
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 10 July 2015
Sec. Global Change and the Future Ocean
Volume 2 - 2015 | https://doi.org/10.3389/fmars.2015.00049
This article is part of the Research Topic Effects of climate change across ocean regions View all 11 articles
 Alexandra Steckbauer1*
Alexandra Steckbauer1* Laura Ramajo1,2
Laura Ramajo1,2 Iris E. Hendriks1
Iris E. Hendriks1 Miriam Fernandez3
Miriam Fernandez3 Nelson A. Lagos2,4
Nelson A. Lagos2,4 Luis Prado2
Luis Prado2 Carlos M. Duarte1,5
Carlos M. Duarte1,5Ocean acidification (OA) and hypoxic events are an increasing worldwide problem, but the synergetic effects of these factors are seldom explored. However, this synergetic occurrence of stressors is prevalent. The coastline of Chile not only suffers from coastal hypoxia but the cold, oxygen-poor waters in upwelling events are also supersaturated in CO2, a study site to explore the combined effect of OA and hypoxia. We experimentally evaluated the metabolic response of different invertebrate species (2 anthozoans, 9 molluscs, 4 crustaceans, 2 echinoderms) of the coastline of central Chile (33°30′S, 71°37′W) to hypoxia and OA within predicted levels and in a full factorial design. Organisms were exposed to 4 different treatments (ambient, low oxygen, high CO2, and the combination of low oxygen and high CO2) and metabolism was measured after 3 and 6 days. We show that the combination of hypoxia and increased pCO2 reduces the respiration significantly, compared to a single stressor. The evaluation of synergistic pressures, a more realistic scenario than single stressors, is crucial to evaluate the effect of future changes for coastal species and our results provide the first insight on what might happen in the next 100 years.
The term Ocean Acidification (OA) is used to describe the decline in seawater pH due to the invasion of ocean waters by anthropogenic CO2 (Caldeira and Wickett, 2003; Caldeira, 2005; Orr et al., 2005; Raven, 2005). About 1/3 of the CO2 released by human activity since the industrial revolution has entered the ocean, leading to a decline in surface pH values by ~0.12 units, with a further decrease of 0.3–0.4 units predicted for a doubling of atmospheric CO2 by the end of the century (Orr et al., 2005; Doney et al., 2009). These decreases in pH are expected to have negative but variable effects specifically on calcifying organisms as altered carbonate chemistry directly affects the deposition and dissolution rates of the CaCO3 used for structures (Gattuso and Buddemeier, 2000; Orr et al., 2005; Raven, 2005; Kleypas et al., 2006; Gazeau et al., 2007; Fabry et al., 2008; Range et al., 2011; Andersson and Gledhill, 2013; Kroeker et al., 2013). Calcifying organisms, such as corals (Marubini and Davies, 1996; Gattuso et al., 1998; Marubini and Atkinson, 1999; Langdon et al., 2000), coral reef communities (Langdon et al., 2000, 2003; Leclercq et al., 2000), and planktonic organisms (Bijma, 1991; Riebesell et al., 2000), are known to be among the most vulnerable. Nevertheless, it has been shown in several studies, that some corals and molluscs were able to calcify and grow even faster when transplanted along carbonate saturation gradients (Rodolfo-Metalpa et al., 2011). Even under low pH some species are able to maintain, or even increase, their net calcification, indicating that the use of carbonate saturation state is inconsistent to predict marine calcification (Wood et al., 2008; Cohen et al., 2009; Ries et al., 2009; Rodolfo-Metalpa et al., 2010). As reviewed by Hendriks et al. (2015), these organisms have different mechanism to cope with OA. Close to the organism's surface the pH can be higher through metabolic activity since rate limiting transport in the Diffusive Boundary Layer (DBL) prevents a direct equilibration with the water column (Hendriks et al., 2015). In foraminifera and diatoms the pH in the DBL ranges from 8.0 to 9.1 (Köhler-Rink and Kühl, 2005; Kühn and Raven, 2008), which allows them to create a microenvironment with increased pH (by 0.5 units) compared to ambient seawater. Moreover, calcifying organisms are able to control the pH in extracellular fluids, or control the deposition in a regulated, intracellular environment. Tissues and external organic layers play a major role in protecting shells and skeletons from corrosive sea water, limiting dissolution, and allowing organisms to calcify (Ries, 2011; Trotter et al., 2011). Some organisms can benefit from symbiotic relationships, e.g., coral symbionts remove CO2 and increase pH due to photosynthesis, enhance conditions for calcification and growth (Gattuso and Jaubert, 1990; Muscatine, 1990).
Whereas increasing atmospheric CO2 clearly drives OA in the open ocean, drivers of changes in pH and the carbonate system in coastal systems are far more complex (Duarte et al., 2013; Waldbusser and Salisbury, 2014). Coastal ecosystems, unlike the surface waters of the open ocean, may display a diversity of pH trajectories, affected by emissions from volcanic vents, watershed processes, eutrophication, upwelling, and changes in ecosystem structure and metabolism (Duarte et al., 2013). Therefore, the carbon system of the coastal ocean is more dynamic and complex than that of the open ocean (Borges and Gypens, 2010; Cai, 2011), and thereby, a general prediction of the trajectories of pH for coastal systems is difficult to make, as regional differences will be important (Duarte et al., 2013). In these shallow environments benthic engineering species, such as corals, seagrass, macroalgae, salt marshes, mangroves, sponges, and oyster reefs, have the capacity to affect the chemical and physical conditions of the ecosystem (Gutierrez et al., 2011), and exert metabolic control on coastal seawater pH values and variability (Duarte et al., 2013).
Coastal ecosystems are also progressively affected by hypoxia, with a current rate of increase of 5.5 ± 0.2% year−1 in coastal areas (Vaquer-Sunyer and Duarte, 2008), and predicted of faster increase in the future (Conley et al., 2009). Hypoxia, is a condition characterized by oxygen levels below a threshold where marine organisms show atypical behavior (Riedel et al., 2013) and eventually leads to mass mortality (Diaz and Rosenberg, 1995; Vaquer-Sunyer and Duarte, 2008). It is typically triggered by respiratory consumption of oxygen to remineralize the excess of organic matter produced in eutrophic coastal systems (Gray et al., 2002). Accordingly, hypoxic coastal waters are characterized by low O2 concentrations and elevated CO2, and, therefore, low pH (Pörtner et al., 2005). This is also the case of coastal areas affecting by upwelling of oxygen-poor, corrosive waters, such as the Oregon and Washington coasts (Feely et al., 2008; Gruber et al., 2012) and much of the Chilean coast (Mayol et al., 2012). Yet, the bulk of the literature on the impacts of hypoxia on marine invertebrates focuses on the role of low oxygen, and the impact of concurrent reduced pH has been generally ignored.
The Respiration Index (RI) was proposed by Brewer and Peltzer (2009) to capture the combined effects of hypoxia and high CO2 on the efficiency of aerobic respiration, by using the basic oxic respiration equation and the free-energy relation. The RI is a simple numeric constraint linearly related to the available energy to support respiration:
where RI ≤ 0 corresponds to the thermodynamic aerobic limit, a formal dead zone; at RI = 0 to 0.4, aerobic respiration does not occur; the range RI = 0.4–0.7 represents the practical limit for aerobic respiration, and the range RI = 0.7–1.0 delimits the aerobic stress zone (Brewer and Peltzer, 2009). The RI links hypoxia and CO2, implying that the thermodynamic constraints for aerobic organisms do not depend on O2 alone, but also on CO2. The implication is that high CO2, by lowering RI, affects the vulnerability of marine organisms to hypoxia.
Considering the impact of CO2 on respiration suggests that the distribution and spatial extent of ocean dead zones will rise, even if the oxygen levels as such do not decline, as a result of rising CO2 concentrations (Brewer and Peltzer, 2009), which will increase the stress to aerobic organisms and raise the O2 thresholds for hypoxia. Rising CO2 concentrations will induce metabolic depression in invertebrate species, reduce the rate of gas exchange across respiratory epithelia, deplete the internal oxygen stores, and accumulate respiratory CO2 (Pörtner et al., 2005) and, thereby, decrease the buffering capacity in hypoxic bottom water (Hagens et al., 2015).
However, the RI index has not been experimentally tested and the underlying expectations have been criticized. Seibel and Childress (2013) argue that CO2 could never reach concentrations that would limit the thermodynamics of this reaction, because of the large standard free energy change for organic carbon oxidation (ΔG° = −686 kcal mol−1), and that a PCO2:PO2 ratio of 10503 would be required to reach equilibrium (equilibrium constant, Keq = 10503; where ΔG = 0). Thus, they argued that a RI of −503 would be the real thermodynamic limit to aerobic life. Although it has been shown that in crabs and catfish the pCO2 in plasma dropped to 45 and 56 mm Hg, respectively, when exposed to elevated CO2, Pörtner et al. (2005), Seibel and Childress (2013), and Cameron and Iwama (1989) argue that cellular respiration and oxygen provision are kinetically controlled and environmental oxygen and CO2 concentrations exert little control on intracellular concentrations. Yet, evidence for synergistic effects of low O2 and high CO2 includes increased bacterial infections in the pacific white shrimp Litopenaeus vannamei (Burgents et al., 2005), inhibition of growth and metamorphosis in the early life stage of bivalves (bay scallops, Argopecten irradians, and hard clams, Mercenaria mercenaria, Gobler et al., 2014), depressed growth rates for juvenile red abalone (Haliotis rufescens, Kim et al., 2013) and synergistic metabolic depression via the effect of adenosine on central nervous functions of the marine invertebrate Sipunculus nudus (Reipschläger et al., 1997; Pörtner et al., 2005). In field studies hypoxia and OA seasonally may occur simultaneously in shallow water tidal creeks and lead to sub-lethal effects on organismal and populational levels and reduce oxygen uptake in blue crabs Callinectes sapidus (Hypes, 1999). Regardless of the accuracy of the thresholds of RI proposed by Brewer and Peltzer (2009), it is clear that the efficiency of aerobic respiratory processes is dependent on the ratio of the partial pressures of both O2 and CO2, suggesting that threats from hypoxia will also be aggravated by increasing CO2 (Brewer and Peltzer, 2009). This is particularly important, as hypoxic and high CO2 stresses are likely to co-occur (Mayol et al., 2012), with both stresses forecasted to increase in the future (Orr et al., 2005; Vaquer-Sunyer and Duarte, 2008).
Here we evaluate the combined effects of hypoxia and OA on the survival and metabolic rates of benthic invertebrate populations in Central Chile. The invertebrates of the coastline along Chile may be regularly exposed to both stressors, as the Humboldt Current System (HCS) is one of the largest naturally hypoxic areas of the word's oceans (Levin et al., 2002; Thiel et al., 2007; Ulloa and Pantoja, 2009). The HCS is a quite complex dynamic region, characterized by the presence of a system of along-slope currents that brings waters of both tropical and subpolar origin, and by upwelling of cold, oxygen-poor waters supersaturated in CO2 (Torres et al., 2002; Mayol et al., 2012). Hence, invertebrates in the HCS coastal region may regularly experience high CO2 and low O2 and are expected to be adapted to these stressors. All except anthozoans are calcifying species, believed to be particularly vulnerable to OA (Kroeker et al., 2013). We experimentally tested the effect of these stressors on invertebrate species by exposing them to 4 different treatments (high O2 and low CO2, low O2 and low CO2, high O2 and high CO2, and both low O2 and high CO2) and measuring survival and respiration rate after 3 and 6 days.
The experiments were conducted between October 17 and December 13, 2012 at the ECIM marine station in Las Cruces, Chile. Organisms were collected during low tide from two sites, the surrounding coastal area of the ECIM marine reserve at Las Cruces and El Tabo, both located on the coastline of central Chile (33°30′S, 71°37′W).
A total of 17 species out of 4 taxonomic groups were tested at control and 3 treatment conditions (Table 1). The selected invertebrate species included 2 anthozoa, 9 molluscs, 4 crustaceans, and 2 echinoderms (Table 2) collected along the coastline of Las Cruces and El Tabo during low tide. These species were selected because of their abundance and significance along the coast, often including a commercial use (e.g., Tegula atra, Prisogaster niger, and Concholepas concholepas). Individuals were acclimated in 25L-tanks with aeration and running seawater, allowing conditions to follow the natural fluctuations occurring in the sea (average ± SD; pH ~ 7.596 ± 0.040, oxygen ~ 8.60 ± 1.10 mg L−1, temperature ~ 15.44 ± 0.07 °C, salinity = 34.26 ± 0.089, see Ramajo et al., 2013; Lardies et al., 2014) for at least 2 days, before being placed into experimental aquaria. Previous to experiments, predators were fed every 1–2 days with bivalves and gastropods, which were collected at the same sites.
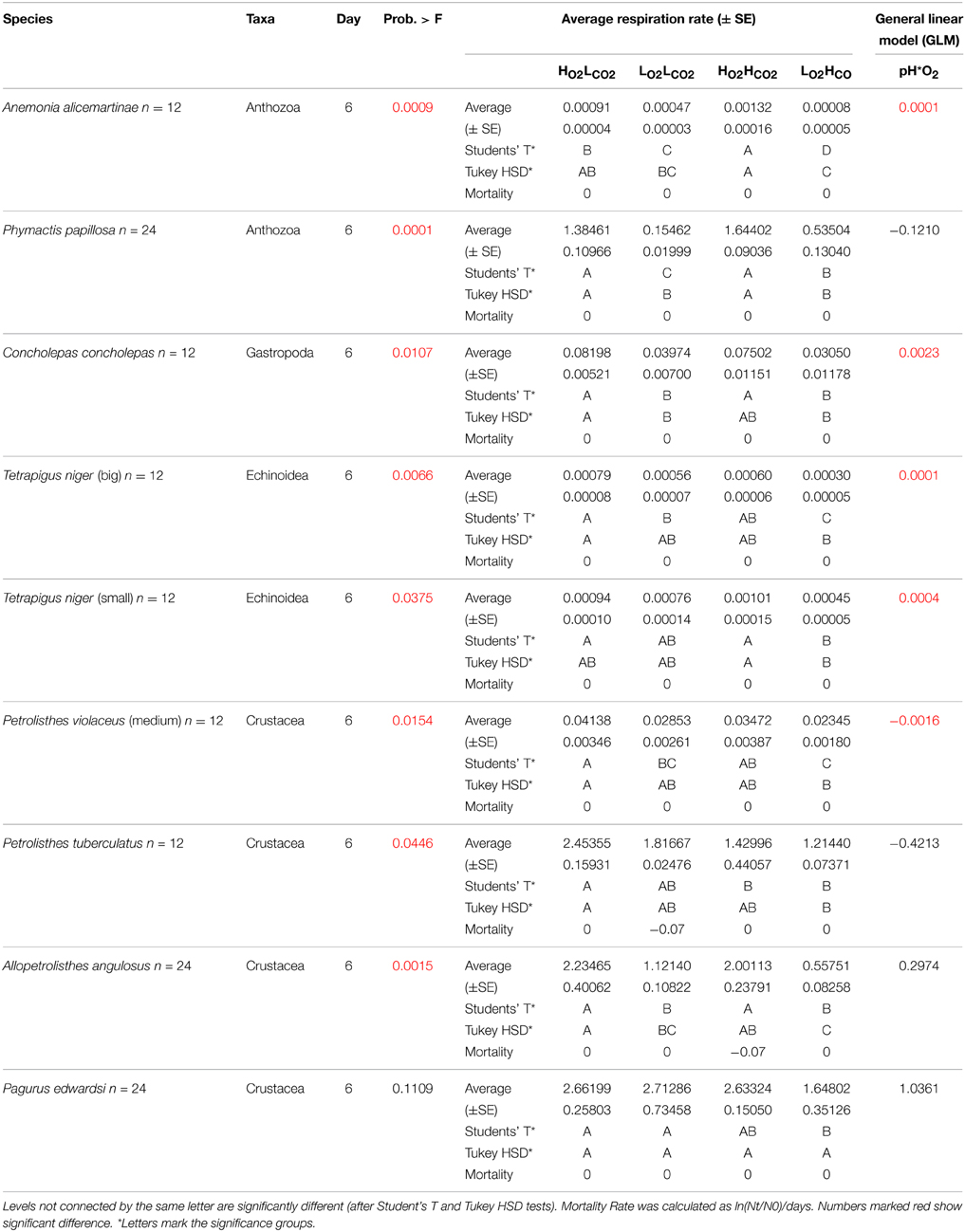
Table 2. Respiration rate (± SE) and results of the General Linear Model off all tested species after 6 days.
Four experimental conditions were used, involving two different levels of pH and oxygen: (1) HO2LCO2—involving pH corresponding to atmospheric equilibrium (380 ppm) and saturated oxygen (20% oxygen in the gas mixture); (2) LO2LCO2—pCO2 corresponding to atmospheric equilibrium (380 ppm) and low oxygen (4% oxygen in the gas mixture); (3) HO2HCO2—a treatment with elevated CO2 (low pH), corresponding to atmospheric levels expected by the end of the century (1000 ppm, Orr et al., 2005) and saturated oxygen; and (4) LO2HCO2—treatment with low O2 (4% oxygen in the gas mixture) and high CO2 (1000 ppm) and low pH. These four experimental conditions conform to an RI gradient, ranging from 0.81 ± 0.01 RI, indicative of aerobic stress (LO2HCO2treatment) to an RI of 1.69 ± 0.02, without limits for aerobic respiration (HO2LCO2 conditions). The respiration index was calculated after Equation (1) following Brewer and Peltzer (2009) from the average of the daily pO2 and pCO2 measurements of the four treatments.
To reach the treatment conditions the aquaria were bubbled with a mixture of nitrogen and air to lower the oxygen content, and with pre-determined pCO2 levels. To set the CO2 content of the air, ambient air was collected via pumps and passed through soda-lime columns to strip the air of CO2. Precise volumes of CO2-stripped air and pure CO2 gas from a commercial 50 L-bottle were administrated using mass-flow controllers (MFCs; Aalborg GFC-17) and mixed in a container filled with marbles to increase mixing efficiency by increasing surface area to achieve pCO2 concentrations of 380 ppm (HO2LCO2, LO2LCO2) and 1000 ppm (HO2HCO2 and LO2HCO2). To reach hypoxic conditions, nitrogen was added to the air-CO2 mixture to reduce the oxygen in the water, maintaining the DO between 2.0 and 3.5 mg L−1, corresponding to sublethal hypoxic levels as defined by (Vaquer-Sunyer and Duarte, 2008; Steckbauer et al., 2011).
Aquaria were filled water filtered over 20 μm filters, equilibrated to the treatment conditions, and placed in temperature-controlled tanks set to ambient temperature. Three replicas were used per treatment, resulting in a total of 12 experimental aquaria per species. We used an optic fiber oxygen-meter (Microx TX3, PreSens, Germany), with diameter tips of 20–50 um. Zero calibration was performed using a sodium sulfite (Na2SO3) solution (0% saturation) and 100% was calibrated using vigorously air-bubbled seawater. Experimental pH was measured at 5 min intervals with pHNBS sensors (Metrohm and Hanna Instruments), connected to a Consort D130 datalogger. At least once per week, pH in total scale was measured using a pH–meter (pH mobile 826, Metrohm), connected to a combined electrode (double juncture), calibrated using buffers Tris (pH = 8.089) y 2–Aminopiridine (pH = 6.786) at 25°C in a temperature controlled water bath (Torres et al., 2011). Water samples for alkalinity analyses were taken at least once per week, fixed with 20 μL HgCl2 and analyzed within 3 months, using a Metrohm Titrando 808 after Dickson et al. (2007). pHNBS, temperature, alkalinity and salinity values were used to calculate pCO2, the saturation state of aragonite (ΩAr) and calcite (ΩCa) in each treatment using CO2SYS (Pierrot et al., 2006), with K1 and K2 constants from Mehrbach et al. (1973), as revised by Dickson and Millero (1987), and the KHSO4 constant from Dickson (1990).
After 3 and 6 days, individuals were transferred to 300 or 1000 mL air-tight vessels and incubated in treatment water for 1–5 h, depending on the size of the animal and vessel, to measure oxygen consumption at 14°C. Temperature was stabilized using a temperature-controlled water bath (JioTech, Co). Oxygen was measured using calibrated PreSens micro-optodes at the beginning and the end of the incubation and the difference was used to calculate the consumption rate using dry weight (DW) and size (in mm) as mg O2g−1 DW min−1 and mg O2 mm−1 min−1. After the experiment, the body size (maximum length, mm) and wet weight (g) of the animals were measured, and the organisms were kept frozen until further processing. To evaluate the dry weight, organisms were dried for at least 24 h at 60°C and weighted. For gastropods, shell and soft parts were treated separately.
To compare the results of the 3 treatments to the HO2LCO2 data across species ranging broadly in size and other traits, we calculated the log “effect size” after Hedges et al. (1999) and Gurevitch and Hedges (1999). Response ratios quantify the proportional change resulting from experimental manipulations and ln-transformed response ratios are commonly used because of their robust statistical properties and ease of biological interpretation (Hedges et al., 1999; Kroeker et al., 2010). The effect of the different water conditions on the oxygen consumption was measured for each treatment as the ln-transformed response ratio,
where XE and XC are the mean values of the response variable in the experimental and HO2LCO2 treatments, respectively. As our goal is to test the effects of low O2 and high CO2 as stressors we designated high O2 and low CO2 as the control treatment, even though ambient values in the ecosystem where the organisms grow are closer to the high O2 low CO2 treatment (see below).
Three-Way ANOVA's were conducted to test the effect of species, treatment and time (i.e., difference in the responses measured between day 3 and 6) on the respiration rate. A One-Way ANOVA was used to test for differences in respiration rate between treatments for each species. Where the respiration showed significant differences, a Student's t-test and post-hoc Tukey HSD test were conducted to resolve which treatments resulted in different respiration rates. Moreover, a General Linear Model (GLM) was used to quantify response to changes in pH, oxygen and their interaction. If the interaction term was significant and positive, then there were synergistic effects between the stressors, and if the interaction term was significant and negative the effects were antagonistic. All analyses were done using RStudio (version 0.97.336) and JMP (version 10.0) with the level for significance set at 0.05.
Seawater temperature averaged (±SE) 16.31 ± 0.06°C during the experimental period and did not differ among treatments (Table 1). Mean dissolved oxygen concentration varied from 9.77 ± 0.12 mg O2 L−1 in the normoxic treatment to 2.86 ± 0.08 mg O2 L−1 in the hypoxic treatments, respectively (Table 1), and were significant different from each other (p < 0.001, ANOVA). Mean pH was 8.03 ± 0.01 in the ambient and 7.75 ± 0.01 in the high CO2 treatments (p < 0.001, ANOVA), respectively. The average alkalinity was 2219.0 ± 18.7 μmol kg−1 throughout the treatments and experimental duration. The mean pCO2 in the water was 562 ± 17 μatm in the normal and 1142 ± 25 μatm in the high CO2 treatments, respectively. ΩAr and ΩCa averaged 1.86 ± 0.04 and 2.89 ± 0.05 in the normal and 1.05 ± 0.02 and 1.63 ± 0.03 in the high CO2 treatments, respectively (Table 1). The RI averaged 1.69 ± 0.02 for the HO2LCO2, 1.12 ± 0.03 for the LO2LCO2, 1.31 ± 0.01 for the high CO2 and 0.81 ± 0.01 for the LO2HCO2 treatment (Table 1). The RI values for the hypoxic and high CO2 treatment were similar as the differences in pO2 and pCO2 had a similar effect on RI. All treatments matched the target values and were held to an acceptable level and variability within each treatment (Table 1).
The animals held at HO2LCO2 conditions of high oxygen and normal pH did not experience mortality, indicating that mortality observed in the LO2LCO2, HO2HCO2, and LO2HCO2 treatments was due to the low pH and/or low DO concentration and not to other potential. Yet, survival rates were very high, with only 10 individuals dying out of a total of 320 specimens tested in the experiment after 3 or 6 days. As most of the individuals survived 3 days even in the LO2HCO2 treatment, they were kept in the aquaria up to 6 days. The species were mortality was observed were limpets Fisurella sp. (1x HO2HCO2 and 1x LO2HCO2 on day 4), the polyplacophora Chiton granosus (1x LO2HCO2 on day 3) and Tonica sp. (2x LO2LCO2 and 1x LO2HCO2 on day 3); and the anomura crustaceans Petrolisthes violaceus (1x LO2HCO2 on day 3), Petrolisthes tuberculatus (1x LO2LCO2 on day 3), and Allopetrolisthes angulosus (2x HO2HCO2 on day 3), respectively. However, survival rates were higher than 97% across treatments and species (Table 2), indicating that the experimental conditions represented sublethal stresses.
After the exposition to experimental conditions, the metabolic rate differed between species and taxa. Generally, echinoderms displayed lower respiration rates and the gastropod species Tegula atra and Diloma nigerrima the highest (Table S1). There were significant differences in metabolic rates between treatments (p < 0.001) and species (p < 0.001) but not with the duration of the experiment (p = 0.69; Table 3). The majority of species (65%) showed metabolic depression, which was reflected in reduced respiration rates, when exposed to hypoxia, high CO2 or both stressors (Figure 1). Their negative responses increased over time, although not significant. The fraction of species showing a significant difference in respiration rate with high CO2 increased from 41% after 3 days to 60% after 6 days and those showing significant responses to hypoxia increased from 65% after 3 days to 90% after 6 days, with 100% of the species showing significant responses to both stressors acting together already after 3 and 6 days (Figure 1 and Figure S1). Anthozoans where the most sensitive taxa as there was a significant difference in all 3 treatments compared to the HO2LCO2 (Figure 2). For all other taxa, the result was significantly different for the LO2LCO2 and LO2HCO2 treatment but not always for the HO2HCO2, respectively (Figure 2). The combination of both stressors (LO2HCO2) led to a greater metabolic depression than either stressor alone (Figure 3).
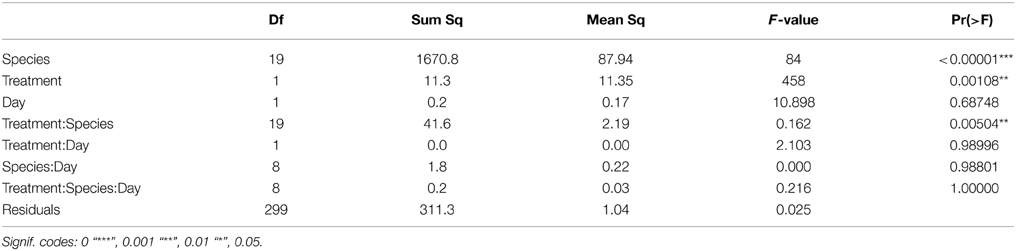
Table 3. Results of the Three-Way ANOVA describing the effects of species, treatment and day on the respiration rate.
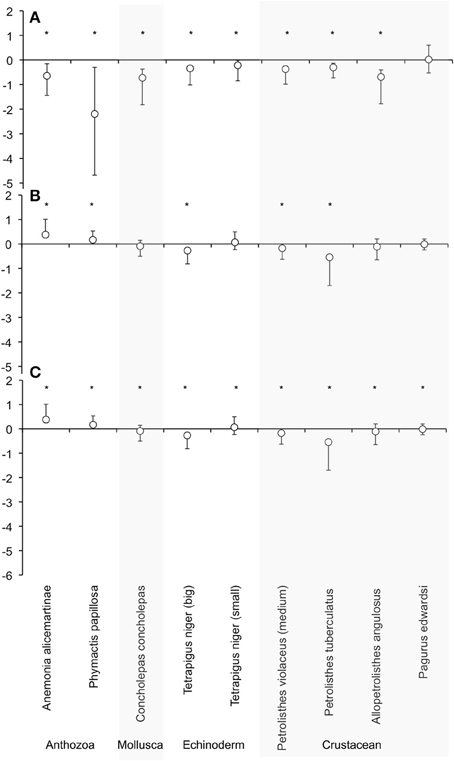
Figure 1. Effect size off of the respiration rate of each species calculated with dry weight after 6 days. Where (A) is LO2LCO2, (B) HO2HCO2, and (C) LO2HCO2 treatment vs. HO2LCO2. LnRR = ln(treatment - HO2LCO2) ± Confidence interval (after Kroeker et al., 2010). *Marks the significant difference between HO2HCO2 and experimental treatments of the effect size.
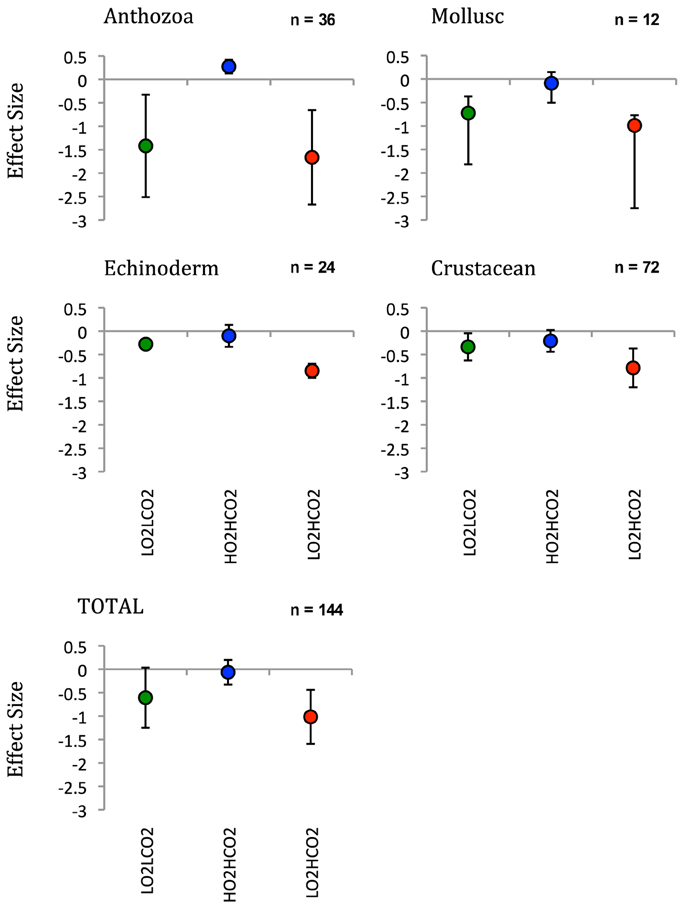
Figure 2. Effect size off of the respiration rate of taxonomic groups calculated with dry weight after 6 days. LnRR = ln(treatment - HO2LCO2) ± Confidence interval (after Kroeker et al., 2010).

Figure 3. Effect size off of the respiration rate of each species calculated with dry weight after 6 days. (A) LO2HCO2 vs. LO2LCO2 and (B) LO2HCO2 vs. HO2HCO2. LnRR = ln(treatment - HO2LCO2) ± Confidence interval (after Kroeker et al., 2010).
Regression analysis of respiration rate in the presence of stressors vs. that in the HO2LCO2 treatment showed that the effects of hypoxia and high CO2 were additive (Figure 4), as the deviations of the slope of the regression line for the LO2HCO2treatment from 1 (1 – slope LO2HCO2 = 0.48 ± 0.11) does not differ from that resulting from the sum of those of the individual treatments (1 – slope LO2LCO2 = 0.24 ± 0.17; 1 – slope HO2HCO2 = 0.15 ± 0.20; expected LO2HCO2 = 0.39 ± 0.18). This finding shows that, overall, the metabolic responses to the stressors tested was additive, and not synergistic or antagonistic, as also confirmed by the general linear model of the metabolic responses of the individual species to the stressors, where after 6 days only in 4 out of the 9 species tested (4 out of 17 species after 3 days, Table S2) showed a significant interaction term between the two stressors (Table 2). The most tolerant taxa after 6 days was the Crustacea, as 3 out of 4 species didn't show significant differences and in Petrolisthes violaceus a significant antagonistic effect was observed.
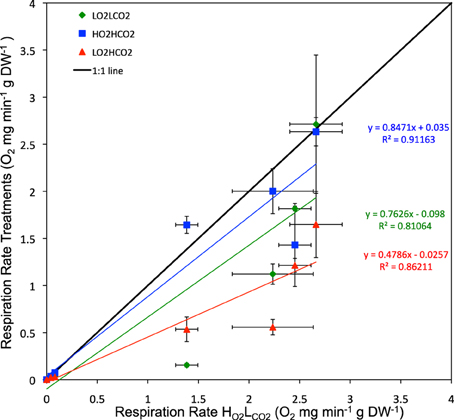
Figure 4. Respiration rate (Average ± SE) after 6 days of all tested species: green – LO2LCO2, blue – HO2HCO2 and red – LO2HCO2.
The respiration rates decreased with decreasing RI in all species as expected (Figure 5), although the relationships between metabolic rates and RI was relatively weak within taxa, due to the different intensity of metabolic rate.
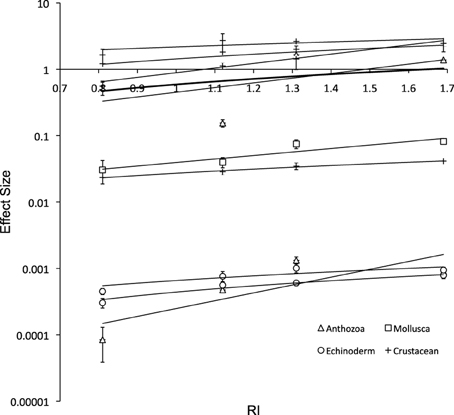
Figure 5. Respiration rate vs. Respiration Index after 6 days (± SE; y = 0.63x – 0.0385, R2 = 0.04). Solid lines show the fitted least squares regression lines for Anthozoa (y = 0.637x – 0.32; R2 = 0.1), Mollusca (y = 0.064x – 0.022; R2 = 0.85), Echinoderm (y = 0.001x – 0.000; R2 = 0.61), Crustacean (y = 1.083x + 0.079; R2 = 0.12), and Total (bold line; y = 0.630x – 0.039; R2 = 0.04); and include the ± SE of the slope, and the P-value from the F-test for each regression.
The tested benthic invertebrates from the central Chilean coast were relatively resistant to hypoxia, high CO2 and their combined effects, as the mortality rate was low across species and metabolic depression, while present, was relatively modest (Table 2). Anthozoans and Crustaceans were relatively vulnerable to hypoxia, while Molluscs and Echinoderms were tolerant. This is consistent with results from Vaquer-Sunyer and Duarte (2008), who showed Molluscs and Echinoderms to be particularly tolerant to hypoxic events compared to Crustaceans. The organisms were comparatively resistant to high CO2 as they showed no significant mortality or metabolic depression when exposed to high CO2. Indeed, exposure to high CO2 showed increased respiration rate in Anthozoans and Echinoderms, as also reported in a recent meta-analysis (Kroeker et al., 2013), rather than a metabolic depression. Although it has been reported that food supply and not pCO2 appears to be the primary factor driving biomass and biogenetic CaCO3 production (Melzner et al., 2011; Thomsen et al., 2013), the effect on respiration rate is controversial (Lampert, 1984). Animals were fed previously, but not during the experiments, as feeding previously to the oxygen measurements was shown to increase respiration rate compared to starved animals. In the HO2HCO2 treatment the concentration of aragonite (ΩAr) was under-saturated (ΩAr < 1) and in the LO2HCO2 treatment close to under-saturation (ΩAr = 1.14 ± 0.15), where calcifiers are expected to be under physiological stress (Doney et al., 2009). Molluscs, depositing mostly aragonite, are expected to be more vulnerable to high CO2 (Porter, 2007) than Echinoderms and Crustaceans, which deposit calcite (Raup, 1959; Raabe et al., 2005).
Most importantly, our results showed that hypoxia and high CO2 have additive effects and revealed no consistent synergetic or antagonistic effect for these stressors. Moreover, the observation of very low mortality rates and relatively modest metabolic depression (on average 52% reduction compared with the values in HO2LCO2 treatments) with both stressors reveals that the Chilean invertebrate species tested are relatively resistant to these stressors. The resistance of invertebrates in the central Chilean coast to hypoxia and high CO2 is nonetheless not surprising as these organisms may experience these conditions in their natural habitat. Whereas pCO2 of 1200 ppm as tested here are used in OA experiments to characterize values expected beyond year 2100 (Kroeker et al., 2013), these values are reached regularly in the Chilean coast (Torres et al., 2011; Mayol et al., 2012). Indeed, in the year preceding this experiments high pCO2 values, of the order of those used in the high treatment here, were found twice, associated with upwelling conditions (N. Lagos, unpubl. data). Moreover, oxygen and pCO2 are closely correlated in the water mass along the Chilean coast (Mayol et al., 2012), so that upwelling events leading to pCO2 values around 1200 ppm are associated with oxygen values of ~2 mg L−1 (Mayol et al., 2012). Hence, the hypoxia and high CO2 treatments used here represent stresses already experienced by these organisms. Comparison of the CO2 and O2 conditions in the treatments with those experienced by the organisms in their habitat shows that the treatment best representing their environment is involving both high O2 and low pH (Figure 6). Indeed, the pH environment in their environment is even lower than that imposed in the high CO2 treatment in our experiment. Shall the organisms be vulnerable to high CO2 they would have been already been sieved from the community and would not occur in this ecosystem. Indeed, the prevalence of high CO2 in coastal waters (e.g., Borges, 2005) suggest that the use of CO2 levels close to present atmospheric equilibrium as HO2LCO2 (cf. Hendriks et al., 2010) may not represent ambient conditions in many coastal ecosystems (Duarte et al., 2013), possibly confounding the interpretation of results. We suggest that the variability in responses to OA and hypoxia experiments (cf. Vaquer-Sunyer and Duarte, 2008; Kroeker et al., 2013, respectively) should be re-examined in terms of the conditions experienced in situ by the population from which the individuals were derived.

Figure 6. The experimental range of variables from this experiment compared to the ecosystems ambient range of oxygen and pH from the study sites in central and southern Chile during November 2009 and January 2010 (Ramajo et al., 2013).
The fact that the ecosystem supports healthy populations of these invertebrate species despite regular upwelling events already suggests that they must be relatively resistant to at least short term exposure to these conditions. Indeed, exposure to such extreme conditions during upwelling events is typically in the order of 3–7 days (Narváez et al., 2004), the time scale to evaluate responses used here. That the previous history of exposure to the stressors affects the resistance of the organisms was shown experimentally by Brady and Targett (2013), who showed that previous diel-cycle hypoxia lowers the avoidance threshold from <2.8 mg O2 L−1 (in saturation-acclimated fish) to ~1.4 mg O2 L−1 (in diel-cycling hypoxia acclimated fish) in the juvenile weakfish Cynoscion regalisthey, showing that they become more resistant to hypoxia.
Whereas hypoxia and high CO2 are expected to co-occur in nature (Brewer and Peltzer, 2009; Mayol et al., 2012), the responses of marine organisms to these stressors has been largely studied in isolation where either hypoxia (Vaquer-Sunyer et al., 2012) or high CO2 (Doney et al., 2009; Hendriks et al., 2010; Kroeker et al., 2013) are tested. High CO2 and hypoxia in the environment, affect the metabolic rates as they lead to a shift in the steady state acid-base equilibrium (Pörtner and Grieshaber, 1993; Pörtner and Heisler, 1998; Pörtner et al., 2005). The combination of hypoxia and increasing CO2 reduces the rates of relevant trans-membrane ion exchange (Pörtner et al., 2000) and causes a synergistic metabolic depression via the effect of adenosine on central nervous functions if anoxia occurs (Reipschläger et al., 1997). Nevertheless, the examination of the responses to combined hypoxia and high CO2 is based on a limited set of studies thus far. Kim et al. (2013) exposed juvenile abalone (Haliotis rufescens) to short term (3–6 h to 24 h) hypoxia and low pH and found that hypoxia had the greater influence on mortality (pH 7.5 vs. 8.0), but growth was lowest when both stressors where combined. Frieder et al. (2014) showed that low O2 in combination with low pH did not affect the development and size of 2 mytilid mussels from the Scripps Institution of Oceanography pier (Mytilus californianus) and San Diego Bay (M. galloprovincialis), USA. Gobler et al. (2014) reported that the bay scallop, Argopecten irradians, showed additive responses on survivorship, growth and metamorphosis to low O2 in combination with low pH, consistent with our findings. However, Gobler et al. (2014) reported that the later stages of the hard clam Mercenaria mercenaria were resistant to hypoxia or acidification separately but experienced significantly reduced growth rates when exposed to both conditions simultaneously. This indicates that responses to hypoxia, high CO2 and their combined effects might be species specific.
The additive nature of the effects of hypoxia and high CO2 lends weight to the use of the Respiration Index, RI, to reflect their combined stress on metabolic processes. Whereas the merit of the RI has been challenged recently (Seibel and Childress, 2013) no experimental test had been reported to date. Our results show that metabolic rates decline with decreasing RI, as expected (Brewer and Peltzer, 2009), confirming that the RI holds power as a predictor of effects, separate or combined, of hypoxia and high CO2 on metabolic rates. However, our results also support the criticisms of Seibel and Childress (2013) to the predictive power of the thresholds proposed by Brewer and Peltzer (2009). The lowest RI we reached in our experiment was 0.81 ± 0.06, reached in the LO2HCO2 treatment. This is within the range of 0.7–1.0 where Brewer and Peltzer (2009) propose that aerobic respiration must be severely compromised. Yet, we observed little or no mortality, suggesting that the RI thresholds for marine invertebrates are well below those postulated by Brewer and Peltzer (2009). The test provided here is, to the best of our knowledge, the first experimental test, and more tests are required to confirm the merit of the RI index and to establish reliable thresholds for marine organisms. Moreover, in future studies measurement of calcification rates would be a good addition to assemble more data on the effects of future scenarios on marine invertebrates.
In summary, marine invertebrates inhabiting the upwelling ecosystems of the Chilean coast show additive but negative responses to hypoxia and high CO2 and are relatively resistant to the combined effects of these stressors. We suggest that responses to the combined effects of hypoxia and high CO2 are likely to be dependent on the conditions previously experienced by marine invertebrate populations and that organisms in upwelling-affected areas, such as those along the Chilean coast, are likely adapted, at least to brief exposures, to the occurrence of both stressors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research is a contribution to ASSEMBLE (grant agreement no. 227799; funded by the European Community: Research Infrastructure Action under the FP7 “Capacities” Specific Program), ESTRESX project funded by the Spanish Ministry of Economy and Innovation (ref. CTM2012-32603), LINCGlobal (funded by The Spanish National Research Council (CSIC) and The Pontificia Universidad Católica de Chile (PUC) to facilitate interaction between Latin American and Spanish researchers in the field of global change). AS was funded by a fellowship from the Government of the Balearic Islands (Department on Education, Culture and Universities) and the EU (European Social Fund), LR was funded by a fellowship from the Government of Chile (CONICYT, Becas Chile Program) and IH was funded by a JAE-DOC fellowship from the Spanish Government. NAL acknowledged funds by Fondecyt 1090624 during the experimental phase and the Millennium Nucleus Center for the Study of Multiple-drivers on Marine Socio-Ecological Systems (MUSELS) by MINECON Project NC120086 also supported this work during the final stages. We thank Sylvain Faugeron and Ricardo Calderón for their help and support.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2015.00049
Andersson, A. J., and Gledhill, D. (2013). Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Annu. Rev. Mar. Sci. 5, 321–348. doi: 10.1146/annurev-marine-121211-172241
Bijma, J. (1991). “Lunar pulses of carbonate output by spinose planktonic Foraminifera,” in Protozoa and Their Role in Marine Processes, eds P. C. Reid, C. M. Turley, and P. H. Burkill (Plymouth: Elsevier), 353–354.
Borges, A. V (2005). Do we have enough pieces of the jigsaw to integrate CO2 fluxes in the coastal ocean? Estuaries 28, 3–27. doi: 10.1007/BF02732750
Borges, A. V, and Gypens, N. (2010). Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification. Limnol. Oceanogr. 55, 346–353. doi: 10.4319/lo.2010.55.1.0346
Brady, D., and Targett, T. (2013). Movement of juvenile weakfish Cynoscion regalis and spot Leiostomus xanthurus in relation to diel-cycling hypoxia in an estuarine tidal tributary. Mar. Ecol. Prog. Ser. 491, 199–219. doi: 10.3354/meps10466
Brewer, P. G., and Peltzer, E. T. (2009). Limits to marine life. Science 324, 347–348. doi: 10.1126/science.1170756
Burgents, J. E., Burnett, K. G., and Burnett, L. E. (2005). Effects of hypoxia and hypercapnic hypoxia on the localization and the elimination of Vibrio campbellii in Litopenaeus vannamei, the Pacific white shrimp. Biol. Bull. 208, 159–168. doi: 10.2307/3593148
Cai, W.-J. (2011). Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Ann. Rev. Mar. Sci. 3, 123–145. doi: 10.1146/annurev-marine-120709-142723
Caldeira, K. (2005). Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. 110, 1–12. doi: 10.1029/2004jc002671
Caldeira, K., and Wickett, M. E. (2003). Anthropogenic carbon and ocean pH. Nature 425, 365. doi: 10.1038/425365a
Cameron, J. N., and Iwama, G. K. (1989). Compromises between ionic regulation and acid–base regulation in aquatic animals. Can. J. Zool. 67, 3078–3084. doi: 10.1139/z89-432
Cohen, A. L., McCorkle, D. C., de Putron, S., Gaetani, G. A., and Rose, K. A. (2009). Morphological and compositional changes in skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification. Geochem. Geophys. Geosyst. 10, 1e12. doi: 10.1029/2009gc002411
Conley, D. J., Carstensen, J., Vaquer-Sunyer, R., and Duarte, C. M. (2009). Ecosystem thresholds with hypoxia. Hydrobiologia 629, 21–29. doi: 10.1007/s10750-009-9764-2
Diaz, R. J., and Rosenberg, R. (1995). Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 33, 245–303.
Dickson, A. (1990). Thermodynamics of the dissociation of boric-acid in potassium-chloride solutions form 273.15 K to 318.15 K. J. Chem. Thermodyn. 22, 113–127. doi: 10.1016/0021-9614(90)90074-Z
Dickson, A. G., Sabine, C. L., and Christian, J. R. (eds.). (2007). Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication 3. Sidney, BC: PICES.
Dickson, A., and Millero, F. (1987). A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep. Res. 34, 1733–1743. doi: 10.1016/0198-0149(87)90021-5
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/annurev.marine.010908.163834
Duarte, C. M., Hendriks, I. E., Moore, T. S., Olsen, Y. S., Steckbauer, A., Ramajo, L., et al. (2013). Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36, 221–236. doi: 10.1007/s12237-013-9594-3
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. J. Mar. Sci. 65, 414–432. doi: 10.1093/icesjms/fsn048
Feely, R. A., Sabine, C. L., Hernandez-Ayon, J. M., Ianson, D., and Hales, B. (2008). Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320, 1490–1492. doi: 10.1126/science.1155676
Frieder, C. A., Gonzalez, J. P., Bockmon, E. E., Navarro, M. O., and Levin, L. A. (2014). Can variable pH and low oxygen moderate ocean acidification outcomes for mussel larvae? Glob. Chang. Biol. 20, 754–764. doi: 10.1111/gcb.12485
Gattuso, J. P., and Buddemeier, R. W. (2000). Ocean biogeochemistry: calcification and CO2. Nature 407, 311–313. doi: 10.1038/35030280
Gattuso, J.-P., Frankignoulle, M., Bourge, I., Romaine, S., and Buddemeier, R. W. (1998). Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet. Change 18, 37e46. doi: 10.1016/s0921-8181(98)00035-6
Gattuso, J.-P., and Jaubert, J. (1990). Effect of light on oxygen and carbon dioxide fluxes and on metabolic quotients measured in situ in a zooxanthellate coral. Limnol. Oceanogr. 35, 1796e1804. doi: 10.4319/lo.1990.35.8.1796
Gazeau, F., Quiblier, C., Jansen, J. M., Gattuso, J.-P., Middelburg, J. J., and Heip, C. H. R. (2007). Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett. 34, 1–5. doi: 10.1029/2006GL028554
Gobler, C. J., DePasquale, E. L., Griffith, A. W., and Baumann, H. (2014). Hypoxia and acidification have additive and synergistic negative effects on the growth, survival, and metamorphosis of early life stage bivalves. PLoS ONE 9:e83648. doi: 10.1371/journal.pone.0083648
Gray, J. S., Wu, R. S., and Or, Y. Y. (2002). Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 238, 249–279. doi: 10.3354/meps238249
Gruber, N., Hauri, C., Lachkar, Z., Loher, D., Frölicher, T. L., and Plattner, G.-K. (2012). Rapid progression of ocean acidification in the California Current System. Science 337, 220–223. doi: 10.1126/science.1216773
Gurevitch, J., and Hedges, L. V (1999). Statistical issues in ecological meta-analyses. Ecology 80, 1142–1149. doi: 10.1890/0012-9658(1999)080[1142:SIIEMA]2.0.CO;2
Gutierrez, J. L., Jones, C. G., Byers, J. E., Arkema, K. K., Berkenbusch, K., Commito, J. A., et al. (2011). “Physical ecosystem engineers and the functioning of estuaries and coasts,” in Treatise on Estuarine and Coastal Science, Vol. 7, eds E.Wolanski and D. S McLusky, (Waltham, MA: Academic Press), 53e81.
Hagens, M., Slomp, C. P., Meysman, F. J. R., Seitaj, D., Harlay, J., Borges, A. V., et al. (2015). Biogeochemical processes and buffering capacity concurrently affect acidification in a seasonally hypoxic coastal marine basin. Biogeosciences 12, 1561–1583. doi: 10.5194/bg-12-1561-2015
Hedges, L. V, Gurevitch, J., Curtis, P. S., and Jun, N. (1999). The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. doi: 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2
Hendriks, I. E., Duarte, C. M., and Álvarez, M. (2010). Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Estuar. Coast. Shelf Sci. 86, 157–164. doi: 10.1016/j.ecss.2009.11.022
Hendriks, I. E., Duarte, C. M., Olsen, Y. S., Steckbauer, A., Ramajo, L., Moore, T. S., et al. (2015). Biological mechanisms supporting adaptation to ocean acidification in coastal ecosystems. Estuar. Coast. Shelf Sci. 152, A1–A8. doi: 10.1016/j.ecss.2014.07.019
Hypes, S. R. (1999). “Sub-lethal effects of hypoxia/hypercapnia on Callinectes sapidus in the York River Estuary,” Theses and Dissertations Paper (Virginia, VA).
Kim, T. W., Barry, J. P., and Micheli, F. (2013). The effects of intermittent exposure to low pH and oxygen conditions on survival and growth of juvenile red abalone. Biogeosciences Discuss. 10, 3559–3576. doi: 10.5194/bgd-10-3559-2013
Kleypas, J., Feely, R., Fabry, V., Langdon, C., Sabine, C., and Robbins, L. (2006). “Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research,” in Report of a Workshop Held 18–20 April 2005. 2006, NSF, NOAA, and the U.S. Geological Survey (St. Petersburg, FL), 88.
Köhler-Rink, S., and Kühl, M. (2005). The chemical microenvironment of the symbiotic planktonic foraminifer Orbulina universa. Mar. Biol. Res. 1, 68–78. doi: 10.1080/17451000510019015
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., et al. (2013). Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 19, 1884–1896. doi: 10.1111/gcb.12179
Kroeker, K. J., Kordas, R. L., Crim, R. N., and Singh, G. G. (2010). Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x
Kühn, S. F., and Raven, J. A. (2008). Photosynthetic oscillation in individual cells of the marine diatom Coscinodiscus wailesii (Bacillariophyceae) revealed by microsensor measurements. Photosynt. Res. 95, 37e44. doi: 10.1007/s11120-007-9221-x
Lampert, W. (1984). “The measurement of respiration,” in A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters, eds J. A. Downing and F. H. Rigler (Oxford: Blackwell Scientific), 413–468.
Langdon, C., Broecker, W. S., Hammond, D. E., Glenn, E., Fitzsimmons, K., et al. (2003). Effect of elevated CO2 on the community metabolism of an experimental coral reef. Glob. Biogeochem. Cycles 17, 1011. doi: 10.1029/2002GB001941
Langdon, C., Takahashi, T., Sweeney, C., Chipman, D., Goddard, J., et al. (2000). Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles 14, 639–654. doi: 10.1029/1999GB001195
Lardies, M. A., Belén, M., Josefina, M., and Bacigalupe, L. D. (2014). Heritability of hsp 70 expression in the beetle Tenebrio molitor?: ontogenetic and environmental effects. J. Insect Physiol. 67, 70–75. doi: 10.1016/j.jinsphys.2014.06.005
Leclercq, N., Gattuso, J. P., and Jaubert, J. (2000). CO2 partial pressure controls the calcification rate of a coral community. Glob. Chang. Biol. 6, 329–334. doi: 10.1046/j.1365-2486.2000.00315.x
Levin, L., Gutierrez, D., Rathburn, A., Neira, C., Sellanes, J., Munoz, P., et al. (2002). Benthic processes on the Peru margin: a transect across the oxygen minimum zone during the 1997-98 El Nino. Prog. Oceanogr. 53, 1–27. doi: 10.1016/S0079-6611(02)00022-8
Marubini, F., and Atkinson, M. J. (1999). Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser. 188, 117–121. doi: 10.3354/meps188117
Marubini, F., and Davies, P. S. (1996). Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar. Biol. 127, 319–328. doi: 10.1007/BF00942117
Mayol, E., Ruiz-Halpern, S., Duarte, C. M., Castilla, J. C., and Pelegri, J. L. (2012). Coupled CO2 and O2-driven compromises to marine life in summer along the Chilean sector of the Humboldt Current System. Biogeosciences 9, 1183–1194. doi: 10.5194/bg-9-1183-2012
Mehrbach, C., Culberson, C., Hawley, J., and Pytkowicz, R. (1973). Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907. doi: 10.4319/lo.1973.18.6.0897
Melzner, F., Stange, P., Trübenbach, K., Thomsen, J., Casties, I., Panknin, U., et al. (2011). Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PLoS ONE 6:e24223. doi: 10.1371/journal.pone.0024223
Muscatine, L. (1990). “The role of symbiotic algae in carbon and energy flux in reef corals,” in Ecosystems of the World, Coral Reefs, Vol. 25, ed Z. Dubinsky (Amsterdam: Elsevier Science Publishing Company, Inc), 550.
Narváez, D. A., Poulin, E., Leiva, G., Hernández, E., Castilla, J. C., and Navarrete, S. A. (2004). Seasonal and spatial variation of nearshore hydrographic conditions in central Chile. Cont. Shelf Res. 24, 279–292. doi: 10.1016/j.csr.2003.09.008
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., et al. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686. doi: 10.1038/nature04095
Pierrot, D., Lewis, E., and Wallace, D. W. R. (2006). MS Excel Program Developed for CO2 System Calculations. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy.
Porter, S. M. (2007). Seawater chemistry and early carbonate biomineralization. Science 316, 1302. doi: 10.1126/science.1137284
Pörtner, H. O. A. R., and Heisler, N. (1998). Acid-base regulation, metabolism and energetics in sipunculus nudus as a function of ambient carbon dioxide level. J. Exp. Biol. 201(Pt 1), 43–55.
Pörtner, H. O., Bock, C., and Reipschläger, A. (2000). Modulation of the cost of pHi regulation during metabolic depression: a 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J. Exp. Biol. 203, 2417–2428.
Pörtner, H. O., and Grieshaber, M. K. (1993). “Critical PO2(s) in oxyconforming and oxyregulating animals: gas exchange, metabolic rate and the mode of energy production,” in The vertebrate Gas Transport Cascade Adaptations to Environment and Mode of Life, ed J. E. P. W. Bicudo (Boca Raton, FL: CRC Press), 330–357.
Pörtner, H. O., Langenbuch, M., and Michaelidis, B. (2005). Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: from Earth history to global change. J. Geophys. Res. 110, C09S10. doi: 10.1029/2004JC002561
Raabe, D., Sachs, C., and Romano, P. (2005). The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater 53, 4281–4292. doi: 10.1016/j.actamat.2005.05.027
Ramajo, L., Baltanás, Á., Torres, R., Manríquez, P. H., Rodriguez-Navarro, A., and Lagos, N. A. (2013). Geographical variation in shell morphology of juvenile snails (Concholepas concholepas) along the physical–chemical gradient of the Chilean coast. J. Mar. Biol. Assoc. U.K. 93, 2167–2176. doi: 10.1017/S0025315413000891
Range, P., Chícharo, M. A., Ben-Hamadou, R., Piló, D., Matias, D., Joaquim, S., et al. (2011). Calcification, growth and mortality of juvenile clams Ruditapes decussatus under increased pCO2 and reduced pH: variable responses to ocean acidification at local scales? J. Exp. Mar. Bio. Ecol. 396, 177–184. doi: 10.1016/j.jembe.2010.10.020
Raven, J. (2005). Ocean Acidification due to Increasing Atmospheric Carbon Dioxide. London: The Royal Society of London.
Reipschläger, A., Nilsson, G. E., and Pörtner, H. O. (1997). A role for adenosine in metabolic depression in the marine invertebrate Sipunculus nudus. Am. J. Physiol. 272, R350–R356.
Riebesell, U., Zondervan, I., Rost, B., Tortell, P. D., Zeebe, R. E., and Morel, F. M. (2000). Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407, 364–367. doi: 10.1038/35030078
Riedel, B., Pados, T., Pretterebner, K., Schiemer, L., Steckbauer, A., Haselmair, A., et al. (2013). Effect of hypoxia and anoxia on invertebrate behaviour: ecological perspectives from species to community level. Biogeosciences Discuss. 10, 14333–14438. doi: 10.5194/bgd-10-14333-2013
Ries, J. (2011). A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 75, 4053–4064. doi: 10.1016/j.gca.2011.04.025
Ries, J., Cohen, A., and McCorkle, D. (2009). Marine calcifiers exhibit mixed responses to CO2 -induced ocean acidification. Geology 37, 1131–1134. doi: 10.1130/G30210A.1
Rodolfo-Metalpa, R., Houlbrèque, F., Tambutté, É., Boisson, F., Baggini, C., Patti, F. P., et al. (2011). Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312. doi: 10.1038/nclimate1200
Rodolfo-Metalpa, R., Lombardi, C., Cocito, S., Hall-Spencer, J. M., and Gambi, M. C. (2010). Effects of ocean acidification and high temperatures on the bryozoan Myriapora truncata at natural CO2 vents. Mar. Ecol. 31, 447–456. doi: 10.1111/j.1439-0485.2009.00354.x
Seibel, B. A., and Childress, J. J. (2013). The real limits to marine life: a further critique of the Respiration Index. Biogeosciences 10, 2815–2819. doi: 10.5194/bg-10-2815-2013
Steckbauer, A., Duarte, C. M., Carstensen, J., Vaquer-Sunyer, R., and Conley, D. J. (2011). Ecosystem impacts of hypoxia: thresholds of hypoxia and pathways to recovery. Environ. Res. Lett. 6:025003. doi: 10.1088/1748-9326/6/2/025003
Thiel, M., Macaya, E. C., Acuna, E., Arntz, W. E., Bastias, H., Brokordt, K., et al. (2007). The Humboldt Current System of northern and central Chile: oceanographic processes, ecological interactions and socioeconomic feedback. Ocean. Mar. Biol 45, 195–344. doi: 10.1201/9781420050943.ch6
Thomsen, J., Casties, I., Pansch, C., Körtzinger, A., and Melzner, F. (2013). Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob. Change Biol. 19, 1017–1027. doi: 10.1111/gcb.12109
Torres, R., Pantoja, S., Harada, N., González, H. E., Daneri, G., Frangopulos, M., et al. (2011). Air-sea CO2 fluxes along the coast of Chile: From CO2 outgassing in central northern upwelling waters to CO2 uptake in southern Patagonian fjords. J. Geophys. Res. 116, C09006. doi: 10.1029/2010JC006344
Torres, R., Turner, D., Rutllant, J., Sobarzo, M., Antezana, T., and Gonzalez, H. E. (2002). CO2 outgassing off central Chile (31–30°S) and northern Chile (24–23°S) during austral summer 1997: the effect of wind intensity on the upwelling and venti- lation of CO2-rich waters. Deep. Res. Pt. I 49, 1413–1429. doi: 10.1016/S0967-0637(02)00034-1
Trotter, J. A., Montagna, P., McCulloch, M. T., Silenzi, S., Reynaud, S., Mortimer, G., et al. (2011). Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 303, 163e173. doi: 10.1016/j.epsl.2011.01.030
Ulloa, O., and Pantoja, S. (2009). The oxygen minimum zone of the eastern South Pacific. Deep Sea Res. Part II Top. Stud. Oceanogr. 56, 987–991. doi: 10.1016/j.dsr2.2008.12.004
Vaquer-Sunyer, R., and Duarte, C. M. (2008). Thresholds of hypoxia for marine biodiversity. PNAS 105, 15452–15457. doi: 10.1073/pnas.0803833105
Vaquer-Sunyer, R., Duarte, C. M., Jordà, G., and Ruiz-Halpern, S. (2012). Temperature dependence of oxygen dynamics and community metabolism in a shallow mediterranean macroalgal meadow (Caulerpa prolifera). Estuaries Coasts 35, 1182–1192. doi: 10.1007/s12237-012-9514-y
Waldbusser, G. G., and Salisbury, J. E. (2014). Ocean acidification in the coastal zone from an organism's perspective: multiple system parameters, frequency domains, and habitats. Ann. Rev. Mar. Sci. 6, 221–247. doi: 10.1146/annurev-marine-121211-172238
Keywords: hypoxia, ocean acidification, Chile, invertebrates, respiration rate
Citation: Steckbauer A, Ramajo L, Hendriks IE, Fernandez M, Lagos NA, Prado L and Duarte CM (2015) Synergistic effects of hypoxia and increasing CO2 on benthic invertebrates of the central Chilean coast. Front. Mar. Sci. 2:49. doi: 10.3389/fmars.2015.00049
Received: 31 March 2015; Accepted: 26 June 2015;
Published: 10 July 2015.
Edited by:
Elvira S. Poloczanska, Commonwealth Scientific and Industrial Research Organisation, AustraliaReviewed by:
Jason Michael Hall-Spencer, Plymouth University, UKCopyright © 2015 Steckbauer, Ramajo, Hendriks, Fernandez, Lagos, Prado and Duarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra Steckbauer, Global Change Department, Instituto Mediterráneo de Estudios Avanzados IMEDEA (CSIC-UIB), C/ Miquel Marqués 21, Esporles, Balearic Islands, 07190, Spain,YXN0ZWNrYmF1ZXJAaW1lZGVhLnVpYi1jc2ljLmVz;c3RlY2tiYXVlci5vY2VhbkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.