
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 07 April 2016
Sec. Marine Conservation and Sustainability
Volume 3 - 2016 | https://doi.org/10.3389/fmars.2016.00043
 Vanessa F. Jaiteh1,2*
Vanessa F. Jaiteh1,2* Steve J. Lindfield3
Steve J. Lindfield3 Sangeeta Mangubhai4,5
Sangeeta Mangubhai4,5 Carol Warren2
Carol Warren2 Ben Fitzpatrick6
Ben Fitzpatrick6 Neil R. Loneragan1,2
Neil R. Loneragan1,2Fisheries are complex social-ecological systems, where managers struggle to balance the socio-economic interests of fishing communities with the biology and ecology of fisheries species. Spatial closures are a popular measure to address conservation and fisheries management goals, including the protection of shark populations. However, very little research has been published on the effectiveness of shark-specific closures to protect sharks, or their impacts on fisher behavior. Situated within the global center of tropical marine biodiversity, Indonesia's shark fishery contributes more to the international shark fin trade than any other nation. Here we evaluate the effect of shark-specific closures on sharks and other species of interest, as well as shark fishers' responses to losing access to their former fishing grounds. We assessed shark diversity and abundance in an open access zone (OAZ) and two No-Take Zones (NTZs) of a Marine Protected Area within the recently established shark sanctuary in Raja Ampat, Indonesia, where sharks have high monetary value as a tourism attraction. Shark abundance was significantly higher in the privately managed NTZs than in the OAZ. Across all management zones, neither zone size, depth nor reef complexity explained variations in shark abundance, suggesting that governance is the main driver of successful shark conservation areas. These trends were also reflected in species targeted by small-scale reef fisheries, including snappers, emperor, groupers, tunas, mackerels, and large-bodied wrasse and parrotfish. Interviews with shark fishers who lost access to their primary fishing grounds when the shark sanctuary was established showed that while most fishers (88%) knew that sharks were protected in Raja Ampat, many were unsure about the purpose of the sanctuary. Few fishers felt that the agencies implementing fishing bans understood their livelihood needs. We found that shark fishers adapted to the loss of former fishing grounds by shifting fishing effort to other locations or diversifying their livelihoods, including illegal petrol transport. While conserving sharks for tourism can be effective, it may inadvertently result in displacing fishing effort to unprotected regions. We propose that effective shark conservation in Indonesia will need to combine strategic spatial protection with efforts to support livelihood security and diversification.
Shark and ray populations have experienced widespread declines in recent years (Baum et al., 2003; Robbins et al., 2006; Ferretti et al., 2008; Ward-Paige et al., 2010; Worm et al., 2013). While the extent of these declines is debated (Burgess et al., 2005; Heupel et al., 2009; Braccini, 2015), there is general consensus that they are primarily caused by elevated fishing mortality through targeted fisheries that supply shark and manta ray products, and bycatch in other fisheries (Friedlander and Demartini, 2002; Dulvy et al., 2008; Davidson et al., 2015). Over the last decade, scientists and conservation practitioners have highlighted the urgent need for improved fisheries management to stem the large-scale exploitation of shark and ray species, many of which are critically important apex predators and valuable marine tourism assets (Heithaus et al., 2010; Gallagher and Hammerschlag, 2011; Vianna et al., 2012; Dulvy et al., 2014). In countries where a large part of the population has a high dependency on marine resources for livelihoods and protein, assessments and management of these fisheries are often hindered by the presence of extensive fleets of unregistered vessels and widespread unregulated small-scale fisheries, as well as a lack of enforcement of existing regulations on registered vessels (Blaber et al., 2009). Moreover, the livelihoods, wellbeing and likely responses of small-scale fishers are often insufficiently accounted for when management measures, such as spatial closures, are implemented (Christie, 2004; West et al., 2006).
Spatial closures, such as multiple use marine protected areas (MPAs) and no-take marine reserves, are one fisheries management strategy that has been implemented to slow and reverse the effects of large-scale overfishing on shark populations (Ward-Paige et al., 2012). Shark protection is increasingly used as a justification for implementing MPAs or expanding No-Take Zones (NTZs) within MPAs, and more recently for establishing areas where shark fishing is explicitly banned (Hoyt, 2014; Gallagher et al., 2015). Known as shark sanctuaries or shark reserves (shark sanctuaries hereafter), they afford sharks blanket protection, often in a jurisdiction's entire Exclusive Economic Zone (EEZ). Palau was the first nation to declare its EEZ a shark sanctuary in 2009, followed by the Marshall Islands, Federated States of Micronesia, French Polynesia, Cook Islands, the Bahamas, and New Caledonia. In many jurisdictions with shark sanctuaries, the contribution of shark fisheries to the local economy is outweighed by the income generated through marine tourism, to which sharks have become important assets (Vianna et al., 2012; Davidson et al., 2015).
The need—and struggle—to balance the socio-economic interests of fishing communities with the ecological goals of spatial protection is well discussed in the literature (Sumaila et al., 2000; Mascia et al., 2010; Pollnac et al., 2010; Ban et al., 2011). In terms of shark-specific closures, however, very little research has been published on their ecological impact on shark populations (White et al., 2015) and the socio-economic dependence and responses of fishers to these fishing ground closures. This may be due to the fact that many shark sanctuaries have been established where live sharks have more value than dead ones, and that research has focused largely on evaluating the economic benefits of shark protection for tourism (Brunnschweiler, 2010; Clua et al., 2011; Vianna et al., 2011) rather than the potential impacts on fishers. However, if sanctuaries are implemented where they are arguably most needed, that is, in regions with significant shark fisheries, exploring potential effects of closures on fishers' behavior is not only important to ensure the welfare of fishing communities, but also to increase the success of shark protection within the closure and beyond its boundaries.
Indonesia has consistently reported annual shark catches of approximately 100,000 tons since the year 2000 (FAO FishStatJ, 2015). Although this is likely an underestimate of true catches, these numbers place Indonesia as the number one exporter of shark products in the world. In many ways, shark fishing is an optimal livelihood for fishers in remote parts of Indonesia as the valuable dried fins can be easily stockpiled in the absence of power and refrigerated transport (Momigliano et al., 2014). The shark fin industry is so lucrative that it has transformed several remote coastal communities in Eastern Indonesia from predominantly subsistence-based fishing villages to cash-based economies (Mangubhai et al., 2012). This departure from subsistence fishing makes it difficult for shark fishers to engage in alternative livelihoods, since there are few legal, marine-based alternatives offering similar financial profit (Whitcraft et al., 2014).
The Raja Ampat regency in far Eastern Indonesia lies in the heart of the Coral Triangle, which encompasses the world's most biodiverse coral reefs (Veron et al., 2009; Allen and Erdmann, 2012). The richness of these reefs has attracted the attention of local and regional fishers as well as divers and conservation groups from around the world. In the early 2000s, as shark fishers from neighboring provinces increasingly focused their fishing efforts on Raja Ampat (Jaiteh, unpublished data), private tourism entrepreneurs, the provincial government and non-government organizations (NGOs) also began promoting Raja Ampat as a world-class dive tourism destination and invested significant efforts to protect the biodiversity of these reefs both for tourism and to support local Papuan fisheries. In 2006, a network of MPAs was established within Raja Ampat. In 2005 and 2010, the Misool Eco Resort (MER) in Southeast Misool MPA (hereafter “SE Misool MPA,” Figure 1) created two large NTZs to protect sharks and other fishery-targeted species through lease agreements with local communities (Table 1). Following an international petition led by MER and US-based NGO Shark Savers, The Nature Conservancy and Conservation International worked with the Raja Ampat regency to develop a decree that was passed by parliament in November 2012 and banned commercial and artisanal fishing for all sharks and mobulid rays throughout the regency (Table 1).
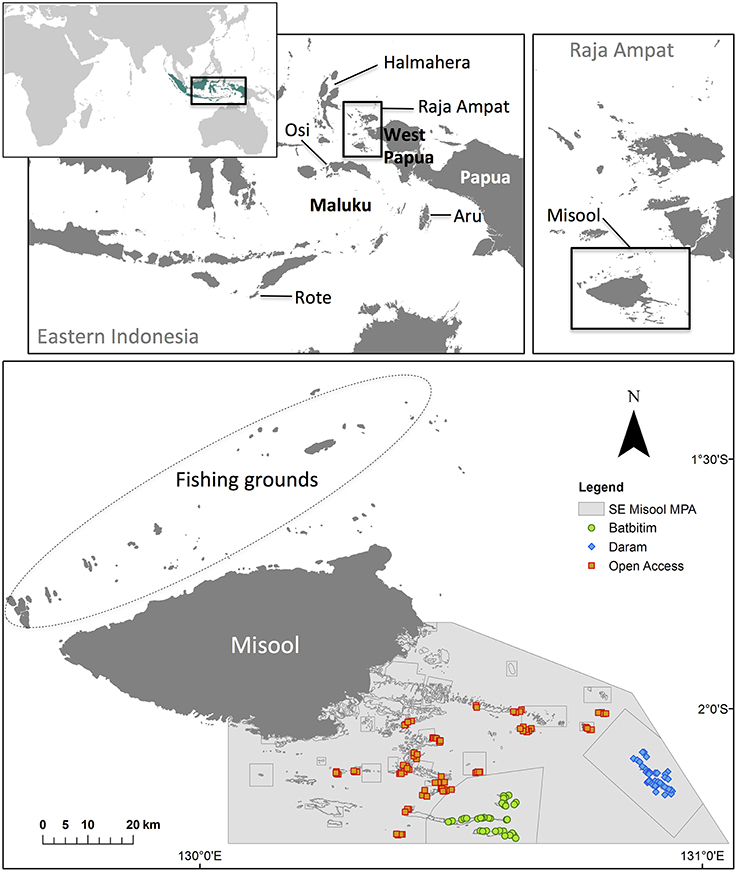
Figure 1. Map of Misool island, showing the boundaries of the Batbitim and Daram No-Take Zones (NTZs) within the SE Misool Marine Protected Area, and the fishing grounds in northern Misool from which catch data were obtained. Colored symbols represent baited remote underwater video systems (BRUVs) replicates. Inset: The provinces of West Papua, Papua and Maluku in Eastern Indonesia and locations of shark fishing villages where interviews were conducted (Osi island, Rote island, and Aru Archipelago), and Halmahera island in North Maluku province.
The Raja Ampat shark sanctuary is unique because it is the only one in the Coral Triangle and within Indonesia, the number one shark fishing nation in the world. We investigated the effects of shark-specific spatial closures on shark populations and on fishers' perceptions and behaviors. Our main hypothesis was that appropriately sized and enforced spatial closures could mitigate against declines in shark populations in a region of intense fishing pressure. By identifying factors that best explain the success of the NTZs in SE Misool MPA in protecting sharks, our aim was to identify key ecological and social considerations that could assist with successful governance of the shark sanctuary. Three assumptions provided the foundation for our hypothesis: (1) that the value of sharks to dive tourism revenue acted as a strong incentive for the establishment and enforcement of shark sanctuaries; (2) that effective enforcement of protected sites would result in higher relative abundance of sharks compared to fished sites; and (3) that the inclusion of shark fisher perceptions and practices in our assessment would allow for a better understanding of the wider implications of spatial closures for shark fishing livelihoods.
This study was carried out under animal and human ethics permits approved by the Research Ethics and Integrity Committee within the Division of Research and Development at Murdoch University, Western Australia. All human participants gave written and/or oral (in case of inability to provide signature) informed consent to be interviewed. Prior to being interviewed, every respondent was informed of the purpose of the interview, the confidentiality of information provided, and the right to omit uncomfortable questions or withdraw from the interview at any stage.
Misool (1°52′40.52″S, 130°06′52.38″E) is the southernmost island in the Raja Ampat regency of West Papua province (Figure 1). Situated on the eastern boundary of the Indonesian archipelago, this region experiences two main seasons; the northeastern monsoon (or rainy season) between May and September, and the southwestern monsoon (dry season) between November and March (Mangubhai et al., 2012). Access to many of the islands off the mainland is restricted or closed during the northeastern monsoon.
In 2006, the SE Misool MPA covering 3432 km2 was established by local communities with technical support from The Nature Conservancy (TNC) and the endorsement of the Raja Ampat regency government (Table 1). Similar to the other six MPAs in the regency, the intention was to establish a multiple use zoning and management plan for SE Misool MPA.
A year earlier, in 2005, MER had established a 425 km2 NTZ around Batbitim island where the resort is located, and its surrounding waters (Table 1), following observations of shark and blast fishing and low numbers of sharks in the area (A. Miners, pers comm.). A lease for the Babitim NTZ was negotiated with the communities traditionally fishing the reefs in the area, conferring management rights to MER for 25 years. Under the lease agreement, communities agreed to a no-harvesting rule, with the exception of controlled collection of commercially valuable trochus shells (Trochus spp) and green snails (Turbo marmoratus) down to free diving depths. These collections are conducted in accordance with sasi, an Eastern Indonesian system for managing natural resources whereby harvest is temporally and spatially limited (McLeod et al., 2009). In 2010, a second NTZ covering 403 km2 was established by MER around Daram island in the southeastern corner of SE Misool MPA, again through a lease agreement with the community that holds traditional access rights to Daram (Table 1). Similar to Babitim, the Daram NTZ is opened for 2 weeks every 2 years to allow local harvesting of permitted invertebrate species. The Batbitim and Daram NTZs are patrolled by Misool Baseftin, a local NGO founded by MER that employs local community members.
Fieldwork was conducted in SE Misool MPA from 25 April to 13 May 2012. We surveyed sites both within the existing NTZs, and control areas within the MPA that had no management in place at the time. Although the zoning plan has now been finalized and the MPA is divided into several no-take and traditional-use zones (where traditional fishing is permitted), we refer to the area outside of the Batbitim and Daram NTZs as an open access zone (OAZ) throughout this paper, as this reflects the management status of the area at the time of study.
Pulau Osi (3°01′22.04″S, 128°04′25.60″E) is a small (~900 × 450 m) sandy island 2 km off Seram in Maluku province (Figure 1). At the time of this study, 963 people lived in the community, with most families dependent on one or more types of small-scale fishing. The majority of Osi's population descended from its original inhabitants who arrived by boat from Buton in southeast Sulawesi. Since the advent of shark fishing however, many a wife from every corner of the archipelago has been brought back to Osi, increasing the ethnic diversity of the exclusively Muslim community. In the early 1990s, Osi fishers earned a reputation throughout the region as far-ranging shark fishers, their fishing grounds extending north to Halmahera in North Maluku, east to Biak off the east coast of Papua, west to Bali and south toward, and sometimes past, the Australian border. Around the turn of the millennium, they began focusing their main fishing grounds to the numerous small islands off Misool's northern coast, where several Osi fishers met their wives and settled, creating family connections between Osi and northern Misool. To catch sharks, Osi fishers exclusively use multiple un-baited, bottom-set gillnets bottom-set gillnets, each 120 m in length, with a stretched a stretched mesh size of 19–23 cm, which are set at night and soaked for 10–12 h before they are manually retrieved. Fishing trips average 6 weeks but range between 2 and 8 weeks (Jaiteh, unpublished survey data). In this time, fishers cover distances of approximately 600 km for a roundtrip to Misool, up to 1000 km if they fish other fishing grounds in Raja Ampat, and over 2000 km if they fish in Halmahera province.
Socio-economic and fishery data were collected on Osi between 3 March and 31 August 2012. At that time, the majority of the community relied almost exclusively on income from shark fishing in Raja Ampat, particularly northern Misool. While this study was underway, a fishing boat from Osi was intercepted by a patrol boat in Wayag MPA in northern Raja Ampat, on its way from Misool to Halmahera after 6 weeks of fishing. Catch and gear were confiscated and the crew sent home with a warning; the shark sanctuary was not yet legally in force at the time. This incident provided an opportunity to examine the fishers' perceptions of shark conservation and their responses to the first signs of enforcement.
We used baited remote underwater video systems (BRUVs) as a fishery-independent survey method to assess the abundance and diversity of sharks and other coral reef species targeted by local fishers in the OAZ and the two NTZs within SE Misool MPA in Raja Ampat (Figure 1). BRUVs were deployed at 40 sites within the three management zones: 10 sites in Batbitim NTZ, 10 sites in Daram NTZ, and 20 sites within the OAZ. Sites were selected to reflect the diversity of habitats while covering the spatial extent of each management zone. At each site, we deployed four BRUVs over a depth range of 1–34 m, with replicates spaced at least 500 m apart.
BRUVs were selected from a number of fishery-independent survey methods as they are more cost-effective than diver video surveys and provide greater statistical power to detect changes over space and time (Langlois et al., 2010). Furthermore, they have been shown to be an adequate sampling method for studying the relative abundance of sharks inside and outside MPAs (Bond et al., 2012; Goetze and Fullwood, 2012; Rizzari et al., 2014). Given the logistical constraints on transport to and from SE Misool MPA, a compact BRUVs system was developed which could be carried, assembled, deployed and retrieved by one person (Figures 2A,B). We achieved this by constructing frames made from light acrylic to which two GoPro Hero 2 cameras were mounted at a 3° angle to each other. The legs of each frame consisted of plastic drainpipes that could be disassembled from the frame and used as a protective cover for the cameras during transport. BRUVs were deployed with six dive weights using 5 mm nylon rope attached to a swim float at the surface, and retrieved manually (Figure 2C). For each replicate, 1 kg of cut and crushed skipjack tuna (Katsuwonus pelamis) was placed in a bait bag that was made from plastic wire mesh and extended 1.2 m from the camera with plastic conduit pipe. In total, we deployed 160 BRUVs, each for a minimum of 45 min.
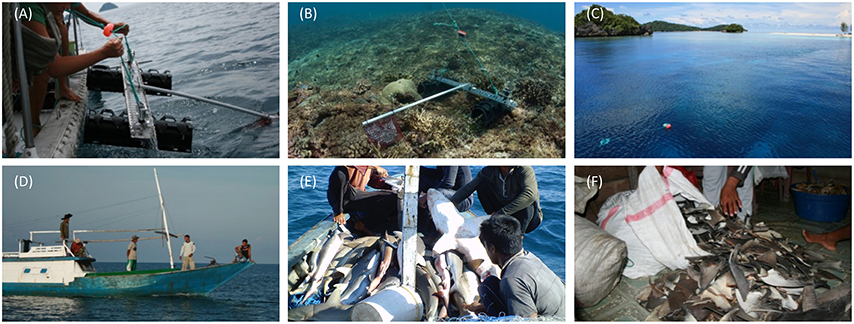
Figure 2. Different means of data collection used in this study. Top row—fishery-independent data collection: (A) deploying lightweight baited remote underwater video systems (BRUVs) with GoPro cameras; (B) BRUVs in situ; (C) Surface floats help to locate and retrieve BRUVs. Bottom row—fishery-dependent data collection: (D) Fishers from Osi island setting out for what was to be their last fishing trip to Raja Ampat; (E) shark catch from Misool, Raja Ampat; (F) dried shark fins from which tissue samples were taken to verify the fishers' species identification of sharks they recorded for this study.
Video footage was analyzed using EventMeasure software (Seager, 2014). The maximum number (MaxN) of any one species seen at once during the recording period was used as a measure of relative abundance. As recording times varied between deployments (mean deployment time ± 1 SD = 54 min ± 8.2 min), we standardized the relative abundance to MaxN per hour, which is consistent with previous studies of shark abundance from BRUVs (e.g., Espinoza et al., 2014). Where possible, sharks were recorded to species level, sexed, and conservatively classified as juveniles if their total length was ~70 cm or less (Figures 3A,B,C). Other taxa of interest were also counted from BRUVs footage. This included reef fish that are targeted by small-scale reef fisheries, such as snappers, emperor, and groupers, two globally threatened species (humphead wrasse Cheilinus undulatus and bumphead parrotfish Bolbometapon muricatum), and species of importance to dive tourism (turtles, rays, and moray eels) which were recorded to at least genus level (Table 2, Figures 3D,E,F).
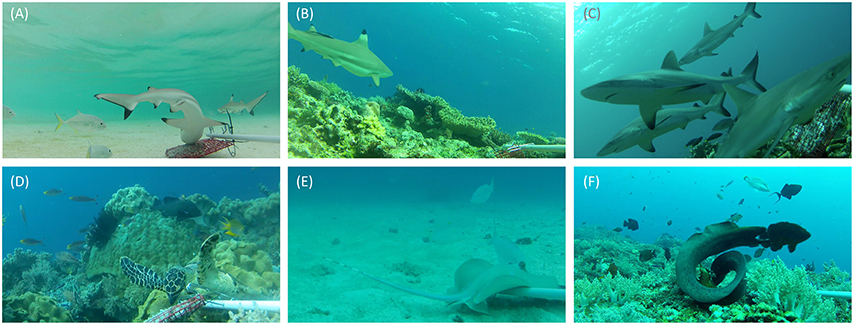
Figure 3. Images captured on baited remote underwater video systems. Top row—sharks: (A) juvenile blacktip reef sharks Carcharhinus melanopterus in a shallow bay in Daram No-Take Zone (NTZ); (B) presumably pregnant C. melanopterus, Daram NTZ; (C) a group of gray reef sharks Carcharhinus amblyrhynchos investigate the bait bag in Batbitim NTZ. Bottom row—species of high conservation or tourism value: (D) hawksbill turtle Eretmochelys imbricata feeding on bait, Batbitim NTZ; (E) bluespotted stingray Neotrygon kuhlii feeding on bait, Open Access Zone; (F) moray eel Gymnothorax javanicus guarding the bait bag, Batbitim NTZ.
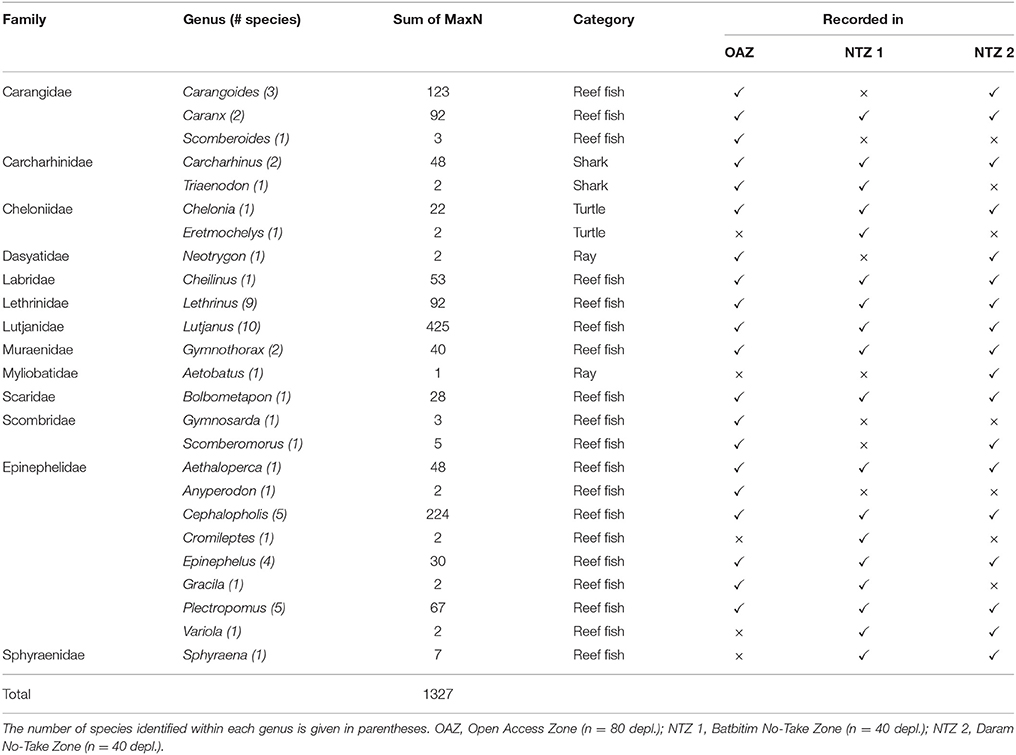
Table 2. Taxa recorded on baited remote underwater video stations during 160 deployments (depl.) within the Southeast Misool Marine Protected Area, Raja Ampat.
Habitat metrics for each BRUV replicate were defined by grading measures of the structural complexity, reef slope and the benthic cover of five habitat types; live coral, macroalgae, turf algae, crustose coralline algae (CCA), and unconsolidated sediment. These measures were visually estimated from the imagery of the video cameras. Estimates of structural complexity followed those used by Wilson et al. (2007) where 0 = no vertical relief, 1 = low and sparse relief, 2 = low but widespread relief, 3 = moderately complex, 4 = very complex with numerous fissures and caves, 5 = exceptionally complex with numerous caves and overhangs. Reef slope was also estimated on a six-point scale from flat to vertical wall. Benthic cover for the five habitat types were graded where 0 = trace (0%), 1 = sparse (1–10%), 2 = low (10–25%), 3 = medium (25–50%), 4 = dense (50–75%), 5 = very dense (>75%).
To test the hypothesis that the abundance of sharks and fishery targeted reef fish differed between management zones (i.e., NTZs, OAZ), we used univariate permutational analysis of variance (PERMANOVA) on the factor Management area (fixed effect with three levels—OAZ, Batbitim NTZ, and Daram NTZ), while including Site (random effect nested within Management area) to account for spatial variation. To account for potential variation in habitat between management areas, we first performed a principal components analysis (PCA) on the seven habitat variables for all BRUVs replicates. This acted as a data reduction tool to determine gradients in habitat and reduce collinearity between the measured variables. The PCA summarized the habitat variation into three components (Principal component axes, PCA1, PCA2, and PCA3), which together explained 83% of the variance between BRUVs replicates. Abundance data were log (x+1) transformed and we included the three habitat components and the continuous factor depth (1–34 m) as covariates in the permutational ANOVAs (Table 3). Where differences were found between management areas, we conducted post-hoc pairwise t-tests and corrected the resulting P-values for multiple comparisons using the Bonferroni adjustment.
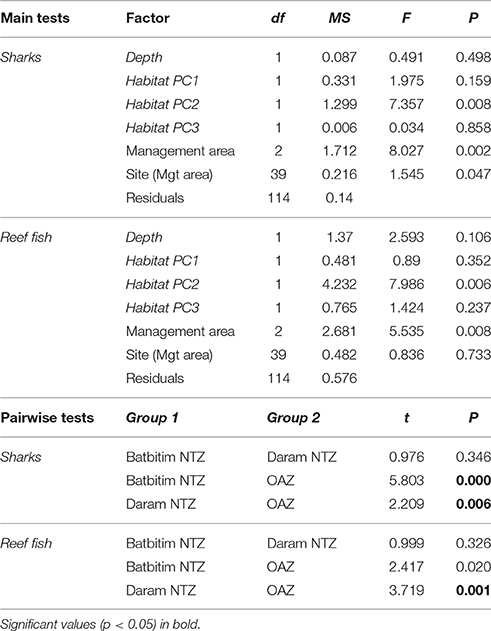
Table 3. Permutational ANOVAs examining the relative abundance of sharks and targeted reef fish between management areas (No-Take Zones 1 and 2, Open Access Zone) and sites along with habitat co-variables (in italics). Post-hoc pairwise t-tests between management areas are shown below using a Bonferroni adjusted significance level of α = 0.017.
To examine how groups of recorded taxa varied between samples, we performed a principal coordinate (PCO) analysis. Prior to the analysis we applied a fourth root transformation to the abundance data of each group and created a Bray–Curtis dissimilarity matrix between every pair of observations (Anderson and Willis, 2003). Spearman rank correlations of |r| > 0.3 were used to show relationships between individual species and the PCO axes. All analyses were performed using the PERMANOVA+ add-on package for PRIMER v6 (Anderson et al., 2008).
During 5 months between March and August 2012, we studied the fishing practices and livelihoods of the community on Osi through biological data collection, participant observation and semi-structured interviews with community members (outlined below). Fishers that set out on a fishing trip were given datasheets and asked to record the location of fishing grounds and the local species names of up to 10 sharks caught per gillnet set (Figures 2D,E). To verify the local species names, we asked fishers to collect small (~5–10 mm) tissue samples from the undersides of dried pectoral fins of a subset of recorded sharks (Figure 2F). No financial reward was offered for data collection to ensure that participating fishers were interested in the data collection itself. Three vessels contributed catch data; the first vessel had left for northern Misool in late January and returned 6 weeks later, shortly before the second vessel left Osi in mid-March to fish in northern Misool. The third and last vessel to leave Osi in that season was at sea for 5 weeks before it was intercepted by water police in Wayag (northern Raja Ampat) on 3 May 2012. Datasheets were returned from this trip but as the police confiscated the catch, no tissue samples were available.
Upon the fishers' return, their species names were matched to scientifically recognized species using an identification guide (White et al., 2006). Tissue samples were stored in a NaCl saturated solution containing 20% dimethyl sulphoxide and 0.25 M ethylenediaminetetraacetic acid. The samples were genetically barcoded in July-August 2014 (Jaiteh and Momigliano, 2015) and incorporated into a larger study on the catch composition of shark fisheries in Eastern Indonesia (Jaiteh et al., unpublished data). The catch data presented here allow for a comparison with the species diversity recorded by the BRUVs in the same geographic region.
Interviews with fishers were guided by a 125 question survey covering a broad range of topics related to shark fishing livelihoods, with 20 questions directly relevant to this study (Supplementary Table S1). Interviews with non-fishing community members were based on a sub-sample of 28 questions that focused on perceptions about sharks and the importance of shark fishing to the wider community. The questionnaire contained mostly closed questions that arose repeatedly in conversations with, and during observations of fishers and their work. The questionnaire was field tested on three active and two retired fishers before data were collected for this study.
Interviews were conducted during the last month of fieldwork in August 2012, when the researcher and community had established mutual trust through informal conversations, participation at community events, and collaborative data collection. The respondents comprised at least 30 active and 30 retired shark fishers, and 20 non-fishers. Active fishers were fishing in the current season, or had fished last season and were currently resting but with either no intention or no means of changing to a new income source. Retired fishers had stopped fishing for sharks and retired altogether, or had permanently switched to a different livelihood with no intention of returning to shark fishing, irrespective of fluctuations in fin prices or other factors. Non-fishers were community members that held central positions in the community, such as cultural leaders, teachers, shop owners, traditional healers, and heads of community organizations such as the papalele (women who clean and sell fish on land). While all fishers were men, the non-fishers group comprised women. Together, these 80 respondents represented almost 10% of the population of Osi. Respondents were chosen randomly, according to availability and only one member of a household was interviewed. All of the potential respondents we approached agreed to be interviewed. They were informed of the intent of the research, their right to skip uncomfortable questions or withdraw from the interview at any stage, and that no names would be used in publications arising from the research. Interviews were conducted in Indonesian by the lead author and four assistants who were native speakers and had been given prior training to ensure consistency of methods. Additional information was gleaned from observations and informal conversations with groups and individuals during the 5-month case study in Osi.
The interviews were later repeated with the same number and categories of respondents in two other shark fishing communities: Dobo in the Aru Archipelago of Maluku and Pepela on Rote island in East Nusa Tenggara province (Figure 1). Fishers from Dobo mainly fish around the Aru Islands and in the Arafura Sea toward the Papuan mainland and the Australian border. Pepelan fishers rarely traveled to Raja Ampat and their traditional fishing grounds are now part of Australian waters, where many have been arrested for illegally fishing outside of officially allocated boundaries. A subset of data from these latter two communities is presented here to complement the views and perceptions of Osi fishers with regards to the measures aimed at protecting sharks.
From 160 BRUVs deployments, 50 individual sharks were recorded of which 48 (96%) were recorded within the two NTZs of the SE Misool MPA: 28 in Batbitim and 20 in Daram (Table 2). The average relative shark abundance (mean MaxN/hour) in Batbitim and Daram was 0.8 and 0.6, respectively, compared to 0.03 in the OAZ (Figure 4A). Three reef shark species were identified; gray reef (Carcharhinus amblyrhynchos, n = 21), blacktip reef (C. melanopterus, n = 26), and whitetip reef (Triaenodon obesus, n = 2). One T. obsesus was recorded in the OAZ, the other in Batbitim NTZ. While all juvenile sharks were recorded in Daram NTZ (n = 7; 1 C. amblyrhynchos, 6 C. melanopterus), there was no significant difference in the abundance and percentage contribution of C. amblyrhynchos and C. melanopterus to the species assemblage between the two NTZs (Figure 4A).
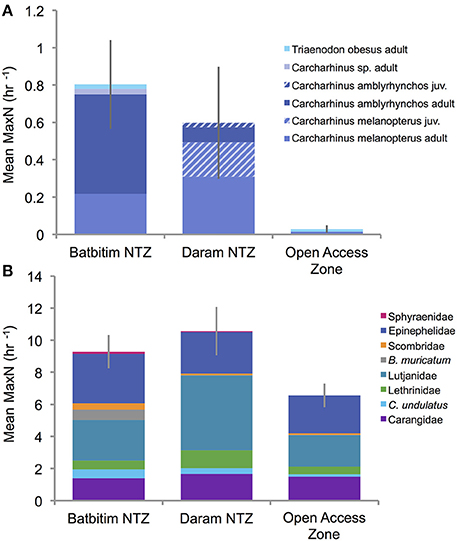
Figure 4. Relative abundance (mean MaxN per hour ± SE) of (A) shark species and life stages, and (B) fishery targeted reef fish families and species recorded in each of the three management areas surveyed in Southeast Misool Marine Protected Area. Juvenile sharks (hatched fields) were only recorded in the Daram No-Take Zone (NTZ).
The relative abundance of sharks was clearly greater within the NTZs compared to the OAZ in the SE Misool MPA (Figure 4A) and management area was highly significant in the PERMANOVA analysis (p < 0.01) after controlling for habitat and depth effects (Table 3). Of the habitat co-variables, reef slope (PC2) was significant with a trend for more sharks associated with a gentle reef slope. Pairwise tests revealed that shark abundance within each of the NTZs differed significantly from the OAZ (p < 0.05), but not between the two NTZs (p = 0.346; Table 3).
The relative abundance of targeted reef fish followed the same trend as that of reef sharks, with greater numbers recorded in the NTZs compared to the OAZ (Figure 4B). Management zone was highly significant (p < 0.01) in the PERMANOVA, with no effect associated with the habitat and depth co-variables (Table 3). Fish abundance was significantly lower in the OAZ than the NTZs (p < 0.05), but did not differ significantly between the two NTZs (Table 3).
In general, the abundance of all recorded taxa of interest was positively correlated with most NTZ sites (to the right of Figure 5), with no taxa showing strong affinity for sites within the OAZ. Particularly the endangered humphead wrasse C. undulatus had a strong correlation in the direction of the NTZs. Reef shark (Carcharhinidae) abundance showed a similar correlation strength and direction as the abundance of other fishery targeted species (Figure 5).
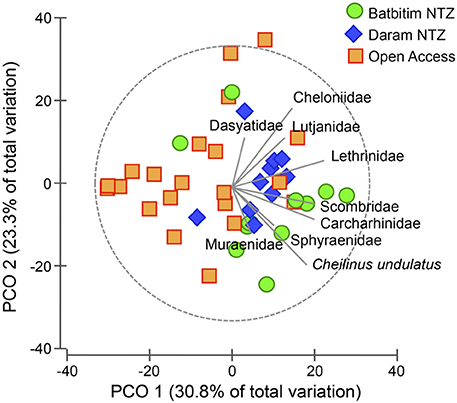
Figure 5. Principal coordinate ordination (PCO) plot for the assemblage of all recorded taxa averaged to the site level. No-Take Zone (NTZ) sites are represented by green circles (Batbitim NTZ) and blue diamonds (Daram NTZ), Open Access sites by orange squares. Correlations of taxa toward sites are indicated by the length and direction of vectors.
Catch data and tissue samples were obtained from three separate fishing trips between March and April 2012, resulting in a total of 474 identified sharks and 173 tissue samples. Genetic analysis of the tissue samples showed a very high percentage of correct species identifications by fishers; over 95% of sharks were identified correctly from dried pelvic fins (Jaiteh, unpublished data). Inconsistencies occurred between some C. amblyrhynchos and silvertip C. albimarginatus sharks, for which one fisher used the same local name, and between the fossil shark Hemipristis elongata and Australian weasel shark Hemigaleus australiensis, because fishers do not distinguish between these two species. These consistent misidentifications were corrected in the recorded catch of the second and third vessel to determine total catch composition, which was calculated from 67 tissue samples from vessel 1, 152 sharks from vessel 2, and 255 sharks from vessel 3 (Figure 6).
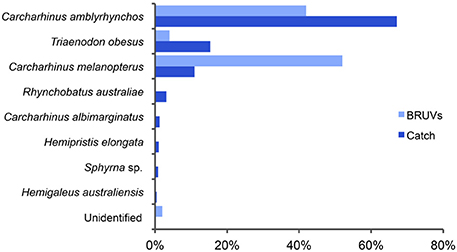
Figure 6. Proportions of shark species captured on baited remote underwater video systems (BRUVs, light blue) and recorded by fishers in their catch (dark blue) in waters surrounding Misool island in Raja Ampat. Both assemblages comprised mainly gray reef (C. amblyrhynchos), whitetip reef (Triaenodon obesus), and blacktip reef (C. melanopterus) sharks.
The species compositions recorded on BRUVs (n = 50) and by Osi fishers during their fishing trips to northern Misool (n = 474) were both dominated by three reef-associated species; C. amblyrhynchos, T. obesus, and C. melanopterus (Figure 6). While C. melanopterus dominated the species assemblage recorded on BRUVs (52%), C. amblyrhynchos was the main species (67%) in the fishers' catch. T. obesus made up 4 and 15% of the BRUVs and catch species composition, respectively. While the species assemblage recorded on the BRUVs consisted entirely of these three species, the fishers recorded five additional species—Rhynchobatus australiae (whitespotted guitarfish), C. albimarginatus, H. elongata, Sphyrna sp. (hammerhead shark) and H. australiensis (Figure 6). Combined, these species made a much smaller contribution (7%) to the catch composition than the three species that also dominated the BRUVs assemblage.
Interviews with 61 shark fishers (30 active, 31 retired fishers) and 20 non-fishing community members from Osi showed that 97% of fishers had fished in Raja Ampat recently or during the last decade, and 66% said they fished there often (Supplementary Table S1). According to 85% of fishers, the main reason for fishing primarily in Raja Ampat was due to the perceived high abundance of sharks there.
While most fishers (88%) knew that shark fishing was banned in Raja Ampat, their perceptions of the purpose of spatial closures (commonly referred to as MPAs in the interviews) or fishing bans varied widely, including “protecting marine resources/sharks and rays/corals and turtles,” “to stop dynamite fishing,” and “for mariculture.” Only two respondents thought their purpose was to protect fishers' livelihoods, and one suggested it was to reduce the income of local people. However, the most frequently stated purpose (48% respondents) was “improving tourism”:
I feel that in Raja Ampat they banned shark fishing because it's a tourism region. Maybe if it is not banned and there are no more sharks, the tourists will say, “Where are the sharks? They are not here!” If there are sharks there, it's good. So do you think the shark fishing ban in Raja Ampat is effective?
Yes.
Have you fished in Raja Ampat?
Yes. But not anymore. (Active fisher, Osi, 08/2012)
Sixty-seven percent of respondents in Osi said that the ban on shark fishing in the Raja Ampat shark sanctuary had affected them. For most of those respondents (66%), this meant having less income, usually because they had to change fishing grounds to less productive areas (63%) which resulted in lower catches, and in a few cases resulted in higher fuel costs (5%). Some Osi fishers said that Raja Ampat had become a dangerous fishing ground because of the risk of getting caught, and rather than fishing for sharks elsewhere, they preferred to start fishing for ikan biasa (small reef fish) in the vicinity of their island or look for an alternative to fishing. Through informal follow-up conversations in the year following the interviews, we found that fishers indeed adapted to the loss of former fishing grounds by diversifying their livelihoods, including coastal small-scale fishing and illegal petrol transport, while others simply redirected their shark fishing efforts to areas open to fishing, mostly in Halmahera to the north-west of Raja Ampat (Figure 1). In Dobo and Pepela, the proportion of fishers who had been affected by fishery closures or bans was much lower (21 and 41%, respectively).
The majority of fishers from all three sites did not feel that the people or organizations implementing shark protection understood the livelihood needs of shark fishers (70% across sites). Only 42% of active fishers expressed confidence in NGOs considering shark-fishing livelihoods, followed by retired fishers (50%) and non-fishers (75%). However, the respondents in Osi were the most optimistic, with 42% believing that their livelihood needs were understood, compared to 37% in Pepela and only 16% in Dobo. Almost 70% of the respondents in Osi thought that fishing bans and MPAs were effective, compared to 49% in Pepela and 29% in Dobo. The most frequently stated reason for their failure was fishers ignoring the closures (95%). Nevertheless, only 10% of all respondents in Osi, Pepela, and Dobo said they would continue fishing if they were aware of a fishing ban or closure. Several fishers expressed a clash between an awareness of the value of live sharks to the ecosystem on one hand, and the need for a livelihood on the other hand:
Sharks are important too in the ocean. But I hope one day the government will say “now there are plenty of sharks, go and fish them again.” (Active fisher, Osi, 08/2012)
Are you worried about sharks declining?
Yes, I feel sad. Because this is our livelihood and if they decline, so will our livelihoods. On the other hand, it's clear that they are part of the ecosystem and sharks need to continue to be in the sea. It's just that we have our livelihoods there too, so whether we want to or not, we have to fish them. (Active fisher, Osi, 08/2012)
Although we expected older fishers, having seen more changes in shark populations, to show more concern for the health of the ecosystem, we found no significant difference between the average age of active fishers concerned about the environment (41.7 years) and those not concerned (40.2 years). However, retired fishers generally appeared more concerned over the ecological consequences of declining shark numbers than active fishers:
Do you feel concern seeing sharks decline in the ocean?
Yes. Us humans have to think too: if we keep catching them, how do we think this can continue? They'll eventually finish. That's where my thinking comes from. Most of the sharks we catch have babies [are pregnant]. So we catch one but kill many. My concern comes from that. (Retired fisher, Dobo, 11/2012)
The majority of respondents in Osi (83%) and the other two sites described sharks primarily as an important source of income and hence a financial benefit. All respondents expressed concern over declining shark populations (100% in Osi, 99% across sites), but while fishers were primarily concerned about the resulting declines in shark catch and/or loss of livelihoods (81% of active and 71% of retired fishers), almost half (49%) of all non-fishers were primarily worried about the loss of ecosystem function. When respondents from all categories were asked if they thought that sharks needed protection from fishing, approximately half agreed in each location (Osi: 53%; Pepela: 61%; Dobo: 48%). In relation to this question, several fishers pointed out that the need for a livelihood and the (perceived or real) inability to switch to an alternative can lead to displaced fishing effort if shark catches decline or if fishers are excluded from a fishing ground:
In 2001 I fished in Sorong, in 2002 in Tual and in 2003 I came here [to Dobo]. Us small-scale fishers move to where the conditions are good. If things get worse in a place, we go to a new place. (Active fisher, Dobo, 12/2012)
Our results clearly indicate a higher relative abundance of reef sharks in well-enforced large NTZs compared to areas open to fishing. With equal sampling effort inside and outside the NTZs, 96% of all sharks were recorded within the two NTZs, which suggests that these no-fishing areas provide critical refuge for reef sharks. These findings are consistent with other studies of shark abundance, showing benefits to reef sharks within no-take marine reserves around the world (Bond et al., 2012; Goetze and Fullwood, 2012; Espinoza et al., 2014). Since both NTZs in SE Misool MPA were relatively young at the time of this study (7 and 2 years), we did not expect the magnitude of difference observed (21 and 28 times greater abundance inside NTZs than outside), which is vastly greater than the up to four-fold increases in reef shark numbers reported from 13 year old marine reserves studied by Goetze and Fullwood (2012) in Fiji and by Bond et al. (2012) in Belize. It is unlikely that shark numbers increased by that magnitude in the relatively short time the NTZs had been enforced, given the approximate population doubling times of the observed species (Smith et al., 1998). Furthermore, the relative abundance of sharks recorded within the NTZs (0.6–0.8 sharks per hour in Daram and Batbitim, respectively), is comparable to the values recorded using BRUVs in other unfished reserves, while it was almost an order of magnitude lower in the OAZ than in fished areas elsewhere (Goetze and Fullwood, 2012). However, given anecdotal reports indicating the virtual absence of sharks at diveable depths where the NTZs were later established (pers. comm. A. Miners, MER owner, and S. Heinrichs, Shark Savers), it is also unlikely that shark numbers were naturally higher in the NTZs to begin with. Rather, we suspect that shark numbers were similar throughout the area that was later divided into the NTZs and the OAZ. The observed magnitude of difference is likely due to continued fishing mortality in the OAZ reducing shark numbers to very low densities, while effective protection has provided refuge and increased prey availability for remaining sharks, both within the NTZs and from adjacent areas, and their offspring.
While we suspect that high historical and ongoing fishing mortality is the main reason for the low numbers of sharks outside the NTZs, the large sizes of the NTZs are likely a key factor for their effectiveness, being sufficiently large to protect the home ranges of reef sharks and their prey. Activity spaces for reef sharks have been calculated as 0.55 km2 (over days) to 12.08 km2 (over months) in area and around 4–8 km in length for C. melanopterus (Papastamatiou et al., 2010, 2009), an average of 4.2 km2 in area and 3.6 km in length for C. amblyrhynchos (McKibben and Nelson, 1986), and home ranges of approximately 1 km2 or up to 5 km linear distance for T. obesus (Nelson and Johnson, 1980; Whitney et al., 2012). These core areas of use correspond roughly to the boundary lengths of the NTZs, which suggests they are of sufficient size to provide important refuge areas to sharks (Green et al., 2014). Both NTZs are larger than many of the marine reserves where shark abundance has been assessed (Bond et al., 2012; Goetze and Fullwood, 2012), and, combined with high levels of enforcement, satisfy key criteria for MPAs identified by Edgar et al. (2014), who observed an up to 14-fold increase in shark biomass in MPAs compared to fished areas. Other authors have suggested that greater prey availability may also be a contributing indirect reason for differences in shark abundance inside and outside protected areas (Goetze and Fullwood, 2012; Momigliano et al., 2015). In addition to harboring more sharks than the OAZ, the NTZs also had significantly more reef-fishery targeted species, some of which may represent or regulate prey species for reef sharks and hence provide a vital food source to sustain populations. While our results demonstrate significant spatial differences in shark abundance, it is unclear whether there is temporal variation in shark numbers. Follow-up studies to investigate how shark populations respond to protection over time would help clarify whether the observed differences in abundance reflect population recovery, or whether higher numbers are largely due to an aggregative effect and successful protection of sharks in the protected area.
We found no significant difference in total shark abundance between the two NTZs, despite Batbitim NTZ having been established 5 years earlier than Daram. Given the NTZs' relatively recent establishment, this result is consistent with generation times of the shark species recorded in this study, which reach sexual maturity at around seven (T. obesus) to 10 (C. melanopterus and C. amblyrhynchos) years of age (Robbins, 2006; Chin et al., 2013). Daram had more small sharks than Batbitim, suggesting that its habitat with gentler reef slopes and shallow bays may provide shark nursery habitats that could play a beneficial role in the recovery of fishery-impacted shark populations. While our data are insufficient to establish the presence of nursery areas within the two NTZs, we recommend future studies to determine whether the NTZs or any areas within the OAZ fulfill the criteria for primary or secondary shark nurseries (Heupel et al., 2007) or other critical habitat for various life history stages of sharks.
Although pelagic BRUVs have been established as a valid fishery-independent method to capture representative samples of the species diversity compared to longline fishing (Santana-Garcon et al., 2014), we found the diversity of reef sharks recorded on BRUVs to be lower than the fishery-dependent data from fishers using gillnets in northern Misool (Figure 6). This suggests that, although both methods were deployed on coral reefs within a similar depth range (the majority of nets were set in 2–40 m depth), BRUVs may not capture the full suite of shark species present on coral reefs. The discrepancy in species diversity may be explained by the fact that BRUVs recorded during the day, when some shark species are less active than at night, when fishers set their nets (Bromhead et al., 2012; Speed et al., 2010, 2016). Reef sharks have been reported to undertake vertical diel migrations between depths, typically approaching shallower depths at dawn and dusk (Vianna et al., 2013), when video cameras do not capture sufficient light for clear footage. Alternatively, BRUVs may be biased toward species attracted to K. pelamis or other bait, while gillnets also recruit sharks that are not bait-dependent, and those that are attracted to other species caught in the nets. Furthermore, the nets fishers used to catch sharks had longer soak times (~12 h or overnight) than BRUVs and covered a larger area (95–120 m per net), and were thus more likely to capture sharks during foraging trips.
Fish species commonly targeted in tropical reef fisheries and other species valuable to diving tourism were measured as an alternative indicator of NTZ effectiveness. Indeed the well-enforced NTZs also provided benefits to these species, consistent with studies elsewhere (Edgar et al., 2014). In Raja Ampat, where many local communities engage in subsistence fishing, the likely spillover benefits (e.g., Russ and Alcala, 2011) of NTZs in SE Misool and other MPAs throughout the regency may provide important livelihood benefits to communities and garner local support for spatial closures as tools for biodiversity conservation and fisheries management, which should ultimately benefit both the tourism industry and local fisheries.
The success of the NTZs within SE Misool MPA suggests that innovative partnerships between the private sector and local communities, coupled with effective enforcement, resulted in greater numbers of sharks with direct benefits for dive tourism. The Batbitim and Daram NTZs were established directly as a lease arrangement between MER and the communities with traditional ownership rights over the islands and reefs. The governance arrangement is essentially a payment for ecosystem services (PES), where in return for protecting the islands and surrounding reefs, communities receive lease payments and employment at the resort or as rangers to patrol the NTZs. Because there are immediate tangible benefits to entering into PES arrangements, these NTZs were established more quickly than other NTZs in the larger MPA.
In contrast, the establishment of the SE Misool MPA and the development of its management and zoning plan required significant investment from NGOs and government in education and outreach, and the inclusion of communities in spatial planning (Mangubhai et al., 2015). The final zoning plan for SE Misool MPA explicitly included socio-economic criteria and data, recognized community use and governance of resources, maximized equity and access to traditional fishing grounds, and addressed long-term food security and livelihoods of local communities (Mangubhai et al., 2015). This investment without direct financial benefits to communities resulted in a 5 year planning process, which far exceeded that of the two NTZs. The resulting delay in enforcement of other NTZs within the MPA is reflected in the significantly lower abundance of sharks outside of the Batbitim and Daram NTZs. This finding corresponds to the results of a recent BRUVs study in northern Raja Ampat, which found no significant difference in shark abundance inside and outside NTZs within the Penemu and Dampier Straits MPAs (Beer, 2015). Both MPAs had only been enforced for 15 months at the time of Beer's (2015) study. At 0.42 sharks/hour, the average relative shark abundance within these recently enforced NTZs was almost half that recorded in our study (0.8 and 0.6 sharks/hour in Batbitim and Daram, respectively). In light of these findings, our case study from SE Misool MPA demonstrates the important role the private sector can play in marine conservation, with quicker and more direct benefits to sharks and other species. Determining the long-term viability of the private-public partnership would be an important focus of future research, for example by documenting how local communities perceive the legitimacy and benefits of PES arrangements and tracking the distribution of payments through the communities.
The ability to effectively enforce any spatial closure is of crucial importance to its success (White et al., 2015). Enforcement and compliance may be compromised if biological concerns cannot be reconciled with the socio-economic needs of fishing communities (Hoyt, 2014; Mangubhai et al., 2015). If fishers are not considered in the planning of a sanctuary, they might simply shift effort to other areas or species, or disregard closures altogether (Hoyt, 2014). Arguably, the importance of effective enforcement increases with the number of fishers and scale of the associated fisheries, which in Indonesia are of global significance in terms of landings and exports (Blaber et al., 2009; Momigliano et al., 2014; Dharmadi et al., 2015). Reef shark populations around Misool island in Raja Ampat provide a valued asset for dive eco-tourism as well as important livelihoods for regional fishers from neighboring provinces. Since shark fishing was mainly done by outside fishers from Sulawesi, Seram, and Halmahera (Varkey et al., 2010), compliance from local fishers with the NTZs was high (Muhajir et al., 2012). Conversely, enforcement at the regency level of the shark sanctuary resulted in a change in the behavior of regional fishers from Osi near Seram in the neighboring province of Maluku. A single warning to a shark fishing vessel from Osi and the subsequent confiscation of catch and gear from another vessel by Raja Ampat water police immediately led to a distinct shift in fishing grounds and a decrease in shark fishing by Osi fishers. At the time of our interviews, 4 months after the incident in Raja Ampat, 88% of fishers knew about the shark-fishing ban, even though the sanctuary was not yet legally in force at the time.
After the confiscation of 6 weeks' worth of catch from one of their vessels, all Osi fishers decided that continuing to fish in Raja Ampat was not worth the financial risk, “because of the high operational costs” (Active fisher, Osi, 08/2012). In addition to fuel, gear and food, operational costs included permits that the fishers bought from different levels of government as well as the villages whose reefs they fished. Having paid for what they understood to be legal permits to fish, several Osi fishers remained confident that their vessel was mistakenly apprehended. However, official sources confirmed that any permits obtained for shark fishing in Raja Ampat were invalid. When the fishers from Osi were asked how the fishing ban had affected them, 66% said that it resulted in less income since they were forced to fish in less productive fishing grounds, predominantly along the southeast coast of Halmahera. Informal follow-up conversations in the year following the interviews revealed that several fishers had adapted to the loss of former fishing grounds by diversifying or changing their livelihoods. Some fishers attempted to replace shark fishing with small-scale reef fishing from wooden canoes, while their shark fishing boats were parked on the beach and began to fall apart. Others began using their shark vessels to (illegally) transport and sell fuel to communities in Raja Ampat, where fuel shortages were quite common at the time. These self-initiated alternative livelihoods show that exclusion from fishing grounds does not necessarily result in a rise in illegal fishing, and that even relatively minor enforcement strategies such as confiscation of gear and catch—as opposed to imprisonment and fines, for example—can successfully discourage continued fishing.
However, the responses of shark fishers from Osi also show that spatial closures can have potentially far-reaching impacts on other shark populations as fishers shift their effort to other fishing grounds. The insights from this study suggest that although spatial closures can provide ecological benefits to marine life, they should be nested within a broader conservation strategy to provide fishers with incentives to leave the fishery, and livelihood options that are legal and sustainable. This is particularly important in terms of nation-wide shark conservation and fisheries management efforts, if displaced fishing effort or a shift toward other unsustainable livelihoods is to be prevented. The poverty and remoteness of most Indonesian fishing communities mean that they are severely disadvantaged in terms of access to public health services, education, and markets. Reductions in income are likely to exacerbate these limitations on welfare, which points to the need for increased and better integrated efforts to protect and diversify fishers' livelihoods alongside conservation initiatives to protect threatened species. If fishers' responses and needs are ignored, protection of certain species or ecosystems in one region may well lead to increased exploitation in another.
VJ designed the study, collected the data, processed and analyzed the data, and wrote the manuscript. SL designed the study, analyzed the data and wrote the manuscript. SM designed the study, provided logistical support, and wrote the manuscript. CW, BF, NL designed the study and revised the manuscript. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully acknowledge the generous support and assistance of Misool Eco Resort and field staff of TNC during fieldwork. We thank the fishing communities of Osi, Dobo, and Pepela for their hospitality, help with data collection at sea and their willingness to share their perceptions on sensitive topics. A special thank you to Kate Fraser and Bertha Ronsumbre for their invaluable assistance and companionship in the field. Purwanto (TNC) in Sorong and Endang Jamal (Universitas Pattimura) in Ambon provided logistical advice and answered follow-up questions. Augy Syahailatua sponsored VFJ's Indonesian research permits and provided accommodation in Ambon. Paolo Momigliano facilitated genetic analysis of tissue samples. This study was funded by a Prime Minister's Australia-Asia Endeavour Award, a grant from the Karl Mayer Foundation in Switzerland and an Australian Postgraduate Award for PhD studies to VJ. Data collection and analysis were conducted under animal ethics permits O2484/12 and human ethics permits 2012/010 from Murdoch University and Indonesian research permits 035/SIP/FRP/SM/I/2012 and 13/EXT/SIP/FRP/SM/I/2013 to VJ, issued by RISTEK Indonesia.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2016.00043
Allen, G. R., and Erdmann, M. V. (2012). Reef Fishes of the East Indies, Vol. I–III. Perth: Tropical Reef Research.
Anderson, M., Gorley, R. N., and Clarke, R. K. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: Plymouth Marine Laboratory.
Anderson, M. J., and Willis, T. J. (2003). Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84, 511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2
Ban, N. C., Adams, V. M., Almany, G. R., Ban, S., Cinner, J. E., McCook, L. J., et al. (2011). Designing, implementing and managing marine protected areas: emerging trends and opportunities for coral reef nations. J. Exp. Mar. Bio. Ecol. 408, 21–31. doi: 10.1016/j.jembe.2011.07.023
Baum, J. K., Myers, R. A., Kehler, D. G., Worm, B., Harley, S. J., and Doherty, P. (2003). Collapse and conservation of shark populations in the Northwest Atlantic. Science 299, 389–392. doi: 10.1126/science.1079777
Beer, A. J. E. (2015). Diversity and Abundance of Sharks in No-Take and Fished Sites in the Marine Protected Area Network of Raja Ampat, West Papua, Indonesia, Using Baited Remote Underwater Video (BRUVs). Masters thesis, Royal Roads University.
Blaber, S. J. M., Dichmont, C. M., White, W., Buckworth, R., Sadiyah, L., Iskandar, B., et al. (2009). Elasmobranchs in southern Indonesian fisheries: the fisheries, the status of the stocks and management options. Rev. Fish Biol. Fish. 19, 367–391. doi: 10.1007/s11160-009-9110-9
Bond, M. E., Babcock, E. A., Pikitch, E. K., Abercrombie, D. L., Lamb, N. F., and Chapman, D. D. (2012). Reef sharks exhibit site-fidelity and higher relative abundance in marine reserves on the Mesoamerican Barrier Reef. PLoS ONE 7:e32983. doi: 10.1371/journal.pone.0032983
Braccini, M. (2015). Is a global quantitative assessment of shark populations warranted? Fisheries 40, 492–501. doi: 10.1080/03632415.2015.1080689
Bromhead, D., Clarke, S., Hoyle, S., Muller, B., Sharples, P., and Harley, S. (2012). Identification of factors influencing shark catch and mortality in the Marshall Islands tuna longline fishery and management implications. J. Fish Biol. 80, 1870–1894. doi: 10.1111/j.1095-8649.2012.03238.x
Brunnschweiler, J. M. (2010). The shark reef marine reserve: a marine tourism project in Fiji involving local communities. J. Sustain. Tour. 18, 29–42. doi: 10.1080/09669580903071987
Burgess, G. H., Beerkircher, L. R., Cailliet, G. M., Carlson, J. K., Cortes, E., Goldman, K. J., et al. (2005). Is the collapse of shark populations in the Northwest Atlantic Ocean and Gulf of Mexico real? Fisheries 30, 19–26. doi: 10.1577/1548-8446(2005)30[19:ITCOSP]2.0.CO;2
Chin, A., Simpfendorfer, C., Tobin, A., and Heupel, M. (2013). Validated age, growth and reproductive biology of Carcharhinus melanopterus, a widely distributed and exploited reef shark. Mar. Freshw. Res. 64, 965–975. doi: 10.1071/MF13017
Christie, P. (2004). Marine protected areas as biological successes and social failures in Southeast Asia. Am. Fish. Soc. 42, 155–164.
Clua, E., Buray, N., Legendre, P., Mourier, J., and Planes, S. (2011). Business partner or simple catch? The economic value of the sicklefin lemon shark in French Polynesia. Mar. Freshw. Res. 62, 764–770. doi: 10.1071/MF10163
Davidson, L. N. K., Krawchuk, M. A., and Dulvy, N. K. (2015). Why have global shark and ray landings declined: improved management or overfishing? Fish Fish. doi: 10.1111/faf.12119. [Epub ahead of print].
Dharmadi, Fahmi, Satria, F. (2015). Fisheries management and conservation of sharks in Indonesia. Afr. J. Mar. Sci. 37, 249–258. doi: 10.2989/1814232X.2015.1045431
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world's sharks and rays. Elife 3, 1–35. doi: 10.7554/eLife.00590
Dulvy, N. K., Baum, J. K., Clarke, S., Compagno, L. J. V., Cortés, E., Domingo, A., et al. (2008). You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 18, 459–482. doi: 10.1002/aqc.975
Edgar, G. J., Stuart-Smith, R. D., Willis, T. J., Kininmonth, S., Baker, S. C., Banks, S., et al. (2014). Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220. doi: 10.1038/nature13022
Espinoza, M., Cappo, M., Heupel, M. R., Tobin, A. J., and Simpfendorfer, C. A. (2014). Quantifying shark distribution patterns and species-habitat associations: implications of Marine Park Zoning. PLoS ONE 9:e106885. doi: 10.1371/journal.pone.0106885
FAO FishStatJ (2015). Fisheries and Aquaculture Software. FishStatJ - Software for Fishery Statistical Time Series. Rome: FAO Fisheries and Aquaculture Department.
Ferretti, F., Myers, R. A., Serena, F., and Lotze, H. K. (2008). Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 22, 952–964. doi: 10.1111/j.1523-1739.2008.00938.x
Friedlander, A. M., and Demartini, E. E. (2002). Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 230, 253–264. doi: 10.3354/meps230253
Gallagher, A. J., and Hammerschlag, N. (2011). Global shark currency: the distribution, frequency, and economic value of shark ecotourism. Curr. Issues Tour. 14, 797–812. doi: 10.1080/13683500.2011.585227
Gallagher, A. J., Vianna, G. M. S., Papastamatiou, Y. P., Macdonald, C., Guttridge, T. L., Hammerschlag, N., et al. (2015). Biological effects, conservation potential, and research priorities of shark diving tourism. Biol. Conserv. 184, 365–379. doi: 10.1016/j.biocon.2015.02.007
Goetze, J. S., and Fullwood, L. A. F. (2012). Fiji's largest marine reserve benefits reef sharks. Coral Reefs 32, 121–125. doi: 10.1007/s00338-012-0970-4
Green, A. L., Maypa, A. P., Almany, G. R., Rhodes, K. L., Weeks, R., Abesamis, R. A., et al. (2014). Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol. Rev. 90, 1215–1247. doi: 10.1111/brv.12155
Heithaus, M. R., Frid, A., Vaudo, J. J., Worm, B., and Wirsing, A. J. (2010). “Unraveling the ecological importance of elasmobranchs,” in Sharks and Their Relatives II: Biodiversity, Adaptive Physiology and Conservation, eds J. C. Carier, J. A. Musick, and M. R. Heithaus (Boca Raton, FL: CRC Press), 607–634.
Heupel, M. R., Carlson, J. K., and Simpfendorfer, C. A. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Heupel, M. R., Williams, A. J., Welch, D. J., Ballagh, A., Mapstone, B. D., Carlos, G., et al. (2009). Effects of fishing on tropical reef associated shark populations on the great barrier reef. Fish. Res. 95, 350–361. doi: 10.1016/j.fishres.2008.10.005
Hoyt, E. (2014). “The role of marine protected areas and sanctuaries,” in Sharks: Conservation, Governance and Management, eds E. J. Techera and N. Klein (New York, NY: Routledge), 263–285.
Jaiteh, V. F., and Momigliano, P. (2015). New distribution records of the vulnerable fossil shark Hemipristis elongata from eastern Indonesia call for improved fisheries management. Mar. Biodivers. Rec. 8, 1–5. doi: 10.1017/s1755267215000548
Langlois, T., Harvey, E., Fitzpatrick, B., Meeuwig, J., Shedrawi, G., and Watson, D. (2010). Cost-efficient sampling of fish assemblages: comparison of baited video stations and diver video transects. Aquat. Biol. 9, 155–168. doi: 10.3354/ab00235
Mangubhai, S., Erdmann, M. V., Wilson, J. R., Huffard, C. L., Ballamu, F., Hidayat, N. I., et al. (2012). Papuan Bird's head seascape: emerging threats and challenges in the global center of marine biodiversity. Mar. Pollut. Bull. 64, 2279–2295. doi: 10.1016/j.marpolbul.2012.07.024
Mangubhai, S., Wilson, J. R., Rumetna, L., Maturbongs, Y., and Purwanto (2015). Explicitly incorporating socioeconomic criteria and data into marine protected area zoning. Ocean Coast. Manag. 116, 523–529. doi: 10.1016/j.ocecoaman.2015.08.018
Mascia, M. B., Claus, C. A., and Naidoo, R. (2010). Impacts of marine protected areas on fishing communities. Conserv. Biol. 24, 1424–1429. doi: 10.1111/j.1523-1739.2010.01523.x
McKibben, J. N., and Nelson, D. R. (1986). Patterns of movement and grouping of gray reef sharks, Carcharhinus amblyrhynchos, at Enewetak, Marshall Islands. Bull. Mar. Sci. 38, 89–110.
McLeod, E., Szuster, B., and Salm, R. (2009). Sasi and marine conservation in Raja Ampat, Indonesia. Coast. Manag. 37, 656–676. doi: 10.1080/08920750903244143
Momigliano, P., Harcourt, R., and Stow, A. (2015). Conserving coral reef organisms that lack larval dispersal: are networks of marine protected areas good enough? Front. Mar. Sci. 2:16. doi: 10.3389/fmars.2015.00016
Momigliano, P., Jaiteh, V., and Speed, C. (2014). “Predators in danger: shark conservation and management in Australia, New Zealand, and their neighbours,” in Austral Ark, eds A. Stow, G. Holwell, and N. Maclean (Cambridge, UK: Cambridge University Press), 467–491.
Muhajir, Purwanto, Mangubhai, S. (2012). Marine Resource Use Monitoring in Misool Marine Protected Area, Raja Ampat, West Papua, 2006–2011. Report No. 4/12, The Nature Conservancy, Bali.
Nelson, D. R., and Johnson, R. H. (1980). Behavior of the reef sharks of Rangiroa, French Polynesia. Natl. Geogr. Soc. Res. Rep. 12, 479–499.
Papastamatiou, Y. P., Friedlander, A. M., Caselle, J. E., and Lowe, C. G. (2010). Long-term movement patterns and trophic ecology of blacktip reef sharks (Carcharhinus melanopterus) at Palmyra Atoll. J. Exp. Mar. Bio. Ecol. 386, 94–102. doi: 10.1016/j.jembe.2010.02.009
Papastamatiou, Y. P., Lowe, C. G., Caselle, J. E., and Friedlander, A. M. (2009). Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 90, 996–1008. doi: 10.1890/08-0491.1
Pollnac, R., Christie, P., Cinner, J. E., Dalton, T., Daw, T. M., Forrester, G. E., et al. (2010). Marine reserves as linked social-ecological systems. Proc. Natl. Acad. Sci. U.S.A. 107, 18262–18265. doi: 10.1073/pnas.0908266107
Rizzari, J. R., Frisch, A. J., and Connolly, S. R. (2014). How robust are estimates of coral reef shark depletion? Biol. Conserv. 176, 39–47. doi: 10.1016/j.biocon.2014.05.003
Robbins, W. (2006). Abundance, Demography and Population Structure of the Grey Reef Shark (Carcharhinus amblyrhynchos) and the White Tip Reef Shark (Triaenodon obesus) (Fam. Charcharhinidae). Ph.D. thesis, James Cook University, Townsville.
Robbins, W. D., Hisano, M., Connolly, S. R., and Choat, J. H. (2006). Ongoing collapse of coral-reef shark populations. Curr. Biol. 16, 2314–2319. doi: 10.1016/j.cub.2006.09.044
Russ, G. R., and Alcala, A. C. (2011). Enhanced biodiversity beyond marine reserve boundaries: the cup spillith over. Ecol. Appl. 21, 241–250. doi: 10.1890/09-1197.1
Santana-Garcon, J., Braccini, M., Langlois, T. J., Newman, S. J., McAuley, R. B., and Harvey, E. (2014). Calibration of pelagic stereo-BRUVs and scientific longline surveys for sampling sharks. Methods Ecol. Evol. 5, 824–833. doi: 10.1111/2041-210X.12216
Seager, J. (2014). Event Measure. Measurement Science Specialists. Available online at: http://www.seagis.com.au
Smith, S. E., Au, D. W., and Show, C. (1998). Intrinsic rebound potentials of 26 species of Pacific sharks. Mar. Freshw. Res. 49, 663. doi: 10.1071/MF97135
Speed, C. W., Field, I. C., Meekan, M. G., and Bradshaw, C. J. A. (2010). Complexities of coastal shark movements and their implications for management. Mar. Ecol. Prog. Ser. 408, 275–293. doi: 10.3354/meps08581
Speed, C. W., Meekan, M. G., Field, I. C., McMahon, C. R., Harcourt, R. G., Stevens, J. D., et al. (2016). Reef shark movements relative to a coastal marine protected area. Reg. Stud. Mar. Sci. 3, 58–66. doi: 10.1016/j.rsma.2015.05.002
Sumaila, U. R., Guénette, S., Alder, J., and Chuenpagdee, R. (2000). Addressing ecosystem effects of fishing using marine protected areas. ICES J. Mar. Sci. 57, 752–760. doi: 10.1006/jmsc.2000.0732
Varkey, D. A., Ainsworth, C. H., Pitcher, T. J., Goram, Y., and Sumaila, R. (2010). Illegal, unreported and unregulated fisheries catch in Raja Ampat Regency, Eastern Indonesia. Mar. Policy 34, 228–236. doi: 10.1016/j.marpol.2009.06.009
Veron, J. E. N., DeVantier, L. M., Turak, E., Green, A. L., Kininmonth, S., Stafford-Smith, M., et al. (2009). Delineating the coral triangle. Galaxea (Tokyo). 11, 91–100. doi: 10.3755/galaxea.11.91
Vianna, G., Meeuwig, J., Pannell, D., Sykes, H., and Meekan, M. (2011). The Socio-Economic Value of the Shark-Diving Industry in Fiji. Australian Institute of Marine Science, University of Western Australia.
Vianna, G. M. S., Meekan, M. G., Meeuwig, J. J., and Speed, C. W. (2013). Environmental influences on patterns of vertical movement and site fidelity of grey reef sharks (Carcharhinus amblyrhynchos) at aggregation sites. PLoS ONE 8:e60331. doi: 10.1371/journal.pone.0060331
Vianna, G. M. S., Meekan, M. G., Pannell, D. J., Marsh, S. P., and Meeuwig, J. J. (2012). Socio-economic value and community benefits from shark-diving tourism in Palau: a sustainable use of reef shark populations. Biol. Conserv. 145, 267–277. doi: 10.1016/j.biocon.2011.11.022
Ward-Paige, C. A., Keith, D. M., Worm, B., and Lotze, H. K. (2012). Recovery potential and conservation options for elasmobranchs. J. Fish Biol. 80, 1844–1869. doi: 10.1111/j.1095-8649.2012.03246.x
Ward-Paige, C. A., Mora, C., Lotze, H. K., Pattengill-Semmens, C., McClenachan, L., Arias-Castro, E., et al. (2010). Large-scale absence of sharks on reefs in the greater-caribbean: a footprint of human pressures. PLoS ONE 5:e11968. doi: 10.1371/journal.pone.0011968
West, P., Igoe, J., and Brockington, D. (2006). Parks and peoples: the social impact of protected areas. Annu. Rev. Anthropol. 35, 251–277. doi: 10.1146/annurev.anthro.35.081705.123308
Whitcraft, S., Hofford, A., Hilton, P., O'Malley, M., Jaiteh, V., and Knights, P. (2014). Evidence of Declines in Shark fin Demand, China. San Francisco, CA: WildAid.
White, E. R., Myers, M. C., Flemming, J. M., and Baum, J. K. (2015). Shifting elasmobranch community assemblage at Cocos Island-an isolated marine protected area. Conserv. Biol. 29, 1186–1197. doi: 10.1111/cobi.12478
White, W. T., Last, P. R., Stevens, J. D., Yearsley, G. K., and Fahmi, D. (2006). Economically Important Sharks and Rays of Indonesia. Canberra: Australian Centre for International Agricultural Research.
Whitney, N. M., Pyle, R. L., Holland, K. N., and Barcz, J. T. (2012). Movements, reproductive seasonality, and fisheries interactions in the whitetip reef shark (Triaenodon obesus) from community-contributed photographs. Environ. Biol. Fishes 93, 121–136. doi: 10.1007/s10641-011-9897-9
Wilson, S. K., Graham, N. A. J., and Polunin, N. V. C. (2007). Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 151, 1069–1076. doi: 10.1007/s00227-006-0538-3
Keywords: Coral Triangle, small-scale fishing, shark finning, spatial closures, marine protected areas, reef sharks
Citation: Jaiteh VF, Lindfield SJ, Mangubhai S, Warren C, Fitzpatrick B and Loneragan NR (2016) Higher Abundance of Marine Predators and Changes in Fishers' Behavior Following Spatial Protection within the World's Biggest Shark Fishery. Front. Mar. Sci. 3:43. doi: 10.3389/fmars.2016.00043
Received: 07 December 2015; Accepted: 20 March 2016;
Published: 07 April 2016.
Edited by:
Romuald Lipcius, College of William & Mary, USAReviewed by:
Leslie Cornick, Alaska Pacific University, USACopyright © 2016 Jaiteh, Lindfield, Mangubhai, Warren, Fitzpatrick and Loneragan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vanessa F. Jaiteh, dmFuZXNzYS5qYWl0ZWhAb3V0bG9vay5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.