Mangubhai et al., 2007; Stubler et al., 2016), and initial colonization patterns by these organisms can strongly influence the trajectory of subsequent community development (Stubler et al., 2016). Non-coral settlement patterns may translate into long-term shifts in reef community structure, as variation in the early recruitment rates of non-coral benthos can considerably influence adult abundance of these organisms (Jackson, 1984; Caley et al., 1996; Cowen and Sponaugle, 2009; Zabin, 2015). Further, many non-coral settlers can alternately inhibit or facilitate subsequent colonization by corals (Dunstan and Johnson, 1998; Mangubhai et al., 2007; Birrell et al., 2008; Arnold et al., 2010; Diaz-Pulido et al., 2010). Thus, developing an understanding of the settlement of non-coral benthos can provide valuable insights into the role that recruitment across the wider benthic community may play in affecting the trajectory of initial community development on reefs following disturbance.
Coral reefs in north-eastern Arabia have been subject to widespread and substantial disturbances in recent years. Recurrent bleaching events and disease outbreaks have heavily affected reefs in the southern Persian/Arabian Gulf (hereafter ‘the Gulf’) while reefs in the adjacent Sea of Oman have been impacted by a super-cyclone and a large-scale harmful algal bloom (Bauman et al., 2010; Riegl and Purkis, 2015; Burt et al., 2016). All of these disturbances have caused substantial declines in coral cover and shifts in the composition of the wider benthic community (Bento et al., 2016). Recovery of reef communities in subsequent years has been variable, with a return toward pre-disturbance assemblages observed in some locations but not others (Burt et al., 2008, 2011; Bento et al., 2016). Several studies have suggested that this divergence in recovery patterns can largely be attributed to variation in the abundance and composition of juvenile corals that have recruited to these reefs (Burt et al., 2008; Pratchett et al., 2017). However, it is unclear whether these patterns in juvenile corals (up to 5 cm diameter) were primarily shaped by larval supply or post-settlement processes.
The purpose of this study was to investigate spatial and seasonal variability in settlement of benthic organisms on reefs spanning > 750 km of coastline in the north-eastern Arabian Peninsula. Terra-cotta settlement tiles were deployed seasonally over 1 year on reefs in the highly disturbed Gulf and Sea of Oman, and on relatively undisturbed reefs in the Musandam Peninsula. Comparison of seasonal dynamics provides insights into peak settlement periods for sessile benthic invertebrates, while large-scale spatial comparisons provide insights into regional scale disturbance and recovery dynamics.
This study was performed across eight sites in three regions spanning >750 km of coastline in the north-eastern Arabian Peninsula: three sites in the southern Arabian Gulf (Saadiyat, Dhabiya, and Ras Ghanada), three sites along the Musandam Peninsula (Al Harf, Falcon Rock, and Coral Garden) and two sites in the Sea of Oman (Dibba and Al Aqa) (Figure 1). All sites were fringing reefs at comparable depths (2–8 m) with coral cover varying between ca. 20–60% among reefs. Communities were generally dominated by faviids and poritids in all regions, with other subdominant families differing in relative abundance among sites. A full description of the benthos in each region is provided in Bento et al. (2016).
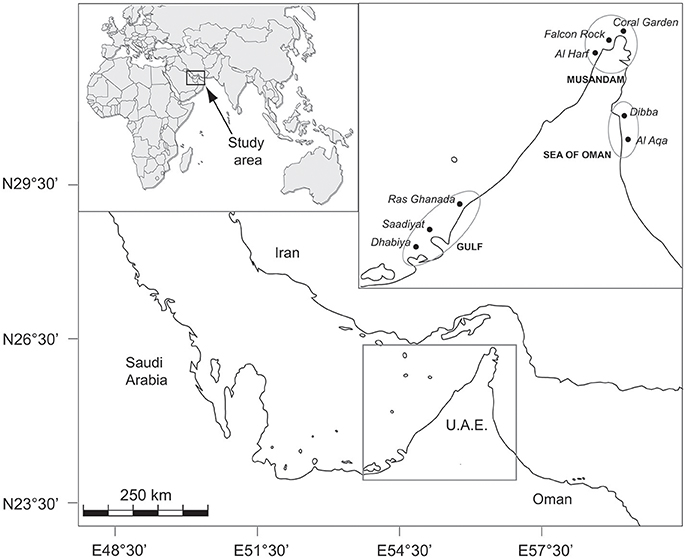
Figure 1. Map of northeastern Arabian Peninsula indicating the location of study sites within each of the three regions.
Settlement of benthic organisms was quantified at all sites using unglazed terracotta settlement tiles (10 × 10 × 1.5 cm) following methods adapted from Mundy (2000). At each site, 30 tiles were attached to the substratum at ca. 5 m depth and spaced 1–3 m apart. Each tile was secured to the reef using a stainless steel stud that was epoxied into the substratum and passed through a 1 cm hole in the center of each tile. A 2 cm plastic washer was placed over each stud to position the tile 2 cm above the reef substratum. The textured (i.e., corrugated) surface of each tile was always positioned facing the substratum, as previous studies have shown that settlement primarily occurs on the underside of tiles and that textured materials generally have higher settlement than smooth surfaces (Burt et al., 2009). To assess seasonal variation in settlement, tiles were deployed and replaced every 3 months over 1 year. Each deployment represented a specific season: (summer: July–September 2012, autumn: October–December 2012, winter: January–March 2013, and spring: April–June 2013), with all tiles deployed/collected across all sites within ca. 7–10 days at the beginning/end of each season. These four seasons were selected based on the periods of highest (summer) and lowest (winter) sea surface temperatures, as well as the transitional spring and autumn periods that are known to be important discrete spawning and/or settlement seasons for a variety of marine fauna in this region (e.g., Bauman et al., 2011; John, 2012; Howells et al., 2014).
Upon retrieval, the bottom of each tile was photographed with a 10 megapixel Nikon D-80 digital camera fitted with a macro lens. Only the bottom surfaces of tiles were analyzed as virtually all settlement in this region occurs on the bottoms of tiles (Burt et al., 2009; Bauman et al., 2014). Percent cover of various benthos on each tile was calculated by image analyses using the software CPC with Excel extension (Kohler and Gill, 2006), with coverage tabulated from 50 random point intercepts per tile. Substratum and benthic community type was categorized into 15 broad groups and several sub-groups: bare tile, coralline algae, coral, ascidiacea (subgroups: colonial ascidian, solitary ascidian), sponge, cnidaria (subgroups: anemone, hydrozoa, zoanthid), arthropoda (subgroup: barnacle), mollusca (subgroups: bivalve, chiton, gastropoda), annelid (subgroup: polychaeta), bryozoa, other live (subgroups: mobile invertebrate, urchin, other), algae (subgroups: algae, turf algae), sand/silt, non-benthos (subgroups: gap, tape, shadow), and unknown taxa. Due to the small size of coral recruits (age: ≤3 mo) and their relatively limited coverage, density of coral juveniles was tabulated separately. Following photography for community analysis, tiles were cleaned of living tissue in bleach for 24 h, rinsed and air dried before tabulating the number of coral recruits on each tile using an Olympus DP-70 stereo-microscope (40X magnification).
Before statistical analyses, percent cover for the various benthic groups was standardized as a proportion of the total living benthos (i.e., relative abundance), and an arcsine-square root transformation was applied to normalize the data, as recommended by Zar (2010). Coral densities were log(x + 1) transformed. Prior to multivariate analyses, benthic categories occurring in <5% of samples were excluded from analyses to avoid the influence of outliers (McCune et al., 2002).
To explore the overall spatial and seasonal structure of settlement assemblages, and to identify which components of the benthos were driving any settlement differences, multivariate analyses were performed using Primer, v6 (Clarke and Gorley, 2006). Non-metric multi-dimensional scaling (nMDS) analyses based on Bray-Curtis distance matrices were used to illustrate the influence of regions and seasons on benthic community composition, with taxa strongly driving divergence along nMDS axes identified using Pearson rank-correlations (r ≥ ±0.5 for either axis). To ease interpretation, each season was manually shaded in the resulting nMDS and the vector plot overlaid. Spatial and seasonal differences in settlement community structure were tested with a partially-nested permutational multivariate analysis of variance (PERMANOVA) on the main effects of seasons and regions with sites nested within regions. A similarity percentages analysis (SIMPER) was used to assess which benthic components contributed most to the observed variation in settlement community structure (Clarke and Gorley, 2006). Spatial and seasonal differences in settlement community structure were tested with a partially-nested permutational multivariate analysis of variance (PERMANOVA) on the main effects of seasons and regions with sites nested within regions based on our a priori hypotheses. Key benthic groups and sub-groups identified by our multivariate analyses and coral settlement densities were then then tested with univariate PERMANOVAs to identify significant settlement differences.
A multiple regression was also employed to determine whether the density of coral spat was associated with percent cover of any other components of the settlement community (including each live benthos category plus bare tile). Before regression analyses, the normality of the residuals and homogeneity of variances were confirmed by plotting residuals against fitted values and using QQ plots (Zuur et al., 2007).
Multivariate ordination of the overall settlement community observed in this study indicated strong differences in community structure between regions, with individual sites clustering as region-specific groups across all seasons with no overlap among regions (Figure 2). Vector plots indicated regional differences were primarily driven by variation in the relative abundance of three benthic groups: coralline algae, colonial ascidians, and barnacles (Figure 2), although other benthos also made strong contributions (Supplementary Table 1). SIMPER analyses showed that settlement communities in the Gulf were primarily characterized by the presence of coralline algae, polychaetes, and bryozoans, which together contributed >80% to similarity in tile assemblage structure in this region (Table 1). In the Musandam region, over half of the similarity in settlement communities was driven by strong contributions from both colonial ascidians and bryozoans, with polychaetes and turf algae making more modest contributions (Table 1). Bryozoans were also the most common component of Sea of Oman settlement communities, where they contributed over a third of the similarity in community structure, followed by barnacles and turf algae also playing important roles. The SIMPER analysis showed higher abundances of coralline algae in the Gulf was the main driver of differences between both Musandam and Sea of Oman communities, where colonial ascidians, bryozoans, and turf algae were more abundant (Table 1). Differences between Musandam and the Sea of Oman settlement communities were largely driven by higher cover of colonial ascidians in the Musandam vs. cover of bryozoans, barnacles, polychaetes and turf algae in Sea of Oman sites (Table 1).
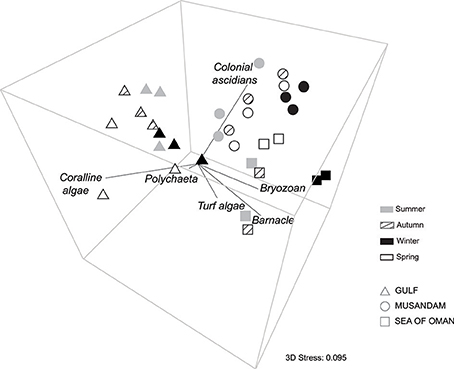
Figure 2. Three-dimensional plot of non-metric multidimensional scaling ordination (nMDS) illustrating the spatial and seasonal variation in benthic community structure across three Arabian water bodies. The vector overlay shows the strength and direction of individual benthos' Pearson rank correlations (restricted to those with r > 0.5 for any axis).
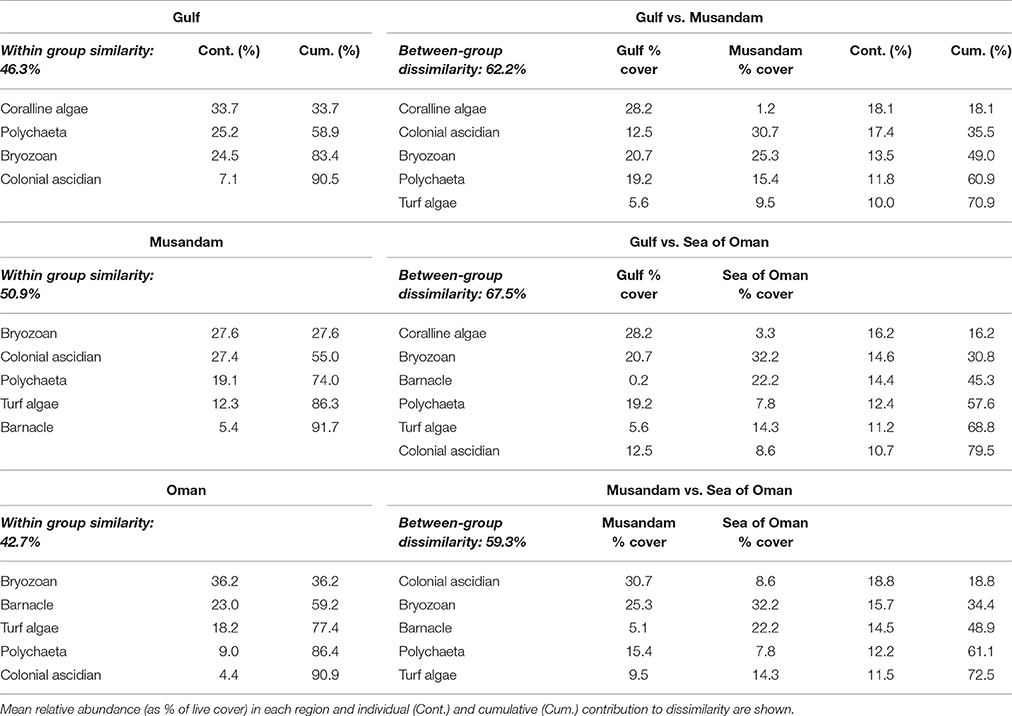
Table 1. Benthic groups responsible for >90% of within-regions similarities and >70% of among-regions dissimilarities based on SIMPER analysis.
While assemblage structure primarily grouped regionally, there were also modest seasonal changes in the settlement community within each region (Figure 2), although these changes were not consistent within regions as shown by a significant interaction between seasons and regions in PERMANOVA (Table 2). In the Gulf the settlement community showed considerable overlap in structure across summer, autumn, and winter, indicating a good degree of similarity across these seasons, with divergence in the remaining season (spring) being primarily due to higher abundances of coralline algae settling into a single site (Ras Ghanada; Figure 2) within this season. In the Musandam the settlement community composition was more similar to Gulf communities during the warm summer season but diverged in the cooler autumn and spring seasons, with the greatest divergence from the Gulf occurring in the cold winter season, when all three Musandam sites had highly convergent community structure, with higher settlement of colonial ascidians (Figure 2). The settlement community in the Sea of Oman varied spatio-temporally. The community at Dibba was fairly consistent across summer, autumn, and spring, while the community at Al Aqa was comparable to that found within Dibba in spring, but diverged markedly in the summer and autumn (Figure 2). In the winter there were dramatic shifts in the settlement community at both of the Sea of Oman sites, with the assemblages at the two sites converging with each other but diverging from the communities observed in all other seasons in this region, due to the settlement of barnacles in both sites. SIMPER analyses (Table 3) showed that the major taxa driving seasonal changes largely reflected the same groups that were associated with regional differences (above; Figure 2), indicating that fluctuations in the relative abundance of these key regional taxa was the primary driver of seasonal shifts in settlement communities.
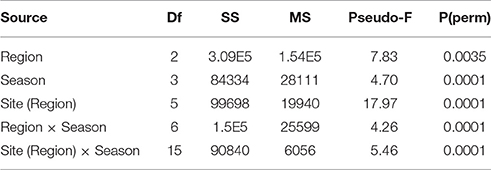
Table 2. Results of the three factor partially nested PERMANOVA analyses assessing differences between regions, seasons and sites (within regions) in benthic community composition.
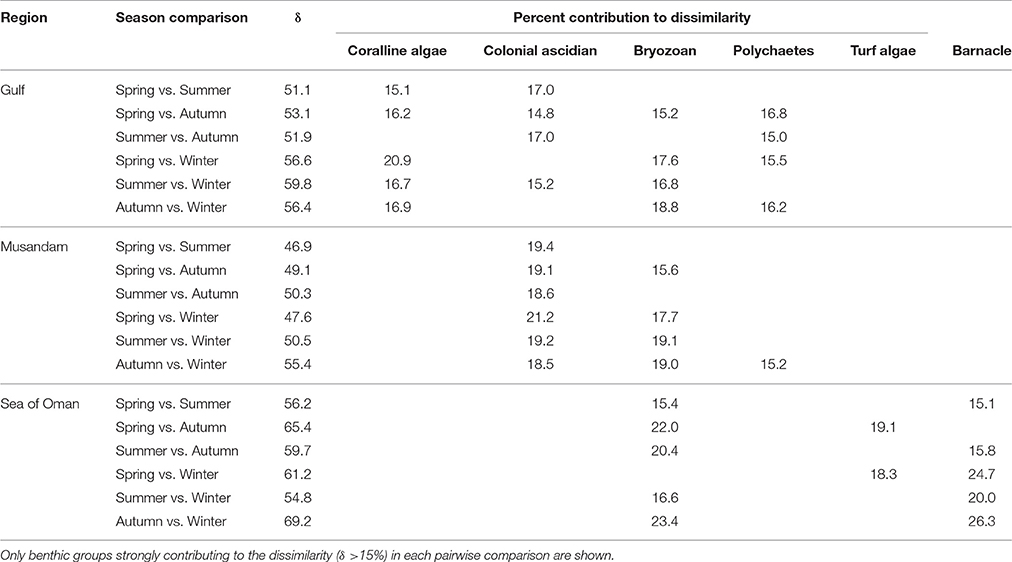
Table 3. Benthic taxa identified as important seasonal drivers of divergence in community structure in SIMPER.
SIMPER analyses were further used to examine the main benthos driving seasonal differences within regional settlement communities (Table 3). The only benthic group that showed wide seasonal variation in all three regions were the bryozoans; all other benthos that were key drivers of seasonal change were important in just one or two regions. In addition to bryozoans, seasonal differences in the Gulf were mainly attributable to variation in the amount of coralline algae, colonial ascidians, and polychaetes, in the Musandam to colonial ascidians, and in the Sea of Oman to barnacles. These seasonal drivers largely reflected the same benthos associated with regional community differences (above; Figure 2).
There was a significant interaction between the main effects of season, region and sites (within region) in structuring settlement of each major benthos (Figure 3), indicating that patterns of change were not consistent among the main effects for any of these benthic groups (PERMANOVA Pseudo- F(15, 824): bryozoans = 11.0, polychaetes = 7.2, turf algae = 5.0, coralline algae = 3.9, barnacles = 7.4, colonial ascidian = 2.7; p < 0.001 for each]. Bryozoans showed strong but inconsistent seasonal fluctuations in all regions, with highest cover in the winter in the Gulf and Musandam (Figures 3A,B), while densities peaked in the autumn in the Sea of Oman when cover was nearly 50% higher than peak densities in the other regions (Figure 3C). While coralline algae cover was negligible in other regions, it was the most abundant benthic component on tiles in the Gulf, and it doubled in cover from winter to summer (mean cover: 16.3% ± 2.2 SE to 31.6% ± 2.7 SE respectively; Figure 3A). Colonial ascidians were considerably more abundant in the Musandam than other regions, explaining its identification as a major distinguishing taxa for tiles in this region in earlier SIMPER analyses, and cover was generally comparable across seasons for this group (Figure 3B). In the Sea of Oman barnacles heavily dominated settlement in winter, when they comprised over half of the benthos at each site and were substantially more widespread than in other seasons; barnacles were uncommon in Musandam and nearly non-existent in the Gulf.
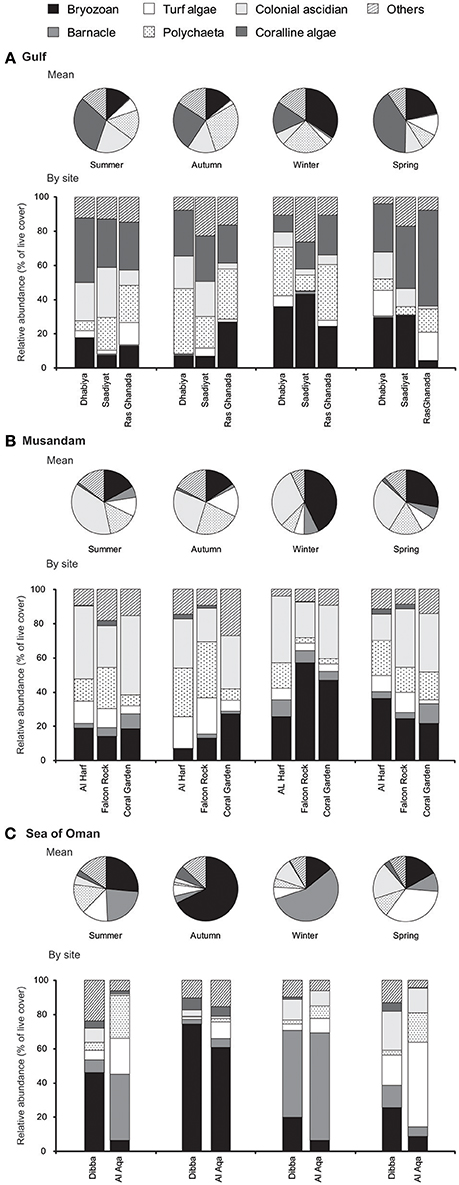
Figure 3. Seasonal changes in benthic community structure: (A) across the Gulf and within each Gulf site, (B) across Musandam and within each Musandam site, (C), across the Sea of Oman and within each Sea of Oman site. Values are shown as relative abundance (percentage of benthic cover).
In total, 216 coral recruits were observed on the 845 tiles deployed across the 8 sites throughout the study. No coral settlers were observed across the entire study in the Gulf of Oman, and there was inconsistent and high variation in coral settlement among sites and seasons within the Gulf and Musandam (Figure 4; Table 4). In the Gulf, mean settlement was highest in autumn but this was mainly due to a large pulse at Saadiyat reef (90% of spat this season); a second peak in settlement also occurred in summer, with a small number of spat recorded in spring as well. In the Musandam, coral settlement mainly occurred in the spring, although spat were observed in low densities in all seasons in at least one site.
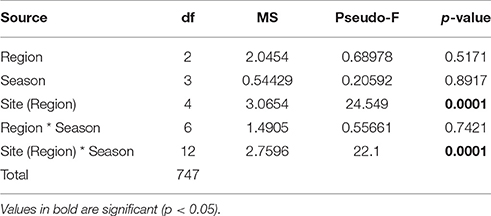
Table 4. Results from PERMANOVA tests comparing mean coral recruits densities among regions, sites and seasons.
Multiple regression revealed that coral density was unrelated to cover of other benthic components [including bare tile space; R2 = 0.27; F(10, 96) = 0.736, p = 0.69], reflecting the considerable variability and low incidence of coral settlement observed in this study.
Coral reef communities in north-eastern Arabia have been subject to various large-scale disturbances in recent years. To date, there had been limited knowledge of the spatial and seasonal patterns of settlement of coral and non-coral benthos, limiting our understanding of the important role that supply may play following disturbance. The results of this study show that settlement of corals was extremely low and that non-coral benthic settlement was highly variable between regions and across sites within regions.
The most striking result of this study was the strong regionally structured patterns of non-coral settlement, with regional differences persisting across seasons throughout the study. These large-scale differences in non-coral settlement mirror the divergence of benthic community structure on reefs across these regions (Bauman et al., 2013; Bento et al., 2016), and is likely driven by the highly divergent environmental conditions among these seas, which have also been subject to varying degrees of disturbance.
The southern Gulf represents one of the most extreme coral reef environments on earth, with sea surface temperatures ranging >25°C annually, daily mean maxima >34°C for several months during summer, and salinities that consistently exceed 44 PSU (Sheppard et al., 1992; Coles, 2003; Foster et al., 2012). In addition, these reefs have experienced numerous large-scale bleaching events over the past two decades (Riegl and Purkis, 2015), resulting in widespread shifts in benthic community structure from which there has been only limited recovery (George and John, 2000; Sheppard and Loughland, 2002; Burt et al., 2008, 2011). In the wake of earlier mass bleaching events, coralline algae dramatically increased in abundance on southern Gulf reefs (George and John, 2000, 2002). In the current study, coralline algae dominated the settlement community on southern Gulf reefs (covering 28% of tile surfaces, on average), and it was the primary differentiator in settlement community structure from the other regions (where it covered <3.5% of tile surfaces in both areas). Thus, the presence of coralline algae as a major component of the settlement community in the southern Gulf appears to be a persistent, long-term characteristic of this highly disturbed, extreme environment.
In contrast to the Gulf, the Sea of Oman has environmental conditions that are more benign. Due to its greater depth and exchange with the wider Indian Ocean, SSTs in the Gulf of Oman are less extreme (mean summer maxima <32°C, range 10°C annually) and salinity is comparable to oceanic conditions (37 PSU) (Foster et al., 2012; Howells et al., 2014). Its waters are also highly productive compared with the Gulf and Musandam as a result of monsoon-induced upwelling (Sheppard et al., 1992). Although reefs in the Sea of Oman have experienced widespread disturbance in the past decade as a result of cyclone storm damage and a hypoxic event associated with an algal bloom (Bauman et al., 2010; Burt et al., 2016), the frequency of impacts to coral reefs here has not been as severe as in the Gulf and there are indications that recovery of reef communities is underway (Bento et al., 2016; Pratchett et al., 2017). Barnacles were one of the primary drivers of the divergence of Sea of Oman reefs from the other regions, with barnacles here covering nearly a quarter of settlement tiles (22%) on average, compared with low abundance in the Musandam (5% cover) and a near absence in the Gulf (0.2% cover). Barnacles are reported to be among the most abundant members of settlement communities in Oman (Wallström et al., 2011; Dobretsov et al., 2013; Polman et al., 2013; Dobretsov, 2015), likely a reflection of their success as filter-feeders in this high-productivity environment (Sheppard et al., 1992), suggesting that the high barnacle abundance we observed is a result of long-term supply dominance in the Sea of Oman.
The Musandam Peninsula sits at the interface between the Arabian Gulf and the Sea of Oman at the Strait of Hormuz. Environmental conditions in the Musandam are generally comparable to the Sea of Oman, although productivity is lower due to a lack of monsoonal upwelling in this area (Sheppard and Salm, 1988; Sheppard et al., 1992; Reynolds, 1993). In addition to having relatively benign environmental conditions, Musandam reefs have also escaped the various large-scale disturbances that affected reefs in the adjacent seas, and reef communities here have among the highest coral cover and diversity in northeastern Arabia and are considered among the most pristine in the region (Sheppard et al., 2010; Bento et al., 2016; Burt et al., 2016). Colonial ascidians were the primary driver of divergence of Musandam reefs from those in the other regions, with these organisms covering nearly a third of Musandam tiles (30.7% cover), nearly triple the coverage in the other regions (8.6–12.5% cover). Ascidians are relatively common members of the benthic community on reefs in the Musandam (R. Bento, unpubl. data), particularly compared to reefs in the southern Gulf or Sea of Oman where they are virtually undetected in benthic surveys (Burt et al., 2011; Grizzle et al., 2016). Given their short pelagic duration and limited larval swimming ability, ascidians generally have a relatively localized dispersal (Shanks et al., 2003; Weersing and Toonen, 2009), suggesting that the high abundance of colonial ascidians observed on Musandam settlement plates likely relates to their high abundance in the wider reef community.
While regional differences primarily structured settlement communities, there were also modest within-region shifts in settlement communities over the course of the year. Much of these within-region shifts were related to fluctuations in the abundance of bryozoans, a dominant member of the community across all regions, as well as to fluctuations in the relative abundance of those same key benthic categories that drove between-region differences. In all three regions the period of peak bryozoan settlement coincided with a reciprocal decline in the abundance of the region-specific key taxa; peak bryozoan abundance in the winter coincided with the lowest annual mean cover of coralline algae in the Gulf and colonial ascidians in the Musandam, while peak bryozoan settlement in the autumn in the Sea of Oman and coincided with a decline of barnacle cover on tiles. In all cases, peak coverage of bryozoans occurred in the season with maximum chlorophyll-a concentrations for every region (Nezlin et al., 2010; Piontkovski et al., 2011; Moradi and Kabiri, 2015), suggesting that while bryozoans settle year round, their success in these particular seasons may be related to enhanced post-settlement growth due to higher planktonic food availability. Together, these observations suggest that while there are distinct regional settlement signatures, seasonality in bryozoan settlement is largely responsible for modulating within-region temporal dynamics. It should be noted, however, that these results represent seasonal settlement data from a single year. While this is useful information given the lack of data available previously, benthic recruitment is highly variable from year to year and it is unknown whether the results of this study are representative of longer-term patterns. A multi-year year settlement study to assess the role of recruitment in sessile benthic population dynamics is warranted.
Coral settlement was low across all three regions in this study. Across the full year of study, coral densities were minimal in the Gulf and Musandam (mean: 38 and 30 coral settlers m−2 year−1, respectively, across the year, and averaging 82 and 70 settlers m−2 year−1 even when considering only the peak settlement season), and corals were entirely absent from settlement tiles in the Sea of Oman throughout the study. The observed densities were substantially lower than has commonly been reported in tropical reef environments (Seychelles: 595 spat m−2 year−1, Chong-Seng et al., 2014; Indonesia: 286–705 m−2 year−1, Sawall et al., 2013; Kenya: 101–908 m−2 year−1, Mangubhai et al., 2007), and less than half of densities reported for comparable high latitude marginal reefs (Eilat: 190 m2 year−1, Glassom and Chadwick, 2006; Solitary Islands: 132 spat m−2 year−1, Harriott and Banks, 1995; Taiwan: 111 spat m−2 year−1, Soong et al., 2003). The observed densities are also low compared with a recent study of coral settlement in Dubai in the southern Gulf where densities of 121 coral settlers m−2 year−1 were reported (Bauman et al., 2014), although those data were mainly collected on breakwaters that have been suggested to entrain eggs, potentially enhancing settlement compared with what would occur on natural reefs (Burt et al., 2009). Overall, the low densities noted in this region are similar to that seen in heavily degraded, highly disturbed reef environments (Singapore: 55 m−2 year−1, Bauman et al., 2015; Florida: 38 m−2 year−1, Van Woesik et al., 2014). Together, the low densities observed in the Gulf and Musandam and the complete absence of coral settlement in the Sea of Oman suggest that there is cause for concern for the regeneration of degraded coral reef communities within the Arabian Peninsula.
The low abundance of coral spat observed here is unlikely to be due to interactions with other members of the settlement community. The abundance of coral recruits was unrelated to cover of any other benthos in our analyses, and bare space was relatively common (ranging from 55% in the Gulf to 20% in the Sea of Oman), suggesting ample availability of habitat for coral settlement. Instead, the low coral settlement likely reflects a limited supply of coral larvae. Low coral settlement has been reported earlier in the southern Gulf (Bauman et al., 2014), and is likely the result of depressed fecundity of corals being exposed to recurrent bleaching events and extreme environmental conditions (Riegl and Purkis, 2015; Howells et al., 2016). Although coral cover is high in the Musandam (Bento et al., 2016; Burt et al., 2016), we observed low levels of coral settlement. This suggests that low coral recruitment is characteristic of reefs in northeastern Arabia, and the high coral cover in the Musandam is likely the result of the relatively low levels of disturbance in this area rather than high larval supply. The implication is that the relatively pristine reefs in the Musandam may be highly vulnerable to any future disturbances that may occur, as any recovery would be potentially limited by low recruitment levels. The absence of coral settlement to tiles in the Sea of Oman was surprising. Reproductive studies occurring near these sites have shown that common coral species were spawning during this study in April 2013 (Howells et al., 2014), and fecundity was substantially higher than conspecifics in the southern Gulf (Howells et al., 2016). Additionally, juvenile surveys on these sites in 2012 showed that corals were recruiting to these reefs, although densities were half of that observed in the southern Gulf (Pratchett et al., 2017), suggesting that recruitment is impaired here. Reefs in the Sea of Oman sites have low coral cover (18%) relative to the southern Gulf and the Musandam (56 and 58%, respectively, Bento et al., 2016), mainly as a result of a algal bloom in 2009 when 50–90% of corals were lost from reefs (Bauman et al., 2010; Foster et al., 2011). As a result, while individual corals here are fecund, reef-wide reproductive output for a variety of dominant coral species is low as a consequence of the limited number of fecund adults in the community (Howells et al., 2016), potentially explaining the absence of coral settlers on tiles and the relatively low overall juvenile recruitment rates observed by Pratchett et al. (2017). It should be noted, however, that recovery on highly disturbed reefs typically takes 10–15 years (Purkis and Riegl, 2005; Burt et al., 2008; Ateweberhan et al., 2011), even in areas where larval supply remains depressed for up to 6 years (Gilmour et al., 2013). As the current study was performed 8 years after the catastrophic HAB event, this suggests that there is hope that recovery may occur in the near future. It is unclear whether the absence of coral settlement observed here extends to other parts of the Sea of Oman. A recent survey of coral reefs 400 km southwest around Muscat, Oman, suggested that many coral reefs there continue to have high coral cover despite showing indications of localized decline in some areas over the past two decades, with available evidence suggesting that declines were primarily due to a recent cyclone rather than the dramic HAB induced loss that impacted the coral communities on reefs studied here (Coles et al., 2015). An assessment of the reproductive capacity and settlement patterns of corals on reefs elsewhere in the Sea of Oman is highly warranted.
Coral reefs around the world are becoming increasingly degraded as a result of climate change and localized anthropogenic impacts (Hughes et al., 2010; Pandolfi et al., 2011) with widespread shifts toward a dominance of non-coral benthos (Colvard and Edmunds, 2011; Kelmo et al., 2013, 2014). While many studies have focused efforts on understanding the dynamics of coral settlement following disturbance (Glassom et al., 2004; Abelson et al., 2005; Green and Edmunds, 2011; Sawall et al., 2013; Bauman et al., 2014), few studies have explored the role that non-coral benthos may play in affecting early recovery dynamics on reefs (Colvard and Edmunds, 2011; Luter et al., 2016). Non-coral benthos are among the first colonists to settle on substrates opened by disturbance, and typically reach an abundance and coverage that greatly exceeds that of coral spat (Dunstan and Johnson, 1998; Díaz-Castañeda and Almeda-Jauregui, 1999; Glassom et al., 2004; Mangubhai et al., 2007; Stubler et al., 2016). Given that many of these non-coral benthos can alternatively facilitate or inhibit subsequent coral recruitment in the space opened by disturbance, their presence could dramatically impact the trajectory of subsequent recovery on disturbed reefs. Our results showed that the initial settlement community was highly region-specific, with non-coral benthos being the primary members of the settlement community in all areas. In some regions, these initial communities were dominated by taxa known to facilitate settlement of coral larvae (for example, over a quarter of Gulf tile space was covered by crustose coralline algae, a well-known inducer of larval coral settlement (Ritson-Williams et al., 2014; Tebben et al., 2015), suggesting conditions favorable to coral colonization following disturbance. In others, however, early settlement communities were dominated by fauna known to inhibit coral larval settlement (e.g., ascidians covered a third of tiles in the Musandam, and various ascidians are known to produce allelochemicals that can inhibit coral settlement (Chadwick and Morrow, 2011). Our knowledge of the types of benthic organisms and the mechanisms that they use to interact with coral larvae is relatively under-developed (Ritson-Williams et al., 2009), but our results here suggest that such information is essential if we are to understand how the initial settlement community may affect the trajectory of early community development. Initial settlement communities will, of course, be shaped by post-settlement processes that will likely lead to mature assemblages that are markedly different in their structure. But because this first stage of development provides the foundation open which all post-settlement processes can act, it represents a critical bottleneck that has important implications for whether or not a disturbed reef has the capacity to recover.
RB: Responsible for data collection, analysis and writing process. DF and AH: Responsible for reviewing the data analysis and writing. JB: Responsible for data analysis and writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AH declared a past co-authorship with one of the authors DF to the handling Editor, who ensured that the process met the standards of a fair and objective review.
This research was supported by the Ford Environmental Grant, Biosphere Expeditions, New York University Abu Dhabi and the Australian Research Council (DE130100688). We thank Fujairah Municipality, Dibba Municipality, and Oman Ministry of Environment and Climate Affairs for research permits. We would also like to thank the NYU Abu Dhabi Institute for field support, and particular gratitude are extended to Grace Vaughan and Dain McParland for field support.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00305/full#supplementary-material
Abelson, A., Olinky, R., and Gaines, S. (2005). Coral recruitment to the reefs of Eilat, Red Sea: temporal and spatial variation, and possible effects of anthropogenic disturbances. Mar. Pollut. Bull. 50, 576–582. doi: 10.1016/j.marpolbul.2005.02.021
Adjeroud, M., Michonneau, F., Edmunds, P. J., Chancerelle, Y., De Loma, T. L., Penin, L., et al. (2009). Recurrent disturbances, recovery trajectories, and resilience of coral assemblages on a South Central Pacific reef. Coral Reefs 28, 775–780. doi: 10.1007/s00338-009-0515-7
Arnold, S. N., Steneck, R., and Mumby, P. J. (2010). Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar. Ecol. 414, 91. doi: 10.3354/meps08724
Ateweberhan, M., Feary, D. A., Keshavmurthy, S., Chen, A., Schleyer, M. H., and Sheppard, C. R. (2013). Climate change impacts on coral reefs: synergies with local effects, possibilities for acclimation, and management implications. Mar. Pollut. Bull. 74, 526–539. doi: 10.1016/j.marpolbul.2013.06.011
Ateweberhan, M., McClanahan, T. R., Graham, N. A. J., and Sheppard, C. R. C. (2011). Episodic heterogeneous decline and recovery of coral cover in the Indian Ocean. Coral Reefs 30:739. doi: 10.1007/s00338-011-0775-x
Bauman, A. G., Baird, A. H., Burt, J. A., Pratchett, M. S., and Feary, D. A. (2014). Patterns of coral settlement in an extreme environment: the southern Persian Gulf (Dubai, United Arab Emirates). Mar. Ecol. Prog. Ser. 499, 115–126. doi: 10.3354/meps10662
Bauman, A. G., Burt, J. A., Feary, D. A., Marquis, E., and Usseglio, P. (2010). Tropical harmful algal blooms: an emerging threat to coral reef communities? Mar. Pollut. Bull. 60, 2117–2122. doi: 10.1016/j.marpolbul.2010.08.015
Bauman, A. G., Feary, D. A., Heron, S. F., Pratchett, M. S., and Burt, J. A. (2013). Multiple environmental factors influence the spatial distribution and structure of reef communities in the northeastern Arabian Peninsula. Mar. Pollut. Bull. 72, 302–312. doi: 10.1016/j.marpolbul.2012.10.013
Bauman, A. G., Guest, J. R., Dunshea, G., Low, J., Todd, P. A., and Steinberg, P. D. (2015). Coral settlement on a highly disturbed equatorial reef system. PLoS ONE 10:e0127874. doi: 10.1371/journal.pone.0127874
Bauman, A., Baird, A., and Cavalcante, G. (2011). Coral reproduction in the world's warmest reefs: southern Persian Gulf (Dubai, United Arab Emirates). Coral Reefs 30, 405–413. doi: 10.1007/s00338-010-0711-5
Bell, J. J. (2008). The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 79, 341–353. doi: 10.1016/j.ecss.2008.05.002
Bento, R., Hoey, A. S., Bauman, A. G., Feary, D. A., and Burt, J. A. (2016). The implications of recurrent disturbances within the world's hottest coral reef. Mar. Pollut. Bull. 105, 466–472. doi: 10.1016/j.marpolbul.2015.10.006
Birrell, C., Mccook, L., Willis, B., and Diaz-Pulido, G. (2008). Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. 46, 25–63. doi: 10.1201/9781420065756.ch2
Bruno, J. F. (2013). Coral reefs: building a better crystal ball. Curr. Biol. 23, R473–R475. doi: 10.1016/j.cub.2013.04.042
Burt, J. A., Coles, S., Van Lavieren, H., Taylor, O., Looker, E., and Samimi-Namin, K. (2016). Oman's coral reefs: a unique ecosystem challenged by natural and man-related stresses and in need of conservation. Mar. Pollut. Bull. 105, 498–506. doi: 10.1016/j.marpolbul.2015.11.010
Burt, J., Al-Harthi, S., and Al-Cibahy, A. (2011). Long-term impacts of coral bleaching events on the world's warmest reefs. Mar. Environ. Res. 72, 225–229. doi: 10.1016/j.marenvres.2011.08.005
Burt, J., Bartholomew, A., and Usseglio, P. (2008). Recovery of corals a decade after a bleaching event in Dubai, United Arab Emirates. Mar. Biol. 154, 27–36. doi: 10.1007/s00227-007-0892-9
Burt, J., Bartholomew, A., Bauman, A., Saif, A., and Sale, P. F. (2009). Coral recruitment and early benthic community development on several materials used in the construction of artificial reefs and breakwaters. J. Exp. Mar. Biol. Ecol. 373, 72–78. doi: 10.1016/j.jembe.2009.03.009
Caley, M. J., Carr, M. H., Hixon, M. A., Hughes, T. P., Jones, G. P., and Menge, B. A. (1996). Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 27, 477–500. doi: 10.1146/annurev.ecolsys.27.1.477
Carpenter, K. E., Abrar, M., Aeby, G., Aronson, R. B., Banks, S., Bruckner, A., et al. (2008). One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. doi: 10.1126/science.1159196
Chadwick, N. E., and Morrow, K. M. (2011). “Competition among sessile organisms on coral reefs,” in Coral Reefs: An Ecosystem in Transition, eds Z. Dubinsky and N. Stambler (Dordrecht: Springer), 347–371. doi: 10.1007/978-94-007-0114-4_20
Chong-Seng, K., Graham, N., and Pratchett, M. (2014). Bottlenecks to coral recovery in the Seychelles. Coral Reefs 33, 449–461. doi: 10.1007/s00338-014-1137-2
Clarke, K. R., and Gorley, R. N. (2006). PRIMER V6: User Manual-Tutorial. Plymouth, UK: Plymouth Marine Laboratory.
Coles, S. L. (2003). Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: a comparison to the Indo-Pacific Region. Atoll Res. Bull. 507, 1–19. doi: 10.5479/si.00775630.507.1
Coles, S., Looker, E., and Burt, J. (2015). Twenty-year changes in coral near Muscat, Oman estimated from manta board tow observations. Mar. Environ. Res. 103, 66–73. doi: 10.1016/j.marenvres.2014.11.006
Colvard, N. B., and Edmunds, P. J. (2011). Decadal-scale changes in abundance of non-scleractinian invertebrates on a Caribbean coral reef. J. Exp. Mar. Biol. Ecol. 397, 153–160. doi: 10.1016/j.jembe.2010.11.015
Cowen, R. K., and Sponaugle, S. (2009). Larval dispersal and marine population connectivity. Ann. Rev. Mar. Sci. 1, 443–466. doi: 10.1146/annurev.marine.010908.163757
De Goeij, J. M., Van Oevelen, D., Vermeij, M. J., Osinga, R., Middelburg, J. J., De Goeij, A. F., et al. (2013). Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342, 108–110. doi: 10.1126/science.1241981
De'ath, G., Fabricius, K. E., Sweatman, H., and Puotinen, M. (2012). The 27-year decline of coral cover on the great barrier reef and its causes. Proc. Natl. Acad. Sci. U.S.A. 109, 17995–17999. doi: 10.1073/pnas.1208909109
Díaz-Castañeda, V., and Almeda-Jauregui, C. (1999). Early benthic organism colonization on a Caribbean coral reef (Barbados, West Indies): a plate experimental approach. Mar. Ecol. 20, 197–220.
Diaz-Pulido, G., Harii, S., Mccook, L., and Hoegh-Guldberg, O. (2010). The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs 29, 203–208. doi: 10.1007/s00338-009-0573-x
Dobretsov, S. (2015). Biofouling on artificial substrata in Muscat waters. J. Agric. Mar. Sci. 19, 24–29. doi: 10.24200/jams.vol20iss0pp24-29
Dobretsov, S., Abed, R. M., and Voolstra, C. R. (2013). The effect of surface colour on the formation of marine micro and macrofouling communities. Biofouling 29, 617–627. doi: 10.1080/08927014.2013.784279
Dunstan, P. K., and Johnson, C. R. (1998). Spatio-temporal variation in coral recruitment at different scales on heron reef, southern great barrier reef. Coral Reefs 17, 71—81. doi: 10.1007/s003380050098
Enochs, I. C., and Glynn, P. W. (2017). “Trophodynamics of eastern pacific coral reefs,” in Coral Reefs of the Eastern Tropical Pacific, eds P. Glynn, D. Manzello, and I. Enochs (Dordrecht: Springer Science + Business Media, B. V.), 291–314.
Ferrario, F., Beck, M. W., Storlazzi, C. D., Micheli, F., Shepard, C. C., and Airoldi, L. (2014). The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat. Commun. 5:3794. doi: 10.1038/ncomms4794
Foster, K. A., Foster, G., Tourenq, C., and Shuriqi, M. K. (2011). Shifts in coral community structures following cyclone and red tide disturbances within the gulf of oman (United Arab Emirates). Mar. Biol. 158, 955–968. doi: 10.1007/s00227-010-1622-2
Foster, K., Foster, G., Al-Cibahy, A. S., Al-Harthi, S., Purkis, S. J., and Riegl, B. M. (2012). Environmental setting and temporal trends in southeastern gulf coral communities. Coral Reefs Gulf 3, 51–70. doi: 10.1007/978-94-007-3008-3_4
George, D., and John, D. (2000). “The status of coral reefs and associated macroalgae in Abu Dhabi (UAE) after recent coral bleaching events,” in Proceedings of the International Symposium on the Extent and Impact of Coral Bleaching in the Arabian Region (Riyadh).
George, J., and John, D. (2002). “Is it Curtains for Coral Reefs in the Southern Arabian Gulf,” in Proccesdings of International Society of Reef Studies in European Meeting (Cambridge).
Gilmour, J. P., Smith, L. D., Heyward, A. J., Baird, A. H., and Pratchett, M. S. (2013). Recovery of an isolated coral reef system following severe disturbance. Science 340, 69–71. doi: 10.1126/science.1232310
Glassom, D., and Chadwick, N. (2006). Recruitment, growth and mortality of juvenile corals at Eilat, northern Red Sea. Mar. Ecol. Prog. Ser. 318, 111–122. doi: 10.3354/meps318111
Glassom, D., Zakai, D., and Chadwick-Furman, N. E. (2004). Coral recruitment: a spatio-temporal analysis along the coastline of Eilat, northern Red Sea. Mar. Biol. 144, 641–651. doi: 10.1007/s00227-003-1243-0
Glynn, P. W., and Enochs, I. C. (2011). “Invertebrates and their roles in coral reef ecosystems,” in Coral Reefs: An Ecosystem in Transition, eds Z. Dubinsky and N. Stambler (Dordrecht: Springer), 273–325. doi: 10.1007/978-94-007-0114-4_18
Green, D. H., and Edmunds, P. J. (2011). Spatio-temporal variability of coral recruitment on shallow reefs in St. John, US Virgin Islands. J. Exp. Mar. Biol. Ecol. 397, 220–229. doi: 10.1016/j.jembe.2010.12.004
Grizzle, R. E., Ward, K. M., Alshihi, R. M., and Burt, J. A. (2016). Current status of coral reefs in the United Arab Emirates: distribution, extent, and community structure with implications for management. Mar. Pollut. Bull. 105, 515–523. doi: 10.1016/j.marpolbul.2015.10.005
Guannel, G., Arkema, K., Ruggiero, P., and Verutes, G. (2016). The Power of three: coral reefs, seagrasses and mangroves protect coastal regions and increase their resilience. PLoS ONE 11:e0158094. doi: 10.1371/journal.pone.0158094
Guest, J. R., Tun, K., Low, J., Verges, A., Marzinelli, E. M., Campbell, A. H., et al. (2016). 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Sci. Rep. 6:36260. doi: 10.1038/srep36260
Halford, A., Cheal, A. J., Ryan, D., and Williams, D. M. B. (2004). Resilience to large-scale disturbance in coral and fish assemblages on the great barrier reef. Ecology 85, 1892–1905. doi: 10.1890/03-4017
Harriott, V. J., and Banks, S. A. (1995). Recruitment of scleractinian corals in the solitary islands marine reserve, a high latitude coral-dominated community in Eastern Australia. Mar. Ecol. Prog. Ser. 123, 155–161. doi: 10.3354/meps123155
Howells, E. J., Abrego, D., Vaughan, G. O., and Burt, J. A. (2014). Coral spawning in the Gulf of Oman and relationship to latitudinal variation in spawning season in the northwest Indian Ocean. Sci. Rep. 4:7484. doi: 10.1038/srep07484
Howells, E. J., Ketchum, R. N., Bauman, A. G., Mustafa, Y., Watkins, K. D., and Burt, J. A. (2016). Species-specific trends in the reproductive output of corals across environmental gradients and bleaching histories. Mar. Pollut. Bull. 105, 532–539. doi: 10.1016/j.marpolbul.2015.11.034
Hughes, T. P., Graham, N. A., Jackson, J. B., Mumby, P. J., and Steneck, R. S. (2010). Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642. doi: 10.1016/j.tree.2010.07.011
Hughes, T. P., Kerry, J. T., Álvarez-Noriega, M., Álvarez-Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Jackson, J. (1984). Ecology of cryptic coral reef communities. III. Abundance and aggregation of encrusting organisms with particular reference to cheilostome Bryozoa. J. Exp. Mar. Biol. Ecol. 75, 37–57. doi: 10.1016/0022-0981(84)90022-4
John, D. (2012). “Marine algae (seaweeds) associated with coral reefs in the Gulf,” in Coral reefs of the Gulf: adaptation to climatic extremes Vol. Coral Reefs of the World 3, eds B. Riegl and S. Purkis (Dordrecht: Springer Science+Business Media, B. V.), 309–335.
Kelmo, F., Bell, J. J., and Attrill, M. J. (2013). Tolerance of sponge assemblages to temperature anomalies: resilience and proliferation of sponges following the 1997-8 El-Nino southern oscillation. PLoS ONE 8:e76441. doi: 10.1371/journal.pone.0076441
Kelmo, F., Bell, J. J., Moraes, S. S., Gomes Rda, C., Mariano-Neto, E., and Attrill, M. J. (2014). Differential responses of emergent intertidal coral reef fauna to a large-scale El-Nino southern oscillation event: sponge and coral resilience. PLoS ONE 9:e93209. doi: 10.1371/journal.pone.0093209
Kohler, K. E., and Gill, S. M. (2006). Coral Point Count with Excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
Lillis, A., Bohnenstiehl, D., Peters, J. W., and Eggleston, D. (2016). Variation in habitat soundscape characteristics influences settlement of a reef-building coral. Peer J. 4:e2557. doi: 10.7717/peerj.2557
Luter, H. M., Duckworth, A. R., Wolff, C. W., Evans-Illidge, E., and Whalan, S. (2016). Recruitment variability of coral reef sessile communities of the far north great barrier reef. PLoS ONE 11:e0153184. doi: 10.1371/journal.pone.0153184
Maina, J., De Moel, H., Zinke, J., Madin, J., Mcclanahan, T., and Vermaat, J. E. (2013). Human deforestation outweighs future climate change impacts of sedimentation on coral reefs. Nat. Commun. 4:1986. doi: 10.1038/ncomms2986
Mallela, J. (2007). Coral reef encruster communities and carbonate production in cryptic and exposed coral reef habitats along a gradient of terrestrial disturbance. Coral Reefs 26, 775–785. doi: 10.1007/s00338-007-0260-8
Mangubhai, S., Harrison, P. L., and Obura, D. O. (2007). Patterns of coral larval settlement on lagoon reefs in the mombasa marine national park and reserve, kenya. Mar. Ecol. Prog. Ser. 348, 149–159. doi: 10.3354/meps07090
McCune, B., Grace, J. B., and Urban, D. L. (2002). Analysis of Ecological Communities. Gleneden Beach, OR: MjM Software Design.
Moradi, M., and Kabiri, K. (2015). Spatio-temporal variability of SST and Chlorophyll-a from MODIS data in the Persian Gulf. Mar. Pollut. Bull. 98, 14–25. doi: 10.1016/j.marpolbul.2015.07.018
Mumby, P. J. (2016). Stratifying herbivore fisheries by habitat to avoid ecosystem overfishing of coral reefs. Fish Fish. 17, 266–278. doi: 10.1111/faf.12078
Mundy, C. N. (2000). An appraisal of methods used in coral recruitment studies. Coral Reefs 19, 124–131. doi: 10.1007/s003380000081
Nezlin, N. P., Polikarpov, I. G., Al-Yamani, F. Y., Subba Rao, D. V., and Ignatov, A. M. (2010). Satellite monitoring of climatic factors regulating phytoplankton variability in the Arabian (Persian) Gulf. J. Mar. Sys. 82, 47–60. doi: 10.1016/j.jmarsys.2010.03.003
Pandolfi, J. M., Connolly, S. R., Marshall, D. J., and Cohen, A. L. (2011). Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422. doi: 10.1126/science.1204794
Piontkovski, S., Al-Azri, A., and Al-Hashmi, K. (2011). Seasonal and interannual variability of chlorophyll-a in the Gulf of Oman compared to the open Arabian Sea regions. Int. J. Remote Sens. 32, 7703–7715. doi: 10.1080/01431161.2010.527393
Pollock, F. J., Lamb, J. B., Field, S. N., Heron, S. F., Schaffelke, B., Shedrawi, G., et al. (2014). Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 9:e102498. doi: 10.1371/journal.pone.0102498
Polman, H., Verhaart, F., and Bruijs, M. (2013). Impact of biofouling in intake pipes on the hydraulics and efficiency of pumping capacity. Desalinat. Water Treat. 51, 997–1003. doi: 10.1080/19443994.2012.707371
Pratchett, M. S., Baird, A. H., Bauman, A. G., and Burt, J. A. (2017). Abundance and composition of juvenile corals reveals divergent trajectories for coral assemblages across the United Arab Emirates. Mar. Pollut. Bull. 114, 1031–1035. doi: 10.1016/j.marpolbul.2016.11.036
Purkis, S., and Riegl, B. (2005). Spatial and temporal dynamics of Arabian Gulf coral assemblages quantified from remote-sensing and in situ monitoring data. Mar. Ecol. 287, 99–113. doi: 10.3354/meps287099
Reynolds, R. M. (1993). Physical oceanography of the Gulf, Strait of Hormuz, and the Gulf of Oman–Results from the Mt Mitchell expedition. Mar. Pollut. Bull. 27, 35–59. doi: 10.1016/0025-326X(93)90007-7
Riegl, B., and Purkis, S. (2015). Coral population dynamics across consecutive mass mortality events. Glob. Chang. Biol. 21, 3995–4005. doi: 10.1111/gcb.13014
Riegl, B., Berumen, M., and Bruckner, A. (2013). Coral population trajectories, increased disturbance and management intervention: a sensitivity analysis. Ecol. Evol. 3, 1050–1064. doi: 10.1002/ece3.519
Ritson-Williams, R., Arnold, S. N., Fogarty, N. D., Steneck, R. S., Vermeij, M. J., and Paul, V. J. (2009). New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson. Contrib. Mar. Sci. 38, 437–457. doi: 10.5479/si.01960768.38.437
Ritson-Williams, R., Arnold, S., Paul, V., and Steneck, R. (2014). Larval settlement preferences of Acropora palmata and Montastraea faveolata in response to diverse red algae. Coral Reefs 33, 59–66. doi: 10.1007/s00338-013-1113-2
Roff, G., Doropoulos, C., Zupan, M., Rogers, A., Steneck, R. S., Golbuu, Y., et al. (2015). Phase shift facilitation following cyclone disturbance on coral reefs. Oecologia 178, 1193–1203. doi: 10.1007/s00442-015-3282-x
Sawall, Y., Jompa, J., Litaay, M., Maddusila, A., and Richter, C. (2013). Coral recruitment and potential recovery of eutrophied and blast fishing impacted reefs in Spermonde Archipelago, Indonesia. Mar. Pollut. Bull. 74, 374–382. doi: 10.1016/j.marpolbul.2013.06.022
Shanks, A. L., Grantham, B. A., and Carr, M. H. (2003). Propagule dispersal distance and the size and spacing of marine reserves. Ecol. App. S159–S169. doi: 10.1890/1051-0761(2003)013[0159:PDDATS]2.0.CO;2
Sheppard, C. R. C., and Loughland, R. (2002). Coral mortality and recovery in response to increasing temperature in the southern Arabian Gulf. Aqua. Ecossys. Health Manage. 5, 395–402. doi: 10.1080/14634980290002020
Sheppard, C. R. C., and Salm, R. V. (1988). Reef and coral communities of Oman, with a description of a new coral species (Order Scleractinia, genusAcanthastrea). J. Nat. Hist. 22, 263–279. doi: 10.1080/00222938800770201
Sheppard, C., Al-Husiani, M., Al-Jamali, F., Al-Yamani, F., Baldwin, R., Bishop, J., et al. (2010). The Gulf: a young sea in decline. Mar. Pollut. Bull. 60, 13–38. doi: 10.1016/j.marpolbul.2009.10.017
Sheppard, C., Price, A., and Roberts, C. (1992). Marine Ecology of the Arabian Region: Patterns and Processes in Extreme Tropical Environments. Toronto, ON: Academic Press.
Soong, K., Chen, M.-H., Chen, C.-L., Dai, C.-F., Fan, T.-Y., Li, J.-J., et al. (2003). Spatial and temporal variation of coral recruitment in Taiwan. Coral Reefs 22, 224–228. doi: 10.1007/s00338-003-0311-8
Spalding, M. D., and Brown, B. E. (2015). Warm-water coral reefs and climate change. Science 350, 769–771. doi: 10.1126/science.aad0349
Stubler, A. D., Stevens, A. K., and Peterson, B. J. (2016). Using community-wide recruitment and succession patterns to assess sediment stress on Jamaican coral reefs. J. Exp. Mar. Biol. Ecol. 474, 29–38. doi: 10.1016/j.jembe.2015.09.018
Tebben, J., Motti, C. A., Siboni, N., Tapiolas, D. M., Negri, A. P., Schupp, P. J., et al. (2015). Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 5:10803. doi: 10.1038/srep10803
Van Woesik, R., Scott, W. J., and Aronson, R. B. (2014). Lost opportunities: coral recruitment does not translate to reef recovery in the Florida Keys. Mar. Pollut. Bull. 88, 110–117. doi: 10.1016/j.marpolbul.2014.09.017
Wallström, E., Jespersen, H. T., and Schaumburg, K. (2011). A new concept for anti-fouling paint for Yachts. Prog. Org. Coat. 72, 109–114. doi: 10.1016/j.porgcoat.2011.03.001
Wear, S. L., and Thurber, R. V. (2015). Sewage pollution: mitigation is key for coral reef stewardship. Ann. N.Y. Acad. Sci. 1355, 15–30. doi: 10.1111/nyas.12785
Weersing, K., and Toonen, R. J. (2009). Population genetics, larval dispersal, and connectivity in marine systems. Mar. Ecol. Progr. Ser. 393, 1–12. doi: 10.3354/meps08287
Zabin, C. J. (2015). Patterns of adult abundance vary with recruitment of an invasive barnacle species on Oahu, Hawaii. J. Exp. Mar. Biol. Ecol. 464, 44–51. doi: 10.1016/j.jembe.2014.12.009
Zhang, S. Y., Speare, K. E., Long, Z. T., Mckeever, K. A., Gyoerkoe, M., Ramus, A. P., et al. (2014). Is coral richness related to community resistance to and recovery from disturbance? PeerJ. 2:e308. doi: 10.7717/peerj.308
Keywords: Arabian Peninsula, benthic communities, coral recruits, settlement tiles, seasonality, sessile invertebrates
Citation: Bento R, Feary DA, Hoey AS and Burt JA (2017) Settlement Patterns of Corals and other Benthos on Reefs with Divergent Environments and Disturbances Histories around the Northeastern Arabian Peninsula. Front. Mar. Sci. 4:305. doi: 10.3389/fmars.2017.00305
Received: 28 May 2017; Accepted: 05 September 2017;
Published: 20 September 2017.
Edited by:
Verena Schoepf, University of Western Australia, AustraliaReviewed by:
Steve L. Coles, Bernice P. Bishop Museum, United StatesCopyright © 2017 Bento, Feary, Hoey and Burt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Bento, cml0YXNhbm1pZ3VlbEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.