
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 13 April 2017
Sec. Coral Reef Research
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00102
This article is part of the Research Topic Optics and Ecophysiology of Coral Reef Organisms View all 15 articles
Reef building corals can host different symbiont genotypes (clades), and form distinct holobionts in response to environmental changes. Studies on the functional significance of genetically different symbionts have focused on the thermal tolerance rather than on the nutritional significance. Here, we characterized the nitrogen and carbon assimilation rates, the allocation patterns of these nutrients within the symbiosis, and the trophic condition of two distinct holobionts of Stylophora pistillata: one associated with Symbiodinium clade A in shallow reefs and the other one associated with clade C in mesophotic reefs. The main findings are that: (1) clade C-symbionts have a competitive advantage for the acquisition of carbon at low irradiance compared to clade A-symbionts; (2) light is however the primary factor that determines the positive relationship between the amount of carbon fixed in photosynthesis by the symbionts and the amount of carbon translocated to the host; and (3) by contrast, the dominant Symbiodinium type preferentially determines a negative relationship between rates of coral feeding and nitrogen assimilation, although light still plays a relevant role in this relationship. Clade C-holobionts had indeed higher heterotrophic capacities, but lower inorganic nitrogen assimilation rates than clade A-holobionts, at all light levels. Broadly, our results show that the assimilation and translocation rates of inorganic carbon and nitrogen are clade and light-dependent. In addition, the capacity of S. pistillata to form mesophotic reefs in the Red Sea relies on its ability to select Symbiodinium clade C, as this symbiont type is more efficient to fix carbon at low light.
Coral reefs support one of the most diverse and productive biological communities on Earth. Their success owe from the symbiotic relationship that corals build with photosynthetic dinoflagellate algae, commonly named zooxanthellae, that can sustain up to 95% of the daily energetic demand of the symbiotic association (Muscatine et al., 1984; Muscatine, 1990), also called holobiont (Rohwer et al., 2002). While symbionts need light for photosynthesis and constrain their host to mainly develop in the first 30 m depth, corals can however form large structures called mesophotic reefs in much deeper waters. Mesophotic reefs therefore refer to deep but light-limited coral ecosystems (Hinderstein et al., 2010), ranging between 30 and 80 m in the Gulf of Mexico (Kahng, 2010), to more than 100 m in some locations with exceptionally clear waters, such as the Red Sea (Lesser et al., 2009; Winters et al., 2009). Although, photosynthesis is limited by the low light levels, these reefs host a wide diversity of taxa due to numerous upwelling events and internal waves, that deliver significant amounts of inorganic nutrients and particulate organic matter (Leichter and Genovese, 2006; Lesser et al., 2009).
In response to decreased light intensity, corals can either present host (Brazeau et al., 2013) and/or symbiont genetic diversity (Lesser et al., 2010) as well as adaptations to grow under low irradiance conditions. For example, in order to increase and optimize the light capture and rates of autotrophic carbon production at depth >30 m, corals undergo morphological changes (Mass et al., 2007; Einbinder et al., 2009; Nir et al., 2011) as well as modifications in the photo-physiological traits of their symbionts (Wyman et al., 1987; Lesser et al., 2000, 2009). They also compensate their low photosynthate production by increasing the acquisition of heterotrophic nutrients (Muscatine et al., 1989; Mass et al., 2007; Alamaru et al., 2009; Einbinder et al., 2009; Lesser et al., 2010; Crandall et al., 2016). Corals can finally harbor different Symbiodinium clades depending on the depth at which they thrive (Finney et al., 2010; Lesser et al., 2010). In the Caribbean, although some colonies can present a mix of symbiont clades with a zonation pattern (Kemp et al., 2015), Symbiodinium A and B are predominant in tissue exposed to high irradiance (surface waters) while Symbiodinium C is dominant in shaded tissue of deep corals (Rowan and Knowlton, 1995; Rowan et al., 1997). In the northern Red Sea, Stylophora pistillata shifts from hosting clade A in shallow waters to clade C below 40 m depth (Mass et al., 2007; Winters et al., 2009). Although, numerous studies have now acknowledged the diversity and flexibility of Symbiodinium under different light environments, the functional significance of these associations remains unexplored. It has been suggested that physiological diversification in Symbiodinium species can confer growth advantages to their associated coral host, by presenting different thermal and light tolerances as well as capacities in nutrient acquisition (Little et al., 2004; Abrego et al., 2008; Stat et al., 2008; Venn et al., 2008; Jones and Berkelmans, 2010; Lesser et al., 2013). Among studies on the functional significance of the clades, most have focused on their capacity to tolerate thermal stress (Berkelmans and van Oppen, 2006; Howells et al., 2012; Silverstein et al., 2015; Hume et al., 2016) while their nutritional capacities are still poorly known, although it has important physiological consequences for the host (Loram et al., 2007; Baker et al., 2013; Starzak et al., 2014; Leal et al., 2015). Baker et al. (2013) and Pernice et al. (2015) showed that the acquisition of nitrogen and carbon by Acroporidae species (Acropora tenuis and Isopora palifera, respectively) was clade—(Baker et al., 2013; Pernice et al., 2015) and temperature dependent (Baker et al., 2013). Other studies on sea anemones also demonstrated that Symbiodinium genotypic diversity affects the quantity and quality of photosynthates translocated to the animal host (Loram et al., 2007; Starzak et al., 2014; Leal et al., 2015), as well as the heterotrophic capacities of the host (Leal et al., 2015). Overall, these studies suggest that symbiont identity directly relates to the trophic plasticity of the holobiont. Because acquisition of both auto-and heterotrophic nutrients is critical for coral growth and reproduction (Muscatine and Porter, 1977; Muscatine et al., 1989; Ferrier-Pages et al., 2000; Houlbreque and Ferrier-Pagès, 2009), a better understanding of the nutritional capacities of different holobionts will help predict their resilience to stress. Until now, no direct simultaneous comparison exists between auto-and heterotrophic performance of shallow and deep corals associated with different Symbiodinium genotypes, although deep reefs host a huge biodiversity (Hovland, 2008).
Here we compare the trophic and photosynthetic capacities of two distinct holobionts of the scleractinian coral S. pistillata living in shallow and mesophotic reefs of Eilat (Red Sea). S. pistillata, harbors clade A1 in shallow waters (<40 m) and clade C in deeper environments (>40 m), forming two different holobionts of the same species (Byler et al., 2013). We measured, under the light conditions of surface and deep waters, the heterotrophic capacities of each holobiont, as well as their rates of photosynthesis and acquisition of inorganic carbon and nitrogen using stable isotopes [NaH13CO3 (13C), 15NH4Cl (NH4), and 15NaNO3 (NO3)]. Inorganic carbon and nitrogen acquisition by the symbionts, together with host prey capture, provide direct measures to score nutritional plasticity of these symbioses. Getting new insights into the trophic behavior and the metabolic processes associated with mesophotic corals is essential to understand the ecology and biology of these unique habitats as well as the vertical connectivity existing between shallow and deep environments, which may be of great importance for the future health and resilience of their associated ecosystems.
Experiments were performed in November 2014 at the IUI, in the Gulf of Eilat (Israel). While light conditions decreased from 200 to 400 μmol quanta m−2 s−1 at 5 m depth to 20 to 40 μmol quanta m−2 s−1 at 50 m depth, the seawater temperature (24 ± 0.5°C) and nutrient concentrations remained stable throughout the investigated depth range (Bednarz et al., 2017). The above environmental data were provided by the Israel National Monitoring Program at the Gulf of Eilat (http://www.iui-eilat.ac.il/Research/NMPMeteoData.aspx) and represented average values collected close to our study site. Ten mother colonies of the Red Sea coral S. pistillata were thus sampled directly on the reef, five colonies from 5 m depth and five others from 50 m depth. Field collection of animals complied with a permit issued by the Israel Nature and Parks Authority (2015/40780). Colonies were enclosed in different bags underwater, to keep track of each genotype, and brought back to the laboratory, located few meters from the reef, were they were properly identified. The main Symbiodinium genotype hosted by each colony was checked according to the protocol of Santos et al. (2002). As repeatedly observed (Mass et al., 2007; Lampert-Karako et al., 2008; Winters et al., 2009; Nir et al., 2014), the main clade associated to shallow colonies was A1, while C1 and C3 (simplified to clades A and C throughout the manuscript) populated the deep colonies (Table S5). Fifteen nubbins of about 3 cm long were then cut from each mother colony, tagged, and distributed in 4 open tanks for clade A and four other tanks for clade C. Aquarium system was set up in outdoor water tables, continuously supplied with unfiltered oligotrophic seawater from the reef at a temperature of 24°C ± 0.5. Coral colonies were exposed to photon flux densities (PFD) similar to those found at 50 and 5 m at the month of collection (mean daily PFDs were equivalent to 13.8 mol photons m−2 d−1 for surface and 0.8 mol photons m−2 d−1 for deep holobionts). Proper light intensity was calibrated by the use of a light meter (Li-Cor, LI-250A, USA) and UV-filters were disposed above aquaria to protect deep corals against UVR. Coral nubbins were maintained for 2 days prior to the measurements described below, which have been performed (i) at the “natural” light level of each population/holobiont (subsequently called “high-light” for the shallow population and “low light” for the deep population) but also (ii) at the other corresponding light (low light for the shallow population and high light for the deep one). This last measurement was performed to assess the ability of each holobiont to quickly adjust its response to changes in irradiance.
Changes in oxygen production were monitored for 60 min on 5 nubbins per holobiont (A and C; from 5 mother colonies) at the two mean irradiances corresponding to the surface and deep PFD levels: 350 μmol photons m−2 s−1 (shallow, High light, HL) and 20 μmol photons m−2 s−1 (deep, Low light, LL), respectively. Respiration rates (R) were assessed in the dark after the illumination period. Nubbins were individually placed in glass chambers, filled with a known volume of 0.45 μM filtered seawater (FSW) which were stirred using a magnetic stirrer. Glass chambers were maintained at 24°C by the use of a water bath equipped with a PreSense optode (oxygen-sensitive minisensor) connected to the Oxy-4 (4 Channel oxygen meter, PreSense, Regensburg, Germany). Optodes were calibrated against air-saturated and dinitrogen-saturated water for the 100 and 0% oxygen, respectively. Estimations of net photosynthesis (Pn) at LL and HL and respiration (R) were measured by regressing oxygen production against time. Gross photosynthesis (Pg) was assessed by adding R to Pn. At the end of each incubation, coral nubbins were frozen at −20°C prior to the subsequent determination of total chlorophyll content. Briefly, coral tissue was removed from its skeleton in 10 mL filtered seawater (FSW) with an air-brush, homogenized with a potter grinder and transferred into a 15 mL tube before being centrifuged at 8,000 g for 10 min at 4°C. The pellet was re-suspended into 5 mL 100% acetone and stored at 4°C overnight. The next day, samples were centrifuged at 11,000 g for 15 min at 4°C before assessing the chlorophyll content according to the protocol of Jeffrey and Humphrey (1975). Data were normalized to the surface area (cm2) of each nubbin using the wax-dipping technique (Davies, 1989). Conversions of oxygen fluxes to carbon equivalents, based on molar weights, were calculated according to Anthony and Fabricius (2000) and Gattuso and Jaubert (1990). P:R ratio, which shows the autotrophic capability of an organism to self-maintenance was obtained with the following equation: P:R = (PC × 12)/(RC × 24), where PC = Pg × 12/PQ and RC = R × (12 × RQ). PC represents the amount of carbon acquired during photosynthesis; RC is the amount of carbon consumed by respiration; 12 is the C molecular mass; PQ and RQ are the photosynthetic and respiratory quotient respectively and vary according to the in situ light intensity experienced by the holobionts (Gattuso and Jaubert, 1990; Lesser, 2013). Net photosynthesis was considered to be efficient for 11 h light (length of the day in November in Eilat, http://www.iui-eilat.ac.il/NMP/Default.aspx) and respiration for 24 h. P:R ratio was calculated for a period time of 24 h.
Additional nubbins (n = 5 per condition from 5 mother colonies) were used to assess the respiration rates of freshly isolated symbionts. For this, tissues were extracted from live coral samples in 10 mL of FSW and centrifuged at the corresponding temperature. After centrifugation, pellets were re-suspended in 20 mL FSW and disposed for 20 min in aquaria before processing to the respiration rate measurements as previously exposed. Data were normalized to the surface area of each nubbin.
The following incubations were performed according to Tremblay et al. (2012) and Grover et al. (2002) (Figure 1). For each holobiont (A and C), 40 coral nubbins (8 per colony) were placed in individual beakers filled with 200 mL FSW. Beakers and corals were transferred back to the outdoor water tables; 20 beakers were incubated under ≪ low light ≫ while the other 20 beakers were maintained under ≪ high light ≫ for 5 h (T0), between 11 a.m. and 4 p.m., to cover the maximal irradiances received at each depth. In addition, for each light level, 10 beakers were enriched with 0.6 mM NaH13CO3 (98 atom %13C, #372382, Sigma-Aldrich, St-Louis, MO, USA) and 3 μM 15NH4Cl (98 atom %15N, #24858029, Sigma-Aldrich, St-Louis, MO, USA) while 10 beakers received 0.6 mM NaH13CO3 and 3 μM 15NaNO3 (98 atom %15N, #24862444, Sigma-Aldrich, St-Louis, MO, USA). The concentrations were chosen to provide sufficient enrichment for further analysis. Additional nubbins were incubated in non-enriched water as control samples (natural isotopic abundance). At the end of the 5 h incubation, half nubbins were sampled for T0 while the other half were transferred into non-enriched seawater for one chase period of 24 h (T24). All nubbins were frozen at −20°C at the end of each time point (T0 and T24) before tissue extraction (air pick), separation of the symbionts from the animal tissue by centrifugation and freeze-drying of the extracts for conservation.
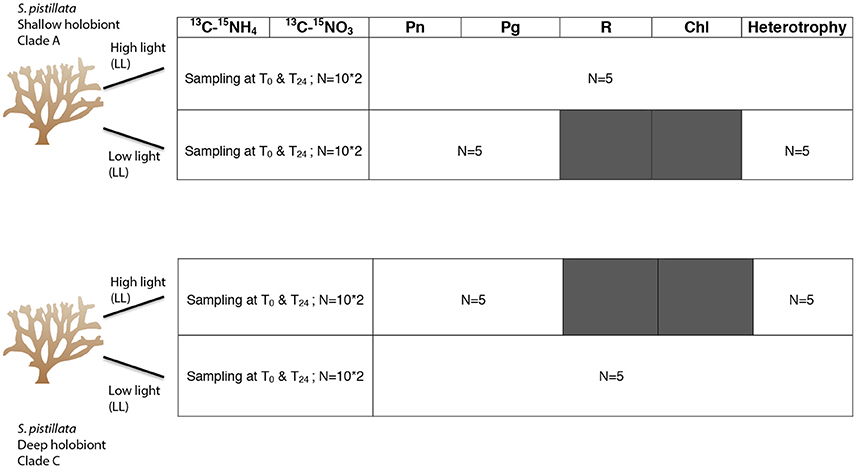
Figure 1. Detailed table summarizing the experimental set up and the different parameters studied according to the number of nubbins used per timesteps [T0 (5 h incubation) and T24 (chase)]. Modified according to: http://ian.umces.edu/imagelibrary/displayimage-77-6640.html.
The subsequent determination of %13C enrichment, %15N enrichment, C and N measurements in the algal and animal compartments were performed with a Delta plus Mass spectrometer (Thermofisher Scientific, Bremen, Germany) coupled to a C/N analyser (Flash EA, Thermofisher Scientific). The 15N or 13C enrichments were calculated from 15N-13C values obtained from samples exposed to 15N or 13C enriched seawater and from control samples incubated without isotope addition. From the difference between these two measurements, the enrichment was calculated and considered significant when it was at least three times higher than the standard deviation obtained on control samples. From there, the nitrogen and carbon assimilation rates were calculated according to Montoya et al. (1996) based on the particulate nitrogen or carbon content of the sample, the incubation time, the theoretical 15N and 13C enrichment of the incubation water at the beginning and the skeletal surface area of the corals.
The carbon incorporation rates in the symbionts and animal tissue was thus calculated according to Tremblay et al. (2012):
Where ρ is the carbon incorporation rate in the algal or animal fraction, Cmeas and Cnat are the percentages of 13C measured in the samples, Cinc is the percent 13C enrichment in the incubation medium, Ms is the mass of the freeze-dried sample (mg) and Mc the mass of carbon per milligram of symbiont or animal tissues (μg mg−1); S is the surface area of the nubbin (cm2) and Tpulse and Tchase are the incubation time periods (h) in the light in the enriched and non-enriched medium. Cinc varies during the pulse-chase period and can be calculated as following:
Where Cpulse and Cchase are the percent 13C enrichment of the enriched and non-enriched incubation media, respectively (Cchase = 1.1%).
Furthermore, the amount of photosynthesized carbon translocated from the symbionts to the host can be calculated using the following equation:
Where Pc is the total amount of autotrophic carbon produced by the symbionts; ρs is the carbon assimilation rate and Rs the respiration rate of the symbionts.
Assimilation rates of ammonium and nitrate ρ were calculated according to Grover et al. (2002, 2003):
Where Nmes is the %15N measured in the sample; Nnat is the natural 15N abundance in control nubbin; Nenr is the 15N enrichment of the incubation medium; Tinc is the incubation time (h) and S is the surface area expressed in cm2; Ms is the total mass of the sample (mg); MN is the particulate nitrogen mass (mg).
For more detailed information regarding the calculations, please refer to Tremblay et al. (2012) and Grover et al. (2002). Data were normalized to the coral surface area.
Feeding rates were determined at each light level (LL, HL) on 10 nubbins per holobiont (from 5 colonies) following Houlbrèque et al. (2004), by incubating individual fragments in small 400 mL plexiglas chambers equipped with a motor-driven propeller to insure a proper flow speed of 4 cm s−1 as described in Ferrier-Pagès et al. (2010). Chambers were kept in a water bath to maintain the temperature constant. After the full expansion of the polyps, the experiment started by delivering 1,000 Artemia salina nauplii L−1 (previously quantified using a Bogorov counting chamber). Artemia concentration was then determined in triplicate after 0, 15, 30, 45, and 60 min and nauplii were directly replaced in the chamber after counting. Three additional chambers without coral were used to account for a possible decrease in nauplii concentration due to natural death. Feeding rates were calculated as the decrease in the number of preys during the incubation, and were normalized to the nubbin surface area. The concentration of A. salina nauplii used in this study (2,000 cells/L) is high compared to the zooplankton concentration usually measured in the reefs (7 cells/L). However, it is low compared to the concentration of nanoplankton prey (106–107 cells/L). It has only been used to assess the heterotrophic capacities of each coral population for a same amount of food.
Statistical analyses were performed using Statistica 10 (Statsoft, Chicago, IL). All data were expressed as mean ± standard error. Normality and homoscedasticity of the data residuals were tested using Kolmogorov–Smirnov (using Lilliefors corrections) and Levene tests. A one-way ANOVA was used to test the difference in chlorophyll content and respiration rates of the clade A and C-symbionts. Two-way ANOVAs were performed to test (i) the effects of the holobiont (A/C) and the light level (LL/HL) on the net and gross photosynthesis rates, the grazing rates and the P:R and (ii) to test the effect of holobiont (A/C) and fraction (host/symbiont) on the structural C:N. A factorial analysis of variance (ANOVA) was performed to test the effects of time (T0 and T24), light level, holobiont and fraction on the rates of carbon and nitrogen (NH4 and NO3) assimilation, the rates and percentage of photosynthate translocation, and the structural C and N contents (μmol C or N mg−1). Moreover, there was no significant difference between 13C measurements obtained in presence of 15NH4 or 15NO3 either for A and C holobionts (ANOVA, p > 0.05), therefore data were pooled for analysis. Percentages of photosynthate translocation were log-transformed prior analyses. When there were significant differences, the analyses were followed by a posteriori testing (Tukey's test). Finally, Pearson correlations were used to attest the relationships between (i) carbon fixation and translocation and (ii) feeding rates and NH4 or NO3 assimilation rates in the algal fraction after 5 h incubation. P-values were considered for p < α, α = 0.05.
Chlorophyll concentration, which was only dependent on the in situ growth conditions of the holobionts (i.e., mainly light) was significantly lower in clade A than clade C-holobionts (Figure 2A; Table S1, ANOVA, p < 0.05). Net rates of photosynthesis (Pn), significantly increased with light for both holobionts (Figure S1A; Tukey HSD, p < 0.001). In addition, while Pn was not different between holobionts under high light, clade A-holobionts presented a lower Pn under low light (Figure S1A; Tukey HSD, p < 0.001). Respiration rates were significantly lower for clade C-holobionts (Figure S1B; Tukey HSD, p < 0.01). Therefore, clade A-holobionts reduced their P:R values with decreasing light intensities from 1.00 ± 0.08 to 0.11 ± 0.1 (Tukey HSD, p = 0.003) while the P:R ratio of clade C-holobionts remained unchanged (6.85 ± 1.5–4.3 ± 1.7 at high and low light respectively, Tukey HSD, p > 0.05), and was higher than the P:R of clade A-holobionts (Tukey HSD, p < 0.02). Feeding rates were significantly lower in clade A than clade C-holobionts, under their natural light level (Figure 2B; Table S1, ANOVA, p < 0.05 and p = 0.02).
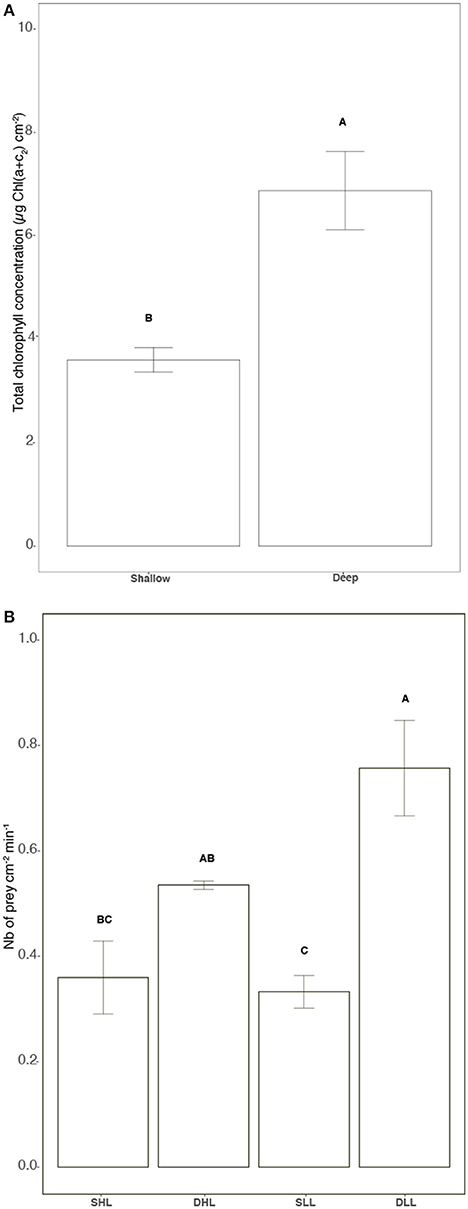
Figure 2. (A) Total chlorophyll concentration [μg Chl (a+c2) cm−2], (B) Grazing rates (Number of prey cm−2 mn−1). Statistically significances are indicated in bold letters, p-values are considered for p < 0.05.
The carbon budget of each holobiont was similar in presence of and for each light level (ANOVA, p > 0.05). Therefore, data represent the mean and standard error of the two measurements.
The amounts of carbon fixed by the symbionts and translocated to the host were significantly affected by both the light level and the clade hosted in each holobiont (Table S2; ANOVA, p < 0.002). Higher rates were indeed observed under high light for both holobionts (Figures 3A,B; Tukey HSD; p < 0.01). However, while clade A fixed and translocated 30% more carbon to the host than clade C under high light (Figures 3A,B; Table S2;Tukey HSD, p < 0.001), the reverse was observed under low light, especially for carbon translocation at T0 (Figure 3B; Tukey HSD, p < 0.05). The rates of carbon incorporation into the symbionts were generally significantly higher than those measured for the host (Figures 4A,B; Tukey HSD, p < 0.03). Higher rates were also generally observed under high light both in the animal and algal fractions of both holobionts (Figures 4A,B; Tukey HSD, p < 0.03).
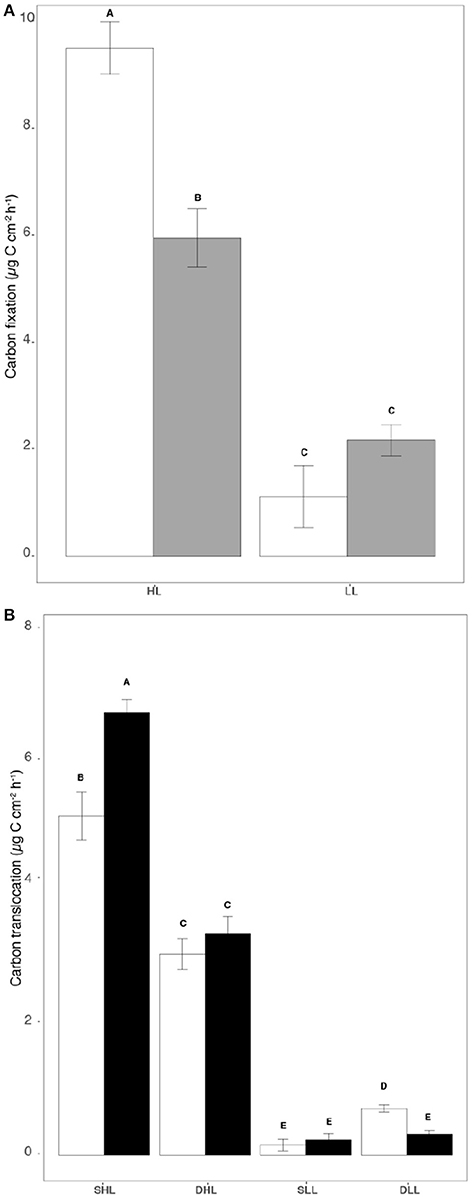
Figure 3. Rates of (A) Carbon fixation (μg C cm−2 h−1) and (B) Carbon translocation (μg C cm−2 h−1) for clade A and C-holobionts according to the light intensity. Statistically significances are indicated in bold letters, p-values are considered for p < 0.05.
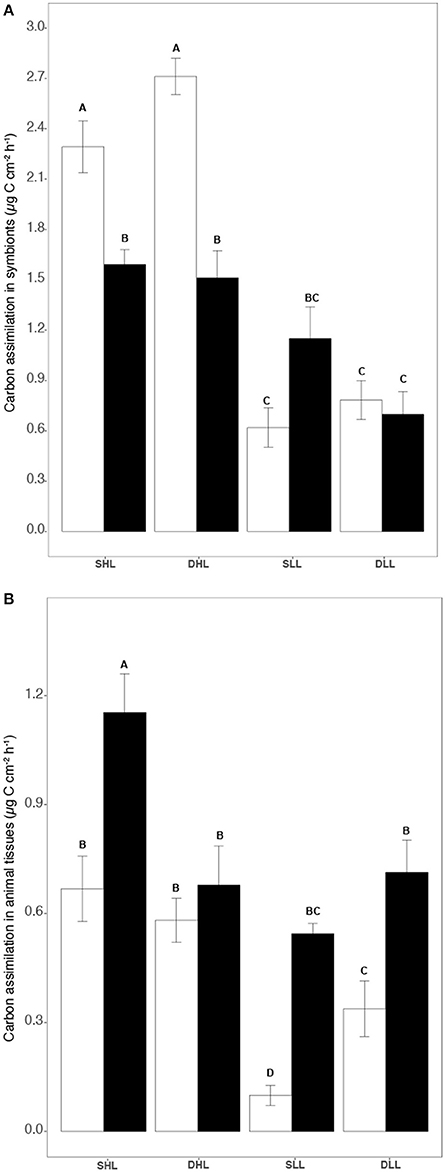
Figure 4. (A) Carbon incorporation rates (μg C cm−2 h−1) in symbionts and (B) in host for both clade A and C-holobionts according to the different fractions (algal and animal tissue), light intensities (LL/HL), and timesteps (T0, T24). Statistically significances are indicated in bold letters, p-values are considered for p < 0.05.
The assimilation of NH4 within the symbiosis was affected by both the clade present in each holobiont, the fraction (host/symbionts) considered and the length of the incubation (Table S3, ANOVA, p < 0.0002). Clade effect was very important as clade A-symbionts assimilated ca. 31 and 95% more NH4 than clade C-symbionts depending on the light level (Tukey HSD, p < 0.03). Clade A symbionts also retained 10–20 times more NH4 than their host (Figures 5A,B; Table S3; Tukey HSD, p < 0.0001), while clade C symbionts retained the same amount (Table S3; Tukey HSD, p > 0.05). The difference between T0 and T24 in the assimilation rates showed that clade A symbionts translocated to their host 6.2% of the NH4 assimilated while clade C symbionts translocated 47.5%.
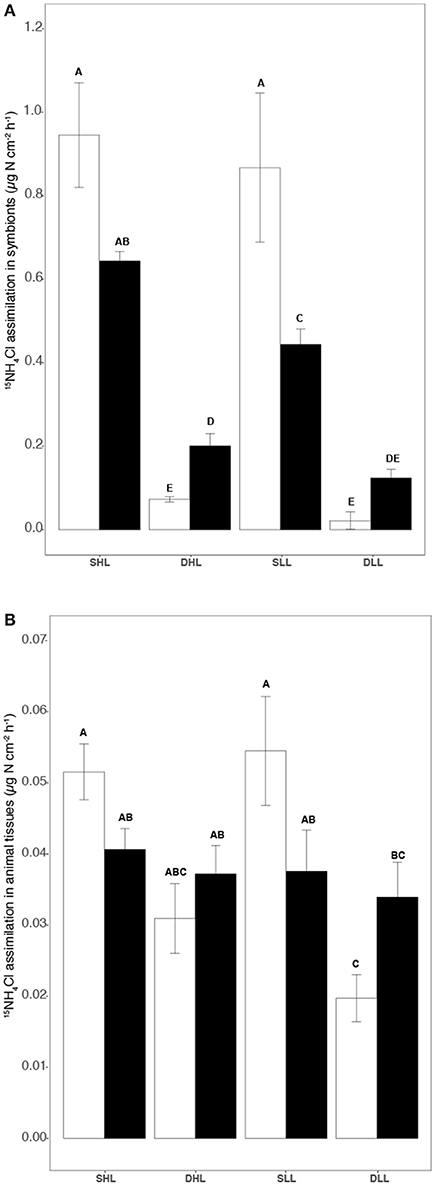
Figure 5. Rates of (A) 15NH4 assimilation in symbionts and (B) 15NO3 host (μg N h−1 cm−2) according to the different fractions (algal and animal tissue), light intensities (LL/HL), and timesteps (T0, T24). Statistically significances are indicated in bold letters, p-values are considered for p < 0.05.
The assimilation of NO3 was affected by both the clade present in each holobiont, and the fraction (host/symbionts) considered (Table S3, ANOVA, p < 0.001). Higher assimilation rates were observed in the symbiont fraction compared to the host tissue for both holobionts at any timesteps and light conditions (Figures 6A,B; Table S3; Tukey HSD, p < 0.0001). Clade effect was also very important as clade A-symbionts assimilated ca. 38 and 67% more NO3 than clade C-symbionts depending on the fraction and time of incubation (Table S3, ANOVA, p < 0.001). In addition, the difference between T0 and T24 in the assimilation rates showed that clade A symbionts translocated 14.3% of the NO3 assimilated while clade C symbionts translocated 19.4%. An increase in the rate of NO3 assimilation was also observed between T0 and T24 in both clade C and clade A symbionts at any timesteps and light intensities (Table S3; Tukey HSD, p < 0.05), except for clade A symbionts exposed to low light (Tukey HSD, p > 0.05).
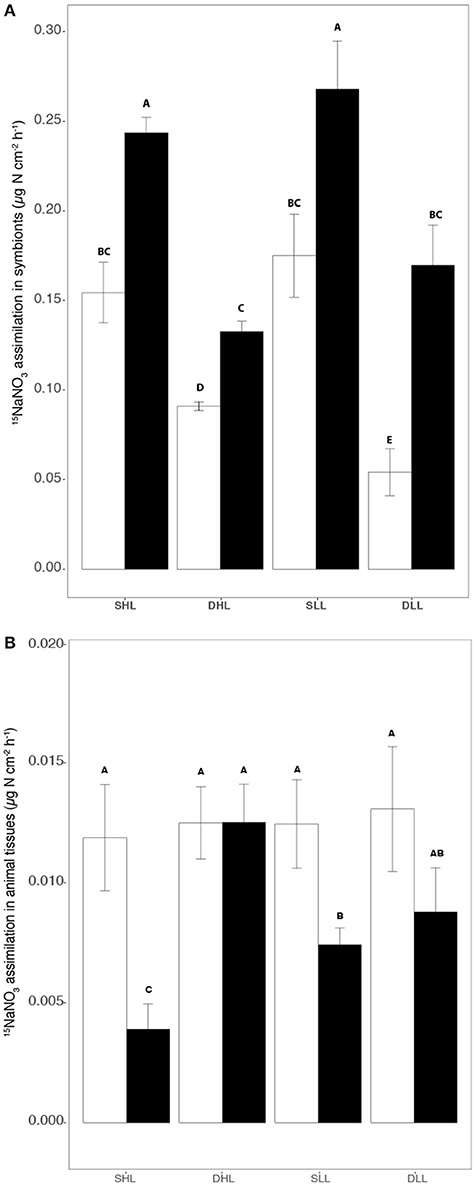
Figure 6. Rates of (A) 15NO3 assimilation in symbionts and (B) host (μg N h−1 cm−2) according to the different fractions (algal and animal tissue), light intensities (LL/HL), and timesteps (T0, T24). Statistically significances are indicated in bold letters, p-values are considered for p < 0.05.
A significant interaction was observed between the fractions (host/symbionts) and the symbiont clade for the structural C:N ratio (Figure S5, Table S4, ANOVA, p < 0.001). The observed difference in C:N between holobionts were due to (1) lower carbon content (μmol C mg−1) of clade C holobionts, mainly in the host (Tukey HSD, p < 0.05; Figure S2); and to (2) lower nitrogen content (μmol N mg−1) of clade C- holobionts in both the host and symbionts (Figures S3, S4;Tukey HSD, p < 0.05). In addition, clade C symbionts showed higher C:N ratio compared to clade A symbionts (Table S4; Tukey HSD, p < 0.05).
A significant positive correlation was observed between the carbon fixation and translocation rates (Figure 7A; r = 0.918; d.f. = 18; p < 0.05). Significant negative correlations were observed between (a) the NH4 assimilation and feeding rates (Figure 7B, r = −0.737; d.f = 18; p < 0.05), (b) the NO3 assimilation and feeding rates (Figure 7C; r = −0.853; d.f = 18; p < 0.05).
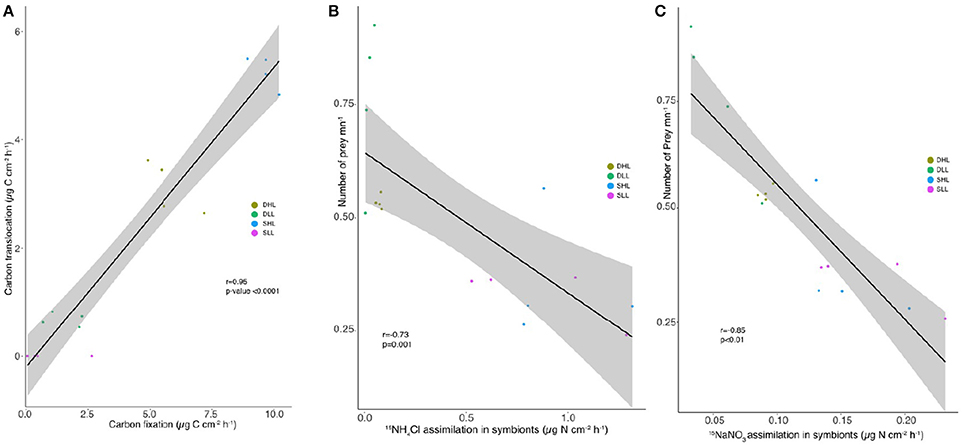
Figure 7. Relationships between (A) rates of carbon fixation (μg C cm−2 h−1) and translocation (μg C cm−2 h−1), feeding and (B) 15NH4 or (C) 15NO3 assimilation rates for clade A and C-holobionts according to the light intensity after 5 h incubation.
This study assessed several aspects of the trophic ecology of the Red Sea coral S. pistillata associated with Symbiodinium clade A in shallow waters and clade C in deep environments (Winters et al., 2003; Mass et al., 2007) forming two different holobionts (Rohwer et al., 2002). The main finding is that clade C-holobionts presented a competitive advantage for the acquisition of carbon at low irradiance compared to clade A-holobionts. This observation is critical to explain the capacity of S. pistillata to colonize deep environments in the Red Sea. In addition, light was the primary factor that determined a positive relationship between the amount of carbon fixed in photosynthesis by the symbionts and the amount of carbon translocated to the host. Symbiodinium genotype was the primary factor influencing nitrogen assimilation rates, although light still played a relevant role in this relationship. Lastly, we observed a negative relationship between feeding rates and inorganic nitrogen assimilation rates. Overall, our results suggest that hosting different symbiont types allows the scleractinian species S. pistillata to colonize both shallow and deep environments in the Red Sea.
The Symbiodinium genotype associated with shallow and deep S. pistillata was one of the primary factors influencing the rates of nitrogen assimilation and translocation. Clade A-holobionts indeed presented higher NH4 and NO3 assimilation rates than clade C-holobionts, independently of the irradiance received during the incubation, and for the same nutrient history (comparable inorganic nutrient levels at 5 and 50 m depth in situ, during the sampling month). Consistent with this finding, two recent studies (Baker et al., 2013; Pernice et al., 2015) found that rates of nitrogen acquisition by Acroporidae species (A. tenuis and I. palifera, respectively) were also clade dependent.
Light intensity/history was the second important factor explaining differences in nitrogen assimilation rates between the two holobionts. The importance of light was evidenced by the fact that (i) clade A-holobionts, which came from the highly-lit shallow reef, and which presented a high metabolic activity, showed higher rates of both NO3 and NH4 assimilation than clade C-holobionts, sampled in deep environment; (ii) NH4 assimilation by clade C-holobionts was higher at 350 μmol photons m−2 s−1 than at 20 μmol photons m−2 s−1. According to previous observations made on other photosynthetic organisms (Canvin and Atkins, 1974; Amory et al., 1991) or on coral symbionts in culture (Rodríguez-Román and Iglesias-Prieto, 2005) and in hospite (Grover et al., 2002), light can have a direct effect on nitrogen assimilation; this later process is indeed closely dependent on the amount of electrons that can be supplied by the ferredoxin, through the linear electron flow between photosystems I and II. Light can also have an indirect effect on nitrogen assimilation, by changing the metabolic activity of the holobionts. The low C and N contents of clade C-holobionts compared to clade A-holobionts are indicative of a lower metabolic activity, which in turn can lead to lower nitrogen requirements. On the contrary, survival in high light environments for clade A-holobionts can increase both their metabolic activity and their nitrogen demand to repair light-damaged photosystems (Dubinsky and Jokiel, 1994; Dubinsky and Falkowski, 2011).
In addition, the higher assimilation of inorganic nitrogen by clade A-holobiont may compensate for the lack of zooplankton, which is known to be much less abundant in shallow waters compared to deep environments (Schmidt, 1973; Lesser et al., 2009). Conversely, nitrogen can be acquired by clade C-holobionts in deep waters through heterotrophy (Crandall et al., 2016) or through dinitrogen fixation (Lesser et al., 2007). This is confirmed by their higher grazing rates compared to shallow holobionts and by the inverse relationship between heterotrophy and inorganic nitrogen acquisition. This negative correlation suggests that nitrogen assimilation by the holobiont needs to be supported by heterotrophic feeding when the activity of the symbionts is reduced, either due to a reduction in light availability or when the dominant symbiont type presents a reduced ability for nitrogen assimilation.
Additionally, clade A and C-holobionts of S. pistillata had unequal translocation rates and allocation patterns of nitrogen within the symbiosis, likely suggesting different needs of the symbionts and animal host in nitrogen. Consistent with previous results on many cnidarian species, clade A-holobionts showed a higher uptake of NH4 compared to NO3 (Wilkerson and Trench, 1986; Wilkerson and Kremer, 1993; Grover et al., 2002, 2003; Pernice et al., 2012), and very low translocation rates to the host. Clade A symbionts thus acted as a sink for nitrogen within the symbiosis (Wilkerson and Kremer, 1993; Grover et al., 2002; Pernice et al., 2012; Kopp et al., 2013). In contrast, clade C-symbionts exhibited similar rates of NH4 and NO3 assimilation, and much higher translocation rates to the host. As a consequence, nitrogen was evenly shared between the host and its symbionts.
The depths at which S. pistillata colonies were sampled (5 and 50 m) account for extreme living conditions because holobionts were exposed either to high irradiance (>350 μmoles photons m−2 s−1) or to <30 μmol photons m−2 s−1 (Cohen and Dubinsky, 2015). By establishing the carbon budget of shallow and deep holobionts of S. pistillata, this study first shows that clade C-holobionts have a competitive advantage for the acquisition of autotrophic carbon at low irradiance compared to clade A-holobionts, and that each holobiont has a different environmental optimum along the irradiance gradient. This study thus confirms previous observations, which concluded that S. pistillata acclimates to depth through photo-adaptive mechanisms linked to the Symbiodinium genotype hosted at each depth distribution (Cohen and Dubinsky, 2015). Overall, many coral species associate with more than one algal taxon, whose relative abundance has been correlated with irradiance gradients (Rowan and Knowlton, 1995; Rowan et al., 1997; Iglesias-Prieto et al., 2004). These changes allow the host to extend its depth range while remaining ecologically dominant. Consistent also with previous observations on deep corals (McCloskey and Muscatine, 1984; Levy et al., 2006; Mass et al., 2007; Cohen and Dubinsky, 2015), low respiration rate was another adaptation of clade C-holobionts to their deep environment. This reduction in respiratory needs maintained the P:R of clade C-holobionts, measured at low light (4.3 ± 1.7), similar to the P:R of clade A-holobionts, measured under high light (1.00 ± 0.08), suggesting that the amount of photosynthates was sufficient to cover the respiratory needs of the deep water colonies. All together, these observations indicate a niche specialization among holobionts of S. pistillata, allowing a maximal acquisition and utilization of carbon throughout the depth profile.
The carbon budget also showed that carbon translocation was closely related to carbon fixation in photosynthesis by the symbionts and was therefore more closely related to light availability than to clade identity. This observation is in agreement with a previous study performed on two scleractinian corals (Tremblay et al., 2015), but challenges the hypothesis that carbon translocation is clade dependent (Leal et al., 2015). Further studies should be carried out, by taking into account different light levels and clade genotypes, to better understand this relationship.
Collectively, the results presented in this study indicate that the vertical distribution pattern of S. pistillata in the Red Sea, and its ability to form mesophotic reefs, can be explained by the presence of specific symbionts adapted to different light regimes. The differential use of light by clade A and C symbionts constitutes an important axis for niche diversification, controlling the abundance and distribution of S. pistillata along the depth gradient. Our results also show that the amount of solar energy that the holobionts absorb drives their metabolic activity, nitrogen assimilation rates and needs as well as their structural C:N content.
LE, CF, MF, and RG conceived the experiment. LE, CF, and MF performed the experiments. LE and CF made the extractions. JM performed the mass spectrometry analyses. LE, CF, RG, and JM analyzed the data. LE, CF, RG, MF, and JM wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a shared affiliation, though no other collaboration, with one of the authors MF and states that the process nevertheless met the standards of a fair and objective review.
We would like to sincerely thank all the staff at the IUI in Eilat (IL) as well as Dr. JO Irisson from the Observatoire Océanologique de Villefranche-sur-mer (LOV) for his constructive comments and help with the statistical analyses, Vanessa Bednarz and Jérôme Durivault from the CSM. Many thanks to Prof. Denis Allemand, Director of the Centre Scientifique de Monaco for scientific support. Lastly, we would like to thank three reviewers for their constructive comments and time spent on revising our manuscript. This project was supported by the IUI, the Israel Science Foundation for MF, the CSM and the Institut Européen de la mer. Financial support to LE was provided by the Centre Scientifique de Monaco.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00102/full#supplementary-material
Abrego, D., Ulstrup, K. E., Willis, B. L., and van Oppen, M. J. (2008). Species–specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc. R. Soc. Lond. B Biol. Sci. 275, 2273–2282. doi: 10.1098/rspb.2008.0180
Alamaru, A., Loya, Y., Brokovich, E., Yam, R., and Shemesh, A. (2009). Carbon and nitrogen utilization in two species of Red Sea corals along a depth gradient: insights from stable isotope analysis of total organic material and lipids. Geochim. Cosmochim. Acta 73, 5333–5342. doi: 10.1016/j.gca.2009.06.018
Amory, A. M., Vanlerberghe, G. C., and Turpin, D. H. (1991). Demonstration of both a photosynthetic and a nonphotosynthetic CO2 requirement for assimilation in the green alga Selenastrum minutum. Plant Physiol. 95, 192–196. doi: 10.1104/pp.95.1.192
Anthony, K. R. N., and Fabricius, K. E. (2000). Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Bio. Ecol. 252, 221–253.
Baker, D. M., Andras, J. P., Jordán-Garza, A. G., and Fogel, M. L. (2013). Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J. 7, 1248–1251. doi: 10.1038/ismej.2013.12
Bednarz, V. N., Grover, R., Maguer, J.-F., Fine, M., and Ferrier-Pagès, C. (2017). The assimilation of diazotroph-derived nitrogen by scleractinian corals depends on their metabolic status. Mbio 8:e02058–16. doi: 10.1128/mBio.02058-16
Berkelmans, R., and van Oppen, M. J. (2006). The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. Lond. B Biol. Sci. 273, 2305–2312. doi: 10.1098/rspb.2006.3567
Brazeau, D. A., Lesser, M. P., and Slattery, M. (2013). Genetic structure in the coral, Montastraea cavernosa: assessing genetic differentiation among and within mesophotic reefs. PLoS ONE 8:e65845. doi: 10.1371/journal.pone.0065845
Byler, K. A., Carmi-Veal, M., Fine, M., and Goulet, T. L. (2013). Multiple symbiont acquisition strategies as an adaptive mechanism in the coral Stylophora pistillata. PLoS ONE 8:e59596. doi: 10.1371/journal.pone.0059596
Canvin, D. T., and Atkins, C. A. (1974). Nitrate, nitrite and ammonia assimilation by leaves: effect of light, carbon dioxide and oxygen. Planta 116, 207–224. doi: 10.1007/BF00390228
Cohen, I., and Dubinsky, Z. (2015). Long term photoacclimation responses of the coral Stylophora pistillata to reciprocal deep to shallow transplantation: photosynthesis and calcification. Front. Mar. Sci. 2:45. doi: 10.3389/fmars.2015.00045
Crandall, J., Teece, M., Estes, B., Manfrino, C., and Ciesla, J. (2016). Nutrient acquisition strategies in mesophotic hard corals using compound specific stable isotope analysis of sterols. J. Exp. Mar. Biol. Ecol. 474, 133–141. doi: 10.1016/j.jembe.2015.10.010
Davies, P. S. (1989). Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395. doi: 10.1007/BF00428135
Dubinsky, Z., and Falkowski, P. (2011). “Light as a source of information and energy in zooxanthellate corals,” in Coral Reefs: An Ecosystem in Transition, eds Z. Dubinsky and N. Stambler (Springer), 107–118.
Dubinsky, Z., and Jokiel, P. L. (1994). Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pac. Sci. 48, 313–324.
Einbinder, S., Mass, T., Brokovich, E., Dubinsky, Z., Erez, J., and Tchernov, D. (2009). Changes in morphology and diet of the coral Stylophora pistillata along a depth gradient. Mar. Ecol. Prog. Ser. 381, 167–174. doi: 10.3354/meps07908
Ferrier-Pages, C., Gattuso, J.-P., Dallot, S., and Jaubert, J. (2000). Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19, 103–113. doi: 10.1007/s003380000078
Ferrier-Pagès, C., Rottier, C., Beraud, E., and Levy, O. (2010). Experimental assessment of the feeding effort of three scleractinian coral species during a thermal stress: effect on the rates of photosynthesis. J. Exp. Mar. Biol. Ecol. 390, 118–124. doi: 10.1016/j.jembe.2010.05.007
Finney, J. C., Pettay, D. T., Sampayo, E. M., Warner, M. E., Oxenford, H. A., and LaJeunesse, T. C. (2010). The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb. Ecol. 60, 250–263. doi: 10.1007/s00248-010-9681-y
Gattuso, J. P., and Jaubert, J. (1990). Effect of light on oxygen and carbon dioxide fluxes and on metabolic quotients measured in situ in a zooxanthellate coral. Limnol. Oceanogr. 35, 1796–1804. doi: 10.4319/lo.1990.35.8.1796
Grover, R., Maguer, J.-F., Allemand, D., and Ferrier-Pages, C. (2003). Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol. Oceanogr. 48, 2266–2274. doi: 10.4319/lo.2003.48.6.2266
Grover, R., Maguer, J.-F., Reynaud-Vaganay, S., and Ferrier-Pages, C. (2002). Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol. Oceanogr. 47, 782–790. doi: 10.4319/lo.2002.47.3.0782
Hinderstein, L. M., Marr, J. C. A., Martinez, F. A., Dowgiallo, M. J., Puglise, K. A., Pyle, R. L., et al. (2010). Theme section on “Mesophotic coral ecosystems: characterization, ecology, and management”. Coral Reefs 29, 247–251. doi: 10.1007/s00338-010-0614-5
Houlbreque, F., and Ferrier-Pagès, C. (2009). Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17. doi: 10.1111/j.1469-185X.2008.00058.x
Houlbrèque, F., Tambutté, E., Allemand, D., and Ferrier-Pagès, C. (2004). Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Biol. 207, 1461–1469. doi: 10.1242/jeb.00911
Hovland, M. (2008). Deep-Water Coral Reefs: Unique Biodiversity Hot-Spots. Springer Science & Business Media.
Howells, E., Beltran, V., Larsen, N., Bay, L., Willis, B., and van Oppen, M. (2012). Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2, 116–120. doi: 10.1038/nclimate1330
Hume, B. C. C., Voolstra, C. R., Arif, C., D'Angelo, C., Burt, J. A., Eyal, G., et al. (2016). Ancestral genetic diversity associated with the rapid spread of stress-tolerant coral symbionts in response to Holocene climate change. Proc. Natl. Acad. Sci. U.S.A. 113, 4416–4421. doi: 10.1073/pnas.1601910113
Iglesias-Prieto, R., Beltran, V., LaJeunesse, T., Reyes-Bonilla, H., and Thome, P. (2004). Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. Lond. B Biol. Sci. 271, 1757–1763. doi: 10.1098/rspb.2004.2757
Jeffrey, S., and Humphrey, G. (1975). New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz BPP 167, 191–194. doi: 10.1016/S0015-3796(17)30778-3
Jones, A., and Berkelmans, R. (2010). Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant vs. sensitive symbiont types. PLoS ONE 5:e10437. doi: 10.1371/journal.pone.0010437
Kahng, S., Garcia-Sais, J. R., Spalding, H. L., Brokovich, E., Wagner, D., Weil, E., et al. (2010). Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29, 255–275. doi: 10.1007/s00338-010-0593-6
Kemp, D. W., Thornhill, D. J., Rotjan, R. D., Iglesias-Prieto, R., Fitt, W. K., and Schmidt, G. W. (2015). Spatially distinct and regionally endemic Symbiodinium assemblages in the threatened Caribbean reef-building coral Orbicella faveolata. Coral Reefs 34, 535–547. doi: 10.1007/s00338-015-1277-z
Kopp, C., Pernice, M., Domart-Coulon, I., Djediat, C., Spangenberg, J. E., Alexander, D. T. L., et al. (2013). Highly dynamic cellular-level response of symbiotic coral to a sudden increase in environmental nitrogen. MBio 4:e00052–13. doi: 10.1128/mBio.00052-13
Lampert-Karako, S., Stambler, N., Katcoff, D. J., Achituv, Y., Dubinsky, Z., and Simon-Blecher, N. (2008). Effects of depth and eutrophication on the zooxanthella clades of Stylophora pistillata from the Gulf of Eilat (Red Sea). Aquat. Conserv. 18, 1039. doi: 10.1002/aqc.927
Leal, M. C., Hoadley, K., Pettay, D. T., Grajales, A., Calado, R., and Warner, M. E. (2015). Symbiont type influences trophic plasticity of a model cnidarian–dinoflagellate symbiosis. J. Exp. Biol. 218, 858–863. doi: 10.1242/jeb.115519
Leichter, J. J., and Genovese, S. J. (2006). Intermittent upwelling and subsidized growth of the scleractinian coral Madracis mirabilis on the deep fore-reef slope of Discovery Bay, Jamaica. Mar. Ecol. Prog. Ser. 316, 95–103. doi: 10.3354/meps316095
Lesser, M. (2013). Using energetic budgets to assess the effects of environmental stress on corals: are we measuring the right things? Coral Reefs 32, 25–33. doi: 10.1007/s00338-012-0993-x
Lesser, M., Stat, M., and Gates, R. (2013). The endosymbiotic dinoflagellates (Symbiodinium sp.) of corals are parasites and mutualists. Coral Reefs 32, 603–611. doi: 10.1007/s00338-013-1051-z
Lesser, M. P., Falcón, L. I., Rodríguez-Román, A., Enríquez, S., Hoegh-Guldberg, O., and Iglesias-Prieto, R. (2007). Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar. Ecol. Prog. Ser. 346, 143–152. doi: 10.3354/meps07008
Lesser, M. P., Mazel, C., Phinney, D., and Yentsch, C. S. (2000). Light absorption and utilization by colonies of the congeneric hermatypic corals Montastraea faveolata and Montastraea cavernosa. Limnol. Oceanogr. 45, 76–86. doi: 10.4319/lo.2000.45.1.0076
Lesser, M. P., Slattery, M., and Leichter, J. J. (2009). Ecology of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 375, 1–8. doi: 10.1016/j.jembe.2009.05.009
Lesser, M. P., Slattery, M., Stat, M., Ojimi, M., Gates, R. D., and Grottoli, A. (2010). Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology 91, 990–1003. doi: 10.1890/09-0313.1
Levy, O., Achituv, Y., Yacobi, Y., Dubinsky, Z., and Stambler, N. (2006). Diel ‘tuning’ of coral metabolism, physiological responses to light cues. J. Exp. Biol. 209, 273–283. doi: 10.1242/jeb.01983
Little, A. F., Van Oppen, M. J., and Willis, B. L. (2004). Flexibility in algal endosymbioses shapes growth in reef corals. Science 304, 1492–1494. doi: 10.1126/science.1095733
Loram, J., Trapido-Rosenthal, H., and Douglas, A. (2007). Functional significance of genetically different symbiotic algae Symbiodinium in a coral reef symbiosis. Mol. Ecol. 16, 4849–4857. doi: 10.1111/j.1365-294X.2007.03491.x
Mass, T., Einbinder, S., Brokovich, E., Shashar, N., Vago, R., Erez, J., et al. (2007). Photoacclimation of Stylophora pistillata to light extremes: metabolism and calcification. Mar. Ecol. Prog. Ser. 334, 93–102. doi: 10.3354/meps334093
McCloskey, L., and Muscatine, L. (1984). Production and respiration in the Red Sea coral Stylophora pistillata as a function of depth. Proc. R. Soc. Lond. B Biol. Sci. 222, 215–230. doi: 10.1098/rspb.1984.0060
Montoya, J. P., Voss, M., Kahler, P., and Capone, D. G. (1996). A simple, high-precision, high-sensitivity tracer assay for N (inf2) fixation. Appl. Environ. Microbiol. 62, 986–993.
Muscatine, L. (1990). The role of symbiotic algae in carbon and energy flux in reef corals. Ecosyst. World 25, 75–87.
Muscatine, L., Falkowski, P., Dubinsky, Z., Cook, P., and McCloskey, L. (1989). The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc. R. Soc. Lond. B Biol. Sci. 236, 311–324. doi: 10.1098/rspb.1989.0025
Muscatine, L., Falkowski, P., Porter, J., and Dubinsky, Z. (1984). Fate of photosynthetic fixed carbon in light-and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc. R. Soc. Lond. B Biol. Sci. 222, 181–202. doi: 10.1098/rspb.1984.0058
Muscatine, L., and Porter, J. W. (1977). Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460. doi: 10.2307/1297526
Nir, O., Gruber, D., Einbinder, S., Kark, S., and Tchernov, D. (2011). Changes in scleractinian coral Seriatopora hystrix morphology and its endocellular Symbiodinium characteristics along a bathymetric gradient from shallow to mesophotic reef. Coral Reefs 30, 1089–1100. doi: 10.1007/s00338-011-0801-z
Nir, O., Gruber, D. F., Shemesh, E., Glasser, E., and Tchernov, D. (2014). Seasonal mesophotic coral bleaching of Stylophora pistillata in the Northern Red Sea. PLoS ONE 9:e84968. doi: 10.1371/journal.pone.0084968
Pernice, M., Meibom, A., Van Den Heuvel, A., Kopp, C., Domart-Coulon, I., Hoegh-Guldberg, O., et al. (2012). A single-cell view of ammonium assimilation in coral–dinoflagellate symbiosis. ISME J. 6, 1314–1324. doi: 10.1038/ismej.2011.196
Pernice, M., Dunn, S. R., Tonk, L., Dove, S., Domart-Coulon, I., Hoppe, P., et al. (2015). A nanoscale secondary ion mass spectrometry study of dinoflagellate functional diversity in reef?building corals. Environ. Microbiol. 17, 3570–3580. doi: 10.1111/1462-2920.12518
Rodríguez-Román, A., and Iglesias-Prieto, R. (2005). Regulation of photochemical activity in cultured symbiotic dinoflagellates under nitrate limitation and deprivation. Mar. Biol. 146, 1063–1073. doi: 10.1007/s00227-004-1529-x
Rohwer, F., Seguritan, V., Azam, F., and Knowlton, N. (2002). Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 243, 1–10. doi: 10.3354/meps243001
Rowan, R., and Knowlton, N. (1995). Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc. Natl. Acad. Sci. U.S.A. 92, 2850–2853. doi: 10.1073/pnas.92.7.2850
Rowan, R., Knowlton, N., Baker, A., and Jara, J. (1997). Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388, 265–269. doi: 10.1038/40843
Santos, S. R., Taylor, D. J., Kinzie, R. A. III, Hidaka, M., Sakai, K., and Coffroth, M. A. (2002). Molecular phylogeny of symbiotic dinoflagellates inferred from partial chloroplast large subunit (23S)-rDNA sequences. Mol. Phylogenet. Evol. 23, 97–111. doi: 10.1016/S1055-7903(02)00010-6
Schmidt, H.-E. (1973). The vertical distribution and diurnal migration of some zooplankton in the Bay of Eilat (Red Sea). Helgoländer Wiss. Meeresunters. 24, 333–340. doi: 10.1007/BF01609523
Silverstein, R. N., Cunning, R., and Baker, A. C. (2015). Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Chang. Biol. 21, 236–249. doi: 10.1111/gcb.12706
Starzak, D. E., Quinnell, R. G., Nitschke, M. R., and Davy, S. K. (2014). The influence of symbiont type on photosynthetic carbon flux in a model cnidarian–dinoflagellate symbiosis. Mar. Biol. 161, 711–724. doi: 10.1007/s00227-013-2372-8
Stat, M., Morris, E., and Gates, R. D. (2008). Functional diversity in coral–dinoflagellate symbiosis. Proc. Natl. Acad. Sci. U.S.A. 105, 9256–9261. doi: 10.1073/pnas.0801328105
Tremblay, P., Grover, R., Maguer, J. F., Legendre, L., and Ferrier-Pagès, C. (2012). Autotrophic carbon budget in coral tissue: a new 13C-based model of photosynthate translocation. J. Exp. Biol. 215, 1384–1393. doi: 10.1242/jeb.065201
Tremblay, P., Maguer, J. F., Grover, R., and Ferrier-Pagès, C. (2015). Trophic dynamics of scleractinian corals: stable isotope evidence. J. Exp. Biol. 218, 1223–1234. doi: 10.1242/jeb.115303
Venn, A., Loram, J., Trapido-Rosenthal, H., Joyce, D., and Douglas, A. (2008). Importance of time and place: patterns in abundance of Symbiodinium clades A and B in the tropical sea anemone Condylactis gigantea. Biol. Bull. 215, 243–252. doi: 10.2307/25470708
Wilkerson, F. P., and Kremer, P. (1993). DIN, DON and PO4 flux by a medusa with algal symbionts. Mar. Ecol. Prog. Ser. 90, 237–237. doi: 10.3354/meps090237
Wilkerson, F., and Trench, R. (1986). Uptake of dissolved inorganic nitrogen by the symbiotic clam Tridacna gigas and the coral Acropora sp. Mar. Biol. 93, 237–246. doi: 10.1007/BF00508261
Winters, G., Beer, S., Zvi, B. B., Brickner, I., and Loya, Y. (2009). Spatial and temporal photoacclimation of Stylophora pistillata: zooxanthella size, pigmentation, location and clade. Mar. Ecol. Prog. Ser. 384, 107–119. doi: 10.3354/meps08036
Winters, G., Loya, Y., Rottgers, R., and Beer, S. (2003). Photoinhibition in shallow-water colonies of the coral Stylophora pistillata as measured in situ. Limnol. Oceanogr. 48, 1388–1393. doi: 10.4319/lo.2003.48.4.1388
Keywords: light acclimation, carbon isotopes, nitrogen isotopes, clades, nitrate, ammonium, scleractinian coral, mesophotic coral
Citation: Ezzat L, Fine M, Maguer J-F, Grover R and Ferrier-Pagès C (2017) Carbon and Nitrogen Acquisition in Shallow and Deep Holobionts of the Scleractinian Coral S. pistillata. Front. Mar. Sci. 4:102. doi: 10.3389/fmars.2017.00102
Received: 14 December 2016; Accepted: 27 March 2017;
Published: 13 April 2017.
Edited by:
Noga Stambler, Bar-Ilan University, IsraelReviewed by:
Susana Enríquez, National Autonomous University of Mexico, MexicoCopyright © 2017 Ezzat, Fine, Maguer, Grover and Ferrier-Pagès. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leïla Ezzat, bGVpbGFAY2VudHJlc2NpZW50aWZpcXVlLm1j
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.