- Population Ecology Division, Fisheries and Oceans Canada, Bedford Institute of Oceanography, Dartmouth, NS, Canada
Understanding high-use areas for highly migratory species and their movements within these areas may provide insight into behaviors such as foraging and mating. In the Western Atlantic, the leatherback sea turtle, Dermochelys coriacea, has a broad geographic range extending from nesting beaches at low latitudes to foraging areas off the coast of Eastern Canada. Biotelemetry has revealed much about the movements and habitats of leatherbacks. However, the timing and location of leatherback mating behavior remains unclear. We conducted spatial analyses of the movements of reproductive female leatherbacks prior to their first seasonal nesting events. Using kernel density estimates, high-use areas for seven female turtles originally tagged in Canadian waters were revealed from 50% volume contours depicting pre-nesting movements (120 days prior to confirmed nesting events) and inferred mating behavior (45 days prior to confirmed nesting events). All individuals initially remained offshore within a relatively small range of latitude (10–15° N) before transiting to and residing in coastal waters adjacent to nesting beaches in Colombia (n = 2), Trinidad (n = 3), Guyana (n = 1), and French Guiana (n = 1). Comparison of these movement patterns to those of mature male leatherbacks (n = 12) revealed similarities. Male and female residency within this offshore high-use area may be indicative of prey exploitation prior to the energetically costly nesting season. While the offshore residency period of three males and one female extended into the interval in which mating is expected to occur, most males and females transited to coastal waters where they resided throughout this period. High-use areas determined through kernel density analysis support and corroborate previous telemetry work indicating that mature male leatherbacks exhibit seasonal residency adjacent nesting beaches for the early portion of the nesting season, presumably to exploit mating opportunities. Fine-scale analyses of fisheries interactions in both coastal and offshore waters and estimation of accompanying mortality rates is required to evaluate fishery threats to this population during the pre-nesting interval.
Introduction
The use of satellite telemetry has provided valuable insight into the distributions and habitat use of many highly migratory species. In the marine realm, satellite telemetry has revealed the distributions and movements of threatened species such as sharks, whales, sea birds, and turtles (Weimerskirch and Robertson, 1994; Godley et al., 2002; Baumgartner and Mate, 2005; Stevens et al., 2010). The ability to remotely observe animals throughout their migrations expands not only our knowledge of their life histories, but may also help identify potential threats that populations face at each phase of their migratory cycle (Hays et al., 2003). In some cases, the use of satellite telemetry has led to the implementation of effective conservation measures such as time-area closures of recreational and industrial fisheries (Domeier, 2006; Jensen et al., 2010). For pelagic species such as most sea turtles, telemetry has provided insight into otherwise enigmatic oceanic movements and residency areas. Advancing telemetry methods have aided in the investigation of mating behavior for populations of loggerhead (Henwood, 1987; Hays et al., 2010; Schofield et al., 2010), green (Balazs and Ellis, 2000), and Kemp's ridley turtles (Shaver et al., 2005).
The leatherback sea turtle, Dermochelys coriacea, is the most widely distributed of all sea turtles, with mature individuals in the North Atlantic making annual migrations from their nesting beaches in the Caribbean and South America to foraging grounds in the Northeast (Witt et al., 2007; Fossette et al., 2010) and Northwest Atlantic (James et al., 2007). The species is classified as endangered in Canada (SARA (Species at Risk Act), 2002). In order to effectively protect this species, their full range of habitats and high-use areas must be understood. Advancements in telemetry have led to discoveries such as leatherback migratory routes (James et al., 2005a,b; Dodge et al., 2014) and northern foraging habitats (James et al., 2006; Jonsen et al., 2007). However, knowledge gaps in the life history of this species remain, including understanding of female pre-nesting and mating behavior. Consistent paternity documented across successive nests suggests that sperm storage occurs from mating event(s) prior to the nesting season (Crim et al., 2002; Stewart and Dutton, 2011; Figgener et al., 2016) and that mating during inter-nesting periods may be rare. Therefore, as mature male and female turtles presumably aggregate to breed in specific areas, identifying where pre-nesting season mating opportunities occur may offer conservation value to the Atlantic leatherback population.
The first published hypothesis regarding timing and location of mating activity in North Atlantic leatherbacks suggested that mating occurred in close proximity temporally and spatially to a female's first oviposition on the nesting beach (Lazell, 1980). An alternate hypothesis was published shortly thereafter, asserting that since there were no reported first-hand observations of mating near nesting beaches, such activity must occur in distant offshore waters (Pritchard, 1982). This topic was revisited again in 1988, when Eckert and Eckert inferred pre-reproductive movements of females through the study of epibionts that colonize females once they arrive in warm, tropical waters. The results of this work suggested that females do not arrive in tropical waters early enough to allow for localized mating near nesting areas, but instead must mate prior to their arrival in tropical waters (Eckert and Eckert, 1988). The advancement of satellite telemetry allowed for these hypotheses to be re-visited in 2005, when James et al. reported that mature male leatherbacks tagged at high-latitude foraging areas migrated to waters adjacent nesting beaches, supporting the earlier hypothesis that mating likely occurs in these areas. However, these findings have yet to be corroborated with mature female movement data.
Inter-nesting and post-nesting movements of mature female leatherbacks have been well documented (Reina et al., 2005; Eckert, 2006; Eckert et al., 2006, 2009; Hays et al., 2006); however, movements of female leatherbacks prior to their first seasonal nesting event have not yet been described. This arises from challenges associated with tracking turtles to their nesting beaches. To investigate behavior prior to the deposition of a female leatherback's first clutch of the season, satellite tags deployed on nesting females must be retained and remain operational for 2–3 years (until their next nesting season), a feat which has not yet been achieved with current technology and tag attachment methods (Hays et al., 2007). Alternatively, transmitters may be deployed on females in northern foraging areas prior to migration to nesting areas. While the required duration of tag retention and operation is considerably shorter in these cases, loss of tags and or transmissions before turtles reach nesting areas is unfortunately the norm, resulting from incidental mortality, biofouling, tag (including battery) failure, and/or tag attachment failure (Hays et al., 2007). These logistic challenges mean that documentation of pre-nesting behavior is exceedingly rare: of 57 mature females satellite tagged off the coast of Atlantic Canada (2000-2016), only six (10.5%) have retained their transmitter through to a confirmed nesting event.
Here we present the first analysis of the movements of female leatherbacks upon their arrival in tropical waters prior to their first confirmed nesting event, and compare their movement patterns to those of mature male turtles. By outlining areas of high-use habitat for both mature male and female turtles prior to the onset of nesting season, we can evaluate mating hypotheses using empirical data.
Methods
Field Sampling
Seven female leatherback sea turtles were equipped with satellite transmitters while foraging in shelf waters off mainland Nova Scotia, Canada (~44°N, 64°W) and Cape Breton Island (~47°N, 60°W) (Table 1). Monel flipper tags (style no. 49, National Band and Tag Company, Newport, KY, USA) were applied to both rear flippers and passive integrated transponders (Avid, Calgary, AB, Canada; Biomark, Boise, ID, USA; Trovan, Douglas, UK) were implanted in the right shoulder. Individuals were equipped with satellite-linked transmitters [Wildlife Computers, Inc., Redmond, WA, USA; models SSC3 (n = 1), MK10-A (n = 1), MK10-AF (n = 2), SPOT5 (n = 2) and SPLASH10 (n = 1)]. All turtles were released immediately after tag attachment. Research and associated protocols were reviewed and approved by Dalhousie University Committee on Laboratory Animals or the Fisheries and Oceans Canada Maritimes Animal Care Committee or the Fisheries and Oceans Canada Maritimes Animal Care Committee to meet standards established by the Canadian Council on Animal Care. Research was conducted under scientific license from Fisheries and Oceans Canada and Species At Risk Act (SARA) Section 73 permits.

Table 1. Summary data for seven mature female leatherback turtles equipped with satellite transmitters off the coast of Nova Scotia, Canada prior to confirmed nesting events.
Spatial Analysis
Location data were acquired via the Argos satellite network1. Locations classified as LC3, LC2, LC1, or LC0 are defined as within 150 m, 150–350 m, 350–1,000 m and >1,000 m of the true location, respectively1. Location class A (LCA), for which Argos does not provide an estimated range of positional accuracy, has been shown to be as accurate, if not more so, than LC0 transmissions (Vincent et al., 2002). Therefore, location classes 3, 2, 1, 0, and A were analyzed in this study. Transmitters deployed in support of several research projects across multiple years had varying programming parameters, including different user-defined transmission intervals. To address this, prior to spatially analyzing these tracks, a daily median position was calculated for each individual, and tracks were linearly interpolated using packages “plyr” (Wickham, 2011) and “zoo” (Zeileis and Grothendieck, 2005) in R 3.0.2 (R Core Team, 2013) (Figure 1A). Linear interpretations assumed constant speed and direction for days in which locations were not generated. The pre-nesting interval was defined as the 120 days prior to each individual's first seasonal nesting event; this interval allowed for discerning marked changes from migratory to residency behavior.
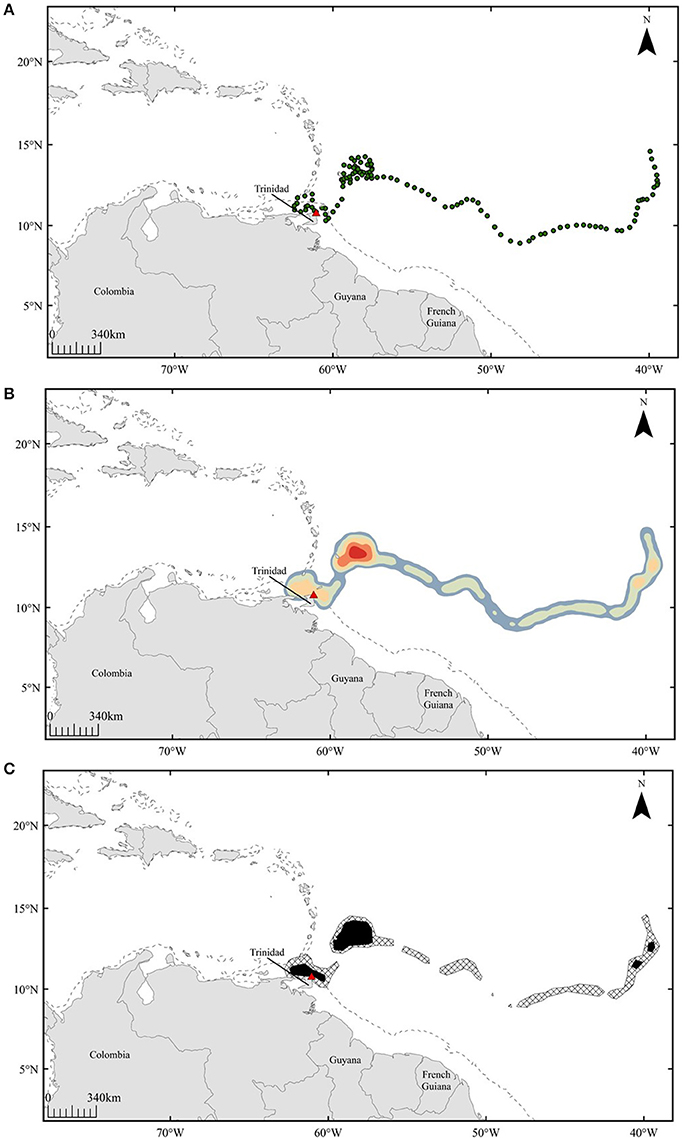
Figure 1. Representative example of spatial analyses conducted for pre-nesting leatherbacks: turtle E. Panels show interpolated positions for 120 days prior to nesting (A), heatmap displaying areas of high density (red) (B), and 50% (black) and 95% (hatched) volume contours generated from kernel density results (C). Dashed line represents 200 m isobath.
To infer potential areas of high use during the pre-nesting interval, interpolated tracks were spatially analyzed in ArcGIS 10.2.2 software (ESRI) using the kernel density tool within the Home Range Tools toolbox (MacLeod, 2013) (Figure 1B). Smoothing parameters for this analysis were calculated using the ad hoc approach in order to minimize fragmentation of potential high-use areas (Kie, 2013; Schuler et al., 2014). From kernel density results, percent volume contours were generated, outlining areas in which 50 and 95% of each individual's locations have the probability of being detected within the 120-day pre-nesting interval (Figure 1C). The 50% volume contours were used to infer high-use areas, while 95% contours showed the range of each individual throughout the pre-nesting interval.
To corroborate leatherback high-use areas derived from female pre-nesting telemetry data, we also considered tracking data from 12 mature male leatherbacks, including data from seven turtles previously analyzed by James et al. (2005a) (Table S1). Male tracking data were analyzed for all dates spanning 120 days prior to the earliest recorded nest (March 22; Female B) through to the onset of each male's northward migration (Figure S2). Male and female high-use areas defined by their 50% volume contours were overlaid to determine areas of overlap using the Clip tool within the Spatial Analysis toolbox (ESRI).
Inference of Mating
Unlike other sea turtles, the timespan between mating and nesting events has not been directly observed in leatherbacks. Therefore, we inferred this period from known follicular development intervals of other sea turtles. Studies of captive green turtles indicate the first oviposition typically occurs ~34–45 days after observed copulation (Simon et al., 1975; Wood and Wood, 1980); however, this interval may reach up to 60 days (Wood and Wood, 1980). The mating period for loggerhead sea turtles has been documented to last up to 42 days (Miller et al., 2003). Leatherbacks are predicted to have a similar timespan between mating and nesting to that recorded for loggerhead and green turtles, as egg incubation and inter-nesting intervals are similar across these species (Hirth, 1980). This interval is also supported by the findings of Eckert and Eckert (1988), who estimated an interval of ~30 days between mating and egg production based on the colonization of tropical epibionts on nesting leatherback females. To remain conservative in our estimation of follicular development time, we assumed the period between mating and first nesting spans 45 days.
To identify potential areas of mating activity, individual female movement datasets were truncated to the inferred mating period (45 days prior to first seasonal nesting events), and spatial analyses applied to the pre-nesting interval (see above) were repeated (Figure S3). Male movement data were analyzed for all dates spanning 45 days prior to the earliest recorded nest (March 22; Female B) through to the onset of each male's northward migration. From kernel density estimates, 50% volume contours were generated for both females (Figure 4) and males (Figure 5). Movement data for turtle F were not analyzed within the mating period (45 days prior to nesting), as transmissions temporarily ceased during this time interval. Nesting events were confirmed through observations of high-quality coastal Argos locations derived from continuous tag transmissions during satellite passes and/or extended surface/dry time logged by tag depth sensors, consistent with turtles coming ashore at known nesting areas (n = 1), or through encounters of tagged animals by collaborating beach monitoring organizations (n = 4). For one female (turtle G), the pre-nesting and mating intervals were estimated based on coastal residency behavior in nearshore waters off Trinidad and Venezuela prior to her fatal entanglement just days before her first predicted nesting event.
Results
Female Movements within the Pre-nesting Interval
All mature female leatherbacks equipped with satellite transmitters off Nova Scotia exhibited seasonal residency in Atlantic Canadian waters from the time of tagging through to late September (n = 2), October (n = 4), or November (n = 1) of their respective deployment years before assuming southward migration. Females traveled southward within a narrow longitudinal range (~35°–50°W). Upon reaching southern waters corresponding roughly to the North Equatorial current (~10–15°N), seven females (turtles A-G) traveled westward toward beaches in Colombia (n = 2), Trinidad (n = 3), Guyana (n = 1), and French Guiana (n = 1) (Figure S1).
Combining the 50% volume contours produced from kernel density estimates for all seven female leatherbacks revealed patterns in female behavior during the 120-day pre-nesting interval (Figure 2). While offshore high-use areas spanned a wide longitudinal range (~40°–60°W), ~75% of activity in these areas (across all corresponding pre-nesting seasons) occurred within a narrow range of latitude (~5°; 10–15°N). After initially departing offshore high-use areas, tracking data from six females (turtles A-E, G) revealed secondary residency areas in coastal waters proximate to their respective nesting beaches, used immediately prior to their respective first nesting events.
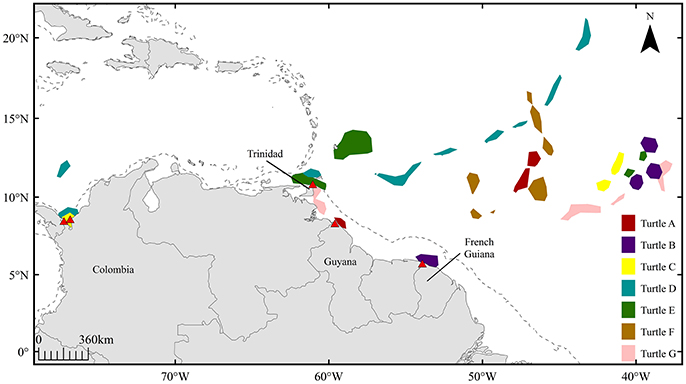
Figure 2. High-use areas defined by 50% volume contours for seven female leatherbacks (turtles A-G) for 120 days prior to first seasonal nesting events; red triangles indicate corresponding nesting sites in Guyana, French Guiana, Trinidad, and Colombia. Dashed line represents 200 m isobath.
Male Movements within the Pre-nesting Interval
Male satellite tracking data (n = 12) also revealed offshore high-use areas within the pre-nesting interval (Figure 3). Similar to patterns observed in female tracking data, 76.4% of total male residency fell within the 10–15°N latitudinal range. Overlap between mature male and female leatherback high-use areas (revealed from 50% volume contours) occurred in offshore areas spanning latitudes of 40–60°W, as well as in coastal waters off Trinidad and French Guiana (Figure 3). Within the 10–15°N latitudinal range, each female exhibited at least one high-use 50% volume contour that corresponded to male residency areas, representing a strong affinity for both males and females for this area over the course of multiple breeding seasons (n = 13).
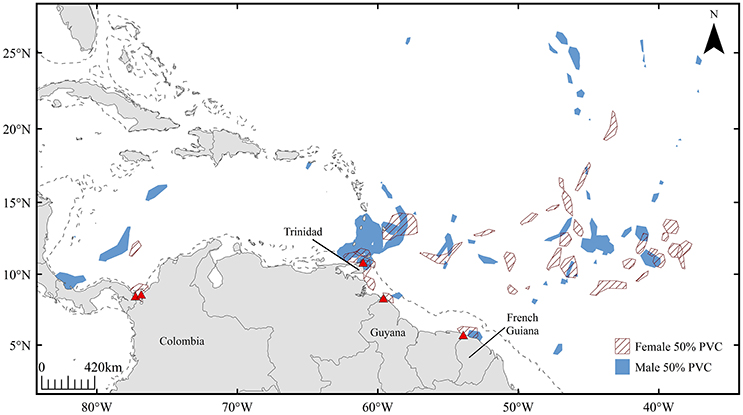
Figure 3. High-use areas defined by 50% volume contours for mature male (n = 12, blue) and female (n = 7, red hatched) leatherbacks throughout the 120-day pre-nesting interval. Dashed line represents 200 m isobath.
Female and Male Movements within the Mating Period
Throughout the inferred mating period, six females (turtles A-E, G; turtle F not included due to transmission gaps) resided in coastal waters proximate to nesting beaches in Trinidad (n = 2), French Guiana (n = 1), Guyana (n = 1), and Colombia (n = 2) (Figure 4). Offshore residency throughout the mating period was observed in one female, turtle E, which occupied waters beyond the 200 m isobath prior to transiting and residing in coastal waters proximate to Trinidad. Each female was present in continental shelf waters (inshore of the 200 m isobath) in the days immediately preceding confirmed nesting events. Mature male tracking data were analyzed to corroborate probable mating areas. Three males exhibited offshore residency throughout the mating period (Figure 5, Figure S4); however, the majority of male high-use areas corresponded to coastal waters directly adjacent nesting beaches in Trinidad, French Guiana, St. Lucia, St. Vincent, Grenada, and Panama prior to the onset of their northward migration.
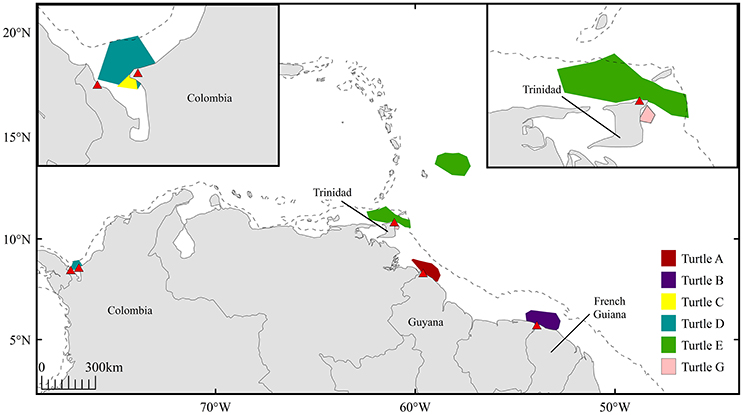
Figure 4. High-use areas defined by 50% volume contours for 6 female leatherbacks (turtles A-E, G) during inferred mating period 45 days prior to confirmed nesting events; red triangles indicate corresponding nesting sites in Guyana (n = 1), French Guiana (n = 1), Trinidad (n = 1), and Colombia (n = 2). Dashed line represents 200 m isobath.
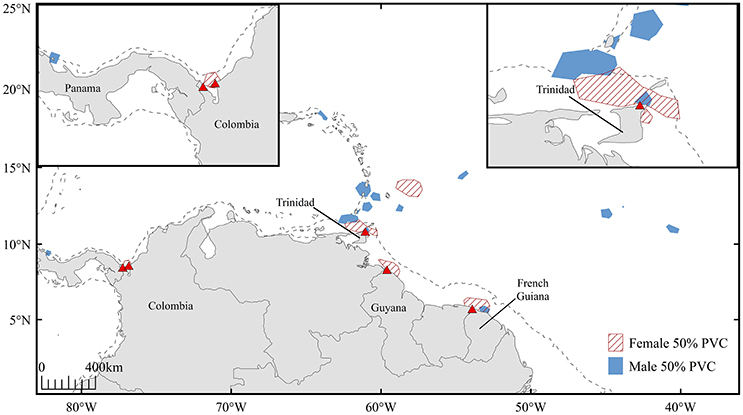
Figure 5. High-use areas defined by 50% volume contours for mature male (n = 12, blue) and female (n = 6, red hatched) leatherbacks throughout the 45-day inferred mating period. Dashed line represents 200 m isobath.
Discussion
Offshore High-Use Areas
Satellite telemetry data from mature leatherbacks tagged in Canadian foraging habitat can be used to identify probable mating areas and provide the first insights into the pre-nesting behavior of this species. Consistent with James et al. (2005a), results of the present spatial analyses showed that mature males spend extended time proximate to nesting beaches within the inferred mating interval. However, this study highlights an additional high-use area for both mature males and females prior to their arrival in coastal waters proximate to nesting beaches (Figure 3). All seven female leatherbacks and seven of 12 males we tracked frequented this offshore area prior to assuming directed movement toward nesting beaches. Residency of reproductively-active females and males in this offshore area over multiple breeding seasons (n = 13) underscores the importance of these waters to the Northwest Atlantic leatherback turtle population.
The fundamental goals of long-distance migrations are often resource driven linked to exploitation of spatially limited food, mates, or shelter, all of which may result in temporary aggregation (Dingle and Drake, 2007). The foraging grounds of Atlantic Canada have been identified as critical habitat for mature leatherbacks (James et al., 2006), and both morphometric and physiological indicators have been used to determine that leatherbacks are capital breeders (James et al., 2005b; Davenport et al., 2011; Plot et al., 2013). Throughout the nesting season, leatherbacks rely on energy stores and become anorexic (Plot et al., 2013). The sub-Equatorial region spanning 5–15°N has previously been posited as an area of foraging success for post-nesting leatherbacks, corroborated by low leatherback travel rates and modeled annual zooplankton biomass (Fossette et al., 2010). It is possible that this offshore high-use area provides foraging opportunities for pre-nesting leatherbacks as well, enabling acquisition of valuable energy reserves prior to the start of the energetically-costly nesting season.
Inferred Mating Behavior
After exhibiting seasonal residency in offshore waters, presumably to exploit available prey, reproductively active male and female leatherbacks transited to coastal waters where they resided throughout the inferred mating period preceding first seasonal nesting events (Figure 5). While three males remained in offshore waters throughout this time interval, six females and nine males exhibited coastal residency during the inferred mating period. Our results corroborate the findings of James et al. (2005a), suggesting that mature leatherbacks frequent coastal waters adjacent to nesting beaches in order to exploit mating opportunities prior to the nesting season.
Coastal mating areas have also been identified for various populations of other sea turtle species. Mature male and female green sea turtles have been documented in coastal waters of Hawaii (Dizon and Balazs, 1982), Costa Rica (Carr et al., 1978), and Australia (Booth and Peters, 1972) prior to the onset of seasonal nesting. Satellite tracking data of male green sea turtles near Ascension Island have revealed temporary residency in the coastal waters of high-density nesting beaches (Hays et al., 2001). Male green turtles have also been observed transiting through coastal waters of multiple high-density nesting sites prior to and during the nesting season, potentially mating in coastal waters of multiple rookeries in Cyprus and Turkey (Wright et al., 2012). Mating pairs of olive ridley sea turtles have been documented offshore from nesting beaches just prior to high-density arribada nesting events (Kalb et al., 1995; Jensen et al., 2006). Similarly, coastal mating has been confirmed in the Laganas Bay population of loggerhead sea turtles, where mating pairs have been directly observed in close proximity to rookeries over the course of multiple breeding seasons (Schofield et al., 2006). Our results suggest that leatherbacks behave similarly to other sea turtle species, with mature males and females residing in coastal waters prior to the onset of seasonal nesting.
Genetic analyses have previously identified reproductively isolated leatherback nesting assemblages within the Northwest Atlantic. Stewart et al. (2013) combined the use of passive identification tagging, satellite telemetry, and mitochondrial DNA analyses to identify the natal origins of 288 leatherbacks sampled off the coast of Atlantic Canada. Results indicated that individuals from the Guiana Shield population (encompassing nesting populations from Trinidad, Guyana and French Guiana) were genetically distinguishable from individuals originating from Costa Rica, Panama, and Colombia. The present telemetry results, also representing turtles sampled in Canadian waters, support the findings of Stewart et al. (2013), as both Colombian-nesting females (turtles C and D) spent their presumed mating period in coastal waters of Colombia. In contrast, females nesting in Trinidad, French Guiana and Guyana (turtles A, B, and E) exhibited longer residency in the North Equatorial Current offshore area throughout their pre-nesting interval, followed by secondary residency periods in waters proximate to their respective nesting beaches throughout the mating period (Figure 4).
A metric of reproductive success among oviparous organisms is the quantity of eggs successfully fertilized (Parker, 1984). As such, reproductively successful males will have morphological or behavioral adaptations that increase their likelihood of fertilizing as many eggs as possible. For male leatherbacks, these adaptations may include the areas they select to intercept females prior to the nesting season. While all species of sea turtle exhibit polyandry (Kichler et al., 1999; Ireland et al., 2003; Jensen et al., 2006; Zbinden et al., 2007; Theissinger et al., 2009; Joseph and Shaw, 2011), multiple paternity in Atlantic leatherback clutches has been observed in low proportions (10–41.7%; Crim et al., 2002; Stewart and Dutton, 2011, 2014; Figgener et al., 2016). Few instances of inter-nesting mating have been identified in leatherbacks (Figgener et al., 2016), and successive nests laid by most females reveal consistent paternities throughout the nesting season, indicative of sperm storage from mating event(s) occurring prior to the nesting season (Crim et al., 2002; Stewart and Dutton, 2011; Figgener et al., 2016). Therefore, male leatherbacks must intercept reproductive females upon arrival in low latitude waters prior to the onset of nesting, highlighting the importance of leatherback high-use areas prior to the nesting season.
Confirmation of leatherback mating interactions during the inter-nesting interval in nearshore waters off Pacific Costa Rica has been achieved through deployments of animal-borne video recorders on nesting turtles (Reina et al., 2005). However, logistical challenges have so far precluded application of this technology on turtles during the pre-nesting period; thus direct confirmation of the times, areas, and behaviors associated with mating remains elusive. With the rapid evolution of animal-borne imaging systems, visual confirmation of pre-nesting mating behavior may eventually be possible.
Fishing Interactions and Significance for Conservation
Identification of high-use areas for leatherbacks is critical to evaluating where this species may be vulnerable to fisheries interactions (James et al., 2005c; Fossette et al., 2014). Artisanal gill net fisheries have been identified as a serious threat to nesting leatherbacks in Trinidad and Tobago (Lee Lum, 2006) and Grenada (Georges et al., 2007), as well as in French Guiana and Suriname (Chevalier et al., 1999; Georges et al., 2007). One female leatherback in this study (turtle G) exhibited pre-nesting behavior consistent with other females (turtles A-F), however, she was confirmed dead, entangled in coastal fishing gear, just prior to the date of her first predicted nesting event. This case highlights the threat coastal fishing gear presents to mature leatherbacks in waters proximate to nesting beaches prior to and during the nesting season.
While artisanal fisheries adjacent to many nesting beaches are an important source of mortality for the Northwest Atlantic leatherback population, this species may also be vulnerable in the additional high-use offshore area identified here, where mature turtles aggregate prior to their arrival in nearshore coastal waters.
Atlantic basin-wide analyses have identified areas where leatherbacks may be vulnerable to interactions with high seas fisheries (Hays et al., 2004; Lewison et al., 2004; Wallace et al., 2010; Fossette et al., 2014). However, where satellite telemetry data has been incorporated into such analyses, mainly post-nesting movements have been considered, potentially missing key areas of reproductive leatherback aggregation including the pre-nesting movements described here. A detailed bycatch analysis quantifying leatherback-fishery interactions and subsequent mortality rates in offshore high-use areas is required to better evaluate the potential impact on the Northwest Atlantic population at this stage of their migratory cycle.
Author Contributions
EPB led the analysis and writing of the manuscript. MCJ conceived the study, conducted fieldwork, collected data, and contributed to the analysis and writing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Canadian Sea Turtle Network field team for assistance deploying satellite transmitters off Nova Scotia and Cape Breton Island. We are grateful to Centre National de la Recherche Scientifique, Conservación Ambiente Colombia Foundation, Grand Riviere Nature Tour Guide Association, Groupo Chibiqui, Nature Seekers, Project GILA, Sea Turtle Conservancy, Université de Strasbourg, and Wider Caribbean Sea Turtle Network for their assistance in transmitter recovery. We thank two reviewers for their helpful comments and suggestions. This project was funded by Canadian Wildlife Federation, Environment and Climate Change Canada, Fisheries and Oceans Canada, Fitzhenry Family Foundation, Gordon and Patricia Gray Animal Welfare Foundation, Habitat Stewardship Program for Species at Risk, Horne Family Foundation, and Individual Donors to the Canadian Sea Turtle Network.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00223/full#supplementary-material
Footnotes
References
Balazs, G. H., and Ellis, D. M. (2000). “Satellite telemetry of migrant male and female green turtles breeding in the Hawaiian Islands,” in Proceedings of the 18th Annual Symposium Sea Turtle Biology and Conservation. NOAA Technical Memoranda NMFS-SEFSC-436 (Miami, FL: United States Department of Commerce) 53–55.
Baumgartner, M. F., and Mate, B. R. (2005). Summer and fall habitat of North Atlantic right whales (Eubalaena glacialis) inferred from satellite telemetry. Can J. Fish Aquat. Sci. 62, 527–543. doi: 10.1139/f04-238
Booth, J., and Peters, J. A. (1972). Behavioural studies on the green turtle (Chelonia mydas) in the sea. Anim. Behav. 20, 808–812. doi: 10.1016/S0003-3472(72)80155-6
Carr, A. F., Carr, M. H., and Meylan, A. B. (1978). The ecology and migrations of sea turtles. 7, The West Caribbean green turtle colony. Bull. AMNH 62:1.
Chevalier, J., Desbois, X., and Girondot, M. (1999). “The reason of decline of leatherback turtles (Dermochelys coriacea) in French Guiana: an hypothesis,” in Current Studies in Herpetology: Proceedings of the Ninth Ordinary General Meeting of the Societas Europaea Herpetologica, eds C. Miaud and R. Guyetant (Le Bourget du Lac: SEH), 79–89.
Crim, J. L., Spotila, L. D., Spotila, J. R., O'connor, M., Reina, R., Williams, C. J., et al. (2002). The leatherback turtle, Dermochelys coriacea, exhibits both polyandry and polygyny. Mol. Ecol. 11, 2097–2106. doi: 10.1046/j.1365-294x.2002.01591.x
Davenport, J., Plot, V., Georges, J. Y., Doyle, T. K., and James, M. C. (2011). Pleated turtle escapes the box–shape changes in Dermochelys coriacea. J Exp Biol. 214, 3474–3479. doi: 10.1242/jeb.057182
Dingle, H., and Drake, V. A. (2007). What is migration? Bioscience 57, 113–121. doi: 10.1641/B570206
Dizon, A. E., and Balazs, G. H. (1982). Radio telemetry of hawaiian green turtles at their breeding colony. Mar. Fish. Revi. 44, 13–20.
Dodge, K. L., Galuardi, B., Miller, T. J., and Lutcavage, M. E. (2014). Leatherback turtle movements, dive behavior, and habitat characteristics in ecoregions of the Northwest Atlantic Ocean. PLoS ONE 9:e91726. doi: 10.1371/journal.pone.0091726
Domeier, M. L. (2006). An analysis of Pacific striped marlin (Tetrapturus audax) horizontal movement patterns using pop-up satellite archival tags. B. Mar. Sci. 79, 811–825. Available online at: http://www.ingentaconnect.com/content/umrsmas/bullmar/2006/00000079/00000003/art00030
Eckert, K. L., and Eckert, S. A. (1988). Pre-reproductive movements of leatherback sea turtles (Dermochelys coriacea) nesting in the Caribbean. Copeia 2, 400–406. doi: 10.2307/1445880
Eckert, K. L., Wallace, B. P., Frazier, J. G., Eckert, S. A., and Pritchard, P. C. H. (2009). Synopsis of the Biological Data on the Leatherback Sea Turtle, Dermochelys coriacea (Vandelli, 1761). US Department of Interior, Fish and Wildlife Service, Biological Technical Publication, 20181-0.
Eckert, S. A. (2006). High-use oceanic areas for Atlantic leatherback sea turtles (Dermochelys coriacea) as identified using satellite telemetered location and dive information. Mar. Biol. 145, 1257–1267. doi: 10.1007/s00227-006-0262-z
Eckert, S. A., Bagley, D., Kubis, S., Ehrhart, L., Johnson, C., Stewart, K., et al. (2006). Internesting and postnesting movements and foraging habitats of leatherback sea turtles (Dermochelys coriacea) nesting in Florida. Chelonian Conserv. Bi. 5, 239–248. doi: 10.2744/1071-8443(2006)5[239:IAPMAF]2.0.CO;2
Figgener, C., Chacón-Chaverri, D., Jensen, M. P., and Feldhaar, H. (2016). Paternity re-visited in a recovering population of Caribbean leatherback turtles (Dermochelys coriacea). J. Exp. Mar. Biol. Ecol. 475, 114–123. doi: 10.1016/j.jembe.2015.11.014
Fossette, S., Hobson, V. J., Girard, C., Calmettes, B., Gaspar, P., Georges, J. Y., et al. (2010). Spatio-temporal foraging patterns of a giant zooplanktivore, the leatherback turtle. J. Mar. Syst. 81, 225–234. doi: 10.1016/j.jmarsys.2009.12.002
Fossette, S., Witt, M. J., Miller, P., Nalovic, M. A., Albareda, D., Almeida, A. P., et al. (2014). Pan-Atlantic analysis of the overlap of a highly migratory species, the leatherback turtle, with pelagic longline fisheries. Proc. R. Soc. Lond. B. Biol. 281:e20133065. doi: 10.1098/rspb.2013.3065
Georges, J. Y., Billes, A., Ferraroli, S., Fossette, S., Fretey, J., Grémillet, D., et al. (2007). Meta-analysis of movements in Atlantic leatherback turtles during nesting season: conservation implications. Mar. Ecol. Prog. Ser. 338, 225–232. doi: 10.3354/meps338225
Godley, B. J., Richardson, S., Broderick, A. C., Coyne, M. S., Glen, F., and Hays, G. C. (2002). Long-term satellite telemetry of the movements and habitat utilisation by green turtles in the Mediterranean. Ecography 25, 352–362. doi: 10.1034/j.1600-0587.2002.250312.x
Hays, G., Broderick, A., Glen, F., Godley, B., and Nichols, W. (2001). The movements and submergence behaviour of male green turtles at Ascension Island. Mar. Biol. 139, 395–400. doi: 10.1007/s002270100580
Hays, G. C., Bradshaw, C. J. A., James, M. C., Lovell, P., and Sims, D. W. (2007). Why do Argos satellite tags deployed on marine animals stop transmitting? J. Exp. Mar. Biol. Ecol. 349, 52–60. doi: 10.1016/j.jembe.2007.04.016
Hays, G. C., Broderick, A. C., Godley, B. J., Luschi, P., and Nichols, W. J. (2003). Satellite telemetry suggests high levels of fishing-induced mortality in marine turtles. Mar. Ecol. Prog. Ser. 262, 305–309. doi: 10.3354/meps262305
Hays, G. C., Fossette, S., Katselidis, K. A., Schofield, G., and Gravenor, M. B. (2010). Breeding periodicity for male sea turtles, operational sex ratios, and implications in the face of climate change. Conserv. Biol. 24, 1636–1643. doi: 10.1111/j.1523-1739.2010.01531.x
Hays, G. C., Hobson, V. J., Metcalfe, J. D., Righton, D., and Sims, D. W. (2006). Flexible foraging movements of leatherback turtles across the North Atlantic Ocean. Ecology 87, 2647–2656. doi: 10.1890/0012-9658(2006)87[2647:ffmolt]2.0.co;2
Hays, G. C., Houghton, J. D., and Myers, A. E. (2004). Endangered species: pan-Atlantic leatherback turtle movements. Nature 429, 522–522. doi: 10.1038/429522a
Henwood, T. A. (1987). Movements and seasonal changes in loggerhead turtle Caretta caretta aggregations in the vicinity of Cape Canaveral, Florida (1978–84). Biol. Conserv. 40, 191–202. doi: 10.1016/0006-3207(87)90085-1
Hirth, H. F. (1980). Some aspects of the nesting behavior and reproductive biology of sea turtles. Am. Zool. 20, 507–523. doi: 10.1093/icb/20.3.507
Ireland, J. S., Broderick, A. C., Glen, F., Godley, B. J., Hays, G. C., Lee, P. L. M., et al. (2003). Multiple paternity assessed using microsatellite markers, in green turtles Chelonia mydas (Linnaeus, 1758) of Ascension Island, South Atlantic. J. Exp. Mar. Biol. Ecol. 291, 149–160. doi: 10.1016/S0022-0981(03)00118-7
James, M. C., Andrea Ottensmeyer, C., and Myers, R. A. (2005c). Identification of high-use habitat and threats to leatherback sea turtles in northern waters: new directions for conservation. Ecol. Lett. 8, 195–201. doi: 10.1111/j.1461-0248.2004.00710.x
James, M. C., Eckert, S. A., and Myers, R. A. (2005a). Migratory and reproductive movements of male leatherback turtles (Dermochelys coriacea). Mar. Biol. 147, 845–853. doi: 10.1007/s00227-005-1581-1
James, M. C., Myers, R. A., and Ottensmeyer, C. A. (2005b). Behaviour of leatherback sea turtles, Dermochelys coriacea, during the migratory cycle. Proc. R. Soc. B. 272, 1547–1555. doi: 10.1098/rspb.2005.3110
James, M. C., Sherrill-Mix, S. A., Martin, K., and Myers, R. A. (2006). Canadian waters provide critical foraging habitat for leatherback sea turtles. Biol. Conserv. 133, 347–357. doi: 10.1016/j.biocon.2006.06.012
James, M. C., Sherrill-Mix, S. A., and Myers, R. A. (2007). Population characteristics and seasonal migrations of leatherback sea turtles at high latitudes. Mar. Ecol. Prog. Ser. 337, 245–254. doi: 10.3354/meps337245
Jensen, M. P., Abreu-Grobois, F. A., Frydenberg, J., and Loeschcke, V. (2006). Microsatellites provide insight into contrasting mating patterns in arribada vs. non-arribada olive ridley sea turtle rookeries. Mol. Ecol. 15, 2567–2575. doi: 10.1111/j.1365-294X.2006.02951.x
Jensen, O. P., Ortega-Garcia, S., Martell, S. J., Ahrens, R. N., Domeier, M. L., Walters, C. J., et al. (2010). Local management of a “highly migratory species”: the effects of long-line closures and recreational catch-and-release for Baja California striped marlin fisheries. Prog. Oceanogr. 86, 176–186. doi: 10.1016/j.pocean.2010.04.020
Jonsen, I. D., Myers, R. A., and James, M. C. (2007). Identifying leatherback turtle foraging behaviour from satellite telemetry using a switching state-space model. Mar. Ecol. Prog. Ser. 337, 255–264. doi: 10.3354/meps337255
Joseph, J., and Shaw, P. W. (2011). Multiple paternity in egg clutches of hawksbill turtles (Eretmochelys imbricata). Conserv. Genet. 12, 601–605. doi: 10.1007/s10592-010-0168-7
Kalb, H., Valverde, R. A., and Owens, D. (1995). “What is the reproductive patch of the olive ridley sea turtle?” in Proceedings of the Twelfth Annual Workshop on Sea Turtle Biology and Conservation. NOAA Technical Memorandum, NMFS-SEFSC-36, eds J. I. Richardson and T. H. Richardson (Jekyll Island), 57–60.
Kichler, K., Holder, M. T., Davis, S. K., Marquez, M. R., and Owens, D. W. (1999). Detection of multiple paternity in the Kemp's ridley sea turtle with limited sampling. Mol. Ecol. 8, 819–830. doi: 10.1046/j.1365-294X.1999.00635.x
Kie, J. G. (2013). A rule-based ad hoc method for selecting a bandwidth in kernel home-range analyses. Anim. Biotel. 1, 1–11. doi: 10.1186/2050-3385-1-13
Lazell, J. D. Jr. (1980). New England waters: critical habitat for marine turtles. Copeia 1980, 290–295. doi: 10.2307/1444006
Lee Lum, L. (2006). Assessment of incidental sea turtle catch in the artisanal gillnet fishery in Trinidad and Tobago, West Indies. Appl. Herpetol. 3, 357–368. doi: 10.1163/157075406778905081
Lewison, R. L., Freeman, S. A., and Crowder, L. B. (2004). Quantifying the effects of fisheries on threatened species: the impact of pelagic longlines on loggerhead and leatherback sea turtles. Ecol. Lett. 7, 221–231. doi: 10.1111/j.1461-0248.2004.00573.x
MacLeod, C. D. (2013). Home Range Tools Toolbox. Available online at: GISInEcology.com/Home_Range_Tools.zip.
Miller, J. D., Limpus, C. J., and Godfrey, M. H. (2003). “Nest site selection, oviposition, eggs, development, hatching, and emergence of loggerhead turtles,” in Loggerhead Sea Turtles, eds A. B. Bolton and B. E. Witherington (Washington, DC: Smithsonian Institution), 125–143.
Parker, G. A. (1984). “Sperm competition and the evolution of animal mating strategies” in Sperm Competition and the Evolution of Animal Mating Systems, ed R. L. Smith (New York, NY: Academic Press), 1–60.
Plot, V., Jenkins, T., Robin, J. P., Fossette, S., and Georges, J. Y. (2013). Leatherback turtles are capital breeders: morphometric and physiological evidence from longitudinal monitoring. Physiol. Biochem. Zool. 86, 385–397. doi: 10.1086/671127
Pritchard, P. C. (1982). Nesting of the leatherback turtle, Dermochelys coriacea in Pacific Mexico, with a new estimate of the world population status. Copeia 1982, 741–747. doi: 10.2307/1444081
R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available onlilne at: http://www.R-project.org/
Reina, R. D., Abernathy, K. J., Marshall, G. J., and Spotila, J. R. (2005). Respiratory frequency, dive behaviour and social interactions of leatherback turtles, Dermochelys coriacea during the inter-nesting interval. J. Exp. Mar. Biol. Ecol. 316, 1–16. doi: 10.1016/j.jembe.2004.10.002
SARA (Species at Risk Act) (2002). An Act Representing the Protection of Wildlife Species in Canada. Available online at: http://laws-lois.justice.gc.ca/eng/acts/s-15.3/page-1.html (Accessed September 12, 2016).
Schofield, G., Hobson, V. J., Lilley, M. K. S., Katselidis, K. A., Bishop, C. M., Brown, P., et al. (2010). Inter-annual variability in the home range of breeding turtles: implications for current and future conservation management. Biol. Conserv. 143, 722–730. doi: 10.1016/j.biocon.2009.12.011
Schofield, G., Katselidis, K. A., Dimopoulos, P., Pantis, J. D., and Hays, G. C. (2006). Behaviour analysis of the loggerhead sea turtle Caretta caretta from direct in-water observation. Endanger. Species Res. 2, 71–79. doi: 10.3354/esr002071
Schuler, K. L., Schroeder, G. M., Jenks, J. A., and Kie, J. G. (2014). Ad hoc smoothing parameter performance in kernel estimates of GPS-derived home ranges. Wildlife. Biol. 20, 259–266. doi: 10.2981/wlb.12117
Shaver, D. J., Schroeder, B. A., Byles, R. A., Burchfield, P. M., Pena, J., Marquez, R., et al. (2005). Movements and home ranges of adult male Kemp's ridley sea turtles (Lepidochelys kempii) in the Gulf of Mexico investigated by satellite telemetry. Chelonian Conserv. Biol. 4, 817–827.
Simon, M. H., Ulrich, G. F., and Parkes, A. S. (1975). The green sea turtle (Chelonia mydas): mating, nesting and hatching on a farm. J. Zool. 177, 411–423. doi: 10.1111/j.1469-7998.1975.tb02242.x
Stevens, J. D., Bradford, R. W., and West, G. J. (2010). Satellite tagging of blue sharks (Prionace glauca) and other pelagic sharks off eastern Australia: depth behaviour, temperature experience and movements. Mar. Biol. 157, 575–591. doi: 10.1007/s00227-009-1343-6
Stewart, K. R., and Dutton, P. H. (2011). Paternal genotype reconstruction reveals multiple paternity and sex ratios in a breeding population of leatherback turtles (Dermochelys coriacea). Conserv. Genet. 12, 1101–1113. doi: 10.1007/s10592-011-0212-2
Stewart, K. R., and Dutton, P. H. (2014). Breeding sex ratios in adult leatherback turtles (Dermochelys coriacea) may compensate for female-biased hatchling sex ratios. PLoS ONE 9:e88138. doi: 10.1371/journal.pone.0088138
Stewart, K. R., James, M. C., Roden, S., and Dutton, P. H. (2013). Assignment tests, telemetry and tag-recapture data converge to identify natal origins of leatherback turtles foraging in Atlantic Canadian waters. J. Anim. Ecol. 82, 791–803. doi: 10.1111/1365-2656.12056
Theissinger, K., FitzSimmons, N. N., Limpus, C. J., Parmenter, C. J., and Phillott, A. D. (2009). Mating system, multiple paternity and effective population size in the endemic flatback turtle (Natator depressus). Conserv. Genet. 10, 329–346. doi: 10.1007/s10592-008-9583-4
Vincent, C., McConnell, B. J., Ridoux, V., and Fedak, M. A. (2002). Assessment of Argos location accuracy from satellite tags deployed on captive gray seals. Mar. Mammal. Sci. 18, 156–166. doi: 10.1111/j.1748-7692.2002.tb01025.x
Wallace, B. P., Lewison, R. L., McDonald, S. L., McDonald, R. K., Kot, C. Y., Kelez, S., et al. (2010). Global patterns of marine turtle bycatch. Conserv. Lett. 3, 131–142. doi: 10.1111/j.1755-263X.2010.00105.x
Weimerskirch, H., and Robertson, G. (1994). Satellite tracking of light-mantled sooty albatrosses. Polar Biol. 14, 123–126. doi: 10.1007/bf00234974
Wickham, H. (2011). The split-apply-combine strategy for data analysis. J. Stat. Softw. 40, 1–29 doi: 10.18637/jss.v040.i01
Wood, J. R., and Wood, F. E. (1980). Reproductive biology of captive green sea turtles Chelonia mydas. Am. Zool. 20, 499–505. doi: 10.1093/icb/20.3.499
Witt, M. J., Broderick, A. C., Johns, D. J., Martin, C., Penrose, R., Hoogmoed, M. S., et al. (2007). Prey landscapes help identify potential foraging habitats for leatherback turtles in the NE Atlantic. Mar. Ecol. Prog. Ser. 337, 231–243.
Wright, L. I., Stokes, K. L., Fuller, W. J., Godley, B. J., McGowan, A., Snape, R., et al. (2012). Turtle mating patterns buffer against disruptive effects of climate change. Proc. R. Soc. Biol. 279, 2122–2127. doi: 10.1098/rspb.2011.2285
Zbinden, J. A., Largiader, C. R., Leippert, F., Margaritoulis, D., and Arlettaz, R. (2007). High frequency of multiple paternity in the largest rookery of Mediterranean loggerhead sea turtles. Mol. Ecol. 16, 3703–3711. doi: 10.1111/j.1365-294X.2007.03426.x
Keywords: Dermochelys coriacea, habitat, pre-nesting, mating, spatial ecology, satellite telemetry, leatherback sea turtle
Citation: Bond EP and James MC (2017) Pre-nesting Movements of Leatherback Sea Turtles, Dermochelys coriacea, in the Western Atlantic. Front. Mar. Sci. 4:223. doi: 10.3389/fmars.2017.00223
Received: 28 February 2017; Accepted: 03 July 2017;
Published: 20 July 2017.
Edited by:
Sara M. Maxwell, Old Dominion University, United StatesReviewed by:
John Roe, University of North Carolina at Pembroke, United StatesSabrina Fossette, Department of Parks and Wildlife, Australia
Copyright © 2017 Bond and James. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael C. James, bWlrZS5qYW1lc0BkZm8tbXBvLmdjLmNh
 Emily P. Bond
Emily P. Bond Michael C. James
Michael C. James