- Biology Program, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates
Climate change is affecting the trophic ecology of reef fishes through changes in reef-associated food availability and fish feeding behavior. The southern Arabian Gulf is a thermally extreme environment, providing an opportunity to study fish diets on reefs with summer temperatures representative of next-century conditions elsewhere. Using 18S metagenomic analyses of stomach contents, we provide the first description of the dietary composition of three abundant reef fishes (Pomacanthus maculosus, Pomacentrus aquilus, and Pomacentrus trichrourus) from the thermally extreme southern Arabian Gulf, with five sampling periods across 1 year used to assess seasonal variation in diet. In total, 146 stomach content samples were sequenced, resulting in 9.6 million filtered reads that aligned to 17 classes in 14 phyla. Corals (Cnidaria, Anthozoa) dominated stomach contents of all three fishes (overall mean: 74.6, 40.6, and 21.2% of stomach reads, respectively), suggesting coral consumption to be characteristic of reef fish diet in the region. Sanger sequencing validated the presence of corals in the stomach contents and identified two common genera in the region, Porites and Platygyra, as part of the diet. Other common phyla included sponges and annelid worms (P. maculosus: 14.9%, 4.1%; P. aquilus: 5.9%, 16.7%; P. trichrourus: 8.2%, 14.7%, respectively), with the remainder comprised of 11 other phyla. Algae were virtually absent in diets of all three species. The P. maculosus diet was consistently coral/sponge dominated across the year, but there was substantial seasonal variation in the damselfishes, with diets dominated by coral in the hottest month (August; P. aquilus: 89.4%, P. trichrourus: 51.5%) but broadest in spring (March, May) when corals became less common (<19.8% each) and bivalves, free living ascidians, and various arthropods increased; parasitic cestodes were also abundant in damselfish stomachs in spring (mean: >16.4%). These results suggest that these fishes have developed a feeding ecology responsive to the fluctuating and extreme environmental conditions of their region. These results broaden our understanding of the diets of these three species and document the nature, complexity and temporal dynamics of reef fish diets in the most thermally extreme coral reef environment on earth.
Introduction
Coral reefs are one of the most diverse, ecologically complex and economically significant ecosystems in the world (Moberg and Folke, 1999). Corals provide essential habitat and food resources for an array of organisms, with coral reef fishes being among the most diverse and functionally important of these reef-associated groups (Moberg and Folke, 1999; Coker et al., 2014). Due to their close association with reefs, fish assemblages are strongly influenced by the topographic complexity and abundance of corals (Graham and Nash, 2013; Rogers et al., 2014), and the amount and variety of food items available on reefs further affects reef fish community structure and functional ecology (Chong-Seng et al., 2012; Glynn et al., 2014; López-Pérez et al., 2016). Reef fishes also influence their habitat, with their feeding activities directly or indirectly affecting the abundance of key benthic groups on reefs, such as algae, sponges and corals (Hixon, 1997; Hill, 1998; Cole et al., 2011). As such, the trophic ecology of reef fishes is strongly interlinked with the functioning of the wider coral reef community.
Climate change represents a growing threat to coral reefs and associated fish communities. Elevated sea surface temperature (SST) in recent decades has been associated with mass coral bleaching events around the world (Hughes et al., 2017), resulting in long-term shifts in the composition of benthic communities on many reefs (Baker et al., 2008; de Bakker et al., 2017). The resultant changes in habitat structure have been shown to strongly influence the composition and abundance of reef fishes (Wilson et al., 2006; Munday et al., 2008; Pratchett et al., 2008), and there is increasing evidence that shifts in the availability of food items on these changing reefs are influencing both the composition of reef fish feeding guilds (Pratchett et al., 2008; Wilson et al., 2008) as well as the items selected as food (Floeter et al., 2004; Berumen and Pratchett, 2006). Increasing temperatures also have direct effects on fish feeding activity itself. Fishes are ectotherms, and so changes of a few degrees Celsius can influence their behavior and metabolism (Wood and McDonald, 1997), affecting feeding-related parameters, such as swimming speed (Johansen and Jones, 2011), bite rates (Smith, 2008), and attack/escape dynamics (Allan et al., 2015), with increased feeding necessary to support the greater metabolic demand at higher temperatures (Nowicki et al., 2012). It is, thus, likely that climate change will have substantial but largely underappreciated effects on the trophic ecology of reef fishes in the future.
The Arabian/Persian Gulf represents a natural laboratory in which to investigate the influence of extreme temperatures on the trophic ecology of reef fishes. Coral reefs in the Arabian Gulf experience the highest summer maxima (>35°C) and the greatest range of temperatures (ca. 20°C annually) known for reefs anywhere in the world (Sheppard and Sheppard, 1991; Coles, 1997, 2003; Hume et al., 2013), with summer conditions in the Gulf today representative of conditions projected for many tropical reef environments in the next century (Riegl, 1999; Collins et al., 2013; Wernberg et al., 2013). As a result of these extreme conditions, coral reefs in the Gulf are less structurally complex and less diverse than in most other parts of the Indo-Pacific (Coles, 2003), with benthic communities distinct from those in even the biogeographically connected Indian Ocean (Sheppard et al., 1992; Bauman et al., 2012). As a result of this extreme environment as well as variation in habitat structure and food resources, reef fish assemblages in the Arabian Gulf are also structurally and functionally distinct from those in more benign reef environments (Feary et al., 2010, 2012), with trophic guild structure that differs markedly from even adjacent seas (Feary et al., 2010; Burt et al., 2011b; Hoey et al., 2016), and broader ranges of food consumed in at least some guilds (Pratchett et al., 2013).
Only limited research is available on the trophic ecology of Arabian Gulf reef fish. Other than studies of diet-related pollutant accumulation in commercially important fish species (e.g., Saei-Dehkordi et al., 2010; Monikh et al., 2012, 2013), only a few other studies have explored reef fish feeding ecology in the Arabian Gulf. Pratchett et al. (2013) used in situ observations of feeding habits to discern selectivity and rates of feeding in corallivorous butterflyfishes, while Burt et al. (2011b) inferred the trophic structure of reef fish communities based on field surveys and literature reviews. Both of these studies suggested that reef fish feeding activities in the Arabian Gulf were distinct from that of less extreme reef environments. Similarly, related studies on the composition of the wider reef fish communities or specific trophic groups have suggested that variation in abundance and composition of certain guilds likely substantially influences trophic ecology in the Arabian Gulf (Feary et al., 2010, 2012; Hoey et al., 2016). However, to date there have been no studies that have directly explored the diets of reef fishes in the Arabian Gulf. Given the environmental conditions and benthic composition of these reefs, such information and more long-term studies would be very useful for developing an understanding of the adaptability of trophic ecology of reef fish communities in other regions to future climate change. In addition, due to reefs here also having the largest range of temperatures known to be experienced by reef fish anywhere in the world (approximately 20°C annually; winter minimum <20°C, summer maximum >35°C) (Sheppard and Sheppard, 1991; Coles, 1997, 2003; Hume et al., 2013), reef fish in the Arabian Gulf also provide an opportunity to understand the plasticity of diets under highly variable environmental conditions (Pratchett et al., 2013).
Traditional techniques for quantifying dietary habits have typically been based on microscopic analysis of stomach, gut or fecal contents (Carreon-Martinez and Heath, 2010). These traditional morphological techniques, however, can be biased and inaccurate as identifiable characters can be lost in digestion and morphological similarities between food items can lead to misidentification (Carreon-Martinez and Heath, 2010; Paquin et al., 2014). To account for these limitations, there has been increasing use of molecular techniques to describe the composition of fish diets (e.g., Budarf et al., 2011; Paquin et al., 2014; Miyake et al., 2015). With recent improvements in technology, high-throughput sequencing techniques now provide a rapid and low cost means by which to characterize diets, with bioinformatic approaches applied to the resultant metagenomic data allowing for a much more robust, reliable and deep understanding of diet composition than had previously been possible using microscopy-based techniques (Clare, 2014). In general, this approach is more qualitative than quantitative. Qualitatively, the metabarcoding approach can reveal dietary items that may not be discoverable from traditional morphological techniques, such as small organisms or highly digested items (Leray and Knowlton, 2015; Albaina et al., 2016; Harms-Tuohy et al., 2016). Quantitatively, however, the number of sequences may not correspond to the abundance of the DNA or the food item in the stomach since the amplicon region may present in different numbers of copies in different organisms (Albaina et al., 2016). Also of note is that the metagenomics approach cannot identify whether a food item is a primary target of the fish feeding or is accidentally ingested in general foraging or indirectly consumed along with the primary items (Harms-Tuohy et al., 2016).
Here we use a metagenomics approach to examine stomach content of three abundant coral reef fishes from the southern Arabian Gulf, Pomacanthus maculosus (Arabian angelfish), Pomacentrus aquilus (dark damsel), and Pomacentrus trichrourus (pale-tail damsel). The goal of this study is to provide an unbiased and detailed account of the diet and its seasonal variation in the three species. The results of this study provide the first record of the diets of reef fishes in this understudied region and revealed important insights into how the diet of reef fishes changes in response to extreme environmental conditions.
Methods
Sample Collection and Processing
Collection permit and IACUC approval were obtained from Environmental Agency Abu Dhabi and New York University Abu Dhabi, respectively. Three reef-associated fish species were collected from Saadiyat reef in Abu Dhabi, UAE (N 24.59900° E054.42150°): the Arabian angelfish, P. maculosus, the dark damsel, P. aquilus, and the pale-tail damsel, P. trichrourus (Figure 1A). These species are among the most abundant coral reef fishes in the southern Arabian Gulf, and among the most important species driving reef fish community divergence from those in adjacent seas (Feary et al., 2010; Burt et al., 2011b, 2013). Although little is known of the dietary habits of these species in the northeastern Arab region, data from other regions suggest that P. maculosus feeds mainly on sponges and algae, P. aquilus primarily on zooplankton, and P. trichrourus mainly on benthic algae (Masuda and Allen, 1993; ter Hofstede, 1998).
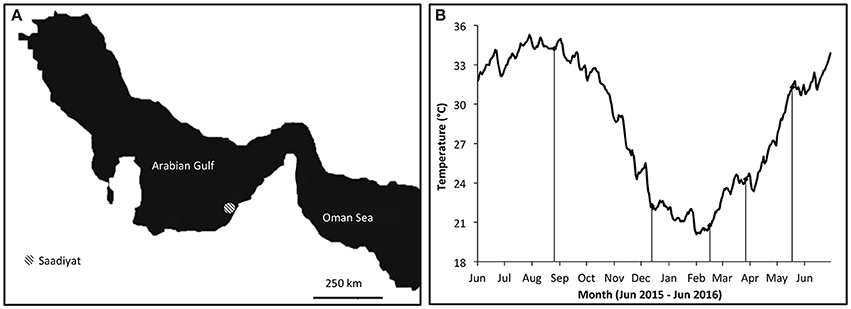
Figure 1. (A) Map of the Saadiyat reef study location (adapted from Howells et al., 2016). (B) Mean daily seawater temperatures on Saadiyat reef. Lines indicate collection periods.
To determine the influence of seasonality on dietary content, collections were performed over five sampling periods across a year from summer (August 2015) to spring (May 2016), when mean monthly sea-bottom temperatures ranged over 15°C (Figure 1B). During each sampling period between 8 and 10 individuals of each species were collected by SCUBA using 30% clove oil (Clove bud oil, Sigma-Aldrich) emulsified with 95% ethanol as an anesthetic for hand-netting (Munday and Wilson, 1997). Anesthetized individuals were immediately transferred to the surface and euthanized in an ice-water slurry before being preserved at −80°C until processing.
To obtain stomach contents, individual fish were thawed and immediately dissected to avoid potential microbial degradation. The gastrointestinal tract was removed and the liver immediately separated and discarded to avoid contaminating the stomach content DNA with any enzymes that could aggravate DNA degradation. The stomach was separated from the intestines and the stomach contents were removed and immediately processed for DNA extraction.
DNA Extraction and Sequencing
DNA extraction was performed on the stomach content using the ZR Soil Microbe DNA MiniPrep (Zymo Research, The Epigenetics Company, USA) following the manufacturer's protocol. DNA samples were eluted in 80 uL of elution buffer. DNA quality was assessed using a Nanodrop 8000 instrument and DNA concentration measured using a Qubit instrument with the High Sensitivity dsDNA assay kit.
A 730 bp 18S rRNA gene fragment was amplified using PCR and purified using Agencourt AMPure XP beads (Beckman Coulter). Purified DNA fragments were then used to construct an 18S library following the Illumina 16S Metagenomic Sequencing Library Preparation workflow adapted for Illumina's MiSeq System (Illumina, 2017). The protocol was adapted for 18S metagenomic profiling by replacing 16S primers with 18S primers 574*f and 1132r (Hugerth et al., 2014; Supplementary Table S1) and using 56°C as annealing temperature:
5′ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCGGTA
AYTCCAGCTCYV 3′,
5′ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCCGT
CAATTHCTTYAART 3′.
Samples amplified using 18S primers were indexed and purified following the Illumina 16S Metagenomic Sequencing Library Preparation protocol. The libraries were quantified using a Qubit instrument and average library size calculated by measuring amplicon size of 4 random samples using a DNA 7500 kit and a 2100 Bioanalyzer (Agilent Technologies). Library concentration was estimated using the following formula:
[(Concentration in ng/μl)/(660 g/mol × average library size)] × 106 = concentration in nM
Equimolar quantities of 18S libraries were then pooled and sequenced using MiSeq Reagent Kit v3 (Illumina) and a MiSeq instrument. The 18S metagenomic data is deposited in BioProject NCBI (Accession: PRJNA393758).
Bioinformatics and Statistical Analyses
Raw sequencing data (fastq files) were subject to quality control following standard protocols recommended by Illumina and were demultiplexed using the bcl2fastq 1.8.4 program (Illumina). Individual sample sequences were trimmed using the program Trimmomatic v0.32 to remove (i) sequencing primers/indexes, (ii) low quality reads and (iii) reads of length below 36 bp (Bolger et al., 2014). Quality control of trimmed reads was performed using FastQC 0.11.4 (Andrews, 2010). Resulting high quality reads (R1 only) were used for downstream analysis, including OTU clustering and taxonomy assignment, using USearch 8.1.1861 (Edgar, 2013), and QIIME 1.9.1 (Caporaso et al., 2010) (based on Pylro et al., 2014). Using USearch the following steps were performed: (i) removal of low quality reads, (ii) dereplication of sequences, (iii) removal of chimeric sequences, and (iv) OTU clustering based on 99% similarity. Taxonomy assignment of the OTUs was performed using QIIME based on a QIIME compatible version of Silva 111. Stringent filtering was done by keeping only OTUs supported by at least two reads and by limiting downstream analysis to OTUs found in at least 50% of a species' stomach samples to ensure that only truly representative dietary items were included in further analyses. To compare relative abundance of OTUs between samples, number of reads were normalized by converting them to fractions over the total number of reads within each individual sample. We note that a number of reads aligned to “Eukaryota, Metazoa, Craniata, and *,” as expected, given that DNA from the fishes themselves would be extracted and amplified. These reads were removed from the analysis prior to normalization. In the overview of the diet at the phylum level, the cutoff for contribution to the stomach content was 90% and the remaining ≤10% were grouped into the category “Other” (Figure 2).
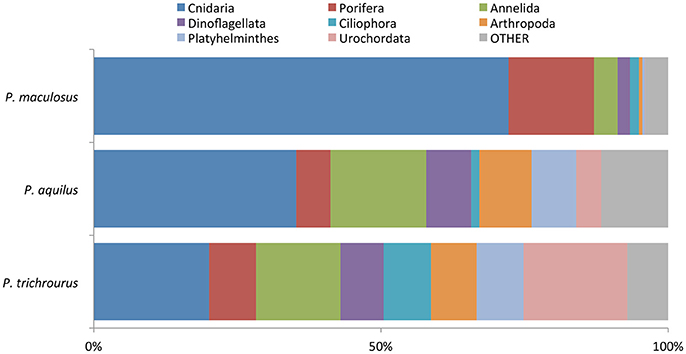
Figure 2. Breakdown of phyla observed in the stomach contents of three reef fish species in Abu Dhabi, UAE, averaged across all sampling periods. The “Other” category is comprised of Cercozoa, Echinodermata, Mollusca, Bryozoa, Apicomplexa and Bolidomonas, each of which was observed in all three fish species at low abundance. See Supplementary Table S3 for means, variances and standard errors.
For the purposes of exploring the taxonomic composition of stomach contents across seasons, the data were aligned and processed at multiple taxonomic levels. Accordingly, statistical and visual analyses were performed on the lowest possible taxonomic classification that did not result in a major loss of data and resolution.
Principal component analysis (PCA) and variance component analysis (VCA) were performed using JMP Genomics 8.0. In all VCAs, the variation required to be explained was across principal components (PC) PC1 to PC3 and the PCAs were based on Pearson correlation with scaled and centered data. SIMPER and PERMANOVA statistical analyses were carried out in Primer-e v7. For these analyses, read proportion data was transformed using the transformation asin[sqrt(Pi)], and abundance values were back-transformed using the formula [sin(Ti)] then converted to percentages. SIMPER analysis was performed with the following parameters: resemblance based on S17 Bray-Curtis similarity and cut off for low contributions was 70.00%. The PERMANOVA parameters were resemblance also based on S17 Bray-Curtis similarity, sums of squares Type III (partial), fixed effects sum to zero for mixed terms, permutation method was permutation of residuals under a reduced model with 999 permutations (PERMANOVA works with any distance measure that is appropriate to the data and uses permutations to make it distribution free; Primer-e, 2017)1. Sampling groups/periods were considered significantly different if p < 0.05 (PERMANOVA). For the alignment of Sanger sequences, the identified coral was based on the top hit in BLAST with the highest E-value (nucleotide collection nr/nt database; megablast).
Results
A total of 146 individual fish samples were collected over the course of this study, including 48 P. maculosus, 50 P. aquilus and 48 P. trichrourus, representing between 8 and 10 individuals per species per sampling period (Supplementary Table S2, S4). Most DNA samples had a yield > 400 ng and a SpeedVac instrument was used to concentrate eight samples that had a yield <20 ng. DNA samples were used as a template for 18S library preparation. Four samples were randomly chosen for amplicon size measurement using a Bioanalyzer 2100 instrument. Average amplicon size was approximately 735.5 bp as expected.
In total, 27,555,995 reads remained after trimming of 28,419,461 raw reads. Approximately 68% of the reads remained after USearch filtering and 35% of reads remained after dereplication. In total, 192,341 Operational Taxonomic Units (OTUs) (at 99% sequence identity) with minimum cluster size of 2 were identified from these filtered sequence reads of which 2,292 remained after applying the “minimum 2” filter and those were used for classification.
Overall, between 3 and 8 phyla accounted for approximately 90% of the stomach content (averaged across all samples in each species), with 6 to 11 rare phyla making up the remaining 10%. Stomach contents in P. maculosus were heavily dominated by animals from the phyla Cnidaria (72.2% of contents), Porifera (14.9%), and Annelida (4.1%), with the remaining 8.8% composed of organisms from 11 other phyla (Figure 2). Cnidarians also dominated the stomach content of P. aquilus, but to a lesser degree (35.3% of contents), with relatively important contributions (totaling 47.3% of contents) from the phyla Annelida, Arthropoda, Dinoflagellata, Platyhelminthes, and Porifera (Figure 2), with 8 additional phyla making up the remaining 17% of the stomach contents. There was a much more even composition of stomach contents in P. trichrourus, with 8 phyla each contributing between 7 and 19% of stomach contents (Figure 2; Cnidaria, Urochordata, Annelida, Ciliophora, Porifera, Platyhelminthes, Arthropoda, and Dinoflagellata), with six additional phyla comprising the remaining 7% (Figure 2). Phyla mean, variance, and standard error values are shown in detail in Supplementary Table S3. To further explore the taxonomic composition of stomach contents across seasons, the data were next processed at the Class level; due to the stringency of the alignment analysis, lower taxonomic classification (i.e., assigning OTUs to Family or Order levels) resulted in loss of resolution.
Seasonal changes in the relative abundance of dietary items in the stomachs of the three fish species is illustrated in Figure 3. P. maculosus showed the highest consistency in diet among individuals and across seasons (Figure 3A). The dominant component of their stomach content across the entire study was Anthozoa (corals), reflecting the heavy weighting of Cnidarians in the Phylum-based analyses above (Figure 2), with corals making up 74.6% of stomach content reads averaged across all seasons. Sponges (Demospongiae) were the second-most common dietary item, but this was mainly due to a spike in sponge abundance in the stomachs of many P. maculosus in December when it made up 48.0% of stomach contents, which coincided with a relative decrease in corals in many individuals (December coral: 44.6% on average). However, excluding December, sponges were a minor component of stomach contents in the remaining sampling periods (mean: 10.4%). Other taxa made very minor contributions to the overall diet for P. maculosus (each class <8.6% of reads in a given season), and mainly represented minor variation among individuals.
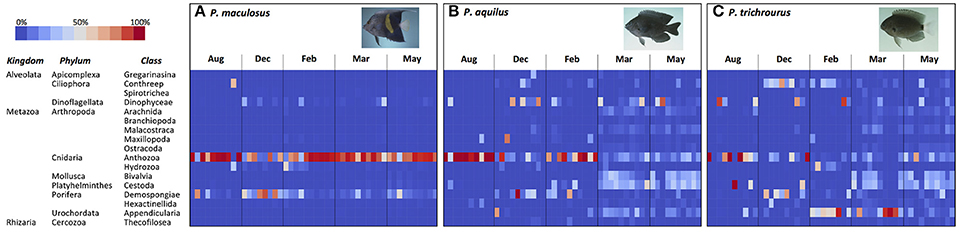
Figure 3. Heatmap illustrating stomach content composition in three species of reef fish (A, P. maculosus; B, P. aquilus; C, P. trichrourus) collected over five sampling periods in Abu Dhabi, UAE. Each cell in the heatmap represents the relative abundance (percent of reads from stomach contents; see key) of a class of organisms in a sample's stomach at a particular sampling period (month). Fish images from http://www.fishbase.org (Randall, unpublished).
The two Pomacentrus species each showed a greater variety of dietary items and a greater degree of temporal variability in stomach contents than were observed in P. maculosus, with more variability among individual fishes (Figure 3). The main contributors to the stomach content of P. aquilus, averaged across the year, were Anthozoa (40.6%), Dinophyceae (12.1%), Cestoda (11.8%), Demospongiae (7.3%), and Bivalvia (7.1%) with all other classes each contributing <5.9% to the overall diet. However, there was considerable temporal variation in stomach content composition (Figure 3B). While corals (Anthozoa) heavily dominated the P. aquilus diet in the hottest month (August) and the coldest month (February) where it made up 89.4 and 67.6% of stomach contents, respectively, it became relatively less common in the transitional months of December, March, and May when other organisms became major dietary contributors (Figure 3B). In December, corals (Anthozoa) declined to 18.8% of contents, while dinoflagellates (Dinophycaeae) and sponges (Demospongiae) became major contributors across the population (22.5 and 20.5% of stomach contents, respectively). Dietary variety was greatest in the spring (March and May), when P. aquilus diets included relatively equal contributions of bivalve molluscs (Bivalvia: 17.7%), dinoflagellates (Dinophyceae: 14.6% of stomach content, averaged across March and May), free-swimming ascidians (Appendicularia: 10%), sea-spiders (Arachnida: 5.2%), and crustaceans (Malacostraca: 4.6%), with corals (Anthozoa) also making an important contribution (13.7%). Interestingly, parasitic flatworms (Cestoda) were very abundant in virtually all P. aquilus individuals in both March and May (25.4 and 24.8% of stomach contents, respectively). Various other Classes of organisms were also present in stomachs at low but meaningful levels, and this dietary heterogeneity was consistent across individuals throughout the P. aquilus population in March and May (Figure 3B).
Like P. aquilus, the stomach content of P. trichrourus had considerable variability in dietary items, and these varied seasonally (Figure 3C). In August, corals (Anthozoa) dominated the stomach content of P. trichrourus (51.5% on average), although a few individual fish had substantial contributions from a small number of other taxa (e.g., Dinophyceae and Demospongiae). As temperatures cooled through the autumn and transitioned into the winter and spring, P. trichrourus diets shifted (Figure 3C). In December, corals became less common (20.2%), while the ciliate protozoan group CONThreeP and the sponges (Demospongiae) became the dominant items in stomachs (24.7 and 24.1% on average, respectively). By February, the coldest month, corals and sponges became minor components of the stomach contents as the free-swimming ascidians, Appendicularia, came to dominate the stomachs of P. trichrourus (49.4%, on average), with CONThreeP remaining a major contributor as well (14.5%; Figure 3C). Similar to what was observed in P. aquilus, the diet of P. trichrourus markedly diversified in the spring, with stomach contents made up of a broader assortment of taxa in generally comparable abundance (Figure 3C). While the free-swimming ascidians (Appendicularia) were among the most common stomach items in March (43.5%), this was mainly due to high abundance in just a few individual fish, with the majority of the fish stomachs containing a diverse assortment of taxa (Figure 3C). By May, there was broader representation among a large number of taxa (9 Classes each had >4% representation in stomach contents), with parasitic flatworms (Cestoda), corals and bivalves being the most common stomach contents (21.5, 19.5, and 16.9%, respectively); cestodes were also relatively common in P. trichrourus stomachs in March (16.4%; Figure 3C).
Next, multivariate analyses were used to explore variation in the composition of stomach content among species and sampling periods. PCA of the full dataset across the three fish species and all sampling periods was performed to examine the correlation structure in the data and relationships between individual fish samples relative to taxonomic status and sampling period (Figure 4A). This analysis revealed a high correlation structure in the data with PC1-3 explaining 64% of total observed variation and highlighted the distinctiveness of P. maculosus‘s stomach content driving the variation observed along PC1 while P. aquilus and P. trichrourus are largely overlapping. The relatively tight grouping of P. maculosus samples reflects a high degree of temporal similarity while the broader spread of samples across both axes for the two Pomacentrus species indicates more variable stomach contents in these species among sampling periods (Figure 4A). VCA was performed to quantify the relative contribution of species, sampling period and their interaction to the observed variance. This analysis revealed that 59.9% of the total variance is explained of these three effects (Figure 4B) with most of the variance explained by the species effect (29.3%) followed by species*sampling period interaction (17.7%) and sampling period (12.9%).
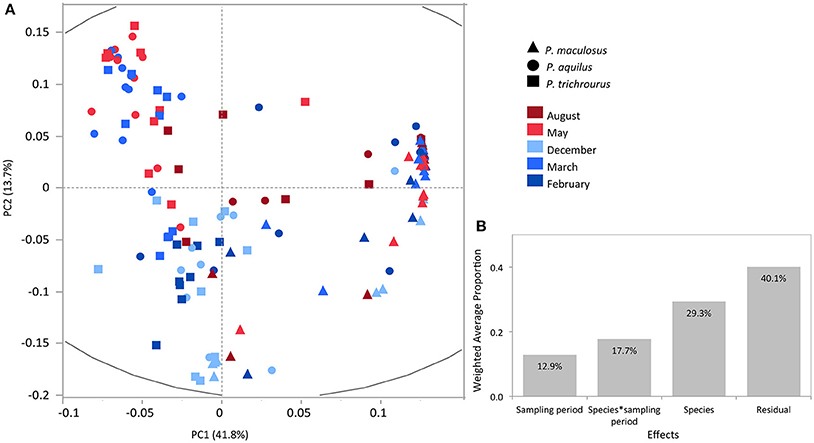
Figure 4. Principal component (PC) and variance component (VC) analyses of the combined dataset. (A) PC1 and PC2 together explain 54.5% of the total variance. (B) VC analysis of PC1-3 for species, sampling period and the interaction between species and sampling period.
Next, PCA was used to quantify the influence of seasonality on stomach contents for each species separately (Figures 5A–C). PC1-3 explain 69, 58, and 53% of the total variation in the data in P. maculosus, P. aquilus, and P. trichrourus, respectively. While the first three PCs explain similar proportions of the variance in each species, PC plots shown in Figures 5A–C highlight both patterns that are shared among the three species as well as species-specific patterns. For example, the March-May samples in each species largely cluster together along one axis (PC1: P. aquilus and P. trichrourus; PC2: P. maculosus). The overall effect of seasonality on each individual species was quantified using VCA and revealed that sampling period accounted for 37.8, 57.7, and 47.1% of the combined PC1-3 variance in P. maculosus, P. aquilus, and P. trichrourus, respectively (Figure 5D). These results are consistent with the patterns visible in Figures 2, 3 further highlighting the more seasonally structured data in P. aquilus and P. trichrourus relative to P. maculosus.
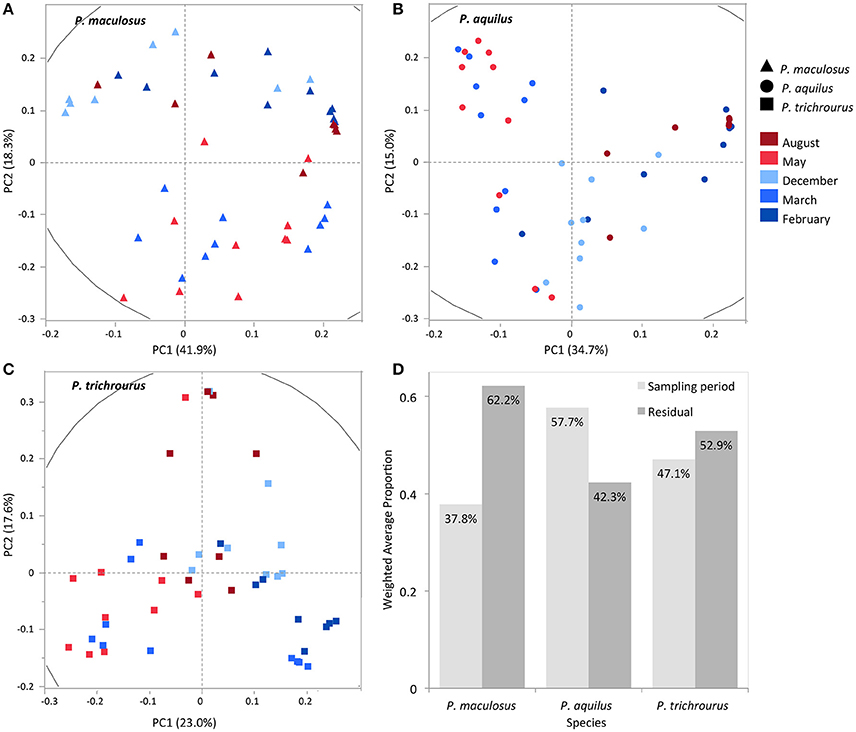
Figure 5. Principal component (PC) and variance component (VC) analysis across each species' dataset. (A) PC1 and PC2 together explain 60.2% of the total variance in P. maculosus, (B) 49.7% in P. aquilus, and (C) 40.6% in P. trichrourus. (D) VC analysis of PC1-3 in each species group for sampling period.
SIMPER analyses were used to determine the diet components driving the observed variation in each species and to quantify their relative contribution. SIMPER showed 72.3% similarity in stomach contents across all seasons in P. maculosus, reflecting relatively limited temporal variability in this species. In P. maculosus there was no difference in stomach composition in the spring sampling periods (March and May) nor between the two most thermally extreme sampling periods (August and February); there was also no difference between February and May (PERMANOVA p > 0.05 each). All other pairwise comparisons between seasons were significant (PERMANOVA p < 0.05) for P. maculosus. primary taxa characterizing P. maculosus stomach content were corals and sponges, which together contributed 83% to the similarity in the stomach contents for this species. The greater overall variability in stomach content in the two Pomacentrus species was reflected with lower similarity values in SIMPER (P. aquilus: 54.7%; P. trichrourus: 42.8%, compared with 72.3% in P. maculosus, Table 1), and each of these species were shown to have significant seasonal shifts in diet with PERMANOVA. In P. aquilus, only August and February samples and March and May samples did not differ significantly (pairwise PERMANOVA p > 0.05 each), while in P. trichrourus all sampling periods differed significantly (pairwise PERMANOVA p < 0.05 each). The primary taxa characterizing P. aquilus were corals, cestodes and bivalves, which together contributed over 54.7% to similarity in stomach content in this species in SIMPER, while corals, appendicularians, cestodes, CONThreeP, and bivalves were strongly associated with P. trichrourus, together contributing 42.8% to similarity.
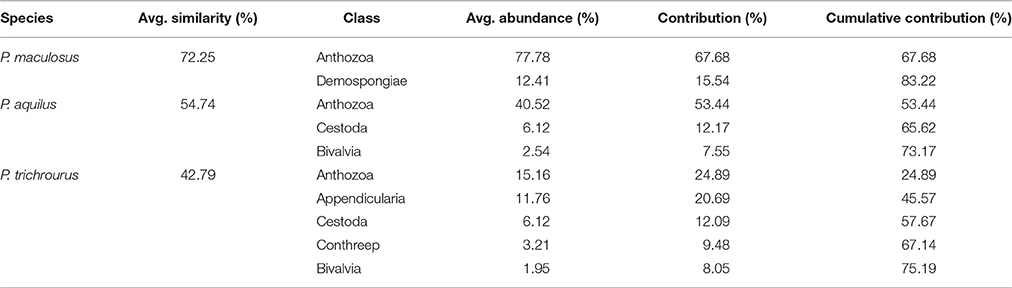
Table 1. Results of SIMPER analysis of similarity within each species group showing top stomach content contributors (cut off 70%).
In addition to exploring taxonomic composition within species, SIMPER was used to identify taxa driving differences in stomach contents between species. All pairwise comparisons showed moderately strong dissimilarity in stomach contents between species (55–68% dissimilarity, Table 2), but the only significant difference was between P. maculosus and P. trichrourus (PERMANOVA p < 0.05, Table 2); stomach contents did not differ between the two Pomacentrus species nor between P. maculosus and P. aquilus (PERMANOVA p > 0.05 each, Table 2). The primary driver of divergence between P. maculosus and P. trichrourus was coral (Anthozoa), which was more than five times more abundant in P. maculosus stomachs, and contributed nearly a third of the dissimilarity between these groups (Table 2). Sponges (Demospongiae) were also four times more common in P. maculosus, while the free-swimming ascidians (Appendicularia) were virtually nonexistent in the angelfish, but made up nearly 12% of the diet of P. trichrourus, with these prey items together contributing another 25% to dissimilarity between these fishes. See Supplementary Tables S5 and S6 for detailed SIMPER and PERMANOVA results in pairs of sampling periods.
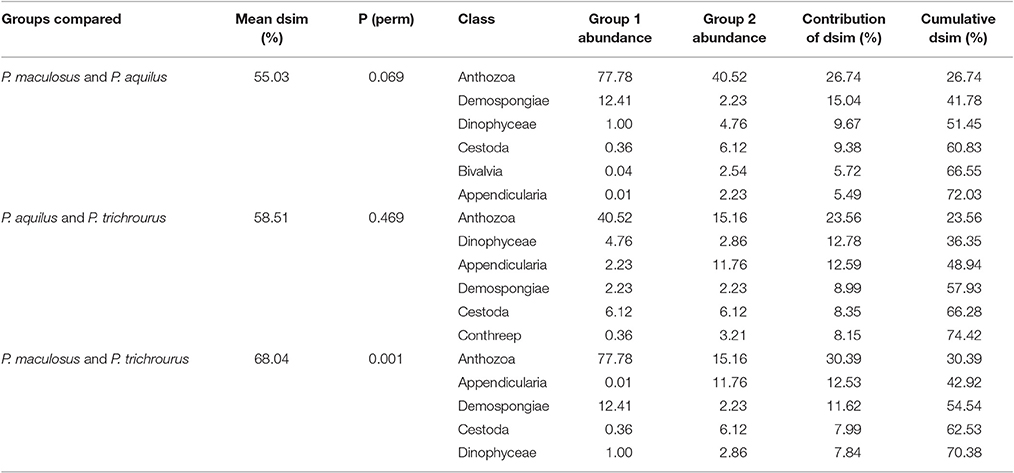
Table 2. Results of SIMPER and PERMANOVA analyses of dissimilarity (dsim) between pairs of groups of species, showing the top contributors to the total dissimilarity in each group (cut off 70%).
One of the surprising observations was the dominance of Anthozoa (corals) in stomach content across the entire study (Figures 2, 3 and SIMPER analysis). To confirm this observation, we performed an independent non-NGS based validation exercise using Sanger sequencing with different primers and genetic loci. DNA was extracted from two P. maculosus stomach samples and two P. aquilus samples, all collected in August. DNA was PCR amplified using two pairs of primers: the first targets the nuclear ribosomal ITS region in Porites corals (Forsman et al., 2009) and the second targets the NAD mitochondrial loci in corals (primer pair ND51a; Concepcion et al., 2006). Amplified PCR fragments were verified using gel electrophoresis and purified using QIAquick Gel Extraction Kit (Qiagen, USA). The amplified fragments had the expected sizes. Forward and reverse sequencing was performed on an ABI PRISM 3730xl Genetic Analyzer (Applied Biosystems, USA) instrument using standard protocols. Sequence files were viewed and cleaned using FinchTV software. All four samples yielded good quality sequences that were subject to BLAST alignment (NCBI). The sequences aligned mainly to Porites and Platygyra species (Table 3). In both P. maculosus samples, the Porites-specific fragment aligned exclusively to Porites lutea, while the fragment from both P. aquilus samples aligned to both P. lutea and “Porites spp.” (N.B. Porites spp. represents a group of Porites that cannot be resolved to species level). Porites spp. were also observed in both fish species using the NAD marker, while P. maculosus was shown to also have Platygyra and/or Montastrea in some stomach samples (the difference cannot be resolved based on the data, but presumably represents the regionally common Platygyra as Montastrea is not known for the Arabian Gulf) (Riegl et al., 2011; Table 3).
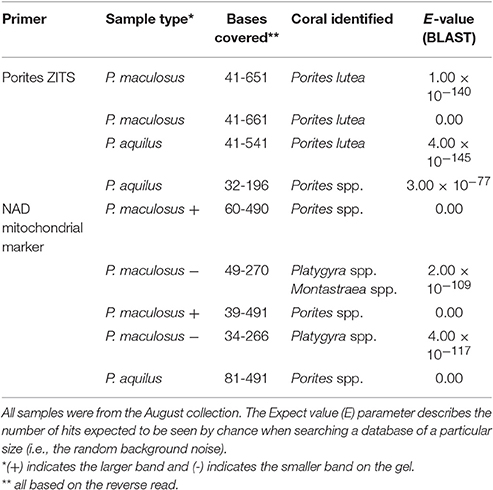
Table 3. Corals identified from Sanger sequencing of two P. maculosus samples and two P. aquilus samples.
Discussion
Coral reefs have undergone considerable change in recent decades as a result of local anthropogenic pressure and SST anomalies (Hughes et al., 2003). Climate change models predict that coral bleaching will increase in both severity and frequency in the future (Diaz-Pulido et al., 2009). Resultant changes in the benthic composition of reefs will likely affect structural and functional attributes of reef fish communities (Wilson et al., 2006; Baker et al., 2008; Munday et al., 2008; Pratchett et al., 2008; de Bakker et al., 2017), with particular effects likely to occur in the feeding ecology of this diverse and important group of organisms (Floeter et al., 2004; Berumen and Pratchett, 2006; Pratchett et al., 2008; Wilson et al., 2008). The Arabian Gulf is the most thermally extreme coral reef environment on earth (Riegl et al., 2011), and as such represents a unique natural laboratory in which to examine the trophic ecology of reef fishes in an extreme environment. The current study is the first to examine the diets of coral reef fishes in the region, with the metagenomics approach used here allowing for a much more comprehensive assessment of the diets of these three reef fish species than has historically been available globally. Our results provide important data on the diet of reef fishes in this thermally extreme environment, which may be useful in shaping further studies and questions on reef fish adaptability to climate change and highly variable seasonal fluctuations in temperature in other regions.
The metagenomics approach employed here allowed for an unbiased and much broader assessment of the dietary items in the stomachs of these fishes than would be possible with traditional approaches. The small size and partially digested nature of stomach contents can lead to bias, inaccuracy and misidentification in traditional microscopy-based stomach content surveys (Carreon-Martinez and Heath, 2010; Paquin et al., 2014). The sequencing-based approach used here, however, requires only minute quantities of genetic material, allowing not only classification of food items that are partially digested, but also items that are individually microscopic and/or unidentifiable but contribute substantially to the overall variety and/or volume of items in stomachs (Budarf et al., 2011; Paquin et al., 2014; Miyake et al., 2015). One limitation of the metagenomics approach is the inability to differentiate between primary food items and incidental food items (Harms-Tuohy et al., 2016). A high abundance of dinoflagellates was observed in the fish diets here, but these are living within corals as well as free-living in sediments so it cannot be determined whether they were consumed as free living or incidentally when consuming corals (Carpenter et al., 1997). Nonetheless, if the item is consistently observed across samples then it is a component of the diet, regardless of the means of consumption. Additionally, while the 18S amplicon approach results in a loss of taxonomic resolution, it allows a much broader coverage that is preferable in diet assessment (Albaina et al., 2016). Limited feeding observations and traditional stomach content analyses from other regions has suggested that P. maculosus feed mainly on sponges and algae, P. aquilus on zooplankton, and P. trichrourus on benthic algae (Allen and Randall, 1980; Masuda and Allen, 1993; ter Hofstede, 1998; Salameh et al., 2012). Our results greatly expand the knowledge of the composition of diets for these species, showing that each of these species is consuming a much wider array of prey items than had previously been appreciated. We observed 17 distinct taxonomic classes across 10 phyla in the stomach contents of the three fish species examined, in addition to 5 phyla where the OTUs did not align to lower taxonomic classifications. Overall, only a small fraction of these food items would have been identifiable under a microscope, with the majority of the taxa occurring in the stomachs extremely difficult or impossible to identify using traditional visual approaches (e.g., a wide variety of microscopic protozoans, heavily fragmented arthropod parts, morphologically indistinct pieces of coral tissue). The better representation of these morphologically indistinct but taxonomically diverse food items validates our use of genomics-based approaches to provide a more comprehensive and taxonomically rich understanding of the food items consumed by these reef fishes.
Perhaps the most interesting observation of this study was that corals are a major dietary component of all three of these species, despite none being considered corallivores, and corals not previously reported as important components of their diet (Masuda and Allen, 1993; Carpenter et al., 1997; ter Hofstede, 1998; Froese and Pauly, 2017). Of note, the genera of corals observed in the diet, as shown using Sanger sequencing, largely reflect the dominant corals observed on reefs in the southern Gulf, namely Porites and Platygyra (70.4% and 13.3% of live coral at Saadiyat reef, respectively; Burt et al., 2011a). Supplementary Figure S1 shows the breakdown of the benthos in the Saadiyat region, where coral makes up over 60% of the overall benthos. Corals likely represent an important but underappreciated nutritional resource for many reef fishes that are not considered corallivores. For example, coral gametes are known to provide substantial benefits to diverse groups of coral reef fishes that opportunistically consume them during coral spawning (Pratchett et al., 2001; McCormick, 2003). In addition, coral mucous coats trap organic detrital material from the surrounding water column, with such enriched material likely representing a substantial food resource for many reef fishes (Wilson et al., 2003). Thus, coral likely represents an important component of the diet of many reef fishes, but its role has previously been underappreciated due to the difficulty of detecting coral in partially digested stomach contents using traditional techniques (Zekeria et al., 2002; Pratchett et al., 2008).
While it could be argued that the prevalence of corals in the stomachs of the fish studied here is due to incidental consumption, with coral tissues being ingested accidentally while foraging for other items, the high proportion of corals in the overall diet and over various sampling periods among virtually all individuals suggests otherwise. Remarkably, P. maculosus in the southern Arabian Gulf appears to be somewhat of a coral-specialist in terms of its diet, with corals making up 45–85% of its diet in a given sampling period, on average. In other regions, the family of Pomacanthidae is generally known to be omnivorous feeders, with sponges and algae dominating their diets (Andréa et al., 2007; Batista et al., 2012), with corals typically making up <2% of their stomach contents (ter Hofstede, 1998). It is also noteworthy that this family is highly diverse in its feeding ecology (Konow and Bellwood, 2011). While our results show that sponges are consumed by P. maculosus throughout the year in the Arabian Gulf, sponges are generally a minor component of the P. maculosus diet (16% overall) relative to coral (75% overall). Sponges are relatively rare in this region (<1% of reef benthos, Burt et al., 2011a), perhaps due to the extreme nature of the environment and/or as a result of overgrazing due to the unusually high abundance of P. maculosus on these reefs (>400 ha−1 in the southern Gulf, Burt et al., 2011b). Regardless of the cause, P. maculosus in the sampled region have focused their diet toward corals, and this is consistent across all individuals and seasons we examined.
The two damselfishes generally had a more varied and flexible diet than the angelfish (within species average similarity of stomach contents across seasons ca. 49%, compared to 72% in the angelfish). Although corals also appear to be a major food source for the damselfish, particularly in summer when it made up >50% of the diet of each damselfish species, there was a shift away from coral dominance and instead a broadening of the food items incorporated into the Pomacentrus diets in December, March and May when corals made up <20% of their diets, suggesting that corals may not be a preferred food source. Corals are generally considered an energy-poor resource (Aeby, 2002), with higher costs related to handling time that further decrease the relative value of these foods for smaller bodied fishes, such as the two damselfish examined here (Tricas, 1989). While these damselfishes may need to rely on coral to supplement their diet in the hot summer months when their metabolic demands will be at their highest (Nilsson et al., 2009), the relative decrease of corals in their diets in December, March and May suggests that their diet is shifting as a result of temperature-related differences in preference and/or food availability. The spring season (March and May) is the time of rapid population growth and spawning of many reef-associated organisms in the southern Gulf (Alsaffar and Lone, 2000; George, 2012; Grandcourt, 2012; Howells et al., 2016), and the broadening of damselfish diets to include large contribution from bivalves, ascidians, sponges and other taxa during these months may simply reflect increased abundance of food items available, or a shift in preference. Interestingly, there is also a notable increase in the parasitic cestode worms in the stomachs of both damselfish species in March and May (P. aquilus: spring mean = 25 vs. 3% mean in all other seasons; P. trichrourus spring mean = 19 vs. 8% mean in all other seasons). While this may represent increased ingestion of cestodes from the surrounding environment, the observed increase in these parasites in stomachs in spring may also reflect weakened host immune systems following several months of cold winter temperatures. A diverse array of cestodes are known to occur in the Arabian Gulf, and the prevalence and intensity of infection in fishes here is generally considered to be high compared with other regions (e.g., 27.2% of individuals infected across a suite of commercial fish species; El-Naffar et al., 1992; Al Kawari et al., 1996). It is unclear why the incidence of cestodes would be substantially higher in the two Pomacentrus species as opposed to the P. maculosus, but earlier surveys of infection rates across various fishes in the Arabian Gulf have shown higher infection rates in species with broader diets (El-Naffar et al., 1992), suggesting that the more coral/sponge-specific diet of P. maculosus may reduce opportunity for infection.
In addition to the general commonness of corals in the diet of these three fish species, the virtual absence of algae in the diets of these fishes is also notable. In other regions, available dietary reports have indicated various algae as contributors to the diet of P. maculosus (ter Hofstede, 1998), and in situ bite observations suggest that algae is a major component of the diet of P. trichrourus (Masuda and Allen, 1993). Algae (mainly turf and CCA) are among the most common benthos on reefs in the southern Gulf, occupying more space than corals on many reefs (Burt et al., 2011a). Various classes of algae (Chlorophyta) were observed in all three fish species in all sampling periods, but at abundance so low that it was removed during the data treatment and filtering process as it did not meet minimum cut-off standards for inclusion in this study (see methods), possibly indicating that while present in abundance on Saadiyat reef it was not being preferentially consumed by any of the fish species examined, suggesting that algae here have low palatability. Various algae are known to produce secondary metabolites that can deter feeding by reef fishes (Hay et al., 1987), and it is possible that algae here are employing such chemical defenses. It is also possible that the heavy sediment loads experienced on these reefs plays a role. Wind-induced sediment resuspension is common on the shallow (<6 m) reefs of the southern Gulf (Riegl, 1999), and these sediments can become trapped in the epilithic algal matrix (EAM). Higher sediment loads have been shown to decrease the energetic value of detrital material trapped in the EAM (Purcell and Bellwood, 2001; Gordon et al., 2016) and reduce reef fish grazing rates (Bellwood and Fulton, 2008; Goatley and Bellwood, 2012), providing another plausible reason for the limited algal consumption by the three fish species examined here.
In the coming century climate change is expected to induce SST increases that will result in many parts of the tropics developing a thermal environment similar to the summer temperatures experienced in the Arabian Gulf today (Riegl, 1999; Collins et al., 2013; Wernberg et al., 2013). These temperature increases are likely to have profound impacts on fish communities, with shifts in benthic communities having indirect influence on the structure and function of fish assemblages (Baker et al., 2008; de Bakker et al., 2017) and thermal stress directly affecting the physiology and behavior of fishes themselves (Floeter et al., 2004; Berumen and Pratchett, 2006; Wilson et al., 2006; Munday et al., 2008; Pratchett et al., 2008). This study examined the diet of three common reef fish in the most thermally extreme environment on earth, providing unique insights into how reef fish have adapted trophically. Overall, our results indicate a greater variety and degree of flexibility in diets in these reef fishes than is generally appreciated, indicating that dietary plasticity has allowed these species to respond to the unique resource and environmental constraints placed on them in the Gulf. Since the metagenomic approach limits the dietary taxonomic resolution, we may also be overestimating their dietary commonality (Longenecker, 2007). Higher taxonomic resolution might demonstrate further trophic diversity and food specialization between fish species (Longenecker, 2007). However, the variety and flexibility presented here also suggests that reef fish species with more flexible and/or generalist diets are likely to be more successful on future reefs in other regions. While the broader flexibility in diets at first glance appears to be of benefit, this flexibility may also come at a cost. For example, our results show that P. maculosus in the Arabian Gulf is feeding primarily on coral, but coral is known to have low nutritional value (Tricas, 1989; Aeby, 2002) and it is unknown whether its focus on corals—rather than the expected sponge/algae diet—has an energetic cost that translates into lower performance at the individual or population level. Additionally, over the long-term the degree of corallivory by the hyper-abundant P. maculosus and the very common P. aquilus and P. trichrourus populations on these reefs (Burt et al., 2011b) may contribute to the further depletion of corals that are already under pressure from recurrent bleaching events and localized development activities (Sheppard et al., 2010; Sale et al., 2011). Further research is needed to examine the broader implications of the unique feeding habits of coral reef fishes living in this extreme environment. In future studies, to compensate for the quantitative and taxonomic shortcomings of the metagenomics approach, it may also be useful to pair the approach with traditional stomach volume observations and a reference amplicon library specific to the region (e.g., Albaina et al., 2016).
Author Contributions
The following contributions were made by authors: Project conception and design: JB, YI, and RS. Sample collection, processing and sequencing: GV, DM, RS, and MD. Data analyses and interpretation: JB, YI, MD, MV, and RS. Writing and revision of manuscript: RS, JB, and YI.
Funding
The study is supported by New York University Abu Dhabi research funds to RS, YI, and JB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer DJB and handling Editor declared their shared affiliation.
Acknowledgments
The authors would like to thank the New York University Abu Dhabi Internal Review Board for IACUC approval and the Environment Agency Abu Dhabi for sampling permits. Sequencing and data processing were supported by the New York University Abu Dhabi Sequencing and Bioinformatics Core teams, and their assistance is greatly appreciated.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00285/full#supplementary-material
Footnotes
1. ^http://www.primer-e.com/permanova.htm (Accessed Jun 28, 2017).
References
Aeby, G. S. (2002). Trade-offs for the butterflyfish, chaetodon multicinctus, when feeding on coral prey infected with trematode metacercariae. Behav. Ecol. Sociobiol. 52, 158–165. doi: 10.1007/s00265-002-0490-2
Albaina, A., Aguirre, M., Abad, D., Santos, M., and Estonba, A. (2016). 18S rRNA V9 metabarcoding for diet characterization: a critical evaluation with two sympatric zooplanktivorous fish species. Ecol. Evol. 6, 1809–1824. doi: 10.1002/ece3.1986
Al Kawari, K. S., Saoud, M. F. A., and Ramadan, M. M. (1996). Biodiversity of helminth parasites of fishes in the Arabian Gulf, with special reference to digenetic trematodes and cestodes. Qatar Univer. Sci. J. 16, 141–153.
Allan, B. J., Domenici, P., Munday, P. L., and McCormick, M. I. (2015). Feeling the heat: the effect of acute temperature changes on predator–prey interactions in coral reef fish. Conserv. Physiol. 3:cov011. doi: 10.1093/conphys/cov011
Allen, G. R., and Randall, J. E. (1980). A review of the damselfishes (Teleostei: Pomacentridae) of the Red Sea. Isr. J. Zool. 29, 1–98. doi: 10.1080/00212210.1980.10688486
Alsaffar, A. H., and Lone, K. P. (2000). Reproductive cycles of Diadema setosum and Echinometra mathaei (Echinoidea: echinodermata) from Kuwait (northern Arabian Gulf). Bull. Mar. Sci. 67, 845–856.
Andréa, B. R., Batista, D., Sampaio, C. L., and Muricy, G. (2007). “Spongivory by juvenile angelfish (Pomacanthidae) in Salvador, Bahia State, Brazil,” in Porifera Research: Biodiversity, Innovation and Sustainability, ed M. R. Custódio (Rio de Janeiro: Museu Nacional), 131–137.
Andrews, S. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (Accessed May 5, 2017).
Baker, A. C., Glynn, P. W., and Riegl, B. (2008). Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471. doi: 10.1016/j.ecss.2008.09.003
Batista, D., Muricy, G. R. D. S., Andréa, B. R., and Villaça, R. C. (2012). High intraspecific variation in the diet of the french angelfish Pomacanthus paru in the south-western Atlantic. Braz. J. Oceanogr. 60, 449–454. doi: 10.1590/S1679-87592012000300015
Bauman, A. G., Feary, D. A., Heron, S. F., Pratchett, M. S., and Burt, J. A. (2012). Multiple environmental factors influence the spatial distribution and structure of reef communities in the northeastern Arabian Peninsula. Mar. Pollut. Bull. 72, 302–312. doi: 10.1016/j.marpolbul.2012.10.013
Bellwood, D. R., and Fulton, C. J. (2008). Sediment mediated suppression of herbivory on coral reefs: decreasing resilience to rising sea levels and climate change?. Limnol. Oceanogr. 53, 2695–2701. doi: 10.4319/lo.2008.53.6.2695
Berumen, M. L., and Pratchett, M. S. (2006). Recovery without resilience: persistent disturbance and long-term shifts in the structure of fish and coral communities at Tiahura Reef, Moorea. Coral reefs 25, 647–653. doi: 10.1007/s00338-006-0145-2
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Budarf, A. C., Burfeind, D. D., Loh, W. K. W., and Tibetts, I. R. (2011). Identification of seagrasses in the gut of a marine herbivorous fish using DNA barcoding and visual inspection techniques. J. Fish Biol. 79, 112–121. doi: 10.1111/j.1095-8649.2011.02999.x
Burt, J., Al-Harthi, S., and Al-Cibahy, A. (2011a). Long-term impacts of coral bleaching events on the world's warmest reefs. Mar. Environ. Res. 72, 225–229. doi: 10.1016/j.marenvres.2011.08.005
Burt, J., Feary, D., Bauman, A., Usseglio, P., Cavalcante, G., and Sale, P. (2011b). Biogeographic patterns of reef fish community structure in the northeastern Arabian Peninsula. ICES J. Mar. Sci. 68, 1875–1883. doi: 10.1093/icesjms/fsr129
Burt, J., Feary, D., Cavalcante, G., Bauman, A., and Usseglio, P. (2013). Urban breakwaters as reef fish habitat in the Persian Gulf. Mar. Pollut. Bull. 72, 342–350. doi: 10.1016/j.marpolbul.2012.10.019
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Carpenter, K. E., Krupp, F., Jones, D., and Zajonz, U. (1997). FAO Species Guide for Fishery Purposes: The Living Marine Resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates. Rome: Food and Agriculture Organization of the United Nations.
Carreon-Martinez, L., and Heath, D. D. (2010). Revolution in food web analysis and trophic ecology: diet analysis by DNA and stable isotope analysis. Mol. Ecol. 19, 25–27. doi: 10.1111/j.1365-294X.2009.04412.x
Chong-Seng, K. M., Mannering, T. D., Pratchett, M. S., Bellwood, D. R., and Graham, N. A. (2012). The influence of coral reef benthic condition on associated fish assemblages. PLoS ONE 7:e42167. doi: 10.1371/journal.pone.0042167
Clare, E. L. (2014). Molecular detection of trophic interactions: emerging trends, distinct advantages, significant considerations and conservation applications. Evol. Appl. 7, 1144–1157. doi: 10.1111/eva.12225
Coker, D. J., Wilson, S. K., and Pratchett, M. S. (2014). Importance of live coral habitat for reef fishes. Rev. Fish Biol. Fish 24, 89–126. doi: 10.1007/s11160-013-9319-5
Cole, A. J., Lawton, R. J., Pratchett, M. S., and Wilson, S. K. (2011). Chronic coral consumption by butterflyfishes. Coral Reefs 30, 85–93. doi: 10.1007/s00338-010-0674-6
Coles, S. (2003). Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: a comparison to the Indo-Pacific region. Atoll Res. Bull. 507, 1–19. doi: 10.5479/si.00775630.507.1
Coles, S. L. (1997). Reef corals occurring in a highly fluctuating temperature environment at Fahal Island, Gulf of Oman (Indian Ocean). Coral Reefs 16, 269–272. doi: 10.1007/s003380050084
Collins, M., Knutti, R., Arblaster, J., Dufresne, J. L., Fichefet, T., Friedlingstein, P., et al. (2013). Long-Term Climate Change: Projections, Commitments and Irreversibility. Cambridge: Cambridge University Press.
Concepcion, G. T., Medina, M., and Toonen, R. J. (2006). Noncoding mitochondrial loci for corals. Mol. Ecol. Resour. 6, 1208–1211. doi: 10.1111/j.1471-8286.2006.01493.x
de Bakker, D. M., van Duyl, F. C., Bak, R. P., Nugues, M. M., Nieuwland, G., and Meesters, E. H. (2017). 40 Years of benthic community change on the Caribbean reefs of Curaçao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs 1–13. doi: 10.1007/s00338-016-1534-9
Diaz-Pulido, G., McCook, L. J., Dove, S., Berkelmans, R., Roff, G., Kline, D. I., et al. (2009). Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4:e5239. doi: 10.1371/journal.pone.0005239
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
El-Naffar, M., Gobashy, A., El-Etreby, S., and Kardousha, M. (1992). General survey of helminth parasite genera of Arabian Gulf fishes (Coasts of United Arab Emirates). Arab Gulf J. Sci. Res. 10, 99–110.
Feary, D. A., Burt, J. A., Bauman, A. G., Usseglio, P., Sale, P. F., and Cavalcante, G. H. (2010). Fish communities on the world's warmest reefs: what can they tell us about the effects of climate change in the future?. J. Fish Biol. 77, 1931–1947. doi: 10.1111/j.1095-8649.2010.02777.x
Feary, D. A., Burt, J. A., Cavalcante, G. H., and Bauman, A. G. (2012). “Extreme physical factors and the structure of Gulf fish and reef communities,” in Coral Reefs of the Gulf, Coral Reefs of the World, Vol. 3, eds B. M. Riegl and S. J. Purkis (Dordrecht: Springer), 163–170.
Floeter, S. R., Ferreira, C. E. L., Dominici-Arosemena, A., and Zalmon, I. R. (2004). Latitudinal gradients in Atlantic reef fish communities: trophic structure and spatial use patterns. J. Fish Biol. 64, 1680–1699. doi: 10.1111/j.0022-1112.2004.00428.x
Forsman, Z. H., Barshis, D. J., Hunter, C. L., and Toonen, R. J. (2009). Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol. Biol. 9:45. doi: 10.1186/1471-2148-9-45
Froese, R., and Pauly, D. (2017). FishBase. Available online at: http://www.fishbase.org (Accessed Feb 8, 2017).
George, J. D. (2012). “Reef-associated macroinvertebrates of the SE Gulf,” in Coral Reefs of the Gulf, Coral Reefs of the World, Vol. 3, eds B. M. Riegl and S. J. Purkis (Dordrecht: Springer), 253–308.
Glynn, P. W., Enochs, I. C., Afflerbach, J. A., Brandtneris, V. W., and Serafy, J. E. (2014). Eastern Pacific reef fish responses to coral recovery following El Ni-o disturbances. Mar. Ecol. Prog. Ser. 495, 233–247. doi: 10.3354/meps10594
Goatley, C. H., and Bellwood, D. R. (2012). Sediment suppresses herbivory across a coral reef depth gradient. Biol. Lett. 8, 1016–1018. doi: 10.1098/rsbl.2012.0770
Gordon, S. E., Goatley, C. H., and Bellwood, D. R. (2016). Low-quality sediments deter grazing by the parrotfish Scarus rivulatus on inner-shelf reefs. Coral Reefs 35, 285–291. doi: 10.1007/s00338-015-1374-z
Graham, N. A. J., and Nash, K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326. doi: 10.1007/s00338-012-0984-y
Grandcout, E. (2012). “Reef fish and fisheries in the Gulf,” in Coral Reefs of the Gulf, Coral Reefs of the World, Vol. 3, eds B. M. Riegl and S. J. Purkis (Dordrecht: Springer), 127–161.
Harms-Tuohy, C. A., Schizas, N. V., and Appeldoorn, R. S. (2016). Use of DNA metabarcoding for stomach content analysis in the invasive lionfish Pterois volitans in Puerto Rico. Mar. Ecol. Prog. Ser. 558, 181–191. doi: 10.3354/meps11738
Hay, M. E., Fenical, W., and Gustafson, K. (1987). Chemical defense against diverse coral-reef herbivores. Ecology 68, 1581–1591. doi: 10.2307/1939850
Hill, M. S. (1998). Spongivory on Caribbean reefs releases corals from competition with sponges. Oecologia 117, 143–150. doi: 10.1007/s004420050642
Hixon, M. A. (1997). Effects of Reef Fishes on Corals and Algae. Life and Death of Coral Reefs. New York, NY: Chapman and Hall. 230–248.
Hoey, A. S., Feary, D. A., Burt, J. A., Vaughan, G., Pratchett, M. S., and Berumen, M. L. (2016). Regional variation in the structure and function of parrotfishes on Arabian reefs. Mar. Pollut. Bull. 105, 524–531. doi: 10.1016/j.marpolbul.2015.11.035
Howells, E. J., Ketchum, R. N., Bauman, A. G., Mustafa, Y., Watkins, K. D., and Burt, J. A. (2016). Species-specific trends in the reproductive output of corals across environmental gradients and bleaching histories. Mar. Pollut. Bull. 105, 532–539. doi: 10.1016/j.marpolbul.2015.11.034
Hugerth, L. W., Muller, E. E., Hu, Y. O., Lebrun, L. A., Roume, H., Lundin, D., et al. (2014). Systematic design of 18S rRNA gene primers for determining eukaryotic diversity in microbial consortia. PLoS ONE 9:e95567. doi: 10.1371/journal.pone.0095567
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., et al. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. doi: 10.1126/science.1085046
Hughes, T. P., Kerry, J. T., Álvarez-Noriega, M., Álvarez-Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Hume, B., D'angelo, C., Burt, J., Baker, A. C., Riegl, B., and Wiedenmann, J. (2013). Corals from the Persian/Arabian Gulf as models for thermotolerant reef-builders: prevalence of clade C3 Symbiodinium, host fluorescence and ex situ temperature tolerance. Mar. Pollut. Bull. 72, 313–322. doi: 10.1016/j.marpolbul.2012.11.032
Illumina (2017). MiSeq Applications and Methods. Available online at: https://www.illumina.com/systems/sequencing-platforms/miseq/applications.html (Accessed May 5, 2017).
Johansen, J. L., and Jones, G. P. (2011). Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob. Chang. Biol. 17, 2971–2979. doi: 10.1111/j.1365-2486.2011.02436.x
Konow, N., and Bellwood, D. R. (2011). Evolution of high trophic diversity based on limited functional disparity in the feeding apparatus of marine angelfishes (f. Pomacanthidae). PLoS ONE 6:e24113. doi: 10.1371/journal.pone.0024113
Leray, M., and Knowlton, N. (2015). DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc. Natl. Acad. Sci. U.S.A. 112, 2076–2081. doi: 10.1073/pnas.1424997112
Longenecker, K. (2007). Devil in the details: high-resolution dietary analysis contradicts a basic assumption of reef-fish diversity models. Copeia 2007, 543–555. doi: 10.1643/0045-8511(2007)2007[543:DITDHD]2.0.CO;2
López-Pérez, A., Guendulain-García, S., Granja-Fernández, R., Hernández-Urraca, V., Galván-Rowland, L., Zepeta-Vilchis, R., et al. (2016). Reef community changes associated with the 2009–2010 El Ni-o in the southern Mexican Pacific 1. Pac. Sci. 70, 175–190. doi: 10.2984/70.2.4
Masuda, H., and Allen, G. R. (1993). Meeresfische der Welt-Groß-Indopazifische Region. Melle: Tetra Verlag, Herrenteich. 528.
McCormick, M. I. (2003). Consumption of coral propagules after mass spawning enhances larval quality of damselfish through maternal effects. Oecologia 136, 37–45. doi: 10.1007/s00442-003-1247-y
Miyake, S., Ngugi, D. K., and Stingl, U. (2015). Diet strongly influences the gut microbiota of surgeonfishes. Mol. Ecol. 24, 656–672. doi: 10.1111/mec.13050
Moberg, F., and Folke, C. (1999). Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233. doi: 10.1016/S0921-8009(99)00009-9
Monikh, F. A., Peery, S., Karami, O., Hosseini, M., Bastami, A. A., and Ghasemi, A. F. (2012). Distribution of metals in the tissues of benthic, Euryglossa orientalis and Cynoglossus arel., and Bentho-Pelagic, Johnius belangerii., Fish from three estuaries, Persian Gulf. Bull. Environ. Contam. Toxicol. 89, 489–494. doi: 10.1007/s00128-012-0747-z
Monikh, F. A., Safahieh, A., Savari, A., and Doraghi, A. (2013). Heavy metal concentration in sediment, benthic, benthopelagic, and pelagic fish species from Musa Estuary (Persian Gulf). Environ. Monit. Assess. 185, 215–222. doi: 10.1007/s10661-012-2545-9
Munday, P. L., Jones, G. P., Pratchett, M. S., and Williams, A. J. (2008). Climate change and the future for coral reef fishes. Fish Fish. 9, 261–285. doi: 10.1111/j.1467-2979.2008.00281.x
Munday, P. L., and Wilson, S. K. (1997). Comparative efficacy of clove oil and other chemicals in anaesthetization of Pomacentrus amboinensis, a coral reef fish. J. Fish Biol. 51, 931–938. doi: 10.1111/j.1095-8649.1997.tb01532.x
Nilsson, G. E., Crawley, N., Lunde, I. G., and Munday, P. L. (2009). Elevated temperature reduces the respiratory scope of coral reef fishes. Glob. Chang. Biol. 15, 1405–1412. doi: 10.1111/j.1365-2486.2008.01767.x
Nowicki, J. P., Miller, G. M., and Munday, P. L. (2012). Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J. Exp. Mar. Biol. Ecol. 412, 46–51. doi: 10.1016/j.jembe.2011.10.020
Paquin, M. M., Buckley, T. W., Hibpshman, R. E., and Canino, M. F. (2014). DNA-based identification methods of prey fish from stomach contents of 12 species of eastern North Pacific groundfish. Deep Sea Res. I Oceonogr. Res. Pap. 85, 110–117. doi: 10.1016/j.dsr.2013.12.002
Pratchett, M. S., Gust, N., Goby, G., and Klanten, S. O. (2001). Consumption of coral propagules represents a significant trophic link between corals and reef fish. Coral Reefs 20, 13–17. doi: 10.1007/s003380000113
Pratchett, M. S., Hoey, A. S., Feary, D. A., Bauman, A. G., Burt, J. A., and Riegl, B. M. (2013). Functional composition of Chaetodon butterflyfishes at a peripheral and extreme coral reef location, the Persian Gulf. Mar. Pollut. Bull. 72, 333–341. doi: 10.1016/j.marpolbul.2012.10.014
Pratchett, M. S., Munday, P., Wilson, S. K., Graham, N. A., Cinner, J. E., Bellwood, D. R., et al. (2008). Effects of climate-induced coral bleaching on coral-reef fishes. Ecol. Econ. Consequences Oceanogr. Mar. Biol. Ann. Rev. 46, 251–296. doi: 10.1201/9781420065756.ch6
Purcell, S. W., and Bellwood, D. R. (2001). Spatial patterns of epilithic algal and detrital resources on a windward coral reef. Coral Reefs 20, 117–125. doi: 10.1007/s003380100150
Pylro, V. S., Roesch, L. F. W., Ortega, J. M., do Amaral, A. M., Tótola, M. R., Hirsch, P. R., et al. (2014). Brazilian microbiome project: revealing the unexplored microbial diversity—challenges and prospects. Microb. Ecol. 67, 237–241. doi: 10.1007/s00248-013-0302-4
Riegl, B. (1999). Corals in a non-reef setting in the southern Arabian Gulf (Dubai, UAE): fauna and community structure in response to recurring mass mortality. Coral Reefs 18, 63–73. doi: 10.1007/s003380050156
Riegl, B. M., Purkis, S. J., Al-Cibahy, A. S., Abdel-Moati, M. A., and Hoegh-Guldberg, O. (2011). Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS ONE 6:e24802. doi: 10.1371/journal.pone.0024802
Rogers, A., Blanchard, J. L., and Mumby, P. J. (2014). Vulnerability of coral reef fisheries to a loss of structural complexity. Curr. Biol. 24, 1000–1005. doi: 10.1016/j.cub.2014.03.026
Saei-Dehkordi, S. S., Fallah, A. A., and Nematollahi, A. (2010). Arsenic and mercury in commercially valuable fish species from the Persian Gulf: influence of season and habitat. Food Chem. Toxicol. 48, 2945–2950. doi: 10.1016/j.fct.2010.07.031
Salameh, P., Sonin, O., Edelist, D., and Golani, D. (2012). The first substantiated record of the Yellowbar Angelfish, Pomacanthus maculosus (Actinopterygii: Perciformes: Pomacanthidae) in the Mediterranean. Acta Ichthyol. Piscatoria 42, 74–75. doi: 10.3750/AIP2011.42.1.10
Sale, P. F., Feary, D. A., Burt, J. A., Bauman, A. G., Cavalcante, G. H., Drouillard, K. G., et al. (2011). The growing need for sustainable ecological management of marine communities of the Persian Gulf. AMBIO 40, 4–17. doi: 10.1007/s13280-010-0092-6
Sheppard, C., Al-Husiani, M., Al-Jamali, F., Al-Yamani, F., Baldwin, R., Bishop, J., et al. (2010). The Gulf: a young sea in decline. Mar. Pollut. Bull. 60, 13–38. doi: 10.1016/j.marpolbul.2009.10.017
Sheppard, C., Price, A., and Roberts, C. (1992). Marine Ecology of the Arabian Region: Patterns and Processes in Extreme Tropical Environments. Toronto: Academic Press.
Sheppard, C. R. C., and Sheppard, A. L. S. (1991). Corals and coral communities of Arabia. Fauna Arab. 12, 3–170.
Smith, T. B. (2008). Temperature effects on herbivory for an Indo-Pacific parrotfish in Panamá: implications for coral–algal competition. Coral Reefs 27, 397–405. doi: 10.1007/s00338-007-0343-6
ter Hofstede, R. (1998). Feeding Ecology of the Angelfish Species Pomacanthus Asfur and Pomacanthus maculosus. MSc thesis, University of Groningen.
Tricas, T. C. (1989). Prey selection by coral-feeding butterflyfishes: strategies to maximize the profit. Environ. Biol. Fishes 25, 171–185. doi: 10.1007/BF00002210
Wernberg, T., Smale, D. A., Tuya, F., Thomsen, M. S., Langlois, T. J., De Bettignies, T., et al. (2013). An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang 3, 78–82. doi: 10.1038/nclimate1627
Wilson, S. K., Bellwood, D. R., Choat, J. H., and Furnas, M. (2003). “Detritus in the epilithic algal matrix and its use by coral reef fishes,” in Oceanography and Marine Biology: An Annual Review, Vol. 41, eds R. N. Gibson and R. J. A. Atkinson (London: Taylor & Francis), 279–309.
Wilson, S. K., Fisher, R., Pratchett, M. S., Graham, N. A. J., Dulvy, N. K., Turner, R. A., et al. (2008). Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Chang. Biol. 14, 2796–2809. doi: 10.1111/j.1365-2486.2008.01696.x
Wilson, S. K., Graham, N. A. J., Pratchett, M. S., Jones, G. P., and Polunin, N. V. C. (2006). Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob. Chang Biol. 12, 2220–2234. doi: 10.1111/j.1365-2486.2006.01252.x
Wood, C. M., and McDonald, D. G. (1997). Global Warming: Implications for Freshwater and Marine Fish. Cambridge: Cambridge: University Press.
Keywords: 18S rRNA, angelfish, damselfish, Arabian Gulf, diet, Persian Gulf, trophic
Citation: Shraim R, Dieng MM, Vinu M, Vaughan G, McParland D, Idaghdour Y and Burt JA (2017) Environmental Extremes Are Associated with Dietary Patterns in Arabian Gulf Reef Fishes. Front. Mar. Sci. 4:285. doi: 10.3389/fmars.2017.00285
Received: 05 May 2017; Accepted: 22 August 2017;
Published: 05 September 2017.
Edited by:
Emma Camp, University of Technology, Sydney, AustraliaReviewed by:
David John Booth, University of Technology, Sydney, AustraliaCarlos Eduardo Leite Ferreira, Federal Fluminense University, Brazil
Copyright © 2017 Shraim, Dieng, Vinu, Vaughan, McParland, Idaghdour and Burt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youssef Idaghdour, eW91c3NlZi5pZGFnaGRvdXJAbnl1LmVkdQ==
John A. Burt, am9obi5idXJ0QG55dS5lZHU=
 Rasha Shraim
Rasha Shraim Mame M. Dieng
Mame M. Dieng Manikandan Vinu
Manikandan Vinu Youssef Idaghdour
Youssef Idaghdour John A. Burt
John A. Burt