
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 27 September 2016
Sec. Coastal Ocean Processes
Volume 3 - 2016 | https://doi.org/10.3389/fmars.2016.00186
 Grant C. Pitcher1,2*
Grant C. Pitcher1,2* Trevor A. Probyn1
Trevor A. Probyn1The dynamics of O2 depletion in exceptional dinoflagellate blooms, often referred to as red tides or harmful algal blooms (HABs), was investigated in St. Helena Bay in the southern Benguela upwelling system in 2013. The transition to bloom decay and anoxia was examined through determination of O2-based productivity and respiration rates. Changes in O2 concentrations in relation to bloom metabolism were tracked by fast response optical sensors following incubation of red tide waters in large volume light-and-dark polycarbonate carboys. Concurrent measurements of nutrients and nutrient uptake rates served to assess the role of nutrient stressors in community metabolism and bloom mortality. The estimates of community productivity and respiration are among the highest values recorded. Nutrient concentrations were found to be low and were unlikely to meet the demands of the bloom as dictated by the rates of nutrient uptake. Ratios of community respiration to gross production were particularly high ranging from 0.6 to 0.73 and are considered to be a function of the inherently high cellular respiration rates of dinoflagellates. Nighttime community respiration was shown to be capable of removing as much as 17.34 ml O2 l−1 from surface waters. These exceptional rates of O2 utilization are likely in some cases to exceed the rate of O2 replenishment via air-sea exchange thereby leading overnight to conditions of anoxia. These conditions of nighttime anoxia and nutrient starvation are likely triggers of cell death and bloom mortality further fueling the microbial foodweb and consumption of O2.
In coastal oceans zones of oxygen (O2) depletion, often referred to as dead zones, are spreading with serious consequences for ecosystem functioning (Diaz and Rosenberg, 2008; Gilbert et al., 2010; Rabalais et al., 2014). The term dead zone most often applies to near-bottom coastal waters where stratification is a key requirement in the formation of hypoxia or anoxia in that it serves to separate O2-rich upper layers from lower less-oxygenated layers. The elevated surface O2 concentrations are attributed to phytoplankton production, while the consequent sinking of labile organic matter to bottom waters fuels microbial respiration and the consumption of O2. The trend of spreading dead zones has therefore been linked in many cases to coastal eutrophication and enhanced primary production, which adds to the flow of organic matter to the seabed (Kemp et al., 2009; Rabalais et al., 2014). Increasing temperatures as dictated by climate change are also likely to promote the extension of dead zones as the capacity of coastal waters to hold O2 will diminish through reduced solubility, while enhanced stratification of the water column will reduce the transport of O2 to bottom waters (Keeling et al., 2010; Gruber, 2011).
Less well-studied events of O2 depletion in the coastal zone include incidents of complete stripping of O2 from the water column of nearshore environments following the decay of blooms of exceptional biomass, in some cases referred to as red tides or harmful algal blooms (HABs; e.g., Vicente et al., 2001; Pitcher and Probyn, 2011). Bloom mortality follows one or another death pathway thought to be triggered in many cases by nutrient stressors (Bidle, 2014). The consequent production of large amounts of phytodetritus is considered to fuel the microbial foodweb leading to high O2 consumption within the confined environment of the nearshore. The resulting episodic hypoxia or anoxia is often associated with major mortalities of marine life (e.g., Cockcroft et al., 2000). Numerous examples world-wide of increases in red tides or HABs attributed to increased nutrient loading of the coastal environment indicate a likely global increase in these events of episodic O2 depletion (Anderson et al., 2002; Heisler et al., 2008). The dynamics of O2 depletion within these high biomass nearshore blooms nevertheless remains poorly established. This limited understanding is a likely consequence of the difficulties in studying these events due to their often local and transient character and owing to the influence of air-sea exchange in determining O2 concentrations in shallow water environments. Specifically the causes and timing of bloom mortality that may lead to a very rapid shift from net autotrophy to net heterotrophy are unknown.
In St. Helena Bay in the Benguela eastern boundary upwelling system the regular appearance of red tides provides an opportunity for investigation of the processes that serve to mediate bloom-to-post-bloom transitions important in determining the biogeochemical fate of the bloom and the consequent onset of anoxia. Here dinoflagellate blooms develop typically during late summer and early autumn and red tides are formed following inshore accumulation of blooms under conditions of downwelling (Pitcher and Probyn, 2011; Pitcher et al., 2014). Under conditions of persistent downwelling bloom decay and the resultant depletion of O2 is the regular cause of large mortalities of marine life with major impacts on economically important marine resources, specifically the west coast rock lobster Jasus lalandii (Matthews and Pitcher, 1996; Cockcroft et al., 2000, 2008).
In the autumn of 2013 we investigated the transition to bloom decay and anoxia in red tide waters collected from St. Helena Bay through determination of O2-based productivity and respiration rates. Changes in O2 concentrations in relation to bloom metabolism were tracked by fast response optical sensors following incubation of red tide waters in large volume light-and-dark polycarbonate carboys. These methods served our aim of assessing the role of nighttime respiration in driving the transition from oxygen saturated to anoxic conditions. Concurrent measurements of nutrients and nutrient uptake rates served to assess the role of nutrient stressors in community metabolism and bloom mortality.
In March 2013 surface samples of red tide were collected for incubation from four localities in St. Helena Bay [15th March (32 18.312 S; 18 19.307 E), 17th March (32 20.516 S; 18 18.327 E), 18th March (32 23.895 S; 18 19.569 E), and 19th March (32 22.732 S; 18 19.197 E); Figure 1]. Water temperatures were determined at each locality using a SBE-19 Seacat CTD. Water samples of 40 l were collected by NIO bottles and transported to the laboratory in darkened containers. Subsamples for Chlorophyll a (Chl-a) determination, corrected for phaeopigments, were analyzed fluorometrically following extraction in 90% acetone (Parsons et al., 1984). For the analysis of phytoplankton assemblages subsamples were fixed in buffered formalin and enumerated by the Utermöhl method (Hasle, 1978). Subsamples were also filtered through Whatman GF/F filters for manual analysis of nutrients. Ammonium () was analyzed according to the methods described in Koroleff (1983), nitrate (), and nitrite () followed the procedure of Nydahl (1976) and phosphate () and silicate () that of Grasshoff et al. (1983).
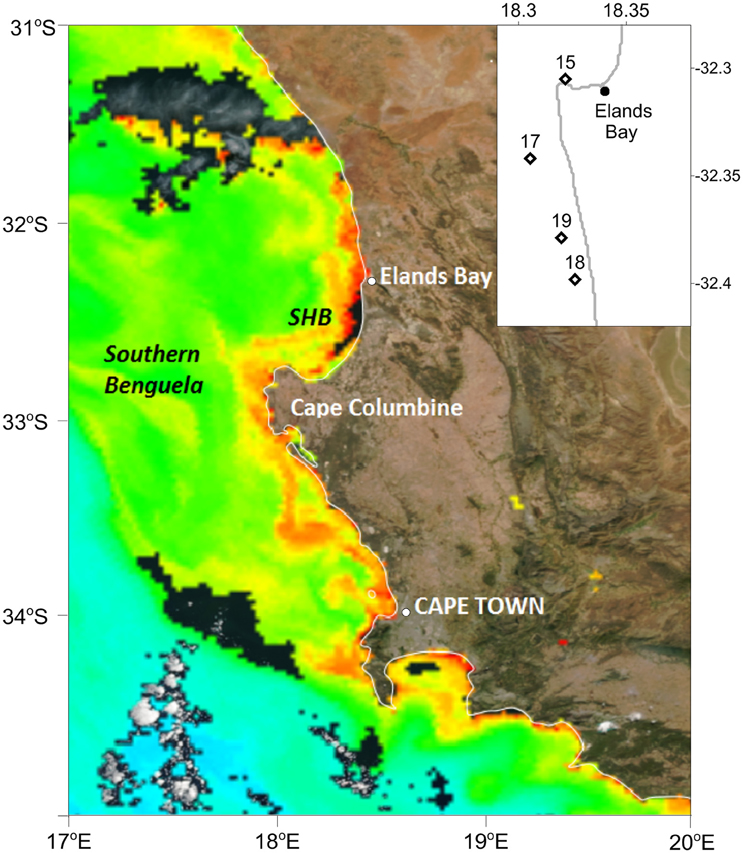
Figure 1. Station positions in St. Helena Bay (SHB) sampled on the 15, 17, 18, and 19 March 2013 inserted on a Chl a ocean color image generated by the NOAA/NESDIS Center for Satellite Applications and Research. Data acquired from the Visible Infrared Imaging Radiometer Suite (VIIRS) on board the Suomi National Polar-orbiting Partnership satellite on the 19 March 2013.
Uptake rates for and were measured using 15N incorporation according to the protocol of Dugdale and Wilkerson (1986). Incubations were carried out in 250 ml tissue culture flasks spiked with either 15NH4Cl or Na15NO3. Final spike concentrations were 0.1–0.2 μmol l−1 for and 0.012–0.2 μmol l−1 for . Flasks were incubated submerged in water recirculated through chillers for temperature control. Experiments were terminated after 2.5–3.5 h by filtration onto 25 mm Whatman GF/F filters. Filters were thoroughly rinsed under vacuum with artificial seawater to flush dissolved isotopes and inorganic C from the filter matrix and were subsequently dried at 75°C for storage. Particulate 15N enrichment, C and N concentrations were measured on a Finnegan MAT mass spectrometer (Department of Archeometry, University of Cape Town). Uptake of was not corrected for regeneration and is thus underestimated. However, isotope dilution is considered to play a relatively minor role given the short incubation periods employed here. uptake was measured as a concentration decrease in ambient concentration over time.
For determination of O2-based productivity and respiration rates samples were incubated for approximately 24 h in large volume (10 l) light-and-dark polycarbonate carboys. The carboys were incubated submerged, in full sunlight, with cooling water recirculated through chiller units to maintain temperature at in situ levels. Prior to incubation initial measurements of O2 were determined in triplicate by Winkler analysis as described by Carpenter (1965). Changes in O2 concentrations in the carboys were logged every 10 min by means of fast response RINKO-1 optical sensors (Sasano et al., 2011; Figure 2). The increasing O2 concentrations in the light carboy during the day provided estimates of hourly net community production (hourly-NCP). Decreasing O2 concentrations in the light carboy at night provided hourly estimates of respiratory losses (hourly-Rn). These estimates were considerably higher than the respiratory losses observed in the dark carboy (hourly-Rd). Estimates of hourly-NCP expressed in terms of carbon were determined through application of a photosynthetic quotient of 1.2. Estimates of daily gross community production (daily-GCP) were calculated as the sum of the daily net community production (daily-NCP), as determined from the increment in O2 concentration over 24 h, and daily estimates of respiration (daily-R = hourly-Rn × 24; respiration during the day was assumed to be equal to respiration during the night [hourly-Rn]).
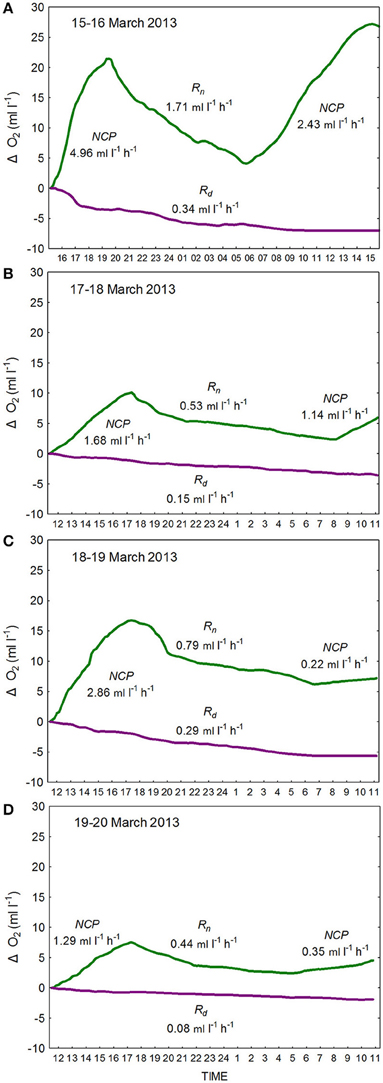
Figure 2. Changes in O2 concentrations as determined from continuous measures (10-min intervals) of oxygen in large-volume (10 l) light- (green line) and dark- (purple line) polycarbonate carboys as logged by RINKO-1 sensors from (A) 15–16 March 2013, (B) 17–18 March 2013 (C) 18–19 March 2013, and (D) 19–20 March 2013. Hourly rates of (1) net community production (hourly-NCP) were determined from increases in O2 in the light bottle during the day, of (2) community respiration (hourly-Rn) as determined from the decrease in O2 concentration in the light bottle at night, and of (3) net community respiration (hourly-Rd) as determined from the decrease in oxygen in the dark bottle.
To establish the likelihood of nighttime community respiration leading to either hypoxia or anoxia estimates of O2 replenishment via air-sea exchange were determined from the equation: F = k ([O2]w − [O2]s), where F is the flux of oxygen across the air-sea interface (mmol m−2 h−1), k is the transfer velocity (cm h−1) and [O2]w and [O2]s (mmol l−1) are the measured oxygen concentrations in the surface layer and its corresponding saturation concentration. The gas transfer velocity was calculated using the updated wind speed gas exchange relationship of Wanninkhof (2014): k = 0.251 <U2> (Sc/660)−0.5, where U the wind speed (m s−1) and Sc the Schmidt number calculated from coefficients given in Wanninkhof (2014).
Surface water temperature in St. Helena Bay on the 15, 17, 18, and 19 March 2013 ranged from 15.65° to 17.21°C (Table 1). A Chl-a ocean color image generated by the NOAA/NESDIS Center for Satellite Applications and Research provides an indication of the bloom distribution on the 19 March 2013 (Figure 1). The phytoplankton assemblages within these waters were dominated by dinoflagellates ranging in concentrations from 1.64 × 106 to 17.99 × 106 cells l−1 (Table 1). The dinoflagellates Ceratium furca and Prorocentrum micans were the most abundant species. Several other dinoflagellates were prominent including the toxic species Alexandrium catenella. Phytoplankton biomass as represented by Chl-a concentrations ranged from 100.6 to 1208.8 mg m−3 (Table 1). There was a strong correlation between dinoflagellate concentration and the biomass parameters of Chl-a, POC, and PON (Figure 3A).
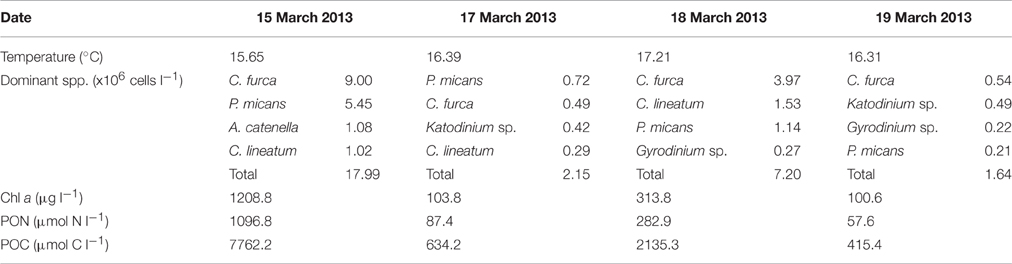
Table 1. Date of surface water collection, surface water temperature, the dominant dinoflagellate species, and cell concentrations, and estimates of Chl-a, PON, and POC concentrations.
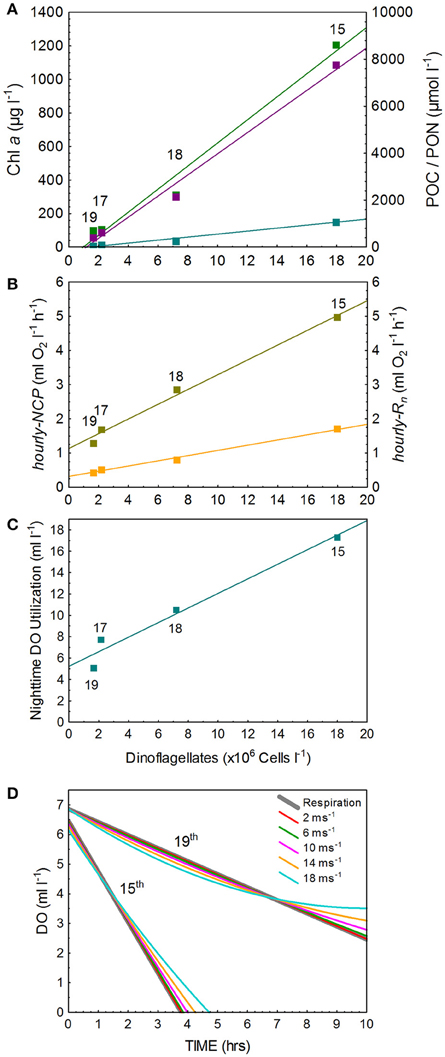
Figure 3. The relationships between dinoflagellate concentration (DC) and (A) the bloom biomass parameters of Chl a, POC, and PON (Chl a = 68.7*DC—66.3 [r2 = 0.98]; POC = 449.9*DC—522.4 [r2 = 0.99]; PON = 63.7*DC—80.6 [r2 = 0.98]), (B) hourly-NCP and hourly-Rn (hourly-NCP = 0.219*DC + 1.13 [r2 = 0.99]; hourly-Rn = 0.076*DC + 0.31 [r2 = 0.98]), and (C) nighttime utilization of O2 (O2 = 0.68*DC + 5.23 [r2 = 0.97]) and (D) nighttime depletion of O2 under the high and low respiration rates measure on the 15 and 19 March 2013 after accounting for air-sea exchange at varying wind speeds.
Dissolved nutrient concentrations and uptake rates for the four sample dates are given in Table 2. Nutrient concentrations were particularly low in waters of highest phytoplankton biomass, except for . The excess in all samples is consistent with a largely dinoflagellate-dominated phytoplankton community. uptake tended also to be lowest in waters of higher biomass, uptake varied independently of biomass and exhibited relatively similar rates of uptake on each sample date. Measured uptake rates in waters of highest biomass, i.e., on 15 and 18 March 2013, were sufficient to have removed all three nutrients in under 3 and 4 h, respectively. This conclusion ignores probable recycling of both and and diffusion of but does serve to illustrate the unlikelihood of observed nutrient concentrations meeting the nutrient requirements of the blooms of highest biomass. The fact that nutrient concentrations generally decreased over the time course of the uptake experiments further indicates that regeneration rates within the same water body could not keep pace with uptake demands. The f -ratio ranged broadly from 0.14 to 0.68 indicating a variable dependence of blooms on .
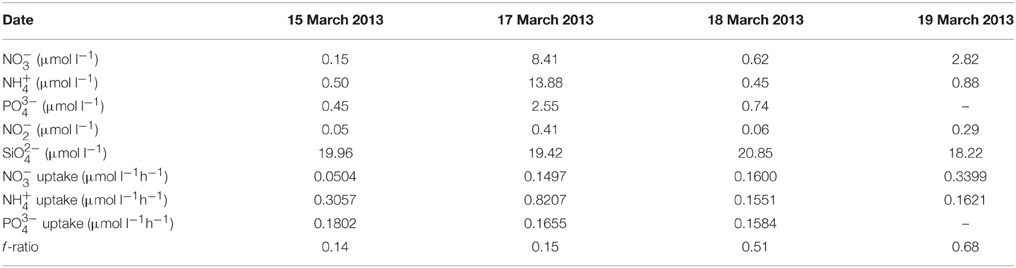
Table 2. Date of surface water collection, ,, , , and concentrations, the rates of uptake of , , and , and the f -ratio.
Observed changes in O2 concentrations in the light-and-dark carboys as measured by the RINKO-1 sensors are plotted for each of the red tide incubations (Figure 2). Productivity and respiration rates determined from these changes in O2 concentrations are provided in Table 3. O2 concentrations in surface waters at the start of the incubations were supersaturated ranging in concentration from 6.33 to 7.54 ml l−1. Hourly-NCP determined from initial increases in O2 concentration in the light carboy ranged from 1.29 to 4.96 ml O2 l−1 h−1 with values increasing in waters of higher biomass and increasing dinoflagellate concentrations (Figure 3B). Values of hourly-NCP expressed in terms of carbon ranged from 576 to 2215 mg C m−3 h−1. Assimilation indices normalized to Chl-a ranged from 1.83 to 7.23 mg C [mg Chl-a]−1 h−1 and were lowest in waters of highest biomass. Hourly-Rn as determined from declining DO concentrations in the light carboy at night ranged from 0.44 to 1.71 ml O2 l−1 h−1 and were considerably higher than the respiratory losses observed in the dark carboy (hourly-Rd) that ranged from 0.08 to 0.34 ml O2 l−1 h−1. As with estimates of production respiratory losses increased in waters of higher biomass and increasing dinoflagellate cell concentrations (Figure 3B). Cell-specific rates of hourly-Rn ranged from 4.22 to 12.20 pmol O2 cell−1 h−1 and Chl-a specific rates of hourly-Rn ranged from 0.06 to 0.23 μmol O2 [μg Chl-a]−1 h−1 and were lowest in waters of highest cell concentrations or biomass. The total nighttime utilization of DO attributed to community respiration ranged between 5.09 and 17.34 ml l−1 and was strongly correlated with dinoflagellate concentration (Figure 3C). Daily-NCP varied between 4.54 and 27.21 ml O2 l−1 d−1, daily-R between 10.56 and 41.04 ml O2 l−1 d−1 and daily-GCP between 15.10 and 68.25 ml O2 l−1 d−1 all of which increased with increasing biomass. The ratio of daily-R: daily-GCP varied between 0.60 and 0.73.
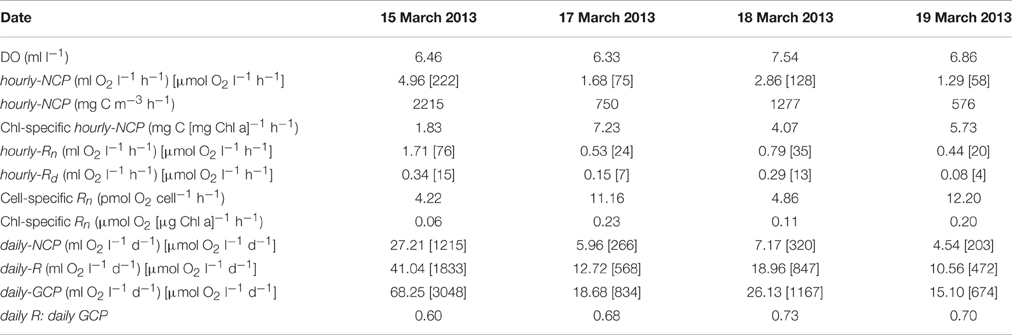
Table 3. Date of surface water collection, DO concentrations prior to incubation, hourly estimates of net community production (hourly-NCP), including estimates expressed in terms of carbon and normalized to Chl-a, hourly estimates of community respiration (hourly-Rn) as determined in the light carboy, hourly estimates of community respiration as determined in the dark carboy (hourly-Rd), cell, and Chl-a specific values of hourly-Rn, daily estimates of net community production (daily-NCP) as determined from the increment in DO concentration in the light bottle over 24 h, daily estimates of respiration (daily-R = hourly-Rn × 24; respiration during the day was assumed to be equal to respiration during the night), daily estimates of gross community production (daily-GCP = daily-NCP + daily-R), and the ratio of daily-R: daily-GCP.
The maximum hourly-Rn measured on 15 March 2013 translates to an O2 consumption rate of 381 mmol O2 m2 h−1 for a 5 m upper mixed water column. At the supersaturated O2 concentrations measured on 15 March 2013, calculated O2 transfer across the air-sea interface following the onset of darkness would initially constitute a loss to the atmosphere, but as respiration reduces ambient O2 levels so the flux of O2 into the water column will increase. However, the maximum flux of 5.46 mmol O2 m2 h−1, calculated at zero ambient concentrations and conditions of low wind speed (3 m s−1), remains considerably less than consumption by respiration suggesting rapid progression toward anoxia. These calculations ignore horizontal and vertical exchange of O2. In this highly simplified system nighttime respiration could reduce ambient O2 to zero in 3.5 h. Increased wind speed will delay the onset of anoxia but only marginally (Figure 3D). At the lower hourly-Rn measured on 19 March 2013, anoxia would not develop over the night period (10 h) and wind speed effects are more pronounced (Figure 3D).
The observed composition and cell concentrations of the red tides sampled during this study are typical of those observed in St. Helena Bay during the latter half of the upwelling season and also typify the blooms that lead to events of anoxia and consequent mortalities (Pitcher and Calder, 2000; Pitcher and Weeks, 2006). The high surface temperatures in St. Helena Bay at the time of sample collection (15.65° to 17.21°C) are indicative of the stratified conditions that develop at this time of the year when red tides are often formed by the shoreward advection and accumulation of dinoflagellate blooms under conditions of downwelling (Pitcher and Nelson, 2006). As expected under these stratified conditions nutrient concentrations were found to be low and nutrient uptake particularly during periods of very high biomass was shown to be capable of exhausting nutrients in a matter of hours. The relatively high f -ratios in some cases suggest that can be important under bloom conditions, although the bloom of highest biomass was largely dependent. Likewise Seeyave et al. (2009) have shown HABs in the southern Benguela to vary in their potential for reduced or oxidized N utilization, dependent on both species composition and state of upwelling. Somewhat contrary to expectations, most upwelling HAB taxa exhibit a comparable or greater capability to utilize over (Kudela et al., 2010). However, nutrient utilization by HAB species remains poorly studied and clear patterns of N utilization strategies are not apparent.
The estimates of productivity and respiration as determined in this study from changes in O2 in red tide waters are among the highest values recorded. The conventional estimation of daily-GCP from initial and end point measurements in light-and-dark BOD bottles as described by Gaarder and Gran (1927) assumes that respiration in the dark bottle is representative of that in the light bottle. The observations in this study of higher respiration at night in the light bottle (hourly-Rn) compared to that in the dark bottle (hourly-Rd) are supportive of the opinion that phytoplankton respiration is closely tied to photosynthesis and that respiratory losses are enhanced in the light bottle (e.g., Bender et al., 1987; Williams and del Giorgio, 2005). It has been shown that in the light bottle, respiration during the day is likely to be greater than that at night through stimulation of heterotrophic activity by autotrophic production (Pringault et al., 2007, 2009), while the photo-dependent degradation of organic matter during the day can also fuel enhanced bacterial respiration (Gustavson et al., 2000). Furthermore, measurement of respiration during the night will result in the exclusion of the Mehler reaction and the RUBISCO oxidase function resulting in a further underestimate of respiration (Williams and del Giorgio, 2005). In the present study evidence of higher respiration during the day is provided by observations of an initial exponential decline in O2 in the light bottle following the onset of darkness (Figure 2). This enhanced respiration near sundown is consistent with maximum utilization of recent photosynthate at the end of the day, and with declining substrate for respiration through the night (Markager and Sand-Jensen, 1989; Fukushima et al., 2000). Therefore, despite our use of hourly-Rn rather than hourly-Rd in the calculation of daily-R, our estimates of respiratory losses, and consequently of daily-GCP are likely to be underestimated.
While rates of production and respiration have been well-characterized in many coastal and oceanic communities this is not the case for the dinoflagellate-dominated blooms responsible for red tides. Of the few measurements most are based on the uptake of 14C which provides a metric close to net primary production attributed to autotrophic metabolism (Marra, 2009). Prior measurements of the productivity of red tides in St. Helena Bay include those of Walker and Pitcher (1991) and Mitchell-Innes et al. (2000) both of which assessed the productivity of blooms dominated by C. furca. Walker and Pitcher (1991) provided a productivity estimate of 517 mg C m−3 h−1 as measured by 14C uptake in waters with C. furca concentrations of 1.3 × 106 cells l−1. The Chl-a concentration of these waters was 139 mg m−3 and the corresponding assimilation index 3.7 mg C [mg Chl-a−1] h−1. Mitchell-Innes et al. (2000) estimated productivity by means of natural fluorescence measurements and reported values >500 mg C m−3 h−1 in waters with Chl-a concentrations >175 mg m−3. Other measurements of productivity of exceptional dinoflagellate blooms in the southern Benguela include those of Brown et al. (1979) for a bloom of an unknown species of Gymnodinium responsible for fish mortalities in False Bay. Their estimate of net production of 405 mg C m−3 h−1 represents the only prior measurement of the productivity of a local dinoflagellate bloom based on changes in O2. The above estimates of productivity and biomass are similar to some of the measurements made during this study, but are considerably lower than the maximum values recorded on the 15 March 2013 (Table 1). Red tide forming dinoflagellate blooms of similar magnitude have also been reported for other eastern boundary upwelling systems (e.g., Blasco, 1975) and reported assimilation indices of 4.3 mg C [mg Chl-a]−1 h−1 for a bloom of Gonyaulax polyedra in the Californian upwelling system and of 5.0 mg C [mg Chl-a]−1 h−1 for a bloom of Gymnodinium splendens in the Peruvian upwelling system (Blasco, 1975) are similar to the assimilation indices recorded in the present study, which ranged from 1.83 to 7.23 mg C [mg Chl-a]−1 h−1. These measures of red tide productivity in eastern boundary upwelling systems are all considerably higher than the estimates of net community production made for Karenia-dominated blooms in coastal waters of West Florida (USA; Hitchcock et al., 2010).
The ratio of community respiration to gross community production widely taken in the past to be of the order 0.1 is now known to significantly underestimate the actual respiratory loss to water column production as this ratio does not typically take into account light respiration, which can be considerably higher than dark respiration (Langdon, 1993). The particularly high ratios of daily-R: daily GCP measured in the dinoflagellate-dominated waters of St. Helena Bay, which ranged from 0.6 to 0.73 are considered to be a function of the inherently high cellular respiration rates of dinoflagellates often attributed to the energetic cost of motility (Langdon, 1993; López-Sandoval et al., 2014). An additional factor influencing community respiration rates in dinoflagellate blooms is that many dinoflagellate species are mixotrophic, and utilize dissolved and particulate nutritional sources to supplement photoautotrophy (Stoecker, 1998). Also, unknown for the present study is the extent to which respiration by the phytoplankton community is augmented by bacterial decomposition of labile organic matter. Bacterioplankton are major respirers and the phasing of autotrophic and heterotrophic plankton metabolism is likely to be strongly influenced by the nature of the links between the phytoplankton and the bacterioplankton (Blight et al., 1995).
The exceptional rates of respiration measured in the present study of between 472 and 1833 μmol O2 l−1 d−1 are placed in perspective through comparison with the reported mean respiration for surface coastal waters of 7.4 μmol O2 l−1 d−1 (Robinson and Williams, 2005) and the range of between 0.05 and 227 (mean 17.8) μmol O2 l−1 d−1 reported for 21 estuarine systems (Hopkinson and Smith, 2005). The Chl-a specific respiration rates reported for St. Helena Bay blooms of between 0.06 and 0.23 μmol O2 [μg Chl-a]−1 h−1 are similar to those reported for dinoflagellates by Langdon (1993) of between 0.10 and 0.29 μmol O2 [μg Chl-a]−1 h−1, which are notably higher than rates measured in other groups of phytoplankton. Cell specific rates of respiration as determined from the St. Helena Bay blooms ranged from 4.22 to 12.20 pmol O2 cell−1 h−1 and also showed good correspondence with previously reported values for dinoflagellates including the value of 6.25 pmol O2 cell−1 h−1 reported for Ceratium tripos in the New York Bight (Falkowski et al., 1980).
Large diurnal fluctuations in O2 between daytime supersaturation and nighttime hypoxia have been observed in various shallow eutrophic waters (e.g., D'Avanzo and Kremer, 1994; Shen et al., 2008; Tyler et al., 2009). In such environments the level of O2 surplus produced during the day is important in offsetting nighttime respiration. Observations during the present study show typical daytime O2 levels within the high biomass dinoflagellate blooms of St. Helena Bay to be supersaturated because of strong photosynthetic production of O2. However, nighttime community respiration was shown to have the potential of removing as much as 17.34 ml O2 l−1 from surface waters and it is considered likely that these exceptional rates of O2 utilization may lead overnight to conditions of anoxia. For this to occur the biological consumption of O2 needs also to exceed O2 replenishment via air-sea exchange.
O2 fluxes across the air-sea interface are governed by the disequilibrium between the concentrations of O2 in the ocean surface layer and the atmosphere, and a rate term known as the gas transfer coefficient, which refers to the rate at which the disequilibrium is removed (Wanninkhof et al., 2009). Several environmental conditions associated with the development of red tides are likely to reduce the gas transfer coefficient thereby retarding O2 replenishment and increasing the likelihood of anoxia. A reduction of near-surface turbulence owing to the reduced winds associated with the development of red tides is likely to be a primary cause of reduced nighttime oxygenation through reduction of the gas transfer coefficient. Further, the typical warming of surface waters during bloom events will decrease the solubility of O2 in the surface waters thereby driving O2 from these waters. Also of likely importance are phytoplankton exudates produced within the bloom which serve as potent surfactants that further dampen turbulence and suppress gas exchange (Frew et al., 1990).
Our calculations show that the maximum respiration rate measured in St. Helena Bay far exceeds the potential for oxygenation through exchange with the atmosphere. Commonly used gas exchange models such as employed here are based on empirical relationships with wind speed despite the fact that processes other than wind speed (e.g., turbulence, bubbles, waves, etc.) are known to influence air-sea gas exchange (Garbe et al., 2014). A number of these interfacial processes, however, are intimately linked to the wind, which explains the success of many of these models. Much of the uncertainty in wind speed based models resorts in the high wind regime where turbulence and bubbles can impact gas transfer velocities significantly (Wanninkhof, 2014). However, the effect of wind speed on gas transfer velocities is likely to be small when red tides are present in St. Helena Bay owing to the low wind conditions associated with their development. Under these conditions diurnal heating cycles, by forcing daytime stratification and nighttime buoyancy fluxes, may be more important than wind in facilitating gas exchange at the sea surface (McGillis et al., 2004). Despite these considerations and despite the model shortcomings and assumptions applied here, such as the absence of vertical or horizontal mixing or advection, the extraordinary community respiration rates measured in the red tides present in St. Helena Bay are very likely to overwhelm oxygenation during the night thereby driving the water column to anoxia.
Shallow coastal waters tend to have high O2 because they are well-ventilated, despite typically higher rates of O2 utilization. However, within red tides the extreme levels of utilization associated initially with the high rates of respiration of the dinoflagellate-dominated blooms followed by the high rates of oxidation of organic matter following bloom death are likely to exceed O2 supply despite the relative rapidity of air-sea gas exchange. The formation of red tides during periods of relative calm and increasing waters temperatures serves to decrease both the O2 solubility in surface waters and the rate of O2 transfer from the atmosphere thereby increasing the likelihood of hypoxic or anoxic conditions.
It is now known that phytoplankton die spontaneously upon encountering adverse abiotic or biotic environmental conditions and various forms of autocatalytic cellular self-destruction have been identified (Bidle, 2014). Also, viruses have been shown to be intimately locked mechanistically with these cell death pathways, fueling microbial food-webs through the lysis of phytoplankton host cells. A variety of nutrient stressors are considered a common trigger for cell death and as demonstrated in this study the low nutrients in red tides relative to the high demand are a likely cause of bloom demise. However, our study further serves to demonstrate the potential role of nighttime community respiration in the development of overnight anoxia, an environmental condition also likely to trigger bloom mortality. Further, understanding of respiration-regulating factors within red tides will facilitate better prediction of nighttime declines in O2.
GP contributed to experimental design, field work, data analysis, and was primarily responsible for writing the paper. TP contributed to experimental design, field work, data analysis, and contributed to writing the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank André du Randt and Lisa Mansfield for their assistance in the collection of field samples and Marie Smith for her assistance in the preparation of Figure 1.
Anderson, D. A., Glibert, P. M., and Burkholder, J. M. (2002). Harmful algal blooms and eutrophication: nutrient sources, composition and consequences. Estuaries 25, 704–726. doi: 10.1007/BF02804901
Bender, M., Grande, K., Johnson, K., Marra, J., Williams, P. J. LeB., Sieburth, J., et al. (1987). A comparison of four methods for determining planktonic community production. Limnol. Oceanogr. 32, 1085–1098. doi: 10.4319/lo.1987.32.5.1085
Bidle, K. D. (2014). The molecular ecophysiology of programmed cell death in marine phytoplankton. Annu. Rev. Mar. Sci. 7, 341–375. doi: 10.1146/annurev-marine-010213-135014
Blasco, D. (1975). “Red tides in the upwelling regions,” in Proceedings of the First International Conference on Toxic Dinoflagellate Blooms, ed V. R. LoCicero (Wakefield, MA: Massachusetts Science and Technology Foundation), 113–119.
Blight, S. P., Bentley, T. L., Lefevre, D., Robinson, C., Rodrigues, R., Rowlands, J., et al. (1995). The phasing of autotrophic and heterotrophic plankton metabolism in a temperate coastal ecosystem. Mar. Ecol. Prog. Ser. 128, 61–75. doi: 10.3354/meps128061
Brown, P. C., Hutchings, L., and Horstman, D. (1979). A red-water outbreak and associated fish mortality at Gordon's Bay near Cape Town. Fish. Bull. S. Afr. 11, 46–52.
Carpenter, J. H. (1965). The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol. Oceanogr. 10, 141–143. doi: 10.4319/lo.1965.10.1.0141
Cockcroft, A. C., Schoeman, D. S., Pitcher, G. C., Bailey, G. W., and van Zyl, D. L. (2000). “A mass stranding, or walkout, of west coast rock lobster Jasus lalandii in Elands Bay, South Africa: causes, results and implications,” in The Biodiversity Crisis and Crustacea, eds J. C. von Kaupel Klein and F. R. Schram (Rotterdam: A.A. Balkema), 673–688.
Cockcroft, A. C., van Zyl, D., and Hutchings, L. (2008). Large-scale changes in the spatial distribution of South African West Coast rock lobsters: an overview. Afr. J. Mar. Sci. 30, 149–159. doi: 10.2989/AJMS.2008.30.1.15.465
D'Avanzo, C., and Kremer, J. N. (1994). Diel oxygen dynamics and anoxic events in an eutrophic estuary of Waquoit Bay, Massachusetts. Estuaries 17, 131–139.
Diaz, R. J., and Rosenberg, R. (2008). Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. doi: 10.1126/science.1156401
Dugdale, R. C., and Wilkerson, F. P. (1986). The use of 15N to measure nitrogen uptake in eutrophic oceans; experimental considerations. Limnol. Oceanogr. 31, 673–689. doi: 10.4319/lo.1986.31.4.0673
Falkowski, P. G., Hopkins, T. S., and Walsh, J. J. (1980). An analysis of factors affecting oxygen depletion in the New York Bight. J. Mar. Res. 38, 479–506.
Frew, N. M., Goldman, J. C., Dennett, M. R., and Johnson, A. S. (1990). Impact of phytoplankton-generated surfactants on air-sea gas exchange. J. Geophys. Res. 95, 3337–3352. doi: 10.1029/JC095iC03p03337
Fukushima, T., Matsushige, K., and Weisburd, R. S. J. (2000). Characteristics of nighttime respiration in outdoor mesocosms. Limnology 1, 159–170. doi: 10.1007/s102010070002
Gaarder, T., and Gran, H. H. (1927). Investigations of the production of plankton in the Oslo Fjord. Rapp. P.-V. Réun. Cons. Int. Explor. Mer. 42, 1–48.
Garbe, C. S., Rutgersson, A., Boutin, J., de Leeuw, G., Delille, B., Fairall, C. W., et al. (2014). “Transfer across the air-sea interface,” in Ocean-Atmosphere Interactions of Gases and Particles, eds P. S. Liss and M. T. Johnson (Heidelberg: Springer Earth System Sciences), 55–112.
Gilbert, D., Rabalais, N. N., Díaz, R. J., and Zhang, J. (2010). Evidence for greater oxygen decline rates in the coastal ocean than in the open ocean. Biogeosciences 7, 2284–2296. doi: 10.5194/bg-7-2283-2010
Grasshoff, K., Ehrhardt, M., and Kremling, K. (1983). Methods of Seawater Analysis, 2nd Edn. Weinheim: Verlag Chemie.
Gruber, N. (2011). Warming up, turning sour, losing breath: ocean biogeochemistry under global change. Philos. Trans. A Math. Phys. Eng. Sci. 369, 1980–1996. doi: 10.1098/rsta.2011.0003
Gustavson, K., Garde, K., Wängberg, S.-A., and Selmer, J.-S. (2000). Influence of UV-B radiation on bacterial activity in coastal waters. J. Plank. Res. 22, 1501–1511. doi: 10.1093/plankt/22.8.1501
Hasle, G. R. (1978). “The inverted microscope method,” in Phytoplankton Manual, ed A. Sournia (Paris: UNESCO), 88–96.
Heisler, J., Glibert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C., et al. (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. doi: 10.1016/j.hal.2008.08.006
Hitchcock, G. L., Kirkpatrick, G., Minnett, P., and Palubok, V. (2010). Net community production and dark community respiration in a Karenia brevis (Davis) bloom in West Florida coastal waters, USA. Harmful Algae 9, 351–358. doi: 10.1016/j.hal.2010.01.002
Hopkinson, C. S., and Smith, E. M. (2005). “Estuarine respiration: an overview of benthic, pelagic, and whole system respiration,” in Respiration in Aquatic Systems, eds P. A. del Giorgio and P. J. LeB. Williams (New York, NY: Oxford University Press), 122–146.
Keeling, R. F., Körtzinger, A., and Gruber, N. (2010). Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2, 199–229. doi: 10.1146/annurev.marine.010908.163855
Kemp, W. M., Testa, J. M., Conley, D. J., Gilbert, D., and Hagy, J. D. (2009). Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences 6, 2985–3008. doi: 10.5194/bg-6-2985-2009
Koroleff, F. (1983). “Determination of ammonia,” in Methods of Seawater Analysis, 2nd Edn, eds K. Grasshoff, E. Ehrhardt, and K. Kremling (Weinheim: Verlag Chemie), 150–157.
Kudela, R. M., Seeyave, S., and Cochlan, W. P. (2010). The role of nutrients in regulation and promotion of harmful algal blooms in upwelling systems. Prog. Oceanogr. 85, 122–135. doi: 10.1016/j.pocean.2010.02.008
Langdon, C. (1993). The significance of respiration in production measurements based on oxygen. ICES Mar. Sci. Symp. 197, 69–78.
López-Sandoval, D. C., Rodríguez-Ramos, T., Cermeño, P., Sobrino, C., and Marañón, E. (2014). Photosynthesis and respiration in marine phytoplankton: relationship with cell size, taxonomic affiliation, and growth phase. J. Exp. Mar. Biol. Ecol. 457, 151–159. doi: 10.1016/j.jembe.2014.04.013
Markager, S., and Sand-Jensen, K. (1989). Patterns of night-time respiration in a dense phytoplankton community under a natural light regime. J. Ecol. 77, 49–61. doi: 10.2307/2260915
Marra, J. (2009). Net and gross productivity: weighing in with 14C. Aquat. Microb. Ecol. 56, 123–131. doi: 10.3354/ame01306
Matthews, S. G., and Pitcher, G. C. (1996). “Worst recorded marine mortality on the South African coast,” in Harmful and Toxic Algal Blooms, eds T. Yasumoto, Y. Oshima, and Y. Fukuyo (Paris: Intergovernmental Oceanographic Commission of UNESCO), 89–92.
McGillis, W. R., Edson, J. B., Zappa, C. J., Ware, J. D., McKenna, S. P., Terray, E. A., et al. (2004). Air-sea CO2 exchange in the equatorial Pacific. J. Geophys. Res. Oceans 109, C08S02. doi: 10.1029/2003JC002256
Mitchell-Innes, B. A., Pitcher, G. C., and Probyn, T. A. (2000). Productivity of dinoflagellate blooms on the west coast of South Africa, as measured by natural fluorescence. S. Afr. J. mar. Sci. 22, 273–284. doi: 10.2989/025776100784125762
Nydahl, F. (1976). On the optimum conditions for the reduction of nitrate to nitrite by cadmium. Talanta 23, 349–357. doi: 10.1016/0039-9140(76)80047-1
Parsons, T. R., Maita, Y., and Lalli, C. M. (1984). A Manual of Chemical and Biological Methods for Seawater Analysis. New York, NY: Pergamon.
Pitcher, G. C., and Calder, D. (2000). Harmful algal blooms of the southern Benguela current: a review and appraisal of monitoring from 1989 to 1997. S. Afr. J. mar. Sci. 22, 255–271. doi: 10.2989/025776100784125681
Pitcher, G. C., and Nelson, G. (2006). Characteristics of the surface boundary layer important to the development of red tide on the southern Namaqua shelf of the Benguela upwelling system. Limnol. Oceanogr. 51, 2660–2674. doi: 10.4319/lo.2006.51.6.2660
Pitcher, G. C., and Probyn, T. A. (2011). Anoxia in southern Benguela during the autumn of 2009 and its linkage to a bloom of the dinoflagellate Ceratium balechii. Harmful Algae 11, 23–32. doi: 10.1016/j.hal.2011.07.001
Pitcher, G. C., Probyn, T. A., du Randt, A., Lucas, A. J., Bernard, S., Evers-King, H., et al. (2014). Dynamics of oxygen depletion in the nearshore of a coastal embayment of the southern Benguela upwelling system. J. Geophys. Res. Oceans 119, 2183–2200. doi: 10.1002/2013JC009443
Pitcher, G. C., and Weeks, S. J. (2006). “The variability and potential for prediction of harmful algal blooms in the southern Benguela ecosystem,” in Benguela: Predicting a Large Marine Ecosystem, Vol. 14, Large Marine Ecosystems Series, eds V. Shannon, G. Hempel, P. Malanotte-Rizzoli, C. Moloney and J. Woods (Amsterdam: Elsevier), 125–146.
Pringault, O., Tassas, V., and Rochelle-Newall, E. (2007). Consequences of respiration in the light on the determination of production in pelagic systems. Biogeosciences 4, 105–114. doi: 10.5194/bg-4-105-2007
Pringault, O., Tassas, V., and Rochelle-Newall, E. (2009). Respiration in the light and bacterio-phytoplankton coupling in a costal environment. Microb. Ecol. 57, 321–334. doi: 10.1007/s00248-008-9422-7
Rabalais, N. N., Cai, W.-J., Carstensen, J., Conley, D. J., Fry, B., Hu, X., et al. (2014). Eutrophication-driven deoxygenation in the coastal ocean. Oceanography 27, 172–183. doi: 10.5670/oceanog.2014.21
Robinson, C., and Williams, P. J. LeB. (2005). “Respiration and its measurement in surface marine waters,” in Respiration in Aquatic Systems, eds P. A. del Giorgio and P. J. LeB. Williams (New York, NY: Oxford University Press), 147–180.
Sasano, D., Ishii, M., Midorikawa, T., Nakano, T., Tokieda, T., and Uchida, H. (2011). Testing a new quick response oxygen sensor, “RINKO.” Meteorol. Geophys. 62, 63–73. doi: 10.2467/mripapers.62.63
Seeyave, S., Probyn, T. A., Pitcher, G. C., Lucas, M. I., and Purdie, D. A. (2009). Nitrogen nutrition in assemblages dominated by Pseudo-nitzschia spp., Alexandrium catenella and Dinophysis acuminata off the west coast of South Africa. Mar. Ecol. Prog. Ser. 379, 91–107. doi: 10.3354/meps07898
Shen, J., Wang, T., Herman, J., Mason, P., and Arnold, G. L. (2008). Hypoxia in a coastal embayment of the Chesapeake Bay: a model diagnostic study of oxygen dynamics. Estuar. Coast. 31, 652–663. doi: 10.1007/s12237-008-9066-3
Stoecker, D. K. (1998). Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications. Eur. J. Protistol. 34, 281–290. doi: 10.1016/S0932-4739(98)80055-2
Tyler, R. M., Brady, D. C., and Targett, T. E. (2009). Temporal and spatial dynamics of diel-cycling hypoxia in estuarine tributaries. Estuar. Coast. 32, 123–145. doi: 10.1007/s12237-008-9108-x
Vicente, H. J., Gaid, R. D., Dejarme, H. E., Roa, E. C., and Azanza, R. V. (2001). Harmful algal bloom in Iligan Bay, Southern Philippines. Sci. Diliman 14, 59–65.
Walker, D. R., and Pitcher, G. C. (1991). The dynamics of phytoplankton populations, including a red-tide bloom, during a quiescent period in St. Helena Bay, South Africa. S. Afr. J. Mar. Sci. 10, 61–70. doi: 10.2989/02577619109504620
Wanninkhof, R. (2014). Relationship between wind speed and gas exchange over the ocean revisited. Limnol. Oceanogr. Methods 12, 351–362. doi: 10.4319/lom.2014.12.351
Keywords: red tides, dinoflagellates blooms, community production, nighttime community respiration, bloom mortality
Citation: Pitcher GC and Probyn TA (2016) Suffocating Phytoplankton, Suffocating Waters—Red Tides and Anoxia. Front. Mar. Sci. 3:186. doi: 10.3389/fmars.2016.00186
Received: 06 May 2016; Accepted: 09 September 2016;
Published: 27 September 2016.
Edited by:
Anas Ghadouani, University of Western Australia, AustraliaReviewed by:
José Pinho, University of Minho, PortugalCopyright © 2016 Pitcher and Probyn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grant C. Pitcher, Z3JhbnRwQGRhZmYuZ292Lnph
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.