- 1Department of Environment and Energy Systems, Graduate School of Science and Technology, Shizuoka University, Shizuoka, Japan
- 2Research Institute of Green Science and Technology, Shizuoka University, Shizuoka, Japan
- 3Department of Geoscience, Shizuoka University, Shizuoka, Japan
- 4Department of Chemistry, Biology and Marine Science, University of the Ryukyus, Okinawa, Japan
Increasing seawater temperatures and CO2 levels associated with climate change affect the shallow marine ecosystem function. In this study, the effects of elevated seawater temperature and partial pressure of CO2 (pCO2) on subtropical sediment systems of mangrove, seagrass, and coral reef lagoon habitats of Okinawa, Japan, were examined. Sediment and seawater from each habitat were exposed to four pCO2-temperature treatments, including ambient pCO2- ambient temperature, ambient pCO2-high temperature (ambient temperature + 4°C), high pCO2 (936 ppm)-ambient temperature, and high pCO2-high temperature. Parameters including primary production, nutrient flux, pigment content, photosynthetic community composition, and bacterial abundance were examined. Neither high temperature nor high pCO2 alone impacted mangrove and seagrass sediment primary production significantly (Tukey's test, P > 0.05). However, the combined stress significantly (Tukey's test, P < 0.01) increased primary production in these two habitats. In sediments from the coral reef lagoon, single and combined stress treatments induced a shift from heterotrophy to autotrophy. Significant increases in net primary production (Tukey's test, P < 0.01), and gross primary production (Tukey's test, P < 0.05) under the combined stress suggested that benthic microalgae in mangrove and seagrass sediments were more responsive to high temperature and pCO2 conditions than those in coral reef lagoon sediments. Additionally, combined stress significantly increased the sediment chlorophyll a content (Tukey's test, P < 0.05) in all habitats. These increases were associated with increased net primary production, indicating that combined stress stimulates primary production activity by the photosynthetic benthic microalgae in all habitats. Diatom activity increased, as silicate uptake increased in all habitats. Microbial abundance significantly increased under the combined stress treatment (Tukey's test, P < 0.01), but did not exceed autotrophic activity. Respiration did not change significantly in any of the three habitats (Tukey's test, P > 0.05) under the combined stress, suggesting that heterotrophic processes were less affected by the combined stress than autotrophic processes. In summary, mangrove and seagrass sediments minimize the negative impacts of elevated temperature and pCO2 via increased primary production and carbon storage. Lagoonal sediments also act as a carbon sink under temperature and ocean acidificationstress.
Introduction
The of marine ecosystems in mitigating the impacts of climate change has generated considerable interest owing to the capacity of these ecosystems to trap and sequester carbon (Mcleod et al., 2011; Miyajima et al., 2015; Ricart et al., 2015). Mangroves, tidal salt marshes, seagrass beds, and coral reefs are key ecosystems that serve important roles in protecting coastal communities worldwide from storm surges, sea level rise, sediment run-off, and food insecurity (Beck et al., 2001; Mumby, 2006; Barbier et al., 2011; Sifleet et al., 2011; Cullen-Unsworth and Unsworth, 2013; Robertson and Alongi, 2013). These ecosystems help to mitigate the effects of environmental change (e.g., increasing atmospheric and oceanic CO2 and temperature) via carbon sequestration, and the storage of atmospheric and seawater carbon (Duarte et al., 2005, 2010; Bouillon et al., 2008; Kennedy et al., 2010; Donato et al., 2012; Pendleton et al., 2012; Chmura, 2013). Although, shallow marine ecosystems are currently subjected to a combination of global and regional stressors, few experimental studies have examined shallow benthic areas to understand the consequences of increased temperature and ocean acidification (OA) (Connell et al., 2013; Johnson et al., 2013). Additionally, it is difficult to conclusively determine the impact of different environmental stressors in shallow-water sediment systems (Alsterberg et al., 2011, 2012) since the combinations of environmental stressors may have synergistic (Aswani et al., 2015) or antagonistic effects, resulting in unexpected patterns of variation (Folt et al., 1999). Warming can negatively decouple (i.e., mismatch) the relationship between phytoplankton and zooplankton, and it causes an earlier peak of primary production (Winder and Schindler, 2004; Woodward et al., 2010). While some studies have suggested that many marine autotrophs are insensitive to changes in pCO2 (Engel et al., 2007), others have revealed that some seagrasses (Zimmerman et al., 1997), macroalgae (Gao et al., 1993), microalgae (Kayanne et al., 2005), diatoms, coccolithophorids (Riebesell et al., 2000; Zondervan et al., 2001; Casareto et al., 2009), and cyanobacteria (Qiu and Gao, 2002) exhibit high rates of photosynthesis under elevated pCO2 conditions (Delille et al., 2005).
Major ecosystem functions in shallow water sediments are primarily controlled by microorganisms; accordingly, the present study focused on benthic microalgal and microbial communities, and their key ecosystem functions (i.e., primary production and nutrient flux). Primary production, microbial activity, oxygen flux, and carbon and nitrogen at the sediment–seawater interface are indicators of the integrated net responses of sediment communities to environmental and anthropogenic stressors (Alsterberg and Sundbäck, 2013). Mangrove ecosystems are characterized by high primary productivity, a permanent exchange with terrestrial and marine ecosystems (Jennerjahn and Ittekkot, 2002) and efficient nutrient filtration between land and sea (Bouillon et al., 2008). Seagrass ecosystems are important for their carbon storage capacity (Miyajima et al., 2015) and high primary productivity (Kennedy et al., 2010; Ricart et al., 2015). Benthic microalgae in seagrass sediments, such as benthic diatoms and mats of filamentous cyanobacteria, maintain net autotrophic conditions under increased temperatures (Alsterberg et al., 2011). Some studies have examined the impact of high temperatures on seagrass sediment systems, but the influence of OA is still unclear. Lagoonal sediment responses under stress are not known owing to limited sediment system studies in coral reef lagoon environments. Additionally, many studies have emphasized the effects of warming on single species or trophic levels (Montoya and Raffaelli, 2010), but not on whole communities.
Most sediment system studies have considered the impact of single (Alsterberg et al., 2011; Johnson et al., 2013) or multiple stressors (Alsterberg et al., 2013) in a single habitat. Whether sediment from different subtropical habitats (e.g., mangroves, seagrasses, and coral reef lagoons) show different patterns under elevated pCO2, temperature stress, and their combination, and whether such differences can be observed when studying primary production, nutrient fluxes, photosynthetic community composition, pigment content, and bacterial dynamics also need to be investigated. Answering these questions will provide opportunities to assess shallow marine sediment system's responses under elevated temperature and OA.
In the present study, we exposed subtropical sediments from mangrove, seagrass, and coral reef habitats of Okinawa, Japan to four pCO2-temperature treatments, including ambient pCO2-ambient temperature, ambient pCO2-high temperature (ambient temperature + 4°C), high pCO2 (936 ppm)-ambient temperature, and high pCO2-high temperature. Parameters including primary production, nutrient fluxes, pigment content, photosynthetic community composition, and bacterial abundance were examined. The following questions were addressed. (1) Do sediments from the three habitats respond differently with respect to primary sediment production under individual and combined stress? (2) What is the effect of stress on nutrient flux? (3) How do sediment and seawater microbial abundances change under elevated temperatures and OA? To address these questions, incubation experiments were performed using sediment and seawater samples from the mangrove, seagrass, and coral reef habitats exposed to elevated temperature and pCO2 treatments.
Methodology
Site Description
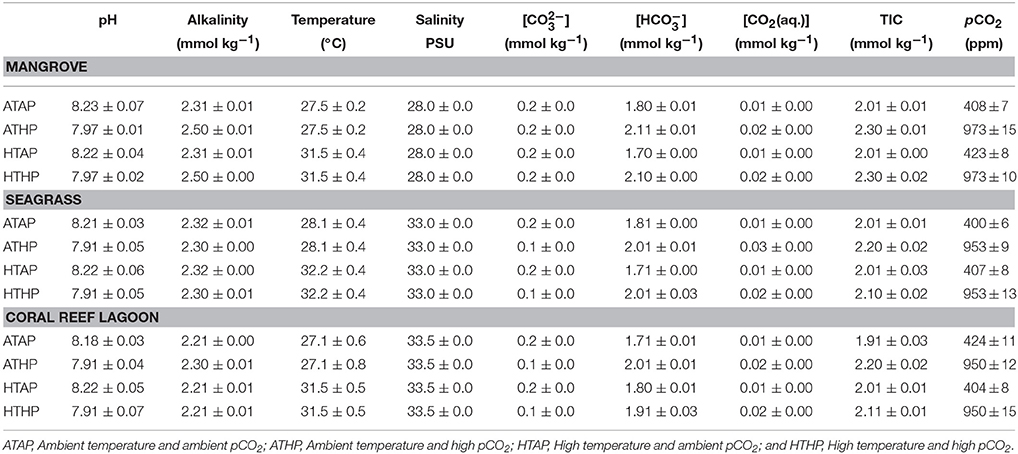
The study was conducted in a subtropical mangrove area (station 1) at Yagachi Island (26°38′N and 128°00′E), a seagrass area (station 2) at Bise (26°42′N and 127°52′E), and a coral reef lagoon area (26°39″N and 127°51′E) at Sesoko reef in Sesoko Island (station 3), Okinawa, Japan (Figure 1). The tidal flat of Yagachi Island was dominated by Bruguiera gymnorrhiza. During high tide, the water level rises to 0.6 m in the mangrove area. Thalassia hemprichii dominated the seagrass area of Bise, which included the intertidal zone covered by a seagrass meadow on the back reef (1.5 m at high tide and 0.5 m at low tide). In Sesoko reef, the lagoonal bed was covered by 10% living corals, 50% coral rubble, 30% sand, and 10% microalgae and turf algae (Nakano and Nakai, 2008). Depths during sampling were 0.6, 0.5, and 0.5 m for mangrove (high tide), seagrass (low tide), and coral reef lagoon (low tide) sediment and seawater. In Yagachi, Bise, and Sesoko, in situ temperature, salinity, pH, and alkalinity were measured during the week of the experiments (Table 1, ambient temperature and ambient pCO2 [ATAP]).
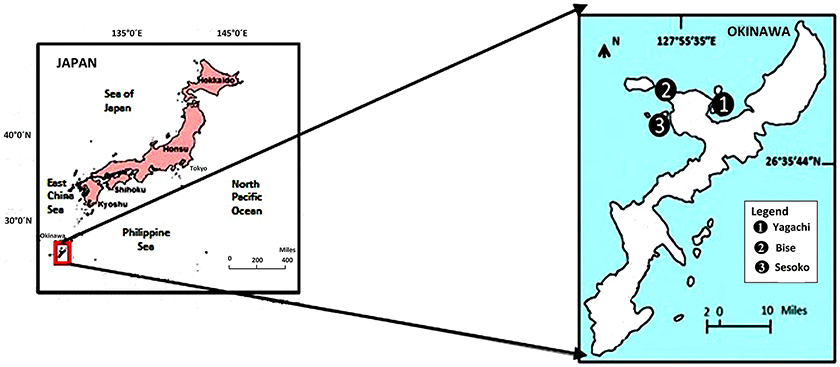
Figure 1. Study area and sampling sites in Okinawa, Japan. Yagachi, Bise, and Sesoko represent mangrove, seagrass, and coral reef lagoon habitats respectively.
Incubation Conditions
Incubation experiments were performed with subtropical sediment and seawater samples obtained from each habitat (mangrove, seagrass, and coral reef lagoon) in the laboratory. Mangrove sediments were generally more fine-grained, with a higher porosity, a higher organic carbon content and a higher carbon-nitrogen ratio than seagrass and coral reef lagoon sediments (Tukey's test, P < 0.05; Supplementary Table 1). Sediment porosity and organic carbon content were measured following the methods of Buchanan (1984) and Miyajima (2015), respectively. Sediment samples were collected using sediment collection cores (4.5 cm radius). Samples were treated following Cornwell et al. (2014) to adjust for sampling disturbances. Intact sediment was transferred to incubation chambers, maintaining their natural heterogeneity and complexity. In each incubation chamber (chamber radius, 5 cm), the upper 3 cm of sediment was kept in the bottom and 1 L of seawater sampled at the same location was added. Levels of pCO2 were adjusted by adding CO2-saturated seawater (100%) to the incubation chambers at different volumes until the desired pH values equivalent to the desired pCO2 levels were obtained (Supplementary Table 2). CO2 saturated seawater was prepared following the methods of Casareto et al. (2009); briefly, CO2 gas was bubbled into natural filtered (0.2 μm) seawater for about 1 h. The computer program CO2 SYS was used to estimate the , , CO2(aq.), TIC, and pCO2 from the pH, temperature, total alkalinity, and salinity (Lewis and Wallace, 1998). Table 1 shows the estimates of pCO2. Four pCO2-temperature treatments, including ambient pCO2–ambient temperature, ambient pCO2-high temperature (ambient temperature + 4°C), high pCO2 (936 ppm)-ambient temperature, and high pCO2-high temperature (Table 1, Supplementary Figure 1), with three replicates per treatment were used. pCO2 conditions of 400 ppm (in-situ pCO2) and 936 ppm (Intergovernmental Panel on Climate Change (IPCC), 2013), were chosen as present day and near-future scenarios, respectively. 27.5, 28.1, and 27.1°C were chosen as ambient temperatures (AT), which were similar to in-situ temperatures in Yagachi, Bise, and Sesoko, respectively, during the incubation experiments (Table 1), and ambient (in-situ temperature) + 4°C (Intergovernmental Panel on Climate Change (IPCC), 2013) was chosen as the stress temperature. Around Okinawa, the average temperature for the month preceding the experiment was also 27°C (unpublished data). Incubations were performed in triplicate for each condition and habitat. Incubation vessels were maintained in water baths under four different incubation conditions (Table 1, Supplementary Figure 1) and run for 24 h following natural illumination (276–0 μmol m−2 s−1 during 12:12 h light: dark cycle) during summer (August–September, 2014). Heating and cooling systems were used to keep the temperature conditions in the water baths stable.
The pH (NBS scale) was measured with an Orion 4 Star pH sensor and an 8156BNUWP pH electrode (Thermo Scientific, Inc., Waltham, MA, USA) calibrated with NIST (NBS)-scaled buffer solutions (Mettler pH 10.012, 6.865, and 4.008 buffers) at 25°C. The accuracy of pH measurements was ±0.005 pH units (1 σ, n = 10). Seawater samples for total alkalinity (TA) measurements were collected from the field and each incubation chamber before starting the incubation. Collected seawater was filtered with 0.45-μm cellulose acetate filters. TA was determined via titration with 0.1 mol L−1 HCL using a TA titrator (ATT-05; Kimoto, Japan), and computed using the Gran plot method (Stumm and Morgan, 1981). Titrations were performed on 30.000 g of filtered seawater that was weighed on a high-precision balance (GH-202, A&D, Tokyo, Japan, 0.00001 accuracy). The precision of the TA measurements was ±2 μmol kg−1 (1 σ, n = 10). The accuracy was within 10 μmol kg−1, as verified using certified reference material (CRM Batch # 50) distributed by A. Dickson of the Marine Physical Laboratory, University of California (San Diego, CA, USA). The carbonate system in seawater was calculated using the apparent equilibrium constants K'0 from Weiss (1974), and K'1 and K'2 from Mehrbach et al. (1973), as described by Millero (1979). The constants are among the suitable combinations for the calculation of carbonate systems with the NBS pH scale. Recently, the Tris buffer pH scale with an acid dissociation constant for the total hydrogen ion concentration has been recommended for the precise calculation for seawater carbonate systems (Dickson et al., 2007). However, it has been suggested that the pCO2 value from the NBS scale is very close to that of the Tris scale when an appropriate combination of equilibrium constants is used (Millero, 1979).
Measurements
Nutrients ( + , , , and Si(OH)4), tracer pigments (chlorophyll a, chlorophyll b, chlorophyll c2, fucoxanthin, zeaxanthin, and β-carotene), net primary production (NPP), gross primary production (GPP), respiration (R), and bacterial abundance were analyzed. Samples for pigment analyses and bacterial abundances were obtained at the initial (0 h) and final (24 h) stages. Dissolved oxygen (DO) and nutrients were measured at the initial (0 h), middle (after 12 h of light), and final (24 h) stages. Parameter changes (Δ values) were calculated by subtracting the initial values from the final values.
Nutrients
To measure nutrients, seawater samples were collected (from the field and each replicate incubation chamber) in previously acid-washed 100-mL polyethylene bottles and were preserved at -20°C until measurement. Pore waters were collected following the methods of Brito et al. (2010). The concentrations of nitrate () + nitrite (), ammonium (), phosphate (), and silicate (Si(OH)4) were analyzed using an auto analyzer (TRAACS 2000; Bran and Luebbe, Norderstedt, Germany) according to the methods of Hansen and Koroleff (2007). The detection limits were 0.043 μM for + , 0.01 μM, for , 0.020 μM for , and 0.310 μM for Si(OH)4. Reproducibility (precision) of nutrient analyses were ±0.2% for + , ±1.2% for , ±0.7% for , and ±0.5% for Si(OH)4.
Pigments and Benthic Microalgal Observations
To assess the pigment concentration, three sediment cores were collected from each replicate incubation chamber using a 1-mL plastic syringe with a cut-off tip. Additionally, to quantify changes in the chlorophyll a concentration at the sediment surface inside the incubation chamber between initial conditions and combined stress conditions, 1 g of sediment was collected at radii of 0, 2.5, and 5 cm (total radius, 5 cm) for angles (θ) of 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, and 360°. Samples were kept in separate pre-combusted GF/F filters and preserved at −20°C until used for measurements. Pigment analyses were carried out following the methods of Zapata et al. (2000) using HPLC (high-performance liquid chromatography) (LC-30AD; Shimadzu, Kyoto, Japan). The HPLC system was equipped with a ZORBAX Eclipse Plus C8 column (2.1 × 50 mm; Agilent Technologies, Santa Clara, CA, USA). Pigments were extracted using a combination (1:3) of dry sediment and acetone (90% final concentration; Alsterberg et al., 2011), sonicated for 15 min, and maintained overnight in the dark at −30°C. Extracts were filtered using a syringe filter (0.2 μm, Millex-LG; Millipore, Bedford, MA, USA; Suzuki et al., 2015) and 1.0 mL of each extract was mixed with 0.2 mL of deionized water (Milli-Q water, Millipore) immediately before injections (Zapata et al., 2000) in the HPLC system. Separated pigments were detected spectrophotometrically using a photodiode array detector (SPD-M30AD; Shimadzu, Kyoto, Japan) with an optical resolution of 1.2 nm across the 320–720 nm bandwidth. For micro-algal observations, sediment samples were obtained from each incubation chamber using a 2.5-mL plastic syringe with a cut-off tip and kept frozen (−20°C) until analysis. After thawing, the samples were diluted with filtered seawater following the methods of Sundback et al. (2010). Epifluorescence microscopy (Eclipse/E6000; Nikon, Tokyo, Japan) and an inverted microscope (Nikon Bk-201) were used to identify the dominant photosynthetic community following Chihara and Murano (1997).
Bacterial Abundance
Bacterial abundance was measured for both seawater and sediment. For seawater, 50 mL of sample was collected from each of the replicate incubation chambers and preserved with 25% glutaraldehyde (1% final concentration). For sediment, three sediment cores were collected from each replicate incubation chamber using a 1-mL plastic syringe with a cut-off tip. To estimate bacterial abundance within the whole incubation chamber at initial conditions and after treatment, 1 g of sediment was collected at the center and radii of 2.5 and 5 cm (total radius, 5 cm) in lines separated by a 30° (θ) angle. Sediments were preserved with 25% glutaraldehyde (4% final concentration) until analysis. Sediments were diluted with artificial seawater (1:99 combinations) and pre-filtered with 0.8-μm polycarbonate filters to remove large particles. Heterotrophic bacteria from seawater and sediment samples were collected on 0.2-μm black polycarbonate filters by filtering 10-mL aliquots of seawater that were previously stained with 4′,6-diamidino-2-phenylindole (DAPI) (Porter and Feig, 1980). Filters were mounted on slides and cells were counted under UV excitation using epifluorescence microscopy (Eclipse/E6000; Nikon). Growth rates and doubling were calculated following the methods of Stanier et al. (1979).
Primary Production
NPP and R were measured based on oxygen flux during day (light) and night (dark) conditions, respectively. Initial (0 h), middle (after 12 h of light), and final (24 h) seawater samples were obtained using sterile plastic syringes. Dissolved oxygen (DO) was measured with an Orion 4 Star sensor and a polarographic DO Probe on a 5-ft Cable (Thermo Scientific, Inc., Waltham, MA, USA). NPP was estimated as DO production during 12 h of illumination. R was estimated as the night-time changes in DO. It is considered that R in the light equals R in the dark (Del Giorgio and Williams, 2005; Alsterberg et al., 2011), such that R = R (12 h of dark) × 2. GPP was calculated as follows: GPP = NPP + R. All estimates of GPP, NPP, and R were converted to carbon (C) using the Redfield–Richards empirical formula (Libes, 2009).
Statistical Analysis
Two-way analyses of variance (ANOVA) were used for comparisons, and incubation conditions (ambient temperature and ambient pCO2 [ATAP], ambient temperature and high pCO2 [ATHP], high temperature and ambient pCO2 [HTAP], and high temperature and high pCO2 [HTHP]) and habitats (mangrove, seagrass, and coral reef lagoon) were used as fixed factors. Significant differences between means were assessed using post-hoc Tukey's tests. A regression analysis was used to evaluate relationships between variables. The homogeneity of the variances among replicates was assessed by applying Levene tests. Statistical analyses were performed using SPSS (version 19).
Results
Abiotic Parameters
In Yagachi, in situ temperature, salinity, pH, and alkalinity during the week of the experiments were 27.5 ± 0.2°C, 28 PSU, 8.23 ± 0.07, and 2.38 ± 0.01 mmol kg−1, respectively. Similarly, in Bise, in situ temperature, salinity, pH, and alkalinity were 28.1 ± 0.4°C, 33 PSU, 8.21 ± 0.03, and 2.37 ± 0.02 mmol kg−1, respectively. In Sesoko, in situ temperature, salinity, pH and alkalinity were 27.1 ± 0.2°C, 33.5 PSU, 8.18 ± 0.03, and 2.28 ± 0.01 mmol kg−1, respectively.
Nutrient Fluxes
Nutrients were generally taken up during incubation in both light and dark periods, with the exception of ammonium and phosphate (Figure 2), and the rate of uptake differed significantly depending on habitat (ANOVA, P < 0.01; Table 2). The seagrass and mangrove samples had comparatively high amounts of silicate initially (Tukey's test, P < 0.05; Supplementary Table 3). Though it was not statistically significant (Tukey's test, P > 0.05), nitrate + nitrite and silicate uptake increased under the HTHP stress during both light and dark periods, especially in mangrove and seagrass samples (Figure 2). The highest nitrate concentration was observed in the seagrass habitat (Tukey's test, P < 0.05; Supplementary Table 3), but the rate of uptake in seagrass did not differ significantly among incubation conditions (Tukey's test, P > 0.05). In lagoonal sediment, the rate of nitrate + nitrite uptake seemed to increase under HTAP and HTHP conditions during light. Additionally, this sediment seemed to release ammonium under ATHP and HTHP conditions during both light and dark periods.
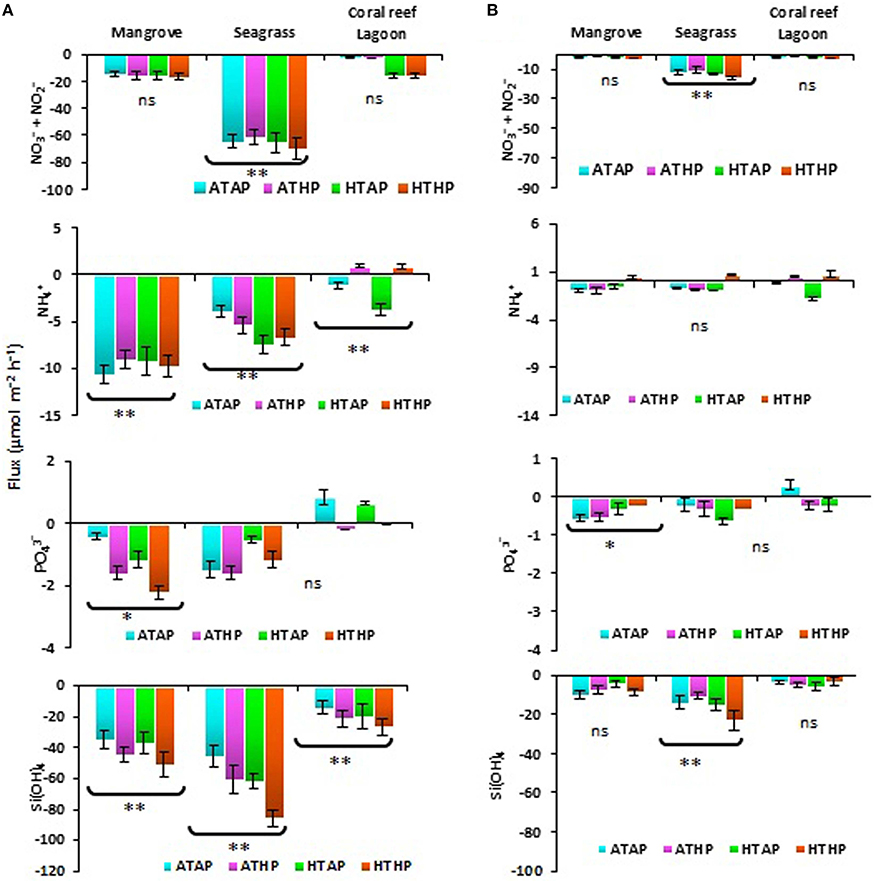
Figure 2. Sediment-water fluxes of nutrients during light (A) and dark (B) periods. *, ** Indicate significant differences at P < 0.01 and P < 0.001 based on the least significant difference test (post-hoc Tukey's test) depending on habitat (mangrove, seagrass, and coral reef lagoon). Lack of significance is indicated by ns. ATAP, ambient temperature and ambient pCO2; ATHP, ambient temperature and high pCO2; HTAP, high temperature and ambient pCO2; and HTHP, high temperature and high pCO2.
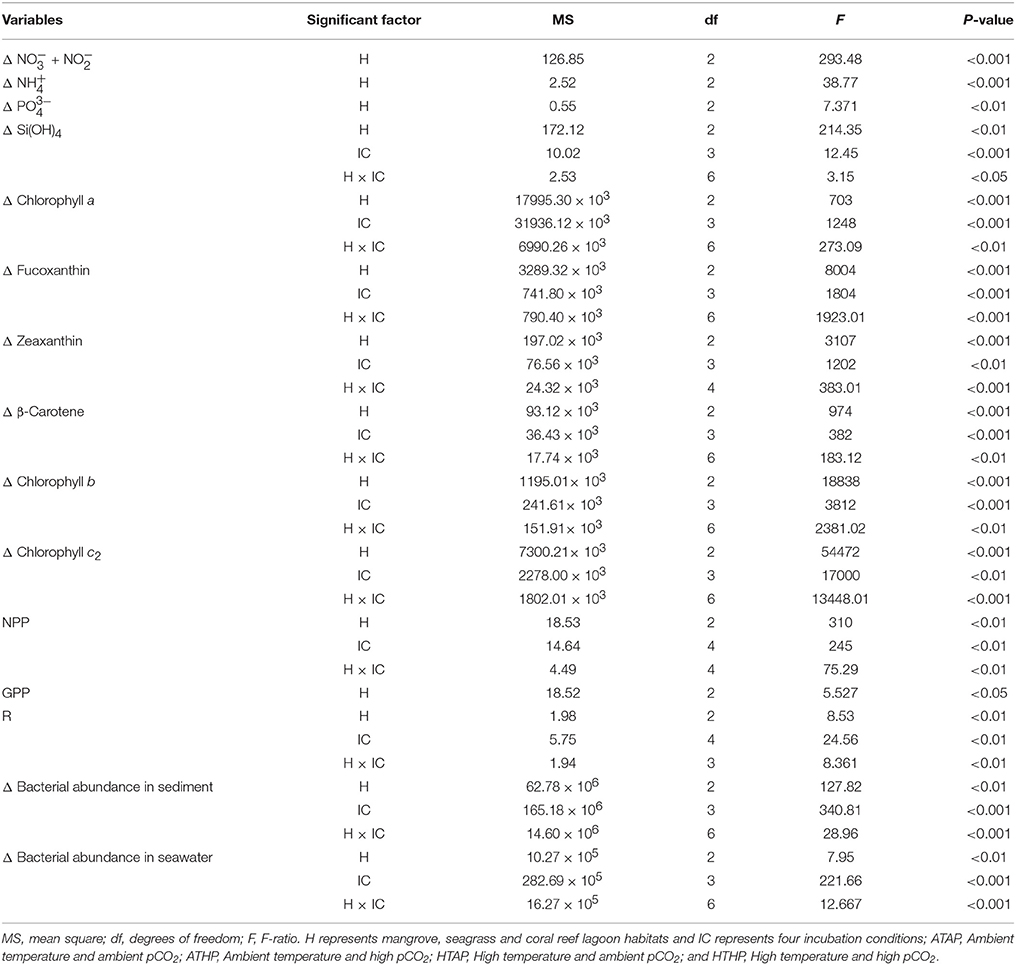
Table 2. The effects of different habitats (H) and incubation conditions (IC) on environmental parameters based on two-way ANOVA.
Photosynthetic Community
Based on tracer pigments and microscopic observations, a well-developed benthic microalgal community was detected in all habitats. The photosynthetic community was dominated by pennate diatoms and filamentous cyanobacteria. Centric diatoms (in mangrove and seagrass sediments), euglenoids (in seagrass and coral reef lagoon sediments), and flagellates (in all sediments) were occasionally observed. Nano-sized (5–20 μm) benthic diatoms (Supplementary Table 4), especially naviculoids, were frequently found in all sediment types. Additionally, Bacillariophyceae (in all sediments) and Coscinodiscophyceae (in seagrass and coral reef sediments) were identified. Pico-sized (< 2 μm) diatoms were abundant in mangrove and seagrass sediments. A higher abundance of photosynthetic community taxa was evidenced by a higher concentration of chlorophyll a, fucoxanthin, and zeaxanthin in mangrove sediment (Tukey's test, P < 0.05; Figure 3, Table 2, Supplementary Figure 2) than in seagrass and lagoonal sediments. Under HTHP stress, chlorophyll a increased in the surface sediment, representing the bulk of the photosynthetic community in mangrove and seagrass incubations (Figure 4). Changes in the concentrations of other pigments under HTHP stress also indicated an increase in the photosynthetic community; however, in the reef lagoon, some degradation of pigments was observed (Figure 3). The highest concentration of chlorophyll a was found in incubated mangrove sediment (Tukey's test, P < 0.05; Figure 3). The chlorophyll a concentration in the surface sediments increased after HTHP treatment in all habitats, and this change was greater in mangrove sediments and lower in coral reef lagoon sediments. Under HTHP treatment chlorophyll a concentration increased 1550–2400, 750–1455, and 620–860 ng g−1 in mangrove, seagrass, and coral reef lagoon sediments, respectively (Figure 4).
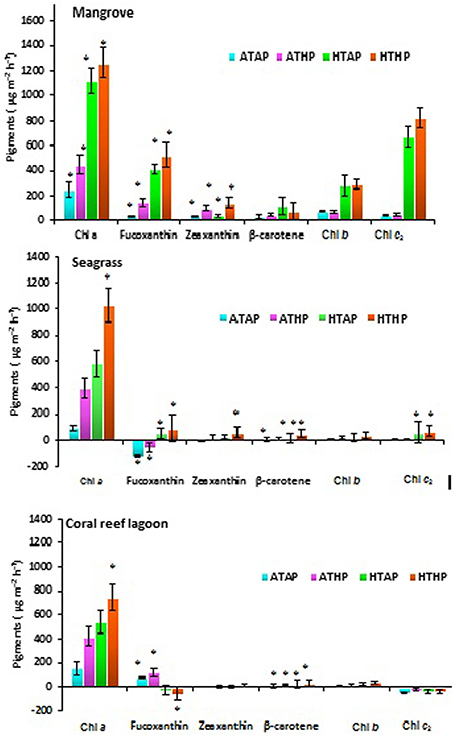
Figure 3. Changes in pigment concentration. *, ** Indicate significant differences at P < 0.05 and P < 0.01 determined using least significant difference tests (post-hoc Tukey's test) depending on incubation conditions (ATAP, ATHP, HTAP, and HTHP) and habitat (mangrove, seagrass, and coral reef lagoon). Lack of significance is indicated by ns. ATAP, ambient temperature and ambient pCO2; ATHP, ambient temperature and high pCO2; HTAP, high temperature and ambient pCO2; and HTHP, high temperature and high pCO2.
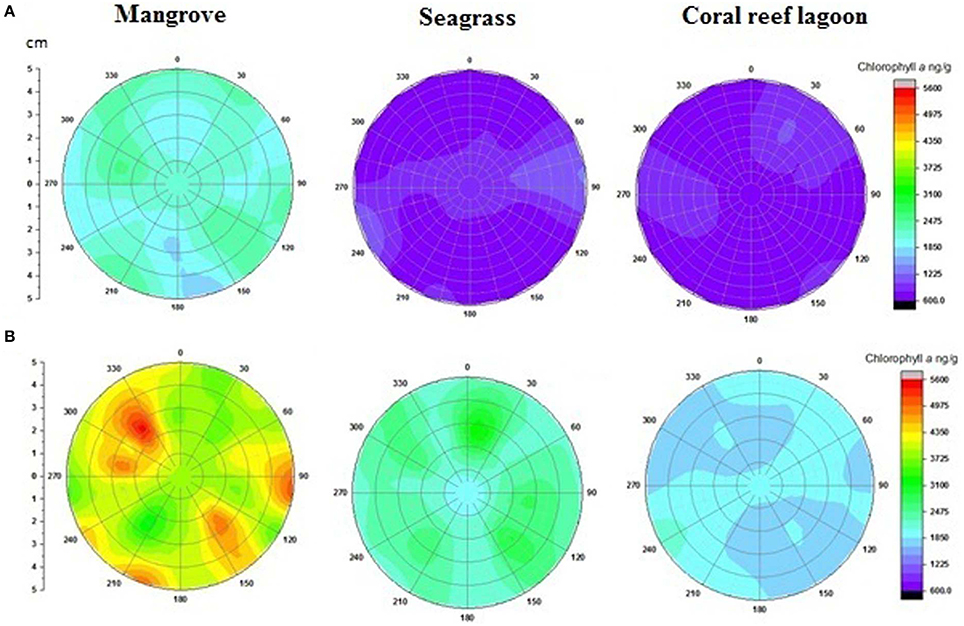
Figure 4. Changes in surficial chlorophyll a concentration in sediment. Initial (A) and after incubation in HTHP (high temperature and high pCO2) condition (B). The graph was derived from r (radius of the incubation chamber), θ (angles of sediment samples collection), and z (chlorophyll a concentration) data. Chlorophyll a concentrations in the incubation chambers' sediment surface are shown with colors. Corresponding chlorophyll a value for each color is presented on the color scale. Colors from purple to red represent lower to higher chlorophyll a concentrations.
Sediment Primary Production and Respiration
Oxygen fluxes increased significantly under HTHP stress during light periods in mangrove and seagrass habitats (Tukey's test, P < 0.001; Figure 5, Supplementary Table 5). In coral reef lagoons, stress increased oxygen fluxes. NPP and R varied depending on incubation conditions and habitat (ANOVA, P < 0.01; Table 2). NPP was comparatively higher under HTHP stress in mangrove (Tukey's test, P < 0.001) and seagrass (Tukey's test, P < 0.01) incubations than in the coral reef lagoon (Figure 6). Additionally, GPP and NPP in seagrass incubations were lower than in mangroves (Tukey's test, P < 0.01), but higher than in coral reef lagoon incubations. Neither high temperature nor high pCO2 alone had a significant impact on primary production (Tukey's test, P > 0.05; Figure 6), but temperature had a slightly greater influence. Respiration did not differ significantly under ATHP, HTAP, or HTHP treatment (Tukey's test, P > 0.05), with the exception of the coral reef lagoon (Tukey's test, P < 0.05; Table 2, Figure 6). Lagoonal sediment showed higher respiration (Tukey's test, P < 0.05) in the ATAP treatment, but it decreased under single or combined stresses, and this was reflected in the increased primary production.
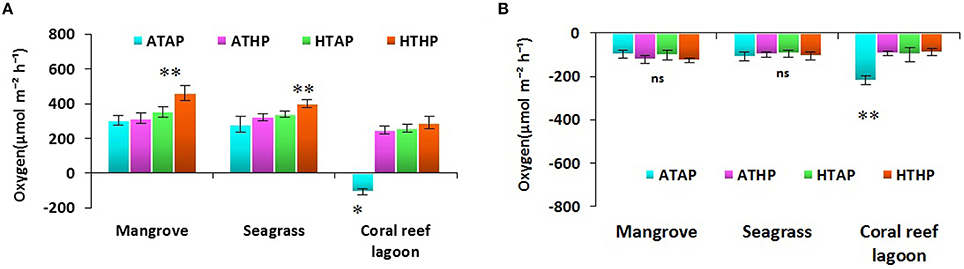
Figure 5. Sediment-water fluxes of oxygen during light (A) and dark (B) periods. *, ** Indicate significant differences at P < 0.01 and P < 0.001 based on the least significant difference test (post-hoc Tukey's test) depending on habitats (mangrove, seagrass, and coral reef lagoon). Lack of significance is indicated by ns. ATAP, ambient temperature and ambient pCO2; ATHP, ambient temperature and high pCO2; HTAP, high temperature and ambient pCO2; and HTHP, high temperature and high pCO2.
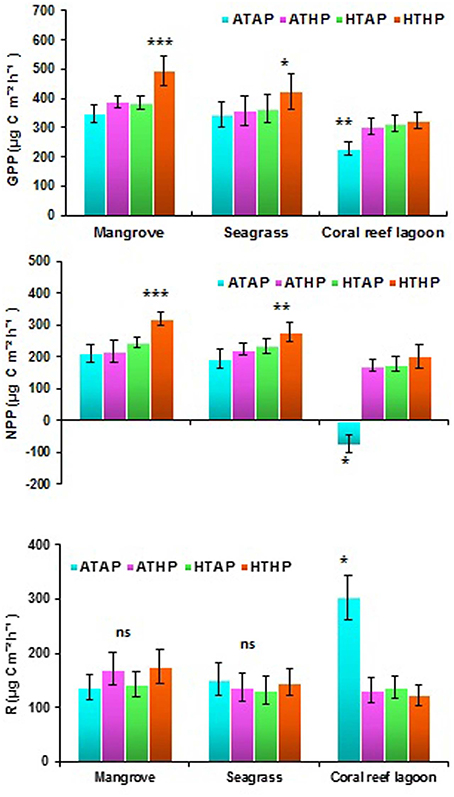
Figure 6. Gross primary production (GPP), net primary production (NPP), and respiration (R) in mangrove, seagrass, and coral reef sediment. *, **, and *** Indicate significant differences at P < 0.05, P < 0.01, and P < 0.001 determined using least significant difference tests (post-hoc Tukey's test) depending on incubation conditions (ATAP, ATHP, HTAP, and HTHP). Lack of significance is indicated by ns. ATAP, ambient temperature and ambient pCO2, ATHP, ambient temperature and high pCO2, HTAP, high temperature and ambient pCO2, and HTHP, high temperature and high pCO2.
Bacterial Abundance
Initial bacterial abundance in sediment was highest (Tukey's test, P < 0.05) in seagrass (19 ± 0.12 × 106 cells cm−2 in mangrove samples, 21 ± 1.1 × 106 cells cm−2 in seagrass samples, and 7 ± 1.0 × 106 cells cm−2 in coral reef sediments). Additionally, bacterial abundance in mangrove seawater was higher than other seawater samples (22 ± 2.0 × 105 cells mL−1 in the mangrove, 21 ± 1.1 × 105 cells mL−2 in the seagrass, and 5 ± 0.5 × 105 cells mL−1 in the coral reef lagoon). Bacterial abundance in sediment and seawater increased significantly by HTHP stress(Tukey's test, P < 0.01, Figures 7, 8), except in seagrass sediments. The impacts of ATHP and HTAP conditions were not significant in the cases of seagrass sediment and seawater, and coral reef lagoon seawater (Tukey's test, P > 0.05, Figure 8). In mangrove seawater and sediments, the impacts of HTAP stress were significantly greater than the impact of ATHP stress (Tukey's test, P < 0.01). Bacterial abundance in the surficial sediments increased after HTHP stress in all habitats, and this change was greater in mangrove sediments and lower in coral reef lagoon sediments. Under HTHP stress bacterial abundance increased 14 × 105–23 × 105 cells g−1, 8 × 105–16 × 105 cells g−1, and 5 × 105–8 × 105 cells g−1 in mangrove, seagrass, and coral reef lagoon sediments, respectively (Figure 7). In mangrove sediment and seawater, growth rate and doublings of bacteria increased significantly under HTHP stress (Tukey's test, P > 0.05, Supplementary Table 6). Similarly, in seagrass and coral reef lagoon seawater, bacterial growth rate and doublings were significantly impacted by HTHP stress (Tukey's test, P > 0.05, Supplementary Table 6).
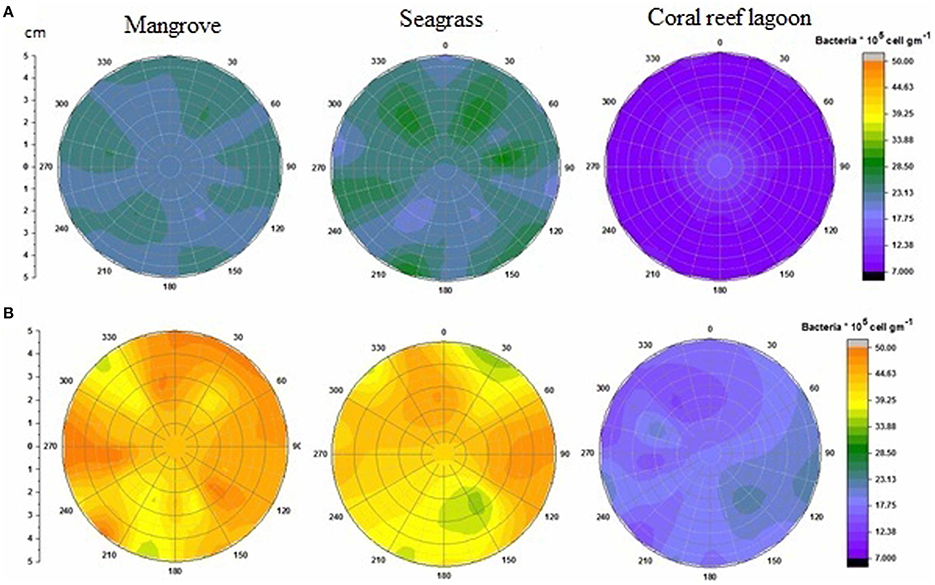
Figure 7. Changes in surficial bacterial abundance in sediment. Initial (A) and after incubation in HTHP (high temperature and high pCO2) conditions (B). The graph was derived from r (radius of the incubation chamber), θ (angles of sediment samples collection), and z (bacterial abundance) data. Bacterial abundances in the incubation chambers' sediment surface are shown. Corresponding bacterial abundance value for each color is presented in color scale. Purple to orange colors represent relatively low to high bacterial abundance.
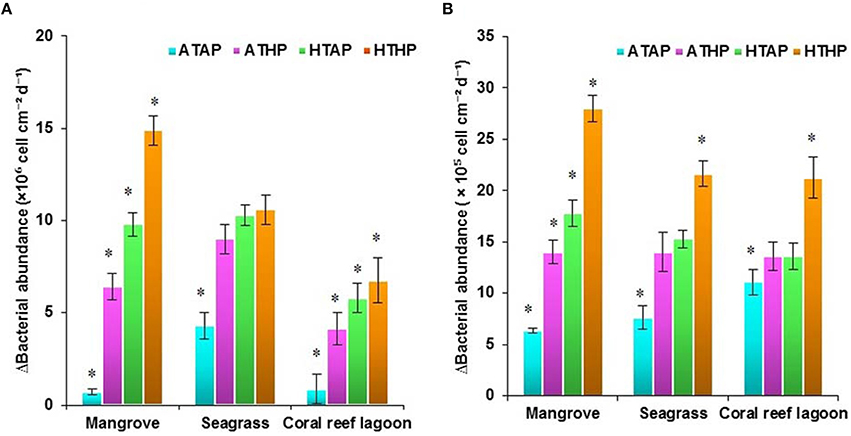
Figure 8. Changes in bacterial abundance in sediment (A) and seawater (B). *Indicates a significantly difference at P < 0.01 determined using a least significant difference test (post-hoc Tukey's test) depending on incubation conditions (ATAP, ATHP, HTAP, and HTHP). ATAP, ambient temperature and ambient pCO2; ATHP, ambient temperature and high pCO2; HTAP, high temperature and ambient pCO2; and HTHP, high temperature and high pCO2.
Discussion
The responses of subtropical shallow marine sediment systems to elevated temperature and pCO2 varied depending on the habitats from which the sediments were collected. In this study, we used sediments collected with cores to test the responses of relatively undisturbed sediments. Changes in primary production in response to a temperature increase of 4°C and 936 ppm pCO2 were detected. The following general responses were observed in this study. (1) Under HTHP stress, sediment primary production rates were higher in mangrove and seagrass environments than in the coral reef lagoon. (2) Nutrients flux was lower in the coral reef lagoon. Silicate and nitrate + nitrite were noticeably consumed in the mangrove and seagrass. (3) Bacterial abundance in sediment and seawater generally increased under ATHP, HTAP, and HTHP. Growth rate and doublings indicated increased the activity of bacteria in seawater (mangrove, seagrass, and coral reef lagoon) and sediment (mangrove) under HTHP conditions.
Effect of Stressors on Primary Production and Bacterial Abundance
Previous studies have suggested that the combination of warming and OA positively stimulate benthic microalgal primary production in seagrass habitats, and benthic microalgae appear to be resilient to future environmental changes (Alsterberg et al., 2013). Our incubation experiments indicated increased NPP and GPP at the sediment–water interface under HTHP stress, with the exception of the coral reef lagoon. OA may cause stimulated diatom activity in benthic assemblages (Johnson et al., 2013). The impacts of temperature (HTAP) on NPP, GPP, and R were slightly, but not significantly higher than those of pCO2 (ATHP). Significant differences in NPP (Tukey's test, P > 0.01) and GPP (Tukey's test, P > 0.05) under the HTHP stress suggested that benthic microalgae in mangrove and seagrass sediments are more responsive to HTHP stress than those in coral reef lagoon sediments. Previously, it has been suggested that microphytobenthic assemblages, including cyanobacteria, phototrophic bacteria, and microalgal mats, increase rapidly in shallow marine environments, such as intertidal flats, coastal embayments, and lagoons (MacIntyre et al., 1996). GPP in tropical mangrove sediments was much higher during the warm season owing to the greater seasonal temperature range (Alongi, 1994). Moreover, phototrophic microphytobenthos are important primary producers, contributing almost half of the primary production in estuarine ecosystems (Underwood and Kromkamp, 1999). Our results suggest that only HTHP stress significantly stimulated sediment primary production in mangrove and seagrass sediments. In contrast, sediment primary production in the coral reef lagoon was stimulated by stress, but the result was not statistically significant; however, decreased R and dark oxygen flux under ATHP, HTAP, and HTHP treatments in lagoonal incubations caused a shift from heterotrophy to autotrophy. Nonsignificant changes in respiration in different habitats under HTHP stress suggest that heterotrophic processes were less affected by HTHP stress than autotrophic processes. Similar results were observed by Alsterberg et al. (2012) for oxygen flux in seagrass sediment under elevated temperature. Furthermore, Johnson et al. (2013) suggested that future changes in CO2 and bicarbonate () availability could stimulate photosynthesis in marine autotrophs.
Previously, it has been suggested that microphytobenthic productivity is correlated with chlorophyll a content (MacIntyre et al., 1996). Based on our primary production data, photosynthetic community activity was stimulated by high temperatures and high pCO2. However, not only high temperature (Alsterberg et al., 2012), but also high pCO2 significantly increased the chlorophyll a content at the sediment surface. These increases are associated with increased NPP, indicating that HTHP conditions stimulate primary production activity by the photosynthetic community in all habitats. Tracer pigment composition and microscopic observations indicated dominance of diatoms in all sediment types. This result was consistent with those of Hendrarto and Nitisuparjo (2011). CO2 enrichment can cause significant increases in chlorophyll a concentrations and in diatom abundance (Johnson et al., 2013). In our incubation experiments, significant increases in pigment concentrations (chlorophyll a, fucoxanthin, and zeaxanthin) were observed, except in the coral reef lagoon. These results revealed that photosynthetic communities inhabiting mangroves and seagrasses more strongly fuel sediment primary production compared to those of coral reef lagoons. These patterns indicate that new organic matter was produced during incubation. The highest nutrient consumption (e.g., ammonium, phosphate, and silicate) and the greatest increase in bacterial abundance were observed in mangrove sediments (Figure 8); these results are supported by the increased nutrient uptake during dark periods in these sediments. Increased bacterial abundance under HTHP conditions may be a direct effect of warming (Sander and Kalff, 1993). However, increased bacterial abundance could be partially dependent on bottom-up effects, since bacteria are considered to use dissolved organic matter produced by benthic microalgae (Evrard et al., 2008; Casareto et al., 2012).
Nutrient Dynamics
Microphytobenthos and mat-forming macroalgae have different direct and indirect effects on nitrogen-associated processes via consumption and the inhibition or stimulation of microbial activity (Bartoli et al., 2012). The rate of nutrient uptake, particularly of silicate, ammonium (not in the coral reef lagoon), and nitrate + nitrite, seemed to be released under HTHP conditions. Higher uptake of silicate in seagrass and mangrove incubations (Figure 2) was associated with a higher initial concentration (Supplementary Table 3). A previous study suggested that a relatively higher abundance of silicate compared to nitrate and phosphate occurs in fresh water-influenced coastal zones (Cloern et al., 1985). However, the consumption of silicate reflected the predominant activity of benthic diatoms. This finding is consistent with those of Alsterberg et al. (2011) and Alsterberg and Sundbäck (2013). Diatoms benefit from increasing CO2 via a reduction in the energetic costs of their carbon-concentrating mechanism (Hopkinson et al., 2011). The tracer pigment data indicated an increased abundance of fucoxanthin (Figure 3) and zeaxanthin, indicating stimulated activity of diatoms and cyanobacteria in mangrove and seagrass incubations. In coral reef incubations, the activity of cyanobacteria increased as evidenced by an increase in the zeaxanthin concentration; slightly higher uptake of silicate under ATHP, HTAP, and HTHP treatments indicated stimulated activity of diatoms in lagoonal incubations. The release of ammonium in lagoonal incubations under ATHP and HTHP treatments indicated increased nitrogen mineralization, consistent with the results of Alsterberg et al. (2012). Initially higher concentrations of nutrients in pore water than that of overlying seawater implied that biological processes could be more important than physical diffusion in determining nutrient fluxes. Therefore, microbes may play an important role consuming nutrients (Arístegui et al., 2009; Miyajima, 2015) in all habitats, and an increase in bacterial abundance could suggest increased nutrient uptake (Ye et al., 2012).
Tentative Biogeochemical Implications
Our results suggest that HTHP stress has a major impact on primary production, especially on carbon cycling. Sediments from mangrove and seagrass habitats are more productive than those from coral reef lagoons, suggesting that they act as potential carbon sinks. Significantly increased uptake of nutrients (particularly silicate) in mangrove and seagrass sediments may enhance the role of sediments as nutrient sinks. Oxygen fluxes and increased bacterial abundance suggest that microbial community activity increased in these sediments, but did not exceed that of autotrophic compartments. In mangroves, community respiration may increase slightly, but not enough to increase heterotrophy owing to the capacity of primary producers to photosynthesize under stressful conditions. Significantly enhanced sediment primary production in shallow marine mangroves and seagrass sediments compared to coral reef lagoon sediments provided evidence for positive responses to HTHP stress with concomitant carbon storage.
Conclusions
The combined impact of increased temperature and pCO2 associated with climate change differs significantly from the impact of individual stressors, and can result in nonlinear effects and unexpected ecological patterns (Segner et al., 2014). To our knowledge, this is the first demonstration of differences in subtropical sediment systems between mangrove, seagrass, and coral reef lagoon habitats of Okinawa, Japan under high temperature and OA. A synergistic increase in sediment primary production was observed under the combination of stressors (HTHP), except in the coral reef lagoon. Elevated temperature and pCO2 stimulated primary production, autotrophic activity, bacterial abundance, and nutrient uptake. Greater carbon fixation in mangrove and seagrass sediments suggests that they have increased resilience compared to lagoonal sediment. However, lagoonal sediment also responded positively via a shift from heterotrophy to autotrophy under single (ATHP, HTAP) or combined stressors (HTHP). These results provide a basis for further studies to understand the responses of different shallow marine habitats to environmental stress. However, further efforts are needed to assess whether the roles proposed for mangroves, seagrass, and coral reef lagoon habitats within this study can be applied to a broader spatiotemporal scale.
Author Contributions
Conceived and designed the experiments: RS, BC, HF, and YS. Performed the experiments: RS, BC, TS, and YS. Analyzed the data: RS, BC, RS, TS and MA. Contributed reagents/materials/analysis tools: RS, BC, RS, TS, MA, HF, and YS. Wrote the paper: RS, BC, RS, TS, MA, HF, and YS.
Funding
This study was supported by the Environmental Leaders Program of Shizuoka University (ELSU), a Grant-in-aid for Scientific Research on Innovative Areas “Coral reef science for symbiosis and coexistence of humans and ecosystems under combined stresses” (No. 20121003) of the Ministry of Education. Culture, Sports, Science and Technology (MEXT), Japan and the Global Coral Reef Conservation Project (GCRCP) of the Mitsubishi Corporation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful for the support of the staff at Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, Japan for providing necessary facilities during this experiment. The authors are also thankful to the members of Marine Ecosystem and Biogeochemistry Laboratory, Shizuoka University, Japan. We are grateful for the help of Dr. Tomihiko Higuchi, The University of Tokyo, Japan during sample analysis. We thank Dr. Mohan Prasad Niraula, Tribhuvan University, Nepal for providing constructive comments on earlier versions of this manuscript. The authors would like to thank Mr. S. Uehara for his help with fieldwork and sample collection. Special thanks to Mr. K. Toyoda who helped with benthic microalgal identification. We thank two anonymous reviewers for constructive comments.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2016.00100
References
Alongi, D. M. (1994). Zonation and seasonality of benthic primary production and community respiration in tropical mangrove forests. Oecologia 98, 320–327. doi: 10.1007/BF00324220
Alsterberg, C., Eklöf, J. S., Gamfeldt, L., Havenhand, J. N., and Sundbäck, K. (2013). Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proc. Natl. Acad. Sci. U.S.A. 110, 8603–8608. doi: 10.1073/pnas.1303797110
Alsterberg, C., Hulth, S., and Sundback, K. (2011). Response of a shallow-water sediment system to warming. Limnol. Oceanogr. 56, 2147–2160. doi: 10.4319/lo.2011.56.6.2147
Alsterberg, C., and Sundbäck, K. (2013). Experimental warming and toxicant exposure can result in antagonistic effects in a shallow-water sediment system. Mar. Ecol. Prog. Ser. 488, 89–101. doi: 10.3354/meps10357
Alsterberg, C., Sundbäck, K., and Hulth, S. (2012). Functioning of a shallow-water sediment system during experimental warming and nutrient enrichment. PLoS ONE 7:e51503. doi: 10.1371/journal.pone.0051503
Arístegui, J., Mendonc, A., Vilas, J. C., Espino, M., Polo, I., Montero, M. F., et al. (2009). Plankton metabolic balance at two North Atlantic seamounts. Deep Sea Res. II 56, 2646–2655. doi: 10.1016/j.dsr2.2008.12.025
Aswani, S., Mumby, P., Baker, A. C., Christie, P., McCook, L. J., Steneck, R. S., et al. (2015). Scientific frontiers in the management of coral reefs. Front. Mar. Sci. 2:50. doi: 10.3389/fmars.2015.00050
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. doi: 10.1890/10-1510.1
Bartoli, M., Castaldelli, G., Nizzoli, D., and Viaroli, P. (2012). Benthic primary production and bacterial denitrification in a Mediterranean eutrophic coastal lagoon. J. Exp. Mar. Biol. Ecol. 438, 41–51. doi: 10.1016/j.jembe.2012.09.011
Beck, M. W., Heck, K. L., Able, K. W., Childers, D. L., Eggleston, D. B., Gillanders, B. M., et al. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51, 633. doi: 10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Bouillon, S., Borges, A. V., Castaneda-Moya, E., Diele, K., Dittmar, T., Duke, N. C., et al. (2008). Mangrove production and carbon sinks: a revision of global budget estimates. Glob. Biogeochem. Cycles 22, 12. doi: 10.1029/2007GB003052
Brito, A., Newton, A., Tett, P., and Fernandes, T. F. (2010). Sediment and water nutrients and microalgae in a coastal shallow lagoon, Ria Formosa (Portugal): implications for the Water Framework Directive. J. Environ. Monit. 12, 318–328. doi: 10.1039/b909429f
Buchanan J. B. (1984). Sediment Analysis, in Methods for the Study of Marine Benthos, eds. N. A. Holme, and A. D. McIntyre (London: Blackwell Scientific Publications), 41–65.
Casareto, B. E., Niraula, M. P., Fujimura, H., and Suzuki, Y. (2009). Effects of carbon dioxide on the coccolithophorid Pleurochrysis carterae in incubation experiments. Aquat. Biol. 7, 59–70. doi: 10.3354/ab00182
Casareto, B. E., Niraula, M. P., and Suzuki, Y. (2012). Dynamics of organic carbon under different inorganic nitrogen levels and phytoplankton composition. Estuar. Coast. Shelf Sci. 102-103, 84–94. doi: 10.1016/j.ecss.2012.03.019
Chihara, M., and Murano, M. (1997). An Illustrated Guide to Marine Plankton in Japan. Tokyo: Tokai University Press.
Chmura, G. L. (2013). What do we need to assess the sustainability of the tidal salt marsh carbon sink? Ocean Coast. Manage. 83, 25–31. doi: 10.1016/j.ocecoaman.2011.09.006
Cloern, J. E., Cole, B. E., Wong, R. L. J., and Alpine, A. E. (1985). Temporal dynamics of estuarine phytoplankton: a case study of San Francisco Bay. Hydrobiologia 129, 153–176. doi: 10.1007/BF00048693
Connell, S. D., Kroeker, K. J., Fabricius, K. E., Kline, D. I., and Russell, B. D. (2013). The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Proc. R. Soc. B Biol. Sci. 368, 1–9. doi: 10.1098/rstb.2012.0442
Cornwell, J. C., Glibert, P. M., and Owens, M. S. (2014). Nutrient fluxes from sediments in the San Francisco Bay delta. Estuar. Coast 37, 1120–1133. doi: 10.1007/s12237-013-9755-4
Cullen-Unsworth, L., and Unsworth, R. (2013). Seagrass meadows, ecosystem services, and sustainability. Environ. Sci. Policy Sus. Dev. 55, 14–28. doi: 10.1080/00139157.2013.785864
Del Giorgio, P., and Williams, P. (2005). Respiration in Aquatic Ecosystems. New York, NY: Oxford University Press.
Delille, B., Harlay, J., Zondervan, I., Jacquet, S., Chou, L., Wollast, R., et al. (2005). Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorid Emiliania huxleyi. Glob. Biogeochem. Cycles 19, GB2023. doi: 10.1029/2004GB002318
Dickson, A. G., Sabine, C. L., and Christian, J. R. (2007). Guide to Best Practices for Ocean CO2 Measurements. PICES special publication 3, IOCCP Report No.8.
Donato, D. C., Kauffman, J. B., Mackenzie, R. A., Ainsworth, A., and Pfleeger, A. Z. (2012). Whole-island carbon stocks in the tropical Pacific: implications for mangrove conservation and upland restoration. J. Env. Manage. 97, 89–96. doi: 10.1016/j.jenvman.2011.12.004
Duarte, C. M., Marbà, N., Gacia, E., Fourqurean, J. W., Beggins, J., Barrón, C., et al. (2010). Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Glob. Biogeochem. Cycles 24, 1–8. doi: 10.1029/2010GB003793
Duarte, C. M., Middelburg, J. J., and Caraco, N. (2005). Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2, 1–8. doi: 10.5194/bg-2-1-2005
Engel, A., Schulz, K., Riebesell, U., Bellerby, R., Delille, B., and Schartau, M. (2007). Effects of CO2 on particle size distribution and phytoplankton abundance during a mesocosm bloom experiment (PeECE II). Biogeosciences 5, 509–521. doi: 10.5194/bg-5-509-2008
Evrard, V., Cook, P. L. M., Veuger, B., Huettel, M., and Middelburg, J. J. (2008). Tracing carbon and nitrogen incorporation and pathways in the microbial community of a photic subtidal sand. Aquat. Microb. Ecol. 53, 257–269. doi: 10.3354/ame01248
Folt, C. L., Chen, C. Y., Moore, M. V., and Burnaford, J. (1999). Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. doi: 10.4319/lo.1999.44.3_part_2.0864
Gao, K., Aruga, Y., Asada, K., and Kiyohara, M. (1993). Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J. Appl. Phycol. 5, 563–571. doi: 10.1007/BF02184635
Hansen, H. P., and Koroleff, F. (2007). Determination of nutrients, in Methods of Seawater Analysis, eds K. Grasshoff, K. Kremling, and M. Ehrhardt (Weinheim: Wiley-VCH Verlag GmbH), 159–228.
Hendrarto, I. B., and Nitisuparjo, M. (2011). Biodiversity of benthic diatom and primary productivity of benthic micro-flora in mangrove forests on central Java. J. Coast. Dev. 14, 131–140. Available online at: http://www.omicsonline.com/open-access/biodiversity-of-benthic-diatom-and-primary-productivity-of-benthic-microflora-in-mangrove-forests-on-central-java-1410-5217-14-309.pdf
Hopkinson, B. M., Dupont, C. L., Allen, A. E., and Morel, F. M. M. (2011). Efficiency of the CO2-concentrating mechanism of diatoms. Proc. Nat. Acad. Sci. U.S.A. 108, 3830–3837. doi: 10.1073/pnas.1018062108
Intergovernmental Panel on Climate Change (IPCC) (2013). Climate Change 2013 The Physical Science Basis Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 2013.
Jennerjahn, T. C., and Ittekkot, V. (2002). Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwissenschaften 89, 23–30. doi: 10.1007/s00114-001-0283-x
Johnson, V. R., Brownlee, C., Rickaby, R. E. M., Graziano, M., and Milazzo, M. (2013). Responses of marine benthic microalgae to elevated CO2. Mar. Biogl. 160, 1813–1824. doi: 10.1007/s00227-011-1840-2
Kayanne, H., Hata, H., Kudo, S., Yamano, H., Watanabe, A., Ikeda, Y., et al. (2005). Seasonal and bleaching-induced changes in coral reef metabolism and CO2 flux. Glob. Biogeochem. Cycles 19, GB3015. doi: 10.1029/2004GB002400
Kennedy, H., Beggins, J., Duarte, C. M., Fourqurean, J. W., Holmer, M., Marbá, N., et al. (2010). Seagrass sediments as a global carbon sink: isotopic constraints. Glob. Biogeochem. Cycles 24, 1–8. doi: 10.1029/2010GB003848
Lewis, E., and Wallace, D. W. R. (1998). Program developed for CO2 system calculations. ORNL/CDIAC-105, in Carbon Dioxide Information Analysis Center (Oak Ridge, TN: Oak Ridge National Laboratory, U.S. Department of Energy).
Libes, S. (2009). Organic matter: Production and destruction, in Introduction to Marine Biogeochemistry, ed S. Libes (Elsevier Inc.), 207–220.
MacIntyre, H. L., Geider, R. J., and Miller, D. C. (1996). Microphytobenthos: the ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. I. Distribution, abundance and primary production. Estuaries 19, 186–201. doi: 10.2307/1352224
Mcleod, E., Chmura, G. L., Bouillon, S., Salm, R., Björk, M., Duarte, C. M., et al. (2011). A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9:4. doi: 10.1890/110004
Mehrbach, C., Culberso, C. H., Hawley, J. E., and Pytkowic, R. M. (1973). Measurement of apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907. doi: 10.4319/lo.1973.18.6.0897
Millero, F. J. (1979). The thermodynamics of the carbonate system in seawater. Geochim. Cosmochim. Acta 43, 1651–1661. doi: 10.1016/0016-7037(79)90184-4
Miyajima, T. (2015). Abiotic versus biotic immobilization of inorganic nitrogen in sediment as a potential pathway of nitrogen sequestration from coastal marine ecosystems. Geochem. J. 49, 453–468. doi: 10.2343/geochemj.2.0370
Miyajima, T., Hori, M., Hamaguchi, M., Shimabukuro, H., Adachi, H., Yamano, H., et al. (2015). Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows. Glob. Biogeochem. Cycles 29, 397–415. doi: 10.1002/2014GB004979
Montoya, J. M., and Raffaelli, D. (2010). Climate change, biotic interactions and ecosystem services. Phil. Trans. R. Soc. Lond. B 365, 2013–2018. doi: 10.1098/rstb.2010.0114
Mumby, P. J. (2006). Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales. Biol. Cons. 128, 215–222. doi: 10.1016/j.biocon.2005.09.042
Nakano, Y., and Nakai, T. (2008). Succeeding to consider reef and humans: a comprehensive view of the enlightenment for coral reef conservation. J. Jpn. Coral Reef Soc. 10, 105–115. doi: 10.3755/jcrs.10.105
Pendleton, L., Donato, D. C., Murray, B. C., Crooks, S., Jenkins, W. A., Sifleet, S., et al. (2012). Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 7:e43542. doi: 10.1371/journal.pone.0043542
Porter, K. G., and Feig, Y. S. (1980). The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25, 943–948. doi: 10.4319/lo.1980.25.5.0943
Qiu, B., and Gao, K. (2002). Effects of CO2 enrichment on the bloomforming cyanobacterium Microcystis aeruginosa (Cyanophyceae): physiological responses and relationships with the availability of dissolved inorganic carbon. J. Phycol. 38, 721–729. doi: 10.1046/j.1529-8817.2002.01180.x
Ricart, A. M., York, P. H., Rasheed, M. A., Pérez, M., Romero, J., Bryant, C. V., et al. (2015). Variability of sedimentary organic carbon in patchy seagrass landscapes. Marine Poll. Bull. 100, 476–482. doi: 10.1016/j.marpolbul.2015.09.032
Riebesell, U., Zondervan, I., Rost, B., Tortell, P. D., Zeebe, R. E., and Morel, F. M. (2000). Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407, 364–367. doi: 10.1038/35030078
Robertson, A. I., and Alongi, D. M. (2013). Tropical Mangrove Ecosystems. Washington, DC: American Geophysical Union.
Sander, B. B., and Kalff, J. (1993). Factors controlling bacterial production in marine and freshwater sediments. Microb. Ecol. 26, 79–99. doi: 10.1007/BF00177045
Segner, H., Schmitt-Jansen, M., and Sabater, S. (2014). Assessing the impact of multiple stressors on aquatic biota: the receptor's side matters. Environ. Sci. Technol. 48, 7690–7696. doi: 10.1021/es405082t
Sifleet, S., Pendleton, L., and Murray, B. C. (2011). State of the Science on Coastal Blue Carbon a Summary for Policy Makers. (Durham, NC: Nicholas Institute for Environmental Policy Solutions).
Stanier, R. Y., Adelverg, E. A., Ingraham, J. L., and Wheelis, M. L. (1979). Microbial growth, in Introduction to the Microbial World, (Englewood Cliffs, NJ: Prentice-Hall).
Stumm, W., and Morgan, J. (1981). Aquatic Chemistry: an Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd Edn. New York, NY: Wiley Interscience.
Sundback, K., Alsterberg, C., and Larson, F. (2010). Effects of multiple stressors on marine shallow-water sediments: response of microalgae and meiofauna to nutrient-toxicant exposure. J. Exp. Mar. Biol. Ecol. 388, 39–50. doi: 10.1016/j.jembe.2010.03.007
Suzuki, T., Casareto, B. E., Shioi, Y., Ishikawa, Y., and Suzuki1, Y. (2015). Finding of 132, 173-cyclopheophorbide a enol as a degradation product of chlorophyll in shrunk zooxanthellae of the coral Montipora digitata. J. Phycol. 51, 37–45. doi: 10.1111/jpy.12253
Underwood, G. J. C., and Kromkamp, J. (1999). Primary production by phytoplankton and microphytobenthos in estuaries. Adv. Ecol. Res. 29, 93–153. doi: 10.1016/S0065-2504(08)60192-0
Weiss, R. (1974). Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar. Chem. 2, 203–215. doi: 10.1016/0304-4203(74)90015-2
Winder, M., and Schindler, D. E. (2004). Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85, 2100–2106. doi: 10.1890/04-0151
Woodward, G., Perkins, D. M., and Brown, L. E. (2010). Climate change and freshwater ecosystems: impacts across multiple levels of organization. Phil. Trans. R. Soc. Lond. B 365, 2093–2106. doi: 10.1098/rstb.2010.0055
Ye, L., Shi, X., Wu, X., and Kong, F. (2012). Nitrate limitation and accumulation of dissolved organic carbon during a spring–summer cyanobacterial bloom in Lake Taihu (China). J. Limnol. 71, 67–71. doi: 10.4081/jlimnol.2012.e6
Zapata, M., Rodríguez, F., and Garrido, J. L. (2000). Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 195, 29–45. doi: 10.3354/meps195029
Zimmerman, R. C., Kohrs, D. G., Steller, D. L., and Alberte, R. S. (1997). Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant Physiol. 115, 599–607.
Keywords: primary production, temperature, pCO2, nutrients and bacterial abundance
Citation: Sultana R, Casareto BE, Sohrin R, Suzuki T, Alam MS, Fujimura H and Suzuki Y (2016) Response of Subtropical Coastal Sediment Systems of Okinawa, Japan, to Experimental Warming and High pCO2. Front. Mar. Sci. 3:100. doi: 10.3389/fmars.2016.00100
Received: 04 March 2016; Accepted: 06 June 2016;
Published: 28 June 2016.
Edited by:
Hajime Kayanne, University of Tokyo, JapanReviewed by:
David I. Kline, University of California, San Diego, USAAtsushi Watanabe, Tokyo Institute of Technology, Japan
Copyright © 2016 Sultana, Casareto, Sohrin, Suzuki, Alam, Fujimura and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beatriz E. Casareto, ZGNiZWF0ckBpcGMuc2hpenVva2EuYWMuanA=; Y2FzYXJldG9iZUBnbWFpbC5jb20=
 Rumana Sultana
Rumana Sultana Beatriz E. Casareto
Beatriz E. Casareto Rumi Sohrin
Rumi Sohrin Toshiyuki Suzuki
Toshiyuki Suzuki Md. Shafiul Alam
Md. Shafiul Alam Hiroyuki Fujimura
Hiroyuki Fujimura Yoshimi Suzuki
Yoshimi Suzuki