- 1School of Environmental Engineering, Technical University of Crete, Chania, Greece
- 2School of Mineral Resources Engineering, Technical University of Crete, Chania, Greece
- 3Department of Civil, Chemical, Environmental and Materials Engineering (DICAM), Alma Mater Studiorum—University of Bologna, Bologna, Italy
The fragmentation of high-density polyethylene (HDPE) films from single-use supermarket plastic bags to microplastics under laboratory-simulated onshore and nearshore conditions was investigated for a period of 6 months. The weathering process of the plastic strips either on beach sand or in seawater under direct natural sunlight was monitored by tensile strength, molecular weight measurements, FTIR, weight loss, and image processing of photographs of the plastic strips before and after mild mechanical stress was applied. The latter represents a novel method proposed for determining the onset of fragmentation through the application of mild mechanical stress on the weathered plastic samples emulating the action of sand and wind on a beach. It was found that 12 h of application of mild mechanical stress in rotating glass bottles filled partially with sand was sufficient time to reach the maximum degree of fragmentation that could occur for the weathered plastics samples being tested. For example, applied mechanical stress yielded an area loss of almost 14% for samples weathered for a period of 5 months and about 16.7% after 5.5 months. While tensile strength tests and molecular weight measurements were rather inconclusive till the very last month when the onset of fragmentation was identified; FTIR measurements revealed that samples under ultraviolet irradiation were gradually modified chemically until fragmentation commenced. After 6 months of weathering, molecular weight measurements showed a 60% reduction for sample SMB-1 whereas for sample SMB-2 the measurement was not possible due to extensive fragmentation. The onset of fragmentation for SMB-1 and SMB-2 samples occurred at a cumulative luminance of 5.3 × 106 lux•d and in the presence of atmospheric oxygen whereby the polymer films broke down partially to microplastics. When the UV exposure reached 7.2 × 106 lux•d the weathered plastic strips broke down fully to microplastics with the application of a mild mechanical stress. Samples placed in seawater proved to be resistant to fragmentation compared to those on sand over the 6-month period of the weathering experiment. The direct implication of this work is that beached macroplastic debris should be regularly collected from the seashore before they are weathered by sunlight and returned to the sea as microplastics by the action of high waves or strong winds.
Introduction
Marine litter is any persistent, manufactured, or processed solid material discarded, disposed of or abandoned in the marine or coastal environment. In general it consists of man-made items that have been deliberately discarded or unintentionally lost in the sea or on beaches, and it includes materials transported from land by rivers, draining or sewage systems, or winds (Cheshire et al., 2009). Globally, the annual input of marine debris in the marine environment has been estimated to be nearly 6.4 million tons (Gregory, 2009), while 103,247,609 items were collected between 1989 and 2007 in 12 marine regions worldwide (Cheshire et al., 2009). Considering the low degradation rates, the effects of accumulation can be observed on both marine life and the human well-being (Barnes et al., 2009). Floating debris patches can act as a means of alien species dispersal in marine ecosystems, while debris ingestion and entanglement of marine organisms in debris, especially plastics, has caused injuries and even death to a multitude of species (Gregory, 2009). Apart from the aesthetic nature of marine litter pollution, it has also been reported to be the cause of injuries and other health concerns besides adverse economic and social impacts (Depledge et al., 2013; Brower, 2016).
Although, marine litter is found in all marine compartments, from surface waters and beaches to deep seas, their composition and quantity varies greatly among locations, due to hydrographic, geomorphologic, and anthropogenic factors (Pham et al., 2014). In the English Channel, 10 to more than 100 items/km2 of debris could be observed floating on the water surface, while in the Atlantic and Pacific oceans concentrations of 0–20 items/km2 and up to 36 items/km2, have been reported accordingly. The values noted for the Mediterranean Sea are much lower, between 1.5 and 25 items/km2. Seafloor densities were much higher, ranging from 50 items/km2 in Indonesia to 1935 items/km2 in the northwestern Mediterranean. Coastline data revealed concentrations of 15–29,000 items per km of shore (Cheshire et al., 2009; UNEP, 2014).
The most abundant of components of marine litter is plastic, and due to the ease with which it can be transferred, it can be found among litter collected from even the remotest locations (Ryan et al., 2009). Enclosed areas, such as the Mediterranean Sea, and oceanic gyres appear to be more heavily polluted, while plastic pollution has been detected even in the Antarctic. Uncontrollable discard of plastics and landfill leaks have been recognized as the most contributing land-based sources of marine pollution (Barnes et al., 2009; Ryan et al., 2009), while less contributing marine-based sources are related to professional activities related to the sea (fishing, offshore oil production, recreational activities, etc.) and to population accumulation in coastal areas. Lost, abandoned, or otherwise discarded fishing gear is the result of a variety of fishing activities both commercial and recreational. Derelict fishing gear, the so-called “ghost-nets” have been identified as a serious environmental problem, due to its negative impact on marine biota (Watson et al., 2006; Cheshire et al., 2009).
Accounting for almost 30% of the yearly demand for plastics in the European Union, Polyethylene is the most widely used type of plastic. Based on density, it can be found in two forms; Low Density Polyethylene (LDPE), used for the production of reusable bags, trays, containers, agricultural film and food packaging film, and High Density Polyethylene (HDPE), used as a manufacturing material for films, toys, bottles, pipes, house-ware, etc. (PlasticsEurope, 2015). It also represents 79% of the polymers in microplastics sampled at sea or in the marine environment (Hidalgo-Ruz and Thiel, 2013). Plastic carrier bags, usually composed of PE, represent a substantial portion of plastic pollution. In the EU alone, 98.6 billion plastic bags were used, corresponding to 198 plastic bags per citizen annually. A devastating 89% of plastic carrier bags are characterized as single-use, with half of the discarded bags ending up in landfills. Throughout the EU, measures have been taken by individual members for the reduction of single use plastic carrier bags, while the Commission policy will enforce pricing of plastic carrier bags, in order to discourage their use. This measure has been proven effective during its effect in member states such as Ireland, France, Portugal, and Spain (Directive of the European Parliament and of the Council Amending Directive, 2013; Martinho et al., 2017).
There is substantial bibliographic evidence of large debris accumulation in European seas, sea floors, and coasts. More specifically, carrier bags accounted for 73% of plastic waste collected of the coast of Tuscany and 70% of total debris collected around French cities, while in the UK, one plastic bag was sampled every 23 m of beach (Directive of the European Parliament and of the Council Amending Directive, 2013). Apart from the effects that marine debris can have on marine life discussed above, plastic bags pose an even greater threat. The effect of environmental factors such as radiation, heat and mechanical stress, leads to fragmentation into small pieces known as secondary microplastics (Andrady, 2011). In the most popular definition of microplastics, they are referred as plastic items with a maximum dimension smaller than 5 mm (Arthur et al., 2009). Besides the secondary microplastics, primary microplastics are also found in the marine environment and represent all polymer products that have been produced at a nominal size <5 mm. Primary microplastics include pellets as raw polymeric materials, cosmetic micro-beads, sandblasting plastic micro-beads (Cole et al., 2011). Secondary microplastics are the most dominant type of plastic found in marine environments and are usually made of polyethylene, with sizes ranging from 0.01 to 1 μm (UNEP, 2015; da Costa et al., 2016).
The generation of microplastics through fragmentation of larger pieces of plastic debris in the marine environment is a complex process, depending on numerous factors, such as luminance, temperature, oxygen level, as well as characteristics inherent to the nature of the degrading material including molecular weight distribution and the presence of additives. It is very important to have estimates of fragmentation rates of the plastics present in the marine environment if we wish to develop reliable models for predicting the future environmental status of our oceans and seas. However, fragmentation rates cannot be described by classical chemical kinetics; as the plastic is weathered, it remains intact while polymer chains are broken and oxygen is incorporated through oxidation. It is only after sufficient weathering that the onset of fragmentation is reached when small fragments start breaking off with the application of mild mechanical stress. As weathering proceeds further, a higher portion of the original piece of plastic is turned into microplastics. The weathering of polyethylene has been previously studied in both real and simulated marine and coastal environments, utilizing mechanical stress and chemical techniques for the testing of degradation. Several studies have shown changes in mechanical properties, although their results are often contradicting. Structural modifications and brittleness, although a usual observation, do not always correspond to mechanical strength and molecular weight changes (Andrady, 1990; Jabarin and Lofgren, 1994; Tidjani, 2000; Carrasco et al., 2001). O'Brine and Thompson (2010) observed statistically significant decreases of tensile strength for four different types of plastic carrier bags in marine conditions. Area loss reported from the same team was insignificant (2%) for standard PE bags, while compostable bag samples had totally deteriorated after 16 weeks. Weight loss of almost 40% has been observed in plastics buried in soil (Accinelli et al., 2012), while Müller et al. (2012) estimated degradation rates between 3 and 9% for biodegradable bags in simulated sea turtle gastrointestinal conditions. Microorganisms have also been used for the biodegradation of weathered plastics (Shah et al., 2008), including polyethylene (Artham et al., 2009; Restrepo-Flórez et al., 2014; Kumar Sen and Raut, 2015).
Yet as of today, no published work has identified the onset of fragmentation. In this paper we determine experimentally the onset of fragmentation as well as the level of weathering that leads to complete fragmentation of PE films of specific thickness. Among the various plastics, we focused on high-density polyethylene (HDPE) films used in single-use supermarket carrier bags where both these weathering thresholds are identified. The study utilized standard techniques, such as tensile strength measurements, molecular weight measurements, FTIR, combined with weight loss and an optical quantification method of fragmentation through image analysis. An innovative protocol to determine the onset of fragmentation by the application of mild mechanical stress is also proposed.
Materials and Methods
Experimental Setup
Plastic bags from two local supermarket stores were used to investigate their fragmentation to microplastics, as such carrier bags represent a usual macroplastic debris found on sandy beaches. The supermarket bags, SMB-1 and SMB-2, consisted of HDPE and had a thickness of 0.1 mm. This is the typical thickness of single-use supermarket plastic bags. Additional samples from bags with a thickness of 0.7 mm were also examined but no fragmentation could be observed after 6 months of irradiation. The results for these samples can be found in the Supplementary Material. All chosen bags were cut into strips of similar dimensions (1 × 20 cm) with an industrial concrete cutter kindly provided by Plastika Kritis SA (Heraklion, Crete, Greece).
Each supermarket bag (SMB) underwent four treatments, each representing different environmental conditions: Treatment A, an indoor sand bed (depth 35 cm, diameter 130 cm), representing the control for the weathering of plastics on a beach shore, with no exposure to UV light. Treatment B consisted of 2 similar sand beds placed outdoors and under direct sunlight emulating the conditions on a sandy beach. In parallel, three aquarium tanks filled with equal quantities of seawater and equipped with air pumps for mixing and oxygenation purposes, were used to observe potential fragmentation in seawater. One tank (the control) was placed indoors (Treatment C), while two were placed outdoors (Treatment D) under direct sunlight. All the sand beds contained the same quantity of sand, circa 188 kg and the tanks were filled with seawater 30 cm deep. About 50 plastic strips from both types of SMB were placed in each sand bed and seawater tank (shown in Figure 1). The experiment lasted 6 months in total, from 6/2/2015 to 2/8/2015. Samplings were performed on a biweekly basis, removing 5 samples per treatment for further examination. Monthly samplings were taken for the molecular weight and FTIR measurements.
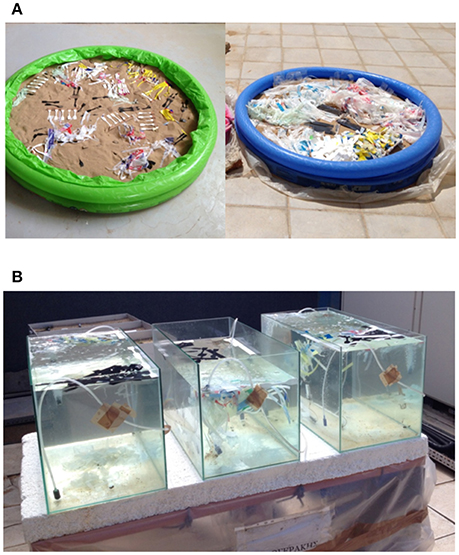
Figure 1. Experimental setup. (A) Simulation of coastal conditions indoors (treatment A) and outdoors (treatment B), (B) simulation of marine conditions outdoors (treatment D).
The environmental conditions (temperature and sunlight intensity) were monitored in 5 min intervals, throughout the duration of the experiment, using one HOBO Temperature Light 3,500 DP Logger per treatment. The data from the loggers were collected monthly and processed, using MS Excel.
Experimental Procedures
Tensile Strength Measurements
Both new and weathered strips underwent tensile strength measurements, using an INSTRON (Norwood, MA, USA) apparatus, enabling the calculation (details given in Supplementary Materials, Figure S1) of the Young's Modulus (E), the Yield Strength (σy), and the Ultimate Elongation (εu). For this procedure, an initial length Lo = 2 cm was used, instead of the whole length of the plastics strips. The end part of each sample strip was first wrapped with paper, preventing slipping to occur during the testing (later, instead of using paper, the sample ends were covered with Teflon to avoid of slipping), and then attached to a mechanism of revolving screws applying the tensile forces. Tensile test samples followed the specific process every time and the velocity of the instrument was set at 0.005 mm/s. Each test was repeated five times and the values of E, σy, and εu were determined using the classical stress strain diagram (Figure S1).
Molecular Weight Estimation—Melt Index
An extrusion plastometer/melt flow index (MFI) tester (ZWICK ROELL, Ulm, Germany) was used to calculate the relative changes in the molecular weight of the samples. Operating conditions, such as temperature, the time required to complete each measurement, and the amount of plastic sample exiting the line were used in combination with basic methodologies (shear flow curve, molecular weight distribution, and melting of polyethylene) to produce the estimates. The procedure consists of the melting of the plastic sample at specific temperature, depending mainly on the type of the polymer tested, followed by extrusion. The measurement required 7 g samples and is repeated 5 times. The extruded plastic mass is measured with a 0.001 g precision scale. The temperature used for all samples was 190°C and the melt flow time was 10 min.
The assessment of the changes in the molecular weight (MW) were obtained indirectly through viscosity measurements and in particular through the MFI computed as the mass of the melt flow over a period of 10 min. From the fundamental equation relating viscosity to MW:
The viscosity (η) is inversely proportional to the MFI:
The MFI is essentially the mass of the melt (m) passing through over a period of 10 min, and hence, by considering the above equations for samples taken at time 0 and at time t:
Finally, the change in molecular weight is estimated from the measured melt mass initially (time 0) and at the requested point in time:
where mt is the average mass of the initial sample (at time 0) from 5 replicates and m0 is the average mass of the sample tested (at time t) from 5 replicates.
Fourier Transform Infrared Spectroscopy (FTIR)
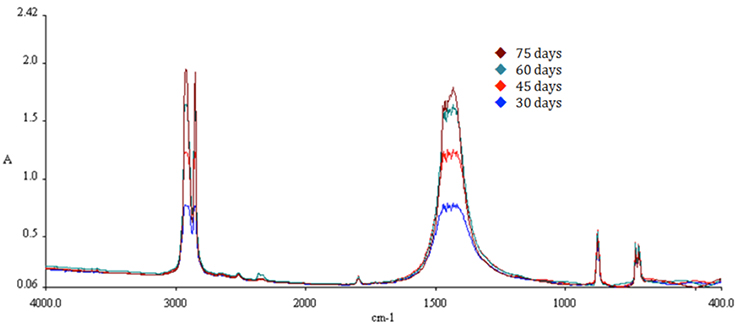
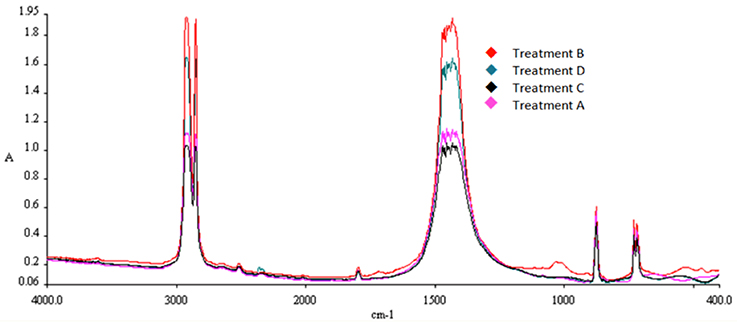
In FTIR, infrared radiation is passed through the sample for the generation of the absorbance or transmittance spectrum. FTIR is preferred in this case due to the measurement of the whole wavelength range at once. Infrared frequency identical to the vibrational frequency of a bond results in absorption, creating a spectrum acting as a molecular “fingerprint” of the sample. The position, shape, and intensity of peaks in this spectrum reveal details about the molecular structure of the sample. All samples were washed twice in ultrasound for 5 min with ultra-pure water and left to dry naturally for 1 day. The spectrum range for the analysis was the middle infrared, 4,000 to 400 cm−1; the spectral resolution was set at 4.0 cm−1 with a step of 2.0 cm−1 every 20 ns. Particular attention was paid for absorbance at 1,471 cm−1 over time where the oxidation of polyethylene is indicated.
Image Processing to Monitor Fragmentation and “Invisible” Plastics
Protocol for image processing of microscope images of new and weathered plastics
Image processing allows for a more detailed observation of the fragmentation, disintegration, and the overall deterioration of the surface of the plastic samples. Inspired by the image analysis performed by O'Brine and Thompson (2010) on weathered plastic bags, a protocol was developed that enables the confirmation and quantification of weathering (i.e., loss of material due to fragmentation or biodegradation) of intact pieces of plastic. The removed pieces due to fragmentation correspond to the so-called “invisible plastics” (maximum length <100 μm), as they are not degraded however they cannot be readily seen with naked eye. Certain samples can even be presented in three-dimensional form. The sample images are stored in a defined folder, also containing a set of initial variables, defined or automatically inferred from a MATLAB-based algorithm, including the gray scale and the size threshold over which pixels are classified as deterioration (i.e., removal of plastic due to fragmentation). The algorithm automatically acquires each image and proceeds to thresholding, in order to acquire deteriorated sections. A cleaning of “salt and pepper” type minor thresholded sections is followed by exclusion of microscope image borders. The overall area of deterioration and the number of deterioration sections are quantitatively measured for each image and finally the average deterioration area and the number of sections for all samples included in the folder are quantitatively estimated. Furthermore, the algorithm computes the degree of deterioration (%) as the ratio of deteriorated area to the total area, and the number of fragments in case distinct pieces of plastic can be identified in the picture, based on the identified area of deterioration. A selected sample (strip of supermarket plastic bag) exposed to sunlight outdoors on a sand bed for a period of 5 months compared to the initial condition when the samples were placed outdoors is shown in Figure 2.
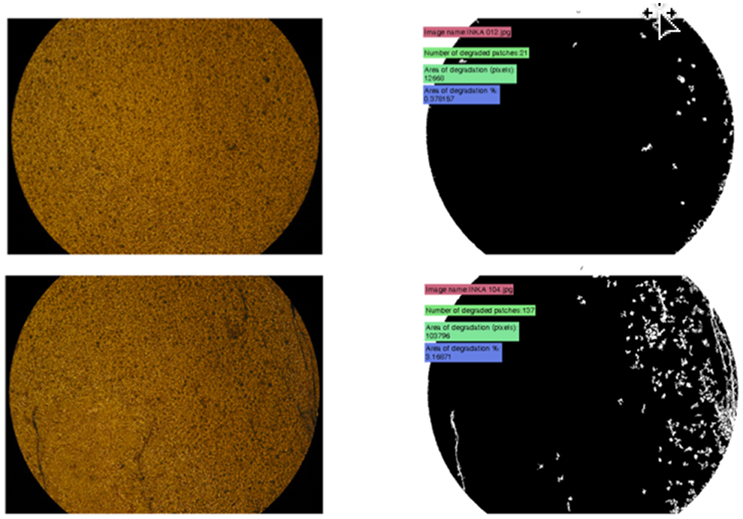
Figure 2. New sample, initial image vs. processed image (above) and older sample, after 5 months of weathering, initial image vs. processed image (below).
The MATLAB-based algorithm used in this study can be found in the Supplementary Materials.
Protocol for image processing of fragmented plastic strips to quantify fragmentation
After a longer weathering period, loss of plastic material could be visually observed especially at the loose edges of the plastic samples (strips). Additionally, the color of the samples often faded away and they became more brittle. The proposed protocol commences with the measurement of the weathered samples from a specific distance from the edge and continues with weighing using a six decimal precision (i.e., 0.000001 g) scale. Parallel placing of the strips on a piece of black paper is followed by the placement of a non-reflecting glass panel, allowing the photographing of the samples with a digital camera. Following that, the images are processed in a manner similar to the one used for the microscopic images. Sample strips or chunks are isolated during image thresholding, followed by noise removal and quantification of the area decrease for each sample over the initial intact sample and quantification of number of pieces (visible fragments) per sample. The algorithm performs the aforementioned steps for samples, strips, or even larger pieces of plastic. The image processing of the photograph of the weather plastic strips (locations 2, 3, and 4) and the initial plastic (location 1) are shown in Figure 3. The missing surface compared to strip in location 1 provides an estimate of the decrease and hence, it can be related to material loss.
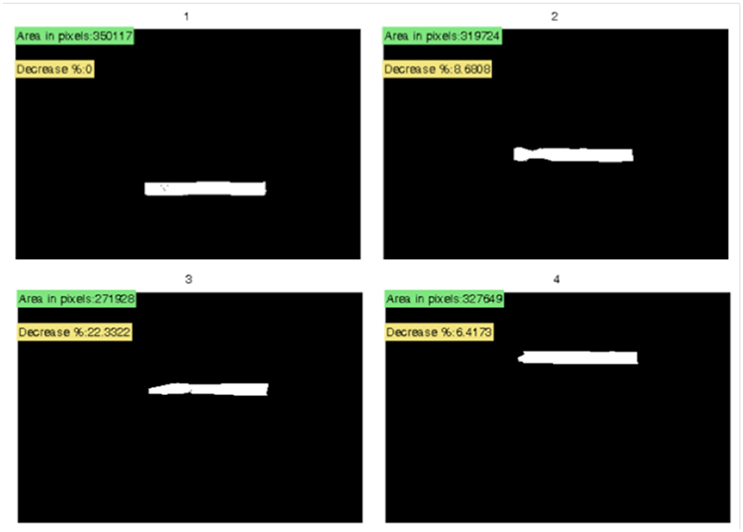
Figure 3. Plastic strips along with area in pixels, and percent (%) area decrease of initial sample.
Novel Method to Determine the Onset of Fragmentation—Application of Mild Mechanical Stress on Weathered Plastics
Weathering of plastics due to exposure to UV light in the presence of atmospheric oxygen results in the breakage of the polymer chains, which ultimately results in fragmentation of the plastic into many pieces; however, in the field, the breakage into pieces is observed following the application of mild mechanical stress by the prevailing winds, movement of the sand, or wave action. If the later is not applied, the plastic looks intact and the fragmentation cannot be quantified. Here we propose for the first time, a simple method for estimating the onset of fragmentation, namely the degree of weathering of the plastic material beyond which visually observed fragmentation takes place. We propose the application of mild mechanical stress to determine if a plastic strip will fragment into smaller pieces. This can be done in many different ways. In our case, we used the following procedure: plastic strips are placed in roller bottles filled 50% with sand, turning at a constant temperature and rotational speed. In our laboratory, we used 250 mL culture bottles that were placed in a Hybridization Incubator (Model#7601, Gesellschaft manufacturer für Labortechnik, Burgwedel, Germany), where the bottles were secured horizontally at specific positions and were left rotating for 24 h at 30°C and rotating at 13 rpm. This particular device holds up to 8 bottles, allowing the simultaneous testing of multiple samples. After 24 h of mild mechanical stress, the weathered samples were removed from the bottles and emptied in an aluminum tray, where all visible plastic pieces created during this procedure were collected with forceps. Care was taken so that any pieces of plastic adhering to the bottle cap were also collected. The plastic pieces were then weighed with a high precision balance and placed on black hardboard paper to be photographed. The weight difference between the original strip prior to weathering and the weight of the collected fragments provided an estimate of the amount of “invisible” plastics, namely microplastics that are small enough not to be visible by naked eye (typically <0.1 mm). A MATLAB algorithm similar to the one used earlier, thresholds the digitally processed photographs for the isolation of sample strips or chunks and removes the noise from the images. A classification process follows, grouping the isolated chunks for each sample, based on a directional erosion process, so that the area decrease for each sample over the initial intact sample and quantification of number of pieces (visible fragments) can be conducted.
In an effort to minimize the time allocated for the application of mild mechanical stress, a series of experiments were conducted with additional HDPE samples (carrier bags and pure HDPE films) which were irradiated with artificial UV-A (wavelength 315–400 nm) for a period of 6 months, then rotated in the incubator for 4, 8, 12, and 24 h, respectively. This experiment revealed the minimum period of time that rotation should take place in order to reach the maximum fragmentation of the plastic samples for the given degree of weathering was 12 h. Hence, a 12 h period is proposed for future experiments.
Results
Environmental Conditions
Temperature variations were observed over time during the experiment and they were due to seasonal temperature changes (winter to summer period). The mean, minimum, and maximum daily temperatures during sunlight exposure times for the periods between samplings and the cumulative luminance exposure on sampling days for all four treatments are shown in Table 1. The mean indoor temperatures (control treatments A and C) are consistently lower than the outdoors (treatments B and D). The minimum temperatures for both indoors control treatments were quite similar, ranging from 4.9 to 20.1°C for treatment A (on sand) and from 5.4 to 20.1°C for treatment C (in seawater). The outdoor samples on beach sand (Treatment B) experienced greater temperature fluctuations, with minimum temperatures from 1.0 to 33.1°C, while the smallest range was observed for the outdoor aquarium tank (treatment D), from 2.6 to 17.4°C. Correspondingly, maximum temperatures of 19.6 to 33.0°C and 17.1 to 28.6°C were measured for treatments A and C (indoors), while the outdoors maximum temperatures for treatment B (33.1–65.3°C) were nearly double those of treatment D (16.1–39.5°C).
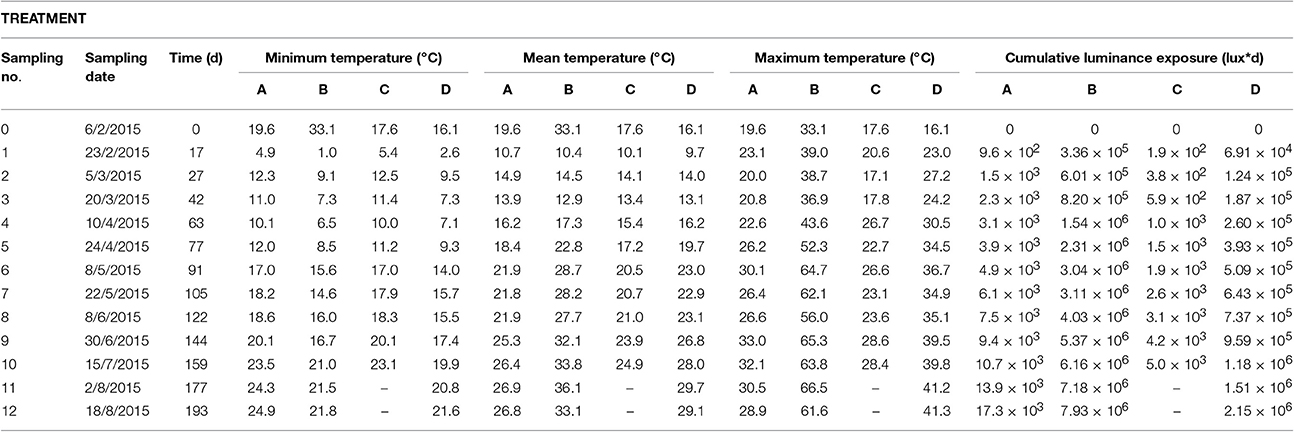
Table 1. Mean, minimum, and maximum temperature during exposure time and cumulative luminance exposure values for all treatments as a function of time.
As expected, both control treatments A and C were exposed to significantly less irradiation (0.15–0.45%) compared to the outdoors treatments B and D, respectively (Table 1). Furthermore, among the outdoor treatments, on 30/6/2015 treatment B (samples placed on beach sand) had a cumulative luminance of 5.37 × 106 lux•d whereas treatment D (samples placed in seawater) had a cumulative luminance of 9.59 × 105 lux•d which is about 18% of the value measured in treatment B. By 2/8/2015 the corresponding values were 7.18 × 106 and 1.51 × 106, respectively as seen in Table 1.
Tensile Strength Tests
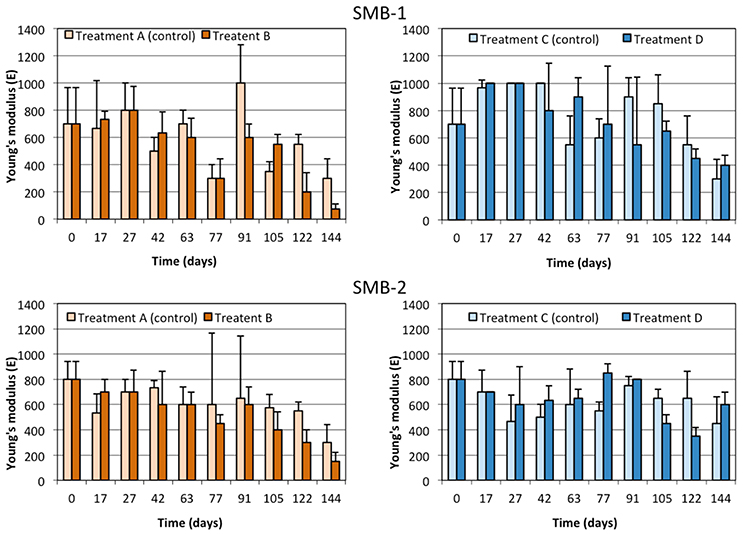
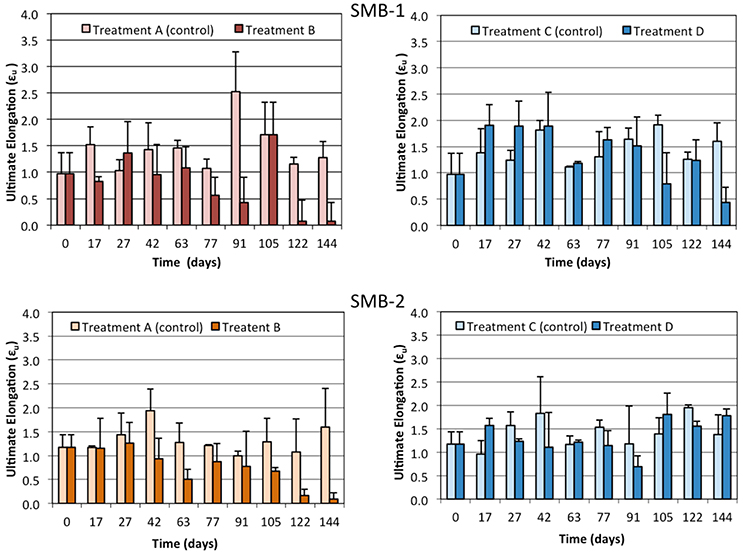
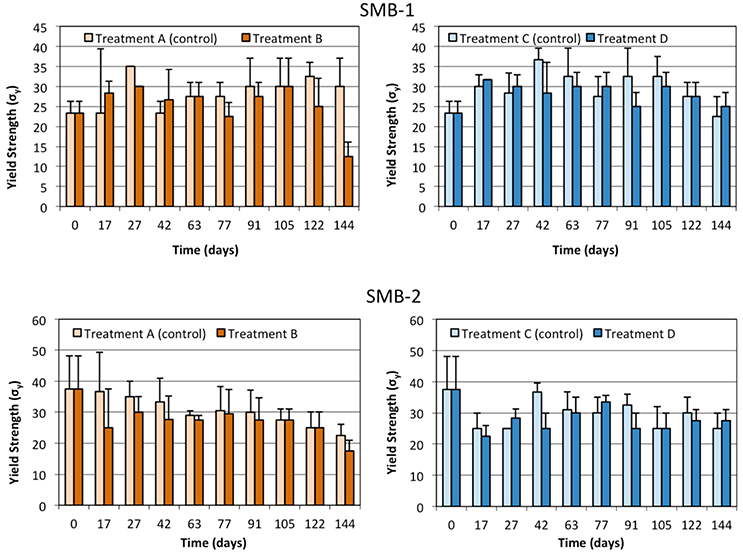
Tensile strength tests were performed, in order to determine whether the weathering of the samples could be linked to changes in their mechanical properties. Results obtained for SMB-1 and SMB-2 were similar as shown in Figures 4–6. More specifically, Young's modulus (E) changes over time (Figure 4) do not show a statistically significant difference between treatments A and B or C and D at all sampling times; namely, the weathering of the plastic film indoors and outdoors did not affect significantly Young's modulus in both media (on sand and in seawater). Similar results can be observed for the yield strength in Figure 5 and the ultimate elongation in Figure 6. Yet, one can make an interesting observation for treatments A and B (on beach sand). The values of ultimate elongation for treatment B are not statistically different; however, they are consistently lower than the control (treatment A), namely 8 out of 10 measurements for SMB-1 and 10 out of 10 for SMB-2. Using arguments from statistical process control, it is noticed that even though there is no significant difference between the data at each sampling time, the overall behavior is statistically significant. For example, under the null hypothesis for SMB-2 that there is no difference in the values of ultimate elongation between the two treatments, the probability of observing 10 times treatment A 10 to be higher than treatment B is (1/2)10 ≈ 0.00098 which is highly improbable (i.e., in an industrial production process an alarm would have been set much earlier). Similar arguments apply for Young's modulus measurements as well (Figure 4). This means that there is a definite decrease in values of ultimate elongation and in Young's modulus due to weathering, however, it is very difficult to reliably observe it with very few measurements; only if many measurements are considered together, one may observe a statistically significant reduction in these two parameters. The measurements in seawater (treatments C and D) do not show any trend. This is due to the lower degree of weathering of the plastic samples that took place in seawater where a significant amount of UV light is absorbed by the seawater. Finally, it should be noted that samples could not be analyzed in the last month of the experiment for treatment B due to breakage during sample handling as the strips became very brittle.
Molecular Weight Changes
Molecular weight is a weathering indicator of great significance. Only samples from the most heavily weathered treatment (B) were analyzed and the results are shown in Figure 7. As seen, extensive UV exposure leads to reduction in molecular weight over time for both SMB-1 and SMB-2, due to breakage of the polymer chains. In the last month the MW of SMB-1 was reduced to 60% of its initial value whereas SMB-2 could not be measured as it fragmented into pieces and several were lost by the wind action and it was not feasible to collect the 7 g of material required for the test.
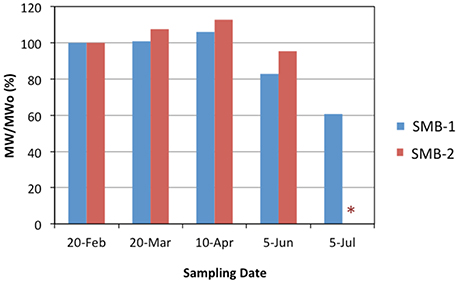
Figure 7. Molecular weight changes (%) of SMB-1 and SMB-2 over time for Treatment B (*measurement could not be done due to excessive fragmentation of sample SMB-2).
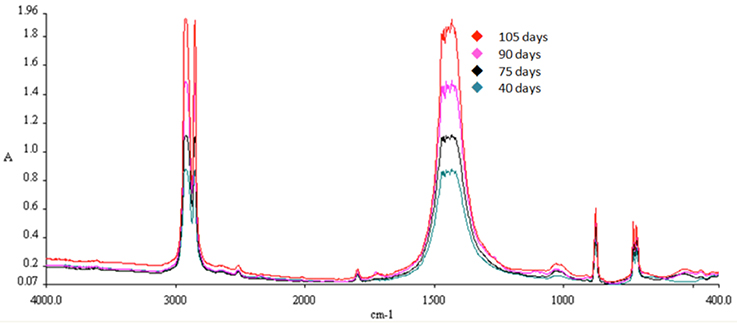
FTIR Spectra
The characteristic peaks for PE detection with FTIR are 2,923, 2,849, 1,471, and 719 cm−1. Increase in absorption at 1,471 cm−1 over time indicates increased oxidation of the polymer chains. A gradual increase of absorption at 1,471 cm−1 was observed in all samples exposed to solar radiation (Treatment B), implying gradual oxidation of the polymer with time. The spectra for SMB-1 can be observed in Figures 8–10. The characteristic peak for PE at 1,471 cm−1 was found to be higher with increasing weathering in all treatments and samples. A difference can be observed even after a short period time of 30 days (Figures 8, 9). Combination with absorption data from samples left in seawater both indoors and outdoors for the same period of time (Figure 10), reveals that the oxidation process is much more pronounced when the samples have been left outside (Treatment B) rather than inside (Treatment A), and the water absorbs a significant amount of UV radiation, thus limiting significantly the weathering process. In addition, the reduced availability of oxygen as dissolved oxygen in seawater compared to atmospheric oxygen also limits the oxidation to process via UV irradiation (i.e., peaks for Treatments A and B are higher than those for Treatments C and D).
Determination of the Onset of Fragmentation
The quantification of fragmentation cannot be effectively represented by a usual kinetic rate equation for fragmentation rates. It is obvious by the aforementioned tensile strength tests, molecular weight measurements, and FTIR analysis that although the weathering process proceeds, the fragmentation rates cannot be quantified as no fragmentation occurs. Only when the degree of weathering is above a certain threshold that can be quantified by the cumulative exposure to UV light, the polymer starts to break down to microplastics. Upon further exposure to UV light, the polymer films become very brittle and break down to “invisible” microplastics.
For modeling purposes, it is vital to be able to identify the onset of fragmentation, which represents the degree of weathering after which fragmentation to microplastics occurs. When the onset of fragmentation has been reached, only a minimal amount of mechanical stress is required for fragmentation of a portion of the polymer film. The procedure to apply mild mechanical stress was specifically designed for the determination of the actual moment when the fragmentation process commences.
Following the application of mild mechanical stress to weathered strips (treatment B) in bottles containing 125 mL of sand, the collected plastic fragments are shown in Figure 11A, and after photographing (Figure 11B) and applying the MATLAB-based algorithm, the percentile decrease in area of each weathered strip was computed. The corresponding weight loss for SMB-1 and SMB-2 following the application of mild mechanical stress, are presented in Table 2. As seen, after 5 months of weathering on the sand beds outside (Treatment B), and after application of mild mechanical stress, SMB-1 and SMB-2 yielded a weigh loss of 13.9 and 13.7%, respectively. After 5.5 months, SMB-2 presented a 16.7% weight loss. The evolution of weathering for treatment B is shown in Figure 12. Samples from the seawater tanks (Treatment D) were also subjected to the application of mild mechanical stress, however, no weight loss was observed due to the insufficient weathering achieved.
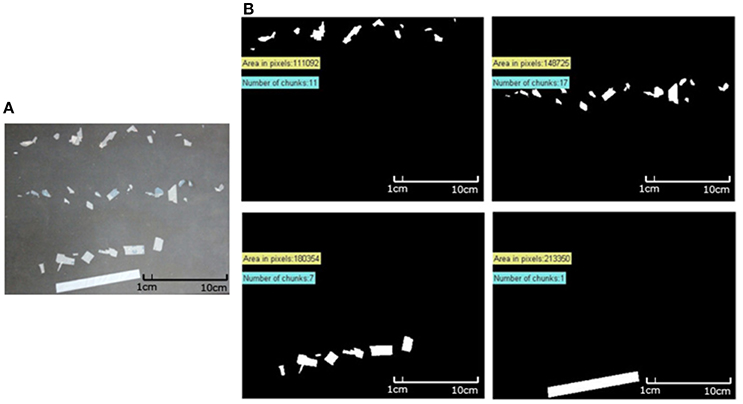
Figure 11. (A) Collected fragments of three different strips from SMB-2 placed in parallel lanes on black paper. (B) Processed images of SMB-2 strips following fragmentation with mild mechanical stress; also shown the number of fragments with their total area in pixels and the area decrease (%) from initial sample.
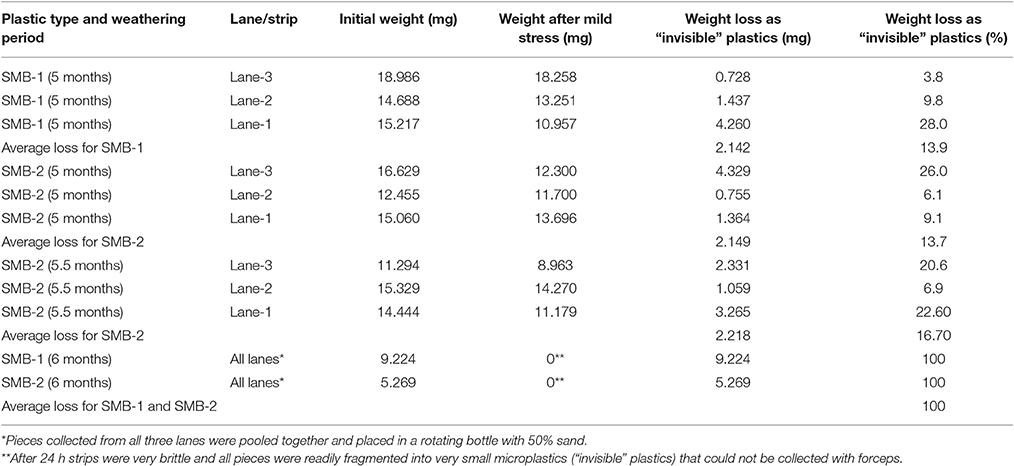
Table 2. Average weight loss after application of mild mechanical stress due to fragmentation to “invisible” microplastics (Treatment B).
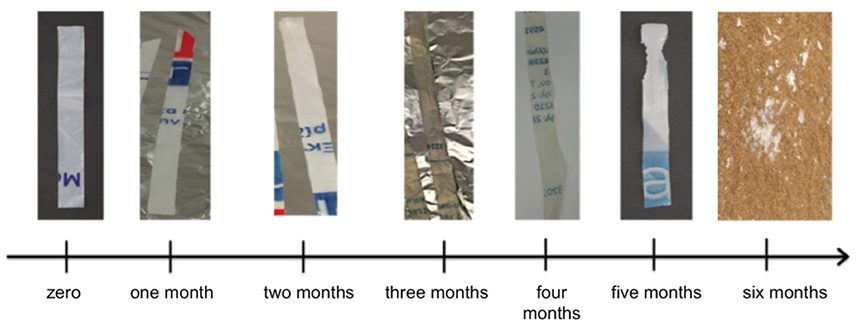
Figure 12. Pictures of SMB-1 plastic strips weathered outdoors on sand bed (treatment B) with respect to time (last picture taken on sand).
Repetition of the mild mechanical stress procedure to supermarket carrier bag samples for residence times ranging from 4 to 24 h, illustrated that sufficient fragmentation of weathered samples was achieved after 12 h of rotation and there was no need to keep the procedure for 24 h.
Complete Fragmentation to Microplastics
For HDPE films of thickness 0.1 mm from supermarket bags without any additives to protect against UV exposure, the onset of fragmentation coincided with a cumulative luminance of 5.3 × 106 lux·d in the presence of atmospheric oxygen whereby the polymer films broke down partially to microplastics. As more UV irradiation is absorbed, the plastic pieces that broke off become smaller in size with the exertion of the slightest mechanical stress. When the UV exposure reached 7.2 × 106 lux·d the weathered plastic strips broke down fully to “invisible” microplastics with the application of a mild mechanical stress. The arrows in Figure 13 indicate the two boundaries of the cumulative UV irradiation. Obviously, these boundaries are indicative, apply only to the specific composition and thickness of the plastic bags and for exposure to similar ambient temperatures over the weathering period.
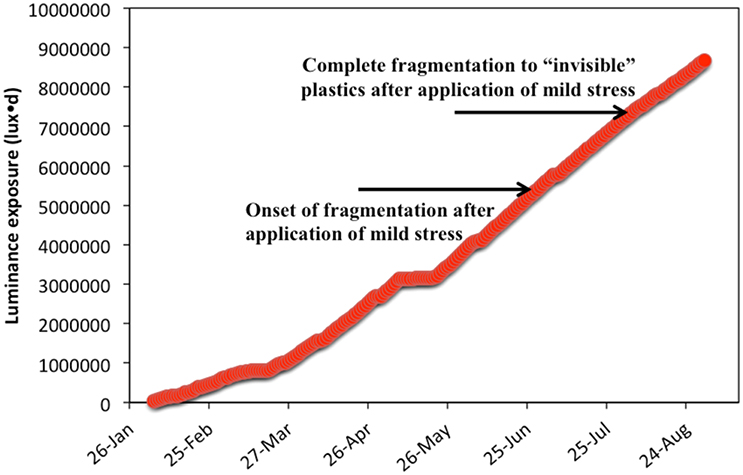
Figure 13. Cumulative luminance exposure and onset of fragmentation for thin HDPE films of thickness 0.1 mm used in supermarket bags (Treatment B—outdoors on sand).
Finally it should be noted that the weathering was accompanied by discoloration of the plastic strips, with the samples of Treatment B ending up almost white at the end of the 6-month experiment, while samples from Treatments A, C, and D were affected to a much less degree.
Discussion
Exposure of polyethylene films to solar radiation induces weathering, altering the molecular weight and physicochemical properties of the polymer, through reactions with oxygen, cross-linking, and chain scission reactions (Abdelhafidi et al., 2015). Several studies on polymer weathering have used mechanical properties tests to measure the degree of weathering achieved. Recently, studies have focused on the potential for effective biodegradation of PE (Restrepo-Flórez et al., 2014; Kumar Sen and Raut, 2015).
Generally, tensile strength has been found to be inadequate in the determination of fragmentation (Andrady et al., 1993). Young's modulus (E) was used successfully by Carrasco et al. (2001); however, in our case it did not yield statistically significant changes when single measurements were considered. Furthermore, the ultimate elongation (εu) has been used many times as a measure of brittleness. Carrasco et al. (2001) reported a decrease of ultimate elongation from 231 to 7.4% in 120 days. Similarly, from our results the ultimate elongation appears to decrease in a statistically significant manner only when all measurements were considered together or at the very end of the weathering period when fragmentation has already begun. Essentially, at this degree of weathering no measurement is possible due to sample breakage (Andrady et al., 1993; Jabarin and Lofgren, 1994; Tidjani, 2000).
FTIR has been frequently used to measure abiotic or biotic degradation of polymer pieces (Ioakeimidis et al., 2016) as well as to support mechanical properties test results and visual observations, for verifying changes in the chemical structure of the examined polymers. Higher peaks and slightly broader bands in the characteristic areas of the 2,923 and 1,471 cm−1 attest to the fact that the polymer has undergone oxidation and chemical alteration, dependent on the period of time the sample was exposed to UV radiation (Figures 8–10). However, the FTIR results can only be used qualitatively. Jabarin and Lofgren (1994), using FTIR to measure crystallinity were able to deduce that absorbance bands tended to be wider with higher peaks with exposure time, in a similar manner to Tidjani (2000). Increasing ratios of oxidized to non-oxidized carbon, associated with surface oxidation of polymers exposed to natural sunlight, can be related to polymer chain scission reactions, leading to polymer deterioration (Stark and Matuana, 2004). An increase in carbonyl index followed by a decrease was considered as an indication of biodegradation by Artham et al. (2009) which was verified by a weight loss of 1.6% for HDPE (1.9% for LDPE) after 12 months.
Changes in the molecular weight can also be linked to polymer deterioration. Jabarin and Lofgren (1994) reported that the molecular weight of HDPE decreased from 147,000 in the original sample to 34,100 after 6 months of exposure to artificial UV light. Both increase and decrease of molecular weight have been observed in PE biodegradation experiments. This is not surprising as abiotic fragmentation leads to a decrease in the average molecular weight whereas biodegradation results in an increase or marginal changes in the average molecular weight, because microbes prefer to attack first the shorter polymeric chains. This behavior was also observed in our lab during the biodegradation of PE films. Other measurement methods, such as rheology and size exclusion chromatography have been proposed as alternatives to molecular weight measurements (Restrepo-Flórez et al., 2014). Although, early molecular weight measurements in this study were rather inconclusive in denoting the gradual weathering of the plastic strips, the fact that the molecular weight of SMB-1 decreased significantly (by 60%) only after 5 months of weathering, it suggests such measurements are of limited value as they can verify significant deterioration only fragmentation can also be visually conformed.
Polyethylene films degradation is faster and more effective in the presence of atmospheric oxygen than in the presence of seawater. Despite the fact that sorbed water molecules can accelerate degradation rates by increasing the accessibility to oxygen in the matrix and enabling the leaching of stabilizers, the amount of light available for light-induced oxidation is significantly lower in the water. Additionally, the temperatures on land are higher, allowing for further deterioration of the polymer possibly due to thermo-oxidation. The effect of fouling could also be decisive for the rate of degradation, since it implies the build-up of dark material on the surface of the polymer, thus making less amounts of light available for photo-oxidation. Likewise, fouling could cause a weight increase of the material, decreasing buoyancy and leading it deeper in the water, where it is harder for light to reach, despite the fact that biodegradation could occur, further deteriorating it (Andrady, 1990; Ho et al., 1999). Andrady (1990) reports that statistically significant changes in ultimate elongation of samples weathered on land vs. samples weathered in seawater where after twice as long (12 months) no fragmentation was observed. Similarly, our work showed only marginal weathering of SMB-1 and SMB-2 in both seawater treatments (C and D). Apart from the higher UV light exposure and temperatures shown in Table 1, these results may also be influenced by the difference in availability of oxygen in the two matrices. While ambient air contains 21% oxygen, the dissolved oxygen in water is usually 8–10 mg/L in the top layers, thus making the oxidation process less effective. Hydrolysis could be playing a role in the degradation of plastics in the marine environment, but it is a very slow process whereby the plasticizers are leached out first.
Mechanical stress exerted on weathered stranded plastics in the marine environment can accelerate the fragmentation process and this is particularly true for lost or abandoned fishing gear. However, the application of mild mechanical stress that has been used in this work to detect the onset of fragmentation is totally different. If the plastic sample being tested in not sufficiently weathered, no changes can be observed with the application of the mechanical forces excreted by the rotating sand. Further experiments performed after the 6-month experiment reported here, 12 h was found as sufficient period of time to have the maximum possible fragmentation for the current degree of weathering.
Brandon et al. (2016) examined FTIR spectra of long-term weathered plastics and found that chemical bonds exhibited some non-linear changes with environmental exposure and hence, they can potentially approximate the weathering time of HDPE in particular. Combination of land conditions (temperature, UV, mechanical stress) can lead to relative rapid transformation of polyethylene films into microplastics, which are much harder to detect and collect from the seawater (da Costa et al., 2016). In general, secondary microplastics resulting from fragmentation, are harder, if not impossible, to collect (Andrady, 2011). They tend to accumulate over time and pose a threat to wildlife through ingestion (Gregory, 2009) or organic pollutant transfer (Teuten et al., 2009; Tanaka et al., 2013). Shopping bag samples exposed to sea turtle gastrointestinal fluids for 49 days showed no deterioration. Biodegradable bags exposed to the same conditions only lost 3–9% of their mass, as opposed to the 100% degradation suggested by the manufacturers (Müller et al., 2012).
Our results suggest that weathering of plastics in the marine environment is significantly higher onshore compared to plastics floating in seawater. Hence, it suggests that in the Mediterranean Sea where there is a plethora of small islands with many kilometers of beach zone, secondary microplastics are generated primarily after they are washed for a period of time on the shore. Particularly in the summer months, the degree of weathering plastics undergo in a couple of months under high temperatures could be sufficient to bring the plastic to the onset of fragmentation. Any further exposure will result in appreciable amount of microplastics being generated. Research should be encouraged on every aspect of plastic pollution, while legislation should rather sooner than later be implemented for its prevention and mitigation.
Finally, it is crucial to emphasize that this experiment is a springboard for further research, due to its simplicity in both setup and number of parameters investigated. The fragmentation of various polymer types should be examined, but most importantly in situ measurements with plastics floating in seawater should be performed. There are many challenges to be faced in the marine environment, but the prognosis is that floating plastic items become populated by microbes and suspended solids from the sea and as a result their density changes and start sinking well before they are weathered to the point of fragmentation. As a result, most plastic items, if they are not washed on a beach, they will most likely sink before they turn into microplastics due to weathering. A critical question that should also be answered is when do naturally weathered plastics like polyethylene films become biodegradable and in particular whether biodegradation rates are high enough prior to the onset of fragmentation. At the present time, we are systematically examining this question in our labs, yet we have recently obtained very encouraging results on the biodegradation of naturally weathered HDPE and LDPE plastics as they are readily degraded by consortia of special cultures with the indigenous bacteria or even with indigenous consortia only, well before the fragmentation to microplastics commences.
Concluding Remarks
In order to address the problem of quantification of macroplastics fragmentation to microplastics in the marine environment, a simple and easily reproducible test procedure was developed for polymer films based on weathering with sunlight and application of mild mechanical stress to pinpoint the onset of fragmentation. For polymer films, like supermarket plastic bags, it is the cumulative luminance exposure that appears to be the main indicator of the degree of weathering and it can be linked to the onset of fragmentation. A simple procedure to apply reliably and repeatedly mild mechanical stress to weathered strips has been proposed.
It is very important to have estimates of fragmentation rates of the plastics present in the marine environment if we wish to develop reliable models forecasting the environmental status of our oceans and seas.
Comparing the results from sunlight exposure on beach sand or in seawater near the surface, it is seen that fragmentation onshore is much faster. Hence, over a short period, beached plastic debris can be turned into microplastics and with the first event of high waves or strong winds, the generated microplastics are returned to the seawater where they are much more difficult to collect. Therefore, local authorities, NGOs and interested parties in general, should make every effort to regularly collect plastic debris from the beaches (especially at known debris accumulation areas) before they turn into microplastics and are returned to the sea.
Author Contributions
KK helped in the analysis of the data and wrote the first draft of the manuscript, GK designed and conducted the experiments and the instrumental analysis and helped in the analysis of the data; ET conducted the experiments and the instrumental analysis and helped in the preparation of the manuscript; AG supervised the experimental measurements for the characterization of the polymer films; PP developed the image processing code in MATLAB and supervised the image analysis; FF critically reviewed the design of the whole project; NK supervised the whole project, helped in the data analysis and contributed in the write up of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding by the European Union FP-7 project BIOCLEAN (grant agreement No. 312100) is highly appreciated.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00084/full#supplementary-material
References
Abdelhafidi, A., Babaghayou, I. M., Chabira, S. F., and Sebaa, M. (2015). Impact of solar radiation effects on the physicochemical properties of polyethylene (PE) plastic film. Procedia-Social Behav. Sci. 195, 2922–2929. doi: 10.1016/j.sbspro.2015.09.002
Accinelli, C., Saccà, M. L., Mencarelli, M., and Vicari, A. (2012). Deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere 89, 136–143. doi: 10.1016/j.chemosphere.2012.05.028
Andrady, A. L. (1990). “Environmental degradation of plastics under land and marine exposure conditions,” in Second International Conference on Marine Debris (April 1989) (Honolulu, HI), 848–869.
Andrady, A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605. doi: 10.1016/j.marpolbul.2011.05.030
Andrady, A. L., Pegram, J. E., and Tropsha, Y. (1993). Changes in carbonyl index and average molecular weight on embrittlement of enhanced-photodegradable polyethylenes. J. Environ. Polym. Degrad. 1, 171–179. doi: 10.1007/BF01458025
Artham, T., Sudhakar, M., Venkatesan, R., Madhavan Nair, C., Murty, K. V. G. K., and Doble, M. (2009). Biofouling and stability of synthetic polymers in sea water. Int. Biodeterior. Biodegradation 63, 884–890. doi: 10.1016/j.ibiod.2009.03.003
Arthur, C., Baker, J., and Bamford, H. (2009). Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. Silver Spring, MD.
Barnes, D. K. A., Galgani, F., Thompson, R. C., and Barlaz, M. (2009). Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. 364, 1985–1998. doi: 10.1098/rstb.2008.0205
Brandon, J., Goldstein, M., and Ohman, M. D. (2016). Long-term aging and degradation of microplastic particles: comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 110, 299–308. doi: 10.1016/j.marpolbul.2016.06.048
Brower, R. (2016). The social costs of beach litter along european coasts. Ocean Coast. Manage. 138, 38–49. doi: 10.1016/j.ocecoaman.2017.01.011
Carrasco, F., Pagès, P., Pascual, S., and Colom, X. (2001). Artificial aging of high-density polyethylene by ultraviolet irradiation. Eur. Polym. J. 37, 1457–1464. doi: 10.1016/S0014-3057(00)00251-2
Cheshire, A., Adler, E., Barbière, J., and Cohen, Y. (2009). UNEP/IOC Guidelines on Survey and Monitoring of Marine Litter. UNEP Regional Seas Reports and Studies, No. 186; IOC Technical Series. Available online at: http://www.unep.org/regionalseas/marinelitter/publications/docs/Marine_Litter_Survey_and_Monitoring_Guidelines.pdf
Cole, M., Lindeque, P., Halsband, C., and Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597. doi: 10.1016/j.marpolbul.2011.09.025
da Costa, J. P., Santos, P. S., Duarte, A. C., and Rocha-Santos, T. (2016). (Nano)plastics in the environment - Sources, fates and effects. Sci. Tot. Environ. 566–567, 15–26. doi: 10.1016/j.scitotenv.2016.05.041
Depledge, M. H., Galgani, F., Panti, C., Caliani, I., Casini, S., and Fossi, M. C. (2013). Plastic litter in the sea. Mar. Environ. Res. 92, 279–281. doi: 10.1016/j.marenvres.2013.10.002
Directive of the European Parliament of the Council (2013). Directive of the European Parliament of the Council Amending Directive 94/62/EC on Packaging and Packaging Waste to Reduce the Consumption of Lightweight Plastic Carrier Bag. Available online at: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:52013SC0444
Gregory, M. R. (2009). Environmental implications of plastic debris in marine settings–entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2013–2025. doi: 10.1098/rstb.2008.0265
Hidalgo-Ruz, V., and Thiel, M. (2013). Distribution and abundance of small plastic debris on beaches in the SE Pacific (Chile): a study supported by a citizen science project. Mar. Environ. Res. 87–88, 12–18. doi: 10.1016/j.marenvres.2013.02.015
Ho, K.-L. G., Pometto, A. L. III, and Hinz, P. N. (1999). Effects of temperature and relative humidity on polylactic acid plastic degradation. J. Polym. Environ. 7, 83–92. doi: 10.1023/A:1021808317416
Ioakeimidis, C., Fotopoulou, K. N., Karapanagioti, H. K., Geraga, M., Zeri, C., Papathanassiou, E., et al. (2016). The degradation potential of PET bottles in the marine environment: an ATR-FTIR based approach. Sci. Rep. 6:23501. doi: 10.1038/srep23501
Jabarin, S. A., and Lofgren, E. A. (1994). Photooxidative effects on properties and structure of high-density polyethylene. J. Appl. Polym. Sci. 53, 411–423. doi: 10.1002/app.1994.070530404
Kumar Sen, S., and Raut, S. (2015). Microbial degradation of low density polyethylene (LDPE): a review. J. Environ. Chem. Eng. 3, 462–473. doi: 10.1016/j.jece.2015.01.003
Martinho, G., Balaia, N., and Pires, A. (2017). The Portuguese plastic carrier bag tax: the effects on consumers' behavior. Waste Manage. 61, 3–12. doi: 10.1016/j.wasman.2017.01.023
Müller, C., Townsend, K., and Matschullat, J. (2012). Experimental degradation of polymer shopping bags (standard and degradable plastic, and biodegradable) in the gastrointestinal fluids of sea turtles. Sci. Tot. Environ. 416, 464–467. doi: 10.1016/j.scitotenv.2011.10.069
O'Brine, T., and Thompson, R. C. (2010). Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull. 60, 2279–2283. doi: 10.1016/j.marpolbul.2010.08.005
Pham, C. K., Ramirez-Llodra, E., Alt, C. H., Amaro, T., Bergmann, M., Canals, M., et al. (2014). Marine litter distribution and density in European seas, from the shelves to deep basins. PLoS ONE 9:e95839. doi: 10.1371/journal.pone.0095839
PlasticsEurope (2015). Plastics - the Facts 2014/2015: An Analysis of European Plastics Production, Demand and Waste Data. Brussels: PlasticsEurope.
Restrepo-Flórez, J. M., Bassi, A., and Thompson, M. R. (2014). Microbial degradation and deterioration of polyethylene - a review. Int. Biodeter. Biodegradation 88, 83–90. doi: 10.1016/j.ibiod.2013.12.014
Ryan, P. G., Moore, C. J., van Franeker, J. A., and Moloney, C. L. (2009). Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1999–2012. doi: 10.1098/rstb.2008.0207
Shah, A. A., Hasan, F., Hameed, A., and Ahmed, S. (2008). Biological degradation of plastics: a comprehensive review. Biotechnol. Adv. 26, 246–265. doi: 10.1016/j.biotechadv.2007.12.005
Stark, N. M., and Matuana, L. M. (2004). Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy. Polym. Degrad. Stabil. 86, 1–9. doi: 10.1016/j.polymdegradstab.2003.11.002
Tanaka, K., Takada, H., Yamashita, R., Mizukawa, K., Fukuwaka, M. A., and Watanuki, Y. (2013). Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 69, 219–222. doi: 10.1016/j.marpolbul.2012.12.010
Teuten, E. L., Saquing, J. M., Knappe, D. R., Barlaz, M. A., Jonsson, S., Björn, A., et al. (2009). Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2027–2045. doi: 10.1098/rstb.2008.0284
Tidjani, A. (2000). Comparison of formation of oxidation products during photo-oxidation of linear low density polyethylene under different natural and accelerated weathering conditions. Polym. Degrad. Stabil. 68, 465–469. doi: 10.1016/S0141-3910(00)00039-2
United Nations Environment Programme (UNEP) (2014). Plastic Debris in the Ocean. UNEP Year Book 2014 Emerging Issues Update (Nairobi), 48–53.
United Nations Environment Programme (UNEP) (2015). Plastic in Cosmetics [Fact Sheet]. Nairobi. 1.24, 1–3.
Keywords: microplastics, plastics, fragmentation, polyethylene, HDPE, weathering
Citation: Kalogerakis N, Karkanorachaki K, Kalogerakis GC, Triantafyllidi EI, Gotsis AD, Partsinevelos P and Fava F (2017) Microplastics Generation: Onset of Fragmentation of Polyethylene Films in Marine Environment Mesocosms. Front. Mar. Sci. 4:84. doi: 10.3389/fmars.2017.00084
Received: 30 December 2016; Accepted: 13 March 2017;
Published: 28 March 2017.
Edited by:
Francois Galgani, French Research Institute for Exploitation of the Sea, FranceReviewed by:
Fabienne Lagarde, University of Maine, FranceChristos Ioakeimidis, United Nations Environment Programme Mediterranean Action Plan (UNEP/MAP), Greece
Copyright © 2017 Kalogerakis, Karkanorachaki, Kalogerakis, Triantafyllidi, Gotsis, Partsinevelos and Fava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolas Kalogerakis, bmljb2xhcy5rYWxvZ2VyYWtpc0BlbnZlbmcudHVjLmdy
 Nicolas Kalogerakis
Nicolas Kalogerakis Katerina Karkanorachaki
Katerina Karkanorachaki G. Calypso Kalogerakis
G. Calypso Kalogerakis Elisavet I. Triantafyllidi1
Elisavet I. Triantafyllidi1 Alexandros D. Gotsis
Alexandros D. Gotsis Panagiotis Partsinevelos
Panagiotis Partsinevelos